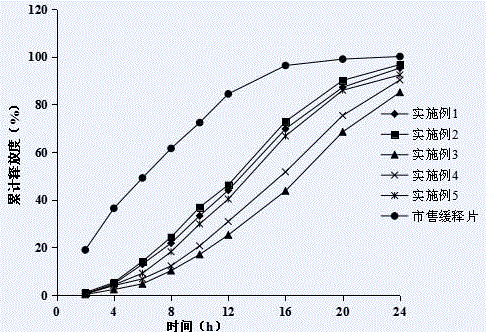
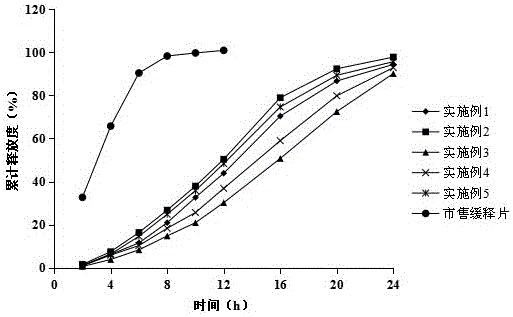
Fluvastatin sodium micro-porous osmotic pump controlled release tablet and preparing method thereof
A technology of osmotic pump controlled release and fluvastatin sodium, which is applied in osmotic delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc. It can solve the problems of short effective drug action time, fluctuation of blood drug concentration, and reduced sustained release effect, etc. problems, achieve the effects of slowing down the drug release rate, stabilizing the blood drug concentration, and prolonging the action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Prescription composition:
[0035] (1) Chip composition:
[0036]
[0037] (2) Composition of semipermeable controlled-release coating film:
[0038]
[0039]
[0040] (3) Composition of the outer film coating:
[0041] Component Weight / g
[0042] Opadry (stomach-soluble type, model: 95F620004) 6.75
[0043] Preparation Process:
[0044](1), tablet core preparation: mix the fluvastatin sodium, starch, dextrin, polyoxyethylene WSR-301NF, potassium chloride, and potassium bicarbonate in the prescribed amount through a 60-mesh sieve, and add an appropriate amount of absolute ethanol to make soft material, granulated with a 20-mesh sieve, dried at 40°C, granulated with a 18-mesh sieve, added with micropowder silica gel, mixed evenly, and pressed into tablets to obtain tablet cores;
[0045] (2) Controlled-release coating film: dissolve the prescribed amount of cellulose acetate, triethyl citrate and PEG600 in a mixed solution of acetone-purified water (98:2 by...
Embodiment 2
[0048] Prescription composition:
[0049] (1) Chip composition:
[0050]
[0051] (2) Composition of semipermeable controlled-release coating film:
[0052]
[0053]
[0054] (3) Composition of the outer film coating:
[0055] Component Weight / g
[0056] Opadry (stomach-soluble type, model: 95F620004) 10.10
[0057] Preparation Process:
[0058] (1), tablet core preparation: mix the prescription amount of fluvastatin sodium, microcrystalline cellulose, lactose, polyoxyethylene WSRN-60KNF, sodium chloride, and potassium bicarbonate through a 60-mesh sieve, and add an appropriate amount of absolute ethanol to prepare Soft material, granulated with a 20-mesh sieve, dried at 40°C, granulated with a 18-mesh sieve, added magnesium stearate, mixed evenly, and pressed into tablets to obtain tablet cores;
[0059] (2) Controlled-release coating film: dissolve the prescribed amount of cellulose acetate, diethyl phthalate and PEG400 in a mixed solution of acetone-purified w...
Embodiment 3
[0062] Prescription composition:
[0063] (1) Chip composition:
[0064]
[0065]
[0066] (2) Composition of semipermeable controlled-release coating film:
[0067]
[0068] (3) Composition of the outer film coating:
[0069] Component Weight / g
[0070] Opadry (stomach-soluble type, model: 95F620004) 10.10
[0071] Preparation Process:
[0072] (1) Preparation of tablet cores: Mix the prescribed amount of fluvastatin sodium, pregelatinized starch, lactose, hypromellose K15M, sodium chloride, glucose, and potassium bicarbonate through a 60-mesh sieve, and add an appropriate amount of anhydrous Soft material made of ethanol, granulated with a 20-mesh sieve, dried at 40°C, granulated with a 18-mesh sieve, added with talc powder and magnesium stearate, mixed evenly, and pressed into tablets to obtain tablet cores;
[0073] (2) Controlled-release coating film: dissolve the prescribed amount of cellulose acetate, triglycerides and mannitol in a mixed solution of aceto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com