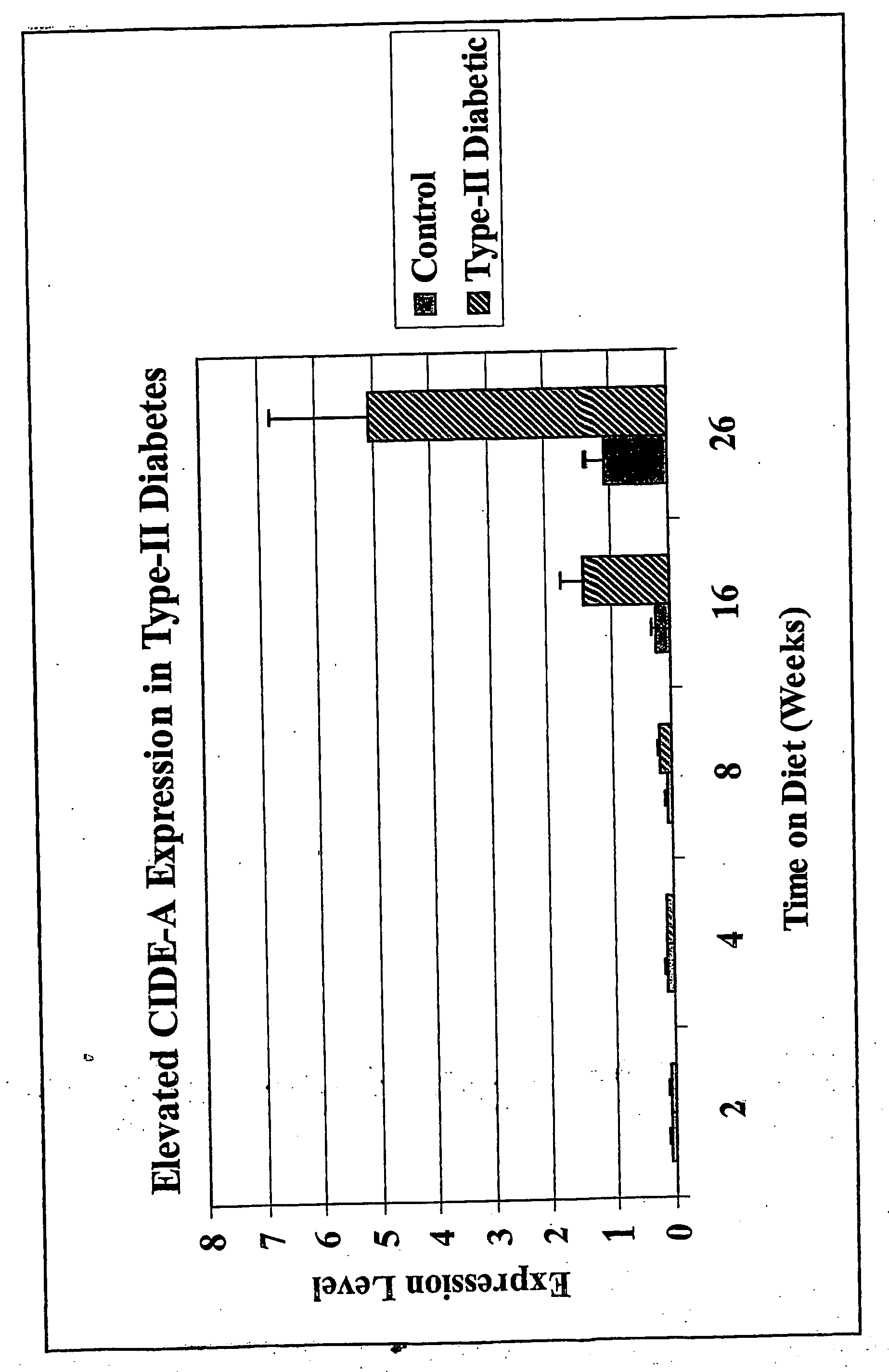
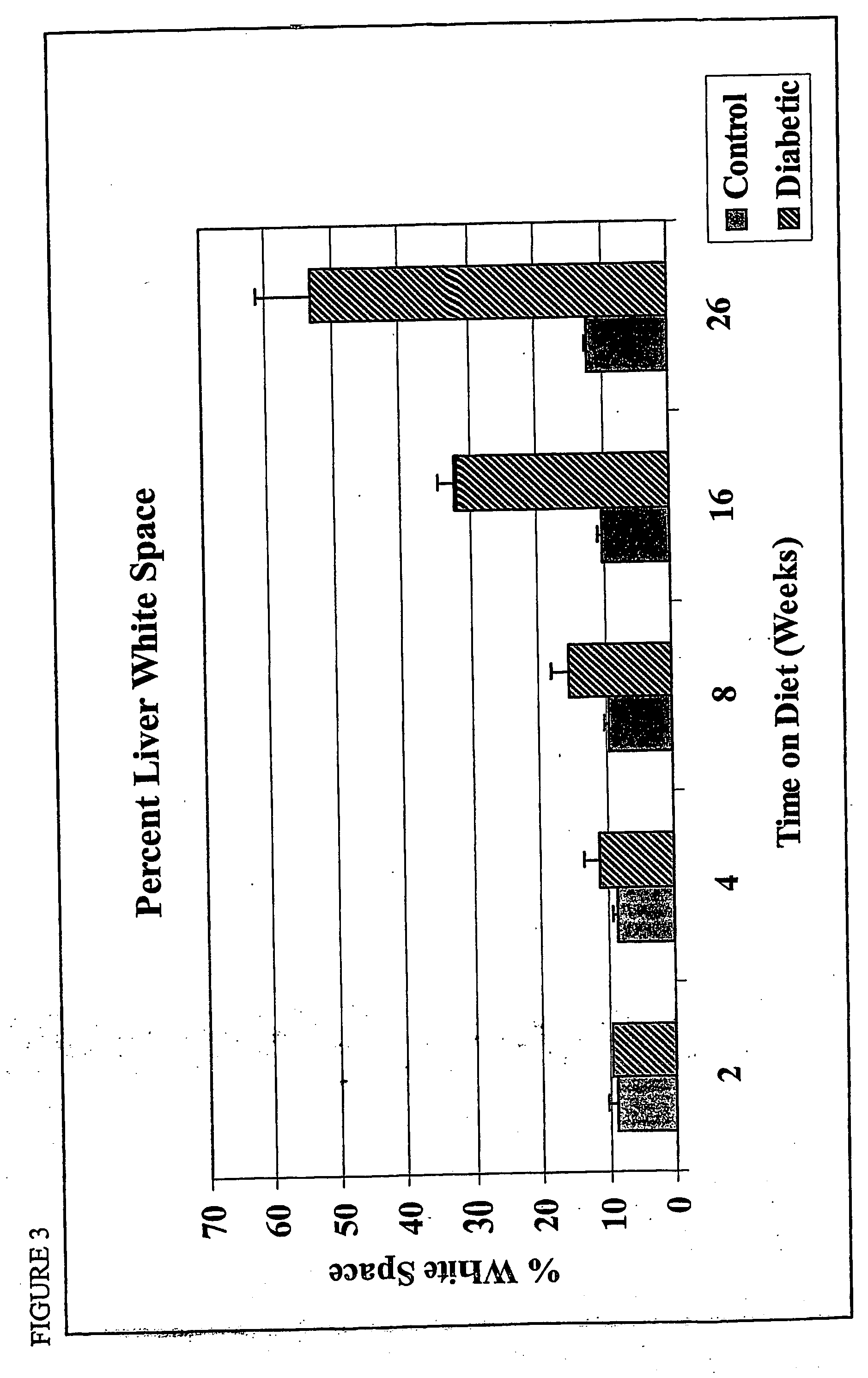
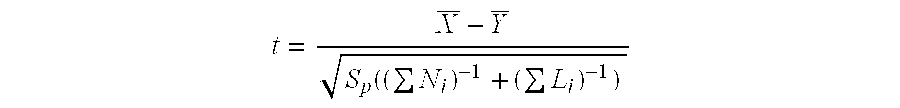Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
286 results about "Genes human" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The human genome is the complete set of nucleic acid sequences for humans, encoded as DNA within the 23 chromosome pairs in cell nuclei and in a small DNA molecule found within individual mitochondria. Human genomes include both protein-coding DNA genes and noncoding DNA.
Method for diagnosing non-small cell lung cancers
InactiveUS20060024692A1Reduce the overall heightTargeted optimizationMicrobiological testing/measurementNon-small cell lung cancer (NSCLC)Differentially expressed genes
Disclosed are methods for detecting, diagnosing, treating and preventing non-small cell lung cancer using differentially expressed genes. Furthermore, novel human genes, whose expression is elevated in non-small cell lung cancer compared to non-cancerous tissues, are provided. Also disclosed are agents for treating and preventing non-small cell lung cancer as well as methods for identifying further compounds for treating and preventing non-small cell lung cancer.
Owner:ONCOTHERAPY SCI INC
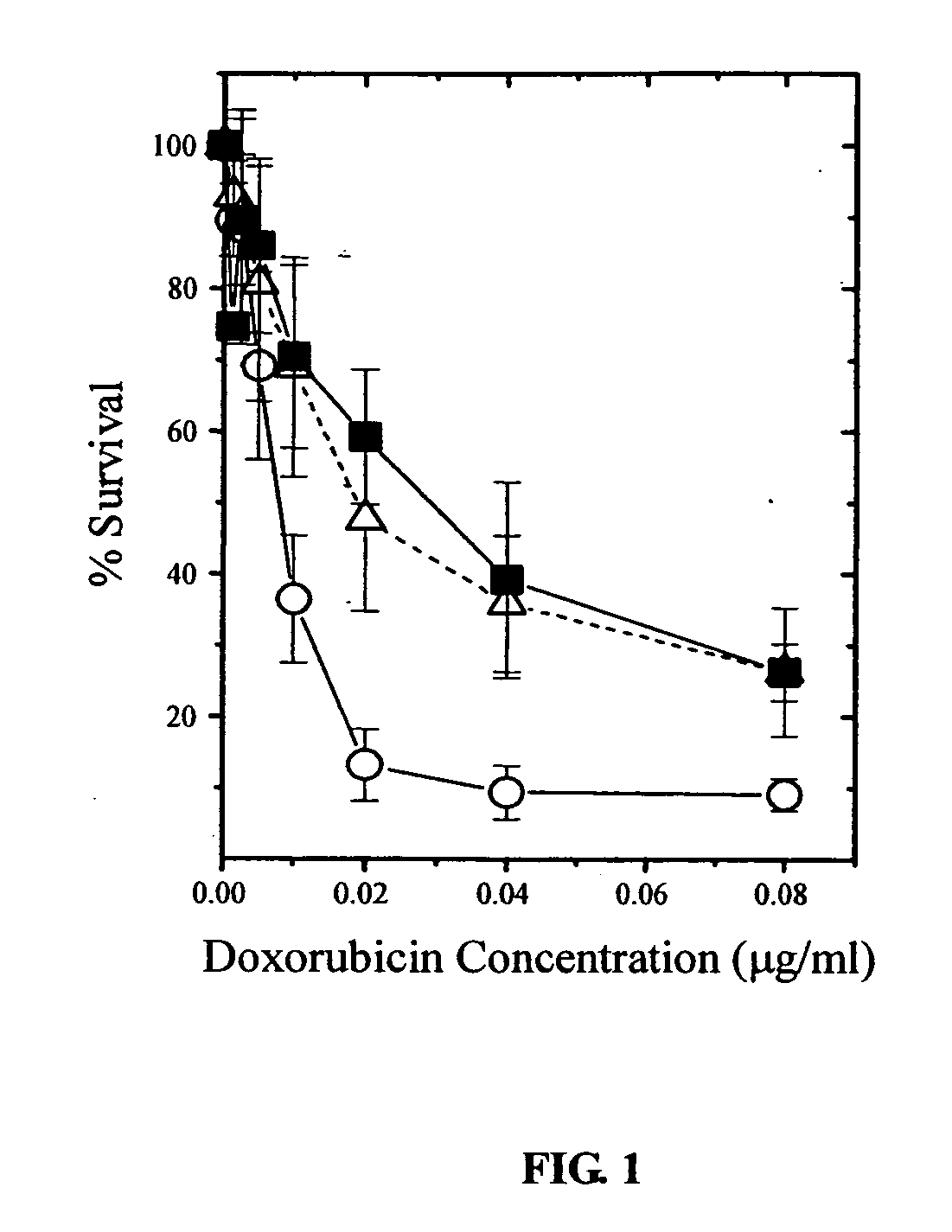
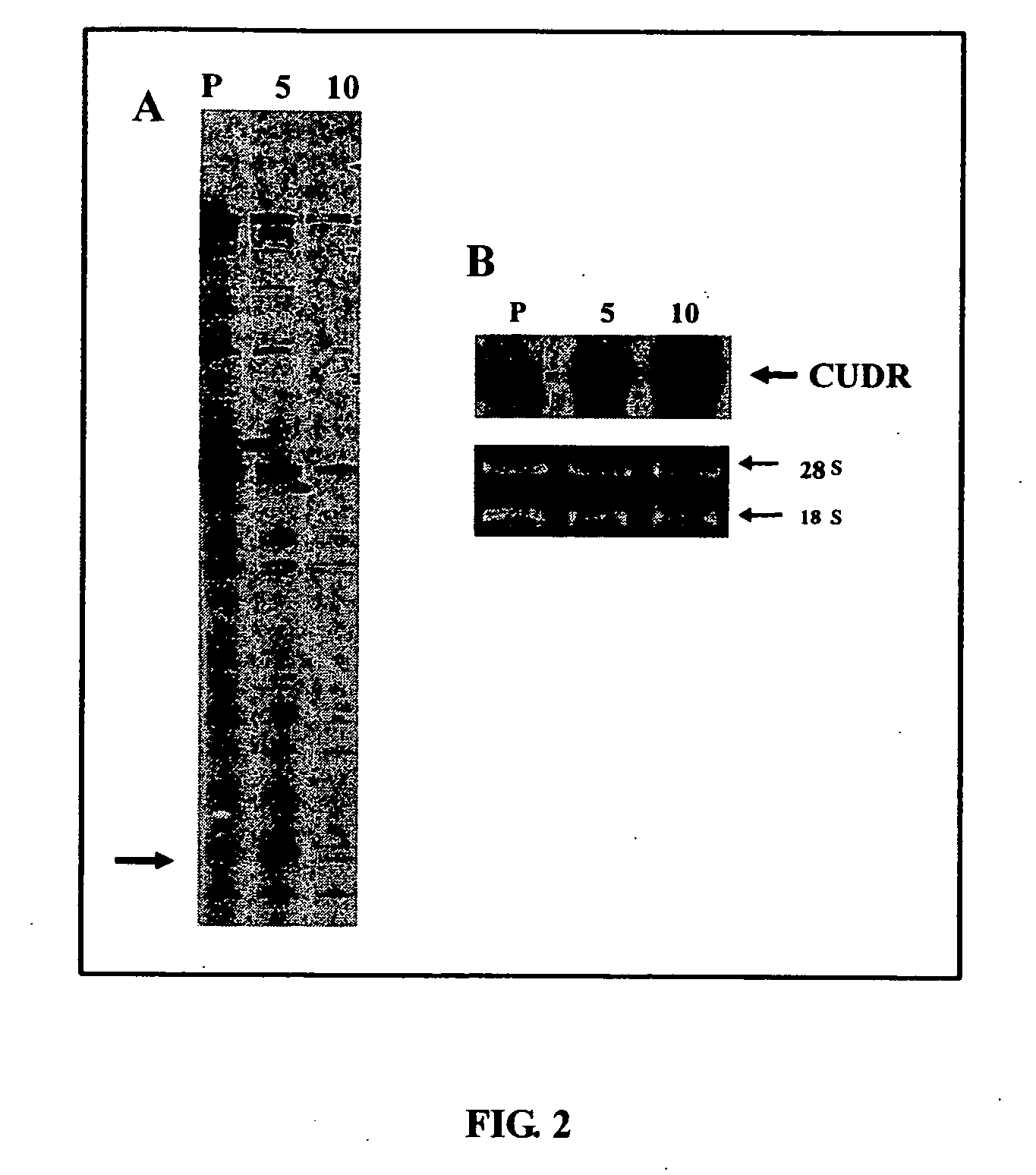
CUDR as biomarker for cancer progression and therapeutic response
InactiveUS20080044828A1Sugar derivativesMicrobiological testing/measurementHuman cancerCancer therapy
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Mutations in human MLH1 and human MSH2 genes useful in diagnosing colorectal cancer
Variant human MLH1 and MSH2 genes are provided. Methods of using these variant genes to diagnose hereditary non-polyposis colorectal cancer (HNPCC) and / or determine a patient's susceptibility to developing HNPCC are also provided. Methods and compositions for identifying new variant MLH1 of MSH2 genes are also provided. In addition, experimental models for hereditary non-polyposis colorectal cancer comprising these variant genes are provided.
Owner:DIADEXUS
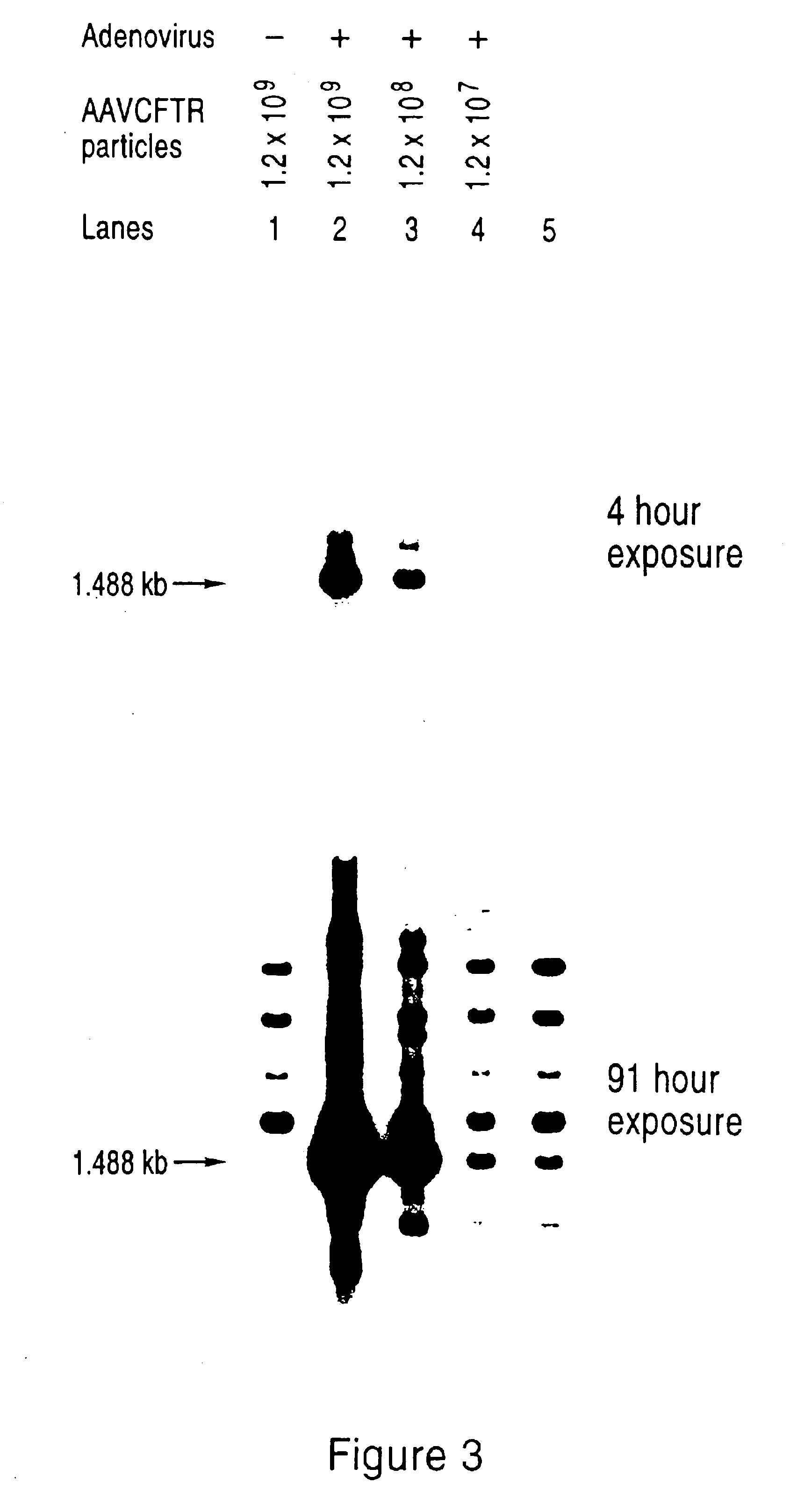
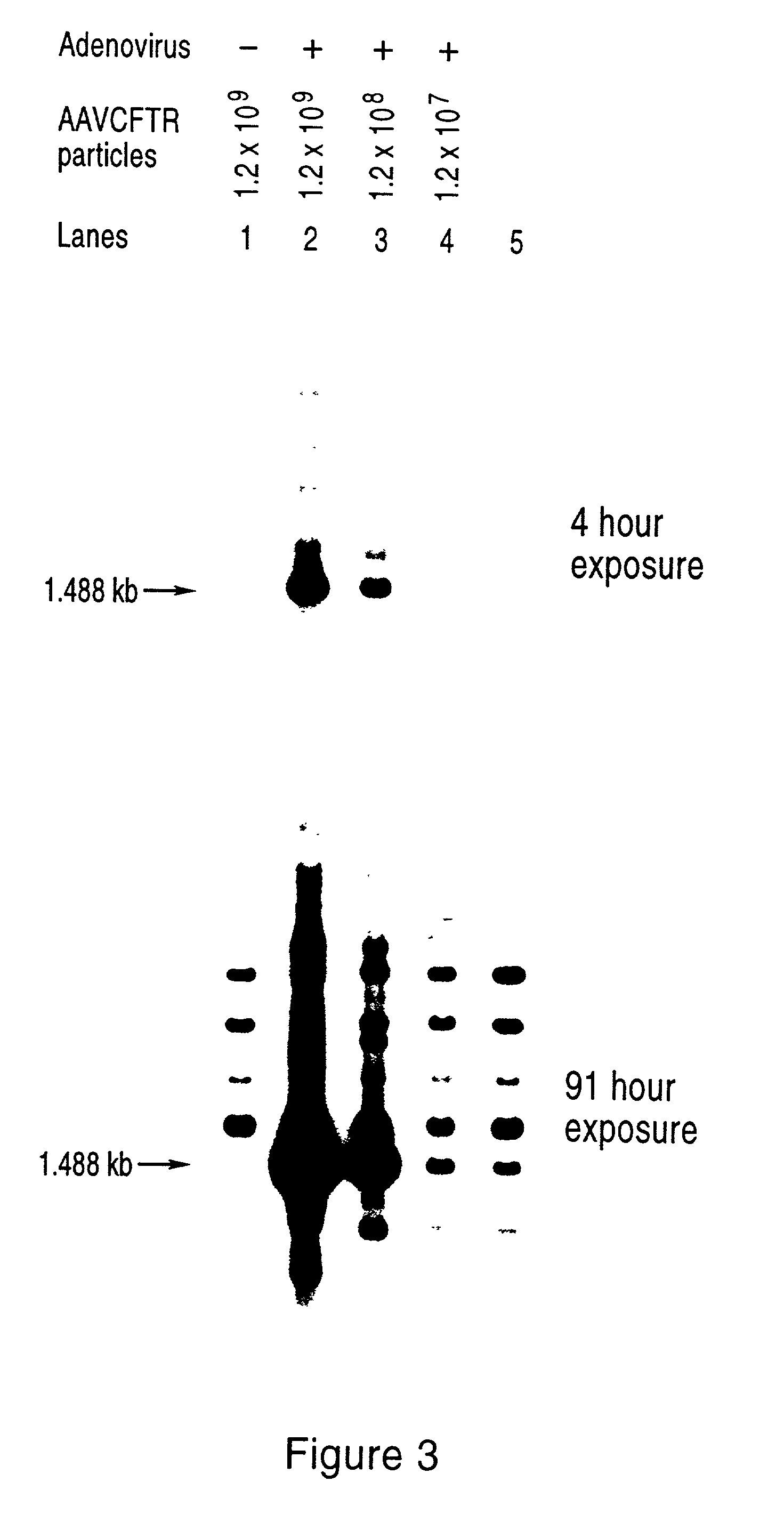
Packaging cell lines for generation of high titers of recombinant AAV vectors
InactiveUS6924128B2Not be easily manipulatedIncrease diversityBiocidePeptide/protein ingredientsGenes humanCell biology
AAV vectors may have utility for gene therapy but heretofore a significant obstacle has been the inability to generate sufficient quantities of such recombinant vectors in amounts that would be clinically useful for human gene therapy application. Stable AAV packaging cell lines have been elusive, mainly due to the activities of Rep protein, which down-regulates its own expression and can negatively affect the host cell. This invention provides packaging systems and processes for packaging AAV vectors that effectively circumvent these problems and that allow for substantially increased packaging efficiency.
Owner:TARGETED GENETICS CORPORTION
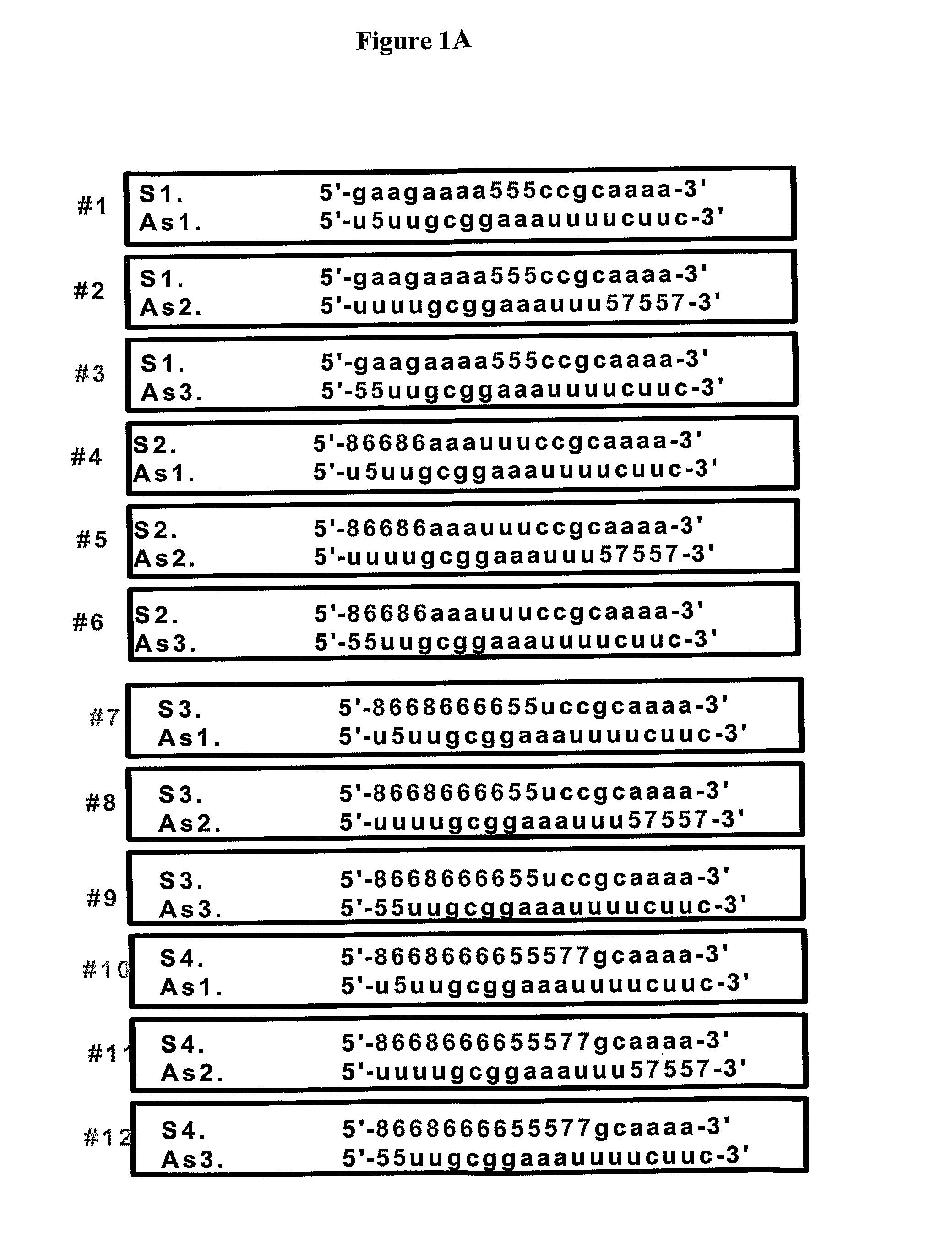
Novel sirna structures
The invention relates to siRNA compounds possessing novel sequences and structural motifs which down-regulate the expression of specific human genes. The invention also relates to pharmaceutical compositions comprising such compounds and a pharmaceutically acceptable carrier. The present invention also provides a method of treating and / or preventing the incidence or severity of various diseases or conditions associated with the genes and / or symptoms associated with such diseases or conditions comprising administering to a subject in need of treatment for such disease or condition and / or symptom the compound or the pharmaceutical composition in a therapeutically effective dose so as to thereby treat the subject.
Owner:QUARK FARMACUITIKALS INC
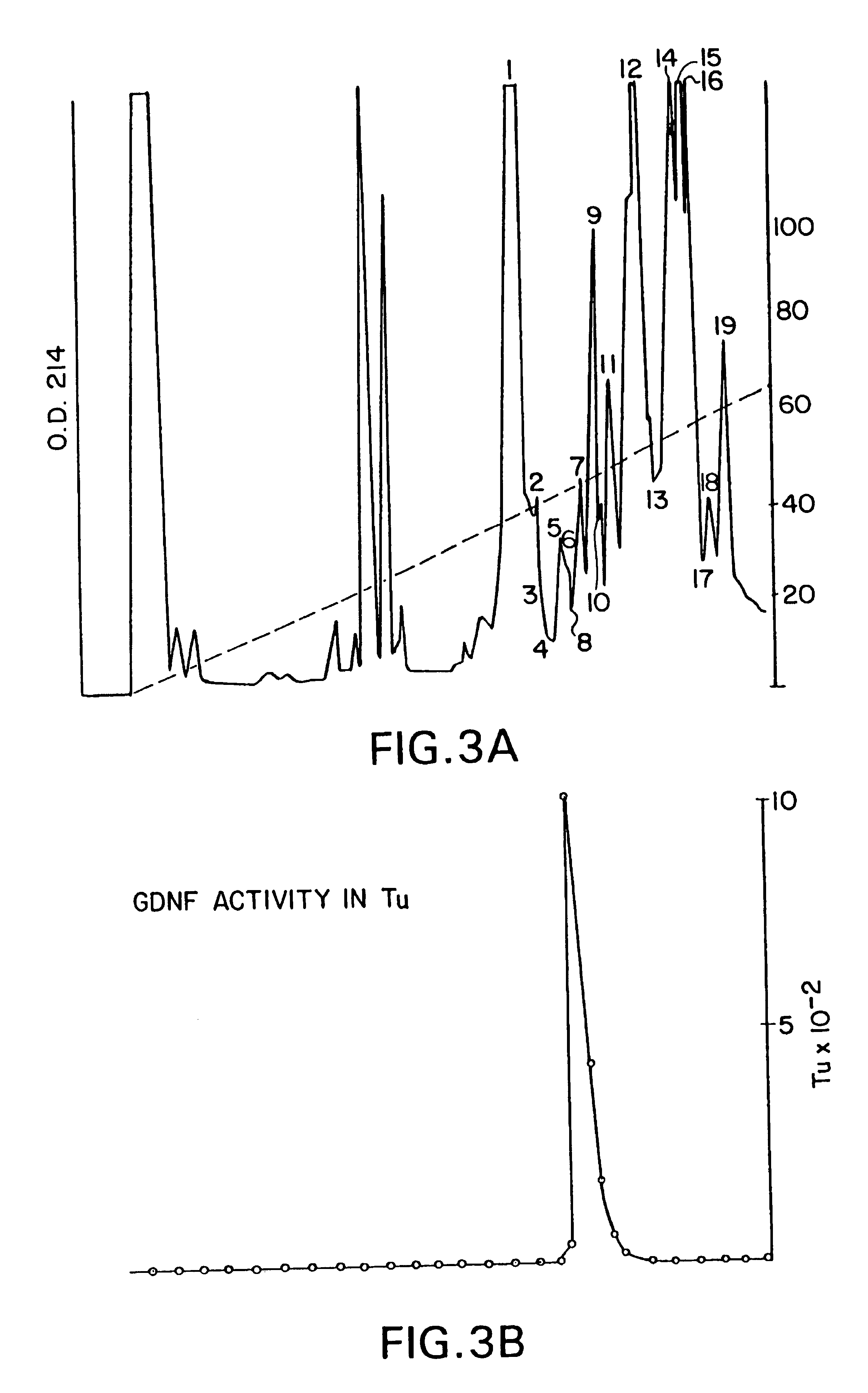
Nucleic acids encoding glial cell line-derived neurotrophic factor (GDNF)
ActiveUS7226758B1Prevent nerve damageOrganic active ingredientsNervous disorderGlial cell line-derived neurotrophic factorSerum free
A novel neurotrophic factor referred to as glial cell line-derived neurotrophic factor (GDNF) has been identified and isolated from serum free growth conditioned medium of B49 glioblastoma cells. Rat and human genes encoding GDNF have been cloned and sequenced. A gene encoding GDNF has been subcloned into a vector, and the vector has been used to transform a host cell in order to produce biologically active GDNF in a recombinant DNA process.
Owner:AMGEN INC
Compositions, kits, and methods relating to the human FEZ1 gene, a novel tumor suppressor gene
InactiveUS20070072230A1Increase stringencyInhibiting tumorigenesisSugar derivativesPeptide/protein ingredientsTumour suppressor geneHuman tumor
Owner:THOMAS JEFFERSON UNIV
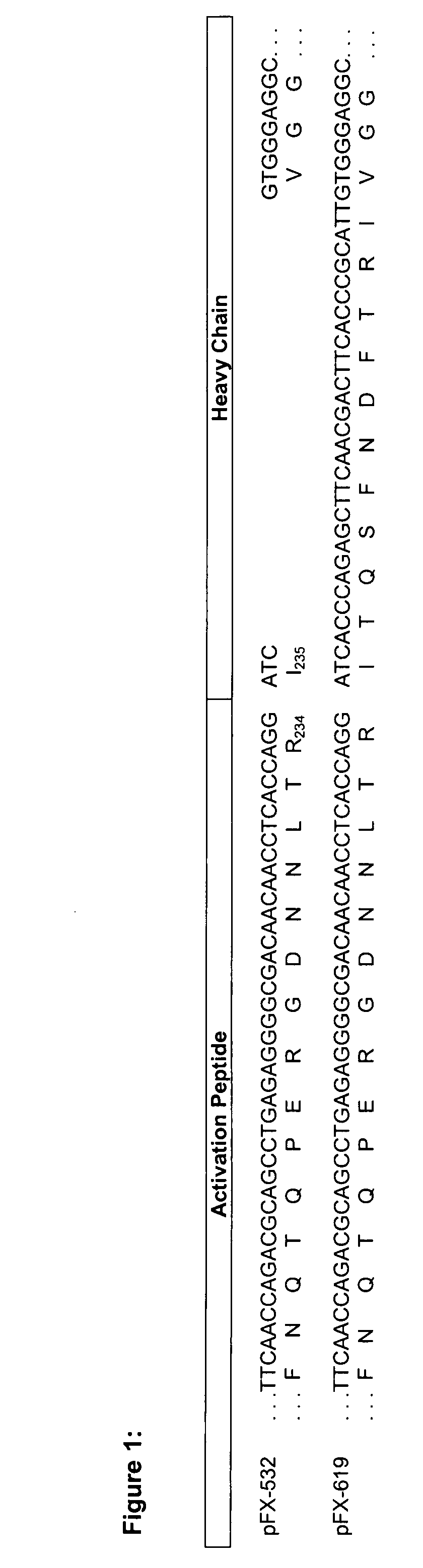
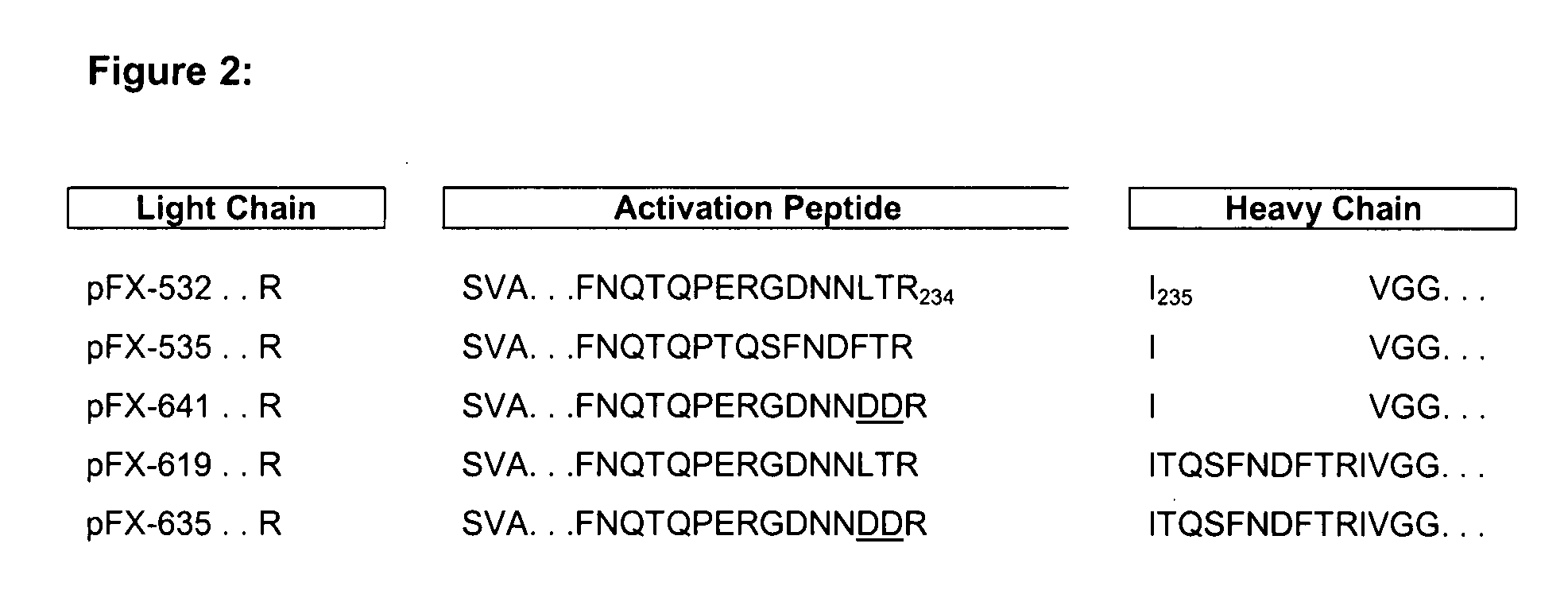
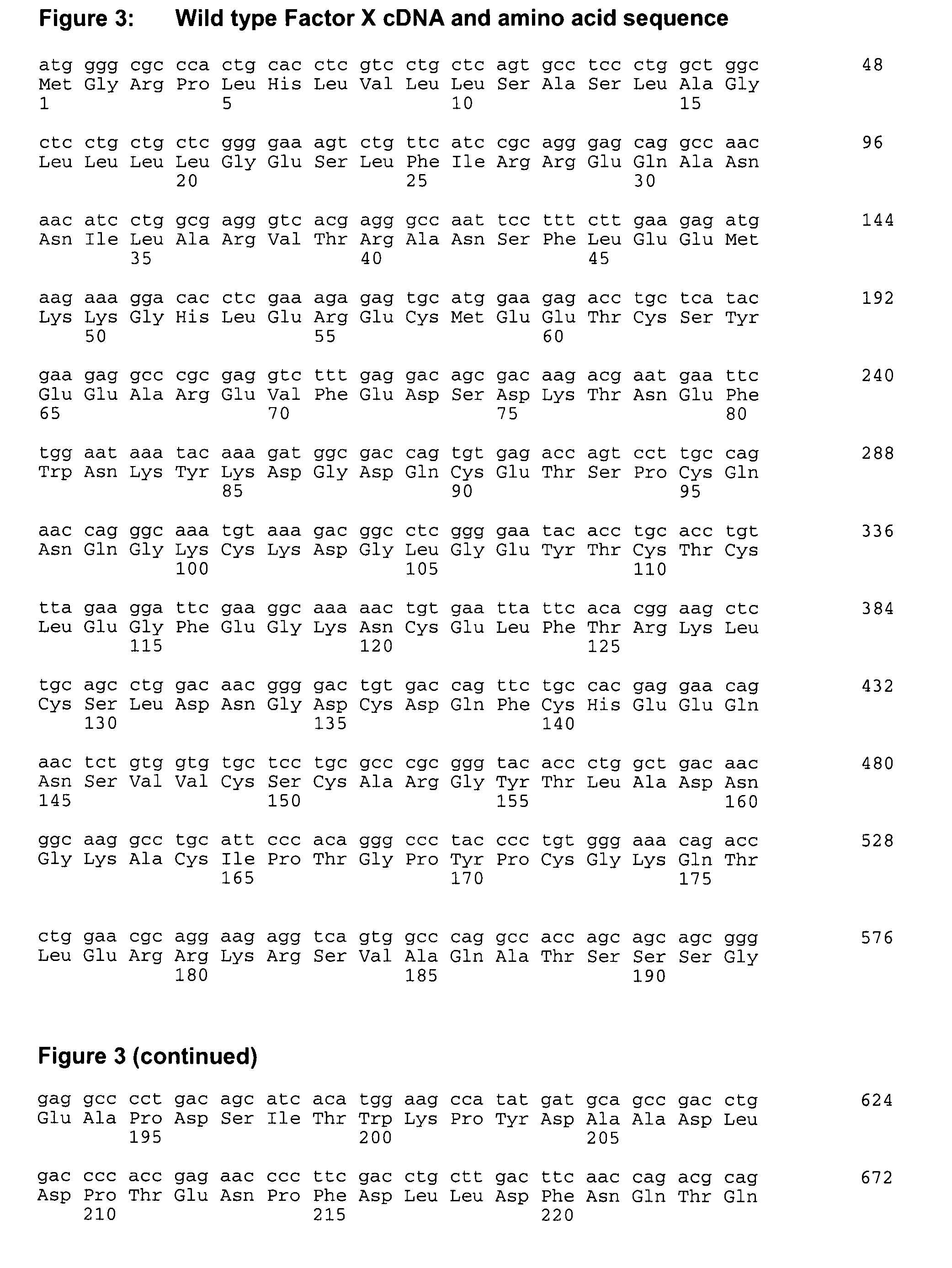
Coagulation factor x polypeptides with modified activation properties
The present invention relates to modified cDNA sequences coding for human Factor X and their derivatives with improved stability and modified activation sequences, recombinant expression vectors containing such cDNA sequences, and host cells transformed with such recombinant expression vectors. The invention also relates to recombinant factor X polypeptides and derivatives which have biological activities of the unmodified wild type protein but with improved stability and processes for the manufacture of such recombinant proteins and their derivatives. The invention also covers a transfer vector for use in human gene therapy, which comprises such modified DNA.
Owner:CSL BEHRING GMBH
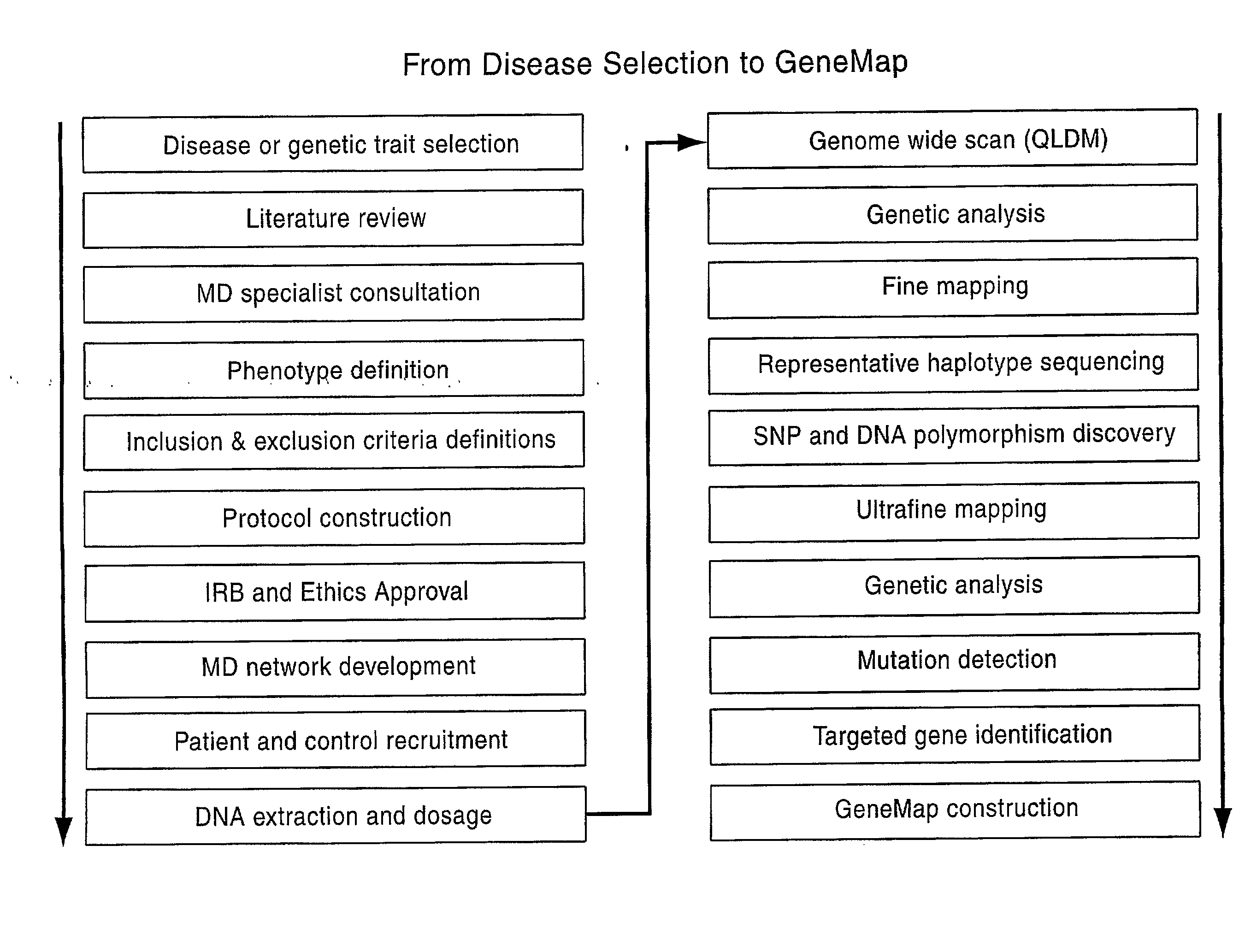
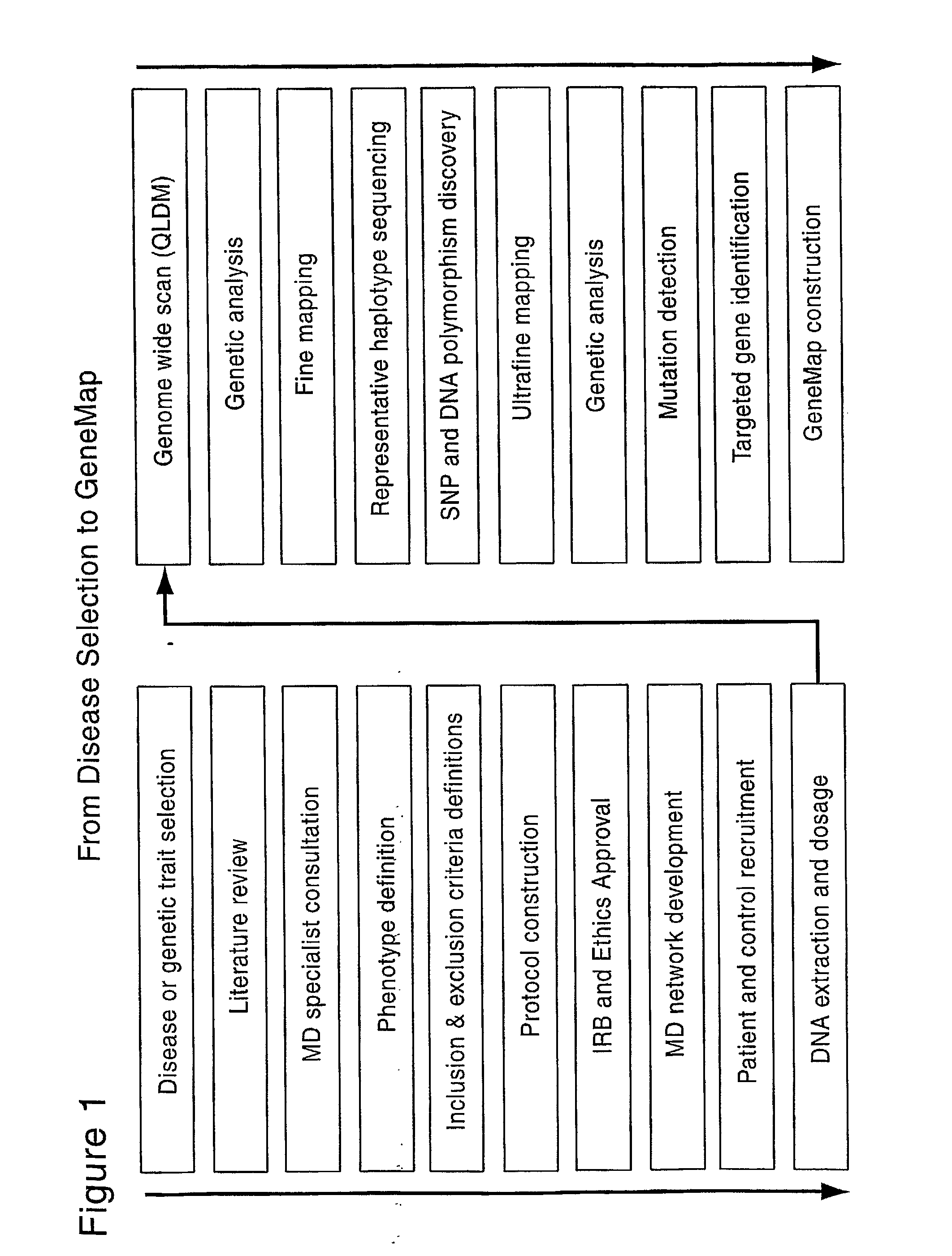
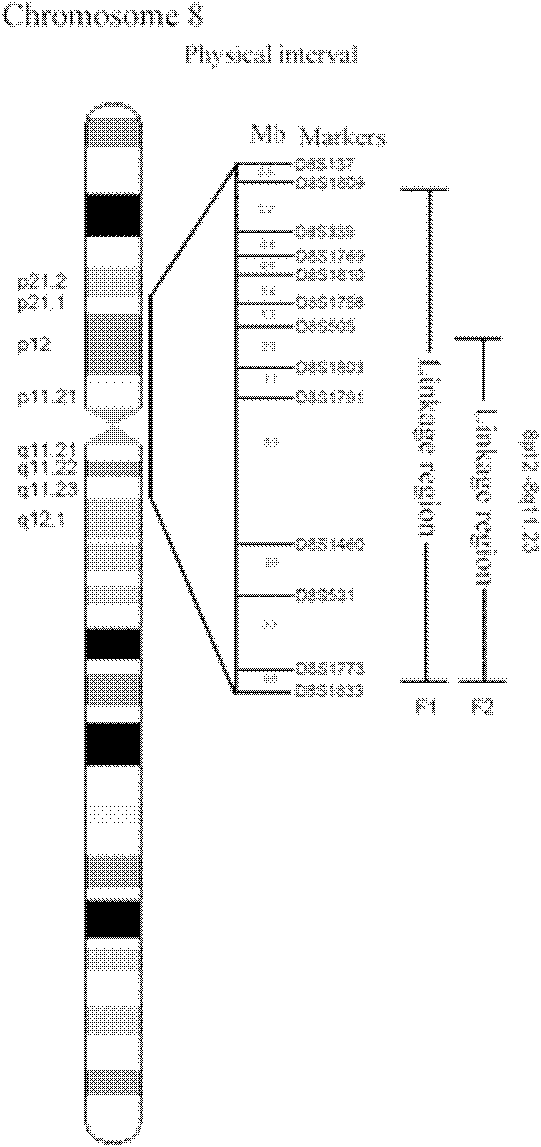
Genemap of the human genes associated with longevity
InactiveUS20090305900A1Electrolysis componentsVolume/mass flow measurementGenomicsLinkage Disequilibrium Mapping
The present invention relates to the selection of a set of SNP markers for use in genome wide association studies based on linkage disequilibrium mapping. In particular, the invention relates to the fields of pharmacogenomics, diagnostics, patient therapy and the use of genetic haplotype information to predict an individual's longevity, their protection against age-related diseases and / or their response to a particular drug or drugs.
Owner:BELOUCHI ABDELMAJID +12
Genemap of the human genes associated with crohn's disease
InactiveUS20090081658A1Reduce frequencyTreatment safetyMicrobiological testing/measurementGenomicsLinkage Disequilibrium Mapping
The present invention relates to the selection of a set of polymorphism markers for use in genome wide association studies based on linkage disequilibrium mapping. In particular, the invention relates to the fields of pharmacogenomics, diagnostics, patient therapy and the use of genetic haplotype information to predict an individual's susceptibility to Crohn's disease and / or their response to a particular drug or drugs.
Owner:GENIZON BIOSCI
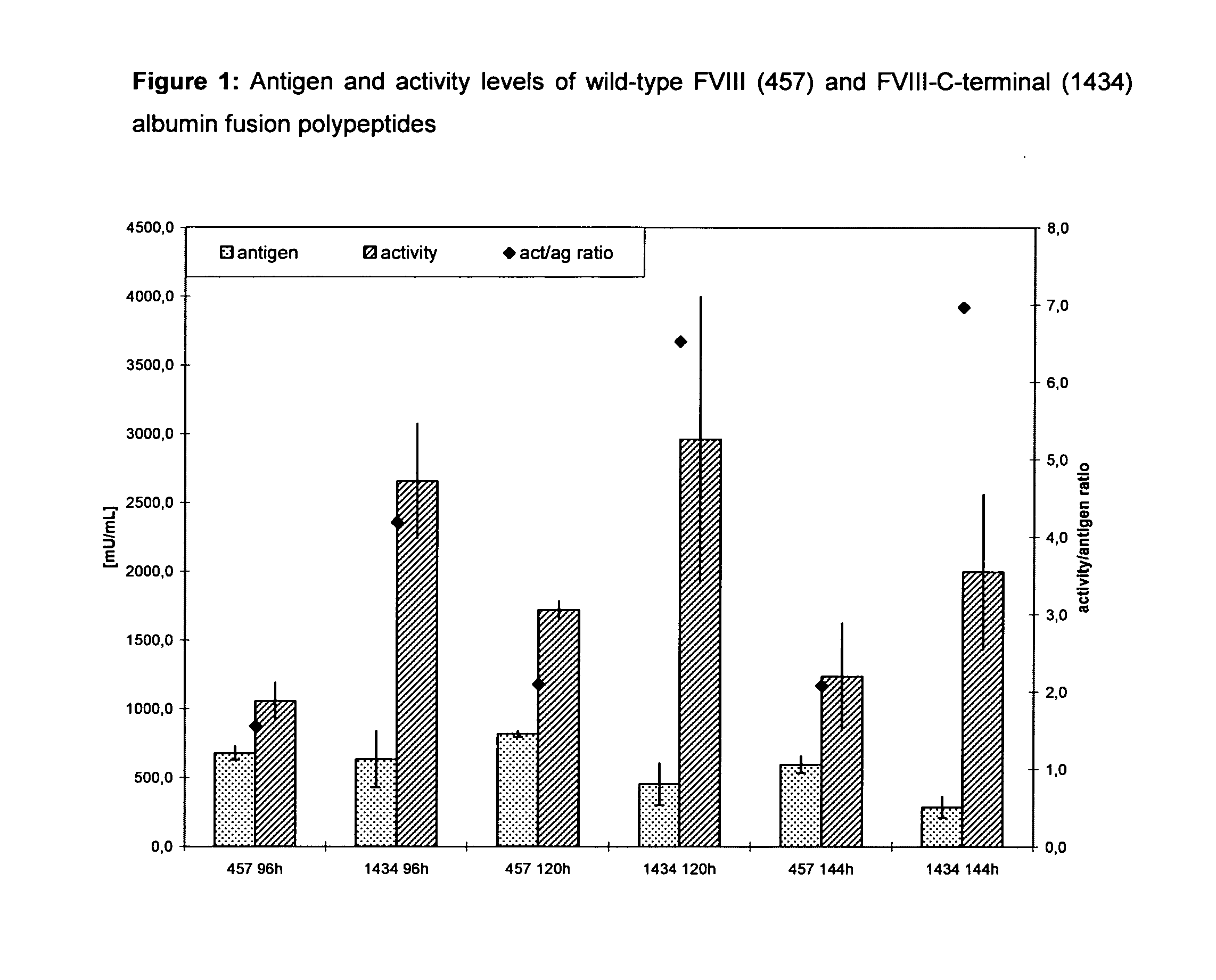
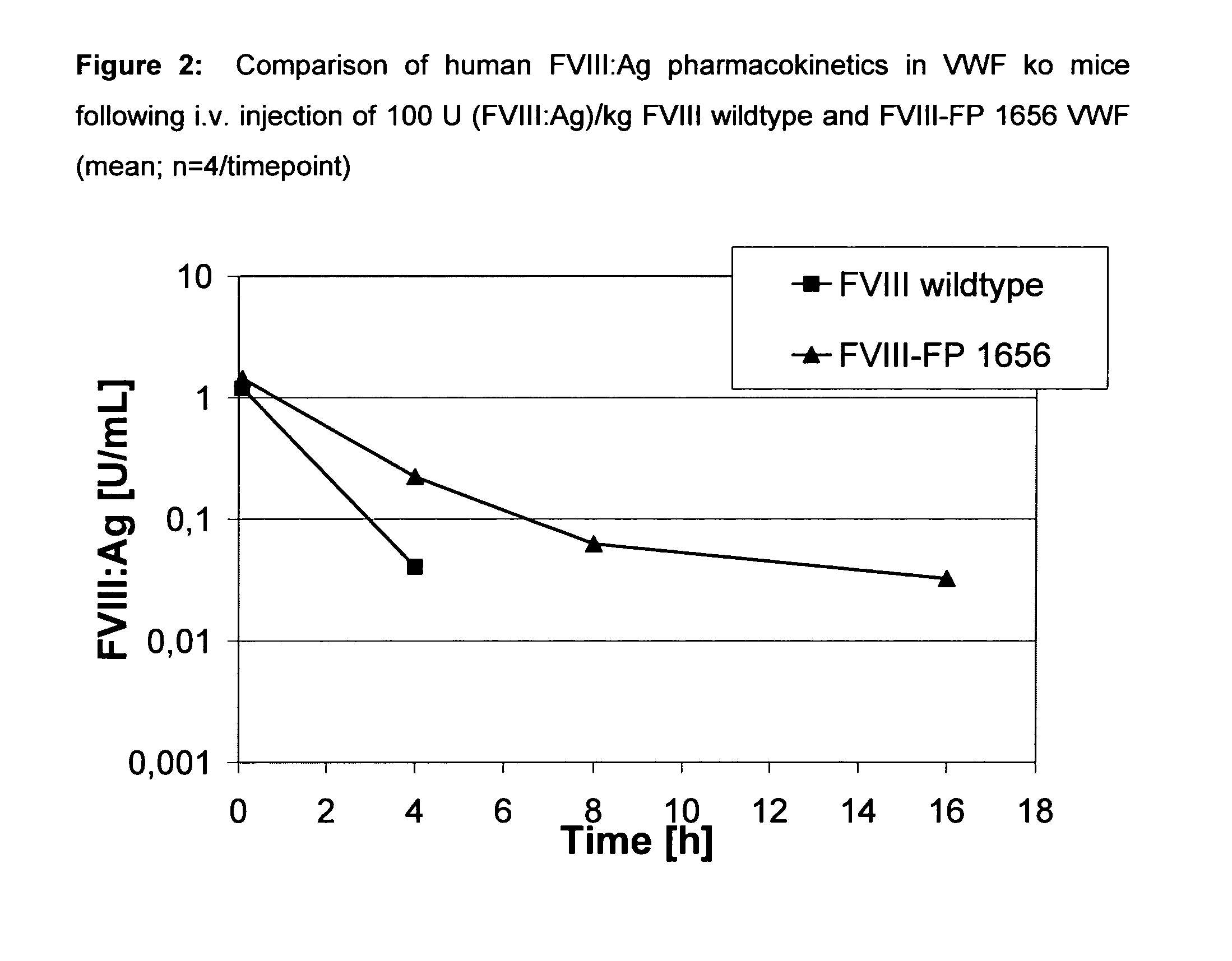
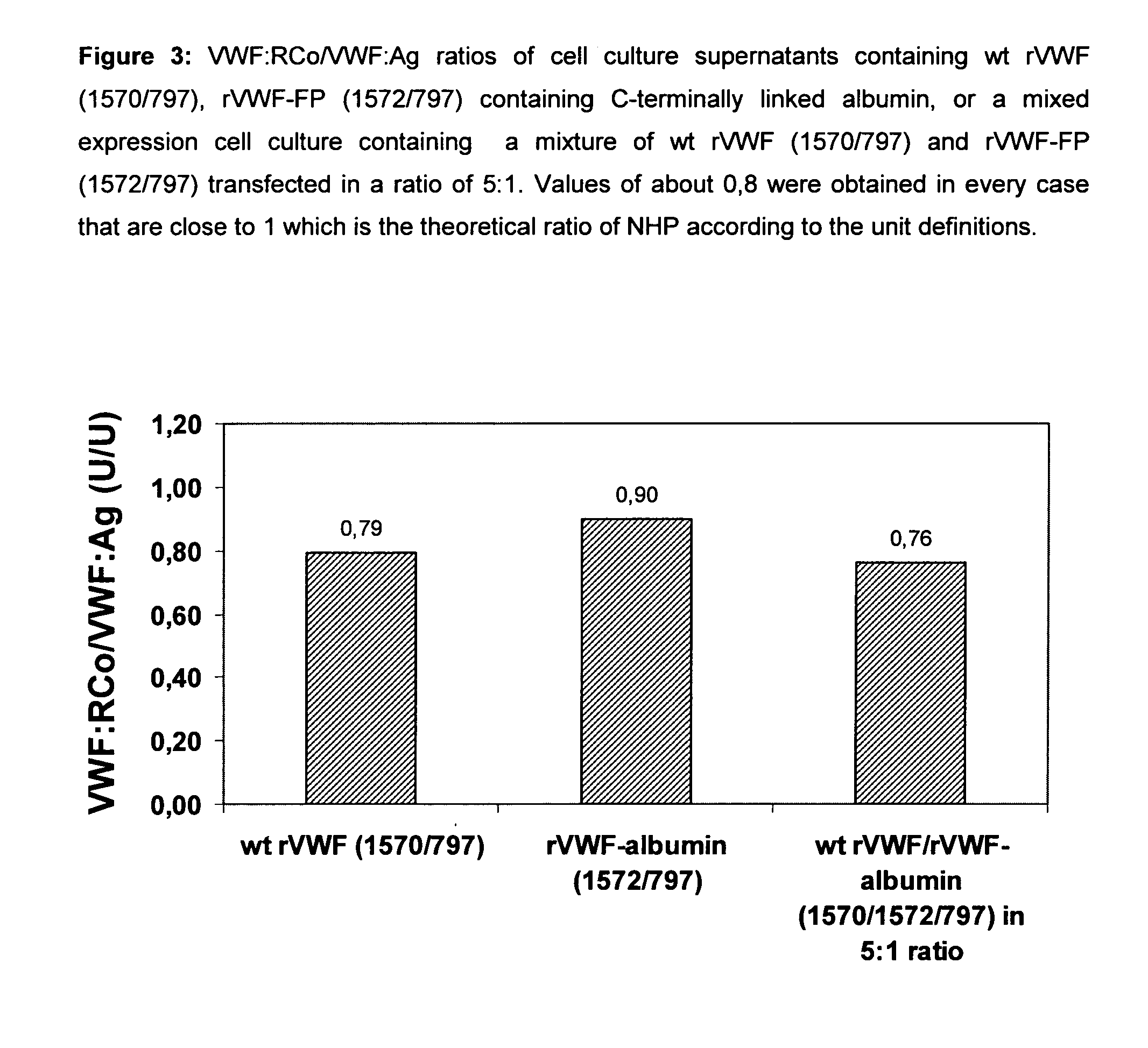
Factor viii, von willebrand factor or complexes thereof with prolonged in vivo half-life
ActiveUS20110183907A1Retain biological activityPromote recoveryOrganic active ingredientsFactor VIIFactor VIII vWFNucleic acid sequencing
The present invention relates to modified nucleic acid sequences coding for coagulation factor VIII (FVIII) and for von Willebrand factor (VWF) as well as complexes thereof and their derivatives, recombinant expression vectors containing such nucleic acid sequences, host cells transformed with such recombinant expression vectors, recombinant polypeptides and derivatives coded for by said nucleic acid sequences which recombinant polypeptides and derivatives do have biological activities together with prolonged in vivo half-life and / or improved in vivo recovery compared to the unmodified wild-type protein. The invention also relates to corresponding FVIII sequences that result in improved expression yield. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such modified nucleic acid sequences.
Owner:CSL BEHRING GMBH
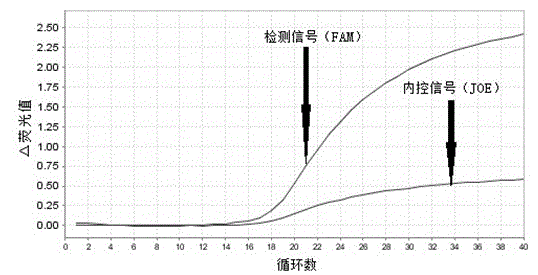
Primers, probes, kit and method for detecting human EGFR (epidermal growth factor receptor) gene mutations
ActiveCN102747157AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationWild type
The invention provides primers, probes, kit and method for detecting human EGFR (epidermal growth factor receptor) gene mutations. The method comprises the following steps of: (1) providing the primers and the probes; (2) processing detected samples and extracting DNA (deoxyribonucleic acid); (3) carrying out fluorescent quantitation on components of a PCR (polymerase chain reaction) system; (4) amplifying target sequences of an EGFR gene mutation to be detected; and (5) distinguishing wild type genes and mutant type genes by utilizing ARMS (amplification refractory mutation system) primers and judging results via the fluorescence intensity of an FAM and a JOE. The primers, probes and method provided by the invention have the beneficial effects that 29 kinds of mutations on the EGFR genes can be simultaneously detected, so that the sensitivity is high, the specificity is strong and the detection speed is high; and the whole detection process only takes 60 minutes.
Owner:武汉海吉力生物科技有限公司
Genemap of the human genes associated with psoriasis
The present invention relates to the selection of a set of polymorphism markers for use in genome wide association studies based on linkage disequilibrium mapping. In particular, the invention relates to the fields of pharmacogenomics, diagnostics, patient therapy and the use of genetic haplotype information to predict an individual's susceptibility to psoriasis disease and / or their response to a particular drug or drugs.
Owner:GENIZON BIOSCI
Novel siRNAS and methods of use thereof
InactiveUS20090156524A1Effective treatmentInhibit expressionSenses disorderNervous disorderDiseaseAntiendomysial antibodies
The invention relates to compounds, in particular siRNAs, which inhibit the expression of specific human genes. The invention also relates to pharmaceutical compositions comprising such compounds and a pharmaceutically acceptable carrier. The present invention also provides a method of treating and / or preventing the incidence or severity of various diseases or conditions associated with the genes and / or symptoms associated with such diseases or conditions comprising administering to a subject in need of treatment for such disease or condition and / or symptom the compound or the pharmaceutical composition in a therapeutically effective dose so as to thereby treat the subject. The invention also provides antibodies which inhibit specified human polypeptides and pharmaceutical compositions comprising one or more such antibodies.
Owner:FEINSTEIN ELENA +4
Method for detecting the risk of and for treatment of type 2 diabetes
InactiveUS20060040293A1Less-effective and ineffectiveAvoid complicationsMetabolism disorderMicrobiological testing/measurementDiseaseBiology
A role of the human EXT2 gene in metabolic conditions such as T2D is disclosed. Methods and test kits for diagnosis, T2D risk prediction and prediction of clinical course of a metabolic condition using biomarkers related to the EXT2 gene are disclosed. Novel methods for prevention and treatment of metabolic diseases based on EXT2 gene, polypeptides and EXT2 related pathways are also disclosed.
Owner:JURILAB
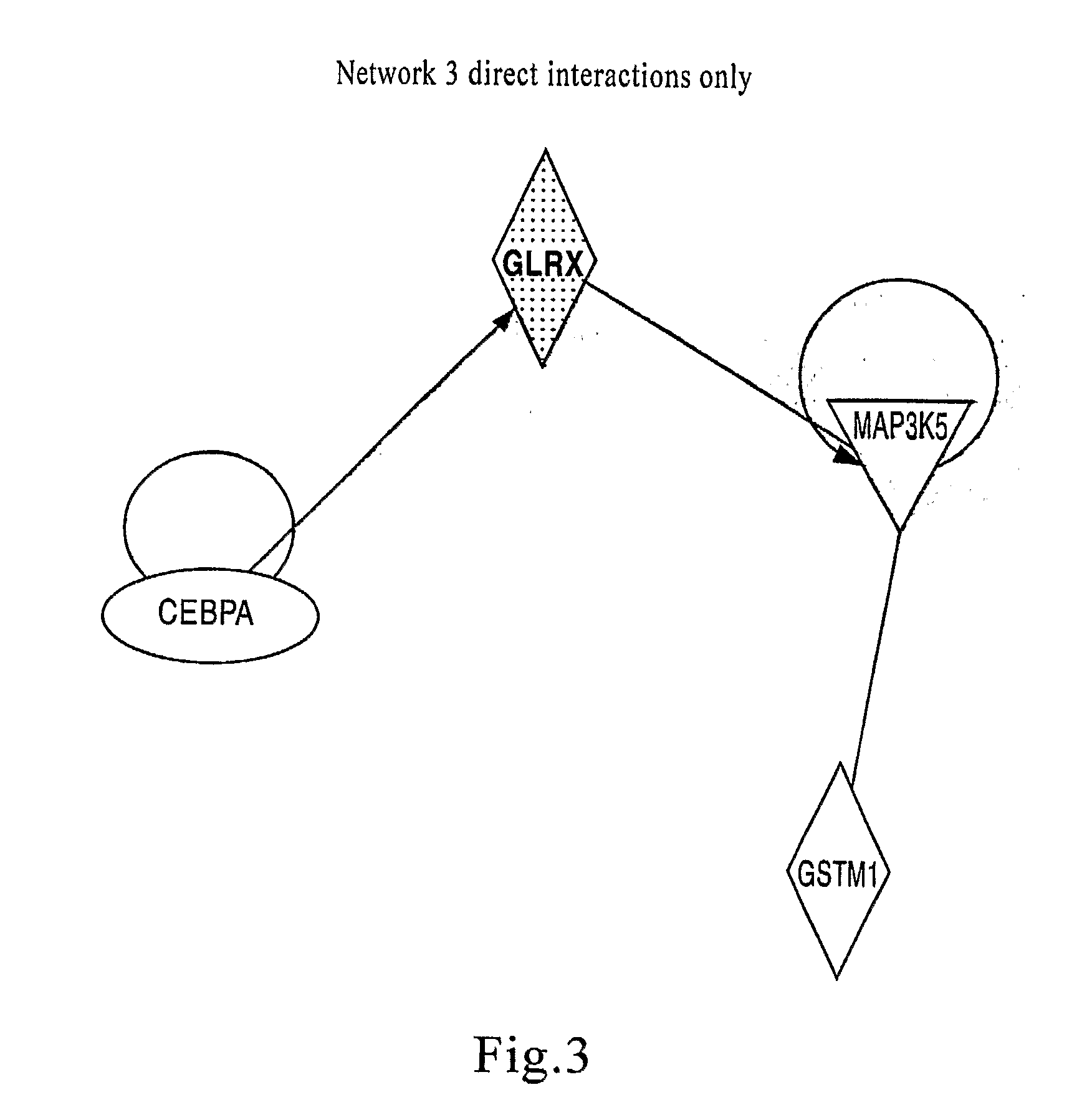
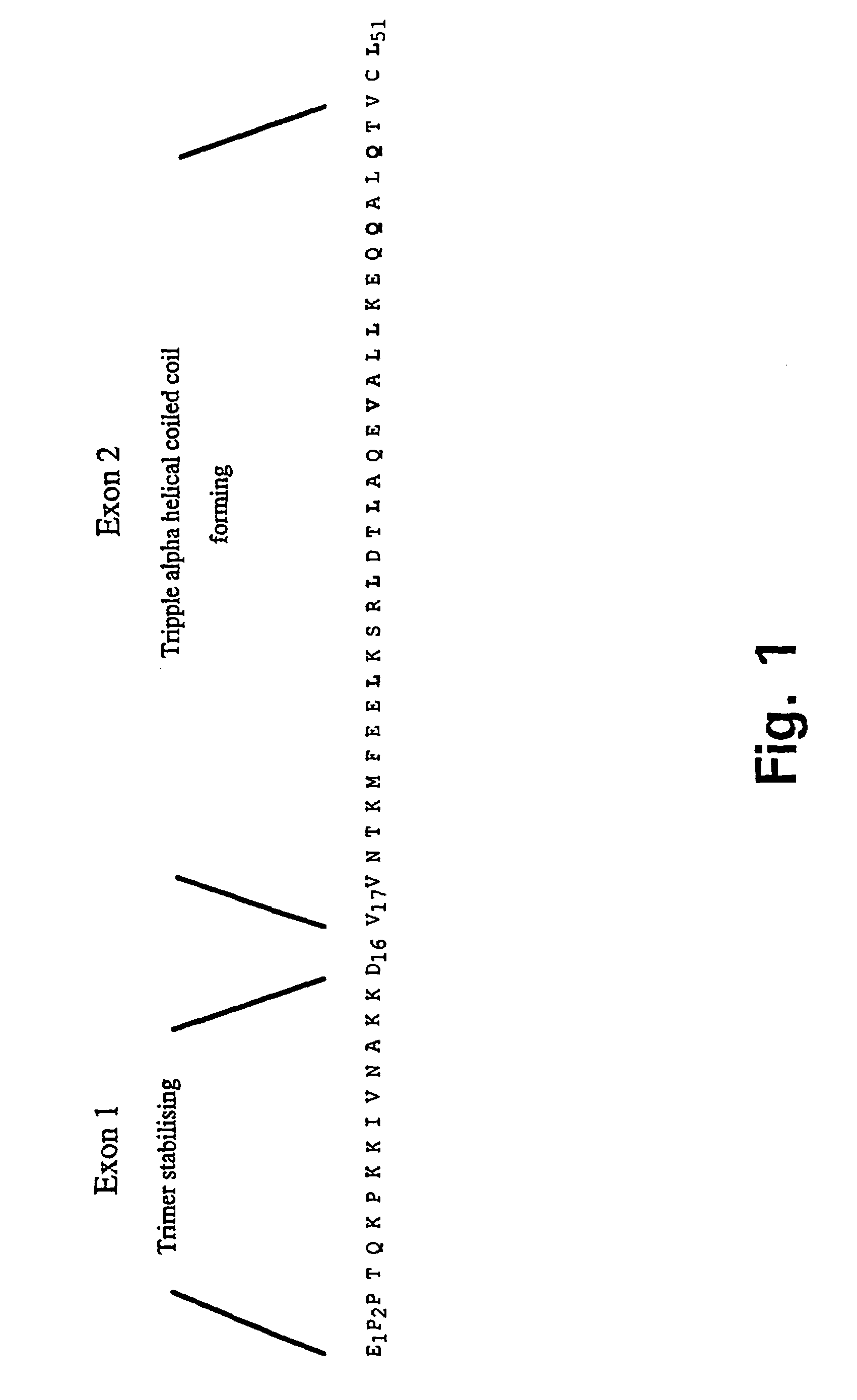
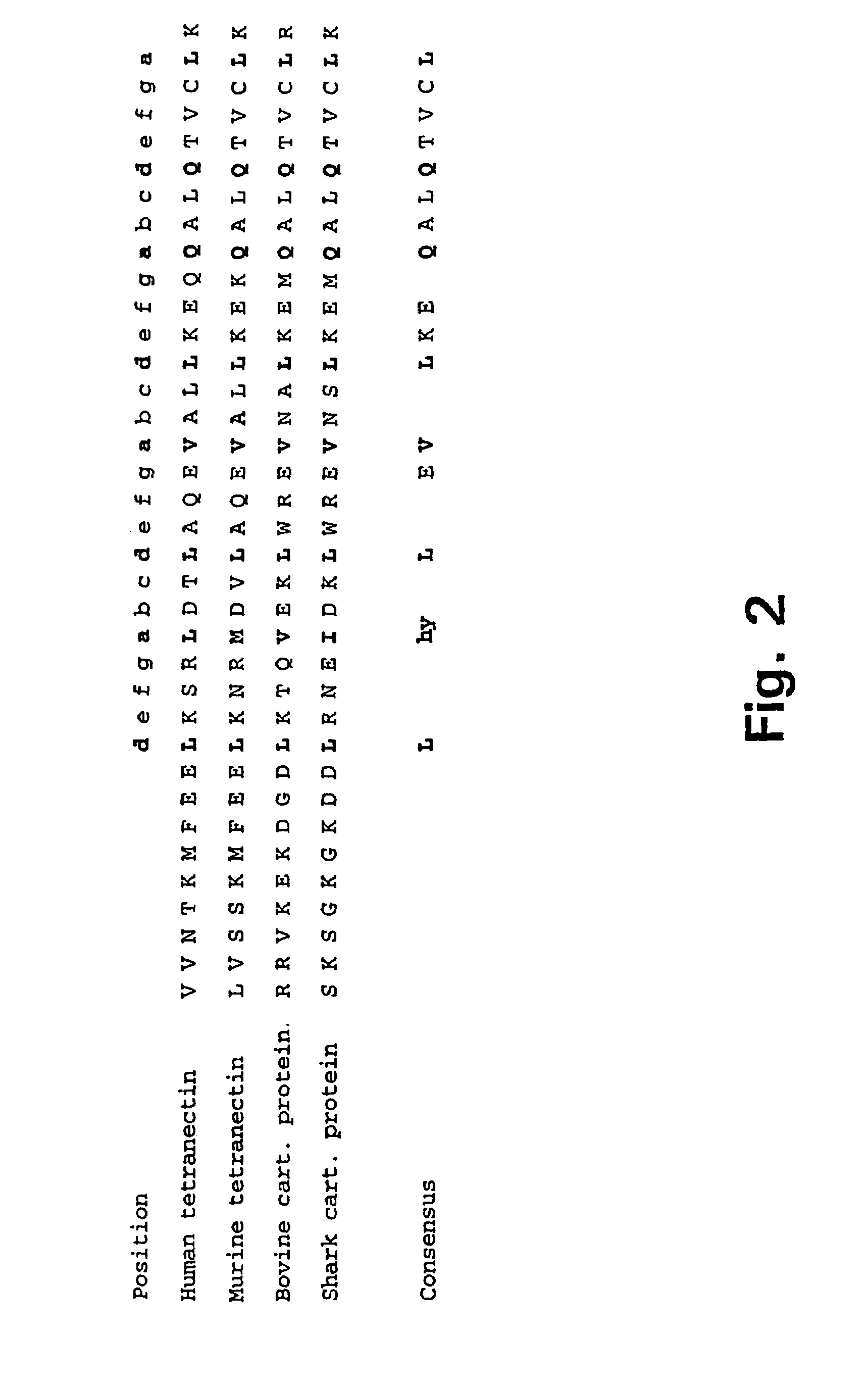
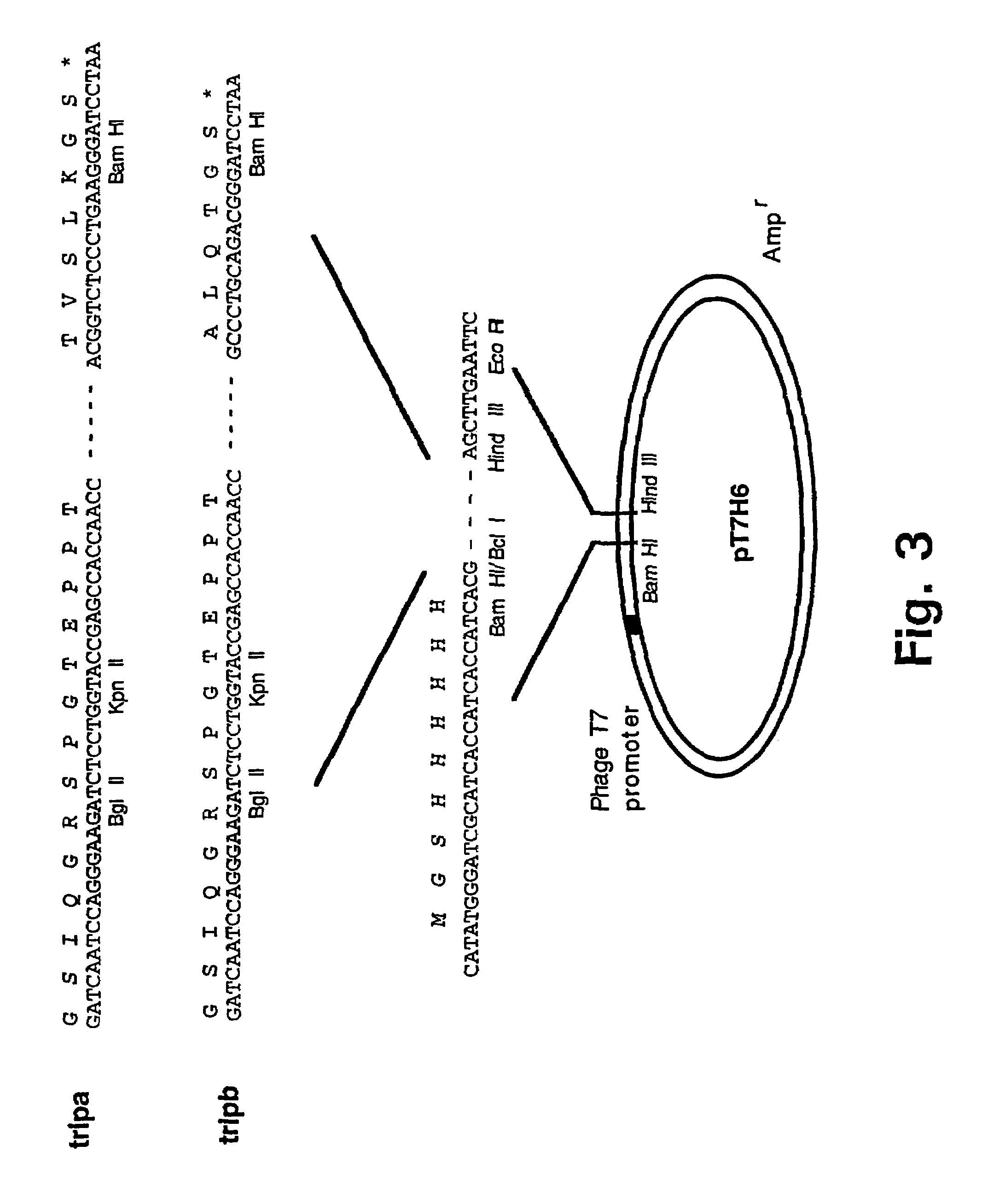
Trimerising module
The present invention relates to the design of trimeric polypeptides using polypeptide structural elements derived from the tetranectin protein family, and their use in rational de novo design and production of multi-functional molecules including the application of the multi-functional molecules in protein library technology, such as phage display technology, diagnostic and therapeutic systems, such as human gene therapy and imaging. The trimeric polypeptides being constructed as a monomer polypeptide construct comprising at least one tetranectin trimerising structural element (TTSE) which is covalently linked to at least one heterologous moiety, said TTSE being capable of forming a stable complex with two other TTSEs; or as an oligomer which is comprised of two monomer polypeptide constructs as mentioned above, and which comprises three TTSEs or a multiplum of three TTSEs, or which is comprised of three monomer polypeptide constructs.
Owner:ANAPHORE INC +1
Novel lentiviral vectors for site-specific gene insertion
ActiveUS20080200663A1Easy to reorganizeSugar derivativesGenetic material ingredientsGene deliveryTherapy Trial
Murine leukemia virus (MLV) and lentivirus vectors have been used previously to deliver genes to hematopoietic stem cells (HSCs) in human gene therapy trials. However, these vectors integrate randomly into the host genome, leading to disruption or inactivation of vital host genes. The present invention discloses a novel lentiviral vector system that overcomes this problem by integrating into a host genome in a site-specific manner.
Owner:CITY OF HOPE
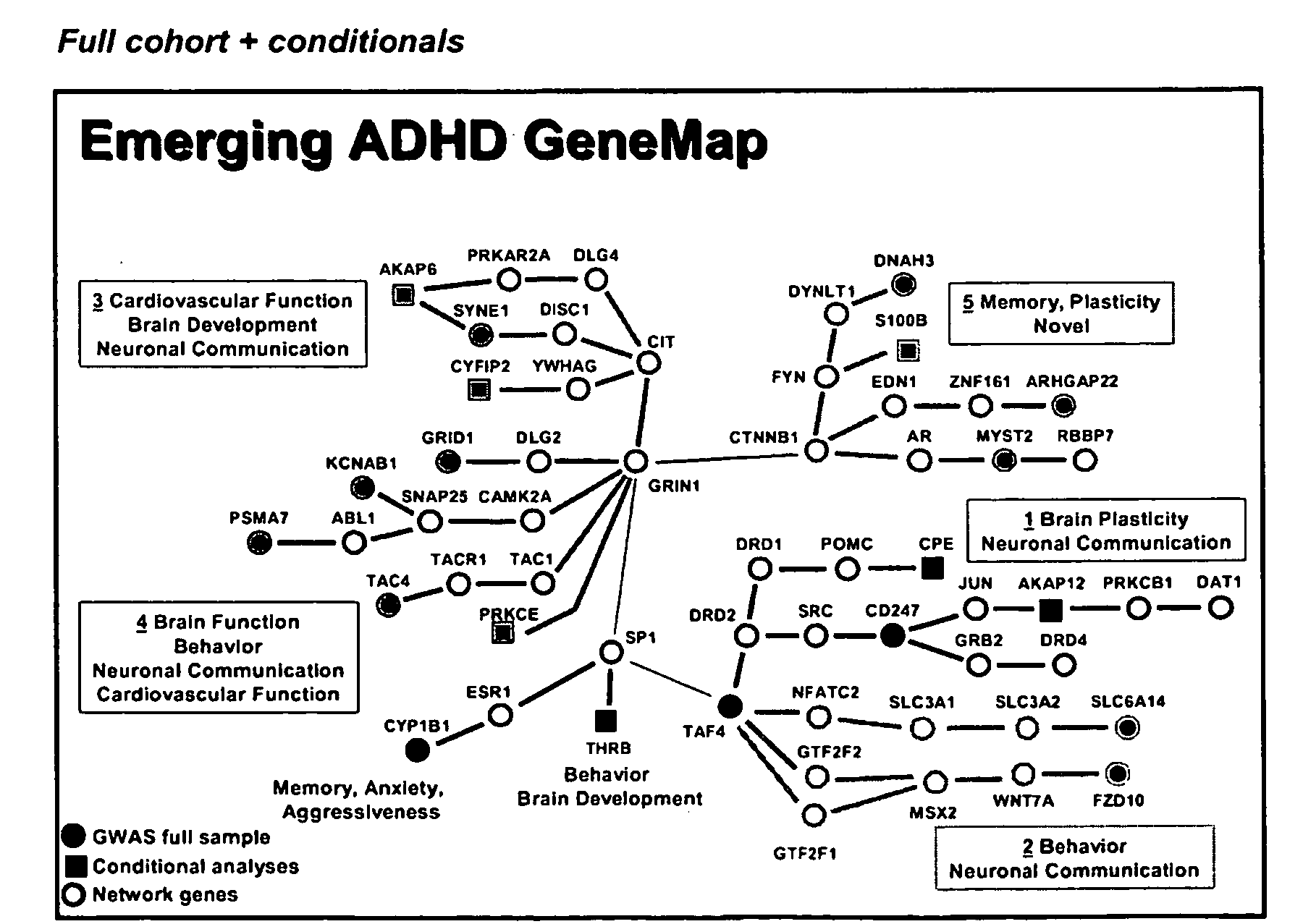
Genemap of the human genes associated with adhd
InactiveUS20100120628A1Easy to understandConvenient treatmentMicrobiological testing/measurementLibrary screeningGenomicsDisease
The present invention relates to the selection of a set of polymorphism markers for use in genome wide association studies based on linkage disequilibrium mapping. In particular, the invention relates to the fields of pharmacogenomics, diagnostics, patient therapy and the use of genetic haplotype information to predict an individual's susceptibility to ADHD disease and / or their response to a particular drug or drugs.
Owner:GENIZON BIOSCI
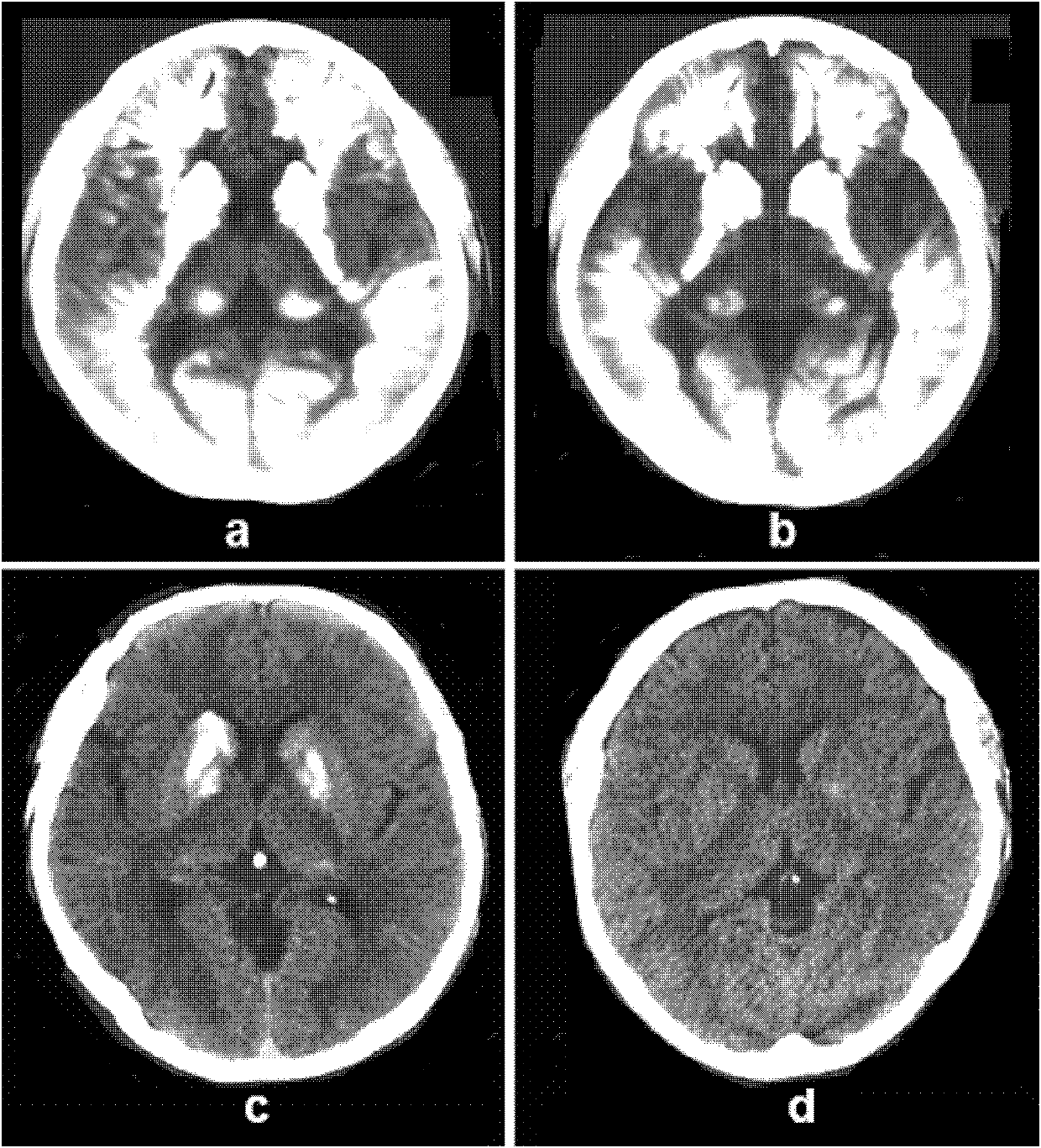
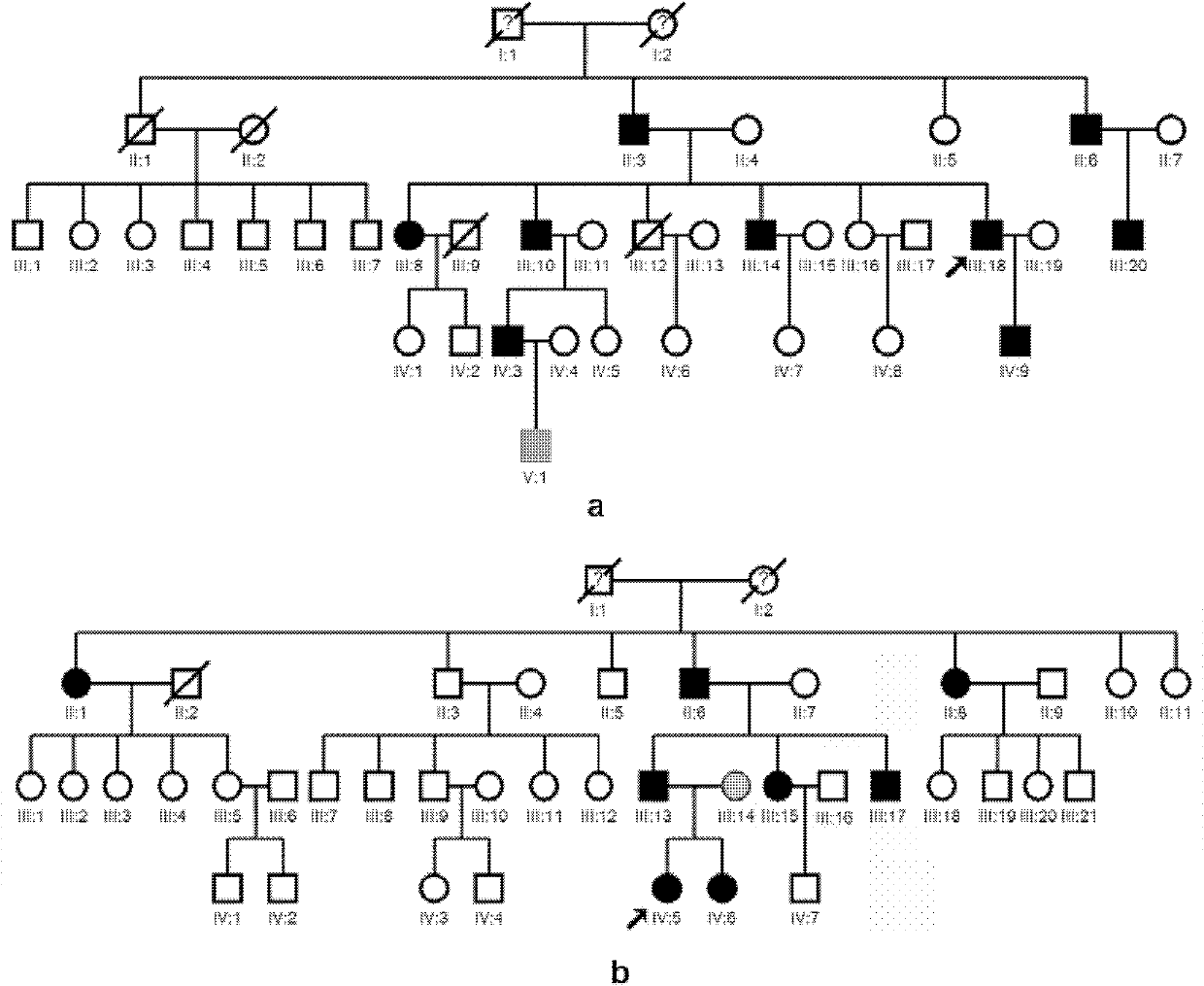
Human idiopathic basal ganglia calcification disease-causing gene and coding protein thereof
InactiveCN102888406AAccurate diagnosisReduce absorptionMicrobiological testing/measurementFermentationProtein insertionDrug target
The invention provides a variant human SLC20A2 gene and discloses that the variant human SLC20A2 gene is idiopathic basal ganglia calcification disease-causing gene. By utilizing the variant human SLC20A2 gene, diagnosis and risk evaluation can be carried out on idiopathic basal ganglia calcification disease. The invention also provides a protein coded by the variant human SLC20A2 gene, and the protein can be taken as a drug target for treating basal ganglia calcification. Besides, the invention also provides a kit used for diagnosing the idiopathic basal ganglia calcification disease and an application of the variant human SLC20A2 gene in preparation of a human idiopathic basal ganglia calcification disease gene diagnosis chip.
Owner:WUHAN TOAZHI LIFE TECH
Tumor necrosis factor receptor 5
InactiveUS20010021516A1Reduce capacityIncreased apoptosisBacteriaPeptide/protein ingredientsTRAIL ReceptorsAgonist
The present invention relates to a novel human gene encoding a polypeptide which is a member of the TNF receptor family, and has now been found to bind TRAIL. More specifically, an isolated nucleic acid molecule is provided encoding a human polypeptide named tumor necrosis factor receptor-5, sometimes referred to as "TNFR-5" or "TR5," and now referred to hereinafter as "TRAIL receptor without intracellular domain" or "TRID." TRID polypeptides are also provided, as are vectors, host cells, and recombinant methods for producing the same. The invention further relates to screening methods for identifying agonists or antagonists of TRAIL polypeptide activity. Also provided are diagnostic and therapeutic methods utilizing such compositions.
Owner:HUMAN GENOME SCI INC
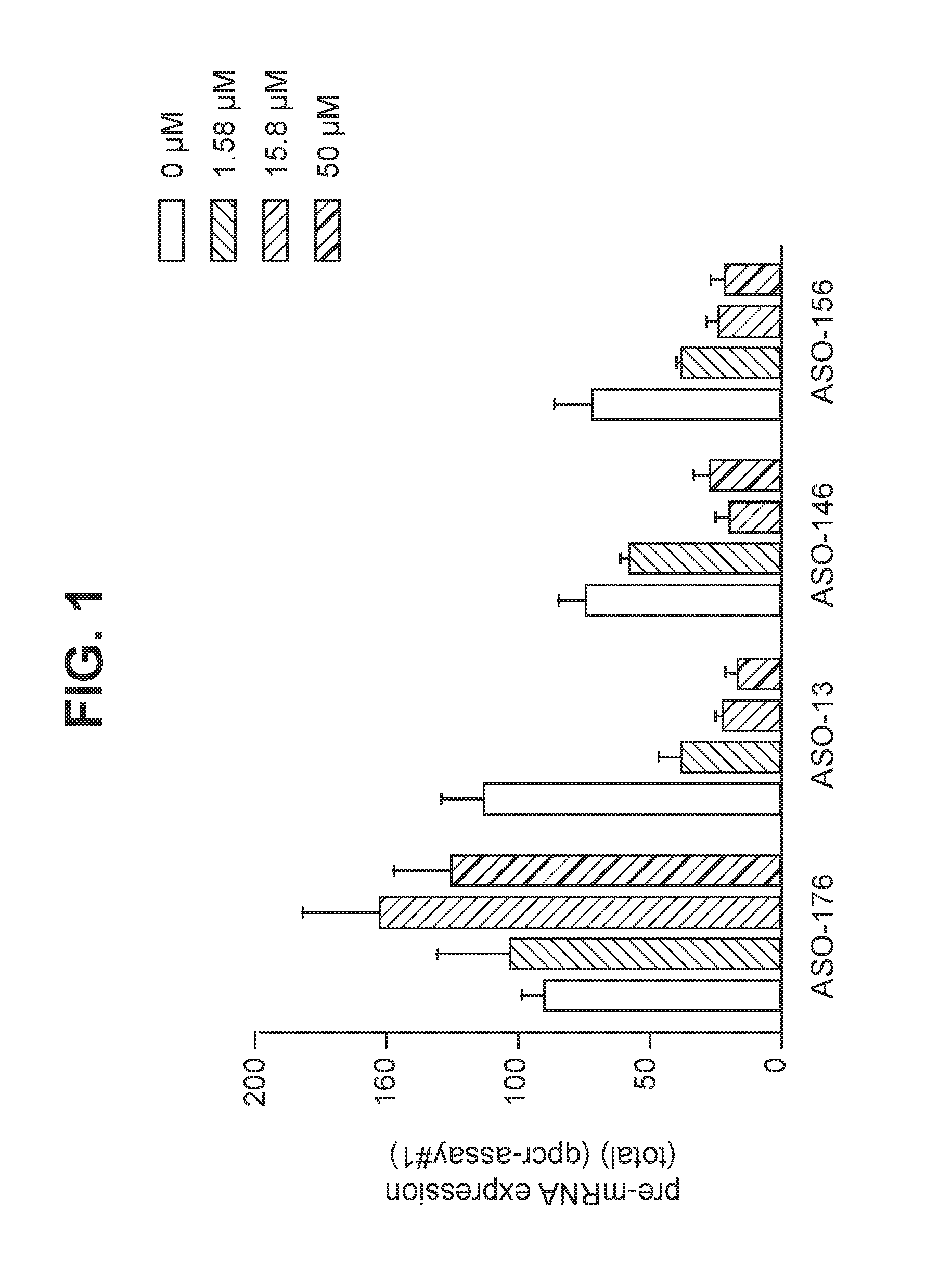
Oligomers targeting hexanucleotide repeat expansion in human c9orf72 gene
InactiveUS20160108396A1Reduce the amount requiredReduce expressionSugar derivativesPolymorphism usesOligomerNucleotide
The disclosure relates to oligomers capable of targeting RNA expressed from the human C9ORF72 gene containing a pathogenic hexanucleotide repeat expansion. Such oligomers are useful for, among other things, reducing or eliminating C9ORF72 RNA and / or proteins translated therefrom, and treating or preventing diseases or disorders caused by, or associated with, hexanucleotide repeat expansion, including familial frontotemporal dementia (FTD) and familial amyotrophic lateral sclerosis (ALS).
Owner:PFIZER INC
Genemap of the human genes associated with crohn's disease
InactiveUS20090181380A1Microbiological testing/measurementAnalogue computers for chemical processesGenomicsLinkage Disequilibrium Mapping
The present invention relates to the selection of a set of polymorphism makers for use in genome wide association studies based on linkage disequilibrium mapping. In particular, the invention relates to the fields of pharmacogenomics, diagnostics, patient therapy and the use of genetic haplotype information to predict an individual's susceptibility to IBD (ex: Chrohn's disease) and / or their response to a particular drug or drugs.
Owner:BELOUCHI ABDELMAJID +12
Nucleic acids encoding tumor necrosis factor receptor 5
InactiveUS6261801B1Reduce capacityIncreased apoptosisBacteriaPeptide/protein ingredientsLine of therapyNucleic acid molecule
The present invention relates to a novel human gene encoding a polypeptide which is a member of the TNF receptor family, and has now been found to bind TRAIL. More specifically, an isolated nucleic acid molecule is provided encoding a human polypeptide named tumor necrosis factor receptor-5, sometimes referred to as "TNFR-5" or "TR5", and now referred to hereinafter as "TRAIL receptor without intracellular domain" or "TRID." TRID polypeptides are also provided, as are vectors, host cells, and recombinant methods for producing the same. The invention further relates to screening methods for identifying agonists or antagonists of TRAIL polypeptide activity. Also provided are diagnostic and therapeutic methods utilizing such compositions.
Owner:HUMAN GENOME SCI INC
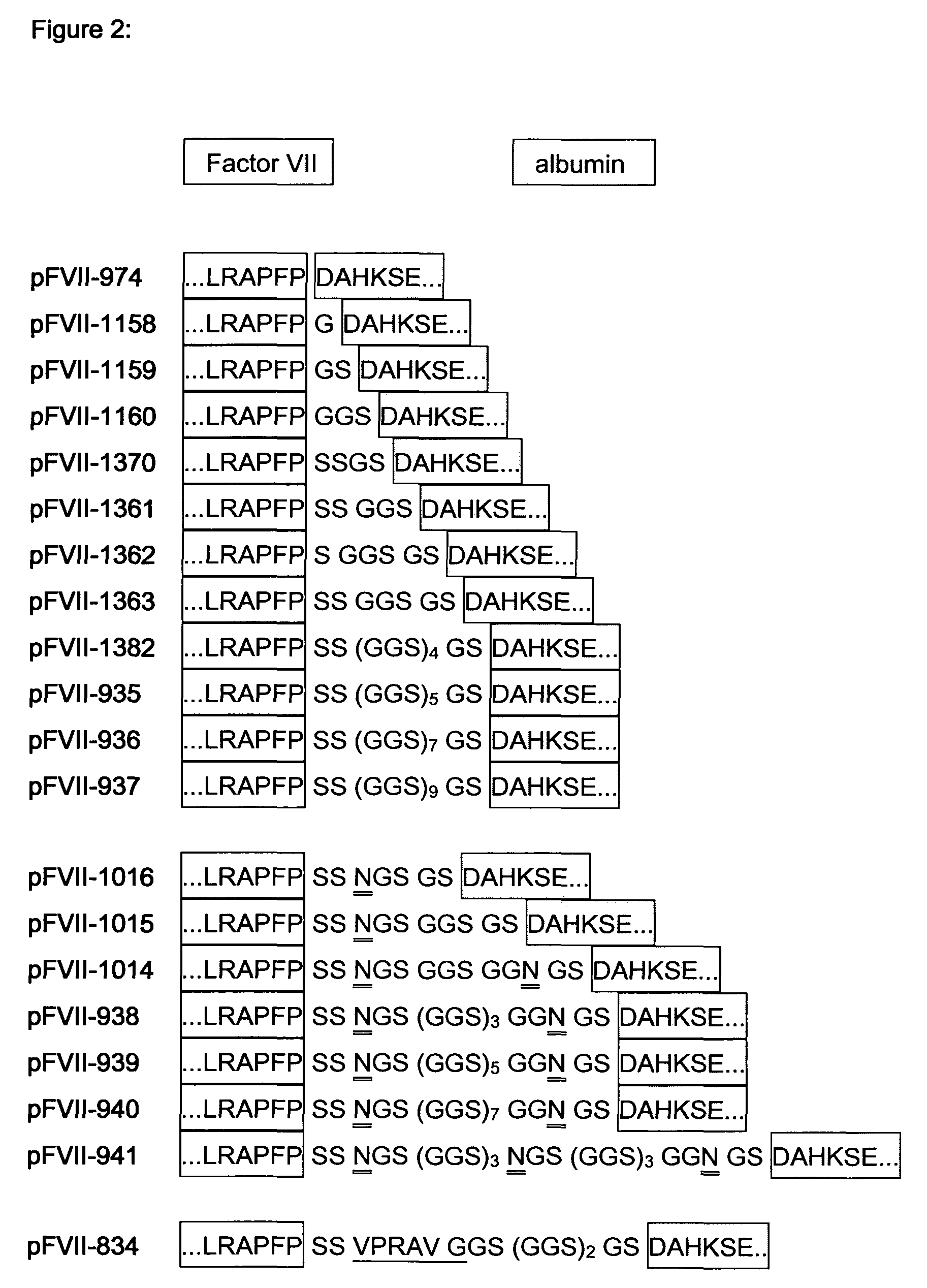
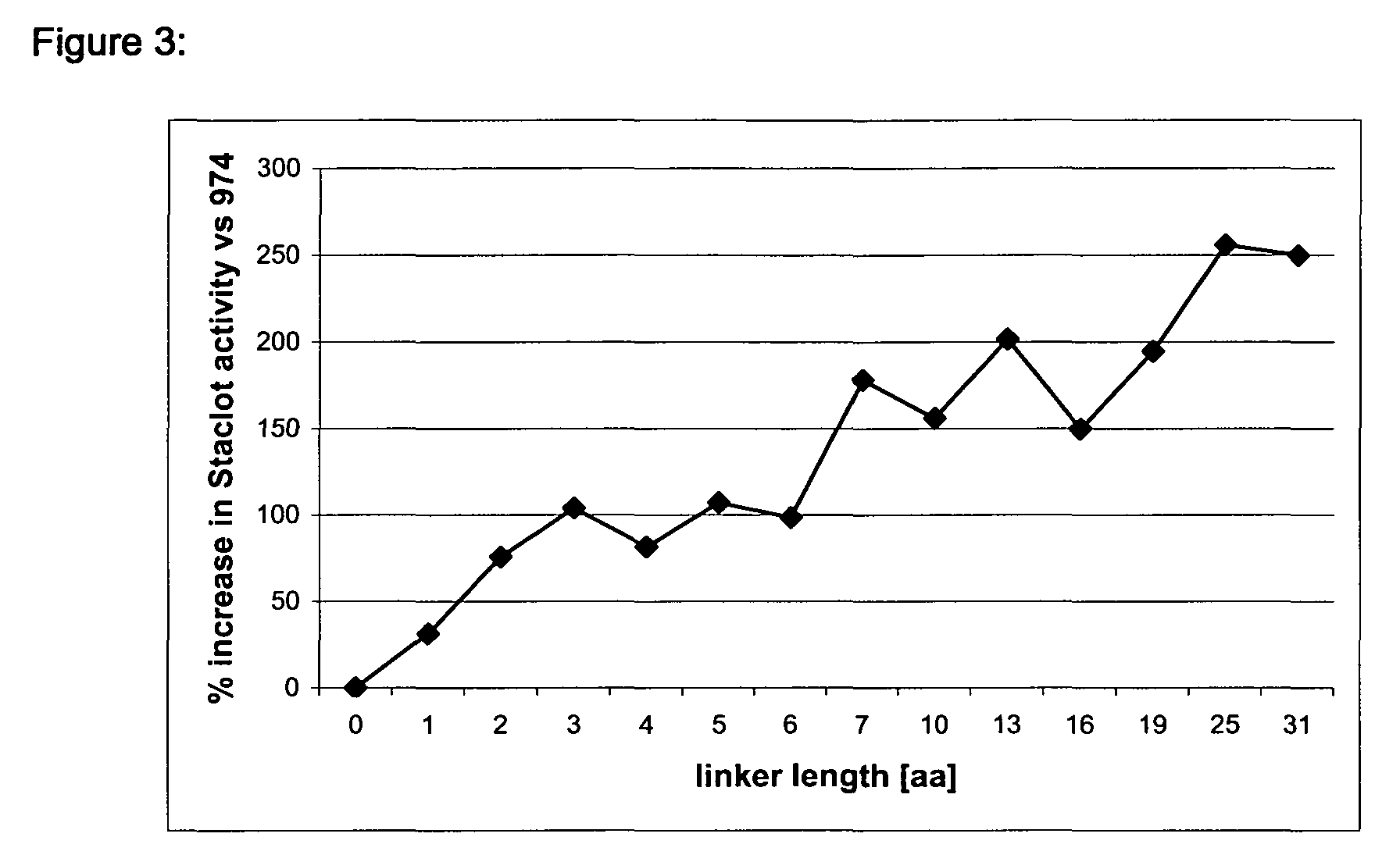
Modified Coagulation Factor VIIa With Extended Half-Life
InactiveUS20090298760A1Specific activityIncrease ratingsOrganic active ingredientsHydrolasesHalf-lifeAlbumin
The present invention relates to the fields of Factor VII (FVII) and Factor VIIa (FVIIa) albumin linked polypeptides. More specifically, the invention relates to cDNA sequences coding for human Factor VII and Factor VIIa and derivatives genetically fused to a cDNA coding for human serum albumin which may be linked by oligonucleotides which code for intervening peptidic linkers such encoded derivatives exhibiting improved stability and extended functional plasma half-life, recombinant expression vectors containing such cDNA sequences, host cells transformed with such recombinant expression vectors, recombinant polypeptides and derivatives which do have biological activities of the unmodified wild type protein but having improved stability and prolonged shelf-life and processes for the manufacture of such recombinant proteins and their derivatives. The invention also covers a transfer vector for use in human gene therapy, which comprises such modified DNA sequences.
Owner:CSL BEHRING GMBH
Genemap of the human genes associated with crohn's disease
InactiveUS20100081129A1Genetic material ingredientsMicrobiological testing/measurementLinkage Disequilibrium MappingPharmacogenomic Study
The present invention relates to the selection of a set of polymorphism markers for use in genome wide association studies based on linkage disequilibrium mapping. In particular, the invention relates to the fields of pharmacogenomics, diagnostics, patient therapy and the use of genetic haplotype information to predict an individual's susceptibility to Crohn's disease and / or their response to a particular drug or drugs.
Owner:GENIZON BIOSCI
Novel sequences and their use
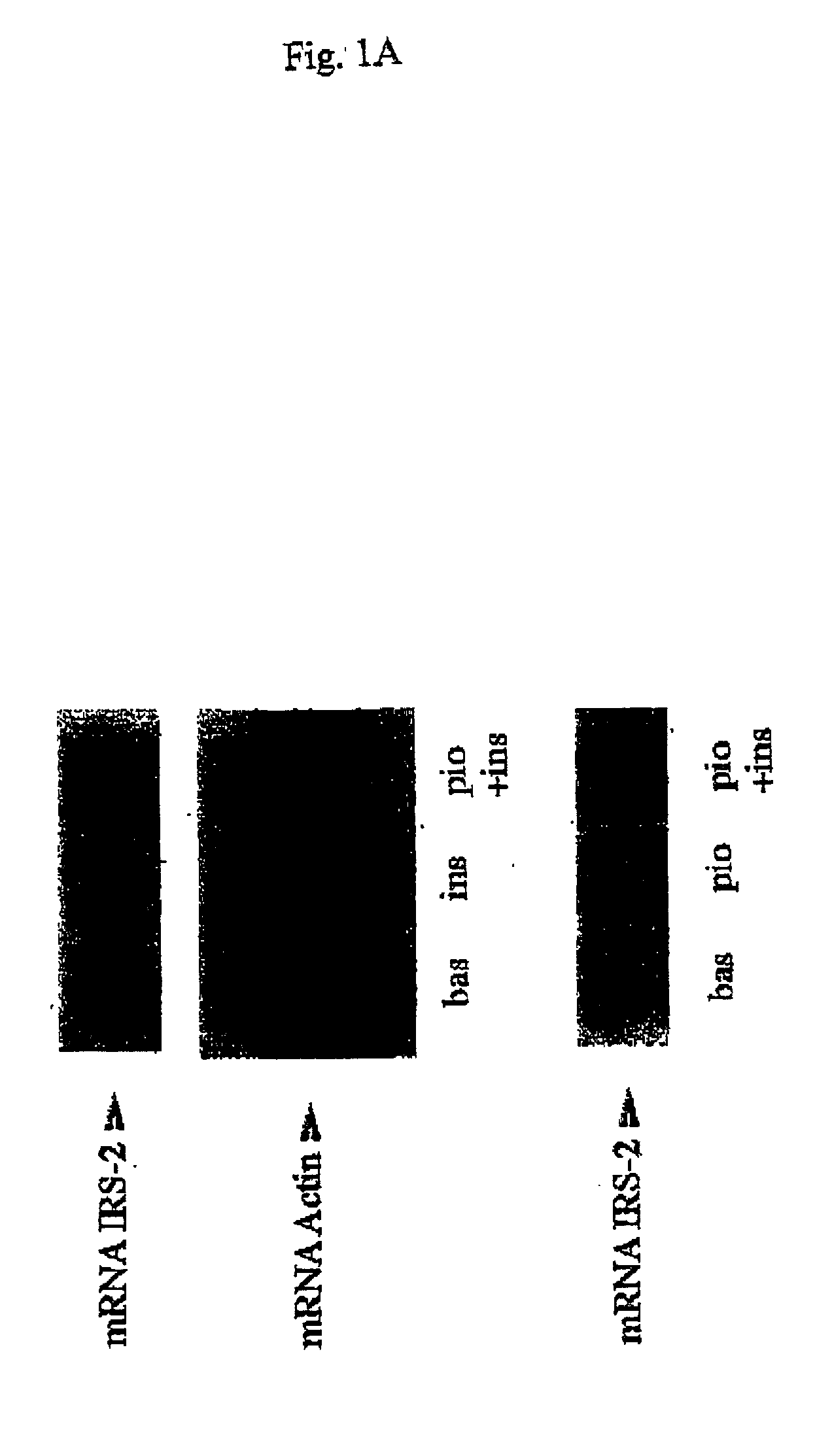
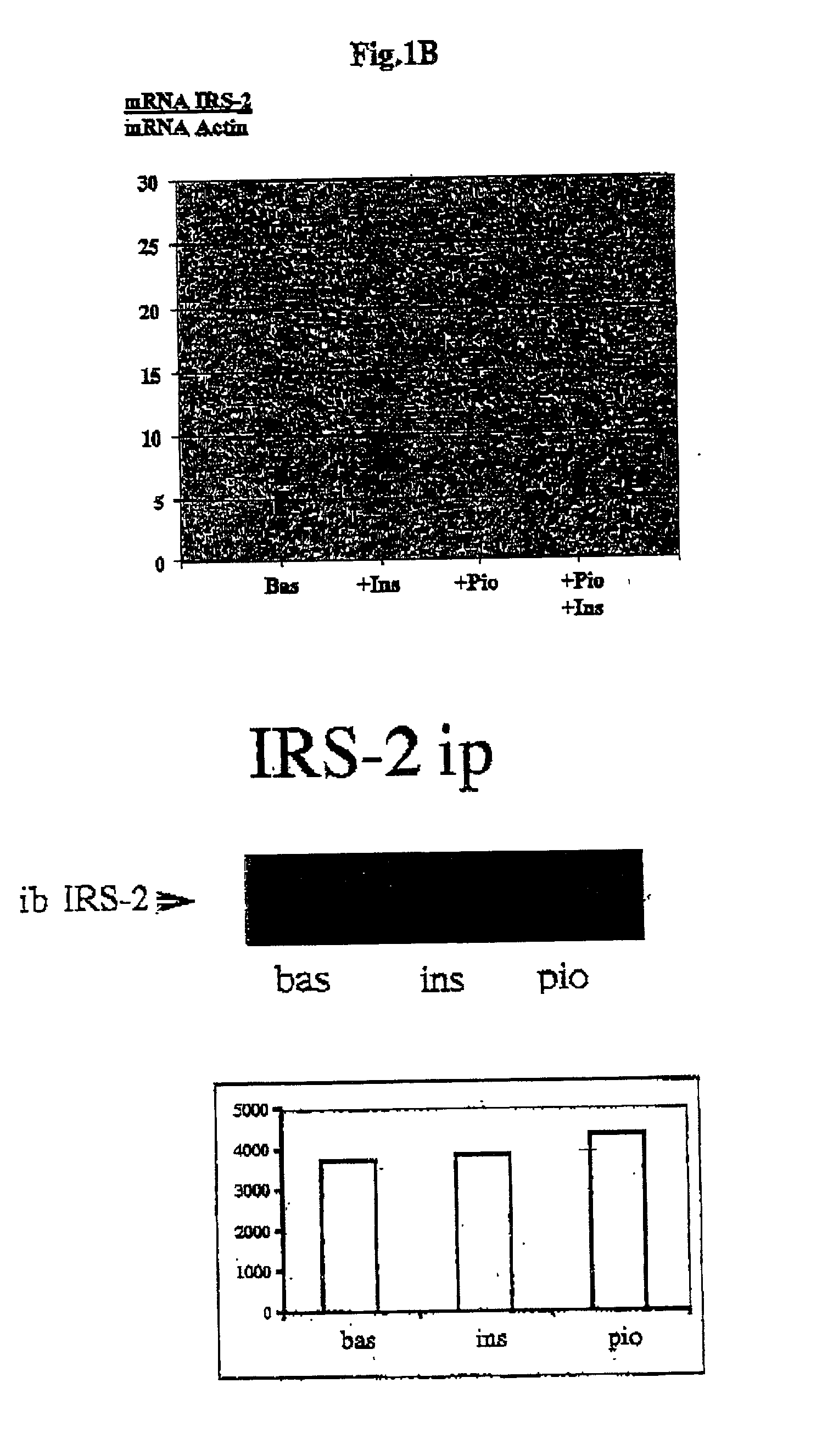
Novel non-coding sequences isolated upstream of the human IRS-2 gene are disclosed as markers for the prediction and / or diagnosis of IRS-2 related metabolic disorders or diseases, such as diabetes. The sequences also fiction as markers in a method and assay for evaluating the insulin regulating, i.e. insulin sensitizing or inhibiting properties of drug candidate substances, e.g. a method and assay for high throughput screening. The sequences and / or information derived therefrom can also be used for influencing the expression of the IRS-2 gene, e.g. in the therapy of IRS-2 related metabolic disorders, such as diabetes.
Owner:METCON MEDICIN
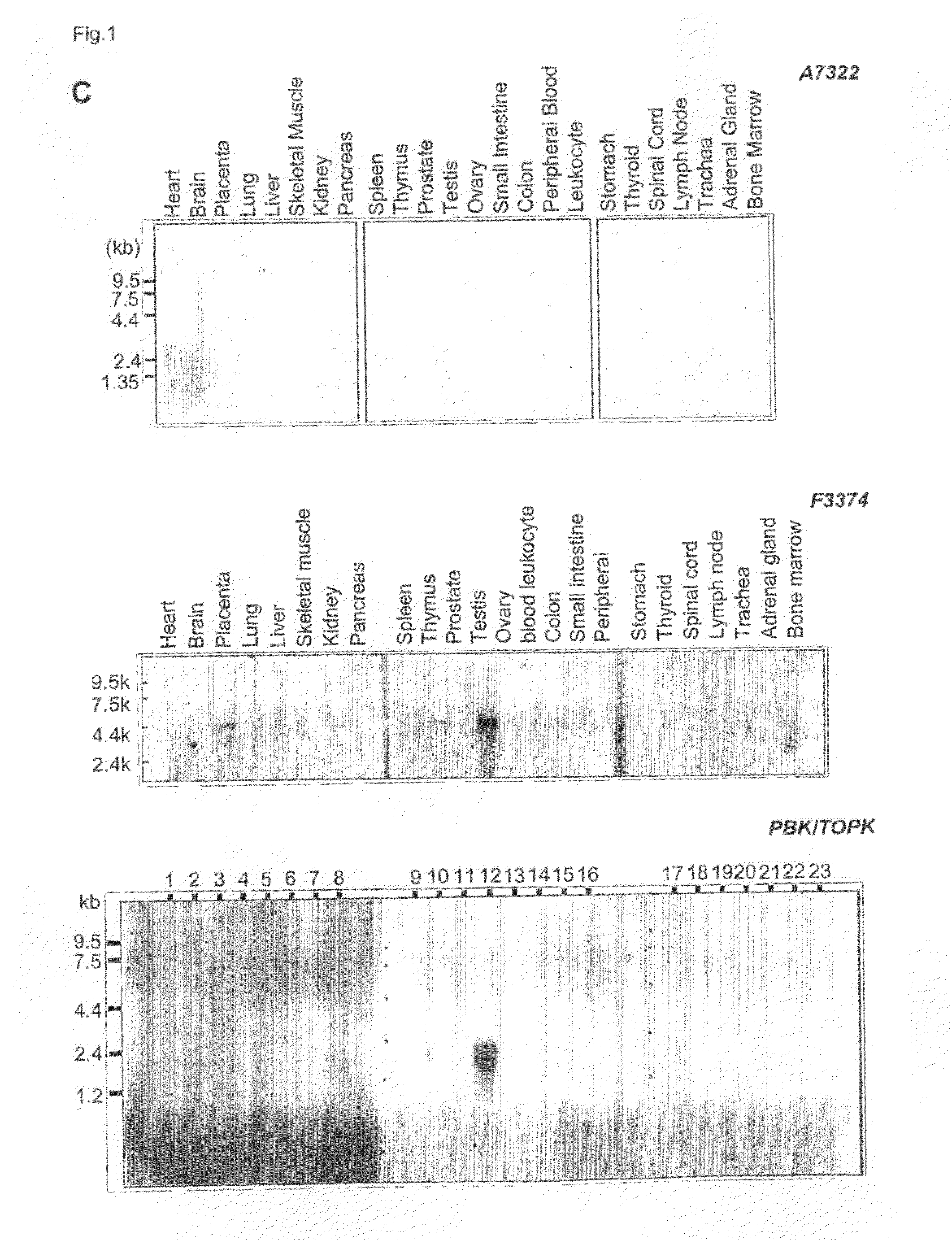
Genes and polypeptides relating to breast cancers
InactiveUS20110135647A1Expanding population of cellExpand the populationOrganic active ingredientsMicroorganismsKinase activityFhit gene
The present application provides novel human genes A7322, whose expression is markedly elevated in breast cancer. The present application also provides human genes F3374 whose expression is markedly elevated in breast cancer. These genes and polypeptides encoded thereby can be used, for example, in the diagnosis of breast cancer, and as target molecules for developing drugs against breast cancer. The invention features methods of screening for modulators of the kinase activity of PBK / TOPK. The invention further provides methods of screening for agents to prevent or treat cancer, such as breast cancer.
Owner:ONCOTHERAPY SCI INC
Packaging cell lines for generation of high titers of recombinant AAV vectors
InactiveUS20030175974A1Improve packaging efficiencyNot be easily manipulatedBiocidePeptide/protein ingredientsGenes humanCell biology
AAV vectors may have utility for gene therapy but heretofore a significant obstacle has been the inability to generate sufficient quantities of such recombinant vectors in amounts that would be clinically useful for human gene therapy application. Stable AAV packaging cell lines have been elusive, mainly due to the activities of Rep protein, which down-regulates its own expression and can negatively affect the host cell. This invention provides packaging systems and processes for packaging AAV vectors that effectively circumvent these problems and that allow for substantially increased packaging efficiency.
Owner:TARGETED GENETICS CORPORTION
Treatment of genetic disorders associated with DNA repeat instability
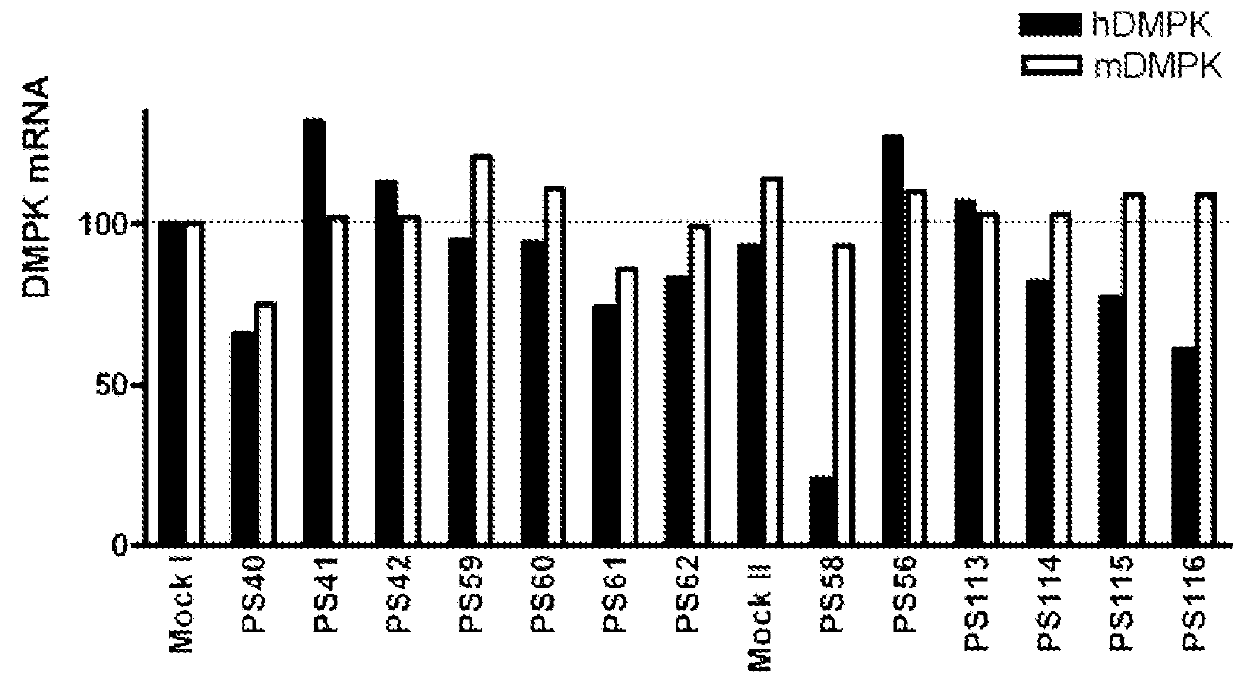
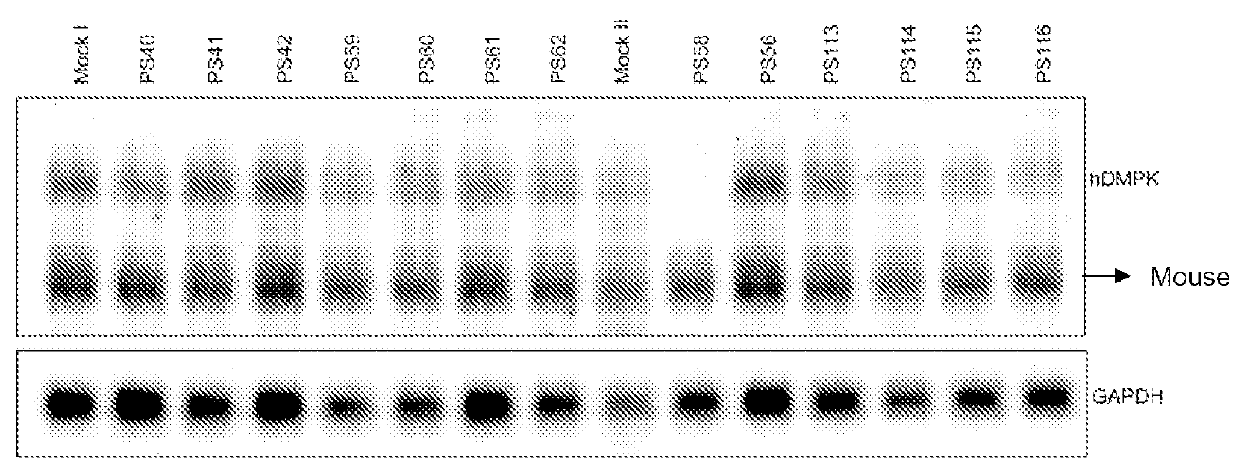
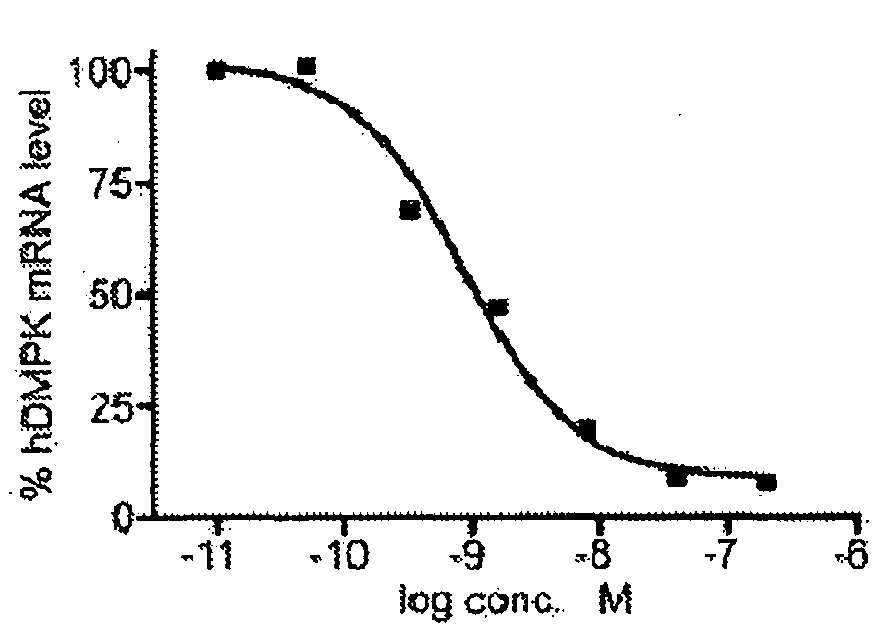
ActiveUS20160053254A1Lower Level RequirementsAltered RNA processing and/or splicingNervous disorderGenetic material ingredientsInstabilityGene transcript
The current invention provides for methods and medicaments that apply oligonucleotide molecules complementary only to a repetitive sequence in a human gene transcript, for the manufacture of a medicament for the diagnosis, treatment or prevention of a cis-element repeat instability associated genetic disorders in humans. The invention hence provides a method of treatment for cis-element repeat instability associated genetic disorders. The invention also pertains to modified oligonucleotides which can be applied in method of the invention to prevent the accumulation and / or translation of repeat expanded transcripts in cells.
Owner:VICO THERAPEUTICS BV
Diagnosis and treatment methods related to aging, especially of liver
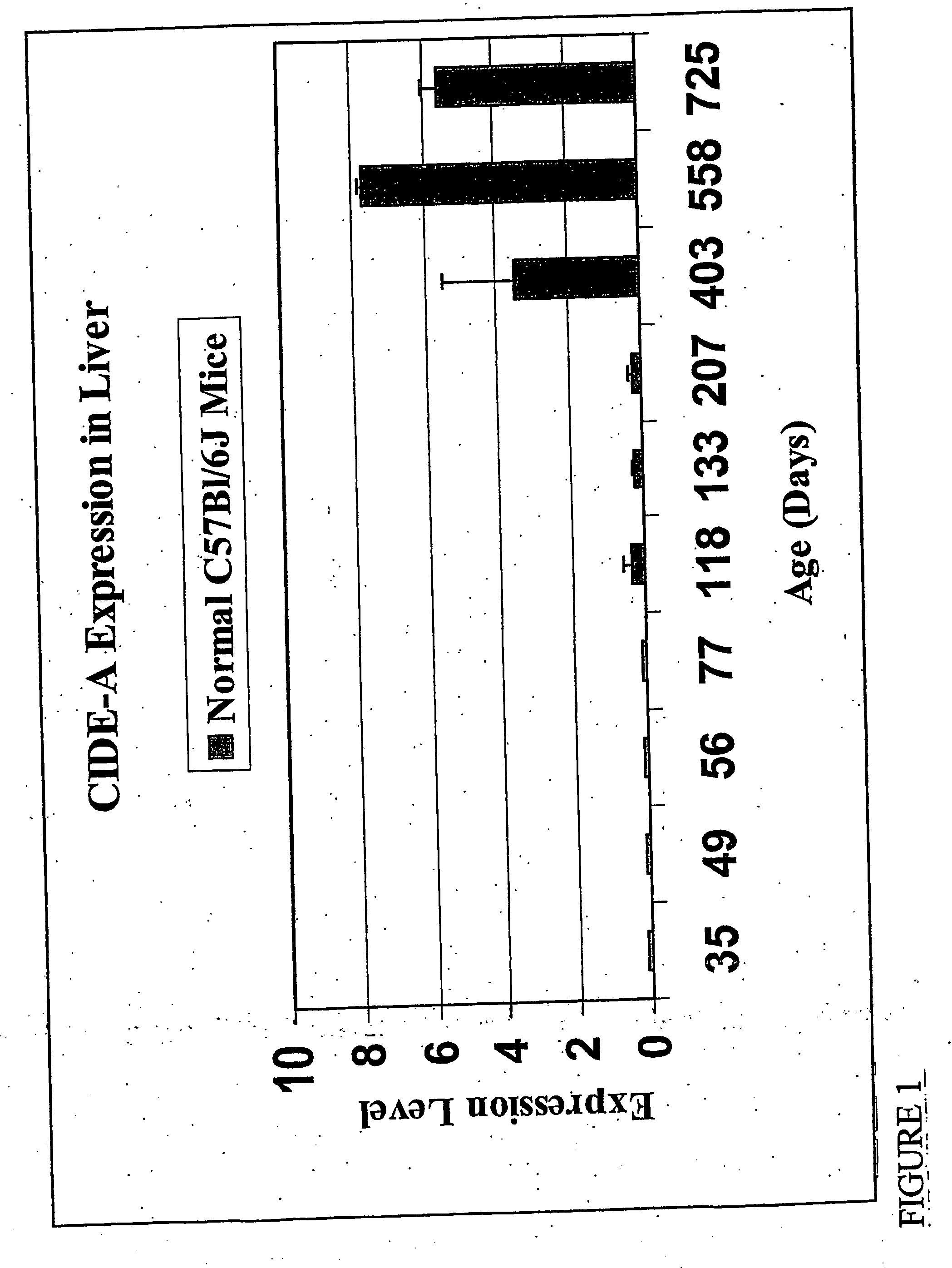
InactiveUS20070111933A1Reduce probabilityReduce severityPeptide/protein ingredientsGenetic material ingredientsHepaticaGene
Mouse genes differentially expressed in comparisons of older and younger livers by gene chip analysis have been identified, as have corresponding human genes and proteins. The human molecules, or antagonists thereof, may be used for protection against faster-than-normal biological aging, or to achieve slower-than-normal biological aging. The human molecules may also be used as markers of biological aging.
Owner:OHIO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com