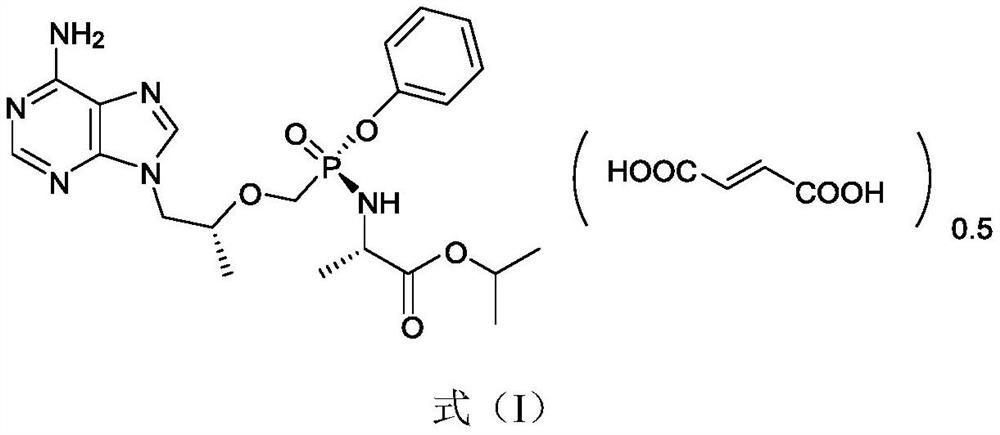
Crystal of tenofovir alafenamide hemifumarate and preparation method thereof
A technology of tenofovir alafenamide and hemifumarate, which is applied in the field of crystallization and preparation of tenofovir alafenamide hemifumarate, and can solve problems such as crystal form dominance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 tenofovir alafenamide
[0037] Add 20kg of (R)-(+)-9-[2-(hydroxyphenoxyphosphorylmethoxy)propyl]adenine into the reaction kettle, add 140L of ethylene glycol dimethyl ether, 13.2kg of chlorinated Sulfoxide, heated to about 70-80°C, reacted for 24 hours, evaporated the solvent under reduced pressure, added 100L of toluene to obtain an acid chloride toluene solution. Add 28kg L-alanine isopropyl ester hydrochloride and 115kg dichloromethane into the reaction kettle, cool down to 0°C, add 20kg triethylamine under stirring, stir for 3 hours under nitrogen protection, filter, add 4A molecular sieve to the filtrate Dry overnight, filter, add 5.6kg of triethylamine, nitrogen protection, cool down to -20~-30°C, add acid chloride toluene solution dropwise, rise to room temperature for 30min reaction, use 100L×3 10% sodium dihydrogen phosphate solution Wash 3 times, 100L 15% KHCO 3 Wash once, wash once with 100L water, dry the organic phase with ...
Embodiment 2
[0038] Example 2 Preparation of tenofovir alafenamide hemifumarate seed crystals
[0039] Add 100mL of acetonitrile, 10g of tenofovir alafenamide, and 1.3g of fumaric acid into the reaction flask, heat to reflux to dissolve, filter, evaporate the solvent under reduced pressure, add 75mL of isopropanol and 25mL of ethanol, and crystallize at room temperature for 24 hours , dried under reduced pressure at 50°C for 4 hours to obtain 1.5 g of off-white solid.
Embodiment 3
[0040] Example 3 Preparation of tenofovir alafenamide hemifumarate crystals
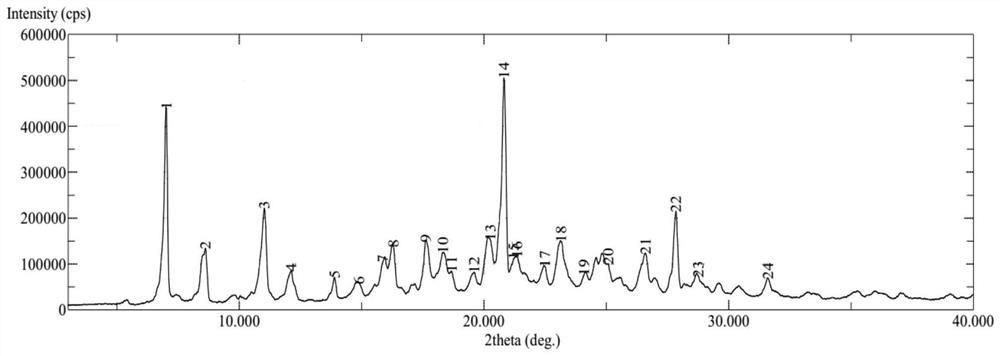
[0041] Add 750ml of isopropanol, 250ml of ethanol, 100g of tenofovir alafenamide, and 13g of fumaric acid into the reaction flask, heat to 50-60°C to dissolve, filter, add a small amount of seed crystals, crystallize at room temperature for 6 hours, and filter , dried under reduced pressure at 50°C for 4 hours to obtain 103 g of off-white solid. Using Cu-Kα radiation, its X-ray powder diffraction data are shown in Table 1, and its XRD pattern is as follows figure 1 shown.
[0042] Table 1
[0043]
[0044]
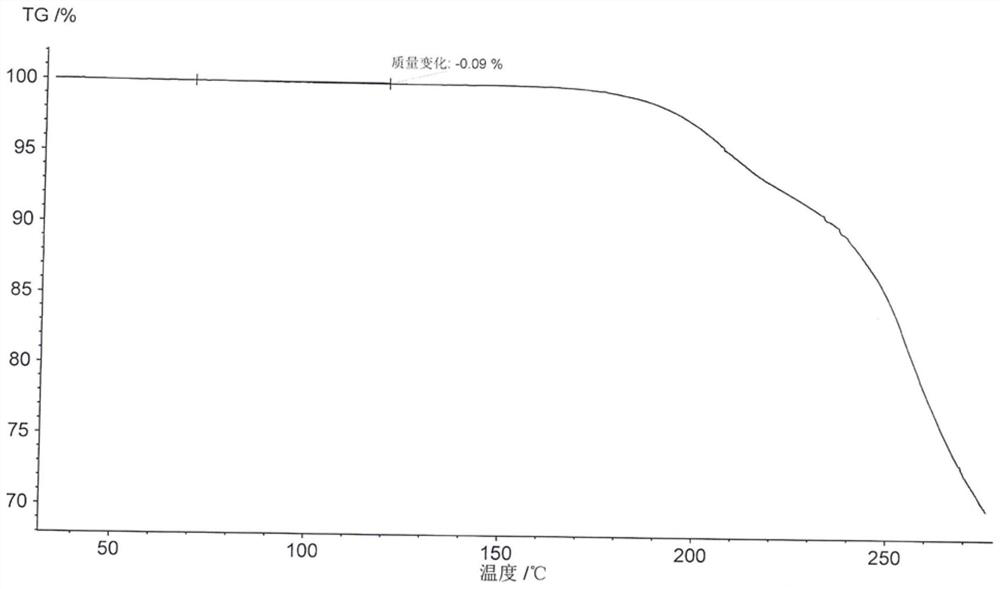
[0045] Its thermogravimetric analysis (TGA) picture is as follows figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com