Green synthesis method of sucralose-6-acetate
A green synthesis, sucralose technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve difficult problems such as processing, and achieve the effect of convenient post-processing, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
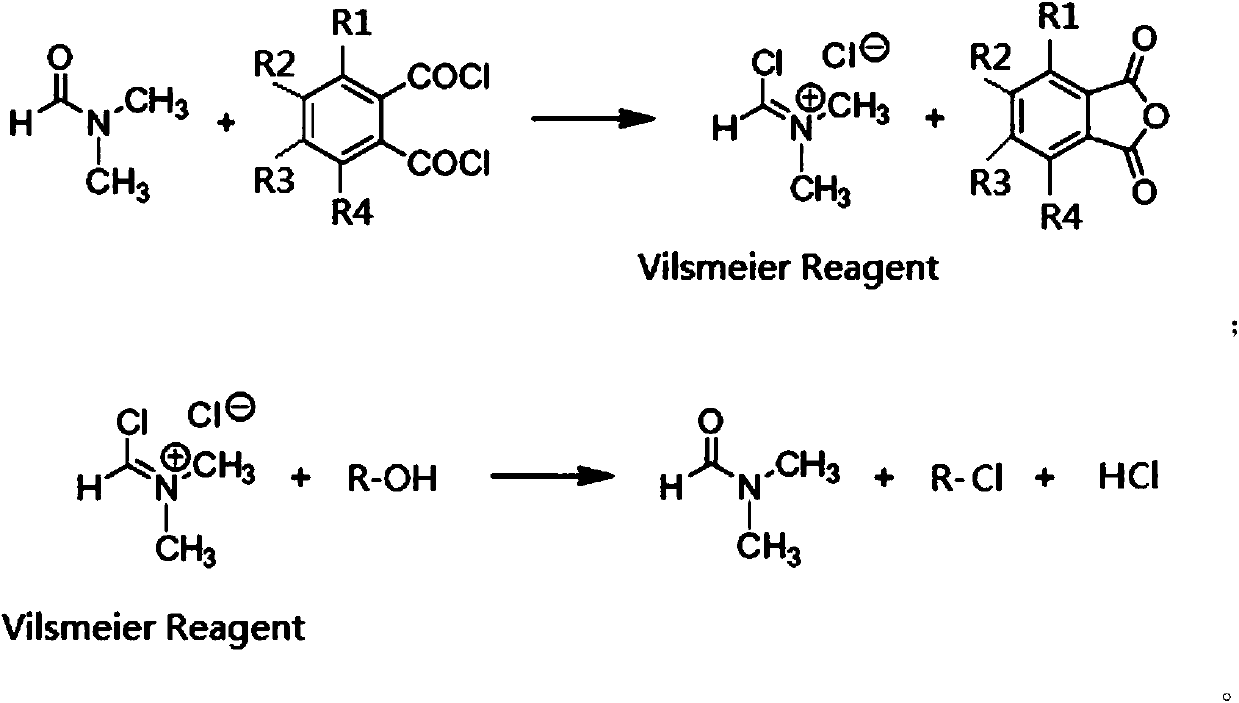
[0023] In 2.5 moles of 1,4-dioxane, add 0.5 moles of DMF and 0.5 moles of phthaloyl chloride, react at 40°C for 3 hours, the generated Vilsmeier salt precipitates, and is filtered and dried to obtain a white solid Vilsmeier salt. Yield 98%.
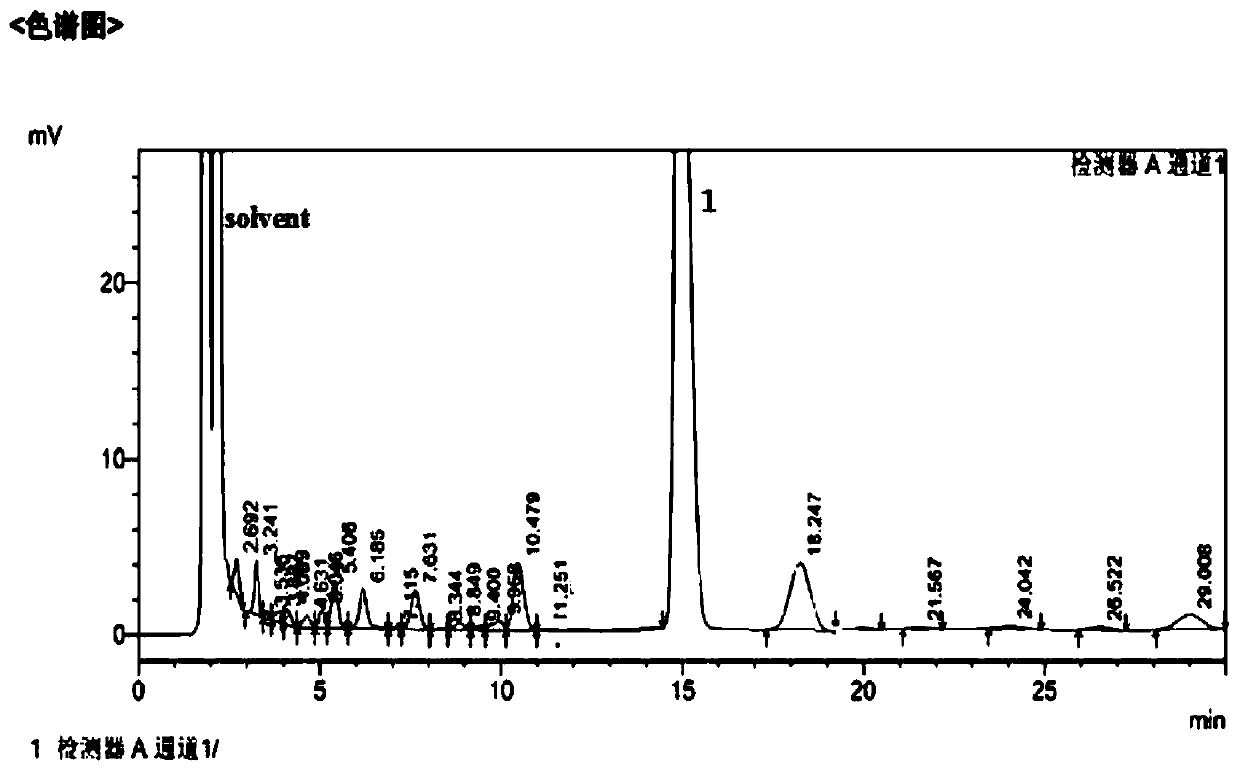
[0024] Add 9 moles of Vilsmeier salt to 4000 mL of 1,1,2-trichloroethane solvent to make a suspension, then add dropwise to the pre-synthesized 1 mole of sucrose-6-acetate dry syrup, control the temperature below 0°C and stir. After the dropwise addition, the temperature was naturally raised to room temperature, and then slowly raised to 113° C. over 10 hours, and kept for 2.5 hours. After the reaction was completed, the crude product of sucralose-6-acetate was obtained through neutralization, solvent removal, water dissolution, decolorization and crystallization, with a yield of 94%. The liquid chromatogram of this crude product sees figure 1 .
Embodiment 2
[0026] In 4 moles of tetrahydrofuran, 0.5 moles of DMF and 0.5 moles of 3-chlorophthaloyl chloride were added and reacted at 30°C for 4 hours. The resulting Vilsmeier salt precipitate was filtered and dried to obtain white solid Vilsmeier salt with a yield of 98%.
[0027] Add 8 moles of Vilsmeier salt to 4000 mL of DMF solvent to make a solution, then dropwise add it to the pre-synthesized 1 mole of sucrose-6-acetate dry syrup, control the temperature below 0°C and stir. After the dropwise addition, the temperature was naturally raised to room temperature, and then slowly raised to 110° C. over 12 hours, and kept for 1.5 hours. After the reaction was completed, the crude product of sucralose-6-acetate was obtained through neutralization, solvent removal, water dissolution, decolorization and crystallization, with a yield of 92%.
Embodiment 3
[0029] In 3 moles of methyl tert-butyl ether, add 0.5 moles of DMF and 0.5 moles of 3,5-dimethylphthaloyl chloride, and react at 45°C for 2.5 hours. The resulting Vilsmeier salt precipitate is filtered and dried to obtain a white solid Vilsmeier salt, yield 97%.
[0030] Add 10 moles of Vilsmeier salt to 4000 mL of toluene solvent to make a suspension, then add dropwise to the pre-synthesized 1 mole of sucrose-6-acetate dry syrup, control the temperature below 0 ° C and stir. After the dropwise addition, the temperature was naturally raised to room temperature, and then slowly raised to 115° C. over 8 hours, and kept for 3 hours. After the reaction was completed, the crude product of sucralose-6-acetate was obtained through neutralization, solvent removal, water dissolution, decolorization and crystallization with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com