Preparation method of gene-recombination human thymosin beta 4
A gene recombination and thymosin technology, applied in the field of bioengineering, can solve the problems of high production cost and low purity of thymosin β4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
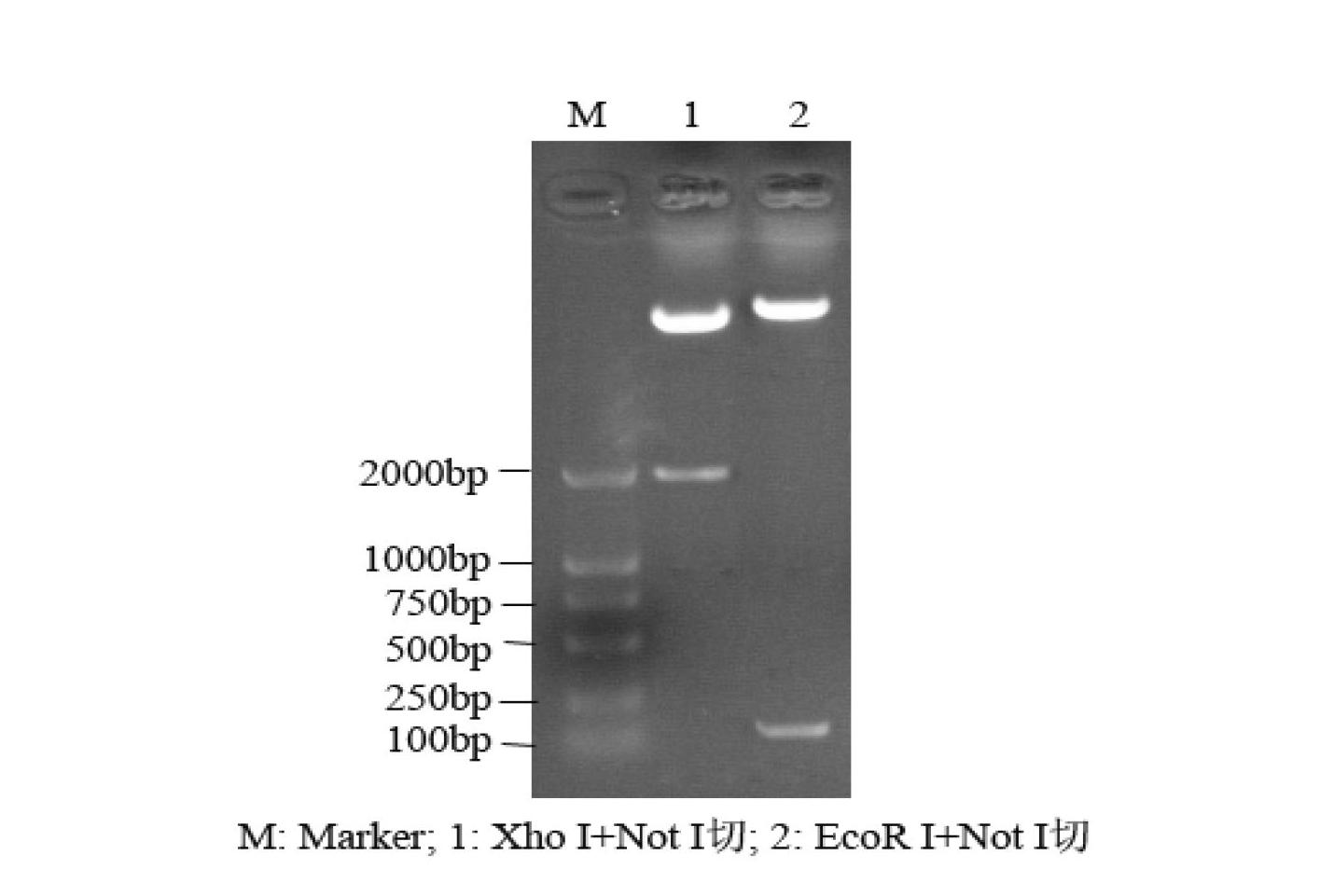
[0126] A recombinant human thymosin β4 fusion protein that uses type I human collagen as a leader peptide to guide the expression of human thymosin β4. The nitrogen terminal is a leader peptide consisting of 600 amino acids of type I human collagen, and the carbon terminal is 43 Amino acids, an enterokinase cleavage site, glutamic acid and phenylalanine for connection are added between the two amino acid sequences, after secreted expression, the recombinant human thymosin β4 is obtained by cutting with enterokinase in vitro, and the recombinant human The amino acid sequence of thymosin β4 is as described above.
[0127] The preparation method of the above-mentioned gene recombinant human thymosin β4 comprises the following steps:
[0128] 1. Acquisition of genes
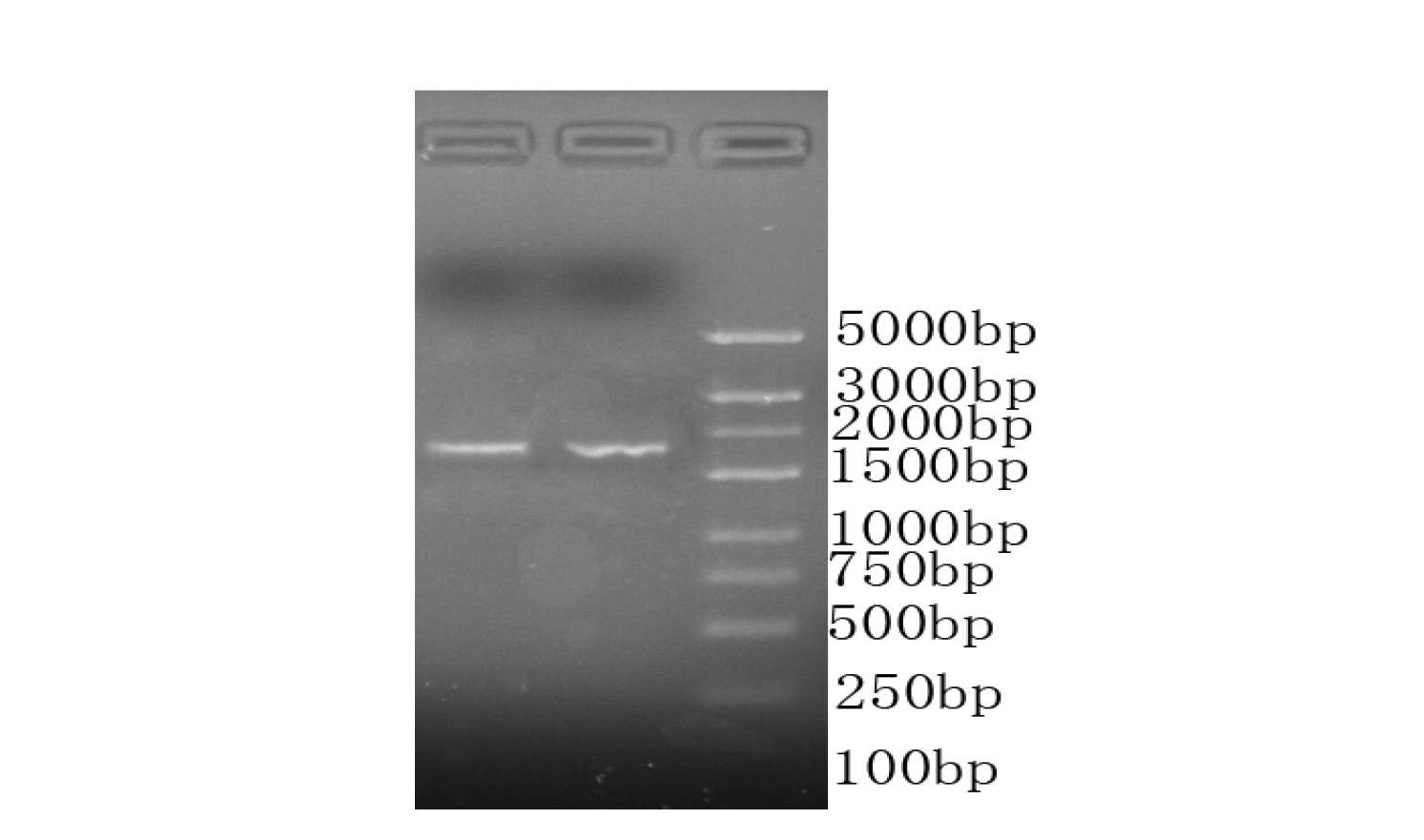
[0129] Total RNA was extracted from isolated neonatal placental tissue, and the GeneCopoeia First-Strand cDNA synthesis Kit kit was used for reverse transcription according to the instructions in the instructions to...
Embodiment 2
[0241] In the purification step 6 of the genetically recombinant human thymosin β4 in Example 1, the fusion protein was cleaved by the enterokinase cleavage method. The enterokinase cleavage method was: the protein concentration was determined by the Coomassie brilliant blue method, diluted to 5 mg / ml, and added Recombinant enterokinase, cut 5 mg of fusion protein per unit of recombinant enterokinase, pH 6.0, cut at 16°C for 18 hours.
[0242] Ultrafiltration with an ultrafiltration membrane with a molecular weight of 10000D, collecting the filtrate, and desalting with an ultrafiltration membrane with a molecular weight of 1000D to obtain human thymosin β4 crude protein, other steps in this step are the same as in Example 1. The other steps were the same as in Example 1, and the recombinant human thymosin β4 was prepared.
Embodiment 3
[0244] In the purification step 6 of the genetically recombinant human thymosin β4 in Example 1, the fusion protein was cleaved by the enterokinase cleavage method. The enterokinase cleavage method was: the protein concentration was determined by the Coomassie brilliant blue method, diluted to 5 mg / ml, and added Recombinant enterokinase, cut 5 mg of fusion protein per unit of recombinant enterokinase, pH 7.0, cut at 16°C for 18 hours.
[0245] Ultrafiltration with an ultrafiltration membrane with a molecular weight of 40000D, collecting the filtrate, and desalting with an ultrafiltration membrane with a molecular weight of 1000D to obtain human thymosin β4 crude protein, other steps in this step are the same as in Example 1. The other steps were the same as in Example 1, and the recombinant human thymosin β4 was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com