Method for preparing rifampicin crystal form II
A technology of rifampicin and vested rifampicin, applied in the field of rifampicin crystal forms, can solve the problems such as unfavorable recovery and reuse of crystallization solvent, low yield, increased production cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]Under the temperature condition of 25°C and stirring speed of 60r / min, within 30min, add 200g of crude rifampicin raw material into 2000mL acetone in three batches, and stir at this temperature for 3h. Dry under reduced pressure (vacuum degree: 0.08MPa) for 3 hours to obtain 186.3 g of rifampicin crystal form product with a yield of 93.15%. The purity is 98.2%.
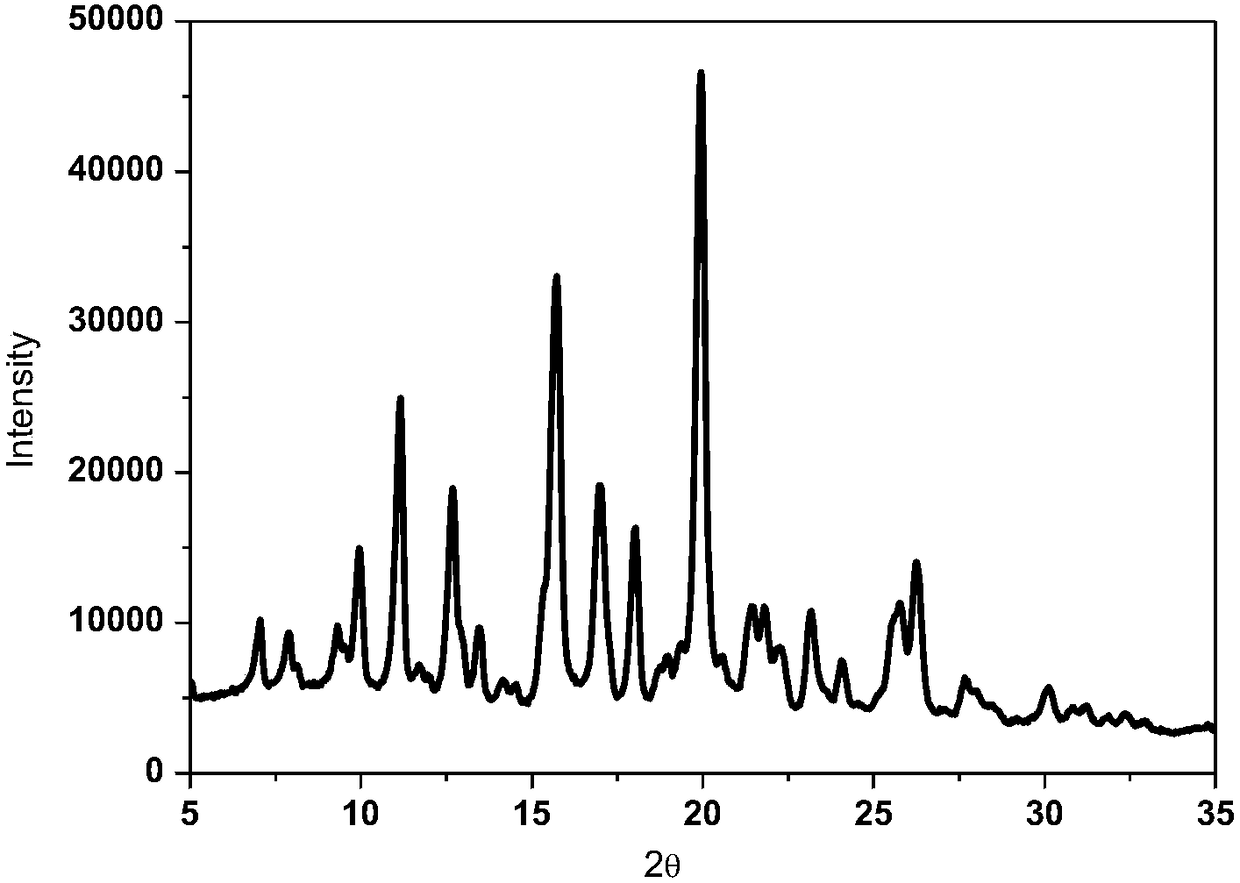
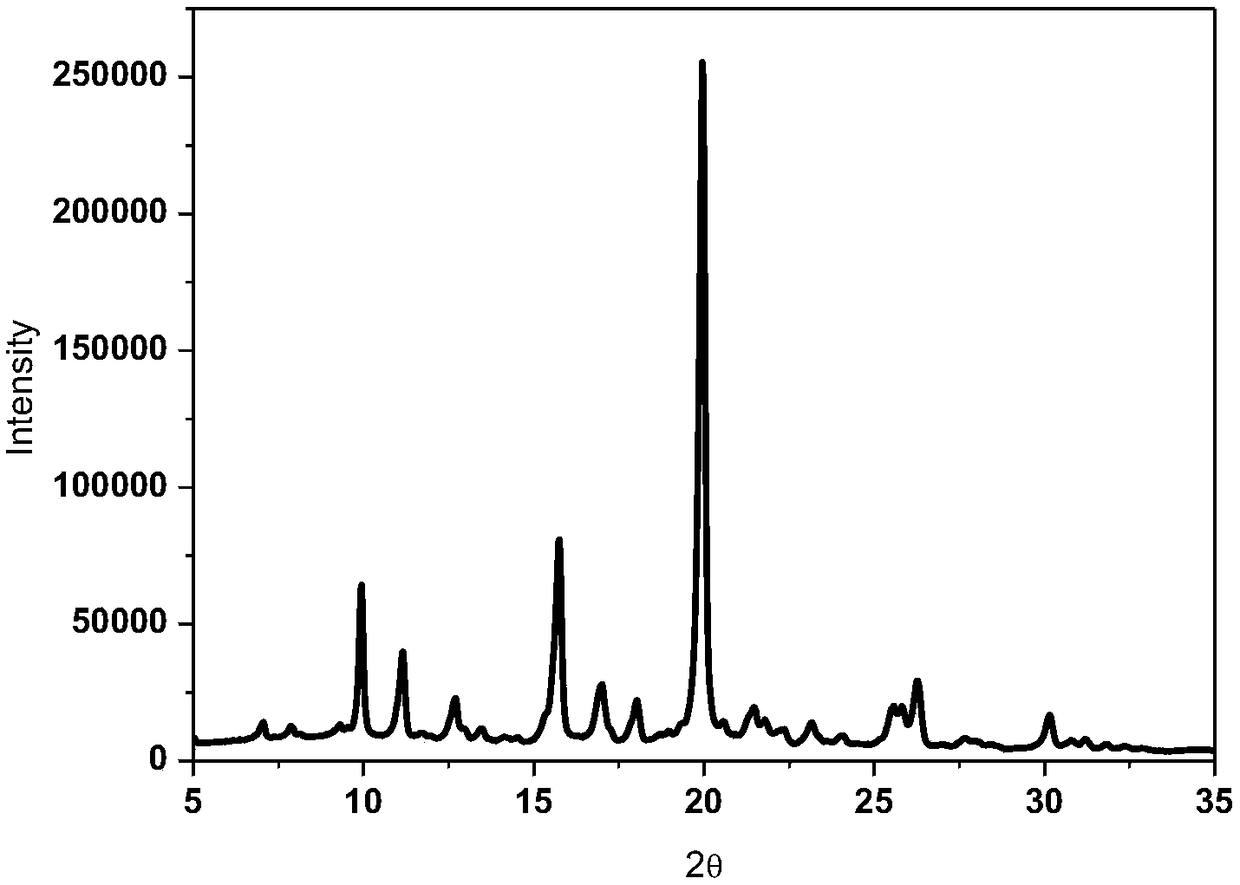
[0025] Test instrument conditions: Bruker D2 PHASER powder diffractometer is used for normal temperature test, the test conditions are: Cu Ka It is the light source, the voltage is 30kV, the current is 10mA, the test step is 0.014°, the scanning speed is 0.1s / step, and the scanning range is 5-40° (2θ). After testing, the X-ray powder diffraction pattern of the rifampicin crystal product prepared in Example 1 of the present invention is as follows: figure 1 As shown, the diffraction angle 2θ is at 5.0°, 7.1°, 7.9°, 9.3°, 9.9°, 11.2°, 12.7°, 12.9°, 13.5°, 14.1°, 15.7°, 17.0°, 18.0°, 19.0°, 20.0 °, 21.1°, 21.4°...
Embodiment 2
[0028] At a temperature of 35°C and a stirring speed of 45r / min, add 250g of crude rifampicin into 1250mL of acetone in two batches within 30min, and stir at this temperature for 2h. Dry under reduced pressure (vacuum degree: 0.06MPa) for 1 hour to obtain 225.5 g of rifampicin crystal product with a yield of 90.2%. The product purity is 98.0%.
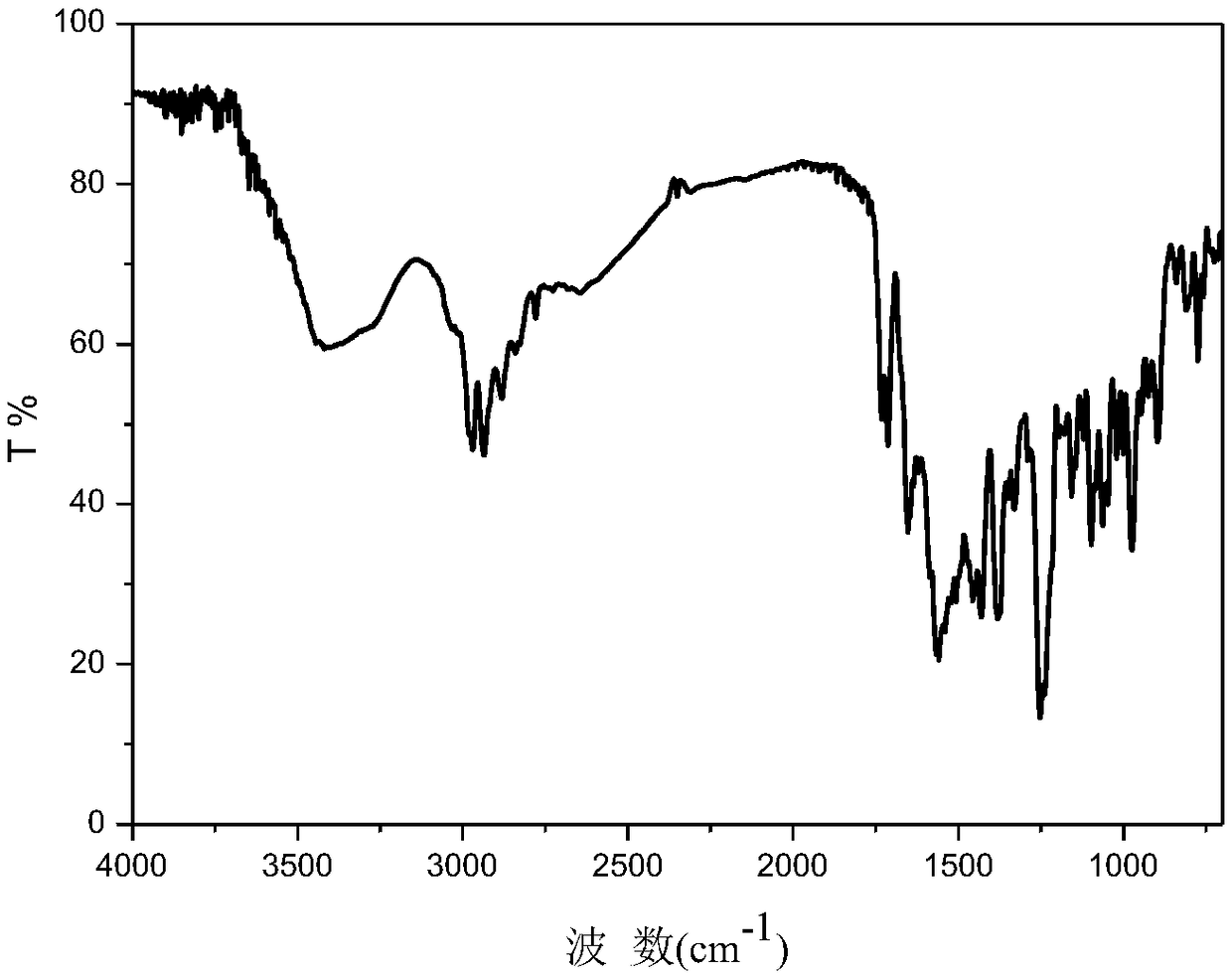
[0029] The rifampicin crystal prepared in Example 2 was tested with reference to the test method of Example 1, and the results showed that the diffraction angle 2θ was 5.0 ± 0.1 °, 7.0 ± 0.1 °, 7.9 ± 0.1 °, 9.3 ± 0.1 °, 9.9 ± 0.1 °, 11.1±0.1°, 12.6±0.1°, 12.8±0.1°, 13.4±0.1°, 14.1±1°, 15.7±0.1°, 17.0±0.1°, 18.0±0.1°, 18.9±0.1°, 19.9±0.1°, There are characteristic diffraction peaks at 21.0±0.1°, 21.4±0.1°, 22.2±0.1°, 23.1±0.1°, 24.0±0.1°, 25.7±0.1°, 26.2±0.1°, 27.6±0.1°, 30.1±0.1°. The infrared wavelength of the product is 773, 810, 840, 895, 974, 1000, 1023, 1047, 1096, 1118, 1158, 1255, 1375, 1427, 1559, 1643, 1714, 1731, 2883, 2936...
Embodiment 3
[0031] At a temperature of 32°C and a stirring speed of 65r / min, add 190g of crude rifampicin into 2850mL of acetone in two batches within 30min, and stir at this temperature for 1.5h. Dry under reduced pressure (vacuum degree is 0.1MPa) for 2 hours to obtain 173.66 g of rifampicin crystal product with a yield of 91.4%. The product purity is 98.3%.
[0032] The rifampin crystal prepared in Example 3 was tested with reference to the test method of Example 1, and the results showed that the diffraction angle 2θ was 5.0 ± 0.1 °, 7.0 ± 0.1 °, 7.9 ± 0.1 °, 9.3 ± 0.1 °, 9.9 ± 0.1 °, 11.1±0.1°, 12.6±0.1°, 12.8±0.1°, 13.4±0.1°, 14.1±1°, 15.7±0.1°, 17.0±0.1°, 18.0±0.1°, 18.9±0.1°, 19.9±0.1°, There are characteristic diffraction peaks at 21.0±0.1°, 21.4±0.1°, 22.2±0.1°, 23.1±0.1°, 24.0±0.1°, 25.7±0.1°, 26.2±0.1°, 27.6±0.1°, 30.1±0.1°. The infrared wavelength of the product is 774, 807, 841, 896, 973, 998, 1021, 1051, 1096, 1122, 1156, 1254, 1376, 1428, 1560, 1643, 1716, 1730, 2883, 29...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com