Ischemia modified albumin liquid stabilization kit
A kit and albumin technology, which is applied in the field of medical testing and determination, can solve the problems of short stability time of aqueous solution refrigeration or cryopreservation, affecting the large-scale clinical application of reagents, and the validity period of only 2 weeks, meeting the requirements of clinical testing, The effect of reducing the rate of oxidation and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The components and concentrations of reagent 1 (R1) are:
[0039] Tris buffer (pH 7.5) 0.1 M
[0040] Cobalt chloride 50 mg / L
[0041] Tween 20 0.1 g / L
[0042] Sodium Fluoride 0.1 g / L
[0044] The components and concentrations of reagent 2 (R2) are:
[0045] Citric acid-trisodium citrate buffer (pH 7.0) 1 M
[0046] Dithiothreitol 0.5 g / L
[0047] Bridger-35 1 g / L
[0049] The preparation method of Reagent 1 and Reagent 2 is a conventional method, that is, the components of Reagent 1 and Reagent 2 are added to distilled water and then mixed and stirred evenly.
Embodiment 2
[0051] The components and concentrations of reagent 1 are:
[0052] 3-Trimethylolmethylamine-2-hydroxypropanesulfonic acid buffer (pH 8.0) 0.2 M
[0053] Cobalt chloride 20 mg / L
[0054] Sodium Fluoride 5 g / L
[0055] Citric acid 0.7 g / L
[0056] Tween 20 1 g / L
[0057] Sodium azide 1 g / L
[0058] The components and concentrations of reagent 2 are:
[0059] Acetic acid-sodium acetate buffer (pH 7.5) 2 M
[0060] Dithiothreitol 10 g / L
[0061] Tween 80 5 g / L
[0062] Proclin300 0.1 g / L
[0063] The preparation method of the kit in Example 2 is the same as in Example 1.
Embodiment 3
[0065] The components and concentrations of reagent 1 are:
[0066] 4-Hydroxyethylpiperazine propanesulfonic acid buffer (pH 8.5) 0.2 M
[0067] Cobalt chloride 200 mg / L
[0068] Glycine 2.5 g / L
[0069] Tween 80 2 g / L
[0070] Proclin300 1g / L
[0071] The components and concentrations of reagent 2 are:
[0072] Imidazole buffer (pH 8.0) 10 M
[0073] Dithiothreitol 30 g / L
[0074] Triton 100 1 g / L
[0075] Proclin300 1g / L
[0076] Example 3 The preparation method of the kit is the same as that in Example 1.
[0077] The testing conditions for the kit of the present invention to measure IMA in samples are as follows: temperature: 30-37° C.; optical path of cuvette: 1.0 cm. The main detection wavelength is 510 nm, and the secondary wavelength is 700 nm.
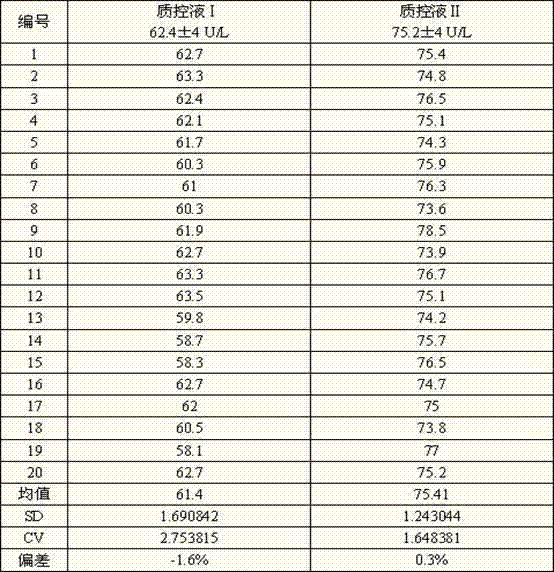
[0078] The method of using the IMA determination kit of the present invention to determine the IMA in the sample is as follows: add R1 to the sample (calibration tube as the sample) and mix well, incubate at 30-37°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com