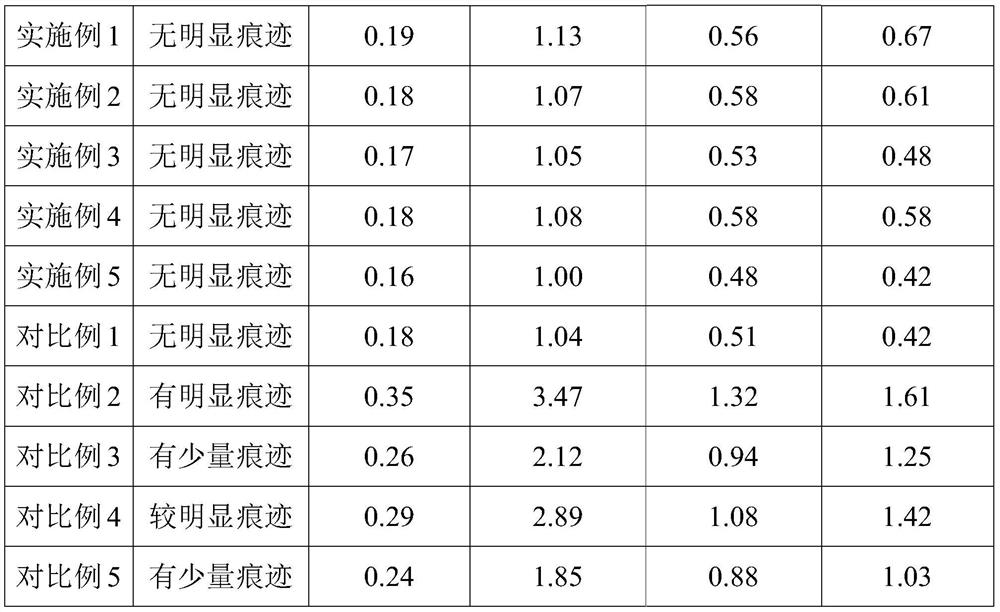
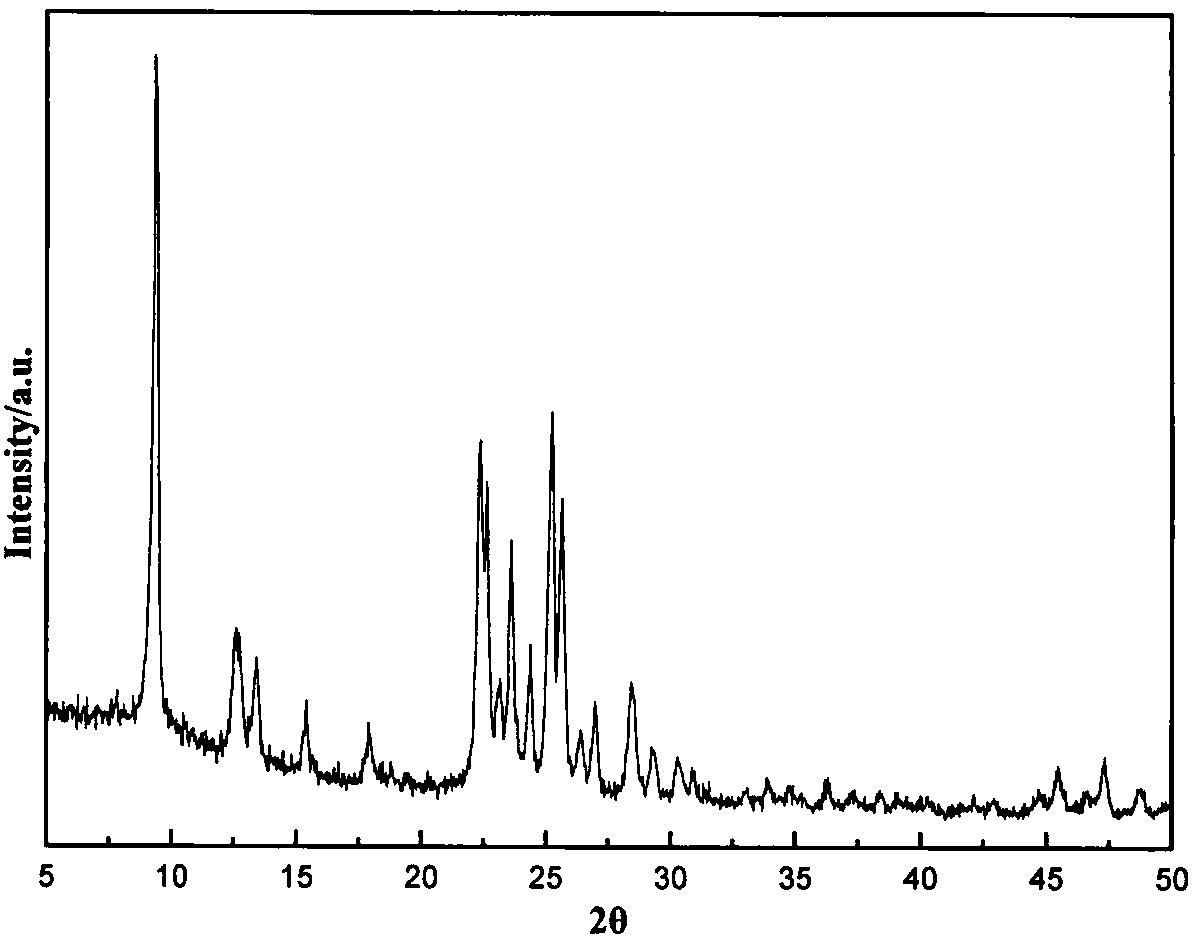
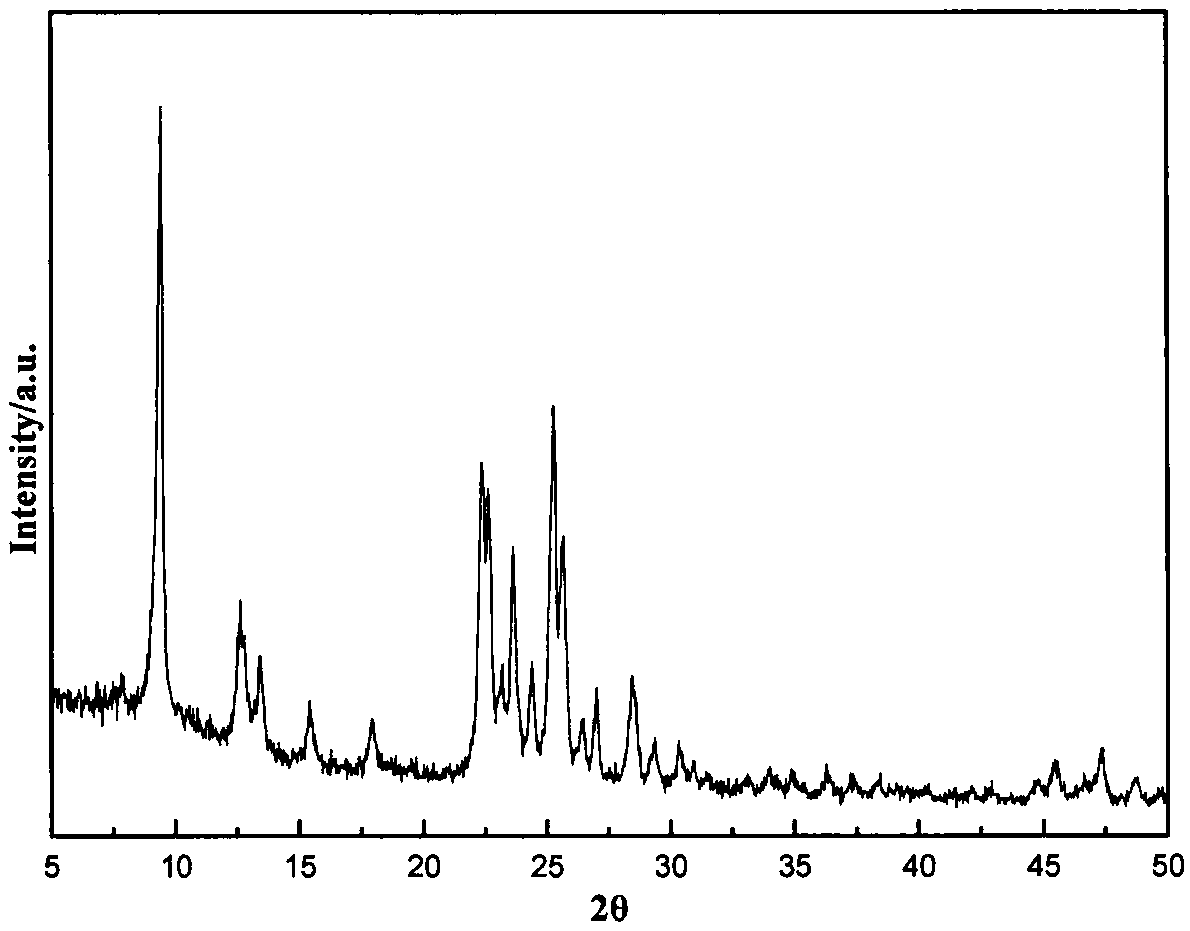
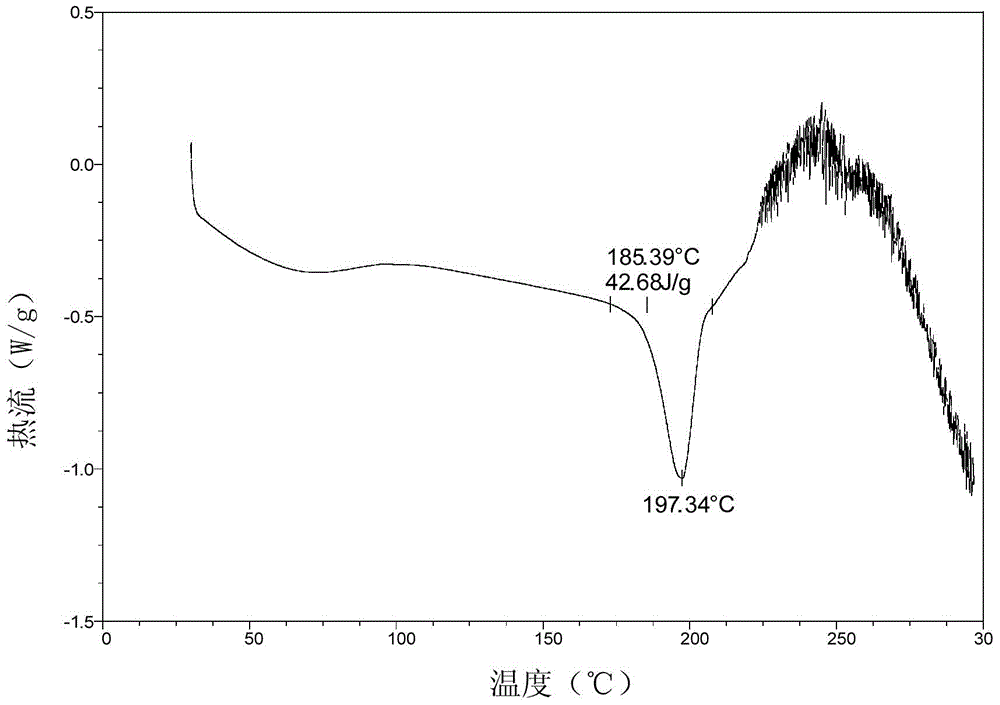
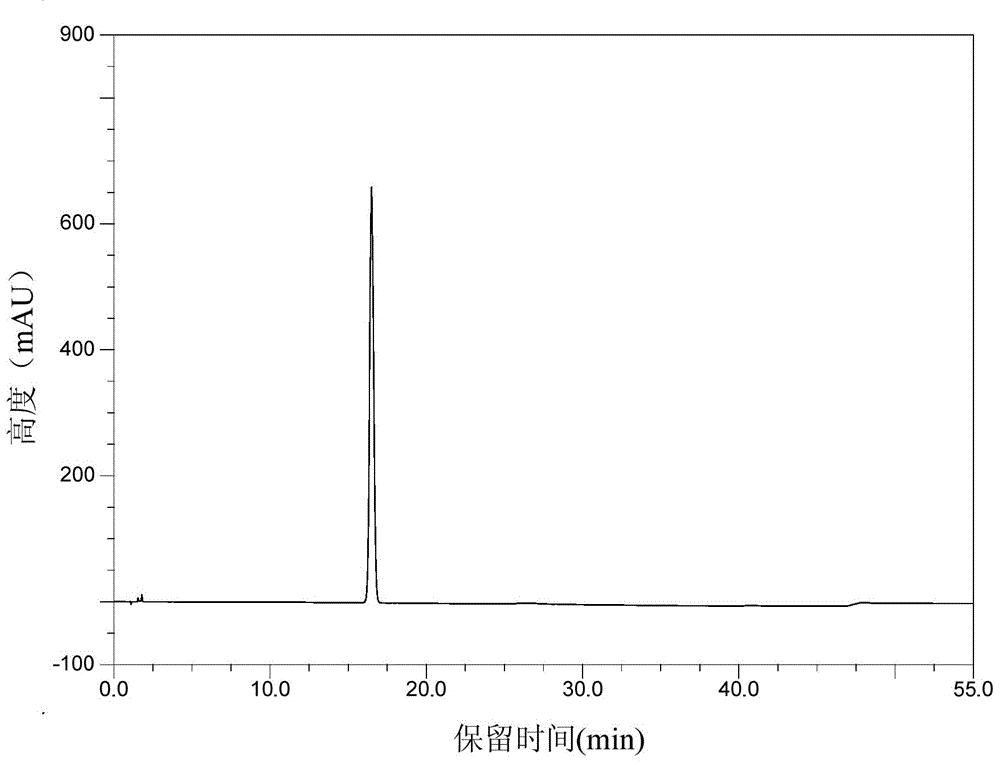
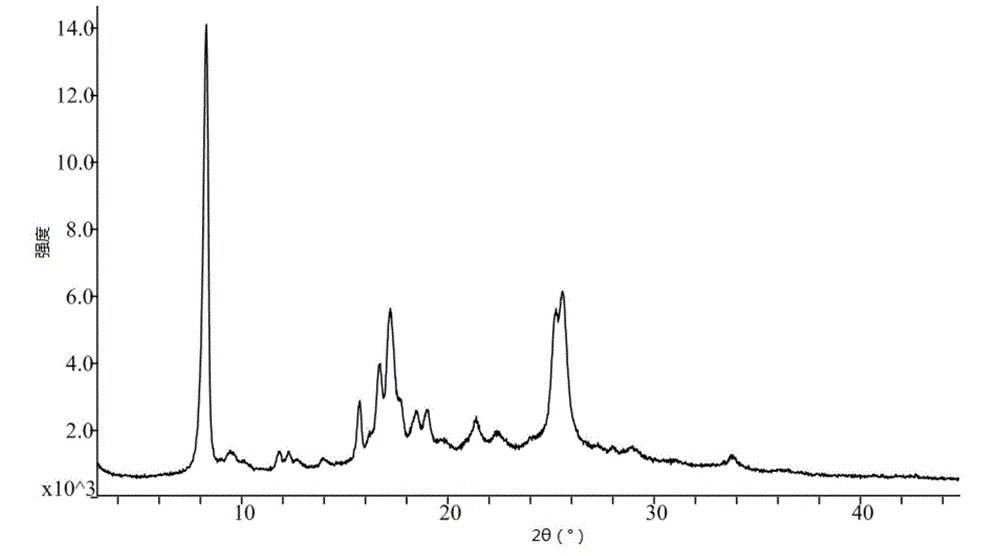
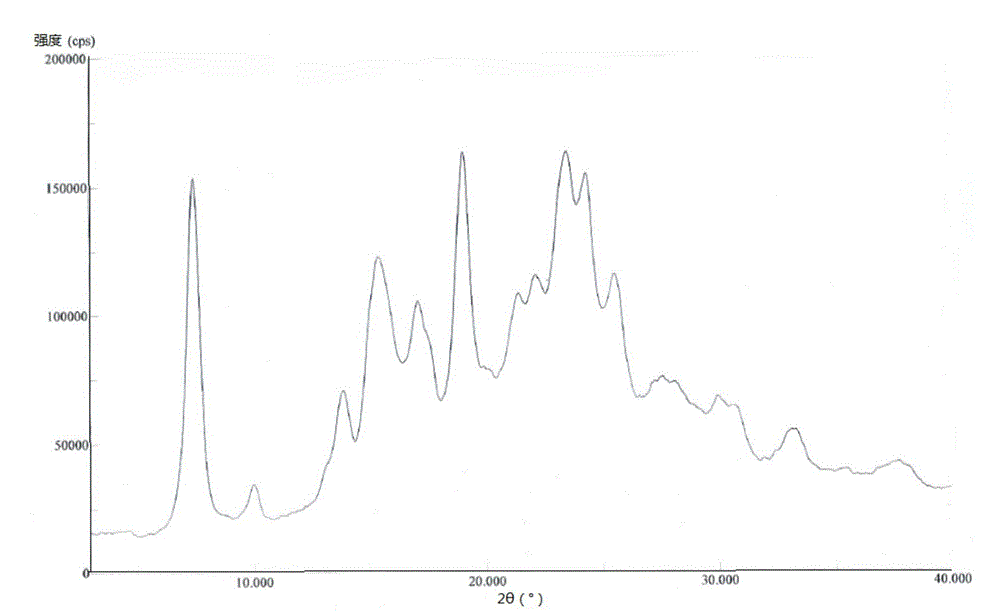
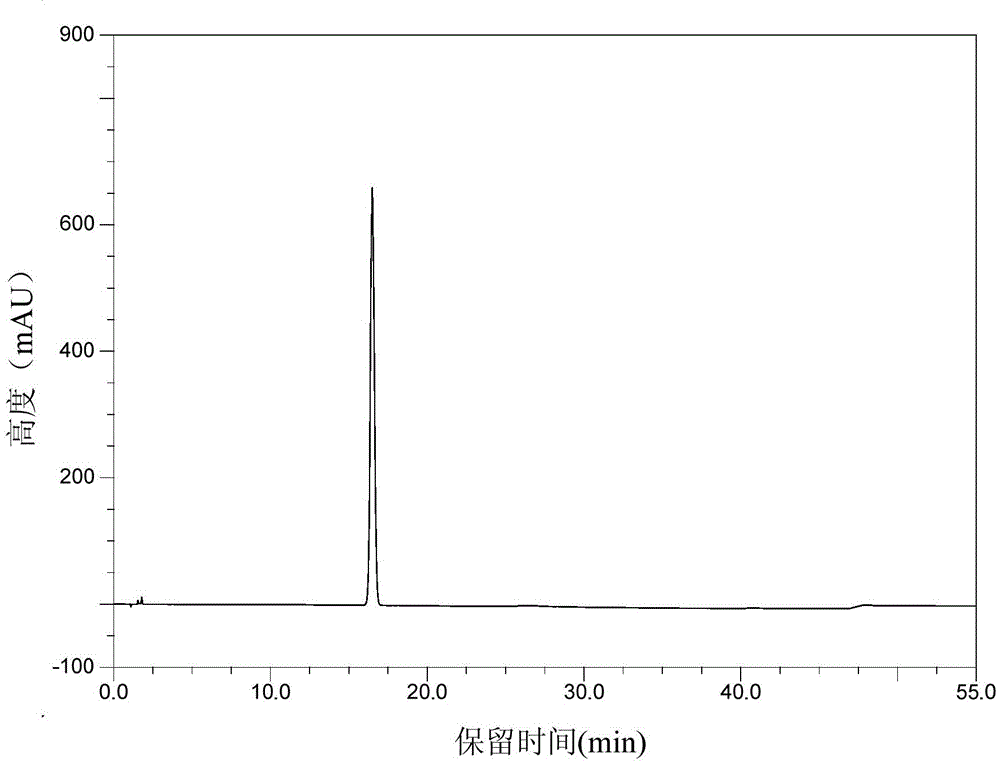
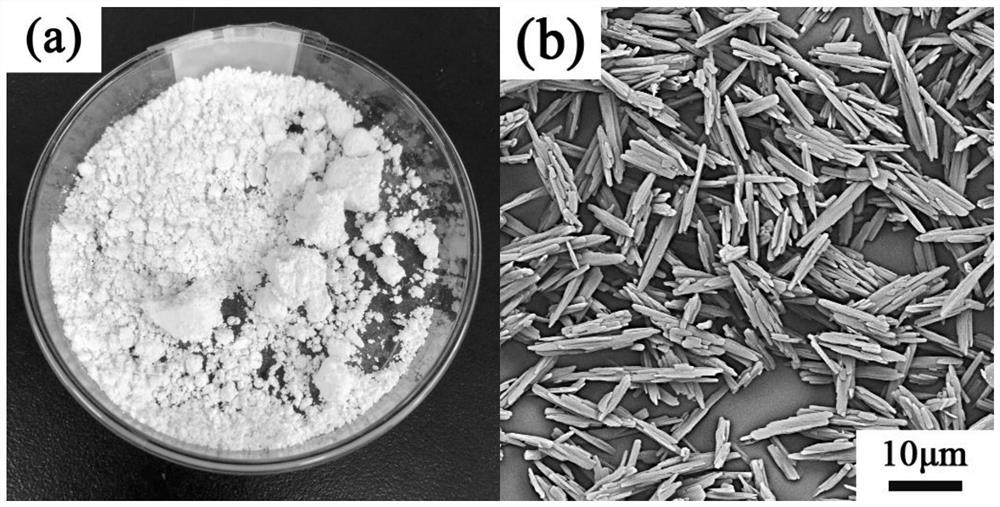
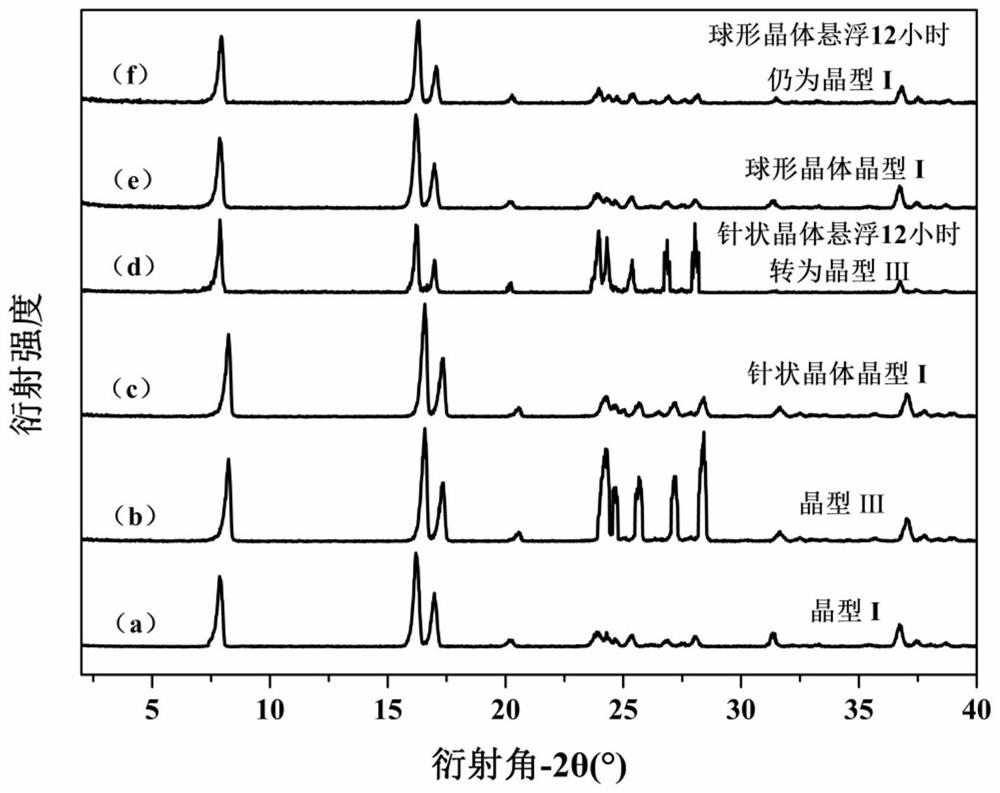
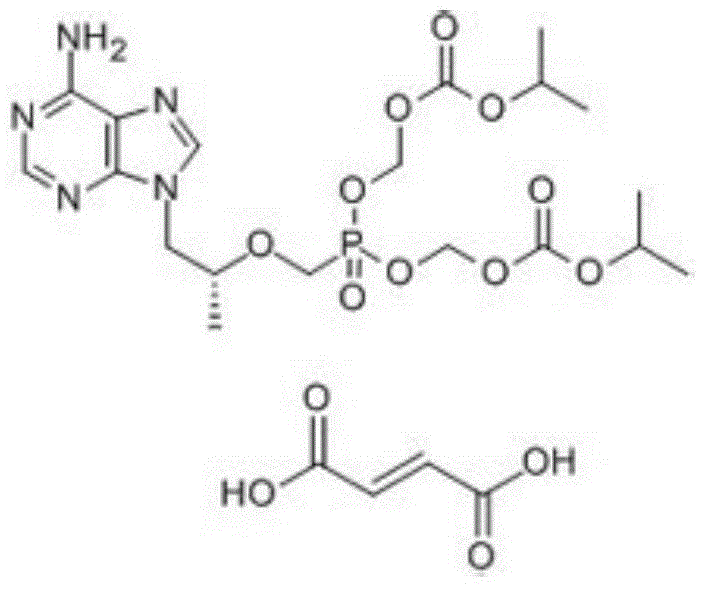
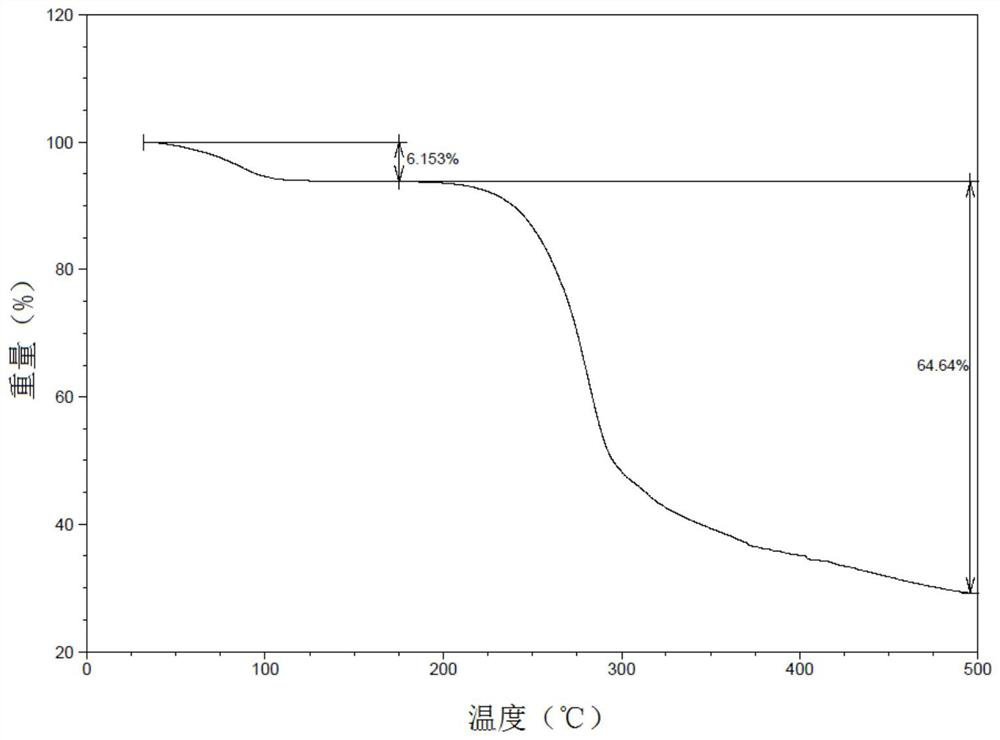
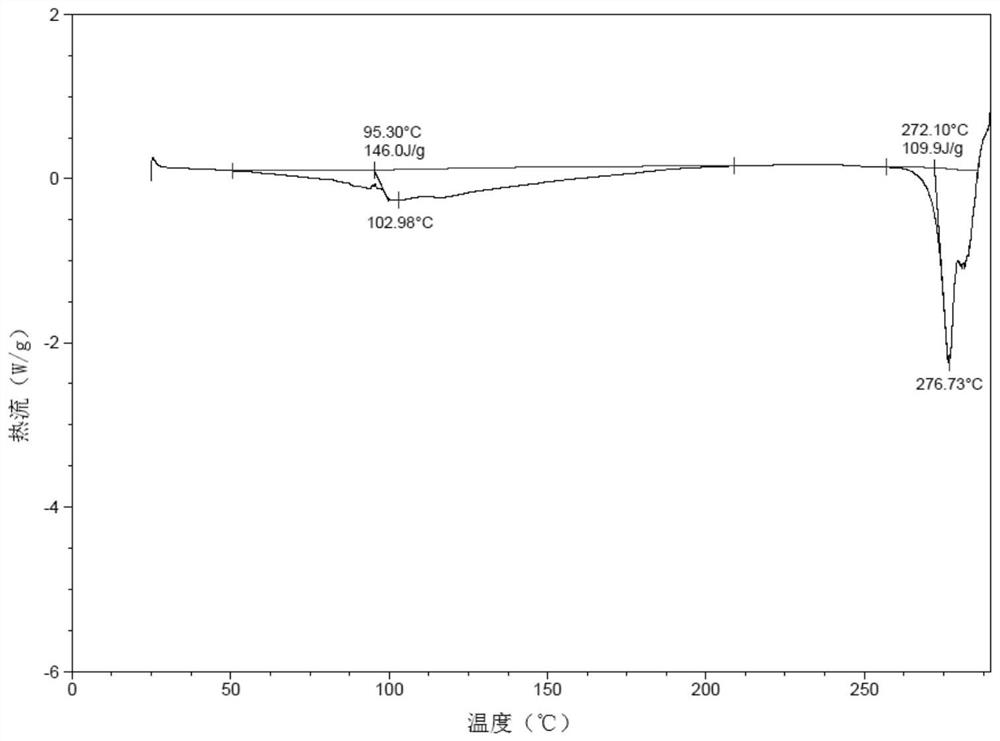
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31results about How to "Mild conditions for crystallization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Crystal form of sorafenib tosylate, and preparation method thereof
InactiveCN104761492AHigh crystal purityHigh crystallinityOrganic chemistry methodsSulfonic acids salts preparationX-rayPowder diffraction
The invention provides a crystal form of sorafenib tosylate, and a preparation method thereof. The X-ray powder diffraction pattern of sorafenib tosylate of crystal form B has diffraction peaks when the values of 2theta are about 7.34DEG, 18.03DEG, 20.15DEG, 20.77DEG, 22.04DEG, 22.43DEG and 23.21DEG; and the X-ray powder diffraction pattern of sorafenib tosylate of crystal form C has diffraction peaks when the values of 2theta are about 7.61DEG and 13-33DEG. The sorafenib tosylate of crystal form C is prepared through reduced pressure heating of the sorafenib tosylate of crystal form B. The invention also provides a preparation method of amorphous crystals of sorafenib tosylate through reduced pressure heating of the sorafenib tosylate of crystal form B or C. The sorafenib tosylate crystals prepared in the invention have the advantages of good stability, high crystallization purity, simple preparation process, and suitableness for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
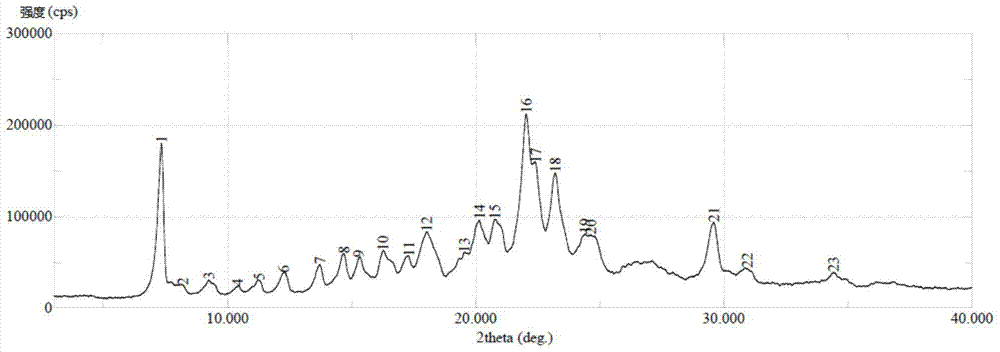
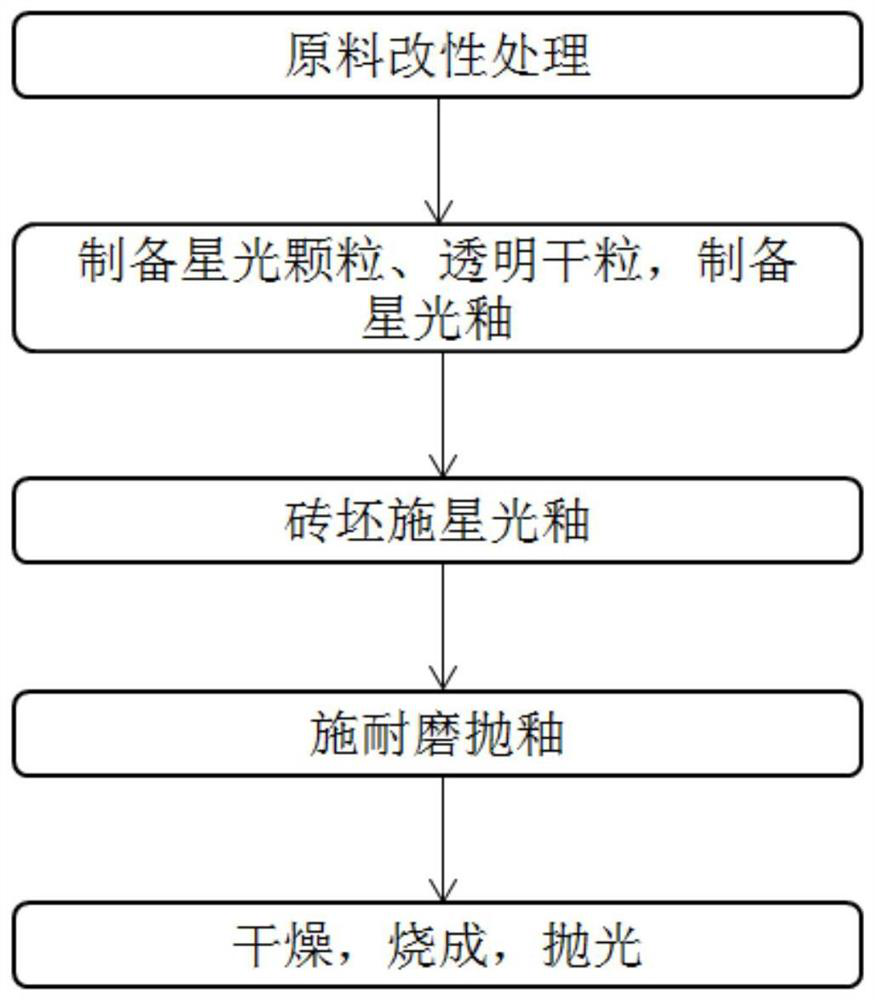
Wear-resistant antifouling ceramic starlight glazed brick and preparation method thereof
ActiveCN112979349AWide firing temperature rangeMild conditions for crystallizationBrickWear resistance
The invention relates to the technical field of preparation of ceramic glazed bricks, and provides a wear-resistant antifouling ceramic starlight glazed brick and a preparation method thereof. The glittering effect is achieved through multiple reflection, dispersion and refraction methods of crystals in the glaze, a glaze layer of the glazed brick has the multi-layer glittering effect and the three-dimensional decoration effect, and the grade and decoration of the product are improved; and an excellent flashing effect can be achieved only by polishing according to a conventional polishing process of a glazed product. The glazed brick disclosed by the invention has relatively high hardness and excellent antifouling property and wear resistance.
Owner:江西金唯冠建材有限公司
Layered nanosheet ferrierite molecular sieve as well as preparation method and application thereof
PendingCN108793189AThe synthesis method is simpleMild conditions for crystallizationMaterial nanotechnologyHydrocarbon by isomerisationChemistryLow-density polyethylene
The invention discloses a layered nanosheet ferrierite molecular sieve. The nanosheet ferrierite molecular sieve is characterized in that the molecular sieve is a high-crystallinity pure-phase ferrierite molecular sieve; the thickness of the sheet is between 30nm to 80nm; the specific surface area is larger than or equal to 340m<2> / g; the mesoporous surface area is larger than or equal to 60 m<2> / g; the crystallinity reaches 109% or more and is larger than or equal to 106%. The invention also relates to a preparation method of the layered nanosheet ferrierite molecular sieve and application ofthe layered nanosheet ferrierite molecular sieve to alkene oligomerization, low-density polyethylene cracking and skeleton isomerization reaction in n-butene and n-pentene.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
Crystal form B of tetrahydrothienopyridine compound as well as preparation method, composition and application thereof
ActiveCN111943958ACrystal stableEasy to prepareOrganic active ingredientsOrganic chemistry methodsChemical compoundInduced platelet aggregation
The invention discloses a crystal form B of a tetrahydrothienopyridine compound with a structure as shown in a formula I, and a preparation method, a composition and application thereof. The inventionaims to solve the problems of many impurities, low content, poor crystal form stability and incapability of forming a medicine in the prior art. When the crystal form B is subjected to Cu-K alpha radiation, in an X-ray powder diffraction pattern expressed by a 2theta angle, characteristic peaks occur at 11.21 + / - 0.2 degrees, 12.61 + / - 0.2 degrees, 14.69 + / - 0.2 degrees, 16.14 + / - 0.2 degrees, 17.81 + / - 0.2 degrees, 20.22 + / - 0.2 degrees and 22.10 + / - 0.2 degrees. The crystal form B provided by the invention has the advantages of few impurities, good stability, good crystallinity and reproducibility, and is suitable for industrial production; and unexpected stronger ADP-induced platelet aggregation resistance and better flowability are also achieved.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
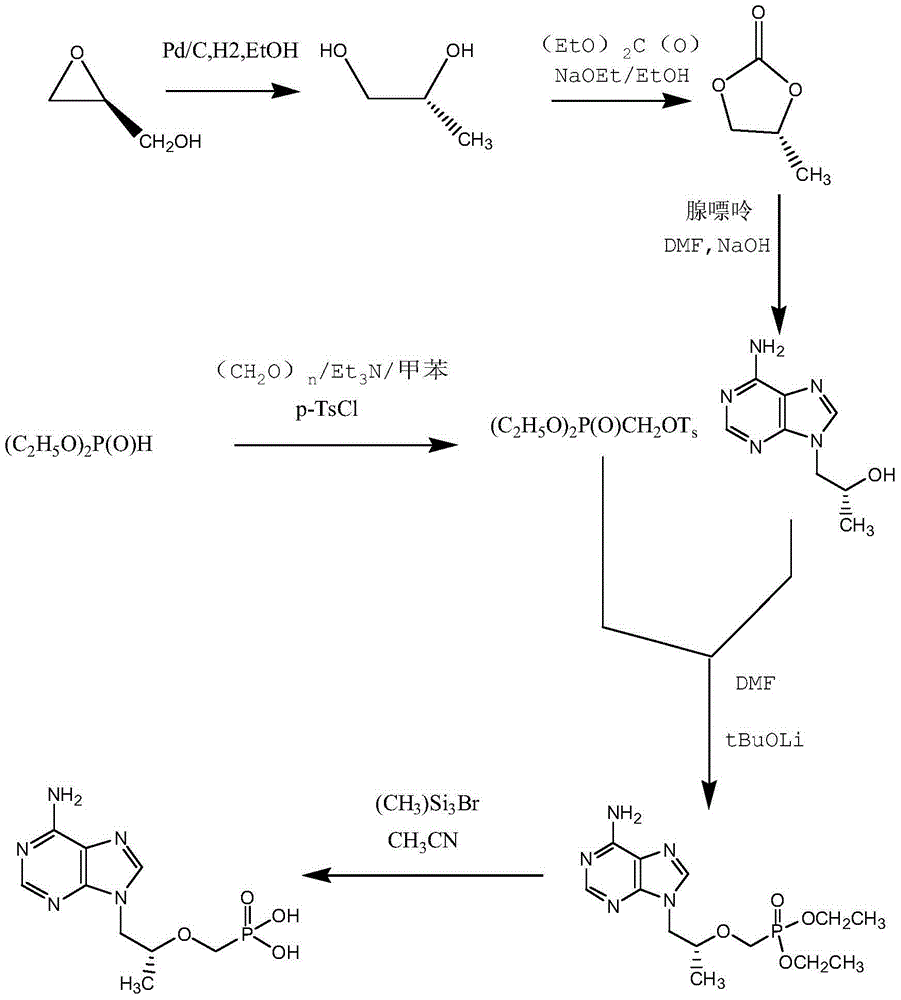
Tenofovir preparation method suitable for industrialized production
ActiveCN104098605AMild conditions for crystallizationMild reaction conditionsGroup 5/15 element organic compoundsLithiumFiltration
The invention provides a tenofovir preparation method suitable for industrialized production. According to the method, tenofovir is obtained through catalyzing and hydrolyzing a raw material intermediate (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine via trimethylbromosilane, wherein the raw material intermediate (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine is obtained through the following steps: firstly, obtaining (R)-9-(2-hydroxypropyl)adenine via the ring-opening condensation of adenine and (R)-epoxypropane under an alkaline condition; secondly, catalyzing the (R)-9-(2-hydroxypropyl)adenine through lithium t-butoxide; thirdly, conducting etherification on the catalyzed (R)-9-(2-hydroxypropyl)adenine and p-benzenesulfonyloxymethyl phosphoric acid diethylester, so as to obtain the (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine. The method is characterized in that purified water of appropriate proportion is added in a mixed product obtained through catalyzing and hydrolyzing the (R)-9-[2-(diethylphosphonomethoxy)propyl] adenine via trimethylbromosilane; the tenofovir is obtained through spontaneous crystallization at an appropriate temperature and cooling rate, as well as at an appropriate stirring speed. According to the invention, the obtained tenofovir product has the characteristics of high yield, strong indissolubility in operation under room temperature, simplicity in filtration and collection due to large particles, short production time, low energy consumption, and the like.
Owner:FUJIAN COSUNTER PHARMA CO LTD
Crystals of anilinopyrimidine compound with indole substituted by trifluoroethyl group and salts thereof
ActiveCN107973782AHigh purityHigh crystallinityOrganic active ingredientsOrganic chemistry methodsEGFR inhibitorsBis indole
The invention provides crystals of an anilinopyrimidine compound with indole substituted by a trifluoroethyl group and salts thereof, wherein the anilinopyrimidine compound is used as an EGFR inhibitor. Specifically, the invention relates to a crystal of the compound as shown in a formula I which is described in the specification, a crystal of a monomesylate of the compound as shown in the formulaI or a dimesylate of the compound as shown in the formula I, and further relates to preparation methods for the crystals, crystal compositions containing the crystals, and pharmaceutical compositionscontaining the crystals or the crystal compositions and medical application thereof. The crystals provided by the invention have the advantages of high purity, high crystallization degree, good stability, etc.
Owner:INVENTISBIO CO LTD +1
Crystal transformation method of aluminum hydride
ActiveCN106957047AHigh crystal conversion efficiencyMild conditions for crystallizationMetal hydridesAir atmosphereFiltration
The invention discloses a crystal transformation method of aluminum hydride. The crystal transformation method of the aluminum hydride comprises the following steps: adding the aluminum hydride into a solvent under the atmosphere of dry nitrogen, stirring, performing suction filtration and removing insoluble impurities to obtain an aluminum hydride solution; performing ultrasonic treatment; performing rotary evaporation to obtain aluminum hydride solid granules; putting the aluminum hydride solid granules into the air, dissolving, filtering, performing rotary evaporation and vacuum-drying the product. According to the crystal transformation method of the aluminum hydride, the aluminum hydride, which exists in beta and gamma crystal forms, in the aluminum hydride is converted into alpha-aluminum hydride with higher stability, and the impurity aluminum and the rest aluminum hydride which exist in an unstable crystal form are removed from the aluminum hydride. The crystal transformation method provided by the invention is high in crystal transformation efficiency, mild in crystal transformation condition and easy to operate and control. The purity of the alpha-aluminum hydride obtained after crystal transformation can reach above 99 percent, and the alpha-aluminum hydride can exist stably in the air atmosphere.
Owner:河南纳宇新材料有限公司
A cobalt-containing modified aluminum phosphate molecular sieve flue gas adsorption material and its preparation method and application
ActiveCN107416857BHighly selective adsorptionStructure orderOther chemical processesTobacco smoke filtersMolecular sieveCrotonaldehyde
The invention relates to a cobalt-containing modified aluminophosphate molecular sieve smoke adsorbing material as well as a preparation method and application thereof, belonging to the technical field of cigarettes. The preparation method of the material comprises the following processing steps of preparation, crystallization, drying and calcination of CoAPO-11 initial gel; preparing the cobalt-containing modified aluminophosphate molecular sieve smoke adsorbing material which is excellent in crystallization; adding the prepared material in the middle of a cigarette filter, and testing the smoke absorbing effect of the material. The result indicates that the material has an excellent adsorbing effect on hydrogen cyanide and crotonaldehyde. According to the preparation method provided by the invention, the raw materials are low in cost, the crystallization condition is relatively mild, and the energy consumption is low. The prepared material can be used for effectively reducing the release amount of the hydrogen cyanide and the crotonaldehyde in the smoke after lowering the cigarette filter, and the harm reduction effect is outstanding.
Owner:CHINA TOBACCO YUNNAN IND
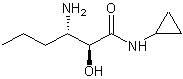
Chiral intermediate (S, S)-3-amino-N-cyclopropyl-2-hydroxyalkanamide or its salt and preparation method thereof
InactiveCN102659618AMild conditions for crystallizationReduce energy consumptionCarboxylic acid amide separation/purificationAminalHydroxyhexamide
The invention relates to a chiral intermediate (S, S)-3-amino-N-cyclopropyl-2-hydroxyalkanamide or its salt and a preparation method thereof. The chiral intermediate (S, S)-3-amino-N-cyclopropyl-2-hydroxyalkanamide is an important intermediate for preparing a hepatitis C-resistant drug Telaprevir. The preparation method utilizes D-tartaric acid as a resolving agent to resolve 3-amino-N-cyclopropyl-2-hydroxyalkanamide so that the chiral intermediate (S, S)-3-amino-N-cyclopropyl-2-hydroxyalkanamide or its salt is obtained.
Owner:PORTON FINE CHEM
Preparation method of insulin crystals and products thereof
PendingCN113896784ARich sourcesFlexible formPeptide preparation methodsInsulinsOrganic solventMedicine
The invention belongs to the technical field of protein crystals, and relates to a preparation method of insulin crystals and a products thereof. Specifically, the method comprises the following steps: 1) mixing insulin, citric acid, phenols, zinc substances, water and an optional organic solvent to obtain crystalline liquid; 2) adjusting the pH value of the crystalline liquid by using a pH regulator, and crystallizing to obtain insulin crystals. The molar ratio among insulin, citric acid, phenols and zinc substances is 1: (127.3-1272.9): (3.1-123.0): (4.3-85.6). The method has the advantages of easiness in crystal formation, mild crystallization conditions, large crystallization temperature range, and short crystallization time. The insulin hexahedral crystals obtained by the method have the advantages of small sedimentation volume, uniformity, stability, high production efficiency, and easiness in industrial production.
Owner:HEFEI TIANMAI BIOTECH DEV
M-aminobenzoic acid spherical crystal and preparation method thereof
ActiveCN112358409AEasy to recycleStrong adhesionOrganic compound preparationAmino-carboxyl compound preparationOrganic solventPhysical chemistry
The invention relates to an m-aminobenzoic acid spherical crystal and a preparation method thereof. The method comprises the following steps: dissolving m-aminobenzoic acid I crystal form raw materials in an organic solvent at 45-65 DEG C under the stirring action to prepare an m-aminobenzoic acid solution, cooling the solution to 5-20 DEG C, continuously stirring until crystals emerge, continuously stirring for 30-60 minutes to coalesce the crystals into balls, separating solid and liquid crystal slurry, filtering, washing and drying a wet solid product to obtain m-aminobenzoic acid sphericalcrystals. The spherical crystal prepared by the invention can effectively improve the chemical stability and the crystal form stability of the m-aminobenzoic acid I crystal form. The purity of the spherical crystals is greater than 98%, the volume average particle size is 0.55-1.50 mm, the crystal particles are round, the fluidity is high, the repose angle is 24.3-27.7 degrees, the caking rate is3.0-6.5%, the tap density is 0.28-0.35 g / cm<3>, and the specific surface area is 0.14-0.23 m<2> / g.
Owner:TIANJIN UNIV
Method for preparing rifampicin crystal form II
InactiveCN108101927AMild conditions for crystallizationPrevent oxidation and discolorationOrganic chemistry methodsSlurryAcetone
The invention provides a method for preparing rifampicin crystal form II. The method comprises the steps: under the condition of 25 DEG C to 35 DEG C, dissolving a rifampicin crude product into acetone at a stirring speed as 45r / min to 65r / min to be prepared into a solution with a concentration as 0.04g / mL to 0.2g / mL; then under the condition of 25 DEG C to 35 DEG C, continuing stirring for 0.5 to3h at 45r / min to 65r / min; filtering, washing and drying under reduced pressure obtained rifampicin crystal slurry to obtain the rifampicin crystal form II product. A product yield is larger than 90%,and a purity is 98% or more. The method disclosed by the invention has the advantages of stable technology, simpleness in operation, moderate condition, high product yield and purity, good fluidity,low cost, suitability for industrial production and good application prospect.
Owner:TIANSHENG PHARMA GROUP
Refining method of aspirin-lysine
PendingCN114394910AReduce contentMild conditions for crystallizationOrganic compound preparationCarboxylic acid esters preparationAspirinAlcohol ethyl
The invention belongs to the technical field of pharmaceutical chemicals, and particularly discloses a refining method of aspirin-lysine. The refining method comprises the following steps: dissolving aspirin-DL-lysine in an ethanol water solution, adding absolute ethyl alcohol, cooling and crystallizing to prepare a refined aspirin-DL-lysine product. Experiments show that the content of free salicylic acid is greatly reduced after the aspirin-lysine is refined, so that the problem that the content of the free salicylic acid in the aspirin-lysine is relatively high due to sudden abnormal production conditions or improper storage conditions can be effectively solved. The method disclosed by the invention is simple in process, mild in crystallization condition, easy to control and low in cost, and has relatively high industrial application value.
Owner:BENGBU BBCA MEDICINE SCI DEV
A kind of crystal transformation method of aluminum hydride
ActiveCN106957047BStable energy characteristicsEnergy characteristics do not changeMetal hydridesAir atmosphereFiltration
The invention discloses a crystal transformation method of aluminum hydride. The crystal transformation method of the aluminum hydride comprises the following steps: adding the aluminum hydride into a solvent under the atmosphere of dry nitrogen, stirring, performing suction filtration and removing insoluble impurities to obtain an aluminum hydride solution; performing ultrasonic treatment; performing rotary evaporation to obtain aluminum hydride solid granules; putting the aluminum hydride solid granules into the air, dissolving, filtering, performing rotary evaporation and vacuum-drying the product. According to the crystal transformation method of the aluminum hydride, the aluminum hydride, which exists in beta and gamma crystal forms, in the aluminum hydride is converted into alpha-aluminum hydride with higher stability, and the impurity aluminum and the rest aluminum hydride which exist in an unstable crystal form are removed from the aluminum hydride. The crystal transformation method provided by the invention is high in crystal transformation efficiency, mild in crystal transformation condition and easy to operate and control. The purity of the alpha-aluminum hydride obtained after crystal transformation can reach above 99 percent, and the alpha-aluminum hydride can exist stably in the air atmosphere.
Owner:河南纳宇新材料有限公司
3-((L-valyl)amino)-1-propanesulfonic acid crystal form, preparation method and applications thereof
PendingCN111170901AHigh purityHigh thermodynamic stabilityOrganic active ingredientsNervous disorderPropylsulfonic acidAmino radical
The invention relates to a crystal form of a compound 3-((L-valyl)amino)-1-propanesulfonic acid, a preparation method and applications thereof.
Owner:RISEN SUZHOU PHARMA TECH CO LTD
Crystal form of pralatrexate, pharmaceutical composition containing pralatrexate, and preparation method and application of pralatrexate
ActiveCN104418859AHigh purityImprove stabilityOrganic active ingredientsOrganic chemistryAnti solventX-ray
The invention discloses a crystal form of pralatrexate, a pharmaceutical composition containing the pralatrexate, and a preparation method and an application of the pralatrexate. According to the crystal form, in an X-ray powder diffraction pattern using a radiation source as Cu-Kalpha, characteristic absorption peaks are formed at the diffraction angles 2theta of 8.29 degrees, 15.73 degrees, 16.72 degrees, 17.21 degrees, 18.46 degrees, 19.01 degrees, 21.38 degrees, 25.25 degrees and 25.56 degrees; and the error range of 2theta is + / -0.2 degrees. The preparation method of the crystal form disclosed by the invention comprises the following steps: heating and dissolving pralatrexate into a good solvent; and dropwise adding an anti-solvent until turbidity is achieved, naturally cooling, and devitrifying, so as to obtain the crystal form. The crystal form of the pralatrexate disclosed by the invention has the advantages of high purity, good stability and the like.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
High-purity Pralatrexate solid and preparation method thereof
ActiveCN105272983AHigh purityImprove securityOrganic active ingredientsOrganic chemistryPralatrexateKetone
The invention provides a high-purity Pralatrexate solid and its preparation method. The high-purity Pralatrexate solid provided by the invention is prepared by recrystallization of an aqueous solution of lower alkyl ketone and has advantages of high purity, low individual impurity content, good stability, high safety and the like. Meanwhile, the preparation method of the high-purity Pralatrexate solid provided by the invention can be adopted to effectively remove IN0222(2,4-diamido-6-chloromethylpteridine) or its analogue and raise safety of medicine. In addition, the preparation method of the high-purity Pralatrexate solid is simple, a solvent is cheap and easily available, and crystallization condition is mild. The preparation method is suitable for industrial production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
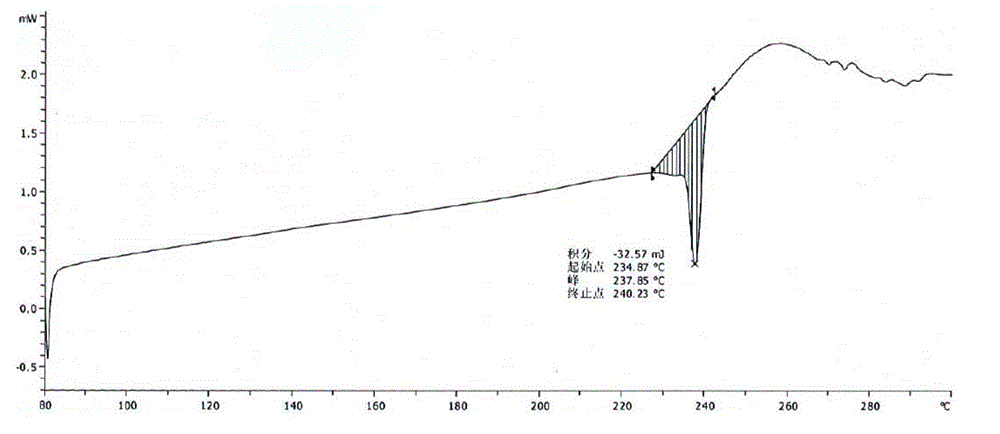
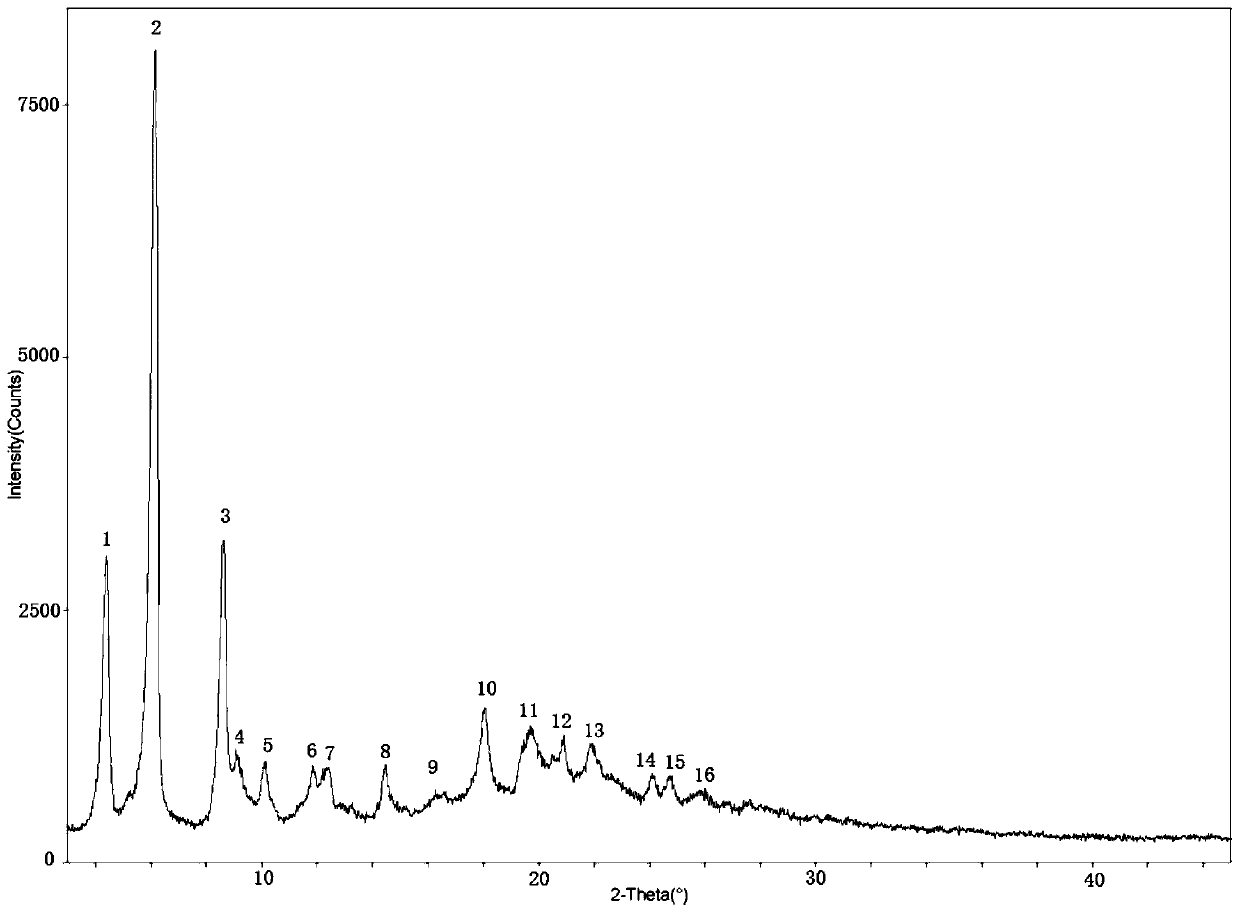
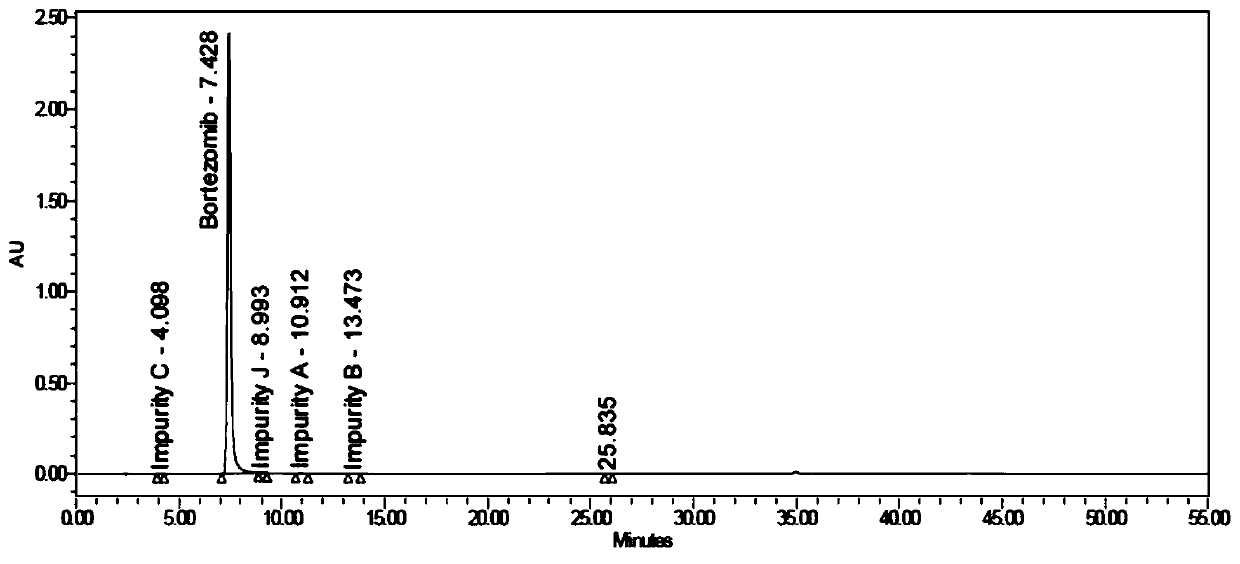
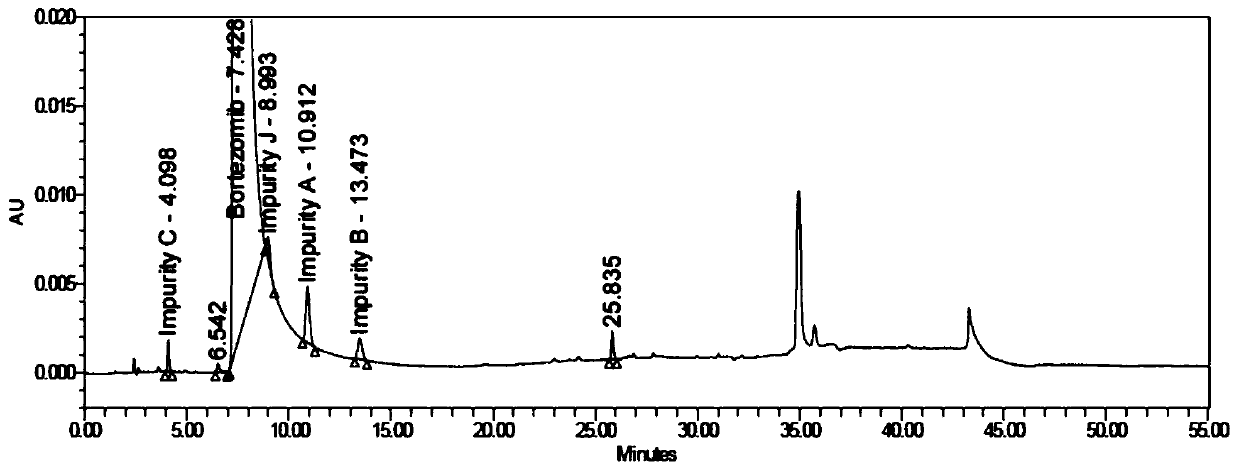
Bortezomib crystal form M, and preparation method and application thereof
InactiveCN110642881AReduce the overall heightEasy to useOrganic chemistry methodsBoron compound active ingredientsMedicinePhysical chemistry
The invention relates to the technical field of pharmaceutical chemistry, in particular to a bortezomib crystal form M, and a preparation method and application thereof. The X-ray powder diffraction pattern of the crystal form M has characteristic peaks at positions of 2theta+ / -0.2 degrees, wherein the values of the 2theta are 4.4, 6.2, 8.6, 9.1, 10.1, 11.8, 12.4, 14.5, 18.1, 19.7, 20.9 and 21.9.The crystal form M has the advantages of high stability, simple preparation, low cost and high purity.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Crystal of aniline pyrimidine compound and salt thereof substituted by trifluoroethyl indole
ActiveCN107973782BHigh purityHigh crystallinityOrganic active ingredientsOrganic chemistry methodsPharmaceutical drugEthyl group
Owner:INVENTISBIO CO LTD +1
Crystal of tenofovir alafenamide hemifumarate and preparation method thereof
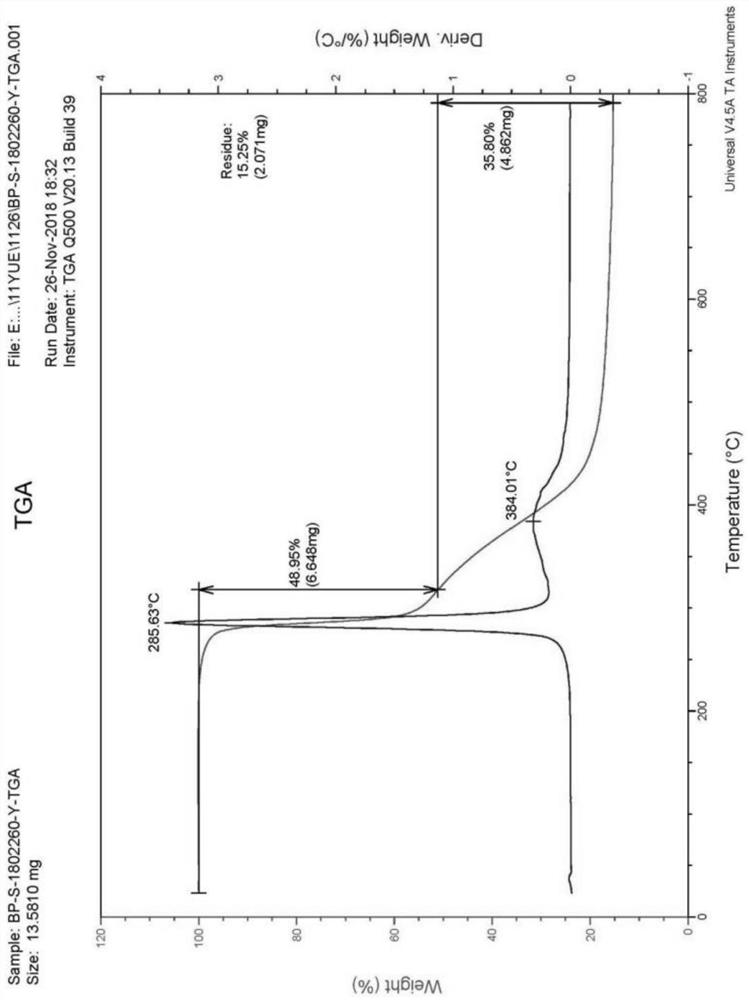
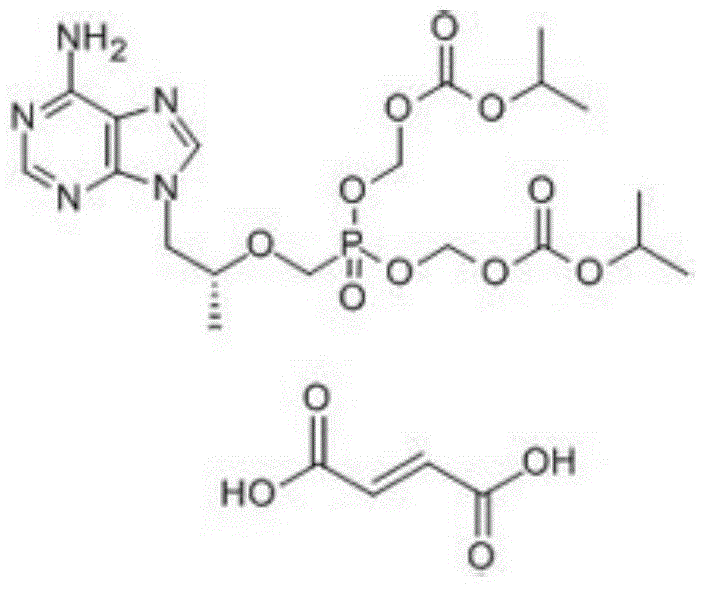
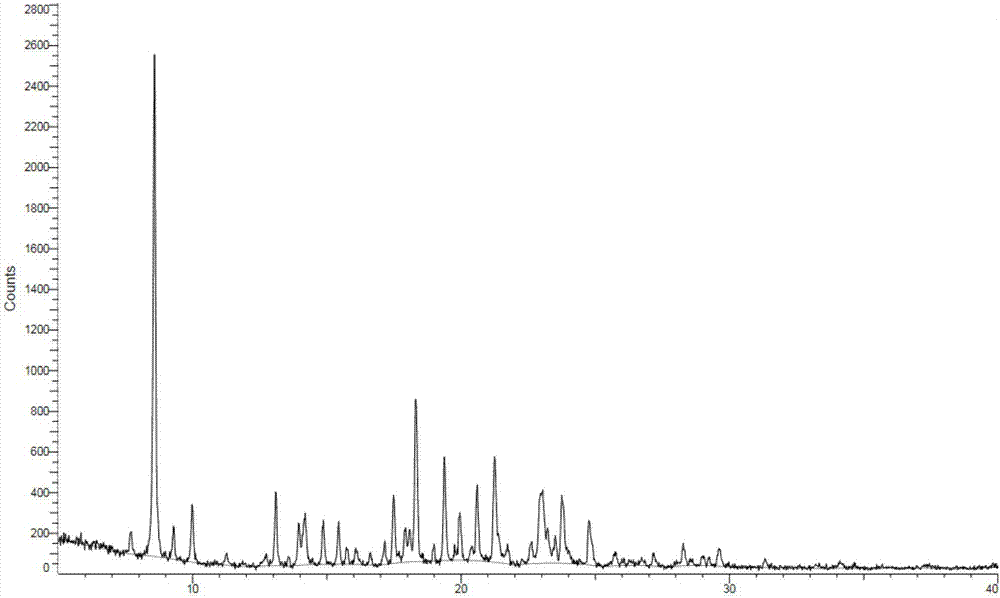
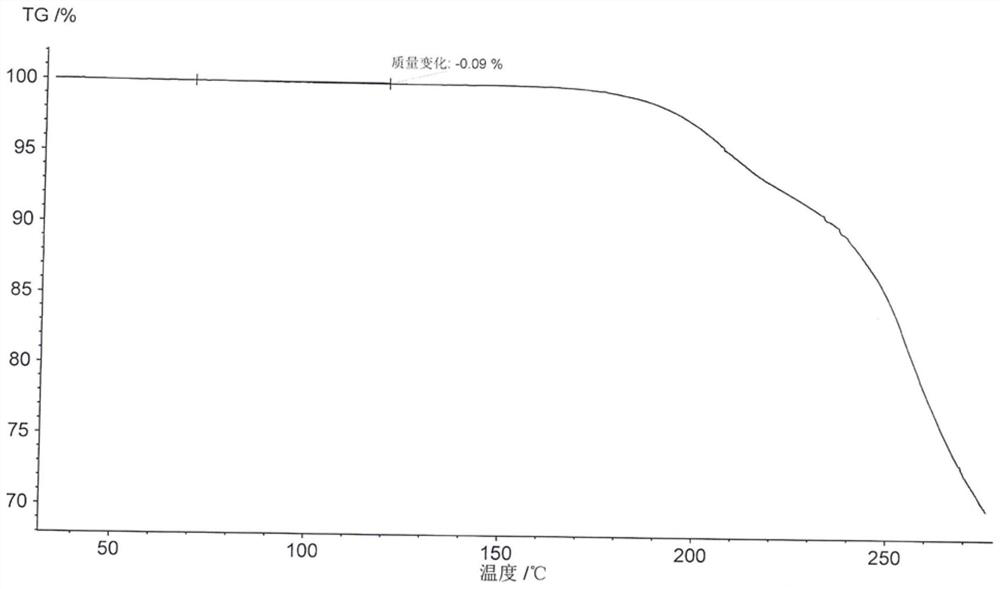
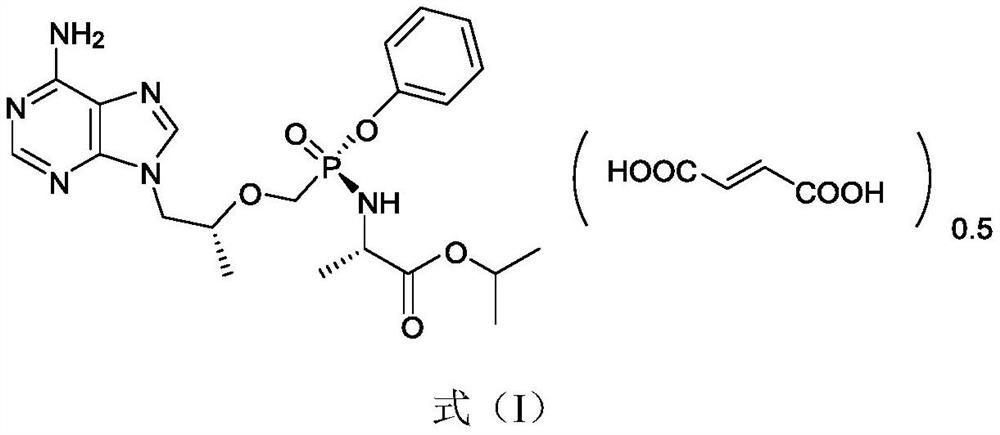
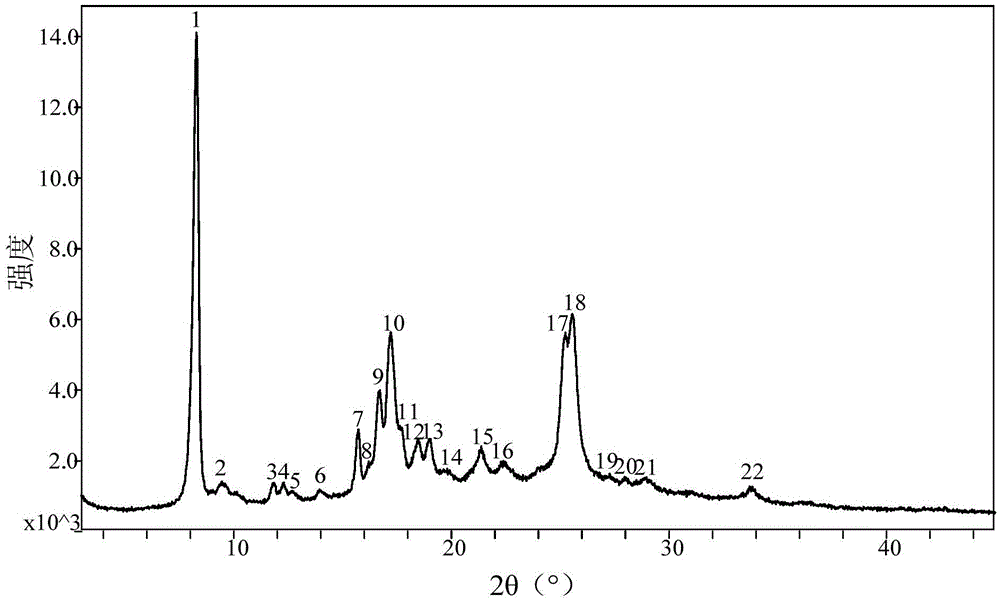
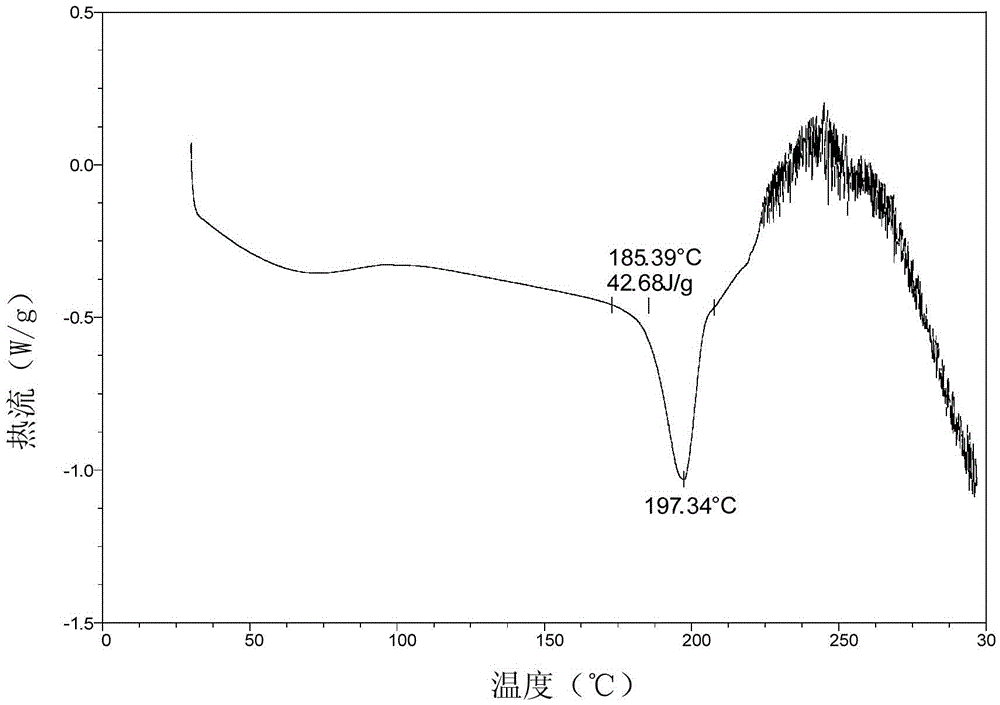
PendingCN113072583AHigh purityHigh crystallinityOrganic active ingredientsGroup 5/15 element organic compoundsMedicinePhysical chemistry
The invention relates to the field of medicines, and discloses a crystal of tenofovir alafenamide hemifumarate and a preparation method thereof. The X-ray powder diffraction of the crystal has characteristic peaks at the following 2 theta angle positions: 7.02 degrees, 8.62 degrees, 11.02 degrees, 12.12 degrees, 13.90 degrees, 15.86 degrees, 16.30 degrees, 17.62 degrees, 18.32 degrees, 20.26 degrees, 20.82 degrees, 23.16 degrees, 26.60 degrees and 27.84 degrees. The tenofovir alafenamide hemifumarate crystal prepared by the invention has the advantages of good fluidity, low moisture absorption and stable crystal form, and is especially suitable for pharmaceutical preparations.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Crystal form of Pralatrexate, pharmaceutical composition containing same, preparation method and application thereof
ActiveCN104418859BHigh purityImprove stabilityOrganic active ingredientsOrganic chemistryAnti solventX-ray
The invention discloses a crystal form of pralatrexate, a pharmaceutical composition containing the pralatrexate, and a preparation method and an application of the pralatrexate. According to the crystal form, in an X-ray powder diffraction pattern using a radiation source as Cu-Kalpha, characteristic absorption peaks are formed at the diffraction angles 2theta of 8.29 degrees, 15.73 degrees, 16.72 degrees, 17.21 degrees, 18.46 degrees, 19.01 degrees, 21.38 degrees, 25.25 degrees and 25.56 degrees; and the error range of 2theta is + / -0.2 degrees. The preparation method of the crystal form disclosed by the invention comprises the following steps: heating and dissolving pralatrexate into a good solvent; and dropwise adding an anti-solvent until turbidity is achieved, naturally cooling, and devitrifying, so as to obtain the crystal form. The crystal form of the pralatrexate disclosed by the invention has the advantages of high purity, good stability and the like.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Preparation method of mycophenolate sodium
The invention belongs to the field of medicines, and particularly relates to a preparation method of mycophenolate sodium. According to the invention, mycophenolic acid is used as a raw material to prepare an M2 crystal form mycophenolate sodium bulk drug suitable for a preparation. According to the preparation method of the mycophenolate sodium, mycophenolic acids with various purities are used as raw materials and can be commercially purchased, and the raw materials are easy to obtain; according to the method, methyl isobutyl ketone which is low in toxicity is adopted as a solvent and is easy to recycle, the production cost is low, meanwhile, methyl isobutyl ketone serves as a crystallization solvent, the crystallization condition is mild, the obtained mycophenolate sodium is in an M2 crystal form, the stacking density ranges from 0.3 g / mL to 0.5 g / mL, and use of pharmaceutical preparations is facilitated. According to the preparation method of the mycophenolate sodium, the crystallization yield reaches up to 93% or above, the process is simple, and industrial production is facilitated.
Owner:GUANGDONG BLUE TREASURE PHARMA
A kind of m-aminobenzoic acid spherical crystal and preparation method thereof
ActiveCN112358409BImprove storage and transportation efficiencyImprove stabilityOrganic compound preparationAmino-carboxyl compound preparationOrganic solventPhysical chemistry
The invention relates to a spherical crystal of m-aminobenzoic acid and a preparation method thereof. Under stirring at 45~65 ℃, the raw material of m-aminobenzoic acid crystal form I was dissolved in an organic solvent to prepare a m-aminobenzoic acid solution, the solution was cooled to 5~20 ℃, and the stirring was continued until the crystals appeared, and the stirring was continued for 30 minutes. ~60min, the crystals are coalesced into balls, then the solid liquid crystal slurry is separated, and the wet solid product is filtered, washed and dried to obtain m-aminobenzoic acid spherical crystals. The spherical crystal prepared by the invention can effectively improve the chemical stability and crystal stability of the crystal form I of m-aminobenzoic acid. The purity of spherical crystals is more than 98%, the volume average particle size is 0.55-1.50mm, the crystal particles are round, the fluidity is high, the angle of repose is 24.3-27.7°, the agglomeration rate is 3.0-6.5%, and the tap density is 0.28-0.35g / cm3, the specific surface area is 0.14 ~ 0.23m2 / g.
Owner:TIANJIN UNIV
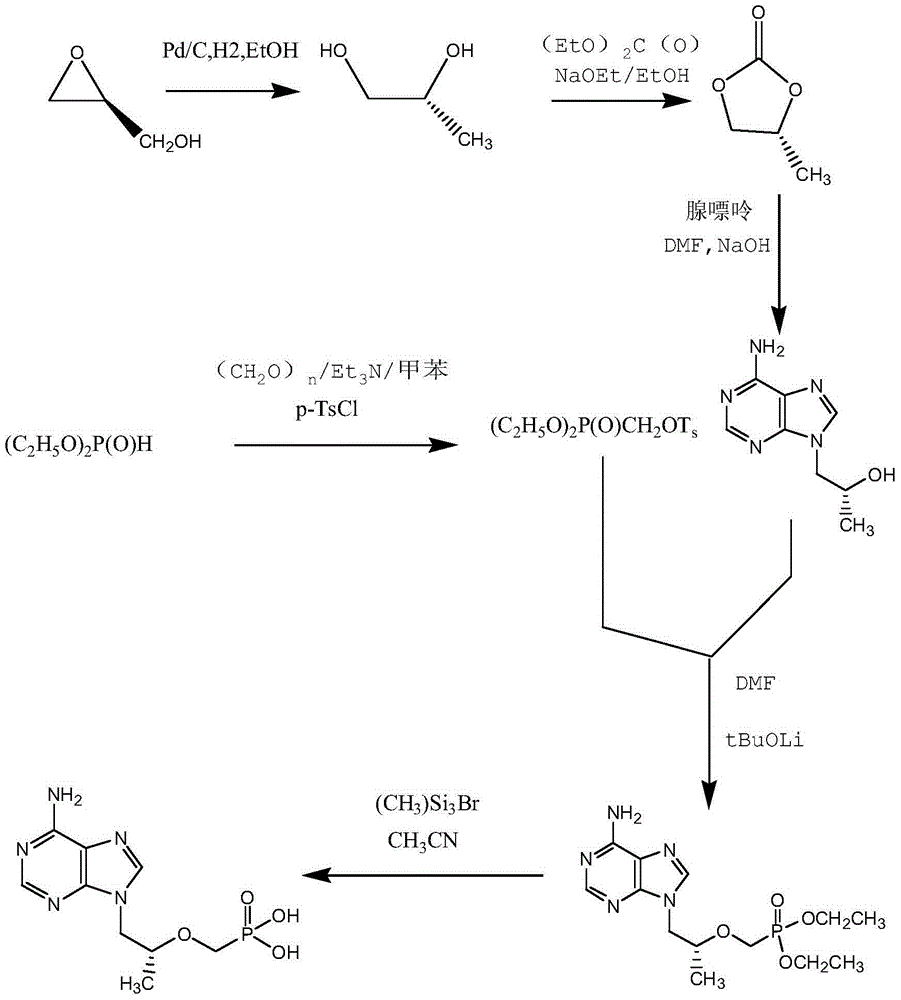
A kind of tenofovir preparation method suitable for industrialized production
ActiveCN104098605BMild conditions for crystallizationMild reaction conditionsGroup 5/15 element organic compoundsLithiumFiltration
The invention provides a tenofovir preparation method suitable for industrialized production. According to the method, tenofovir is obtained through catalyzing and hydrolyzing a raw material intermediate (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine via trimethylbromosilane, wherein the raw material intermediate (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine is obtained through the following steps: firstly, obtaining (R)-9-(2-hydroxypropyl)adenine via the ring-opening condensation of adenine and (R)-epoxypropane under an alkaline condition; secondly, catalyzing the (R)-9-(2-hydroxypropyl)adenine through lithium t-butoxide; thirdly, conducting etherification on the catalyzed (R)-9-(2-hydroxypropyl)adenine and p-benzenesulfonyloxymethyl phosphoric acid diethylester, so as to obtain the (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine. The method is characterized in that purified water of appropriate proportion is added in a mixed product obtained through catalyzing and hydrolyzing the (R)-9-[2-(diethylphosphonomethoxy)propyl] adenine via trimethylbromosilane; the tenofovir is obtained through spontaneous crystallization at an appropriate temperature and cooling rate, as well as at an appropriate stirring speed. According to the invention, the obtained tenofovir product has the characteristics of high yield, strong indissolubility in operation under room temperature, simplicity in filtration and collection due to large particles, short production time, low energy consumption, and the like.
Owner:FUJIAN COSUNTER PHARMA CO LTD
Azoxystrobin acetic acid solvate and preparation method thereof
ActiveCN108947914BHigh crystallinityReduce energy consumptionBiocideOrganic chemistry methodsAcetic acidAzoxystrobin
The invention relates to azoxystrobin acetic acid solvate and a preparation method thereof. Its X-ray powder diffraction pattern is at diffraction angle 2θ=7.40±0.20°, 8.28±0.20°, 13.26±0.20°, 14.07±0.20°, 14.52±0.20°, 18.08±0.20°, 18.42±0.20°, 18.86±0.20 °, 20.46±0.20°, 21.18±0.20°, 22.14±0.20°, 22.68±0.20°, 24.46±0.20°, 26.88±0.20°, 28.60±0.20°, etc. have characteristic peaks, of which 7.40±0.20° is the starting point peak, the relative intensity of the characteristic peak at 21.18±0.20° is 100%. The preparation method is a constant-temperature suspension crystallization method, and the operation is simple, the reproducibility is good, the product has good fluidity, is not easy to coalesce, and is easy to be industrialized.
Owner:TIANJIN UNIV
A kind of crystal form of bortezomib, its preparation method and its pharmaceutical composition and application
ActiveCN104693271BResidue reductionLess irritatingDipeptide ingredientsPeptide preparation methodsSolubilityMedicine use
The invention relates to the field of medicinal chemistry and particularly relates to a new crystal form of the bortezomib. The invention additionally discloses a preparation of the crystal form and an application in the antineoplastic medicine. The crystal form disclosed by the invention solves the technical problems that the current bortezomib crystal form has bad solubility and weak antioxidant ability and is not good for liquid formulation preparation, and has the advantages of good solubility, high stability and strong antioxidant ability; simultaneously the crystal form prepared by the method disclosed by the invention is high in crystal form purity and safe in medicine use.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Crystal or amorphous substance of steroid derivative fxr agonist, preparation method and use thereof
ActiveCN110869382BHigh purityHigh crystallinityOrganic active ingredientsMetabolism disorderDiseasePharmaceutical drug
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
A kind of preparation method of high-content geniposide crystal
ActiveCN105273014BReduce solubilityEasy to controlSugar derivativesSugar derivatives preparationFiltrationToxic material
The invention discloses a preparation method of high-content geniposide crystals. The preparation method comprises the steps that cape jasmine fruits are smashed, extracted with ethyl alcohol, adsorbed with kieselguhr and then concentrated to obtain geniposide extracting extractum; extracting is performed with a mixed solution of ethyl acetate and methyl alcohol to obtain crude geniposide; the crude geniposide is dissolved with a methyl alcohol solution to obtain a saturated solution, cooling is performed after immediate filtration is performed, analytically pure ethyl acetate is added for first-time stirring crystallizing, solution adding is stopped, standing is performed for several hours, the analytically pure ethyl acetate is added for second-time stirring crystallizing until acicular crystals stop growing, a crystallizing system is obtained, and after filtering and reduced-pressure drying are performed, the snowy white acicular geniposide crystals are obtained. According to the crystals obtained through the method, it is detected that the geniposide purity can be more than 98.8 percent, and no toxic substance residue exists. The preparation method is mild in condition, low in cost and high in speed, has the advantages of being safe in production process, easy and convenient to operate, low in cost, short in cycle, high in product quality and stable in quality, and is suitable for industrialized production.
Owner:YUNNAN CHANONG BIOLOGICAL IND
A kind of high-purity pralatrexate solid and preparation method thereof
ActiveCN105272983BHigh purityImprove securityOrganic active ingredientsOrganic chemistryPharmaceutical drugPralatrexate
The invention provides a high-purity Pralatrexate solid and its preparation method. The high-purity Pralatrexate solid provided by the invention is prepared by recrystallization of an aqueous solution of lower alkyl ketone and has advantages of high purity, low individual impurity content, good stability, high safety and the like. Meanwhile, the preparation method of the high-purity Pralatrexate solid provided by the invention can be adopted to effectively remove IN0222(2,4-diamido-6-chloromethylpteridine) or its analogue and raise safety of medicine. In addition, the preparation method of the high-purity Pralatrexate solid is simple, a solvent is cheap and easily available, and crystallization condition is mild. The preparation method is suitable for industrial production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Polymorphic substance of oxycodone hydrochloride as well as preparation method and application of polymorphic substance
ActiveCN114276358AEasy to prepareMild conditions for crystallizationOrganic active ingredientsNervous disorderDrugs preparationsMedicinal chemistry
The invention relates to the field of medicines, in particular to a polymorphic substance of oxycodone hydrochloride as well as a preparation method and application of the polymorphic substance. The oxycodone hydrochloride polymorphic substance provided by the invention has the following crystal forms: crystal forms alpha, beta, gamma or delta, the polymorphic substance with the crystal forms alpha and delta can be obtained by volatilizing a solvent at a constant temperature, and the polymorphic substance with the crystal forms beta and gamma can be obtained by performing crystal transformation on the polymorphic substance with the crystal form alpha under a high-humidity or high-temperature condition. The oxycodone hydrochloride polymorphic substance obtained by the invention is relatively high in purity and excellent in stability, the preparation method is simple, the working time is short, the crystallization condition is mild, the used reagent is cheap and easy to obtain, the cost is low, and the oxycodone hydrochloride polymorphic substance is suitable for industrial production and preparation of related pharmaceutical preparations.
Owner:北京华素制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com