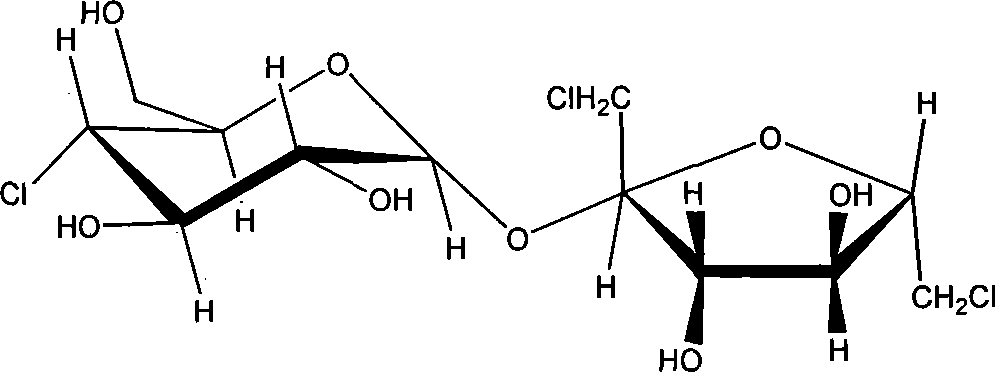
Synthesis of trichloio-sugar
A technology of sucralose and synthesis process, which is applied in the direction of sugar derivatives, sugar derivatives, chemical instruments and methods, etc. It can solve the problems of short life, easy deactivation, poor catalyst stability, etc., and achieve low production cost and high catalytic activity. High, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] SO 4 2- / TiO 2 / C 4+ Catalyst preparation
[0021] Add 20g TiCl to a 1000ml round bottom flask 4 Hydrolyze with dilute ammonia water until the solution is alkaline, and obtain white metatitanic acid precipitate. After standing for 24 hours, separate and remove the supernatant, wash the precipitate until there is no chloride ion, dry it at 110°C, grind it into powder, and pass through a 120-mesh sieve to obtain white powder TiO 2 . Dissolve cerium sulfate in 500mmol / L sulfuric acid solution to form a 1mol / L (molar concentration of cerium) solution, and sieve the white powder TiO 2 Soak in it for 14h, filter with suction, dry the solid in an oven at 110°C, and roast in a muffle furnace to obtain about 25g of rare earth solid superacid catalyst SO 4 2- / TiO 2 / C 4+ .
Embodiment 2
[0023] For the synthesis of sucrose-6-acetate, the molar ratio of sucrose to acetic anhydride is 1:0.9; the catalyst is 2% of the weight of sucrose.
[0024] Add 50g (0.146mol) sucrose, 350g N,N-dimethylformamide, entrainer cyclohexane 100g, acetic anhydride 13.4g in the 1000ml three-necked flask equipped with magnetic stirrer, water separator, reflux condenser (0.131mol), 1g catalyst SO 4 2- / TiO 2 / C 4+, stirred and heated to 85°C for 5 hours, cooled to normal temperature, filtered to recover the catalyst, and recovered N,N-dimethylformamide under reduced pressure to obtain 62g of syrup, which contained 80.1% of sucrose-6-acetate as measured by HPLC , sucrose diacetate was 5.2%, and residual sucrose was 10.5%.
Embodiment 3
[0026] For the synthesis of sucrose-6-acetate, the molar ratio of sucrose to acetic anhydride is 1:1; the catalyst is 2% of the weight of sucrose.
[0027] Add 50g (0.146mol) sucrose, 350g N, N-dimethylformamide, entrainer cyclohexane 100g, acetic anhydride 14.9g in the 1000ml three-necked flask equipped with magnetic stirrer, water separator, reflux condenser (0.146mol), 1g catalyst SO 4 2- / TiO 2 / C 4+ , stirred and heated to 85°C for 5 hours, cooled to normal temperature, recovered the catalyst by filtration, and recovered N,N-dimethylformamide under reduced pressure to obtain 64g of syrup, which contained 84.1% of sucrose-6-acetate as measured by HPLC , sucrose diacetate was 6.2%, and residual sucrose was 4.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com