Misfolded protein sensor method in body fluids
a protein sensor and body fluid technology, applied in the field of catalytic conformational sensor methods, can solve the problems of large amount of infectious sample, large financial investment in equipment, and time-consuming, and achieve the effects of rapid and cost-effective analytical, easy detection, and immediate interpretation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
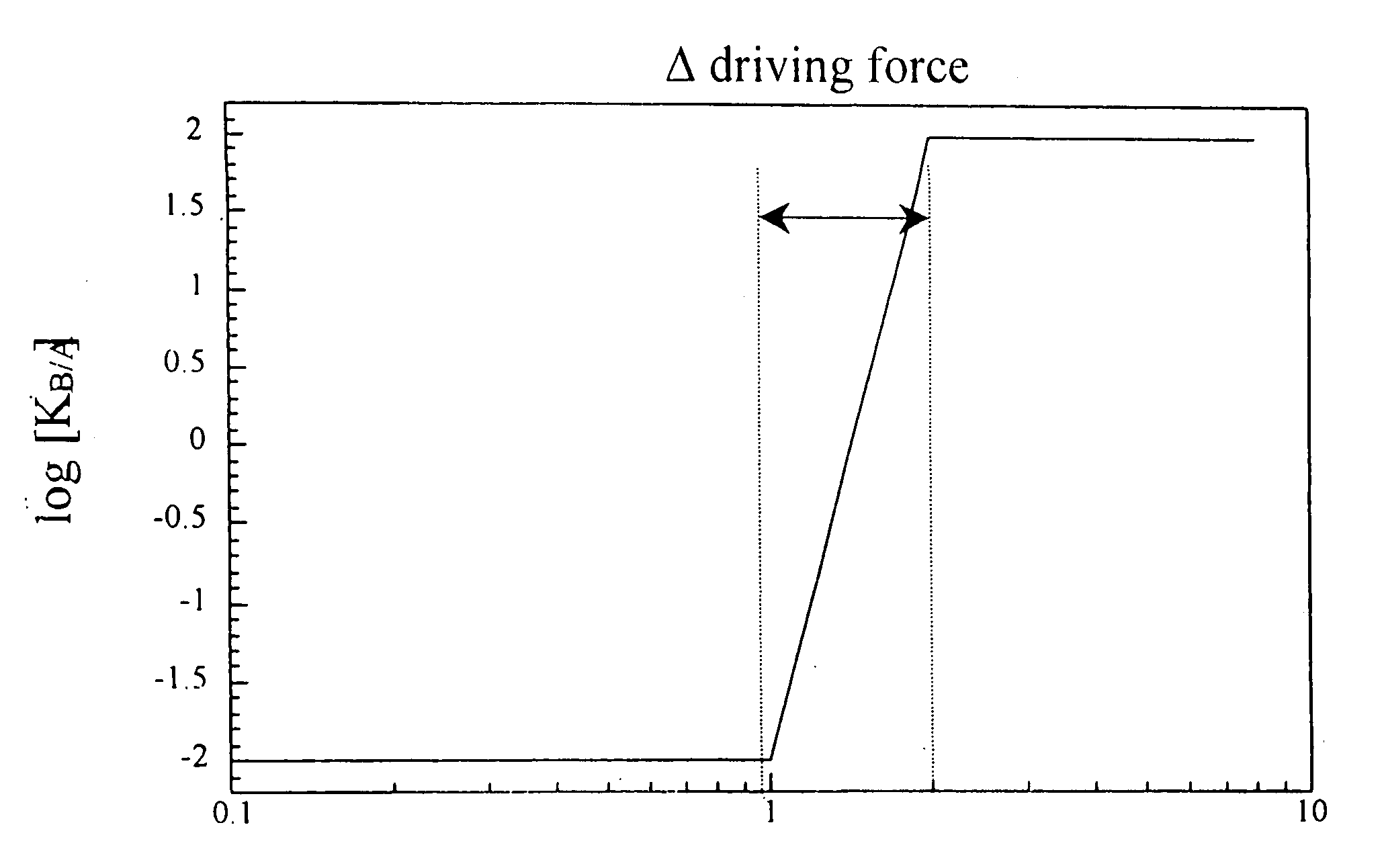
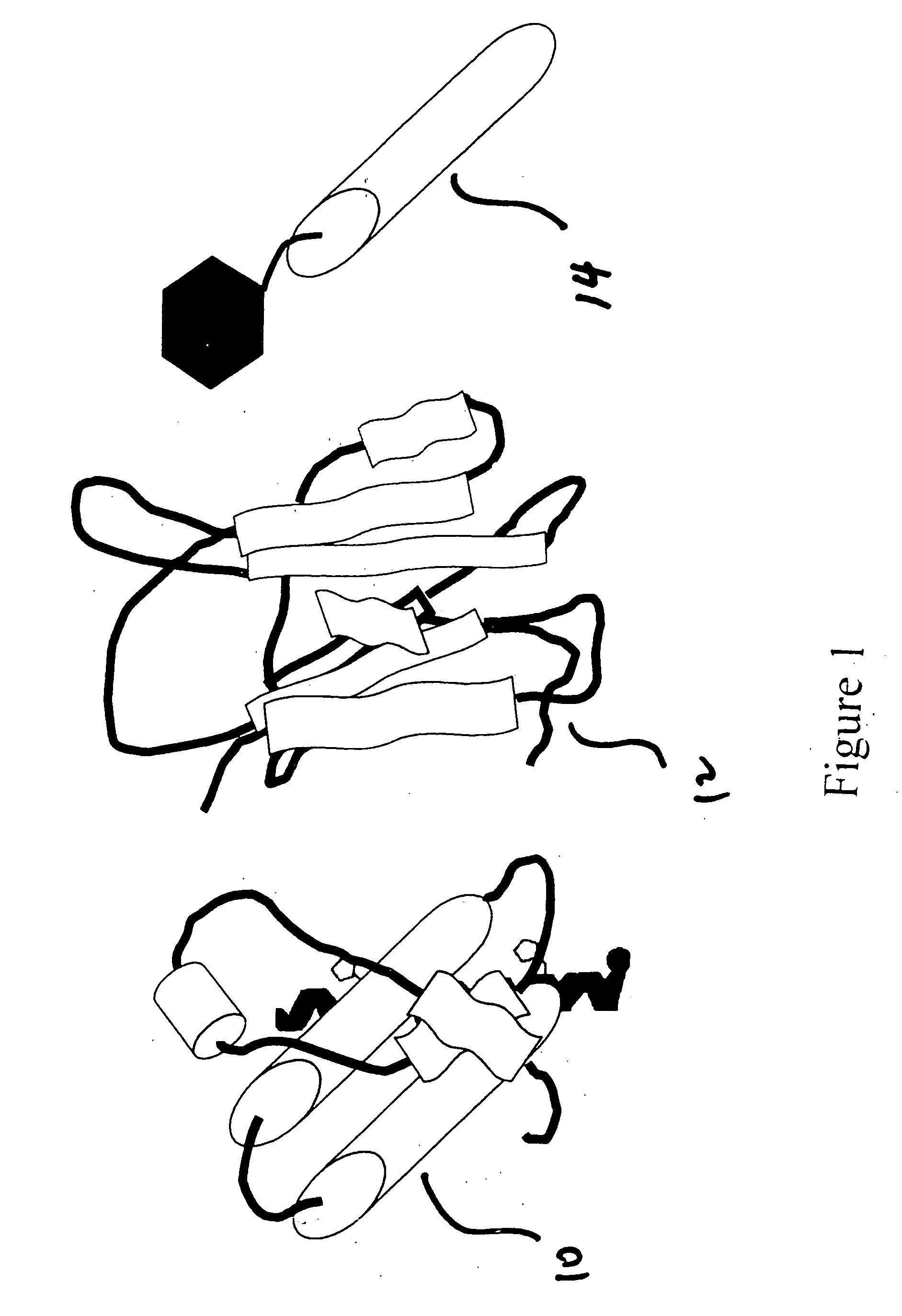
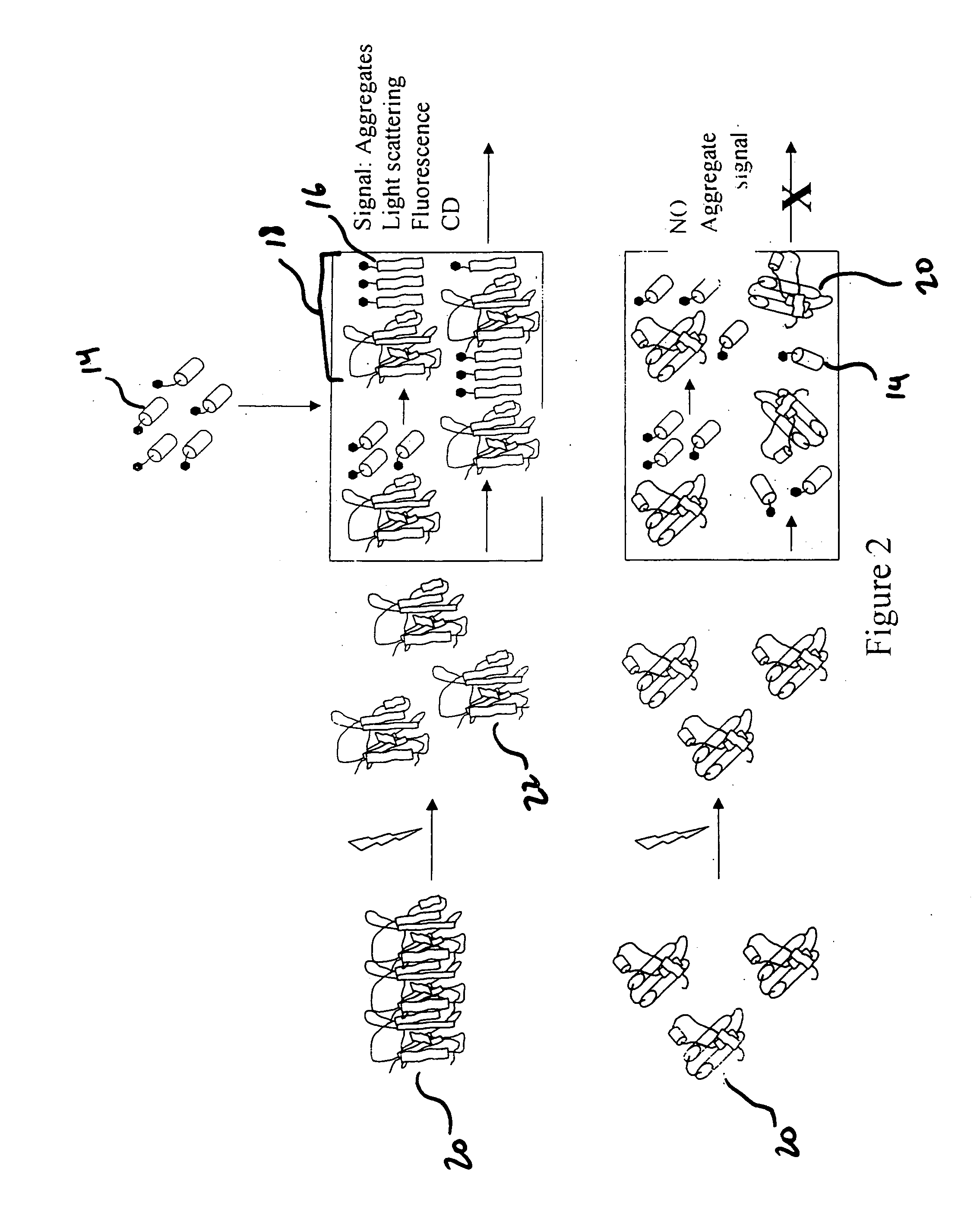
[0024] The present invention detects the presence of abnormal proteins and proteinaceous particles based on a method that utilizes catalytic propagation. Upon interaction of a sample, containing abnormal proteins or proteinaceous particles, with a peptide probe of the invention, the peptide probe undergoes conformational changes resulting in the formation of aggregates. The addition of the abnormal proteins and proteinaceous particles catalyzes the formation of the aggregates and causes further propagation of this conformational transition. The resulting aggregates are then easily detected using common analytical instrumentation and techniques.
[0025] The abnormal proteins and proteinaceous particles on which the invention focuses are proteins, protein based chemical structures such as prions and protein subunits such as peptides that are capable of conformational changes that lead to the formation of aggregates and ultimately to disease states.
[0026] These proteins and proteinaceo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| secondary structure | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
| morphology | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com