Application of PLpro protein inhibitor in medicines for treating or preventing novel coronavirus infection
A technology for protein inhibitors and coronaviruses, which can be used in antiviral agents, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., and can solve problems such as high risk of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Induced expression and purification of PLpro protein
[0066] Transform the PLpro prokaryotic expression plasmid (see SEQ ID NO.1 for the amino acid sequence of the expressed PLpro protein) into BL21 (DE3) Escherichia coli competent cells, and grow in LB medium at 37°C until the OD600 is 0.6-0.8 Time. Add 0.5mM IPTG to induce protein expression and add 1mM zinc chloride (ZnCl 2 ), and then grow E. coli overnight at 18°C. After collection by centrifugation, resuspend the cell pellet with 50mM Tris-HCl, 150mM NaCl, 10mM Imidazole, 2mM DTT, and buffer solution of pH=8.5, break it by ultrasonic, and centrifuge at 18000rpm to get the supernatant. Purified with His-TRAPTM chromatographic column, finally washed out with buffer (50mM Tris-HCl, 150mM NaCl, 250mM midazole, 1mM DTT, pH 7.4), further purified on gel chromatographic column (Superdex 20016 / 60, GE), The SEC buffer is 20 mM Tris-HCl, 100 mM NaCl, 1 mMDTT, and its pH is 7.4. Purified PLpro protein was con...
Embodiment 2
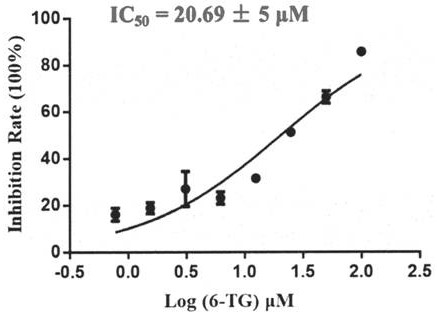
[0067] Embodiment 2: In vitro experiments measure the activity (IC50) of thioguanine (6-TG) inhibiting PLpro protein
[0068] The reaction system was 50 μL, in which the concentration of PLpro was 30 nM, the concentration of the substrate Ub-AMC (Boston Biochem) was 250 nM, the reaction buffer was 50 mM Hepes (pH=7.5), 0.01% Trixton X-100, 0.1 mg / ml BSA and 2 mMDTT . Add gradient concentration (100, 50, 25, 12.5, 6.25, 3.125, 1.56, 0.78μM) of inhibitor thioguanine (6-TG), and then use a microplate reader to measure the fluorescence emission intensity (excitation: 340nm; emission: 430nm ), the activity (IC50) of thioguanine (6-TG) inhibiting PLpro enzyme was calculated according to the fluorescence intensity, and the inhibition curve was fitted using GraphPad Prism. For fitting results see figure 2 .
Embodiment 3
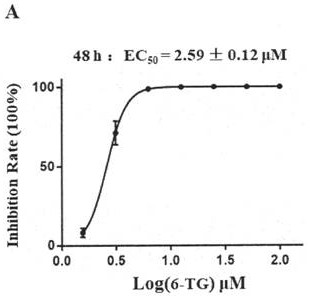
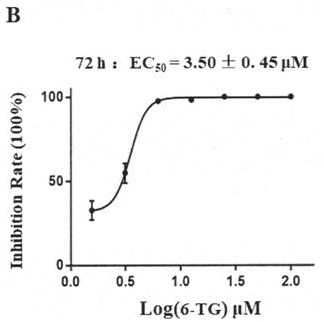
[0069] Embodiment 3: P3 laboratory tests the inhibitory activity of thioguanine (6-TG) to new coronavirus
[0070] (1) Compound cytotoxicity test:
[0071] Will 1×10 4 The Vero cells (source: VERO cells are from the Cell Resource Center of the Shanghai Institute of Biological Sciences, Chinese Academy of Sciences) were inoculated in 96-well plates, and three replicate wells were set up, grown at 37 degrees for 20-24 hours, the medium was removed, and 100 μL containing different concentration Compounds (100, 50, 25, 12.5, 6.25, 3.125, 1.56, 0 μM) were grown in new media for 72 hours, using DMSO as a control, using MTS / CCK8 reagents (the above reagents were purchased from: Beijing Suo Lai Bao Technology Co., Ltd., Product No. CK04-500T), measure the cell viability, and calculate the cytotoxicity (CC50) of the compound.
[0072] (2) Compound inhibitory virus activity test:
[0073] Will 1×10 4 Vero cells were seeded in a 96-well plate, and three replicate wells were set up, g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com