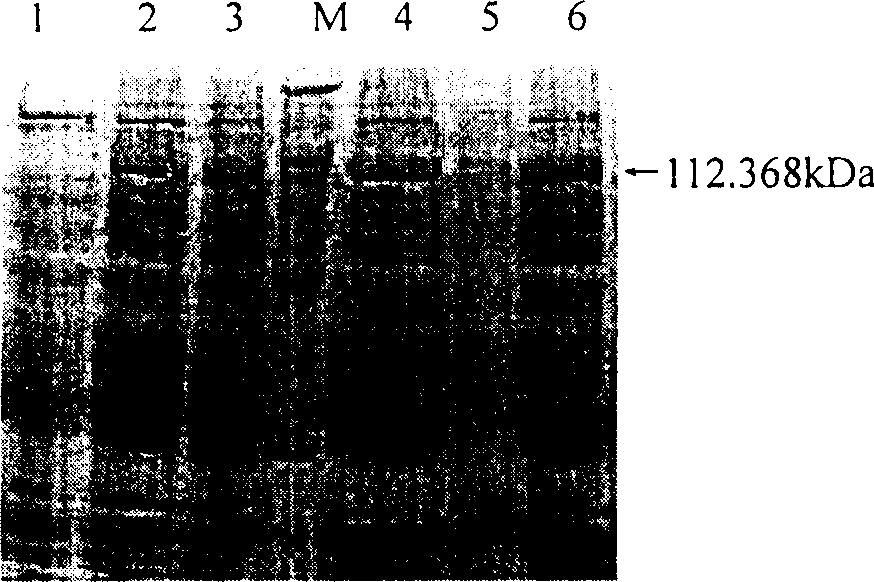
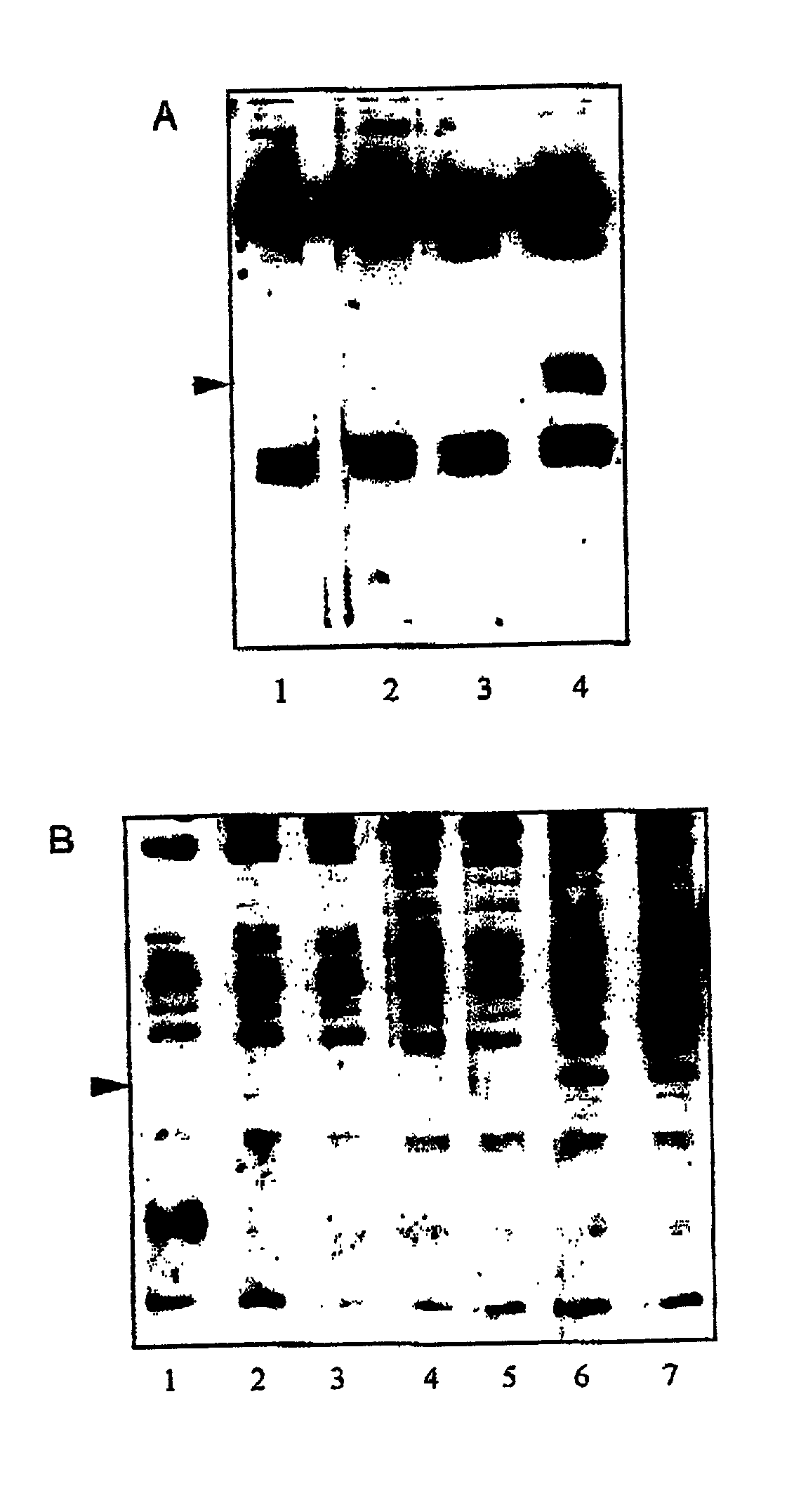
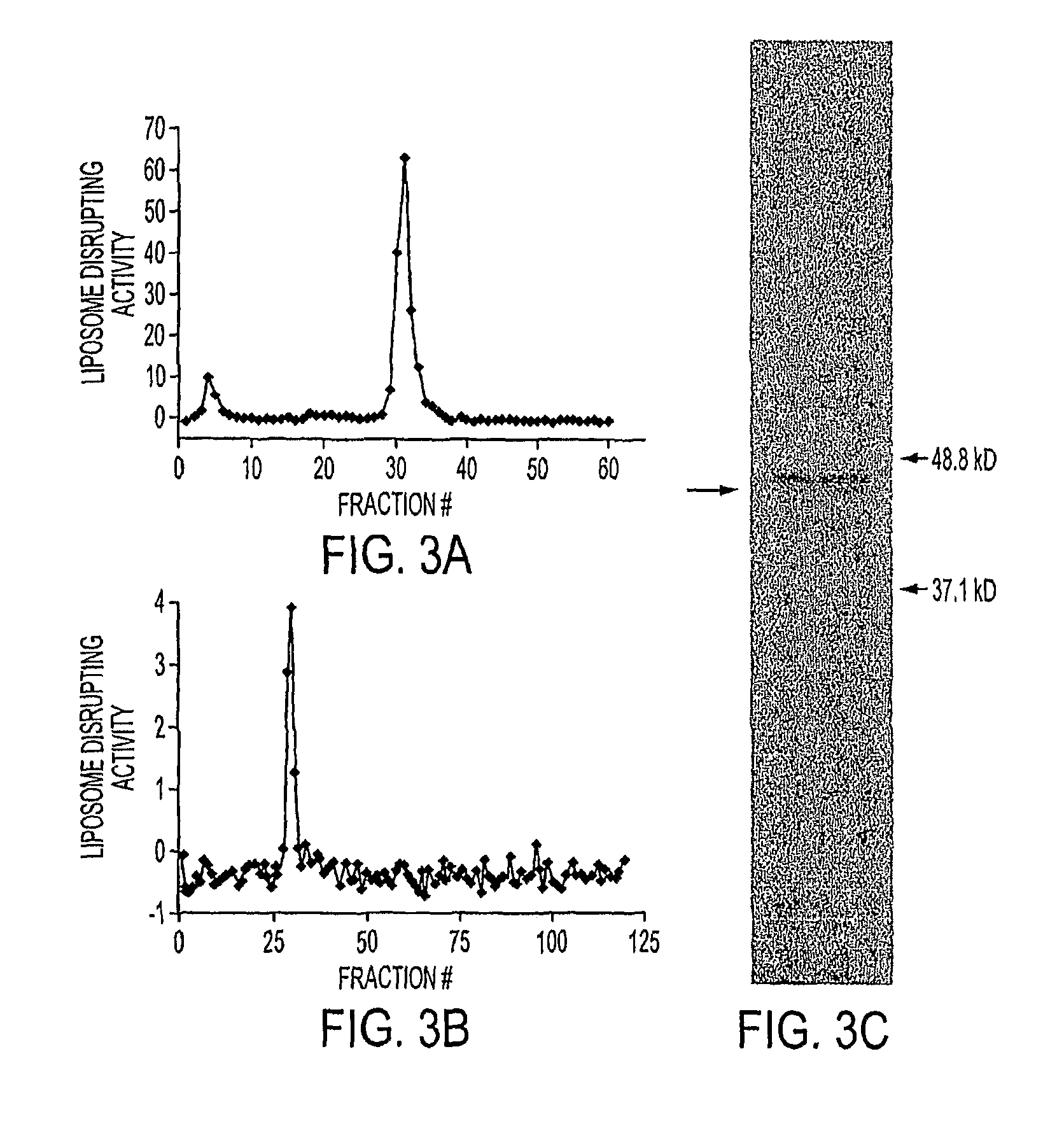
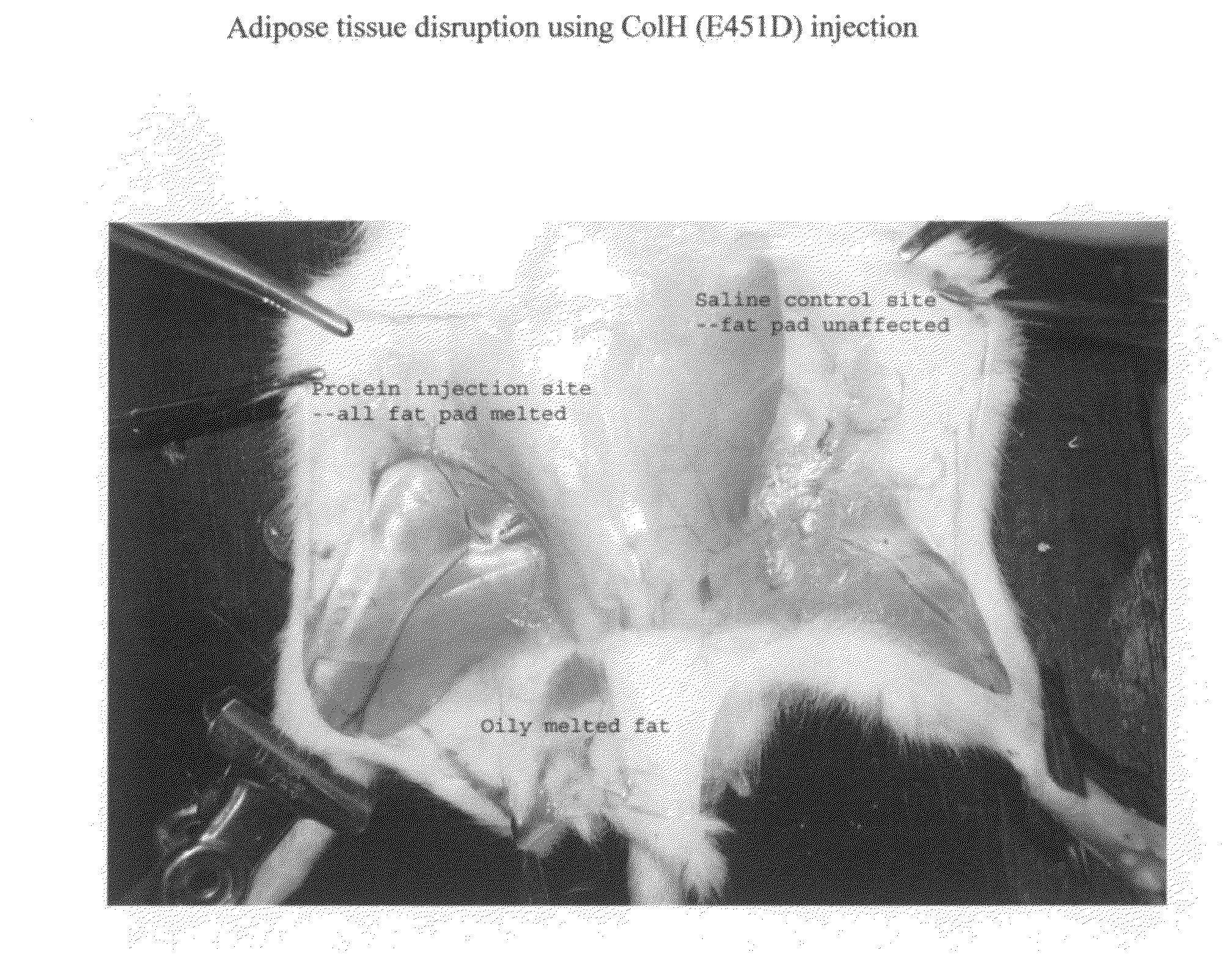Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Clostridium organisms" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
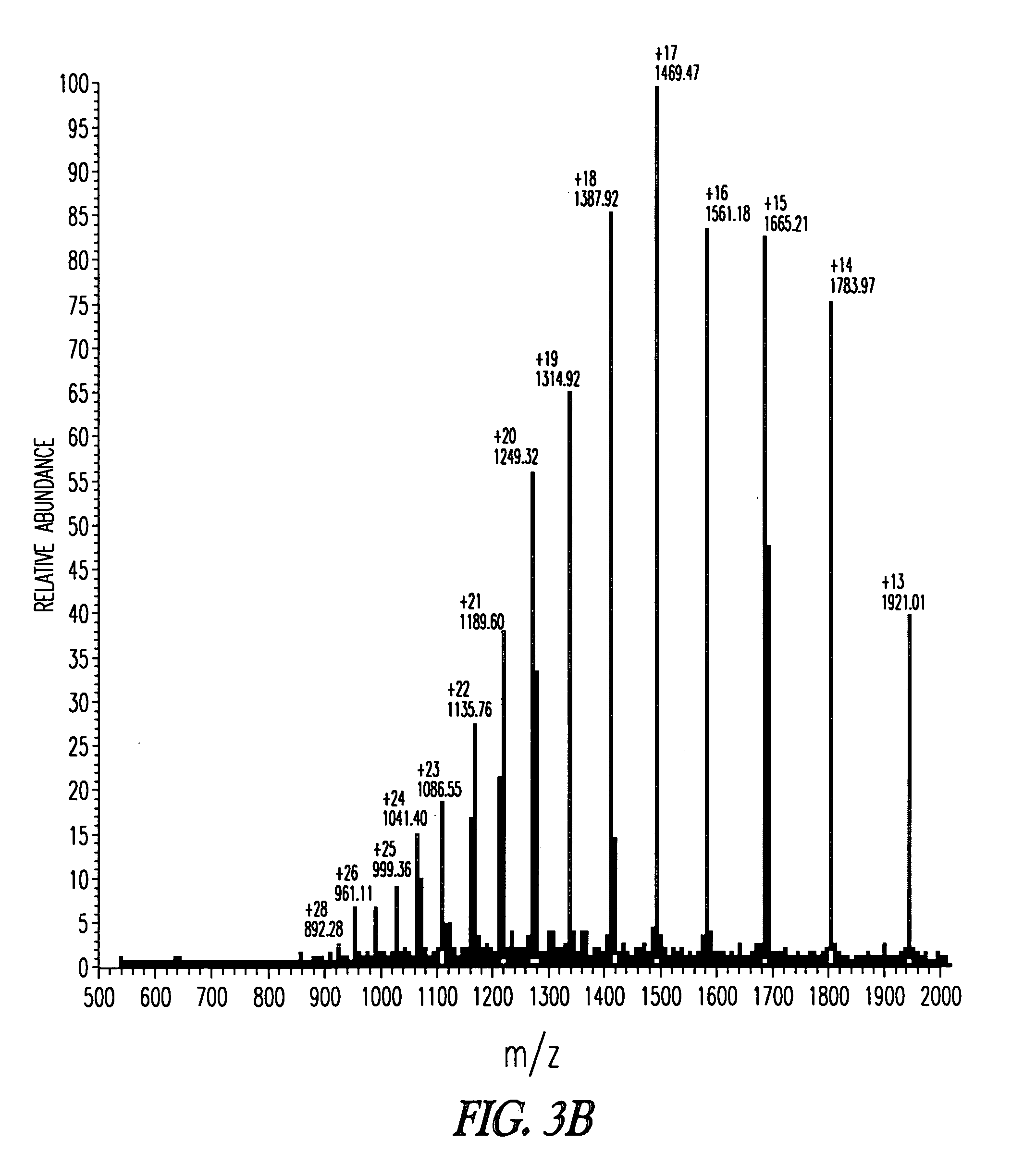
In vivo production of a clostridial neurotoxin light chain peptide
A method of producing in a cell in vivo a clostridial neurotoxin light chain peptide by delivering into the cell in vivo a nucleic acid construct. The nucleic acid construct comprises (a) a nucleic acid encoding a clostridial neurotoxin light chain peptide and (b) a regulatory sequence operably linked to the nucleic acid to allow expression of the nucleic acid. The expression of the nucleic acid produces the clostridial neurotoxin light chain peptide in the cell in vivo.
Owner:THE CLEVELAND CLINIC FOUND
SYNTHESIS OF HUMAN SECRETORY IgA AND IgM AND THE FORMATION OF A MEDICAMENT THEREFROM
A composition for treating a subject is provided. The composition includes antigen specific dimeric secretory IgA and pentameric IgM therapeutic. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the modification of antigen specific dimeric secretory IgA and pentameric IgM with secretory component to form a antigen specific dimeric secretory IgA and pentameric secretory IgM therapeutic. The antigen specific dimeric secretory IgA and the pentameric secretory IgM therapeutic is then mixed with formulating agents to create a capsule, tablet, liquid or suppository dosing form. The therapeutic is amenable to enrobement directly through microencapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R +2
Nano antibody of clostridium difficile glutamate dehydrogenase, and encoding sequence, screening method and application thereof
ActiveCN109336979ASimple and fast operationReduced operating requirementsFermentationVector-based foreign material introductionScreening methodCoat protein
The invention provides a nano antibody of clostridium difficile glutamate dehydrogenase, and an encoding sequence, a screening method and the application thereof and belongs to the technical field ofimmunology. The invention provides an amino sequence of the nano antibody of clostridium difficile glutamate dehydrogenase. By using a phage display technology, a sequence with a constant region specific gene is inserted into a vector of a phage encoding coat protein, after recombination expression, an exogenous gene expression product is fused with the phage encoding coat protein and demonstratedon the surface of phage to form a phage demonstration library, phage monocloning of the nano antibody is expressed through screening, and the nano antibody is prepared through sequencing. Phage-ELLSAidentification shows that the phage that the nano antibody is expressed on the surface can be specifically combined with clostridium difficile glutamate dehydrogenase antigen and has a good developing property, the result shows that the nano antibody has a good combination property with glutamate dehydrogenase, therefore, the nano antibody can be adopted to replace a common antibody applied to aclostridium difficile immunodetection kit.
Owner:NINGXIA MEDICAL UNIV
High-level expression of tetanus toxin receptor binding domain Hc in Escherichia coli and application
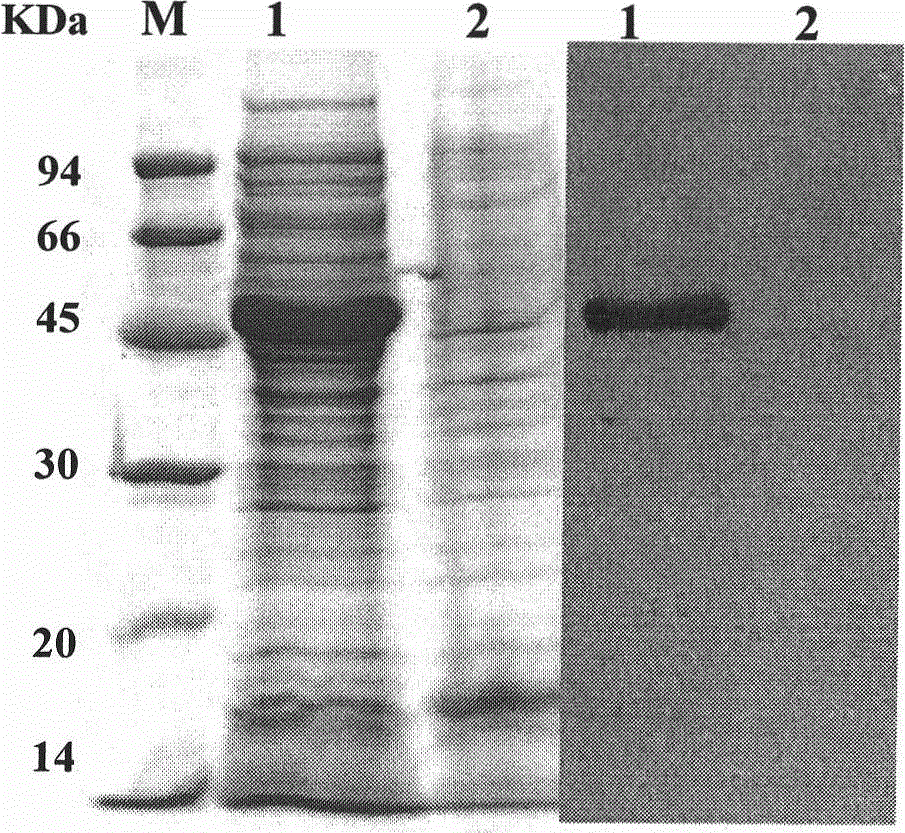
The invention relates to a method for a tetanus toxin receptor binding domain Hc to be subjected to high-level soluble expression in Escherichia coli through nucleotide sequence optimization. According to the sequencing result of a domestic C.Tetani virulent strain CMCC64008, the tetanus toxin receptor binding domain Hc sequence is analyzed and optimized, the optimized sequence is SEQ ID No.1 and the coded protein sequence is SEQ ID No.2. The synthesized Hc gene is linked into an expression vector pET32a(+) after undergoing double enzyme digestion, the recombinant Hc is subjected to high soluble expression in Escherichia coli and the target protein accounts for about 46% of the total protein in the supernatant undergoing bacteriociasis. After QFF column purification, phenyl hydrophobic column purification and SP column purification, the purity of the target protein can be more than 95% and the yield thereof is more than 300mg / L. The recombinant protein prepared by the method of the invention has good immunogenicity, can induce the mice to produce high-titre protective antibodies and can resist attack of high-dose lethal toxins. The method has extensive application prospect in large-scale high-level preparation of the tetanus toxin recombinant subunit vaccine Hc.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
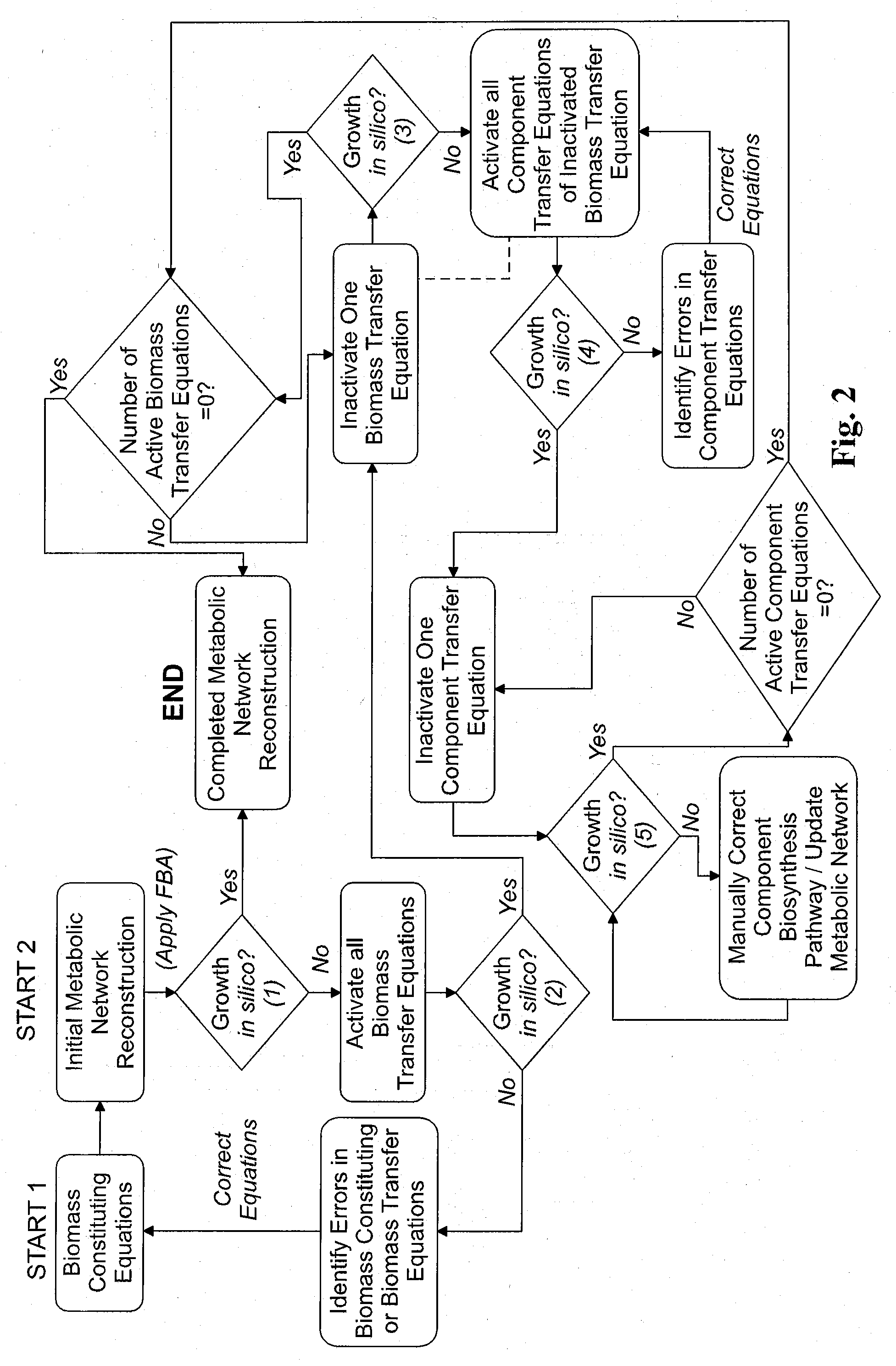
Reverse engineering genome-scale metabolic network reconstructions for organisms with incomplete genome annotation and developing constraints using proton flux states and numerically-determined sub-systems
InactiveUS20090259451A1Reduce in quantityAnalogue computers for chemical processesBiological testingUnderdetermined systemIn silico
A genome-scale metabolic network reconstruction for Clostridium acetobutylicum (ATCC 824) was created using a new semi-automated reverse engineering algorithm. This invention includes algorithms and software that can reconstruct genome-scale metabolic networks for cell-types available through the Kyoto Encyclopedia of Genes and Genomes. This method can also be used to complete partial metabolic networks and cell signaling networks where adequate starting information base is available. The software may use a semi-automated approach which uses a priori knowledge of the cell-type from the user. Upon completion, the program output is a genome-scale stoichiometric matrix capable of cell growth in silico. The invention also includes methods for developing flux constraints and reducing the number of possible solutions to an under-determined system by applying specific proton flux states and identifying numerically-determined sub-systems. Although the model-building and analysis tools described in this invention were initially applied to C. acetobutylicum, the novel algorithms and software can be applied universally.
Owner:UNIVERSITY OF DELAWARE
18-membered macrocycles and analogs thereof
The present invention relates generally to the 18-membered macrocyclic antimicrobial agents called Tiacumicins, specifically, OPT-80 (which is composed almost entirely of the R-Tiacumicin B), pharmaceutical compositions comprising OPT-80, and methods using OPT-80. In particular, this compound is a potent drug for the treatment of bacterial infections, specifically C. difficile infections.
Owner:MERCK SHARP & DOHME LLC
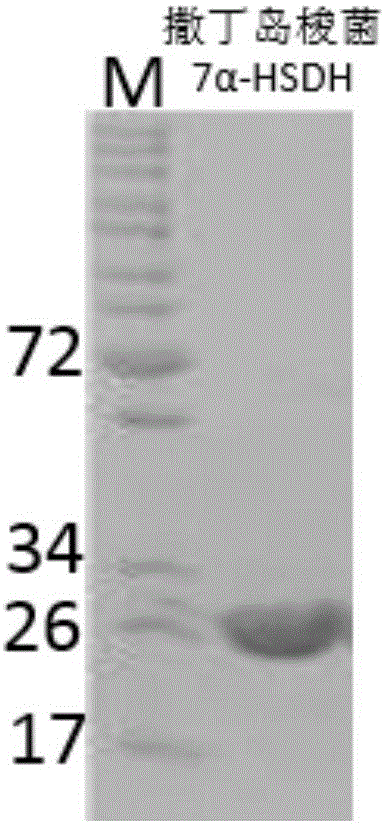
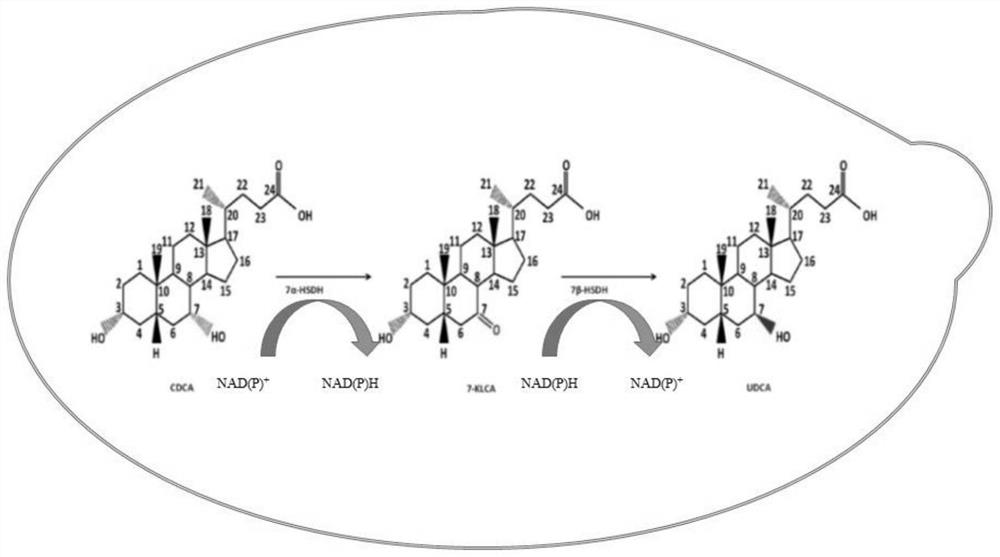
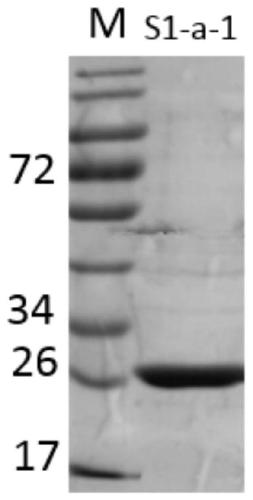
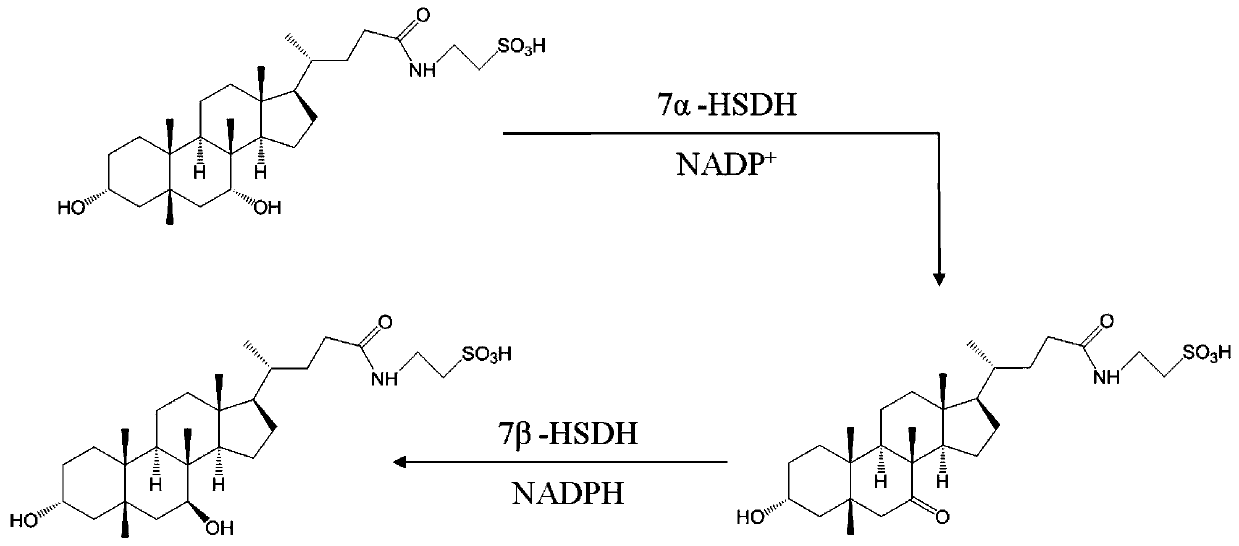
Gene S1-a-1 of novel 7 alpha-HSDH (hydroxysteroid dehydrogenase)
ActiveCN106701707ACatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to HSDH (hydroxysteroid dehydrogenase), in particular to a gene S1-a-1 of novel 7 alpha-HSDH. A nucleotide sequence of the gene is shown as SEQ ID NO.2, the novel 7 alpha-HSDH is encoded, a nucleotide sequence of the novel 7 alpha-HSDH is shown as SEQ ID NO.1, the novel 7 alpha-HSDH can be used for catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (taurine-7-ketone lithocholic acid), wherein the catalytic activity of the novel 7 alpha-HSDH for CDCA is about 5 times of that of 7 alpha-HSDH of Sardinia clostridium, and the catalytic activity of the novel 7 alpha-HSDH for TCDCA is about over 2.5 times of that of 7 alpha-HSDH of Sardinia clostridium, therefore, the novel 7 alpha-HSDH has a great industrial application value.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
Modified mutant collagenase and it's use in fat melting and in scar reduction
ActiveUS20120164131A1Reduce the amount presentReduction and removal of lipomaHydrolasesPeptide/protein ingredientsSide effectLiposuction
A Clostridium histolyticum collagenase ColH (Glu451Asp) melts adipose tissue when injected into selected regions of the body. This protein product melts fat pads effectively in fat rat experiments, with very little side effects. Very little hemorrhage was observed. We also invent a new version of ColH mutant by linking a peptide motif CKGGRAKDC-G(varying from 2 to 6 Gs) (SEQ ID NO: 2) in front of ColH (Glu451Asp) (called topical ColH-FM), which can target to white fat vasculature. By combining with novel transdermal technology (such as Hydroxysome technology), we develop a topical protein cream that can melt fat. This product can be used as cellulite cream and for chemical liposuction. This topical ColH-FM can also be injected into adipose tissue as a replacement for liposuction or as an adjunct method with liposuction. Since raise scar is formed by overgrowth of collagen, our topical ColH-FM cream is shown to have application in scar reduction.
Owner:REJUVEN DERMACEUTICAL CO LTD
Recombinant alpha protein for inhibiting clostridium perfringens infection and preparation method and application thereof
InactiveCN106008684AHigh expressionOptimizing expression conditionsAntibacterial agentsBacterial antigen ingredientsSolubilityProtein tag
The invention discloses recombinant alpha protein for inhibiting clostridium perfringens infection and a preparation method and application thereof. The recombinant alpha protein is shown in (a) or (b) or (c), wherein the protein in (a) is composed of amino acid sequences shown as SEQ ID No.2; the protein in (b) is composed of amino acid sequences shown at the sites No.51-No.353 of SEQ ID No.2; the fusion protein in (c) is obtained by fusing protein tags at carboxyl terminals or / and amino terminals of the protein shown in (a) or (b). The recombinant alpha protein enables animals to have a higher serum antibody level and resist attack of clostridium perfringens after the animals are immunized with the recombinant alpha protein. The recombinant alpha protein is good in solubility and easy to purify and can serve as a diagnostic antigen to be prepared into a monoclonal antibody or be used for further research on protein functions and conformation relations.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
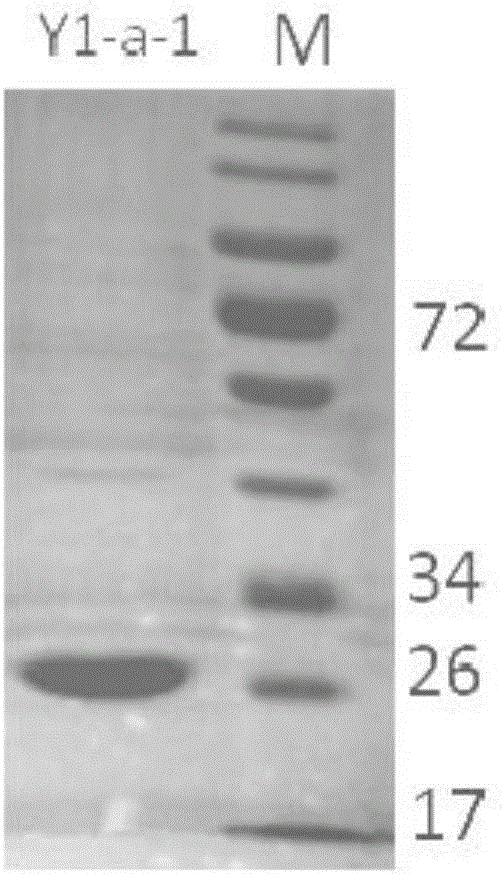
New 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1
ActiveCN106701708AHigh industrial application valueBetter than activityBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to hydroxysteroid dehydrogenase, in particular to a new 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1. The nucleotide sequence of the gene is as shown in SEQ ID NO. 2. New 7alpha-hydroxysteroid dehydrogenase is coded, and the amino acid sequence of new 7alpha-hydroxysteroid dehydrogenase is as shown in SEQ ID NO.1; new 7alpha-hydroxysteroid dehydrogenase can catalyze chenodeoxycholic acid (CDCA) and taurochenodeoxycholic acid (TCDCA) to generate 7-ketone lithocholic acid (7K-LCA) and tauro 7-ketone lithocholic acid (T7K-LCA); the catalysis activity for CDCA or TCDCA is more than two times that of existing Sardinia clostridium 7alpha-HSDH, and the new 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1 has an extremely large industrial application value.
Owner:CHONGQING UNIV
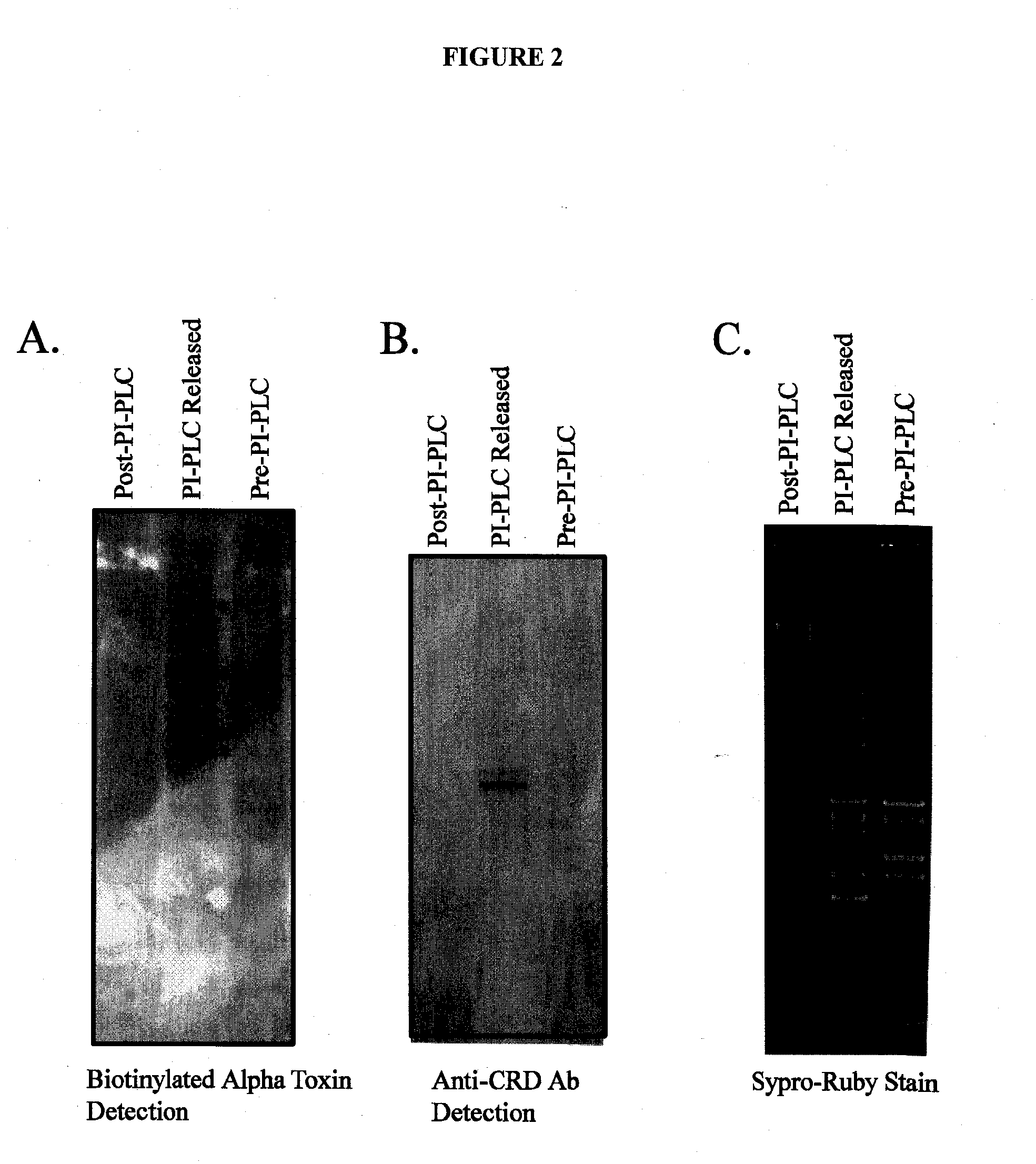
Alpha toxin detection of gpi anchored proteins
InactiveUS20140315212A1Cell receptors/surface-antigens/surface-determinantsMicrobiological testing/measurementDiseaseProstate cancer
The present invention relates to a method for the purification, concentration and identification of glycosylphosphatidylinositol anchored proteins (GPI-APs) from a biological sample (cells, tissues and / or blood / serum) in a patient or subject, including a human patient or subject. A new method to separate GPI-anchored glycoproteins, a class of glycoproteins found in all animal cells and fluids including serum, from other glycoproteins and proteins for the purpose of identifying potential biomarkers for various diseases, including cancer, especially breast cancer, vaginal cancer, endometrial cancer, uterine cancer, cervical cancer, pancreatic cancer and prostate cancer. The method uses the alpha-toxin from Clostridium septicum to separate GPI-anchored glycoproteins for identification and optionally quantification. The GPI-APs so obtained may be used to raise antibodies for inclusion in an immunosorbent assay for the diagnosis or the monitoring of therapy of cancer in a patient.
Owner:UNIV OF GEORGIA RES FOUND INC
Recombinant yeast chassis cell transformation for efficiently converting chenodeoxycholic acid and construction and application of recombinant strain
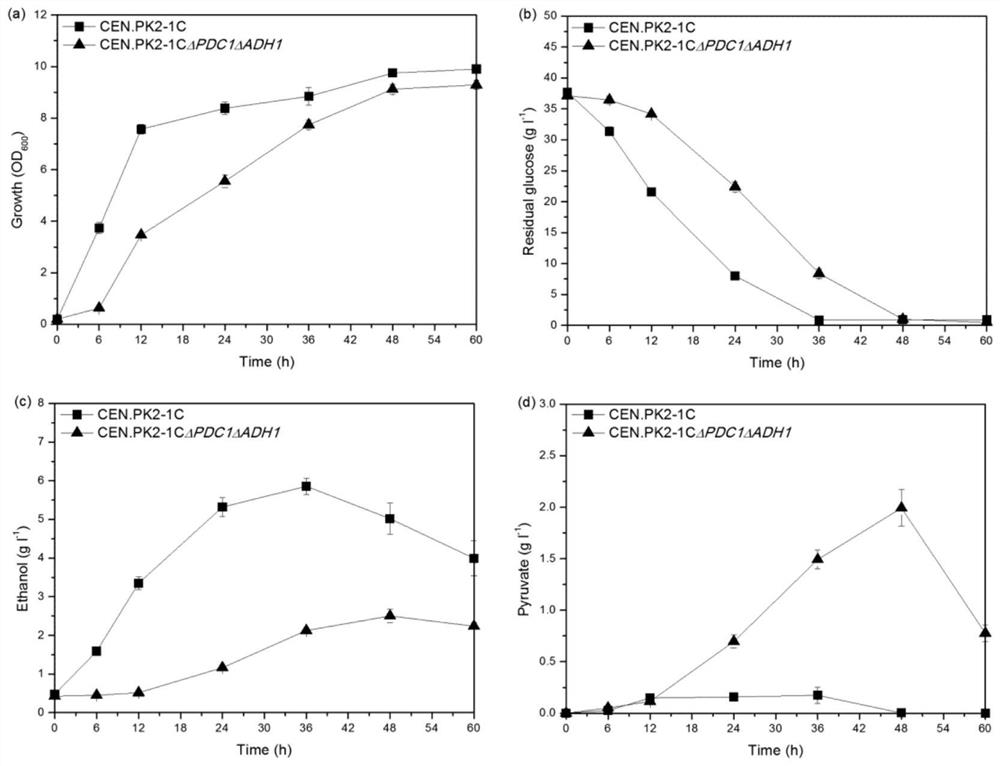
The invention discloses recombinant yeast chassis cell transformation for efficiently converting chenodeoxycholic acid and recombinant strain construction and application, a saccharomyces cerevisiae strain S. Cerevisiae CEN.PK21C is used as a chassis cell of a recombinant yeast strain, a mutant yeast strain is obtained by knocking out target genes PDC1 and ADH1, and weakening of an ethanol synthesis path is realized. On the basis, 7 alpha HSDH and 7 beta HSDH coding genes from clostridium are heterologously expressed, and the purpose of biosynthesis of UDCA by taking CDCA as a substrate is achieved. At present, the conversion rate of the substrate CDCA reaches 90%.
Owner:JIANGNAN UNIV
High-level expression of tetanus toxin receptor binding domain Hc in Escherichia coli and application
The invention relates to a method for a tetanus toxin receptor binding domain Hc to be subjected to high-level soluble expression in Escherichia coli through nucleotide sequence optimization. According to the sequencing result of a domestic C.Tetani virulent strain CMCC64008, the tetanus toxin receptor binding domain Hc sequence is analyzed and optimized, the optimized sequence is SEQ ID No.1 and the coded protein sequence is SEQ ID No.2. The synthesized Hc gene is linked into an expression vector pET32a(+) after undergoing double enzyme digestion, the recombinant Hc is subjected to high soluble expression in Escherichia coli and the target protein accounts for about 46% of the total protein in the supernatant undergoing bacteriociasis. After QFF column purification, phenyl hydrophobic column purification and SP column purification, the purity of the target protein can be more than 95% and the yield thereof is more than 300mg / L. The recombinant protein prepared by the method of the invention has good immunogenicity, can induce the mice to produce high-titre protective antibodies and can resist attack of high-dose lethal toxins. The method has extensive application prospect in large-scale high-level preparation of the tetanus toxin recombinant subunit vaccine Hc.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
7α-hydroxysteroid dehydrogenase gene s1-a-1
ActiveCN106701707BCatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesCholic acidChenodeoxycholic acid
The invention relates to HSDH (hydroxysteroid dehydrogenase), in particular to a gene S1-a-1 of novel 7 alpha-HSDH. A nucleotide sequence of the gene is shown as SEQ ID NO.2, the novel 7 alpha-HSDH is encoded, a nucleotide sequence of the novel 7 alpha-HSDH is shown as SEQ ID NO.1, the novel 7 alpha-HSDH can be used for catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (taurine-7-ketone lithocholic acid), wherein the catalytic activity of the novel 7 alpha-HSDH for CDCA is about 5 times of that of 7 alpha-HSDH of Sardinia clostridium, and the catalytic activity of the novel 7 alpha-HSDH for TCDCA is about over 2.5 times of that of 7 alpha-HSDH of Sardinia clostridium, therefore, the novel 7 alpha-HSDH has a great industrial application value.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
Pichia pastoris surface co-display system based on cellulosome as well as construction method and application of pichia pastoris surface co-display system
InactiveCN110229220AAchieve multi-protein co-displayReduce manufacturing costFungiBacteriaPichia pastorisCellulosomes
The invention provides a pichia pastoris surface co-display system based on cellulosome as well as a construction method and application of the pichia pastoris surface co-display system and belongs tothe technical fields of molecular biology, food and gene engineering. The system comprises anchoring protein and polyprotein display chassis protein, wherein the anchoring protein is saccharomyces cerevisiae cell wall protein Sed1p; and the polyprotein display chassis protein is artificial mini scaffold protein MixA2 of clostridium cellulosome. The pichia pastoris surface co-display system basedon cellulosome can realize polyprotein co-display on cell surfaces; heterologous protein for fusion expression of dockerin is prepared from recombinant bacteria, purification is not needed, multiple proteins can be displayed on cell surfaces by direct specific binding of dockerin on recombinant protein and cohesion on MixA2, and the preparation cost of recombinant protein can be saved effectively.
Owner:NANJING FORESTRY UNIV
Fixable oxycarbide and microorganism capable of performing fermentation reaction and preparation method and application thereof
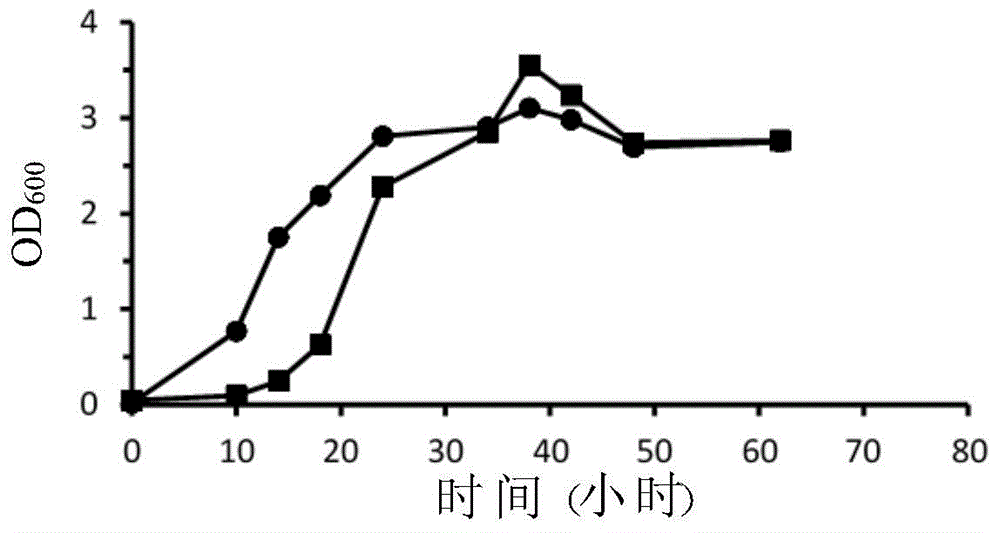
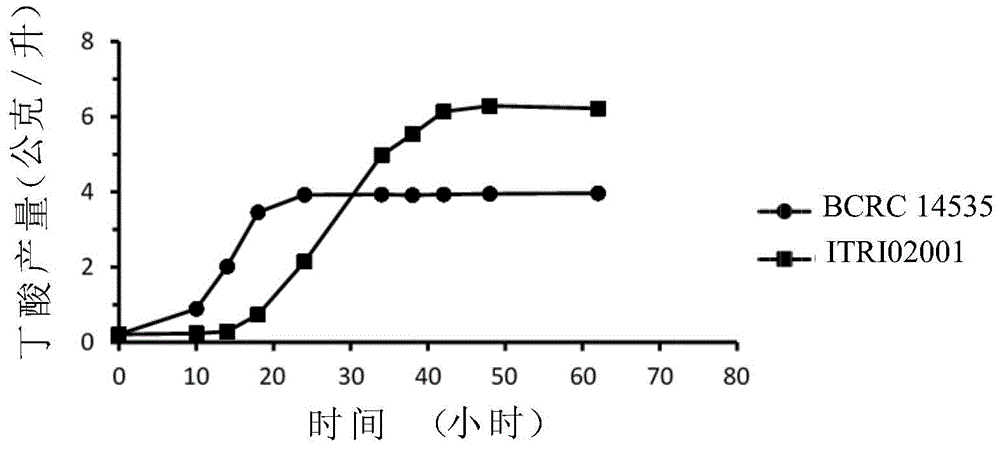
A fixable oxycarbide and a microorganism capable of performing fermentation reaction and a preparation method and application thereof are characterized in that the preparation method includes: (a) providing a first parental generation bacterial strain capable of fixing the oxycarbide; (b) providing a second parental generation bacterial strain capable of performing the fermentation reaction; (c) respectively preparing the first parental generation bacterial strain and the second parental generation bacterial strain into a first protoplast and a second protoplast; (d) fusing the first protoplast and the second protoplast so as to provide a fused bacterial strain; and (e) cultivating the fused bacterial strain so as to obtain an object bacterial strain which can be used for producing organic compounds (such as organic acids and alcohols). A specific sample of the object bacterial strain is Colstridium tyrobutyricum strian ITRI02001 and is stored in Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ). The storage number is DSM 32077.
Owner:GREEN CELLULOSITY
7alpha-oxhydryl sterol dehydrogenase and encoding gene and application thereof
The invention relates to 7alpha-oxhydryl sterol dehydrogenase and an encoding gene and application thereof, and belongs to the technical field of biology. The 7alpha-oxhydryl sterol dehydrogenase is protein shown in SEQ ID NO.1 or protein with the same functions, obtained through substitution and / or deletion and / or addition of one or more amino acid residues of an amino acid sequence shown in theSEQ ID NO.1. The 7alpha-oxhydryl sterol dehydrogenase can catalyze an asymmetric reduction reaction of carbanyl groups of C7alpha-oxhydryl groups of taurocholic acid, glycocholic acid, taurochenodeoxycholic acid and glycochenodeoxycholic acid and catalyze an asymmetric reduction reaction of carbanyl groups and oxhydryl groups in benzoyl groups of ethyl benzoylformate. Compared with 7alpha-oxhydrylsterol dehydrogenase in clostridium sardiniense, the 7alpha-oxhydryl sterol dehydrogenase has higher catalytic activity and thermostability, and has very high industrial application value.
Owner:CHONGQING UNIV
Nano antibody for resisting glycosyl transferase A subunit and application of nano antibody
ActiveCN112812189ASmall molecular weightHigh affinityAntibacterial agentsImmunoglobulins against bacteriaAntiendomysial antibodiesComplementarity determining region
The invention discloses a nano antibody for resisting glycosyl transferase A subunit and application of the nano antibody. A variable region of the nano antibody is provided with three complementary determining regions, namely CDR-1, CDR-2 and CDR-3, the CDR-1 is composed of an amino acid sequence as shown in SEQ ID NO.1, the CDR-2 is composed of an amino acid sequence as shown in SEQ ID NO.2, and the CDR-3 is composed of an amino acid sequence as shown in SEQ ID NO.3. The nano antibody for resisting the glycosyl transferase A subunit has the activity of being specifically bonded to the glycosyl transferase A subunit, neutralizes the activity of the glycosyl transferase A, can be used for detecting clostridium difficile and can also be used for preparing drugs for treating and resisting the clostridium difficile; the nano antibody also has the characteristics of small molecular weight, high affinity, stable structure and performance and the like; and the nano antibody has the advantages of being capable of tolerating the gastric acid environment and being not easily degraded by pepsin and the like.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Recombinant alpha protein for inhibiting Clostridium perfringens infection and its preparation method and application
InactiveCN106008684BHigh expressionOptimizing expression conditionsAntibacterial agentsBacterial antigen ingredientsProtein tagBacillus perfringens
The invention discloses recombinant alpha protein for inhibiting clostridium perfringens infection and a preparation method and application thereof. The recombinant alpha protein is shown in (a) or (b) or (c), wherein the protein in (a) is composed of amino acid sequences shown as SEQ ID No.2; the protein in (b) is composed of amino acid sequences shown at the sites No.51-No.353 of SEQ ID No.2; the fusion protein in (c) is obtained by fusing protein tags at carboxyl terminals or / and amino terminals of the protein shown in (a) or (b). The recombinant alpha protein enables animals to have a higher serum antibody level and resist attack of clostridium perfringens after the animals are immunized with the recombinant alpha protein. The recombinant alpha protein is good in solubility and easy to purify and can serve as a diagnostic antigen to be prepared into a monoclonal antibody or be used for further research on protein functions and conformation relations.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
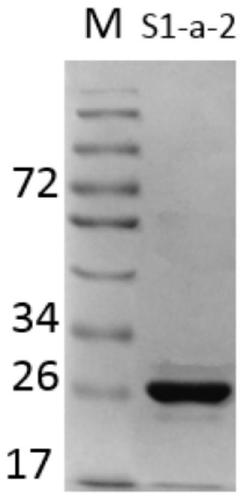
7α-hydroxysteroid dehydrogenase gene s1-a-2
ActiveCN106676079BCatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesCholic acidChenodeoxycholic acid
The invention relates to hydroxysteroid dehydrogenase, in particular to a novel 7alpha-hydroxysteroid dehydrogenase gene S1-a-2. A nucleotide sequence of the gene is shown as SEQ ID NO.2. A novel 7alpha-hydroxysteroid dehydrogenase with an amino acid sequence shown as SEQ ID NO.1 is coded and is capable of catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (tauro-7-keto lithocholic acid), catalytic activity of the novel 7alpha-hydroxysteroid dehydrogenase for the CDCA or TCDCA is about twice of that of Clostridium sardiniense 7alpha-HSDH for the same, and accordingly the novel 7alpha-hydroxysteroid dehydrogenase is high in industrial application value.
Owner:CHONGQING UNIV
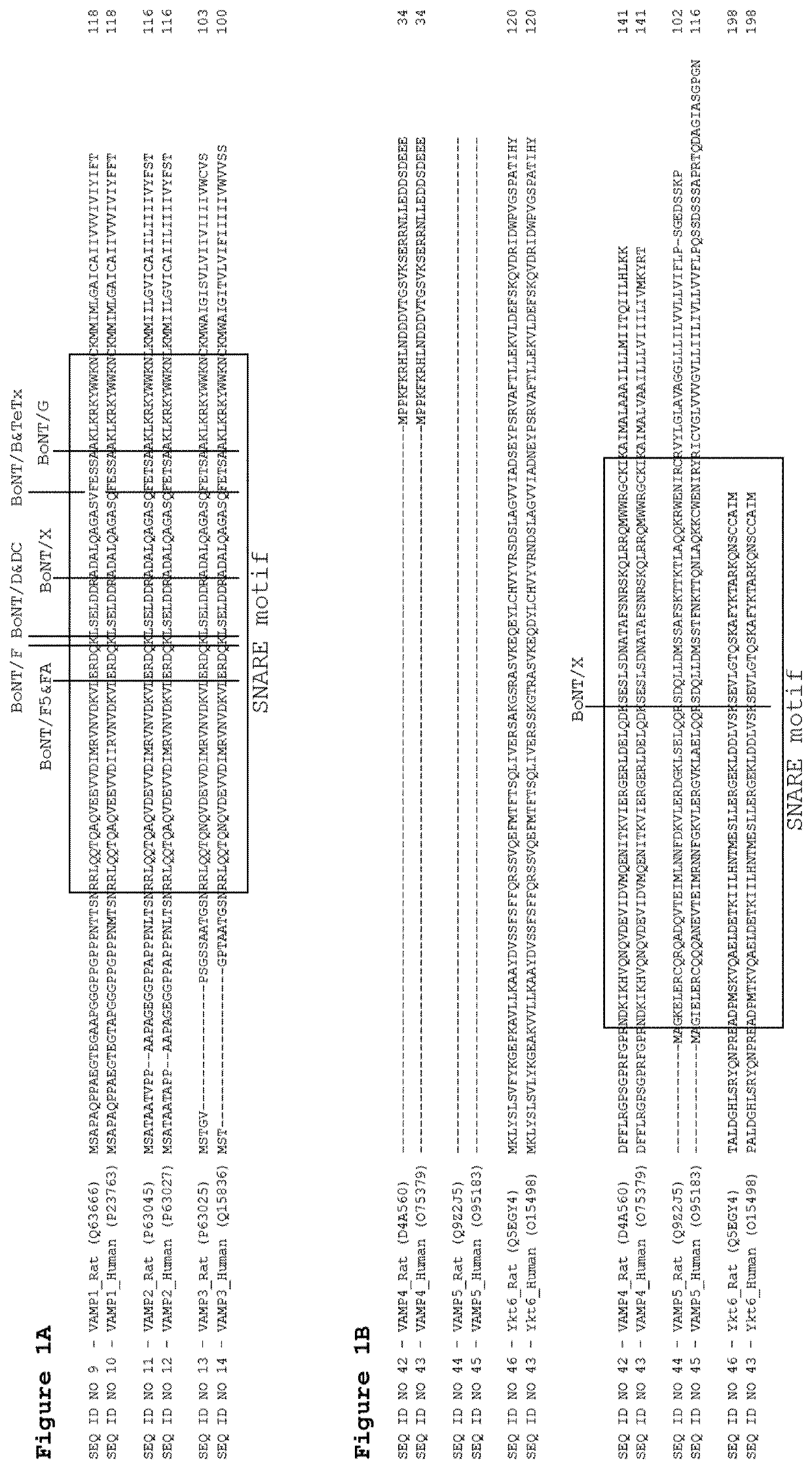
Cellular vamp cleavage assay
ActiveUS11193931B2Antibody mimetics/scaffoldsMicrobiological testing/measurementEpitopeAntiendomysial antibodies
A VAMP epitope suitable for generating an antibody against a VAMP C-terminal neurotoxin cleavage product. Method of using such an epitope to generate an antibody against cleaved VAMP. Method of using such an antibody to assay for cleavage of a VAMP by clostridial neurotoxin.
Owner:IPSEN BIOPHARM LTD
Food-poisoning fungus concatenated fusion mycinamicin and use thereof
InactiveCN1818066AImprove featuresImprove responseImmunoglobulins against bacteriaBacteria peptidesBacteroidesResponse sensitivity
The invention is about the recombined toxin of the bacteria which can lead the food poisoning. The Hc-VT1B-SEA-VT2B-SEB linked by the linker sequence is from the gene Hc of the botulin A, the A gene: SEA and the B gene: SEB of Staphylococcus aureus, the VT1B and VT2B of E.Colli O 157. The serum antibody can anti above five bacteria toxin. So it can be used to produce the reagent box of recombined toxin to detect the food.
Owner:JILIN UNIV
Clostridium difficile vaccine
InactiveUS20030054009A1Strict controlAntibody mimetics/scaffoldsSnake antigen ingredientsDiseaseClostridium difficile
A vaccine for the treatment or prophylaxis of C. difficile associated disease comprises a C. difficile gene or a C. difficile peptide / polypeptide or a derivative or fragment or mutant or variant thereof which is immunogenic in humans. The gene encodes a C. difficile surface layer protein, SlpA or variant or homologue thereof. The peptide / polypeptide is a C. difficile surface layer protein, SlpA or variant or homologue thereof. The vaccine may comprise a chimeric nucleic acid sequence.
Owner:TRINITY COLLEGE DUBLIN
Method for the detection and identification of a variant C. difficile strain in a sample
ActiveUS8889363B2Low costSave costs-theMicrobiological testing/measurementImmunoglobulins against bacteriaReference patternsGroup ii
The method for detecting and identifying a variant C. difficile strain in a sample is characterized by the following steps: (a) obtaining a sample of excreta or tissue from a human or animal patient body; (b) bringing the sample into contact with at least one antibody from each of at least three of the antibody groups group I, group II, group III, group IV, group V and group VI; (c) detecting the antibody reaction and constructing the reaction pattern; (d) comparing the reaction pattern obtained in (c) with reference patterns from known C. difficile strains and C. difficile strains the presence of which is to be tested in the investigation test; (e) assessing the agreement between the reaction pattern obtained in (c) and the reference pattern as indicative of the presence of the C. difficile strain of the reference pattern concerned in the sample.
Owner:BIODICS
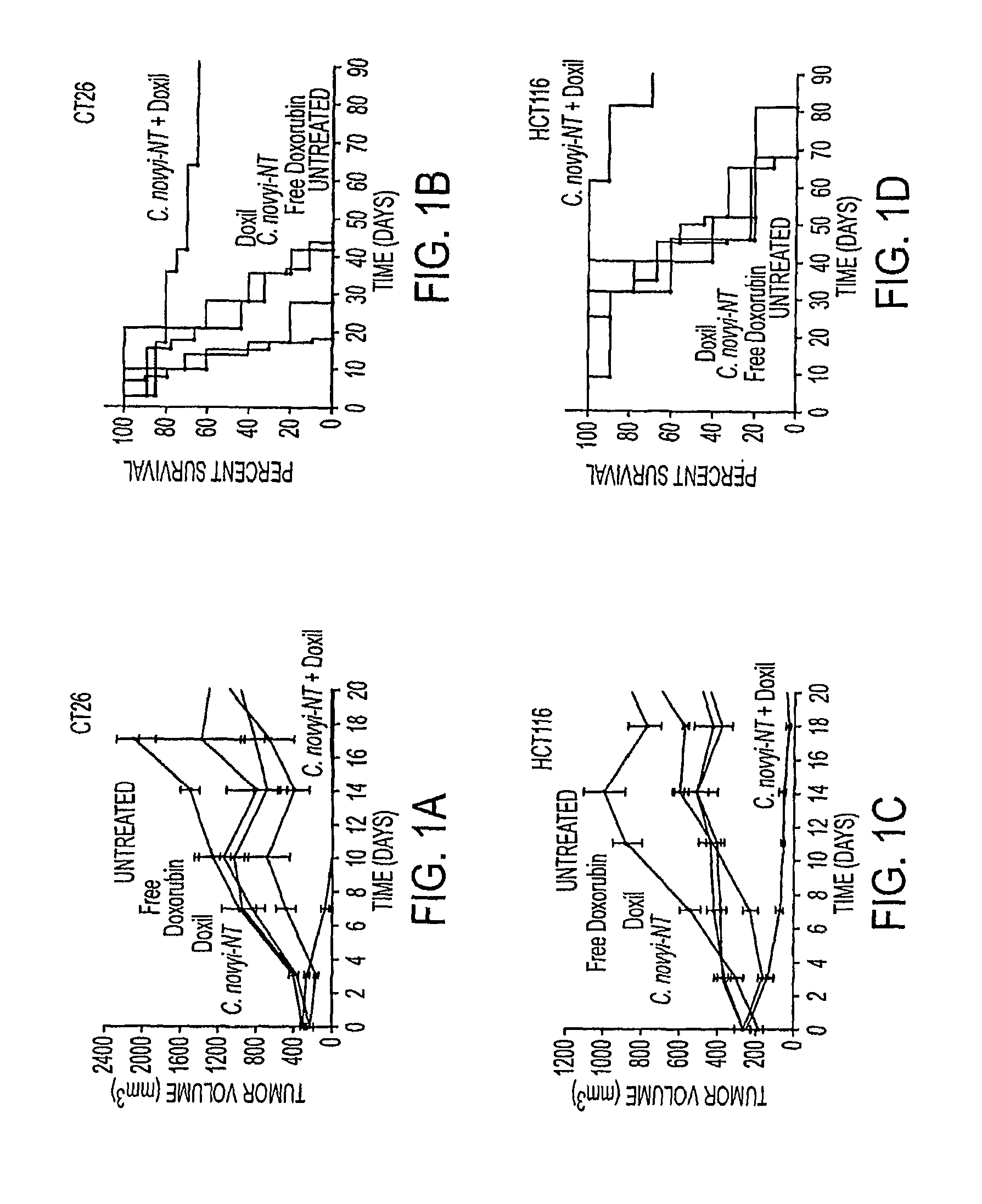
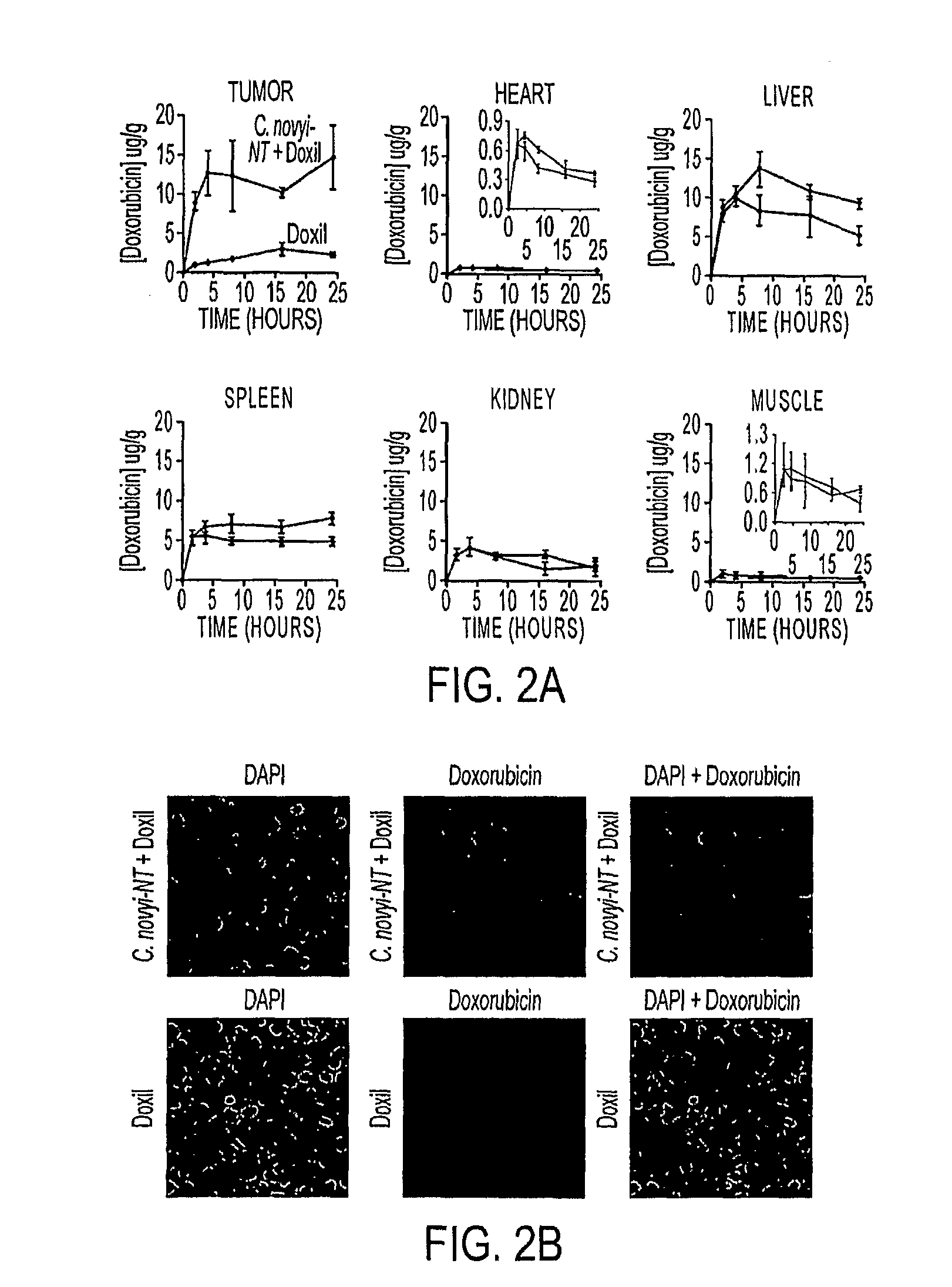
Tumor specific delivery of therapeutic agents via liposomase
Clostridium novyi is an obligate anaerobe that can infect hypoxic regions within experimental tumors. We found that mice bearing large, established tumors were often cured when treated with C. novyi plus a single dose of liposomal doxorubicin. The secreted factor responsible for this phenomenon was identified and, surprisingly, proved to be a member of the lipase family. The gene encoding this protein, called liposomase, has the potential to be incorporated into diverse therapeutic methods to deliver specifically a variety of chemotherapeutic agents to tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for improving fermenting property of solventogenic clostridia
The invention provides a method for improving the fermenting property of solventogenic clostridia. Specifically, the invention provides a nucleic acid construct first, wherein the nucleic acid construct contains amino acid sequences shown as coding SEQ ID NO:1,2,3 and 4, or polynucleotide sequences or the complementary sequences thereof of amino acid sequences which are subjected to substitution, deletion or adding of one or multiple amino acids in the amino acid sequences shown as the SEQ ID NO:1,2,3 and 4, respectively maintain the biological activity of the SEQ ID NO:1,2,3 and 4 and are respectively derived by the SEQ ID NO:1,2,3 and 4, and an optional promoter sequence creatively connected with the above polynucleotide sequences or the complementary sequences thereof. The invention also discloses the solventogenic clostridia, a method for fermentation by the solventogenic clostridia and a method for improving the growing ability and / or the solvent production ability of the solventogenic clostridia.
Owner:CAS CENT FOR EXCELLENCE IN MOLECULAR PLANT SCI
7β-hydroxysteroid dehydrogenase gene y1-b-1
ActiveCN107058250BCatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesCholic acidChenodeoxycholic acid
The invention relates to a hydroxysteroid dehydrogenase and particularly relates to a novel 7beta-hydroxysteroid dehydrogenase gene Y1-b-1. A nucleotide sequence of the gene is as shown in SEQ ID NO.2; a novel 7beta-hydroxysteroid dehydrogenase is encoded and an amino acid sequence thereof is as shown in SEQ ID NO.1. Epimerization of 7-hydroxy of ursodesoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA) can be catalyzed to generate intermediates 7-ketone lithocholic acid (7K-LCA) and taurine 7-ketone lithocholic acid (T7K-LCA) of chenodeoxycholic acid (CDCA) and taurochenodeoxycholic acid (TCDCA). The specific activity of the novel 7beta-hydroxysteroid dehydrogenase gene Y1-b-1 on the UDCA and the TUDCA is equivalent to that of existing clostridium sardiniense 7beta-HSDH, but the novel 7beta-hydroxysteroid dehydrogenase gene Y1-b-1 has better heat stability and a great industrial application prospect.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
Process for the biological production of n-butanol with high yield
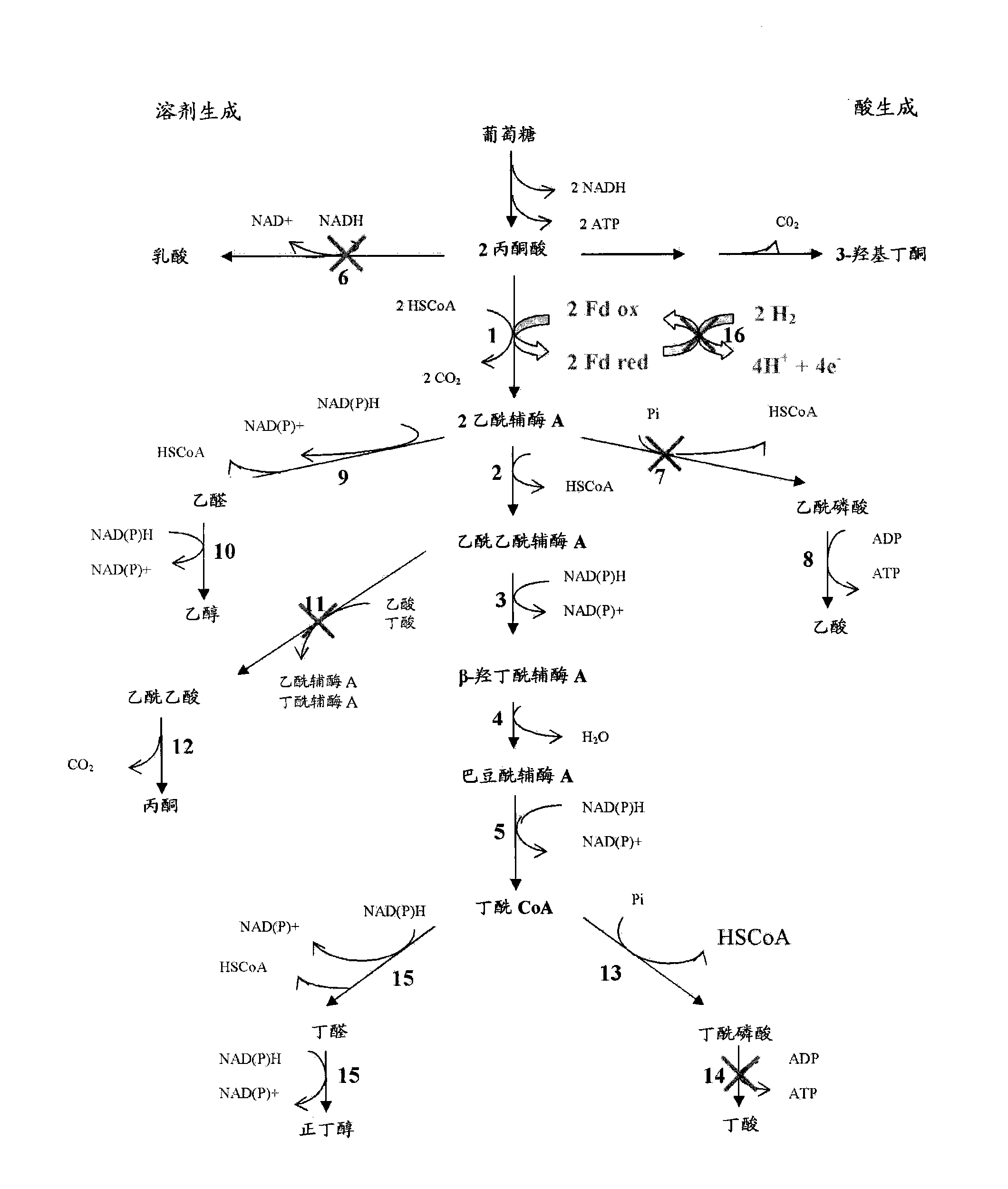
The present invention provides a method for the biological production of n-butanol at high yield from a fermentable carbon source. In one aspect of the present invention, a process for the conversion of glucose to n-butanol is achieved by the use of a recombinant organism comprising a host C. acetobutlicum transformed i) to eliminate the butyrate pathway ii) to eliminate the acetone pathway iii) to eliminate the lactate pathway and iv) to eliminate the acetate pathway. In another aspect of the present invention, the hydrogen flux is decreased and the reducing power redirected to n-butanol production by attenuating the expression of the hydrogenase gene. Optionally the n-butanol produced can be eliminated during the fermentation by gas striping and further purified by distillation.
Owner:METABOLIC EXPLORER
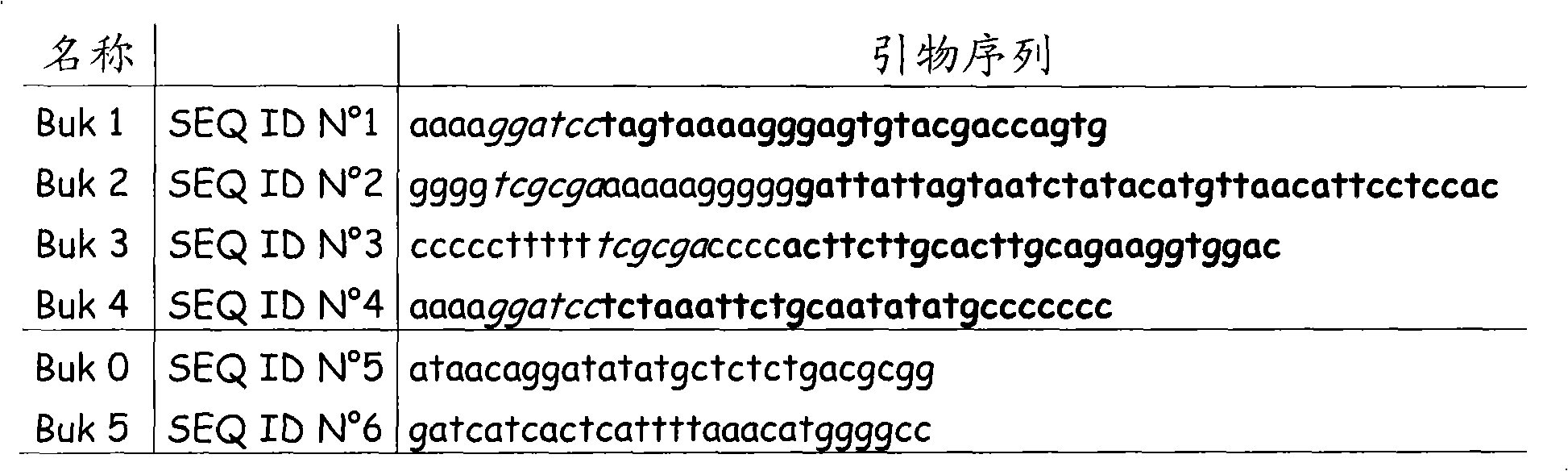
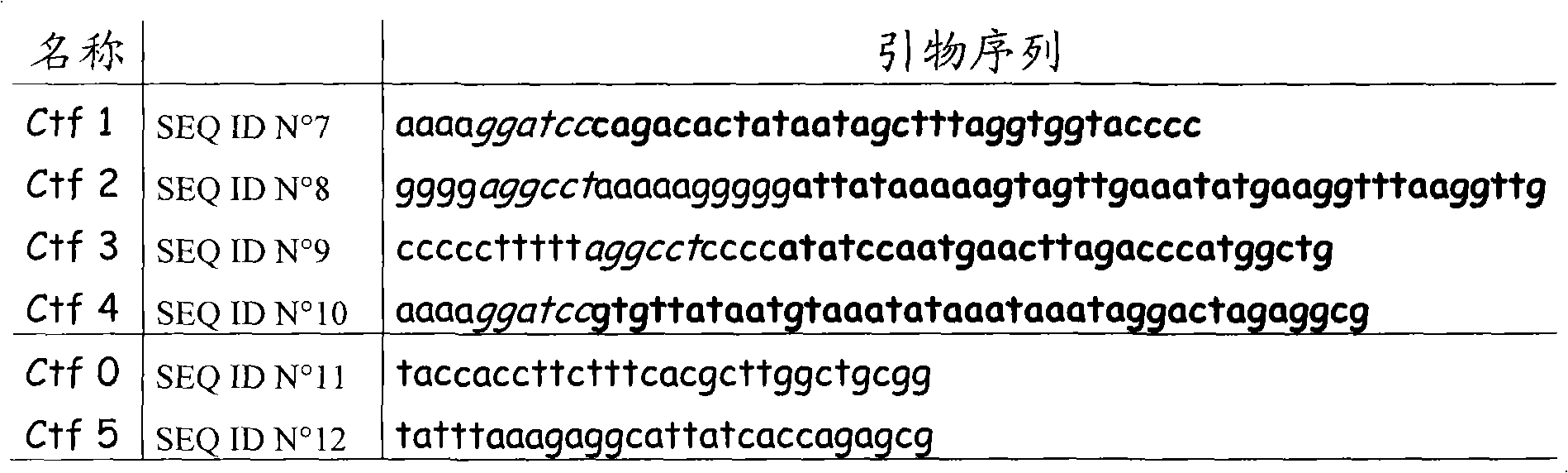
Anti-c.difficile toxin antibodies and associated methods
InactiveUS20150104454A1Antibacterial agentsBacterial antigen ingredientsEpitopeClostridium difficile toxin A
Embodiments of the invention are directed to a composition comprising a recombinant protein in soluble form wherein said recombinant protein comprises a portion of the Clostridium difficile toxin B sequence that comprises an epitope for anti-toxin B antibody. Other embodiments of the invention are directed to the generation of antibodies using peptide fragments of C. difficile toxin B.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com