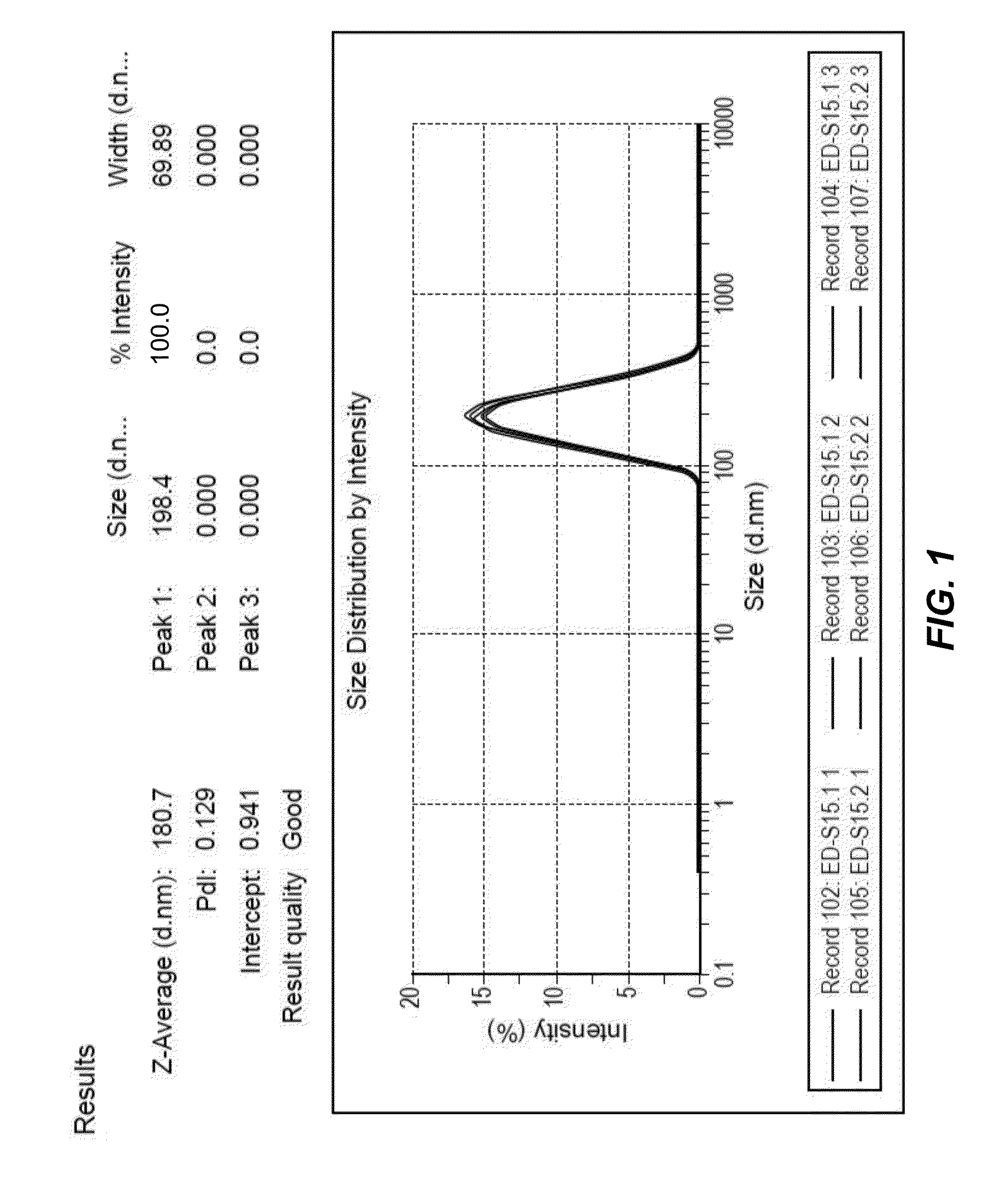
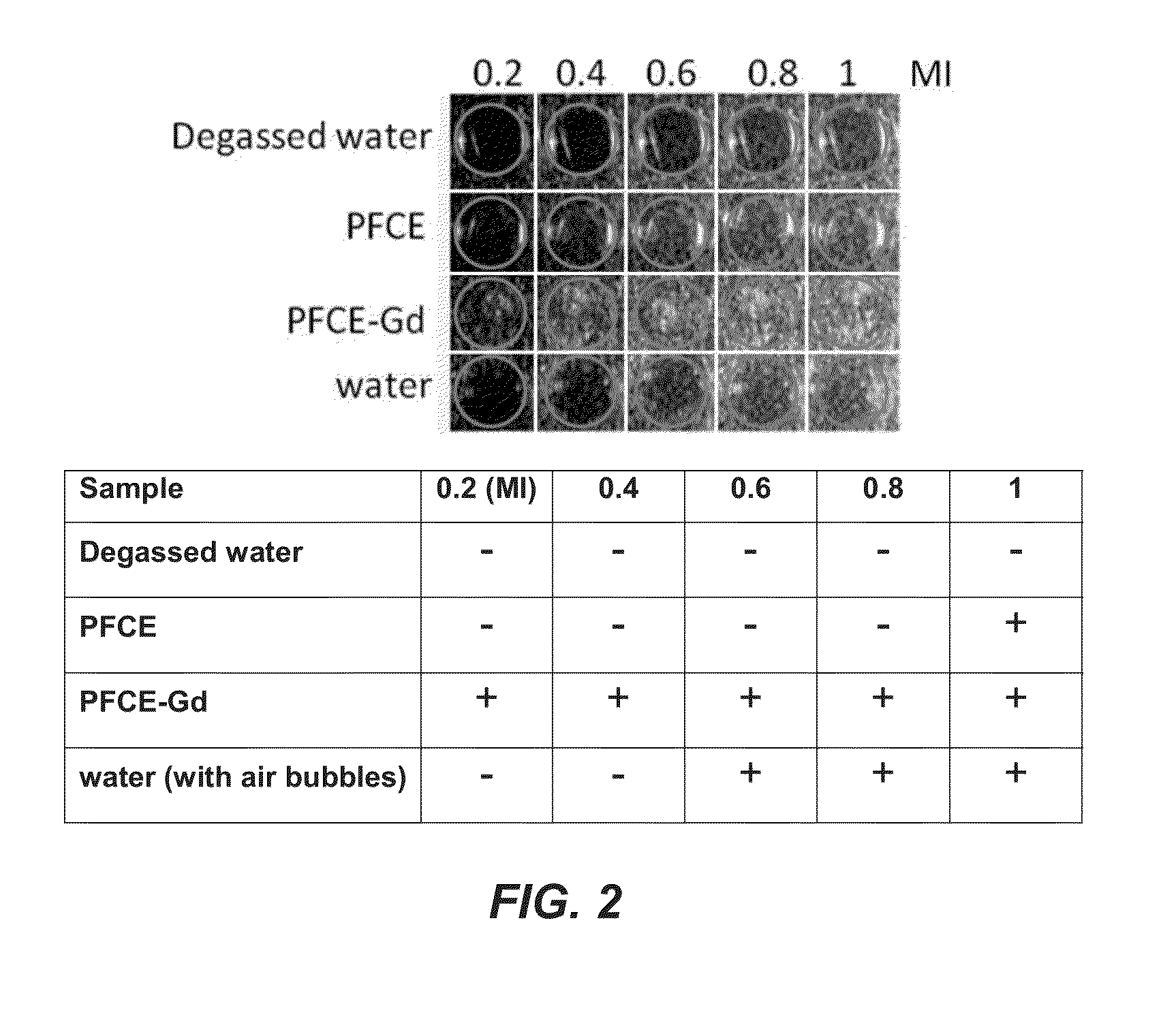
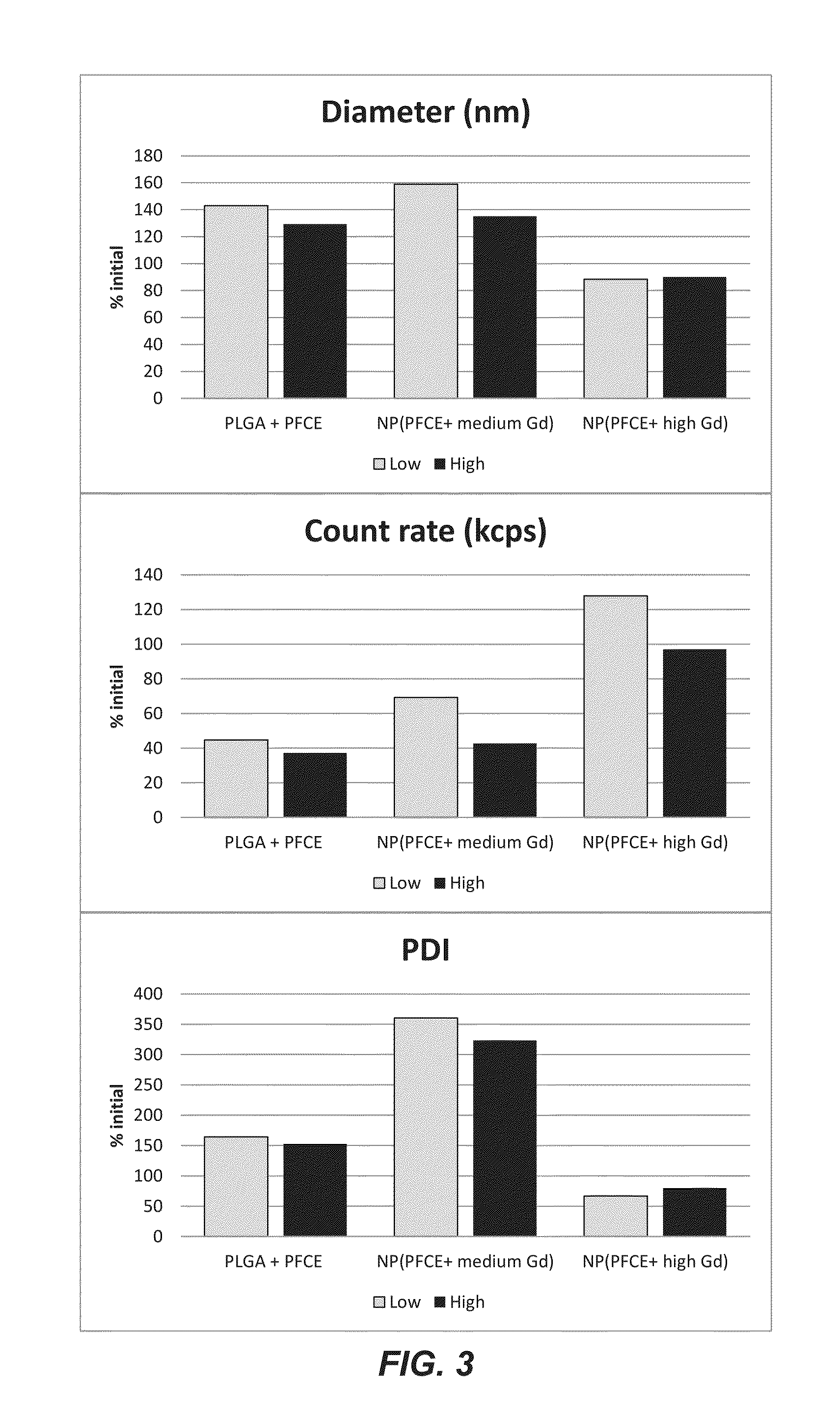
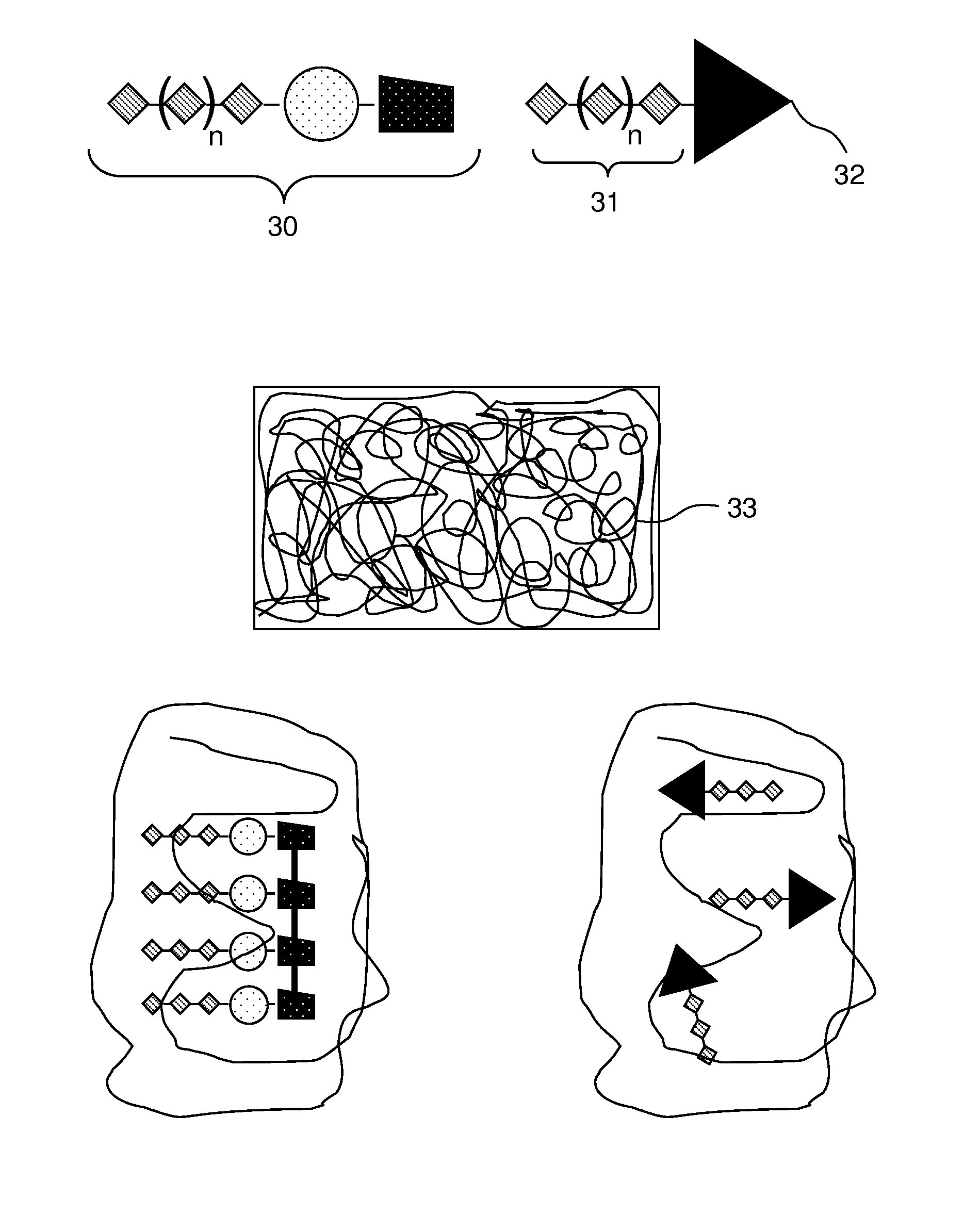
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Scintigraphy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Scintigraphy (from Latin scintilla, "spark"), also known as a Gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally and the emitted gamma radiation is captured by external detectors (gamma cameras) to form two-dimensional images in a similar process to the capture of x-ray images. In contrast, SPECT and positron emission tomography (PET) form 3-dimensional images, and are therefore classified as separate techniques to scintigraphy, although they also use gamma cameras to detect internal radiation. Scintigraphy is unlike a diagnostic X-ray where external radiation is passed through the body to form an image.
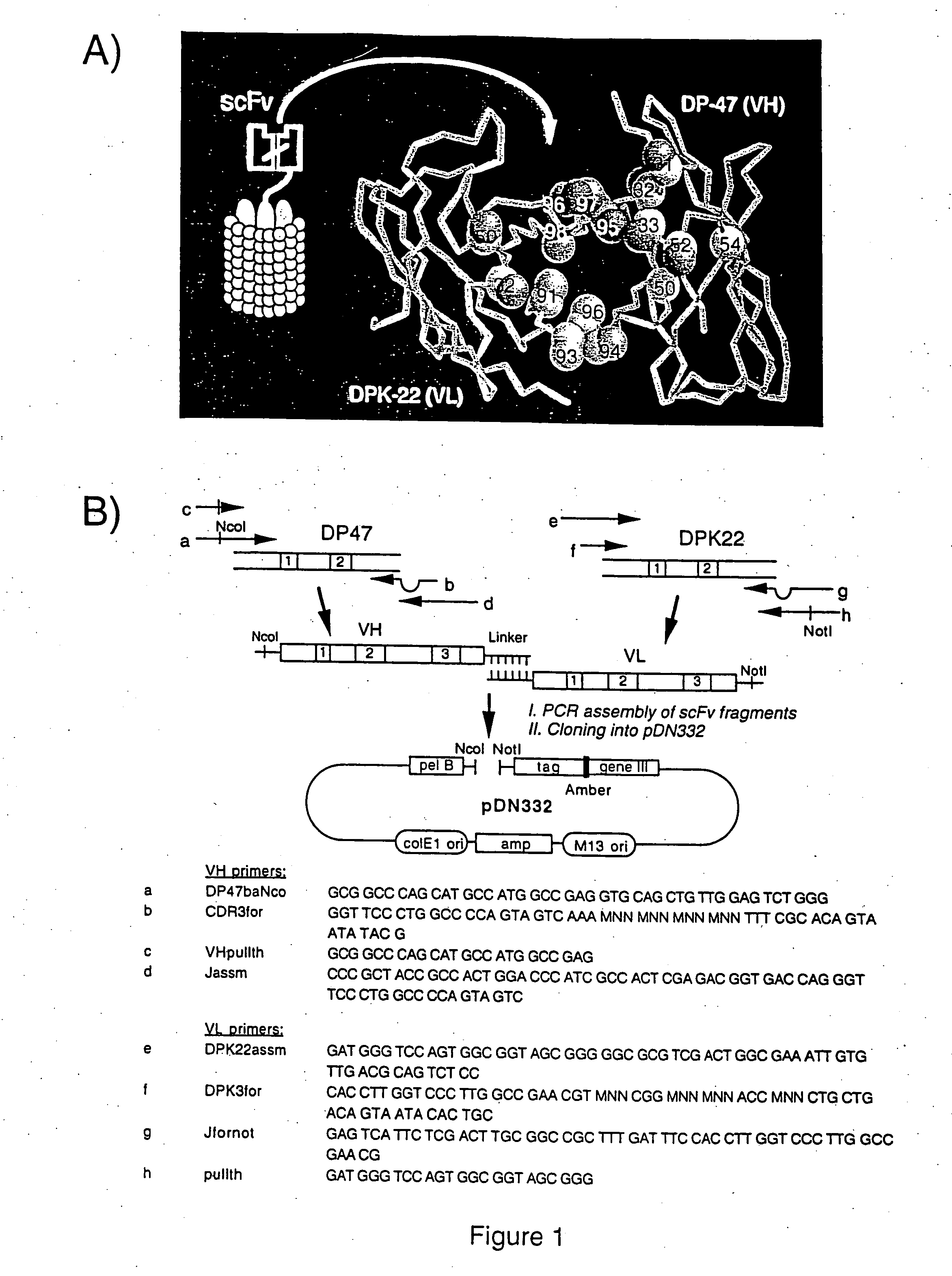
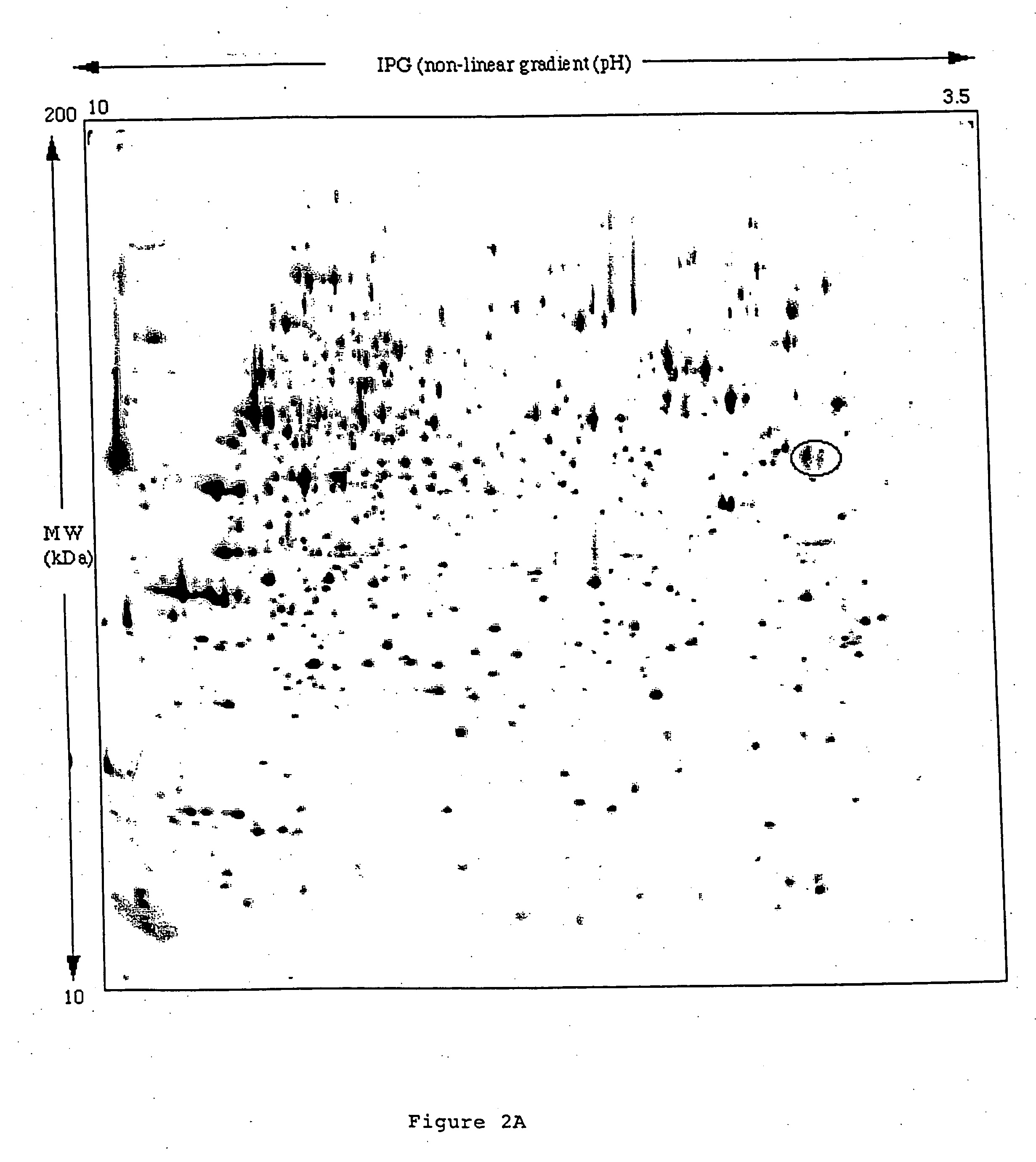
Specific binding molecules for scintigraphy, conjugates containing them and therapeutic method for treatment of angiogenesis
InactiveUS20070189963A1Promote apoptosisPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopePhotosensitizer
The present invention relates to antibodies with sub-nanomolar affinity specific for a characteristic epitope of the ED-B domain of fibronectin, a marker of angiogenesis Furthermore, it relates to the use of radiolabeled high affinity anti ED-B antibodies for detecting new-forming blood vessels in vivo and a diagnostic kit comprising comprising said antibody. Furthermore, it relates to conjugates comprising said antibodies and a suitable photoactive molecules (e.g. a judiciously chosen photosensitizer), and their use for the selective light-mediated occlusion of new blood vessels.
Owner:NERI DARIO +3
Methods of testing digestive functions using both a breath test and a scintigraphy test, and methods of using a breath test as an overall digestive health assessment
ActiveUS20080281194A1In-vivo radioactive preparationsPerson identificationLipid formationGastric emptying
Owner:ADVANCED BREATH DIAGNOSTICS
Fucoidans as Ligands for the Diagnosis of Degenerative Pathologies
InactiveUS20120183475A1In-vivo radioactive preparationsSugar derivativesAbnormal tissue growthReperfusion injury
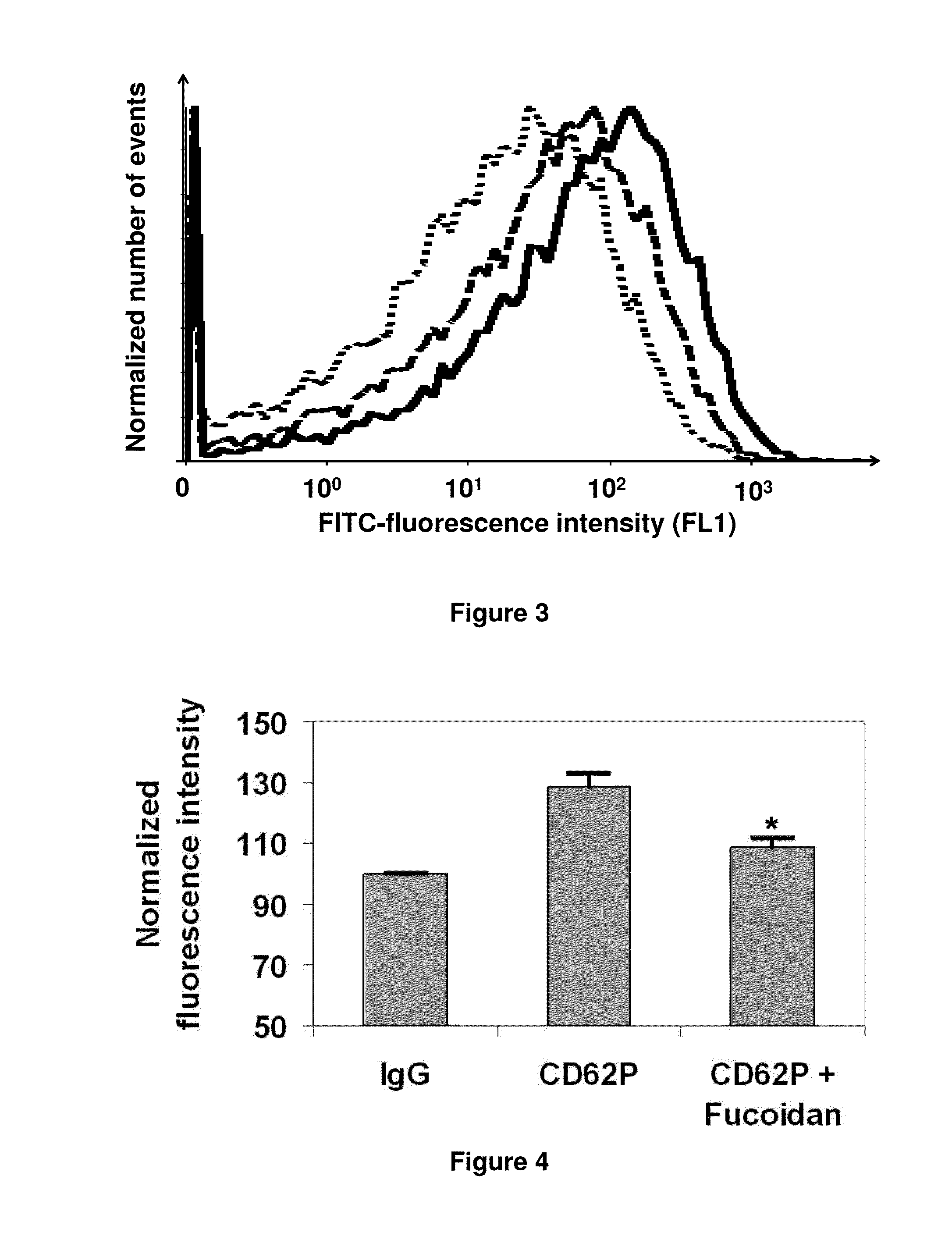
The present invention relates to the diagnosis of clinical conditions characterized by undesirable and / or abnormal selectin expression. In particular, the invention provides for the use of fucoidans for the detection of selectins using imaging techniques including ultrasonography, scintigraphy and MRI. Selectin-targeted imaging agents are provided that comprise at least one fucoidan moiety associated with at least one detectable moiety. Methods and kits are described for using these imaging agents in the diagnosis of clinical conditions such as thrombosis, myocardial ischemia / reperfusion injury, stroke and ischemic brain trauma, neurodegenerative disorders, tumor metastasis and tumor growth, and rheumatoid arthritis.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
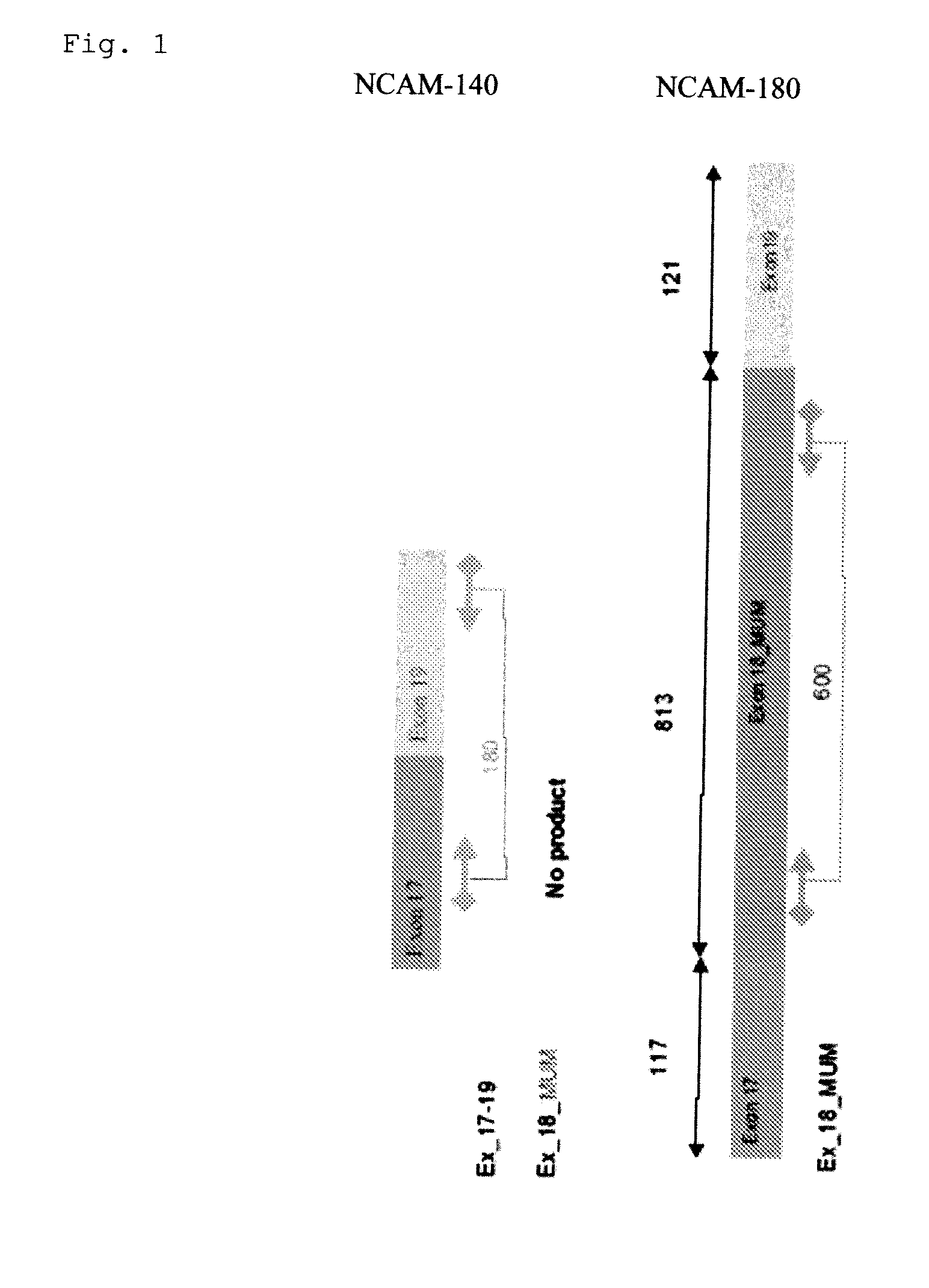
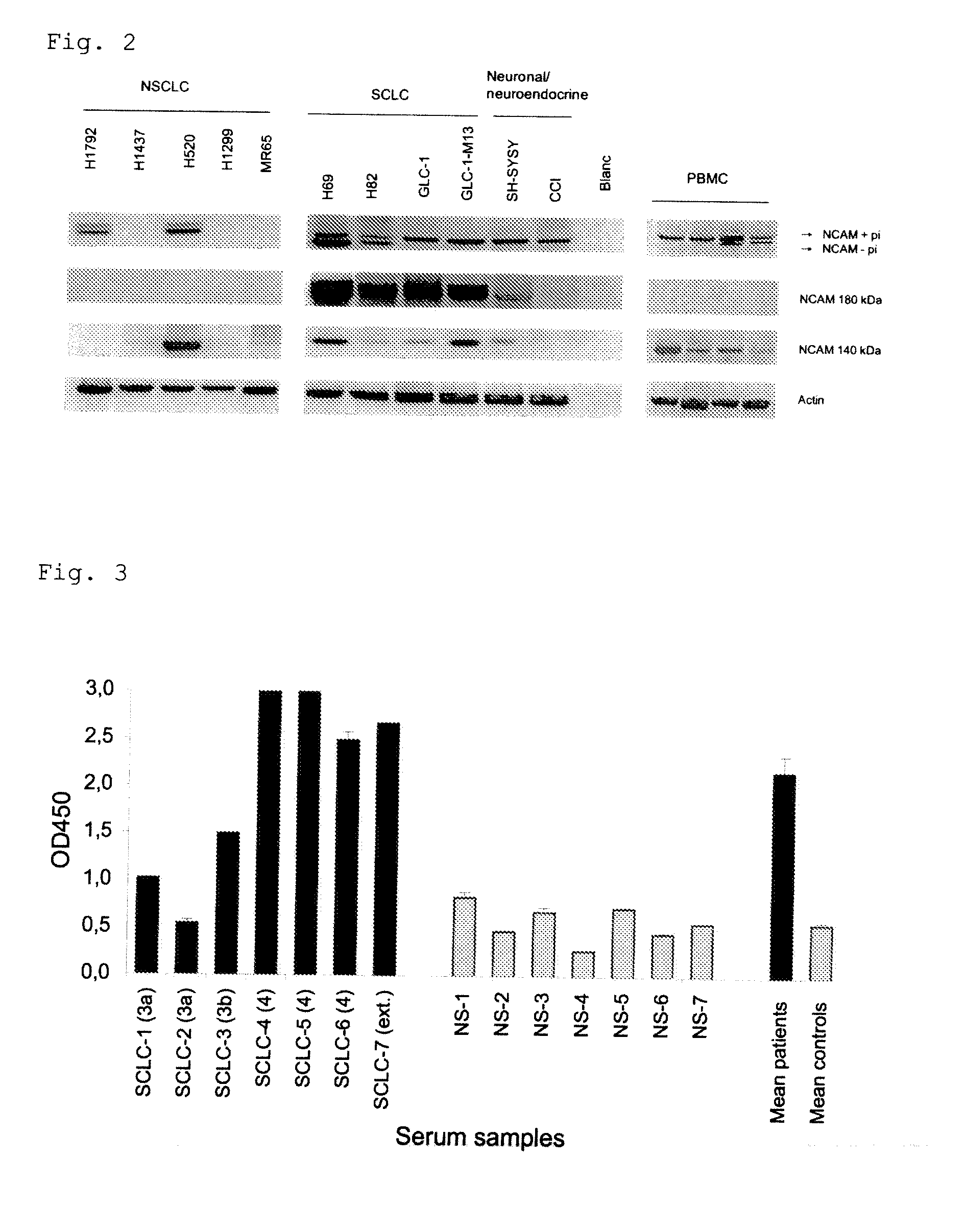
Small cell lung carcinoma biomarker panel
The invention relates generally to the field of cancer detection, diagnosis, subtyping, staging, prognosis, treatment and prevention. More particularly, the present invention relates to methods for the detection, and / or diagnosing and / or subtyping and / or staging of lung cancer in a patient. Based on a particular panel of biomarkers, the present invention provides methods to detect, diagnose at an early stage and / or differentiate small cell lung cancer (SCLC) from non-small cell lung cancer (NSCLC) and within NSCLC to differentiate between squamous cell carcinomas (SCC), adenocarcinomas (AC), within SCC to discriminate G2 and G3 stage and within lung cancer to differentiate for lung cancers with or without neuroendocrine origin. It further provides the use of said panel of biomarkers in monitoring disease progression in a patient, including both in vitro and in vivo imaging techniques. The in vitro imaging techniques typically include an immunoassay detecting protein or antibody of the biomarkers on a sample taken from said patient, e.g. serum or tissue sample. The in vivo imaging techniques typically include chest radiographs (X-rays), Computed Tomography (CT) imaging, spiral CT, Positron Emission Tomography (PET), PET-CT and scintigraphy for molecular imaging and diagnosis and to monitor disease progression and treatment response in patients. It is accordingly a further aspect to provide a kit to perform the aforementioned diagnosing and / or subtyping and / or staging assay and the imaging techniques, comprising reagents to determine the gene expression or protein level of the aforementioned panel of biomarkers for in vitro and in vivo applications.
Owner:MUBIO PRODS BV
Small cell lung carcinoma biomarker panel
InactiveUS20110053156A1Peptide/protein ingredientsMicrobiological testing/measurementBiomarker panelCompanion animal
The invention relates generally to the field of cancer detection, diagnosis, subtyping, staging, prognosis, treatment and prevention. More particularly, the present invention relates to methods for the detection, and / or diagnosing and / or subtyping and / or staging of lung cancer in a patient. Based on a particular panel of biomarkers, the present invention provides methods to detect, diagnose at an early stage and / or differentiate small cell lung cancer (SCLC) from non-small cell lung cancer (NSCLC) and within NSCLC to differentiate between squamous cell carcinomas (SCC), adenocarcinomas (AC), within SCC to discriminate G2 and G3 stage and within lung cancer to differentiate for lung cancers with or without neuroendocrine origin. It further provides the use of said panel of biomarkers in monitoring disease progression in a patient, including both in vitro and in vivo imaging techniques. The in vitro imaging techniques typically include an immunoassay detecting protein or antibody of the biomarkers on a sample taken from said patient, e.g. serum or tissue sample. The in vivo imaging techniques typically include chest radiographs (X-rays), Computed Tomography (CT) imaging, spiral CT, Positron Emission Tomography (PET), PET-CT and scintigraphy for molecular imaging and diagnosis and to monitor disease progression and treatment response in patients. It is accordingly a further aspect to provide a kit to perform the aforementioned diagnosing and / or subtyping and / or staging assay and the imaging techniques, comprising reagents to determine the gene expression or protein level of the aforementioned panel of biomarkers for in vitro and in vivo applications.
Owner:MUBIO PRODS BV
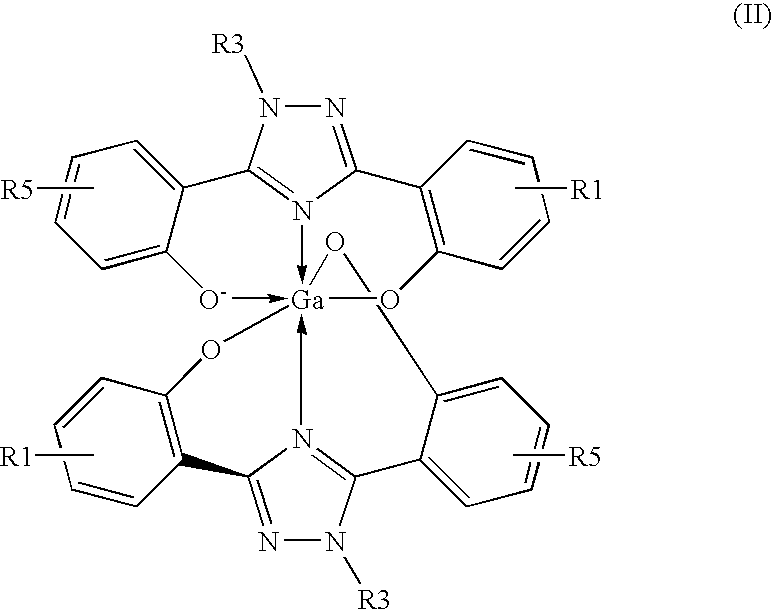
Method of Scintigraphy
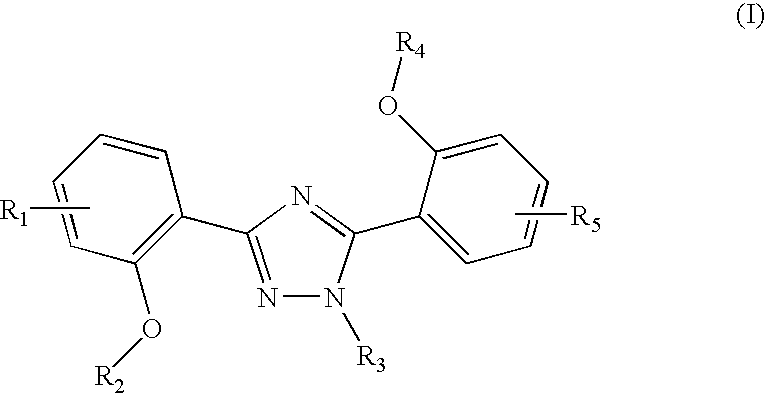
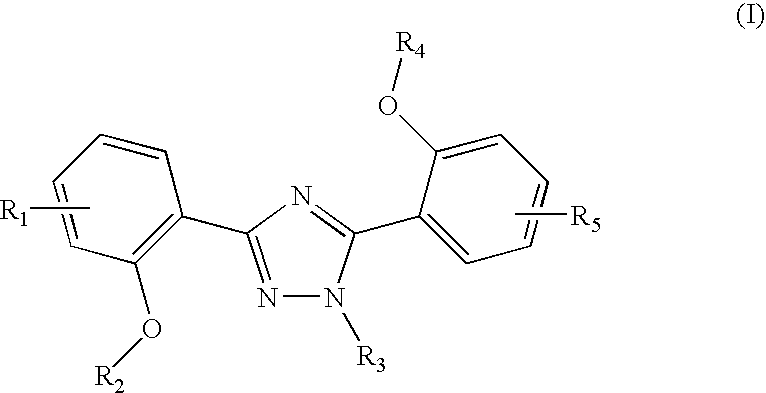
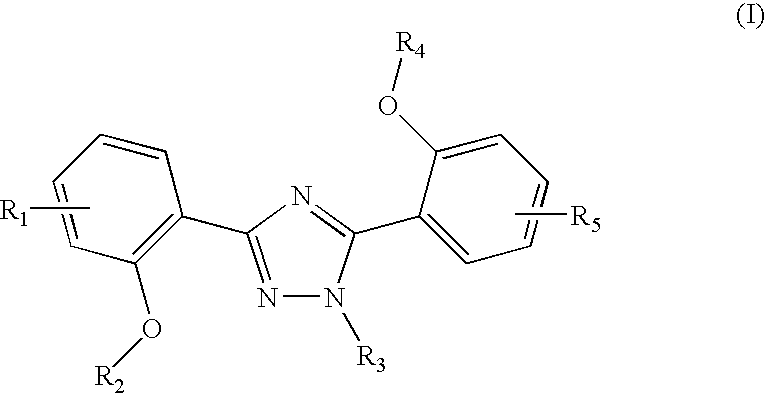
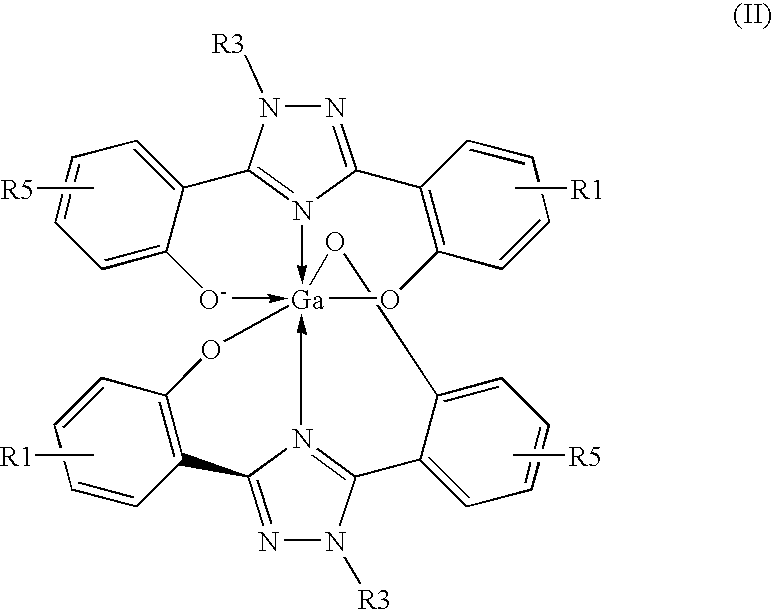
The invention relates to combination comprising a pharmaceutically acceptable preparation of a compound of formula (I), or a pharmaceutically acceptable salt thereof, and a gallium and its use in diagnosis. The invention also pertains to the use of a compound of formula I or a pharmaceutically acceptable salt thereof, for the preparation of a medicament for the treatment of an excess iron overload in the human or animal body whereby said body is undergoing gallium scintigraphy and whereby the treatment removing said excess of iron is interrupted for a period of 2 to 10 days prior to the gallium scintigraphy and resumed after the gallium scintigraphy readings.
Owner:NOVARTIS AG
Fucoidans as Ligands for the Diagnosis of Degenerative Pathologies
InactiveUS20120093725A1High affinityHigh selectivityNervous disorderAntipyreticAbnormal tissue growthReperfusion injury
The present invention relates to the diagnosis of clinical conditions characterized by undesirable and / or abnormal selectin expression. In particular, the invention provides for the use of fucoidans for the detection of selectins using imaging techniques including ultrasonography, scintigraphy and MRI. Selectin-targeted imaging agents are provided that comprise at least one fucoidan moiety associated with at least one detectable moiety. Methods and kits are described for using these imaging agents in the diagnosis of clinical conditions such as thrombosis, myocardial ischemia / reperfusion injury, stroke and ischemic brain trauma, neurodegenerative disorders, tumor metastasis and tumor growth, and rheumatoid arthritis.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Contrast agent and its use for imaging
ActiveUS20150250905A1Increase awarenessImprove performanceUltrasonic/sonic/infrasonic diagnosticsPowder deliveryUltrasonographyFluorescence
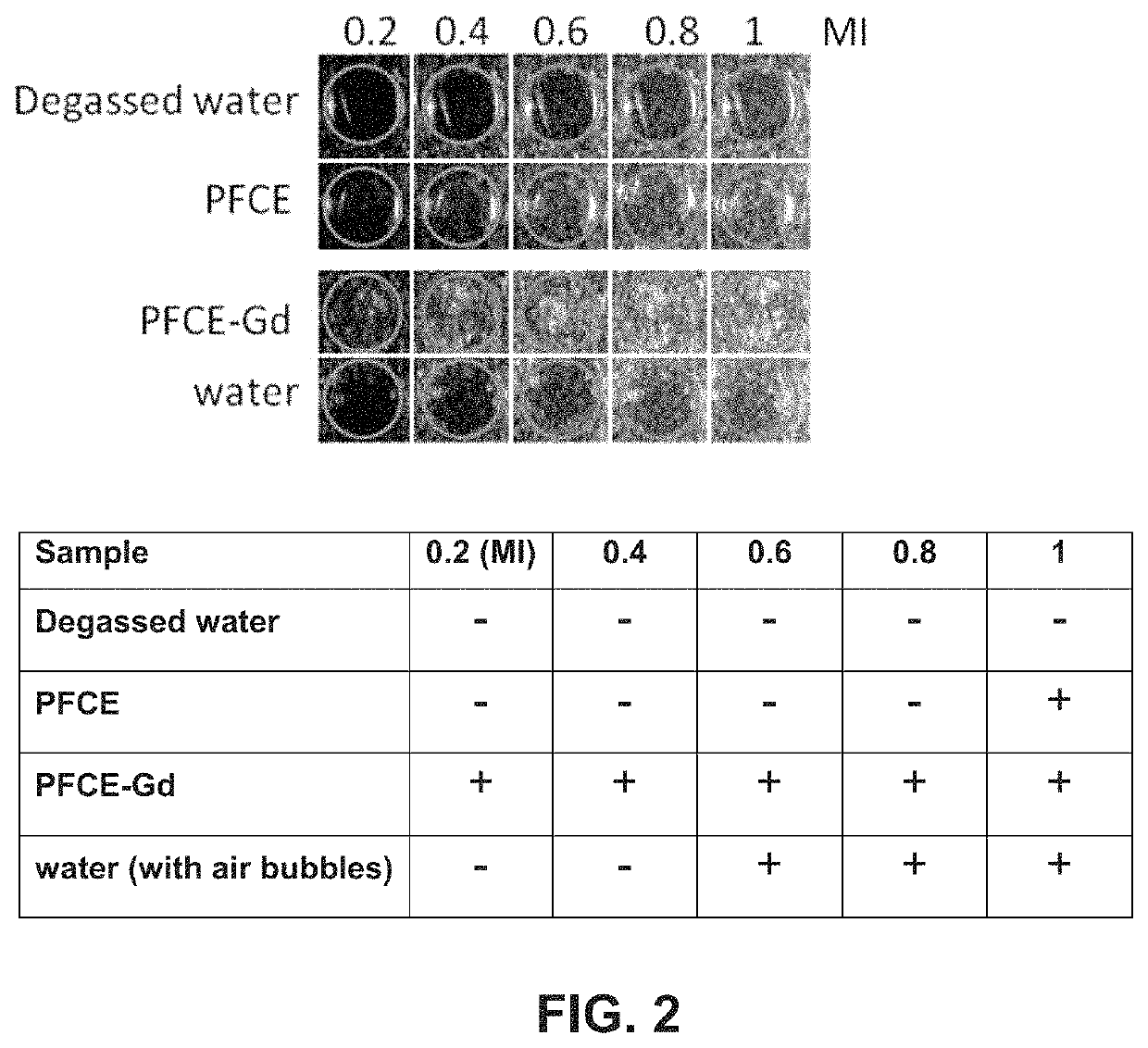
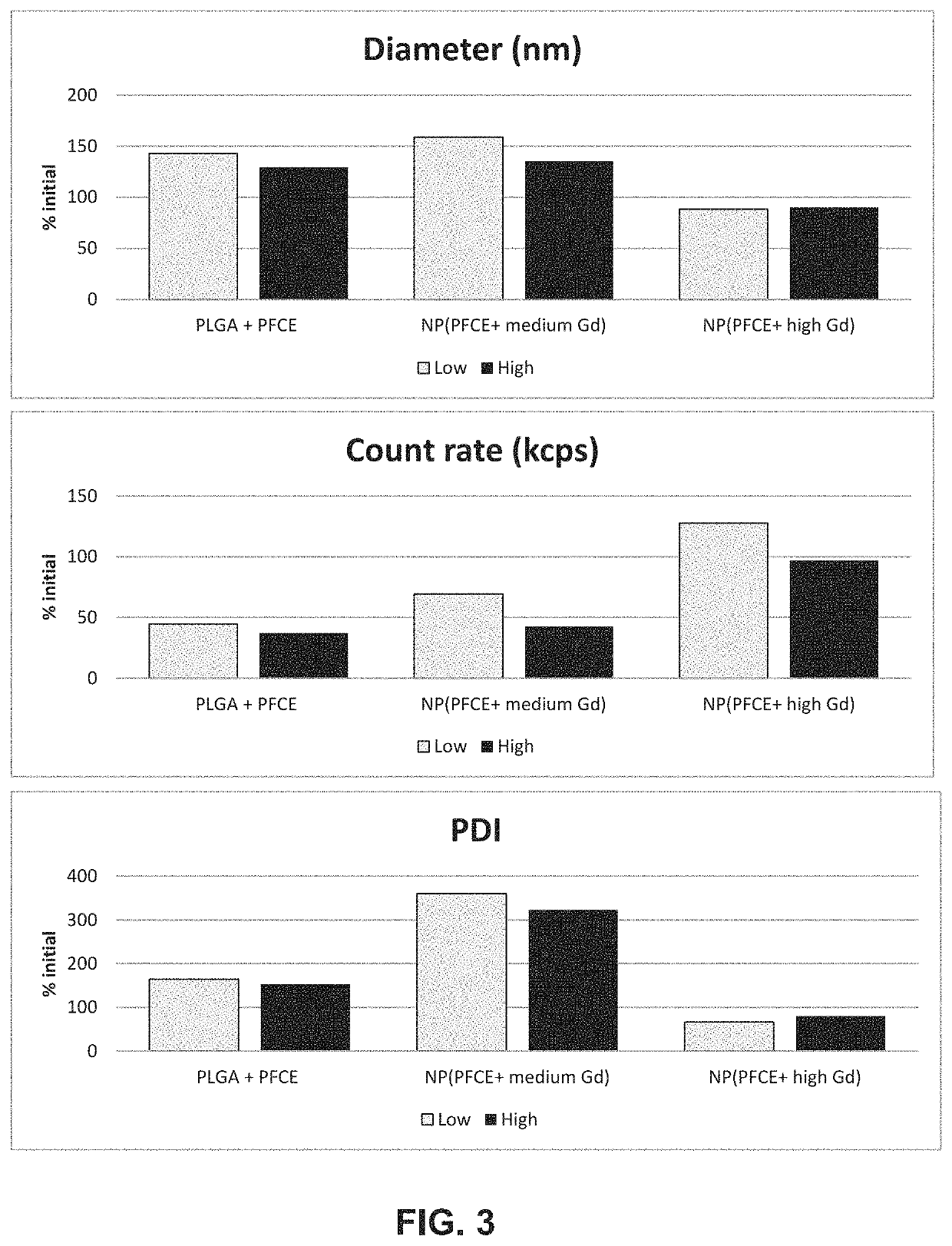
The present invention relates to contrast agent enhanced medical ultrasound imaging. In particular, the contrast agents provided are useful for cell imaging and cell therapy, as well as in vivo targeting, drug delivery and perfusion or vascular imaging applications. More specifically, it provides a particle comprising a fluorinated organic compound and a metal. Such particles may be advantageously employed in qualitative or quantitative imaging such as acoustic imaging including photoacoustic and ultra-sound imaging, MRI imaging, such as 19F imaging, 1H imaging including T1 and T2 weighted imaging, SPECT, PET, scintigraphy, fluorescence imaging and optical coherence imaging and tomographic applications. This may then be employed in cell labeling, microscopy, histology or for imaging vasculature or perfusion in vivo and in vitro.
Owner:STICHTING RADBOUD UNIVERSITAIR MEDISCH CENT
Method for the production of labelled scaffolds, comprising at least one reactive fluorinated surfactant, and scaffold produced therewith
InactiveUS20110243852A1Improve solubilityPrevent evaporationIn-vivo testing preparationsProsthesisUltrasound imagingCombinatorial chemistry
The present invention is related to scaffolds for tissue engineering and organ engineering. More particularly, the present invention is related to a method for the production of a labelled scaffold for tissue and / or organ engineering comprising a reactive fluorinated surfactant, which serve as imaging label for medical imaging means, like CT, MRI, PET, scintigraphy and / or ultrasound imaging and the like.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Fucoidans as Ligands for the Diagnosis of Degenerative Pathologies
ActiveUS20140134102A1Ultrasonic/sonic/infrasonic diagnosticsIn-vivo radioactive preparationsReperfusion injuryImaging agent
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
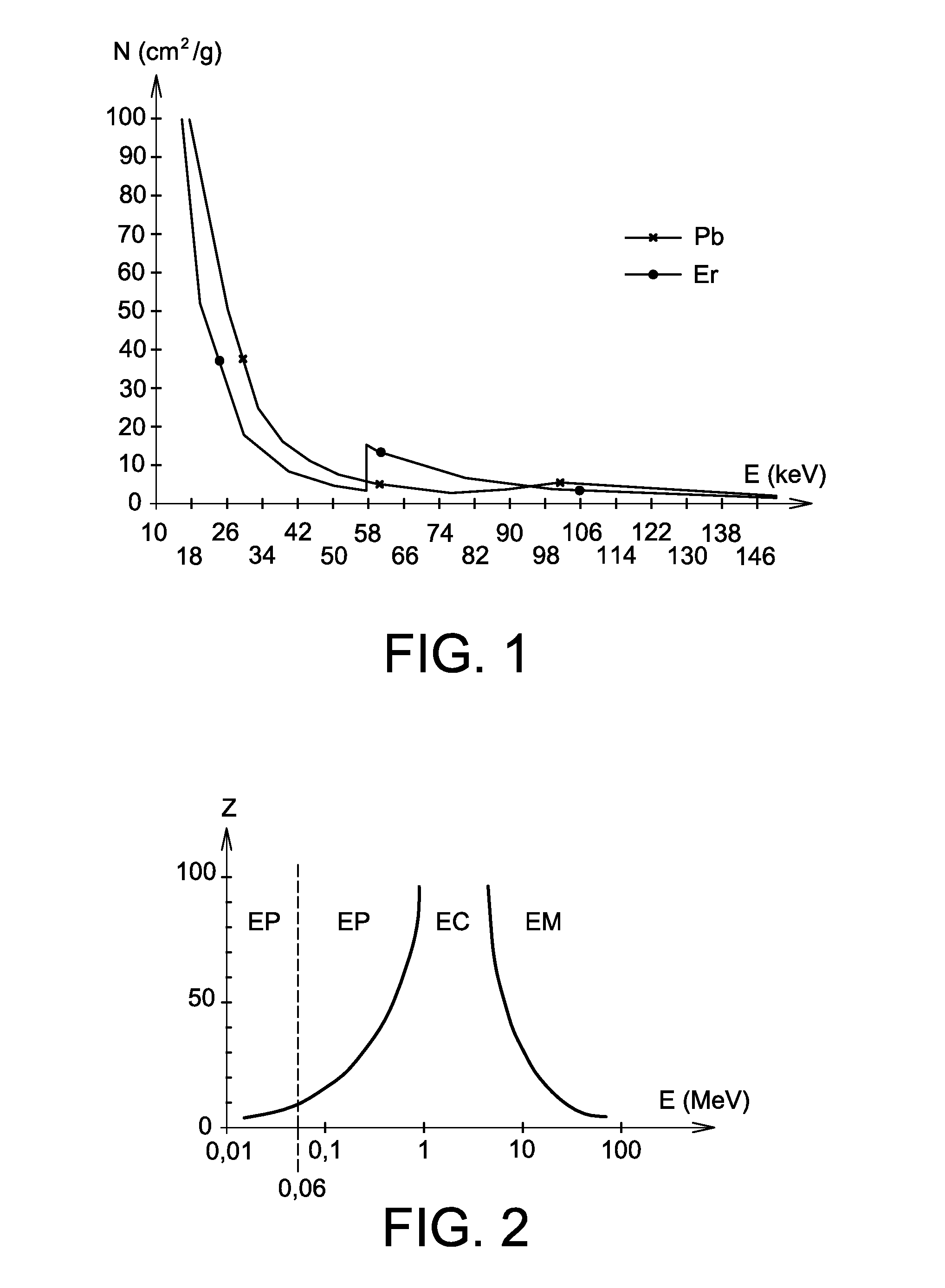
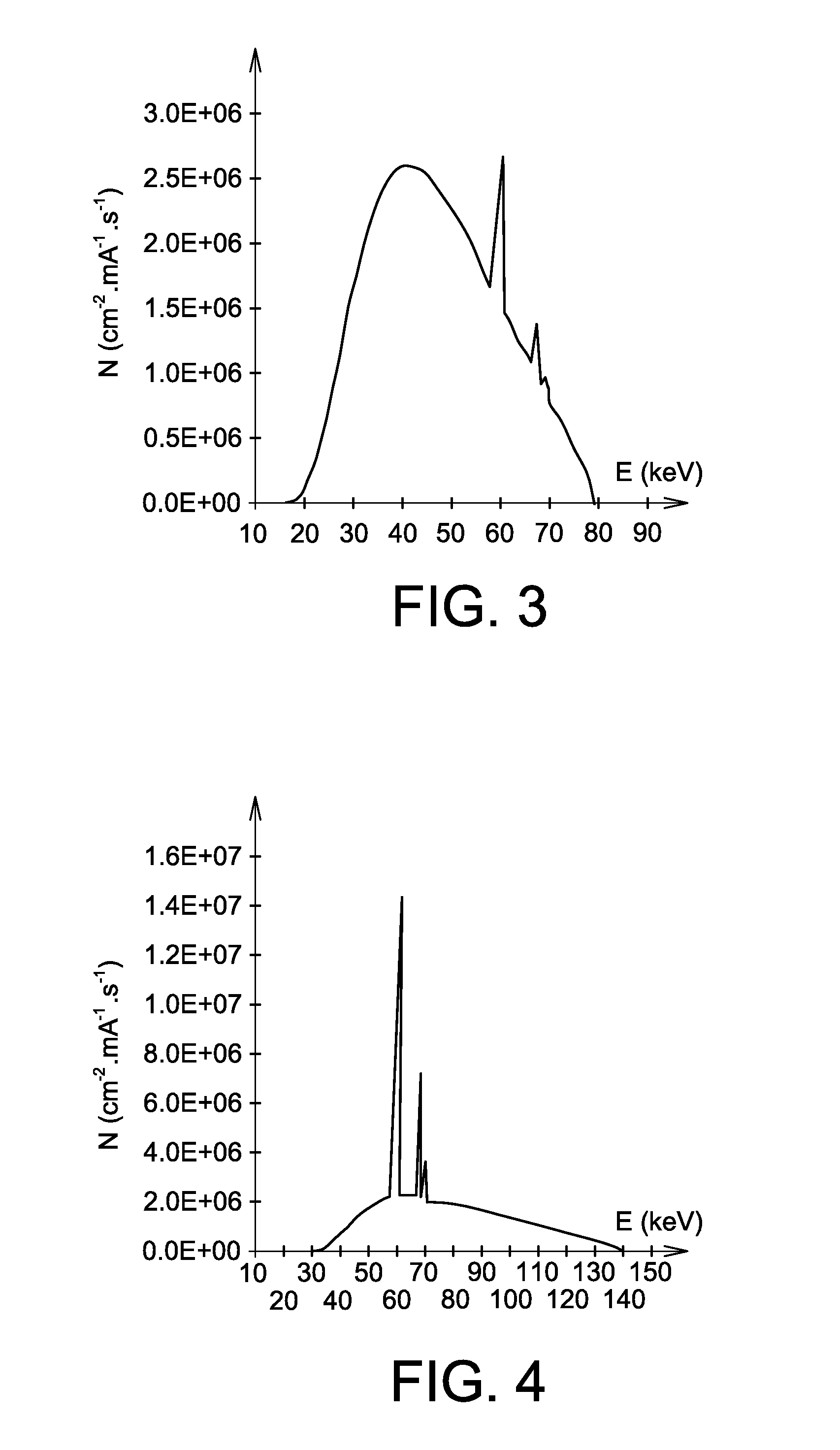
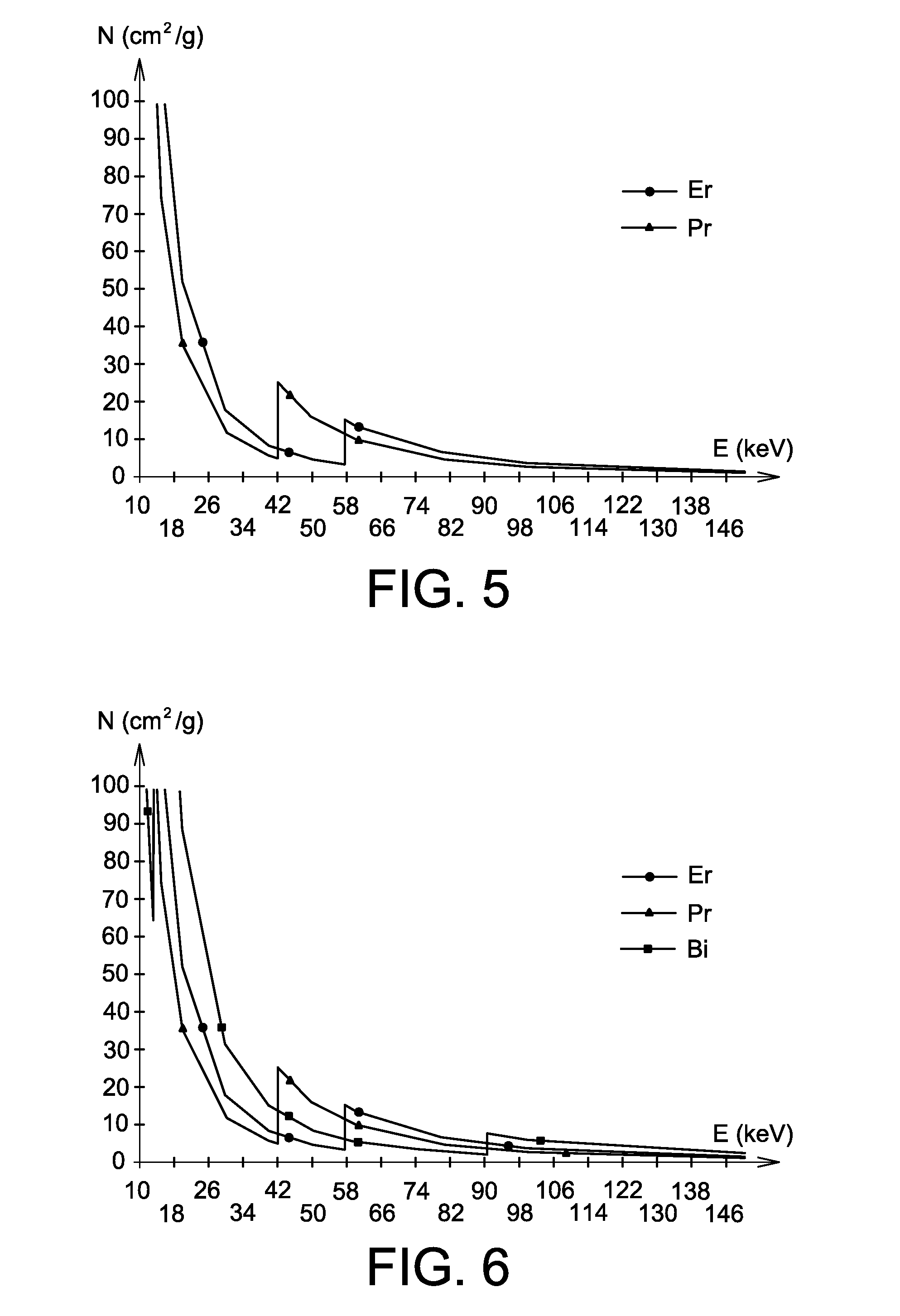
Use of a mixture comprising erbium and praseodymium as a radiation attenuating composition, radiation attenuating material, and article providing protection against ionising radiation and comprising such a composition
ActiveUS9006695B2Restore energyImprove protectionRadiation/particle handlingElectrode and associated part arrangementsMedical imagingRadiation damping
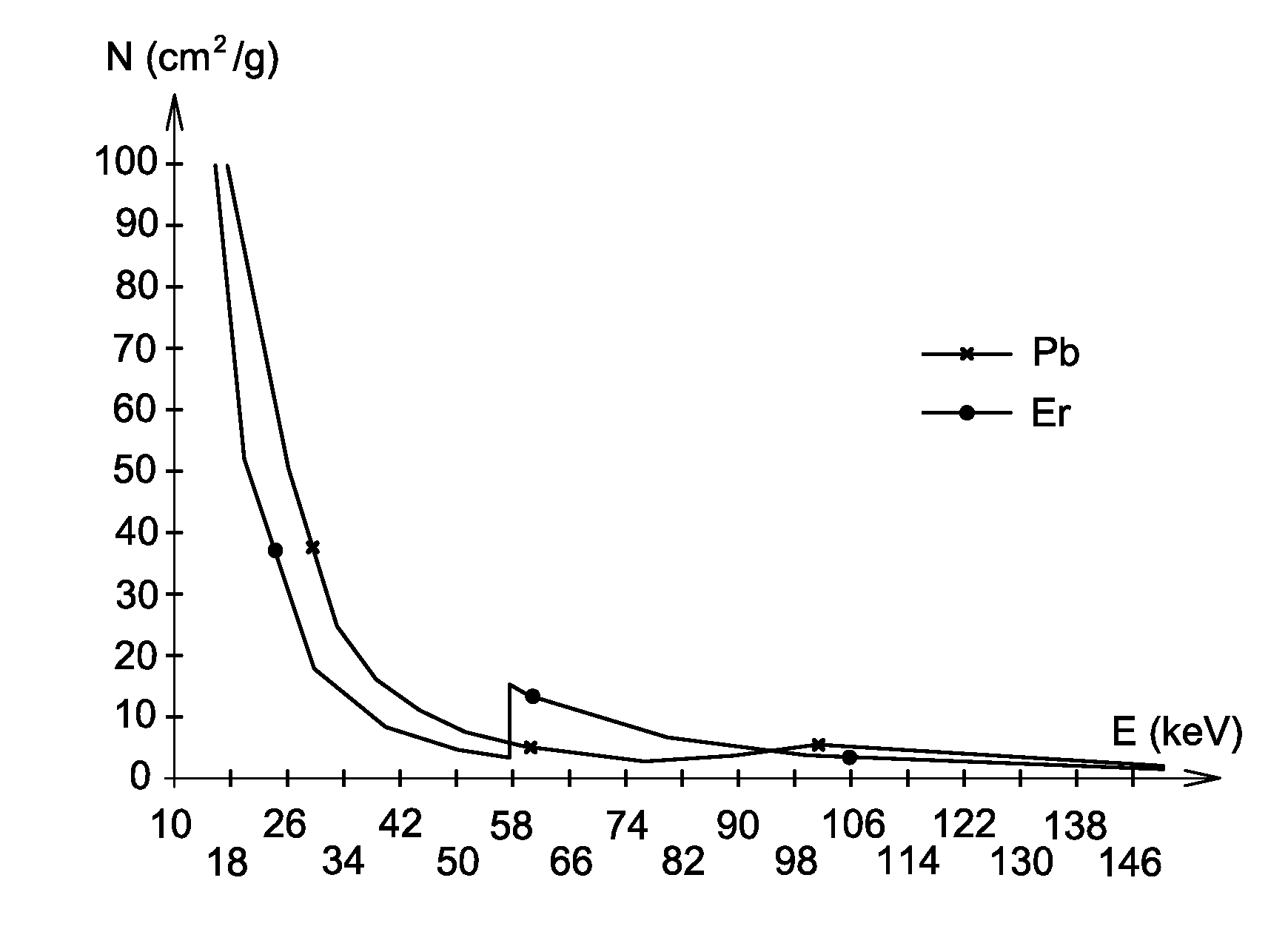
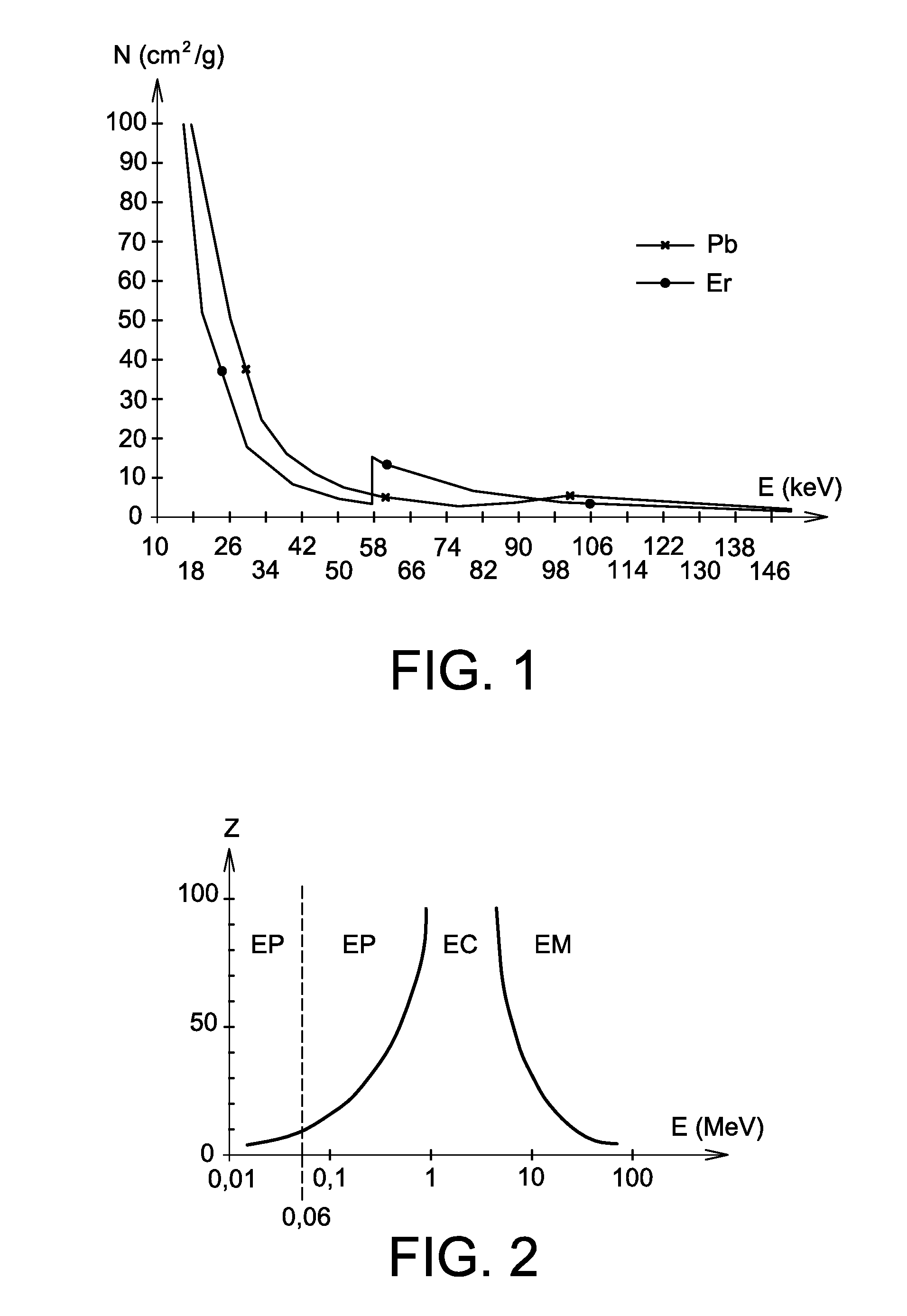
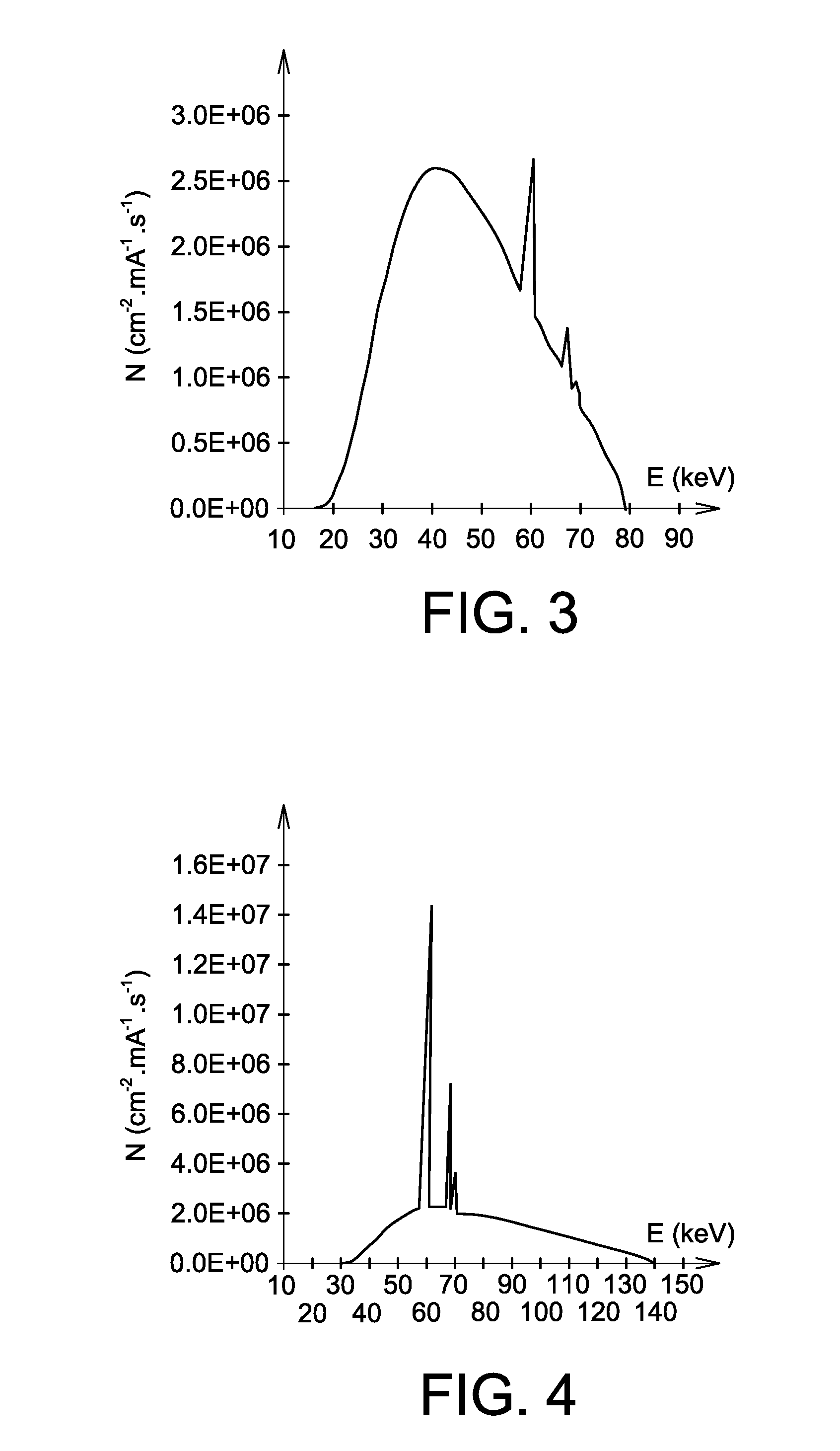
The invention relates to the use of a mixture comprising erbium and praseodymium as a radiation attenuating composition, i.e. as a composition that can attenuate ionizing radiation, in particular X- and gamma-type electromagnetic radiation.The invention also relates to a radiation attenuating material comprising an erbium- and praseodymium-based composition, as well as a protective article which provides group or individual protection against ionizing radiation and comprises said material.The invention is suitable for use in nuclear medicine (scintigraphy, radiotherapy, etc.), radiology, medical imaging, the nuclear industry, etc.
Owner:ORANO RECYCLAGE
Specific binding molecules for scintigraphy, conjugates containing them and therapeutic method for treatment of angiogenesis
InactiveUS20060133994A1Reduce nephrotoxicityHigh selectivitySenses disorderPeptide/protein ingredientsEpitopePhotosensitizer
The present invention relates to antibodies with sub-nanomolar affinity specific for a characteristic epitope of the ED-B domain of fibronectin, a marker of angiogenesis. Furthermore, it relates to the use of radiolabelled high affinity anti ED-B antibodies for detecting new-forming blood vessels in vivo and a diagnostic kit comprising comprising said antibody. Furthermore, it relates to conjugates comprising said antibodies and a suitable photoactive molecules (e.g. an appropriately chosen photosensitizer or radionuclide), and their use for the selective light-mediated occlusion of new blood vessels.
Owner:ETH ZZURICH
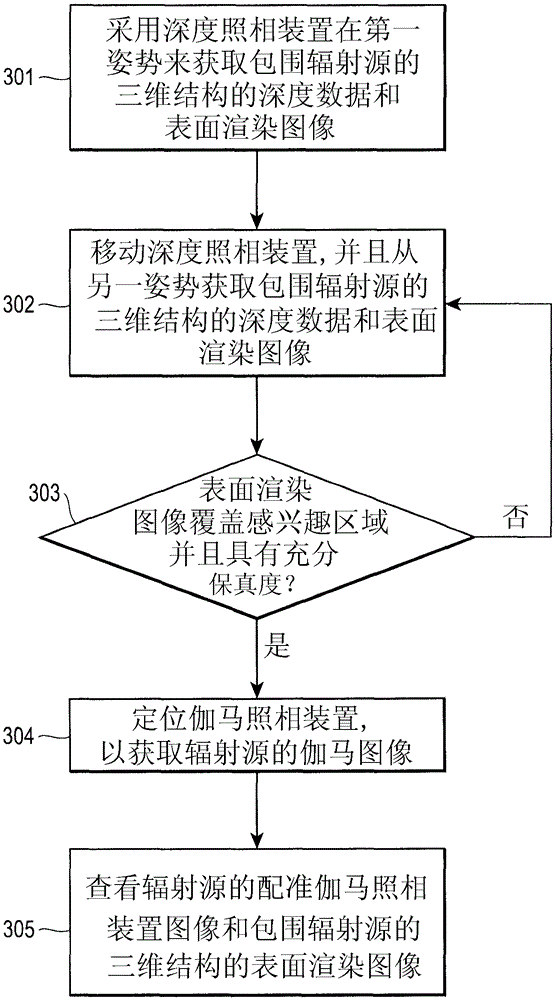
Combined radiationless automated three dimensional patient habitus imaging with scintigraphy
InactiveCN105264403ANo increase in radiation doseSurgical navigation systemsDiagnostics using fluorescence emissionRadioactive tracerPhysics
An apparatus and method to map the body habitus, without the use of ionizing radiation, and to simultaneously track the position of an ionizing radiation imaging detector with respect to the body habitus map so that the radiotracer distribution of the patient can be fused with the body habitus map and thus provide an anatomical reference for the radiotracer distribution within the patient. A depth camera, capable of imaging a 3-dimensional surface, is attached to an ionizing radiation imaging detector where the relative position between the two is known.
Owner:NOVADAG TECH INC
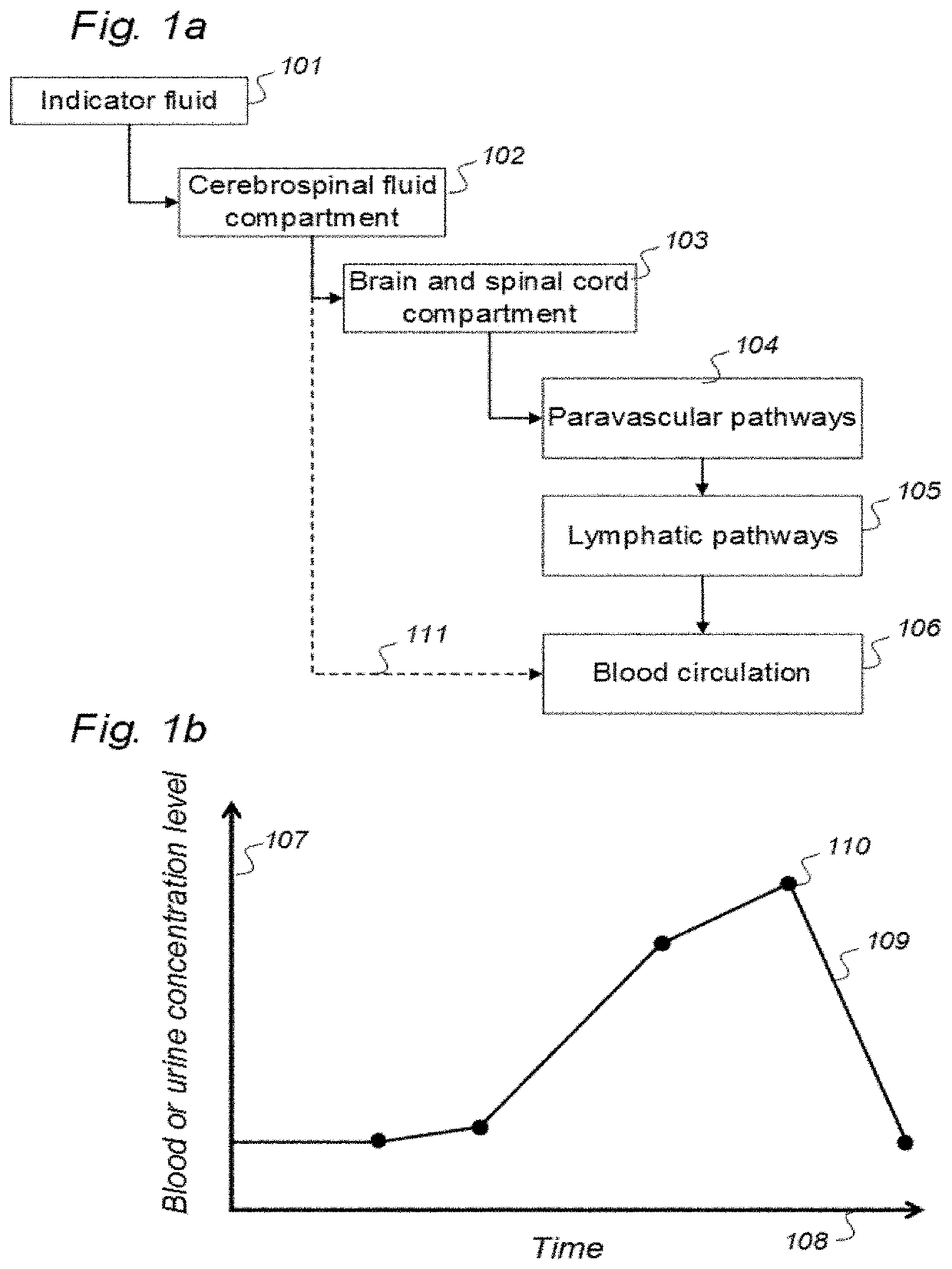
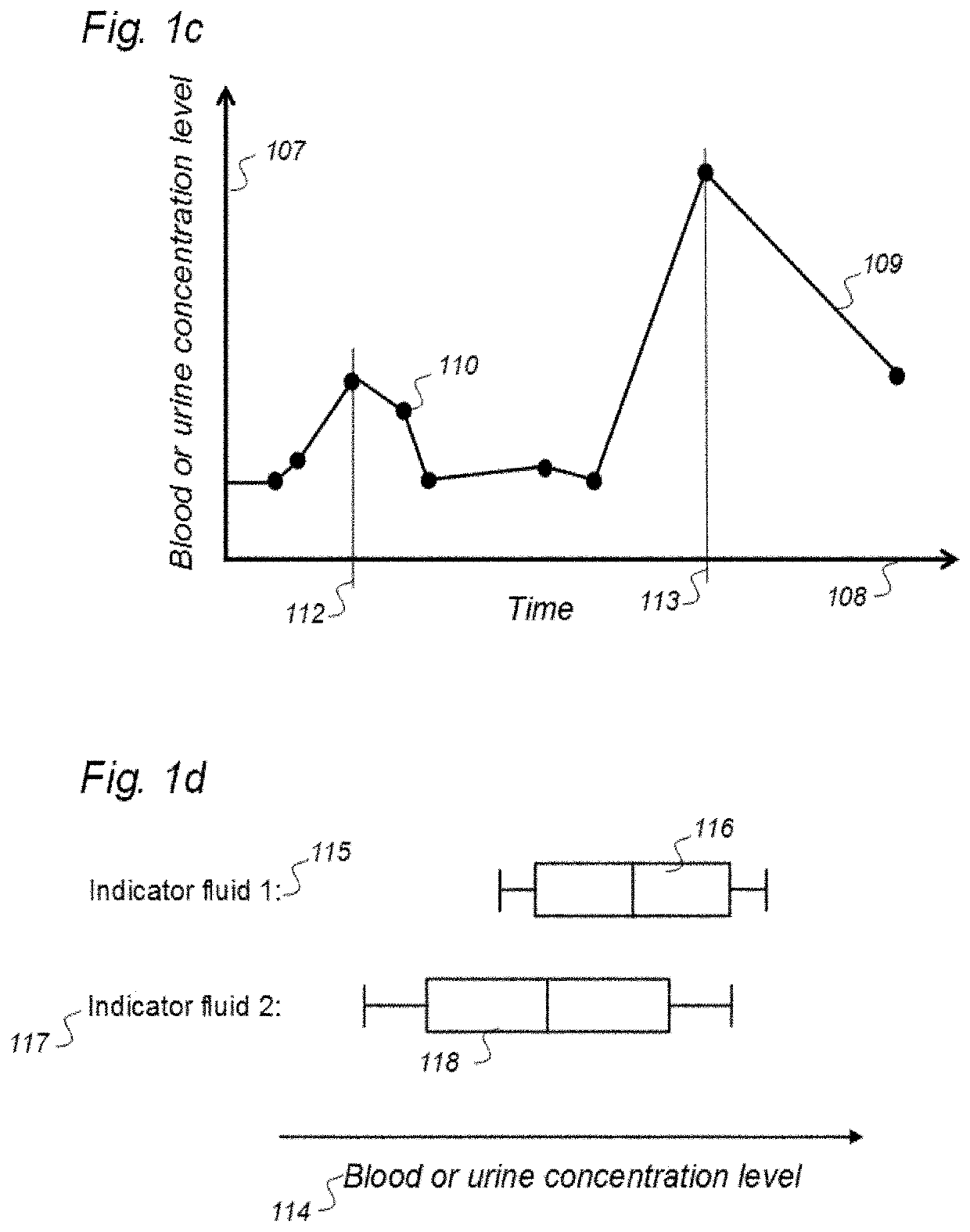
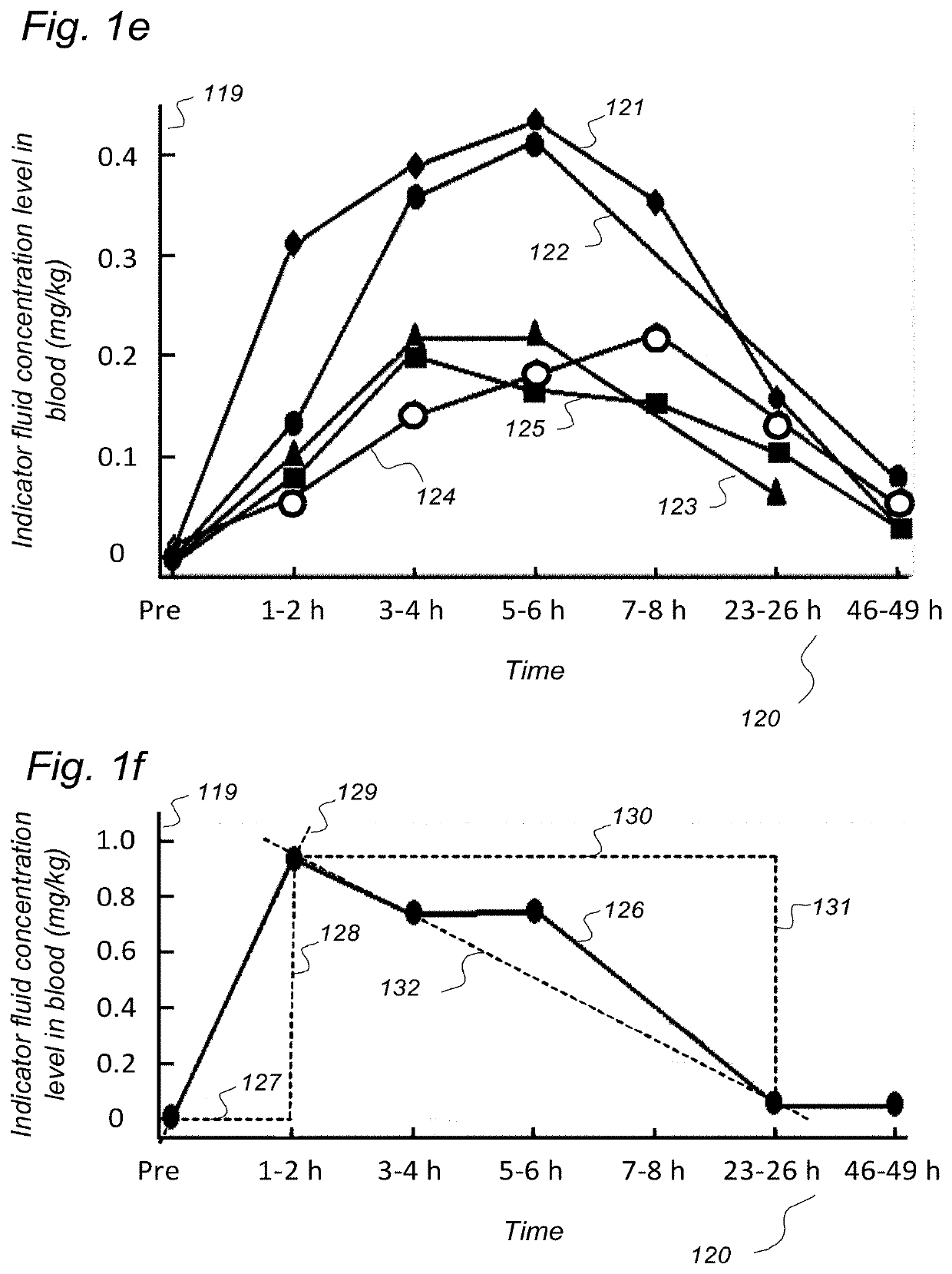
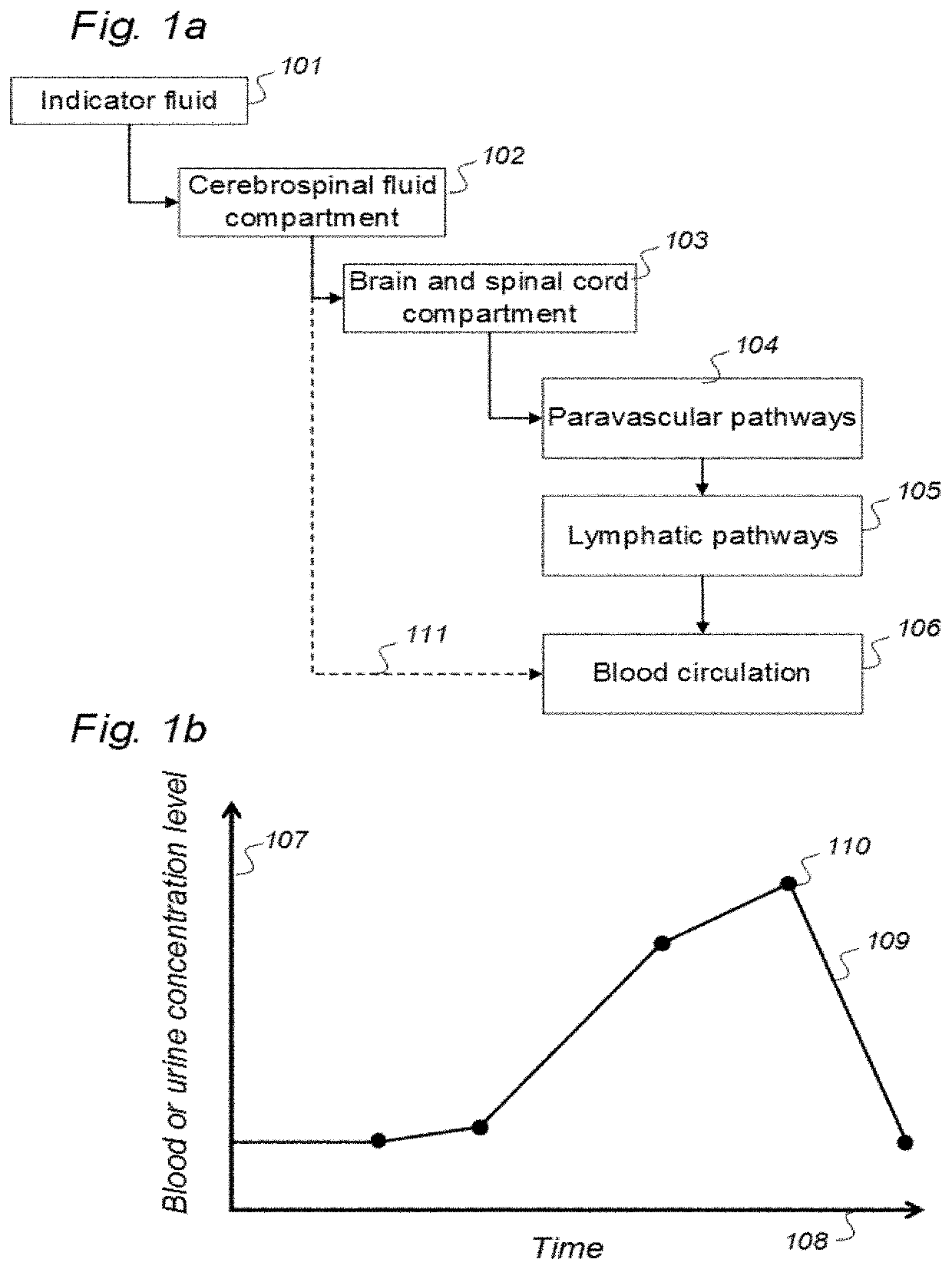
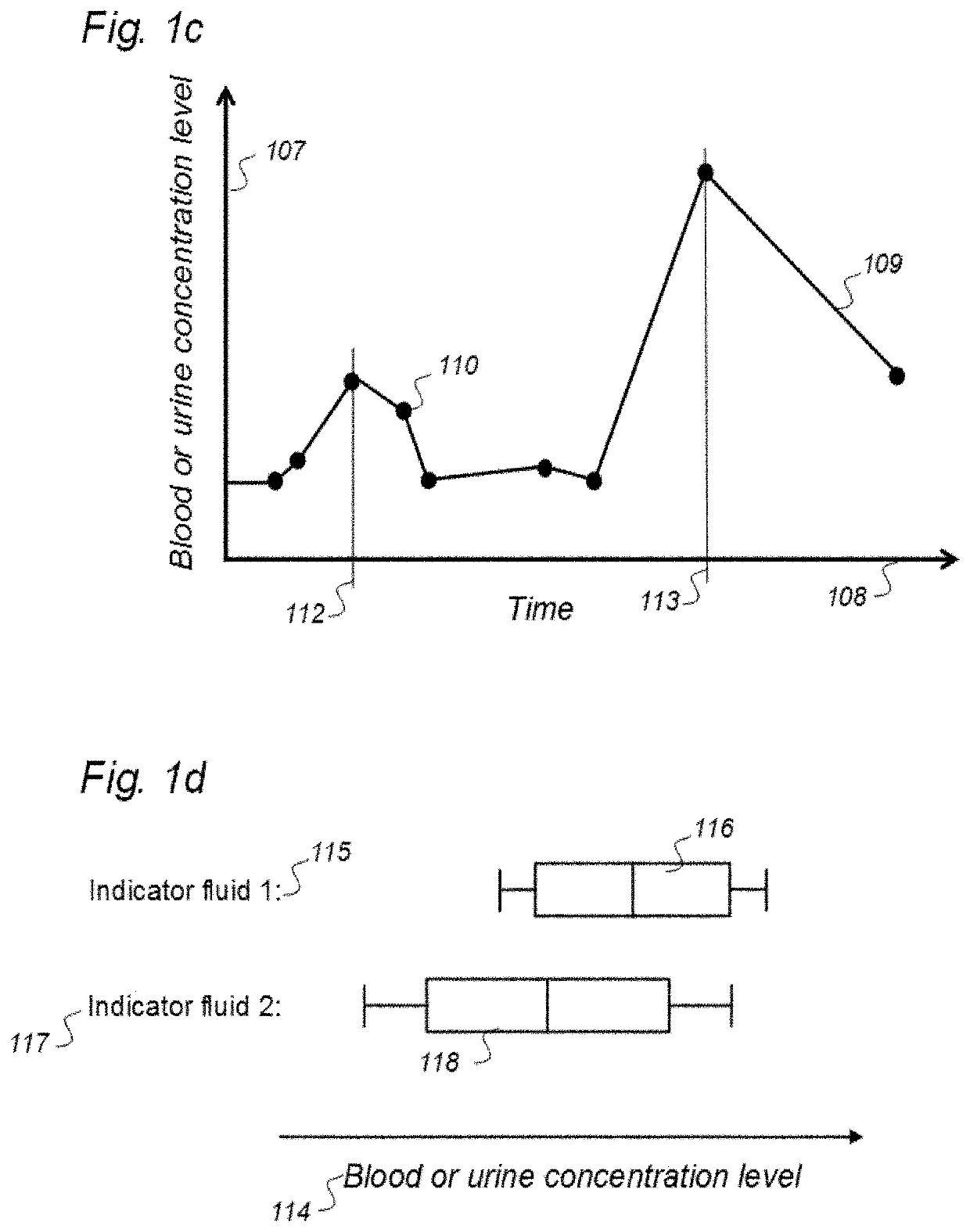
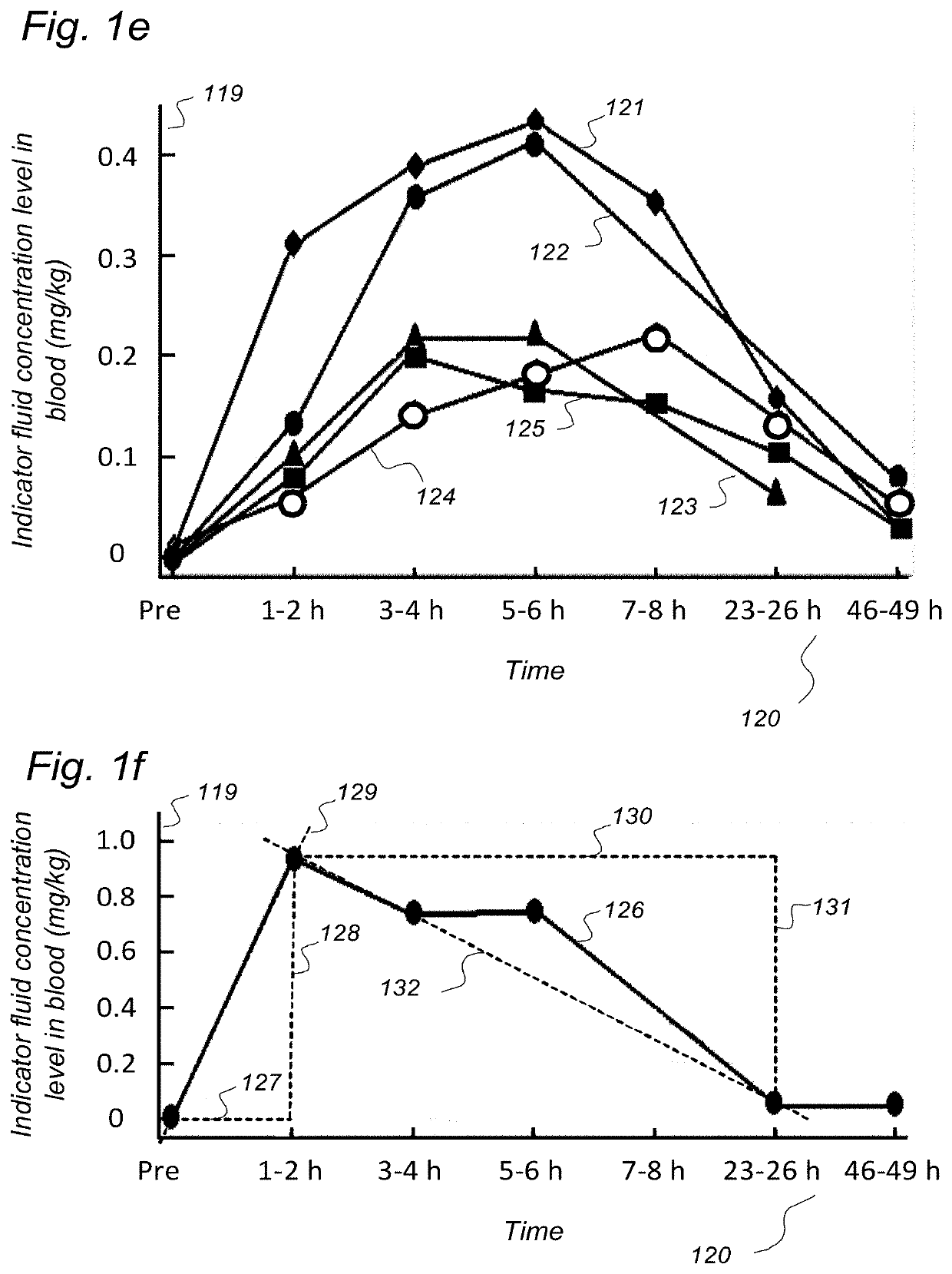
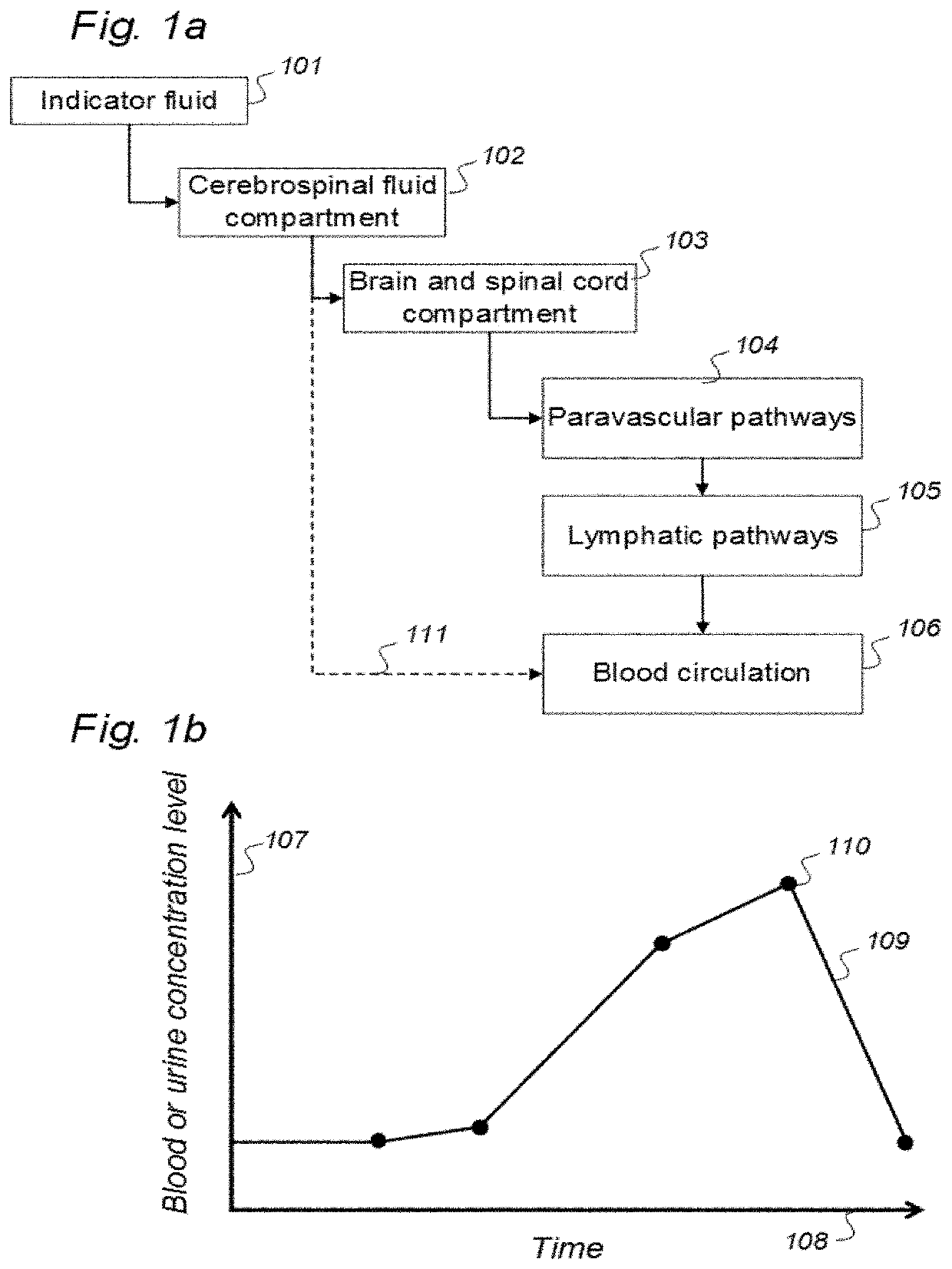
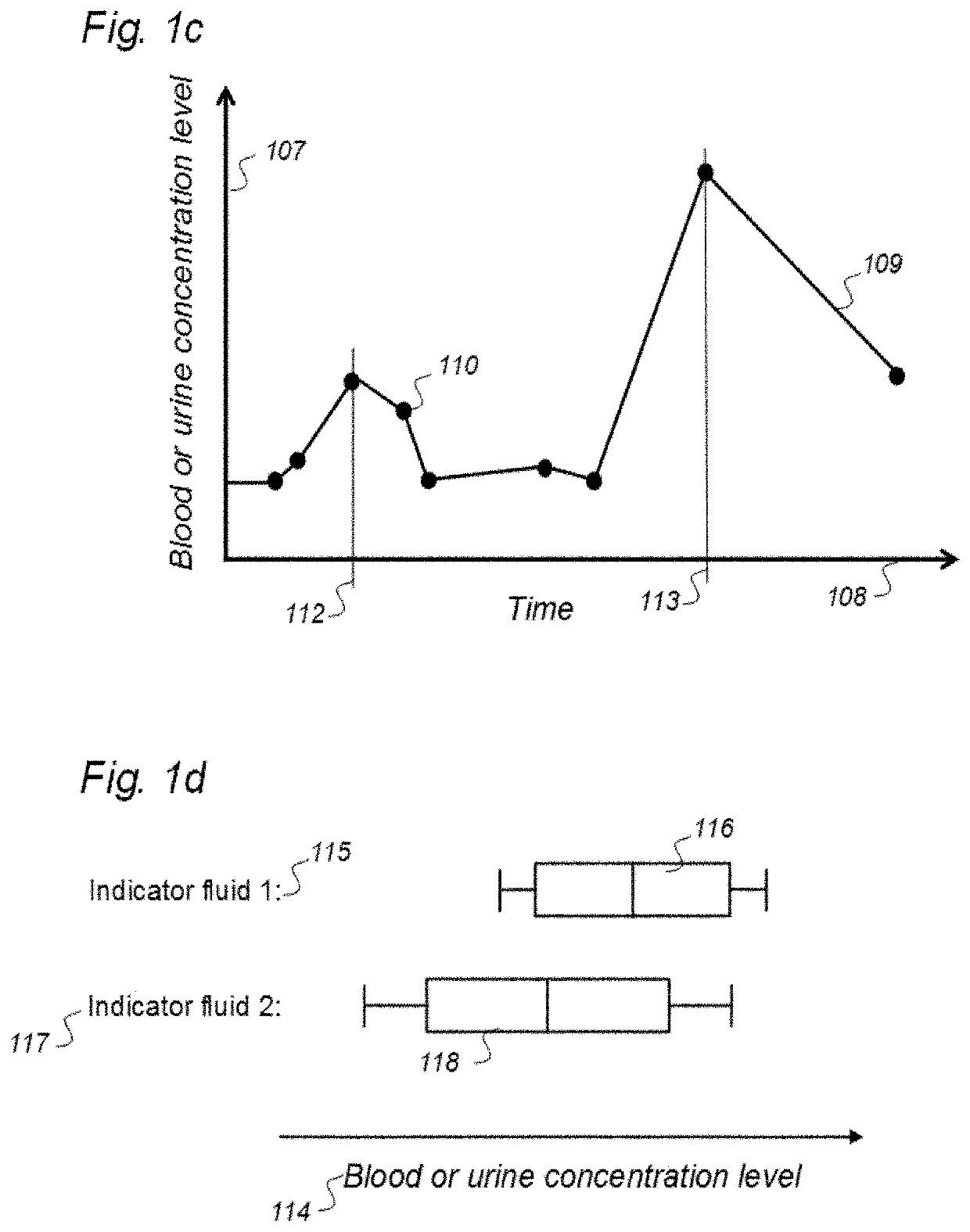
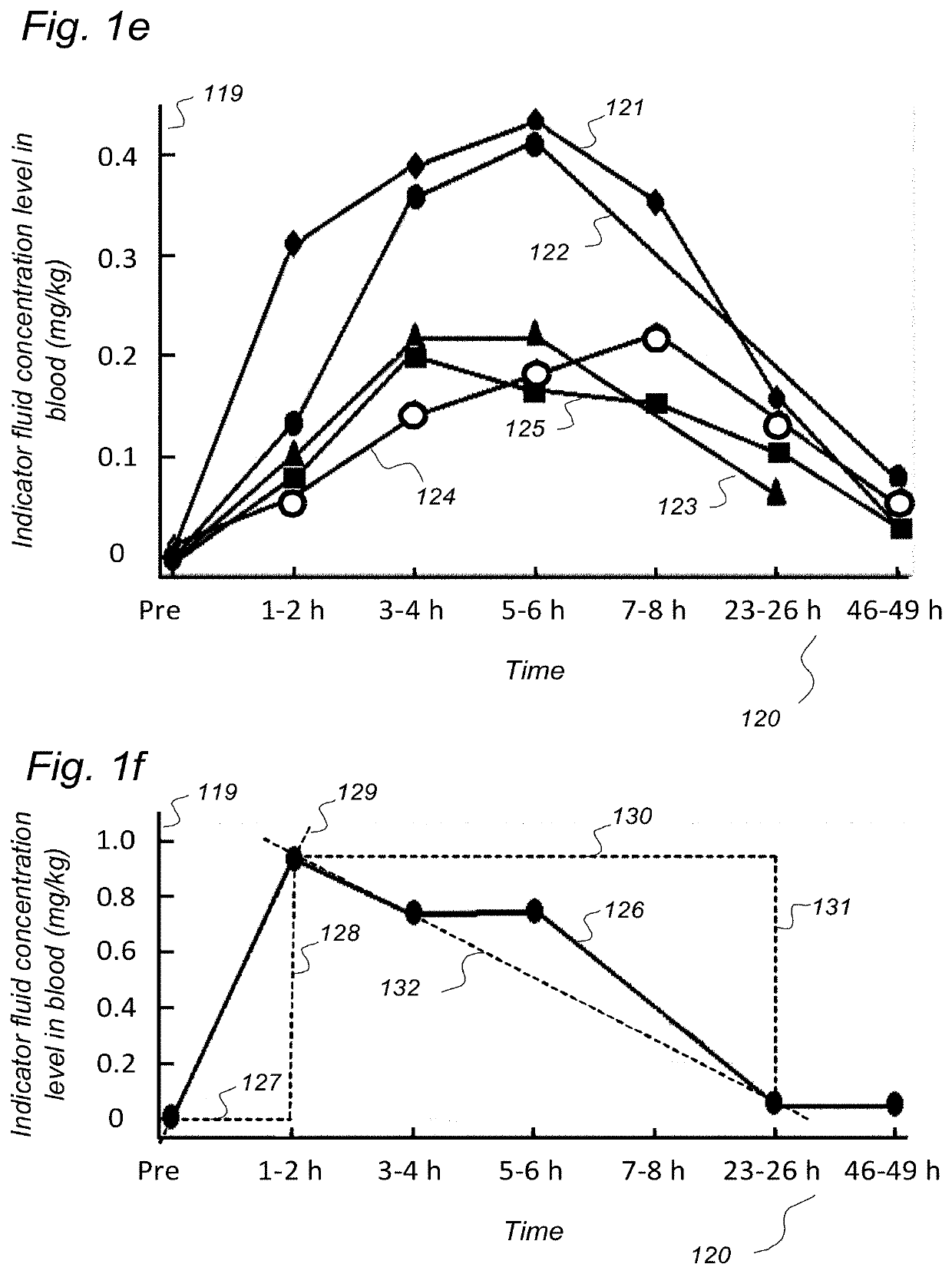
Indicator Fluids, Systems, and Methods for Assessing Movement of Substances Within, To or From a Cerebrospinal Fluid, Brain or Spinal Cord Compartment of a Cranio-Spinal Cavity of a Human
ActiveUS20200170509A1Accurate comparisonCompounds screening/testingMedical imagingPhoton emissionRadioactive agent
The present invention discloses indicator fluids, reference indicator fluid, and usage thereof, and systems and methods for assessing movement of molecular substances within, to or from a cerebrospinal fluid, brain or spinal cord compartment of a human cranio-spinal cavity. Indicator fluid moving from the cerebrospinal fluid compartment enables measurements of levels of indicator fluid in blood or urine and assessment of the cranio-spinal cavity's ability to remove molecular substances. The indicator fluids may be contrast agents used for imaging, such as by computed tomography imaging, and magnetic resonance imaging, or imaging utilizing radioactive substances by positron emission tomography, single-photon emission computed tomography or scintigraphy. Using these imaging modalities, the invention describes indicator fluids, systems and methods enabling assessment of movement of substances within, to or from a cerebrospinal fluid, brain or spinal cord compartment of a cranio-spinal cavity, and from the human cranio-spinal cavity to lymphatic pathways or kidneys.
Owner:BRAINWIDESOLUTIONS AS
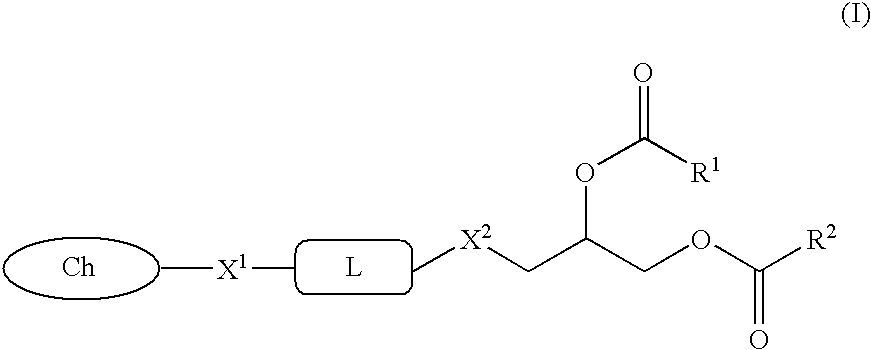
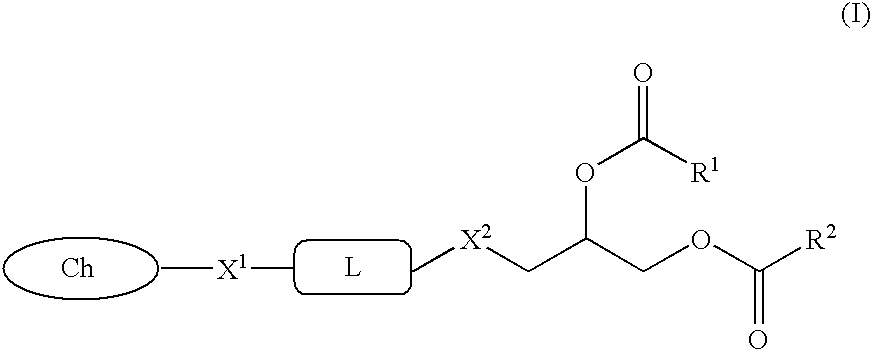
Glycerol ester derivative having metal chelate structure
A compound represented by the following general formula (I):wherein R1 and R2 represent an alkyl group or alkenyl group; X1 and X2 represent —O—, or —N(Z)-; Z represents hydrogen atom, or an alkyl group; L represents a divalent bridging group comprising an alkylene group, an alkenylene group, —CO—, —O— and the like wherein number of atoms constituting a chain length of the bridging group is 1 to 10; and Ch represents a partial structure containing 3 or more nitrogen atoms and capable of forming a chelate, and a chelate compound comprising the compound are provided. A vascular lesion can be selectively contrasted by MRI or scintigraphy imaging using a contrast medium comprising liposomes containing said compound or said chelate.
Owner:FUJIFILM CORP
Use of a Mixture Comprising Erbium and Praseodymium as a Radiation Attenuating Composition, Radiation Attenuating Material, and Article Providing Protection Against Ionising Radiation and Comprising Such a Composition
ActiveUS20140361199A1Improve protectionOther chemical processesShieldingMedical imagingRadiation damping
The invention relates to the use of a mixture comprising erbium and praseodymium as a radiation attenuating composition, i.e. as a composition that can attenuate ionising radiation, in particular X- and gamma-type electromagnetic radiation.The invention also relates to a radiation attenuating material comprising an erbium- and praseodymium-based composition, as well as a protective article which provides group or individual protection against ionising radiation and comprises said material.The invention is suitable for use in nuclear medicine (scintigraphy, radiotherapy, etc.), radiology, medical imaging, the nuclear industry, etc.
Owner:ORANO RECYCLAGE
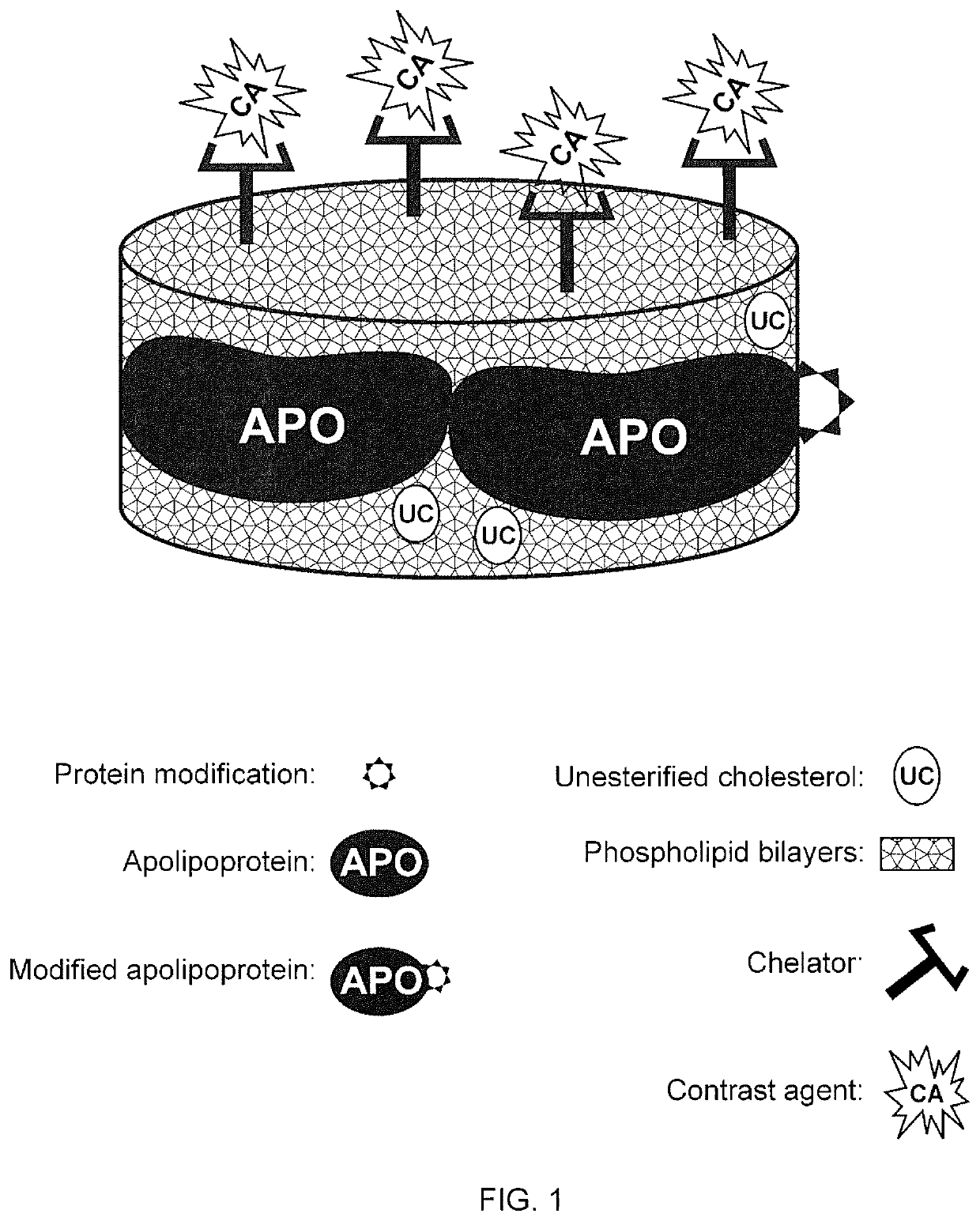
Methods And Compositions For Targeted Imaging
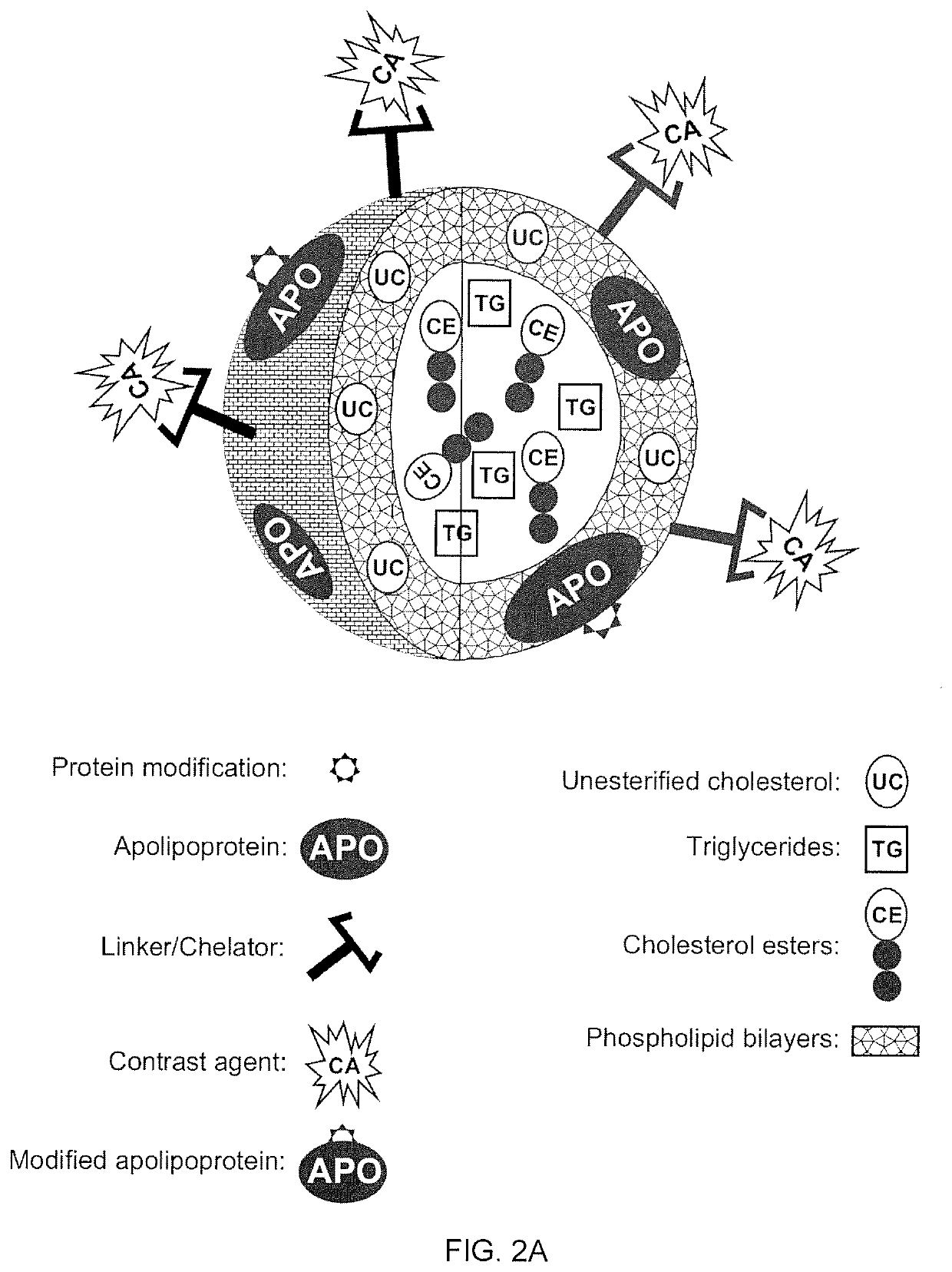
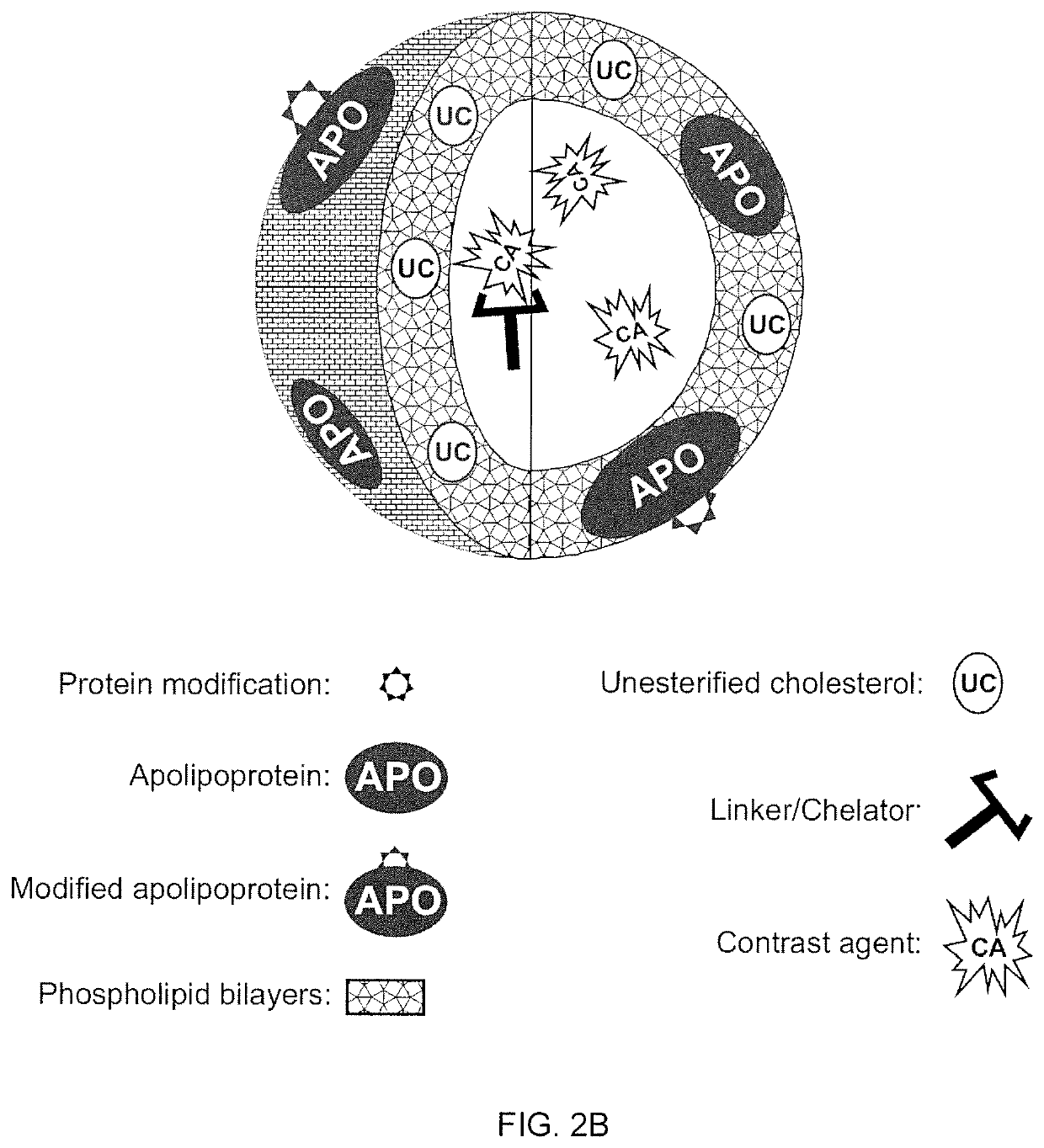
ActiveUS20200101178A1Dispersion deliveryPharmaceutical non-active ingredientsPhoton emissionA lipoprotein
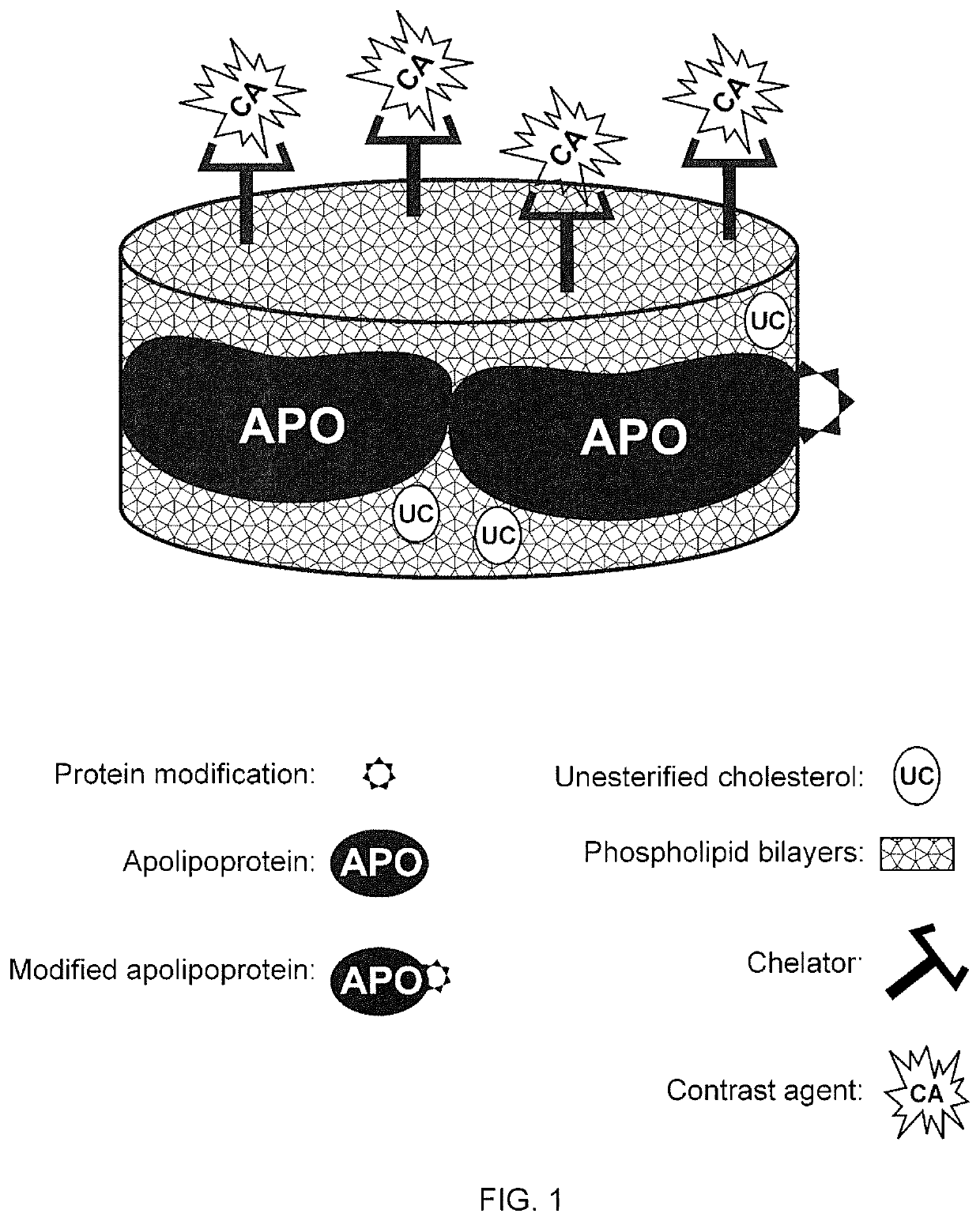
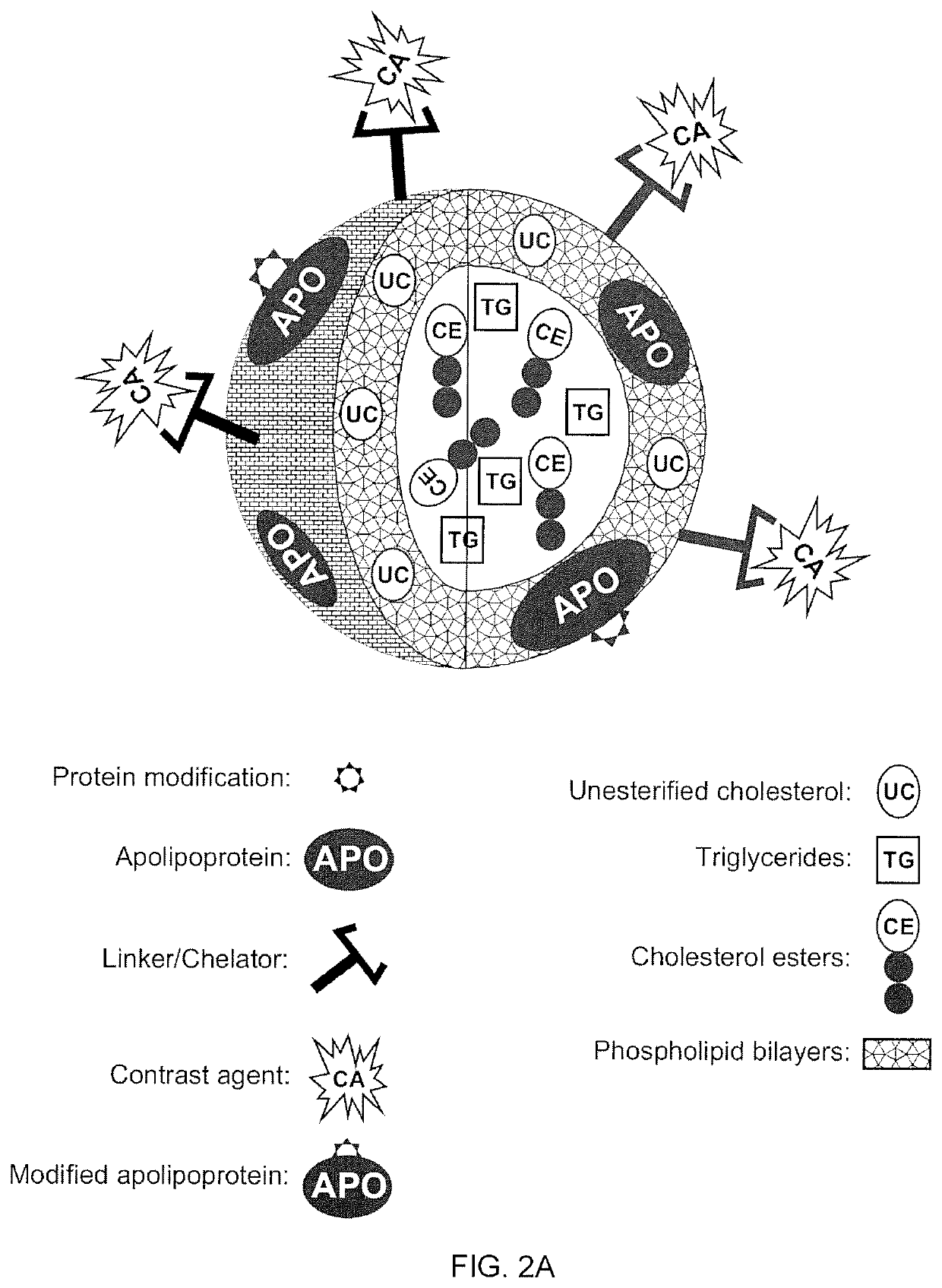
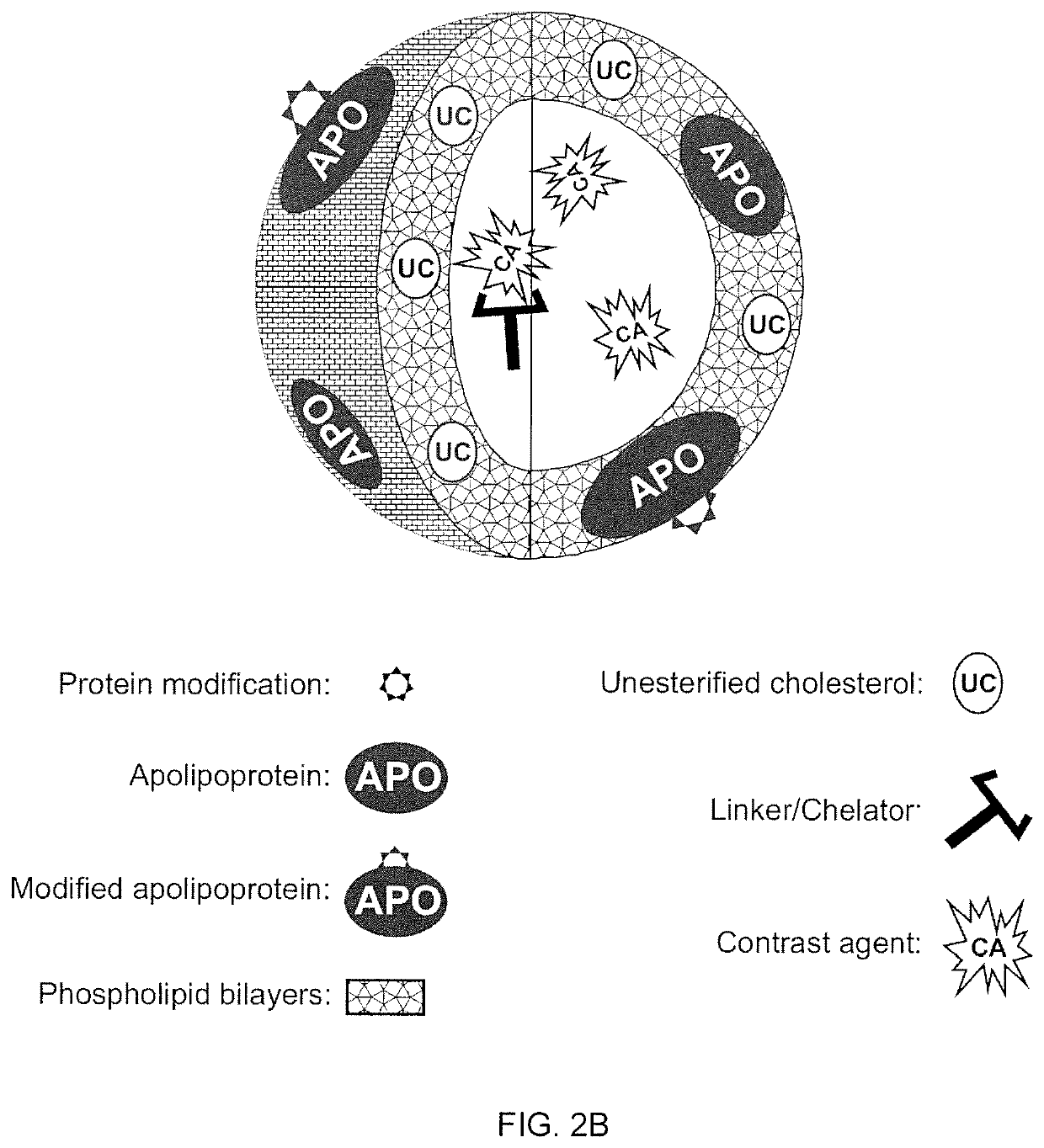
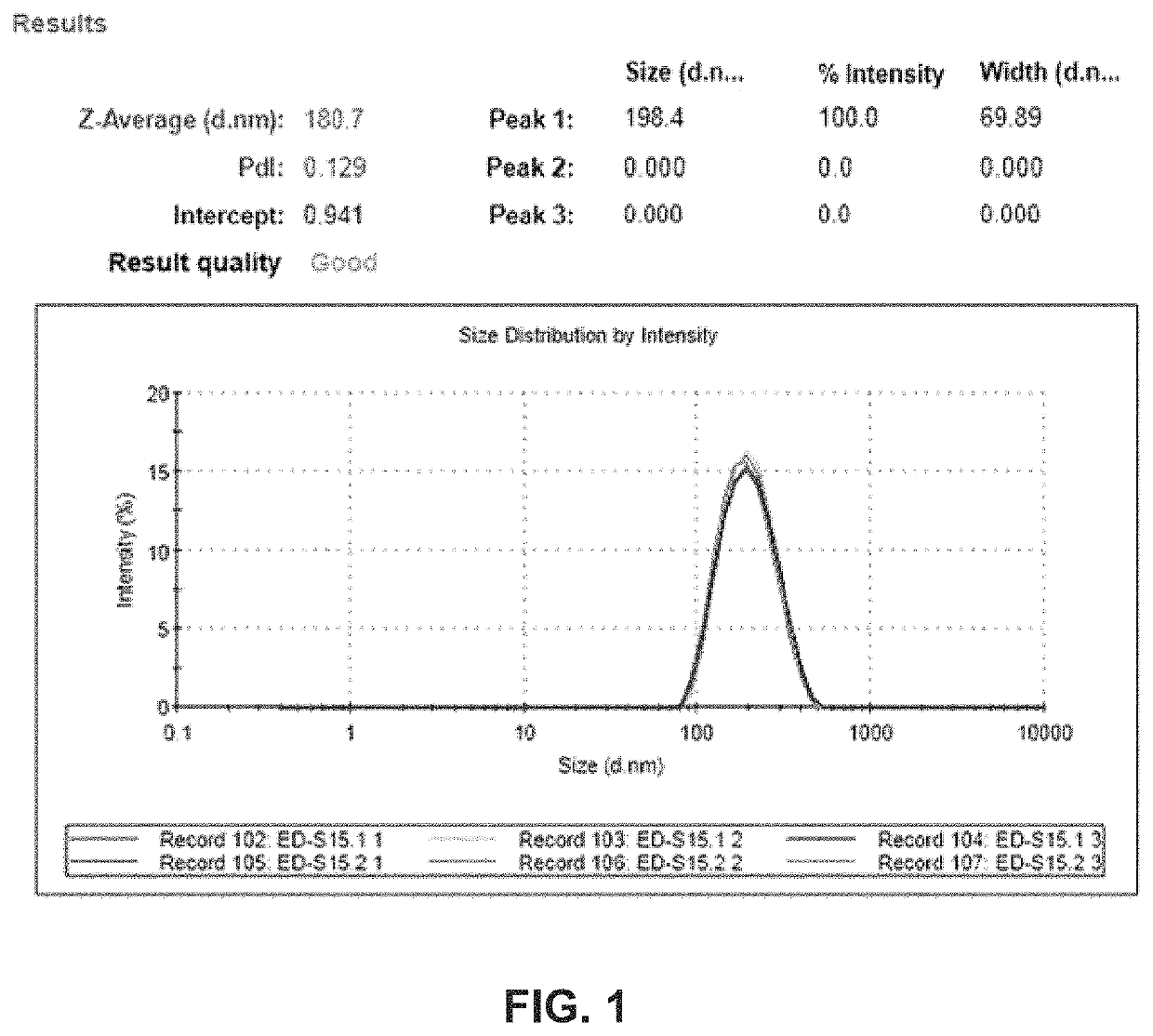
A new approach to targeting imaging agents to macrophage-rich sites of interest is disclosed. Compositions of the invention are rHDL and HDL-like liposomal compositions, protein constituents of which, apolipoproteins A-I and / or A-II or fragments thereof are used not only as structural but also as targeting agents. This is achieved by certain controlled chemical or enzymatic modification of apolipoproteins A-I or A-II or fragments thereof. Such modification converts these apolipoproteins to substrates for macrophage scavenger receptors and results in the improvement of contrast agent-(HDL / modified apolipoprotein)-particle association with macrophages and / or absorption (uptake) by macrophages when compared to that of the contrast agent-(HDL / apolipoprotein)-particle constructed with non-modified naturally occurring apo A-I. The compositions can be used for noninvasive specific in vivo molecular detection and localization of macrophage-rich sites of interest using imaging techniques such as computed tomography (CT), gamma-scintigraphy, positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI).
Owner:SIGNABLOK
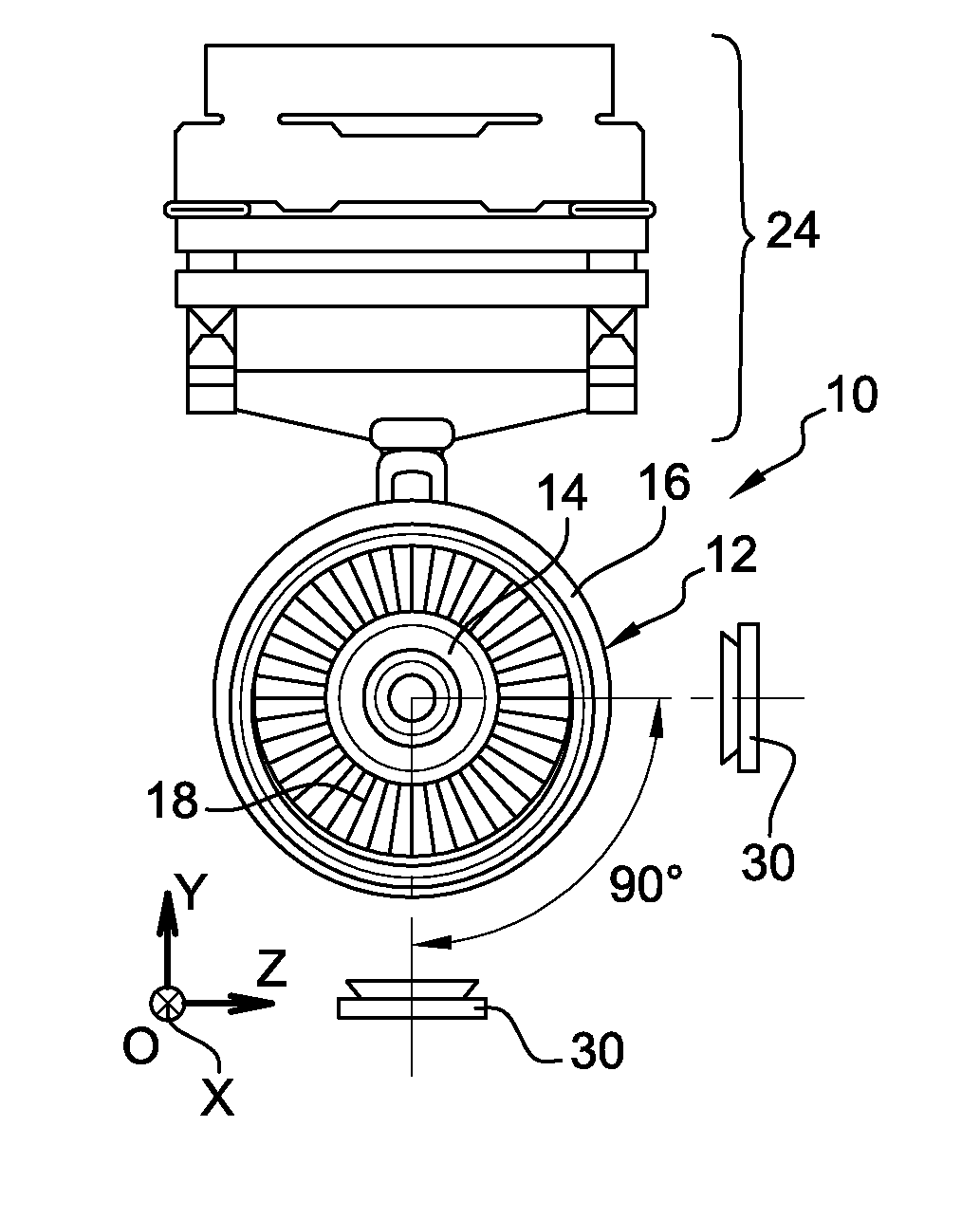
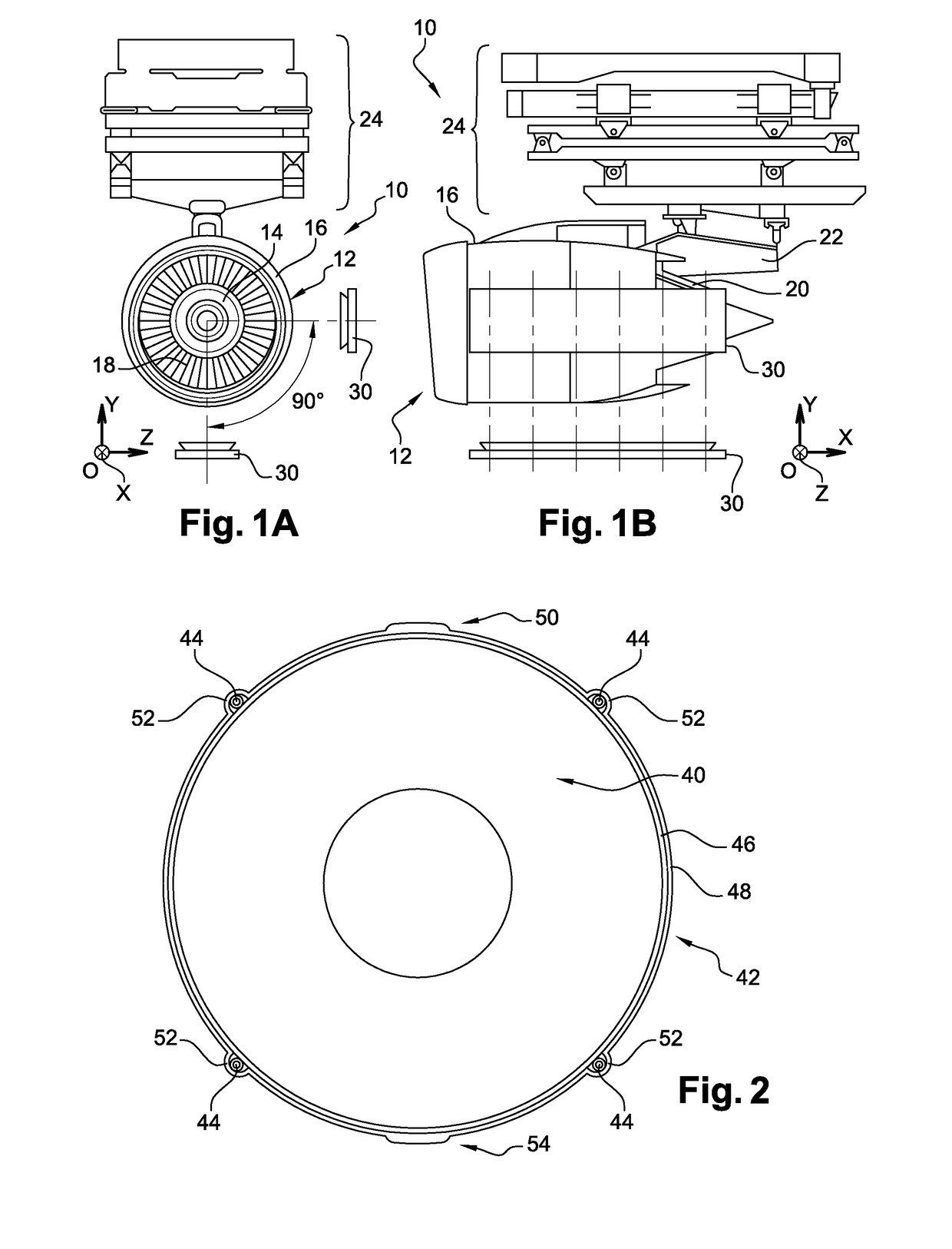
Method for measuring the kinematics of at least one turbomachine rotor
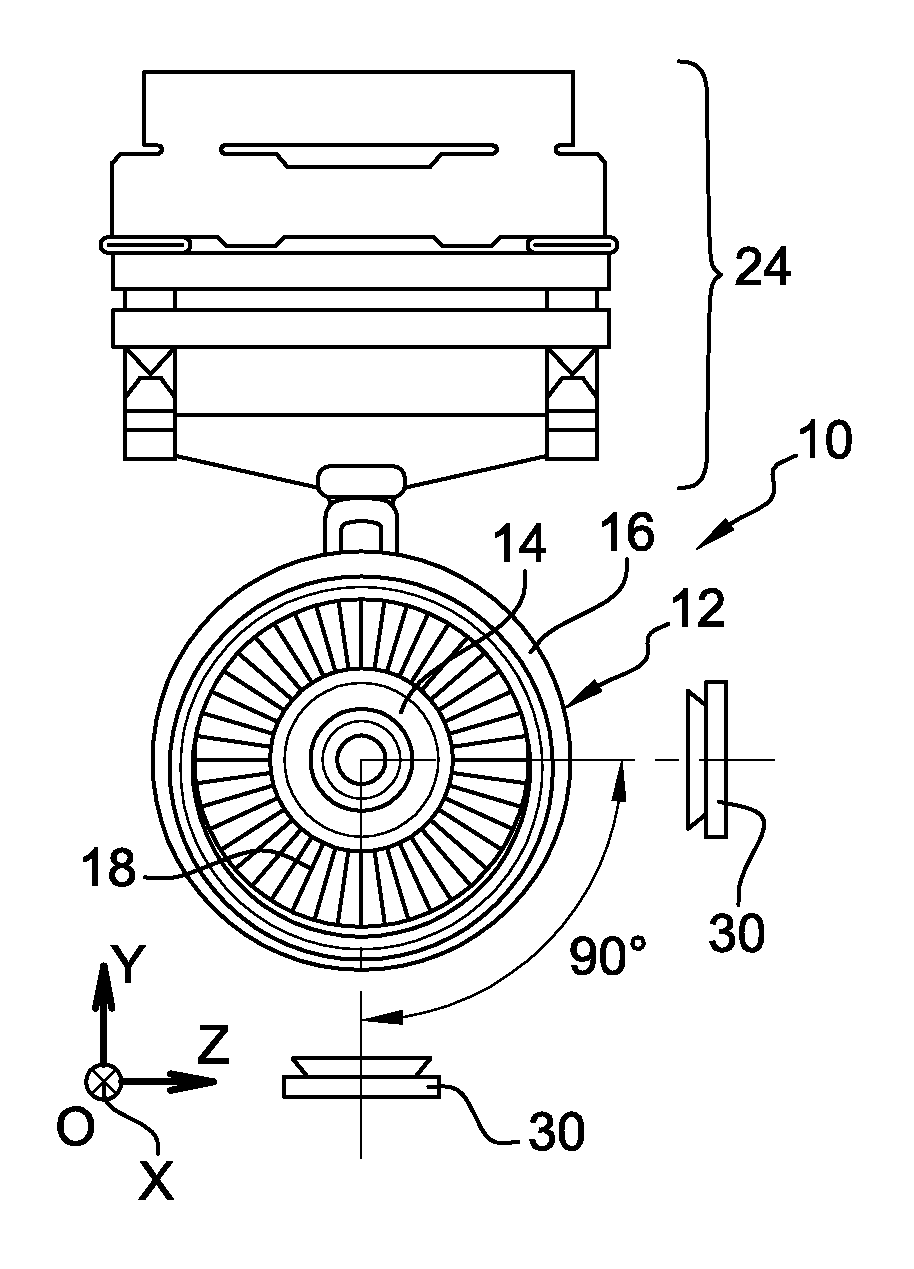
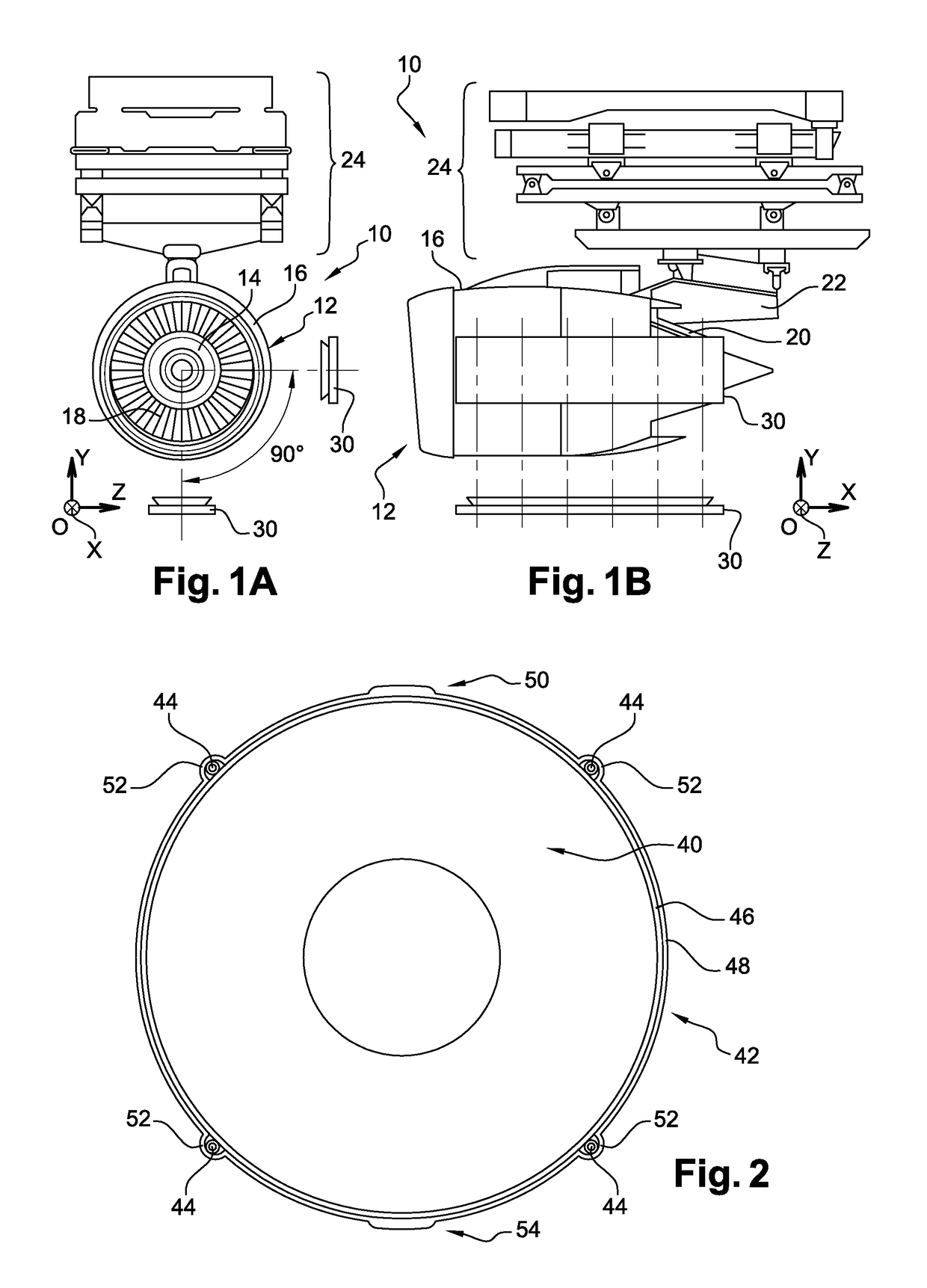
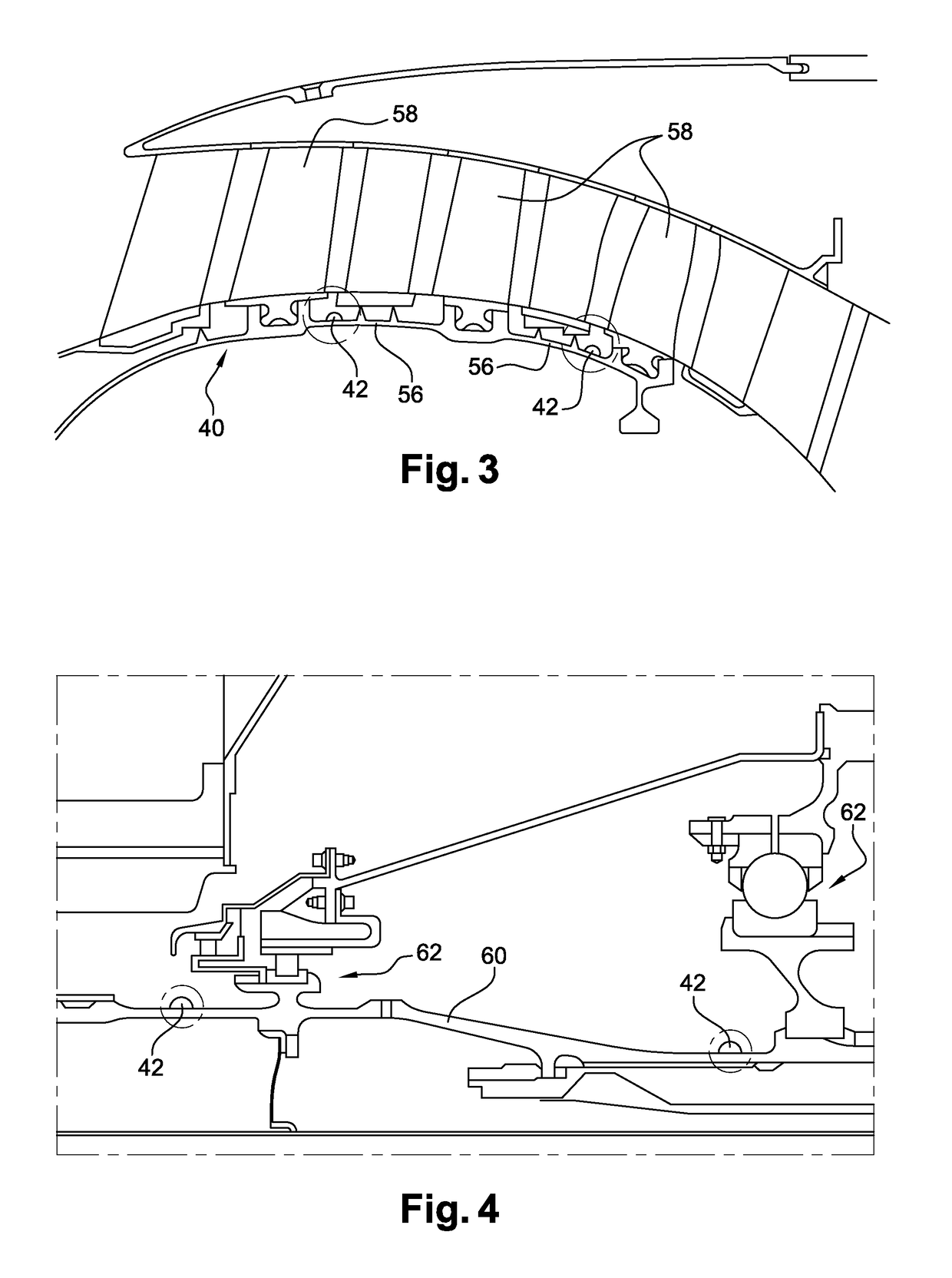
ActiveUS9851371B2Controlling the riskReduce riskGas-turbine engine testingLinear/angular speed measurementRadioactive tracerKinematics
The invention relates to a method for measuring the kinematics of at least one rotor of an engine (14), especially a turbomachine, characterized in that the measurement is performed by gamma ray scintigraphy, the method comprising steps consisting in providing the rotor with radioactive tracers, and, during the operation of the engine, detecting the gamma rays emitted by said tracers by means of at least two gamma cameras (30).
Owner:SN DETUDE & DE CONSTR DE MOTEURS DAVIATION S N E C M A
Indicator Fluids, Systems, and Methods for Assessing Movement of Substances Within, To or From a Cerebrospinal Fluid, Brain or Spinal Cord Compartment of a Cranio-Spinal Cavity of a Human
ActiveUS20220142479A1Accurate comparisonCompounds screening/testingMedical imagingPhoton emissionRadioactive agent
The present invention discloses indicator fluids, reference indicator fluid, and usage thereof, and systems and methods for assessing movement of molecular substances within, to or from a cerebrospinal fluid, brain or spinal cord compartment of a human cranio-spinal cavity. Indicator fluid moving from the cerebrospinal fluid compartment enables measurements of levels of indicator fluid in blood or urine and assessment of the cranio-spinal cavity's ability to remove molecular substances. The indicator fluids may be contrast agents used for imaging, such as by computed tomography imaging, and magnetic resonance imaging, or imaging utilizing radioactive substances by positron emission tomography, single-photon emission computed tomography or scintigraphy. Using these imaging modalities, the invention describes indicator fluids, systems and methods enabling assessment of movement of substances within, to or from a cerebrospinal fluid, brain or spinal cord compartment of a cranio-spinal cavity, and from the human cranio-spinal cavity to lymphatic pathways or kidneys.
Owner:BRAINWIDESOLUTIONS AS
Method for the production of labelled scaffolds, comprising at least one reactive fluorinated surfactant, and scaffold produced therewith
Owner:KONINK PHILIPS ELECTRONICS NV
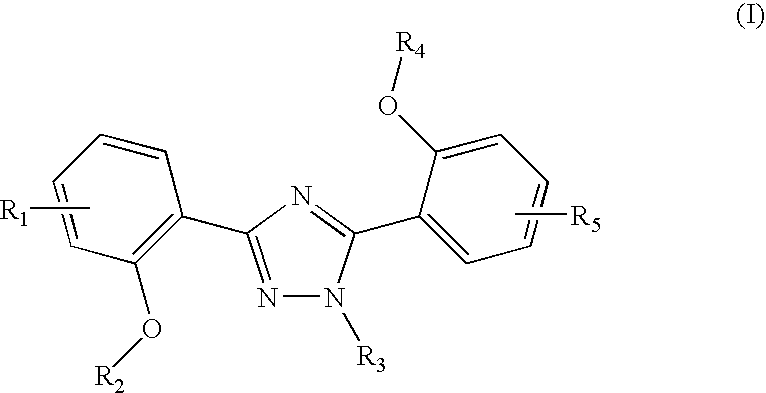
Method of scintigraphy
The invention relates to combination comprising a pharmaceutically acceptable preparation of a compound of formula (I), or a pharmaceutically acceptable salt thereof, and a gallium and its use in diagnosis. The invention also pertains to the use of a compound of formula I or a pharmaceutically acceptable salt thereof, for the preparation of a medicament for the treatment of an excess iron overload in the human or animal body whereby said body is undergoing gallium scintigraphy and whereby the treatment removing said excess of iron is interrupted for a period of 2 to 10 days prior to the gallium scintigraphy and resumed after the gallium scintigraphy readings.
Owner:NOVARTIS AG
Methods and compositions for targeted imaging
ActiveUS10894098B2Dispersion deliveryPharmaceutical non-active ingredientsPhoton emissionA lipoprotein
A new approach to targeting imaging agents to macrophage-rich sites of interest is disclosed. Compositions of the invention are rHDL and HDL-like liposomal compositions, protein constituents of which, apolipoproteins A-I and / or A-II or fragments thereof are used not only as structural but also as targeting agents. This is achieved by certain controlled chemical or enzymatic modification of apolipoproteins A-I or A-II or fragments thereof. Such modification converts these apolipoproteins to substrates for macrophage scavenger receptors and results in the improvement of contrast agent-(HDL / modified apolipoprotein)-particle association with macrophages and / or absorption (uptake) by macrophages when compared to that of the contrast agent-(HDL / apolipoprotein)-particle constructed with non-modified naturally occurring apo A-I. The compositions can be used for noninvasive specific in vivo molecular detection and localization of macrophage-rich sites of interest using imaging techniques such as computed tomography (CT), gamma-scintigraphy, positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI).
Owner:SIGNABLOK
Contrast agent and its use for imaging
ActiveUS11185600B2Increase awarenessImprove performanceUltrasonic/sonic/infrasonic diagnosticsPowder deliveryUltrasonographyAcoustical imaging
The present invention relates to contrast agent enhanced medical ultrasound imaging. In particular, the contrast agents provided are useful for cell imaging and cell therapy, as well as in vivo targeting, drug delivery and perfusion or vascular imaging applications. More specifically, it provides a particle comprising a fluorinated organic compound and a metal. Such particles may be advantageously employed in qualitative or quantitative imaging such as acoustic imaging including photoacoustic and ultrasound imaging, MRI imaging, such as 19F imaging, 1H imaging including T1 and T2 weighted imaging, SPECT, PET, scintigraphy, fluorescence imaging and optical coherence imaging and tomographic applications. This may then be employed in cell labeling, microscopy, histology or for imaging vasculature or perfusion in vivo and in vitro.
Owner:STICHTING RADBOUD UNIVERSITAIR MEDISCH CENT
Method for measuring the kinematics of at least one turbomachine rotor
ActiveUS20170097374A1Controlling the riskReduce riskGas-turbine engine testingLinear/angular speed measurementRadioactive tracerKinematics
The invention relates to a method for measuring the kinematics of at least one rotor of an engine (14), especially a turbomachine, characterised in that the measurement is performed by gamma ray scintigraphy, the method comprising steps consisting in providing the rotor with radioactive tracers, and, during the operation of the engine, detecting the gamma rays emitted by said tracers by means of at least two gamma cameras (30).
Owner:SN DETUDE & DE CONSTR DE MOTEURS DAVIATION S N E C M A
Infusion of a Mixture of Autologous Bone Marrow-Derived Mononuclear Cells and Autologous or Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells for Treating Myocardial and/or Cardiovascular Disorders
InactiveUS20110044950A1Effective and enduring myocardialEffective and enduring and cardiovascular replacement therapyBiocidePharmaceutical delivery mechanismDamages tissuePerfusion
The present invention is a method for improving cardiac and / or cardiovascular functions in living subjects after the occurrence of a myocardial and / or cardiovascular disorder, involving tissue damage and / or an ischemic event. The method is a combination stem cell therapy involving a mixture of bone marrow-derived mesenchymal stem cells and bone marrow-derived mononuclear cells surgically implanted by using either a direct or catheter-mediated injection into damaged tissue. Studies have shown that the implant improves blood perfusion in an ischemic tissue and thus contributes to the recovery of cardiac and / or cardiovascular function as assessed by methods of choice, including magnetic resonance imaging (“MRI”), echocardiography, angiography and 99mTc-TF perfusion scintigraphy.
Owner:TCA CELLULAR THERAPY
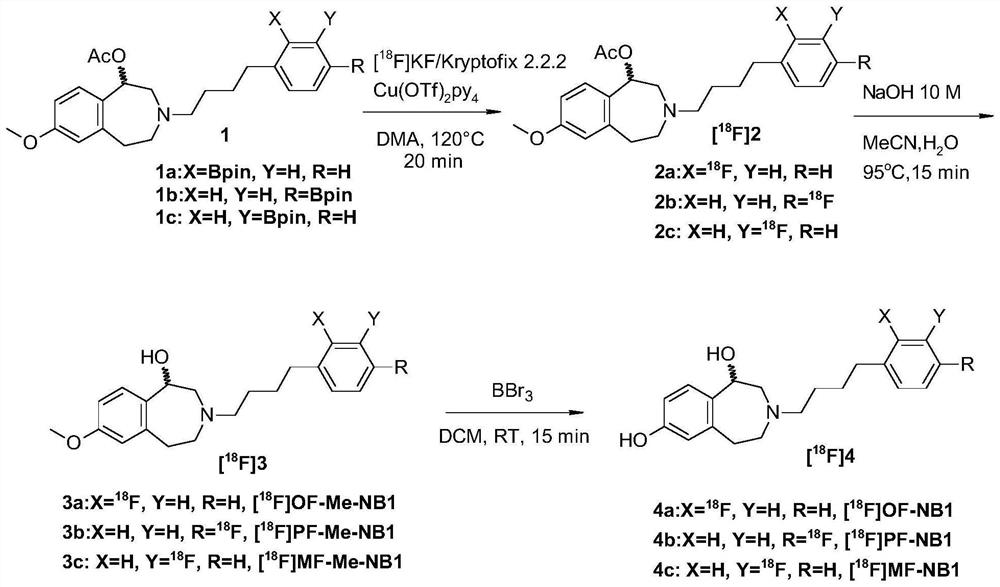
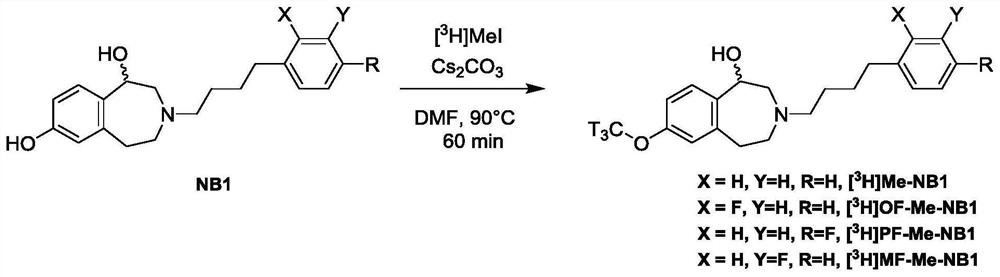
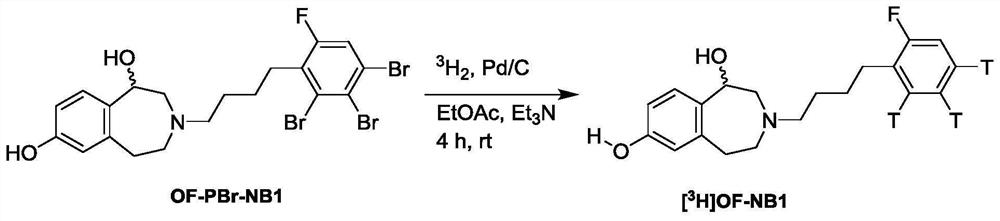
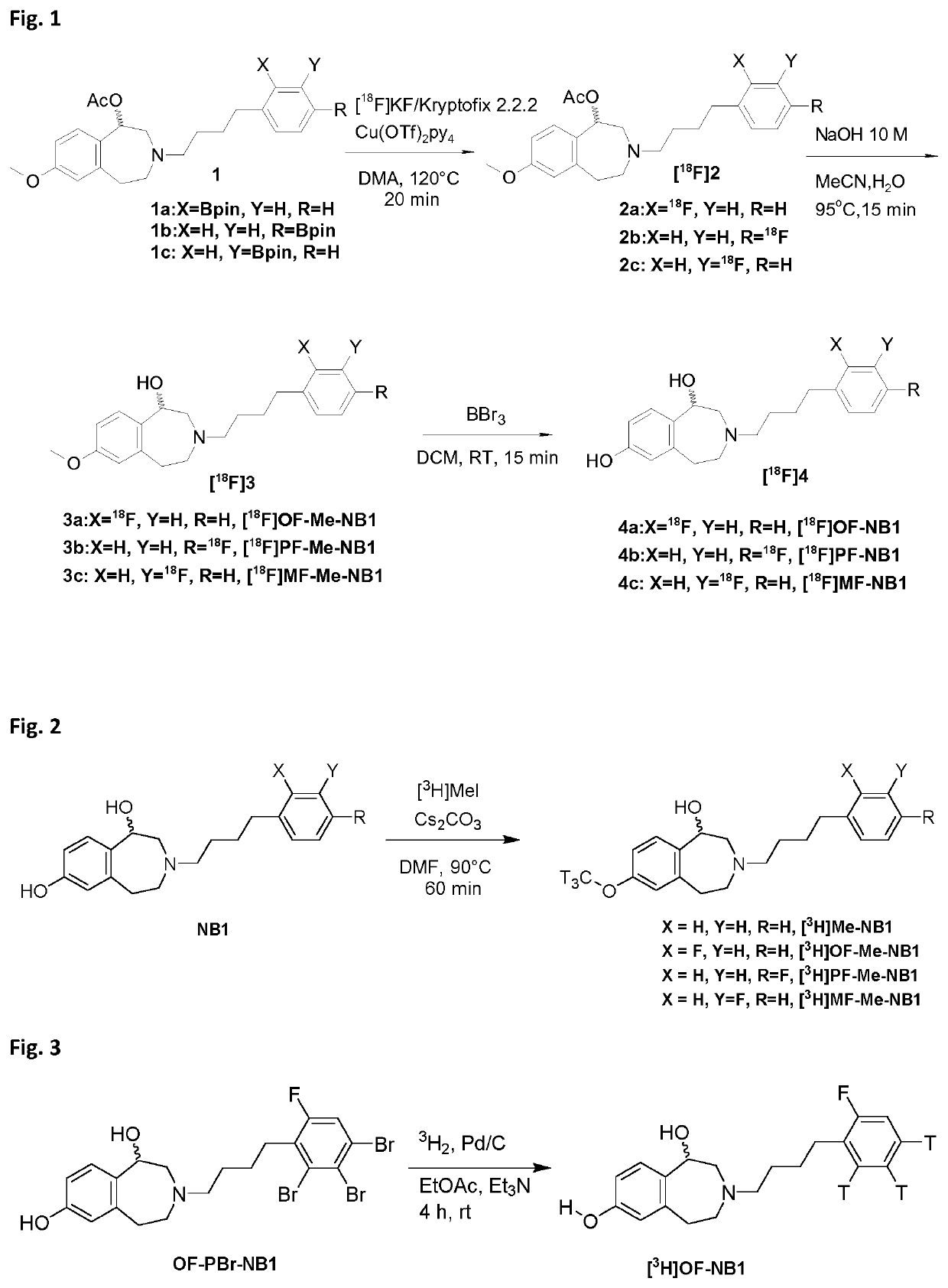
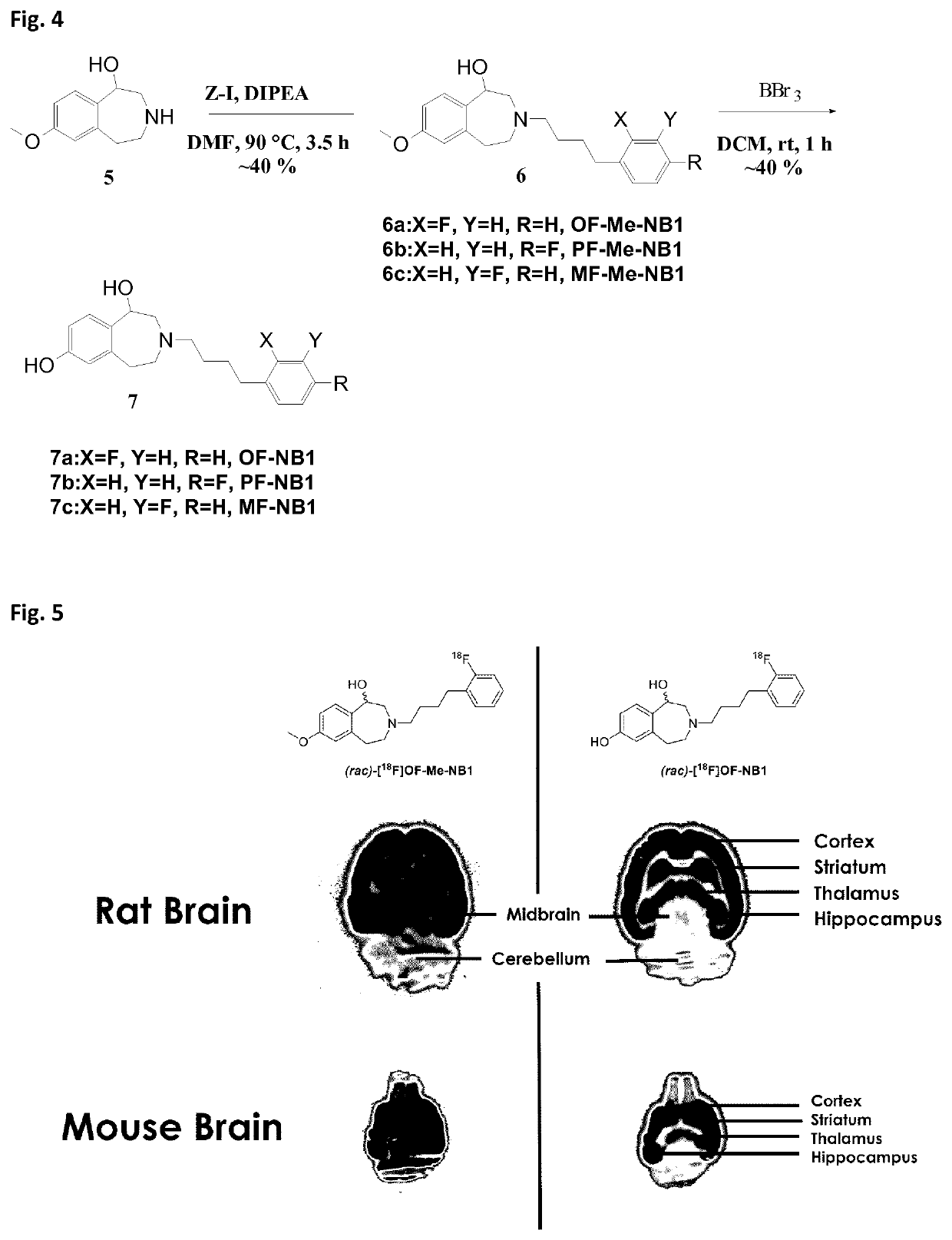
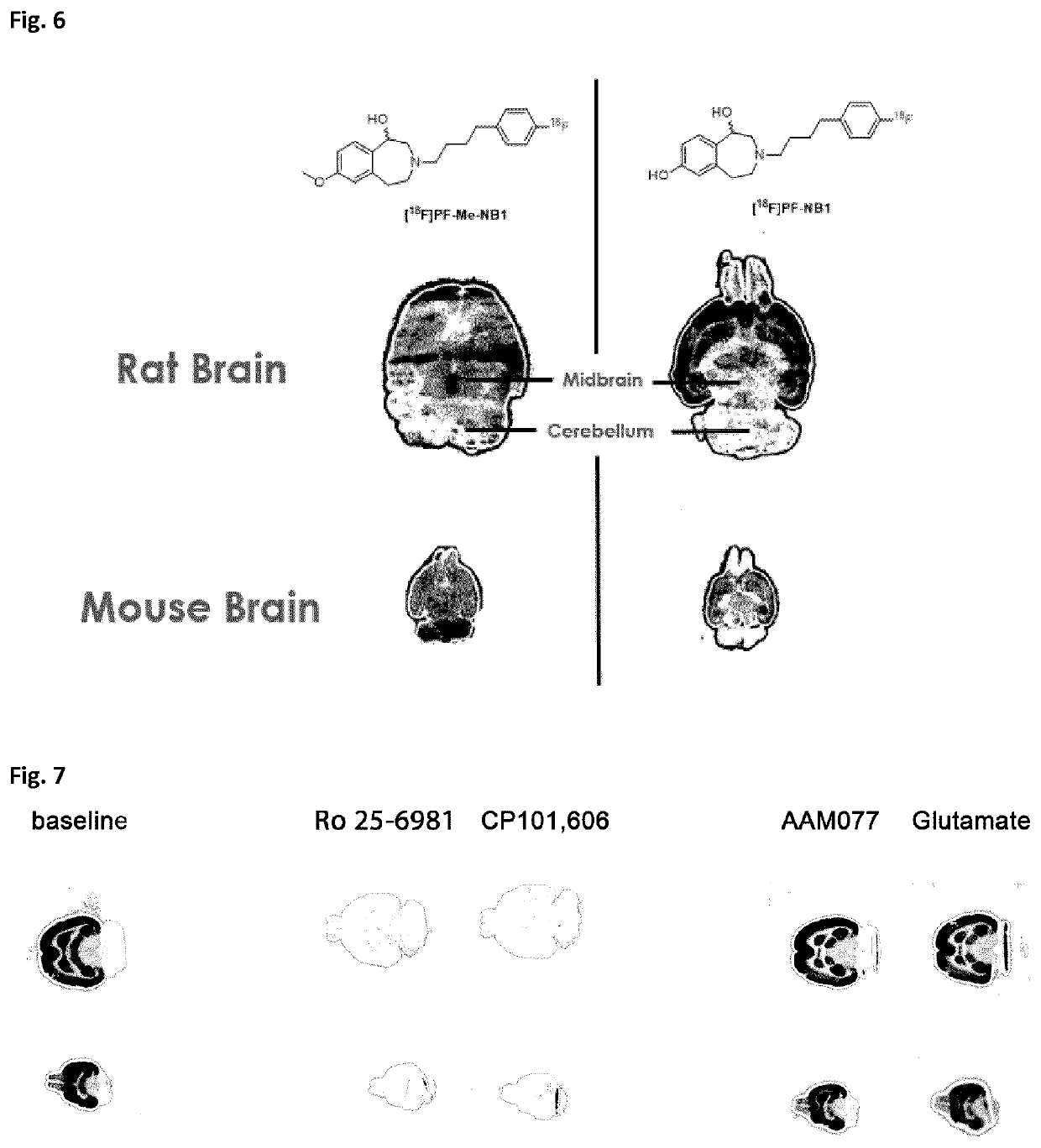
Benzazepin-l,7-diol-derived radiolabeled ligands with high in vivo NMDA specificity
PendingCN113166066ALow costImprove securityCompounds screening/testingNervous disorderDiseaseNR1 NMDA receptor
The present invention is directed to benzazepin-1,7-diol-derived compounds (I) for use in the diagnosis of NMDA (N-methyl-D-aspartate) receptor-associated diseases or disorders by positron emission tomography (PET), single-photon emission computed tomography (SPECT), liquid based-scintillation- and / or autoradiography-based assays. The invention also relates to a method for the diagnosis of NMDA receptor-associated diseases or disorders by administering to a patient or a sample of a patient in need of such diagnosis a compound of the invention in an amount effective for PET imaging, SPECT imaging, liquid based-scintillation- and / or autoradiography-based assays of NMDA receptors, recording at least one PET or SPECT scan, liquid based-scintillation or autoradiography result, and diagnosing an NMDA receptor-associated disease or disorder from an abnormal NMDA receptor expression pattern on the PET or SPECT scan, in the liquid based-scintillation or autoradiography result. The present invention also provides a method for evaluating a putative NMDA-receptor antagonist in a liquid scintigraphy detection assay or an autoradiography assay using the compounds of the present invention.
Owner:ETH ZZURICH
Indicator fluids, systems, and methods for assessing movement of substances within, to or from a cerebrospinal fluid, brain or spinal cord compartment of a cranio-spinal cavity of a human
ActiveUS11272841B2Accurate comparisonCompounds screening/testingMedical imagingPhoton emissionRadioactive agent
The present invention discloses indicator fluids, reference indicator fluid, and usage thereof, and systems and methods for assessing movement of molecular substances within, to or from a cerebrospinal fluid, brain or spinal cord compartment of a human cranio-spinal cavity. Indicator fluid moving from the cerebrospinal fluid compartment enables measurements of levels of indicator fluid in blood or urine and assessment of the cranio-spinal cavity's ability to remove molecular substances. The indicator fluids may be contrast agents used for imaging, such as by computed tomography imaging, and magnetic resonance imaging, or imaging utilizing radioactive substances by positron emission tomography, single-photon emission computed tomography or scintigraphy. Using these imaging modalities, the invention describes indicator fluids, systems and methods enabling assessment of movement of substances within, to or from a cerebrospinal fluid, brain or spinal cord compartment of a cranio-spinal cavity, and from the human cranio-spinal cavity to lymphatic pathways or kidneys.
Owner:BRAINWIDESOLUTIONS AS
Simultaneous blood glucose monitoring and gastric emptying scintigraphy
Systems and methods are provided for providing improved information, diagnoses and therapies for dyspepsia and gastric emptying disorders based on gastric protein emptying and gastric carbohydrate emptying data.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Benzazepin-l,7-diol-derived radiolabeled ligands with high in vivo NMDA specificity
PendingUS20220118118A1Ease of radioactivity handlingLess expenseCompounds screening/testingNervous disorderDiseaseNR1 NMDA receptor
The present invention is directed to benzazepin-1,7-diol-derived compounds (I) for use in the diagnosis of NMDA (N-methyl-D-aspartate) receptor-associated diseases or disorders by positron emission tomography (PET), single-photon emission computed tomography (SPECT), liquid based-scintillation- and / or autoradiography-based assays. The invention also relates to a method for the diagnosis of NMDA receptor-associated diseases or disorders by administering to a patient or a sample of a patient in need of such diagnosis a compound of the invention in an amount effective for PET imaging, SPECT imaging, liquid based-scintillation- and / or autoradiography-based assays of NMDA receptors, recording at least one PET or SPECT scan, liquid based-scintillation or autoradiography result, and diagnosing an NMDA receptor-associated disease or disorder from an abnormal NMDA receptor expression pattern on the PET or SPECT scan, in the liquid based-scintillation or autoradiography result. The present invention also provides a method for evaluating a putative NMDA-receptor antagonist in a liquid scintigraphy detection assay or an autoradiography assay using the compounds of the present invention.
Owner:ETH ZZURICH
Methods of testing digestive functions using both a breath test and a scintigraphy test, and methods of using a breath test as an overall digestive health assessment
ActiveUS8317718B2In-vivo radioactive preparationsPerson identificationLipid formationGastric emptying
Methods for assessing an overall digestive health of a patient are provided, which include administering a breath test that uses a label incorporated into proteins, carbohydrates, and lipids and further incorporating the labeled material into a meal which, when cooked, facilitates the binding of the label material to the solid phase matrix of the meal. Methods for assessing digestive functions of a patient are also provided, which includes concurrently administering a breath test and a scintigraphy test that measure gastric emptying in a stomach.
Owner:ADVANCED BREATH DIAGNOSTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com