Crystals of anilinopyrimidine compound with indole substituted by trifluoroethyl group and salts thereof
A compound and crystallization technology, applied in the field of medicinal chemistry, can solve problems such as the reduction of the binding rate between TKI and kinase domain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
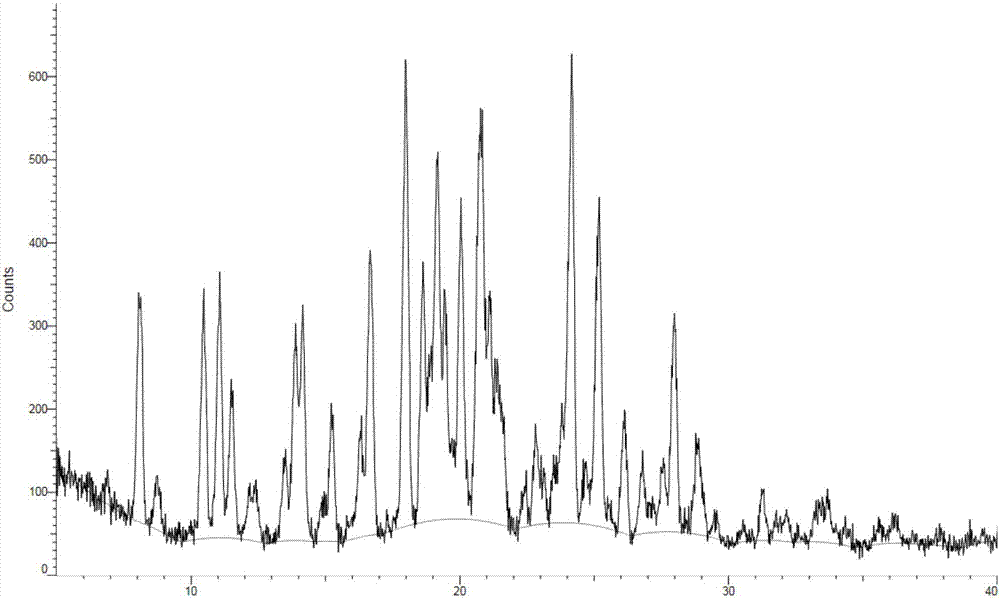
[0148] Compound shown in embodiment 1 amorphous formula I
[0149]
[0150] The compound shown in Formula 2 (18.9g, 0.037mol), DIEA (9.6g, 0.074mol) and DCM (400mL) were added to a 1L three-necked flask, stirred and dissolved by magnetic force, the air in the system was replaced with nitrogen, and the temperature of the reaction solution was cooled to -20 At ~-10°C, add acryloyl chloride (3.4g, 0.037mol) dropwise. After the dropwise addition, react at -20~-10°C for 20min. After the reaction was completed, water (300 mL) was added to the reaction system for liquid separation, and the organic phase was dried with saturated brine (200 mL*2), anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated to obtain a residue purified by column chromatography (DCM / MeOH =50 / 1~20 / 1) to obtain the compound represented by compound formula I (10.0 g, 47.6%).
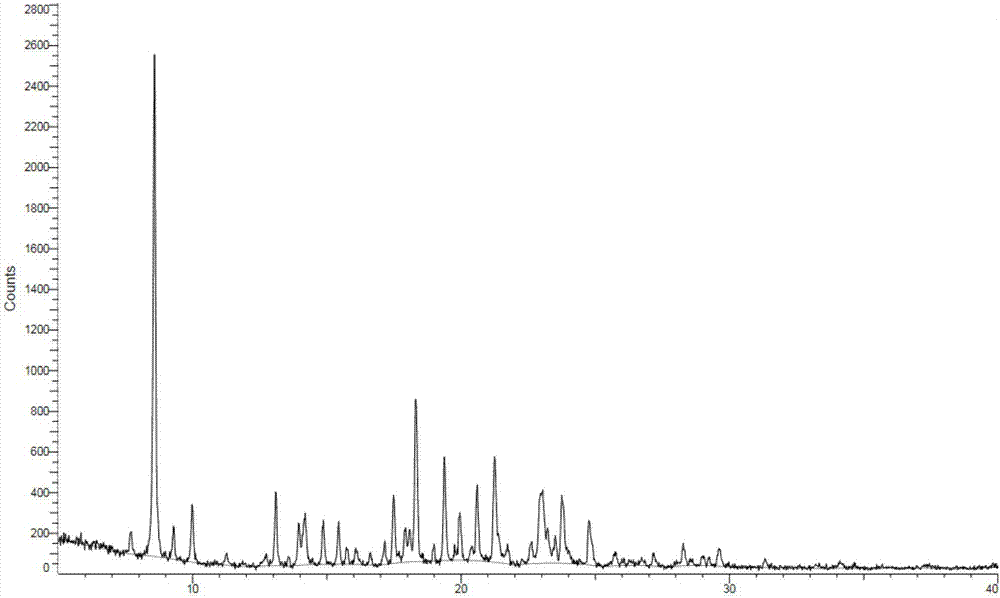
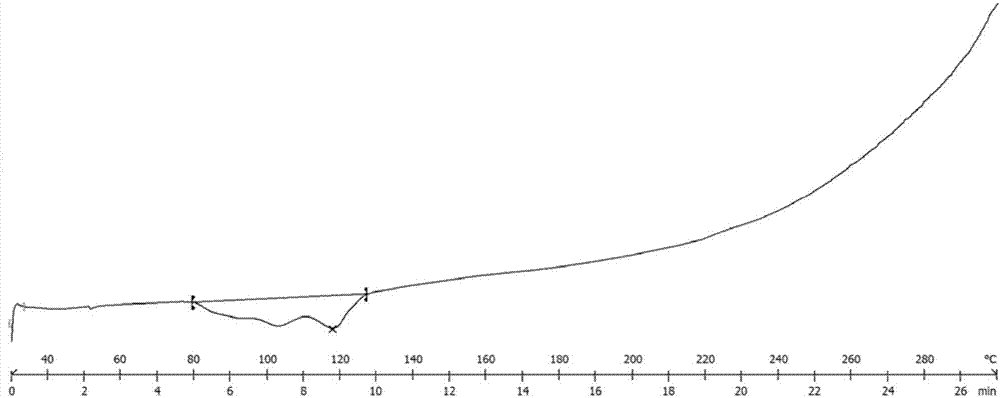
[0151] Compound crystal A shown in embodiment 2 formula I
[0152] Add the product obtained in Example 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com