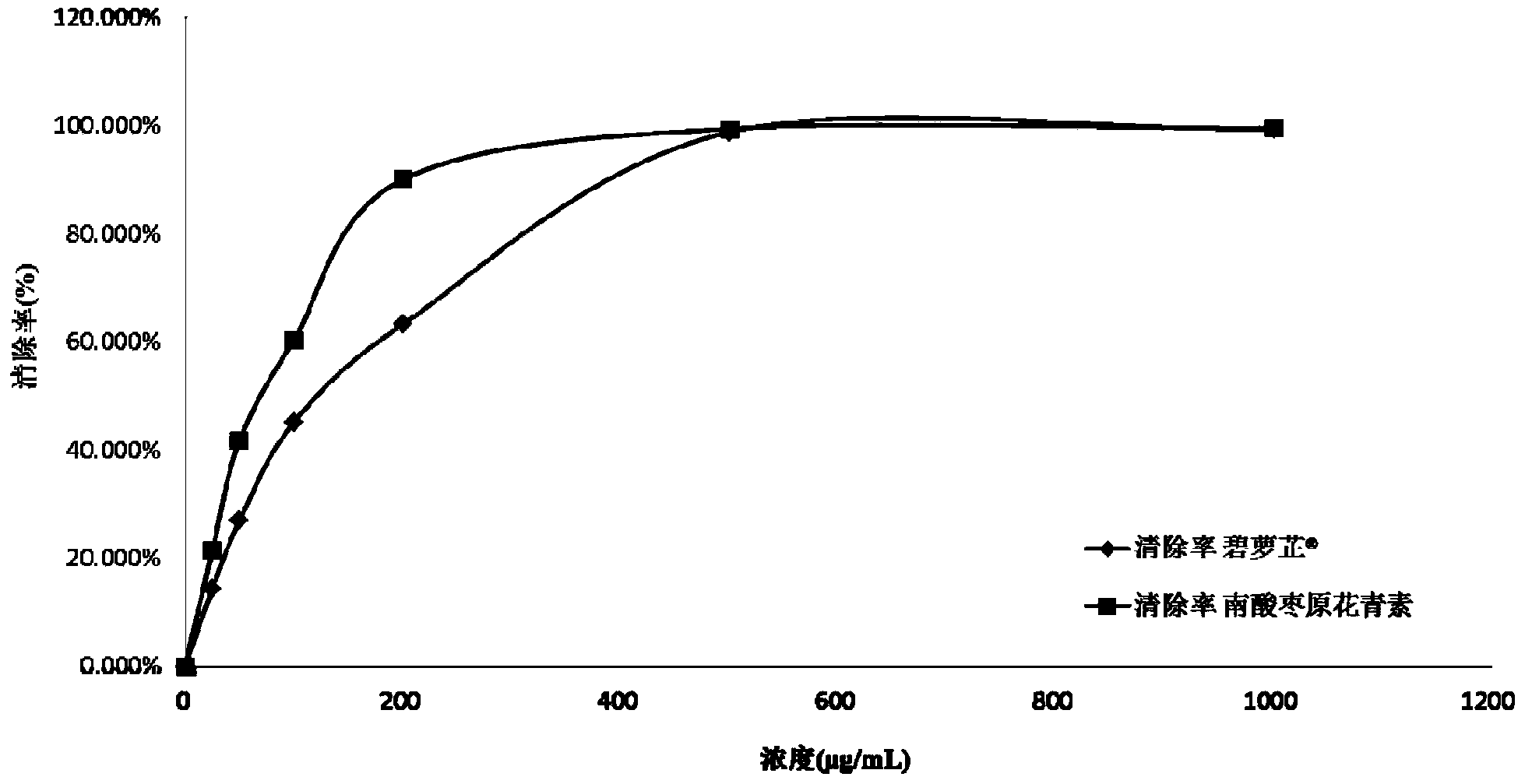
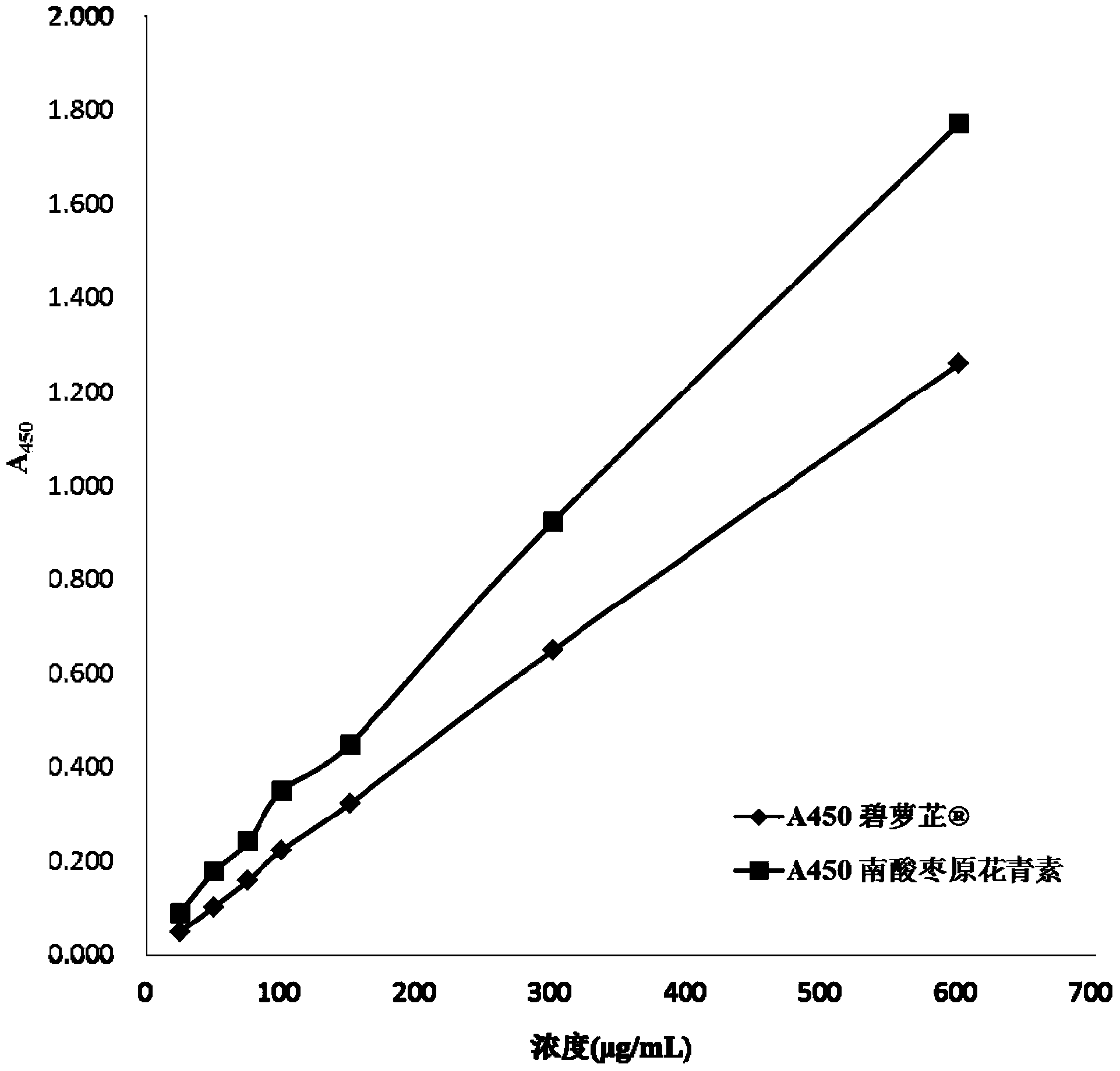
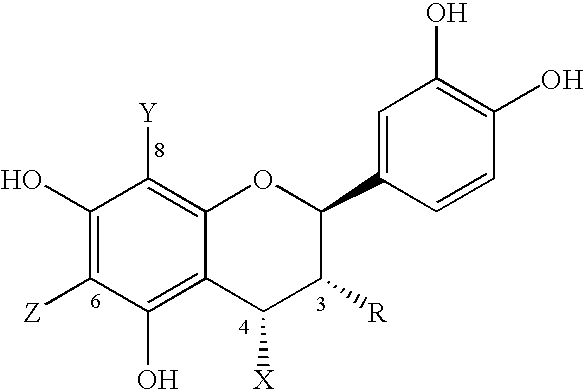
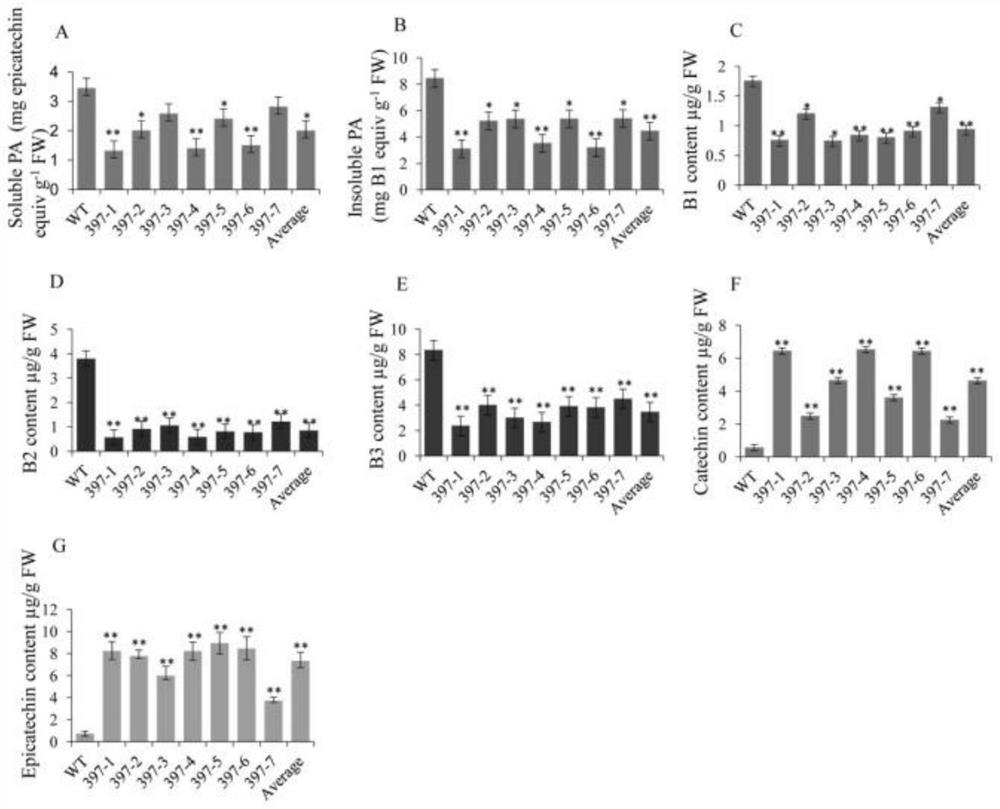
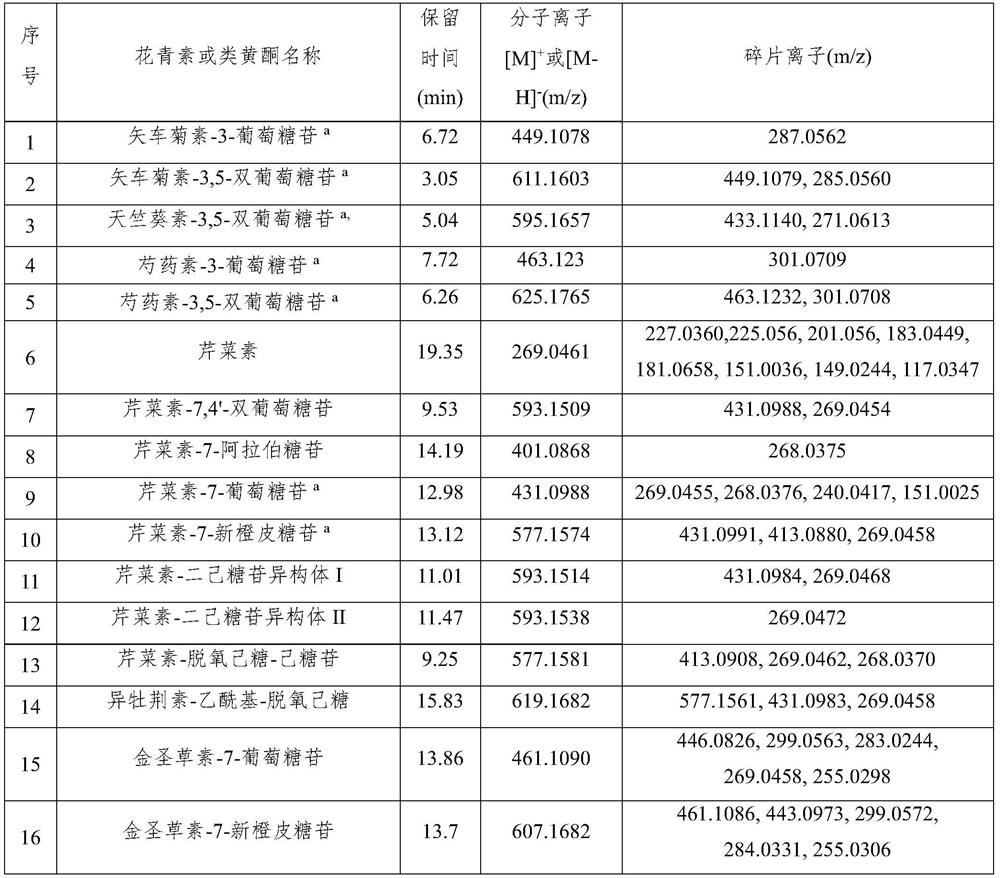
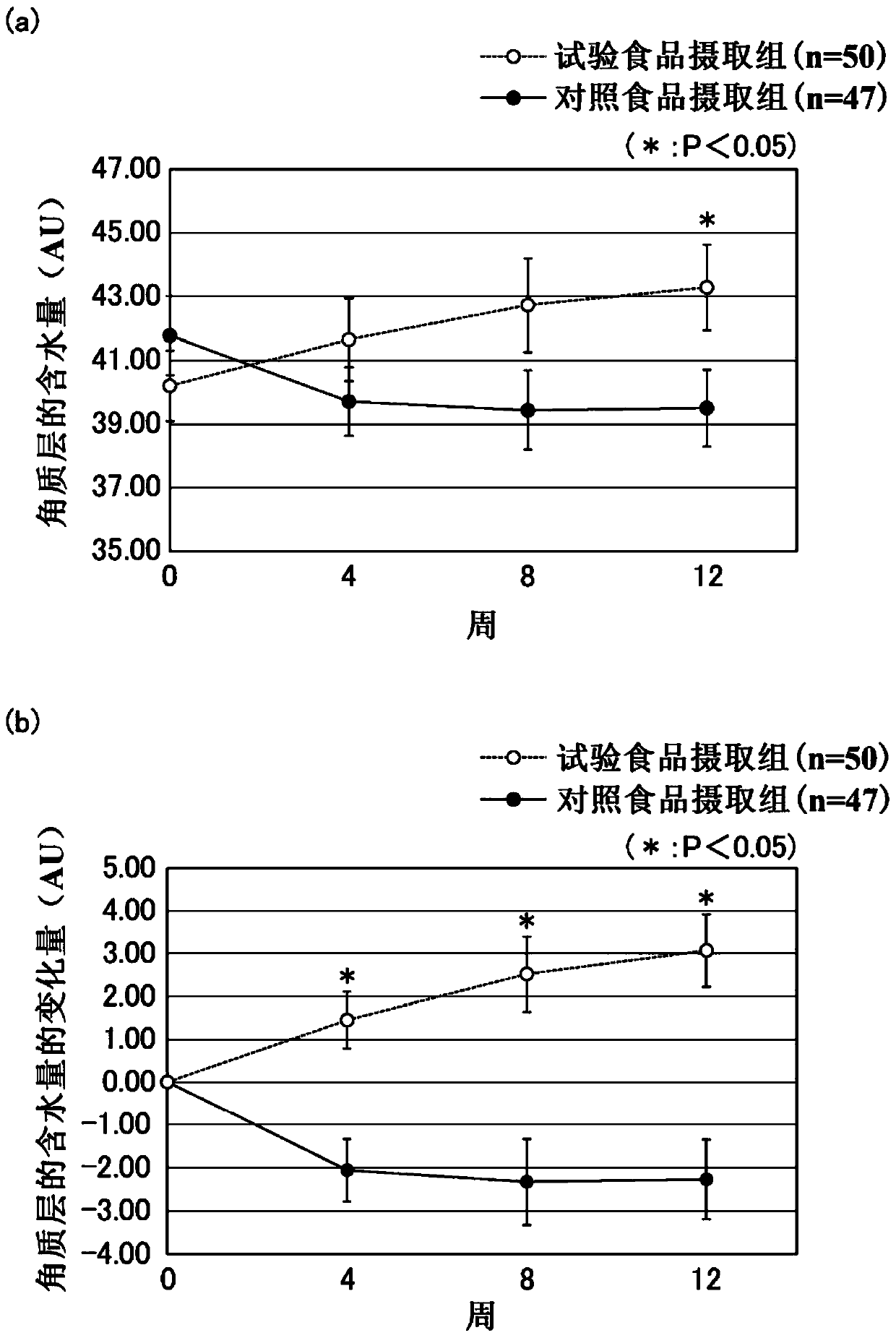
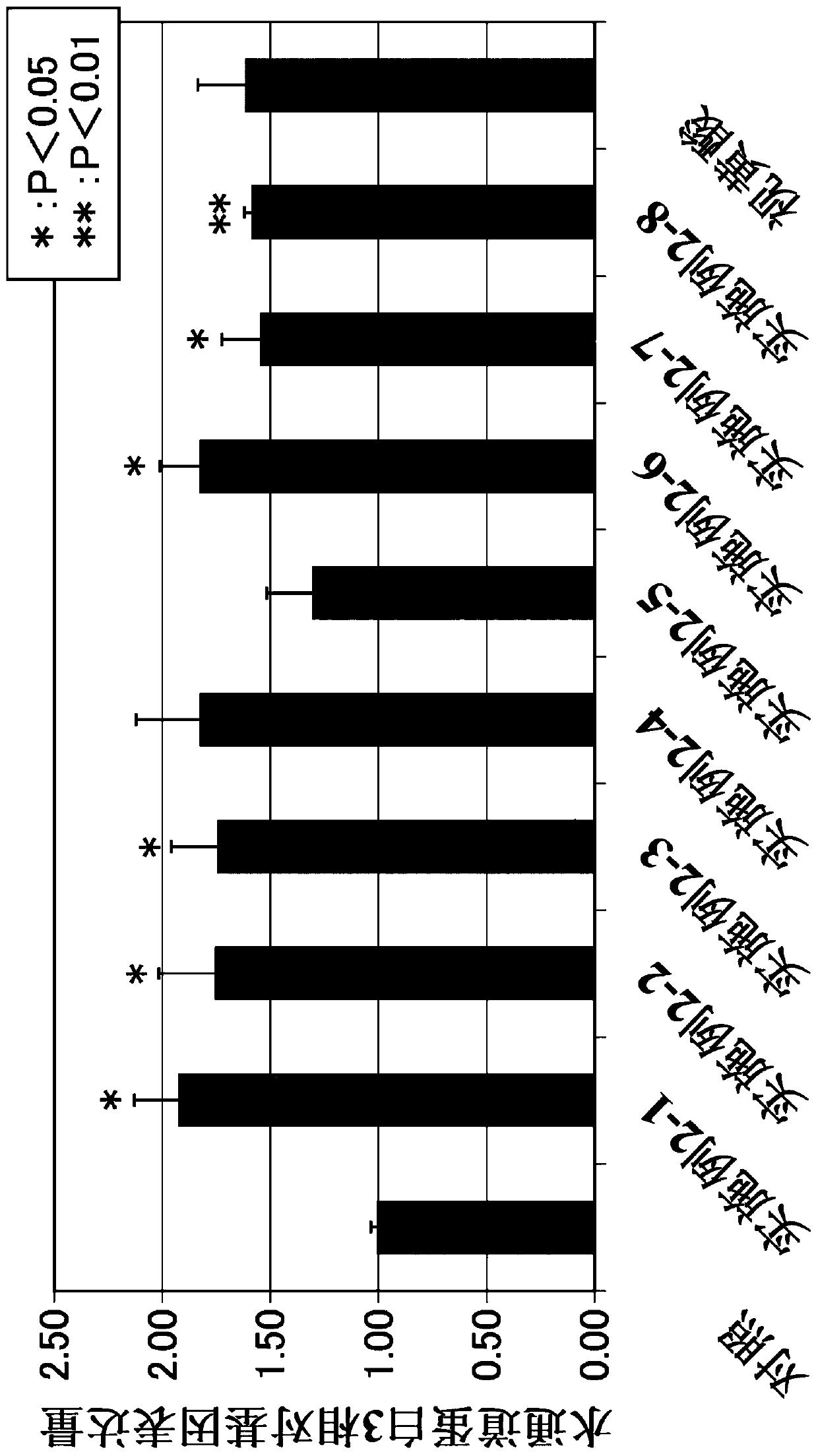
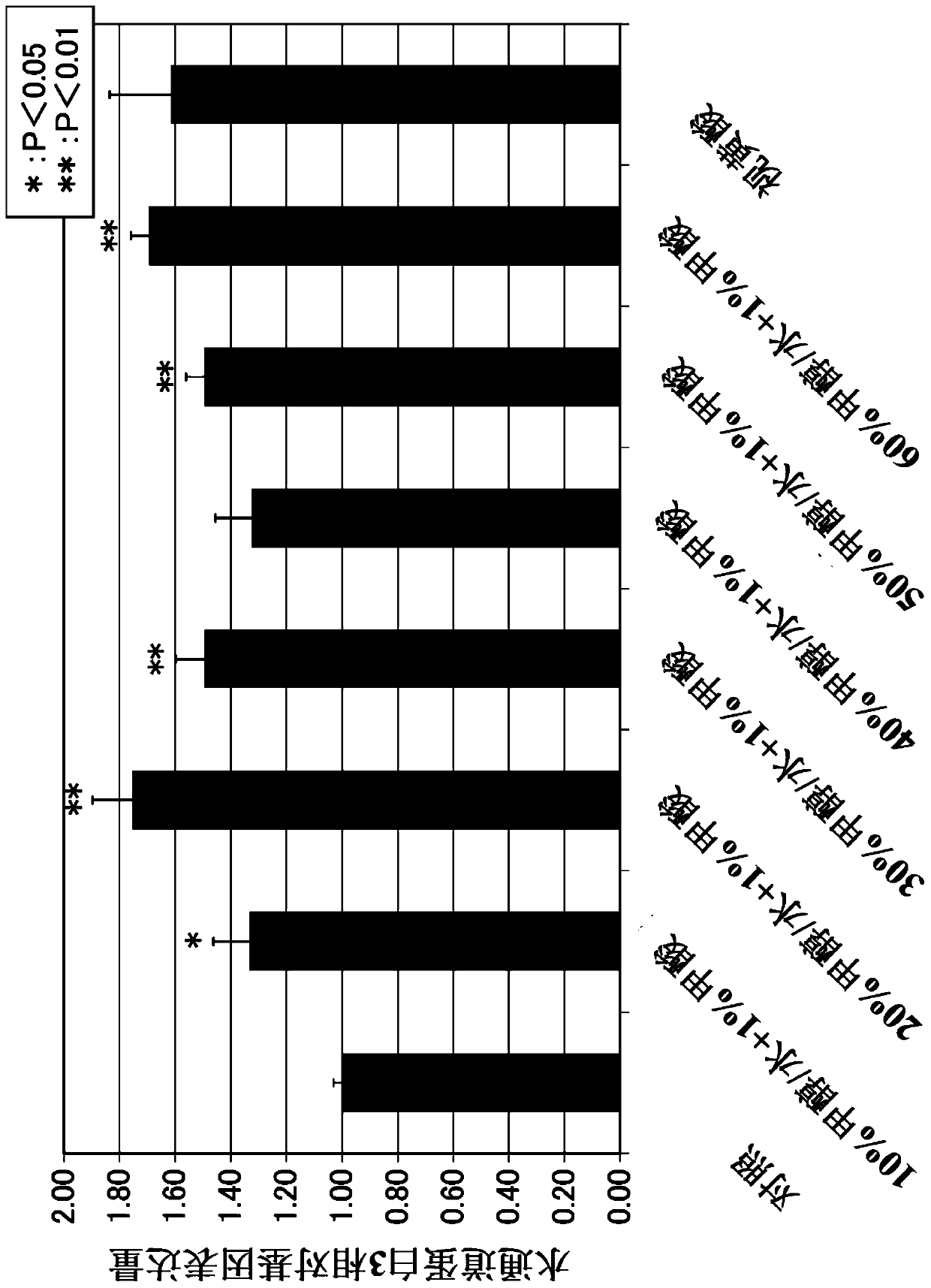
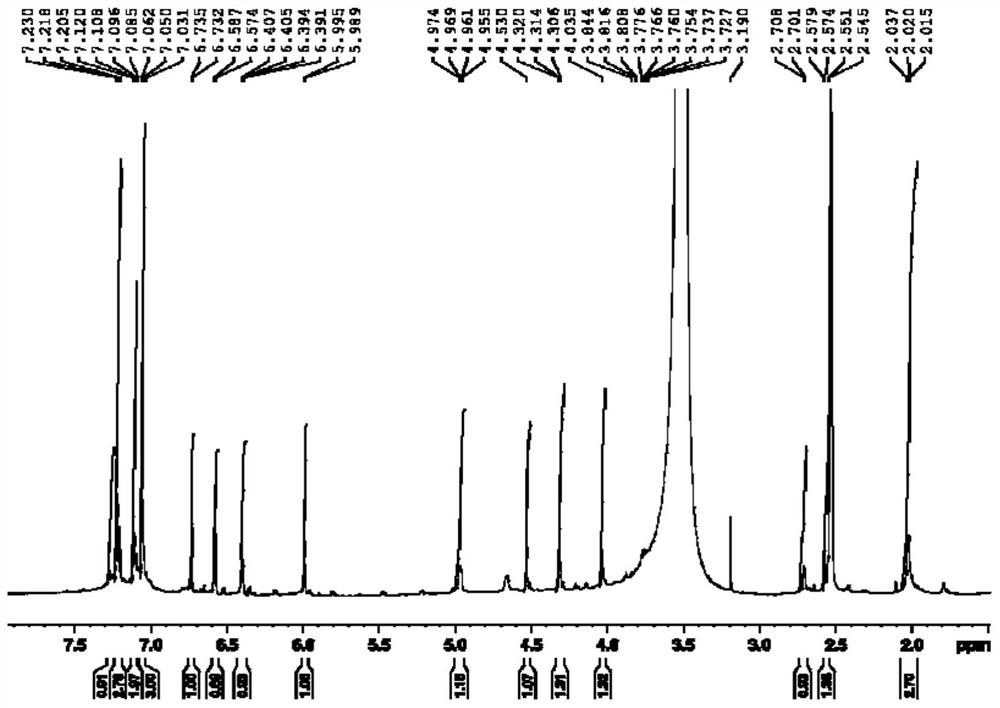
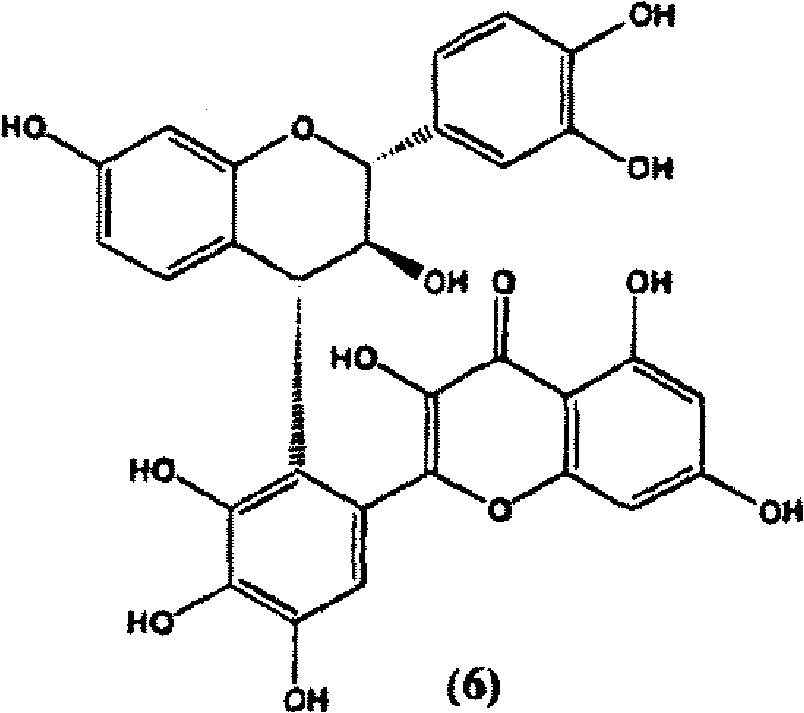
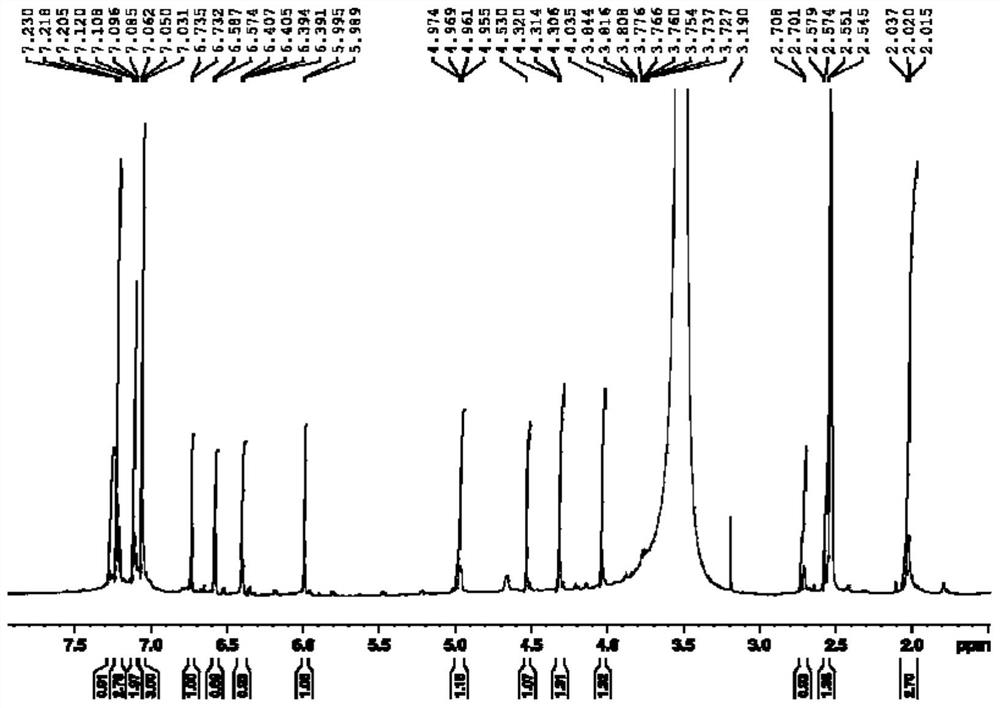
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Flavanolol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for procyanidin analysis
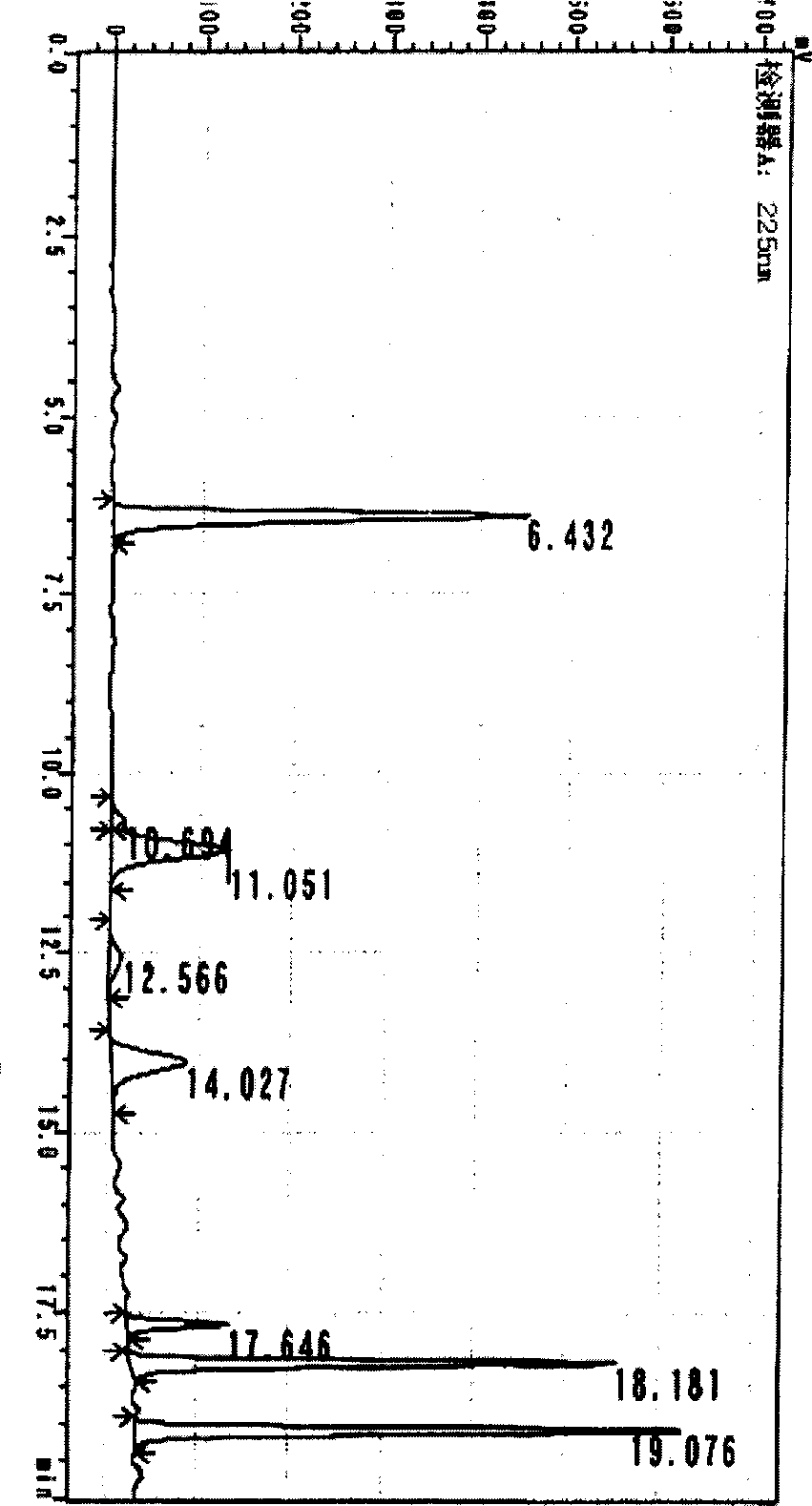
The present invention relates to a novel method for quantifying procyanidin and flavan-3-ols contained in natural products, foods and drinks, pharmaceuticals and / or cosmetics. The present invention provides a method for quantifying procyanidin (a generic name for a mixture of catechin n-mer; n >= 1) contained in test samples such as natural products, foods and drinks, pharmaceuticals and / or cosmetics, which comprises a) pretreating a test sample using a column to remove contaminants which affect the analysis of procyanidin and flavan-3-ol; and b) effecting high performance liquid chromatography (HPLC) to separate and quantify procyanidin and flavan-3-ols in the solution treated to remove contaminants during the above pretreatment step a).
Owner:SUNTORY HLDG LTD
Synergistic Neuroprotective, Combinations Of Flavanols, Hydroxystilbenes, Flavanones, Flavones, Flavonols, Proanthocyanidins And Anthocyanidins
InactiveUS20080026086A1Delay and reduce and prevent age related decline in cognitive functionReduce or prevent age related decline in cognitive functionBiocideNervous disorderNeuronal degenerationProanthocyanidin
A synthetic composition is provided comprising (a) one or more compounds selected from the group consisting of flavanols and hydroxystilbenes; and (b) one or more compounds selected from the group consisting of flavanones, flavones, flavonols, proanthocyanidins and anthocyanidins. Also provided is a method of reducing neuronal degeneration in an individual which method comprises administering to the individual said composition.
Owner:CONOPCO INC D B A UNILEVER
Photinia serrulata procyanidine as well as preparation method and application thereof
The invention discloses photinia serrulata procyanidine as well as a preparation method and an application thereof. The photinia serrulata procyanidine is prepared by extracting and purifying rachis of a photinia plant as an effective component. According to the photinia serrulata procyanidine as well as the preparation method and application, the branches, leaves and fruits of the photinia plant contain extremely high procyanidine like substances, and the matured rachis contain the most procyanidine; the plant extractive prepared through the rachis of the photinia plant by purifying has the antioxidant activity under low concentration higher than that as specified in the specification under the same mass concentration; and the chemical component analysis sheet shows that the contents of flavonoid components, phenol components and symbolic flavane-3-alcohol antioxidant components in the extractive are higher than that as specified in the specification. The raw materials of the extractive are easily obtained, the extracting process is simple, the cost is small, and wide market prospect and high economic value are brought.
Owner:NORTHWEST A & F UNIV
Flavanol (isobutene flavanol) urease inhibitor and synthesis and application thereof
InactiveCN102503922AStrong inhibitory activityOrganic active ingredientsOrganic chemistryFlavanololGastritis
The invention discloses a type of flavanol (isobutene flavanol) compounds. The structural formulae of the compounds are shown in the specifications. The compounds have good inhibiting actions on urease, and can be used for preparing medicaments for resisting gastritis, gastric ulcer, lithangiuria and the like. The invention further discloses a preparation method of the compounds.
Owner:JISHOU UNIVERSITY
Methods of treating muscular dystrophies
The invention relates to methods of treating Duchenne muscular dystrophy with flavonoids. The methods may include (a) providing a pharmaceutical composition comprising a therapeutically effective amount of flavonoid, and (b) administering the composition to a human patient, wherein the flavonoid comprises an isoflav-4-one with at least one of the carbons located at positions 8, 7, 6, 5, 2, 2′, 3′, 4′, 5′, or 6′ modified by an alcohol group. Alternatively, the flavonoid may comprise (1) a flavan-3-ol with at least one of the carbons located at positions 8, 7, 6, 5, 4, 2′, 3′, 4′, 5′, or 6′ modified by an alcohol group, or (2) a Free-B-Ring flavone with at least one of the carbons located at positions 8, 7, 6, 5 or 3 modified by an alcohol group and with at least one of the carbons located at positions 8, 7, 6, 5 or 3 modified by a glycoside group.
Owner:PRIMUS PHARM INC
Cortex choerospondias proanthocyanidins and preparation method and application thereof
The invention discloses a cortex choerospondias proanthocyanidins and a preparation method and application thereof. The cortex choerospondias proanthocyanidins is extracted and purified from an effective part of cortex choerospondias. The invention discloses that cortex choerospondias contains high content of procyanidins. The plant extract prepared from cortex choerospondias by purification has antioxidant activity at a low concentration higher than that of Pycnogenol in the same concentration. Chemical composition analysis shows that the extract the contents of antioxidant components such as flavonoids, phenolic compounds and iconic flavanone-3-alcohol are higher than thos in Pycnogenol. The extract has the advantages of easily available raw materials, simple extraction process and low cost, and has a very broad market prospects and economic values.
Owner:NORTHWEST A & F UNIV
Inducing peripheral blood vesel vasodilation
InactiveUS20070037872A1Reduce the valueIncrease valueBiocideCarbohydrate active ingredientsVascular diseaseMedicine
The invention relates to compositions, and methods of use thereof, containing polyphenols such as flavanols, procyanidins, their derivatives and epimers thereof, for inducing peripheral blood vessel vasodilation, for example for treating or preventing peripheral vascular diseases.
Owner:MARS INC
Isoflavanol compound and preparation method and application thereof
The invention provides an isoflavanol compound and a preparation method and application thereof. The structure of the isoflavanol compound is reported for the first time, and the isoflavanol compoundis derived from suberect spatholobus stem, and can serve as a standard product for identifying isoflavanol substances and / or flavone substances in the suberect spatholobus stem. The isoflavanol compound provided by the invention is separated from an ethyl acetate extract of the suberect spatholobus stem salable product, by adopting the same preparation method, the compound is not separated from other suberect spatholobus stem local medicinal materials, and therefore, the isoflavanol compound is further expected to serve as a fingerprint compound for identifying the suberect spatholobus stem salable product and the local medicinal materials.
Owner:ZHUZHOU QIANJIN PHARMA
Methods for biologically synthesizing and detecting catechin intermediates
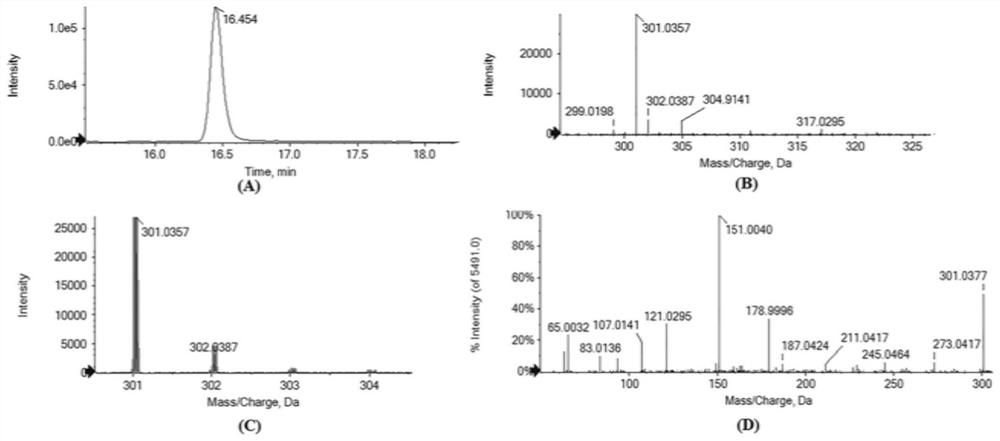
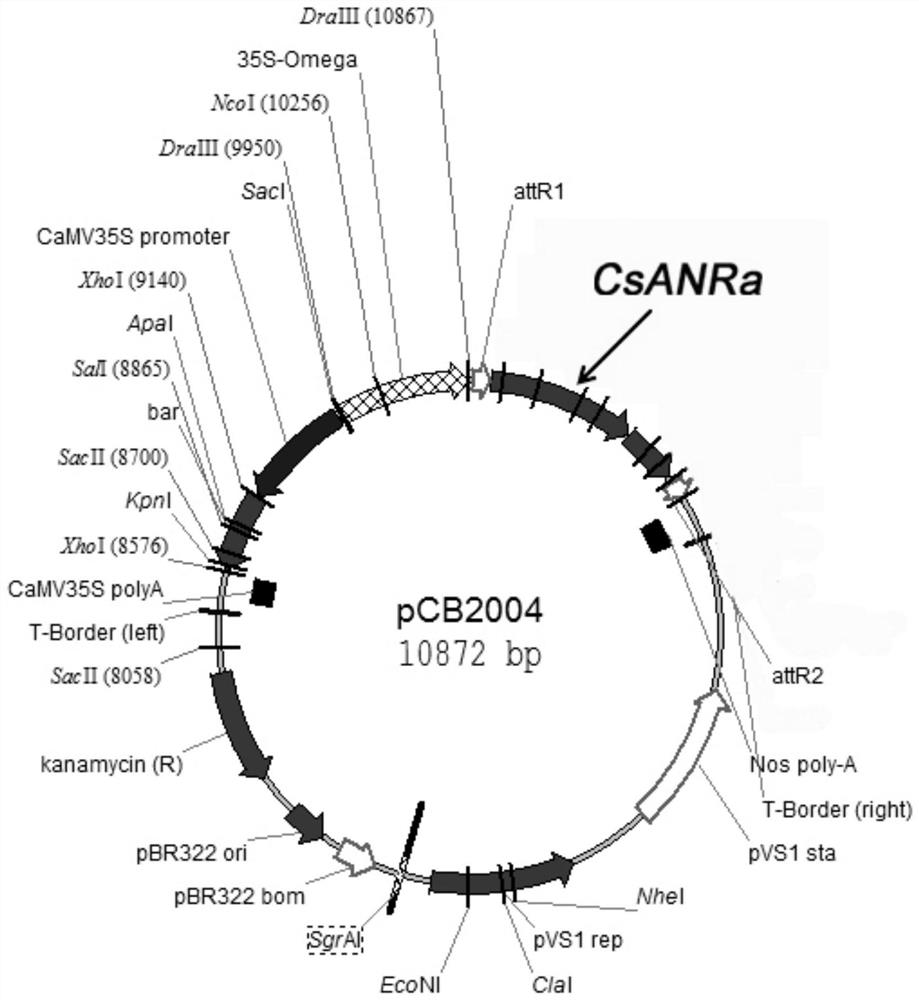
ActiveCN108410886ALow priceEasy to operateOrganic chemistryComponent separationAlcoholOrganic solvent
The invention discloses methods for synthesizing and detecting catechin intermediates. The methods include acquiring CsANRa genes from tea trees by means of cloning and then constructing binary expression vectors; transforming the binary expression vectors into plant bodies to obtain transgenic plants; extracting polyphenols substances such as flavane-3-alcohol intermediates in the transgenic plants by the aid of extracting solution which is 80% methanol and hydrochloric acid mixed solution so as to obtain the catechin intermediates; qualitatively and quantitatively authenticating catechin intermediate products by means of UPLC-QQQ-MS / MS (ultra-performance liquid chromatography-triple quadrupole-mass spectrometry / mass spectrometry). Compared with the prior art, the methods have the advantages that the methods are easy to operate, and organic solvents are inexpensive and are low in cost; the catechin intermediates acquired by the aid of the corresponding method do not contain catechin and are relatively simple, accordingly, the corresponding method is suitable to be used for preparing catechin intermediates with high purity, and the methods for synthesizing and detecting the catechin intermediates are reported for the first time.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Gene SmLAC1 and application of gene SmLAC1 in regulation and control of synthesis of procyanidine
The invention provides a gene SmLAC1, which is a salvia miltiorrhiza laccase gene, and the sequence of the salvia miltiorrhiza laccase gene SmLAC1 is as shown in SEQ ID NO. 1; the sequence of the SmLAC1 protein is as shown in SEQ ID NO. 2; an expression vector carrying the SmLAC1 is transformed into a living body, so that the content of procyanidine in a plant body can be increased; and organisms comprise microorganisms and plants, and the proanthocyanidins are dimers of proanthocyanidins B1, B2 and B3. The gene is cloned from a salvia miltiorrhiza plant for the first time, and the encoded protein can catalyze flavan-3-ol to polymerize into the procyanidine, has application value in the aspect of production of effective active substances, provides a new way for large-scale production of the procyanidine, and also lays a solid foundation for industrial production of other related procyanidine.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Flavone-metal complex microsphere and preparation method and application thereof
InactiveCN101891783BEasy to shapeSimple structureSugar derivativesCopper organic compoundsPhenolic content in teaEthyl acetate
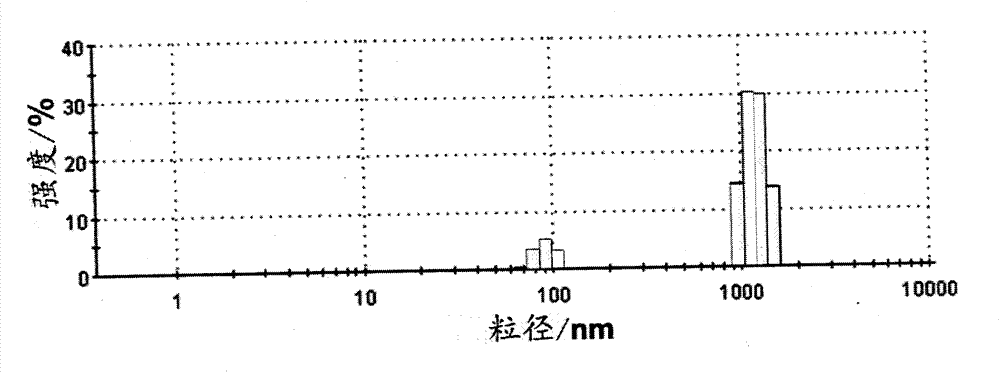
The invention relates to a flavone-metal complex microsphere which is in a spherical shape and in the structure of a solid sphere or a hollow sphere or a porous sphere, and the particle size of the sphere is 50nm-5000nm. The flavone-metal complex microsphere is formed by complexing flavonoid substances such as flavanol, anthocyanidin, tea polyphenol and the like with metal, and the preparation process comprises the following sequential steps: (1) preparing a flavone solution, (2) reacting and synthesizing, and (3) collecting and drying the product. In the reaction for synthesis, glycerin or glycol is used as an assistant, and the reaction temperature is 60 DEG C-70 DEG C for obtaining the solid microsphere. In the reaction for synthesis, ethyl acetate or vinyl alcohol is used as an assistant, and the reaction temperature is 90 DEG C-150 DEG C for obtaining the hollow microsphere. In the reaction for synthesis, ethanol is used as an assistant, and the reaction temperature is 70 DEG C-90 DEG C for obtaining the porous microsphere. The flavone-metal complex hollow microsphere can be used as a drug carrier or fluorescence molecular carrier for application.
Owner:SICHUAN UNIV
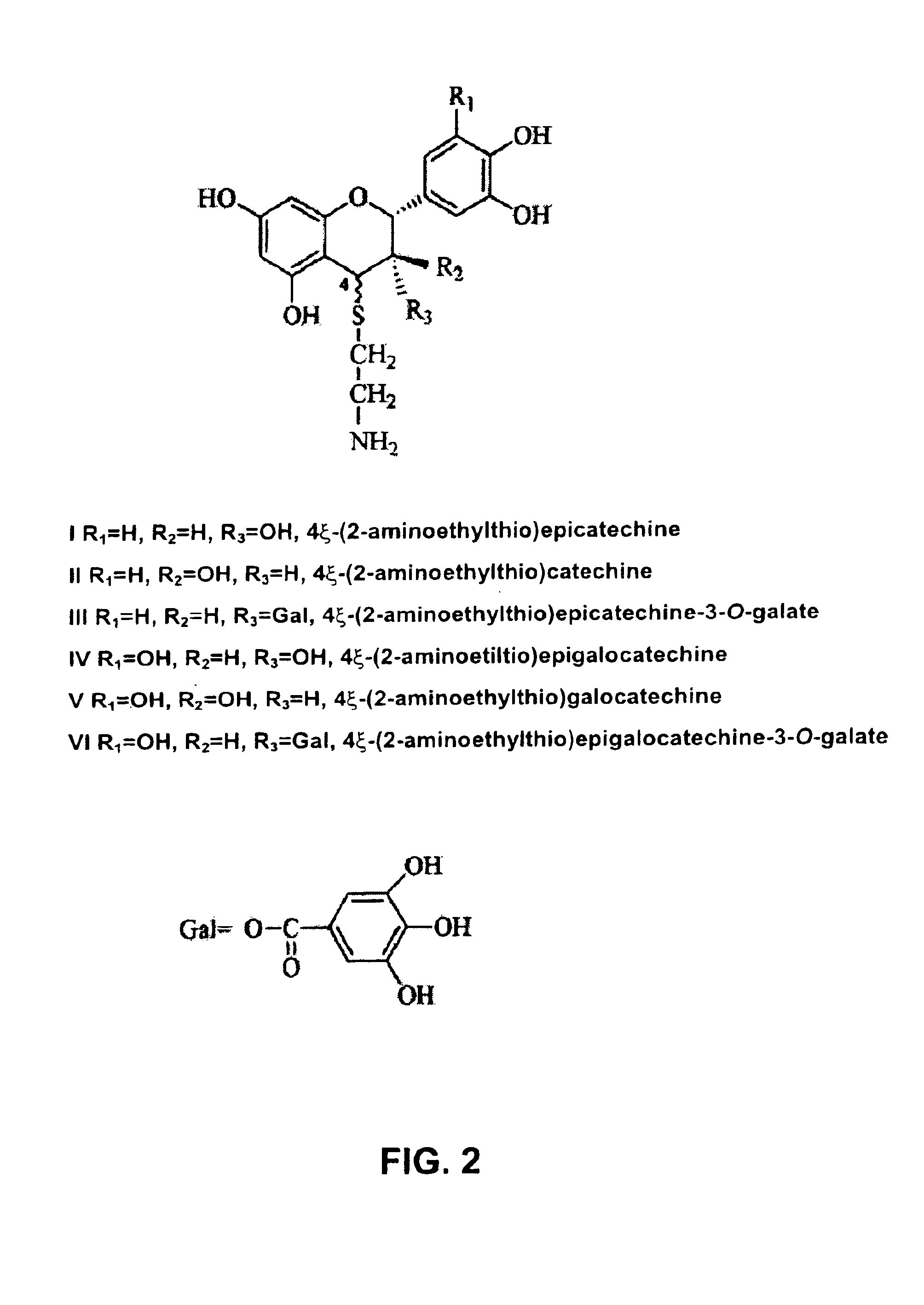
Flavanol and cysteamine conjugates
The present invention refers to new products generated by the conjugation of flavanols with molecules that contain the thiol group. New molecules are obtained from polyphenolic plant extracts rich in oligomeric and polymeric procyanidines and prodelfinidins. In this way, new products are generated with antioxidant properties for application as protective agents for the organism against disorders such as cancer, cardiovascular diseases and premature aging. The invention also refers to obtaining these new agents from waste material generated by the agroalimentary industry. Since these waste materials are highly complex mixtures a simple and effective method is described to isolate and purify these, based on the physico-chemical characteristics of the new molecules.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
Flavanoid compounds as chemotherapeutic, chemopreventive, and antiangiogenic agents
InactiveUS7329687B2Potent inhibitor of angiogenesisSimple molecular structureBiocideOrganic compound preparationAntiangiogenic agentsFlavanone
Compounds useful as chemotherapeutic, chemopreventive, and antiangiogenic agents are provided. The compounds are flavanoids, including flavanones, flavanols, and chalcones. The compounds have the structure of formula (I)wherein R1 through R3 and R5 through R11 are defined herein, and α, β, and γ are optional bonds, providing that when α is absent, β is present, and when β is absent, α is present. When α is present, preferred R4 moieties are selected from O, S, NH and CH2, and when α is absent, preferred R4 groups are selected from OH, SH, NH2 and CH3. When γ is present, the preferred R5 substituent is O, while when γ is absent, the preferred R5 substituent is OH. Pharmaceutical compositions are provided as well, as are methods of synthesis and use.
Owner:SRI INTERNATIONAL
Method for analyzing proanthocyanidin structure through combination of hydrophilic interaction and reversed-phase liquid chromatography
The invention discloses a method for analyzing a proanthocyanidin structure through combination of hydrophilic interaction and reversed-phase liquid chromatography. The method comprises the following steps: analyzing a flavan-3-alcohol monomer; carrying out acid degradation on the proanthocyanidin to obtain an acid degradation product to be analyzed; analyzing the acid degradation product to be analyzed by using reversed-phase liquid chromatography (RPLC), analyzing the type and content of terminal units of proanthocyanidin according to the difference of the type and content of flavane-3-alcohol analyzed by the RPLC and Hilic, and analyzing the type and content of extension units of proanthocyanidin according to the flavane-3-alcohol-phloroglucinol addition product analyzed by the RPLC and the anthocyanidin; and calculating the average polymerization degree of the proanthocyanidin according to the mole number of the proanthocyanidin extension unit and the mole number of the terminal unit. According to the scheme, the step of directly removing flavane-3-alcohol in a sample in a traditional method (time consumption is long) is replaced, and the analysis speed of the terminal unit of the proanthocyanidin is greatly increased.
Owner:ZHEJIANG UNIV
Beverage composition containing green tea solids, electrolytes and carbohydrates to provide improved cellular hydration and drinkability
InactiveCN1075715CImproved Physiological ResponseResponsiveTea extractionFood preparationPotassium ionsGlucose polymers
This invention relates to a composition, preferably in the form of a beverage, whereby cellular hydration and drinkability are enhanced by the combination of green tea solids with selected levels and types of electrolytes and carbohydrates. The compositions comprise (a) from about 0.01 % to about 0.35 % flavanols; (b) from about 0.01 % to about 0.3 % sodium ions; (c) from about 0.005 % to about 0.08 % potassium ions; (d) from about 0.1 % to about 20 % of a carbohydrate which provides: (i) from about 0.05 % to about 10.0 % fructose; (ii) from about 0.05 % to about 10.0 % glucose; and (e) water.
Owner:THE PROCTER & GAMBLE COMPANY
Flavanol-fatty alcohol hybrids as well as pharmaceutical composition, preparation method and application thereof
PendingCN111978330AStrong inhibitory activityOrganic active ingredientsOrganic chemistryFlavanololAlglucerase
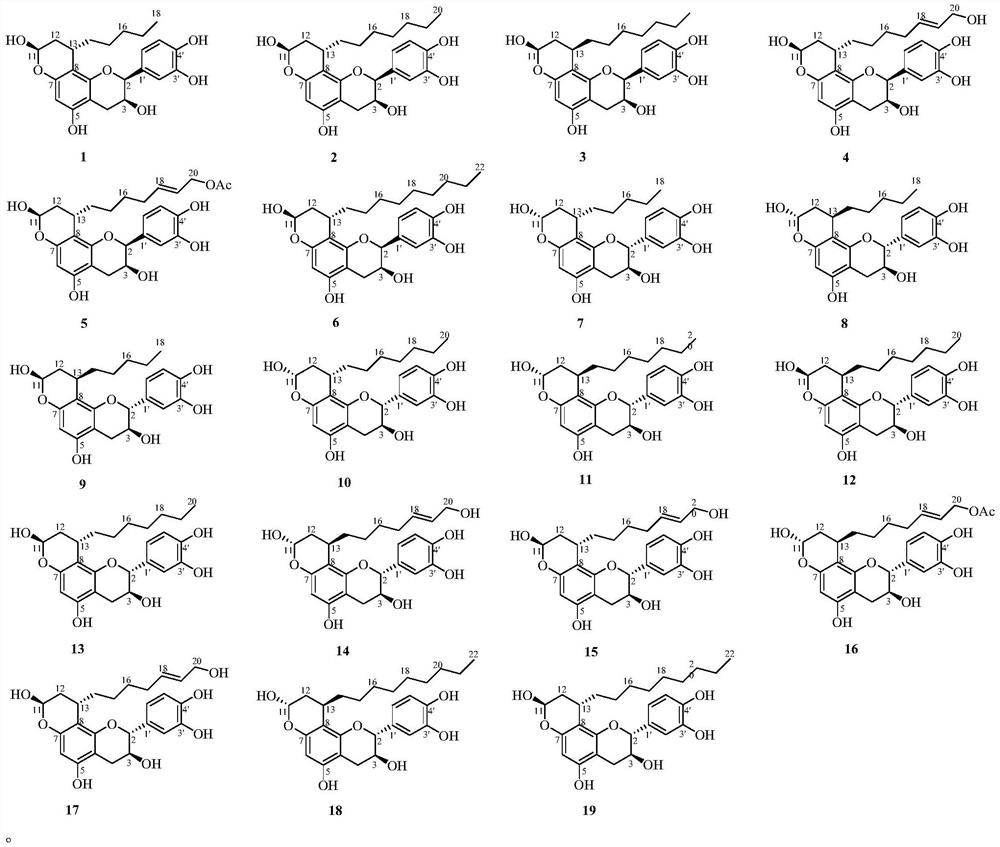
The invention provides 19 novel flavanol-fatty alcohol hybrids, as shown in a structural formula (I), tsaokoflavanols A-S 1-19, as well as a preparation method, pharmaceutical composition and application thereof, and relates to the technical field of medicines. The compound disclosed by the invention has obvious inhibitory activity on PTP1B and alpha-glucosidase, can form the pharmaceutical composition together with a medicinal carrier or an excipient, and can be used for preparing PTP1B and alpha-glucosidase inhibitor drugs, hypoglycemic drugs or health-care foods.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Composition for improving intestinal barrier function
InactiveUS20210015850A1Improve intestinal barrier functionPrevention and amelioration of conditionOrganic active ingredientsMetabolism disorderGallic acid esterAcyl group
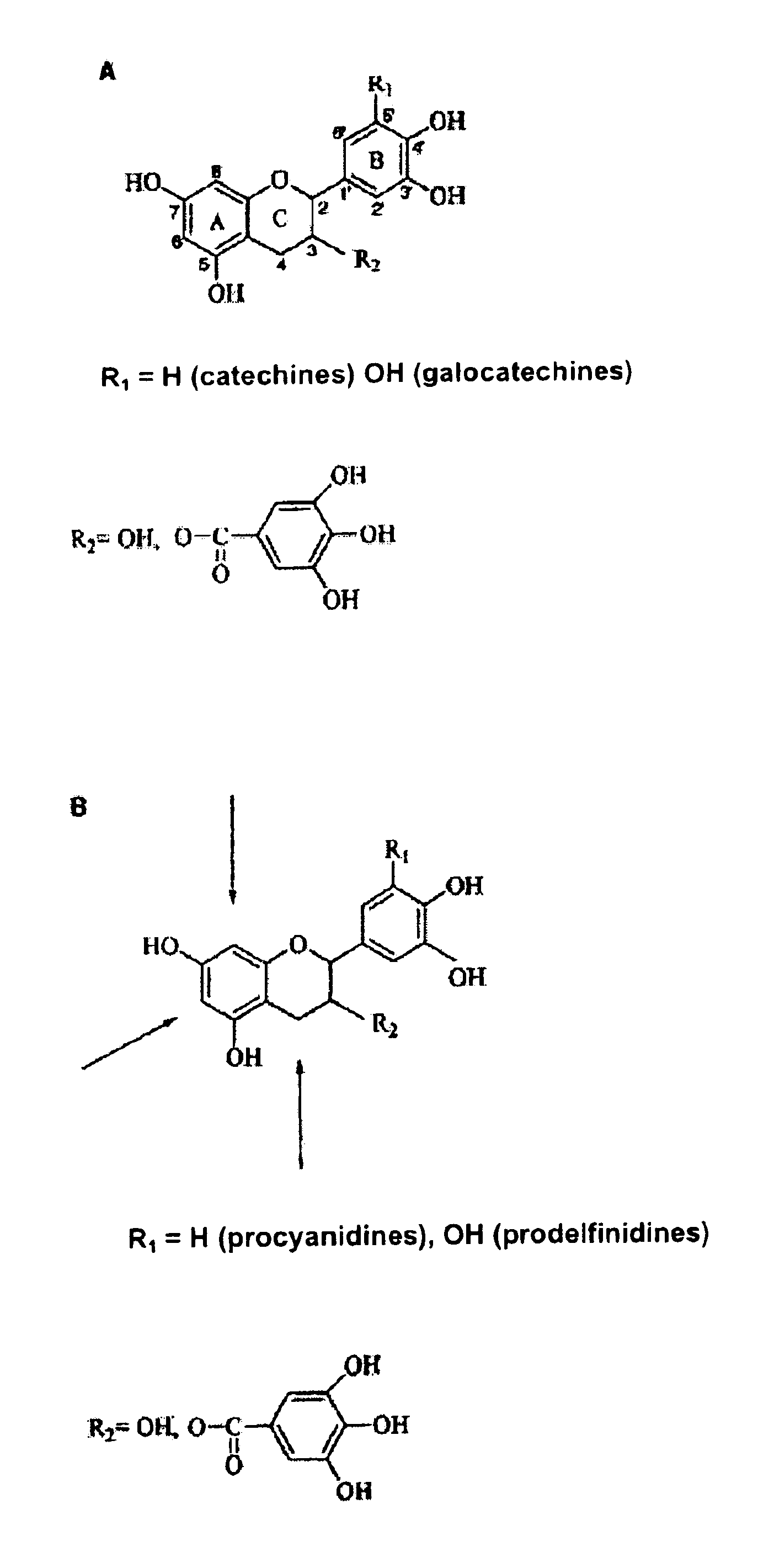
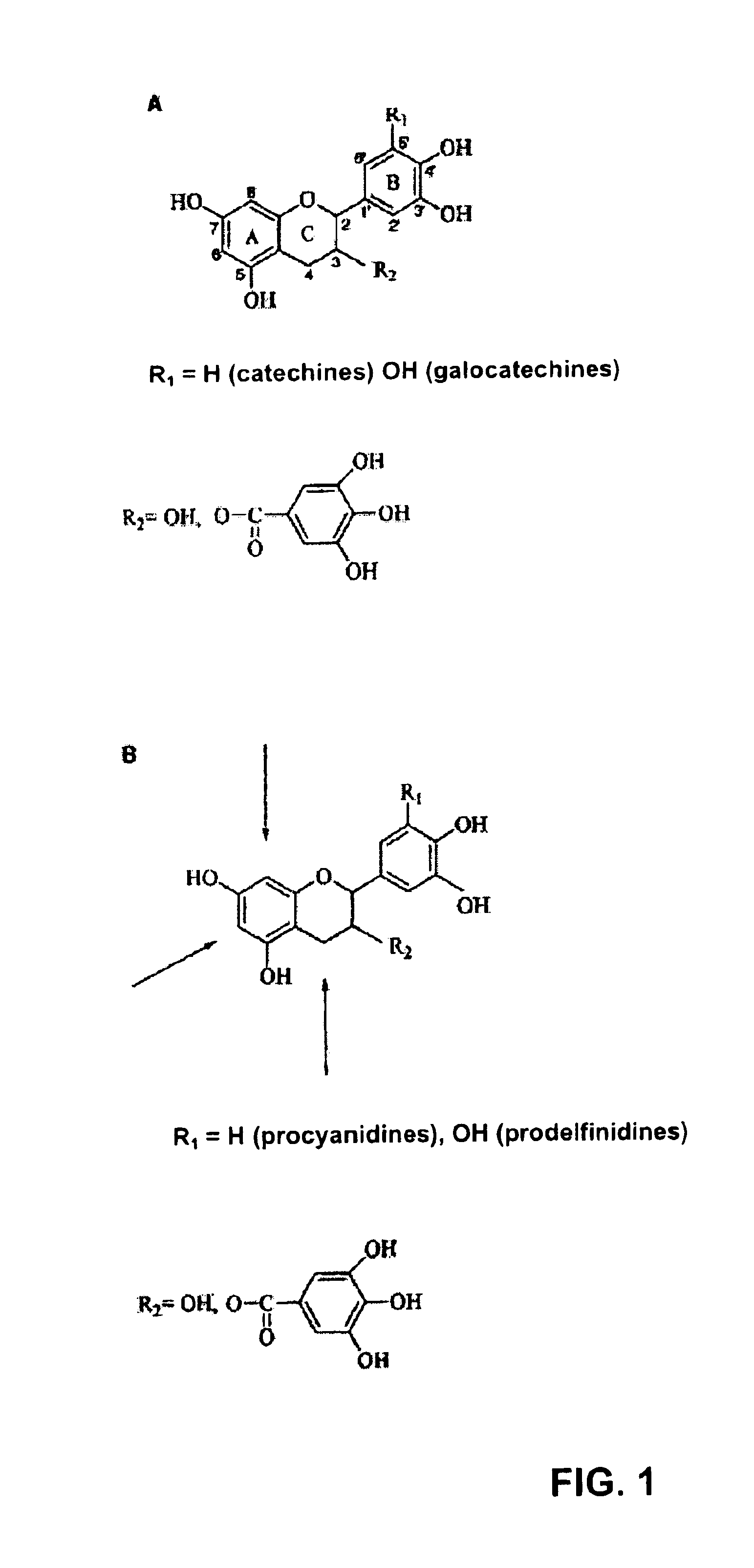
The present invention relates to a composition for improving intestinal barrier function, the composition containing a compound having a gallic acid residue as an active ingredient, wherein the compound having a gallic acid residue is at least one compound selected from the group consisting of the following (A1) to (A3): (A1) a flavan-3-ol polymer having a galloyl group; (A2) a hydrolyzable tannin; and (A3) at least one compound selected from the group consisting of catechin gallate, epicatechin gallate, and gallocatechin gallate.
Owner:SUNTORY HLDG LTD
Process for extracting active component from haw fruit
InactiveCN1141039CMake the most ofEfficient separationSolid solvent extractionFood scienceCelluloseActive component
A process for extracting the active biological components (including haw flavanol, edible cellulose and haw juice) from has is disclosed.
Owner:承德瑞泰食品有限公司
A kind of heather proanthocyanidin and its preparation method and application
InactiveCN104189140BHigh activityEasy to getCosmetic preparationsToilet preparationsBiotechnologyFlavanolol
The invention discloses photinia serrulata procyanidine as well as a preparation method and an application thereof. The photinia serrulata procyanidine is prepared by extracting and purifying rachis of a photinia plant as an effective component. According to the photinia serrulata procyanidine as well as the preparation method and application, the branches, leaves and fruits of the photinia plant contain extremely high procyanidine like substances, and the matured rachis contain the most procyanidine; the plant extractive prepared through the rachis of the photinia plant by purifying has the antioxidant activity under low concentration higher than that as specified in the specification under the same mass concentration; and the chemical component analysis sheet shows that the contents of flavonoid components, phenol components and symbolic flavane-3-alcohol antioxidant components in the extractive are higher than that as specified in the specification. The raw materials of the extractive are easily obtained, the extracting process is simple, the cost is small, and wide market prospect and high economic value are brought.
Owner:NORTHWEST A & F UNIV
Hydrophilic interaction and reversed-phase liquid chromatography coupled to analyze the structure of proanthocyanidins
The invention discloses a method for analyzing the structure of proanthocyanidins by combining hydrophilic interaction and reversed-phase liquid chromatography, comprising the following steps: analyzing flavan-3-alcohol monomers; Acid degradation product; the acid degradation product to be analyzed is analyzed by reversed-phase liquid chromatography (RPLC), and the type and content of proanthocyanidin end units are analyzed according to the difference in the type and content of flavan-3-alcohol analyzed by RPLC and Hilic, according to RPLC The analyzed flavan-3-alcohol-phloroglucinol addition products and anthocyanins were analyzed for the type and content of proanthocyanidin extension units; then the average degree of polymerization of proanthocyanidins was calculated according to the number of moles of proanthocyanidin extension units and terminal units. This solution replaces the step of directly removing flavan-3-alcohols in the sample (which takes a long time) in the traditional method, and greatly improves the analysis speed of the terminal units of proanthocyanidins.
Owner:ZHEJIANG UNIV
A method for detecting flavonoids in peony petals
The invention relates to a method for detecting a flavonoids component in peony petals. The method comprises the following steps: preparing a flavonoids extracting solution; setting a chromatographiccondition, a mass spectrum condition and a detection wavelength of a diode array detector; detecting the flavonoids extracting solution by adopting an ultra performance liquid chromatograph-diode array detector-triple quadrupole flight time tandem mass spectrometry and obtaining a detection result; carrying out structure identification on a flavonoids substance through analyzing the detection result; reckoning the structure of the flavonoids component. The method provided by the invention has the advantages of simple operation steps, rapid detection speed and high accuracy and sensitivity andcan be used for detecting the flavonoids component with the extremely less content; identification species are comprehensive and 67 types of main flavone, flavonol, flavanone and flavanol compounds and 5 types of main anthocyanin compounds are totally identified from the peony petals; the blank of identifying the flavonoids component in the peony petals is filled up and the method has important meanings on in analysis of nutrient components of the peony petals with different varieties.
Owner:城发投资集团有限公司 +1
Composition for promoting expression of aquaporin 3
InactiveCN111182798AHigh expressionIncrease moistureCosmetic preparationsOrganic active ingredientsFlavanololPolymer science
The purpose of the present invention is to provide a specific composition for promoting expression of aquaporin 3, and the use of the composition. The present invention pertains to a composition for promoting expression of aquaporin 3, said composition including, as essential ingredients, a flavan-3-ol monomer having a galloyl group and / or a galloyl-group-containing flavan-3-ol polymer.
Owner:SUNTORY HLDG LTD
A method for detecting flavonoid components in peony leaves
The invention relates to a method for detecting the flavonoid components in peony leaves. The method comprises the following steps of preparing a flavonoid extracting solution; setting chromatographicconditions, mass spectrum conditions and the detection wavelength of a diode array detector; adopting the super-efficient liquid chromatogram-diode array detector-triple quadrupole rod flight time tandem mass spectrum linkage technology for detecting the flavonoid extracting solution, and obtaining a detecting result; performing structure identification on flavonoid substances by analyzing the detecting result, and speculating the structure of the flavonoid compound. The method is simplified in operation steps, rapid in detection and high in accuracy and sensitivity, the micro content of theflavonoid components can be detected, the identification variety is comprehensive, 45 main flavone, flavonol, flavanone and flavanol compounds are identified in the peony leaves, a gap for flavonoid component identification in the peony leaves is filled up, and the method is of great significance in analysis of different varieties of peony leaf nutrient contents.
Owner:INST OF VEGETABLE & FLOWERS CHINESE ACAD OF AGRI SCI +1
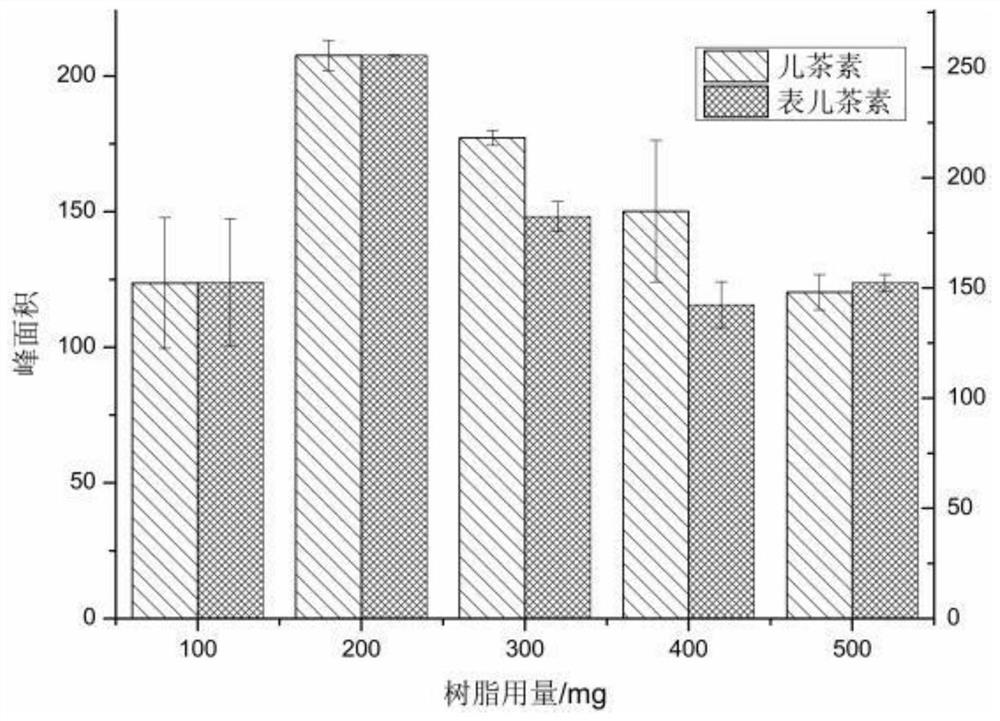
SPE-DES-HPLC method for simultaneously determining two flavanol substances in Shanxi mature vinegar
ActiveCN113567578AOptimal adsorption timeAchieve separationComponent separationPhosphatePhosphoric acid
The invention belongs to the technical field of flavanol substance detection, and discloses an SPE-DES-HPLC method for simultaneously determining two flavanol substances in Shanxi mature vinegar. According to the method, a Dimonsil C18 chromatographic column is selected, and the flow velocity is 1mL / min; the sample size is 10 [mu] L; the detection wavelength is 280 nm; a mobile phase is a 0.1% phosphate buffer and acetonitrile; and gradient elution is carried out. Through an optimization experiment, when the dosage of the activated XAD-2 macroporous adsorption resin is finally determined to be 188mg, the volume of the eutectic solvent (tetraethylammonium chloride-n-caprylic acid in a molar ratio of 1: 3) is 400mu L, the adsorption time is 11min, and the analysis time is 20min, the enrichment effect of the two flavanol substances is optimal. Under the condition, the linear relation of catechin and epicatechin is sound (R2 > 0.99), and compared with the tedious process of traditional solid-phase extraction, the detection limit, the quantitation limit, the intraday and interday relative standard deviation and the adding standard recovery rate show that the method is simpler, more convenient, high in sensitivity and small in matrix interference, and is suitable for enrichment determination of catechin and epicatechin in Shanxi mature vinegar.
Owner:神池县海子园醋业有限公司
A kind of biosynthesis and detection method of catechin intermediate
The invention discloses methods for synthesizing and detecting catechin intermediates. The methods include acquiring CsANRa genes from tea trees by means of cloning and then constructing binary expression vectors; transforming the binary expression vectors into plant bodies to obtain transgenic plants; extracting polyphenols substances such as flavane-3-alcohol intermediates in the transgenic plants by the aid of extracting solution which is 80% methanol and hydrochloric acid mixed solution so as to obtain the catechin intermediates; qualitatively and quantitatively authenticating catechin intermediate products by means of UPLC-QQQ-MS / MS (ultra-performance liquid chromatography-triple quadrupole-mass spectrometry / mass spectrometry). Compared with the prior art, the methods have the advantages that the methods are easy to operate, and organic solvents are inexpensive and are low in cost; the catechin intermediates acquired by the aid of the corresponding method do not contain catechin and are relatively simple, accordingly, the corresponding method is suitable to be used for preparing catechin intermediates with high purity, and the methods for synthesizing and detecting the catechin intermediates are reported for the first time.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Active compound for inhibiting insulin resistance and application thereof
ActiveCN114099493AInhibition of resistance activityInhibition of immune activityOrganic active ingredientsMetabolism disorderFlavanololPancreatic hormone
The invention relates to a compound for inhibiting insulin resistance activity and application thereof. The compound is selected from one or a combination of hyperoside and 5, 7, 3 ', 4'-tetrahydroxyflavanol. Hyperoside and 5, 7, 3 ', 4'-tetrahydroxyflavanol have the activity of inhibiting insulin resistance, especially when the hyperoside and the 5, 7, 3 ', 4'-tetrahydroxyflavanol are combined according to a specific proportion, the effect of synergistically inhibiting the insulin resistance activity of the hyperoside and the 5, 7, 3 ', 4'-tetrahydroxyflavanol can be achieved, and the application has important significance for providing a novel medicine for treating type 2 diabetes mellitus.
Owner:黑龙江护理高等专科学校
A kind of gelatin-based food packaging film and preparation method thereof
ActiveCN111073308BImprove securityGood physical and mechanical propertiesFlexible coversWrappersGlycerolCross linker
The invention discloses a preparation method of a gelatin-based food packaging film, which comprises the following process steps: 1) mixing and soaking 16-30 parts of gelatin and 100 parts of distilled water according to the mass parts of raw materials for 0.5-1.5 hours to obtain a gelatin matrix; 2. ) Add 15 to 25 parts of an aqueous solution of a titanium compound with a concentration of 3 to 10 g / L into the gelatin matrix, then add 15 to 25 parts of an aqueous solution of high-polymerization flavanols with a concentration of 0.5 to 2 g / L, and then add 3 to 6 parts of aqueous sodium hydroxide solution with a concentration of 0.1-1.2 mol / L and 3-6 parts of glycerin are cast into a film and dried to obtain a finished product. The present invention uses natural polyflavanol and high-safety titanium ion as a composite crosslinking agent, utilizes the principle that protein reactive sites increase after the coordination compound is formed by titanium ion and polyflavanol, and prepares high-safety Gelatin-based food packaging film with excellent physical and mechanical properties.
Owner:SHANTOU UNIV
Flavane-3-alcohol acylpropanoid heterocomplex and preparation method and application thereof
The invention relates to a flavane-3-alcohol acylpropanoid heterocomplex and a preparation method and application thereof, and belongs to the technical field of natural pharmaceutical chemistry and medicine. The inventor of the invention separates a new flavane-3-alcohol acylpropanoid heterocomplex from the whole herb of mentha piperita, and accidentally finds that the compound has an obvious anti-inflammatory effect in a further pharmacodynamic research process. The flavane-3-alcohol acylpropanoid heterocomplex disclosed by the invention has a clinical application prospect of an anti-inflammatory drug.
Owner:FENGXIAN CENT HOSPITAL
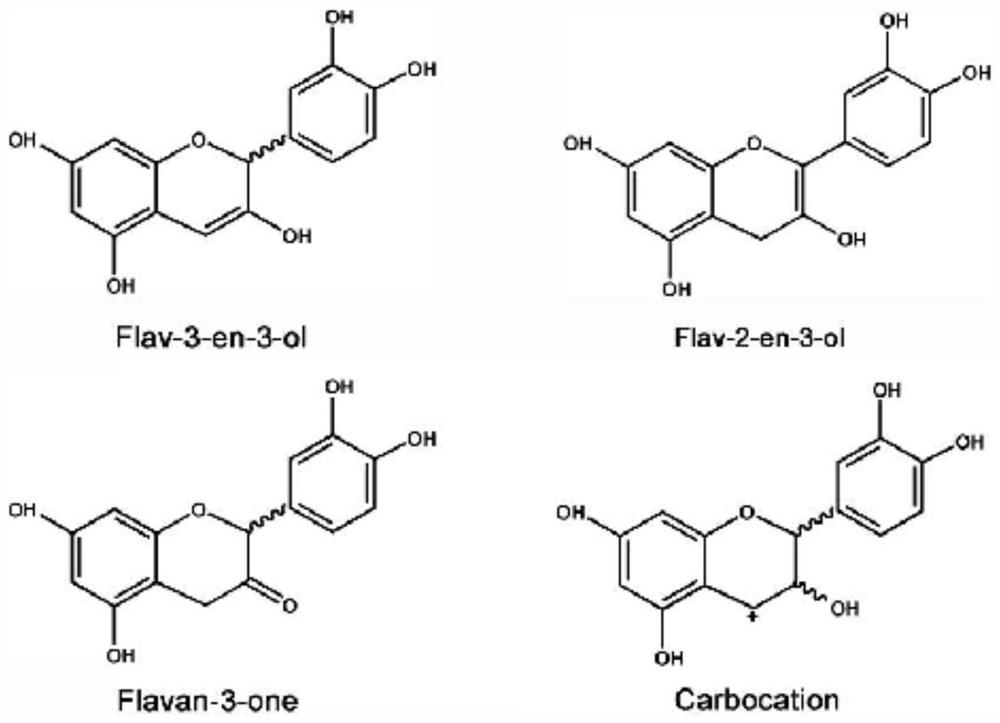
Synthesis of C-3 coupled biflavonoids and C-3 coupled biflavonoid analogues
The invention relates to methods for the preparation of an optically inactive and optically active compounds which are selected from the group consisting of C-3 coupled biflavonoids and C-3 coupled biflavonoid analogues from a starting material or intermediate which are respectively selected from the group consisting of optically inactive or optically active flavan-3-ols and optically active flavan-3-ones, the method comprising the steps of (a) providing an optically inactive or active compound having a fIavan-3-ol structure or a compound which is a flavan-3-one, (b) if a compound having a flavan-3-ol structure with a hydroxy group on the C-3 carbon is selected as starting material, converting the hydroxy group on the C-3 carbon of the compound having the flavan-3-ol structure to an oxo group to form a flavan-3-one of that compound, (c); providing a compound having a nucleophilic aromatic moiety, which compound is selected from the group of compounds having a nucleophilic aromatic moiety and which have flavonoid base structures and compounds having a nucleophilic aromatic moiety and which do not have a flavonoid base structure, (d) contacting the flavan-3-one provided by step (a) or obtained by step (b) with the compound containing the nucleophilic aromatic moiety in the presence of a Lewis acid; (e) forming a first intermediate compound wherein the oxo group on the C-3 carbon is converted to a hydroxy group by virtue of nucleophilic addition when the compound containing the nucleophilic aromatic moiety is contacted to the C-3 carbon of the flavan-3-one, (f) subjecting the first intermediate compound to dehydration so as to introduce a double bond between the C-3 carbon and C-4 carbon of the intermediate compound with the concomitant removal of the hydroxy group from the C-3 carbon to form an optically active flavene compound which is substituted by the nucleophilic aromatic moiety on the C-3 carbon, (g).
Owner:UNIVERSITY OF THE FREE STATE
A kind of flavan-3-ol phenylpropanoid hybrid compound, its preparation method and application
Owner:FENGXIAN CENT HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com