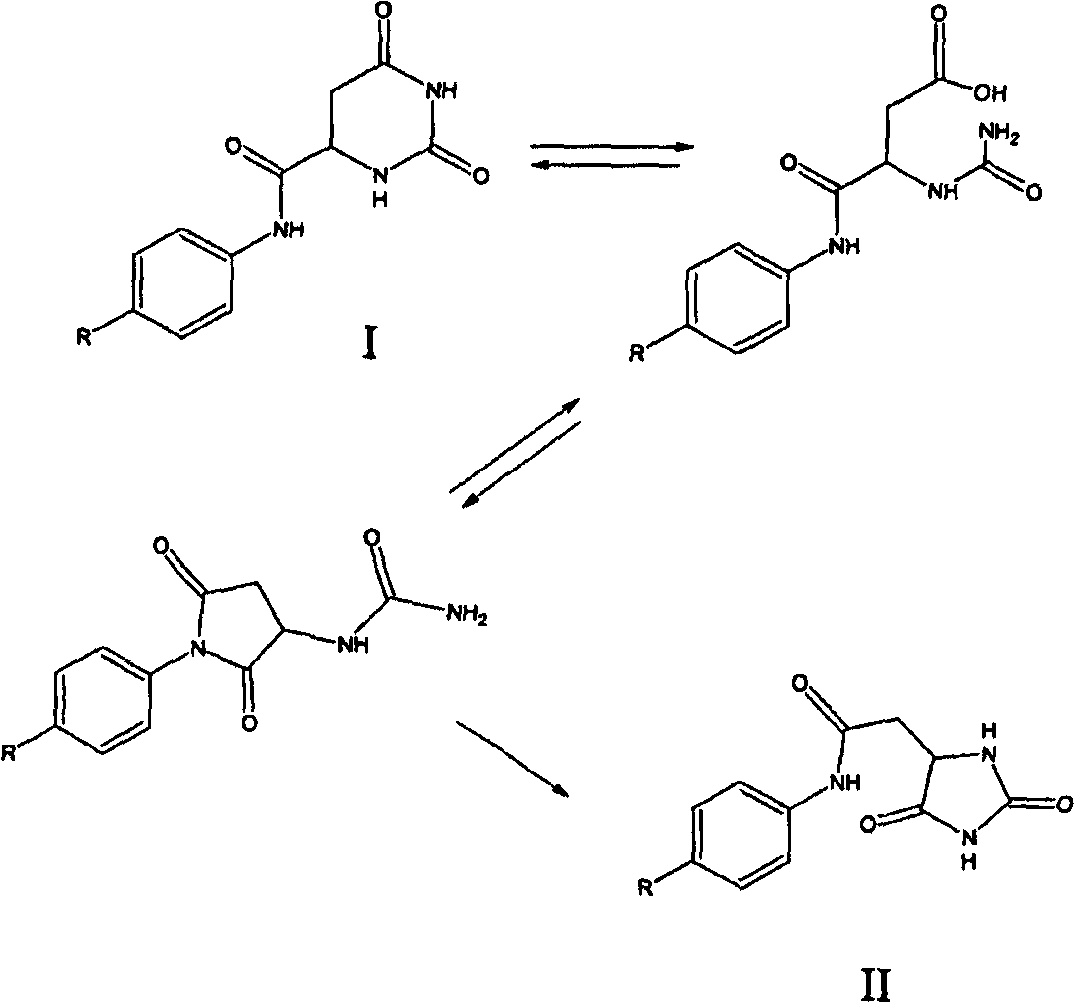
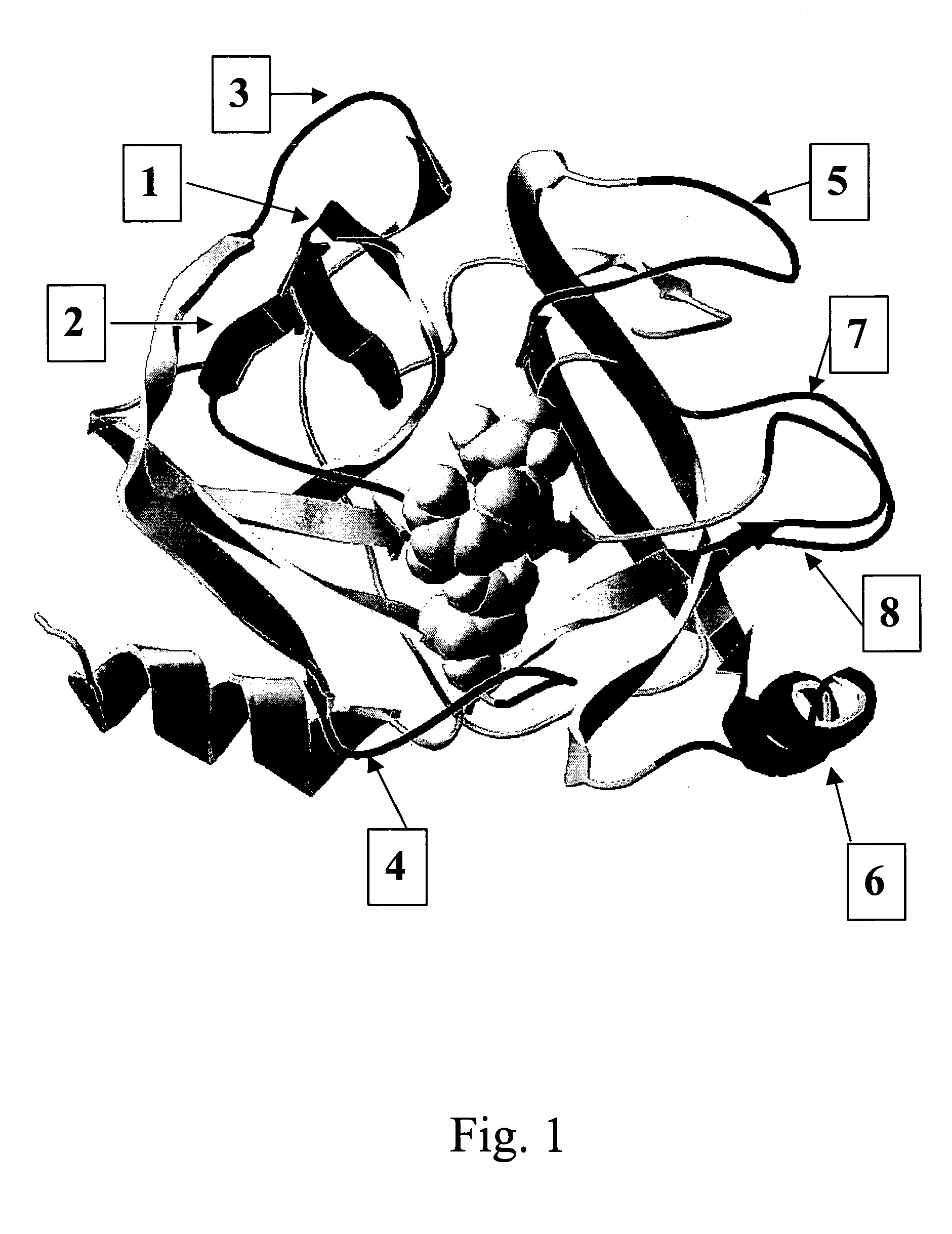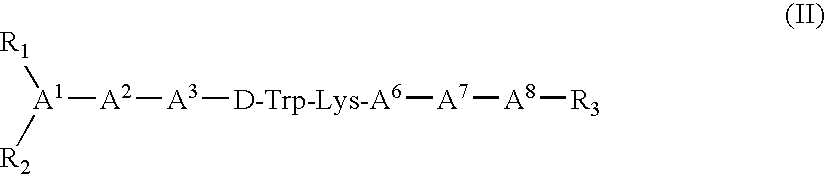Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
574 results about "Peptide bond" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino "acid" and N2 (nitrogen number two) of another along a peptide or protein chain.
Biological entities and the pharmaceutical and diagnostic use thereof
InactiveUS20050175581A1Extended half-lifeEnhanced interactionAntibacterial agentsSenses disorderDiseasePharmaceutical drug
The present invention provides method for the treatment of a disease by applying a medicament comprising a protease with a defined specificity is capable to hydrolyze specific peptide bonds within a target substrate related to such disease. The proteases with such a defined specificity can further be used for related therapeutic or diagnostic purposes.
Owner:DIREVO BIOTECH
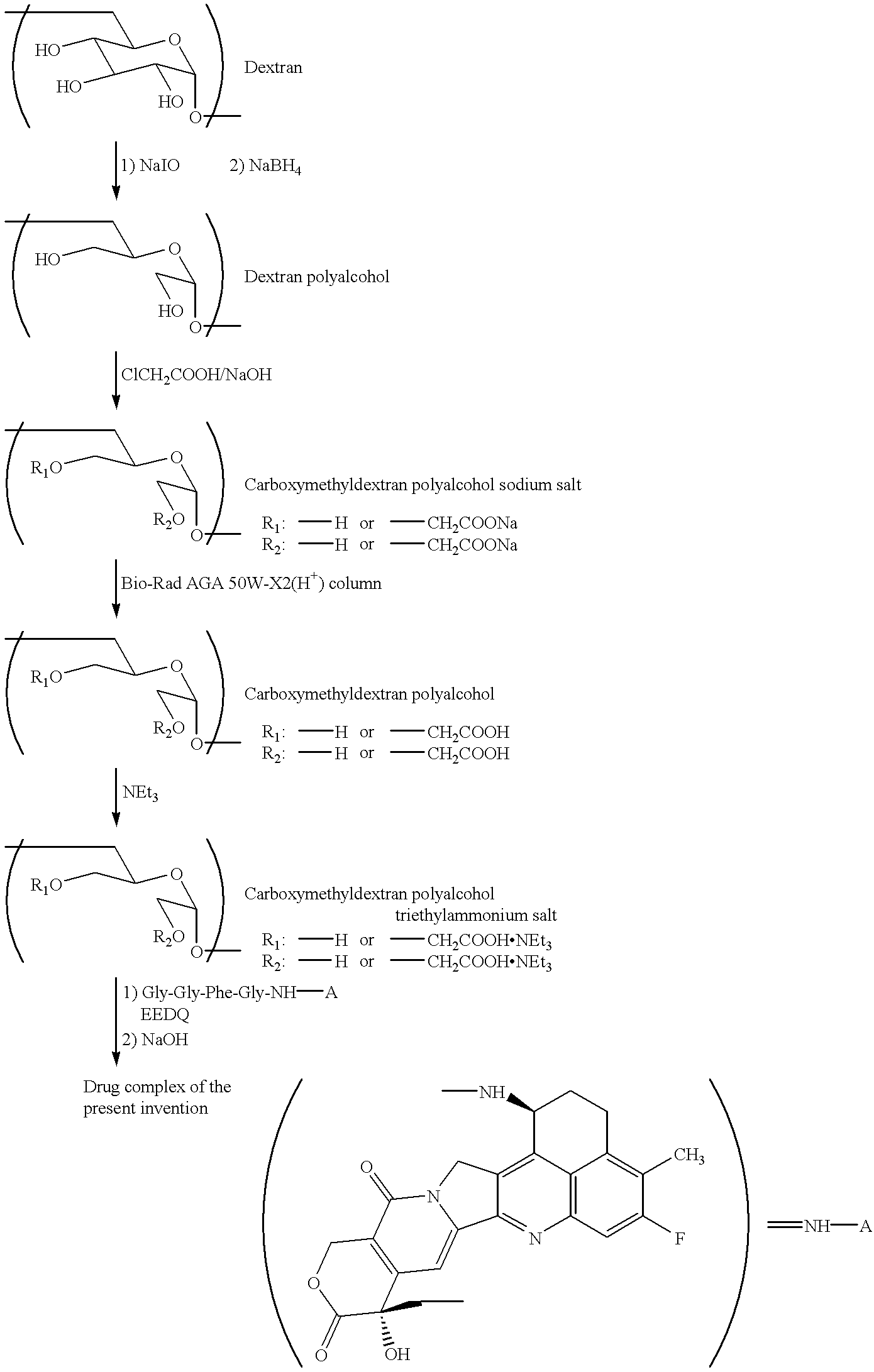
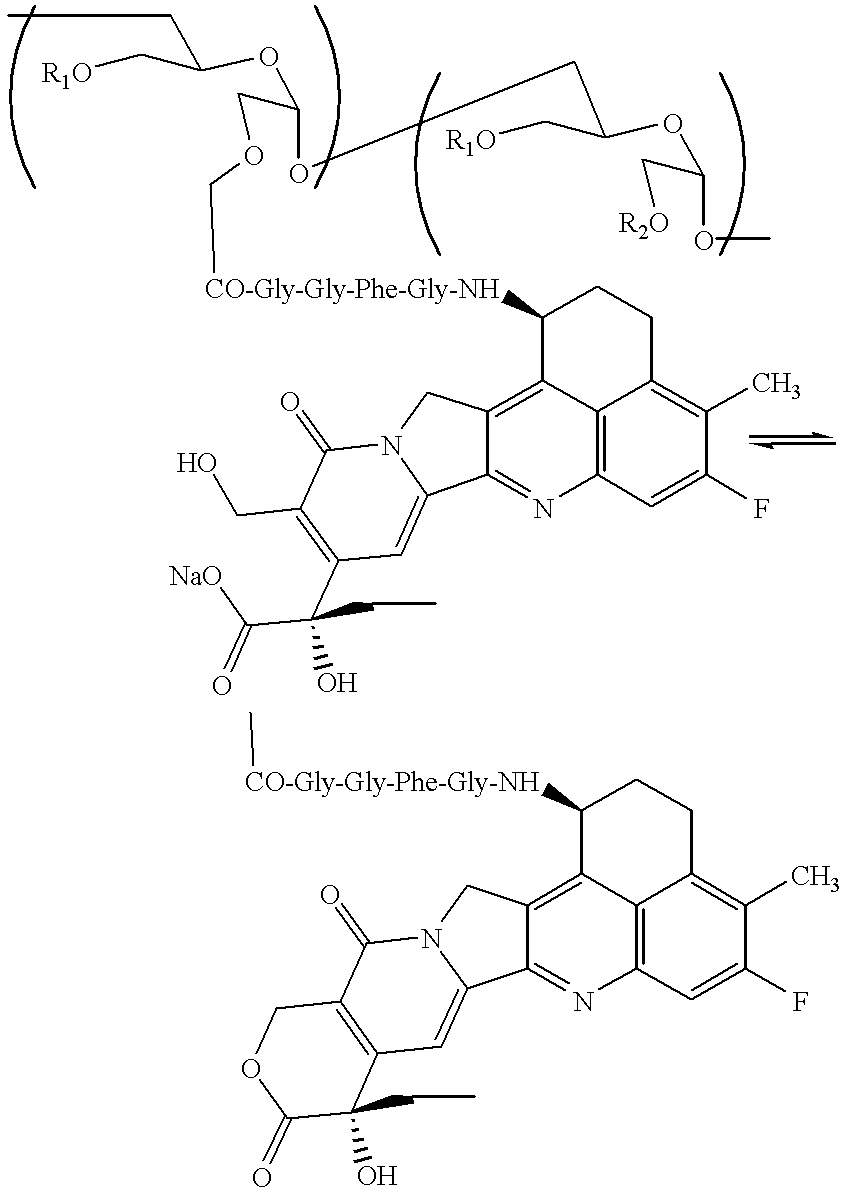
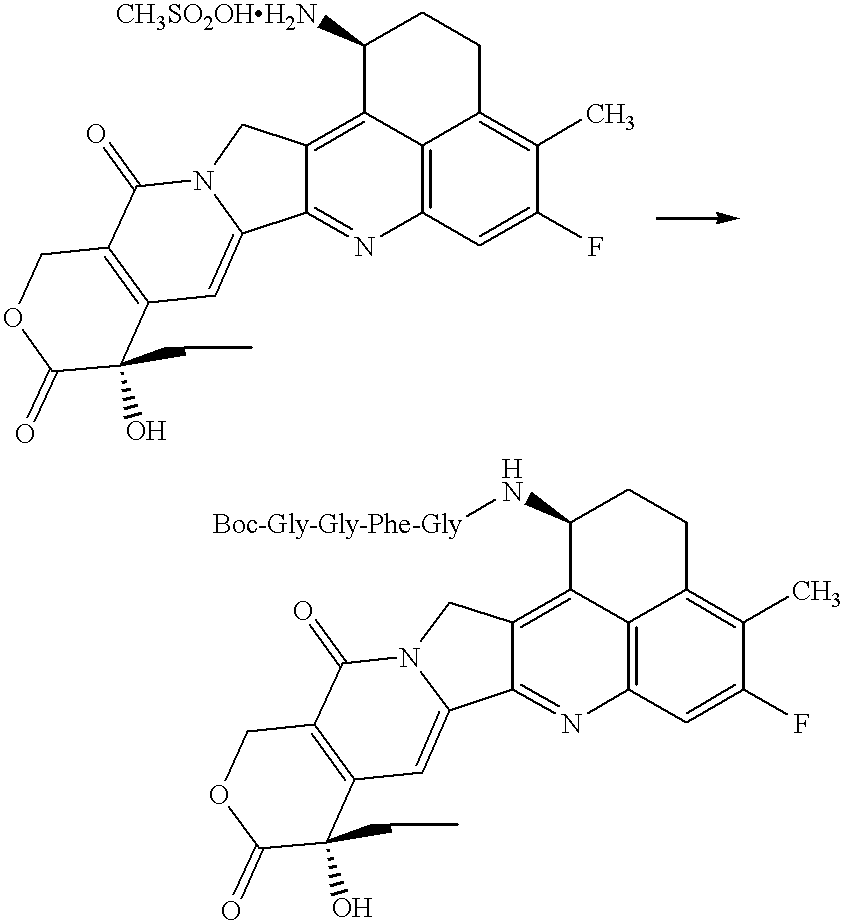
Process for producing drug complexes
InactiveUS6291671B1Efficient productionProduce significantEsterified saccharide compoundsSugar derivativesDrug compoundSide reaction
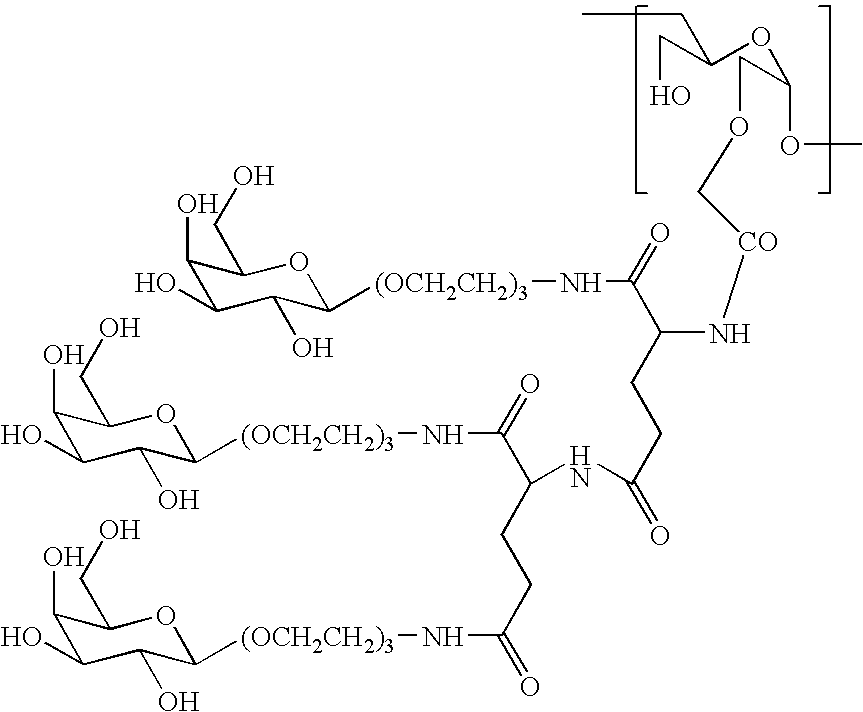
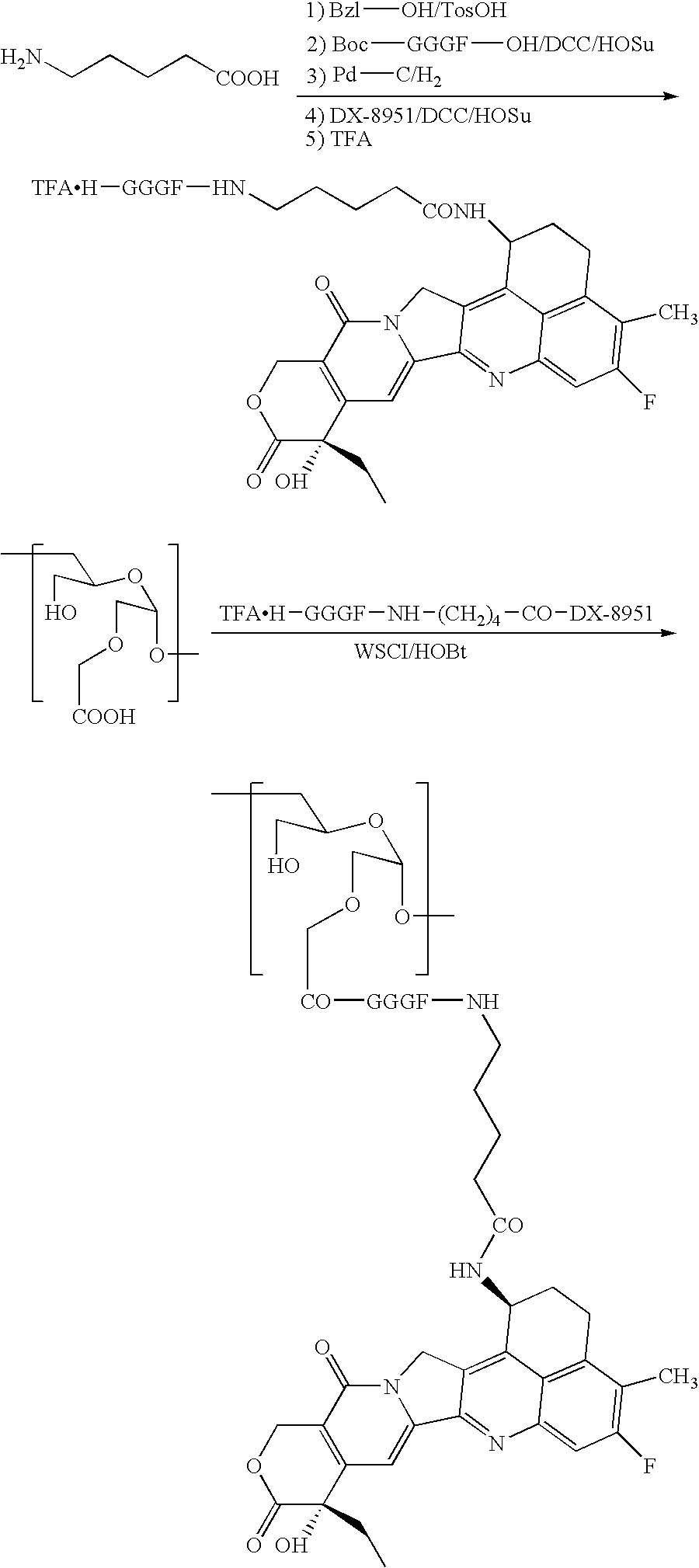
A method for preparing a drug complex in which a polysaccharide derivative having carboxyl groups and a residue of a drug compound are bound to each other by means of a spacer comprising an amino acid or a spacer comprising peptide-bonded 2 to 8 amino acids, or a drug complex in which a polysaccharide derivative having carboxyl groups and a residue of a drug compound are bound to each other without the spacer, characterized in that an organic amine salt of the polysaccharide derivative having carboxyl groups is reacted with the drug compound or the spacer bound to the drug compound in a non-aqueous system. The reaction between the polysaccharide derivative having carboxyl groups and the drug compound bound with the spacer or the like can be carried out in high yields, and when a drug compound having a lactone ring is subjected to the reaction, side reactions can be reduced.
Owner:DAIICHI PHARMA CO LTD
Single-chain polypeptides
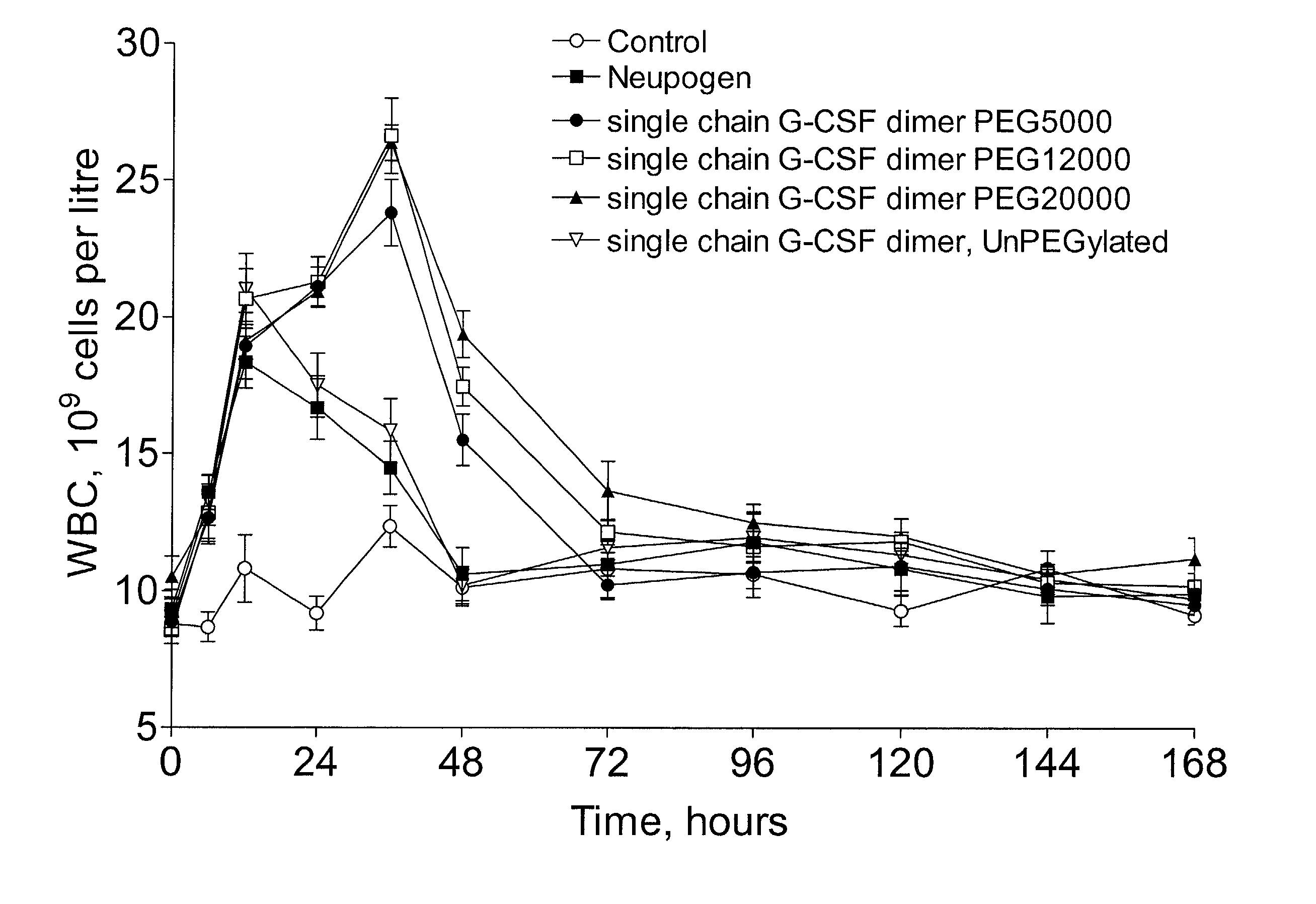
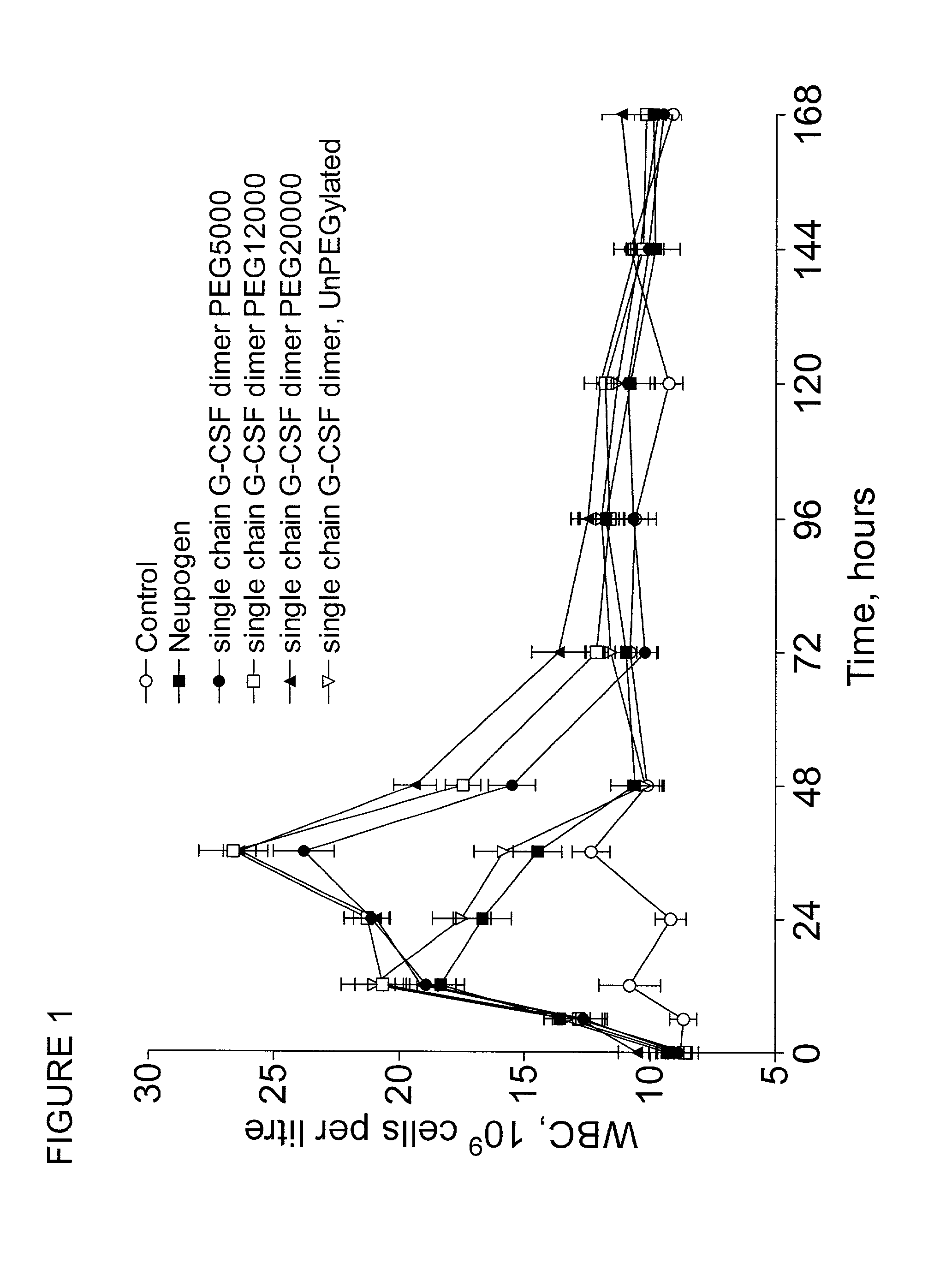
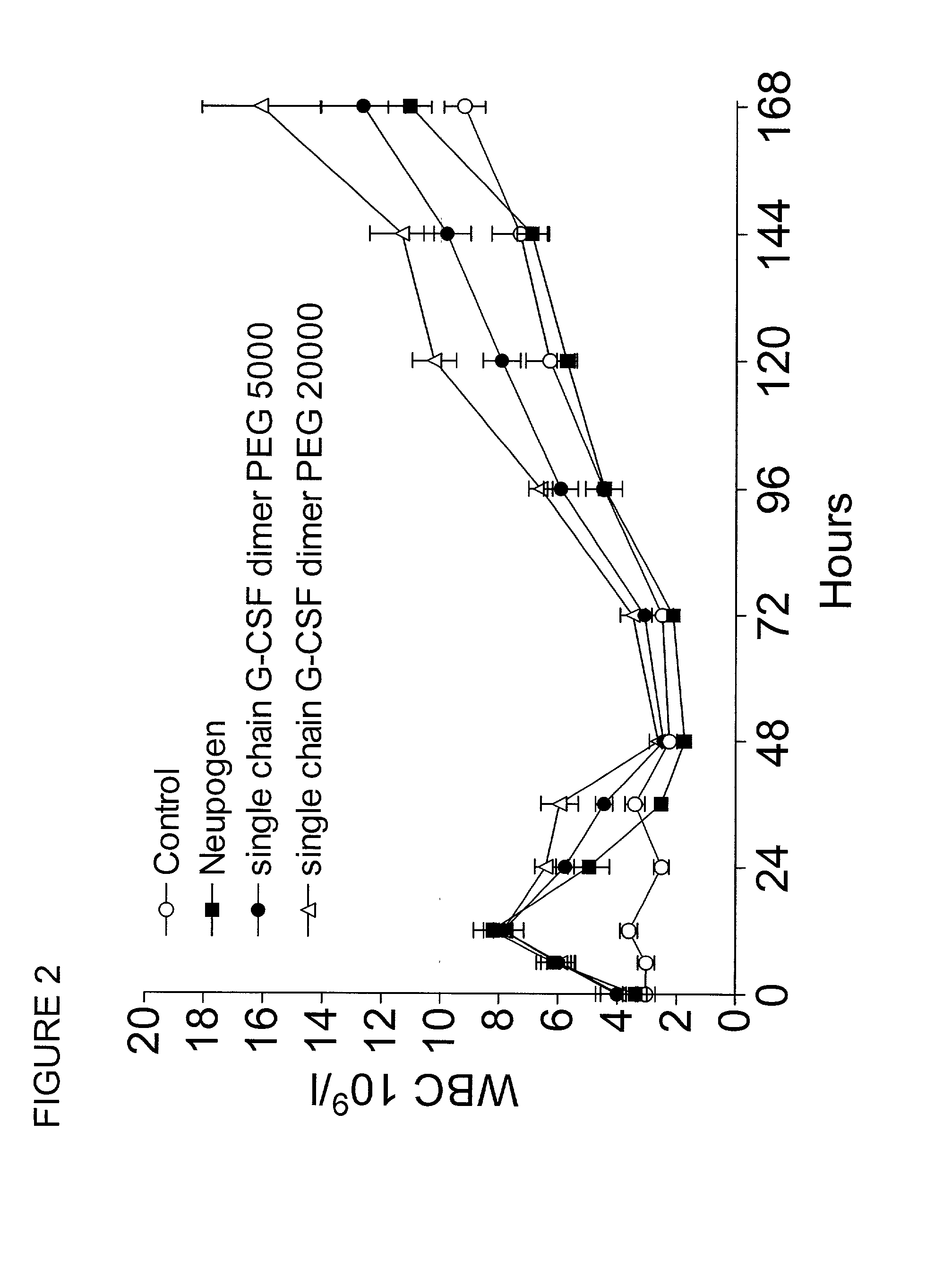
The invention relates to single-chain multimeric polypeptides comprising at least two units of a monomeric polypeptide linked via a peptide bond or a peptide linker, wherein the monomeric polypeptide is of a type that is biologically active in monomeric form, and to polypeptide conjugates having at least one non-polypeptide moiety covalently bound to an attachment group of the polypeptide. The polypeptide is preferably a G-CSF dimer bound to a polymer molecule, preferably to one or more polyethylene glycol molecules.
Owner:MAXYGEN HLDG +1
Biomacromolecule interpenetrating polymer network hydrogel and preparation method thereof
InactiveCN104004231AHigh mechanical strengthGood biocompatibilityAerosol deliveryOintment deliveryPolymer scienceTissue repair
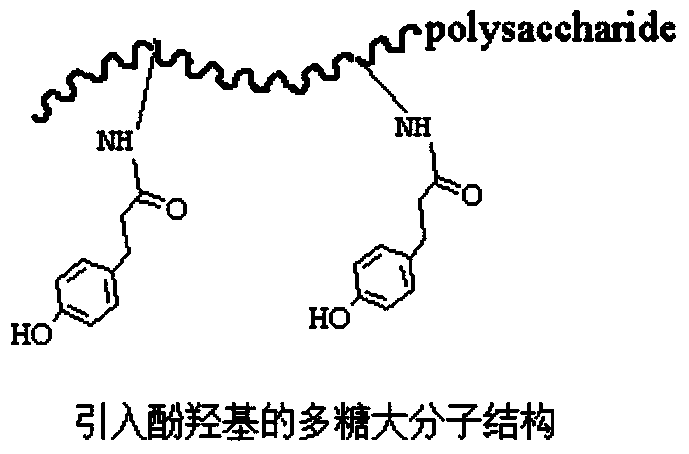
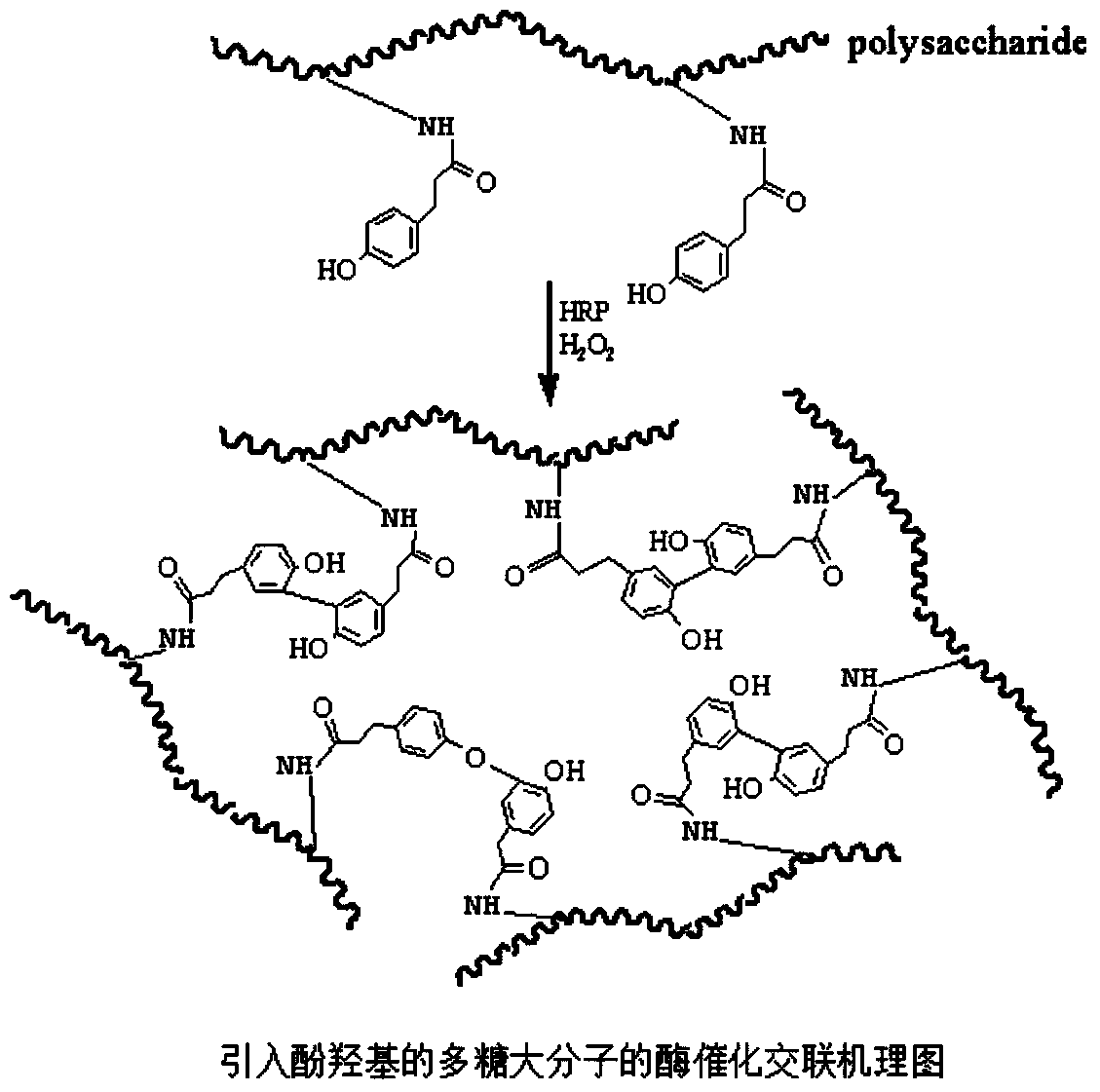
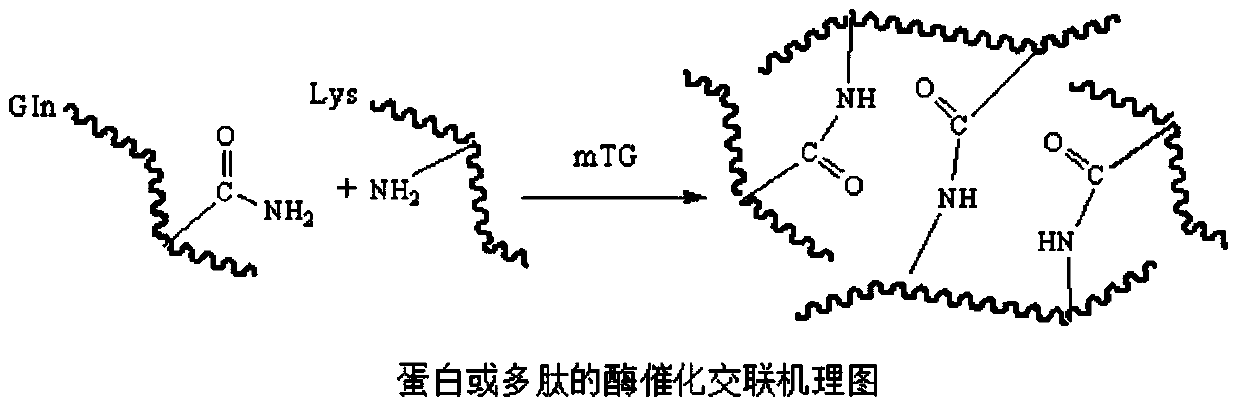
The invention discloses a biomacromolecule interpenetrating polymer network hydrogel and a preparation method of the biomacromolecule interpenetrating polymer network hydrogel. The biomacromolecule interpenetrating polymer network hydrogel is formed by crosslinking two kinds of enzymes in a catalysis mode, one is a polysaccharide macromolecule network formed by crosslinking polysaccharide macromolecules with introduced phenolic hydroxyl groups in an oxidation mode as oxidase and hydrogen peroxide catalyze phenolic hydroxyl groups, and the other is a protein or polypeptide macromolecule network formed crosslinking protein or polypeptide containing amino acid residues as transferase catalyzes peptide bonds. The two networks interpenetrate each other and form the novel interpenetrating polymer network hydrogel without bonding of chemical bonds. No chemical crosslinking agent is used in the hydrogel, and the hydrogel has excellent biocompatibility and mechanical property, can be shaped like a dry or wet film, like porous sponge or fibers, and can serve as a contact lens, a medicine release carrier, a scaffold for tissue engineering or materials for tissue repair.
Owner:SOUTHEAST UNIV
DDS compound and method for measurement thereof
InactiveUS7041818B2High organ selectivityExcellent liver selectivityBiocidePharmaceutical non-active ingredientsPolyolHydrolysate
A DDS compound comprising a carboxy(C1-4)alkyldextran polyalcohol modified with a saccharide compound and a residue of drug compound bound to the carboxy(C1-4)alkyldextran polyalcohol, and a method for measuring a DDS compound in which a polymer carrier and a residue of drug compound are bound to each other by means of a spacer comprising 2 to 8 amino acids linked by peptide bond(s), which comprises the steps of treating the DDS compound with a peptidase, and measuring the resulting hydrolysate.
Owner:DAIICHI PHARMA CO LTD
Diagnostic or therapeutic somatostatin or bombesin analog conjugates and uses thereof
InactiveUS20050070470A1Quick eliminationReduce drug toxicityOrganic active ingredientsSenses disorderDiseaseCytotoxicity
Disclosed are peptide agents and uses thereof that are analogs of biologically active peptides such as somatostatin and bombesin. The compounds of the invention have the general formula X-Y-Z-Q, where X is a cytotoxic agent, therapeutic agent, detectable label or chelating group, and Q is a biologically active peptide. In peptide agents of the invention Y is optionally a hydrophilic polymer or peptide, and Z is a linking peptide bonded to Q at the amino terminus of Q, having two, three, four, or five, amino acid residues selected to link X to Q, while retaining the biological activity of Q. Methods of using these peptide agents in the diagnosis and treatment of diseases are also disclosed.
Owner:TULANE EDUCATIONAL FUND
Peptide capable of binding to nanographite structures
ActiveUS20050277160A1Material nanotechnologyCell receptors/surface-antigens/surface-determinantsCarbon nanotubeBinding peptide
It is intended to provide a peptide or a phage recognizing nanographite structures and thus enabling efficient recognition, binding, separation and alignment of nanographite structures such as carbon nanohoms or carbon nanotubes, an artificial protein or a chimeric molecule comprising the above-described peptide bonded to a functional peptide, a protein, a labeling, etc., and a complex of the above-described peptide molecule, artificial protein or chimeric molecule with a nanographite structure. By panning peptide-presenting phages bonded to nanographite structures, a nanographite structure-binding peptide capable of specifically recognizing nanographite structures such as carbon nanohoms or carbon nanotubes is obtained.
Owner:JAPANESE FOUND FOR CANCER RES +1
Variants of C-type natriuretic peptides
InactiveUS8377884B2Improve the immunityReduce functionPeptide/protein ingredientsPeptide sourcesChemical MoietyDisease
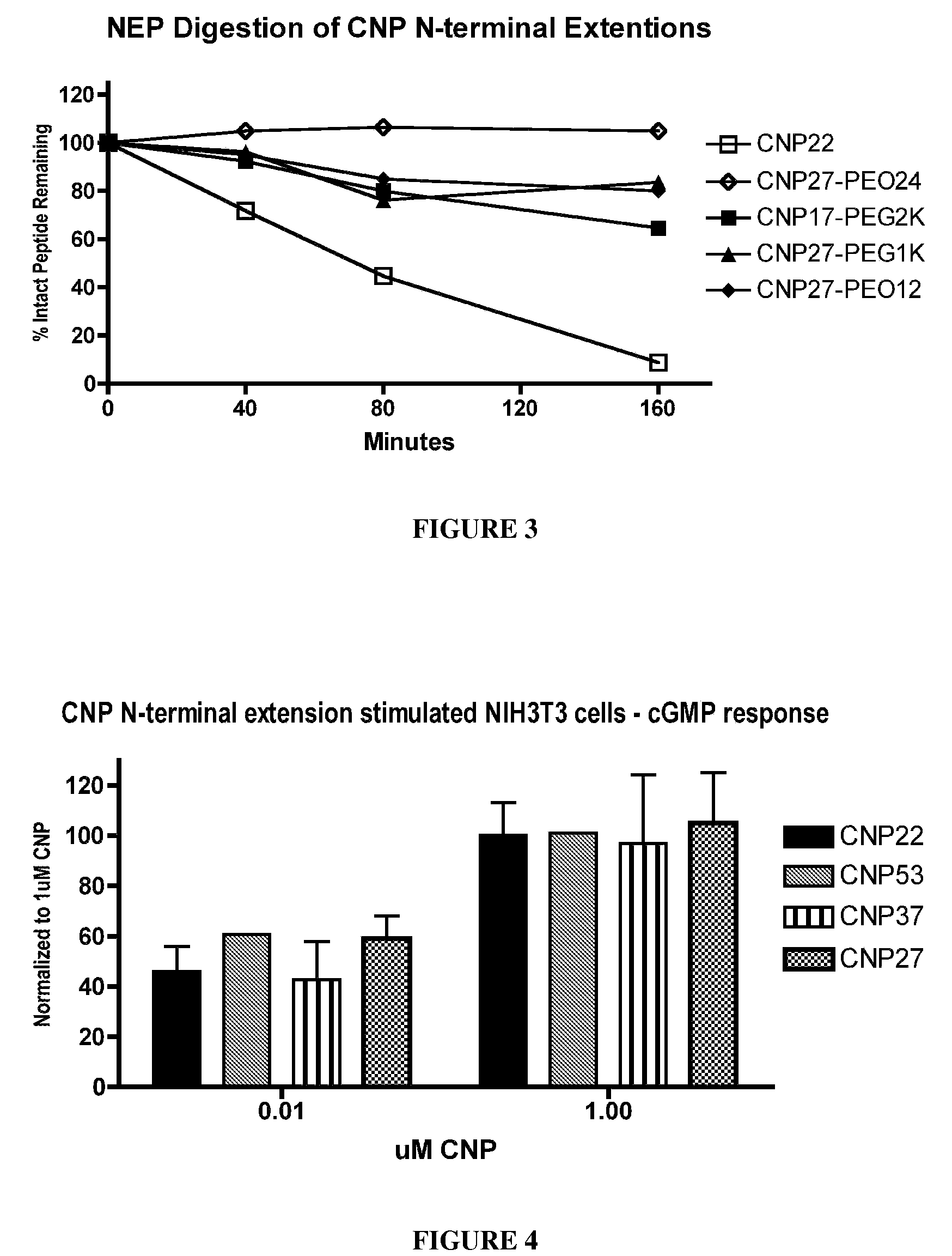
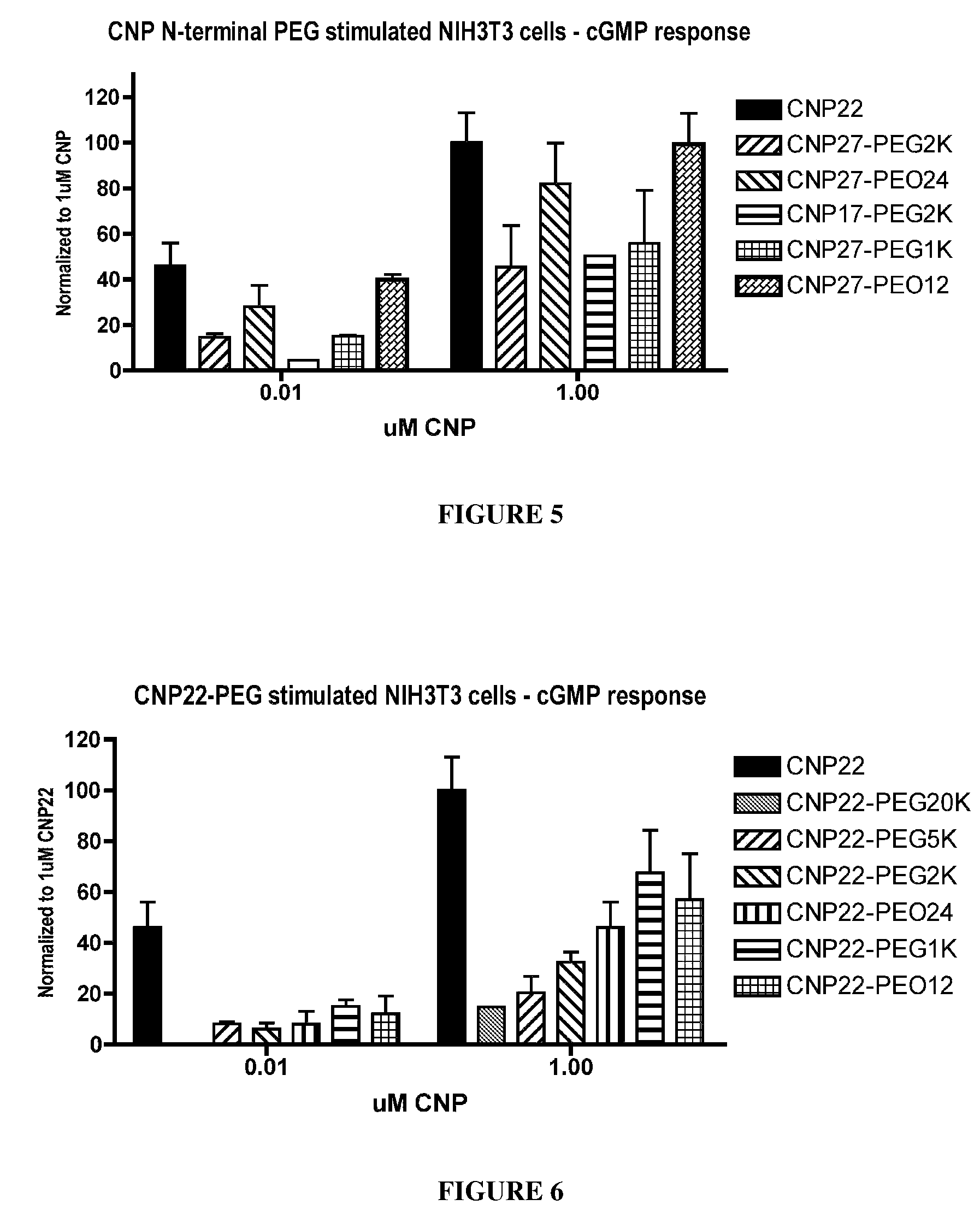
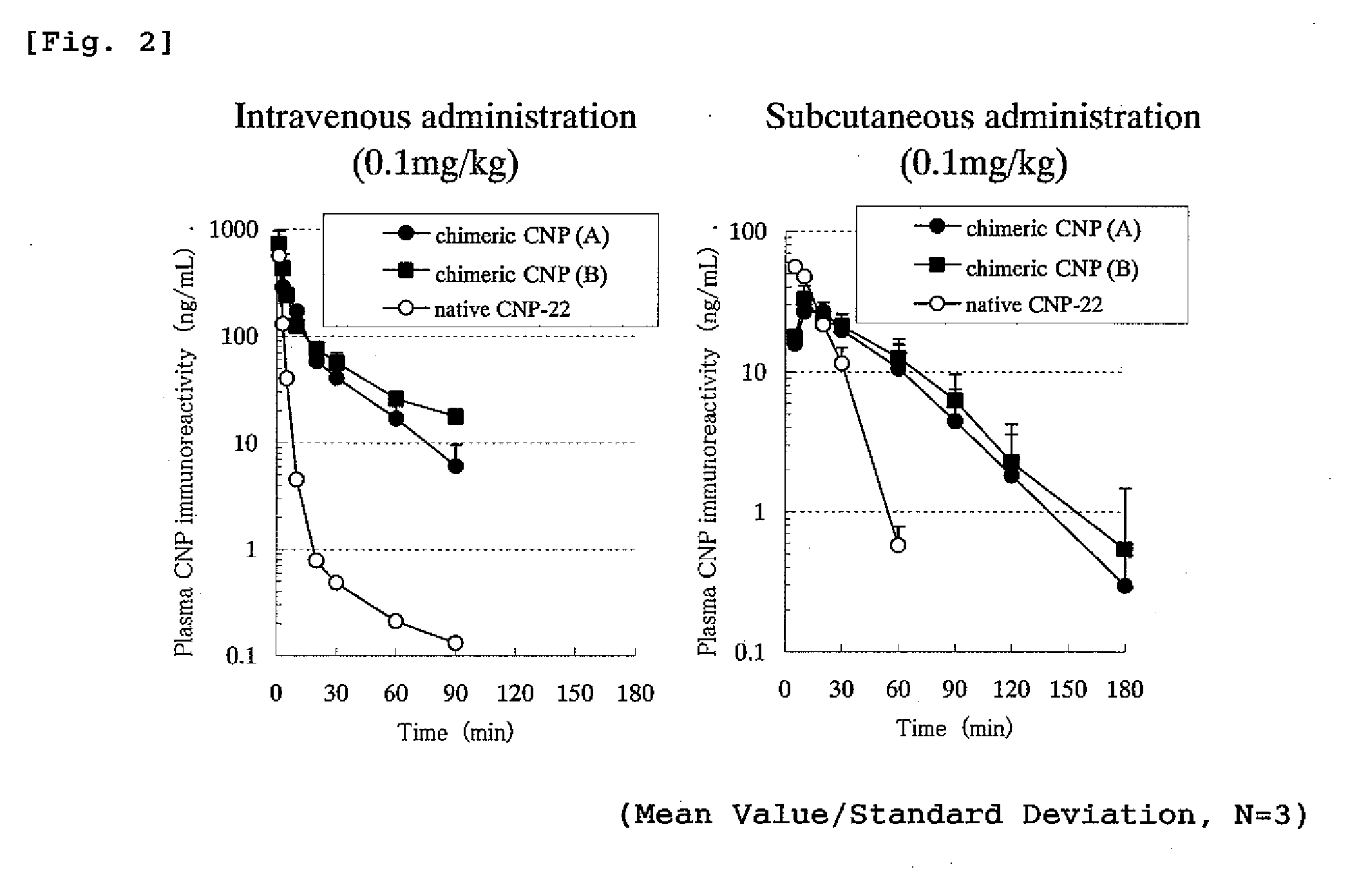
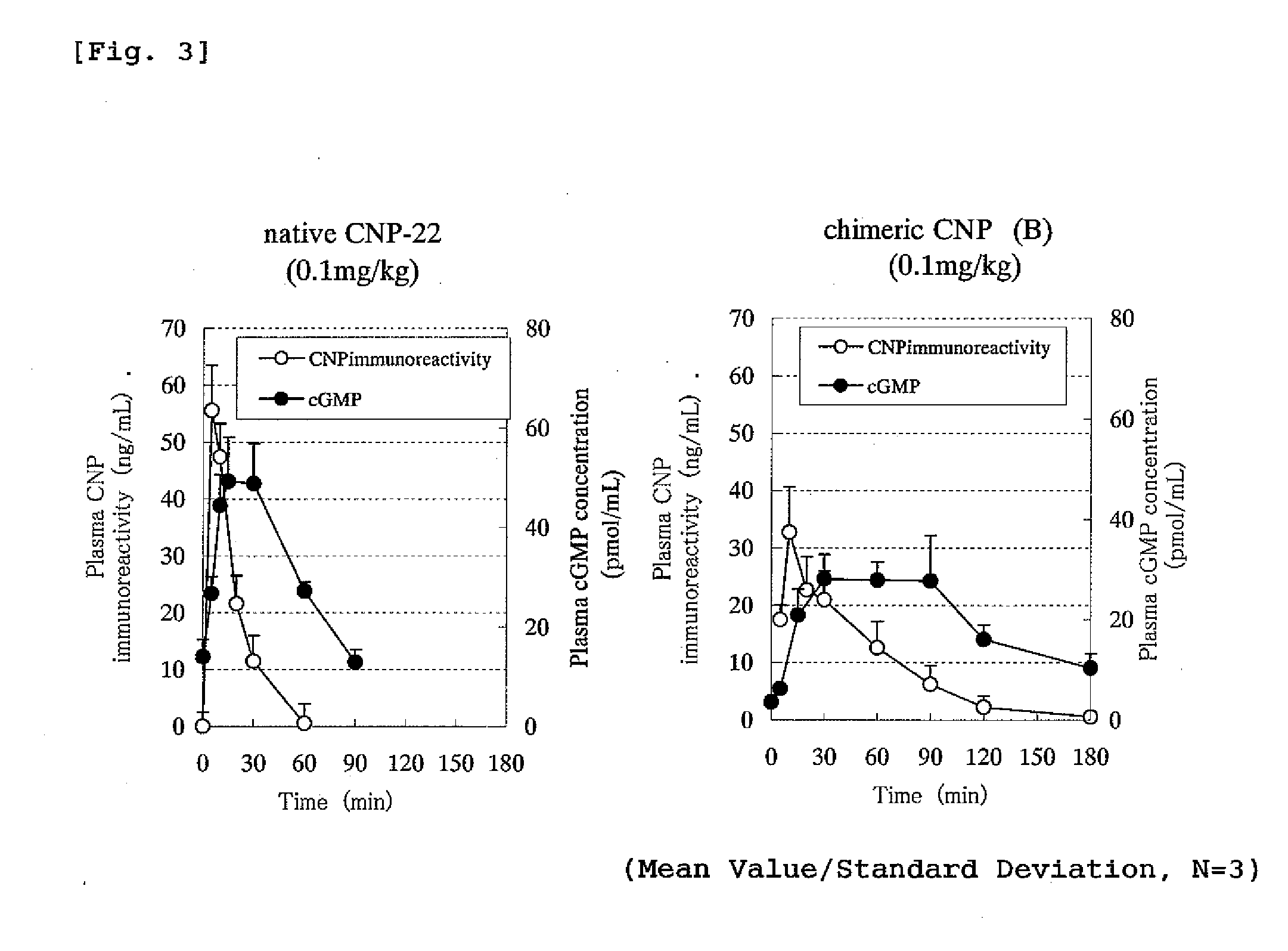
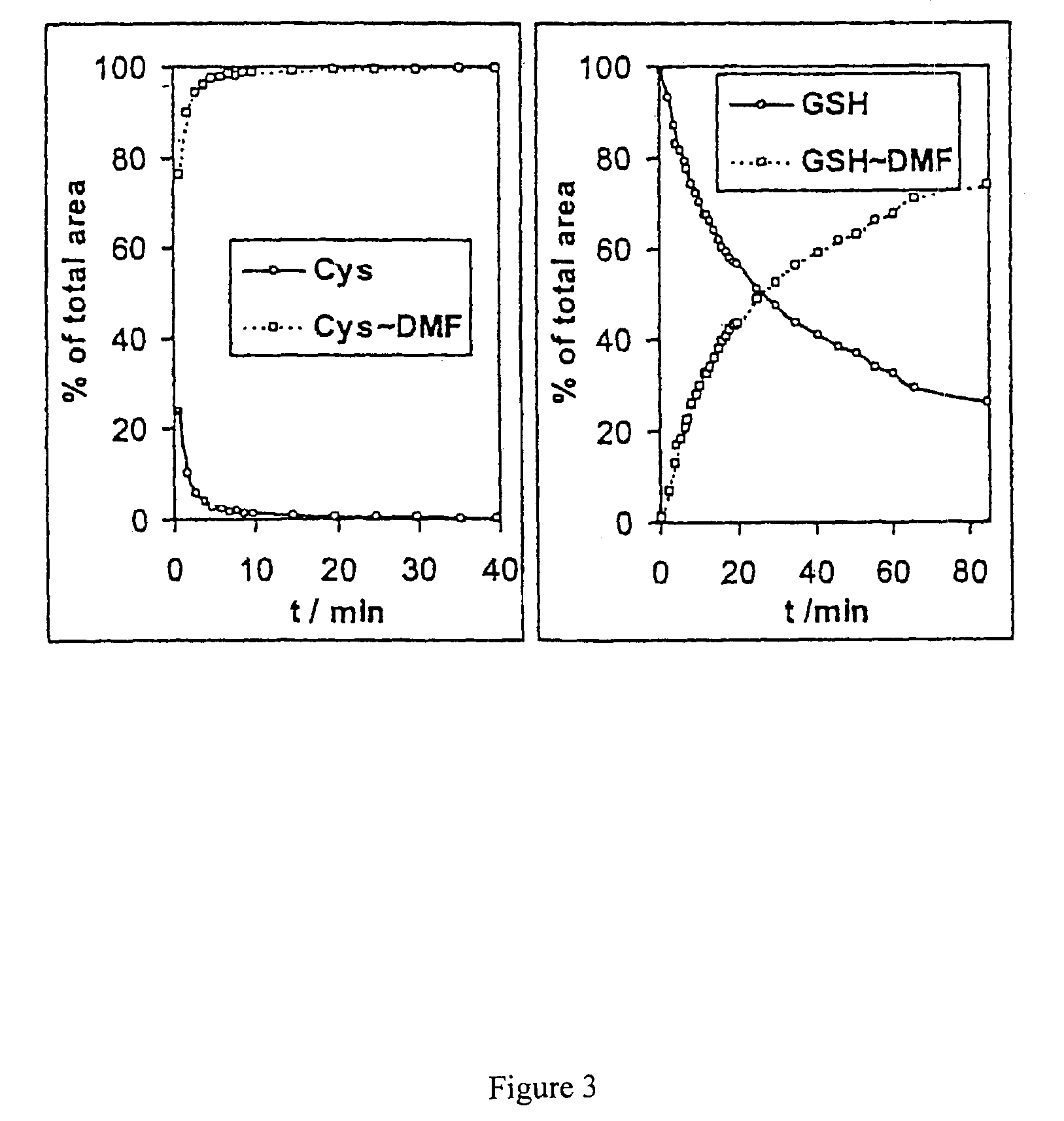
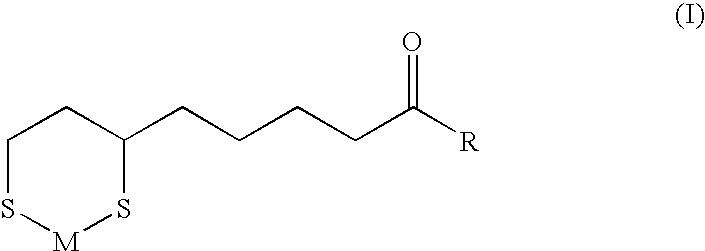
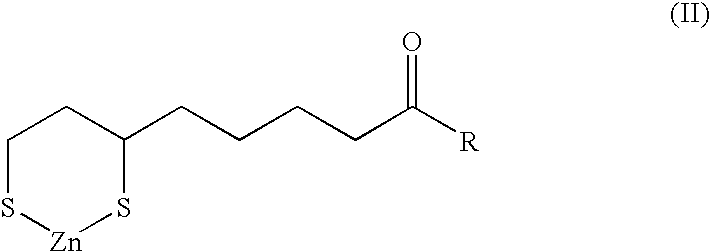
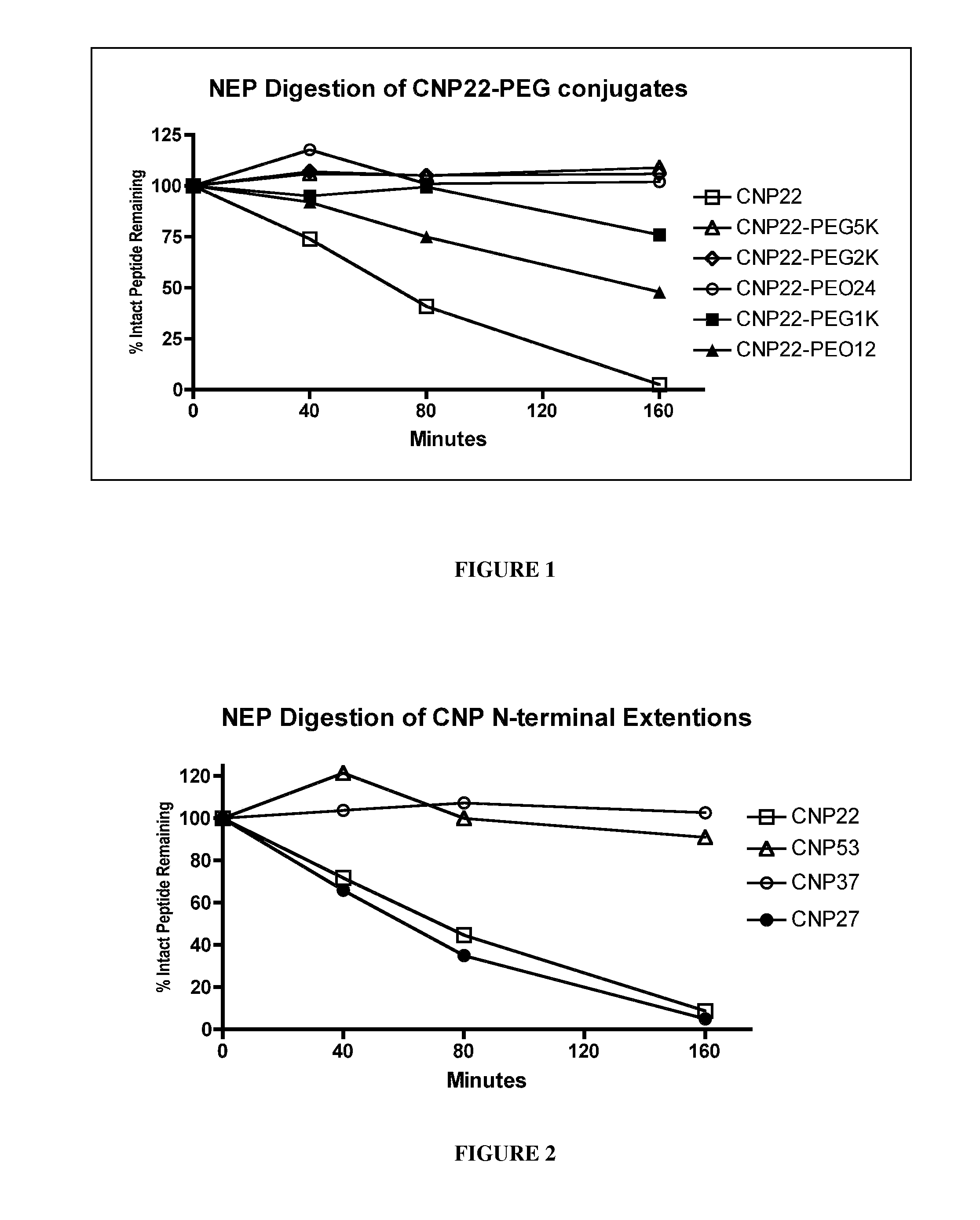
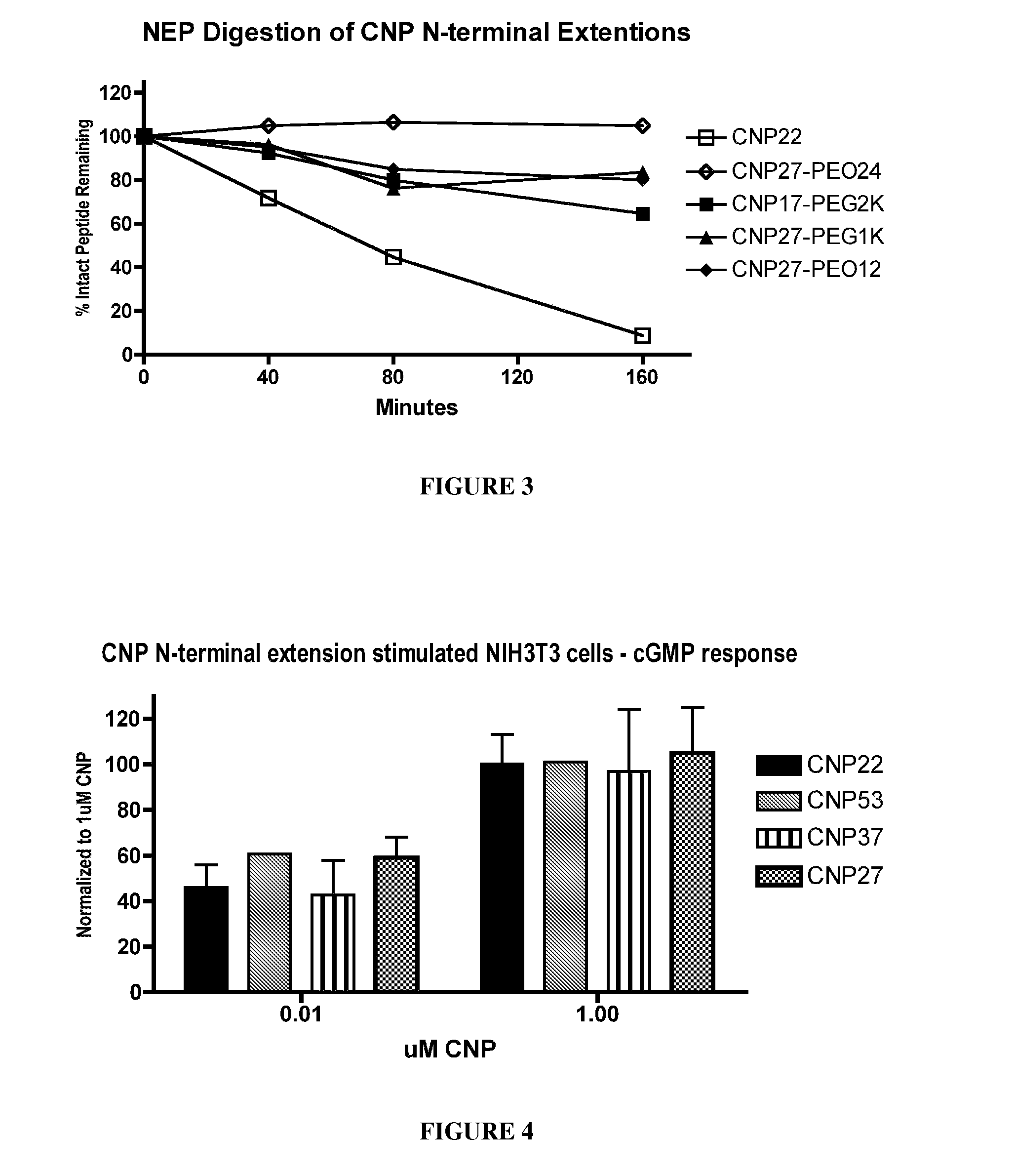
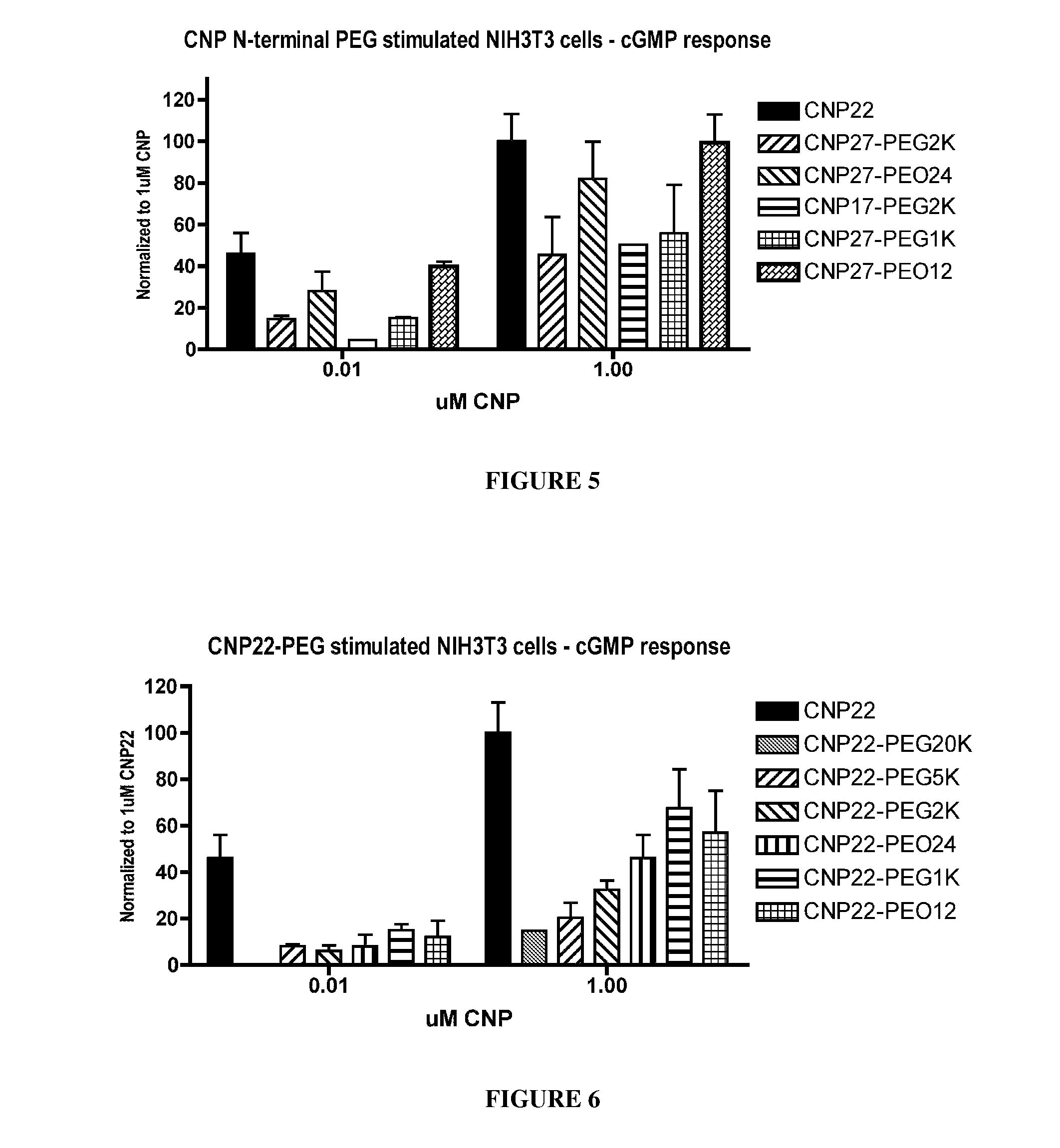
The present invention provides variants of C-type natriuretic peptide (CNP) comprising one or more deletions; additions of and / or substitutions with natural amino acids, unnatural amino acids and / or peptidomimetics (including peptide bond isosteres); amino acid extensions; and / or other chemical moieties such as, e.g., poly(ethylene glycol) and hydrophobic acids. The CNP variants are useful as therapeutic agents for the treatment of diseases responsive to CNP, including but not limited to bone-related disorders such as, e.g., skeletal dysplasias and achondroplasia, and vascular smooth muscle disorders such as, e.g., restenosis and arteriosclerosis.
Owner:BIOMARIN PHARMA INC
Fusion proteins, uses thereof and processes for producing same
InactiveUS20080014208A1Efficient expressionAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class IAmino acid
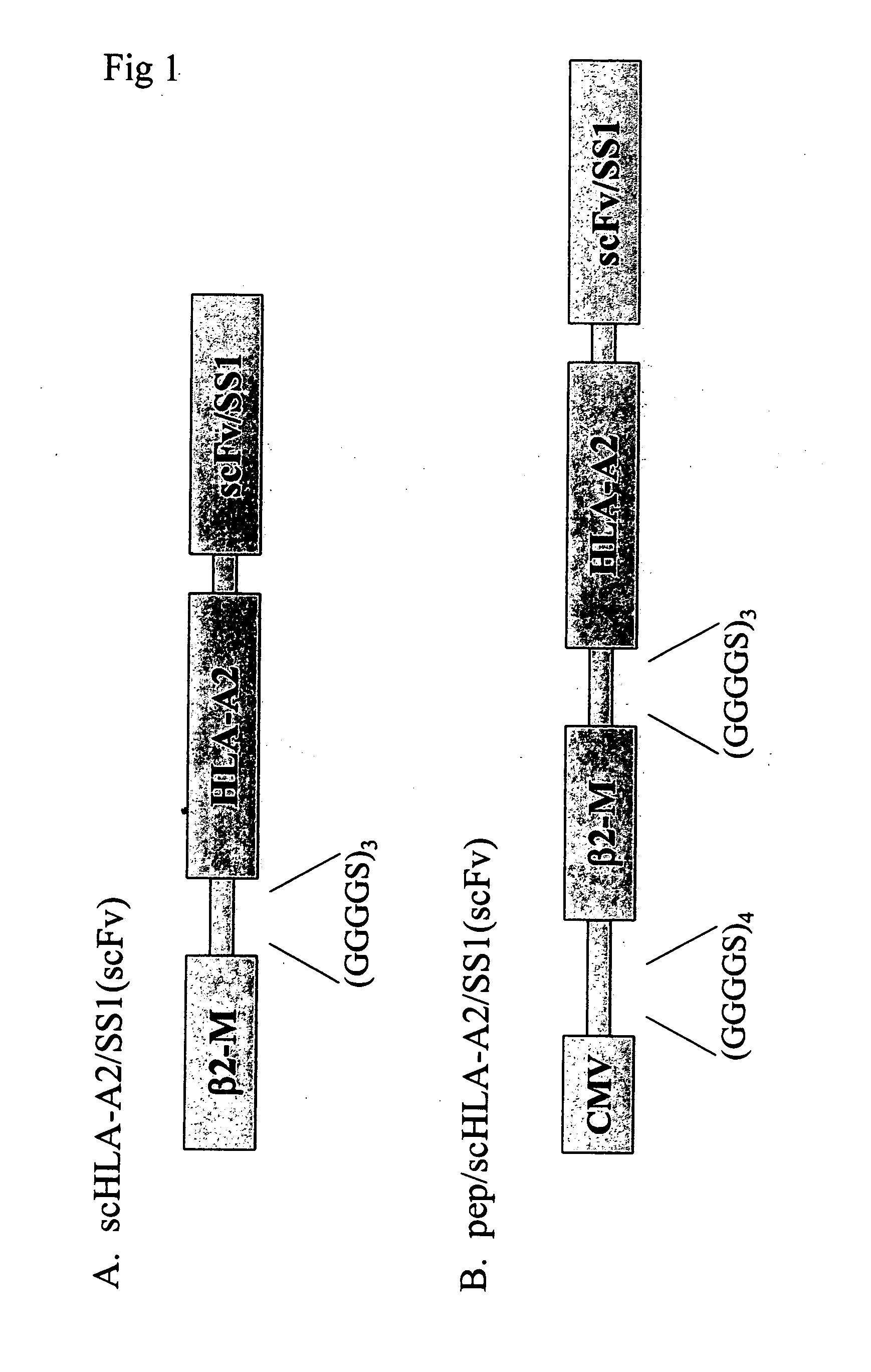
This invention provides fusion proteins comprising consecutive amino acids which beginning at the amino terminus of the protein correspond to consecutive amino acids present in (i) a cytomegalovirus human MHC-restricted peptide, (ii) a first peptide linker, (iii) a human β-2 microglobulin, (iv) a second peptide linker, (v) a HLA-A2 chain of a human MHC class I molecule, (vi) a third peptide linker, (vii) a variable region from a heavy chain of a scFv fragment of an antibody, and (viii) a variable region from a light chain of such scFv fragment, wherein the consecutive amino acids which correspond to (vii) and (viii) are bound together directly by a peptide bond or by consecutive amino acids which correspond to a fourth peptide linker, wherein the antibody from which the scFv fragment is derived specifically binds to mesothelin. This invention provides nucleic acid constructs encoding same, processes for producing same, compositions, and uses thereof.
Owner:TECHNION RES & DEV FOUND LTD
Peptide pharmaceutical for oral delivery
ActiveUS20090317462A1Prevent undesirable effect of acidImproved profilePowder deliveryNervous disorderParticulatesActive agent
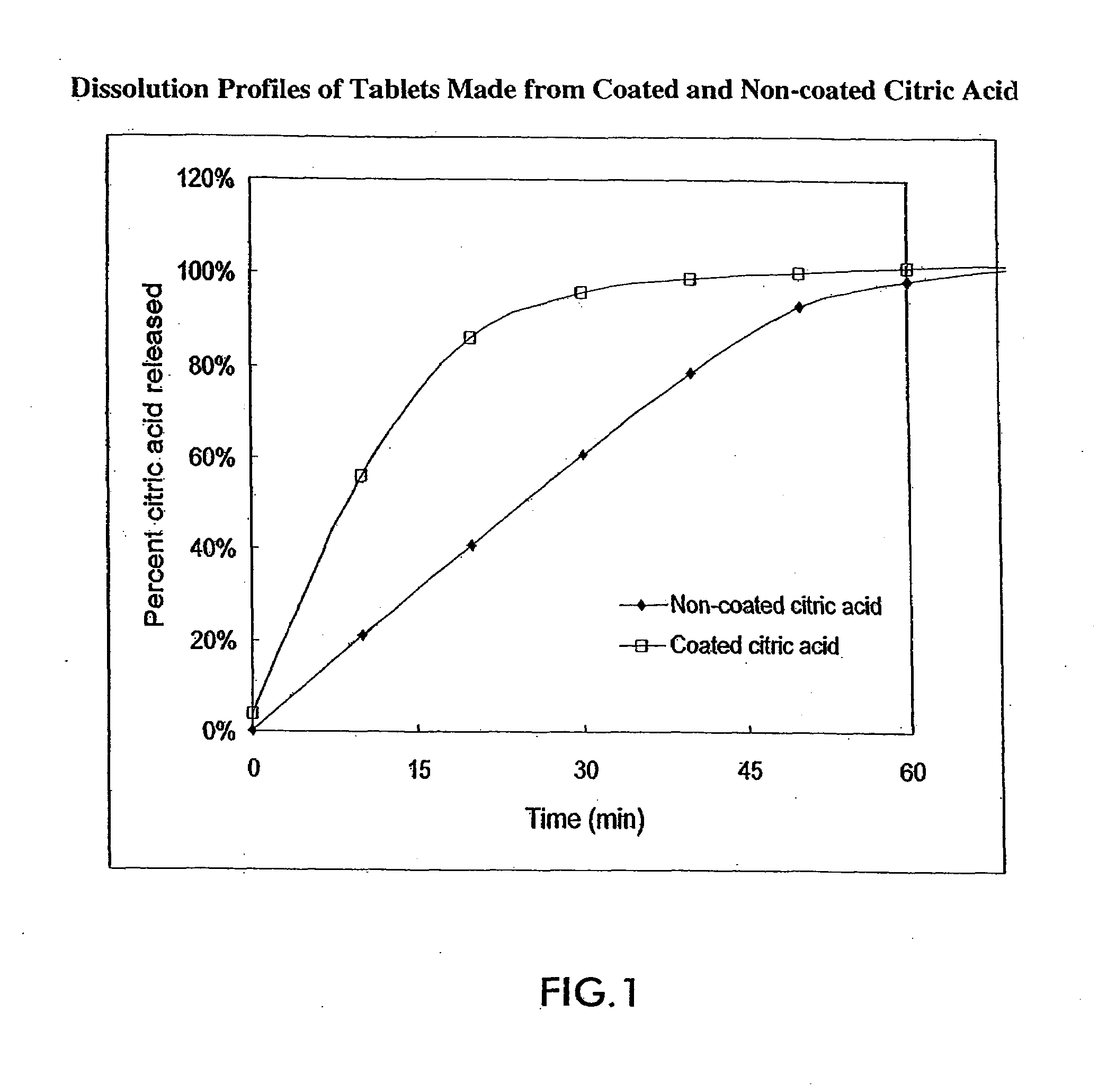
Acid-containing oral pharmaceutical compositions are provided wherein the pharmaceutical active agents are peptide compounds (i.e., those that include a plurality of amino acids and at least one peptide bond in their molecular structures). Certain barrier layers and / or particulate coated acid are used to reduce any adverse interactions that might otherwise occur between the acid of the compositions and other components of the composition. Use of these barrier layers and / or use of particulate coated acid is believed to promote a more simultaneous release of the components of the composition than is achieved by prior art acid-protection techniques, thus enhancing, and making more consistent, the bioavailability of the active peptide compounds.
Owner:ENTERIS BIOPHARMA
Prodrugs activated by plasmin and their use in cancer chemotherapy
InactiveUS7402556B2Low toxicityHigh specificity of actionSugar derivativesTetrapeptide ingredientsPeptide substrateOligopeptide
The product of the invention is a modified form of a therapeutic agent and comprises a therapeutic agent, an oligopeptide having a plasmin peptide substrate of 2-4 amino acids and mono- or di-peptide linkage, a stabilizing group and, optionally, a linker group. The prodrug is cleavable by plasmin. Also disclosed are methods of making and using the prodrug compounds.
Owner:MEDAREX LLC
Peptide having an extending action for half-life of object peptide in plasma
ActiveUS20100305031A1Improve securityLow costPeptide/protein ingredientsAntipyreticHalf-lifeBlood plasma
A peptide of the following (I) or (II).(I) a peptide represented by the formula B, A-B, B-C or A-B-C in which A, B and C each is represented by the following (1), (2) and (3) and, when it is bonded to other object peptide, it is able to extent the half-life in plasma as compared with the object peptide where the physiological activity of the object peptide is still retained.(II) a peptide comprising a reversed sequence of the peptide of (I); a sequence which is represented by A-B in (I) and A or B is reversed; a sequence which is represented by B-C in (I) and B or C is reversed; or a sequence which is represented by A-B-C in (I) and A, B, C, A and B, B and C or A and C is reserved.(1) A is a peptide comprising 1 to 14 of any amino acid(s)(2) B is a peptide represented by the formula 1:(Wk-Xl-Y-Zm-Wn)-(Wo-Xp-Y-Zq-Wr)s(In the formula 1, W is a basic amino acid; X and Z are any amino acids; Y is an acidic amino acid; k is 1 or 2; l is an integer of 4≧l≧0; m is an integer of 2≧m≧0; 4≧l+m≧0; n is 1 or 2; o is 1 or 2; p is an integer of 4≧p≧0; q is an integer of 2≧q≧0; 4≧p+q≧0; r is 1 or 2; and s is 0 or 1.)(3) C is a peptide comprising 2 to 14 of any amino acids.
Owner:DAIICHI SANKYO CO LTD
Heterodimeric fusion proteins useful for targeted immune therapy and general immune stimulation
InactiveUS20050137384A1Extended half-lifeAltering pharmacology and biodistributionPeptide/protein ingredientsAntibody mimetics/scaffoldsWhite blood cellStepwise approach
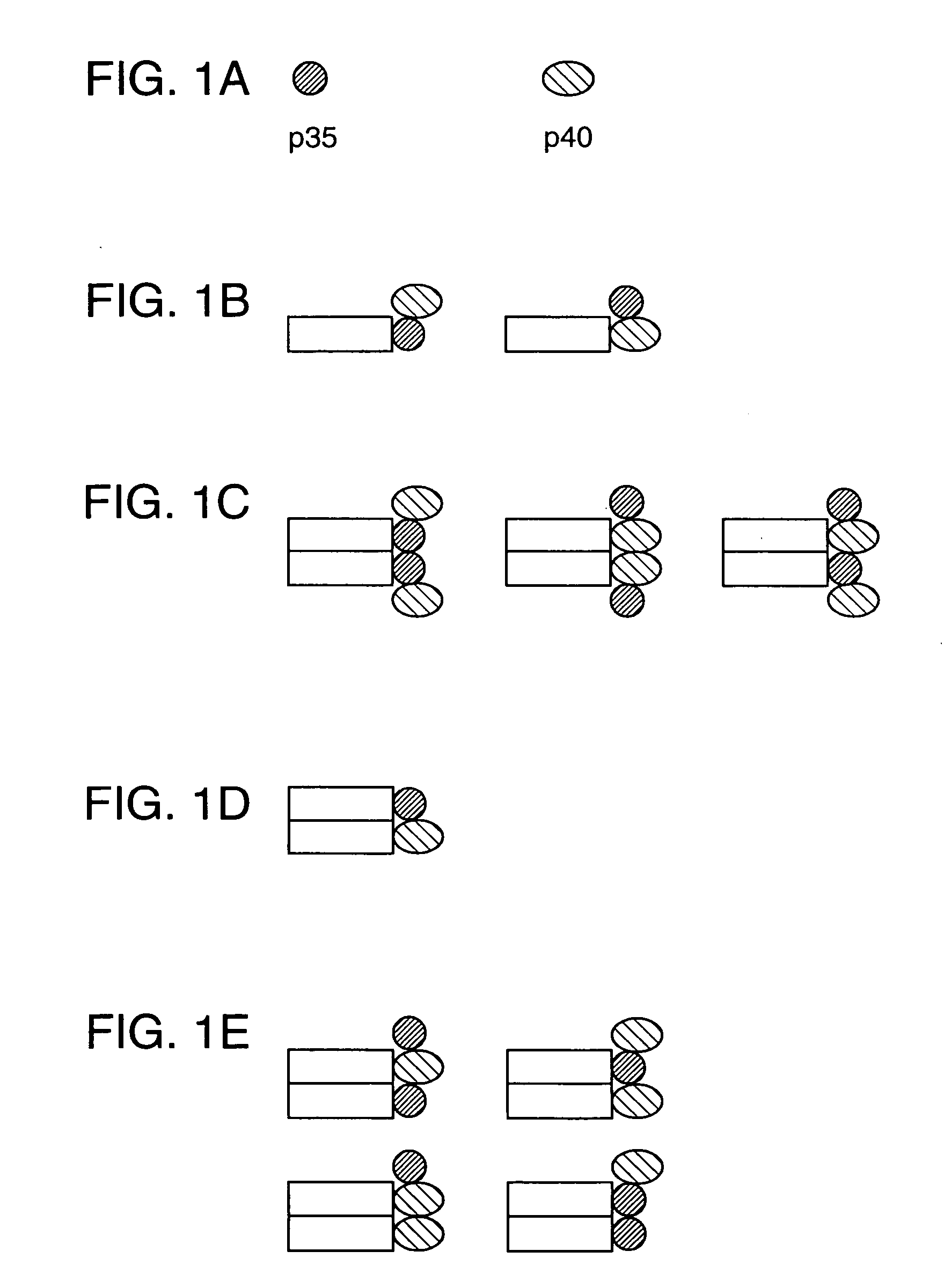
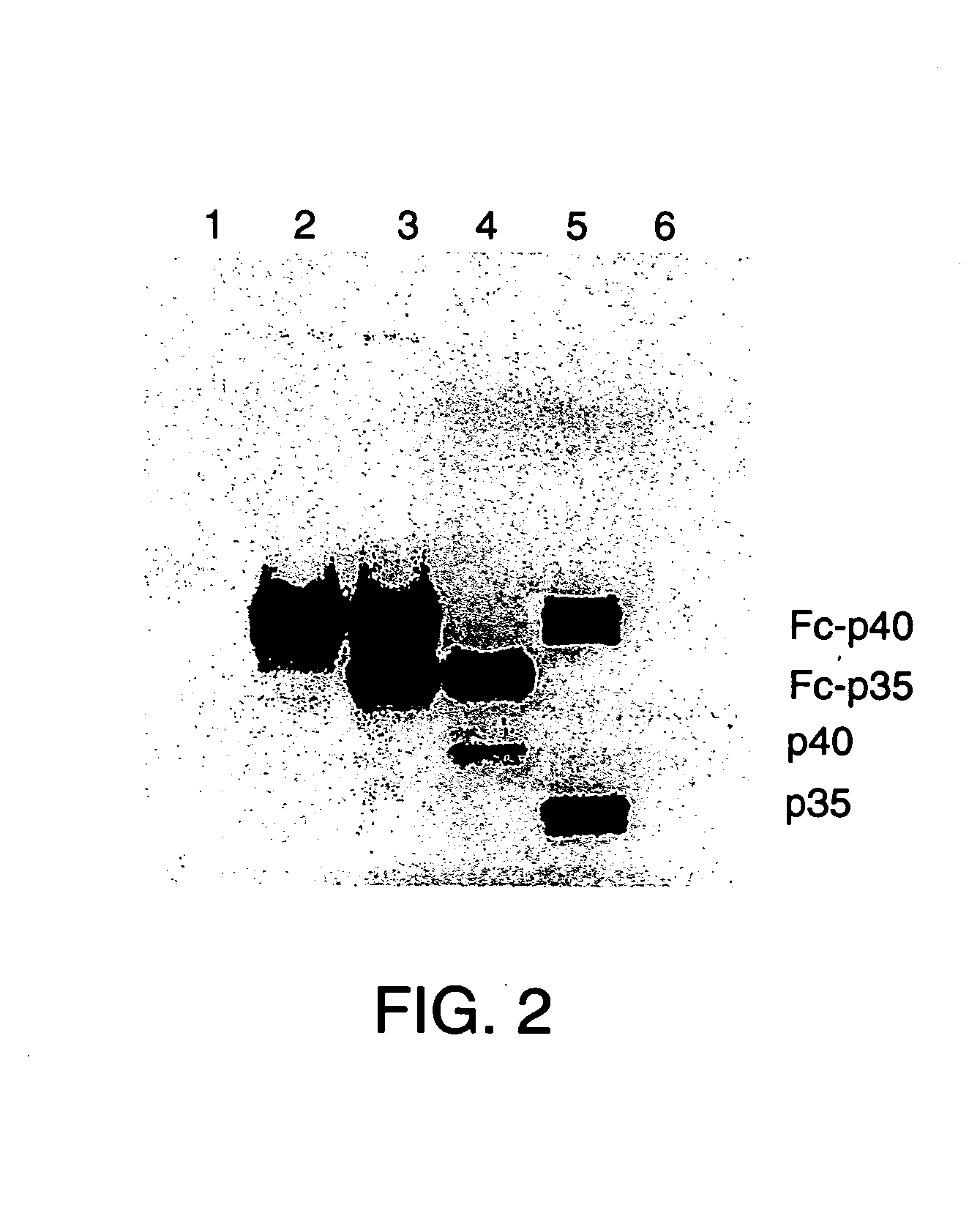
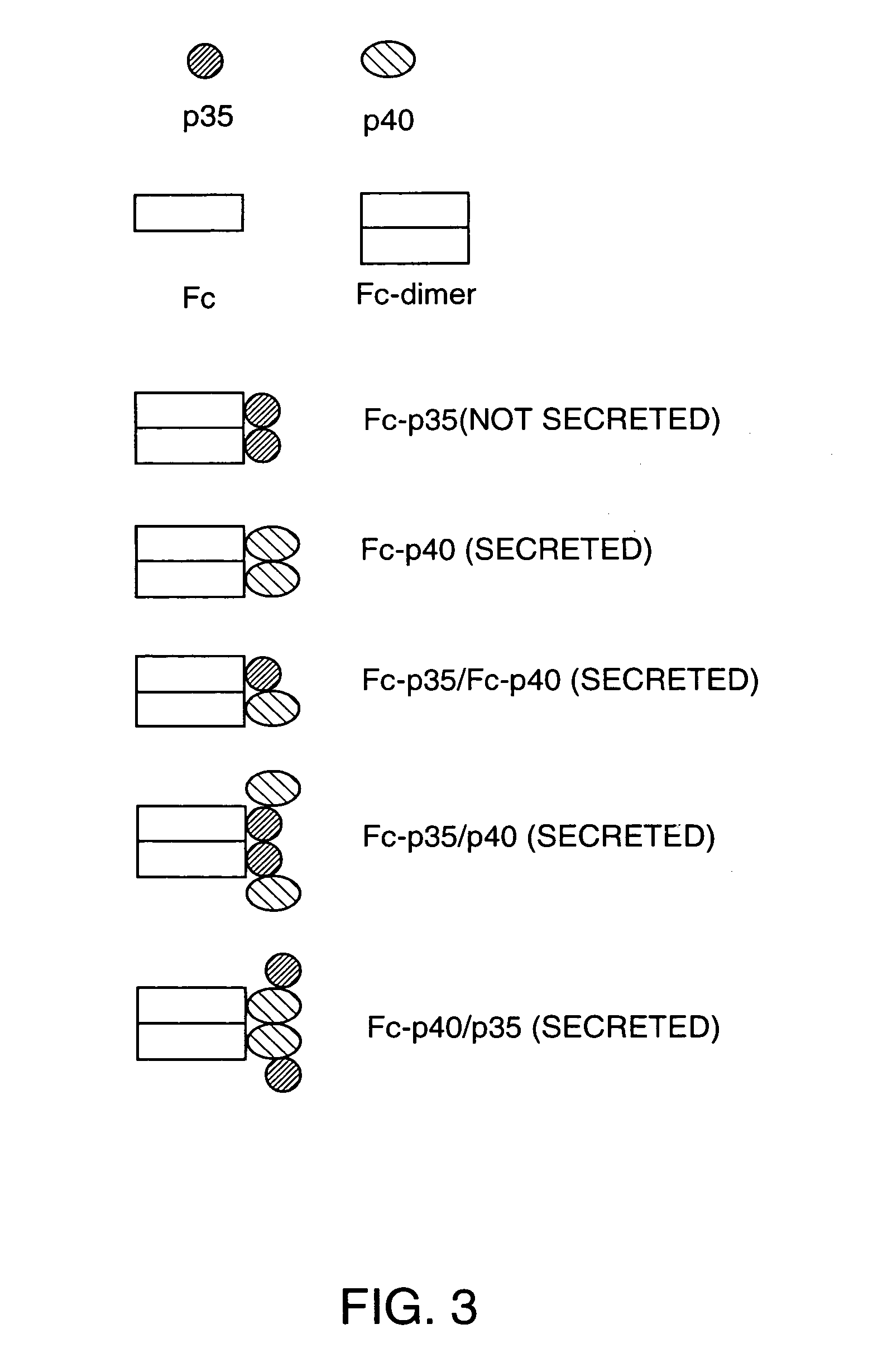
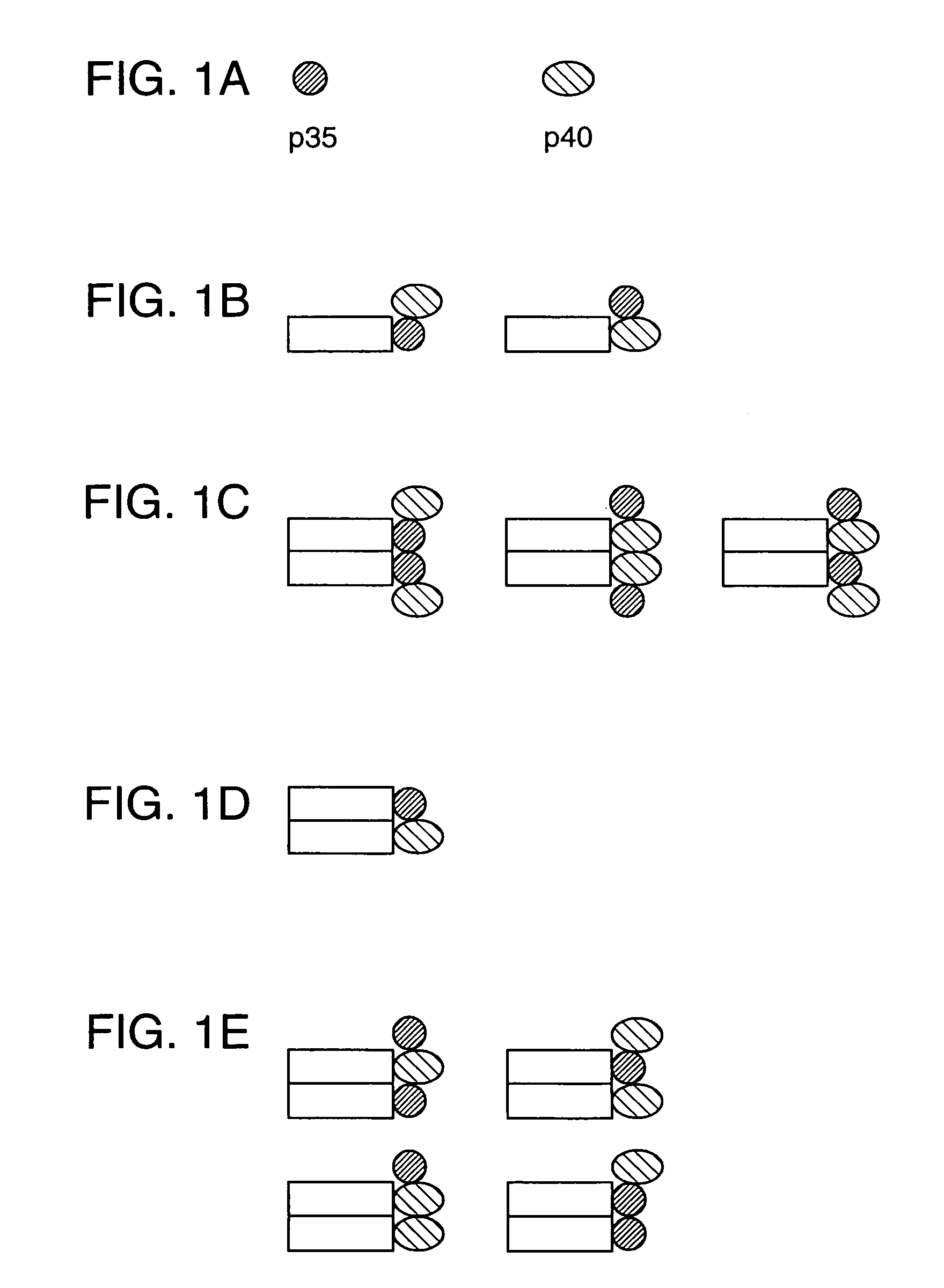
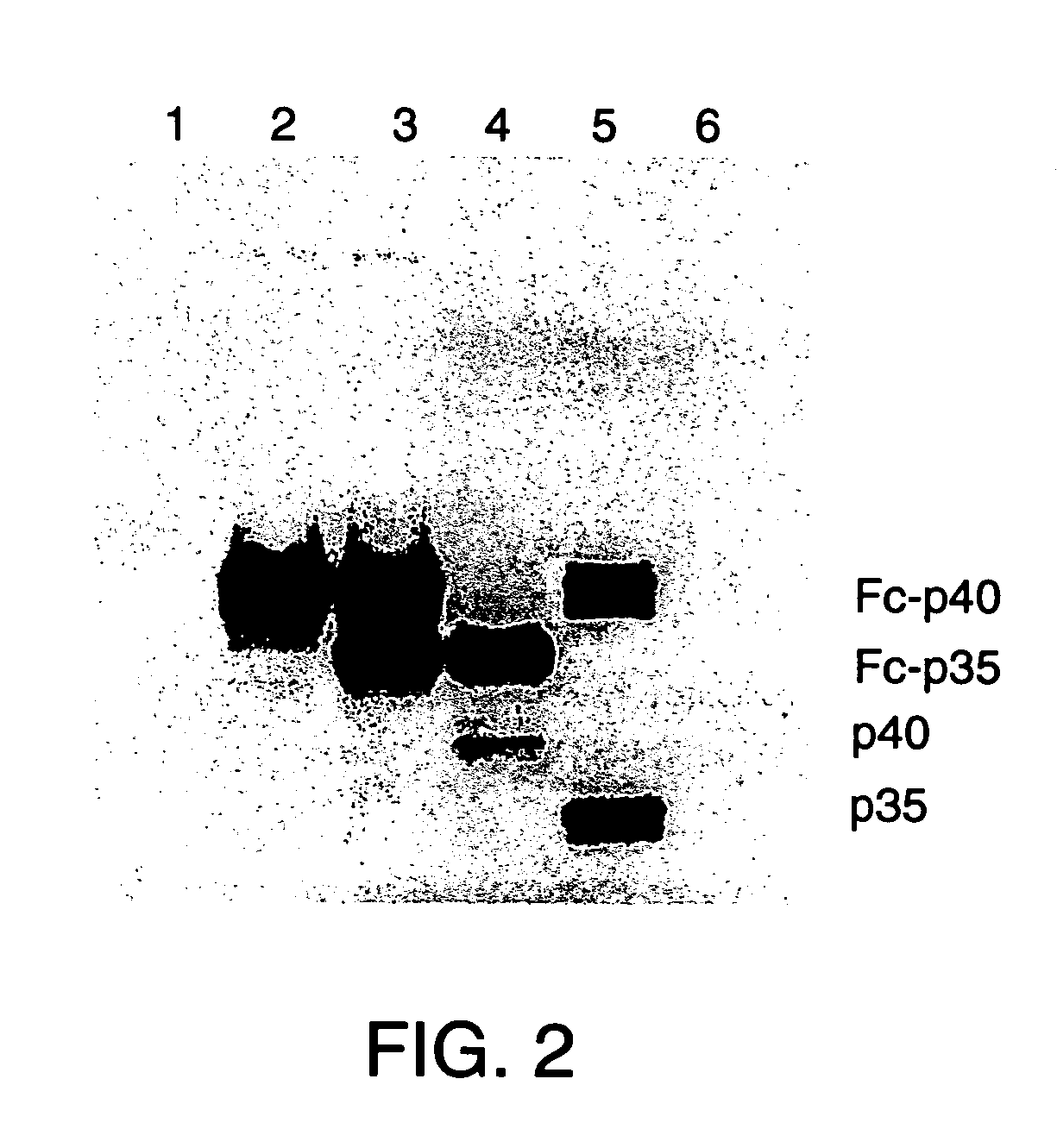
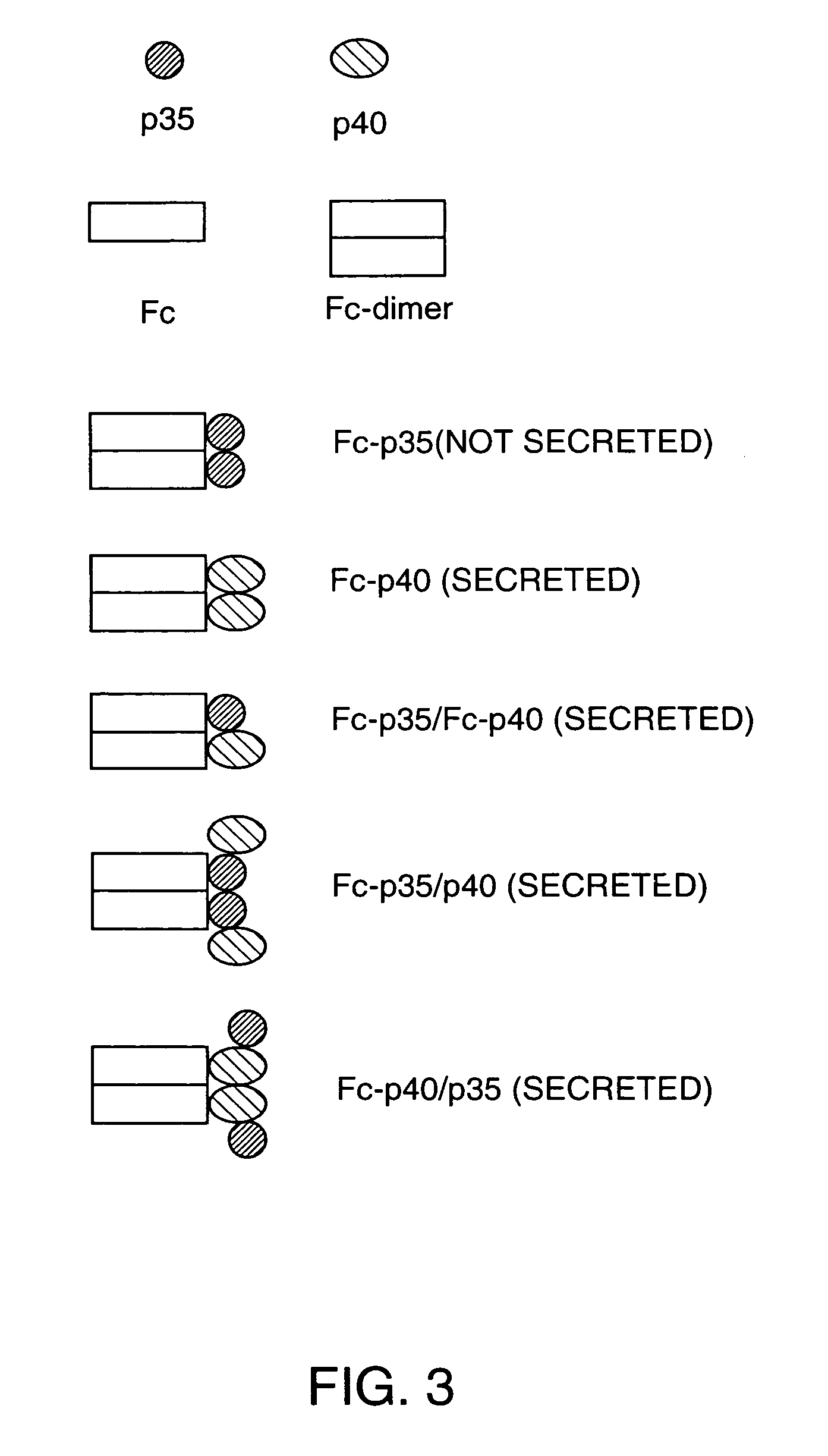
Disclosed are methods for producing fusion proteins with the heterodimeric cytokine, interleukin-12. In order to insure that the proper ratio of fused and non-fused subunits are obtained in the fusion protein, a specific stepwise approach to genetic engineering is used. This consists of first expressing the non-fused p40 IL-12 subunit in a production cell line, followed by or simultaneously expressing in the same cell, a second recombinant fusion protein consisting of the fused polypeptide linked by a peptide bond to the p35 subunit of IL-12. Molecules containing the p35 fusion protein cannot be secreted from the transfected mammalian cell without first complexing in a one to one ratio with the p40 subunit, thus ensuring the production of active heterodimeric fusion proteins.
Owner:MERCK PATENT GMBH
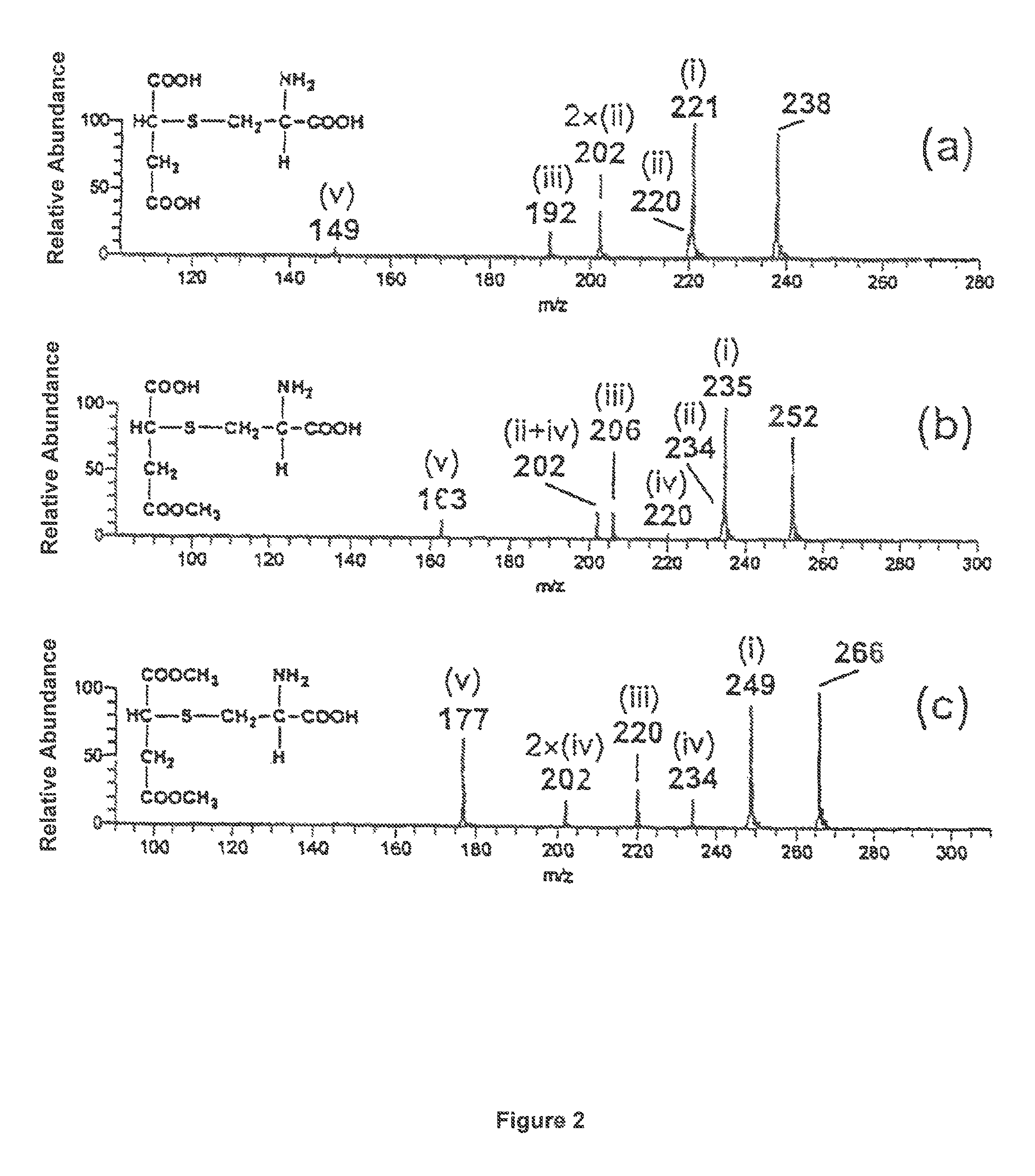
Thiosuccinic Acid Derivatives and the Use Thereof
ActiveUS20090011986A1Similar effectEasy to prepareOrganic active ingredientsBiocideThio-Autoimmune disease
The present invention relates to compounds of the Formula (I), wherein X1 and X2 independently represent O, NH or S; R1 and R2 are independently selected from the group consisting of a C1-C30 hydrocarbyl group, an amino acid bonded via an amide bond or a peptide bonded via an amide bond each having up to 200 amino acids, the conjugated residue X1 or X2 in this case being NH, and hydrogen, both radicals R1 and R2 preferably not being H; and R3 is a residue selected from group consisting of —S—R6, wherein R6 is a C1-C30 hydrocarbyl group, at least one of R1 and R2 not being H when X1 and X2 are oxygen, —S—CH2—CH(NH2)(COOH) (cysteine-S-yl), a homologue or derivative (e.g. N-acetyl cysteine-S-yl) thereof, a peptide having up to 200 amino acids which contains at least one amino acid radical with a thiol group, preferably a cysteine radical, and is bonded via the thio sulfur, preferably via the cysteine sulfur (peptide-S-yl), coenzyme A which is bonded via a thiol group or fragments thereof, acyl carrier protein bonded via a thiol group, and dihydrolipoic acid bonded via a thiol group; and pharmaceutically acceptable salts thereof. The present invention also relates to the use of these compounds for preparing a drug, drugs containing the same and their use for the therapy of diseases such as autoimmune disease, NF-kappaB mediated diseases, psoriasis, psoriatic arthritis, neurodermitis, enteris regionalis Crohn, cardiac insufficiency, chronic obstructive pulmonary diseases and asthma and in transplantation medicine.
Owner:BIOGEN INT
Heterodimeric fusion proteins useful for targeted immune therapy and general immune stimulation
InactiveUS7226998B2Altering pharmacology and biodistributionIncreasing its circulating half-life and its affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsStepwise approachImmune therapy
Disclosed are methods for producing fusion proteins with the heterodimeric cytokine, interleukin-12. In order to insure that the proper ratio of fused and non-fused subunits are obtained in the fusion protein, a specific stepwise approach to genetic engineering is used. This consists of first expressing the non-fused p40 IL-12 subunit in a production cell line, followed by or simultaneously expressing in the same cell, a second recombinant fusion protein consisting of the fused polypeptide linked by a peptide bond to the p35 subunit of IL-12. Molecules containing the p35 fusion protein cannot be secreted from the transfected mammalian cell without first complexing in a one to one ratio with the p40 subunit, thus ensuring the production of active heterodimeric fusion proteins.
Owner:MERCK PATENT GMBH
Thiosuccinic acid derivatives and the use thereof
ActiveUS8067467B2Easy to prepareImprove toleranceOrganic active ingredientsBiocideThio-Coenzyme A biosynthesis
The present invention relates to compounds of the formula (I) wherein X1 and X2 independently represent O, NH or S, R1 and R2 are independently selected from the group consisting of a C1-C30 hydrocarbyl group, an amino acid bonded via an amide bond or a peptide bonded via an amide bond each having up to 200 amino acids, the conjugated residue X1 or X2 in this case being NH, and hydrogen, both radicals R1 and R2 preferably not being H, wherein R3 is a residue selected from group consisting of —S—R6, wherein R6 is a C1-C30 hydrocarbyl group, at least one of R1 and R2 not being H when X1 and X2 are oxygen, —S—CH2—CH(NH2)(COOH) (cysteine-S-yl), a homologue or derivative (e.g. N-acetyl cysteine-S-yl) thereof, a peptide having up to 200 amino acids which contains at least one amino acid radical with a thiol group, preferably a cysteine radical, and is bonded via the thio sulfur, preferably via the cysteine sulfur (peptide-S-yl), coenzyme A which is bonded via a thiol group or fragments thereof, acyl carrier protein bonded via a thiol group, and dihydrolipoic acid bonded via a thiol group, and pharmaceutically acceptable salts thereof. The present invention also relates to the use of these compounds for preparing a drug and drugs containing the same.
Owner:BIOGEN INT
Variants of C-Type Natriuretic Peptides
InactiveUS20100331256A1Improve the immunityReduce functionPeptide/protein ingredientsPeptide sourcesChemical MoietyDisease
The present invention provides variants of C-type natriuretic peptide (CNP) comprising one or more deletions; additions of and / or substitutions with natural amino acids, unnatural amino acids and / or peptidomimetics (including peptide bond isosteres); amino acid extensions; and / or other chemical moieties such as, e.g., poly(ethylene glycol) and hydrophobic acids. The CNP variants are useful as therapeutic agents for the treatment of diseases responsive to CNP, including but not limited to bone-related disorders such as, e.g., skeletal dysplasias and achondroplasia, and vascular smooth muscle disorders such as, e.g., restenosis and arteriosclerosis.
Owner:BIOMARIN PHARMA INC
Chaperonin-target protein complex, method of producing the same, method of stabilizing target protein, method of immobilizing target protein, method of analyzing the structure of target protein, sustained-release formulation, and method of producing antibody against target protein
InactiveUS20070059794A1Excessive immune responseEfficient executionSugar derivativesHydrolasesProtein targetChaperonin
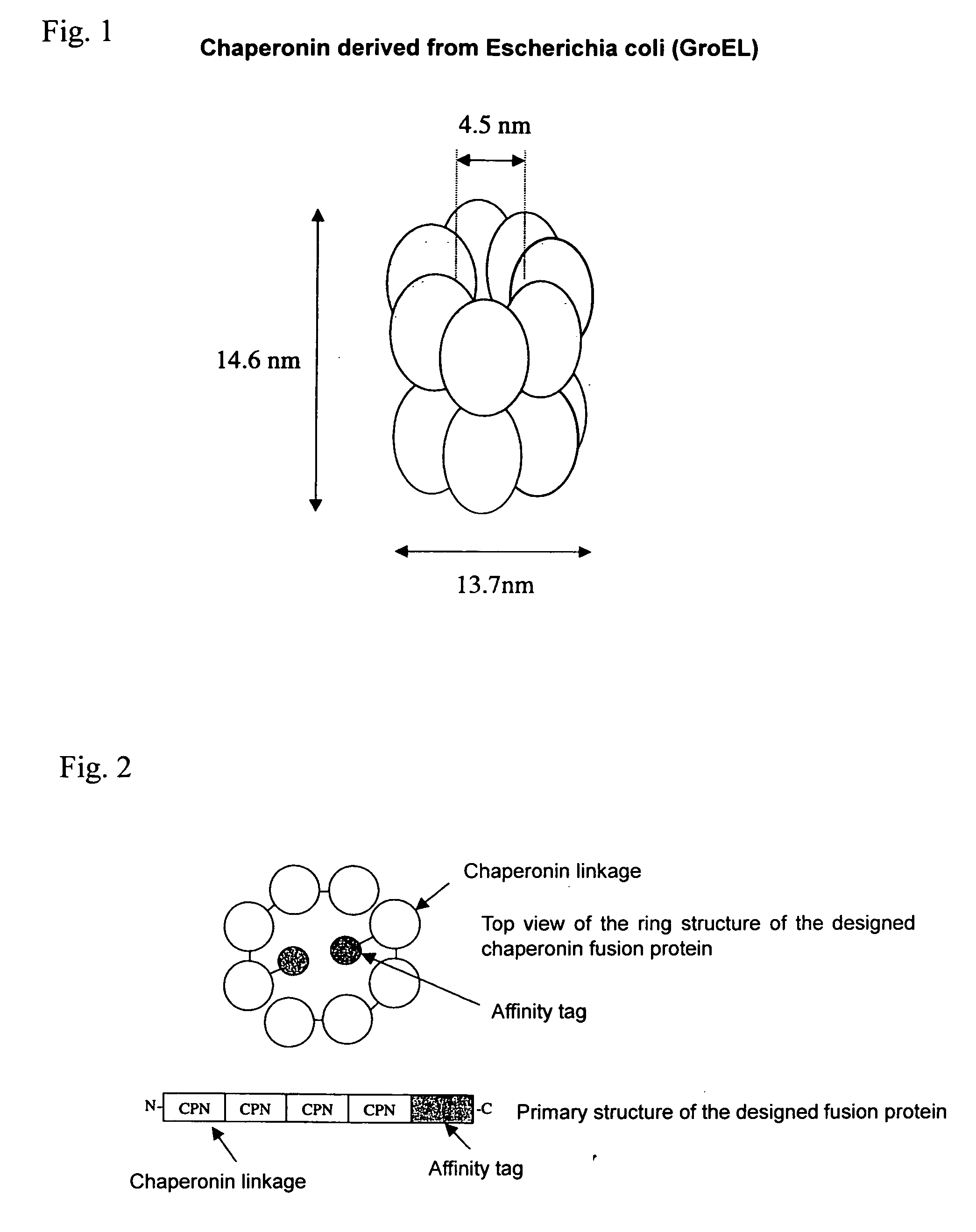
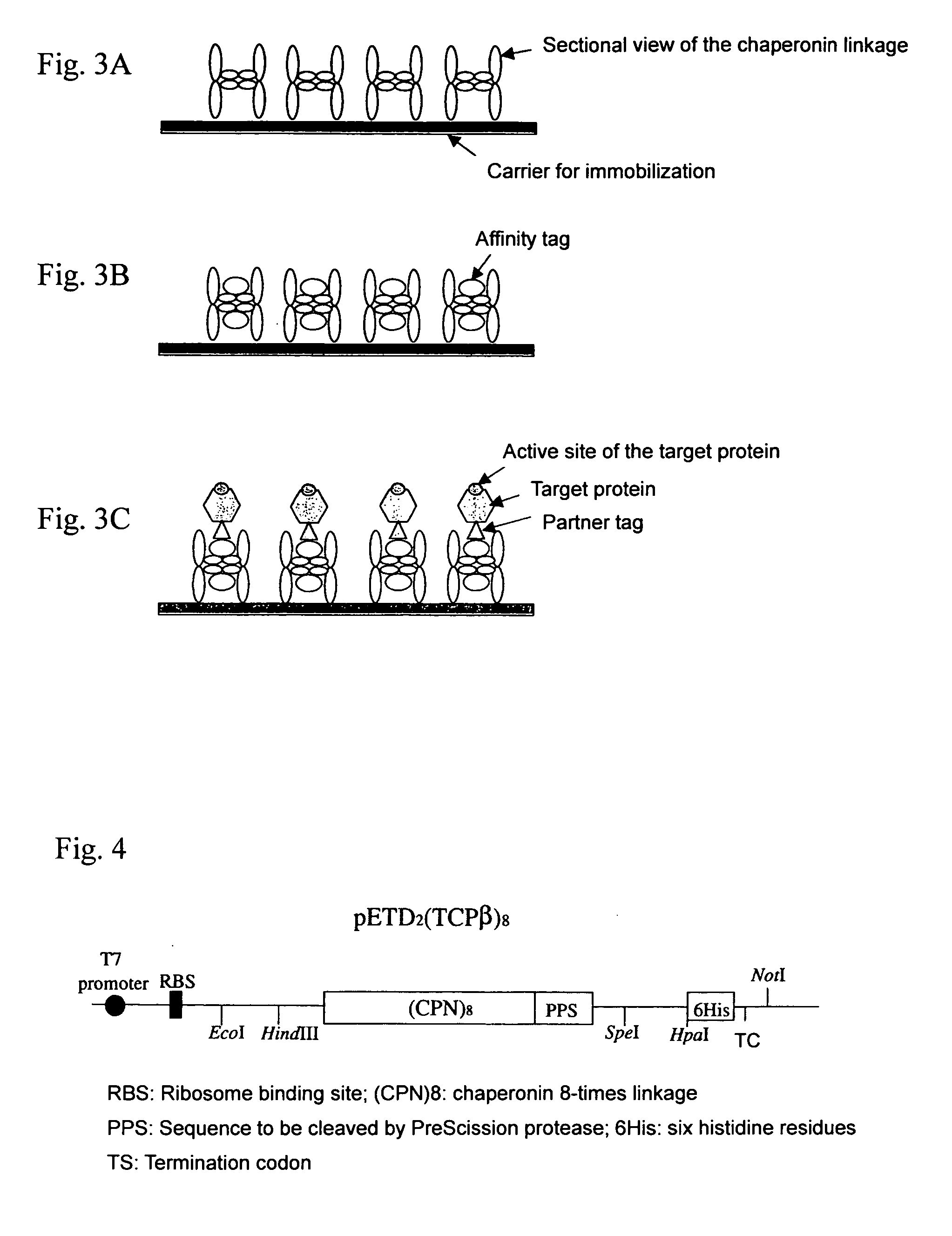
The present invention provides a chaperonin-target protein complex and a method of producing the same, and a method of stabilizing the target protein, a method of immobilizing the target protein, a method of analyzing the structure of the target protein, a sustained-release formulation, and a method of producing an antibody against the target protein. The chaperonin-target protein complex in the present invention includes a fusion protein having a chaperonin subunit and an affinity tag linked to the chaperonin subunit via a peptide bond and a target protein for which the affinity tag shows a specific affinity, wherein the target protein is bound to the affinity tag by means of the specific affinity, thereby forming a chaperonin ring structure consisting of a plurality of chaperonin subunits. The chaperonin-target protein in the present invention stabilizes the target protein and surely immobilize on a carrier without causing any change in its stereostructure.
Owner:SEKISUI CHEM CO LTD
Preparation method of fermented rice sticks
ActiveCN101623065APlay the effect of debranching and straighteningShorten fermentation timeFood preparationAmylaseProteinase activity
The invention relates to a preparation method of fermented rice sticks, which belongs to the field of food fermentation and comprises a pulp making procedure and a pulp steaming procedure. The preparation method of fermented rice sticks is characterized in that 1-1000ml of debranching amylase for per 100kg of rice is added into rice pulp with the weight percent of 5 to 50% before the pulp steaming procedure and after the pulp making procedure, the pH value is regulated to 4.0-5.0, and enzymolysis is carried out for 30-360 min under the temperature of 30-60 DEG C; 1-50 g of protease with proteolysis peptide bonds for per 100kg of rice is added into the rice pulp, and enzymolysis is carried out for 60-360 min under the temperature of 30-60DEG C and the pH value of 4.0-8.0; then, 0.2 ml / kg of zymophyte is added to the pulp and fermented for 360-3600 min under the temperature of 25-30DEG C and the pH value of 5.0-6.0. In the invention, the debranching amylase is utilized to hydrolyze Alpha-D-1, 6 glycoside bonds as the branched chains in starch and dextrine to generate straight-chain compound sugar containing Alpha-D-1, 4 glycoside bonds so as to obtain the effect on debranching and adding straight chains; the protease with proteolysis peptide bonds can be used for hydrolyzing molecular protein and amino acid to provide sufficient amino acid for following mixed fermentation, shorten fermentation time, and improve the quality and the taste of products.
Owner:江西蓓蕾食品有限公司
Somatostatin agonists
Claimed are a series of somatostatin agonists and uses thereof, according to formula (I),A1-cyclo[Cys-A2-D-Trp-A3-A4-Cys]-A5-Y1, (I)wherein A1, A2, A3, A4, A5 and Y1 are as defined in specification provided that the amine nitrogen of at least one peptide bond is substituted with a methyl group or pharmaceutically acceptable salts thereof.
Owner:TULANE EDUCATIONAL FUND THE ADMINISTRATORS +1
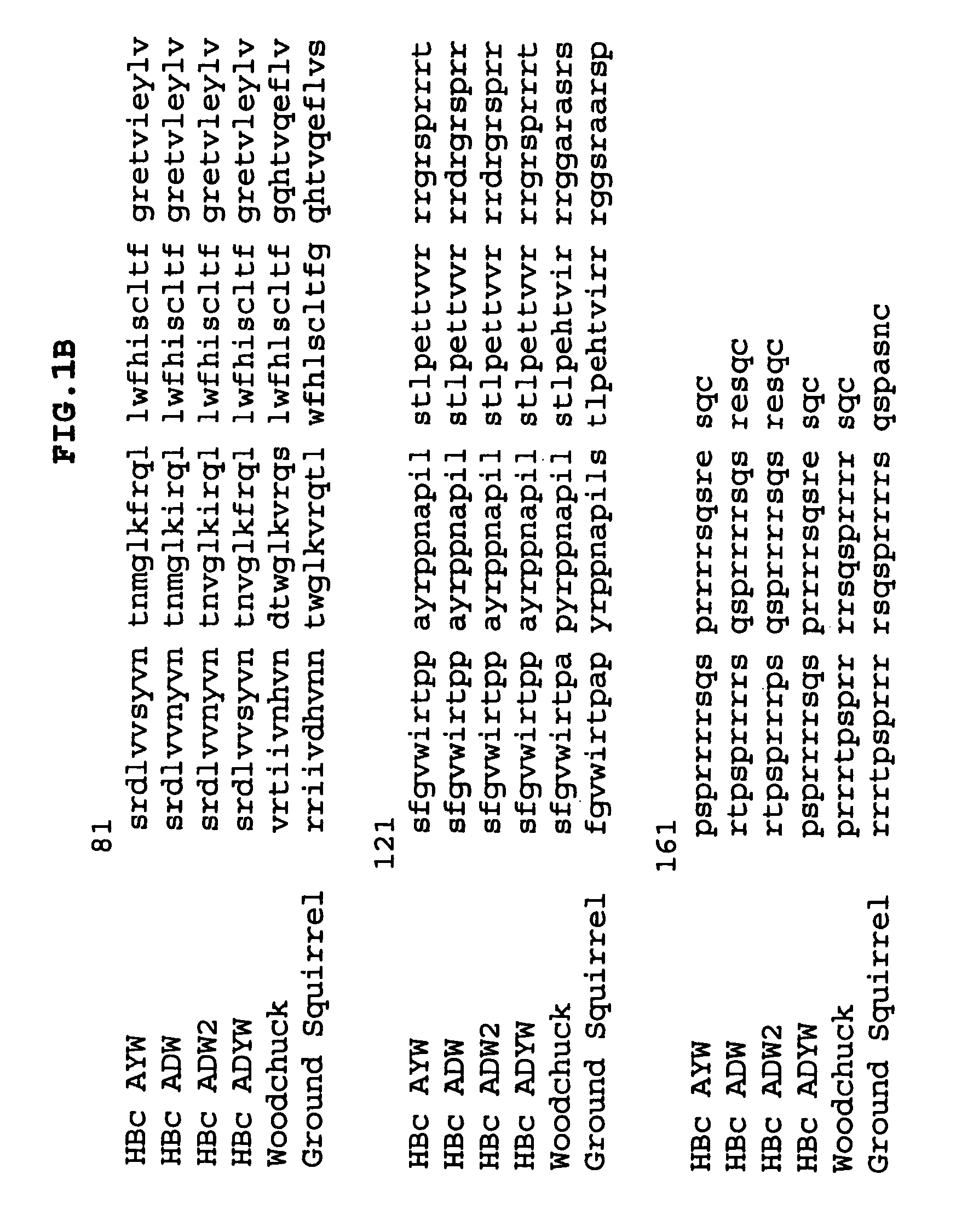
Stabilized HBc chimer particles as immunogens for chronic hepatitis
InactiveUS7351413B2Easy to prepareImprove stabilityAntibody mimetics/scaffoldsAntipyreticProtein moleculesChronic hepatitis
A method of treating chronic hepatitis B is disclosed that comprises administering a T cell-stimulating amount of a vaccine to a patient. The vaccine comprises an immunogenic amount of chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (core) protein (HBc) that is engineered for both enhanced stability of self-assembled particles and the substantial absence of nucleic acid binding by those particles. The chimeric protein molecule can include one or more immunogenic epitopes peptide-bonded to one or more of the N-terminus, the immunogenic loop or the C-terminus of HBc. The enhanced stability of self-assembled particles is obtained by the presence of at least one heterologous cysteine residue near one or both of the amino-terminus and carboxy-terminus of the chimer molecule.
Owner:LORANTIS
Leaf-shaped bean curd and production method thereof
The invention belongs to the field of a food machining technology and particularly relates to a leaf-shaped bean curd and a production method of the leaf-shaped bean curd. The leaf-shaped bean curd comprises the following components: soy isolated protein, vegetable protein, starch, ice cakes, water, plant oil, white granulated sugar, a TG (Triglyceride) enzyme, a coagulator, chicken powder, egg white, table salt, sophora flowers, a flavor enhancer and a sucrose fatty acid ester. The preparation method comprises the following steps of: mixing the TG enzyme with the starch and adding the soy isolated protein, the ice cakes and the water; and pulping, emulsifying, traying, curing and molding to obtain the leaf-shaped bean curd. According to the leaf-shaped bean curd and the production method of the leaf-shaped bean curd disclosed by the invention, the TG enzyme and the starch including potato starch, sweet potato starch and the like are used as auxiliary materials to have synergistic effect so as to catalyze protein molecules to have cross-linking; and the performance of the soy isolated protein is improved so that a network structure is formed between adjacent peptide bonds and the toughness and the mouth feel of the leaf-shaped bean curd are enhanced.
Owner:SHANDONG GAOTANG LANSHAN GRP CORP
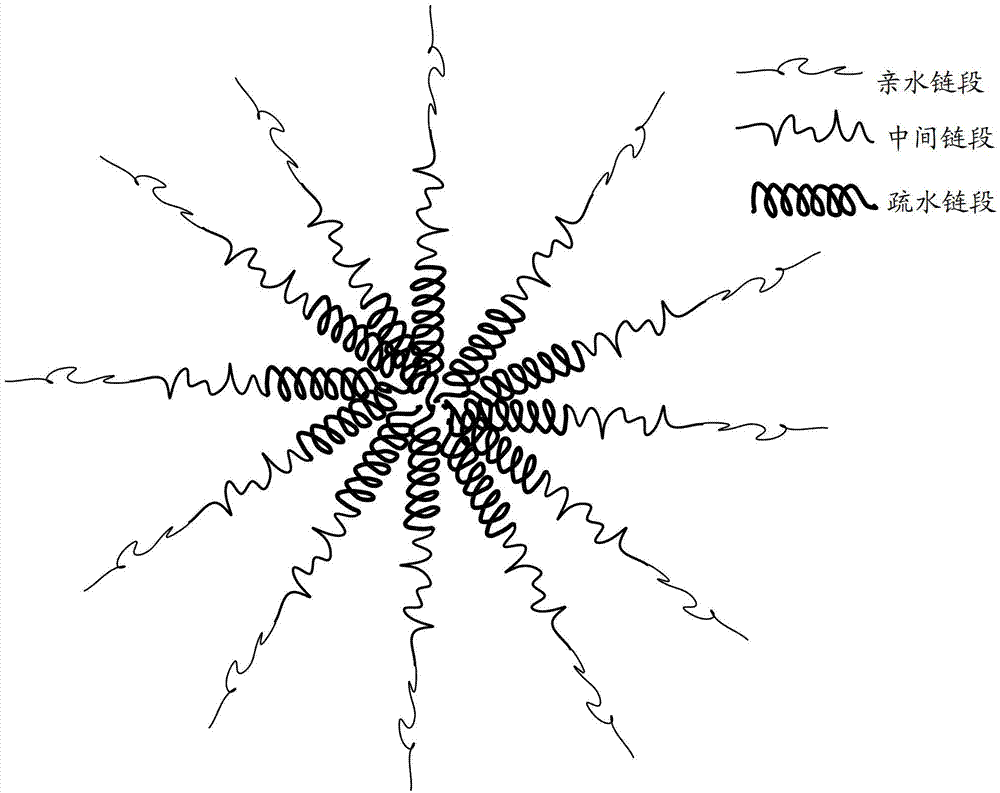
Amphipathy triblock polymer and preparation method and application thereof
ActiveCN103087311ACharge Strength ControlGenetic material ingredientsEmulsion deliveryCyclic processProtein molecules
The invention relates to an amphipathy triblock polymer. The amphipathy triblock polymer is a linear compound comprising a hydrophilic chain section, an intermediate chain section and a hydrophobic chain section, wherein the hydrophilic chain section is a polyethylene glycol derivative; the intermediate chain section is a peptide polymer comprising lysine, leucine and glutamic acid; the hydrophobic chain section is polyphenylalanine; the polyethylene glycol derivative is connected with one end of the intermediate chain section through an amido bond; and the polyphenylalanine is connected with the other end of the intermediate chain section through a peptide bond. The amphipathy triblock polymer provided by the invention can be self-assembled in an aqueous solution to form a polymer nano-micelle with a three-layer structure; the polymer nano-micelle servicing as a vector has the characteristics of high stability, excellent biocompatibility and the like; the amphipathy triblock polymer is hardly removed by the immune system during circulating in a human body, and hydrophobic small molecule drugs, genetic materials and protein molecules can be effectively loaded at the same time, so that the amphipathy triblock polymer has a wide application prospect. In addition, the invention also provides a preparation method of the amphipathy triblock polymer.
Owner:SHENZHEN INST OF ADVANCED TECH
Amphiphilic triblock copolymer, polymer nano-carrier preparation and preparation methods
The invention relates to an amphiphilic triblock copolymer, a polymer nano-carrier preparation and preparation methods. The copolymer is a liner polymer compound containing a polyethylene glycol derivative, poly-lysine and poly-leucine, wherein one end of the poly-lysine is connected with the polyethylene glycol derivative through an amide bond, and the other end of the poly-lysine is connected with the poly-leucine through a peptide bond. The amphiphilic triblock copolymer, namely the polyethylene glycol derivative-poly-lysine-poly-leucine triblock copolymer combines the advantages of polyethylene glycol and poly-amino acid, can form a nano-carrier with a three-layer structure by self-assembly in a water solution, and can simultaneously effectively load a small molecular hydrophobic medicament, gene substances and proteins or polypeptides to form a multifunctional nano-carrier.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Methods of synthesizing insulin polypeptide-oligomer conjugates, and proinsulin polypeptide-oligomer conjugates and methods of synthesizing same
InactiveUS6913903B2Less expensiveHigh yieldPeptide/protein ingredientsDepsipeptidesOligomerProinsulin
Methods for synthesizing proinsulin polypeptides are described that include a contacting a proinsulin polypeptide including an insulin polypeptide coupled to one or more peptides by peptide bond(s) capable of being cleaved to yield the insulin polypeptide with an oligomer under conditions sufficient to couple the oligomer to the insulin polypeptide portion of the proinsulin polypeptide and provide a proinsulin polypeptide-oligomer conjugate, and cleaving the one or more peptides from the proinsulin polypeptide-oligomer conjugate to provide the insulin polypeptide-oligomer conjugate. Methods of synthesizing proinsulin polypeptide-oligomer conjugates are also described as are proinsulin polypeptide-oligomer conjugates. Methods of synthesizing C-peptide polypeptide-oligomer conjugates are also described.
Owner:BIOCON LTD
Method for treating rheumatoid arthritis by inhibiting peptidylarginine deiminase
InactiveUS20050159334A1Organic active ingredientsPeptide/protein ingredientsSide chainRheumatoid arthritis
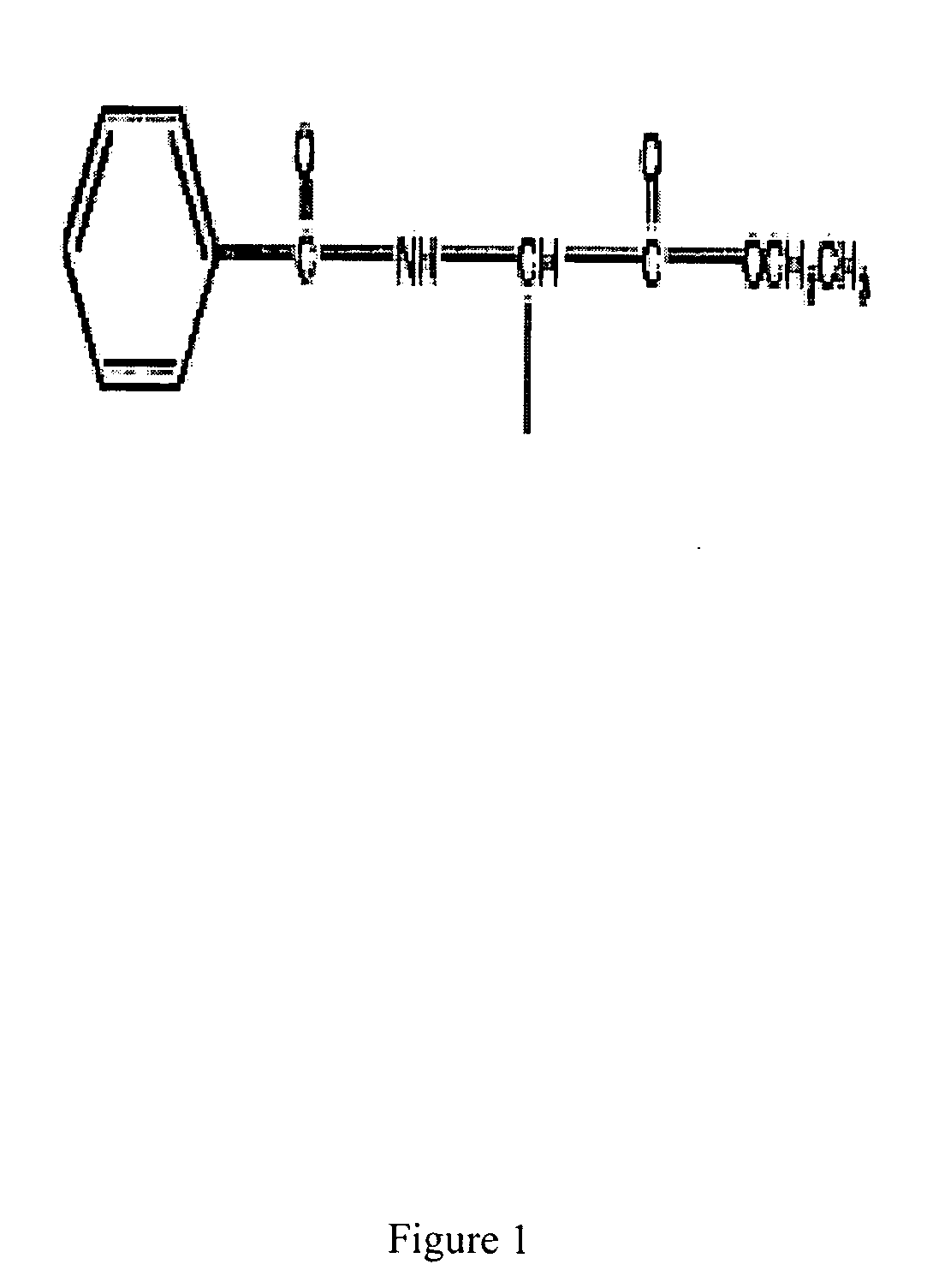
Rheumatoid arthritis is treated with the administration of a therapeutic dose of a therapeutically acceptable PAD inhibitor. Administration can occur after the onset of RA symptoms, or prophylactically before such symptoms present. In one embodiment, the inhibitor has a side chain including a benzamide group to the left and an ester group to the right of a peptide bond.
Owner:GLUCK OSCAR S +1
Method For The Manufacture Of Degarelix
ActiveCN102428097APeptide/protein ingredientsLuteinising hormone-releasing hormoneOrganic solventOrganic base
In a step-wise synthesis of degarelix comprising 0.3 % by weight or less of 4-([2-(5-hydantoyl)]acetylamino)-phenylalanine analog on (solid support)-NH2 a step comprises providing a solution of an amino acid or peptide of which the a-amino group is protected by Fmoc; contacting the support with the solution in the presence of reagent for forming a peptide bond between a carboxyl group of the amino acid or peptide and (solid support)-NH2; removing Fmoc by contacting the support with an organic base, in particular piperidine, in an organic solvent. Also disclosed is degarelix of high purity prepared by the method of the invention and the use of Fmoc in the synthesis of degarelix.
Owner:POLYPEPTIDE LAB AS
Prenatal enzyme replacement therapy
The invention contemplates transplacental enzyme replacement therapy (ERT) for deficiency of a polypeptide such as a tissue-nonspecific alkaline phosphatase (TNSALP) by administering a before-described pharmaceutical composition to a pregnant animal whose fetus or embryo is in need of such therapy. The fusion protein of such a composition comprises a water-soluble TNSALP portion, e.g., C-terminus-truncated TNSALP peptide-bonded to an IgG1 antibody Fc portion.The invention also contemplates a method for treating a metabolic disorder, such as HPP, in a fetus or embryo were a protein is administered to a pregnant mother. The fusion protein comprises a Fc fragment of an IgG1 antibody peptide-bonded to TNSALP. The protein crosses the placenta of the mother and enters the fetal blood stream. The protein is taken up into fetal tissue such that the TNSALP restores normal metabolic activity in the fetus.
Owner:SAINT LOUIS UNIVERSITY
Use of poly-gamma-glutamic acid as fertilizer absorbefacient in agricultural planting
InactiveCN1827560AHigh environmental valueReduce the amount of applicationFertilizer mixturesKilodaltonPotassium
The invention relates to the application of the gamma-polyglutamic acid as the absorption promoting agent of the fertilizer in the agricultural husbandry. The said gamma-polyglutamic acid is a high molecular polymer of D-glutacid and L- glutacid formed by the alpha-amino and the gamma-carboxyl in the manner of peptide bond and there is also a dissociative carboxyl on the alpha-carbon atom of every repetitive unit with average molecular weight of 10-10,000 kilodaltons. The said gamma-polyglutamic acid can be used as the absorption promoting agent of the fertilizer made of single element of nitrogen, phosphor, kalium or their composition. The significance of the invention is: it can be degraded by edaphon and its environmental value is much better in relation to TPA; it can reduce by 10-30 % of the normal usage of the chemical fertilizer by applying 10-50 grams of fertilizer per area and increase by 10-30 % of the crop yield, at the same time it can improve the crop quality and increase the crop content of nitrogen, phosphor and kalium.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com