Papillary thyroid carcinoma serum marker and application
A serum marker, papillary cancer technology, applied in the field of serum markers for papillary thyroid cancer, can solve problems such as unclear diagnosis, and achieve the effects of reducing bleeding, low cost, and improving diagnostic accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
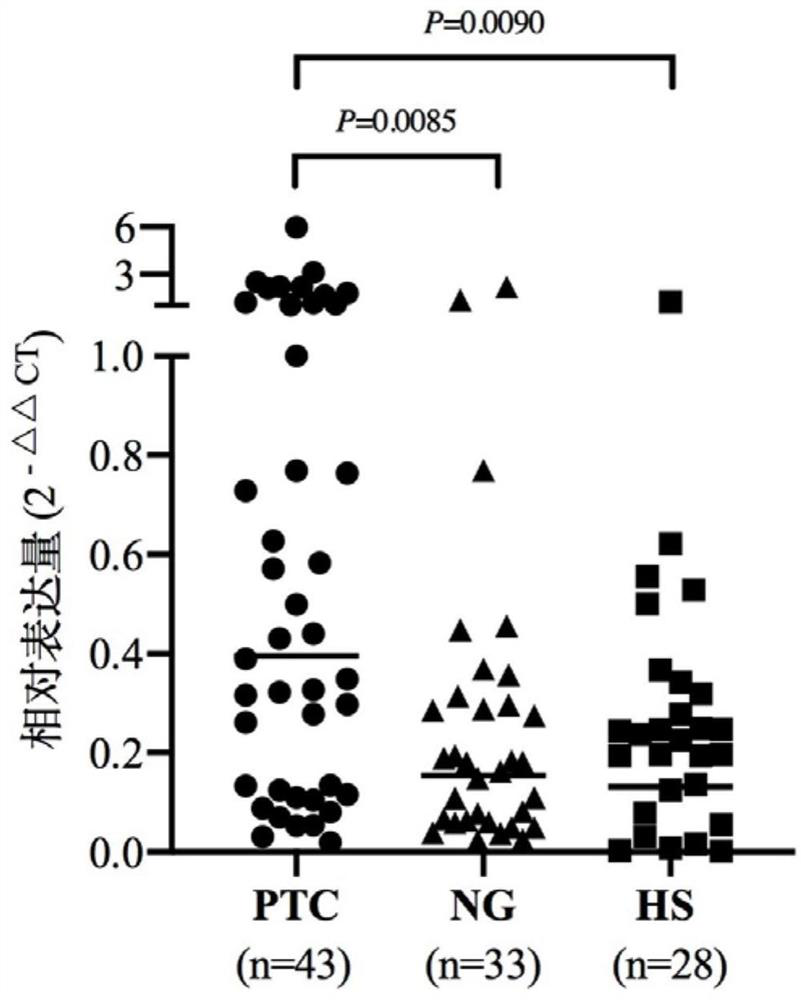
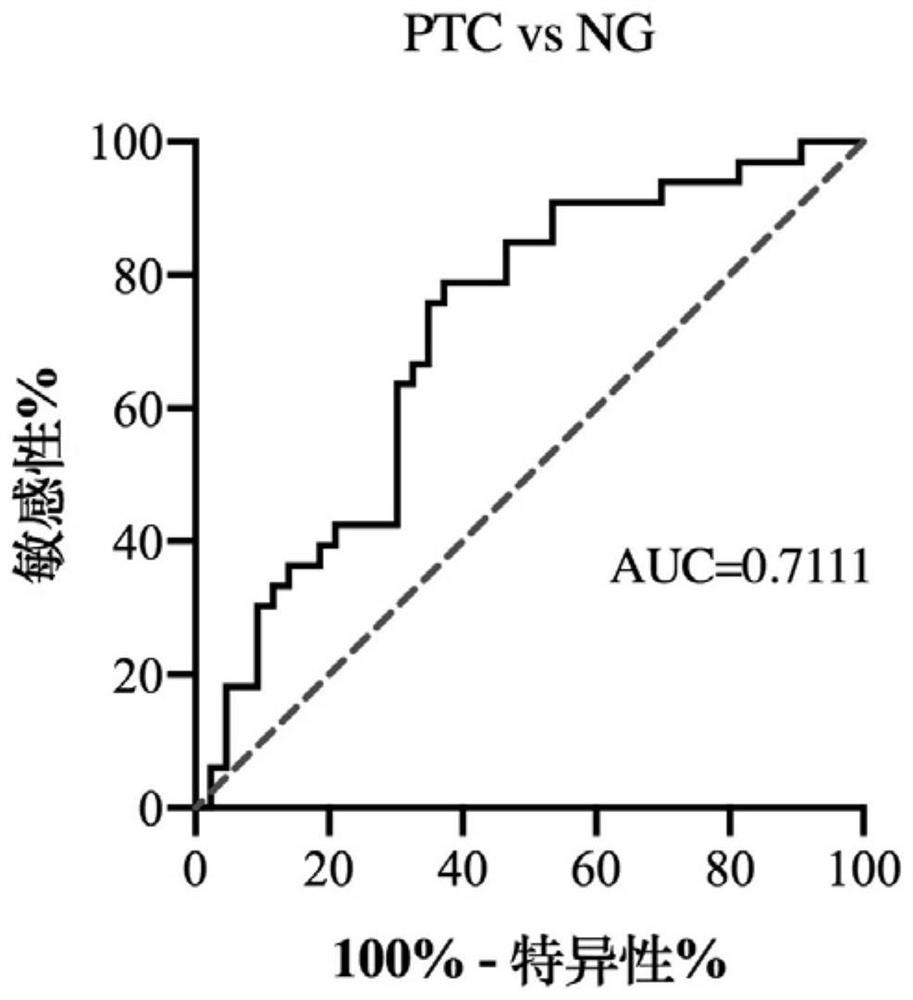
[0021] Example 1: Discovery and proof of tRF-Pro-AGG-018 molecule as a serum marker
[0022] 1. Research object
[0023] Serum samples from 43 patients with PTC, serum samples from 33 patients with benign nodules and 28 normal serum samples.
[0024] 2. Research Methods
[0025] 1. Collect serum samples: Use EDTA anticoagulant tube to collect 5 mL of cubital vein. After blood collection, gently shake the anticoagulant tube to mix the anticoagulant with the blood. After standing at 4°C for 24 hours, centrifuge at 3000×g for 5 minutes at room temperature. Use a micropipette to draw 200 μL of the upper serum and aliquot it into a new 500 μL centrifuge tube, and store it at -80°C for later use.
[0026] 2. RNA extraction
[0027] (1) Homogenate: Take out the serum sample from the -80°C refrigerator, thaw it and centrifuge at 12000×g at 4°C for 10 minutes to remove possible impurities. Take 250uL serum liquid and transfer it to a 1.5mL centrifuge tube. TRIzol LS Reagent (Invitr...
Embodiment 2
[0091] Embodiment 2: Configuration of diagnostic kit for thyroid papillary carcinoma
[0092] According to the results of Example 1, this example provides a preferred kit for the diagnosis of papillary thyroid carcinoma, which includes the following reagents:
[0093] 1. Serum RNA extraction reagent: TRIzol LS Reagent (Invitrogen life technologies), chloroform, isopropanol, 100% ethanol, 75% ethanol (prepared with DEPC-treated water), glacial acetic acid, RNase-free water (Invitrogen life technologies ), RNase-free glycogen (Invitrogen life technologies).
[0094] 2. Reverse transcription reagent: rtStar TM tRF&tiRNA Pretreatment Kit (Cat#AS-FS-005, Arraystar),
[0095] rtStar TM First-Strand cDNA Synthesis Kit (3' and 5' adapter) (Cat#AS-FS-003, Arraystar).
[0096]3. Fluorescence quantitative PCR reagent: 2X PCR master mix (Arraystar); tRF-Pro-AGG-018 specific primer: primer F: 5'-TACAGTCCGACGATCGGCTC-3', as shown in SEQ ID NO.2; primer R: 5 '-CTCTTCCGATCTCCTAGACCAAC-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com