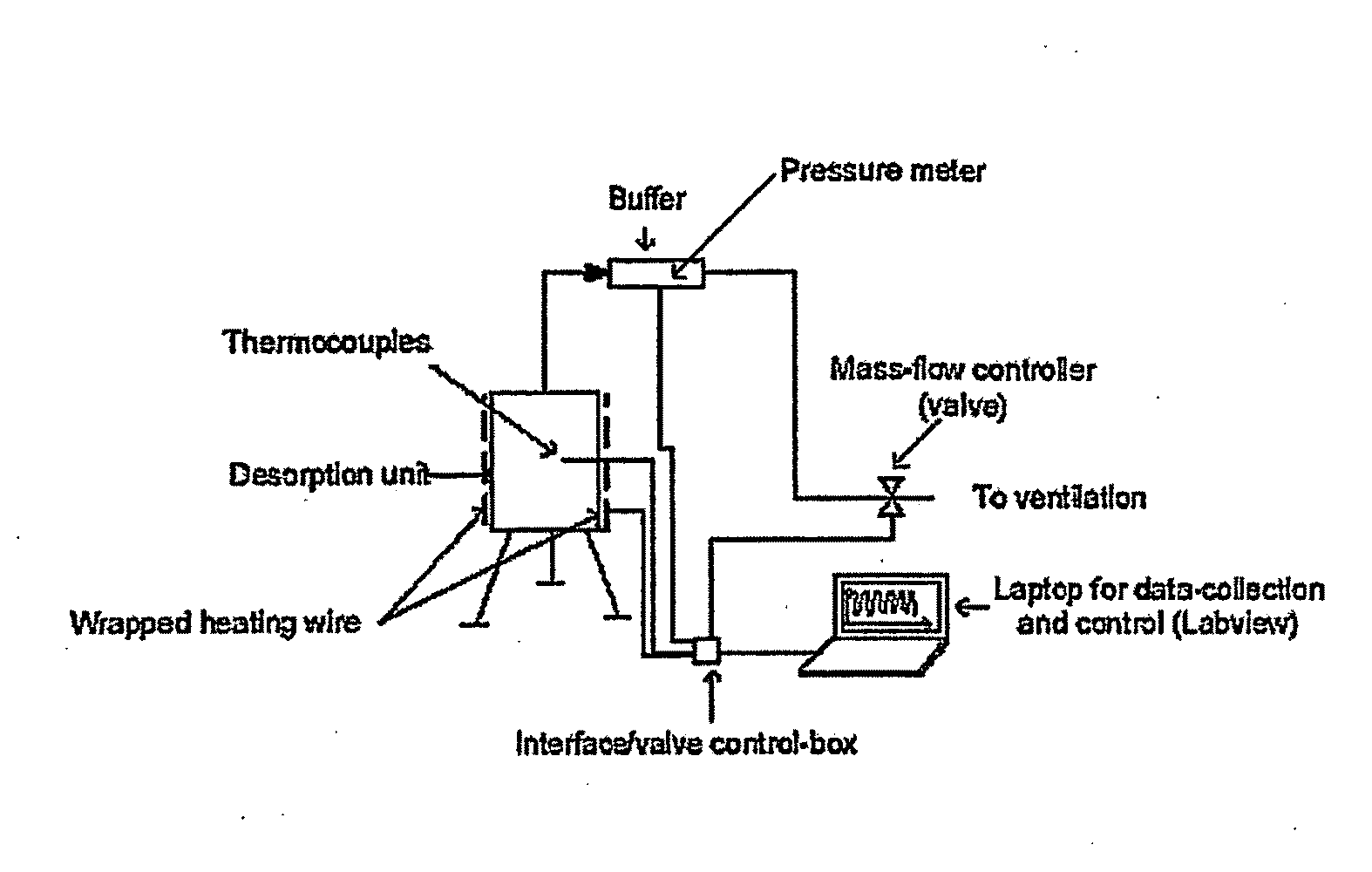
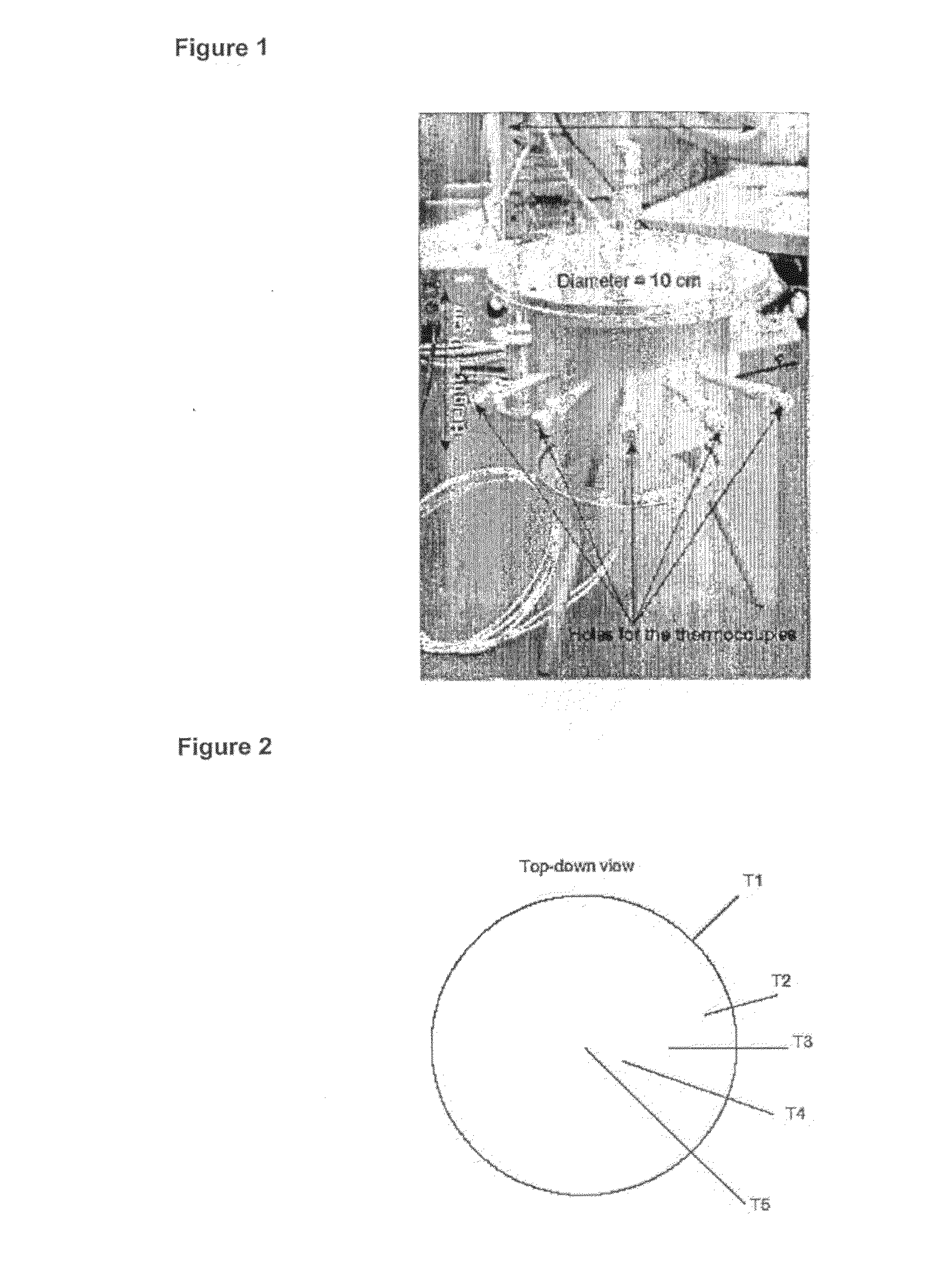
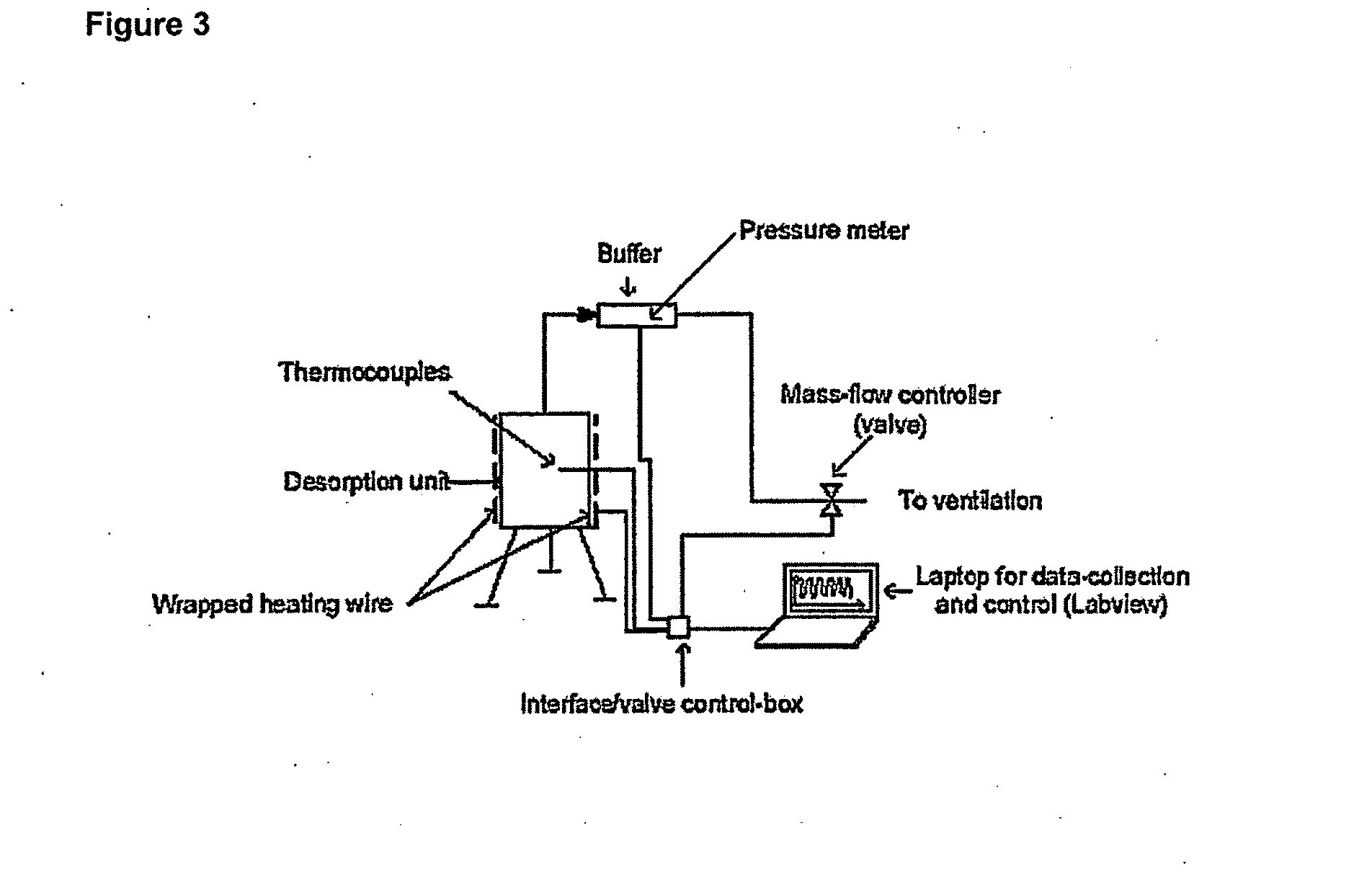
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
98results about "Ammonia handling/storage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
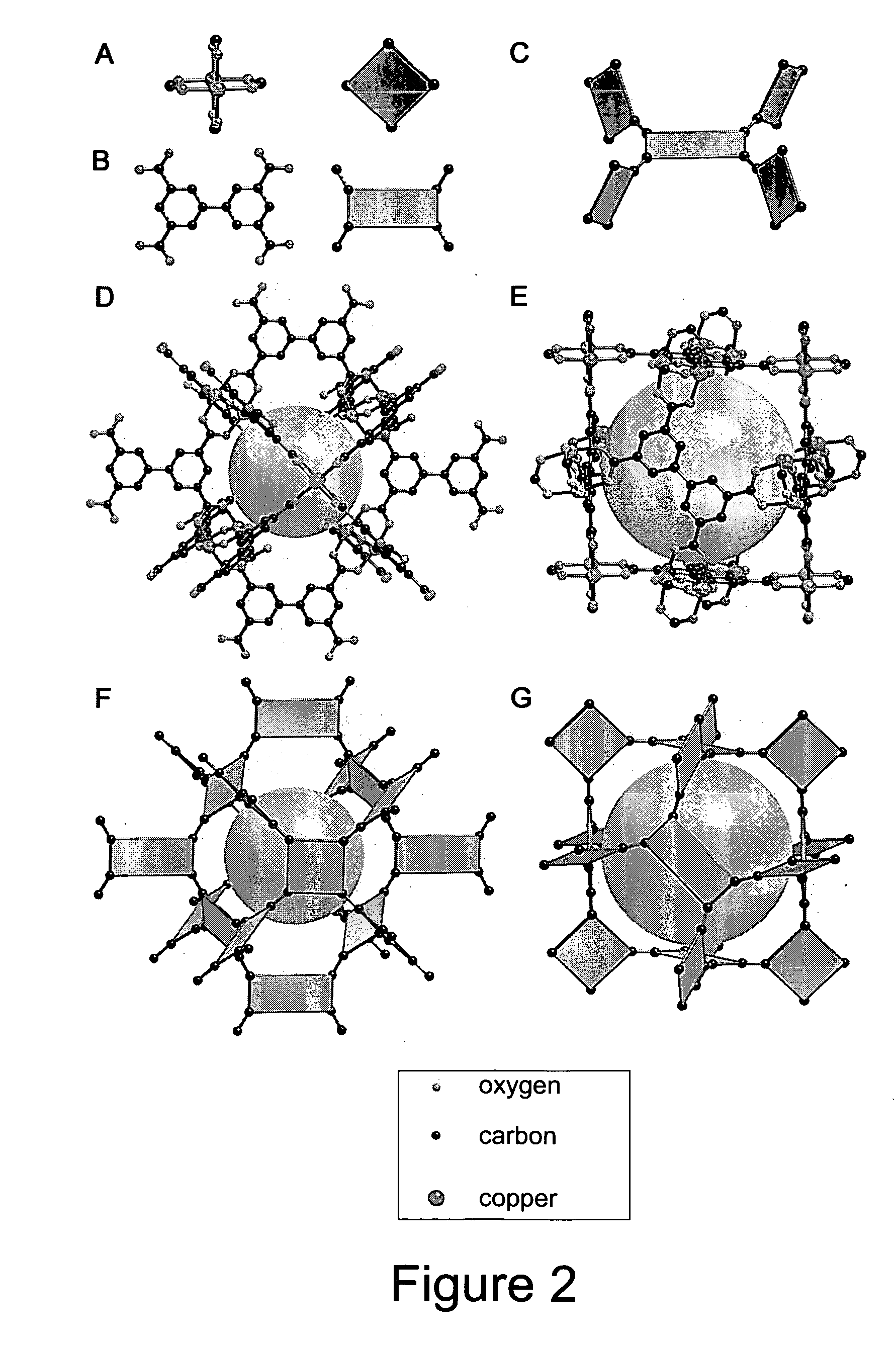
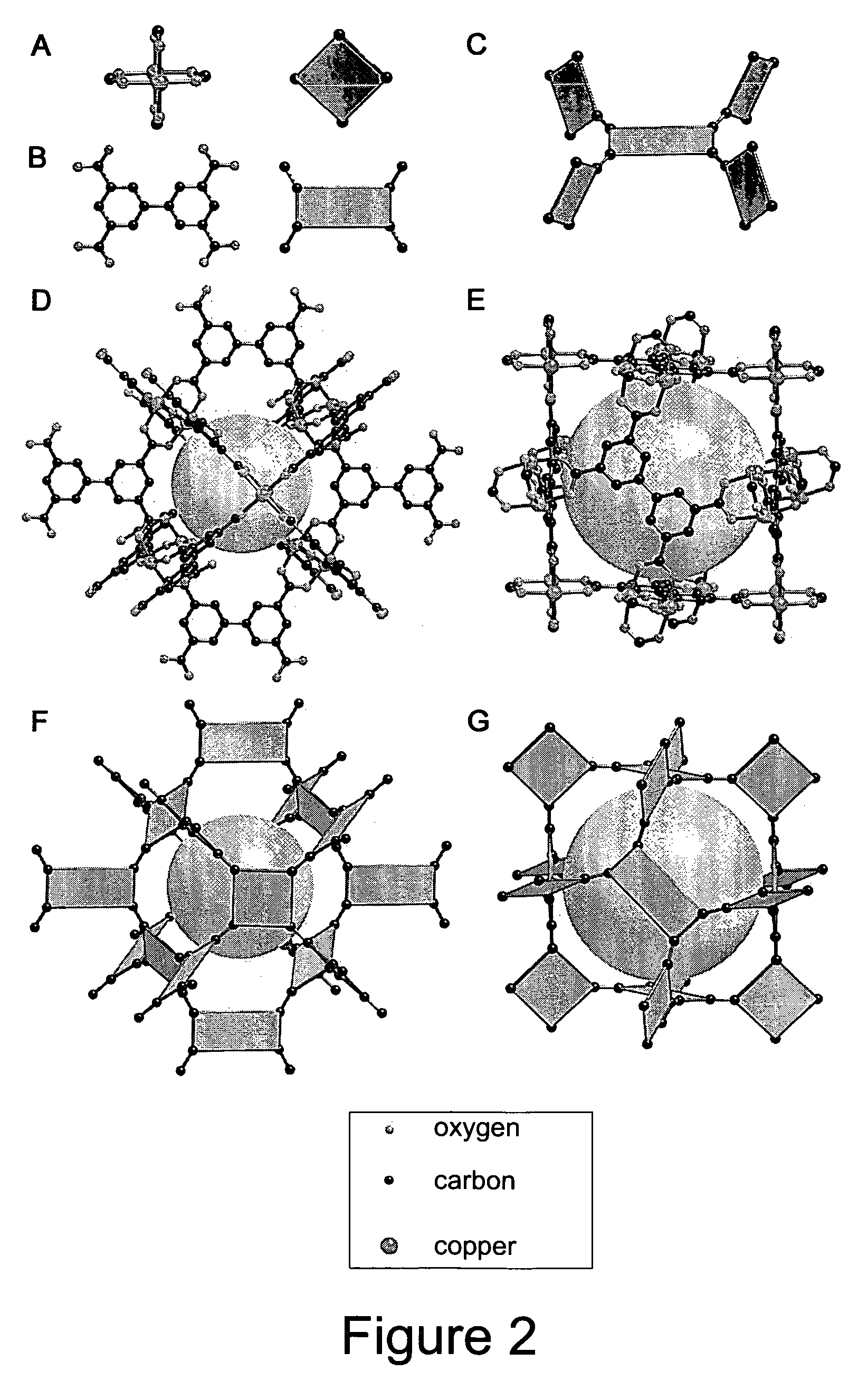
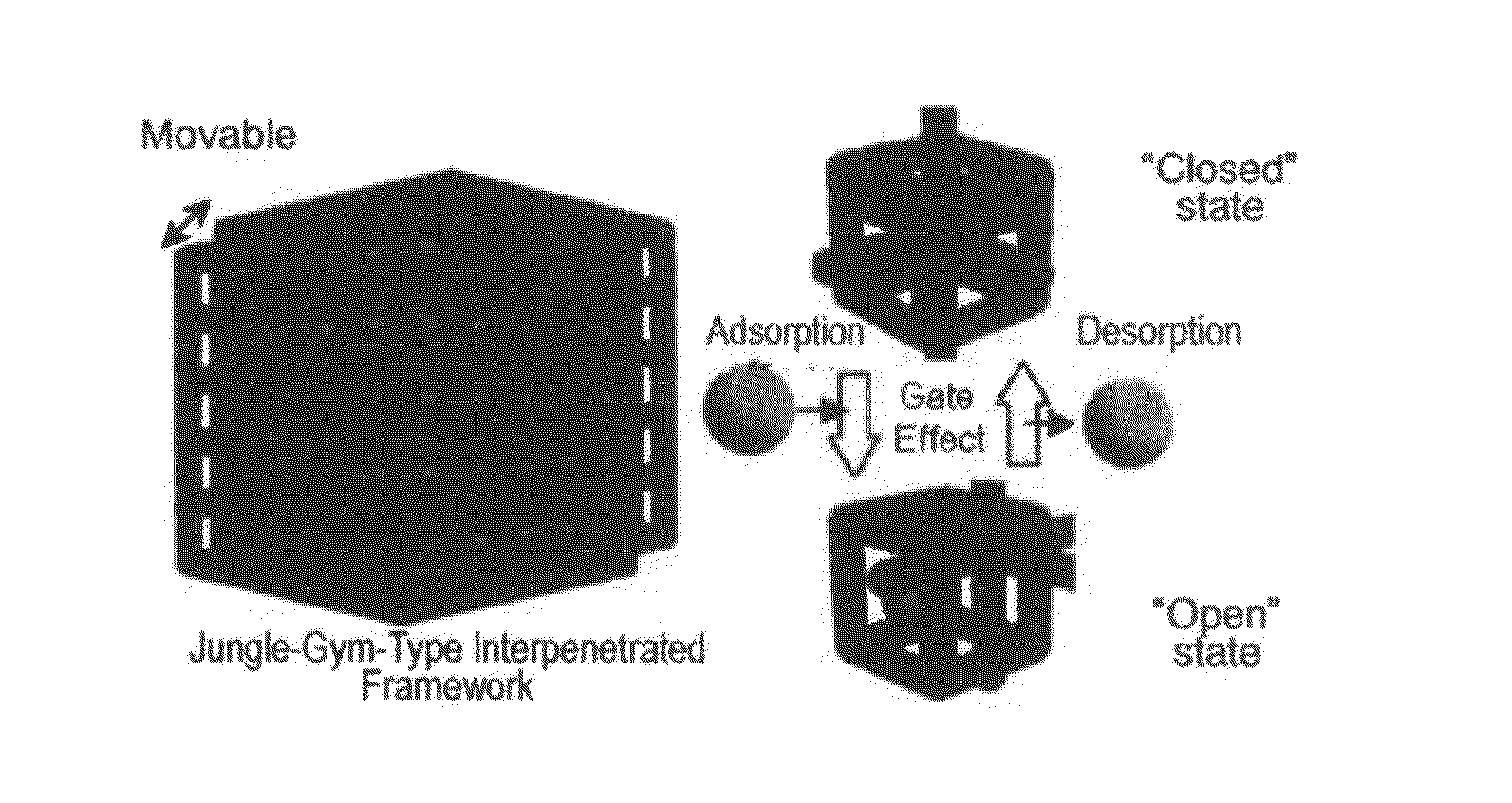
High gas adsorption in a microporous metal-organic framework with open-metal sites
A gas storage material contains a metal-organic framework that includes a plurality of metal clusters and a plurality of charged multidentate linking ligands that connect adjacent metal clusters. Each metal cluster includes one or more metal ions and at least one open metal site. The metal-organic framework includes one or more sites for storing molecular hydrogen. A hydrogen storage system using the hydrogen storage material is provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
High gas adsorption metal-organic framework
A gas storage material contains a metal-organic framework that includes a plurality of metal clusters and a plurality of charged multidentate linking ligands that connect adjacent metal clusters. Each metal cluster includes one or more metal ions and at least one open metal site. The metal-organic framework includes one or more sites for storing molecular hydrogen. A hydrogen storage system using the hydrogen storage material is provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Adsorption based ammonia storage and regeneration system
One aspect of the invention relates to a device for storing ammonia for use in SCR on board a vehicle. The device comprises an adsorption bed with a high capacity for storing ammonia. The device can be designed to hold a long-lasting charge of ammonia comparable to a urea tank, but will not release substantial amounts of ammonia into the environment even if the device is accidentally ruptured. In one embodiment, the devices are charged at stationary locations. In another embodiment, the devices are charged by vehicle-mounted ammonia synthesis plants. The device facilitate the use of small ammonia synthesis plants that operate at low pressures and give low conversions. Preferably, the devices are operated through temperature swing adsorption.
Owner:EATON CORP
Use of an ammonia storage device in production of energy
An electric power generating unit comprising (i) an ammonia storage device in the form of a container comprising an ammonia absorbing and releasing salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkali metals, alkaline earth metals, and transition metals such as Li, K, Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu or Zn, X is one or more anions selected from fluoride, chloride, bromide, iodide, nitrate, thiocyanate, sulphate, molybdate, phosphate, and chlorate ions, a is the number of cations per salt molecule, Z is the number of anions per salt molecule, and n is the coordination number of 2 to 12. (ii) means for heating said container and ammonia absorbing and releasing salt for releasing ammonia gas and (iiia) a fuel cell for converting ammonia directly into electric power; or (iiib1) a reactor for dissociating ammonia into hydrogen and nitrogen and (iiib2) a fuel cell for converting hydrogen into electric power.
Owner:AMMINEX
Energy conversion system
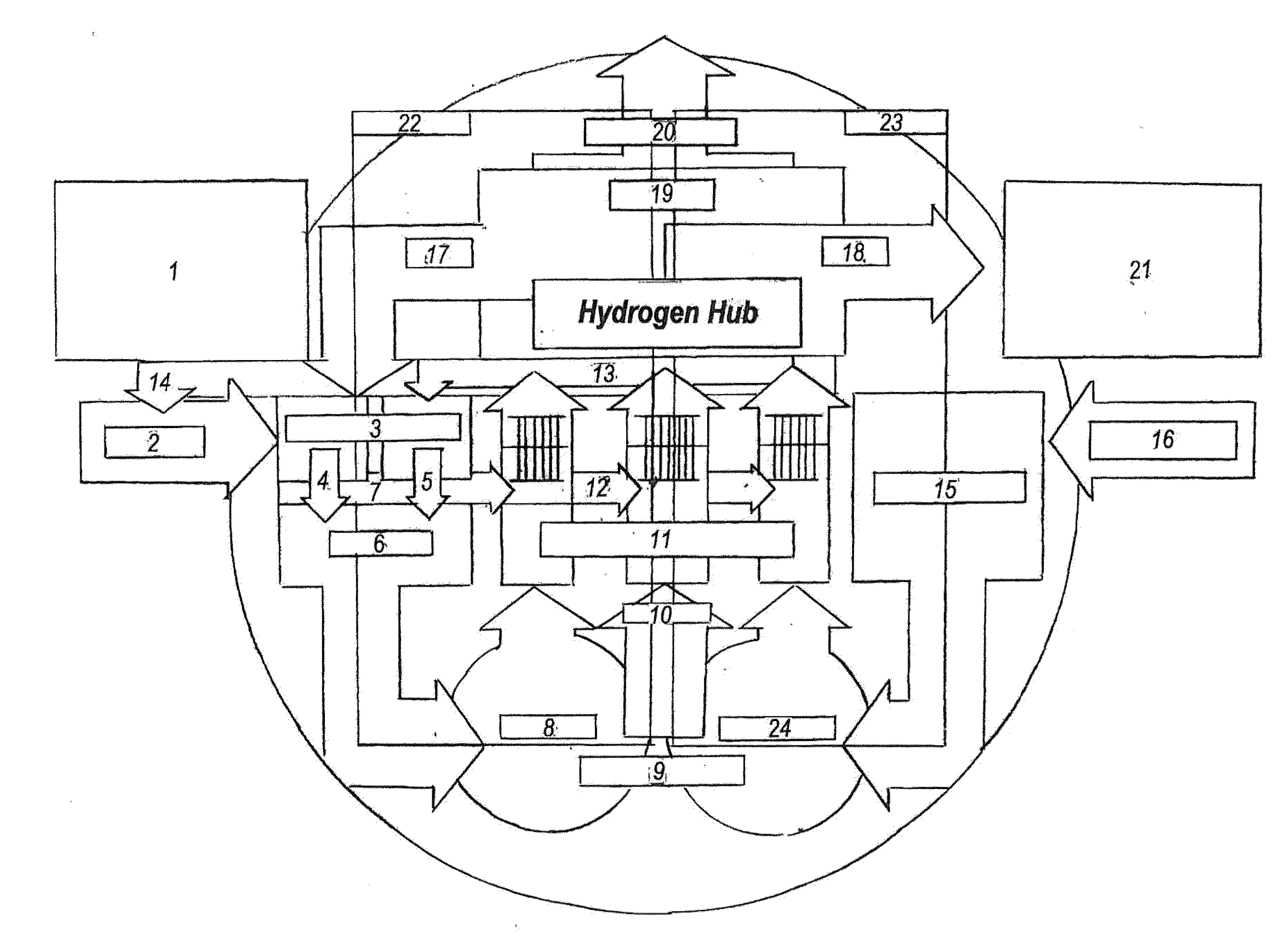
InactiveUS20160006066A1More energyEasy to operateElectrolysis componentsGeneral water supply conservationNuclear engineeringElectric power system
An improved system of hardware and controls, known as a Hyper Hub, that absorbs electric power from any source, including hydropower, wind, solar, and other renewable energy resources, chemically stores the power in hydrogen-dense anhydrous ammonia, then reshapes the stored energy to the power grid with zero emissions by using anhydrous ammonia to fuel diesel-type, spark-ignited internal combustion, combustion turbine, fuel cell or other electric power generators, and for other purposes.
Owner:ROBERTSON JOHN S
Ammonia storage for on-vehicle engine
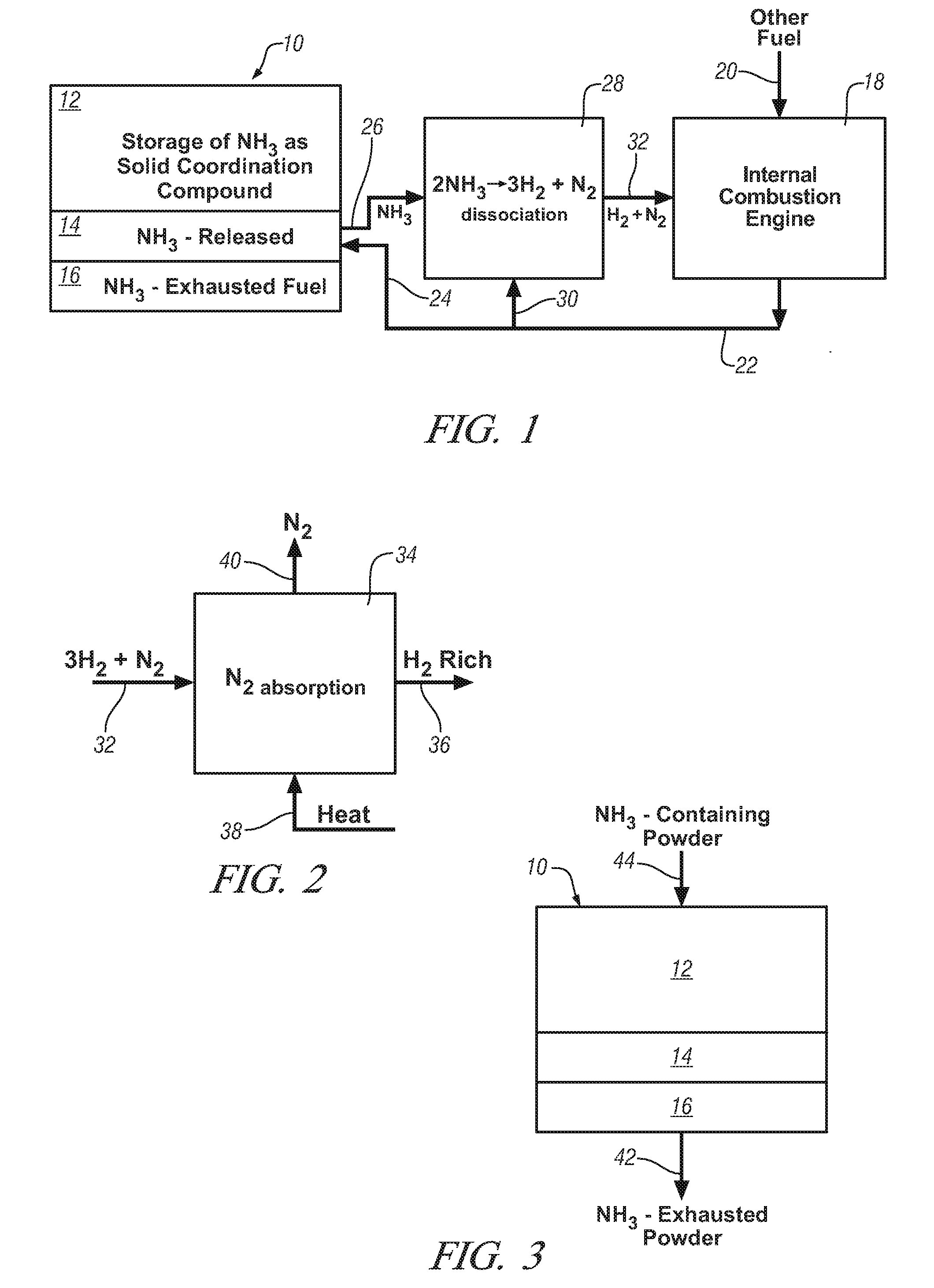
InactiveUS20080241033A1Efficient and reversible processCobalt ammonia complexesCyanogen compoundsAmmonia storageExternal combustion engine
Ammonia is used as precursor source of hydrogen fuel in an on-vehicle internal combustion engine. Ammonia is stored as, for example, a ligand in an on-vehicle transition metal composition. Upon demand for hydrogen by the vehicle's engine control system, ammonia is expelled as a gas from some of the composition and the ammonia gas is dissociated into a mixture of hydrogen and nitrogen and delivered as a fuel-containing mixture to the engine. In a preferred embodiment, the hydrogen is used as a supplement to gasoline as a fuel for engine operation.
Owner:GM GLOBAL TECH OPERATIONS LLC
Use Of An Ammonia Storage Device In Production Of Energy
InactiveUS20070207351A1Reactant parameters controlMagnesium halidesAlkaline earth metalAmmonia storage
An electric power generating unit comprising (i) an ammonia storage device in the form of a container comprising an ammonia absorbing and releasing salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkali metals, alkaline earth metals, and transition metals such as Li, K, Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu or Zn, X is one or more anions selected from fluoride, chloride, bromide, iodide, nitrate, thiocyanate, sulphate, molybdate, phosphate, and chlorate ions, a is the number of cations per salt molecule, Z is the number of anions per salt molecule, and n is the coordination number of 2 to 12. (ii) means for heating said container and ammonia absorbing and releasing salt for releasing ammonia gas and (iiia) a fuel cell for converting ammonia directly into electric power; or (iiib1) a reactor for dissociating ammonia into hydrogen and nitrogen and (iiib2) a fuel cell for converting hydrogen into electric power is useful for large stationary energy producing facilities, but also for use for is useful for large stationary energy producing facilities, but also for use for small rechargeable and / or replaceable power supply units for micro-fabricated or miniaturized ammonia decomposition reactors for use in mobile units and portable devices may be used for large energy producing facilities, and by use of small rechargeable and / or replaceable ammonia storage decomposition reactors, it is also possible to provide energy for mobile units and portable devices.
Owner:AMMINEX
Method and device for ammonia storage and delivery using in situ re-saturation of a delivery unit
InactiveUS20100062296A1Partially be recoveredInternal combustion piston enginesExhaust apparatusAmmonia storageAbsorbent material
Disclosed is a method for storing and delivering ammonia, wherein a first ammonia adsorbing / absorbing material having a higher vapor pressure at a given temperature than a second ammonia adsorbing / absorbing material is used as an ammonia source for said second ammonia adsorbing / absorbing material when said second adsorbing / absorbing material is depleted of ammonia by consumption, and a device for performing the method.
Owner:AMMINEX
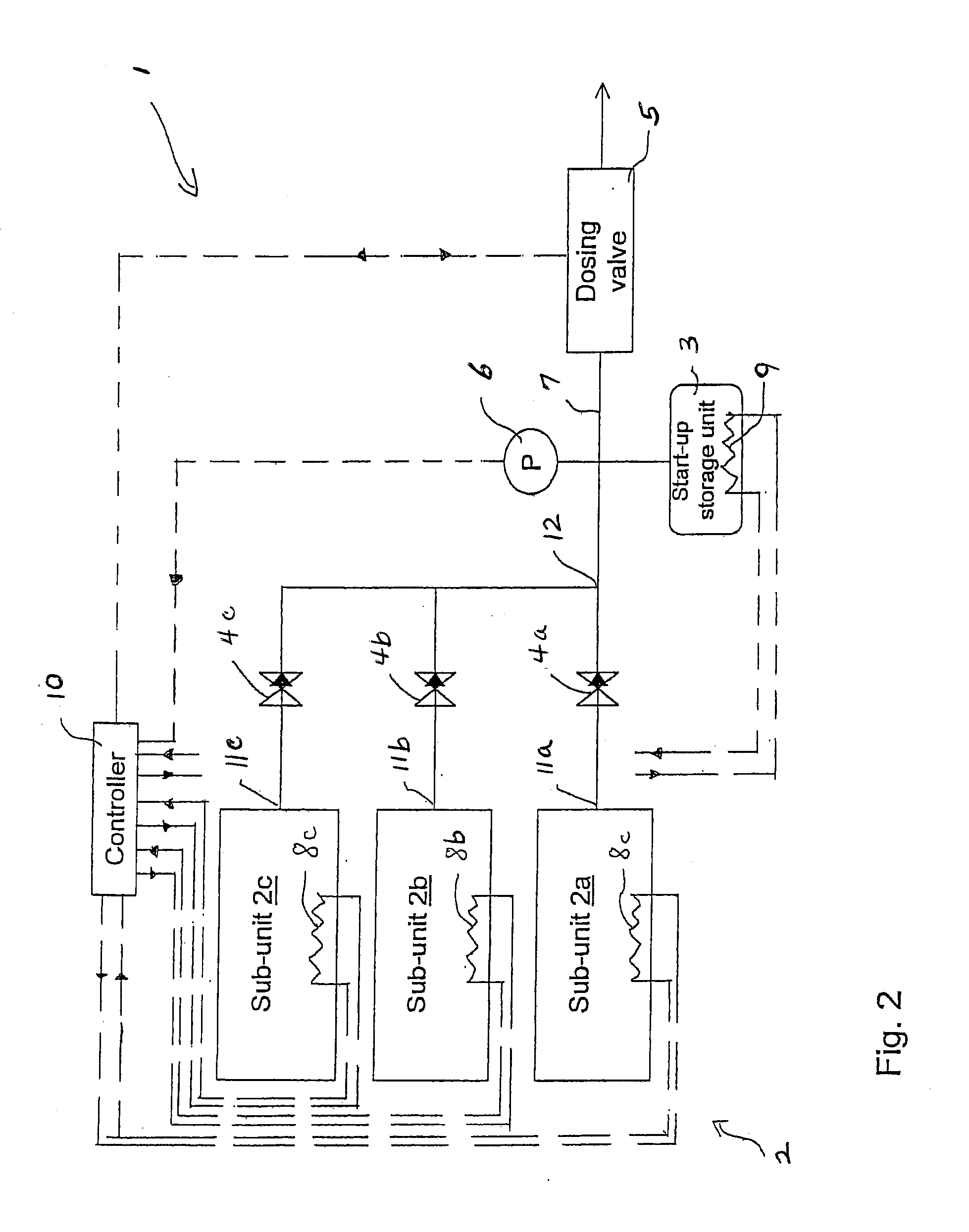
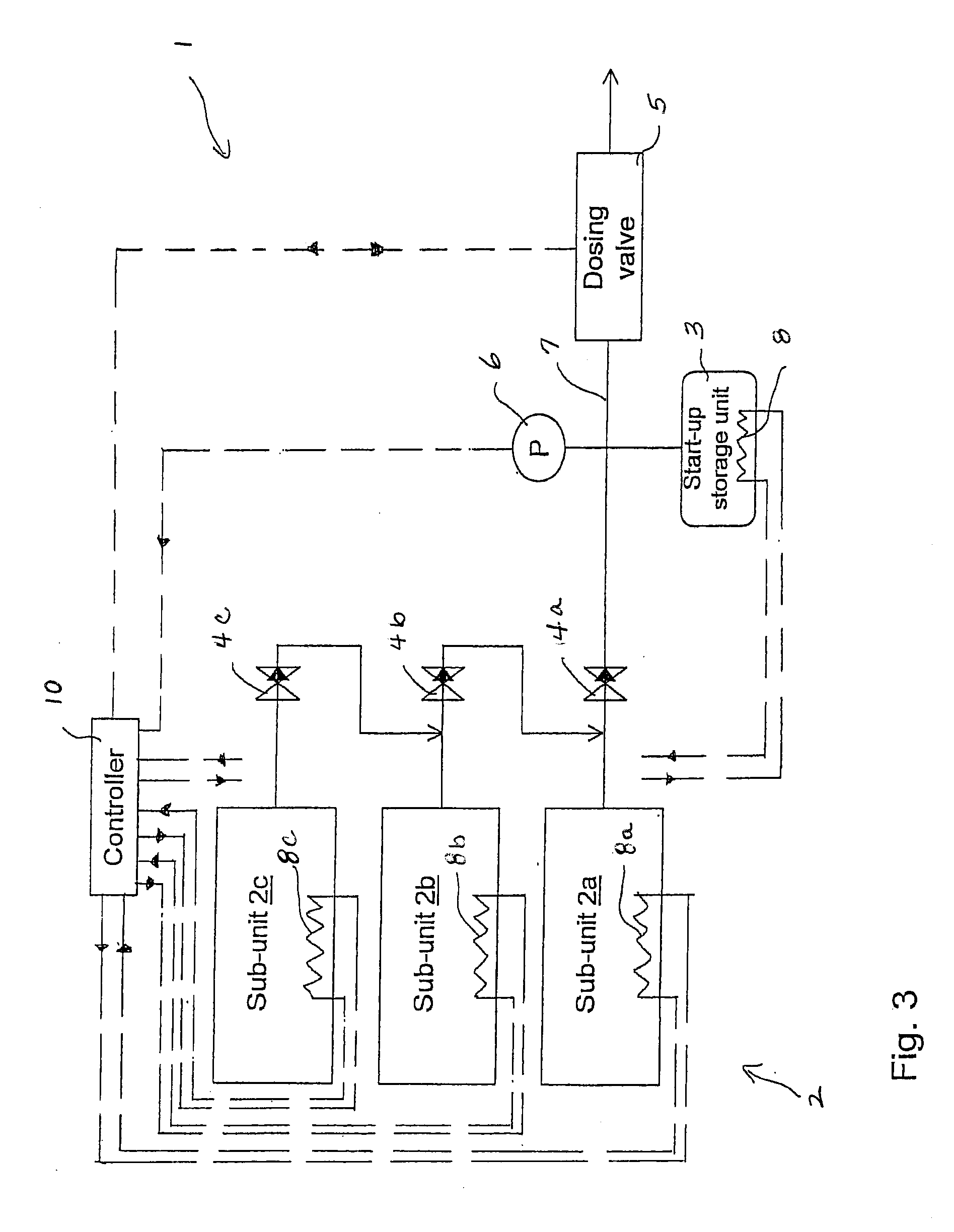
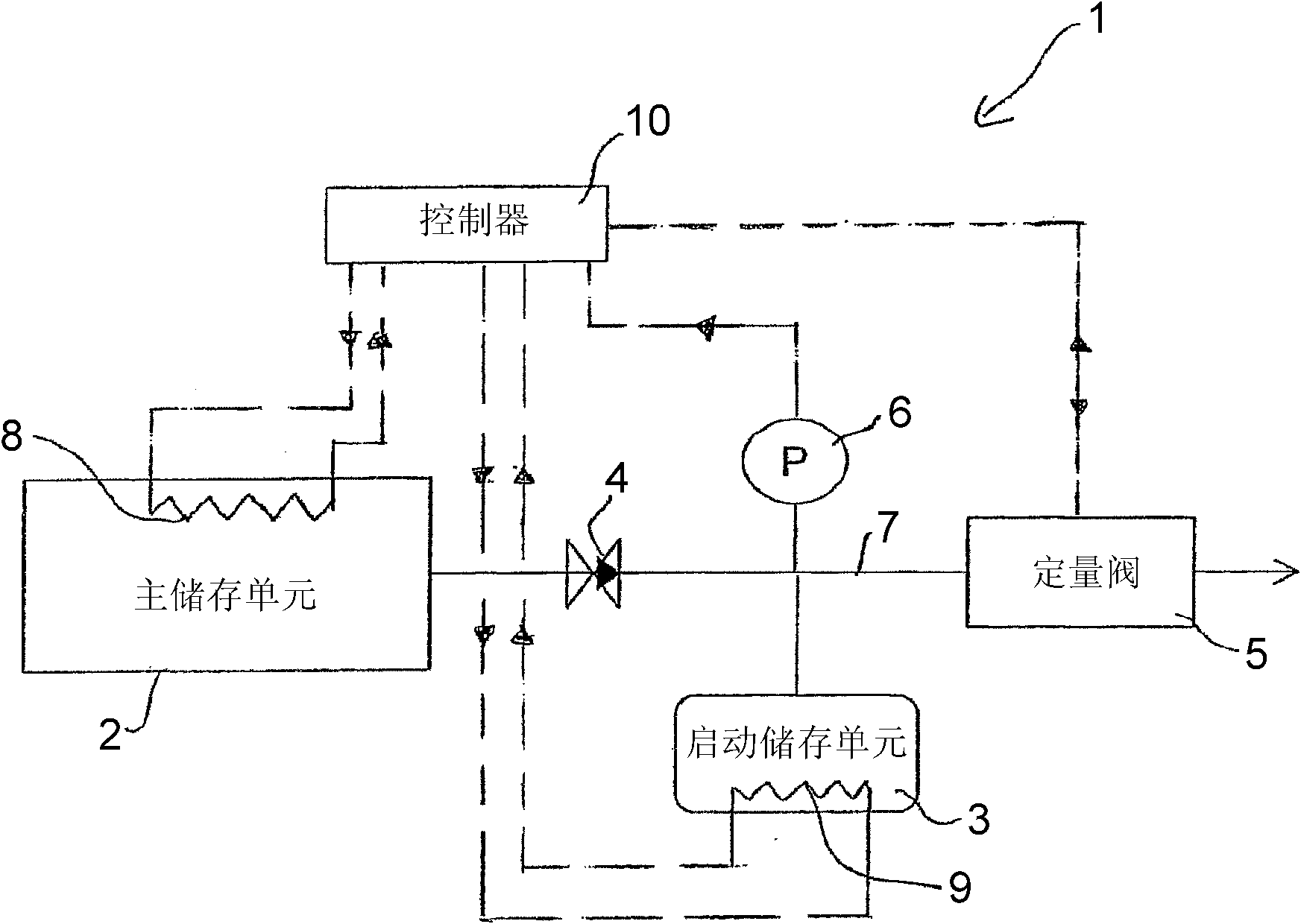
Release of Stored Ammonia at Start-Up
ActiveUS20100086467A1Demand for it variesPrevent backflowInternal combustion piston enginesExhaust apparatusAmmonia storageGaseous ammonia
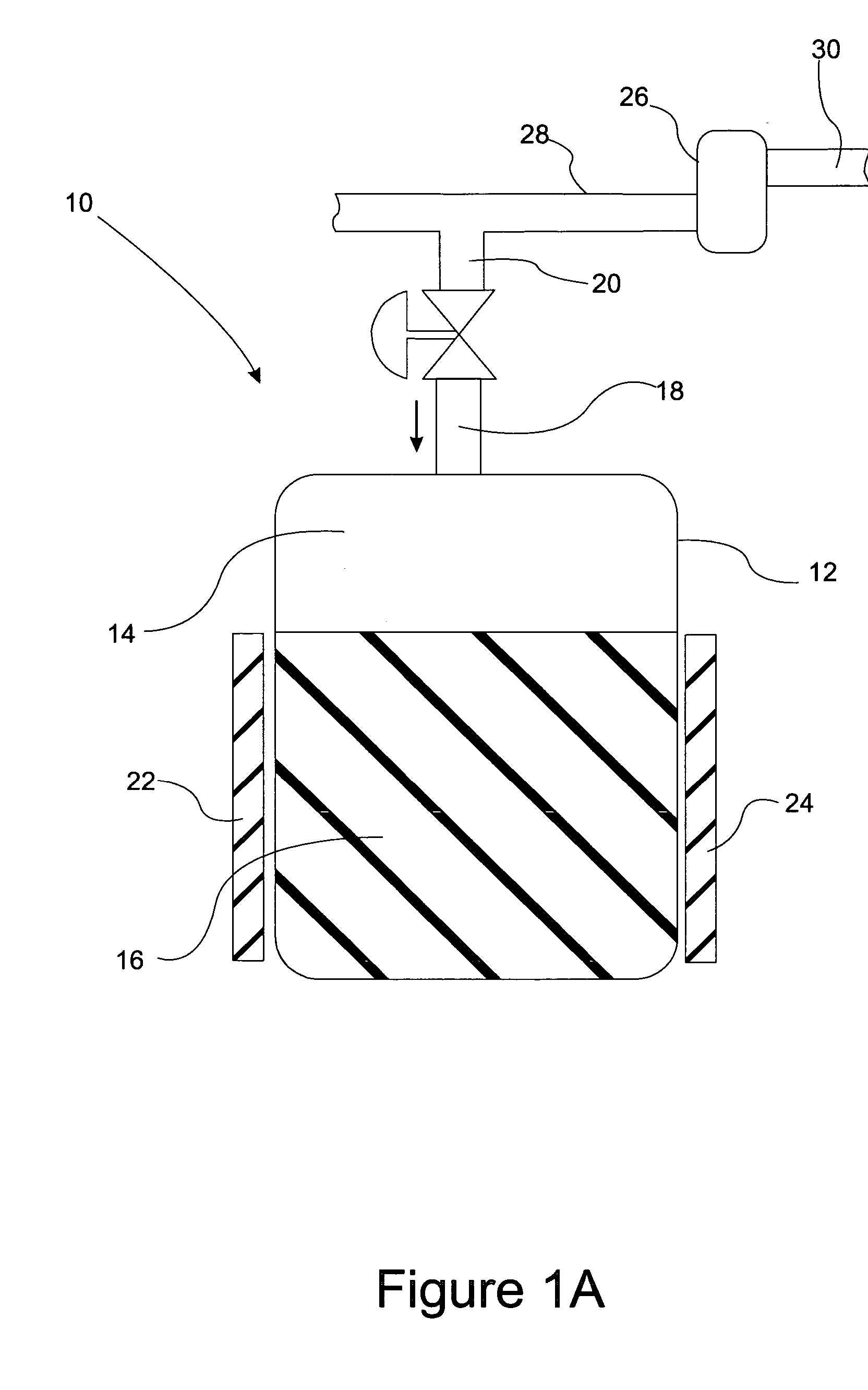
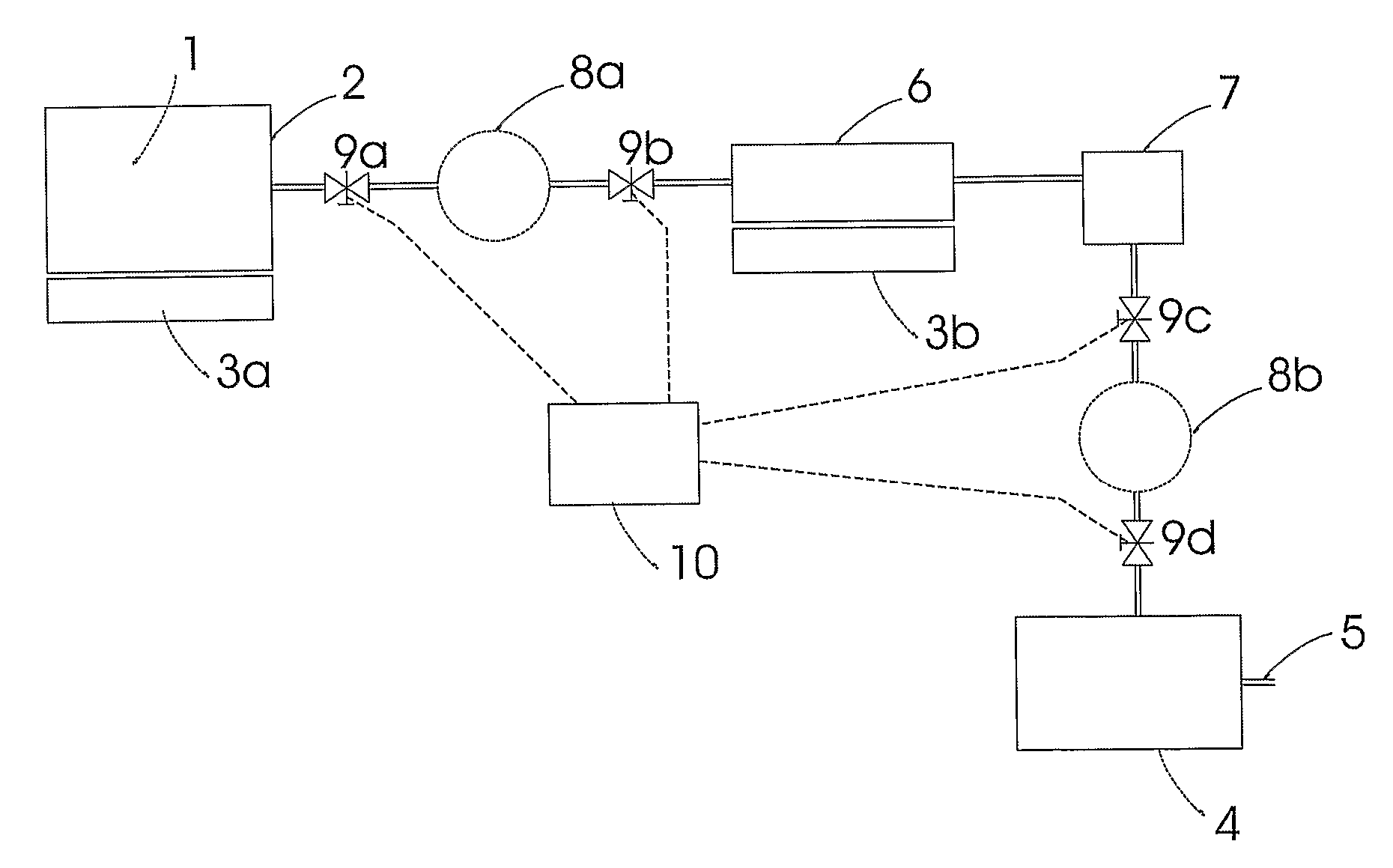
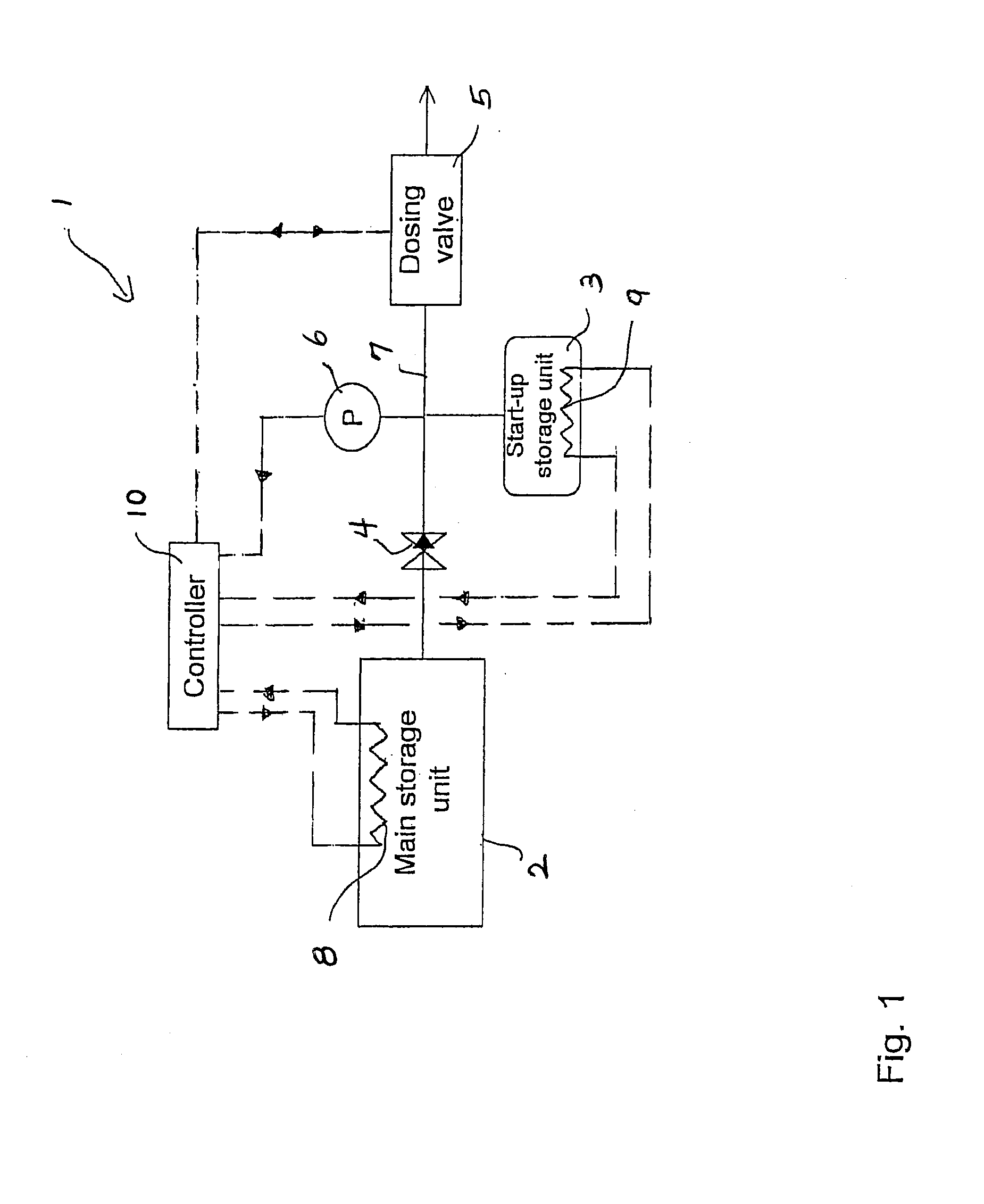
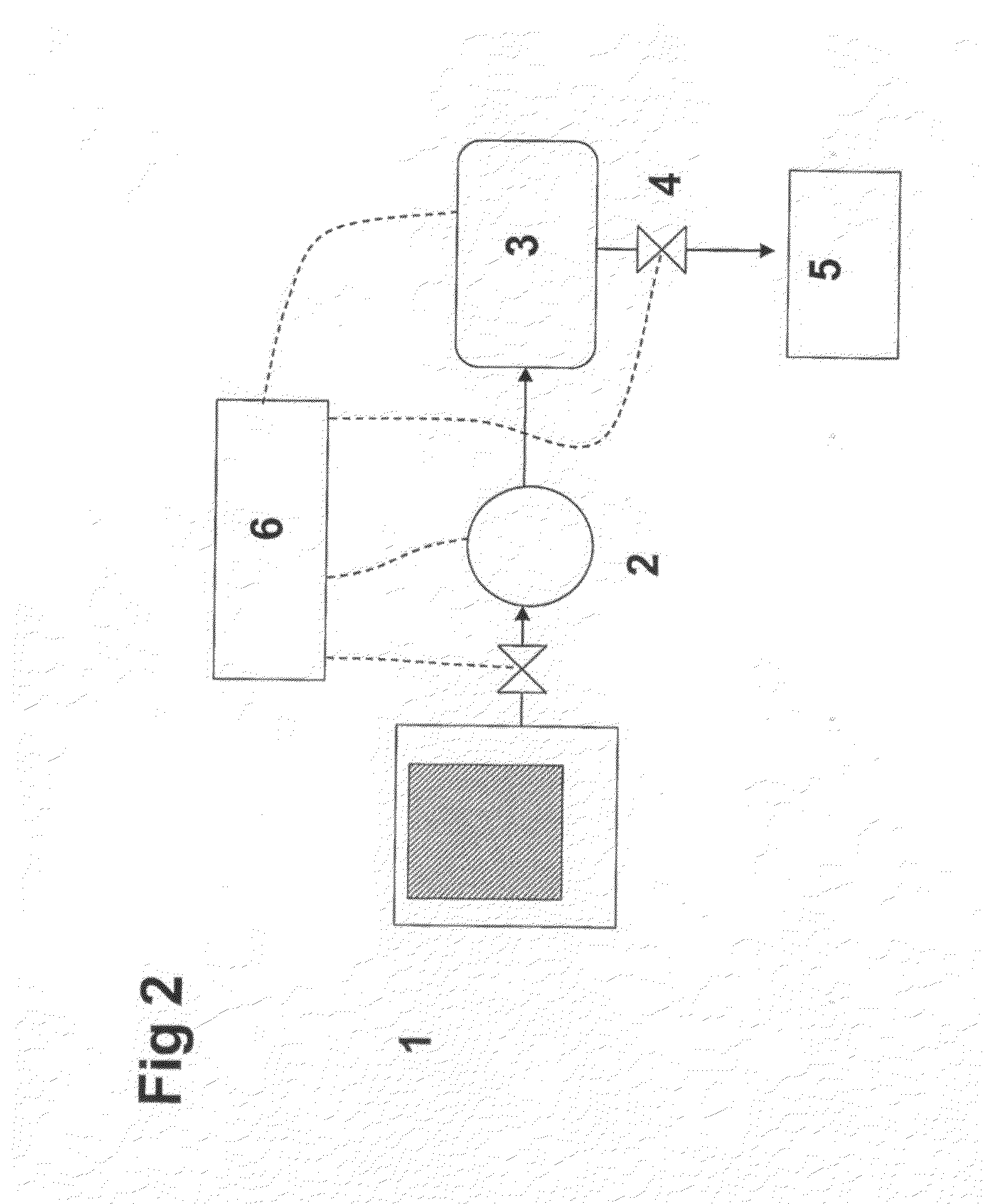
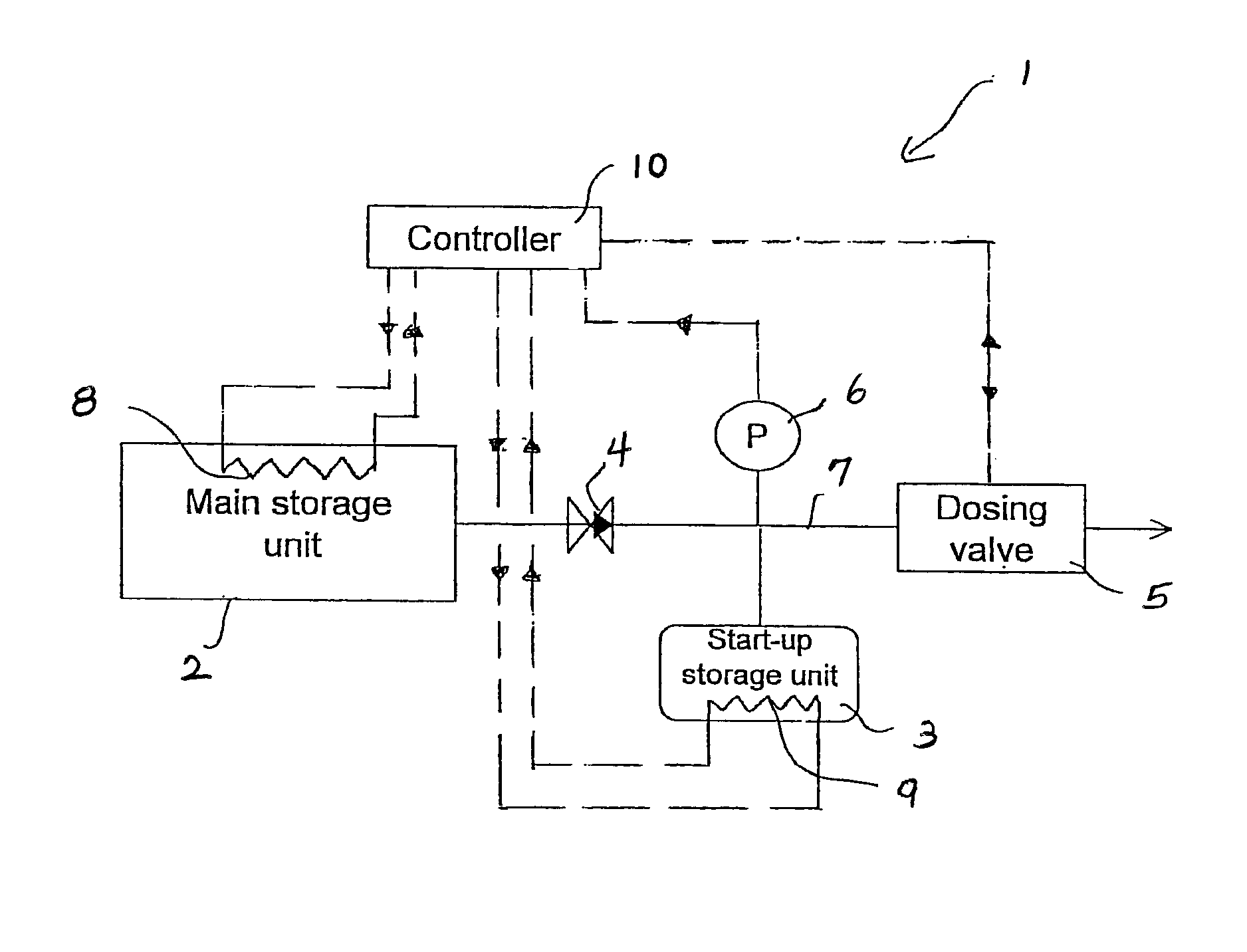
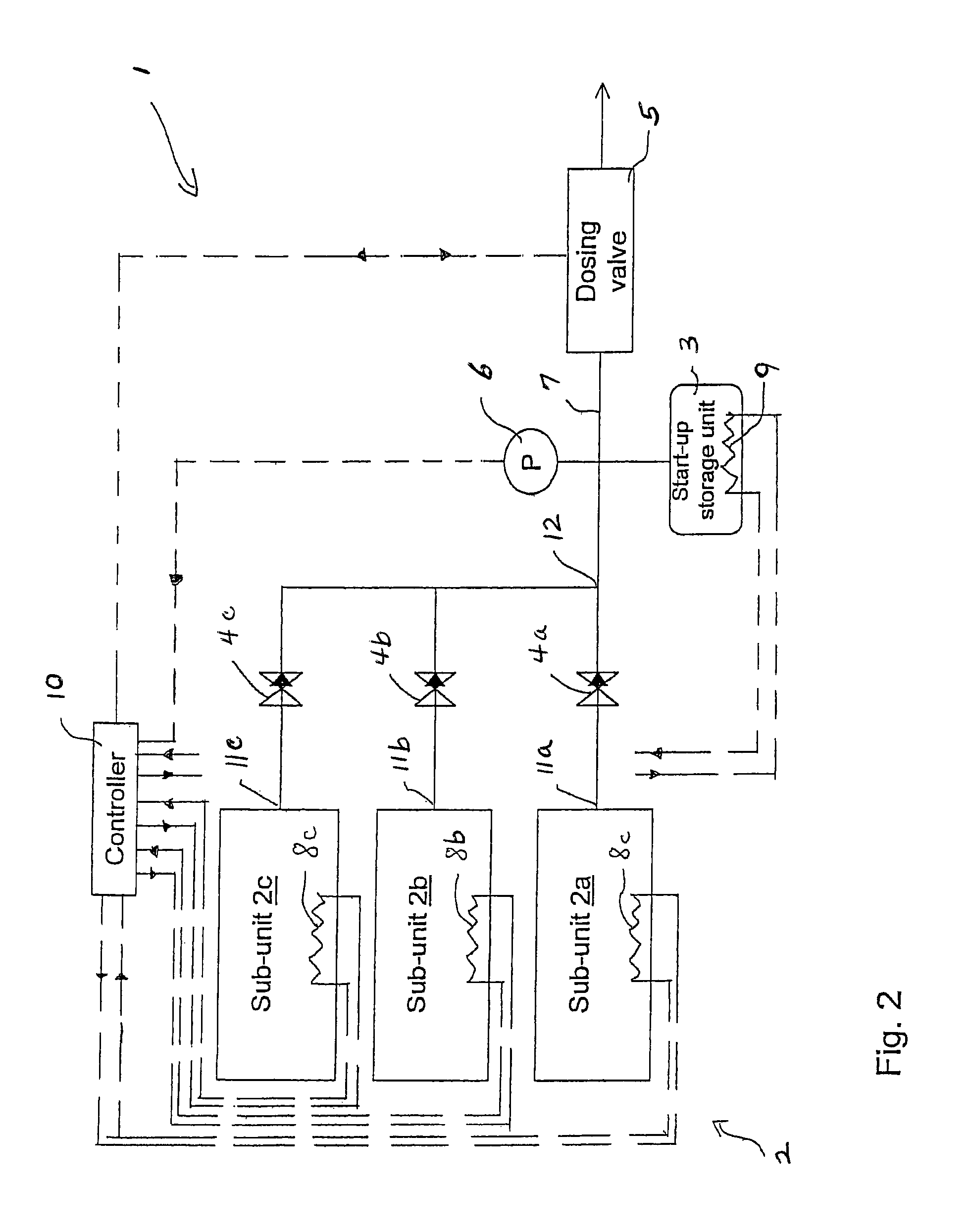
A system for storage and dosing of ammonia, including a solid ammonia storage material capable of binding and releasing ammonia reversibly by adsorption / absorption. The system is able to release ammonia gradually according to a demand that can vary over time with intermediate periods of no ammonia demand. A main storage unit and a start-up storage unit are provided. The storage units hold ammonia storage material. At least one one-way valve is provided via which the one main storage unit is in communication with the start-up storage unit. The one-way valve prevents any back-flow of ammonia from the start-up storage unit to the main storage unit. Heating devices are arranged to heat the main storage unit and the start-up storage unit separately to generate gaseous ammonia by thermal desorption from the solid storage material. A controller controls the heating power of the main storage unit and the start-up storage unit, thereby enabling ammonia release from at least one of the start-up and / or the main storage unit. A dosing valve controls ammonia flow from the storage units according to a demand.
Owner:AMMINEX EMISSIONS TECH
Method and device for safe and controlled delivery of ammonia from a solid ammonia storage medium
InactiveUS20100293927A1Facilitated releaseReduce the temperatureInternal combustion piston enginesExhaust apparatusGas phaseDesorption
Solid metal ammine complexes are applied for safe and high-density storage of ammonia to be released for use as reducing agent in selective catalytic reduction of NOx in exhaust gases. The compositional formula of the metal ammine complexes is M(NH3)nXz, where MZ+ represents one or more metal ions capable of binding ammonia, X represents one or more anions, n is the coordination number (from 2 to 12), and z the valency of the metal ion (and thus the total number of compensating anion charges). Ammonia is released by generating a reduced gas-phase pressure of ammonia inside the container, which is below the equilibrium desorption pressure of ammonia at the operating temperature of the storage material thus enabling the unit to be operated is a safe manner with lower operating temperature and pressure.
Owner:AMMINEX
System and method for delivery of a vapor phase product to a point of use
Provided are a novel system and method for delivery of a vapor phase product to a point of use, as well as a novel on-site chemical distribution system and method. The system for delivery of a vapor phase product includes a storage vessel containing a liquid chemical under its own vapor pressure, a column connected to receive the chemical in liquified state from the storage vessel, wherein the chemical is fractionated into a contaminated liquid heavy fraction and a purified light vapor fraction and a conduit connected to the column for removing the purified light vapor fraction therefrom. The system is connected to the point of use for introducing the purified vapor fraction thereto. Particular applicability is found in semiconductor manufacturing in the delivery of electronic specialty gases to one or more semiconductor processing tools.
Owner:AIR LIQUIDE AMERICA INC
Method and Device for Safe Storage and Use of Volatile Ammonia Storage Materials
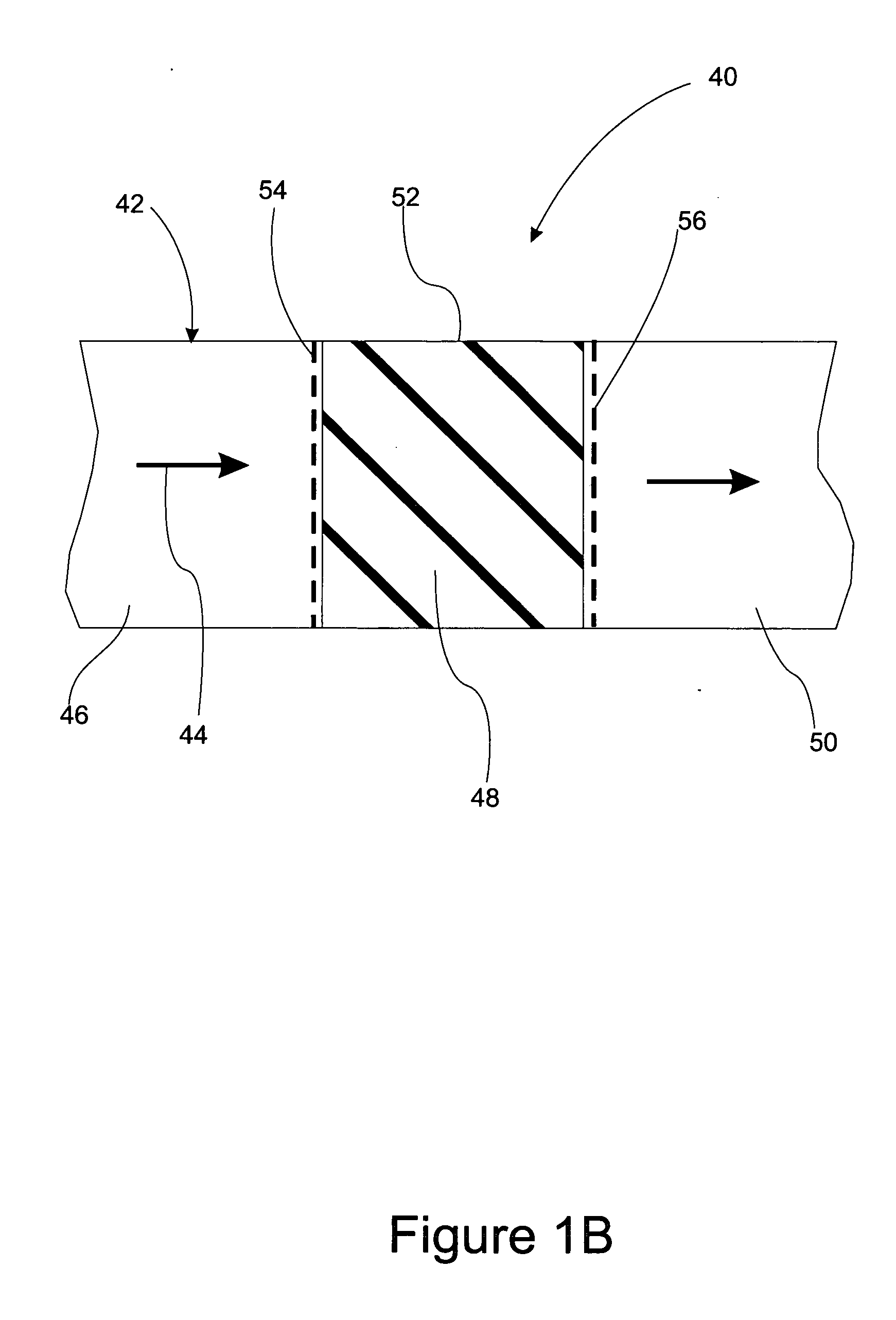
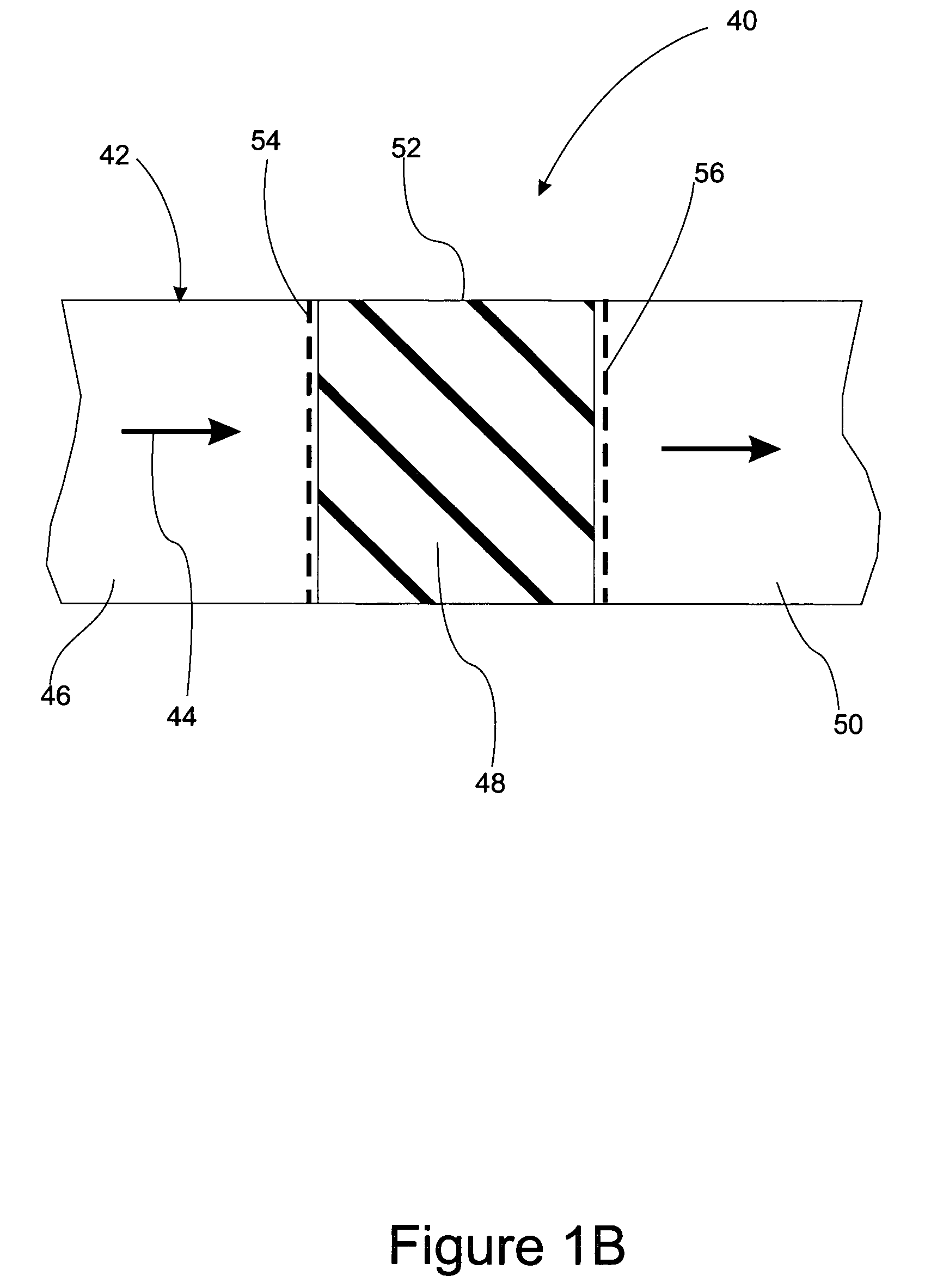
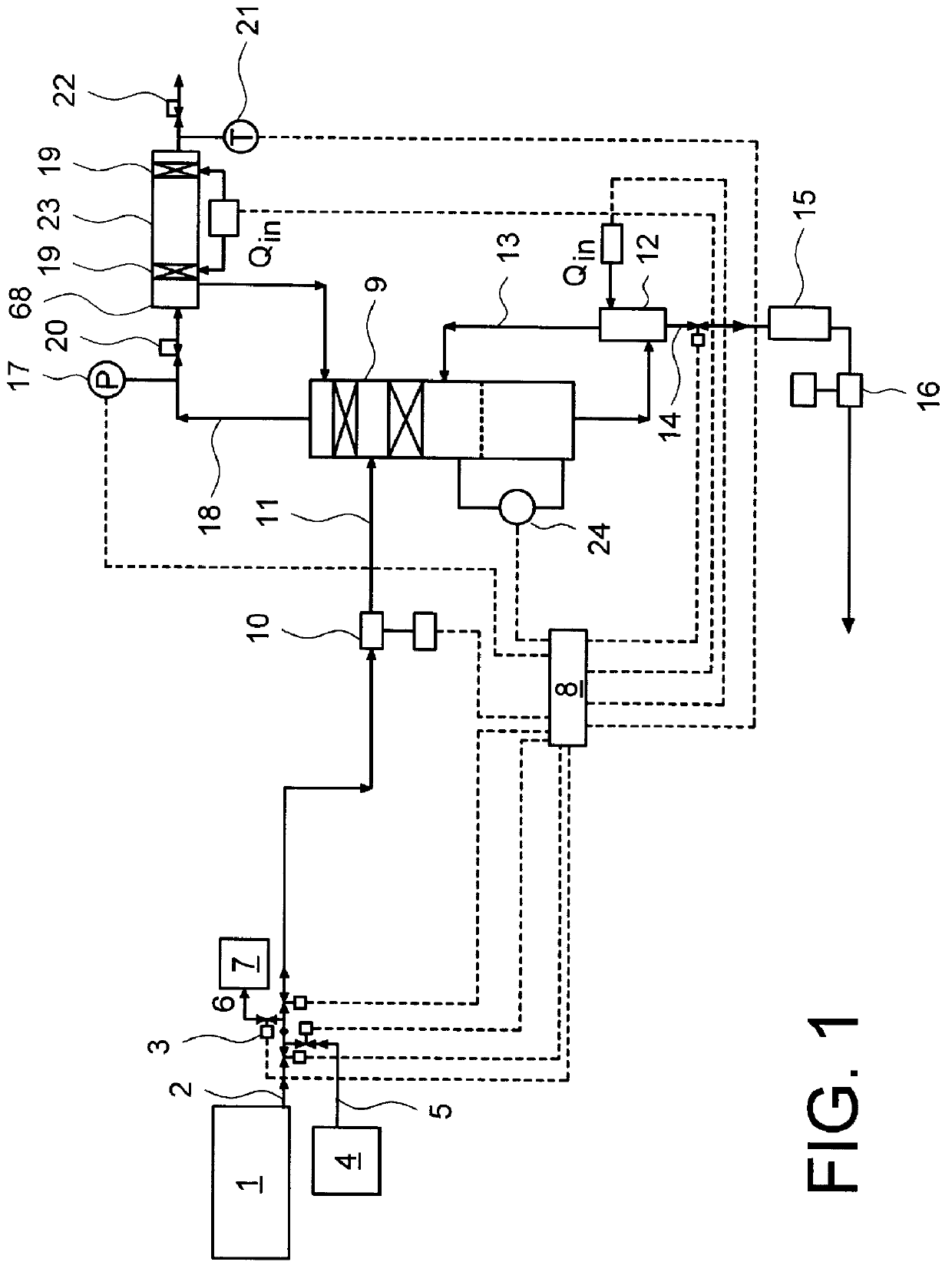
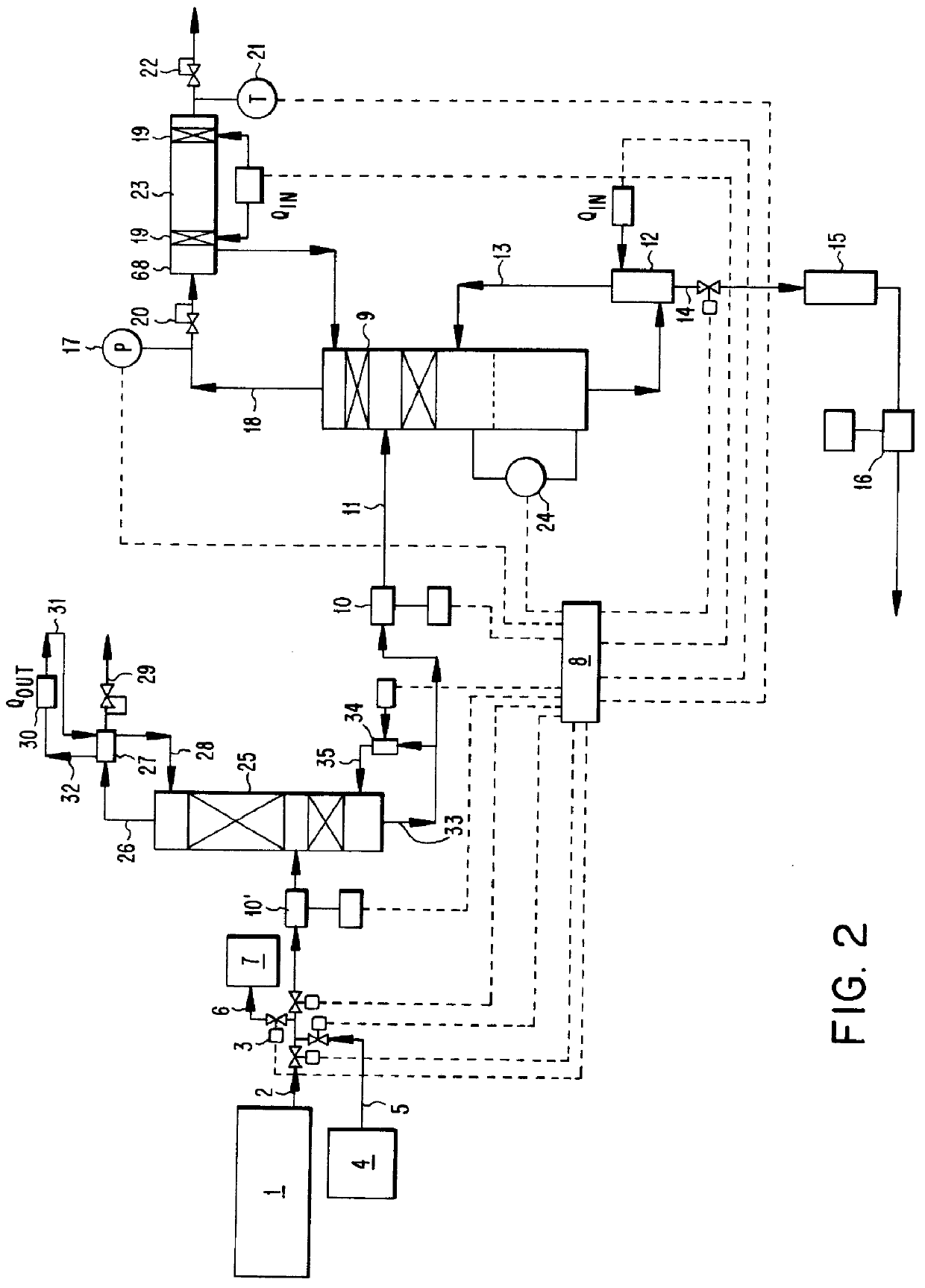
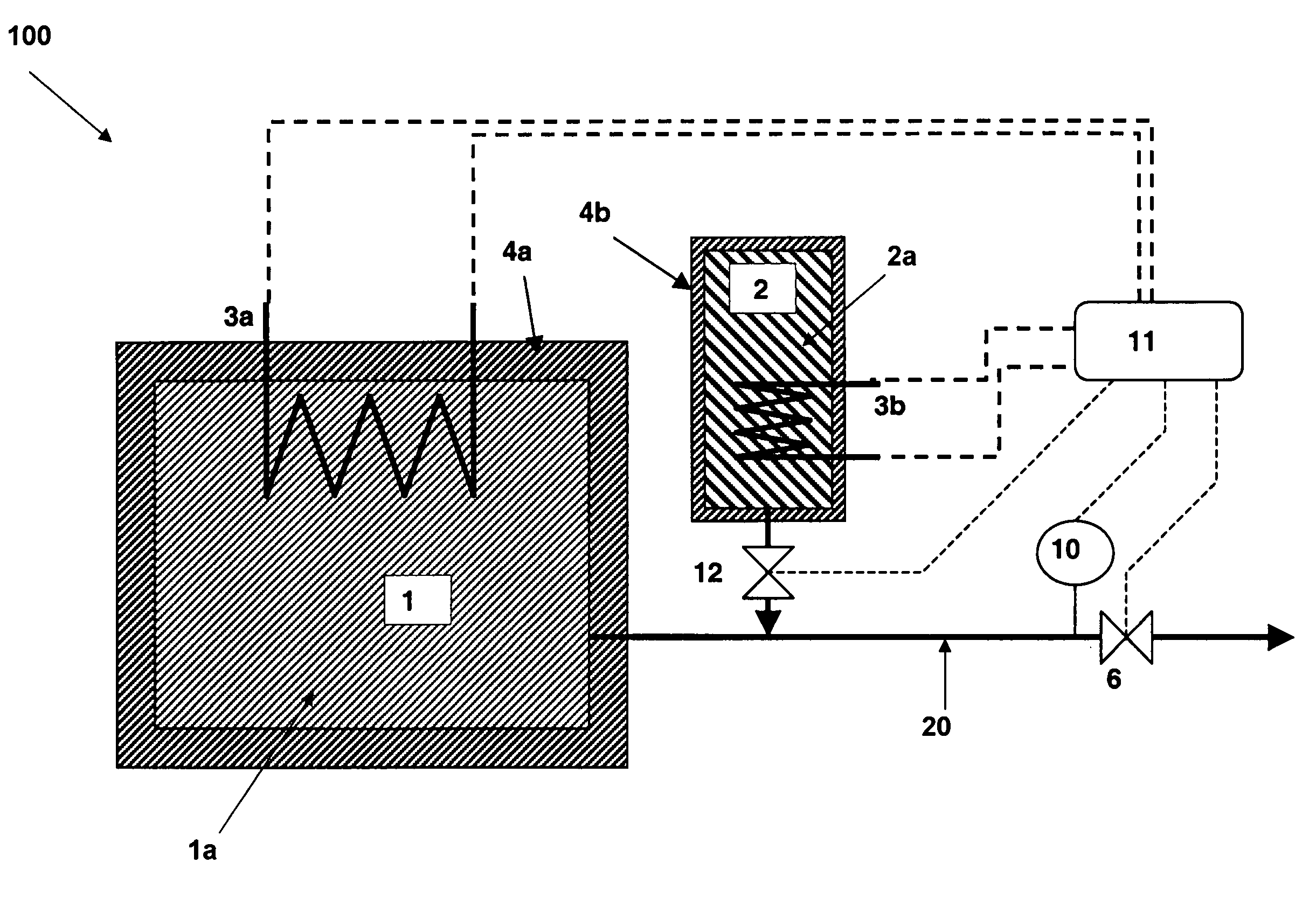
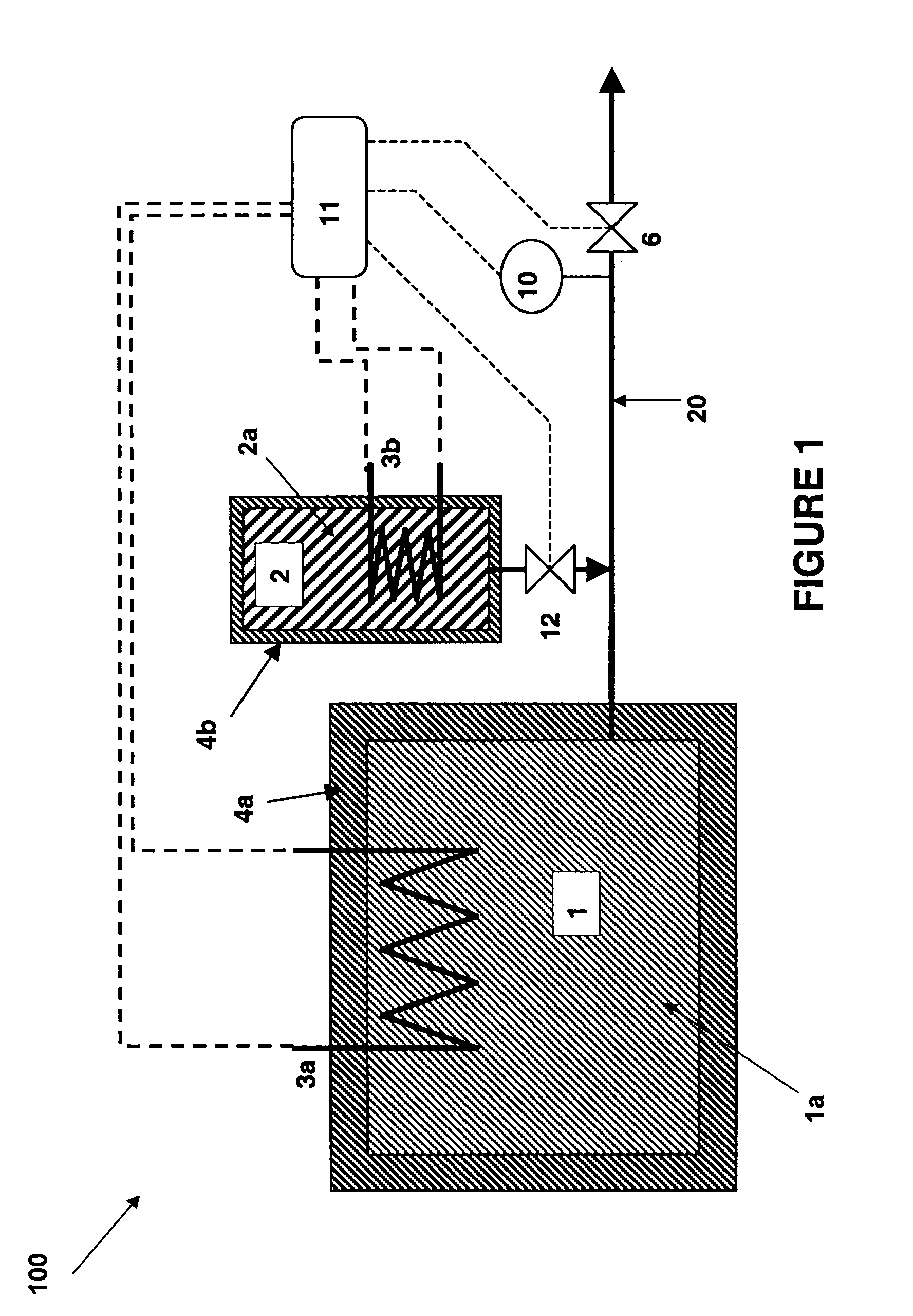
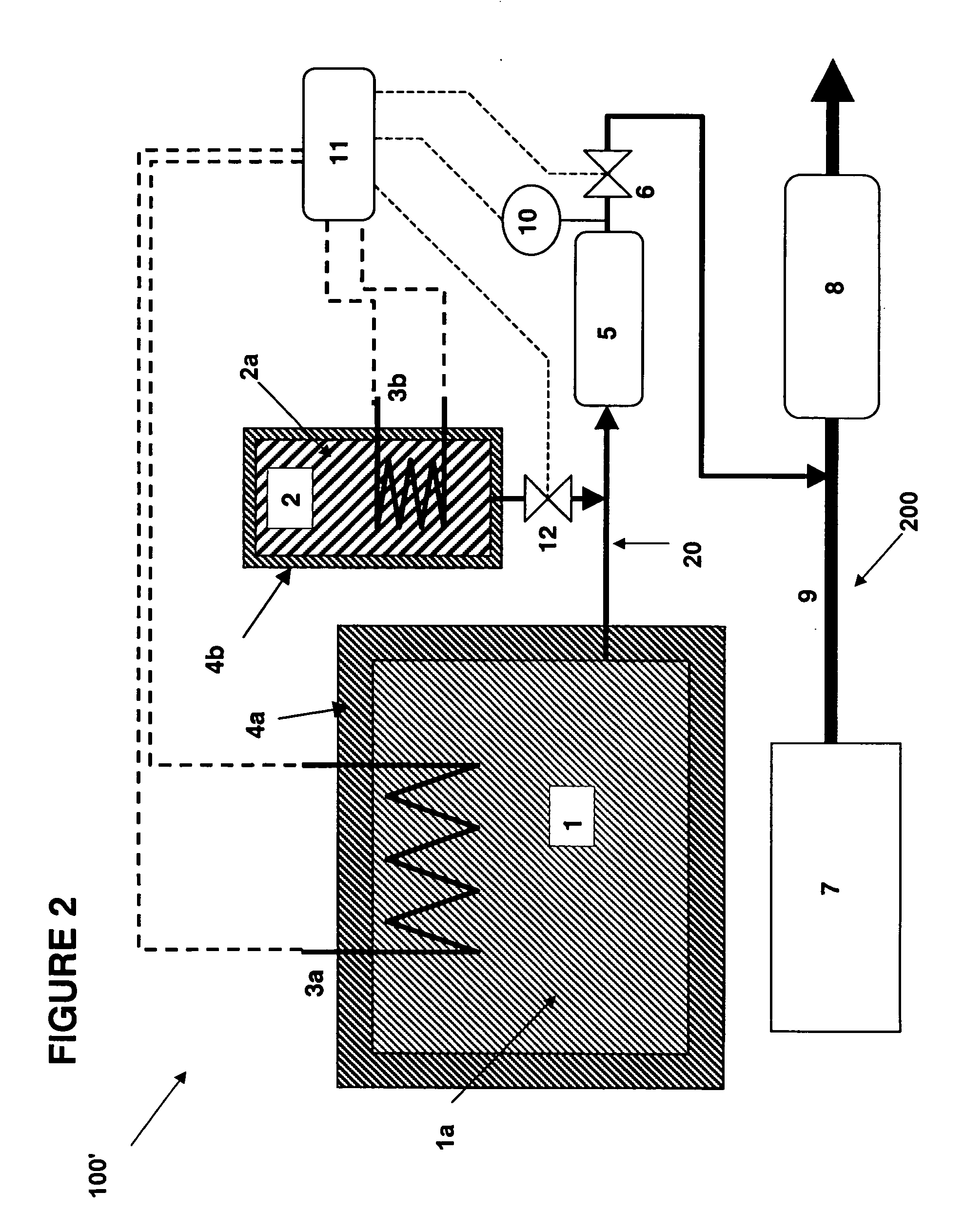
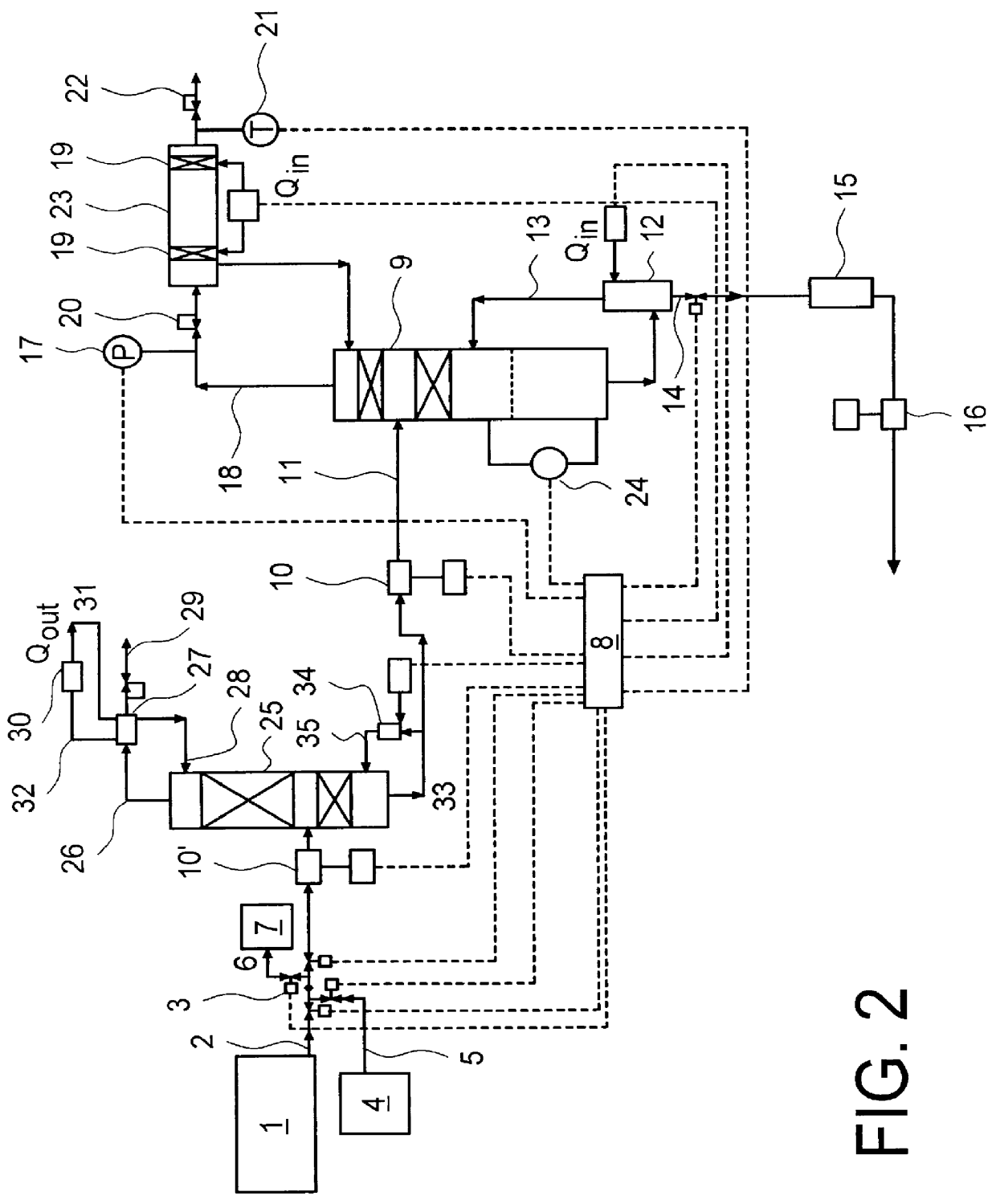
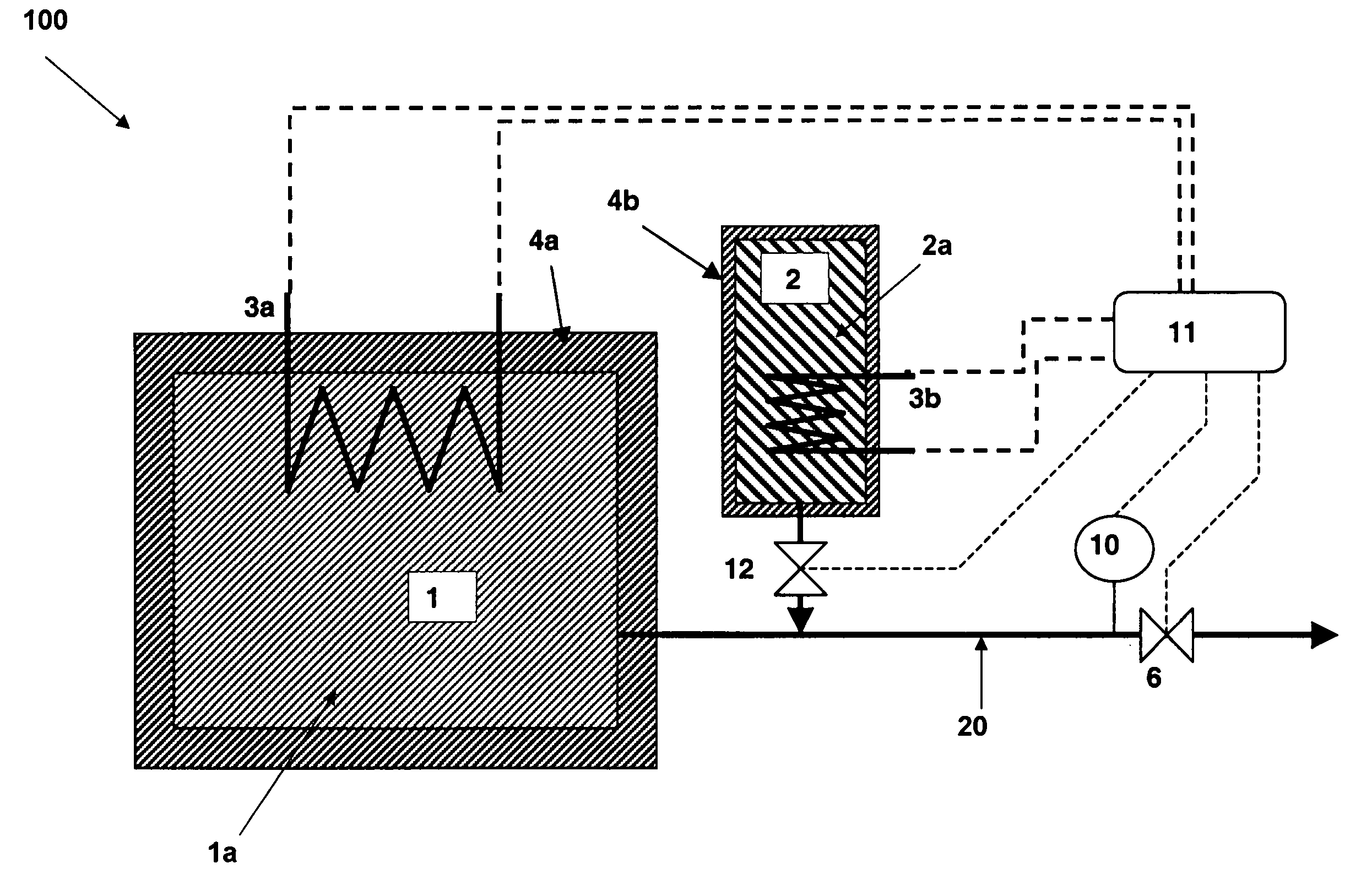
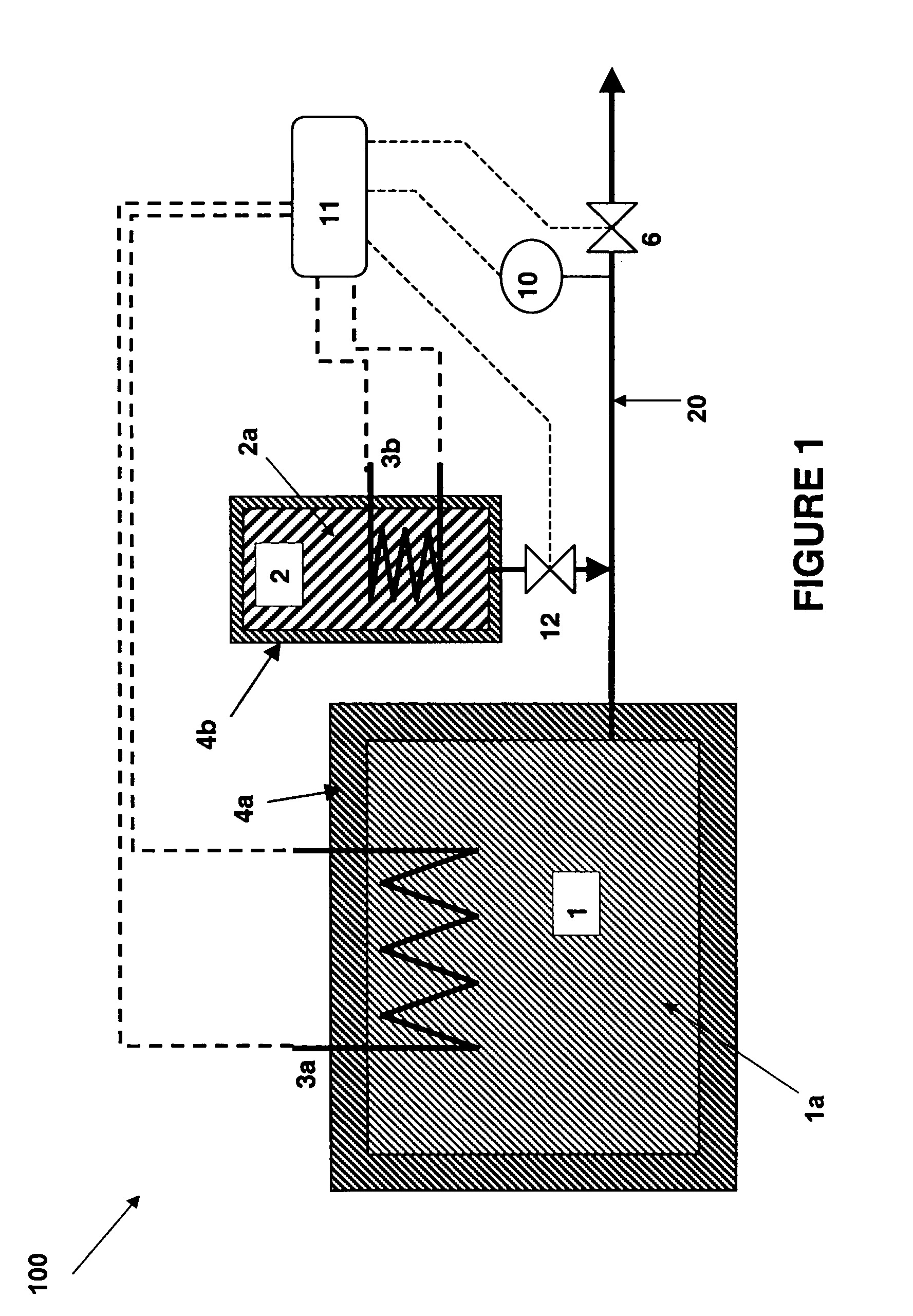
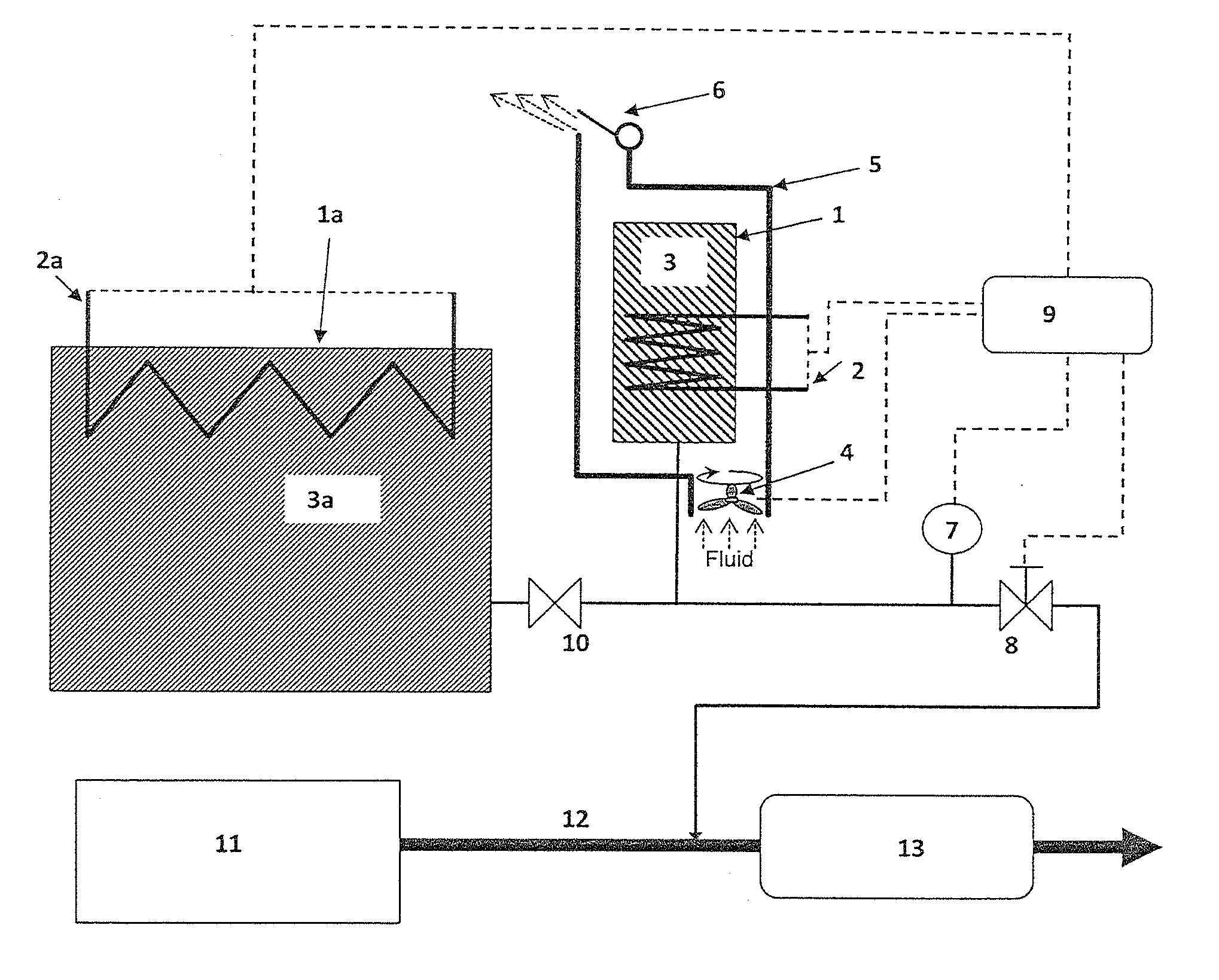
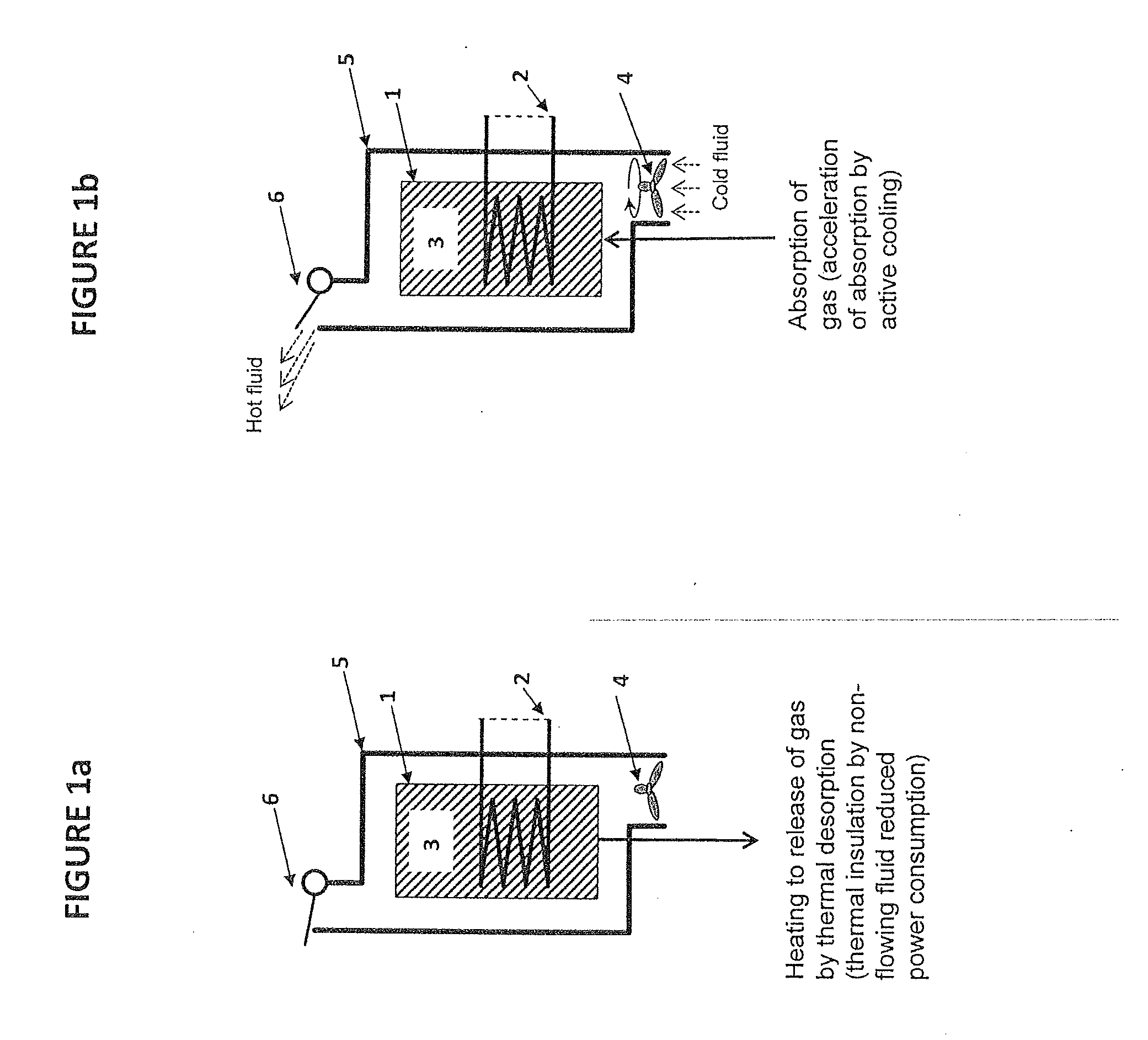
There is described a method and apparatus (100, 100′) for storing and delivering ammonia wherein at least two ammonia storage materials (1a, 2a) capable of reversibly adsorbing or absorbing ammonia having different ammonia vapor pressures are used. Ammonia storage material (2a) having a lower vapor pressure, which is only partially saturated with ammonia or void of ammonia, is brought into fluid communication with ammonia storage material (1a) having a higher ammonia vapor pressure to adsorb or absorb ammonia released from the ammonia storage material (1a) having a higher ammonia vapor pressure when the latter is higher than a pressure threshold. An automotive NOx treatment system (200) comprising such apparatus (100, 100′) is also described.
Owner:AMMINEX
System and method for delivery of a vapor phase product to a point of use
Provided are a novel system and method for delivery of a vapor phase product to a point of use, as well as a novel on-site chemical distribution system and method. The system for delivery of a vapor phase product includes a storage vessel containing a liquid chemical under its own vapor pressure, a column connected to receive the chemical in liquified state from the storage vessel, wherein the chemical is fractionated into a contaminated liquid heavy fraction and a purified light vapor fraction and a conduit connected to the column for removing the purified light vapor fraction therefrom. The system is connected to the point of use for introducing the purified vapor fraction thereto. Particular applicability is found in semiconductor manufacturing in the delivery of electronics specialty gases to one or more semiconductor processing tools.
Owner:AIR LIQUIDE AMERICA INC
Method and device for safe storage and use of volatile ammonia storage materials
There is described a method and apparatus (100, 100′) for storing and delivering ammonia wherein at least two ammonia storage materials (1a, 2a) capable of reversibly adsorbing or absorbing ammonia having different ammonia vapor pressures are used. Ammonia storage material (2a) having a lower vapor pressure, which is only partially saturated with ammonia or void of ammonia, is brought into fluid communication with ammonia storage material (1a) having a higher ammonia vapor pressure to adsorb or absorb ammonia released from the ammonia storage material (1a) having a higher ammonia vapor pressure when the latter is higher than a pressure threshold. An automotive NOx treatment system (200) comprising such apparatus (100, 100′) is also described.
Owner:AMMINEX
Solid ammonia storage and delivery material
InactiveUS20090280047A1Cobalt ammonia complexesNitrogen compoundsAlkaline earth metalAmmonia storage
A solid ammonia storage and delivery material A solid ammonia storage material comprising: an ammonia absorbing salt, wherein the ammonia absorbing salt is an ionic salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkaline earth metals, and / or one or more transition metals, such as Mn, Fe, Co, Ni, Cu, and / or Zn, X is one or more anions, a is the number of cations per salt molecule, z is the number of anions per salt molecule, and ri is the coordination number of 2 to 12, wherein M is Mg provides a safe, light-weight and cheap compact storage for ammonia to be used in the automotive industry.
Owner:AMMINEX EMISSIONS TECH
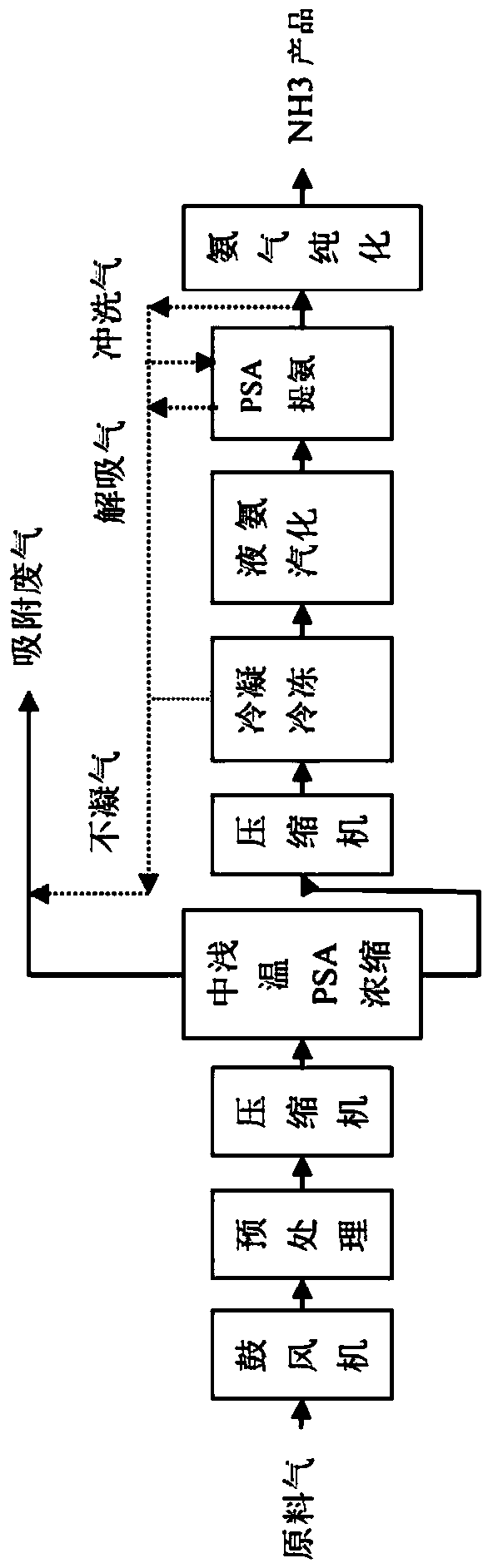
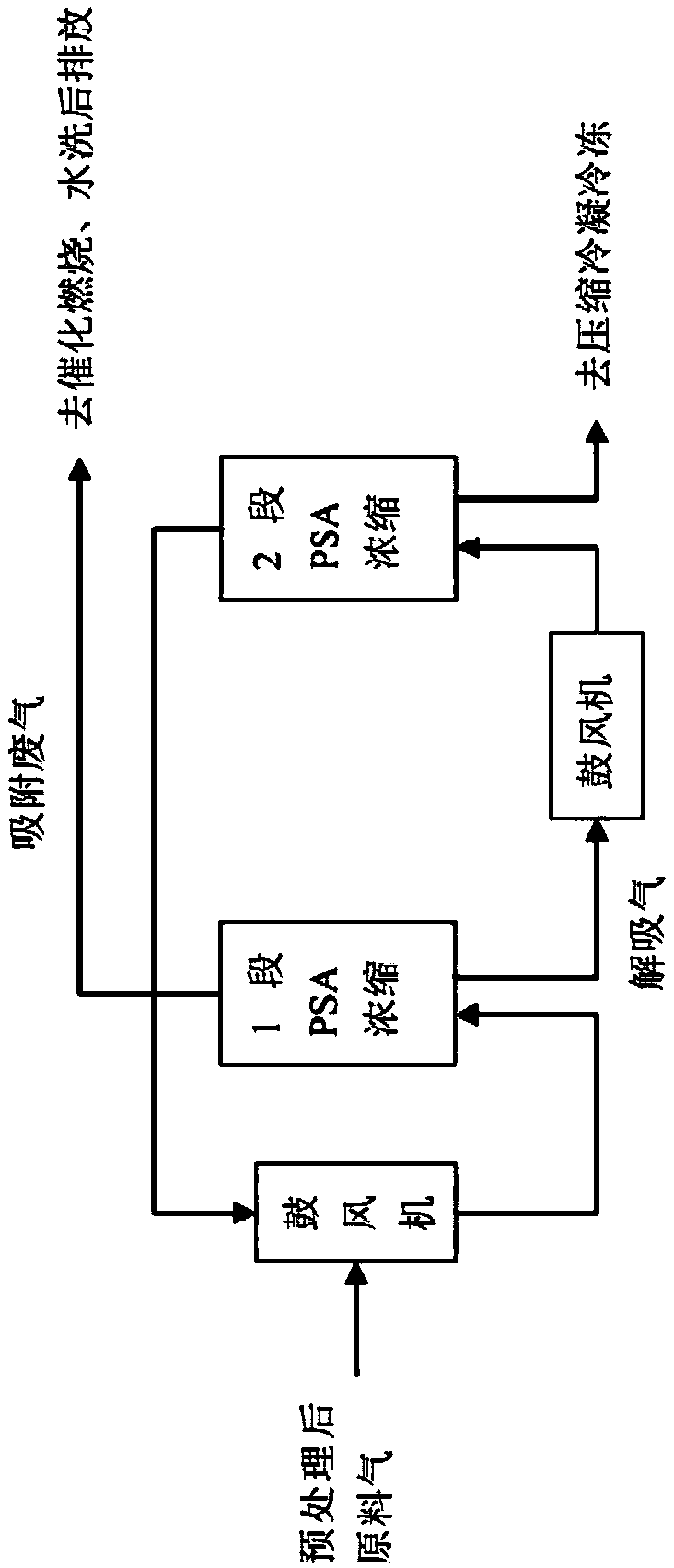
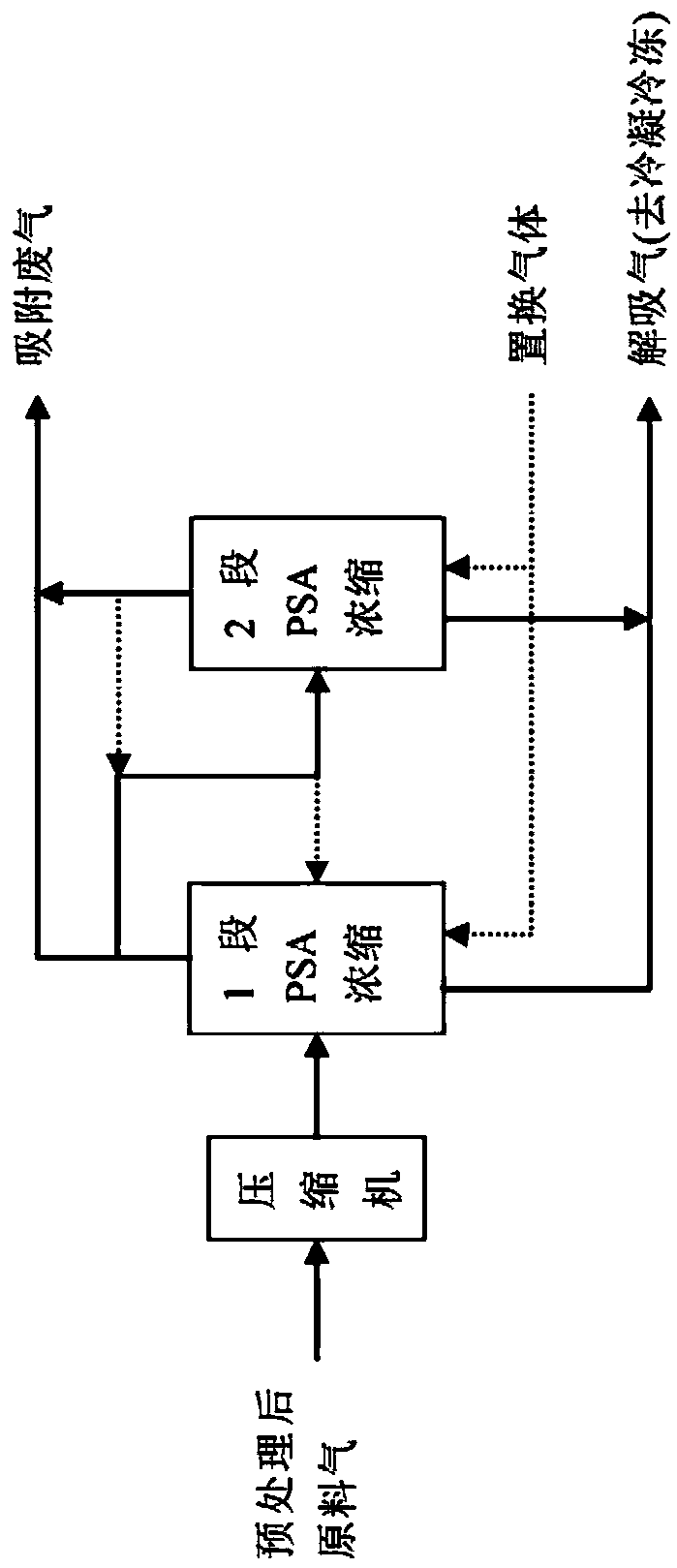
Method for full temperature range PSA (pressure swing adsorption) ammonia purification and recycling of waste gas from LED-MOCVD (metal-organic chemical vapor deposition) process
ActiveCN108744882ARealize the recycling and reuse of all componentsEmission reductionGas treatmentAuxillary pretreatmentVaporizationCircular economy
The invention discloses a method for full temperature range PSA (pressure swing adsorption) ammonia purification and recycling of waste gas from LED-MOCVD (metal-organic chemical vapor deposition) process. By procedure of pretreatment, medium and shallow temperature PSA concentration, condensation and freezing, liquid ammonia vaporization, PSA ammonia extraction and ammonia purification, the ammonia-containing waste gas from the LED-MOCVD process is purified to conform to the standard of electronic grade ammonia gas required by the LED-MOCVD process, and the waste gas is recycled; the ammoniagas yield is higher than or equal to 70%-85%. The technical problem that atmospheric or low pressure ammonia-containing waste gas from the LED-MOCVD process cannot be returned to the LED-MOCVD processfor use is solved, and the gap in green LED industry and circular economy development is filled in.
Owner:ZHEJIANG TIANCAIYUNJI TECH CO LTD
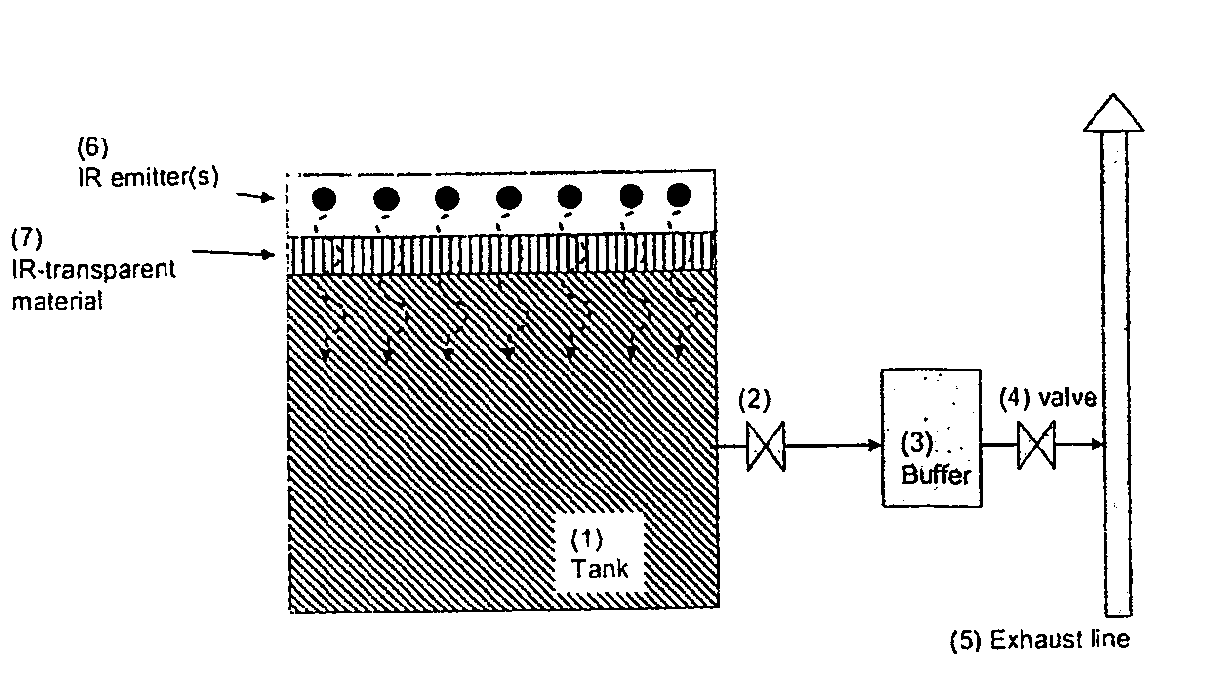
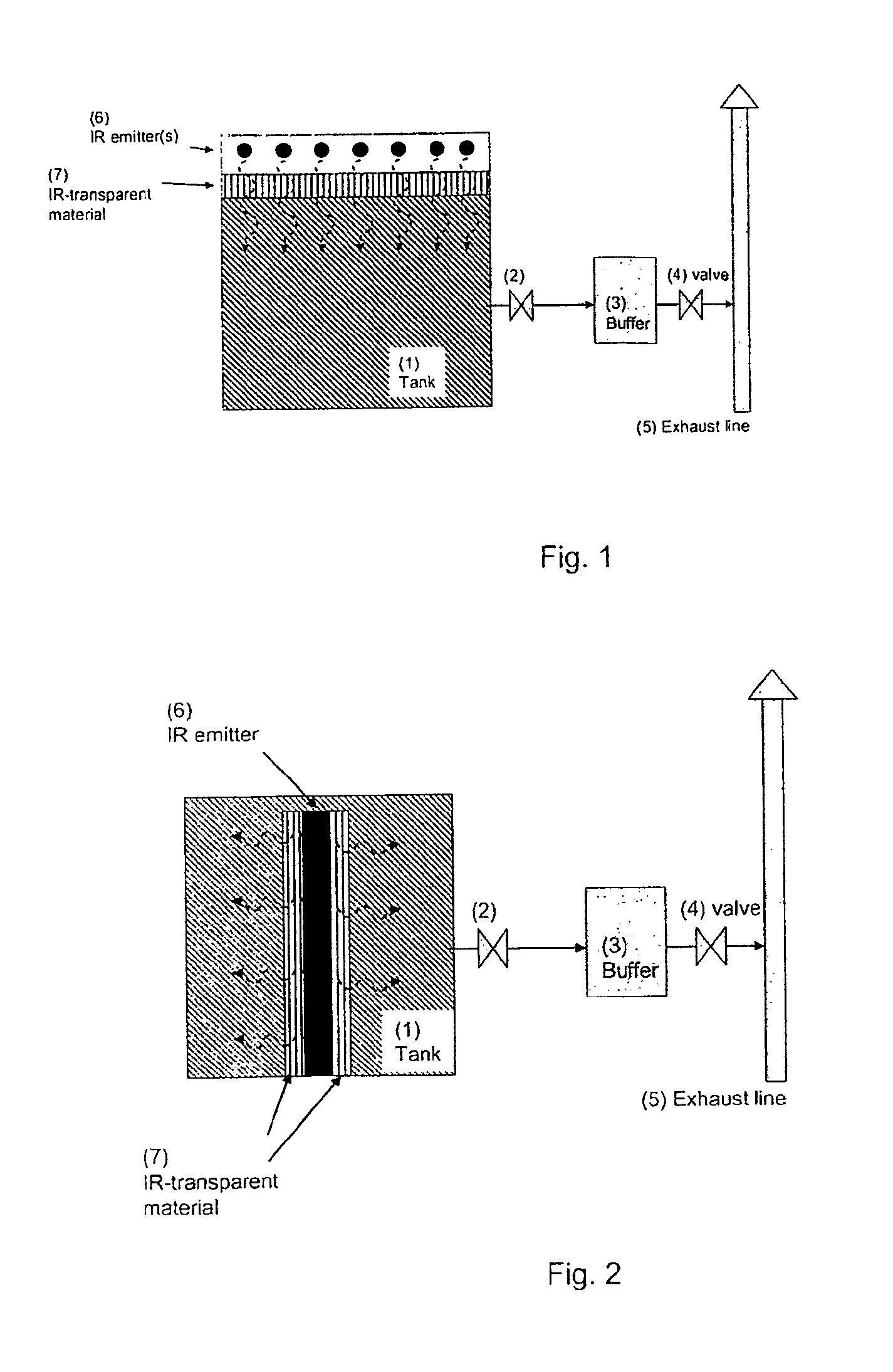
Method of Storing and Delivering Ammonia and the Use of Electromagnetic Radiation for Desorption of Ammonia from a Chemical Complex
InactiveUS20090313976A1High NOx ConversionMinimizing ammonia slipNitrous oxide captureCombination devicesDesorptionBiological activation
A method of storing and delivering ammonia and the use of electromagnetic radiation for desorption of ammonia from a chemical complex. Solid metal ammine complexes are applied for safe and high-density storage of ammonia to be released for use as reducing agent in selective catalytic reduction of NOx in exhaust gases. The compositional formula of the metal ammine complexes is M(NH3)nXz, where M2+ represents one or more metal ions capable of binding ammonia, X represents one or more anions, n is the coordination number (from 2 to 12), and z the valency of the metal ion (and thus the total number of compensating anion charges). Ammonia is released non-thermally by photon-activation using electromagnetic irradiation of the complex bond between ammonia coordinated to the metal ion
Owner:AMMINEX EMISSIONS TECH
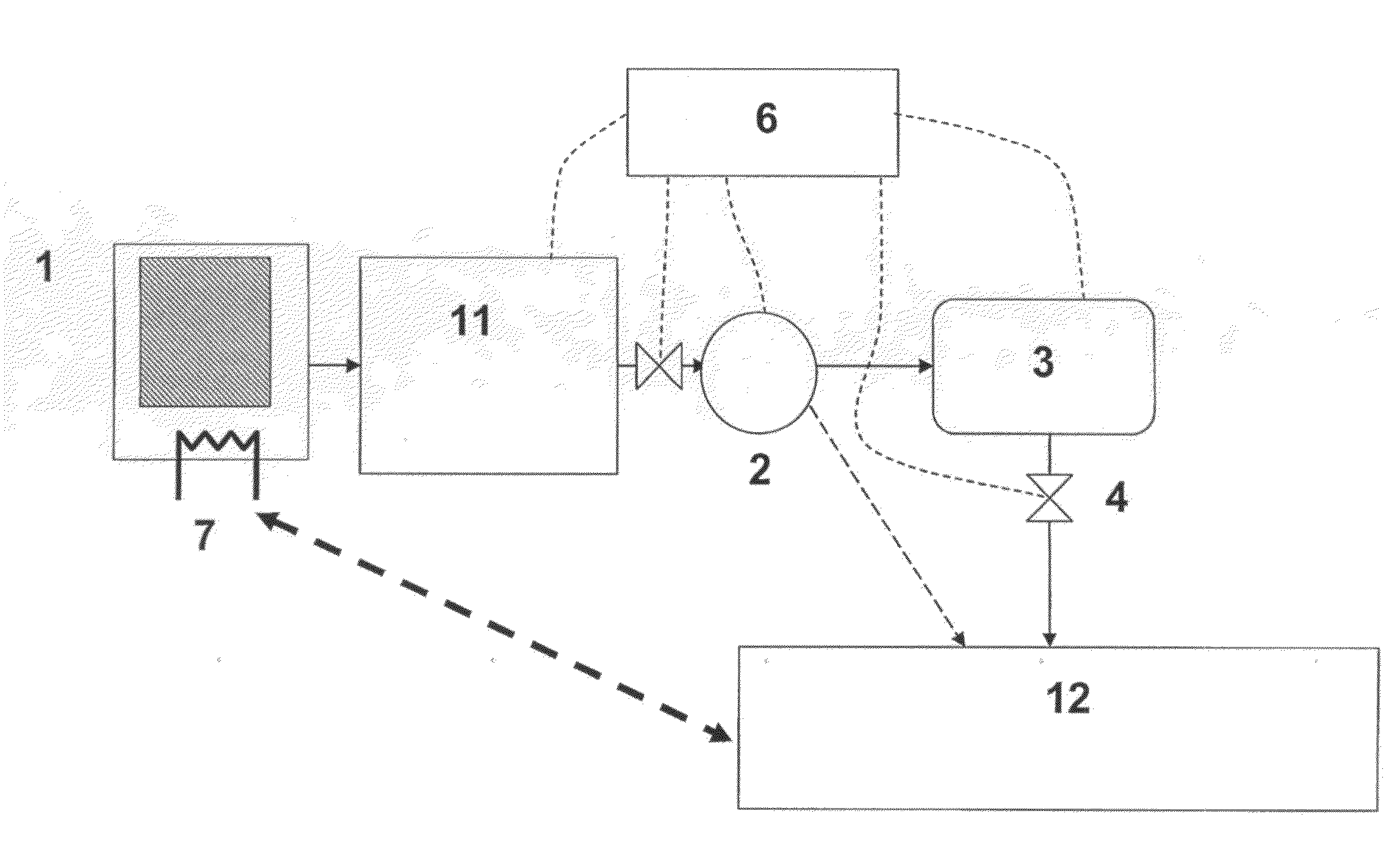
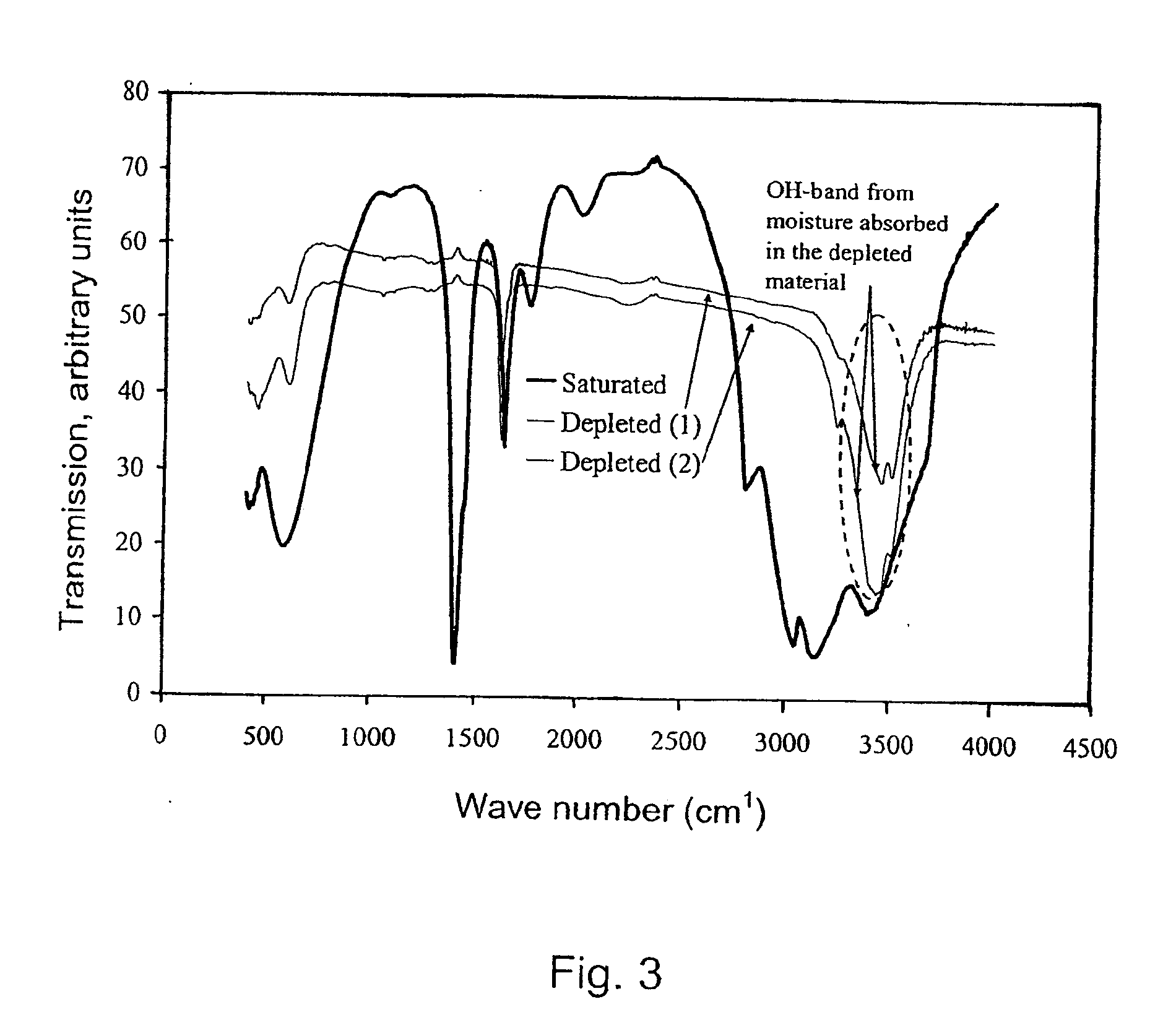
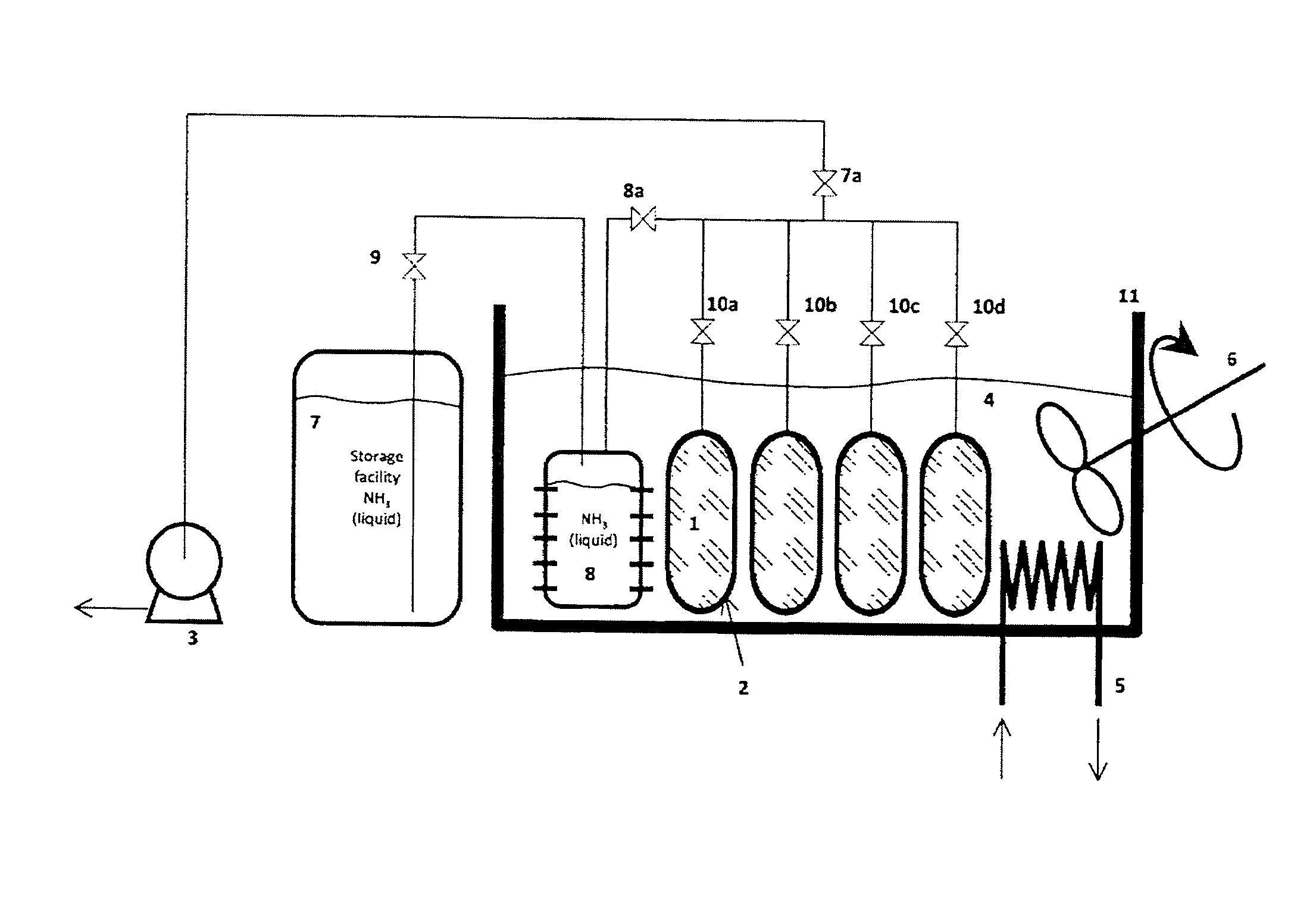
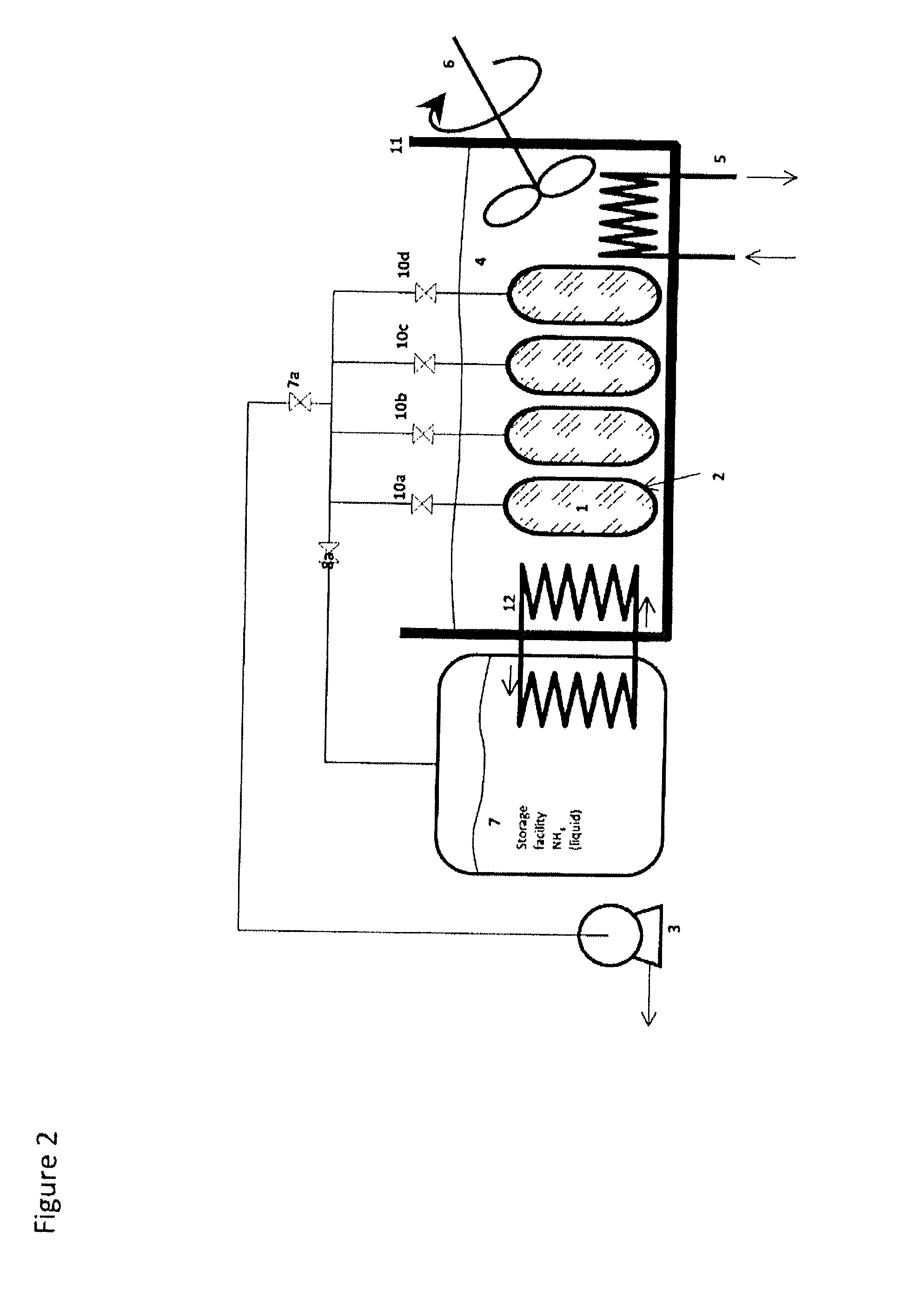
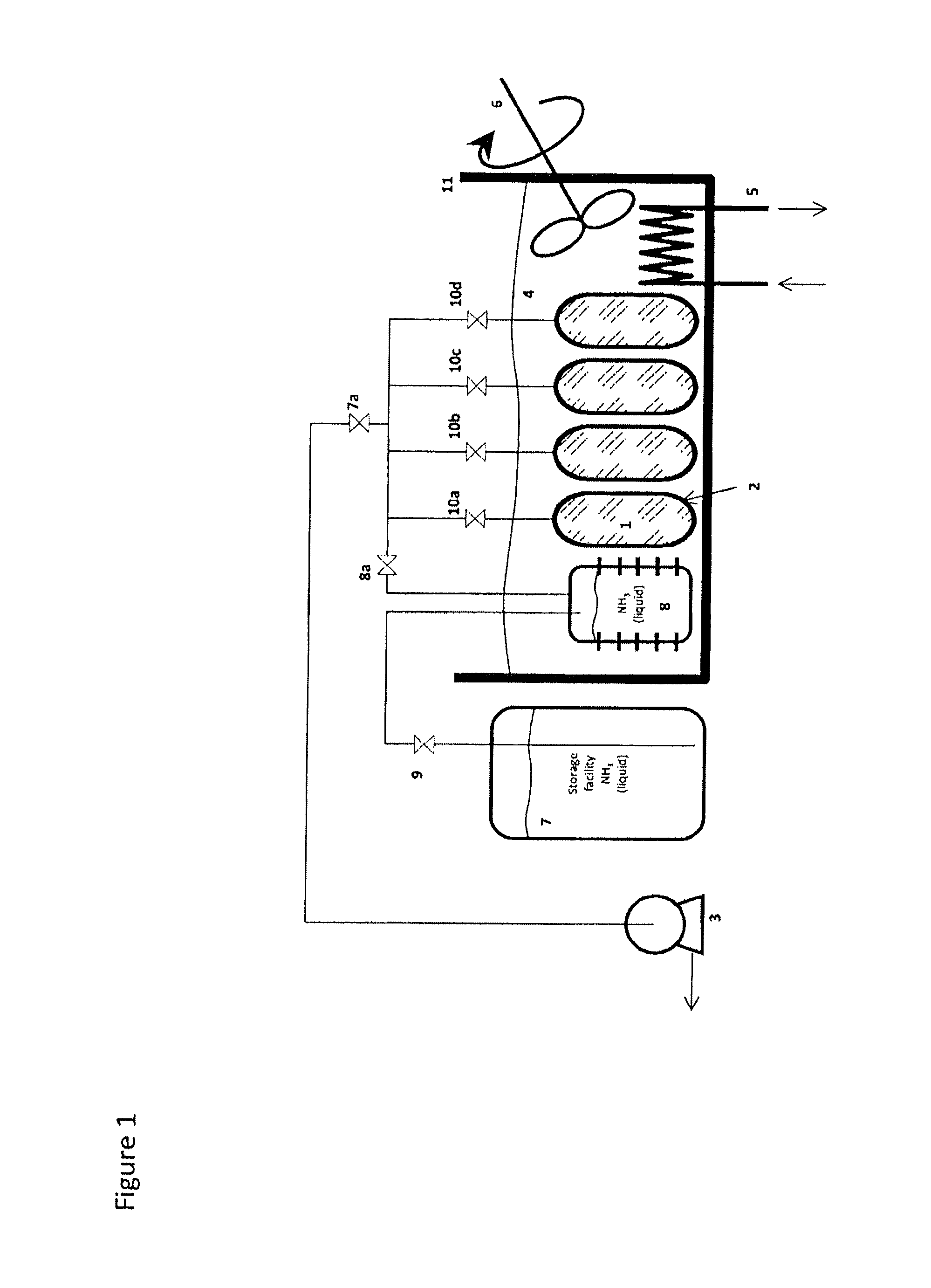
Method for Saturating and Re-Saturating Ammonia Storage Material in Containers
A method for saturating or re-saturating ammonia storage material (1) capable of reversibly absorbing and desorbing ammonia in one or more storage containers (2), wherein said material is partly or fully depleted of ammonia, with ammonia to a predetermined saturation degree comprises a. placing the storage container(s) (2) in direct or indirect contact with a thermostatting medium (4) at a temperature level TT≦ about 65° C.; and b. connecting the storage container(s) (2) to a source of gaseous ammonia wherein at least during a part of the method the gaseous ammonia during saturating or re-saturation of the ammonia storage material (1) is at a pressure PS≦ about PT, wherein PS is the ammonia pressure during saturating or re-saturating of the ammonia storage material (1) and PT is the equilibrium vapor pressure of liquid ammonia at the temperature level TT.
Owner:AMMINEX EMISSIONS TECH
Method for saturating and re-saturating ammonia storage material in containers
A method for saturating or re-saturating ammonia storage material (1) capable of reversibly absorbing and desorbing ammonia in one or more storage containers (2), wherein said material is partly or fully depleted of ammonia, with ammonia to a predetermined saturation degree comprises a. placing the storage container(s) (2) in direct or indirect contact with a thermostatting medium (4) at a temperature level TT≦ about 65° C.; and b. connecting the storage container(s) (2) to a source of gaseous ammonia wherein at least during a part of the method the gaseous ammonia during saturating or re-saturation of the ammonia storage material (1) is at a pressure PS≦ about PT, wherein PS is the ammonia pressure during saturating or re-saturating of the ammonia storage material (1) and PT is the equilibrium vapor pressure of liquid ammonia at the temperature level TT.
Owner:AMMINEX EMISSIONS TECH
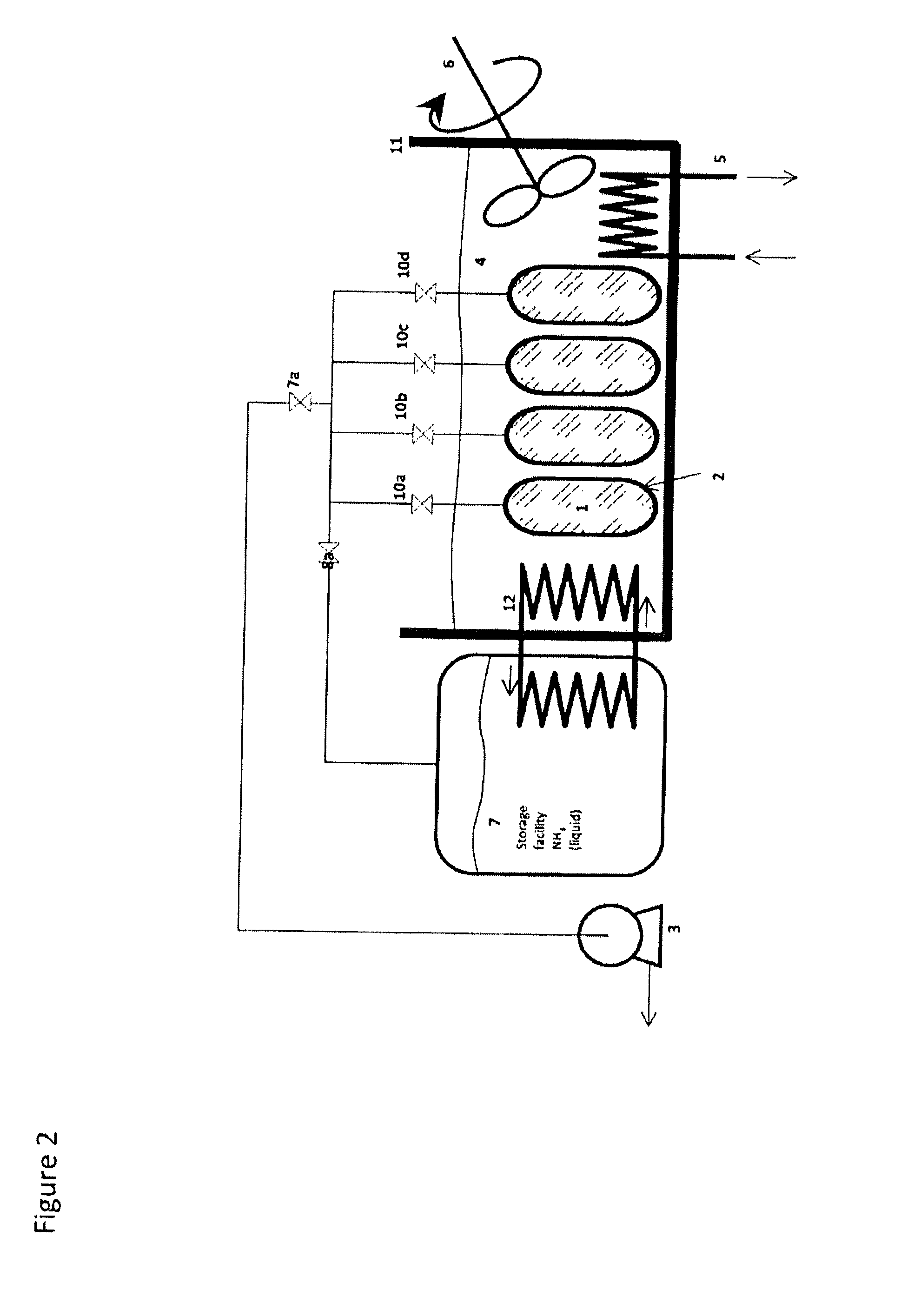
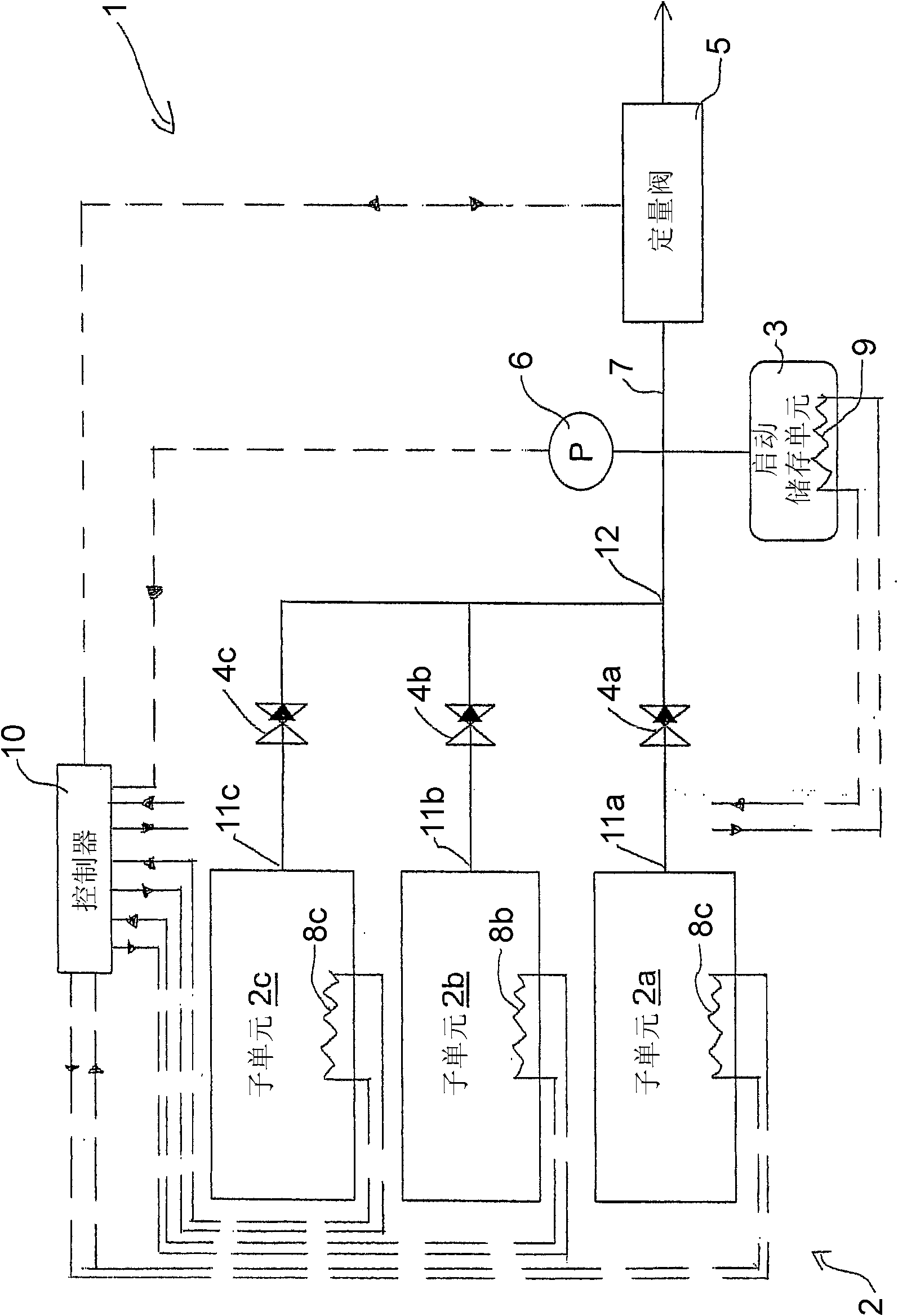
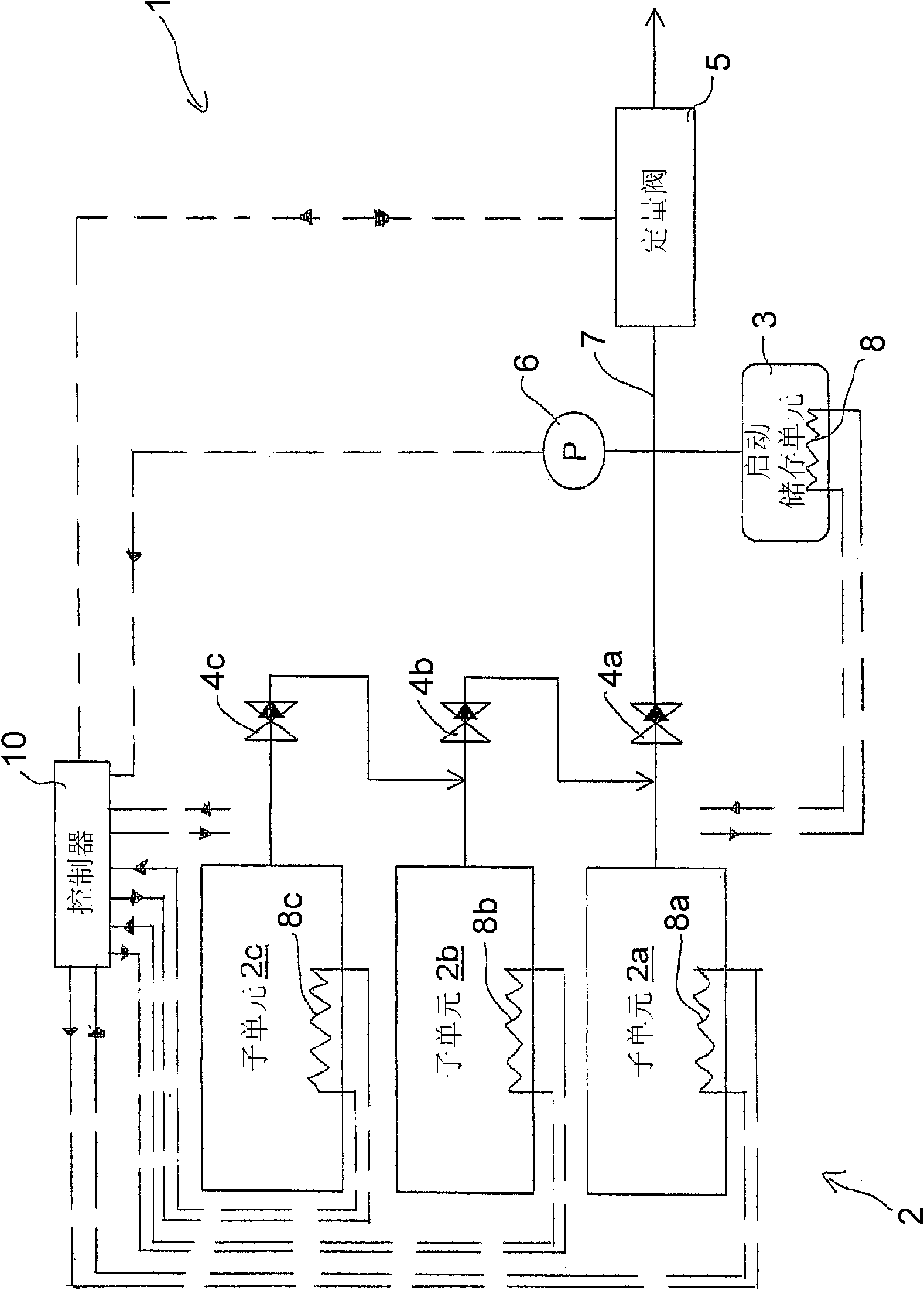
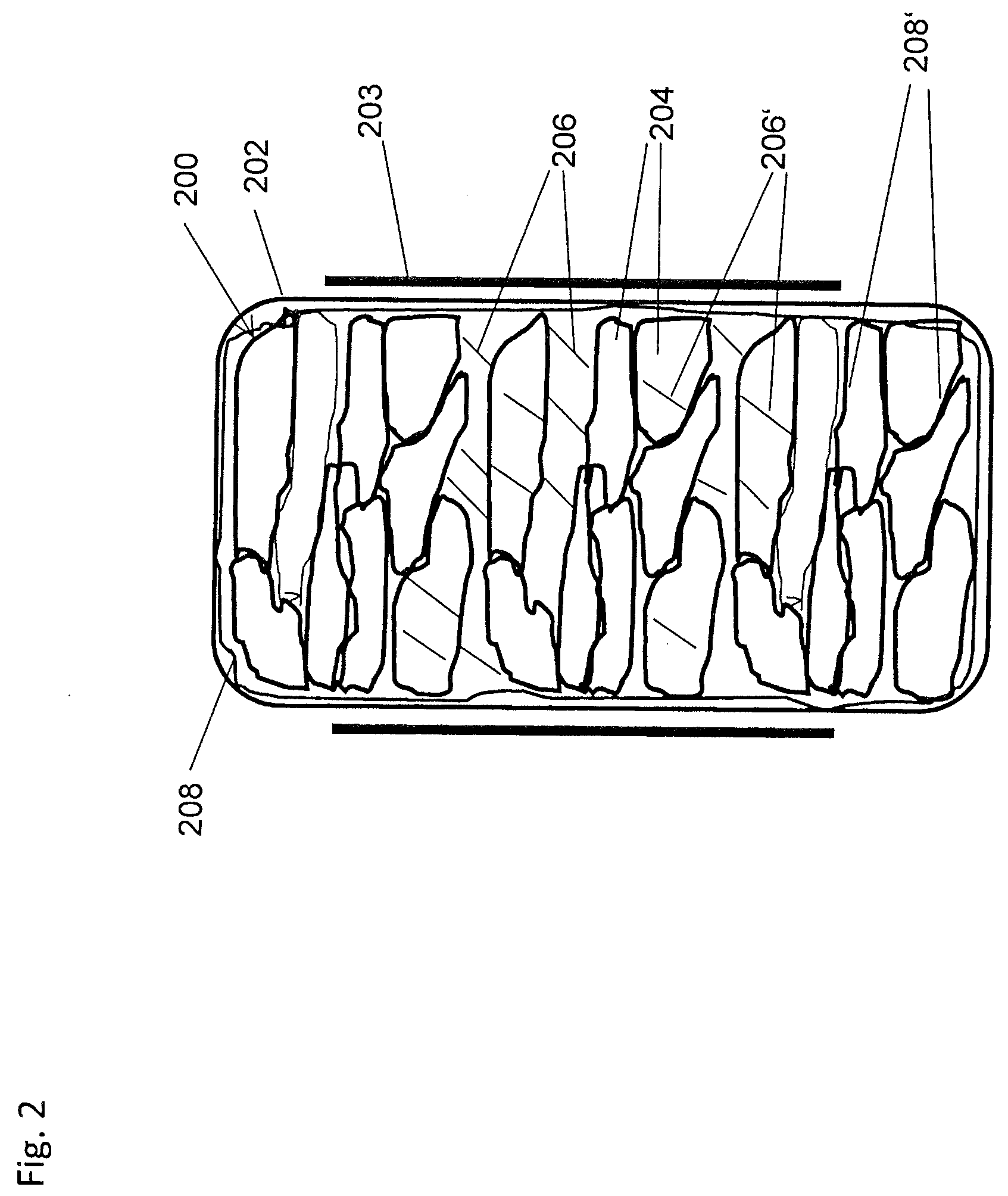
Ammonia Storage Cartridge With Optimized Filling Time, in Particular for a Motor Vehicle Gas Exhaust system
ActiveCN104234796AShorten filling timeExhaust apparatusSilencing apparatusMobile vehicleAmmonia storage
An ammonia storage cartridge includes_an ammonia storage member having a storage material capable of absorbing or adsorbing ammonia. The storage member extends along a longitudinal axis. A heating element heats the storage member, and a hermetic tank houses the storage member. A tubular ammonia circulation element is arranged coaxially to the storage member, and includes_a first surface at least partially delimiting, with an element chosen from among the heating element and the hermetic tank, a circulation duct for the fluid ammonia. A second surface is arranged at least partially in contact with the storage member, and-at least one orifice passes radially through, allowing the circulation of fluid between the circulation duct and the storage member.
Owner:FAURECIA SYST DECHAPPEMENT
Release of stored ammonia at start-up
A system for storage and dosing of ammonia, includes a solid ammonia storage material capable of binding and releasing ammonia reversibly by adsorption / absorption. The system is able to release ammonia gradually according to a demand that can vary over time with intermediate periods of no ammonia demand. A main storage unit and a start-up storage unit are provided. The storage units hold ammonia storage material. At least one one-way valve is provided via which the one main storage unit is in communication with the start-up storage unit. The one-way valve prevents any back-flow of ammonia from the start-up storage unit to the main storage unit. Heating devices are arranged to heat the main storage unit and the startup storage unit separately to generate gaseous ammonia by thermal desorption from the solid storage material. A controller controls the heating power of the main storage unit and the start-up storage unit, thereby enabling ammonia release from the start-up and / or the main storage units. A dosing valve controls ammonia flow from the storage units according to a demand.
Owner:AMMINEX
Ammonia supply device, ammonia supply method and exhaust gas purification system
An ammonia supply device includes an ammonia absorber, a conductive element, a mixture, a tank and an electrode. The ammonia absorber is in powder or granular form. Ammonia is stored in the ammonia absorber and released from the ammonia absorber. The conductive element in paste or liquid form has a conductive property and a nonreactive property with ammonia. The mixture is made by mixing the ammonia absorber and the conductive element. The tank holds the mixture. The electrode includes a pair of first and second electrode elements for applying voltage to the mixture.
Owner:TOYOTA IND CORP
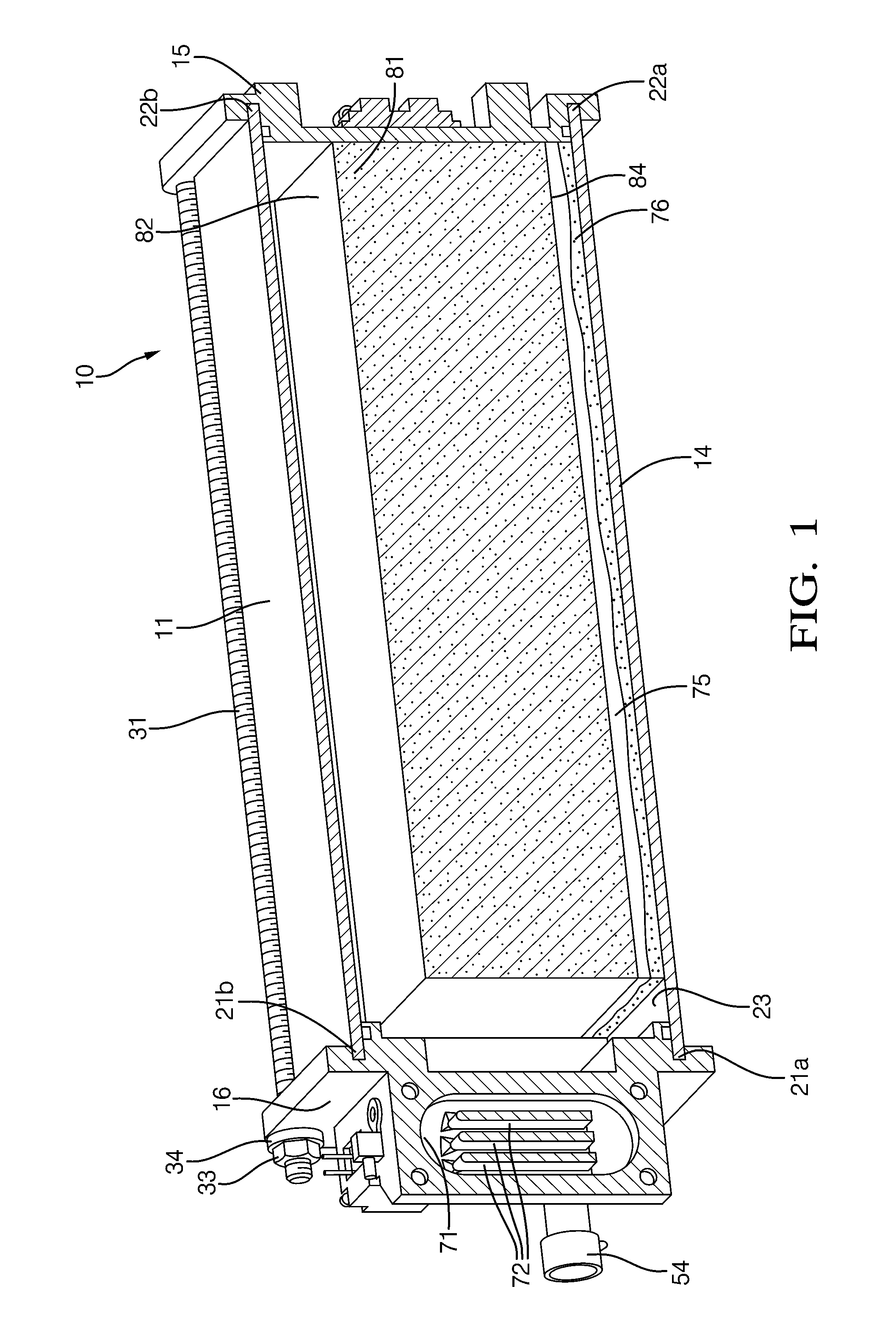
Connected heat conducting structures in solid ammonia storage systems
ActiveUS20110073806A1Other chemical processesCalcium/strontium/barium compoundsAmmonia storageHeat conducting
A compacted block of material constructed of one or more units consisting of matter comprising an ammonia-saturated material capable of reversibly desorbing and ad- or absorbing ammonia surrounded by a gas-permeable, flexible material having a thermal conductivity of at least five times the thermal conductivity of said ammonia-saturated material at −70° C. to 250° C. and methods for producing the same are described.
Owner:AMMINEX EMISSIONS TECH
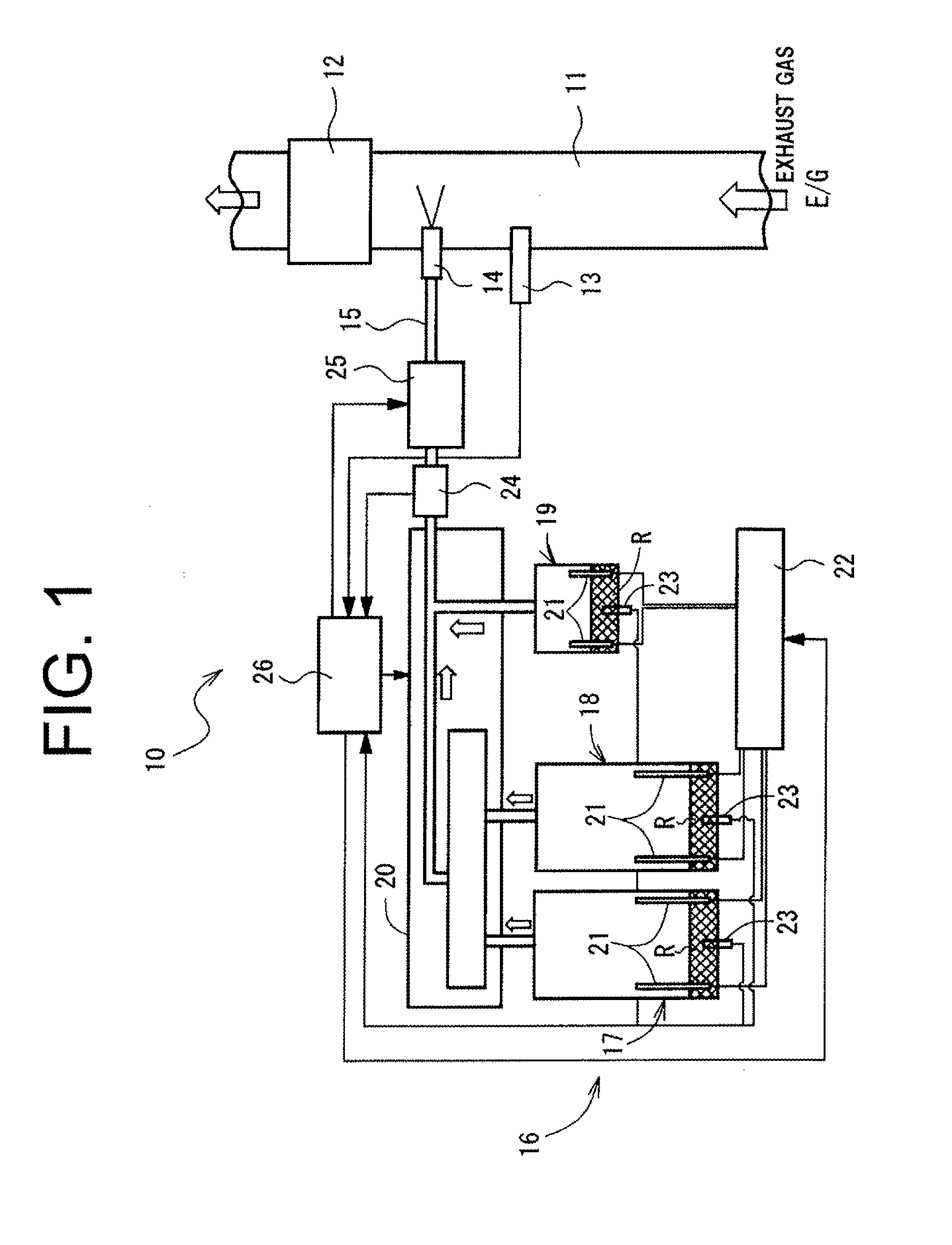
Flash heat ammonia generator
An ammonia producing device for an exhaust system of an engine is provided. It includes a pressure vessel having a cavity for storage of pressurized gases. The pressure vessel includes insulation located at least partially about the cavity for limiting heat transfer from within the cavity. A flash heater is disposed within the cavity and adjacent a solid ammonia gas producing material. An outlet port extends from the pressure vessel and has a valve located therein for providing egress of pressurized gases from within the pressure vessel.
Owner:DELPHI TECH HLDG S A R L
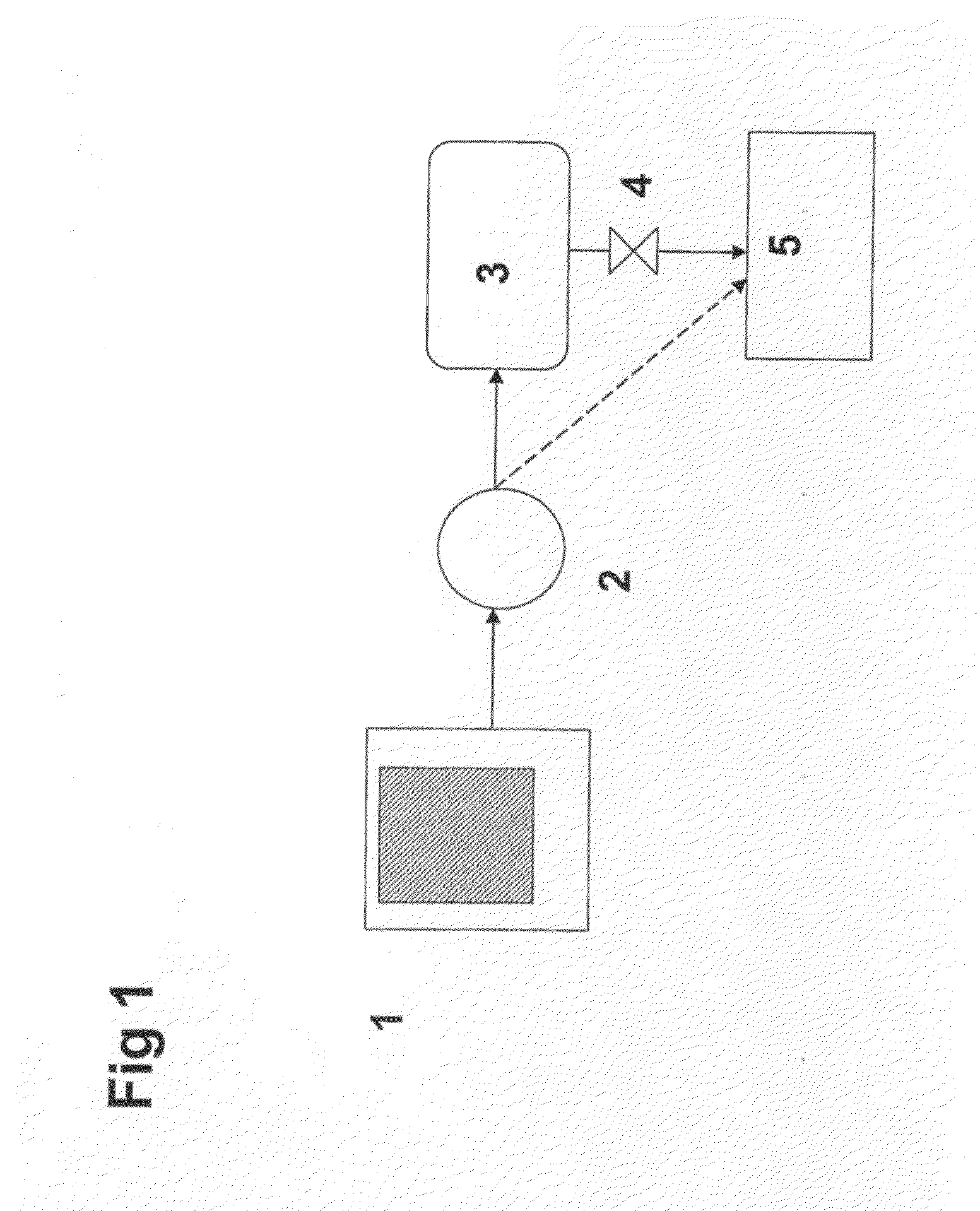
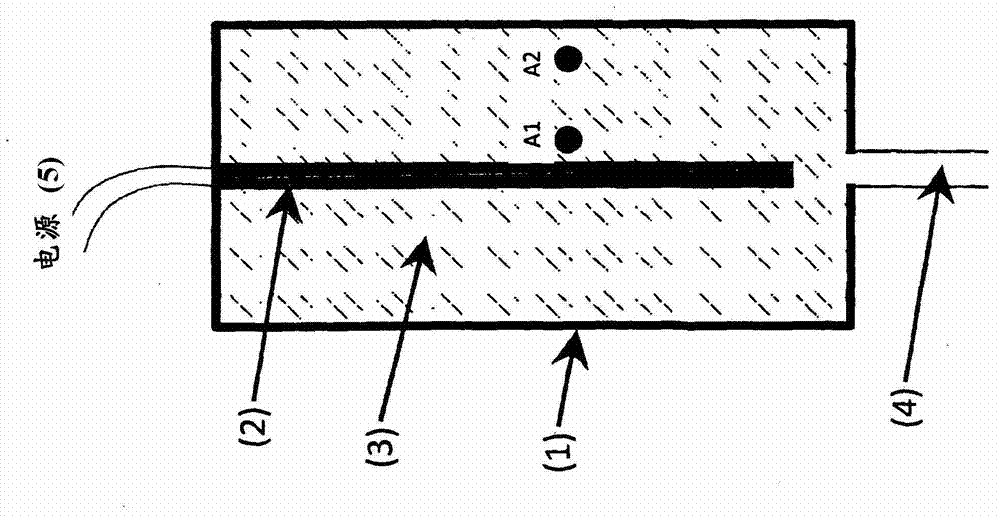
Method for determining the degree of saturation of solid ammonia storage materials in containers
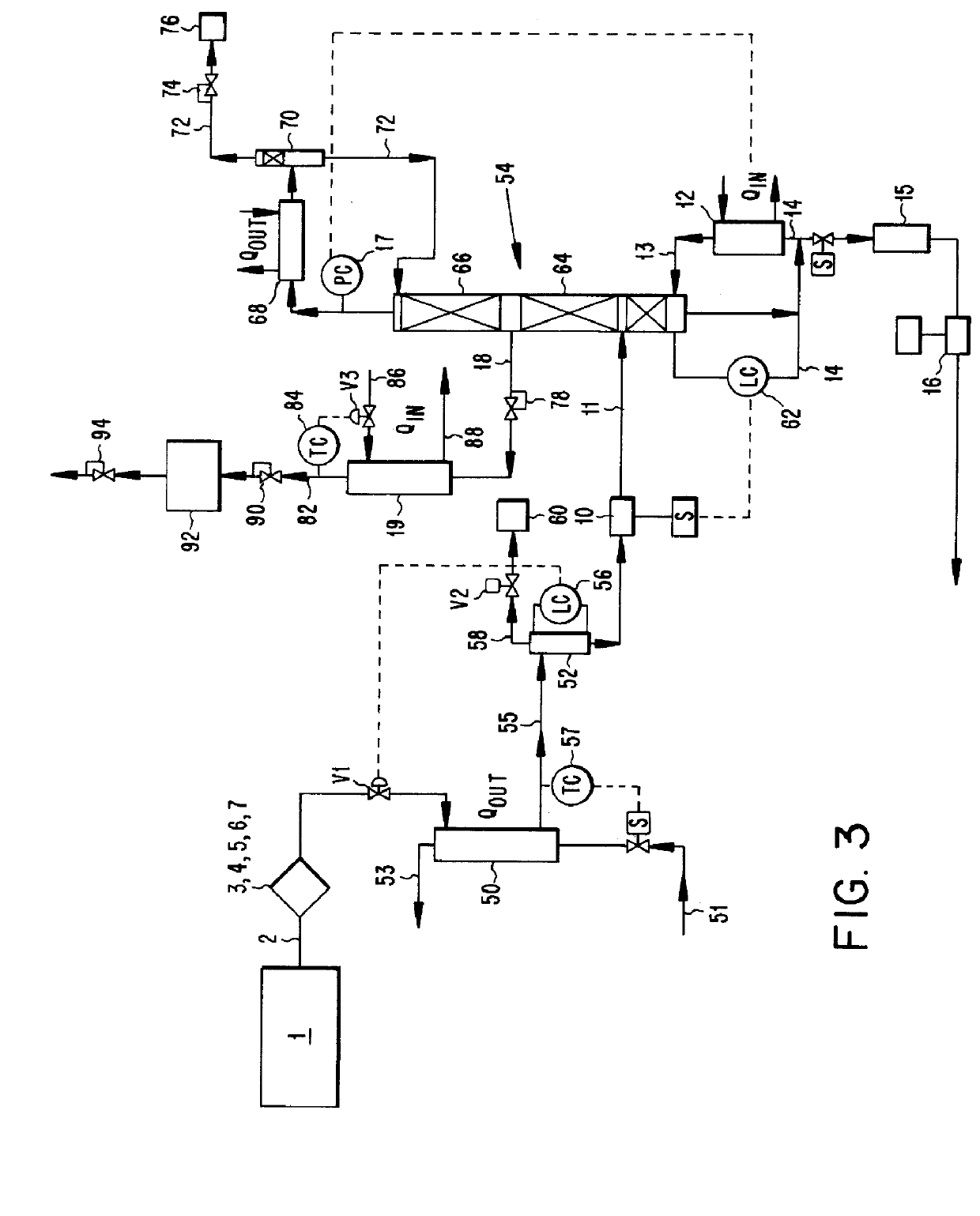
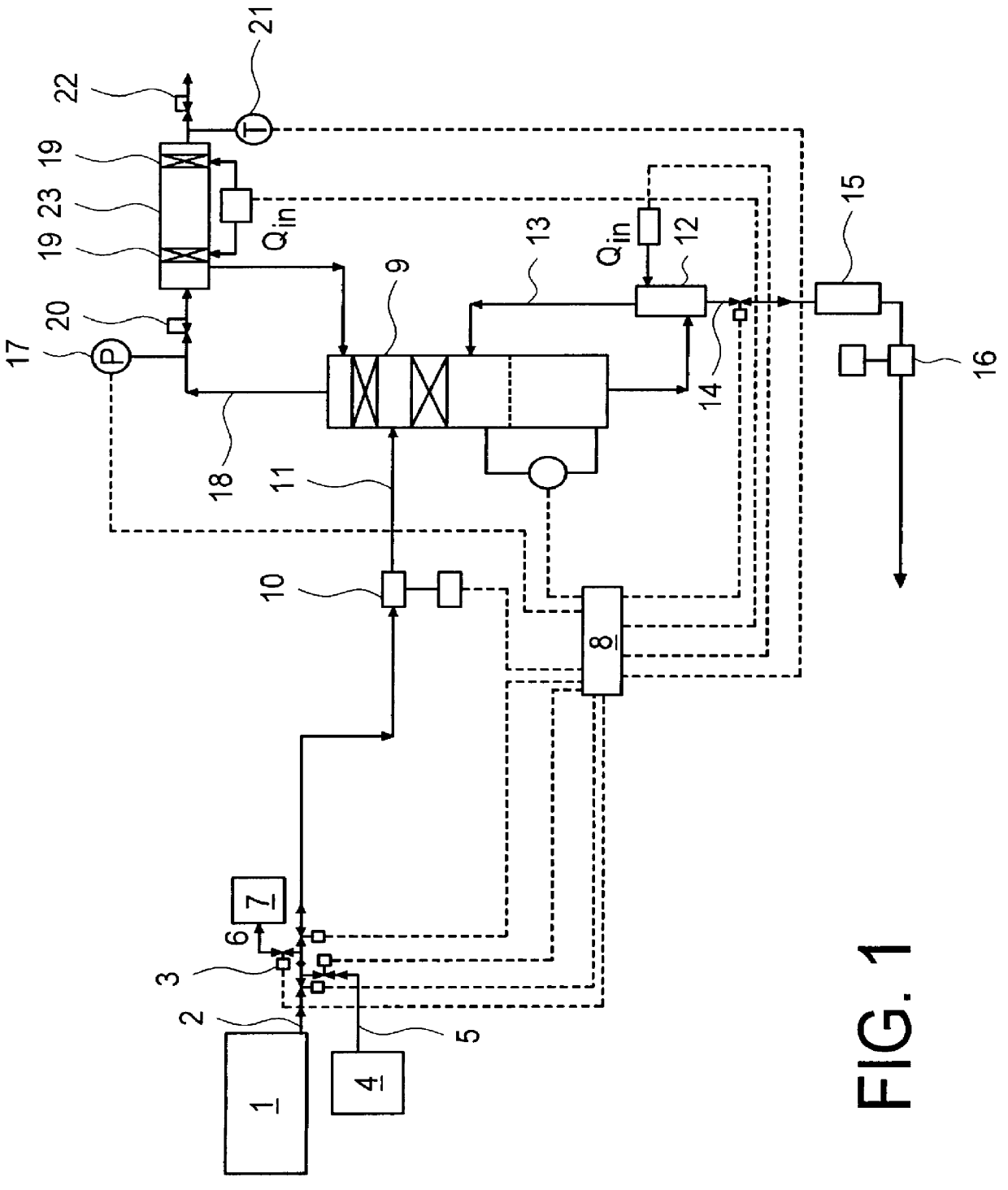
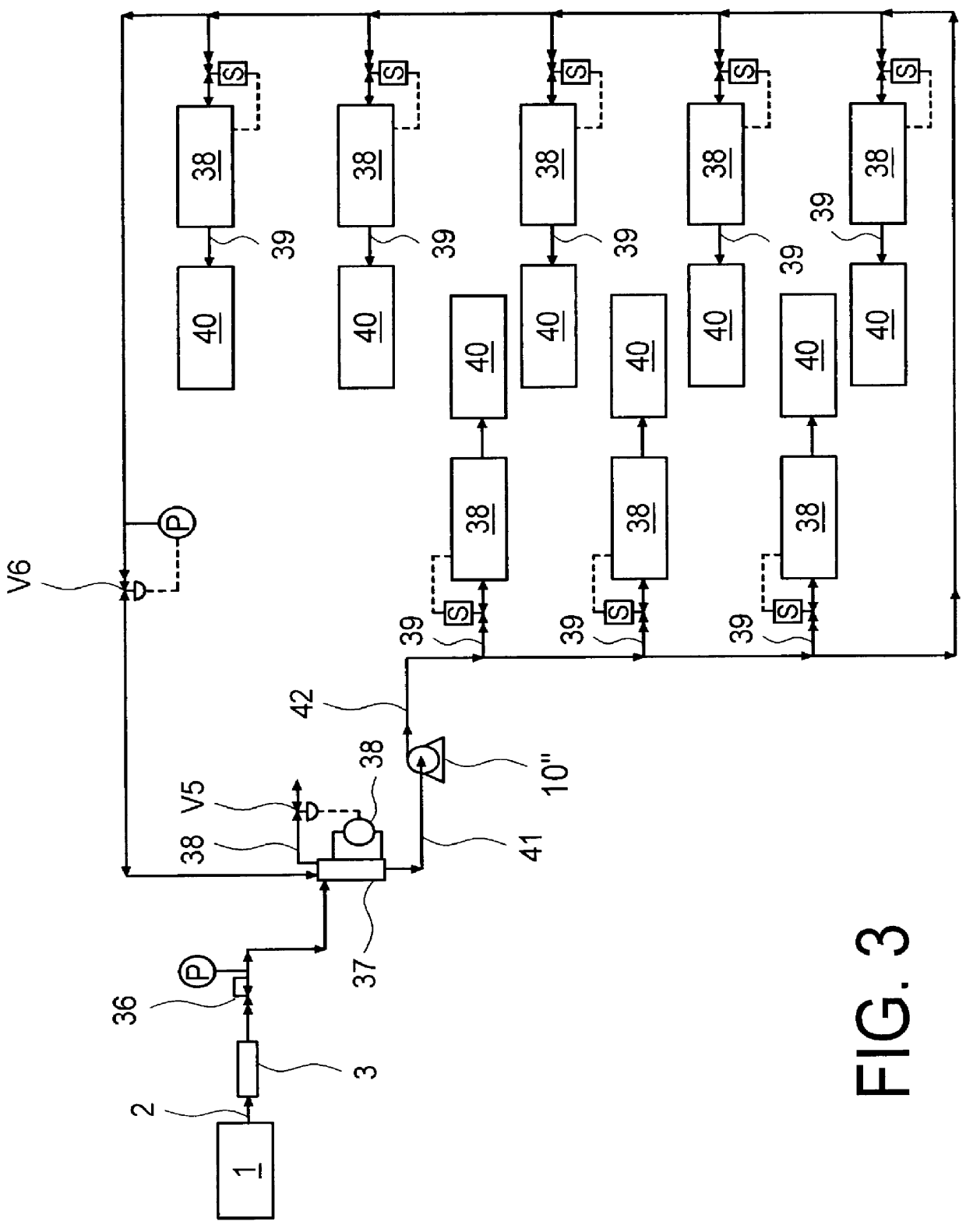
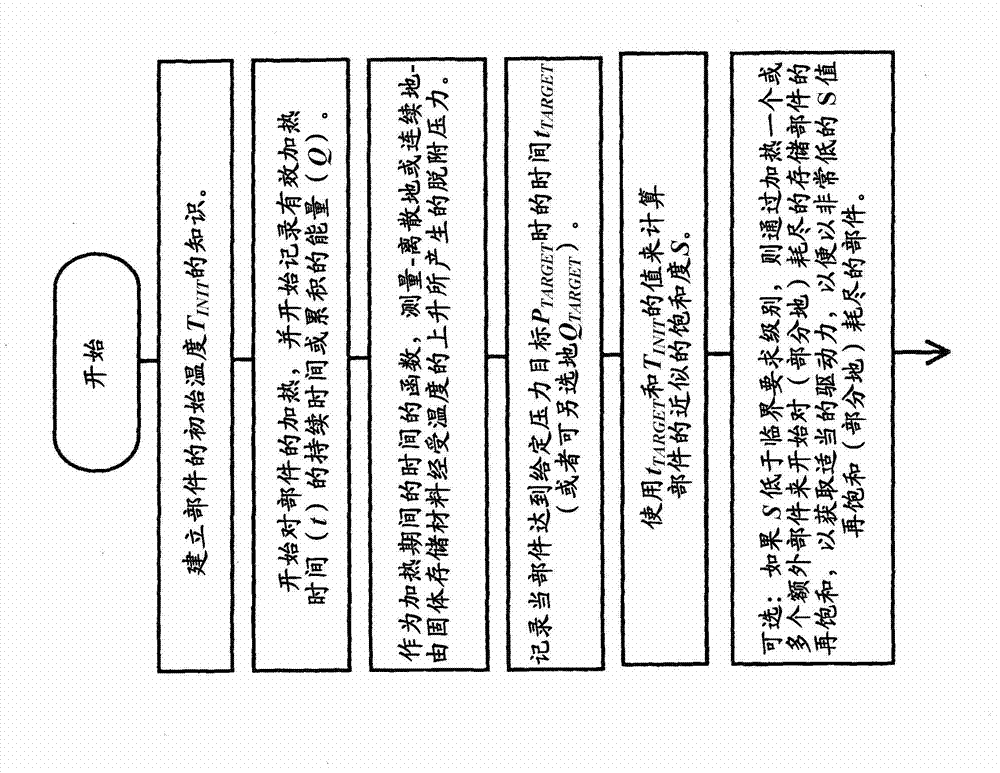
The invention relates to a method for determining the degree of saturation of solid ammonia storage materials in containers. A method is provided for estimating the degree of saturation (S) of a reversible solid ammonia storage material (3) in a storage unit (1). The storage unit (1) is equipped with a heater (2) to release ammonia and a connected tube (4) for ammonia flow. The initial temperature (TINIT) is measured with a sensor (9) in or around the storage unit (1) before any heating is initiated. Heating is initiated while recording the active time of heating (t) or the amount of energy (Q) released by the heater. The desorption pressure created by solid storage material in the storage unit (1) is measured via a pressure sensor (8) in fluid communication with the storage unit (1). The time (t TARGET), or the heat (Q TARGET) where the pressure reaches a certain target pressure (PTARGET) is recorded.; The values of the target-pressure time (t TARGET), or the target-pressure heat (Q TARGET), and the initial temperature (TINIT) are used to compute an approximate degree of saturation (S).
Owner:AMMINEX
Release of stored ammonia at start-up
ActiveUS8491842B2Prevent backflowReactant parameters controlInternal combustion piston enginesThermodynamicsAmmonia storage
A system for storage and dosing of ammonia, including a solid ammonia storage material capable of binding and releasing ammonia reversibly by adsorption / absorption. The system is able to release ammonia gradually according to a demand that can vary over time with intermediate periods of no ammonia demand. A main storage unit and a start-up storage unit are provided. The storage units hold ammonia storage material. At least one one-way valve is provided via which the one main storage unit is in communication with the start-up storage unit. The one-way valve prevents any back-flow of ammonia from the start-up storage unit to the main storage unit. Heating devices are arranged to heat the main storage unit and the start-up storage unit separately to generate gaseous ammonia by thermal desorption from the solid storage material. A controller controls the heating power of the main storage unit and the start-up storage unit, thereby enabling ammonia release from at least one of the start-up and / or the main storage unit. A dosing valve controls ammonia flow from the storage units according to a demand.
Owner:AMMINEX EMISSIONS TECH
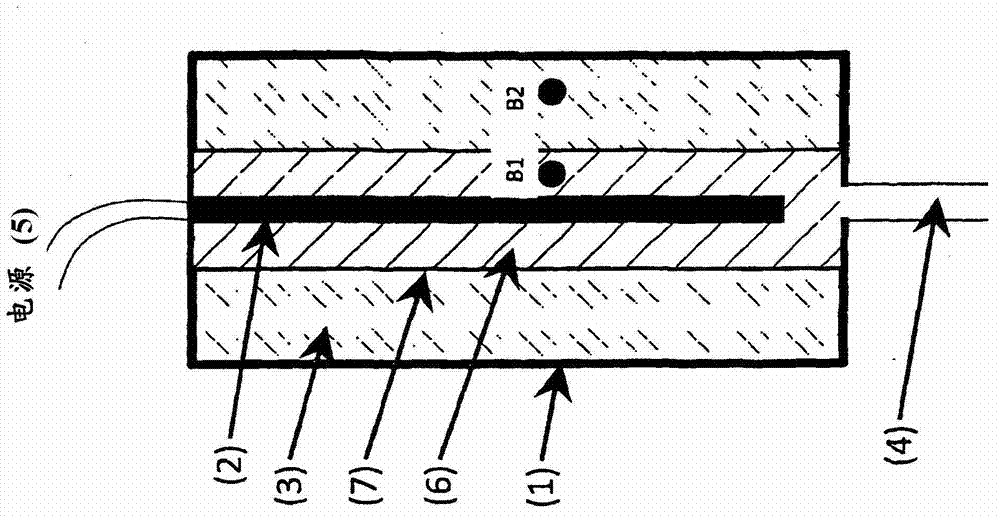
Method for determining the degree of saturation of solid ammonia storage materials in containers
InactiveUS8834603B2Reduce stepsImprove resaturationInternal combustion piston enginesDispersed particle filtrationActive timeDesorption
A method is provided for estimating the degree of saturation (S) of a reversible solid ammonia storage material (3) in a storage unit (1). The storage unit (1) is equipped with a heater (2) to release ammonia and a connected tube (4) for ammonia flow. The initial temperature (TINIT) is measured with a sensor (9) in or around the storage unit (1) before any heating is initiated. Heating is initiated while recording the active time of heating (t) or the amount of energy (Q) released by the heater. The desorption pressure created by solid storage material in the storage unit (1) is measured via a pressure sensor (8) in fluid communication with the storage unit (1). The time (tTARGET), or the heat (QTARGET) where the pressure reaches a certain target pressure (PTARGET) is recorded. The values of the target-pressure time (tTARGET), or the target-pressure heat (QTARGET), and the initial temperature (TINIT) are used to compute an approximate degree of saturation (S).
Owner:AMMINEX EMISSIONS TECH
Method and device for controlling effective heat transfer in a solid gas storage system
A method for controlling the effective heat transfer from a storage unit (1). During gas release from storage material (3) in the storage unit the storage material is heated by a heater (2). During re-saturation of the storage material (3) with gas the heater is off. Controlling of the effective heat transfer from the storage unit (1) is performed, during gas release, by ceasing convection of a convection gas and, during re-saturation, by performing or enabling convection of a convection gas to cool the storage unit (1).
Owner:AMMINEX EMISSIONS TECH
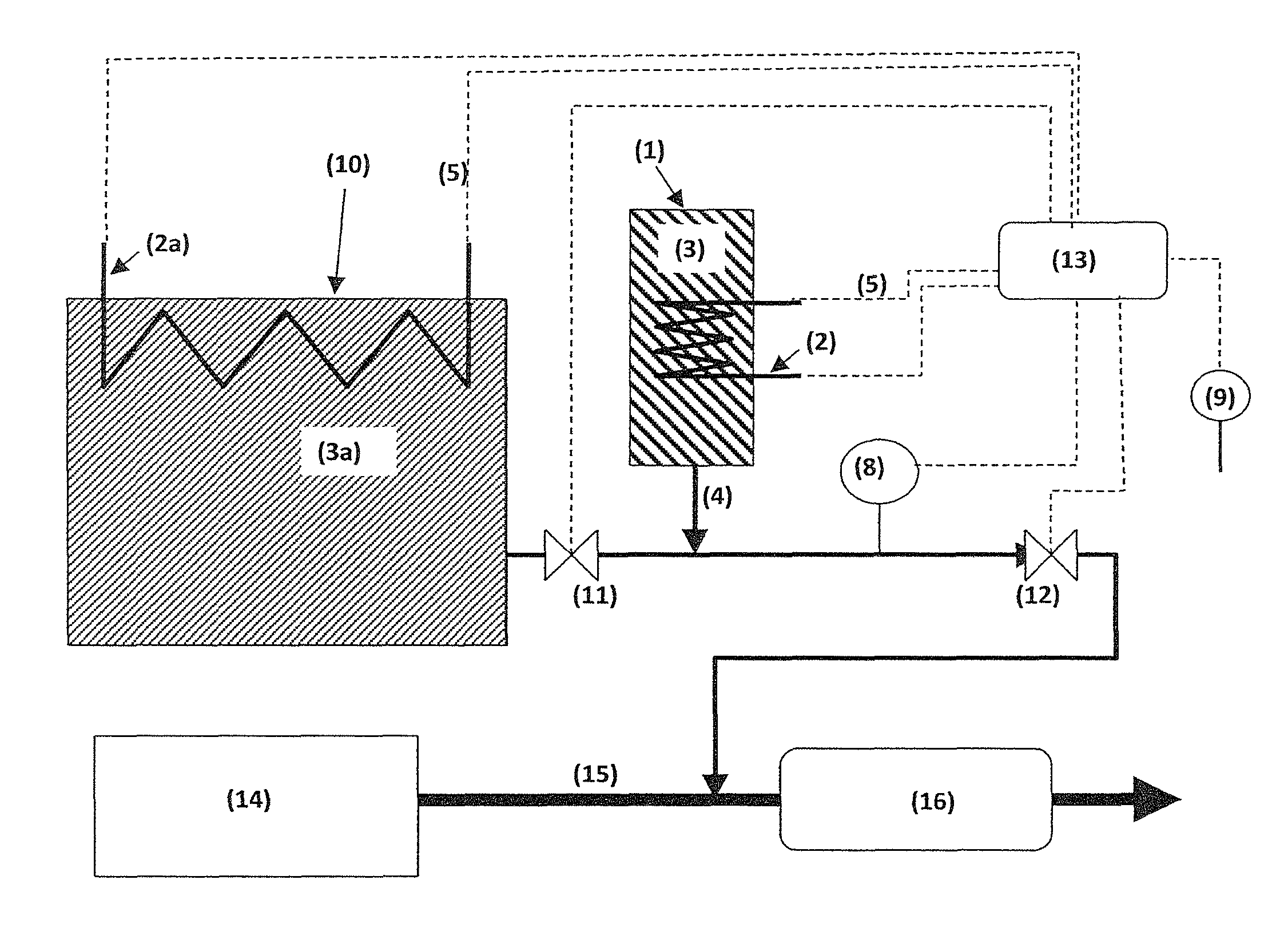
Method for storing and delivering ammonia from solid storage materials using a vacuum pump
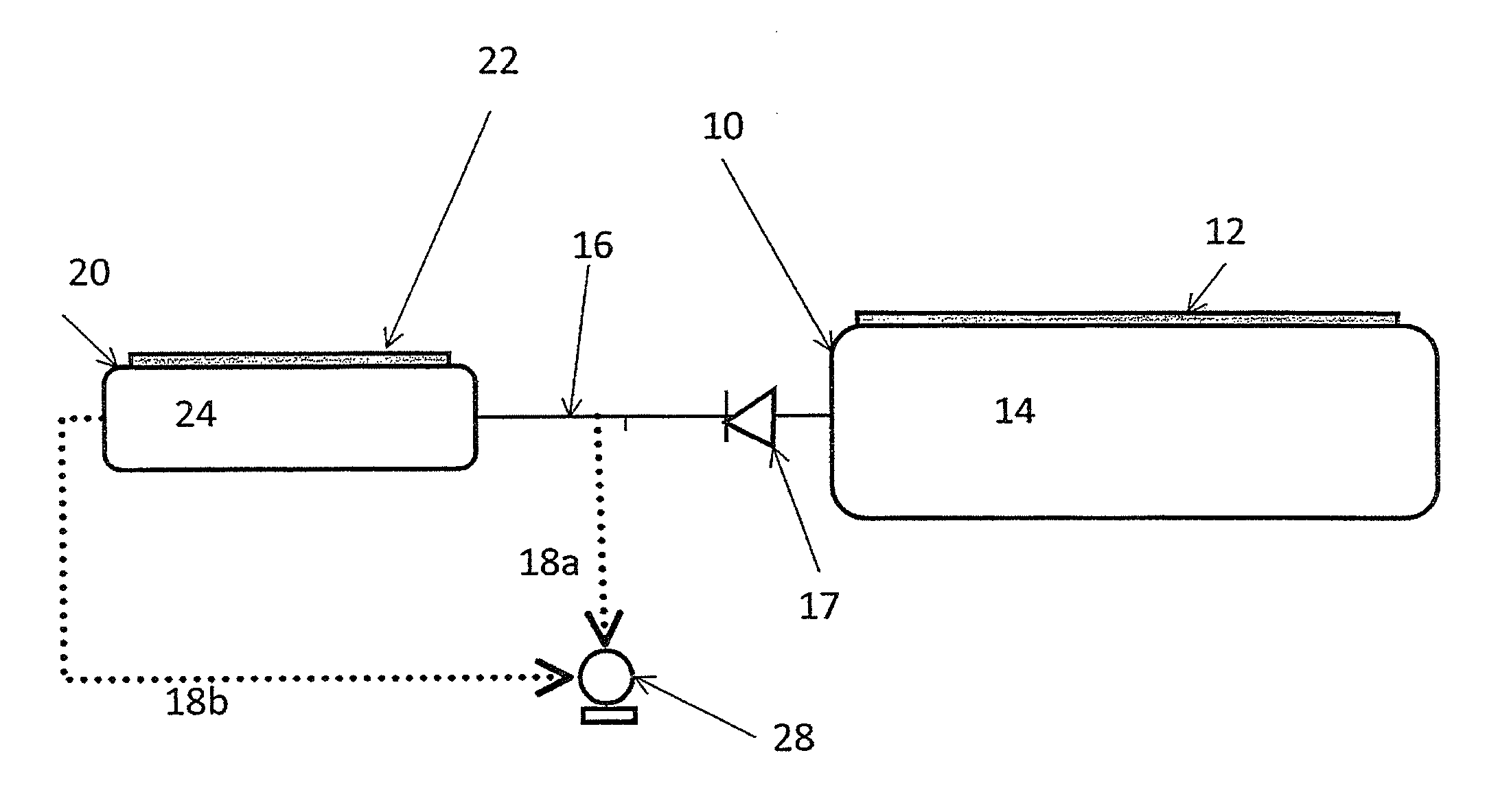
InactiveUS8551219B2Reduce pressureNitrous oxide captureCombination devicesDesorptionPressure threshold
In a method of storing and releasing gaseous ammonia from solid storage materials a first solid storage material (14) capable of releasing ammonia by desorption in a first container (10) and a second solid storage material (24) capable of ad- or absorbing ammonia reversibly and having a higher affinity for ammonia than the first storage material (14) in a second container (20) smaller than said first container (10) are in fluid communication. The pressure in at least the first container (10) is kept below the equilibrium pressure between ammonia and the storage material contained therein by means of a pump (28). When the pressure in the first container (10) is below a pressure threshold where the first storage material (14) does not release an amount of ammonia required by an ammonia consuming device connected with the containers via the vacuum pump (28), the second storage material (24) is heated such that the ammonia pressure of the second material (24) is higher than the ammonia pressure of the first material (14). The ammonia released by the second material (24) is continuously pumped off so as to deliver sufficient ammonia to said ammonia consuming unit. A device for carrying out the method is also described.
Owner:AMMINEX EMISSIONS TECH
Adsorbent
ActiveUS20140224120A1Improve adsorption capacityEasy to storeMethane captureNitrogen compoundsElastomerSorbent
An object of the present invention is to provide an adsorbent material having excellent gas storage performance and gas separation performance.This object can be achieved by an adsorbent material comprising a composition comprising a metal complex (A) and an elastomer (B), the metal complex (A) containing an anionic ligand and at least one metal ion selected from ions of metals belonging to Groups 1 to 13 of the periodic table, the metal complex (A) being capable of undergoing a volume change upon adsorption, and the mass ratio of the metal complex (A) and the elastomer (B) being within the range of 1:99 to 99:1.
Owner:KURARAY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com