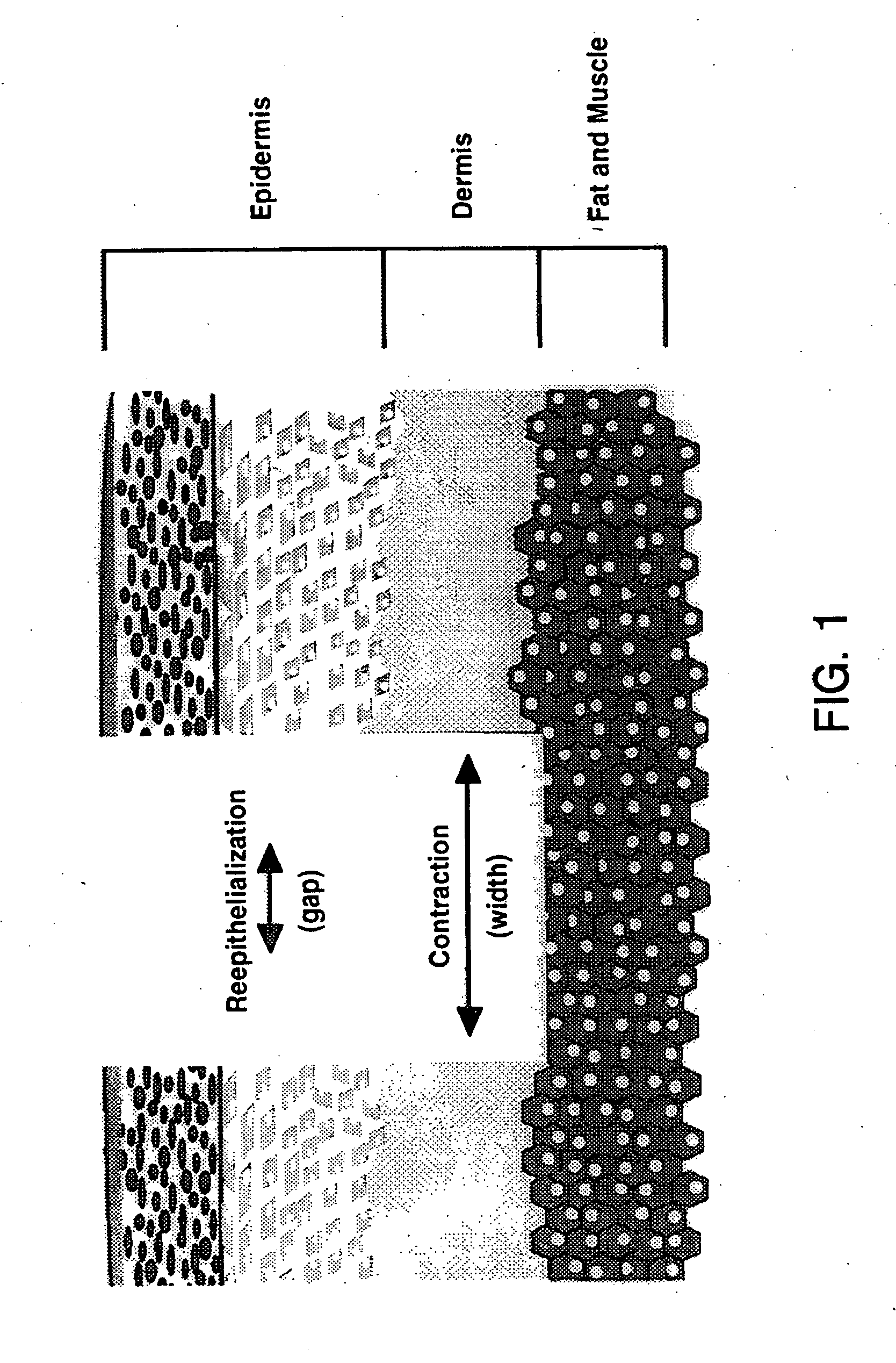
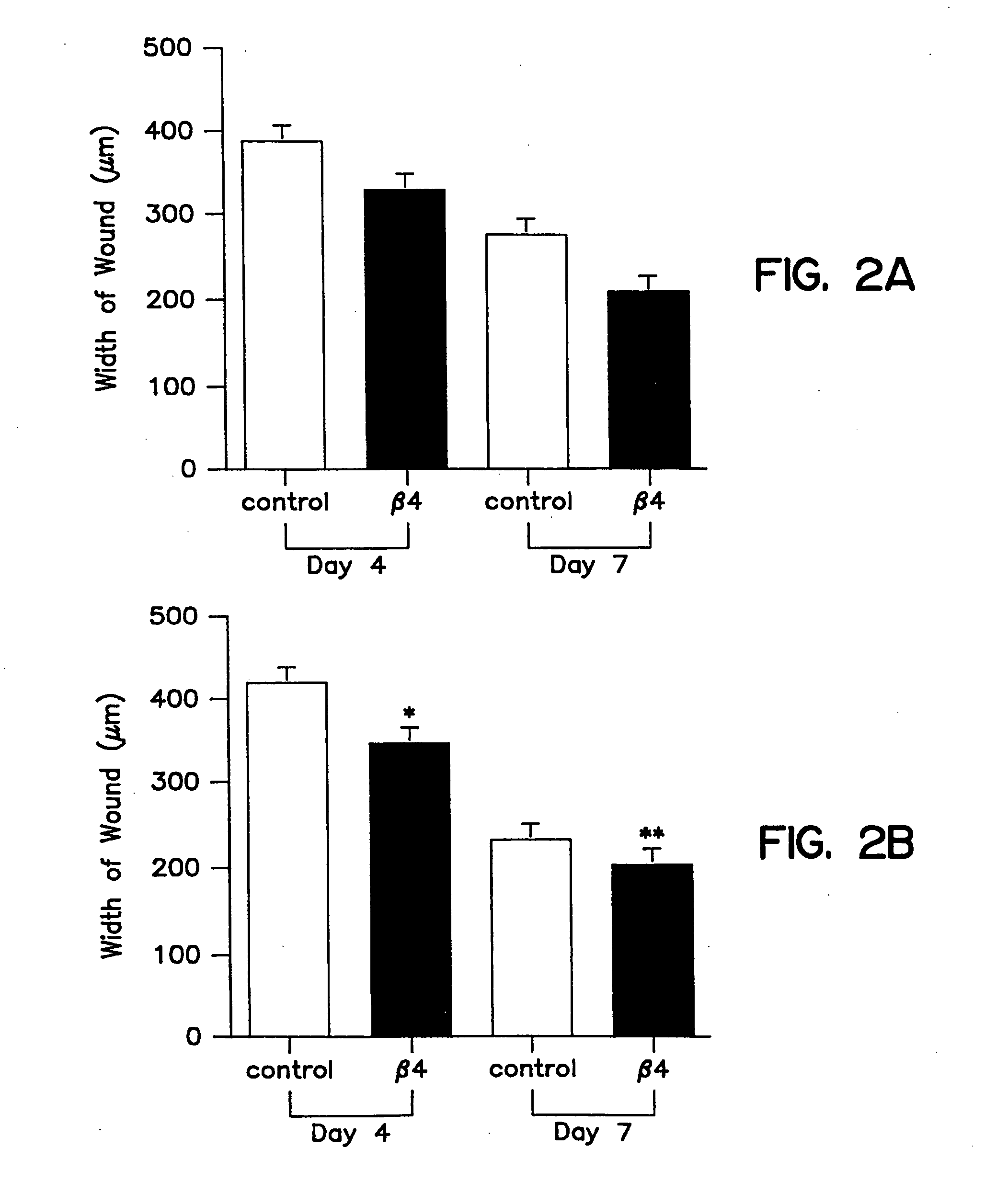
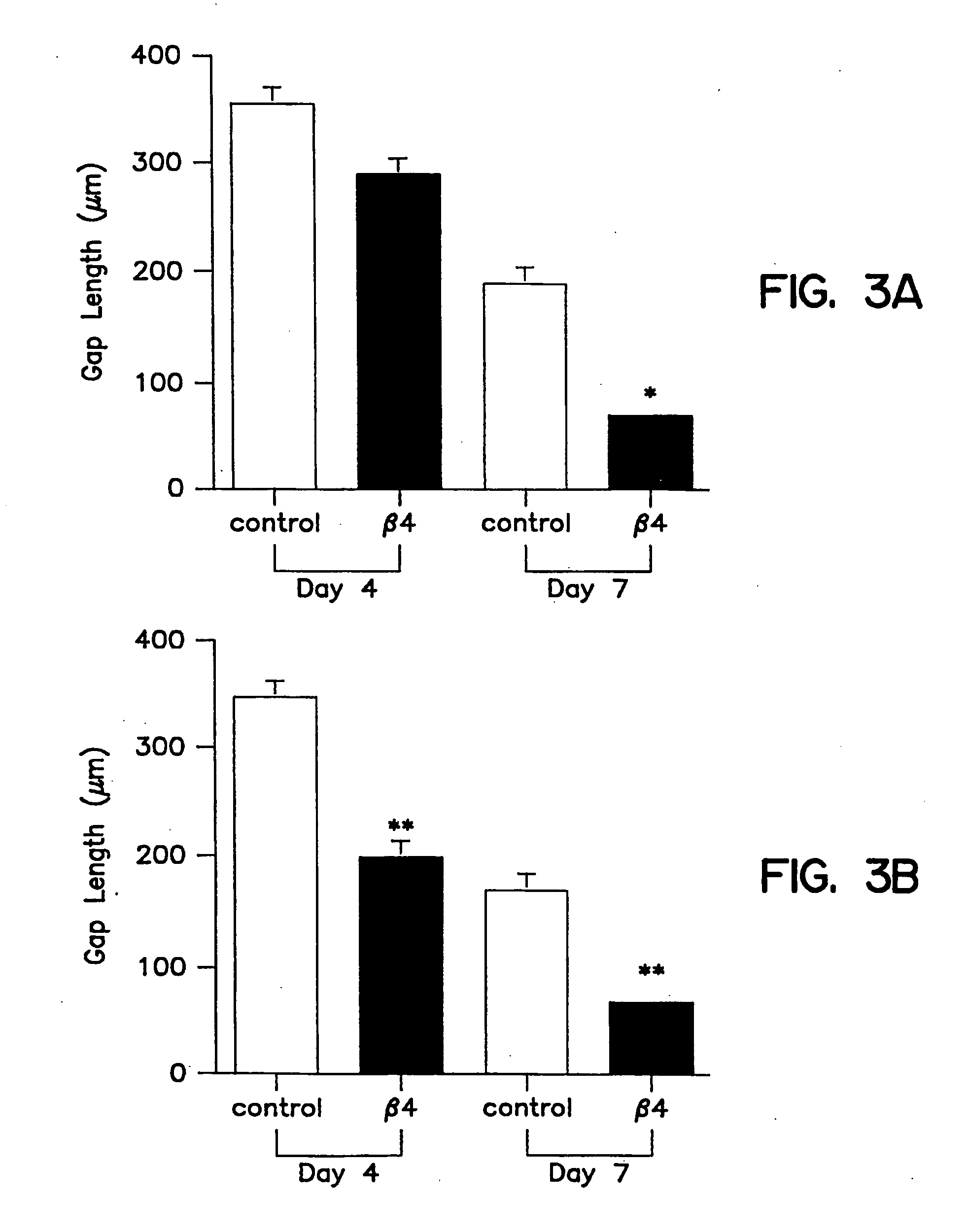
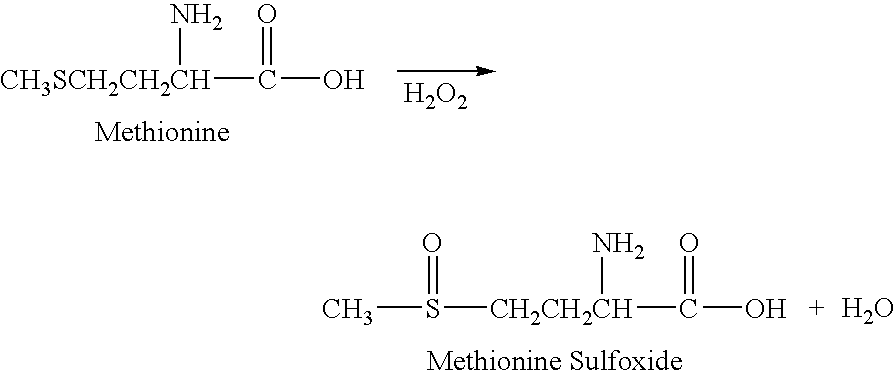
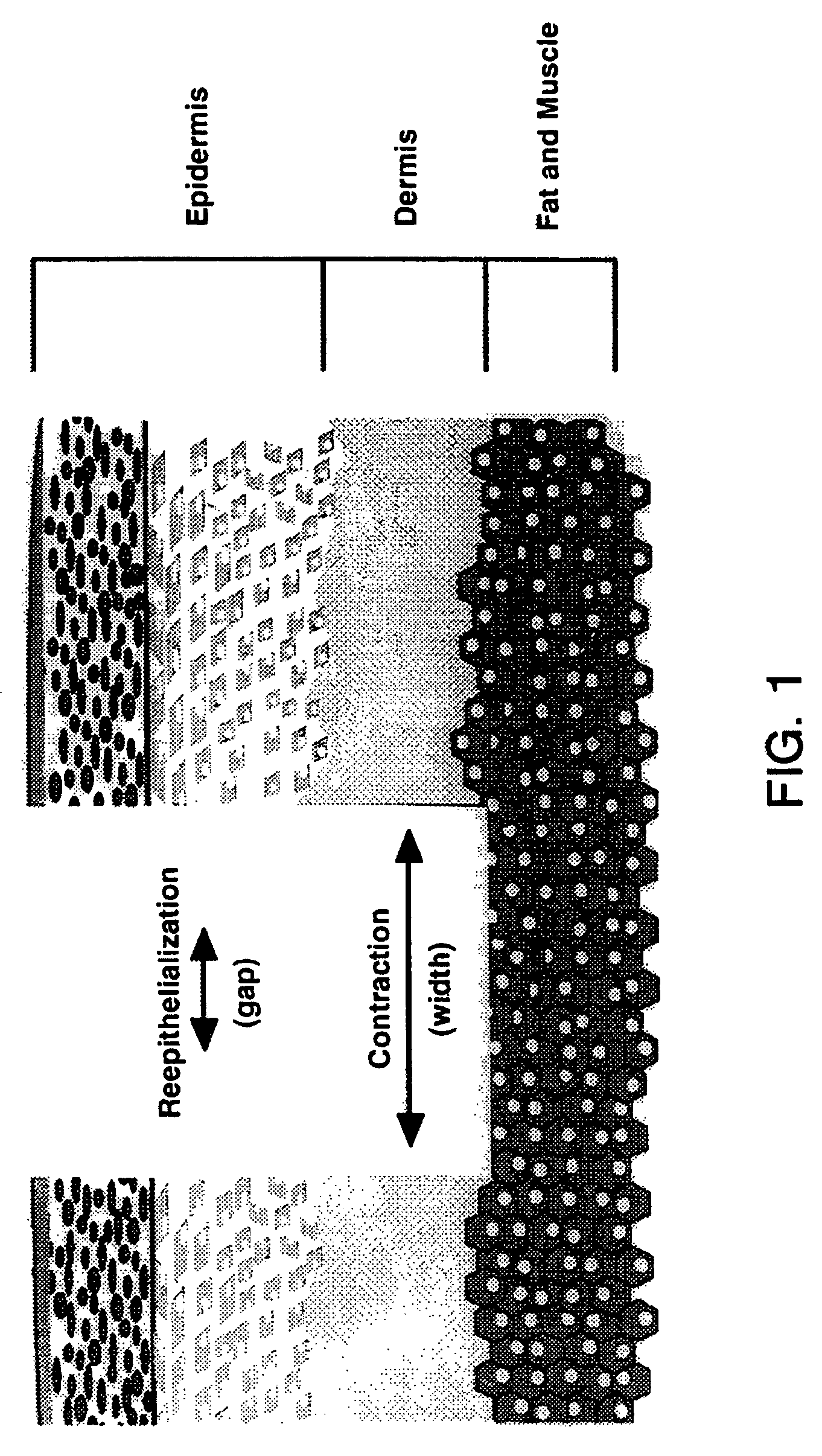
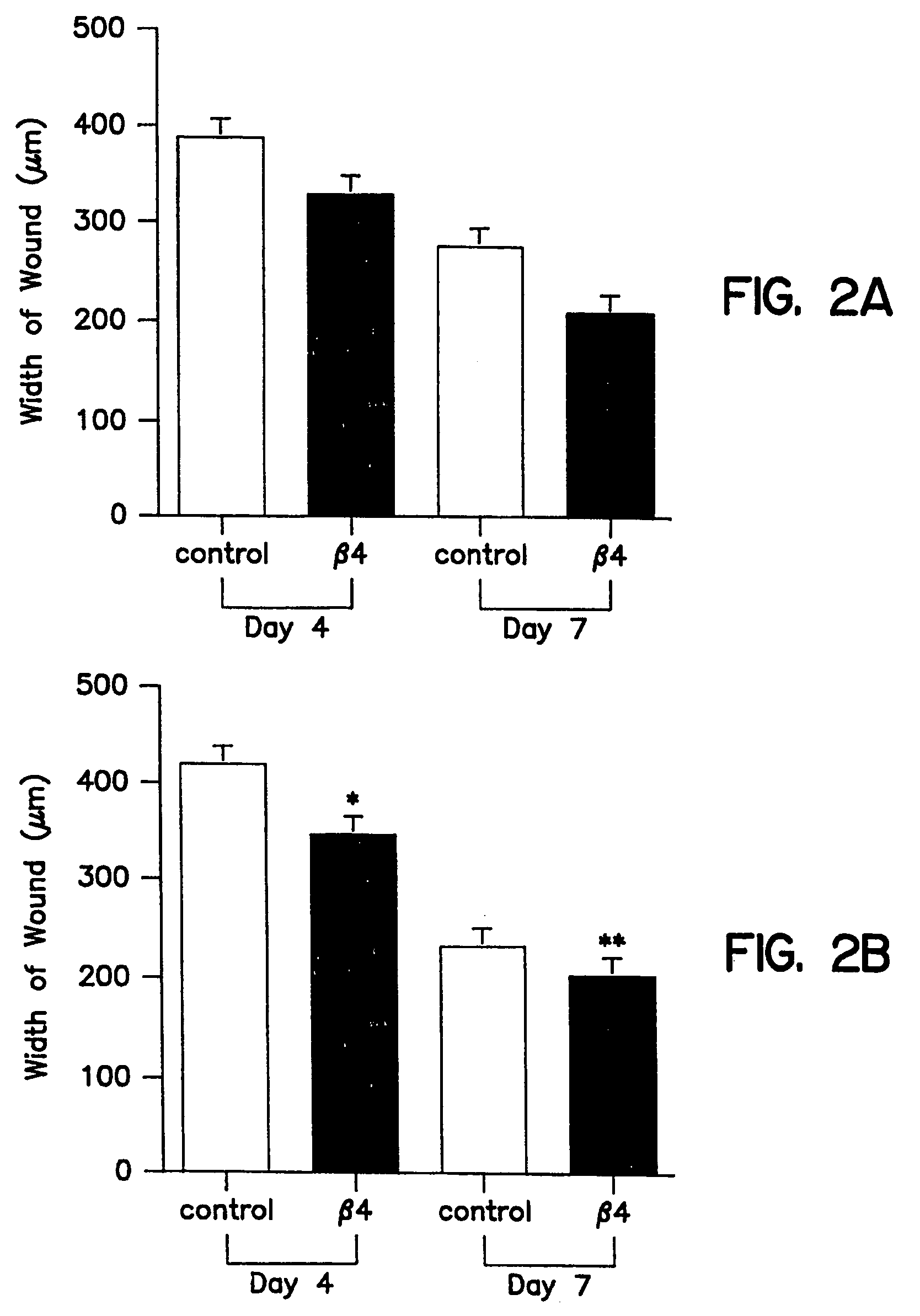
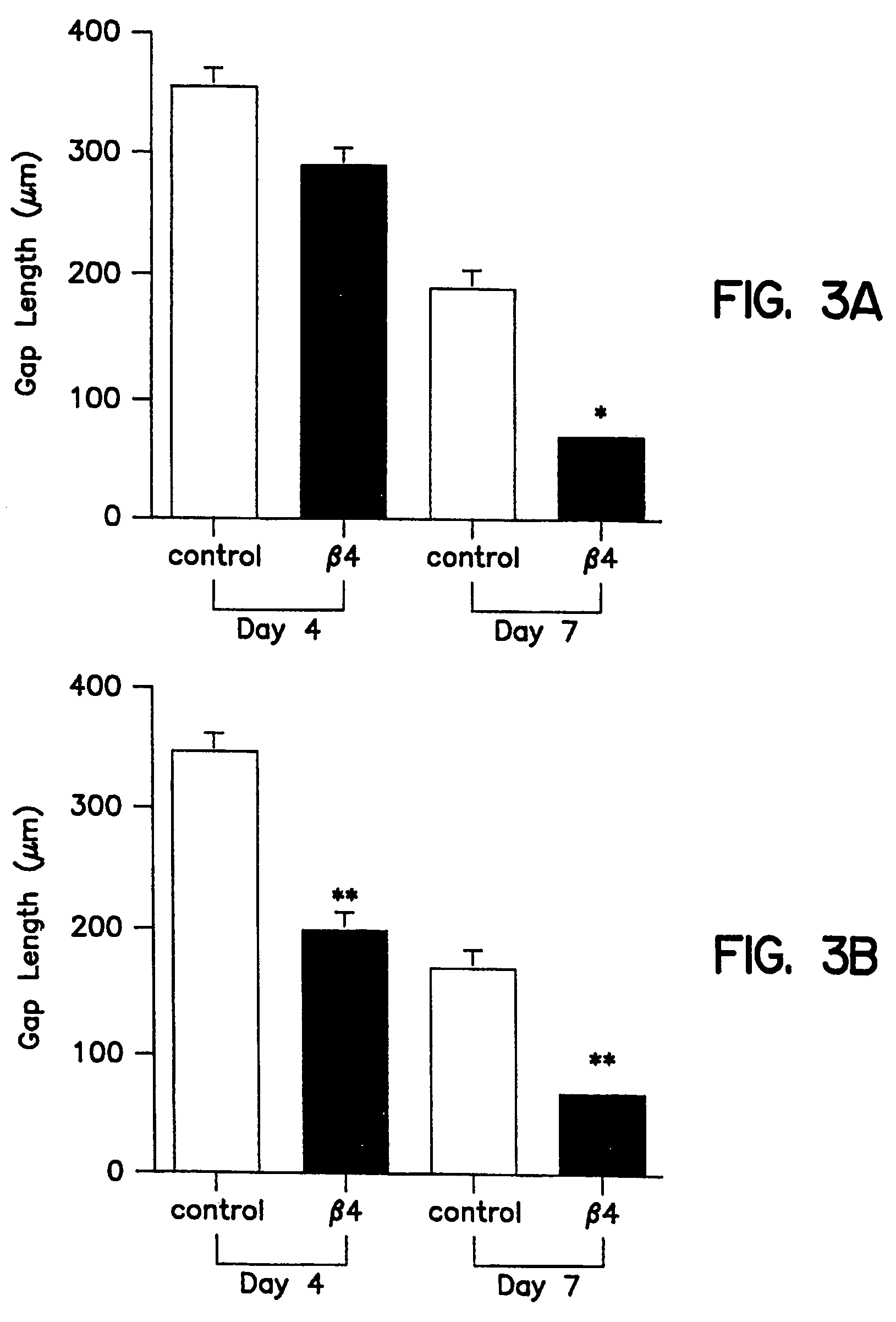
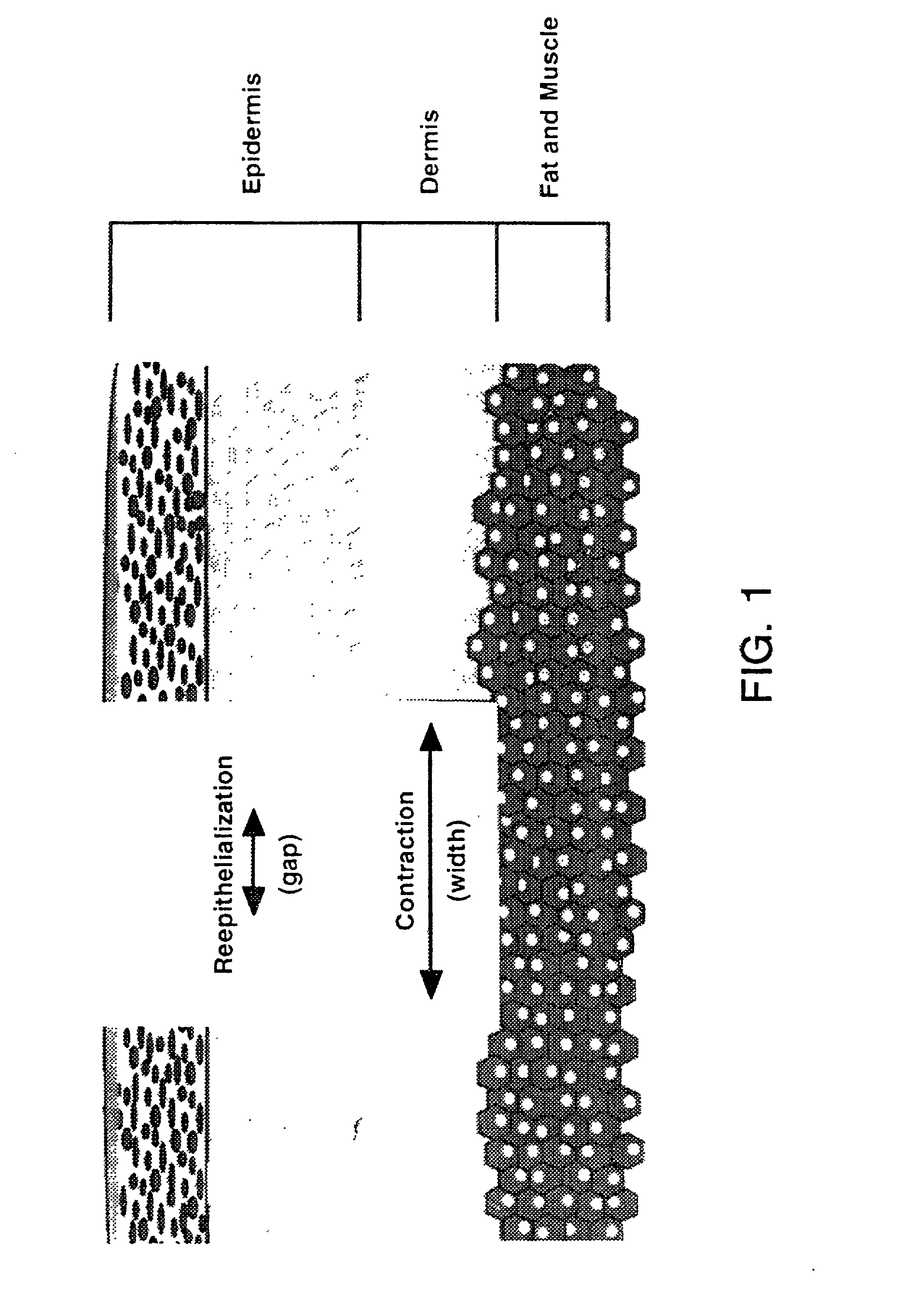
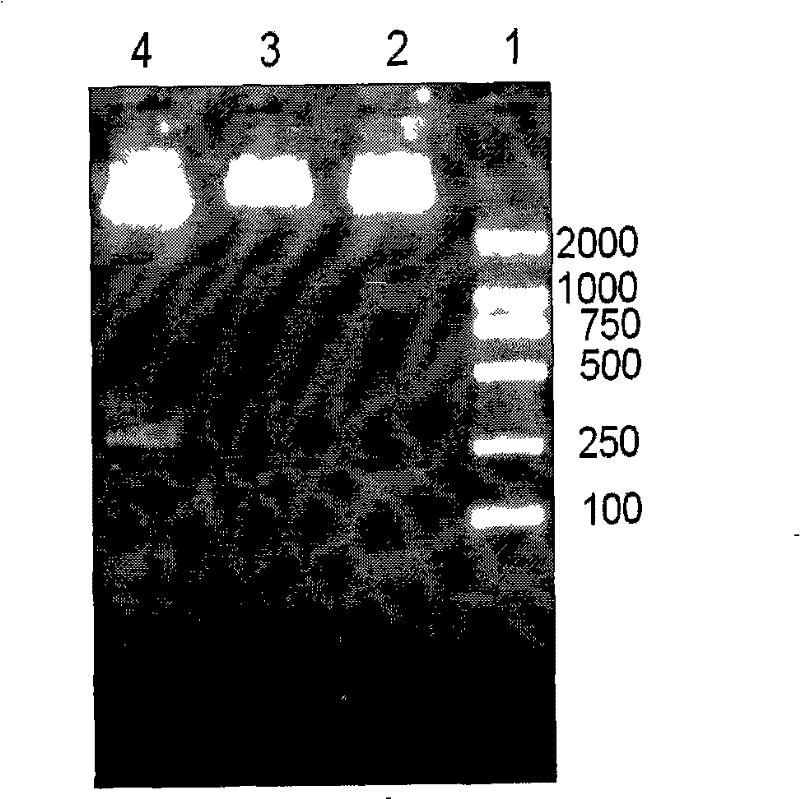
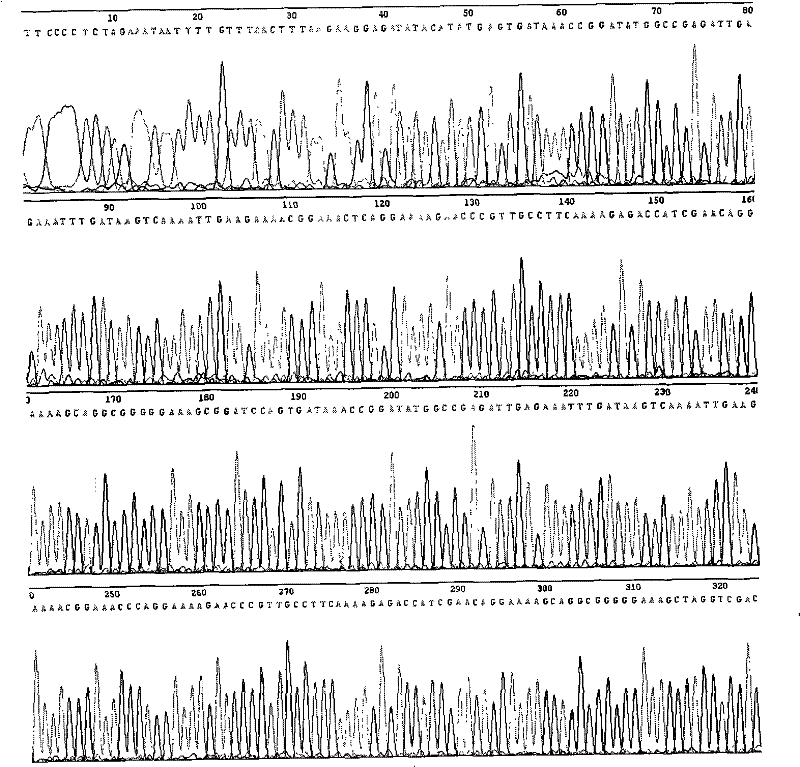
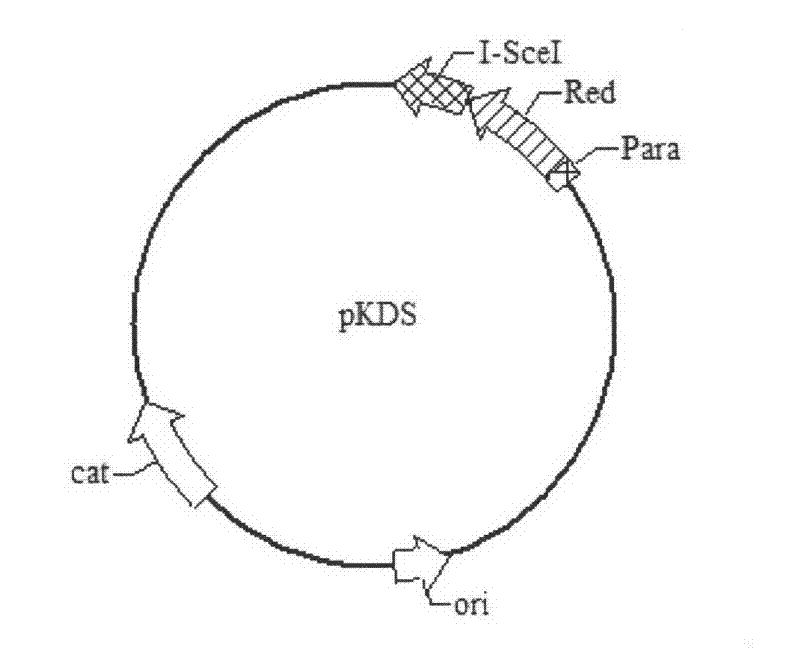
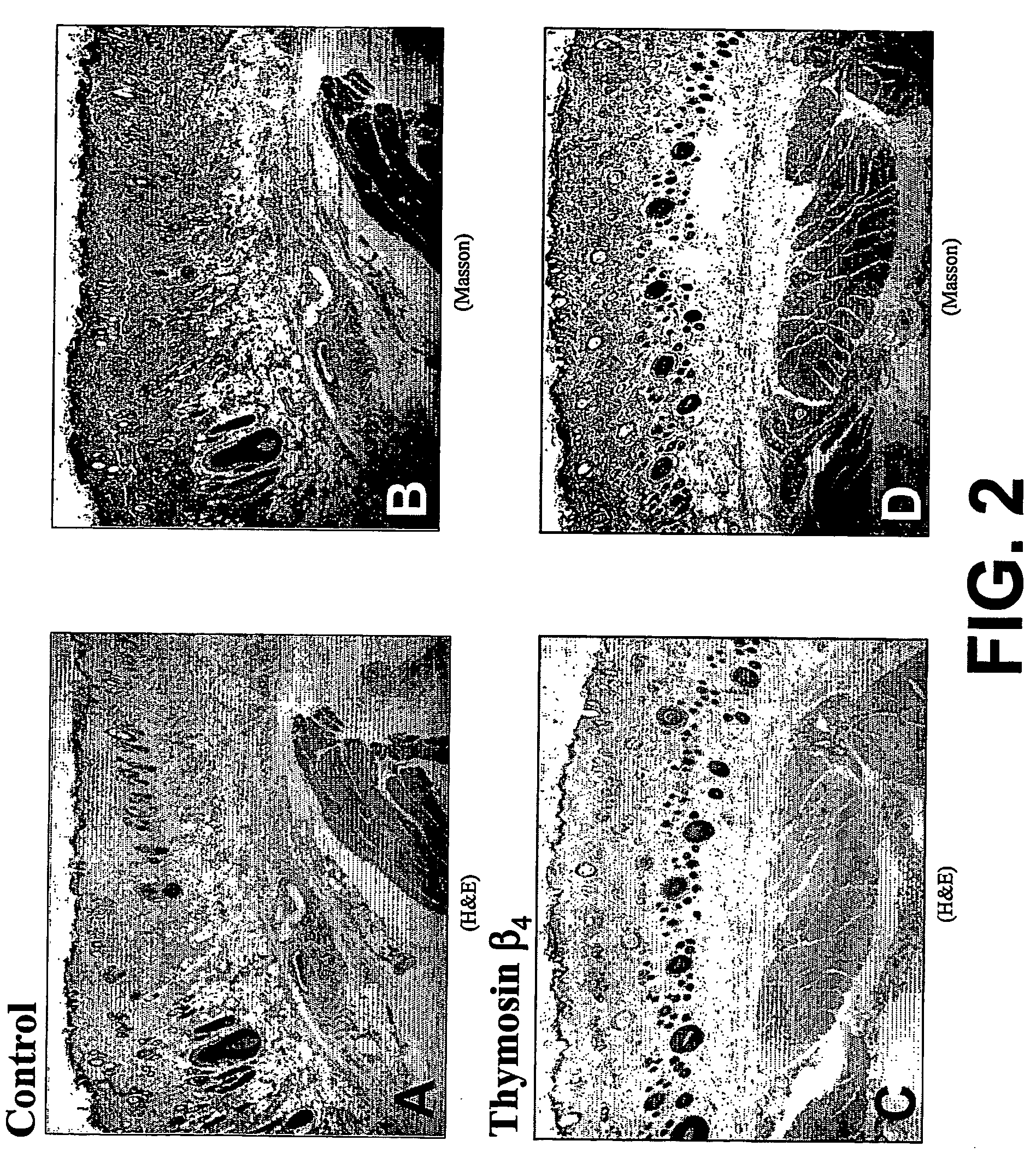
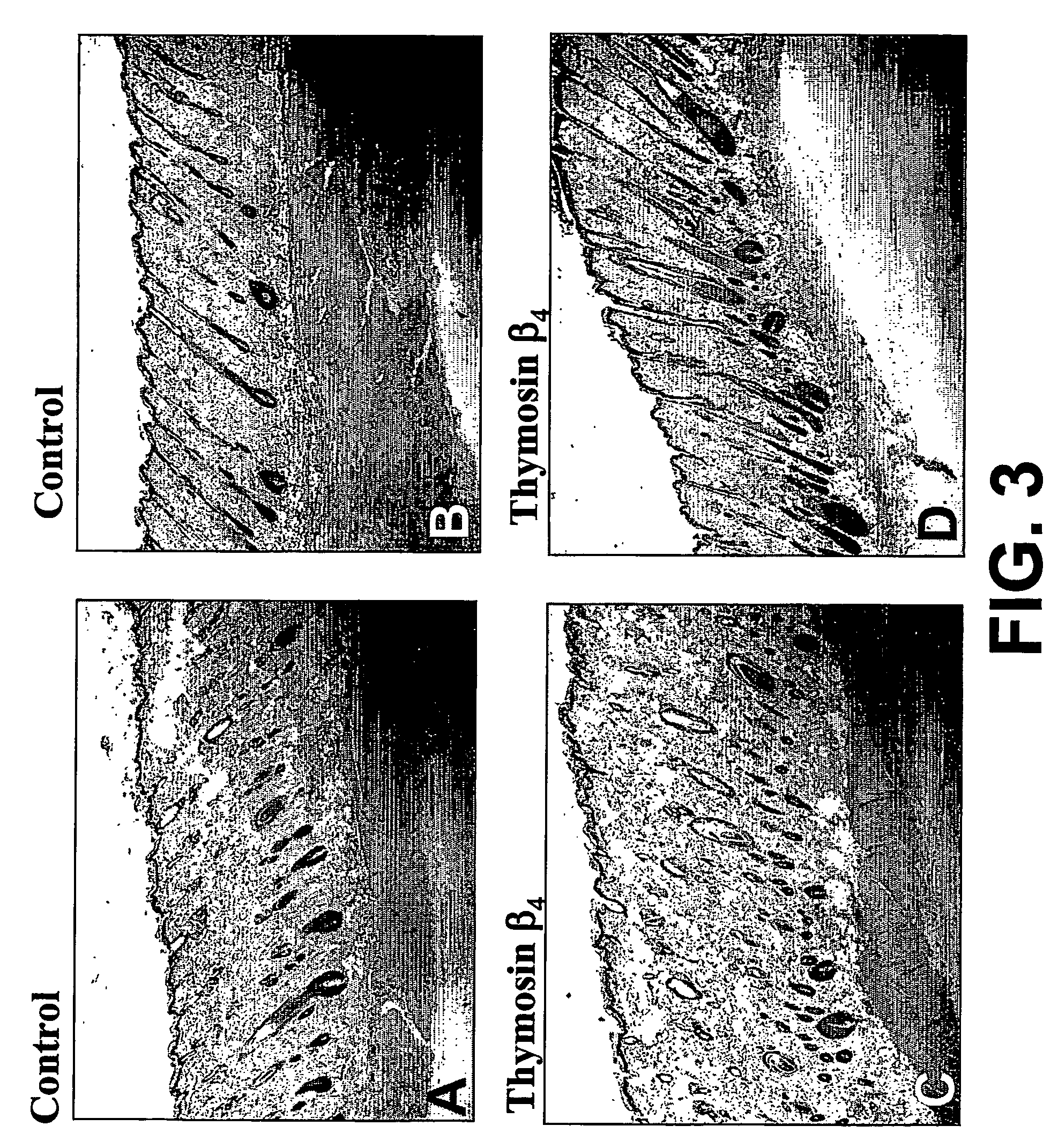
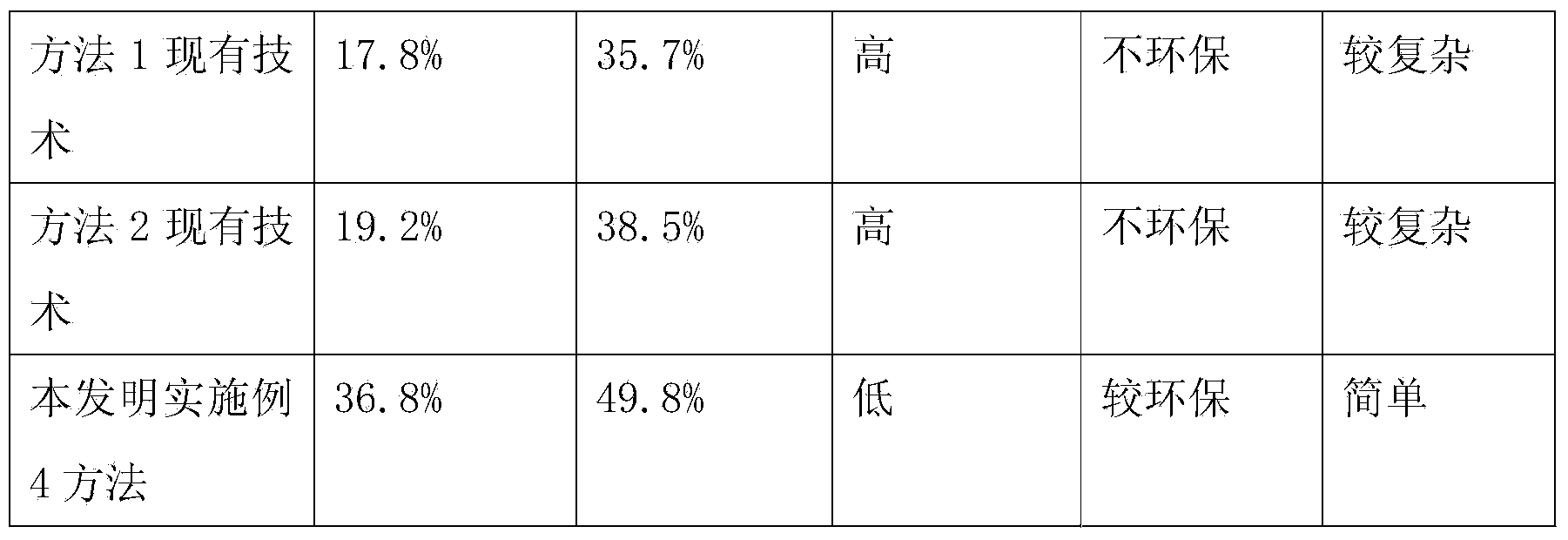Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
64 results about "Thymosin β4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
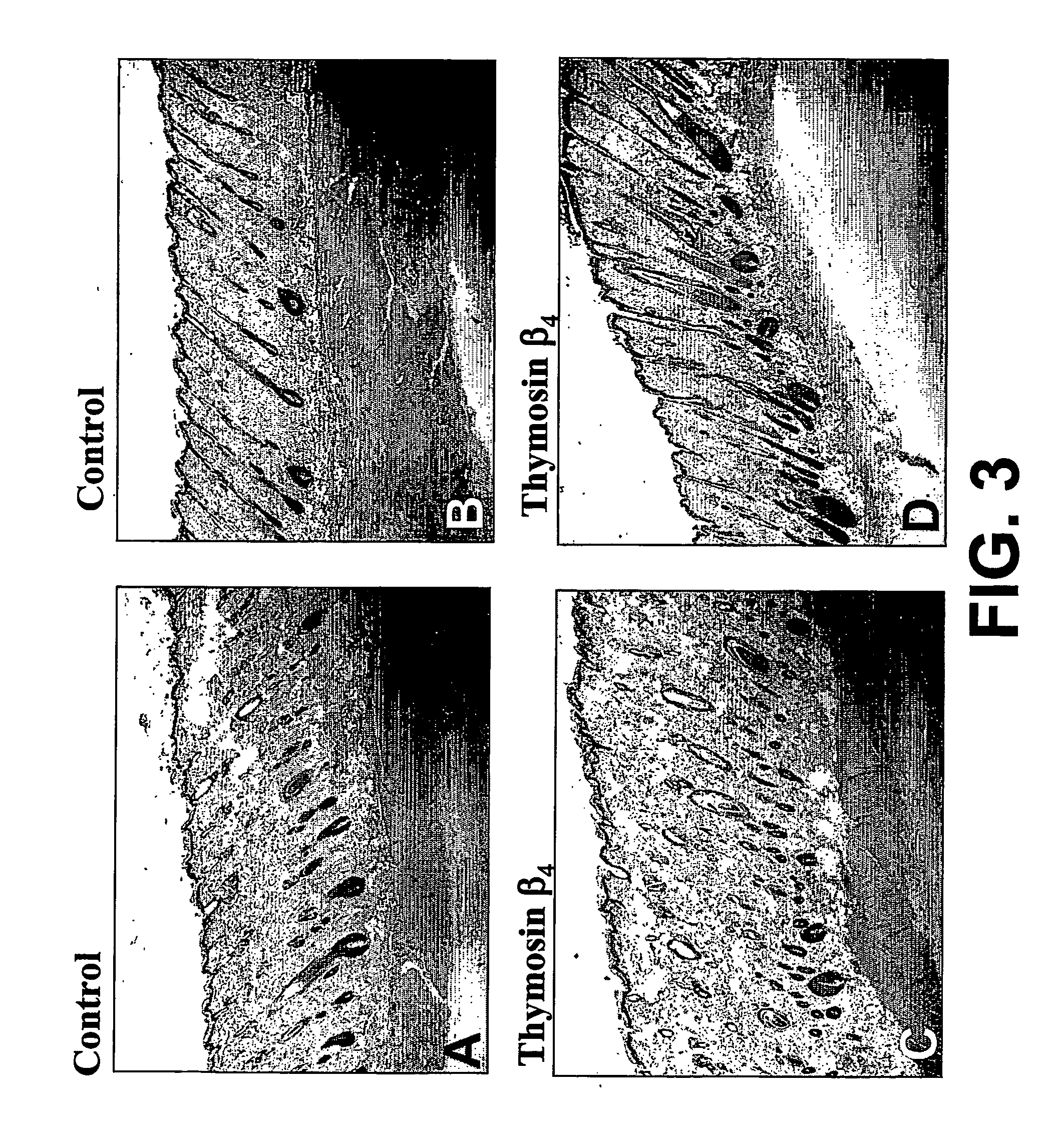
Treatment of skin, and wound repair, with thymosin beta 4
InactiveUS20070015698A1Promote wound healingPromote wound repairBiocideCosmetic preparationsDermatologyThymosin beta-4
Owner:REGENERX BIOPHARMACEUTICALS INC +1
Methods of treating disorders of the eye and surrounding tissue with thymosin beta4 (tb4) analogues, isoforms and other derivatives
InactiveUS20040131626A1Reduce dry eye syndromeSenses disorderPeptide/protein ingredientsDiseaseMyokine
Owner:REGENERX BIOPHARMACEUTICALS INC
Anti-inflammatory and wound healing effects of lymphoid thymosin beta-4
The invention relates to a method of treating inflammatory conditions in a subject comprising administering to a subject a composition comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant. The invention also provides a method of promoting wound healing in a subject comprising administering to the subject a composition comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant. The invention also relates to methods of treating the above mentioned conditions in a subject comprising administering to the subject a nucleic acid encoding a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4 polypeptide variant. The invention also relates to pharmaceutical compositions comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant, or salt thereof, and a pharmaceutically acceptable carrier.
Owner:KING'S COLLEGE LONDON +1
Treatment of skin, and wound repair, with thymosin beta 4
Owner:UNITED STATES OF AMERICA +1
Thymosin beta4 compositions
Owner:REGENERX BIOPHARMACEUTICALS INC +1
METHODS OF TREATING DISORDERS OF THE EYE AND SURROUNDING TISSUE WITH THYMOSIN BETA 4 (Tbeta4), ANALOGUES, ISOFORMS AND OTHER DERIVATIVES
InactiveUS20080096817A1Promoting reversal of and inhibiting eye degenerationHormone peptidesSenses disorderDiseaseOphthalmology
Owner:REGENERX BIOPHARMACEUTICALS INC
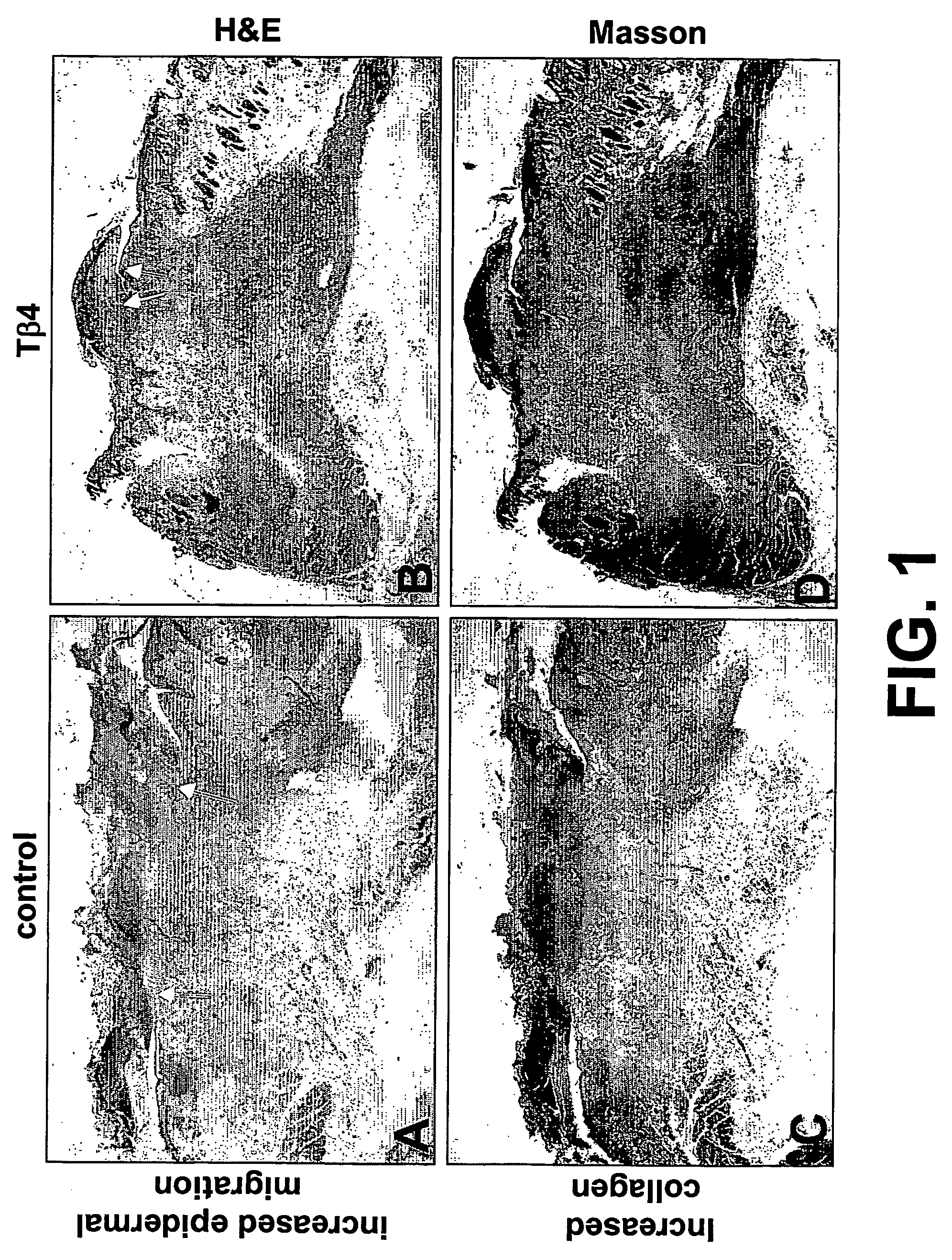
Compositions and methods for promoting wound healing and tissue repair
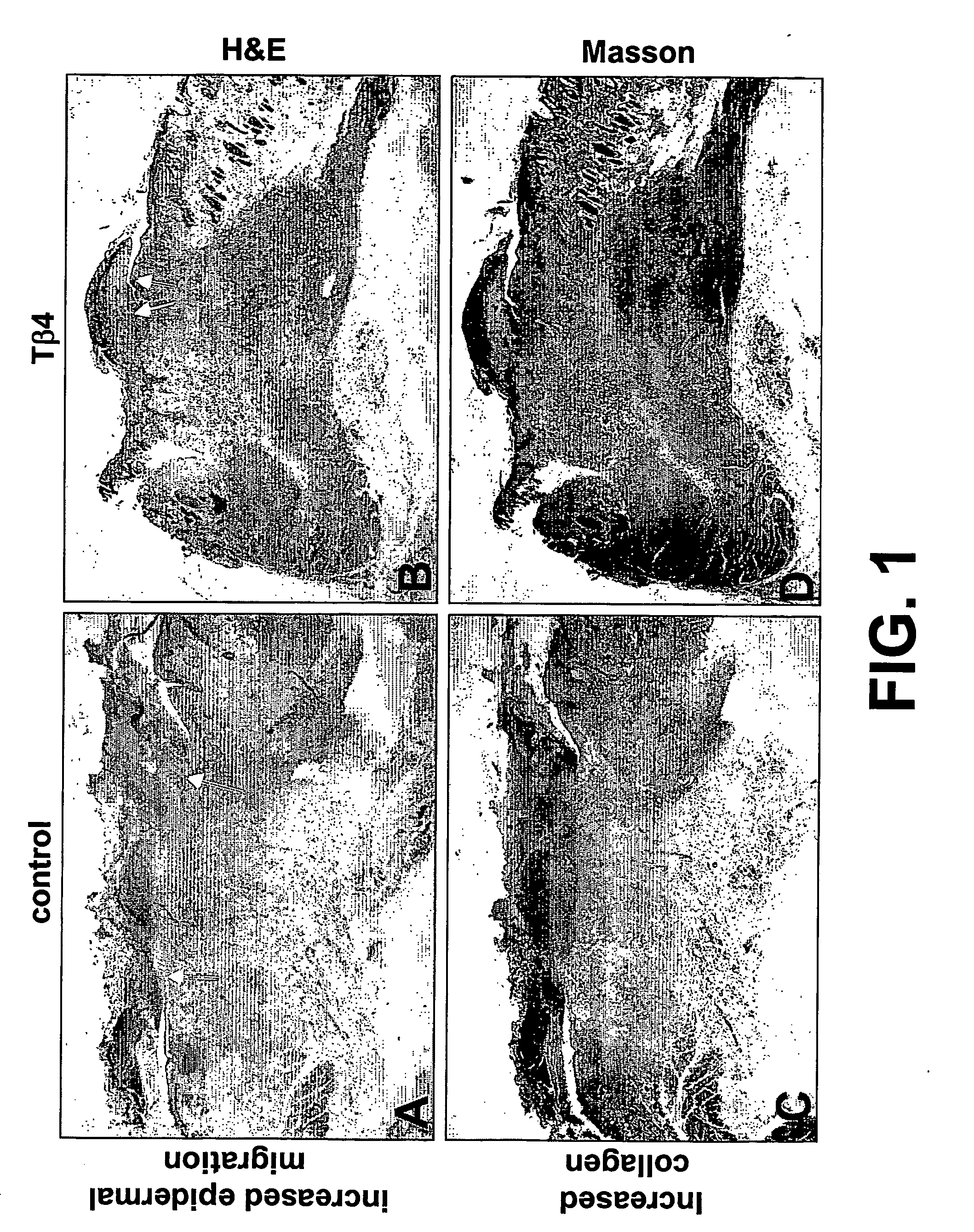
The present invention relates to methods for promoting tissue repair, angiogenesis and cell migration. The method of the invention utilizes thymosin β4 (Tβ4) peptide to promote tissue repair, angiogenesis and cell migration. The invention further relates to modulating Tβ4 activity in tissues.
Owner:UNITED STATES OF AMERICA +1
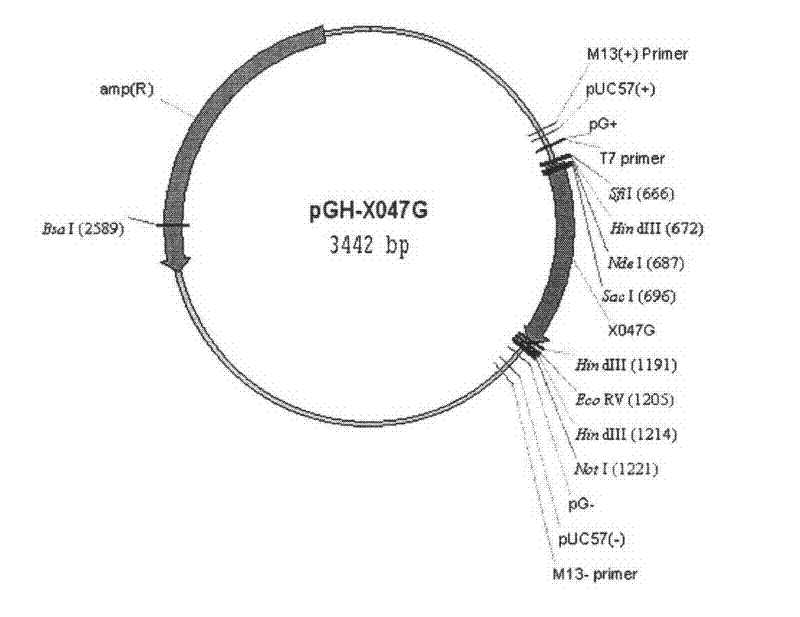
Recombinant thymosin beta 4 two repeat protein and preparation thereof
InactiveCN101434651ALow immunogenicityOvercoming the defect of unstable expressionThymopoietinsPeptide preparation methodsEscherichia coliMouse Lymphocyte
The invention relates to recombinant thymosin Beta 4 two-repeat protein and a preparation method thereof. Human thymosin Beta 4 two-repeat gene expression vector pET-22b(+)-T Beta (2) is constructed through recombinant human thymosin Beta 4 full length cDNA combined with PCR technology, human thymosin Beta 4 that can not be expressed directly in colibacillus is highly actively expressed in colibacillus in the form of two-repeat and purified human thymosin Beta 4 two-repeat protein has biologic activity, can promote multiplication of lymphocyte of mice, has low immunogenicity and lays a foundation for the further research and wide application of human thymosin Beta 4.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Stabilized external preparation comprising thymosin beta 4 as an active ingredient
ActiveUS20180280479A1MinimizationMaintain biological activityPeptide/protein ingredientsInorganic non-active ingredientsStable stateMedicine
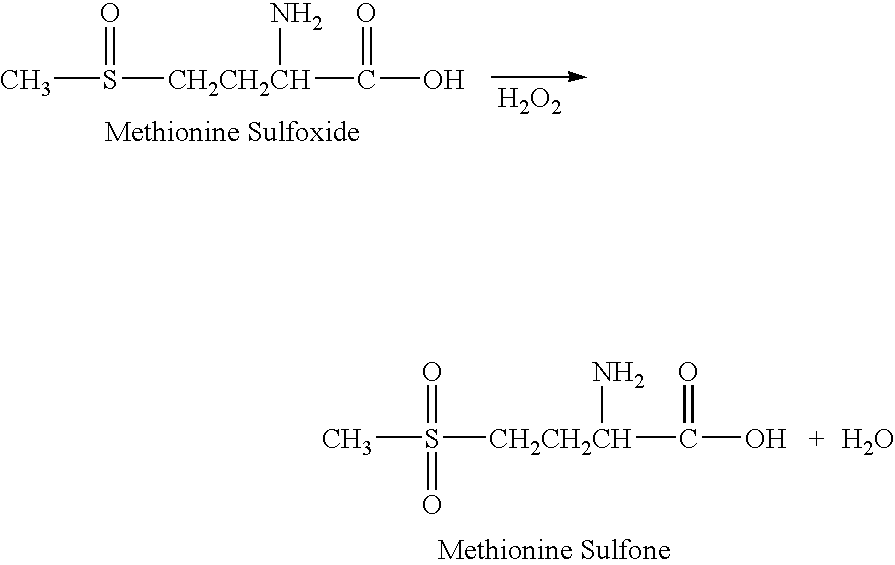
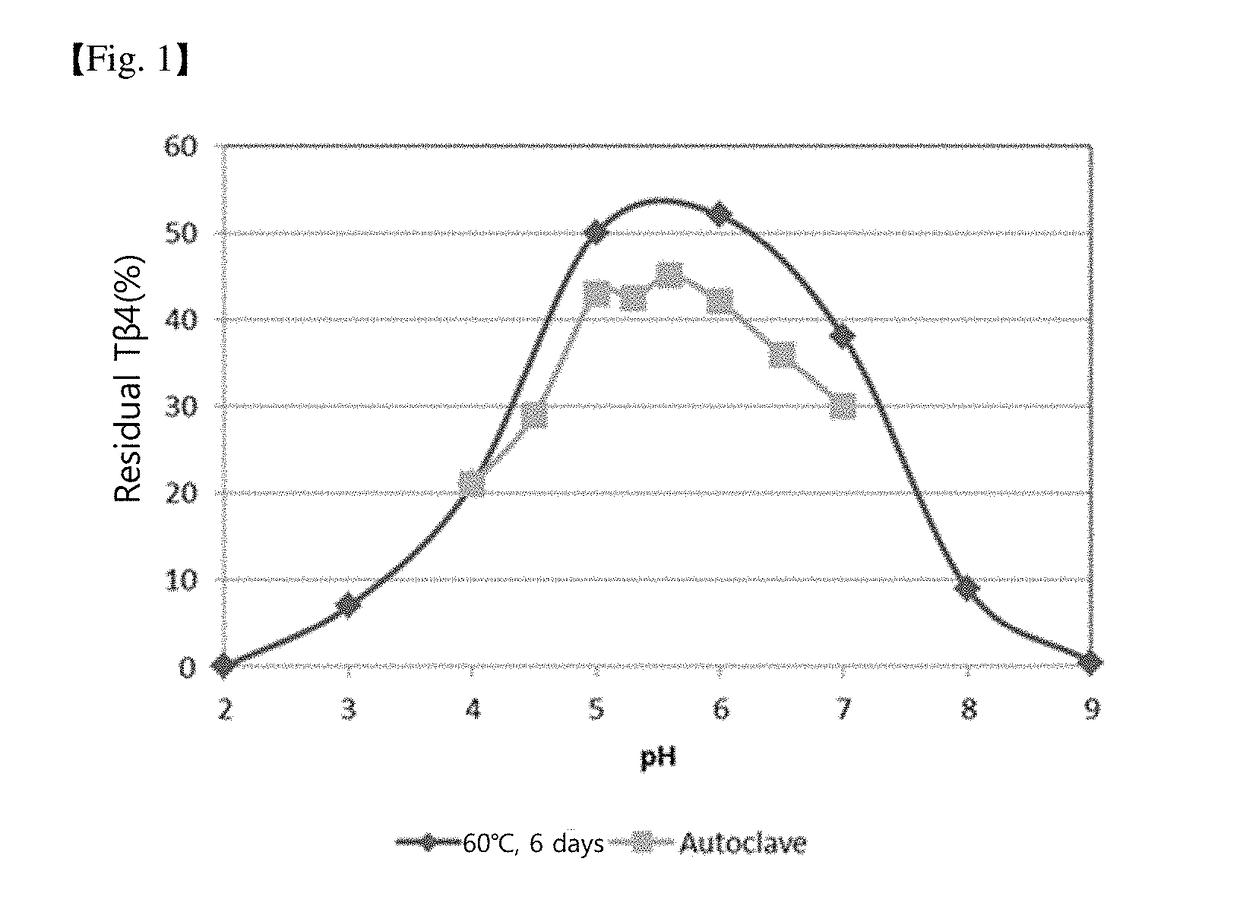
The present invention relates to a stabilized external preparation comprising thymosin beta 4 (Tβ4) as an active ingredient. More specifically, the present invention relates to a therapeutically effective external preparation with improved stability and biological activity of Tβ4. The preparation according to the present invention provides Tβ4 in a stable state by maintaining the biological activity of Tβ4 and minimizing the generation of Tβ4 sulfoxide through oxidization reactions and multimers through aggregation.
Owner:HLB THERAPEUTICS CO LTD
Medicine for treating eye diseases and composition of medicine
The invention provides application of a pharmaceutical composition containing recombinant human prothymosin beta4 in preparation of a medicine for treating eye diseases and particularly application in the treatment of xerophthalmia. The pharmaceutical composition is characterized in that the concentration of the recombinant human prothymosin beta4 is 1ug / mL-1000ug / mL. The invention further provides the pharmaceutical composition containing pharmaceutically acceptable carriers.
Owner:BEIJING NORTHLAND BIOTECH
Methods and compositions for the promotion of hair growth utilizing actin binding peptides
InactiveUS20050239697A1Promote growthHigh activityCosmetic preparationsHormone peptidesBinding peptideHair growth
Disclosed herein are methods and compositions suitable for the promotion of hair growth on humans and other animals. Disclosed embodiments include compositions comprising actin-binding peptides. In some embodiments, the actin-binding peptides comprise fragments of thymosin β4. In other embodiments, the disclosure provides compositions comprising fragments of thymosin β4 and / or other actin-binding peptides that are suitable for the treatment of alopecia and other conditions associated with hair loss. In still further embodiments, the disclosure provides compositions comprising the sequence of approximately six or seven amino acids of the thymosin β4 sequence that bind actin.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF +1
Method and special engineering bacteria for producing N-terminal acetyl protein or polypeptide
ActiveCN101979505AOvercoming the inability to acetylateOvercomes the disadvantage of only partial acetylationHormone peptidesBacteriaNucleotideAmino acid
The invention discloses a method and special engineering bacteria for producing N-terminal acetyl protein or polypeptide. The recombinant engineering bacteria capable of expressing transferase of archaebacteria on chromosomes are obtained by integrating the expression box of the transferase of archaebacteria onto the chromosomes of a host; the expression box for expressing the transferase of archaebacteria comprises a promoter and an archaebacteria transferase gene connected with the downstream of the promoter; and the nucleotide sequence of the archaebacteria transferase gene is represented by the sequence No.1 in a sequence list, and the amino acid sequence of the archaebacteria transferase gene is represented by a sequence No.2 in a sequence table. The engineering bacteria of the invention can directly produce complete N-terminal acetyl protein or polypeptide, such as thymosin extrasin alpha 1 and thymosin extrasin beta 4, the drawback of incompetence of acetylation or partial acetylation in a conventional genetic engineering technique is overcome, the production of N-terminal acetyl thymosin extrasin by the genetic engineering technique is realized completely, and the method and the special engineering bacteria are very practical.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
BIOMARKERS FOR IgA NEPHROPATHY AND APPLICATIONS THEREOF
InactiveUS20110183349A1Microbiological testing/measurementDisease diagnosisSecreted Phosphoprotein 1Butyrophilin
The present invention provides a method for diagnosis or prognosis of IgA nephropathy in a subject based on detection of the expression level of one or more biomarker genes selected from the group consisting of thymosin β4 (Tmsb4), serine or cysteine proteinase inhibitor clade E member 2 (Serpine2), secreted phosphoprotein 1 (OPN), butyrophilin-like-2 (BTNL2), S100 calcium binding protein A8 (S100A8), Cystatin C (CysC), and any combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Oral nanoparticle polypeptide composition tablets and preparation method thereof
The invention relates to a medicinal preparation and a preparation method thereof, and in particular relates to a series of polypeptide thymopentin, thymalfasin and thymosin beta4 combined enzyme inhibitors capable of improving the immunity. An oral preparation is prepared by adopting a nanoparticle technology. A pharmaceutically-acceptable biodegradable high polymer material is coated with a polypeptide medicament for improving the immunity to prepare nanoparticles, and tablets are made by tableting. The medicament-loading nanoparticles prepared by using the preparation method have high bioavailability.
Owner:深圳市健翔生物制药有限公司
Thymosin beta4 derivative and itsuse
The invention relates to one section of thymosin beta 4 derivatives with high bioactivity which can be used to repair cornea, skin, and heart injury.
Owner:BEIJING NORTHLAND BIOTECH
Methos of healing or preventing inflammation, damage and other changes that occur prior to, during or immediately after a myocardial event with thymosin beta 4, analogoues, isoforms and other derivatives
ActiveUS20040258680A1Promote healing preventionLower Level RequirementsHormone peptidesPeptide/protein ingredientsCardiac muscleAngiogenesis growth factor
Inflammation or damage associated with myocardial events is treated or prevented by administration of an angiogenesis-inducing, anti-inflammatory peptide such as Thymosin beta4, an isoform of Thymosin beta4 or oxidized Thymosin beta4.
Owner:REGENERX BIOPHARMACEUTICALS INC
Immunomodulator polypeptide slow-release microsphere preparation and preparation method thereof
InactiveCN103961320AImprove adaptabilityEasy to acceptPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectMicrosphere
The invention belongs to the field of medical preparations, and relates to an immunomodulator polypeptide slow-release microsphere preparation and a preparation method thereof. Particularly, the immunomodulator polypeptide comprises thymopentin, thymalfasin and thymosin beta4. The slow-release microsphere comprises 0.1-30% (w / w) of immunomodulator polypeptide, 60-80% of biodegradable high polymer material with biocompatibility, of which the molecular weight is 5,000-200,000 Dalton, and 0.1-20% of other pharmaceutical acceptable auxiliary materials on the basis of the total weight of the microsphere. According to the slow-release microsphere disclosed by the invention, the mean grain size is 5-60mu m; the encapsulation efficiency is greater than 90%; the slow release period of the slow-release microsphere can be up to a few days and months, the medication times is obviously reduced, the bioavailability is improved, the toxic and side effects of the medicine are reduced, and clinical treatment is facilitated. The product is good in production process repeatability and good in feasibility.
Owner:SHENZHEN JYMED TECH
Methods for improving neurological outcome after neural injury and neurodegenerative disease
ActiveUS20120021992A1Promote resultsEasy to migrateNervous disorderPeptide/protein ingredientsProgenitorDisease
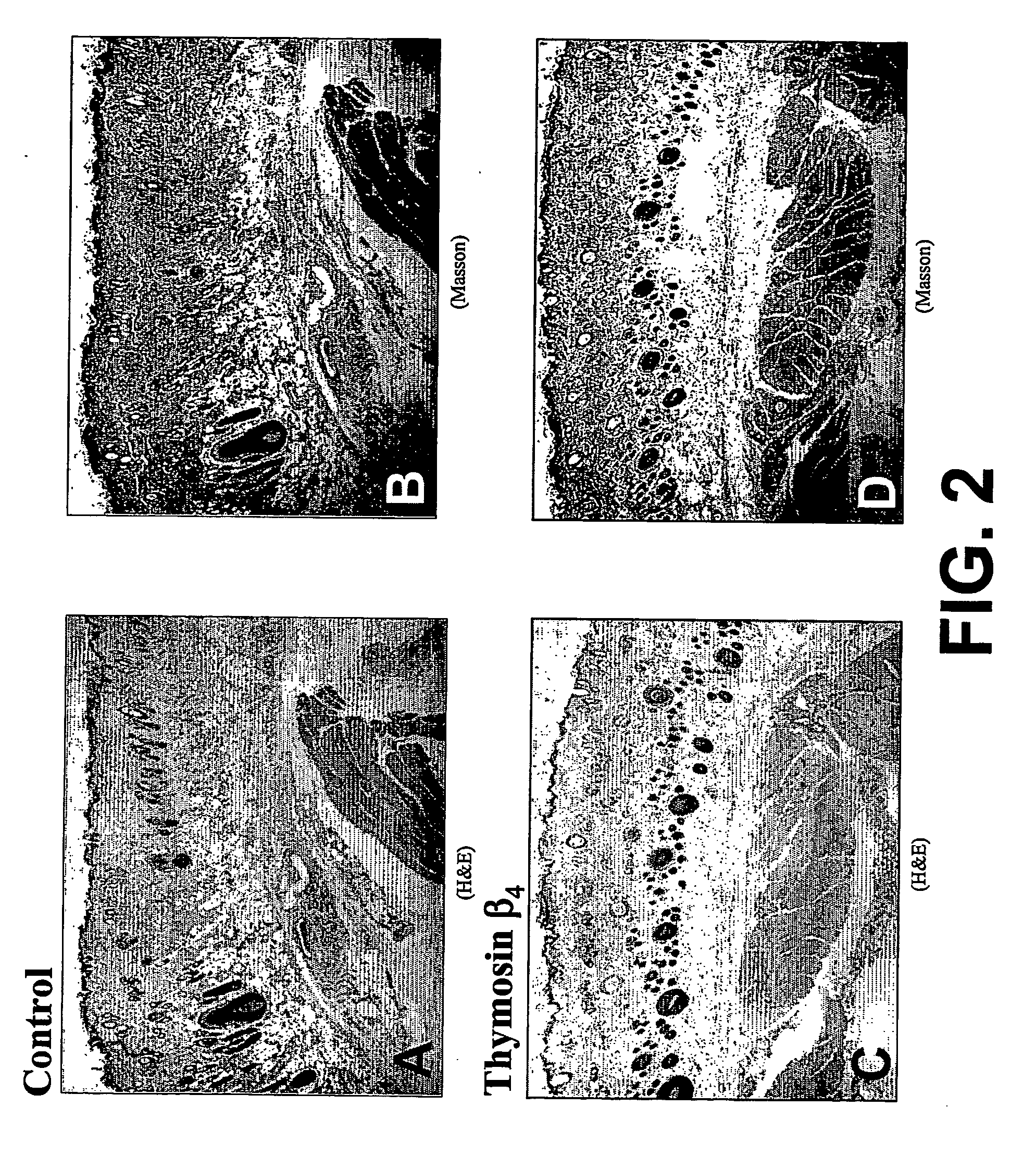
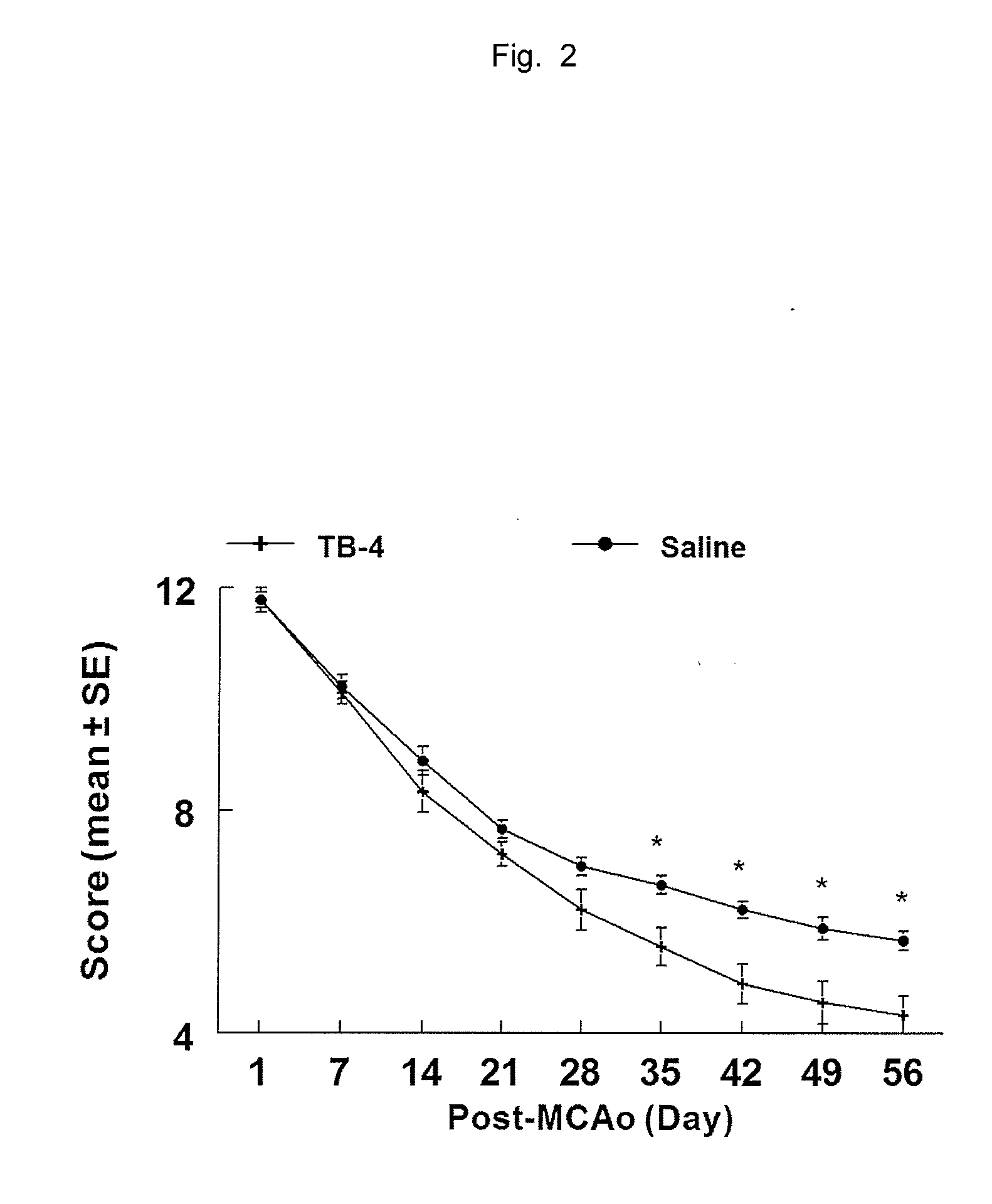
Thymosin β4 can be used to treat neuronal and brain injuries that are accompanied by neuronal cell death or injury, including injuries caused by stroke or trauma and injuries caused by neurological and neurodegenerative disease. In particular, stroke and multiple sclerosis are examples of conditions which may be ameliorated by treatment with thymosin β4. Thymosin β4 has been found to restore neurological tissue through several effects on several neurological parameters which are improved by administration of thymosin β4 to a subject in need of neurological tissue restoration. For example, thymosin β4 improves axonal myelination, migration of neural progenitor cells, neural progenitor cell proliferation, differentiation of neural progenitor cells into mature neurons, differentiation of neural progenitor cells into mature glia, nerve regeneration, and brain remodeling at locations of brain injury.
Owner:HENRY FORD HEALTH SYST
Methods of treating disorders of the eye and surrounding tissue with thymosin beta 4 (TB4), analogues, isoforms and other derivatives
InactiveUS20110020449A1Reduce decreaseDecrease corneal stromal edemaPowder deliverySenses disorderDiseaseIrritation
Pain or irritation of the eyes, caused by injury due to dry eye syndrome, chemical burns or the like can be accompanied by corneal stromal edema. It has been discovered that administration of thymosin β4 and / or oxidized thymosin β4 to cornea in need of treatment of corneal stromal edema is a useful treatment for decreasing such corneal stromal edema.
Owner:REGENERX BIOPHARMACEUTICALS INC
Thymosin beta4 gene expression inhibiting RNA and use thereof
InactiveCN101591657APrevent invasionPrevent proliferationMicroorganismsGenetic material ingredientsSingle-Stranded RNAFhit gene
The invention discloses a thymosin beta4 gene expression inhibiting RNA and use thereof. The shRNA provided by the invention is a single-chain RNA having a stem-and-loop structure consisting of a stem I, a loop and a stem II; the sequence of the stem I is represented by the sequence 1 in a sequence table; and the sequence of the stem II is represented by the sequence 2 in the sequence table. The RNA of the invention can be used to construct recombinant plasmids and recombinant lentiviruses and obviously inhibit the invasion by tumor cells, the propagation of tumor cells and the expression of the thymosin beta4 gene in cells, thereby having medicinal and pharmaceutical applications and profound significance.
Owner:PEKING UNIV THIRD HOSPITAL
Recombinant thymosin beta 4 two repeat protein and preparation thereof
InactiveCN101434651BLow immunogenicityOvercoming the defect of unstable expressionThymopoietinsPeptide preparation methodsEscherichia coliMouse Lymphocyte
The invention relates to recombinant thymosin Beta 4 two-repeat protein and a preparation method thereof. Human thymosin Beta 4 two-repeat gene expression vector pET-22b(+)-T Beta (2) is constructed through recombinant human thymosin Beta 4 full length cDNA combined with PCR technology, human thymosin Beta 4 that can not be expressed directly in colibacillus is highly actively expressed in colibacillus in the form of two-repeat and purified human thymosin Beta 4 two-repeat protein has biologic activity, can promote multiplication of lymphocyte of mice, has low immunogenicity and lays a foundation for the further research and wide application of human thymosin Beta 4.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Treatment or prevention of damage due to radiation exposure.
A method of treatment or prevention of damage due to ionizing radiation exposure involves administering to a subject in need of such treatment an effective amount of a composition containing 1) a compound including a radiation damage-inhibiting polypeptide containing amino acid sequence LKKTET (such as Thymosin beta4), a conservative variant of LKKTET, an actin-sequestering agent, an anti-inflammatory agent; 2) an agent which stimulates production of the compound in the subject; 3) an agent which regulates the compound in the subject; or 4) an antagonist of the compound, so as to inhibit radiation damage in the subject.
Owner:REGENERX BIOPHARMACEUTICALS INC
Method and special engineering bacteria for producing N-terminal acetyl protein or polypeptide
The invention discloses a method and special engineering bacteria for producing N-terminal acetyl protein or polypeptide. The recombinant engineering bacteria capable of expressing transferase of archaebacteria on chromosomes are obtained by integrating the expression box of the transferase of archaebacteria onto the chromosomes of a host; the expression box for expressing the transferase of archaebacteria comprises a promoter and an archaebacteria transferase gene connected with the downstream of the promoter; and the nucleotide sequence of the archaebacteria transferase gene is represented by the sequence No.1 in a sequence list, and the amino acid sequence of the archaebacteria transferase gene is represented by a sequence No.2 in a sequence table. The engineering bacteria of the invention can directly produce complete N-terminal acetyl protein or polypeptide, such as thymosin extrasin alpha 1 and thymosin extrasin beta 4, the drawback of incompetence of acetylation or partial acetylation in a conventional genetic engineering technique is overcome, the production of N-terminal acetyl thymosin extrasin by the genetic engineering technique is realized completely, and the method and the special engineering bacteria are very practical.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Preparation method of gene-recombination human thymosin beta 4
ActiveCN102660550AAvoid problemsHigh molecular weightHormone peptidesPeptide preparation methodsPichia pastorisCollagen i
The invention discloses a preparation method of gene-recombination human thymosin beta 4. The preparation method comprises the following steps of: acquisition of genes, connection of a carrier p PIC (positive-impedance converter) 9k and human-like collagen I and human thymosin beta 4 through pichia pastoris electrotransformation, selection of multi-copy insertion recombinants, fermentation of fusion protein of the gene-recombination human thymosin beta 4 with the pichia pastoris, and purification of the gene-recombination human thymosin beta 4. According to the method, the characteristic of high expression of the human-like collagen I in the pichia pastoris is utilized to guide the stable and efficient expression of the human thymosin beta 4 in the pichia pastoris. As enterokinase cuttingsites are introduced between leading peptide of the human-like collagen I of the fusion protein and the human thymosin beta 4, the problems, caused by small molecular weight, of the human thymosin beta 4 in the process of expression and purification are solved, the expression index is increased, the purification procedure is simplified, and the extraction and purification efficiency of the product is improved. The preparation method can be used for preparing the gene-recombination human thymosin beta 4.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Methods of Treating or Preventing Tissue Damage Caused by Increased Blood Flow
InactiveUS20090169538A1Avoid tissue damageNervous disorderPeptide/protein ingredientsBinding peptideActinin binding
A method of treating or preventing tissue damage occurring subsequent to affecting an increase in blood flow through a blood vessel which is in communication with the tissue, by administering an effective amount of a composition including a tissue damage-reducing or -preventing polypeptide including at least one of Thymosin beta 4 (TB4), an isoform of TB4, an N-terminal fragment of TB4, a C-terminal fragment of TB4, TB4 sulfoxide, an LKKTET [SEQ ID NO: 1] peptide, an LKKTNT [SEQ ID NO: 2] peptide, an actin-sequestering peptide, an actin binding peptide, an actin-mobilizing peptide, an actin polymerization-modulating peptide, or a conservative variant thereof having tissue damage-reducing activity. The composition is administered to the tissue before, during and / or after affecting the increase in blood flow.
Owner:REGENERX BIOPHARMACEUTICALS INC
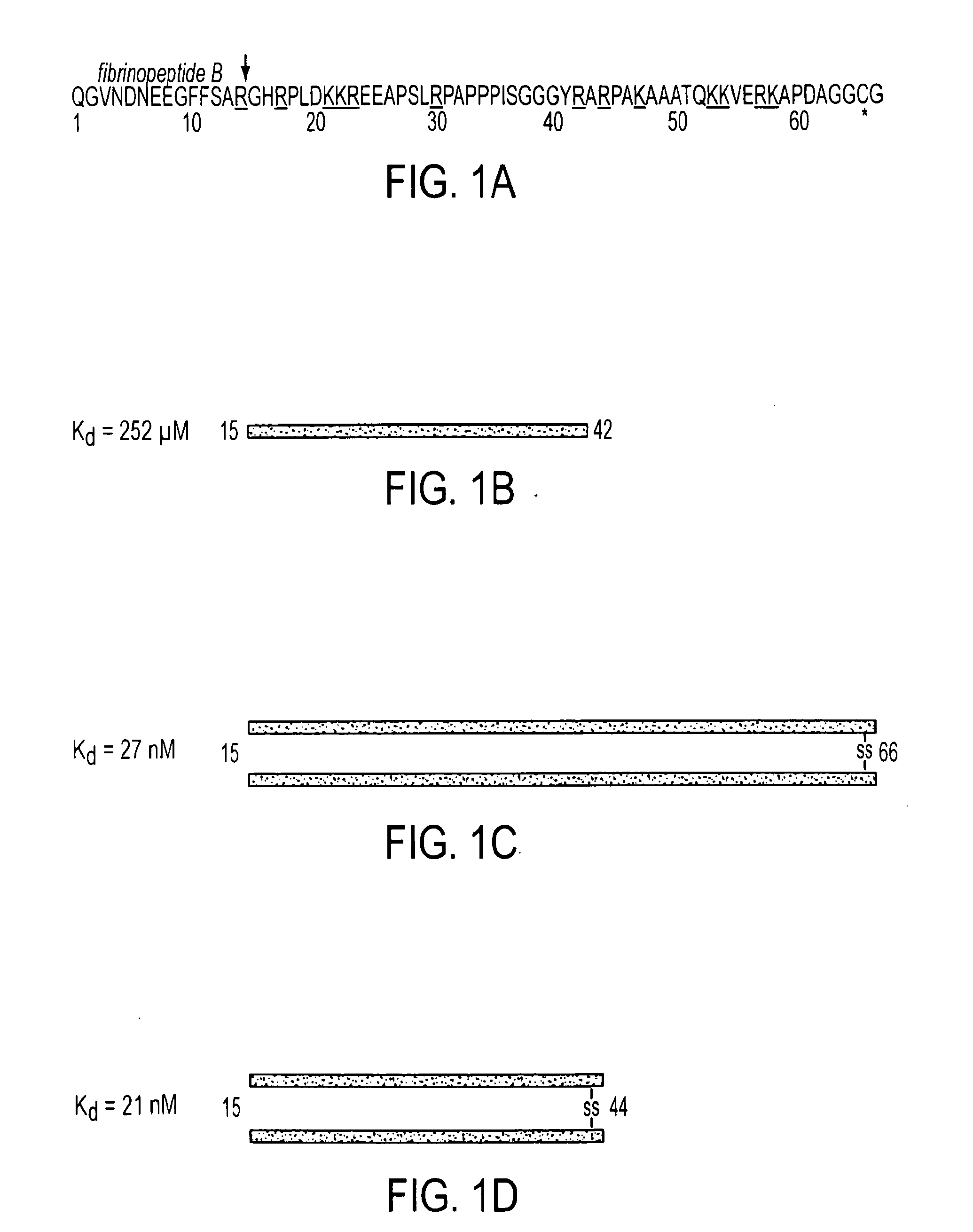
Compositions and Methods Utilizing Fibrin Beta Chain Fragments
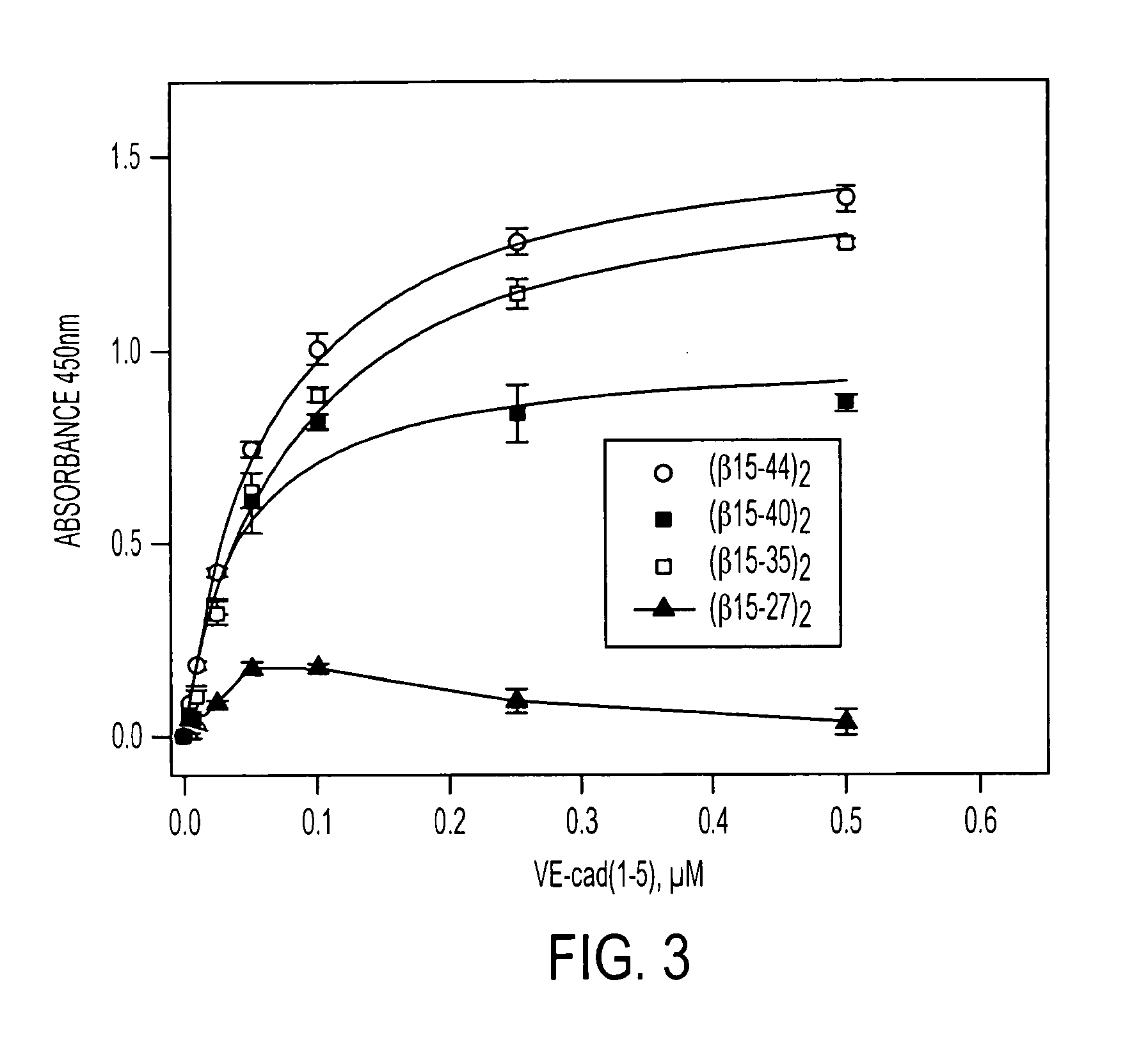
A composition including a peptide sequence of the formula βX1-X2, the peptide sequence corresponding to an amino acid sequence of a fibrin beta chain fragment of a Bbeta chain of fibrinogen, wherein X1 represents an N-terminal end of the peptide sequence, and X2 represents a C-terminal end of the peptide sequence, wherein the peptide sequence includes additional amino acids between X1 and X2, wherein the peptide sequence may contain a non-naturally occurring amino acid residue, wherein the peptide sequence is other than a wild-type β15-42 monomer sequence per se, and wherein the peptide sequence is other than (β15-66)2 dimer having two chains with each chain limited to wild type amino acids β15-65 and each chain further including a non-naturally occurring Gly at position 66 of each chain. Methods for treatment and pharmaceutical combinations may include a polypeptide agent such as Thymosin beta 4. In such methods and combinations, a dimer of the peptide sequence may include amino acids 15-66 of the fibrin beta chain.
Owner:UNIV OF MARYLAND BALTIMORE
Method for pyrolysis of thymosin beta-4
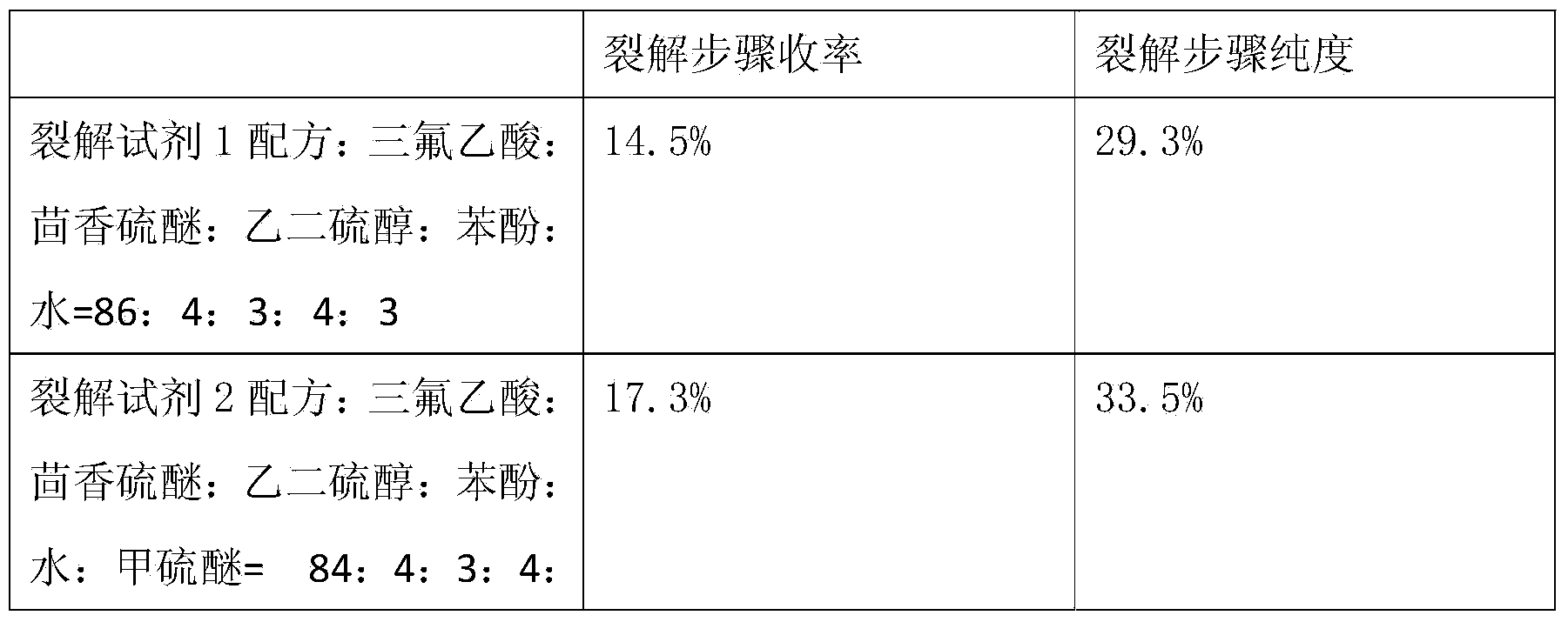
ActiveCN103724422AEfficient removalPrevent oxidationThymosin peptidesPeptide preparation methodsSolventPhenol
The invention discloses a method for pyrolysis of thymosin beta-4. The method comprises the step of carrying out pyrolysis on thymosin beta-4 peptide resin to obtain crude peptide, wherein a mixed solvent of trifluoroacetic acid, thioanisole, dithioglycol, phenol, water, dimethyl sulfide and ammonium iodide is used as a pyrolysis reagent in the pyrolysis process. According to the method for pyrolysis of thymosin beta-4, the purity of crude peptide is greatly improved, meanwhile the total yield is greatly increased, the production cost is reduced, and the method is simple in process and convenient for industrial production.
Owner:哈尔滨吉象隆生物技术有限公司
Sustained-release microsphere preparation of goserelin composition
InactiveCN104436169AHigh acceptanceReduce dosing frequencyPeptide/protein ingredientsGranular deliverySide effectMedicine
The invention belongs to the field of a pharmaceutical preparation, and relates to a sustained-release microsphere preparation of a goserelin composition and a preparation method of the sustained-release microsphere preparation. Specifically, the goserelin composition includes goserelin and a polypeptide composition capable of enhancing immunity, wherein the polypeptide capable of enhancing immunity comprises thymalfasin, thymopentin and thymosin beta4. The sustained-release microsphere consists of 0.1-40% (w / w) of goserelin and polypeptide capable of enhancing immunity in terms of the total weight of the microsphere, 60-99.9% of a biodegradable and biocompatible high polymer material which is 5,000-200,000Dalton in molecular weight in terms of the weight of the microsphere, and 0-10% of other pharmaceutically acceptable accessories in terms of the weight of the microsphere. The sustained-release microsphere disclosed by the invention is 5-20microns in average grain size and encapsulation efficiency is more than 80%. The sustained-release duration of the sustained-release microsphere can last for several days or several months, so that administration frequency is obviously reduced, bioavailability is improved, the toxic and side effects of medicine are reduced, and the sustained-release microsphere is conducive to clinic treatment. The production process of the finished product is good in reproducibility and good in feasibility.
Owner:SHENZHEN JYMED TECH
Methods and compositions for the promotion of a hair growth utilizing actin binding peptides
InactiveUS7563766B2Promote growthHigh activityHormone peptidesCosmetic preparationsBinding peptideHair growth
Disclosed herein are methods and compositions suitable for the promotion of hair growth on humans and other animals. Disclosed embodiments include compositions comprising actin-binding peptides. In some embodiments, the actin-binding peptides comprise fragments of thymosin β4. In other embodiments, the disclosure provides compositions comprising fragments of thymosin β4 and / or other actin-binding peptides that are suitable for the treatment of alopecia and other conditions associated with hair loss. In still further embodiments, the disclosure provides compositions comprising the sequence of approximately six or seven amino acids of the thymosin β4 sequence that bind actin.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com