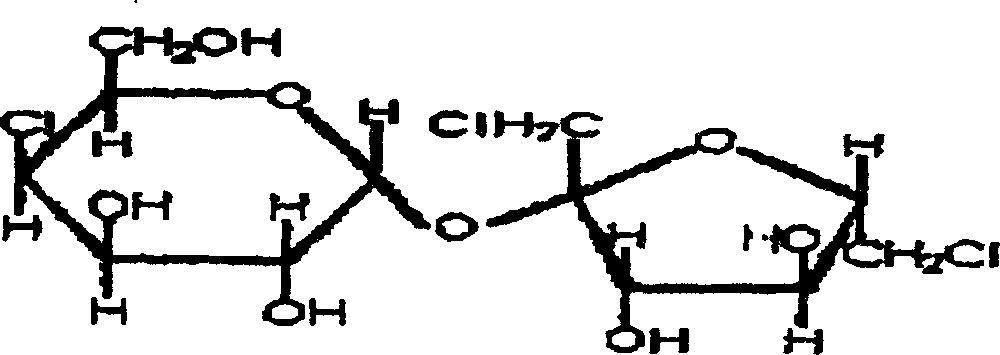
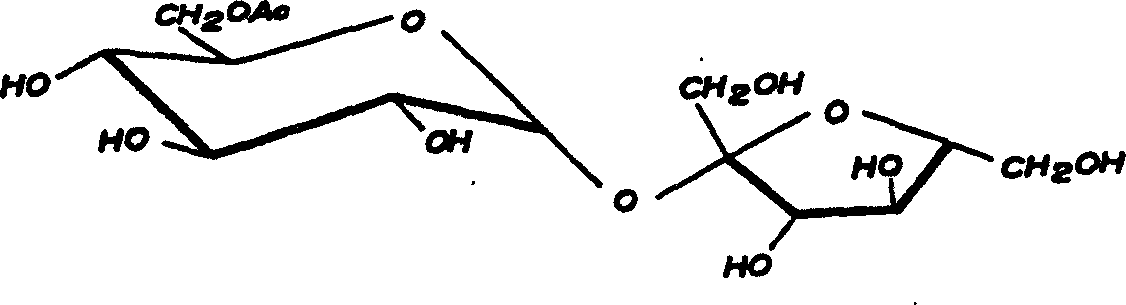
Synthesis of trichlorosucrose
A synthesis method and technology of sucralose, applied in chemical instruments and methods, disaccharides, sugar derivatives and other directions, can solve the problems of complex process and high production cost, and achieve the effects of simple process, low production cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] ①Synthesis of sucrose-6-acetate
[0015] Add 60g of sucrose, 250ml of N,N-dimethylformamide, 100ml of ethyl acetate, 1g of cerium sulfate-silica gel (1:1) into a 1000ml three-necked flask, heat to 80°C and react for 4 hours under stirring, cool to 5 DEG C, the catalyst was recovered by filtration, ethyl acetate was recovered from the mother liquor under normal pressure, and DMF was recovered under reduced pressure to obtain 68 g of syrup, which contained 83.4% of sucrose-6-acetate as measured by HPLC (equipped with a differential detector).
[0016] ②Synthesis of sucralose-6-acetate
[0017] Take the above 68g syrup and dissolve it with 400ml N, N-dimethylformamide, cool to -20°C, add 100ml of thionyl chloride dropwise, control the rate of addition, keep the temperature below 0°C during the dropping process, , and stirred at 0° C. for 30 minutes, then slowly raised the temperature to 110° C., and kept at 110° C. for 4 reaction hours. The reaction solution was cooled t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com