Kit for combined quantitative detection of five cardiac markers and preparation method thereof
A combined detection and kit technology, applied in the field of clinical medical testing, can solve the problems of inability to achieve risk assessment, low detection efficiency, waste of consumables, etc., and achieve high yield, easy automation, and obvious advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
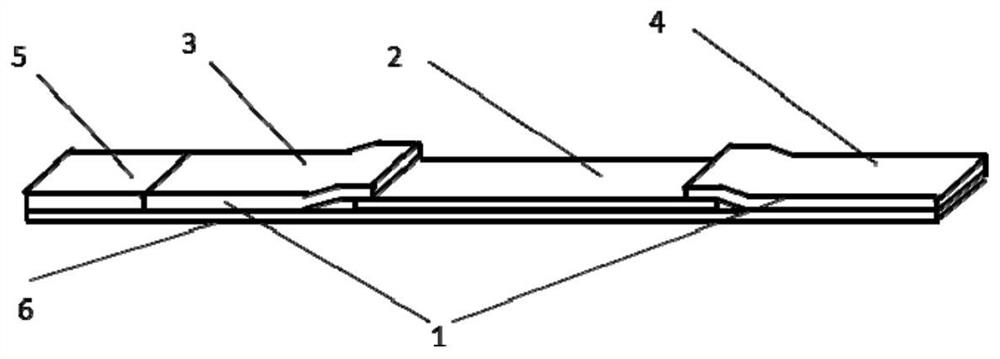
[0044] (1) Preparation of sample pad
[0045] Dissolve BSA and NaCl with pH 8.0 sodium bicarbonate buffer until the final concentration of BSA is 2% and the final concentration of NaCl is 0.1M, then add the surfactant Tween20 to the final concentration of 0.5%, adjust the pH to 8.0, press the glass fiber The water absorption capacity of the plain film is 60uL / cm2, and the above buffer solution is evenly spread on the glass cellulose film, and dried at 37°C for 8 hours to obtain a sample pad. Store at 4°C for later use.
[0046] (2) Preparation of bonding pad
[0047] The monoclonal antibody labeled with fluorescent microspheres is evenly spread on the sample pad prepared in step (1), freeze-dried in a vacuum, sealed, and stored at room temperature for use. The preparation process of the monoclonal antibody labeled with fluorescent microspheres is as follows:
[0048] a. Preparation of hs-CRP monoclonal antibody labeled with fluorescent microspheres
[0049] Take 0.5 mL of the fluores...
Embodiment 2
[0068] Detection of hypersensitivity C-reactive protein, myeloperoxidase, lipoprotein phospholipase A2, oxidized low-density lipoprotein and F2-isoprostaglandin fluorescence immunochromatographic detection kit.
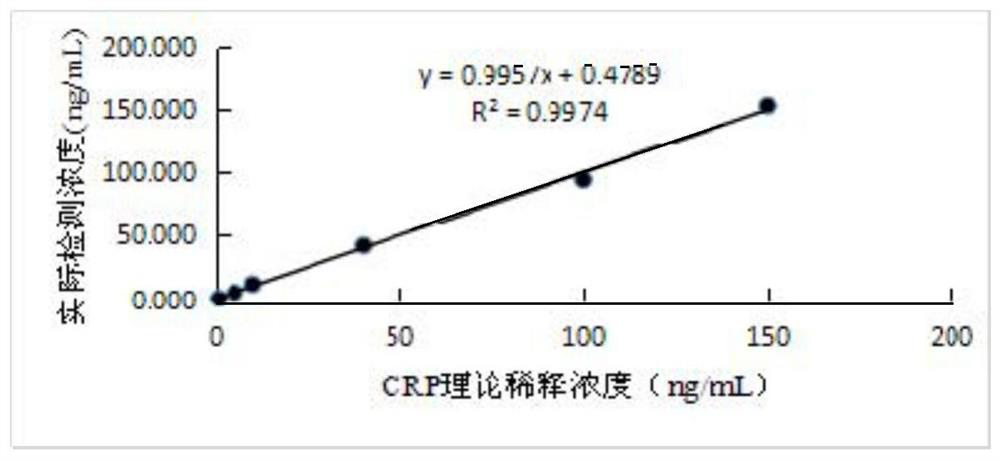
[0069] (1) Draw a standard curve
[0070] Different concentrations of hypersensitive C-reactive protein, myeloperoxidase, lipoprotein phospholipase A2, oxidized low-density lipoprotein, and F2-isoprostaglandin antigen standards were added to the sample pads of the kit prepared according to Example 1. With 7 different concentrations, the standards of hypersensitive C-reactive protein antigen are 0, 0.5, 5, 10, 40, 100, 150 ng / mL, and the standards of myeloperoxidase antigen are 0, 10, 50, 100, 200, 500, 1000 ng / mL, lipoprotein phospholipase A2 antigen standards were 0, 50, 100, 200, 500, 1000 and 1500 ng / mL, oxidized low density lipoprotein antigen standards were 0, 0.5, 1, 2, 4, 8, 16 and 20μg / mL, the standard F2-isoprostaglandin antigen is 0, 30, 50, 200, 400, 800 and 15...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com