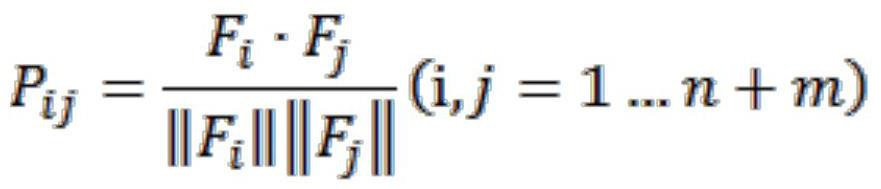Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Corpus vertebrae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
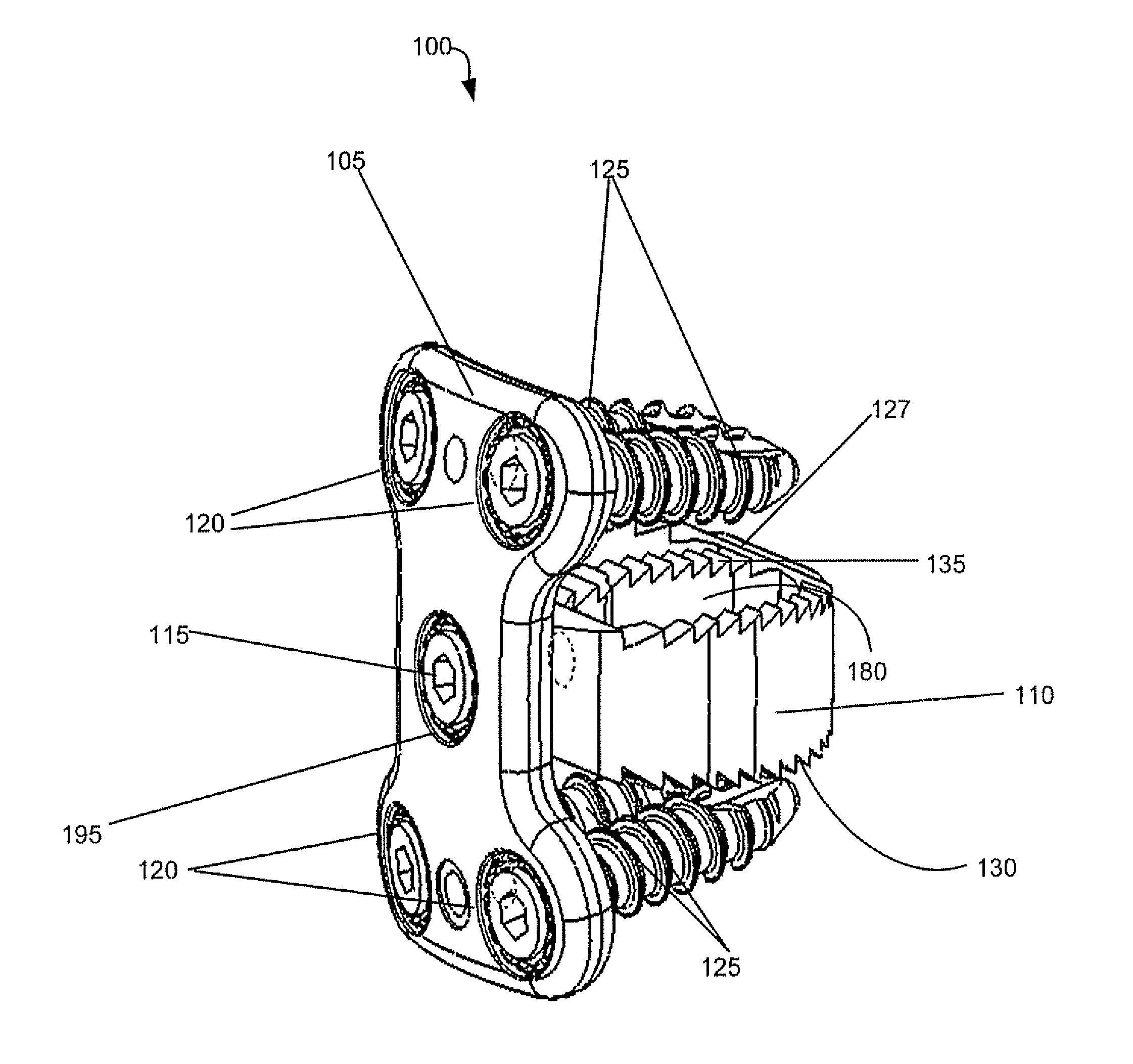
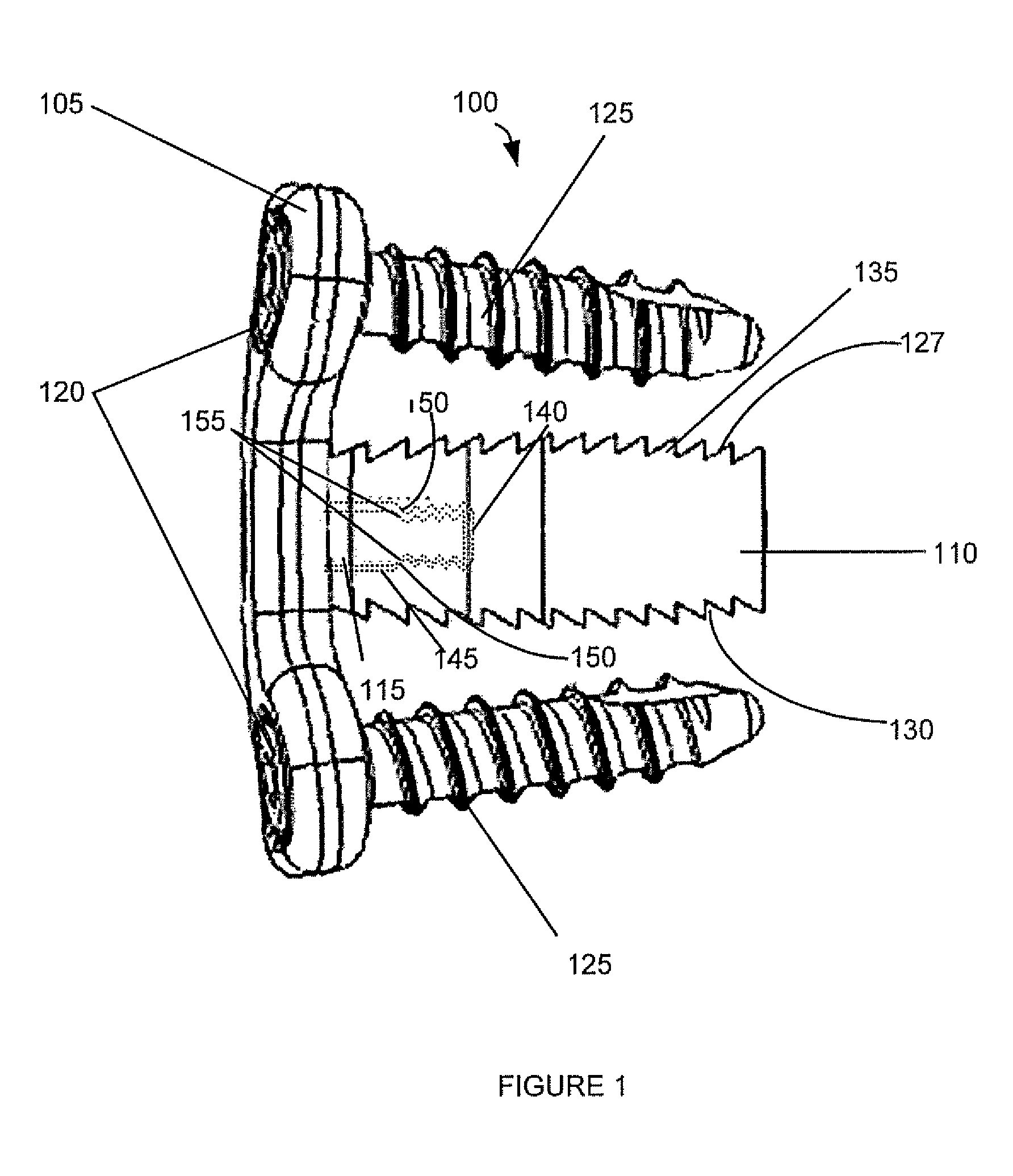
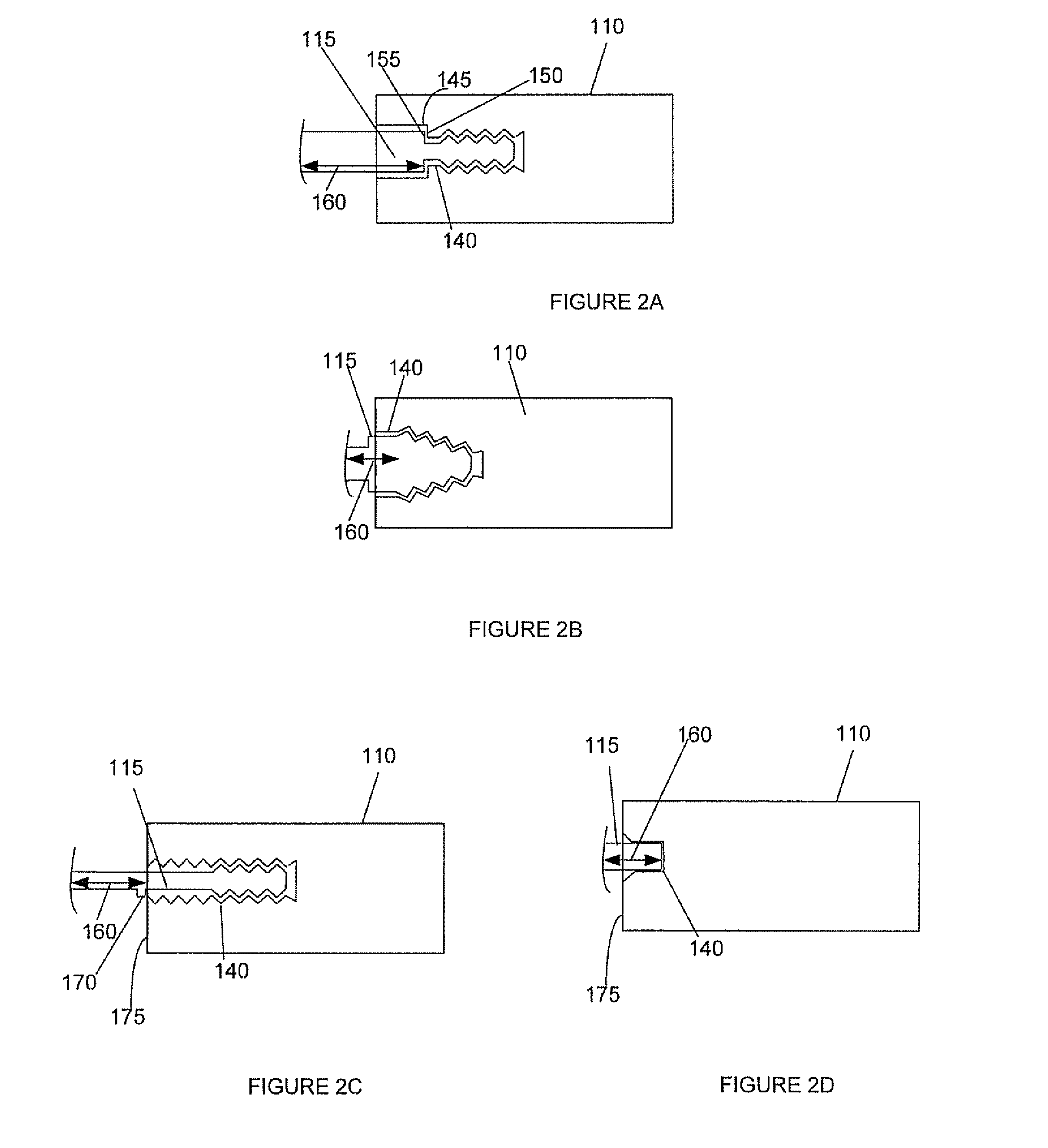
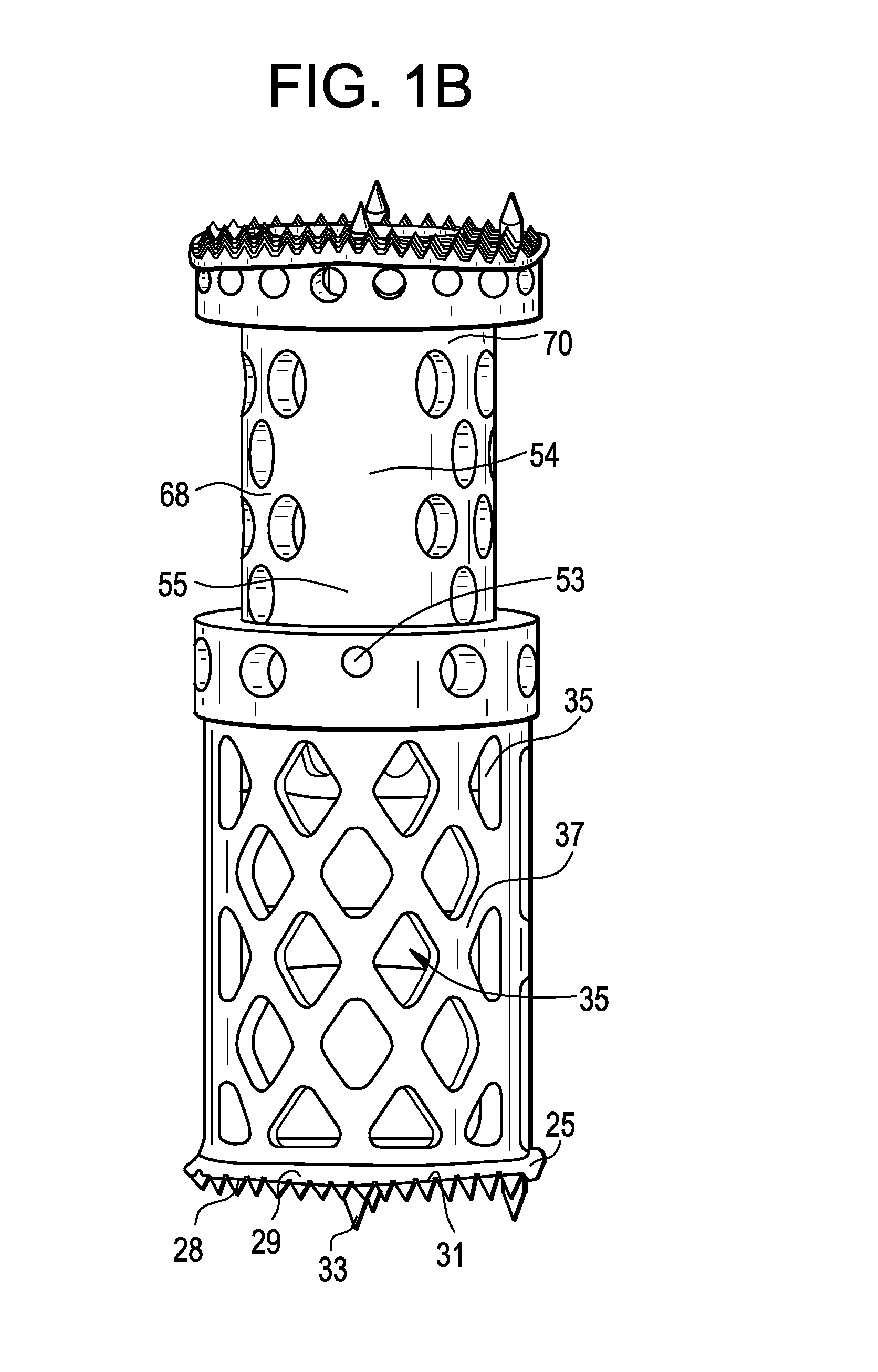
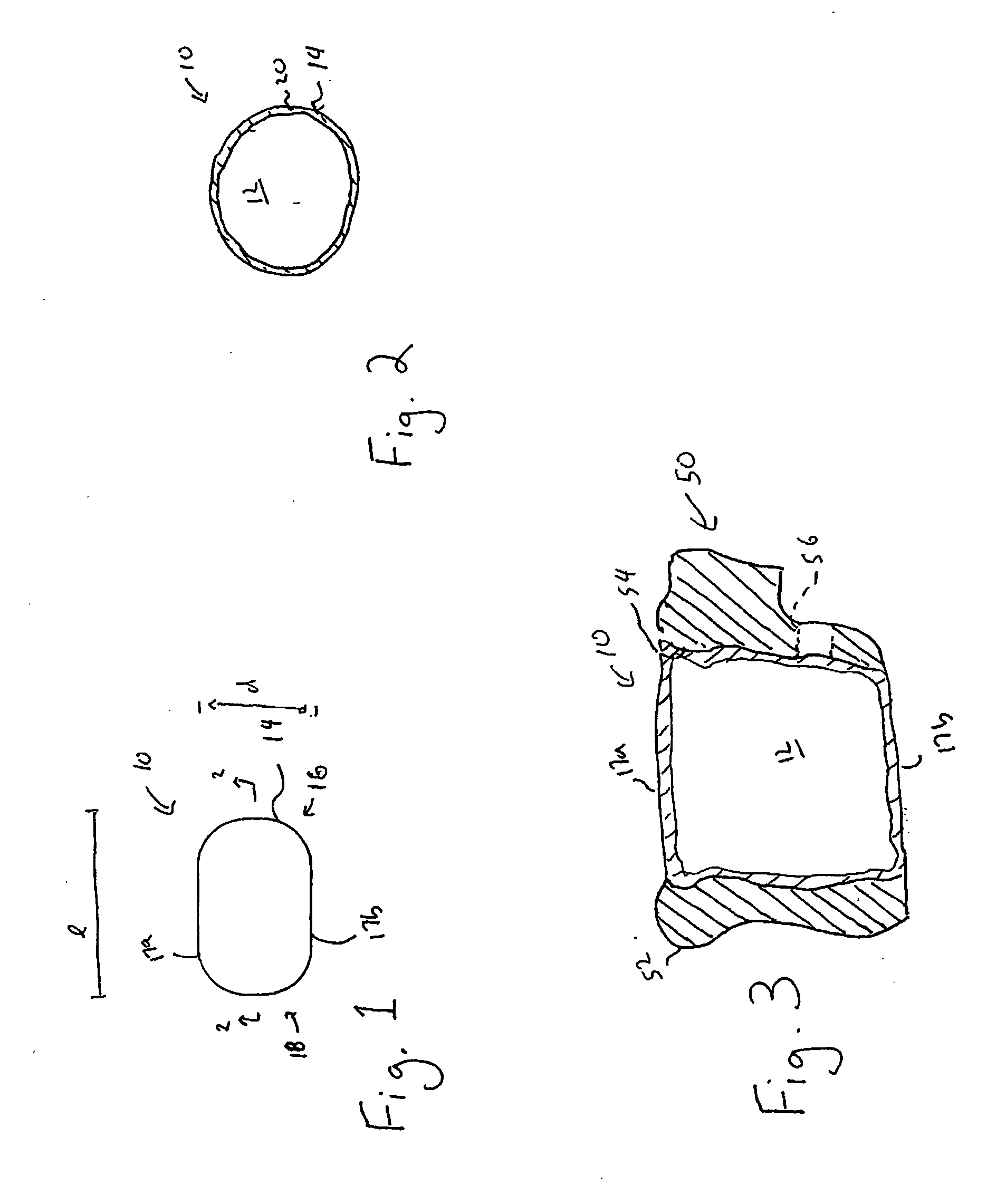
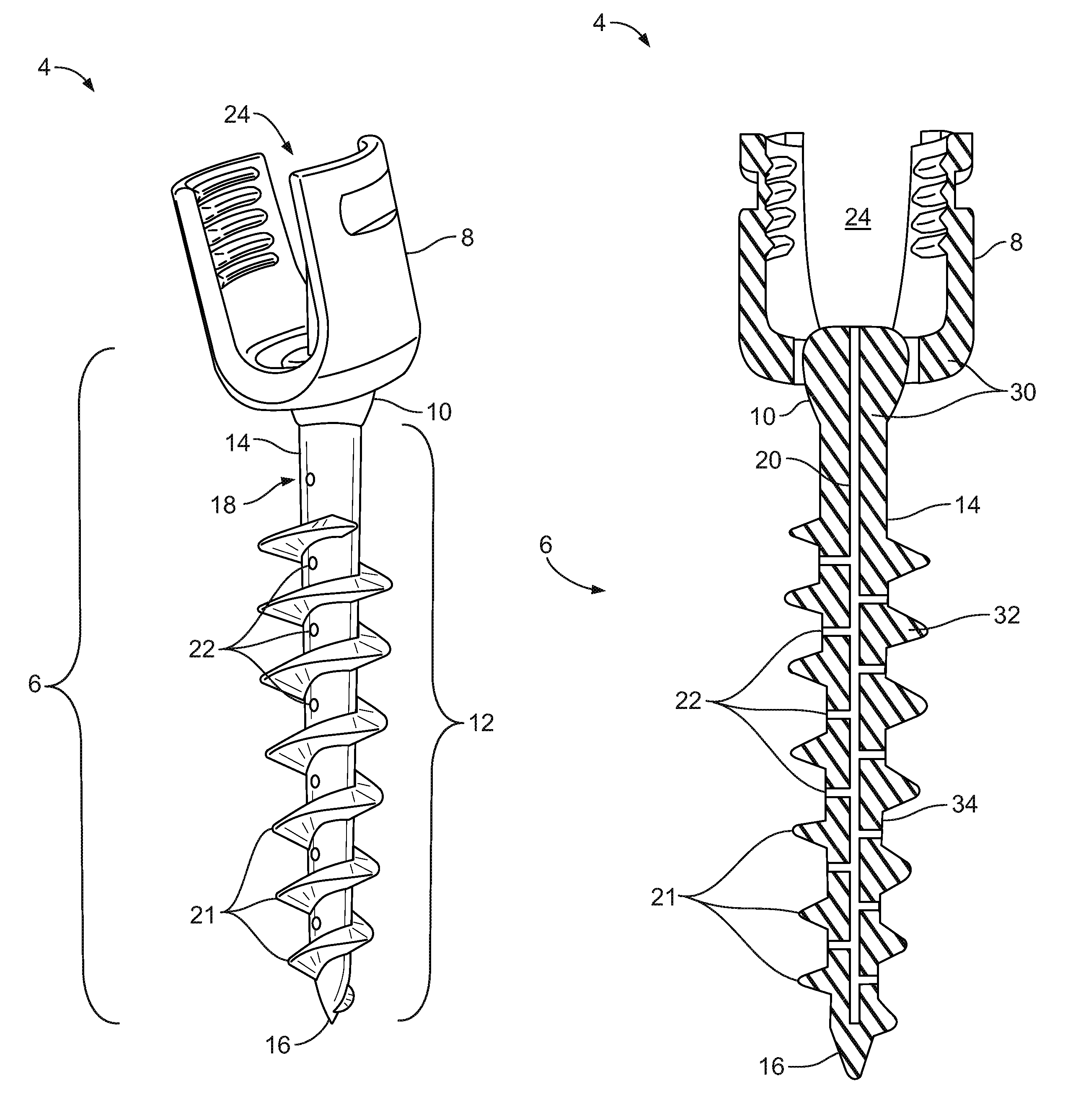
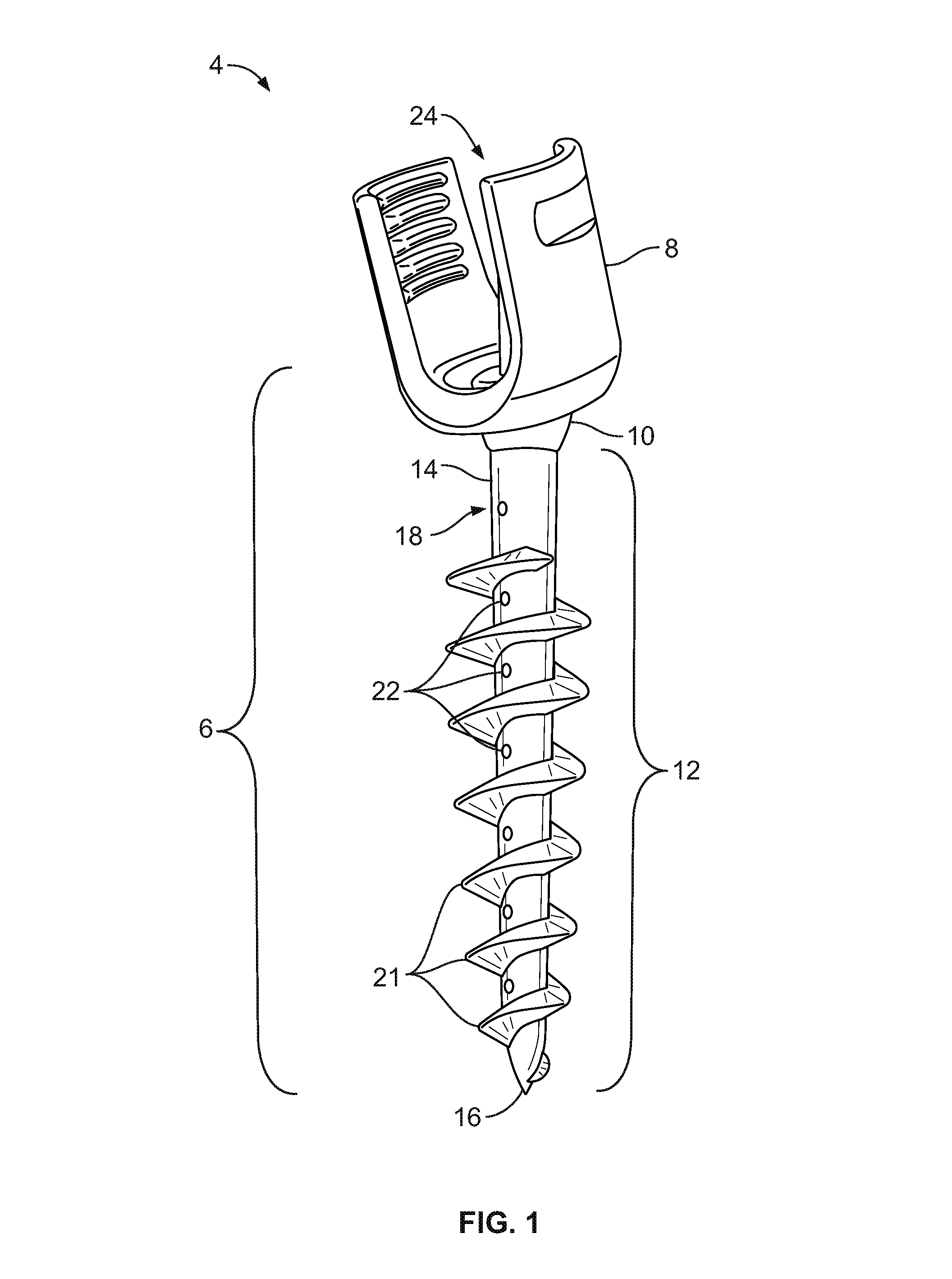
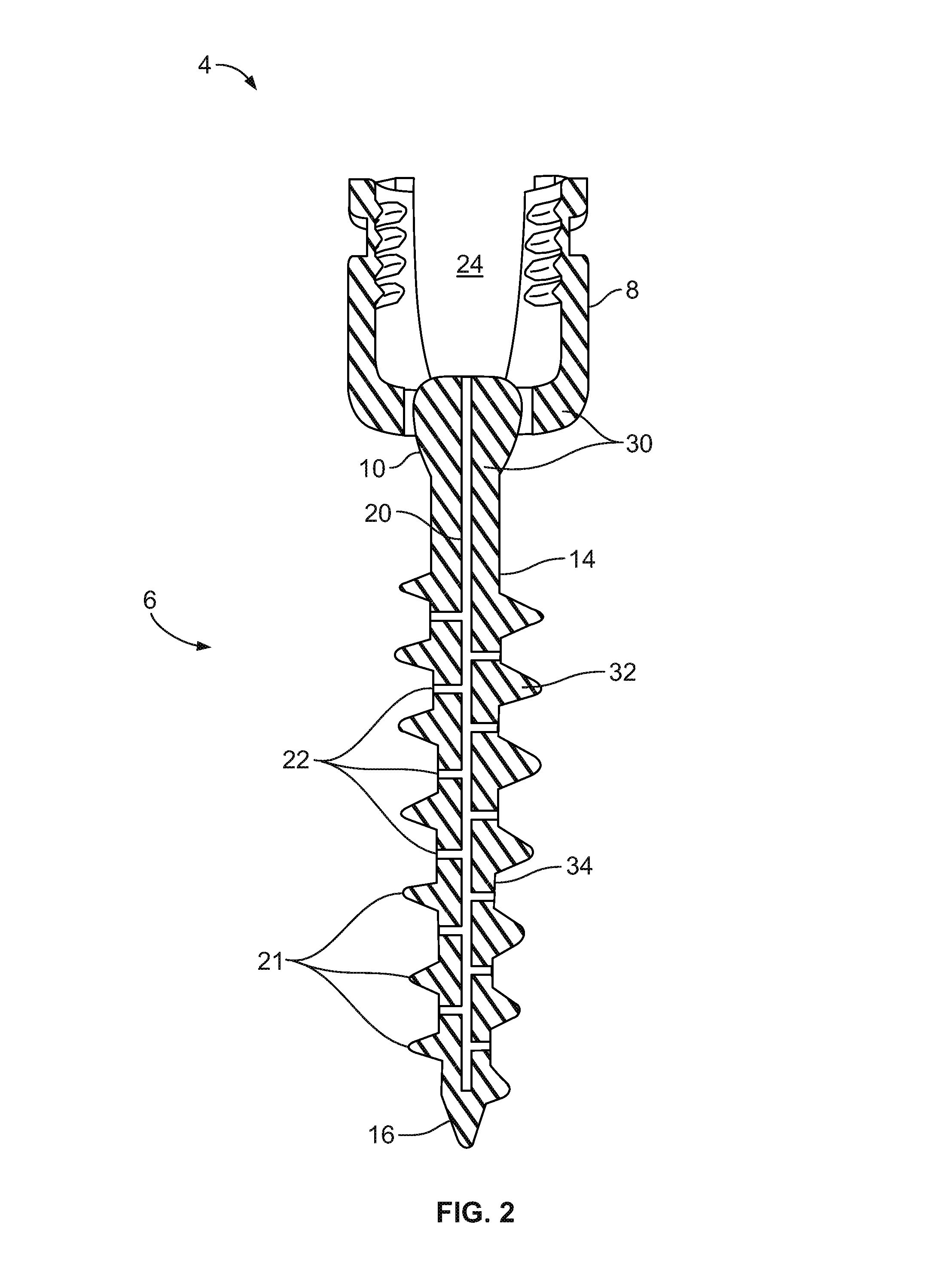
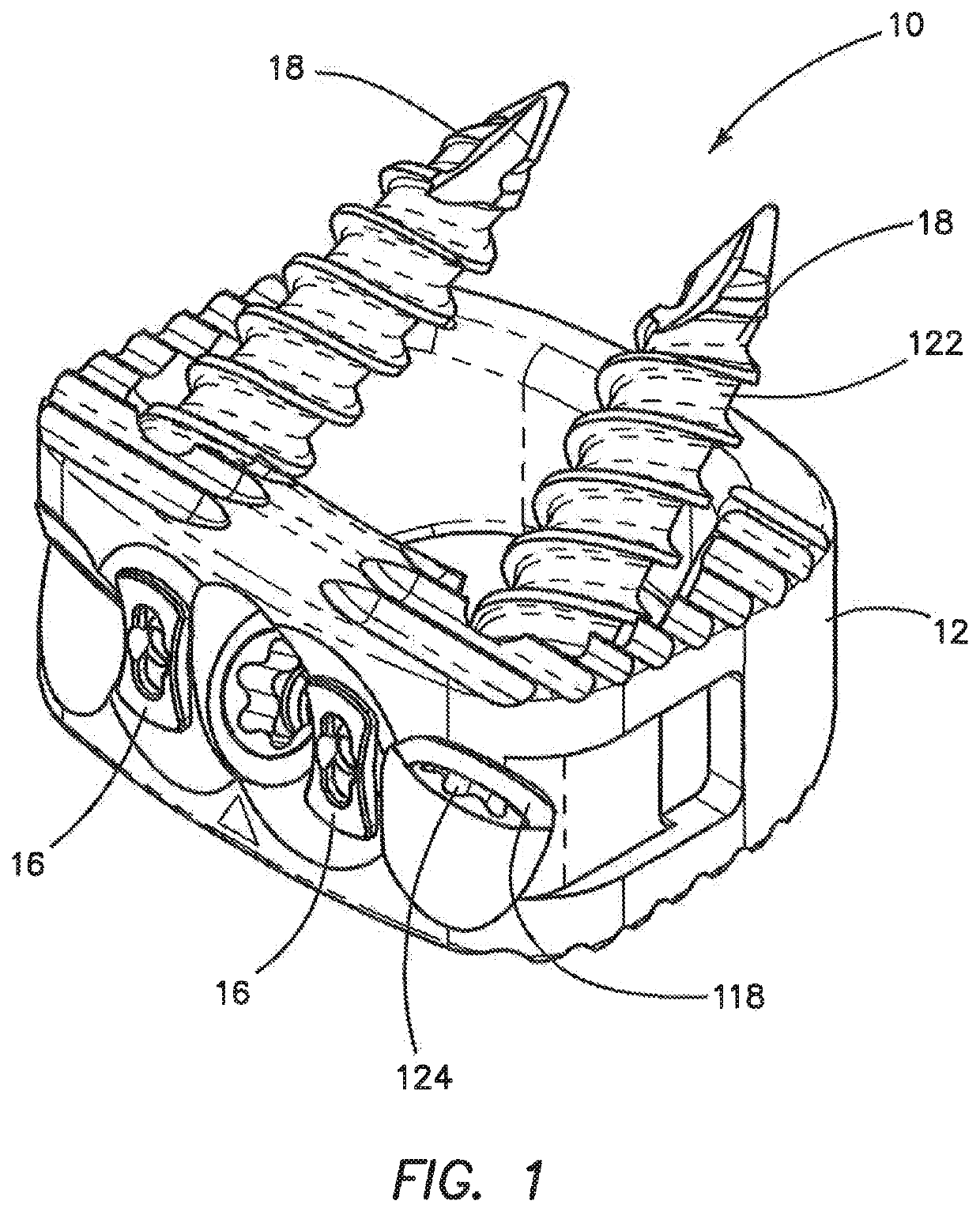
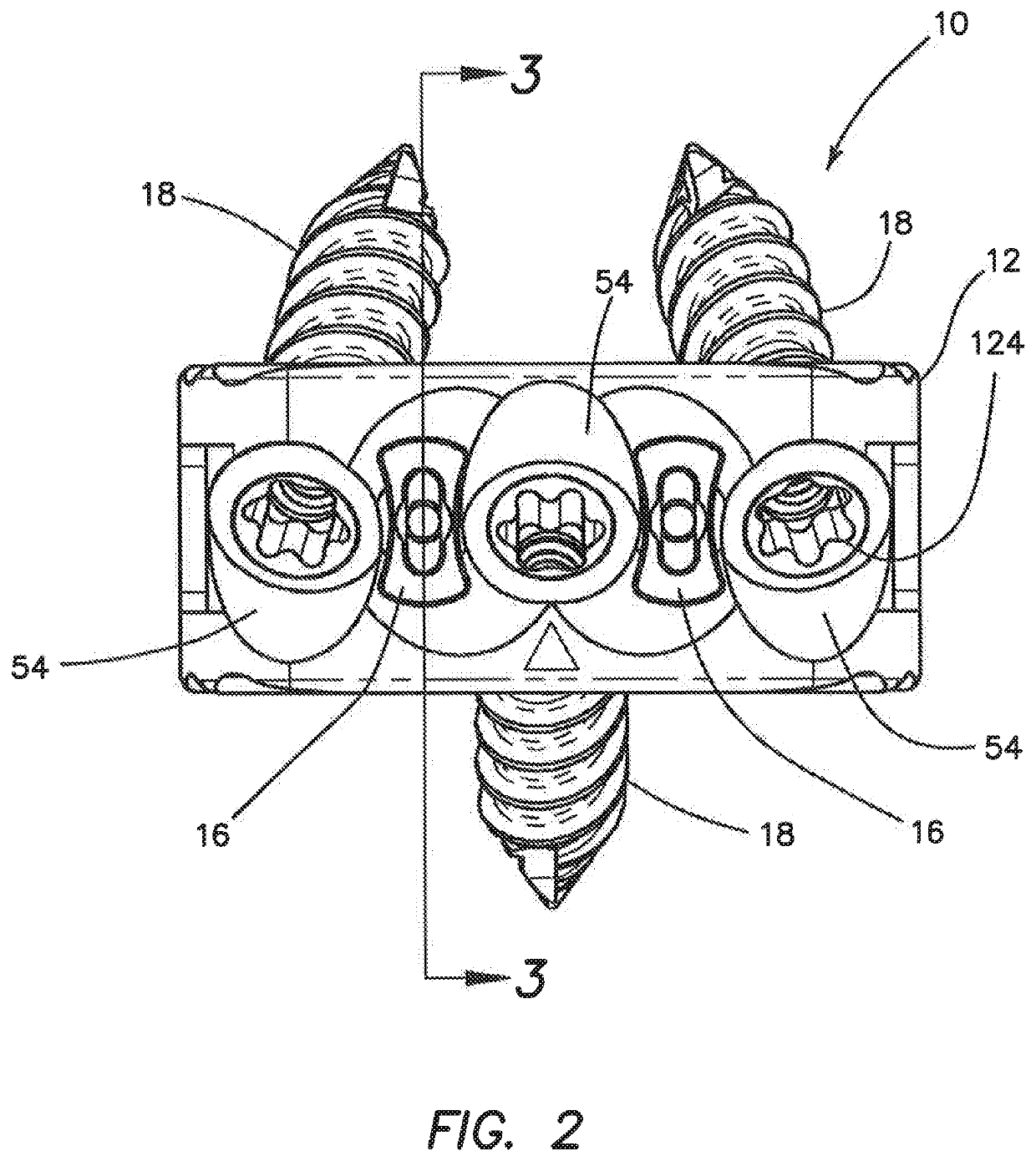
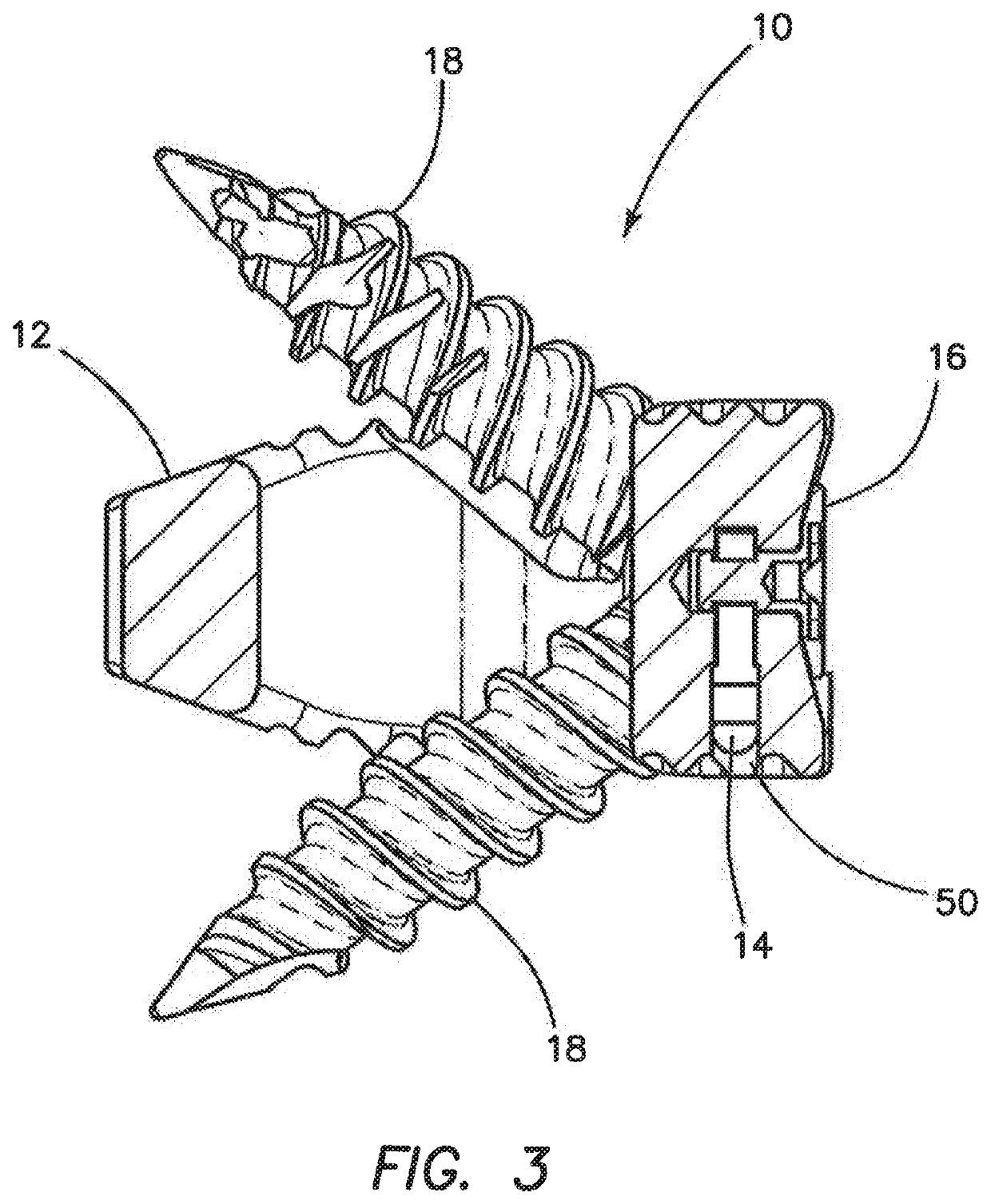
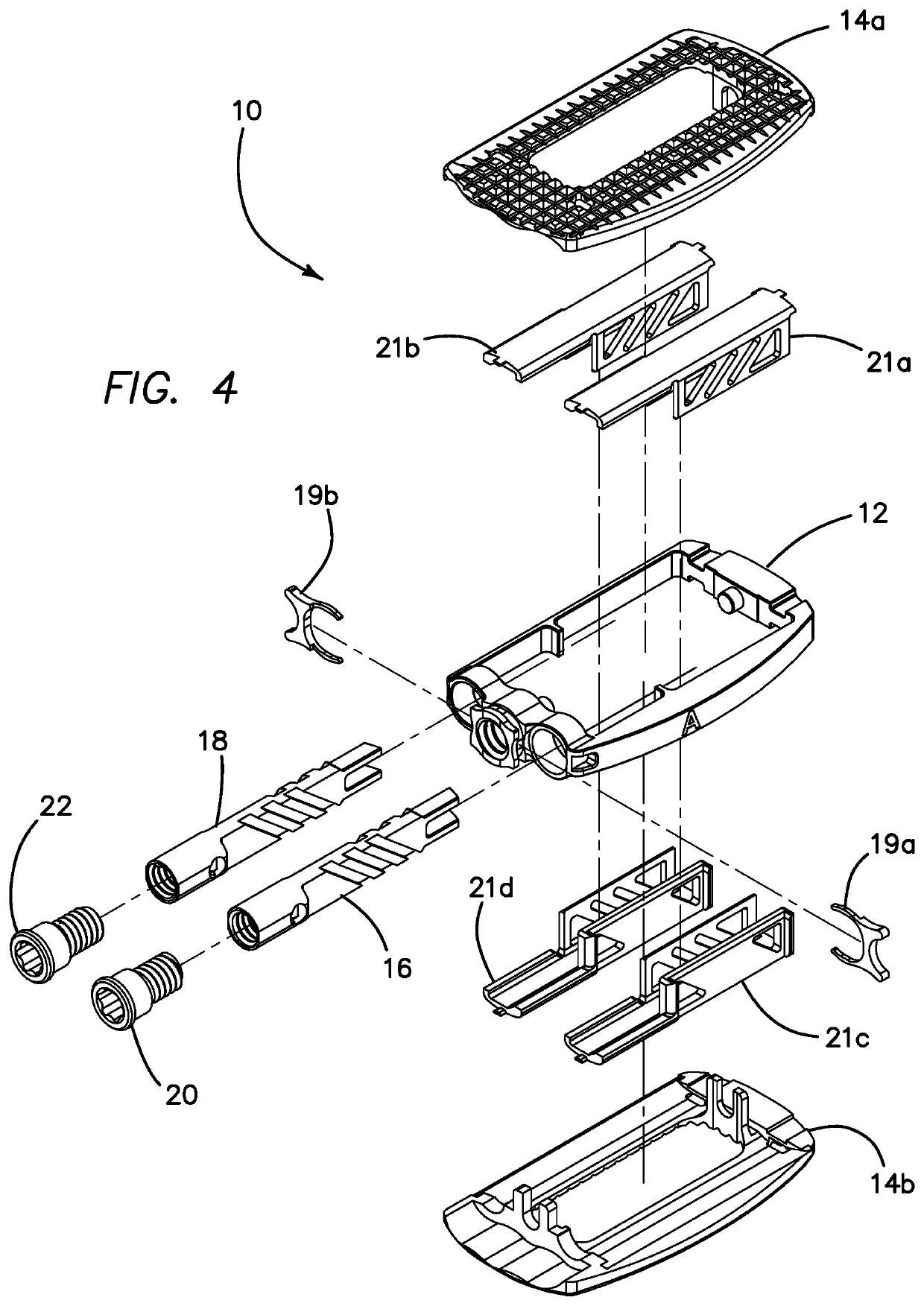
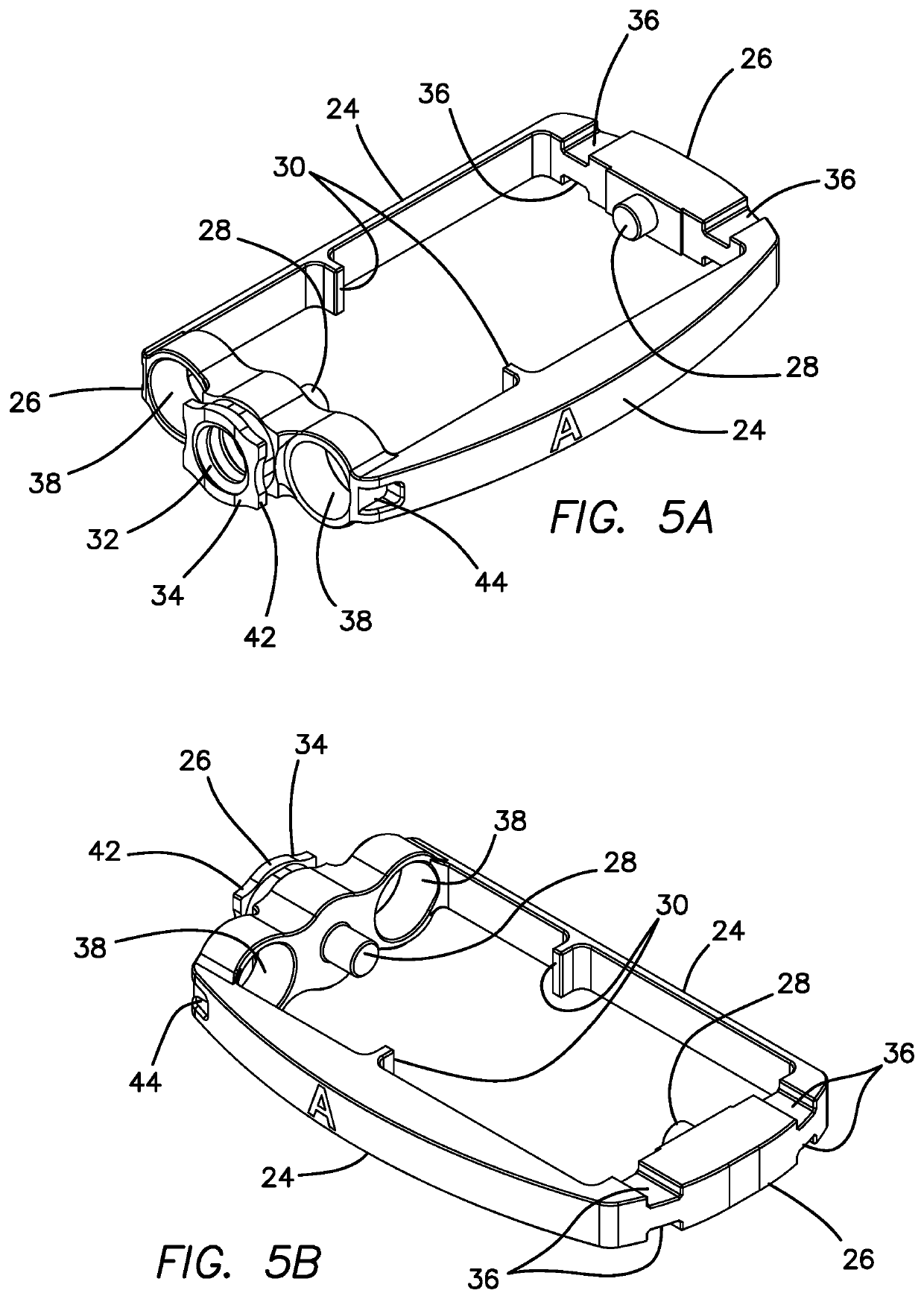
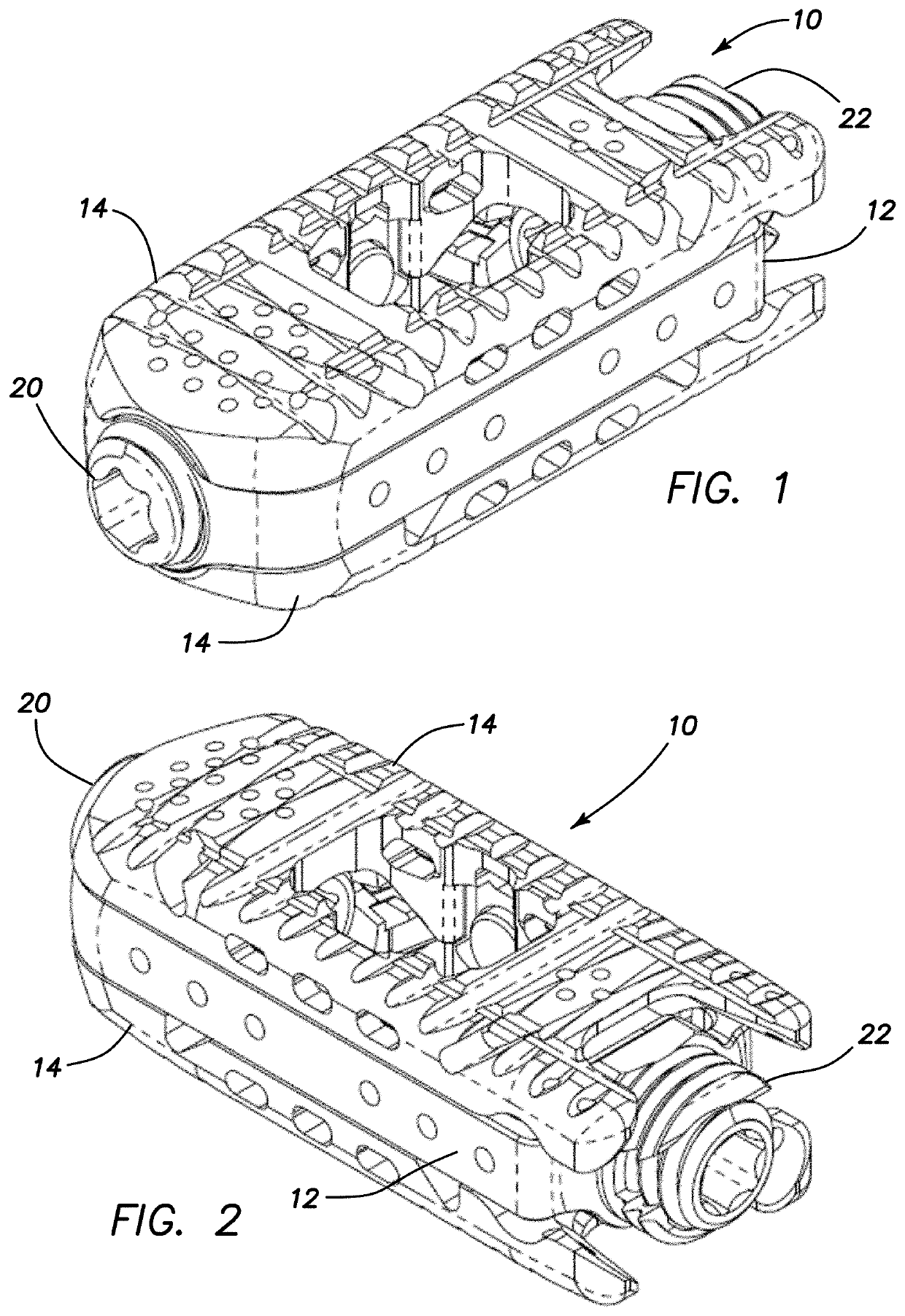
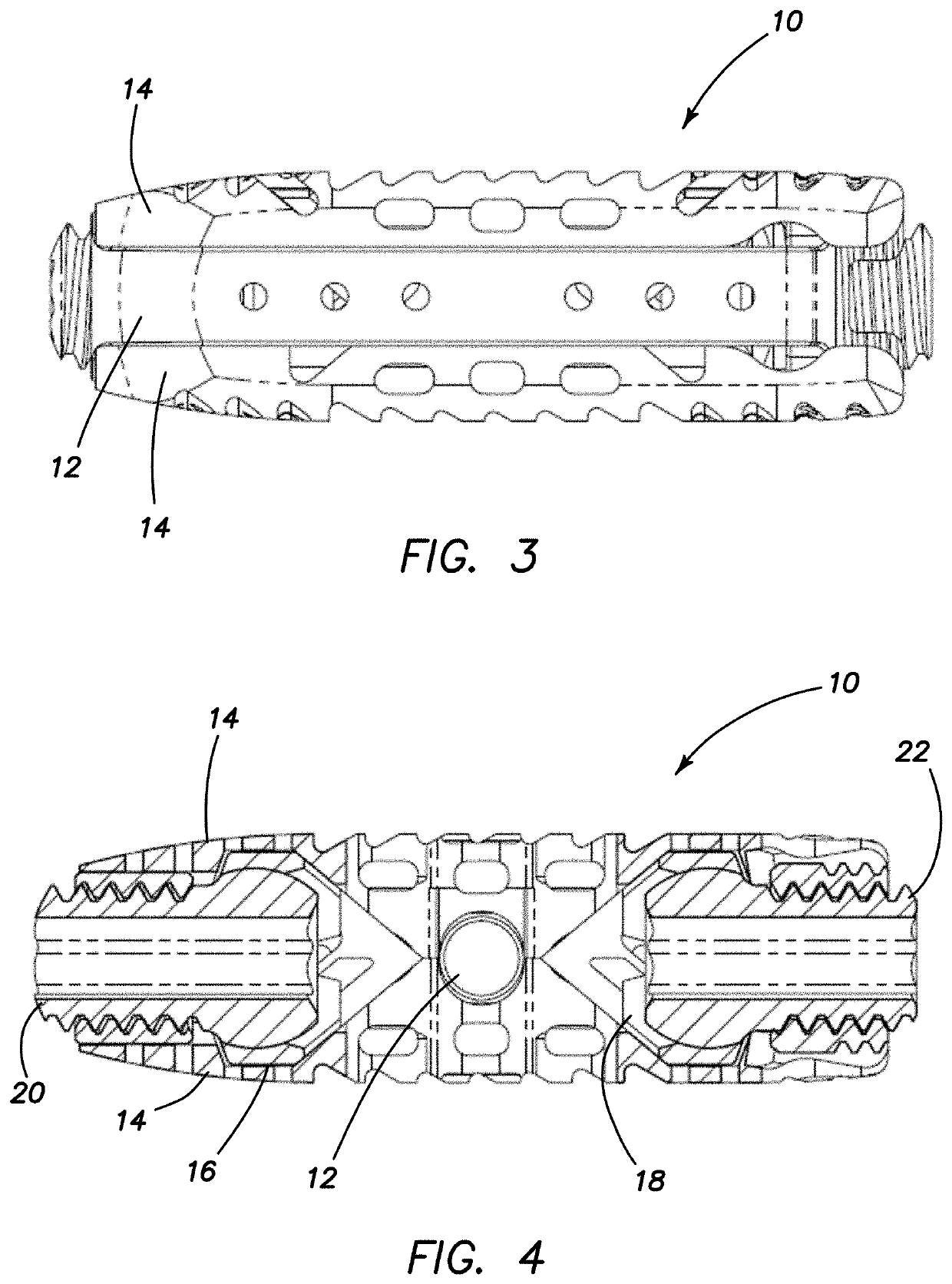
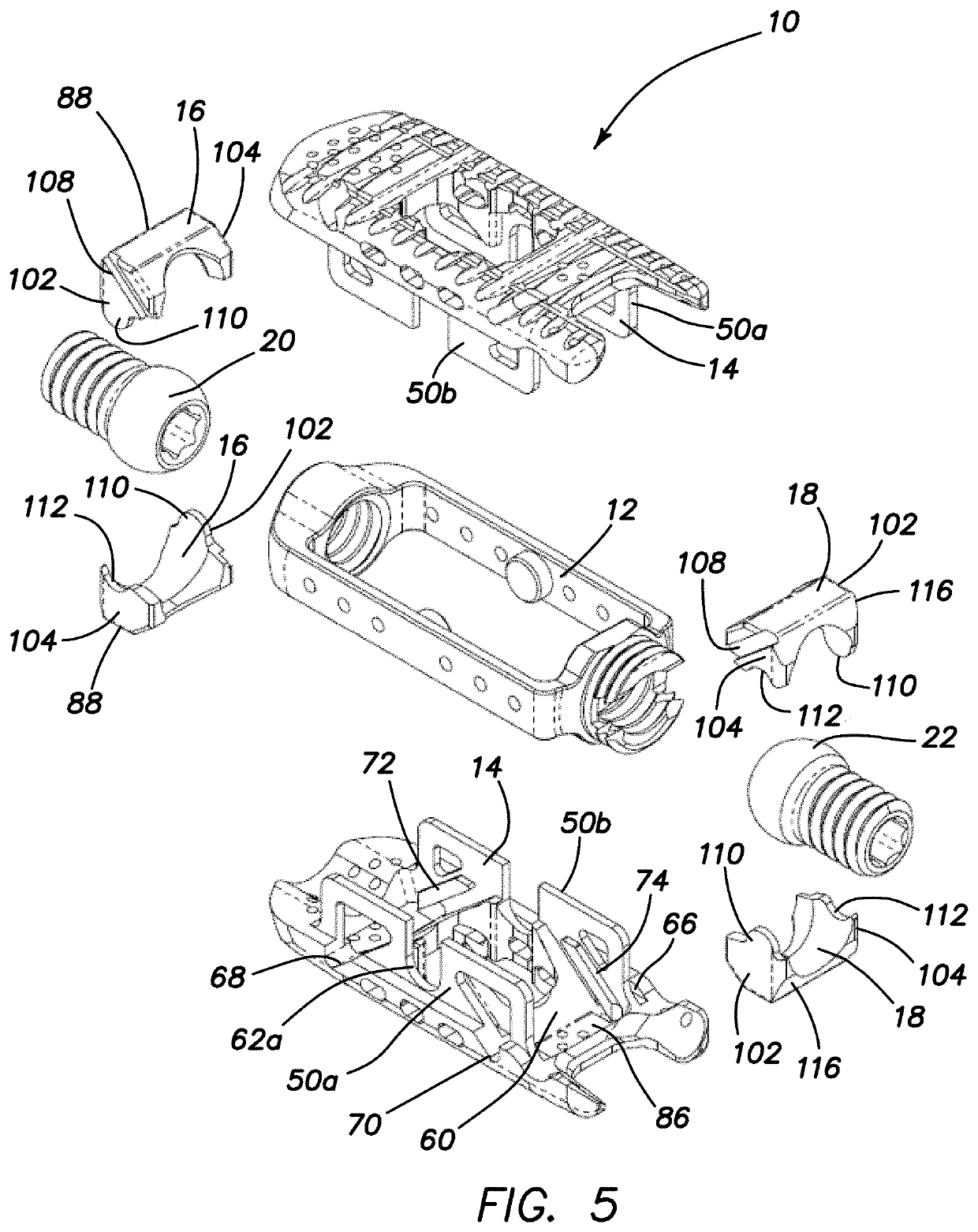
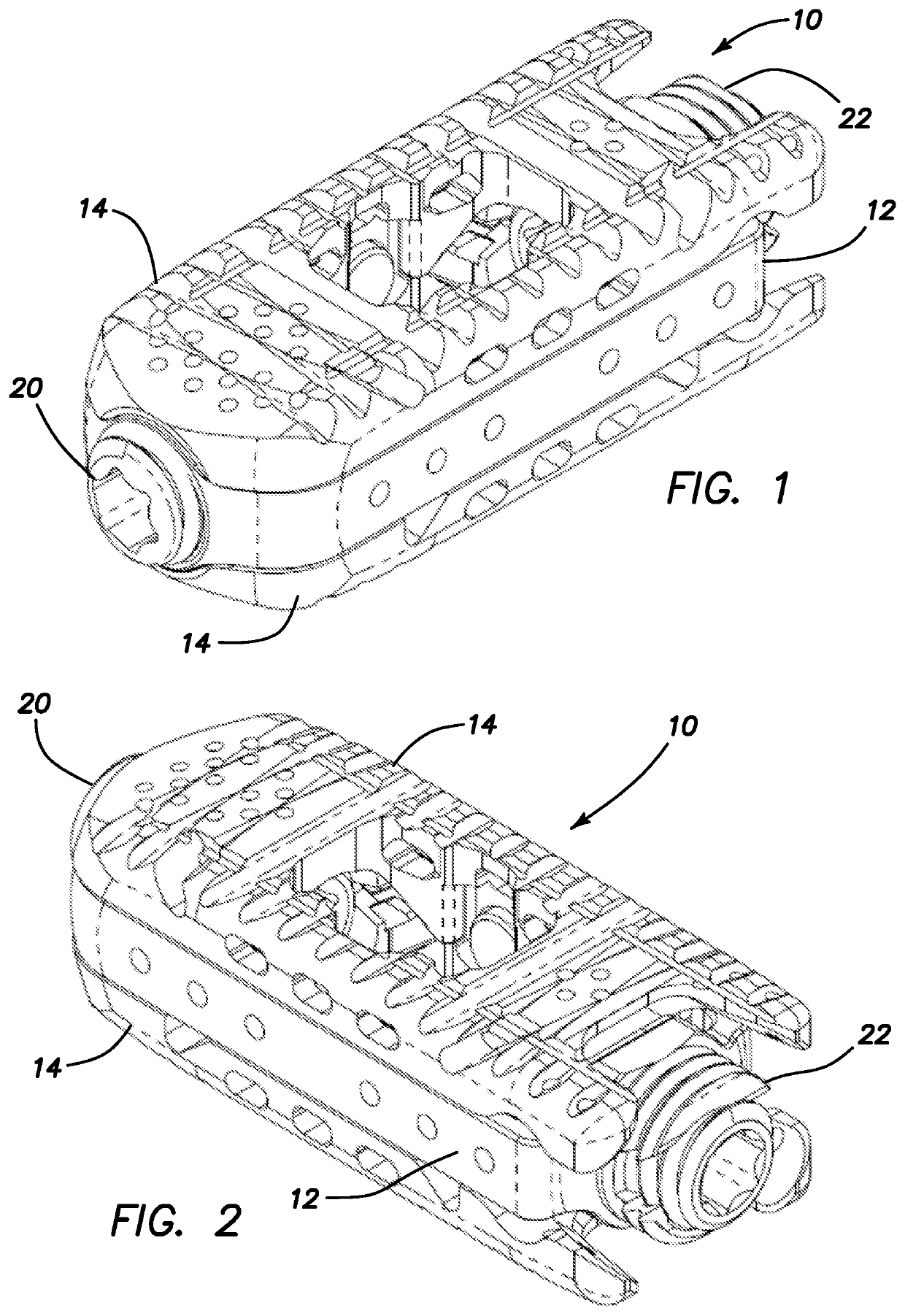
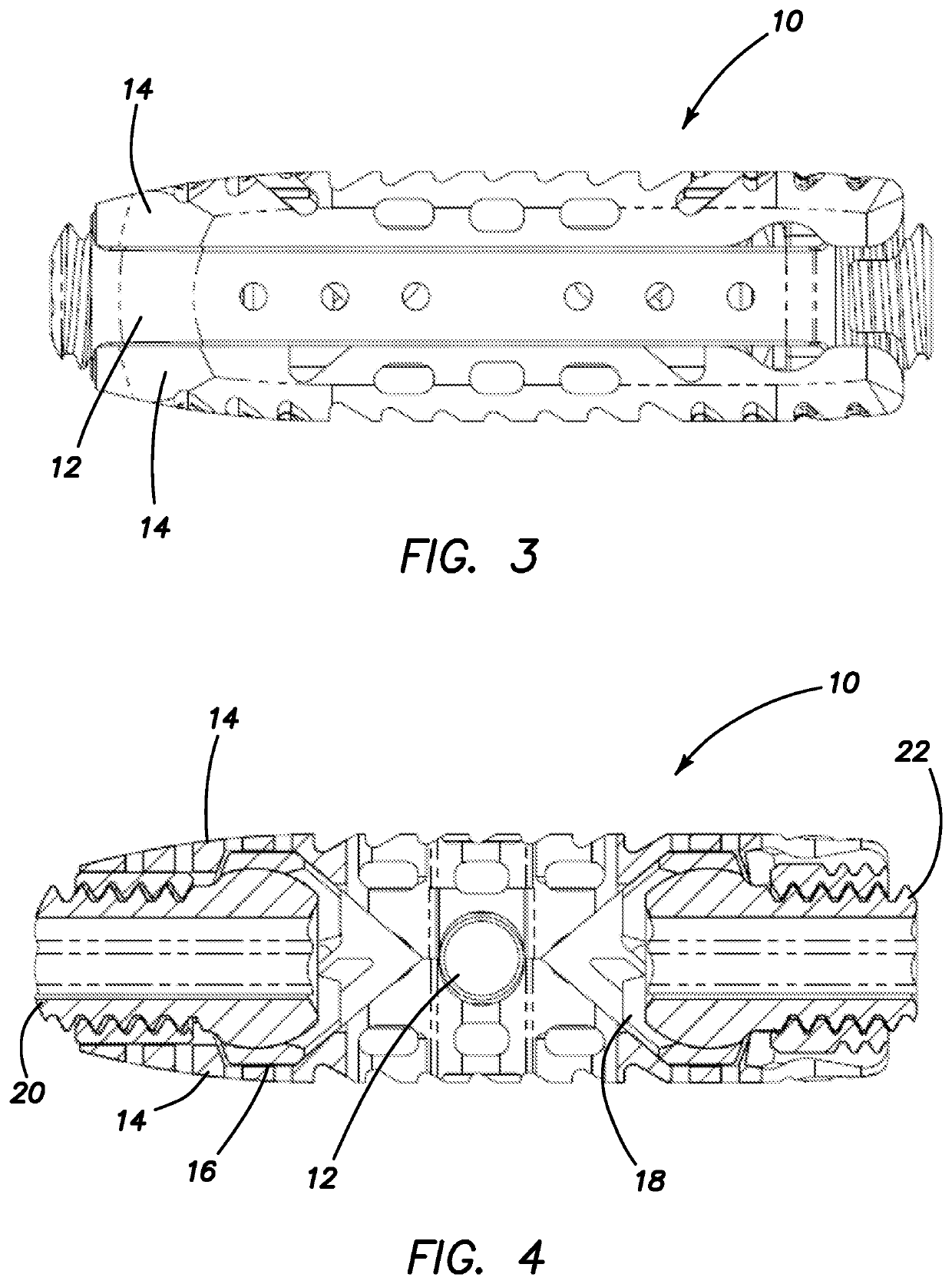
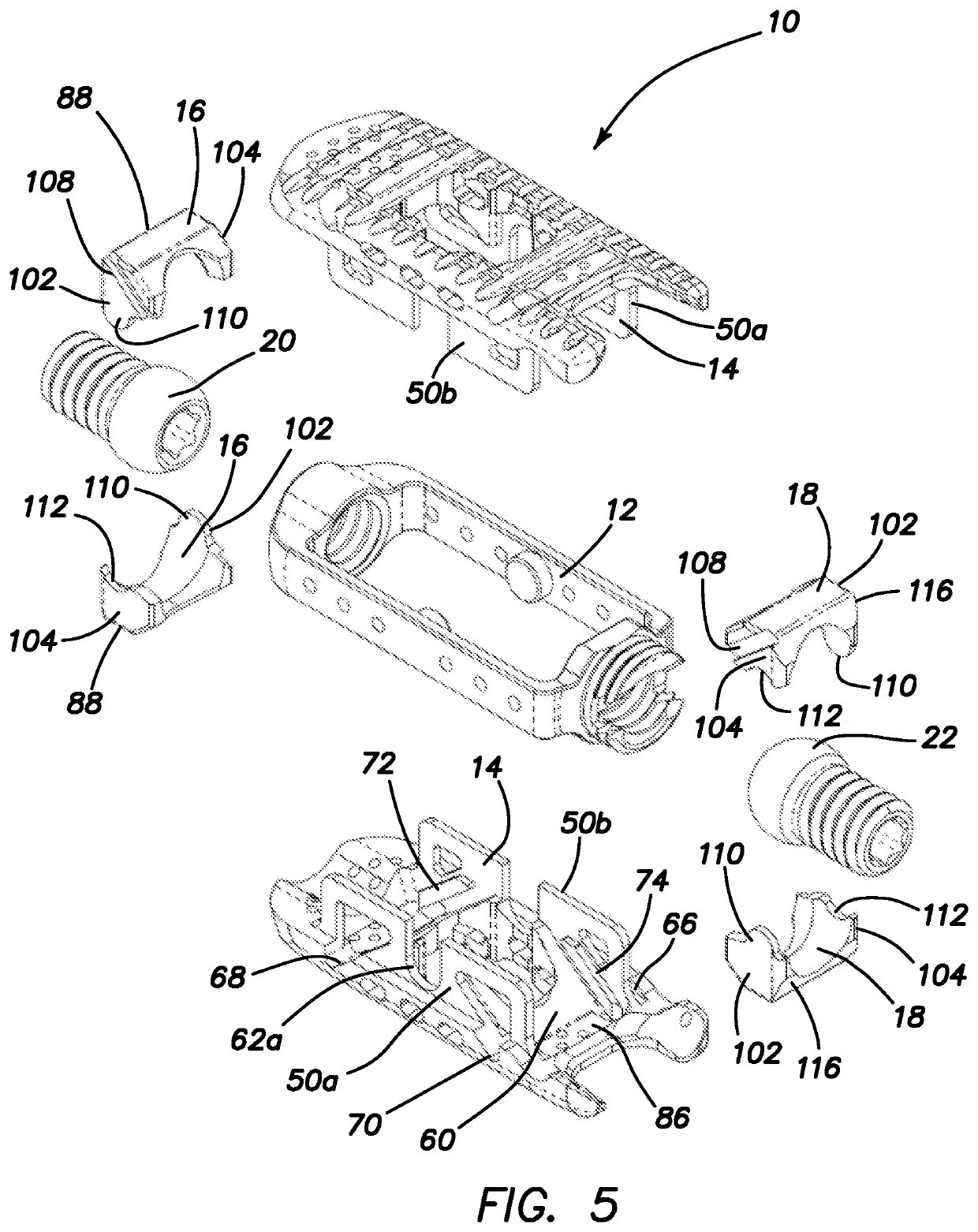
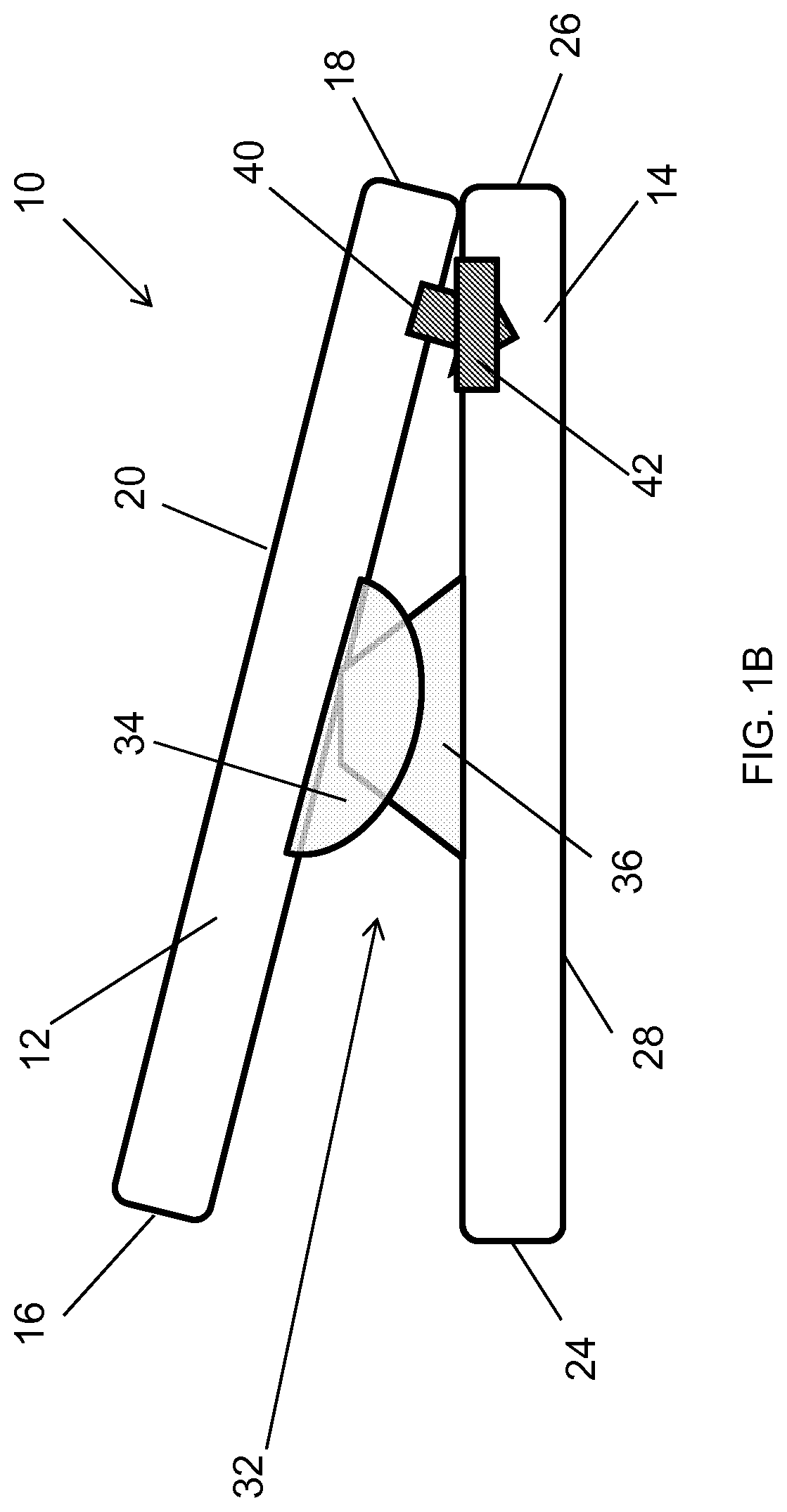
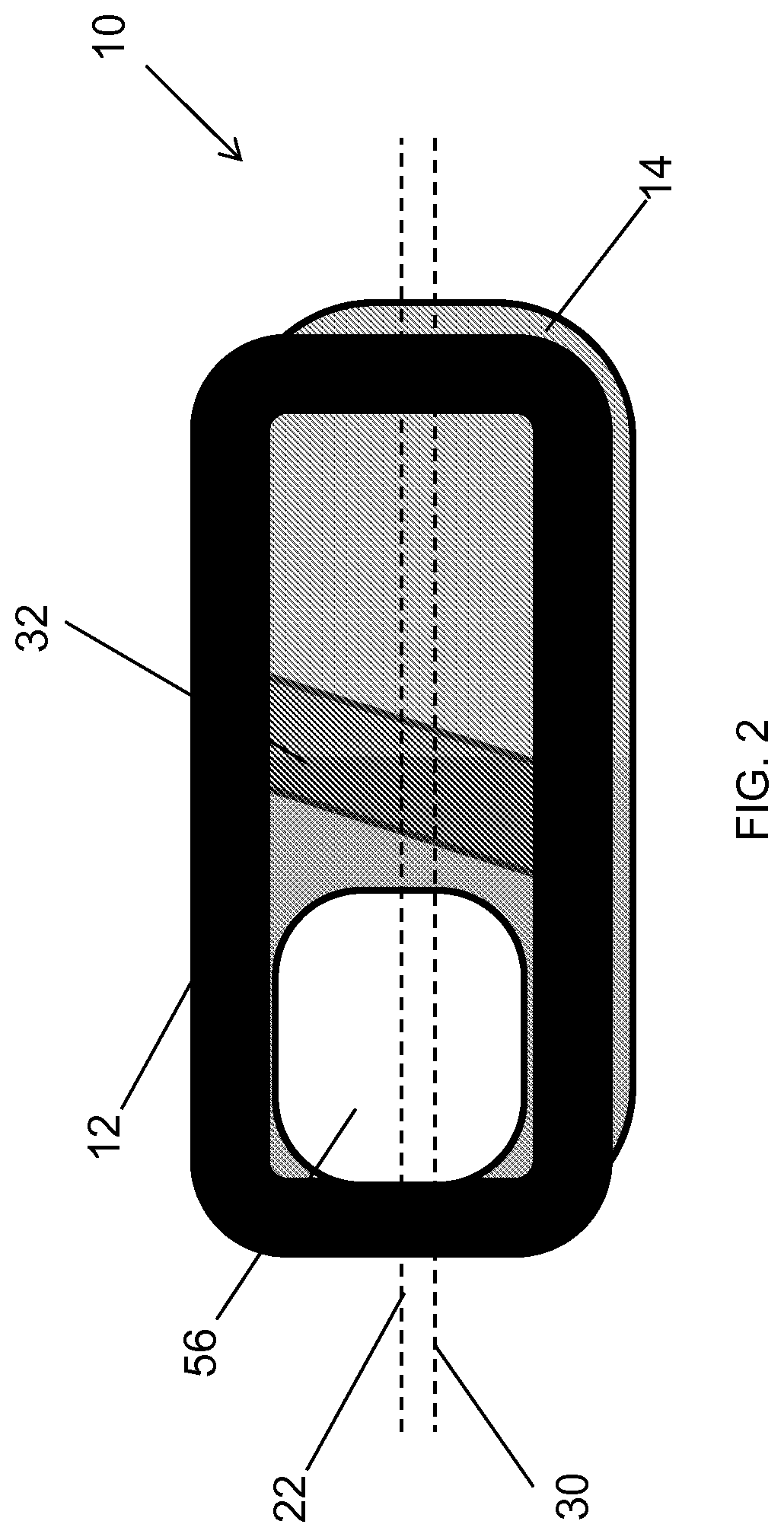
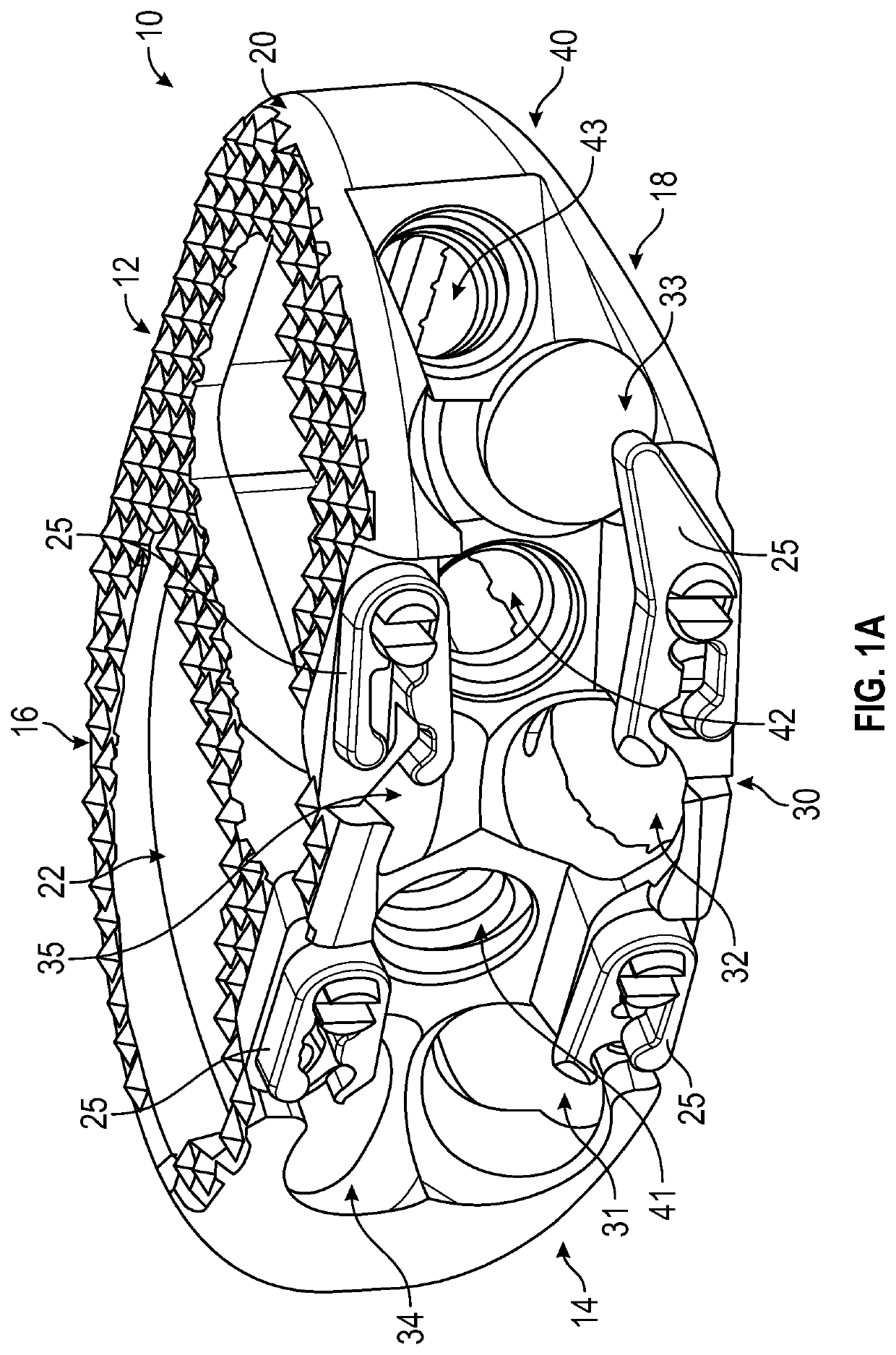
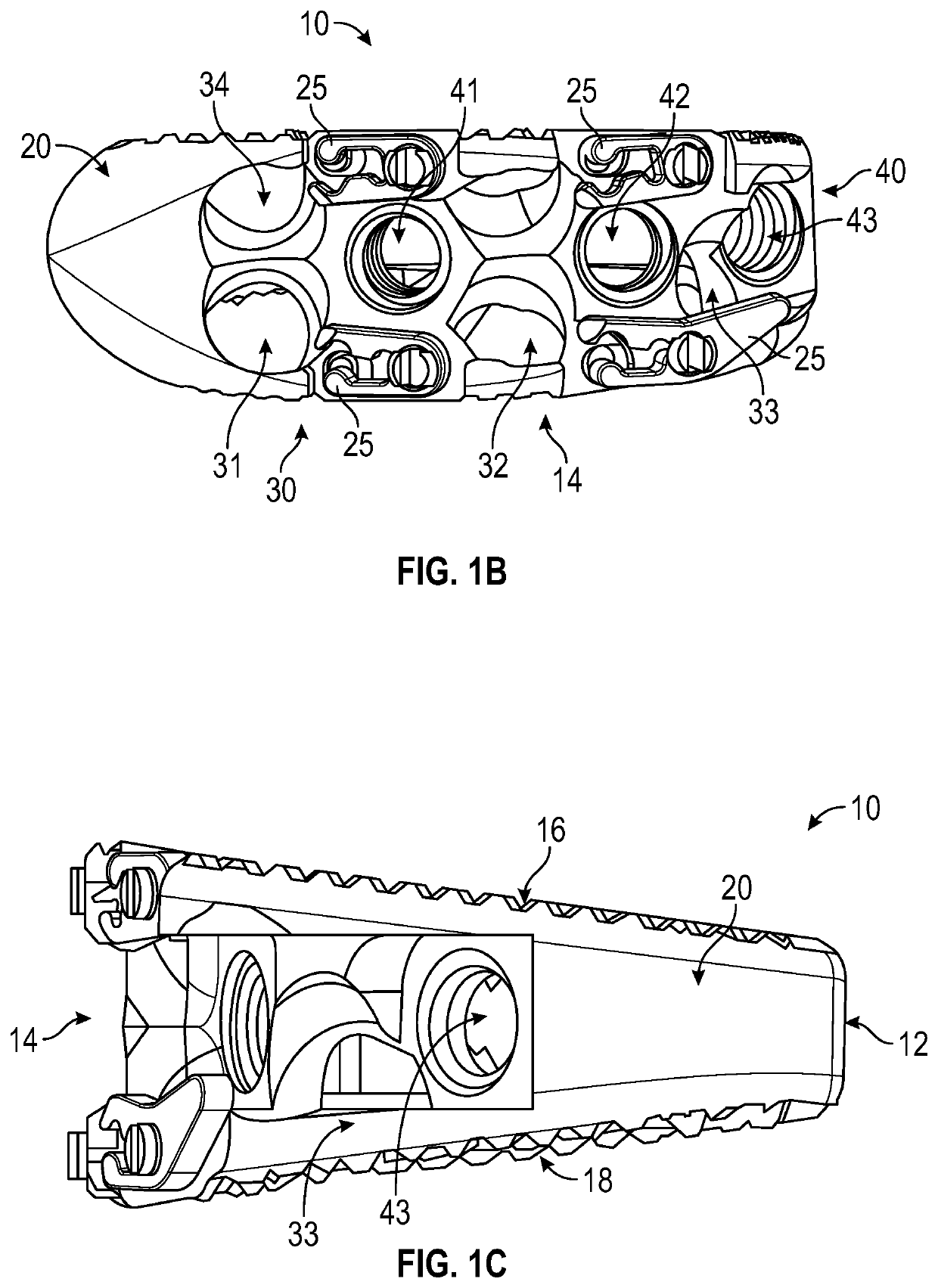
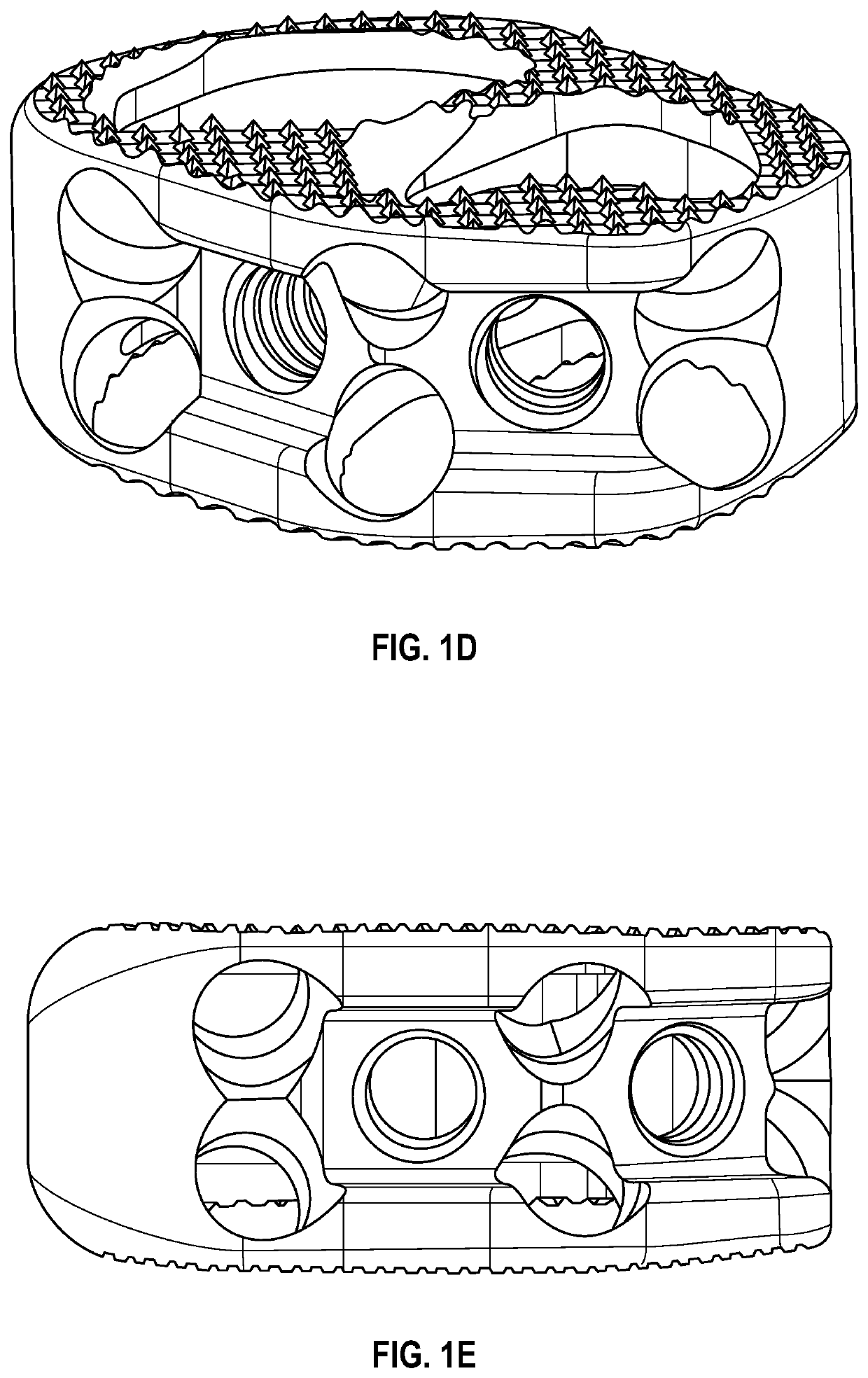
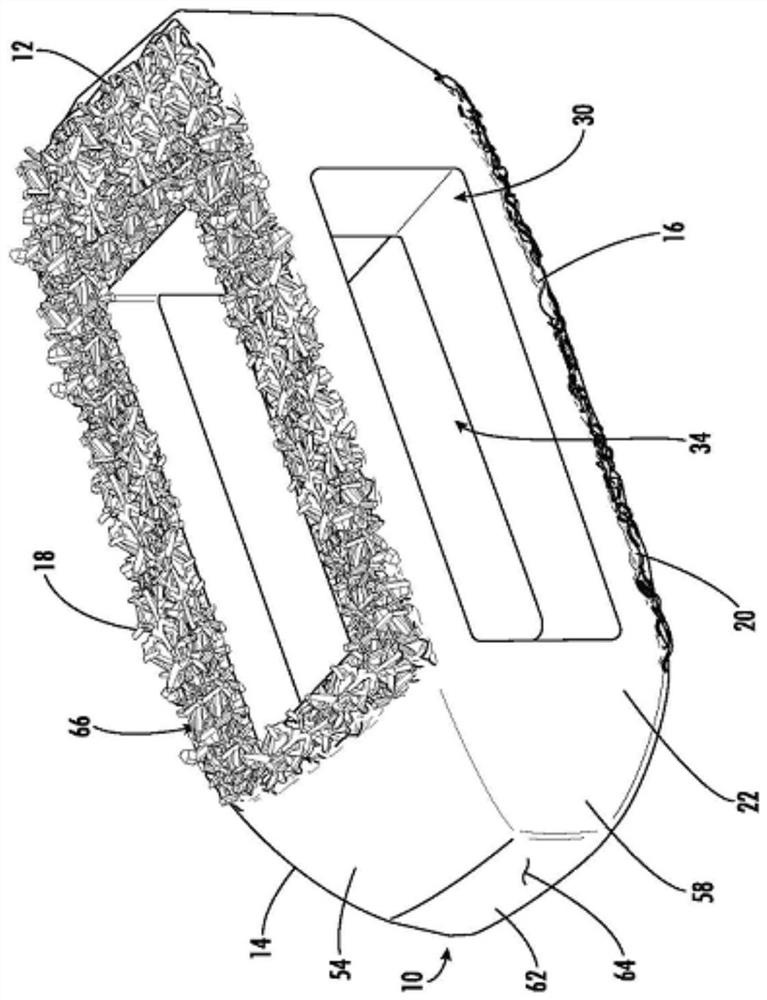
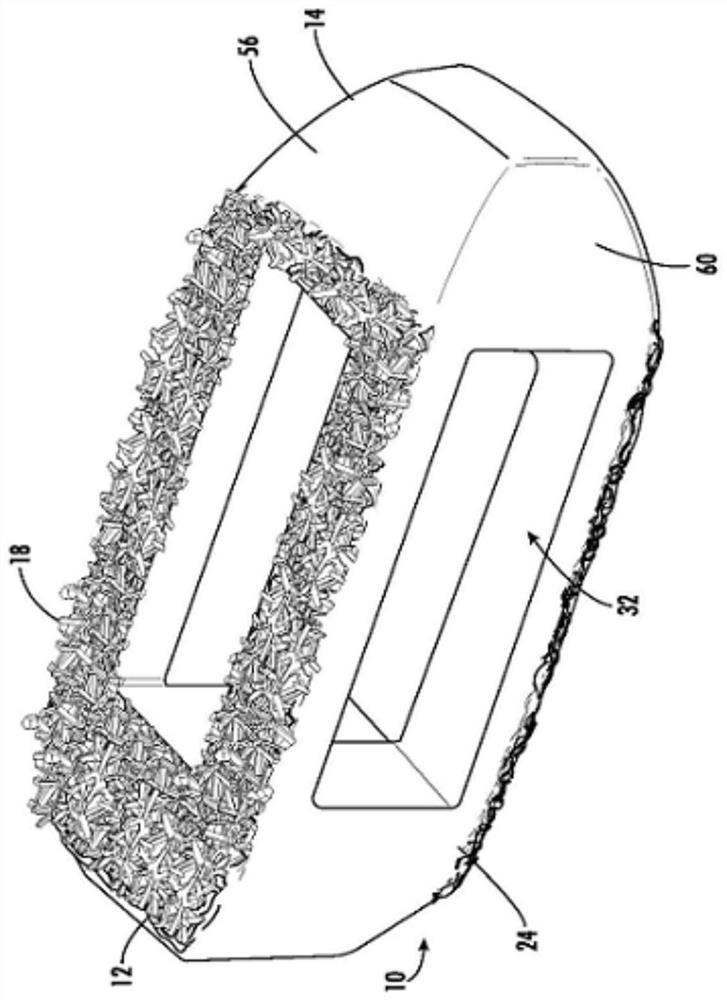
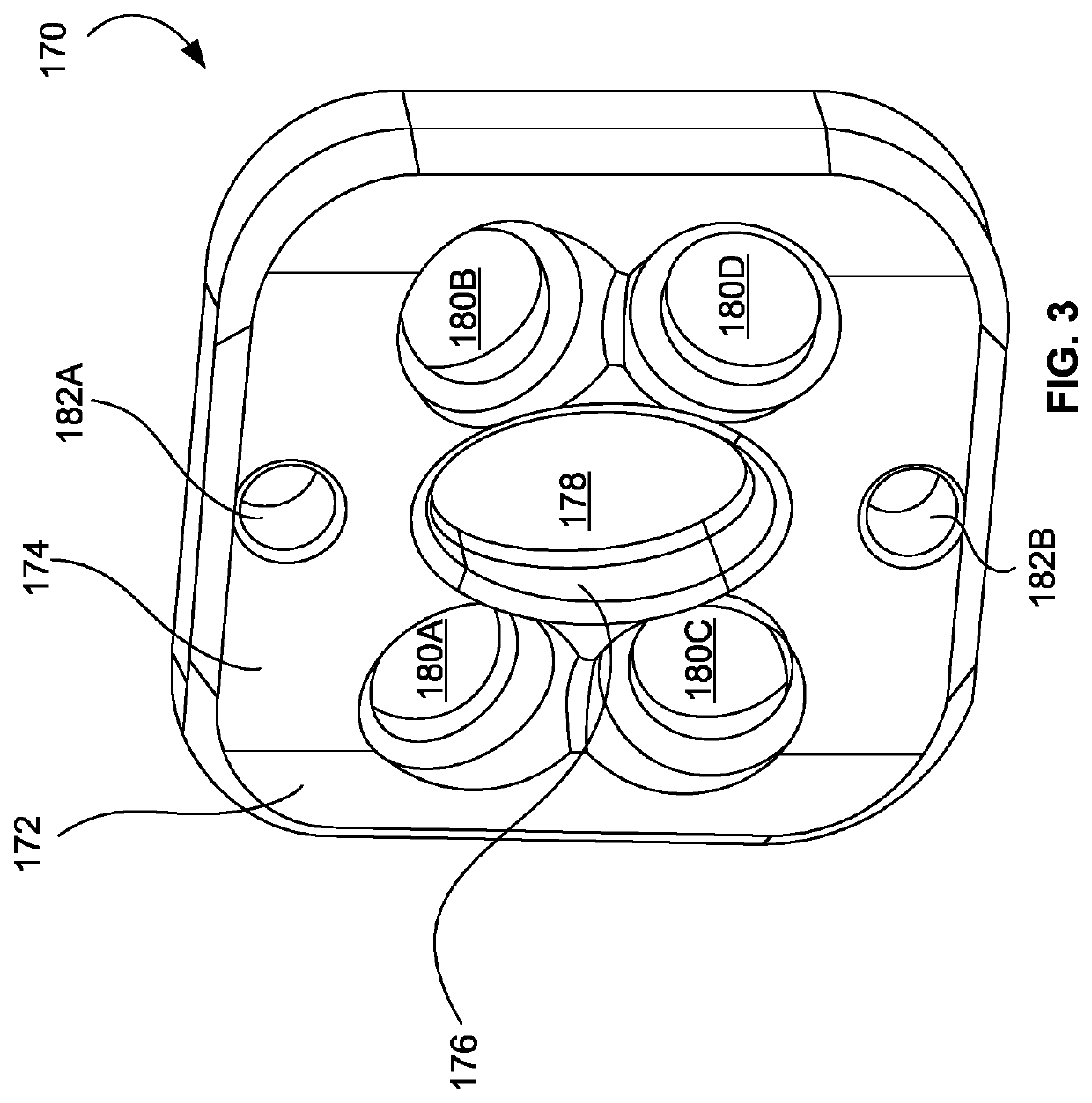
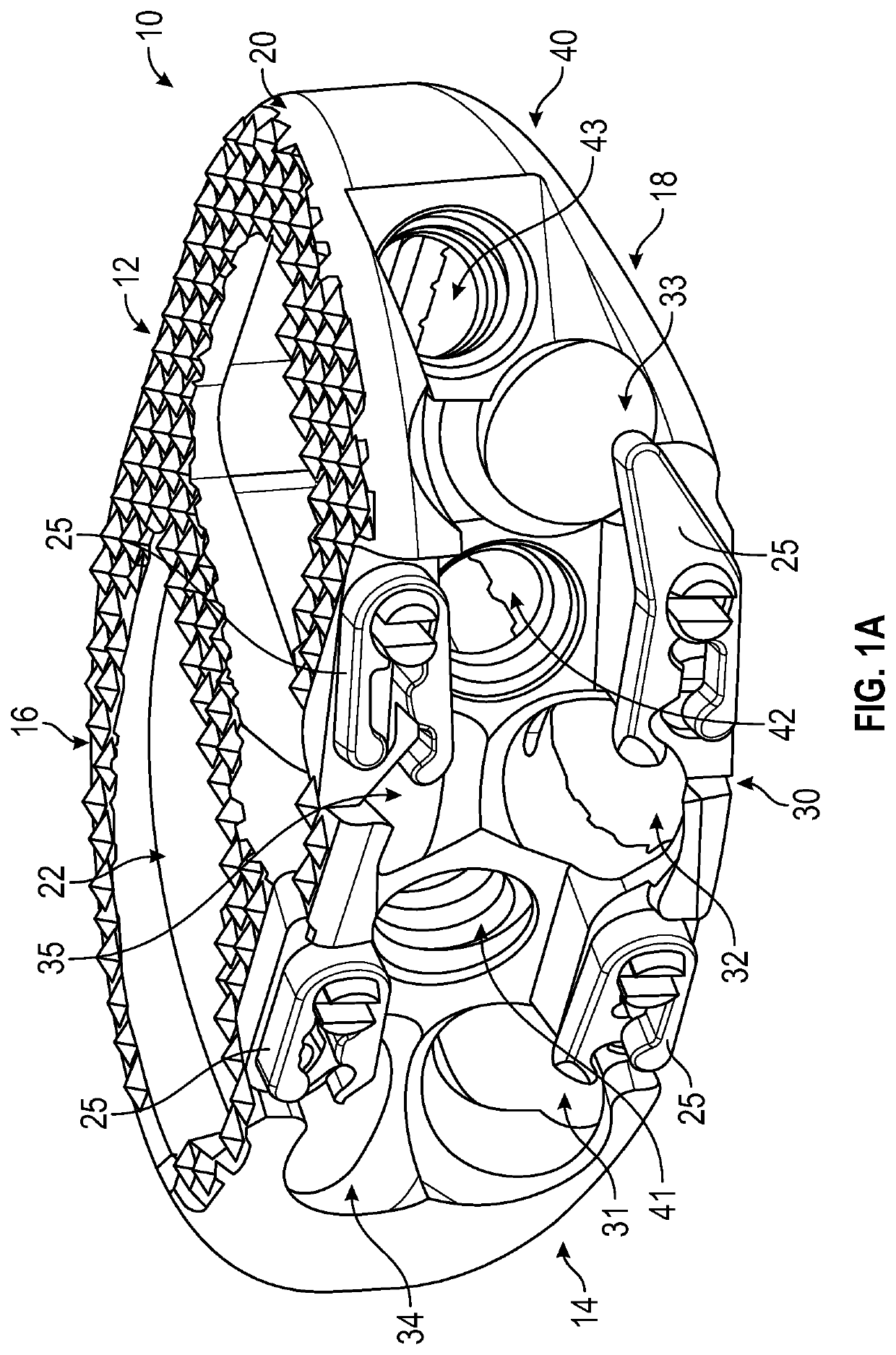
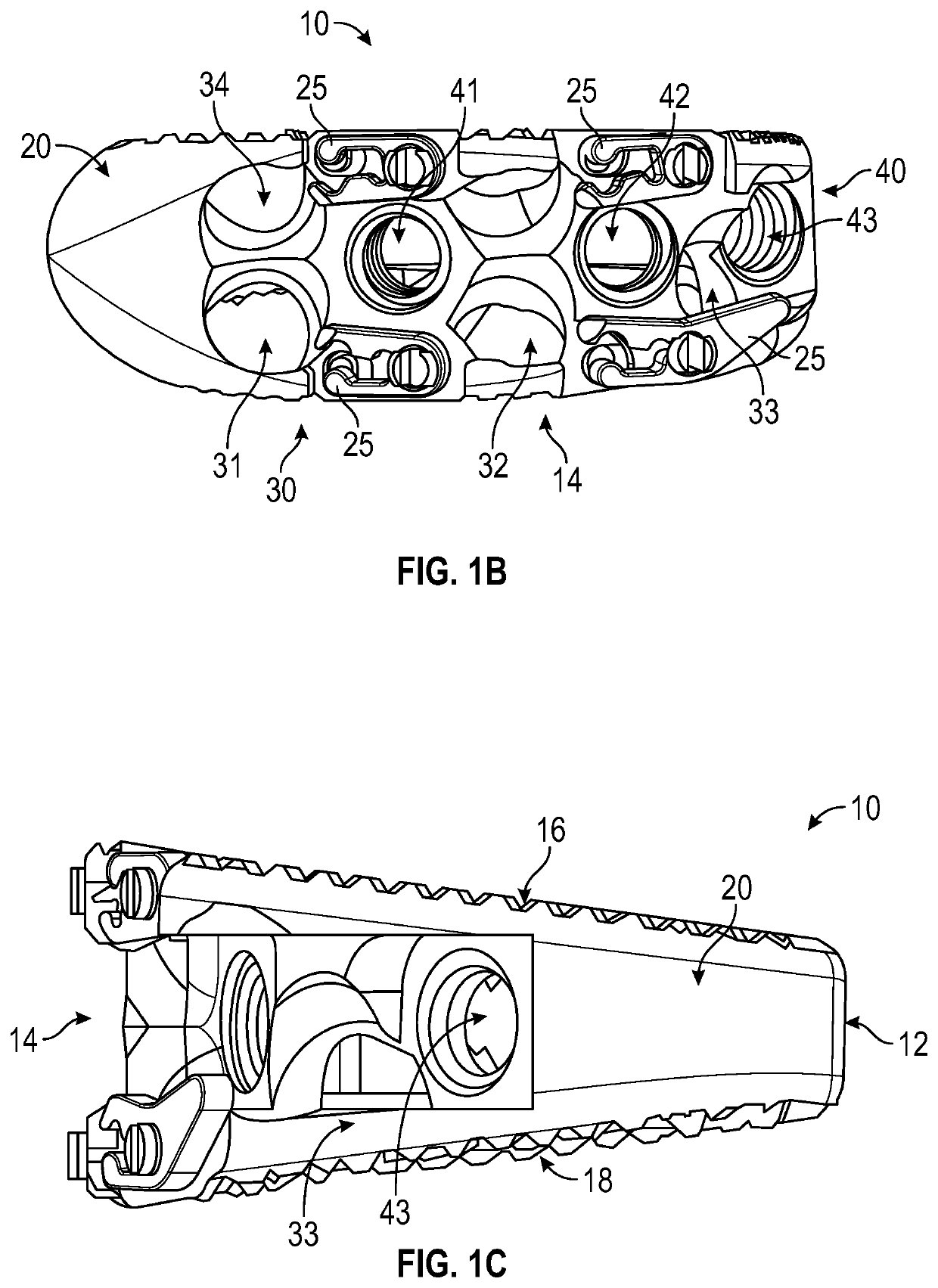
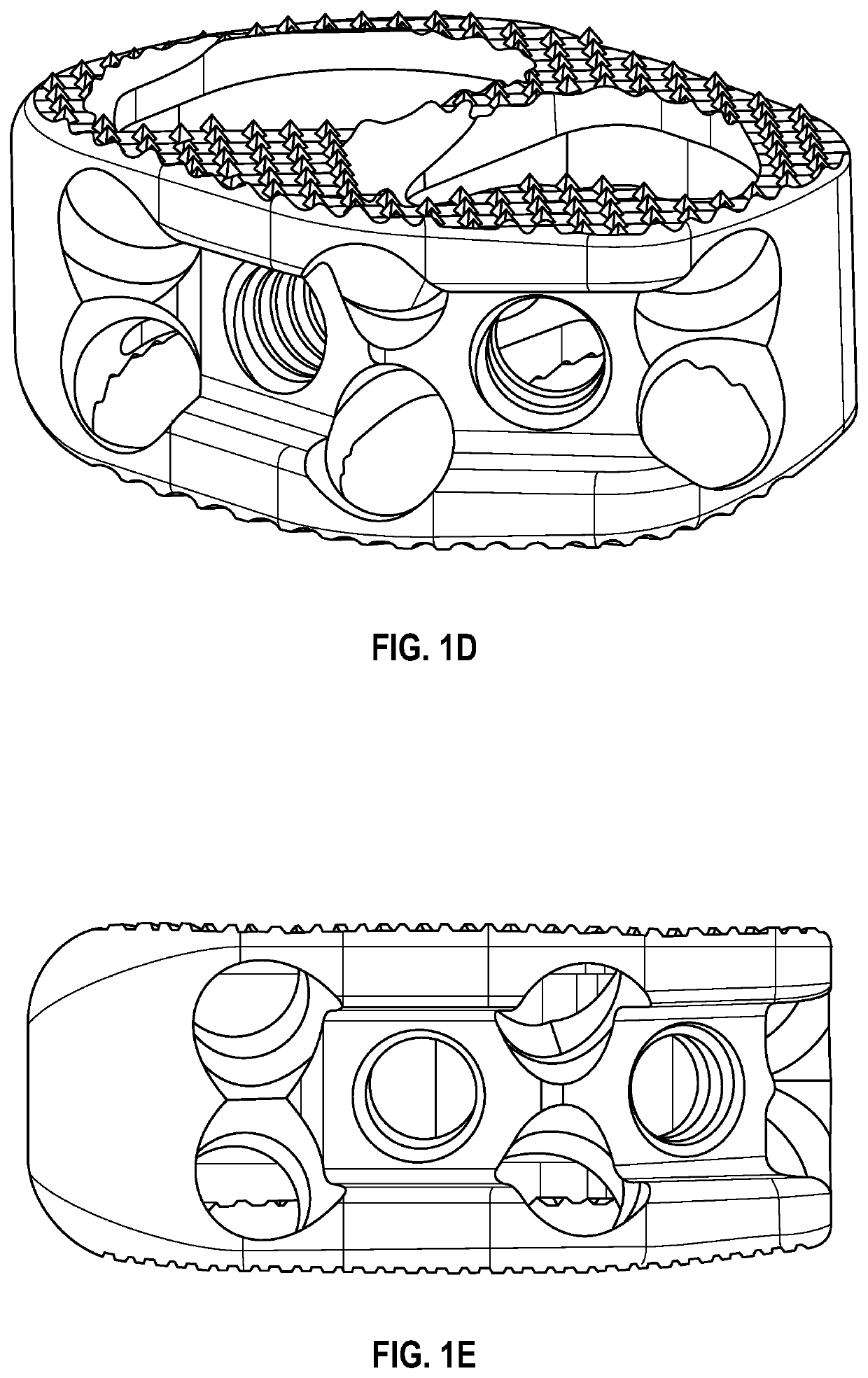
Spinal interbody system and method
ActiveUS8216312B2Avoid expulsionLimiting reducing and limiting impactInternal osteosythesisBone implantCouplingIntervertebral disk
Embodiments of the present invention provide spinal implant systems and methods. According to one embodiment, a plate attached to one or more vertebrae can prevent expulsion of an interbody device from a disc space. The plate and interbody device can be coupled by an attachment member. According to one embodiment, the attachment member is coupled to the plate and includes a portion inserted in a threaded or non-threaded cavity. Preferably, the coupling between the plate and attachment member allows rotation in three dimensions thereby allowing the plate to rotate relative to the interbody device. This allows the plate to be better positioned for attachment to the spine during an operation.
Owner:ZIMMER BIOMET SPINE INC
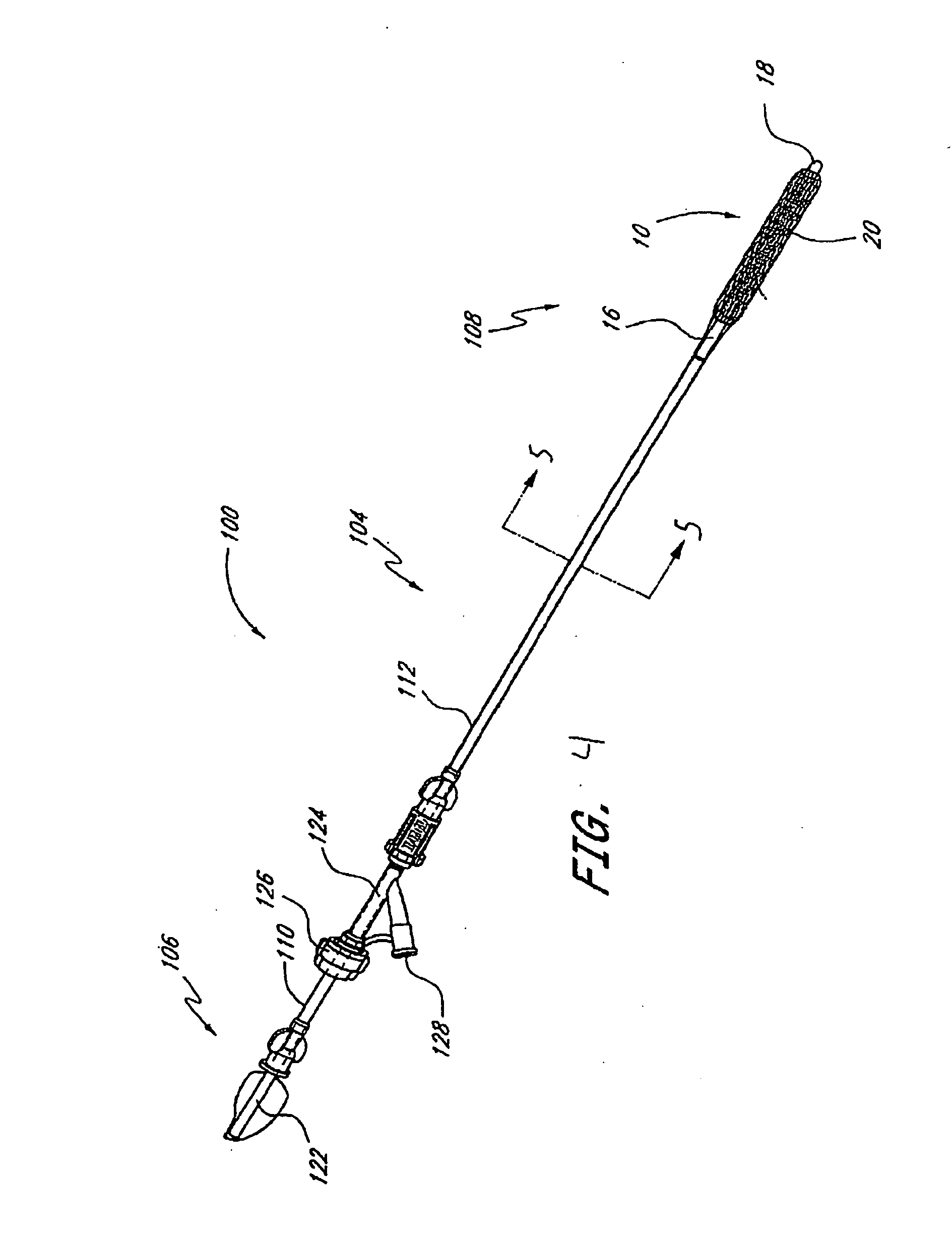
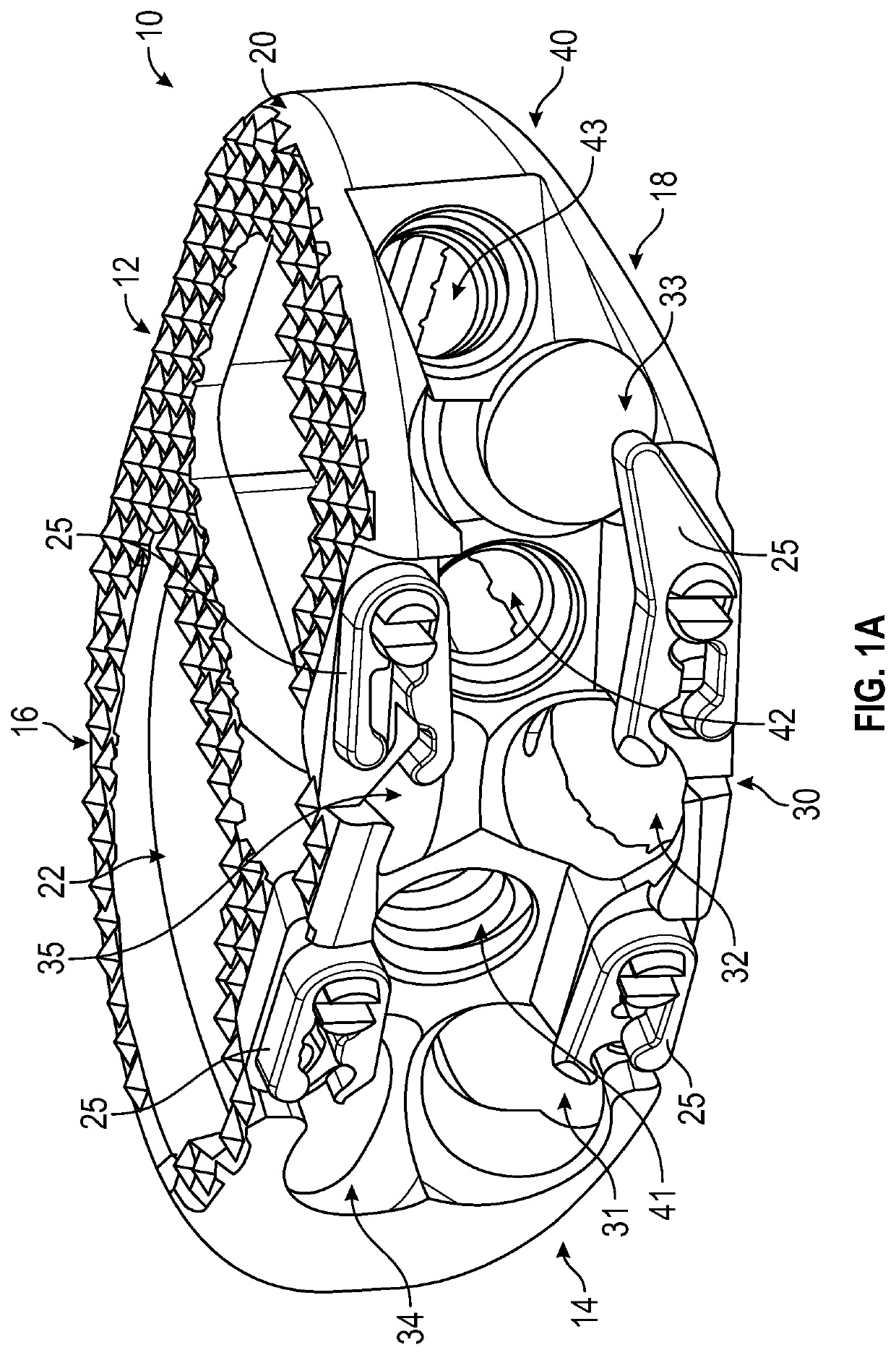
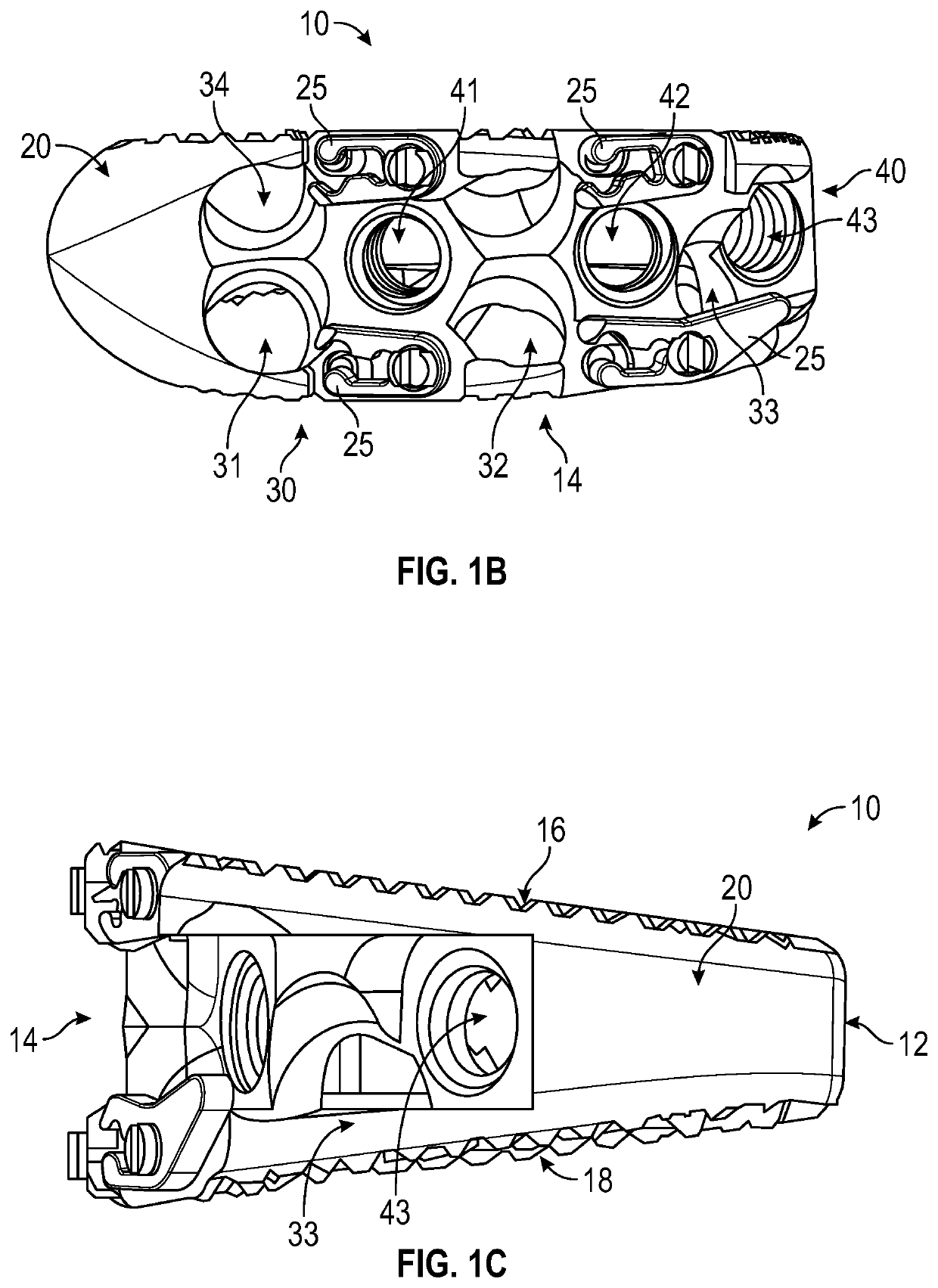
Instrument and method for implanting an interbody fusion device
A holder is provided which couples to the spine. In an embodiment, the holder has two conduits into which sleeves may be inserted during a spinal fusion procedure. The holder may have a distractor extending from the bottom of the holder. The distractor secures the holder to the spine and maintains a proper separation distance between adjacent vertebrae. The sides of the distractor may be serrated to better secure the holder to the spine. The sleeves and conduits serve as alignment guides for instruments and implants used during the procedure. In an embodiment, the holder may include holes for fasteners that fixably secure the holder to vertebrae adjacent to a disc space. A flange may be placed around the holder to shield surrounding tissue and to provide a placement location for adjacent blood vessels during the spinal fusion procedure.
Owner:ZIMMER SPINE INC
Systems, devices and methods for posterior lumbar interbody fusion
InactiveUS20090292323A1Easy to cutEasy to shapeJoint implantsSpinal implantsSurgical operationIntervertebral disc
Described herein are stabilization devices, systems and methods to aid in posterior lumbar interbody fusion (PLIF) surgeries. The stabilization devices (“devices”) described herein are typically self-expanding devices that may be implanted into an intervertebral disc and packed with a bone graft or biologic or synthetic material to promote anchoring of the stabilization device and fusion of the vertebrae adjacent to the intervertebral disc.
Owner:SPINEALIGN MEDICAL
Formed in place corpectomy device
An inflatable corpectomy device comprises a balloon, which defines a cavity for receiving a hardenable media. The device can be inserted in an un-inflated form into a space formerly occupied by a diseased or damaged vertebrae bone. When inflated with a hardenable material, the device expands and fills the space. Various bone anchors or plates can be used in combination with the inflatable device to bridge the space and stabilize the spine while the device hardens.
Owner:WARSAW ORTHOPEDIC INC
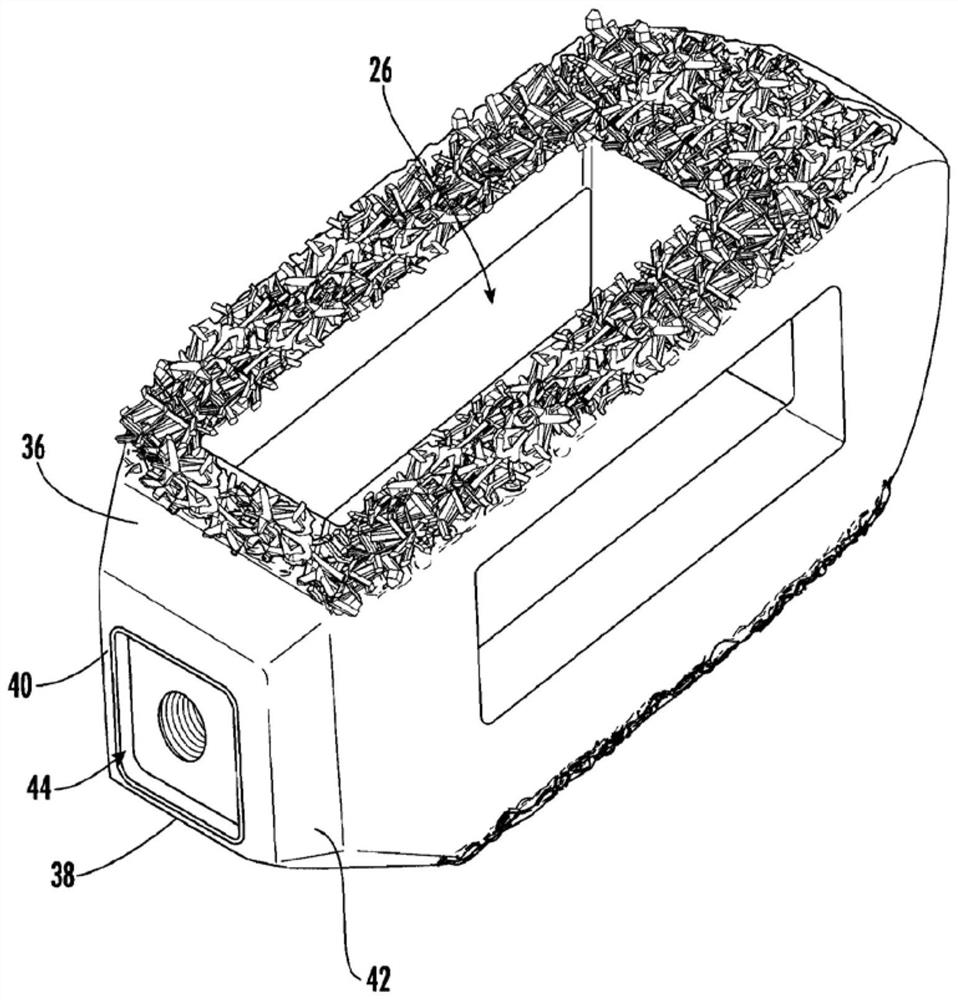
Implants having internal features for graft retention and load transfer between implant and vertebrae
InactiveUS20120303127A1Easy to insertPositively influenceDecorative surface effectsBone implantBone growthBone healing
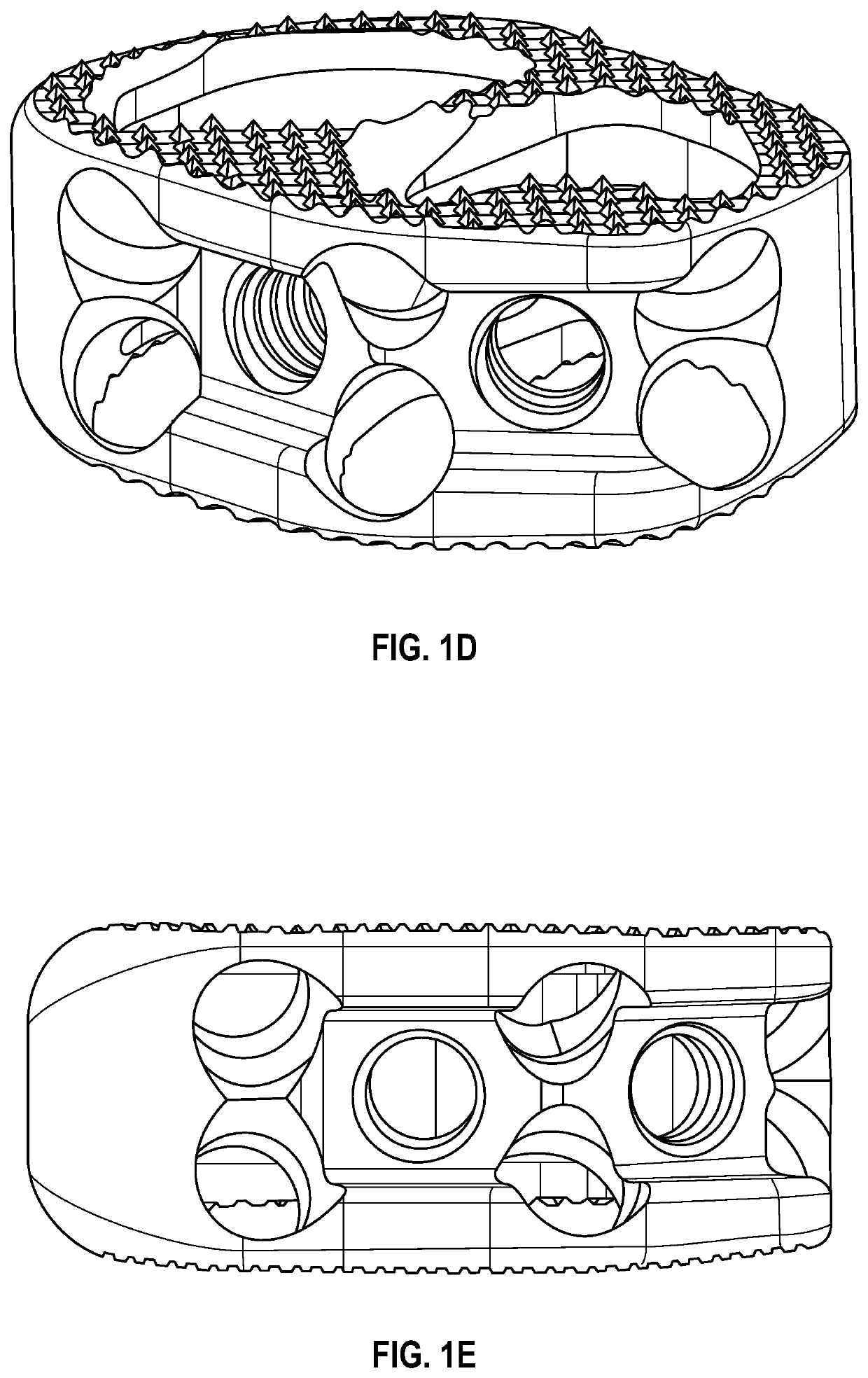
An interbody spinal implant, such as a solid-body or composite implant, includes at least one graft contact surface as one or more of the internal surfaces of the implant. The graft contact surface, for example, having at least one ridge or groove is designed to contact and promote retention and stabilization of bone growth-inducing materials placed within the internal openings of the implant body. In addition, the ridges or grooves may influence the biological processes to promote bone healing and fusion. Also disclosed are processes of fabricating the graft contact surfaces and other surface topographies on the implant.
Owner:TITAN SPINE
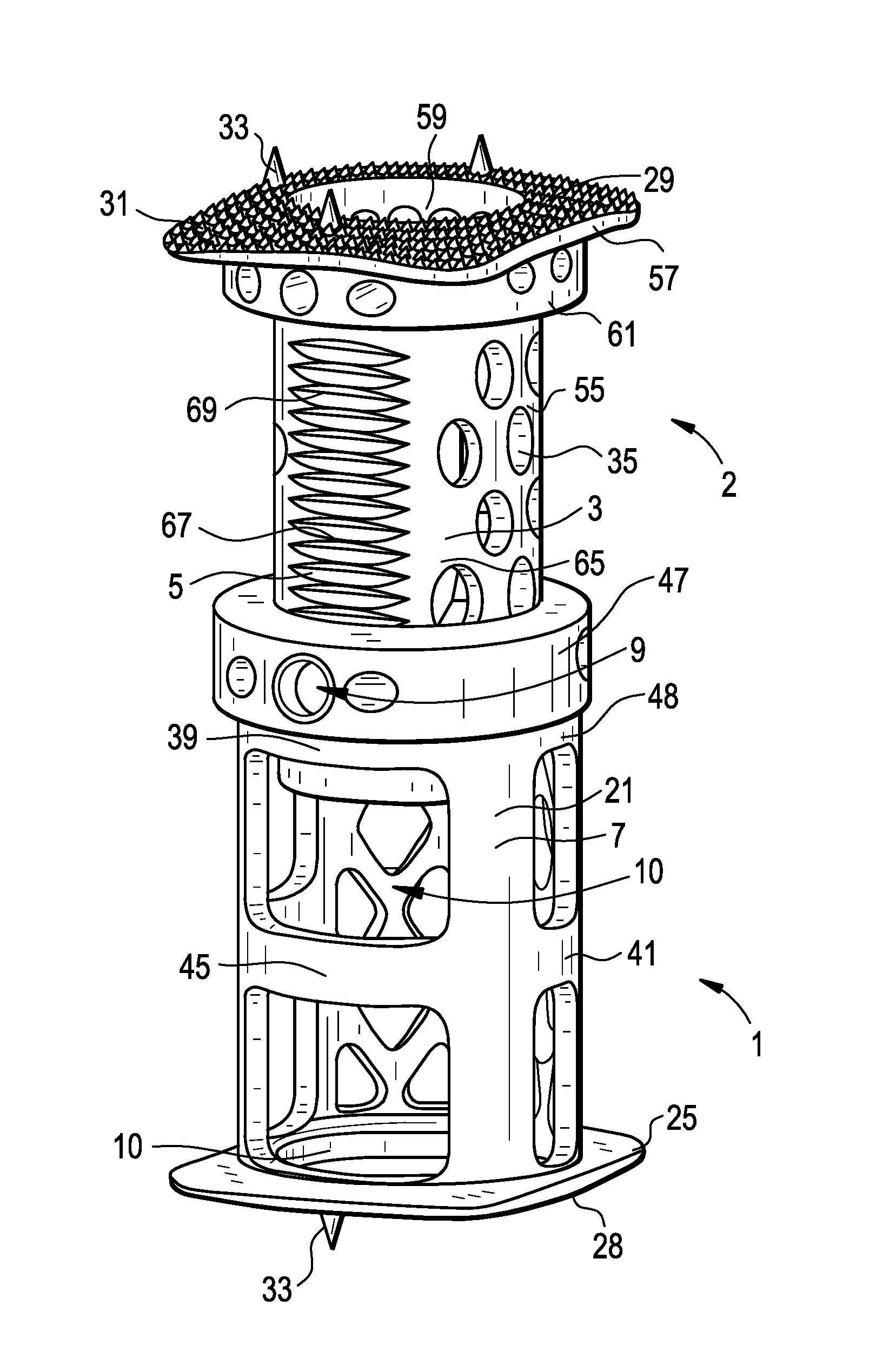
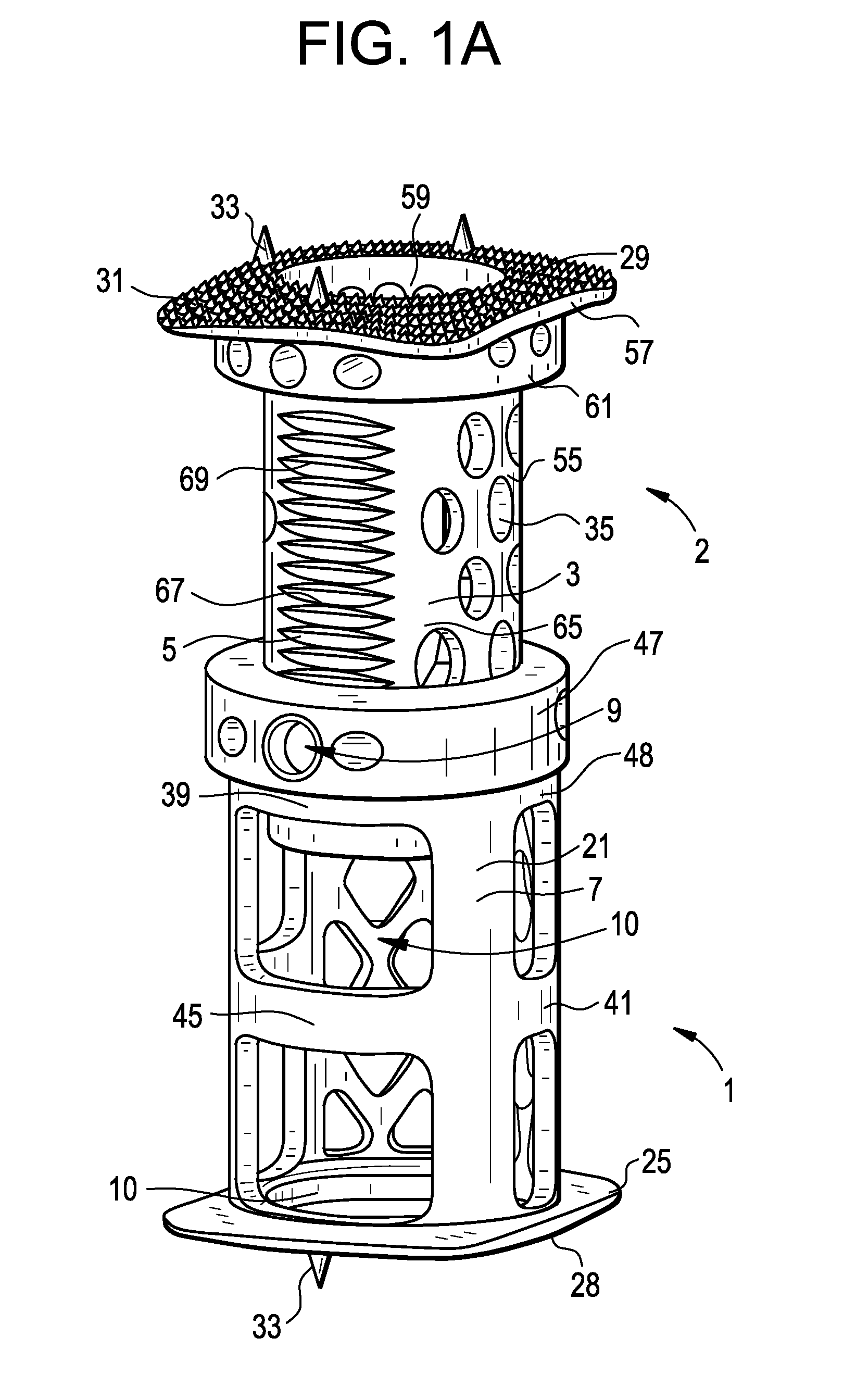
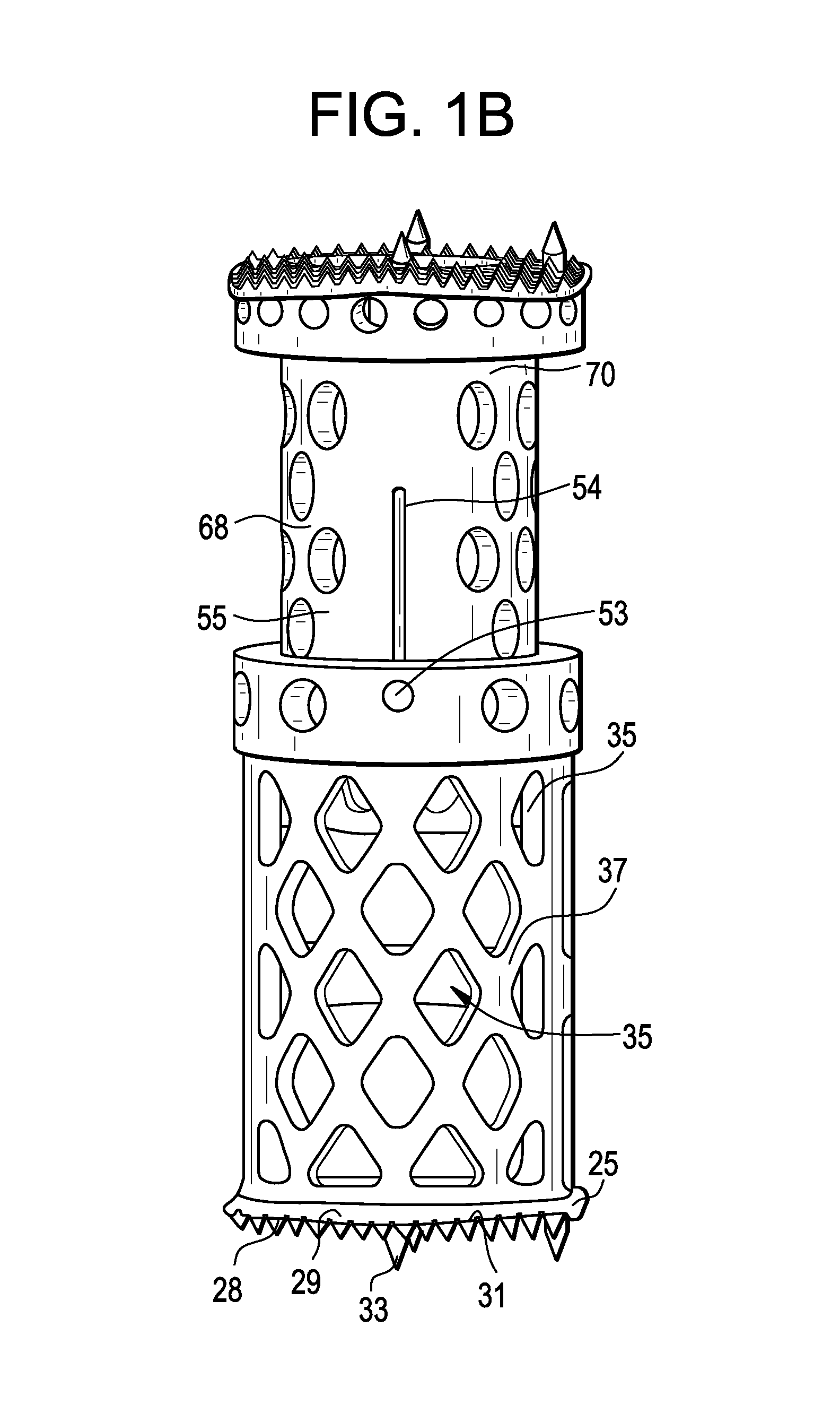
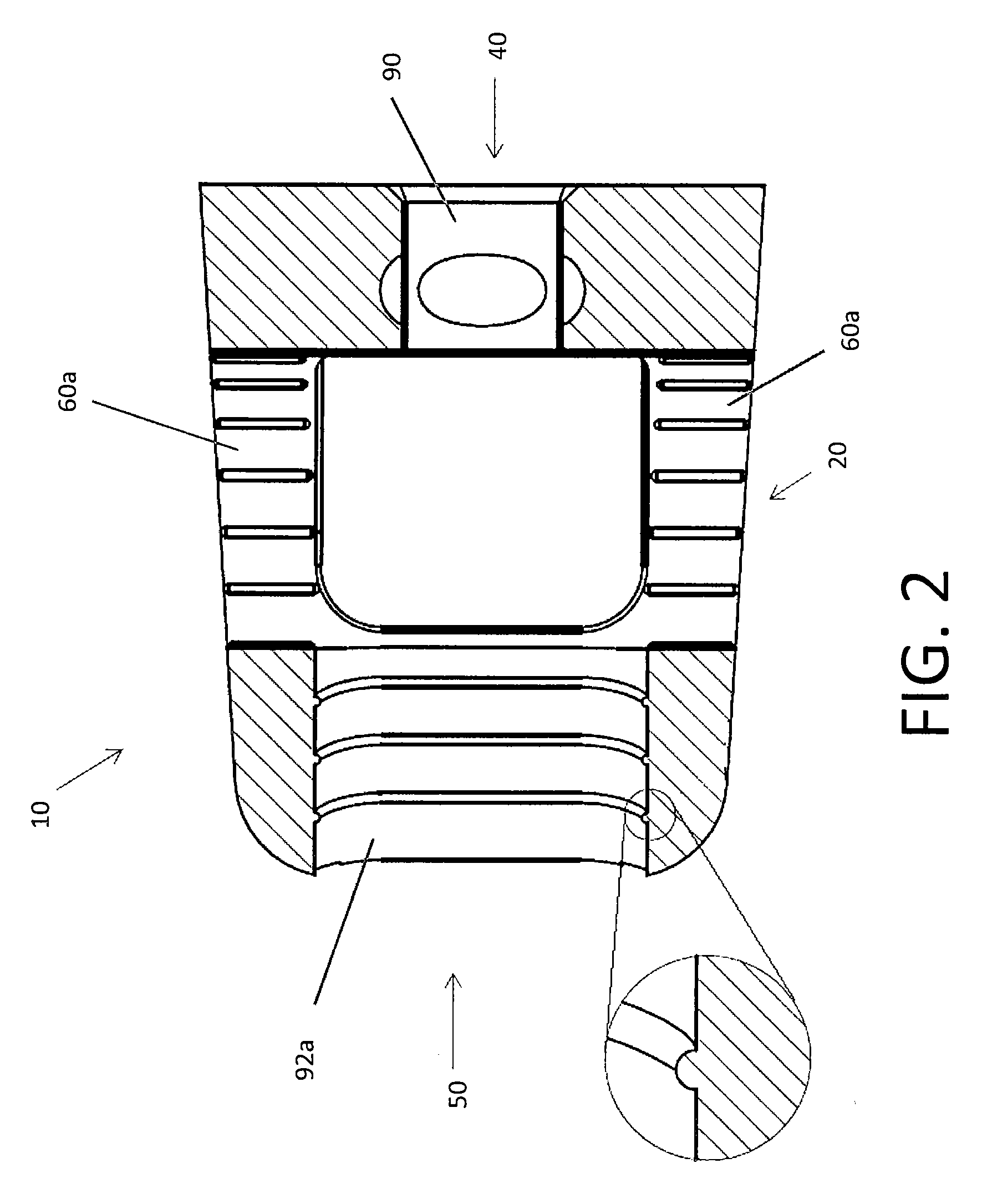
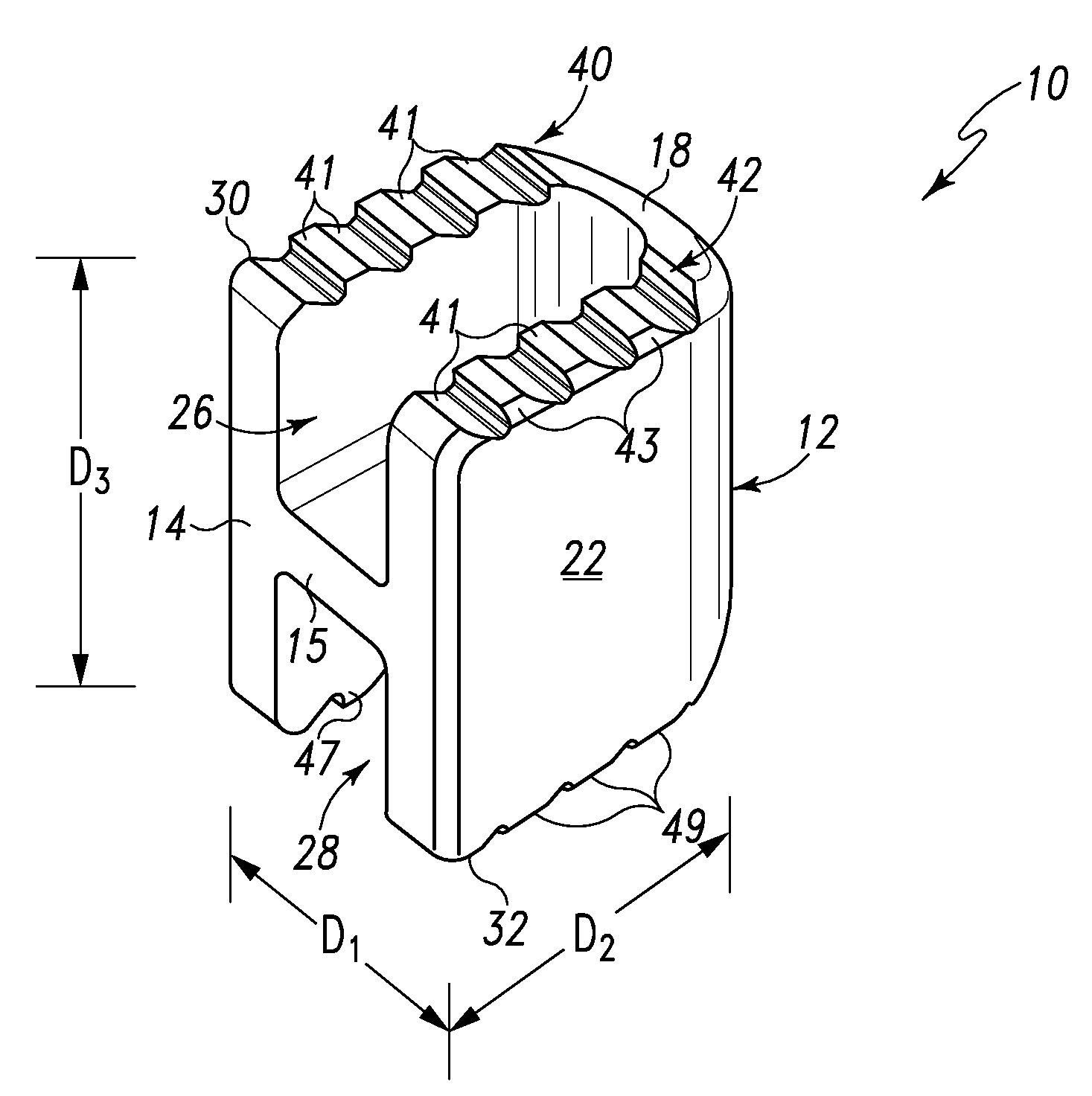
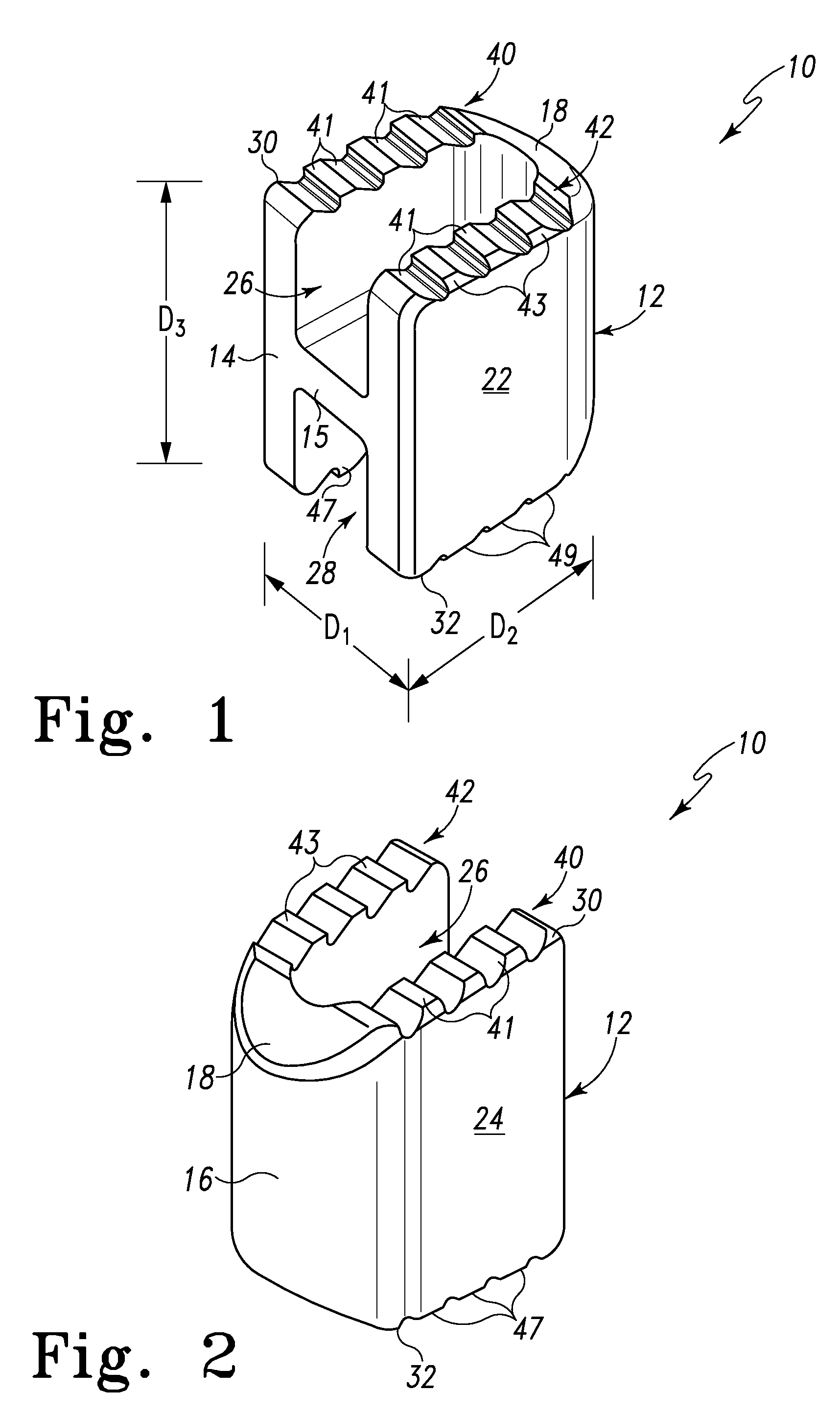
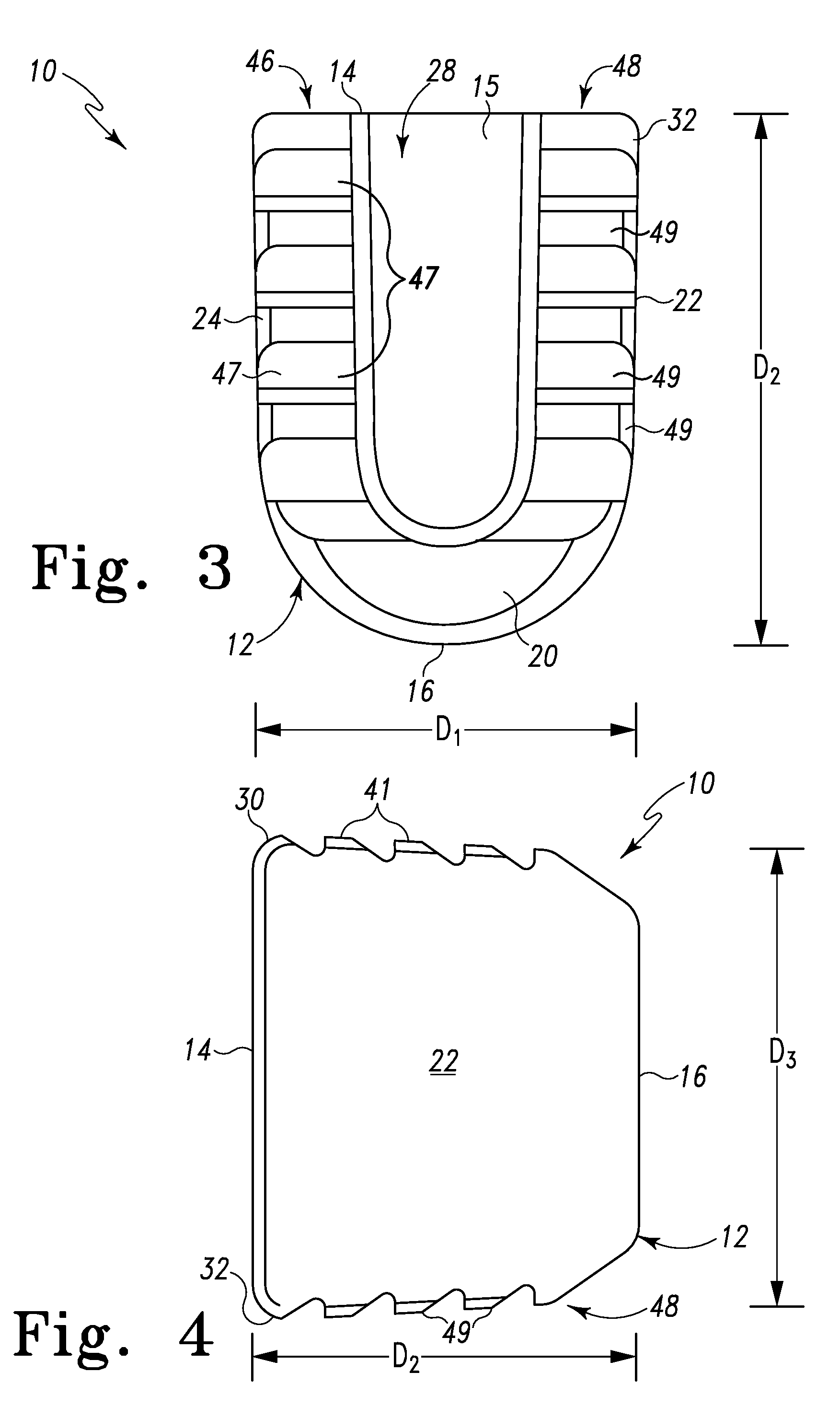
Spinal Interbody Distractor
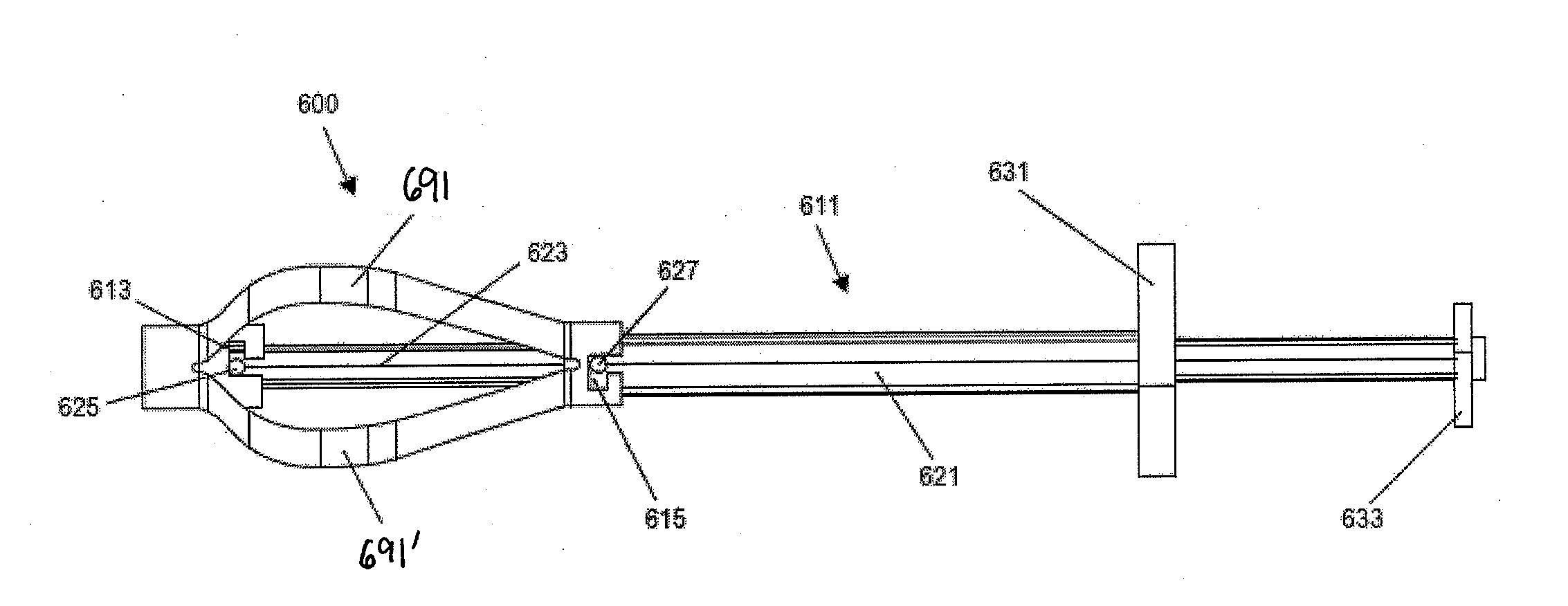
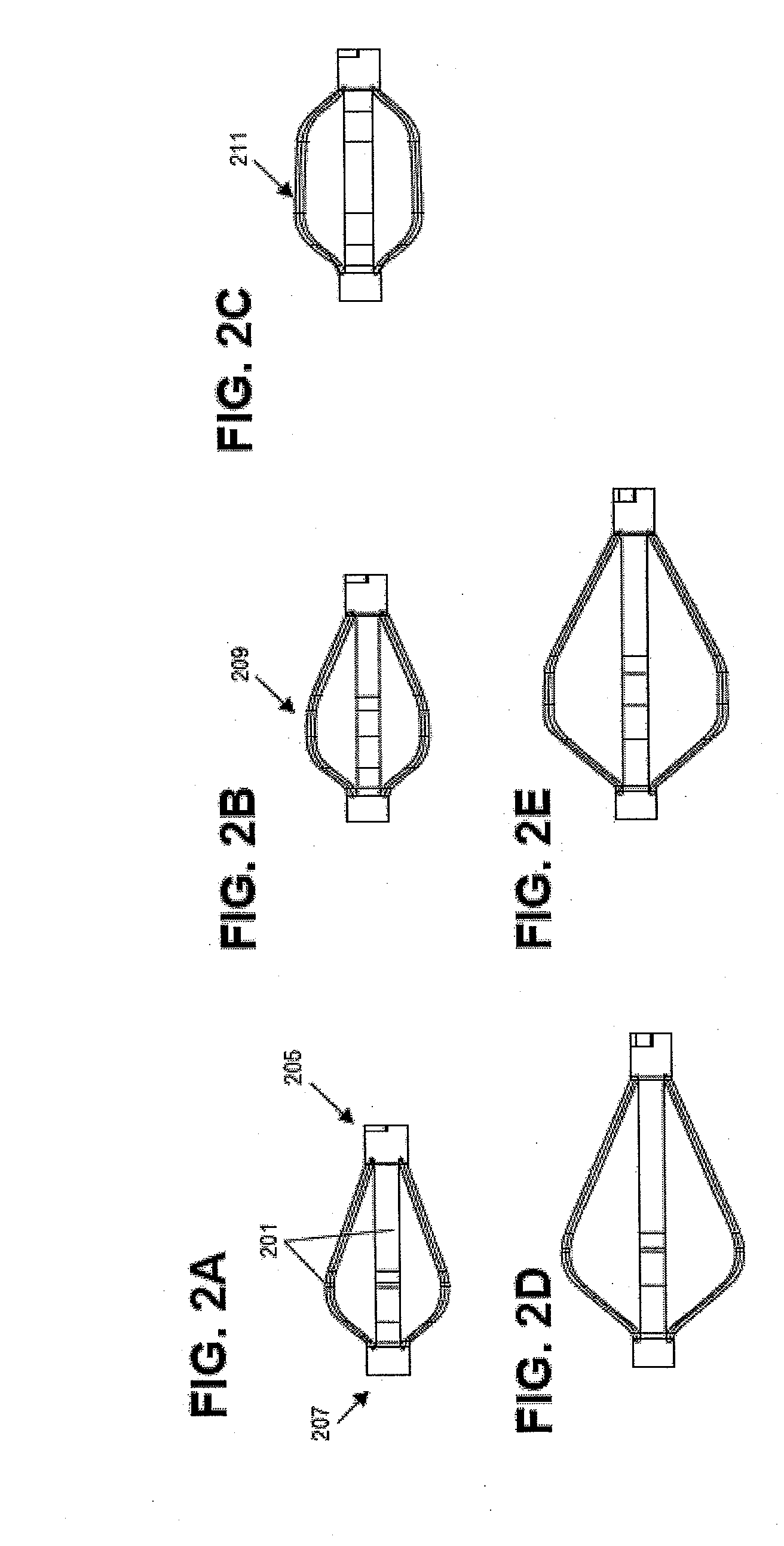
A spinal interbody distractor is provided that is configured to provide distraction of adjacent vertebrae after implantation within a spinal disc cavity through manual rotation of the distractor once implanted. Such implantation is preferably, but not necessarily, through minimally invasive surgical techniques. In one form, the distractor is configured to be rotated 90° to 180° after implantation to provide distraction of adjacent vertebrae. The distractor has a lateral side to lateral side height of a first length and a superior end to inferior end height of a second length that is greater than the first length. This configuration allows the distractor to be inserted into a spinal disc cavity having an opening with a height that is smaller than an end height of the spinal disc cavity after implantation thereof, thereby providing distraction of adjacent vertebrae. The spinal interbody distractor may have one or more cavities, areas, openings and / or bores for spinal fusion material when the distractor is used as a fusion body. The spinal interbody distractor is made from a biocompatible material such as a thermoplastic (e.g. PEEK), a polymer, metal, combination thereof or otherwise, such as desired and / or is appropriate.
Owner:LIFE SPINE INC
Composite metal and bone orthopedic fixation devices
ActiveUS9173692B1Promote healingImprove bio-integrationInternal osteosythesisBone implantFiberOrthopedic devices
Composite orthopedic devices that facilitate spine stabilization, such as: bone screws, rods, plates, interbodies, and corpectomy cages are disclosed. They are designed to provide both strength and load carrying capabilities, while increasing bio-integration of the devices with the surrounding bone tissue. They are constructed of composite layers of allograft and / or autograft bone and a structural material, such as titanium alloy or carbon / graphite fiber composite. Cannulations within the device are loaded with a mixture of stem cells, particles of allograft and / or autograft bone, and bone growth factors, such as BMP-2. The cannulations are connected to the surface of the device via multiple fenestrations that provide pathways to supply the bone / stem cell mixture to the surface, allowing living bone tissue to grow and insure bio-integration. The devices can also have radiofrequency (RF) stimulation implantation within the structure of the implanted device, capable of responding to external RF stimulation of enhanced bone growth.
Owner:STC UNM
Method of using instruments and interbody spinal implants to enhance distraction
ActiveUS8545568B2Add seatsImprove visualizationBone implantJoint implantsSpinal columnPhysical medicine and rehabilitation
A method of using an interbody spinal implant by implanting the spinal implant into a patient in need of the spinal implant. The method includes accessing the disc space of the patient and locating the center of the disc space. The disc space is incised by making a window in the annulus of the disc space for insertion of the spinal implant. The endplates are cleaned of all cartilage and the disc structure, which is encapsulated by the annulus, is removed while avoiding damage to the endplate structure of the vertebrae. Optionally a size-specific rasp is selected and the disc space is cleared of all soft tissue and cartilage. Optionally the disc space is distracted by sequentially expanding it with distractors of progressively increasing heights. A spinal implant having a pre-determined size sufficient to balance frictional fit and elongation of the annulus is selected and seated in the disc space.
Owner:TITAN SPINE
Spine detection method and device, electronic equipment and storage medium
PendingCN112233083AReduce workloadImprove work efficiencyImage enhancementImage analysisCorpus vertebraeRadiology
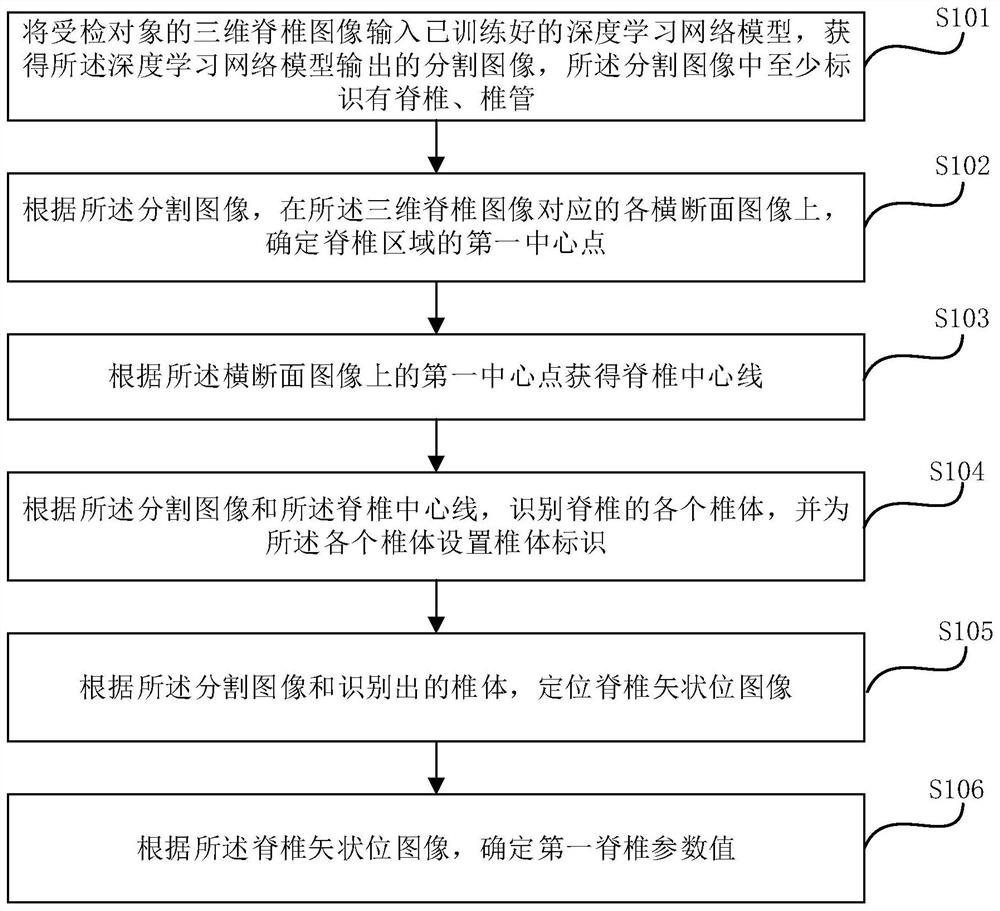
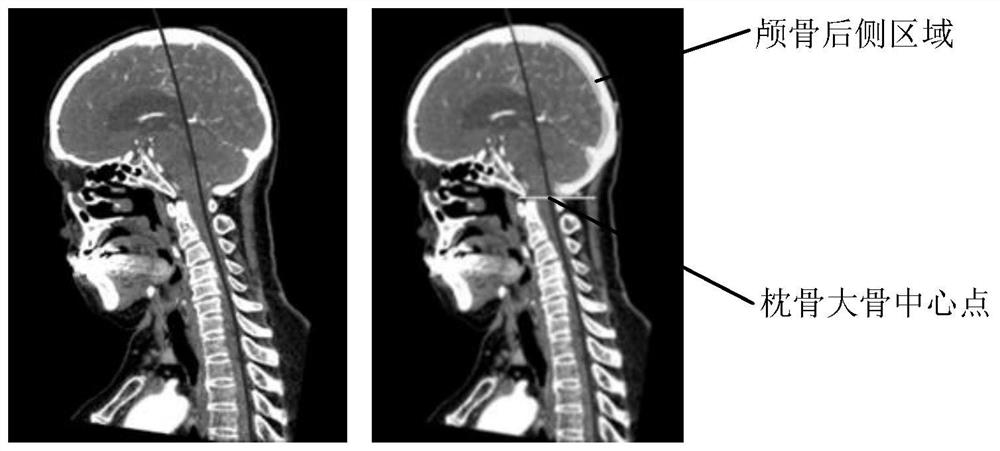
The embodiment of the invention provides a spine detection method and device, electronic equipment and a storage medium. According to the embodiment of the invention, the method comprises the steps: inputting a three-dimensional spine image into a deep learning network model, obtaining a segmented image outputted by the deep learning network model, and determining a first central point of a spineregion on each cross section image corresponding to the three-dimensional spine image according to the segmented image, obtaining a spine center line according to a first center point on the cross section image, identifying each spine body of the spine according to the segmentation image and the spine center line, setting a spine body identifier for each spine body, positioning a spine sagittal image according to the segmentation image and the identified spine body, and determining a first spinal parameter value according to the spine sagittal image. According to the method and device, the spine parameters can be measured in a full-automatic mode, and the working efficiency is improved.
Owner:沈阳先进医疗设备技术孵化中心有限公司
Expandable interbody spacer
An expandable interbody spacer for the spine is provided. The interbody spacer includes a housing, upper and lower endplates, an anterior actuator, a posterior actuator, an anterior drive screw, and a posterior drive screw. The anterior and posterior drive screws are independently or simultaneously rotated with respect to each other by a driver to wedge one or more of the anterior and posterior actuators between the endplates moving them into parallel and / or angular expansion.
Owner:NEUROSTRUCTURES
Interbody spacer
Owner:NEUROSTRUCTURES
Expandable interbody spacer
ActiveUS20220183854A1Increase and decrease heightIncrease or decrease heightJoint implantsSpinal implantsSpinal columnCorpus vertebrae
An expandable interbody spacer for the spine is provided. The spacer includes upper and lower endplates simultaneously movable with respect to a housing along an axis traverse to a longitudinal axis to increase or decrease the height of the spacer selectably along both an anterior side and a posterior side for uniform expansion / contraction of the endplates or only along the anterior side for angular expansion / contraction of the endplates. A spacer deployment instrument is provided that is selectable to effect uniform or angular expansion / contraction. When uniform expansion / contraction is selected a gear on an anterior rod is engaged with a gear on a posterior rod to simultaneously rotate both rods in opposite directions. Opposite threads on actuators of the spacer effect translation of the actuators in the same direction along the longitudinal axis.
Owner:NEUROSTRUCTURES
Expandable interbody spacer
An expandable interbody spacer for the spine is provided. The interbody spacer includes a housing, upper and lower endplates, an anterior actuator, a posterior actuator, an anterior drive screw, and a posterior drive screw. The anterior and posterior drive screws are independently or simultaneously rotated with respect to each other by a driver to wedge one or more of the anterior and posterior actuators between the endplates moving them into parallel and / or angular expansion.
Owner:NEUROSTRUCTURES
Fixed observation device for animal spinal nerve living body imaging and use method thereof
PendingCN113413231AReduce pollutionEasy to observeDiagnostic recording/measuringSensorsSpinal columnCorpus vertebrae
Owner:SHENZHEN PEOPLES HOSPITAL
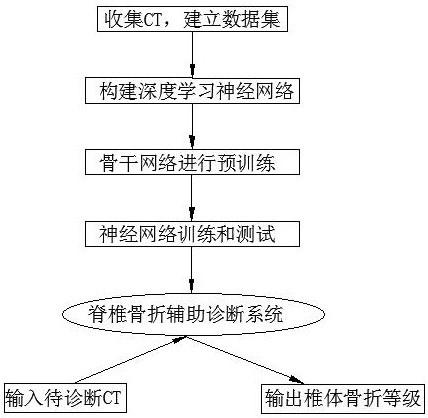
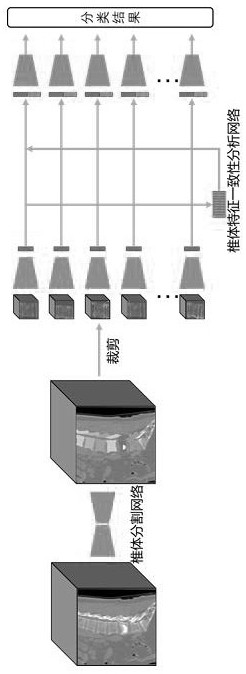
Computed tomography spinal fracture auxiliary diagnosis system
InactiveCN114049955AReduce learning difficultyImprove performanceImage enhancementImage analysisSpinal columnData set
Owner:MEI HOSPITAL UNIV OF CHINESE ACAD OF SCI
Expandable interbody spacer
An expandable interbody spacer for the spine is provided. The interbody spacer includes a housing, upper and lower endplates, an anterior actuator, a posterior actuator, an anterior drive screw, and a posterior drive screw. The anterior and posterior drive screws are independently or simultaneously rotated with respect to each other by a driver to wedge one or more of the anterior and posterior actuators between the endplates moving them into parallel and / or angular expansion.
Owner:NEUROSTRUCTURES
Intervertebral inflatable distractors employing thecal sac retractors, and related systems and methods
InactiveUS20200054314A1Efficient receptionEffective installationInternal osteosythesisDiagnosticsSpinal columnCorpus vertebrae
Intervertebral inflatable distractors employing thecal sac retractors, and related systems and methods are disclosed. Adjacent vertebrae are spaced apart or distracted to prepare for the installation of intervertebral cages during spine surgery. An intervertebral inflatable distractor may include a inflatable portion having first and second inflation modes. In the lower-volume first mode, the inflatable portion may be efficiently received within the intervertebral space, then the higher-volume second mode may be used to abut against vertebrae endplates to urge them apart and thereby provide space to receive the cages. A thecal sac retractor of the intervertebral inflatable distractor may also be used to abut against the thecal sac to provide space for larger cages. In this manner, an interbody cage may be efficiently installed between vertebrae while minimizing injury to the vertebrae and the thecal sac.
Owner:DESIGN ENTERPRISES LLC
Pharmaceutical formulation for use in spinal fusion
ActiveUS10589001B2Peptide/protein ingredientsPharmaceutical delivery mechanismSpinal columnCorpus vertebrae
A pharmaceutical formulation for use in a spinal fusion method, comprising a composition for forming a matrix, a kit comprising the composition, a pharmaceutical product obtainable from the pharmaceutical formulation, and an interbody spinal fusion cage containing the pharmaceutical formulation or the pharmaceutical product are described herein. The composition comprises at least a first matrix material precursor component and a second matrix material precursor component that are able to crosslink to form the matrix under appropriate conditions, a bioactive factor that is biologically active for stimulating bone formation between two vertebrae and for effecting or supporting spinal fusion. The bioactive factor is PTH, optionally a PTH fusion peptide. The bioactive factor is releasably incorporated in the matrix upon crosslinking of the matrix material precursor components.
Owner:KUROS BIOSURGERY AG
Medicinal formula for treating spinal degenerative diseases
InactiveCN107184773ARelieve the strained stateReduce oppressionMuscular disorderSkeletal disorderDrosera peltataMicrocirculation
The invention discloses a medicinal formula for treating spinal degenerative diseases. The medicinal formula comprises the following traditional Chinese medicine components in parts by mass: 18-22 parts of cortex zanthoxyli, 18-22 parts of drosera peltata, 17-20 parts of sambucus williamsii, 24-27 parts of reineckia carnea, 13-17 parts of homalomena occulta, 18-22 parts of fructus psoraleae, 18-22 parts of talinum crassifolium and 18-22 parts of curculigo orchioides. The medicinal formula disclosed by the invention is capable of expelling wind, coldness and dampness, activating blood and removing stasis, regulation of qi and blood, balancing yin and yang, warming tendon and nourishing bone as well as supporting the healthy energy. By adopting the medicinal formula, the partial muscle strain state can be alleviated, spasmodic muscle can be recovered into normal tissue softness, stress of centrums to intervertebral discs is reduced, and besides spine microcirculation is effectively improved, microcirculation blood supply of tissue such as spine intervertebral discs, ligaments and cartilage can be recovered, and spinal degenerative diseases can be effectively retarded.
Owner:梁承志
Spinal fusion apparatus
ActiveUS10945855B2Maximizes anterior heightMinimize heightJoint implantsSpinal implantsSpinal columnCorpus vertebrae
An interbody spinal fusion cage for posterior interbody fusion procedures includes a superior member and an inferior member connected to each other via a joint. The joint allows the interbody spinal fusion cage to achieve lordosis even if implanted non-orthogonal to the sagittal plane. For example, the joint can be a hinge oriented non-normal to a longitudinal axis of the interbody spinal fusion cage, a polyaxial ball joint, and / or a universal joint. Complementary locking mechanisms, such as locking teeth or a ratchet-and-pawl arrangement, can be provided near the posterior ends of the superior and inferior members in order to prohibit the posterior ends of the superior and inferior members from separating from each other in situ. Bone holes can be provided in the superior and inferior members.
Owner:KRAEMER PAUL E
Interbody cage device and methods of use
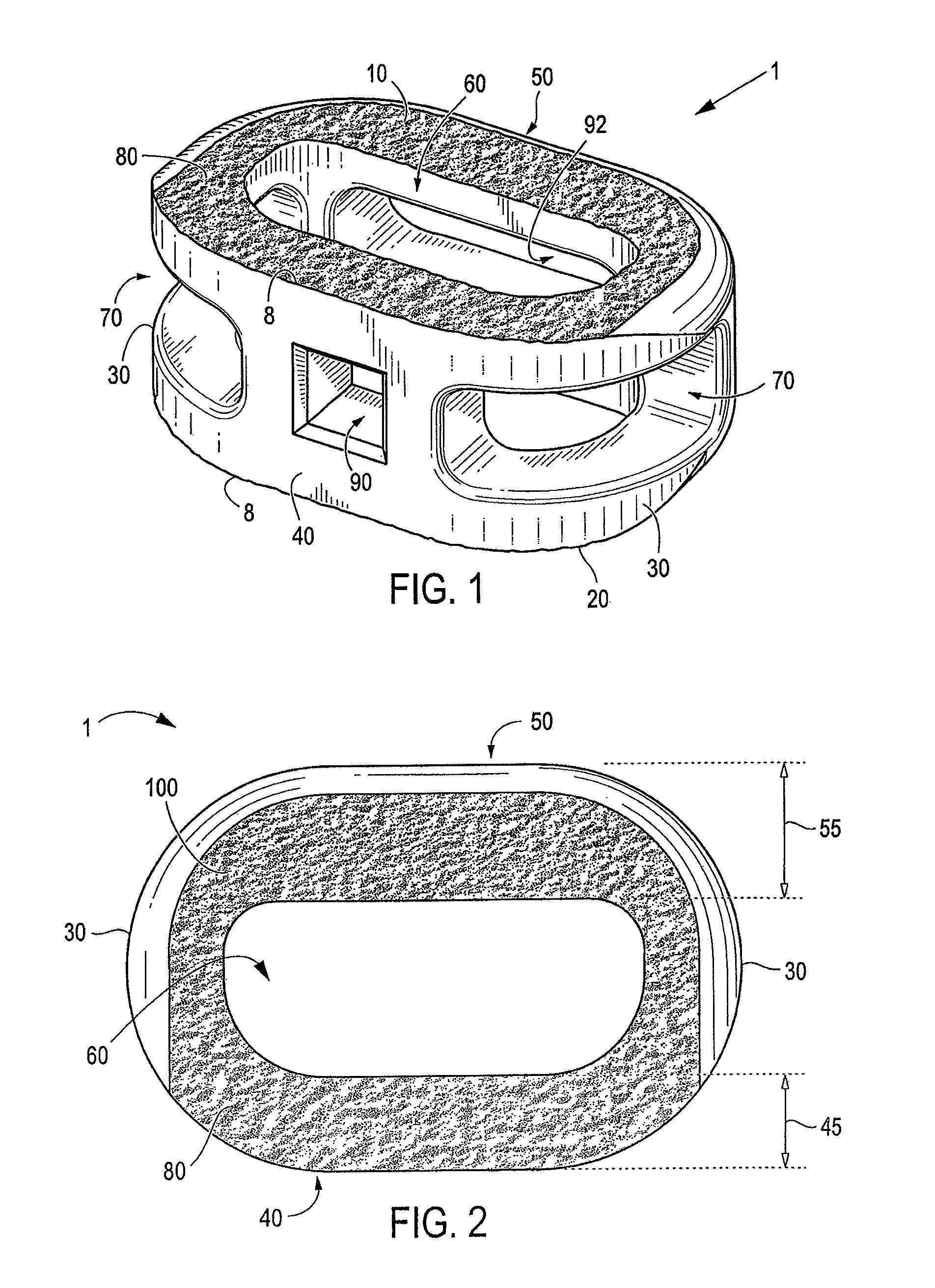
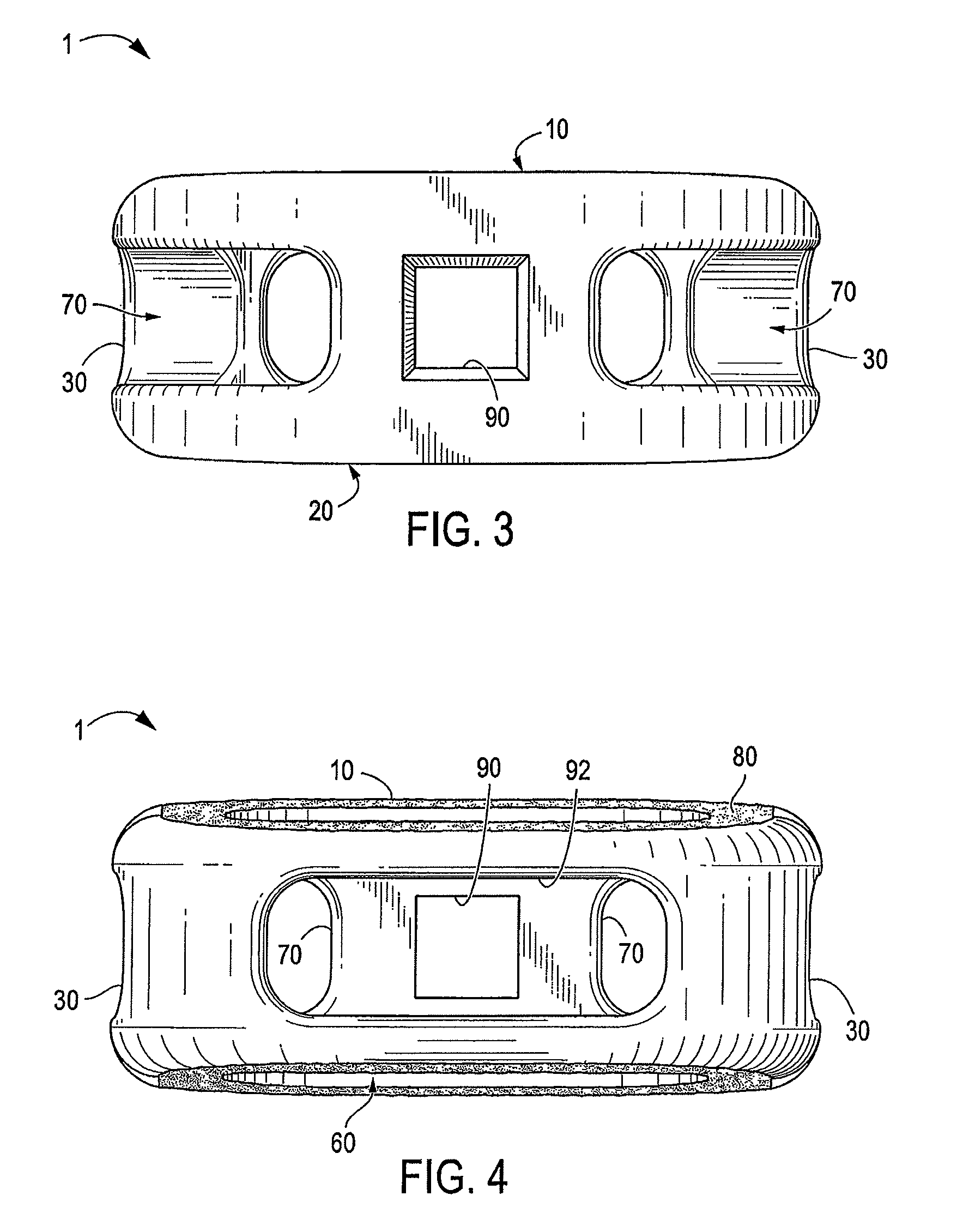
A spinal interbody fusion device for use in a plurality of surgical approaches includes a cage, a top end, a bottom end, and at least a first side representing the width of the cage and at least a second side representing a length of the cage. The cage includes fixation holes and inserter holes, with each fixation hole being configured for accepting a screw or anchor and each inserter hole being accessible for one or more surgical approaches for performing a spinal fusion. Also included are methods for selecting a size of an intervertebral implant and methods of surgically approaching a spine of a patient for spinal surgical procedures.
Owner:NOVAPPROACH SPINE
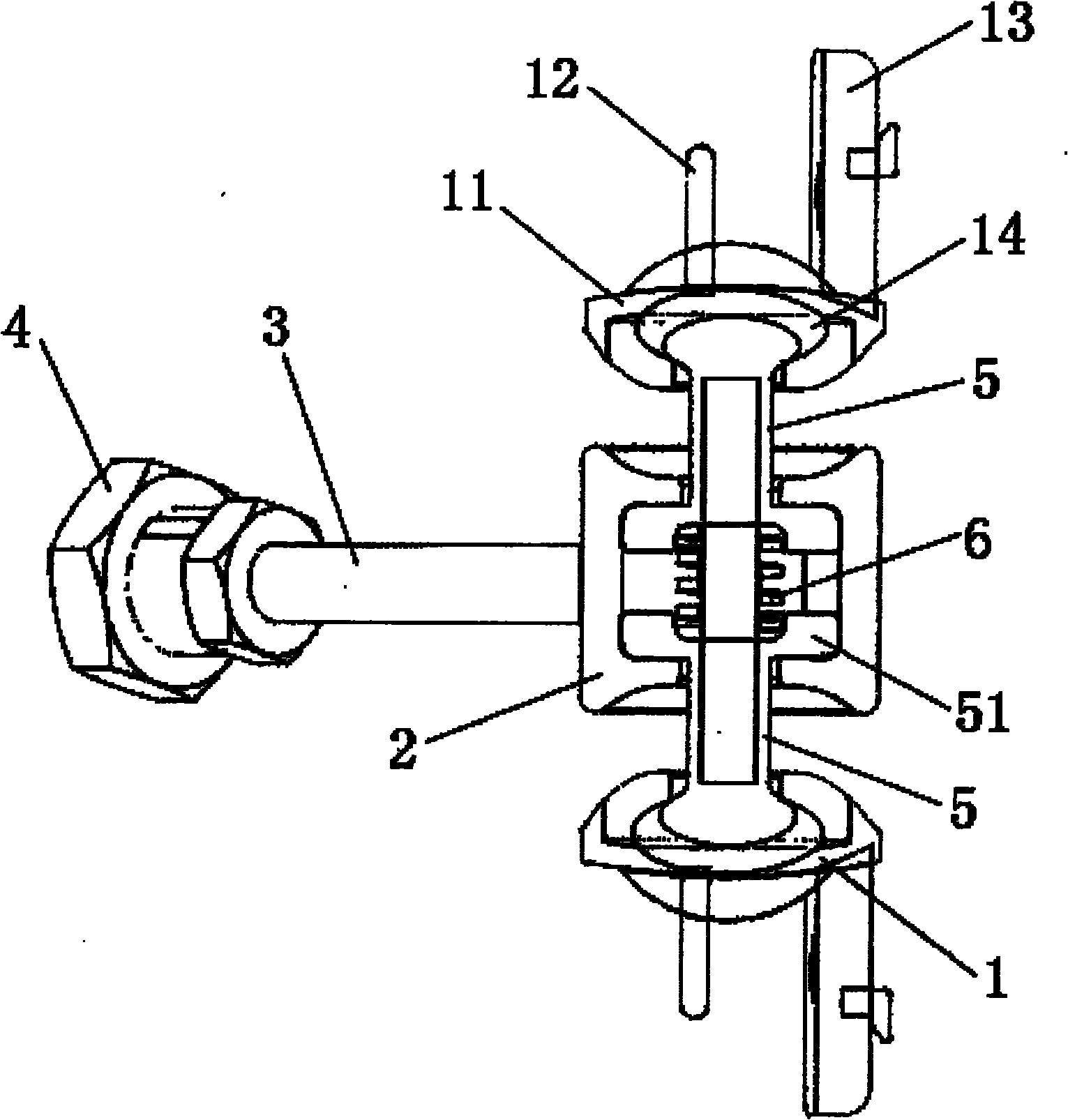
Subtotal ablation of anterior cervical vertebrae continuity multiple corpus vertebraes physiological inner fixing device
InactiveCN101095624BPlay a supporting roleGuaranteed normal movementInternal osteosythesisSpinal implantsCorpus vertebraeMedicine
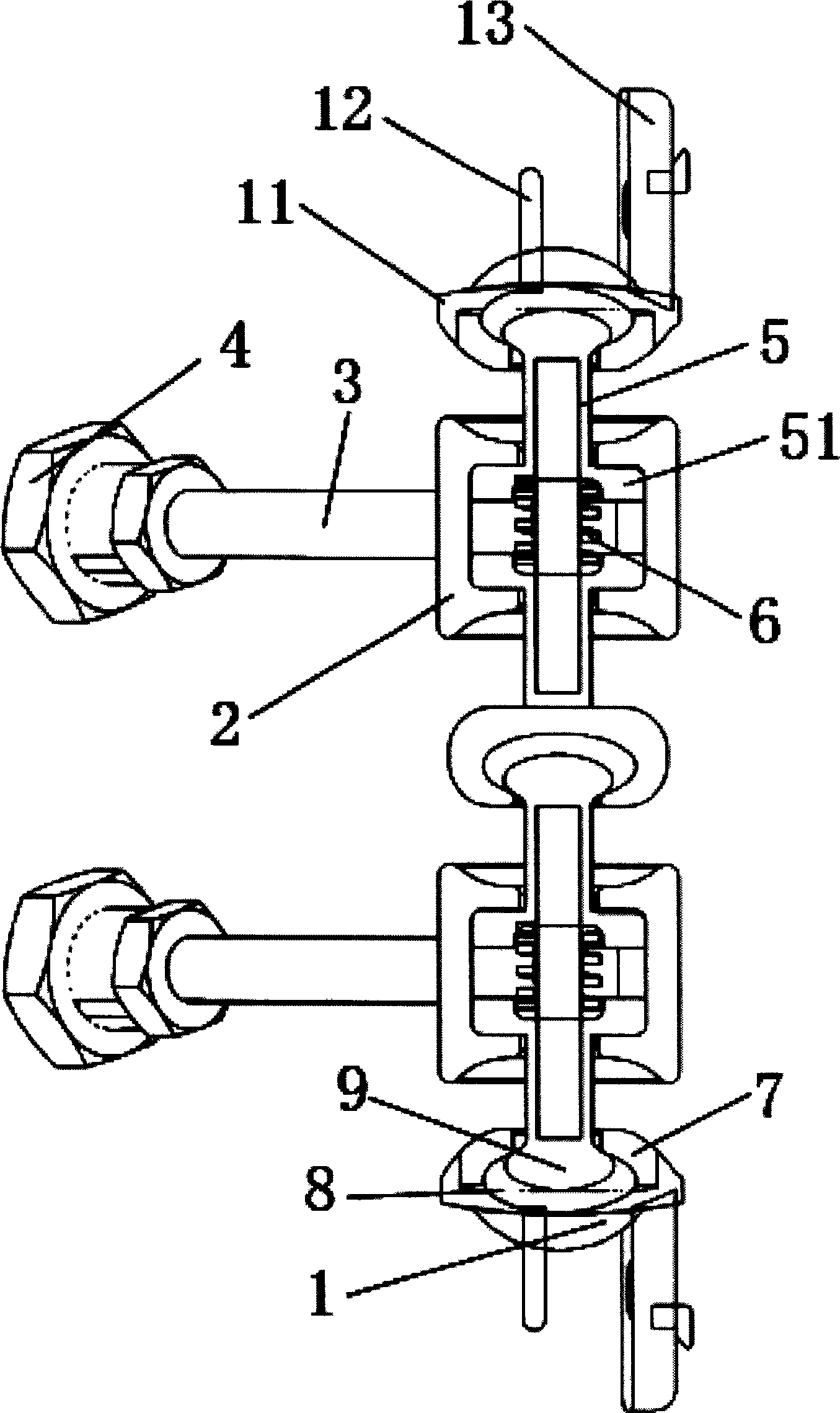
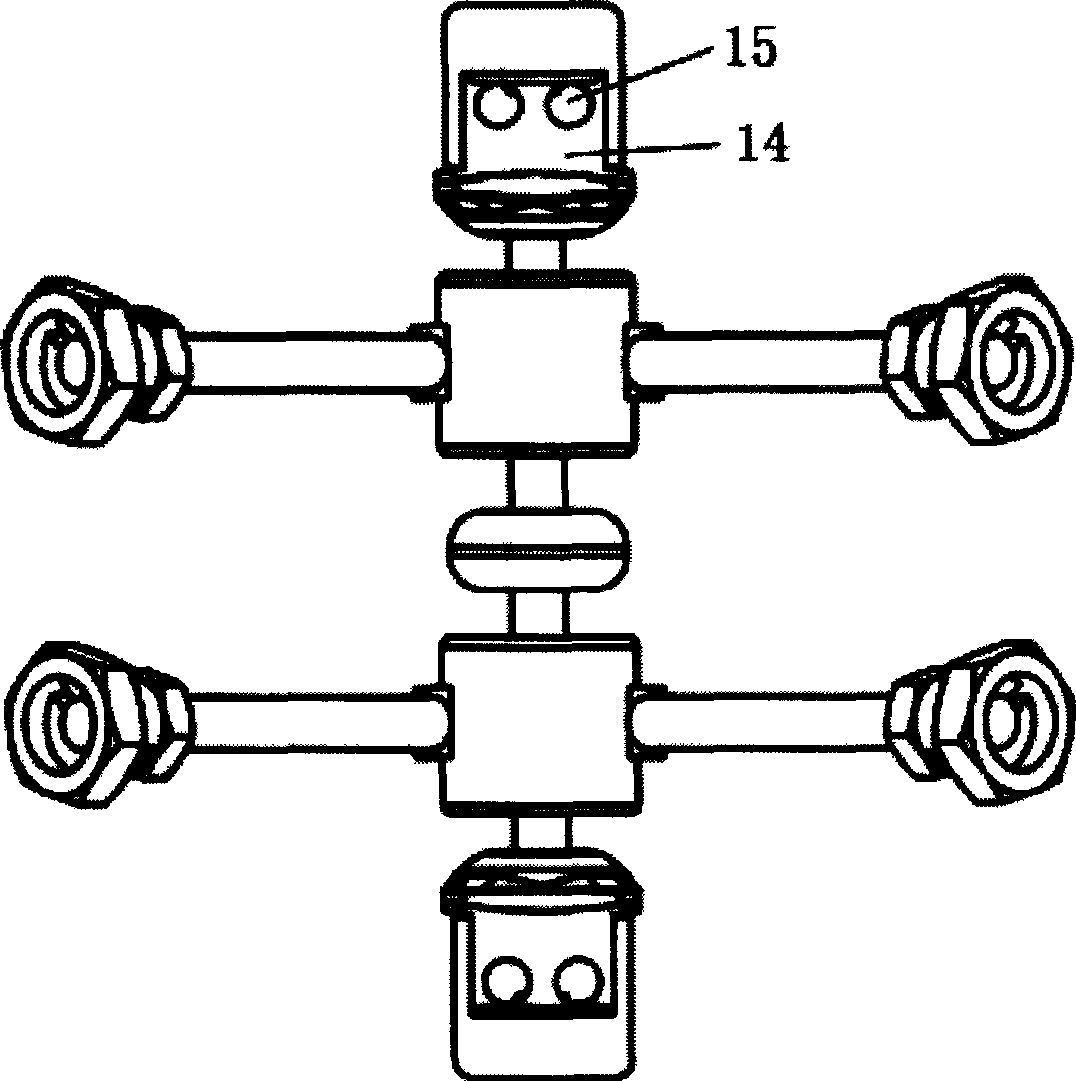
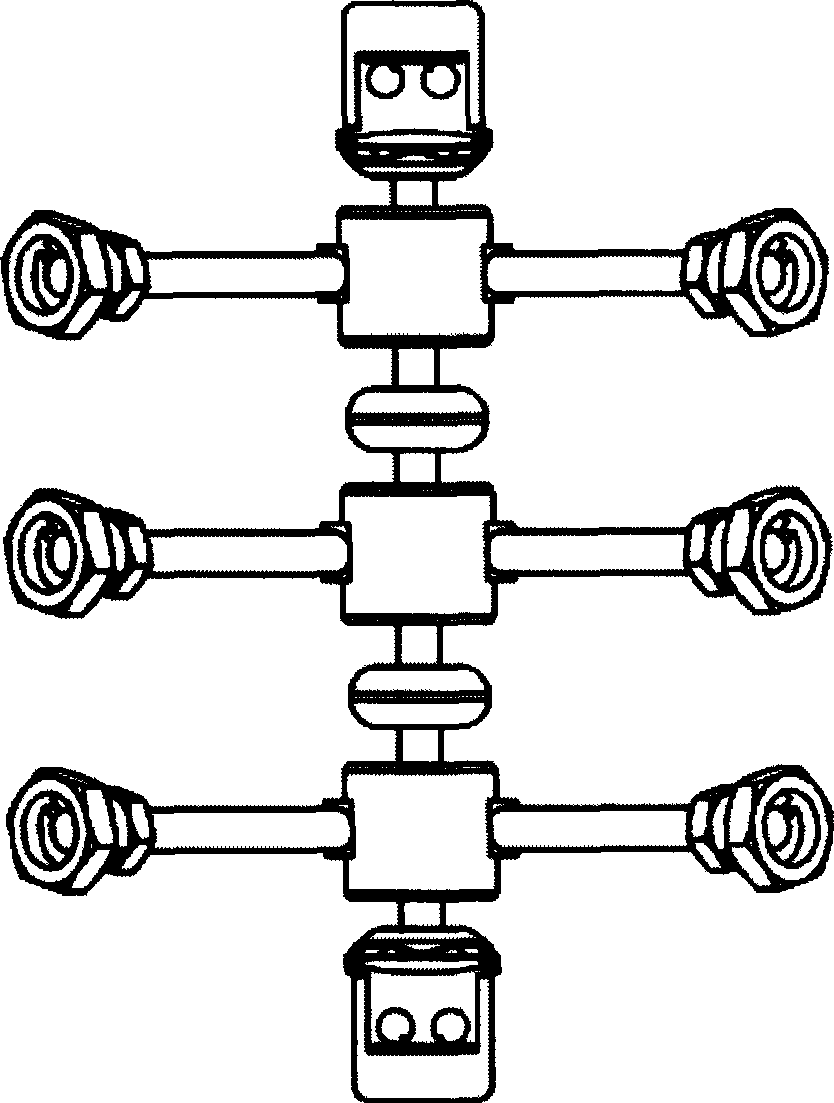
The invention discloses an anterior cervical subtotal subcorpectomy physiological internal fixation, which comprises two retaining brackets on the two ends and a plurality of intermittent fixation units. Each intermittent fixation unit comprises one adjustable mechanism, the two ends of adjustable mechanism are connected with one knuckle arm respectively, the outer part of adjustable device is equipped with one lantern ring, the lantern ring is connected with two anterior pedicle of vertebral arch screws, the end of rear pedicle of vertebral arch screws, the end of knuckle arm is equipped with bulb or ball socket, the two near intermittent units are connected with each other through bulb and ball socket to form movable fit, the intermittent fixation unit is also connected with retaining bracket through bulb and ball socket to form movable fit. The fixation device is suitable for fiaxtoin for a plurality of continuous subtotal subcorpectomys, and maintains the up and down motion between near vertebra, reduces compensation motion for near vertebra and prevents vertebra degeneration.
Owner:李郁松
125I particle-containing kit and application thereof
The invention discloses a 125I particle-containing kit and application thereof. The kit comprises 125I particles, bone cement polymethyl methacrylate and a contrast agent. A Banna minipig test animal model implanted by percutaneous vertebroplasty is established. By the kit, a relationship between irradiation dose and time of radiation myelitis caused by short-distance irradiation of the 125I particles is discussed. By the kit, spinal metastatic tumor patients are treated and researched; and after implantation by the percutaneous vertebroplasty, curative effect is improved and postoperative complications are reduced compared with those by the pure percutaneous vertebroplasty.
Owner:杨祚璋
Interbody cage device and methods of use
A spinal interbody fusion device for use in a plurality of surgical approaches includes a cage, a top end, a bottom end, and at least a first side representing the width of the cage and at least a second side representing a length of the cage. The cage includes fixation holes and inserter holes, with each fixation hole being configured for accepting a screw or anchor and each inserter hole being accessible for one or more surgical approaches for performing a spinal fusion. Also included are methods for selecting a size of an intervertebral implant and methods of surgically approaching a spine of a patient for spinal surgical procedures.
Owner:NOVAPPROACH SPINE
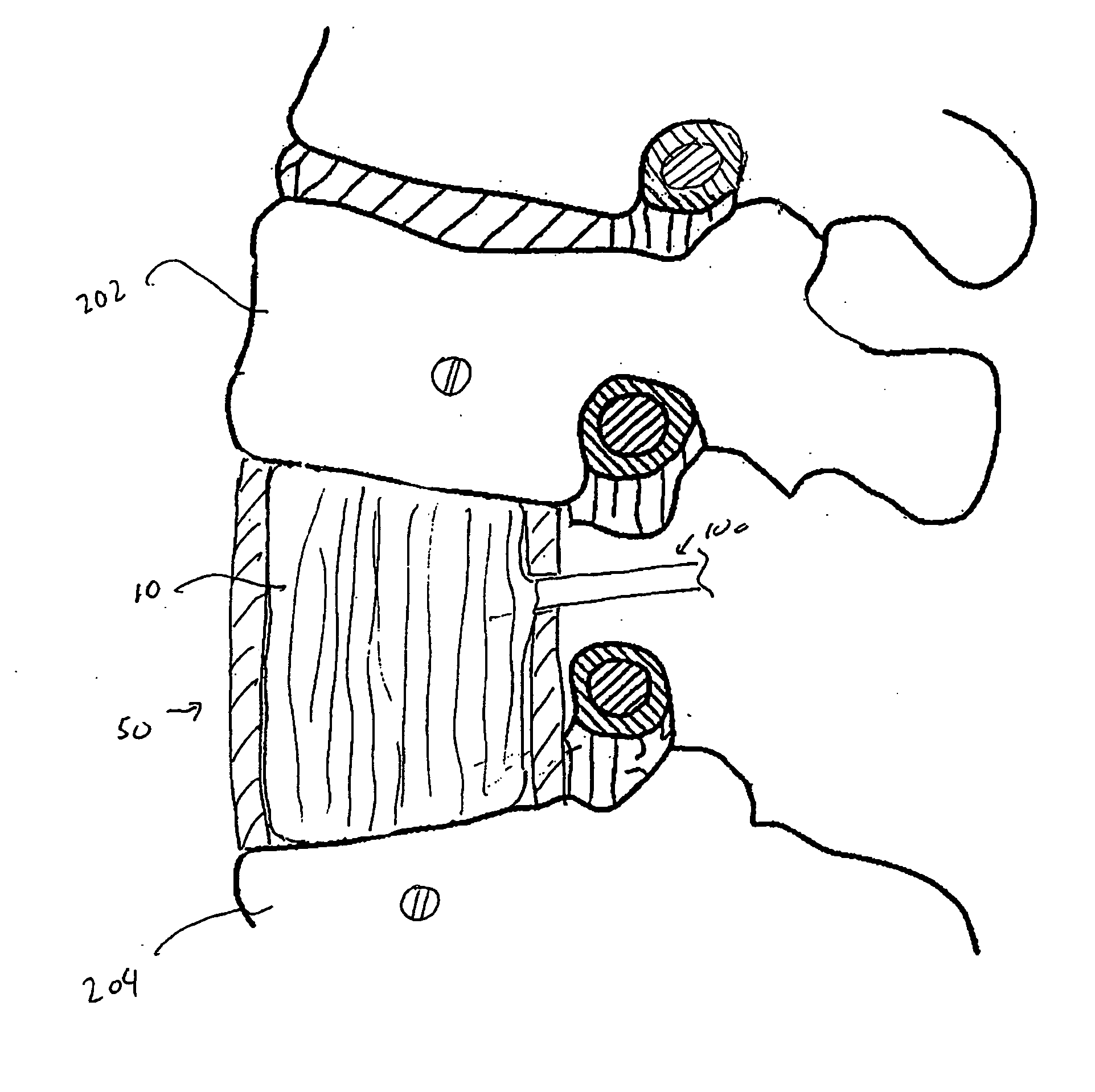
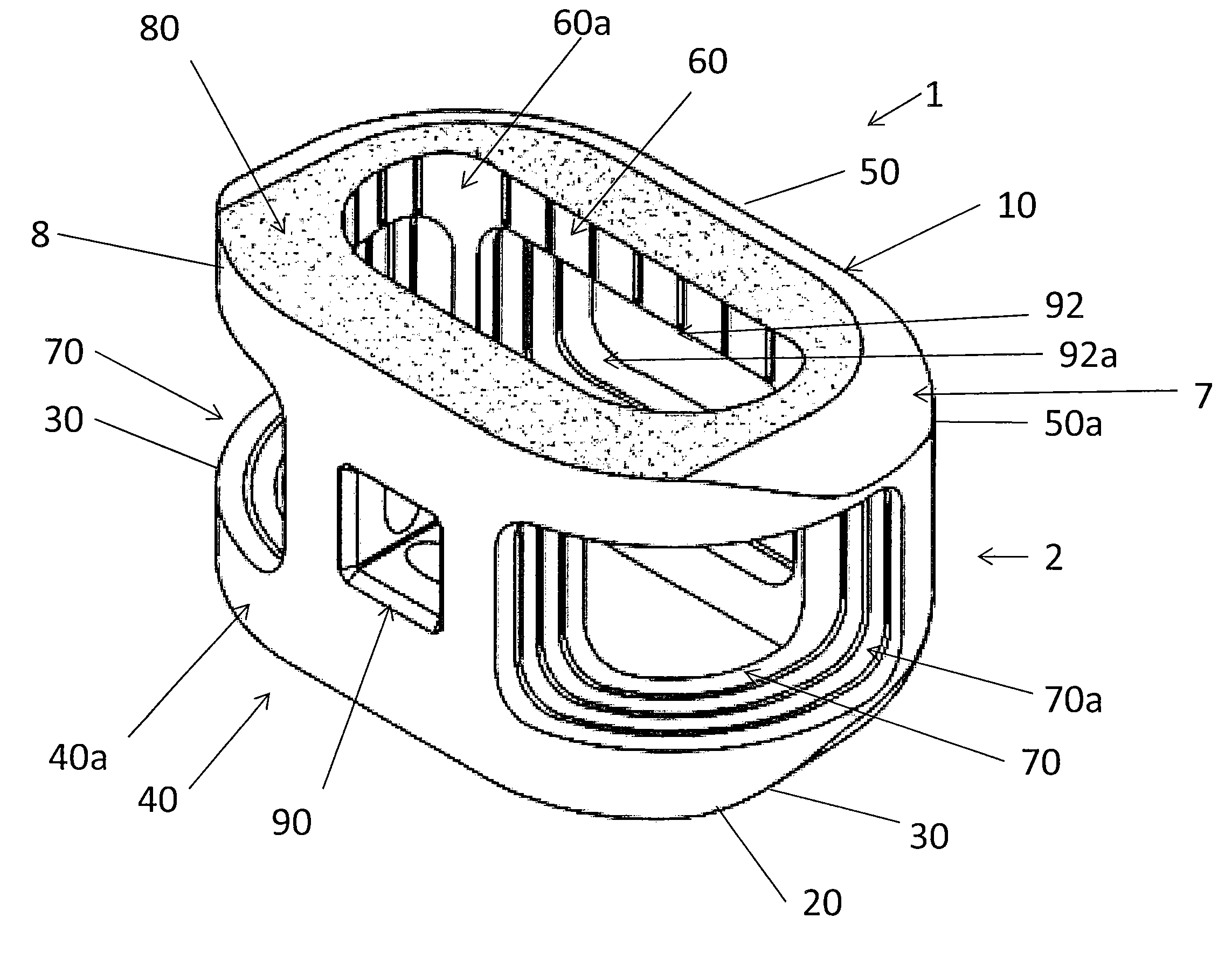
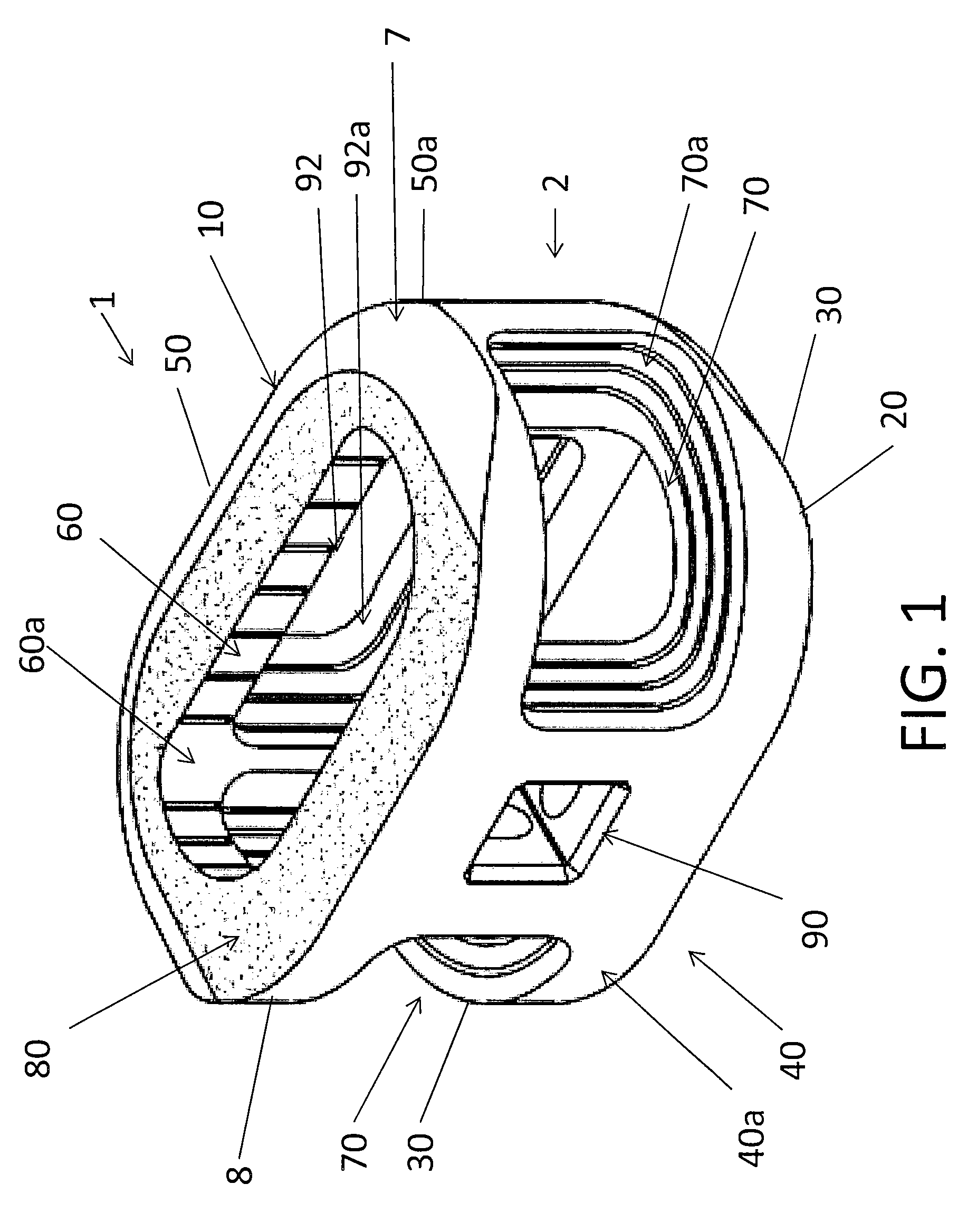
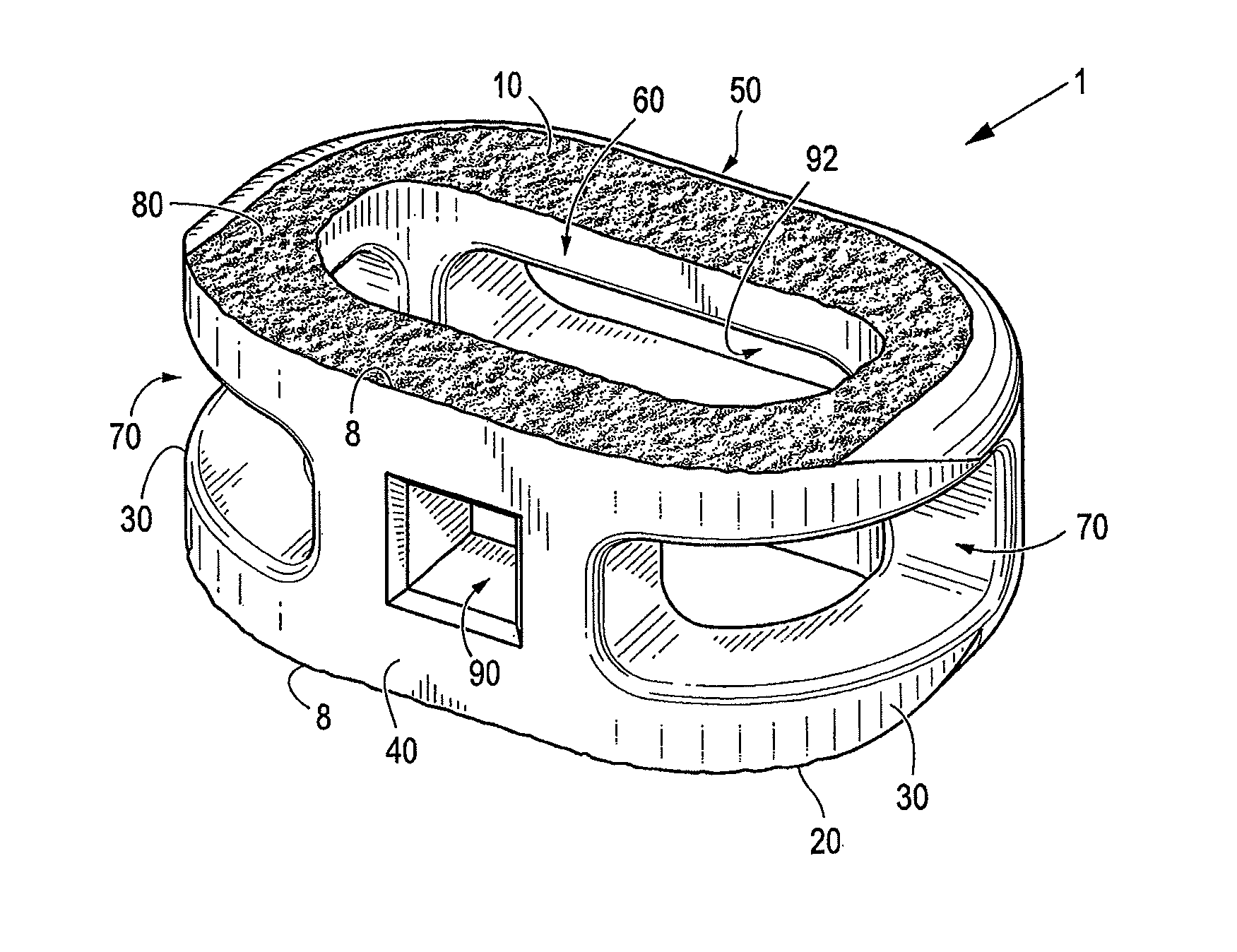
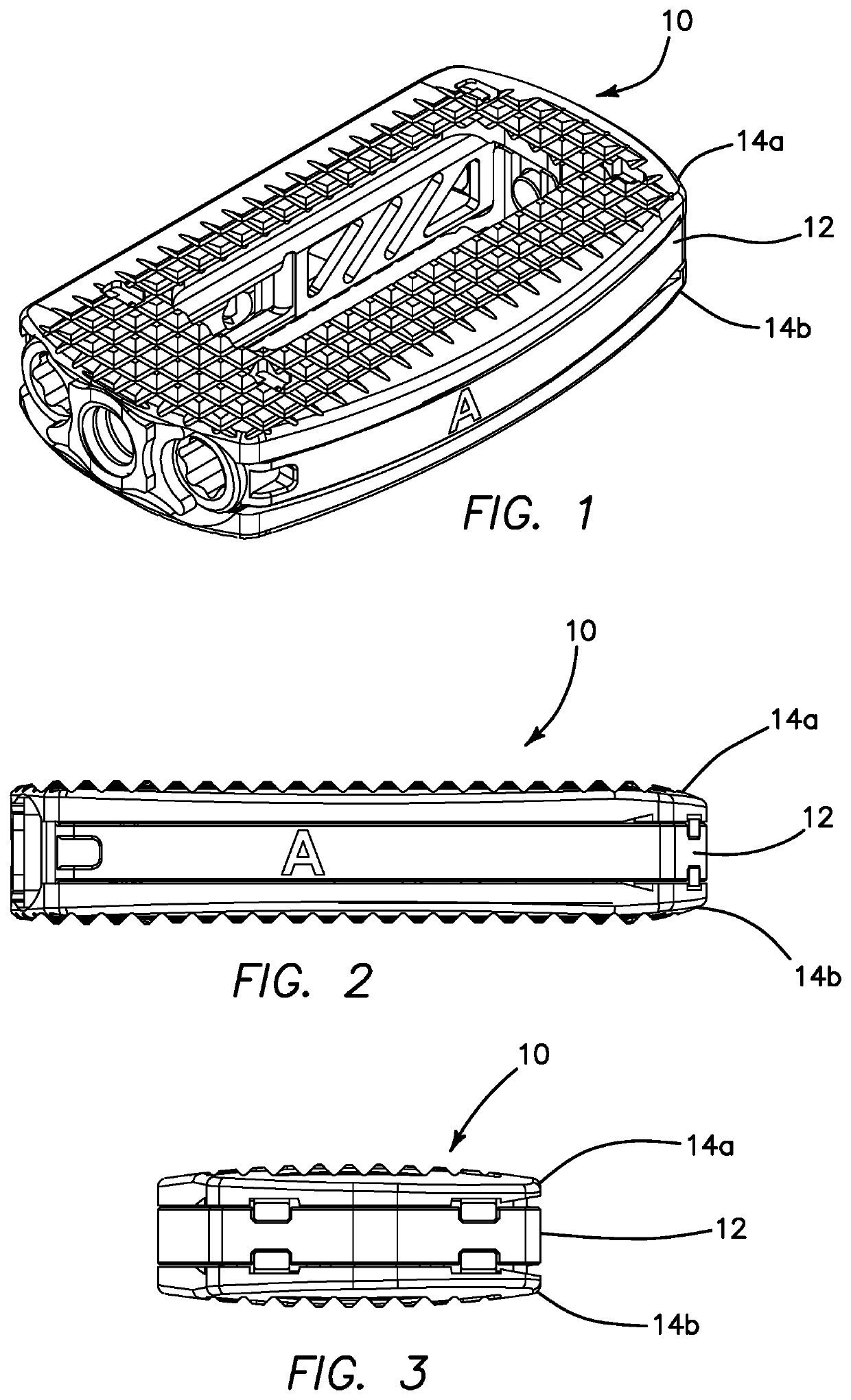
Spinal implant with surface protrusions
The invention relates to an intervertebral spacer for spinal surgery. The intervertebral spacer has one or more surfaces with a unique surface pattern. The interbody spacer is preferably designed for use in spinal fusion procedures in which the portion of the affected disc is removed from between two adjacent vertebrae and replaced with an interbody spacer that provides segment stability, which can correct deformity and allow bone to grow between the two vertebrae, and gaps caused by intervertebral disc resection are bridged. The intervertebral spacer has one or more unique surfaces intended to facilitate bone growth and attachment. The unique surface includes one or more surface protrusions, generally referred to as a pattern or matrix of surface protrusions, which may be arranged to form unique patterns and structures.
Owner:BEACON BIOMEDICAL LLC
Spinal plate selection and positioning system
A spinal plate selection and positioning system is provided for use in a spinal fusion procedure. The system may comprise an elongated guide member that is removably securable to an interbody cage by a holding rod. An interbody plate may be aligned and positioned above the interbody cage installed in a disc space. A drill guide may also be aligned and positioned above the interbody plate. The drill guide may be utilized to drill pilot holes in the vertebrae defining the disc space. Fasteners to secure the interbody plate may also by installed using the drill guide. The use of the guide member ensures that the interbody plate is properly aligned and positioned with respect to the interbody cage.
Owner:RETROSPINE
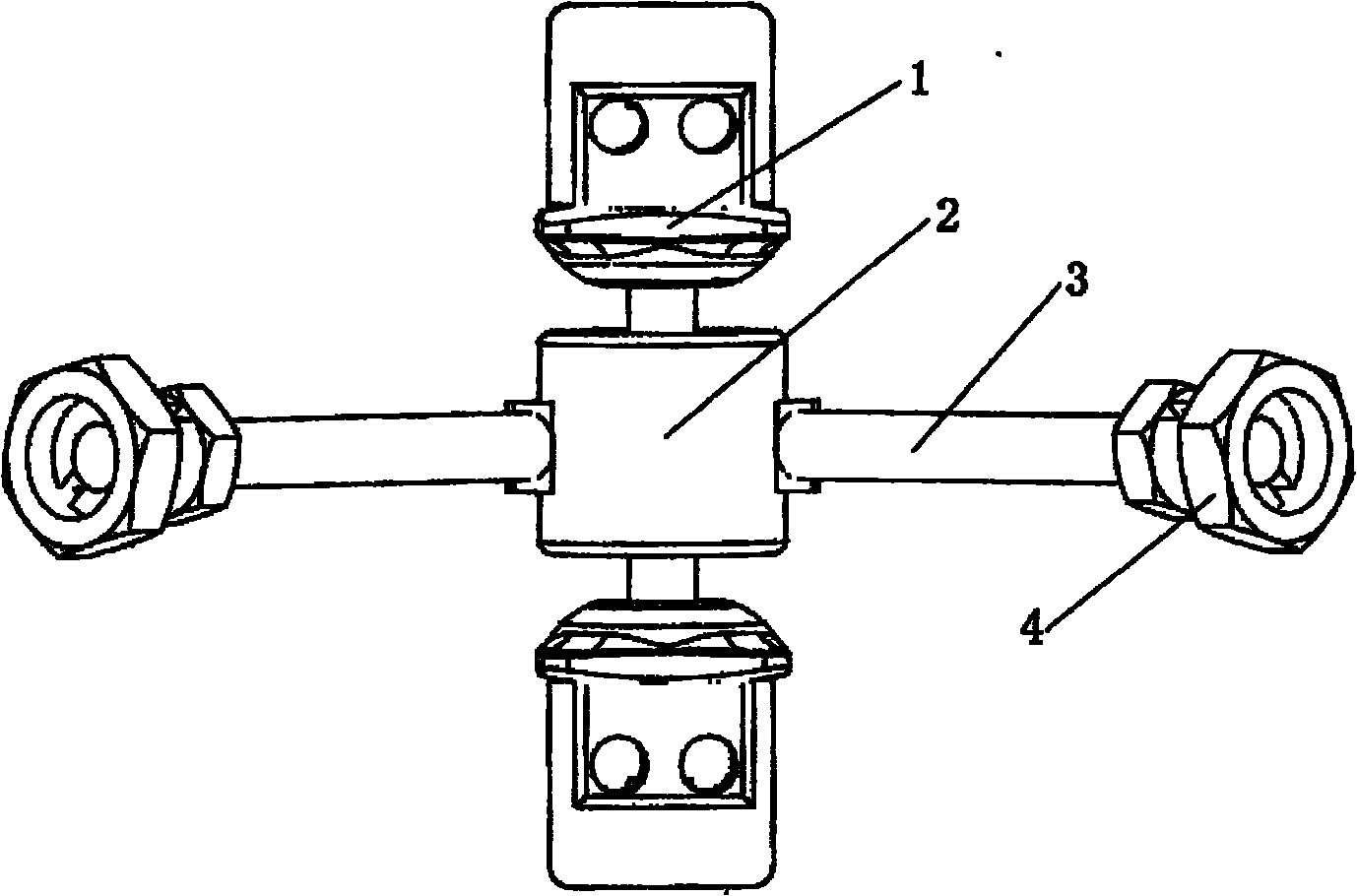
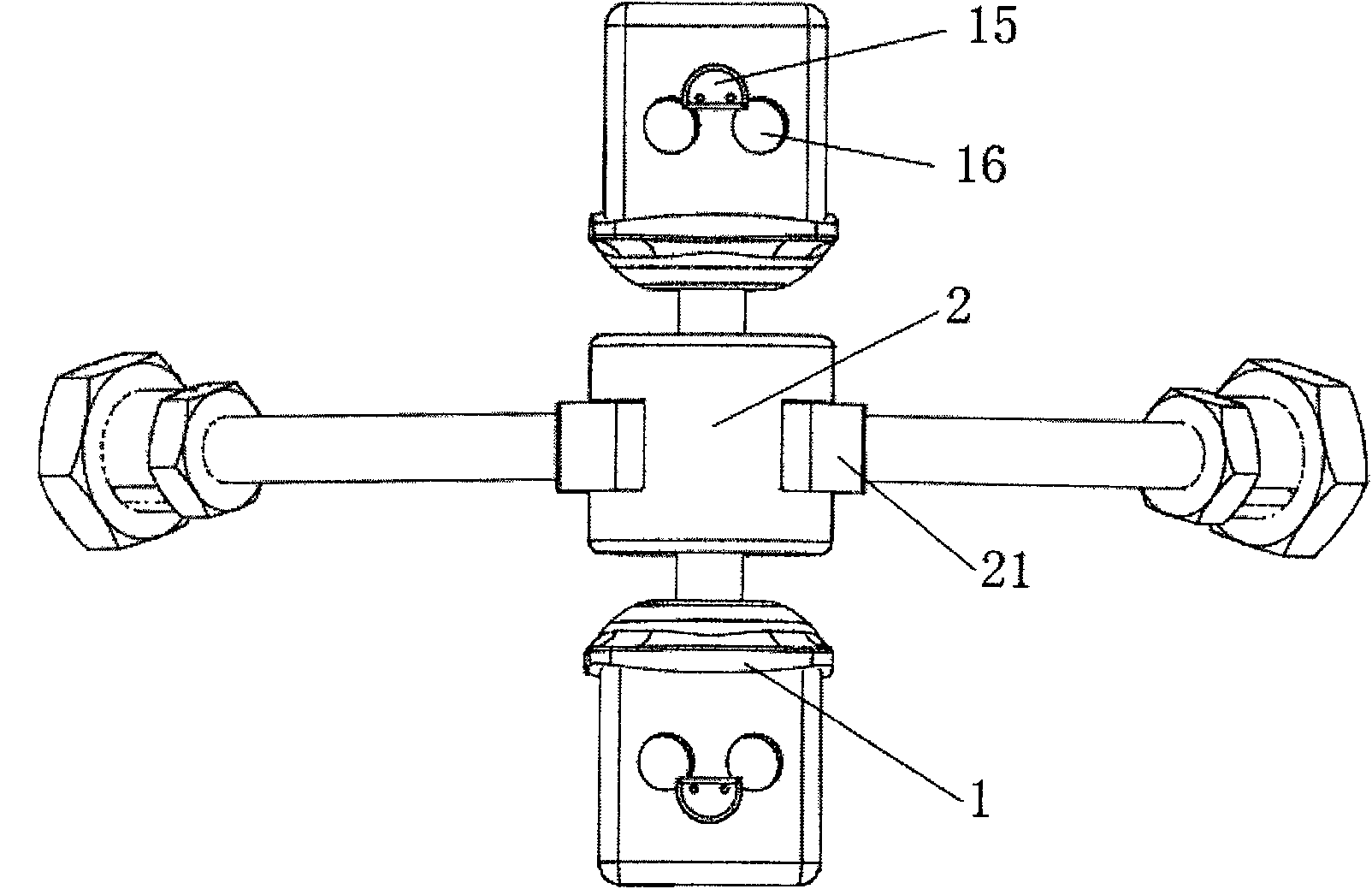
Anterior cervical vertebrae single corpus vertebrae Subtotal ablation physiological inner fixing device
InactiveCN101095626BPlay a supporting roleGuaranteed normal movementInternal osteosythesisSpinal implantsCorpus vertebraeMedicine
The invention discloses an anterior cervical subtotal subcorpectomy physiological fixation device, which comprises two retaining brackets connected with cervical vertebra, the bottom of retaining bracket is equipped with ball socket; it also comprises one adjustable device, the two ends of adjustable device are connected with bulb pole respectively, the end of bulb pole is provided with ball socket moveable junction, the outer part of adjustable device is equipped with lantern ring, which is connected with two anterior pedicle of vertebral arch screws, the end part of pedicle of vertebral arch screws is provided with rear pedicle of vertebral arch screws. The fixation device can be fixed subtotal subcorpectomy vertebra through pedicle of vertebral arch screw, the retaining brackets can befixed with vertebral on the upper and lower part, which ensures the flexion and extension, rotary motion and axial motion between subtotal subcorpectomy vertebra and near vertebral, and thus reduces compensation motion for near vertebra and prevents vertebra degeneration.
Owner:李郁松
Interbody cage device and methods of use
A spinal interbody fusion device for use in a plurality of surgical approaches includes a cage, a top end, a bottom end, and at least a first side representing the width of the cage and at least a second side representing a length of the cage. The cage includes fixation holes and inserter holes, with each fixation hole being configured for accepting a screw or anchor and each inserter hole being accessible for one or more surgical approaches for performing a spinal fusion. Also included are methods for selecting a size of an intervertebral implant and methods of surgically approaching a spine of a patient for spinal surgical procedures.
Owner:NOVAPPROACH SPINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com