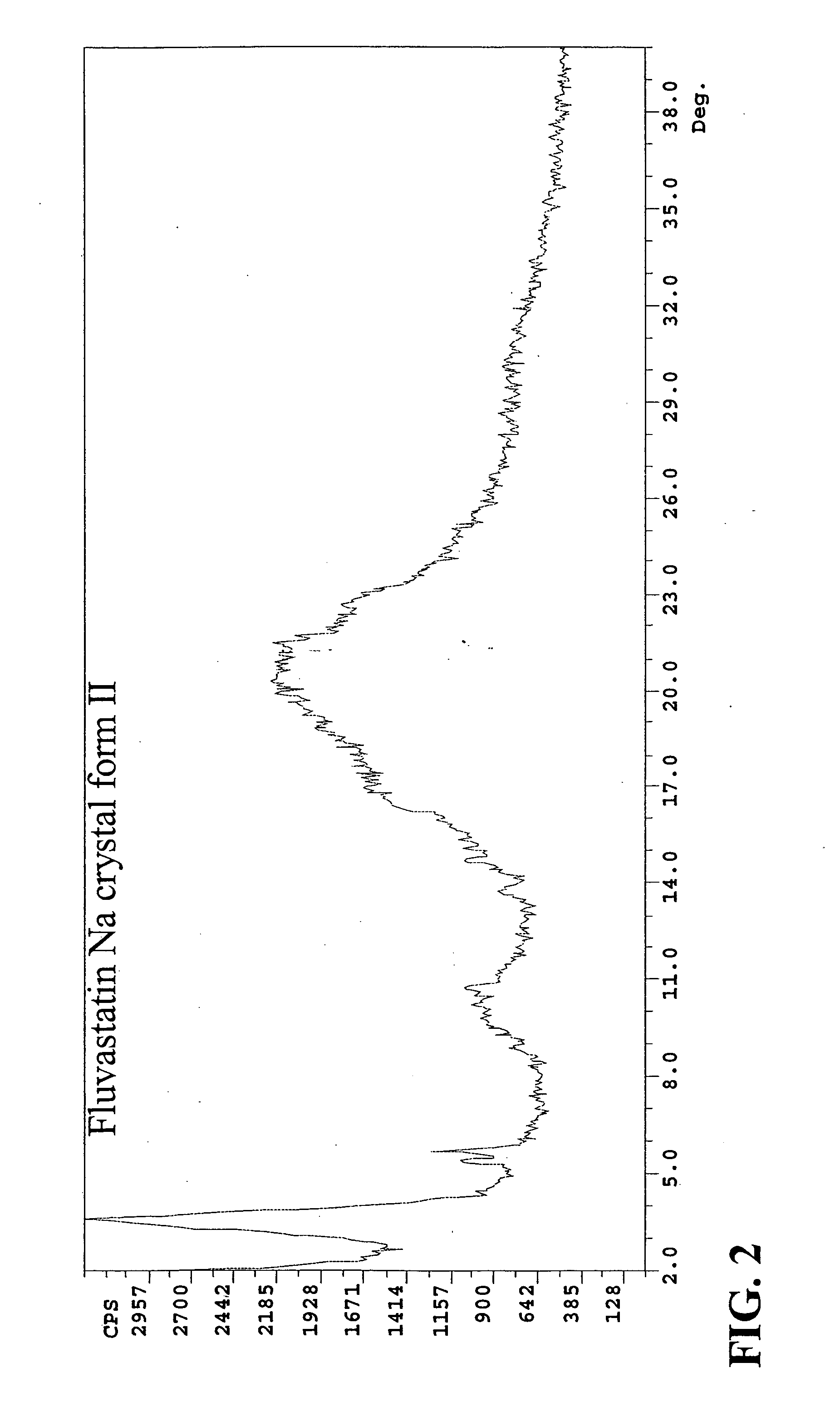
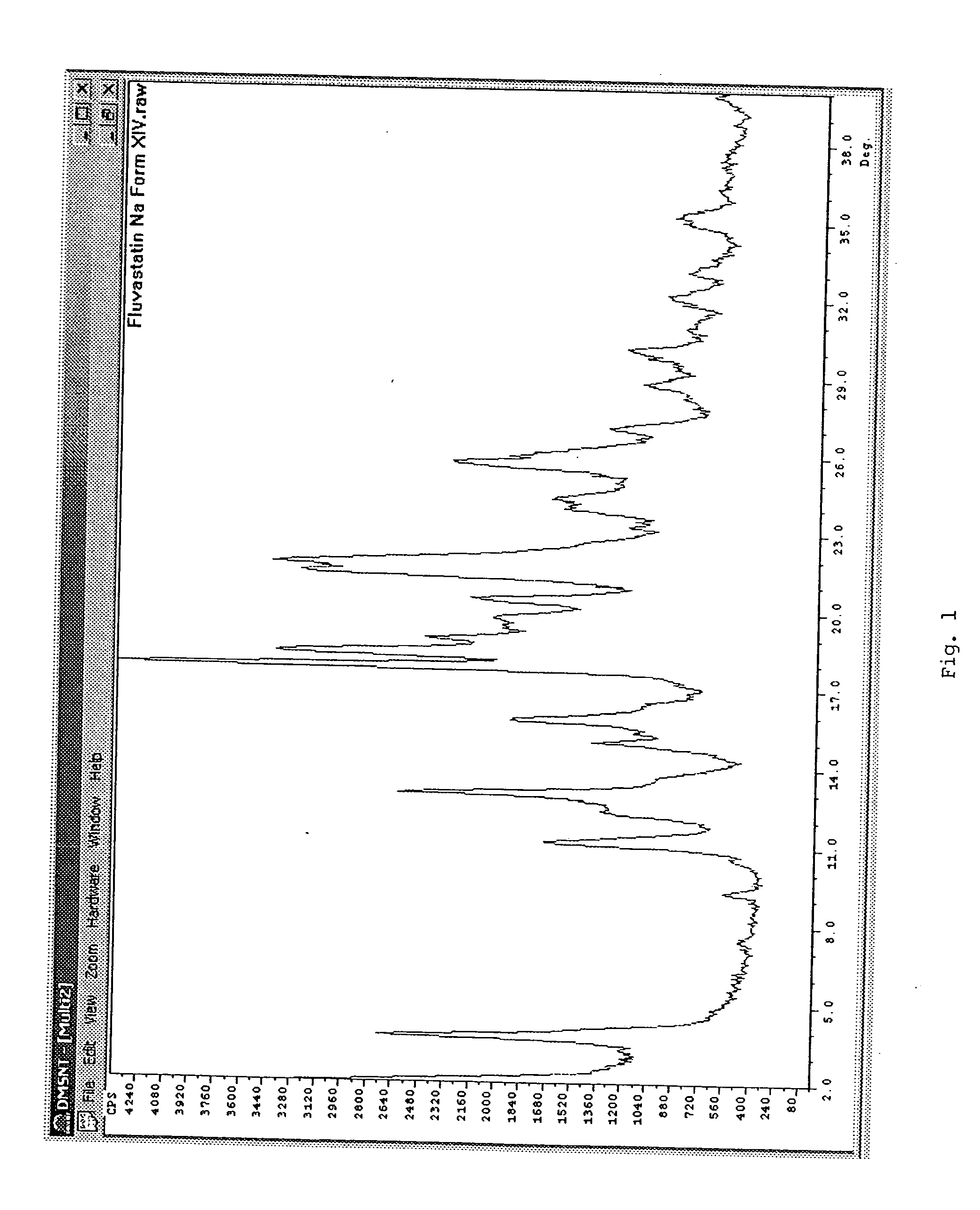
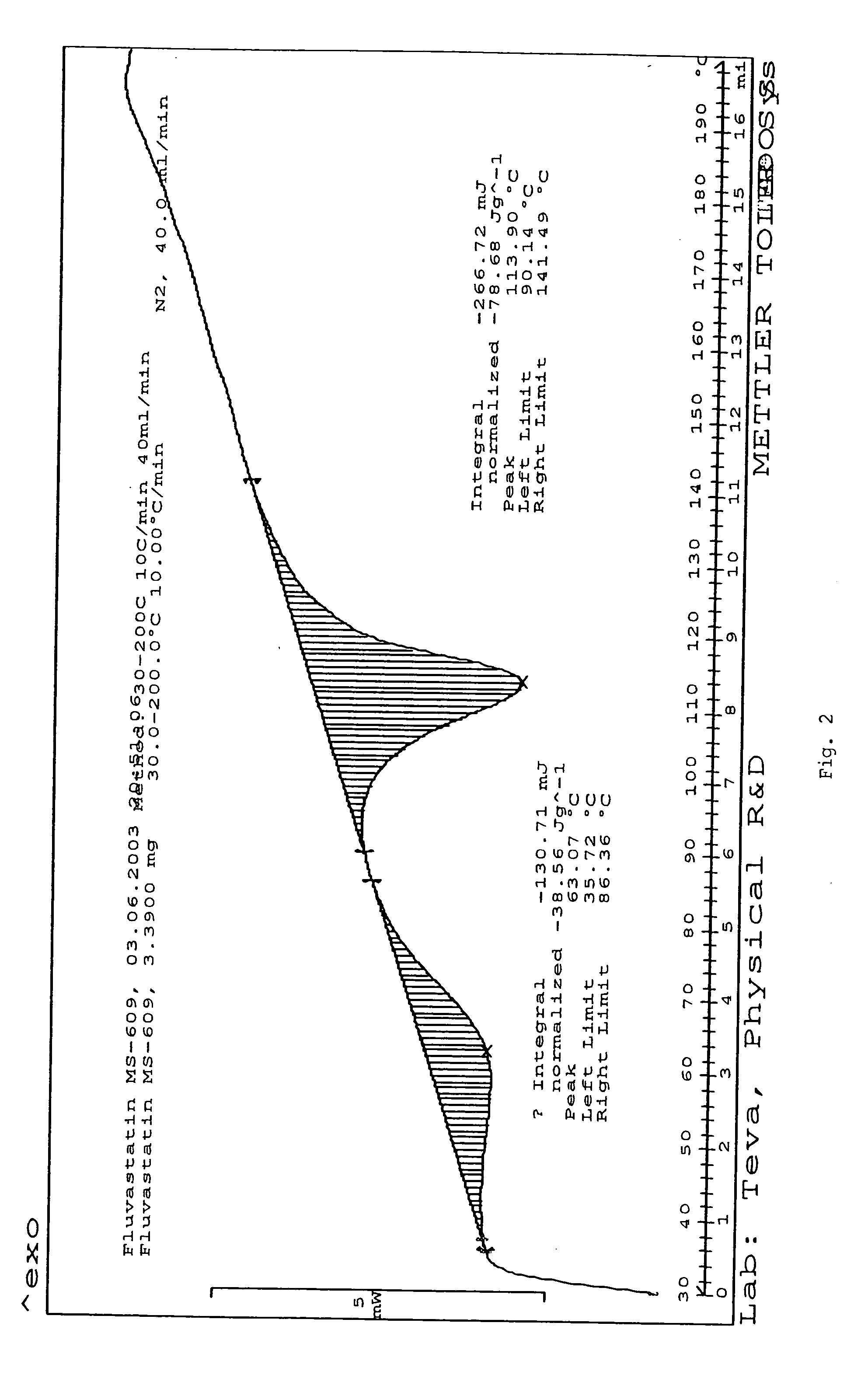
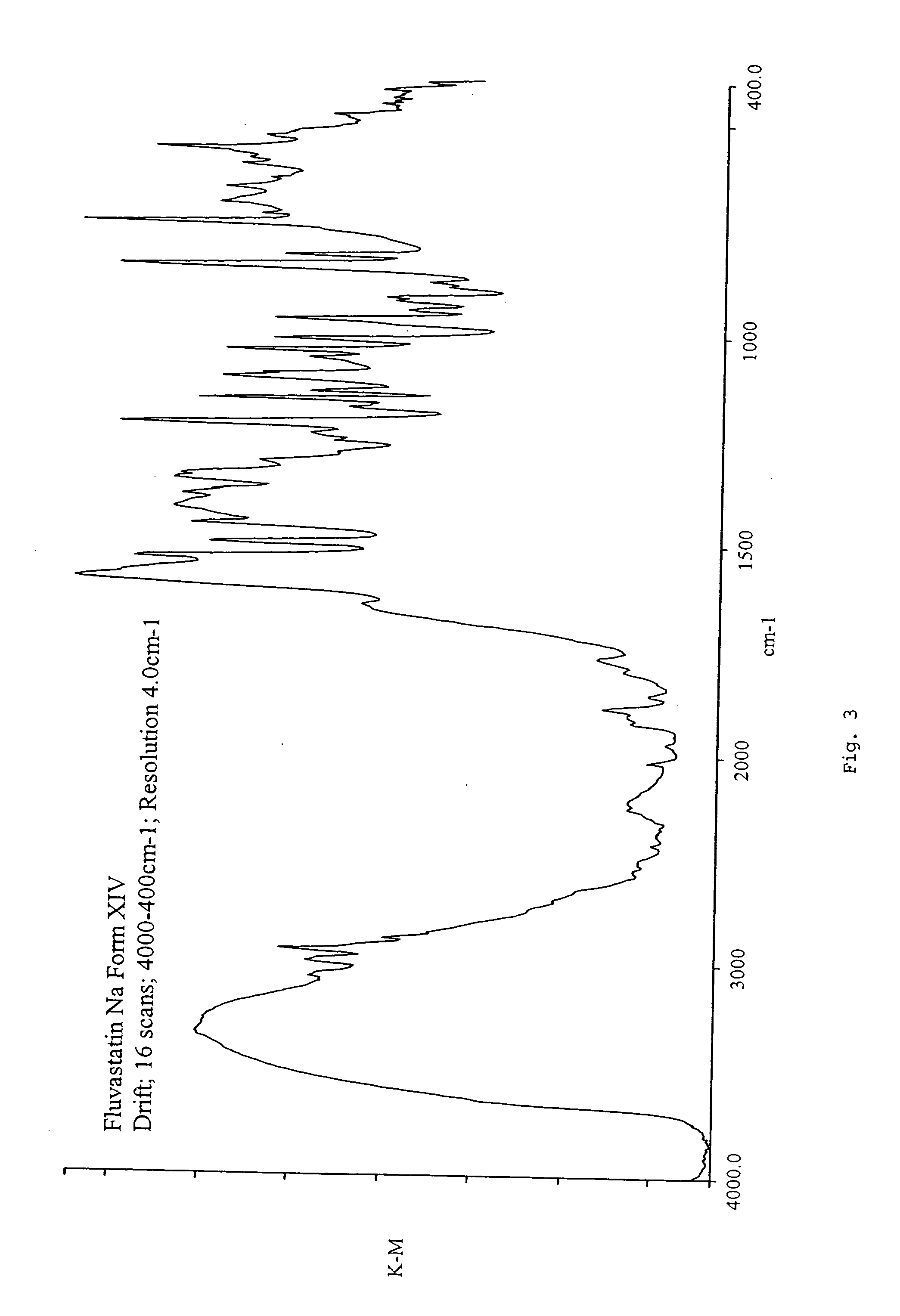
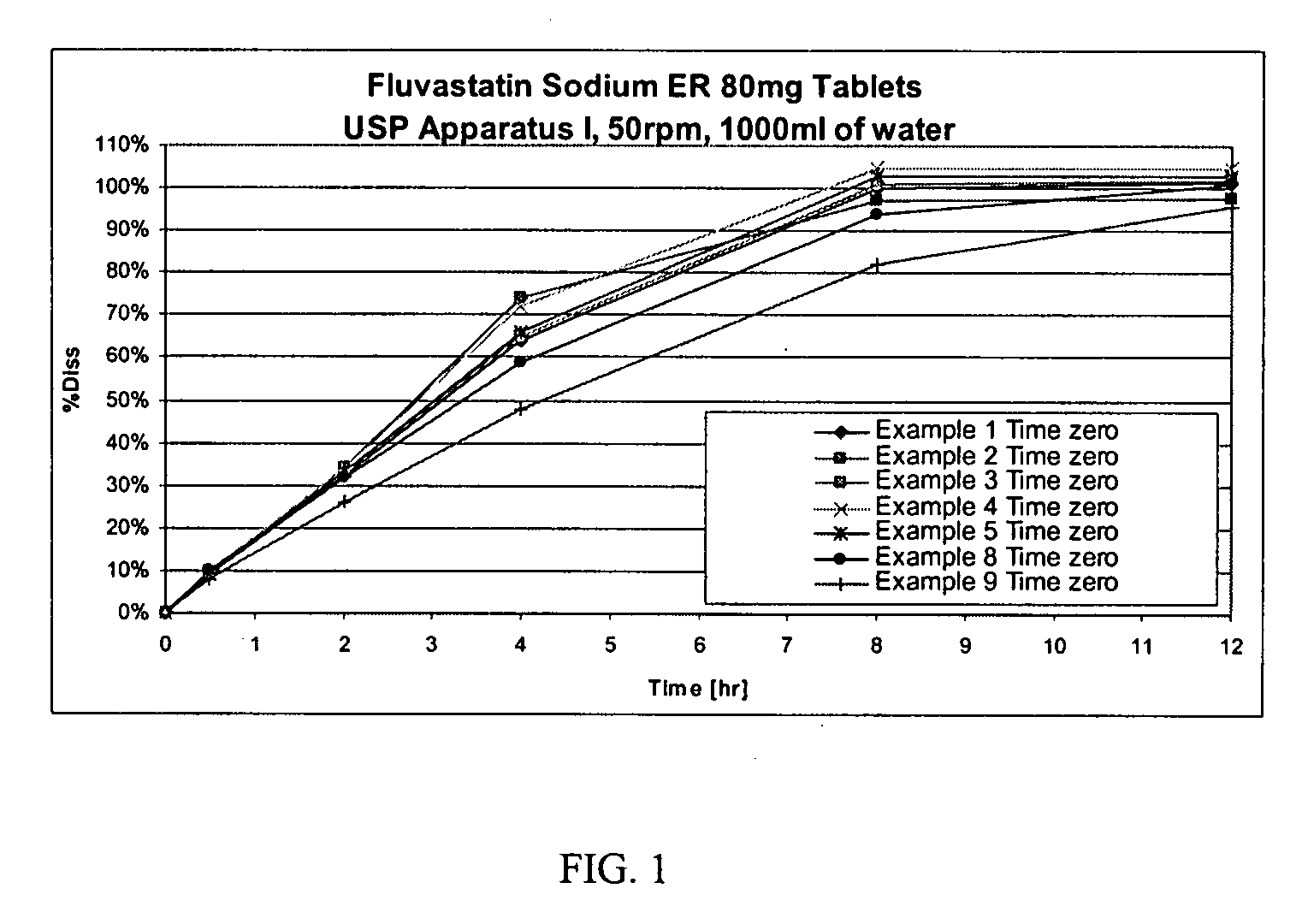
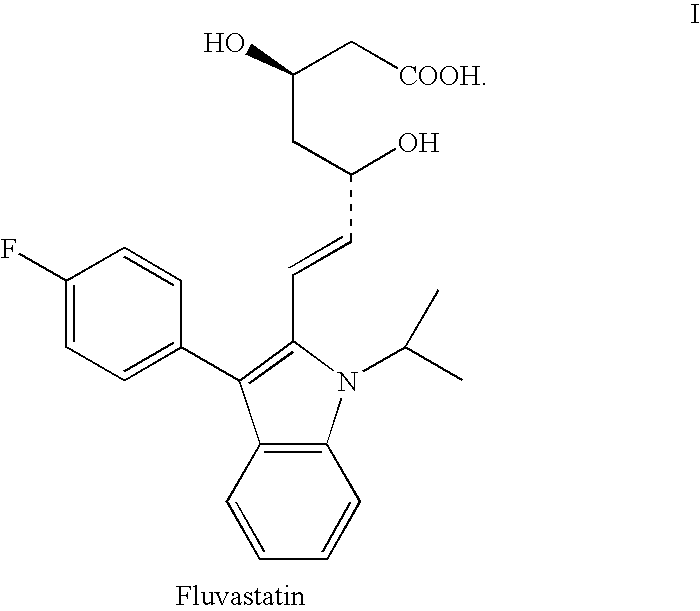
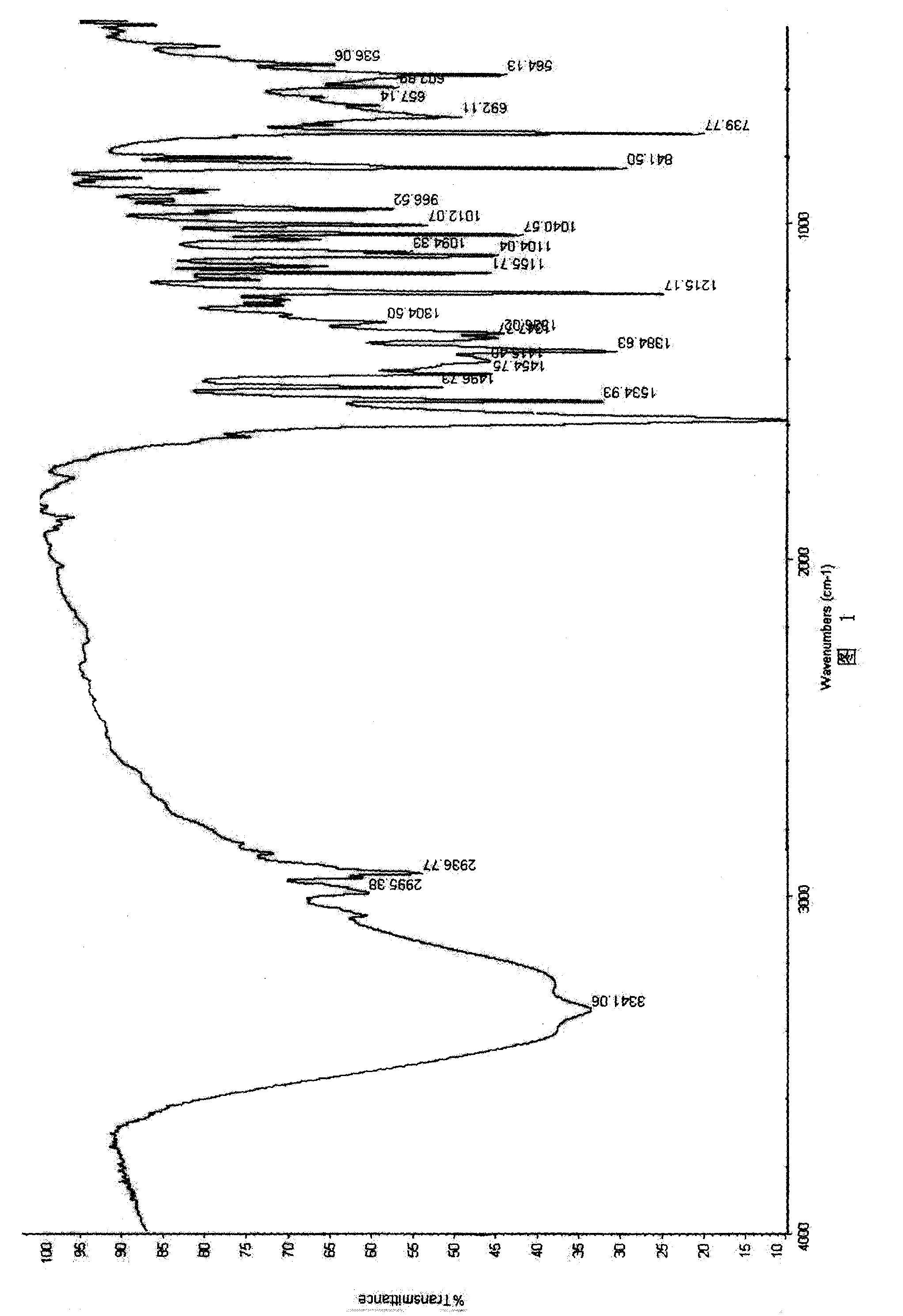
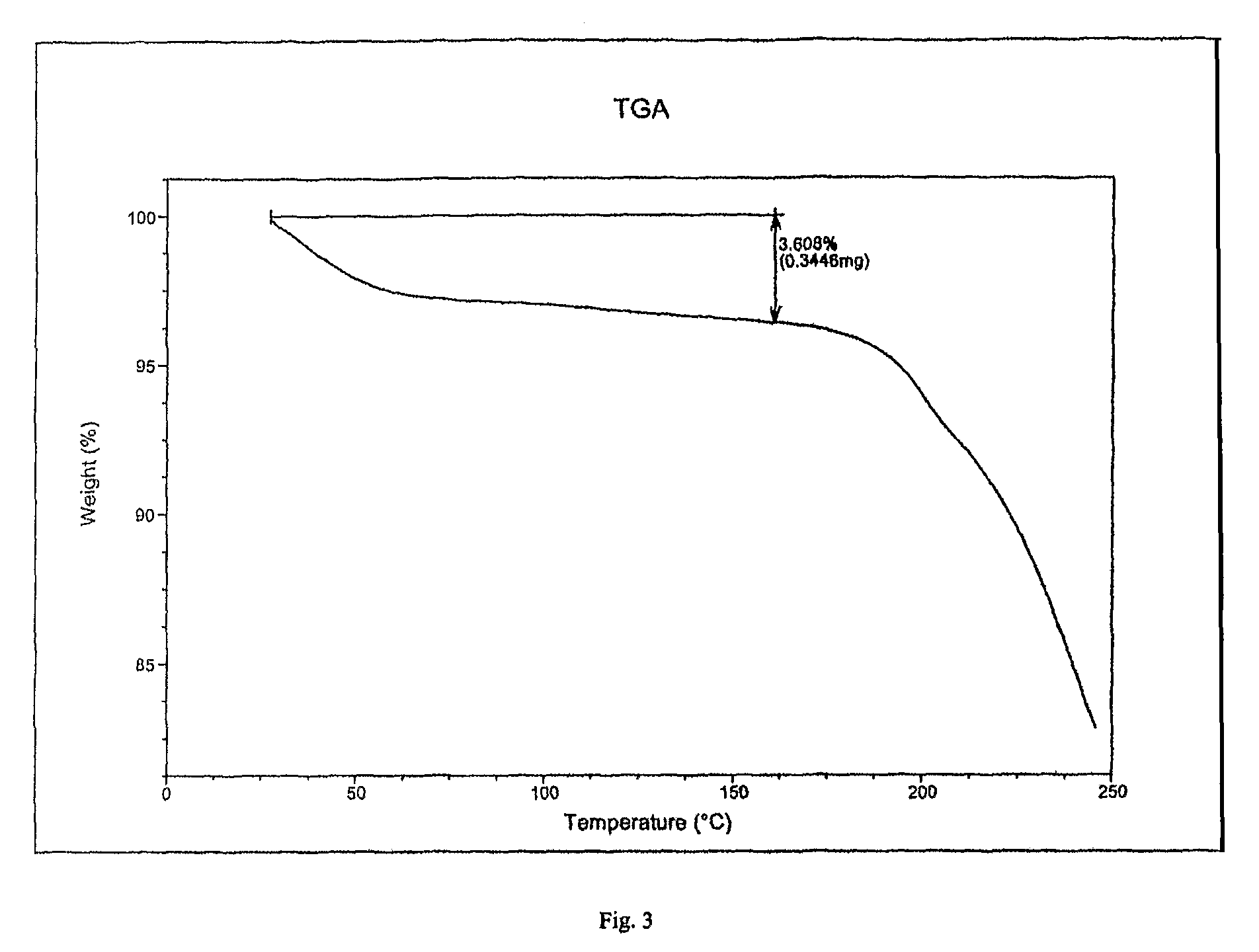
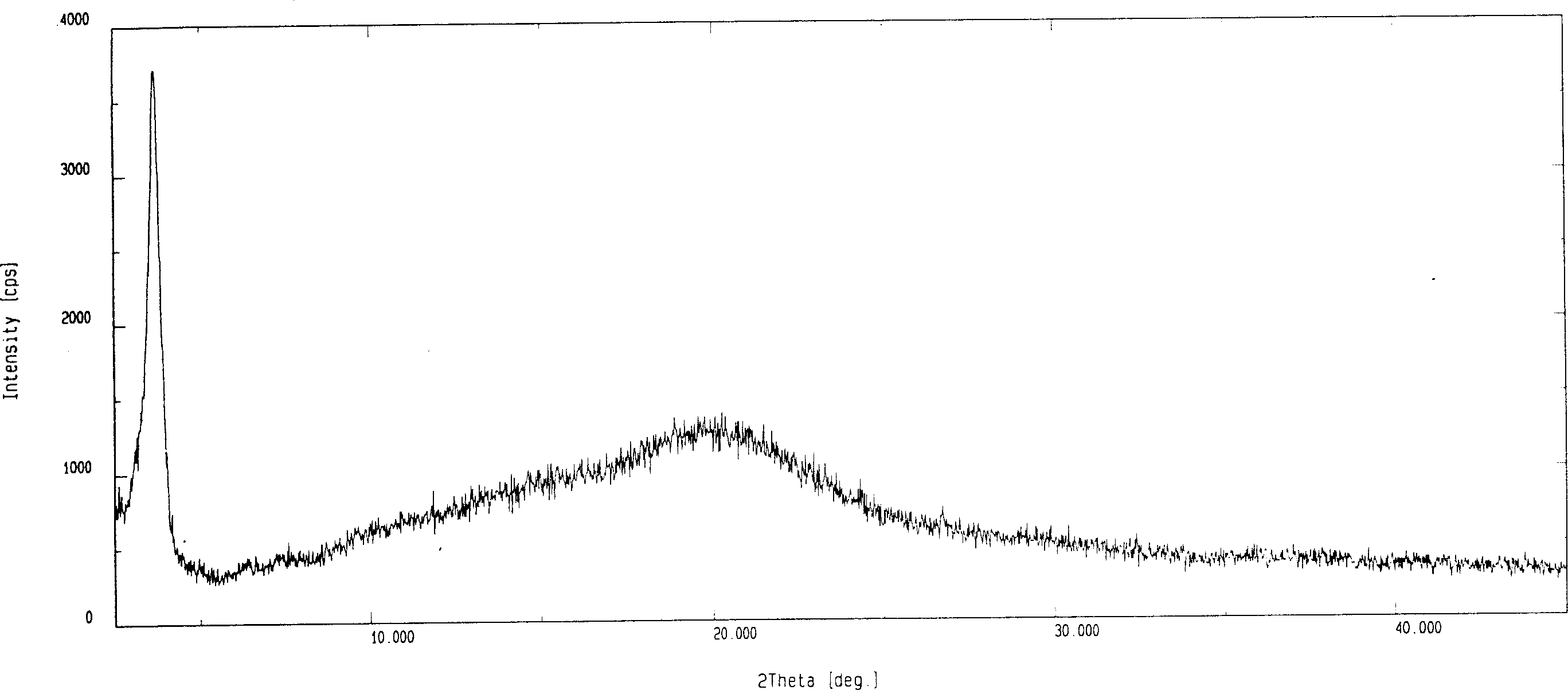
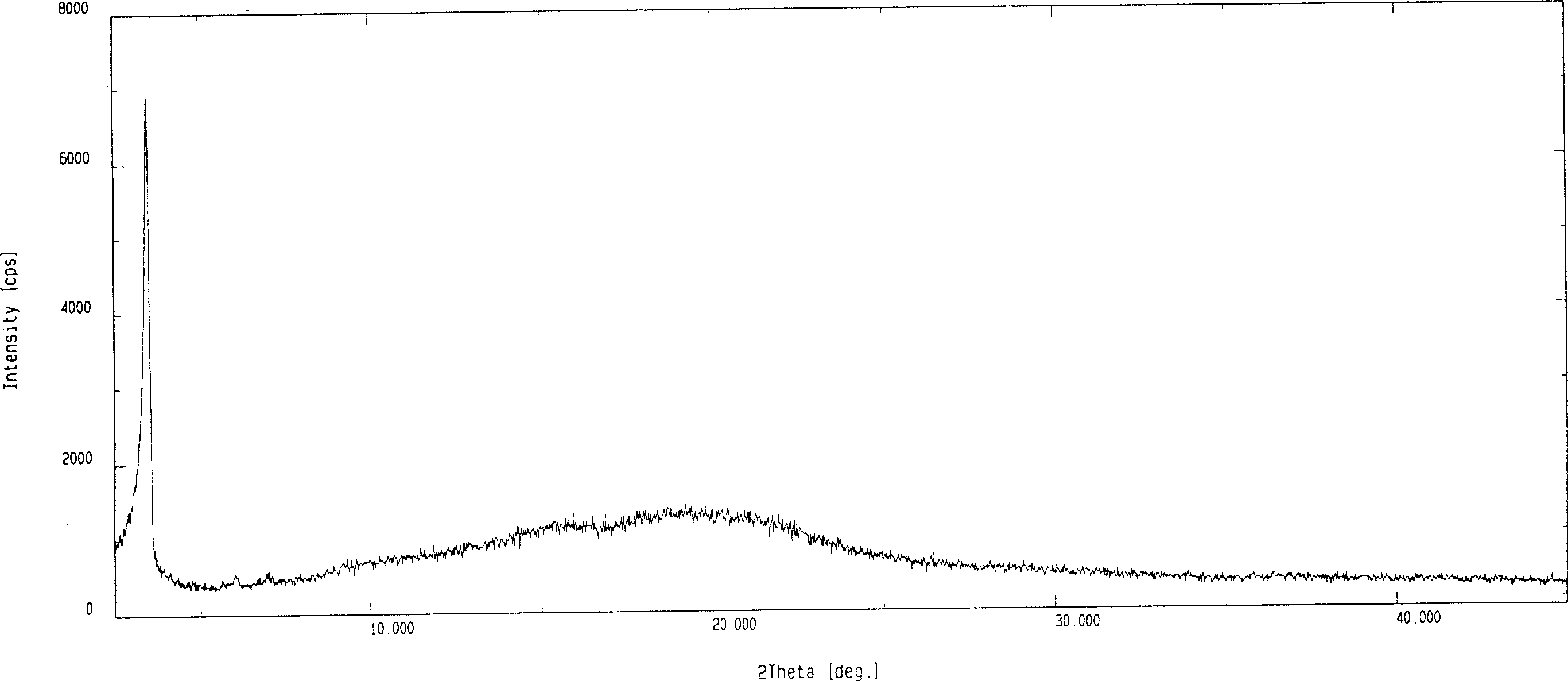
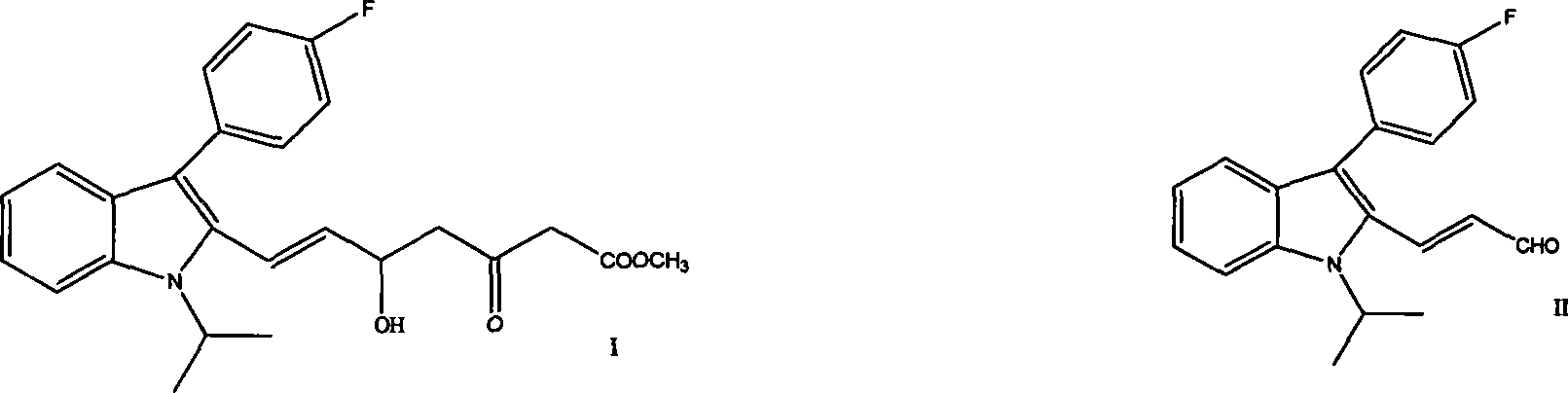Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
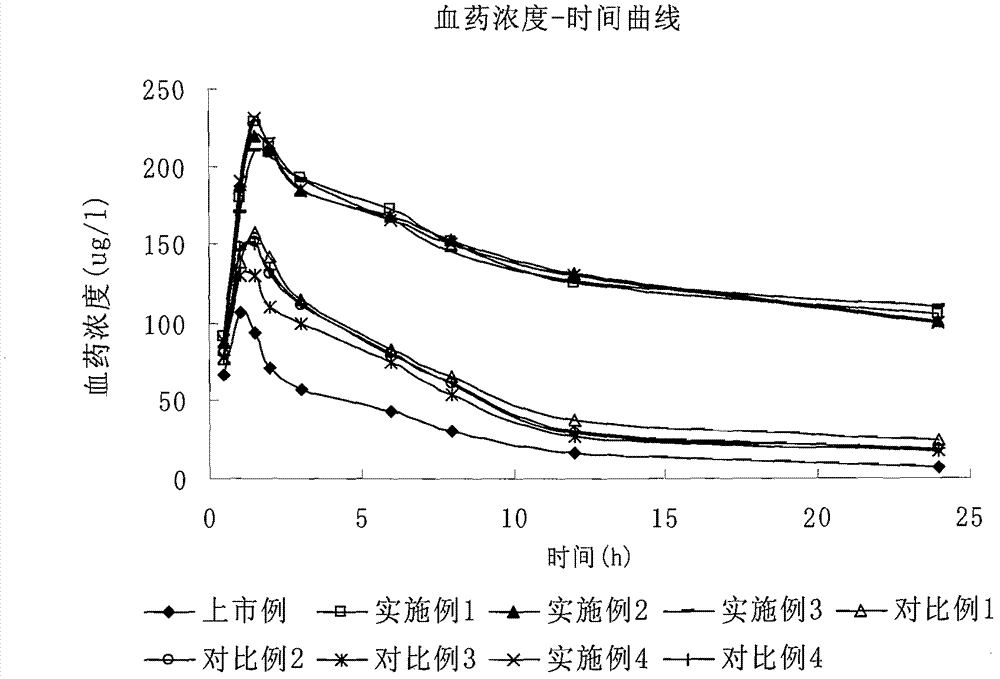
40 results about "Fluvastatin Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
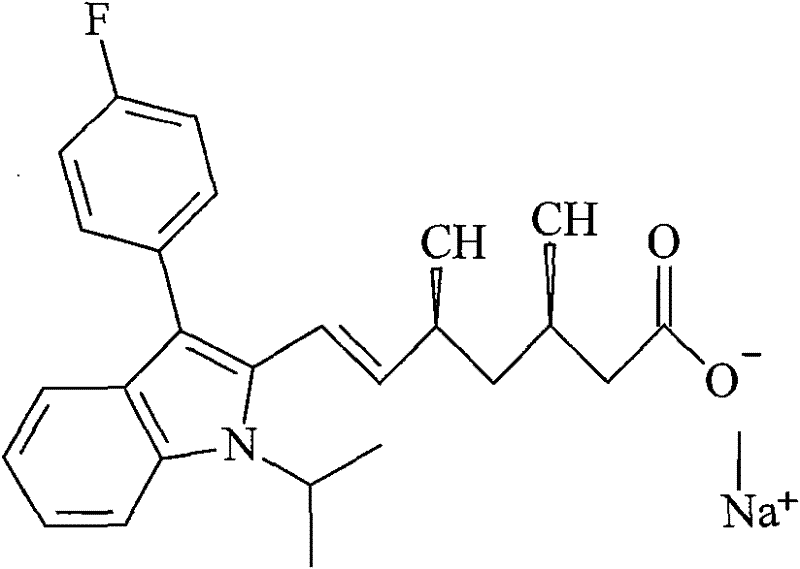
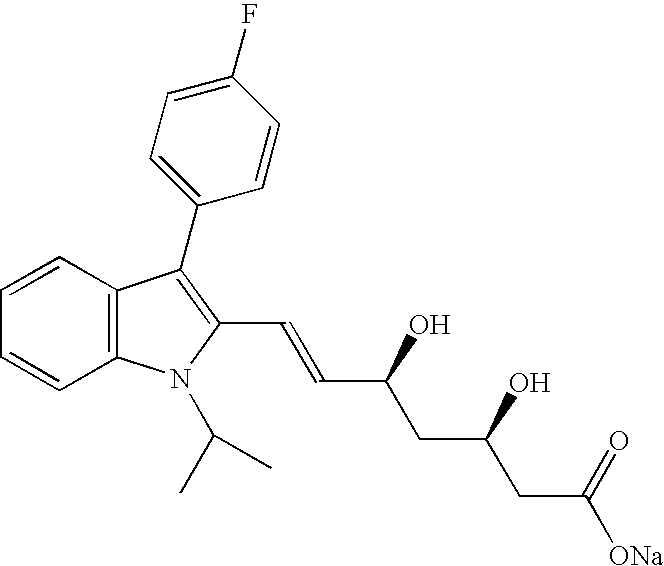
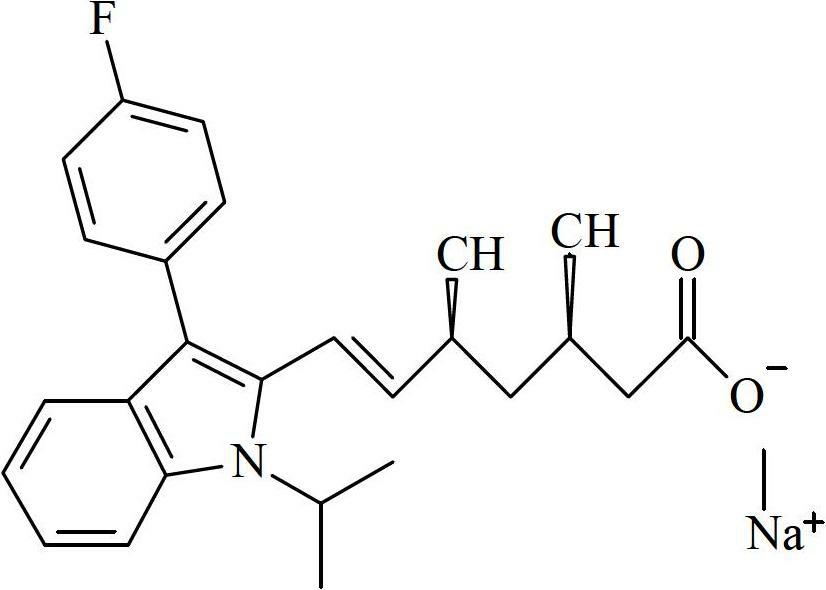
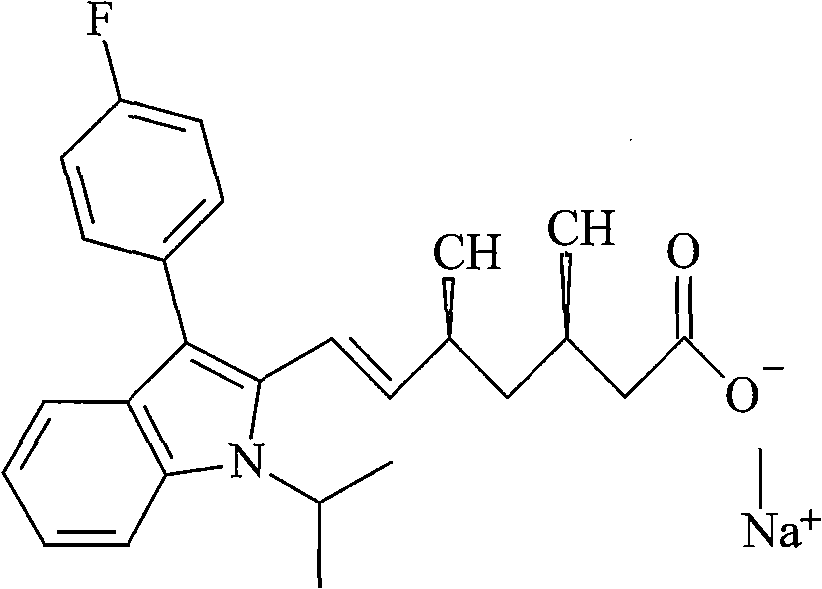
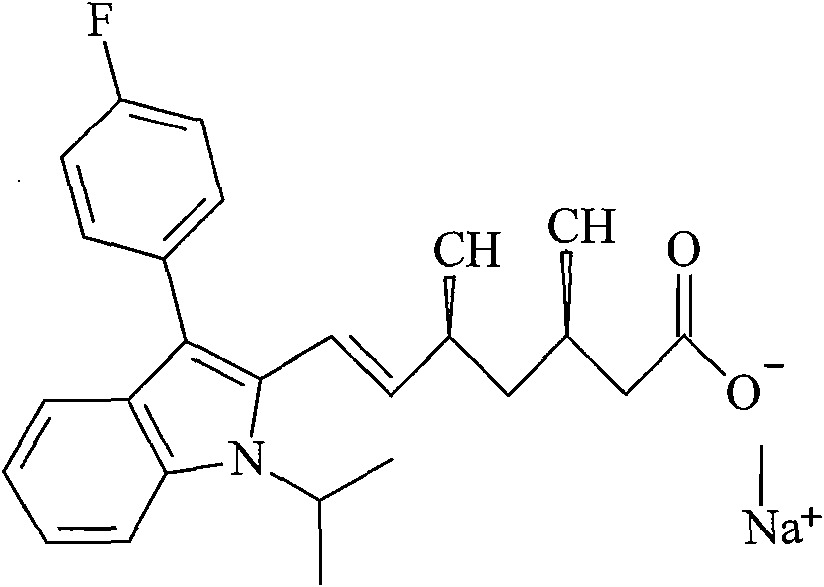
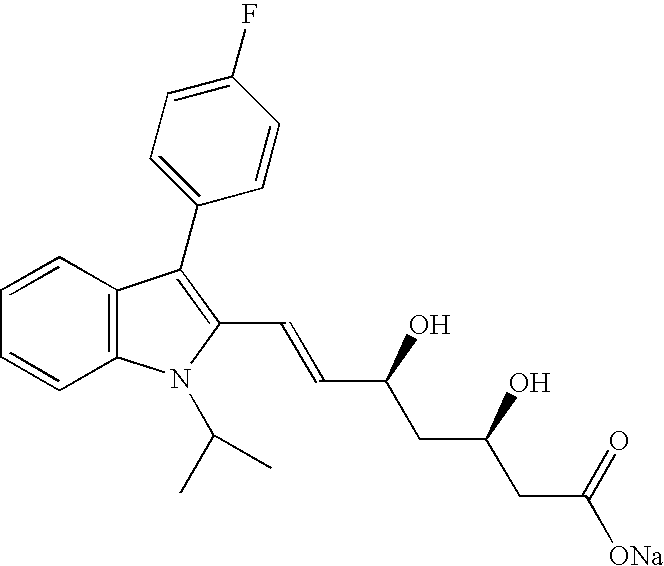
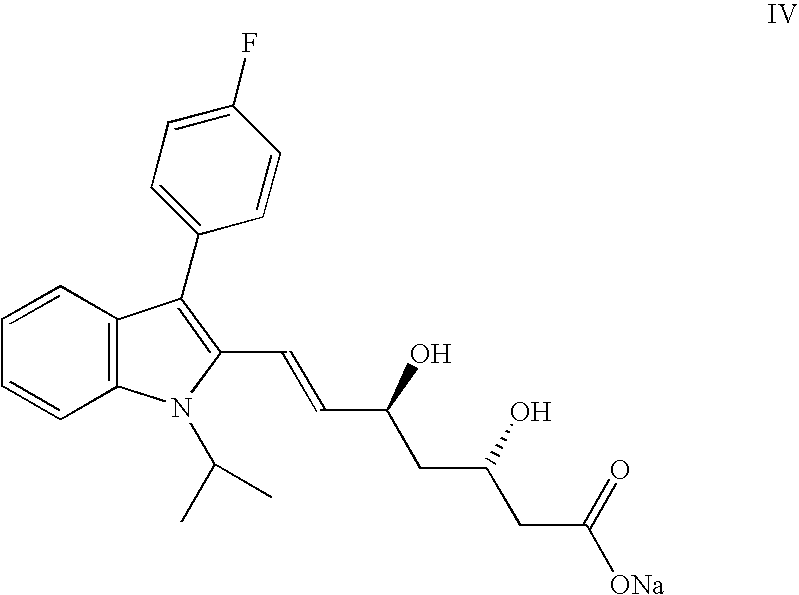
The sodium salt of a synthetic lipid-lowering agent with potential antineoplastic activity. Fluvastatin competitively inhibits hepatic 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, a key step in cholesterol synthesis. This agent lowers plasma cholesterol and lipoprotein levels, and modulates immune responses through the suppression of MHC II (major histocompatibility complex II) on interferon gamma-stimulated, antigen-presenting cells such as human vascular endothelial cells. Through the inhibition of mevalonate synthesis, statins, like fluvastatin, have been shown to inhibit the production of dolichol, geranylpyrophosphate (GPP) and farnesylpyrophosphate (FPP) and the isoprenylation of the intracellular G-proteins Ras and Rho, which may result in antiangiogenic, apoptotic, and antimetastatic effects in susceptible tumor cell populations.
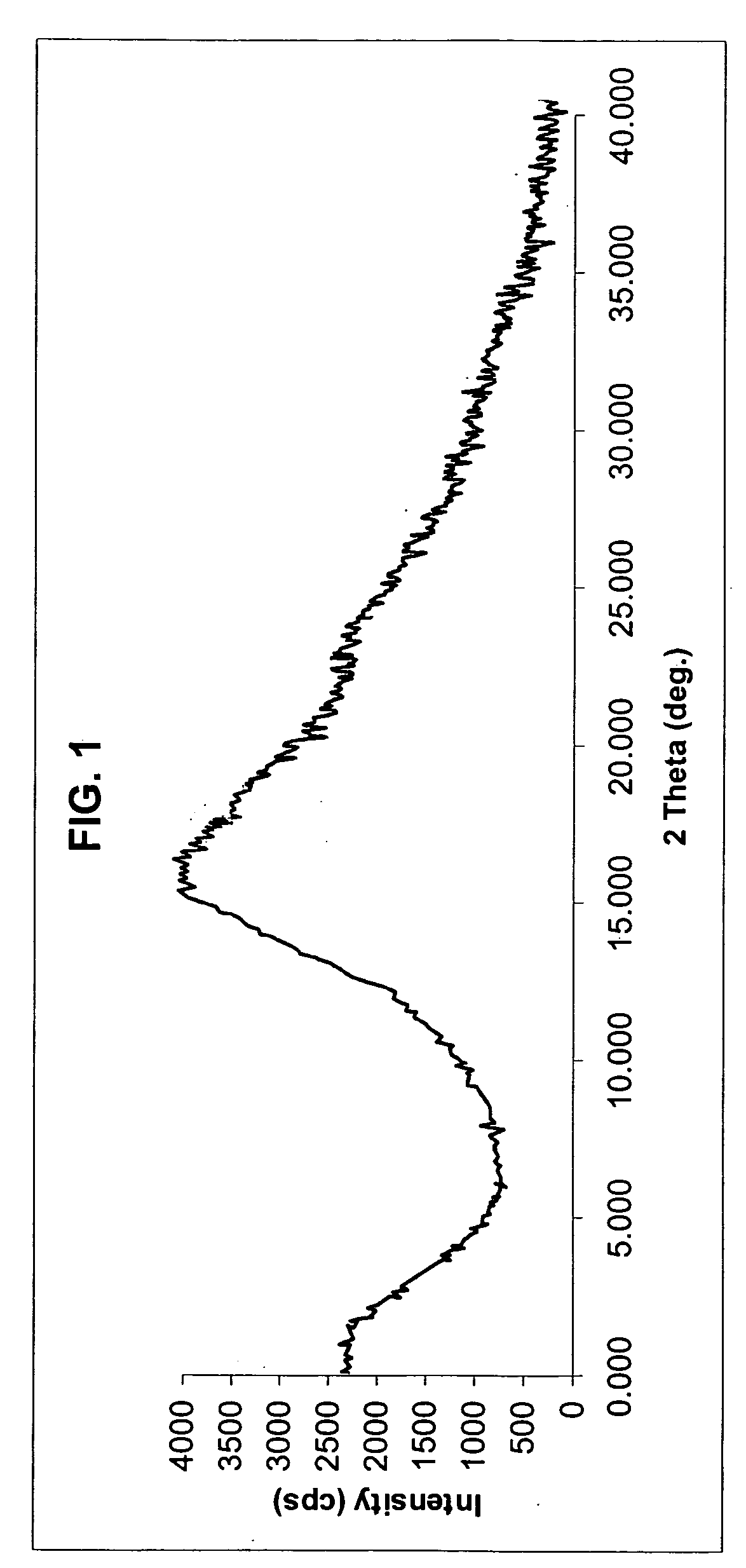
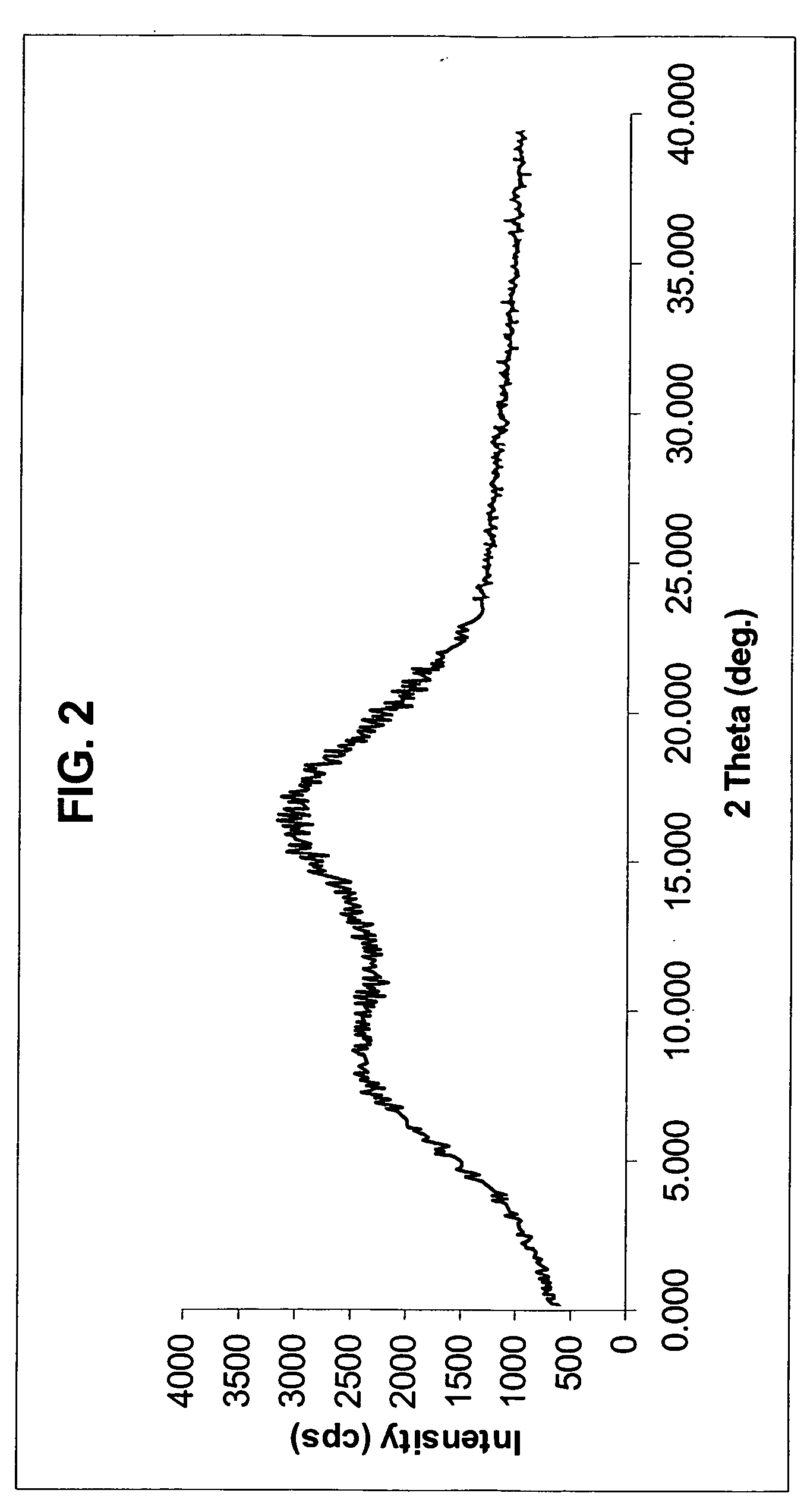
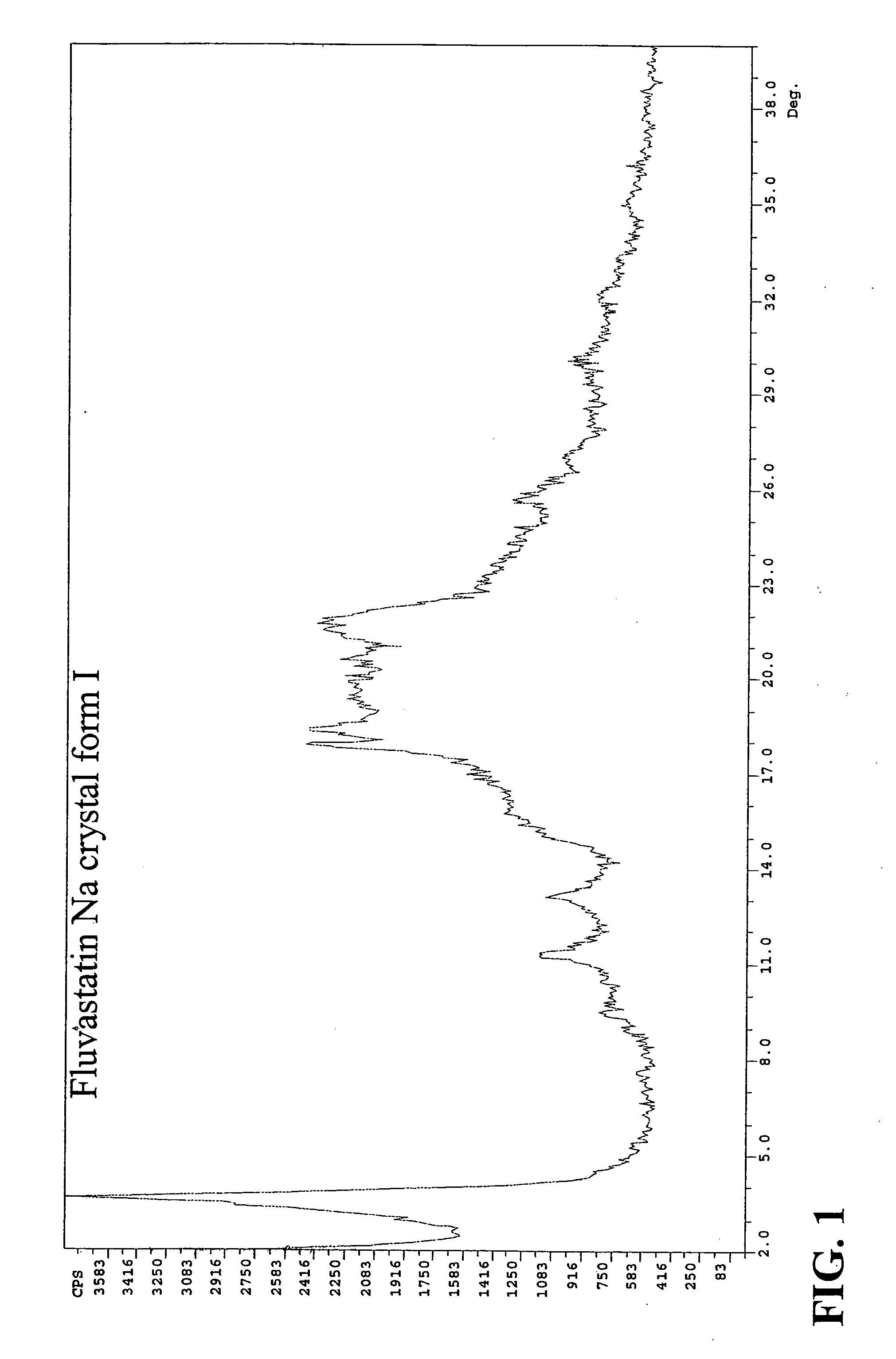
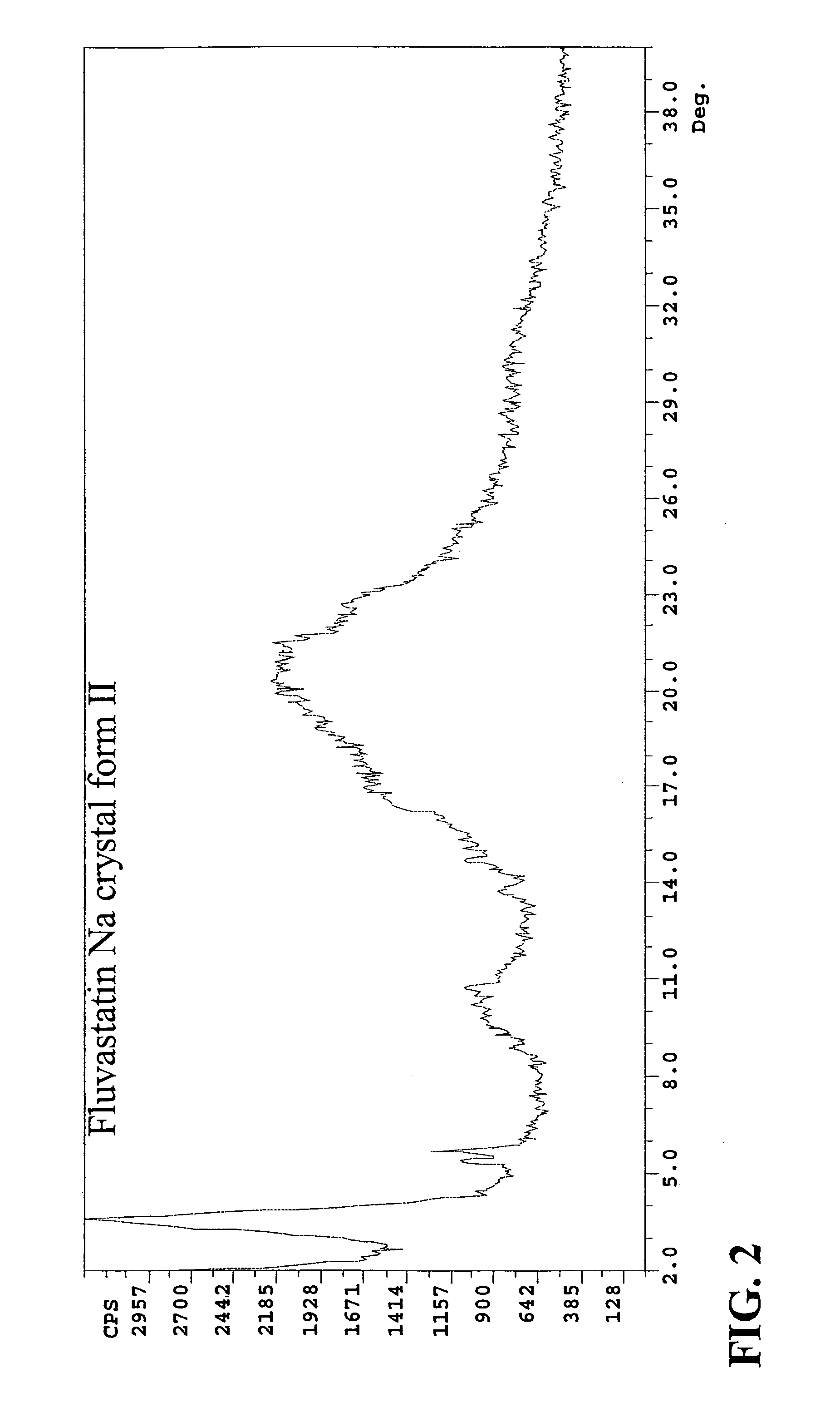
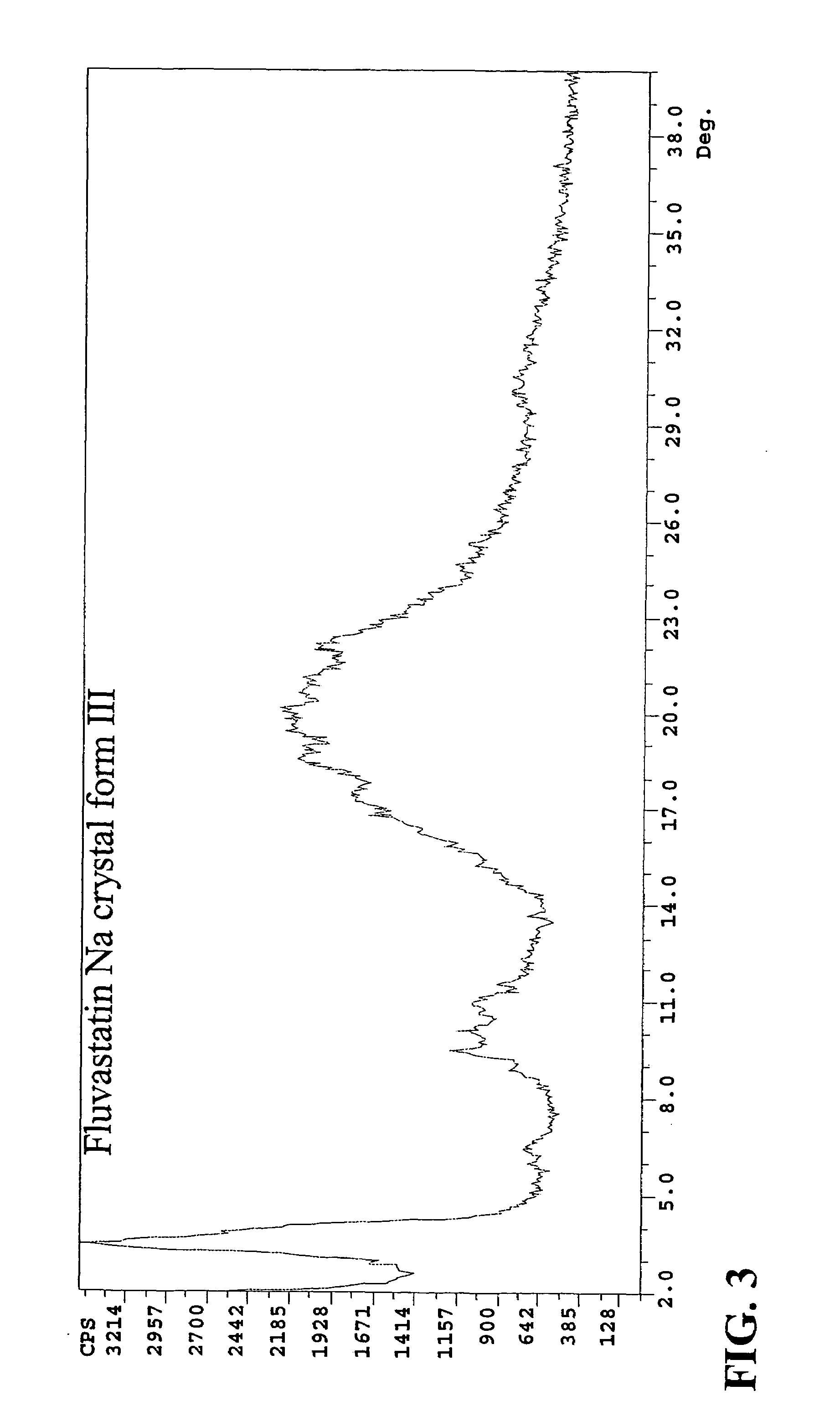
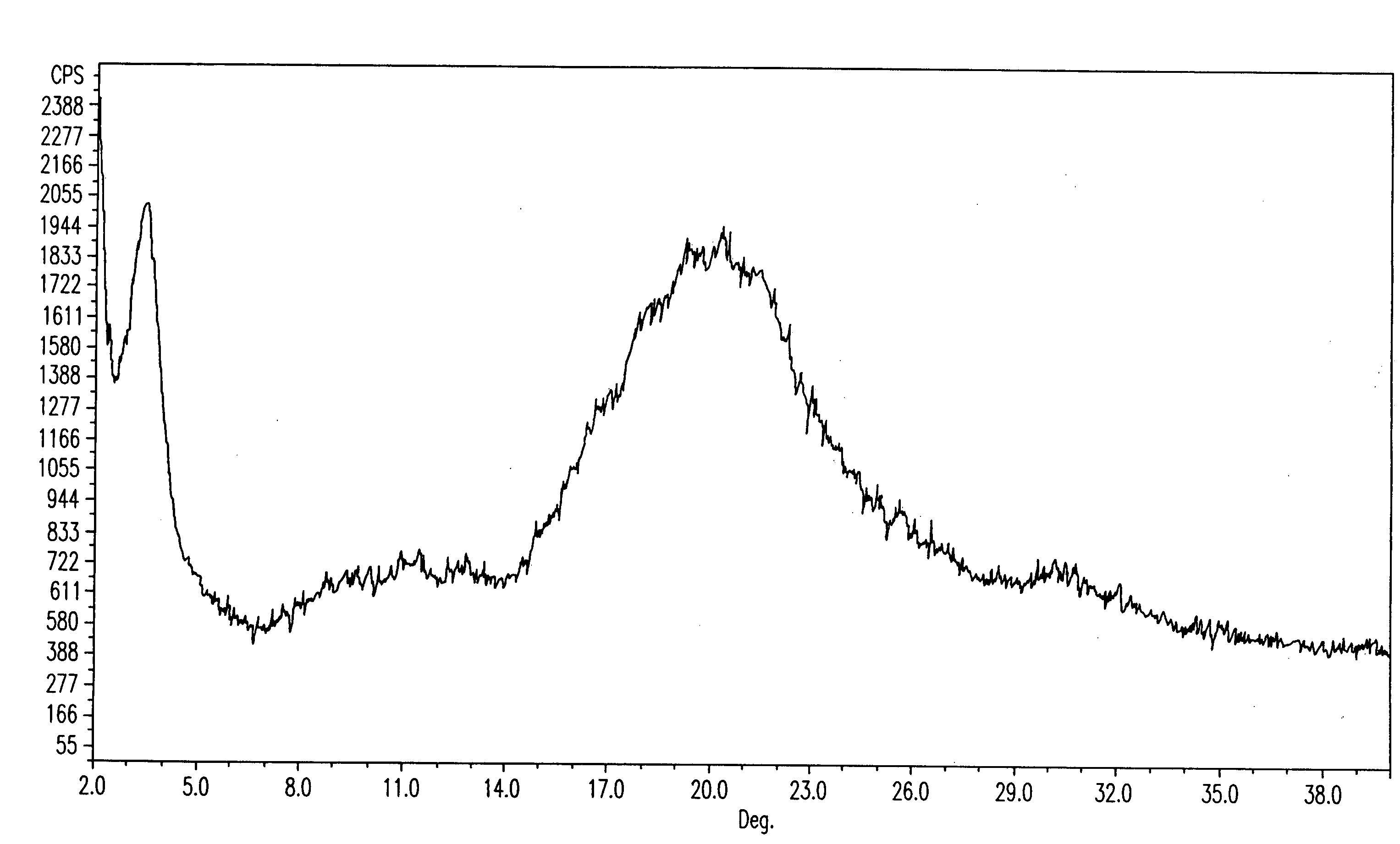
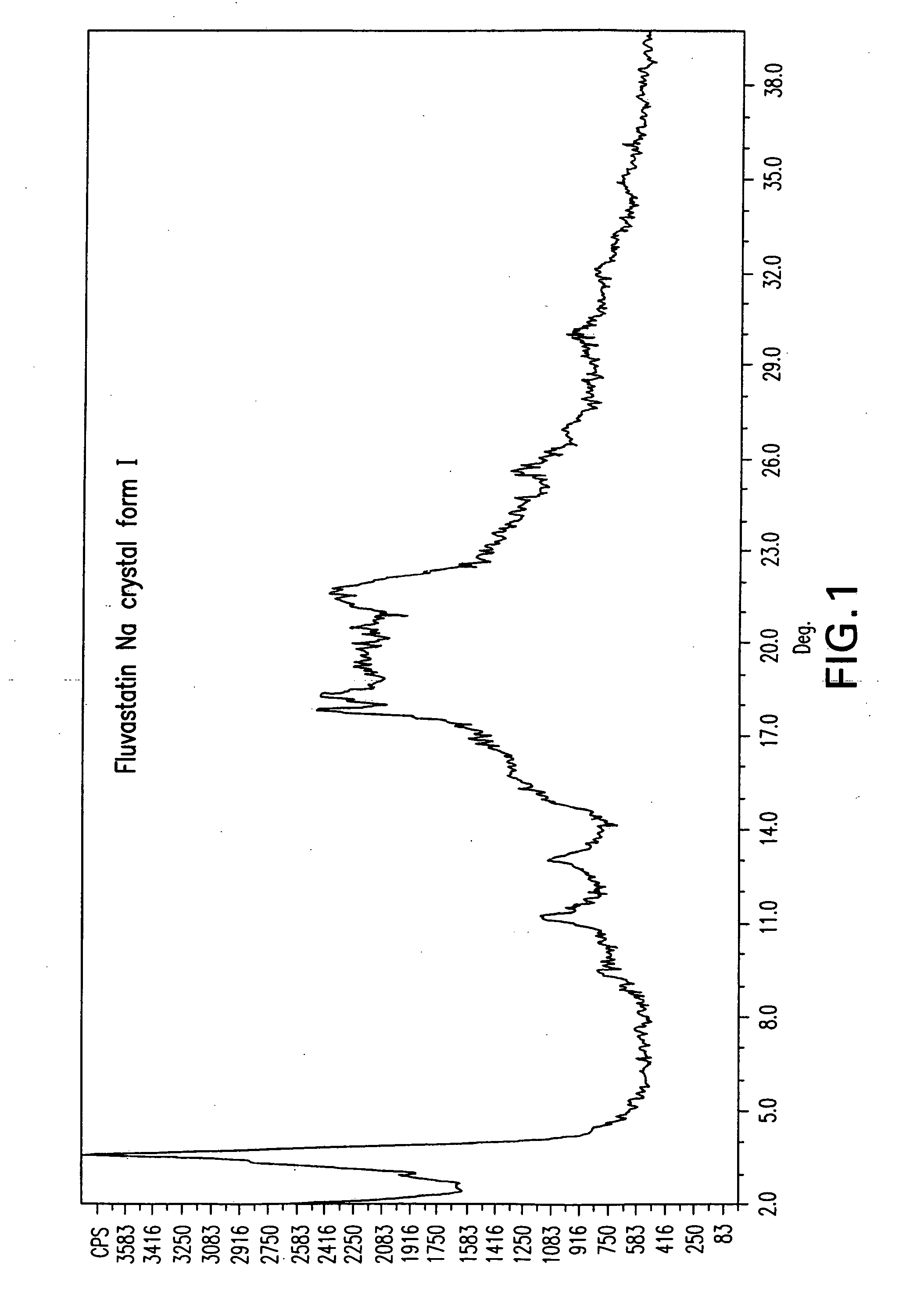
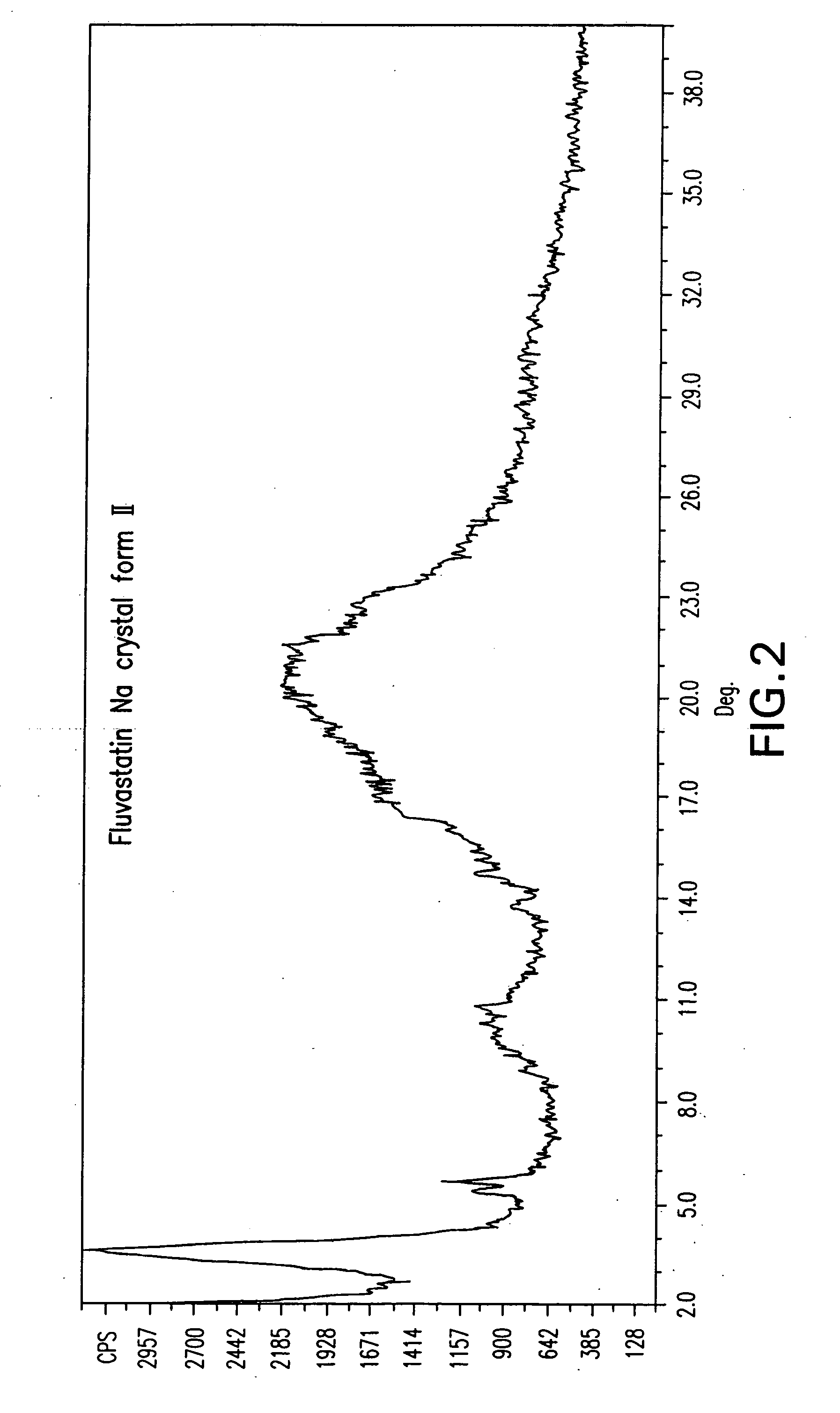
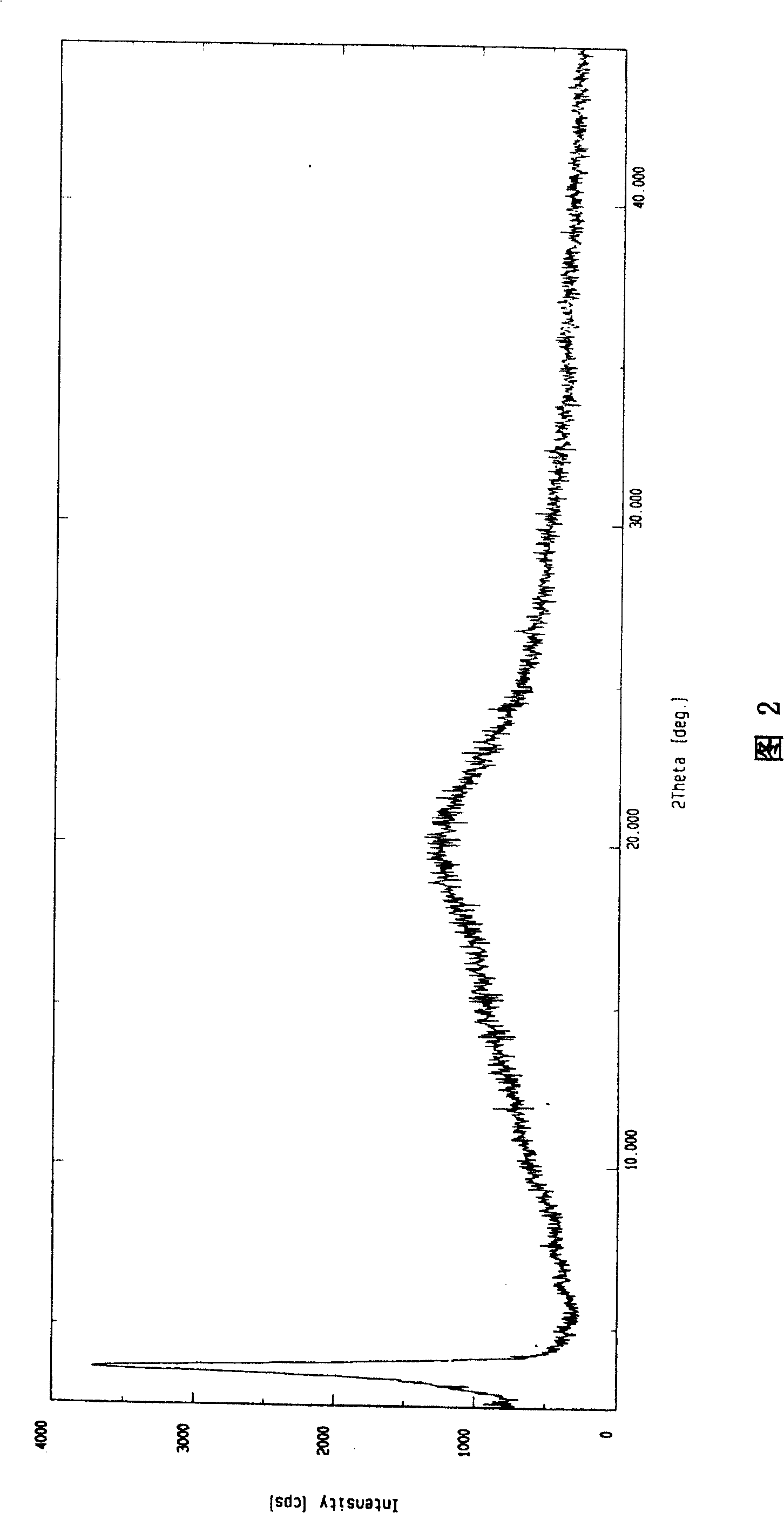
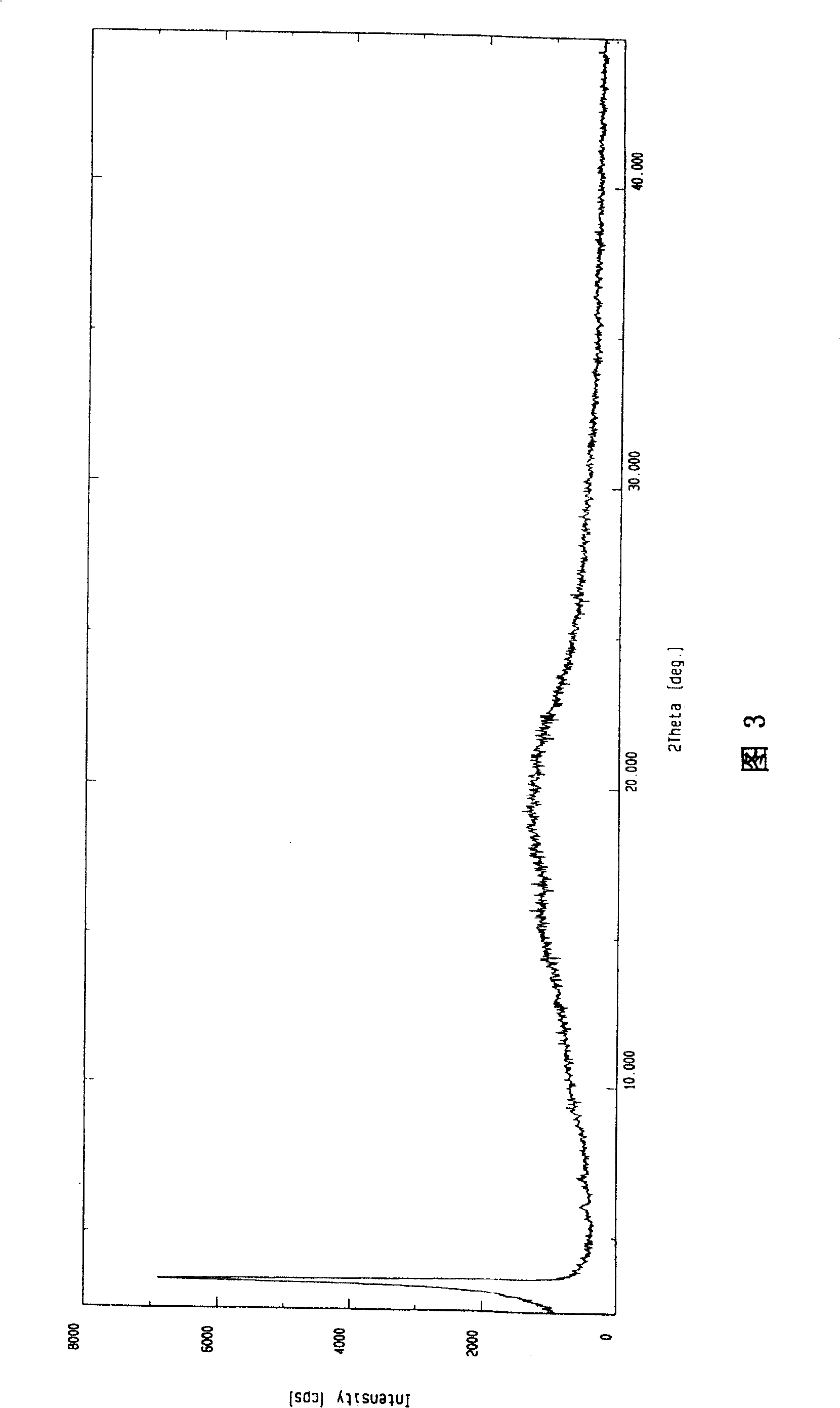
Novel anhydrous amorphous forms of rosuvastatin calcium, pitavastatin calcium and fluvastatin sodium
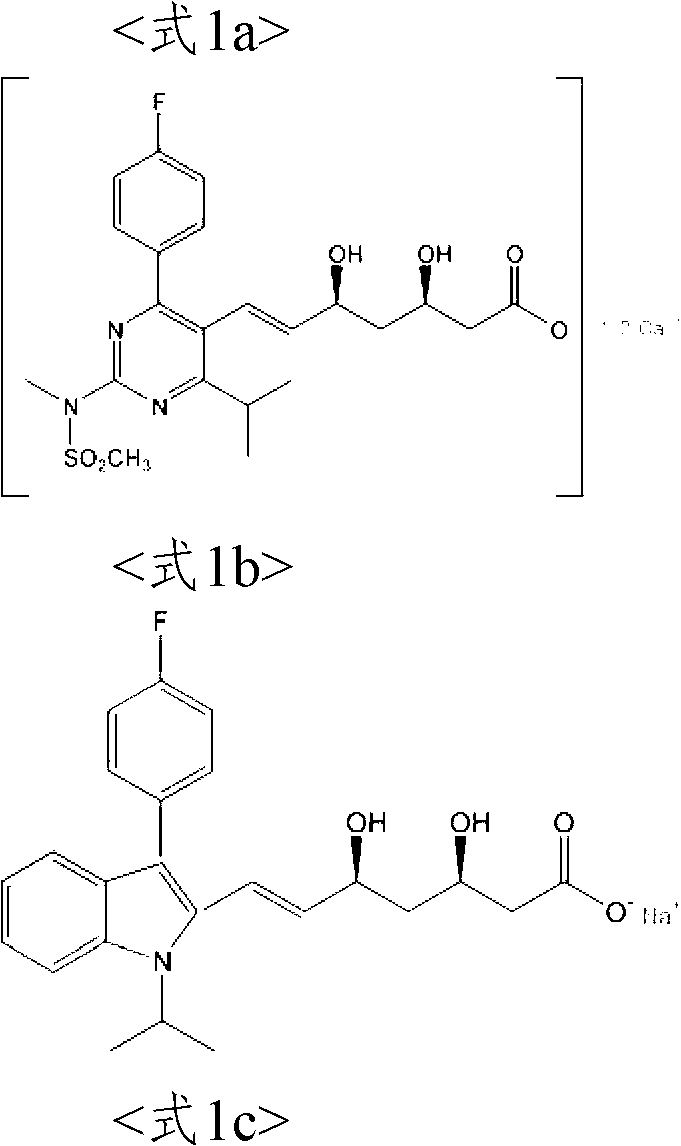
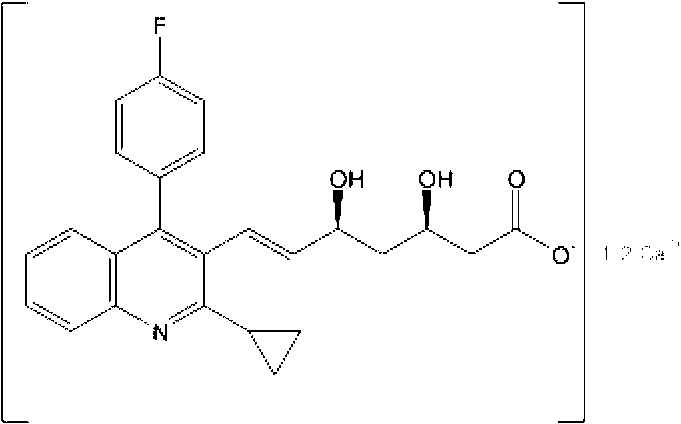
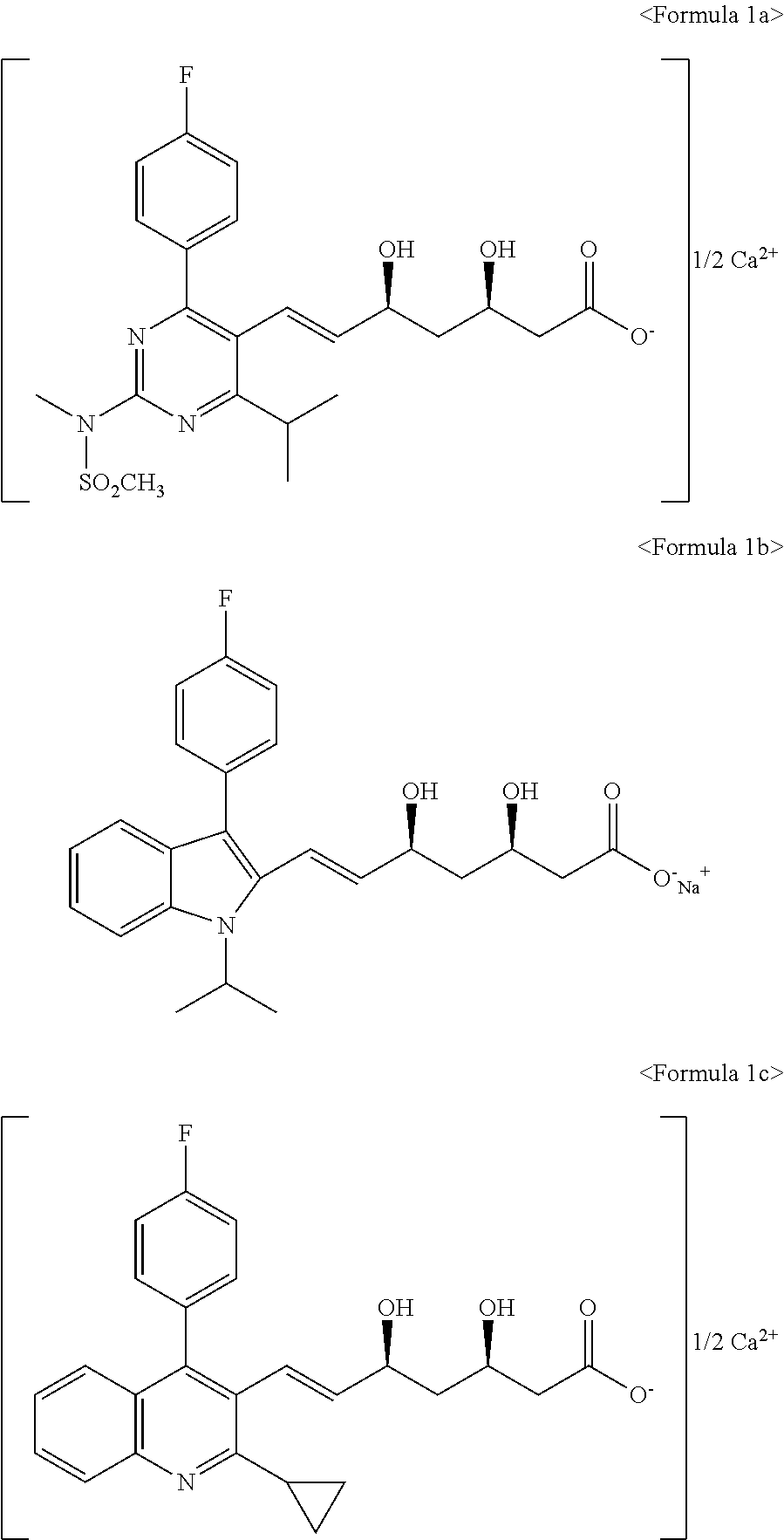
The present invention relates to novel anhydrous amorphous forms of bis[(E)[4-(4-fluorophenyl)isopropyl[methyl(methylsulfonyl)amino]pyrimidinyl](3R,5S)-3,5-dihydroxyhept enoic acid]calcium salt (rosuvastatin calcium), (±)7-(3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl)3,5-dihydroxy heptenoic acid monosodium salt (fluvastatin sodium) and bis[(E)-3,5-dihydroxy-7-[4′-(4″-fluorophenyl)-2′-cyclopropyl-quinolin-3′-hept-6-enoic acid]calcium salt (pitavastatin calcium), to processes for their preparation, to pharmaceutical compositions containing them and to methods of treatment using the same. The rosuvastatin calcium, pitavastatin calcium and fluvastatin sodium obtained are known valuable agents useful in treating hyperlipidemia and hypercholestrolemia.
Owner:MAI DE
Eluvastatin sodium crystal forms, processes for preparing them, compositions containing them and methods of using them
Owner:TEVA PHARM USA INC
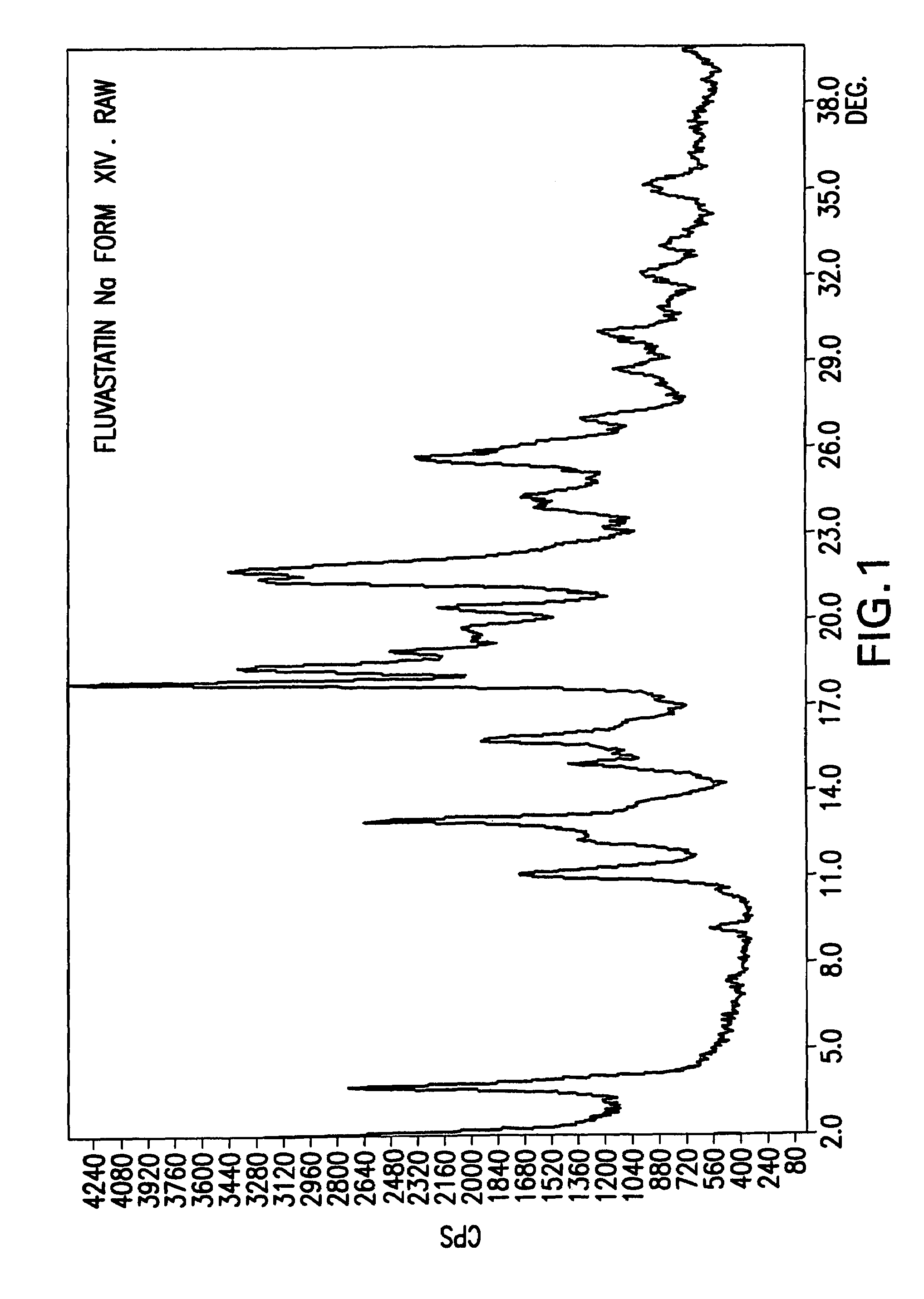
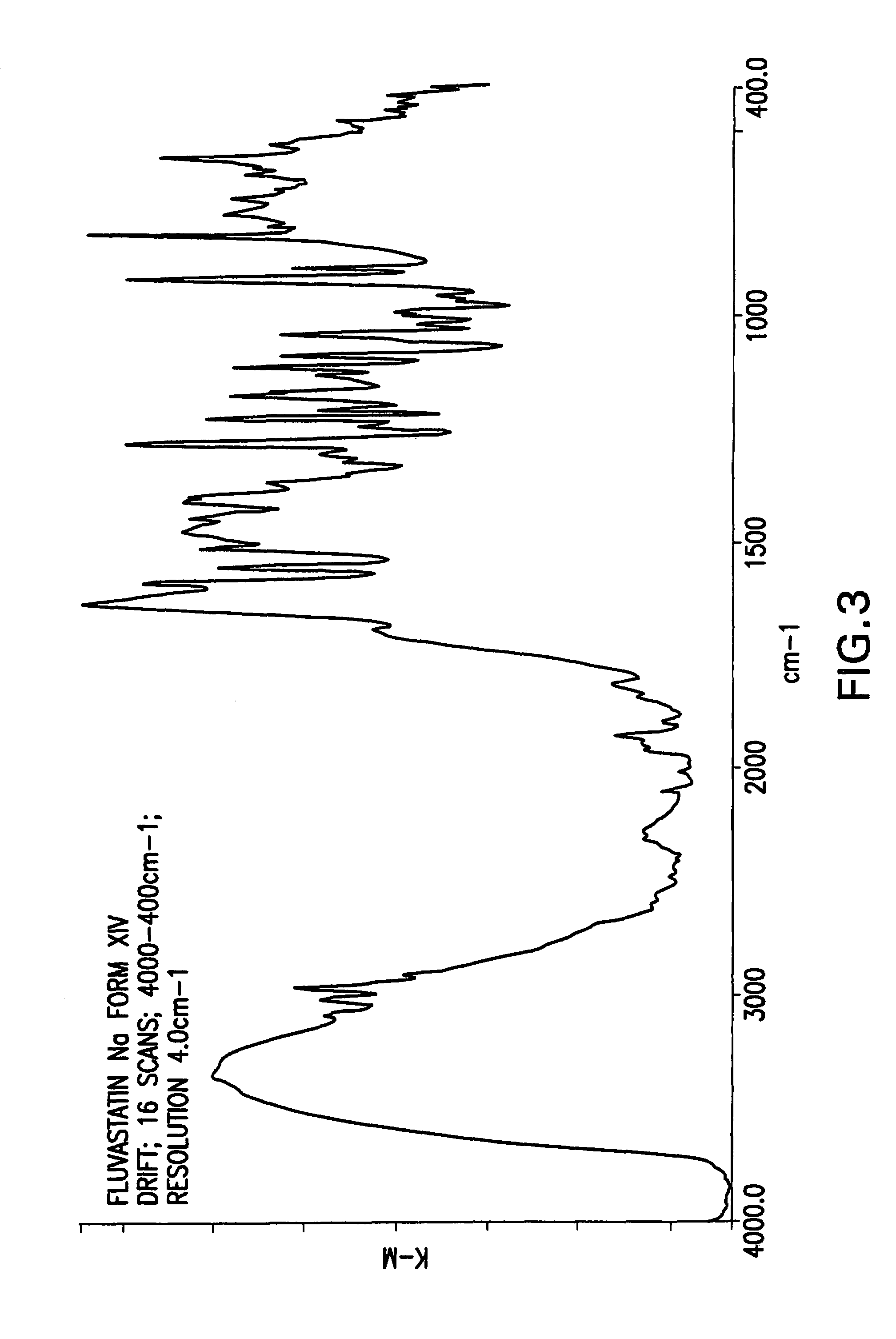
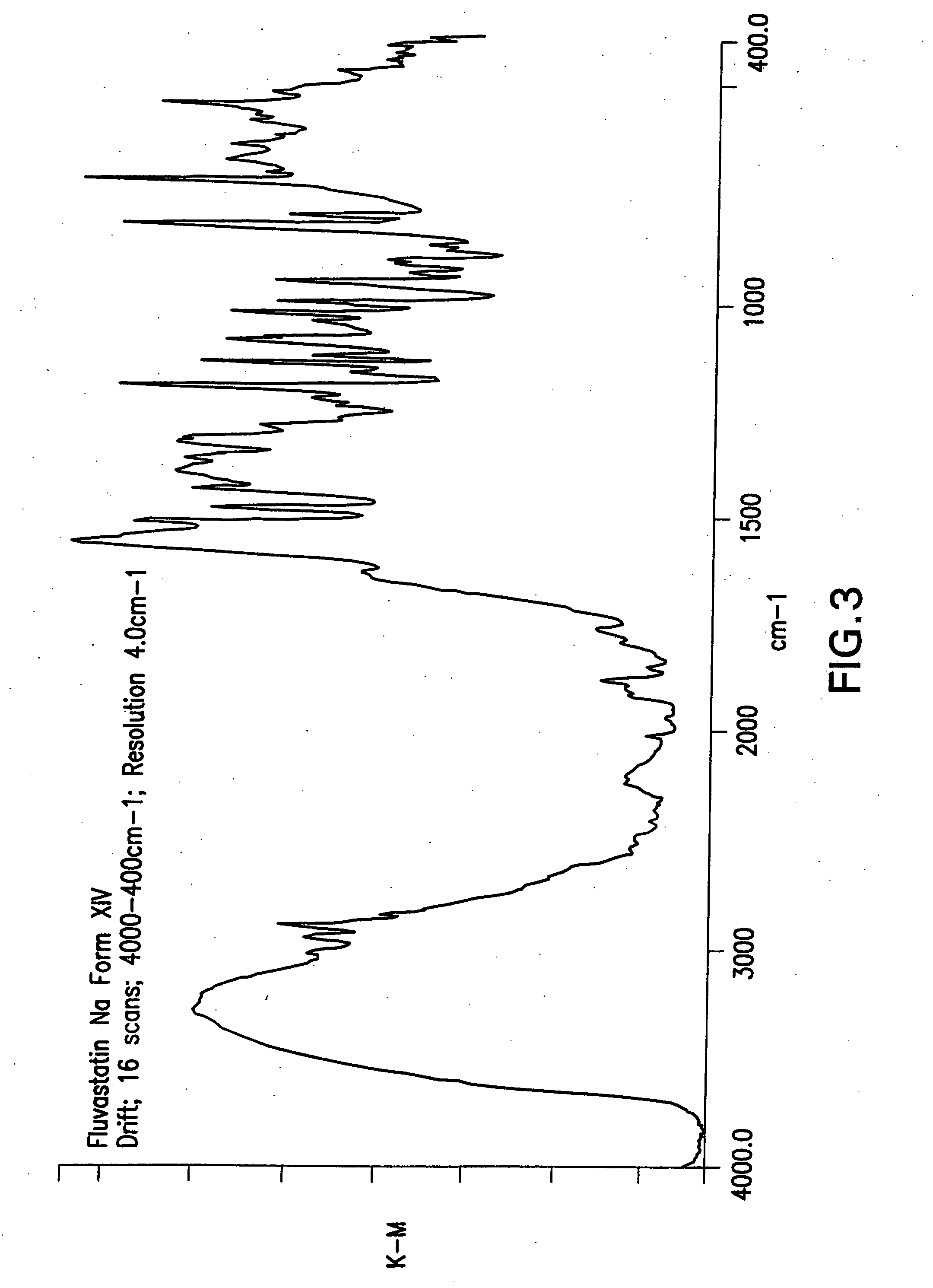
Fluvastatin sodium crystal forms XIV, LXXIII, LXXIX, LXXX and LXXXVII, processes for preparing them, compositions containing them and methods of using them
Owner:TEVA PHARM USA INC
Process For The Preparation Of HMG-COA reductase inhibitors and intermediates thereof
ActiveCN103025727AAvoid formingFew reaction stepsOrganic active ingredientsOrganic chemistryHMG-CoA reductaseRosuvastatin Calcium
Owner:YUHAN
Anhydrous amorphous form of fluvastatin sodium
The present invention relates to novel anhydrous amorphous forms of bis[(E)[4-(4-fluorophenyl)isopropyl[methyl(methylsulfonyl)amino]pyrimidinyl](3R,5S)-3,5-dihydroxyhept enoic acid]calcium salt (rosuvastatin calcium), (±)7-(3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl)3,5-dihydroxy heptenoic acid monosodium salt (fluvastatin sodium) and bis[(E)-3,5-dihydroxy-7-[4′-(4″-fluorophenyl)-2′-cyclopropyl-quinolin-3′-hept-6-enoic acid]calcium salt (pitavastatin calcium), to processes for their preparation, to pharmaceutical compositions containing them and to methods of treatment using the same. The rosuvastatin calcium, pitavastatin calcium and fluvastatin sodium obtained are known valuable agents useful in treating hyperlipidemia and hypercholestrolemia.
Owner:MAI DE
Fluvastatin sodium crystal forms, processes for preparing them, compositions containing them and methods of using them
Owner:TEVA PHARM USA INC
Fluvastatin sodium crystal forms XIV, LXXIII, LXXIX, LXXX and LXXXVII, processes for preparing them, compositions containing them and methods of using them
Owner:TEVA PHARM USA INC
Process for the preparation of HMG-COA reductase inhibitors and intermediates thereof
ActiveUS8476432B2Improve isolationReduce stepsOrganic active ingredientsOrganic chemistryHMG-CoA reductasePitavastatin
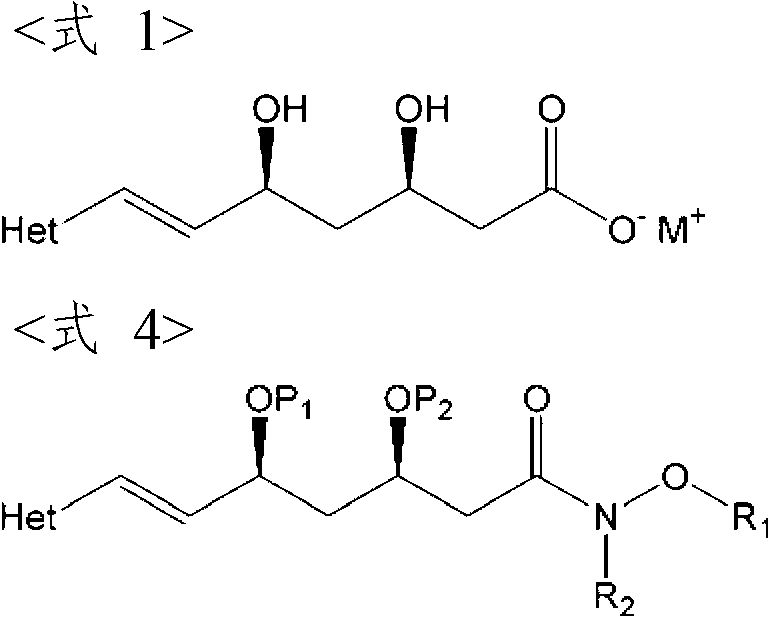
The present invention provides an improved process for preparing HMG-CoA reductase inhibitors such as rosuvastatin calcium, fluvastatin sodium, and pitavastatin calcium under a mild condition, using a novel amide-bond-containing compound having R2—N—O—R1 moiety as a key intermediate. And also, the present invention provides the novel compound, an intermediate useful for the preparation thereof, and a process for the preparation thereof.
Owner:YUHAN
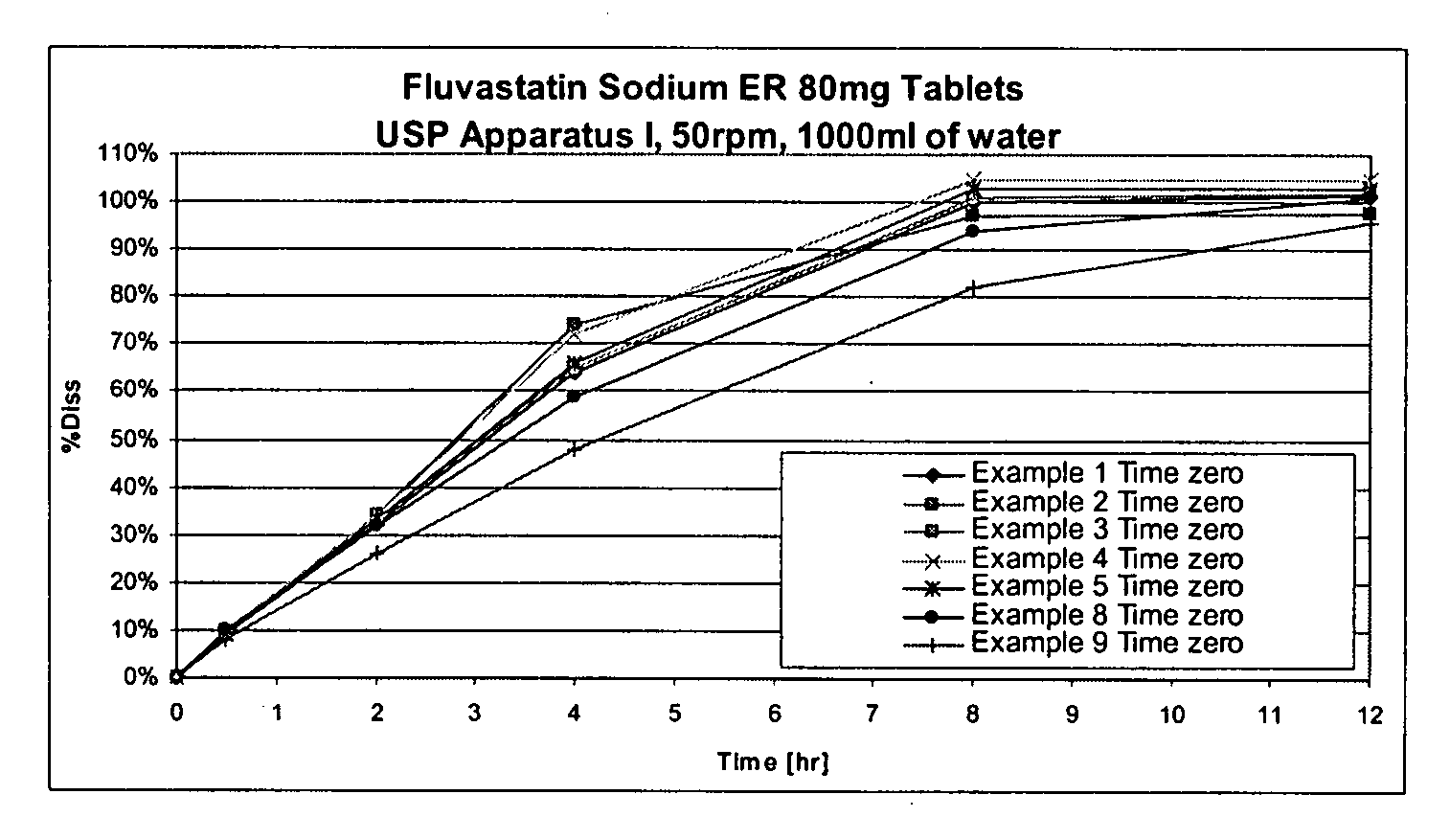
Fluvastatin sodium pharmaceutical compositions
InactiveUS20080033030A1Increase moisture contentPremature release of significant is preventedBiocideMetabolism disorderControlled releaseNon ionic
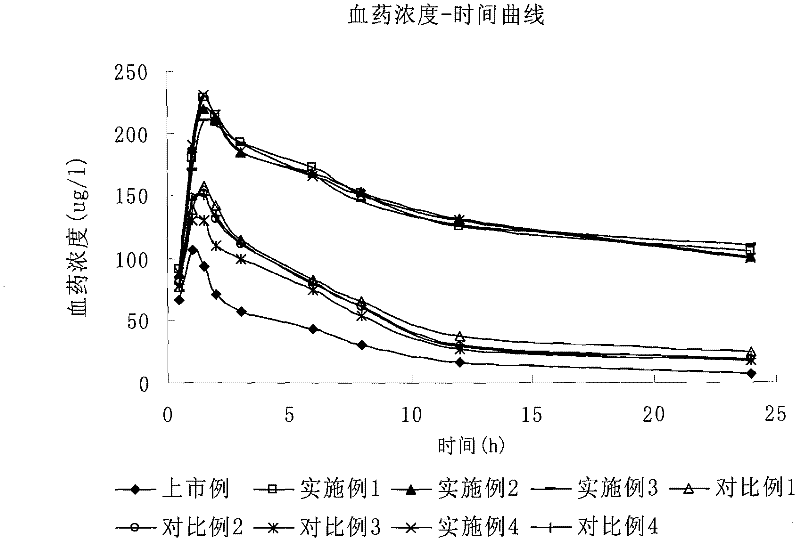
Various fluvastatin compositions and methods for preparing them are described. One example is a controlled release pharmaceutical composition comprising fluvastatin and at least one non-ionic hydrophilic polymer, wherein the composition is substantially free of hydroxypropyl methylcellulose. Another example is a stable pharmaceutical composition comprising fluvastatin, preferably, fluvastatin sodium wherein the composition is substantially free of an alkalizing stabilizing agent. Another example is a stable controlled release pharmaceutical formulation, comprising fluvastatin, preferably, fluvastatin sodium, that is stable with a water content greater than 3.5 percent by weight.
Owner:TEVA PHARM USA INC
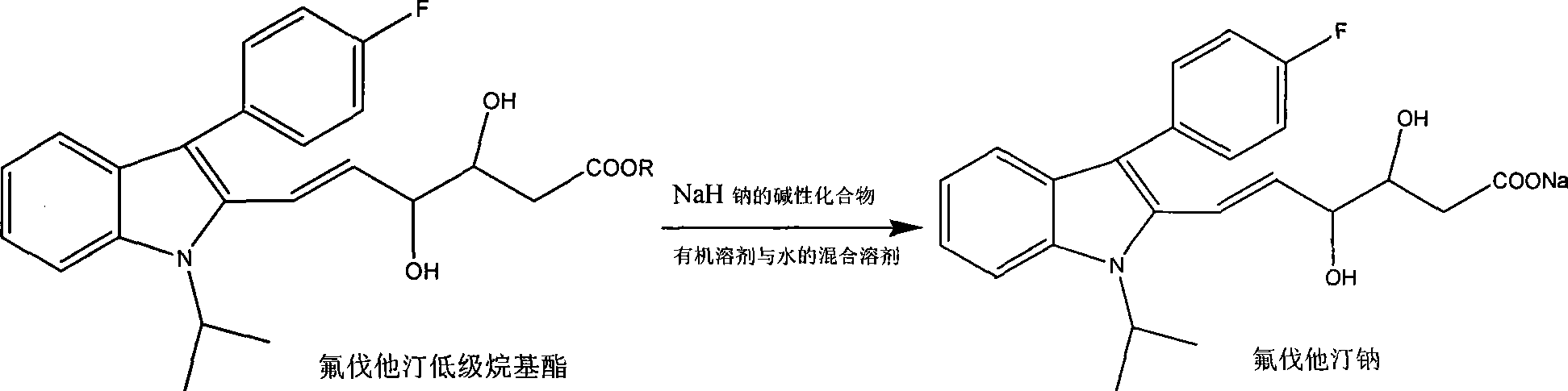
Refining of fluvastatin sodium intermediate and preparation method of fluvastatin sodium
InactiveCN101215257AQuality improvementSuitable for industrial productionOrganic chemistryEthyl esterMethyl acetate
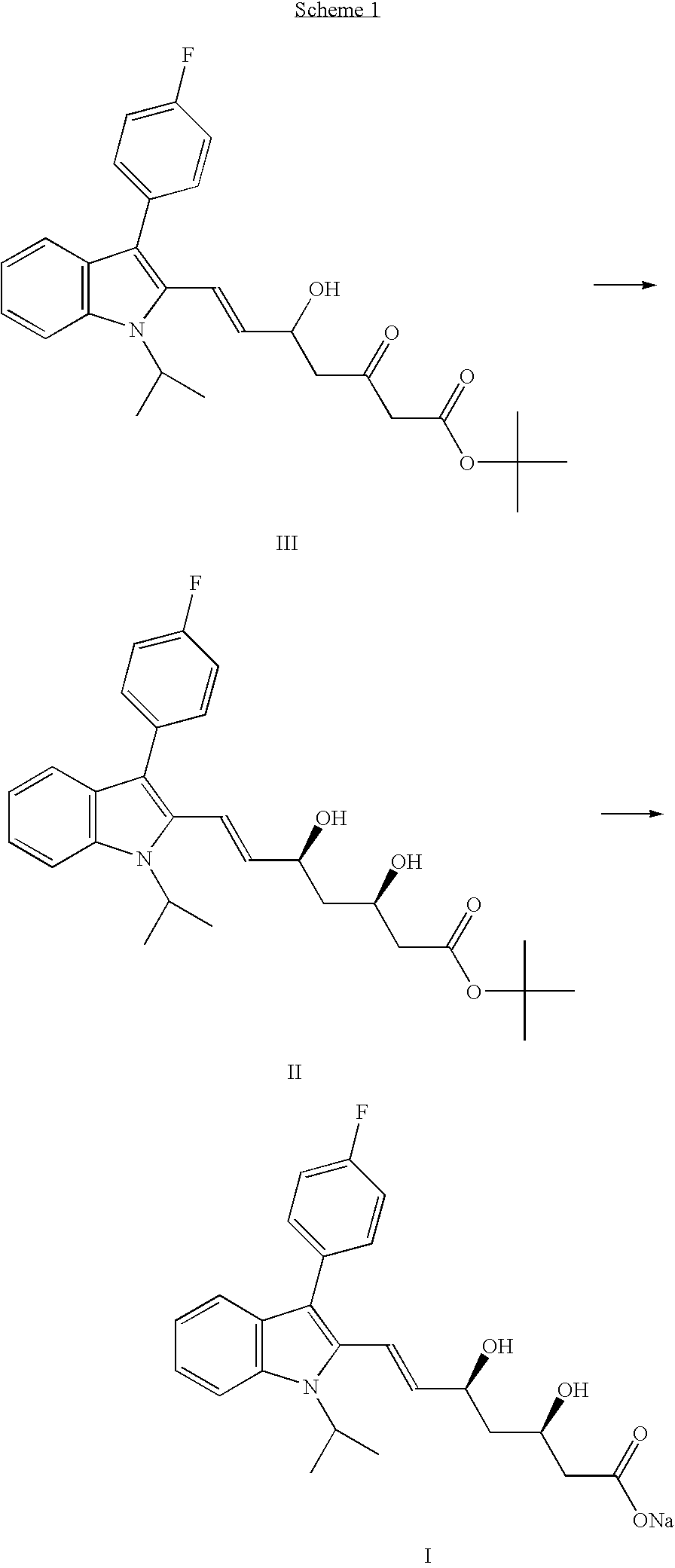
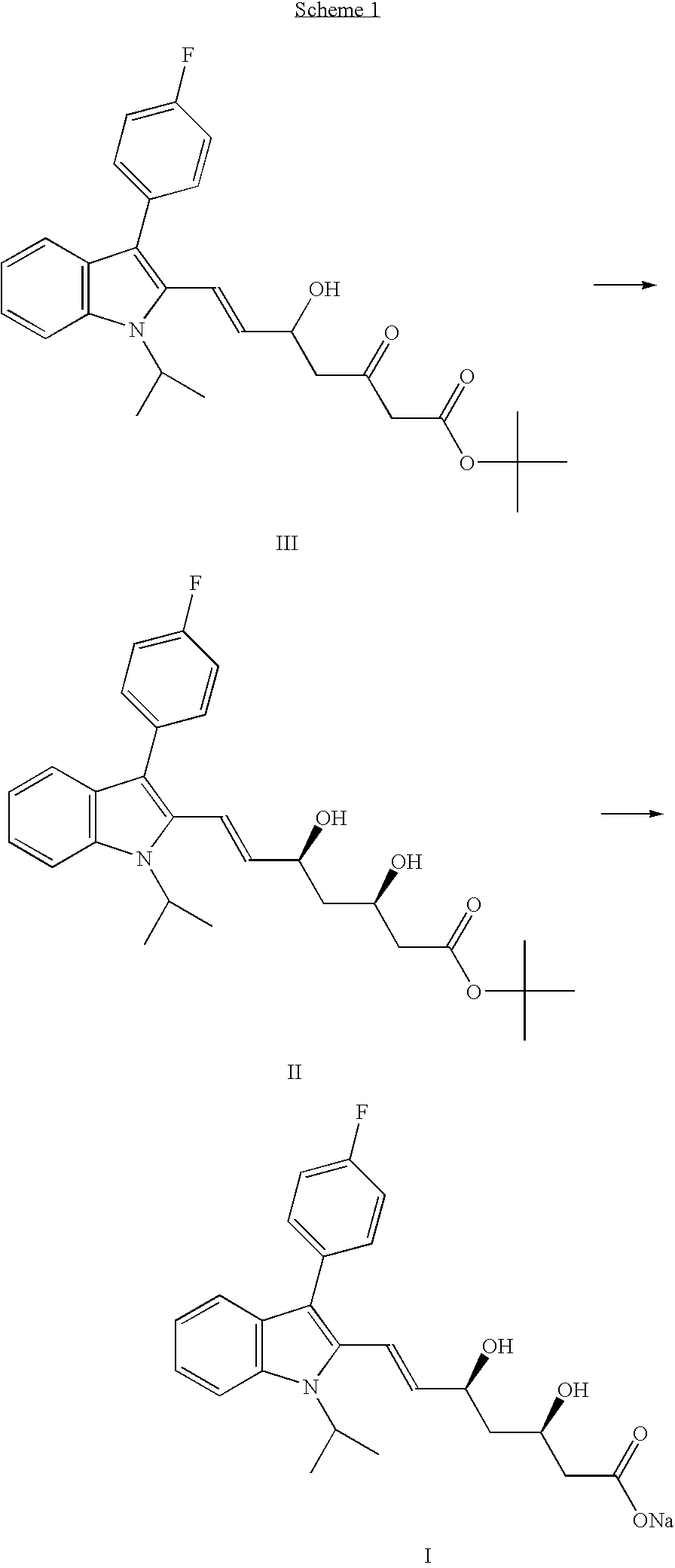
The invention provides a refining process of intermediate I (E)-7-[3-(4-fluorophenyl)-1-(1-methyl ethyl)-1H-indole-2-radicial]-5-hydroxy-3-oxygen-6-heptene methyl acetate. The invention refines fluvastatin sodium intermediate I through adopting acetic acid ethyl ester and hexane solvent system, which increases the purity of product which is refined, and simultaneously avoids the inference of impurities for subsequent preparation fluvastatin sodium. Simultaneously, the invention further provides a preparation process of fluvastatin sodium which contains the intermediate refining steps.
Owner:HUIZHOU XINLITAI PHARMA
Technique for preparing fluvastatin sodium crystal system
The invention provides a preparation method of fluvastatin sodium B type crystal. The invention uses the mixture solvent of organic solvent and water, which has suitable polarity and different solubilities on fluvastatin ester and fluvastatin sodium, therefore the mixture solvent can be used as the reaction system of fluvastatin low class alkyl ester and alkali compound of sodium and can be used as the crystallization system of fluvastatin sodium, to directly precipitate crystal after complete reaction without adding sediment solvent. The whole operation is simple and short, and only needs several hours (5-8h) to prepare the product whose purity is at least 99% and yield is at least 91%. The invention is suitable for industrial production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
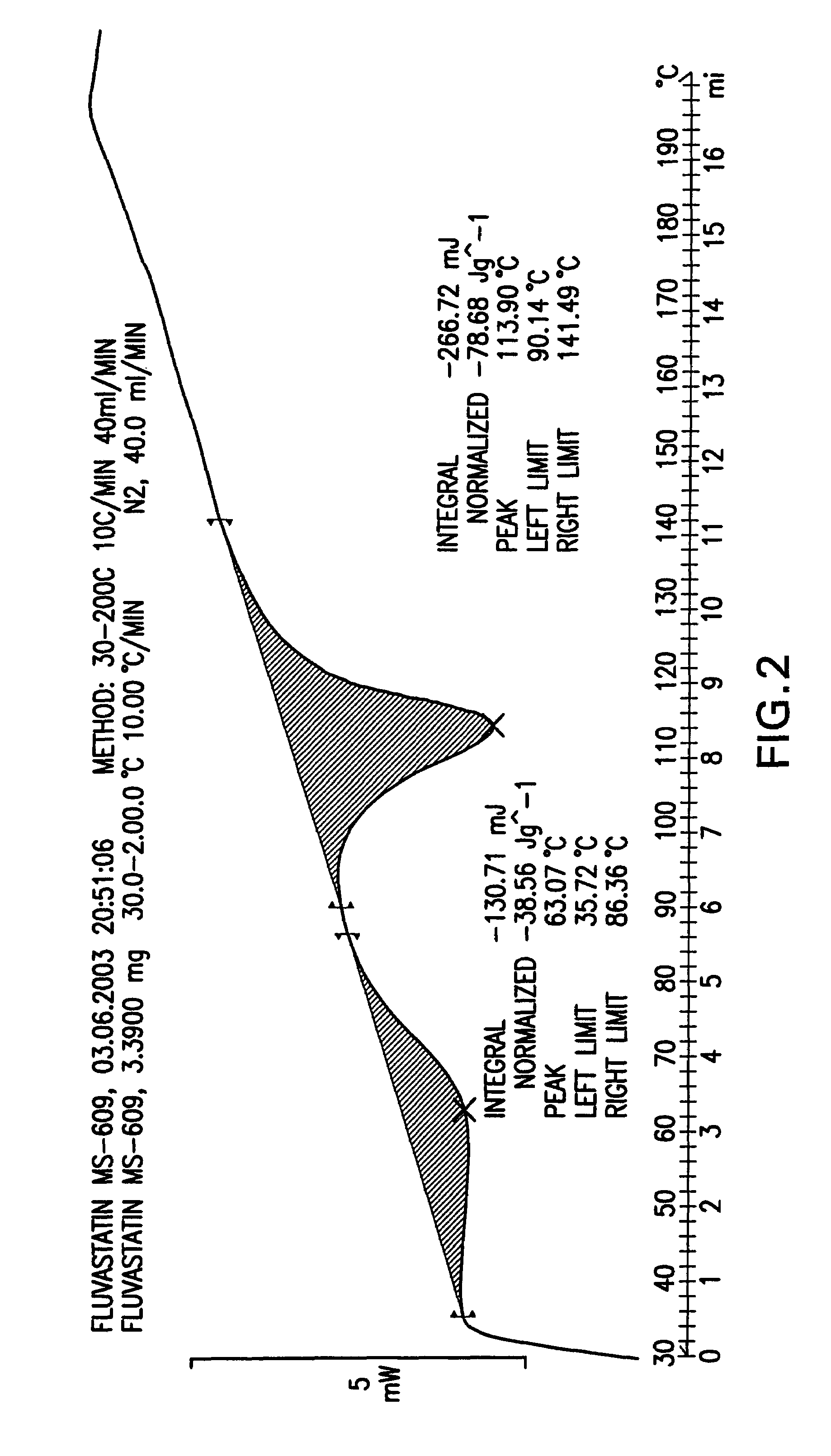
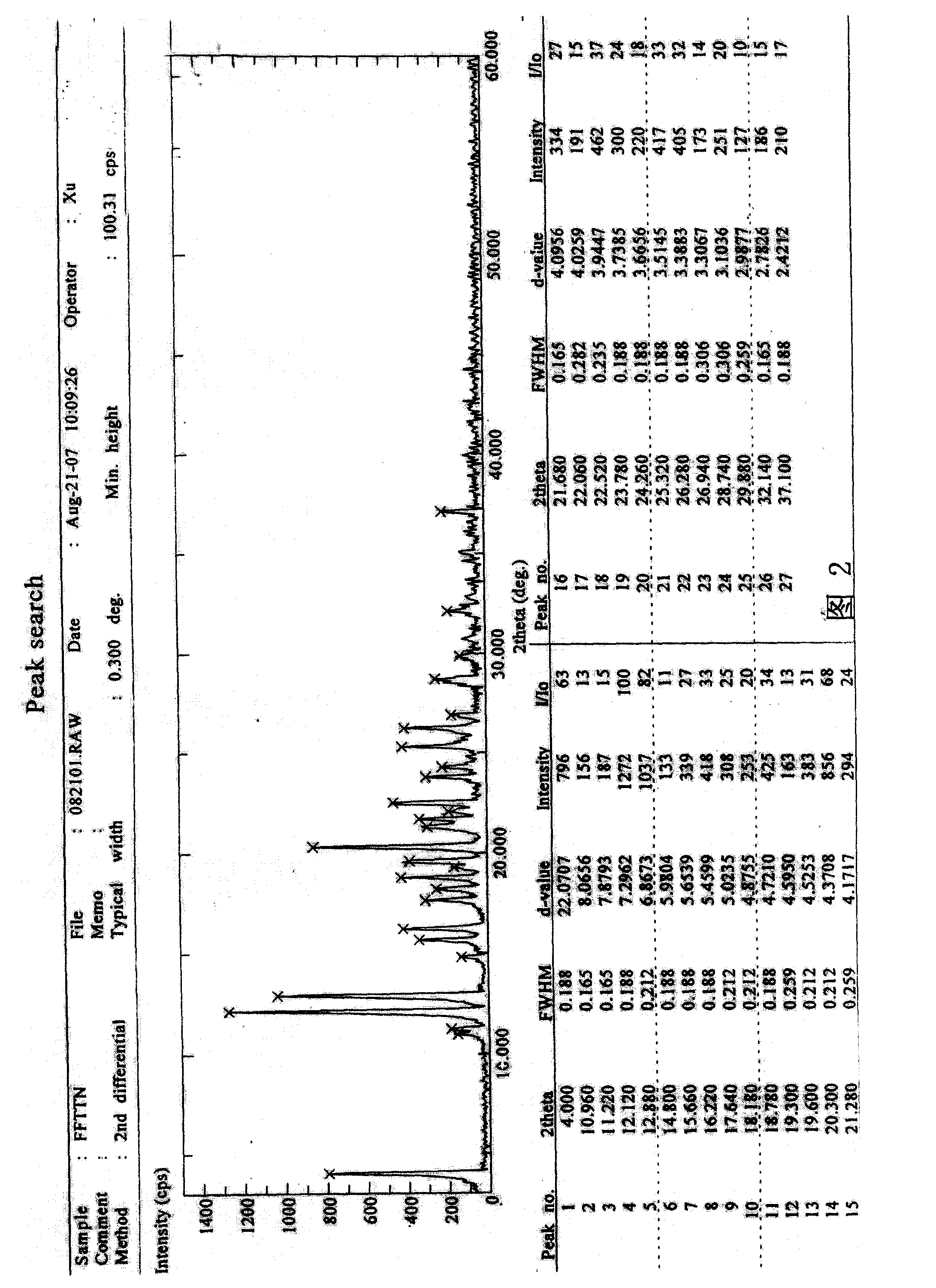
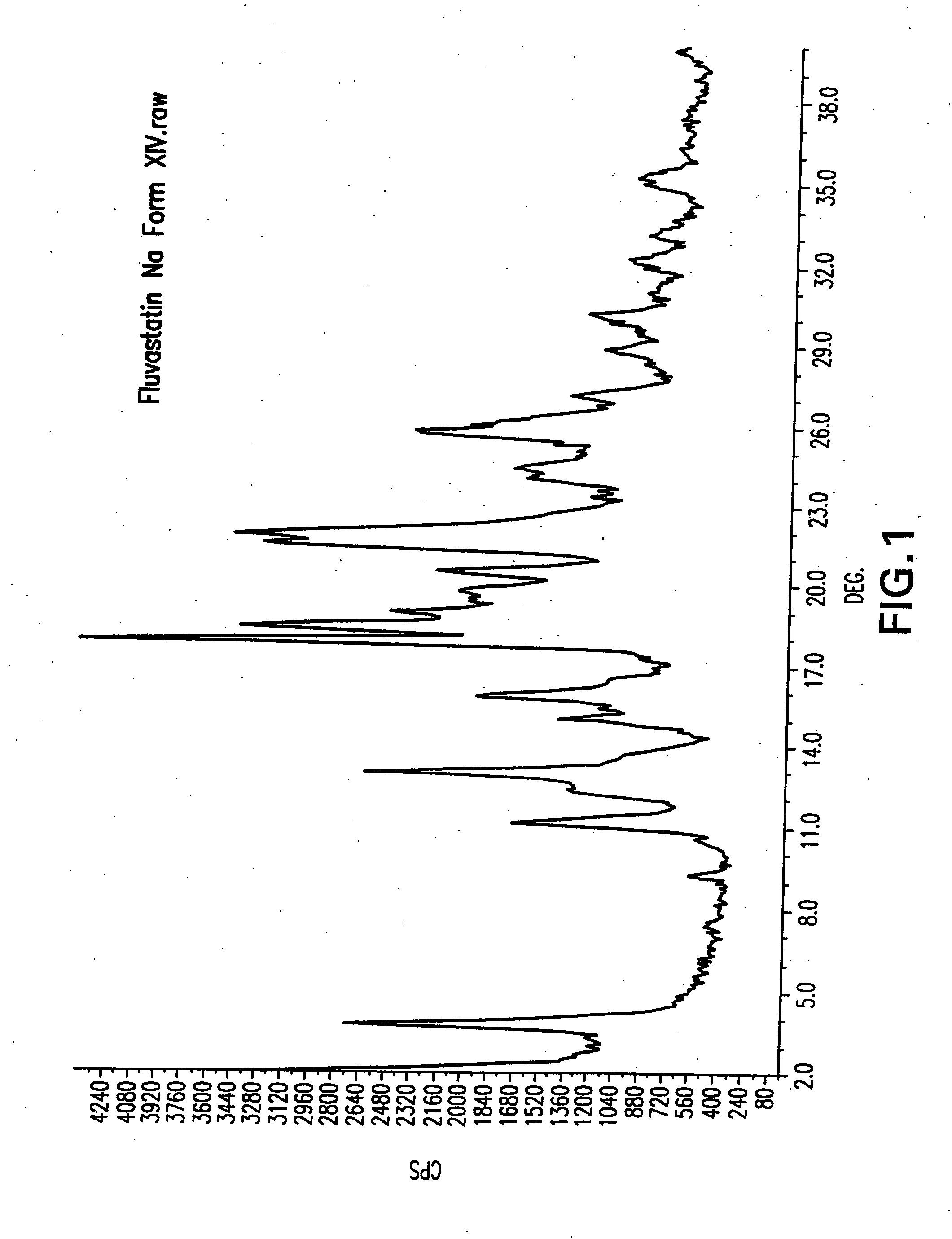
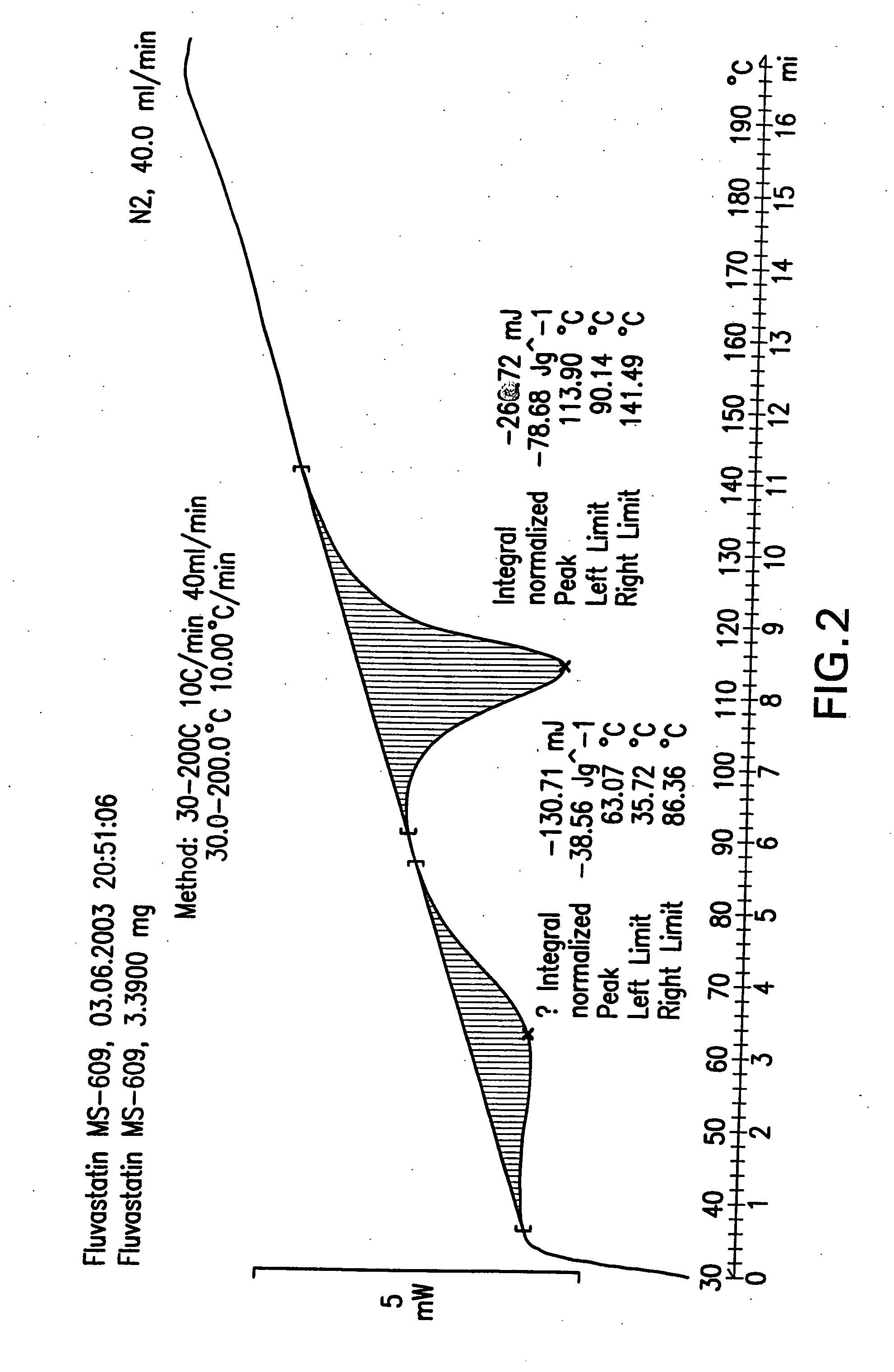
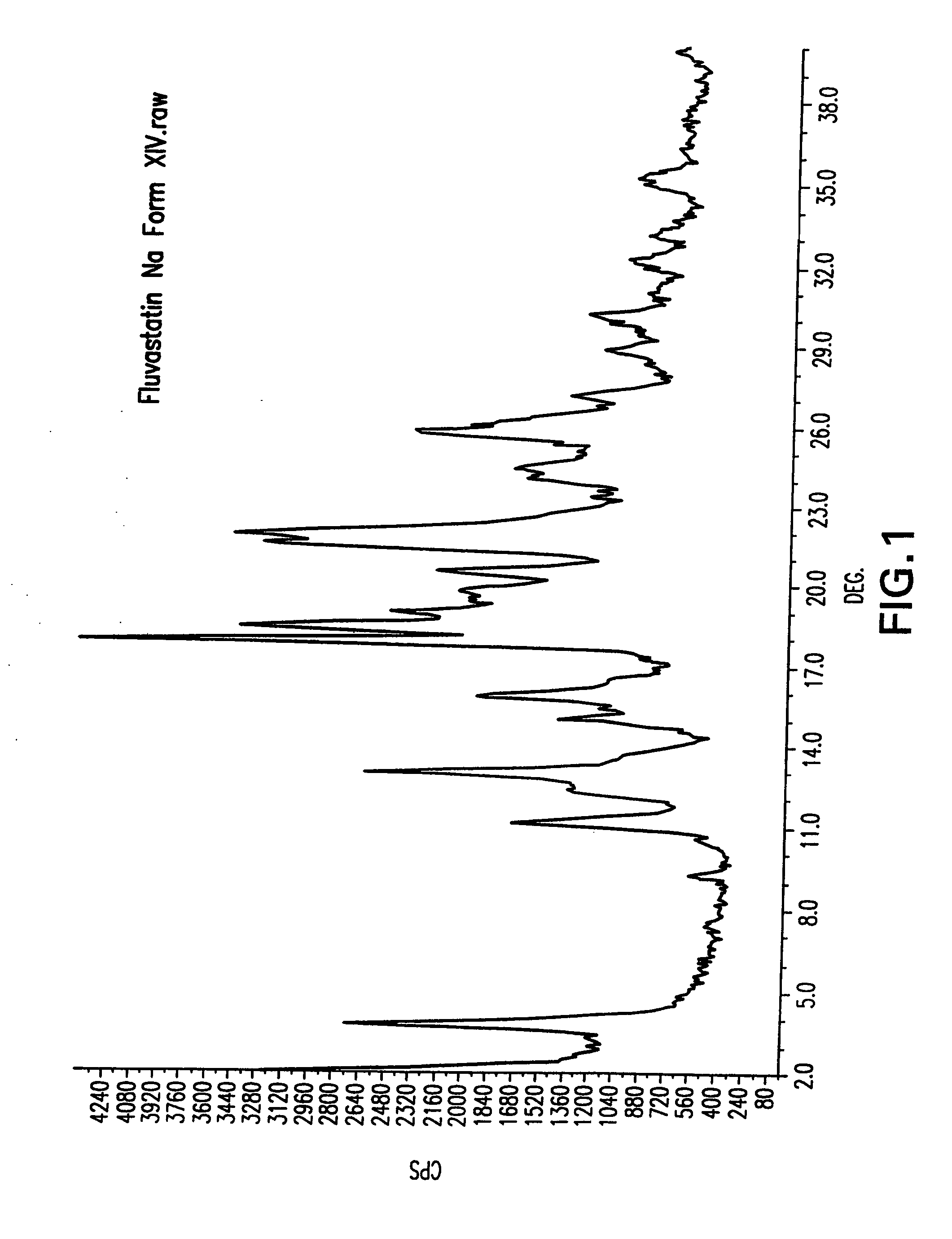
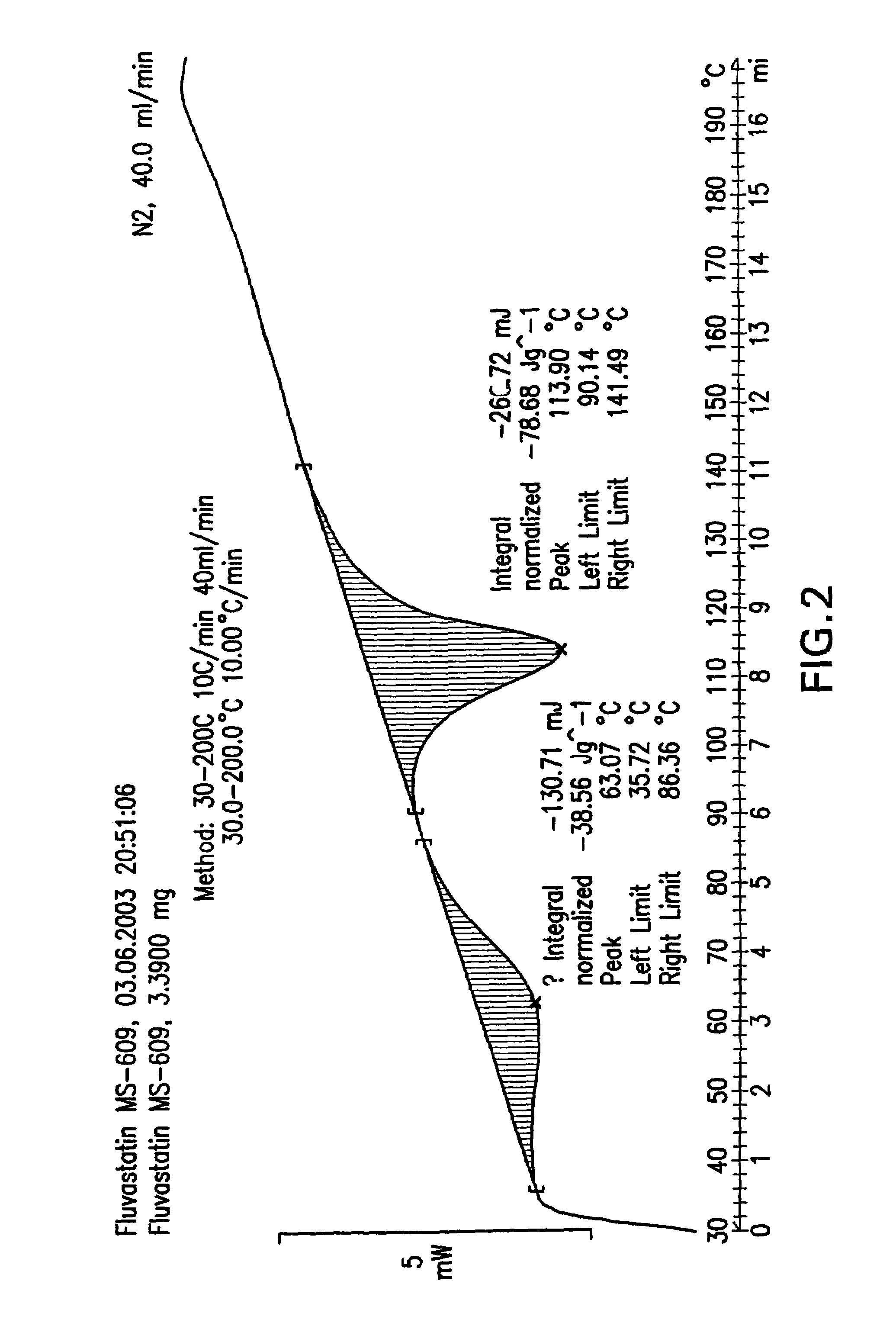
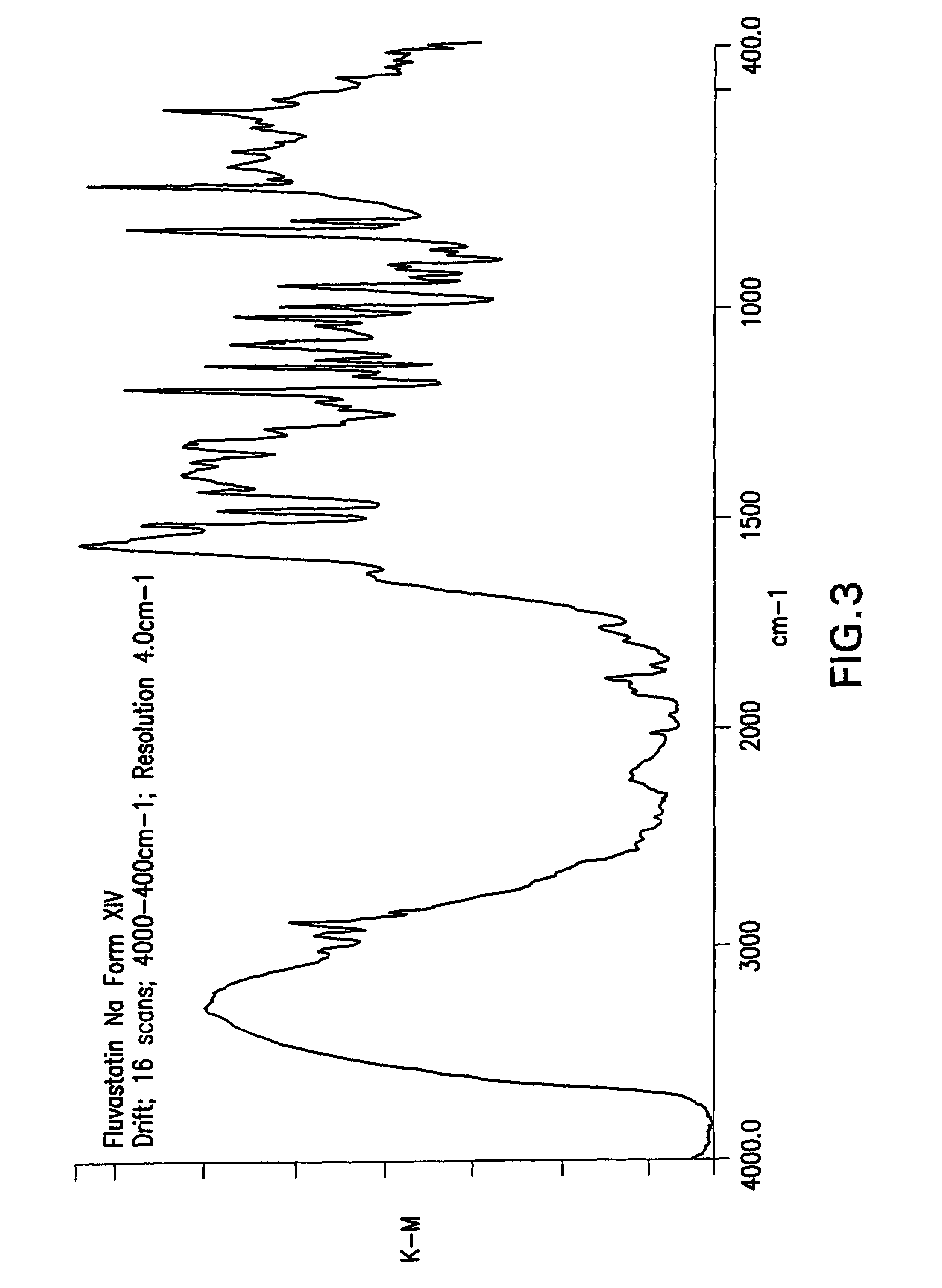
Process for the preparation of fluvastatin sodium crystal form XIV
Provided is a process for preparing a polymorphic form of fluvastatin sodium, particularly Form XIV.
Owner:TEVA PHARM USA INC
Fluvastatin sodium liposome solid preparation
InactiveCN102309452AQuality improvementImprove bioavailabilityOrganic active ingredientsMetabolism disorderSide effectCurative effect
The invention discloses a fluvastatin sodium liposome solid preparation and a preparation method thereof. According to the invention, fluvastatin sodium, soya lecithin, cholesterin, soybean derived sterol and dehydrated sorbitol stearate are used according to a specific weight ratio to prepare fluvastatin sodium liposome with excellent quality, and the fluvastatin sodium liposome is prepared intoa solid preparation by using a common pharmaceutical method. Compared to conventional preparations, the preparation provided in the invention has greatly improved stability and bioavailability, stable drug release, remarkable curative effects, enhanced quality and reduced toxic and side effects.
Owner:HAINAN MEIDA PHARMA
Process for the preparation of Fluvastatin Sodium salt
Owner:F I S FAB ILTALIANA SINTETICI SPA
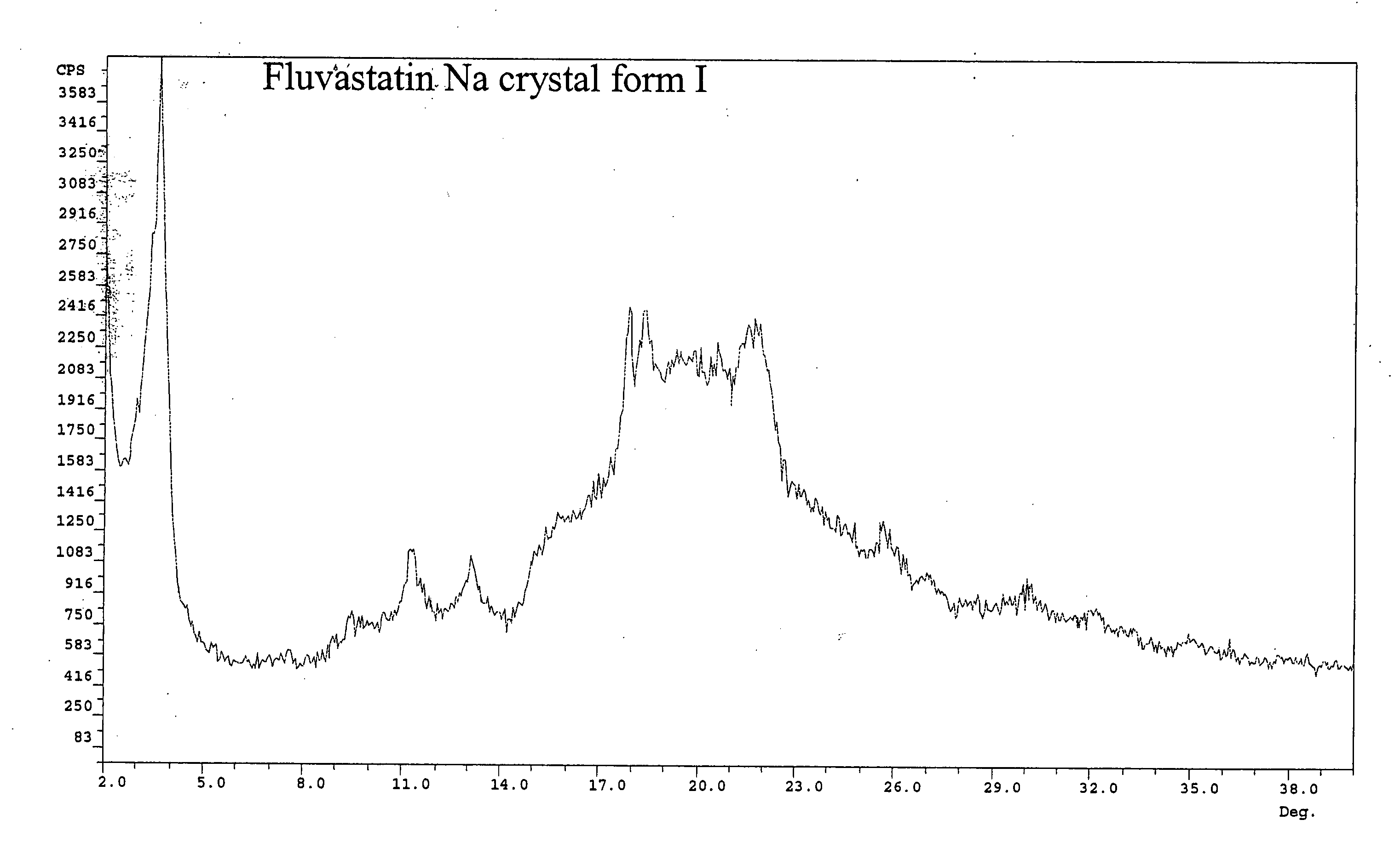
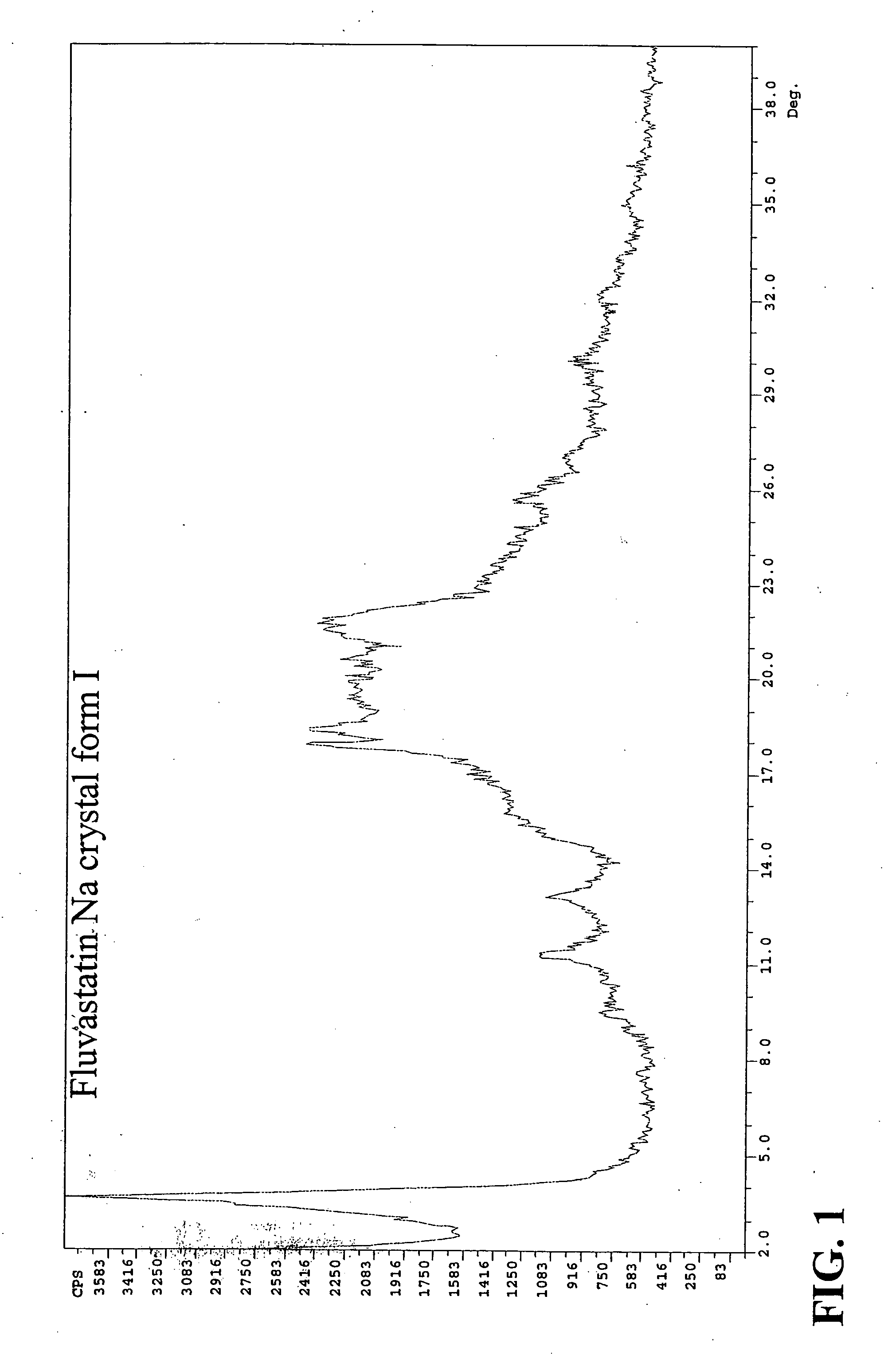
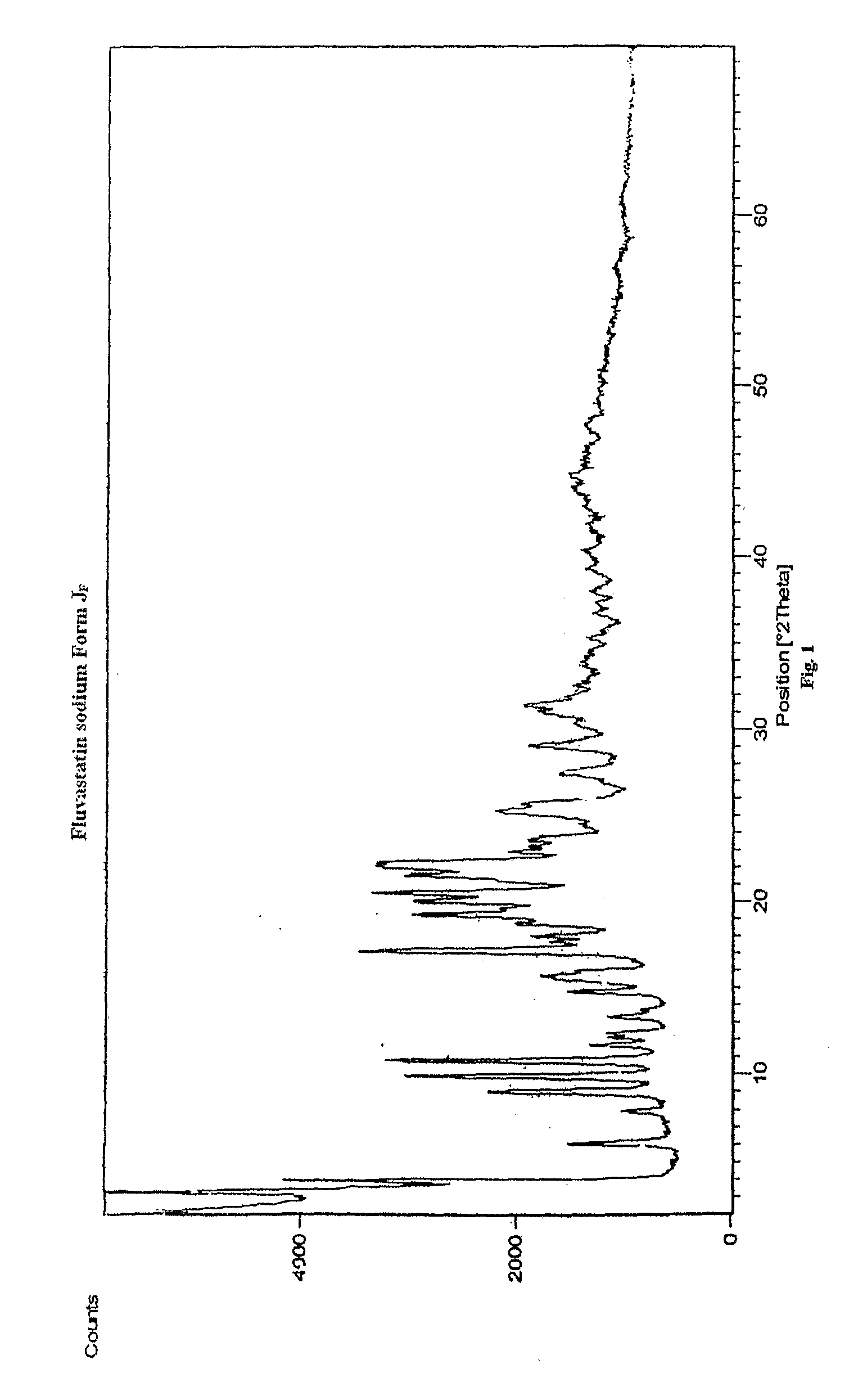
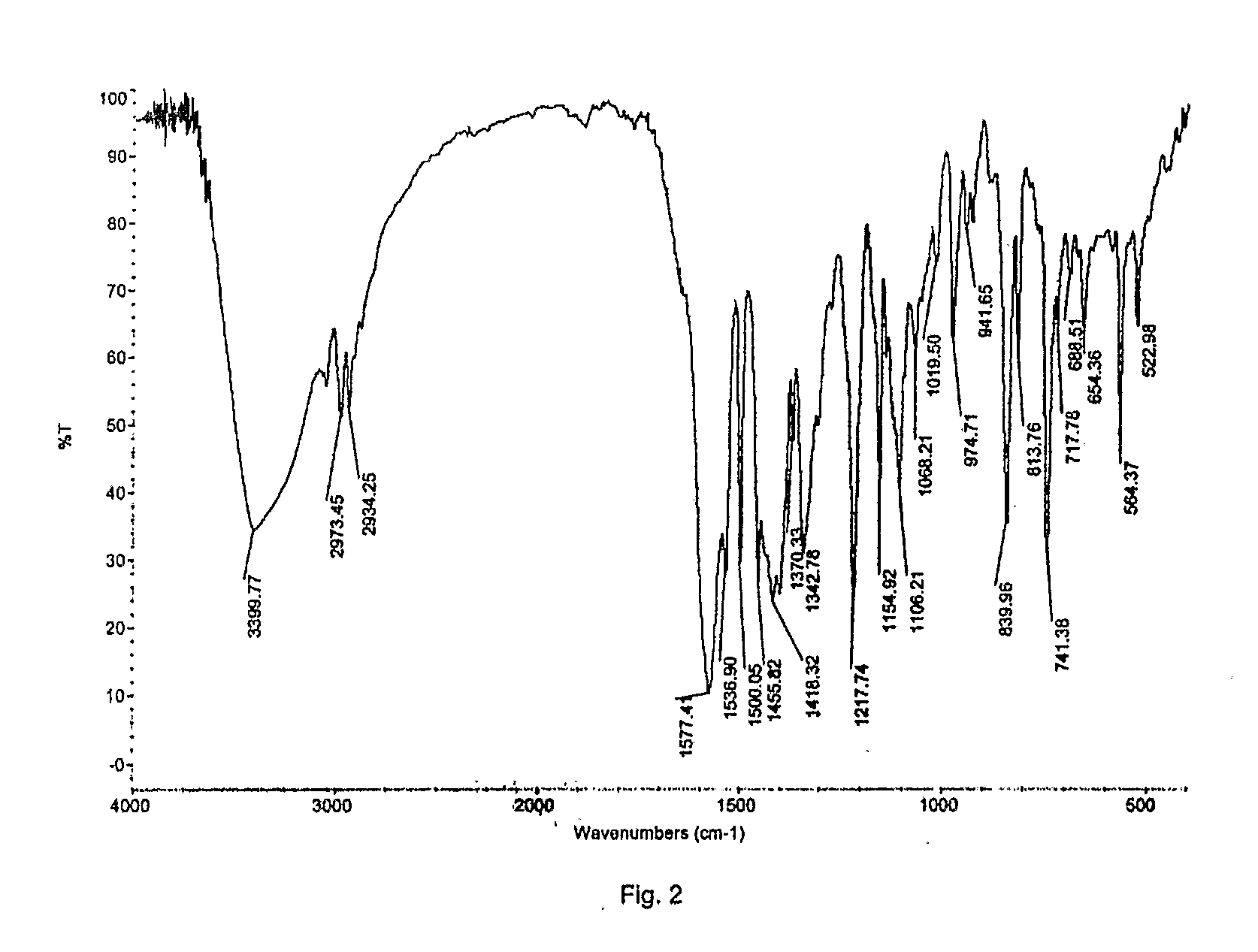
Polymorphic forms of fluvastatin sodium and process for preparing the same
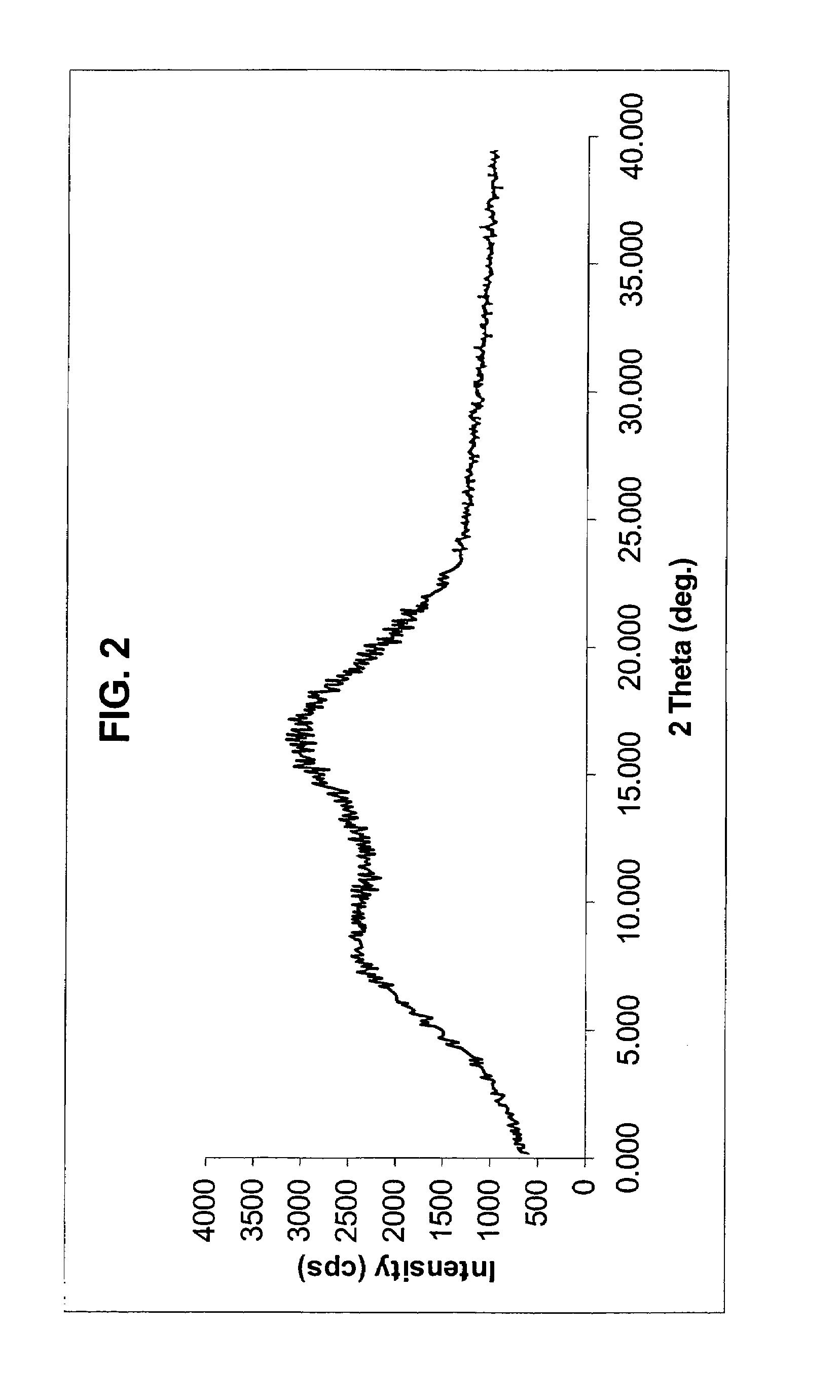
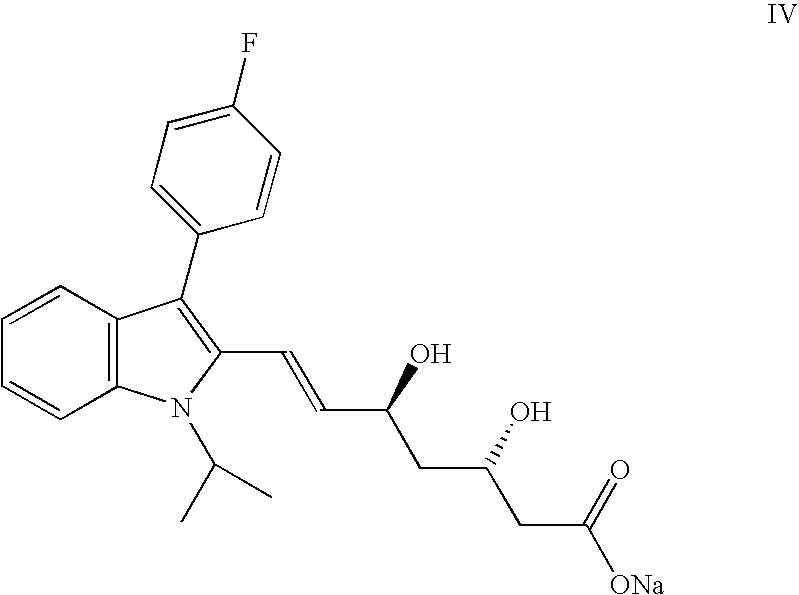
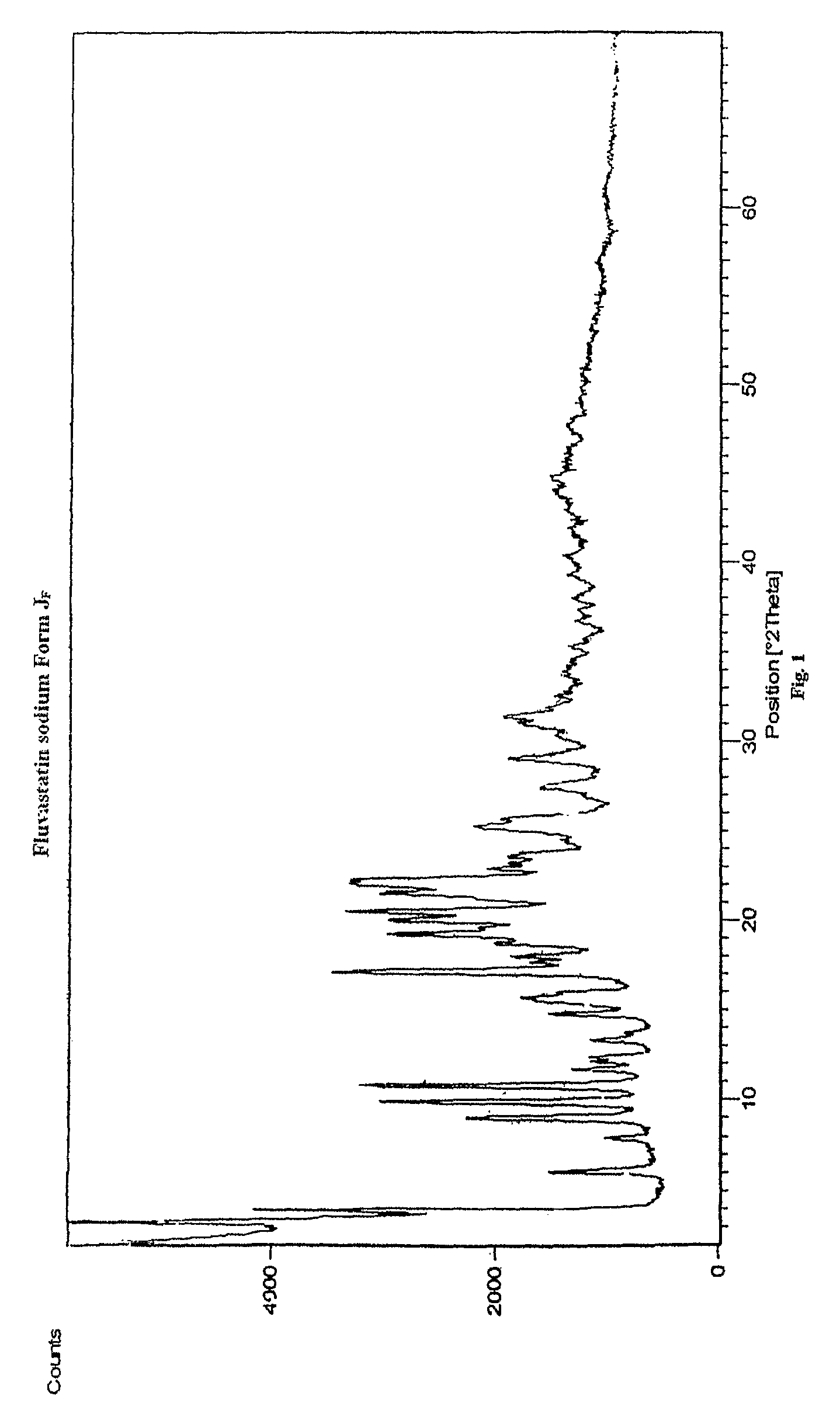
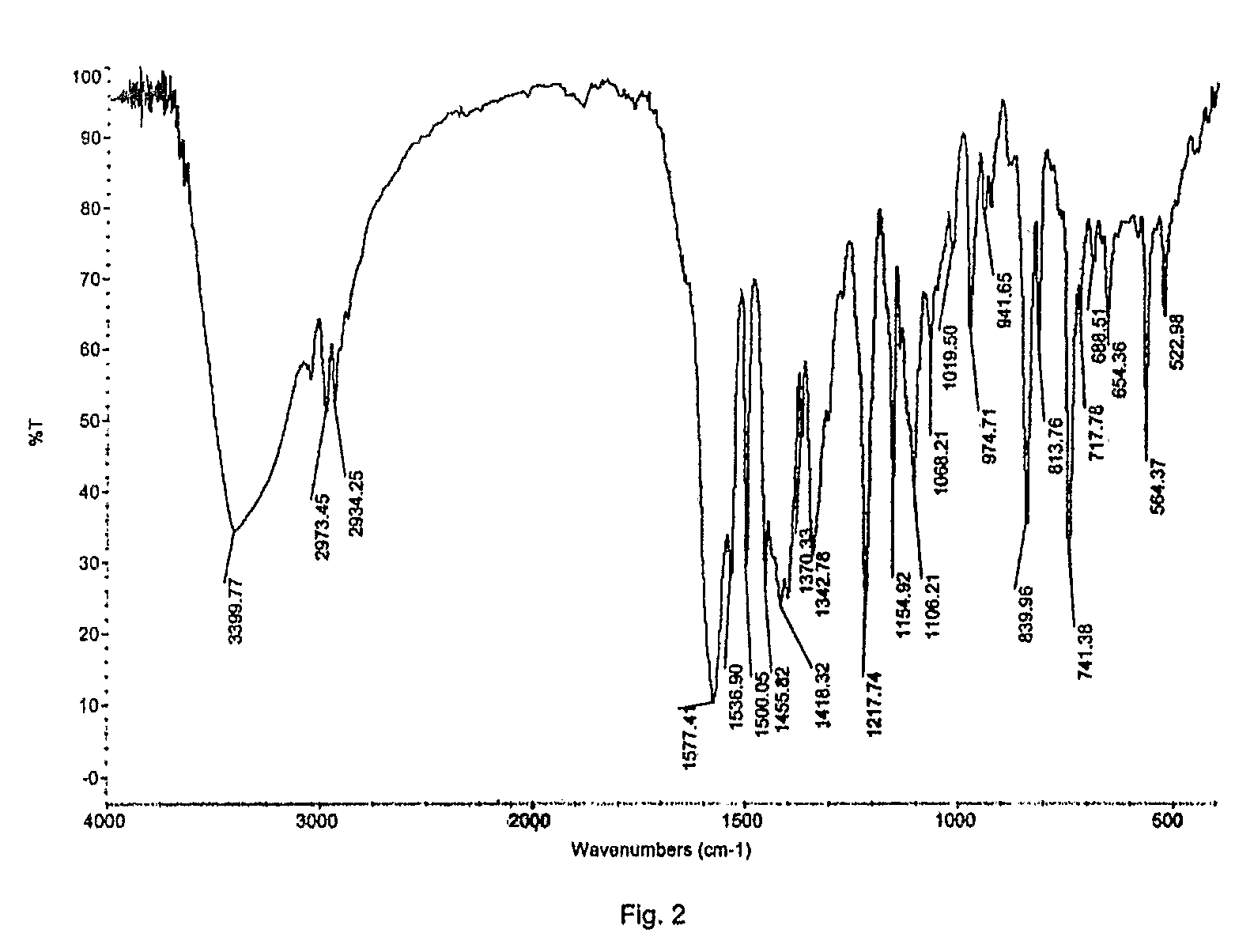
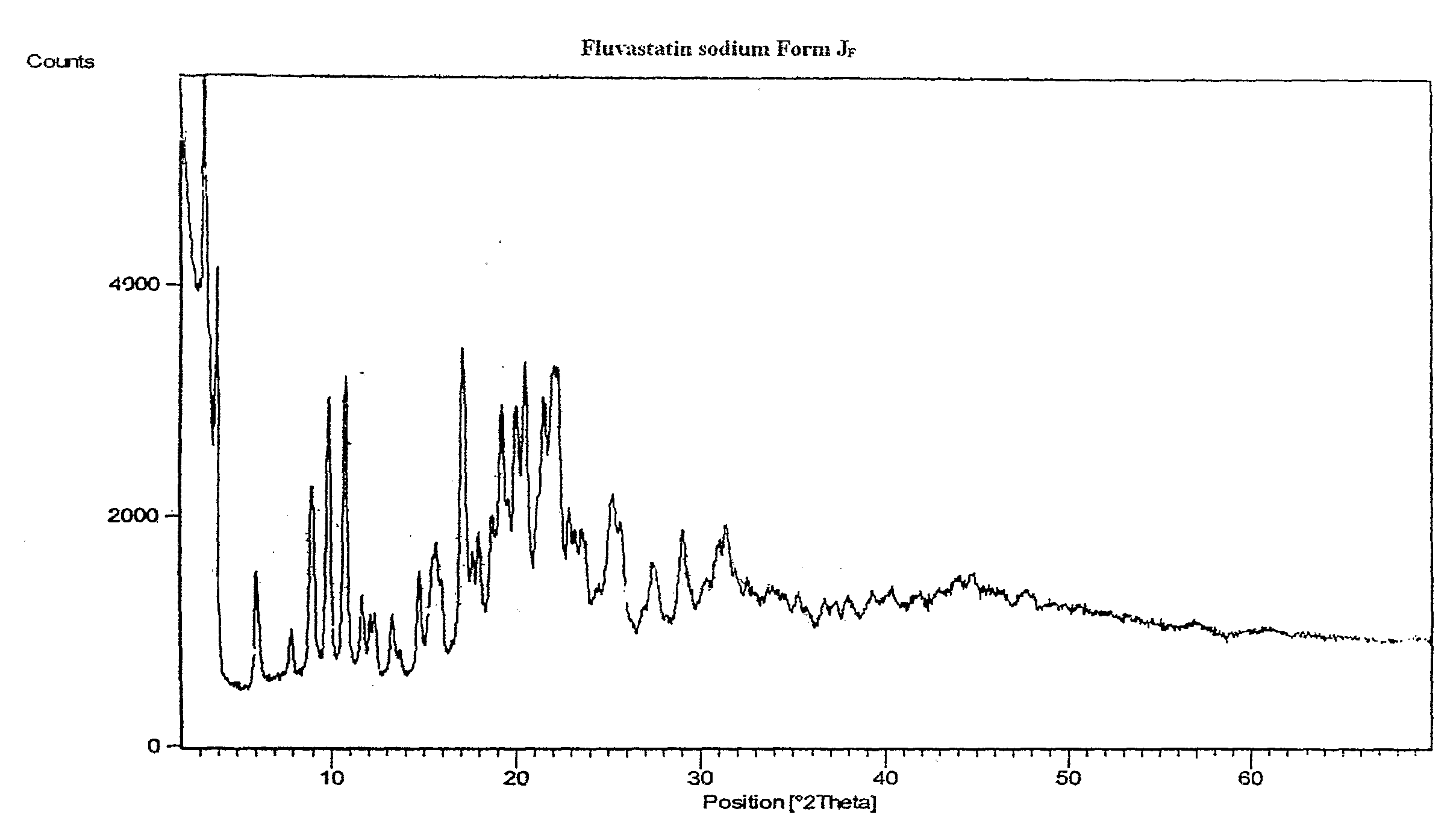
Disclosed herein are novel polymorphic forms of Fluvastatin sodium, wherein said polymorphic forms are designated as JF, JF1, JF2, JF3 and are characterized by their powder X-ray diffraction patterns, Infrared absorption spectrums, thermo gravimetric analysis and differential scanning calorimetry. The processes for preparing said polymorphic forms are also disclosed. The present invention also relates to process for preparing amorphous form of Fluvastatin sodium.
Owner:JUBILANT ORGANOSYS LTD
Preparation method of amorphous fluvastatin sodium
ActiveCN1876630AHigh yieldSimple processOrganic active ingredientsOrganic chemistryMentholState of art
The invention relates the preparing method of non-shaped fluorine-vastatin sodium. The current technology has many defects. The method comprises the following steps: preparing menthol dissolution liquid, dissolving fluorine-vastatin calcium in the liquid, distilling and getting the menthol dissolution liquid which can be recovered. The invention has the advantages of simple technology and high productivity.
Owner:ZHEJIANG LEPU PHARMA CO LTD +1
Fluvastatin sodium crystal forms, processes for preparing them, compositions containing them and methods of using them
InactiveUS20080146817A1Organic active ingredientsMetabolism disorderFluvastatin SodiumStereochemistry
Owner:TEVA PHARMA IND LTD
Controlled release color stable pharmaceutical dosage form of HMG-coa reductase inhibitors, free of alkalizing or buffering agents
InactiveUS20080102134A1BiocidePharmaceutical non-active ingredientsHMG-CoA reductaseControlled release
A color stable controlled release pharmaceutical dosage form comprising HMG CoA reductase inhibitor, rate controlling polymers and one or more pharmaceutical excipients wherein the granules are independent of particle size the pharmaceutical dosage form is free of alkalizing / buffering agents. A color stable controlled release pharmaceutical dosage form comprising Fluvastatin Sodium, hydroxypropyl methylcellulose polymer and hydroxypropyl cellulose, wherein the granules are independent of particle size.
Owner:LUPIN LTD
Fluvastatin sodium compound and preparation method thereof
InactiveCN102351776BChange the status quo of low purityImprove product qualityOrganic active ingredientsOrganic chemistryAlkaline earth metalFiltration
Owner:HAINAN MEIDA PHARMA
Fluvastatin sodium composition capsule and preparation method thereof
ActiveCN103239421BSimple processProcess control parameters are easy to controlOrganic active ingredientsMetabolism disorderHigh absorptionLactose
The invention relates to the field of medicaments, and discloses a fluvastatin sodium composition capsule; the medicament effective components prepared into one thousand capsules include 15 to 40grams of fluvastatin sodium, 20 to 50grams of microcrystalline cellulose, 120-250grams of lactose, 4 to 10grams of hydroxypropylcellulose, 50 to 100grams of 3% starch slurry and 0.5 to 2.5grams of magnesium stearate. The invention also discloses a preparation method of the fluvastatin sodium composition capsule. The fluvastatin sodium composition capsule provided by the invention has the characteristics of low cost, high absorption rate after being taken, excellent therapeutic dose tolerance of patients, light adverse reaction and the like, and is a safe and effective lipid lowering medicament; in the preparation method, the whole process is simple and the process control parameter is easy to control, and therefore the preparation method is suitable for large-scale industrialized popularization.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Fluvastatin sodium liposome solid preparation
InactiveCN102309452BQuality improvementImprove bioavailabilityOrganic active ingredientsMetabolism disorderSide effectCurative effect
The invention discloses a fluvastatin sodium liposome solid preparation and a preparation method thereof. According to the invention, fluvastatin sodium, soya lecithin, cholesterin, soybean derived sterol and dehydrated sorbitol stearate are used according to a specific weight ratio to prepare fluvastatin sodium liposome with excellent quality, and the fluvastatin sodium liposome is prepared intoa solid preparation by using a common pharmaceutical method. Compared to conventional preparations, the preparation provided in the invention has greatly improved stability and bioavailability, stable drug release, remarkable curative effects, enhanced quality and reduced toxic and side effects.
Owner:HAINAN MEIDA PHARMA
Application of fluvastatin sodium in preparation of drugs for treating corona virus disease 2019
The invention discloses an application of fluvastatin sodium in the preparation of drugs for treating corona virus disease 2019. The mechanism by which fluvastatin sodium can alleviate corona virus disease 2019 is to weaken the ability of COVID-19 virus to invade human cells through ACE2, thereby inhibiting replication of COVID-19 virus.
Owner:李成
A composition capable of preventing and controlling hyperlipidemia and its resulting cardiovascular and cerebrovascular or neuron damage, and corresponding preparation and use methods
ActiveCN107260727BSmall doseSmall toxicityNervous disorderHydrocarbon active ingredientsLycoperseneVitamin C
The invention provides a composite capable of preventing and controlling hyperlipidemia and heart vessel, cerebral vessel or neuron damage caused by the hyperlipidemia. The composite mainly comprises the following components in parts by mass: 10-40 parts of lycopene, 100 parts of vitamin C and 5-20 parts of fluvastatin sodium. The invention further provides a preparation method and a use method of the composite. The composite combines the low-dose lycopene, low-dose fluvastatin sodium and the certain-dose vitamin C for the first time based on an effect of the lycopene for efficiently preventing the hyperlipidemia from damaging a heart vessel, a cerebral vessel and a neuron and by virtue of an idea of system biology, so that synergetic effects of reducing blood fat, protecting the heart vessel, the cerebral vessel and the neuron and preventing non-infective chronic diseases are exerted; the dose of statins and the lycopene is reduced; toxic and side effects of a medicine are reduced; the cost of the medicine is lowered; and the composite has good economic and social benefits.
Owner:CENT SOUTH UNIV
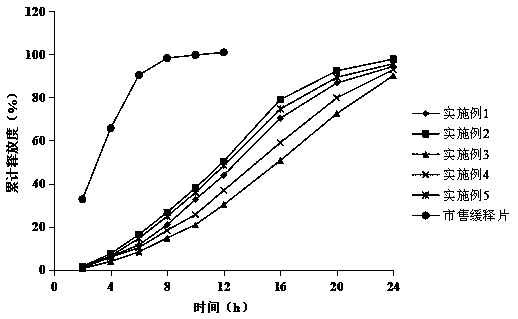
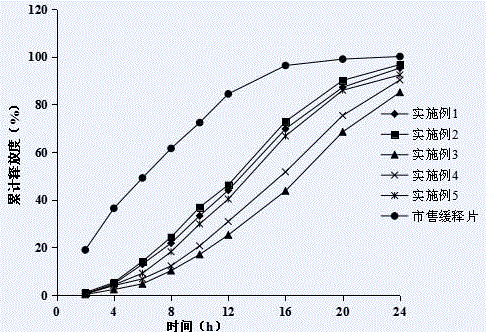
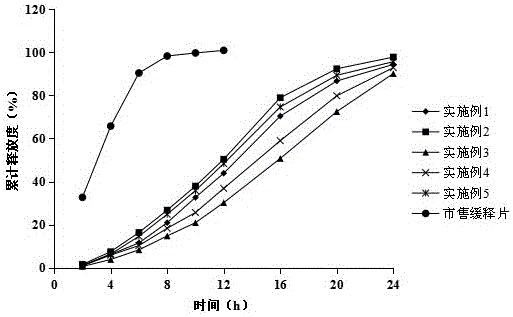
A fluvastatin sodium microporous osmotic pump controlled-release tablet and preparation method thereof
InactiveCN106344535BReduced release rateProlong the action timeOrganic active ingredientsMetabolism disorderCellulose acetatePatient compliance
The invention relates to a fluvastatin sodium microporous osmotic pump controlled-release tablet and a preparation method thereof. The preparation comprises a drug-containing tablet core, a semipermeable controlled-release coating film and a common film coating film wrapped outside the tablet core. The core contains fluvastatin sodium, fillers, blockers, osmotic pressure active substances, alkali stabilizers and lubricants; semipermeable coatings include cellulose acetate, porogens and plasticizers; ordinary film coatings Gastric Opadry for film coating premix. The present invention slows down the release rate of the drug by adding a polymer blocker to the tablet core, and then wraps the tablet core with a semipermeable controlled-release coating and a common coating with a light-shielding effect in order to prepare fluvastatin sodium The microporous osmotic pump controlled-release preparation can maintain constant drug release within 24 hours, the blood drug concentration is more stable, the curative effect is long-lasting, the toxicity and side effects are small, and the patient's compliance is good. The preparation process of the preparation is simple and easy for industrial production.
Owner:JINAN KANGHE MEDICAL TECH
Process for the preparation of fluvastatin sodium crystal from XIV
Provided is a process for preparing a polymorphic form of fluvastatin sodium, particularly Form XIV.
Owner:TEVA PHARM USA INC
Process for the preparation of fluvastatin sodium salt
The present invention is directed to a process for preparing Fluvastatin Sodium salt by basic hydrolysis of its alkyl ester. The reaction is performed in conditions suitable to allow a selective hydrolysis of the desired syn isomer, while the unwanted anti isomer is removed by extraction, thus reducing its content in the final product; this diastereomer is the main impurity of Fluvastatin sodium salt and its ester precursor.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Novel Polymorphic Forms Of Fluvastatin Sodium And Process For Preparing The Same
Disclosed herein are novel polymorphic forms of Fluvastatin sodium, wherein said polymorphic forms are designated as JF, JF1, JF2, JF3 and are characterized by their powder X-ray diffraction patterns, Infrared absorption spectrums, thermo gravimetric analysis and differential scanning calorimetry. The processes for preparing said polymorphic forms are also disclosed. The present invention also relates to process for preparing amorphous form of Fluvastatin sodium.
Owner:JUBILANT ORGANOSYS LTD
Fluvastatin sodium micro-porous osmotic pump controlled release tablet and preparing method thereof
InactiveCN106344535AReduced release rateProlong the action timeOrganic active ingredientsMetabolism disorderCellulose acetatePatient compliance
The invention relates to a fuvastatin sodium micro-porous osmotic pump controlled release tablet and preparing method thereof. The fuvastatin sodium micro-porous osmotic pump controlled release tablet comprises tablet core and semi-transparent controlled-release membrane and ordinary thin membrane wrapping the tablet core. The tablet core comprises fuvastatin sodium, filler, retarder, osmotic-pressure active substance, alkali stabilizer and lubricating agent; the semi-transparent membrane comprises cellulose acetate, pore-foaming agent and plasticizer; and ordinary thin membrane is thin-film-covered premix gastric-soluble opadry. The fuvastatin sodium micro-porous osmotic pump controlled release tablet and preparing method thereof alleviates rate of drug release by adding macromolecular retardant in the tablet core and covers the tablet core by semi-transparent controlled-release membrane and lightproof ordinary membrane in order to prepare the fuvastatin sodium micro-porous osmotic pump controlled release tablet that keeps drug release at constant rate in 24 hours in more steady blood concentration more steady, durable effects, minor toxic and side effect and good patient compliance. Besides, the fuvastatin sodium micro-porous osmotic pump controlled release tablet is in simple preparing technology, facilitating industrialized production.
Owner:JINAN KANGHE MEDICAL TECH
Composite capable of preventing and controlling hyperlipidemia and heart vessel, cerebral vessel or neuron damage caused by hyperlipidemia, and corresponding preparation and use methods
ActiveCN107260727ASmall doseSmall toxicityNervous disorderHydrocarbon active ingredientsLycoperseneVitamin C
The invention provides a composite capable of preventing and controlling hyperlipidemia and heart vessel, cerebral vessel or neuron damage caused by the hyperlipidemia. The composite mainly comprises the following components in parts by mass: 10-40 parts of lycopene, 100 parts of vitamin C and 5-20 parts of fluvastatin sodium. The invention further provides a preparation method and a use method of the composite. The composite combines the low-dose lycopene, low-dose fluvastatin sodium and the certain-dose vitamin C for the first time based on an effect of the lycopene for efficiently preventing the hyperlipidemia from damaging a heart vessel, a cerebral vessel and a neuron and by virtue of an idea of system biology, so that synergetic effects of reducing blood fat, protecting the heart vessel, the cerebral vessel and the neuron and preventing non-infective chronic diseases are exerted; the dose of statins and the lycopene is reduced; toxic and side effects of a medicine are reduced; the cost of the medicine is lowered; and the composite has good economic and social benefits.
Owner:CENT SOUTH UNIV
Preparation method of amorphous fluvastatin sodium
ActiveCN100429202CHigh yieldSimple processOrganic active ingredientsOrganic chemistryMentholState of art
Owner:ZHEJIANG LEPU PHARMA CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com