Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Retinal degeneration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Retinal degeneration is a retinopathy which consists in the deterioration of the retina caused by the progressive death of its cells. There are several reasons for retinal degeneration, including artery or vein occlusion, diabetic retinopathy, R.L.F./R.O.P. (retrolental fibroplasia/ retinopathy of prematurity), or disease (usually hereditary). These may present in many different ways such as impaired vision, night blindness, retinal detachment, light sensitivity, tunnel vision, and loss of peripheral vision to total loss of vision. Of the retinal degenerative diseases retinitis pigmentosa (RP) is a very important example.
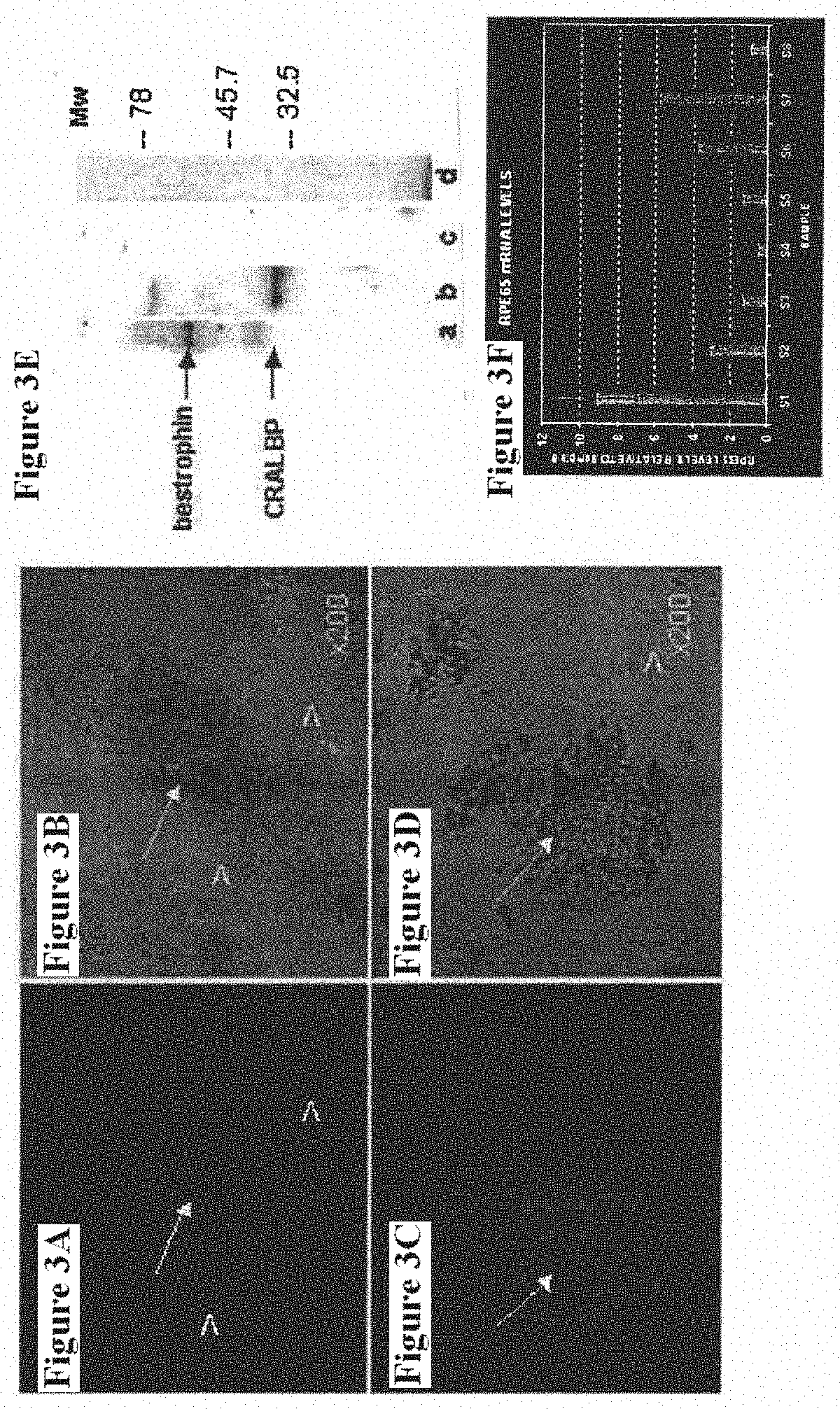
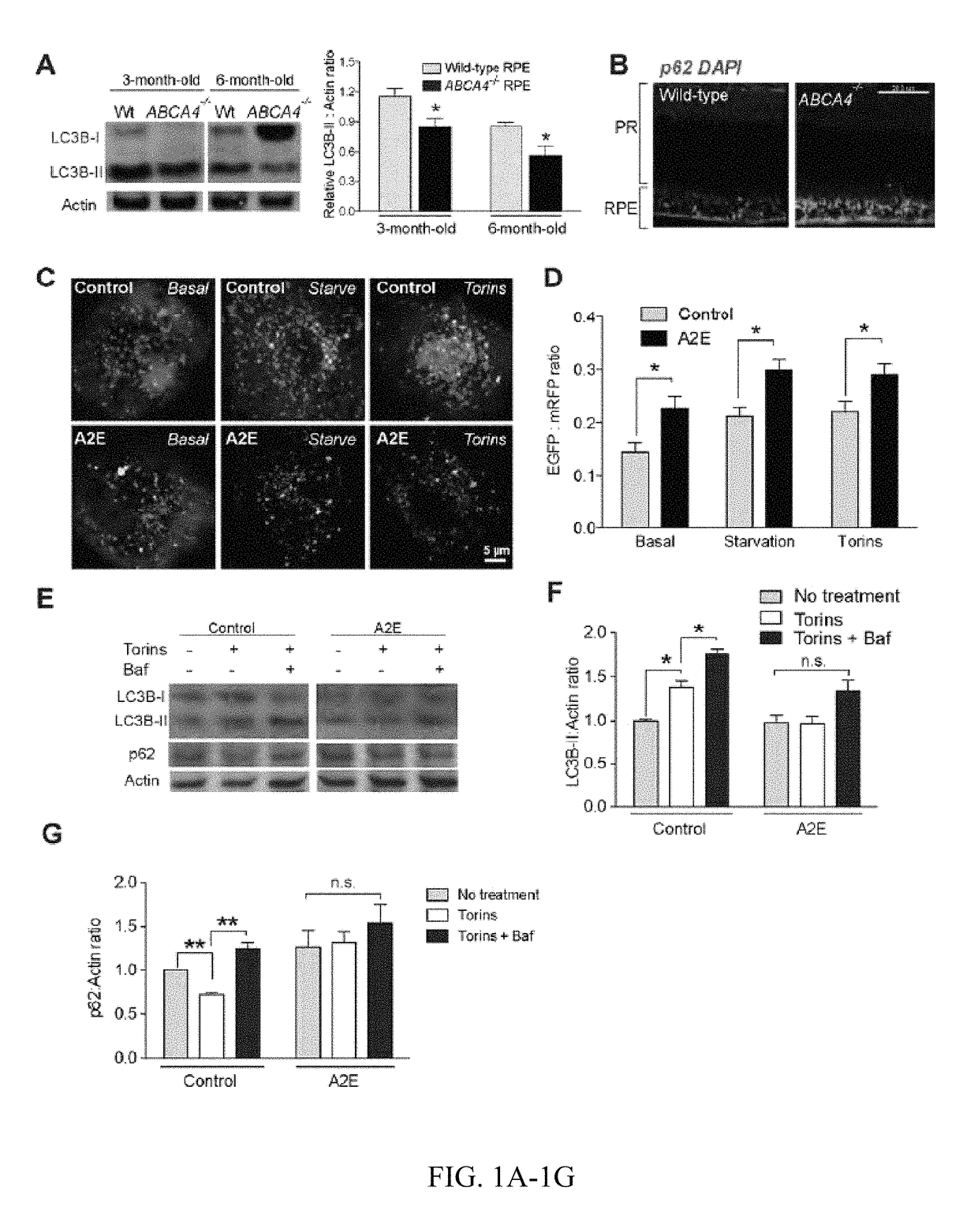
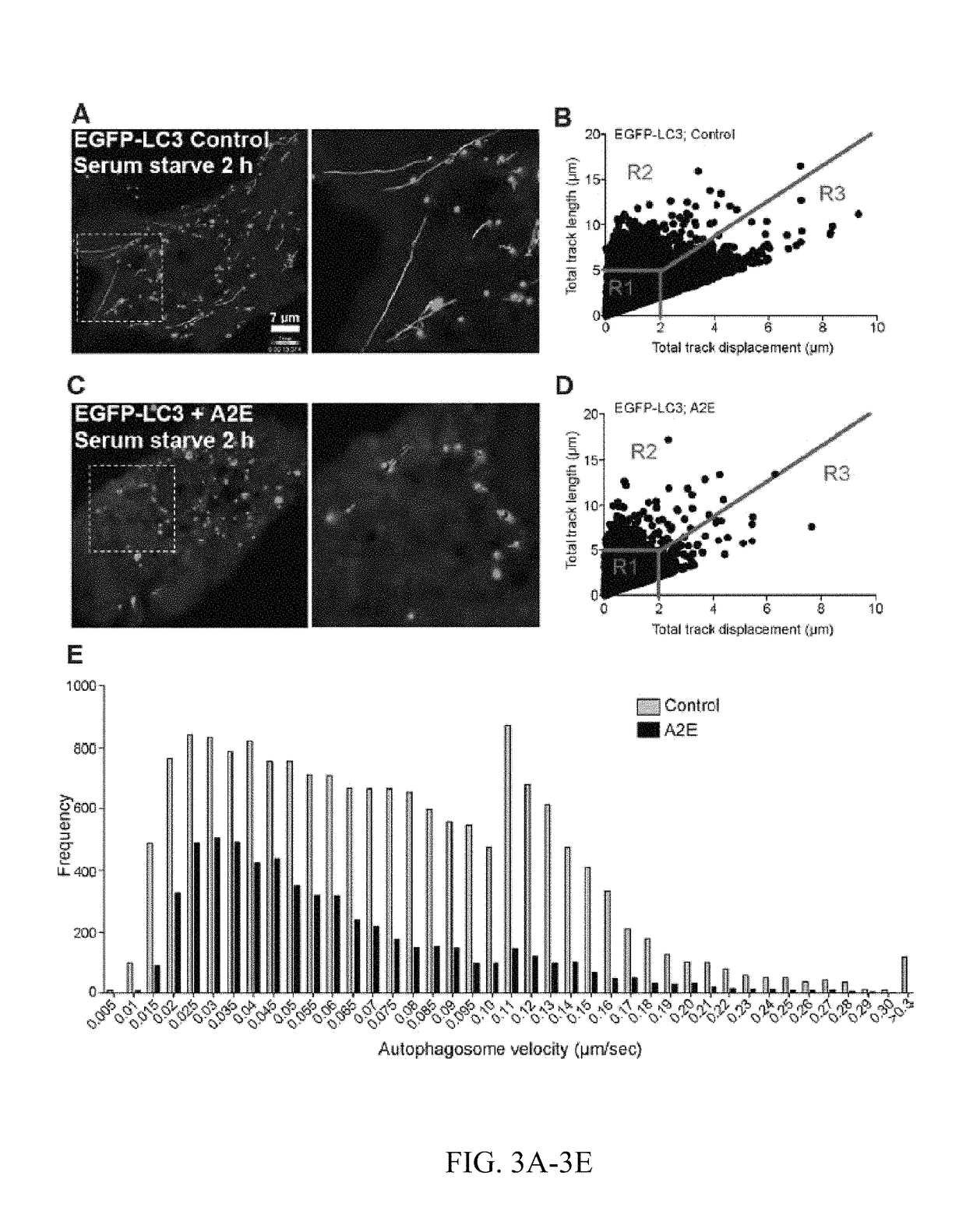
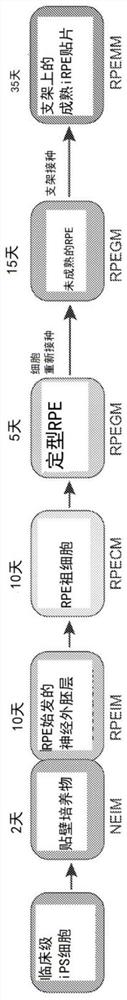
Methods for producing enriched populations of human retinal pigment epithelium cells for treatment of retinal degeneration
ActiveUS7794704B2Quality improvementReduce complexityBiocideSenses disorderMedicineRetinal progenitor
This invention relates to methods for improved cell-based therapies for retinal degeneration and for differentiating human embryonic stem cells and human embryo-derived into retinal pigment epithelium (RPE) cells and other retinal progenitor cells.
Owner:ADVANCED CELL TECH INC
Raav vector compositions and methods for the treatment of choroidal neovascularization
InactiveUS20060193830A1Prevention of variousTreatment of variousBiocideSenses disorderPIGMENT EPITHELIUM-DERIVED FACTORDisease
Disclosed are methods for the use of therapeutic polypeptide-encoding polynucleotides in the creation of transformed host cells and transgenic animals is disclosed. In particular, the use of recombinant adeno-associated viral (rAAV) vector compositions comprising polynucleotide sequences that express one or more mammalian PEDF or anti-angiogenesis polypeptides is described. In particular, the invention provides gene therapy methods for the prevention, long-term treatment and / or amelioration of symptoms of a variety of conditions and disorders in a mammalian eye, including, for example blindness, loss of vision, retinal degeneration, macular degeneration, and related disorders resulting from retinal or choroidal neovascularization in affected individuals.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
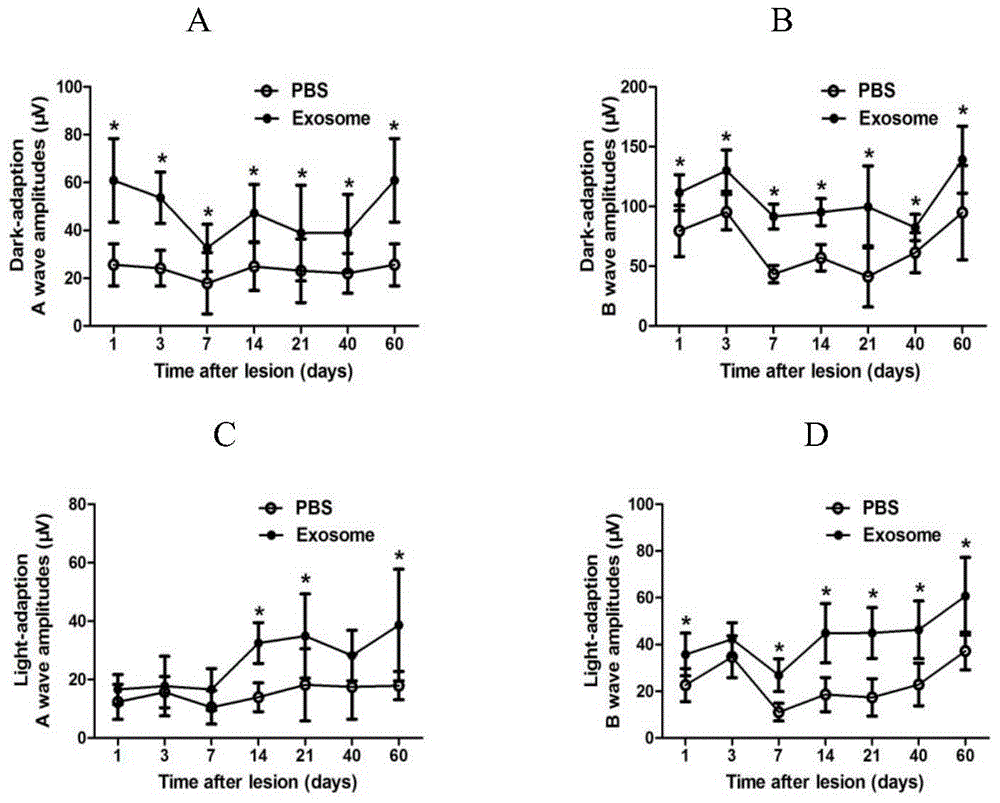
Applications of exosome from mesenchymal stem cells
ActiveCN105267240AReduce retinal damageReduce inflammationSenses disorderUnknown materialsDiseaseUveitis
The invention relates to applications of exosome from mesenchymal stem cells. The concrete applications are applications of exosome from mesenchymal stem cells in preparation of medicines treating ophthalmic diseases. The ophthalmic diseases comprise uveitis, retinal detachment, retinal degeneration, retinal damage caused by various reasons and inflammation. Experiments prove that exosome has nerve nourishing and immunosuppression functions, can relief retina damage caused by various reasons and can reduce inflammatory reactions, therefore retina functions are improved, and a new approach is provided for clinic treatment of ophthalmic diseases.
Owner:天津医科大学眼科医院
Pyrazolopyridazines and methods for treating retinal-degenerative diseases and hearing loss associated with usher syndrome
ActiveUS20140121197A1Useful in treatmentBiocideOrganic chemistryHL - Hearing lossRetinitis pigmentosa
Compounds, compositions and methods for the treatment of retinal degenerative diseases, such as retinitis pigmentosa, Leber's congenital Amaurosis, Syndromic retinal degenerations, age-related macular degeneration and Usher Syndrome, and hearing loss associated with Usher Syndrome are described herein.
Owner:USHER III INITIATIVE
Antioxidant eye drops
ActiveUS9173915B1Reducing oxidative stressReduce oxidative stressBiocideSenses disorderAnti-inflammatoryChemistry
The antioxidant eye drops is a topical ophthalmic formulation that contains four nutraceuticals (namely, astaxanthin, resveratrol, pyruvate and epigallocatechin gallate (EGCG)) having antioxidant, anti-inflammatory, and chelating activity in a carbomer gel carrier. The active ingredients can reduce oxidative stress through free radical scavenging and chelating activity. The carrier is composed of a solution of deionized water containing about 0.4% Carbopol 980 NF, 5.7% sorbitol. 0.01% cetrimide, 0.01% EDTA, and sodium chloride and sodium hydroxide in adequate amounts to adjust both the viscosity and the pH of the topical carrier to pH 7.0-7.4. Use of the formulation may delay the development of cataracts, reduce retinal degeneration, and maintain normal tear flow in dry eye due to the antioxidant, anti-inflammatory, and chelating activity of the active ingredients.
Owner:KADOR PETER F
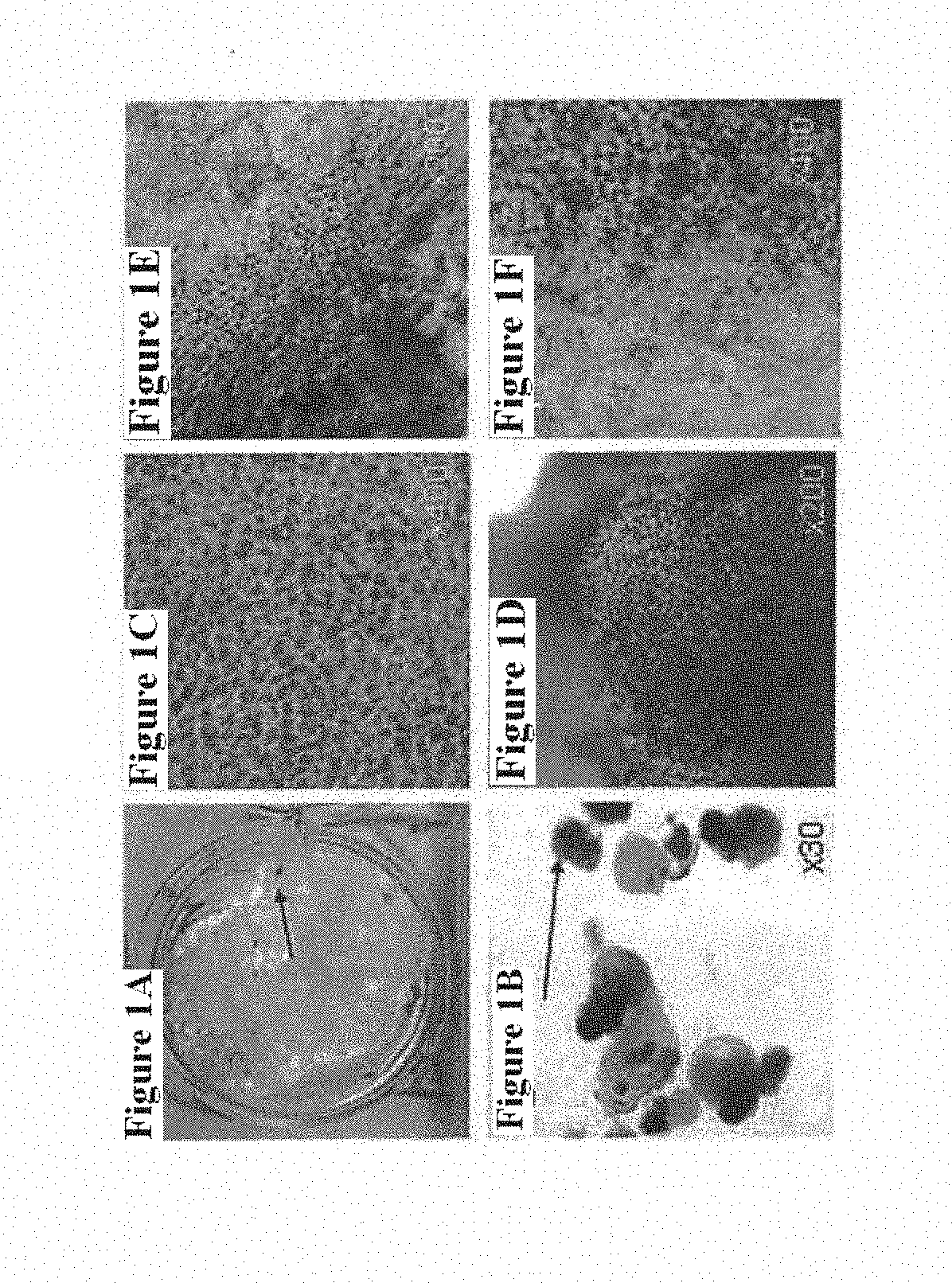
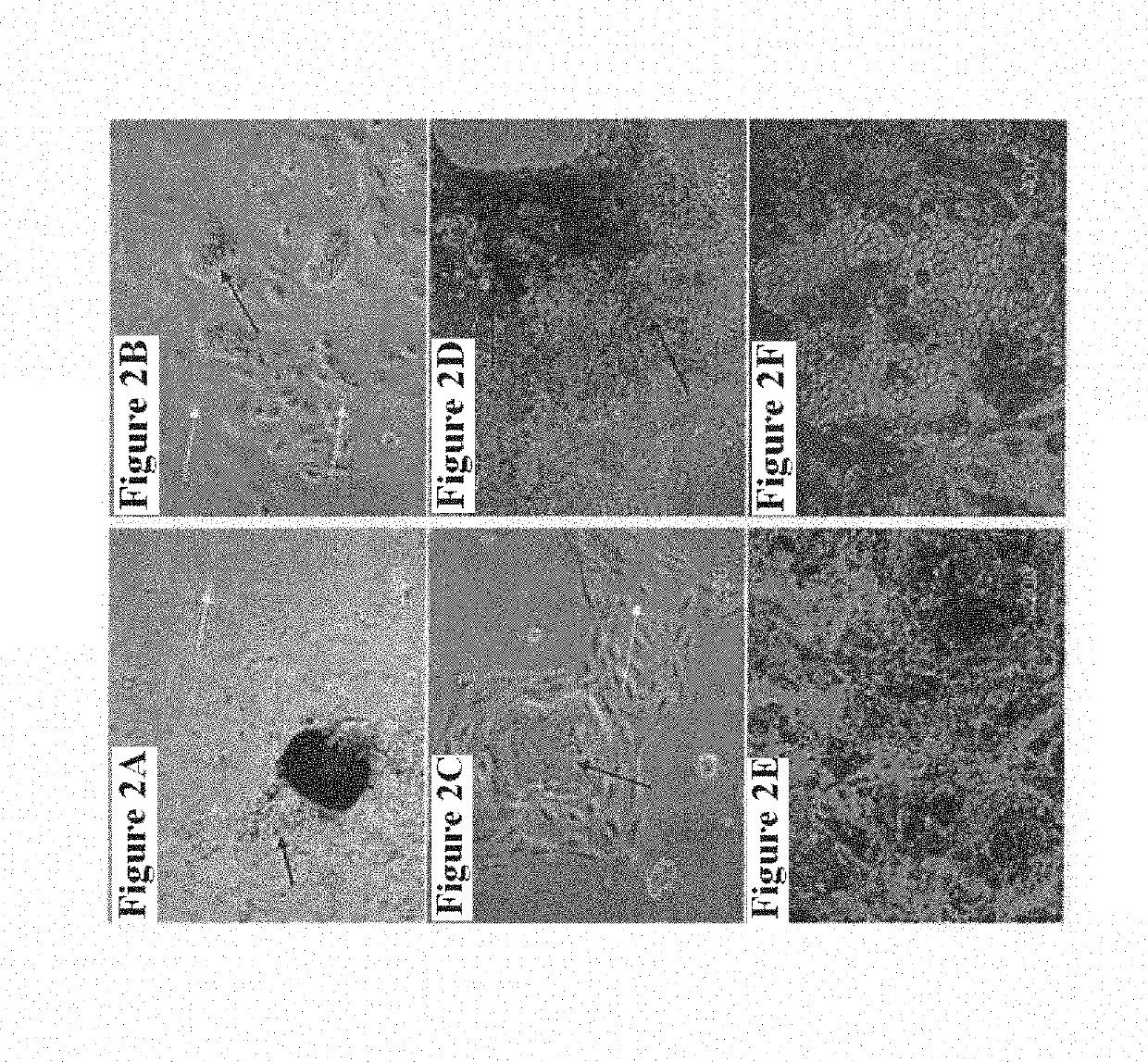
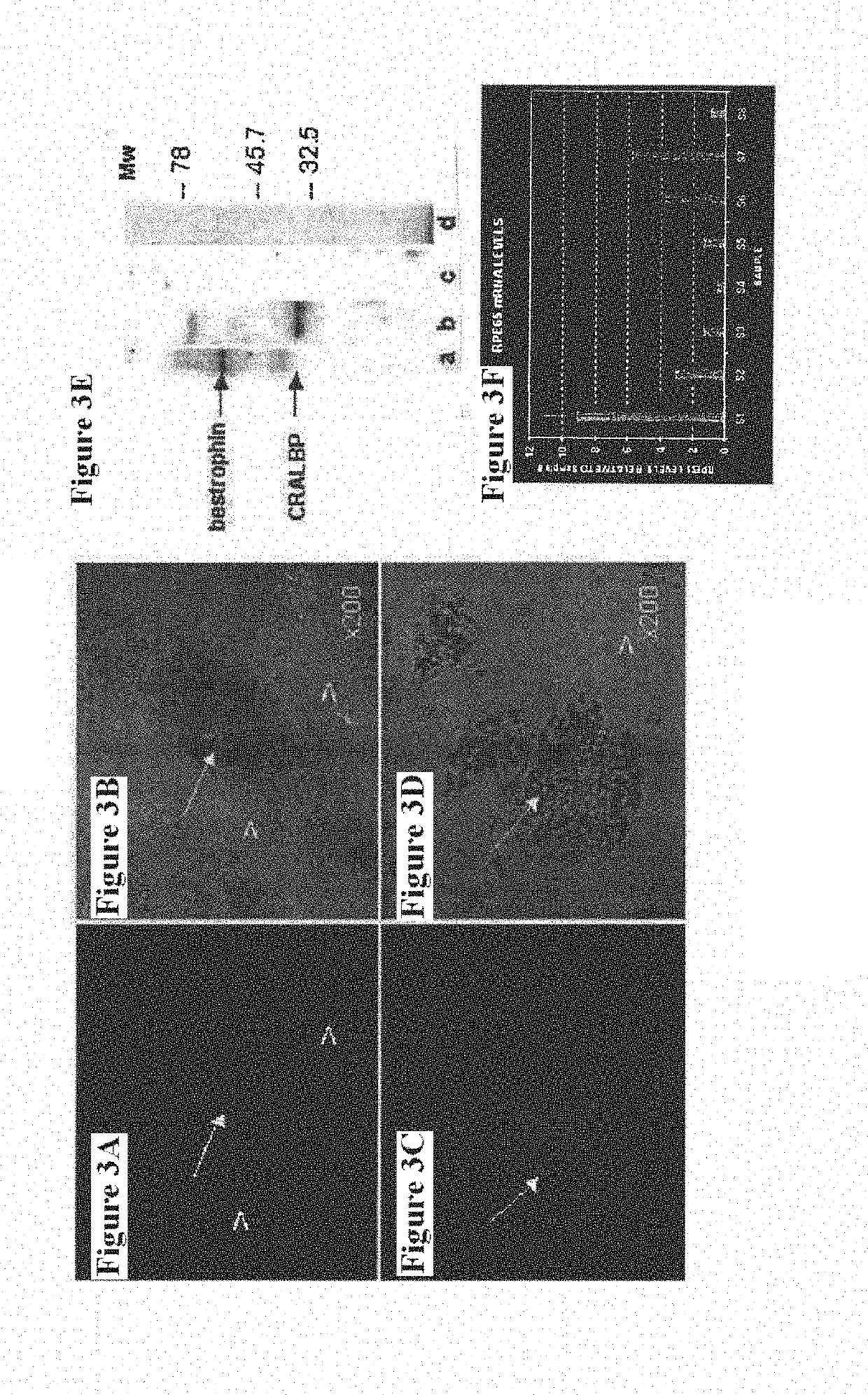
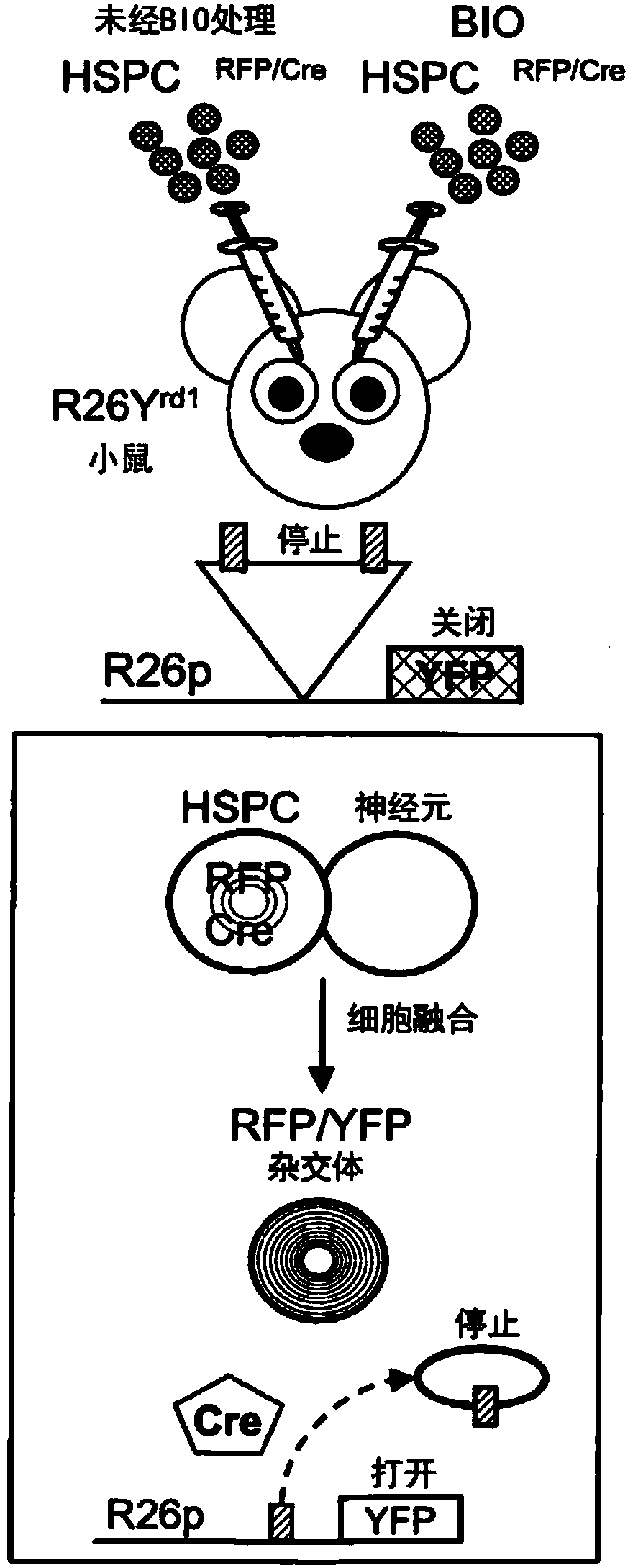
Methods of Treatment of Retinal Degeneration Diseases
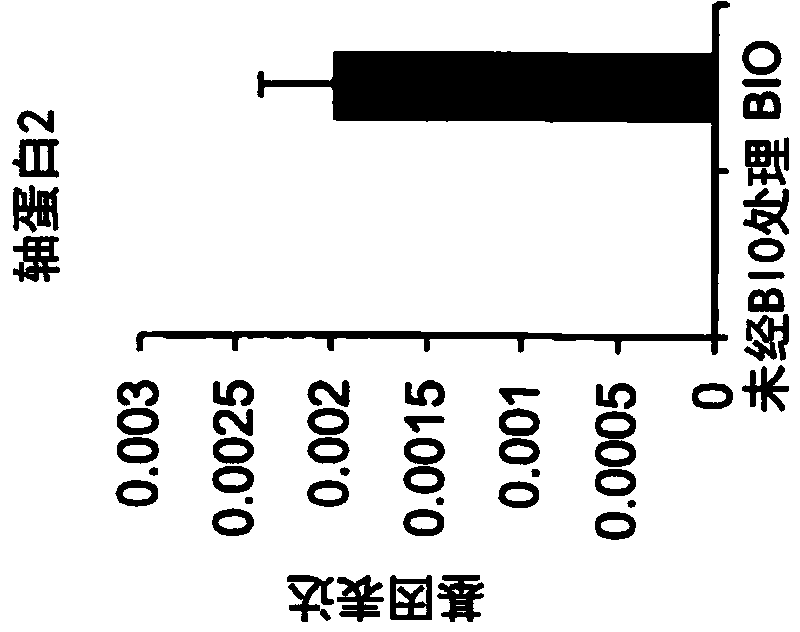
The methods comprise administering cells having properties of stem cells or progenitor cells, to the retina and reprogramming of retinal cells mediated by cell fusion of said cells with said retinal cells, said reprogramming being mediated by activation of the Wnt / β-catenin signalling pathway.
Owner:SANGES DANIELA +1
Analogs of choline for neuroprotection and cognitive enhancement in neurodegenerative disorders
InactiveUS6881738B2Prevent further deteriorationIncrease awarenessBiocideNervous disorderParaneoplastic cerebellar degenerationDementia with Lewy bodies
The present invention relates to novel analogs of choline and methods of use or treatment of neurodegenerative disorders and / or conditions such as Parkinson's disease, Huntington disease, Alzheimer's disease and related disorders such as amyotrophic lateral sclerosis, spinal muscular atrophy, Friedrich's ataxia, Pick's disease, Bassen-Kornzweig syndrome, Refsom's disease, retinal degeneration, Cruetzfelt-Jacob syndrome or prion disease (mad cow disease), dementia with Lewy bodies, schizophrenia, paraneoplastic cerebellar degeneration and neurodegenerative conditions caused by stroke. The present compounds are effective to treat any neurological condition where acetylcholine transmission neurons and their target cells are affected. Compounds according to the present invention are effective to alleviate and / or reverse the effects of a neurodegenerative condition, prevent further deterioration and / or enhance cognition and memory in patients suffering from neurodegenerative disorders, especially Alzheimer's disease.
Owner:AUGUSTA UNIV RES INST INC +1
Method of fast increasing glandular related virus mediating gene in expression of inside of retina cell
This invention relates to the expression method of adeno-associated virus-mediated gene in retinal cells. This invention can significantly improve the transfer efficiency and gene expression level of adeno-associated virus-mediated gene in retinal cells by using a combination of different types of adeno-associated virus, and has no toxic or side effect. Experimental results show that at a certain dosage range, replication-competent adenovirus and replication-defective adenovirus can significantly accelerate AAV2-mediated gene expression, and no cell lesion is observed. This invention can be used for gene treatment of ocular fundus diseases, such as retinosis, maculopathy, diabetic retinopathy, uveitis, choroidal neovascularization, optic nerve degeneration and damage.
Owner:上海交通大学附属第一人民医院
Composition for improving vision and preparation method
InactiveCN102600356AReduce fatigueAvoid harmSenses disorderPlant ingredientsDiseaseRadix Ophiopogonis
The invention relates to a composition for improving vision. The composition is prepared by raw materials consisting of components of butterflybush flower, chrysanthemum, semen cassiae, ginseng, fructus ligustri lucidi, coptis chinensis, radix ophiopogonis, poria cocos and moutan bark. The composition disclosed by the invention can be applied to people which use a computer for a long time, to people which usually drive at night, to people which watch television and use a game machine for a long time, and to people which worry about vision loss simultaneously, and has a remarkable effect on the patients with the diseases such as retinopathy and cataract caused by myopia, hyperopia, presbyopia, retinal degeneration, nyctalopia, macular degeneration, glaucoma, senile cataract and diabetes mellitus. Lots of clinical applications indicate that a purpose of thoroughly radical cure is realized for many patients in a short time, and the cure rate is more than 97.2%.
Owner:李美霖
Modalities for the treatment of degenerative diseases of the retina
InactiveUS20190282622A1Quality improvementReduce complexityPharmaceutical delivery mechanismNervous system cellsRetinal progenitorCell based
This invention relates to methods for improved cell-based therapies for retinal degeneration and for differentiating human embryonic stem cells and human embryo-derived into retinal pigment epithelium (RPE) cells and other retinal progenitor cells.
Owner:ADVANCED CELL TECH INC
Methods Of Treatment Of Retinal Degeneration Diseases
InactiveCN104011203AAchieve regenerationOrganic active ingredientsSenses disorderProgenitorBeta-catenin
The methods comprise administering cells having properties of stem cells or progenitor cells, to the retina and reprogramming of retinal cells mediated by cell fusion of said cells with said retinal cells, said reprogramming being mediated by activation of the Wnt / ss-catenin signalling pathway.
Owner:玛丽亚·皮娅·卡斯马 +1
Use of Inhibitors of Acid Sphingomyelinase to Treat Acquired and Inherited Retinal Degenerations
ActiveUS20150366876A1Efficacy of treatmentLack and slowing of disease progressionBiocideSenses disorderMedicineDisease patient
Owner:WISCONSIN ALUMNI RES FOUND
Aav-mediated gene therapy for rpgr x-linked retinal degeneration
ActiveUS20150202269A1Preventing and arresting progressionPreventing and arresting of and vision lossBiocideSenses disorderGene productNucleic acid sequencing
Described herein are methods of preventing, arresting progression of or ameliorating vision loss and other conditions associated with retinitis pigmentosa and x-linked retinitis pigmentosa in a subject. The methods include administering to said subject an effective concentration of a composition comprising a recombinant adeno-associated virus (AAV) carrying a nucleic acid sequence encoding a normal retinitis pigmentosa GTPase regulator (RPGR gene), or fragment thereof, under the control of regulatory sequences which express the product of the gene in the photoreceptor cells of the subject, and a pharmaceutically acceptable carrier.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Treatment of retinal degeneration using gene therapy
The present invention relates to an improved method of providing photoreceptor function to a cell, for example for use in the treatment of retinal degeneration. The present invention also relates to compositions and kits, in particular for use in such methods.
Owner:UNIV OF MANCHESTER
Modalities for the treatment of degenerative diseases of the retina
InactiveUS20210308187A1Quality improvementReduce complexityPharmaceutical delivery mechanismNervous system cellsAnatomyRetinal pigment epithelial cell
This invention relates to methods for improved cell-based therapies for retinal degeneration and for differentiating human embryonic stem cells and human embryo-derived into retinal pigment epithelium (RPE) cells and other retinal progenitor cells.
Owner:ADVANCED CELL TECH INC
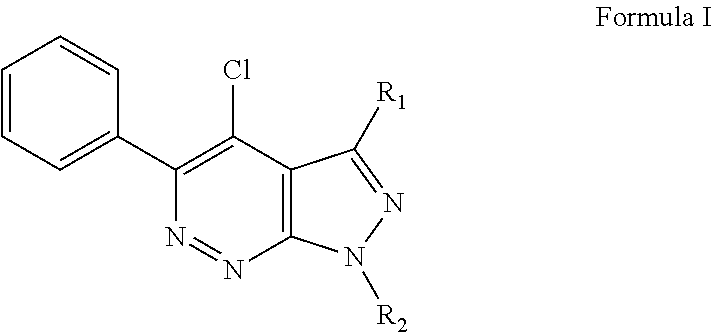
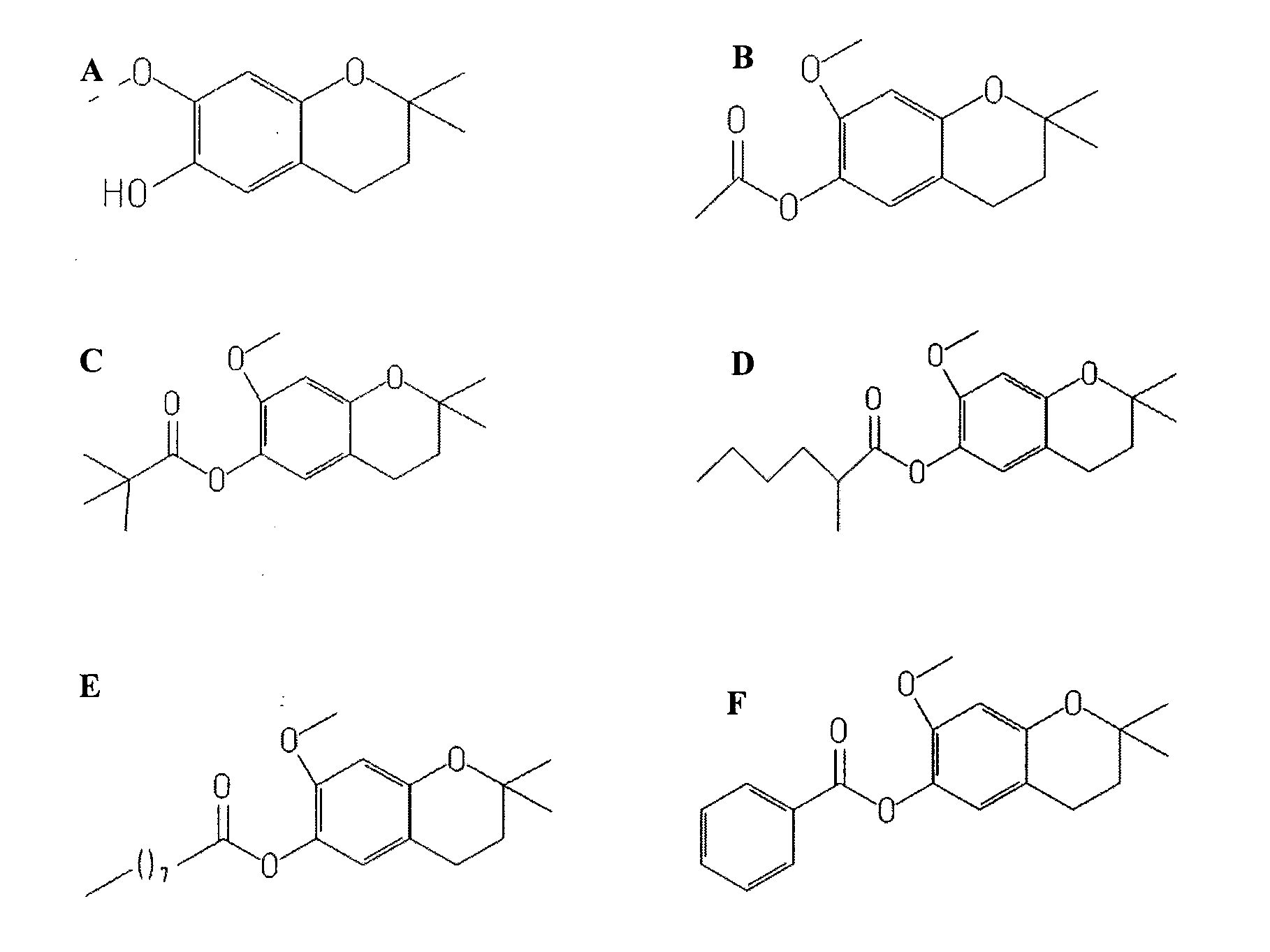
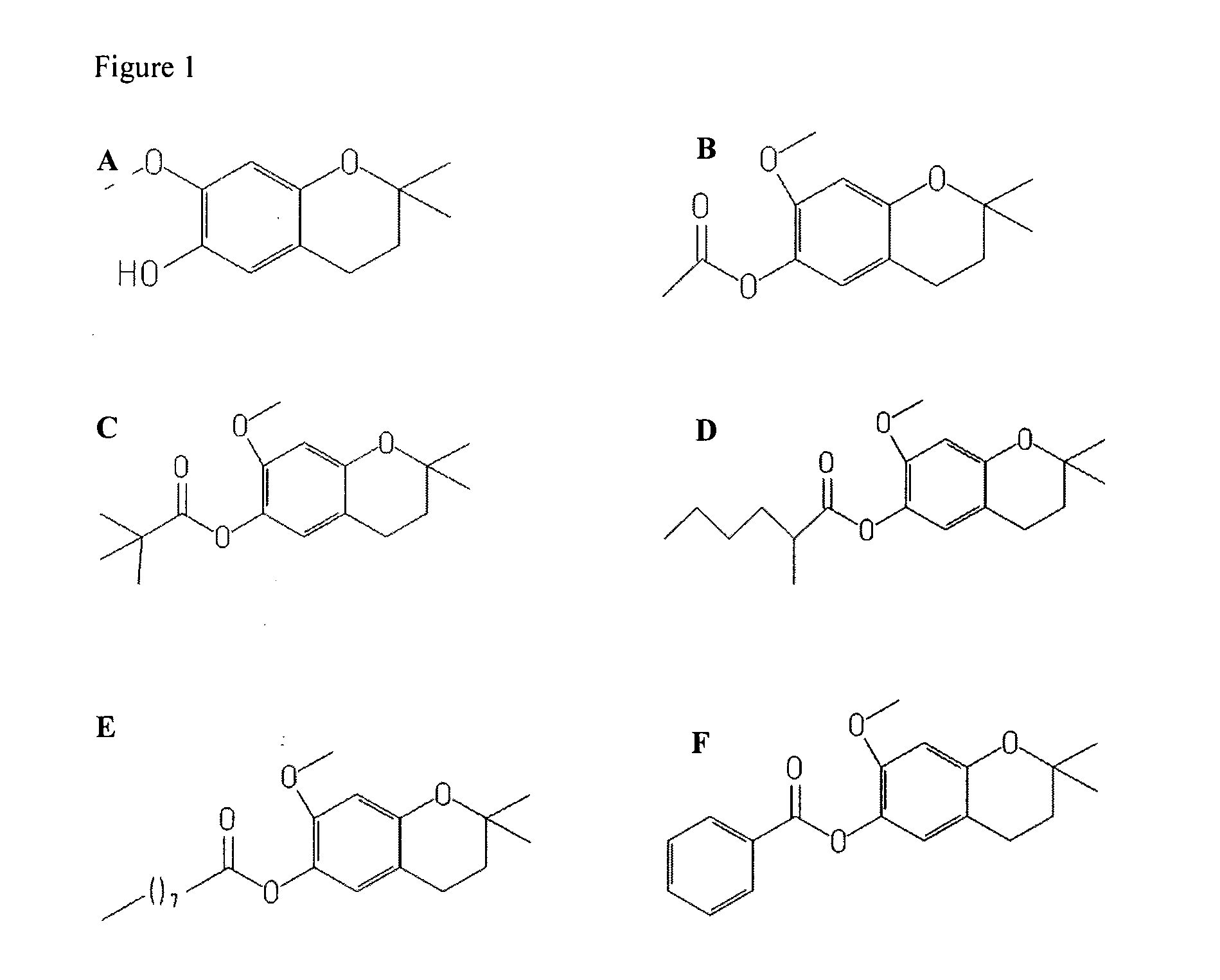
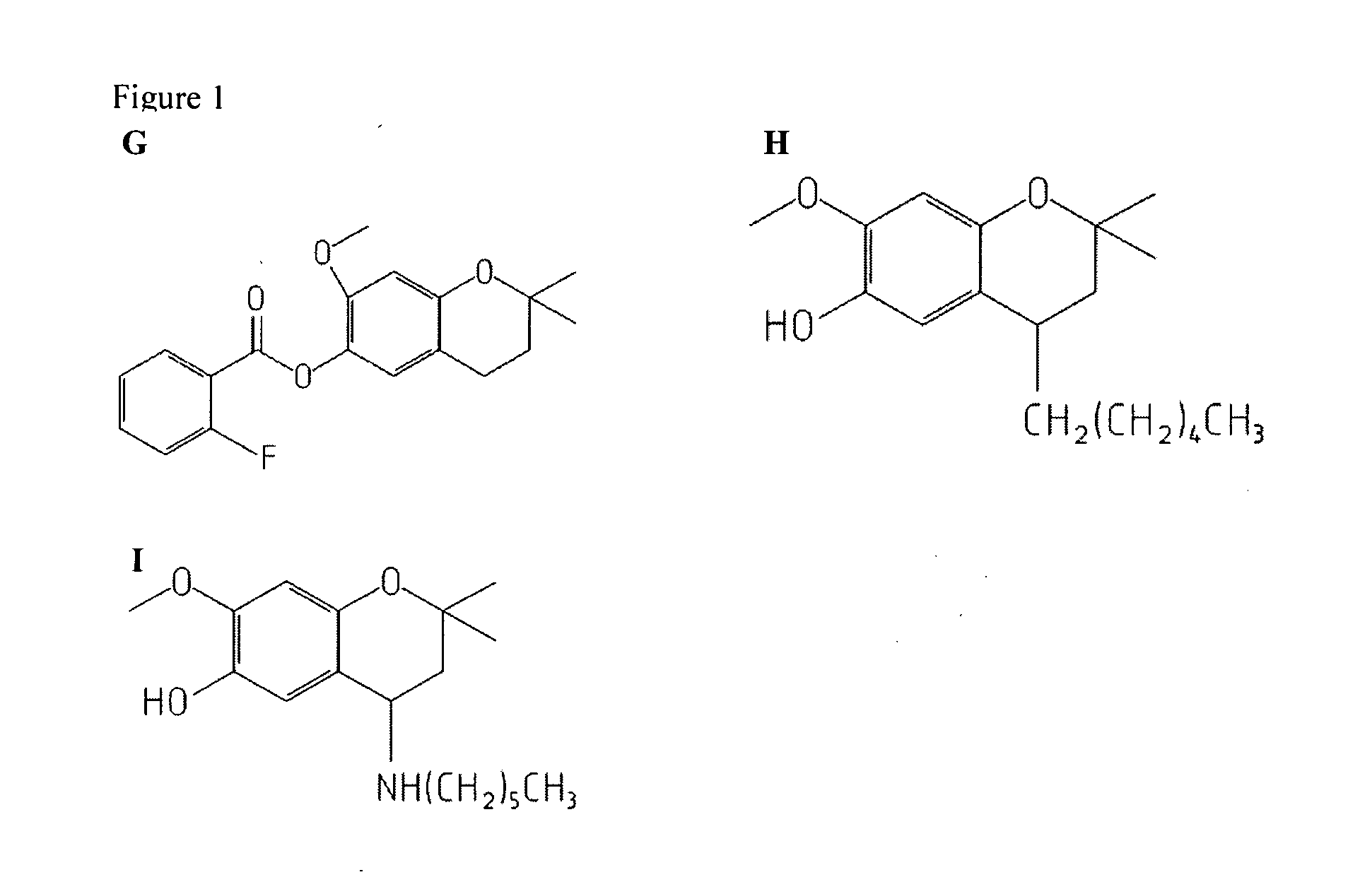
Treatment of retinal degeneration
The use of a compound of general formula (I): or a pharmaceutically acceptable salt thereof, in the manufacture of a medicament for the treatment or prevention of a disease or condition characterised by apoptosis or degeneration of mammalian cells, wherein: R1 is a alkoxy, alkyl, ether or ester group; R2 is H or has the formula wherein Y is linear or branched, saturated or unsaturated, aliphatic group with from 2 to 23 carbon atoms, or a cyclic group, and which can contain substituents selected from the group consisting of hydroxyl, alkoxy, amino, carboxyl, cyano, nitro, alkylsuphonyl or halogen atoms, X is O or S; and R3 is any substituent.
Owner:UNIV COLLEGE CORK NAT UNIV OF IRELAND CORK
Compositions and methods for restoring or preventing loss of vision caused by disease or traumatic injury
PendingCN111511377AOrganic active ingredientsSenses disorderRetinal ganglionRetinal pigment epithelium cell
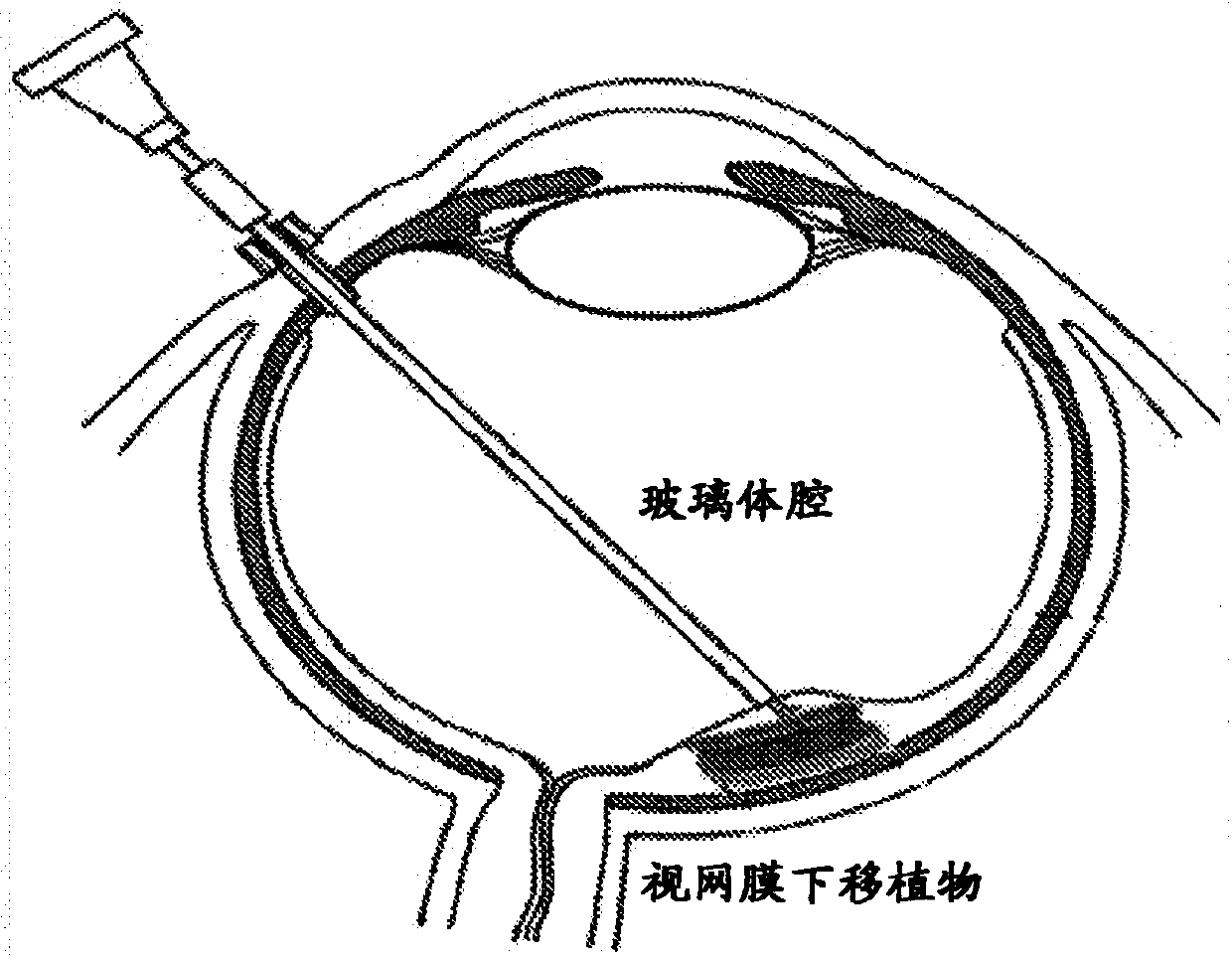
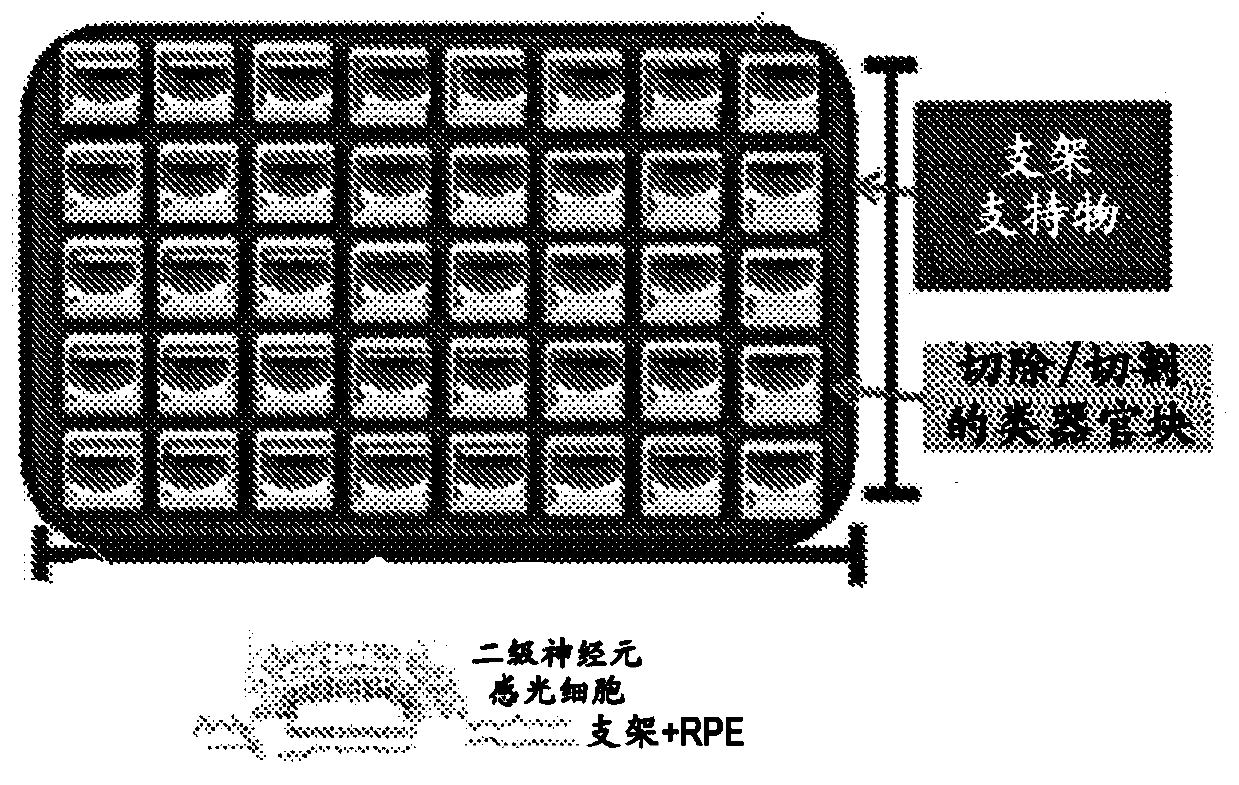
Bioprosthetic retinal grafts (or devices) comprising stem cell derived tissues and / or cells may be used to slow the progression of retinal degenerative disease, slow the progression of retinal degenerative disease after traumatic injury, slow the progression of age related macular degeneration (AMD), prevent retinal degenerative disease, prevent retinal degenerative disease after traumatic injury,prevent AMD, restore retinal pigment epithelium (RPE), photoreceptor cells (PRCs) and retinal ganglion cells (RGCs) lost from disease, injury or genetic abnormalities, increasing RPE, PRCs and RCGs,treat RPE, PRCs and RCG defects in a subject, or for other purposes. Bioprosthetic retinal grafts may comprise a bioprosthetic carrier or scaffold suitable for implantation into the ocular space of asubject's eye, to form a bioprosthetic retinal patch. In certain embodiments, the bioprosthetic retinal patch may comprise multiple pieces of stem cell derived tissues or cells on a carrier or scaffold, which may be used to treat large areas of retinal degeneration or damage, or for other purposes.
Owner:LINEAGE CELL THERAPEUTICS INC
Method for differentiation into retinal cells from stem cells
Disclosed is a method for inducing stem cells to differentiate into retinal cells at high yield within a short period of time, without gene implantation and co-culture with retinal tissues, by implementing a differentiation process similar to the in vivo embryonic development in chemically defined conditions. Also, retinal cells including the photoreceptor cells and their progenitor cells, and various types of other retinal cells, generated according to the method, are disclosed. A composition comprising the retinal cells and a method are provided for treating retinal degeneration-related diseases. The differentiated photoreceptor cells, when transplanted into degenerated or injured retinas, can be engrafted and fused within the retinas to prevent or cure retinal degeneration.
Owner:CLAVISTHERAPEUTICS INC
Druggable target to treat retinal degeneration
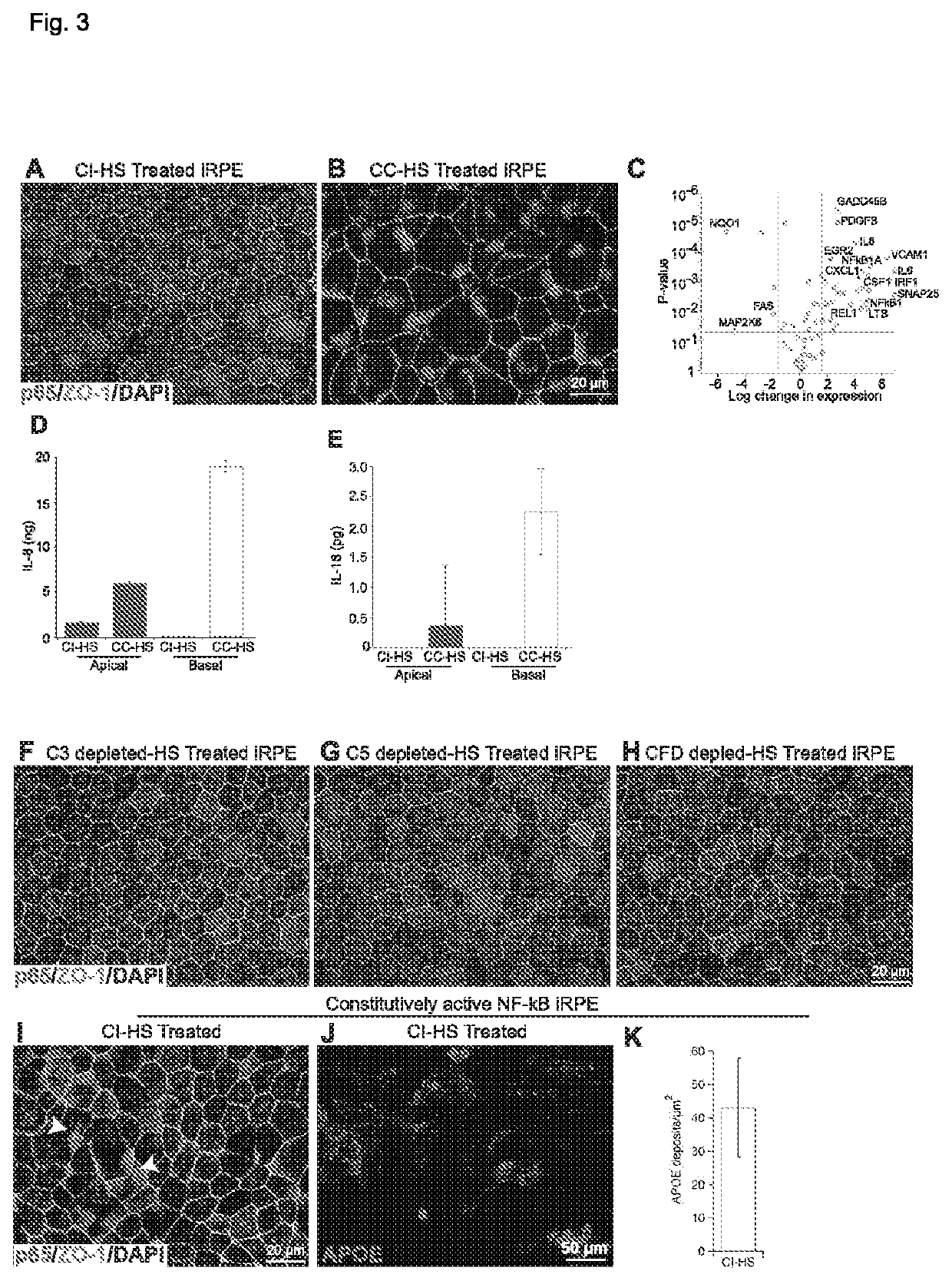
This invention relates to novel method of treating or ameliorating a retinal disease or disorder or retinal degradation in a subject and a novel method of restoring retinal pigment epithelium cell compromising the administration of a one or more compounds which modulate Nox4, formation of radical oxygen species, serine protease, a dopamine receptor, NF-kB, mTOR, AMPK, RPE epithelial to mesenchymal transition, RPE dedifferentiation, or one or more Rho GTPases; and kits for administration of the methods.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
1,7-naphthyridine derivatives
The present invention relates to compounds of general formula I wherein R1, R2, R3 and R4 are as defined herein which maybe used for the treatment of schizophrenia, obsessive-compulsivepersonality disorder, major depression, bipolar disorders, anxiety disorders, normal aging, epilepsy, retinal degeneration, traumatic brain injury, spinal cord injury, post-traumatic stress disorder, panic disorder, Parkinson's disease, dementia, Alzheimer's disease, cognitive impairment, chemotherapy-induced cognitive dysfunction (“chemobrain”), Down syndrome, autism spectrum disorders, hearing loss, tinnitus, spinocerebellar ataxia, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, stroke, and disturbances due to radiation therapy, chronic stress, optic neuropathy or macular degeneration, or abuse of neuro-active drugs, selected from alcohol, opiates, methamphetamine, phencyclidine and cocaine.
Owner:F HOFFMANN LA ROCHE & CO AG
Retinochrome degeneration macaque model construction method based on in-vivo gene knockout
InactiveCN111850044AHigh simulationAvoid expensive feesStable introduction of DNANucleic acid vectorDiseaseMacaque
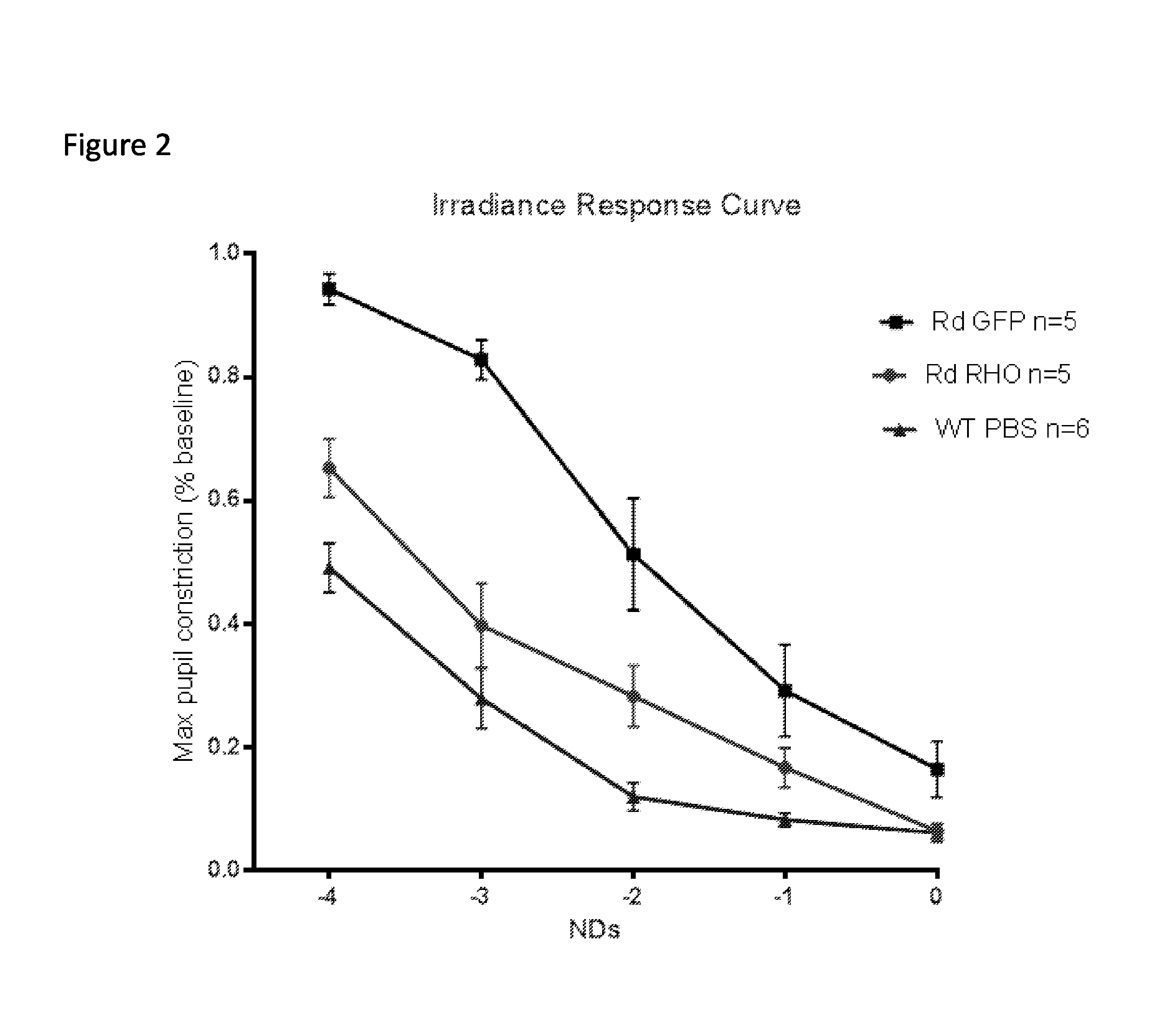
According to the invention, a CRISPR gene editing system and an AAV transduction system are utilized to directly knock out an RHO gene from adult macaque retinal photoreceptor cells so as to simulateretinal degeneration degeneration caused by hereditary RHO mutation, and a macaque retinal pigment degeneration (RP) animal model is constructed within 3-6 months. The model can promote research and treatment of human RP diseases.
Owner:UNIV OF SCI & TECH OF CHINA
Use of inhibitors of acid sphingomyelinase to treat acquired and inherited retinal degenerations
Owner:WISCONSIN ALUMNI RES FOUND
Animal model for the retinal degeneration by targeted pde6b gene mutation and the preparation method thereof
ActiveUS20210144976A1Stably achievedIncreasing the thicknessCompounds screening/testingVector-based foreign material introductionGenes mutationDisease
The present invention relates to a Pde6b-deficient animal model of retinal degeneration produced by engineered endonucleases, and a method for producing the same. In the animal model of retinal degeneration according to the present invention, only a specific target gene can be removed using engineered endonucleases, so that mutagenesis can be stably achieved. In addition, it is possible to produce a congenital animal model through genetic manipulation at the embryonic stage rather than through acquired factors, which allows for production of an animal model that uniformly exhibits symptoms of the disease in question without being influenced by other factors.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION +1
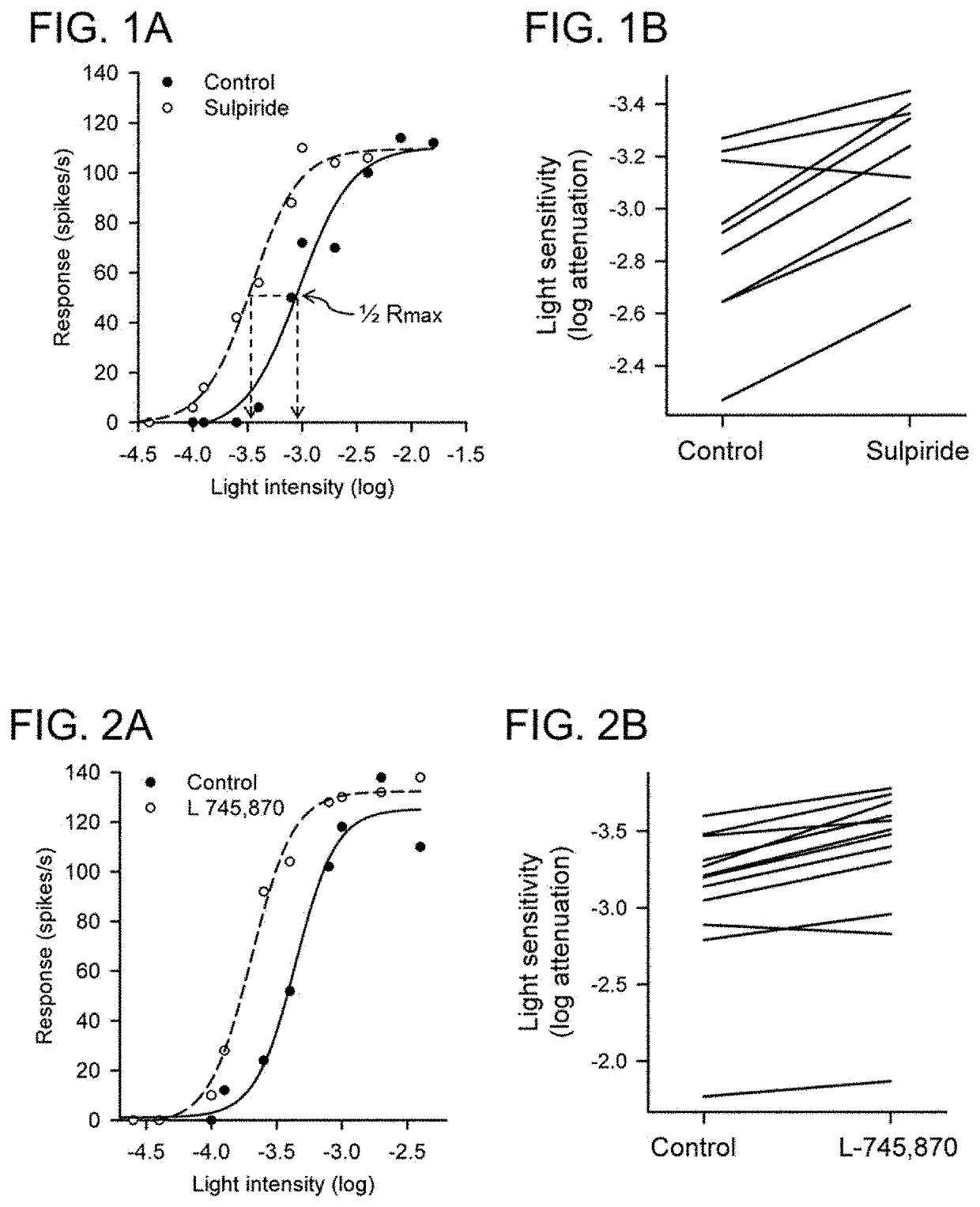
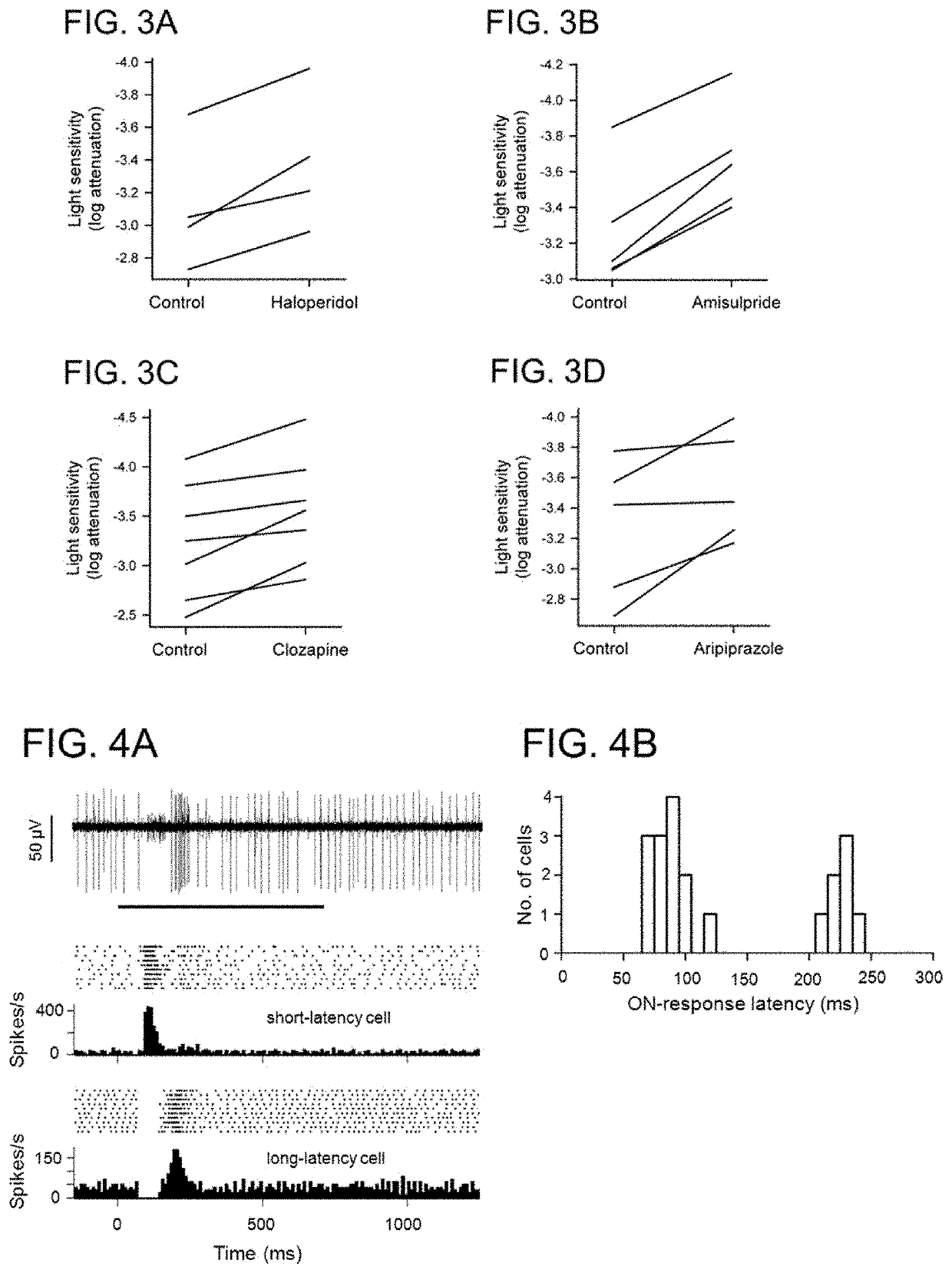
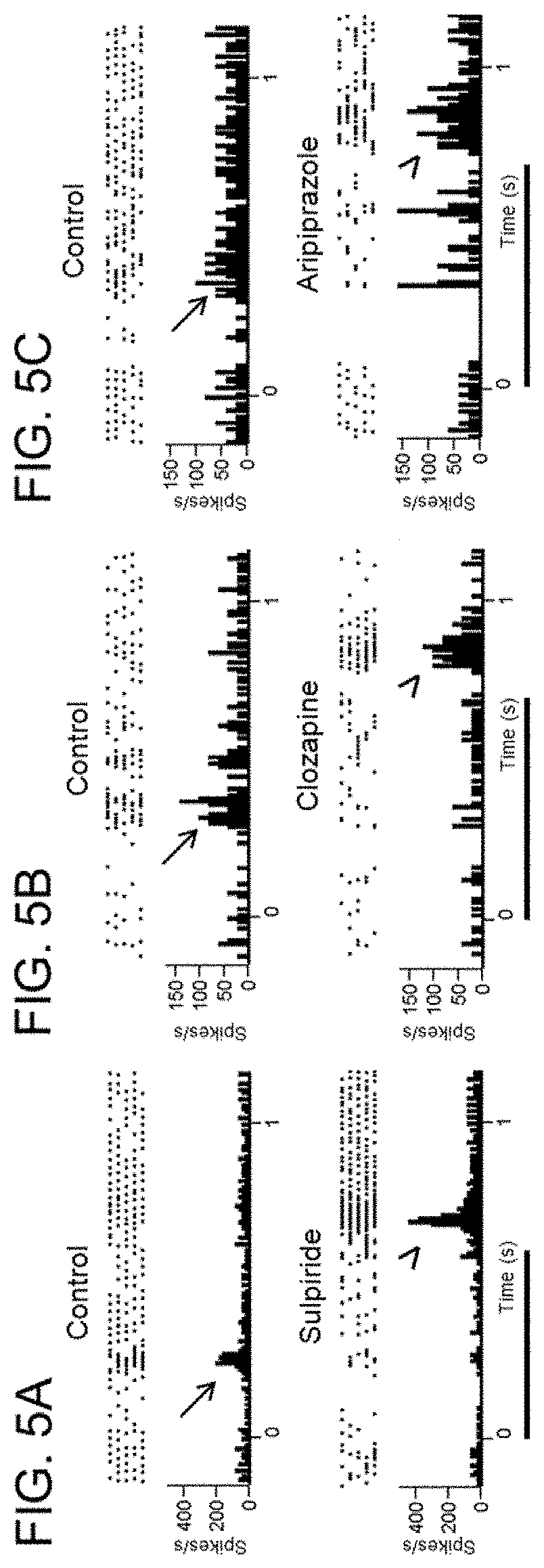
Use of dopamine and serotonin receptor antagonists for treatment in a subject with retinal degeneration
ActiveUS11033554B2Pharmaceutical delivery mechanismHeterocyclic compound active ingredientsSerotoninPharmaceutical drug
Disclosed herein are methods of treating disorders of the retina (e.g., macular degeneration, retinitis pigmentosa, etc.) comprising administering to a subject in need of such treatment a therapeutically effective amount of a compound (for example, an antipsychotic drug) that blocks or diminishes agonist-mediated responses upon binding to either dopamine D2-like receptors or serotonin 5-HT2 receptors.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
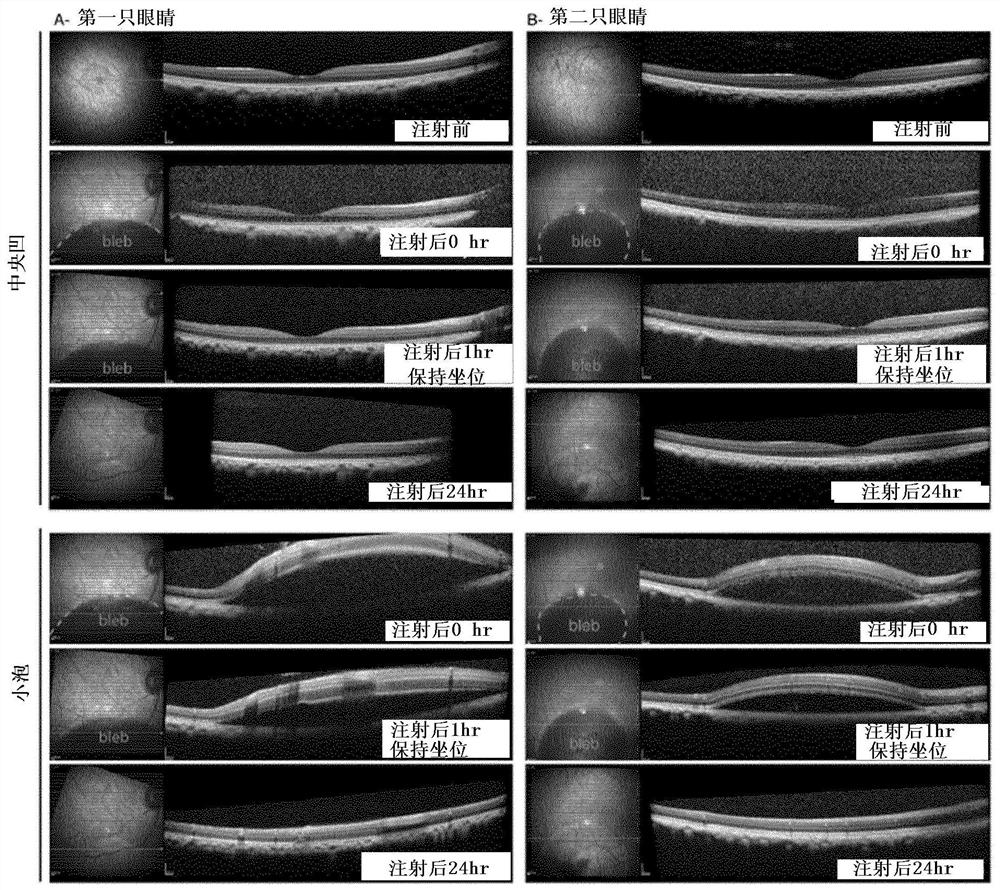
Methods of expressing a polynucleotide of interest in the cone photoreceptors of a subject comprising the subretinal delivery of a therapeutically effective amount of a recombinant aav9-derived vector
Intraocular injection of adeno-associated viral (AAV) vectors has been an evident route for delivering gene drugs into the retina. Currently, the vectors need to be injected into the subretinal spacein order to provide gene delivery to cones. In this approach, gene delivery is limited to cells that contact the local "bleb" of injected fluid. Furthermore, retinal detachment that occurs during subretinal injections is a concern in eyes with retinal degeneration. Here, the inventors establish several new vector-promoter combinations to overcome the limitations associated with AAV-mediated cone transduction in the fovea with supporting studies in mouse models, human induced pluripotent stem cell-derived organoids, post-mortem human retinal explants and living macaques. They show that an AAV9variant provides efficient foveal cone transduction when injected into the subretinal space several millimeters away from the fovea, without detaching this delicate region. The delivery modality relies on a cone-specific promoter and result in high-level transgene expression compatible with optogenetic vision restoration. Accordingly, the present invention relates to method of expressing a polynucleotide of interest in the cone photoreceptors of a subject comprising subretinal delivery of a therapeutically effective amount of a recombinant AAV9-derived vector comprising a VP1 capsid protein asset forth in SEQ ID NO: 11 and the polynucleotide of interest under the control of the pR1.7 promoter as set forth in SEQ ID NO: 12.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS +1
Biodegradable tissue replacement implant and its use
Owner:UNITED STATES OF AMERICA
NOX2 gene defect rd1 mouse model as well as preparation method and application thereof
PendingCN114532296ALoss delayInhibition of activationCompounds screening/testingAnimal husbandryGlial activationKnockout mouse
The invention relates to an NOX2 gene defect rd1 mouse model as well as a preparation method and application thereof, and belongs to the technical field of biology. The invention provides a method for preparing an NOX2 gene defect rd1 mouse model, which comprises the following steps: hybridizing and screening an NOX2 gene knockout mouse and an rd1 mouse to obtain the NOX2 gene defect rd1 mouse model. The loss of retinal outer nuclear layer photoreceptor cells of the NOX2 gene defect rd1 mouse model prepared by using the method is obviously delayed, the activation of microglia cells is obviously inhibited, and the expression of NOX2 in the microglia cells is obviously reduced, so that the NOX2 activation in the microglia cells is further verified to be a pathogenic mechanism of hereditary retinal degeneration; in addition, the invention provides a basis for screening and researching and developing medicines for treating and / or preventing retinal degeneration, which are clearer in action mechanism, stronger in specificity and better in market and clinical application prospect, and has a great application prospect.
Owner:BEIJING TONGREN HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV +1
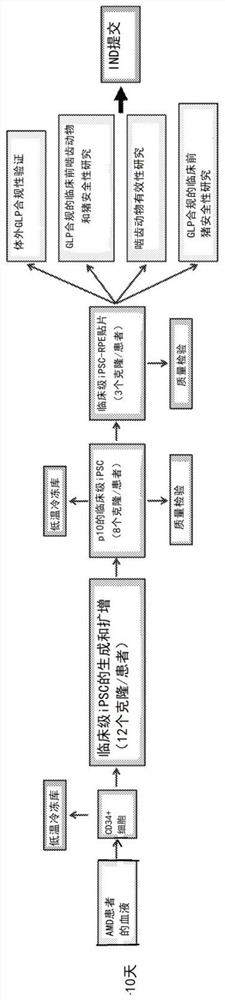
Assays for cell-based therapies or treatments
The present disclosure provides in vitro methods for determining the potency of a cell-based therapy or treatment. In alternative embodiments, provided are compositions, including products of manufacture and kits, and methods, comprising (or comprising use of) quantitative in vitro assays for determining the potency of cell-based therapies or treatments, including those used in the treatment of retinal degeneration.
Owner:RGT UNIV OF CALIFORNIA
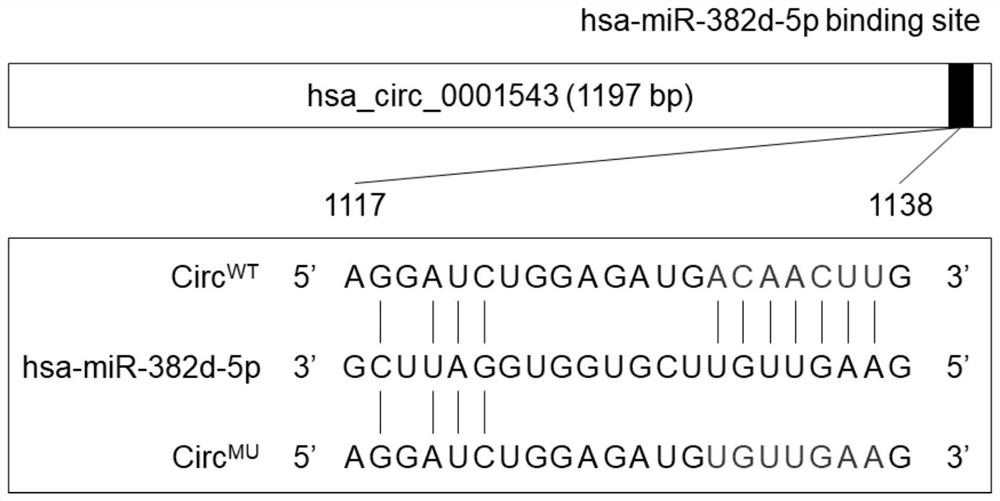
Application of hsa-mir-382-5p in preparation of kits for diagnosing retinal degenerative diseases
ActiveCN110066870BAchieve early diagnosisSenses disorderMicrobiological testing/measurementAids diagnosticsPharmaceutical drug
The invention discloses the application of hsa-miR-382-5p in preparing a kit for diagnosing retinal degenerative diseases. A diagnostic kit for retinal degenerative diseases, comprising a reagent for detecting the expression level of hsa-miR-382-5p. Application of a substance inhibiting the expression of hsa-miR-382-5p in the preparation of a drug for treating retinal degenerative diseases. The study found that the increased expression of hsa-miR-382-5p can be used as an auxiliary diagnosis basis for retinal degenerative diseases and help realize the early diagnosis of retinal degenerative diseases. The damaging effect of hsa-miR-382-5p on the retina suggests that it can be used to prepare and screen drugs for treating retinal degenerative diseases. The invention provides a new detection marker and therapeutic target for the retinal degenerative disease, and provides brand new molecular information and biological basis for the diagnosis and treatment of the disease.
Owner:JIANGSU PROVINCE HOSPITAL THE FIRST AFFILIATED HOSPITAL WITH NANJING MEDICAL UNIV
Ffa1 (GPR40) as a therapeutic target for neural angiogenesis diseases or disorders
ActiveUS20210322414A1Effect be exertIncreasing glucose entry into the retinaSenses disorderMetabolism disorderVascular diseaseNeurophysins
The instant invention provides methods and compositions related to discovery of Free Fatty Acid Receptor 1 (FFA1) as a therapeutic target for treatment or prevention of diseases or disorders of neurons that are characterized by angiogenesis, or of vascular diseases of the eye, retinal degeneration and / or tumors more generally. Therapeutic and / or prophylactic uses and compositions of known FFA1 inhibitors, including small molecules and nucleic acid agents, are described. Methods for identification of novel FFA1 inhibitors are also provided.
Owner:CHILDRENS MEDICAL CENT CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com