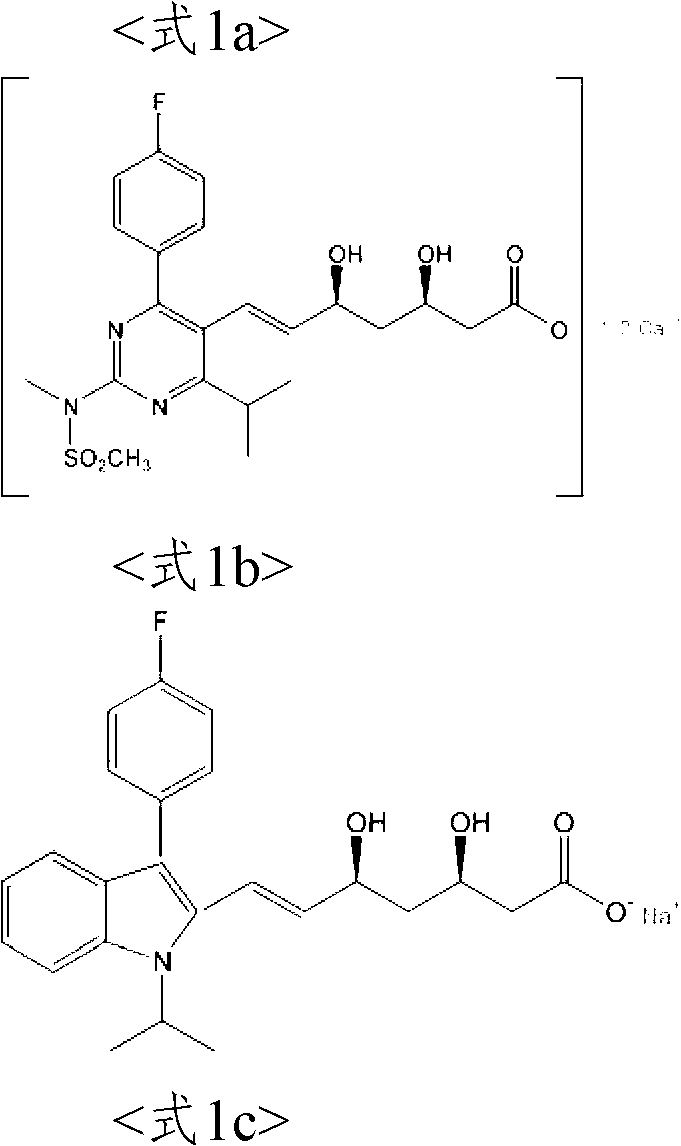
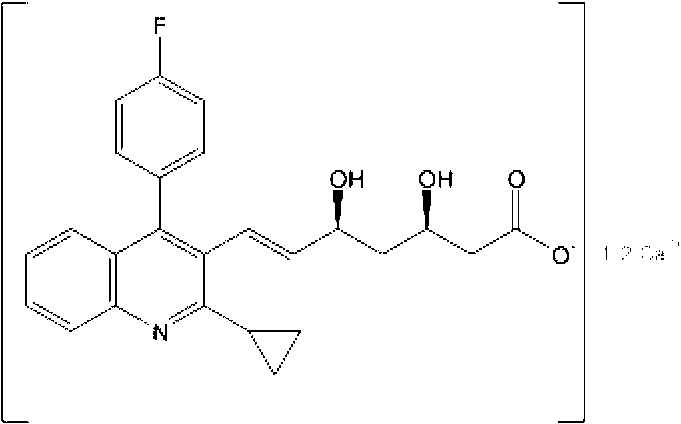
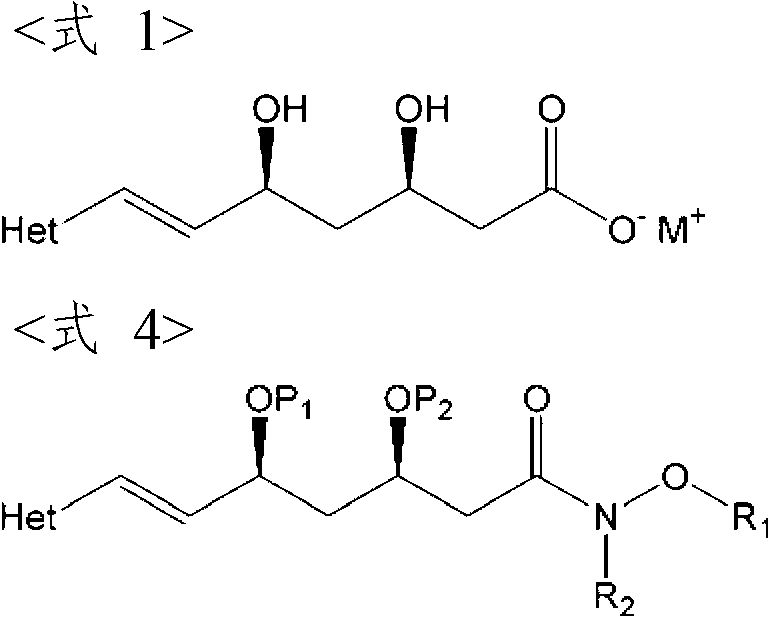
Process For The Preparation Of HMG-COA reductase inhibitors and intermediates thereof
一种C1-C6、-CH2SO2R5的技术,应用在制备HMG-CoA还原酶抑制剂领域,能够解决不适于大规模工业化生产等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] In the preparation method (i.e. deprotection method) of the compound of formula 6, the acid can be selected from hydrochloric acid, sulfuric acid, phosphoric acid, nitric acid, acetic acid, formic acid, sulfonic acid and mixtures thereof; preferably an inorganic acid such as hydrochloric acid, sulfuric acid, phosphoric acid or nitric acid; more preferably hydrochloric acid. The amount of acid used may be a catalytic amount. Typically, the acid is used in an amount of 0.005-0.2 equivalents relative to 1 equivalent of the compound of formula 4, but it is not limited thereto. And, the reaction can be carried out at 20-80°C, preferably 30-50°C, more preferably about 40°C. Therefore, since the method of the present invention can be implemented under mild conditions, it is very suitable for large-scale industrial production. The reaction can be carried out in the presence of a solvent selected from water, C 1 -C 10 Alcohols (such as methanol, ethanol, isopropanol, butanol...
Embodiment 1
[0161] 2-[(4R,6S)-6-Hydroxymethyl-2,2-dimethyl-[1,3]dioxan-4-yl]-N-methoxy-N-methyl-acetamide
[0162] [(4R,6S)-6-Hydroxymethyl-2,2-dimethyl-[1,3]dioxan-4-yl]-acetic acid (50.0 g), acetonitrile (500.0 mL) and chloride Ammonium (1.3 g) was charged to the reactor. The reaction mixture was cooled to 0-5°C, then hexamethyldisilazane (102.1 mL) was slowly added thereto. The reaction mixture was stirred at 30 °C for 1 h, then concentrated under reduced pressure to remove the solvent. Ethyl acetate (250.0 mL) and water (200.0 mL) were added to the obtained residue. The separated organic layer was dehydrated with anhydrous magnesium sulfate (50.0 g), and then filtered under reduced pressure. The filtrate was concentrated under reduced pressure to obtain a colorless transparent oily concentrated residue. Dichloromethane (250.0 mL) was added to the concentrated residue. The reaction mixture was cooled to 0-5°C, and then 1,1'-carbonyldiimidazole (43.7 g) was slowly added thereto. T...
Embodiment 2
[0165] 2-[(4R,6S)-6-Formyl-2,2-dimethyl-[1,3]dioxan-4-yl]-N-methoxy-N-methyl-acetamide
[0166] Under nitrogen atmosphere, 2-[(4R,6S)-6-hydroxymethyl-2,2-dimethyl-[1,3]dioxan-4-yl]-N-methoxy-N - Methyl-acetamide (5.0 g), dichloromethane (30.0 mL) and Dess-Martin oxidant (11.2 g) were charged to the reactor. The reaction mixture was stirred at 20-30 °C for more than 5 h. Water (30.0 mL) was added to the reaction mixture. The separated organic layer was concentrated under reduced pressure. The obtained residue was purified using silica gel column chromatography (ethyl acetate / n-hexane=1:3) to obtain 2-[(4R,6S)-6-formyl-2,2-dimethyl-[1, 3] Dioxan-4-yl]-N-methoxy-N-methyl-acetamide (4.3 g, 87% yield).
[0167] 1 H-NMR, 400MHz, CDCl 3 ,ppm:1.30(m,1H),1.46(s,3H),1.51(s,3H),1.92(d,1H),2.42~2.83(dd,2H),3.19(s,3H),3.69(s ,3H), 4.37(d,1H), 4.48(m,1H), 9.58(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com