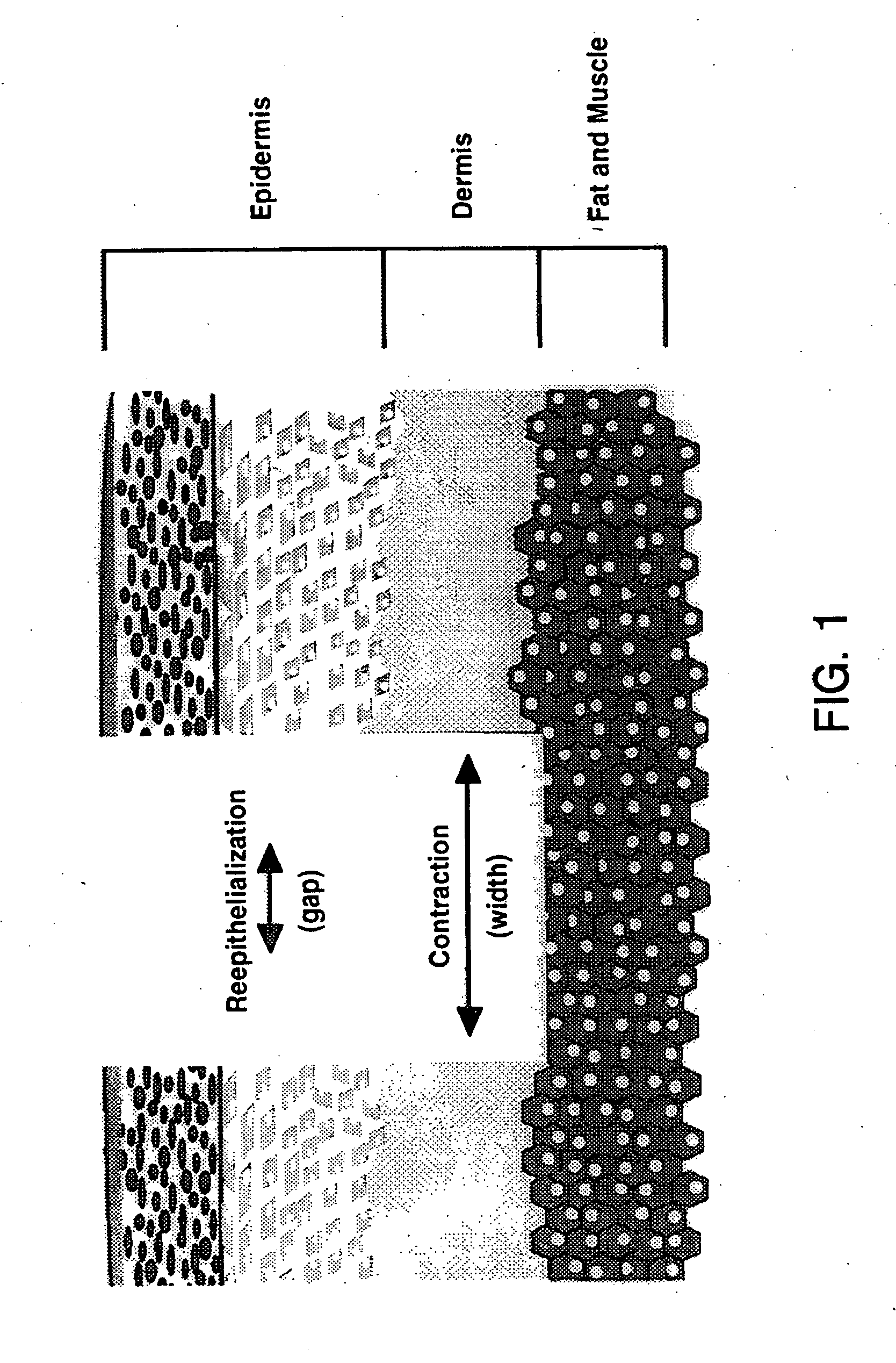
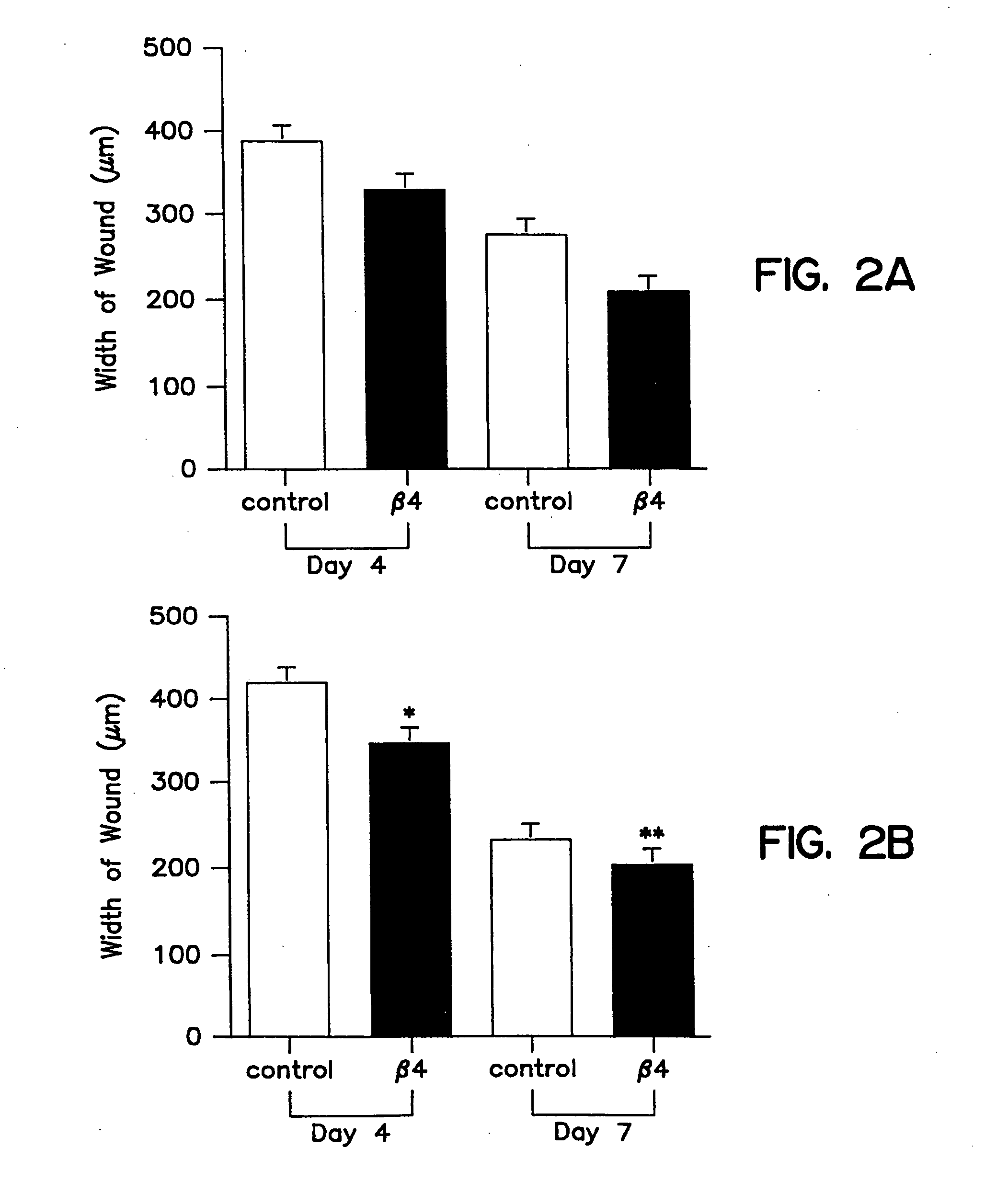
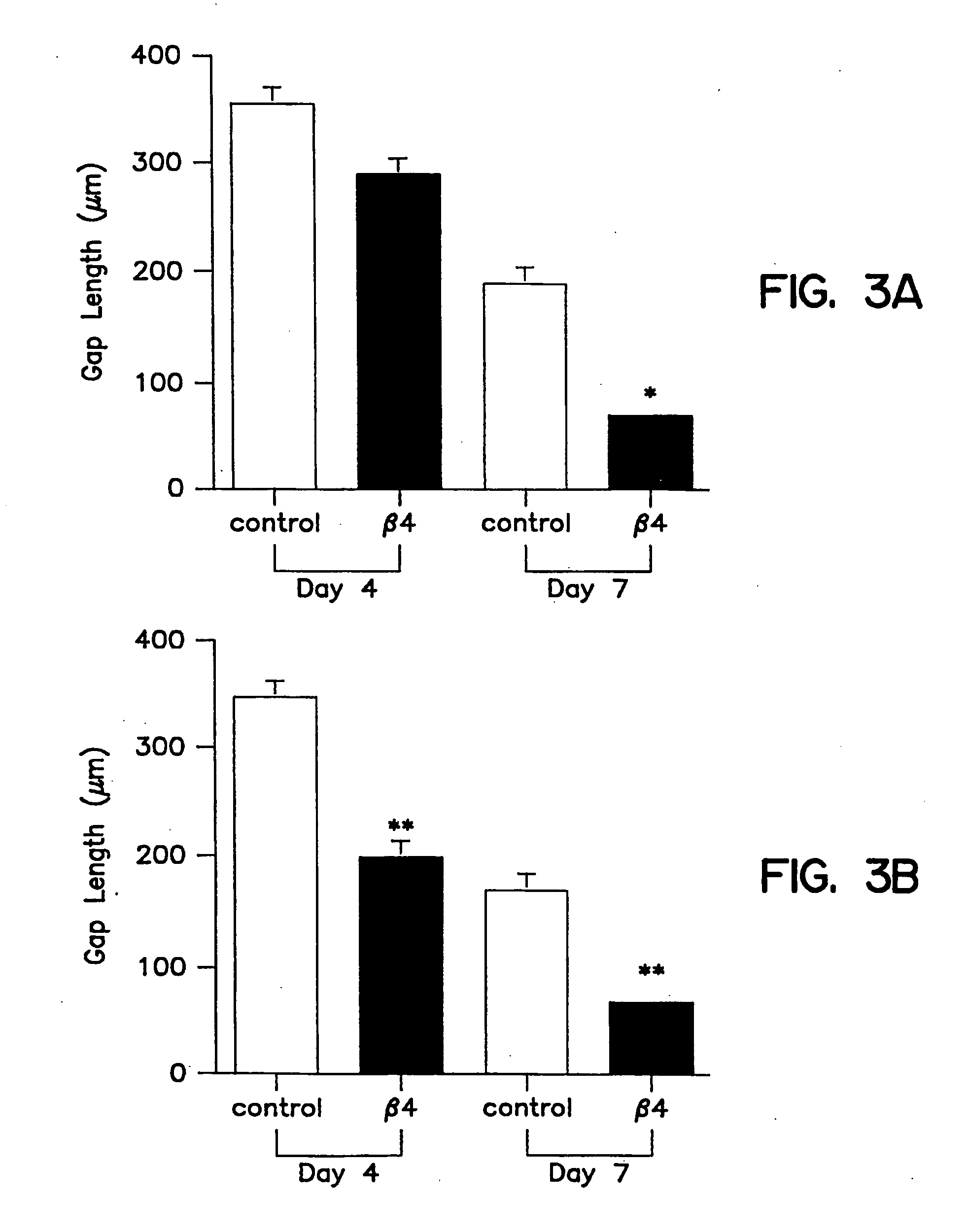
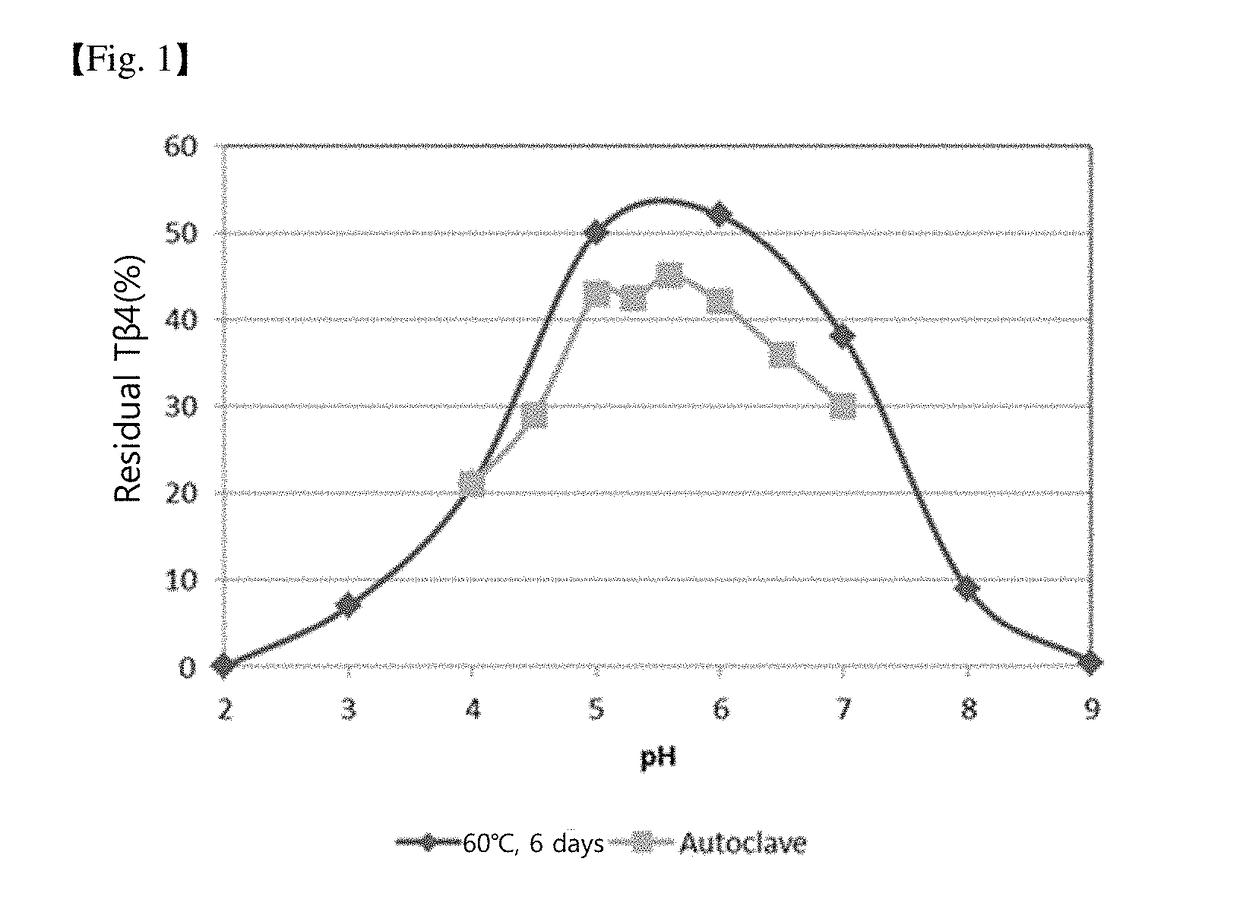
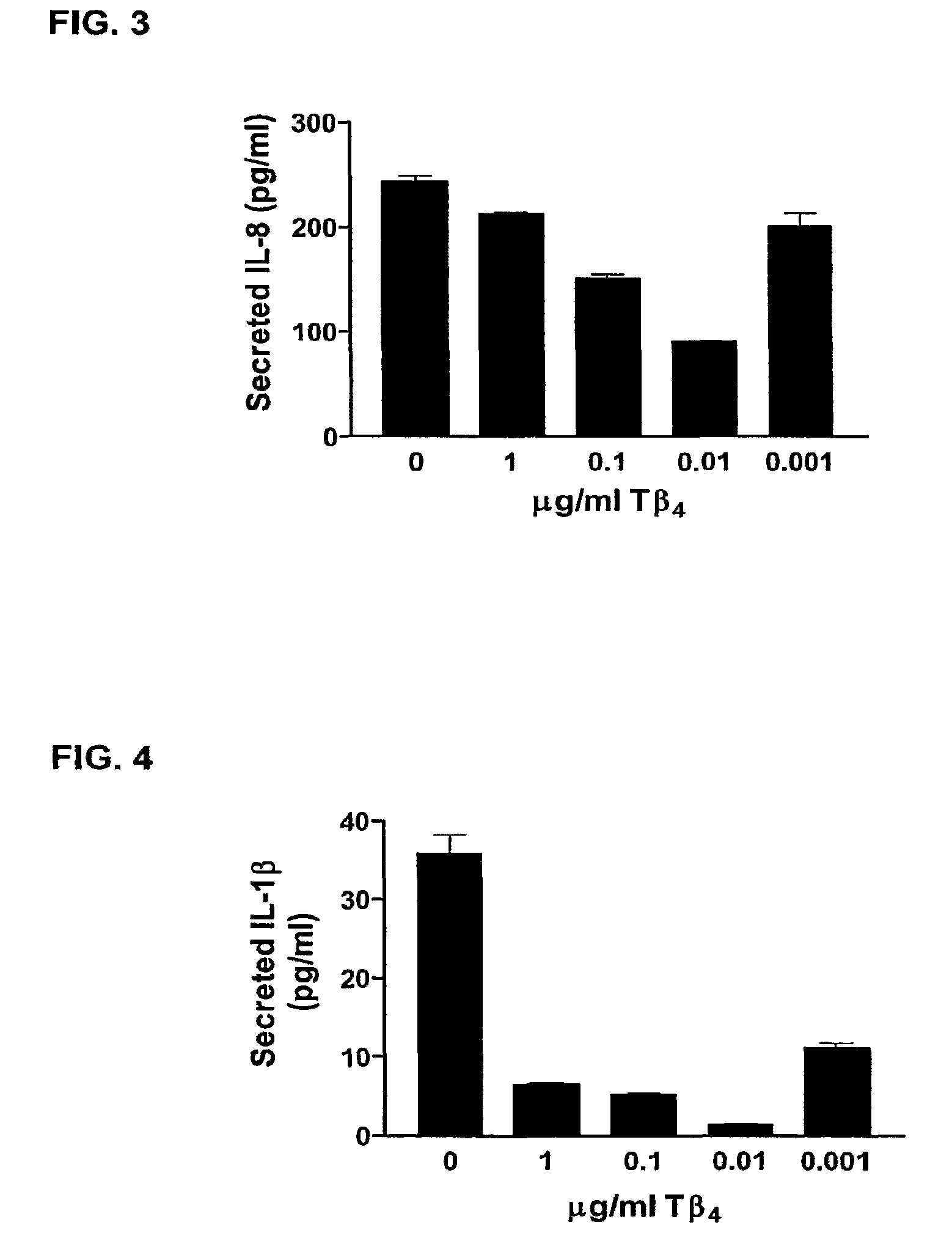
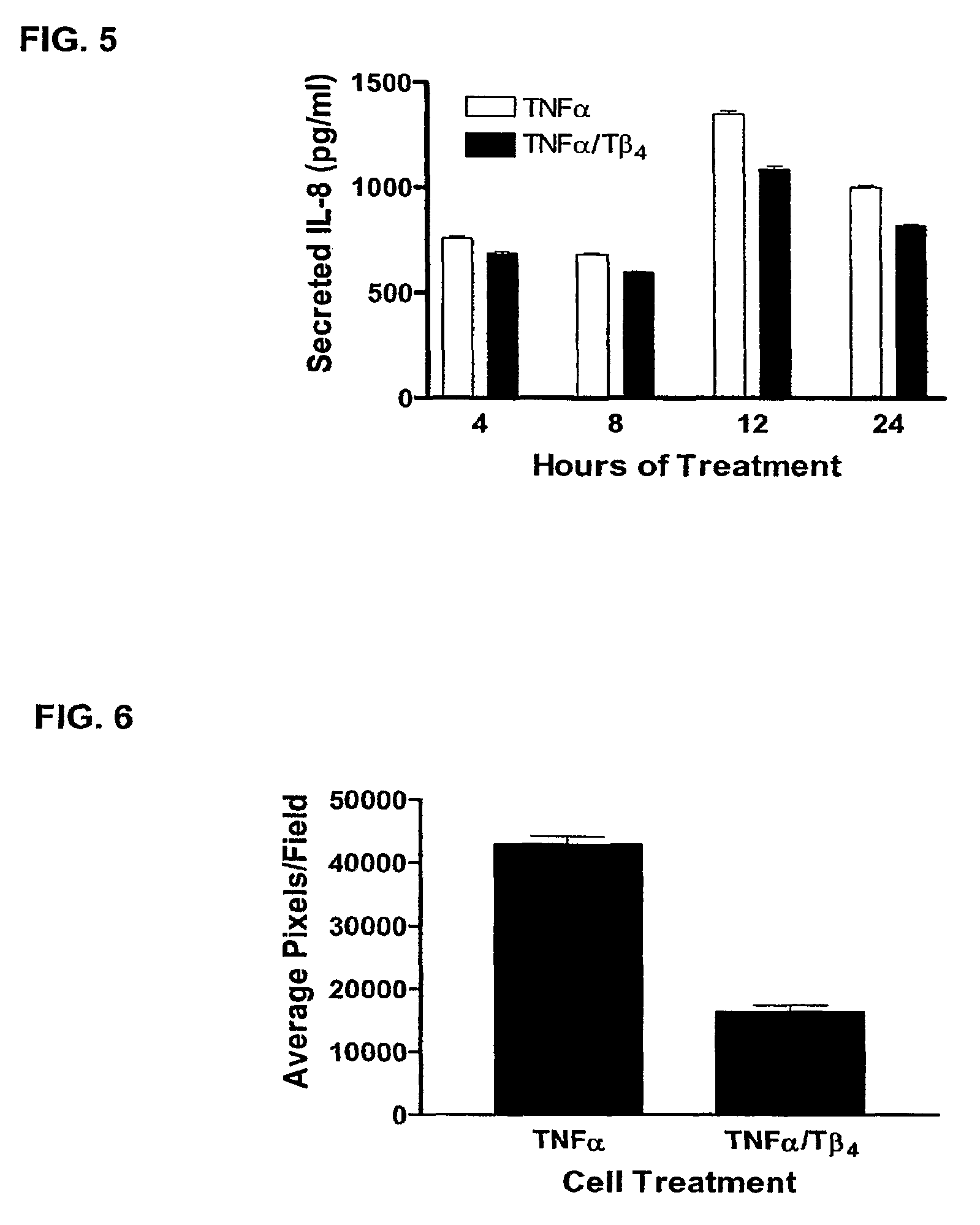
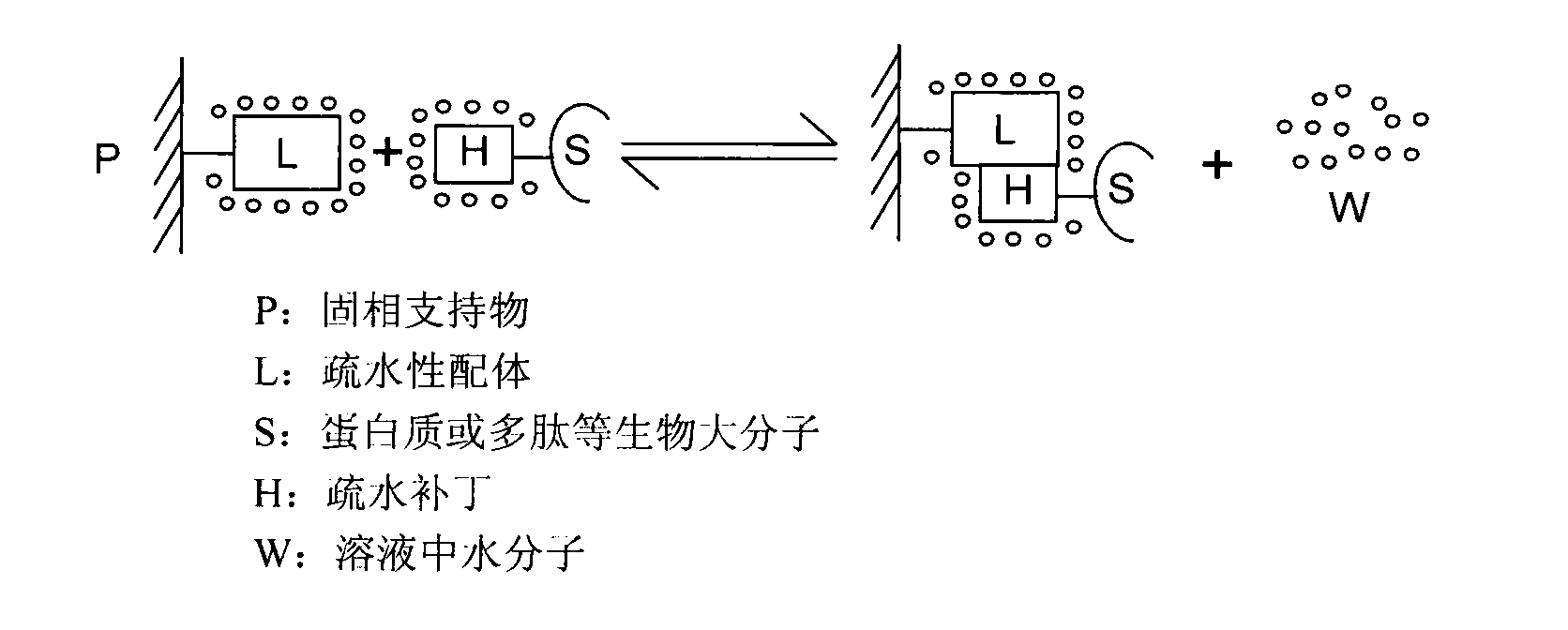
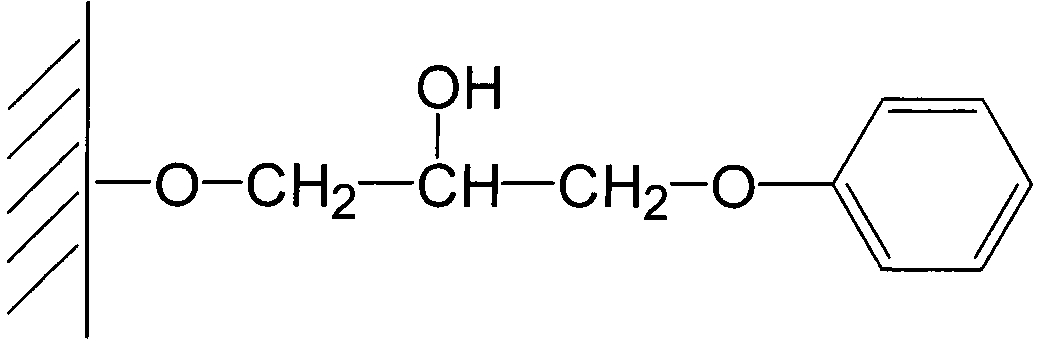
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Thymosin beta-4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thymosin beta-4 is a protein that in humans is encoded by the TMSB4X gene. Recommended INN (International Nonproprietary Name) for thymosin beta-4 is 'timbetasin', as published by the World Health Organization (WHO).
Treatment of skin, and wound repair, with thymosin beta 4
InactiveUS20070015698A1Promote wound healingPromote wound repairBiocideCosmetic preparationsDermatologyThymosin beta-4
Owner:REGENERX BIOPHARMACEUTICALS INC +1
Anti-inflammatory and wound healing effects of lymphoid thymosin beta-4
The invention relates to a method of treating inflammatory conditions in a subject comprising administering to a subject a composition comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant. The invention also provides a method of promoting wound healing in a subject comprising administering to the subject a composition comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant. The invention also relates to methods of treating the above mentioned conditions in a subject comprising administering to the subject a nucleic acid encoding a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4 polypeptide variant. The invention also relates to pharmaceutical compositions comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant, or salt thereof, and a pharmaceutically acceptable carrier.
Owner:KING'S COLLEGE LONDON +1
Treatment of skin, and wound repair, with thymosin beta 4
Owner:UNITED STATES OF AMERICA +1
METHODS OF TREATING DISORDERS OF THE EYE AND SURROUNDING TISSUE WITH THYMOSIN BETA 4 (Tbeta4), ANALOGUES, ISOFORMS AND OTHER DERIVATIVES
InactiveUS20080096817A1Promoting reversal of and inhibiting eye degenerationHormone peptidesSenses disorderDiseaseOphthalmology
Owner:REGENERX BIOPHARMACEUTICALS INC
Recombinant thymosin beta 4 two repeat protein and preparation thereof
InactiveCN101434651ALow immunogenicityOvercoming the defect of unstable expressionThymopoietinsPeptide preparation methodsEscherichia coliMouse Lymphocyte
The invention relates to recombinant thymosin Beta 4 two-repeat protein and a preparation method thereof. Human thymosin Beta 4 two-repeat gene expression vector pET-22b(+)-T Beta (2) is constructed through recombinant human thymosin Beta 4 full length cDNA combined with PCR technology, human thymosin Beta 4 that can not be expressed directly in colibacillus is highly actively expressed in colibacillus in the form of two-repeat and purified human thymosin Beta 4 two-repeat protein has biologic activity, can promote multiplication of lymphocyte of mice, has low immunogenicity and lays a foundation for the further research and wide application of human thymosin Beta 4.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Stabilized external preparation comprising thymosin beta 4 as an active ingredient
ActiveUS20180280479A1MinimizationMaintain biological activityPeptide/protein ingredientsInorganic non-active ingredientsStable stateMedicine
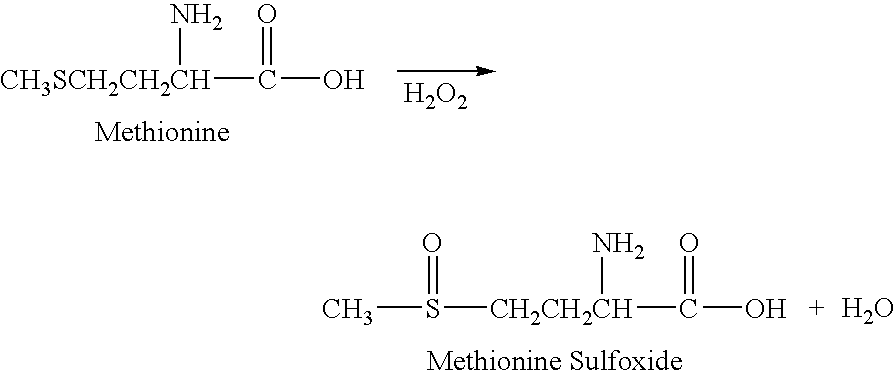
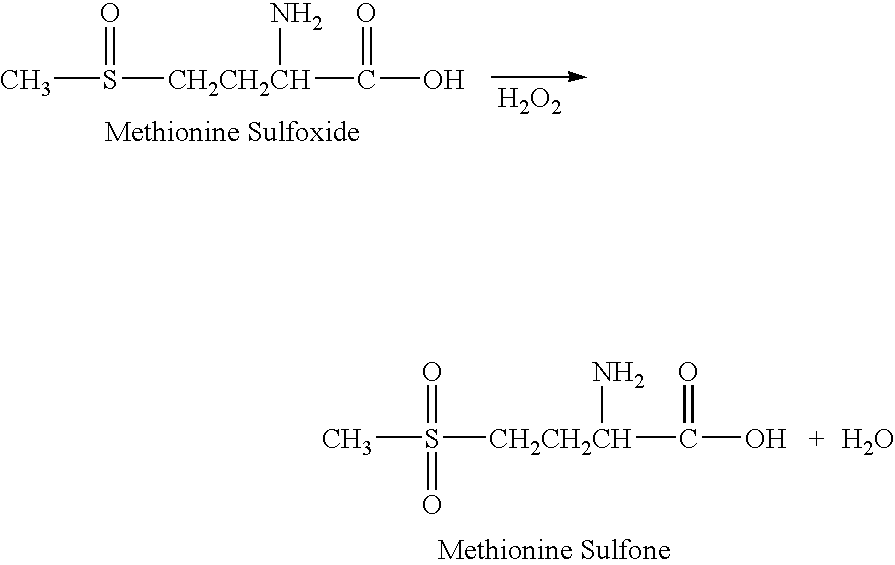
The present invention relates to a stabilized external preparation comprising thymosin beta 4 (Tβ4) as an active ingredient. More specifically, the present invention relates to a therapeutically effective external preparation with improved stability and biological activity of Tβ4. The preparation according to the present invention provides Tβ4 in a stable state by maintaining the biological activity of Tβ4 and minimizing the generation of Tβ4 sulfoxide through oxidization reactions and multimers through aggregation.
Owner:HLB THERAPEUTICS CO LTD
Thymosin beta4 derivative and itsuse
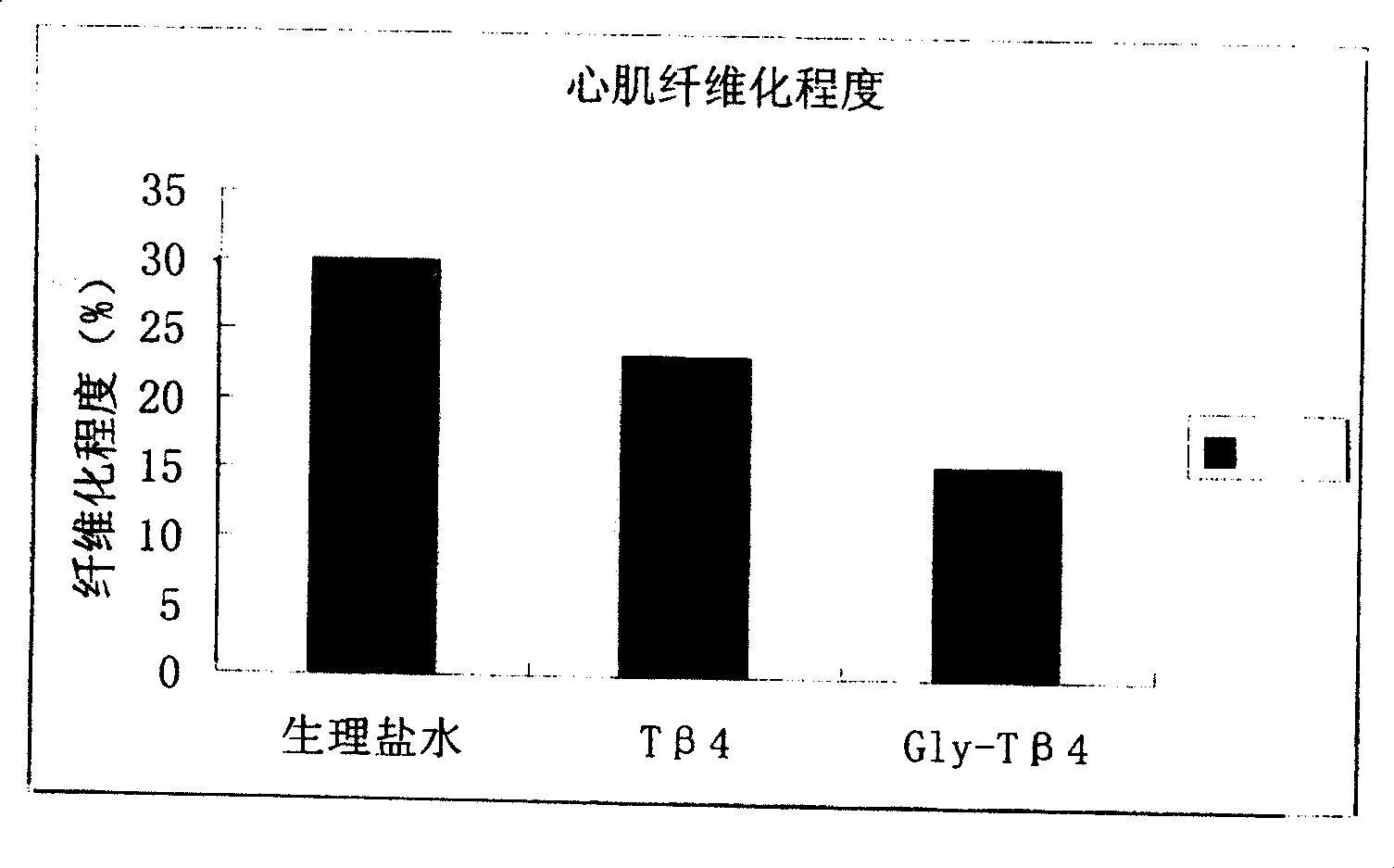
The invention relates to one section of thymosin beta 4 derivatives with high bioactivity which can be used to repair cornea, skin, and heart injury.
Owner:BEIJING NORTHLAND BIOTECH
Methos of healing or preventing inflammation, damage and other changes that occur prior to, during or immediately after a myocardial event with thymosin beta 4, analogoues, isoforms and other derivatives
ActiveUS20040258680A1Promote healing preventionLower Level RequirementsHormone peptidesPeptide/protein ingredientsCardiac muscleAngiogenesis growth factor
Inflammation or damage associated with myocardial events is treated or prevented by administration of an angiogenesis-inducing, anti-inflammatory peptide such as Thymosin beta4, an isoform of Thymosin beta4 or oxidized Thymosin beta4.
Owner:REGENERX BIOPHARMACEUTICALS INC
Methods of treating disorders of the eye and surrounding tissue with thymosin beta 4 (TB4), analogues, isoforms and other derivatives
InactiveUS20110020449A1Reduce decreaseDecrease corneal stromal edemaPowder deliverySenses disorderDiseaseIrritation
Pain or irritation of the eyes, caused by injury due to dry eye syndrome, chemical burns or the like can be accompanied by corneal stromal edema. It has been discovered that administration of thymosin β4 and / or oxidized thymosin β4 to cornea in need of treatment of corneal stromal edema is a useful treatment for decreasing such corneal stromal edema.
Owner:REGENERX BIOPHARMACEUTICALS INC
Preparation method for human thymosin beta 4 two-string body protein
ActiveCN102533911AMicroorganism based processesPeptide preparation methodsChemical synthesisProtein target
The invention belongs to the technical field of medical biology, and particularly relates to a preparation method for a human thymosin beta 4 two-string body protein. A target protein with purity more than 98% can be obtained through the preparation method, the obtained human thymosin beta 4 two-string body protein has biological activity, the activity of the protein is determined through proliferation experiments and migration experiments, and results show that the human thymosin beta 4 two-string body protein can promote proliferation and migration of cells and the activity of the human thymosin beta 4 two-string body protein is superior to that of a chemical synthesis monomer. The method only uses one chromatographic column to complete separation and purification of the target protein,is simple in steps, rapid and low in cost and lays a foundation for further study and wide application of human thymosin beta 4.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Recombinant thymosin beta 4 two repeat protein and preparation thereof
InactiveCN101434651BLow immunogenicityOvercoming the defect of unstable expressionThymopoietinsPeptide preparation methodsEscherichia coliMouse Lymphocyte
The invention relates to recombinant thymosin Beta 4 two-repeat protein and a preparation method thereof. Human thymosin Beta 4 two-repeat gene expression vector pET-22b(+)-T Beta (2) is constructed through recombinant human thymosin Beta 4 full length cDNA combined with PCR technology, human thymosin Beta 4 that can not be expressed directly in colibacillus is highly actively expressed in colibacillus in the form of two-repeat and purified human thymosin Beta 4 two-repeat protein has biologic activity, can promote multiplication of lymphocyte of mice, has low immunogenicity and lays a foundation for the further research and wide application of human thymosin Beta 4.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method of gene-recombination human thymosin beta 4
ActiveCN102660550AAvoid problemsHigh molecular weightHormone peptidesPeptide preparation methodsPichia pastorisCollagen i
The invention discloses a preparation method of gene-recombination human thymosin beta 4. The preparation method comprises the following steps of: acquisition of genes, connection of a carrier p PIC (positive-impedance converter) 9k and human-like collagen I and human thymosin beta 4 through pichia pastoris electrotransformation, selection of multi-copy insertion recombinants, fermentation of fusion protein of the gene-recombination human thymosin beta 4 with the pichia pastoris, and purification of the gene-recombination human thymosin beta 4. According to the method, the characteristic of high expression of the human-like collagen I in the pichia pastoris is utilized to guide the stable and efficient expression of the human thymosin beta 4 in the pichia pastoris. As enterokinase cuttingsites are introduced between leading peptide of the human-like collagen I of the fusion protein and the human thymosin beta 4, the problems, caused by small molecular weight, of the human thymosin beta 4 in the process of expression and purification are solved, the expression index is increased, the purification procedure is simplified, and the extraction and purification efficiency of the product is improved. The preparation method can be used for preparing the gene-recombination human thymosin beta 4.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Methods of Treating or Preventing Tissue Damage Caused by Increased Blood Flow
InactiveUS20090169538A1Avoid tissue damageNervous disorderPeptide/protein ingredientsBinding peptideActinin binding
A method of treating or preventing tissue damage occurring subsequent to affecting an increase in blood flow through a blood vessel which is in communication with the tissue, by administering an effective amount of a composition including a tissue damage-reducing or -preventing polypeptide including at least one of Thymosin beta 4 (TB4), an isoform of TB4, an N-terminal fragment of TB4, a C-terminal fragment of TB4, TB4 sulfoxide, an LKKTET [SEQ ID NO: 1] peptide, an LKKTNT [SEQ ID NO: 2] peptide, an actin-sequestering peptide, an actin binding peptide, an actin-mobilizing peptide, an actin polymerization-modulating peptide, or a conservative variant thereof having tissue damage-reducing activity. The composition is administered to the tissue before, during and / or after affecting the increase in blood flow.
Owner:REGENERX BIOPHARMACEUTICALS INC
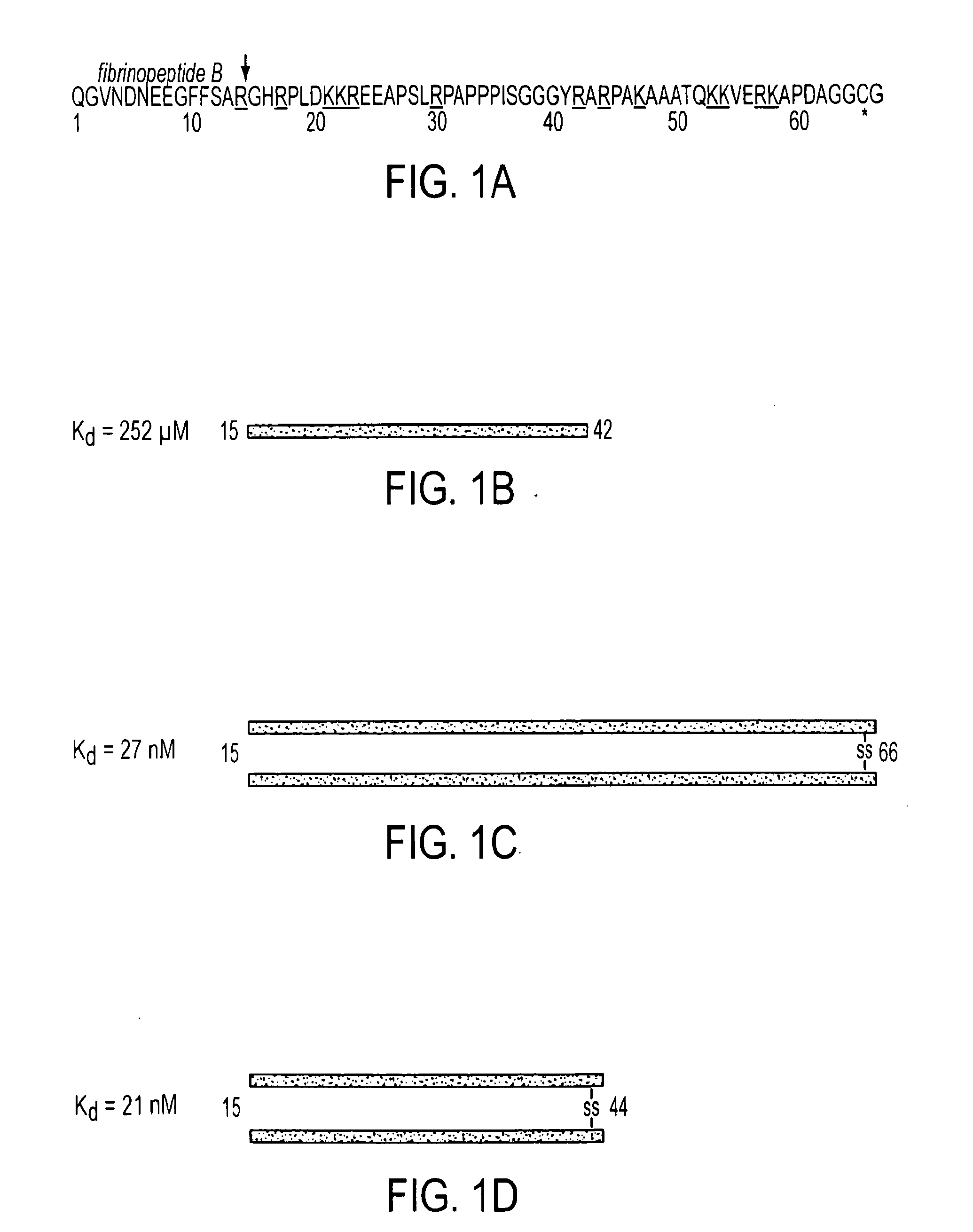
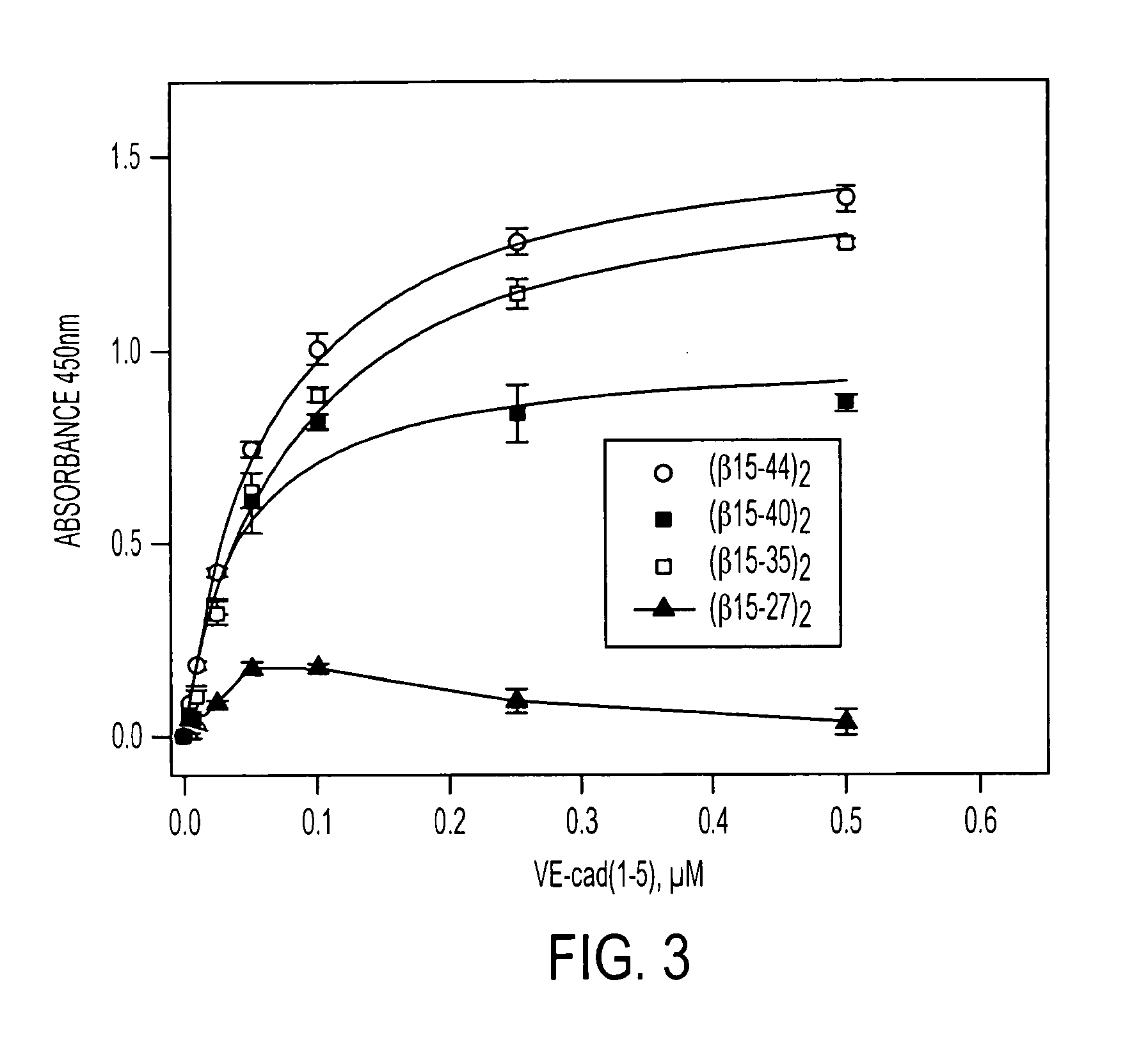
Compositions and Methods Utilizing Fibrin Beta Chain Fragments
A composition including a peptide sequence of the formula βX1-X2, the peptide sequence corresponding to an amino acid sequence of a fibrin beta chain fragment of a Bbeta chain of fibrinogen, wherein X1 represents an N-terminal end of the peptide sequence, and X2 represents a C-terminal end of the peptide sequence, wherein the peptide sequence includes additional amino acids between X1 and X2, wherein the peptide sequence may contain a non-naturally occurring amino acid residue, wherein the peptide sequence is other than a wild-type β15-42 monomer sequence per se, and wherein the peptide sequence is other than (β15-66)2 dimer having two chains with each chain limited to wild type amino acids β15-65 and each chain further including a non-naturally occurring Gly at position 66 of each chain. Methods for treatment and pharmaceutical combinations may include a polypeptide agent such as Thymosin beta 4. In such methods and combinations, a dimer of the peptide sequence may include amino acids 15-66 of the fibrin beta chain.
Owner:UNIV OF MARYLAND BALTIMORE
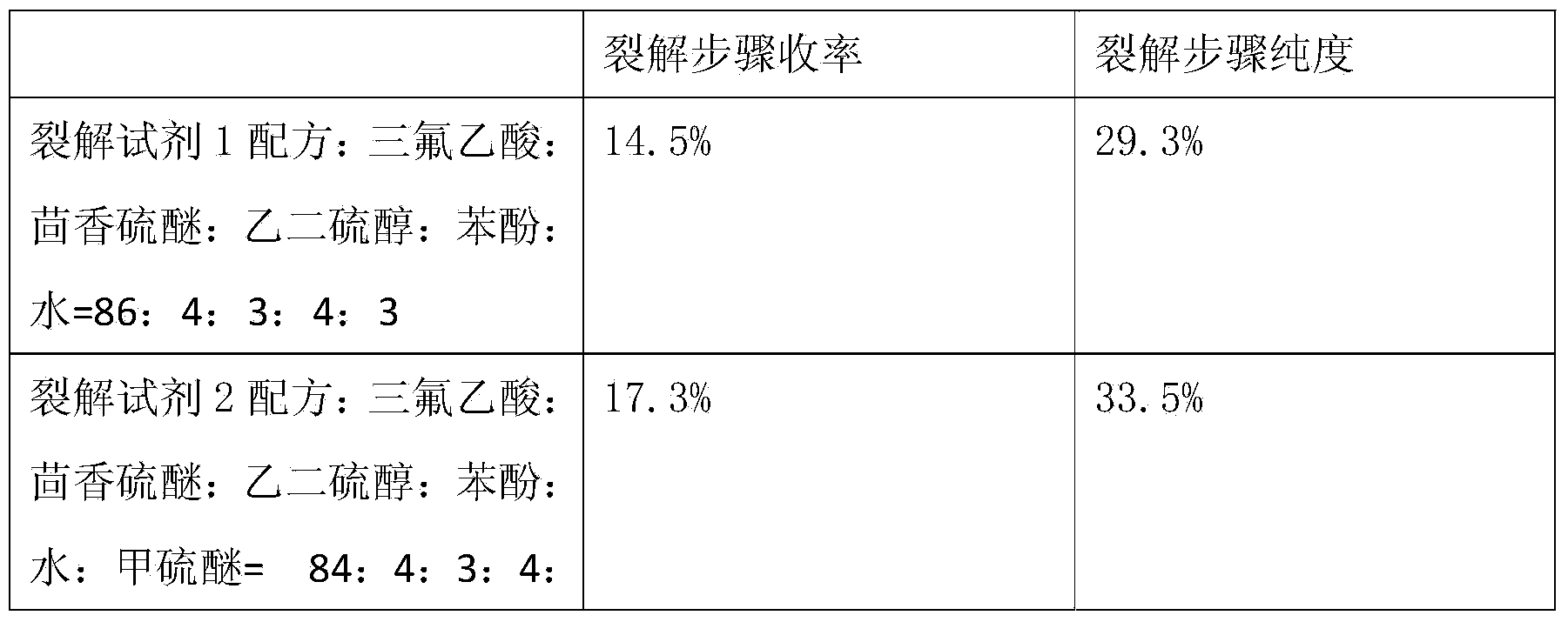
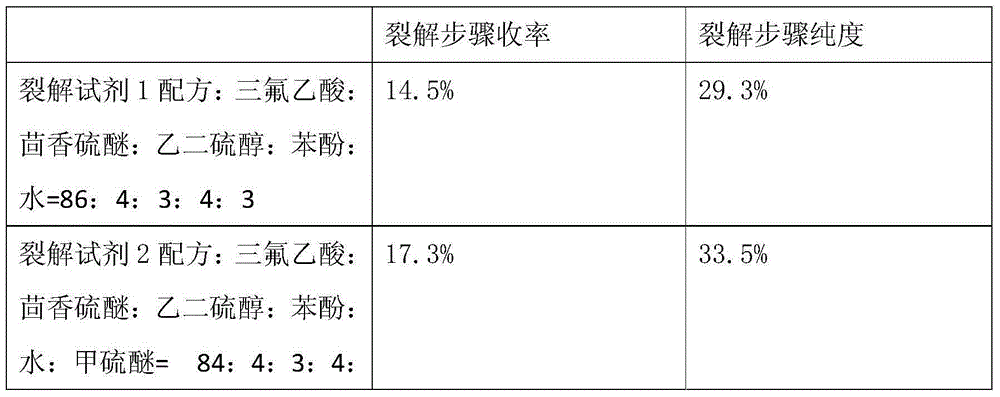
Method for pyrolysis of thymosin beta-4
ActiveCN103724422AEfficient removalPrevent oxidationThymosin peptidesPeptide preparation methodsSolventPhenol
The invention discloses a method for pyrolysis of thymosin beta-4. The method comprises the step of carrying out pyrolysis on thymosin beta-4 peptide resin to obtain crude peptide, wherein a mixed solvent of trifluoroacetic acid, thioanisole, dithioglycol, phenol, water, dimethyl sulfide and ammonium iodide is used as a pyrolysis reagent in the pyrolysis process. According to the method for pyrolysis of thymosin beta-4, the purity of crude peptide is greatly improved, meanwhile the total yield is greatly increased, the production cost is reduced, and the method is simple in process and convenient for industrial production.
Owner:哈尔滨吉象隆生物技术有限公司
Method of treating, preventing, inhibiting or reducing damage to cardiac tissue with thymosin beta 4 fragments
InactiveUS20070191275A1Promote regenerationPromote repairHormone peptidesTetrapeptide ingredientsEndocrinologyThymosin beta-4
A method of treatment for promoting regeneration or repair a damaged cardiovascular tissue, or for preventing damage to cardiovascular tissue, includes administering to the tissue a damage-treating or -preventing fragment of thymosin beta 4 (Tβ4), such as AcSDKP, or a stimulating agent that forms such a fragment of (Tβ4).
Owner:UNIVET ERLANGEN NUERNBERG +1
Composition containing thymosin beta 4, and pharmaceutical formulation comprising same
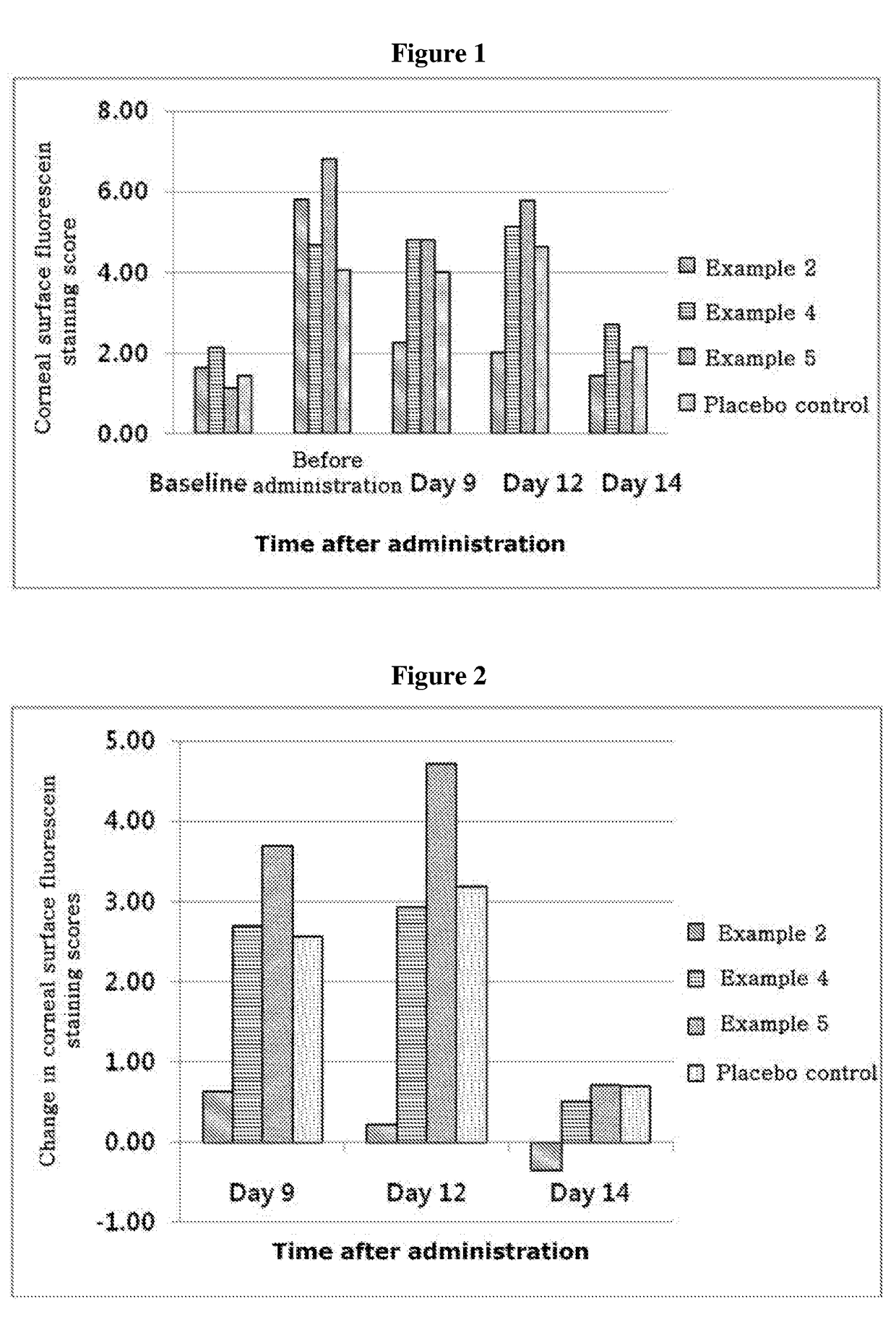
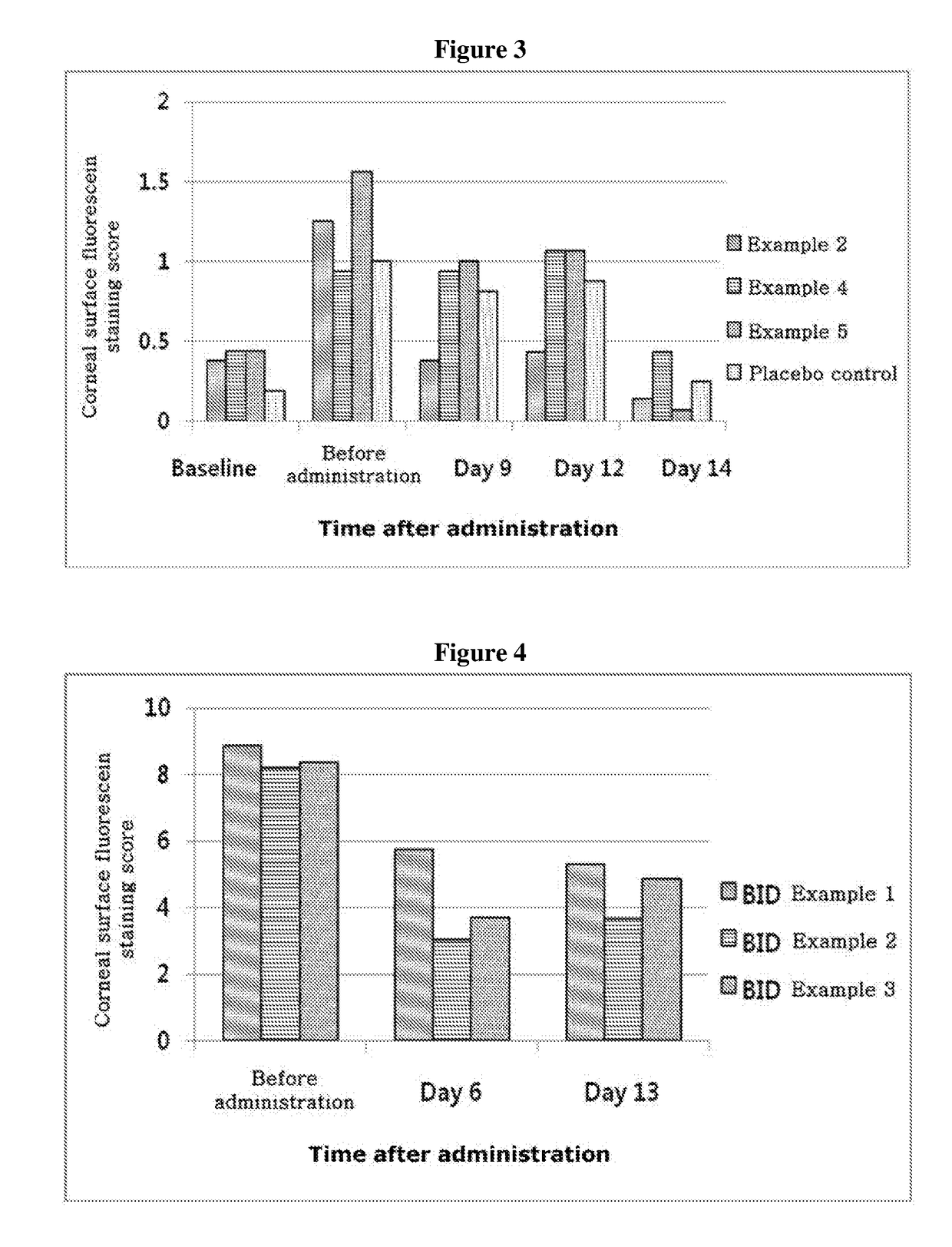
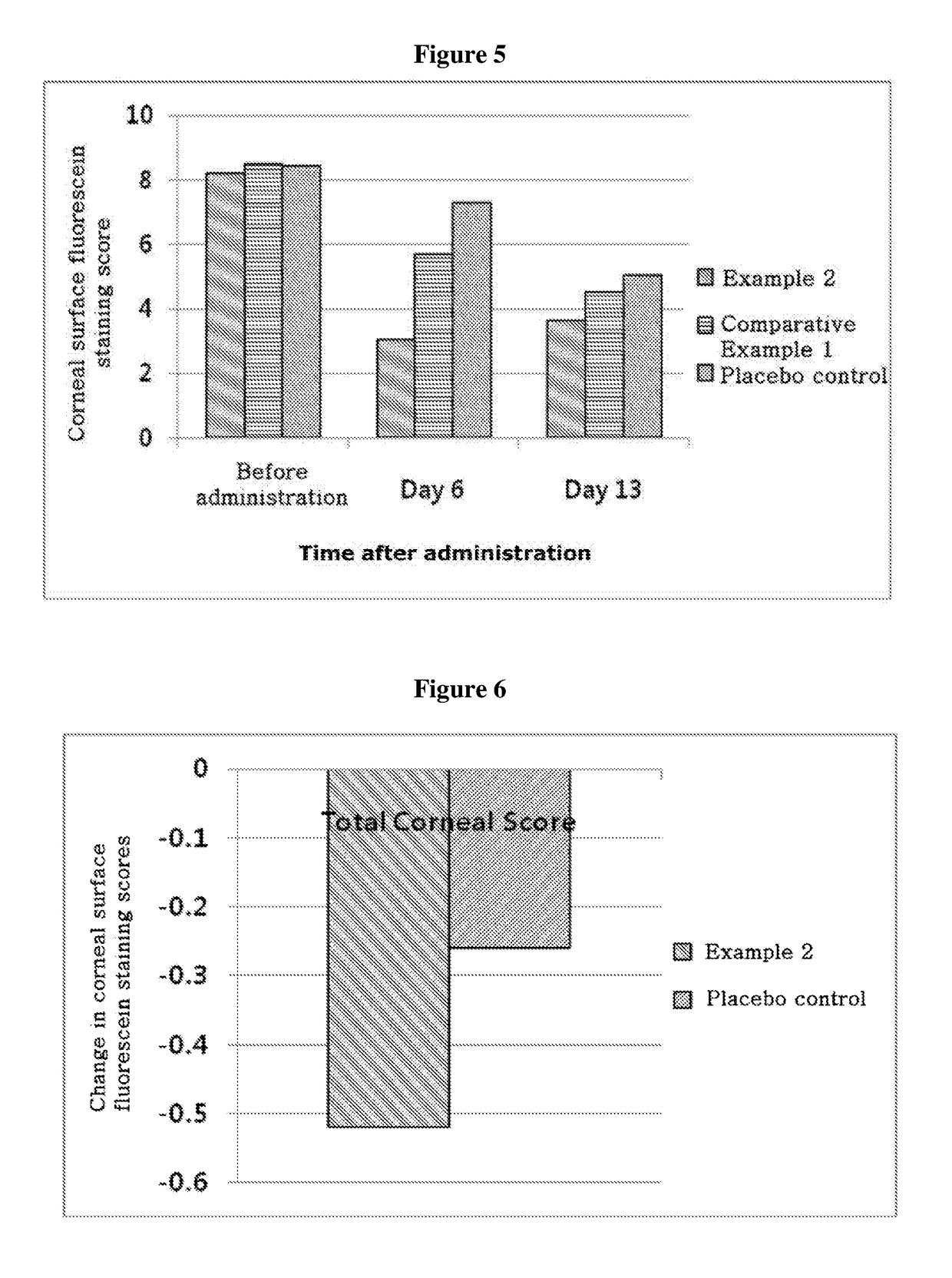
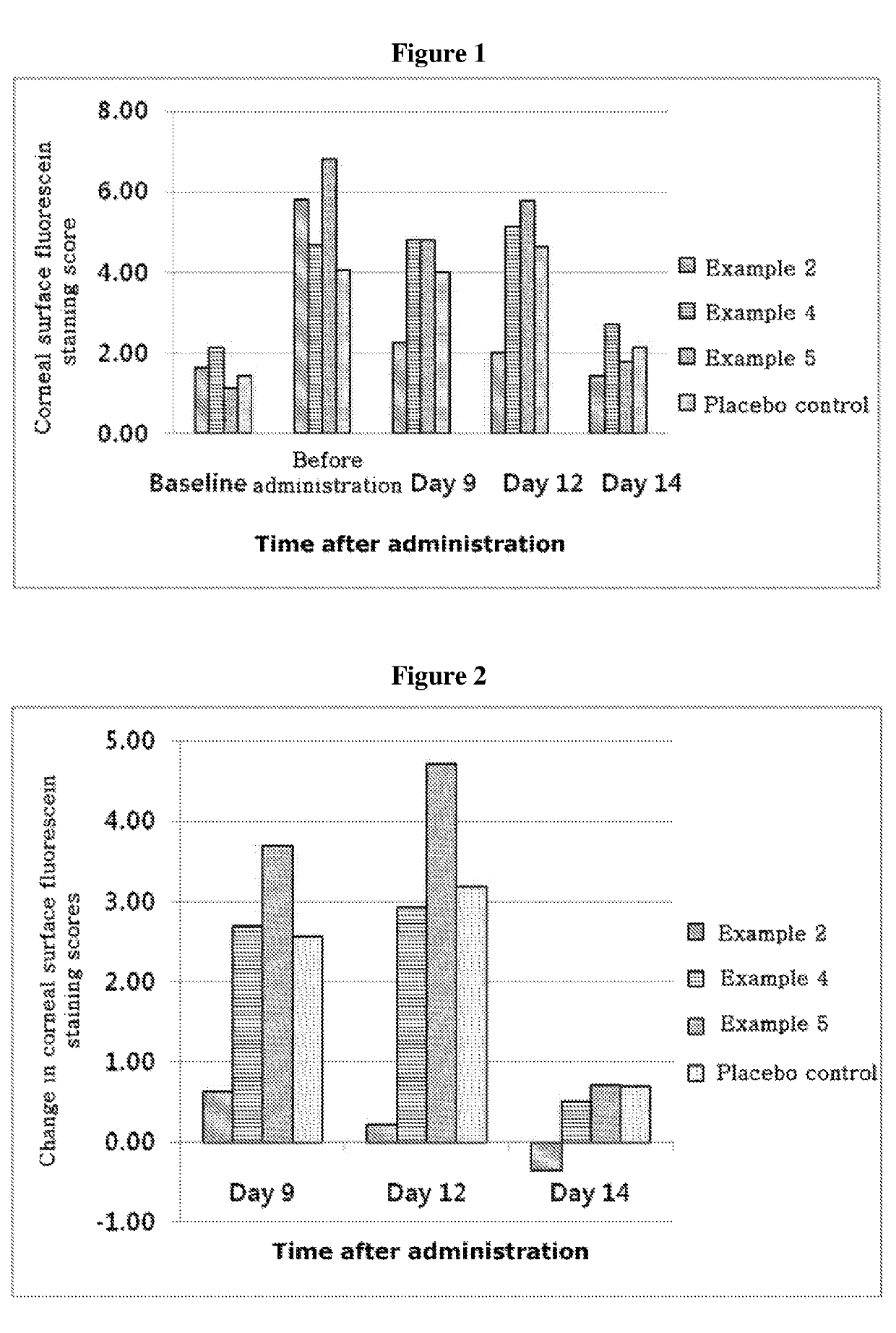
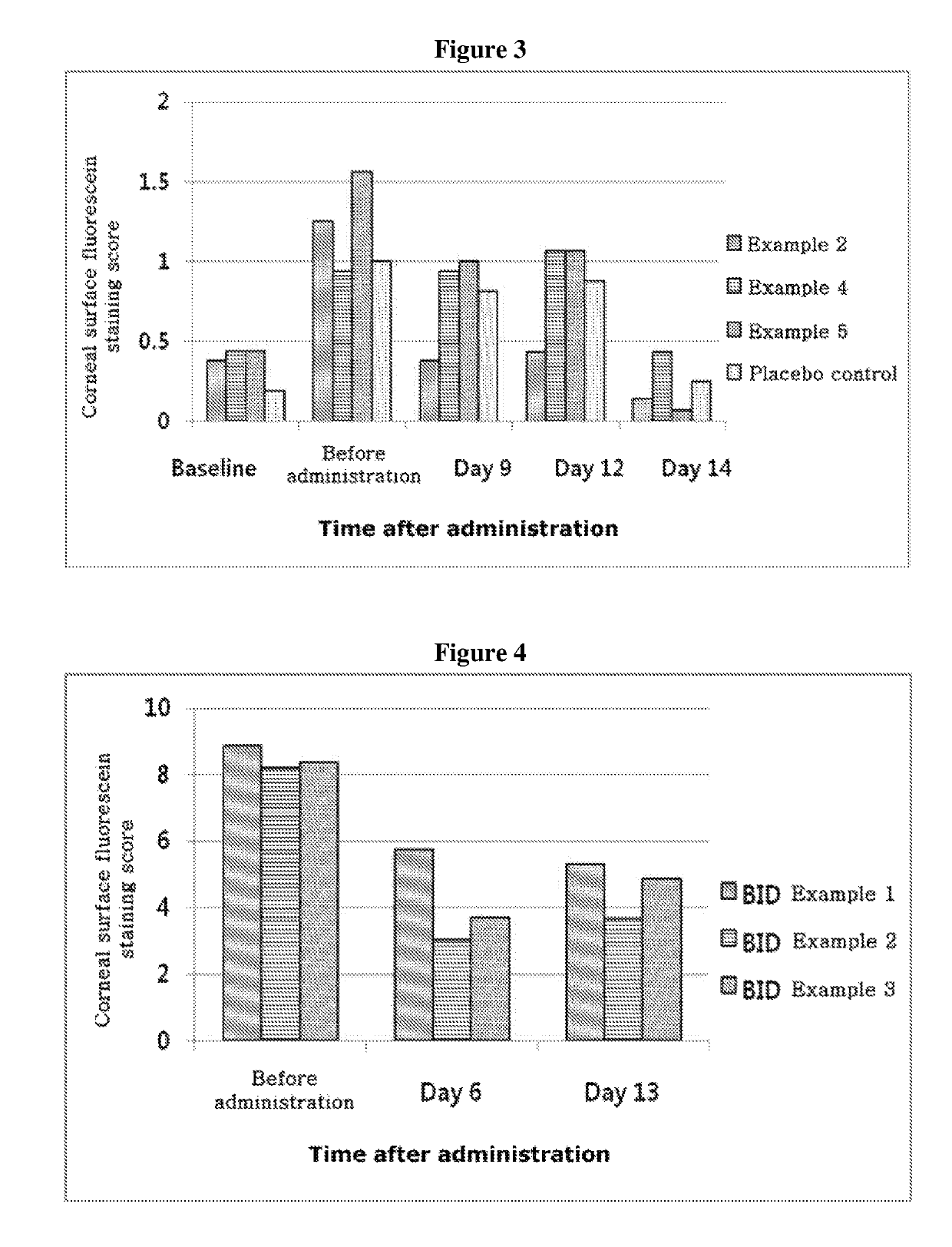
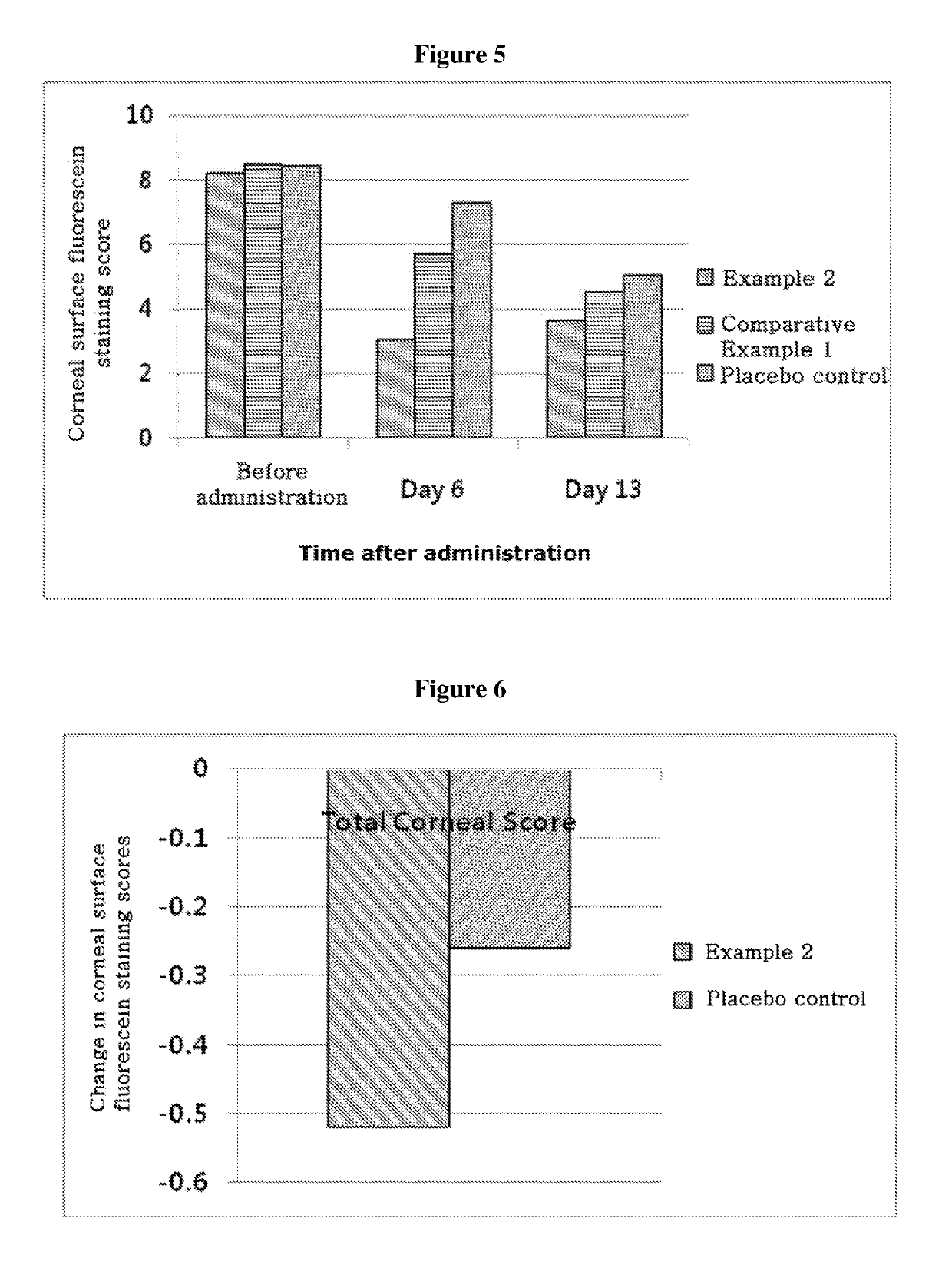
ActiveUS20170333531A1Use minimizedImprovement of dry eye syndromeSenses disorderPeptide/protein ingredientsXerophthalmiaIrritation
The present invention relates to a therapeutic agent for ophthalmic disease containing thymosin beta 4. The present invention is more effective in reducing xerophthalmia than an ophthalmic solution containing cyclosporine A, is less irritant to eyes than conventional ophthalmic solutions and is physiochemically safe.
Owner:HLB THERAPEUTICS CO LTD
Thymosin beta-4 ethosome, and preparation method thereof
ActiveCN108721604AReduce inactivationImprove skin penetrationPeptide/protein ingredientsPharmaceutical non-active ingredientsMedicineCholesterol
The invention discloses a thymosin beta-4 ethosome, and a preparation method thereof. The thymosin beta-4 ethosome comprises 0.022 to 0.043% of thymosin beta-4, 0.054 to 0.084% of phosphatide, 0.018 to 0.028% of cholesterol, 0.02 to 0.04% of an anionic surfactant, 10.10 to 30.30% of ethanol, and the balance distilled water. The thymosin beta-4 ethosome is capable of improving medicine transdermalabsorption effect, and improving the restoration technical effect of thymosin beta-4 on scar skin. According to the preparation method, the anionic surfactant and cholesterol are added to solve a stability problem of thymosin beta-4 ethosome. The thymosin beta-4 ethosome prepared through the preparation method is high in encapsulation efficiency, and stability; the technology is simple; excellenteconomical benefit is achieved, and the invention also discloses applications of the thymosin beta-4 ethosome in the technical field of scar restoration.
Owner:山东源科生物科技股份有限公司
Purpose of modified thymosin beta 4 in respect of treating diabetic peripheral neuropathy
The invention relates to the field of disease treatment, in particular to a purpose of modified thymosin beta 4 for treating diabetic peripheral neuropathy in examinees (such as people), and a purposeof the thymosin beta 4 for preparing a medicine for treating the peripheral neuropathy in examinees (such as people). The invention further relates to a method for treating the peripheral neuropathy.The method comprises the step of applying the modified thymosin beta 4 to the examinee having the need.
Owner:BEIJING NORTHLAND BIOTECH
Method of achieving a thymosin beta 4 concentration in a human patient
The present invention provides embodiments which involve methods of providing a predetermined concentration of thymosin beta 4 (TB4) at a predetermined time, t, in a body portion of a live human patient. The methods can include determining a thymosin beta 4 treatment dosage (D) using Formula I: C=(A)D·t−B, wherein C is the predetermined concentration at time t, in ng / mL, D is the dosage of thymosin beta 4 administered in mg, t is the time elapsed after administration of dosage D in hours, A is about 30 to about 38, and B is about 0.5 to about 1; and administering the dosage (D) of thymosin beta 4 to the patient. Formula I may be, for example,C=(35.6)D·t−0.754 (Formula II).
Owner:REGENERX BIOPHARMACEUTICALS INC
Polyethylene glycol modified human thymosin beta 4 tandem repeat protein, as well as preparation method and application thereof
InactiveCN110964117AEasy to operateLow experimental requirementsThymosin peptidesPeptide/protein ingredientsProtein targetTandem repeat
The invention discloses a polyethylene glycol modified human thymosin beta 4 tandem repeat protein (PEG-rTbeta4), as well as a preparation method and application thereof. The PEG-rTbeta4 is obtained after recombinant human thymosin beta 4 tandem repeat protein is subjected to specific chemical modification with polyethylene glycol, wherein the rTbeta4 has gene sequence codes connected in the following sequence: a HKCDI gene sequence, a thymosin beta4 gene sequence, a GS Linker GSGSG-thymosin beta4 gene sequence and a 6*His label gene sequence. Furthermore, the invention discloses a method forpreparing the protein. The preparation method can be used for preparing target protein having the purity of 90 percent or more, has the advantages of low cost, high bioactivity and the like, and is simple and quick. Experiments prove that the PEG-rTbeta4 protein has the effects of promoting proliferation and migration of myocardial cells, resisting apoptosis, promoting hair growth and angiogenesisand accelerating wound healing. Therefore, the PEG-rTbeta4 protein has a wide application prospect in heart function recovery after myocardial infarction, hair growth promotion and wound healing acceleration.
Owner:HARBIN MEDICAL UNIVERSITY
A method for splitting thymosin β4
ActiveCN103724422BEfficient removalPrevent oxidationThymosin peptidesPeptide preparation methodsSolventPhenol
Owner:哈尔滨吉象隆生物技术有限公司
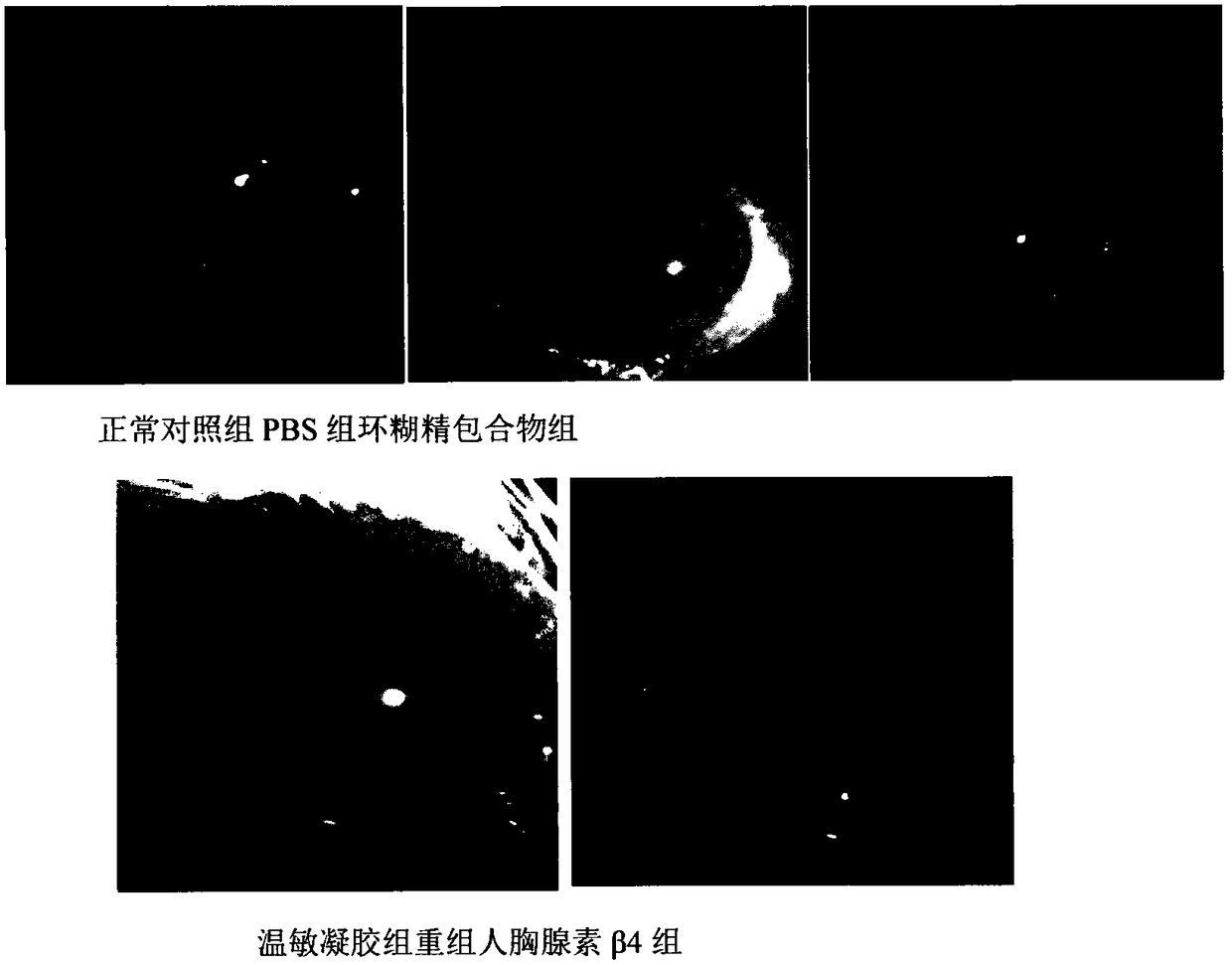
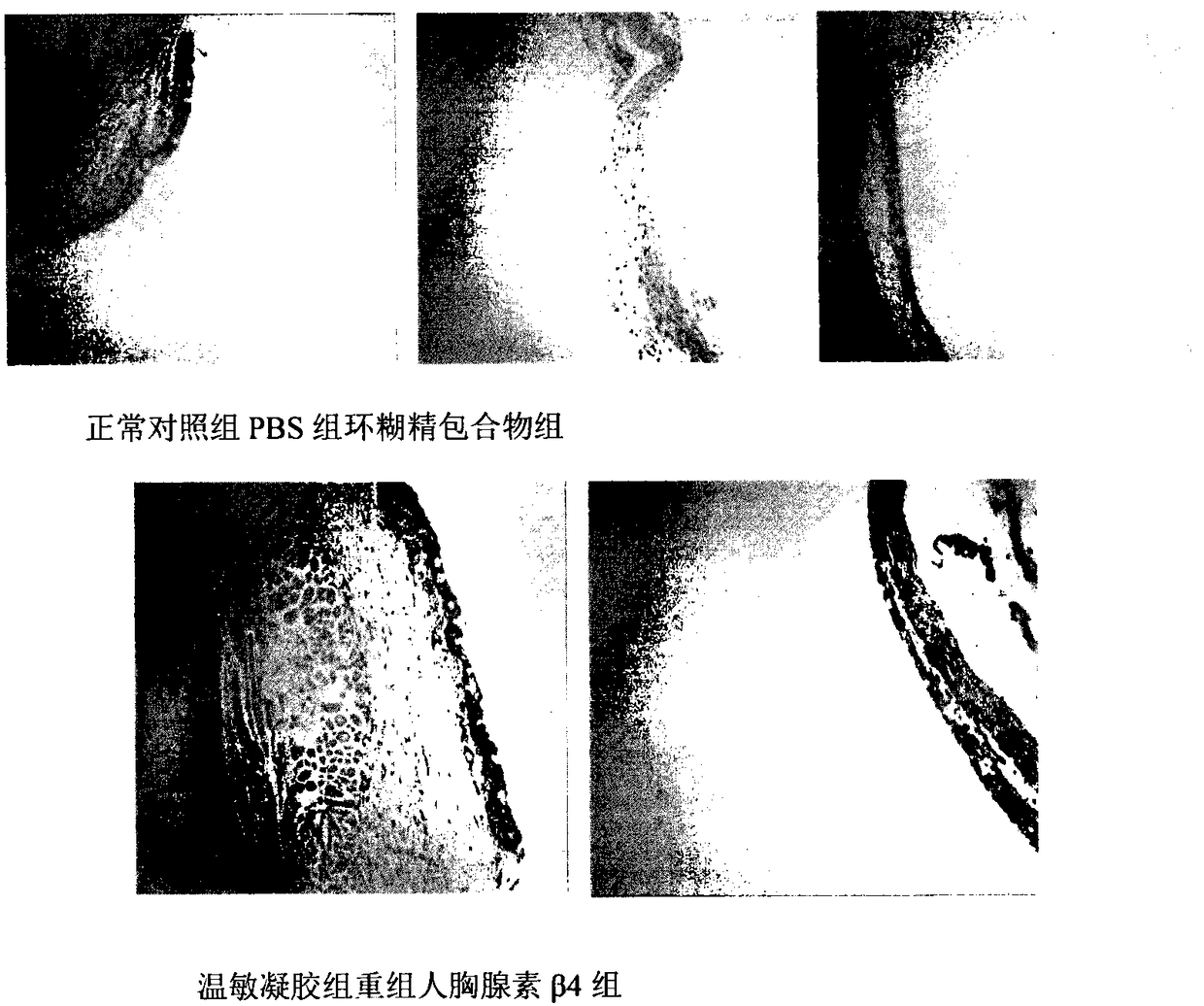
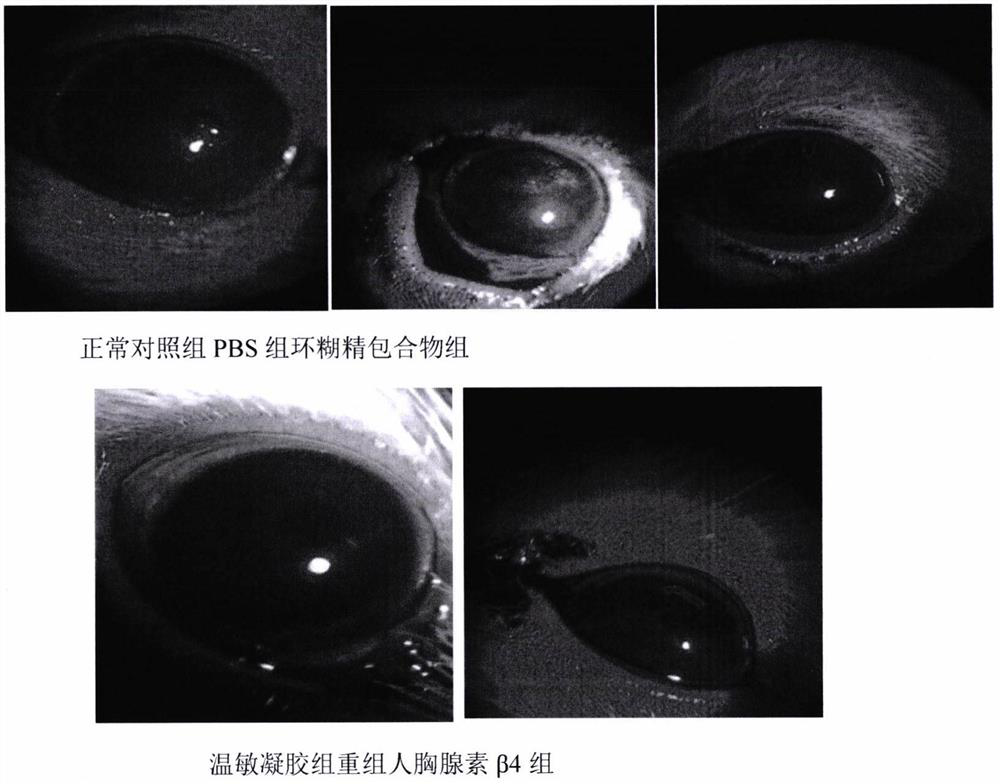
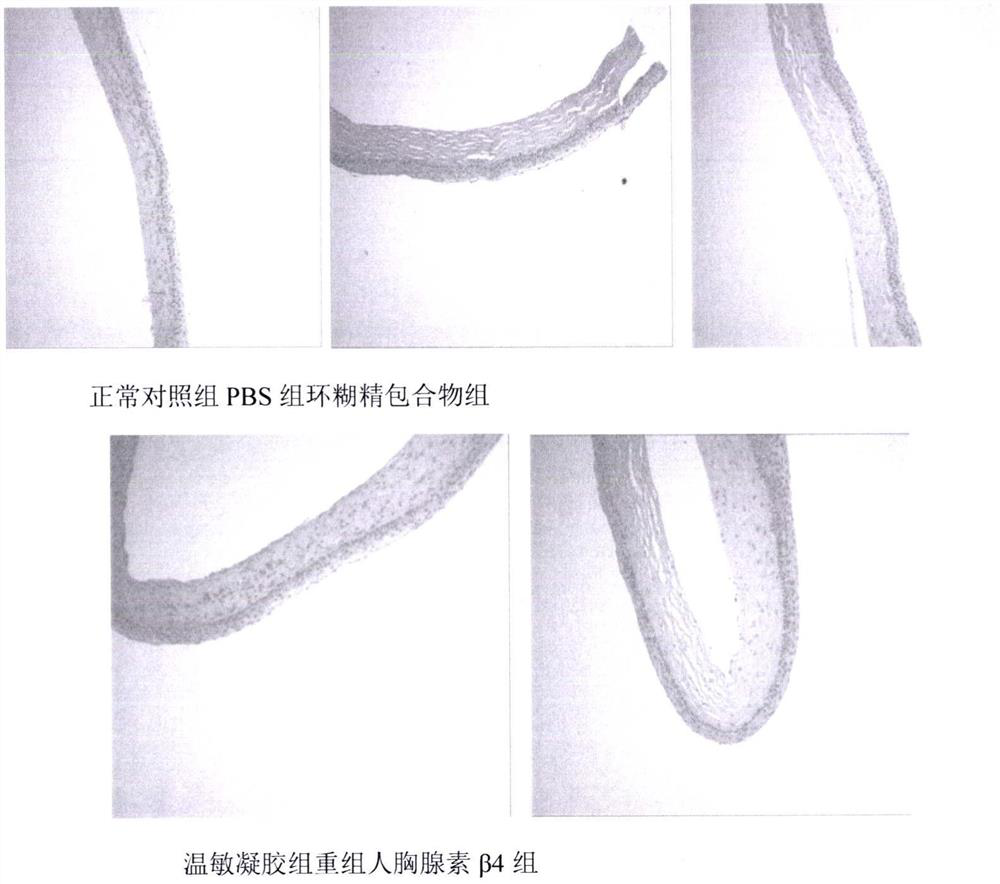
Temperature-sensitive recombinant human thymosin beta 4 eye in-situ gel preparation
The invention discloses a temperature-sensitive eye in-situ gel preparation suitable for phase inversion temperature. The temperature-sensitive eye in-situ gel preparation comprises different types ofpoloxamer 407 and 188. The in-situ gel preparation can be dosed in a liquid state under the condition of room temperature, and gel is formed on the surfaces of eyes. The stability of recombinant human thymosin beta 4 medicines can be enhanced, and the efficacy and the storage life of the medicines are prolonged.
Owner:北京汇恩兰德制药有限公司
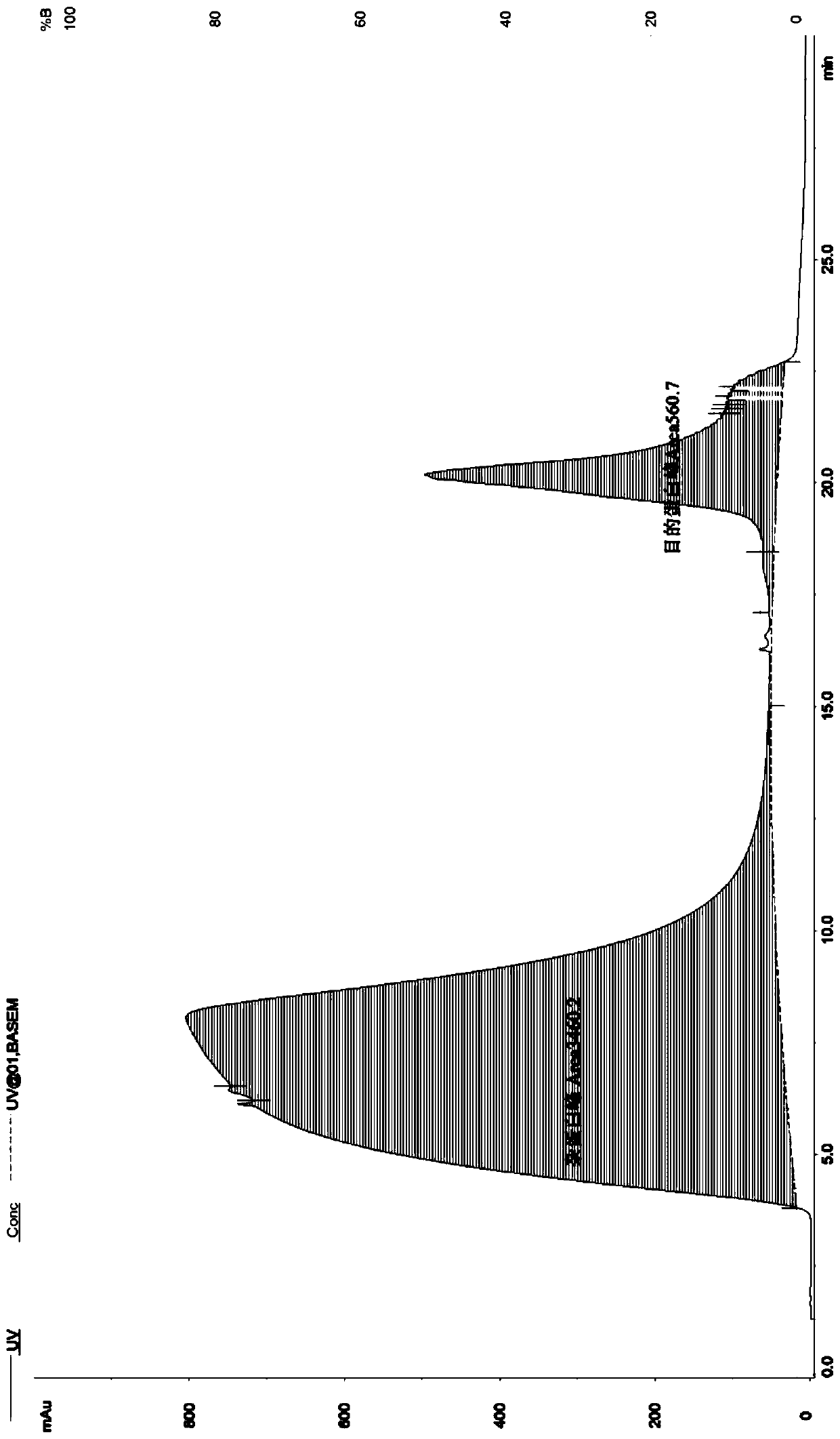
Method for detecting thymosin beta 4 content based on high performance liquid chromatograph
InactiveCN105891399AControl contentSimple and effective content determination methodComponent separationGradient elutionAnalysis method
The invention belongs to the technical field of biological chemistry and provides a high performance liquid chromatograph analysis method for thymosin beta 4 content. In the method, a high performance liquid chromatograph is used, and a gradient elution method is used for content detection. A conventional protein content detecting method for thymosin beta 4 has the defects of being complex in operation, likely to be affected by various factors and the like. Operation is simple, the peak type is good, the separation degree of a main peak and an impurity peak is good, and a simple and reliable analysis method is provided for detecting the thymosin beta 4 content.
Owner:BEIJING NORTHLAND BIOTECH
Method of treating or preventing tissue deterioration, injury or damage due to periodontal disease or disease of oral mucosa, and/or downregulating NF-kappabeta or supressing NF-kappabeta-mediated actions
A method of treatment for treating, at least partly preventing, inhibiting or reducing tissue deterioration, injury or damage due to a periodontal disease or disease of oral mucosa, or for restoring tissue adversely affected by the disease, in a subject, and / or for downregulating NF-kappaB or suppressing NF-kappaB mediated action in a body, organ, tissue or cell, includes administering to a subject, body, organ, tissue or cell an effective amount of a composition including a peptide agent including at least one of Thymosin beta 4 (Tβ4), an isoform of Tβ4, an N-terminal fragment of Tβ4, a C-terminal fragment of Tβ4, Tβ4 sulfoxide, an LKKTET peptide or conservative variant thereof, an LKKTNT peptide or conservative variant thereof, a KLKKTET peptide or conservative variant thereof, an LKKTETQ peptide or conservative variant thereof, Tβ4ala, Tβ9, Tβ10, Tβ11, Tβ12, Tβ13, Tβ14, Tβ15, gelsolin, vitamin D binding protein (DBP), profilin, cofilin, depactin, DnaseI, vilin, fragmin, severin, capping protein, β-actinin, acumentin, an actin-sequestering peptide, an actin binding peptide, an actin-mobilizing peptide, an actin polymerization-modulating peptide, or a stimulating agent that stimulates production of an effective amount of the peptide agent in the subject, body, organ, tissue or cell.
Owner:REGENERX BIOPHARMACEUTICALS INC
Preparation method for human thymosin beta 4 two-string body protein
ActiveCN102533911BMicroorganism based processesPeptide preparation methodsChemical synthesisProtein target
The invention belongs to the technical field of medical biology, and particularly relates to a preparation method for a human thymosin beta 4 two-string body protein. A target protein with purity more than 98% can be obtained through the preparation method, the obtained human thymosin beta 4 two-string body protein has biological activity, the activity of the protein is determined through proliferation experiments and migration experiments, and results show that the human thymosin beta 4 two-string body protein can promote proliferation and migration of cells and the activity of the human thymosin beta 4 two-string body protein is superior to that of a chemical synthesis monomer. The method only uses one chromatographic column to complete separation and purification of the target protein,is simple in steps, rapid and low in cost and lays a foundation for further study and wide application of human thymosin beta 4.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Recombined Tbeta 4-BP5 fusion peptide, gene, engineering bacteria and application
InactiveCN103145854BImprove immunityTo achieve the purpose of preventing and controlling the corresponding diseasesBacteriaMicroorganism based processesEscherichia coliThioredoxin
The invention discloses a recombined Tbeta 4-BP5 fusion peptide, a gene, an engineering bacteria and an application. The recombined fusion peptide is prepared by fusing thymosin beta 4 with Fabricius bursa pentapeptide BP5 through a soft Linker. The recombined Tbeta 4-BP5 fusion peptide gene is inserted into an expression vector to transfer the escherichia coli so as to obtain the gene engineering bacteria for effectively express the Tbeta 4-BP5 fusion peptide; the Tbeta 4-BP5 fusion peptide is prepared through liquid cultivation and purification; and the thioredoxin of the fusion peptide is eliminated by using enterokinase with His tag at the N-end, and the fusion peptide is further subjected to affinity chromatography purification so as to obtain the single recombined Tbeta 4-BP5 fusion peptide. The recombined Tbeta 4-BP5 fusion peptide disclosed by the invention can be used as a novel polypeptide immunologic adjuvant which is used in match with vaccines, can effectively enhance the organism cell immune level and the body liquid immune level, and has wide application prospects.
Owner:HENAN UNIV OF SCI & TECH
Thymosin beta4 derivative and its use
The invention relates to one section of thymosin beta 4 derivatives with high bioactivity which can be used to repair cornea, skin, and heart injury.
Owner:BEIJING NORTHLAND BIOTECH
A temperature-sensitive recombinant human thymosin β4 ophthalmic in situ gel preparation
The invention discloses a temperature-sensitive ophthalmic in-situ gel preparation with a suitable phase transition temperature, which contains different types of poloxamers, and the preparation contains poloxamer 407 and poloxamer 188. The in situ gel can be administered in a liquid state at room temperature and forms a gel on the surface of the eye. The invention can enhance the stability of the recombinant human thymosin β4 drug and prolong the efficacy and shelf life of the drug.
Owner:北京汇恩兰德制药有限公司
Composition containing thymosin beta 4, and pharmaceutical formulation comprising same
ActiveUS10406208B2Use minimizedImprovement of dry eye syndromeSenses disorderPeptide/protein ingredientsDiseaseXerophthalmia
The present invention relates to a therapeutic agent for ophthalmic disease containing thymosin beta 4. The present invention is more effective in reducing xerophthalmia than an ophthalmic solution containing cyclosporine A, is less irritant to eyes than conventional ophthalmic solutions and is physiochemically safe.
Owner:HLB THERAPEUTICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com