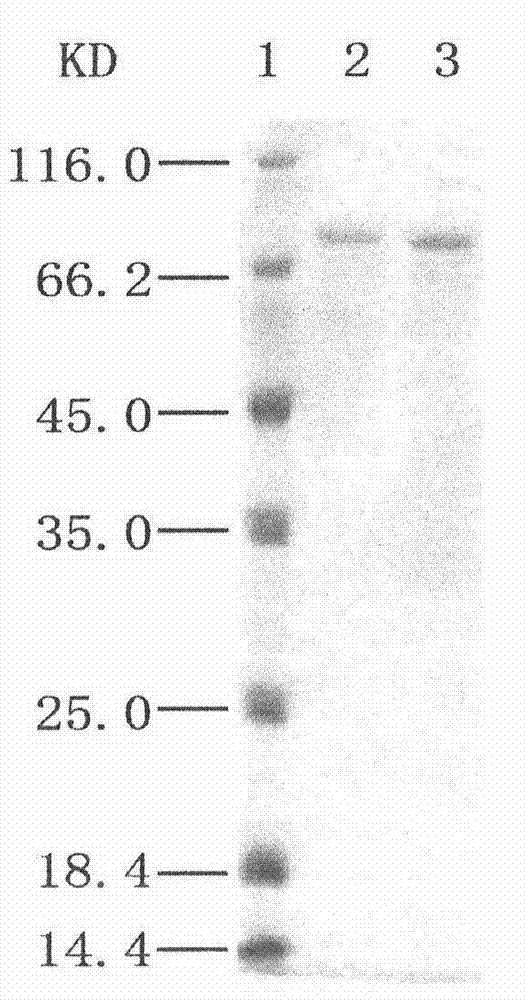
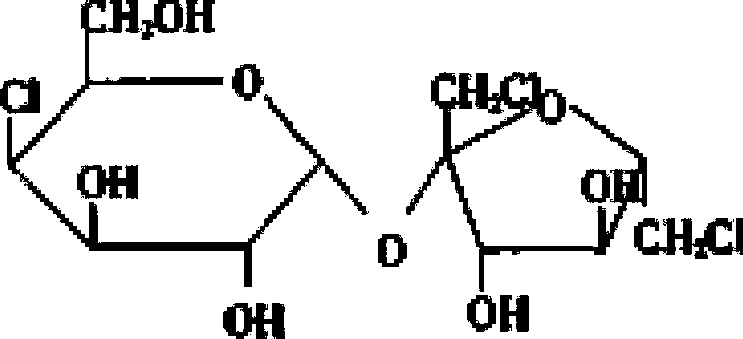
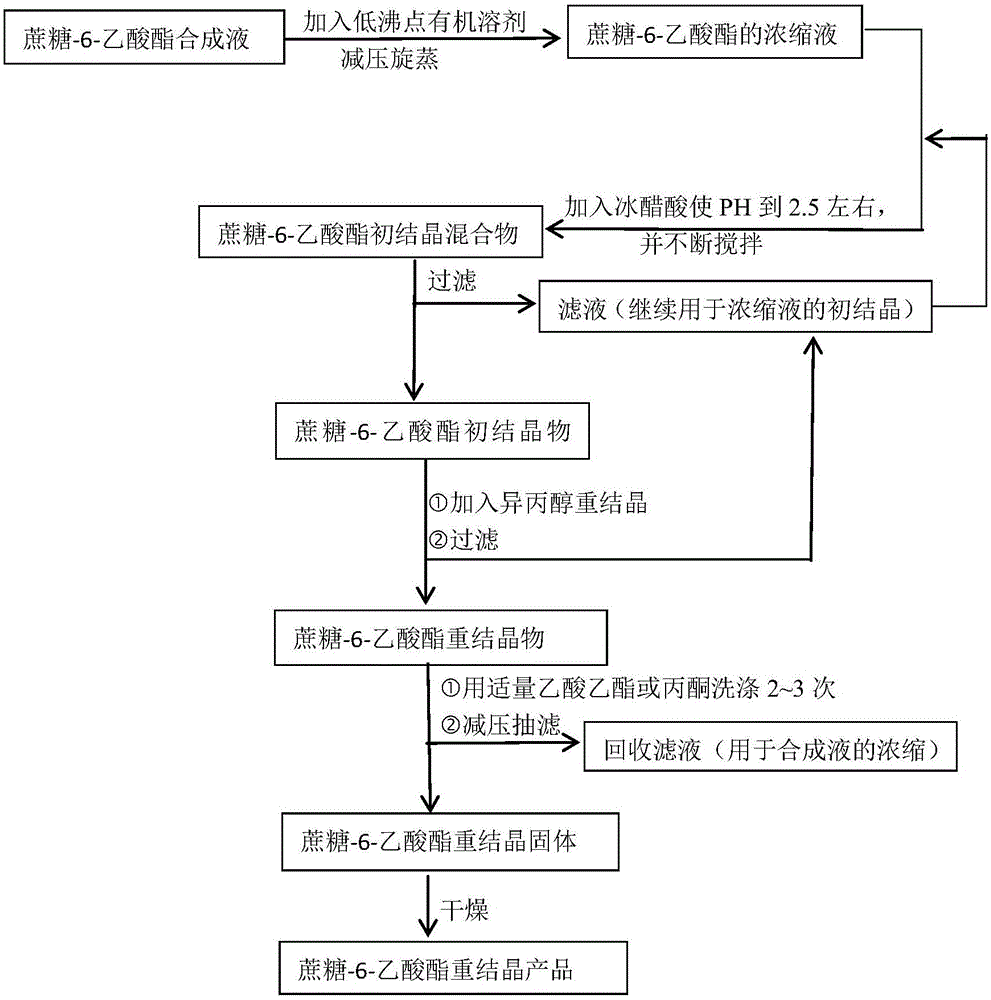
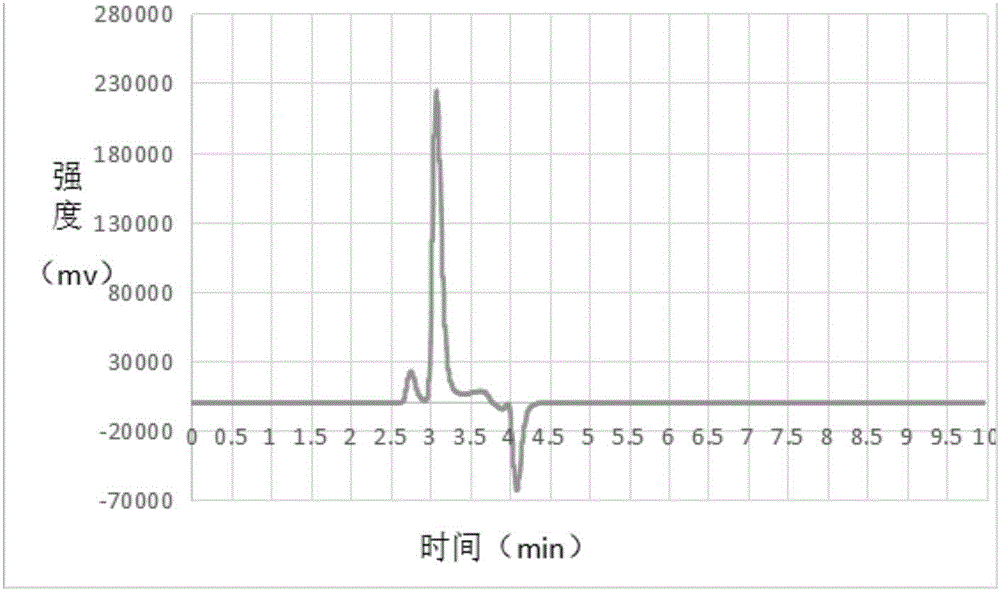
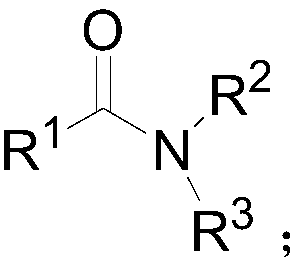
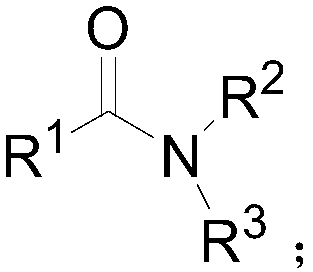
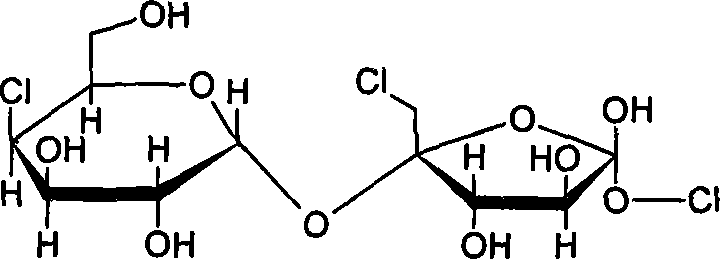
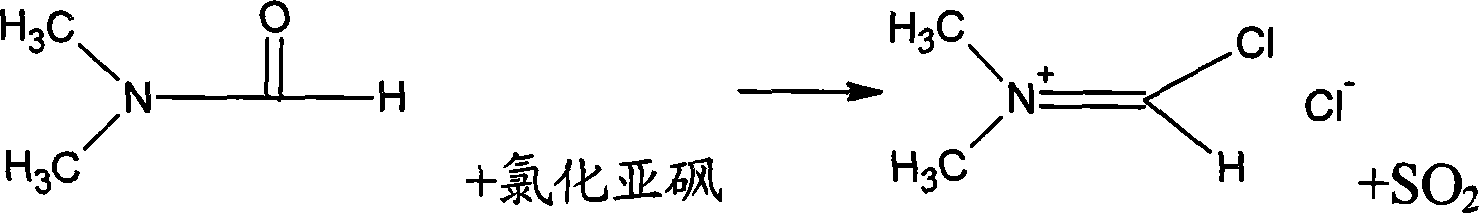
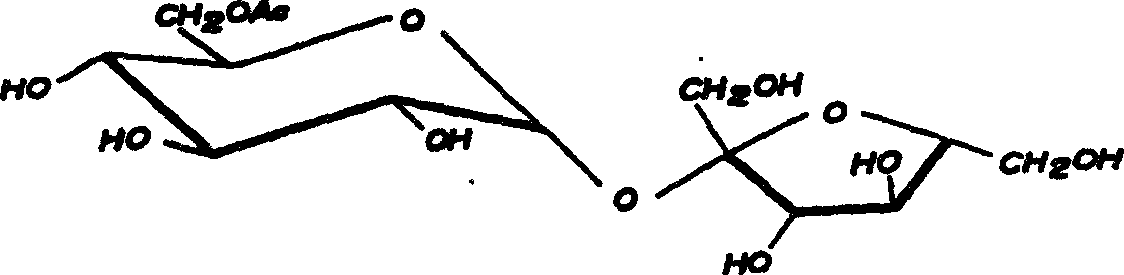Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
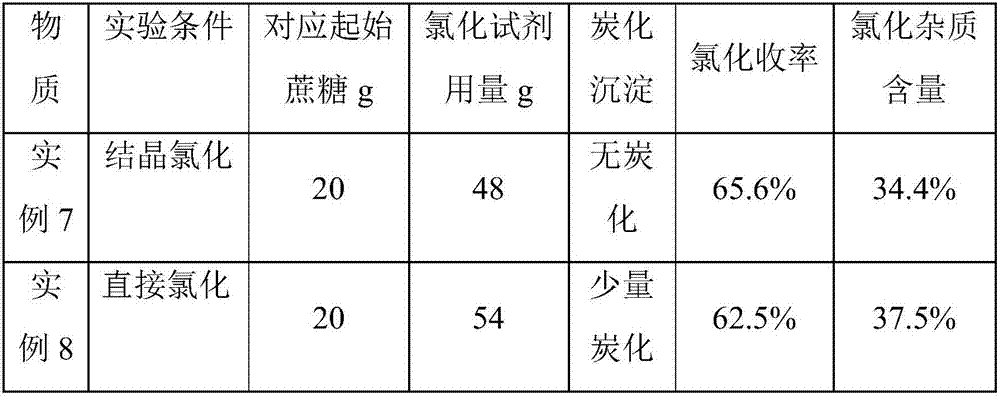
44 results about "Sucrose-6-acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
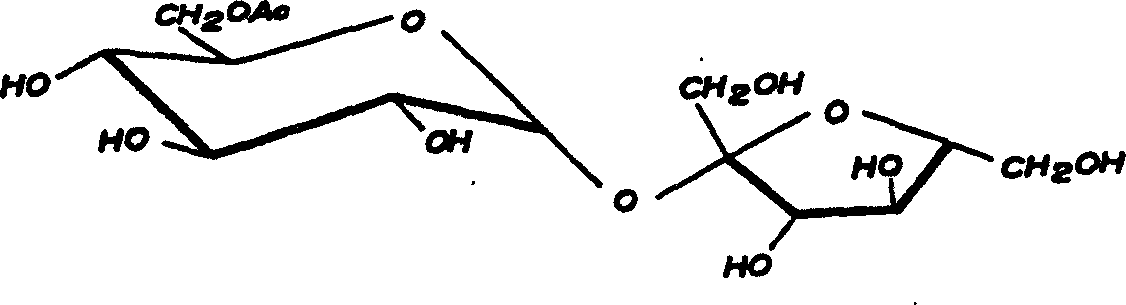
Method for on-line synthesizing sucrose-6-acetate catalyzed by lipase
ActiveCN103184257AReduce usageShort reaction timeBioreactor/fermenter combinationsBiological substance pretreatmentsAcetic acidMicrofluidic channel
The invention discloses a method for on-line synthesizing sucrose-6-acetate catalyzed by lipase. According to the method, sucrose and vinyl acetate with a molar ratio being 1 : 15-20 are used as raw materials; 0.5-1.0 g of the lipase (Lipozyme TLIM) is used as a catalyst and a mixed solvent formed by tert-amyl alcohol and DMSO is used as a reaction solvent. The lipase (Lipozyme TLIM) is uniformly filled in a reaction channel of a microfluidic channel reactor, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, and the length of the reaction channel is 0.5-1.0 m; the raw materials and the reaction solvent are continuously introduced into the reaction channel for an acylation reaction, wherein the temperature of the acylation reaction is controlled at 40-55 DEG C and the time of the acylation reaction is 20-40 min; a reaction liquid is collected on-line; and the sucrose-6-acetate is obtained after the reaction liquid is subjected to conventional post-treatment. The method provided by the invention has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
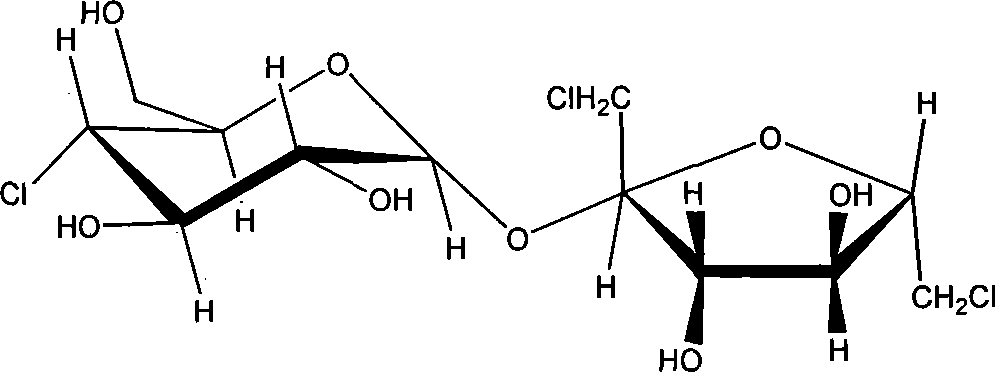
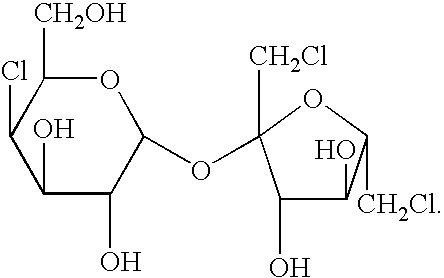
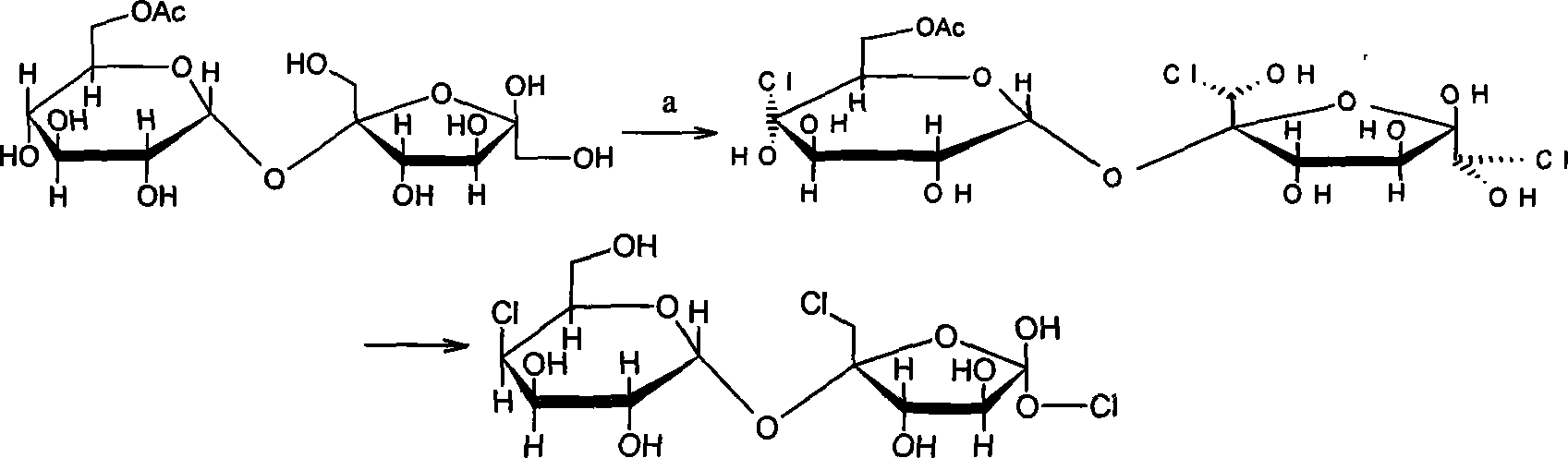
Synthesis of trichloio-sugar
InactiveCN101029062AHigh catalytic activitySimple processSugar derivativesSucrose-6-acetateAcetic anhydride
A process for synthesizing trichlorosucrose from sucrose includes such steps as adding sucrose, azeotropic agnet, solid catalyst and acetic anhydride to the organic solvent of N-amide compound, reacting at 70-95 deg.C to generate sucrose-6-acetate, chorinating, and alcoholyzing.
Owner:淄博联技甜味剂有限公司
Preparation method of sucrose-6-acetate
InactiveCN104098617AEasy to operateHigh yieldEsterified saccharide compoundsSugar derivativesAcetic anhydrideEthyl Chloride
The invention relates to a preparation method of a key intermediate sucrose-6-acetate for a novel sweetener sucralose. The method including the following specific processes: (1) adding the sucrose into an organic solvent N,N-dimethyl formamide, heating to 80-100 DEG C, dissolving, then cooling to 70 DEG C, adding a non-polar organic solvent and an organic tin compound, and conducting reflux and reaction in the presence of water for 5-8 h, wherein the reflux temperature is controlled at 80-100 DEG C; (2) cooling the reactants to below 25 DEG C, then dropwise adding acetic anhydride and reacting for 2-8 h; and (3) adding an appropriate amount of water to the above reaction liquid, extracting the organic tin compound with a non-polar organic solvent, concentrating and recycling the organic tin compound, conducing azeotropy on a raffinate and a non-polar organic solvent in the presence of water to obtain a sucrose-6-acetate solution. The method avoids the problems of solid treatment and low yield in a traditional organic tin process, has the advantages of simple operation, low labor intensity, high reaction conversion rate, and direct usage of the reaction solution without refining separation into a next step of chlorination reaction.
Owner:NANJING UNIV OF TECH
Method for preparation of sucrose-6-acetate from fructosyl transferase
InactiveCN104774889AGood application effectPromote degradationEsterified saccharide compoundsSugar derivativesCross-linkPurification methods
The invention relates to a method for preparation of sucrose-6-acetate from fructosyl transferase. The method includes the steps of: S1. preparation of fructosyl transferase; S2. preparation of immobilized fructosyl transferase; S3. catalytic synthesis of sucrose-6-acetate with fructosyl transferase in a nonaqueous phase reaction system; and S4. separation and purification of sucrose-6-acetate. The invention has the technical characteristics that: the invention establishes a fructosyl transferase separation and purification method; the invention establishes a fructosyl transferase immobilization method, magnetic chitosan microspheres are adopted as the carrier, and genipin is taken as the cross-linking agent; the invention establishes a method for catalytic synthesis of sucrose-6-acetate with immobilized fructosyl transferase, the ionic liquid [Dmin][PF6] and tert-butyl alcohol mixed solvent is taken as the reaction medium, and sucrose and glucose-6-acetate are adopted as the substrate to carry out catalytic synthesis of sucrose-6-acetate, the output and the yield respective reach 57.2g / L and 86.2%; and the invention establishes a separation and purification method for the product sucrose-6-acetate, the purity reaches 99.3%, and the recovery rate reaches 96.2%.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Preparation method of sucralose
InactiveCN103896995AEasy to operateReduce usageSugar derivativesSugar derivatives preparationAntioxidantReaction step
The invention discloses a preparation method of sucralose. The preparation method comprises the following steps: (1) adding sucrose into N,N-dimethyl formamide, adding a catalyst, ortho-acetate, water and tert-butylamine and stirring; and conducting vacuum distillation to obtain a sucrose-6-acetate N,N-dimethyl formamide solution; (2) adding a phase transfer catalyst and a chlorination reagent into N,N-dimethyl formamide to obtain a mixture, and reacting; adding an antioxidant and the solution obtained in the step (1) into the mixture, reacting to obtain a sucralose-6-ester reaction solution; and cooling to room temperature, neutralizing, distilling, extracting, concentrating and crystallizing to obtain sucralose-6-acetate; and (3) dissolving sucralose-6-acetate in methanol, regulating the pH value, reacting, neutralizing to remove impurities, decolourizing, concentrating, crystallizing, recrystallizing and drying to obtain sucralose. The invention simplifies the reaction steps, effectively avoids excessive chlorination and polymeric reaction, improves the reaction efficiency, and is beneficial to industrial production.
Owner:CHANGMAO BIOLOGICAL LIANYUNGANG
Crystallization method for synthesizing sucrose-6-acetate from sucrose and ortho-acetate
ActiveCN106749440AAvoid destructionLower azeotropic temperatureEsterified saccharide compoundsSugar derivativesFiltrationEvaporation
The invention discloses a crystallization method for synthesizing sucrose-6-acetate from sucrose and ortho-acetate. The crystallization method comprises the following steps: putting an appropriate amount of a synthetic solution of the sucrose-6-acetate into a round-bottom flask, and adding a low-boiling organic solvent of which the volume is 0.4 to 0.5 time of that of the synthetic solution; carrying out rotary evaporation, and concentrating the volume of the synthetic solution, thus obtaining a concentrated solution of the sucrose-6-acetate; transferring the concentrated solution into a beaker, and cooling to room temperature; dropwise adding glacial acetic acid in the concentrated solution until the pH (Potential of Hydrogen) value is 2.5, continuously stirring for 25 to 35 minutes under 25 to 30 DEG C and at the speed of 100 r / min, and stopping stirring when much white crystalline precipitation is formed; standing for 30 minutes until complete crystallization of the white crystalline precipitation, and carrying out suction filtration, thus obtaining a primary crystallization product of the sucrose-6-acetate; putting a certain mass of the primary crystallization product in the beaker under the room temperature, forming a saturated solution by adding isopropanol of which the mass is 1.5 to 2.5 times of that of the primary crystallization product, then adding a small amount of the primary crystallization product until completely separating out the sucrose-6-acetate, and carrying out vacuum drying under 40 to 45 DEG C, thus obtaining the sucrose-6-acetate.
Owner:JK SUCRALOSE
Synthesis method of sucrose-6-acetate
InactiveCN101693729ASimple processEasy to operateEsterified saccharide compoundsSugar derivativesAcetic acidRoom temperature
The invention discloses a synthesis method of sucrose-6-acetate, which is characterized by comprising the following steps: adding a solvent in sucrose as raw material, heating until dissolving the sucrose under the action of carbonate as a catalyst and benzyltriethylammonium chloride as a phase transfer catalyst; then cooling to be below room temperature, dropwise adding ethyl acetate for reaction; after the ethyl acetate is added, raising the temperature to 60-95 DEG C; continuously reacting for 2-6h, cooling to room temperature; filtering to remove the catalyst; and distilling over unreacted ethyl acetate to obtain the sucrose-6-acetate. The invention has the advantages of simple process, easy operation, high yield and high product purity.
Owner:李松伦
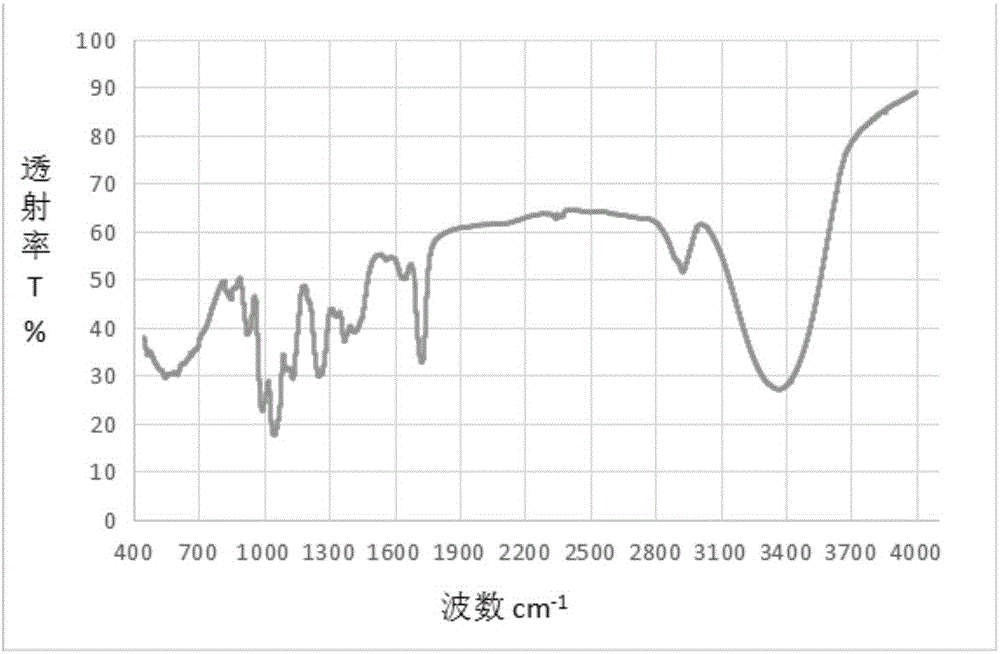
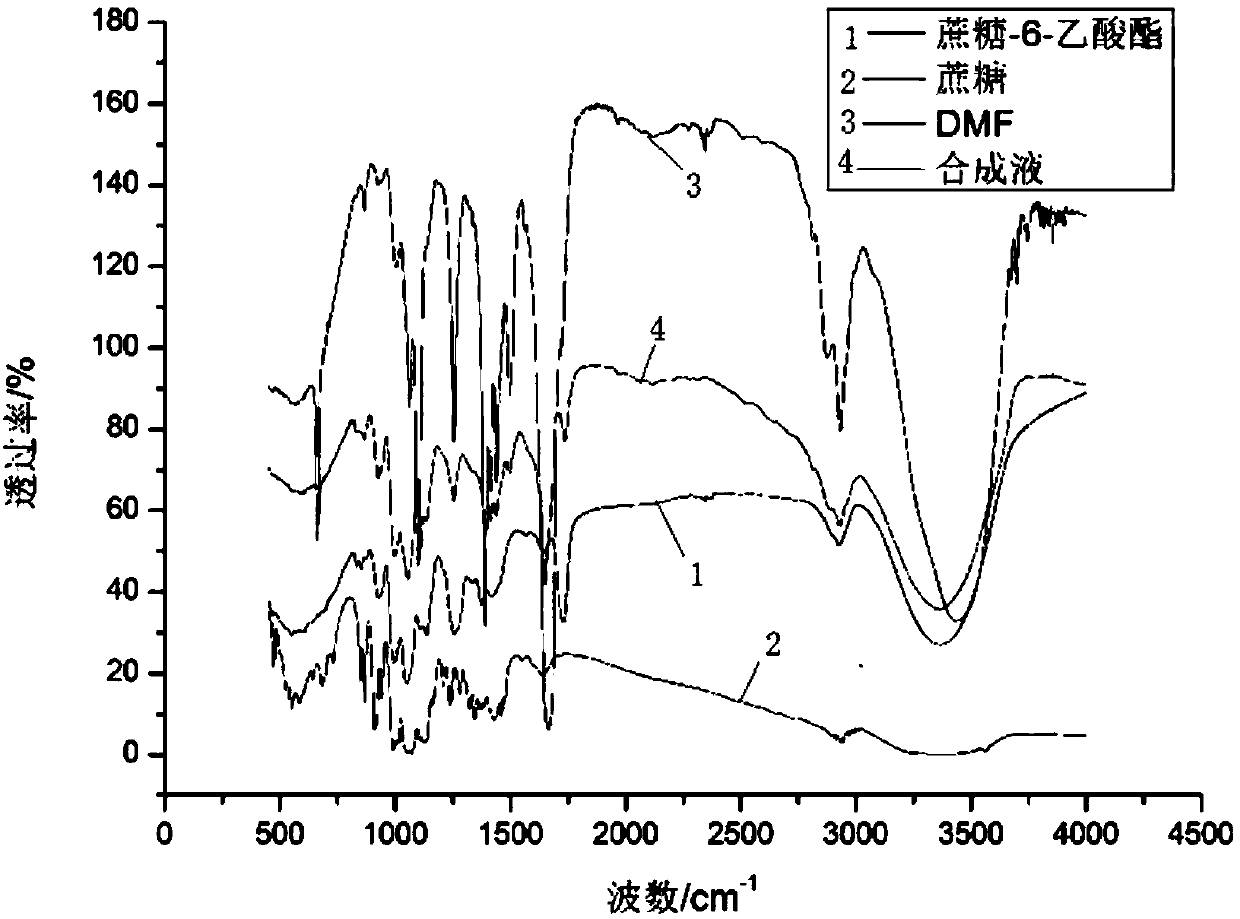
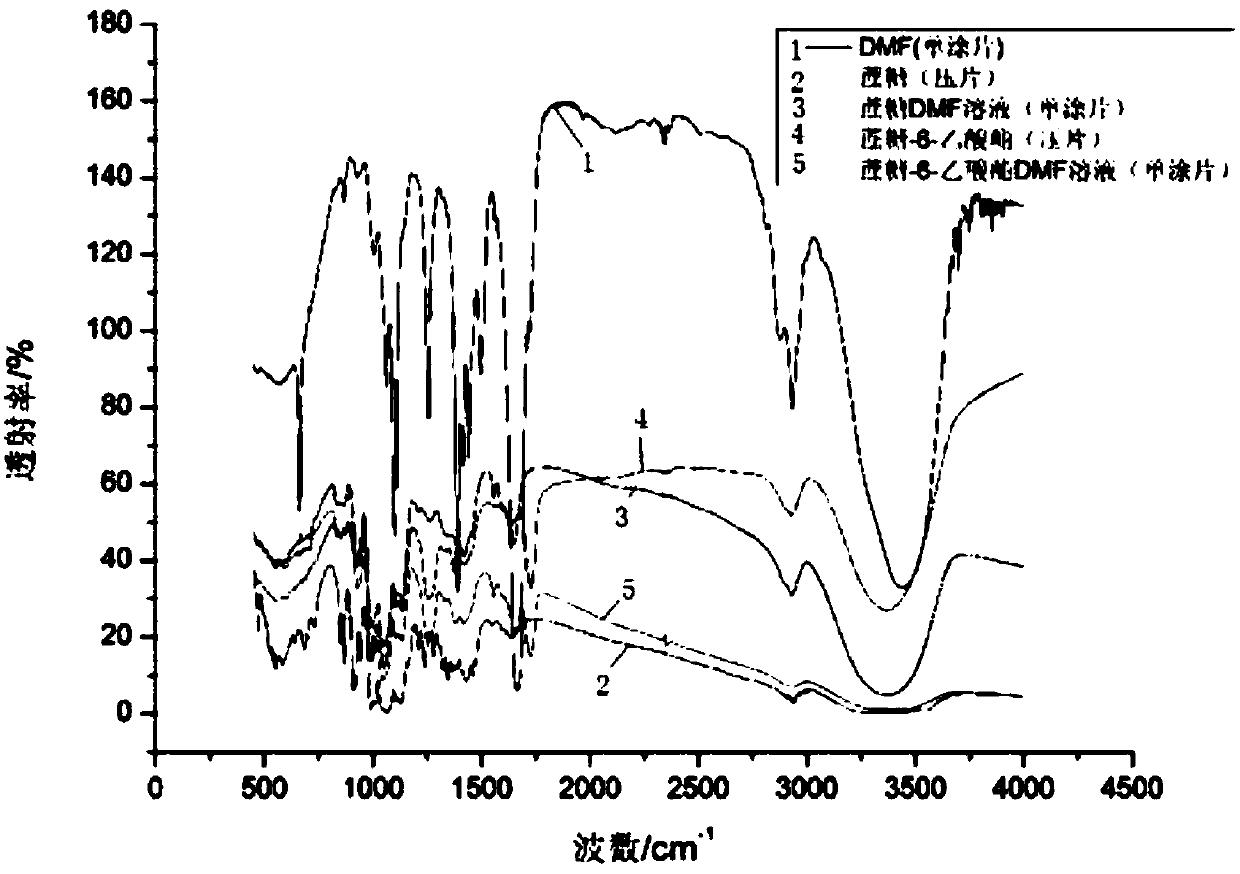
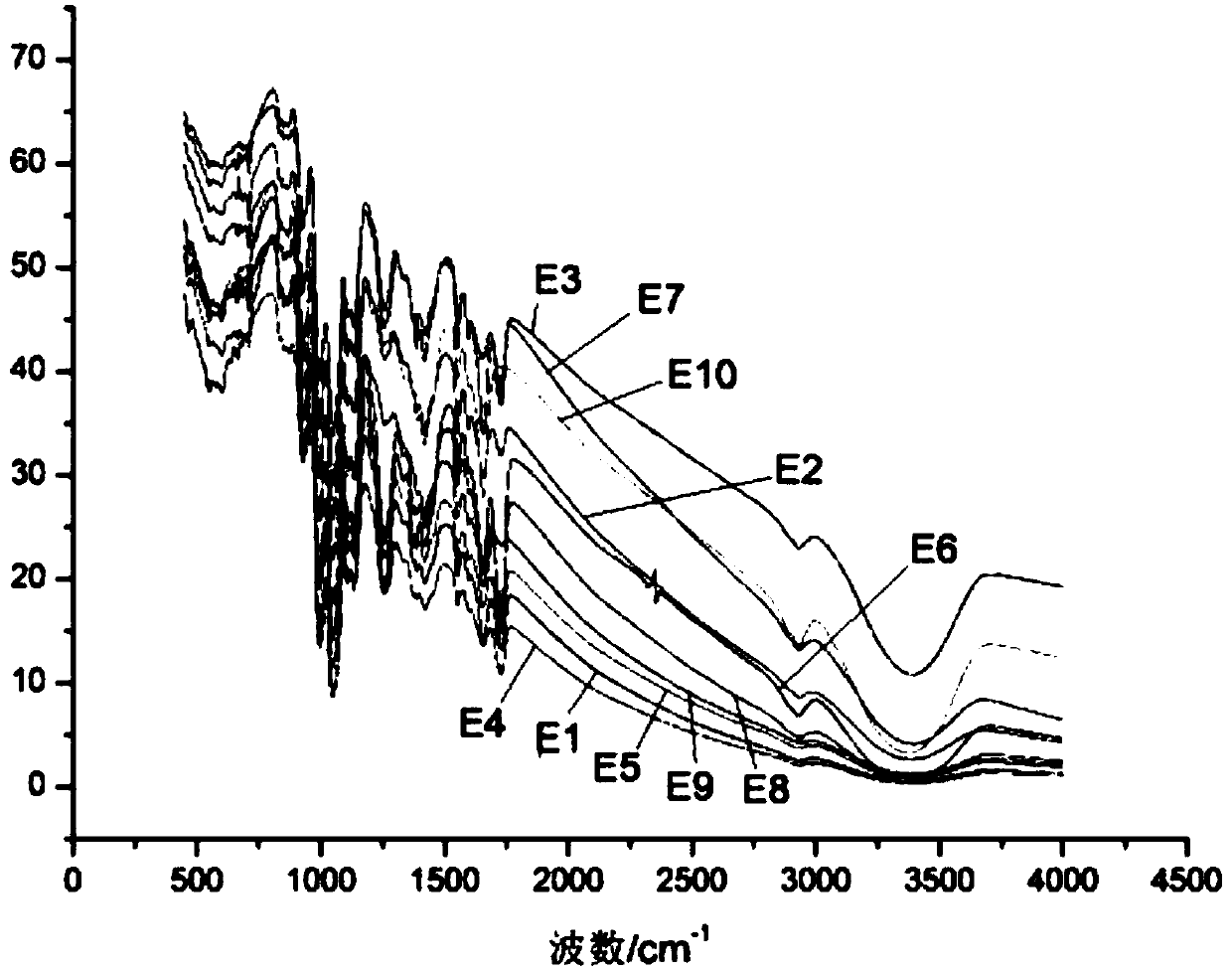
Method for quantitative analysis of sucrose-6-acetate through combination of mid-infrared spectrum and vector angle
ActiveCN107702971AGood linear relationshipEasy to operatePreparing sample for investigationMaterial analysis by optical meansData modelingData treatment
A method for quantitative analysis of sucrose-6-acetate through combination of a mid-infrared spectrum and a vector angle includes a step 1, preparing a series of samples; step 2, collecting the infrared spectrums of the samples, which includes selecting an infrared transmission mode, setting test parameters, including setting the spectrum collection range to be 450-4000 cm<-1>, the resolution tobe 4 cm<-1>, and the data interval to be 1 cm<-1>, and under the condition, collecting mid-infrared spectrums of the series of samples, including a DMF solution of sucrose-6-acetate, a DMF solution ofsucrose, DMF and a mixed solution of sucrose-6-acetate; and step 3, treating data. The method can fast accurately measure the content of sucrose-6-acetate in a synthetic process. The method doesn't require sample pretreatment and big data modeling, is simple, and has the characteristics of independence to spectral signature response, low detection limit and wide application range.
Owner:JK SUCRALOSE
Method of separating and purifying sucrose-6-acetate mother liquor by salt fractionation
ActiveCN103012509AIncrease the effective concentrationEasy to handleEsterified saccharide compoundsSugar derivativesFractionationWastewater
The invention discloses a method of separating and purifying sucrose-6-acetate mother liquor by salt fractionation. A sucrose-6-acetate crystal is obtained by salt fractionation, extracting, decoloration, filtering and crystallizing. The method is simple, quick and efficient; and by utilizing the method, not only the purifying effect obviously is improved, and the product yield and economic benefit are improved, so that actual production requirements are goodly met, but also the environment pressure is reduced, and a new path is provided for the treatment of industrial production waste water.
Owner:TECHNO (FUJIAN) FOOD INGREDIENTS CO LTD
Preparation method of dibutyltin oxide catalyzed sucrose-6-acetate single solvent
InactiveCN107011394AEsterified saccharide compoundsSugar derivativesResource consumptionDibutyltin oxide
The invention relates to a preparation method of dibutyltin oxide catalyzed sucrose-6-acetate. The preparation method is characterized by including: using dibutyltin oxide as the catalyst and trace diacetyl distannoxane as the cosolvent to prepare the sucrose-6-acetate single solvent in a single DMF solvent, and using a simple heating, alkali washing and neutralizing method to recycle the dibutyltin oxide. The preparation method has the advantages that the method is high in sucrose-6-acetate yield, low in resource consumption and high in operability in industrial production, the prepared sucrose-6-acetate is good in quality, and the catalyst recycling method is simple.
Owner:林洪
Synthesis of sucrose-6-acetate
InactiveCN1176095CSimple processHigh purityEsterified saccharide compoundsSugar derivativesAcetic acidSulfate
The present invention discloses the synthesis of sucrose-6-acetate. Sucrose material is mixed with N, N-dimethyl formamide solution, made to produce ester exchange reaction with ethyl acetate under the action of solid sulfate catalyst, which may be adsorbed on polymer carrier, to produce sucrose-6-acetate. Sucrose-6-acetate is the key intermediate for synthesizing trichlorosucrose as sweetening agent. The present invention has the advantages of simple technological process, high product purity, low production cost, etc. and is suitable for industrial production.
Owner:广东广业清怡食品科技股份有限公司
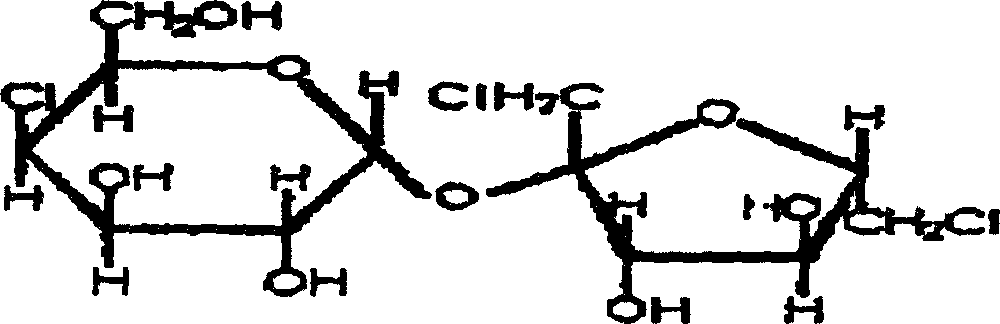
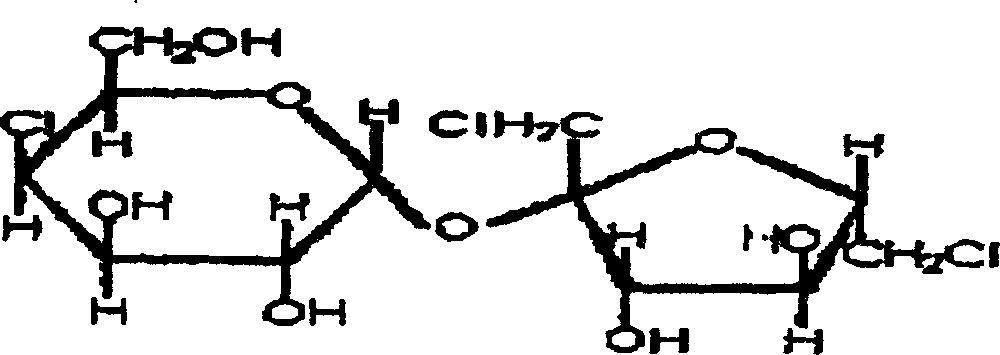
Synthesis of trichlorosucrose
InactiveCN1176094CSimple processHigh purityEsterified saccharide compoundsSugar derivativesAcetic acidSulfate
The present invention discloses the synthesis of trichlorosucrose. Sucrose material is mixed with N, N-dimethyl formamide solution, made to produce ester exchange reaction with ethyl acetate in the action of solid sulfate catalyst, which may be adsorbed on polymer carrier, to produce sucrose-6-acetate. Sucrose-6-acetate is then chlorohydrinated to produce trichlorosucrose. The present invention has the advantages of simple technological process, high product purity, low production cost, etc. and is suitable for industrial production.
Owner:广东广业清怡食品科技股份有限公司
Recrystallization method of sucrose-6-acetate and applications thereof
ActiveCN106946956AOvercome carbonizationLow impurity contentEsterified saccharide compoundsSugar derivativesEthyl ChlorideEther solvent
The invention discloses a recrystallization method of sucrose-6-acetate, and applications thereof. The recrystallization method comprise following steps: a solvent is added into a sucrose-6-acetate mixture obtained via sucrose esterification; and stirring, dissolving, filtering, and drying are carried out so as to obtain sucrose-6-acetate, wherein the solvent is a mixture of a nitrile solvent and an ether solvent. The recrystallization method is capable of increasing sucrose-6-acetate purity, and reducing adverse influences on subsequent chlorination.
Owner:ZHEJIANG NHU CO LTD
Process for synthesizing and purifying sucralose
ActiveUS20100292462A1Simple processHigh puritySugar derivativesChemical recyclingAcetic acidAcetic anhydride
The present invention discloses a process for synthesizing sucralose, which comprises reacting sucrose with acetic anhydride in the solvent of a N-amide compound in the presence of an organic complex alkali metal salt catalyst to produce sucrose-6-acetate, and then chlorinating and deacetylating the sucrose-6-acetate to give sucralose. The present invention also discloses a process for purifying sucralose, which comprises purifying crude sucralose with one or more organic solvents to obtain purified sucralose.
Owner:HANGZHOU XINFU TECH CO LTD +1
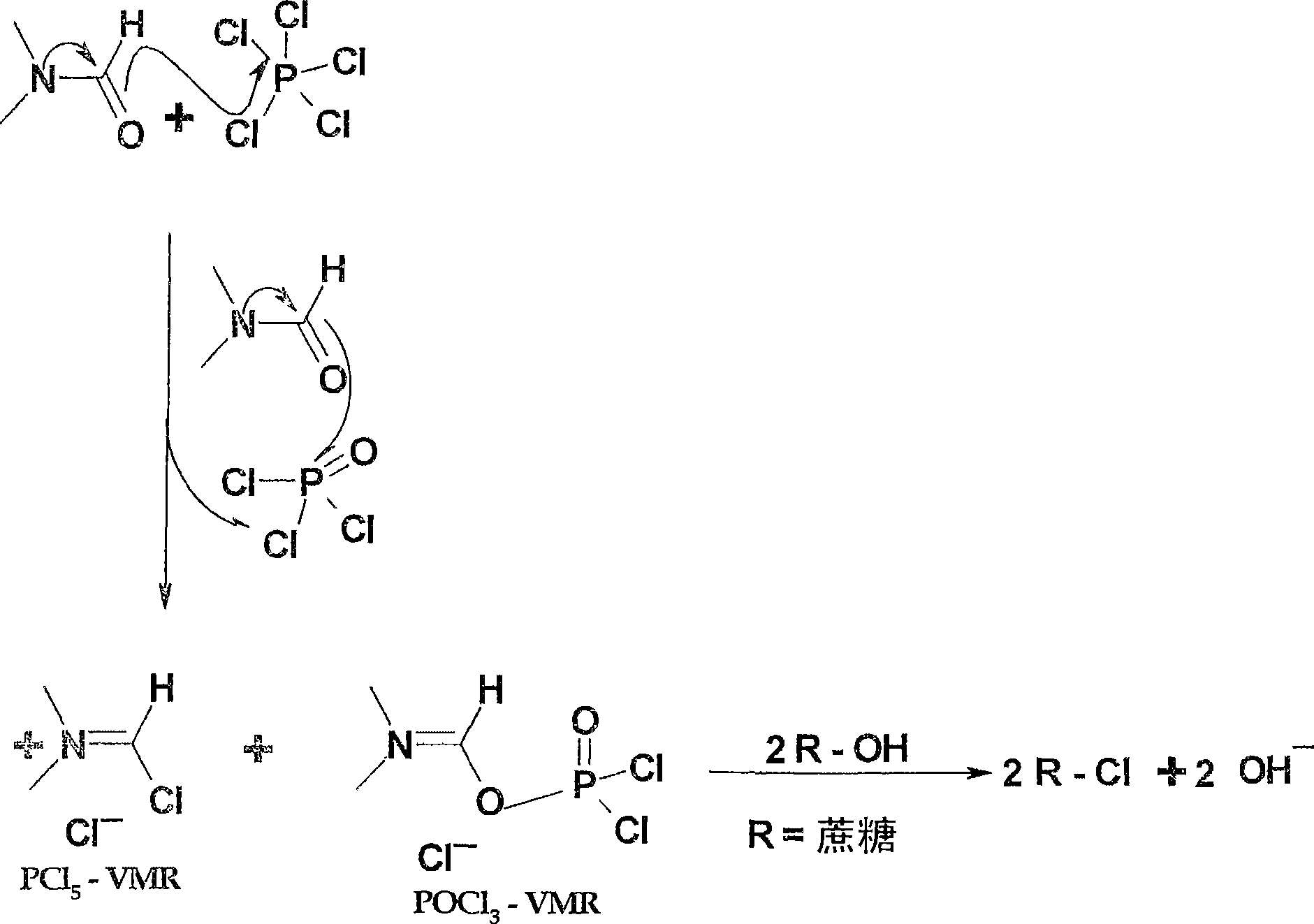
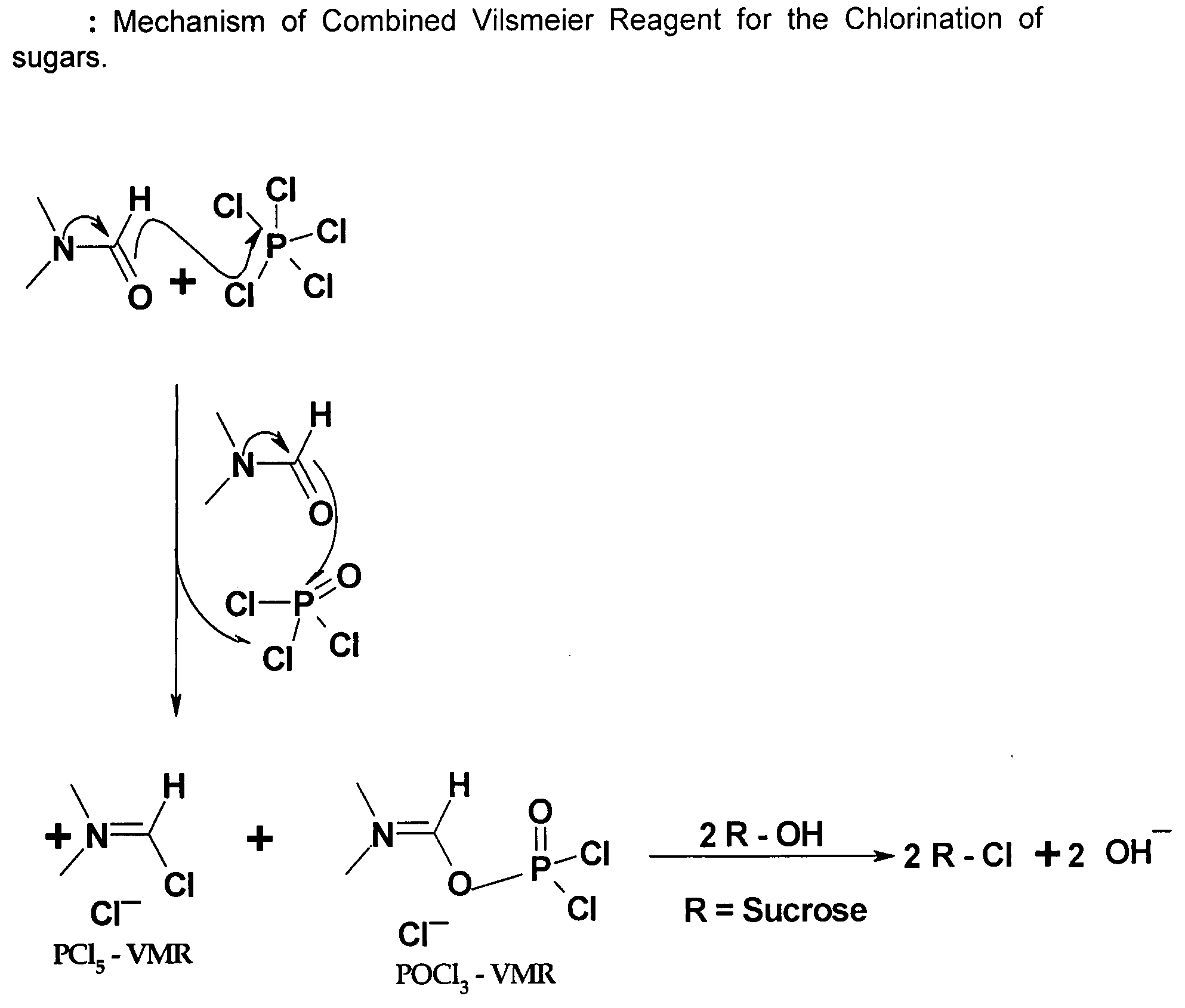
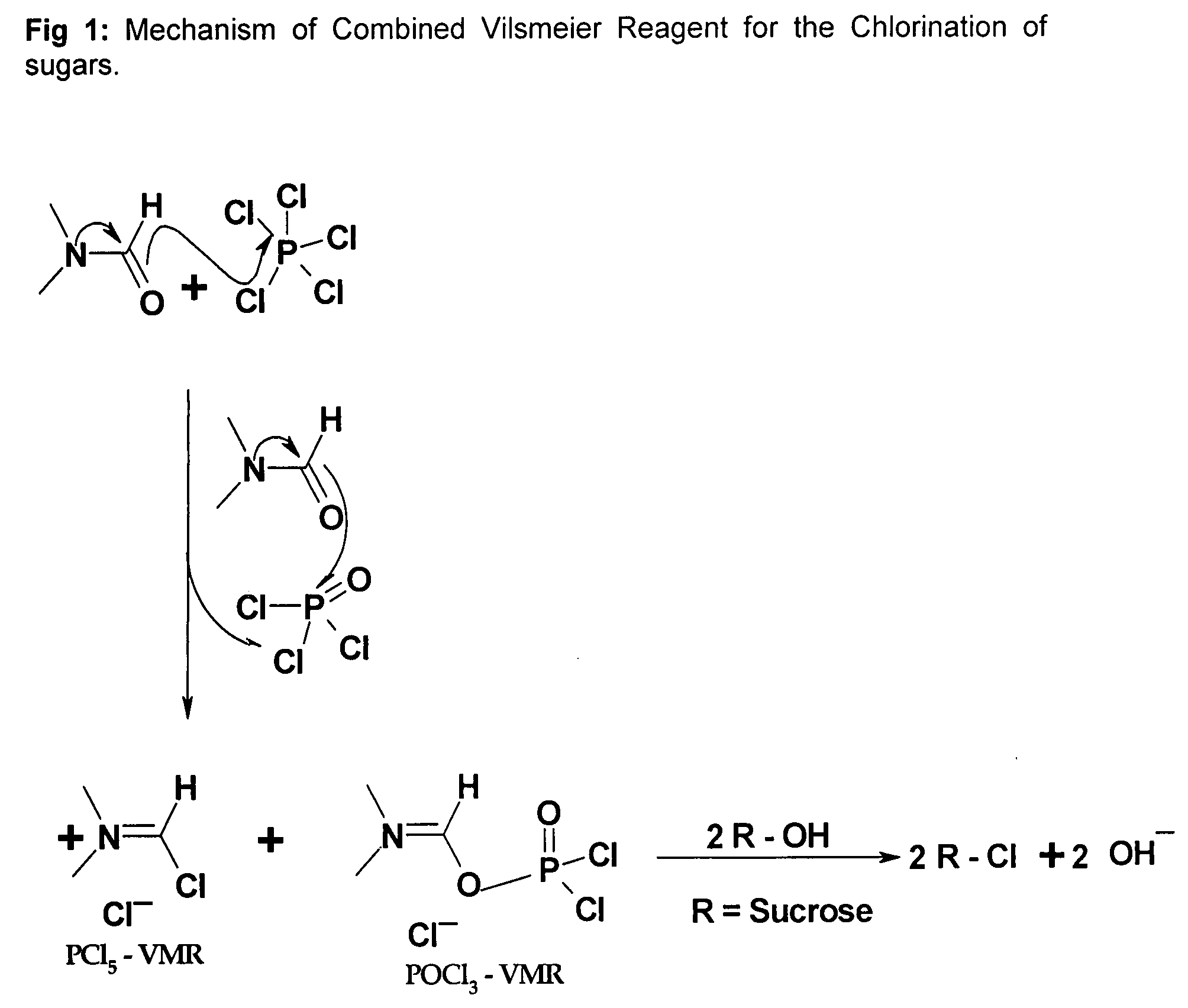
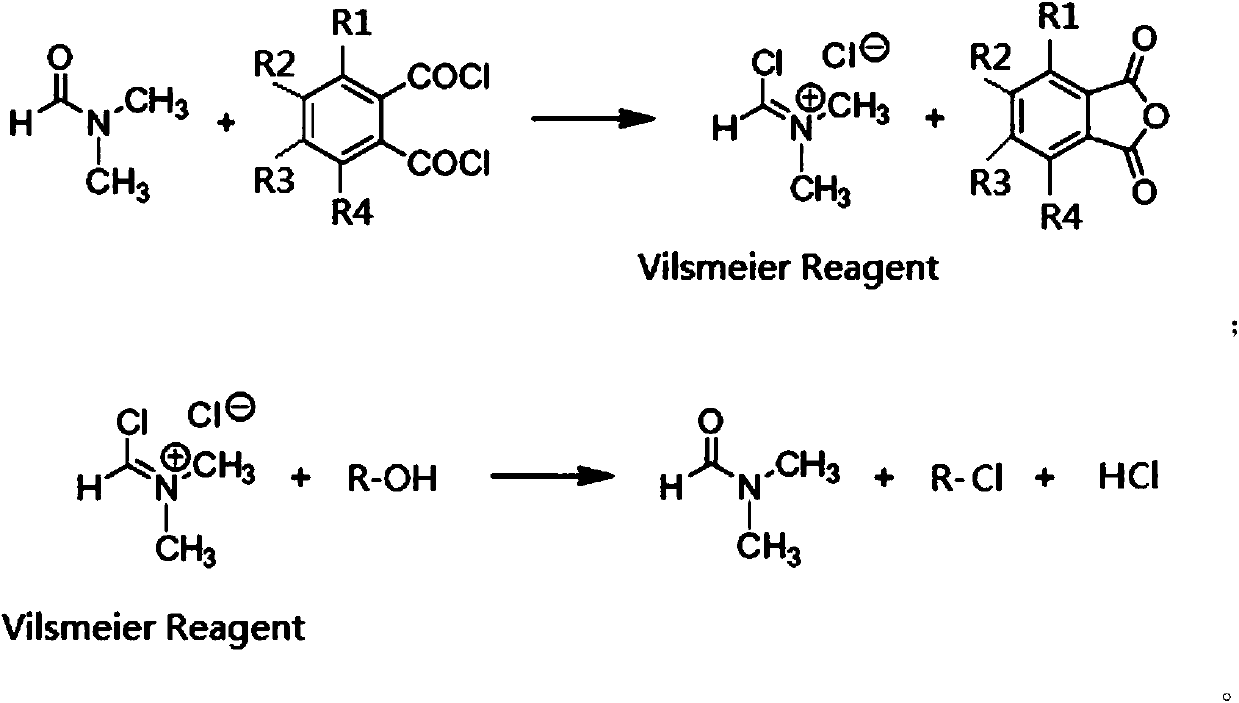
Generation of phosphorus oxychloride as by-product from phosphorus pentachloride and dmf and its use for chlorination reaction by converting into vilsmeier-haack reagent.
InactiveCN101490070AEsterified saccharide compoundsPhosphorus halides/oxyhalidesN dimethylformamidePhosphorus pentachloride
A process is described wherein after formation of first crop of Vilsmeier-Haack reagent, a by-product of the reaction, namely Phosphorus Pentachloride, reacts with N,N-dimethylformamide to form the second crop of Vilsmeier reagent. The PCl5 reacts with N,N-dimethylformamide to obtain the first crop of Vilsmeier reagent as insoluble crystals. The second crop of Vilsmeier reagent is soluble in DMF. This process makes it possible to double the yield of chlorinated substrate, such as sucrose-6-acetate or sucrose-6-benzoate, from the same quantity of Phosphorus Pentachloride.
Owner:V B MEDICARE PVT LTD
Use of Acid Scavengers in Removal of Protons (Acidity) of the Reaction Mass During Chlorination of Sucrose-6-Acetate
InactiveUS20090163704A1Increase productionSugar derivativesSugar derivatives preparationAcetic acidScavenger
A process is described wherein efficiency of chlorination is improved in a process for production of a chlorinated sucrose by scavenging, using an acid scavenger, of excess of acidic protons formed during a chlorination reaction between 6-O-acyl sucrose in dimethylformamide and a chlorinating reagent.
Owner:V B MEDICARE PVT LTD +1
Low-temperature and efficient preparation method of sucrose-6-acetate
InactiveCN106632533AReduce the temperatureThoroughly dehydratedEsterified saccharide compoundsSugar derivativesOrganic solventCarboxylic acid
The invention discloses a low-temperature and efficient preparation method of sucrose-6-acetate. The method comprises the steps of dissolving sucrose into an organic solvent, mixing with an organic tin acylation accelerator containing a cosolvent, removing generated water in vacuum and low-temperature conditions; and adding carboxylic acid anhydride at 0 DEG C for thermal reaction and terminating reaction through adding water to obtain the sucrose-6-acetate. The preparation process of the sucrose-6-acetate is low in temperature, thorough in dehydration and stable in production; the obtained product is high in purity, especially the sucrose residue content is low; the difficulty of subsequently removing 6'-tetradeoxygalactosucrose is reduced; and the yield of the sucrose-6-acetate is high. In addition, the organic tin acylation accelerator can be utilized in cycle without removing the cosolvent, the difficulty of solvent treatment in the preparation process is reduced, and massive production is facilitated.
Owner:TECHNO (FUJIAN) FOOD INGREDIENTS CO LTD
Generation of Phosphorus Oxychloride as by-Product from Phosphorus Pentachloride and DMF and its Use for Chlorination Reaction by Converting Into Vilsmeier-Haack Reagent
InactiveUS20090131653A1Efficient executionEsterified saccharide compoundsIsocyanic acid derivatives preparationAcetic acidN dimethylformamide
A process is described wherein after formation of first crop of Vilsmeier-Haack reagent by reacting Phosphorus Pentachloride with N,N-dimethylformamide to form a first crop of Vilsmeier reagent as insoluble crystals, a by-product of this reaction, the Phosphorus Oxy-Chloride, reacts with N,N-dimethylformamide to give a second crop of Vilsmeier reagent. This second crop of Vilsmeier reagent is soluble in DMF. This process makes it possible to double the yield of chlorinated substrate, such as sucrose-6-acetate or sucrose-6-benzoate, from the same quantity of Phosphorus Pentachloride.
Owner:V B MEDICARE PVT LTD
Method for increasing crystallization yield of trichloro sucrose-6-acetate
InactiveCN108250255AImprove solubilityHigh yieldEsterified saccharide compoundsSugar derivativesAlkaneSolubility
The invention relates to a method for increasing the crystallization yield of trichloro sucrose-6-acetate. The method is characterized by comprising the following production steps: mixing 30.0 parts of a trichloro sucrose-6-acetate crude product with 40.0 parts of a crystallization solvent according to a proportion at room temperature, and adding the obtained mixture into a reaction kettle; then,heating up to 50-70 DEG C and stirring for 1h at the temperature of 50-70 DEG C; after that, stirring at low speed, naturally cooling to room temperature, then cooling the solution to -10 to 10 DEG C,standing and crystallizing for 3h; finally, filtering and drying to obtain the trichloro sucrose-6-acetate crystals. The method has the beneficial effects that a mixed solvent formed by alcohol and alkane is used as a crystallization solvent, and crystallization is performed at the low temperature of -10 to 10 DEG C, so that the product yield is high, and the product purity is good; the mixed solvent used in the method is better in solubility to the trichloro sucrose-6-acetate at the high temperature and poorer in solubility to the trichloro sucrose-6-acetate at the low temperature, so that the dissolution of impurities and the precipitation of the product are facilitated. The method obviously increases the crystallization yield and lowers the product cost under the premise of guaranteeing the product purity.
Owner:SHANDONG KANBO BIOCHEM TECH +1
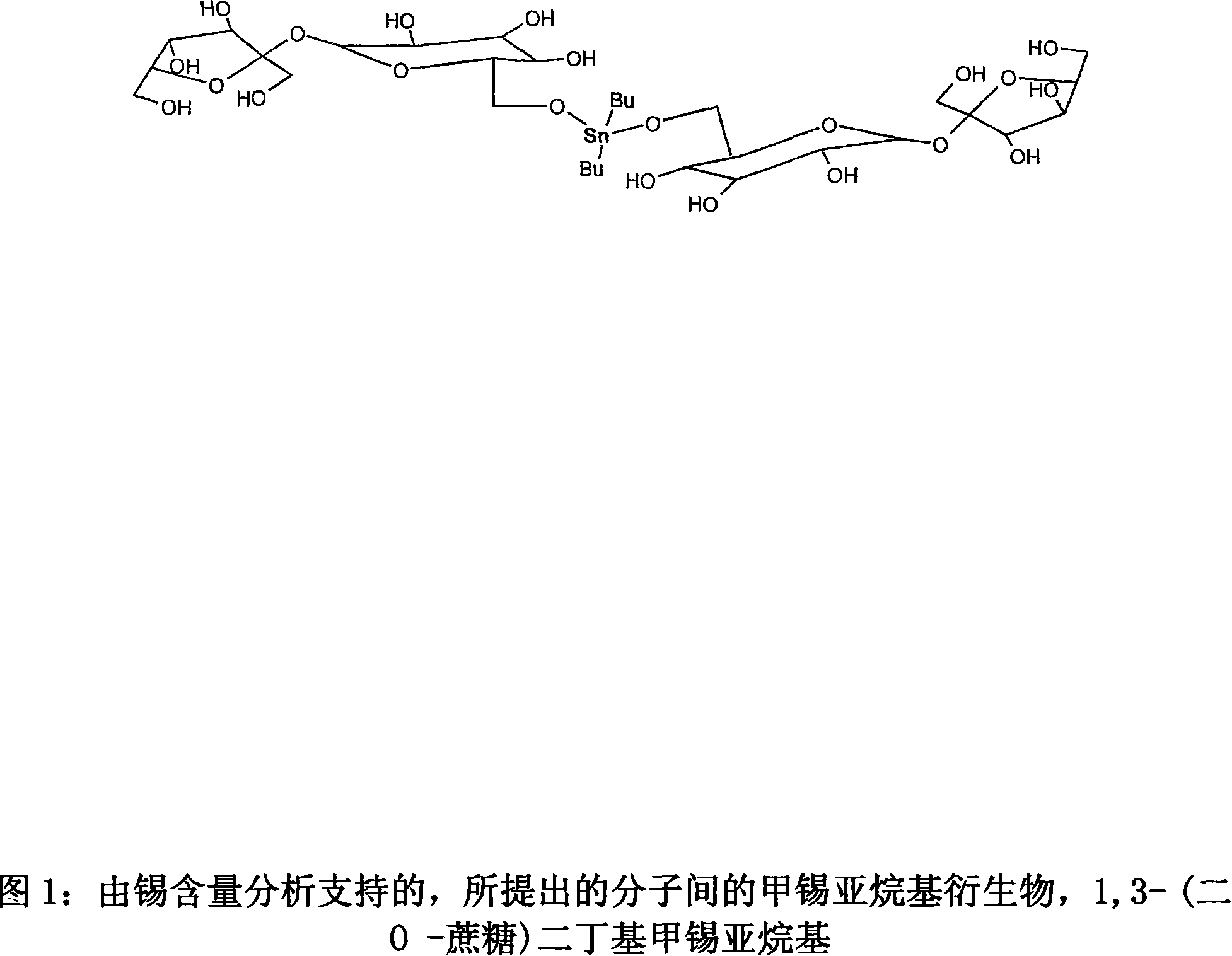
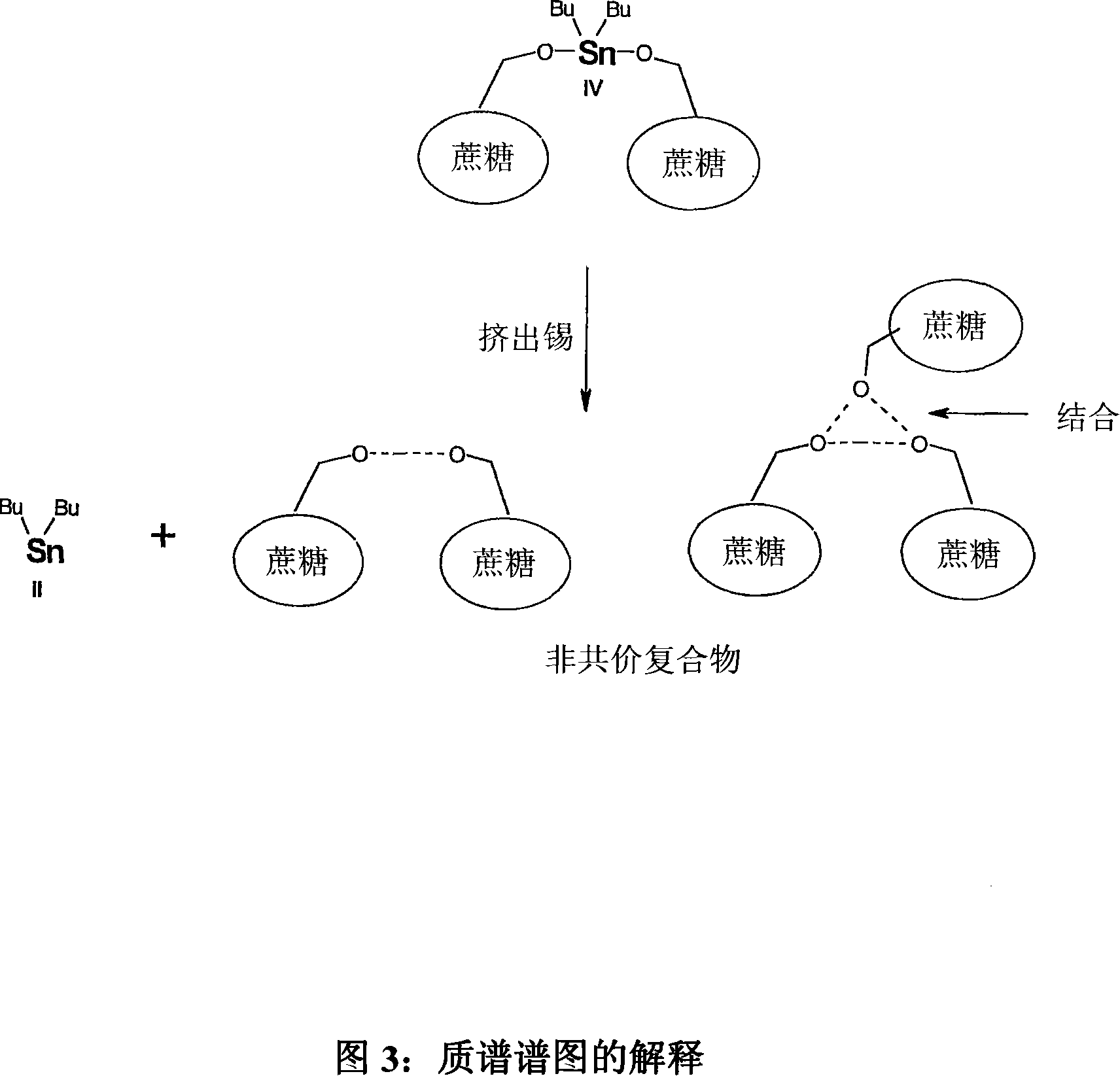
Tin mediated regioselective synthesis of sucrose-6-esters
InactiveCN101132705AEsterified saccharide compoundsSugar derivativesRegioselectivitySaccharophagus degradans
A method is disclosed for regioselective synthesis of sucrose-6-acetate via formation of a novel sucrose-tin adduct using sucrose and DBTO. The novel tin adduct can be represented by a formula (6-O-sucrose) - O - Snbutyl.sub.2 -O- (6-O-sucrose) or as 1,3.( di O-sucrose) dibutyl stannylene. The adduct is acylated to yield sucrose-6-acetate or sucrose-6-benzoate as major product.
Owner:V B MEDICARE PVT LTD
Green synthesis method of sucralose-6-acetate
PendingCN109897075AAvoiding Difficult-to-Separate ProblemsAvoid interferenceEsterified saccharide compoundsSugar derivativesSynthesis methodsEthyl Chloride
The invention discloses a green synthesis method of sucralose-6-acetate. The green synthesis method comprises the steps that step 1, benzene ring substituted phthaloyl chloride and DMF are adopted toreact in a non-polar organic solvent to generate Vilsmeier salt; step 2, the Vilsmeier salt and sucrose-6-acetate are chloridized in a polar organic solvent to obtain the product sucralose-6-acetate.The green synthesis method of the sucralose-6-acetate has the advantages that no sulfur dioxide gas is released in the reaction, the problem that tail gas sulfur dioxide and hydrogen chloride gas aredifficultly separated in a traditional process is avoided, the difficulty of tail gas treatment is greatly reduced, and the method is a novel green chlorination process; the separation purity of the Vilsmeier salt is high, and the reaction yield is high; acid anhydride produced during the preparation of the Vilsmeier salt can be rechlorinated to generate acyl chloride to be recycled, thereby reducing the production cost; the chlorination process is simpler in operation, more convenient in post-treatment and suitable for industrial production.
Owner:JK SUCRALOSE
Purification method for sucrose-6-acetate
ActiveCN103288891AHigh yieldHigh purityEsterified saccharide compoundsSugar derivativesChromatographic separationAcetic acid
The invention discloses a purification method for sucrose-6-acetate, which belongs to the technical field of purification of organic matters. The method comprises the following steps: mixing a crude product of sucrose-6-acetate with petroleum ether to form an emulsion; uniformly mixing the emulsion with silica gel to form a to-be-separated liquid; and adding the to-be-separated liquid into a silica gel-petroleum ether chromatographic column for chromatographic separation by using a developing solvent and subjecting a flow containing sucrose-6-acetate to reduced pressure condensation so as to obtain a finished product of sucrose-6-acetate. The purification method for sucrose-6-acetate provided by embodiments in the invention has the advantages of simple process, high purity (more than 98%) and high yield of the target product, capacity of improving purity and quality of the final product sucralose and reduced production cost. Since the developing solvent and the silica gel used in the process of separation can be reused, cost for purification is effectively reduced.
Owner:HUBEI HONGYUAN PHARMA
A kind of purification method of sucrose-6-acetate
ActiveCN103319548BLow equipment requirementsSuitable for the needs of large-scale industrial productionEsterified saccharide compoundsSugar derivativesAcetic acidPurification methods
The invention provides a purification method for cane sugar-6-acetate. The purification method comprises the specific steps of: adding isoamylol in a cane sugar-6-acetate crude product on the condition of a reaction temperature of 20-80 DEG C; separating out crystals, and filtering; and drying the separated crystals in a vacuum drying box, wherein the volume ratio of isoamylol to the cane sugar-6-acetate crude product is 1: 1 to 5: 1. According to the purification method, isoamylol is used as an extraction agent, isoamylol is a polar solvent, and the polarity of cane sugar is greater than the polarity of cane sugar-6-acetate, so that cane sugar-6-acetate can be separated from isoamylol; moreover, the needed amount is low, the required temperature is low, and the requirement on equipment in industrial production is low; simultaneously, the purification method is high in economic benefits and meets the need of large-scale industrial production.
Owner:天津北方食品有限公司
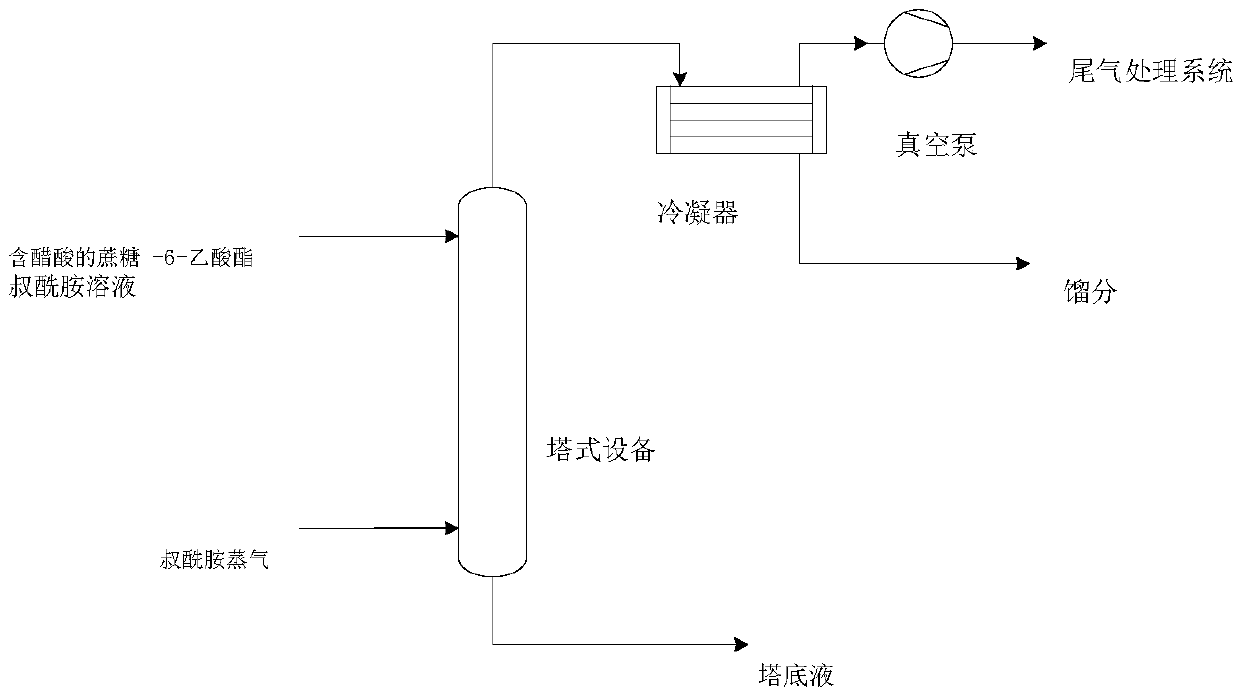
Method for removing acetic acid in sucrose-6-acetate solution and application thereof
ActiveCN108676041AWon't clogFree from destructionEsterified saccharide compoundsSugar derivativesAcetic acidResidence time
The invention discloses a method for removing acetic acid in a sucrose-6-acetate solution and application thereof. The method comprises the following steps: under a certain residence time condition, in tower equipment for removing acetic acid, vapor formed by tertiary amide of certain temperature is in contact with the sucrose-6-acetate solution containing acetic acid in a continuous countercurrent manner, thereby achieving the purpose of removing the acetic acid from the sucrose-6-acetate solution. The method provided by the invention can be applied to the synthesis of sucrose-6-acetate and sucralose, has the advantages of simple process, high acetic acid removal rate, no damage to a sucrose-6-acetate product, continuity and the like, and is very suitable for industrial production.
Owner:SHANDONG NHU FINE CHEM SCI & TECH CO LTD +1
Chlorination method for trichlorosucrose preparation
InactiveCN101239997AMild reaction conditionsControllableSugar derivativesN dimethylformamideOrganic solvent
The invention discloses a chlorination process in preparation of sucralose, including steps of: putting N,N-dimethylformamide into reaction vessels, adding chlorination agent at normal temperature, wherein ratio between chlorination agent and N,N-dimethylformamide is in range of 1:2.5-7.5 (mol ratio), adding sucrose-6-acetate, heating directly while controlling temperature, adding thick sodium hydroxide for neutralisation in normal temperature after reaction, adding organic solution group by group for extraction after a after-treatment; treating organic facies, and refining subsequently so that chlorid of sucralose is obtained. The process is characterized by mild reacting condition, easiness to control, and low energy consumption, thus has a great expectation for development and application.
Owner:江建成
Monoester process of synthesizing trichlorosucrose
The present invention relates to the synthesis of sweet food material. Sucrose is esterified to produce sucrose-6-acetate; sucrose-6-acetate is then chlorinated to produce trichlorosucrose-6-acetate; and trichlorosucrose-6-acetate is reduced to produce trichlorosucrose. The produced trichlorosucrose has the features of high sweetness, low heat value, no toxicity and dental caries resistance. With low production cost, the present invention may be used in beverage, pickled vegetable, composite seasoning, wine, ice cream and other food products.
Owner:李宝才
Process for the preparation of sucralose
ActiveUS20130060018A1Efficient decolourization of TGS-6-acetateSugar derivativesDisaccharidesAcetic acidLactose
The present invention provides a method for preparing colorless sucralose, wherein 4,1′,6′-trichloro-4,1′,6′-trideoxy-galacto sucrose-6- acetate containing colored impurities formed during chlorination of sucrose-6-acetate is treated with sodium hypochlorite, where sodium hypochlorite acts both as a decolorizing agent and as a reagent for the ester hydrolysis.
Owner:DIVI S LAB LTD
A kind of purification method of sucrose-6-acetate
ActiveCN103288891BHigh yieldHigh purityEsterified saccharide compoundsSugar derivativesChromatographic separationAcetic acid
Owner:HUBEI HONGYUAN PHARMA
Method of separating and purifying sucrose-6-acetate mother liquor by salt fractionation
ActiveCN103012509BIncrease the effective concentrationEasy to handleEsterified saccharide compoundsSugar derivativesFractionationWastewater
Owner:TECHNO (FUJIAN) FOOD INGREDIENTS CO LTD
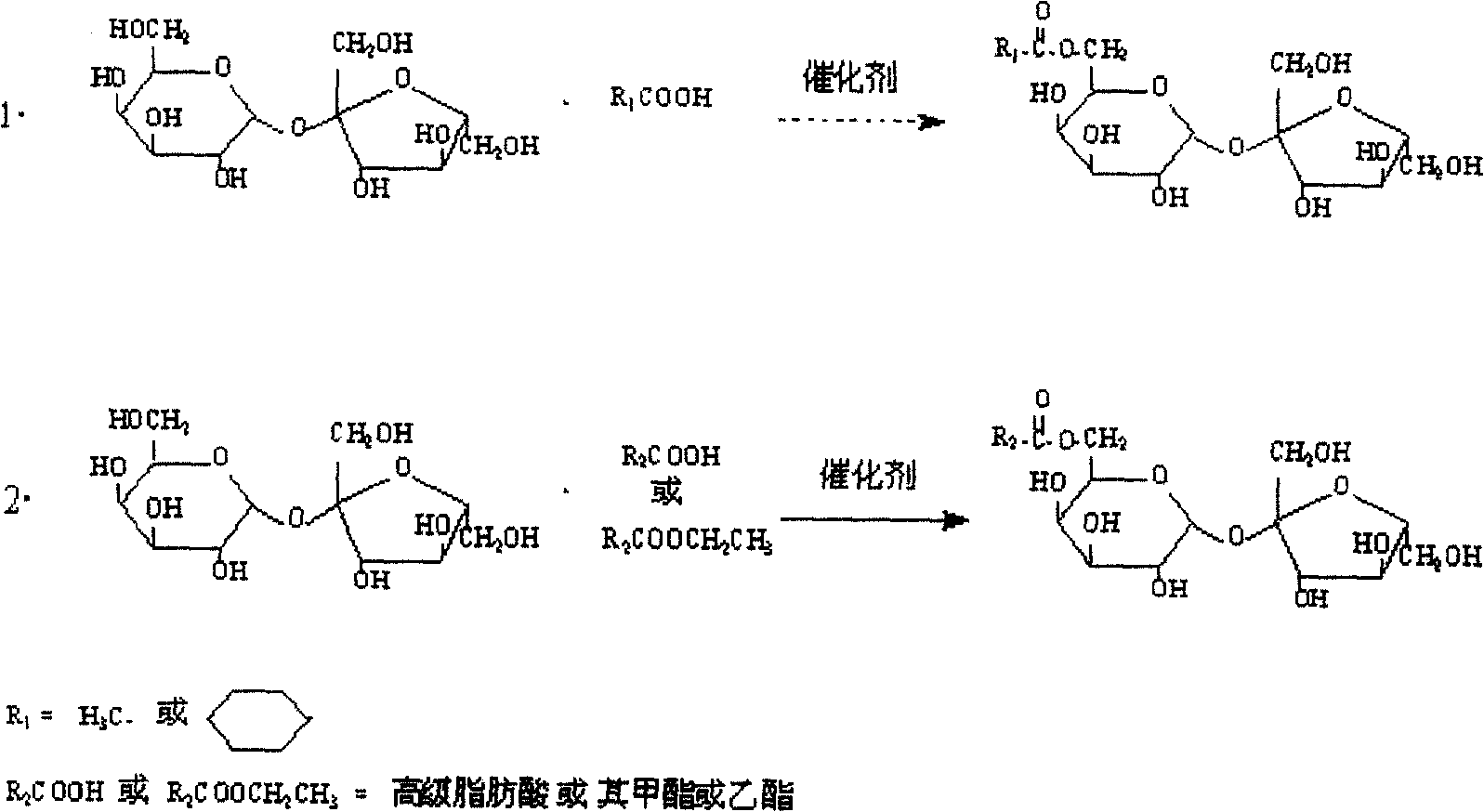
Two-step method oriented synthesis of sucrose-6-higher fatty acid monoester
InactiveCN100451026COrientation is accurateAchieving Directed SynthesisEsterified saccharide compoundsSugar derivativesChemical synthesisStearic acid
The invention relates to a method for using tow-step to oriented synthesis sucrose -6-higher fatty acid monoesters. It is characterized in that the first step: using chemical synthesis method to synthesis the sucrose-6-acetate or benzoate, so that the hydroxyl of the 6 bit of the sucrose actives; the second step: it uses enzyme catalysis, higher fatty acid or methyl ester and ethyl ester and sucrose -6-acetate or benzoate to do ester exchange reaction to directional or cheesed synthesis oriented synthesis sucrose -6-higher fatty acid monoesters.
Owner:GUANGXI UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com