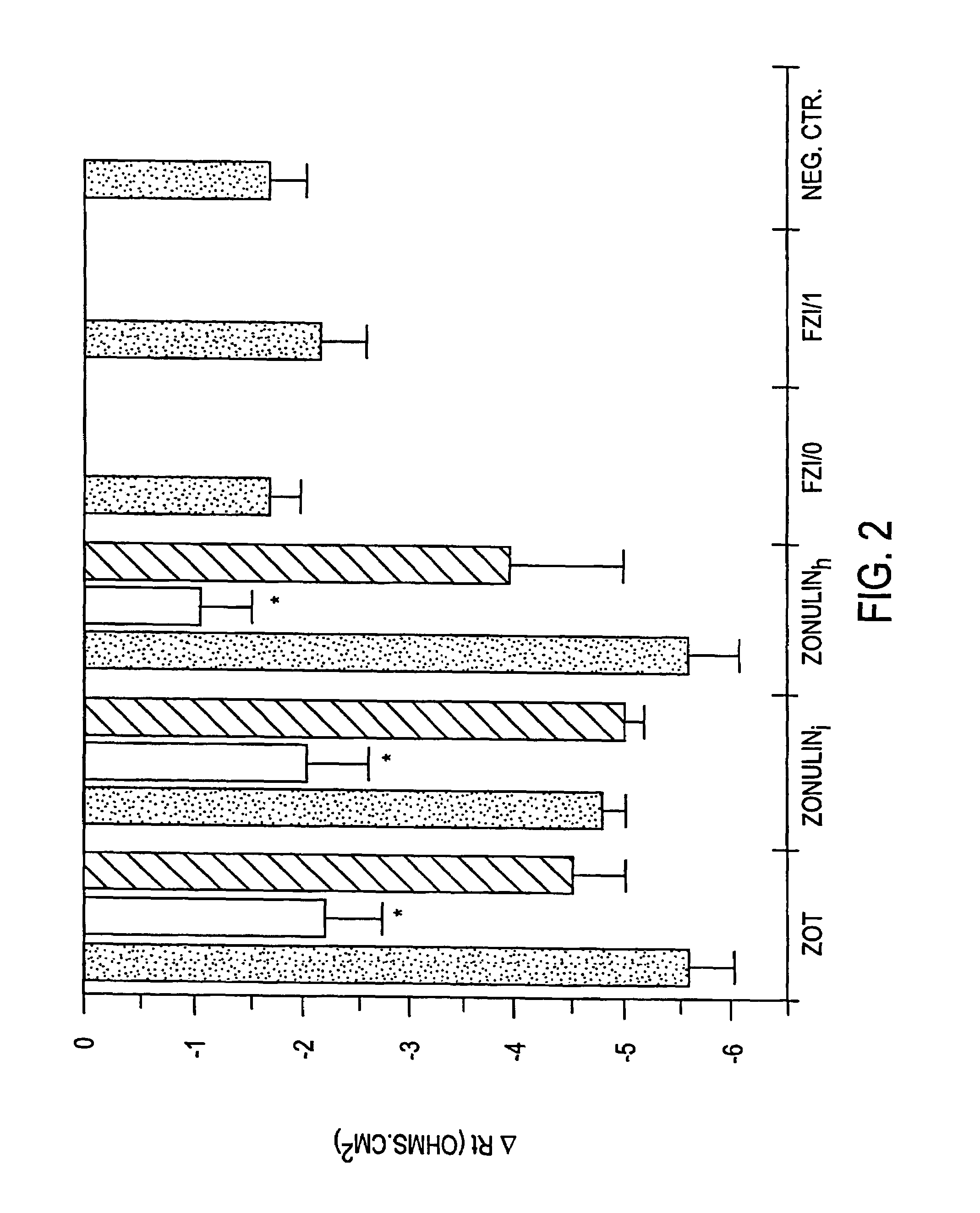
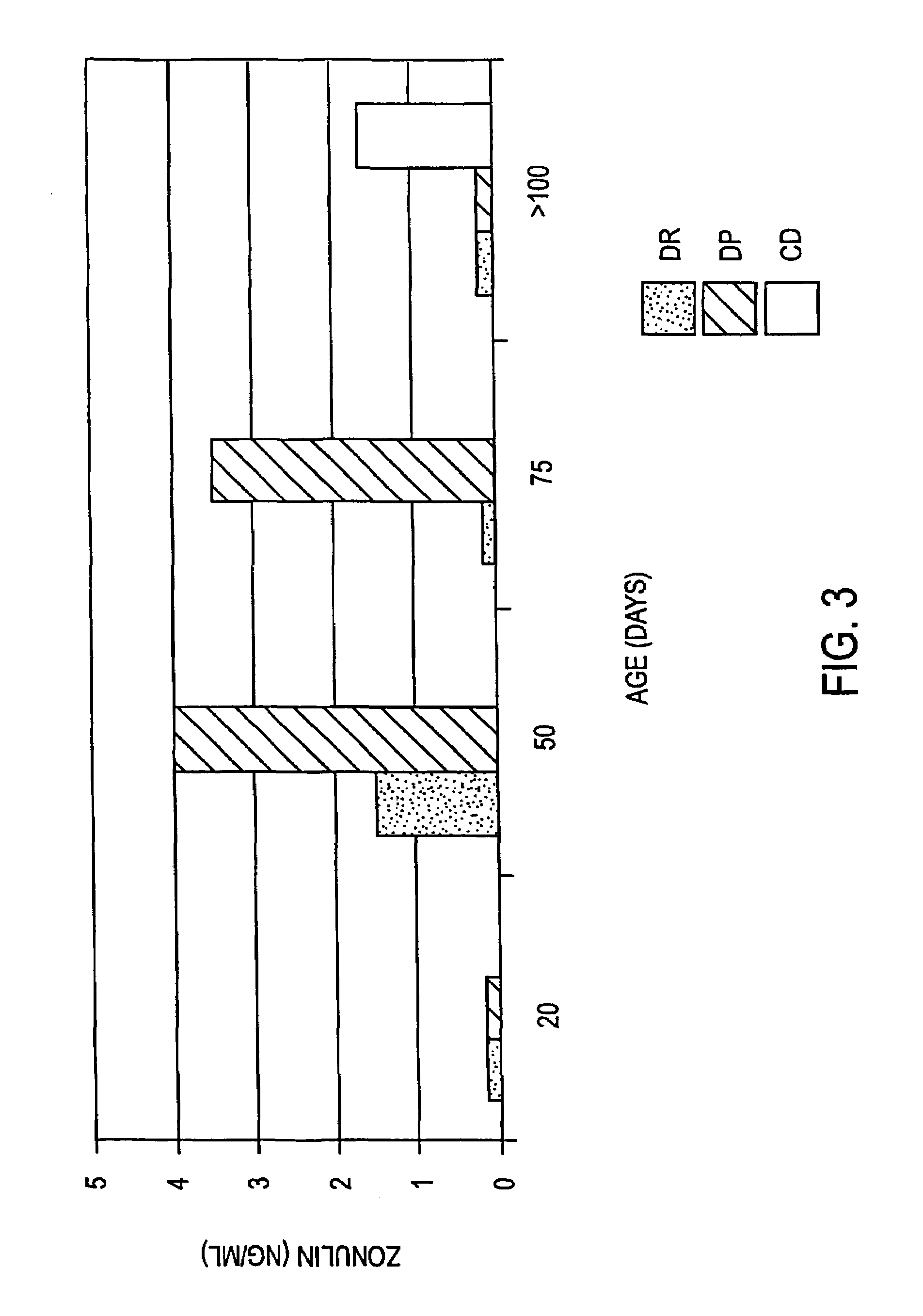
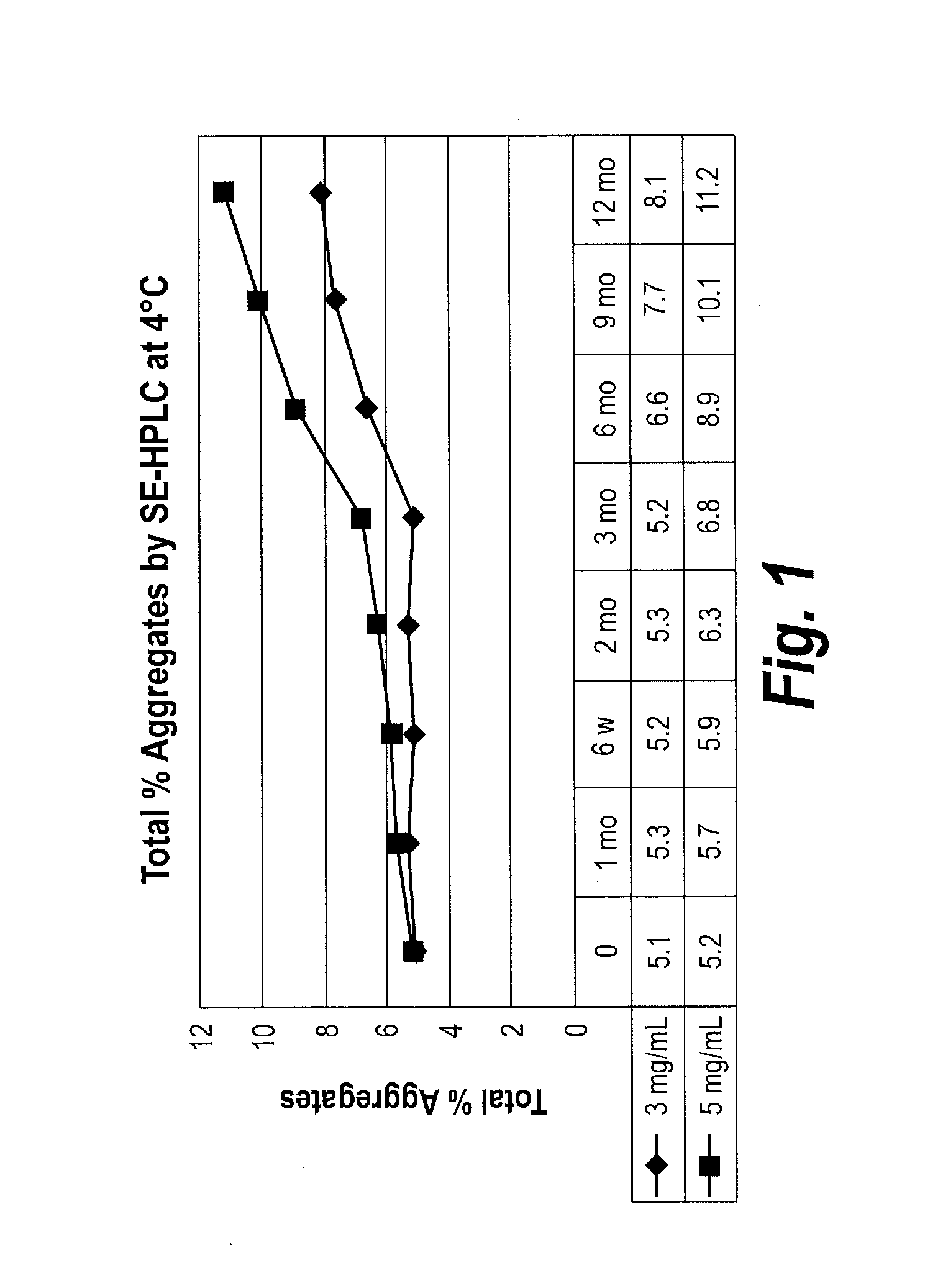
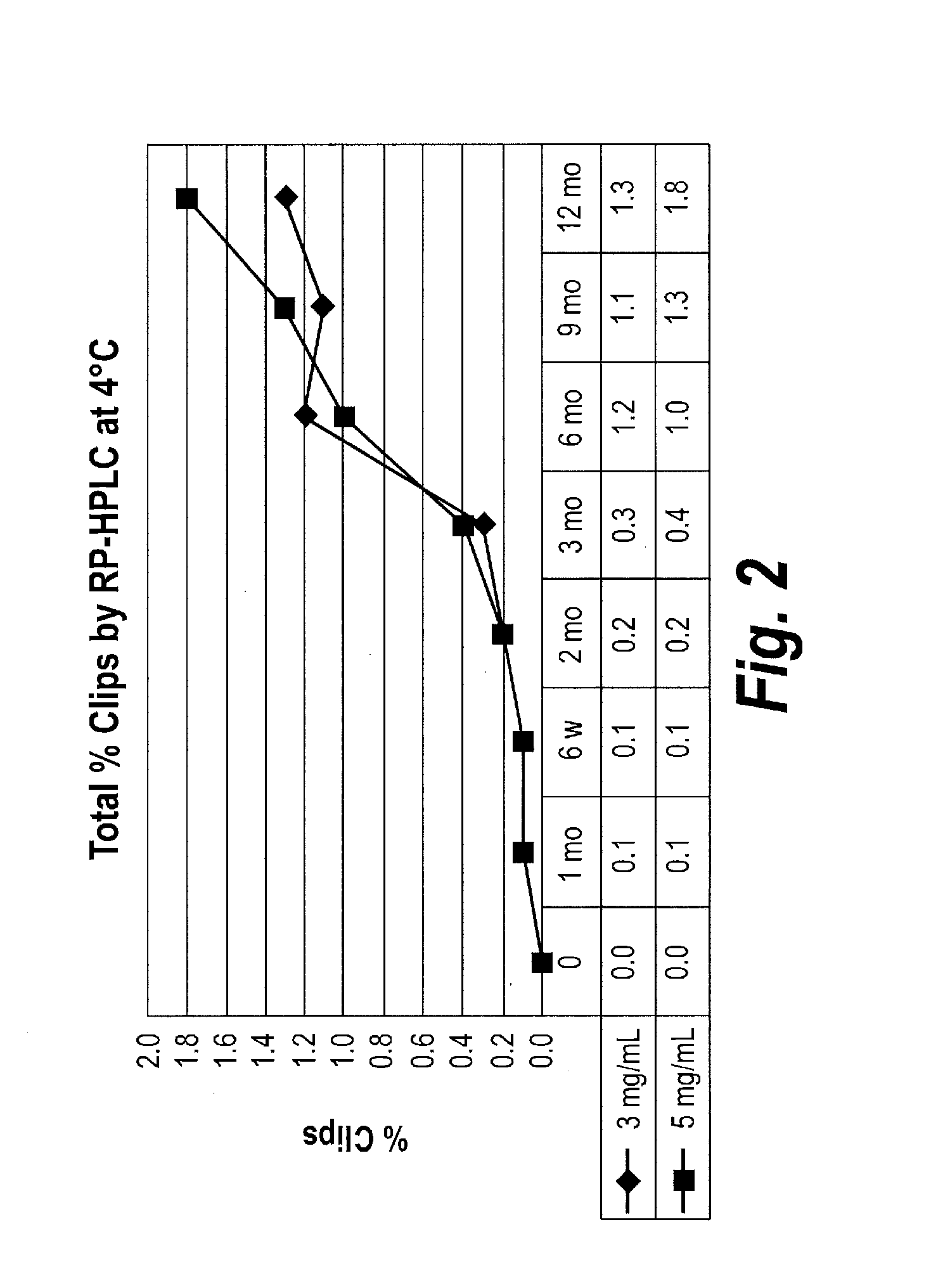
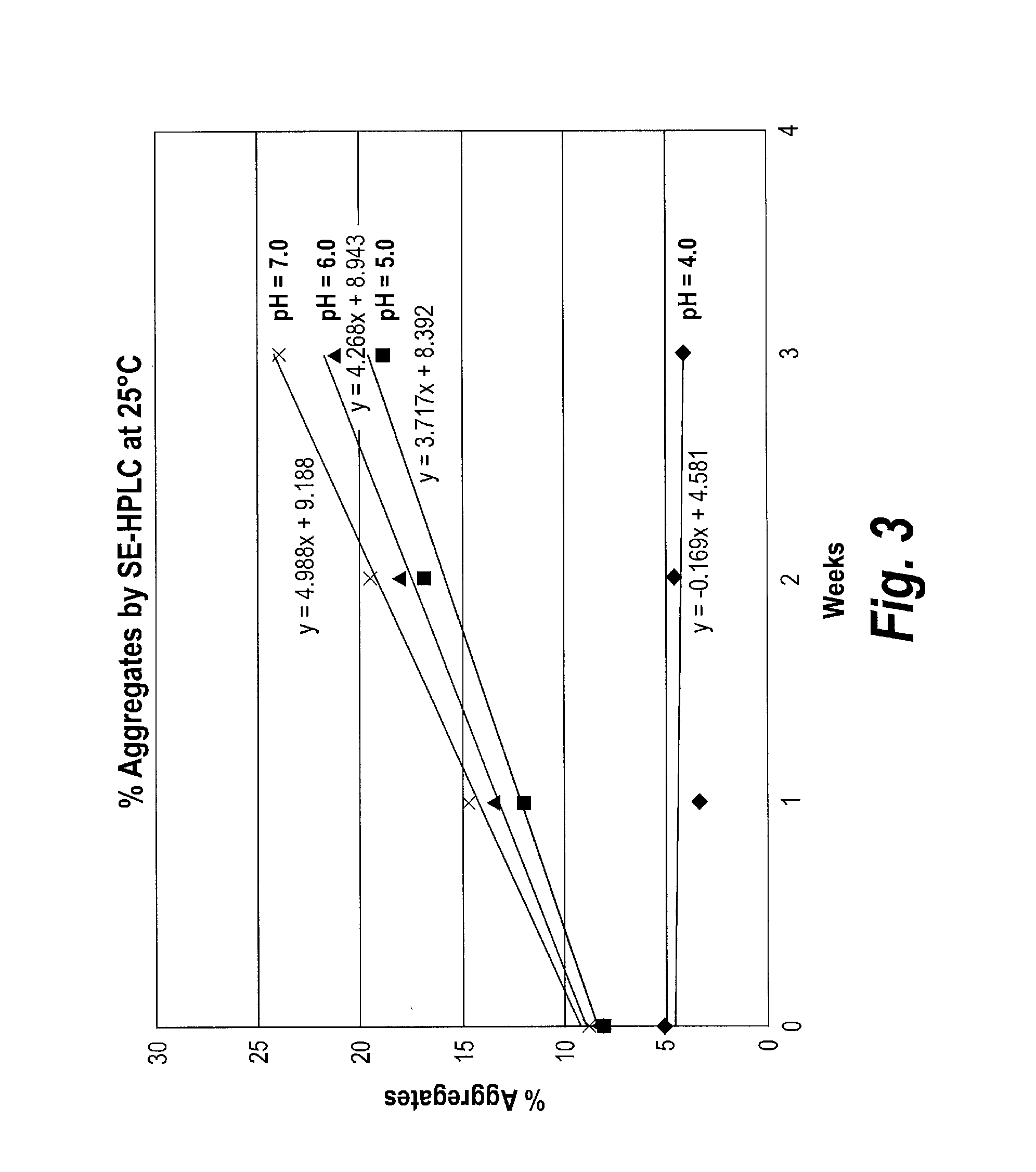
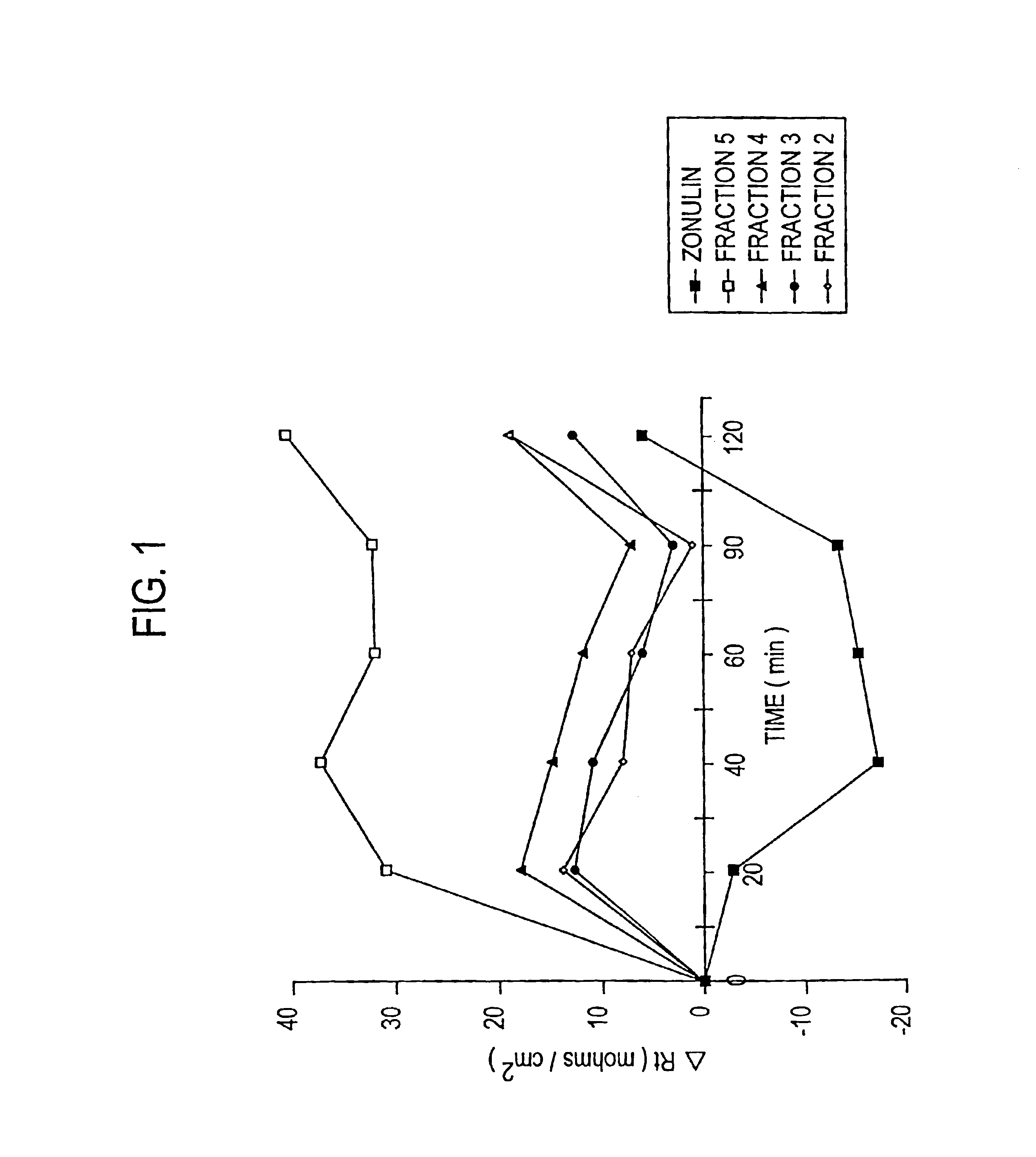
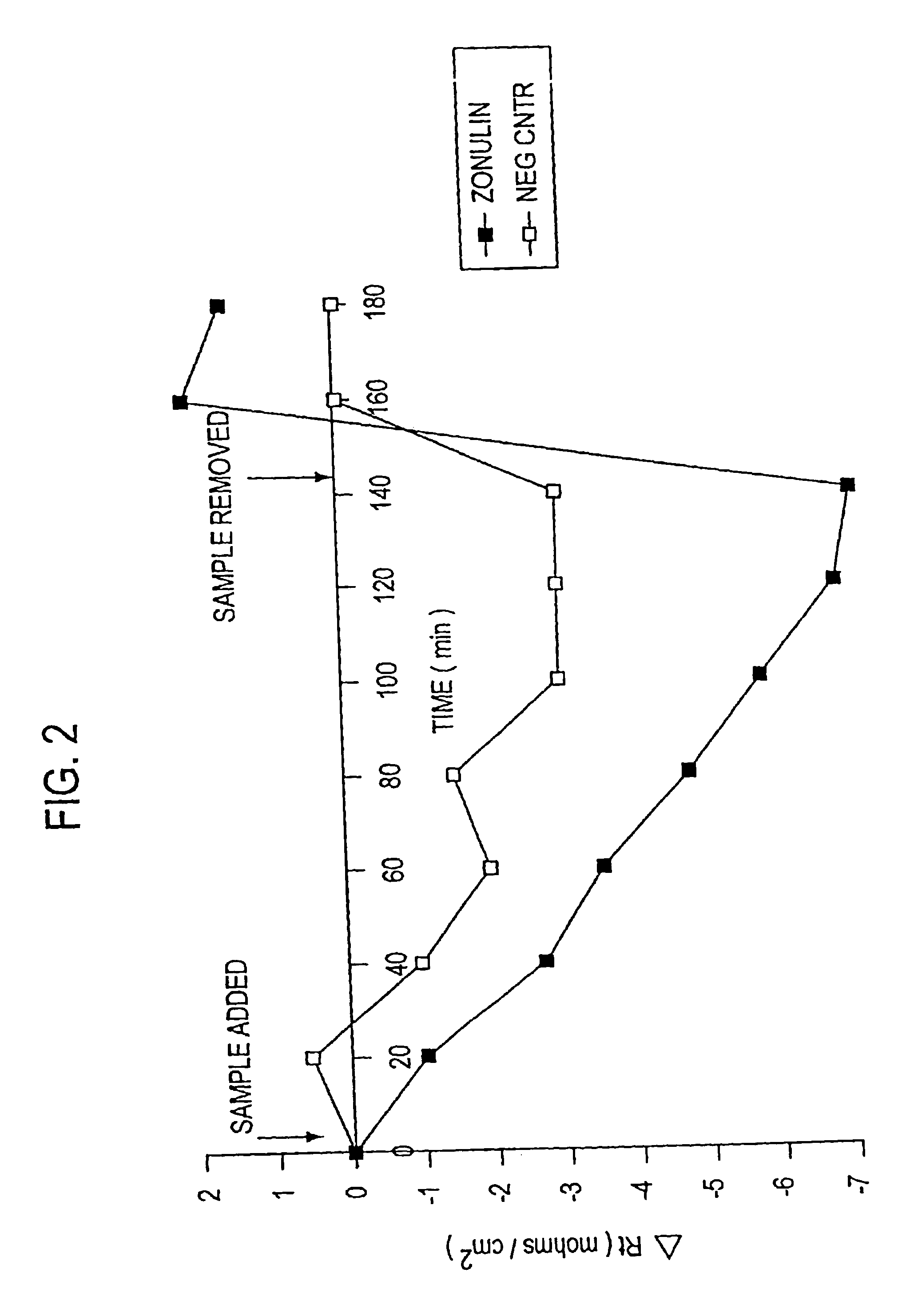
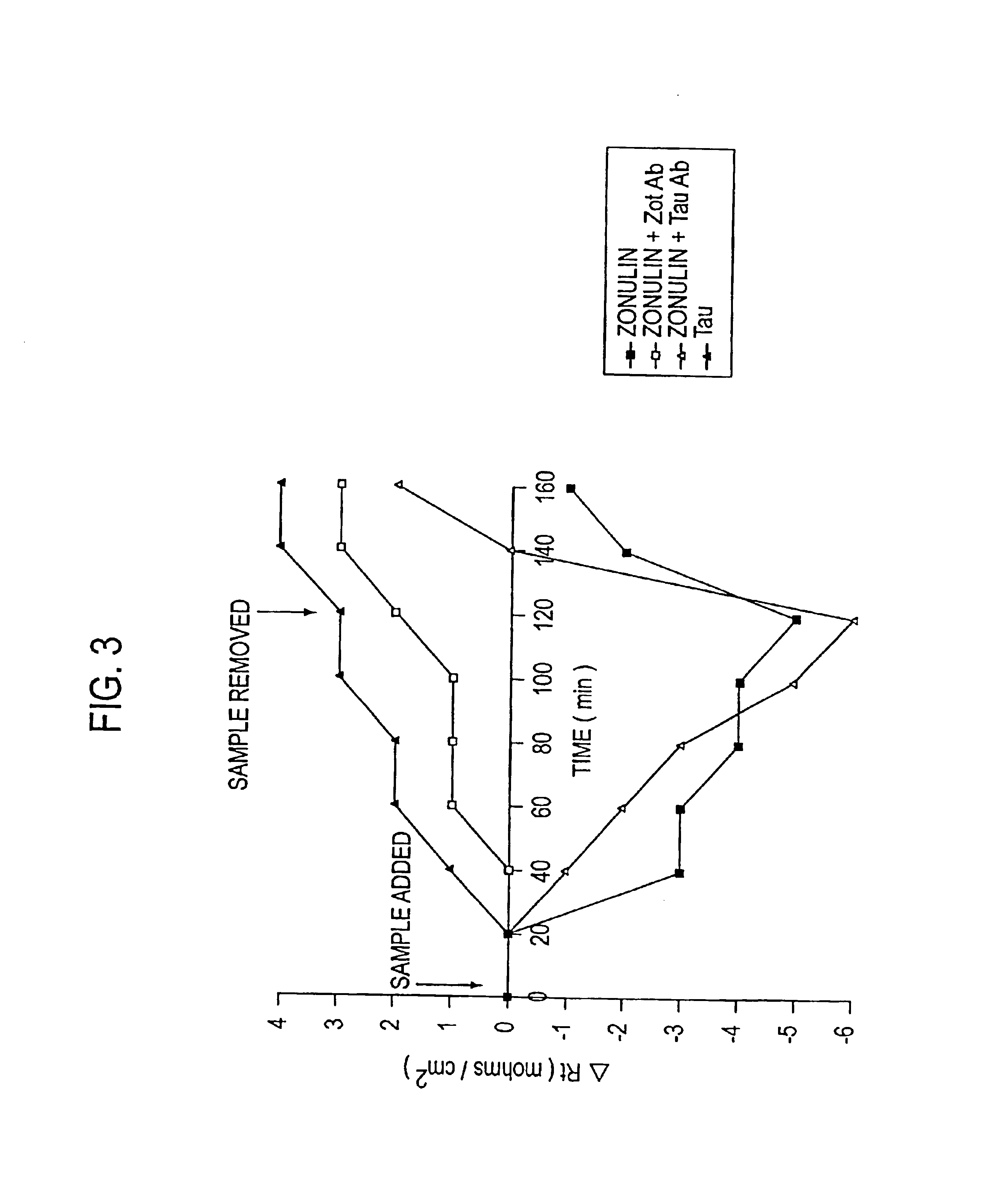
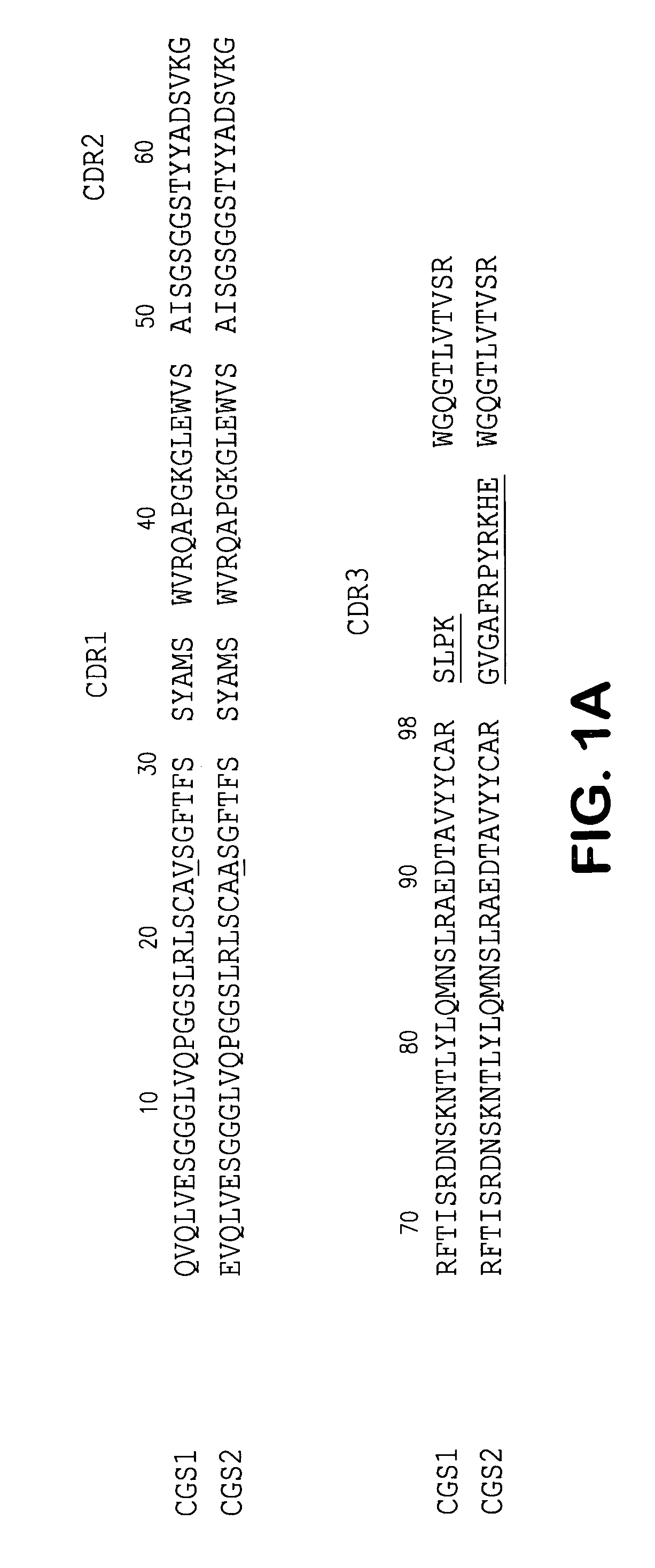
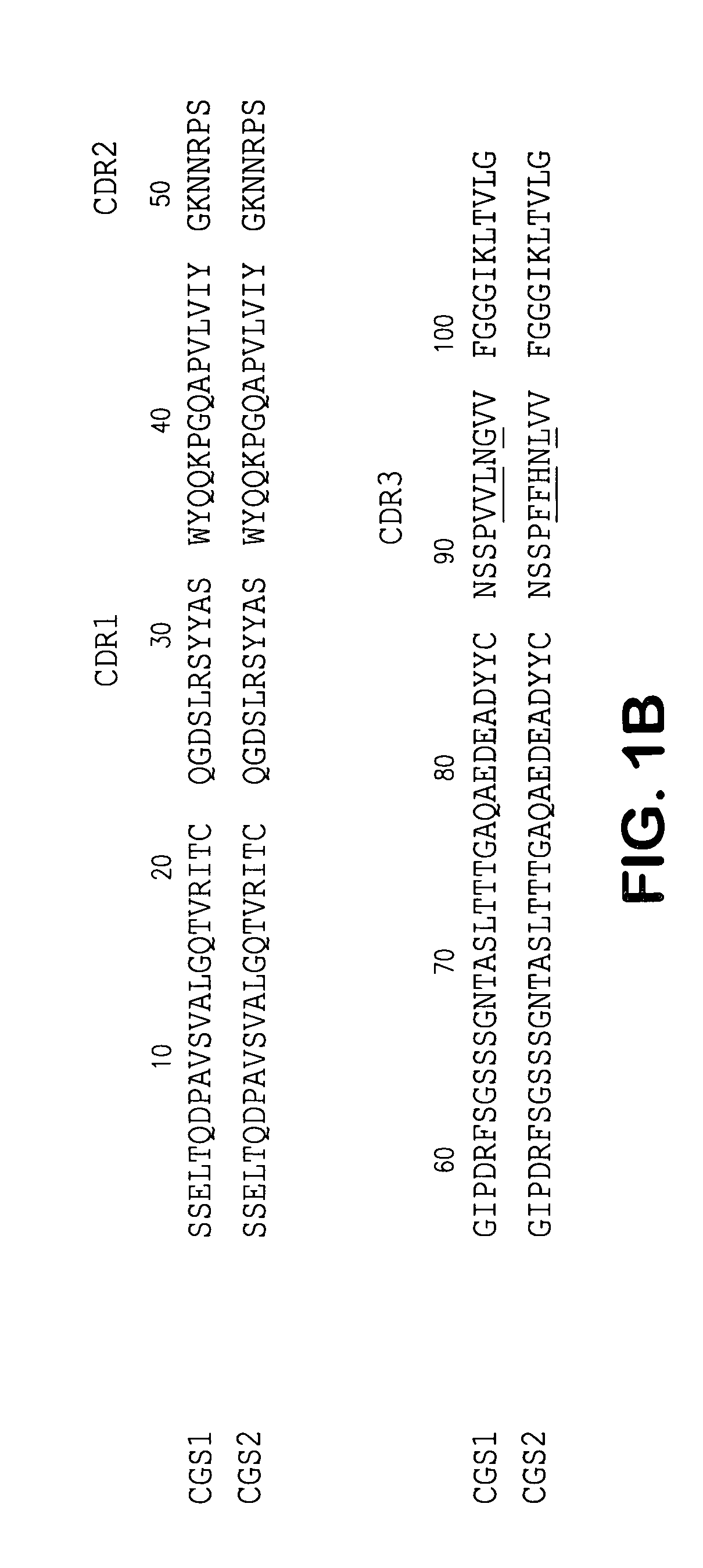
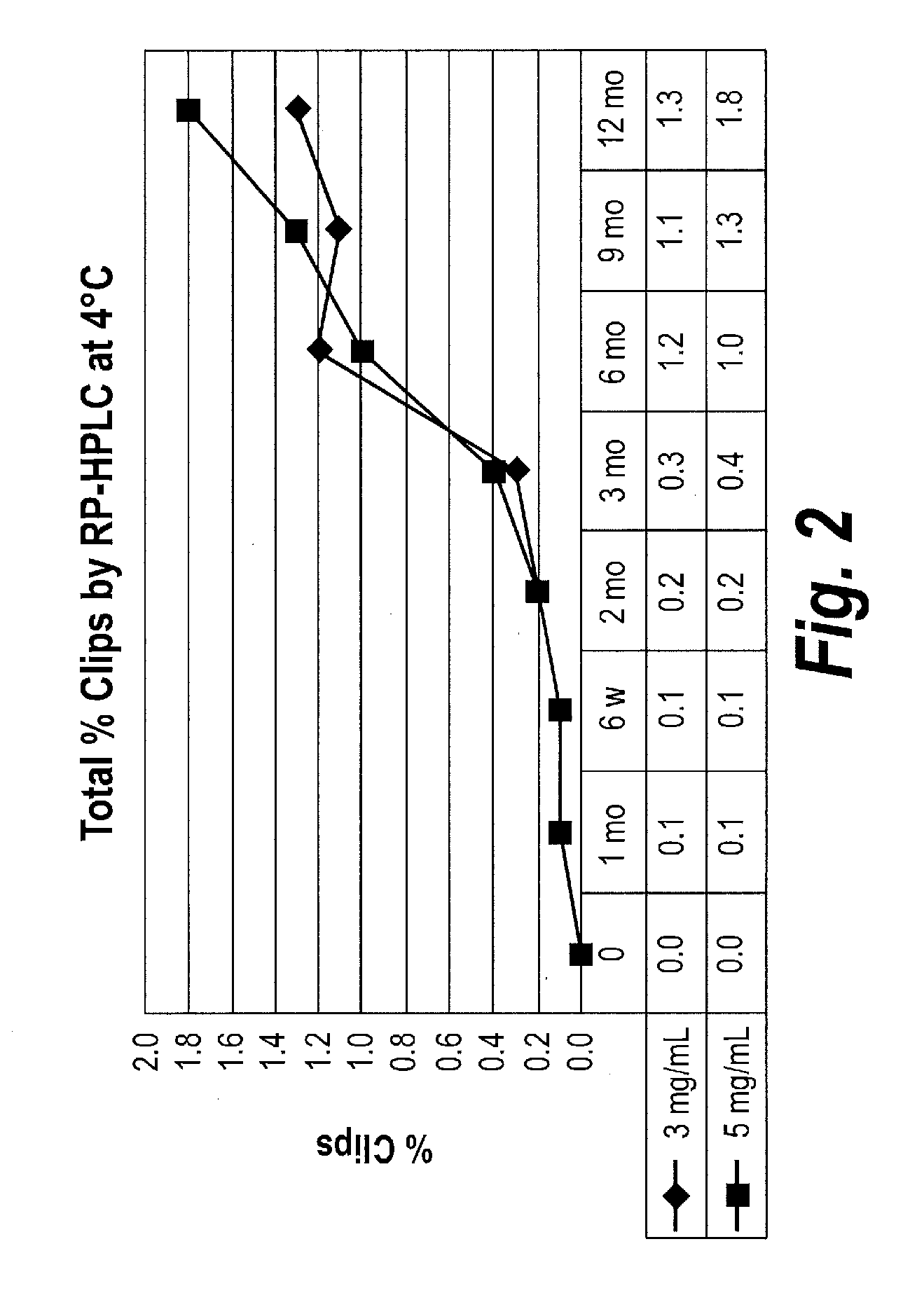
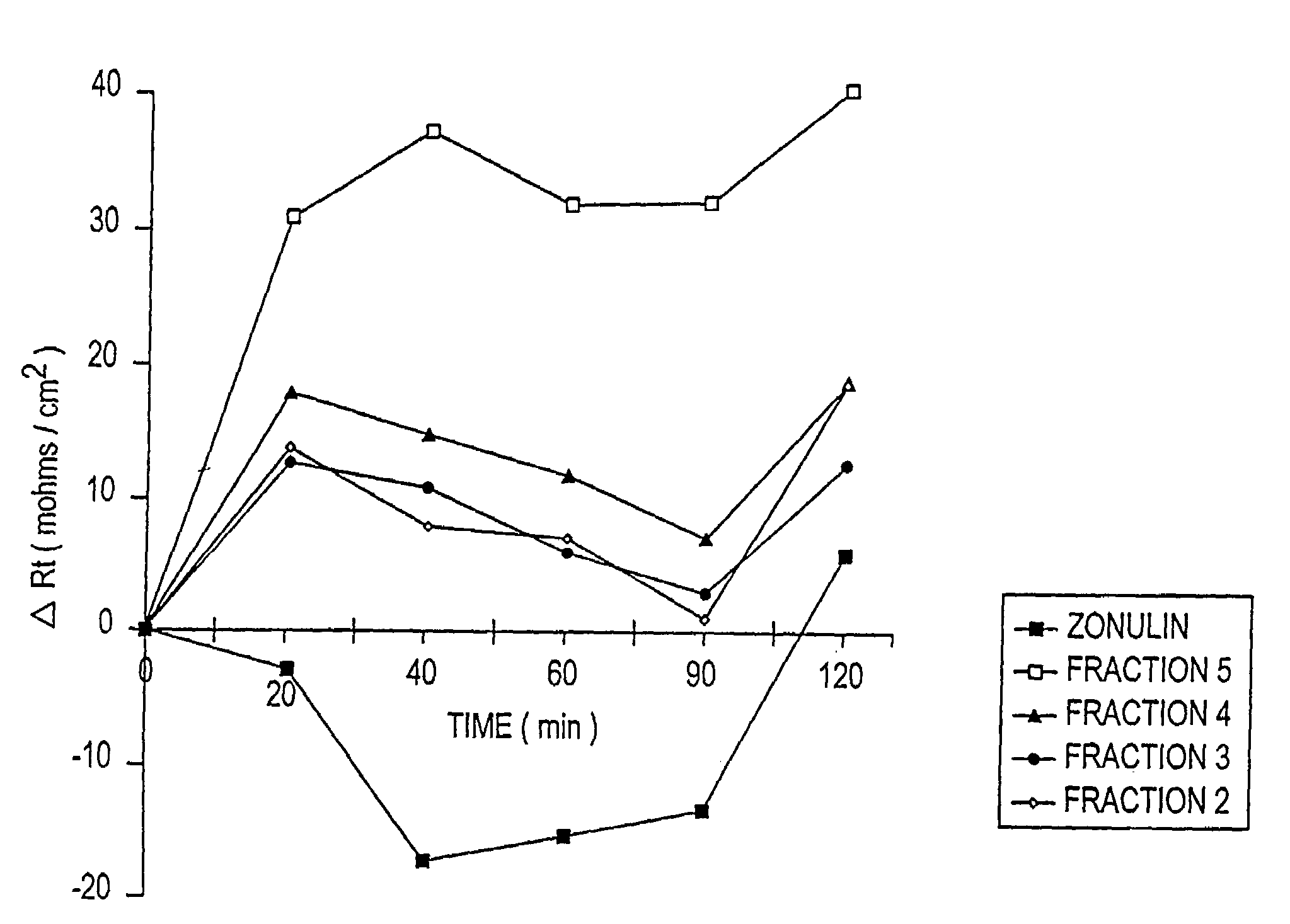
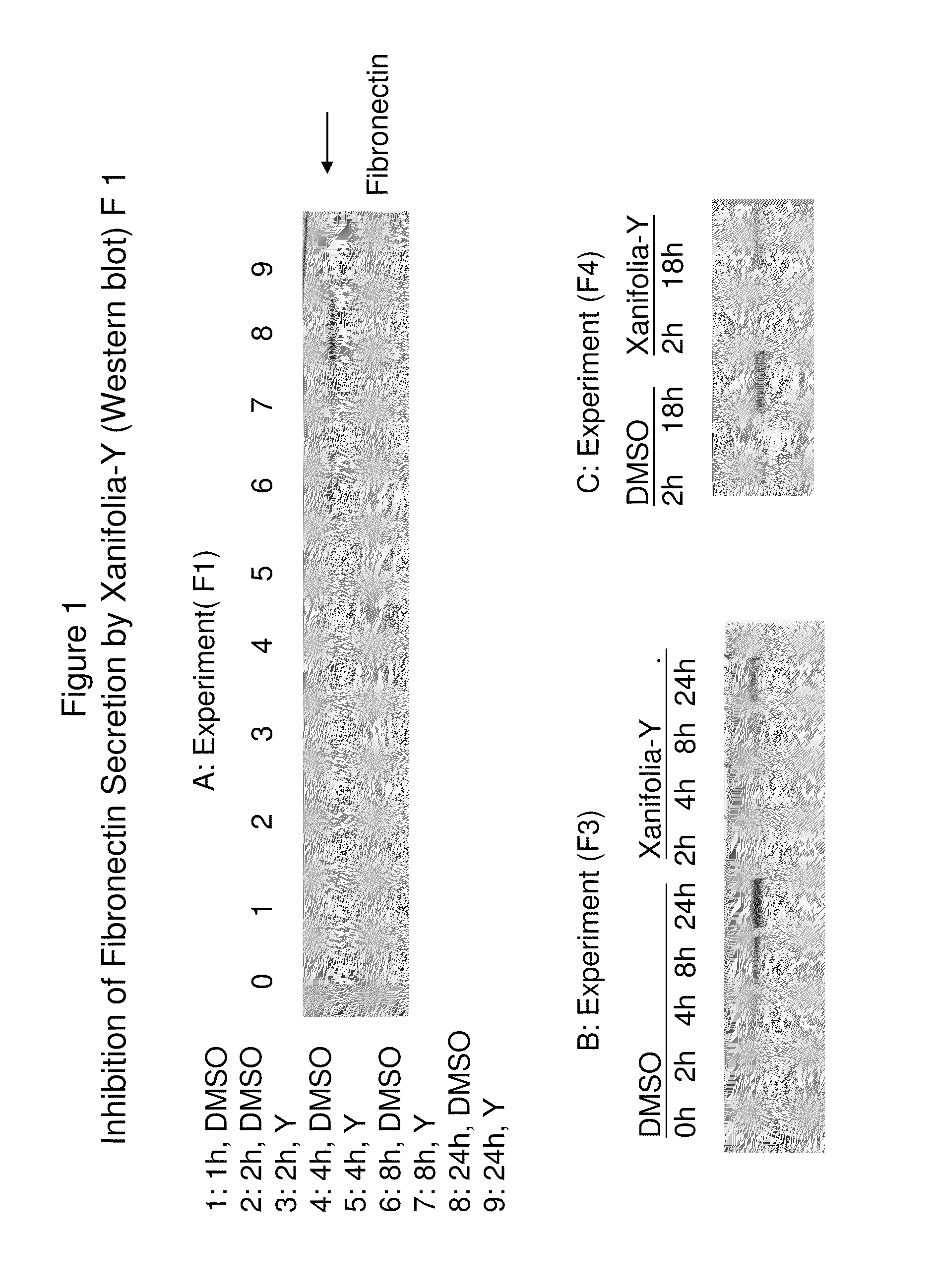
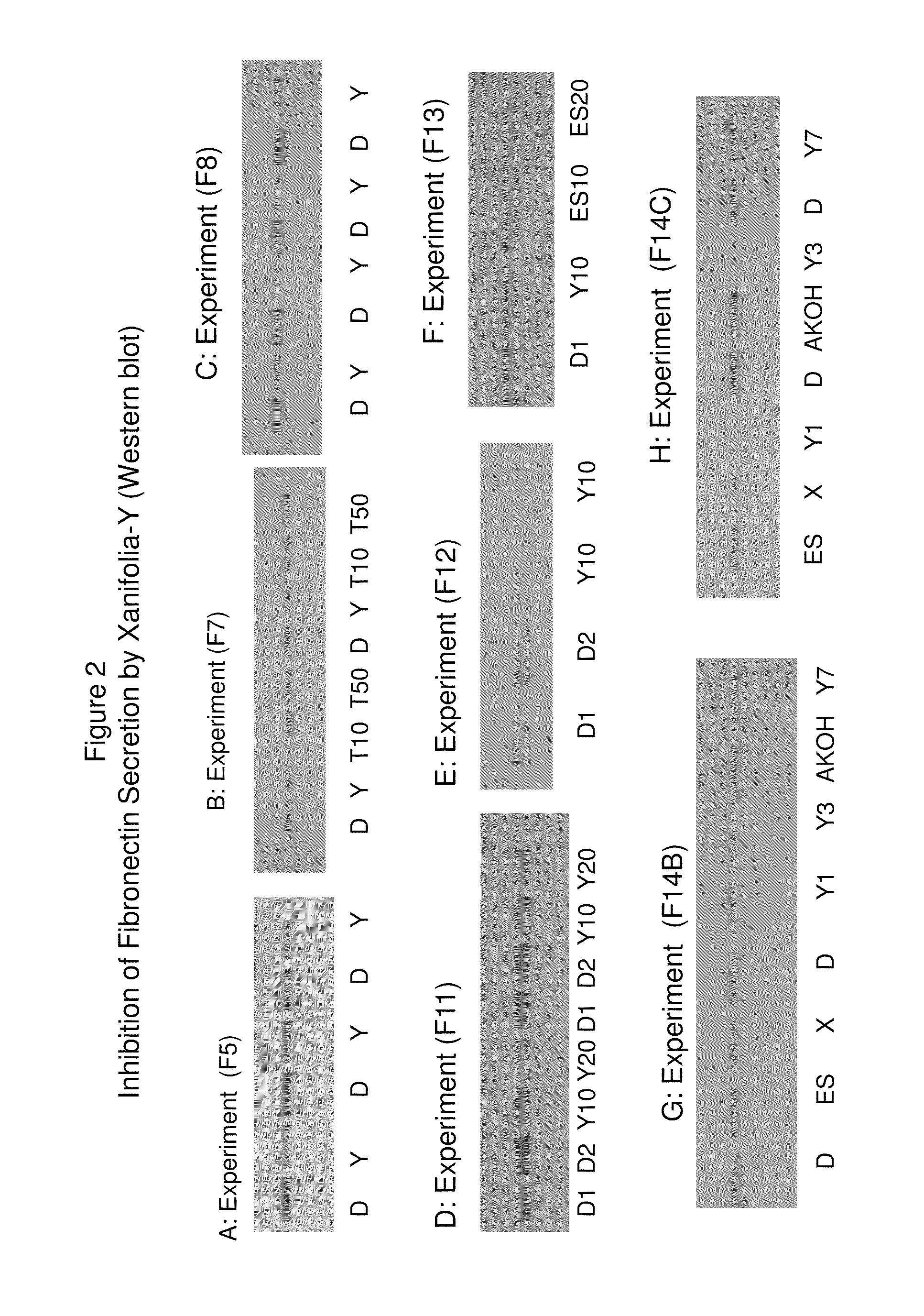
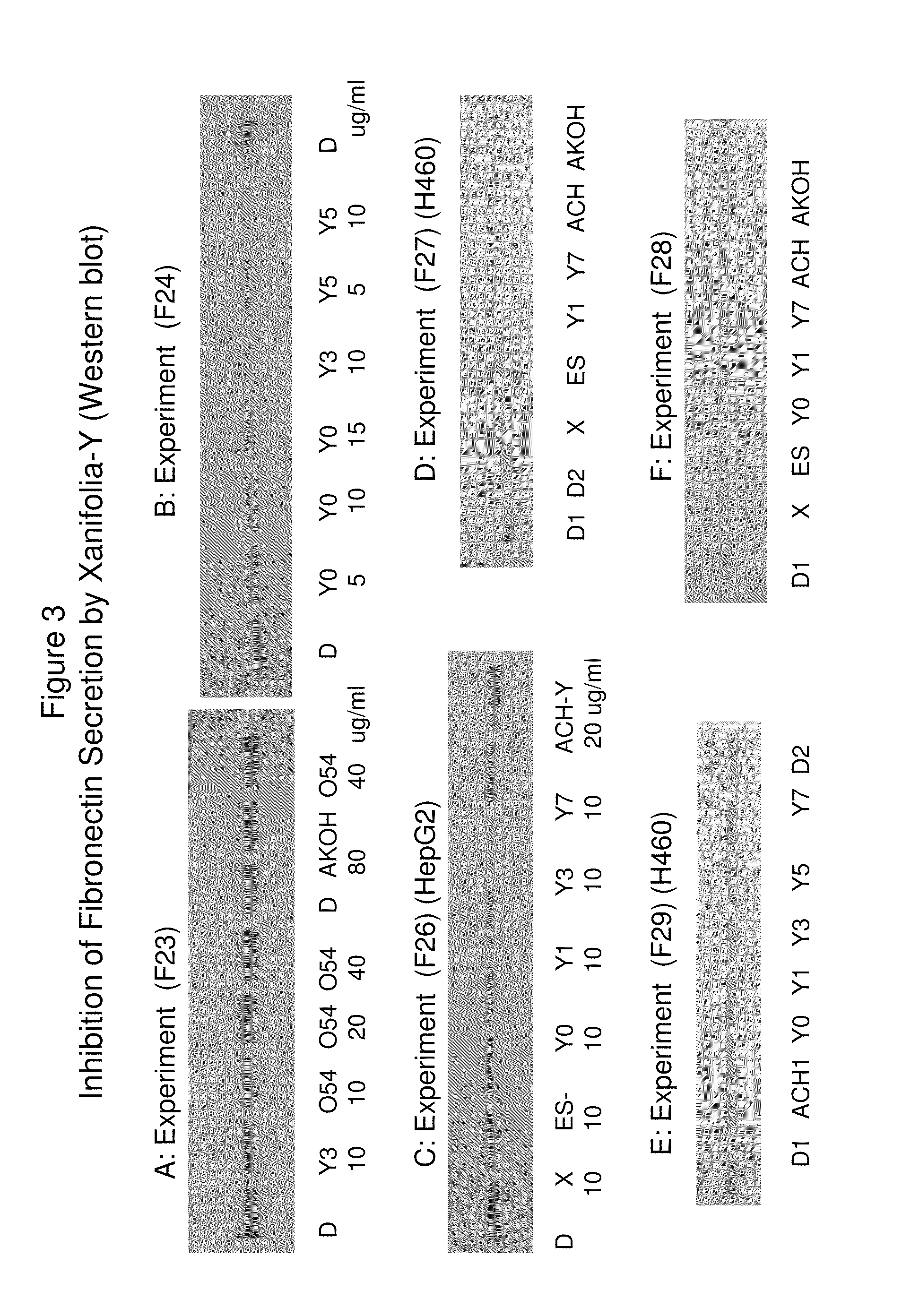
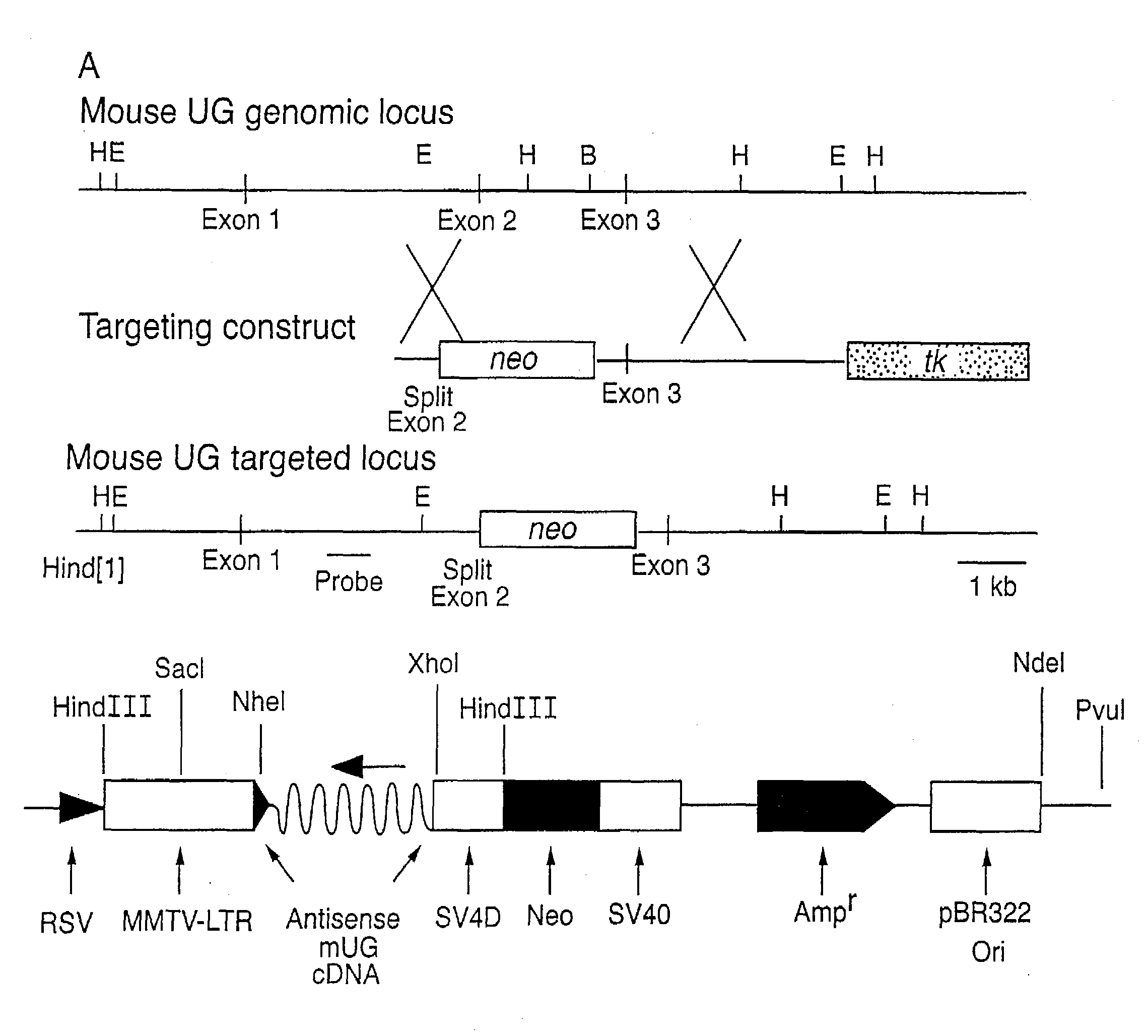
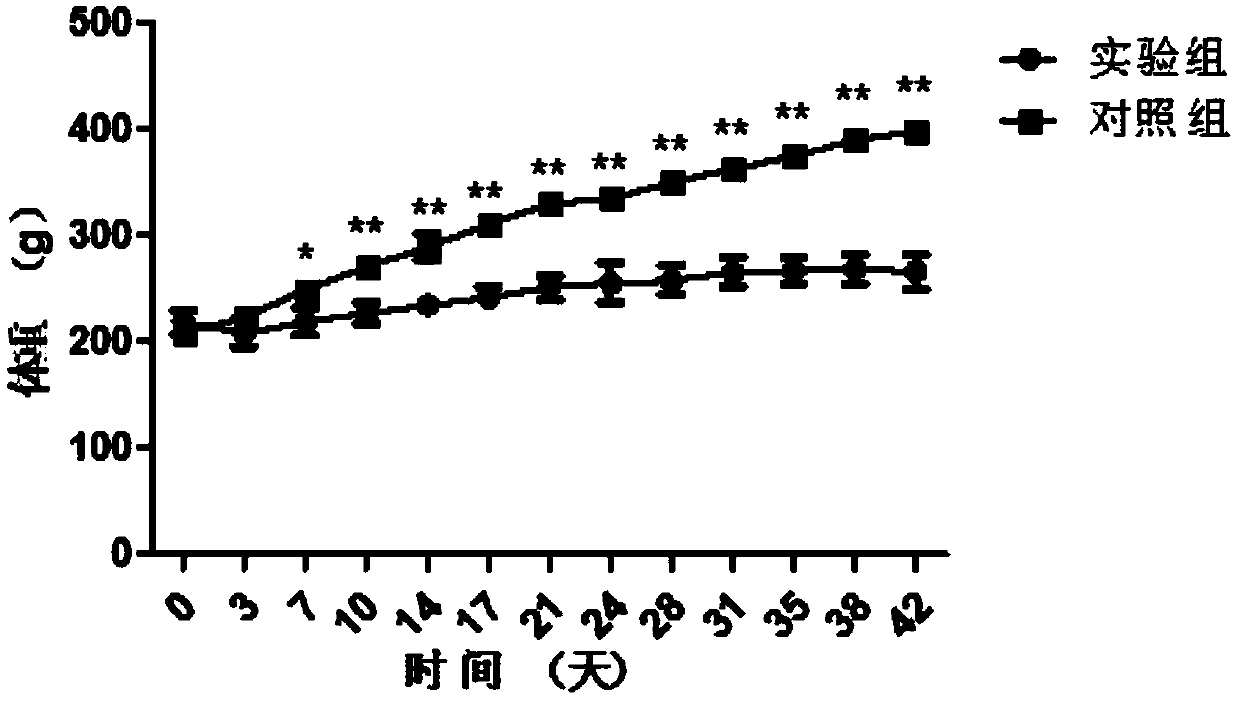
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
92 results about "Zonulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
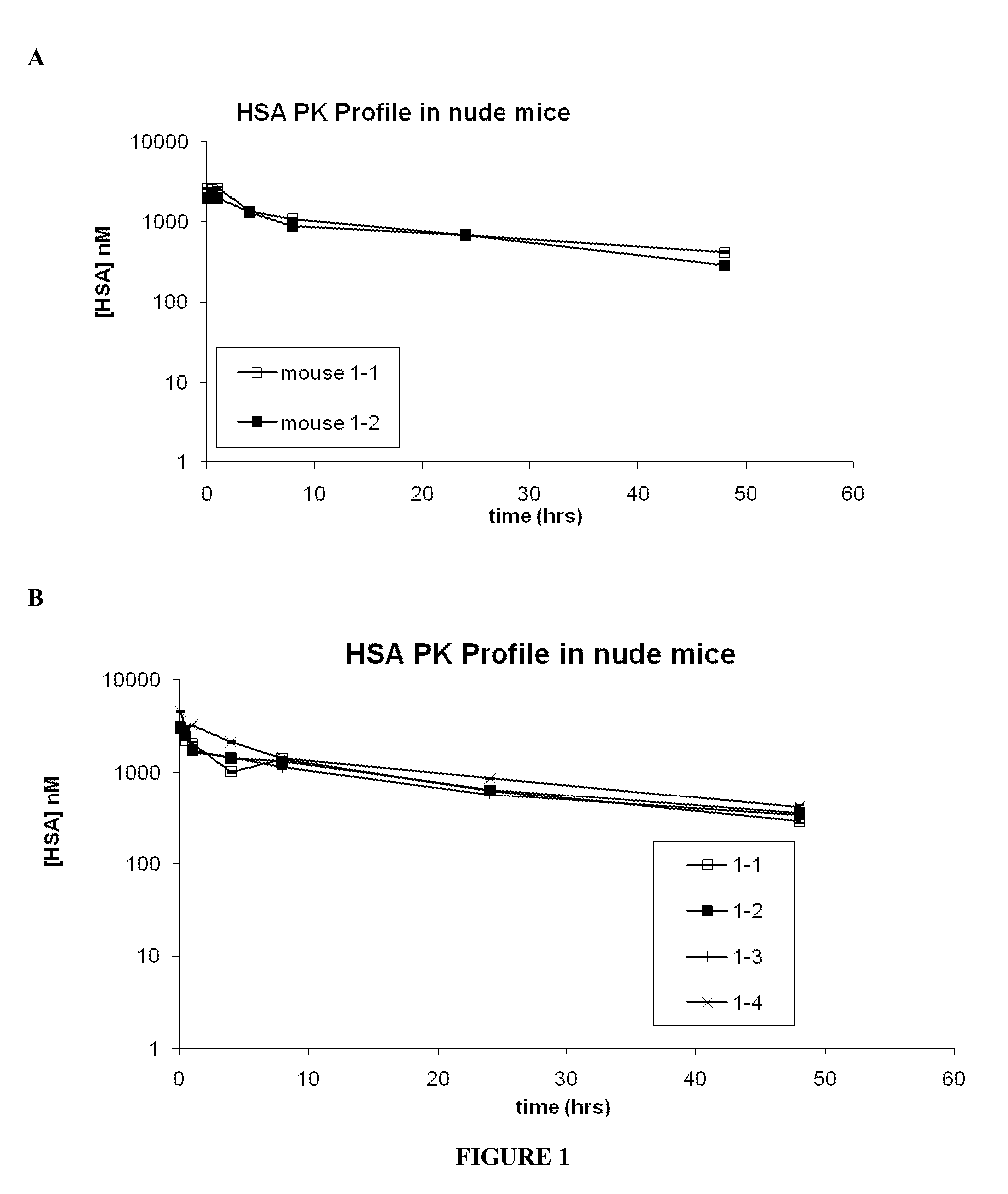
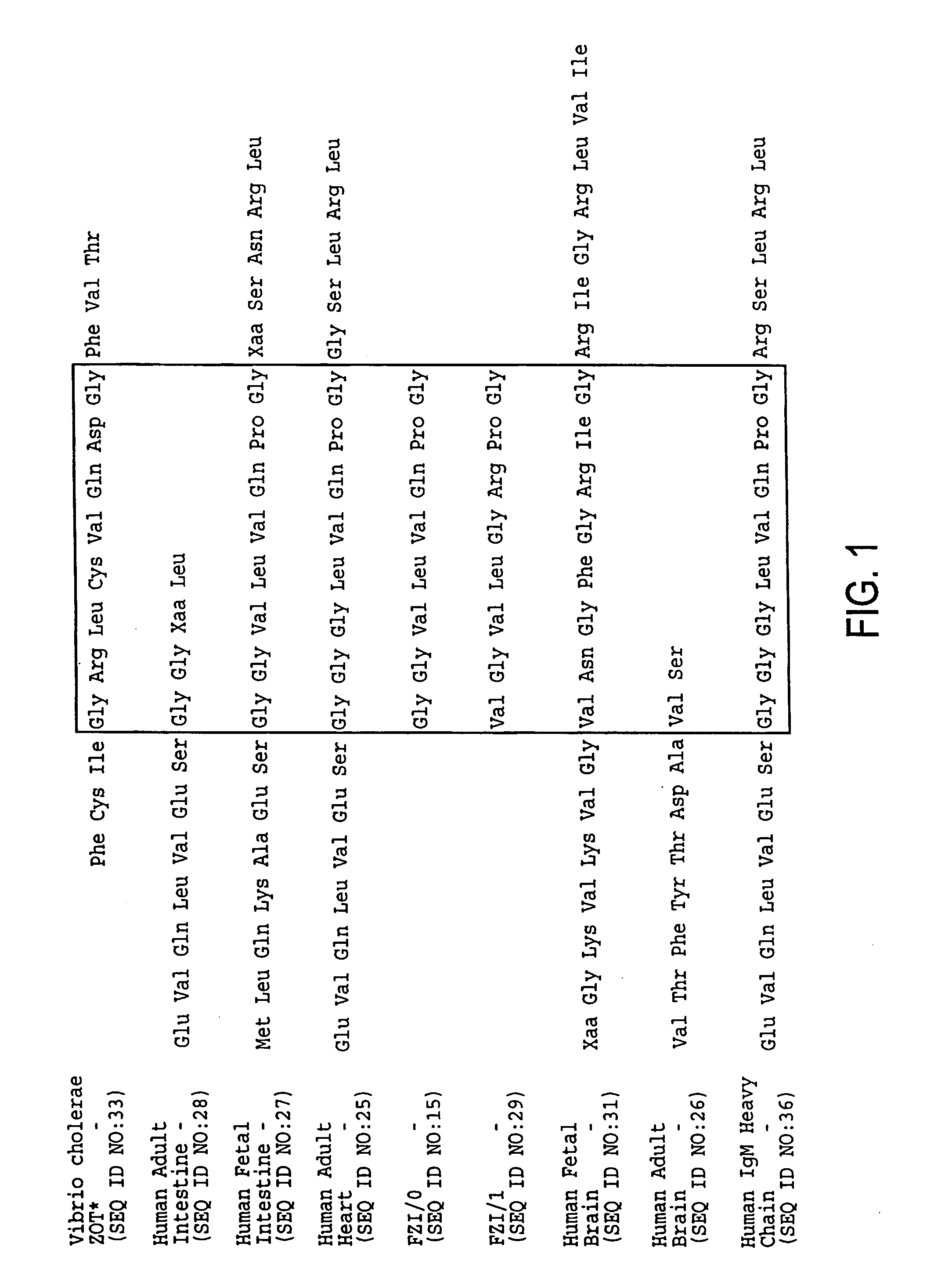
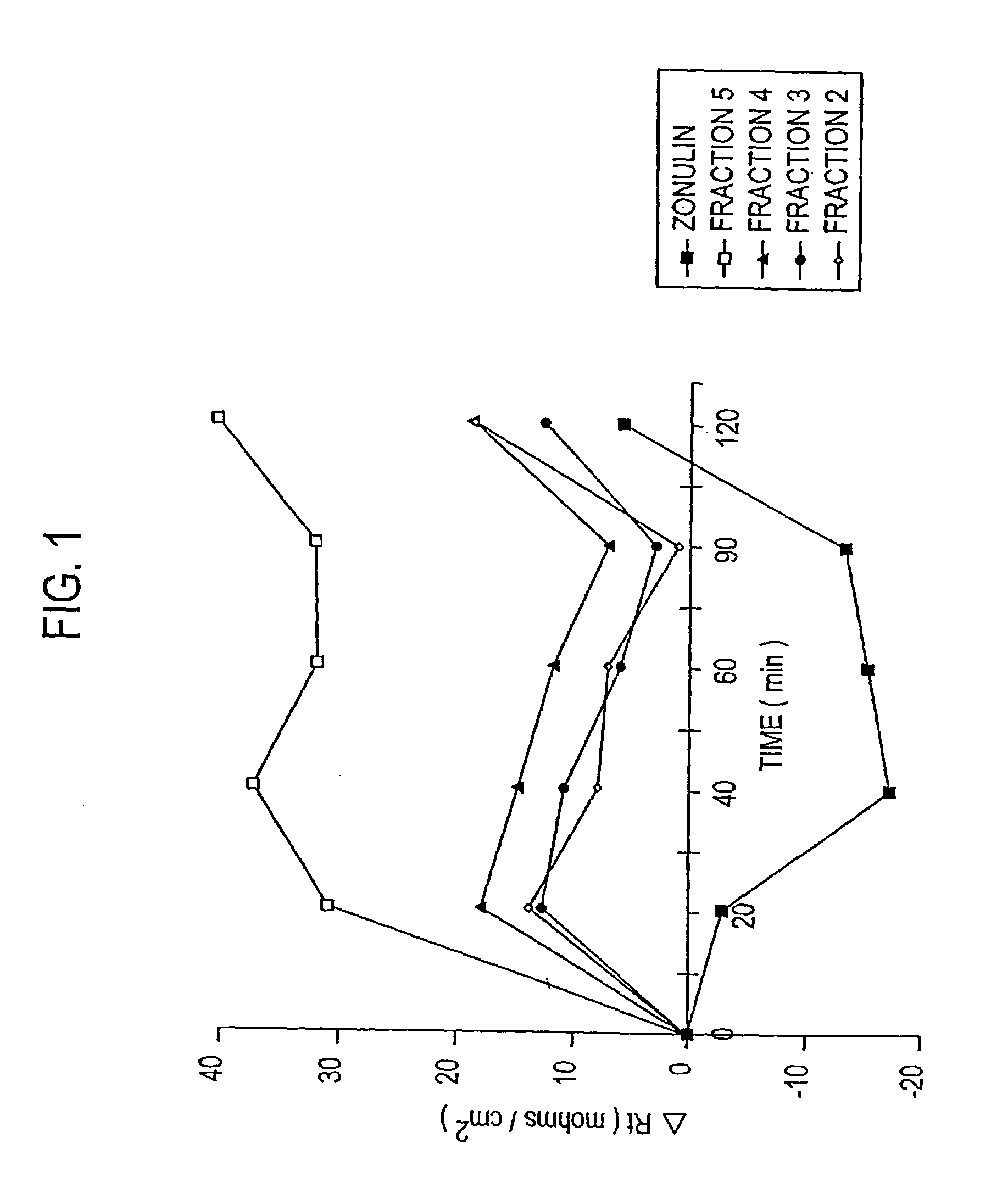
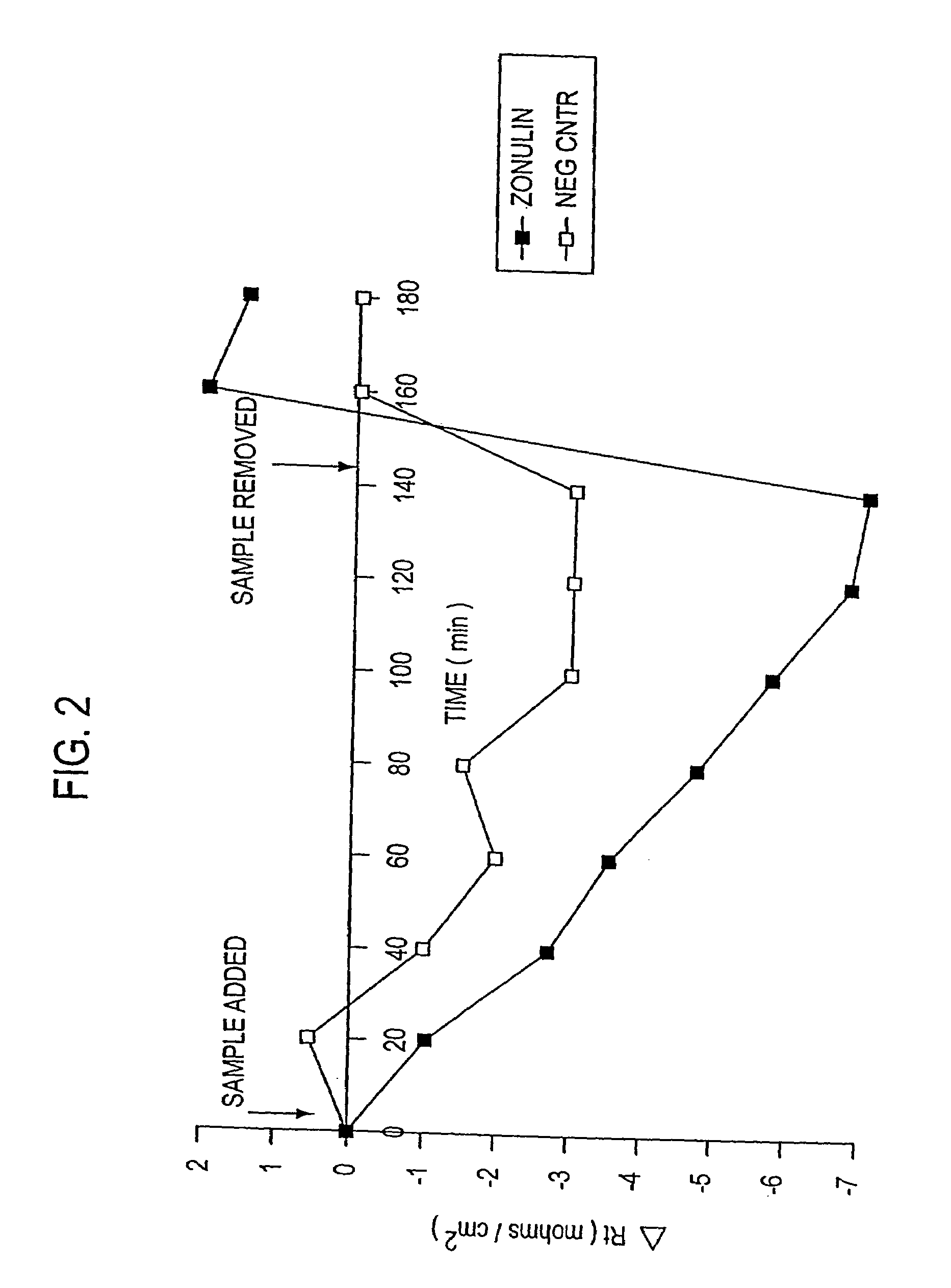
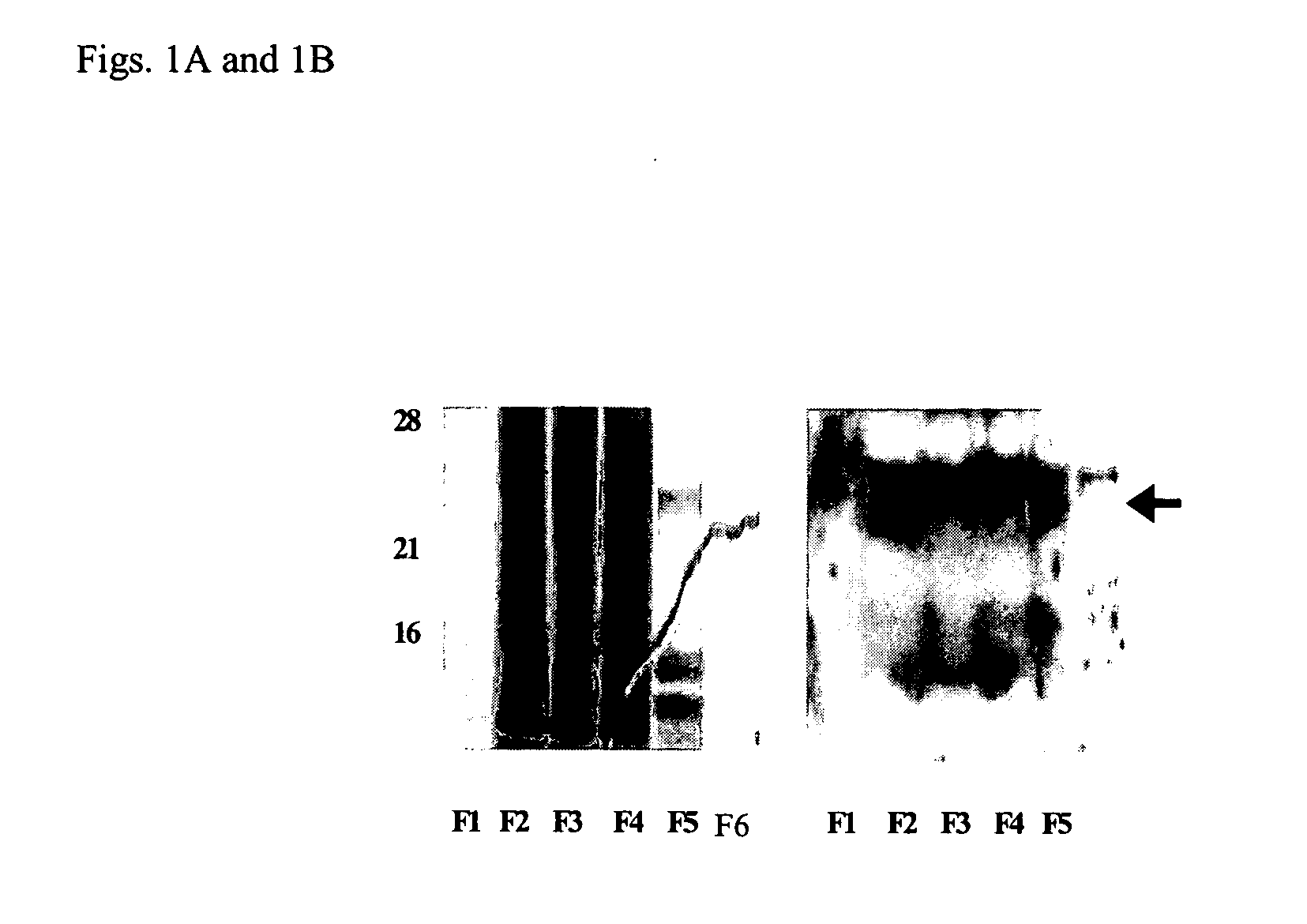
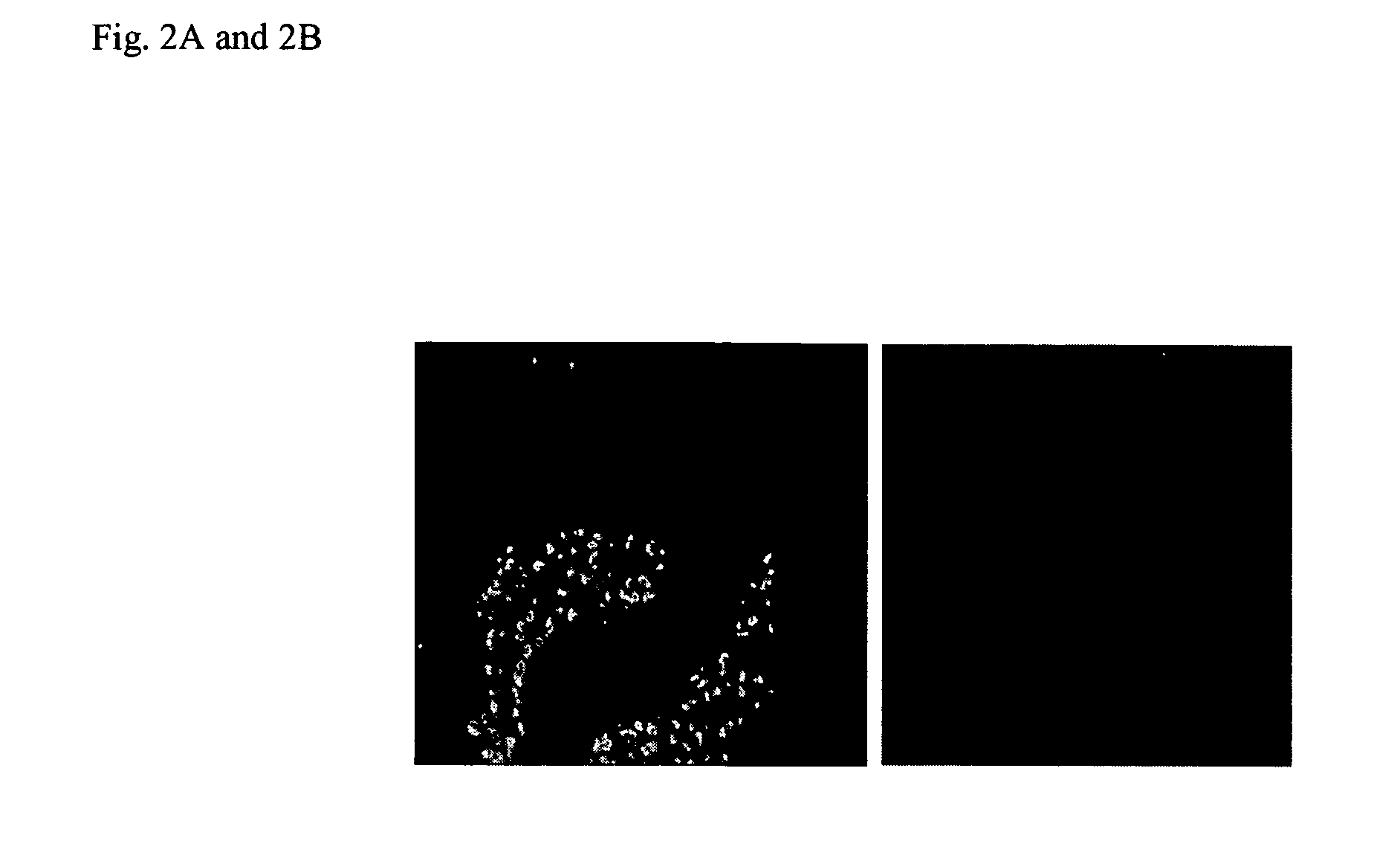
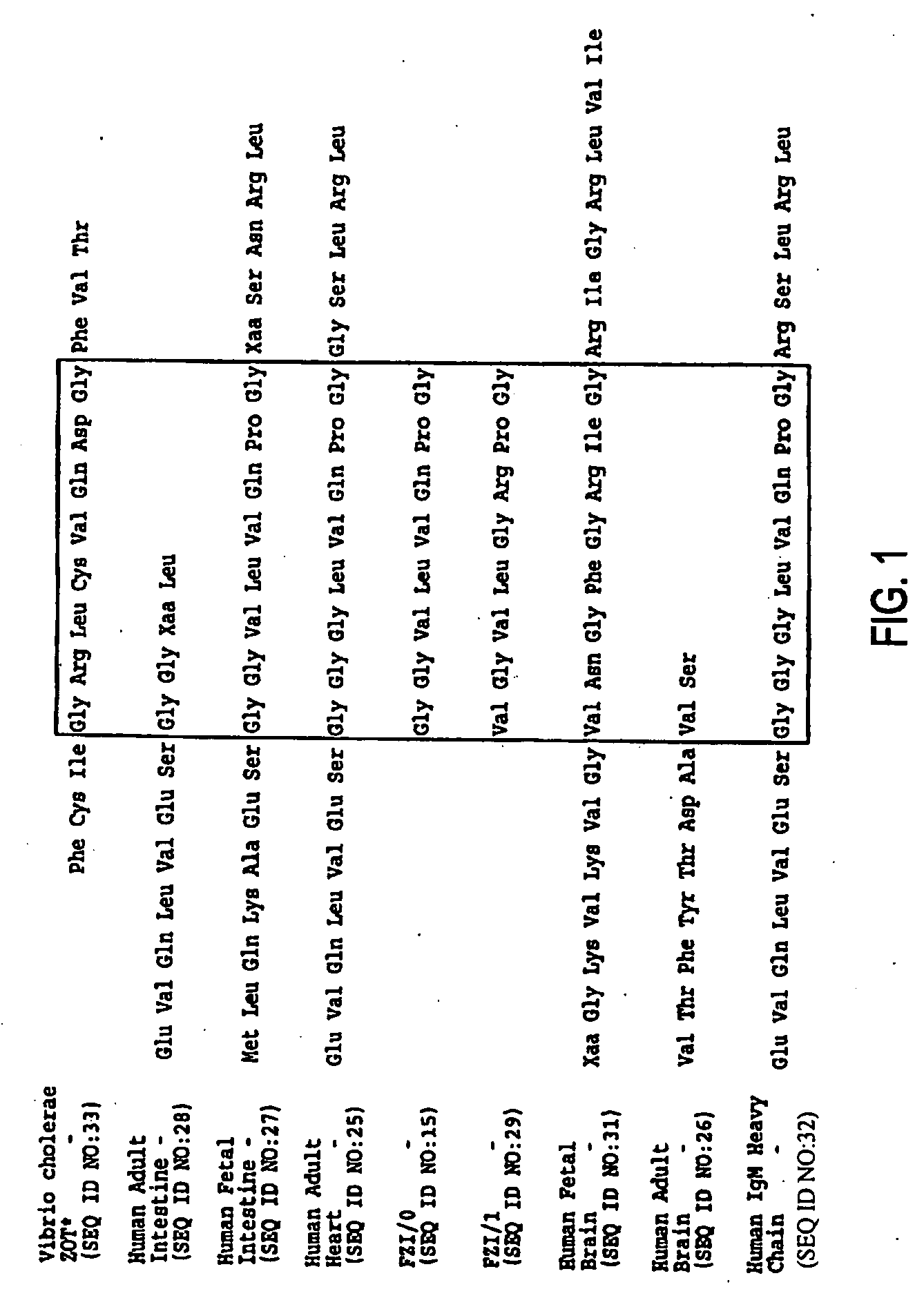
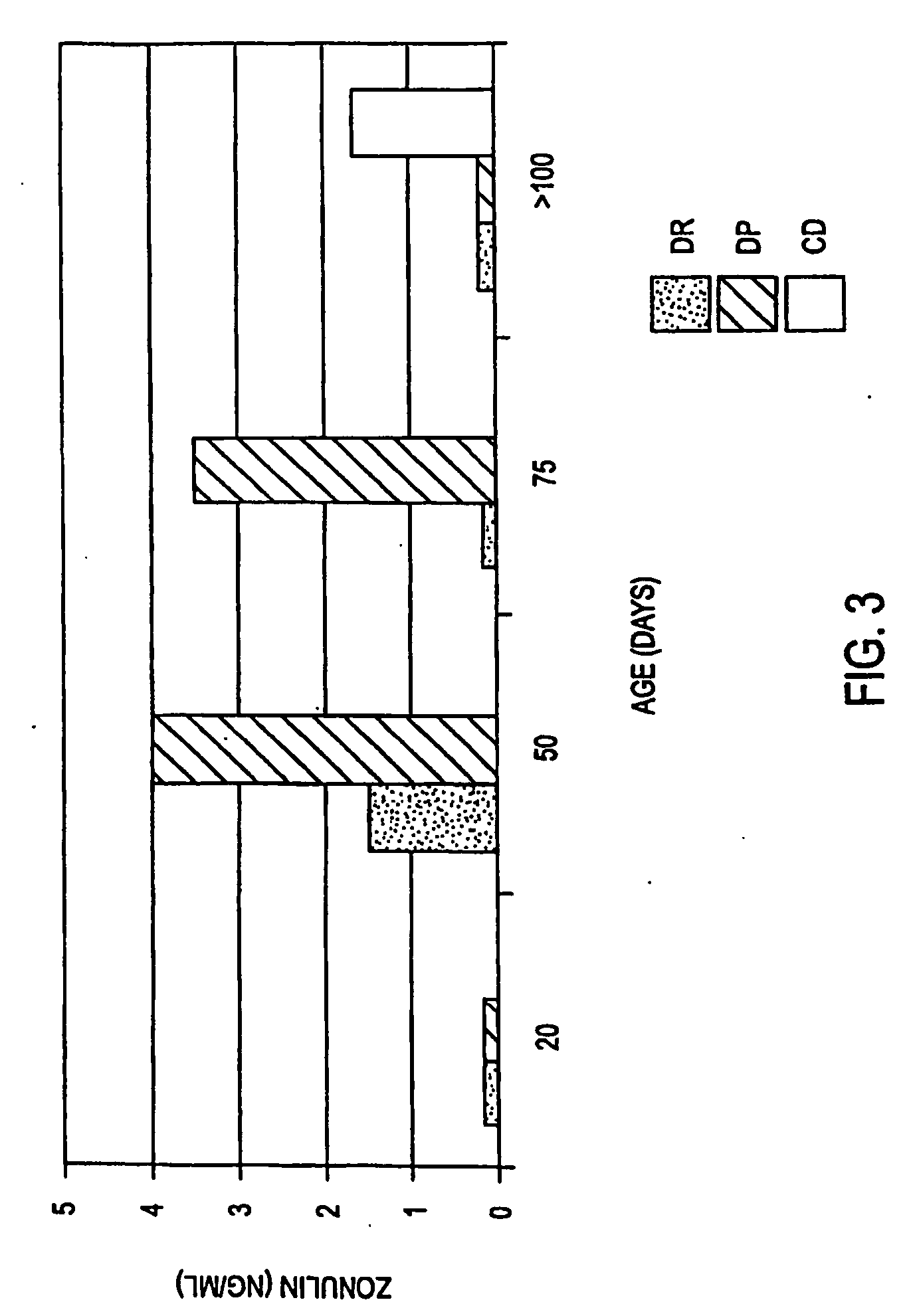
Zonulin (haptoglobin 2 precursor) is a protein that modulates the permeability of tight junctions between cells of the wall of the digestive tract. It was discovered in 2000 by Alessio Fasano and his team at the University of Maryland School of Medicine. As the mammalian analogue of zonula occludens toxin, secreted by cholera pathogen Vibrio cholerae, zonulin has been implicated in the pathogenesis of coeliac disease and diabetes mellitus type 1.
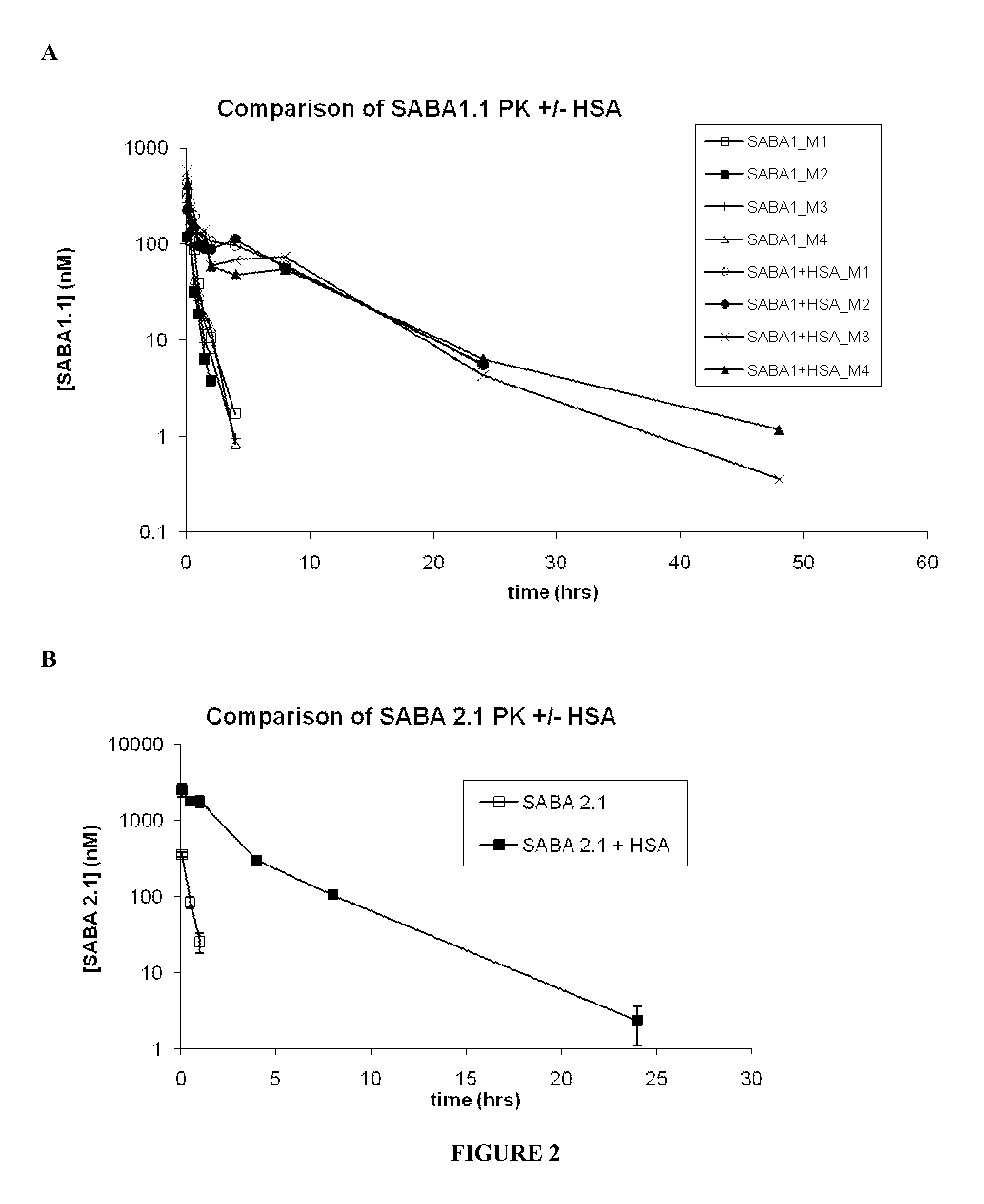
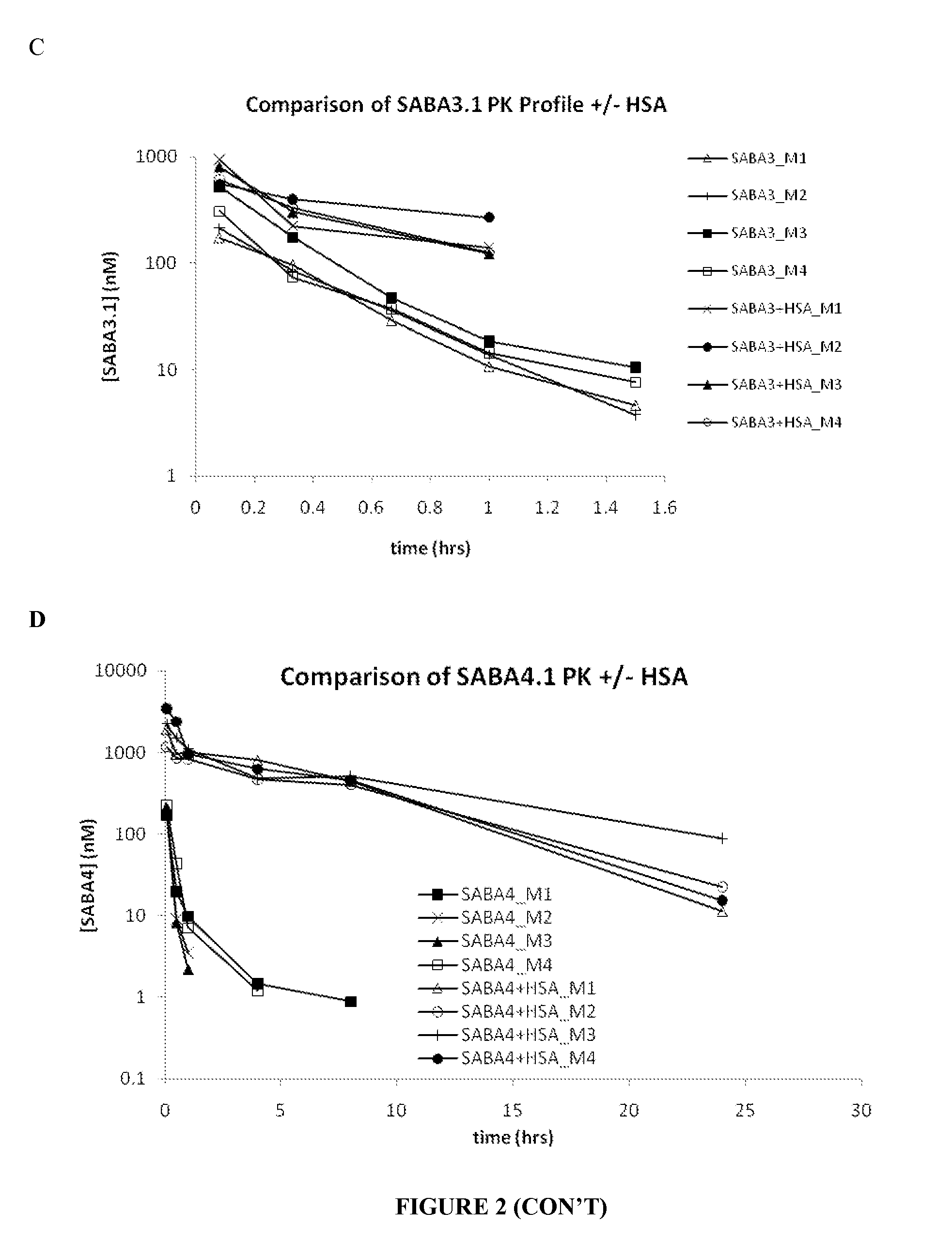
Serum albumin binding molecules
ActiveUS20110305663A1Improve solubilityReduce aggregationBacteriaPeptide/protein ingredientsSerum igeSerum protein albumin
The present invention relates to an antibody-like protein based on the tenth fibronectin type III domain (10Fn3) that binds to serum albumin. The invention further relates to fusion molecules comprising a serum albumin-binding 10Fn3 joined to a heterologous protein for use in diagnostic and therapeutic applications.
Owner:BRISTOL MYERS SQUIBB CO
Method of use of peptide antagonists of zonulin to prevent or delay the onset of diabetes
InactiveUS7026294B2Avoid delayPeptide/protein ingredientsMetabolism disorderDiabetes mellitusZonulin
Owner:UNIV OF MARYLAND
Fibronectin based scaffold proteins having improved stability
ActiveUS20130184212A1Improve stabilityReduce fragmentationBacteriaPeptide/protein ingredientsScaffold proteinProteinoid
The present application provides fibronectin based scaffold proteins associated with improved stability. The application also relates to stable formulations of fibronectin based scaffold proteins and the use thereof in diagnostic, research and therapeutic applications. The application further relates to cells comprising such proteins, polynucleotides encoding such proteins or fragments thereof, and to vectors comprising such polynucleotides.
Owner:BRISTOL MYERS SQUIBB CO
Antibodies to the ED-B domain of fibronectin, their construction and uses
InactiveUS7273924B1High expressionEasy to detectOrganic active ingredientsBacteriaCancer researchFibronectin
A specific binding member is specific for and binds directly to the ED—B oncofoetal domain of fibronectin (FN).
Owner:PHILOGEN SRL LLC
Fibronectin based scaffold proteins having improved stability
ActiveUS9562089B2Improve stabilityReduce fragmentationBacteriaPeptide/protein ingredientsScaffold proteinProteinoid
Owner:BRISTOL MYERS SQUIBB CO
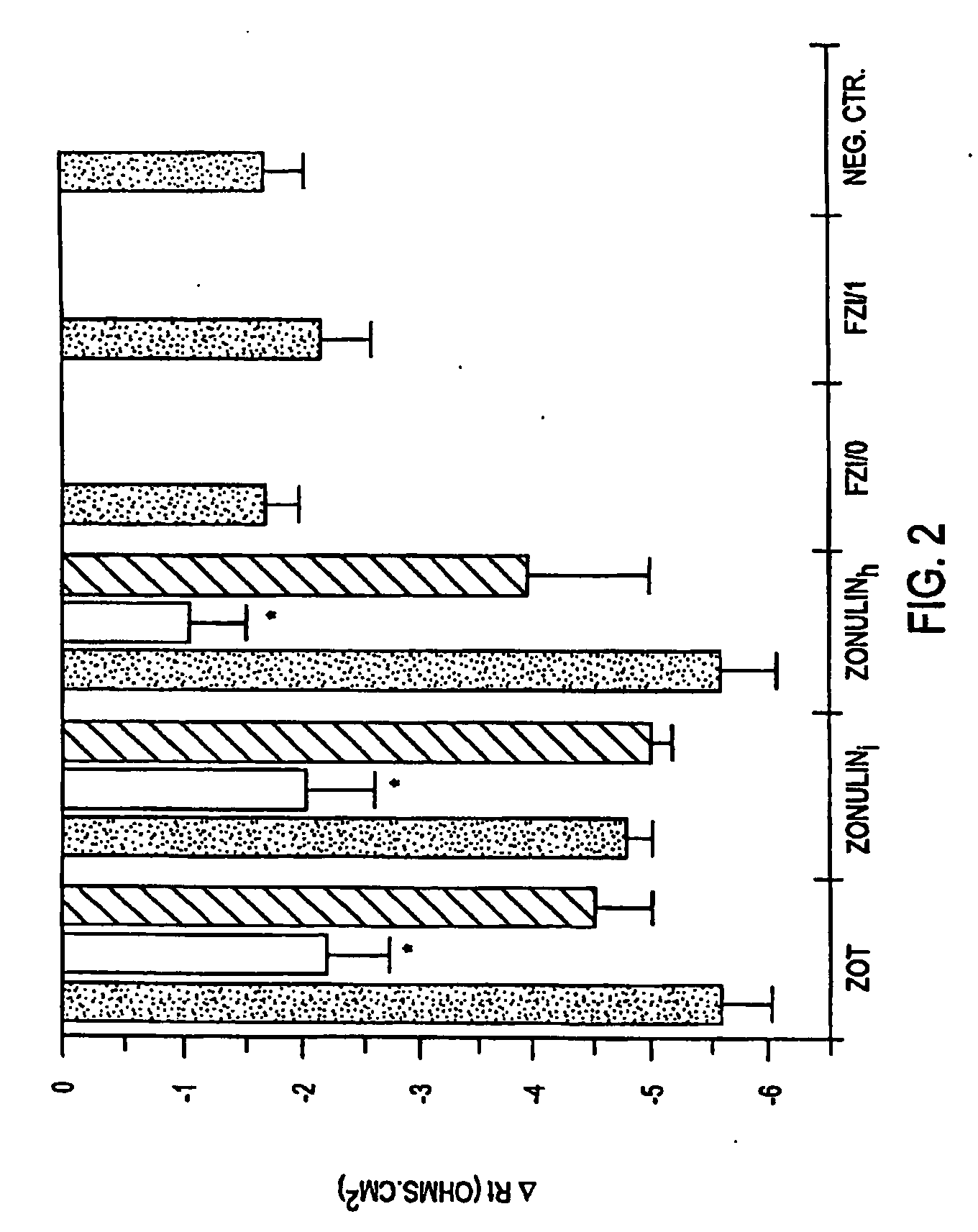
Agonist polypeptide of receptor for Zot and Zonulin
ActiveUS20050059593A1Antibacterial agentsPeptide/protein ingredientsAgonistPharmaceutical formulation
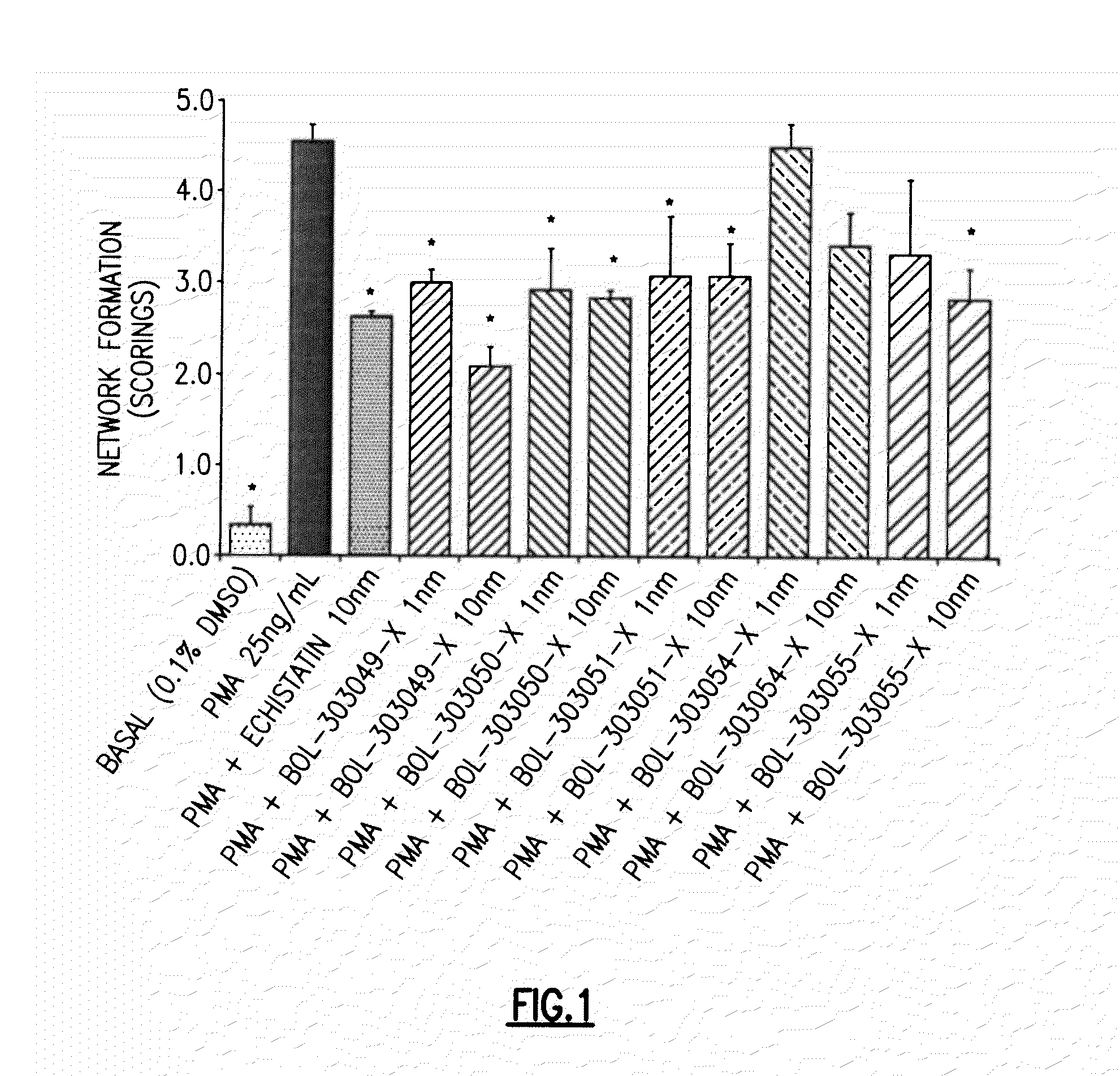
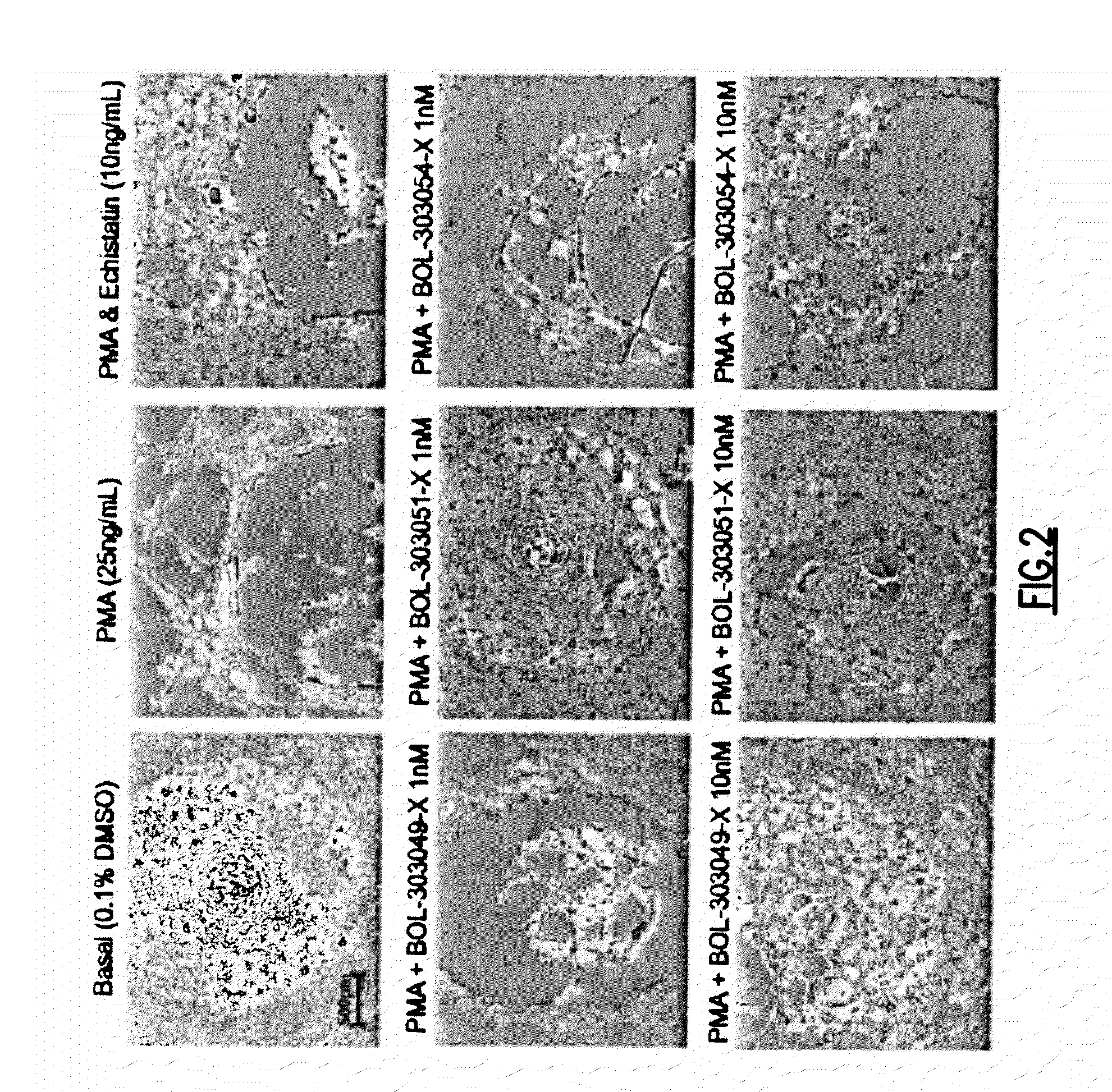
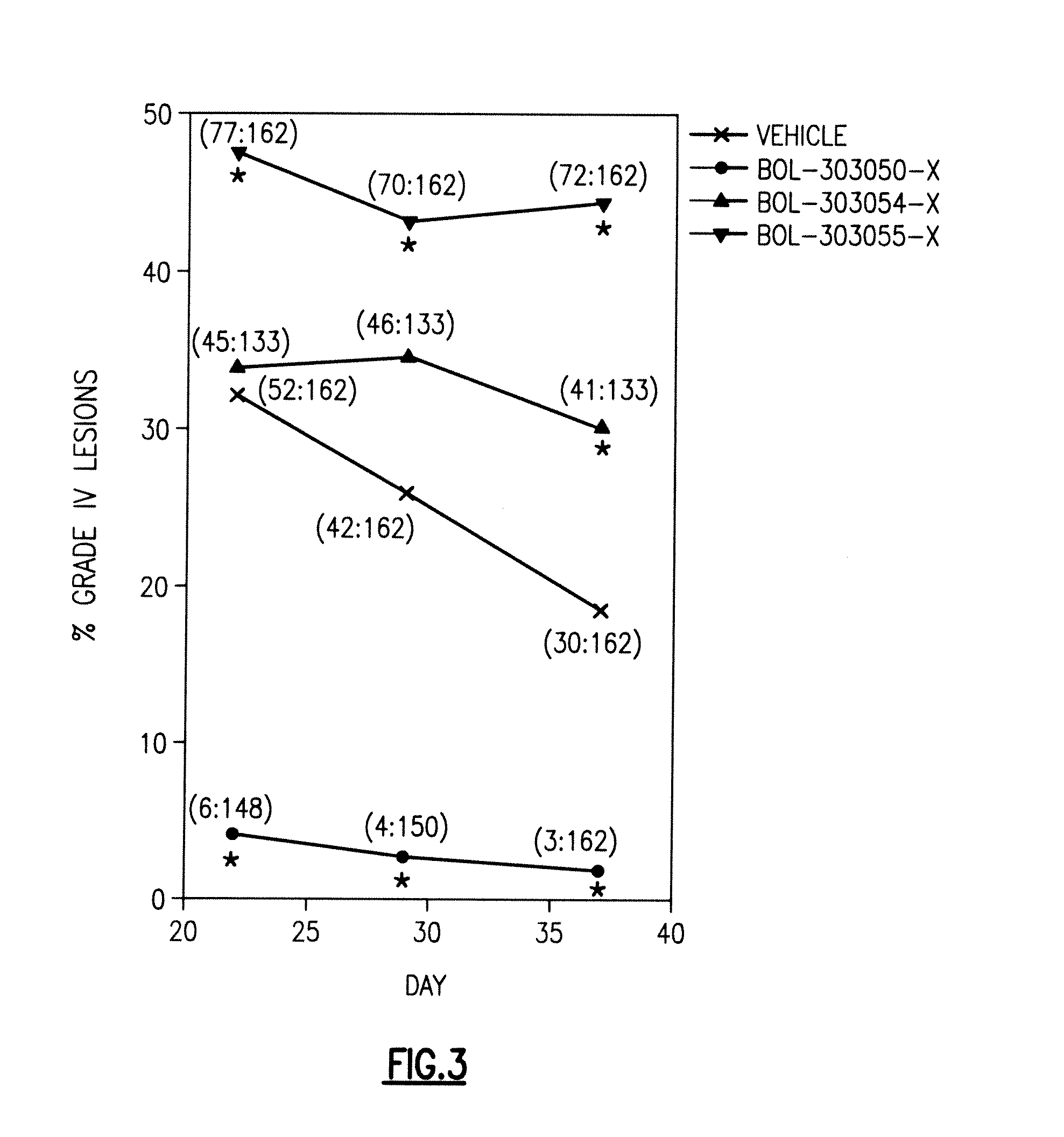
Agonist polypeptide of a receptor protein has been identified. The agonist can be used to facilitate drug and antigen absorption. Suitable routes of administration include oral, nasal, transdermal, and intravenous. Pharmaceutical formulations may comprise a therapeutic agent or an immunogenic agent in combination with the agonist polypeptide.
Owner:MARYLAND UNIV OF BALTIMORE OFFICE OF RES & DEV
Agonist polypeptide of receptor for Zot and Zonulin
Agonist polypeptide of a receptor protein has been identified. The agonist can be used to facilitate drug and antigen absorption. Suitable routes of administration include oral, nasal, transdermal, and intravenous. Pharmaceutical formulations may comprise a therapeutic agent or an immunogenic agent in combination with the agonist polypeptide.
Owner:MARYLAND UNIV OF BALTIMORE OFFICE OF RES & DEV
Oral delivery of therapeutic agents using tight junction agonists
The present invention provides compositions and methods for the administration of the compositions to mammals. The compositions comprise therapeutic agents and an intestinal absorption enhancing amount of one or more tight junction agonists. Tight junction agonists include zonulin and / or ZOT receptor agonists. Methods of the invention include orally administering compositions of the invention.
Owner:UNIV OF MARYLAND
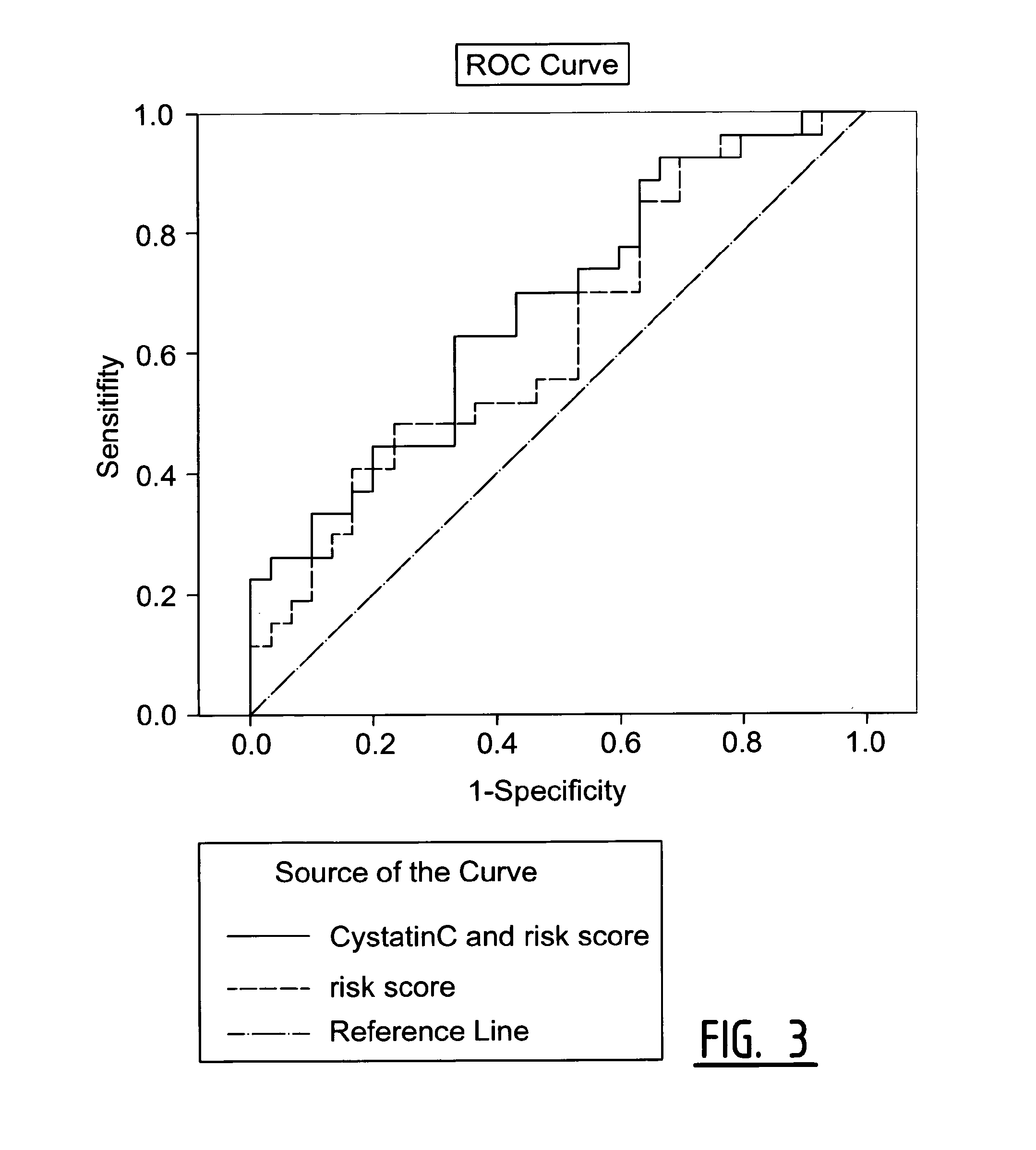
Determination of Exosomel Biomarkers for Predicting Cardiovascular Events
InactiveUS20120309041A1Reduce stepsComplicate to detectMicrobiological testing/measurementImmunoglobulins against animals/humansAmniotic fluidNGAL Protein
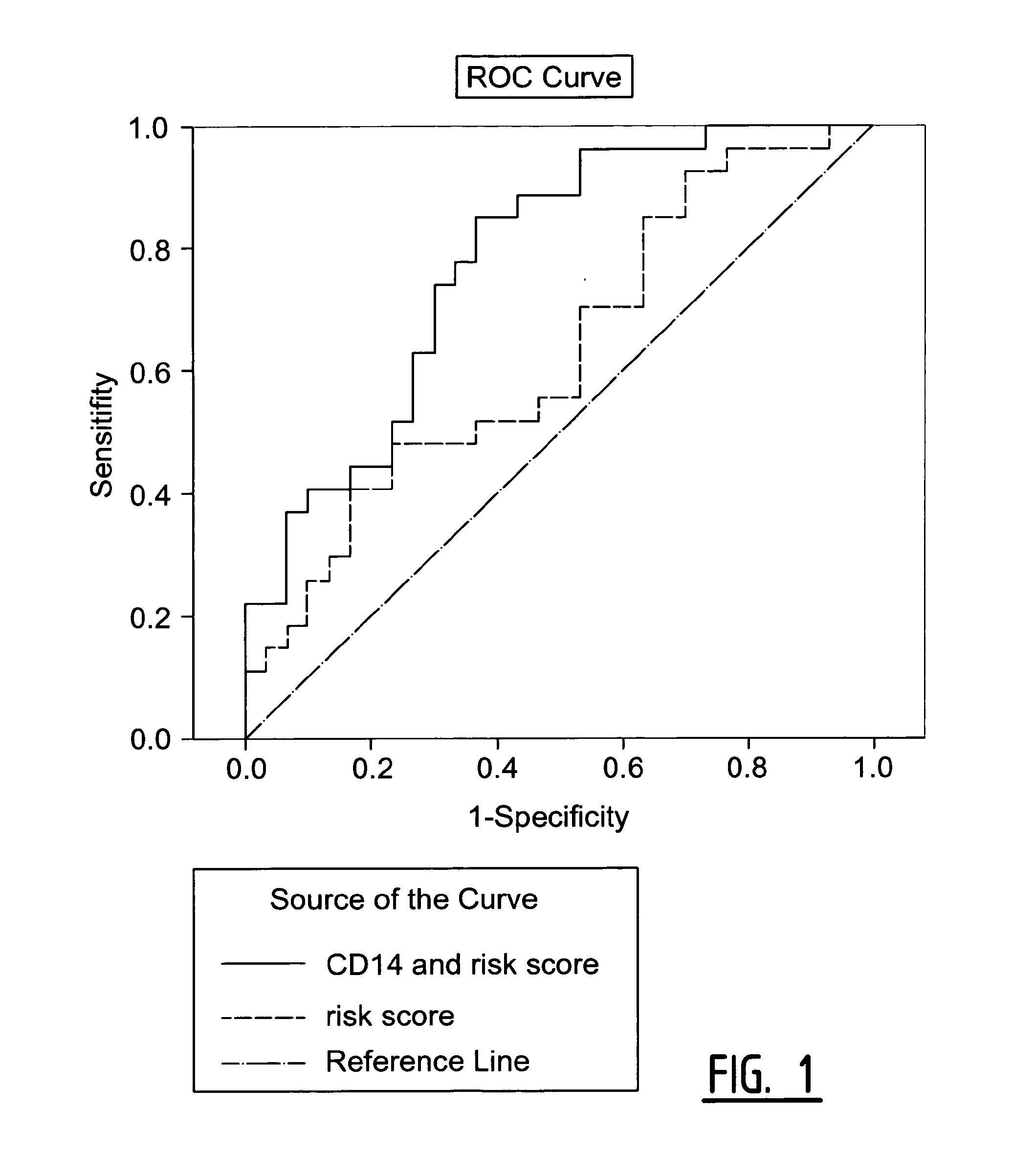
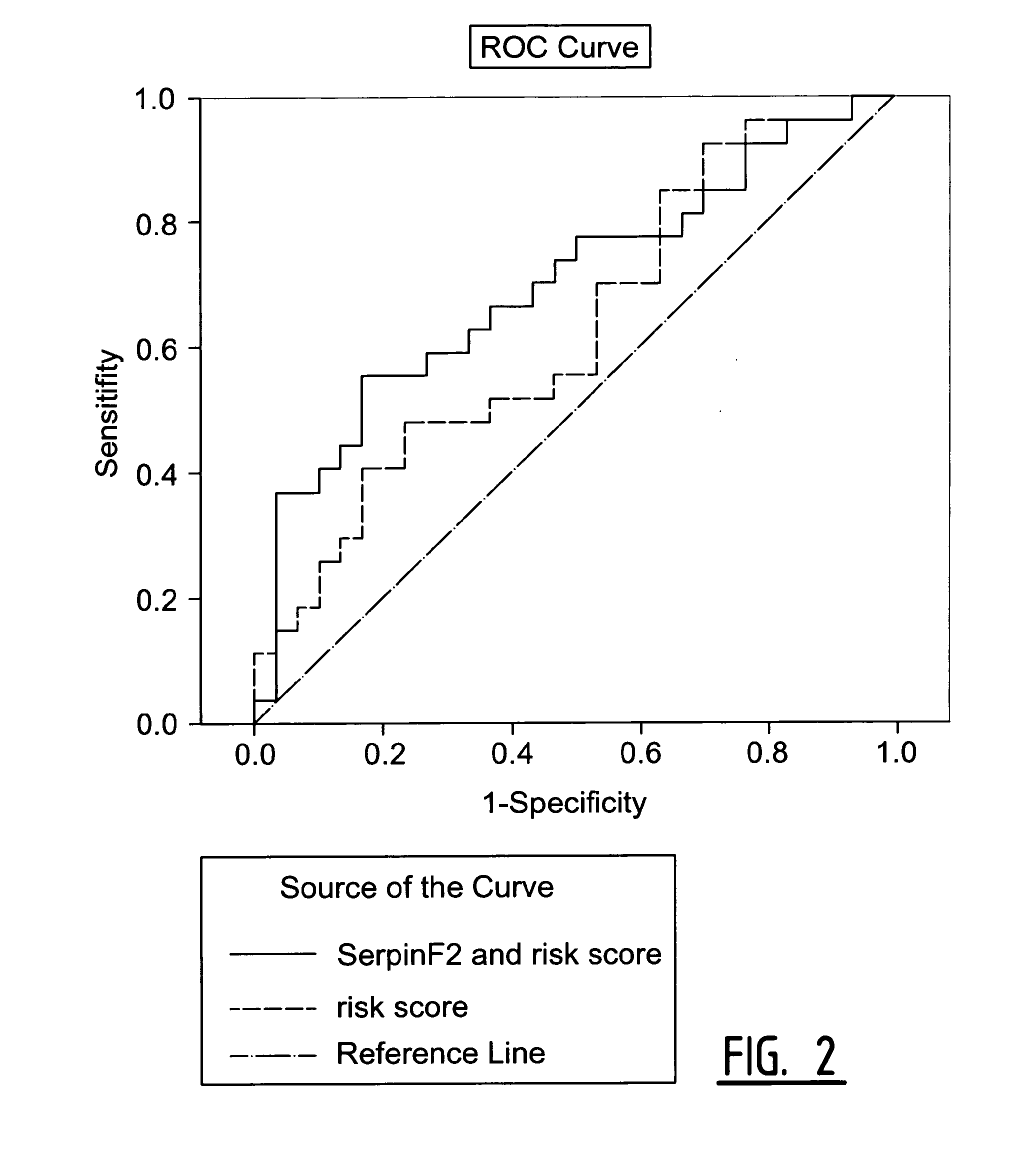
The present invention relates to a method of predicting the risk of a subject developing a cardiovascular event, comprising determining the presence of a biomarker that is indicative of the risk of developing a cardiovascular event in exosomes from the subject. The exosomes are suitably isolated from a body fluid selected from serum, plasma, blood, urine, amniotic fluid, malignant ascites, bronchoalveolar lavage fluid, synovial fluid, breast milk, saliva, in particular serum. The biomarker is selected from the proteins Vitronectin, Serpin F2, CD14, Cystatin C, Plasminogen, Nidogen 2 or any combination of two or more of these proteins.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
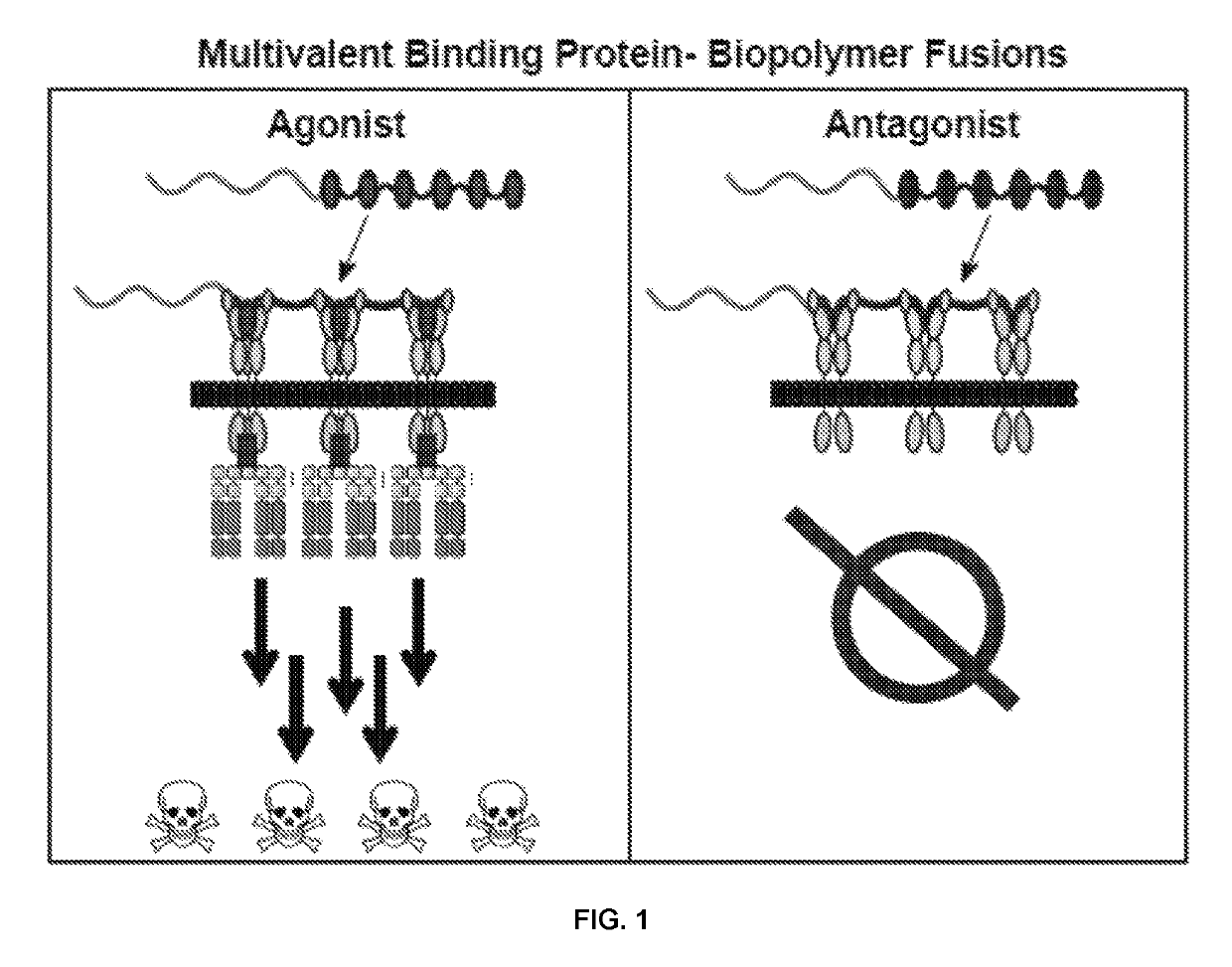
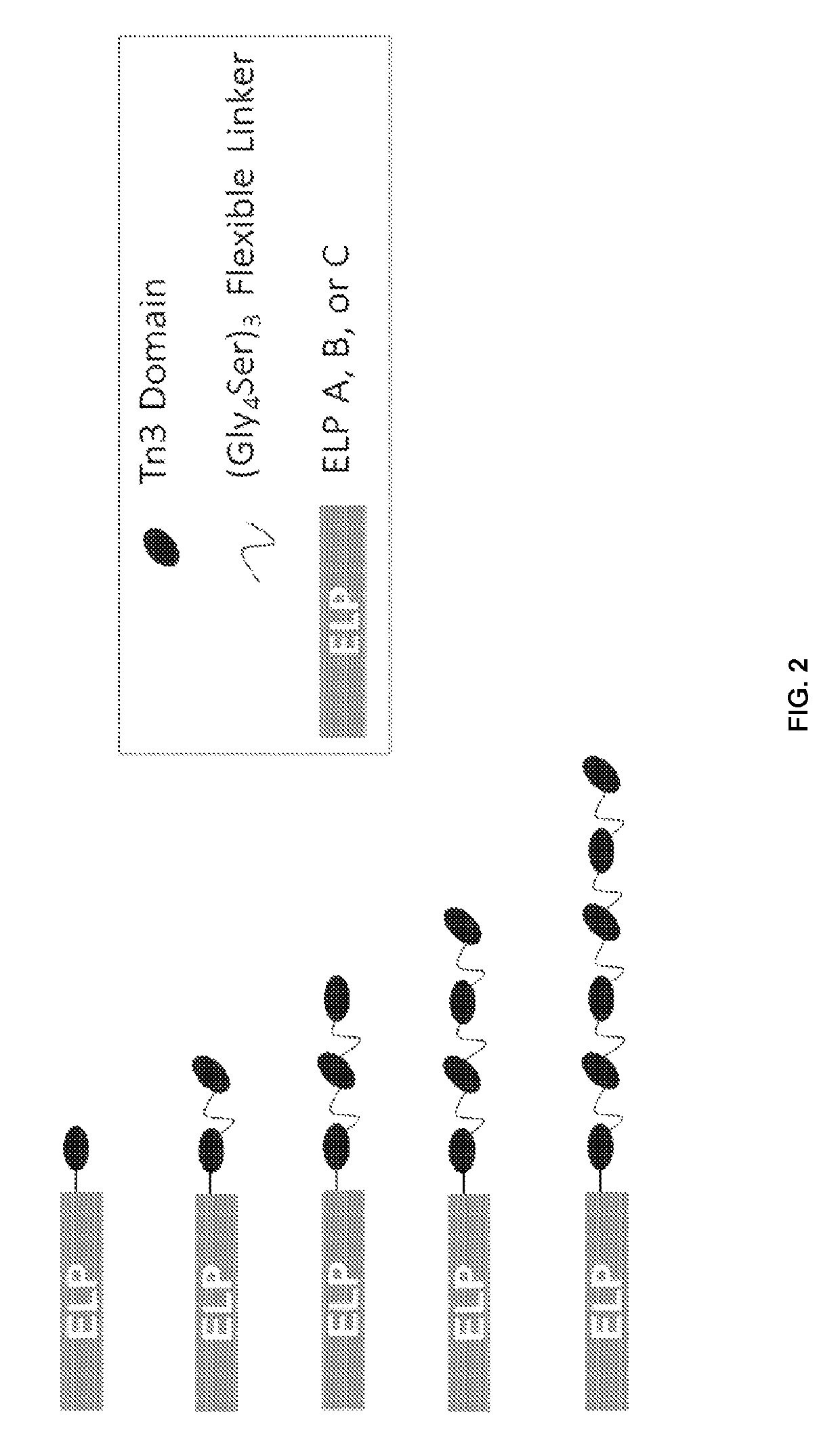
Fibronectin type III domain-based fusion proteins
ActiveUS10385115B2Polypeptide with localisation/targeting motifConnective tissue peptidesDiseaseMedicine
Provided herein are fusion proteins including at least one binding polypeptide and at least one unstructured polypeptide. The fusion protein may further include at least one linker. Further provided are methods for determining the presence of a target in a sample, methods of treating a disease, methods of diagnosing a disease in a subject, and methods of determining the effectiveness of a treatment for a disease in a subject. The methods may include administering to the subject an effective amount of the fusion protein.
Owner:DUKE UNIV
Method of use of antagonists of zonulin to prevent the loss of or to regenerate pancreatic cells
InactiveUS20060287233A1Improve permeabilityImprove breathabilityPeptide/protein ingredientsBlood/immune system cellsDiabetes mellitusProviding material
Owner:FASANO ALESSIO +2
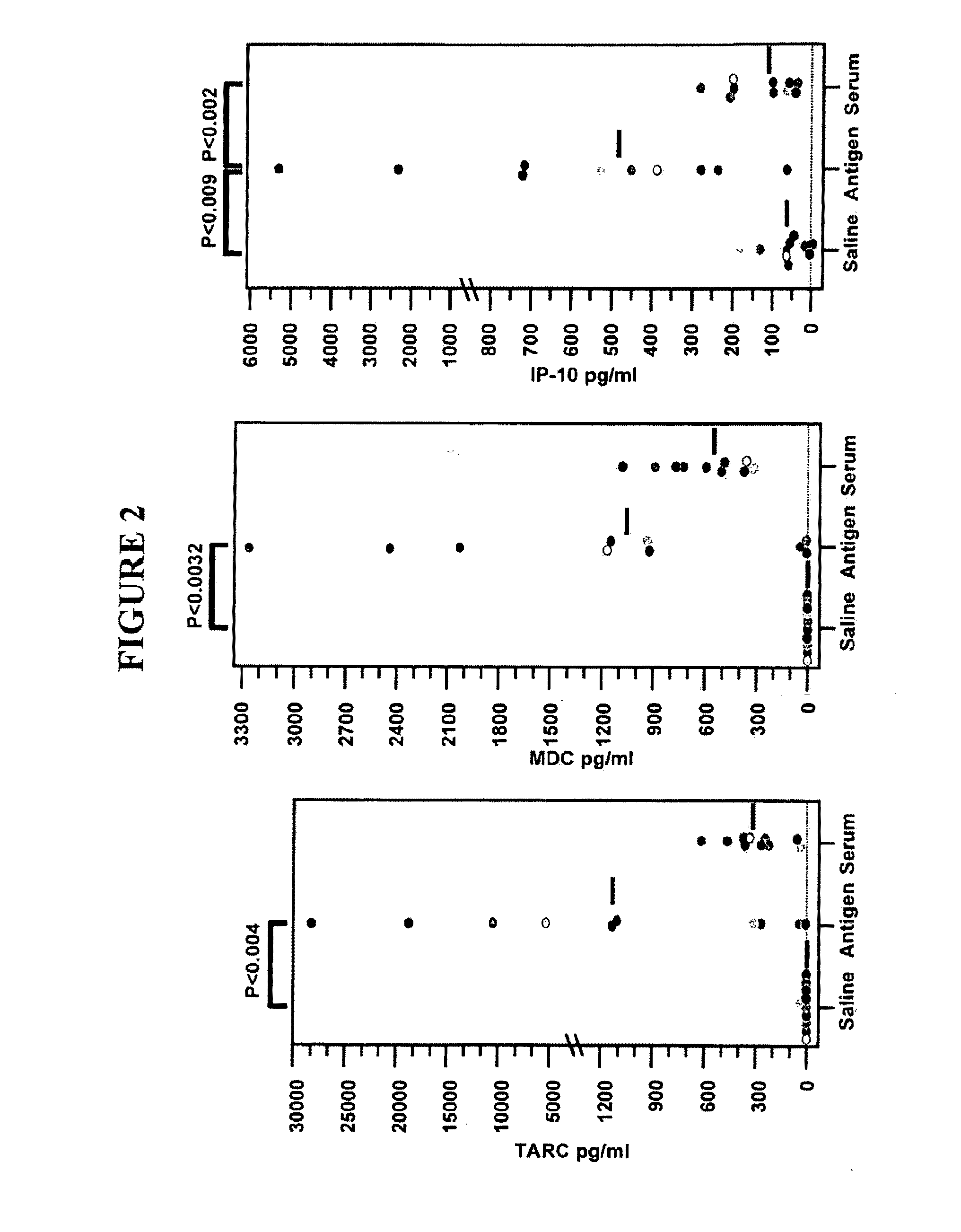
Methods of diagnosing and treating asthma
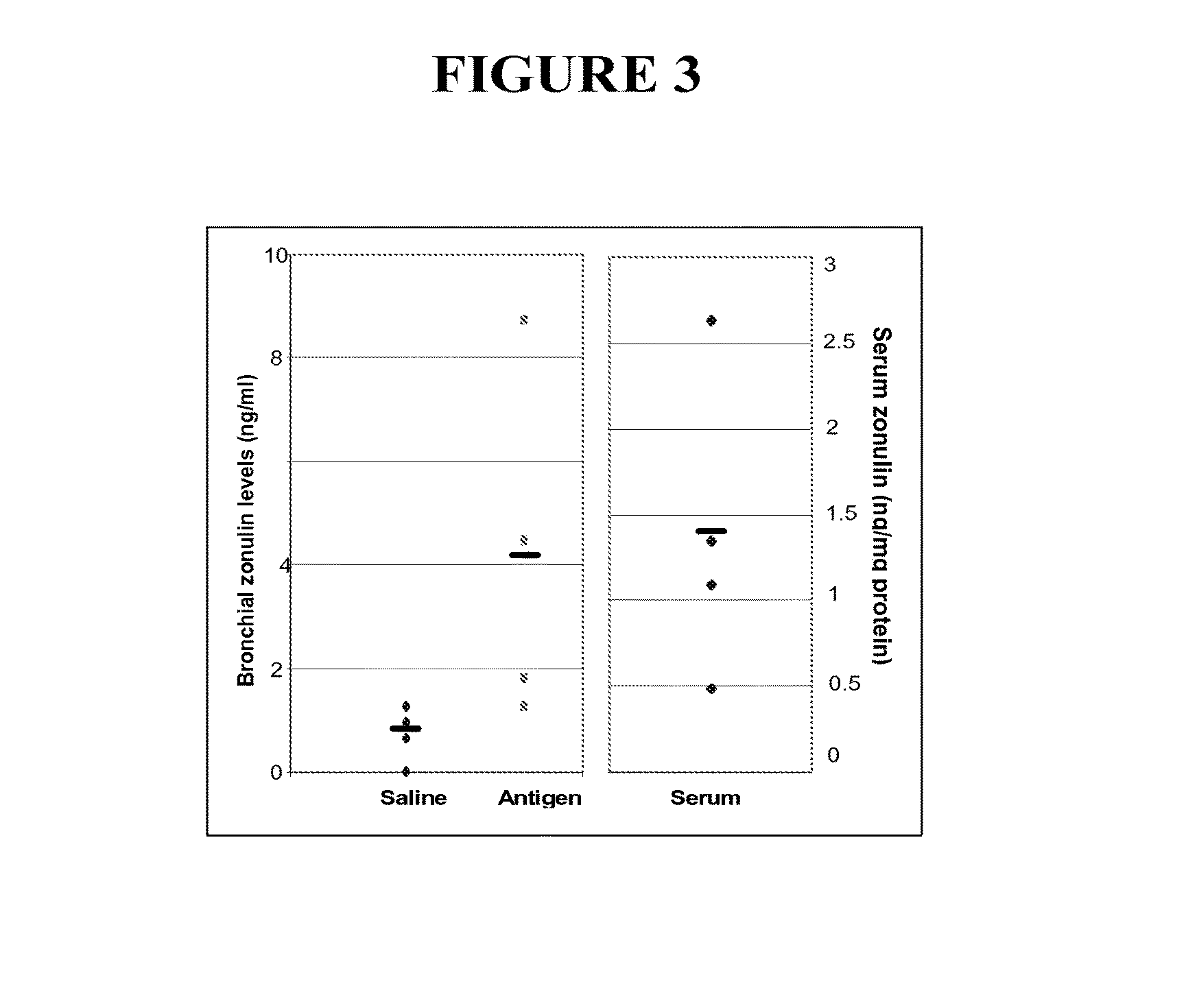
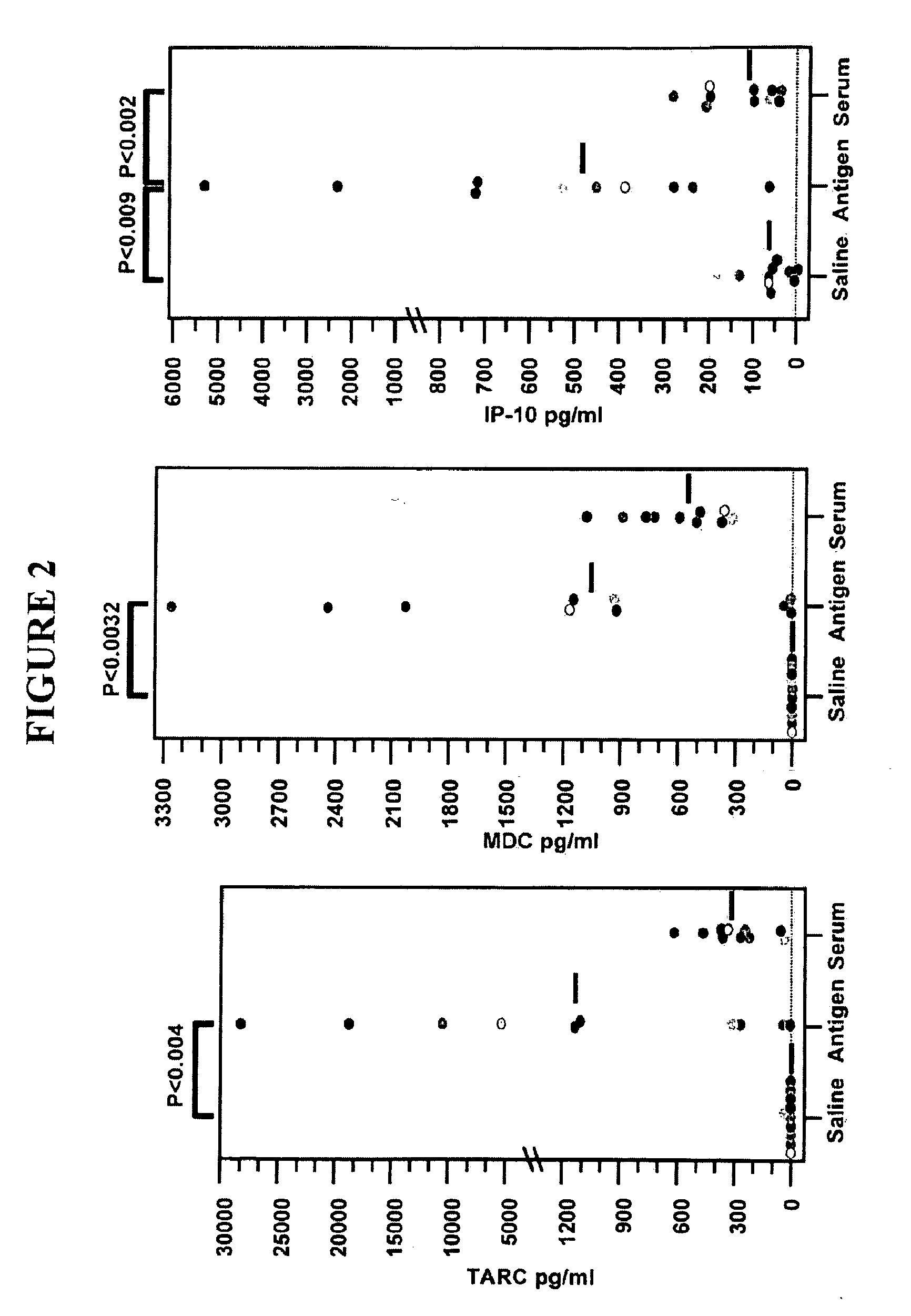
The present invention provides materials and methods to diagnose asthma. In some embodiments, the present invention provides a method of diagnosing asthma by measuring the zonulin level of a subject. The present invention also provides methods and compositions for treating asthma that comprise one or more zonulin antagonist.
Owner:ALBA THERAPEUTICS CORP
Compositions and methods for treating, reducing, ameliorating, alleviating, or inhibiting progression of, pathogenic ocular neovascularization
InactiveUS20110189174A1Improve treatmentDecrease increaseBiocideOrganic active ingredientsVitronectinOcular neovascularization
A composition for treating, reducing, ameliorating, alleviating, or inhibiting the progression of, pathological ocular neovascularization comprises an integrin or vitronectin receptor antagonist having any one of Formulae I-XI, as defined herein. The composition can further comprise a VEGF inhibitor. Such composition is administered to an ocular environment by a method such as topical application, periocular injection, intravitreal injection, or intravitreal implantation. The composition can be administered alone or in combination with another procedure chosen to enhance the outcome of the treatment.
Owner:BAUSCH & LOMB INC
Staphylococcus aureus fusion protein of fibronectin and bindin, its preparation method and uses
InactiveCN1884302AImprove protectionImproving immunogenicityAntibacterial agentsSkeletal disorderStaphylococcus cohniiMicrococcus pyogenes
The invention discloses a fusion protein for Micrococcus pyogenes surface protein, and also discloses the method for preparing the same as well as its usage. The fusion protein is fused by Micrococcus pyogenes fnb and thioredoxin Trx on expression carrier, and the process comprises preparation of Micrococcus pyogenes surface protein fnb coding gene, colony of fnb coding gene into expression carrier and protein fusion. The fusion protein is soluble protein and retains immunity of natural protein. It is detected that said fusion protein is characterized by good response for humoral immunity and cell immunity, enlargement of protection range of protein vaccine and prevention of infection caused by most of Micrococcus pyogenes.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Peptide ligands of the urokinase receptor
InactiveUS7094752B2Peptide/protein ingredientsMicrobiological testing/measurementDiseasePeptide ligand
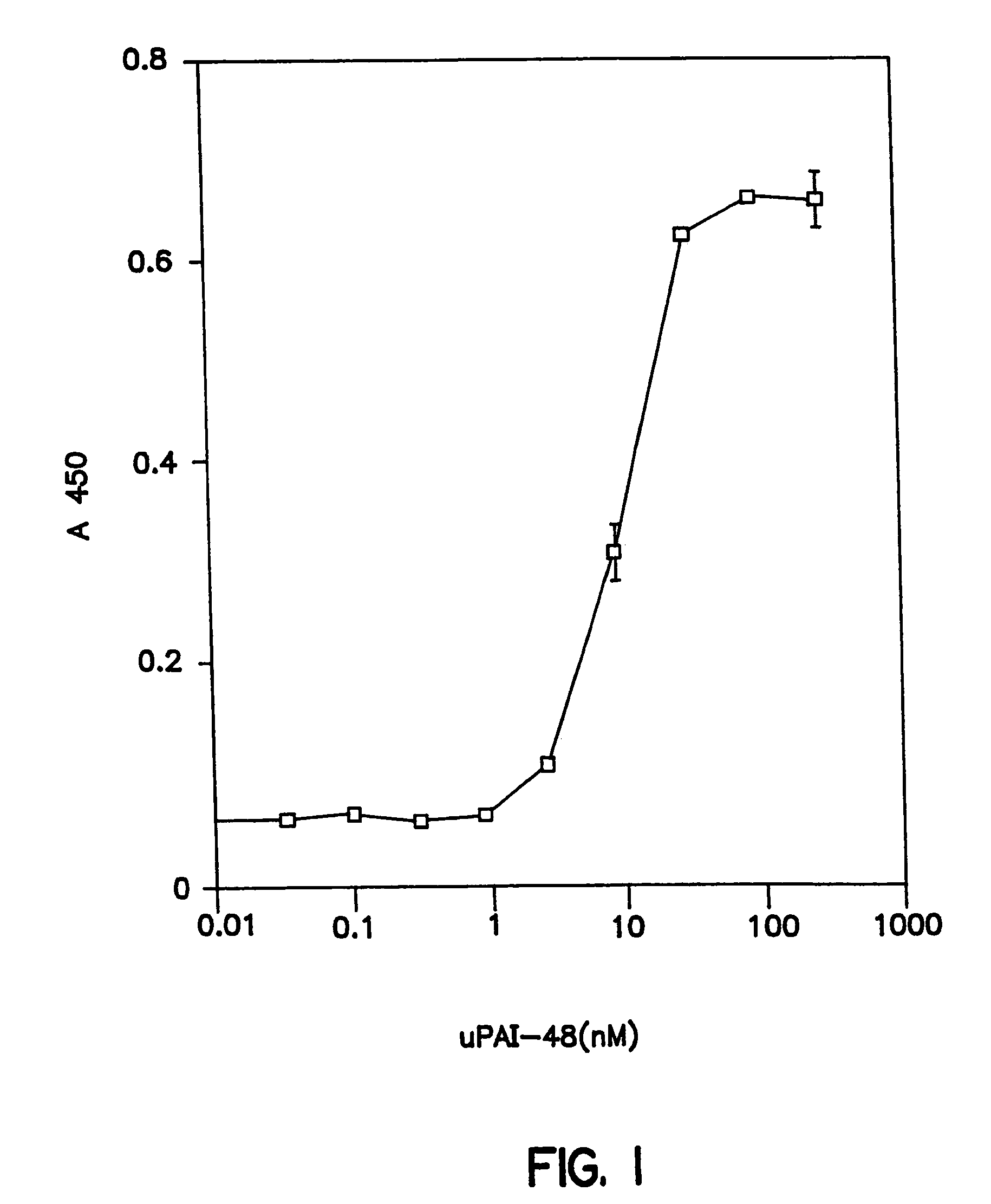
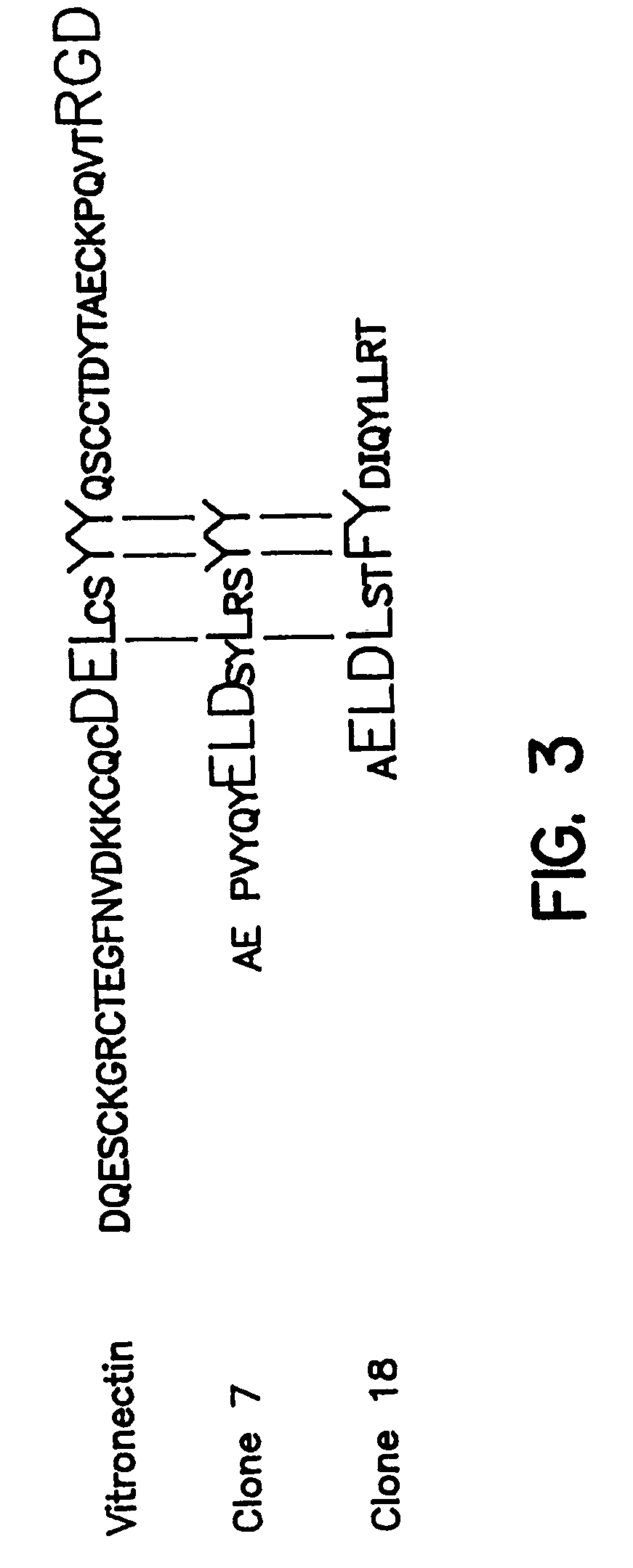
Novel peptides that are capable of binding to uPAR and inhibiting the binding of an integrin and vitronectin are described. Also provided are nucleic acid sequences encoding the novel peptides. Methods for screening for small molecules, other peptides, or peptoids that mimic the antagonistic function of the peptides of the invention are described. The invention has applications in design of therapeutics for treating disorders characterized by upregulation of uPA and uPAR, and cancer and chronic inflammation, cell migration or uPAR: integrin binding interactions, and diagnostical applications to such disorders.
Owner:CHIRON CORP +1
Blocking the migration or metastasis of cancer cells by affecting adhesion proteins and the uses of new compounds thereof
This invention provides methods, processes, compounds and compositions for modulating the gene expression and modulating the secretion, expression, or synthesis of adhesion proteins or their receptors to cure disease, wherein the modulating comprises positive and negative regulating; wherein comprises inhibiting cancer growth, wherein the adhesion proteins or receptors comprise fibronectin, integrins family, Myosin, vitronectin, collagen, laminin, Glycosylation cell surface proteins, polyglycans, cadherin, heparin, tenascin, CD 54, CAM, elastin and FAK; wherein the methods, processes, compounds and compositions are also for anti-angiogenesis; wherein the cancers comprise breast cancer, leukocyte cancer, liver cancer, ovarian cancer, bladder cancer, prostate cancer, skin cancer, bone cancer, brain cancer, leukemia cancer, lung cancer, colon cancer, CNS cancer, melanoma cancer, renal cancer or cervix cancer.
Owner:PACIFIC ARROW
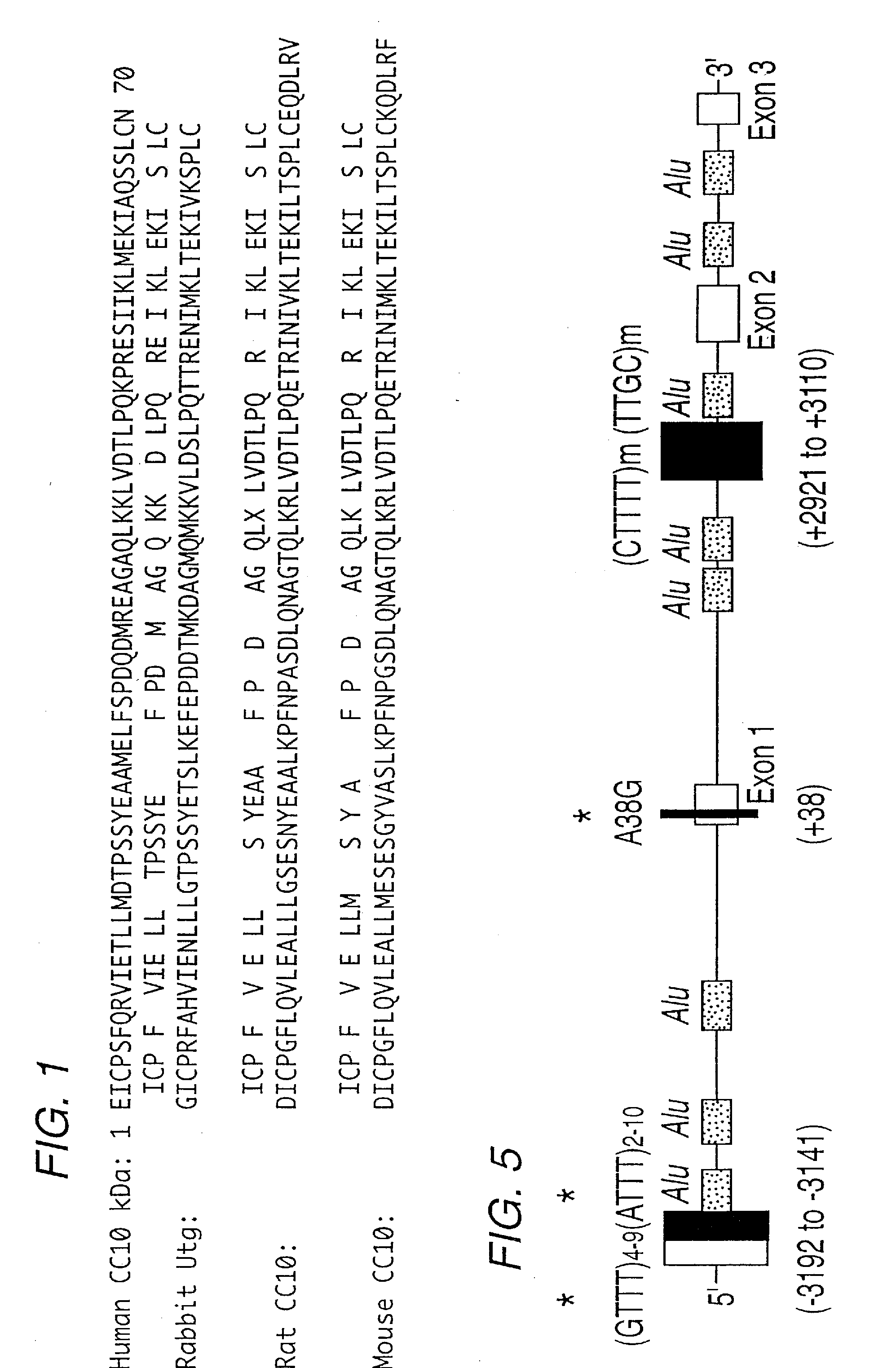
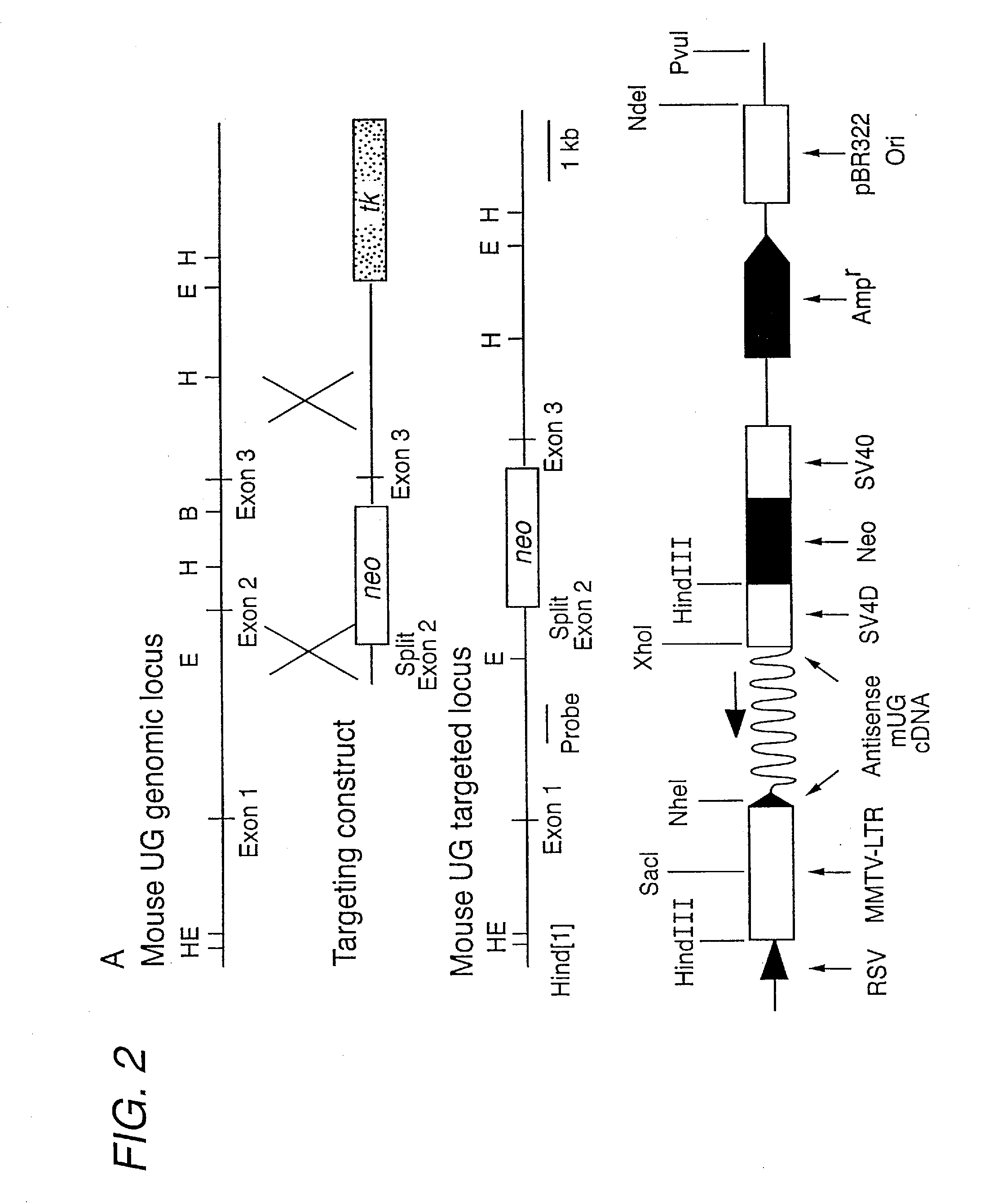
UTEROGLOBIN IN THE TREATMENT OF IgA MEDIATED AUTOIMMUNE DISORDERS
InactiveUS20090029908A1Avoid developmentInhibition formationNervous disorderPeptide/protein ingredientsProper treatmentKnockout animal
Uteroglobin has been discovered to prevent IgA mediated diseases, such as IgA nephropathy, by preventing the deposition of IgA-Fibronectin immunocomplexes in tissues such as the renal glomeruli. The invention therefore includes methods of treating such diseases by administering therapeutically effective amounts of uteroglobin (and variants or mimetics) to prevent or improve the IgA mediated condition. Transgenic uteroglobin knockout animals, and animals in which uteroglobin-protein expression is reduced by antisense technology, also provide systems for studying IgA mediated diseases, and screening for appropriate treatments.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Agonist polypeptide of receptor for ZOT and zonulin
Owner:MARYLAND UNIV OF BALTIMORE OFFICE OF RES & DEV
Altered Fibronectin-Binding Protein of Staphylococcus Aureus
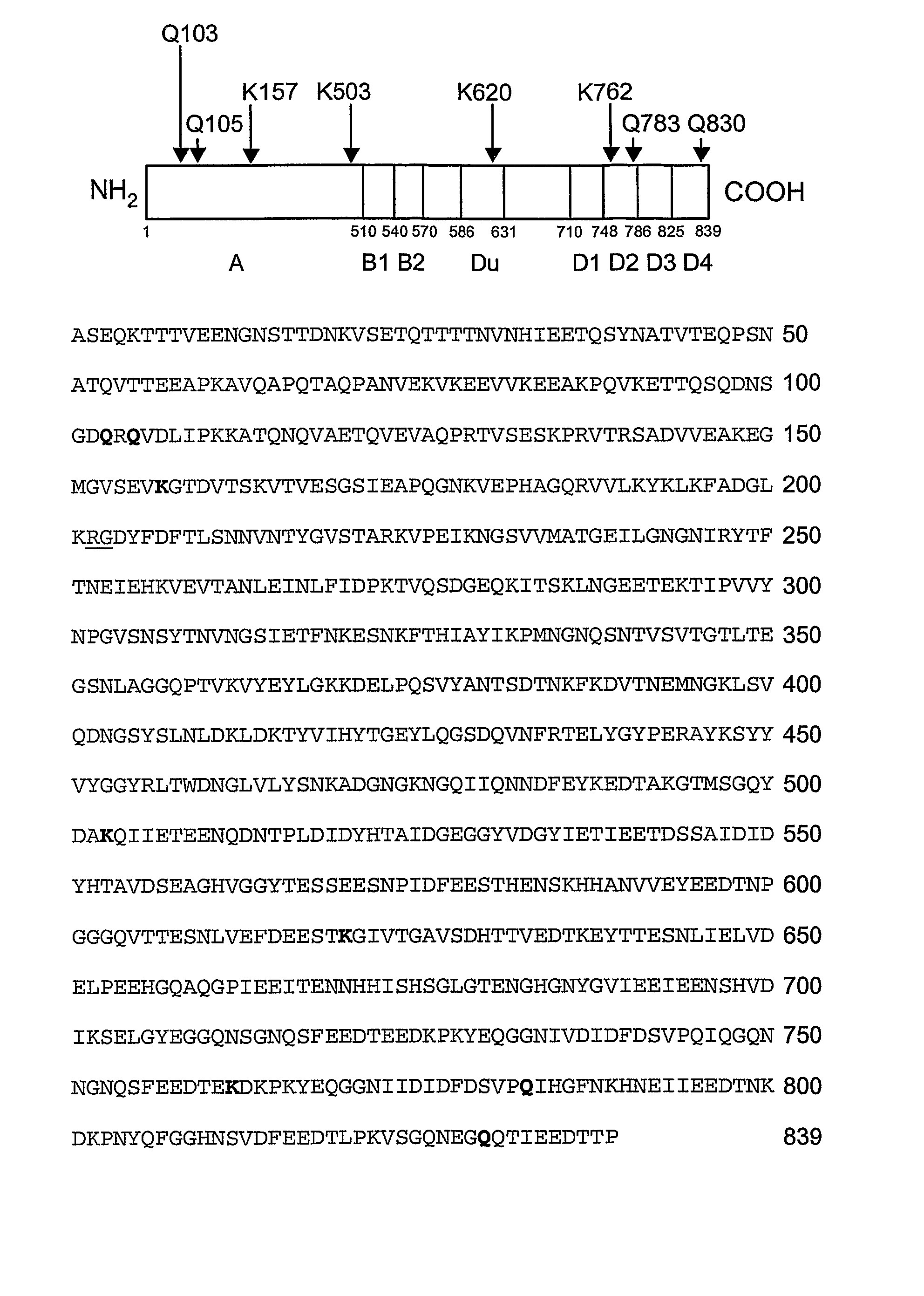
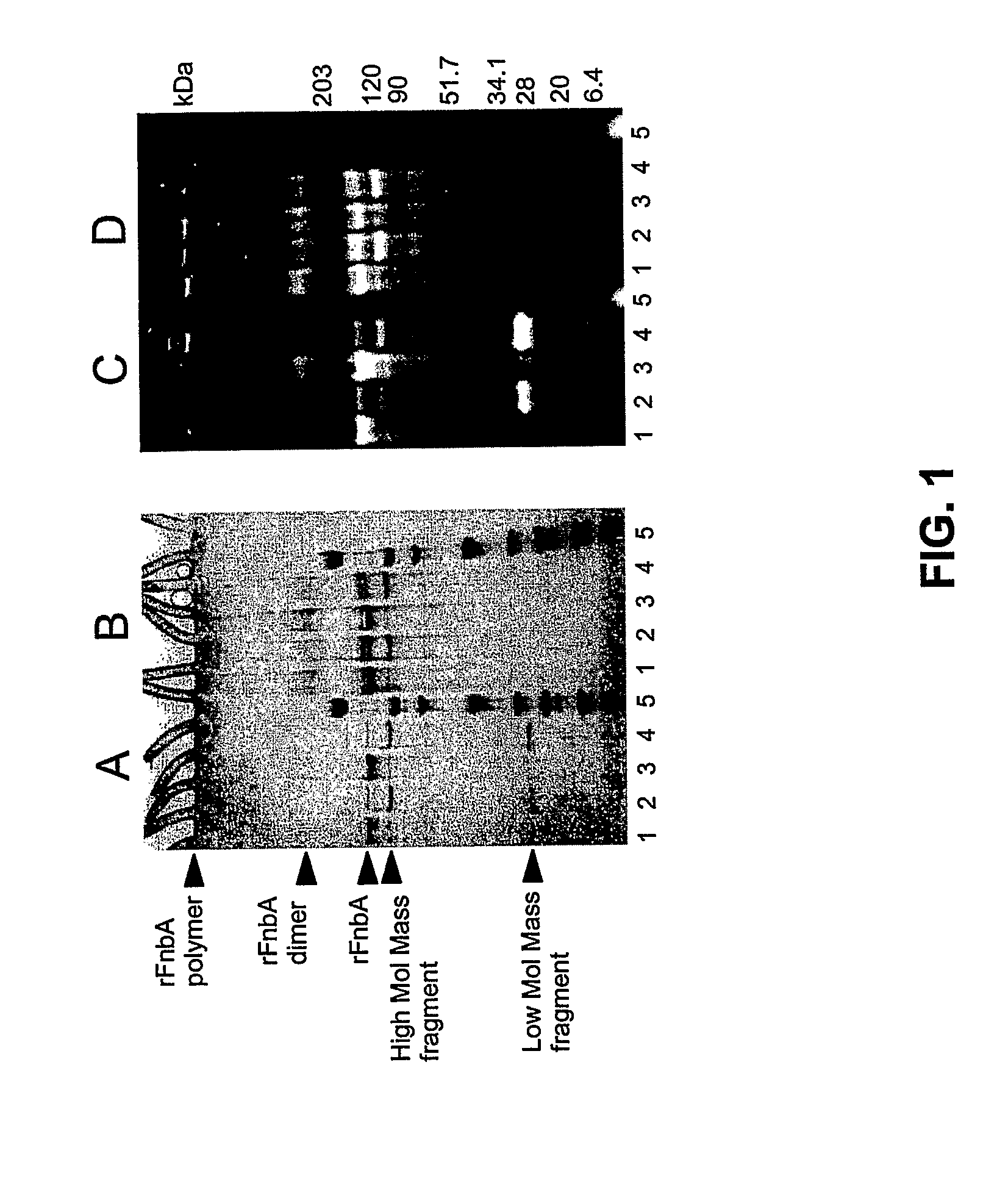
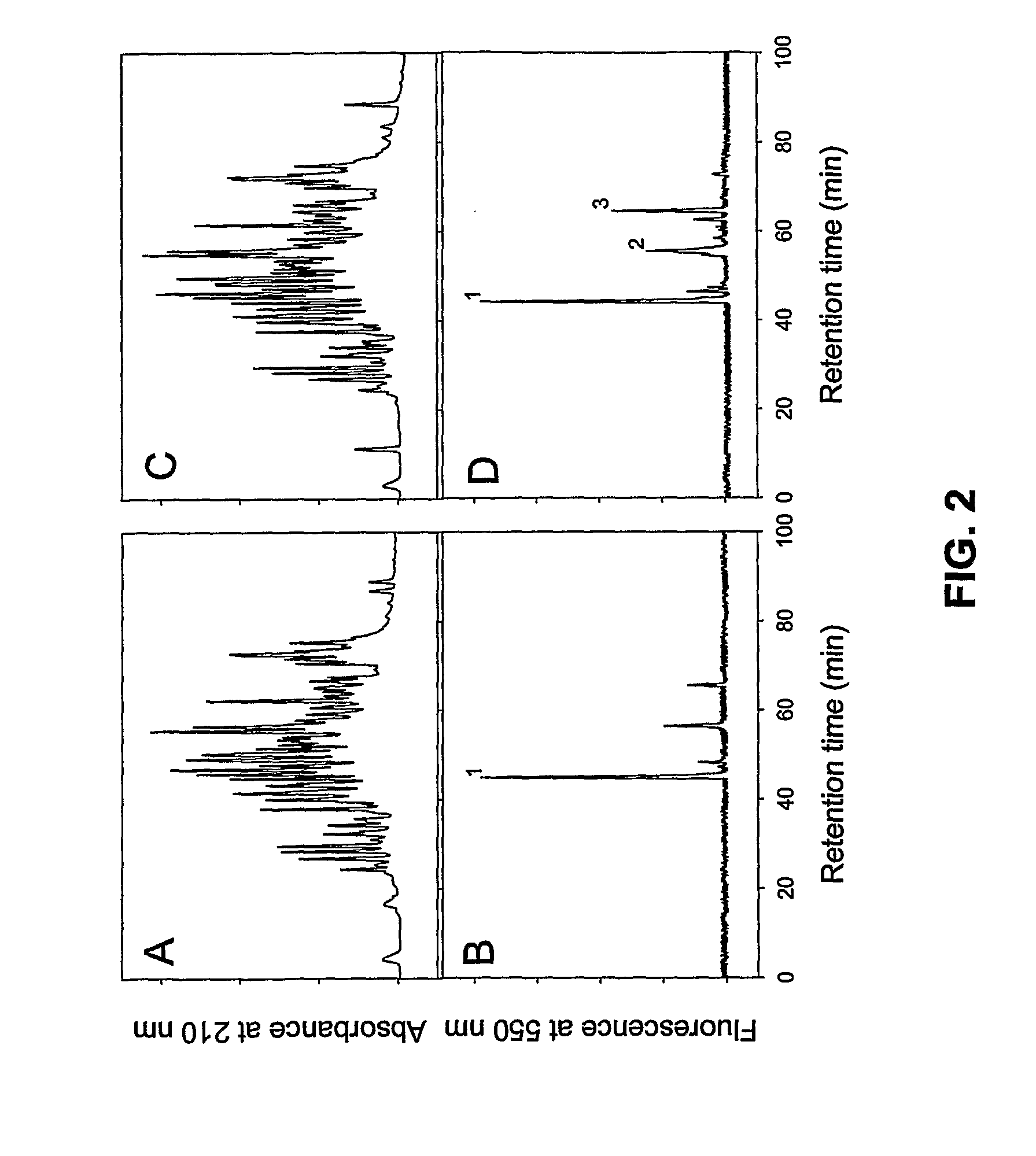
An isolated, altered fibronectin-binding protein (Fnb) of S. aureus having at least one mutation in an amino acid selected from residues corresponding to Gln103, Gln105, Lys157, Lys503, Lys620, Lys702, Lys762, Gln783 and Gln830 of FnbA of S. aureus strain ATCC49525 is described. Replacement of these reactive residues within the fibronectin-binding protein renders this protein less capable than wild-type Fnb of covalently cross-linking with fibronectin and fibrin. The altered fibronectin-binding protein effectively interferes with adhesion of S. aureus to fibronectin and fibrin, and therefore, an immunogenic composition comprising such altered Fnb exhibits improved immunogenic properties and is safer to use.
Owner:WYETH LLC
Laminin chemiluminescence immune analysis determination reagent kit and preparing method thereof
InactiveCN101377508AEfficient use ofGuaranteed SensitivityChemiluminescene/bioluminescenceBiological testingLamininMonoclonal antibody
The invention relates to the medical field of immunoassay, more specially, the invention provides a laminin detection kit and a preparation method thereof. The kit of the invention comprises 1) a laminin calibrator, 2) an avidin-coated vector, 3) a mixture of a biotinylation laminin monoclonal antibody and a laminin polyclonal antibody or a monoclonal antibody enzyme marker, 5) a chemiluminescent substrate and 6) a concentrated washing solution. Further, the preparation method of the kit according to the invention comprises the following steps: 2) preparing the calibrator with laminin, 2) coating the vector with avidin, 3) biotinylating the laminin monoclonal antibody, 4) marking the laminin polyclonal antibody or monoclonal antibody with the enzyme, 5) preparing the chemiluminescent substrate, 6) preparing the concentrated washing solution, 7) packaging the calibrator, the biotinylation laminin, the chemiluminescent sustrate and the concentrated washing solution, and 8) assembling finished products. The kit of the invention has the advantages of simplicity, convenience, sensitivity, stability and the like, and provides a valuable detection means for clinic diagnosis and scientific research.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Urine protein marker for chronic pancreatitis and application thereof
The present application relates to urinary protein markers for chronic pancreatitis and uses thereof. Specifically, this application relates to the use of urine protein markers obtained by using chronic pancreatitis disease models and mass spectrometry analysis in the early diagnosis of human chronic pancreatitis disease and the use of disease course monitoring. The urine protein markers are selected from Igγ‑1 chain C domain, nucleonectin‑2, beta‑2‑microglobulin, interleukin‑4 receptor alpha subunit, phosphoglycerate mutase 1, dipeptidyl peptidase 2, secreted pyrophosphoprotein 24, glycans Core protein, endothelial cell selective adhesion molecule, nestin-2, serum amyloid substance p, nerve cell adhesion molecule 1, etc. In this application, the obtained differential protein in urine provides a non-invasive and convenient option for early diagnosis and subsequent treatment of chronic pancreatitis.
Owner:BEIJING NORMAL UNIVERSITY
EGFR and PAR2 Regulation of Intestinal Permeability
InactiveUS20120107329A1Inhibition releaseReduce penetrationOrganic active ingredientsMicrobiological testing/measurementAutoimmune conditionAllergy
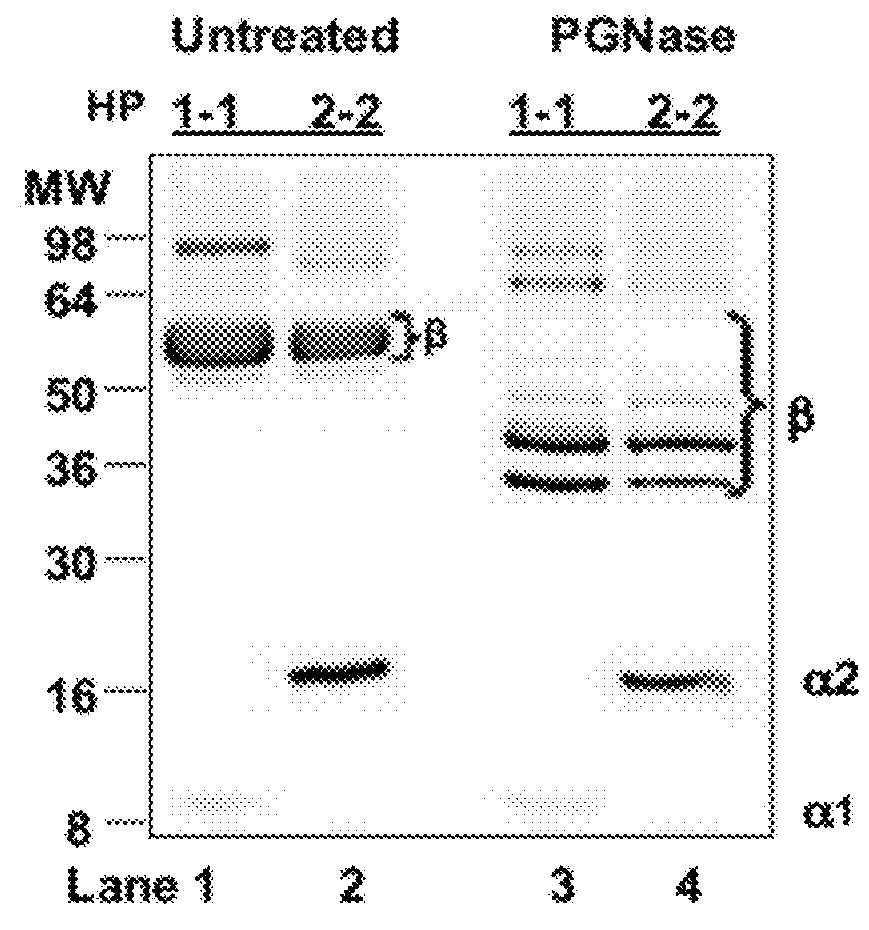
The present invention provides methods for diagnosing and treating an immune-mediated disease, e.g., an autoimmune disease, an allergy or an inflammatory disease. Diagnosis is made by detecting a heterozygous or homozygous genotype of haptoglobin 2 or by detecting and quantifying pre-haptoglobin 2 mRNA or protein. After diagnosis, the disease may be treated by decreasing cell permeability leading to increased transepithelial electrical resistance, for example, by administering an antibody directed against single chain zonulin thereby inhibiting epidermal growth factor receptor and inhibiting proteinase-activated receptor 2 (PAR2).
Owner:UNIV OF MARYLAND
Method of diagnosing and treating asthma
InactiveUS20100144646A1Sufficient affinityPreventing and ameliorating and treating asthmaPeptide/protein ingredientsDisease diagnosisProviding materialZonulin
The present invention provides materials and methods to diagnose asthma. In some embodiments, the present invention provides a method of diagnosing asthma by measuring the zonulin level of a subject. The present invention also provides methods and compositions for treating asthma that comprise one or more zonulin antagonist.
Owner:ALBA THERAPEUTICS CORP
Prostatic cancer markers from prostatic secretion
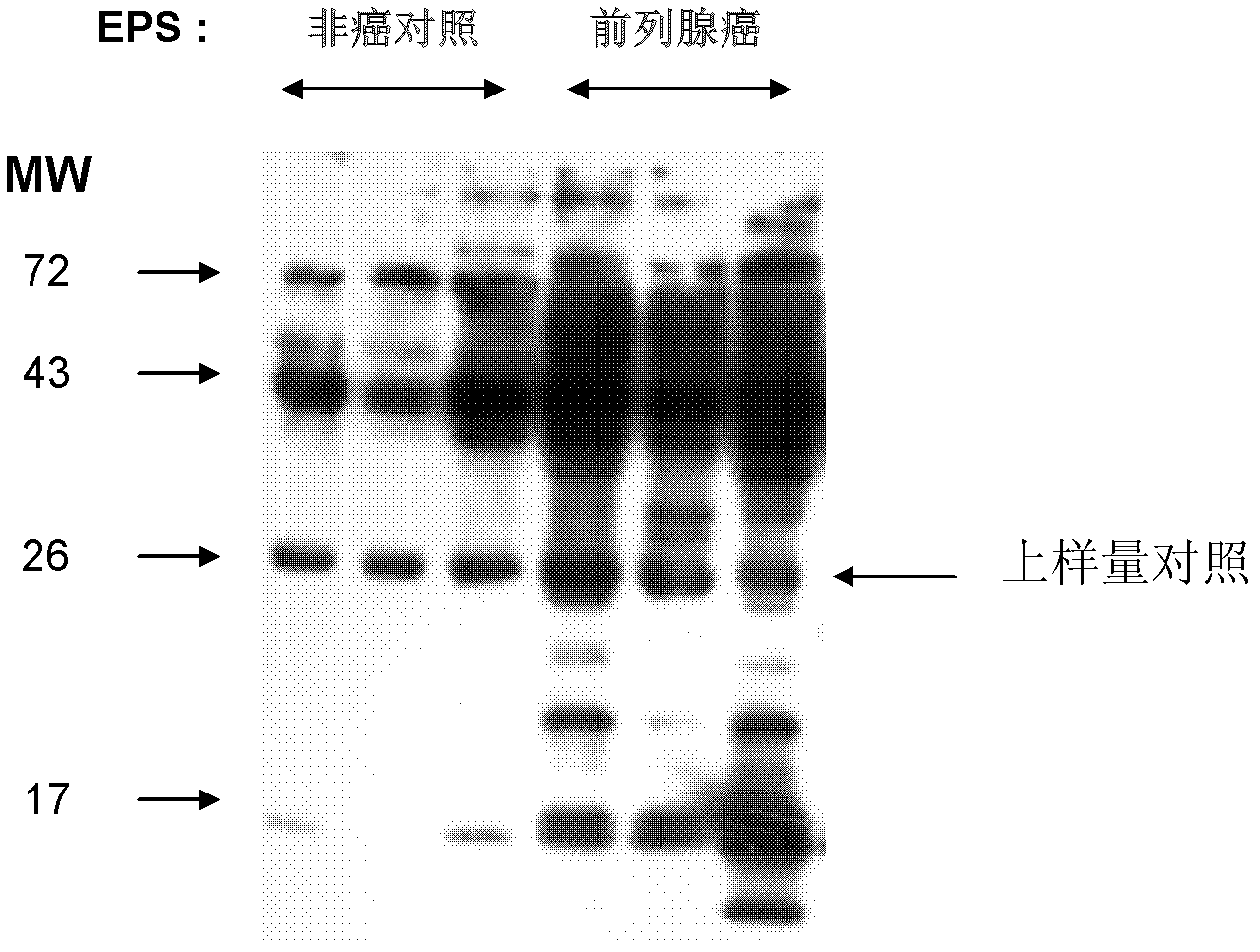
The invention relates to prostatic cancer markers from a prostatic secretion, and discloses a group of new prostatic cancer markers comprising Haptoglobin alpha-chains, Fibronectin1, Albumin and / or SERPINB1. In the prostatic secretion, the expression of the above proteins in prostatic cancer patients is substantially higher than the expression in non-cancer patients, so reagents or kits for the diagnosis, the assessment and the prognosis of the prostatic cancer can be developed based on the proteins.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +1
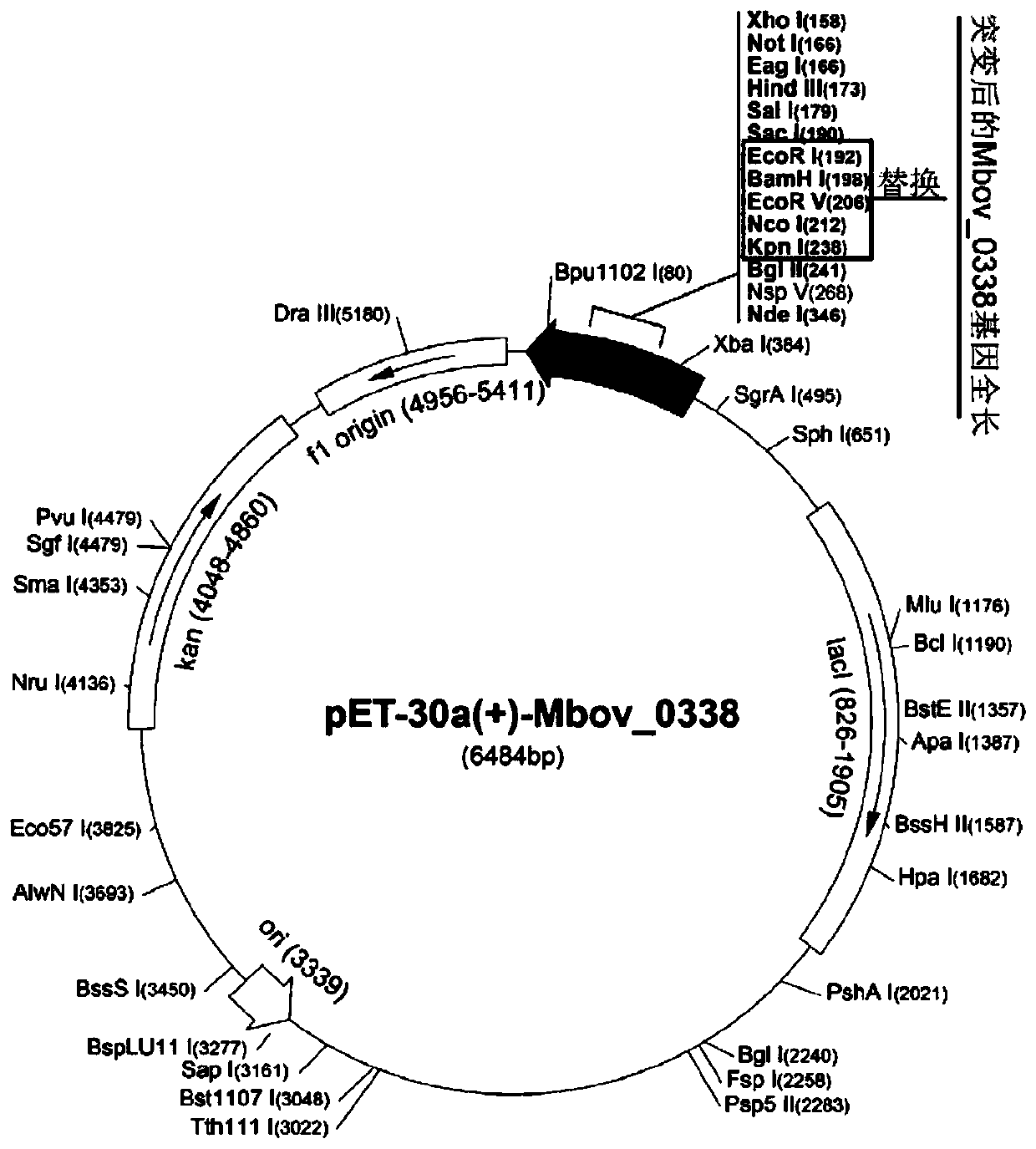
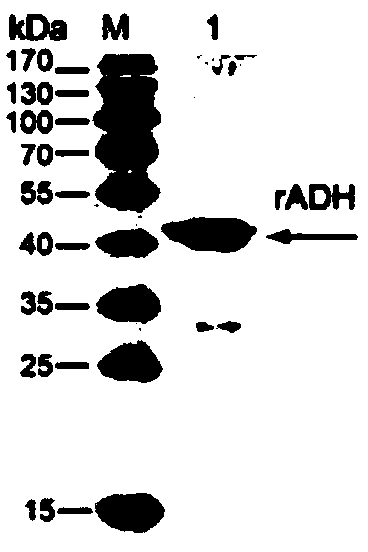
Mycoplasma bovis alcohol dehydrogenase gene and coded protein and application thereof
ActiveCN110257405AHas alcohol dehydrogenase activityAvoid infectionAntibacterial agentsBacteriaVirulent characteristicsNucleotide
The invention discloses a Mycoplasma bovis alcohol dehydrogenase gene, having a nucleotide sequence shown as SEQ ID NO: 1. The invention also discloses a protein coded by the Mycoplasma bovis alcohol dehydrogenase gene; the protein has an amino acid sequence shown as SEQ ID NO: 2 and belongs to the technical field of prevention and treatment of animal infectious diseases and biology. The protein, as a recombinant protein herein, has alcohol dehydrogenase activity, can attach to EBLs (embryonic bovine lung cells), can combine with bovine Fn (fibronectin), has good immunogenicity and reactogenicity, is a virulence-related protein of Mycoplasma bovis, and has a good application prospect in the research on pathogenesis of Mycoplasma bovis, and the research and development of vaccines and diagnostic reagents.
Owner:HUAZHONG AGRI UNIV
Biological protective covering
InactiveCN105879103AMicropores are small and denseImprove breathabilityAbsorbent padsBandagesIntestinal membraneImmunogenicity
The invention discloses a biological protective covering and a preparation method thereof. The biological protective covering is made of a substrate namely animal intestinal membranes, which have been crosslinked and fixed by a non-aldehyde fixing agent and subjected to an antigen removal treatment. An active modification layer, which contains fibronectin, laminin, or vitronectin that can adhere to cells, or an antibacterial sustained-release layer containing antibacterial drugs is arranged on the surface of the substrate. The biological protective covering is made of thin and tough animal intestinal membranes, and is light, soft, and user-friendly. The intestinal membranes are semi-permeable, gas and steam can penetrate the intestinal membranes, but bacteria cannot go through the intestinal membranes. Moreover, the intestinal membranes have been subjected to a multi-aspect antigen removal treatment to effectively remove the immunogenicity, the surface is subjected to active modification, the surface can aggregate epithelial cells and fibroblast to promote wound healing; the antibacterial drugs can be sustained-released, the anti-infection effect is enhanced, and the using performance and protective effect are better than those of dressing or protective covering made of pig skin.
Owner:CHONGQING BAFANGYUAN NETWORK TECH CO LTD
Recombinant human fibronectin III1-C, and preparation method and application thereof
ActiveCN111217903AIncrease productionShorten the production cycleCosmetic preparationsConnective tissue peptidesBALB/cCell adhesion
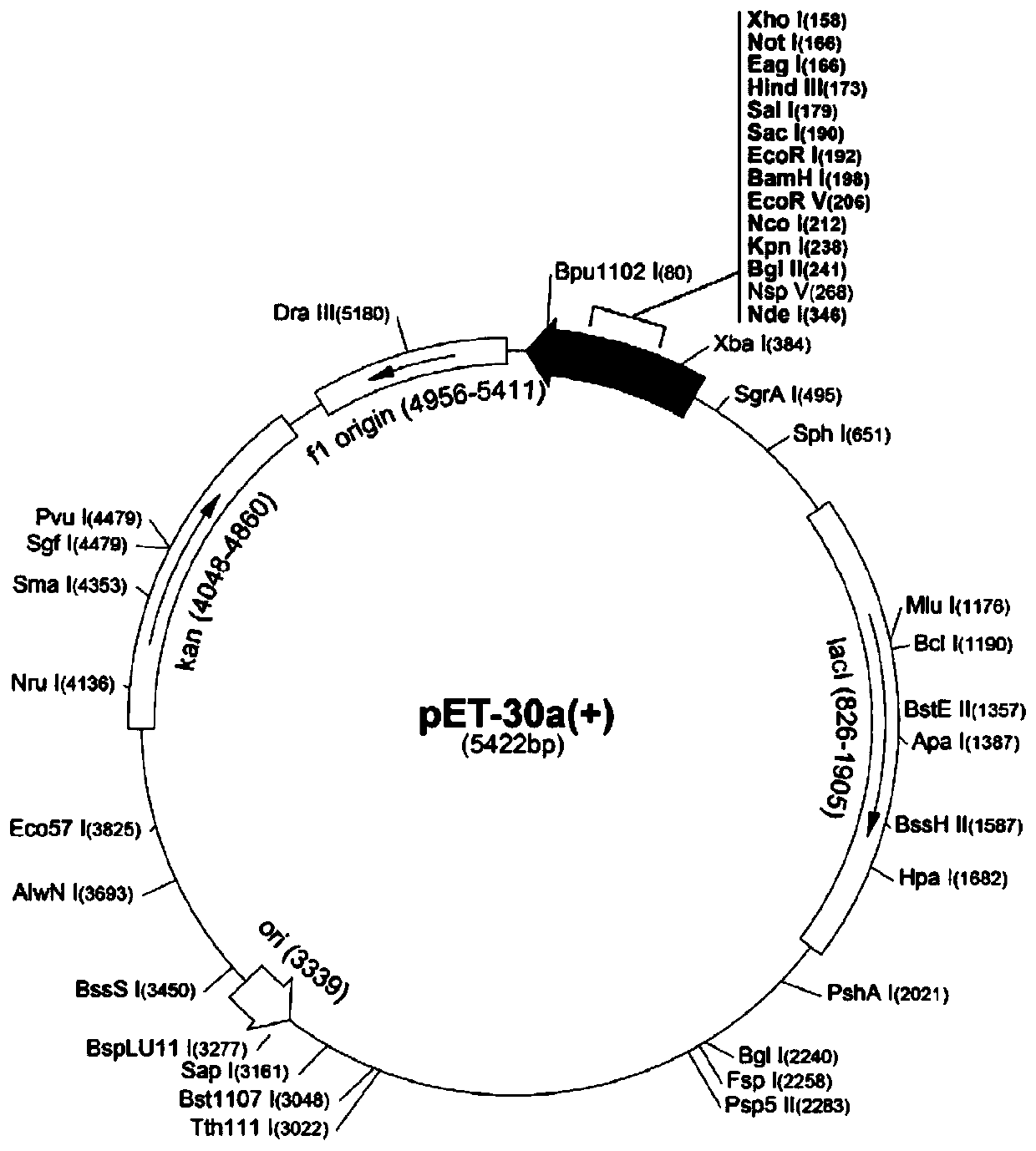
The invention discloses a recombinant human fibronectin III1-C (rhFNIII1-C), and a preparation method and application thereof. The preparation method comprises the following steps: optimizing a targetgene segment of the target protein according to codon expression preference, and inserting the obtained gene segment into pET-32a to construct a recombinant plasmid; transforming and introducing therecombinant plasmid into an expression vector BL21(DE3), and carrying out screening to obtain positive clone bacteria capable of realizing efficient soluble expression; carrying out enlarging fermentation culture on the positive clone bacteria, carrying out induced expression, performing crushing and centrifuging to obtain a supernatant, and carrying out purification through elution with a His-tagaffinity chromatography column, dialysis, filtration and other steps to obtain a high-quality rhFNIII 1-C solution with a protein concentration of 1.0 mg / mL or above and a purity of 90% or above. According to detection and observation results of cell adhesion promoting tests, the rhFNIII1-C has the performance of obviously promoting adherence and adhesion of MDBK cells and Balb / c / 3T3 cells and rapid division and growth of the cells, which indicates that the rhFNIII1-C has huge potential of being used as a raw material and a finished product for cosmeceuticals and medical skincare.
Owner:ANHUI MEDICAL UNIV
Polypeptide face-firming repairing essence and preparation method thereof
InactiveCN112675067AWide variety of sourcesSafe and environmentally friendlyCosmetic preparationsToilet preparationsBiotechnologyArginine
The invention relates to polypeptide face-firming repairing essence and a preparation method thereof. The essence is prepared from the following components of, according to the following percentage, 8% of butanediol, 1% of betaine, 0.9% of glyceryl polyacrylate, 1.1% of glycerinum, 2% of lactobacillus, 2% of rice fermentation products, 1% of radix ginseng rubra (PANAXGINSENG) extract, 0.5% of 3-o-ethyl ascorbic acid, 0.4% of acetyl hexapeptide-8, 0.4% of palmitoyl pentapeptide-4, 0.3% of fullerene, 0.3% of decapeptide-4, 0.3% of glycoprotein, 0.3% of fibronectin, 0.3% of bifida ferment lysate, 0.3% of peony (PAEONISASUFFRUTICOSA) root extract, 0.2% of arginine, 0.2% of lactobacillus ferment lysate, 0.2% of hydrolyzed collagen, 0.2% of euglena gracilis (EUGLENAGRACILIS) polysaccharide, 0.2% of radix gentianae (GENTIANASCABRA) extract, 0.2% of chlorella vulgaris (CHLORELANACABRA) extract, 0.2% of crocus sativus (CROCUSSATIVUS) extract, 0.2% of caprylhydroxamic acid and the balance water. The essence has an obvious moisturizing effect, can be used for a long time, has no side effect, is good in effective component extracting effect, and is suitable for industrial production.
Owner:芯萃生物科技(上海)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com