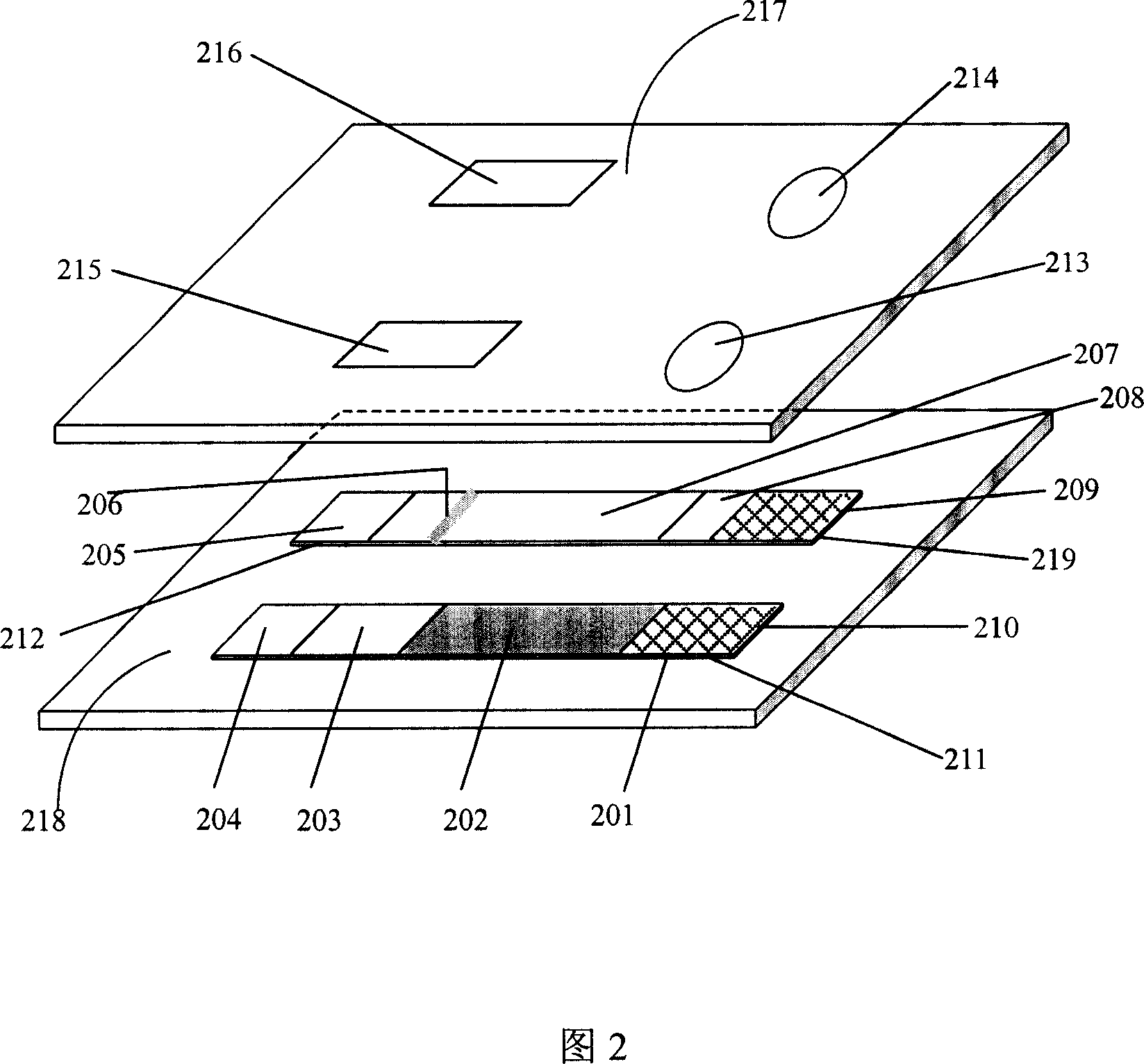
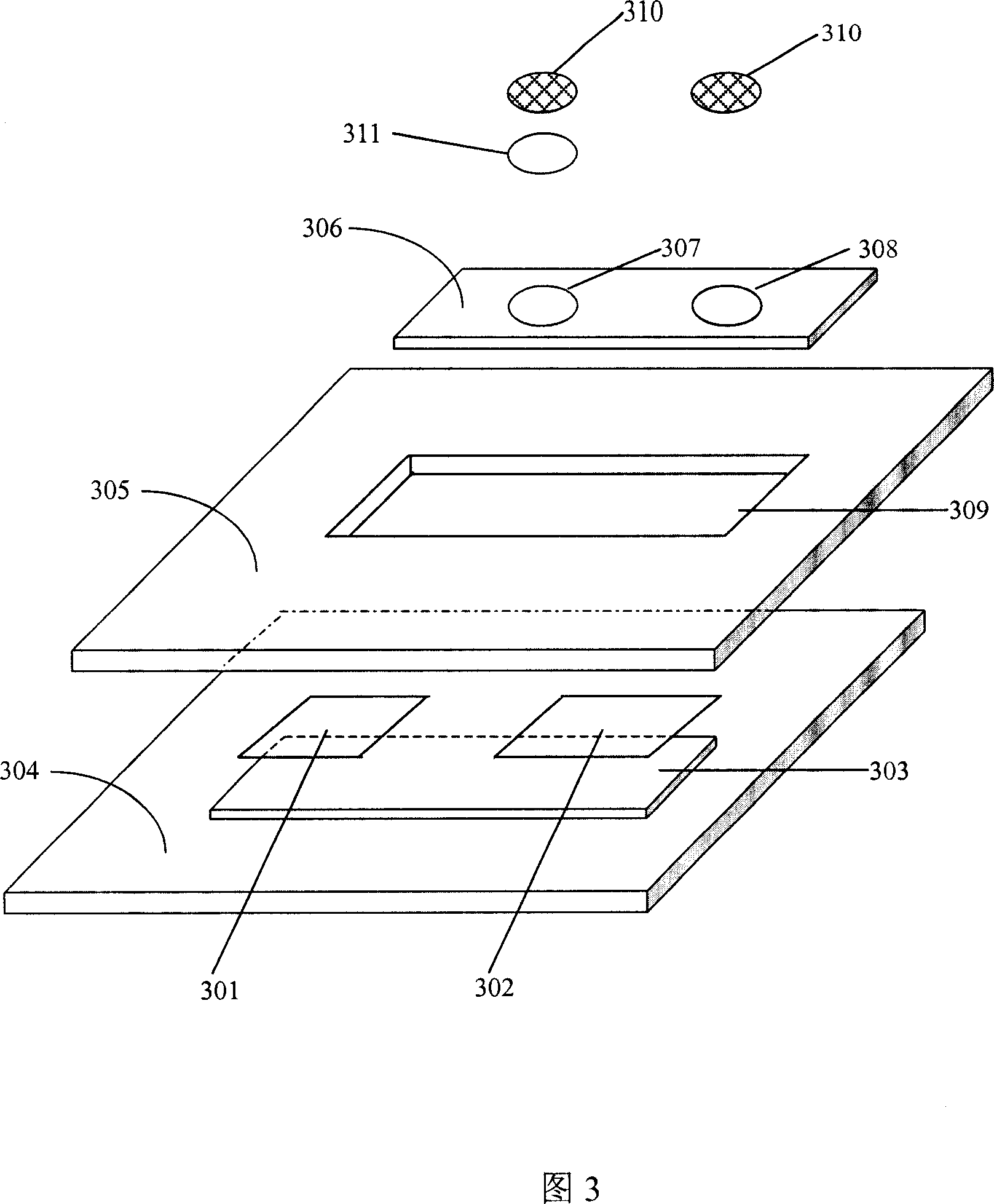
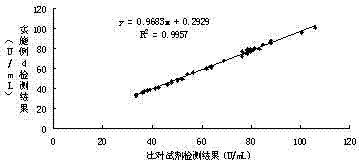
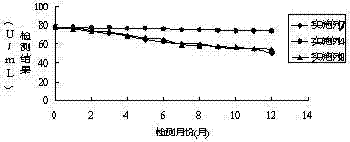
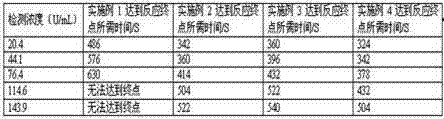
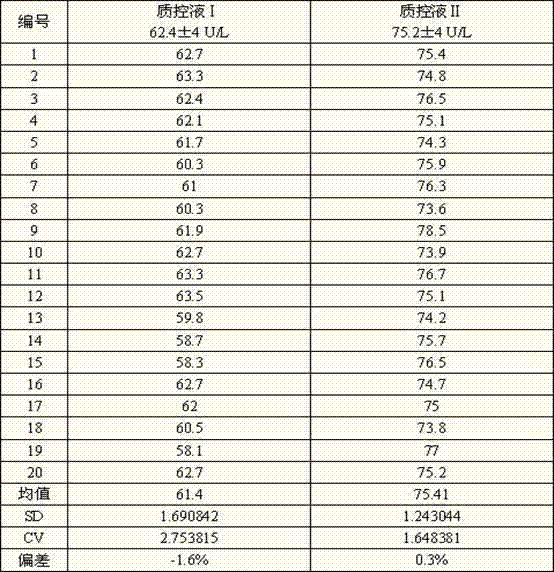
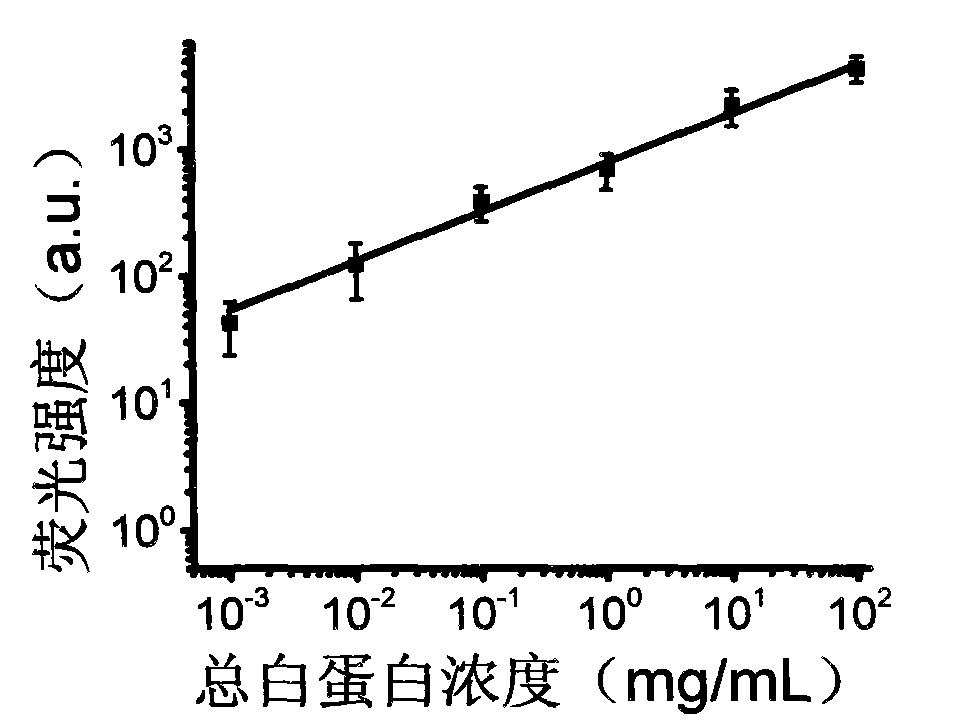
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Ischemia-modified albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The IMA (Ischemia-Modified Albumin) is used in the context of myocardial ischemia, which occurs during a heart attack. During a heart attack there is a decreased supply of oxygen to the heart resulting in biochemical characteristics of albumin protein molecules.
Reagent casing for detecting blood-lacking modification albumin and method thereof
InactiveCN101013137AStrong specificityOD value is trueMaterial analysis by observing effect on chemical indicatorBiological material analysisUltrafiltrationFiltration
It is a detecting ischemia modified albumin (IMA) reagent kit and detection method, and the reagent kit contains different division to remove the background interference material device, which can be centrifugal ultrafiltration IMA detection reagent kit, immunomagnetic beads IMA detection reagent kit, immunochromatography IMA detection reagent kit, immune chromatography membrane IMA detection board reagent kit or immune membrane filtration plate IMA detection reagent kit. After division removal of the background interference material, it can enhance the specific of detection IMA reagent kit and detection method, and using this reagent kit and hospital existing equipments, in about half an hour, it can measure the IMA value to diagnosis myocardial ischemia symptoms. Particularly suitable for bedside rapid detection kit development, ease to use, low cost, it is a very development promising product.
Owner:贺坚慧
Detection kit for ischemia modified albumin
ActiveCN103760357AImprove binding efficiencyGuaranteed accuracyDisease diagnosisColor/spectral properties measurementsNormal albuminMedicine
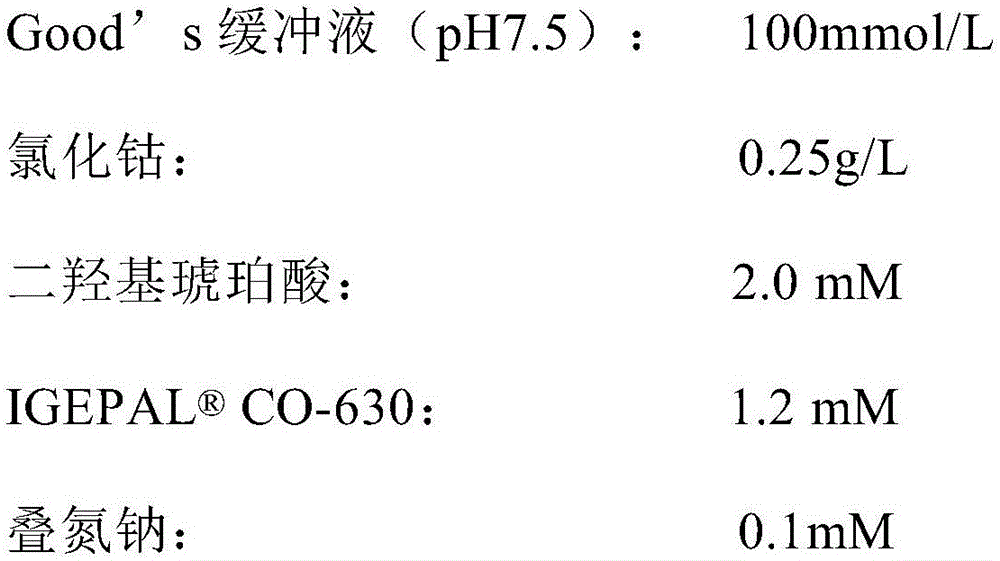
The invention relates to the technical field of detection of ischemia modified albumin content, and in particular to a detection kit for ischemia modified albumin. The kit includes a reagent 1 and a reagent 2. The reagent 1 contains a buffer, cobalt chloride, a stabilizer and a preservative; and the reagent 2 contains a buffer, dithiothreitol, a stabilizer, a reducing protective agent and a preservative. The kit can accelerate the binding efficiency of cobalt ions with normal albumin, and the cobalt ions fully integrate with normal albumin in a certain period of time, so as to ensure the accuracy of the detection result on a sample by the reagent; the kit protects the stability of dithiothreitol in the solution, and does not affect the binding of dithiothreitol with cobalt ions, so as to ensure that an open reagent bottle can be stably kept in a dark place for 30 days at room temperature and a closed reagent bottle can be stably kept at 2-8 DEG C for 12 months, and fully meet the needs of clinical laboratory; and the kit accelerates the combination of dithiothreitol with cobalt ions, so that the reagents reach reaction endpoints as soon as possible, thereby ensuring the efficient detection effect of reagents and significantly improving the accuracy of reagent detection.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Kit for detecting ischemia modified albumin and method for making same
InactiveCN101458257ARapid responseGuaranteed stabilityBiological testingIschemia-modified albuminSURFACTANT BLEND
The invention discloses a kit for detecting ischemia modified albumin. The kit consists of a reagent 1 and a reagent 2. A surfactant is added to accelerate the reaction of the ischemia modified albumin; and a protective agent is selected to ensure the stability of the reagents. The kit has the better clinical application prospect. The invention further provides a preparation method of the kit.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Ischemia modified albumin liquid stabilization kit
ActiveCN102507916AImprove stabilityAvoid interferenceBiological testingIschemia-modified albuminMedicinal chemistry
The invention discloses an ischemia modified albumin liquid stabilization kit. The kit is a liquid dual reagent of a reagent 1 and a reagent 2, wherein the reagent 1 comprises a buffer solution of which the pH is 7.5 to 8.5 and the concentration is 0.05 to 0.5M, cobalt chloride at the concentration of 20 to 200mg / L, an interference removing agent at the concentration of 0.1 to 10g / L, a stabilizing agent at the concentration of 0.1 to 10g / L, and preservative at the concentration of 0.1 to 10g / L; and the reagent 2 comprises a buffer solution of which the pH is 7.0-8.0 and the concentration is 0.5 to 10M, dithiothreitol at the concentration of 0.5 to 50g / L, a stabilizing agent at the concentration of 0.1 to 10g / L, and preservative at the concentration of 0.1 to 10g / L. The kit has the advantages of fully meeting the clinical inspection requirement, along with high accuracy and stability, and can be stabilized for 12 months when stored at the temperature of between 2 and 8 DEG C in a dark place.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Stable ischemia modified albumin testing kit
ActiveCN102147416AImprove stabilityReduce the binding forceColor/spectral properties measurementsBiological testingRoom temperatureOxygen
The invention discloses a stable ischemia modified albumin testing kit which comprises a cobalt ion reagent and a dithiothreitol (DTT) reagent. A small amount of nonionic surfactant is added in the dithiothreitol reagent, thus the combination of DTT in the reagent and free oxygen is greatly reduced, the oxidation speed of DTT is reduced, and the stability of the reagent is remarkably improved. Simultaneously, the concentration of DTT is increased, thus the stability of the kit is improved to a certain extent. The stable ischemia modified albumin testing kit disclosed by the invention can be stored stably in an opened bottle in a dark place at the room temperature for 30 days, and also can be stored stably in a closed bottle at the temperature of 2 to 8 DEG C for 12 months, and can totally meet the requirements for clinical examinations.
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
Reagent kit for detecting mouse ischemia modified albumin (IMA) by red light and detection method thereof
The invention discloses a reagent kit for detecting mouse ischemia modified albumin (IMA) by red light and a detection method thereof. The reagent kit consists of a divalent metal ion cobalt, zinc ornickel solution as a first reagent and a metal ion color development reagent with the pH value of 8-10 as a second reagent, or an IMA test paper tape which is subject to color development by the second reagent. The detection method is as follows: mixing a biological sample and a first reagent solution to form a mixed liquid, then adding the second reagent for developing color or dripping the mixedliquid on the IMA test paper tape for developing color to form a blue or green coordination compound, and measuring the absorbance or the reflectivity of the blue or green coordination compound underthe condition that the wavelength of red light is 640-700 nm, wherein the measured value can be used for diagnosing whether a patient has a ischemia symptom or not. The invention develops a method for rapidly detecting IMA, which is suitable for clinically diagnosing, has simple steps, finishes the diagnosis within only about 15min and avoids the influence of interferents in the sample so that the measured result is closer to the truth value. The reagent kit has low cost, high sensitivity, strong specificity, stable performance and convenient transportation and storage.
Owner:贺坚慧
Steady ischemia modified albumin kit
ActiveCN104198729AImprove test performanceImprove stabilityMaterial analysis by observing effect on chemical indicatorDisease diagnosisSurface-active agentsIschemia-modified albumin
The invention relates to a steady ischemia modified albumin kit, which comprises a cobalt ion reagent and a dithiothreitol reagent, wherein the cobalt ion reagent comprises the following components: 50-500 mmol / L of buffer solution, 0.01-1.0 g / L of cobalt chloride, 1 g / L of surface active agent, and 0.5 g / L of preservative; and the dithiothreitol reagent comprises the following components: 50-300 mmol / L of buffer solution, 0.01-1.0 g / L of dithiothreitol (DTT), 1 g / L of surface active agent, 1-20 g / L of protective agent, and 0.5 g / L of preservative. The kit disclosed by the invention can reach reaction endpoint rapidly and is steady in property; after being opened, the kit can be stably stored in a dark place at room temperature for 30 days; after being closed, the kit can be stably stored at 2 DEG C -8 DEG C for 12 months, and therefore, clinical test requirements can be completely satisfied.
Owner:NINGBO RUI BIO TECH
Ischemia modified albumin detection reagent and detection method thereof
ActiveCN103558397AImprove stabilityAvoid interferenceMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsBetaineM-aminophenol
The invention relates to the technical field of detection of ischemia modified albumin and in particular relates to an ischemia modified albumin detection reagent. A reagent R1 contains a buffer solution, cobalt chloride, dodecyl dimethyl betaine and a preservative; a reagent R2 contains a buffer solution, thiadiazolyazo dimethylaminophenol, dodecyl dimethyl betaine and a preservative. A novel color developing agent, namely the thiadiazolyazo dimethylaminophenol is adopted, so that interference of metal ions such as Pd, Ni, Zn and Cu is effectively avoided, the reagent is stably and easily preserved at low toxicity, and the stability of the reagent is greatly improved. A novel ampholytic surfactant, namely the dodecyl dimethyl betaine is adopted, so that the determination performance is obviously improved, the reagent has obvious effects of maintaining stabilization of the system and avoiding lipid turbidity, and the stability and interference resistance of the reagent are also obviously improved.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Preparation method of quality control serum for ischemia-modified albumin
ActiveCN102539206AReduce in quantitySimple preparation processPreparing sample for investigationBottleIschemia-modified albumin
The invention discloses a preparation method of quality control serum for ischemia-modified albumin; the preparation method comprises the following steps of: (1) adopting the blood serum of a healthy person, and adjusting a pH value to be 7.0-7.5; (2) adding 0.1-100mm of metal ionic salt with a variable valence center or a metal organic composite with molecule planarity to the blood serum, and then adding 0.1-100mm of hydrogen peroxide; (3) reacting for 10-30min under the 35-40 DEG C illumination; (4) dialyzing a solution obtained through the reaction in the step (3) in 0.1-1000mm of a dialysis buffer solution with pH of 7.5-8.0 under the 20-25 DEG C temperature for 12-24h; and (5) adding a preservative according to a conventional technology, subpackaging and determining after mixing uniformly. The preparation method has the advantages of adequate the source of raw materials, easily obtaining of the raw materials, simple preparation technology, few amount of additives, low production cost, small non-specificity interference, no needing for composite dissolving, good homogeneity and small difference between bottles.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Anti-heparan-interference ischemia modified albumin detection reagent
ActiveCN103558398AStrong reductionReduce trimmingDisease diagnosisBiological testingGlycosideActive agent
The invention relates to the technical field of ischemia modified albumin detection and in particular relates to an anti-heparan-interference ischemia modified albumin detection reagent. The reagent comprises a reagent 1 and a reagent 2, wherein the reagent 1 contains 0.1-1 percent of alkyl polyglycoside (APG) 1214 and 0.1-1 percent of Emulgen709. Two special nonionic surfactants, namely the APG1214 and the Emulgen709 are added into the reagent 1, so that the measurement performance is obviously improved, and the effects of maintaining the transparency of the system and preventing white turbidity are obvious. Compared with previously used Tween-80, Tritonx-100 and polyethylene glycol monooleyl ether, the two special nonionic surfactants have better effects, and the heparan interference resistance of the detection reagent is obviously improved.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Ischemic heart disease detection kit and application thereof
InactiveCN103344768AAccurate detectionUndisturbedMaterial analysis using wave/particle radiationBiological testingHeart diseaseBuffer solution
The invention discloses an ischemic heart disease detection kit and application thereof. The kit comprises a human serum albumin (HSA) primary antibody, an HAS secondary antibody compound coupled with QD, Co <2+> and a phosphate buffer solution (PBS) buffering solution. By the kit provided by the invention, a real ischemia modified albumin (IMA) value can be accurately detected, and the interference of ultrahigh or ultralow total HSA concentration is avoided; the detection can be completed within 20-30 minutes at high speed; the kit is particularly suitable for a biological indicator which needs to obtain a result quickly, such as the IMA.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Detection kit for ischemia modified albumin and preparation method of detection kit
ActiveCN106443014AImprove stabilityImprove anti-interference abilityDisease diagnosisBiological testingAnti jammingHalf-life
The invention relates to the field of biotechnologies, and discloses a detection kit for ischemia modified albumin and a preparation method of the detection kit. The kit comprises a reagent R1and a reagent R2, wherein stabilizing agents of the R2 comprise ethanolamine, a nonionic surfactant and 2,2'-diethylamino dithio dihydrochloride, and the massic volume of ethanolamine is 20%-80%. According to the preparation method, by adding ethanolamine, the nonionic surfactant and 2,2'-diethylamino dithio dihydrochloride into the reagent R2, dithiothreitol (DTT) in the reagent R2 of the kit can not be easily oxidized and is long in half-life period, therefore, the detection kit for the ischemia modified albumin is high in stability, good in sample correlation and high in anti-jamming capability.
Owner:SHANGHAI KEHUA BIO ENG
Targets for Detection of Ischemia
InactiveUS20080305550A1Convenient careMonitor progressImmunoglobulins against animals/humansDisease diagnosisDivalent metalFatty acid binding
The subject application comprises methods for determining the occurrence of an ischemic event in a subject by determining an ischemia score based on the amount of at least two ischemia modified albumin markers. The ischemia modified albumin markers include complexes of fatty acids bound to albumin, albumin molecules with open Cys34 sites, albumin molecules that are products of oxidation at Cys34, albumin molecules with altered conformation or altered divalent metal binding due to the conformational change or oxidation at Cys34, and albumin molecules that have been oxidized at the N-terminus. Also included in the invention are ligands to each of the foregoing ischemia modified albumin markers. Further included are methods of determining the occurrence of an ischemic event by determining the amount of fatty acid that is complexed to albumin in a patient sample. In another embodiment, an ischemic event is determined by quantitating the relative amounts of reduced and oxidized forms of albumin Cys34. In an additional embodiment, an ischemic event is determined by observing whether a shift in albumin conformation has occurred which would reflect oxidized Cys34. Further, the invention comprises a method of determining an ischemic event by determining the amount of metal ion bound to the albumin metal ion binding sites.
Owner:ISCHEMIA TECH
Kit for determination of ischemia modified albumin and preparation method thereof
InactiveCN106093017AImprove stabilityImprove measurement accuracyMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsSerum samplesSolvent
The invention discloses a kit for determination of ischemia modified albumin and a preparation method thereof, wherein the kit includes double-liquid components of a reagent R1 and a reagent R2 which are independent with each other and includes the components and the corresponding content: the reagent R1 including 10-200 mmol / L of a buffer liquid, 0.01-1.00 mmol / L of cobalt chloride, 1-10 g / L of a stabilizing agent, and a solvent being purified water; and the reagent R2 including 10-200 mmol / L of Na2HPO4, 0.1-3.0 g / L of a chromogenic agent, 1-10 g / L of a stabilizer, and a solvent being purified water. The preparation method includes the following steps: according to the component content, preparing the reagents; mixing a to-be-tested serum sample with the reagent R1 and the reagent R2, and carrying out full reaction; determining the absorbance difference value after the reaction with a fully automatic biochemical analyzer; and calculating the concentration of the ischemia modified albumin in the sample according to the absorbance change value. The kit has the advantages of high accuracy and the like.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Detection kit of ischemia modified albumins
ActiveCN105445469AThe measurement result is accurateHigh speedMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsSolventIschemia-modified albumin
The invention relates to a detection kit of ischemia modified albumins. The kit concretely comprises cobalt ions and dithiothreitol. A stabilizer is added to the kit, so the storage stability of the kit is increased; and a solubilizer is also added, so pollution of reagents to a colorimetric device is removed.
Owner:BEIJING STRONG BIOTECH INC
Method, reagent and kit for quantitatively detecting ischemia modified albumin
InactiveCN104677898AAccurate detectionSimple and fast operationMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsElectrophoresisIschemia-modified albumin
The invention relates to a reagent for quantitatively detecting the content of ischemia modified albumin in human serum. The reagent comprises a reagent I and a reagent II which are respectively placed, wherein the reagent I comprises sodium dihydrogen phosphate, disodium hydrogen phosphate and cobalt chloride, and the reagent II comprises sodium dihydrogen phosphate, disodium hydrogen phosphate and dithiothreitol. The kit and the detection method only need dozens of microlitres of serum without centrifugal or electrophoresis and other separation treatments, is simple and convenient to operate, can be used for meeting the requirement of full-automatic analysis, and are suitable for timely accurate detection of large-scale samples.
Owner:ZHEJIANG KAICHENG BIOTECH
Biomarkers for assessing liver function
ActiveUS20090280519A1Improves predictive utilityImprove overall utilizationAnalysis using chemical indicatorsMicrobiological testing/measurementLiver functionIschemia-modified albumin
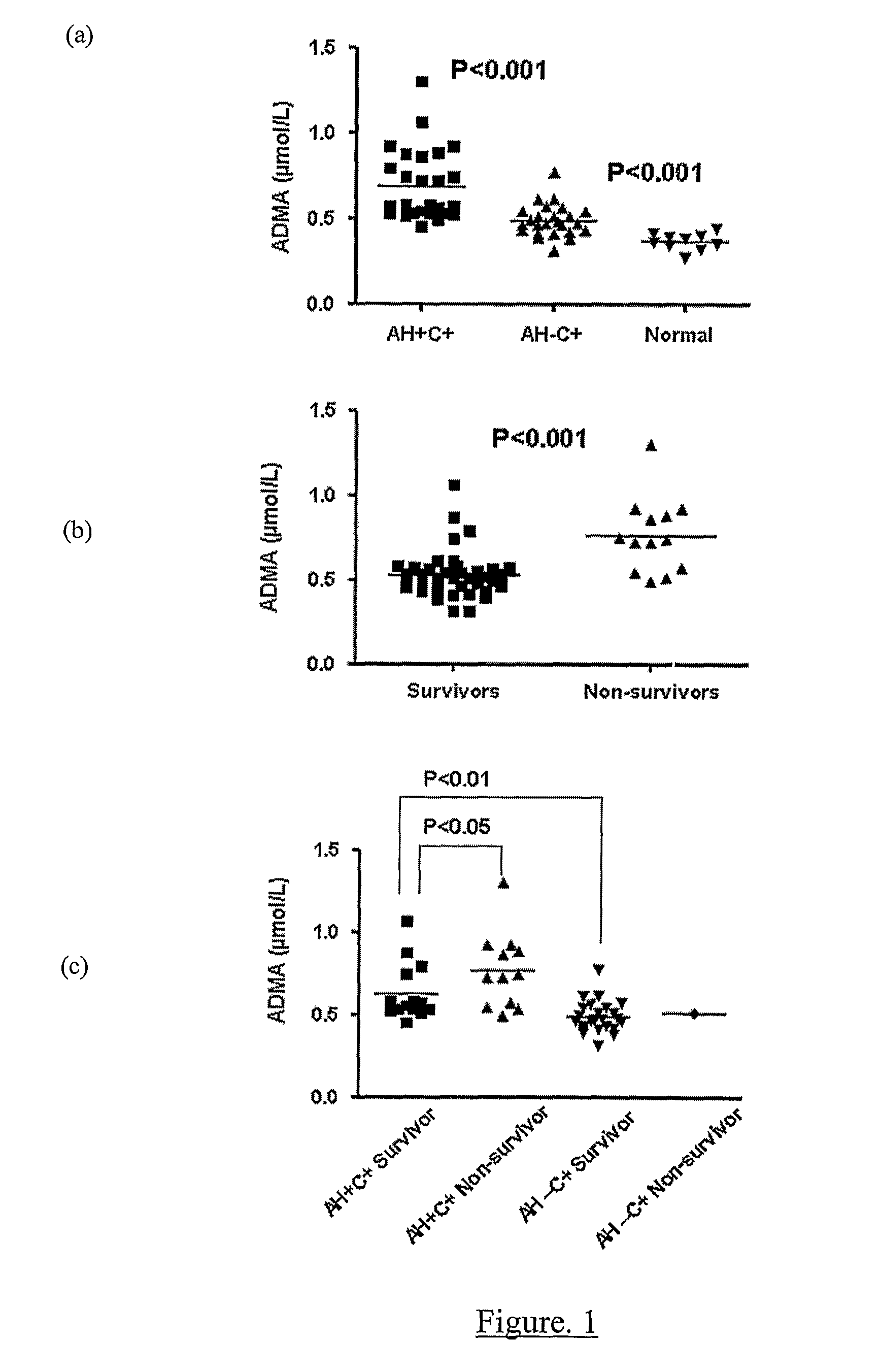
A method for assessing liver function in an individual, which method comprises determining the level of methylarginine(s) (such as ADMA and / or SDMA) and the ratio of ischemia modified albumin (IMA):albumin ratio (IMAR) in the individual, thereby to assess liver function in the individual.
Owner:UCL BUSINESS PLC
Kit for rapidly detecting ischemia modified albumin and application of kit
InactiveCN106706623AEffective Detection SensitivityImprove accuracyMaterial analysis by observing effect on chemical indicatorDipotassium phosphateIschemia-modified albumin
The invention belongs to the technical field of medical examination, and in particular relates to a kit for rapidly detecting ischemia modified albumin and application of the kit. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 mainly consists of a glycine-sodium hydroxide buffer solution, cobalt chloride, hexadecyl trimethyl ammonium chloride and a preservative; and the reagent R2 mainly consists of a trihydroxymethyl aminomethane buffer solution, alpha-pyridine azo-beta-naphthol, hexadecyl trimethyl ammonium chloride, dipotassium phosphate, a stabilizing agent and a preservative. The kit for rapidly detecting ischemia modified albumin, which is provided by the invention, is high in detection result precision, good in repeatability and good in interference resistance, also has the advantages of being good in stability, low in price and easy to preserve, is a relatively ideal ischemia modified albumin detection kit, and is beneficial to clinical application of kits for rapidly detecting ischemia modified albumin.
Owner:GUANGZHHOU HUAHONG BIOLOGICAL TECH
Preparation method of quality control serum for ischemia-modified albumin
ActiveCN102539206BSufficient sourceEasy to getPreparing sample for investigationBottleIschemia-modified albumin
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Ischemia modified albumin determination colour-developing agent and preparation method thereof
ActiveCN102323255AQuick checkSensitive detectionMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsFructoseNitroso
The invention discloses an ischemia modified albumin determination colour-developing agent and a preparation method thereof. The preparation method comprises the steps of: taking 0.8-3.6 parts of 1-nitroso-2-naphthol and 1.2-3.5 parts of sodium hydroxide, dissolving in 100ml of purified water, and preparing into a solution A; taking 0.5-4.0 parts of citric acid, dissolving into 100ml of purified water, and preparing into a solution B; and blending the solution A with the solution B until the pH reaches 7.50-10.00, and preparing into a solution C; taking 100ml of solution C, taking 0.5-3.0 parts of fructose, 0.05-0.5 part of Tween 20 and 0.01-0.06 part of Proclin 300, adding into the solution C, uniformly stirring, and preparing into the ischemia modified albumin determination colour-developing agent. The invention has the advantages that the ischemia modified albumin determination colour-developing agent can be used for detecting ischemia modified albumin more sensitively, components of the ischemia modified albumin are common and are lower in price; and the whole preparation is more convenient and the preparation procedure is simple and easy to operate.
Owner:ZYBIO INC
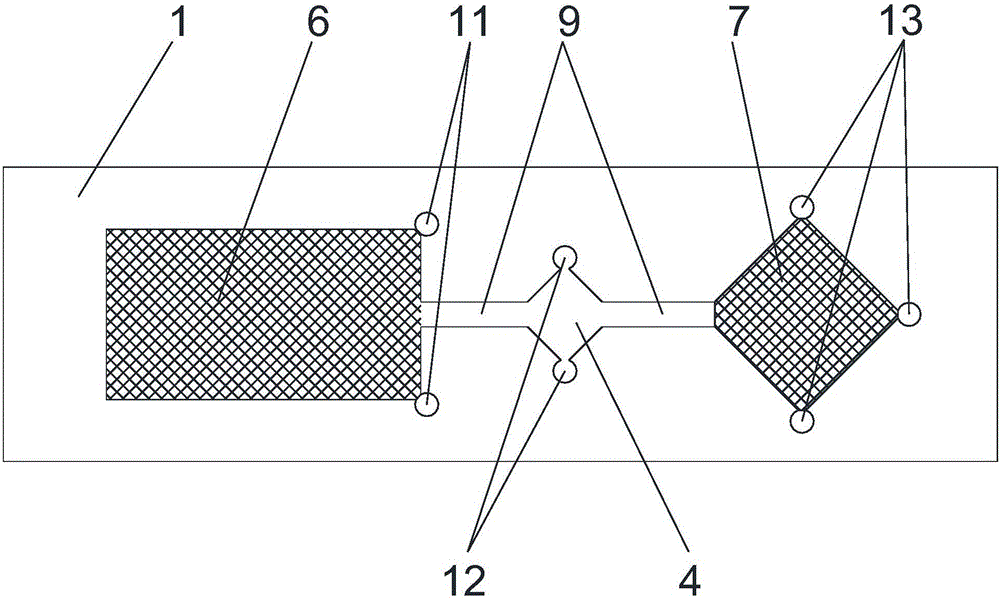
A chip for rapid detection of ischemia-modified albumin in blood
ActiveCN105973887BUniform physical and chemical propertiesMulti-buffer mixing spaceMaterial analysis by observing effect on chemical indicatorMedical unitHospitalized patients
The invention discloses a chip for rapidly detecting ischemia modified albumin in blood. The chip comprises a non-absorbent bottom plate and a non-absorbent cover plate, wherein a sample tank, a mixing tank and a detection tank are arranged on the bottom plate in sequence; micro-pipelines are arranged between the sample tank and the mixing tank and between the mixing tank and the detection tank; the sample tank is internally filled with a sample pad; the sample pad is coated with a cobalt standard product; the detection tank is internally filled with a detection pad; the detection pad is coated with a cobalt indicator; the cover plate is fixedly arranged above the bottom plate; and the cover plate is located above the sample tank and is provided with a sample feeding hole. The chip disclosed by the invention is simple in detection method, and a result is easy to judge; and the chip is suitable for being used in medical units including primary-level hospitals, emergency departments and the like or on bedsides of hospitalized patients, so that medical workers can rapidly judge dangers of the patients by layers and medical resources and health cost are saved.
Owner:西安良升生物科技有限公司
Biomarkers for assessing liver function
ActiveUS8455220B2High incidenceImproves predictive utilityMammal material medical ingredientsDisease diagnosisLiver functionLiver function tests
A method for assessing liver function in an individual, which method comprises determining the level of methylarginine(s) (such as ADMA and / or SDMA) and the ratio of ischemia modified albumin (IMA): albumin ratio (IMAR) in the individual, thereby to assess liver function in the individual.
Owner:UCL BUSINESS PLC
Ischemia modified albumin calibrator and application thereof
InactiveCN109557322ASimple configurationReliable test resultsBiological testingEthylene diamineSodium azide
The invention provides an ischemia modified albumin calibrator and application thereof. The calibrator comprises a horizontal reagent 1 and a horizontal reagent 2. Each of the horizontal reagent 1 andthe horizontal reagent 2 is made of raw materials including ethylene diamine tetraacetic acid, sodium azide, sodium chloride and 3-(N-morpholino) propanesulfonic acid. According to the invention, onthe basis of the reaction principle of the ischemia modified albumin, a novel technology is established; the calibrator is prepared by using chelating agents like the EDTA to achieve the objective andthe traditional calibration formula is abandoned; compared with the traditional technology, the novel formula has advantages of high efficiency and stability and simple preparation; and the cost is lowered. Therefore, the ischemia modified albumin calibrator has the broad application prospects and great market value.
Owner:AILEX TECH GRP CO LTD +1
Chip for rapidly detecting ischemia modified albumin in blood
ActiveCN105973887AUniform physical and chemical propertiesMulti-buffer mixing spaceMaterial analysis by observing effect on chemical indicatorMedical unitHospitalized patients
The invention discloses a chip for rapidly detecting ischemia modified albumin in blood. The chip comprises a non-absorbent bottom plate and a non-absorbent cover plate, wherein a sample tank, a mixing tank and a detection tank are arranged on the bottom plate in sequence; micro-pipelines are arranged between the sample tank and the mixing tank and between the mixing tank and the detection tank; the sample tank is internally filled with a sample pad; the sample pad is coated with a cobalt standard product; the detection tank is internally filled with a detection pad; the detection pad is coated with a cobalt indicator; the cover plate is fixedly arranged above the bottom plate; and the cover plate is located above the sample tank and is provided with a sample feeding hole. The chip disclosed by the invention is simple in detection method, and a result is easy to judge; and the chip is suitable for being used in medical units including primary-level hospitals, emergency departments and the like or on bedsides of hospitalized patients, so that medical workers can rapidly judge dangers of the patients by layers and medical resources and health cost are saved.
Owner:西安良升生物科技有限公司
Stable ischemia modified albumin testing kit
ActiveCN102147416BImprove stabilityReduce the binding forceColor/spectral properties measurementsBiological testingRoom temperatureBottle
The invention discloses a stable ischemia modified albumin testing kit which comprises a cobalt ion reagent and a dithiothreitol (DTT) reagent. A small amount of nonionic surfactant is added in the dithiothreitol reagent, thus the combination of DTT in the reagent and free oxygen is greatly reduced, the oxidation speed of DTT is reduced, and the stability of the reagent is remarkably improved. Simultaneously, the concentration of DTT is increased, thus the stability of the kit is improved to a certain extent. The stable ischemia modified albumin testing kit disclosed by the invention can be stored stably in an opened bottle in a dark place at the room temperature for 30 days, and also can be stored stably in a closed bottle at the temperature of 2 to 8 DEG C for 12 months, and can totally meet the requirements for clinical examinations.
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
Method for detecting ischemia modified albumin and detection kit
ActiveCN108152225ANot easy to sublimateNot easily oxidizedPreparing sample for investigationColor/spectral properties measurementsCobaltAbsorbance
The invention provides a method for detecting ischemia modified albumin. According to the method, the characteristic that the binding capacity of the ischemic modified albumin and the cobalt is weakened is utilized, free cobalt in a reaction system is detected by using chemical reagents, the change of the content of the free cobalt is expressed through the change of absorbance, the concentration of the ischemia modified albumin is indirectly expressed, and a color developing agent used in the detection is 2,4-dimercaptopyrimidine and is used for detecting the free cobalt in the reaction system. The method has the advantages that the stability of the color developing agent of the 2,4-dimercaptopyrimidine is higher, the analysis sensitivity is higher, the price is lower, the detection resultis reported based on the concentration of the free cobalt, and the problem that the absorbance of an IMA (ischemia modified albumin) detection result is unstable due to the fact that the performanceof instruments or reagents is different and unstable when the absorbance is adopted for report can be solved. The invention further provides a detection kit for the ischemia modified albumin. The detection kit comprises the reagents used in the method.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Ischemia modified albumin liquid stabilization kit
ActiveCN102507916BImprove stabilityAvoid interferenceBiological testingBuffer solutionIschemia-modified albumin
The invention discloses an ischemia modified albumin liquid stabilization kit. The kit is a liquid dual reagent of a reagent 1 and a reagent 2, wherein the reagent 1 comprises a buffer solution of which the pH is 7.5 to 8.5 and the concentration is 0.05 to 0.5M, cobalt chloride at the concentration of 20 to 200mg / L, an interference removing agent at the concentration of 0.1 to 10g / L, a stabilizing agent at the concentration of 0.1 to 10g / L, and preservative at the concentration of 0.1 to 10g / L; and the reagent 2 comprises a buffer solution of which the pH is 7.0-8.0 and the concentration is 0.5 to 10M, dithiothreitol at the concentration of 0.5 to 50g / L, a stabilizing agent at the concentration of 0.1 to 10g / L, and preservative at the concentration of 0.1 to 10g / L. The kit has the advantages of fully meeting the clinical inspection requirement, along with high accuracy and stability, and can be stabilized for 12 months when stored at the temperature of between 2 and 8 DEG C in a dark place.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Detection kit for ischemic modified albumin and preparation method thereof
ActiveCN106443014BImprove stabilityGood sample correlationDisease diagnosisBiological testingAnti jammingHalf-life
The invention relates to the field of biotechnologies, and discloses a detection kit for ischemia modified albumin and a preparation method of the detection kit. The kit comprises a reagent R1and a reagent R2, wherein stabilizing agents of the R2 comprise ethanolamine, a nonionic surfactant and 2,2'-diethylamino dithio dihydrochloride, and the massic volume of ethanolamine is 20%-80%. According to the preparation method, by adding ethanolamine, the nonionic surfactant and 2,2'-diethylamino dithio dihydrochloride into the reagent R2, dithiothreitol (DTT) in the reagent R2 of the kit can not be easily oxidized and is long in half-life period, therefore, the detection kit for the ischemia modified albumin is high in stability, good in sample correlation and high in anti-jamming capability.
Owner:SHANGHAI KEHUA BIO ENG
Ischemia modified albumin detection reagent and detection method thereof
ActiveCN103558397BImprove stabilityAvoid interferenceMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsM-aminophenolAssay
The invention relates to the technical field of detection of ischemic modified albumin, in particular to a detection reagent for ischemic modified albumin. The reagent R1 contains buffer, cobalt chloride, dodecyl dimethyl betaine and preservatives; the reagent R2 Contains buffer, thiadiazole azodimethylaminophenol, lauryldimethylbetaine, preservatives. The use of a new chromogenic agent, thiadiazole azodimethylaminophenol, effectively avoids the interference of metal ions such as Pd, Ni, Zn, and Cu, and is more stable, low-toxic, and easy to store, greatly enhancing the stability of the reagent; using a new The amphoteric surfactant, dodecyl dimethyl betaine, not only significantly improves the performance of the assay, but also maintains the stability of the system and prevents fatty turbidity. The effect is obvious, and the stability and anti-interference ability of the reagent are significantly improved.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
A kind of determination method and detection kit of ischemia-modified albumin
ActiveCN108152225BNot easy to sublimateNot easily oxidizedPreparing sample for investigationColor/spectral properties measurementsMedicineIschemia-modified albumin
The invention provides a method for detecting ischemia modified albumin. According to the method, the characteristic that the binding capacity of the ischemic modified albumin and the cobalt is weakened is utilized, free cobalt in a reaction system is detected by using chemical reagents, the change of the content of the free cobalt is expressed through the change of absorbance, the concentration of the ischemia modified albumin is indirectly expressed, and a color developing agent used in the detection is 2,4-dimercaptopyrimidine and is used for detecting the free cobalt in the reaction system. The method has the advantages that the stability of the color developing agent of the 2,4-dimercaptopyrimidine is higher, the analysis sensitivity is higher, the price is lower, the detection resultis reported based on the concentration of the free cobalt, and the problem that the absorbance of an IMA (ischemia modified albumin) detection result is unstable due to the fact that the performanceof instruments or reagents is different and unstable when the absorbance is adopted for report can be solved. The invention further provides a detection kit for the ischemia modified albumin. The detection kit comprises the reagents used in the method.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com