Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Hematologic disorders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Disorders that affect the cells in the blood (blood cells) or proteins in the blood clotting or immune systems are called blood disorders or hematologic disorders.
Methods of using (+)-1,4-dihydro-7-[(3S,4S)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders
Methods of treating, preventing or managing hematologic disorders, such as leukemia are disclosed. The methods encompass the administration of SNS-595. Also provided are methods of treatment using this compound with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy. In certain embodiments, the method of treatment comprise administering SNS-595 in combination with Ara-C. Pharmaceutical compositions and single unit dosage forms suitable for use in the methods are also disclosed.
Owner:SUNESIS PHARMA INC
Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of antecedent hematologic disorders
Methods of treating, preventing or managing antecedent hematologic disorders, such as myelodysplastic syndrome, including chronic myelomonocytic leukemia are disclosed. The methods encompass the administration of SNS-595. Also provided are methods of treatment using this compound with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy. In certain embodiments, the method of treatment comprise administering SNS-595 in combination with cytarabine. Pharmaceutical compositions and single unit dosage forms suitable for use in the methods are also disclosed.
Owner:SUNESIS PHARMA INC
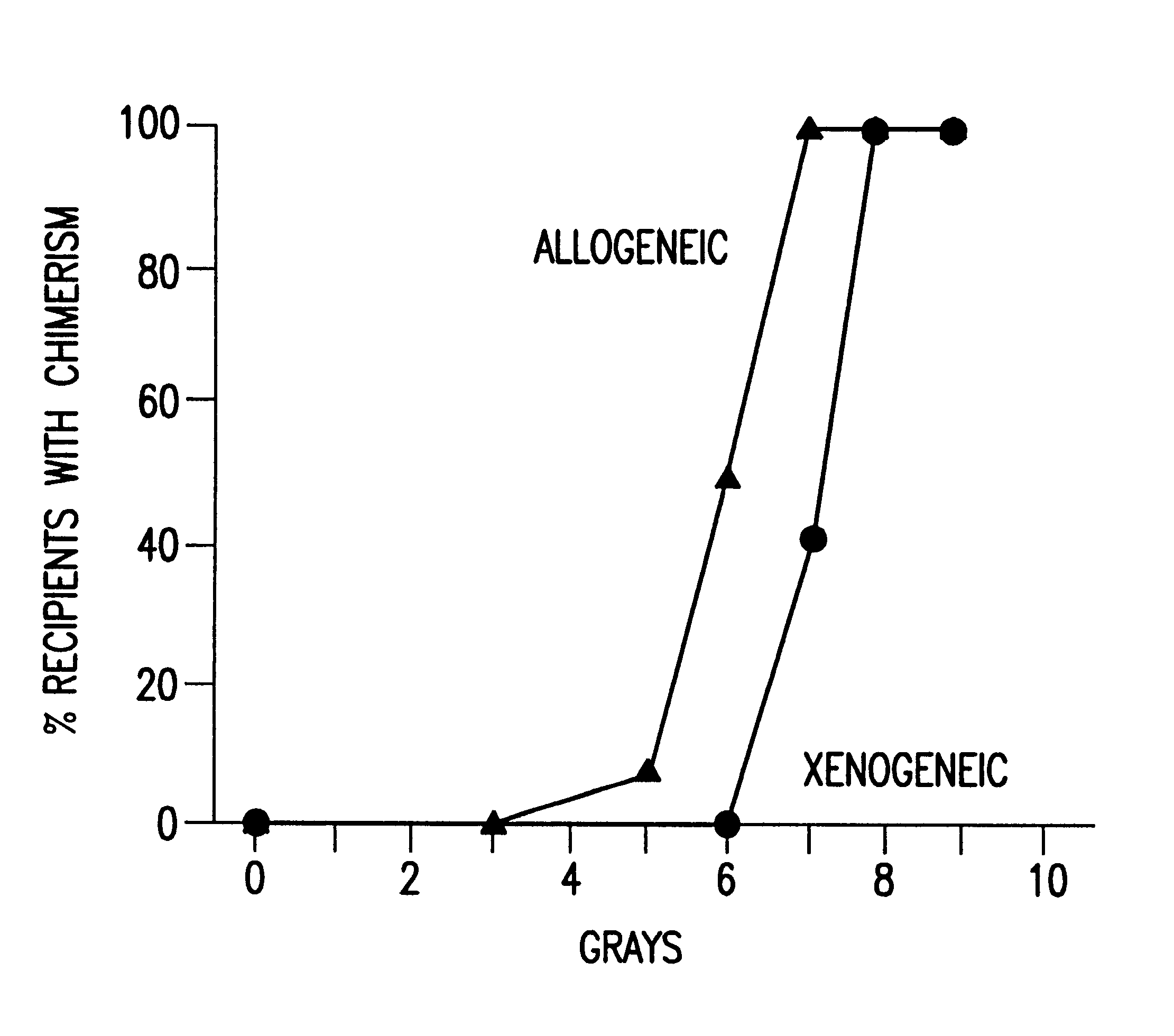
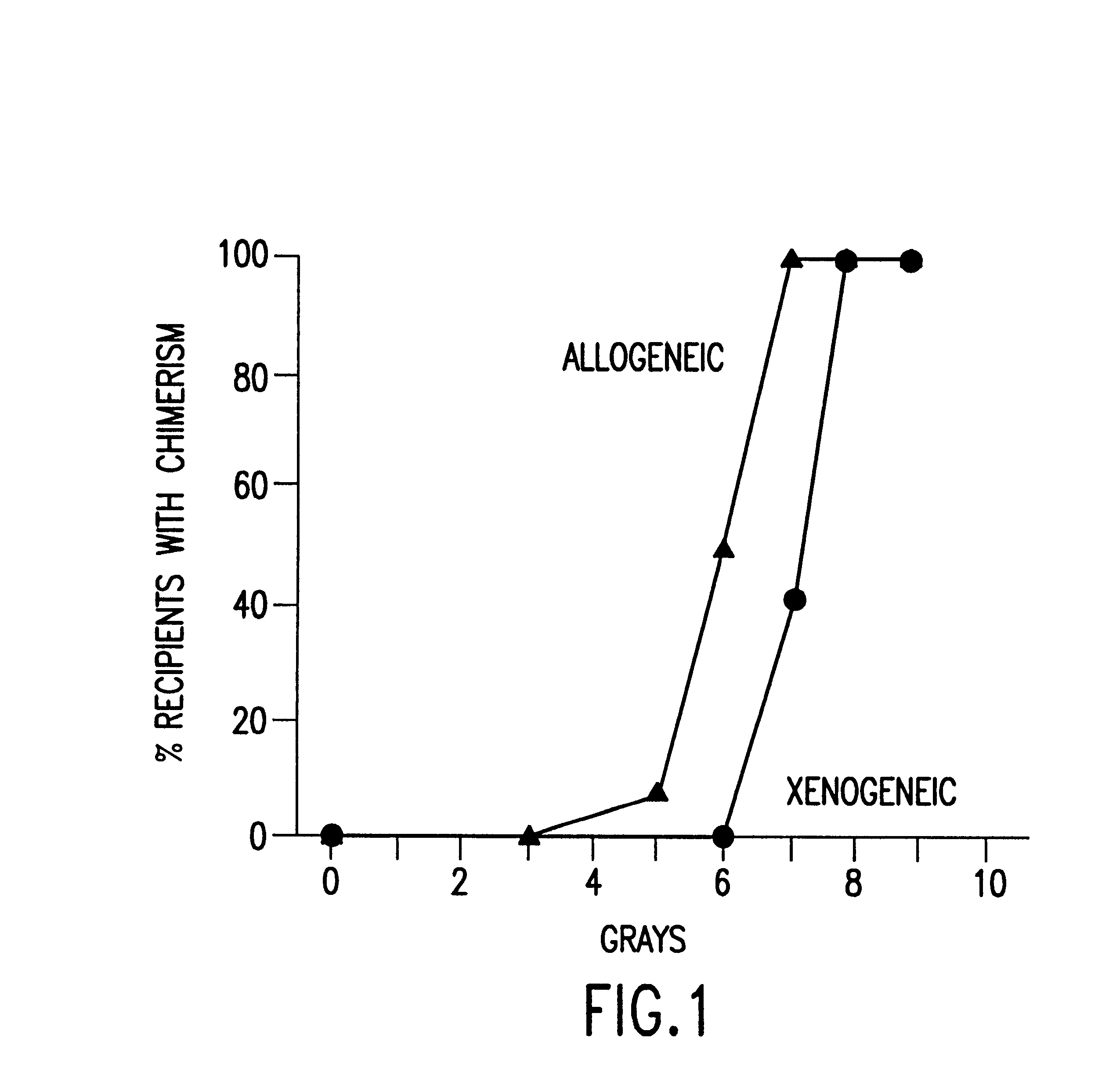
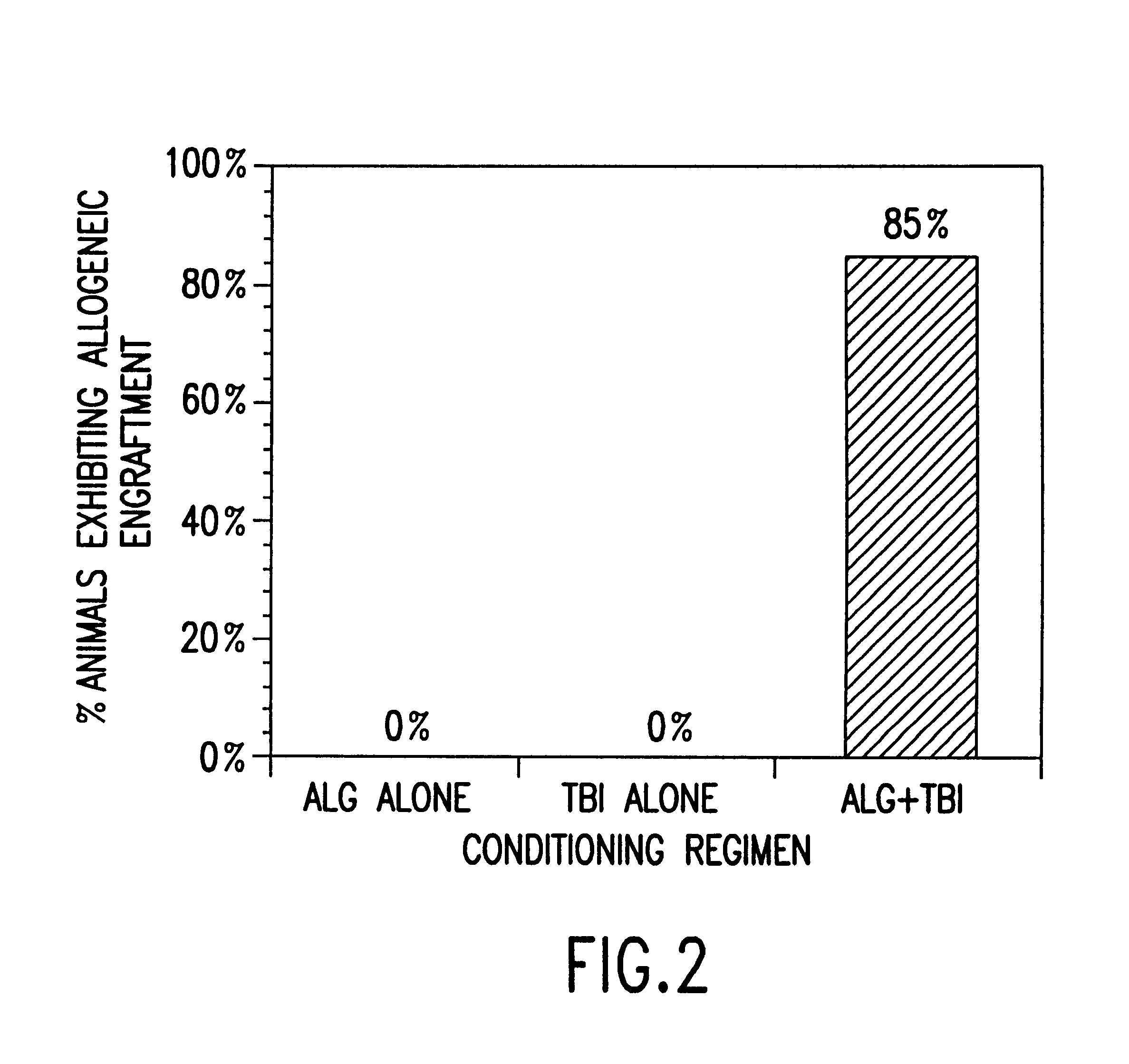
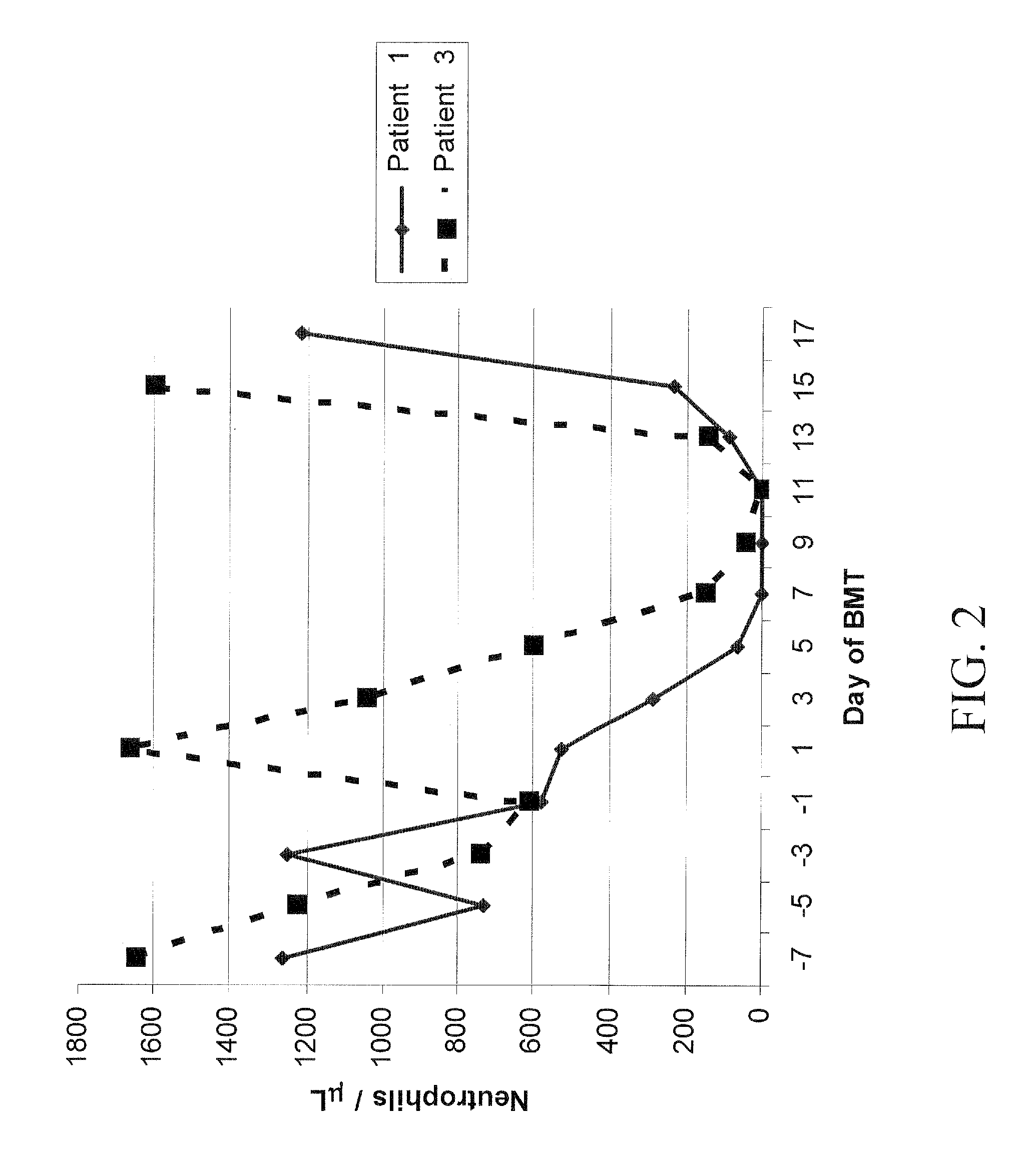
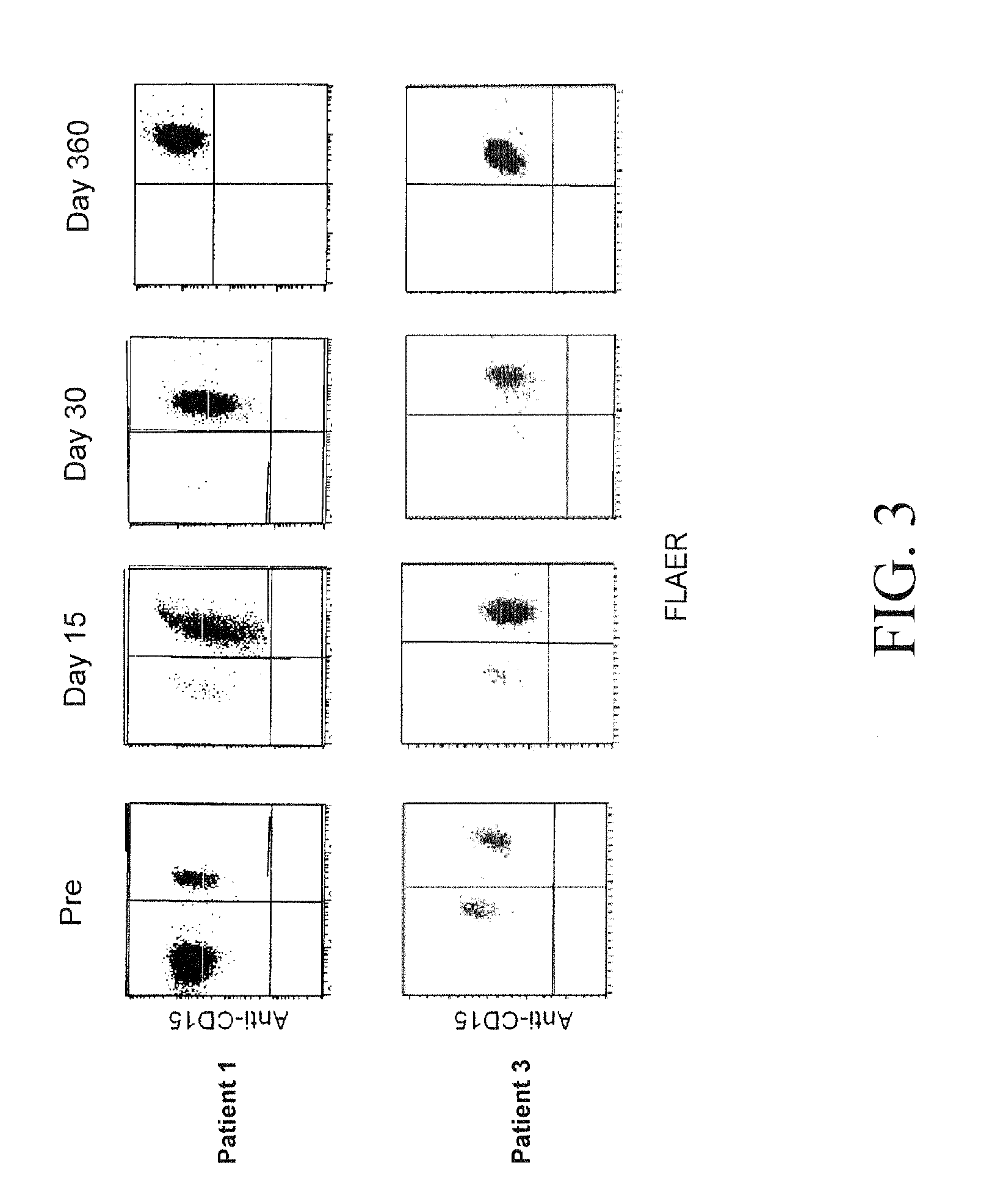
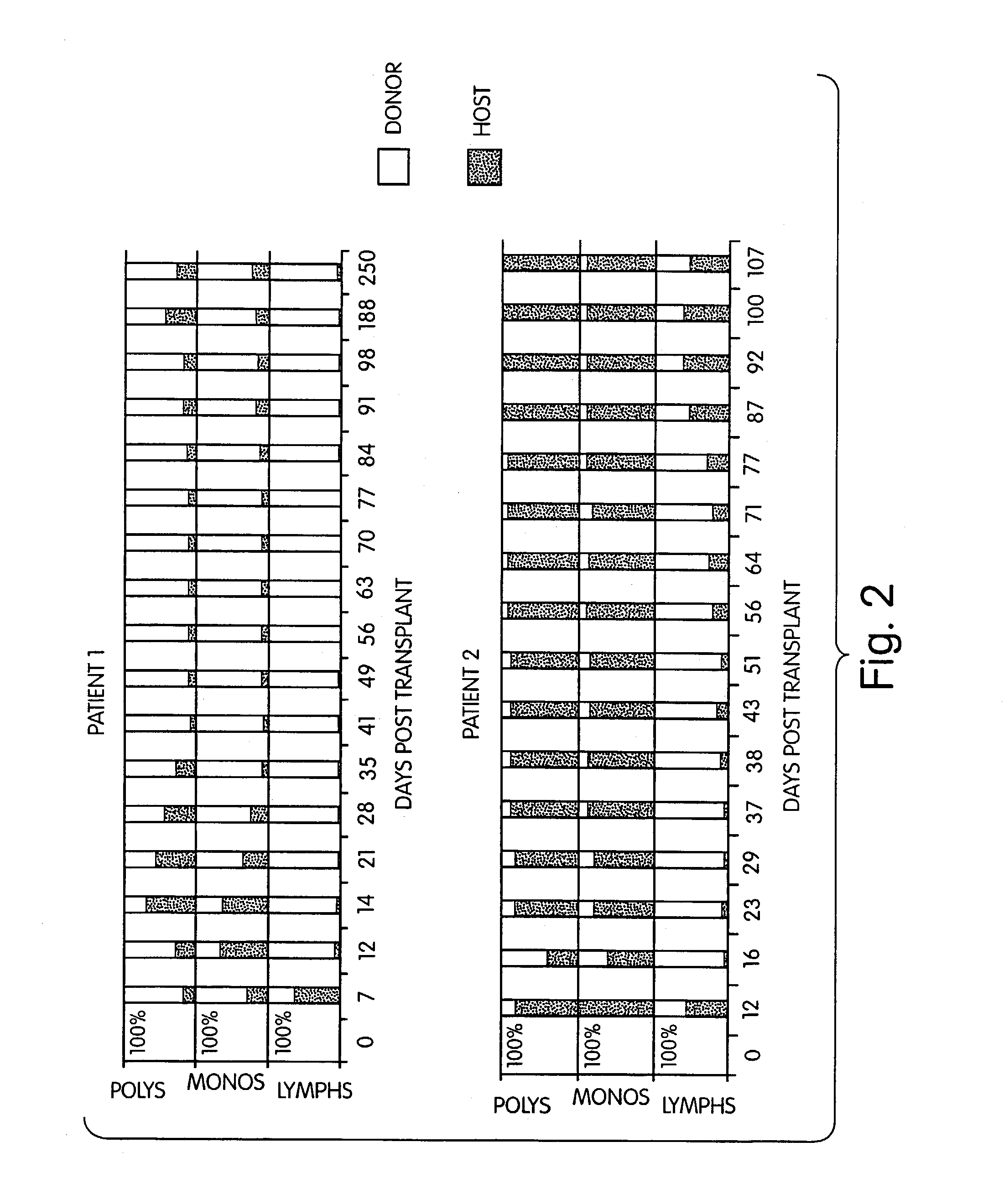
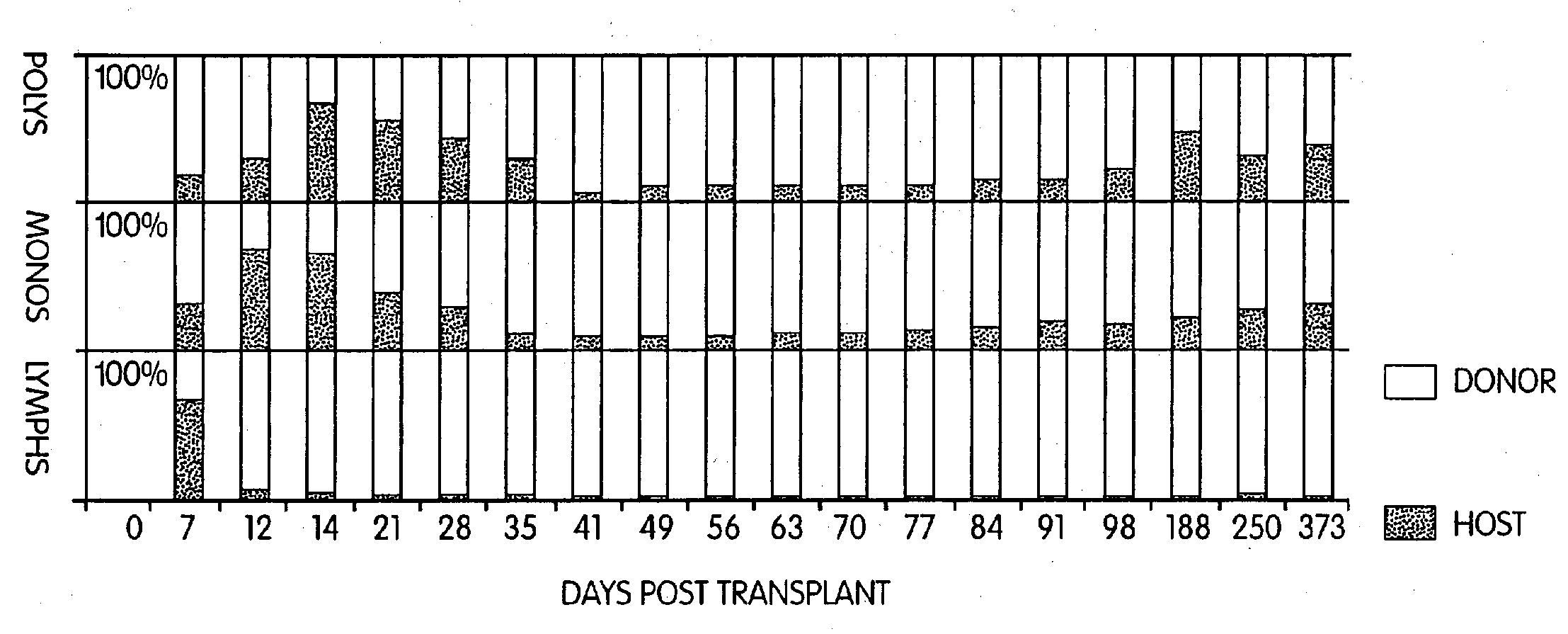
Non-lethal methods for conditioning a recipient for bone marrow transplantation
InactiveUS6217867B1Reduce proliferationDiminished cytotoxic activityBiocideOrganic active ingredientsGackstroemiaAcquired immunodeficiency
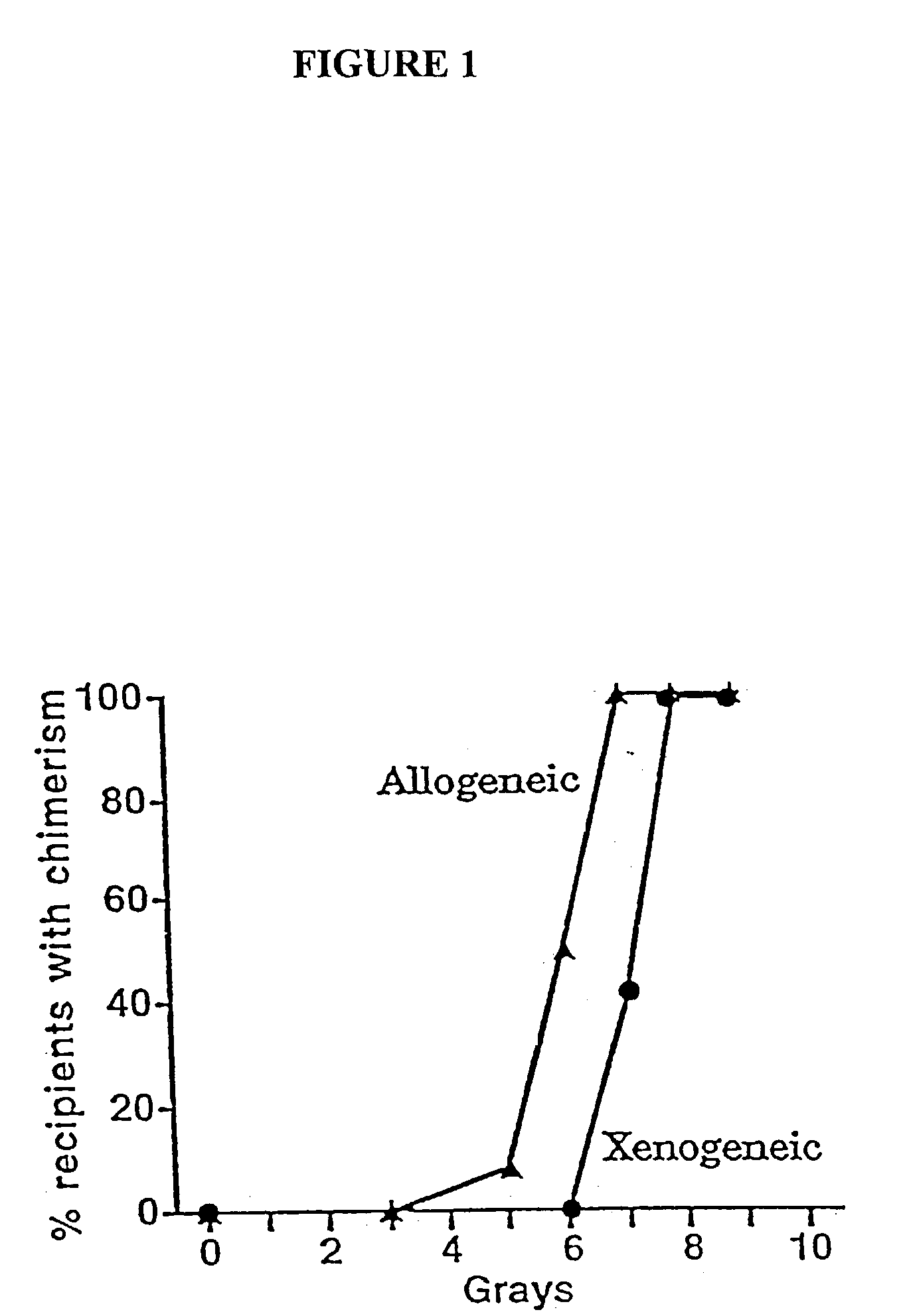
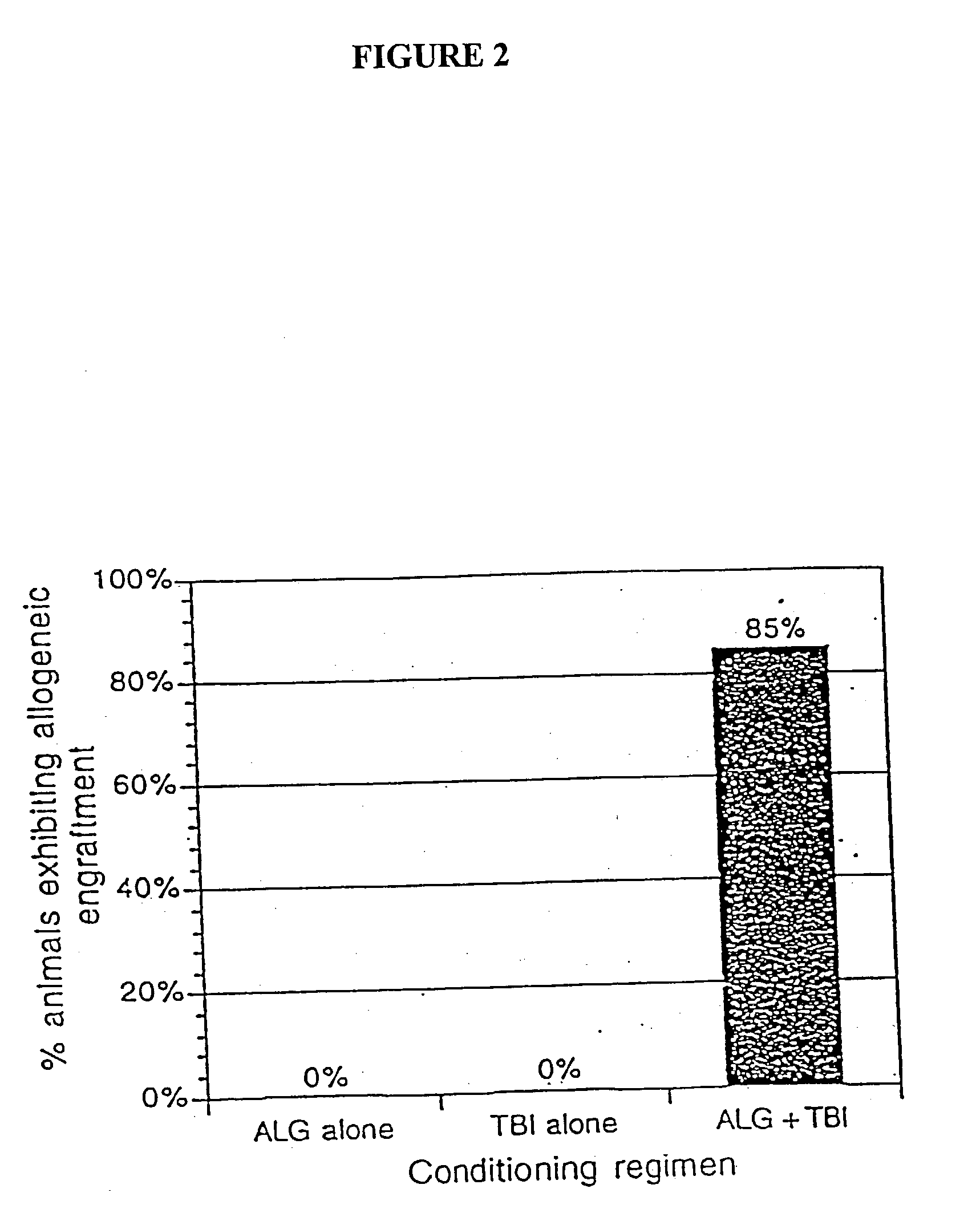
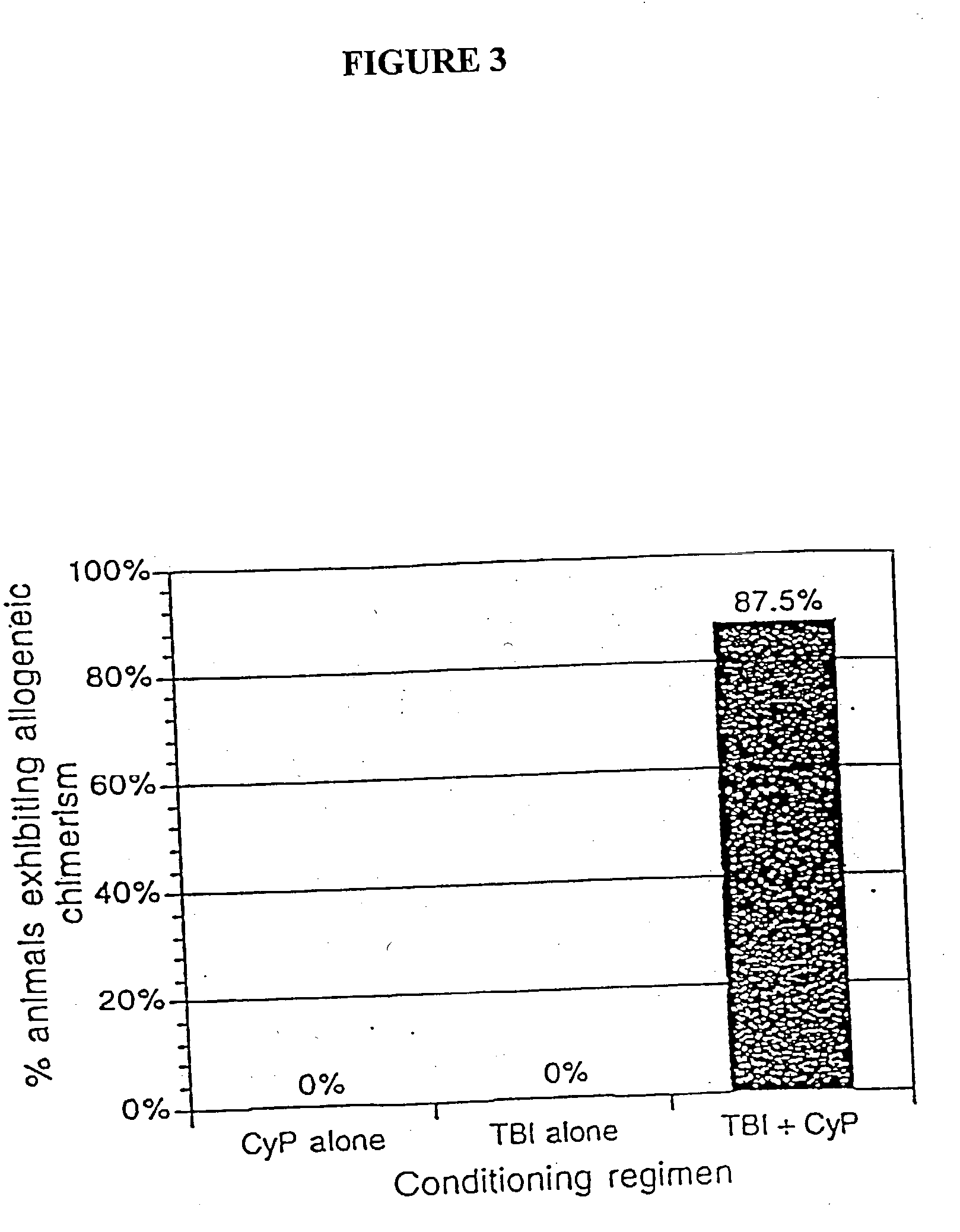
The present invention relates to non-lethal methods of conditioning a recipient for bone marrow transplantation. In particular, it relates to the use of nonlethal doses of total body irradiation, total lymphoid irradiation, cell type-specific or cell marker-specific antibodies, especially antibodies directed to bone marrow stromal cell markers, NK cells, or the CD8 cell marker, cytotoxic drugs, or a combination thereof. The methods of the invention have a wide range of applications, including, but not limited to, the conditioning of an individual for hematopoietic reconstitution by bone marrow transplantation for the treatment of hematologic malignancies, hematologic disorders, autoimmunity, infectious diseases such as acquired immunodeficiency syndrome, and the engraftment of bone marrow cells to induce tolerance for solid organ, tissue and cellular transplantation.
Owner:PITTSBURGH UNIV OF
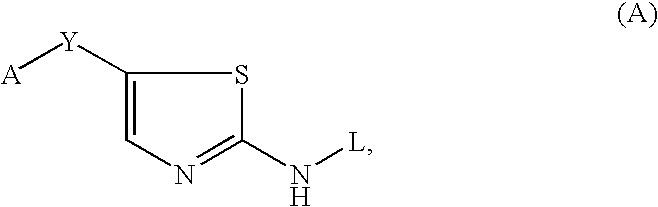
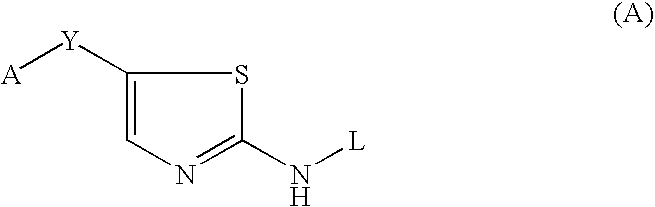
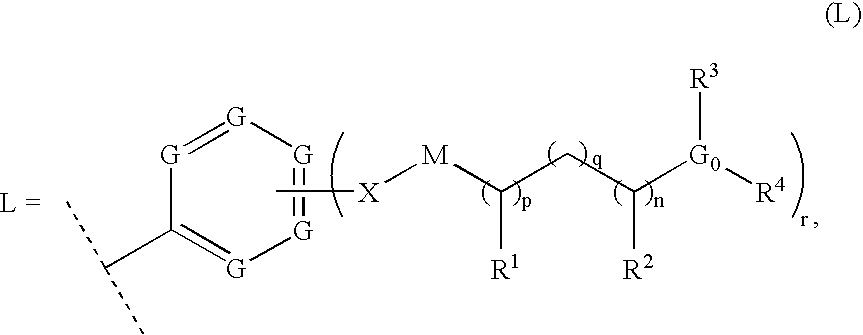
Thiazole inhibitors targeting resistant kinase mutations
A compound is provided, having the general structure (A): wherein A is an aryl or heteroaryl group, Y is a hydrophbic linking moiety, and L is a substitutent. The compound (A) can be used for treatment of various angiogenic-associated or hematologic disorders, such as myeloproliferative disorders in patients who do not respond to kinase-inhibition therapy that comprises administering currently used medications.
Owner:TARGEGEN
Non-lethal methods for conditioning a recipient for bone marrow transplantation
InactiveUS20030165475A1Diminished proliferative and cytotoxic activityDosage of TBI can be further reducedBiocideEnergy modified materialsAcquired immunodeficiencyGackstroemia
The present invention relates to non-lethal methods of conditioning a recipient for bone marrow transplantation. In particular, it relates to the use of nonlethal doses of total body irradiation, total lymphoid irradiation, cell type-specific or cell marker-specific antibodies, especially antibodies directed to bone marrow stromal cell markers or the CD8 cell marker, cytotoxic drugs, or a combination thereof. The methods of the invention have a wide range of applications, including, but not limited to, the conditioning of an individual for hematopoietic reconstitution by bone marrow transplantation for the treatment of hematologic malignancies, hematologic disorders, autoimmunity, infectious diseases such as acquired immunodeficiency syndrome, and the engraftment of bone marrow cells to induce tolerance for solid organ, tissue and cellular transplantation.
Owner:ILDSTAD SUZANNE T
Use of high-dose, post-transplantation oxazaphosphorine drugs for reduction of transplant rejection
InactiveUS20120148577A1Reduce transplant rejectionReduce rejectionBiocidePeptide/protein ingredientsGackstroemiaSickle cell anemia
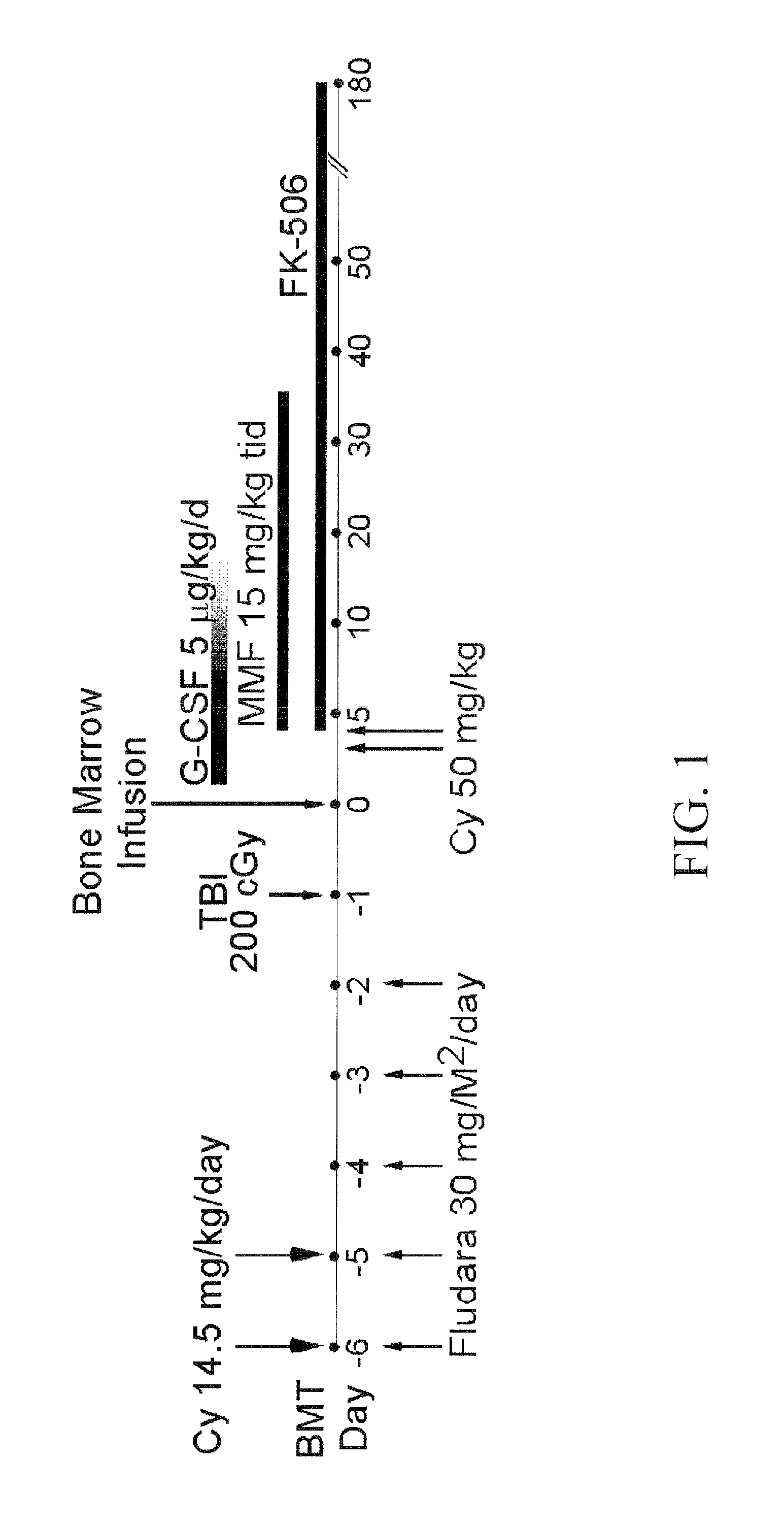
A lymphocytotoxic, but hematopoietic stem cell-sparing, high-dose amount of an oxazaphosphorine drug such as, for example, cyclophosphamide, administered post-transplantation can be used to reduce transplant rejection, including graft-versus-host-disease (GVHD). In some embodiments, the transplants are bone marrow transplants or hematopoietic stem cell transplants carried out for the treatment of hematologic disorders, including hematologic malignancies and non-malignant hematologic disorders. In some embodiments, the transplants are carried out for the treatment of hereditary hemoglobinopathies, such as sickle cell anemia and thalassemia.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Kits for treatment of hematologic disorders
InactiveUS7408039B2Reduce GVHDSignificant graft-versus-leukemia (GVL) effectBiocideGenetic material ingredientsGraft versus leukaemiaHematological Diseases
The inventors have discovered that hematologic disorders, e.g., both neoplastic (hematologic cancers) and non-neoplastic conditions, can be treated by the induction of mixed chimerism using myeloreductive, but not myeloablative, conditioning. Methods of the invention reduce GVHD, especially GVHD associated with mismatched allogeneic or xenogeneic donor tissue, yet provide, for example, significant graft-versus-leukemia (GVL) effect and the like.
Owner:THE GENERAL HOSPITAL CORP
Treatment of hematologic disorders
InactiveUS7892578B2Reduce GVHDRemarkable effectBiocideOrganic active ingredientsGraft versus leukaemiaHematological Diseases
The inventors have discovered that hematologic disorders, e.g., both neoplastic (hematologic cancers) and non-neoplastic conditions, can be treated by the induction of mixed chimerism using myeloreductive, but not myeloablative, conditioning. Methods of the invention reduce GVHD, especially GVHD associated with mismatched allogeneic or xenogeneic donor tissue, yet provide, for example, significant graft-versus-leukemia (GVL) effect and the like.
Owner:THE GENERAL HOSPITAL CORP
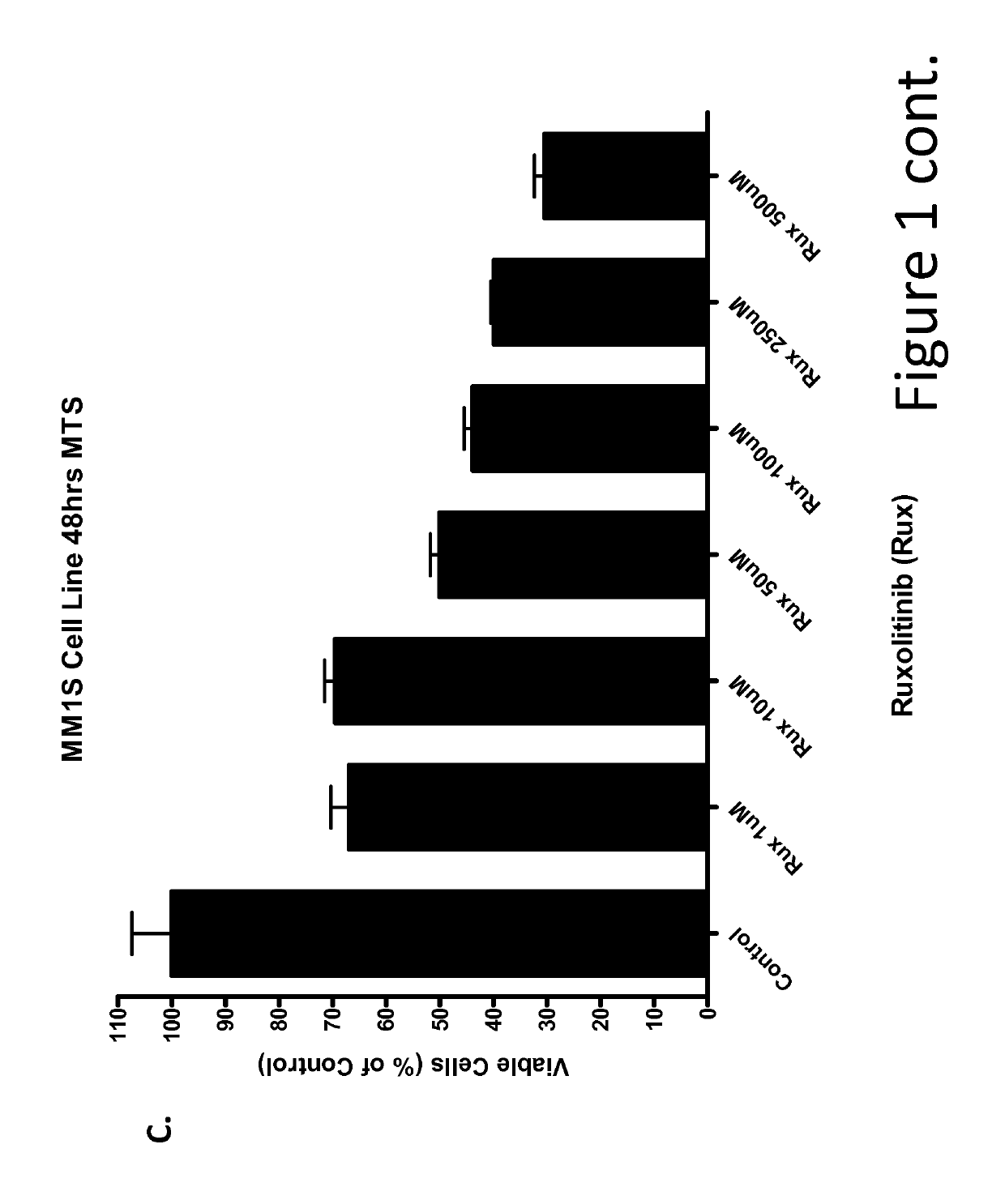
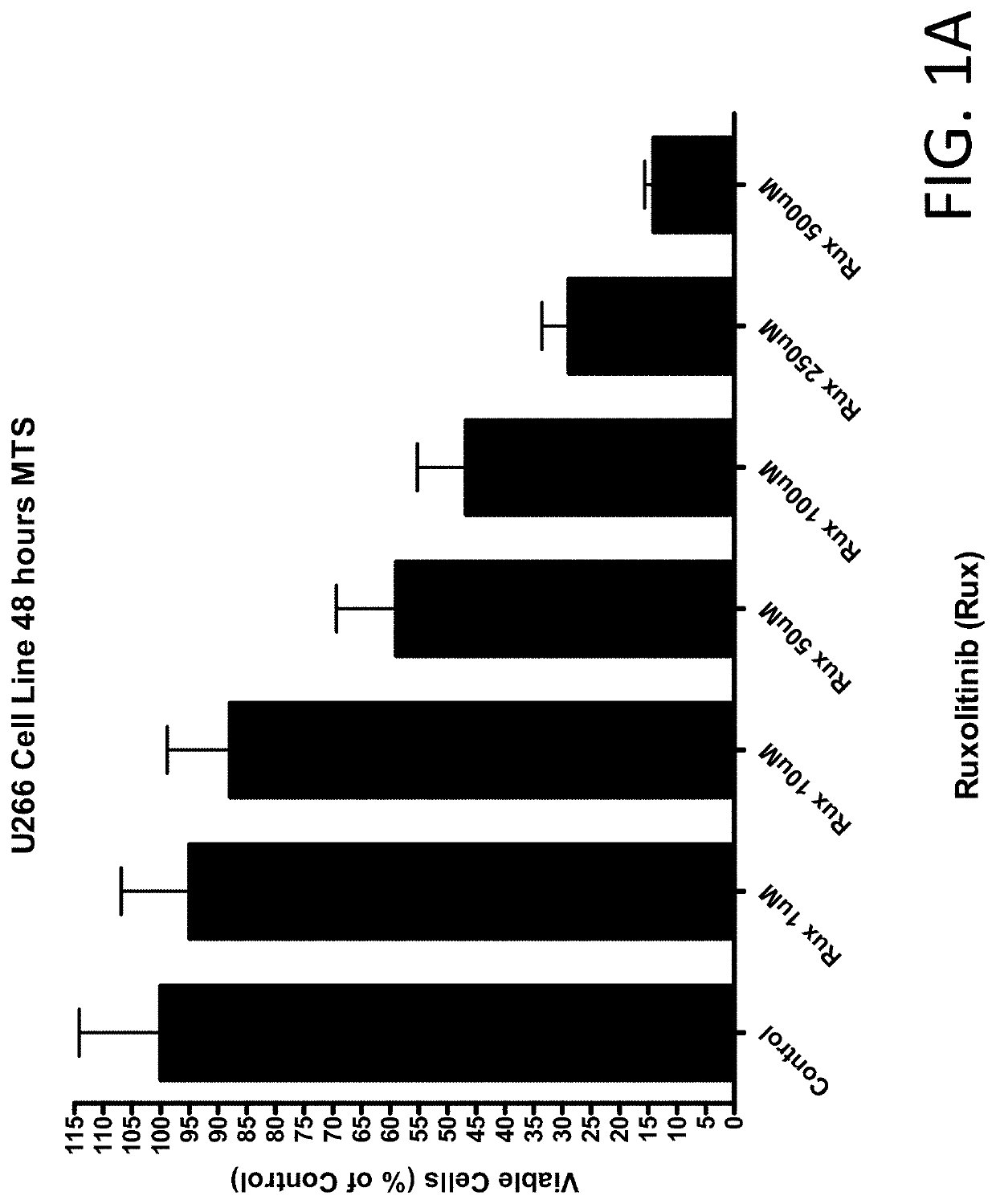
Anti-cancer effects of jak2 inhibitors in combination with thalidomide derivatives and glucocorticoids
ActiveUS20170106003A1Organic active ingredientsPharmaceutical delivery mechanismGlucocorticoidCancer therapy
The present invention provides methods of treatment for hematological malignancies involving synergistic combination of a JAK2 inhibitor and a glucocorticoid or a JAK2 inhibitor, thalidomide or thalidomide derivative, and a glucocorticoid. The compositions and methods provide an unexpected efficacy in the treatment for hematological disorders. The hematological disorders treated by the current invention include multiple myeloma, and may also include hematological disorders that are refractory to prior cancer treatments, or a relapsed hematologic disorder.
Owner:ONCOTRACKER INC
Kit for quantitatively detecting IKZF1 gene mutation
The invention discloses a kit for quantitatively detecting IKZF1 gene mutation. The kit for quantitatively detecting the IKZF1 gene mutation consists of a primer pair and a probe, wherein a primer sequence of the primer pair is shown as SEQ ID No:1; the other primer sequence is shown as SEQ ID No:2; and the sequence of the probe is shown as SEQ ID No:3. The primer pair and the probe can be used for quantitatively detecting the IKZF1 gene mutation level, and the kit is easy, convenient and sensitive, so that the kit is used for auxiliary diagnosis, MRD (Minimal Residual Disease) monitoring, prognostic evaluation and the like on neoplastic hematologic disorder patients (particularly ALL leukemia patients and CML patients) and has significance in the field of medical detection.
Owner:PEOPLES HOSPITAL PEKING UNIV
Method for treating hematologic disorders with water insoluble 20 (S)-camptothecin
InactiveUS20020131997A1Efficient managementOrganic active ingredientsPill deliveryMedicineWater insoluble
A method is provided for treating a patient afflicted with a hematologic disorder such as chronic leukemia and the myelodysplastic syndromes. The method includes administering to the patient an effective amount of a water-insoluble 20(S)-camptothecin compound with a closed lactone ring, a derivative thereof, or a mixture thereof. In a preferred method, the compound administered is 9-nitro-20(S)-camptothecin. The compound can be administered orally, intramuscularly, transdermally, subcutaneously, or parenterally.
Owner:THE STEHLIN FOUND FOR CANCER RES +1
Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of antecedent hematologic disorders
Methods of treating, preventing or managing antecedent hematologic disorders, such as myelodysplastic syndrome, including chronic myelomonocytic leukemia are disclosed. The methods encompass the administration of SNS-595. Also provided are methods of treatment using this compound with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy. In certain embodiments, the method of treatment comprise administering SNS-595 in combination with cytarabine. Pharmaceutical compositions and single unit dosage forms suitable for use in the methods are also disclosed.
Owner:SUNESIS PHARMA INC
Treatment of Hematologic Disorders
InactiveUS20090028870A1Reduce GVHDRemarkable effectBiocideOrganic active ingredientsGraft versus leukaemiaHematological Diseases
Owner:THE GENERAL HOSPITAL CORP
Human-derived CD-19 chimeric antigen receptor T lymphocyte vector and application thereof
InactiveCN108823247AIncreased suicide geneImprove conversion efficiencyGenetically modified cellsMammal material medical ingredientsMalignant lymphomaT-Cell Specificity
The invention discloses a human-derived CD-19 chimeric antigen receptor T lymphocyte vector which is characterized by comprising a plasmid vector for coding single-chain variable region genes of CD19monoclonal antibodies, nucleosomal histone monoclonal antibodies and FNEDB monoclonal antibodies. The vector is a lentiviral vector. The invention further discloses application of the human-derived CD-19 chimeric antigen receptor T lymphocyte vector in preparation of CATR cells. The vector is used for transduction of T lymphocytes, natural killer cells and mononuclear cells, and is applied to treating tumors, preferably treating reoccurrence in malignant lymphoma drugs as well as neoplastic hematologic disorders and lymphomas which are refractory and unsuccessful in bone marrow transplantationmatching. The human-derived CD-19 chimeric antigen receptor T lymphocyte vector disclosed by the invention has the beneficial effects that the vector constructed in the invention can achieve the effects of enhancing the targeted CD19 antigen transgenic T lymphocyte specificity, overcoming the tumor microenvironment inhibited immune cellular function and controlling cytokine storm in clinic treatment.
Owner:山东省医学科学院附属医院 +1
Anti-cancer effects of JAK2 inhibitors in combination with thalidomide derivatives and glucocorticoids
ActiveUS10363260B2Organic active ingredientsPharmaceutical delivery mechanismGlucocorticoidCancer treatment
Owner:ONCOTRACKER INC
Method for leukaemia high-flux medicine screening
PendingCN111118099AGood curative effectLittle side effectsMicrobiological testing/measurementMaterial analysisOncologyChemo therapy
The invention provides a method for leukaemia high-flux medicine screening. The method comprises the following steps of performing separation, extraction, identification and culture on neoplastic hematologic disorder stem cells: performing acquisition on bone marrow samples, performing separation on mononuclear cells, performing separation on leukaemia stem cell groups through an immunomagnetic bead method; and performing subculturing on the cells after separation and purification of magnetic beads; and establishing a medicine library: according to a medicine description and pharmacokinetics,selecting the concentration of medicines, performing mixed culture on the medicines and the neoplastic hematologic disorder stem cells, and analyzing the restraining effect of the medicines on the neoplastic hematologic disorder stem cells. According to the method for leukaemia high-flux medicine screening disclosed by the invention, in accordance with a patient, an appropriate treatment scheme and opinions can be accurately given in an individualizing manner, a valuable treatment opportunity is strived for the recurrent and refractory patient, reagent risk is reduced, the medicine curative effect is increased to the maximum degree, and medicine side effects are reduced to the maximum degree; and besides, pharmaceutical combinations can be performed, in accordance with clinical frequently-used chemotherapeutics combinations, comprehensive marking is performed, and a reference is provided for a doctor in the respect of formulating clinical united medication for the patient.
Owner:HARVEST BIOTECH CO LTD
Method and reagent for improving homing and implantation rate of hematopoietic stem cells
ActiveCN108404112BIncrease usePeptide/protein ingredientsImmunological disordersDiseaseHematopoietic stem cell transplantation
The invention relates to a method of improving the homing and engraftment rate of hematopoietic stem cells and a reagent of the method. By taking small molecule peptides as a main component to performpretreatment on the hematopoietic stem cells, the method provided by the invention improves the homing and engraftment rate of the hematopoietic stem cells in vivo, shortens the time required for reconstruction of a hematopoietic system, improves the success rate of hematopoietic stem cell transplantation and makes up for the status quo of insufficient sources of the hematopoietic stem cells. Inaddition, low-dose intravenous infusion of the small molecule peptides does not have bad influence on the safety of a human body. Thus, the invention actually provides application of the small molecule peptides in preparing a drug for treating diseases of a blood system.
Owner:BEIJING DAXI BIOTECHNOLOGY CO LTD
Method of improving homing and engraftment rate of hematopoietic stem cells and reagent of method
ActiveCN108404112AEnhance response effectIncrease homing and implantation efficiencyPeptide/protein ingredientsImmunological disordersDrugPeptide
The invention relates to a method of improving the homing and engraftment rate of hematopoietic stem cells and a reagent of the method. By taking small molecule peptides as a main component to performpretreatment on the hematopoietic stem cells, the method provided by the invention improves the homing and engraftment rate of the hematopoietic stem cells in vivo, shortens the time required for reconstruction of a hematopoietic system, improves the success rate of hematopoietic stem cell transplantation and makes up for the status quo of insufficient sources of the hematopoietic stem cells. Inaddition, low-dose intravenous infusion of the small molecule peptides does not have bad influence on the safety of a human body. Thus, the invention actually provides application of the small molecule peptides in preparing a drug for treating diseases of a blood system.
Owner:BEIJING DAXI BIOTECHNOLOGY CO LTD
Antibody-drug conjugate of targeted CD45 and preparation and application thereof
PendingCN111617261AReduce the burden onLittle side effectsAntiviralsPharmaceutical non-active ingredientsDrug conjugationAntiendomysial antibodies
The invention discloses an antibody-drug conjugate of targeted CD45 and preparation and application thereof. By coupling a CD45 antibody with a drug, the targeted CD45 is used for highly selectively eliminating normal, diseased and cancerous immune cells. The antibody-drug conjugate of the targeted CD45 can be used for eliminating diseased or cancerous immune cells, replacing chemotherapeutic drugs and treating blood system diseases. The antibody-drug conjugate of the targeted CD45 only aims at the immune cells and cannot kill other active division cells, and the targeted elimination of an immune system can avoid graft-versus-host disease (GVHD), so that a large number of donor mature immune cells can be transplanted at the same time, and the immune system is quickly established.
Owner:乾元康安(苏州)生物科技有限公司
PCR (Polymerase Chain Reaction) primer group for detecting human herpes virus 6B type in human body fluid and droplet type digital PCR kit thereof
PendingCN114807452ARich in genetic informationReduce background noiseMicrobiological testing/measurementMicroorganism based processesDiseaseConserved sequence
The invention discloses a PCR (Polymerase Chain Reaction) primer group for detecting human herpes virus type 6B in human body fluid and a droplet type digital PCR kit thereof. A specific primer and an MGB hydrolysis probe are designed on the basis of a conserved sequence of a human herpes virus 6B genome, so that the microdroplet type digital PCR kit is obtained, free DNA in body fluid of a patient suffering from blood system diseases after being treated by chimeric antigen receptor T cells is extracted, HHV-6B DNA is detected through the microdroplet type digital PCR kit, and the detection sensitivity is high. The method has important guiding significance for assisting clinical understanding of HHV-6B infection conditions, identification of infection of other pathogens and identification of CAR-T cell-related encephalopathy syndromes.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Therapy with cells from human placenta and hematopoietic cells
Provided herein are methods of treatment comprising administering to a subject, e.g., a human subject, mononuclear cells from human placental perfusate and hematopoietic cells, and compositions comprising them, and their uses to establish chimerism, engraft tissue (e.g., blood), reduce the severity or duration of graft versus host disease, and treat or ameliorate symptoms of metabolic disorders and hematologic disorders, such as hematologic malignancies.
Owner:ANTHROGENESIS LLC
A safe 3D printing device with platform cleaning function
ActiveCN108527865BClear fastImprove cleanlinessAdditive manufacturing apparatus3D object support structuresComputer printingLiquid storage tank
The invention relates to a safe 3D printing device with a platform cleaning function, comprising an operation box, a cleaning box, a working box, a screen, a power device and a cleaning mechanism, a driving mechanism is arranged in the cleaning box, and an ash collection box is arranged in the operation box , the cleaning mechanism includes a motor, a screw, a nut, a moving plate, a liquid storage tank, a liquid inlet pipe and a liquid discharge pipe, the driving mechanism includes a compression pipe, a filter unit and a moving unit, and the filter unit includes a filter pipe, an air inlet pipe and an exhaust pipe , the safe 3D printing equipment with platform cleaning function can remove the dust or raw material particles attached to the support platform through the cleaning mechanism, so as to avoid excessive accumulation of dust or raw material particles and cause warping at the bottom of the product. Not only that, through The driving mechanism, the 3D printer can filter out the toxic ultrafine particles released during operation, and prevent the excessive accumulation of toxic ultrafine particles from causing lung diseases and blood system diseases.
Owner:杨锐
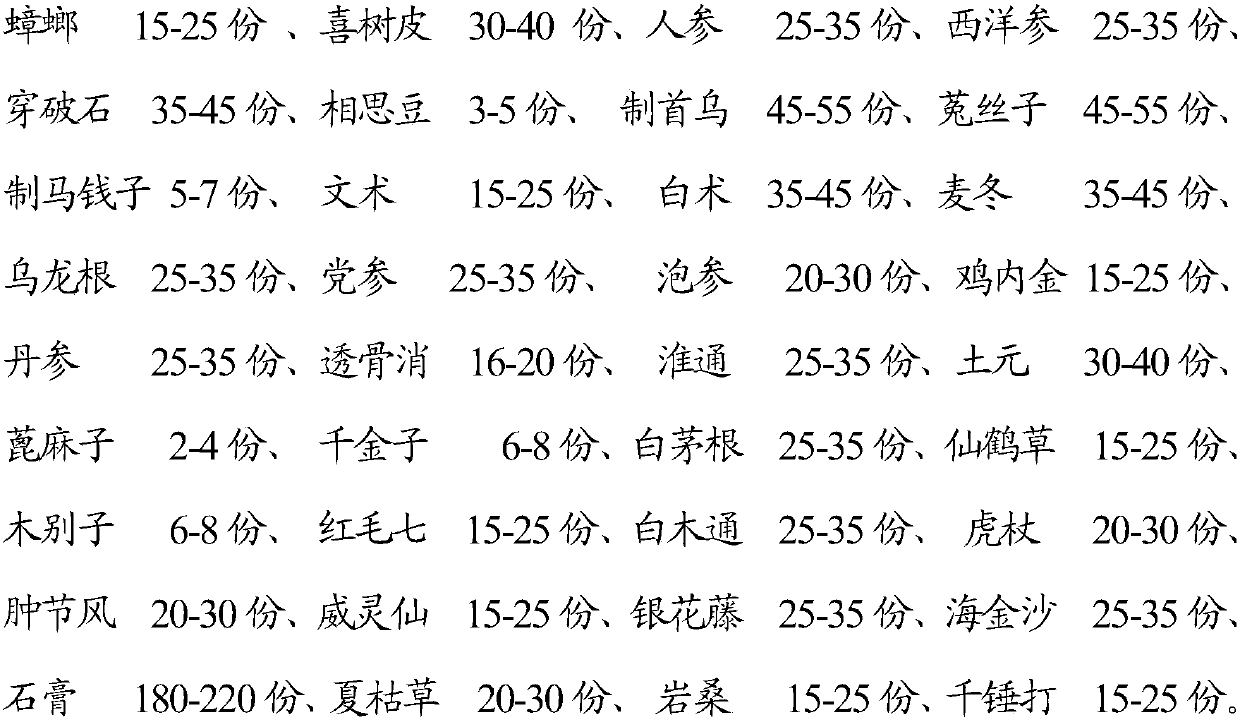
Traditional Chinese medicine composition used for treating malignant diseases of blood system and application
InactiveCN107913373AEfficient killingRelieve bleedingAnthropod material medical ingredientsPteridophyta/filicophyta medical ingredientsSalvia miltiorrhizaMorus australis
The invention discloses a traditional Chinese medicine composition used for treating malignant diseases of the blood system, containing the following 36 components in total: 10-30 parts of cockroach,25-45 parts of camptotheca acuminata decne, 2-6 parts of jequirity bean, 20-40 parts of Wulonggen, 20-40 parts of salvia miltiorrhiza, 25-45 parts of eupolyphaga, 2-5 parts of castor bean, 20-40 partsof lalang grass rhizome, 10-30 parts of hairyvein agrimony, 10-30 parts of caulophyllum robustum, 15-35 parts of glabrous sarcandra herb, 160-240 parts of gypsum, 15-35 parts of prunella vulgaris, 10-30 parts of morus australis, and the like. The composition is applied to malignant diseases of the blood system, can effectively kill cancer cells, and can achieve the clinical cure criterion, and disease cannot relapse after long-term follow-up.
Owner:袁仕忠
Application of targeting STAT1 inhibitor in treatment of immune thrombocytopenia and method
PendingCN113679843ANo radioactivityImprove bioavailabilityImmunological disordersBlood disorderDiseasePhosphorylation Inhibition
The invention discloses application of a targeting STAT1 inhibitor in treatment of immune thrombocytopenia and a method. An STAT1 nuclear inhibitor ivermectin and an STAT1 activation inhibitor fludarabine are adopted, and the number of platelets of an immune thrombocytopenia mouse model is increased by injecting the activation inhibitor or the nuclear inhibitor. In the abovementioned way, the fludarabine is a fluorinated nucleotide analogue of arabinoside, is free of radioactivity and is a small-molecule phosphorylation inhibitor; meanwhile, the ivermectin is a small-molecule inhibitor introduced by an input protein alpha / beta 1 mediated cell nucleus, the ivermectin and the small-molecule inhibitor are high in bioavailability, are widely applied to treatment of various blood system diseases, are good in safety, are applied to treatment of the immune thrombocytopenia and are safe, effective and high in compliance.
Owner:SUZHOU UNIV
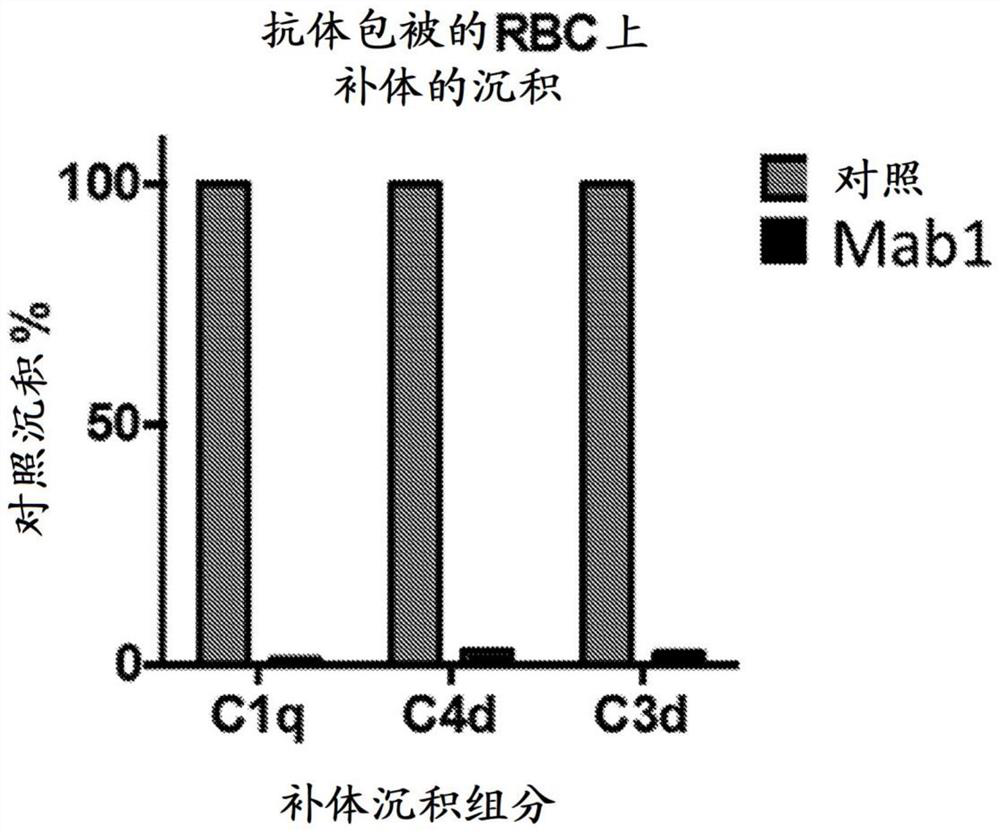
Compositions and methods for treating hemological disorders
PendingCN114746118APreserve complement activityPrevent Blood DisordersImmunoglobulins against animals/humansAntibody ingredientsPhospholipid antibodyDisease
The present disclosure is generally directed to methods of preventing, reducing the risk of developing, or treating a blood disorder (e.g., hematologic disorders, etc. Condensate hemolytic anemia (condensate disease), cold antibody hemolytic anemia, ABO incompatible acute hemolytic reaction, thermal agglutinin hemolytic anemia, warm antibody hemolytic anemia, warm antibody autoimmune hemolytic anemia (WAIHA), autoimmune hemolytic anemia (AIHA), autoimmune thrombocytopenia, antiphospholipid syndrome, Evans syndrome, and the like. Red blood cell isoimmunity, Verpet syndrome, neonatal isoimmune thrombocytopenia, heparin-induced thrombocytopenia (HIT), heparin-induced thrombocytopenia and thrombus (HITT), thrombotic thrombocytopenic purpura (TTP), immune thrombocytopenic purpura (ITP), thrombocytopenia, thrombus, vasculitis, lupus nephritis, systemic lupus erythematosus (SEE), and the like. The present invention relates to methods for treating a subject (e.g., glomerulonephritis, anti-phospholipid antibody syndrome (APS), infection or drug-induced hematological disorder) comprising administering to the subject an inhibitor of the complement pathway.
Owner:ANNEXON
Anti-cancer effects of jak2 inhibitors in combination with thalidomide derivatives and glucocorticoids
ActiveUS20200030343A1Organic active ingredientsPharmaceutical delivery mechanismAnticarcinogenic EffectDisease
The present invention provides methods of treatment for hematological malignancies involving synergistic combination of a JAK2 inhibitor and a glucocorticoid or a JAK2 inhibitor, thalidomide or thalidomide derivative, and a glucocorticoid. The compositions and methods provide an unexpected efficacy in the treatment for hematological disorders. The hematological disorders treated by the current invention include multiple myeloma, and may also include hematological disorders that are refractory to prior cancer treatments, or a relapsed hematologic disorder.
Owner:ONCOTRACKER INC
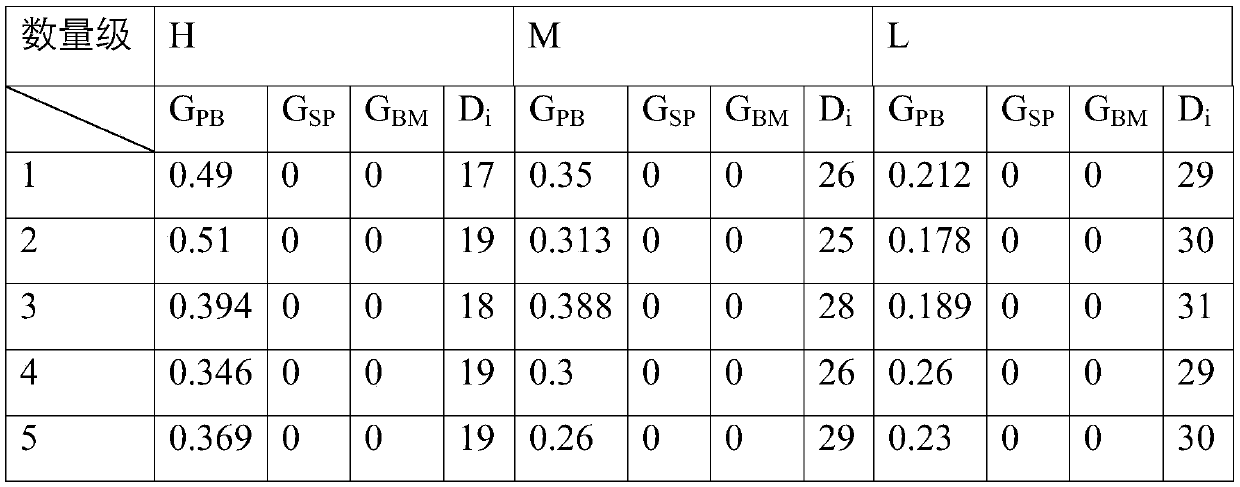
A method for evaluating the degree of immunodeficiency in an immunodeficiency mouse model
ActiveCN105994125BThe degree of immunodeficiency can be quantifiedArtificial cell constructsVertebrate cellsMathematical modelImmunodeficient mouse model
The invention relates to the field of biology, and discloses a method for evaluating immunodeficiency degree of an immunodeficient mice model. The method comprises: through transplanting neoplastic hematologic disorder cells and solid tumor cells in different orders of magnitudes into an immunodeficient mice model, establishing a tumor mice model, detecting the proportion of tumor cells GPB in internal and external blood of the tumor mice model, the proportion of tumor cells GBM in bone marrow, and the proportion of tumor cells GSP in spleen, according to proportion parameter indexes, evaluating the immunodeficiency degree of the immunodeficient mice model. The method is advantaged in that the method can quantitatively evaluate defect level of the immunodeficient mice, the method relates to simplify and summarize a mathematic model and a calculation method which are obtained through relations between tumor transplanting efficiency and mice immunodeficiency degree, and through scores of immunodeficiency degree, defect difference degree among the immunodeficient mice can be compared. The method is simple and feasible, and is easy to operate, and the method has good application prospect.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders
Methods of treating, preventing or managing hematologic disorders, such as leukemia are disclosed. The methods encompass the administration of SNS-595. Also provided are methods of treatment using this compound with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy. In certain embodiments, the method of treatment comprise administering SNS-595 in combination with Ara-C. Pharmaceutical compositions and single unit dosage forms suitable for use in the methods are also disclosed.
Owner:SUNESIS PHARMA INC
Medicine for treating respiratory diseases and preparation method thereof
PendingCN114010689AHigh activityGood antiviral effectAntibacterial agentsAntipyreticHematologic disordersDisease
The invention provides a medicine for treating respiratory diseases and a preparation method thereof, and relates to the technical field of pharmaceutical preparations. The medicine is prepared from radix astragali, Parnassia delavayi Franch., flos lonicerae, radix ginseng, apricot kernel and kernels. The preparation method comprises the following steps: mixing radix astragali, Parnassia delavayi Franch., flos lonicerae, radix ginseng, apricot kernel and kernelsto obtain a mixture, adding water into a mixture, carrying out vacuum distillation, and filtering to obtain the liquid medicine. The medicine disclosed by the invention has a killing effect on various pneumonia and pulmonary tuberculosis viruses, also has a relatively good treatment effect on respiratory system diseases, digestive system diseases and blood system diseases, is not easy to relapse, and has no toxic or side effect. According to the preparation method, a distillation process is adopted, so that the concentration of effective components can be improved, the medicine can better exert the medicine effect, and the treatment effect on diseases is improved.
Owner:杨棕嵌
Methods of making oligopotent and unipotent precursors
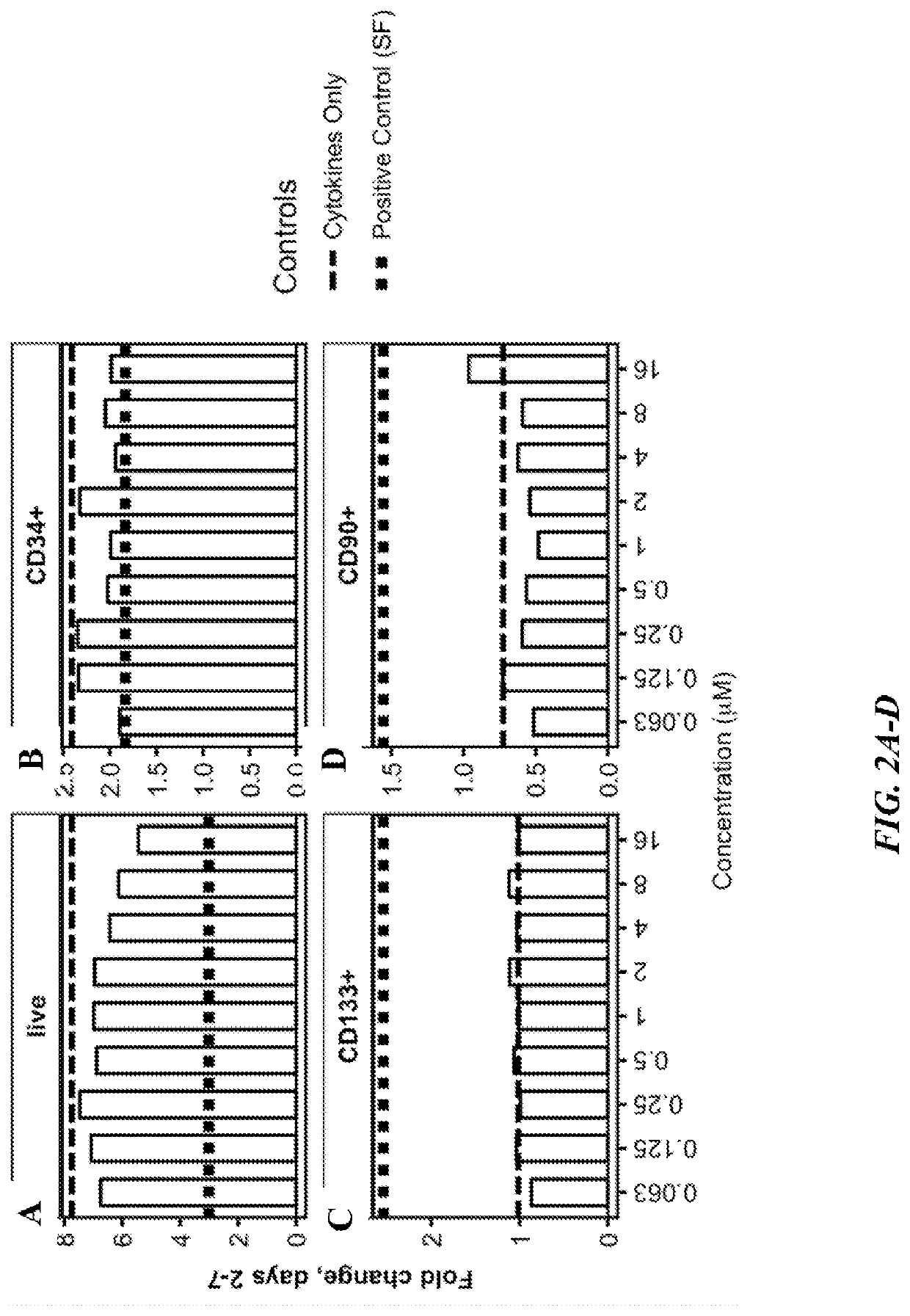
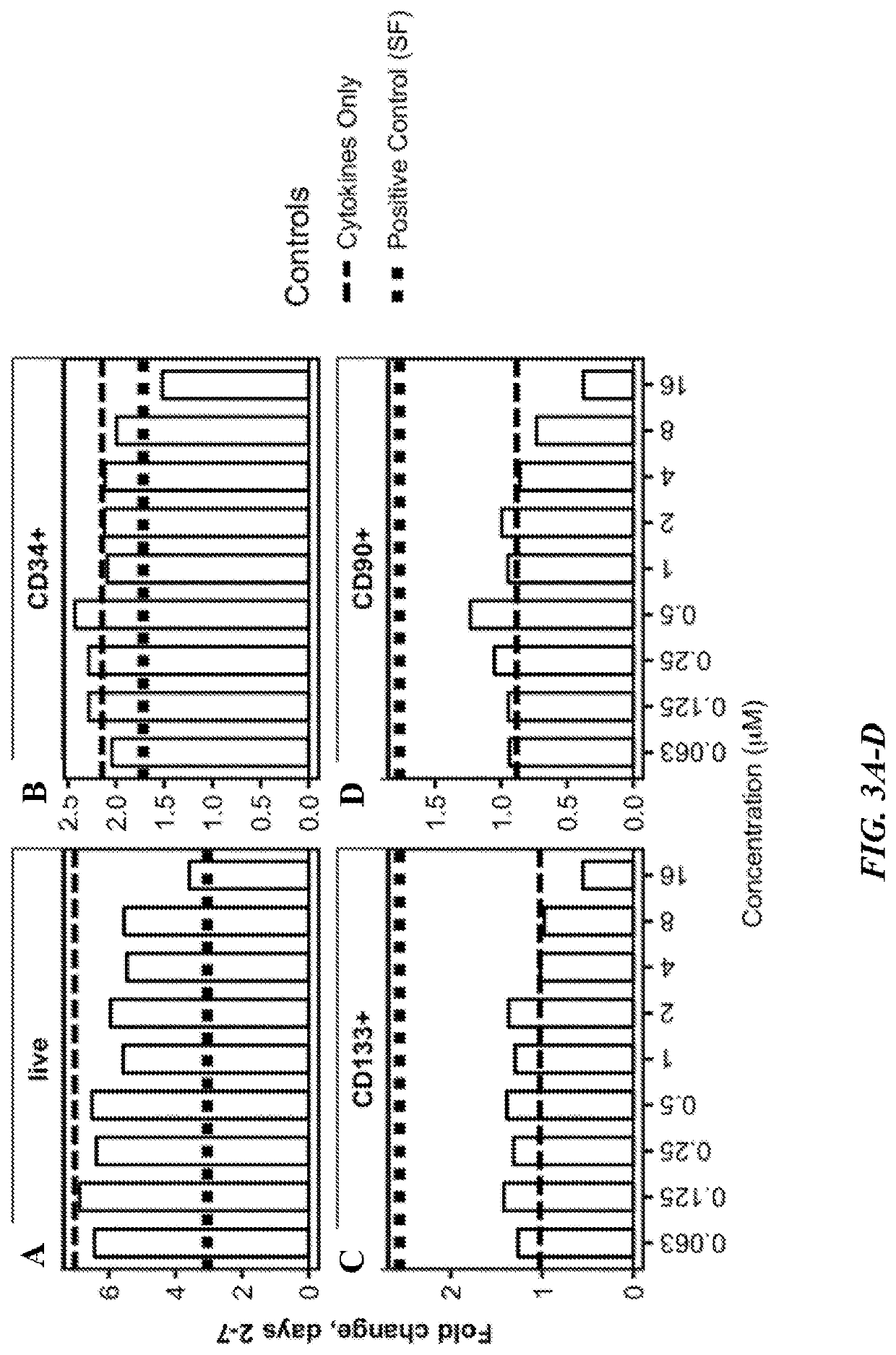
PendingUS20220315895A1Increase the number ofOrganic active ingredientsPeptide/protein ingredientsCD34Hematologic disorders
This disclosure is directed to, inter alia, methods and systems for preparing oligopotent and unipotent progenitor cells of defined lineages in culture from an expanded source of CD34+ cells, media for making the same, and therapeutic compounds and compositions comprising the same for treatment a variety of diseases included, but not limited to, hematologic disorders, immune diseases, cancers, and infectious diseases.
Owner:IMMUNEBRIDGE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Methods of using (+)-1,4-dihydro-7-[(3S,4S)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders Methods of using (+)-1,4-dihydro-7-[(3S,4S)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/408a1ac5-24ef-4a6a-983b-785f6d97d096/US20080063642A1-20080313-D00000.png)
![Methods of using (+)-1,4-dihydro-7-[(3S,4S)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders Methods of using (+)-1,4-dihydro-7-[(3S,4S)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/408a1ac5-24ef-4a6a-983b-785f6d97d096/US20080063642A1-20080313-D00001.png)
![Methods of using (+)-1,4-dihydro-7-[(3S,4S)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders Methods of using (+)-1,4-dihydro-7-[(3S,4S)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/408a1ac5-24ef-4a6a-983b-785f6d97d096/US20080063642A1-20080313-D00002.png)
![Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of antecedent hematologic disorders Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of antecedent hematologic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28a10672-fdc4-4e33-8f21-8d1ac3c58954/US20110008371A1-20110113-C00001.png)









![Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of antecedent hematologic disorders Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of antecedent hematologic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ebfed1e4-d12a-4c82-a865-593916fde792/US20130102559A1-20130425-C00001.png)

































![Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bed19261-1726-47bc-9d80-5ff125a7009c/US20150190380A1-20150709-D00001.PNG)
![Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bed19261-1726-47bc-9d80-5ff125a7009c/US20150190380A1-20150709-D00002.PNG)
![Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders Methods of using (+)-1,4-dihydro-7-[(3s,4s)-3-methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid for treatment of certain hematologic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bed19261-1726-47bc-9d80-5ff125a7009c/US20150190380A1-20150709-D00003.PNG)