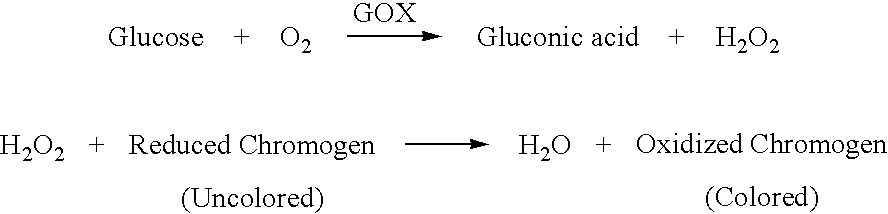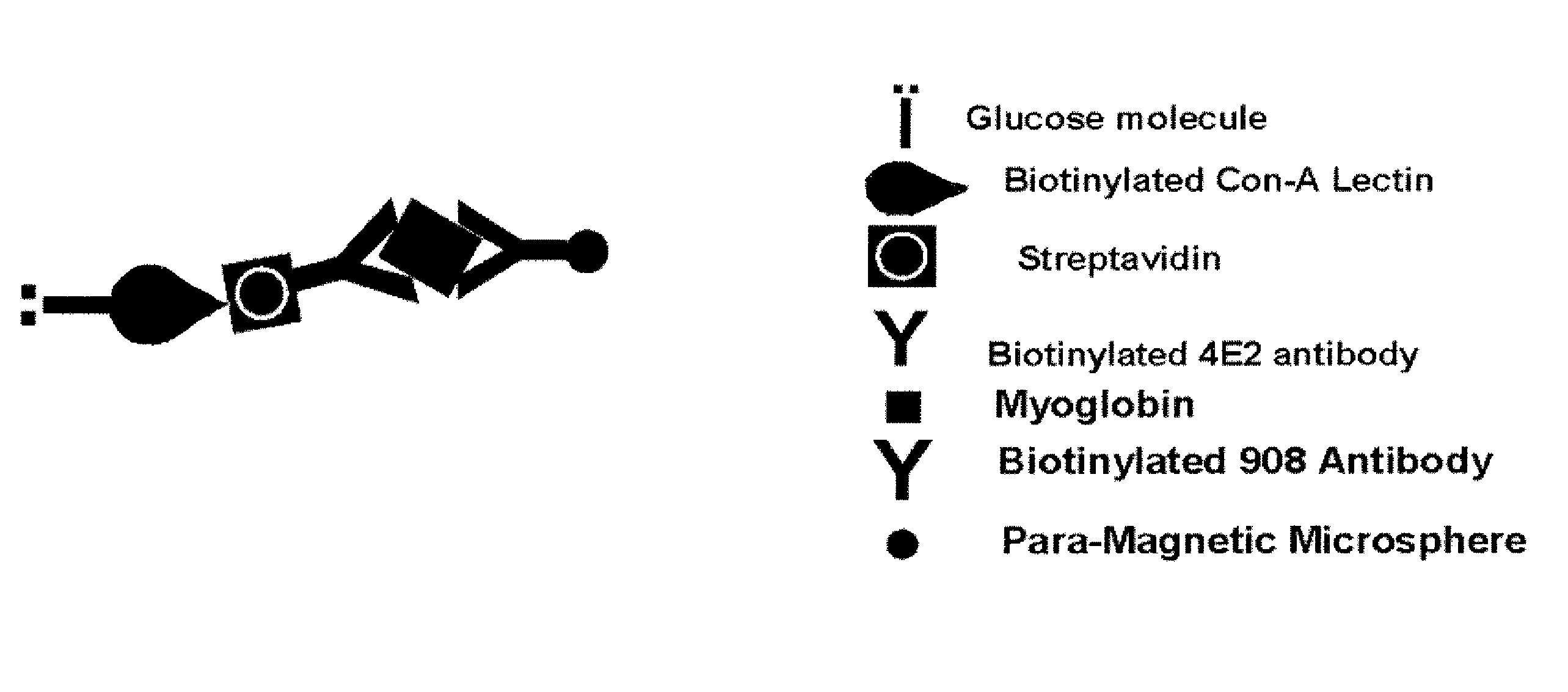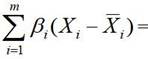Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
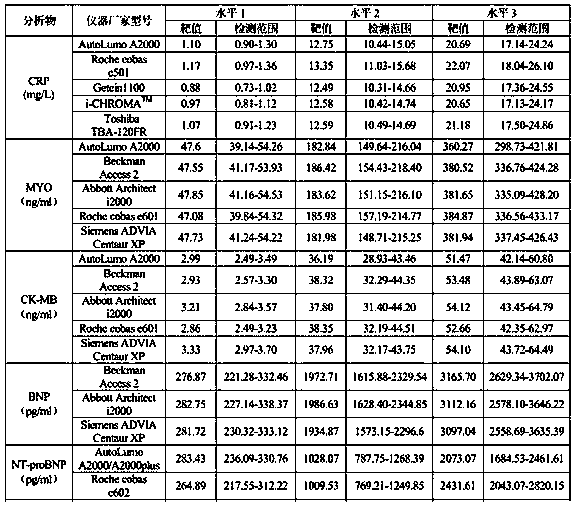
34 results about "Cardiac marker" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
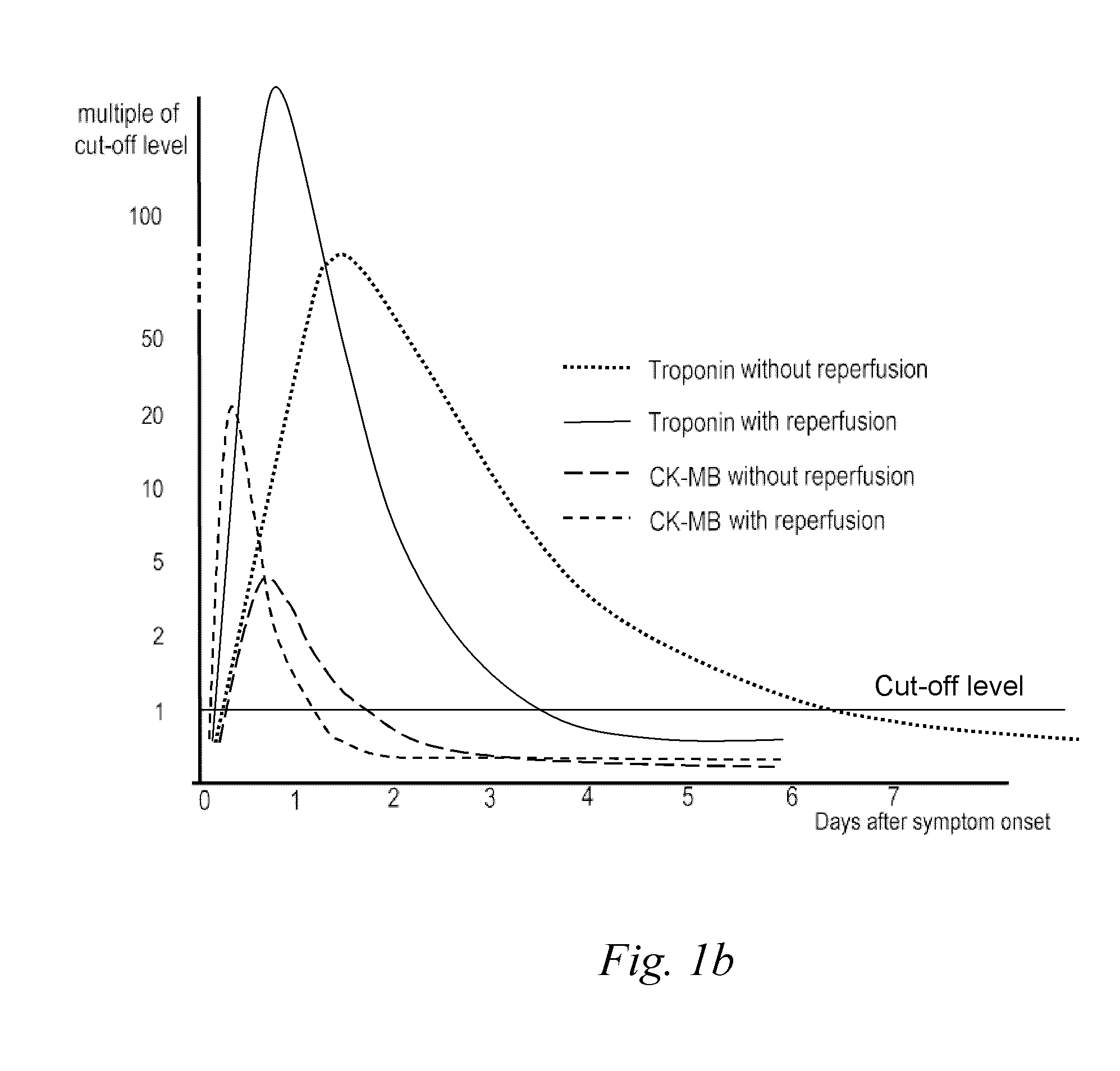
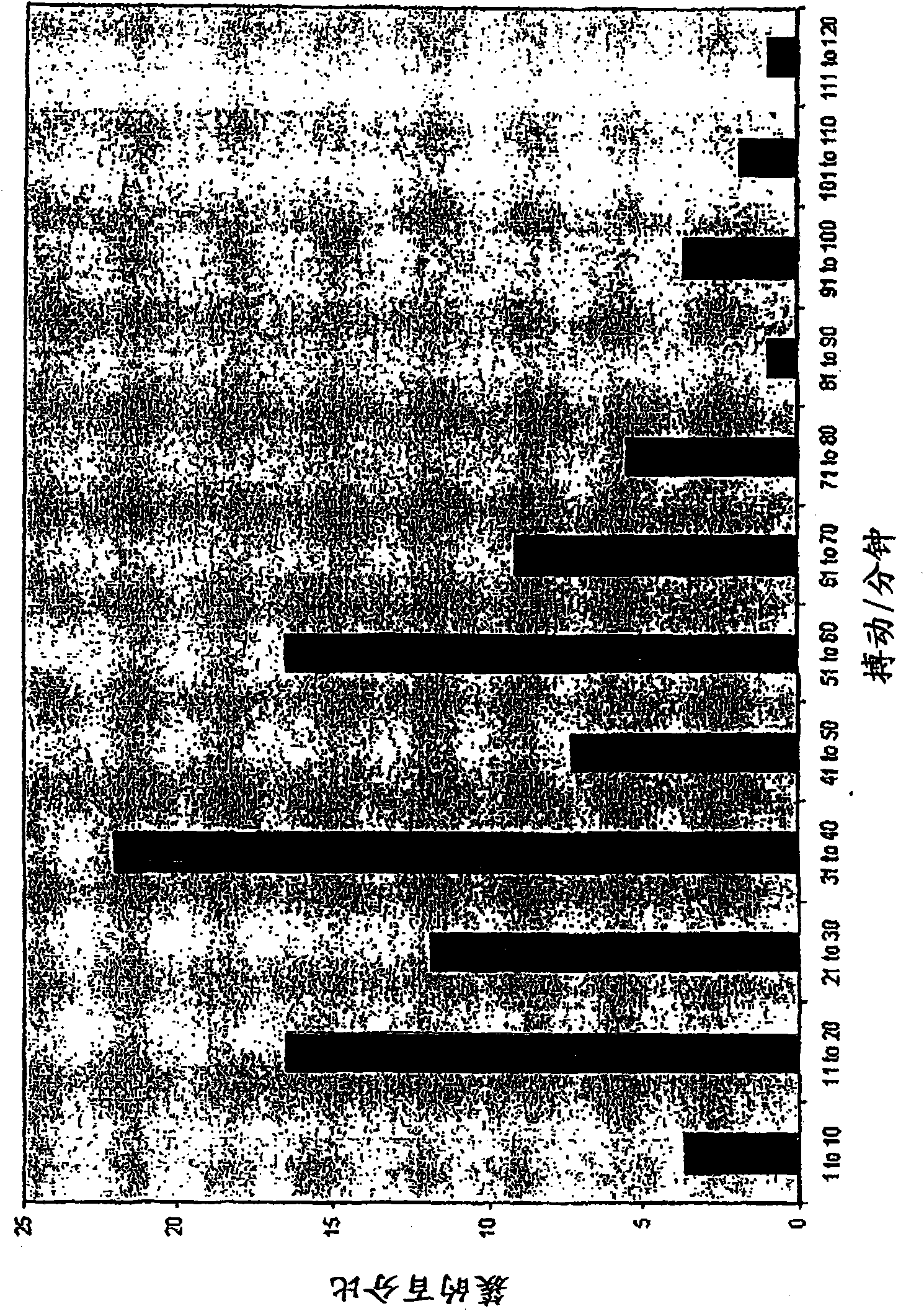
<ul><li>Normal results are inferred when the levels of 'Troponin I' is < 0.35 µg/l, 'Troponin T' is < 0.2 µg/l, Total Creatine Phosphokinase is between 0 to 120 µg/l and Creatine kinase is 0 to 3 µg/l.</li><li>Heart complications may be inferred if the levels fall higher than these values.</li></ul>
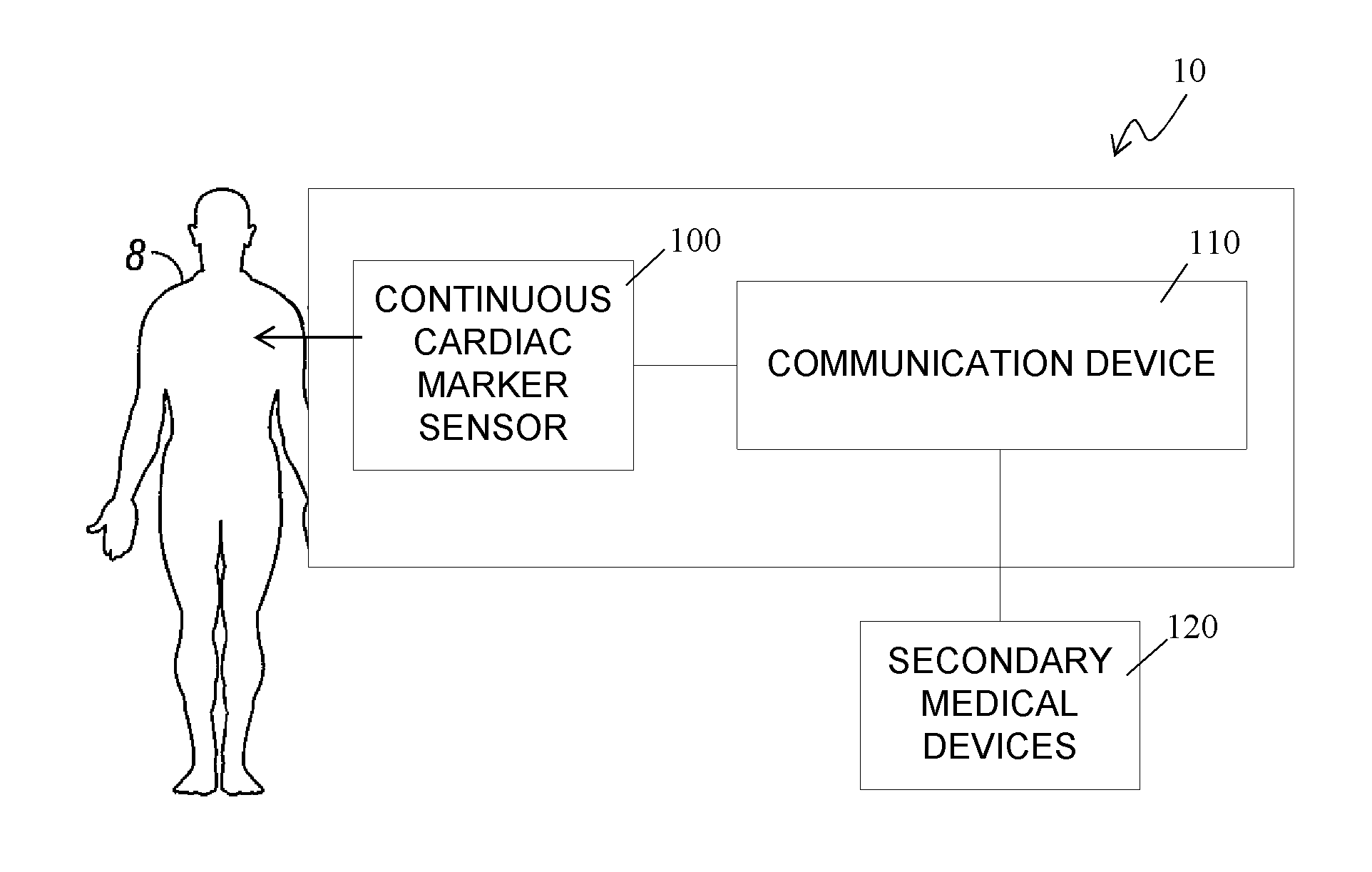
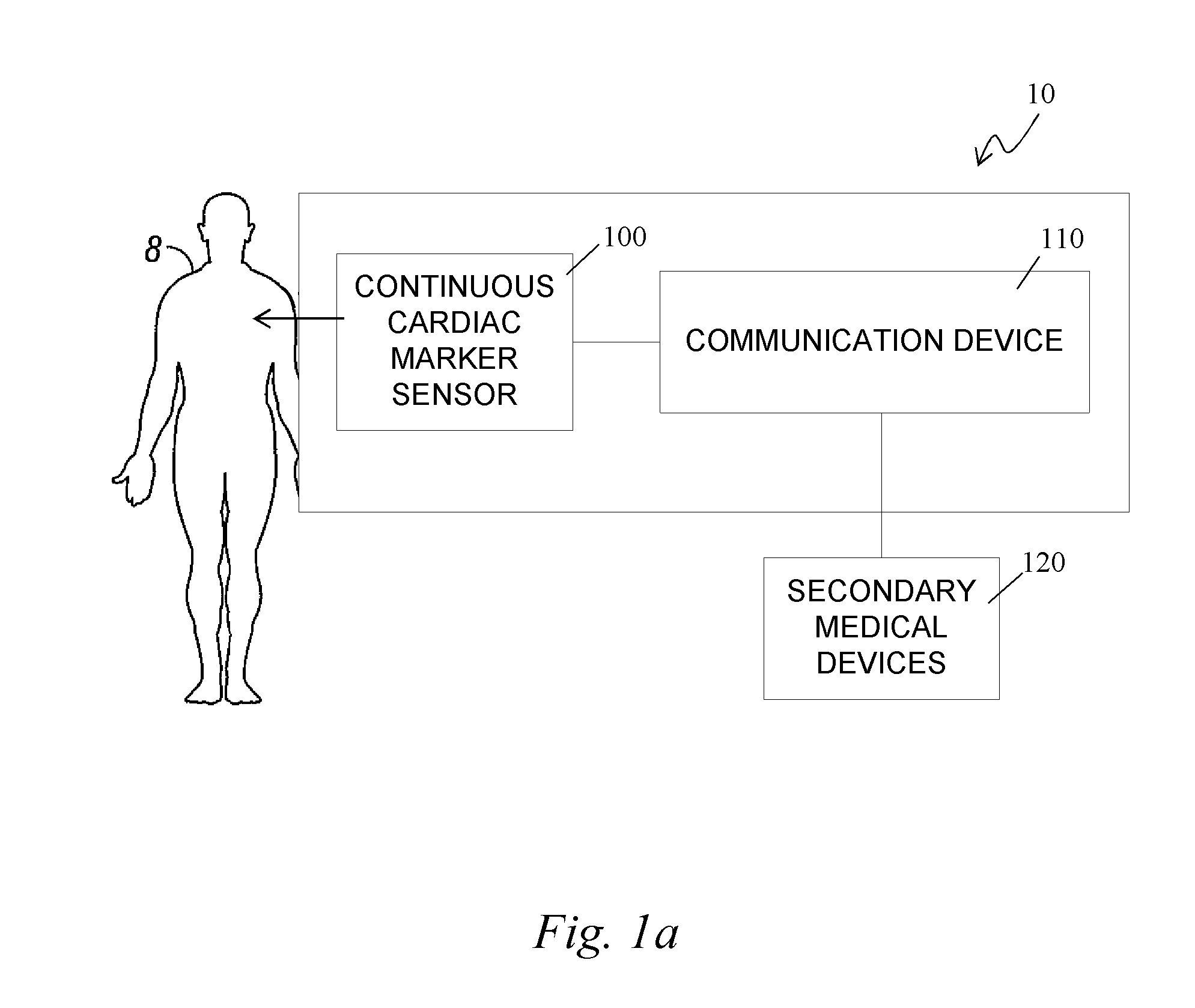
Continuous cardiac marker sensor system
The present invention relates generally to systems and methods for continuous measurement of a cardiac marker in vivo. In some embodiments, the system includes a continuous sensor and a communication device. The continuous sensor is configured to continuously measure a concentration of a cardiac marker in vivo and to provide a signal associated therewith. The communication device includes a processor module configured to process the signal to obtain cardiac information, wherein the communication device is configured to output the cardiac information.
Owner:DEXCOM
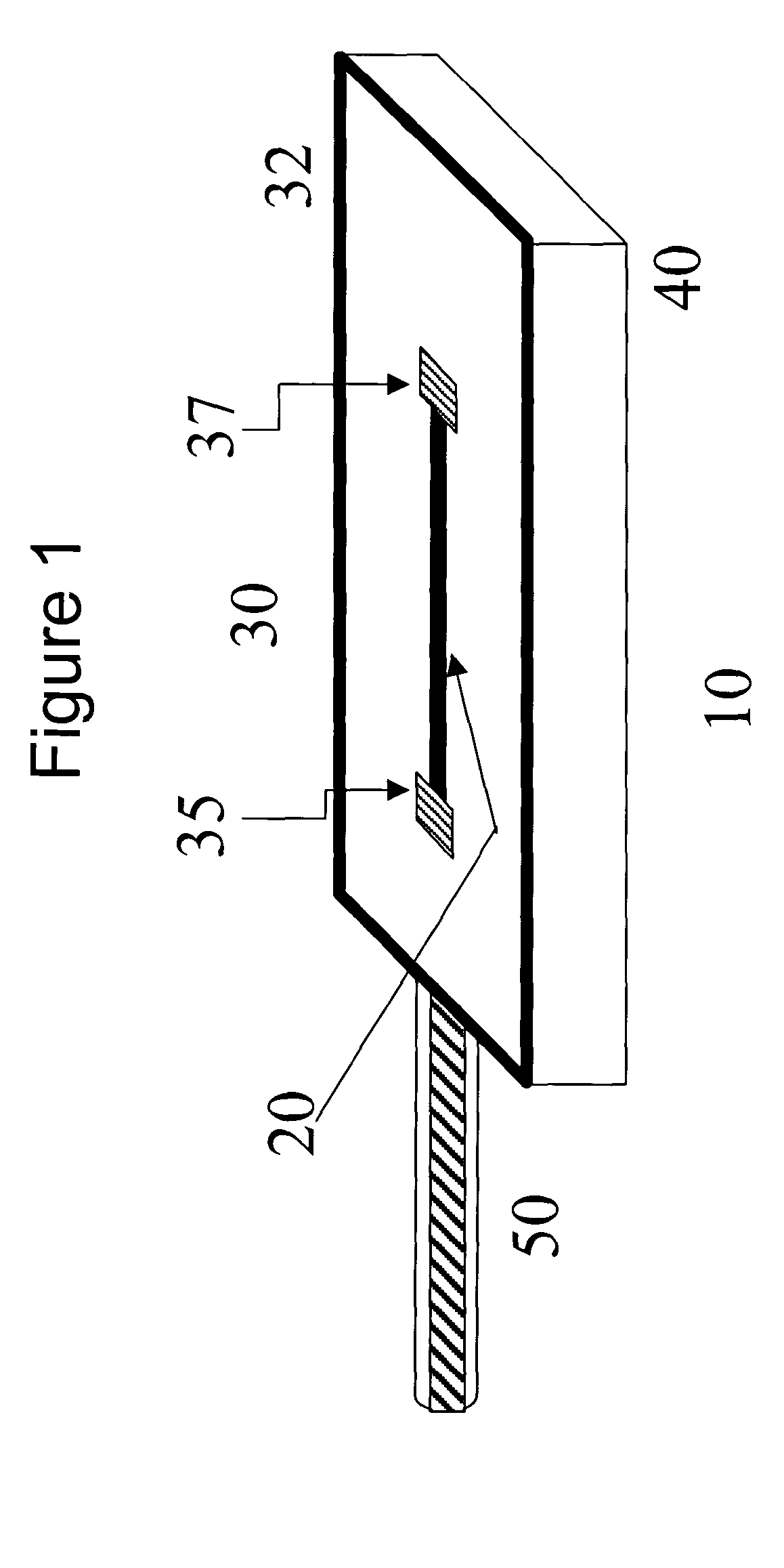
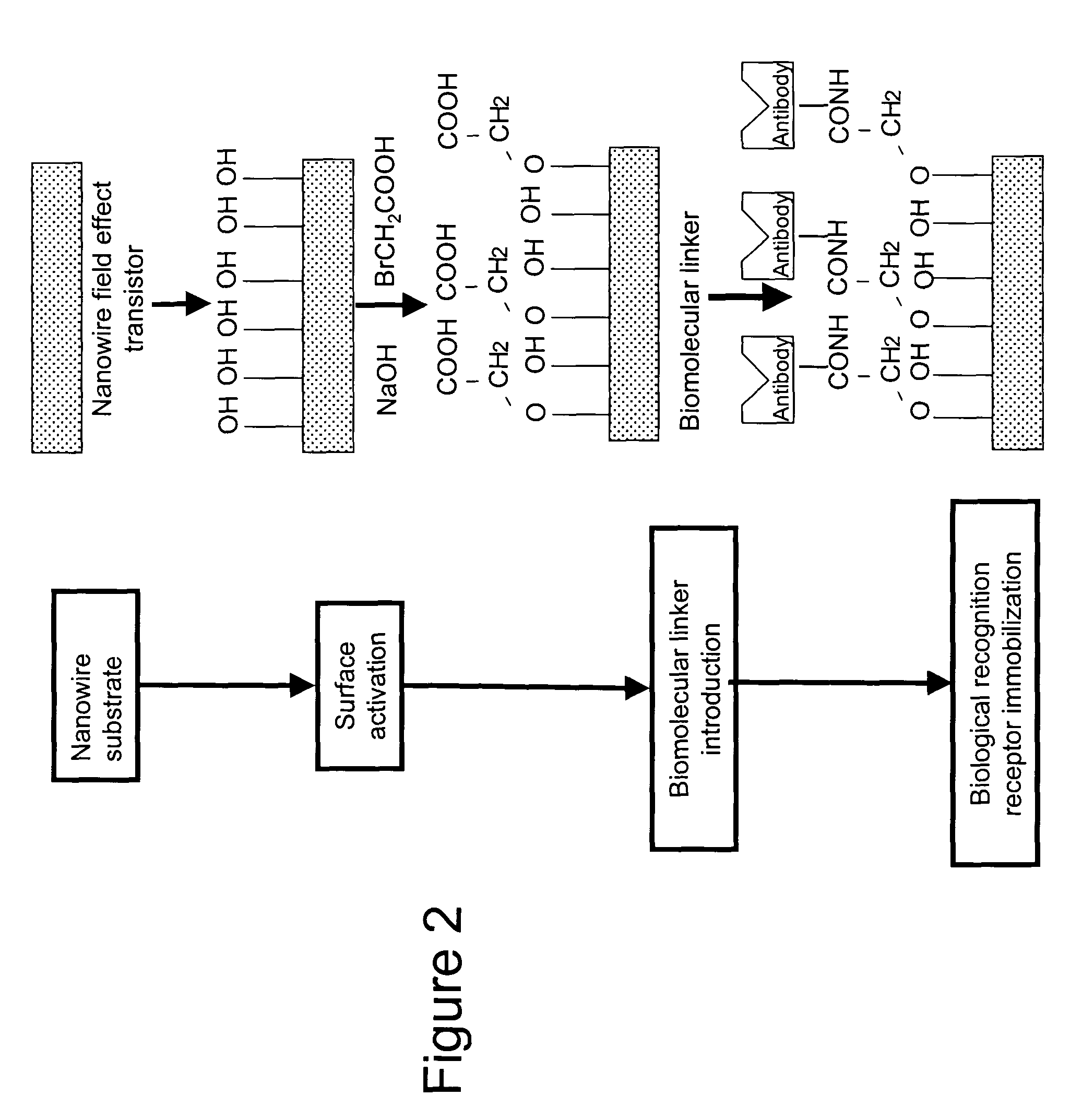
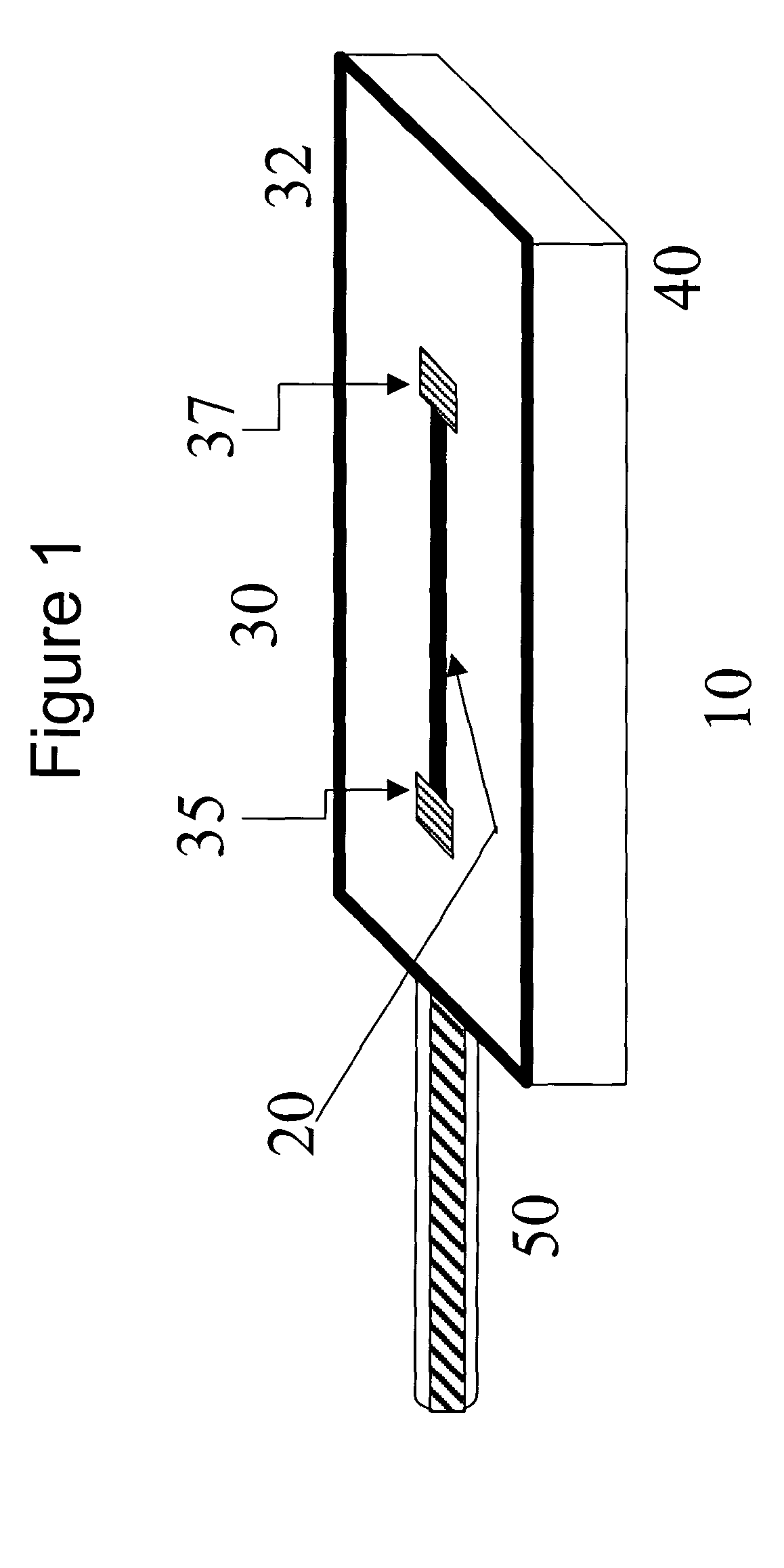
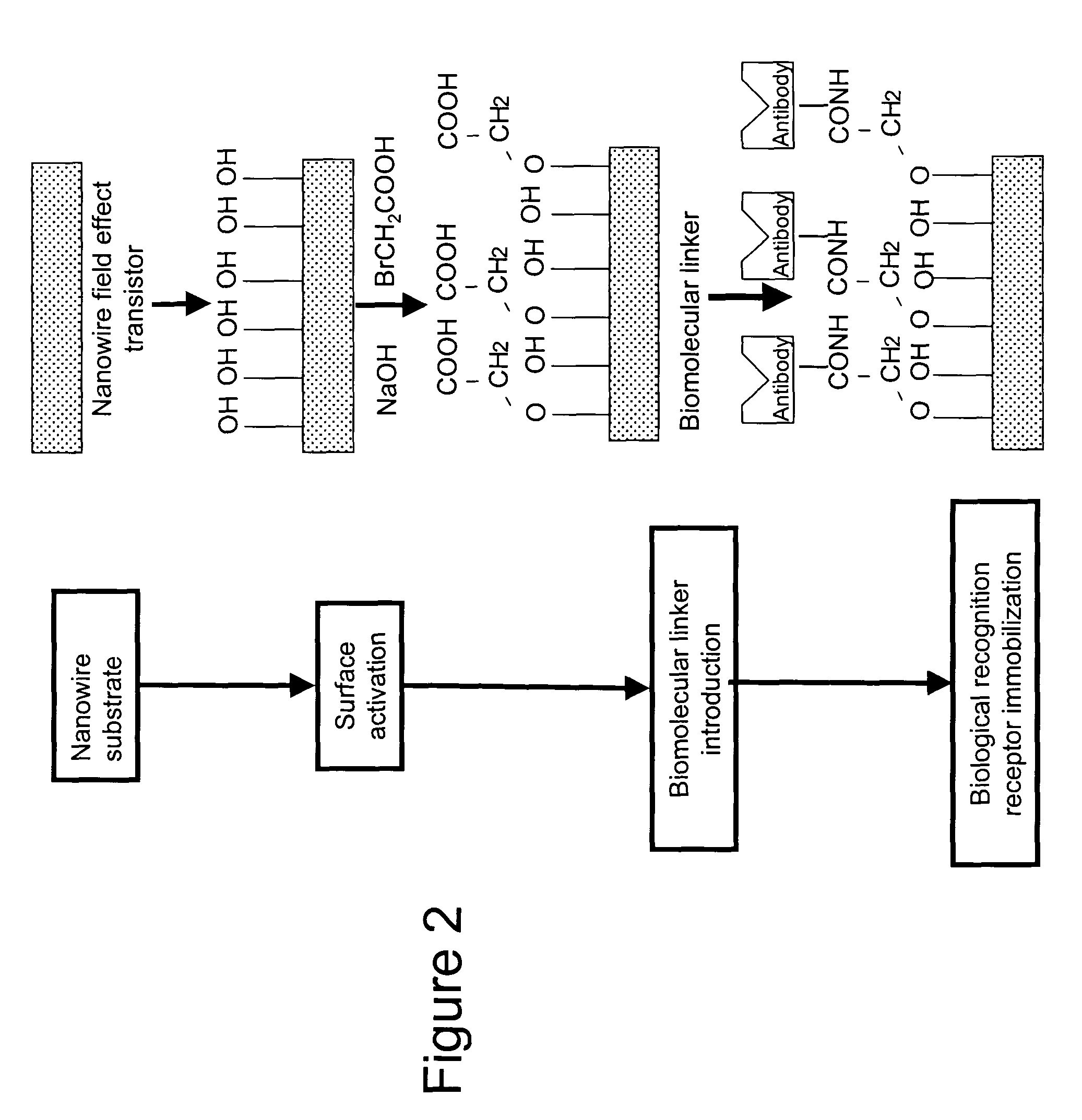
Implantable biosensor devices for monitoring cardiac marker molecules
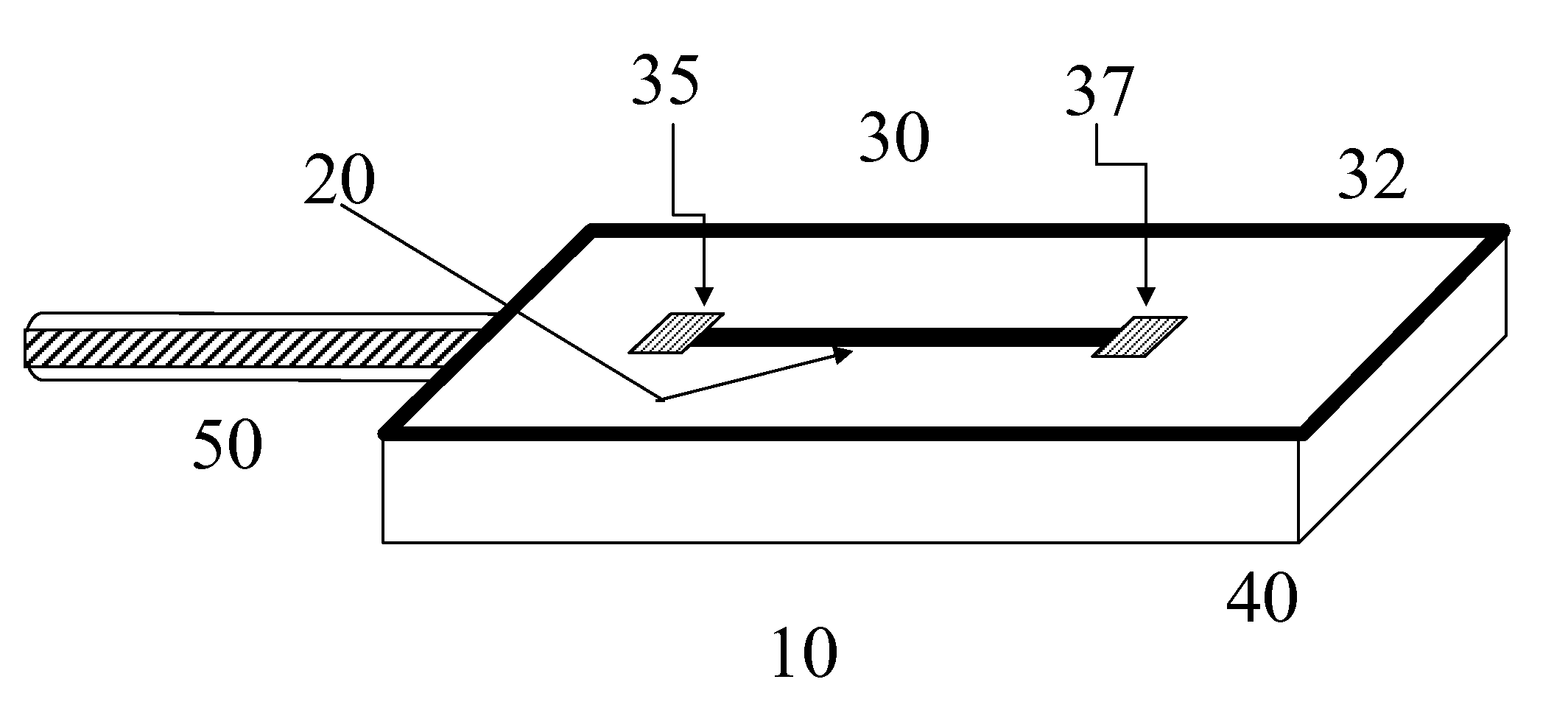
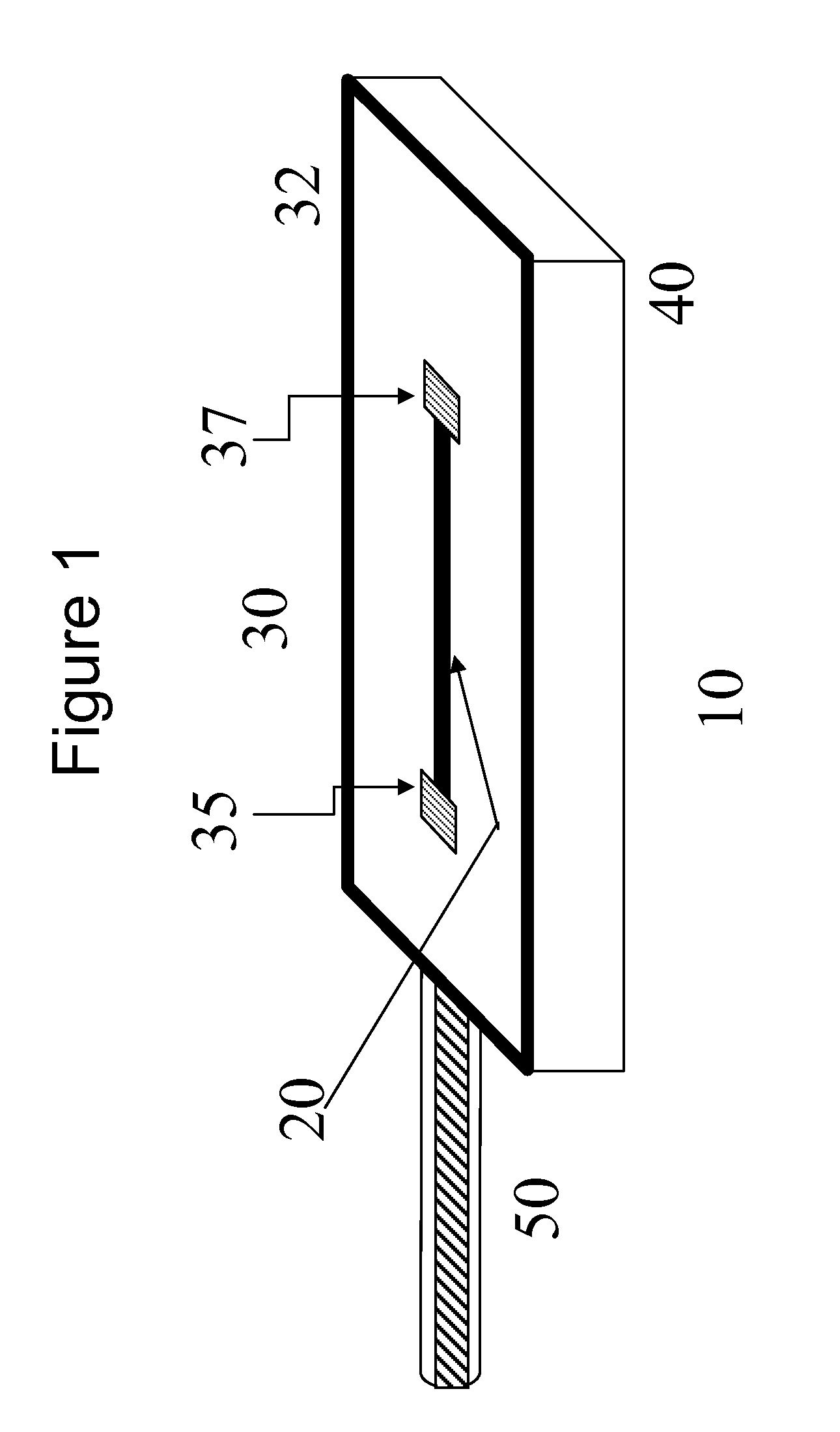
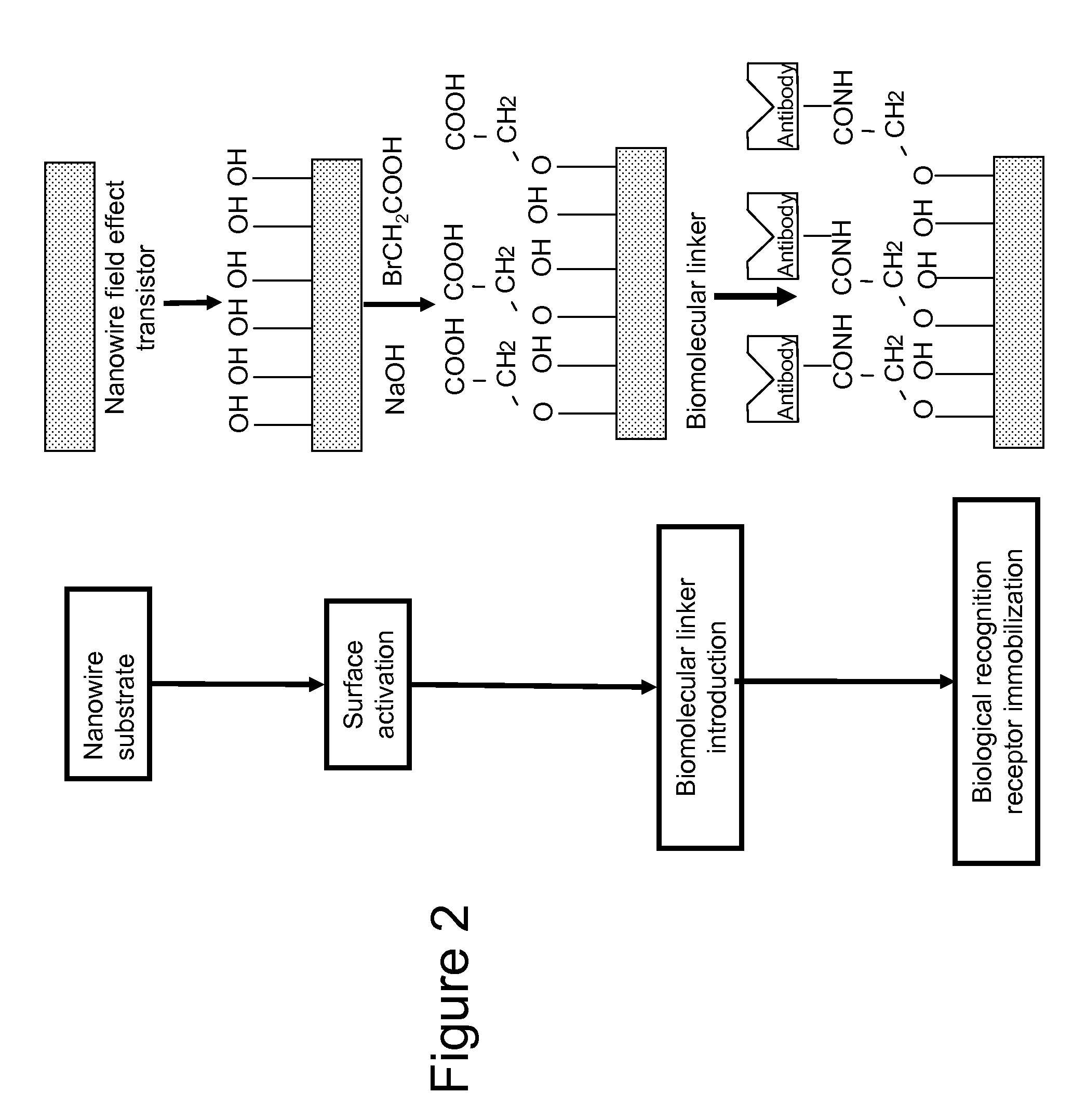
An implantable biosensor system is disclosed for determining levels of cardiac markers in a patient to aid in the diagnosis, determination of the severity and management of cardiovascular diseases. The sensor includes nanowire sensor elements having a biological recognition element attached to a nanowire transducer that specifically binds to the cardiac marker being measured. Each of the sensor elements is associated with a protective member that prevents the sensor element from interacting with the surrounding environment. At a selected time, the protective member may be disabled, thereby allowing the sensor element to begin sensing signals within a living body.
Owner:MEDTRONIC INC
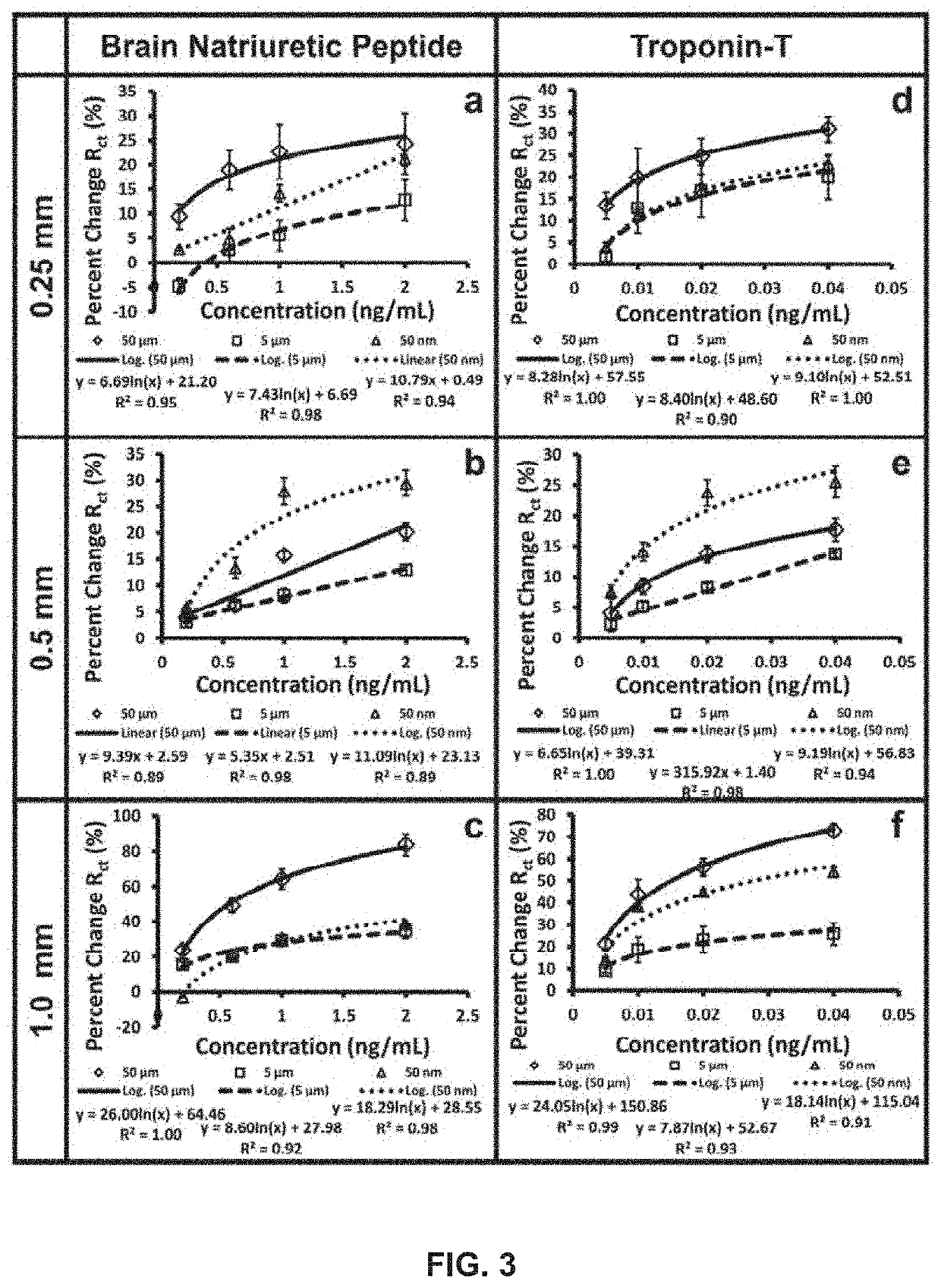
Detection of Cardiac Markers on a Droplet Actuator
InactiveUS20110076692A1Easy to useFacilitates of propertyDisease diagnosisBiological testingActuatorReagent
The present invention provides for the detection of cardiac markers on a droplet actuator. An aspect provides a method of assaying a cardiac marker in a biological sample from a subject, the method including providing a droplet actuator, loading the biological sample and assay reagents on the droplet actuator, executing droplet operations to create sample droplets from the sample and reagent droplets from the reagents on the droplet actuator, and executing droplet operations using the sample droplets and reagent droplets to produce a detectable signal indicative of the quantity of the cardiac marker in the biological sample. Still other aspects are provided.
Owner:DUKE UNIV +1
Methods and apparatus for detecting cardiac injury markers using an acoustic device
InactiveUS20060257945A1Improve the level ofAvoid delayBioreactor/fermenter combinationsMaterial nanotechnologyAnalyteResonant sensor
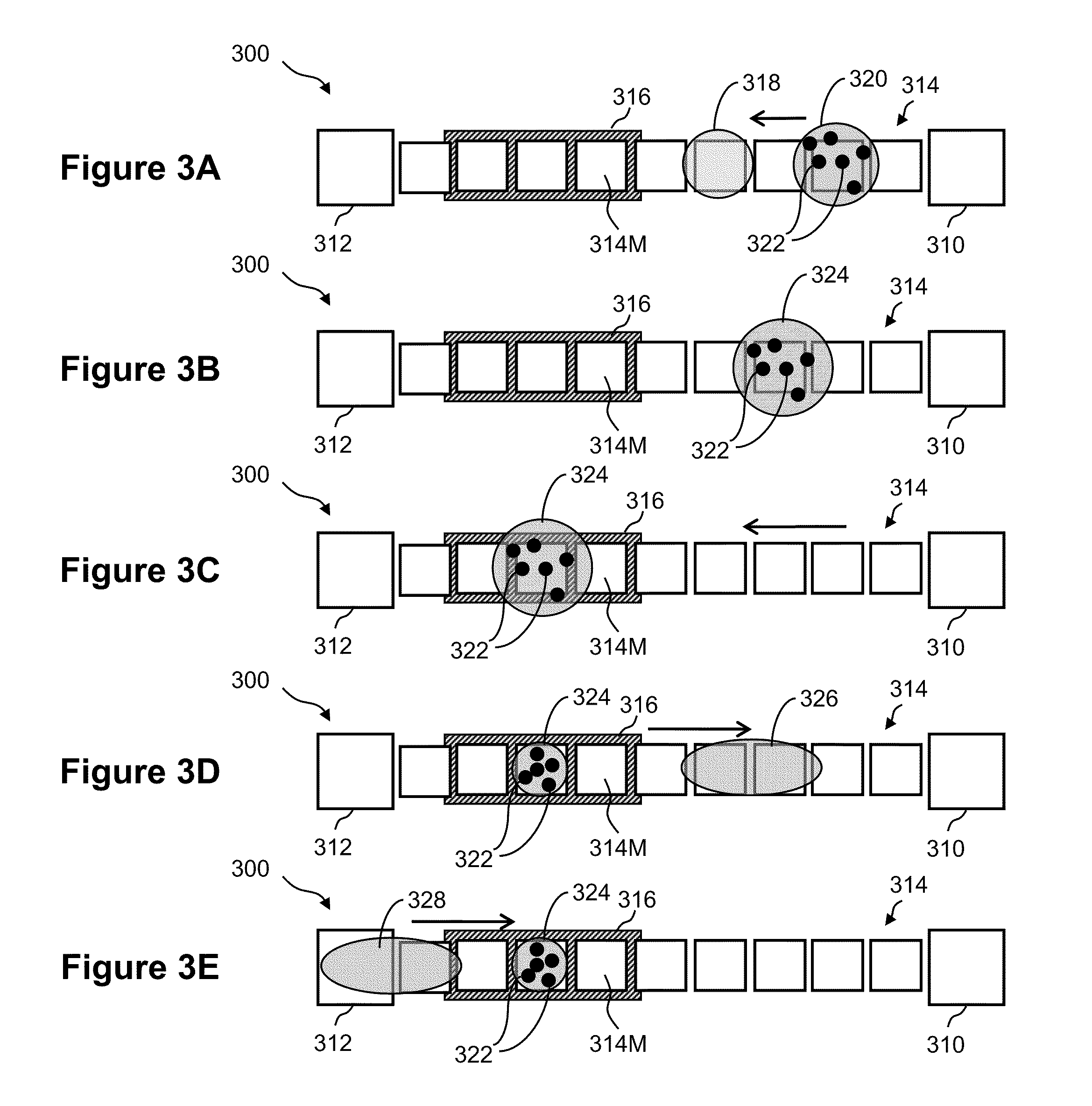
Methods for detecting cardiac injury by detecting one or more cardiac markers are provided. A plurality of particles, each of which is coated with a capture agent having an affinity for a cardiac marker, is combined with the sample to form a plurality of analyte-particle complexes. The system also includes a transport arrangement for transporting the sample to the sensor surface, and optionally a magnetic field inducing structure constructed and arranged to establish a magnetic field at and adjacent to the sensor surface. The resonant sensor produces a signal corresponding to an amount of analyte-particle complexes that are bound to the sensor surface.
Owner:BIOSCALE
Implantable biosensor devices for monitoring cardiac marker molecules
An implantable biosensor system is disclosed for determining levels of cardiac markers in a patient to aid in the diagnosis, determination of the severity and management of cardiovascular diseases. The sensor includes nanowire sensor elements having a biological recognition element attached to a nanowire transducer that specifically binds to the cardiac marker being measured. Each of the sensor elements is associated with a protective member that prevents the sensor element from interacting with the surrounding environment. At a selected time, the protective member may be disabled, thereby allowing the sensor element to begin sensing signals within a living body.
Owner:MEDTRONIC INC
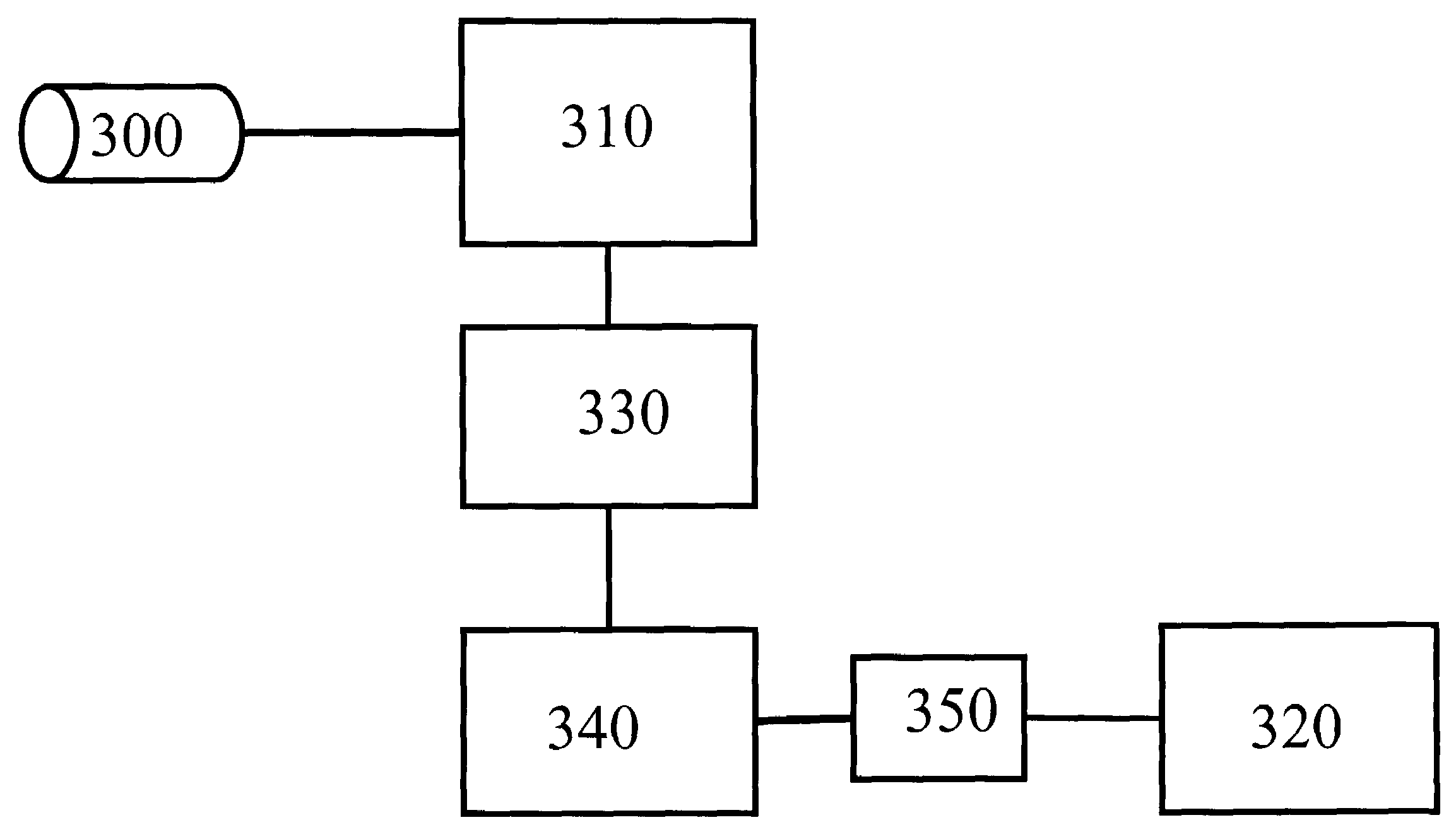
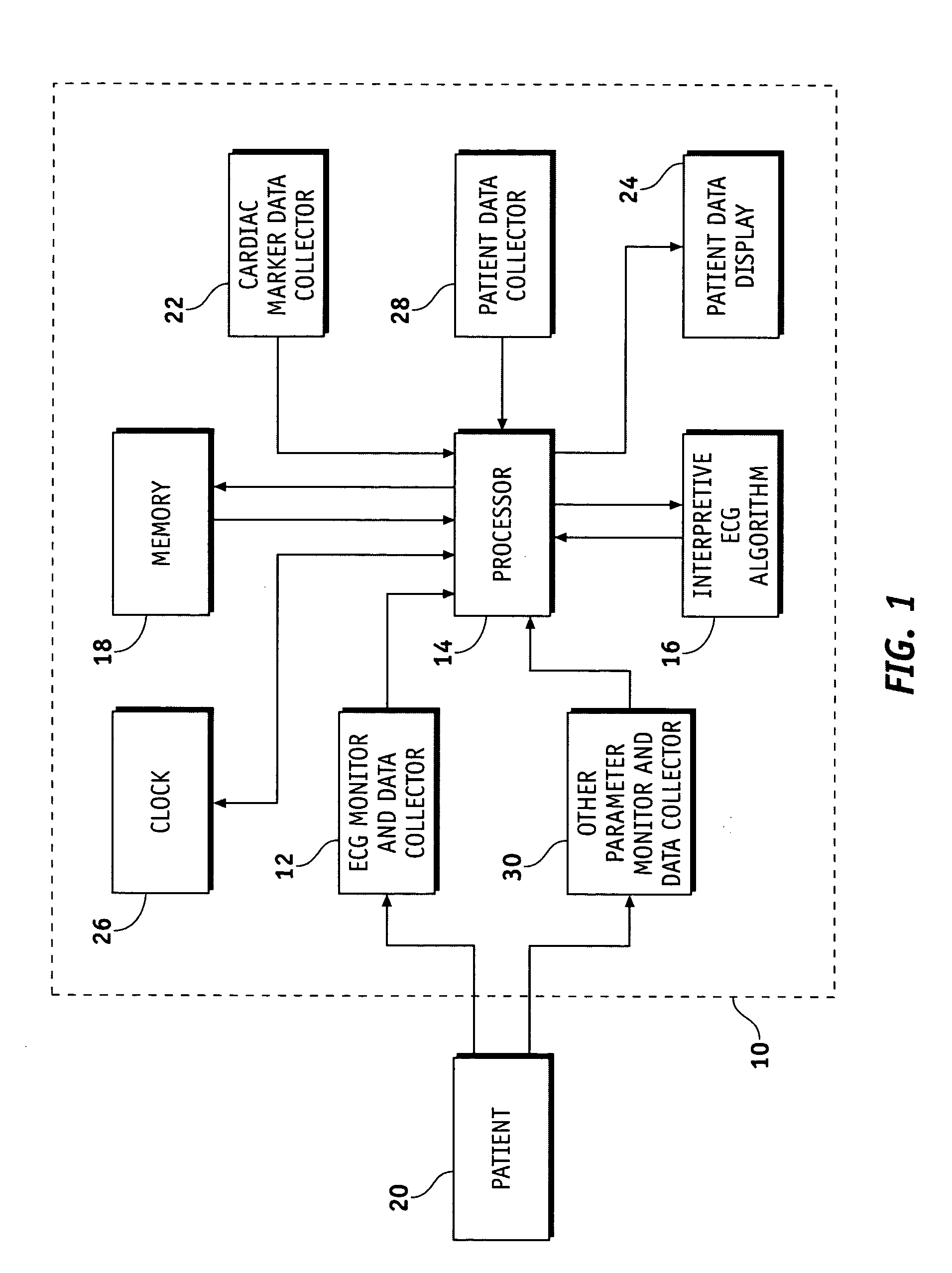
Apparatus and methods for documenting myocardial ischemia
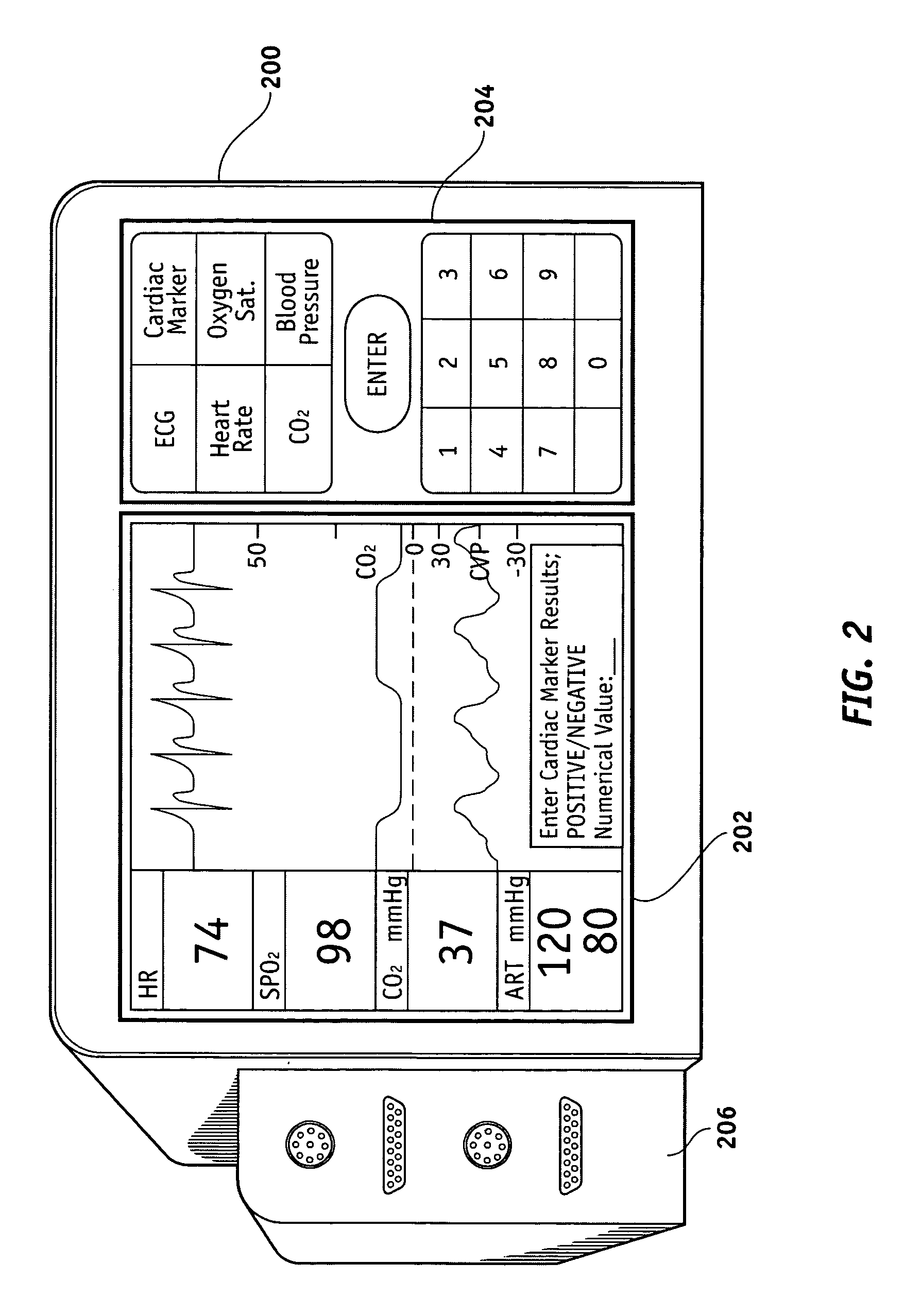
Methods and apparatus are provided for documenting the myocardial ischemia of a patient's heart. The apparatus comprises an ECG monitor and data collector configured to receive electrocardial data about the patient's heart. The apparatus further comprises a cardiac marker data collector configured to receive cardiac marker data about the patient's heart. A data processing and recording module is in electrical communication with the ECG monitor and data collector and the cardiac marker data collector and is configured to record the electrocardial data and the cardiac marker data. The method comprises the steps of obtaining electrocardial data about the patient's heart and receiving results of a cardiac marker test performed on the patient. The electrocardial data and the test results are stored in a patient report, which then may be displayed.
Owner:MEDTRONICS PHYSIO CONTROL MFG
Indirect detection of cardiac markers for assessing acute myocardial infarction
InactiveUS20060040412A1Microbiological testing/measurementDisease diagnosisMeasurement testMicroparticle
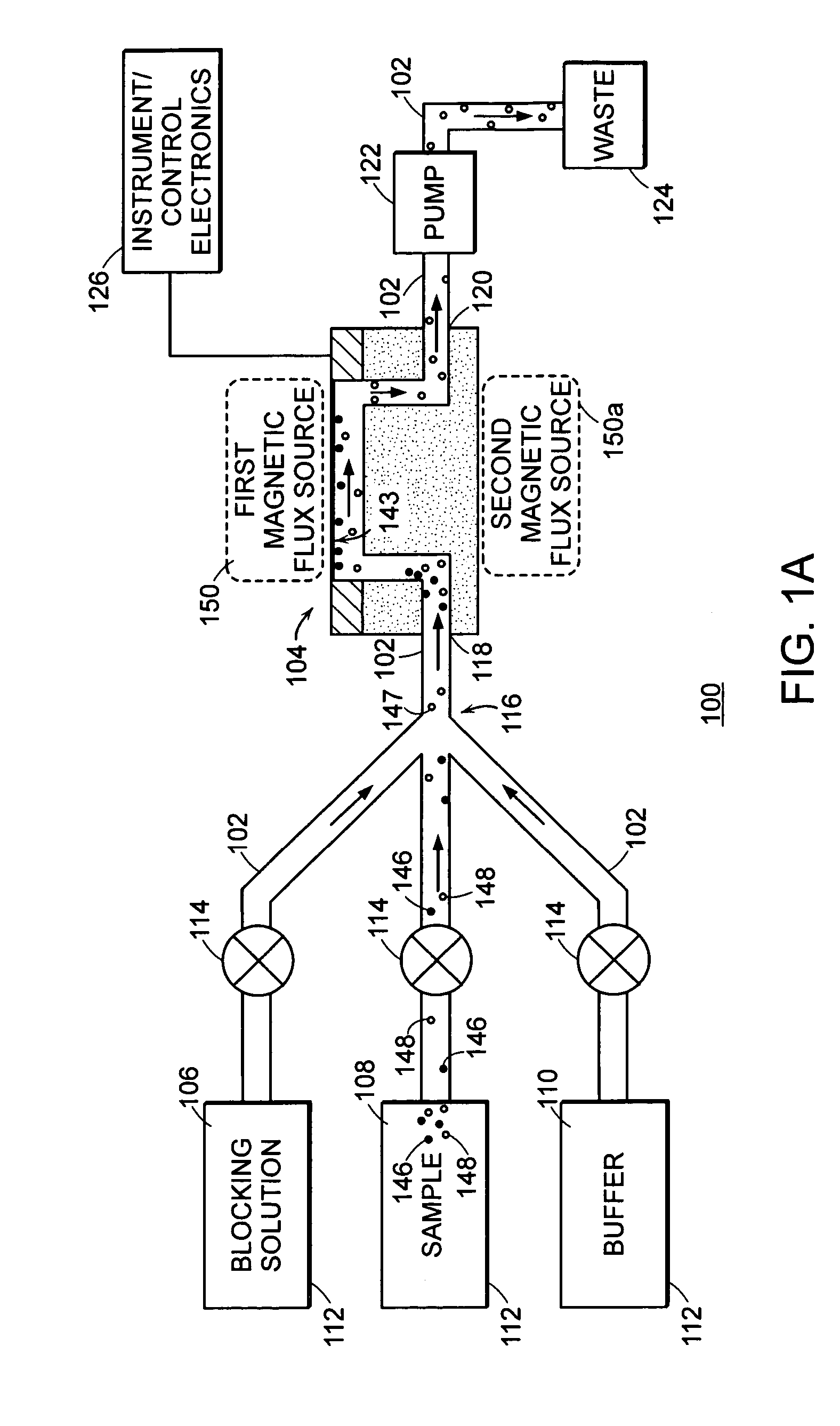
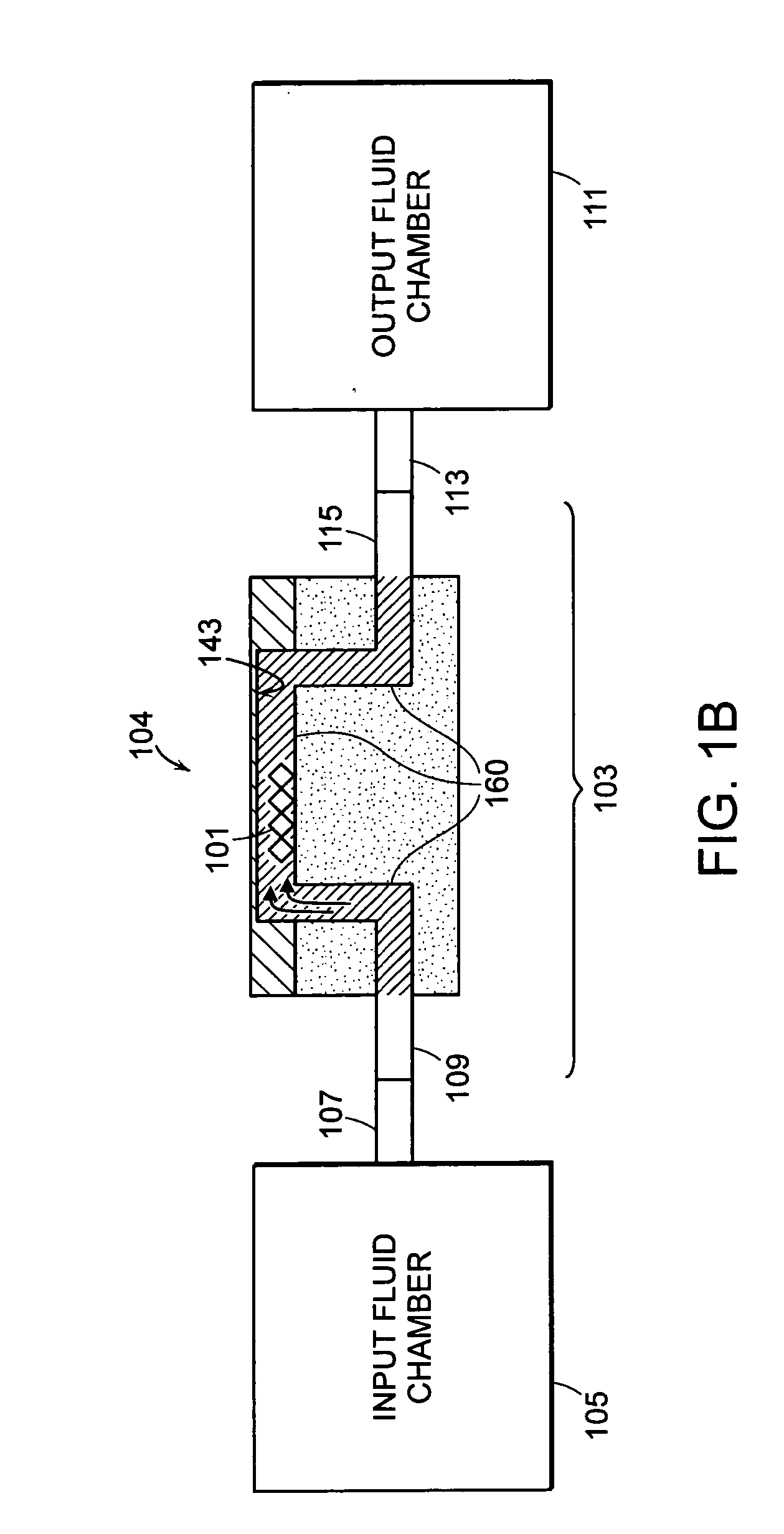
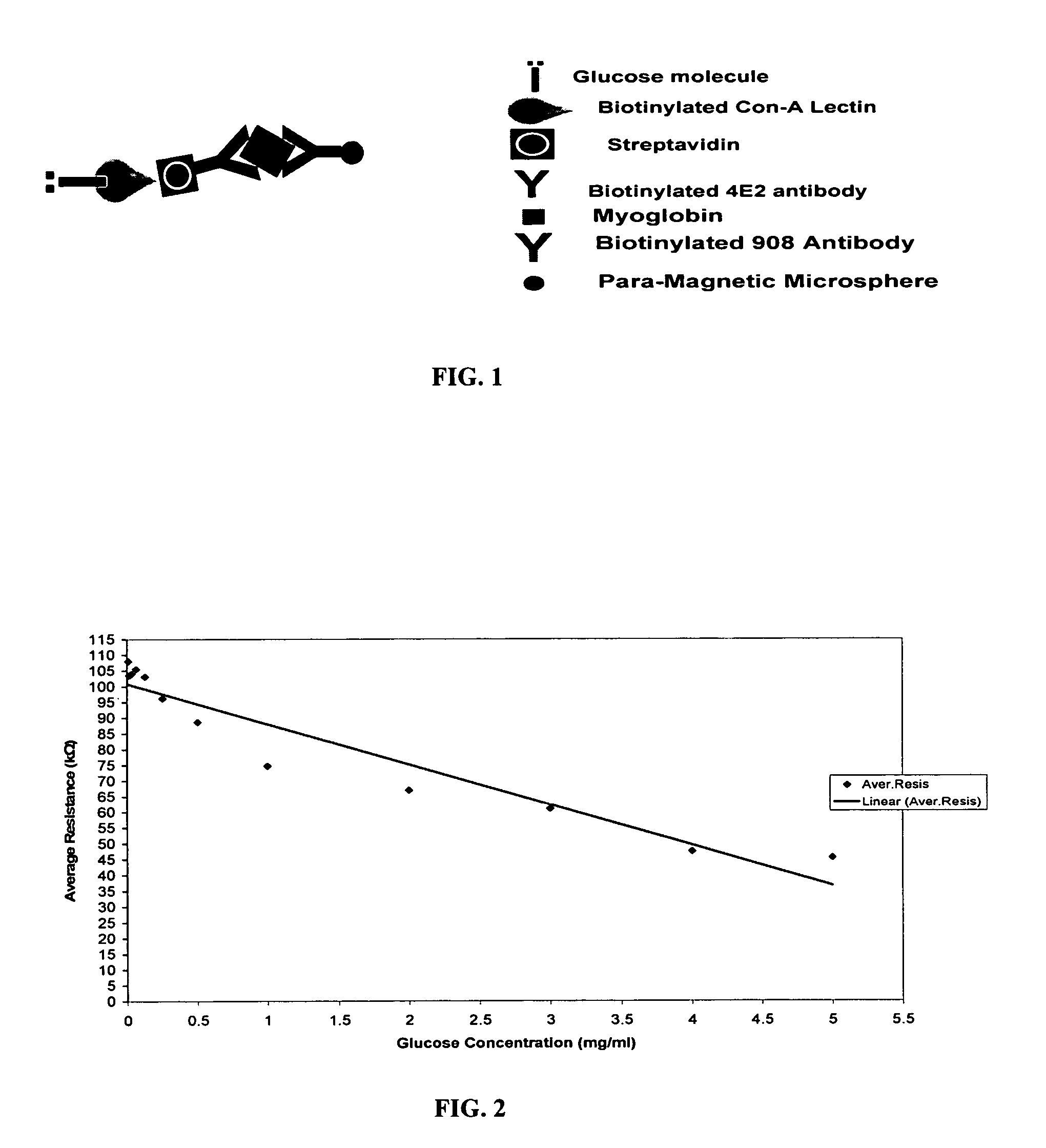
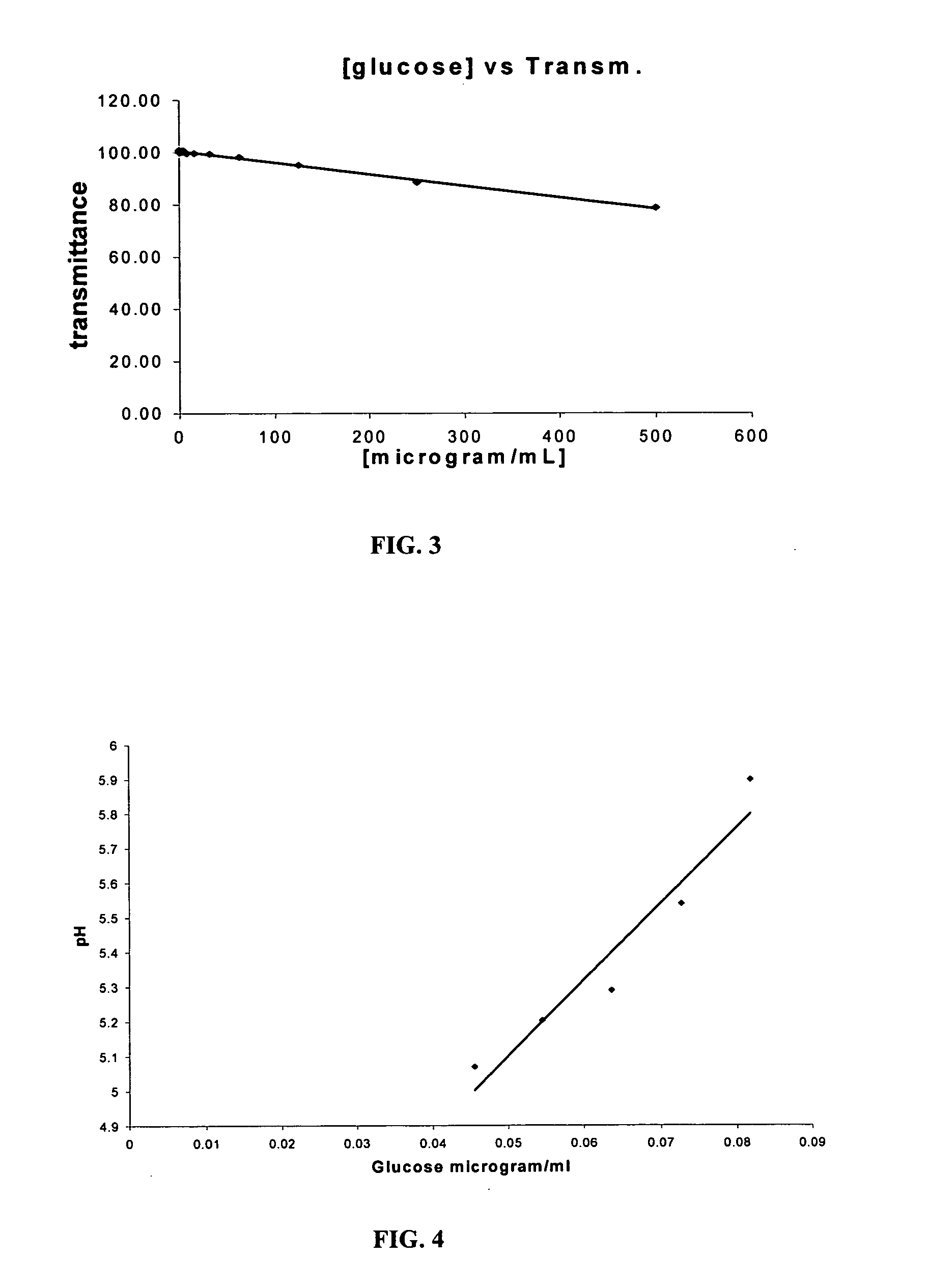
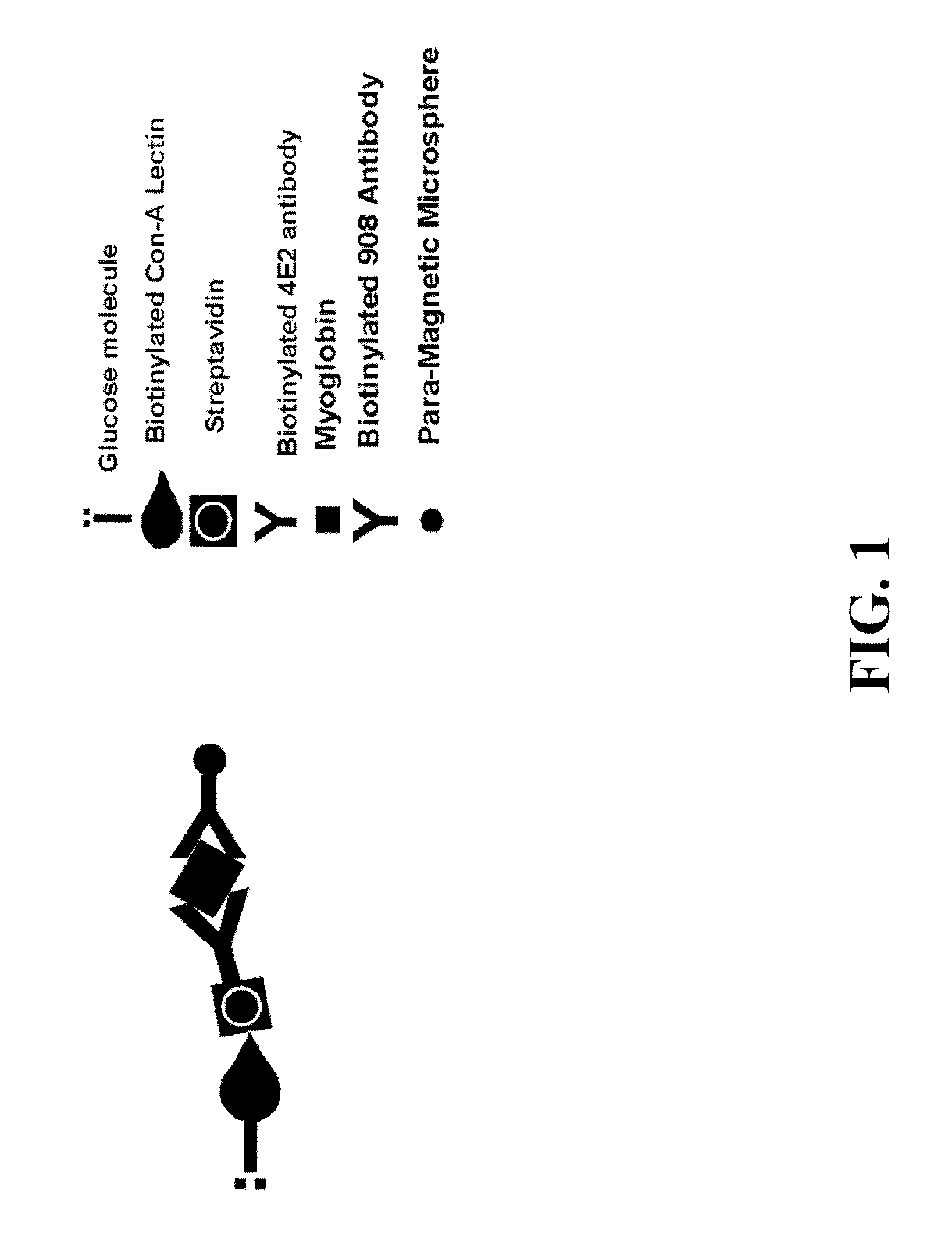
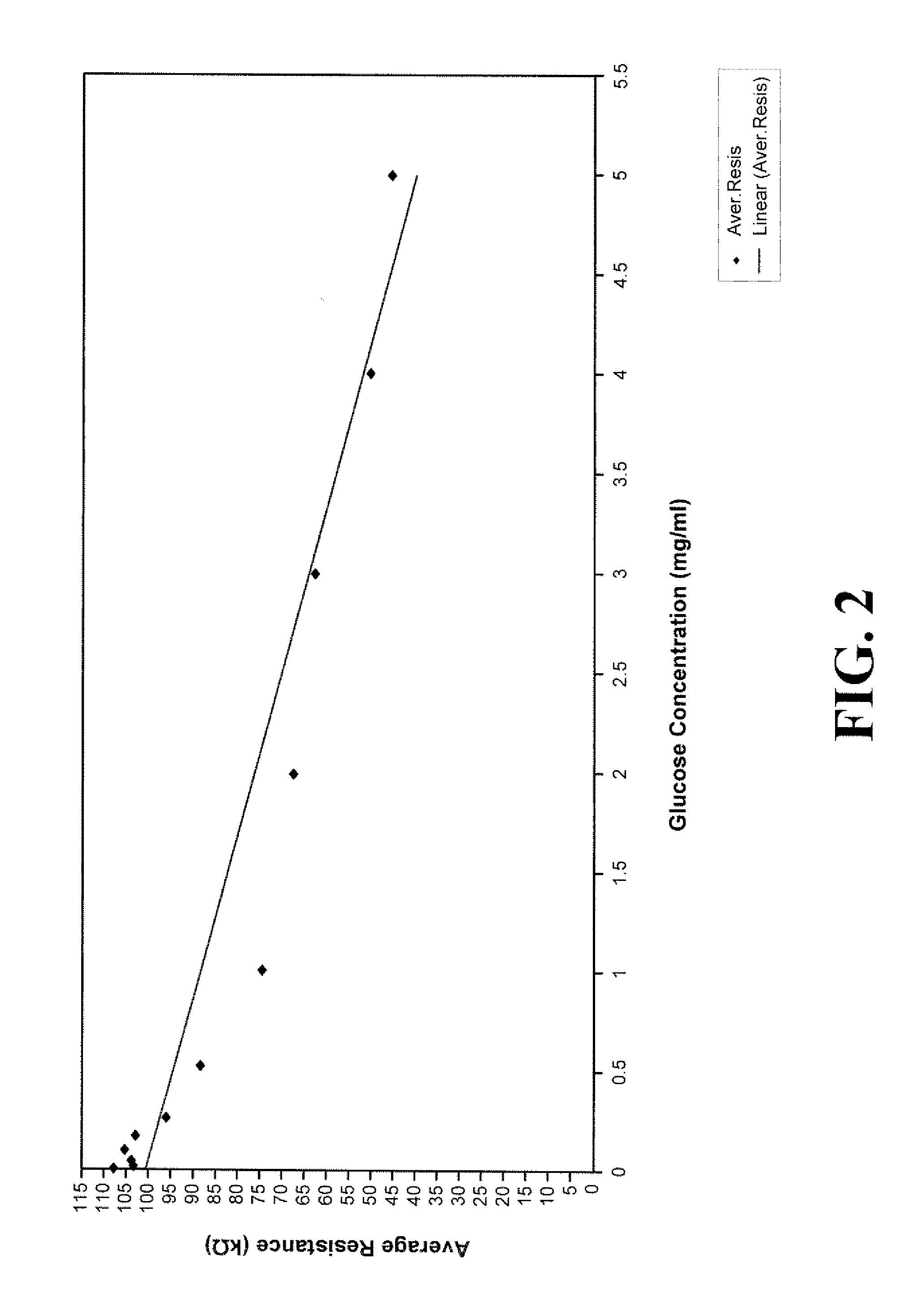
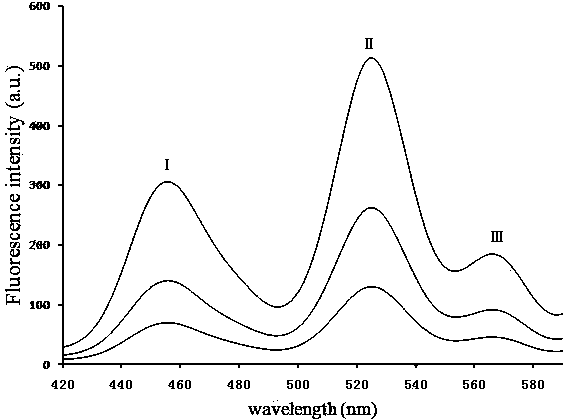
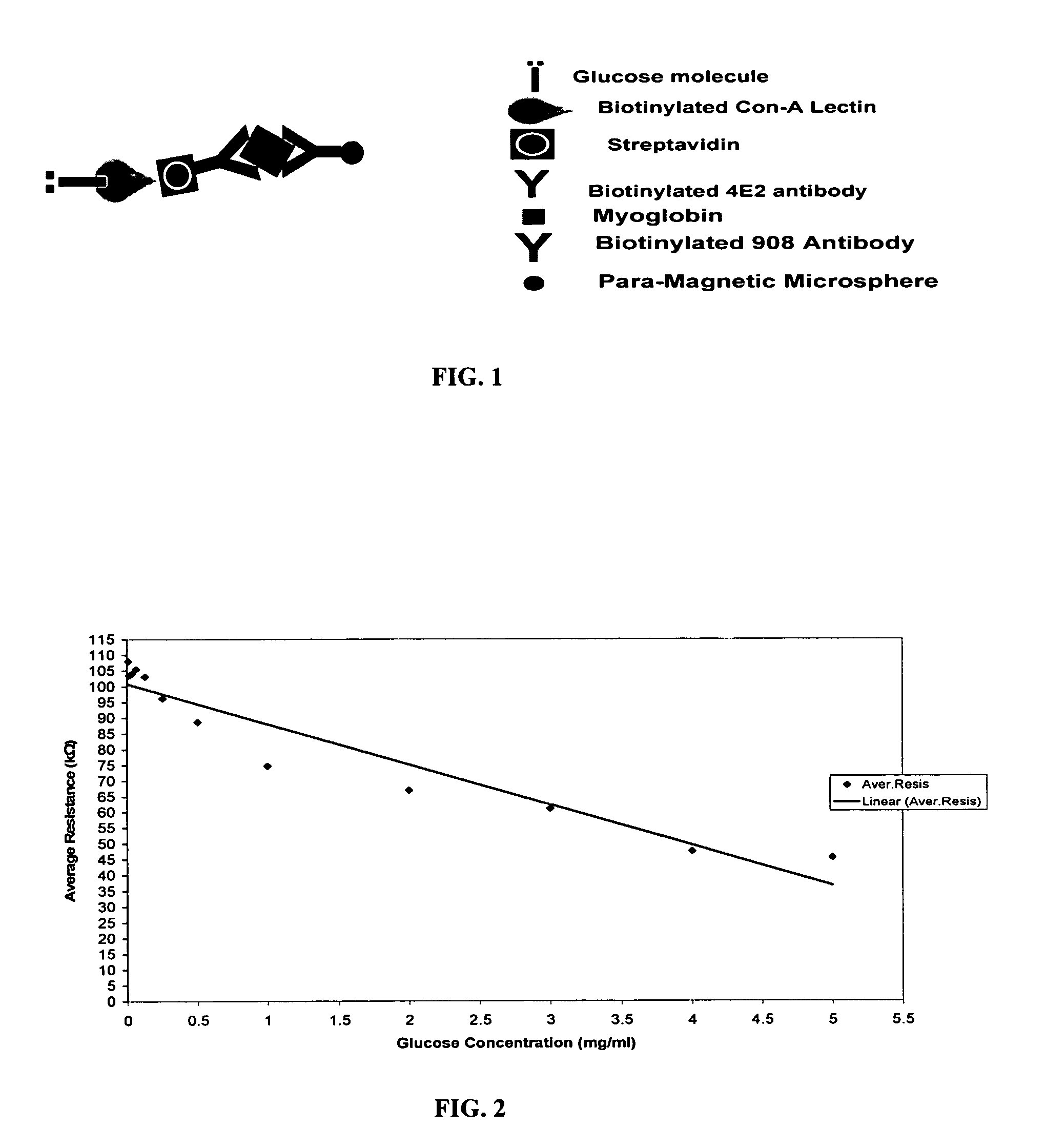
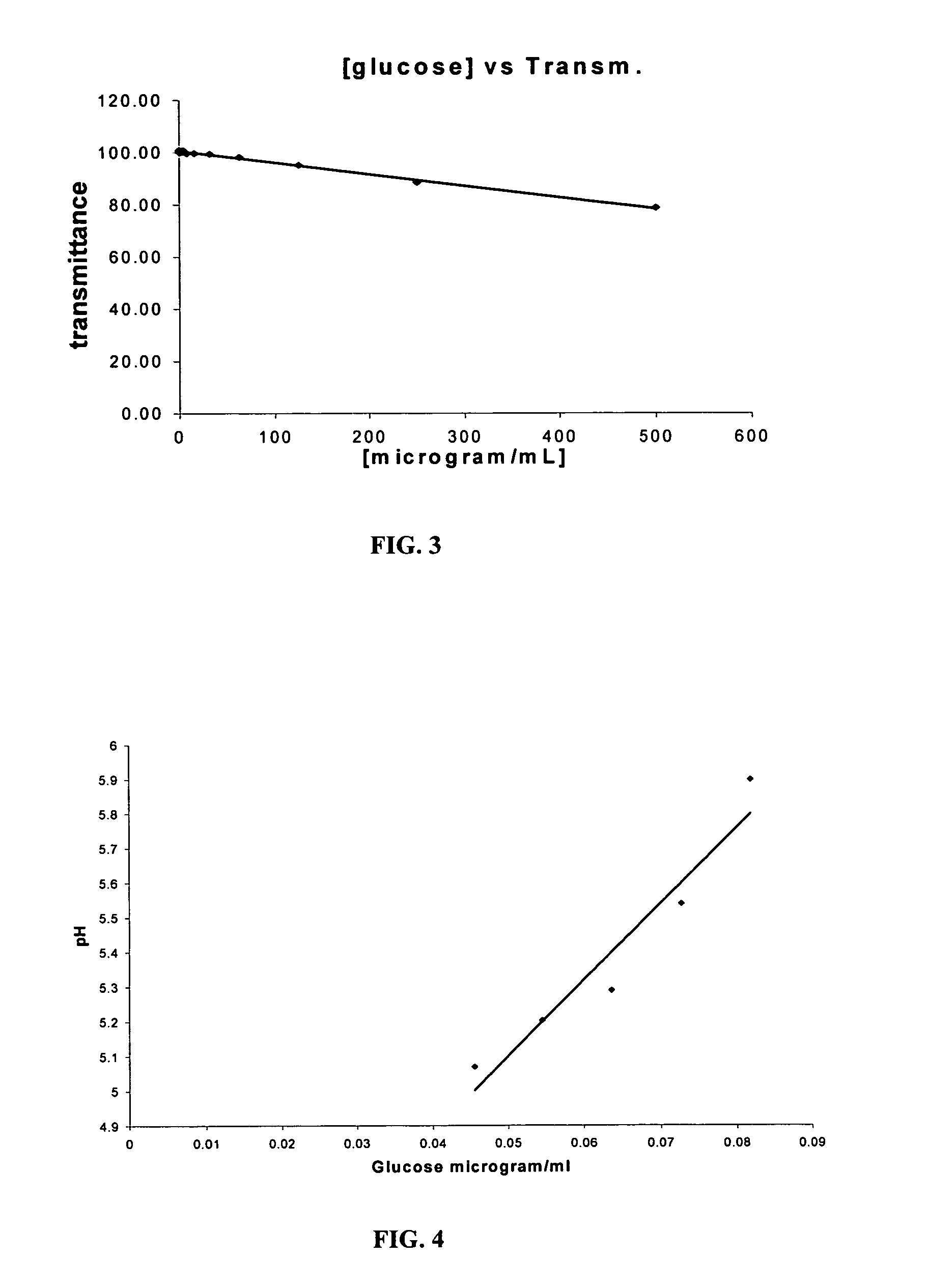
Assay systems and methods are provided for detecting a target antigen in a physiological fluid (e.g., blood, serum, or urine). The method includes linking via a first antibody a magnetic microparticle to the target antigen in the physiological fluid; linking via a second antibody a glucose molecule to the target antigen; utilizing a magnetic field to separate the magnetic microparticle-linked antigen from the physiological fluid to form a test sample; and detecting the glucose in the test sample to determine the concentration of target antigen in the physiological fluid. The target antigen can be a protein or marker resulting from cardiac tissue injury, which can be used to assess acute myocardial infarction. An exemplary target antigen is myoglobin. The glucose detection preferably is one that can be done rapidly, e.g., with a conventional glucometer, and may include measuring the electrical resistance, color, or pH of the test sample.
Owner:FLORIDA STATE UNIV RES FOUND INC
Micro-fluidic chip based on magnetic bead coated antibody and method for capturing cardiac markers
ActiveCN105424922AImprove capture abilityEnsure identificationMaterial analysisMagnetic beadAntigen binding
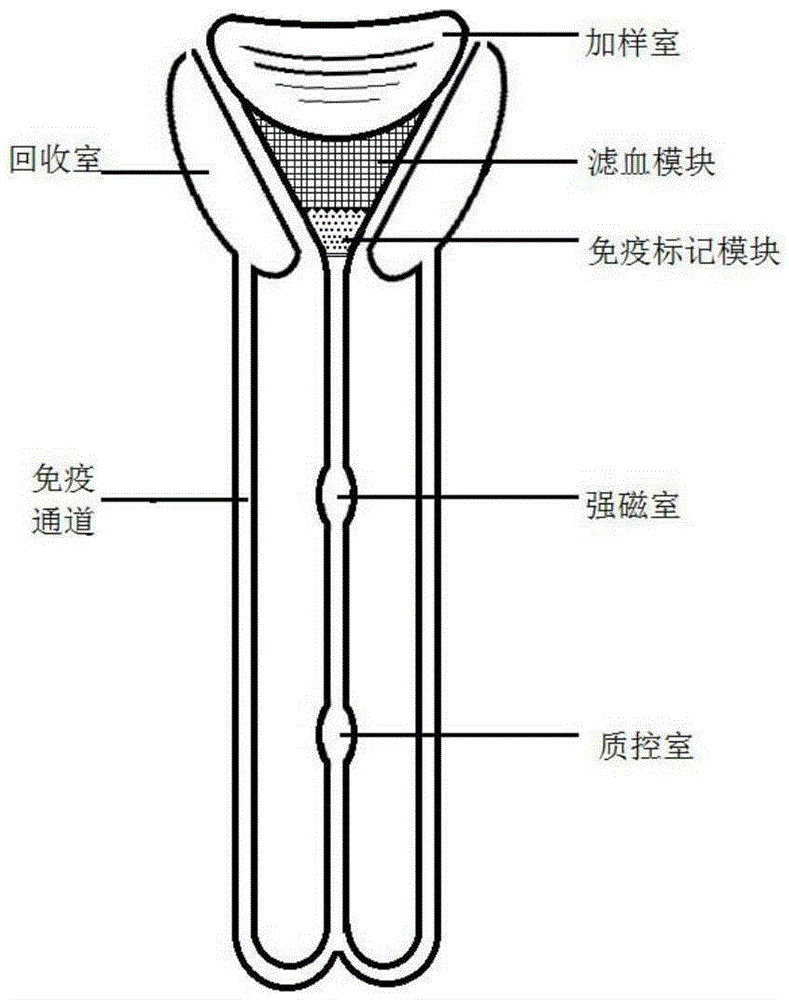
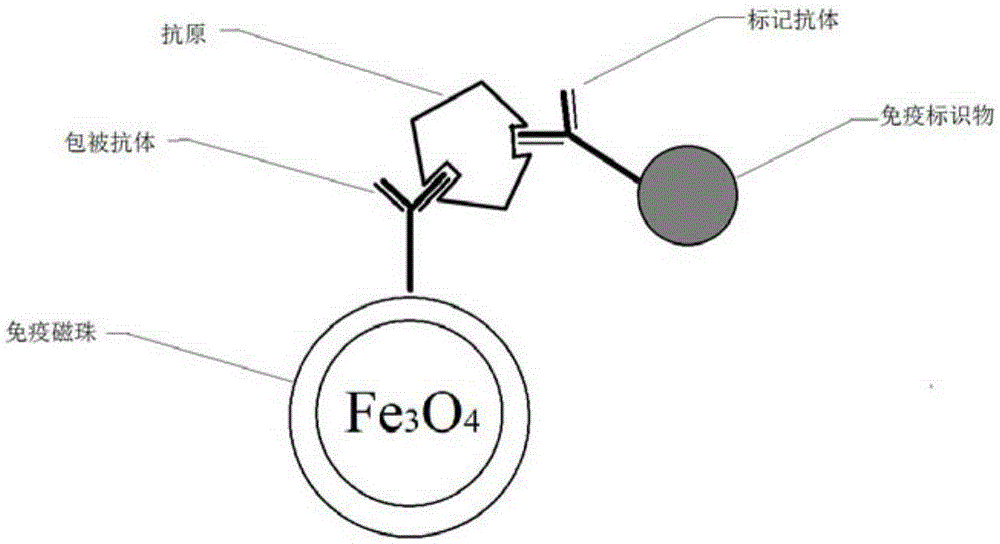
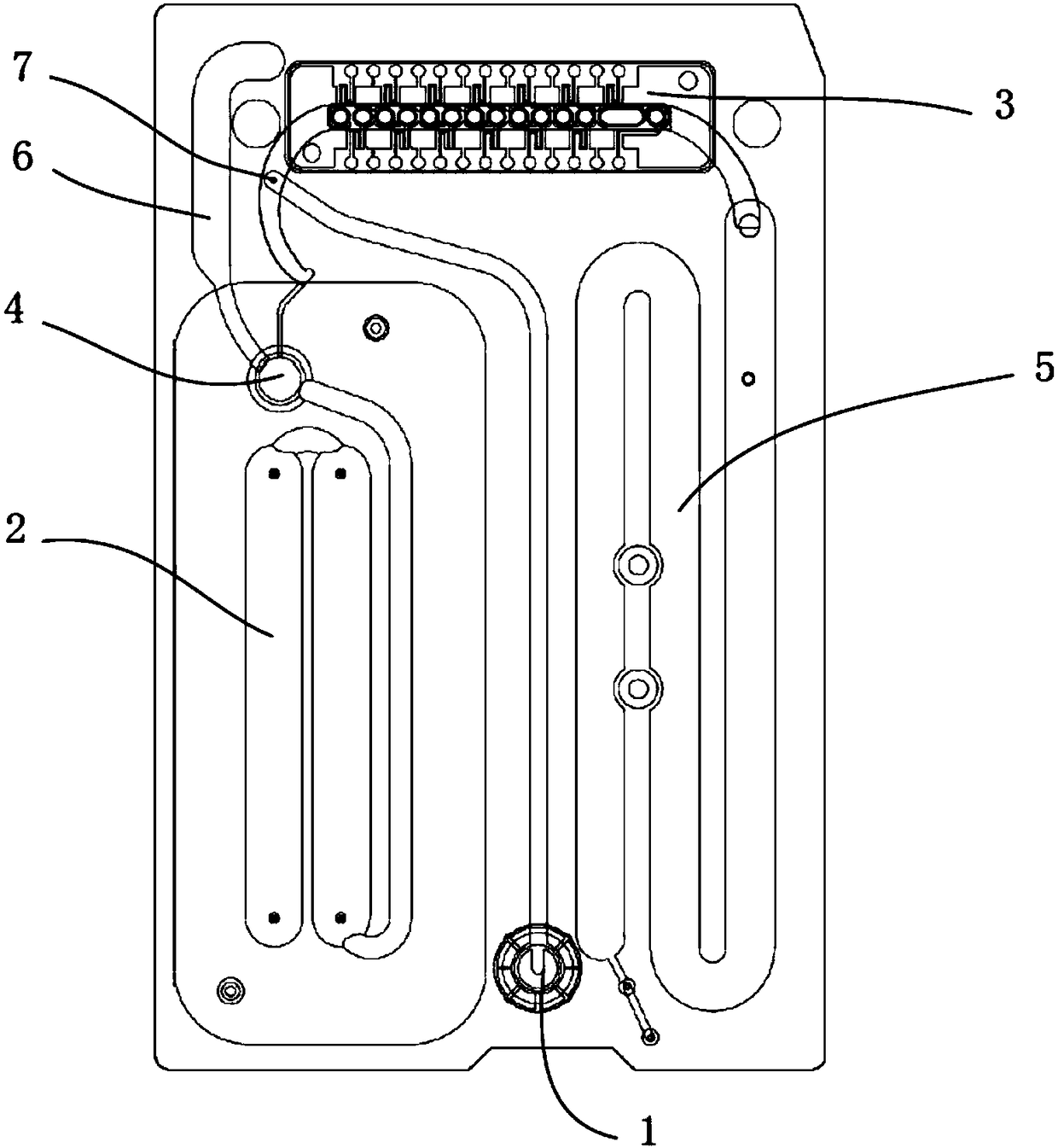
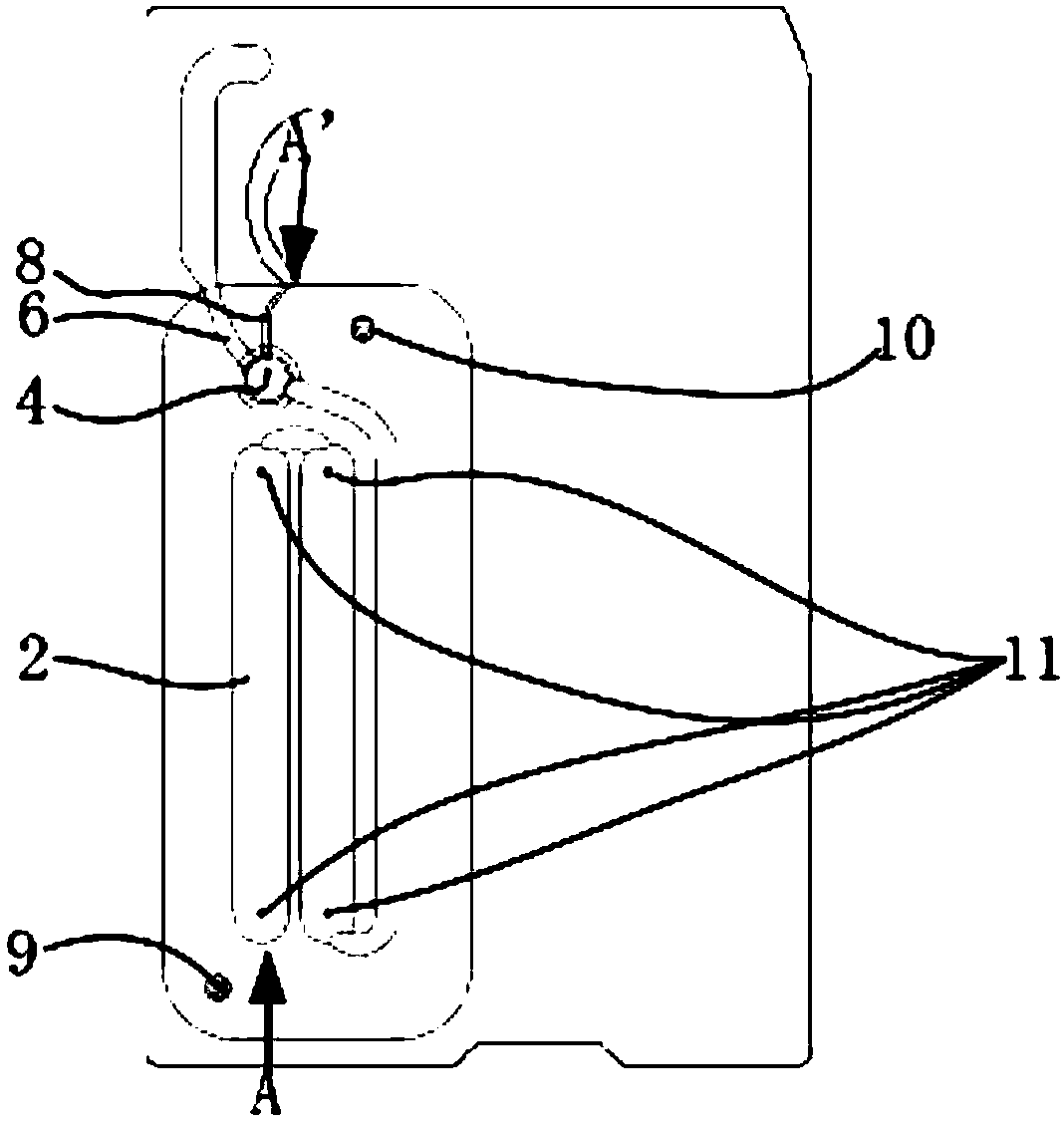
The embodiment of the invention discloses a micro-fluidic chip based on a magnetic bead coated antibody and a method for capturing cardiac markers. The micro-fluidic chip based on the magnetic bead coated antibody and the method for capturing cardiac markers are applied to the field of immunomagnetic bead coated binding antibodies, and prevent coated antibodies from being redissolved in a fluid system or being captured by antigens in fluid and disengaged from a base material. The micro-fluidic chip comprises a sample injection chamber, a Y-shaped structure channel and recycling chambers. The Y-shaped structure channel comprises a funnel-shaped channel at the upper portion and a columnar channel at the lower portion. An immunolabelling module is arranged at the bottom of the funnel-shaped channel. A blood filtering module is arranged at the upper portion of the immunolabelling module. The bottom end of the columnar channel at the lower portion is forked to form a pair of parallel immunity channels. The tail end of each immunity channel is connected with one recycling chamber. The columnar channel is provided with an upper cavity and a lower cavity. The upper cavity is a strong magnetism cavity, and the lower cavity is a quality control cavity.
Owner:北京乐普诊断科技股份有限公司
Method, system and chip test paper for parallel detection on various cardiac markers
InactiveCN102636651AHigh sensitivityShorten detection timeAnalysis by material excitationBiological testingFiberCreatine kinase
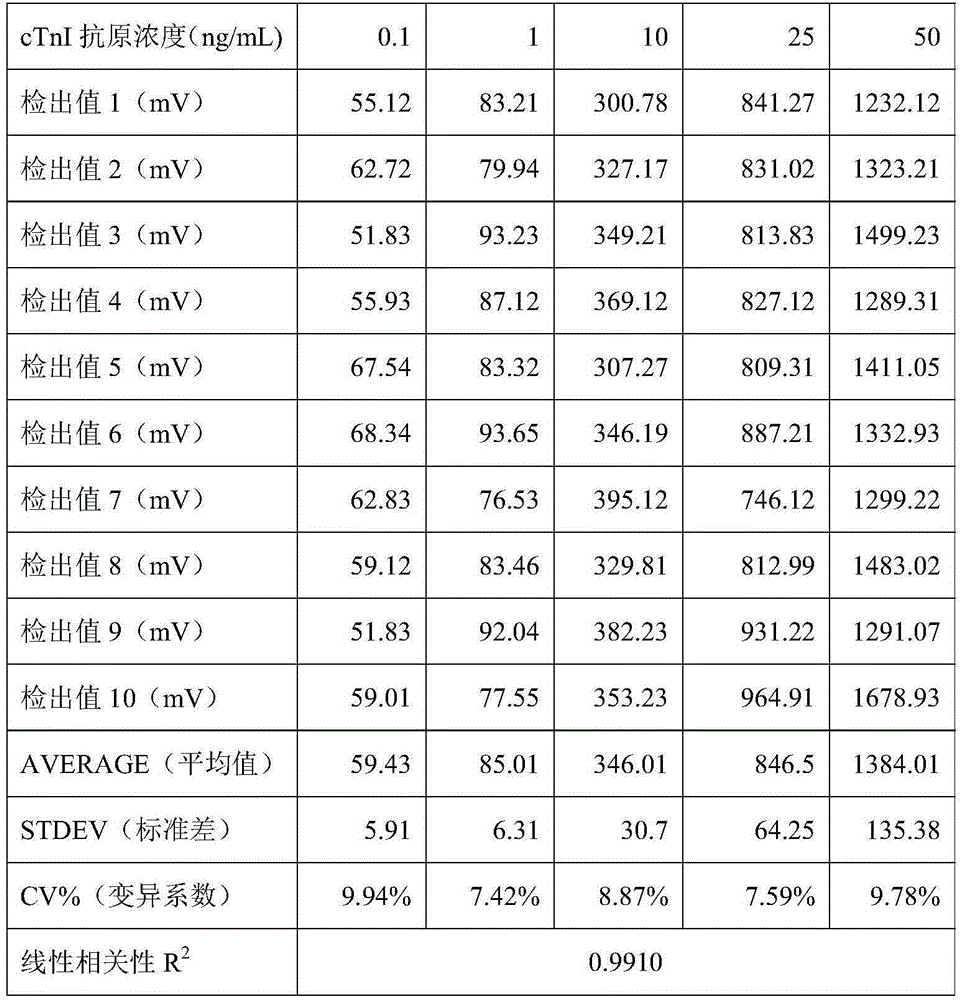
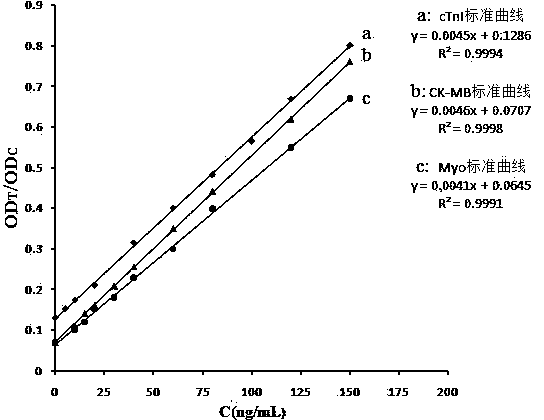
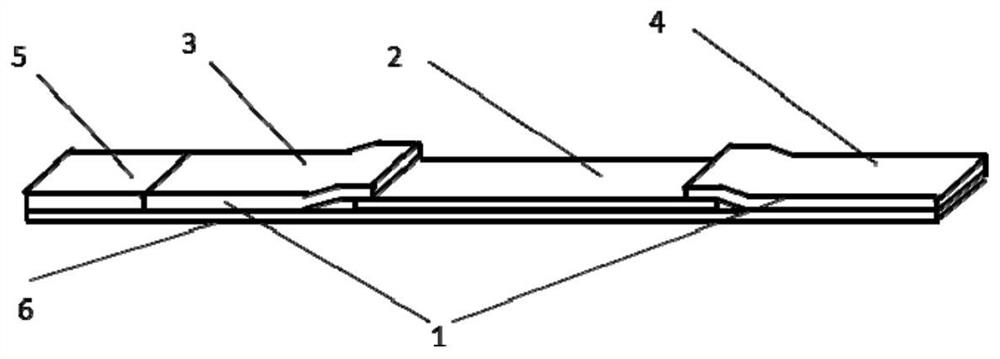
The invention discloses a method, system and chip test paper for parallel detection on various cardiac markers. The chip test paper is used for detecting a part or all of the cardiac markers including cTNI (cardiac troponin I), cTNT (cardiac troponin T), MYO (myoglobin), CK-MB (isoenzymeof creatine kinase containing M and B subunits), BNP (B-type natriuretic peptide), CRP (C-reactive protein) and FABP (fatty acid-binding protein); the chip test paper comprises a first membrane and a second membrane; a ligand An of each marker is arranged on the first membrane, and a ligand Bn coupled with a signal marker is absorbed on the second membrane; and a detected material forming sandwich detection together with the ligands An and Bn is added from a sampling hole, then under the acting force of percolation or other factors, the detected material moves to be combined with the ligands An and Bn respectively to form a composite array of the first membrane-the ligand An-the detected material-the ligand Bn-the signal marker, which is fixed on the first membrane, the composite array and a capture fiber membrane are assembled simultaneously, and a detection hole is reserved. The method, the system and the chip test paper provided by the invention can be used for detecting the various cardiac markers simultaneously and quantitatively; moreover, the detection sensitivity can be improved, and the detection time is saved.
Owner:SHANGHAI LINC BIO SCI
System and assay for detection of cardiac markers for assessing acute myocardial infarction
Assay systems and methods are provided for detecting a target antigen in a physiological fluid (e.g., blood, serum, or urine). The method includes linking via a first antibody a magnetic microparticle to the target antigen in the physiological fluid; linking via a second antibody a glucose molecule to the target antigen; utilizing a magnetic field to separate the magnetic microparticle-linked antigen from the physiological fluid to form a test sample; and detecting the glucose in the test sample to determine the concentration of target antigen in the physiological fluid. The target antigen can be a protein or marker resulting from cardiac tissue injury, which can be used to assess acute myocardial infarction. An exemplar target antigen is myoglobin. The glucose detection preferably is one that can be done rapidly, e.g., with a conventional glucometer, and may include measuring the electrical resistance, color, or pH of the test sample.
Owner:FLORIDA STATE UNIV RES FOUND INC
Detection device and application thereof
ActiveCN108120755AAvoid risk of cloggingImprove convenienceMaterial analysis by electric/magnetic meansMedical wasteHematological test
The invention relates to the field of in-vitro diagnosing medical appliances, particularly relates to a detection device, as well as a preparation method and application thereof, and further disclosesa POCT diagnostic card. The diagnostic card integrates a calibration reagent, a reagent valve, an electrode array chip, a waste liquid storage cavity and the like, so that reagent sampling is not required in the detecting process, no extra medical waste is generated, and all fluids and pipelines are integrated on the test card, so that use convenience is greatly improved, and the risk of fluid path blockage of an instrument can be avoided. The diagnostic card applies the electrochemical principle, can be used for performing in-vitro diagnosis on patients and analyzing certain biological and chemical substances in human blood samples, and can be applied to multiple medical diagnosing fields, such as biochemical item tests, blood-qi item tests, metabolin tests, hematological tests, clottingtests and immune tests (cardiac markers and the like).
Owner:SHENZHEN GOLDSITE DIAGNOSTICS
Fluorescent microsphere combined detection device for cardiac marker and preparation method of fluorescent microsphere combined detection device
InactiveCN109799334ASensitive detectionSpecific detectionBiological testingGlass fiberImmunofluorescence
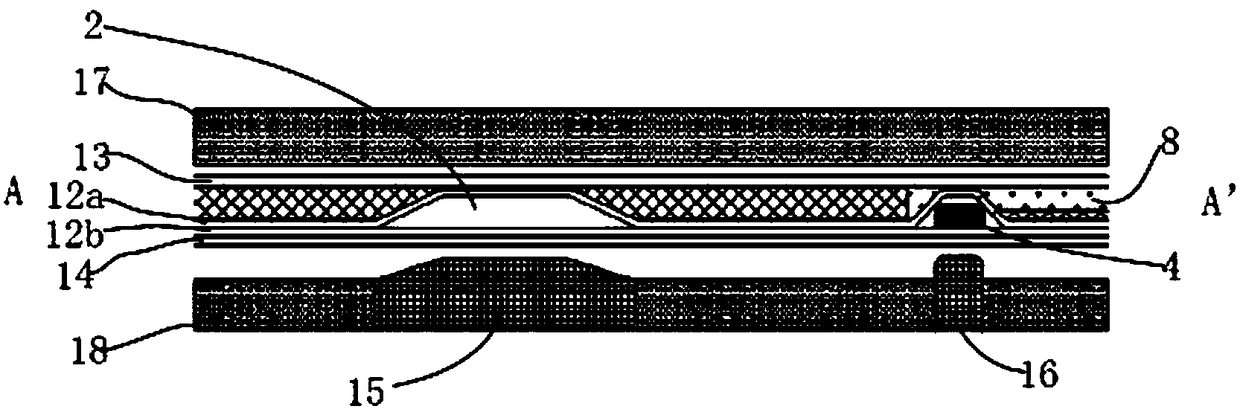
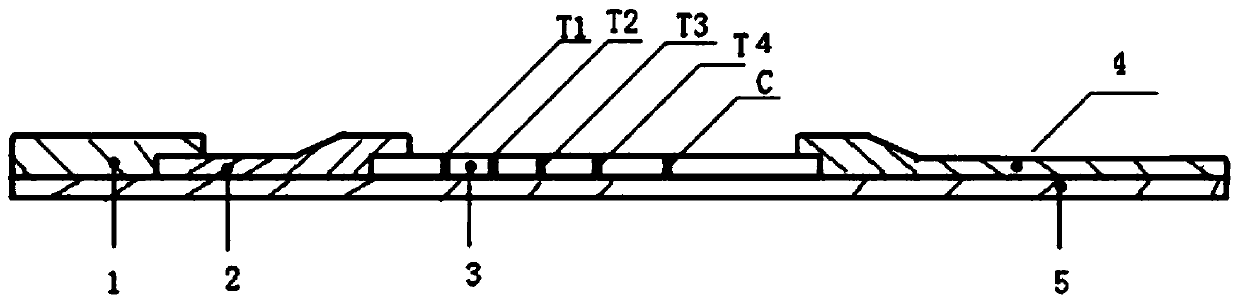
The invention relates to a fluorescent microsphere combined detection device for a cardiac marker and a preparation method of the fluorescent microsphere combined detection device, and belongs to thefield of medical detection equipment. A sample pad, an immunofluorescent antibody glass fiber membrane, a nitrocellulose membrane and an absorption pad respectively adhere to a plastic plate, the twoends of the nitrocellulose membrane are respectively overlapped with the absorption pad and the immunofluorescent antibody glass fiber membrane, the other end of the immunofluorescent antibody glass fiber membrane is overlapped with the sample pad, the nitrocellulose membrane is provided with a first detection line T1 with a solid-phase high-specificity MPO antibody, a second detection line T2 with a solid-phase high-specificity cTnI antibody, a third detection line T3 with a solid-phase high-specificity NT-proBNP antibody, a fourth detection line T4 with a solid-phase high-specificity D dimerantibody and a quality control line C; and a goat anti-mouse IgG polyclonal antibody is sprayed on the quality control line C. The fluorescent microsphere combined detection device for the cardiac marker has the advantages that the sensitivity and the specificity of reaction are effectively improved, the use amount of immune microspheres can further be reduced under the same threshold value, thecost is saved, and the device is simple and convenient to operate and high in practicability.
Owner:JILIN UNIV +1
Quantum dot marking test strip and method for synchronously and quantitatively detecting multiple indices of cardiac markers
InactiveCN104280545ASimultaneous rapid quantitative detectionPrevent passageMaterial analysisTest stripsAntibody
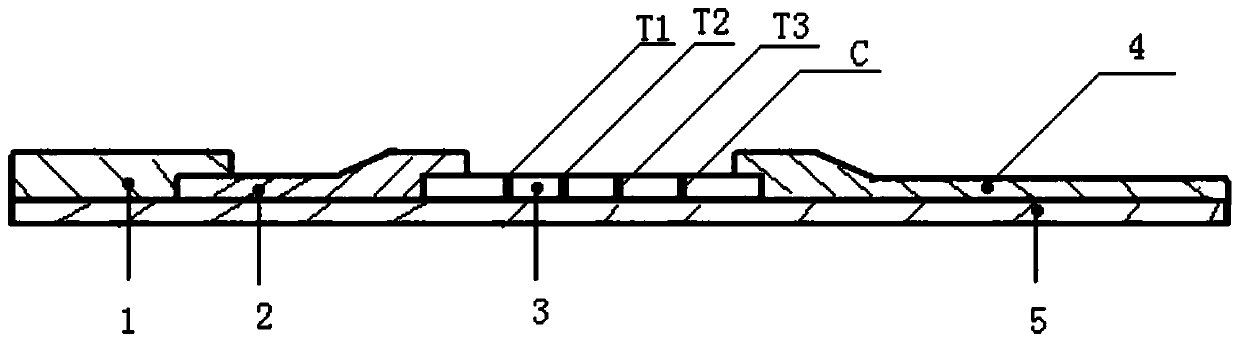
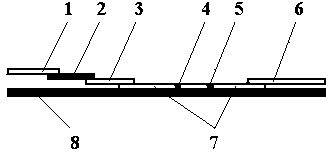
The invention belongs to the field of in-vitro diagnosis and particularly relates to a quantum dot marking test strip and method for synchronously and quantitatively detecting multiple indices of cardiac markers. A marking pad (3) of the test strip is coated by a mixture of all cardiac marker antibodies correspondingly marked by quantum dots with different wavelengths; a zone T (4) of an analysis membrane (7) is coated by a mixture of all cardiac marker antibodies; and a zone C (5) of the analysis membrane (7) is coated by a second antibody. The standard curve of each detected matter is stored by using an electronic tag and is mounted on the test strip. According to the test strip, the standard curve data stored by the electronic tag is read by a detector with a signal detection function, and the concentrations of all the cardiac markers in a sample are synchronously and quantitatively detected by combining fluorescence intensity corresponding to a to-be-detected sample detected by the detector.
Owner:CHENGDU LINGYU BIOTECH
Indirect detection of cardiac markers for assessing acute myocardial infarction
InactiveUS7527980B2Microbiological testing/measurementDisease diagnosisMeasurement testMicroparticle
Owner:FLORIDA STATE UNIV RES FOUND INC
Cardiac marker quality control serum preparation method
InactiveCN109916682ALow viscosityReduce matrix effectPreparing sample for investigationFreeze-dryingBlood plasma
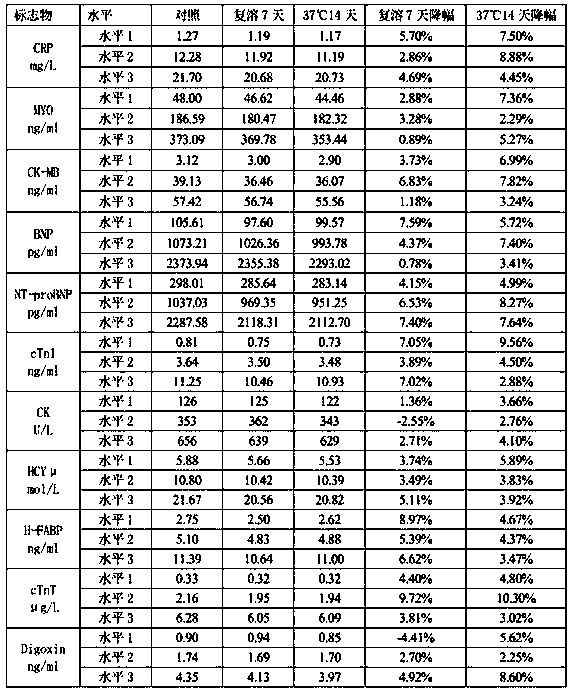
The invention discloses a cardiac marker quality control serum preparation method. Normal human serum is adopted as a substrate liquid, impurities are removed by a filter membrane after a warm bath for 1 to 3 hours, CK, CK-MB, cTnI, cTnT, CRP, BNP, MYO, NT-proBNP, HCY, H-FABP, Dig and other cardiac marker positive substances are added to the obtained quality control serum substrate liquid, qualitycontrol serum solutions with a high, a medium and a low concentration level are prepared, a proper amount of preservative, a protein protective agent and a stabilizer are added and are then mixed uniformly, debugging and determining are carried out, and after the detected result meets a quality standard, the solution is placed in a glass bottle for low temperature vacuum drying to be in a freeze-dried state and is saved at 2 to 8 DEH C. The quality control serum selects normal human serum / normal human plasma / de-hormone human serum / delipidated human serum as the substrate liquid, and the stable-performance multi-item cardiac marker quality control serum prepared according to actual clinical demands has low viscosity, quick redissolving, little substrate effects, excellent clinical applicability, thereby greatly facilitating clinical promotion and development.
Owner:郑州标源生物科技有限公司
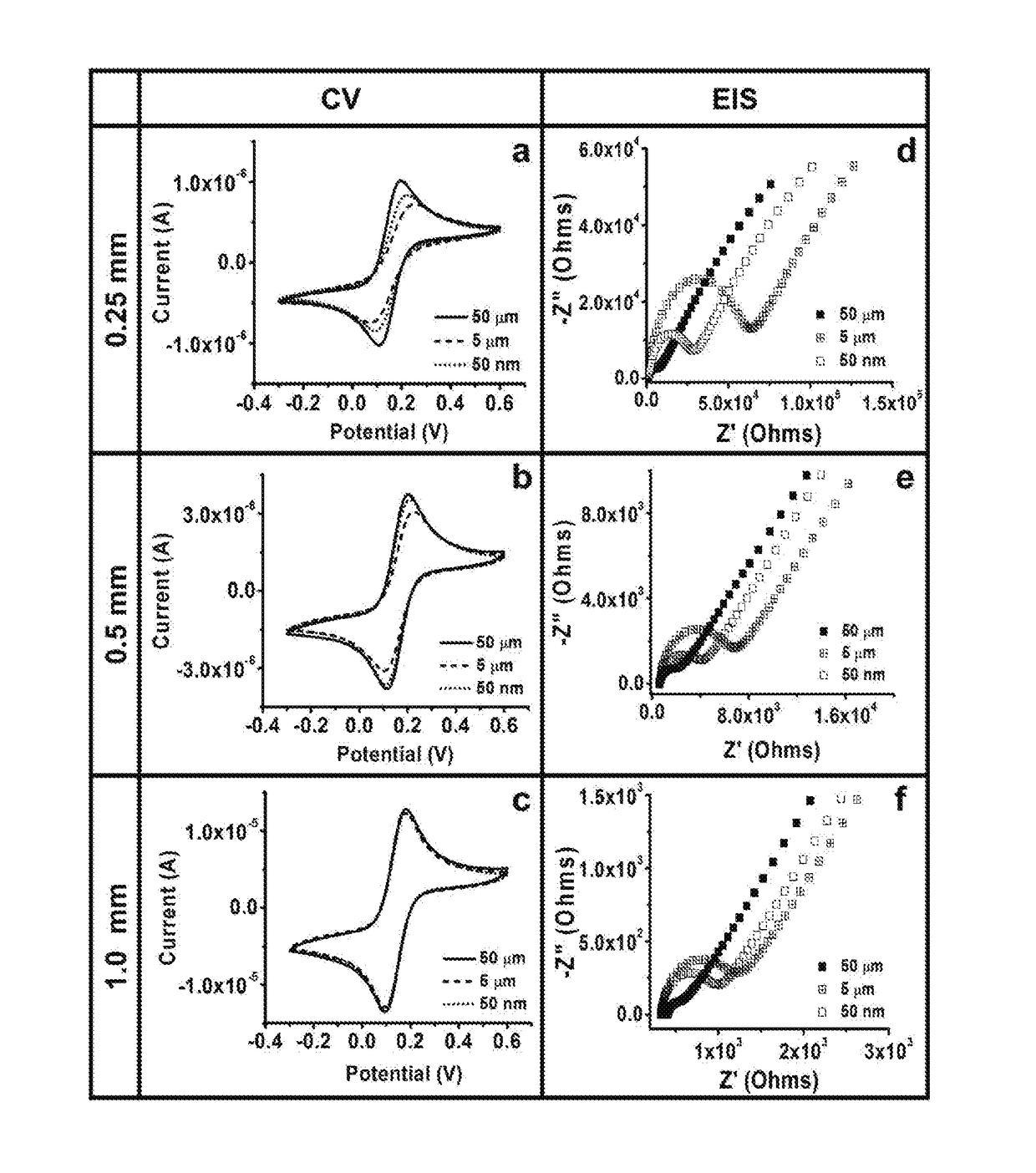
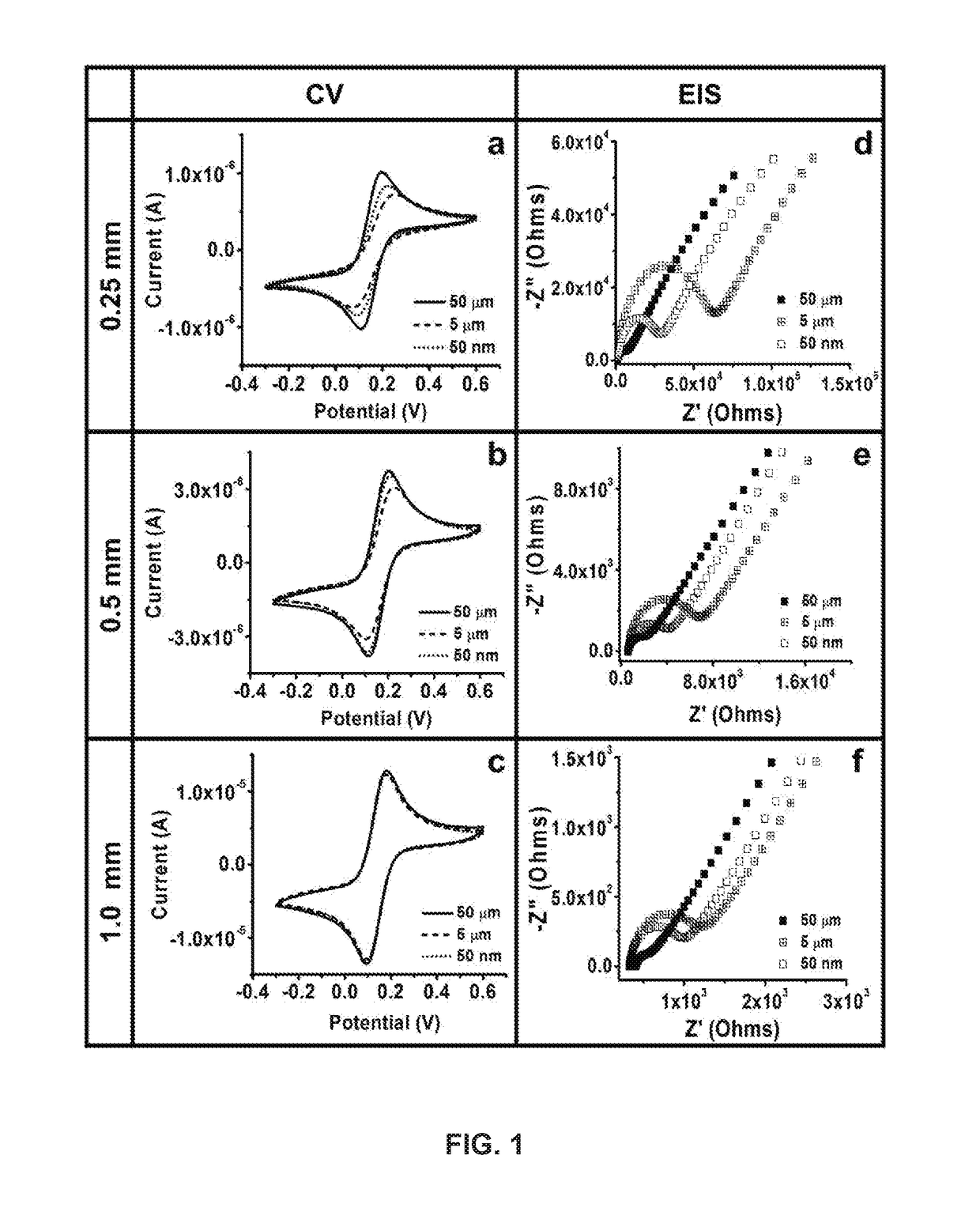
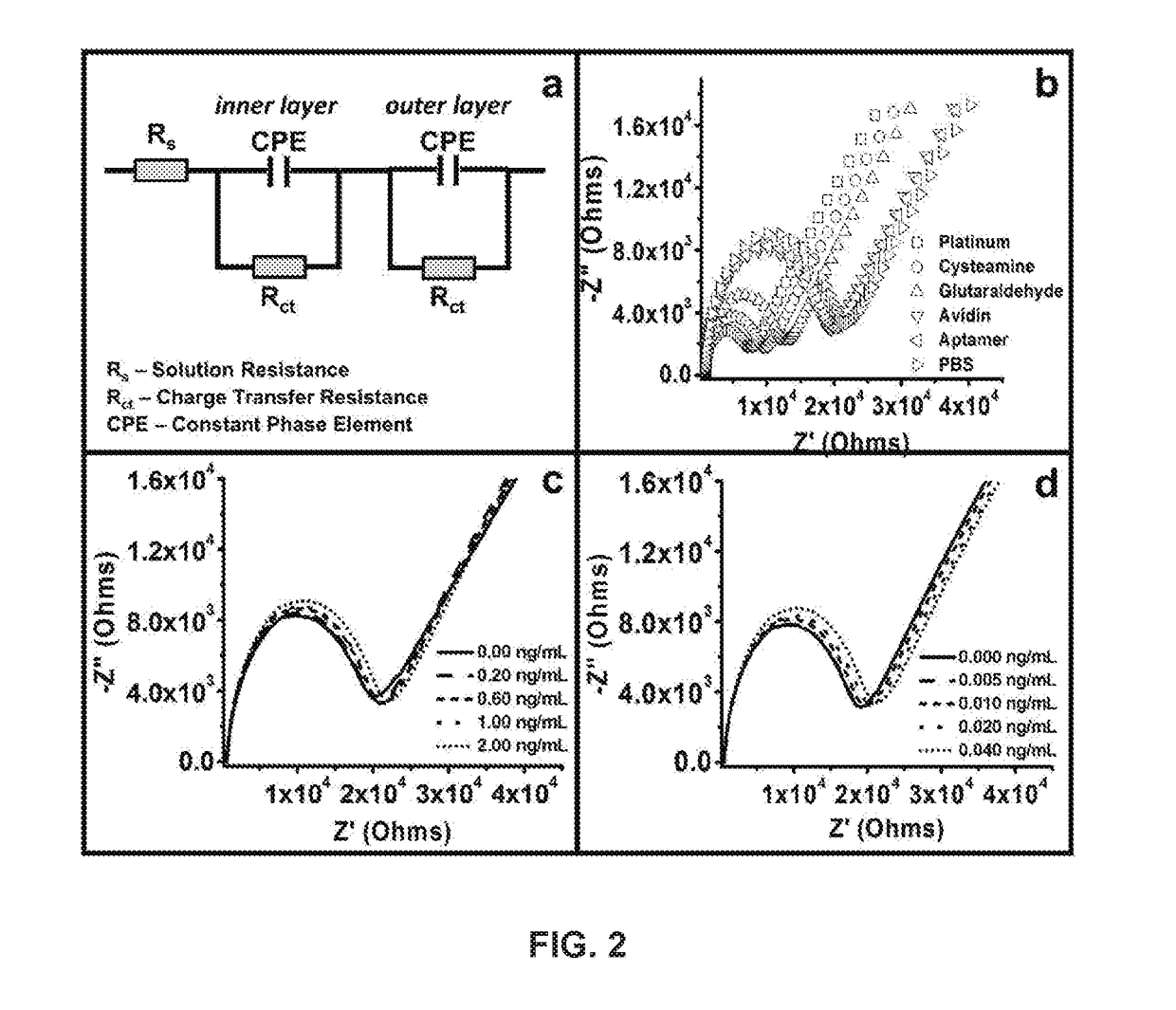
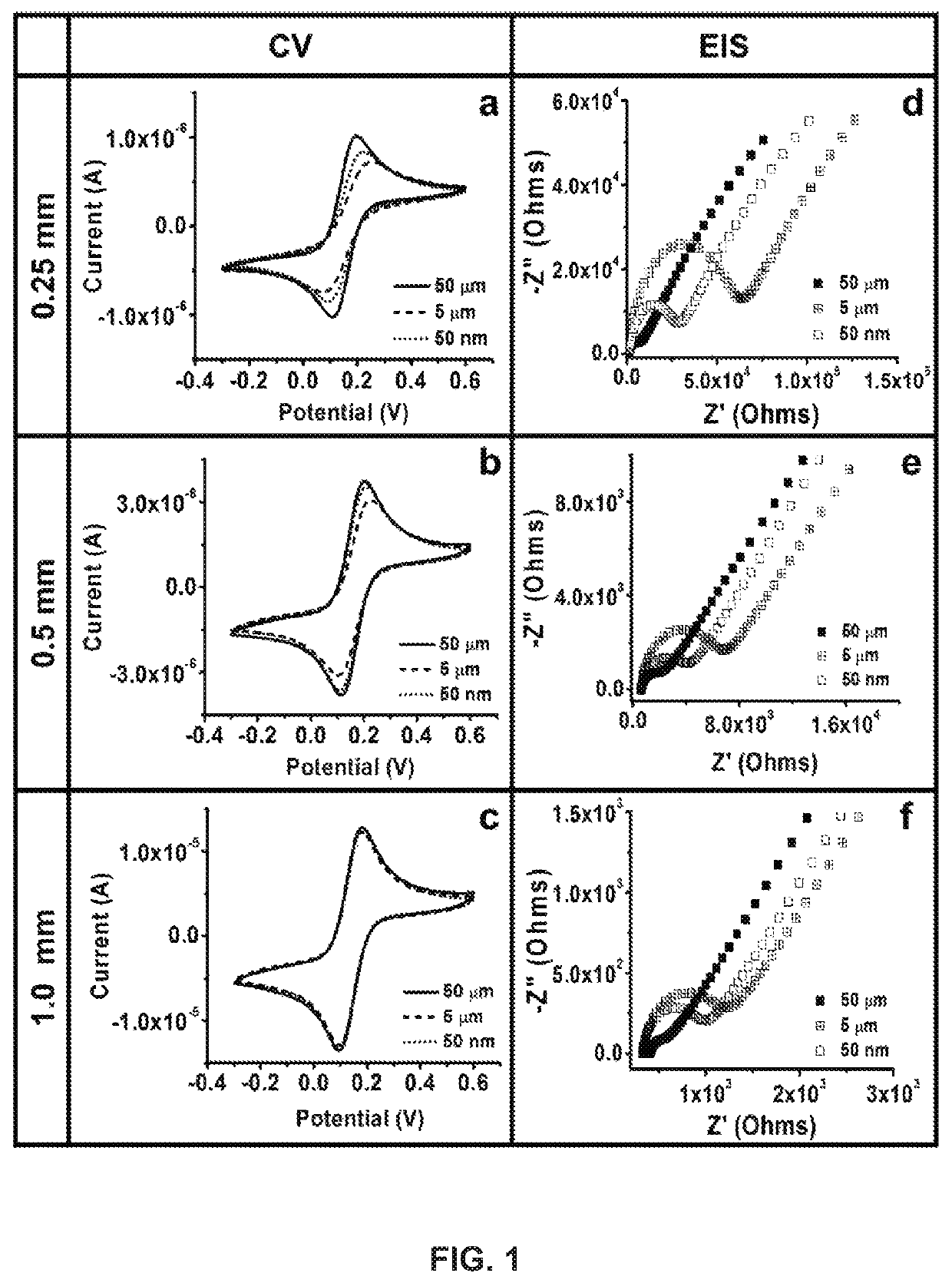
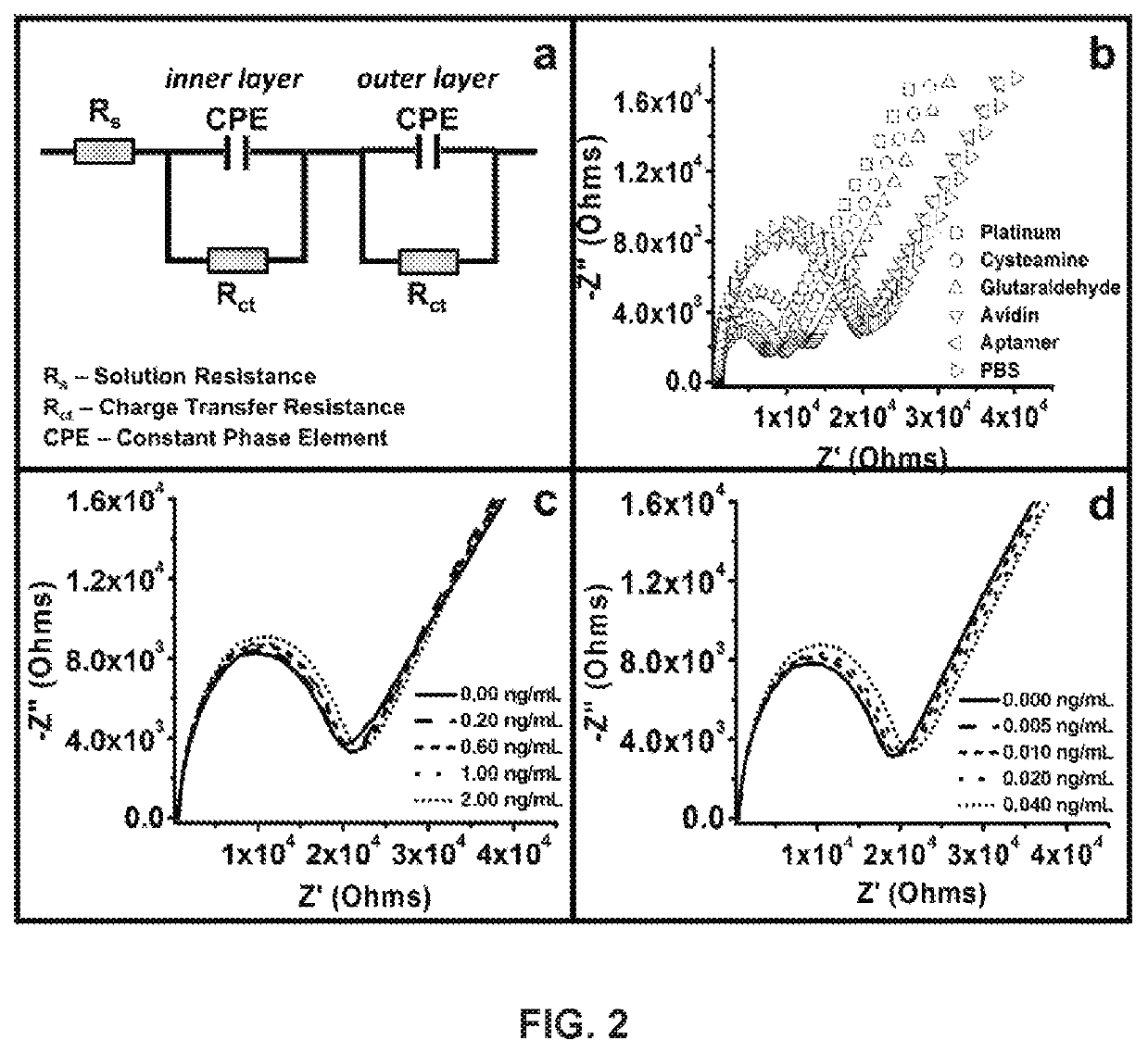
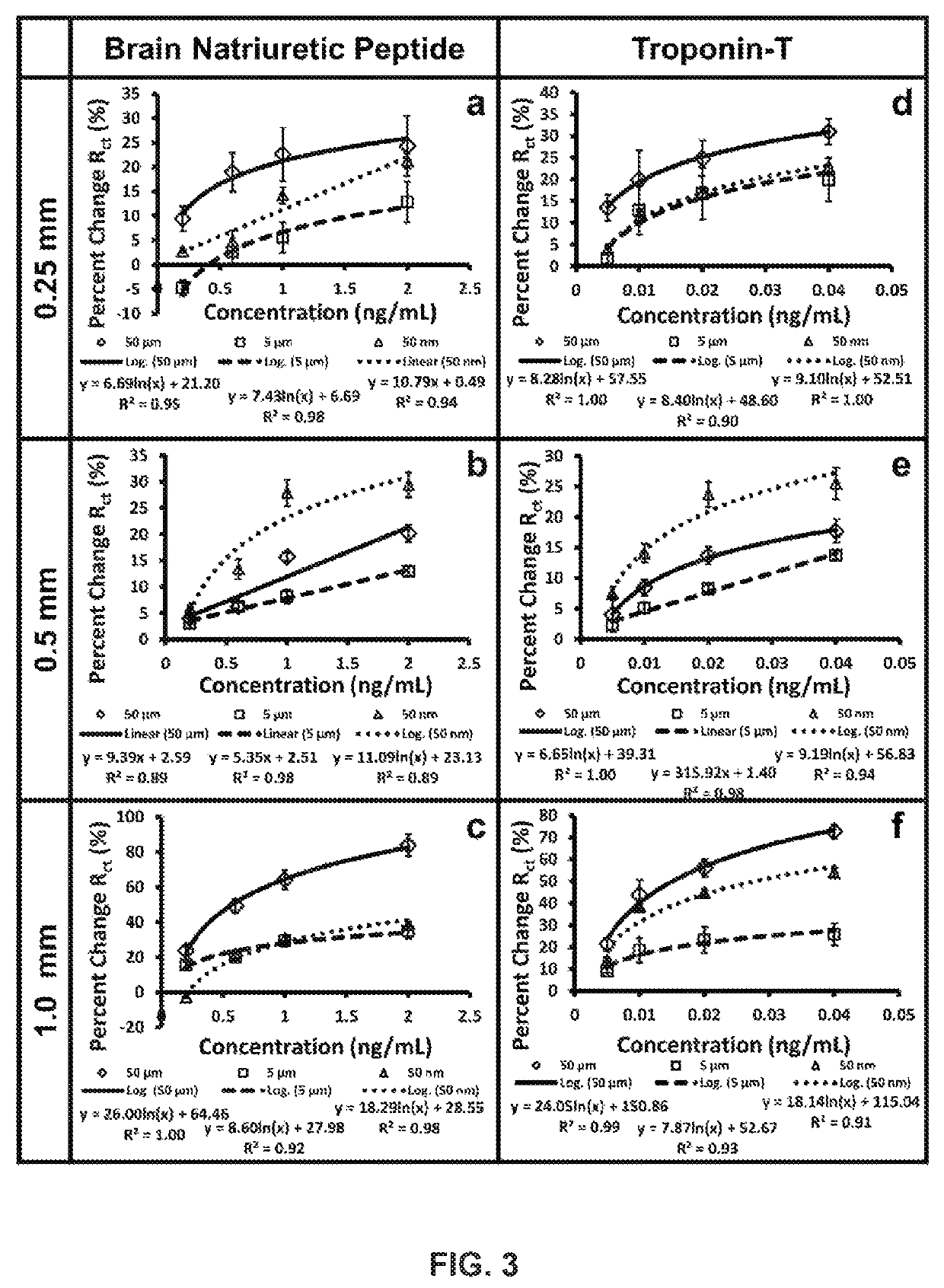
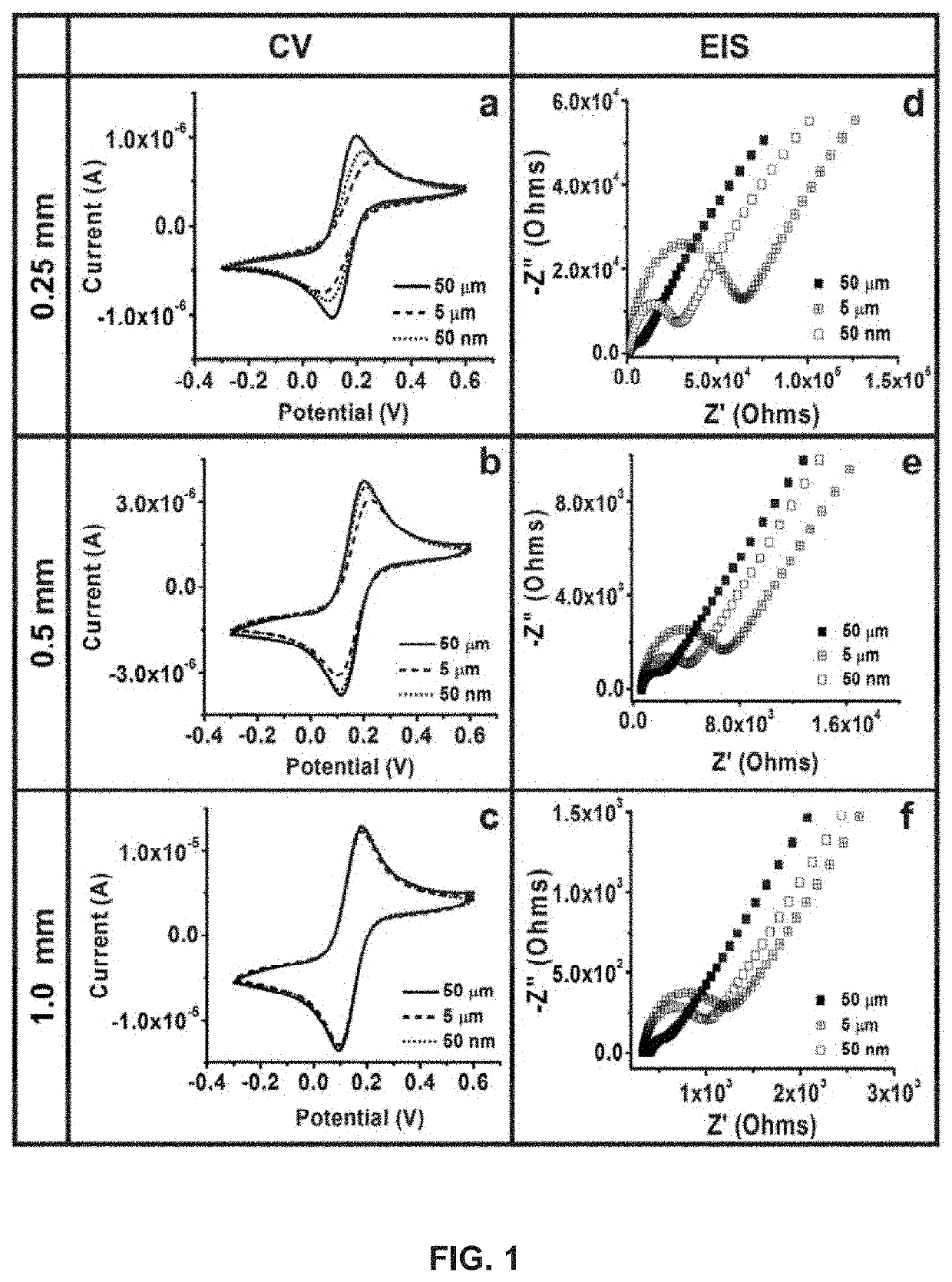
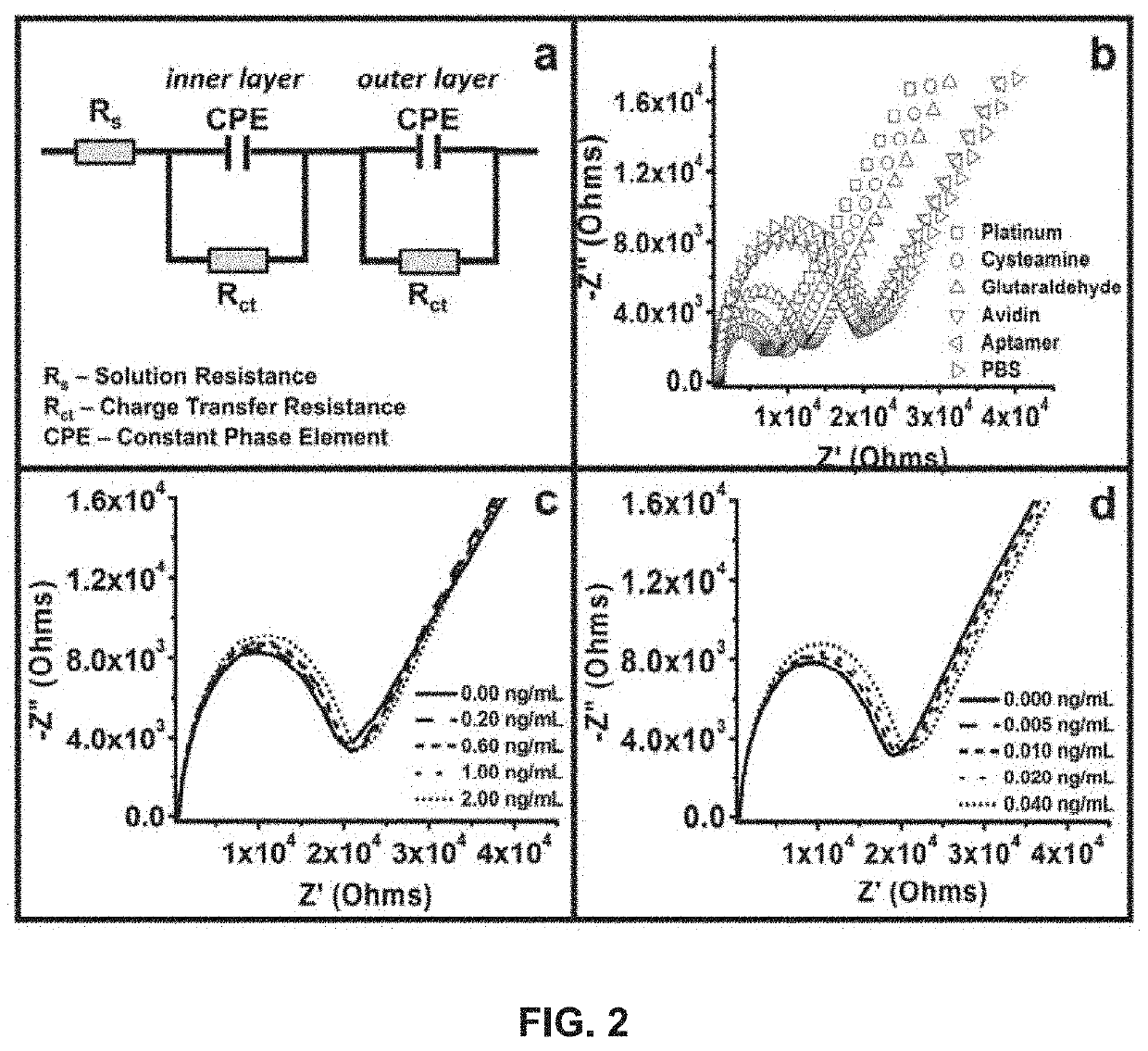
Development and parameter assessment for vertically aligned platinum wire aptasensor arrays for impedimetric detection of cardiac biomarkers
ActiveUS20180292400A1Efficient detectionMaterial analysis by electric/magnetic meansDisease diagnosisPoint of careAptamer
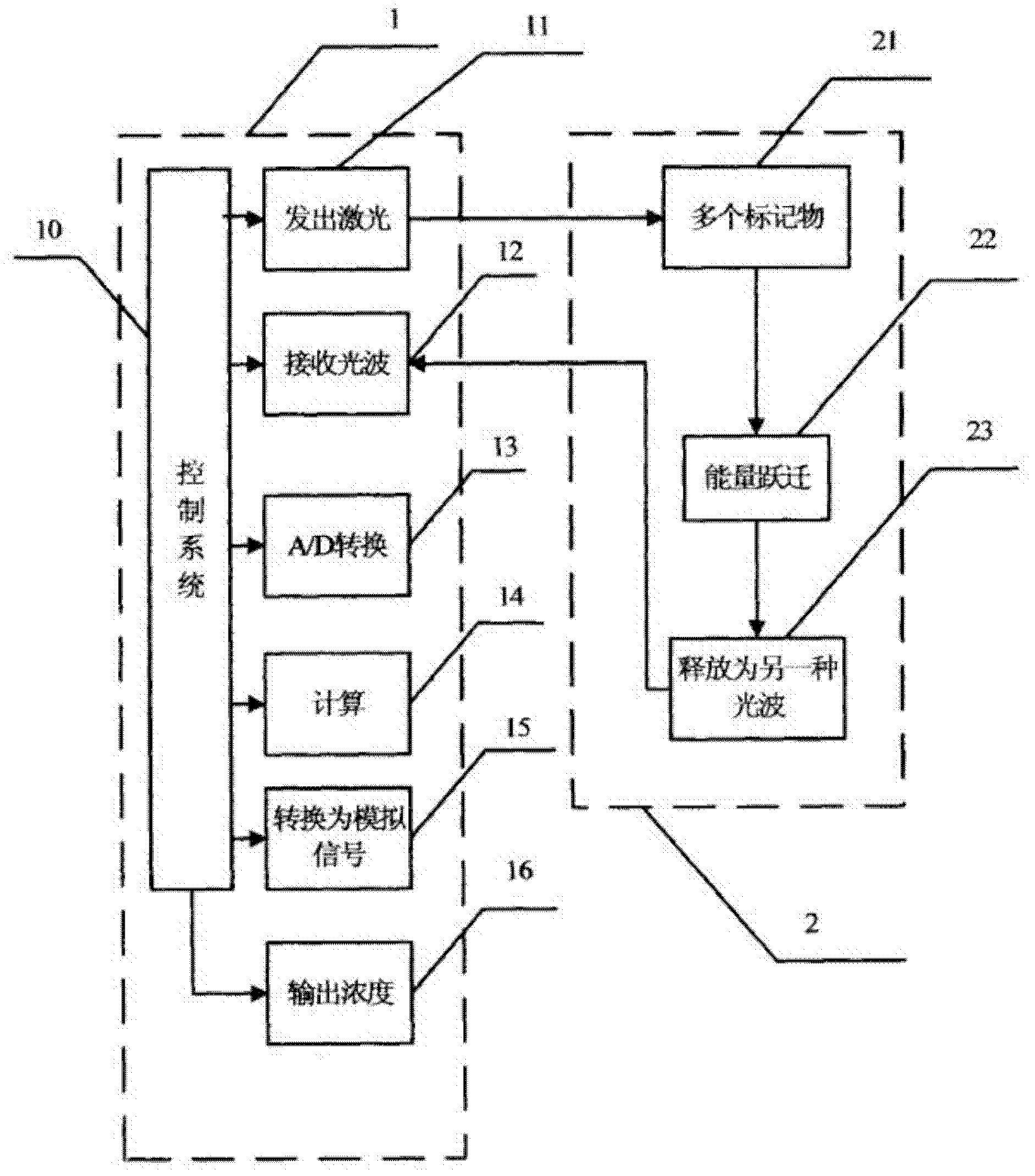
The invention relates to ex-situ biosensors that impedimetrically detect cardiac biomarkers of interest in a bodily fluid sample derived from a patient. The biosensors include a multi-array of vertically aligned platinum wires having immobilized thereon an aptamer that is selected to specifically and selectively bind to the cardiac markers of interest. The biosensors are contacted with a portion of the bodily fluid sample, and the aptamer binds to the cardiac markers of interest in the bodily fluid sample. As a result, an electrochemical impedance signal is generated and therefore, a change in electrochemical impedance is indicative of the presence of the cardiac markers of interest in the bodily fluid sample. The biosensors are point-of-care, on-demand devices that can be used in a medical environment, as well as in domestic settings.
Owner:UNIVERSITY OF PITTSBURGH
Cardiomyocyte-like cell clusters derived from hBS cells
A cluster comprising cardiomyocyte-like cells, wherein the cluster has i) contracting cells, ii) cells that are electrically connected, and expresses iii) cardiac markers including Nkx.2.5, troponin and myosin, iv) markers for functional adrenergic receptors, v) markers for functional muscarinic receptors, vi) markers for functional ion-channels including hERG, Na+, Ca2+ and K+ channels, vii) one or more endodermal markers selected from the group consisting of AFP, TF, APOA2, AHSG, SERPINA1, APOA1, APOC3, TTR1 APOB, and RBP4. A method for preparing the clusters and the use of the clusters in drug discovery and toxicity screenings are described.
Owner:CELLECTIS SA
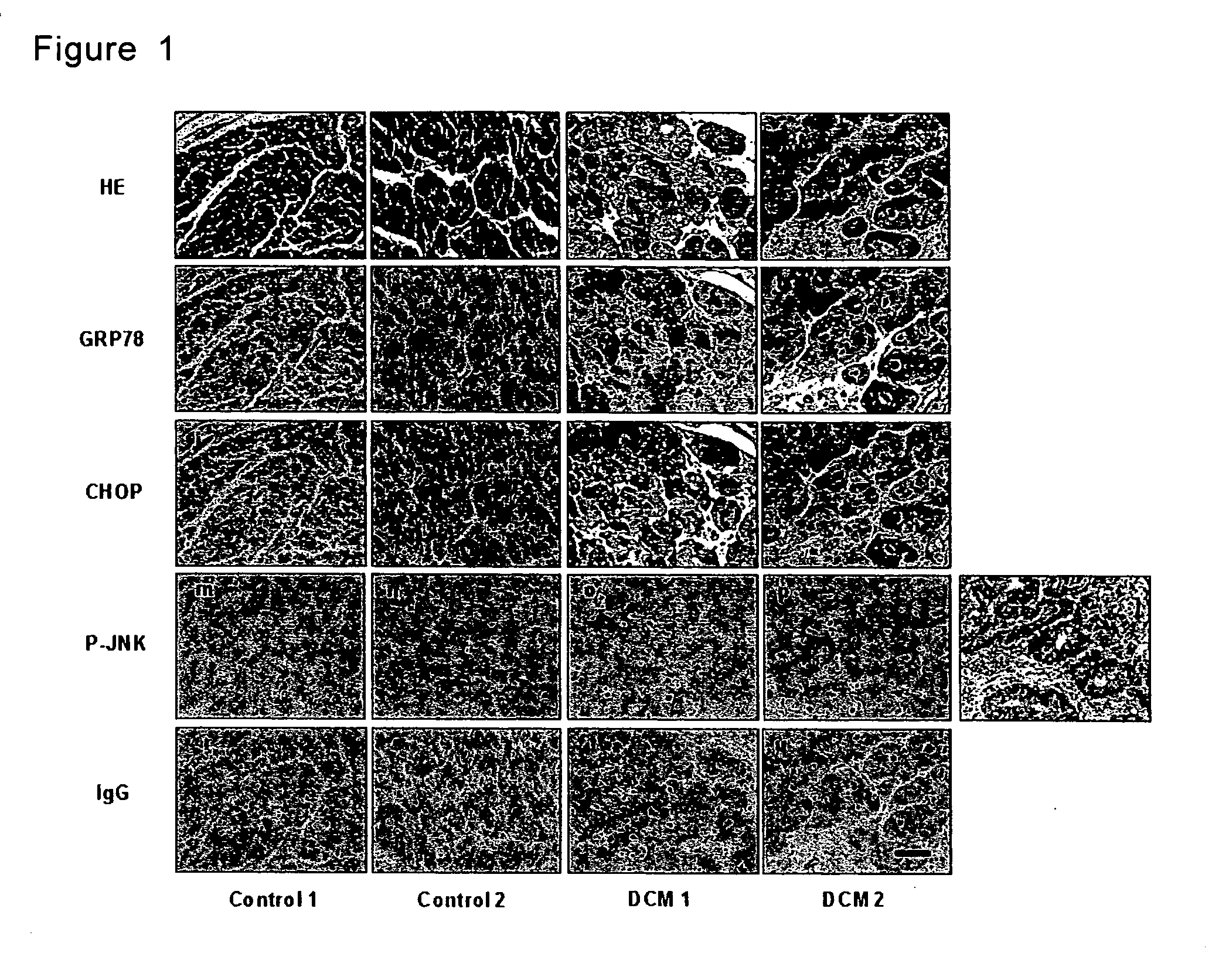
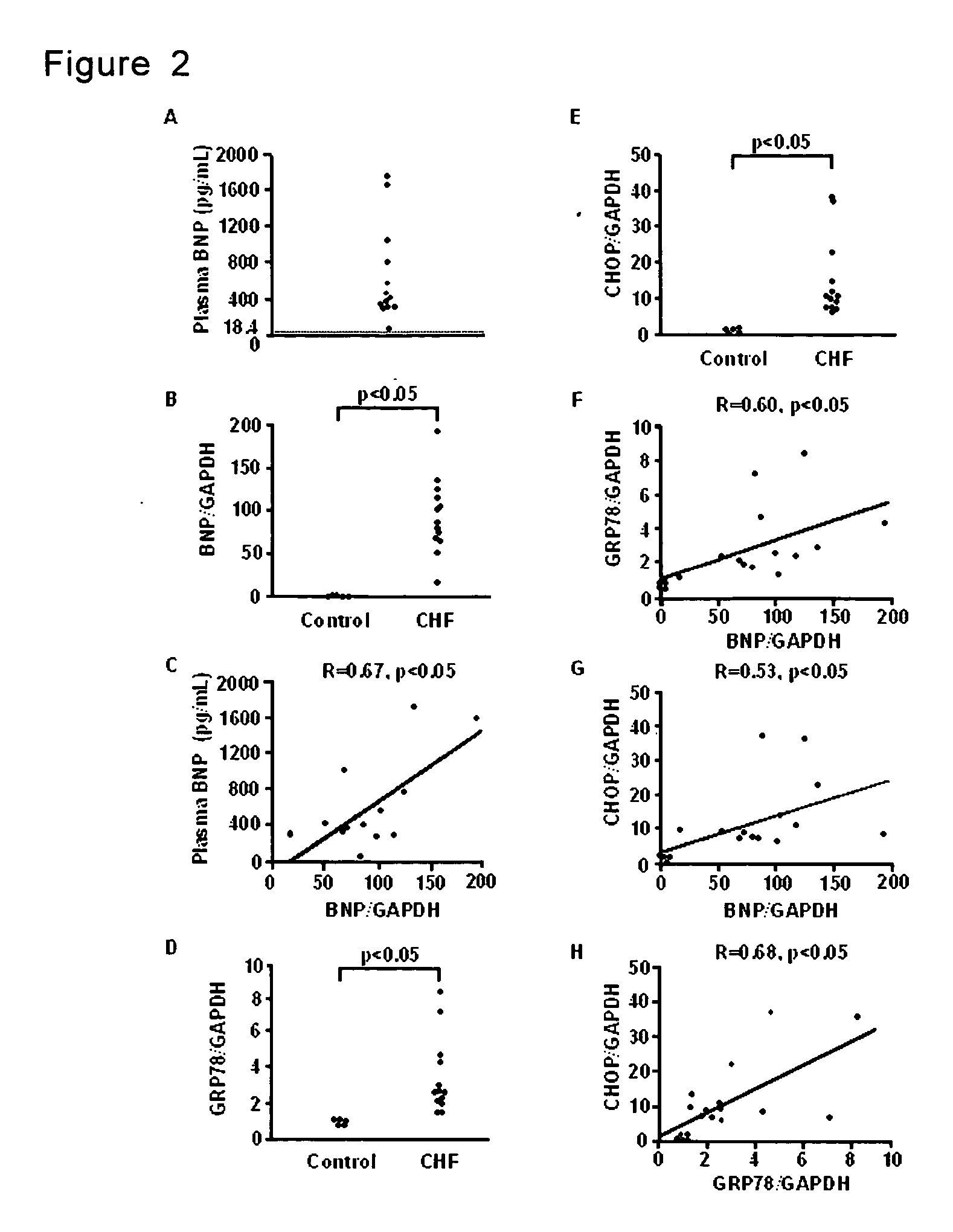
Method for diagnosis of heart failure
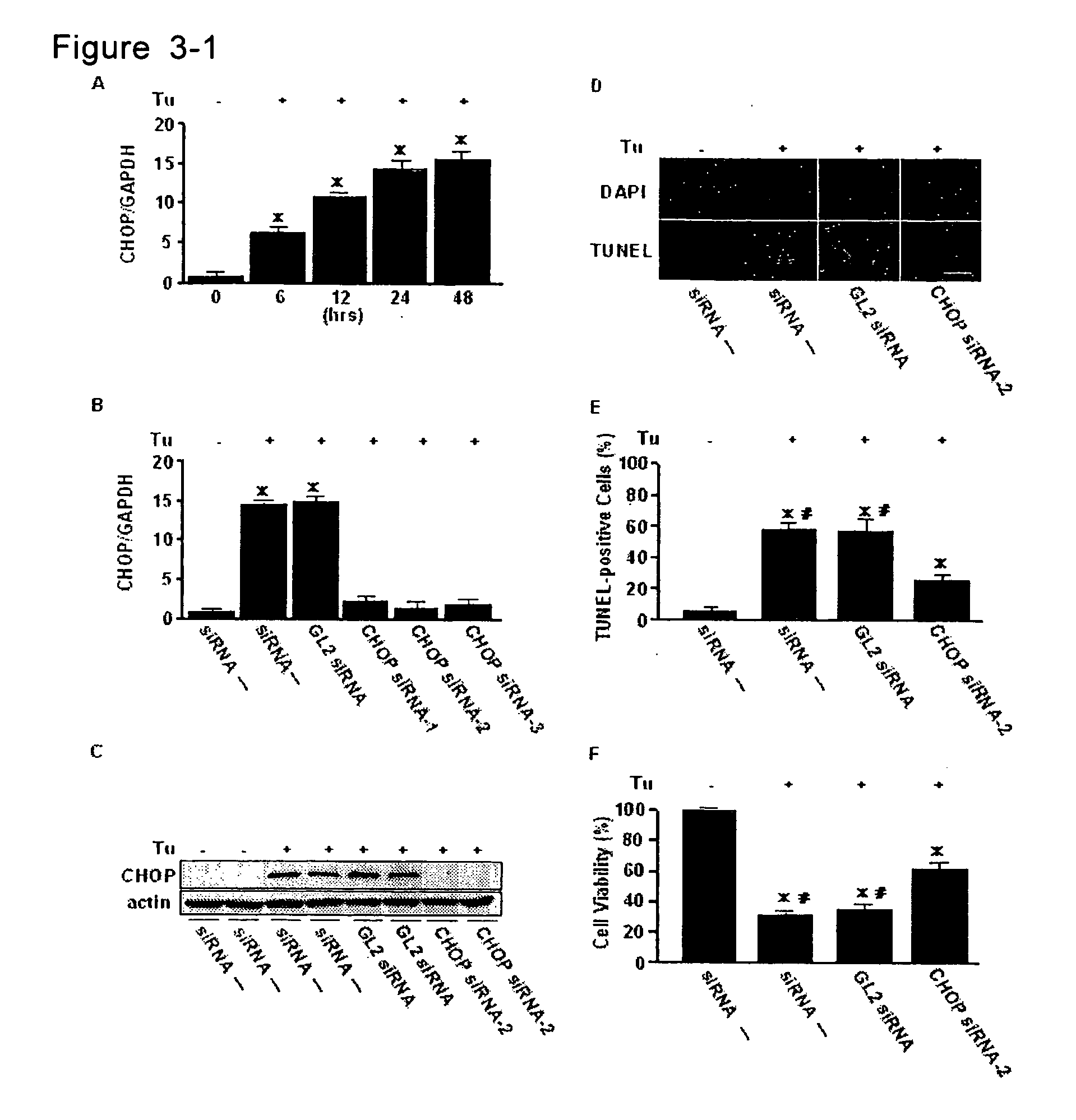
InactiveUS20070065850A1Accurate diagnosisDetermination of severityMicrobiological testing/measurementDisease diagnosisReticulum cellCHOP
A method for diagnosis of heart failure in a human subject, which comprises the step of: measuring an endoplasmic reticulum (ER)-initiated apoptotic signal such as CHOP in a biological sample originated from the subject is disclosed. According to the invention, by employing the new marker in stead of or in addition to a conventional cardiac marker such as BNP, more accurate diagnosis of heart failure can be attained.
Owner:OSAKA UNIV +1
Implantable biosensor devices for monitoring cardiac marker molecules
An implantable biosensor system is disclosed for determining levels of cardiac markers in a patient to aid in the diagnosis, determination of the severity and management of cardiovascular diseases. The sensor includes nanowire sensor elements having a biological recognition element attached to a nanowire transducer that specifically binds to the cardiac marker being measured. Each of the sensor elements is associated with a protective member that prevents the sensor element from interacting with the surrounding environment. At a selected time, the protective member may be disabled, thereby allowing the sensor element to begin sensing signals within a living body.
Owner:MEDTRONIC INC
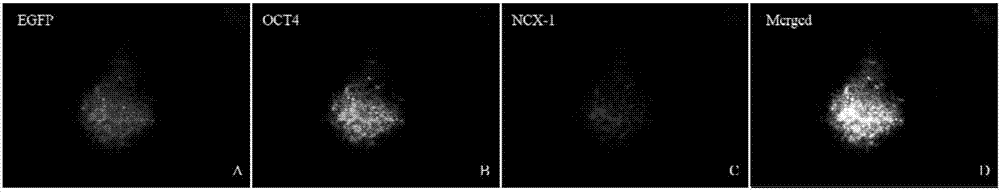
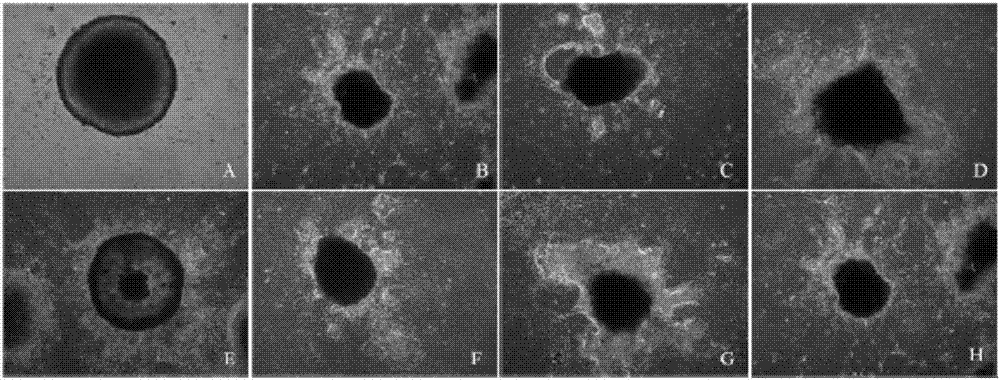
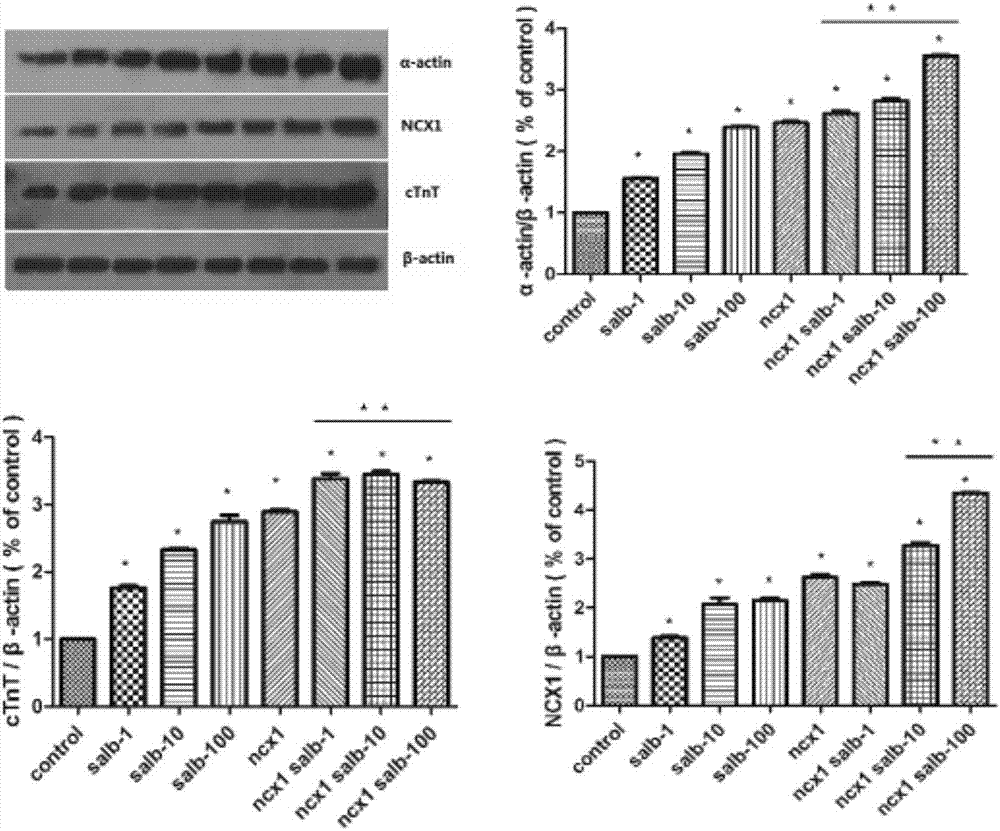
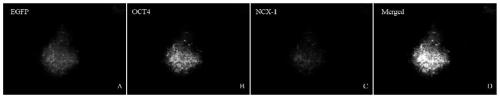
Method of combining salvianolic acid B to induce directed myocardiac differentiation of iPSCs by sodium-calcium exchanger 1 promoter
ActiveCN107012124AHigh expressionForeign genetic material cellsSodium/Calcium Exchanger 1Salvianolic acid B
The invention discloses a method of combining salvianolic acid B to induce directed myocardiac differentiation of iPSCs by a sodium-calcium exchanger 1 promoter and belongs to the technical field of medicines. The method comprises the following steps: constructing NCX1 lentivirus particles and transfecting the NCX1 lentivirus particles to iPSCs; and performing assistance with combined induction of salvianolic acid B. The concentration of the salvianolic acid B is 1-100[mu]M. The method disclosed by the invention verifies that the sodium-calcium exchanger 1 promoter combined with the salvianolic acid B can effectively induce directed myocardiac differentiation of iPSCs. The salvianolic acid B can effectively promote NCX1 expresion and promote remarkable improvement of expressions of cardiac markers alpha-actin and cardiac troponin (cTnT). According to the shape of the iPSCs, fusiform change of a myocardial cell sample can be seen. IPSCs as a seed cell for repairing acute myocardial infarction have a wide application prospect.
Owner:JINAN UNIVERSITY
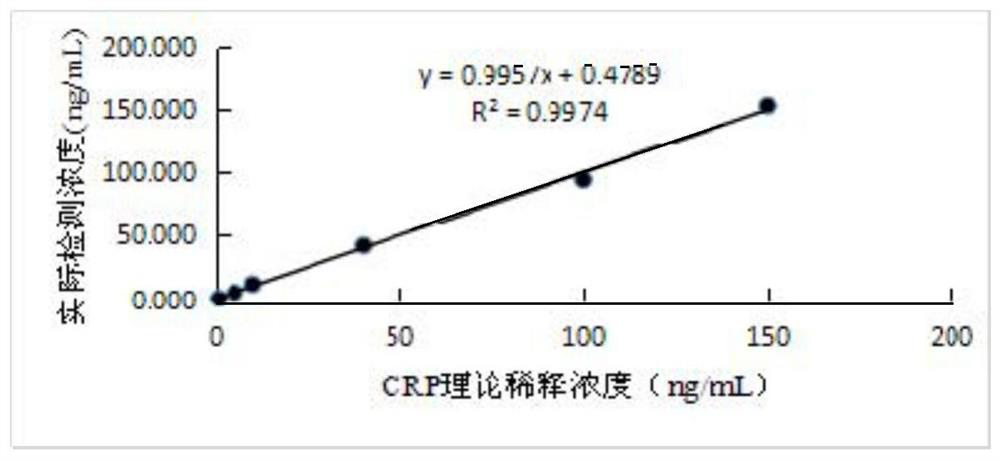
Kit for combined quantitative detection of five cardiac markers and preparation method thereof
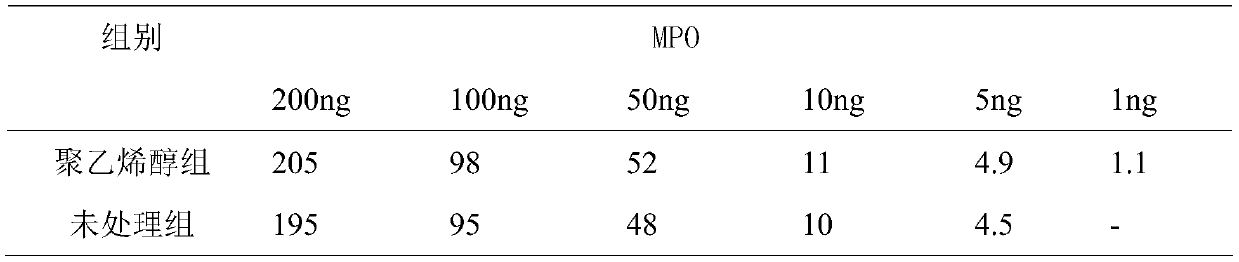
The invention discloses a preparation method of a fluorescence immunochromatographic detection kit for composite quantitative detection of hypersensitive C-reactive protein (hs-CRP), myeloperoxidase (MPO), lipoprotein phospholipase A2 (Lp-PLA2), oxidized low-density lipoprotein (ox-LDL) and F2-isoprostaglandin (F2-isop). The kit comprises a desiccant, a sealing bag and a detection card. The detection card comprises a pentaplet buckle card and a reagent strip, wherein the reagent strip comprises a sample pad, a combination pad, a blood filtering membrane, a nitrocellulose membrane, absorbent paper and a PVC bottom plate; the combination pad is coated with an hs-CRP antibody, an MPO antibody, an Lp-PLA2 antibody, an ox-LDL antibody and an F2-isop antibody; and the antibody is one or more pairing combinations of a monoclonal antibody, a polyclonal antibody, an antibody fragment or a chimeric antibody. The preparation method disclosed by the invention has the outstanding advantages of specificity, sensitivity, high yield, rapidness, simplicity, convenience, good repeatability, easiness in automation, minimization of false positive, high detection efficiency, low detection cost and thelike, and has important practical significance.
Owner:CHANGZHOU BIOWIN BIOPHARM
Method for detecting cardiac troponin I based on CRISPR Cas13d
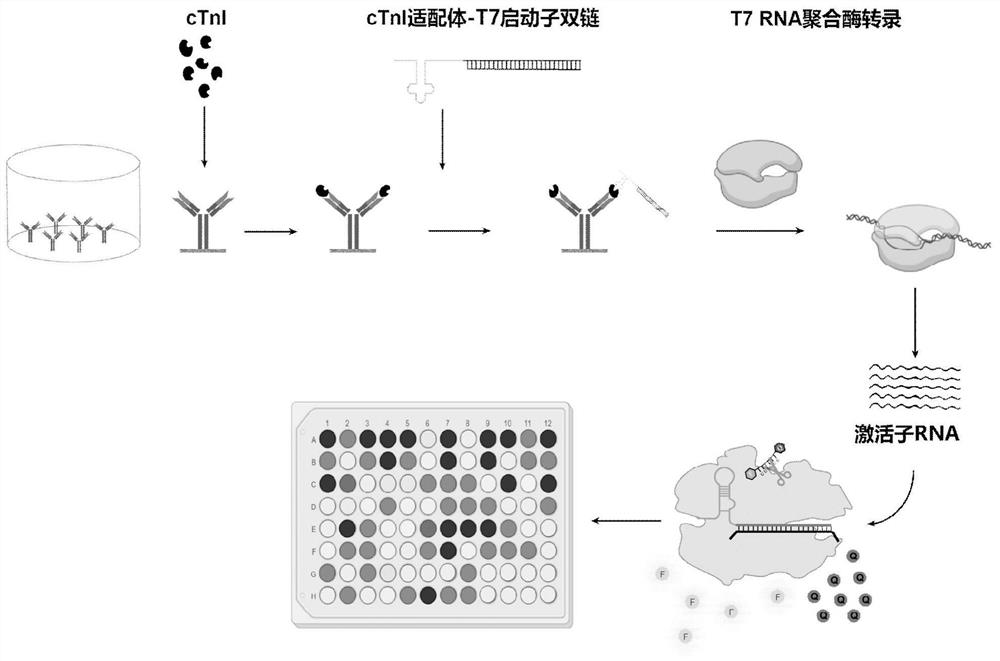
PendingCN114839381AReach detection capabilityAchieving Quantitative DetectionDisease diagnosisBiological testingTranscriptional expressionDouble strand
The invention discloses a method for detecting an acute myocardial infarction marker cTnI (cardiac troponin I) based on CRISPR (clustered regularly interspaced short palindromic repeats) Cas13d gene editing protein. A sample is incubated on an elisa plate to form an antibody-cTnI compound, two ssDNAs are annealed to form a double-chain cTnI DNA aptamer with a T7 promoter sequence, and the aptamer and the cTnI-antibody compound are incubated and combined to form an antibody-cTnI-aptamer ternary sandwich structure. The structure can be recognized by T7 RNA polymerase, transcripts and expresses activator RNA, and can be recognized by a CRISPR Cas13d-crRNA compound, and the side cutting activity generated by activation can effectively cut RNA fluorescent reporter molecules to generate certain fluorescent signals. The Cas13d gene editing protein is applied to accurate detection of the cardiac marker cTnI for the first time.
Owner:NANJING UNIV
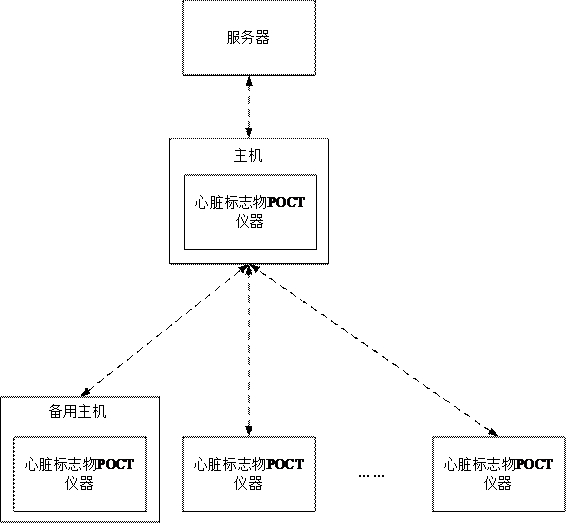
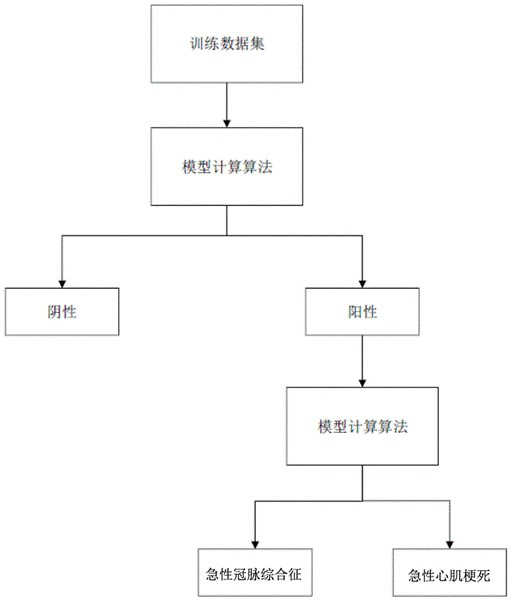
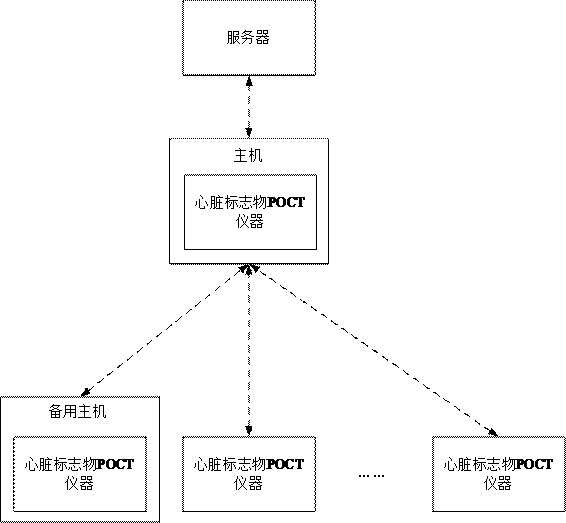
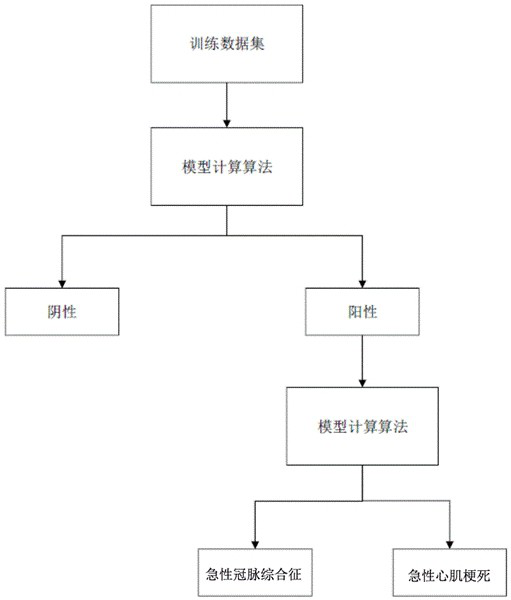
Cardiac marker POCT (point of care testing) system and medical equipment
ActiveCN114388121ARelieve pressureImprove communication efficiencyMedical data miningTelemetry/telecontrol selection arrangementsMedical equipmentPoint-of-care testing
The invention discloses a heart marker POCT (point of care testing) system and medical equipment. The heart marker POCT system comprises a plurality of heart marker POCT instruments, each heart marker POCT instrument comprises a shell, a communication chip and a heart marker detection module; at least one heart marker POCT instrument in the plurality of heart marker POCT instruments is set as a host, biological parameters collected by other heart marker POCT instruments except the host are all transmitted to the host, and the host transmits the received biological parameters to the server. The host is arranged, other instruments communicate with the server through the host, so that each instrument is prevented from communicating with the server, the pressure of the server is relieved, the host adapts to the instruments through the network, the total communication amount of the instruments connected with each host does not exceed the network upper limit of the host, and the purposes of relieving the pressure of the server and improving the communication efficiency are achieved. And the communication efficiency is improved.
Owner:北京盛坤康如医疗器械有限责任公司
Cardiac marker poct system and medical equipment
ActiveCN114388121BRelieve pressureImprove communication efficiencyMedical data miningTelemetry/telecontrol selection arrangementsMedical equipmentNetwork on
Owner:北京盛坤康如医疗器械有限责任公司
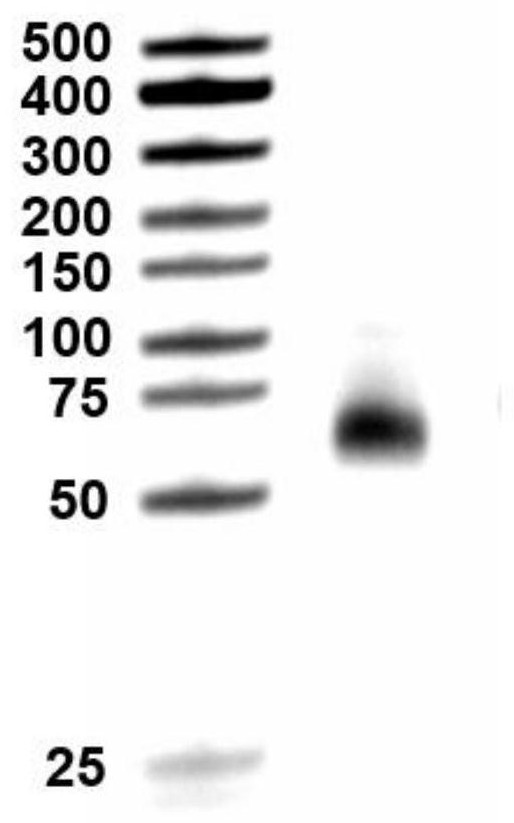
Preparation method of label-free electrochemical sensor for cardiac troponin I and detection method for CTNI
ActiveCN110161100BQuick checkSensitive detectionMaterial analysis by electric/magnetic meansElectron transferCardiac biomarkers
Preparation method of label-free electrochemical sensor for cardiac troponin I and its detection method for cTnI. In this experiment, a new type of label-free sensor was constructed by combining electrochemically active Cu-MOF-74 with biological immune reaction. BSA / anti‑cTnI / Cu‑MOF‑74 / NH 2 ‑rGO / GCE sensor, the sensor is specifically reacted with the target molecule (cardiac marker cTnI) to obtain a cardiac marker antigen‑antibody binding layer, which causes electrochemically active Cu‑MOF‑74 layer "electron transfer disturbance", enabling The output electrochemical signal changes regularly. The electrochemical sensor can be used for rapid and sensitive detection of cardiac biomarkers, providing the possibility for early detection, early diagnosis and early treatment of patients with cardiovascular diseases.
Owner:MINNAN NORMAL UNIV
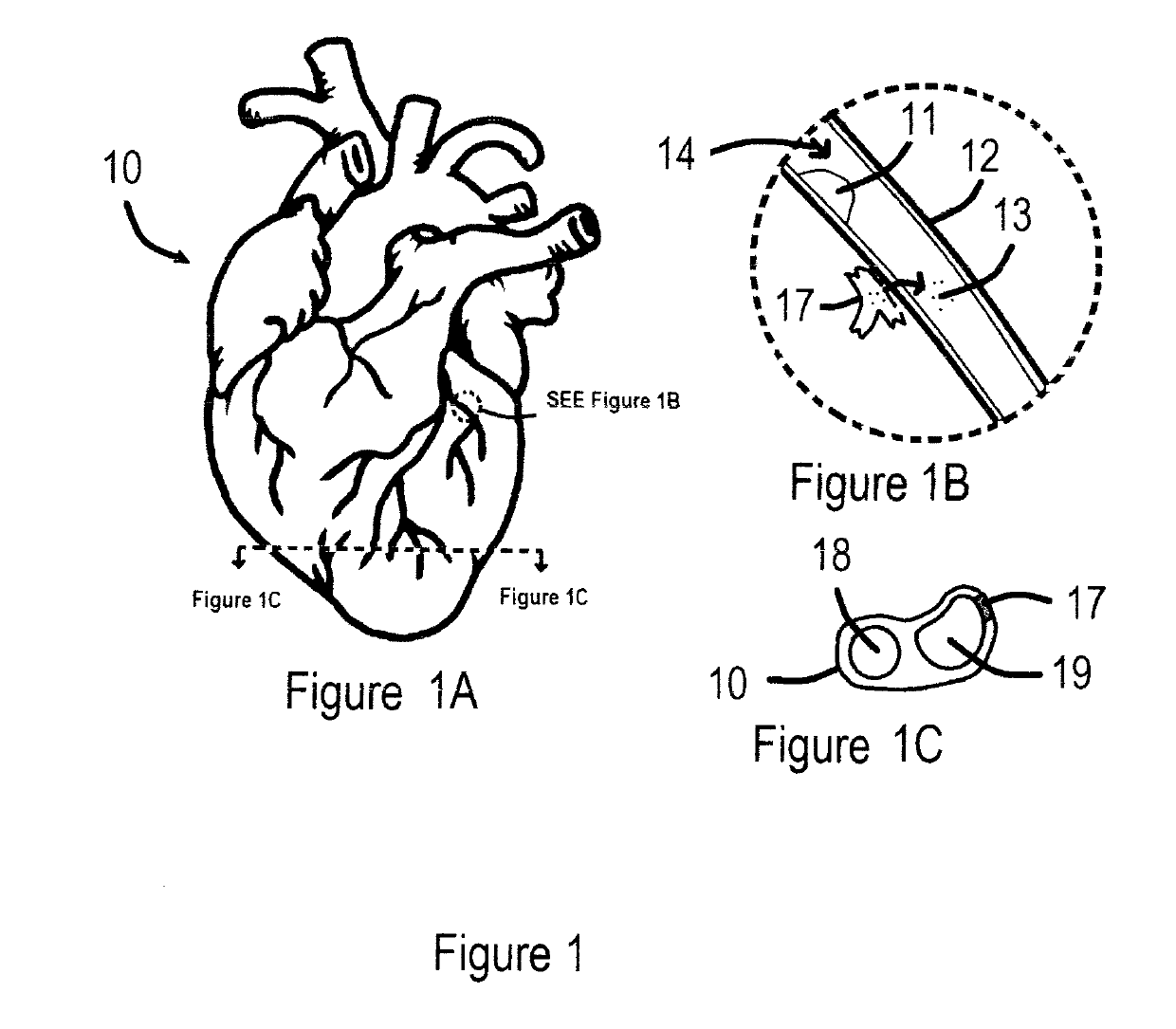
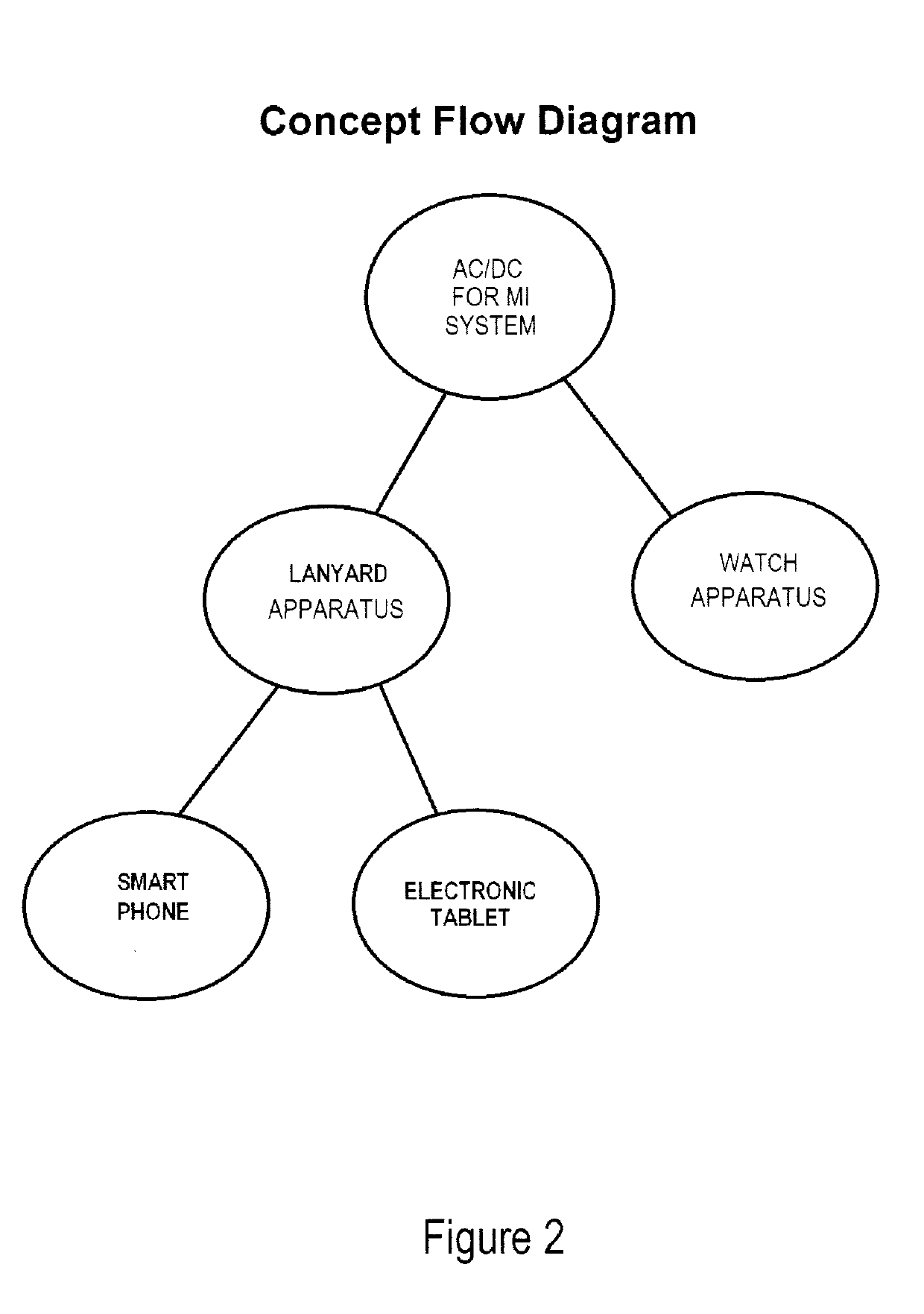
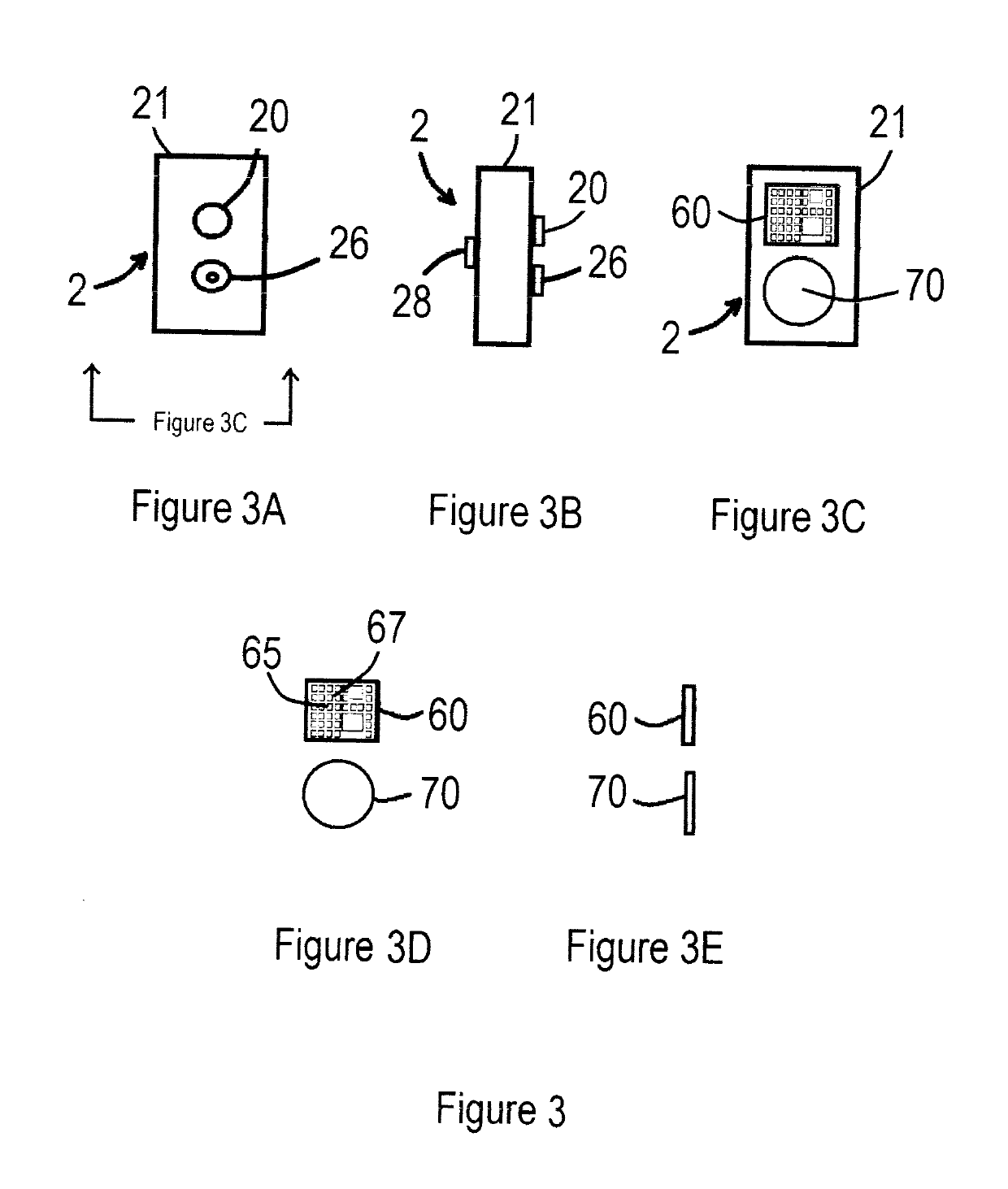
ASSISTED CAPACITY DEVICE CALCULATOR FOR MYOCARDIAL INFARCTION (AC/DC for MI)
InactiveUS20190159721A1Reducing and controlling amountMinimizes problemPhysical therapies and activitiesElectrocardiographyHeart diseaseSAPS II
The assisted capacity device calculator for myocardial infarction system is intended to assist a user during, or recently having had, a myocardial infarction and is intended to aid to help all people, especially those who have are susceptible to having myocardial infarctions, i.e., heart attacks. The apparatuses of the system are predominately mobile but can also be stationary and can be programmed by receiving and selecting pre-set commands to operate and assist a user but is particularly adapted for use when a user is having symptoms of a myocardial infarction. Particularly, the system is capable of prompting the user to respond to myocardial infarction symptoms, by answering various symptom questions, and if necessary, activate electrocardiographic (ECG) components, to test for electro cardio activity and / or a blood analyzer sensors to test for cardiac markers. The apparatuses of the also are provided with various features including an illuminated display panel, a CPS tracking capability, alarms among other things.
Owner:CANDY KATRINA GOFF
Development and parameter assessment for vertically aligned platinum wire aptasensor arrays for impedimetric detection of cardiac biomarkers
ActiveUS11156609B2Efficient detectionMaterial analysis by electric/magnetic meansDisease diagnosisAptamerRat heart
The invention relates to ex-situ biosensors that impedimetrically detect cardiac biomarkers of interest in a bodily fluid sample derived from a patient. The biosensors include a multi-array of vertically aligned platinum wires having immobilized thereon an aptamer that is selected to specifically and selectively bind to the cardiac markers of interest. The biosensors are contacted with a portion of the bodily fluid sample, and the aptamer binds to the cardiac markers of interest in the bodily fluid sample. As a result, an electrochemical impedance signal is generated and therefore, a change in electrochemical impedance is indicative of the presence of the cardiac markers of interest in the bodily fluid sample. The biosensors are point-of-care, on-demand devices that can be used in a medical environment, as well as in domestic settings.
Owner:UNIVERSITY OF PITTSBURGH
Development and parameter assessment for vertically aligned platinum wire aptasensor arrays for impedimetric detection of cardiac biomarkers
PendingUS20210405038A1Efficient detectionMaterial analysis by electric/magnetic meansDisease diagnosisAptamerRat heart
Owner:UNIVERSITY OF PITTSBURGH
The method of sodium-calcium exchanger 1 promoter combined with salvianolic acid b to induce directional myocardial differentiation of iPSCs
ActiveCN107012124BHigh expressionForeign genetic material cellsSodium/Calcium Exchanger 1Salvianolic acid B
The invention discloses a method of combining salvianolic acid B to induce directed myocardiac differentiation of iPSCs by a sodium-calcium exchanger 1 promoter and belongs to the technical field of medicines. The method comprises the following steps: constructing NCX1 lentivirus particles and transfecting the NCX1 lentivirus particles to iPSCs; and performing assistance with combined induction of salvianolic acid B. The concentration of the salvianolic acid B is 1-100[mu]M. The method disclosed by the invention verifies that the sodium-calcium exchanger 1 promoter combined with the salvianolic acid B can effectively induce directed myocardiac differentiation of iPSCs. The salvianolic acid B can effectively promote NCX1 expresion and promote remarkable improvement of expressions of cardiac markers alpha-actin and cardiac troponin (cTnT). According to the shape of the iPSCs, fusiform change of a myocardial cell sample can be seen. IPSCs as a seed cell for repairing acute myocardial infarction have a wide application prospect.
Owner:JINAN UNIVERSITY
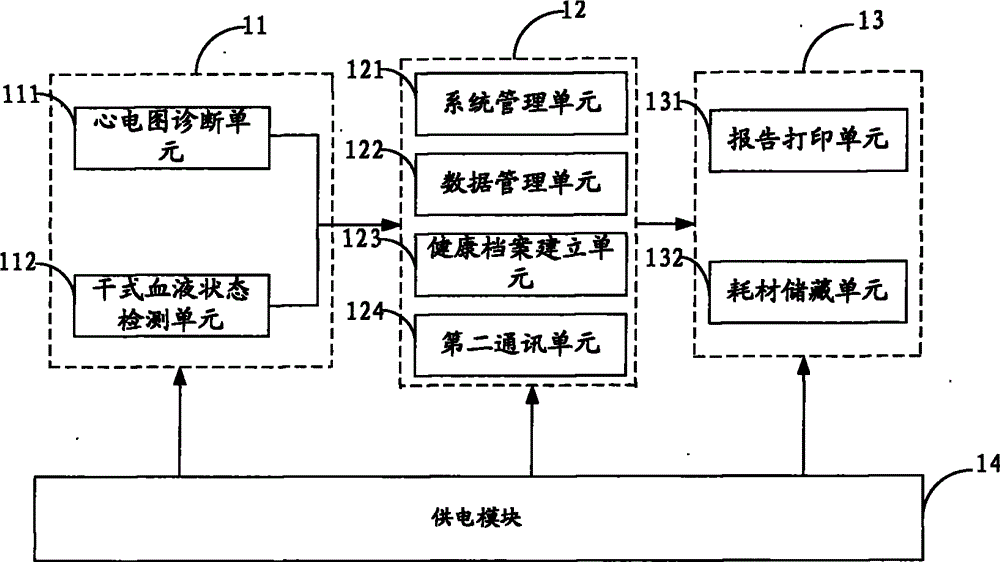
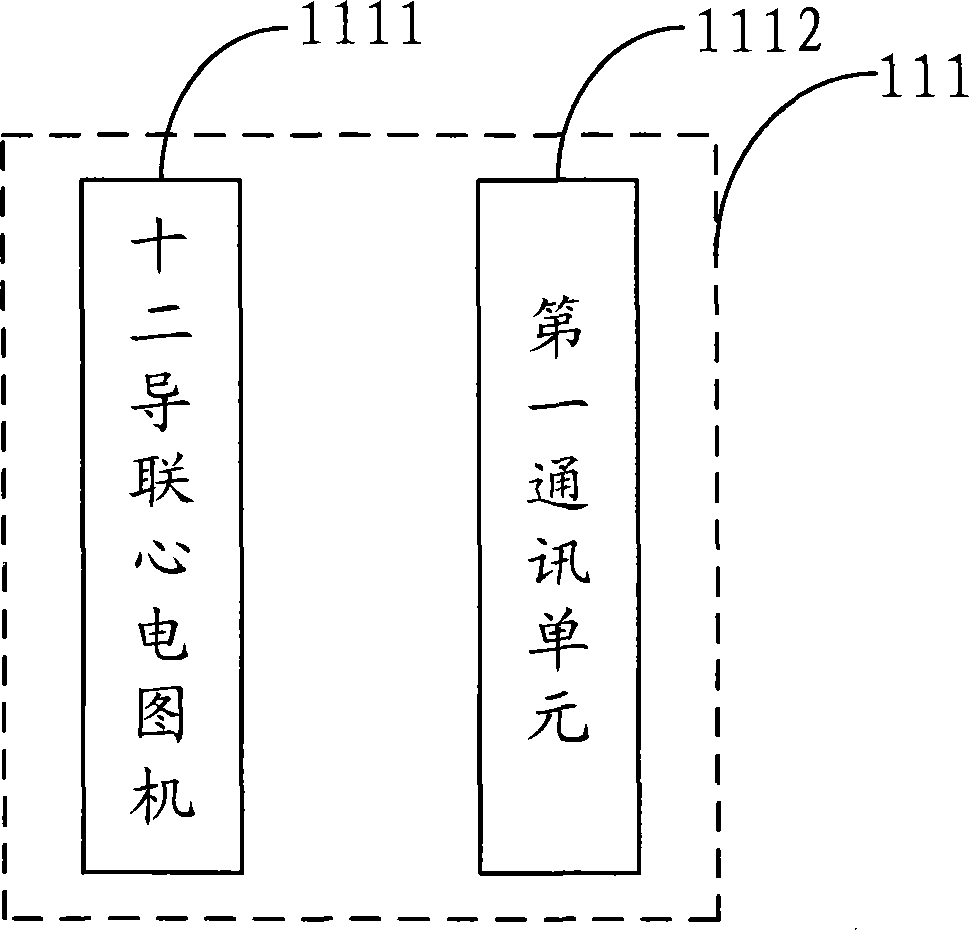
Digitized cardiovascular and cerebrovascular disease early diagnosis system
ActiveCN103142222BImprove accuracyHigh degree of informatizationDiagnostic recording/measuringSensorsMortality rateHospital information system
The invention is suitable for the field of cardiovascular and cerebrovascular disease early diagnosis, and provides a digitized cardiovascular and cerebrovascular disease early diagnosis system. The digitized cardiovascular and cerebrovascular disease early diagnosis system comprises a diagnosis and detection module, an information management module and an auxiliary module, wherein joint inspection and dynamic detection are carried out on an electrocardiogram and a cardiac marker by the diagnosis and detection module, so that the accuracy and timeliness of the cardiovascular and cerebrovascular disease early diagnosis can be effectively promoted; a 12-lead electrocardiograph is provided with a first communication unit, so that remote expert consultation is realized, and the accuracy rate of diagnosis is improved; and the information management module is provided with a second communication unit, so that remote wireless communication of the digitized system and a hospital information system, as well as the digitized system and a laboratory information management system is realized, and the informationization degree of the digitized system is improved. The digitized cardiovascular and cerebrovascular disease early diagnosis system is portable in design, strong in maneuverability, convenient to fold and unfold, can be used at any time, simple to operate and complete in detection items, is suitable for rapidly diagnosing on site, and is capable of assisting medical workers to treat pointing to symptoms, so that the mortality rate of patients is lowered.
Owner:北京倍肯恒业科技发展股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com