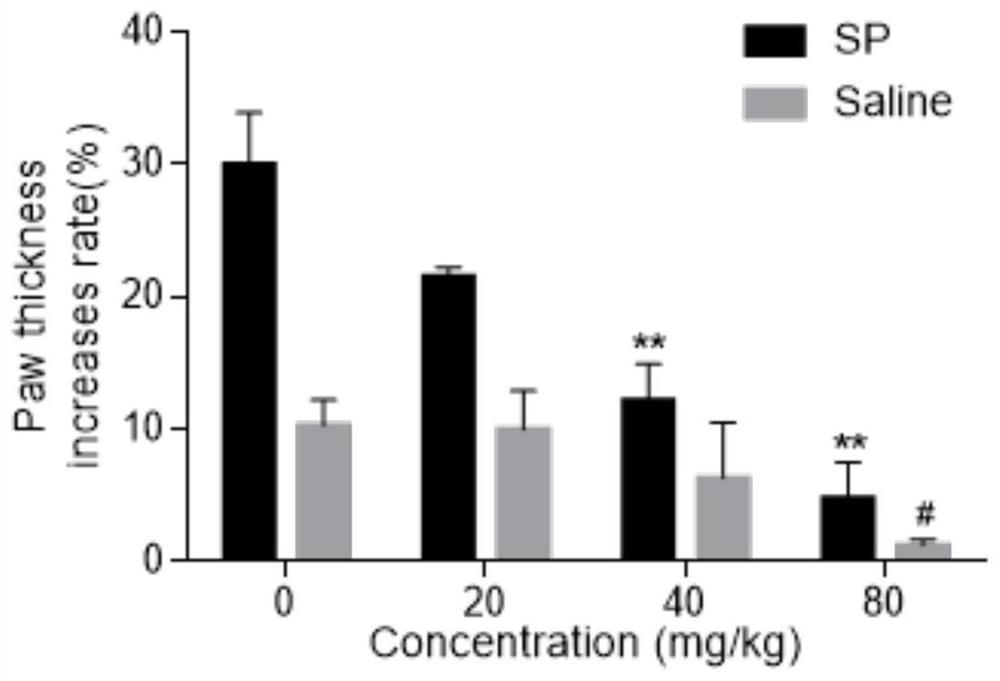
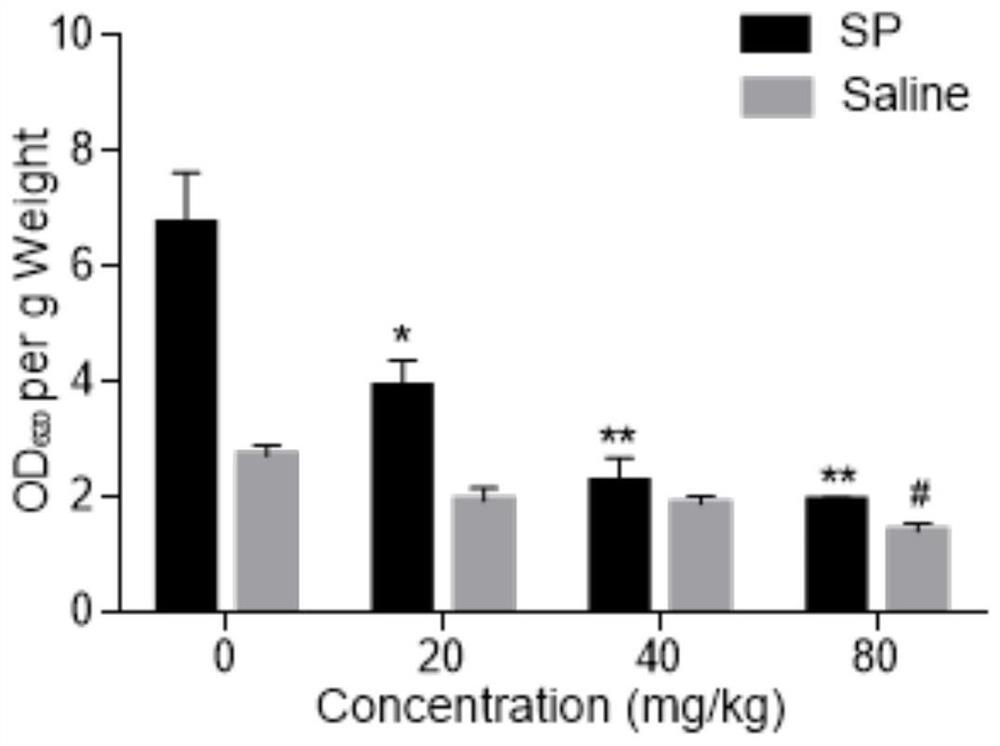
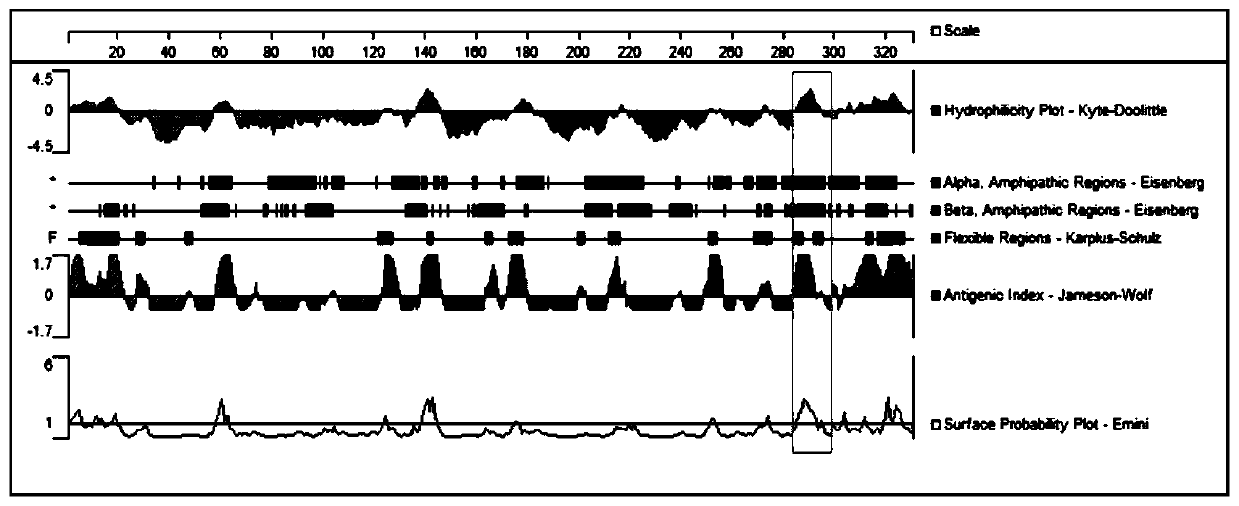
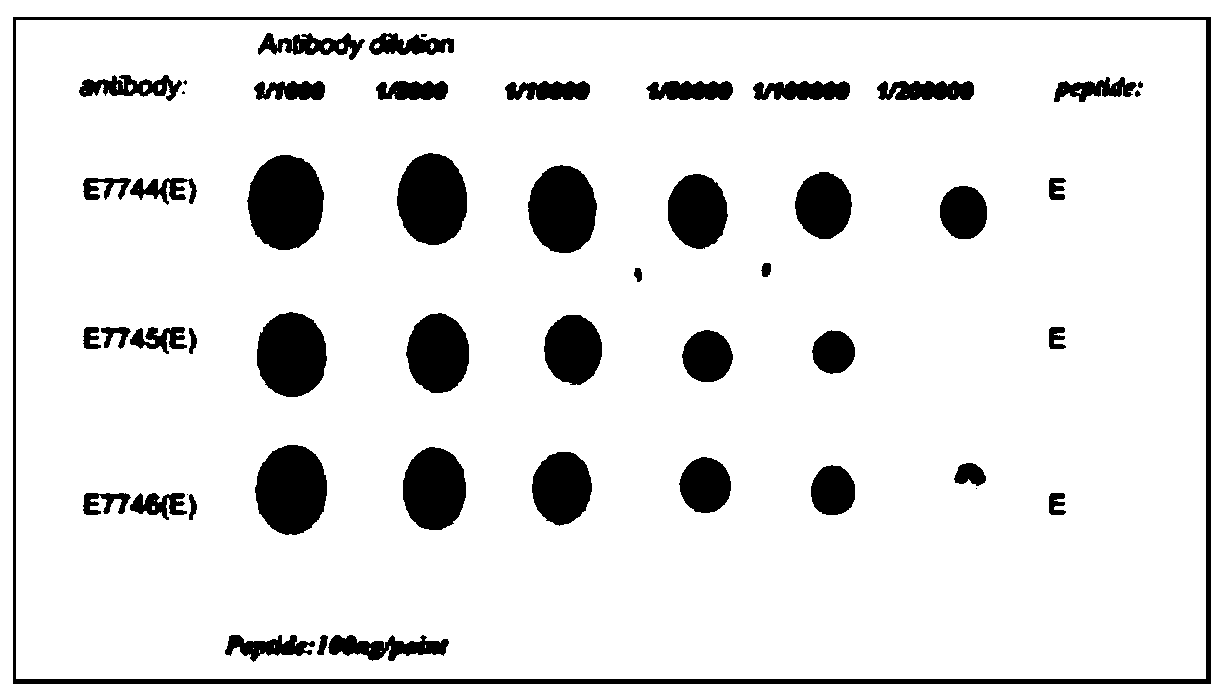
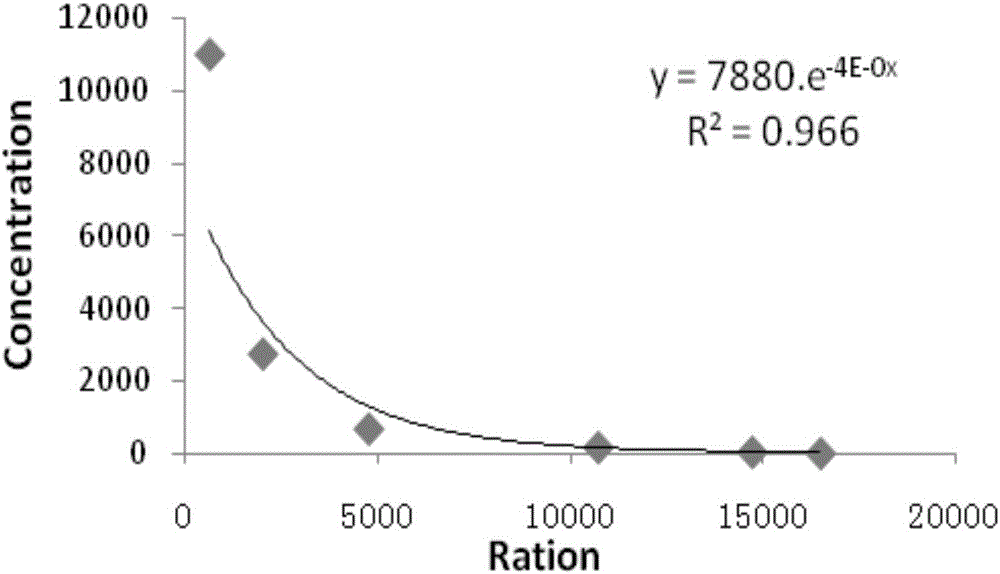
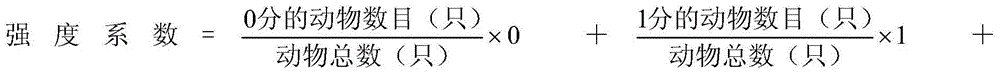Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Anaphylactoid reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
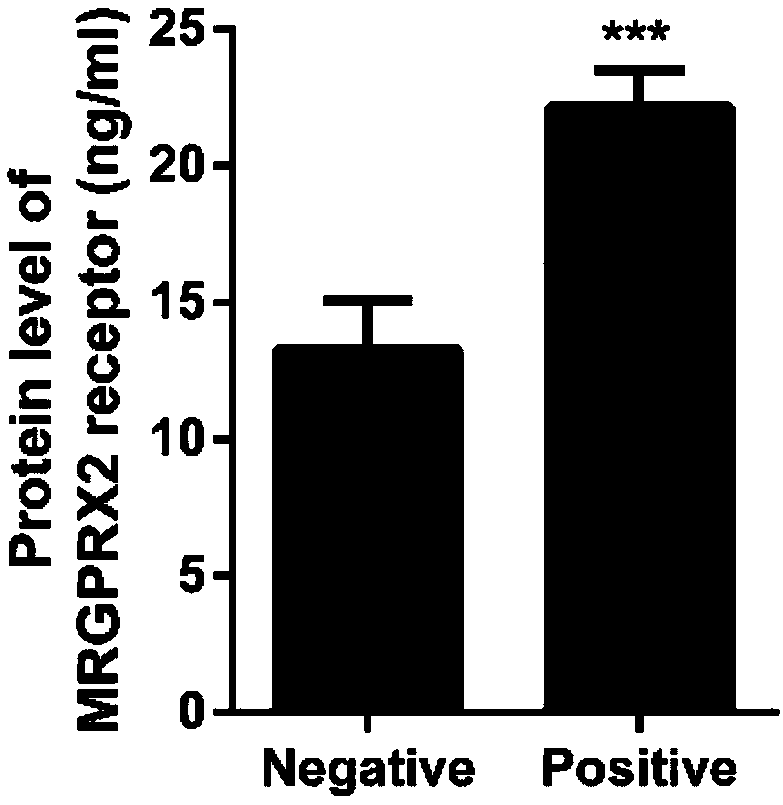
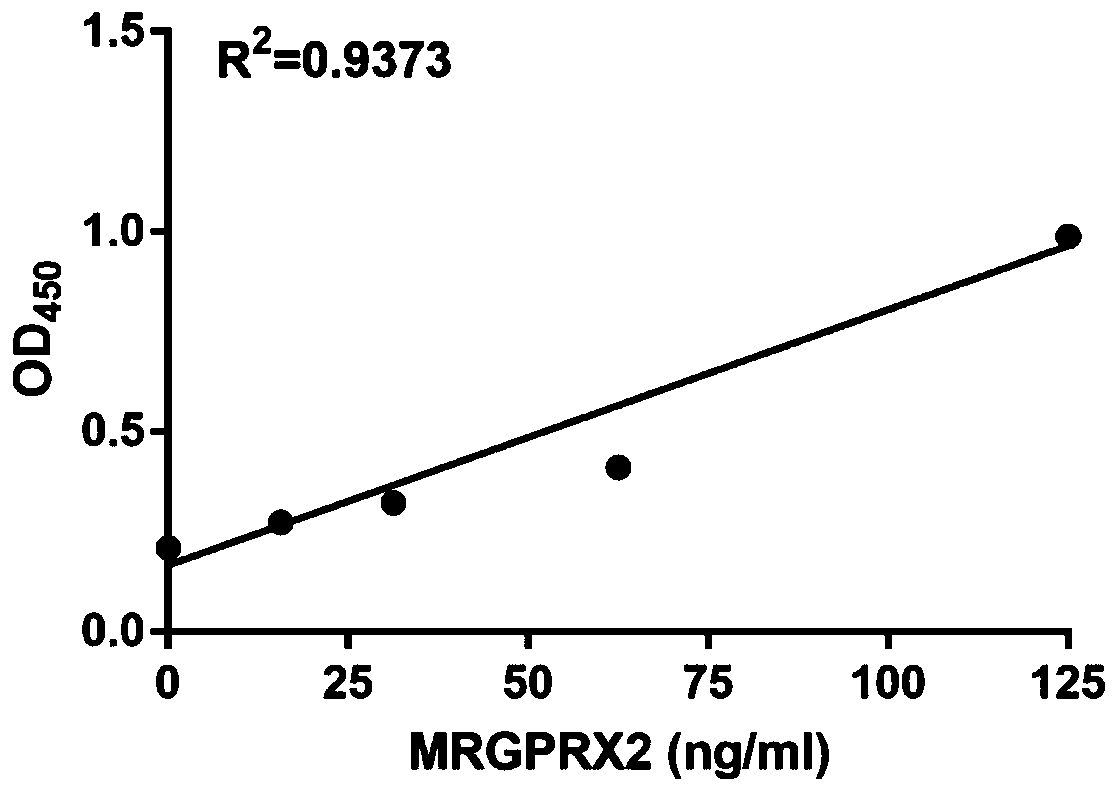
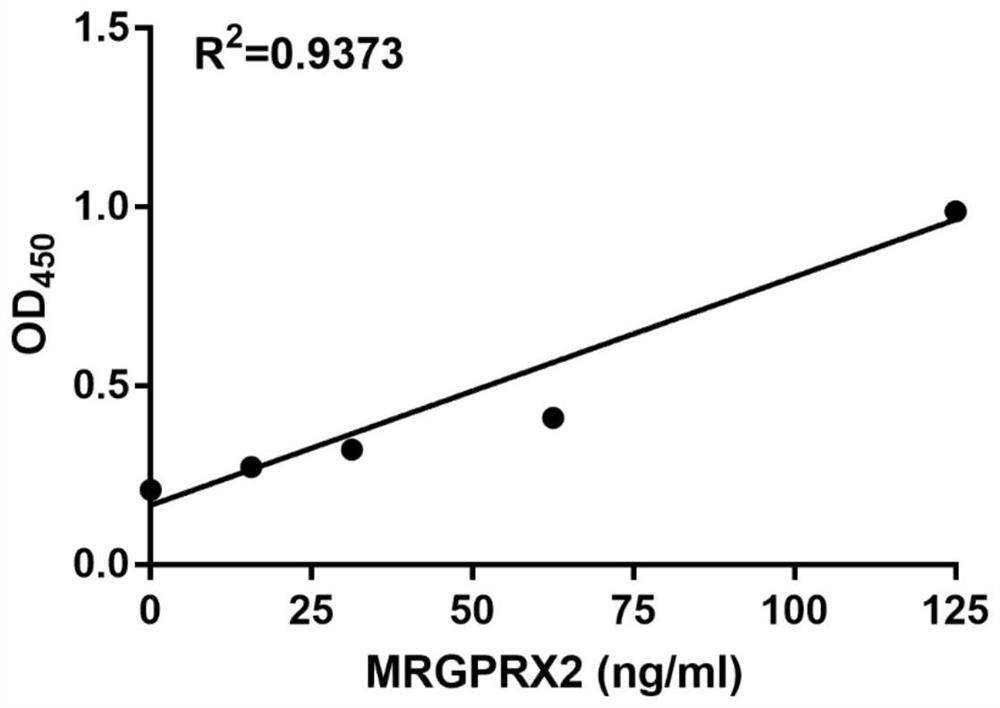
Protein marker and method for screening drug anaphylactoid reaction susceptive groups
InactiveCN107703312APromote early discoveryIncreased sensitivityCell receptors/surface-antigens/surface-determinantsBiological material analysisUse medicationAnaphylactoid reactions
The invention provides an MRGPRX2 protein serving as a protein marker for screening drug anaphylactoid reaction susceptive groups and application thereof. Researches find out that the relative expression of the MRGPRX2 protein in peripheral blood has relevance to drug anaphylactoid reaction susceptivity of testers, and the MRGPRX2 protein can serve as a protein marker for screening the drug anaphylactoid reaction susceptive groups. The relative expression of the MRGPRX2 protein is used for evaluating risk of anaphylactoid reaction of patients occurring in the medication process, and has significance on clinical guiding of pharmacy safety. The method for screening drug anaphylactoid reaction susceptive groups provided by the invention is used for screening occurrence risk of anaphylactoid reaction of the patients and has extremely high susceptivity and specificity. Moreover, since the clinically available peripheral blood is adopted for detection, the method is convenient to use and issuitable for screening of large scale population of patients before medication, advanced discovery of the anaphylactoid reaction susceptive groups is facilitated, and the medication safety is improved.
Owner:XI AN JIAOTONG UNIV
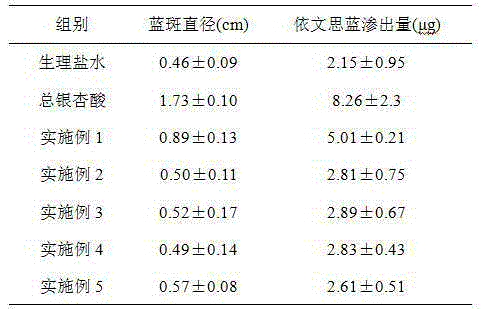
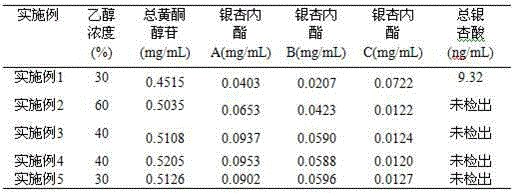
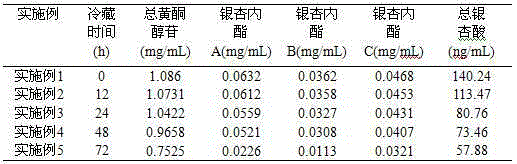
Preparation method of ginkgo leaf extract
InactiveCN105267257AReal-time online analysis and detectionGuaranteed continuityGinkgophyta medical ingredientsReflux extractionAnaphylactoid reactions
The invention discloses a preparation method of a ginkgo leaf extract. The preparation method comprises the following steps: (1) heating for reflux extraction by using ethanol; (2) adjusting the pH to be alkaline, and placing for cold storage; (3) passing through a macroporous resin column; and (4) filtering through a microporous filtering film, and sterilizing to obtain the ginkgo leaf extract, wherein 25% ethanol and a 10% sodium acetate solution are used for eluting impurity peaks in the step (3), and 30-50% ethanol is used for eluting target component peaks. The preparation method disclosed by the invention has the beneficial effects that a harmful component namely total ginkgolic acid is effectively removed, anaphylactoid reaction is reduced, the safety is improved, the purity of a target component is improved, the product quality is improved, and automatic online detection is realized.
Owner:HEILONGJIANG ZBD PHARMA
Method for detecting anaphylactoid reaction of Shengmai liquid preparation
InactiveCN102590492AQuality assuranceHigh sensitivityBiological testingAnaphylactoid reactionsBasophilic Granulocyte
The invention discloses a method for detecting an anaphylactoid reaction of a Shengmai liquid preparation. According to the method, the quality of a finished Shengmai liquid preparation product is detected by inducing the activation amount of basophilic granulocyte through the Shengmai liquid preparation, and the method is a quick detection method for industrial scale production safety evaluation of the Shengmai liquid preparation, so that the quality of the finished Shengmai liquid preparation product is ensured, and the occurrence rate of the anaphylactoid reaction in the clinical application process is reduced.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD
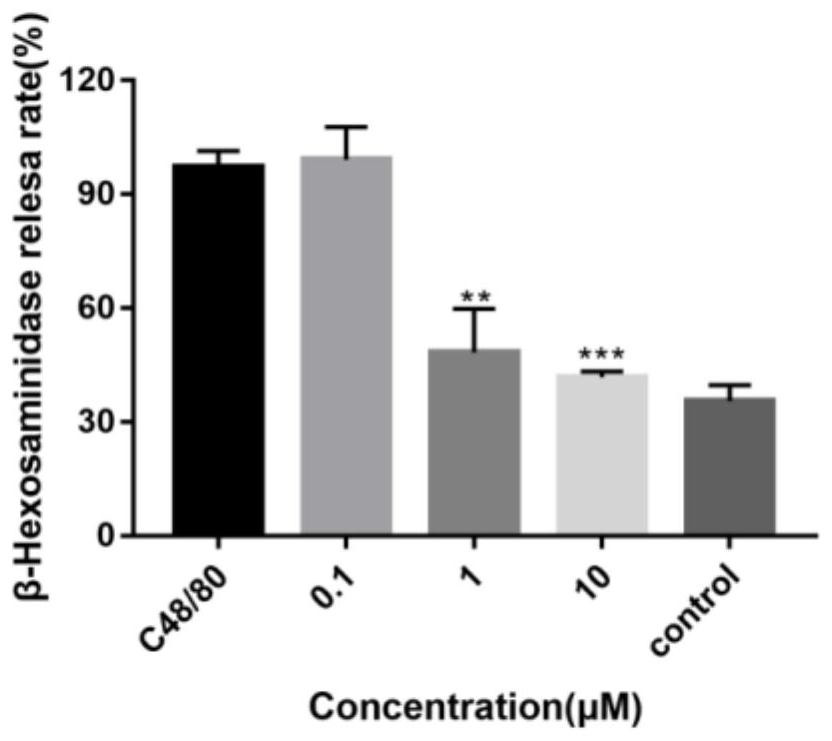
Application of furocoumarins compound in preparing anti-allergic medicine
ActiveCN107661328AAllergic reactions suppressedRich types of anti-allergic drugsImmunological disordersHeterocyclic compound active ingredientsMast cellAnaphylactoid reactions
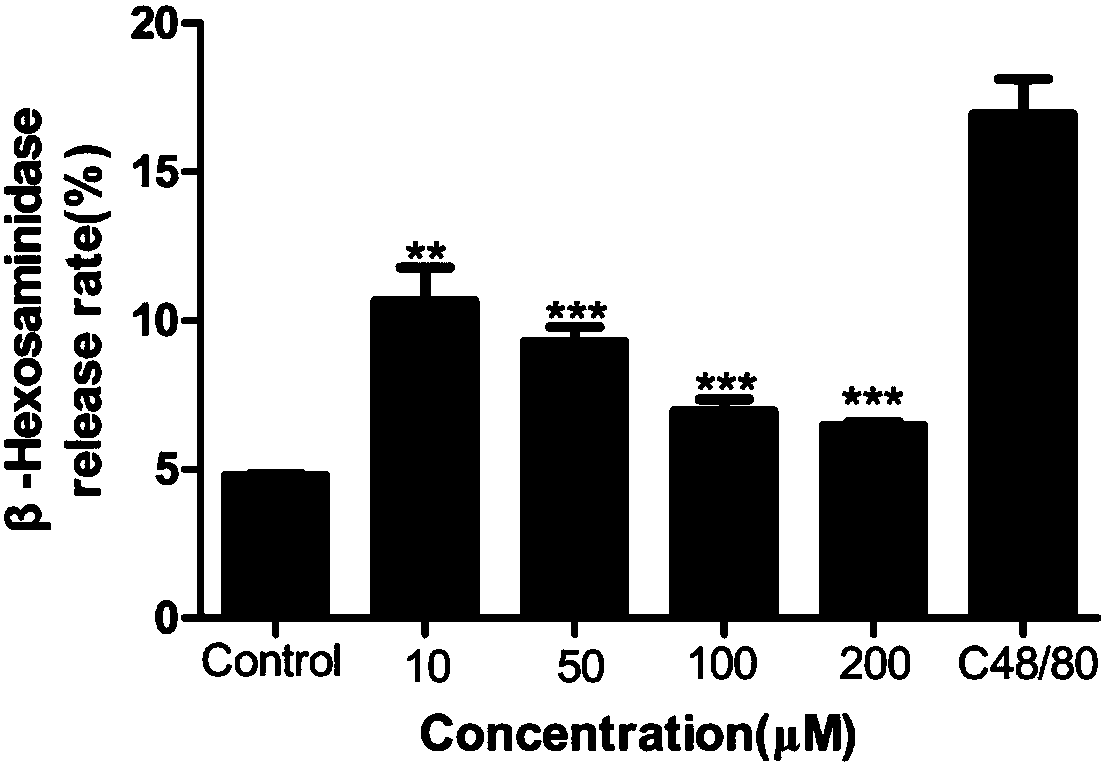
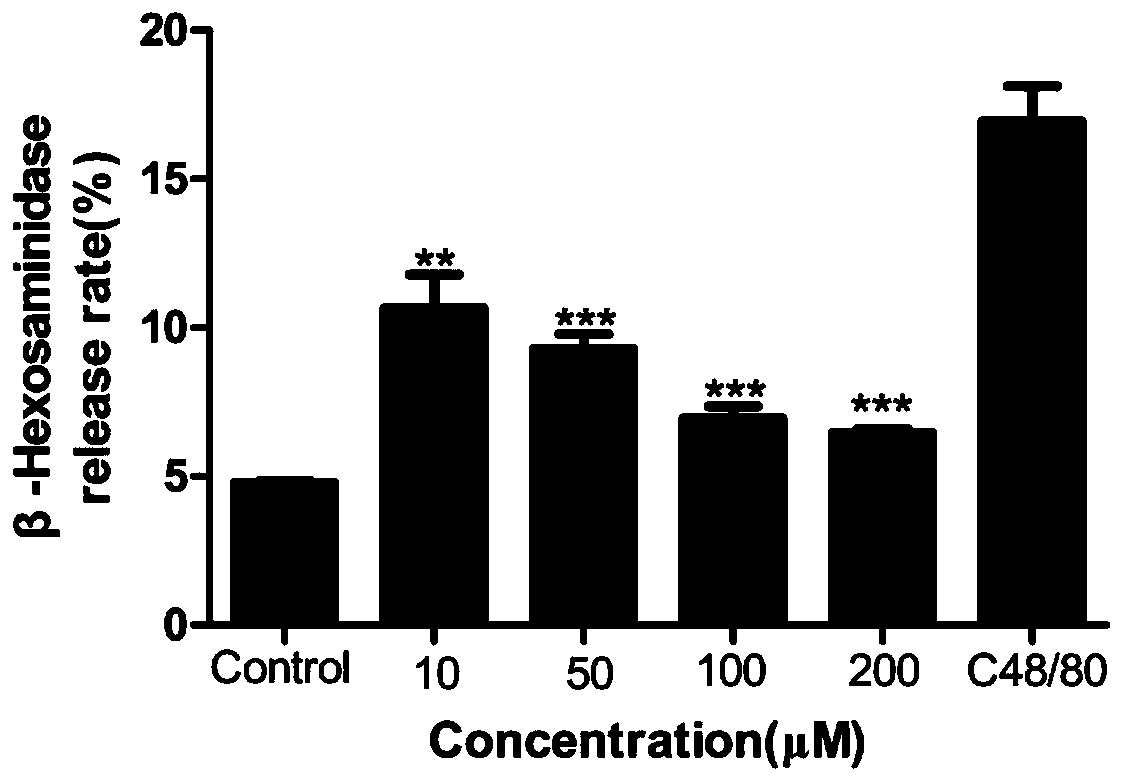
The invention provides application of furocoumarins compound in preparing anti-allergic medicine and belongs to the technical field of biological medicine. The application disclosed by the invention proves that the furocoumarins compound can effectively antagonize beta-hexosaminidase release caused by C48 / 80 of human mast cell KU812 for the first time and further can inhibit anaphylactoid reaction; furthermore, the application also proves that an action receptor of the furocoumarins compound is an MrgprX2 receptor for the first time; when the furocoumarins compound in preparing anti-allergic medicine is prepared into anti-allergic preparation, anti-allergic medicine types can be enriched, and a brand-new choice and a brand-new strategy are provided for anti-allergic treatment.
Owner:XI AN JIAOTONG UNIV
Ginkgo leaf composition and application thereof for preparing ginkgo biloba leaf extract injection
ActiveCN106420850AEfficient removalImprove stabilityMetabolism disorderPharmaceutical delivery mechanismAnaphylactoid reactionsSolubility
The invention relates to a ginkgo leaf composition, in particular to a ginkgo leaf composition and application thereof for preparing a ginkgo biloba leaf extract injection. The ginkgo leaf composition is prepared from 25-43% of total flavonol glycosides, 6.5-17% of ginkgolides, 3-5% of bilobalide and smaller than 3 ppm of ginkgoic acid. According to the preparation method of the ginkgo leaf composition, two kinds of resin of DM130 and AB-8 are adopted for adsorption, the harmful ingredient total ginkgolic acid is effectively removed, and anaphylactoid reaction occurrences are reduced; meanwhile, various extracting methods and composite extracting agent repeated extraction are adopted, the target component content is high, the purity is good, and the product quality is improved. The obtained ginkgo biloba leaf extract injection is clear in compositional ratio, good in stability, good in solubility and stable in curative effect and contains few impurities.
Owner:HEILONGJIANG ZBD PHARMA +1
Method for detecting materials causing anaphylactoid reaction
ActiveCN102154434BHigh sensitivityAddress limitationsMicrobiological testing/measurementAnaphylactoid reactionsCulture cell
Owner:EXPERIMENTAL RES CENT CHINA ACAD OF CHINESE MEDICAL SCI
Polypeptide used for preparing anaphylactoid reaction double-antibody sandwich kit paired antibody and application thereof
ActiveCN110240643AImproving immunogenicityHelp with researchReceptors for hormonesDisease diagnosisAnaphylactoid reactionsPolyclonal antibodies
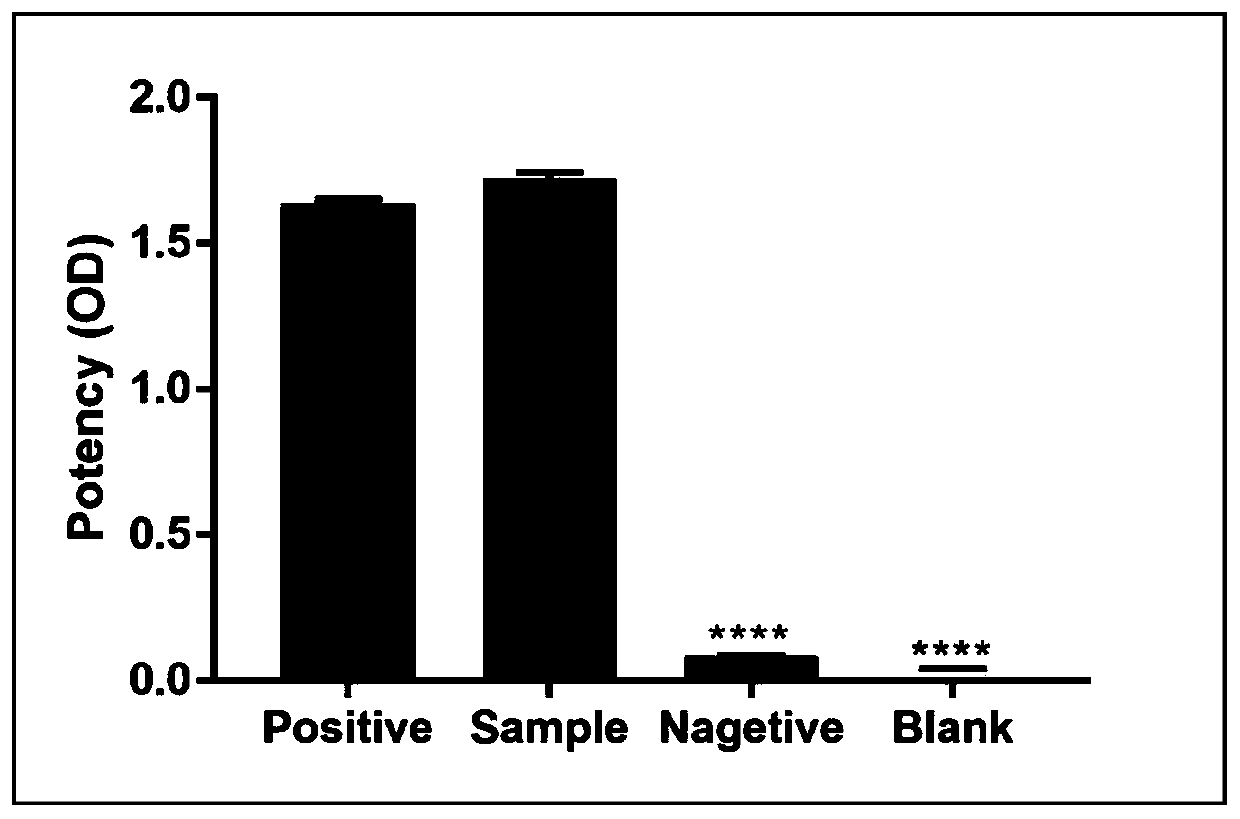
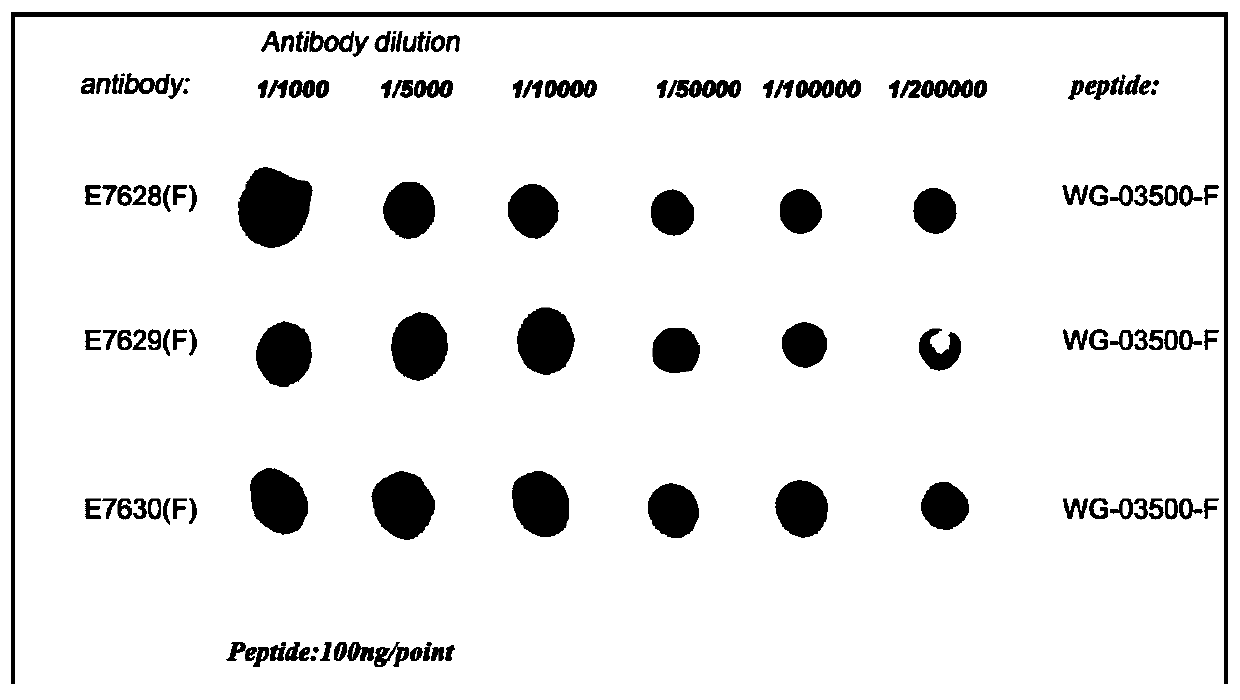
The present invention discloses a polypeptide used for preparing an anaphylactoid reaction double-antibody sandwich kit paired antibody and an application thereof, and belongs to the technical field of biomedicines. The polypeptide of the double-antibody sandwich kit paired antibody comprises a polypeptide used for preparing a mouse monoclonal antibody and a polypeptide used for preparing a rabbit polyclonal antibody; an amino acid sequence of the polypeptide used for preparing the mouse monoclonal antibody is shown in SEQ ID NO:1; and an amino acid sequence of the polypeptide used for preparing the rabbit polyclonal antibody is shown in SEQ ID NO:2. The polypeptide is an immunogenic polypeptide of an anaphylactoid reaction specific receptor MRGPRX2 protein, can be used for preparing an ELISA mouse monoclonal coating antibody and a rabbit polyclonal detection antibody, successfully prepares a double-antibody sandwich ELISA, uses the monoclonal antibody as the coating antibody and the polyclonal antibody as the detection antibody, verifies clinical applications, contributes to research work of the anaphylactoid reaction specific receptor MRGPRX2, and is of great significance for clinically guiding safety of drug use.
Owner:于向东
Detection method of medicine injection anaphylactic and anaphylactoid reactions and new application of adopted dye
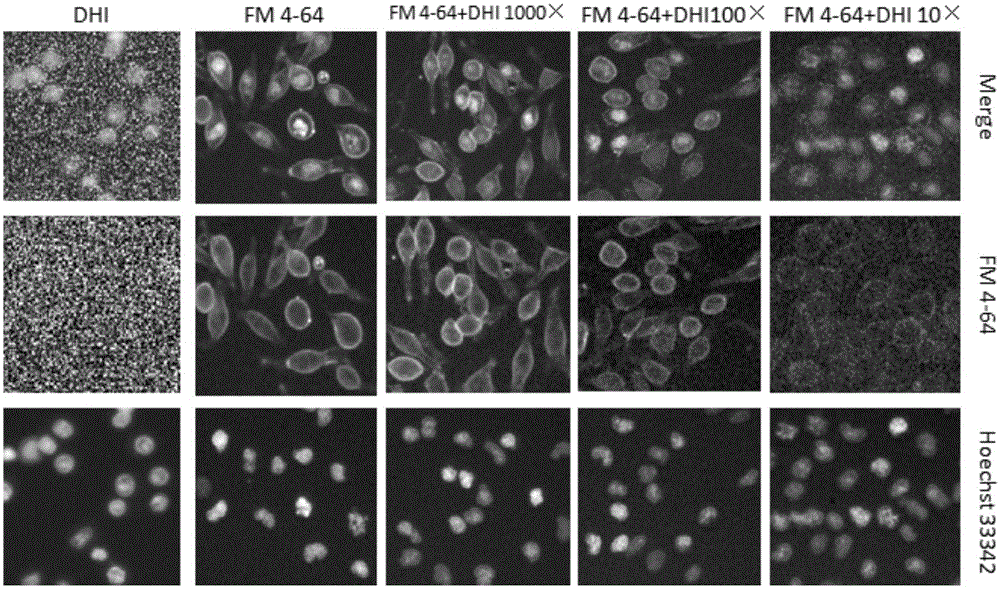
ActiveCN105004705AHigh fluorescence intensityHigh Throughput Screening CapabilitiesMicrobiological testing/measurementFluorescence/phosphorescenceAnaphylactoid reactionsCell membrane
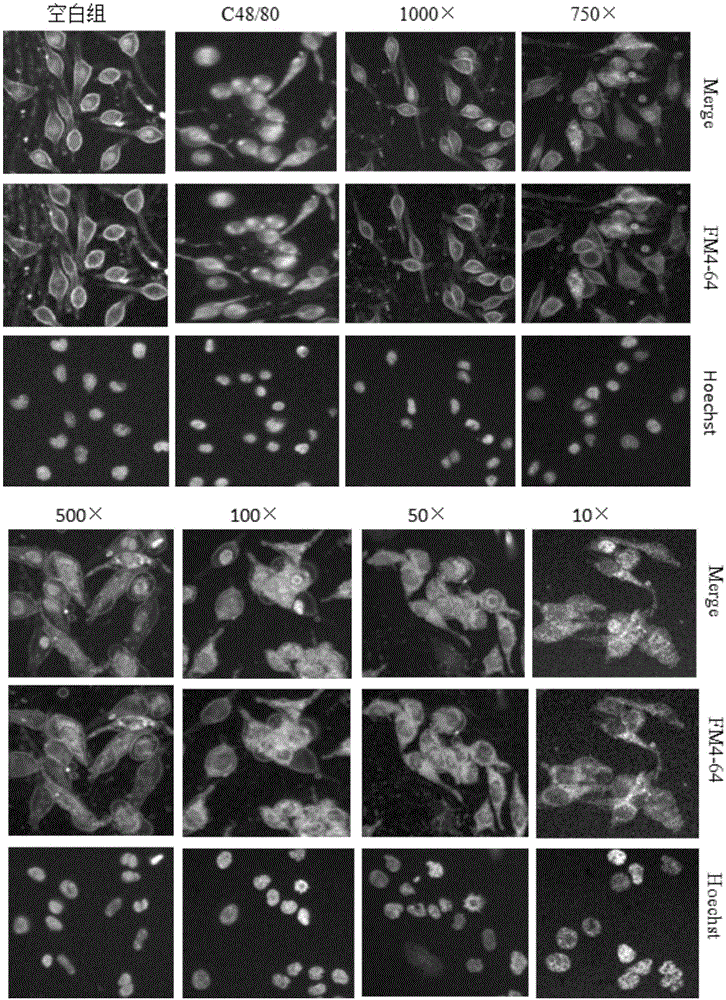
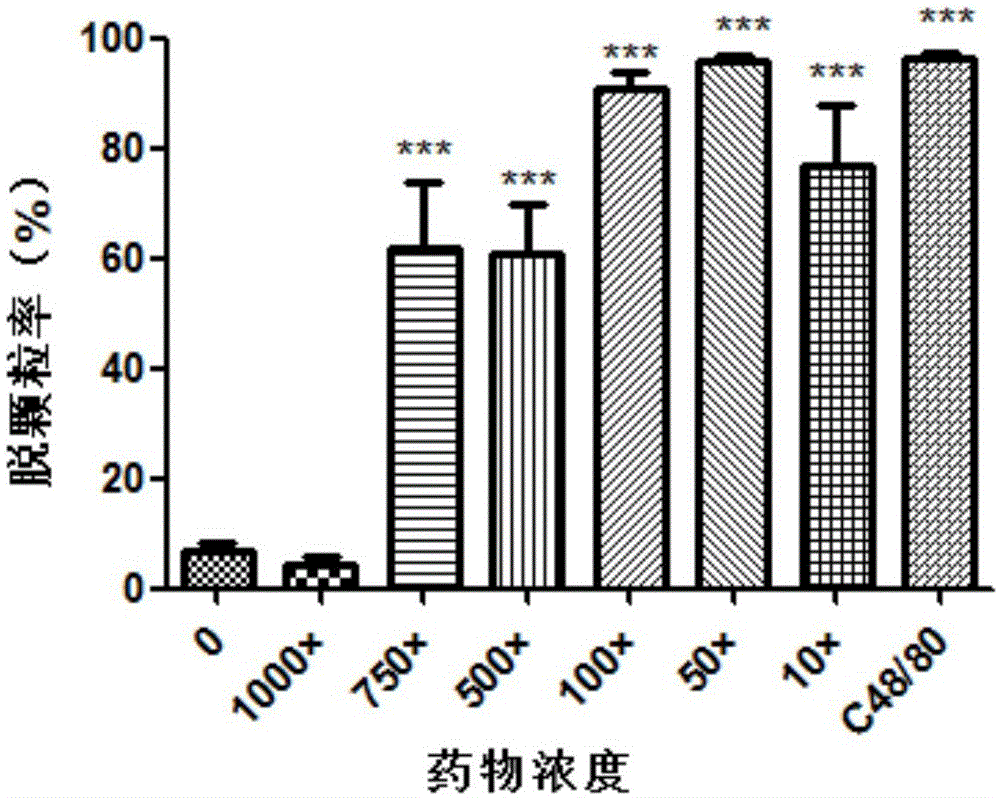
The invention provides a detection method of medicine injection anaphylactic and anaphylactoid reactions and the new application of adopted dye. Cytomembrane fluorescent dye capable of specifically staining cytomembranes and entering cells through endocytosis is adopted for marking the vesica circulating phenomenon on a cell degranulation module, analysis is carried out based on iconography information, a high content system is used as a detection means, intracellular fluorescence enhancement caused by medicine injections is detected on the high content system to reflect the degranulation condition of cells, and the probability that the anaphylactic and anaphylactoid reactions are caused by the medicine injections is predicted by calculating the cell degranulation positive rate through a fluorescence intensity conversion calculation method. The adopted dye comprises FM4-64, FM1-43, AM1-43 or AM4-66. The detection method is easy to operate, the result is objective, and important application value is achieved.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Shuxuening injection and preparation method thereof
ActiveCN106420851AEfficient removalImprove stabilityNervous disorderMetabolism disorderSolubilityAnaphylactoid reactions
The invention relates to a ginkgo leaf composition, and in particular relates to a Shuxuening injection and a preparation method thereof. The Shuxuening injection is made from ginkgo leaf extract which contains 25-40% of total flavonol glycosides, 6.5-16% of ginkgolide, 3-5% of bilobalide and less than 5ppm of ginkgolic acid. The preparation method has the advantages that a harmful ingredient, namely total ginkgolic acid, is effectively removed, and anaphylactoid reactions are reduced. The prepared Shuxuening injection is definite in compositional ratio, good in stability, low in irritability, good in solubility and stable in curative effect.
Owner:HEILONGJIANG ZBD PHARMA +1
Method for analyzing quality safety of traditional Chinese medicine injection and medicine preparation
InactiveCN103751809AControl immune securityImprove the safety of useOrganic active ingredientsImmunological disordersAnaphylactoid reactionsMast cell
The invention belongs to the field of medicines, and particularly relates to a method for analyzing quality safety of a traditional Chinese medicine injection and a medicine preparation. According to the invention, the traditional Chinese medicine injection serves as an object of study, a Qingkailing injection, a Chuanhuning injection and a Shenmai injection serve as representatives, a mast cell membrane stabilizer and other anti-allergic medicines are added into the traditional Chinese medicine injection to inhibit various immune toxicity reactions induced by the traditional Chinese medicine injection, and the capability of inducing various hypersensitivities and anaphylactoid reactions is detected, so that the result proves the feasibility of the method for quality control of immunity safety of the traditional Chinese medicine injection. The method provides a novel solution for the main adverse reaction, namely immunotoxicity, of the traditional Chinese medicine injection applied to clinic. A method for detecting the immunity safety control of the traditional Chinese medicine injection is established to control the clinical adverse reactions of the traditional Chinese medicine injection; the mechanism of action is studied, so that the selection and basis are provided for the method for detecting the immunity safety control of the traditional Chinese medicine injection in the new version of pharmacopeia, and the reference is provided for establishing novel guiding principles of immunity safety evaluation for new drugs in China.
Owner:潘卫松
A kind of allergy test method for Acanthopanax injection
ActiveCN106191208BEasy to operateInspection time is shortMicrobiological testing/measurementMaterial analysisAnaphylactoid reactionsMedicine
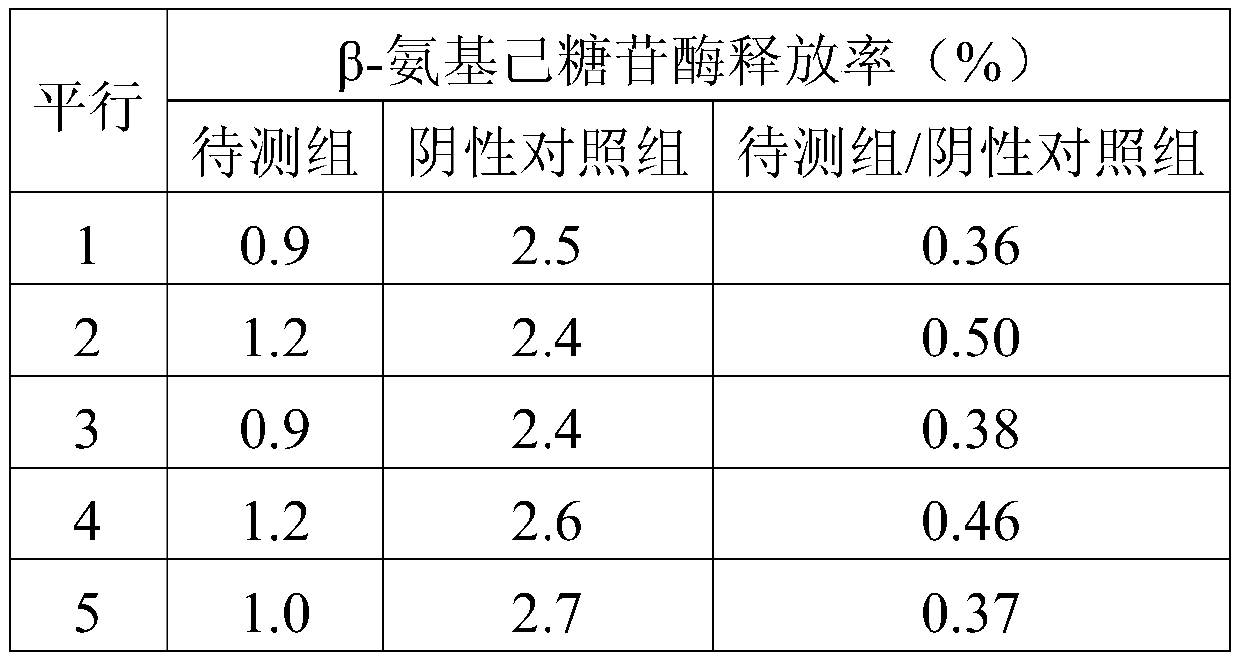
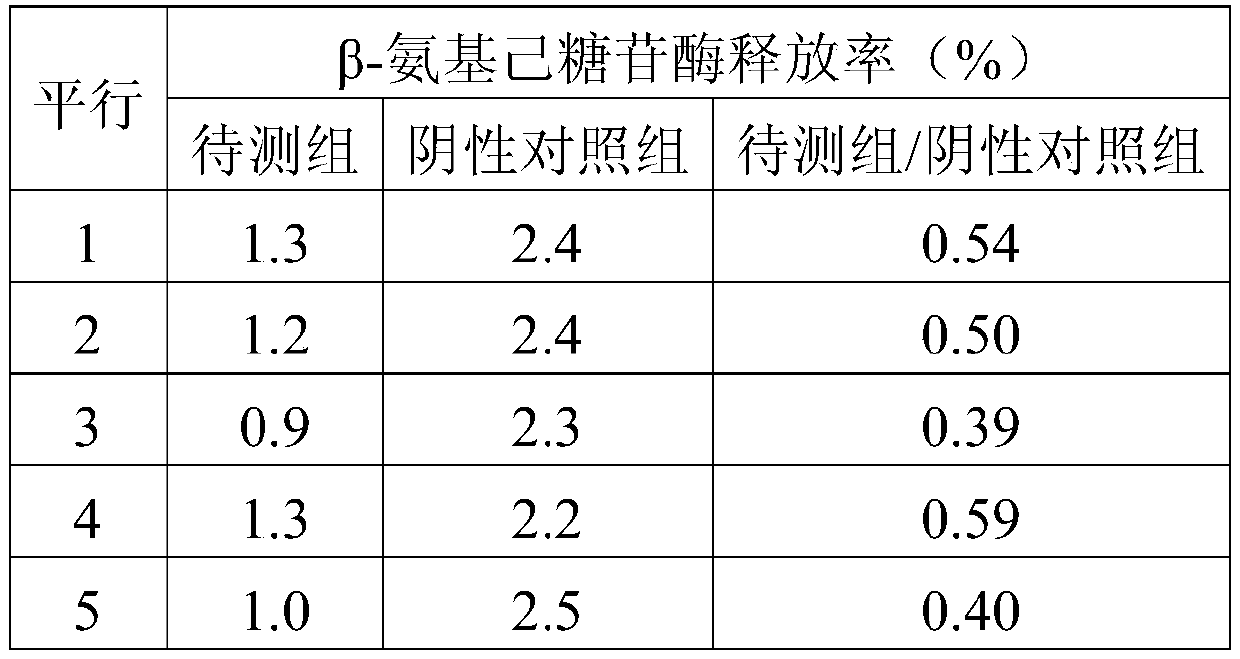
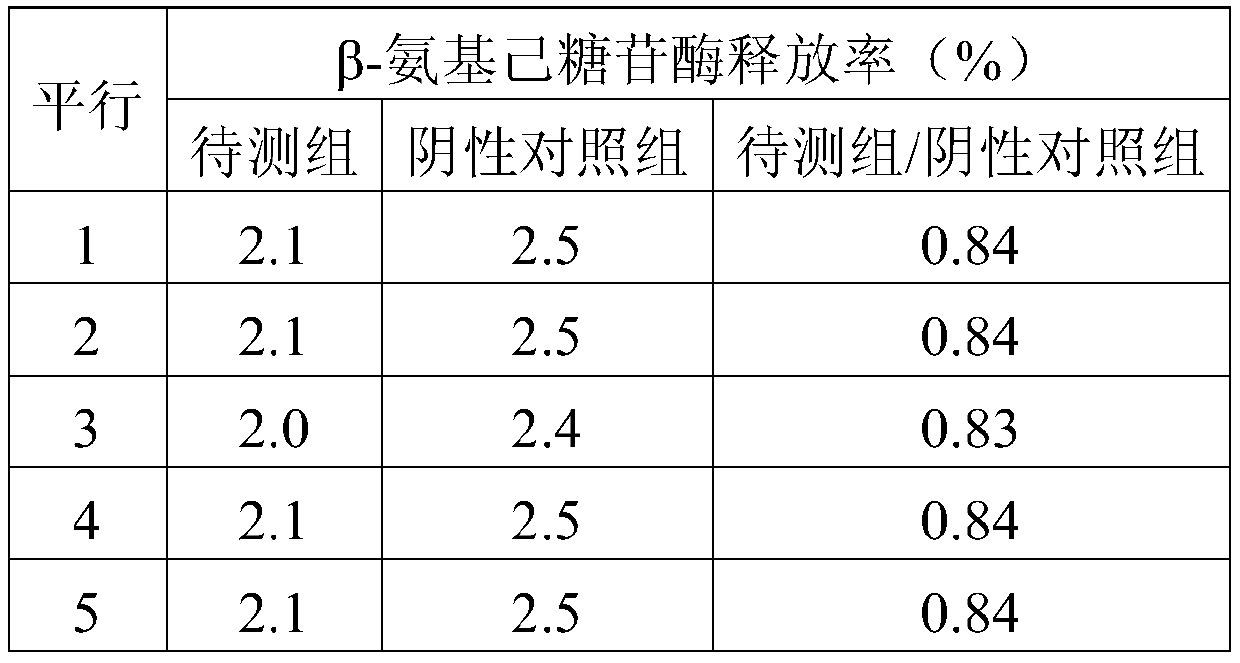
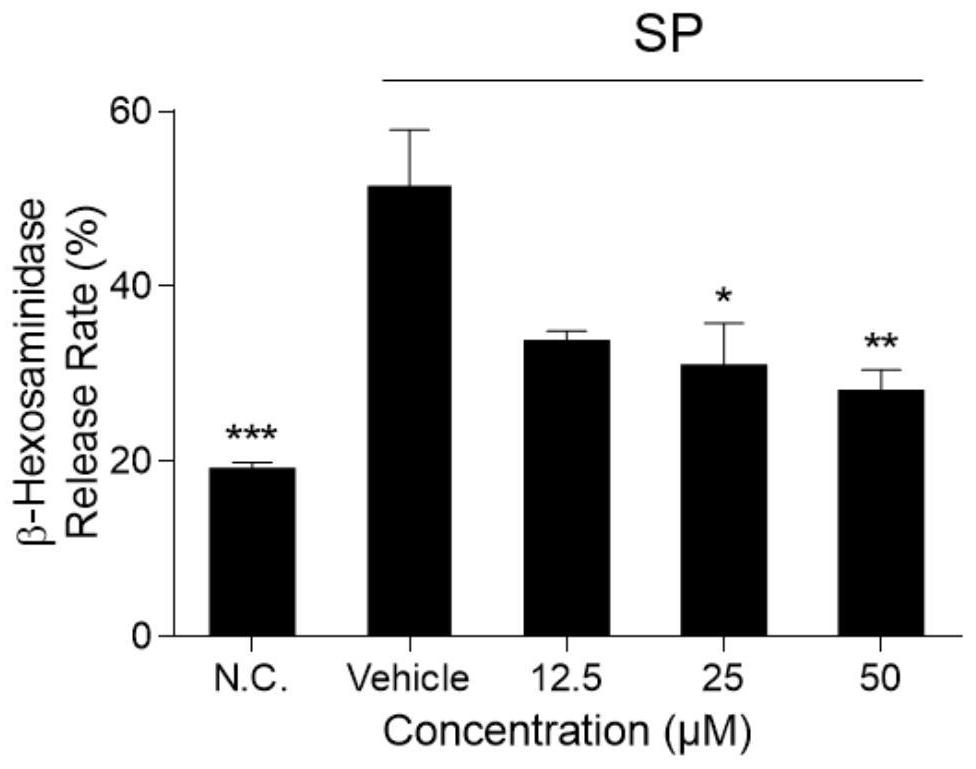
The invention belongs to the field of pharmaceutical preparations, and in particular relates to an allergy-like testing method for Ciwujia injection. The test method provided by the invention comprises the following steps: (1), sample pretreatment; (2), preparation of detection cells; (3), detection of release rate of β-hexosaminidase. Compared with the prior art, the anaphylactoid test method of Acanthopanax injection provided by the present invention is simple to operate, and the test time is short; it makes the evaluation and judgment of the test results more convenient, intuitive and reliable; using the Acanthopanax provided by the present invention The anaphylactoid test method for injection can obtain relatively accurate test and judgment results that are consistent with the actual mouse experiment; it is more stable and reliable, there is no obvious difference in multiple test results for the same sample, and the repeatability is high.
Owner:HARBIN ZHENBAO PHARMA
Traditional Chinese medicine injection with low anaphylactoid reaction
PendingCN114073672AEfficient removalReduce security risksOrganic active ingredientsInorganic non-active ingredientsAnaphylactoid reactionsMacromolecular Substances
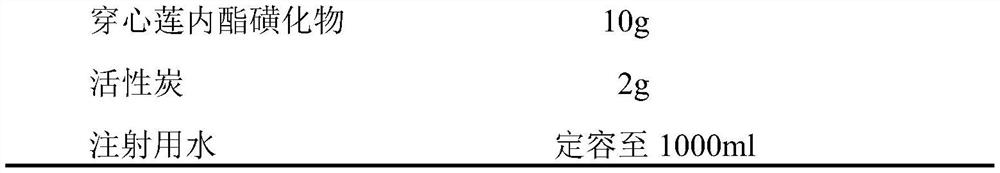
The invention discloses a traditional Chinese medicine injection with low anaphylactoid reaction, which can effectively remove macromolecular substances, pyrogen reactive substances, small-molecular-weight tannin, resin and other impurities in andrographolide sulfonate raw materials, and reduce the anaphylactoid reaction of a traditional Chinese medicine injection product through preparation liquid preparation.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Ginkgo biloba composition and its application in the preparation of Shuxuening injection
ActiveCN106420850BEfficient removalImprove stabilityMetabolism disorderPharmaceutical delivery mechanismAnaphylactoid reactionsSolubility
The invention relates to a ginkgo leaf composition, in particular to a ginkgo leaf composition and application thereof for preparing a ginkgo biloba leaf extract injection. The ginkgo leaf composition is prepared from 25-43% of total flavonol glycosides, 6.5-17% of ginkgolides, 3-5% of bilobalide and smaller than 3 ppm of ginkgoic acid. According to the preparation method of the ginkgo leaf composition, two kinds of resin of DM130 and AB-8 are adopted for adsorption, the harmful ingredient total ginkgolic acid is effectively removed, and anaphylactoid reaction occurrences are reduced; meanwhile, various extracting methods and composite extracting agent repeated extraction are adopted, the target component content is high, the purity is good, and the product quality is improved. The obtained ginkgo biloba leaf extract injection is clear in compositional ratio, good in stability, good in solubility and stable in curative effect and contains few impurities.
Owner:HEILONGJIANG ZBD PHARMA +1
Application of licochalcone A in preparation of anti-anaphylactoid drugs
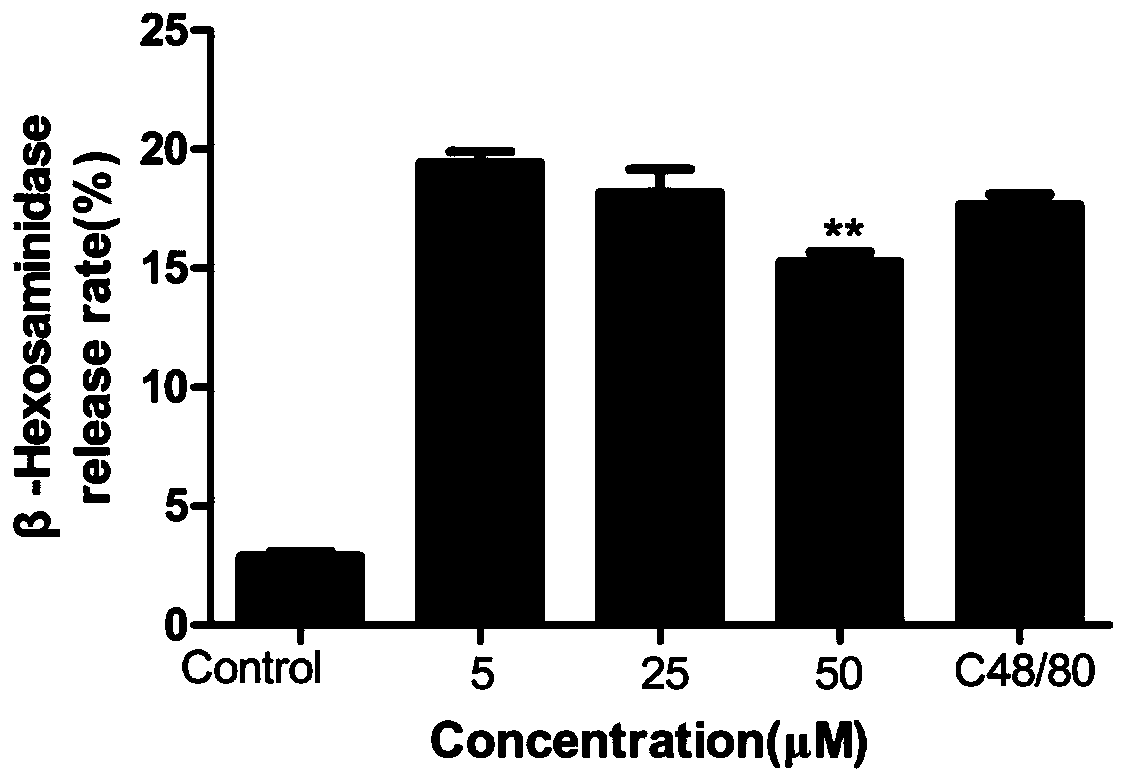
PendingCN113304130AReduced degranulationImprove allergic symptomsKetone active ingredientsImmunological disordersAnaphylactoid reactionsDisease
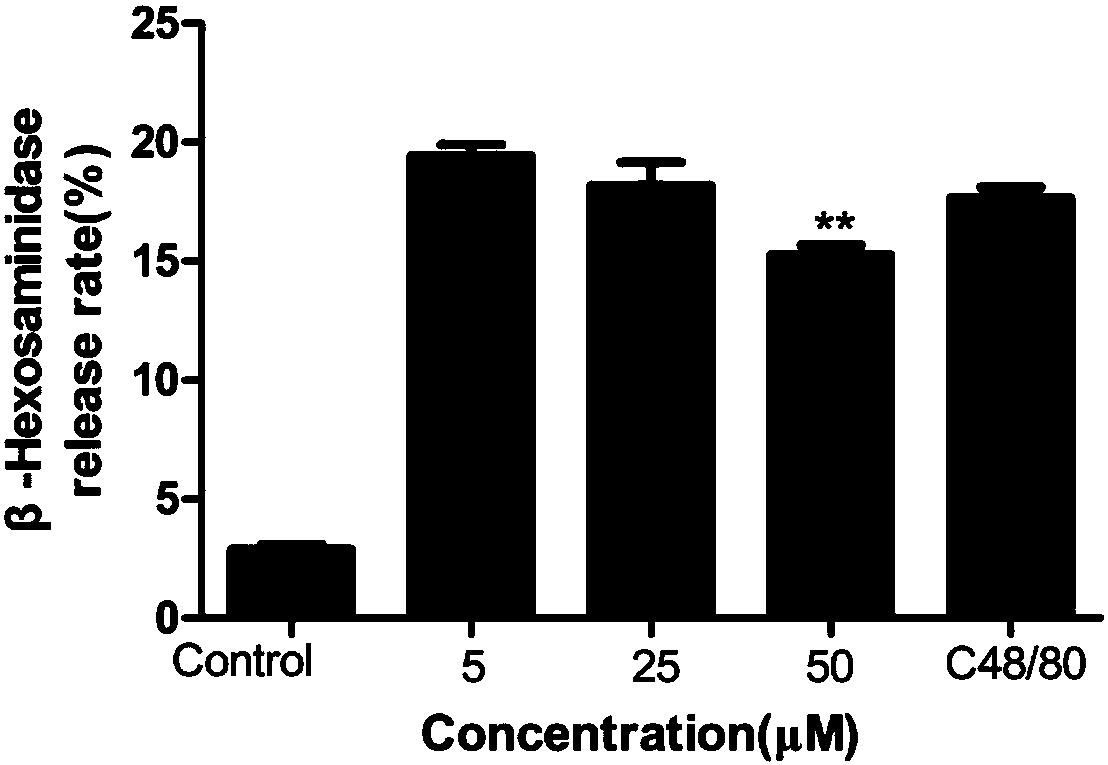
The invention discloses an application of licochalcone A in preparation of an anti-anaphylactoid drug. KU812 cell degranulation, toe swelling degree, toe Evans blue exudation degree and serum TNF-alpha release amount are compared to evaluate a to-be-detected medicine group, a normal saline control group and a normal control group, so that the treatment effect of the medicine on anaphylactoid diseases is evaluated, and the invention determines that the licochalcone A can be used for preparing the anti-anaphylactoid drug. The licochalcone A can effectively relieve local and systemic anaphylactoid reaction symptoms of mice; and the licochalcone A can reduce mast cell degranulation, and remarkably improve symptoms caused by anaphylactoid.
Owner:XI AN JIAOTONG UNIV
Polypeptide for preparing rabbit polyclonal antibody and application thereof
ActiveCN110204606AImproving immunogenicityHelp with researchCell receptors/surface-antigens/surface-determinantsBiological material analysisAnaphylactoid reactionsUse medication
The invention discloses a polypeptide for preparing a rabbit polyclonal antibody and an application thereof, which belong to the technical field of biomedicine. The polypeptide is an immunogenic polypeptide of an allergic reaction-specific receptor MRGPRX2 protein, which can be used for preparing the rabbit polyclonal antibody, an enzyme-linked immunosorbent assay (direct method) is successfully prepared, the polyclonal antibody is a detection antibody, the established method is used to verify the clinical application, which contributes to the research work of the allergic reaction-specific receptor MRGPRX2, and the polypeptide has great significance for clinically guiding the safety of the drug.
Owner:于向东
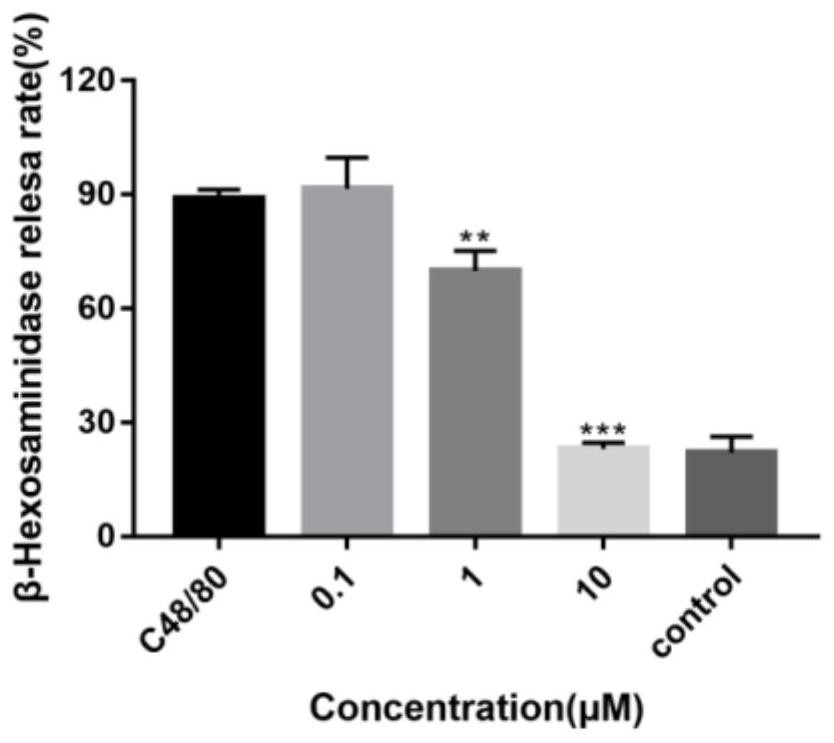
Halogenated diarylurea compounds and application thereof in preparation of antiallergic drugs
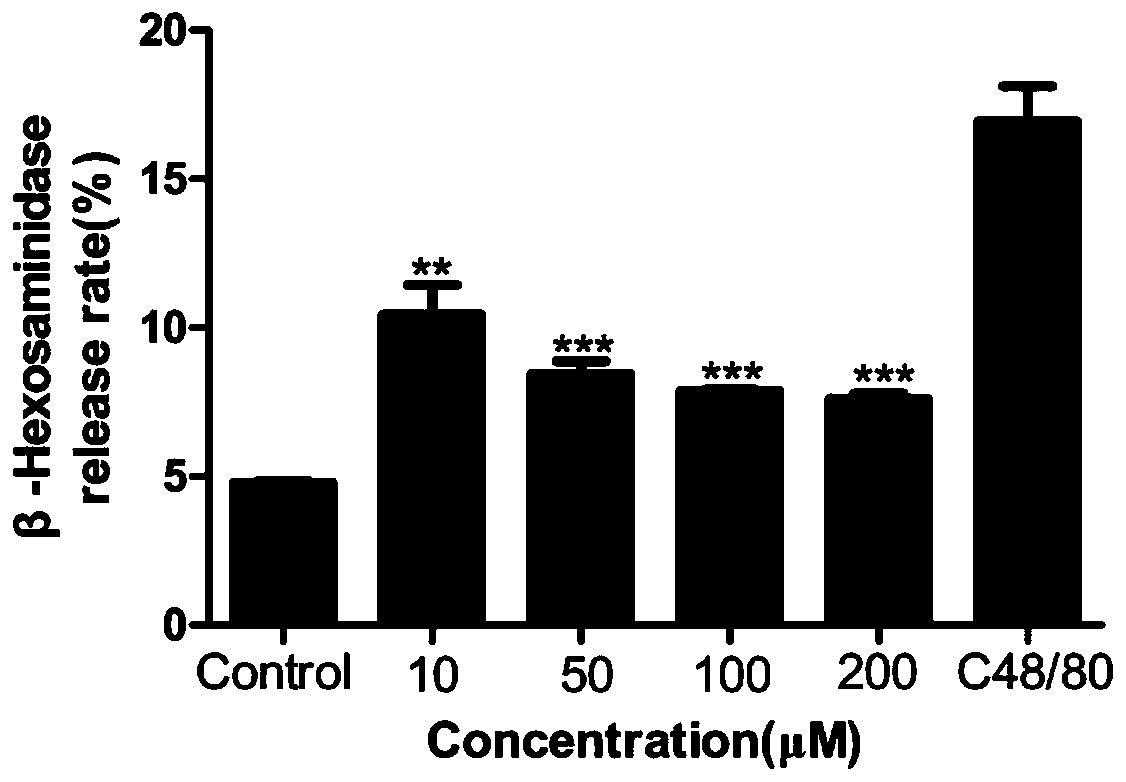
InactiveCN112076185AAllergic reactions suppressedRich types of anti-allergic drugsOrganic chemistryAmide active ingredientsAnaphylactoid reactionsBasophilic Granulocyte
The invention discloses halogenated diarylurea compounds and application thereof in preparation of an antiallergic drug, and discloses for the first time that the halogenated diarylurea compounds caneffectively antagonize beta-aminohexylglycosidase release and histamine release of human basophilic granulocyte KU812 caused by C48 / 80, so as to inhibit anaphylactoid reaction. Therefore, when the halogenated diarylurea compounds are used as an anti-allergic preparation, the types of anti-allergic medicines are enriched, and more possible treatment schemes are provided for clinical anti-allergic treatment.
Owner:XI AN JIAOTONG UNIV
Method for detecting anaphylactoid reaction caused by chlorogenic acids
InactiveCN103255197AReal-time online monitoringSave manpower and material resourcesMicrobiological testing/measurementAnaphylactoid reactionsChlorogenic acid
The invention discloses a method for detecting anaphylactoid reaction caused by chlorogenic acids. The method comprises the following steps of (1) respectively preparing RBL-2H3 cell suspension and chlorogenic acid solution which are different in concentration; (2) determining the optimal cell concentration of the RBL-2H3 cell suspension and the optimal time point of addition of the chlorogenic acid solution when a real-time cell analyzer detects the anaphylactoid reaction caused by chlorogenic acids; and (3) detecting the RBL-2H3 cell growth index values before and after addition of the chlorogenic acid solution by utilizing a real-time cell analyzer. The method has the advantages that growth, extension, morphologic change and death physiological states of the RBL-2H3 cell before and after the chlorogenic acid solution is added can be reflected in real time; and the concentration range of the solution of the chlorogenic acids causing the anaphylactoid reaction can be obtained.
Owner:BIOMEDICAL ANALYSIS CENT OF ACADEMY OF MILITARY MEDICAL SCI +1
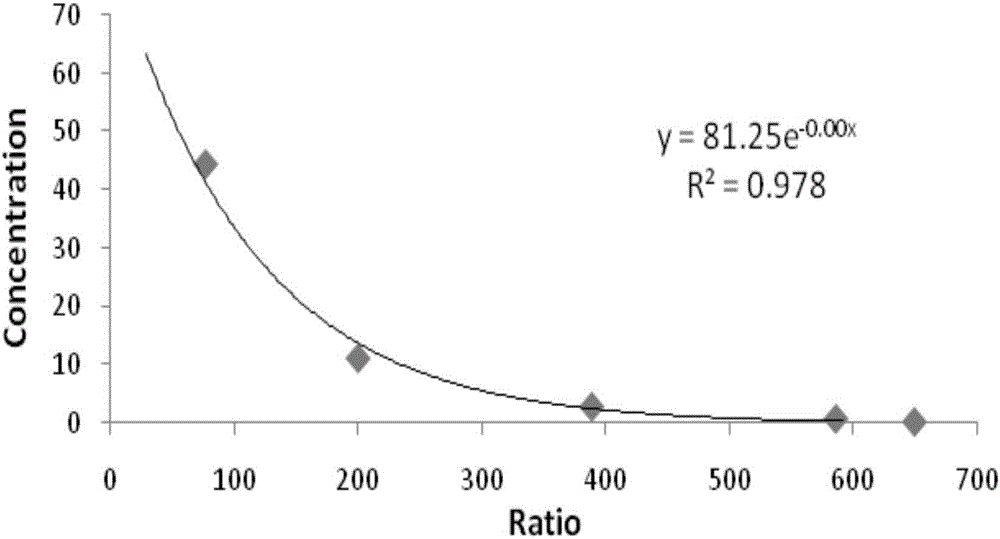
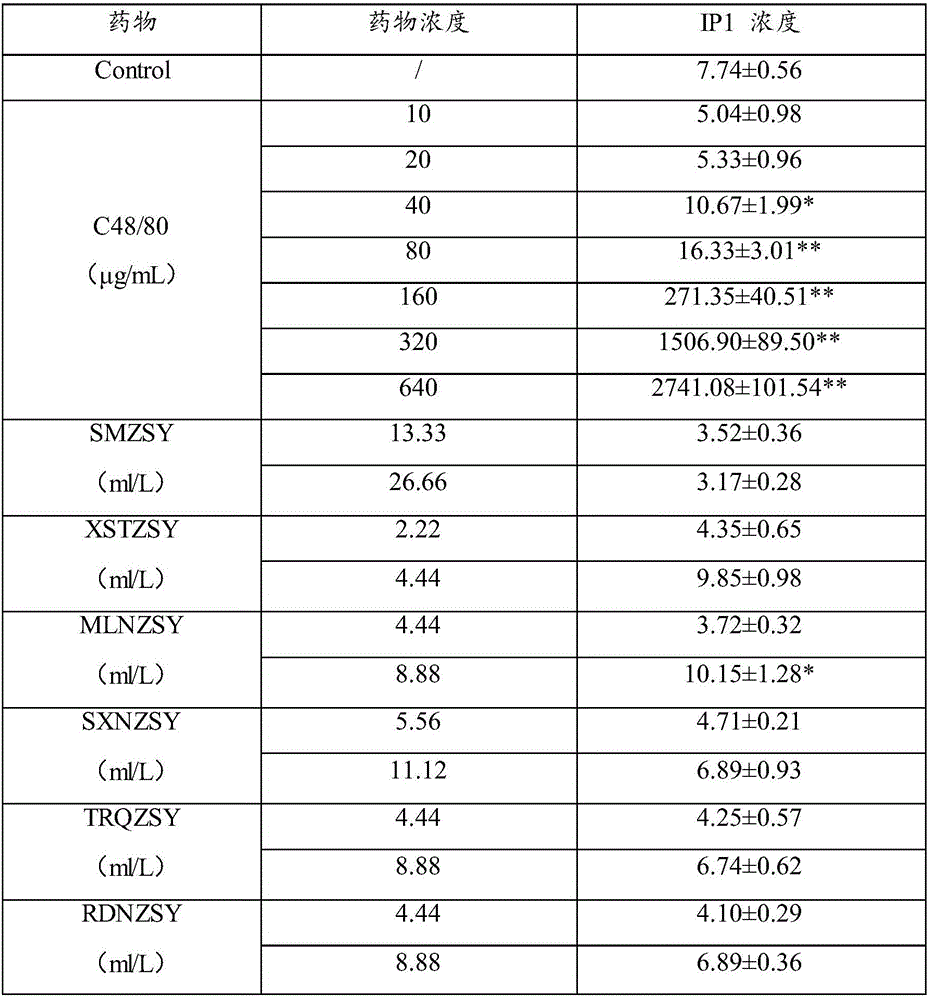
Method for detecting substance inducing anaphylactoid reaction and kit thereof
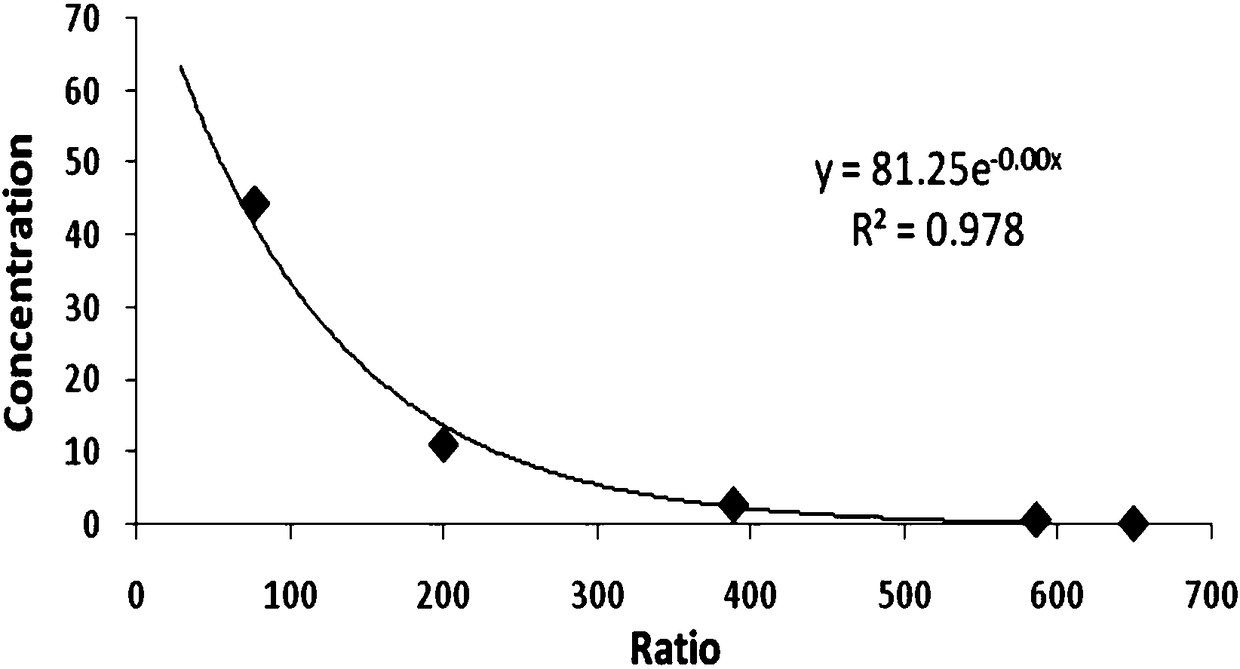
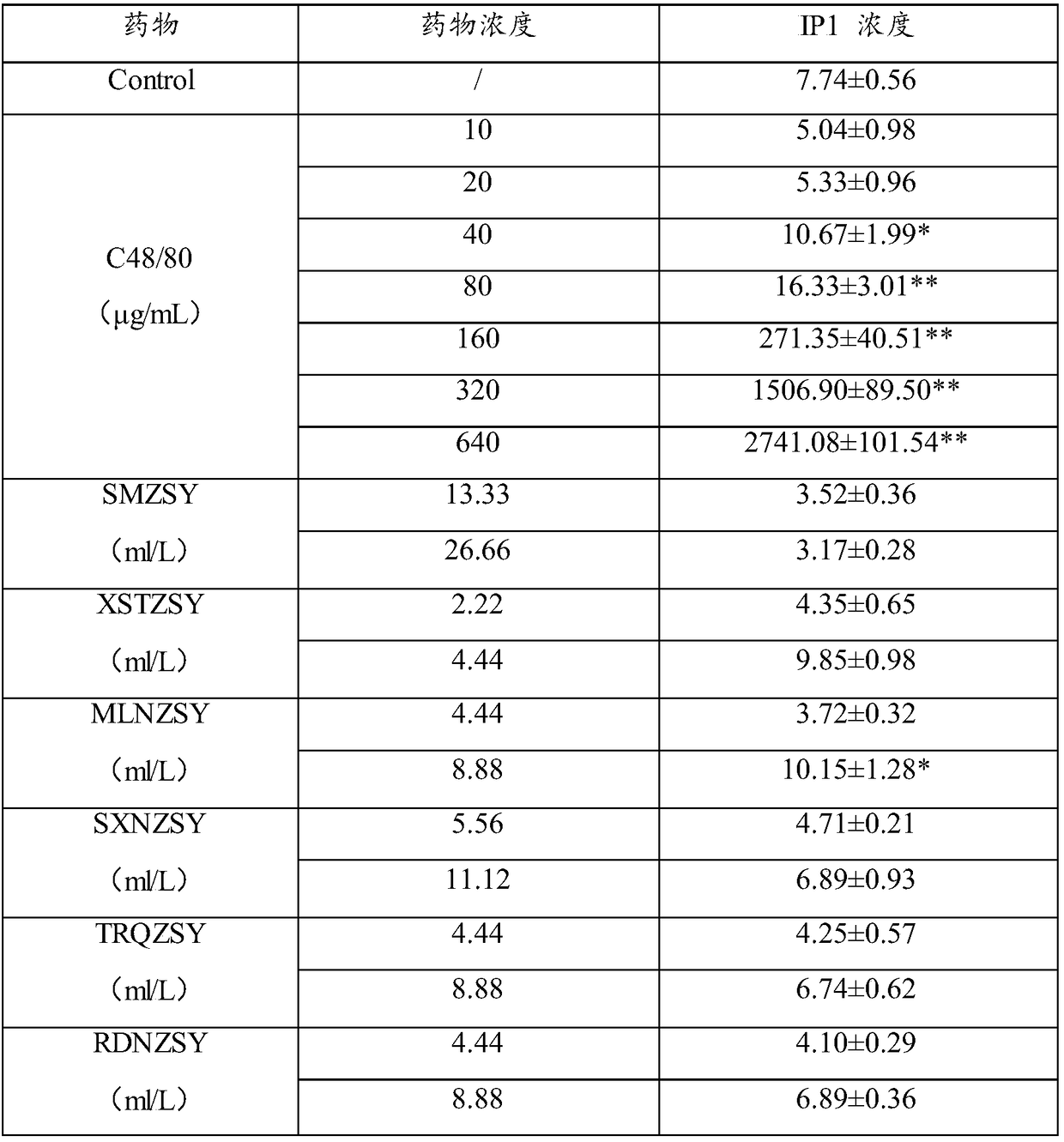
The invention relates to the technical field of drug testing, in particular to a method for detecting a substance inducing anaphylactoid reaction and a kit thereof. The method comprises the following steps: inoculating P815 cells into a culture medium; adding a to-be-detected object and a biological molecule specifically bonded with IP1 and / or cAMP to the culture medium, and detecting concentration of IP1 and / or cAMP; and according to the concentration of IP1 and / or cAMP, determining whether the to-be-detected object is the substance inducing the anaphylactoid reaction or not. The detection method provided by the invention has the advantages that the result is accurate and reliable and is basically consistent with the detection result of the traditional beta-hexosaminidase release rate experiment, but the sensitivity is higher than that of a beta-hexosaminidase release rate detection method; and the detection method provided by the invention is simple and quick, overcomes the defects of the traditional method, can be applied to early stage rapid screening and identification of the substance inducing the anaphylactoid reaction and has extensive value in use.
Owner:JIANGSU KANION PHARMA CO LTD
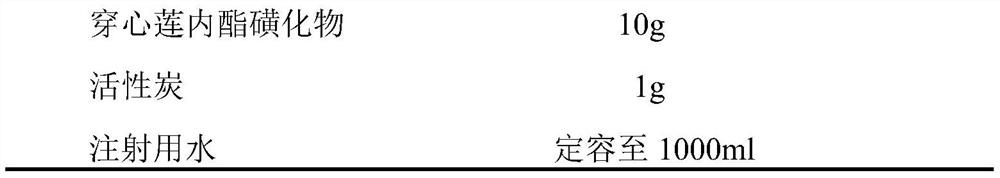
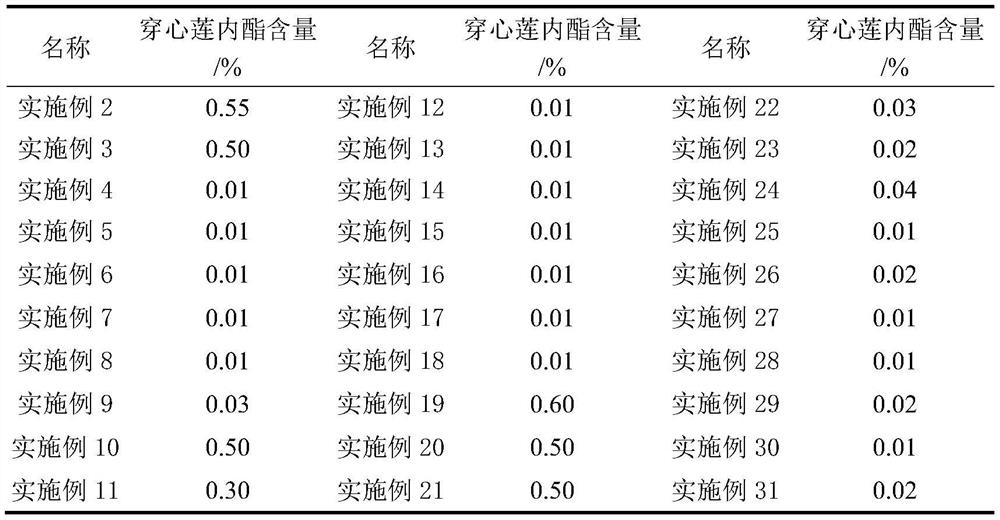
Preparation method of andrographolide sulfonate for controlling anaphylactoid reaction of traditional Chinese medicine injection
PendingCN113861141AImprove securityGuaranteed feasibilityOrganic chemistryAnaphylactoid reactionsSulfonate
The invention discloses a preparation method of an andrographolide sulfonate for controlling anaphylactoid reaction of a traditional Chinese medicine injection. The content uniformity of andrographolide in the andrographolide sulfonate is controlled through the procedures of intermediate filtrate concentration, water precipitation, cooling, refrigeration, filtration, concentration, extraction and drying, so that the anaphylactoid reaction of a traditional Chinese medicine injection is effectively controlled.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
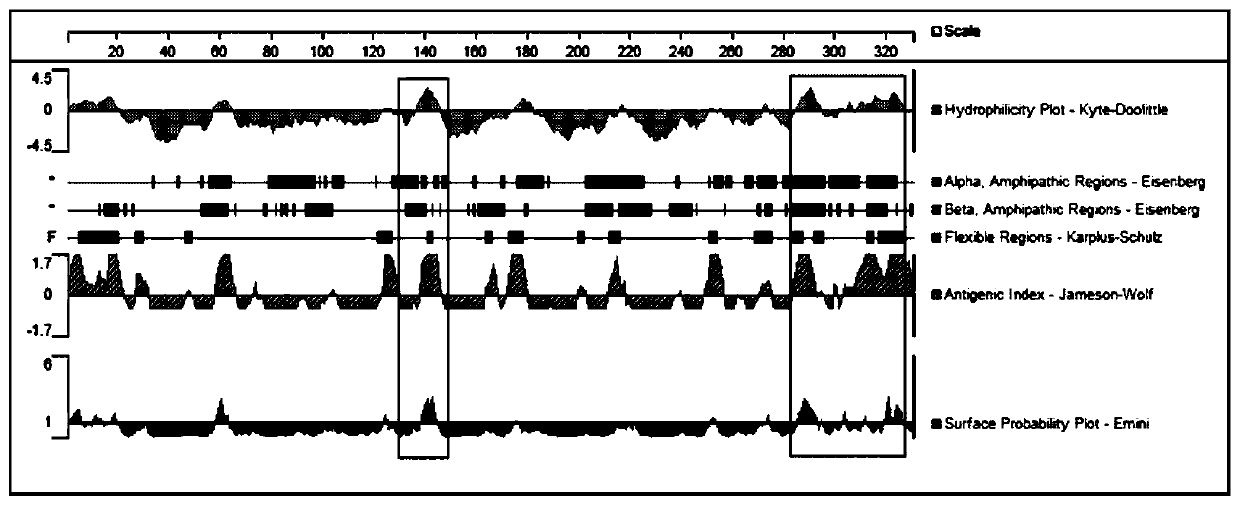
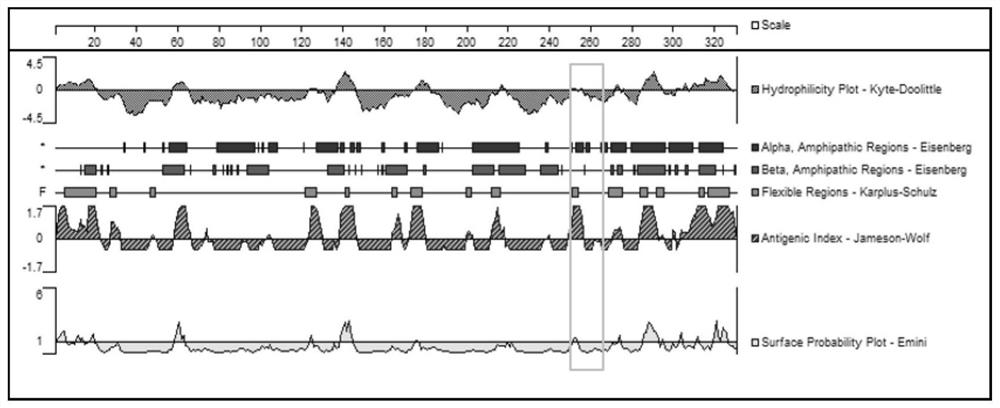
A kind of polypeptide with immunogenicity to anaphylaxis-like specific receptor mrgprx2 protein and application thereof
ActiveCN110229228BImproving immunogenicityHelp with researchBiological material analysisReceptors for hormonesAnaphylactoid reactionsReceptor
Owner:于向东
Freeze-dried powder injection of andrographolide sulfonate or salt thereof with low anaphylactoid reaction
PendingCN114073695AImprove securityImprove stabilityPowder deliveryOrganic active ingredientsAnaphylactoid reactionsSulfonate
The invention discloses a freeze-dried powder injection of andrographolide sulfonate or salt thereof with low anaphylactic reaction, which is characterized by being prepared by sulfonating andrographolide to obtain the andrographolide sulfonate, dissolving the andrographolide sulfonate with a solvent, adding an excipient, and freeze-drying. Compared with the existing solution injection, the freeze-dried powder injection has the advantages of less anaphylactoid and good stability, the method is simple, easy, scientific and reasonable, and the safety and stability of the finished product are improved.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of furanocoumarins in the preparation of antiallergic drugs
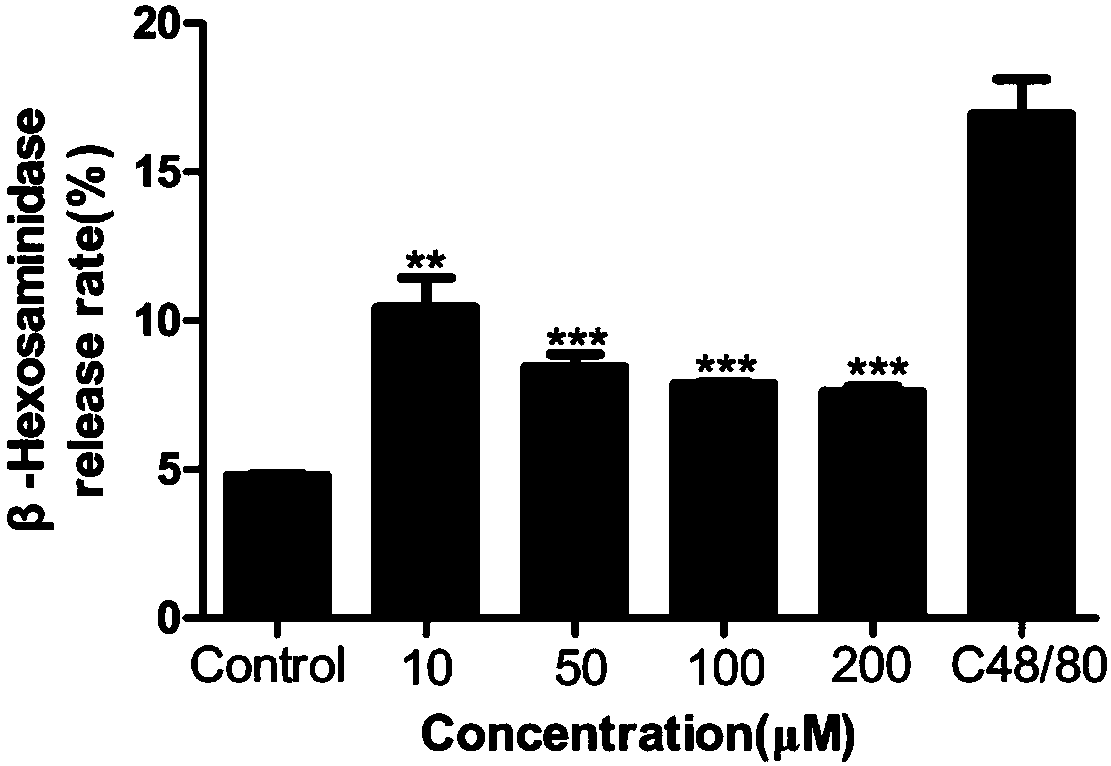
ActiveCN107661328BAllergic reactions suppressedRich types of anti-allergic drugsImmunological disordersHeterocyclic compound active ingredientsAnaphylactoid reactionsAntiallergic drugs
The invention provides application of furocoumarins compound in preparing anti-allergic medicine and belongs to the technical field of biological medicine. The application disclosed by the invention proves that the furocoumarins compound can effectively antagonize beta-hexosaminidase release caused by C48 / 80 of human mast cell KU812 for the first time and further can inhibit anaphylactoid reaction; furthermore, the application also proves that an action receptor of the furocoumarins compound is an MrgprX2 receptor for the first time; when the furocoumarins compound in preparing anti-allergic medicine is prepared into anti-allergic preparation, anti-allergic medicine types can be enriched, and a brand-new choice and a brand-new strategy are provided for anti-allergic treatment.
Owner:XI AN JIAOTONG UNIV
A method and kit for detecting substances causing anaphylactoid reactions
ActiveCN106442963BReliable resultsHigh sensitivityBiological testingAnaphylactoid reactionsP815 cell
The invention relates to the technical field of drug testing, in particular to a method for detecting a substance inducing anaphylactoid reaction and a kit thereof. The method comprises the following steps: inoculating P815 cells into a culture medium; adding a to-be-detected object and a biological molecule specifically bonded with IP1 and / or cAMP to the culture medium, and detecting concentration of IP1 and / or cAMP; and according to the concentration of IP1 and / or cAMP, determining whether the to-be-detected object is the substance inducing the anaphylactoid reaction or not. The detection method provided by the invention has the advantages that the result is accurate and reliable and is basically consistent with the detection result of the traditional beta-hexosaminidase release rate experiment, but the sensitivity is higher than that of a beta-hexosaminidase release rate detection method; and the detection method provided by the invention is simple and quick, overcomes the defects of the traditional method, can be applied to early stage rapid screening and identification of the substance inducing the anaphylactoid reaction and has extensive value in use.
Owner:JIANGSU KANION PHARMA CO LTD
Method for detecting induced anaphylactoid reaction of medicines and application thereof
InactiveCN104415354ASimple test methodThe detection index is objectiveIn-vivo testing preparationsAnaphylactoid reactionsWhole body
The invention relates to the technical field of medical evaluation and detection, and in particular relates to a method for detecting induced anaphylactoid reaction of intravenous injection medicines. The method comprises the following steps: observing anaphylactoid reaction symptoms of experimental animals after being dosed in a certain time by supplying different doses of tested medicines to intravenous injection of the experimental animals, and judging the grade of anaphylactoid reaction strength of intravenous injection administration of the tested medicines according to scale scores of the anaphylactoid reaction symptoms. According to the method disclosed by the invention, by adopting the anaphylactoid reaction symptoms of the animals after being dosed as 'gold standards', the whole process of dynamic reaction of the occurrence of clinical systemic anaphylactoid reaction can be relatively well simulated, so that the method can be applied to the detection and determination of anaphylactoid reaction of the intravenous injection medicines, also can be applied to the screening of anti-anaphylactoid medicines and the research of systemic anaphylactoid pathogenesis and medicine action mechanism, and has the advantages of simplicity and convenience in operation, short experimental period, good detection reproducibility, clinically-close results and the like.
Owner:SHANGHAI UNIV OF T C M
A kind of Shuxuening injection and preparation method thereof
ActiveCN106420851BEfficient removalImprove stabilityNervous disorderMetabolism disorderSolubilityAnaphylactoid reactions
The invention relates to a ginkgo leaf composition, and in particular relates to a Shuxuening injection and a preparation method thereof. The Shuxuening injection is made from ginkgo leaf extract which contains 25-40% of total flavonol glycosides, 6.5-16% of ginkgolide, 3-5% of bilobalide and less than 5ppm of ginkgolic acid. The preparation method has the advantages that a harmful ingredient, namely total ginkgolic acid, is effectively removed, and anaphylactoid reactions are reduced. The prepared Shuxuening injection is definite in compositional ratio, good in stability, low in irritability, good in solubility and stable in curative effect.
Owner:HEILONGJIANG ZBD PHARMA +1
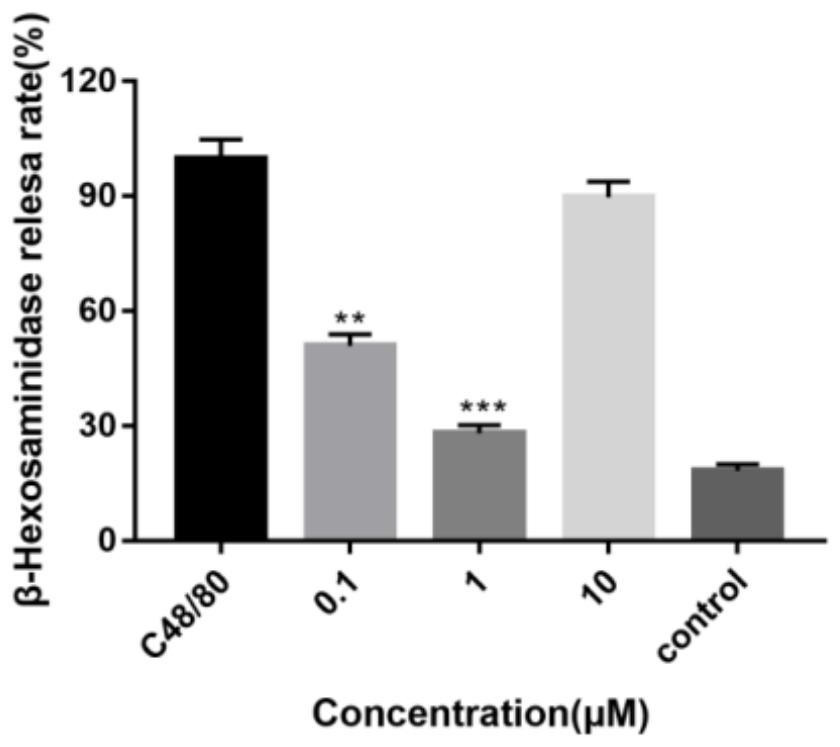
Application of clarithromycin in the preparation of anti-allergic drug
ActiveCN108451967BLittle side effectsPromote absorptionOrganic active ingredientsImmunological disordersAnaphylactoid reactionsDisease
The invention discloses application of clarithromycin in preparation of medicine for resisting anaphylactoid reaction. The medicine has dosage forms such as tablets, controlled release formulations, injection or spray. The clarithromycin has an antagonistic effect of human mast cell released beta-hexosaminidase caused by substance P. Meanwhile, the clarithromycin has an effect of lowering rise ofhistamine in serum of mice caused by the substance P. The clarithromycin has the characteristics of being less in side effects, excellent in absorption, high in stability and the like, is capable of effectively inhibiting mast cell degranulation reaction caused by a substance P activated MRGPRX2 (Mas-Related G-Protein coupled Receptor X2), has effects of reducing mast cell released histamine on acellular level and an animal level, and is applicable to treatment of diseases having long course of treatment. In addition, the clarithromycin is discovered to have the activity of resisting anaphylactoid reaction in theoretical and clinical treatment, and has potential development value of treating immune diseases.
Owner:XI AN JIAOTONG UNIV
Polypeptide for preparing ELISA mouse monoclonal coating antibody and rabbit polyclonal detection antibody and application thereof
ActiveCN110229227AImproving immunogenicityCell receptors/surface-antigens/surface-determinantsBiological material analysisAnaphylactoid reactionsPolyclonal antibodies
The invention discloses a polypeptide for preparing an ELISA mouse monoclonal coating antibody and a rabbit polyclonal detection antibody and application thereof, and belongs to the technical field ofbiomedicine. The polypeptide is a polypeptide which has immunogenicity on an anaphylactoid reaction specific receptor MRGPRX2 protein, and the amino acid sequence of the polypeptide is as shown in SEQ ID NO:1. The polypeptide is a polypeptide which has immunogenicity on an anaphylactoid reaction specific receptor MRGPRX2 protein, and can be used for preparing an ELISA mouse monoclonal coated antibody and a rabbit polyclonal detection antibody. A double-antibody sandwich ELISA method is successfully prepared, wherein the monoclonal antibody is a coating antibody, the polyclonal antibody is a detection antibody, and the application of the method in clinic is verified. The invention is beneficial to the research work of anaphylactoid reaction specific receptor MRGPRX2, and has important significance for clinically guiding the safety of medication.
Owner:于向东
Method to Determine Pseudo-Allergic Reactions
InactiveUS20090017475A1High activationMicrobiological testing/measurementBiological material analysisAnaphylactoid reactionsBasophil cell
Method and kit for determining the possible appearance of adverse reactions (such as anaphylactoid reactions) in patients in need to undergo to an administration of a pharmaceutical compound. The method for determining potential hypersensitivity in a patient to pseudo-allergic reactions comprises adding a predetermined amount of a compound with anaphylatoxic activity to a sample of the patient's blood and determining the amount of activation of the patient's basophil cells in said blood sample. The compound with anaphylatoxic activity is preferably selected from C3a, C5a, analogs of C3a or C5a, derivatives of C3a or C5a, and mixtures thereof.
Owner:BRACCO RES USA
A kind of polypeptide for preparing rabbit polyclonal antibody and its application
ActiveCN110204606BImproving immunogenicityHelp with researchCell receptors/surface-antigens/surface-determinantsBiological material analysisAnaphylactoid reactionsAntiendomysial antibodies
The invention discloses a polypeptide for preparing rabbit polyclonal antibody and its application, belonging to the technical field of biomedicine. The polypeptide is an immunogenic polypeptide similar to the allergic response specific receptor MRGPRX2 protein, and can be used to prepare rabbit polyclonal antibodies. The enzyme-linked immunosorbent method (direct method) has been successfully prepared, wherein the polyclonal antibody is a detection antibody. The established method has verified its clinical application, which will help the research work on the specific receptor for anaphylactoid MRGPRX2, and is of great significance for clinically guiding the safety of drug use.
Owner:于向东
Method for detecting anaphylactoid reaction caused by chlorogenic acids
InactiveCN103255197BReal-time online monitoringSave manpower and material resourcesMicrobiological testing/measurementAnaphylactoid reactionsChlorogenic acid
Owner:BIOMEDICAL ANALYSIS CENT OF ACADEMY OF MILITARY MEDICAL SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com