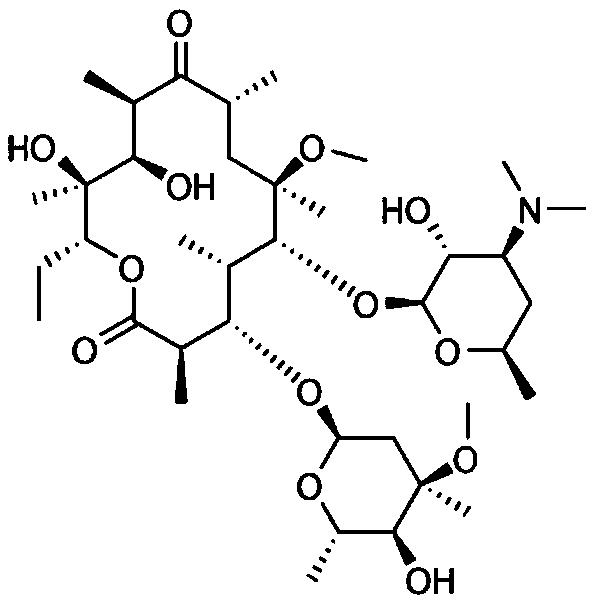
Application of clarithromycin in the preparation of anti-allergic drug
A technology for clarithromycin and allergic reaction, which is applied to the application field of clarithromycin in the preparation of anti-allergic drugs, can solve problems such as increased bioavailability, and achieve the effects of less side effects, good absorption and strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
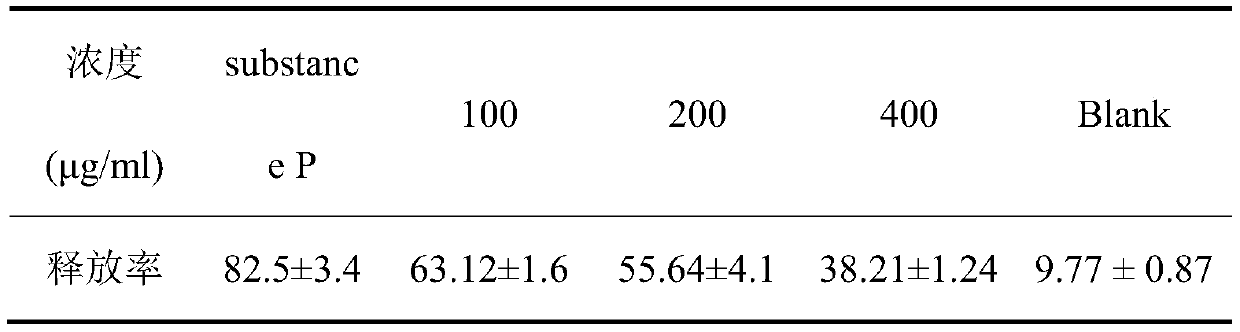
[0024] (1) Antagonize the release of human mast cell β-hexosaminidase induced by substance P
[0025] 1) Reagent
[0026] 0.1% Triton X-100 Lysis Solution: Dissolve 10 μL Triton X-100 in 10 mL PBS to obtain 0.1% Triton X-100 Lysis Solution.
[0027] 0.1mol / L citric acid / sodium citrate buffer solution (pH4.5): Weigh 2.101g of citric acid monohydrate and dissolve it in 100mL triple distilled water to obtain a 0.1mol / L citric acid solution, keep it away from light at 4°C save. Weigh 2.941g of sodium citrate, dissolve it in 100mL of triple distilled water to obtain a 0.1mol / L sodium citrate solution, and store it in the dark at 4°C. Before use, mix according to the ratio of 0.1mol / L citric acid: 0.1mol / L sodium citrate=10.4:9.6 (v / v) to obtain 0.1mol / L citric acid / sodium citrate buffer solution.
[0028] 1mmol / L β-hexosamine solution: Weigh 0.034g of β-hexosamine and dissolve it in 100mL 0.1mol / L citric acid / sodium citrate buffer solution to obtain 1mmol / L β-hexosamine solution...
Embodiment 2
[0047] 1) Reagent
[0048] The preparation of 0.5% CMC-Na solution, 0.5g CMC-Na is added in 100mL physiological saline, ultrasonically dissolves, prepares and obtains 0.5% CMC-Na solution. For the preparation of 60μg / mL substance P solution, weigh 6mg of substance P powder, add 0.1mL of normal saline, and dilute 100 times with normal saline after ultrasonic dissolution to obtain 60μg / mL substance P solution.
[0049] 2) Experimental steps
[0050] Use 0.5% CMC-Na solution to prepare clarithromycin suspension with corresponding concentration.
[0051] 50 C57BL / 6 mice at 7-8 weeks were randomly divided into 5 groups, including 3 different concentration administration groups, negative control group and blank control group, with 10 mice in each group.
[0052] For intragastric administration, 8.125, 16.25 and 32.5 mg / kg clarithromycin suspensions were administered to three different concentration administration groups; 0.2 mL of 0.5% CMC-Na solution was intragastrically administ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com