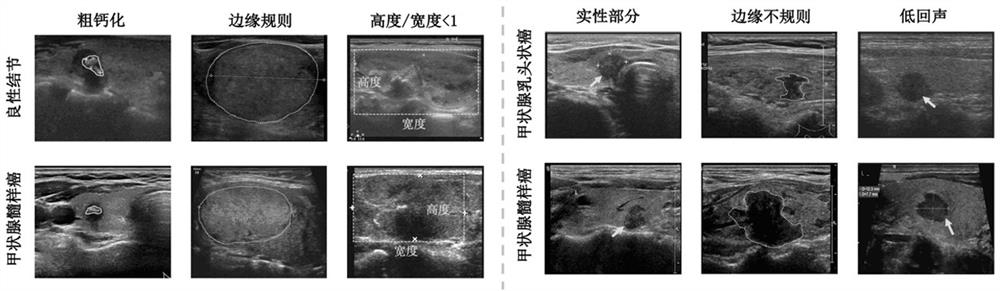
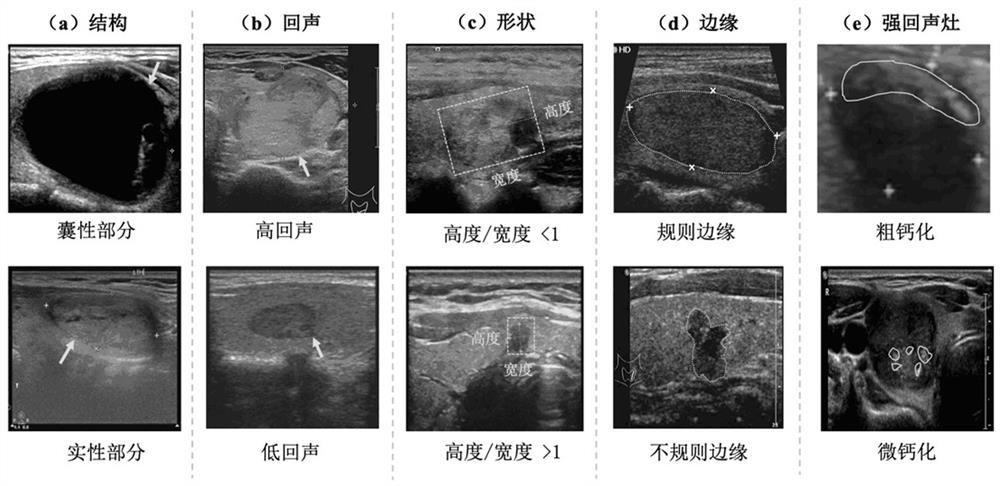
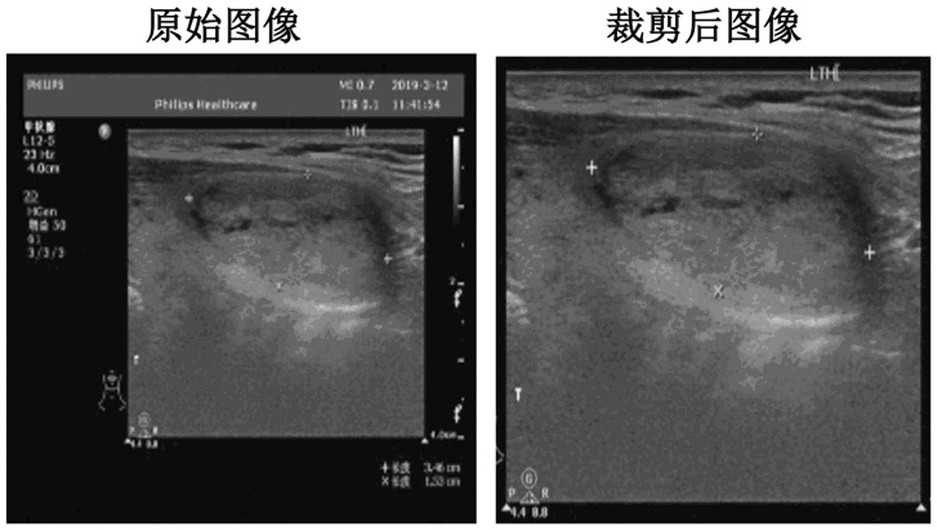
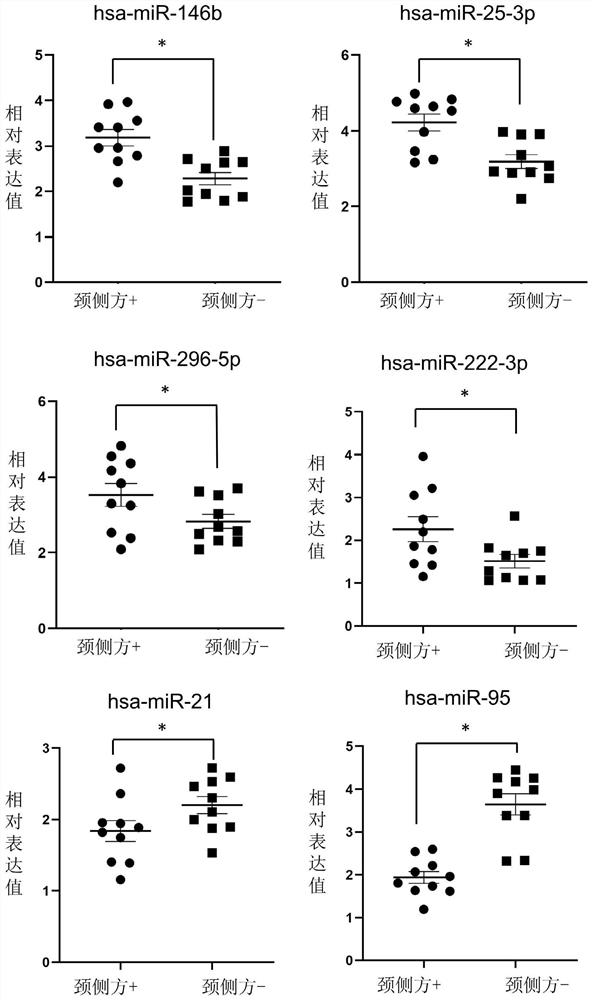
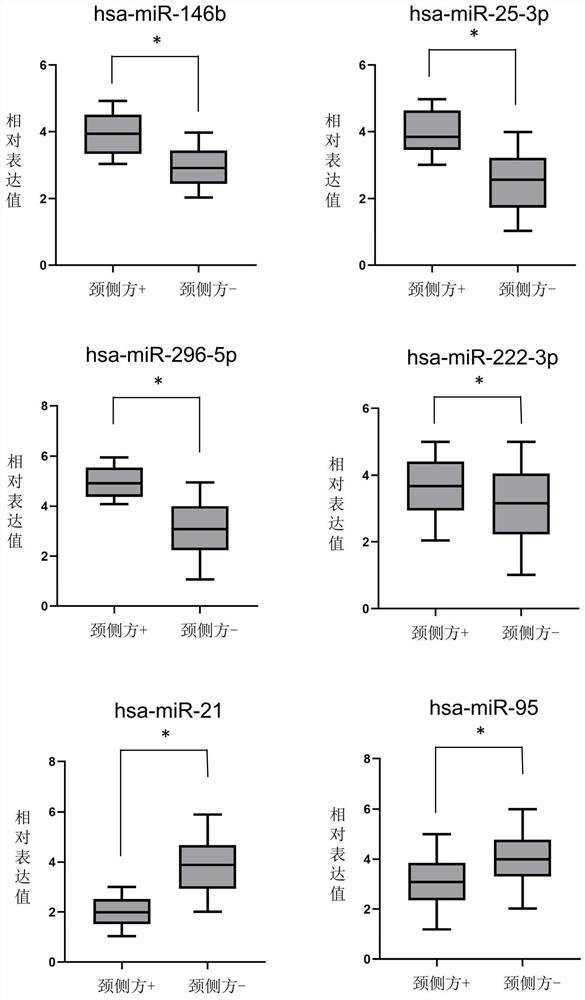
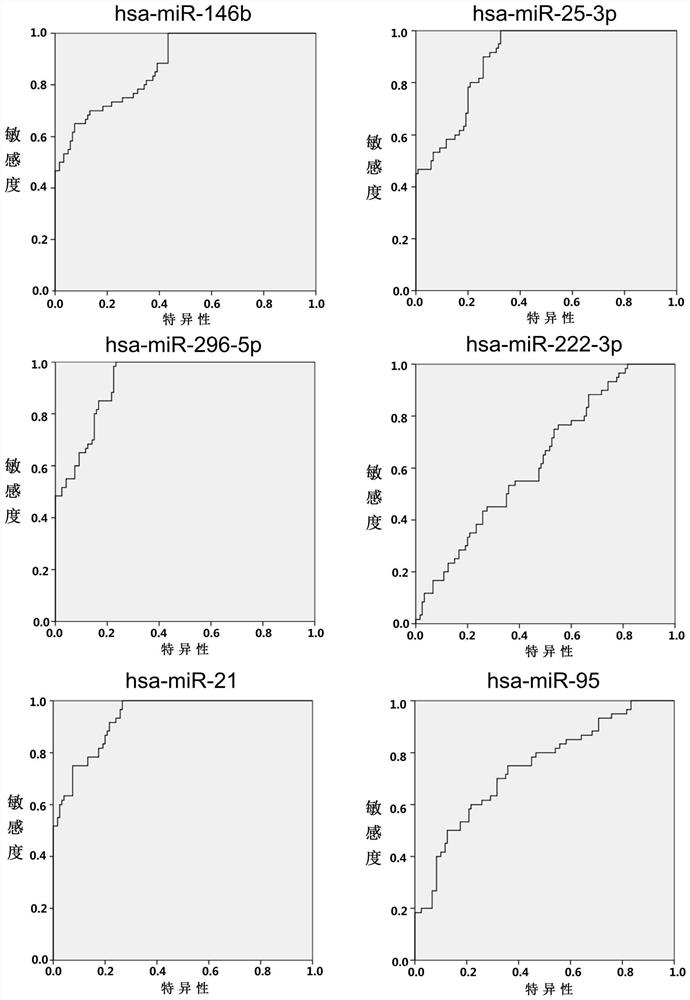
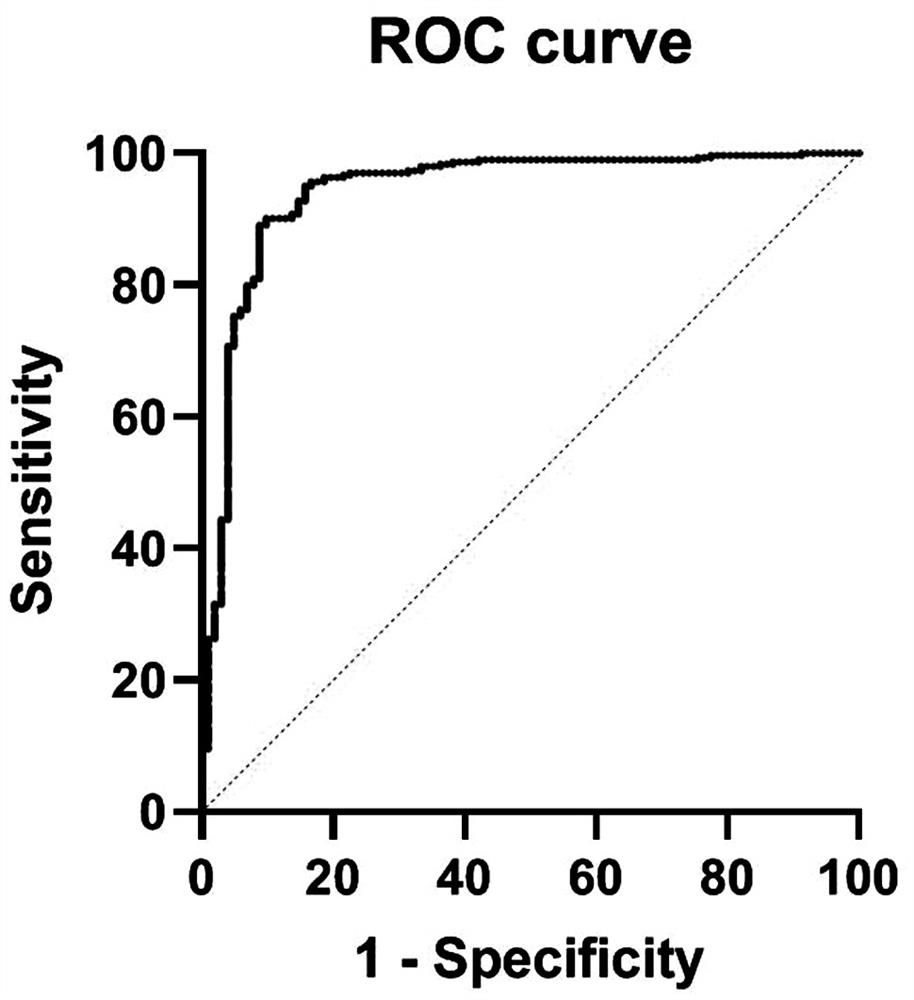
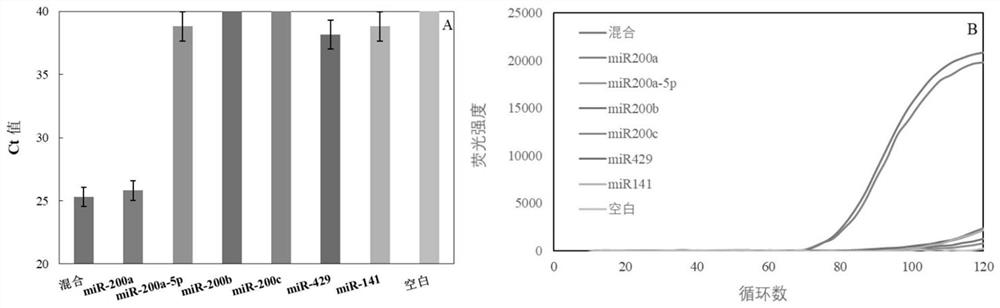
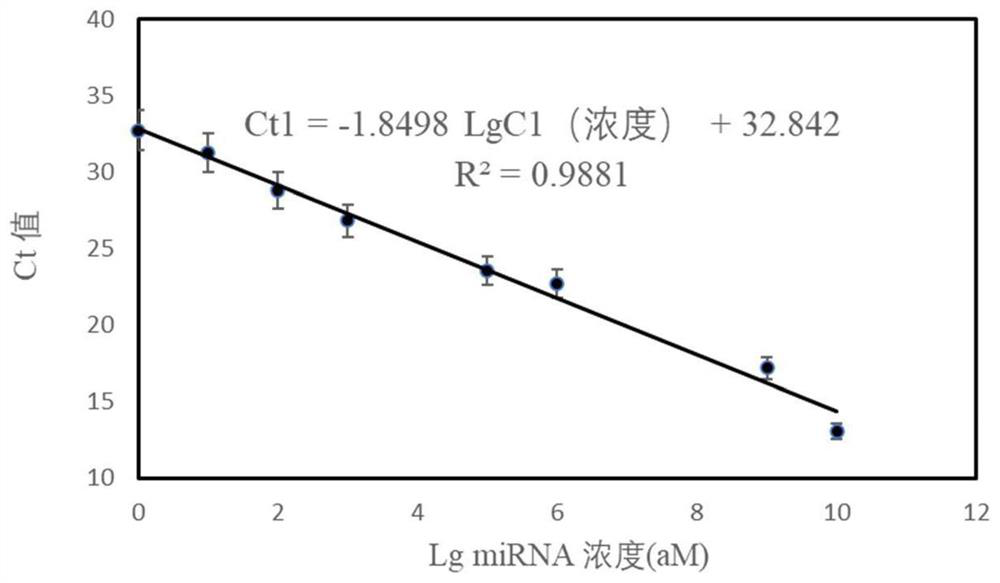
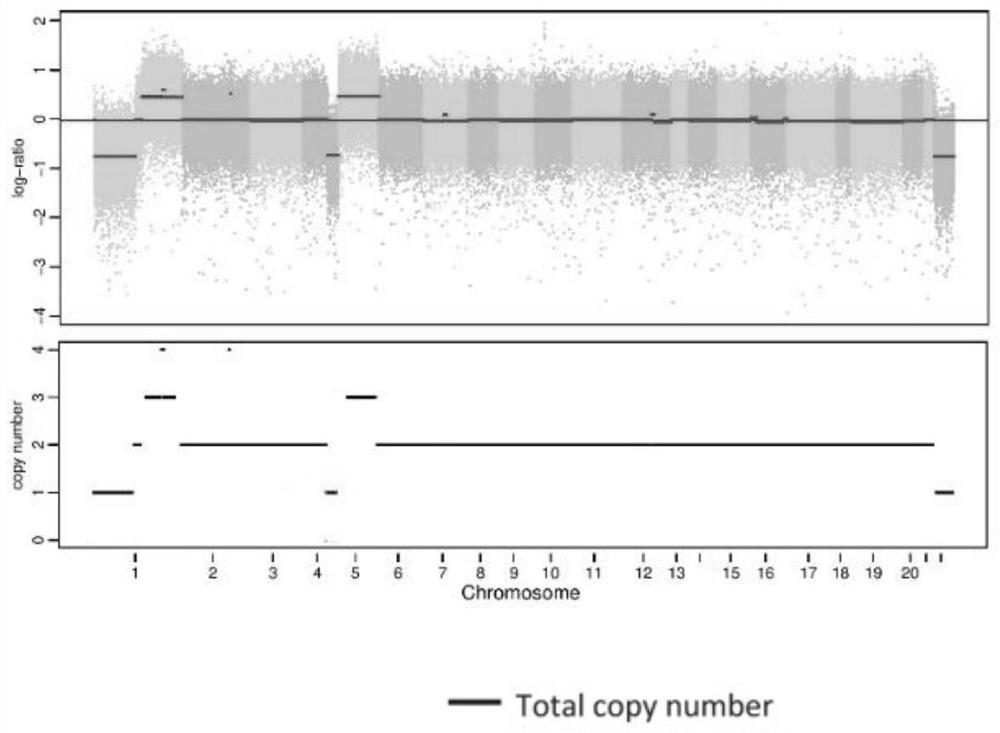
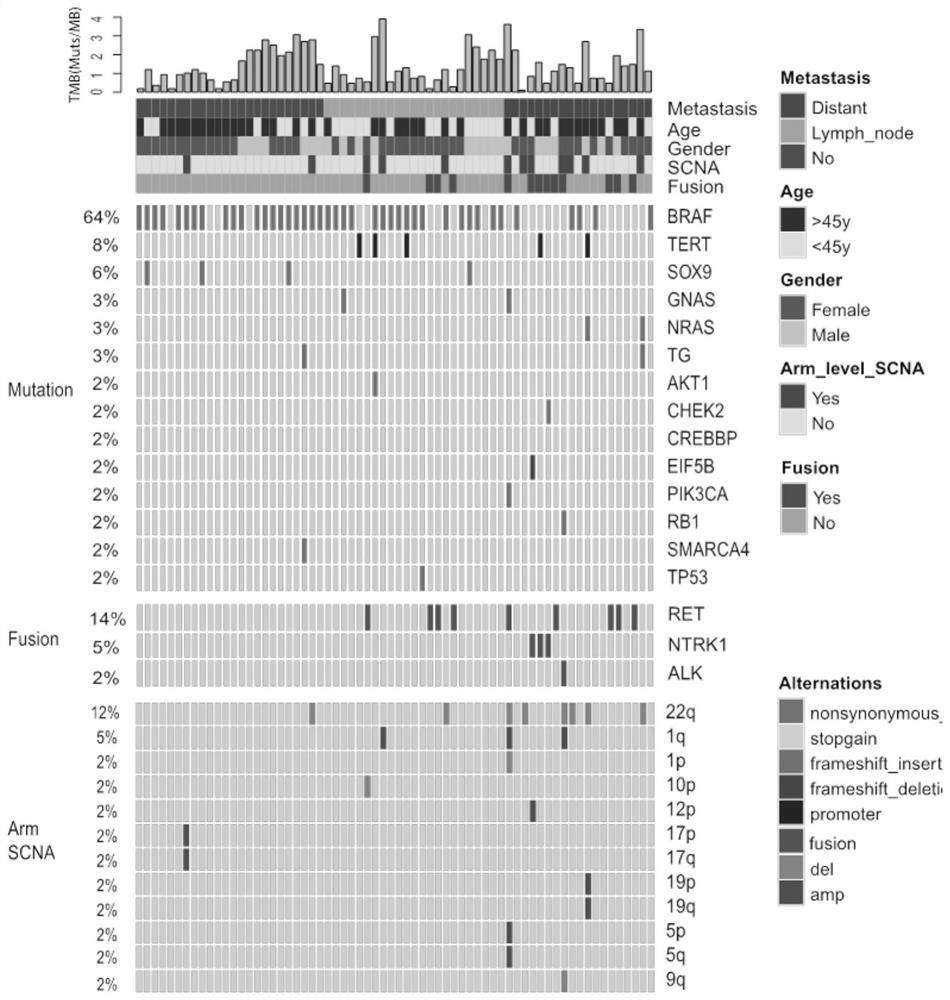
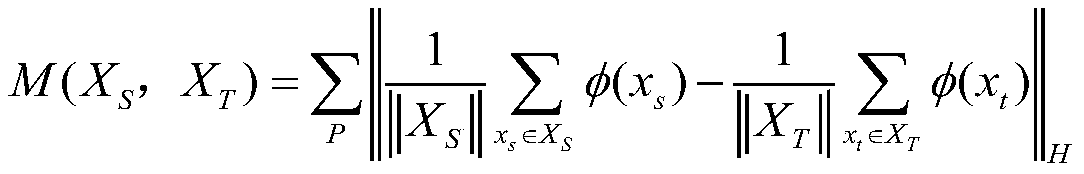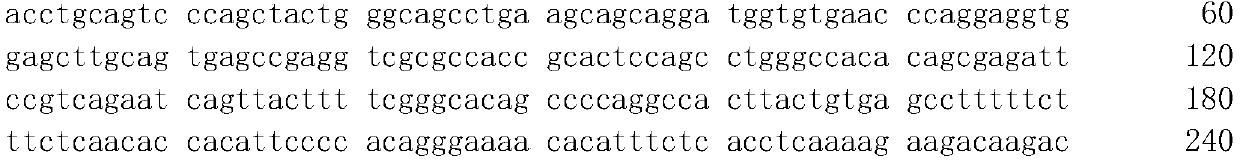Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Thyroid papillary carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
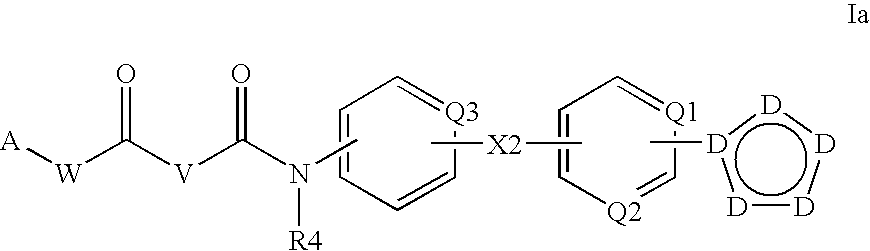
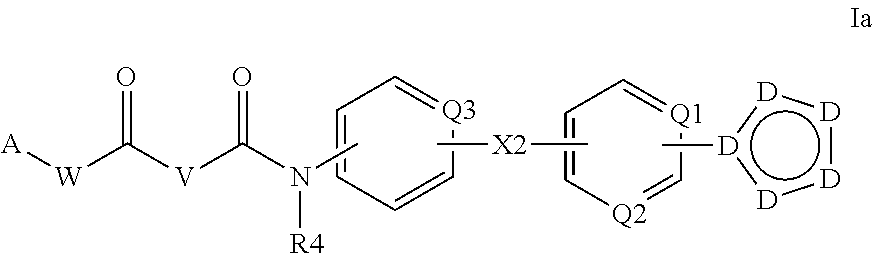
N-acyl ureas exhibiting anti-cancer and anti-proliferative activities
Compounds of the present invention find utility in the treatment of mammalian cancers and especially human cancers including, but not limited to, malignant melanomas, solid tumors, glioblastomas, ovarian cancer, pancreatic cancer, prostate cancer, lung cancers, breast cancers, kidney cancers, hepatic cancers, cervical carcinomas, metastasis of primary tumor sites, myeloproliferative diseases, chronic myelogenous leukemia, leukemias, papillary thyroid carcinoma, non-small cell lung cancer, mesothelioma, hypereosinophilic syndrome, gastrointestinal stromal tumors, colonic cancers, ocular diseases characterized by hyperproliferation leading to blindness including various retinopathies, diabetic retinopathy, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, mastocytosis, mast cell leukemia, and diseases caused by PDGFR-α kinase, PDGFR-β kinase, c-KIT kinase, cFMS kinase, c-MET kinase, and oncogenic forms, aberrant fusion proteins and polymorphs of any of the foregoing kinases.
Owner:DECIPHERA PHARMA LLC
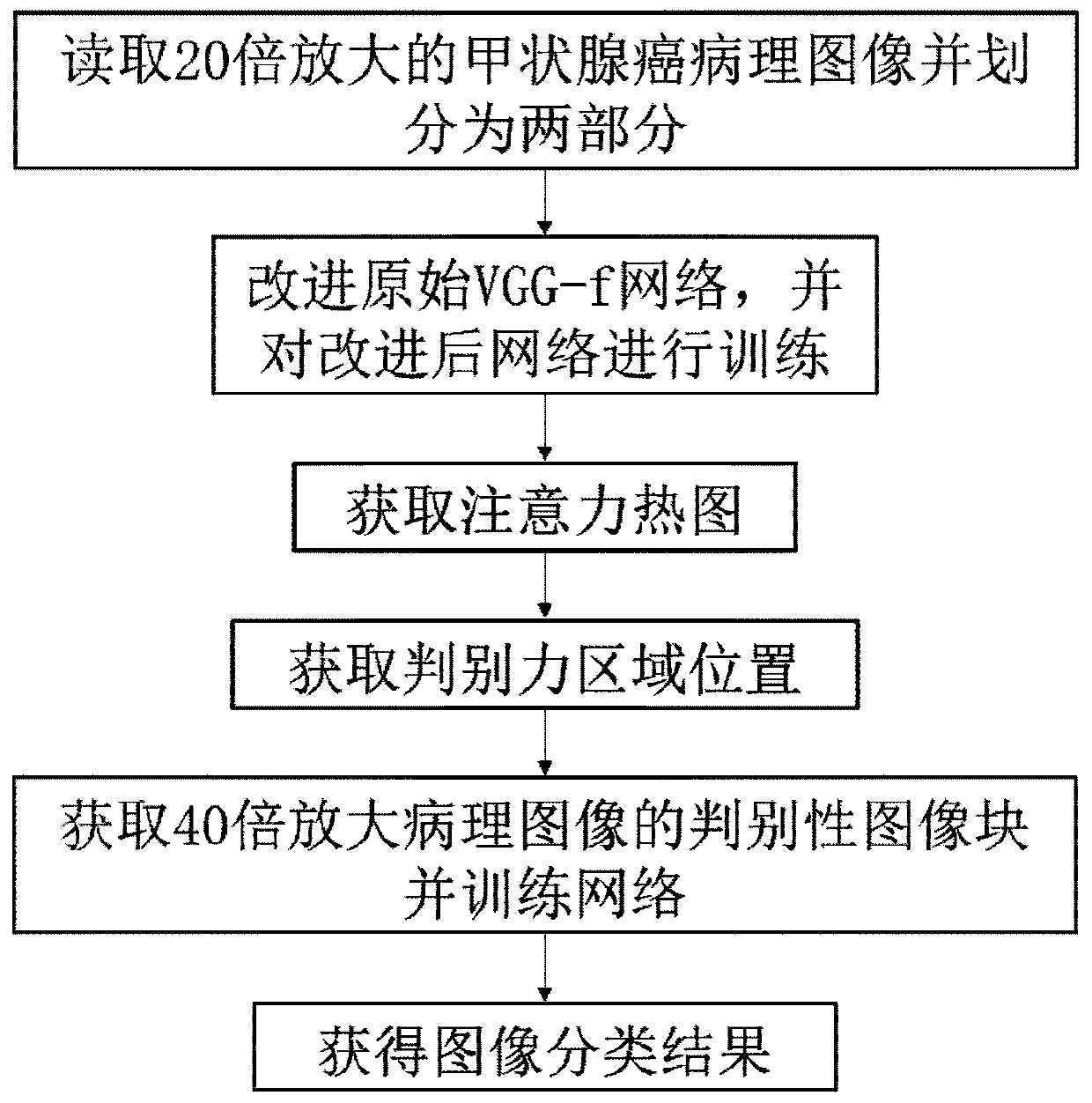
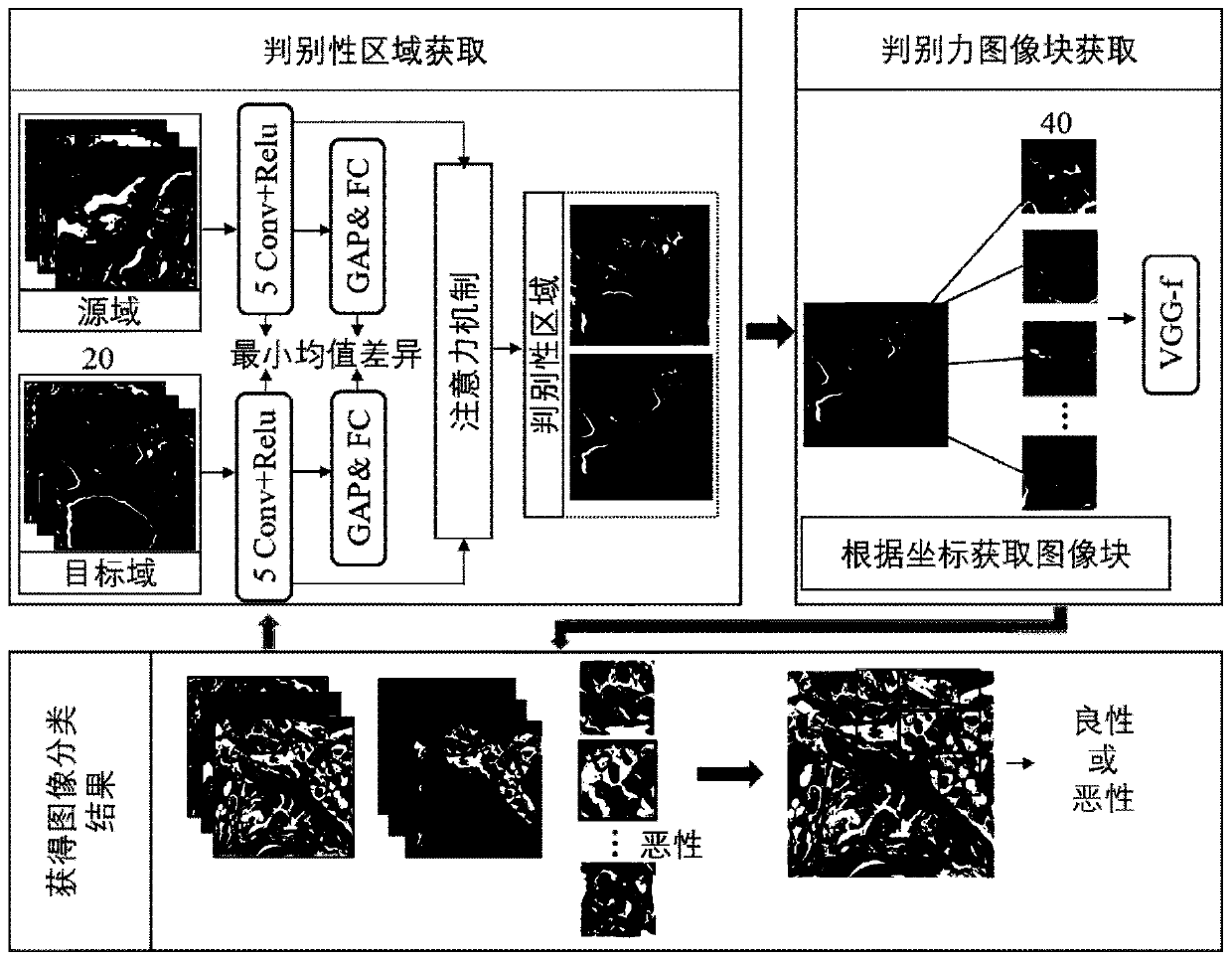
Papillary thyroid cancer pathology image classification method based on deep learning
ActiveCN111079862AImprove classification accuracyCharacter and pattern recognitionNeural architecturesHeat mapOncology
The invention discloses a papillary thyroid cancer pathological image classification method based on deep learning, and mainly solves the problem of poor classification effect on papillary thyroid cancer pathological images in the prior art. According to the scheme, the method comprises the following steps: 1) reading a papillary thyroid cancer pathological section image with an amplification factor of 20, and inputting the papillary thyroid cancer pathological section image into an improved VGG-f convolutional neural network to obtain an attention heat map; 2) normalizing the attention diagram to obtain a discrimination force region position; reading a 40-time amplified thyroid cancer pathological image and obtaining an image block according to the position of the discrimination area; 3)inputting the image blocks into an original VGG-f network, constructing a loss function, and performing supervised training on the network; 4) extracting trained VGG-f network convolution features andperforming classification processing to obtain categories of the image blocks, and 5) judging the categories of the thyroid cancer pathological images according to the categories of the image blocks.The classification accuracy is high, and the method can be used for classifying the thyroid cancer papillary cancer pathological images by a computer.
Owner:XIDIAN UNIV
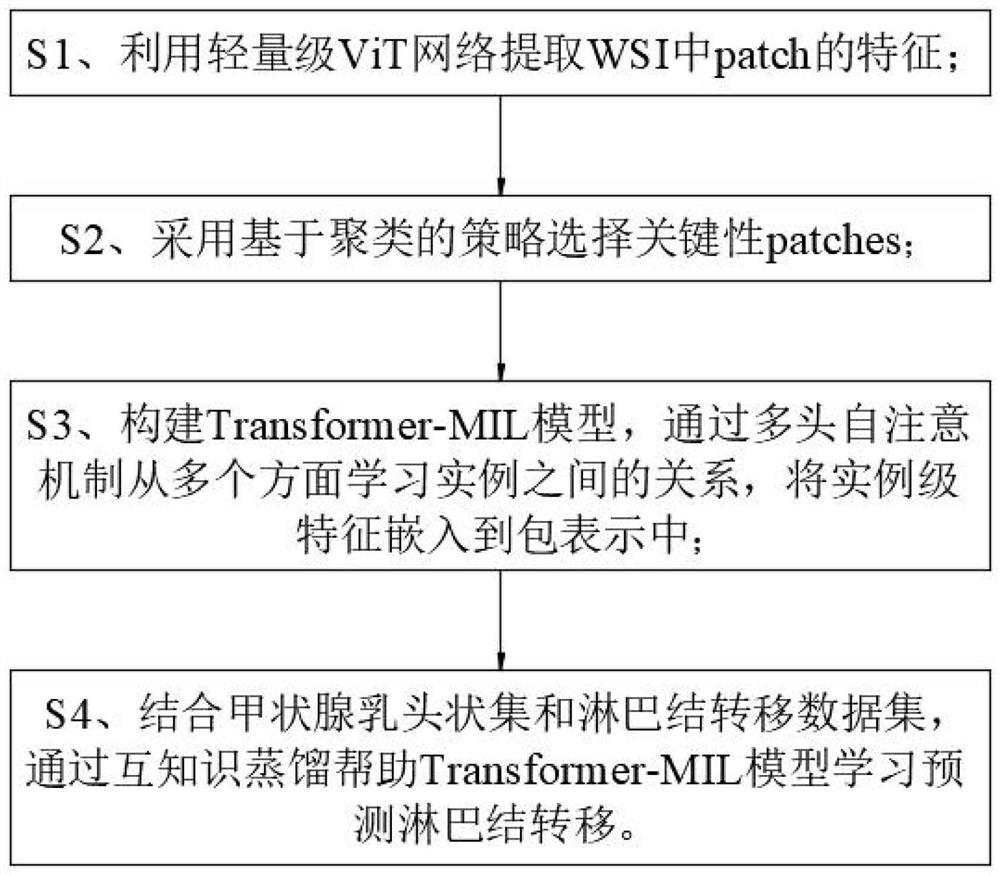
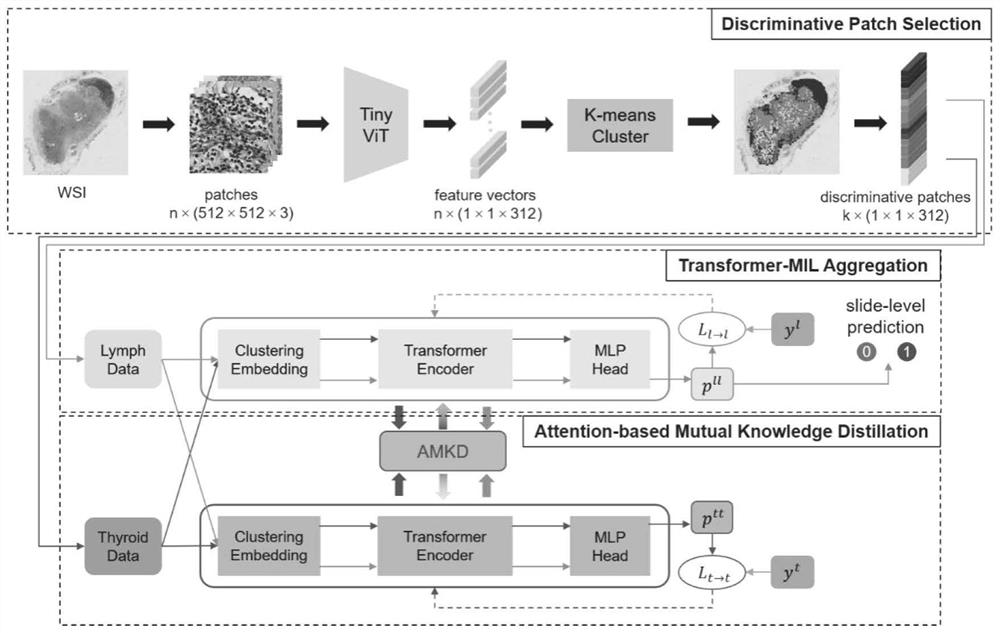
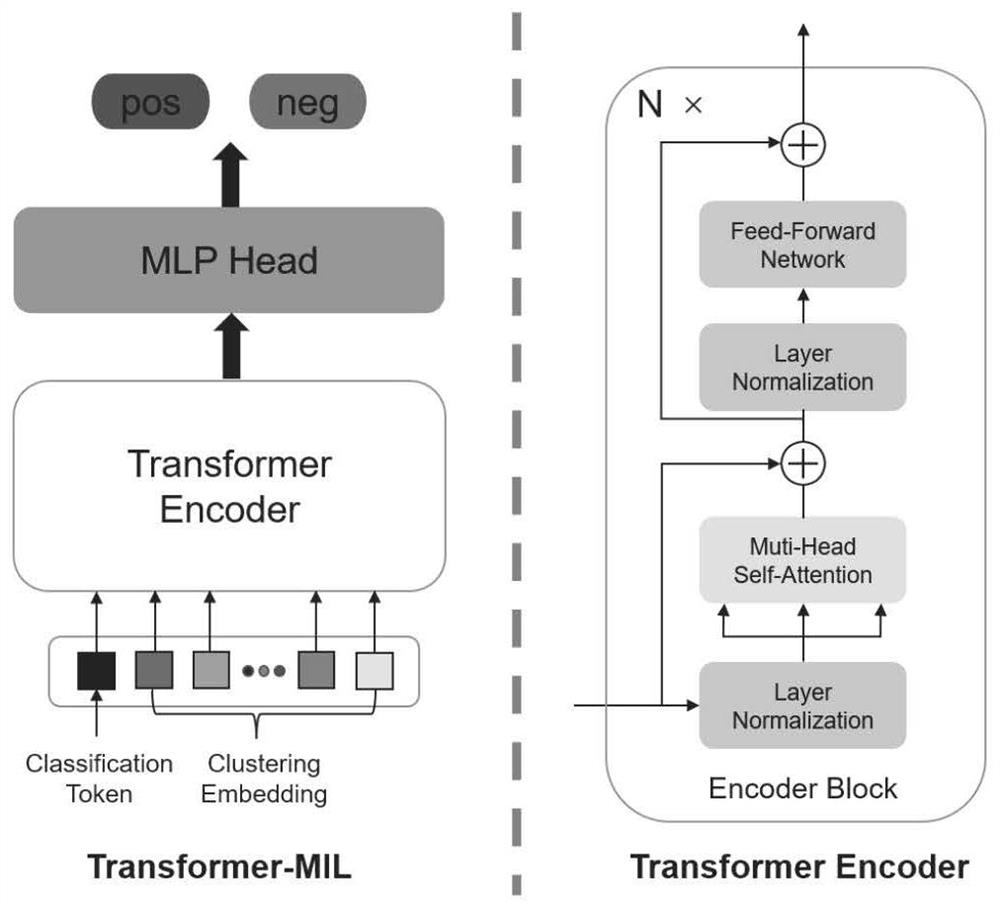
Papillary thyroid carcinoma lymph node metastasis prediction method based on Transform-MIL
PendingCN114188020ASave trainingShorten the timeImage enhancementImage analysisNode metastasisData set
The invention discloses a papillary thyroid carcinoma lymph node metastasis prediction method based on Transform-MIL. The method comprises the following steps: S1, extracting the characteristics of patch in WSI by using a lightweight ViT network; s2, selecting a key path by adopting a clustering-based strategy; s3, constructing a Transform-MIL model, learning relationships among instances from multiple aspects through a multi-head self-attention mechanism, and embedding instance-level features into packet representation; s4, combining a thyroid papillary set and a lymph node metastasis data set, and helping a Transform-MIL model to learn and predict lymph node metastasis through mutual knowledge distillation; according to the method, the Transform-MIL model is constructed, the instance-level features are better embedded into the packet representation, the morphological similarity between the tumor cells and the lymph node metastasis cells is fully utilized, and the knowledge of the relationship between the two data sets is transmitted by taking the attention map as a medium, so that the prediction accuracy of the lymph node metastasis histopathology image is improved.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Separation method of macrophage in thyroid papillary carcinoma tissue
InactiveCN103361312ASeparation succeededIncrease positive rateBlood/immune system cellsPhosphateDigestion
The invention provides a cell separation technology, and particularly relates to a separation method of macrophage in a thyroid papillary carcinoma tissue. The separation method comprises the following separation steps of: placing the collected thyroid papillary carcinoma tissue into D-hanks liquid; flushing; removing D-hanks liquid and connective tissues; cutting the collected thyroid papillary carcinoma tissue into blocks; adding II type collagenase into the blocked tissue; performing digestion reaction for 1 to 3 hours at temperature of 35 to 40 DEG C; adding an 1640 culture medium containing 10% of fetal calf serum for stopping the digestion reaction; filtering; centrifuging; removing supernatant; resuspending and planking cell sap through the 1640 culture medium; transferring into a cell incubator containing 5% of CO2 for incubating; fully washing through PBS (Phosphate Buffer Solution); re-culturing the obtained anchorage-dependent cells; collecting the supernatant; and freezing and storing. According to the separation method, the separated macrophage is circular and can be tightly adhered to a petri dish and shows positive by staining by CD68; the positive rate reaches more than 95%, namely, the macrophage is separated successfully.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Application of apatinib to preparing BRAF V600E protein kinase inhibitors
The invention relates to the technical field of medicines, in particular to application of apatinib to preparing BRAF V600E protein kinase inhibitors and application of the apatinib to preparing medicines for treating BRAF V600E mutation tumor. Melanoma, thyroid papillary carcinoma, borderline ovarian tumor, colorectal cancer, non-small cell lung cancer and hairy cell leukemia can be treated by the medicines. The application has the advantages that novel targets and indication can be provided for the apatinib; novel thinking can be provided for treating tumor such as BRAF V600E mutation melanoma, colorectal cancer and non-small cell lung cancer.
Owner:SECOND AFFILIATED HOSPITAL SECOND MILITARY MEDICAL UNIV
Thyroid myeloid cancer ultrasonic image recognition method based on clinical prior knowledge guidance
PendingCN114119458AImprove generalization abilityImprove accuracyImage enhancementImage analysisMalignancyDepartment radiology
The invention relates to a thyroid myeloid cancer ultrasonic image identification method based on clinical prior knowledge guidance. Firstly, a thyroid nodule area is obtained by designing a cascade segmentation network, then a multi-branch classification network is designed, and according to clinical knowledge guidance of radiologists, important thyroid ultrasound image feature validity and calcification are added as prior knowledge to assist network classification. The method not only can distinguish benign and malignant nodules in the image, but also can accurately distinguish the malignant nodules into myeloid thyroid carcinoma and papillary thyroid carcinoma.
Owner:FUZHOU UNIV
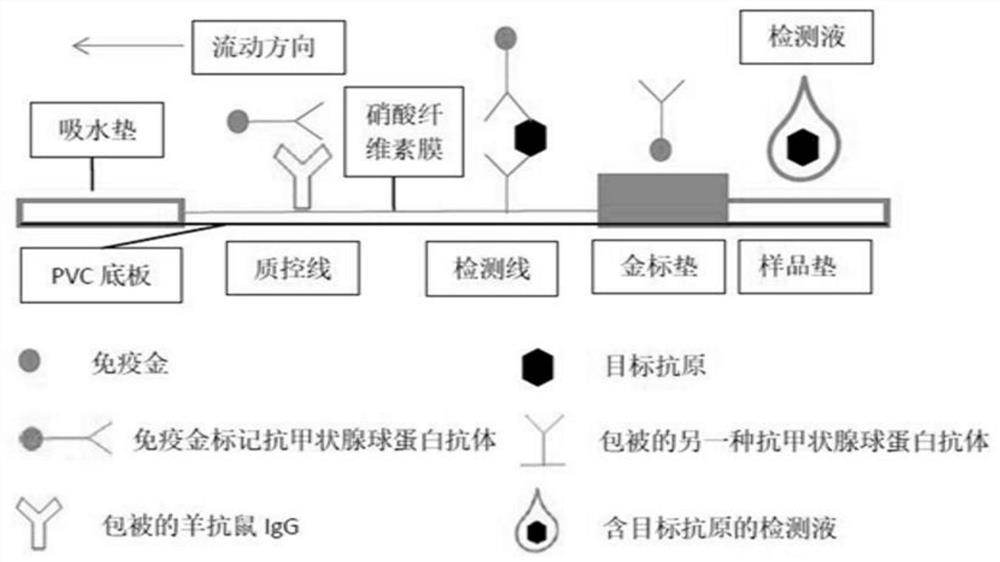
Colloidal gold chromatography test strip for rapidly detecting lymph node metastasis of papillary thyroid carcinoma and preparation method of colloidal gold chromatography test strip
PendingCN114859038AImprove organizational utilizationImprove work efficiencyDisease diagnosisDiseasePolyester
The invention discloses a colloidal gold chromatography test strip for rapidly detecting thyroid papillary carcinoma lymph node metastasis and a preparation method of the colloidal gold chromatography test strip. A sample pad, a combination pad, a nitrocellulose membrane and an absorption pad are sequentially overlapped at one end of a bottom plate from left to right; the binding pad is a polyester film sprayed with a Cyfra21-1 / Braf V600E capture antibody-colloidal gold compound, the nitrocellulose film is coated with a detection line T and a quality control line C, an antibody in the detection line T is a Cyfra21-1 / Braf V600E monoclonal antibody, and the quality control line C is goat anti-mouse IgG. The occurrence rate of false negative is reduced by improving the tissue utilization degree, the detection speed is increased through a chromatography test strip method, finally the purposes of reducing the missed diagnosis rate, relieving the disease burden of a patient and improving the working efficiency of thyroid surgery are achieved, and important clinical significance is achieved.
Owner:THE FIRST AFFILIATED HOSPITAL OF ANHUI MEDICAL UNIV
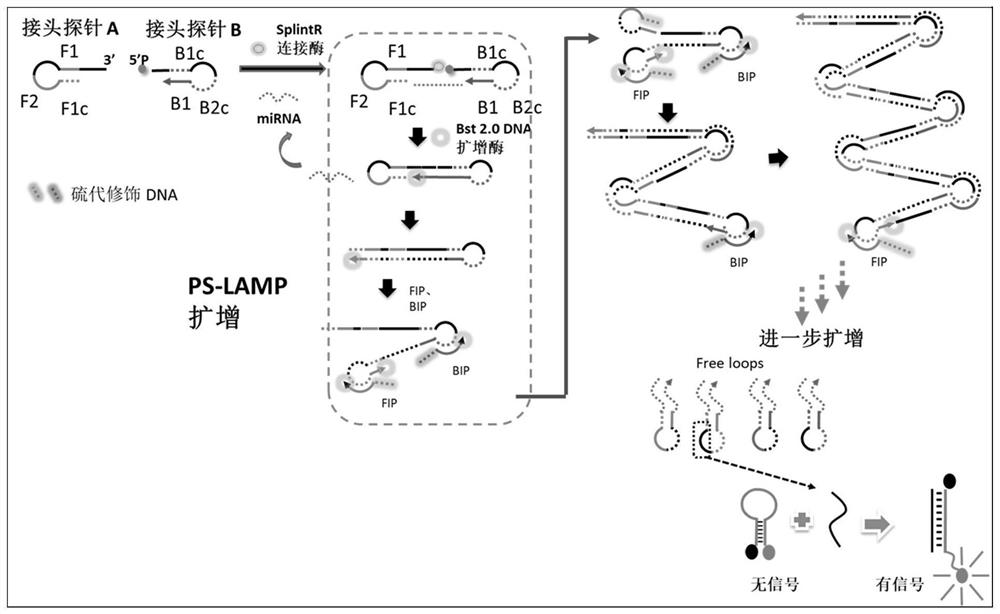
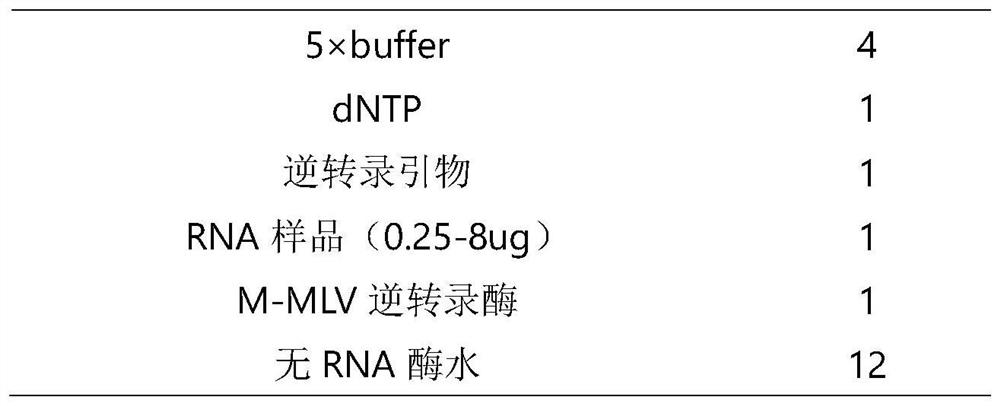
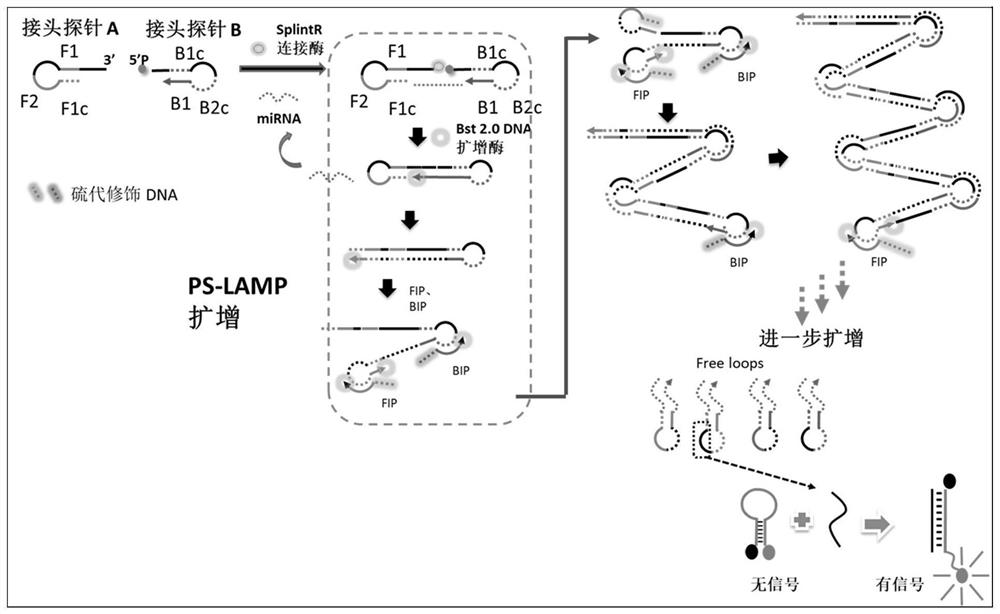
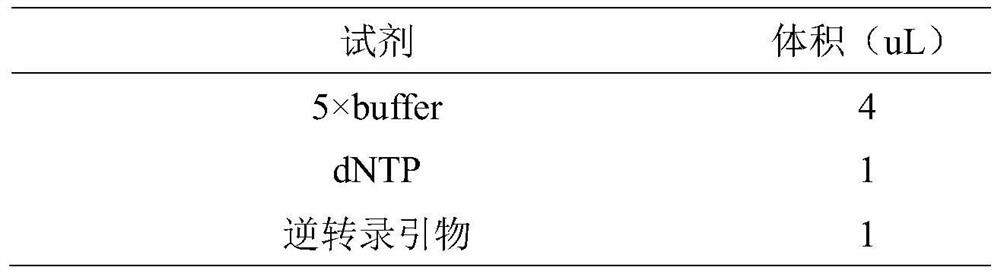
miRNA detection kit based on sulfo-modified loop-mediated isothermal amplification method
ActiveCN111961730AImprove efficiencyImprove stabilityMicrobiological testing/measurementModified dnaQuantitative PCR instrument
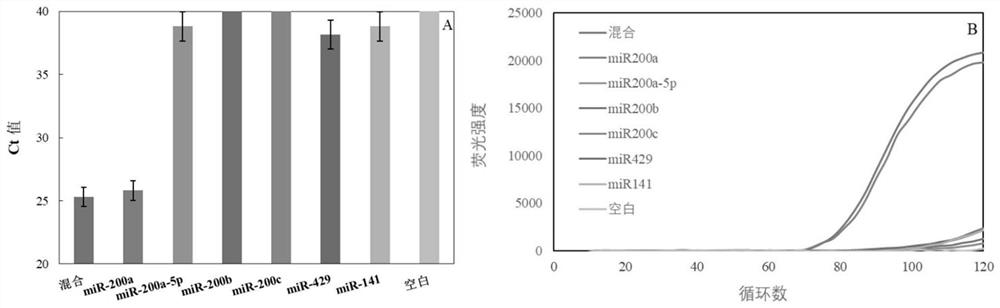
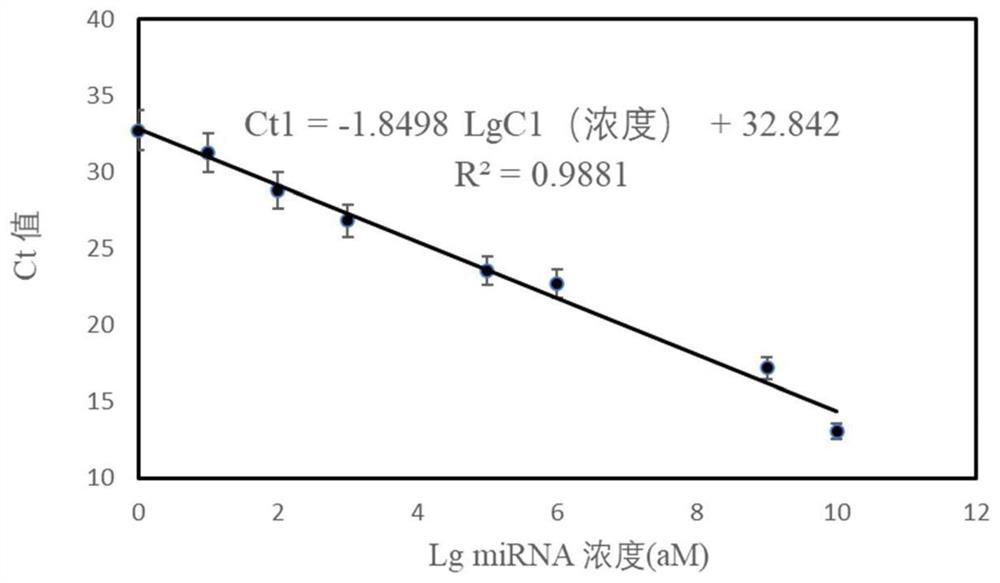
The invention provides an miRNA detection kit based on a sulfo-modified loop-mediated isothermal amplification method. The miRNA detection kit consists of a loop-mediated amplification reaction solution, an enzyme, a primer and probe mixed solution and a standard substance. Dumbbell-shaped DNA formed by connecting a base sulfo-modified linker probe Linker A / B in the presence of target miRNA is used as an amplicon and is combined with the same part of base sulfo-modified FIP / BIP primers to initiate LAMP exponential amplification; a sulfo-modified DNA structure can further enhance the amplification efficiency, the formed amplification product opens a signal probe-molecular beacon to generate a fluorescence signal, and the fluorescence signal of the target product is monitored by a real-timefluorescence quantitative PCR instrument, so that the target miRNA is detected, and the miRNA detection kit has the characteristics of strong stability, good specificity and high sensitivity. The miRNA detection kit can be used for detecting various miRNAs in serum of a papillary thyroid cancer patient, and can be used as a tumor marker for auxiliary diagnosis of papillary thyroid cancer.
Owner:ZHEJIANG UNIV
Papillary thyroid carcinoma serum marker and application
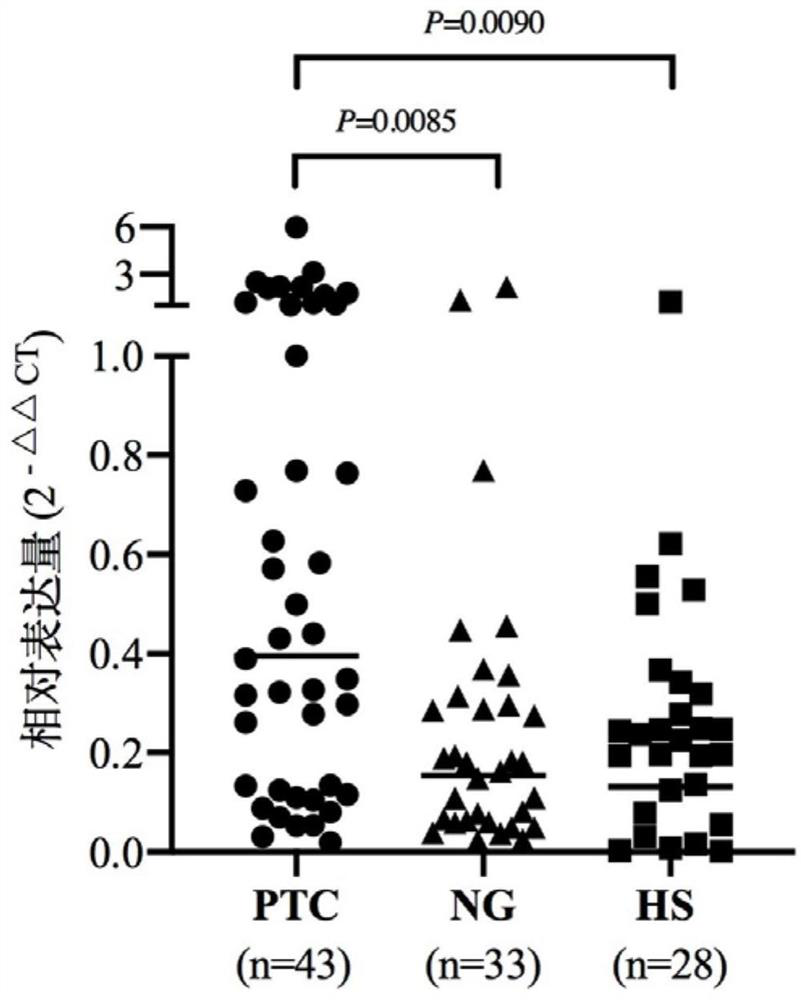
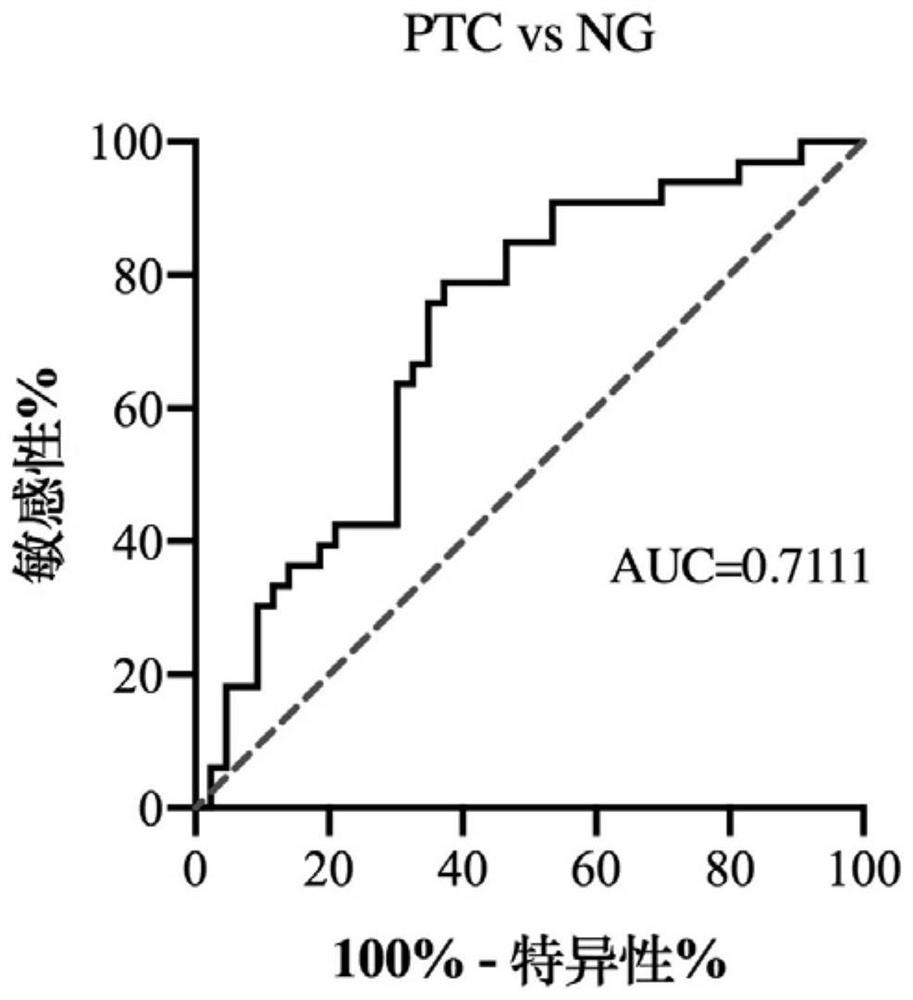
ActiveCN112226507AAvoid differencesAvoid dependencyMicrobiological testing/measurementDNA/RNA fragmentationNucleotideThyroid papillary carcinoma
The invention relates to a serum tRF marker used for PTC diagnosis. Secondly, the invention provides application of a reagent used for detecting an expression quantity of the tRF in the serum in preparation of a PTC diagnosis preparation. Finally, the invention provides a PTC diagnosis kit, the PTC diagnosis kit can measure the content of the tRF in the serum, and the nucleotide sequence of the serum marker is disclosed by SEQ ID NO.1. When a tRF molecule provided by the invention and a specific primer of the tRF molecule are taken as the diagnosis reagent, the diagnosis accuracy of the PTC can be further improved, help is provided for clinical doctors to quickly master the state of an illness of a patient and formulate a diagnosis and treatment scheme, and a foundation is laid for discovering a novel small molecule targeted medicine with a potential treatment value.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
Immune colloidal gold test strip for detecting thyroglobulin and preparation method thereof
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
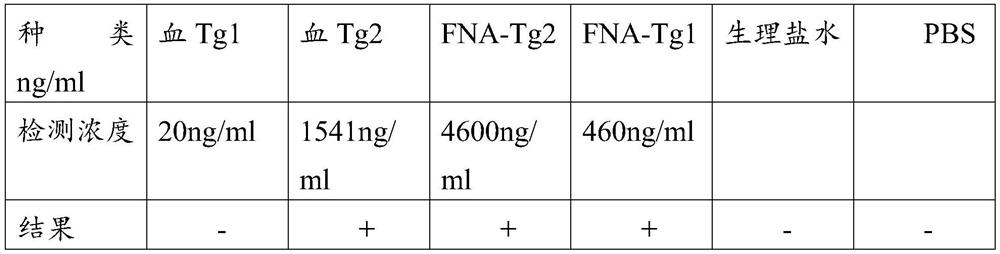
Iodine resistance screening kit for papillary thyroid carcinoma
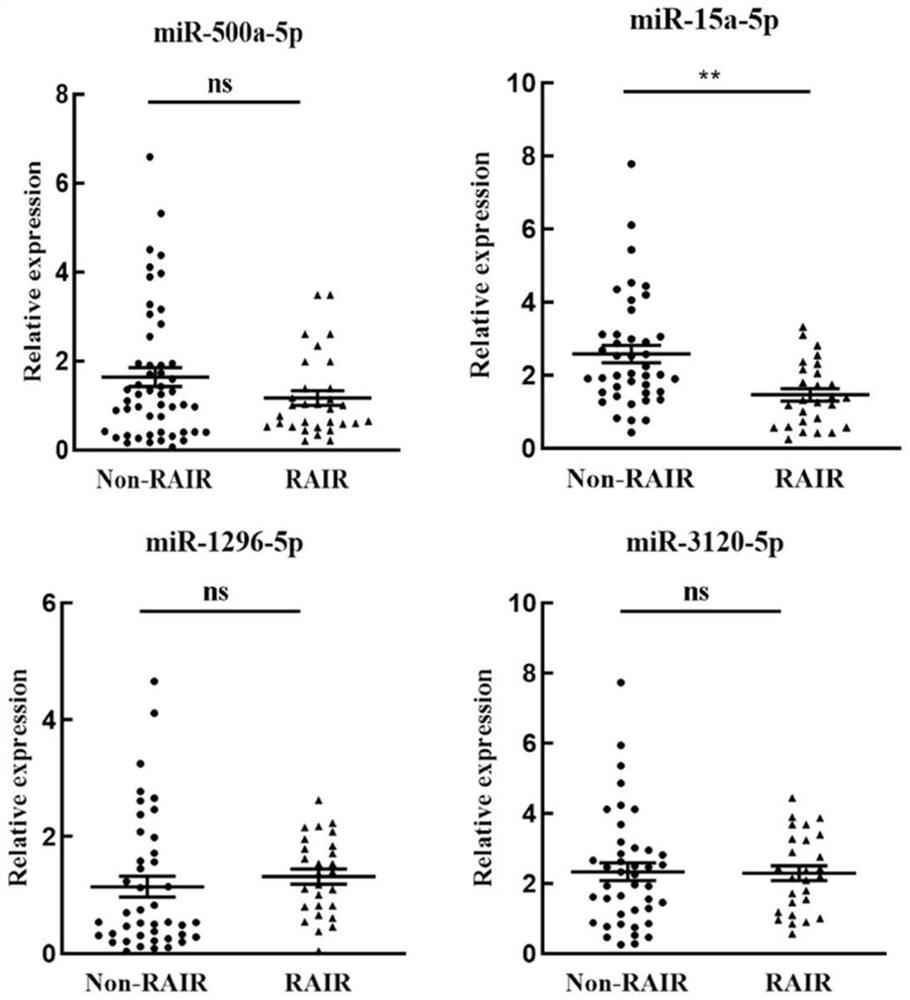
The invention discloses application of a reagent for detecting exosome miR-15a-5p in preparation of a papillary thyroid carcinoma iodine resistance screening kit, and belongs to the field of cancer diagnosis. In the plasma exosome of a patient with iodine-resistant papillary thyroid carcinoma, the level of miR-15a-5p is obviously lower than that of a patient with non-iodine-resistant papillary thyroid carcinoma. According to the discovery, the reagent for detecting the exosome miR-15a-5p is used for preparing the papillary thyroid carcinoma iodine resistance screening kit and has a good application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Method for rapidly identifying thyroid papillary carcinoma lymphonodi cervicales metastasis in operation
The invention discloses fluorescence immunochromatographic test paper for rapidly detecting human thyroglobulin (Tg) so as to rapidly identify thyroid papillary carcinoma lymphonodi cervicales metastasis. According to the test paper, a double-antibody sandwich method and a fluorescence immunochromatography technology are adopted to detect human Tg; and antibodies are prepared through specific antigen epitope peptides. With the method adopted, human Tg in an object to be detected can be rapidly and accurately detected. The test paper has the advantages of simple, convenient and rapid operation, wide detection range, high specificity and high sensitivity.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Cyclopropane amides and analogs exhibiting Anti-cancer and Anti-proliferative activities
Compounds of the present invention find utility in the treatment of mammalian cancers and especially human cancers including, but not limited to, malignant melanomas, solid tumors, glioblastomas, ovarian cancer, pancreatic cancer, prostate cancer, lung cancers, breast cancers, kidney cancers, hepatic cancers, cervical carcinomas, metastasis of primary tumor sites, myeloproliferative diseases, chronic myelogenous leukemia, leukemias, papillary thyroid carcinoma, non-small cell lung cancer, mesothelioma, hypereosinophilia syndrome, gastrointestinal stromal tumors, colonic cancers, ocular diseases characterized by hyperproliferation leading to blindness including various retinopathies, diabetic retinopathy, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, mastocytosis, mast cell leukemia, and diseases caused by PDGFR-α kinase, PDGFR-β kinase, c-KIT kinase, cFMS kinase, c-MET kinase, and oncogenic forms, aberrant fusion proteins and polymorphs of any of the foregoing kinases.
Owner:DECIPHERA PHARMA LLC
Genes Associated with Papillary Thyroid Tumors
ActiveCN104845970BMicrobiological testing/measurementDNA/RNA fragmentationNon-coding RNAGene silencing
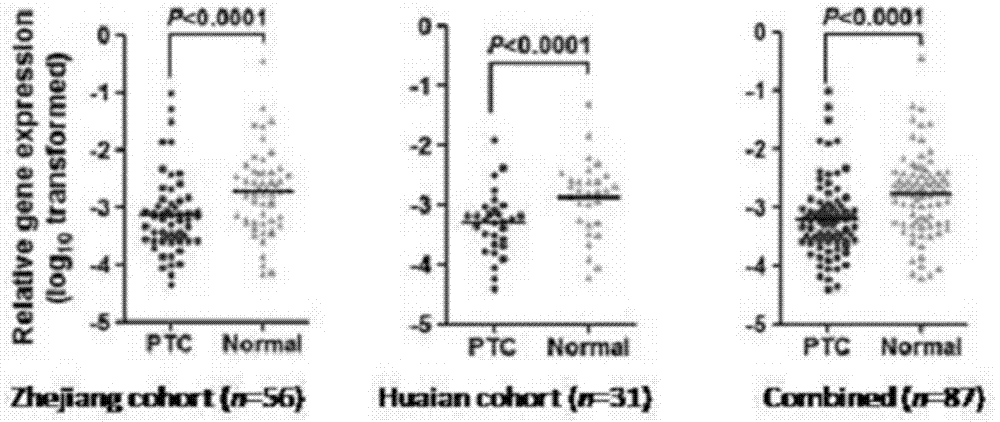
The invention relates to a gene related to papillary thyroid cancer and its application. According to the base sequence of the gene, real-time quantitative PCR primers are designed and synthesized, and the long-chain non-coding transcribed by the gene is detected in clinical case samples of papillary thyroid cancer. The expression level of RNA was found to be significantly down-regulated in papillary thyroid tumor tissue, and gene silencing of the long non-coding RNA could significantly promote the growth of thyroid cancer cells. The invention is expected to prepare preparations for auxiliary diagnosis, gene therapy, curative effect prediction or prognosis judgment of papillary thyroid cancer.
Owner:BOZHOU CITY THE NEW HEALTH TECH CO LTD
Molecular marker for distinguishing papillary thyroid carcinoma from benign thyroid nodules
InactiveCN110964819AMicrobiological testing/measurementDNA/RNA fragmentationRNA SequenceNodular lesion
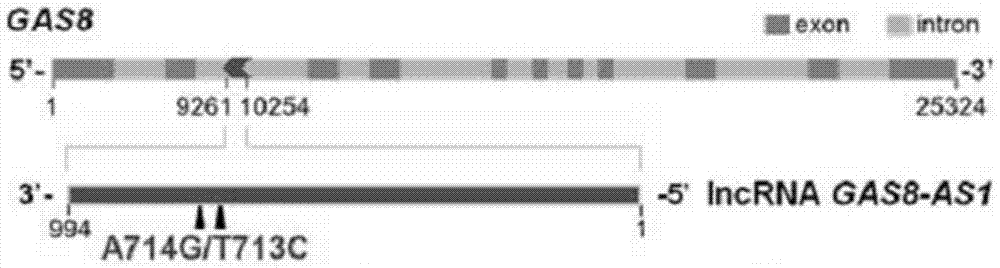
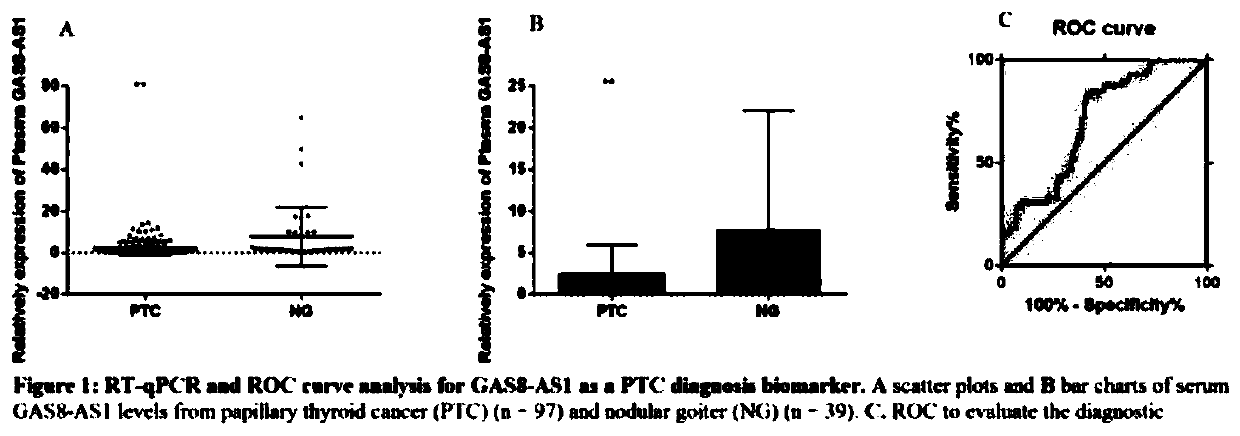
The invention relates to a molecular marker for distinguishing papillary thyroid carcinoma from benign thyroid nodules. The molecular marker is an RNA sequence segment of GAS8-AS1, wherein the RNA segment is as shown in SEQ ID No. 2, and a coding DNA sequence of the RNA sequence segment is as shown in Seq ID No.1. The invention also relates to application of the RNA fragment in preparation of a detection reagent or kit for distinguishing papillary thyroid carcinoma from benign thyroid nodules.
Owner:BEIJING SHIJITAN HOSPITAL CAPITAL MEDICAL UNIVERSTY
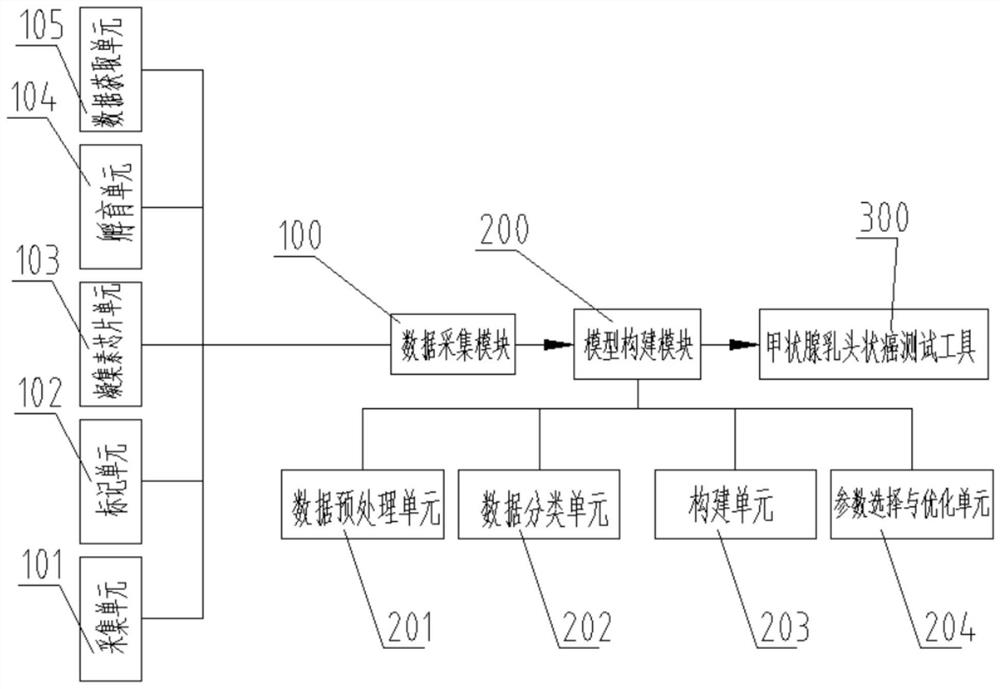
Tool and system for testing papillary thyroid carcinoma
The invention provides a tool and a system for testing papillary thyroid carcinoma. The tool comprises a processor and a storage medium, the storage medium performs data interaction with the processor, and is used for executing the following steps when a program stored in the storage medium is loaded by the processor: identifying lectin chip data of saliva of a patient to be diagnosed through a papillary thyroid carcinoma identification model, and determining whether the patient to be diagnosed is a papillary thyroid carcinoma patient. According to the tool for testing papillary thyroid carcinoma, saliva of a patient to be diagnosed is identified through the papillary thyroid carcinoma diagnosis module, whether a sample to be diagnosed is a papillary thyroid carcinoma sample or not is determined, and the tool has the advantages of being convenient to sample and high in sensitivity; and whether a subject suffers from papillary thyroid carcinoma or not can be quickly identified.
Owner:NORTHWEST UNIV
Application of gene mutation site and mutation site detection method
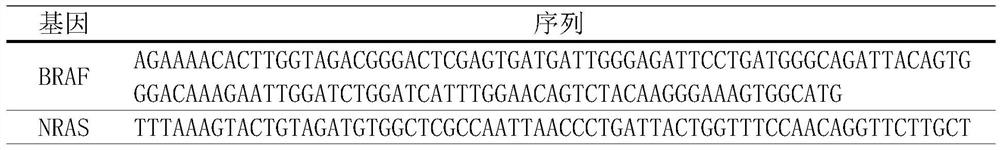
PendingCN113584171AReduce workloadShorten detection timeMicrobiological testing/measurementAntineoplastic agentsRet geneAdjuvant therapy
The invention belongs to the technical field of medicine, and relates to application of a gene mutation site and a mutation site detection method. The invention mainly discloses application of a PTEN-p.P248L gene locus, a PTEN-p.T319fs gene locus, a BRAF gene V600E locus, an NRAS gene Q61R locus, an RET gene M918T locus, an NRAS gene Q61K locus, an HRAS gene Q61R locus and the like in preparation of thyroid cancer prevention and treatment drugs and / or preparation of thyroid cancer detection reagents. The mutant PTEN gene at least comprises one of the following site mutation: PTEN-p.P248L site mutation, and the base change is c.743C > T; the PTEN-p.T319fs site is mutated, and the basic group is changed into c.955_956insAA. The invention further discloses a capture method in high-throughput sequencing. The invention provides the mutant gene which is related to papillary thyroid carcinoma targeted medication and has a high proportion, so that the sites can be purposefully detected and can be better used for adjuvant therapy, the workload of detection and data analysis is effectively reduced, and the detection time and cost are reduced.
Owner:湖北省中医院
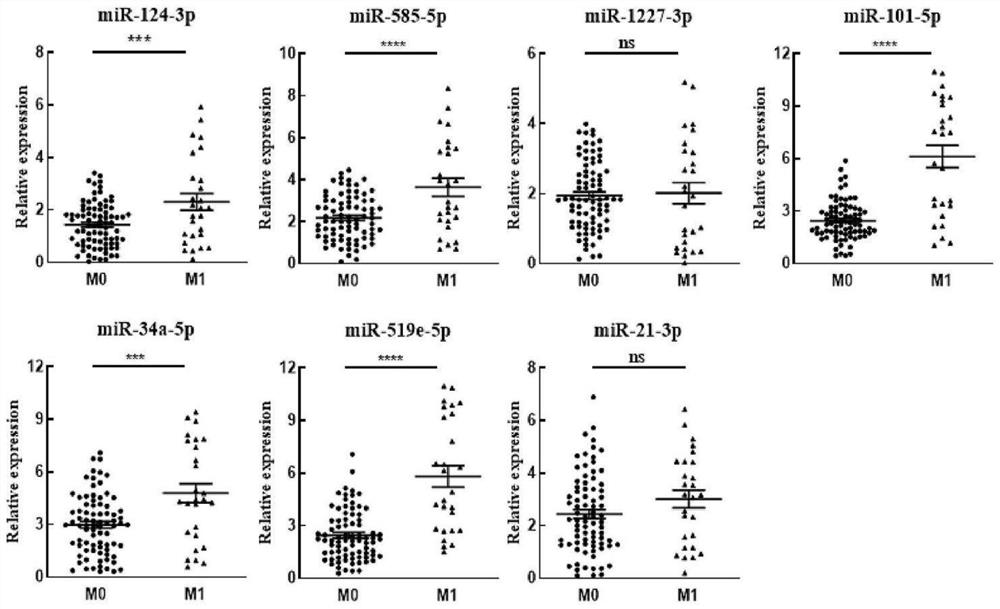
miRNA biomarkers and detection kits for the diagnosis of cervical lymph node metastasis in papillary thyroid carcinoma
ActiveCN113025714BTraumaEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationNode metastasisSerum mirna
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Risk assessment model for papillary cancer of thyroid nodule patient
PendingCN112037919AComprehensive forecastIncrease medical costsMedical simulationMedical data miningDiseaseOncology
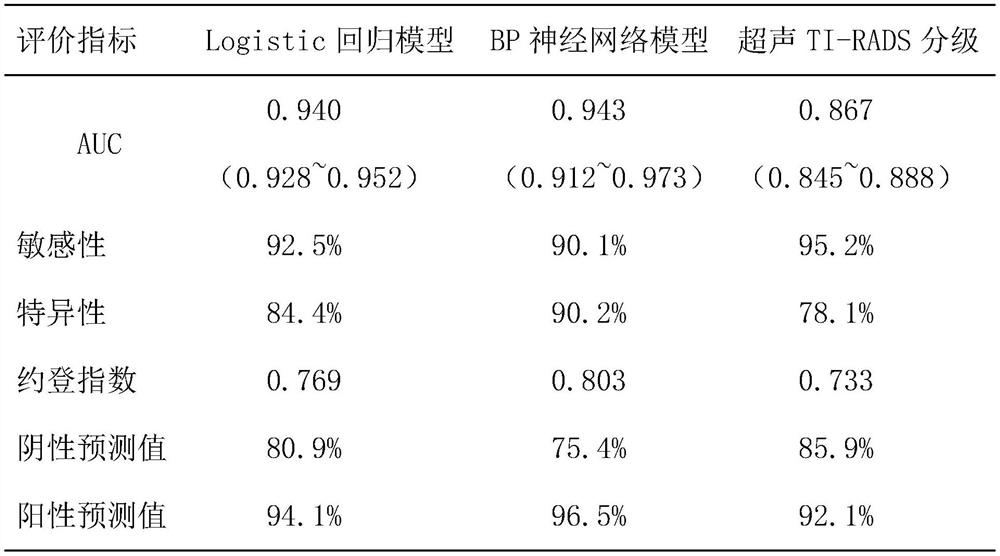
The invention discloses a risk assessment model for papillary cancer of a thyroid nodule patient, and the model is characterized in that the model comprises the steps: carrying out the risk factor analysis of PTC through Logistic regression, and selecting a specific index as an independent variable of the model; constructing a BP neural network model comprising three layers of feedforward neural network structures of an input layer, a hidden layer and an output layer, and training and predicting the BP neural network model by utilizing data information comprising the specific indexes; and comparing a prediction value output by the model with a real value, drawing an ROC curve to obtain an area AUC under the curve, evaluating the diagnosis performance of the model by using the AUC value, and the like, so that the model with the diagnosis performance reaching the standard is a target risk evaluation model. The risk assessment model can be used as a reference for a doctor to diagnose papillary thyroid carcinoma, and the accuracy of diagnosis of papillary thyroid carcinoma diseases is improved.
Owner:NANJING DRUM TOWER HOSPITAL
Thyroid cancer prognosis condition judgment method
ActiveCN113176408AHigh probability of dedifferentiationReduced characteristicsBiological material analysisBiological testingThyroid gland cancerOncology
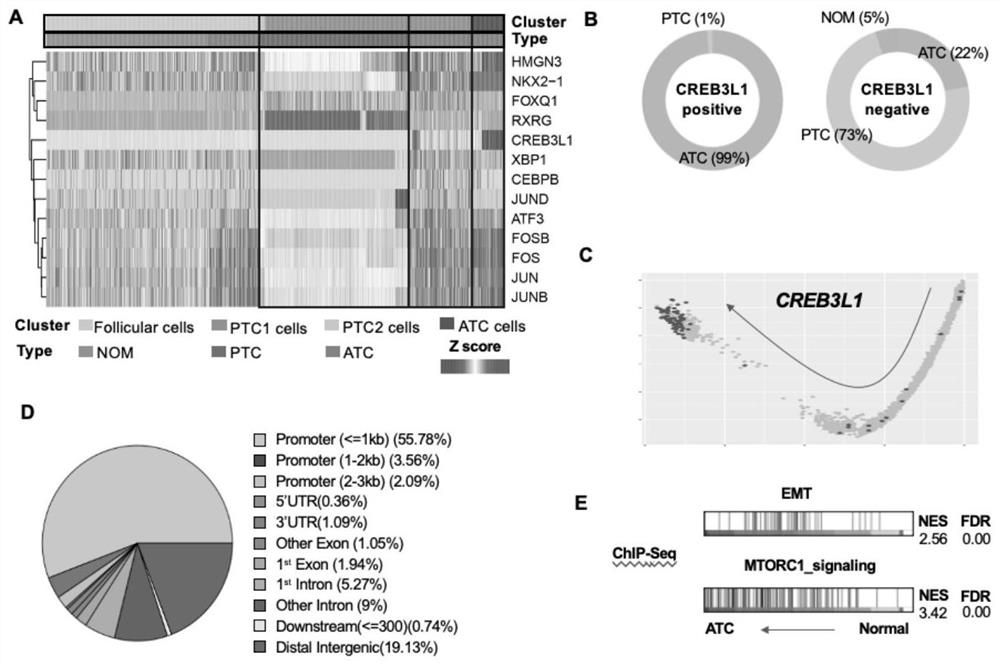
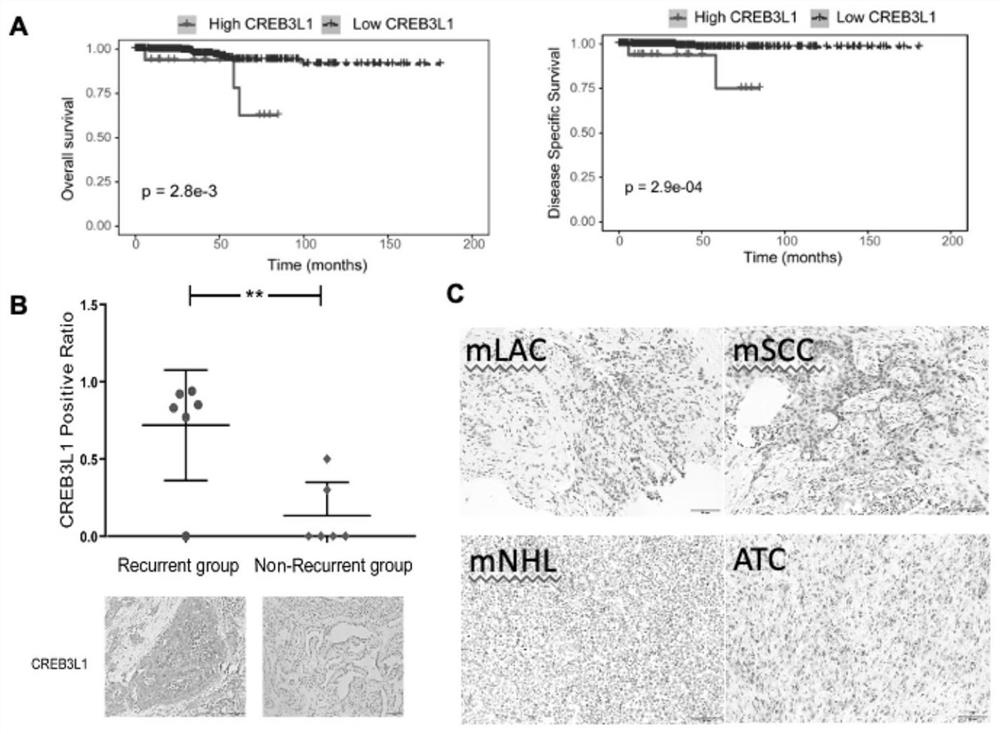
The invention provides a thyroid cancer prognosis condition judgment method and an antibody, particularly relates to an application of a CREB3L1 gene to preparation of a reagent for thyroid papillary cancer prognosis condition judgment, and particularly relates to thyroid papillary cancer prognosis condition judgment.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
miRNA detection kit based on thio-modified ring-mediated isothermal amplification method
ActiveCN111961730BHigh sensitivityShort detection timeMicrobiological testing/measurementModified dnaThio-
The invention provides a miRNA detection kit based on a thio-modified ring-mediated isothermal amplification method, which is composed of a ring-mediated amplification reaction solution, enzyme, primer and probe mixed solution, and a standard product. In the present invention, the dumbbell-shaped DNA formed by ligation of linker probes Linker A / B modified by base thio in the presence of target miRNA is used as an amplicon, and combined with FIP / BIP primers modified by the same part of base thio, triggering a LAMP index Level amplification, the thio-modified DNA structure can further enhance the amplification efficiency, and the formed amplification product turns on the signal probe-molecular beacon to generate a fluorescent signal, and the fluorescent signal of the target product is monitored by a real-time fluorescent quantitative PCR instrument to detect the target miRNA , has the characteristics of strong stability, good specificity and high sensitivity. The invention can be used to detect multiple miRNAs in serum of patients with thyroid papillary carcinoma, and can be used as a tumor marker for auxiliary diagnosis of thyroid papillary carcinoma.
Owner:ZHEJIANG UNIV
Molecular mutation prediction model, method and system for distant metastasis of thyroid papillary carcinoma
The invention provides a papillary thyroid carcinoma distant metastasis mutation predicting model, a predicting model method and a predicting model system. The papillary thyroid carcinoma distant metastasis mutation predicting model realizes high predicting accuracy which is higher than that of a currently reported model. Furthermore the papillary thyroid carcinoma distant metastasis mutation predicting model has high sensitivity and high specificity. Furthermore researching of the invention proves a fact that TERT starter mutation has no evident correlation with distant metastasis, and furthermore chr22q loss and gene fusion are remarkably correlated with distant metastasis. The samples of the invention are obtained from Chinese people, and foreign researching samples are obtained from the Western country people. Therefore the researching result of the invention has higher application value for the Chinese people.
Owner:ZHEJIANG CANCER HOSPITAL
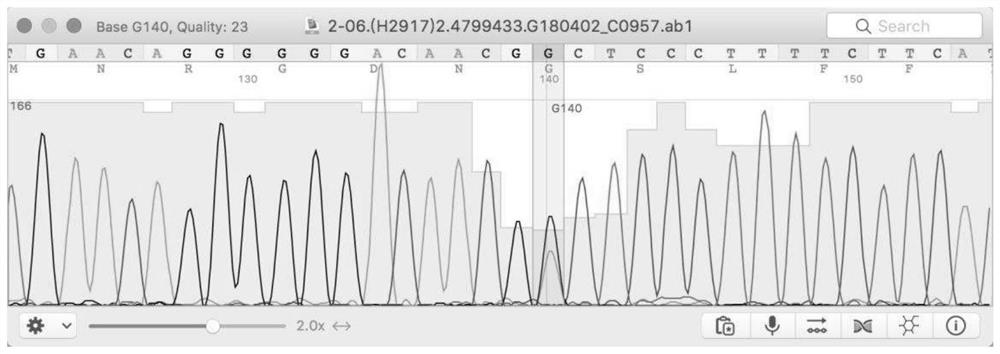
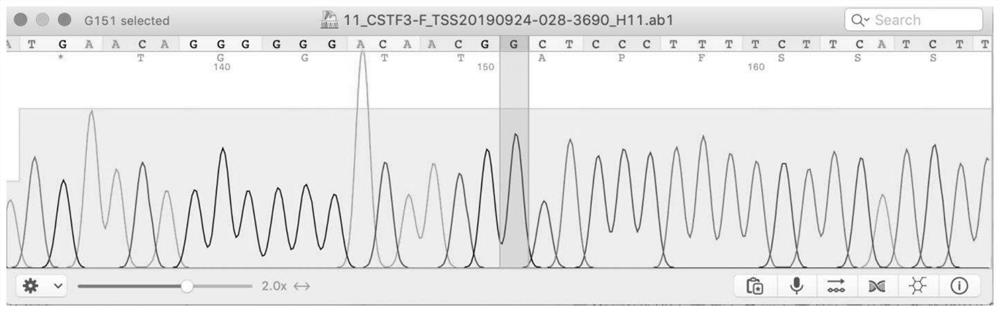
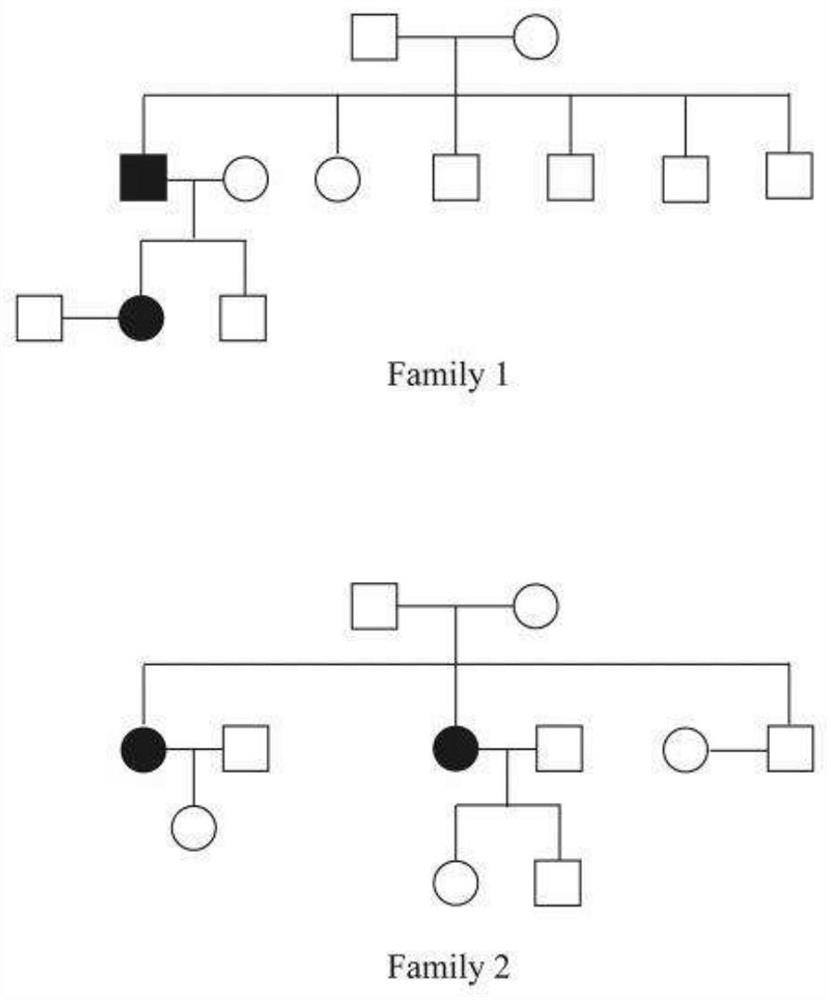
Kit for assisting in screening of familial papillary thyroid carcinoma
The invention discloses a kit for assisting in screening of familial papillary thyroid carcinoma, and belongs to the field of gene polymorphism detection kits. The kit mainly comprises a reagent for detecting the variant human CSTF3 gene, and the gene is the CSTF3 gene corresponding to the 33106691th base of the eleventh chromosome of the genome changed from G to A. The invention further discloses an application of the reagent for detecting the base variation in preparation of a reagent for assisting in screening of familial papillary thyroid carcinoma and a kit for assisting in screening of the familial papillary thyroid carcinoma. The kit disclosed by the invention can be used for assisting in diagnosis of thyroid cancer and has an excellent application prospect.
Owner:四川省三物科技有限公司
Liquid biopsy kit for diagnosis of lymph node metastasis in papillary thyroid carcinoma
ActiveCN111763736BMicrobiological testing/measurementBiological material analysisNode metastasisOncology
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
A method for rapid intraoperative identification of cervical lymph node metastasis in papillary thyroid carcinoma
The invention discloses a fluorescent immunochromatography test paper for rapidly detecting human thyroglobulin (Tg) to rapidly identify cervical lymph node metastasis of papillary thyroid carcinoma. The test paper detects human Tg by double antibody sandwich method and fluorescent immunochromatography technology. Antibodies are prepared using specific epitope peptides. The invention can quickly and accurately detect the human Tg in the object to be tested, and has the advantages of simple and rapid operation, wide detection range, high specificity and good sensitivity.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
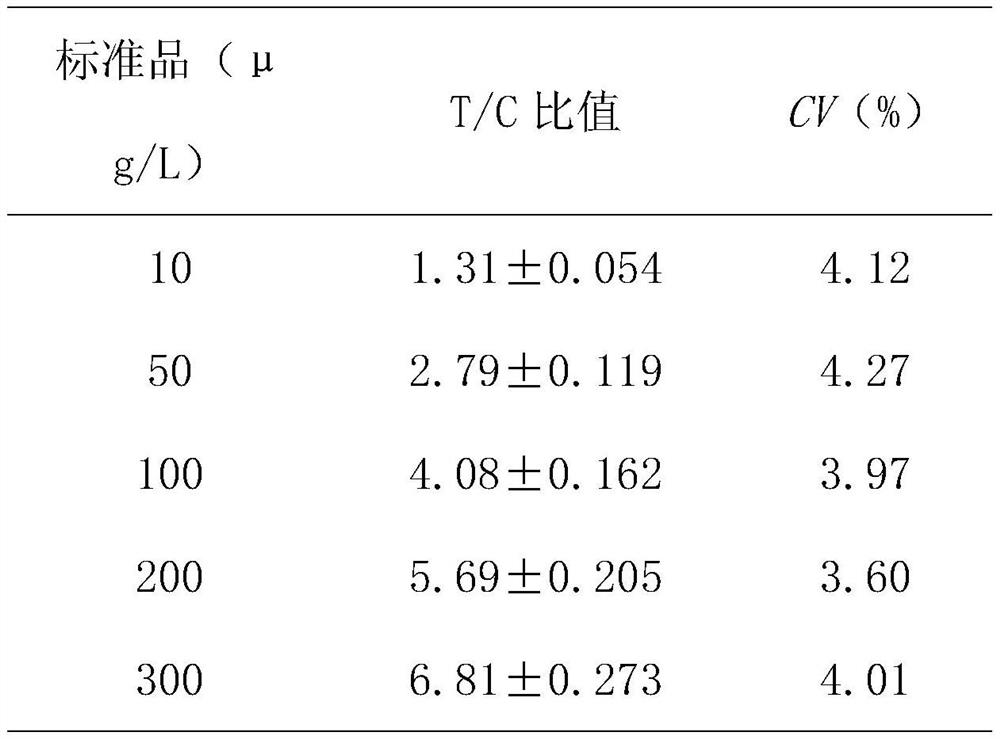
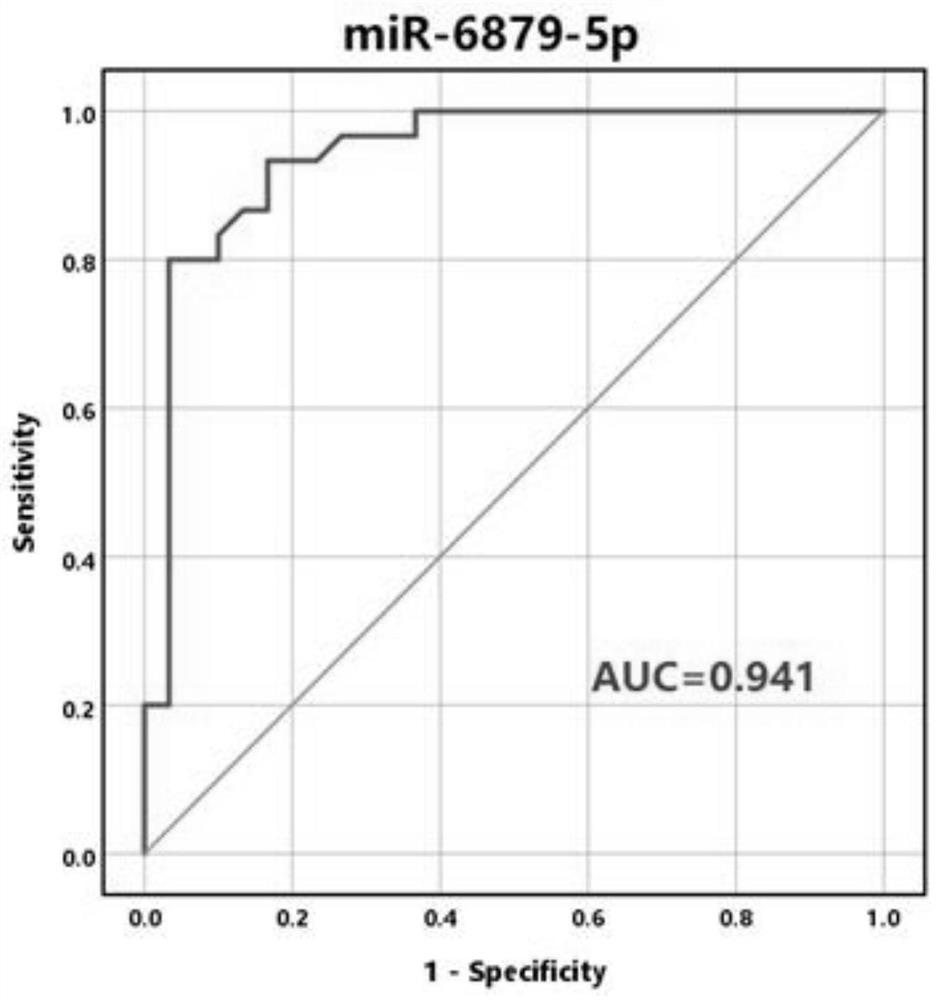
Application of reagent for detecting exosome miR-6879-5p in preparation of thyroid cancer metastasis detection kit
ActiveCN114058698AMicrobiological testing/measurementAgainst vector-borne diseasesNode metastasisCancers diagnosis
The invention discloses application of a reagent for detecting exosome miR-6879-5p in preparation of a thyroid cancer lymph node metastasis detection kit, and belongs to the field of cancer diagnosis. In the plasma exosome of a papillary thyroid carcinoma lymph node metastasis patient, the level of miR-6879-5p is obviously higher than that of a non-lymph node metastasis patient. According to the discovery, the reagent for detecting the exosome miR-6879-5p is used for preparing the thyroid cancer lymph node metastasis detection kit, whether a papillary thyroid cancer patient has lymph node metastasis or not can be accurately judged, and the kit has a good application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
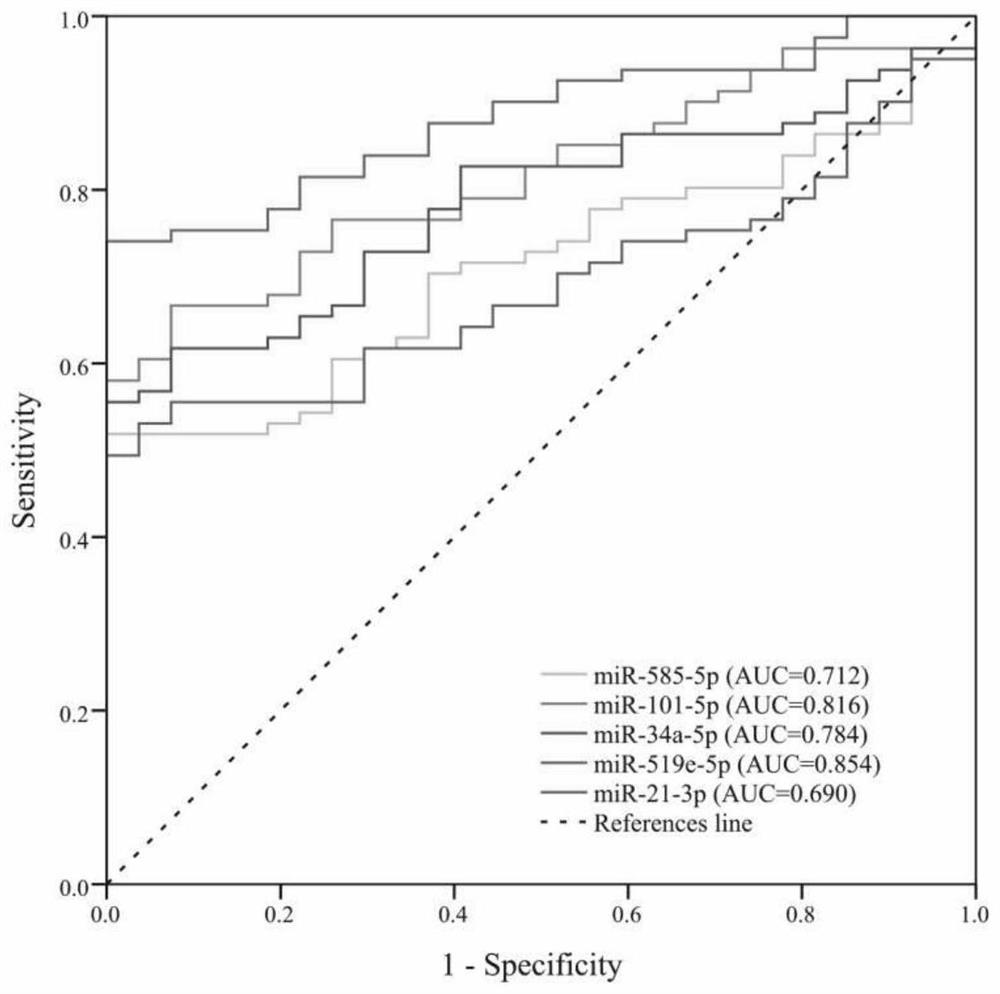
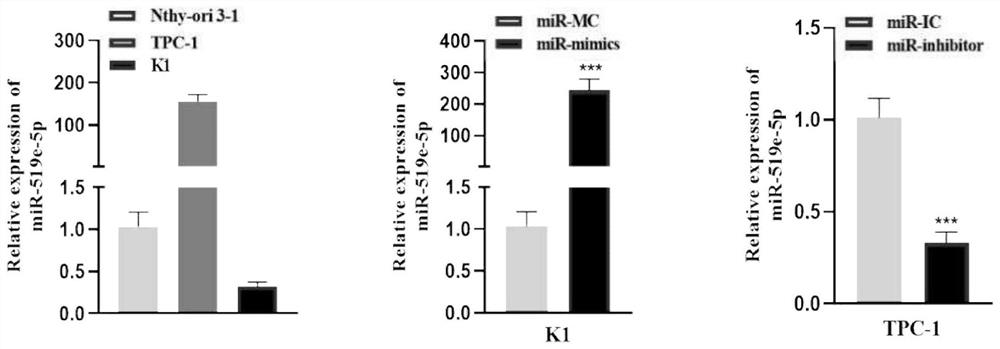
Application of miR-519e-5p as target spot for detecting or treating papillary thyroid carcinoma distant metastasis
ActiveCN114058696AOrganic active ingredientsMicrobiological testing/measurementPharmaceutical drugMetastasis detection
The invention discloses application of miR-519e-5p as a target spot for detecting or treating papillary thyroid carcinoma distant metastasis, and belongs to the field of cancer detection and treatment. The technical scheme of the invention mainly comprises application of a reagent for detecting exosome miR-519e-5p in preparation of a papillary thyroid carcinoma remote metastasis detection kit, and application of an artificial simulant of miR-519e-5p or miR-519e-5 in preparation of a medicine for inhibiting papillary thyroid carcinoma metastasis. According to the kit, the detection process can be simplified, and the pain of a patient is relieved; the medicine can remarkably inhibit mobility, invasiveness and dryness of thyroid cancer cells, and has great application potential.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Application of selfheal extract in medicine preparation
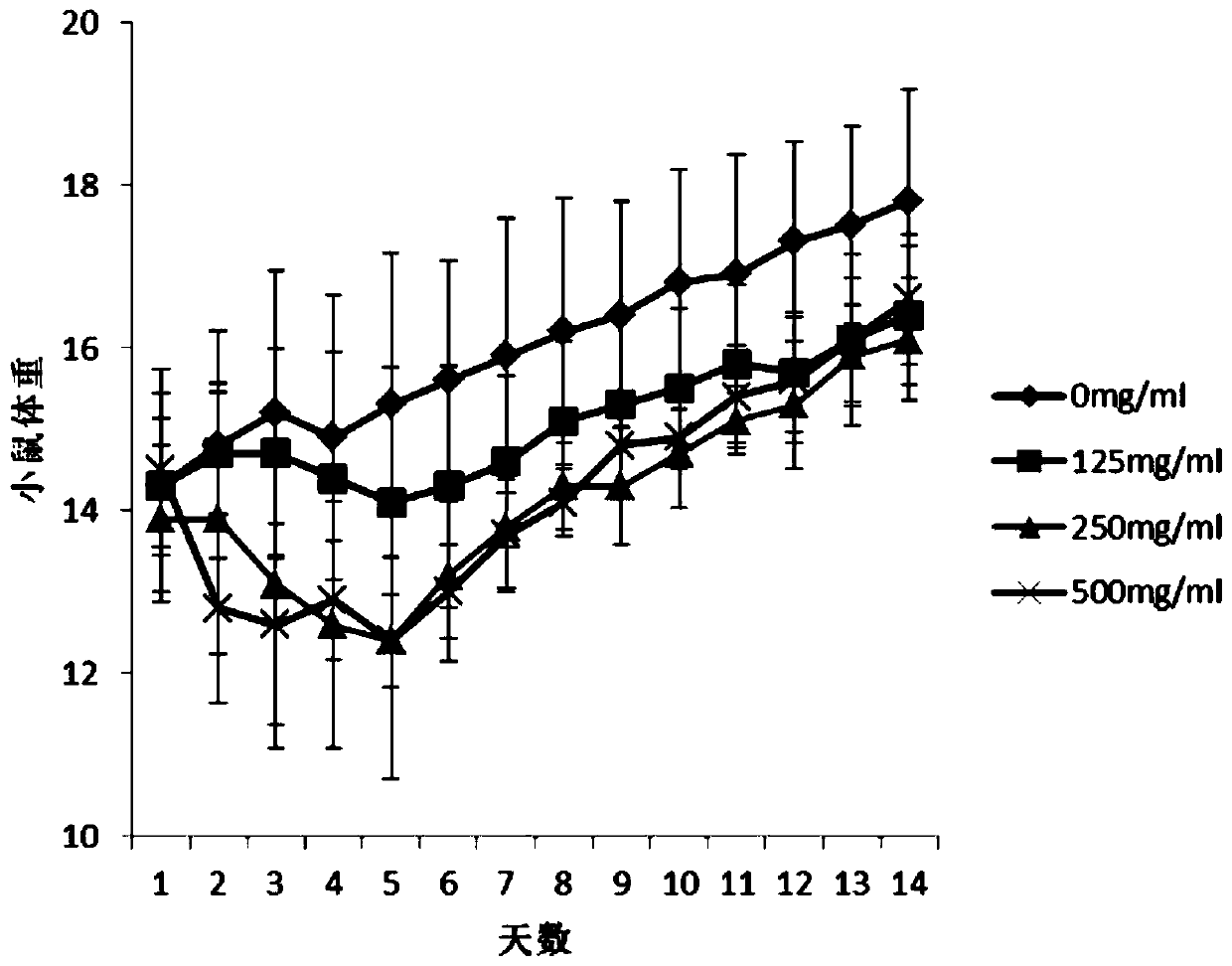
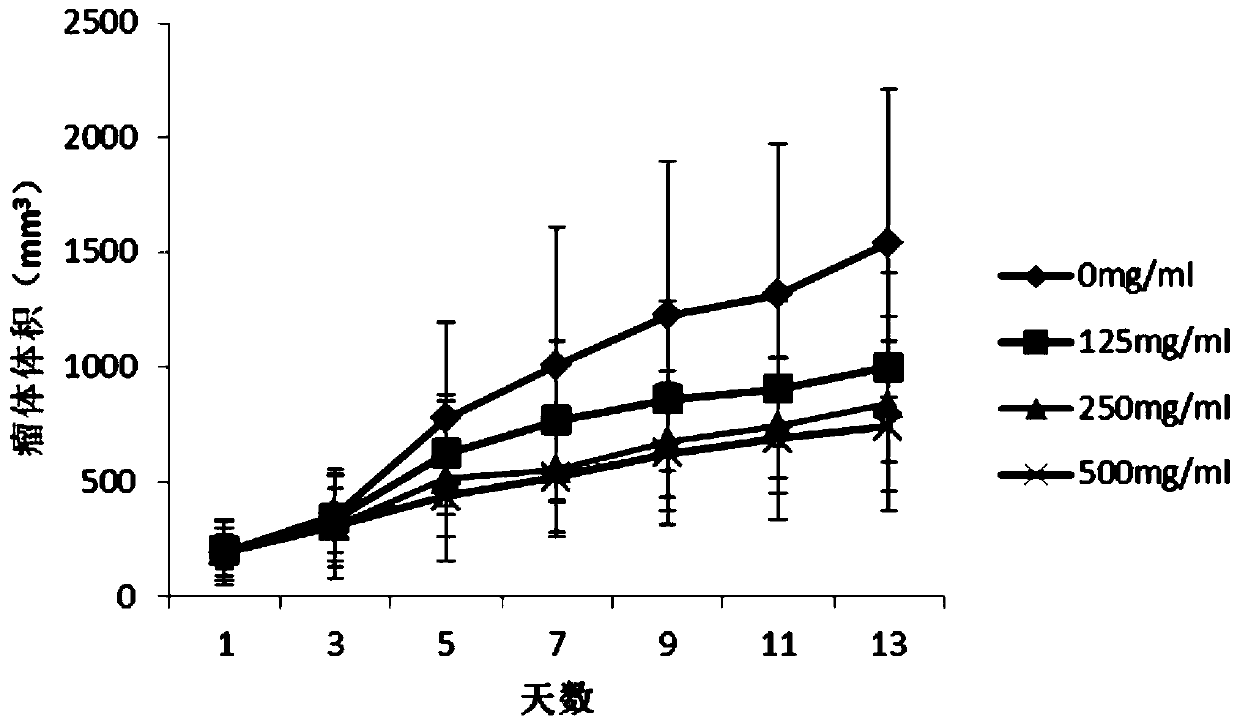
PendingCN111419898AIncrease in sizeStable functionAntineoplastic agentsPlant ingredientsDiseaseThyroid gland cancer
The invention discloses an application of a selfheal extract in medicine preparation, and belongs to the field of traditional Chinese medicines. The selfheal extract is prepared by subjecting selfhealfruit-spike to ultrasonic extraction, macroporous resin column gradient elution and heating concentration. When used for preparing medicines for treating thyroid cancer and preventing recurrence of thyroid cancer after operation, the selfheal extract is used as an active ingredient, the weight percentage content of the total organic acid components is not less than 20%, and the weight percentagecontent of the total flavonoid components is not less than 10%. Animal experiments prove that: the selfheal extract can effectively inhibit the increase of the volume of the thyroid papillary carcinoma, has concentration dependence, and can be used for treating thyroid cancer; and clinical tests prove that the selfheal extract has the effects of stabilizing the thyroid function and other abnormalindexes after the operation of a thyroid cancer patient, can be used for preventing the recurrence of the thyroid cancer, and provides a new medicine way for treating thyroid diseases and preventing the recurrence after the operation.
Owner:GUIYANG XINTIAN PHARMA CO LTD
Methods, kits, and systems for treatment of metastatic papillary thyroid cancer
Provided herein are methods for identifying a subject with an increased likelihood of developing or having metastatic papillary thyroid cancer (PTC), or a subject with an increased likelihood of developing or having recurrent PTC, and the treatment of such a subject.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
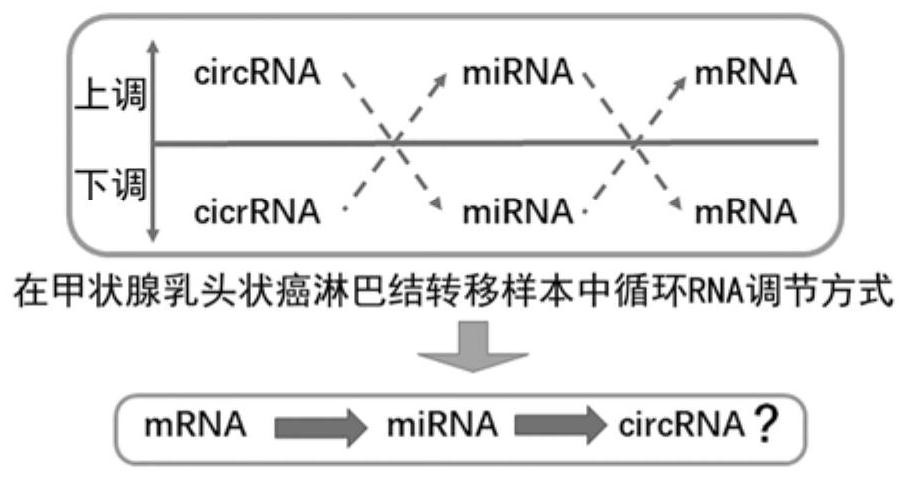
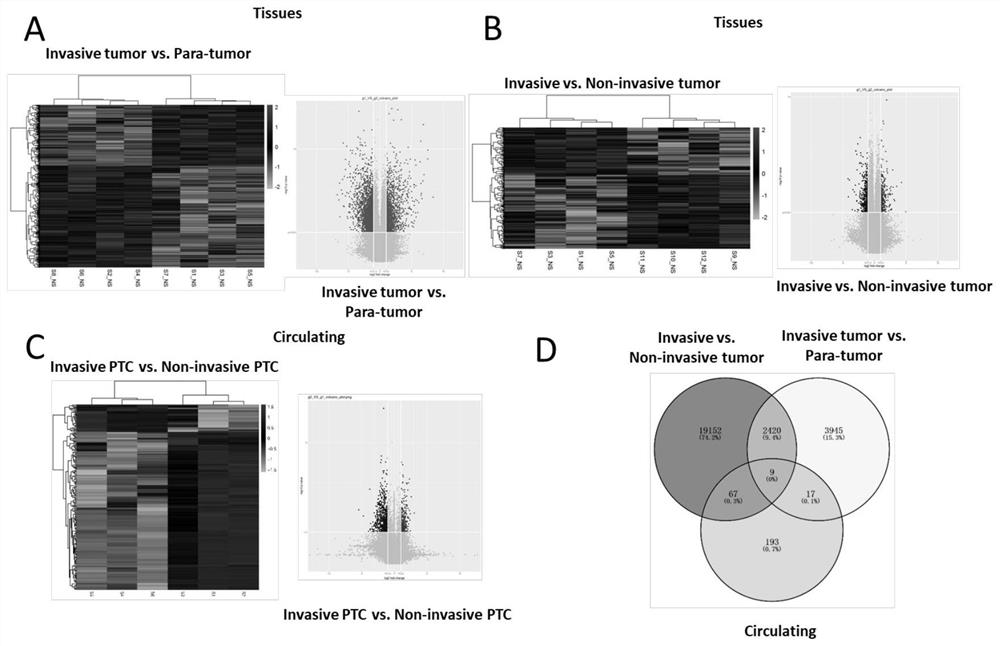
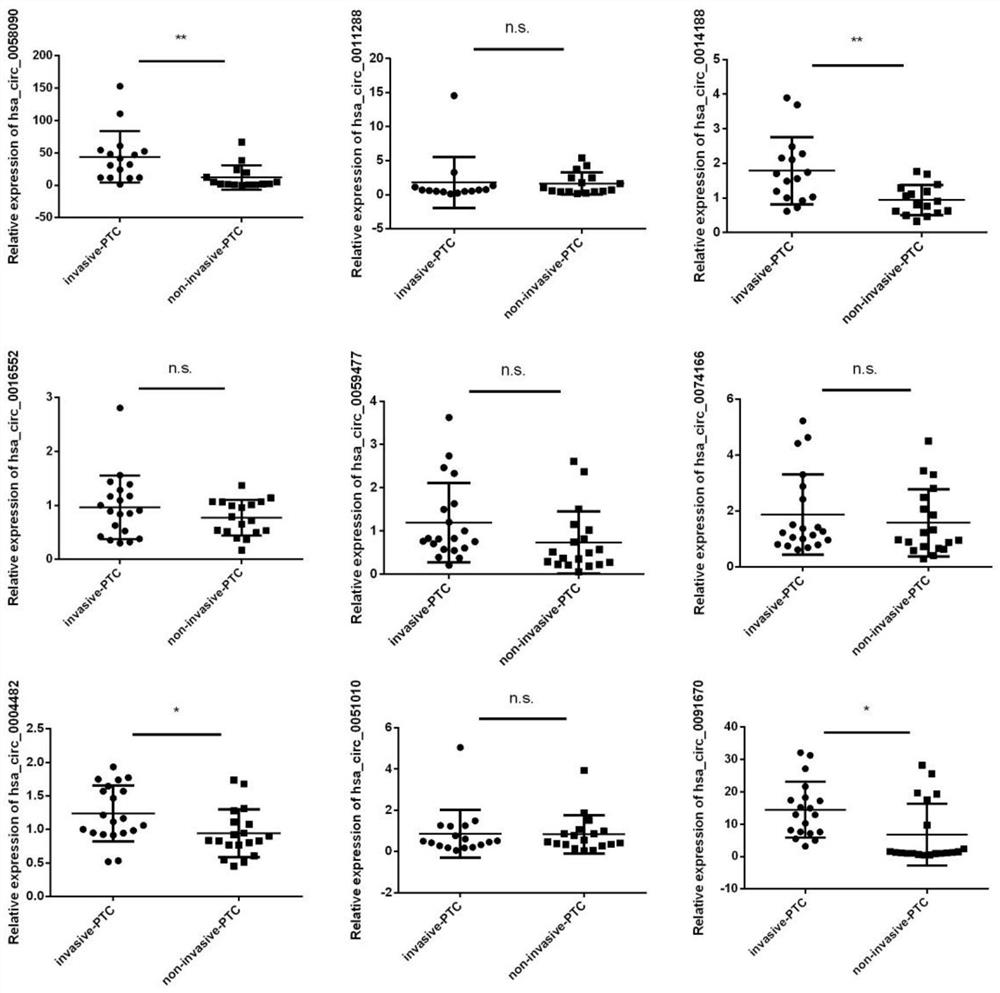
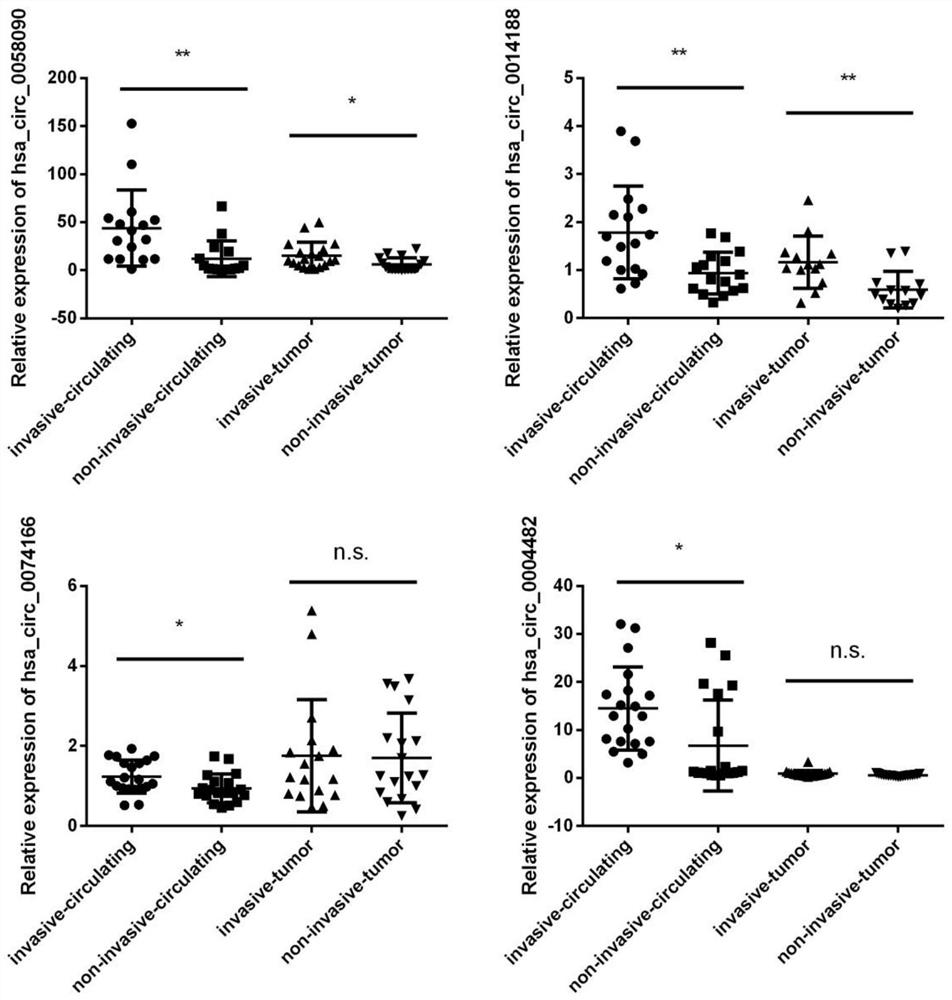
Circulating circular RNA markers and applications for the diagnosis of invasive papillary thyroid carcinoma
ActiveCN110982901BReduce wasteNon-invasiveMicrobiological testing/measurementBiologic markerCircular RNA
The invention belongs to the technical field of biological detection, and specifically discloses a circulating circular RNA marker for diagnosing invasive papillary thyroid carcinoma and its application. The biomarker for diagnosing invasive papillary thyroid carcinoma is circulating circular RNA, The circulating circular RNAs are circ_0058090 and circ_0014188. The biomarker of the invention has high sensitivity and specificity, can be effectively used in the diagnosis of invasive PTC, and can significantly improve the survival of patients.
Owner:JIANGSU CANCER HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com