Thymosin beta-4 ethosome, and preparation method thereof
A technology for thymosin and alcohol plastids, which is applied to thymosin beta-4 alcohol plastids and their preparation technology and application fields, to achieve the effects of prolonging action time, reducing inactivation and strengthening membrane structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The selection of embodiment 1 preparation method
[0049] 1.1 Alcohol phase injection
[0050] Add 1.5mg soy lecithin and 0.25mg cholesterol into 1mL absolute ethanol solution, dissolve 0.50mg thymosin β-4 in 1.75mL distilled water and put it in a pear-shaped bottle, inject the mixed solution of alcohol phase into the pear at 400uL / min In the shaped bottle solution, hydration at 700r / min at 30°C for 15min, after cooling to room temperature, passing through a microporous membrane with a pore size of 450nm, after a certain treatment, the measured EE was 10±4%, and the appearance of the ethanol solution was turbid. The background of the TEM photo is messy, the size of the formed ethanol bodies is different, most of them are ruptured or adhered, and the results are as attached figure 1 shown. 1.2 Water phase injection
[0051] Add 1.5mg soy lecithin and 0.25mg cholesterol to 1mL absolute ethanol solution and put it in a pear-shaped bottle, dissolve 0.50mg thymosin β-4 in...
Embodiment 2
[0052] Embodiment 2 ethosome preparation single factor test
[0053] 2.1 The dosage of cholesterol
[0054] Add 1.5mg soy lecithin and different amounts of cholesterol to 1mL absolute ethanol solution and place it in a pear-shaped bottle, dissolve 0.50mg thymosin β-4 in 1.75mL distilled water, and inject the water-phase mixed solution into the pear-shaped bottle at 400uL / min. In the bottle solution, hydrate at 700r / min for 15min at 30°C, cool to room temperature, pass through a microporous membrane with a pore size of 450nm, and measure EE after a certain treatment. The experimental results are shown in the table below:
[0055] Effect of Cholesterol Dosage on EE (n=3)
[0056]
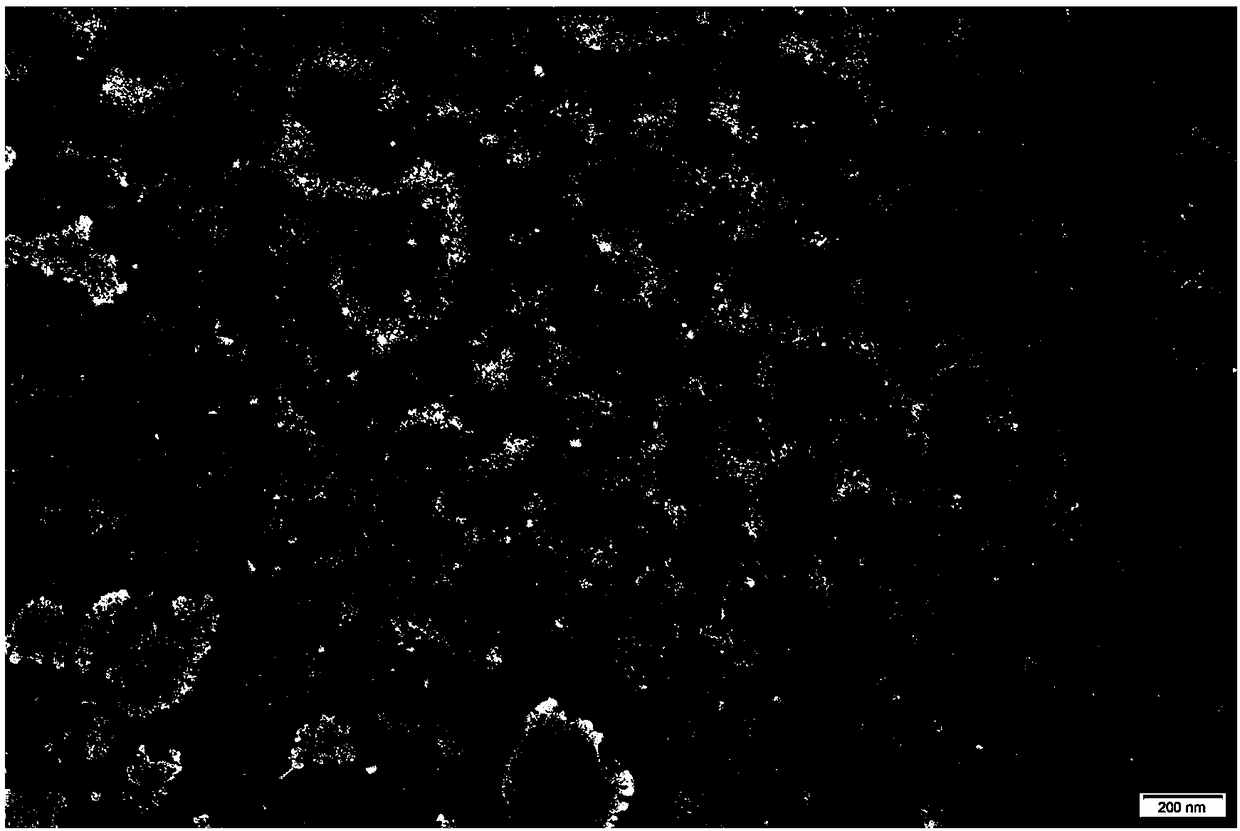
[0057] The amount of cholesterol has a great impact on the EE of ethosomes, especially when no cholesterol is added, the prepared ethosome solution cannot show light blue opalescence, such as image 3 As shown, the TEM pictures show that only a very small amount of ethosomes are formed and the sh...
Embodiment 3
[0093] Embodiment 3 Orthogonal Design Method Optimizing Prescription
[0094]The single factor experiment result shows in embodiment 2, the factor that has bigger influence to ethosome has: the consumption (A) of cholesterol, beta-4 consumption (B), ethanol concentration (C), drop rate (D), by Orthogonal design L9(3^4) table is used as the parameter of investigation to evaluate each prescription, the following table is the investigation result of factor level table and orthogonal design respectively.
[0095] Orthogonal experimental factors and level table
[0096]
[0097]
[0098] Orthogonal Experimental Results
[0099]
[0100] Combined with orthogonal test and single factor experiment to determine the final prescription and process: add 1.5mg soybean lecithin and 0.25mg cholesterol to 1mL absolute ethanol solution, put 0.75mg thymosin β-4 in 1.75mL distilled water and place in pear In the pear-shaped bottle, inject the alcohol-phase mixed solution into the pear...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com