Biological protective covering
A biological and animal technology, applied in the field of medical supplies, can solve problems such as unsatisfactory performance and therapeutic effect, poor toughness and softness, and poor histocompatibility of synthetic materials, so as to increase the anti-infection effect, Good air permeability and vapor permeability, and the effect of promoting wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
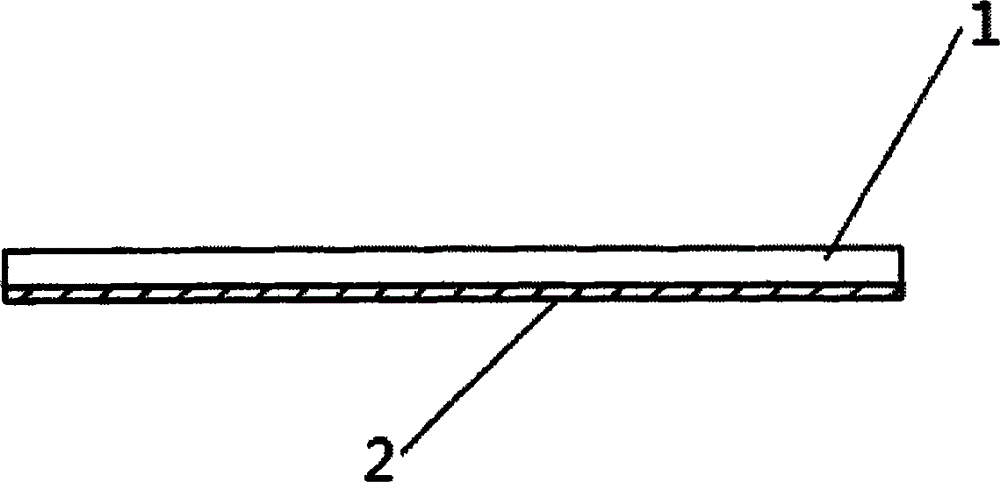
[0018] Such as figure 1 As shown, the bio-type wound protection film is composed of animal intestinal membranes that have been cross-linked and fixed by non-aldehyde fixatives and treated with antigen removal as the substrate 1. On the surface of the substrate 1, cells containing adherent cells are arranged. The active modification layer 2 of fibronectin, laminin or vitronectin or the antibacterial slow-release layer 2 containing antibacterial drugs.
[0019] The preparation method of biotype wound protection membrane of the present invention comprises the following steps:
[0020] (1), material selection: collect fresh animal small intestine;
[0021] (2), pretreatment: cleaning, disinfection, use special tools to scrape off the small intestinal mucosa and lower connective tissue, take a tough film and trim it flat and dry to obtain a film material;
[0022] (3), degreasing: extract the fat and fat-soluble impurities in the membrane material with organic solvent;
[0023] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com