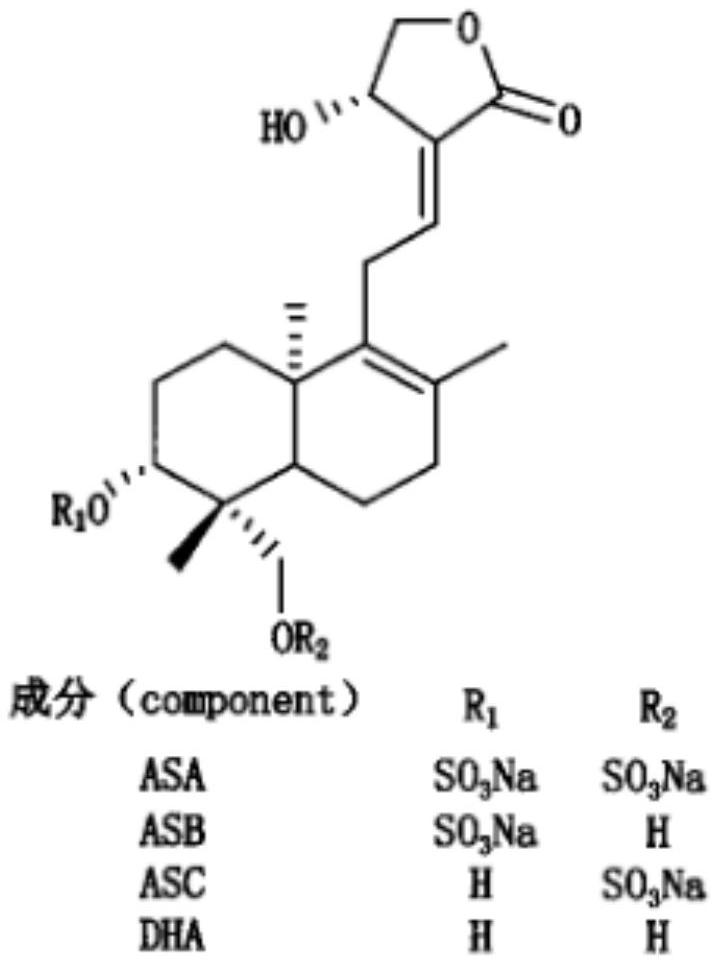
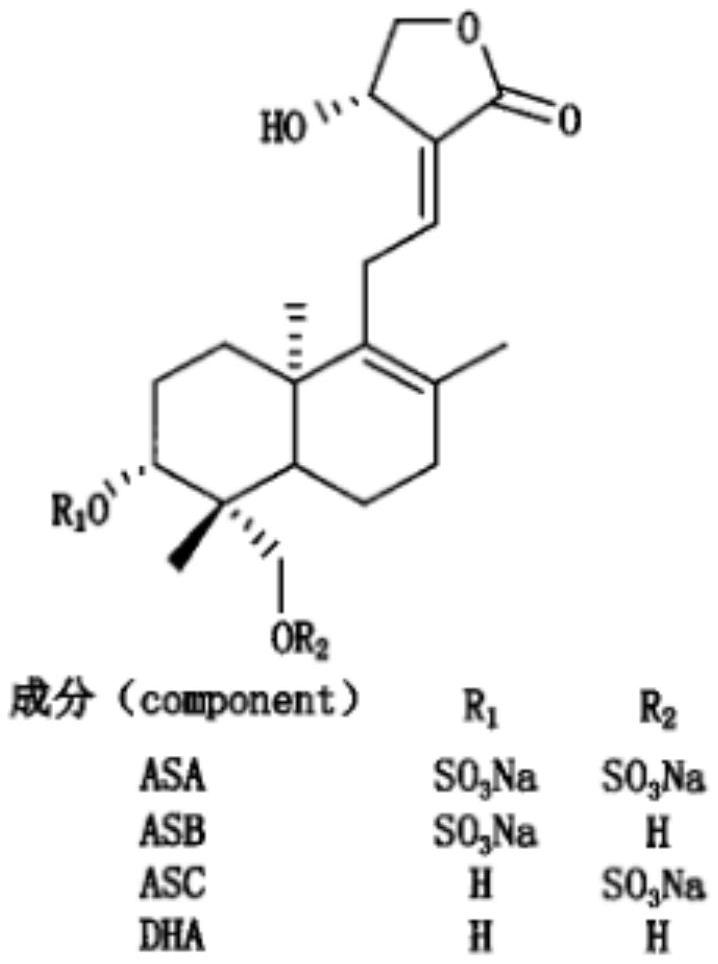
Freeze-dried powder injection of andrographolide sulfonate or salt thereof with low anaphylactoid reaction
An andrographolide, allergic reaction technology, applied in freeze-drying delivery, powder delivery, drug delivery and other directions, can solve the problems of wide molecular weight range, anaphylactic-like stability, and complete removal by ultrafiltration process, and achieve a stable improvement. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 mixed liquor and preparation of andrographolide sulfonate 1
[0034] a Sulfonation: Take absolute ethanol and place it in a reaction vessel, add concentrated sulfuric acid with a chemically pure specific gravity of 1.84 on a cold water bath, add andrographolide powder, stir well, and place at room temperature.
[0035] b Neutralization: After standing for 24 hours, add ethanol and stir evenly, control the temperature on a cold water bath at 25°C, and neutralize with sodium hydroxide solution until the pH value is 6.5.
[0036] c Filtration: add ethanol, stir well, filter after standing for 2 hours, wash the filter residue with ethanol until the lotion is almost colorless, combine the filtrate and lotion to obtain the mixed solution.
[0037] d Recover ethanol: recover ethanol and concentrate, heat and volatilize ethanol to the utmost, then cool to obtain cooling liquid.
[0038] e suction filtration, concentration and drying: add the cooling liquid to acti...
Embodiment 2
[0040] Embodiment 2 preparation of andrographolide sulfonate
[0041] Take the mixed solution of Example 1, detect its conductivity as 1.04ms / cm, pour it into a nanofiltration membrane separation device, add purified water to dilute to ethanol concentration to 50%, and set aside. Turn on the infusion pump, adjust the pressure so that the membrane inlet pressure is 1.0-1.5MPa, and use a molecular weight cut-off of 250Da for nanofiltration enrichment and concentration. During the nanofiltration process, the conductivity of the dialysate was monitored to be 2.92 us / cm. The nanofiltration permeate was collected to detect resins, tannins and allergic reactions, and the alcohol content of the concentrated solution was monitored to 5% to obtain a concentrated solution. Add water for injection of 20 times the amount of andrographolide to the concentrated solution for water precipitation to obtain a supernatant for subsequent use. The supernatant was concentrated in a vacuum of -0.08m...
Embodiment 3
[0042] Embodiment 3 preparation of andrographolide sulfonate
[0043] Take the mixed solution of Example 1, detect its conductivity as 3.00ms / cm, pour it into a nanofiltration membrane separation device, add purified water to dilute it until the concentration of ethanol reaches 50%, and set it aside. Turn on the infusion pump, adjust the pressure so that the membrane inlet pressure is 1.0-1.5MPa, and use a molecular weight cut-off of 300Da for nanofiltration enrichment and concentration. During the nanofiltration process, the conductivity of the dialysate was monitored to be 3.25 us / cm, the permeated liquid was collected to detect resin, tannin and allergic reactions, and the alcohol content of the concentrated solution was monitored to 5% to obtain a concentrated solution. Add water for injection of 20 times the amount of andrographolide to the concentrated solution for water precipitation to obtain a supernatant for subsequent use. The supernatant was concentrated in a vacu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com