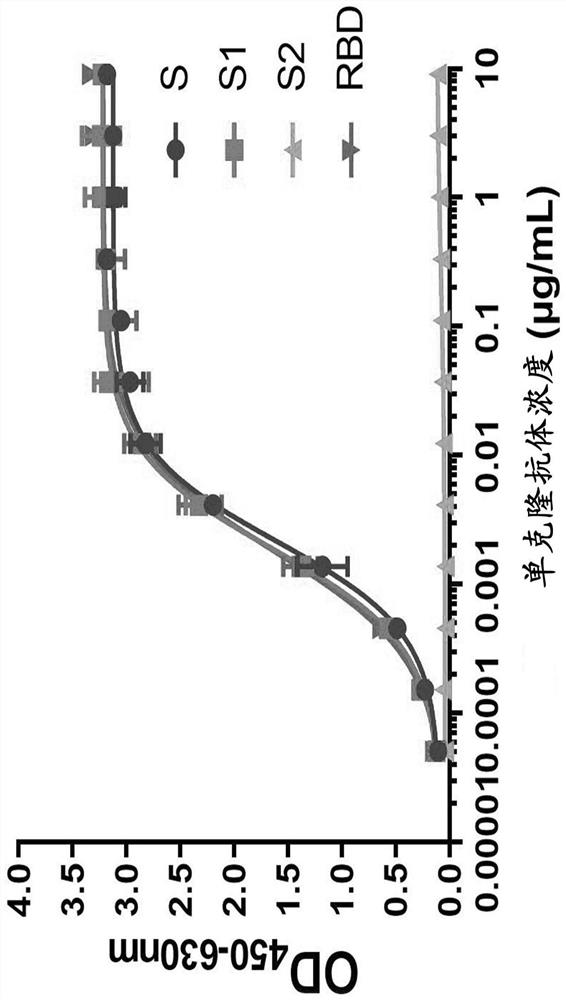
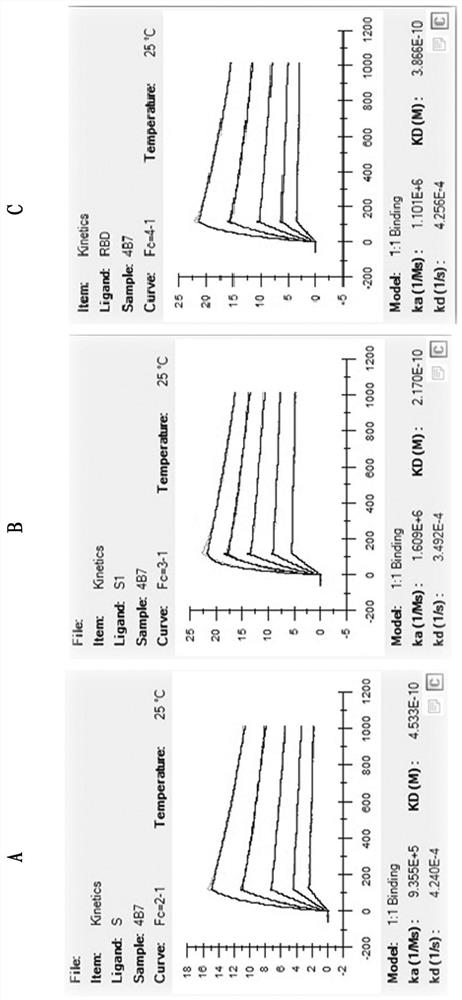
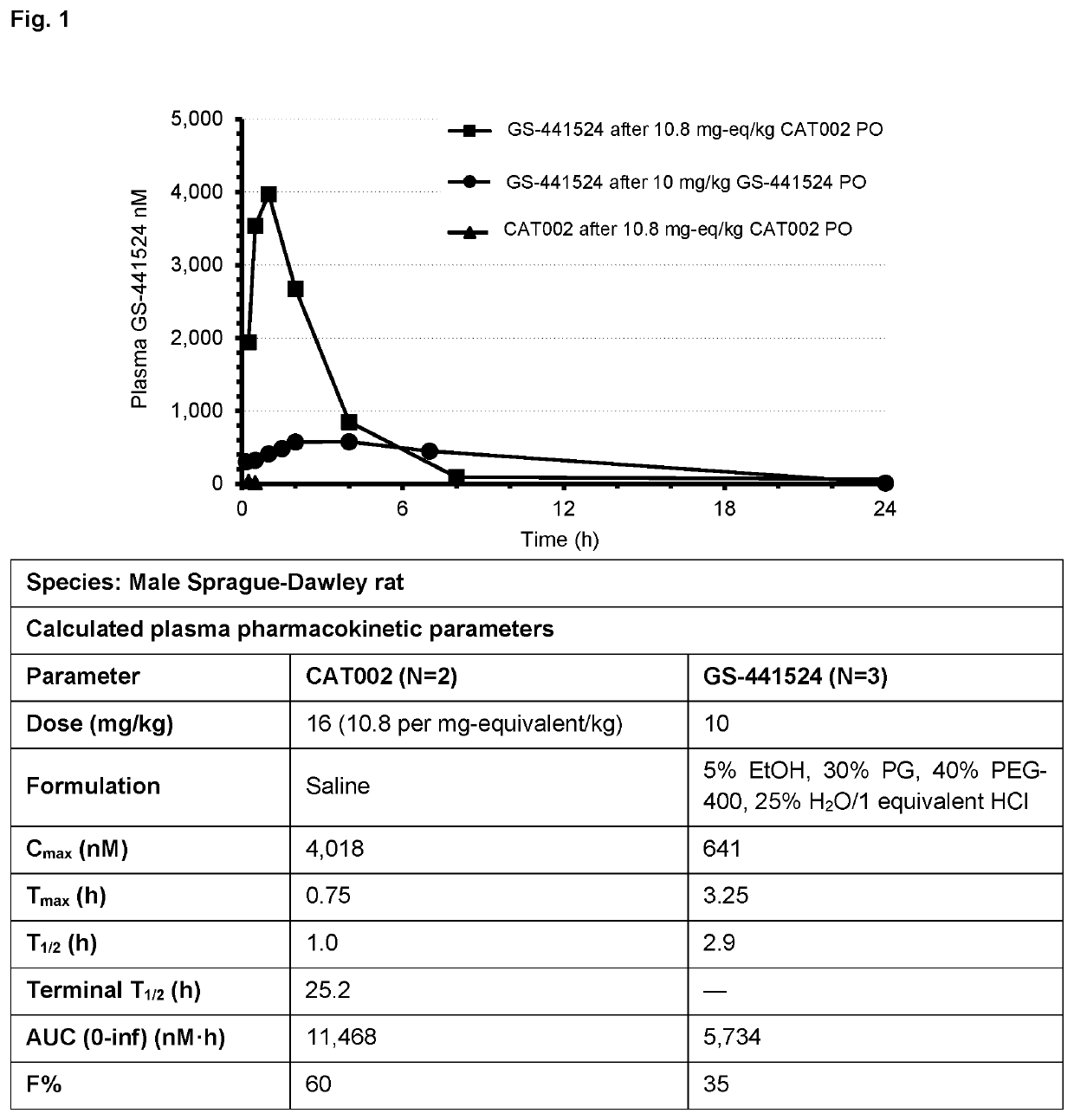
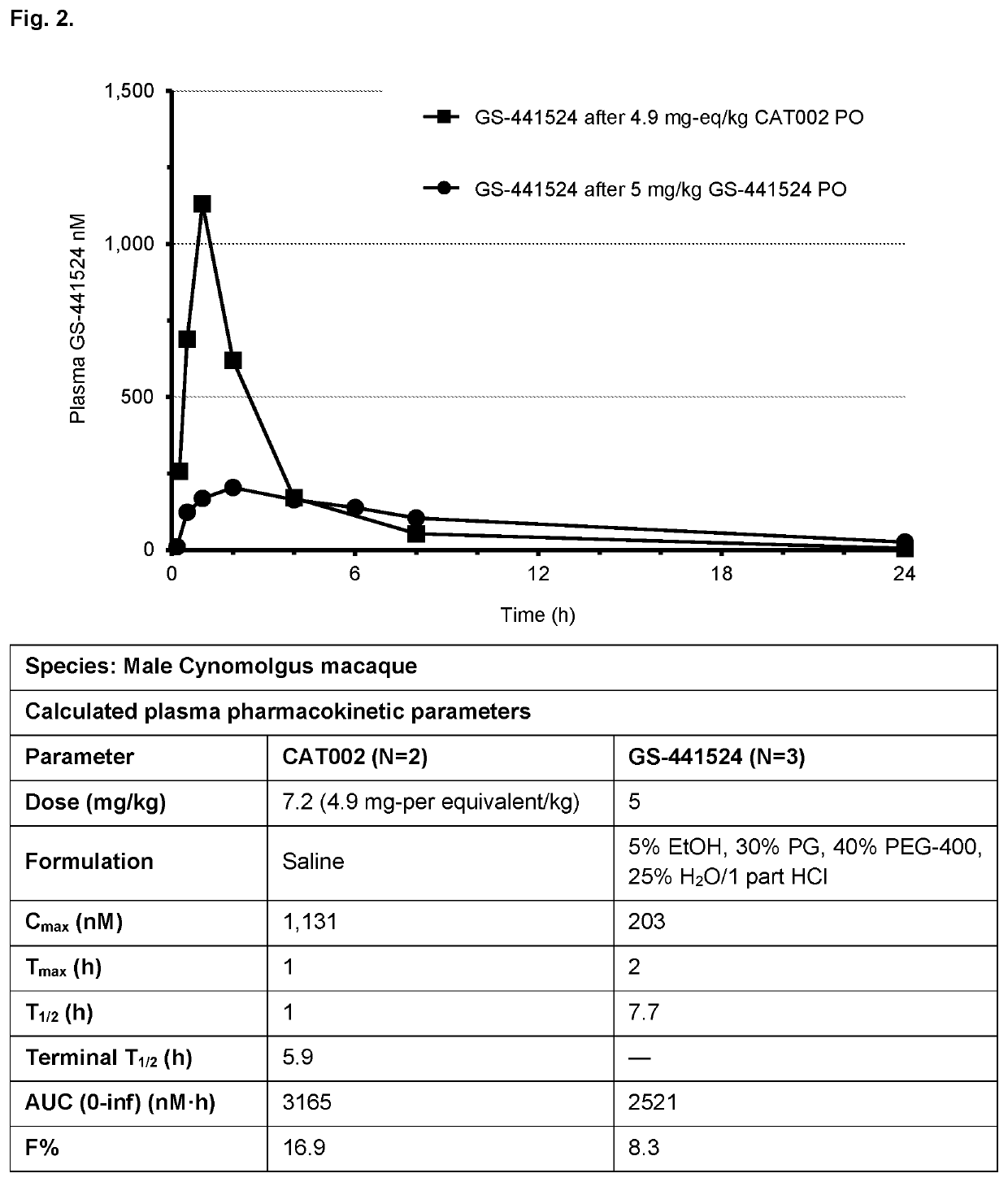
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), previously known by the provisional name 2019 novel coronavirus (2019-nCoV), is a positive-sense single-stranded RNA virus. It is contagious in humans and is the cause of the ongoing 2019–20 coronavirus outbreak, an epidemic of coronavirus disease 2019 (COVID-19) that has been designated a Public Health Emergency of International Concern by the World Health Organization (WHO).
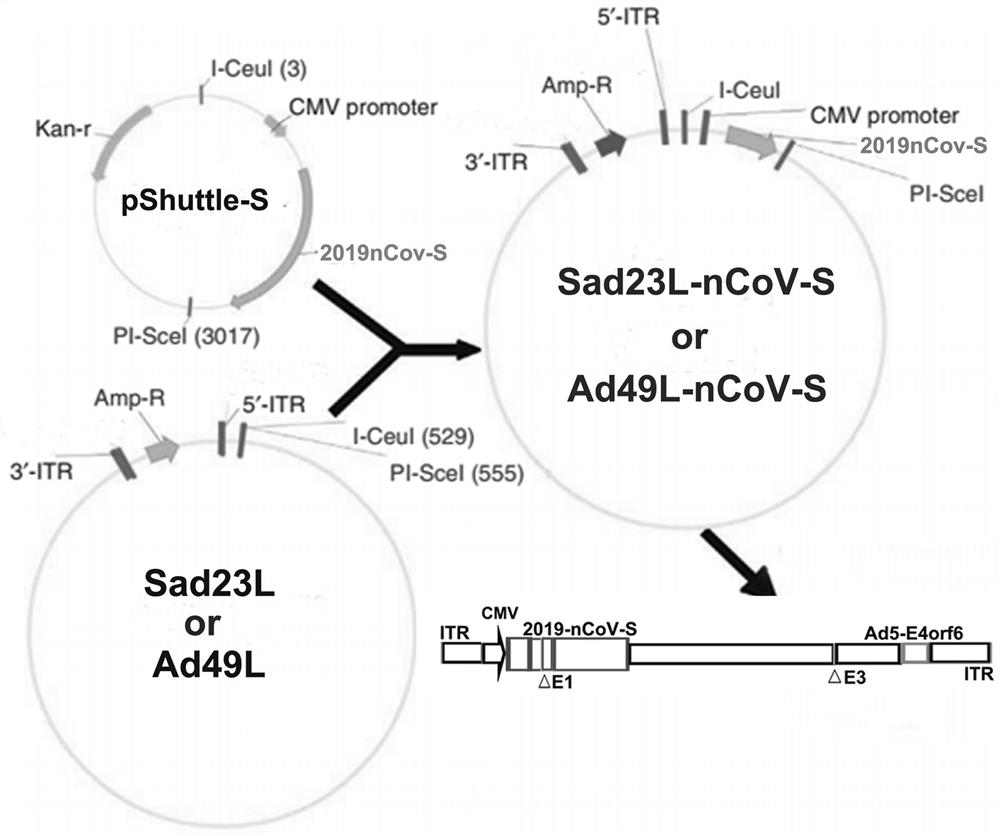
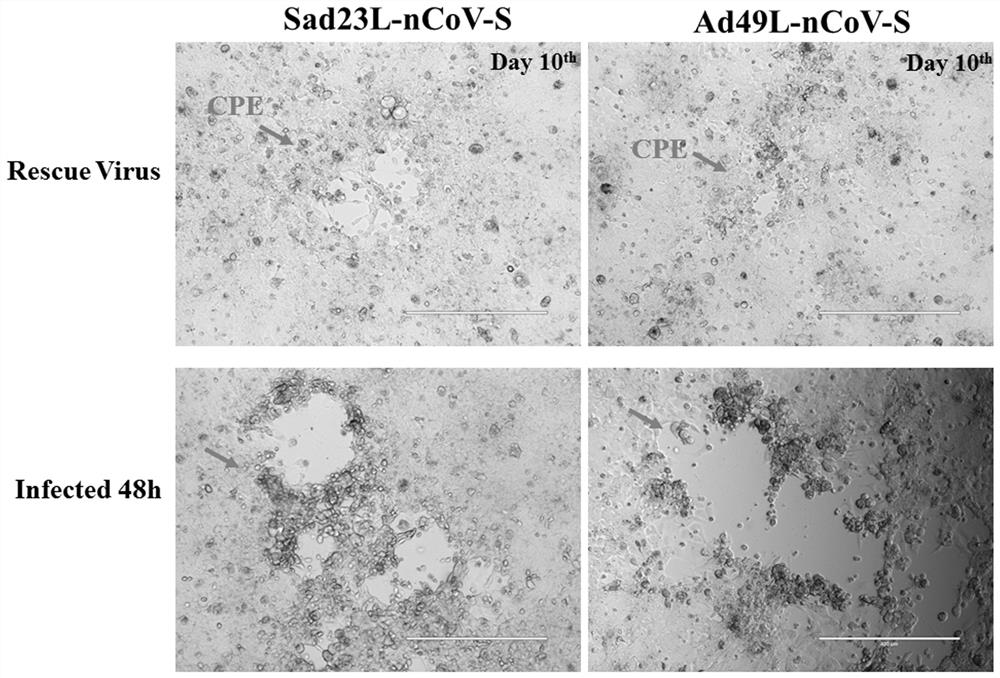
Severe acute respiratory syndrome coronavirus 2 vaccine based on novel adenovirus vectors Sad23L and/or Ad49L
ActiveCN111778264AOptimized nucleotide sequenceSsRNA viruses positive-senseViral antigen ingredientsForeign proteinCell immunity
The invention discloses a corona virus disease COVID-19 vaccine based on novel adenovirus vectors Sad23L and / or Ad49L. An exogenous gene is optimized to enable the expression of a foreign protein to be obviously increased, and besides, human rare or monkey adenoviruses are further attempted to be used as vectors, and a prestored immune reaction for common adenoviruses is avoided. The obtained corona virus disease COVID-19 vaccine can be induced in an animal body to generate high-level body fluid and cellular immunity, and after animal immunization, side effects do not occur. The corona virus disease COVID-19 vaccine is safe and effective, and can be quickly and massively prepared, and therefore, the corona virus disease COVID-19 vaccine is a candidata vaccine for preventing SARS-CoV-2.
Owner:广州佰芮慷生物科技有限公司
Novel severe acute respiratory syndrome coronavirus 2 N proteantigen variant and application thereof to detection of novel severe acute respiratory syndrome coronavirus 2 antibody
PendingCN112028977AEfficient captureReduce false positive problemsSsRNA viruses positive-senseVirus peptidesViral antibodySevere acute respiratory syndrome
The invention provides a novel severe acute respiratory syndrome coronavirus 2 N proteantigen variant and an application thereof to detection of the novel severe acute respiratory syndrome coronavirus2 antibody, and relates to variants of N protein important epitope polypeptide. The variants reduce the non-specific reaction of the novel severe acute respiratory syndrome coronavirus 2 protein as acapture antigen with other coronavirus antibodies such as SARS and human influenza coronavirus antibodies. The polypeptide has coronavirus N protein epitope peptide activity, and cysteine residues are added to the C end of the polypeptide. Amino acid residue sites of the mutation are selected from: lysine at the 342rd site, lysine at the 347 site, lysine at the 387 site and lysine atthe 388 site are mutated into amino acids with relatively low homology. The sequence of the N protein 340-419 polypeptide is shown as SEQ ID NO 7. The invention further discloses an antigen composition, use and method of the variant.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Production method of virus-inactivated plasma for treating COVID-19
InactiveCN111346108AQuick Preparation and AcquisitionHigh cure rateMammal material medical ingredientsAntiviralsVirus inactivationCoronavirus vaccination
The invention relates to a production method of virus-inactivated plasma for treating COVID-19. The method comprises the following steps: (1) collecting convalescent plasma of COVID-19 survivors or plasma immune to a Severe Acute Respiratory Syndrome Coronavirus 2 vaccine (SARS-CoV-2 vaccine), and conducting pretreatment; (2) adding methylene blue, and conducting photo-inactivation on the plasma;and (3) conducting labeling and packaging after the plasma passes tests.
Owner:国药集团武汉血液制品有限公司
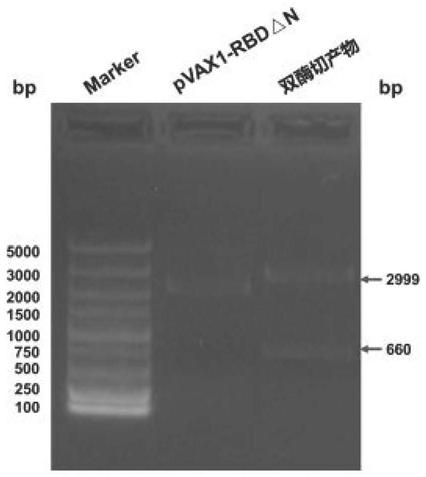
Corona Virus Disease 2019 (COVID-19) DNA vaccine and preparation method and application thereof
InactiveCN111569057AImprove expression levelMaintain antigenicitySsRNA viruses positive-senseViral antigen ingredientsProtein targetReceptor
The invention discloses a Corona Virus Disease 2019 (COVID-19) DNA vaccine and a preparation method and application thereof. According to the DNA vaccine, glycosylated asparaginate on an N-1 site of aconventional Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Receptor Binding Domain (RBD) sequence is deleted, an expression level can be improved, and functionality and antigenicity ofthe DNA vaccine are maintained. RBD triangle N can trigger a high-titer neutralizing antibody for pseudoviruses and live viruses. Meanwhile, an encoding protein uses pVAX1 as vector skeleton molecules, and front and rear base sequences of a gene initial codon ATG are designed and regulated so as to conform to the Kozak rule; and a recombinant of the DNA vaccine transfects 293 cells, and RT-PCR and Western Blot detection shows that a target protein is successfully expressed and an expression product can be identified by standard serum.
Owner:吉林省中科生物工程股份有限公司
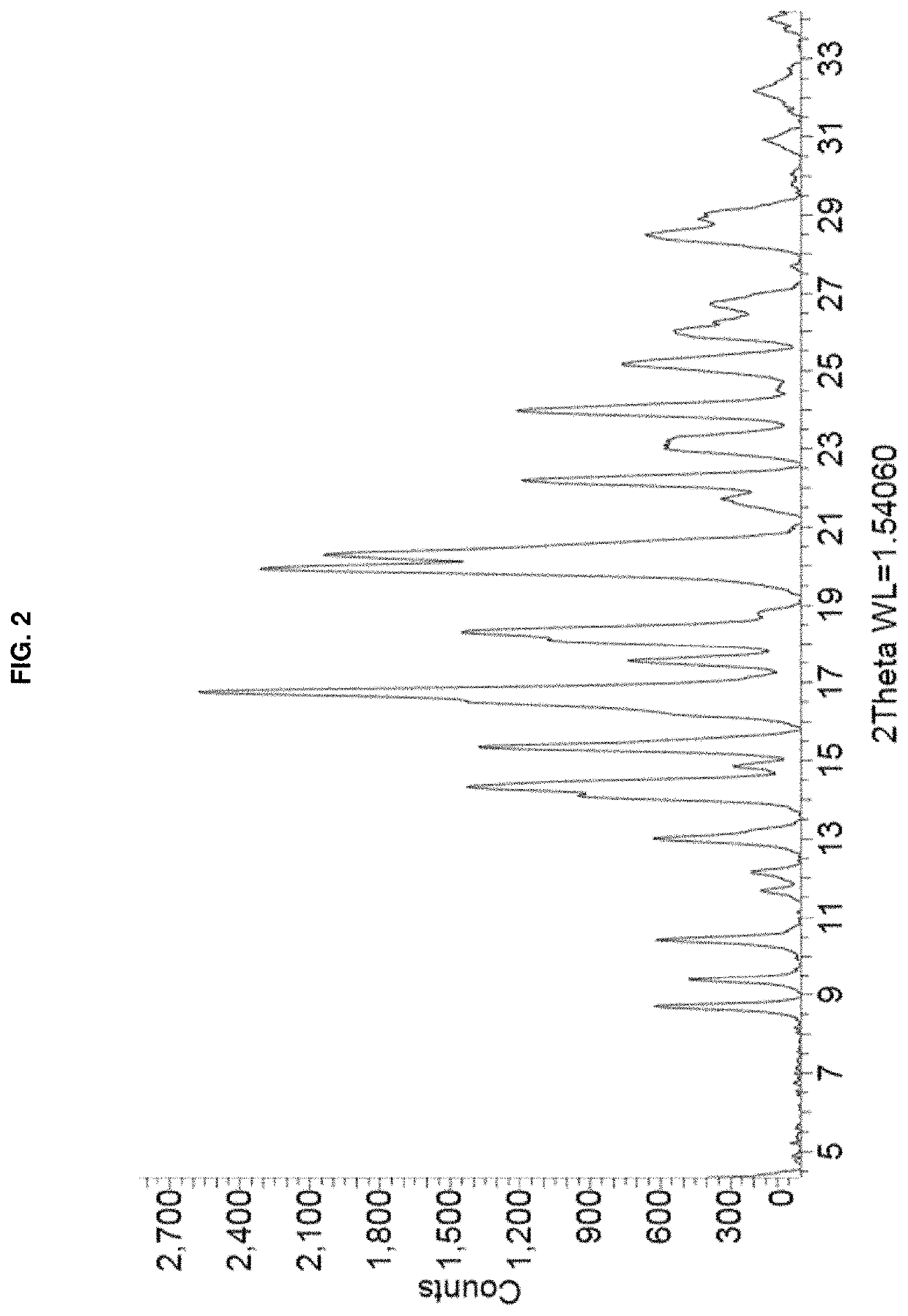
Crystalline form of Remdesivir
ActiveUS11377456B2Established safety recordEasy to useEsterified saccharide compoundsOrganic active ingredientsViral infectionBiomedical engineering
The present invention provides a novel crystalline form of remdesivir, remdesivir Form APO-I, including remdesivir and dimethyl sulfoxide, compositions and processes for the preparation thereof, the use of this crystalline form in the treatment of a viral infection, and methods of treating viral infections using the same, and in particular, a viral infection caused by Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2).
Owner:APOTEX INC
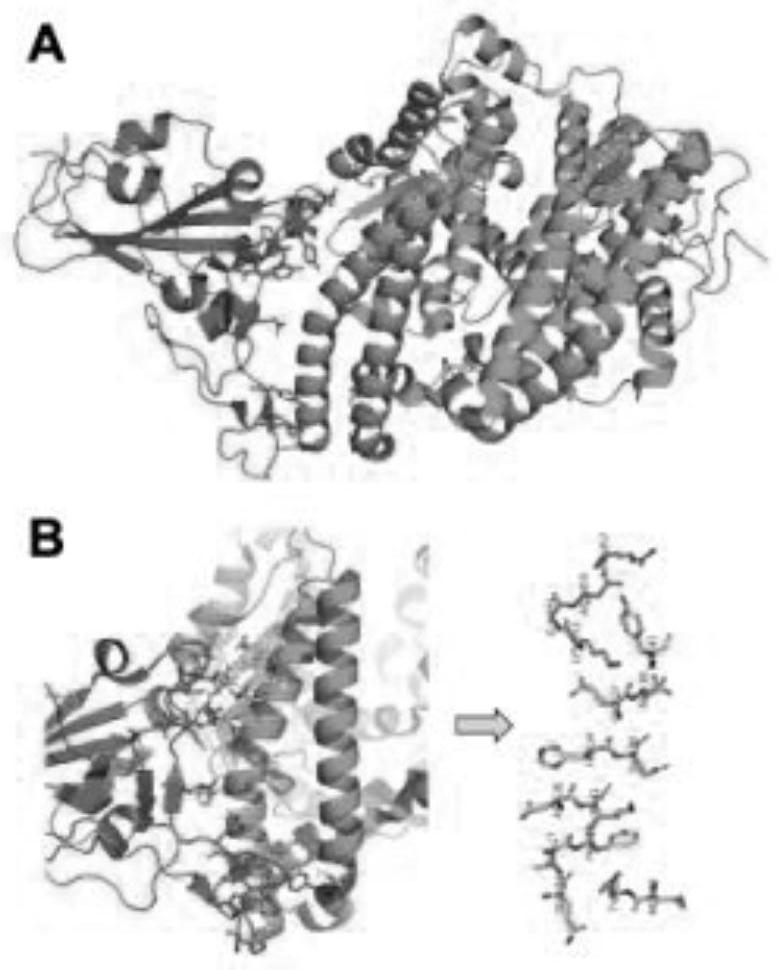
Human angiotensin converting enzyme 2-based affinity polypeptide for severe acute respiratory syndrome coronavirus 2
ActiveCN112375754AAvoid interactionNo effectBiological material analysisPeptide preparation methodsBinding siteVirus Binding
The invention provides a human angiotensin converting enzyme 2-based affinity polypeptide for the severe acute respiratory syndrome coronavirus 2, and relates to the technical field of biological materials. Key human angiotensin converting enzyme 2 amino acid residues of binding sites of human angiotensin converting enzyme 2 and the severe acute respiratory syndrome coronavirus 2 are extracted toreconstruct a peptide library, and the human angiotensin converting enzyme 2-based affinity polypeptide for the severe acute respiratory syndrome coronavirus 2 is obtained by screening; and then, a biological detection reagent is prepared from the affinity polypeptide, and thus, a new method is provided for detection of the severe acute respiratory syndrome coronavirus 2.
Owner:TSINGHUA UNIV
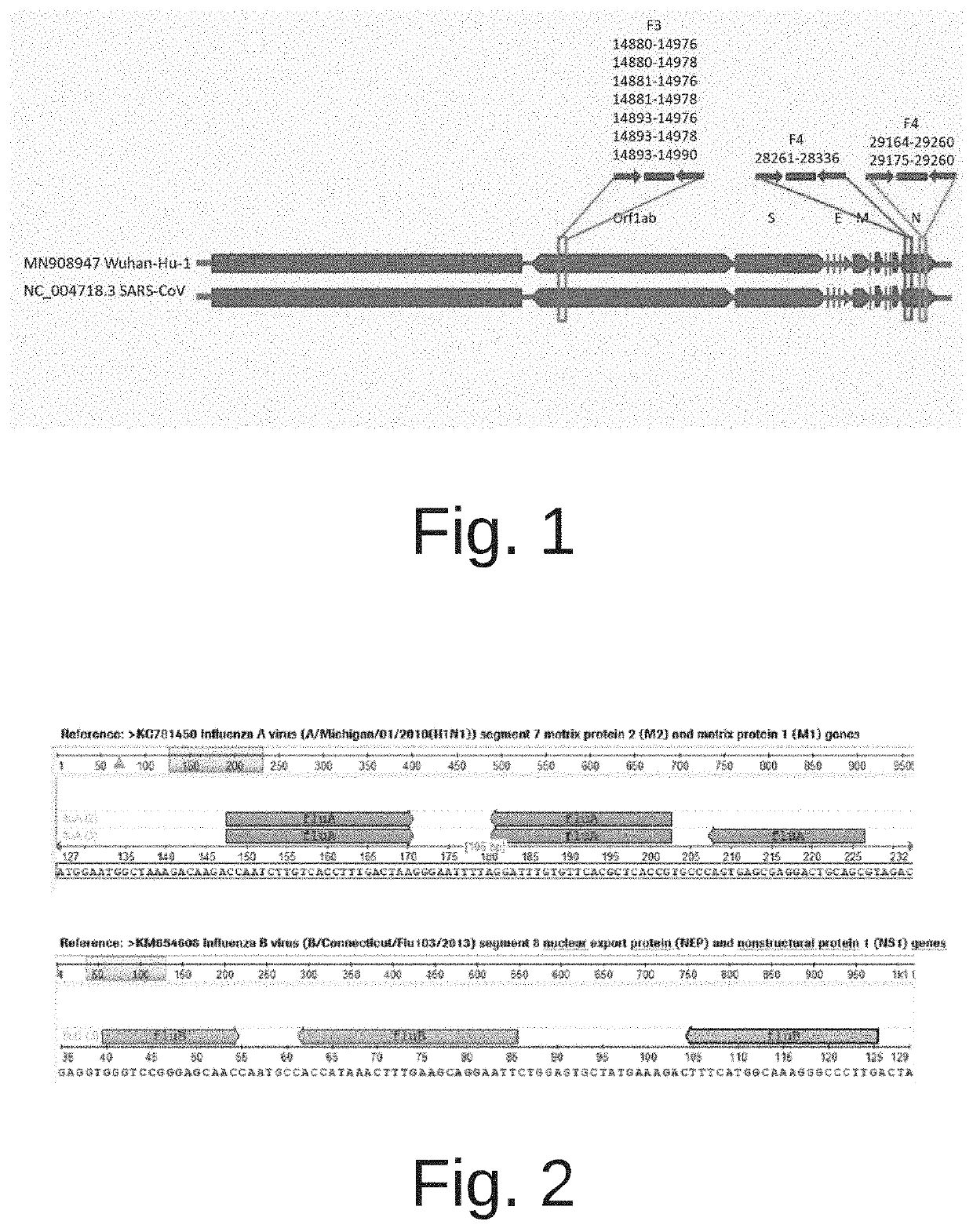
COMPOSITIONS AND METHODS FOR THE SIMULTANEOUS DETECTION OF INFLUENZA A, INFLUENZA B, AND SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-CoV-2)
PendingUS20220042117A1Enlarge regionMinimizing the potential for cross-contaminationMicrobiological testing/measurementInfluenza A antigenCare setting
Methods for the rapid detection of the presence or absence of SARS-CoV-2 in biological or non-biological samples are described. These methods are adapted to be performed rapidly in a point-of-care setting. The methods can include performing an amplifying step, a hybridizing step, and a detecting step. Specifically, primers and probes targeting SARS-CoV-2 are provided that are designed for the detection of this target. Additionally, kits and reaction vessels containing primers and probes targeting SARS-CoV-2 are provided. Additionally, methods, kits and reaction vessels for the simultaneous rapid detection of the presence or absence of SARS-CoV-2, influenza A, and influenza B in biological or non-biological samples are described.
Owner:ROCHE MOLECULAR SYST INC
Compositions and methods for detecting severe acute respiratory syndrome coronavirus 2 (sars-cov-2) variants having spike protein mutations
Methods for the rapid detection of the presence of variants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that contain mutations in the Spike (S) protein gene in a biological or non-biological sample are described. The methods can include performing an amplifying step, a hybridizing step, and a detecting step. Furthermore, primers and probes targeting SARS-CoV-2 variants containing S gene mutations and kits are provided that are designed for the detection of SARS-CoV-2 variants containing S gene mutations.
Owner:ROCHE MOLECULAR SYST INC
SYSTEMS AND PROCESSES TO SCREEN FOR SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-CoV-2) OF 2019 (COVID-19)
PendingUS20210373020A1Culture processImmunoglobulins against virusesSevere acute respiratory syndrome coronavirus 2 (SARS-CoV-2)Virology
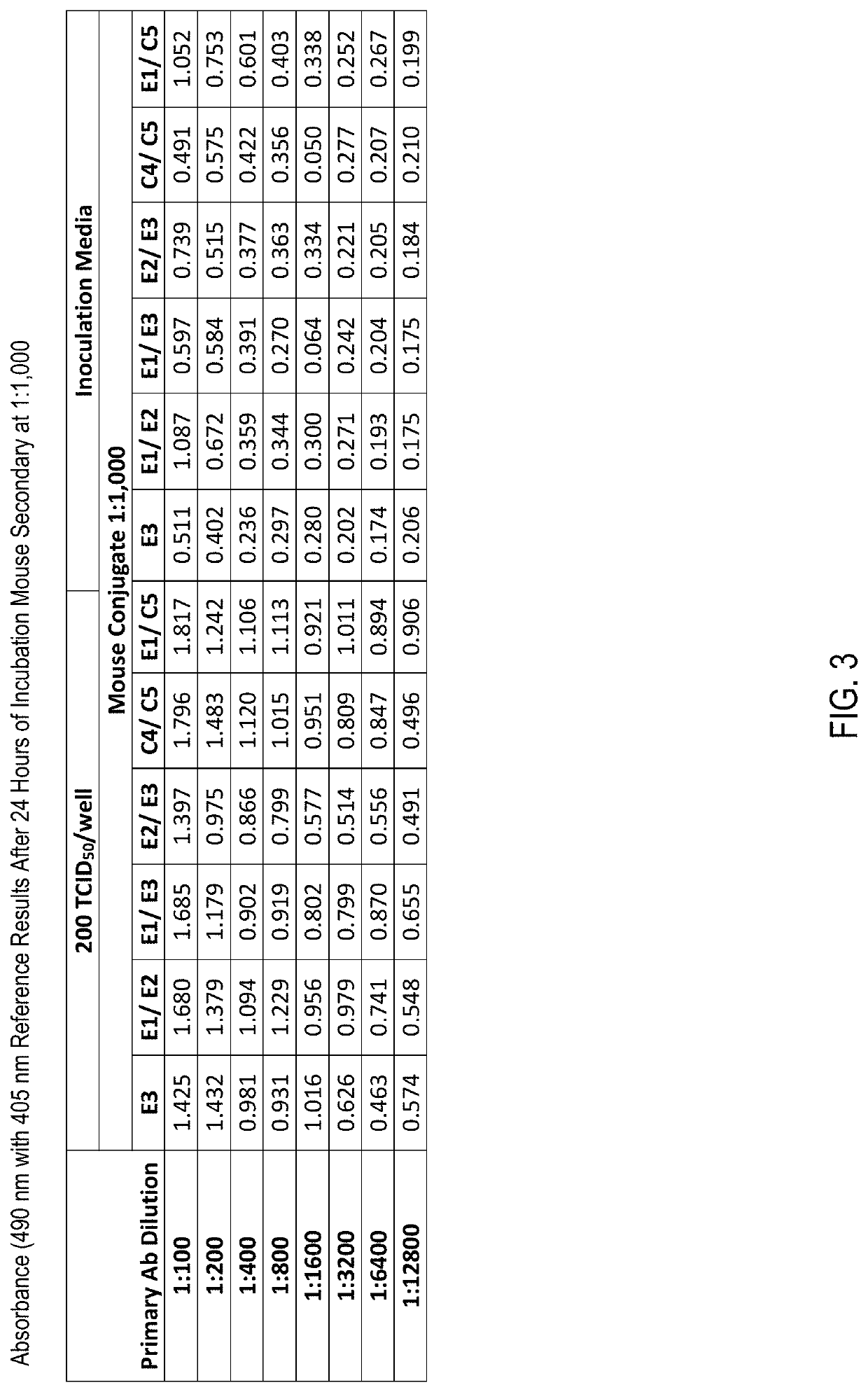
Alternative antibodies to screen for SARS-CoV-2 are disclosed. One alternative antibody is Mouse Species Coronavirus (COVID-19, MERS, and SARS-CoV NP) Antibody, Catalog Number HM1056 (“E3-Antibody” or “E3”), from EastCoast Bio, Inc., PO Box 489, North Berwick, Me. 03906, USA (“EastCoast Bio”). Another alternative antibody is a combination of the E3-Antibody and Mouse Species Coronavirus (COVID-19, MERS, and SARS-CoV NP) Antibody, Catalog Number HM1057 (“E1-Antibody” or “E1”), from EastCoast Bio (the combination of the E1-Antibody and the E3-Antibody is designated as “E1 / E3-Antibody” or simply “E1 / E3”). Yet another alternative antibody is a combination of Mouse Species Anti-SARS-CoV-2 NP mAb, clone 4B21, Catalog Number CABT-CS027 (“C4-Antibody” or “C4”), from Creative Diagnostics, 45-1 Ramsey Road, Shirley, N.Y. 11967, USA (“Creative Diagnostics”), and Mouse Species Anti-SARS-CoV-2 NP mAb, clone 7G21, Catalog Number CABT-CS026 (“C5-Antibody” or “C5”), also from Creative Diagnostics (the combination of the C4-Antibody and the C5-Antibody is designated as “C4 / C5-Antibody” or simply “C4 / C5”).
Owner:BATTELLE MEMORIAL INST
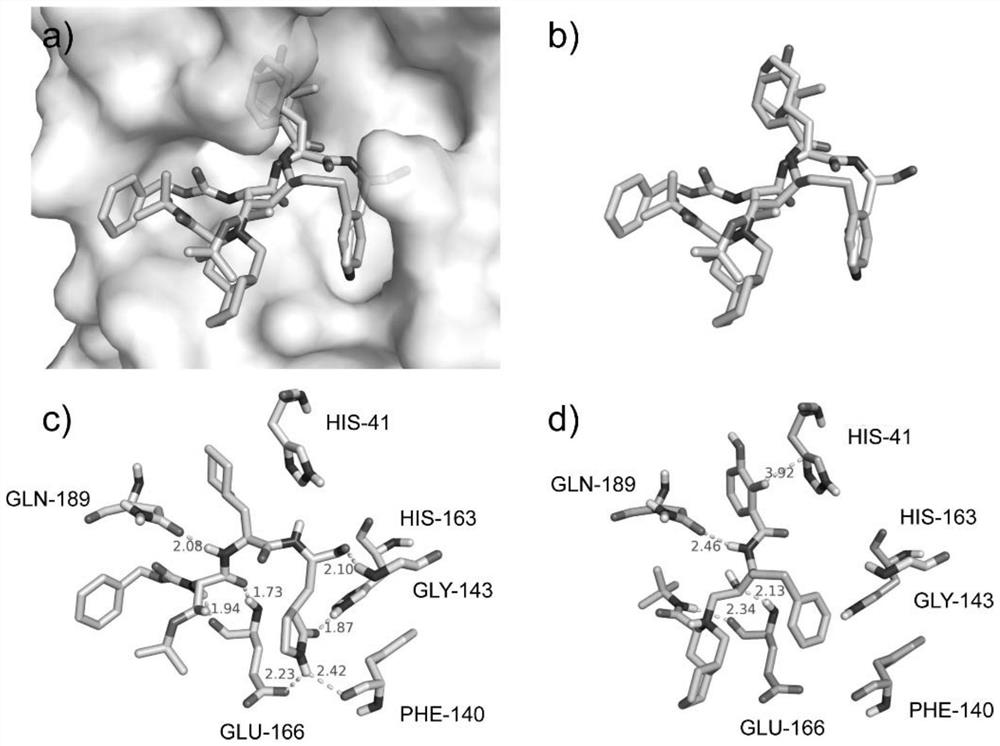
Application of PLpro protein inhibitor in medicines for treating or preventing novel coronavirus infection
The invention relates to an application of a PLpro protein inhibitor in preparation of medicines for treating or preventing diseases related to novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and provides a pharmaceutical composition containing the PLpro protein inhibitor and used for preparing medicines for treating or preventing diseases related to novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL +1
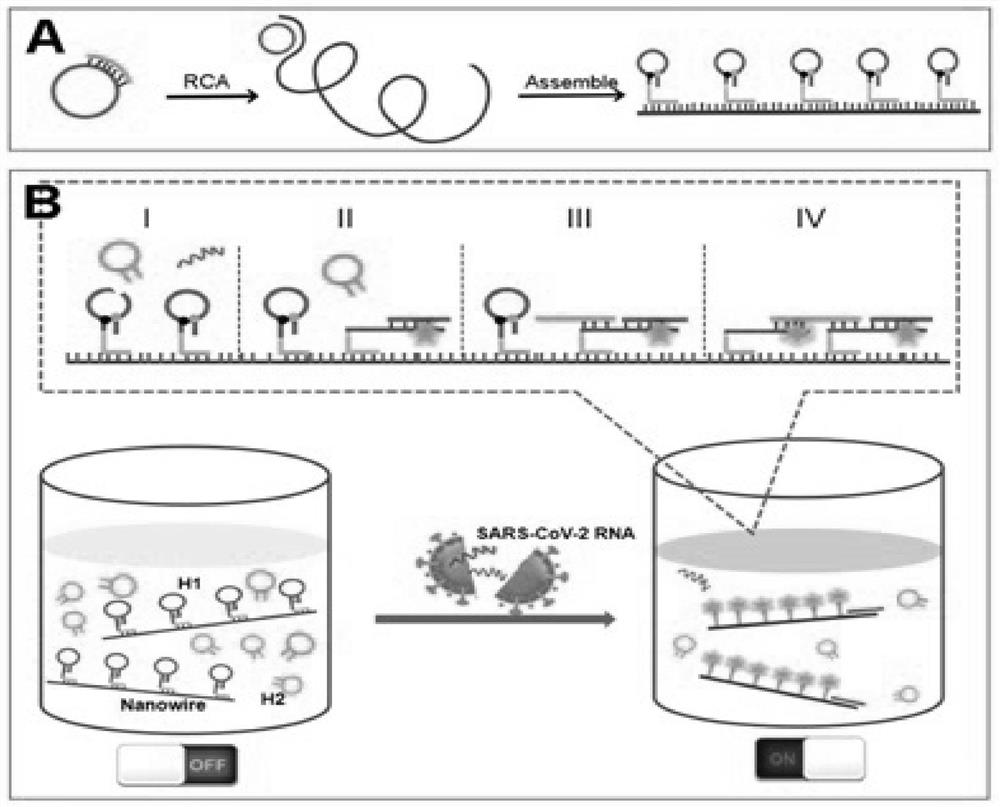
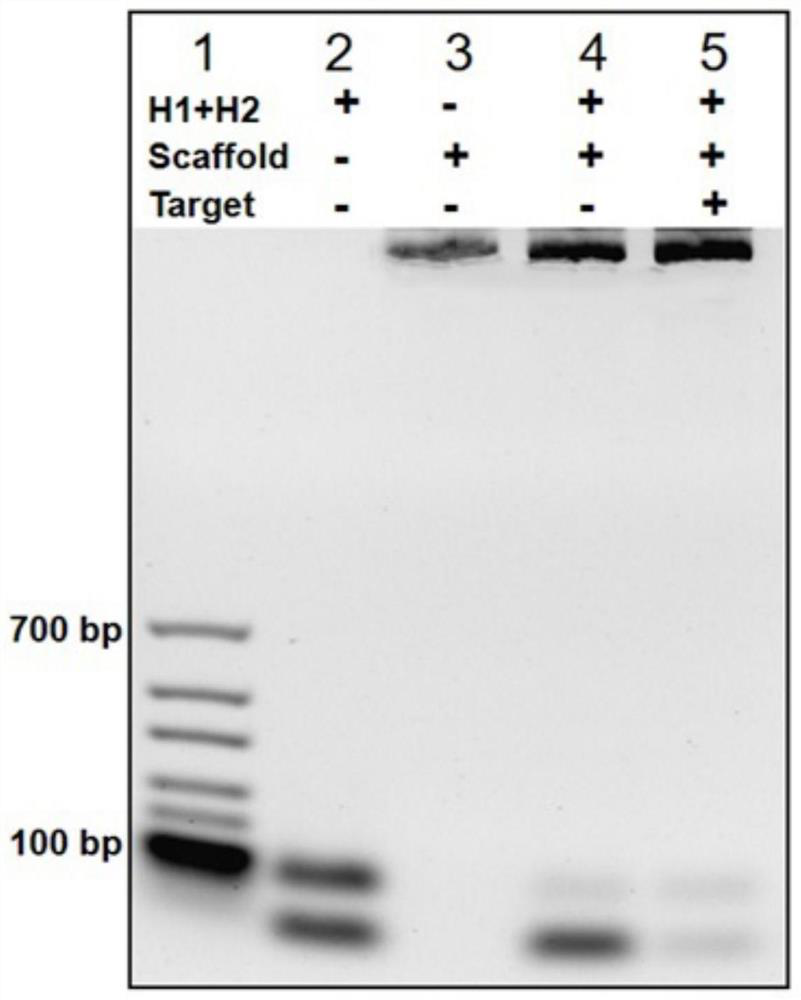
Method for rapidly detecting SARS-CoV-2 based on DNA nano-scaffold
ActiveCN111676317AHigh detection sensitivityIncrease signal gainMicrobiological testing/measurementAgainst vector-borne diseasesDiseaseNano-scaffold
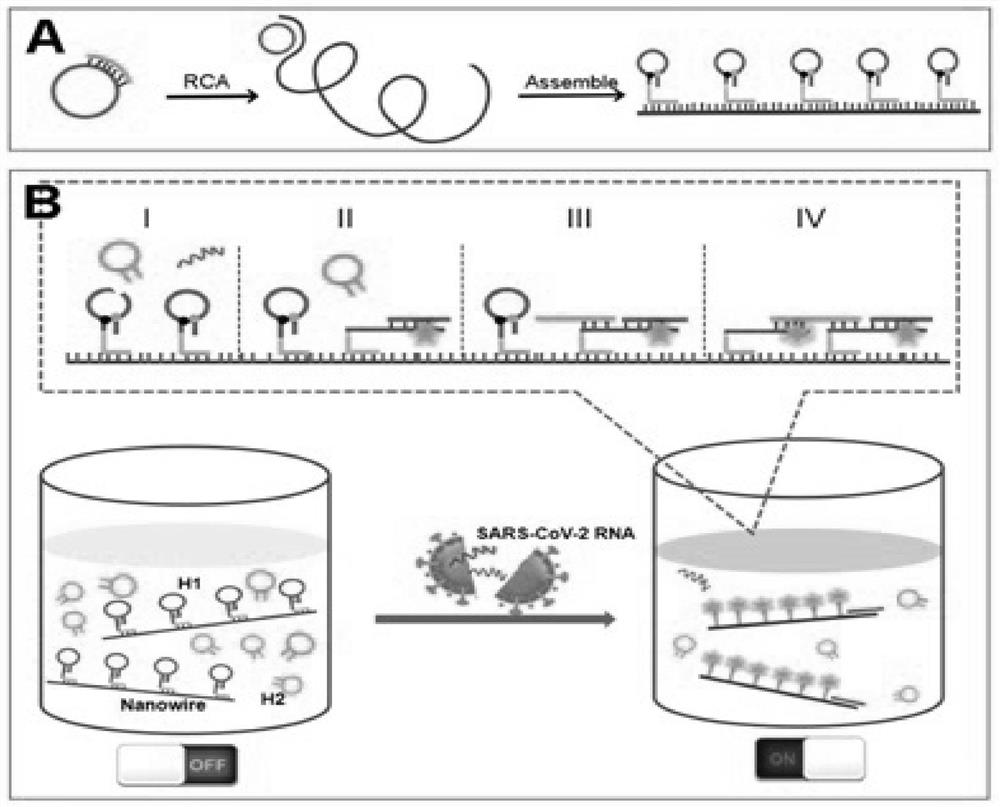
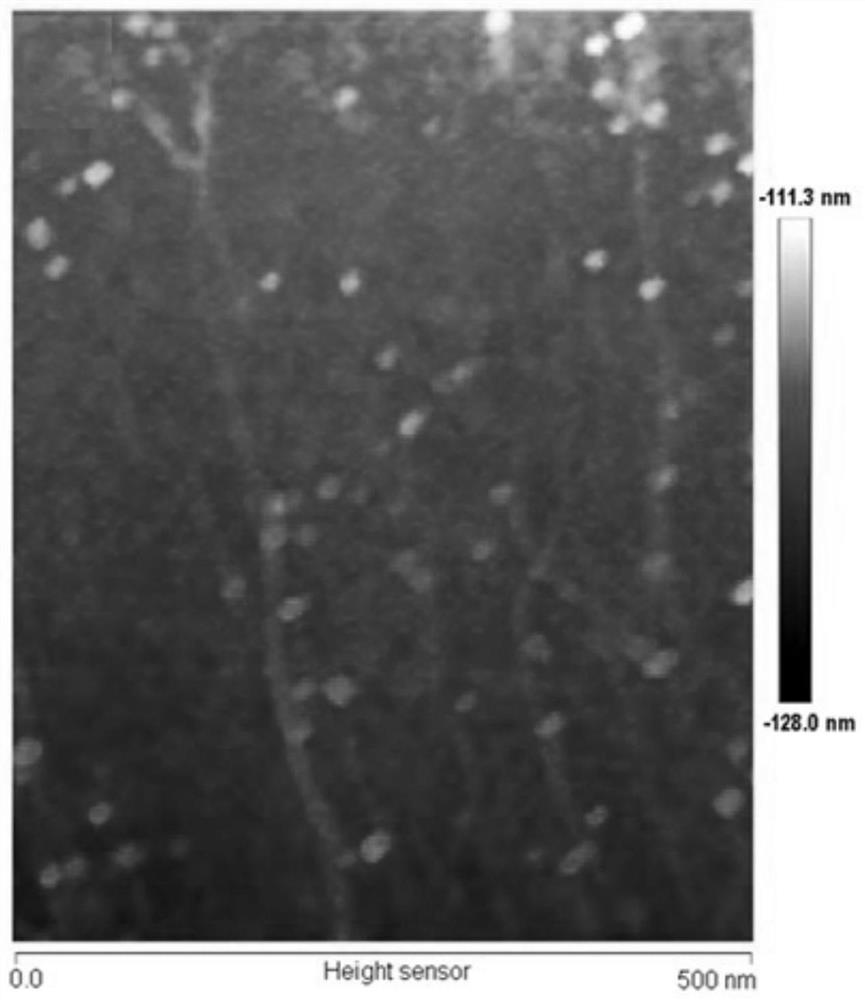
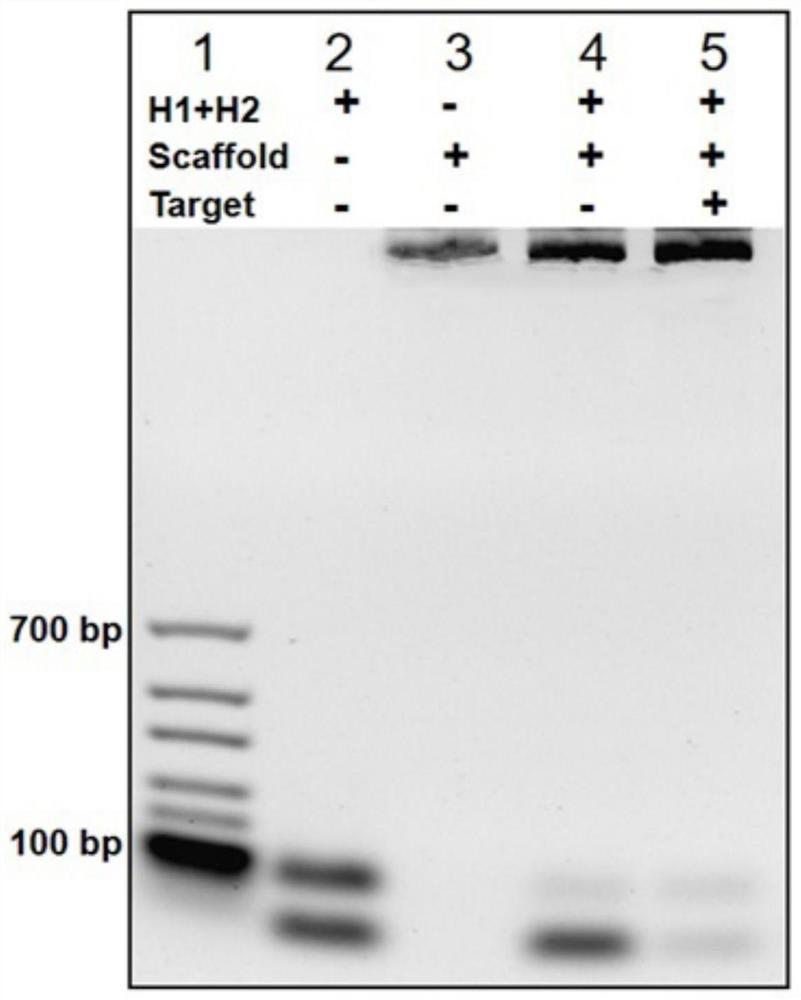
The invention discloses a method for rapidly detecting SARS-CoV-2 based on a DNA nano-scaffold. The method comprises the following steps of (1) constructing the DNA nano-scaffold through a DNA chain self-assembly and self-quenching probe H1, triggering free hairpin probe H2 and hairpin probe H1 to rapidly hybridize along the nano-scaffold by target RNA, and realizing amplification of signals. Theglobal coronavirus disease (COVID-19) in 2019 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The method is high in detection sensitivity, programmable in sequence, high insignal gain, high in specificity, short in reaction time (10 minutes), low in cost and simple to operate.
Owner:NANJING UNIV
Double-antibody composition and application to preparation of COVID-19 (Coronavirus Disease 2019) treatment drugs
ActiveCN112076316AHigh neutralizing activityImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesReceptor
The invention relates to an antibody composition containing a monoclonal antibody specifically bound to an S protein receptor binding domain of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) and a monoclonal antibody specifically bound to an S protein N-terminal structural domain of the SARS-CoV-2. Compared to the monoclonal antibodies separately used, the antibody composition is significantly improved in the aspect of the neutralizing activity on novel coronavirus infected cells, the neutralizing activity is respectively increased to 6.4 times of that of an anti-S protein receptor binding domain and 3.8 times of that of an anti-S protein N-terminal structural domain, and the antibody composition has a remarkable synergistic effect. The invention further relates to application of the antibody composition to preparation of COVID-19 treatment and / or prevention drugs. The antibody composition can be prepared on a large scale by stable engineered strains and an industrial pharmaceutical method, and has a huge industrial prospect.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Method of identifying severe acute respiratory syndrome corona virus 2 (sars-cov-2) ribonucleic acid (RNA)
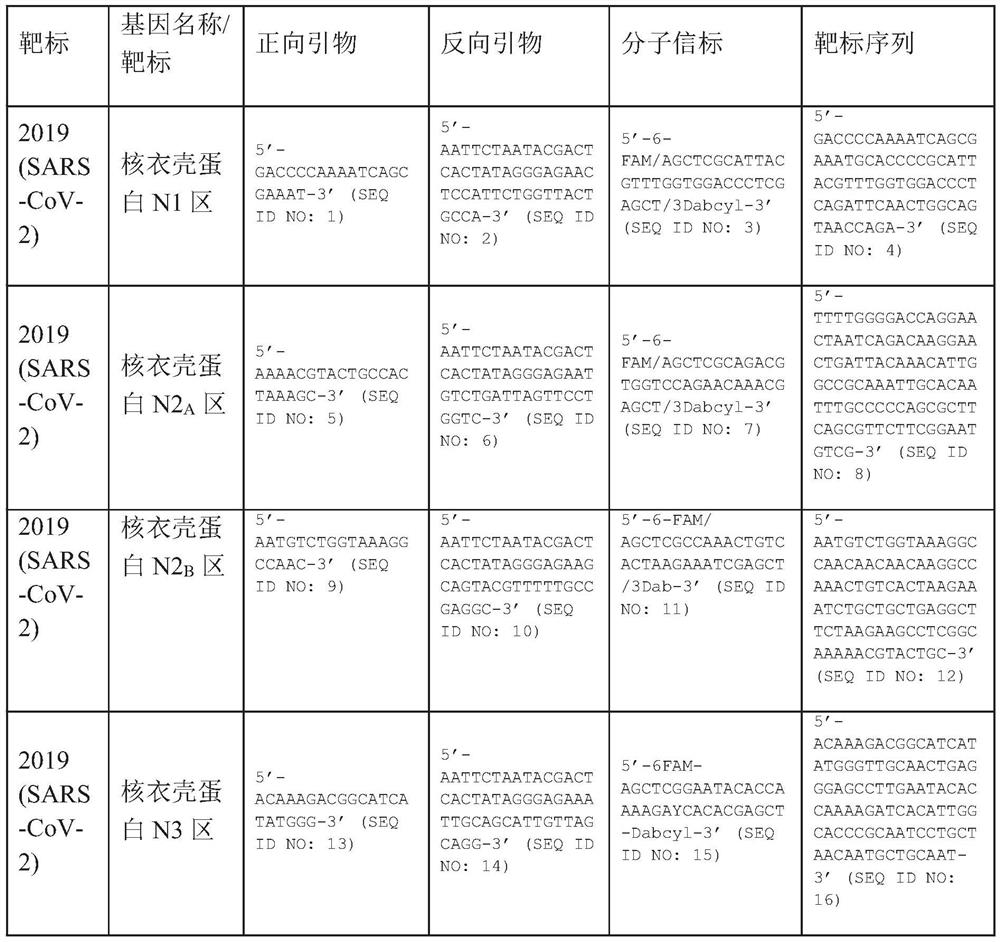
A method for detecting the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a biological sample includes the steps of lysing the biological sample to form a lysate and generating an amplified lysate by performing a nucleic acid sequence-based (NASBA) amplification for a target nucleic acid sequence in the lysate in the presence of: a forward primer having the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 5, SEQ ID NO: 9, SEQ ID NO: 13, and SEQ ID NO: 17; a reverse primer having the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 2, SEQ ID NO: 6, SEQ ID NO: 10, SEQ ID NO: 14, and SEQ ID NO: 18; and a molecular beacon having the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 3, SEQ ID NO: 7, SEQ ID NO: 11, SEQ ID NO: 15, and SEQ ID NO: 19 and a fluorophore. The amplified lysate is exposed to an excitation source. A fluorescence of the fluorophore is detected in the amplified lysate exposed to the excitation source The SARS-CoV-2 is determined to be present in the biological sample in response to detecting the fluorescence of the fluorophore.
Owner:TELEFLEX MEDICAL INC
Prodrugs of 1'-substituted carba-nucleoside analogues for antiviral treatment
The present invention provides novel compounds and pharmaceutically acceptable salts or esters thereof. For example, the compound has the structure of Formula V. Also provided is a pharmaceutical composition comprising the compound or a pharmaceutically acceptable salt or ester thereof and a pharmaceutically acceptable carrier. Further provided a method for inhibiting a polymerase of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (SARS-CoV-2 polymerase) or treating viral infection in a subject in need thereof, comprising administering an effective amount of the pharmaceutical composition to the subject, for example, orally.
Owner:COPYCAT SCI INC
Ratio-type ECL biosensor as well as preparation method and application thereof
ActiveCN113278683AGood receptorMicrobiological testing/measurementAgainst vector-borne diseasesA-DNAElectrochemiluminescence
The invention constructs a ratio electrochemical luminescence (ECL) biosensor, which is used for detecting the RNA-dependent RNA polymerase (RdRp) gene of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). The SARS-COV-2RdRp gene participates in the entropy-driven cyclic amplification reaction, then a piece of Bandage DNA is output, and can be combined with the other two single chains S1 and S2 to form a double-chain DNAWalker, and the next cyclic reaction is started. After the walking process of the double-foot DNA Walker is completed, a hairpin structure at the top of a DNA tetrahedron (TDNAs) is removed. Then, a PEI-Ru@Ti3C2@AuNPs-S7 probe is used for being specifically combined with a TDNAs hairpin part cut off from the surface of Au-g-C3N4, and signal change is achieved through ECL-RET (electrochemiluminescence resonance energy transfer).
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Application of PLpro protein inhibitor in medicine for treating or preventing novel coronavirus infection
The invention relates to application of a PLpro protein inhibitor to preparation of a medicine for treating or preventing novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection related diseases, and provides a pharmaceutical composition which contains the PLpro protein inhibitor and is used for preparing the medicine composition for treating or preventing the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection related diseases.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL +2
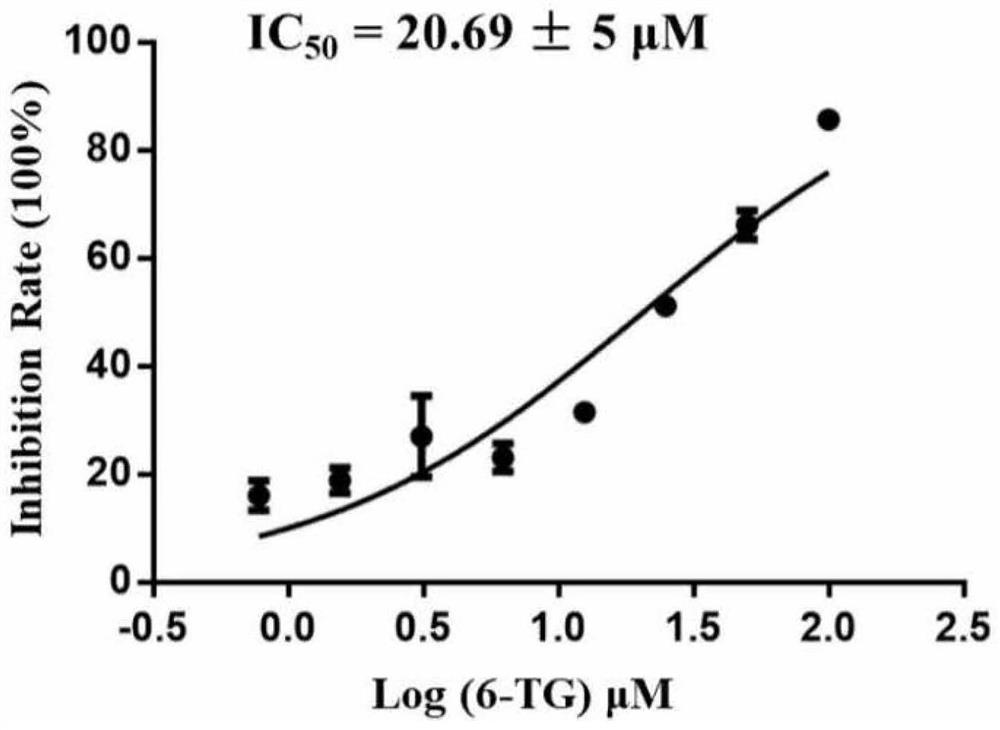
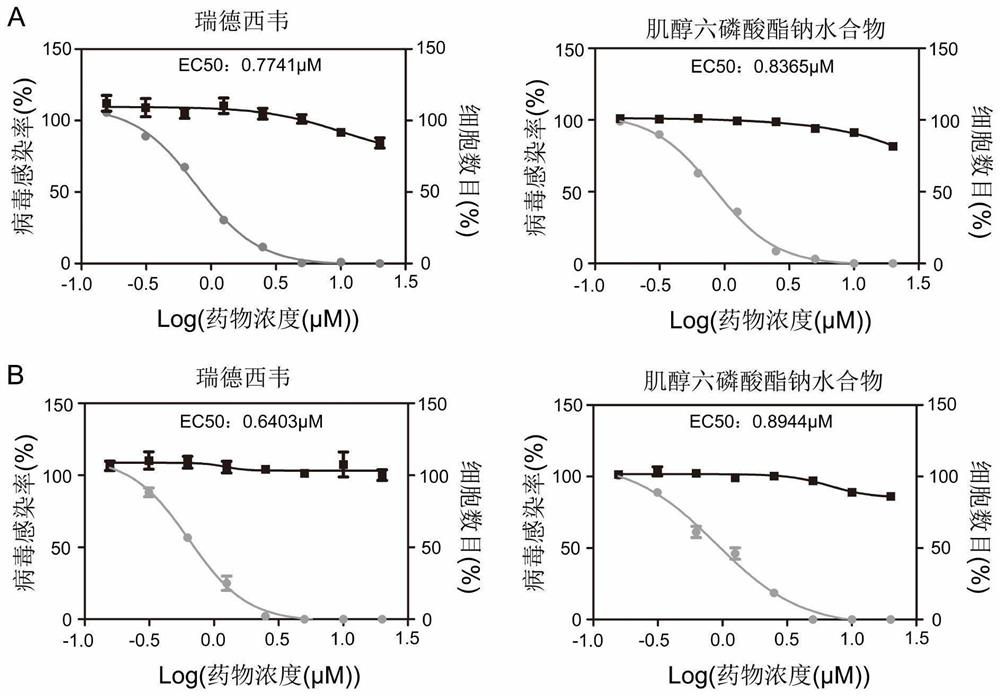
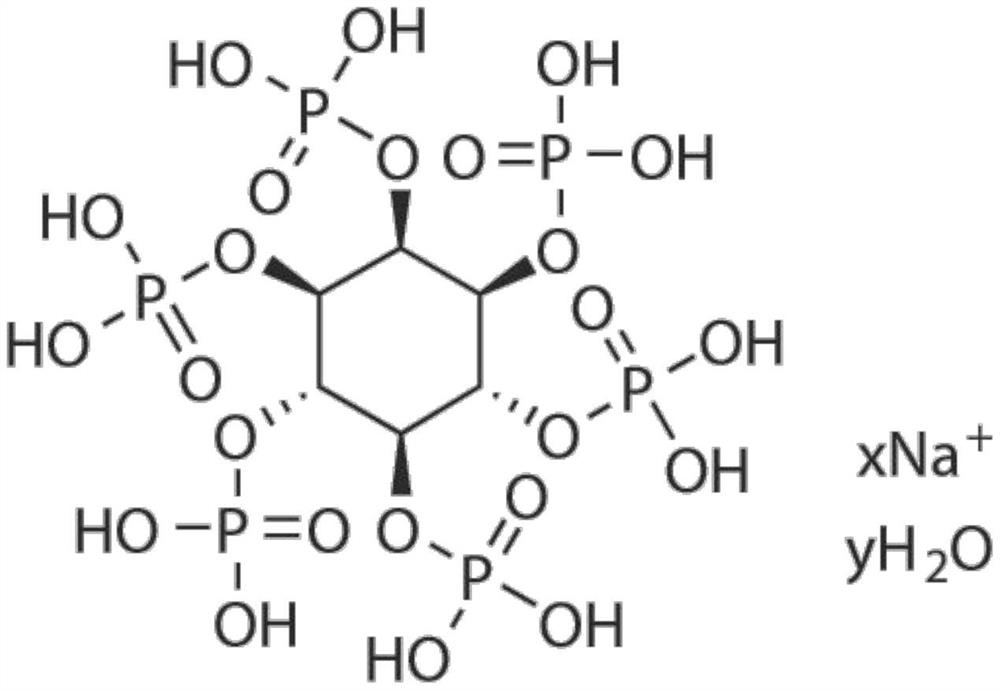
Application of small molecule compound sodium phytate hydrate in preparation of medicine for resisting SARS-CoV-2
ActiveCN113712976ALow toxicityAvoid infectionOrganic active ingredientsAntiviralsGreen monkeyBULK ACTIVE INGREDIENT
The invention discloses application of a small molecule compound sodium phytate hydrate in preparation of a medicine for resisting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); the medicine for resisting the SARS-CoV-2 is a pharmaceutical composition which takes the sodium phytate hydrate as a unique active ingredient or contains the sodium phytate hydrate; and the medicine for resisting the SARS-CoV-2 is a medicine for preventing or treating SARS-CoV-2 infection. According to the invention, an SARS-CoV-2 susceptible cell line, including African green monkey kidney cells Vero E6 and human lung adenocarcinoma cells Calu-3, is utilized to detect the anti-SARS-CoV-2 activity of the sodium phytate hydrate. Experimental results show that the sodium phytate hydrate can effectively inhibit infection of the SARS-CoV-2 on the susceptible cells, is relatively low in cytotoxicity, is expected to be used as a medicine for effectively resisting SARS-CoV-2 infection, and has an application prospect.
Owner:THE NAVAL MEDICAL UNIV OF PLA
Severe acute respiratory syndrome coronavirus 2 affinity peptide based on human angiotensin-converting enzyme 2
ActiveCN112375754BAvoid interactionImprove specific binding abilityBiological material analysisPeptide preparation methodsPhysiologyBinding site
The invention provides a severe acute respiratory syndrome coronavirus 2 affinity polypeptide based on human angiotensin-converting enzyme 2, and relates to the technical field of biological materials. By extracting the key human angiotensin converting enzyme 2 amino acid residues of the binding site of human angiotensin converting enzyme 2 and severe acute respiratory syndrome coronavirus 2, the peptide library was reconstructed, and the severe acute respiratory syndrome coronavirus 2 based on human angiotensin converting enzyme 2 was screened. Acute respiratory syndrome coronavirus 2 affinity polypeptide; and then prepare biological detection reagents through the above affinity polypeptide, which provides a new method for the detection of severe acute respiratory syndrome coronavirus 2.
Owner:TSINGHUA UNIV
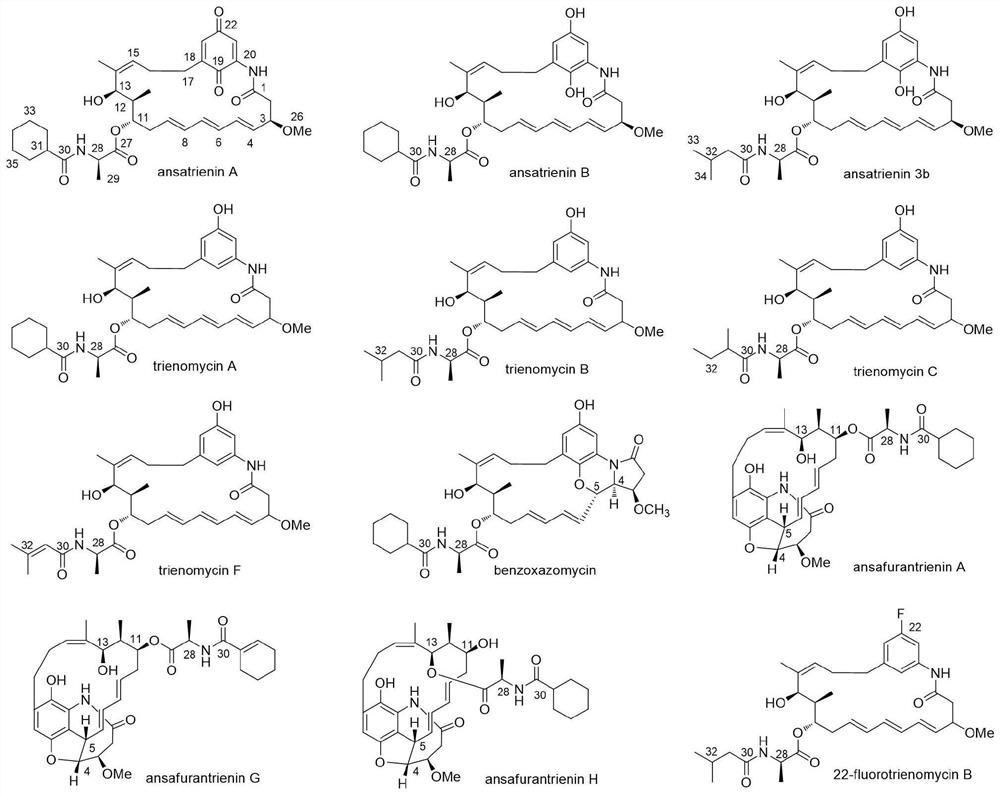
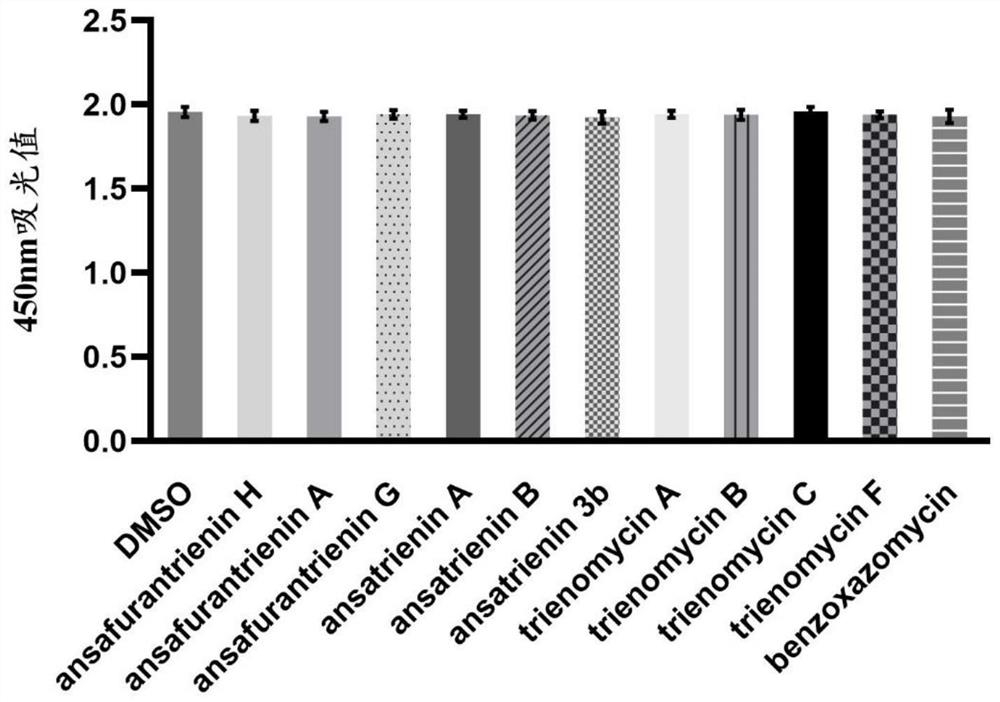
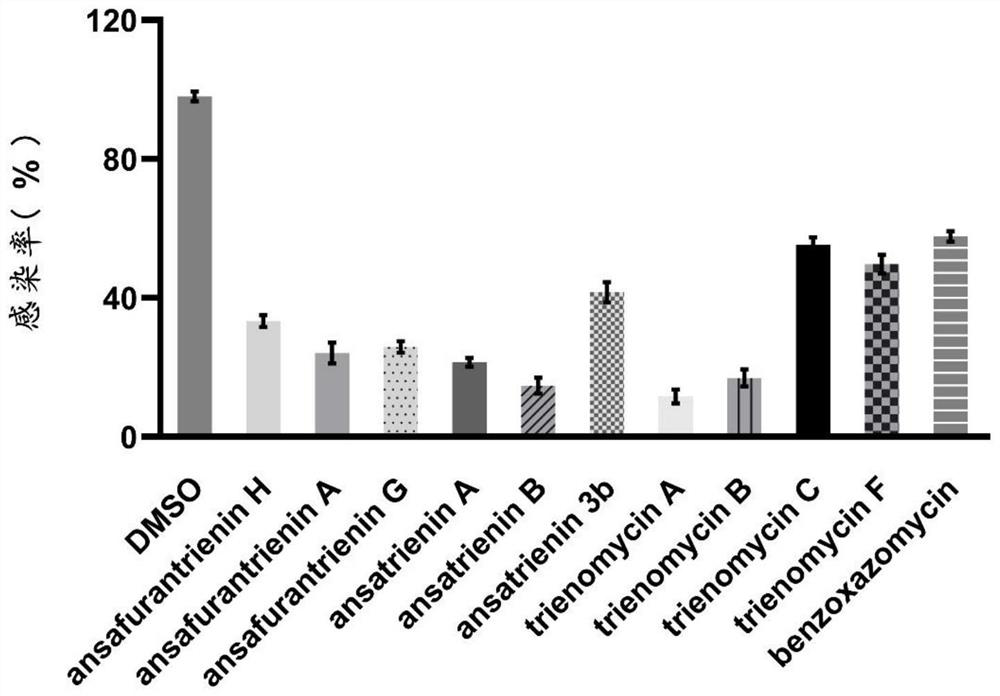
Application of ansatriene compound in antiviral infection medicine and preparation method of ansatriene compound
PendingCN114796204AHas inhibitory effectLow cytotoxicityOrganic active ingredientsAntimycoticsTick-borne encephalitis virus TBEVCoronavirus
The invention relates to the technical field of medicines, and relates to application of an ansatriene compound in preparation of medicines or pharmaceutical compositions for resisting infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), tick-borne encephalitis virus (TBEV), west nile virus (WNV), yellow fever virus (YFV) and chikungunya fever virus (CHIKV) and a preparation method of the ansatriene compound.
Owner:THE NAVAL MEDICAL UNIV OF PLA
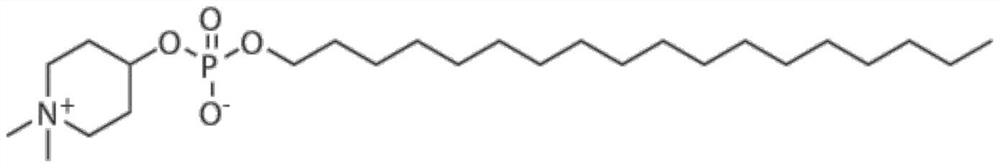
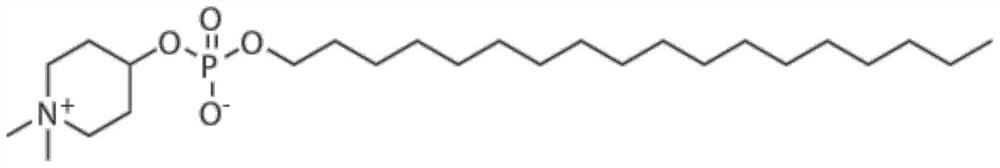
Application of small molecule compound piperifoside in preparation of SARS-CoV-2 resisting medicine
PendingCN114515291ALow toxicityAvoid infectionOrganic active ingredientsAntiviralsCancer cellBULK ACTIVE INGREDIENT
The invention discloses an application of a small molecule compound piperifoside in preparation of a medicine for resisting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the medicine for resisting SARS-CoV-2 is a medicine composition taking piperifoside as a unique active ingredient or containing piperifoside, and the medicine for resisting SARS-CoV-2 refers to a medicine for preventing or treating SARS-CoV-2 infection. According to the invention, a susceptible cell line of SARS-CoV-2, including African green monkey kidney cells Vero E6 and human lung adenocarcinoma cells Clu-3, is utilized to detect the anti-SARS-CoV-2 activity of piperifen. Experimental results show that the piperifoside can effectively inhibit infection of the SARS-CoV-2 on the susceptible cells, is relatively low in cytotoxicity, is expected to be used as a medicine for effectively resisting SARS-CoV-2 infection, and has an application prospect.
Owner:THE NAVAL MEDICAL UNIV OF PLA
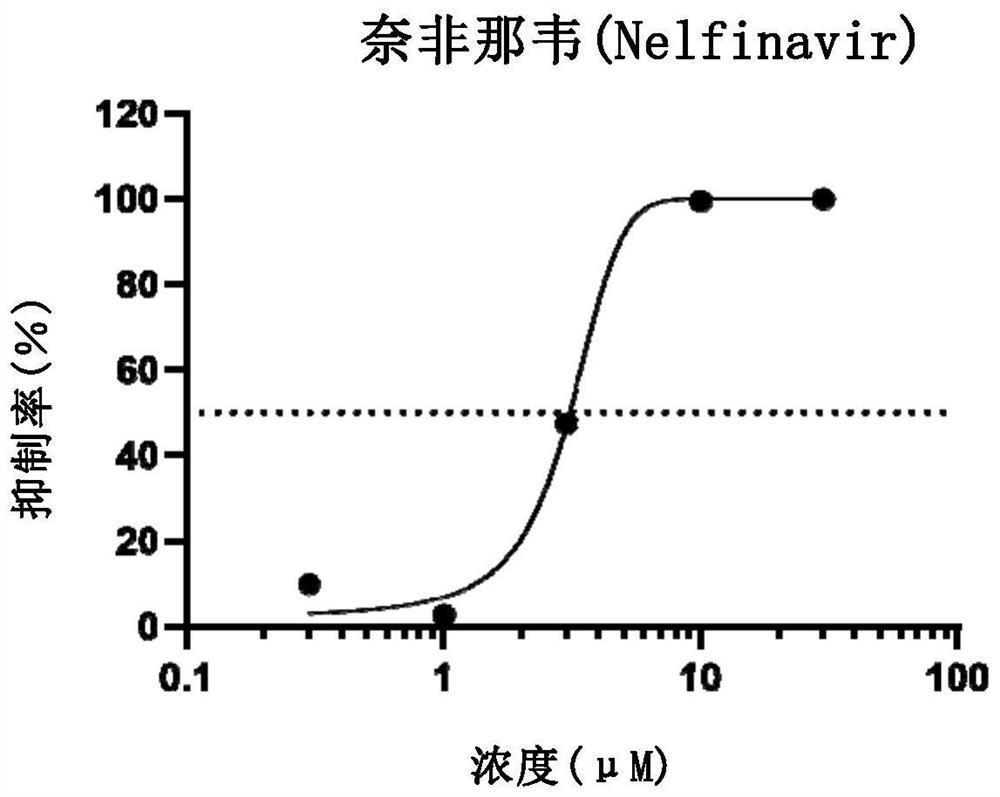
Application of nelfinavir in preparation of medicine for preventing and treating COVID-19
The invention relates to application of nelfinavir in preparation of a medicine for preventing and treating COVID-19. Specifically, the invention relates to application of nelfinavir and a pharmaceutical composition thereof as a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or 2019-nCoV) inhibitor in preparation of drugs for treating and / or preventing and relieving respiratory tract infection, pneumonia and other related diseases caused by 2019-nCoV infection.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +3
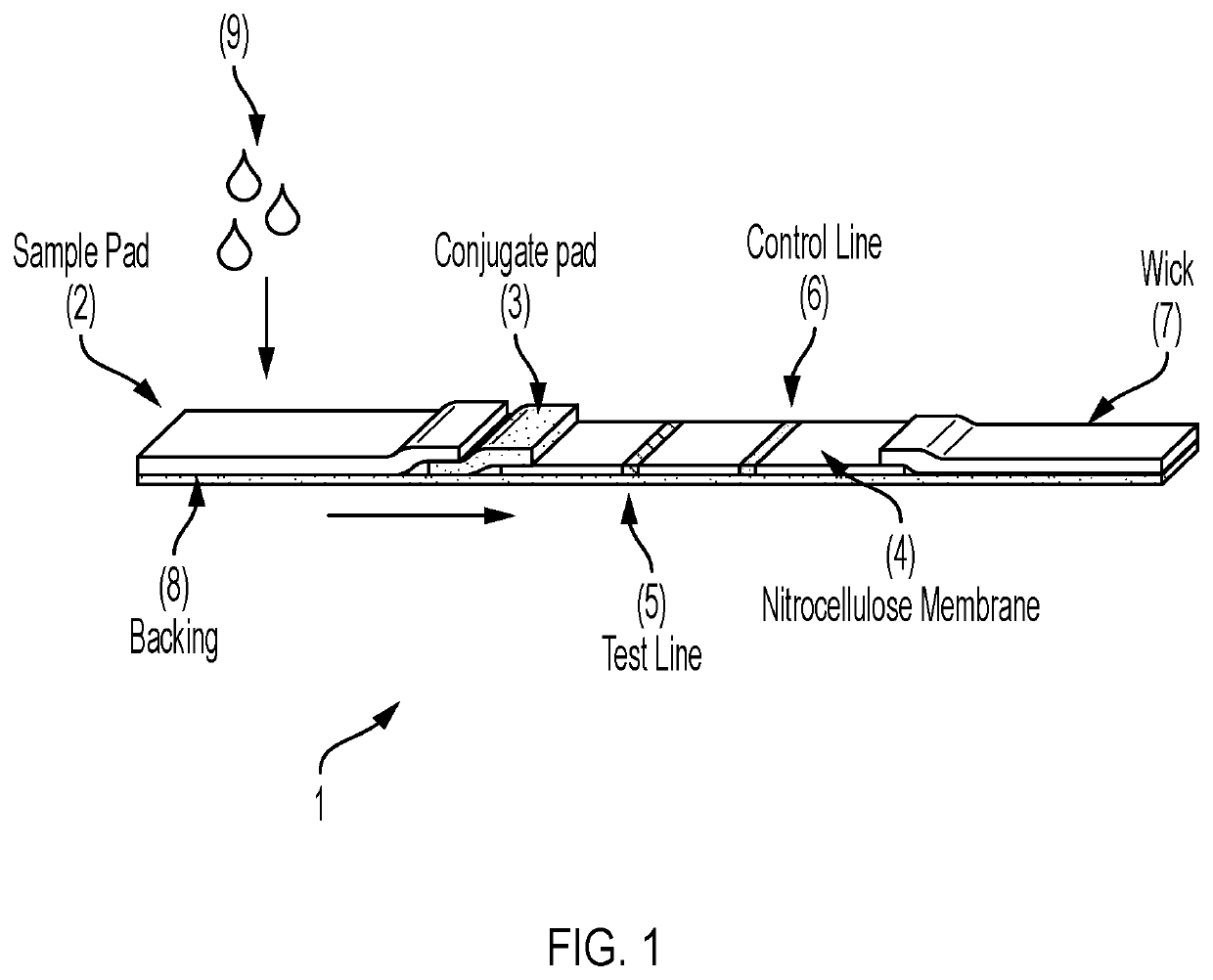
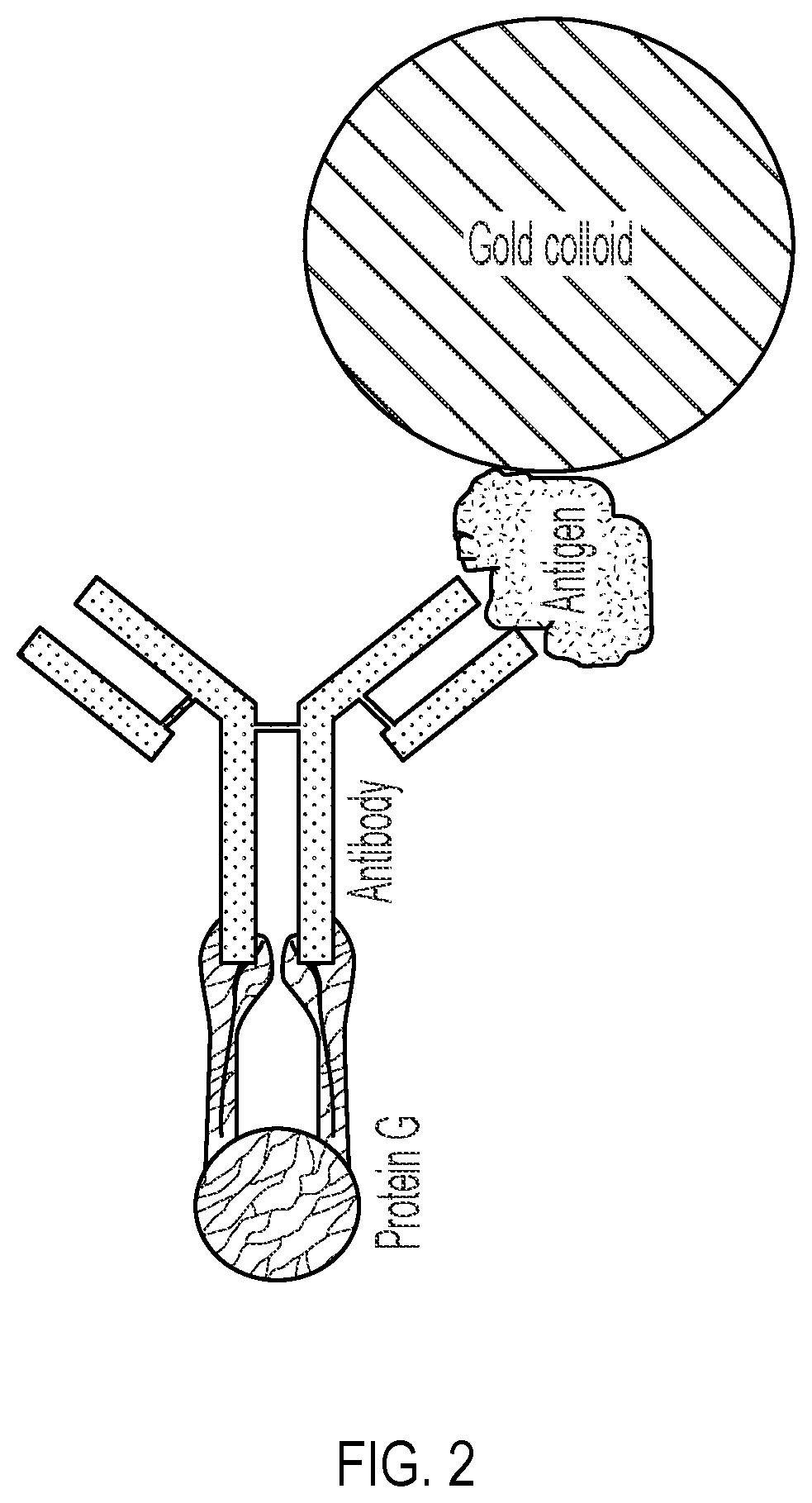
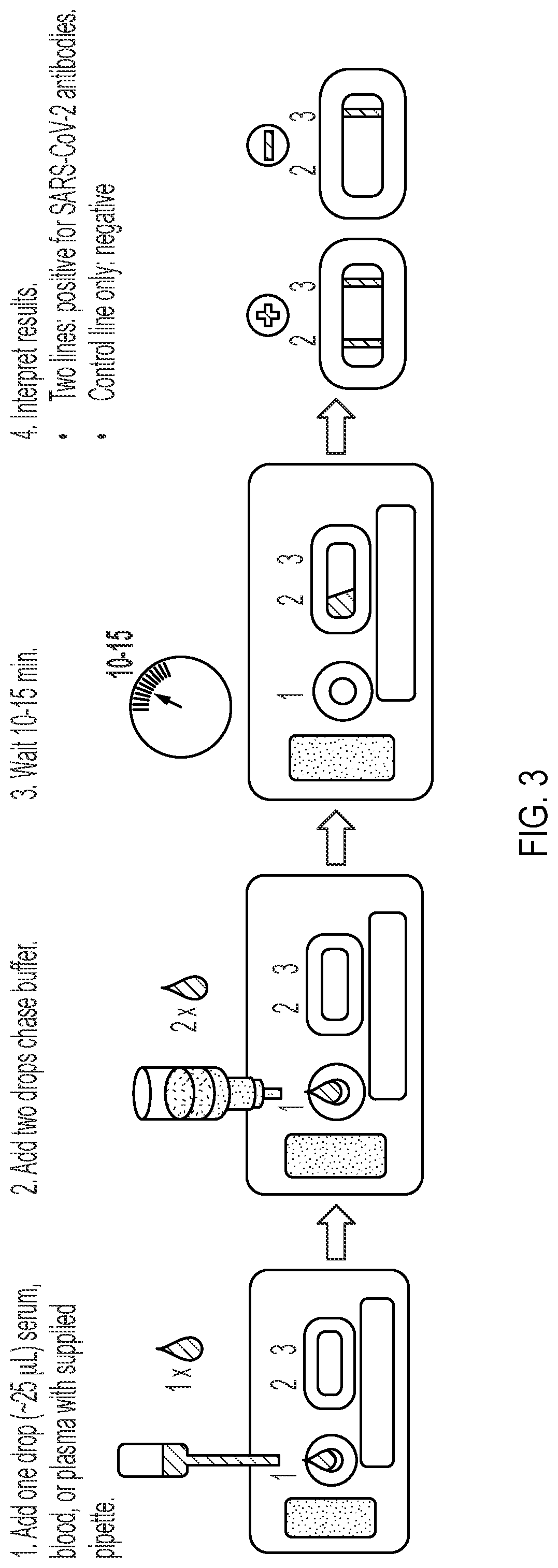
Lateral Flow Device for Detecting SARS-CoV-2 Antibodies in Human and Animal Samples
The invention provides a lateral flow device and methods for the detection of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a sample of bodily fluid of an animal or human. The test methods include contacting the sample with a conjugate comprising a recombinant SARS-CoV-2 spike protein antigen that has been conjugated to a detection agent, wherein an antigen-antibody complex is formed between the SARS-CoV-2 spike protein antigen conjugate and SARS-CoV-2 antibodies present in the sample; capturing the formed antigen-antibody complex with an Fc-binding molecule; and detecting the captured complex.
Owner:ZOETIS SERVICE LLC
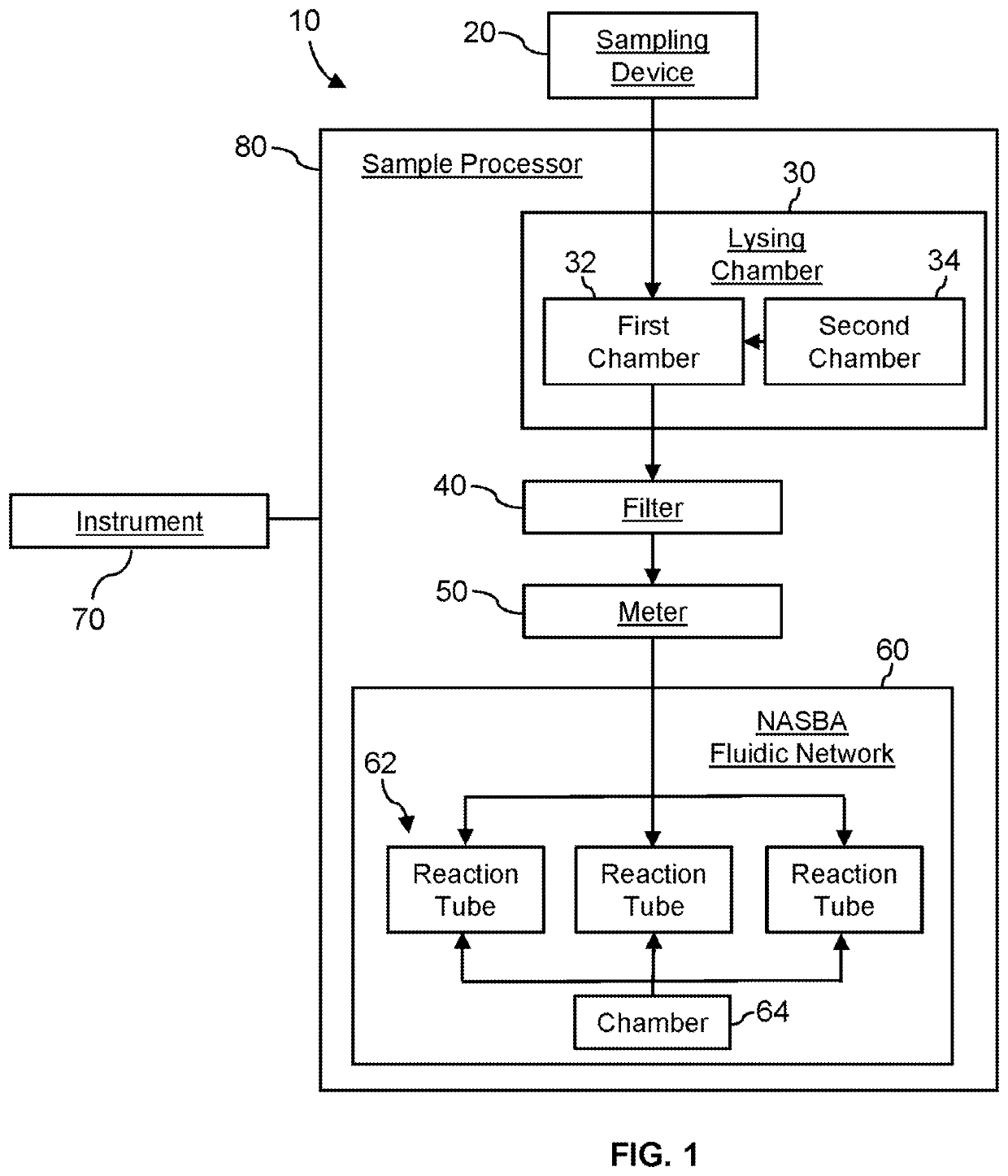
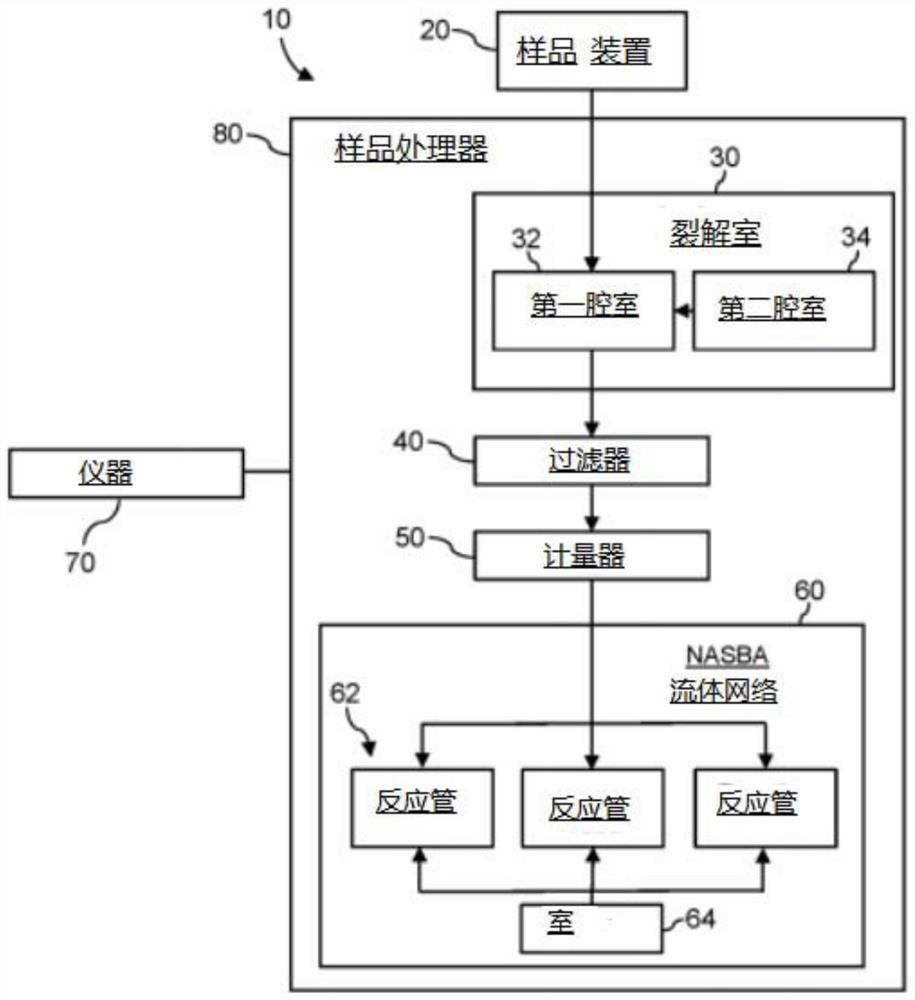
System for identifying severe acute respiratory syndrome corona virus 2 (sars-cov-2) ribonucleic acid (RNA)
InactiveUS20210129144A1Microbiological testing/measurementLaboratory glasswaresForward primerFluorophore
A system for detecting the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a biological sample includes a sampling device, a lysing chamber, a NASBA fluidic network, and an analytical instrument. The sampling device is configured to contain a sample containing a pathogen target sequence for SARS-CoV-2. The lysing chamber is configured to be in fluid communication with the sampling device to receive the sample. The is lysing chamber is configured to lyse the sample into a lysate. The NASBA fluidic network is configured to be in fluid communication with the lysing chamber to receive the lysate. The NASBA fluidic network includes an enzyme, a forward primer, and a reverse primer for amplifying a predetermined genetic sequence in the pathogen target sequence contained within the lysate. The forward primer has the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 5, SEQ ID NO: 9, SEQ ID NO: 13, and SEQ ID NO: 17. The reverse primer has the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 2, SEQ ID NO: 6, SEQ ID NO: 10, SEQ ID NO: 14, and SEQ ID NO: 18. A molecular beacon is configured to attach to the pathogen target sequence. The beacon has the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 3, SEQ ID NO: 7, SEQ ID NO: 11, SEQ ID NO: 15, and SEQ ID NO: 19 and a fluorophore. The analytical instrument is configured to excite the beacon when the molecular beacon is attached to the pathogen target sequence to signal a presence of the pathogen target sequence.
Owner:TELEFLEX MEDICAL INC
A method for rapid detection of SARS-CoV-2 based on DNA nanoscaffolds
ActiveCN111676317BEfficient diagnosis and sensitive detectionEffective diagnosisMicrobiological testing/measurementAgainst vector-borne diseasesDiseaseSignal gain
The invention discloses a method for rapidly detecting SARS-CoV-2 based on DNA nano-stents, comprising the following steps: (1) DNA nano-stents are constructed by DNA chain self-assembly and self-quenching probe H1, and target RNA triggers free Hairpin probe H2 and hairpin probe H1 rapidly hybridize along the nano-scaffold to achieve signal amplification. A global coronavirus disease 2019 (COVID‑19) of the present invention is caused by severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2). The invention has high detection sensitivity, programmable sequence, high signal gain, high specificity, short reaction time (<10 minutes), low cost and simple operation.
Owner:NANJING UNIV
Composition for preventing or treating sars coronavirus 2 infection disease
Disclosed is a pharmaceutical composition for preventing or treating severe acute respiratory syndrome coronavirus 2 infection disease, the composition comprising a compound represented by a Chemical Formula 1, or a pharmaceutically acceptable salt thereof, as an active ingredient.
Owner:シンテカバイオ インコーポレイティッド
Antibody, preparation method thereof, coding nucleic acid and engineering cell
PendingCN114349850AStrong specificityIncreased sensitivityImmunoglobulins against virusesFermentationSevere acute respiratory syndrome coronavirus 2 (SARS-CoV-2)Virology
The present disclosure relates to a method for preparing an antibody, an antibody, a nucleic acid encoding the antibody, and an engineered cell. The antibody provided by the invention can resist severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and is good in specificity, high in sensitivity and excellent in pathogen neutralization effect.
Owner:蒋庆华 +3
Detection system for severe acute respiratory syndrome coronavirus 2
The invention relates to a detection system for severe acute respiratory syndrome coronavirus 2. The detection system comprises a sampling device, a cracking chamber, an NASBA fluid network and an analysis instrument. The sampling device is configured to contain a sample containing a pathogen target sequence for SARS-CoV-2. The NASBA fluid network includes an enzyme, a forward primer and a reverse primer for amplifying a predetermined gene sequence in a pathogen target sequence contained within the lysate. The forward primer has an oligonucleotide sequence selected from the following groups: SEQ ID NO: 1, SEQ ID NO: 5, SEQ ID NO: 9, SEQ ID NO: 13 and SEQ ID NO: 17. The reverse primer has oligonucleotide sequences selected from the following groups: SEQ ID NO: 2, SEQ ID NO: 6, SEQ ID NO: 10, SEQ ID NO: 14 and SEQ ID NO: 18. The molecular beacon is configured to attach to a pathogen target sequence. The beacon has an oligonucleotide sequence selected from the group consisting of SEQ ID NO: 3, SEQ ID NO: 7, SEQ ID NO: 11, SEQ ID NO: 15, and SEQ ID NO: 19 and a fluorophore.
Owner:TELEFLEX MEDICAL INC
Compositions and methods for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection
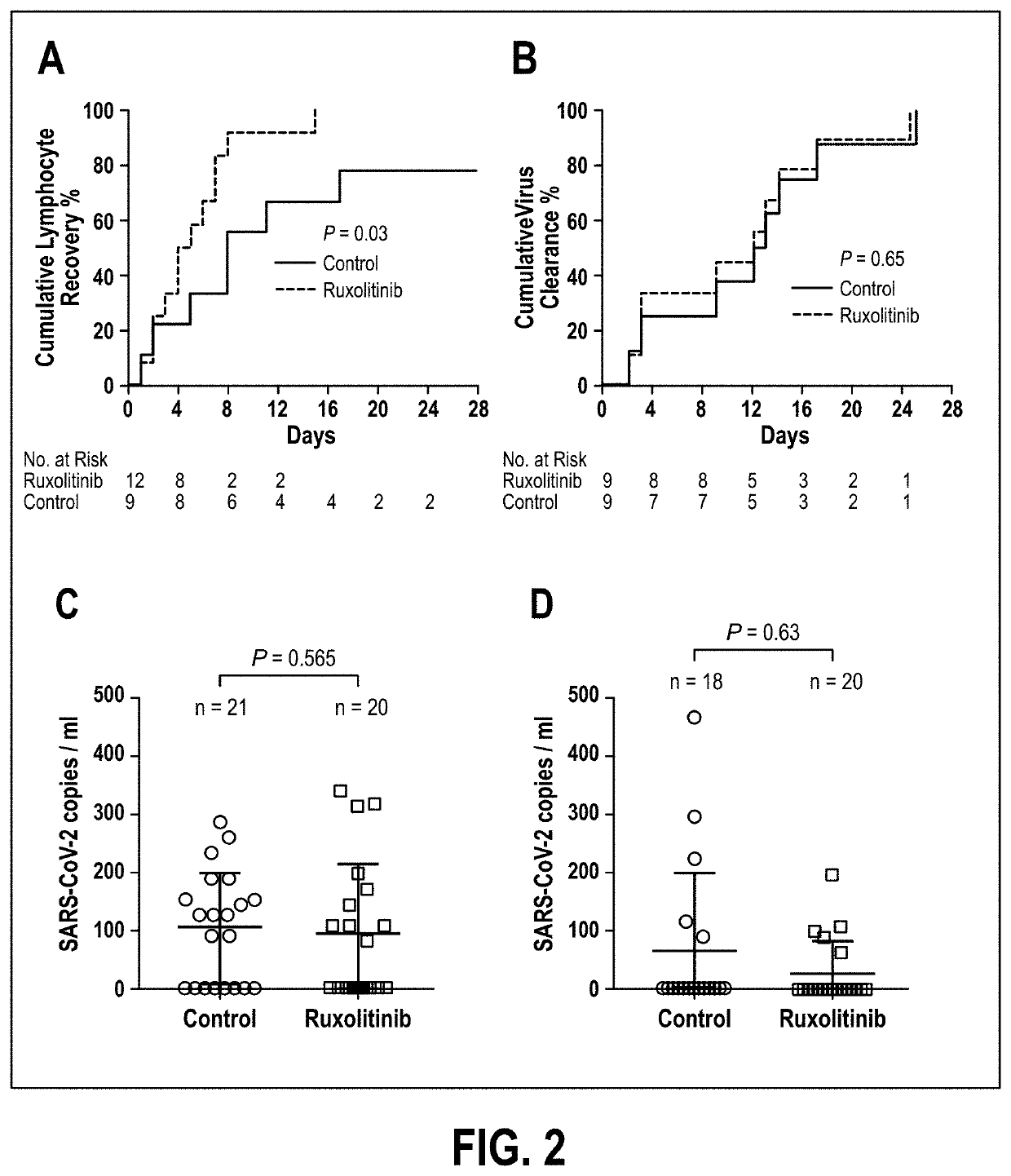
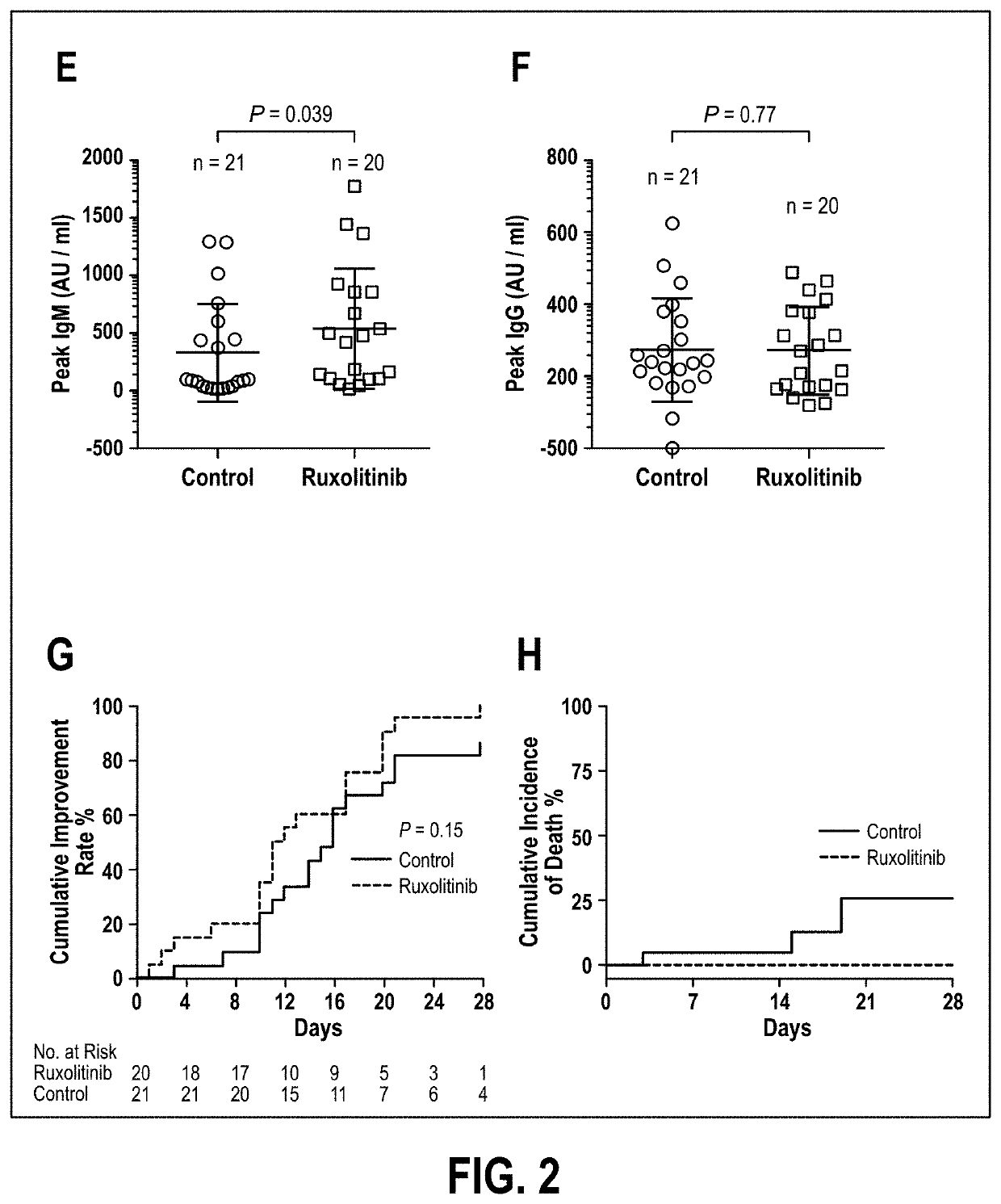
Disclosed herein are methods for treating an individual having a Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. The method may comprise administering a JAK inhibitor, for example ruxolitinib (JAKAFI®), to an individual in need thereof, such individual generally being an individual having, or suspecting of having, SARS-CoV-2 infection. The individual in need thereof may be an individual having, or suspected of having or at risk for developing SARS-CoV-2 infection-related cytokine storm. The individual in need thereof may further be an individual having, or suspected of having SARS-CoV-2 infection-related pneumonia.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Methods to prepare nasopharyngeal and oral material for oral inoculation of covid-19
The instant disclosure relates generally to oral inoculation and specifically to methods to prepare nasopharyngeal and oral material for oral inoculation of COVID-19. Severe acute respiratory syndrome coronavirus 2 (“SARS-CoV-2”) viral particles are collected from at least one of a nasopharyngeal specimen and an oral specimen each derived from a patient infected with SARS-CoV-2 or a SARS-CoV-2 variant. Mammalian cells (e.g., Vero-E6 and / or Vero-CCL81 cells) are infected with the SARS-CoV-2 viral particles to produce infected mammalian cells. The infected mammalian cells are cultured to produce a mammalian cell culture. SARS-CoV-2 viral particles are collected from the mammalian cell culture to produce isolated viral particles. The isolated viral particles are frozen to produce a frozen isolate. The frozen isolate is partitioned into a plurality of tablets each having a therapeutically effective amount of the SARS-CoV-2 viral particles. An enteric coating is applied to each tablet.
Owner:HAYDEN STEVEN MARK
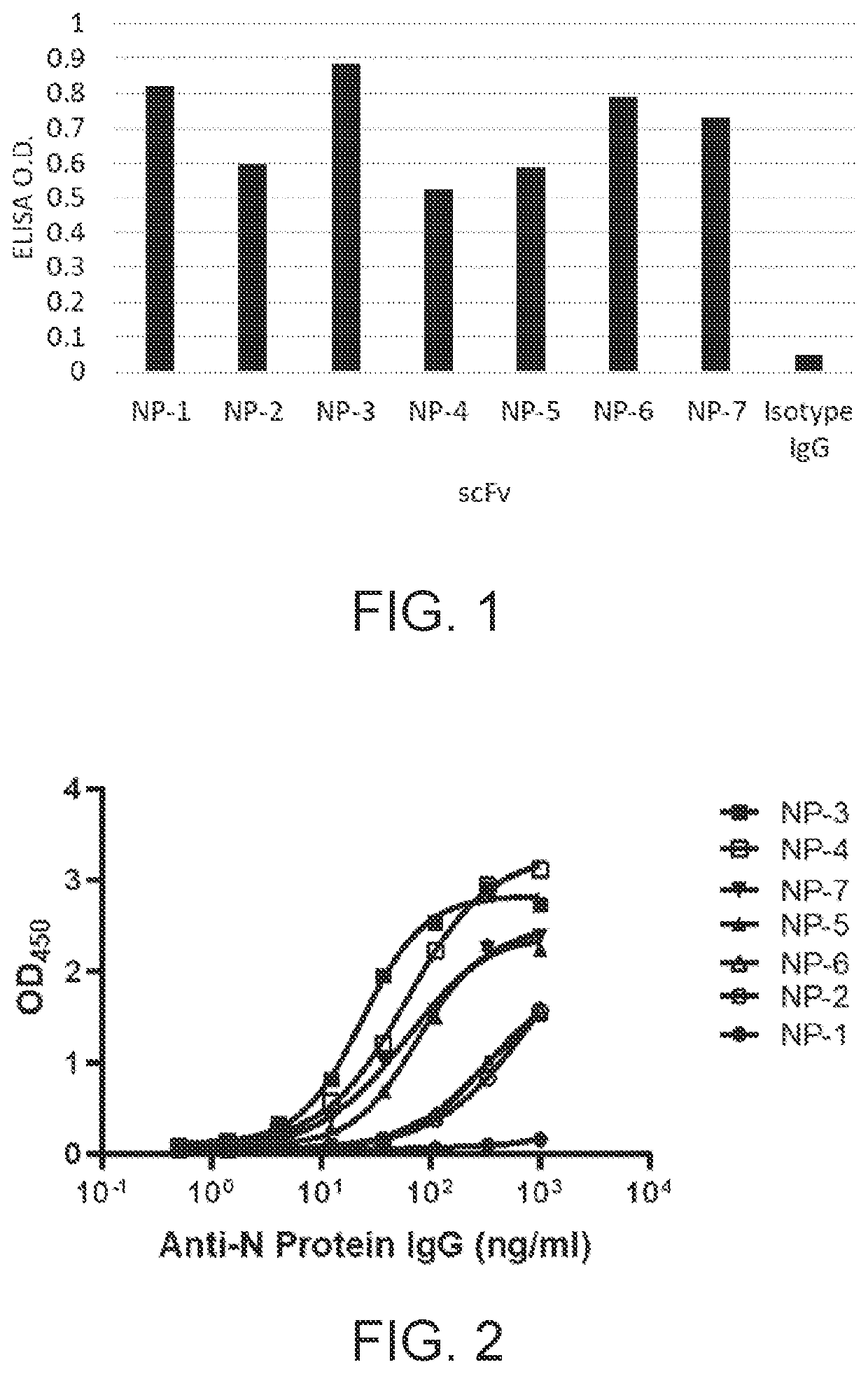
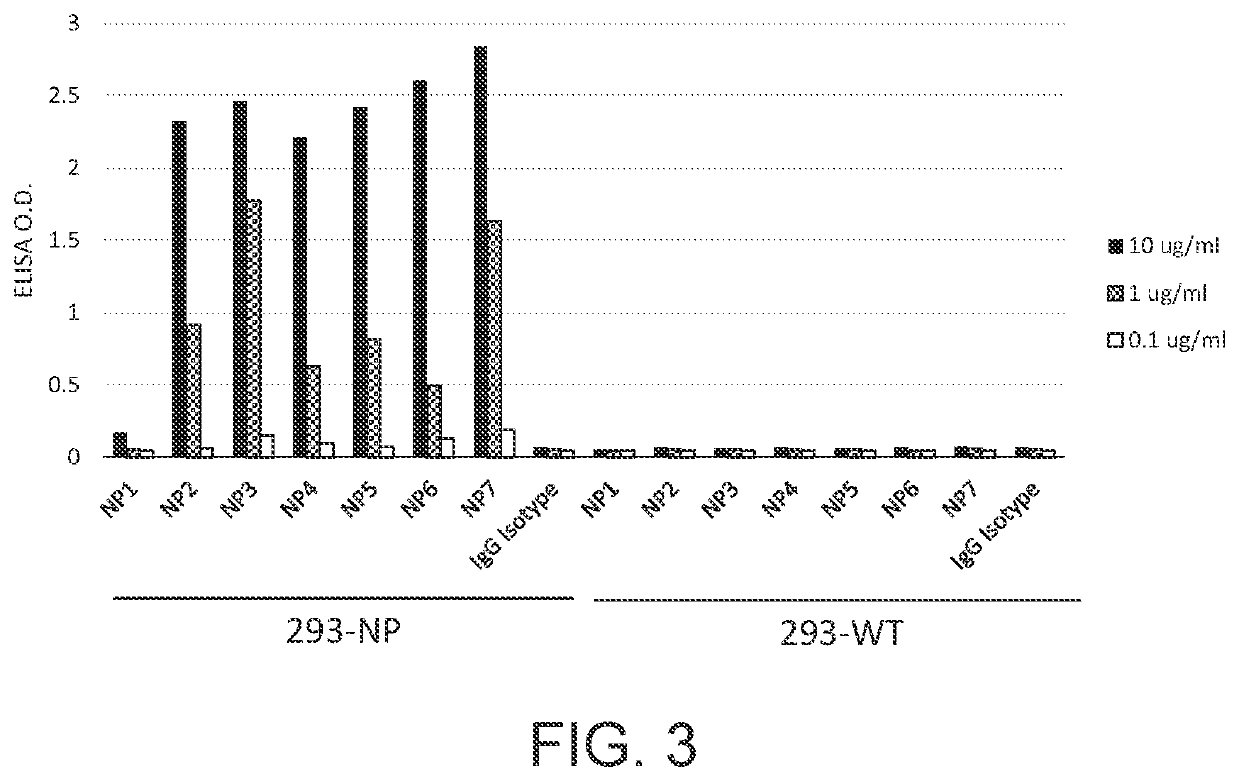
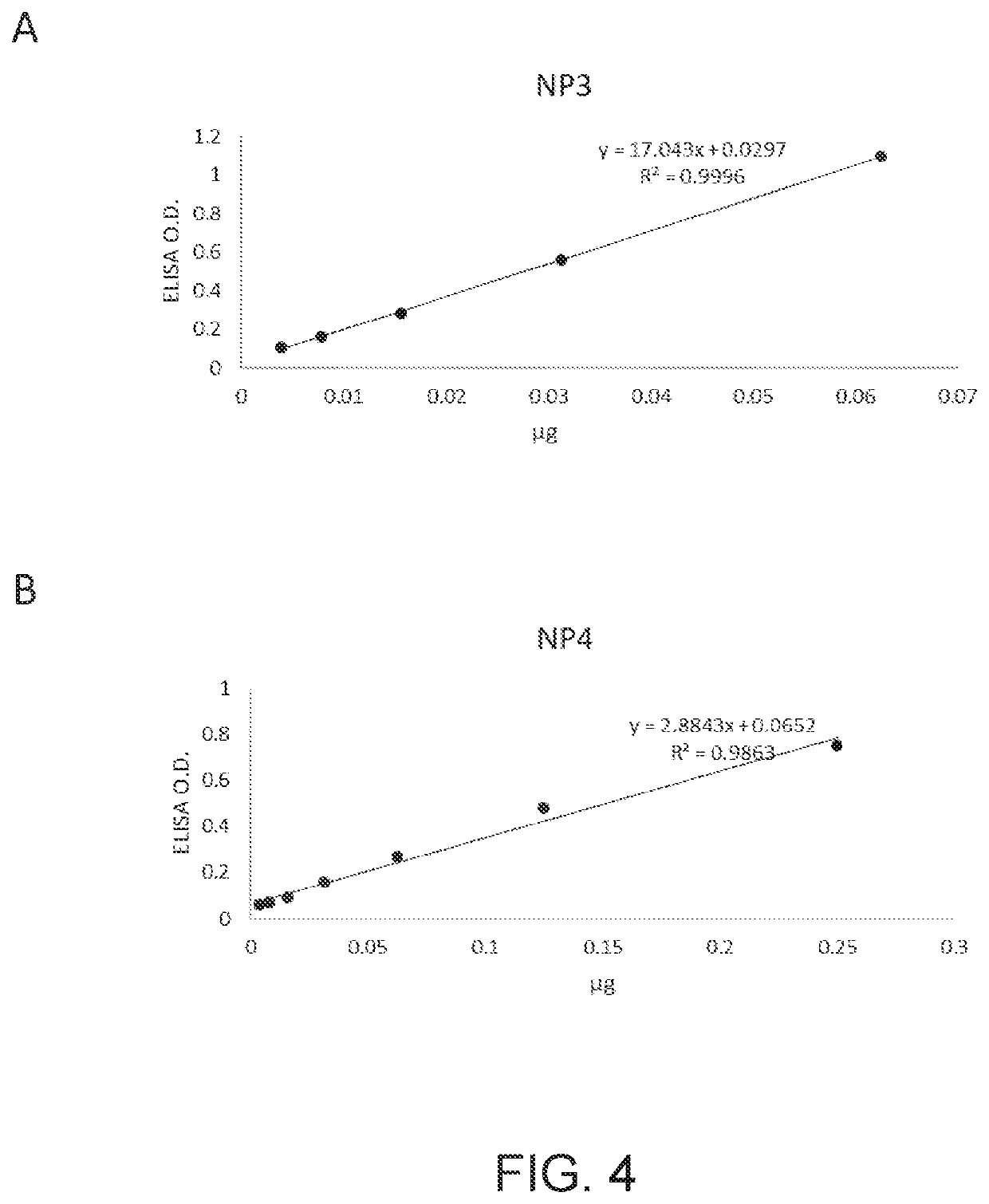
Recombinant antibodies, kits comprising the same, and uses thereof
PendingUS20210341478A1Well formedBiological material analysisImmunoglobulins against virusesAntiendomysial antibodiesCapsid
Disclosed herein is a recombinant antibody against the nucleocapsid protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A kit containing the recombinant antibody for use in coronavirus detection is encompassed in the present disclosure. Also disclosed herein are in vitro methods of detecting coronavirus infection in a subject with the aid of the recombinant antibody.
Owner:ANTAIMMU BIOMED CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com