Crystallization method for synthesizing sucrose-6-acetate from sucrose and ortho-acetate
A technology of orthoacetate and acetate, which is applied in the direction of chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve problems such as difficult suction filtration and drying, complicated and cumbersome operation, poor crystal shape, etc., to achieve The effect of reducing the requirement of ambient temperature, simple operation steps and mild operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
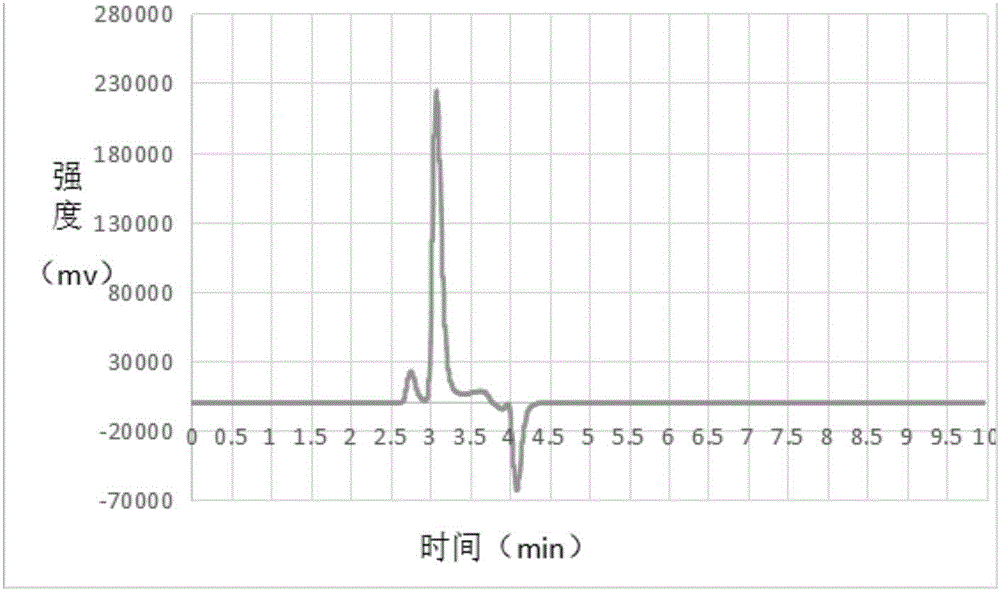
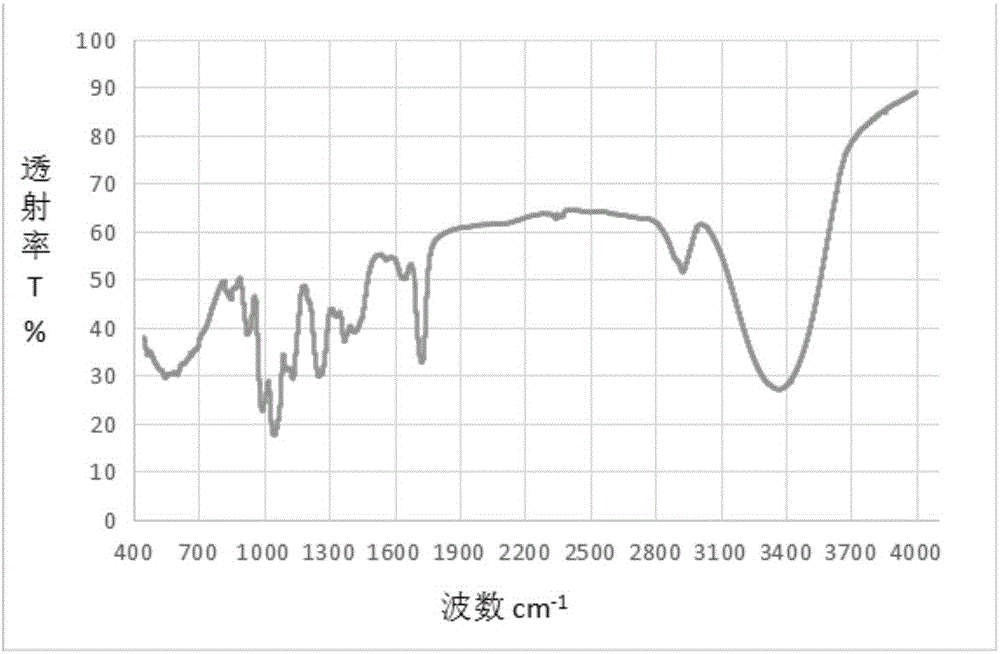
Image
Examples
Embodiment 1
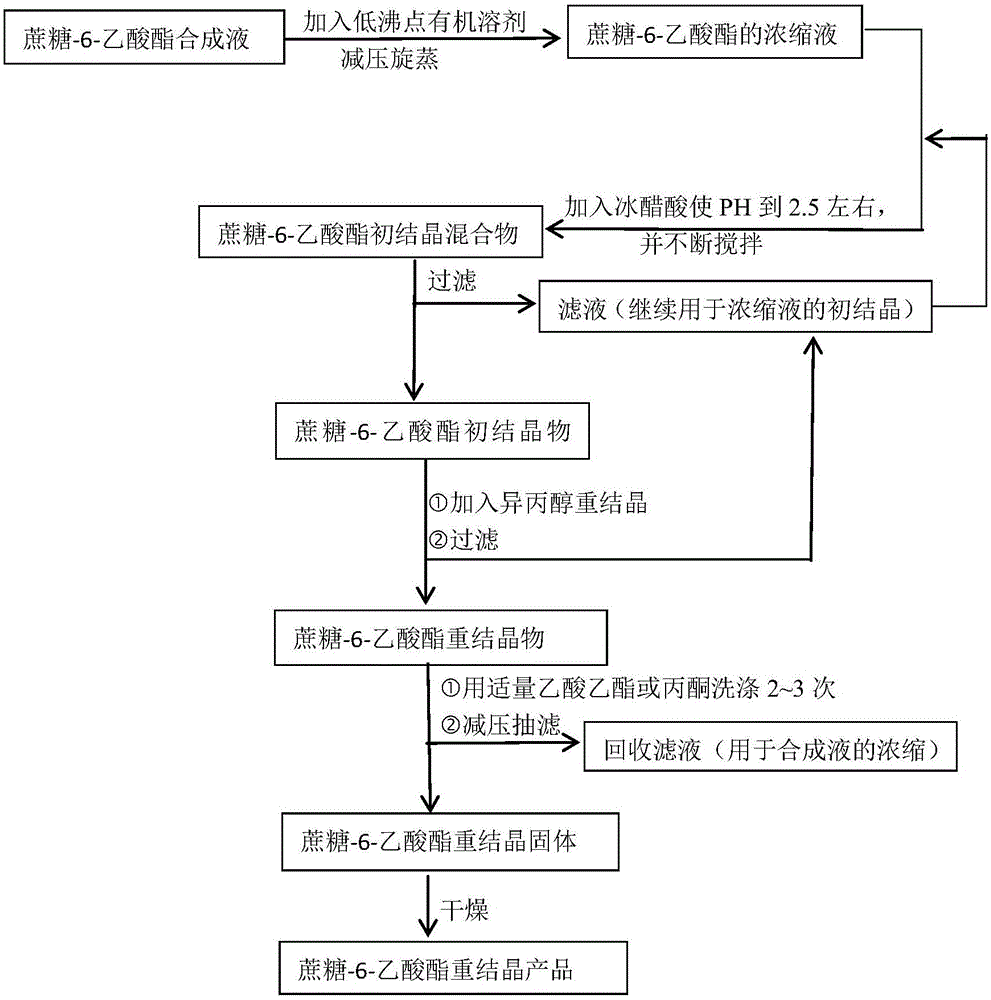
[0047] Take 50 mL of the synthesis solution of sucrose-6-acetate synthesized according to the above method, add 25 mL of ethyl acetate, heat and rotary steam at 55 ° C, -0.1 Mpa for 30 min until the volume of the mixture is 20 mL.
[0048] Add 2mL of ethyl acetate at 30°C, add glacial acetic acid dropwise under continuous stirring until the pH value of the solution reaches 2.6, then add about 20mL of glacial acetic acid dropwise; stir for about 25min to form a large number of white crystals, and filter under reduced pressure to obtain sucrose-6-acetic acid 10.4 g of ester primary crystals.
[0049] Take the primary crystals and place them in a beaker, slowly add isopropanol, and keep stirring until the sucrose-6-acetate crystals are completely dissolved, then add about 0.1g of sucrose-6-acetate primary crystals to make the sucrose-6-acetate -Acetate was recrystallized and precipitated, stood still for 30 minutes, filtered out the sucrose-6-acetate crystals, washed the crystals...
Embodiment 2
[0061] Take 50 mL of the sucrose-6-acetate mixture synthesized according to the above method, add 25 mL of methanol, 50 ° C, -0.1 Mpa, and rotary steam for 30 min until the volume of the mixture is 18 mL.
[0062] Add 2 mL of ethyl acetate at 30°C, add glacial acetic acid dropwise under constant stirring until the pH value of the solution reaches 2.6, then add about 20 mL of glacial acetic acid dropwise; stir for about 25 minutes to form a large number of white crystals, crystallize completely after 30 minutes, and filter under reduced pressure to obtain sucrose - 10.7 g of primary crystallization of 6-acetate.
[0063] Put the primary crystals in a beaker, slowly add isopropanol, and keep stirring until the sucrose-6-acetate crystals are completely dissolved, then add about 0.2g of sucrose-6-acetate primary crystals in batches to make the sucrose-6-acetate 6-Acetate was recrystallized and precipitated, stood still for 30 minutes, filtered out the crystals, washed twice with 3...
Embodiment 3
[0065] Take 50 mL of the synthesis solution of sucrose-6-acetate synthesized according to the above method, add 25 mL of ethyl acetate, heat and rotary steam at 53 ° C, -0.09 Mpa for 30 min until the volume of the mixture is 19 mL.
[0066] Add 2mL of ethyl acetate at 30°C, add glacial acetic acid dropwise under continuous stirring until the pH value of the solution reaches 2.6, then add about 20mL of glacial acetic acid dropwise; stir for about 25min to form a large number of white crystals, and filter under reduced pressure to obtain sucrose-6-acetic acid 10.6g of ester primary crystallization.
[0067] Take the primary crystals and place them in a beaker, slowly add isopropanol, and keep stirring until the sucrose-6-acetate crystals are completely dissolved, then add about 0.2g of sucrose-6-acetate primary crystals to make the sucrose-6-acetate -Acetate recrystallization precipitated, stood still for 30min, filtered out sucrose-6-acetate crystals, then washed the crystals t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com