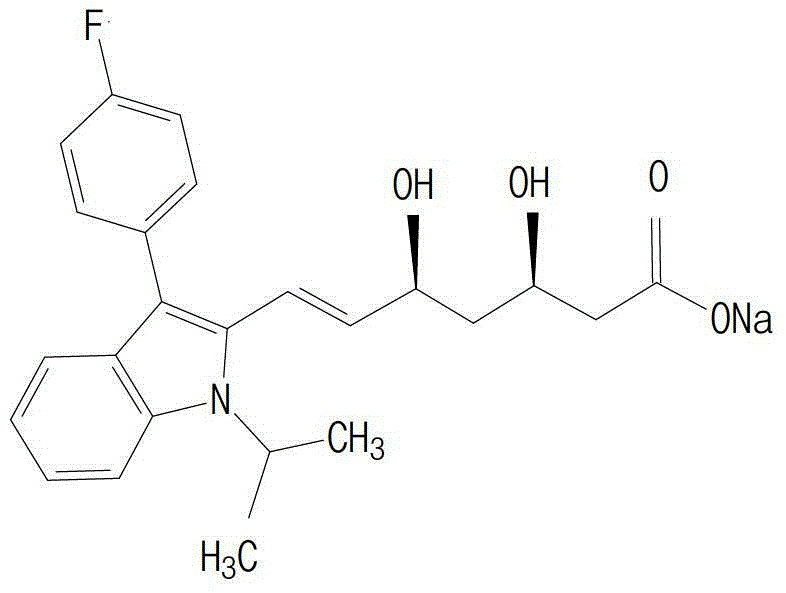
Fluvastatin sodium composition capsule and preparation method thereof
A technology of fluvastatin sodium and its composition, which is applied in the field of fluvastatin sodium composition capsule and its preparation, which can solve the problems of high price and achieve the effects of cheap price, simple process flow and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A fluvastatin sodium composition capsule with a specification of 20 mg.
[0032] Fluvastatin sodium is C 24 h 26 FNO 4 Calculated, the active ingredient of the medicine in the raw material of making 1000 is as follows:
[0033]
[0034]
[0035] The specific preparation method is as follows:
[0036] Step 1: Pass fluvastatin sodium, microcrystalline cellulose, hydroxypropyl cellulose, and lactose through a 100-mesh sieve respectively, and set aside;
[0037] Step 2: Weigh fluvastatin sodium, microcrystalline cellulose, hydroxypropyl cellulose and lactose in the prescribed amount, and mix them uniformly to obtain the first mixture;
[0038] Step 3: Add 3% starch slurry in the prescribed amount to the first mixture to make a soft material, pass through an 18-mesh sieve and granulate to obtain wet granules of the fluvastatin sodium composition.
[0039] Step 4: Dry the wet granules of the fluvastatin sodium composition through a blast drying oven at 50-60°C unti...
Embodiment 2
[0043] A fluvastatin sodium composition capsule with a specification of 40 mg.
[0044] Fluvastatin sodium is C 24 h 26 FNO 4 Calculated, the active ingredient of the medicine in the raw material of making 1000 is as follows:
[0045]
[0046] The specific preparation method is as follows:
[0047] Step 1: Pass fluvastatin sodium, microcrystalline cellulose, hydroxypropyl cellulose, and lactose through a 100-mesh sieve respectively, and set aside;
[0048] Step 2: Weigh fluvastatin sodium, microcrystalline cellulose, hydroxypropyl cellulose and lactose in the prescribed amount, and mix them uniformly to obtain the first mixture;
[0049] Step 3: Add 3% starch slurry in the prescribed amount to the first mixture to make a soft material, pass through an 18-mesh sieve and granulate to obtain wet granules of the fluvastatin sodium composition.
[0050] Step 4: Dry the wet granules of the fluvastatin sodium composition through a blast drying oven at 50-60°C until the water ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com