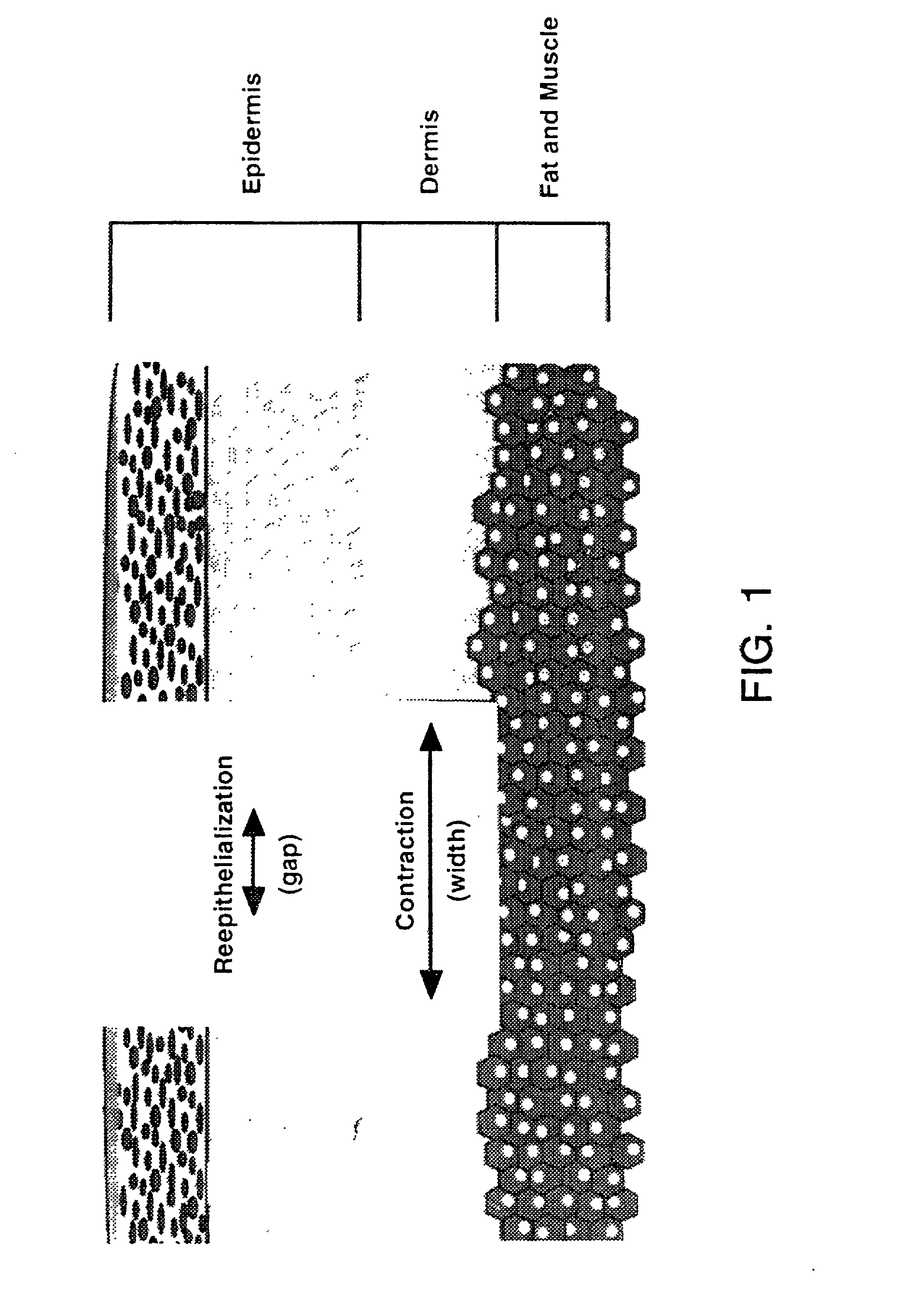
Compositions and methods for promoting wound healing and tissue repair
a tissue repair and composition technology, applied in the field of tissue repair, can solve the problems of reducing the chances of mortality and morbidity, impaired wound healing, and significant health care problems, and achieve the effects of stimulating wound repair, accelerating wound healing, and accelerating wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
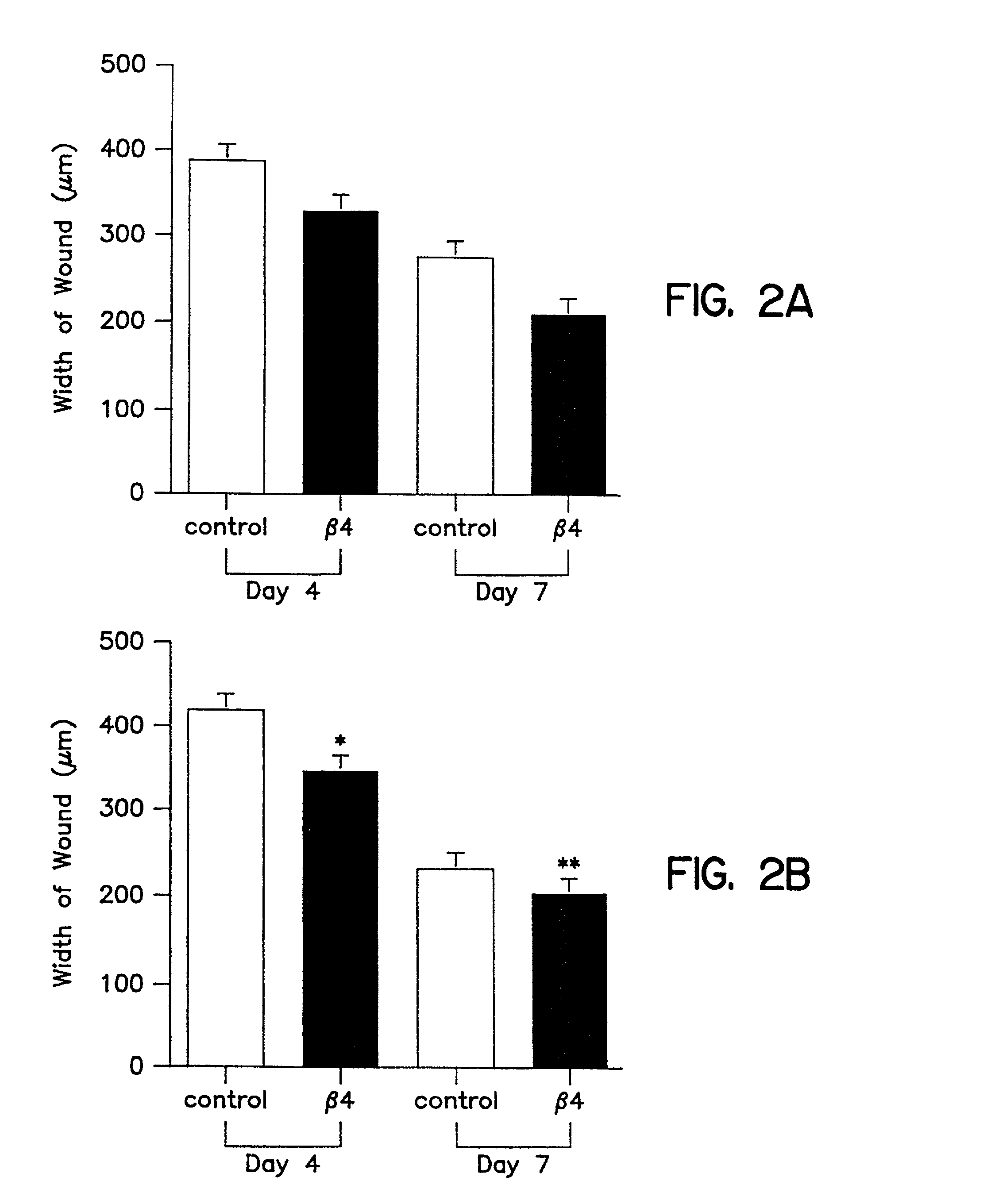
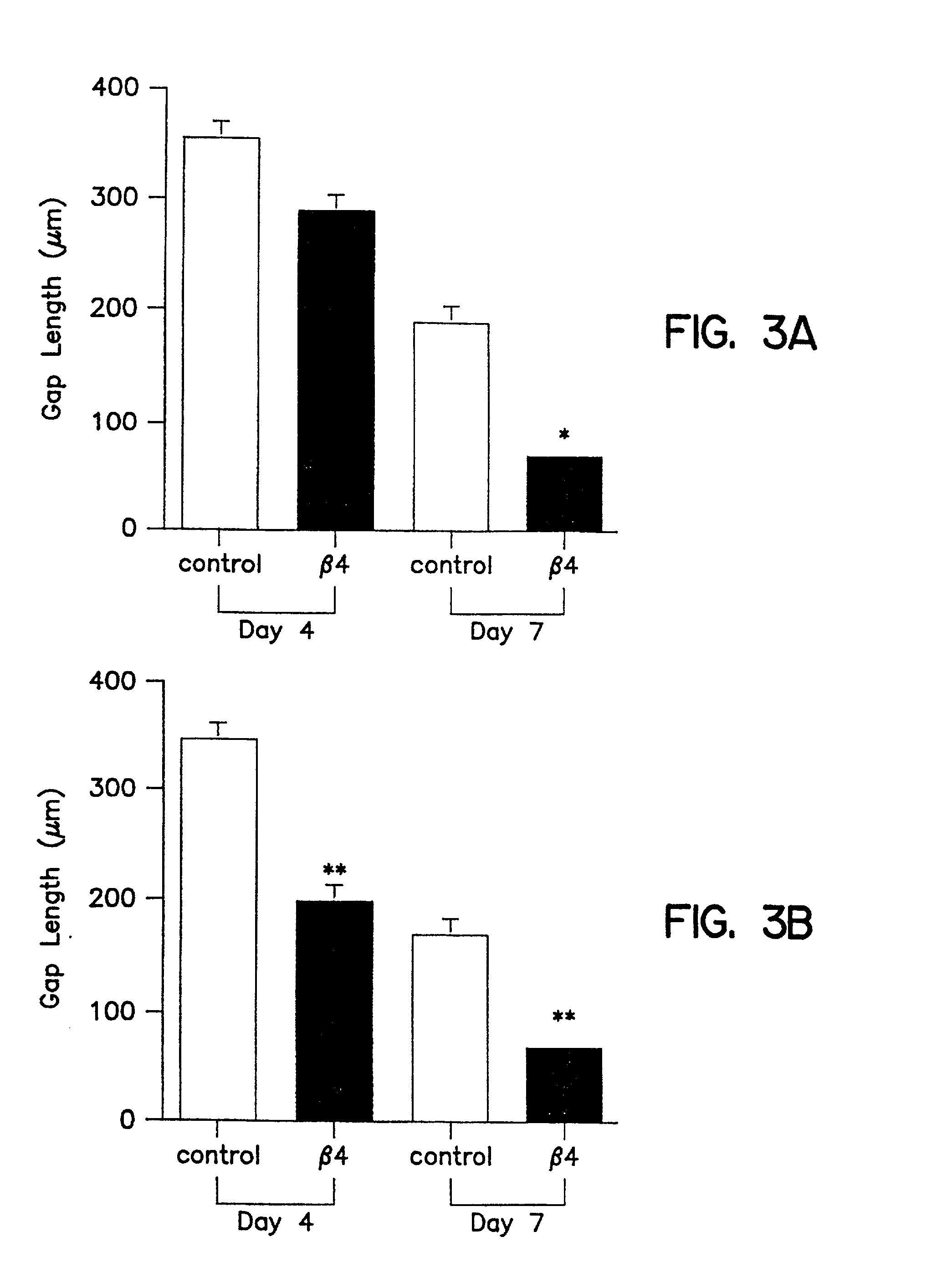
[0096] In vivo wound healing is accelerated by Tβ4
[0097] Tβ4, whether administered topically or intraperitoneal, significantly accelerated wound healing as compared to untreated wounds (FIG. 2 and 3). Full thickness 8 mm punch biopsy wounds were made on the dorsal surface of rats as previously reported (Bhartiya et al., J. Cell. Physiol. 150:312, 1992; Sihhu et al., J. Cell. Physiol. 169:108, 1996) and Tβ4 was given topically at the time of wounding (5 μg in 50 μl) and again after 48 hours. Controls for the topical treatment received identical amounts of saline at the time of wounding and at 48 hours. Additional rats received intraperitoneal injections at the time of wounding (60 μg in 300 μl) and again every other day (e.g., days 0, 2, 4, and 6). Controls for these animals received identical amounts of saline intra-peritoneally on the same injection schedule. On days 4 and 7 post-wounding, measurements were made on the wound size. At days 8 and 9 post-wounding, tissue was collecte...
example 2
[0104] Migration Assays of Keratinocytes
[0105] Primary keratinocytes were prepared from either Balb / c or CD-1 newborn mice as described previously (Dlugosz et al., 1995). Cells were plated in calcium- and magnesium-free Eagle's Minimal Essential Medium (EMEM) containing 8% fetal calf serum treated with 8% Chelex (Bio-Rad Laboratories, Hercules, Calif.), 20 units / ml penicillin-streptomycin, and the calcium concentration was adjusted to 0.25 mM. The following day, cultures were washed with calcium- and magnesium-free phosphate buffered saline, treated briefly with Trypsin (Life Technologies, Gaithersburg, Md.), washed with culture medium and resuspended in EMEM containing 0.05 mM calcium. Cells were used immediately in migration assays.
[0106] Keratinocyte migration assays were carried out in Boyden chamber using 12 μm pore polyester membranes (Poretics, Livermore, Calif.) coated with a 0.1 mg / ml solution of collagen IV in dH20 (Trevigen, Gaithersburg, Md.). Filters were then dried a...
example 3
[0108] Migration Assays of Corneal Epithelial Cells
[0109] Corneal Epithelial Cell migration assays were carried out in Boyden chamber using 12 μm pore polyester membranes (Poretics, Livermore, Calif.) coated with a 0.1 mg / ml solution of collagen IV in dH20 (Trevigen, Gaithersburg, Md.). Filters were then dried at least 1 h. Cells were cultured and resuspended in Eagle's Minimal Essential Medium with 0.05 mM Ca2+. The bottom chamber was loaded with EMEM containing 0.01, 0.1, 10, 100, and 1000 ng / ml of synthetic Tβ4. Conditioned medium from primary dermal fibroblasts and / or keratinocyte growth factor was added to several wells as a positive control. Cells were added to the upper chamber at a concentration of 50,000 cells per well. Chambers were incubated at 35 C / 7% CO2 for 4-5 hours and the filters were then fixed and stained using Diff-Quik (Baxter Healthcare Corporation, McGraw Park, Ill.). The cells that migrated through the filter were quantitated by counting the center of each w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com