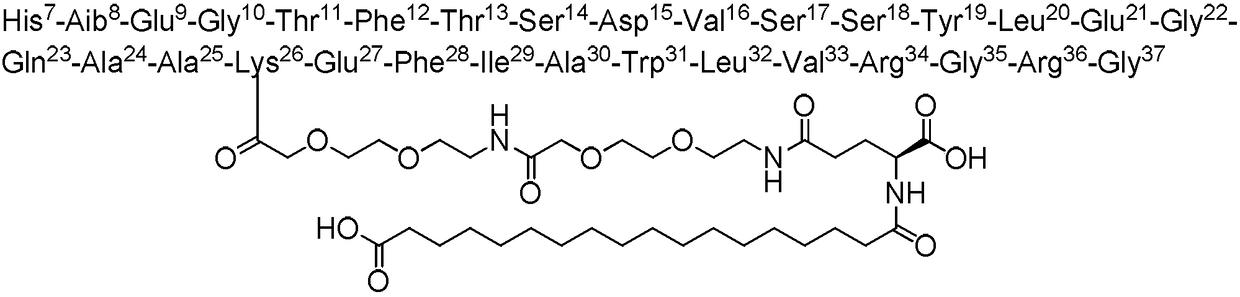
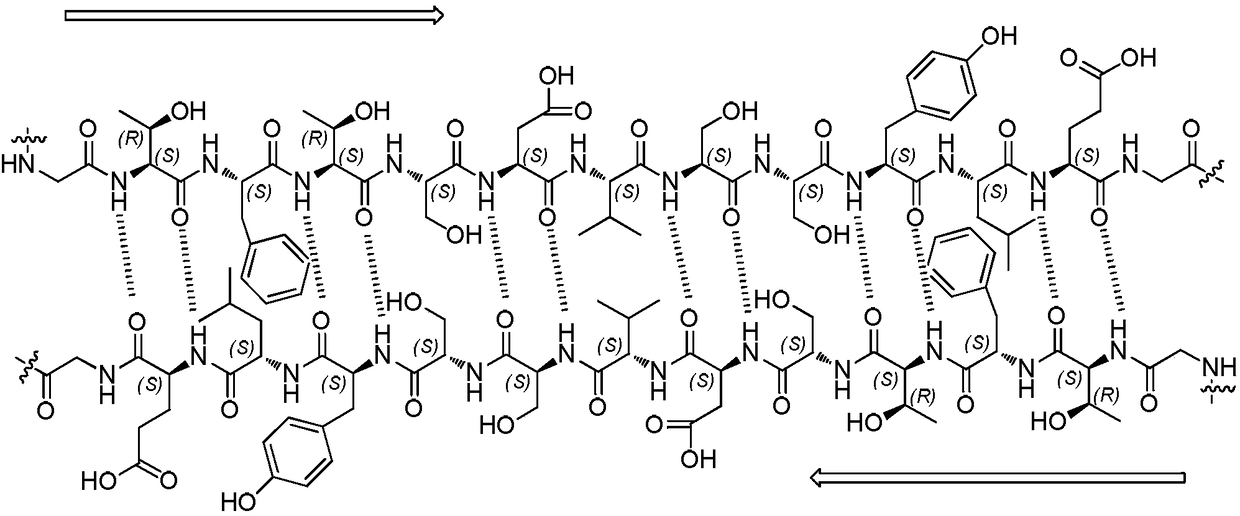
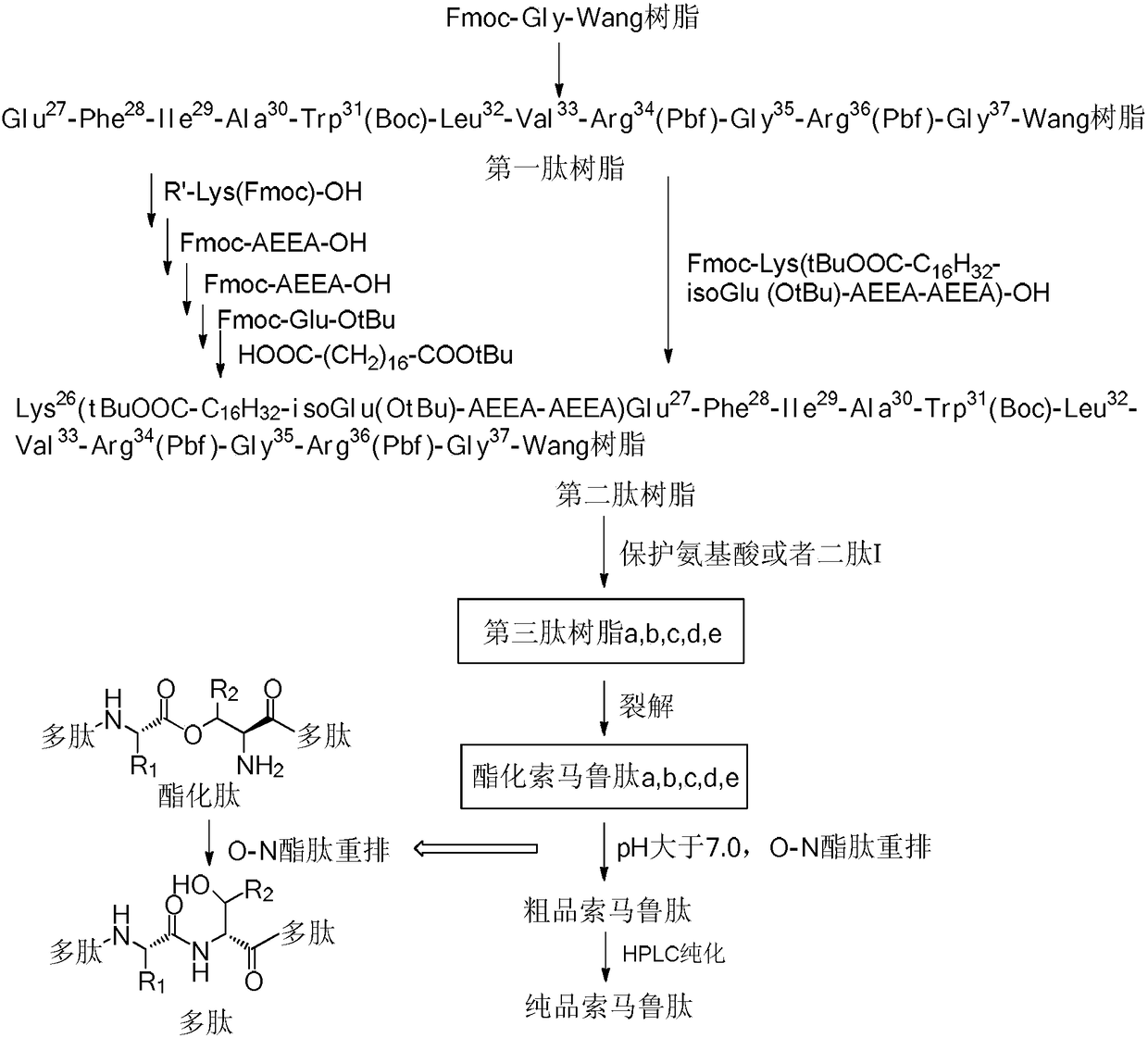
Method used for preparing semaglutide
A peptide resin and resin technology, applied in the field of synthesizing semaglutide, can solve the problems of large steric hindrance, difficulty in synthesizing amino acids and dipeptides, and increasing the difficulty of coupling, achieve efficient coupling reaction, avoid polycondensation problems, reduce Effects of Synthetic Difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Fmoc-Gly 37 -Synthesis of Wang Resin
[0042] Weigh 150 g of Wang resin with a degree of substitution of 0.82 mmol / g, add it to a solid-phase reaction vessel, wash it three times with DMF, add DMF to swell for 30 minutes, mix 36.6 g of Fmoc-Gly-OH, 19.6 g of HOBt, and 72.0 mL of DIC was activated at low temperature for 15 minutes and added. After 5 minutes of reaction with nitrogen gas, 1.9g of DMAP was added. After 2 hours of reaction, it was washed 3 times with DMF and 3 times with DCM, and capped with 480mL of 1:1 acetic anhydride / pyridine solution. After 3 hours, wash the resin 5 times with DMF, wash it 2 times with DCM, add methanol to shrink the resin, and dry to obtain 172.8 g of Fmoc-Gly-OH Wang resin, whose substitution degree is 0.39mmol / g detected by UV spectrophotometer .
Embodiment 2
[0043] Example 2 Synthesis of the first peptide resin
[0044] Weigh 45 g of Fmoc-Gly-Wang resin with a substitution degree of 0.39 mmol / g and add it to a solid-phase reaction vessel, wash it with DMF three times, add DMF to swell for 30 minutes, add 20% piperidine DMF solution, react for half an hour, and use DMF After washing 3 times and washing 3 times with DCM, the ninhydrin detection resin turns blue. After activating Fmoc-Arg(Pbf)-OH (34.1g, 52.65mmol), HOBt (7.15g, 52.65mmol) and DIC (6.7g, 52.65mmol) in low temperature DMF for 15 minutes, they were added to the resin at room temperature After 2 hours of reaction, the ninhydrin method was used to detect that the resin was colorless, indicating that the reaction was complete. After washing 4 times with DMF, it was washed twice with DCM.
[0045] Repeat the above steps of removing Fmoc and adding corresponding amino acid coupling, and complete Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Gly-OH, Fmoc-Arg( Pbf)-OH, Fmoc-Val-OH, F...
Embodiment 3
[0046] Example 3 Using Mtt-Lys(Fmoc)-OH as raw material to synthesize the second peptide resin
[0047] Weigh Mtt-Lys(Fmoc)-OH (43.9g, 70.2mmol), DIC (8.9g, 70.2mmol), HOBt (9.5g, 70.2mmol), after activation at low temperature for 15 minutes, add to the first peptide resin After 2 hours of reaction, the resin was detected to be colorless with ninhydrin, and the reaction was completed. Wash the resin 4 times with DMF and 2 times with DCM, then add 20% piperidine DMF solution, react for half an hour, and wash with DMF After washing 3 times with DCM, the ninhydrin detection resin turns blue. Weigh Fmoc-AEEA-OH (27.0g, 70.2mmol), DIC (8.9g, 70.2mmol), HOBt (9.5g, 70.2mmol), activate at low temperature for 15 minutes, add to the peptide resin, and react for 2 hours Finally, use ninhydrin to detect that the resin is colorless, and the reaction is complete. Wash the resin 4 times with DMF and 2 times with DCM, add 20% piperidine DMF solution, react for half an hour, wash 3 times wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com