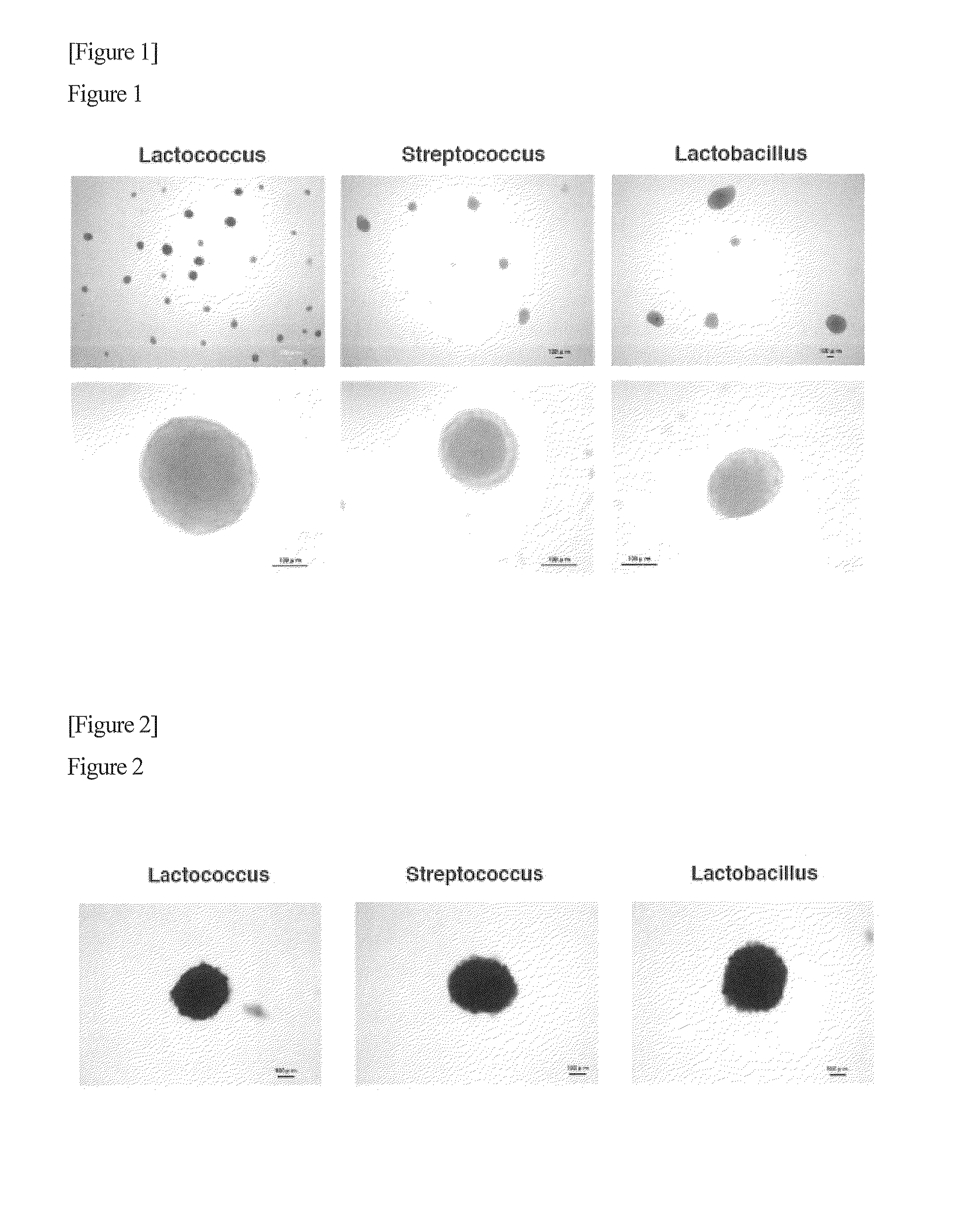
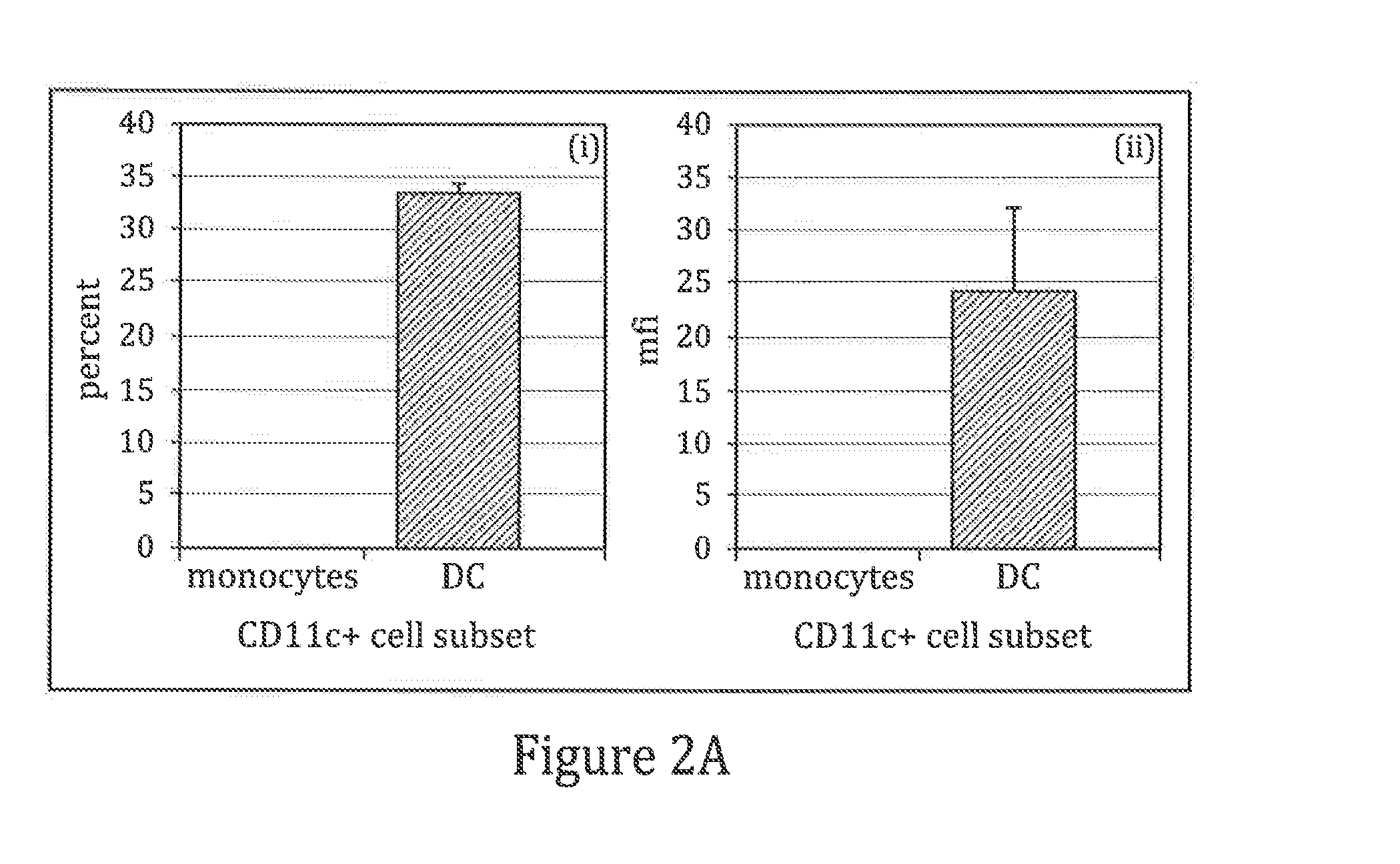
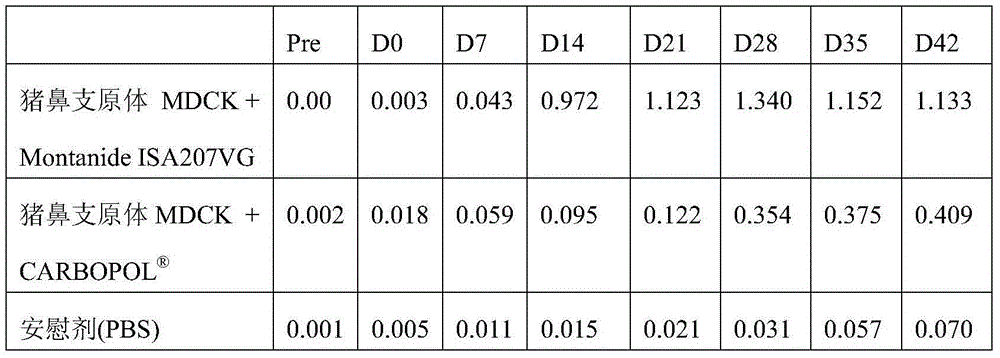
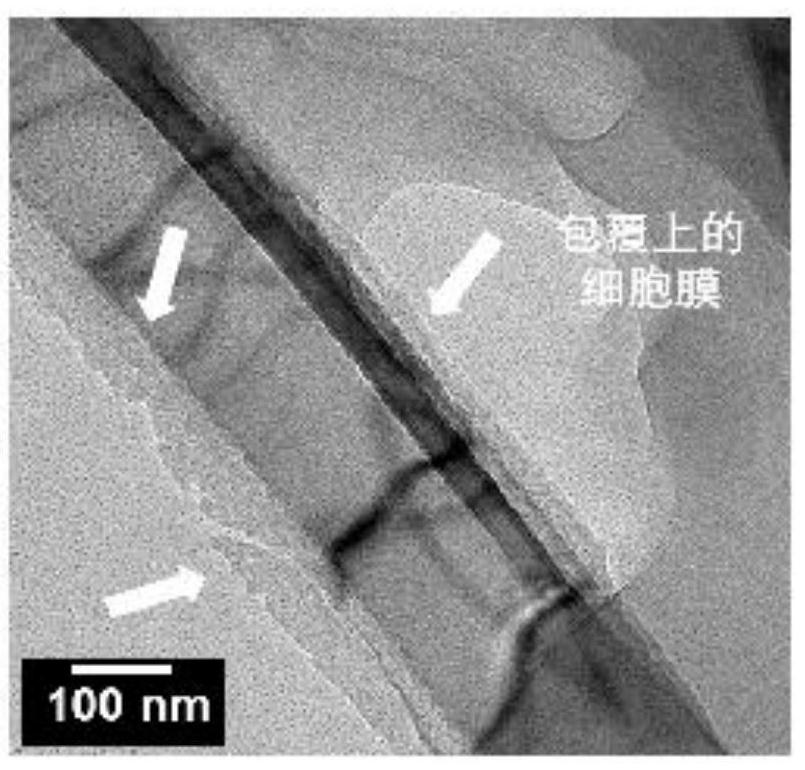
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44results about "Non-animal cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
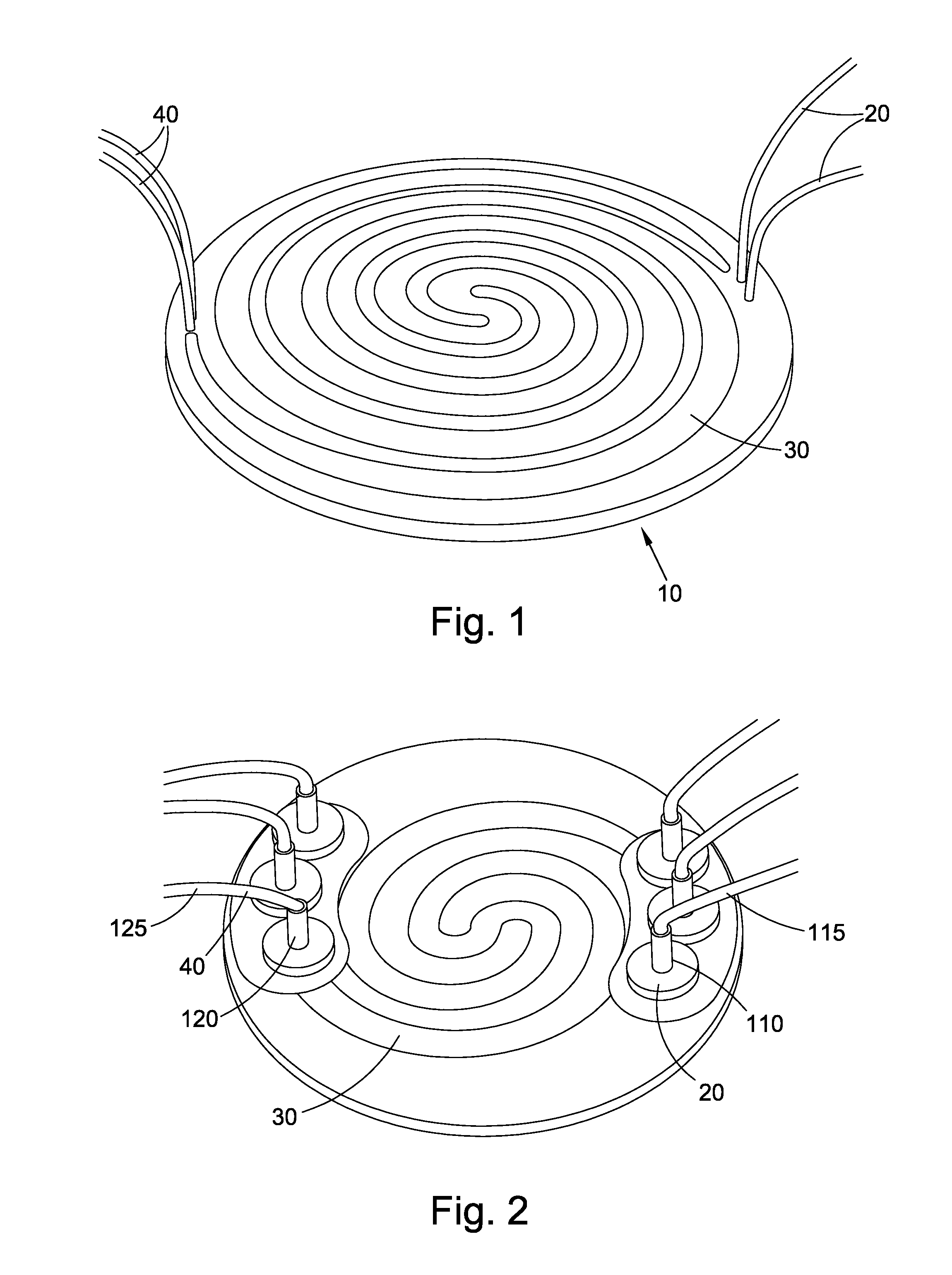
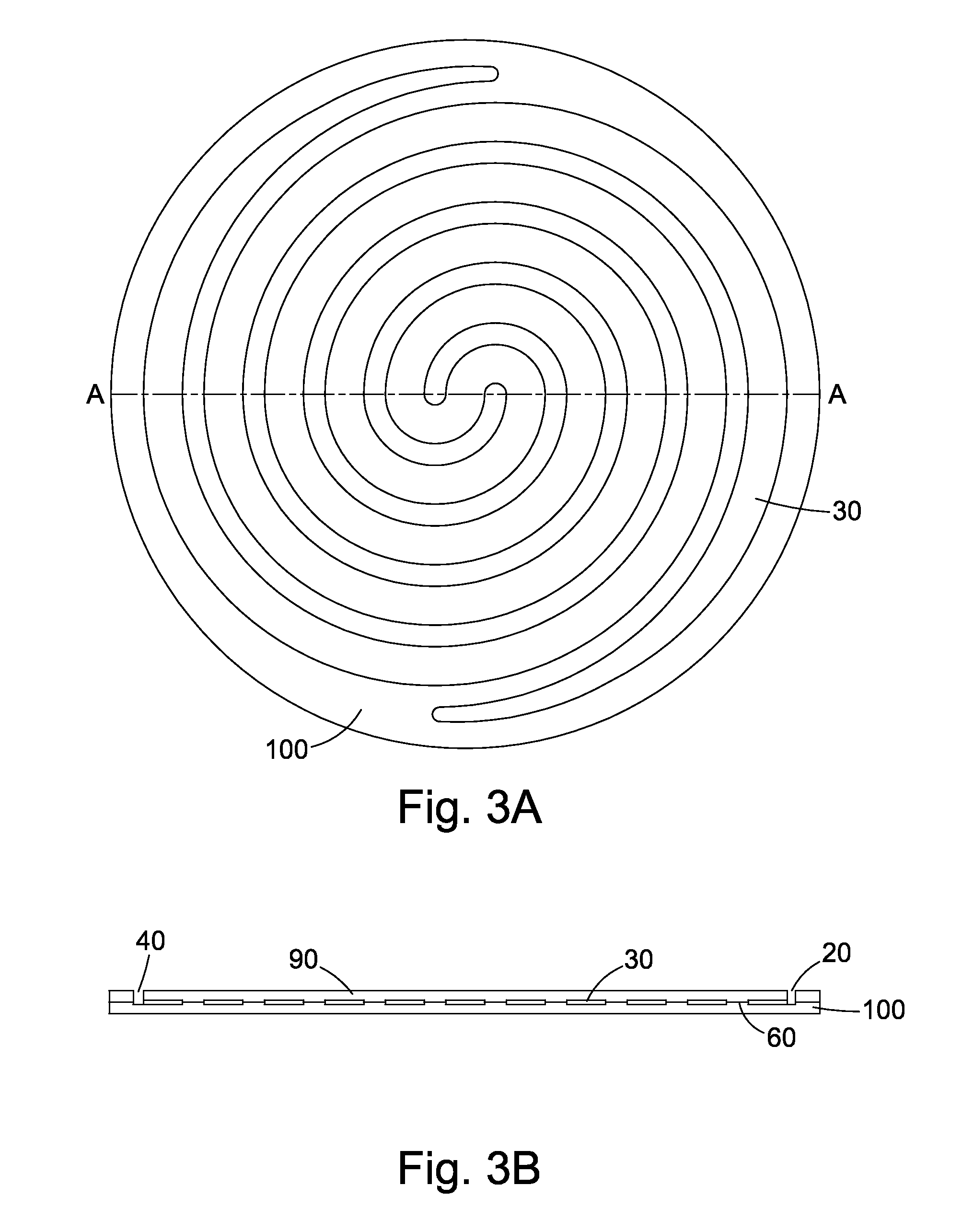
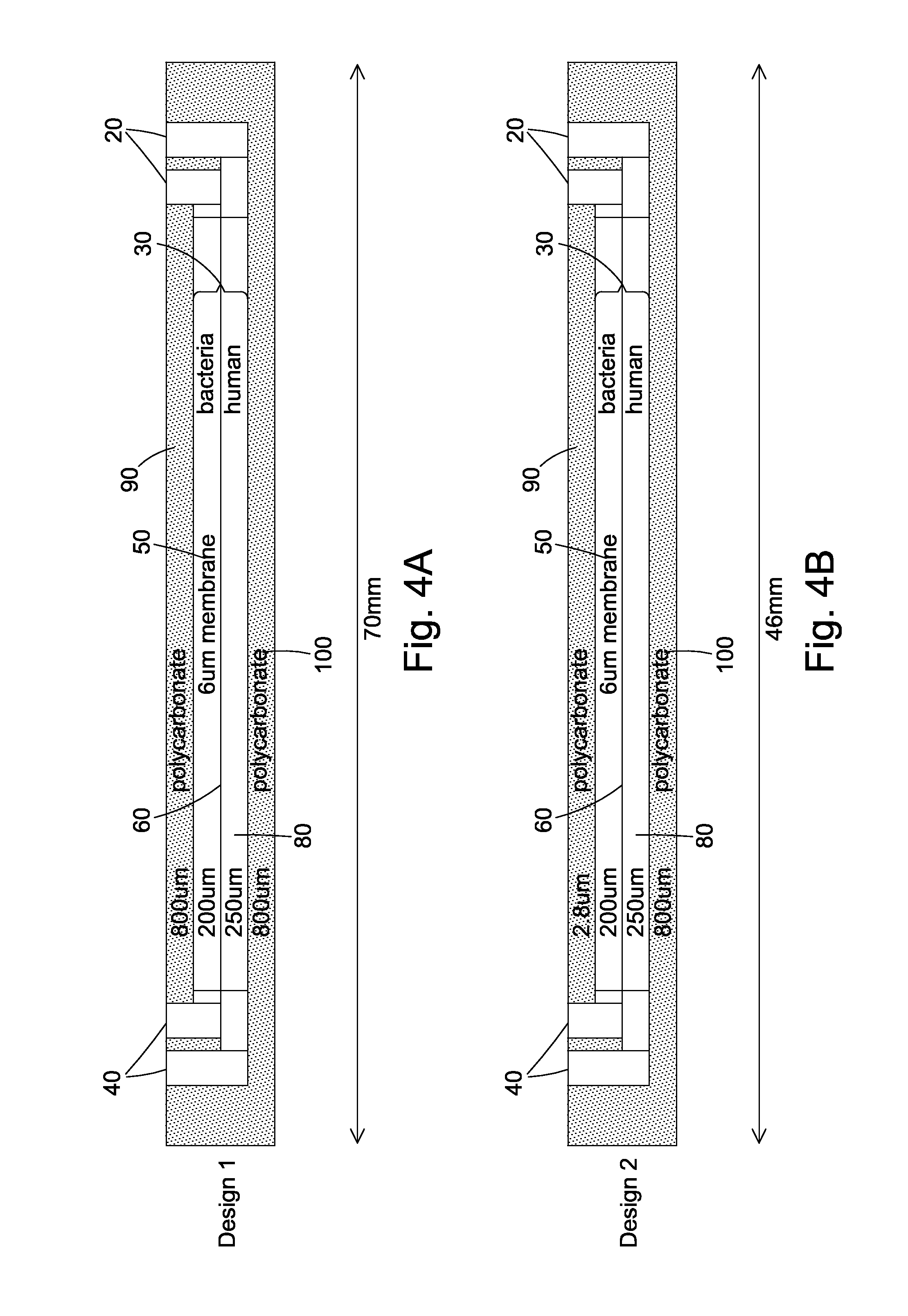
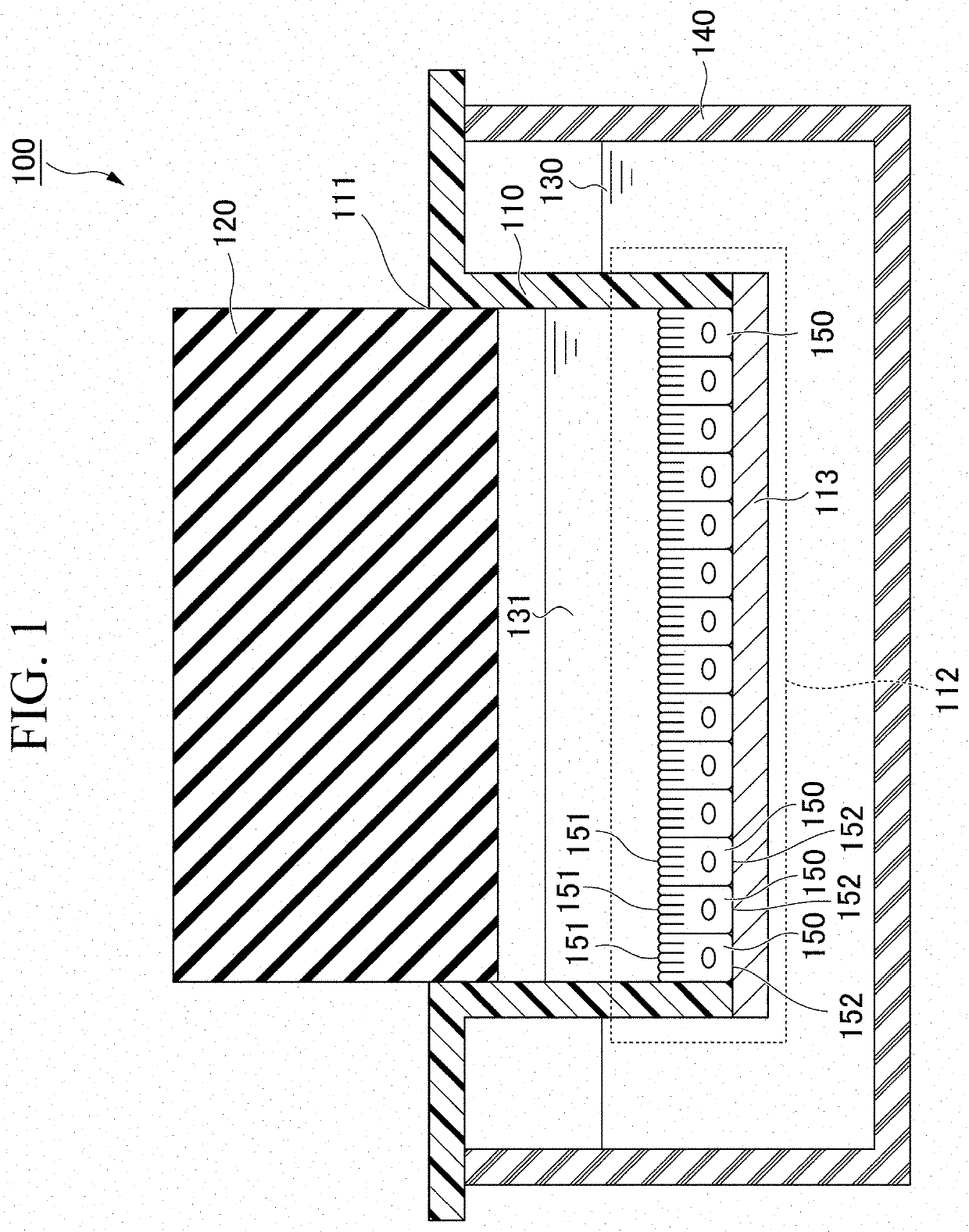
Cell culture apparatus and culture methods using same
InactiveUS20150072413A1Improve surface adhesionEffective medium supplyBioreactor/fermenter combinationsBiological substance pretreatmentsMicrobial fuel cellSemipermeable membrane
Cell culture apparatus comprising at least two adjacent cell cultivation channels separated by a permeable or semipermeable membrane, wherein at least one channel, for the majority of its length, has a cross sectional area of no more than 1 mm2, said channel being provided with entrance and exit means to permit the passage of media therethrough, allows co-culture of separate cell types, e.g. human and microbial cells, without mingling, allowing monitoring of cell cultures and chemical exchanges between the respective cell cultures.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +2
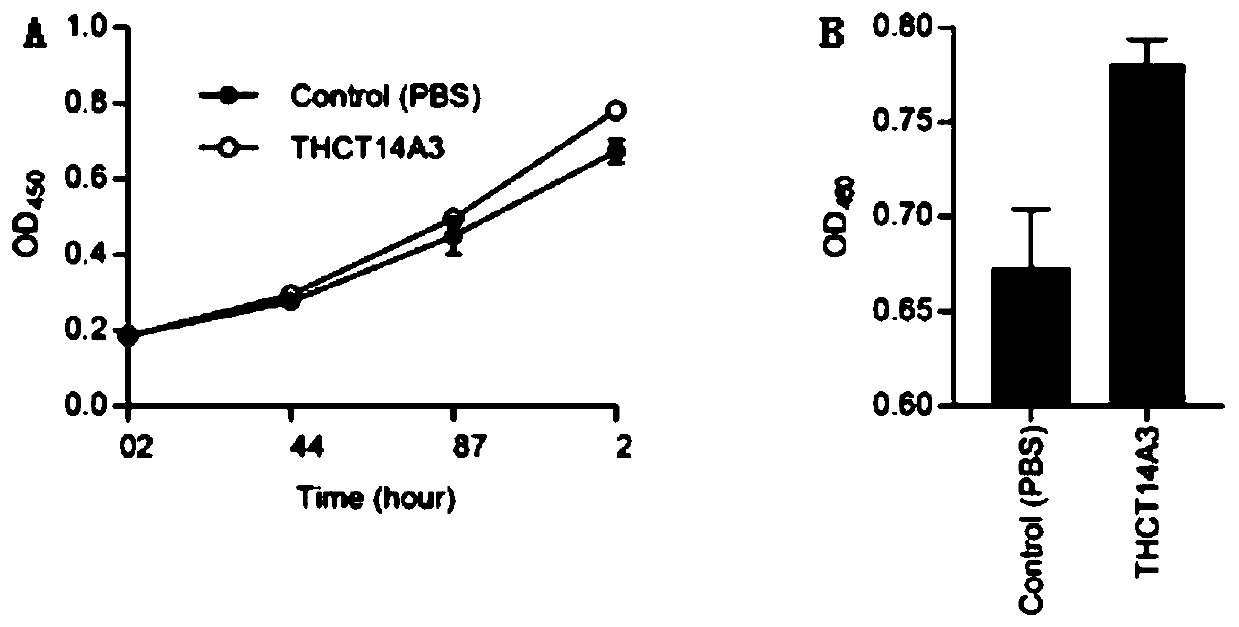
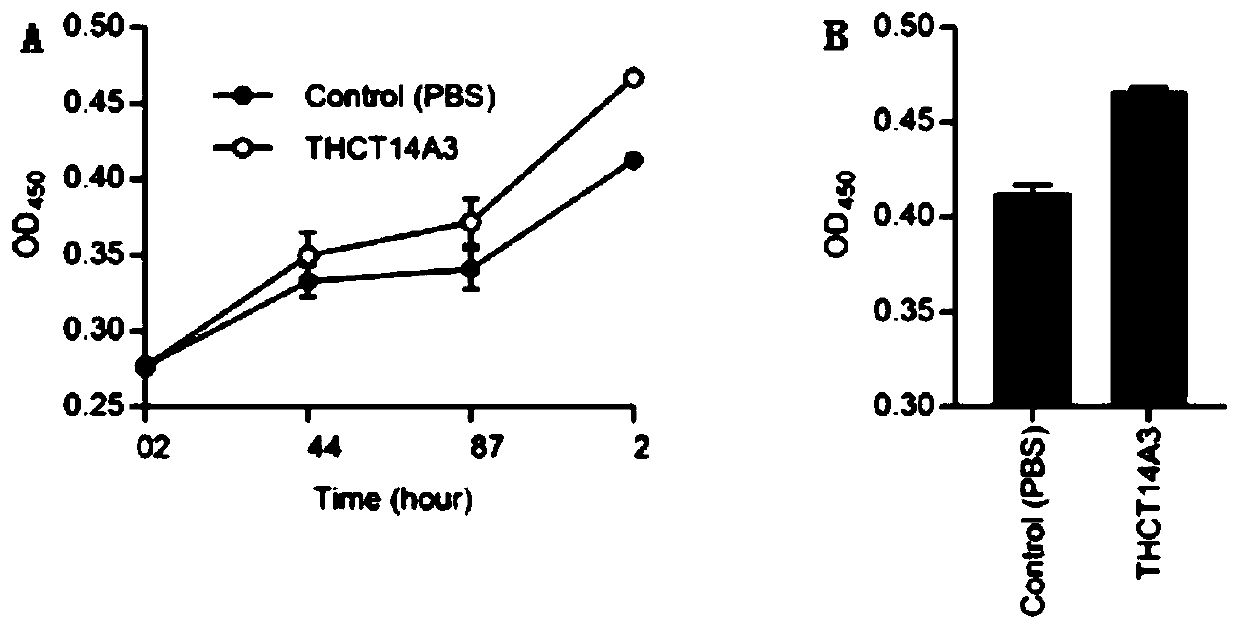
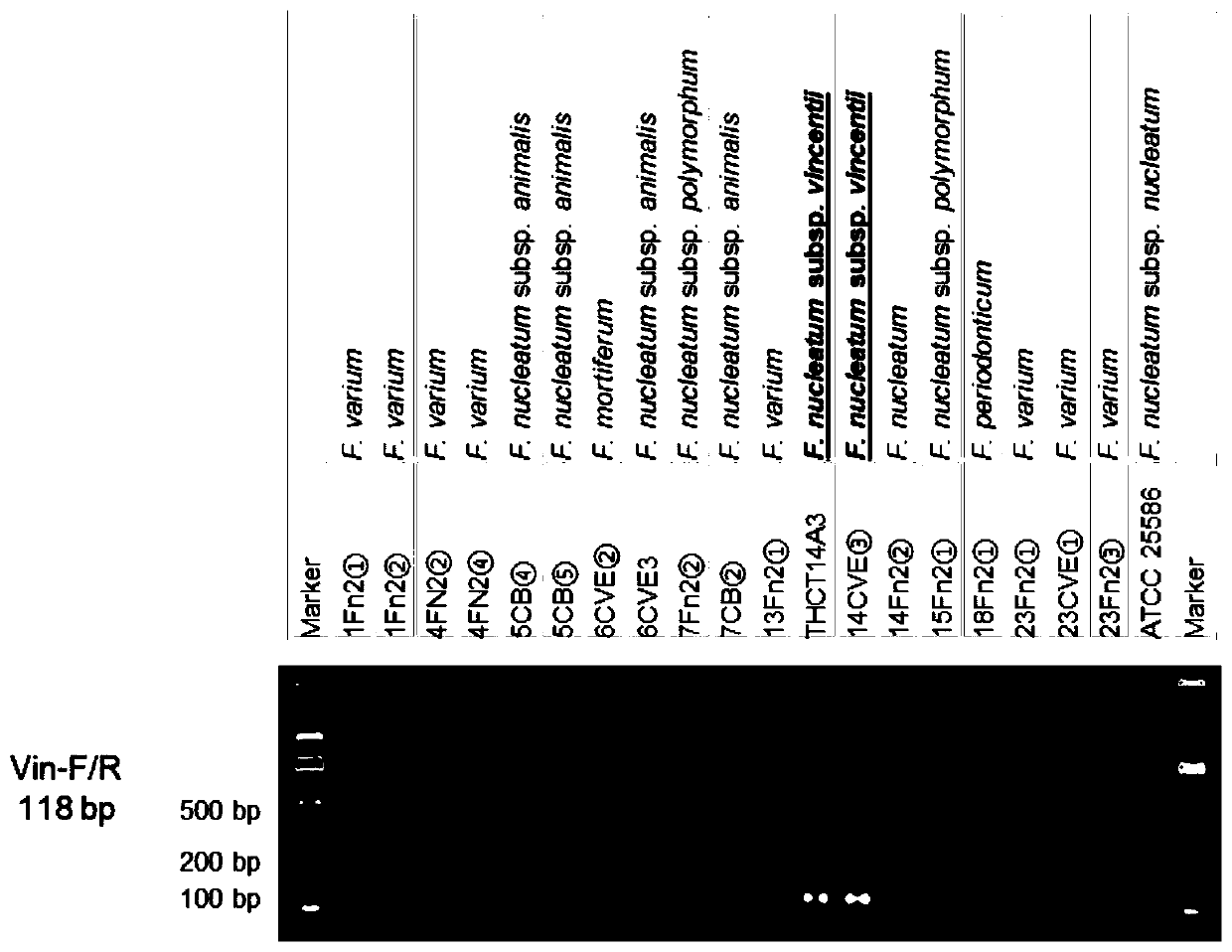
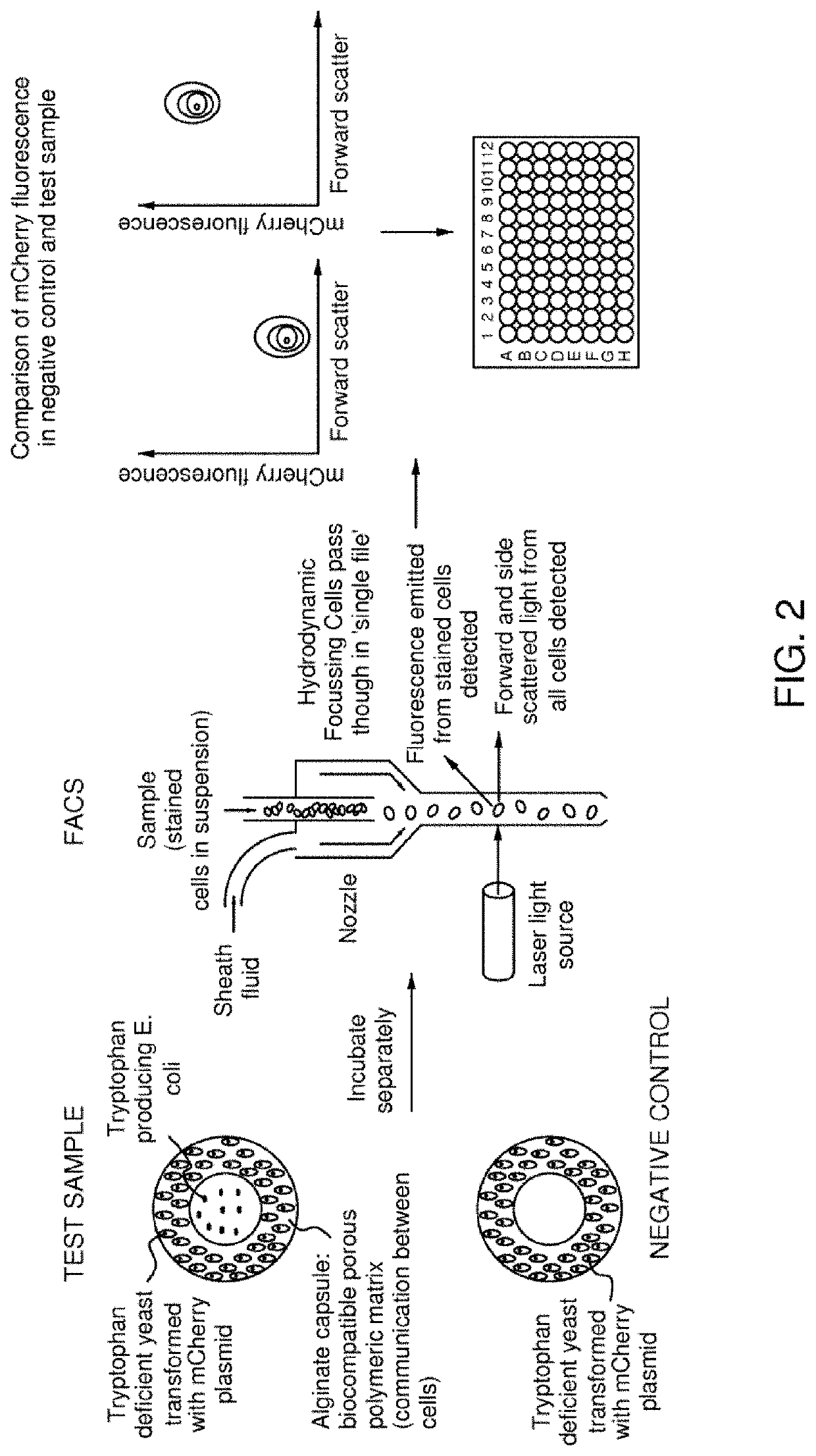
Fusobacterium nucleatum subsp.vincentii strain for intestinal tract separation and application of fusobacterium nucleatum subsp.vincentii strain for intestinal tract separation
The invention provides a fusobacterium nucleatum subsp.vincentii strain THCT14A3 for intestinal tract separation. The classification designation is Fusobacterium nucleatum subsp.vincentii, the preservation number is CCTCC NO:M 2019363, the preservation date is May 17th, 2019, and the preservation unit is China Center for Type Culture Collection. The 16SrRNA gene sequence of the strain is as shownin SEQID NO:1. The invention further provides a specific DNA sequence for identifying the fusobacterium nucleatum subsp.vincentii and a primer sequence corresponding to the specific DNA sequences. Thecolorectal cancer cell line and THCT14A3 co-culture result show that the THCT14A3 can significantly promote colorectal cancer cell proliferation. Therefore, the THCT14A3 provided by the invention canfacilitate micro-ecology research of intestinal tracts suffering from colorectal cancer.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
Generation of dendritic cells from monocytic dendritic precursor cells with GM-CSF in the absence of additional cytokines
ActiveUS8389278B2Minimizing activationInhibition of activationCulture processArtificial cell constructsDiseaseDendritic cell
The present invention it was determined that dendritic cells could be derived from various sources including peripheral blood monocytes in the presence of only GM-CSF without other cytokines if the monocytes were not activated. By preventing activation, such as by preventing binding of the cells to the surface of the culture vessel, the monocytes do not require the presence of additional cytokines, such as IL-4 or IL-13, to prevent differentiation into a non-dendritic cell lineage. The immature DCs generated and maintained in this manner were CD14− and expressed high levels of CD1a. Upon maturation by contact with an agent such as, for example, BCG and IFNγ, the cells were determined to express surface molecules typical of mature dendritic cells purified by prior methods and cultured in the presence of GM-CSF and IL-4. The mature dendritic cells produced from monocytes without activation and cultured in GM-CSF alone are suitable for use in dendritic cell-based immunotherapy methods, such as for use in the treatment of disease, including cancer.
Owner:NORTHWEST BIOTHERAPEUTICS INC
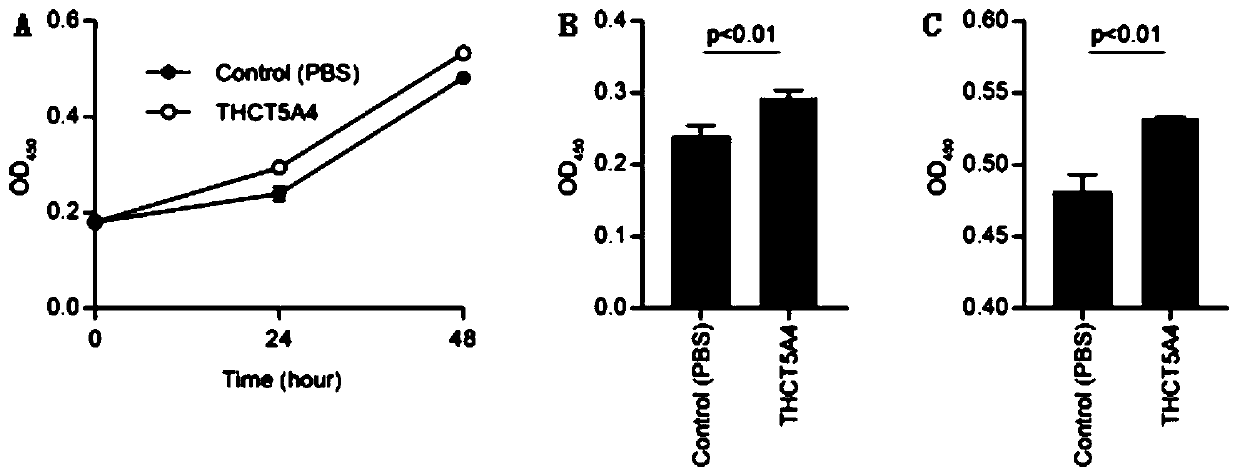
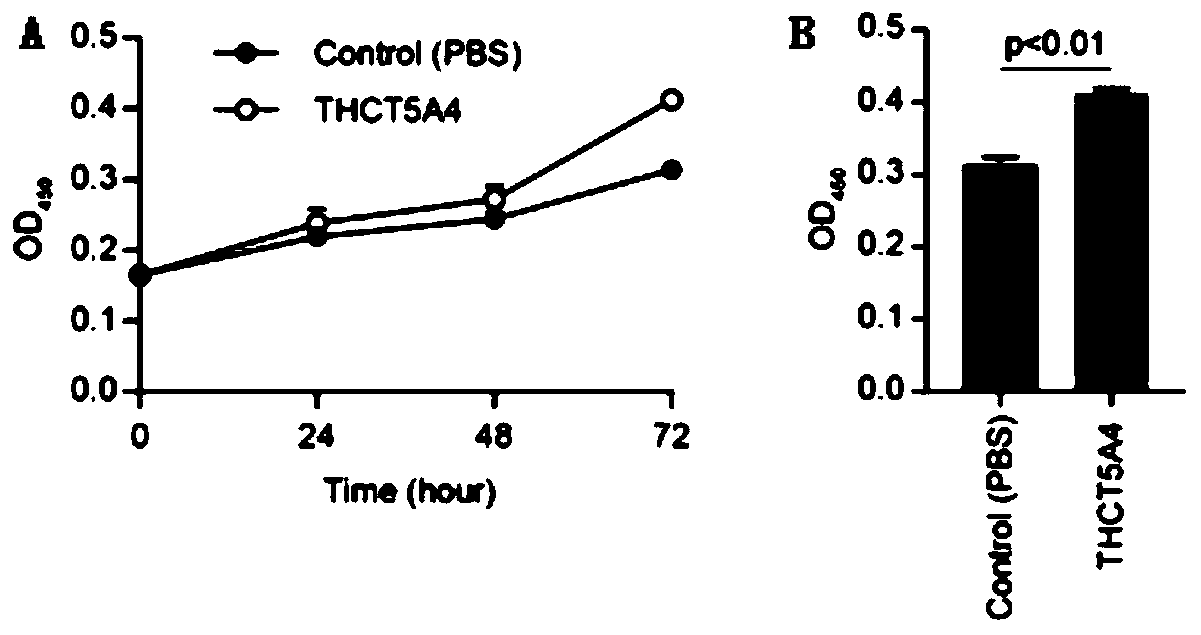
Fusobacterium nucleatum subsp.animalis strain and application thereof
ActiveCN111205994APromote proliferationCompound screeningApoptosis detectionDiseaseColorectal cancer cell line
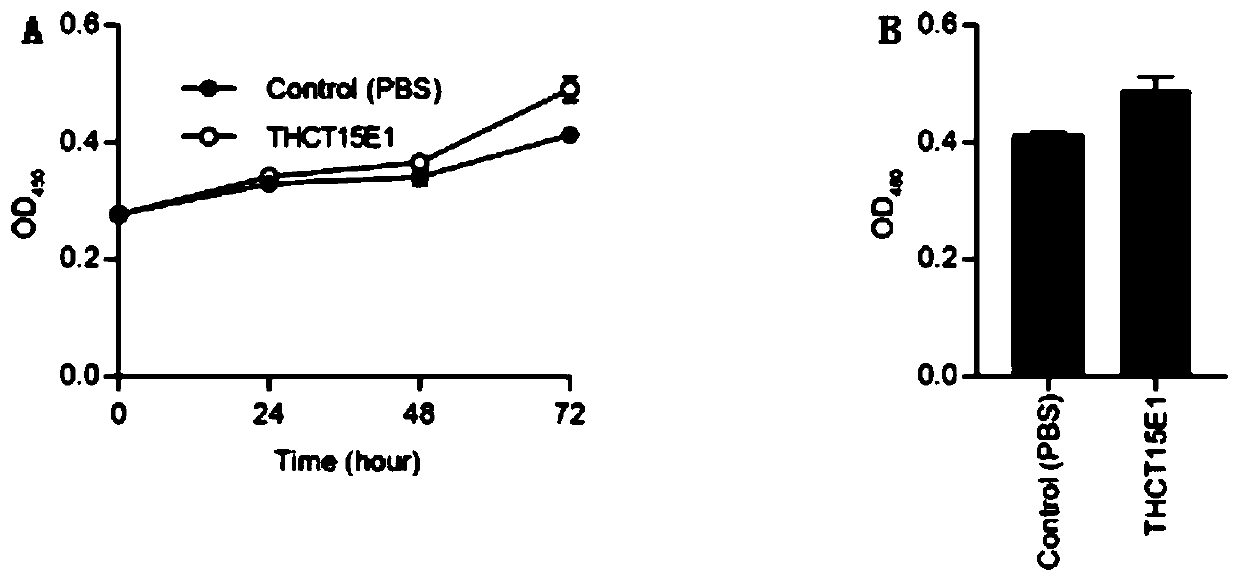
The invention provides a fusobacterium nucleatum subsp.animalis strain THCT5A4 separated from human rectal cancer tumor tissue. A classification name is Fusobacterium nucleatum subsp.animalis THCT5A4,a preservation number of the fusobacterium nucleatum subsp.animalis strain THCT5A4 separated from the human rectal cancer tumor tissue is CCTCC NO: M 2019366, the preservation date is May 17th, 2019,the preservation organization is China General Microbiological Culture Collection Center, and a 16SrRNA gene sequence of the fusobacterium nucleatum subsp.animalis strain THCT5A4 separated from the human rectal cancer tumor tissue is shown in SEQ ID NO:1 as shown in the description. A separation method of the fusobacterium nucleatum subsp.animalis strain is provided. A result of co-culture of a colorectal cancer cell line and the THCT5A4 shows that the THCT5A4 can significantly promote proliferation of colorectal cancer cells, therefore, the THCT5A4 can provide diverse in-vitro or in-vivo experimental conditions for simulating an intestinal environment for studies of colorectal cancer, and a colorectal cancer disease model can be built for screening medicines for treating colorectal cancer.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
Fusobacterium nucleatum polymorphic subspecies isolate and application thereof
ActiveCN111004738APromote proliferationCompound screeningApoptosis detectionDiseasePharmaceutical drug
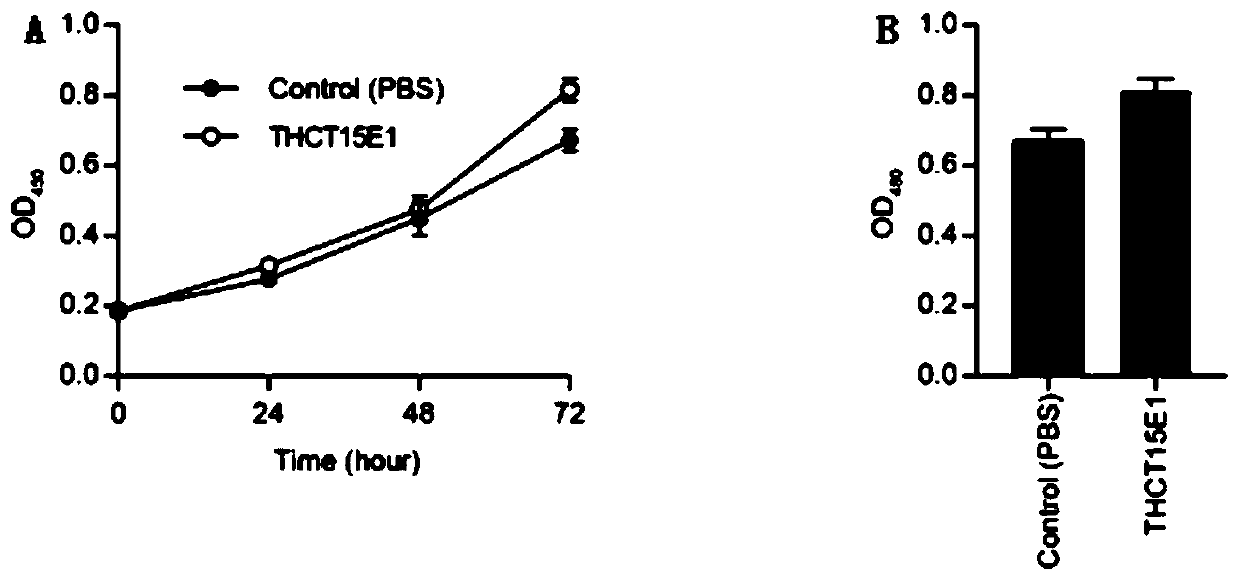
The invention provides a fusobacterium nucleatum polymorphic subspecies isolate THCT15E1, a classification name is Fusobacterium nuucleates subsp.nucleatum THCT15E1, a preservation number of the strain is CCTCC NO: M 2019362, a preservation date is May 17, 2019, a preservation unit is China Center for Type Culture Collection, and the 16SrRNA gene sequence of the strain is shown as SEQ ID NO: 1. The result of co-culture of the colorectal cancer cell line and THCT15E1 shows that THCT15E1 can significantly promote colorectal cancer cell proliferation; therefore, the THCT15E1 can provide diversified experimental conditions for in-vitro or in-vivo intestinal environment simulation for colorectal cancer research, and can also construct a colorectal cancer disease model to screen drugs for treating colorectal cancer.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
Soybean bHLH transcription factor gene GmFER and encoded protein and application thereof
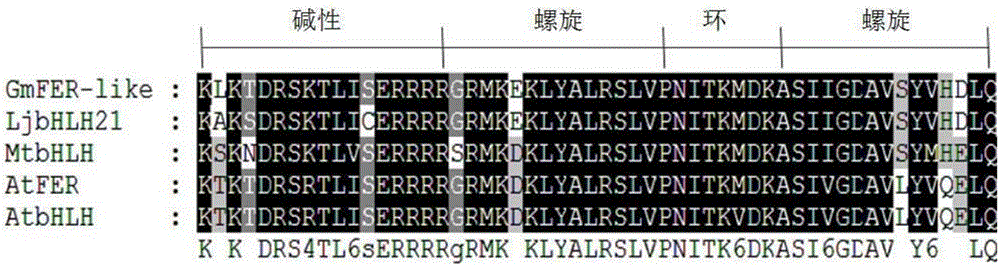
InactiveCN106399326AImprove toleranceHigh expressionGenetically modified cellsNon-animal cellsYeastBiotechnology
The invention discloses a soybean bHLH transcription factor gene GmFER and encoded protein and application thereof. The nucleotide sequence of the gene GmFER is as shown in SEQ ID No.1, and the amino acid sequence of the protein encoded by the gene is as shown in SEQ ID No. 2. A semi-quantitative detecting result shows that the gene GmFER is mainly expressed in soybean roots; the expression quantity of the gene GmFER is increased evidently after Cd stress, that is to say, the gene GmFER is induced by Cd; by overexpressing the gene GmFER in yeast and plants, the Cd tolerance of the yeast and plants can be increased, and the gene GmFER is high in application value.
Owner:JIANGSU ACAD OF AGRI SCI
Microorganism co-culture system and uses of the same
InactiveUS20160068919A1Reducing unnecessary carbon lossHigh yieldBacteriaBiofuelsMicroorganismMetabolite
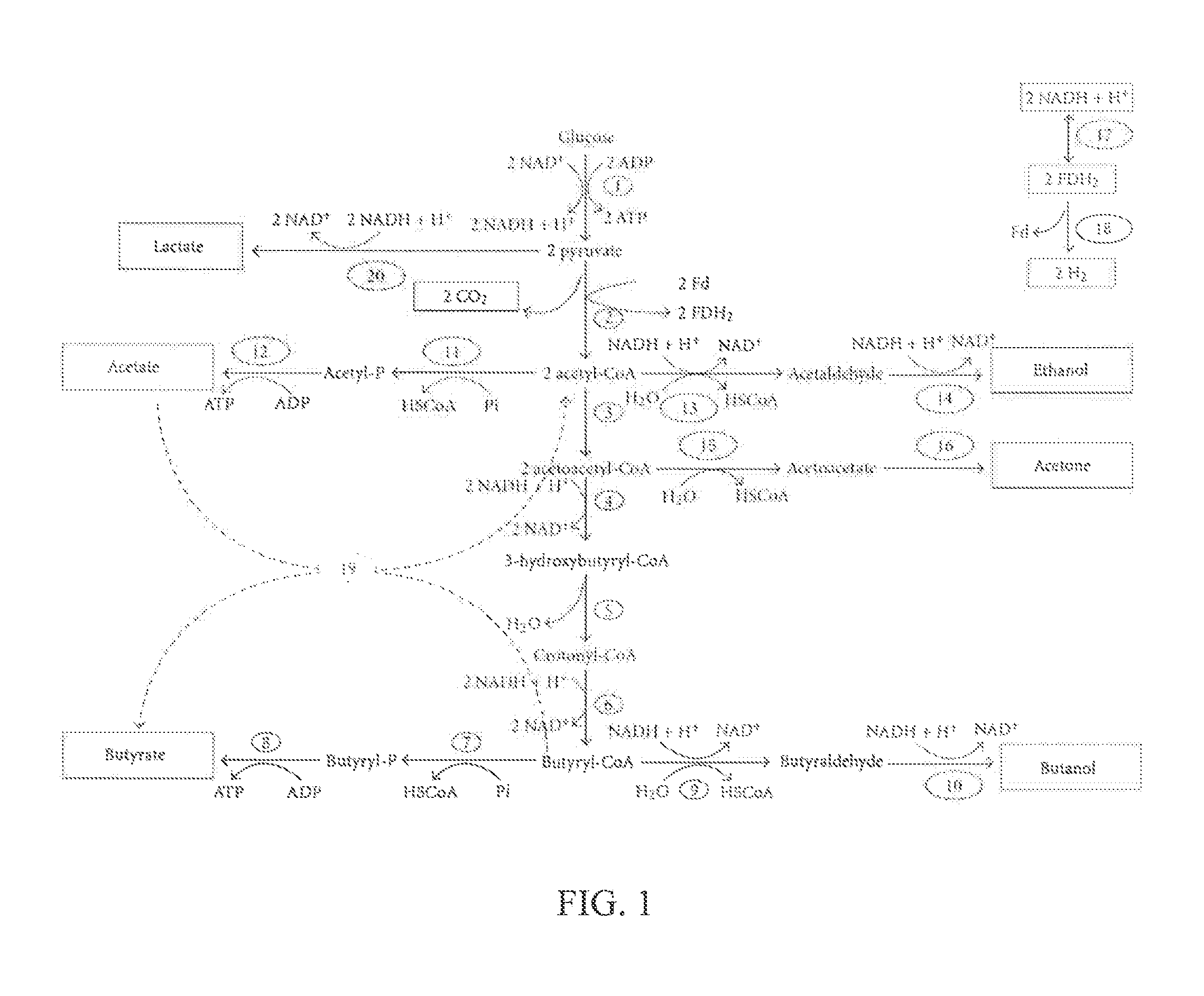
A microorganism co-culture system, comprising:(1) a substrate, comprising a saccharide(2) at least one of a first strain and a second strain, wherein the first strain is able to fix a carbon oxide the second strain is able to fermentatively metabolize an amino acid, and wherein the first strain produces a first metabolite in the fermentation, and the second strain produces a second metabolite in the fermentation; and(3) a third strain, being able to metabolize the saccharide, the first metabolite and the second metabolite in the fermentation to produce butyric acid and / or butanol,wherein, when the second strain is present in the co-culture system, the substrate further comprises an amino acid.
Owner:GREEN CELLULOSITY
Composition for promoting production of hyaluronic acid
PendingCN111163788AProliferation effect is goodGenerate good effectCosmetic preparationsBacteriaHyaluronan synthetaseFibroblast
The purpose of the present invention is to provide a composition for promoting the proliferation of a fibroblast and / or a composition for promoting the expression of a hyaluronic acid synthase gene. The present invention provides a composition containing Lactobacillus plantarum L-137 and used for promoting the proliferation of the fibroblast and / or the composition for promoting the expression of the hyaluronic acid synthase gene.
Owner:เฮ้าส์ เวลล์เนสส์ ฟูดส์ คอร์ปอเรชั่น
Compositions and methods for long term culture of hepatocytes
PendingCN113423818AOrganic active ingredientsMicrobiological testing/measurementCyclaseCell culture media
Provided are compositions for long-term maintenance of functional hepatocytes in culture, a method for improved maintenance of functional hepatocytes in vitro, and functional hepatocytes cultures according to the methods. The culture compositions include at least: one activator of adenylate cyclase, one TGFbeta inhibitor, one Notch inhibitor, one Wnt inhibitor, and / or one BMP inhibitor. The combinations of compounds are added to any hepatocyte cell culture medium in an effective amount to maintain functional hepatocyte function in vitro, long term. The hepatocytes can be used for in vitro drug research and to model liver disease.
Owner:PEKING UNIV
Method for producing pluripotent cell using bacterium having fermentation ability
It is an object of the present invention to provide a method for producing pluripotent cells that are free of the risk of cellular canceration and that can be applied to regenerative medicine with a high degree of safety. The present invention provides a method for producing pluripotent cells from somatic cells comprising a step of bringing bacteria having fermentation ability or a component or secretory product thereof into contact with somatic cells.
Owner:INTEGRICULTURE INC
Generation of Dendritic Cells from Monocytic Dendritic Precursor Cells with GM-CSF in the Absence of Additional Cytokines
ActiveUS20130273654A1Minimizing activationInhibition of activationCulture processNon-animal cellsDiseaseDendritic cell
The present invention it was determined that dendritic cells could be derived from various sources including peripheral blood monocytes in the presence of only GM-CSF without other cytokines if the monocytes were not activated. By preventing activation, such as by preventing binding of the cells to the surface of the culture vessel, the monocytes do not require the presence of additional cytokines, such as IL-4 or IL-13, to prevent differentiation into a non-dendritic cell lineage. The immature DCs generated and maintained in this manner were CD14− and expressed high levels of CD1a. Upon maturation by contact with an agent such as, for example, BCG and IFNγ, the cells were determined to express surface molecules typical of mature dendritic cells purified by prior methods and cultured in the presence of GM-CSF and IL-4. The mature dendritic cells produced from monocytes without activation and cultured in GM-CSF alone are suitable for use in dendritic cell-based immunotherapy methods, such as for use in the treatment of disease, including cancer.
Owner:NORTHWEST BIOTHERAPEUTICS INC
Method for greatly increasing PCV2 cultivation virus titer through streptococcus culture preparation
ActiveCN106520626ARaise culture valenceGood effectBacteriaMicroorganism based processesVirus CultivationStreptococcus agalactiae
The invention discloses a method for greatly increasing the PCV2 cultivation virus titer through a streptococcus culture preparation, and belongs to the technical field of cell engineering and virus cultivation. The streptococcus culture preparation is prepared through high-temperature inactivation of streptococcus-containing supernate which is obtained after co-culture of streptococcus and anchorage-dependent cells in a maintenance culture medium. The streptococcus is streptococcus agalactiae, the cells are PK-15 cells, and the content of the streptococcus in the streptococcus-containing supernate is 100-900 million / mL. The streptococcus culture preparation is added into the maintenance culture medium according to the concentration of 0.1-1% to prepare a special culture medium used for stimulating PCV2 to generate viruses, the cultivation virus titer of the culture medium can be greatly increased by adopting the special culture medium as the maintenance culture medium for culturing PCV2, and the virus titer can reach 10<-7> or above; and a production process is greatly simplified, and the production cost is greatly lowered.
Owner:WUHAN ENG SCI & TECH RESINST +1
A method of co-culturing helicobacter pylori and gastric mucosa epithelial cells
InactiveCN106947732AGuaranteed normal growthAvoid cumbersomeBacteriaGastrointestinal cellsLesionCo culturing
A method of co-culturing helicobacter pylori and gastric mucosa epithelial cells is disclosed. The method includes 1) preparing a helicobacter pylori medium; 2) adding the medium into a sterile cell culture flask and preparing a slant surface; 3) culturing gastric mucosa epithelial cells in the culture flask in which the slant surface is formed and allowing the cells to be adhered to the wall of the flask; 4) centrifuging a helicobacter pylori 48-h culture liquid, and then resuspending a centrifuging product with a cell culture liquid; and 5) adding a resuspended bacterial liquid into the culture flask in which the slant surface and the adherent cells are formed, culturing, and counting the living bacteria and observing cell morphological changes every day. A beneficial effect of the method is that the novel method of co-culturing the helicobacter pylori and the gastric mucosa epithelial cells is provided. In the novel system, the helicobacter pylori can grow normally and continuously stimulate the adherent cells to generate lesions, thus simulating a human gastric mucosa infection situation well. The problem that helicobacter pylori declines or dies and cells are not effectively stimulated because culture liquids are not suitable for growth of the helicobacter pylori in traditional methods in which helicobacter pylori are directly added is overcome.
Owner:LANZHOU UNIVERSITY
Method of making a mycoplasma vaccine
ActiveCN104968365AAntibacterial agentsBacterial antigen ingredientsMycoplasma bacteriaImmunogenicity
The present invention relates to a method for the preparation of an immunogenic composition for the treatment and / or prophylaxis of mycoplasma infections in a subject comprising the cultivation of mycoplasma bacteria in a serum-reduced or swine serum-free, eukaryotic cell system; obtaining an antigen of the mycoplasma bacteria; and addition of a pharmaceutically acceptable carrier. Further, the present invention relates to the immunogenic composition obtainable by said method and a method for immunizing a subject comprising the administration of said immunogenic composition to a subject.
Owner:BOEHRINGER INGELHEIM VETMEDICA GMBH
Methods, compositions, and systems for culturing and characterizing fastidious plant microbes
ActiveUS20170137776A1Easy, rapid and scalable platform to cultureBacteriaMicrobiological testing/measurementDiseaseRhizobium rhizogenes
Numerous plant microbes, including the vascular-limited Candidatus spp.—causal agents of citrus greening and potato zebra chip diseases—are non-culturable. The present disclosure relates, according to some embodiments, to compositions, methods and systems for culturing such organisms. For example, the present disclosure relates to methods for culturing, propagating, and characterizing fastidious vascular-colonizing microbes using a hairy root system (e.g., in vitro, in planta). The present disclosure relates, in some embodiments, to methods for cultivating a fastidious plant microbe including: contacting a plant (e.g., a tomato plant, a potato plant, a citrus plant) colonized by a fastidious plant microbe (e.g., Xylella fastidiosa, Candidatus Liberibacter spp.) with a suspension of R. rhizogenes under conditions that permit induction of hairy roots colonized with the fastidious plant microbe, and propagating the colonized microbial hairy roots.
Owner:TEXAS A&M UNIVERSITY
Preparation method and application of cell membrane coated nano topological structure array
ActiveCN111647952AEfficient captureWith cell membrane fluidityBacteriaMicroorganism based processesNanowireCell membrane
The invention relates to a preparation method and an application of a cell membrane coated nano topological structure array. The preparation method comprises the following steps: stimulating macrophages to form stimulated macrophages, and extracting stimulated macrophage membranes; meanwhile, processing the substrate to form a substrate with nanowires, and processing the substrate with the nanowires to form a nanowire substrate with positive charges; and combining the stimulated macrophage membrane with the nanowire substrate with the positive charges to obtain the macrophage membrane modifiednano topological structure array. The cell membrane coated nano topological structure array is simple in preparation and operation, and can be applied to capture of bacteria.
Owner:SUZHOU UNIV
Inhibiting or alleviating agent for inflammation in the brain
PendingCN113424063ANervous disorderMicrobiological testing/measurementVacciniaBULK ACTIVE INGREDIENT
An inhibiting or alleviating agent for inflammation in the brain comprising an extract from inflamed tissue inoculated with vaccinia virus as the active ingredient. A determination or evaluation method of an extract from inflamed tissue inoculated with vaccinia virus or an agent comprising the extract, characterized in that the inhibition of the expression of pro-inflammatory cytokines and / or NF-kB pathway related proteins induced by the promotion of expression of BDNF in cultivated glial cells is used as an indicator. A use of an extract from inflamed tissue inoculated with vaccinia virus in the production of the inhibiting or alleviating agent for inflammation in the brain.
Owner:刘军 +1
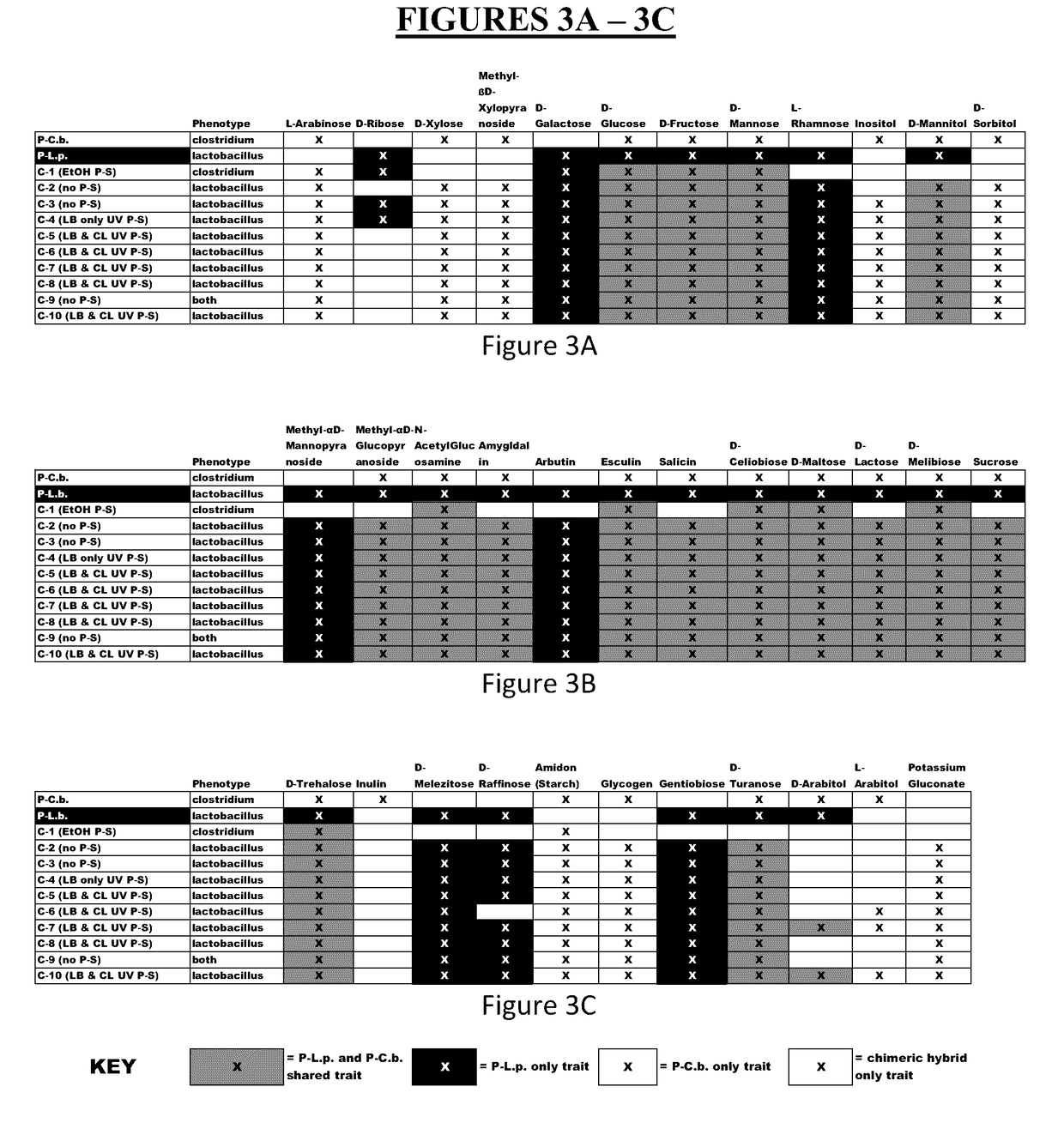
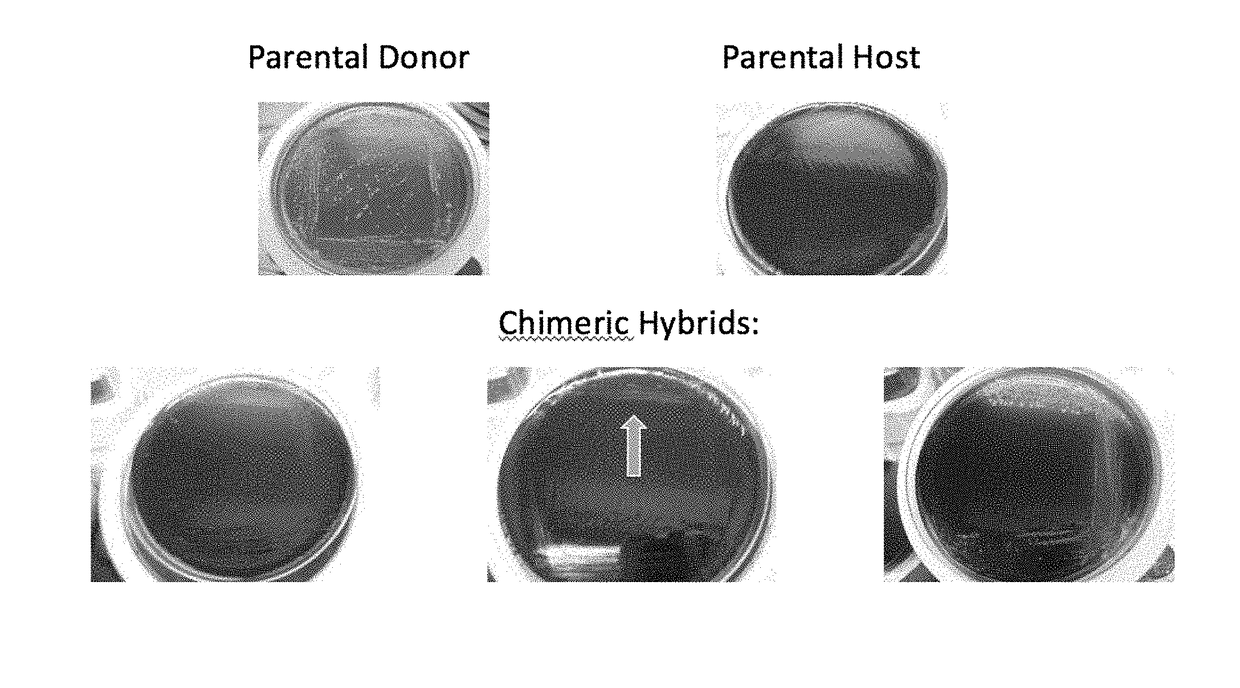
Method for producing chimeric microbial hybrids
Described is a method to transfer chromosomal DNA between two microbial species without genetic engineering or vectors. The strains resulting from this method are chimeric microbial hybrids that can express a combination of genotypes from both parents.
Owner:SCIBAC INC
Employing human adipose-derived stem cells to propagate serum-derived hepatitis c virus and use thereof
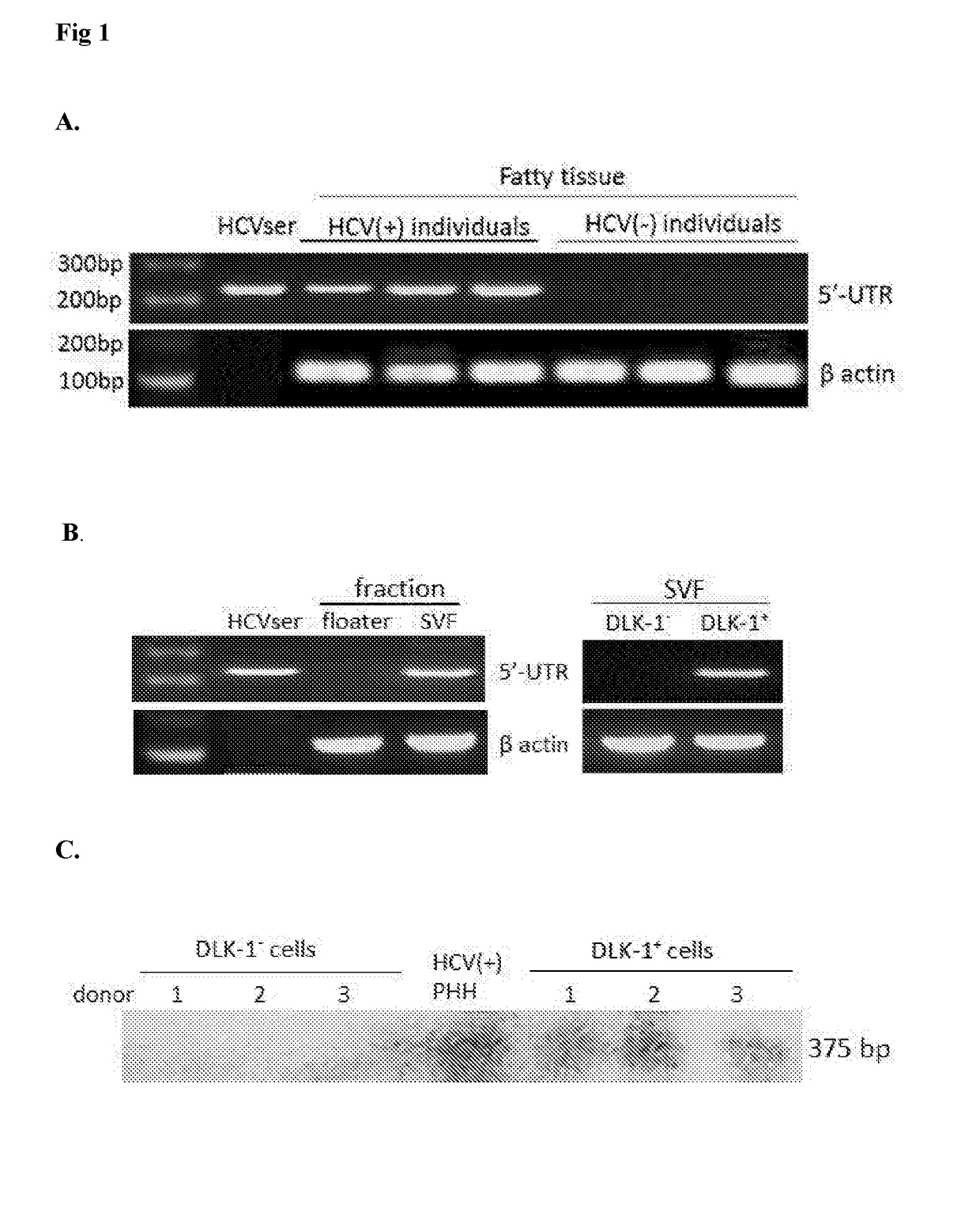
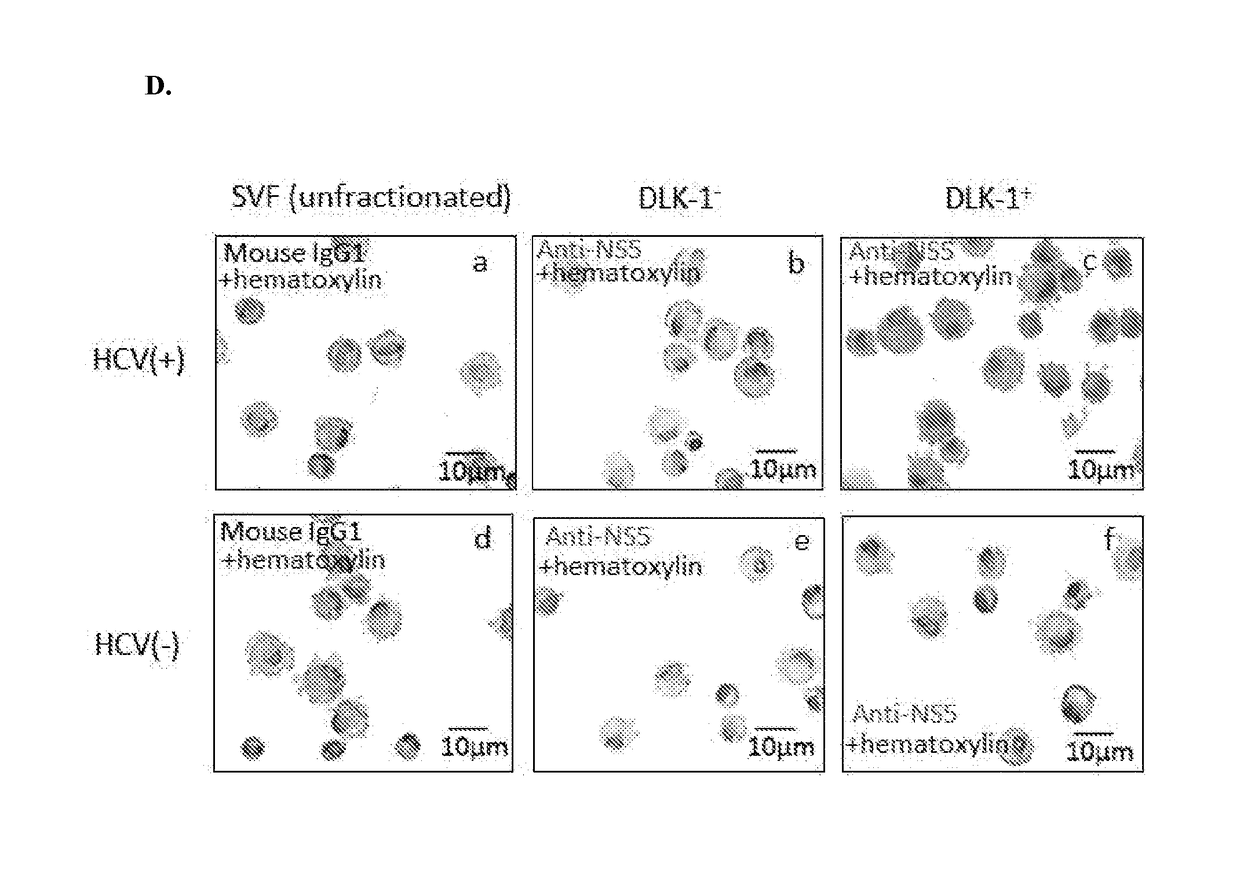
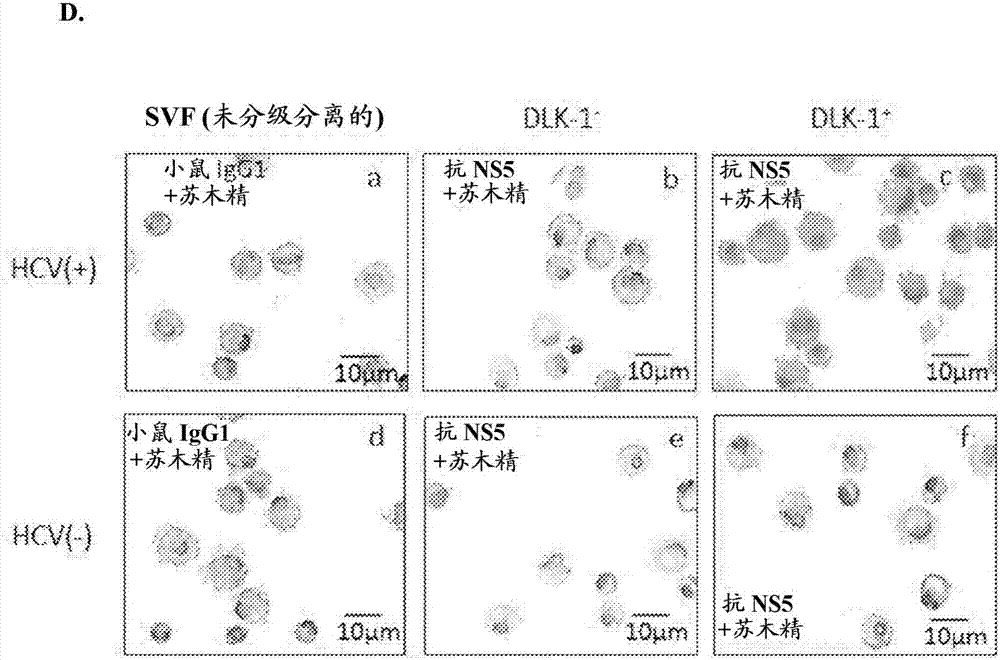
ActiveUS20180023058A1Reduce decreaseReduce the amount requiredSsRNA viruses positive-senseMicrobiological testing/measurementViral transcriptHepatica
Hepatitis C virus replication at extrahepatic sites has been suggested; however, complete viral replication has only been confirmed in hepatocytes. Here we show that human adipogenic DLK-1+ stem cells (hADSC) freshly isolated from HCV-infected individuals contained viral transcripts, replication intermediates and viral antigens in vivo, and viral transcripts increased in supernatants upon prolonged ex vivo culture. Furthermore, naive hADSC isolated from HCV (−) individuals support complete replication of clinical isolates in vitro, and the infection is donor-nonspecific for cells and cross-genotypic for viruses. Viral infection / replication is mediated through CD81, LDL-R, SR-B1, EGFR, Apolipoprotein E, occludin, claudin-1, NPC1L1 and diacylglycerol acetyltransferase-1, and can be inhibited by anti-viral drugs. In addition, the physical properties of hADSC-propagated viral particles resemble clinical isolates more than JFH1 / HCVcc, and viruses propagated by in vitro infected hADSC are infectious to primary human hepatocytes. Therefore, hADSC are an in vivo HCV reservoir and represent a novel venue of clinical virus-host interaction. hADSC can also be exploited as a physiologically relevant primary cell culture system to propagate clinical isolates.
Owner:FRONTIER BIO DRUG DEV LTD
Method for producing pluripotent cell using bacterium having fermentation ability
It is an object of the present invention to provide a method for producing pluripotent cells that are free of the risk of cellular canceration and that can be applied to regenerative medicine with a high degree of safety. The present invention provides a method for producing pluripotent cells from somatic cells comprising a step of bringing bacteria having fermentation ability or a component or secretory product thereof into contact with somatic cells.
Owner:INTEGRICULTURE INC
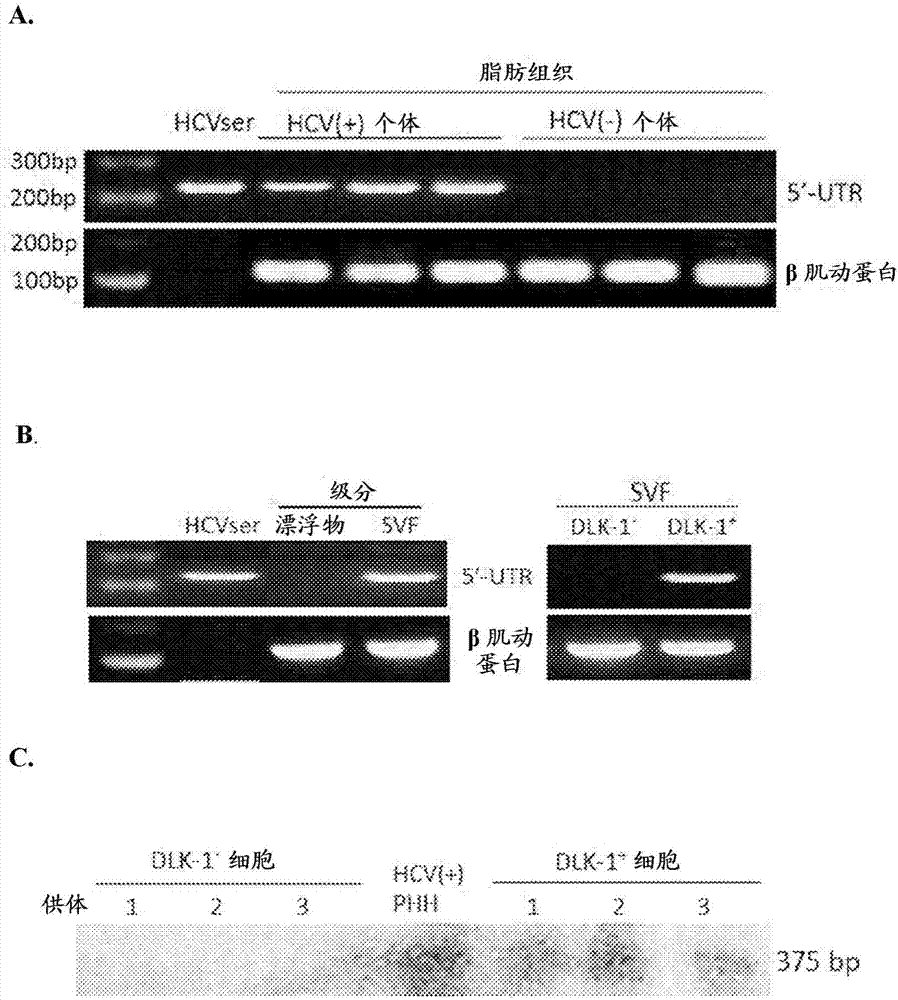
Employing human adipose-derived stem cells to propagate serum-derived hepatitis c virus and use thereof
ActiveCN107208060ASsRNA viruses positive-senseMicrobiological testing/measurementAdipogenesisHepatica
Hepatitis C virus replication at extrahepatic sites has been suggested; however, complete viral replication has only been confirmed in hepatocytes. Here we show that human adipogenic DLK-1+stem cells (hADSC) freshly isolated from HCV-infected individuals contained viral transcripts, replication intermediates and viral antigens in vivo, and viral transcripts increased in supernatants upon prolonged ex vivo culture. Furthermore, naive hADSC isolated from HCV(-)individuals support complete replication of clinical isolates in vitro, and the infection is donor-nonspecific for cells and cross-genotypic for viruses. Viral infection / replication is mediated through CD81, LDL-R, SR-B1, EGFR, Apolipoprotein E, occludin, claudin-1, NPC1L1 and diacylglycerol acetyltransferase-1, and can be inhibited by anti-viral drugs. In addition, the physical properties of hADSC-propagated viral particles resemble clinical isolates more than JFH1 / HCVcc, and viruses propagated by in vitro infected hADSC are infectious to primary human hepatocytes. Therefore, hADSC are an in vivo HCV reservoir and represent a novel venue of clinical virus-host interaction. hADSC can also be exploited as a physiologically relevant primary cell culture system to propagate clinical isolates.
Owner:VANWORLD PHARMA (RUGAO) CO LTD
Method for Producing Chimeric Microbial Hybrids
Described is a method to transfer chromosomal DNA between two microbial species without genetic engineering or vectors. The strains resulting from this method are chimeric microbial hybrids that can express a combination of genotypes from both parents.
Owner:SCIBAC INC
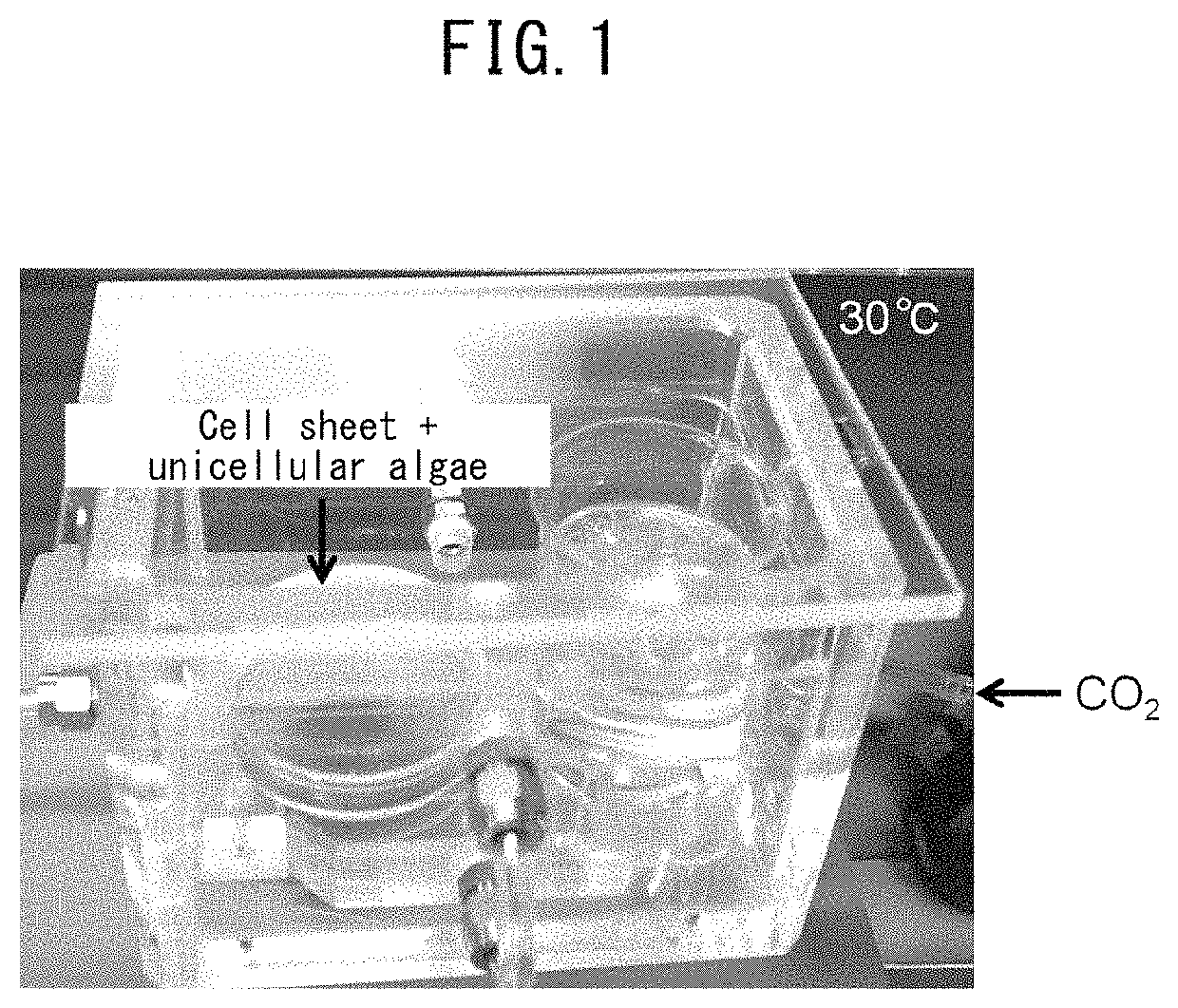
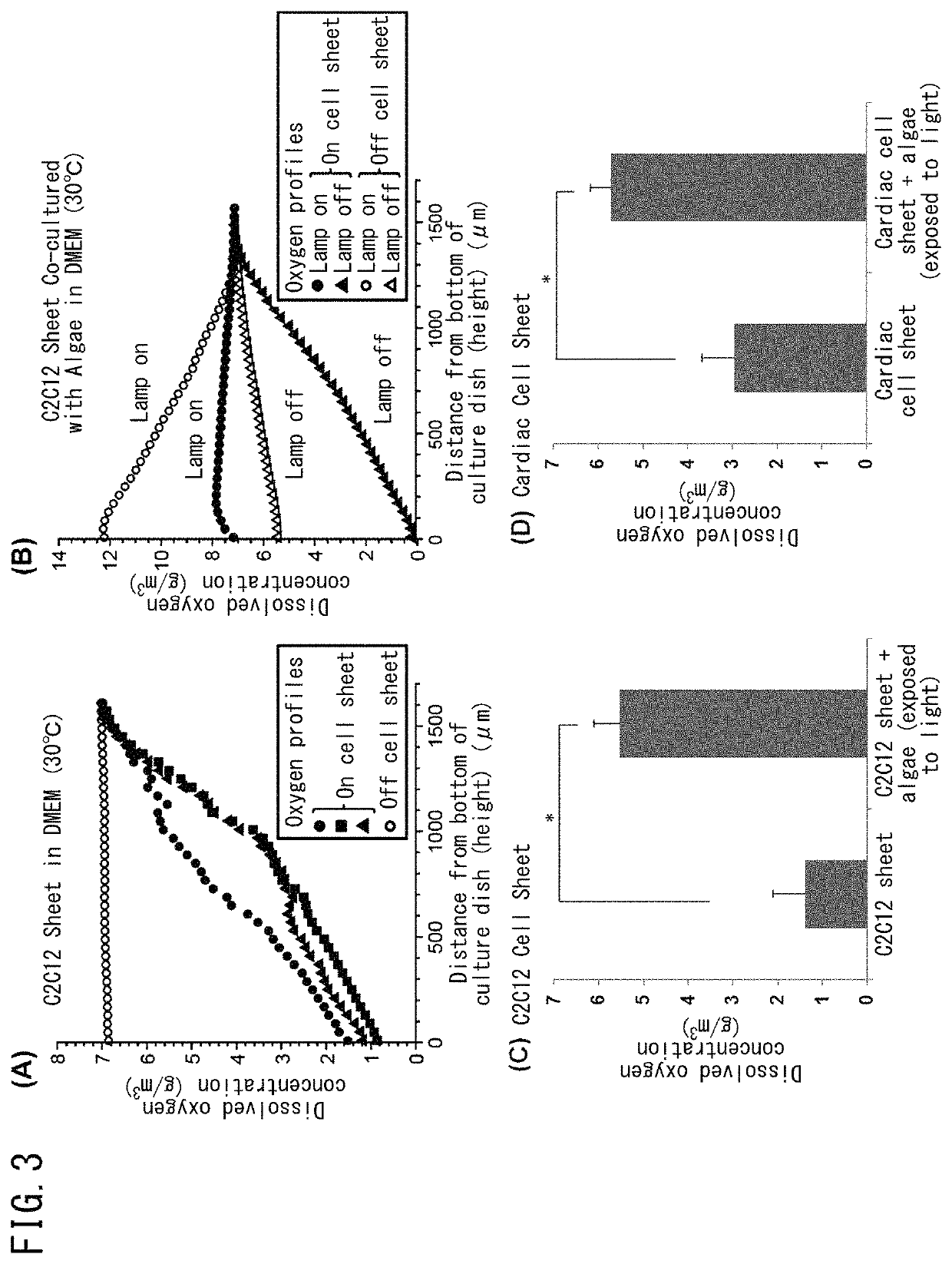
Method for culturing animal cell composition, method for producing animal cell composition using same, and animal cell composition
ActiveUS10883080B2Increase concentrationReduce cell damageUnicellular algaeCulture processBiotechnologyCapillary network
An object of the present invention is to obtain a thicker animal cell composition by a simple and less expensive method. Namely, an object of the present invention is to provide a method for culturing a thicker animal cell composition by eliminating the hypoxia associated with animal cell compositions, a method for producing an animal cell composition containing unicellular algae, and an animal cell composition.The present invention provides a method for culturing an animal cell composition in a culture medium in the presence of unicellular algae and under exposure to light. According to the method of the present invention, oxygen can be continuously supplied in the culture medium, cell damage is alleviated, and a thicker cell composition can be obtained in the absence of a capillary network.
Owner:TOKYO WOMENS MEDICAL UNIV
Culture container for culturing epithelial cells and use thereof
PendingCN113840903AOxygen partial pressure controlBioreactor/fermenter combinationsBiological substance pretreatmentsCell culture mediaBiomedical engineering
A culture container for culturing epithelial cells that comprises an upper container, a lid member air tightly fitting into the opening of the upper container, and a lower container housing the upper container and a cell culture medium therein, wherein; at least a part of an area of the upper container, said area being in contact with the cell culture medium, is formed of a membrane that is permeable to at least some components of the cell culture medium and impermeable to cells; and the material of the lid member has an oxygen permeation coefficient of not more than 1.0x10<-6> cm<3> cm / (cm<2> s.Pa).
Owner:KEIO UNIV
Inhibiting or alleviating agent for inflammation in the brain
PendingUS20220096561A1Reduce inflammationReduce inhibitionNervous disorderMicrobiological testing/measurementVacciniaBULK ACTIVE INGREDIENT
An inhibiting or alleviating agent for inflammation in the brain comprising an extract from inflamed tissue inoculated with vaccinia virus as the active ingredient. In another aspect, the invention relates to a determination or evaluation method of an extract from inflamed tissue inoculated with vaccinia virus or an agent comprising the extract, characterized in that the inhibition of the expression of pro-inflammatory cytokines and / or NF-κB pathway related proteins induced by the promotion of expression of BDNF in cultivated glial cells is used as an indicator. In still another aspect, the invention also relates to a use of an extract from inflamed tissue inoculated with vaccinia virus in the production of the inhibiting or alleviating agent for inflammation in the brain.
Owner:LIU JUN +1
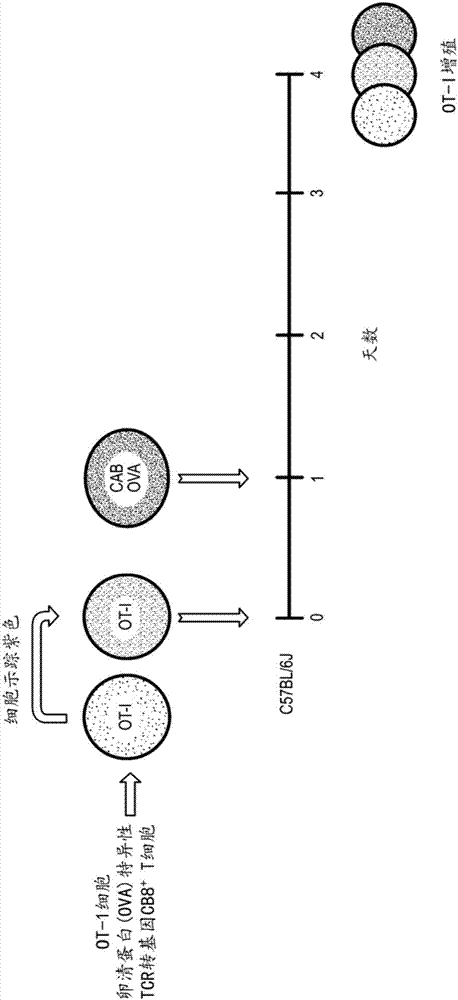
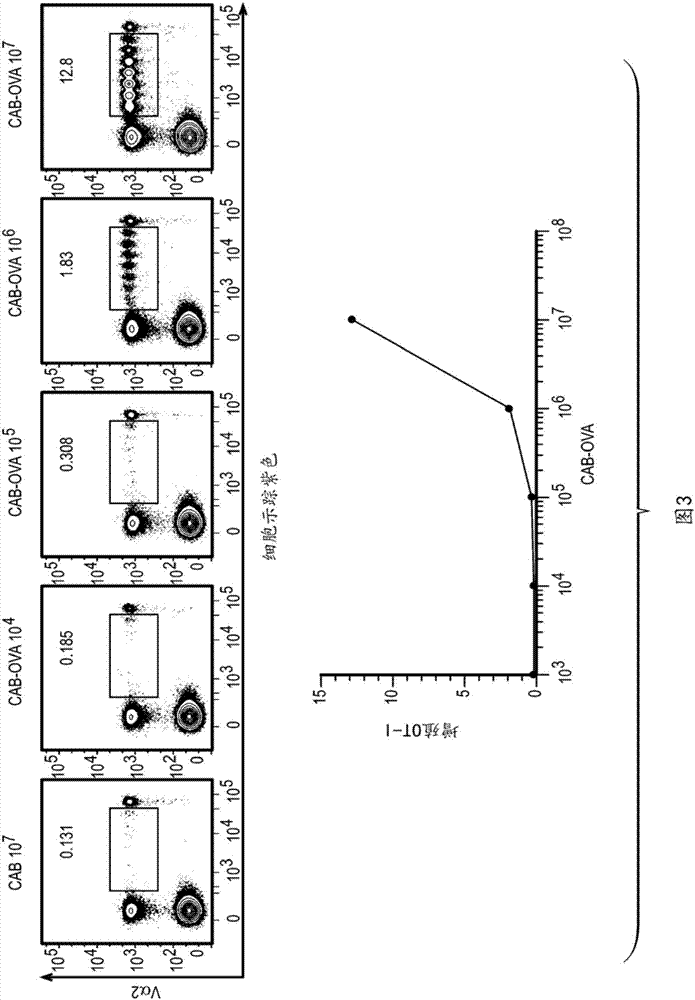
Chlamydia-activated B cell platforms and methods thereof
Disclosed herein is a Chlamydia-activated B cell (CAB) platform. Also disclosed is a method of enhancing a population of B cells, comprising exposing said B cells to Chlamydia spp. under conditions suitable to enhance the population of B cells, such that expansion and differentiation of said B cells takes place, and said B cells are exposed or crosslinked to an antigen. Also disclosed are methods of producing said CABs, and treating a subject in need thereof with said CABs.
Owner:OHIO STATE INNOVATION FOUND
Microparticle for cultivating and testing cells
ActiveUS11448645B2Gastrointestinal cellsMicrobiological testing/measurementChemical compoundMicroparticle
Cultivation and / or test-system comprising at least two cultivation spaces combined in a microparticle, wherein the sperically shaped microparticle comprises, (i) a first cultivation space in the center core of said spherically shaped microparticle, (ii) a second cultivation space in the wall surrounding the core of said microparticle, (iii) wherein the wall surrounding the core allows for the exchange of molecules as, salts, nutrients, peptides, chemicals and other compounds in order for the cells in the first cultivation space to interact with the cells in second cultivation space and vice versa. In the test system, the cells are co-cultivated and the microparticles are then selected based on the phenotype of the cells in the first or second cultivation space.
Owner:BIOMILLENIA SAS
Culture container for culturing epithelial cells and use thereof
PendingUS20220220427A1Easy to controlBioreactor/fermenter combinationsBiological substance pretreatmentsCell culture mediaBiomedical engineering
A culture container for culturing epithelial cells, includes an upper container, a lid member configured to airtightly fit with an opening portion of the upper container, and a lower container configured to accommodate the upper container and a cell culture medium, in which at least a part of an area of the upper container in contact with the cell culture medium is formed of a membrane that is permeable to at least a part of components of the cell culture medium and impermeable to a cell, and a material of the lid member has an oxygen permeability coefficient of 1.0×10−6 cm3 cm / (cm2·sec·atm) or less.
Owner:KEIO UNIV
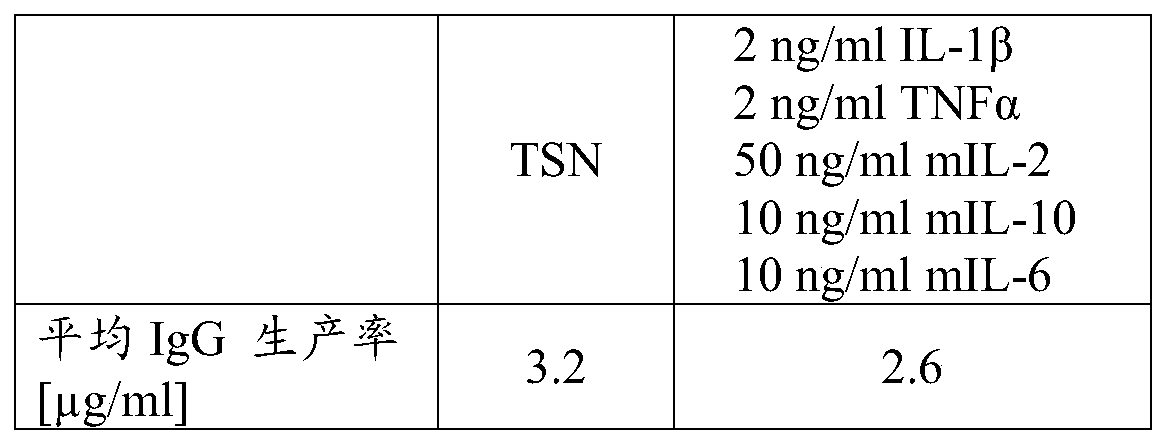
B-cell cultivation method
Herein is reported a method for co-cultivating B-cells in the presence of phorbol myristate acetate, IL-1beta, TNFalpha, IL-2, IL-10 and IL-6.
Owner:F HOFFMANN LA ROCHE & CO AG
Method for Producing Chimeric Microbial Hybrids
Described is a method to transfer chromosomal DNA between two microbial species without genetic engineering or vectors. The strains resulting from this method are chimeric microbial hybrids that can express a combination of genotypes from both parents.
Owner:SCIBAC INC
Popular searches
Apparatus sterilization Biochemistry cleaning apparatus Biomass after-treatment Enzymology/microbiology apparatus Tissue/virus culture apparatus Stress based microorganism growth stimulation Specific use bioreactors/fermenters Microorganism fixing/supporting apparatus Lamination ancillary operations Lamination
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com