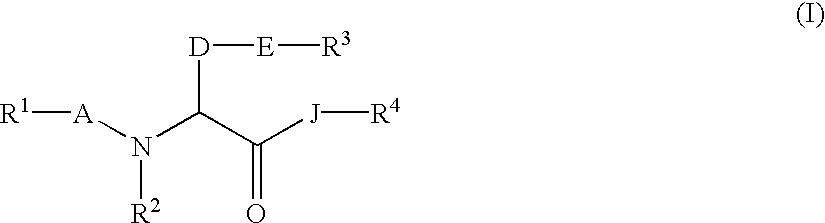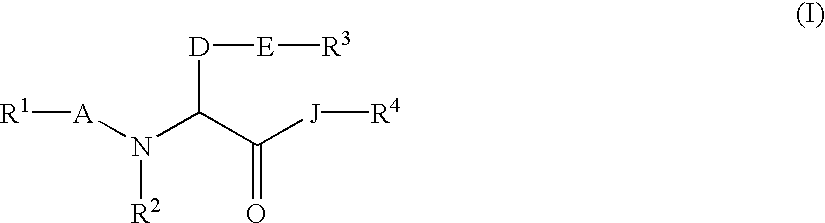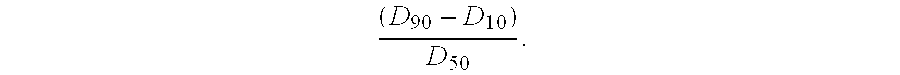Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Brain spinal cord" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Meninges are membranes that cover and protect the brain and spinal cord. There are three layers of meninges: Dura mater (closest to the bone), Arachnoid loosely around the brain, Pia mater is closely attached to the brain and spinal cord surface.
Brain, spinal, and nerve injury treament
InactiveUS20030109417A1Significant morbidityHigh incidenceBiocideNervous disorderSubstance P Receptor AntagonistsSpinal cord
A treatment for brain, spinal and nerve injury comprising use of a substance P receptor antagonist optionally in combination with a magnesium compound. There is also provided a formulation for use in this treatment comprising a substance P receptor antagonist and a magnesium compound.
Owner:EUSTRALIS PHARMA LTD
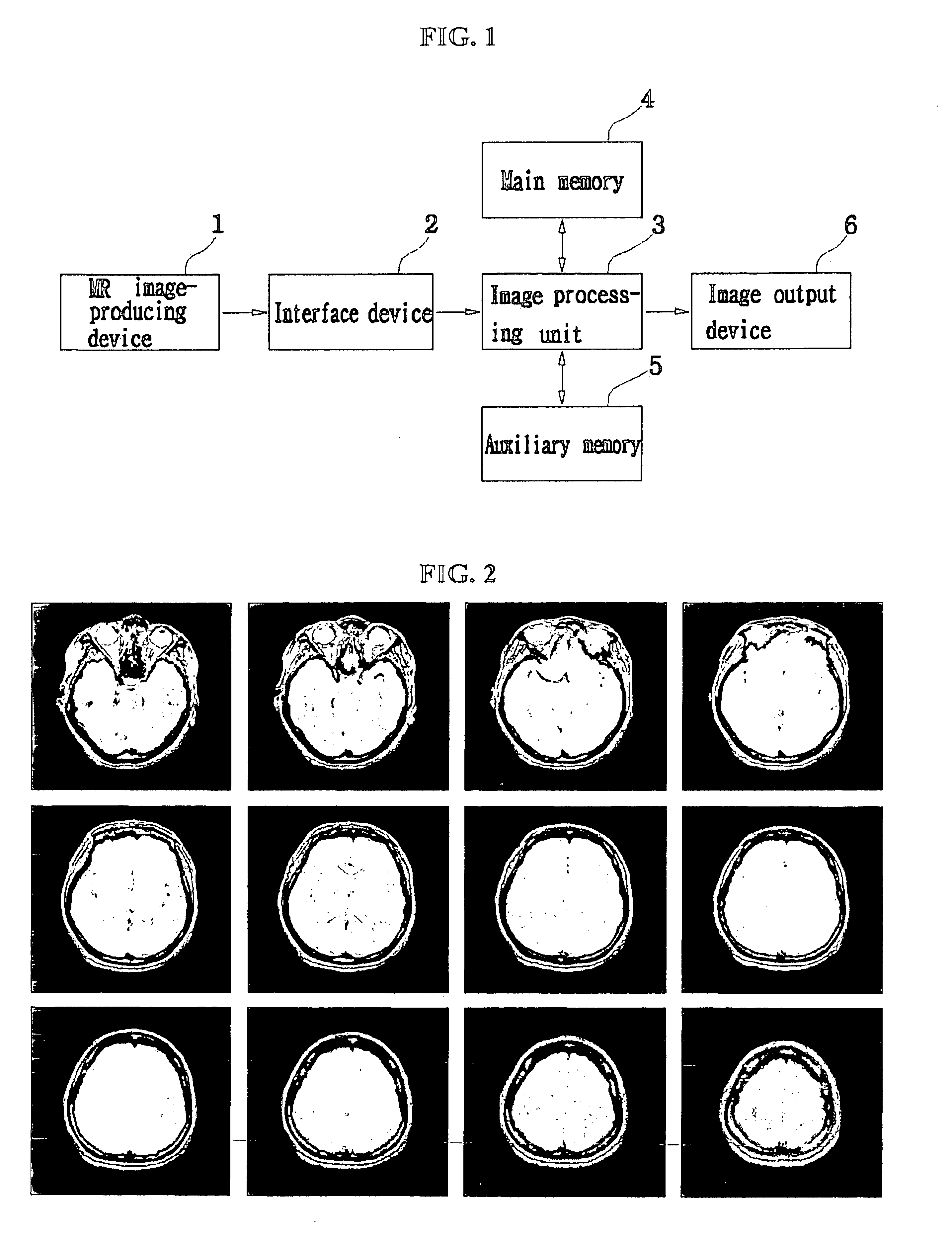
Histogram segmentation of FLAIR images
ActiveUS20030009098A1Automatically measuring the volume of tissueThe classification result is accurateImage enhancementImage analysisFluid-attenuated inversion recoveryLeukoaraiosis
A method for classifying tissue in a magnetic resonance image. and particularly for measuring a volume of pathological tissue such as white tissue hyperintensity (leukoaraiosis) in the brain based on the segmentation of the intensity histogram of fluid attenuated inversion recovery (FLAIR) images is described. A magnetic resonance image of the brain of a subject is acquired, and a pixel intensity histogram is constructed from the image. A statistically-based regression analysis is applied to the histogram to determine upper and lower threshold values, which define different types of brain tissue, particularly normal brain, cerebral spinal fluid (CSF), or lesion.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
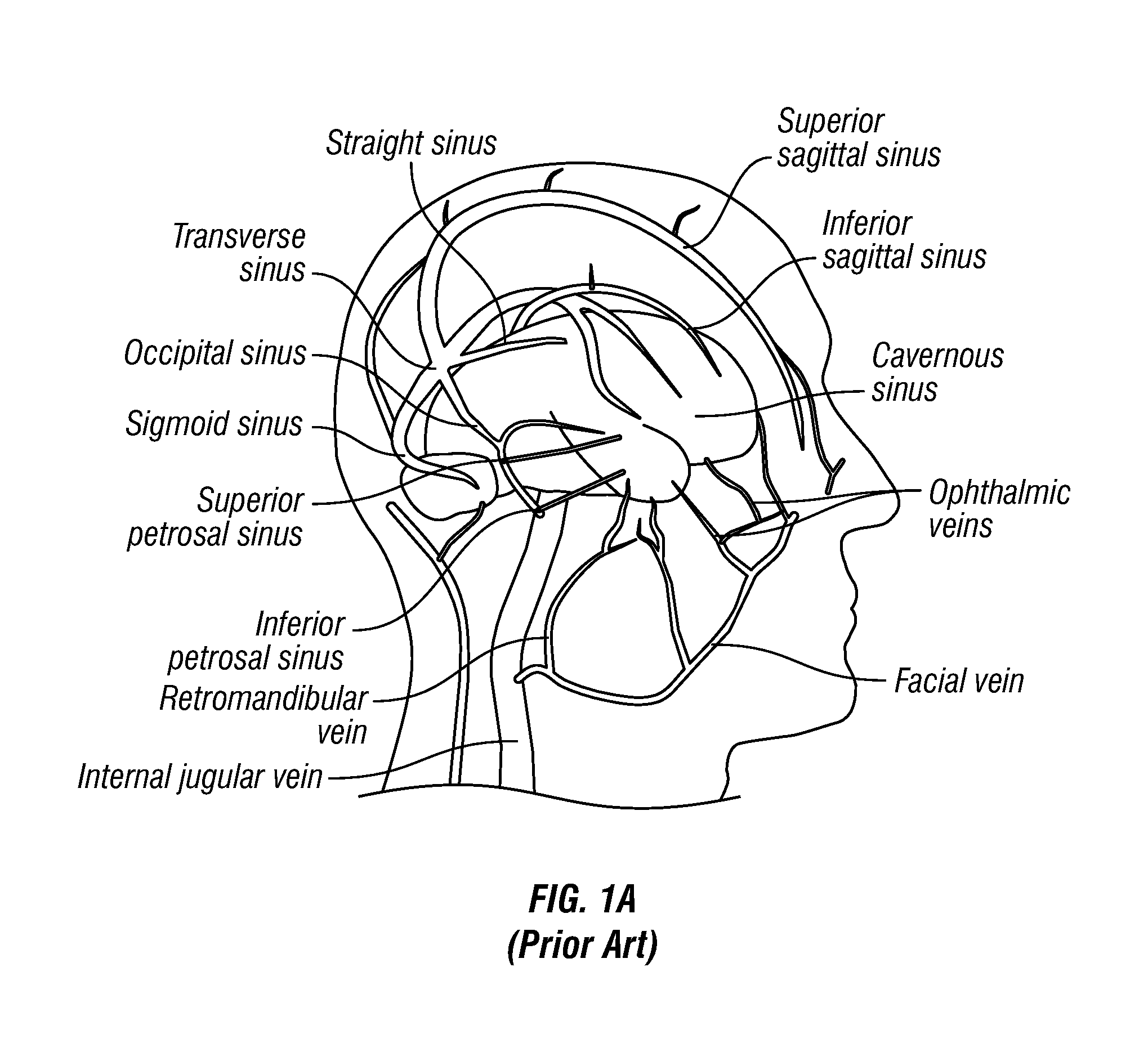
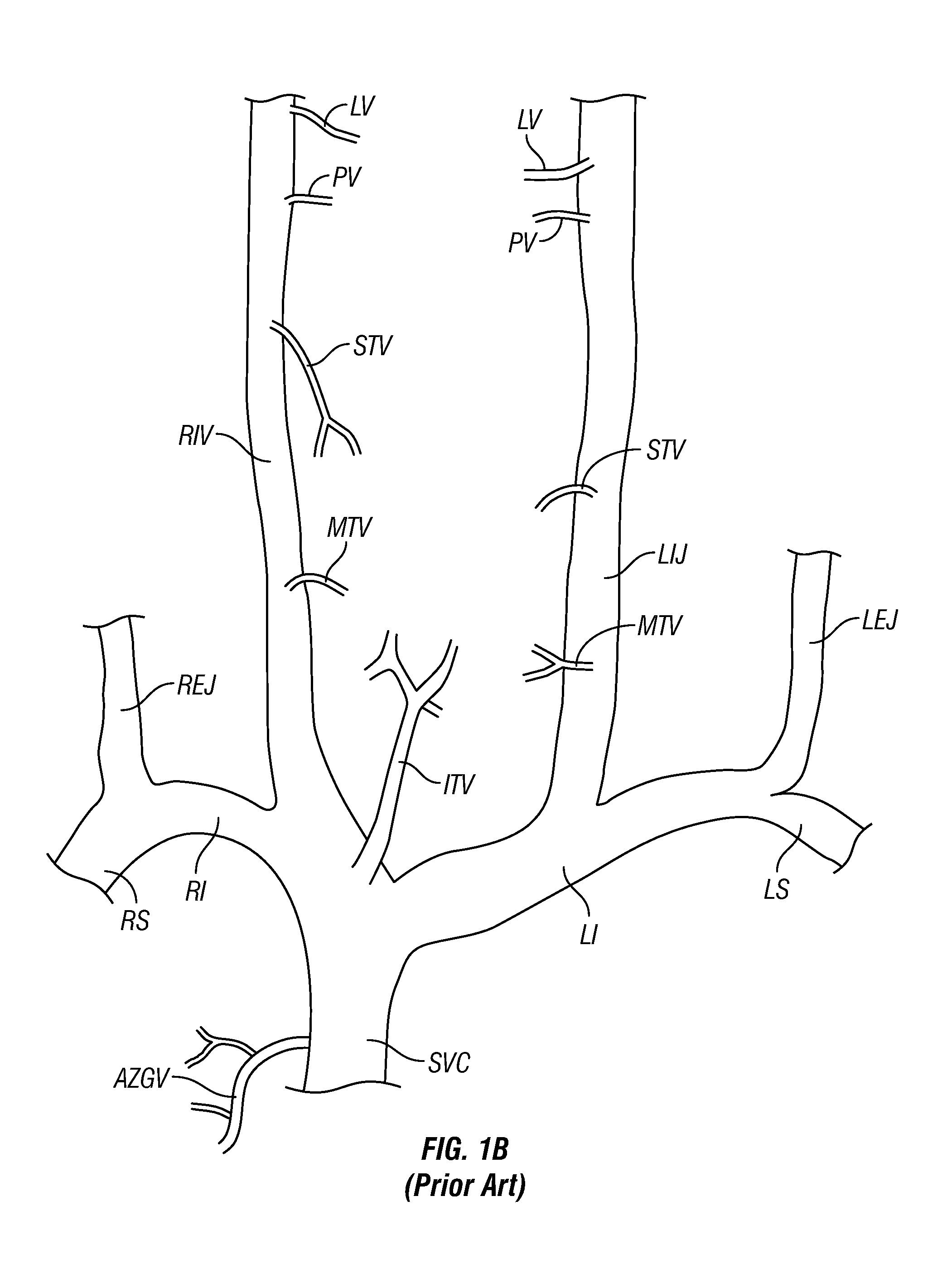
Methods, Systems and Devices for Treatment of Cerebrospinal Venous Insufficiency and Multiple Sclerosis
InactiveUS20130211489A1Speed up circulationEnlargeStentsBlood vesselsCerebellospinal tractVenous occlusion
Methods and devices for relieving stenoses in, or otherwise improving blood flow through, body lumens. Although applicable in a variety of different body lumens, the methods and devices of this invention are specifically useable for relieving stenoses in, or otherwise improving blood flow through, veins which drain blood from the brain for treatment of multiple sclerosis or other neurodegenerative disorders that are caused, triggered or exacerbated by venous occlusion or venous insufficiency.
Owner:EXPLORAMED DEV LLC
Composition and method to prevent and treat brain and spinal cord injuries
A composition and method for treating and preventing injury to central nervous system tissue are provided. The composition is comprised of agents that can increase colloidal osmotic pressure and osmolality. The method comprise of: a). Withdrawing cerebrospinal fluid from the subarachnoid spaces around the tissue to be treated or protected and b). Injecting the composition into subarachnoid spaces.
Owner:WANG YANMING
Graft core for seal and suture anastomoses with devices and methods for percutaneous intraluminal excisional surgery (PIES)
The present invention is a combination anastomosis device that both sutures and seals connections between two native body tubes and a graft—better proof against leaks than prior art of suturing alone or as some propose, by sealing. The invention is also a combination of supporting devices and methods that allow the anastomoses to be performed in seconds rather than the minutes required by present art, causing no more collateral bodily damage than percutaneous entry, requiring no time on a cardiac bypass system, either no heart stoppage or less than a minute thus potentially increasing the population who can tolerate coronary bypass as an out-patient procedure. The tract of application is not limited to coronary but includes vascular, urinary, pulmonary, alimentary, cerebral-spinal or other mammalian tract. May be manufactured of biodegradeable or biocompatible material and graft may be harvested or synthetic.
Owner:SHRIVER EDGAR LOUIS
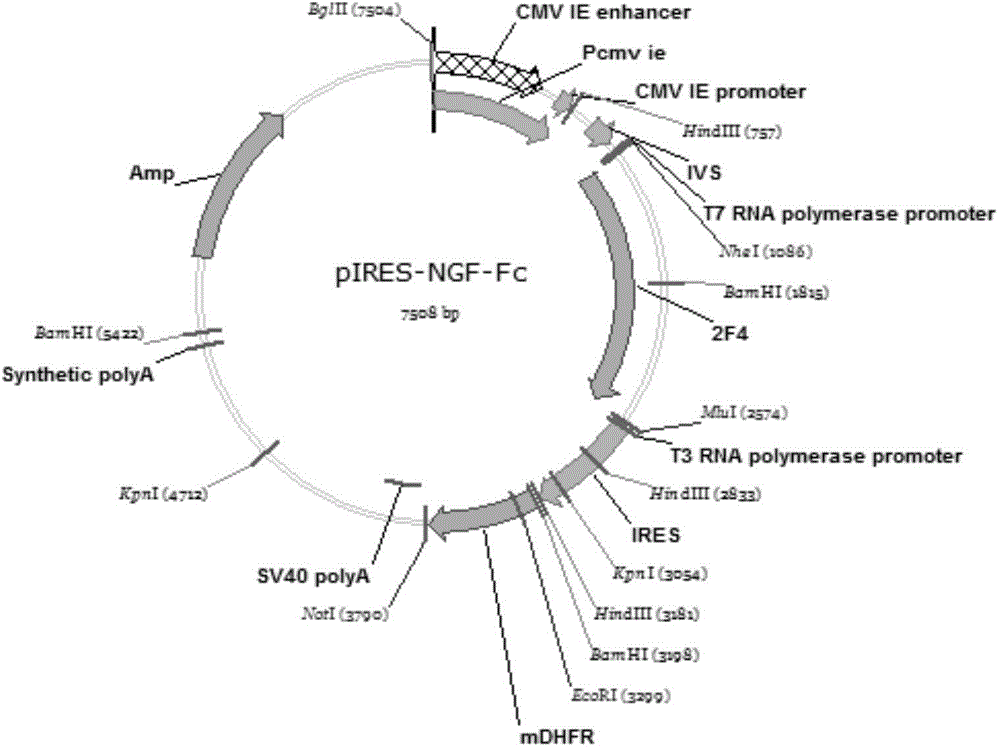
NGF-Fc fusion protein and preparation method thereof
InactiveCN105273087AImprove stabilityExtended half-lifeNervous disorderPeptide/protein ingredientsDiseaseNervous system
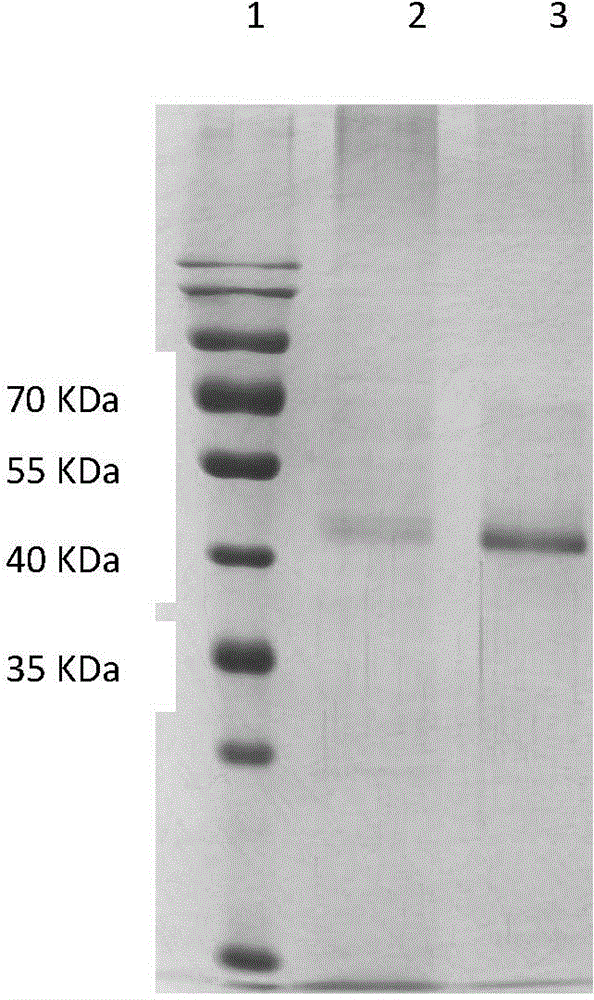
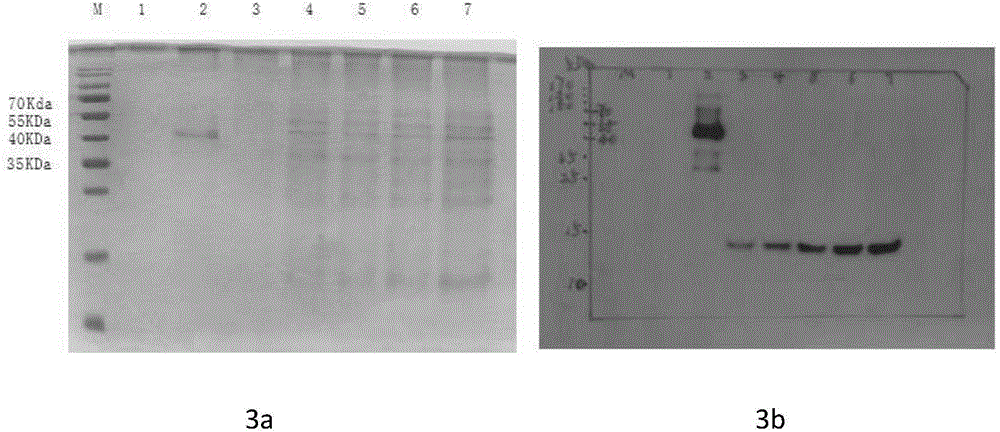
The invention belongs to the technical field of biological engineering, and relates to an NGF-Fc fusion protein and a preparation method thereof. The method comprises the following steps: constructing a human immune globulin IgG Fc and nerve growth factor (NGF) fusion gene expression vector through a genetic engineering means, transferring to a mammal cell to make the transferred mammal cell highly express and produce NGF-Fc fusion proteins, purifying, identifying and carrying out biological activity detection. The expression vector transferred mammal cell constructed in the invention can express bioactive NGF-Fc fusion proteins, so the expression level can be 150mg / L or above, and the obtained protein has good stability and long half life, and can be used to prepare drugs for treating Alzheimer's disease, diabetic peripheral neuropathy, Parkinson's disease, facial neuritis, craniocerebral trauma, trauma of spinal cord, acute cerebrovascular disease, encephalatrophy and other neurological diseases, and peripheral nerve injury acute cerebral vascular central nerve injuries induced by chemical drugs.
Owner:FUDAN UNIV
Amino acid derivatives and drugs containing the same as the active ingredient
The present invention relates to the compounds of the formula (I) and salts thereof (all the symbols are the same meanings as described in the specification).The compounds of the formula (I) possess inhibitory activity of N-type calcium channel, so they are useful as drug for prevention and / or treatment of cerebral infarct, transient ischemic attack, encephalomyelopathy after cardiac operation, spinal angiopathy, hypertension with stress, neurosis, epilepsy, asthma and pollakiuria etc. or agent for the treatment of pain.
Owner:ONO PHARMA
Ketogenic diet formula milk and preparation method thereof
ActiveCN102715240AChange the structure of nutrientsChange structureMilk preparationNervous disorderKetogenic dietLiquid milk
The invention discloses a ketogenic diet formula milk and a preparation method thereof and aims to solve the technical problem that convenience is provided for a patient to have food therapy through ketogenic diet. The ketogenic diet formula milk contains the following components per 100 g: 1.5-12.5 g of fat, 0-5 g of protein, 0-0.5 g of carbohydrate, 0-0.6 g of flavoring, 0-1.2 g of nourishing essence and the balance of liquid milk. The preparation method comprises the step of uniformly mixing fat, protein, carbohydrate, flavoring, nourishing essence and the liquid milk according to the prior art. Compared with the prior art, the ketogeic diet formula milk takes human milk and animal milk of which metabolizable sugar is removed as the main raw material, adopts a nutrient formula close to physiological needs, has a good mouthfeel, is easily accepted by the patient, is convenient to take, is suitable for treating epilepsy, diabetes, all kinds of tumors, behavior disorder, autism, brain and spinal cord injuries, and adiposity, and is also suitable for normal people to lose weight and change the structure of the main nutrients of the body, as well as for animal experiments.
Owner:廖建湘
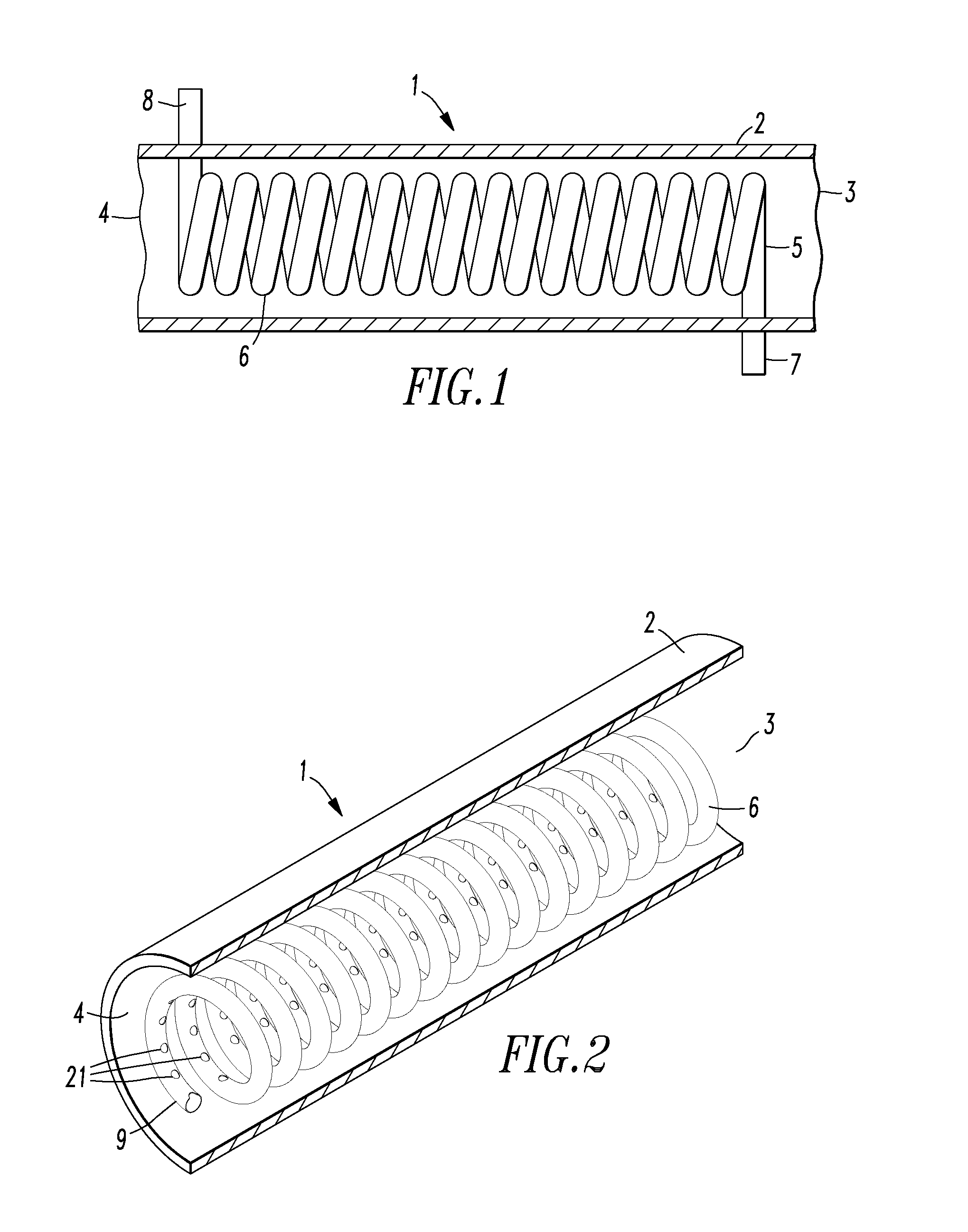
Implantable cerebral spinal fluid flow device and method of controlling flow of cerebral spinal fluid
InactiveUS20060247569A1Reduce decreaseReduce usageWound drainsIntravenous devicesBiomedical engineeringCerebrospinal fluid flow
An implantable cerebral spinal fluid flow device and method. A body has an inlet for the cerebral spinal fluid, an outlet for the cerebral spinal fluid and a first interior cavity fluidly coupled with the inlet and the outlet. A first rotational element is mounted in the first interior cavity in a pathway between the inlet and the outlet. The first rotational element provides resistance to flow of the cerebral spinal fluid from the inlet to the outlet. In the method, the cerebral spinal fluid flow device is implanted. Resistance to rotation of the first rotational element to flow of the cerebral spinal fluid from the inlet to the outlet is provided.
Owner:MEDTRONIC INC
Method for segmentation and volume calculation of white matter, gray matter, and cerebral spinal fluid using magnetic resonance images of the human brain
InactiveUS6895107B2Reduce blurAssisted identificationImage enhancementImage analysisVisual recognitionGrey matter
A fully-automated method for segmentation of white matter, gray matter, and cerebral spinal fluid (CSF) and for calculating their respective volumes is disclosed. The technique preferably uses a T2-weighted scan and a proton-density scan. The T2-weighted scans are used to highlight those portion of the brain corresponding to CSF. Thereafter, the proton density images are separated in CSF-containing and CSF-free portions, in accordance with the T2-weighted images. The CSF-free portion is then segmented into white matter and gray matter portions. The CSF-containing portion is further segmented into pure CSF-regions, and regions containing a mixture of gray and white matter and CSF. After CSF is segmented from this mixture, the white and gray matter and again segmented, and the respective volumes of white matter, gray matter, and CSF are calculated. A technique for determining a threshold gray scale value for segmenting the white and gray matter to assist in the visual identification of such regions is also disclosed.
Owner:PARK JONG WON
Compositions and treatment method for brain and spinal cord injuries
InactiveUS20040142905A1Effective treatment and preventionOrganic active ingredientsBiocideSubarachnoid spacePreventing injury
Owner:ONYX OPTICS
Methods for Detecting Survival Motor Neuron (SMN) Protein in Whole Blood or Cerebral Spinal Fluid
InactiveUS20140367278A1Weather/light/corrosion resistanceChemiluminescene/bioluminescenceElectrochemiluminescenceMotor neuron
A method for determining the level of survival motor neuron (SMN) protein in a whole blood or cerebral spinal fluid (CSF) sample, for example, a whole blood lysate sample or a CSF sample, including obtaining a whole blood or cerebral spinal fluid (CSF) sample from the subject and conducting an electrochemiluminescence immunoassay to determine the level of SMN in the whole blood or cerebral spinal fluid (CSF) sample.
Owner:PHARMOPTIMA
T. Cruzi-derived neurotrophic agents and methods of use therefor
InactiveUS20090117593A1Provide supportCompound screeningApoptosis detectionNeurophysinsNeuronal degeneration
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TUFTS UNIV
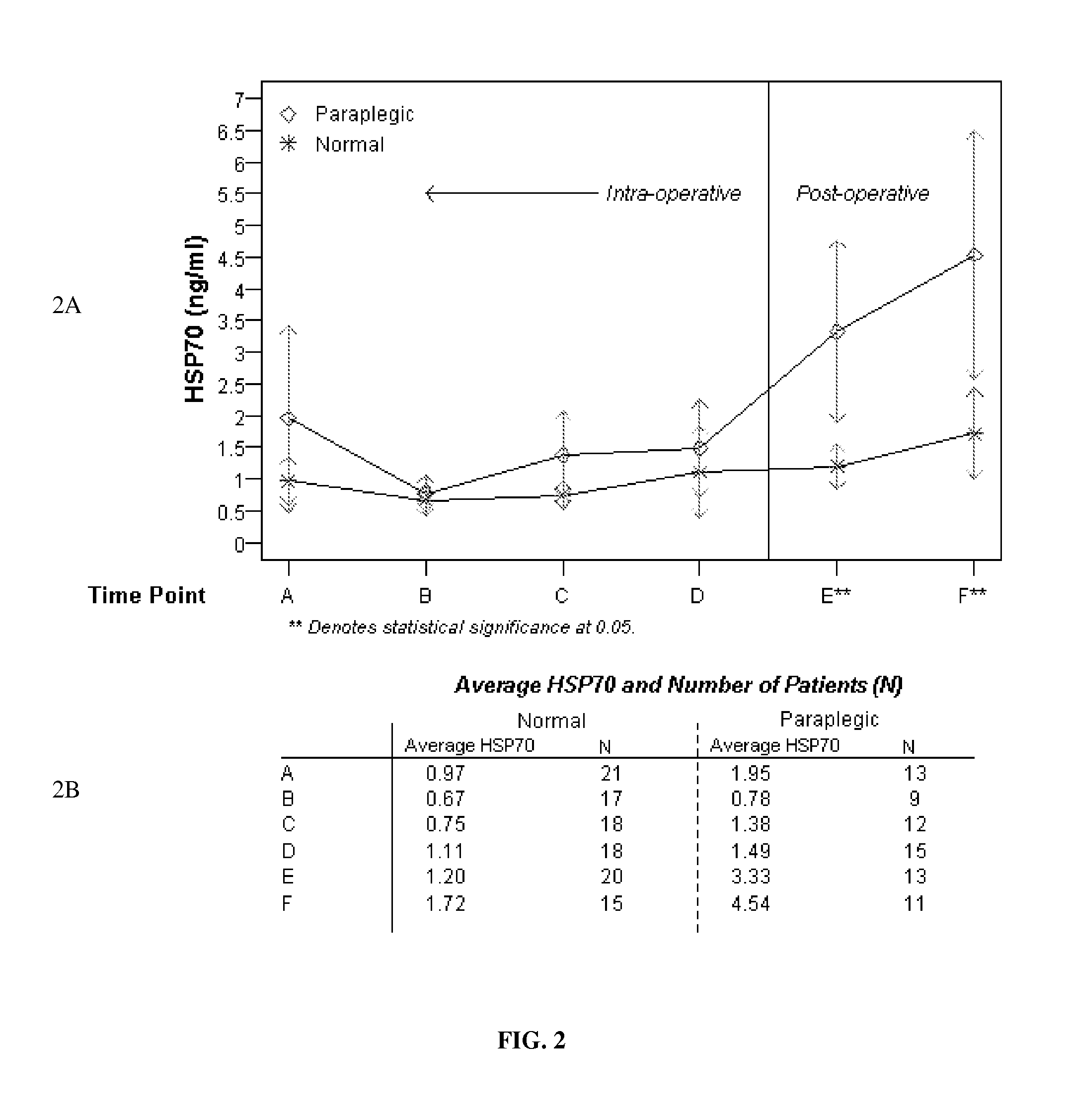
Elevation of Induced Heat Shock Proteins in Patient's Cerebral Spinal Fluid: A Biomarker of Risk/Onset of Ischemia and/or Paralysis in Aortic Surgery
Provided are methods for intra-operatively predicting, detecting or diagnosing the risk or onset of spinal cord ischemia and / or associated permanent paralysis in a patient, based upon the stress-induced elevation of levels of heat shock proteins, specifically HSP70 and / or HSP27 in the cerebral spinal fluid of the patient, as measured during thoracic-aorta surgery, particularly thoracic aneurysm repair surgery, that will permit intra-operative medical intervention to try to prevent or attenuate severe, and often fatal, complications. Further provided are kits, assay devices and methods of analyzing biomarker data for use in pre-, intra- or post-operatively detecting the stress-induced elevations of the measured levels of HSP70 and / or HSP27, and the biomarker itself.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Ceruloplasmin and uses thereof in neurodegenerative related conditions
InactiveUS20030054980A1Effective mean for maintaining and stimulating regenerationImprove efficiencyBiocideNervous disorderNervous systemMedicine
The present invention relates to the use of ceruloplasmin (and / or derivatives thereof) for treating neurodegenerative related conditions. More particularly, the present invention pertains to the use of ceruloplasmin in pharmaceutical neurotrophic compositions, to its use as a carrier for delivering therapeutic agent(s) to tissues of the nervous systems and to its use in methods for culturing neuronal cells in vitro. The present invention is useful for maintaining or stimulating the regeneration of neuronal cells, particularly for the treatment of injuries to the brain, the spinal cord and other central nervous system tissues.
Owner:UNIV DU QUEBEC A MONTREAL
Method for the treatment of multiple sclerosis
InactiveUS20140251917A1Efficacious dialysisOther blood circulation devicesMedical devicesTreatment targetsBody fluid
The present invention relates to a treatment of multiple sclerosis, and includes the extracorporeal treatment of one or more body fluids, such as, for example blood, cerebral-spinal fluid, or lymphatic fluid. A treatment is applied to the extracorporeal body fluid where the treatment targets at least one target multiple sclerosis antigen in the body fluid. The treatment can include creating an antibody-antigen moiety and then removing antibody-antigen moiety from the body fluid before returning the body fluid to a patient.
Owner:MARV ENTERPRISES
Method of treatment and/or prevention of brain, spinal or nerve injury
InactiveUS20110053954A1Improve actionEnhance neuroprotectiveSenses disorderNervous disorderMagnesium saltSpinal cord
The invention relates to a method of treatment and / or prevention of brain, spinal or nerve injury comprising administration to a person in need of such treatment, of a therapeutically effective amount of an NK-1 receptor antagonist compound of the formulawherein the meanings of R, R1, R2, R2′, R3, and R4 are explained in the specification and the pharmaceutically acceptable acid addition salts and the prodrugs thereof either alone or in combination with a magnesium salt. Exemplified is the use of N-(3,5-bis-trifluoromethyl-benzyl)-N-methyl-6-(4-methyl-piperazin-1-yl)-4-o-tolyl-nicotinamide. The invention also relates to pharmaceutical composition comprising one or more such NK-1 receptor antagonists, optionally in combination with a magnesium salt, and a pharmaceutically acceptable excipient for the treatment and / or prevention of brain, spinal or nerve injury.
Owner:HOFFMANN TORSTEN +4
Method of treatment and/or prevention of brain, spinal or nerve injury
InactiveUS20060247240A1Improve actionEnhance neuroprotectiveBiocideSenses disorderMagnesium saltNK 1 Receptor Antagonists
The invention relates to a method of treatment and / or prevention of brain, spinal or nerve injury comprising administration to a person in need of such treatment, of a therapeutically effective amount of an NK-1 receptor antagonist compound of the formula wherein the meanings of R, R1, R2, R2, R3, and R4 are explained in the specification and the pharmaceutically acceptable acid addition salts and the prodrugs thereof either alone or in combination with a magnesium salt. Exemplified is the use of N-(3,5-bis-trifluoromethyl-benzyl)-N-methyl-6-(4-methyl-piperazin-1-yl)-4-o-tolyl-nicotinamide. The invention also relates to pharmaceutical composition comprising one or more such NK-1 receptor antagonists, optionally in combination with a magnesium salt, and a pharmaceutically acceptable excipient for the treatment and / or prevention of brain, spinal or nerve injury.
Owner:HOFFMANN TORSTEN +4
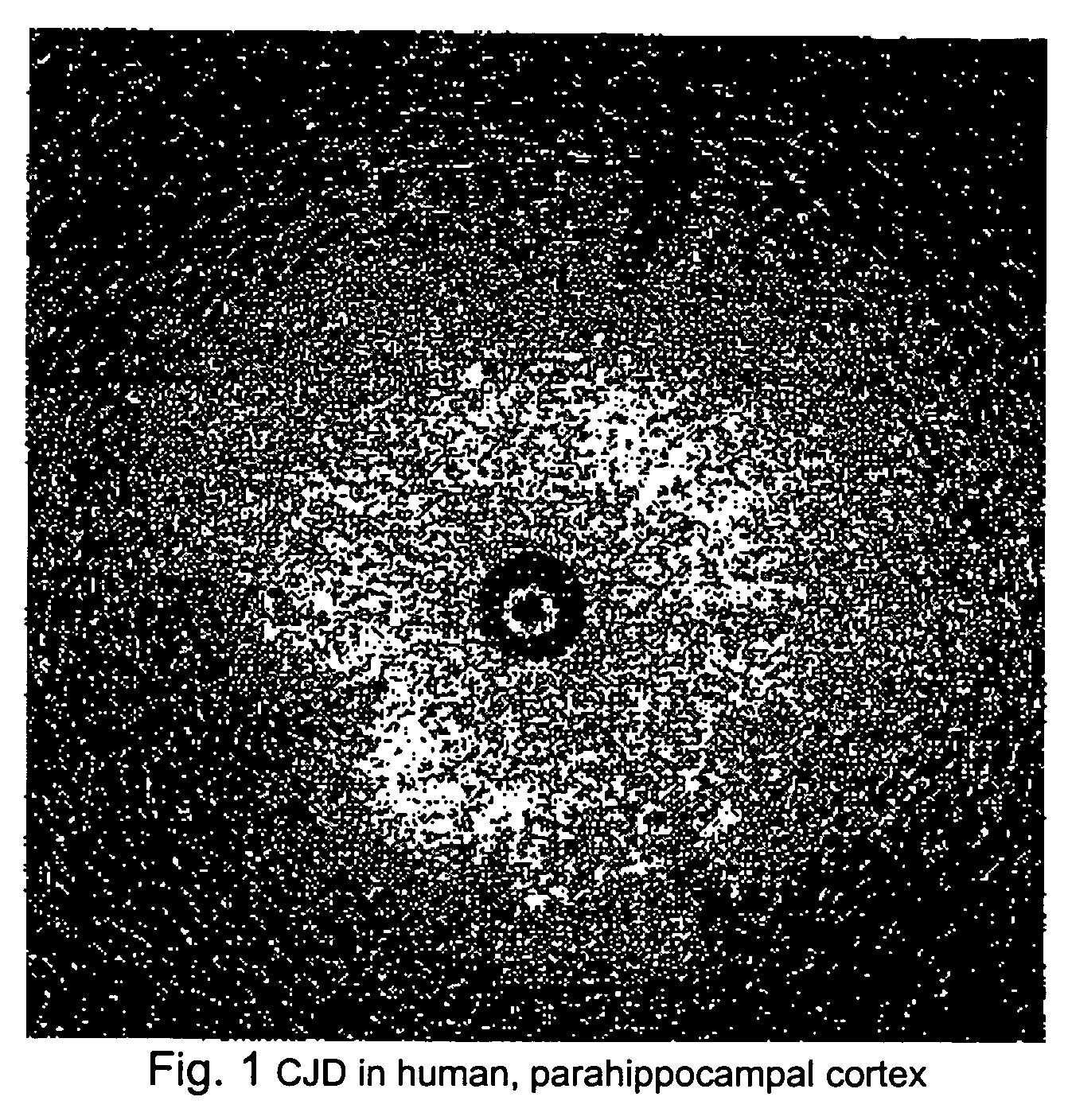
Methods for rapid screening of mad cow disease and other transmissible spongiform encephalopathies
Methods for diagnosing altered neuropathology in an animal are disclosed, wherein said methods comprise imaging brain, spinal cord, or other neural tissue of the animal, analyzing the appearance of the tissue, and determining whether the appearance of the tissue is altered relative to corresponding unaltered tissue. Also disclosed are methods for diagnosing spongiform encephalopathies in an animal, wherein said methods comprise imaging brain, spinal cord, or other neural tissues of the animal, analyzing the appearance of vacuoles in the tissue, and determining whether the appearance of the vacuoles in the tissue is altered relative to corresponding spongiform encephalopathy-free tissue. Also disclosed are automated methods for diagnosing altered neuropathy and spongiform encephalopathies.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
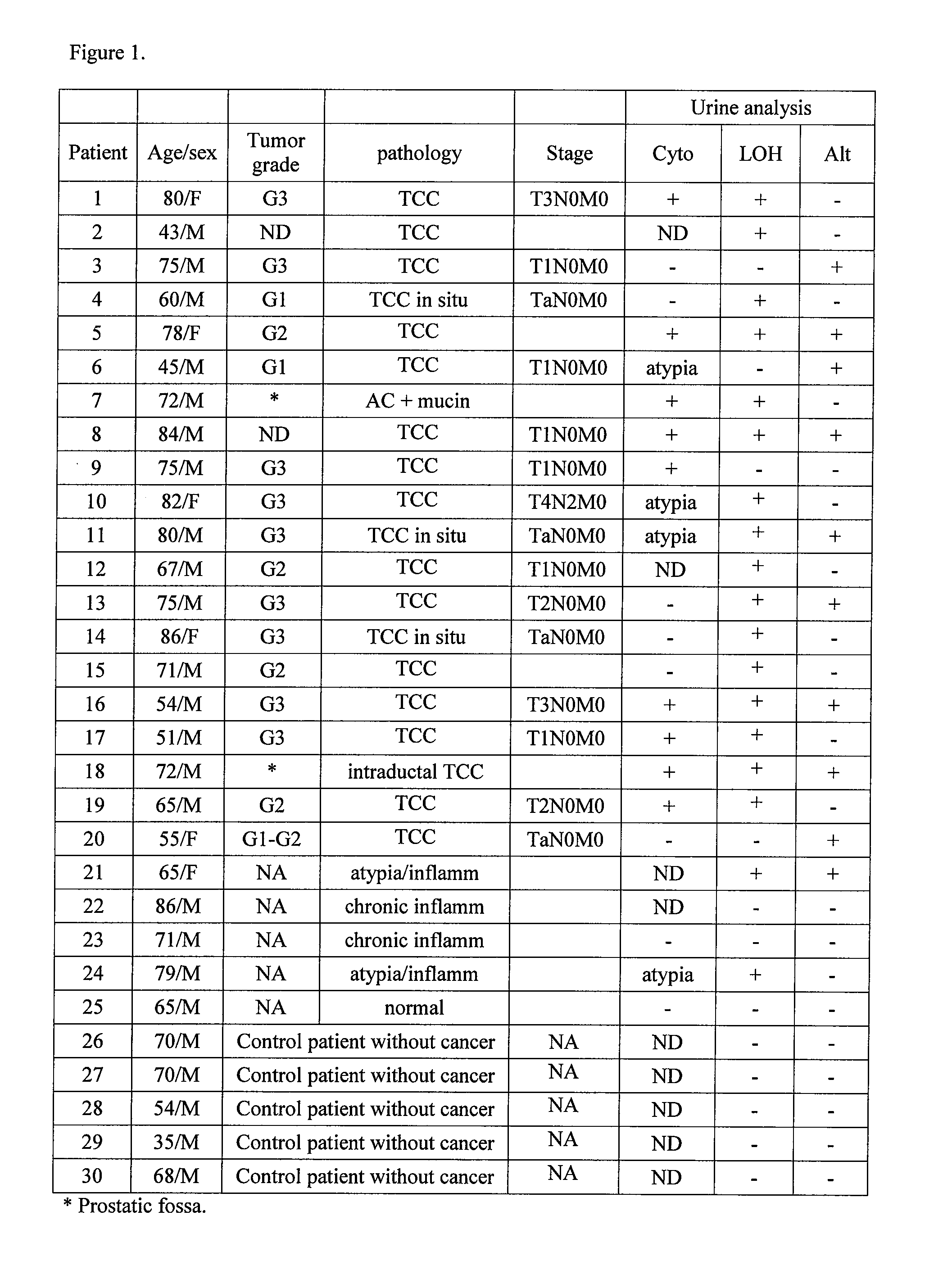
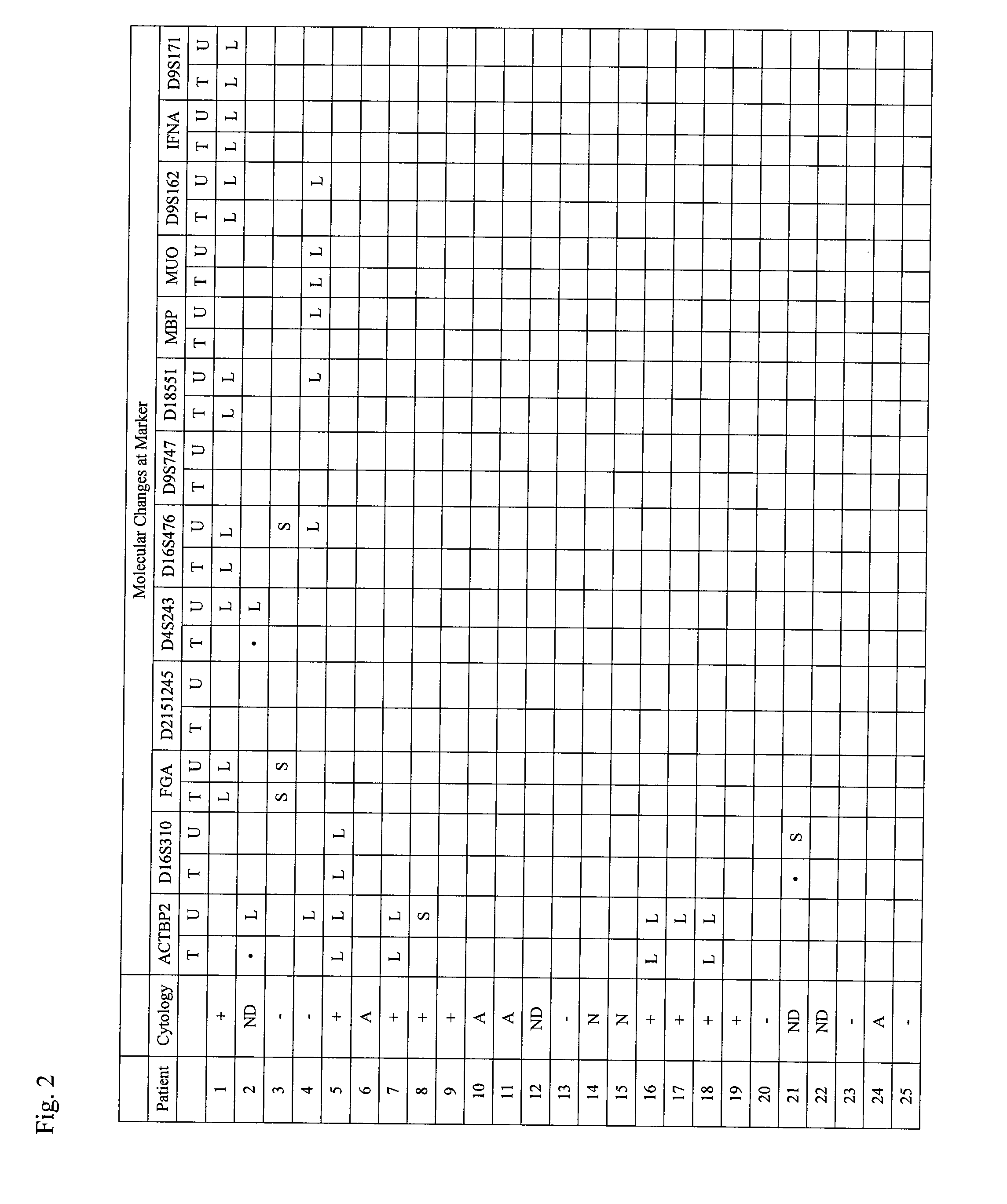
Method for detecting cell proliferative disorders
InactiveUS20090286236A1Fast and reliable and sensitive and non-invasive screeningMicrobiological testing/measurementDiseaseCervical tissue
The present invention relates to the detection of a cell proliferative disorder associated with alterations of microsatellite DNA in a sample. The microsatellite DNA can be contained within any of a variety of samples, such as urine, sputum, bile, stool, cervical tissue, saliva, tears, or cerebral spinal fluid. The invention is a method to detect an allelic imbalance by assaying microsatellite DNA. Allelic imbalance is detected by observing an abnormality in an allele, such as an increase or decrease in microsatellite DNA which is at or corresponds to an allele. An increase can be detected as the appearance of a new allele. In practicing the invention, DNA amplification methods, particularly polymerase chain reactions, are useful for amplifying the DNA. DNA analysis methods can be used to detect such a decrease or increase. The invention is also a method to detect genetic instability of microsatellite DNA. Genetic instability is detected by observing an amplification or deletion of the small, tandem repeat DNA sequences in the microsatellite DNA which is at or corresponds to an allele. The invention is also a kit for practicing these methods.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Wireless and noninvasive epidermal electronics
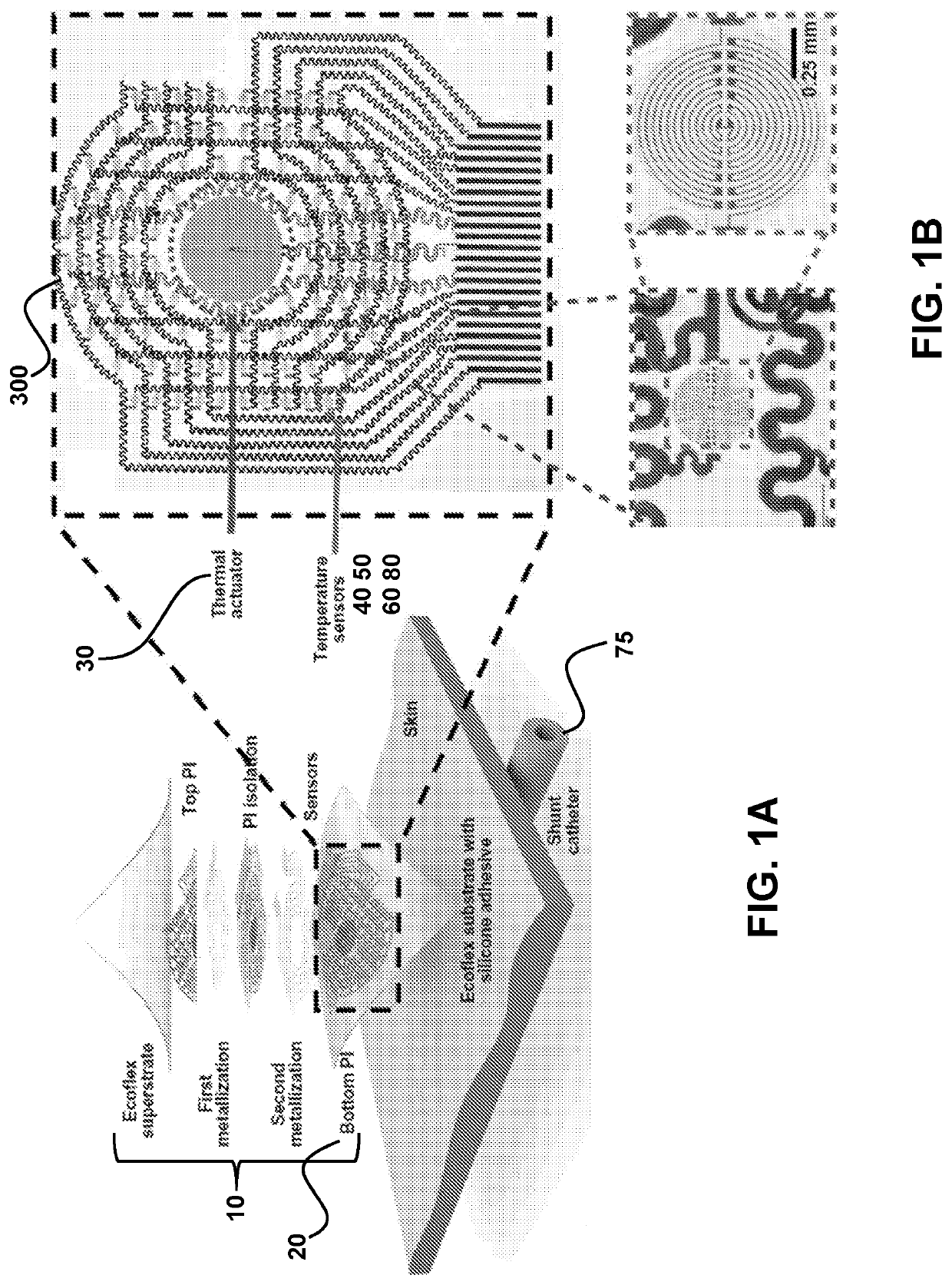
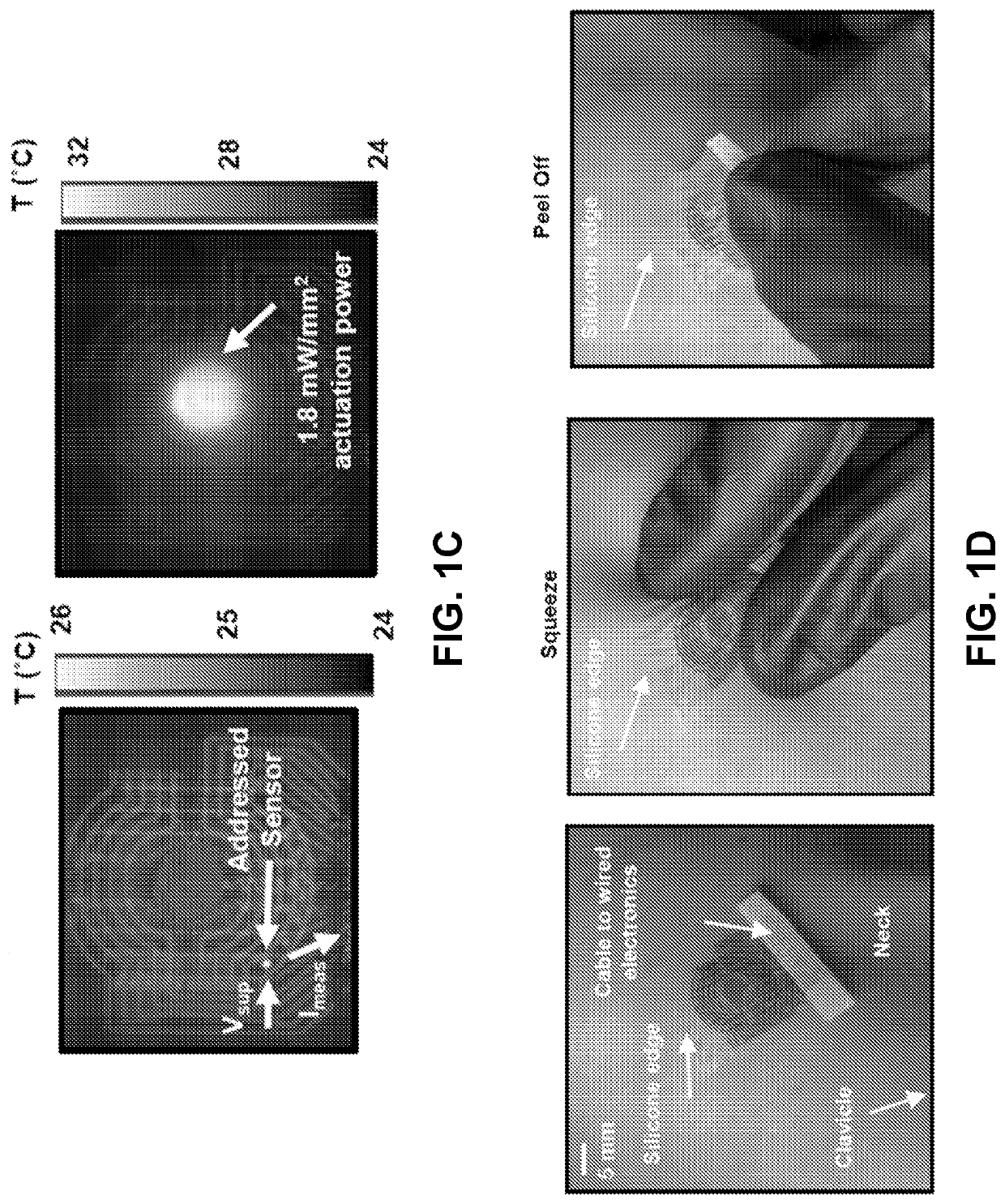
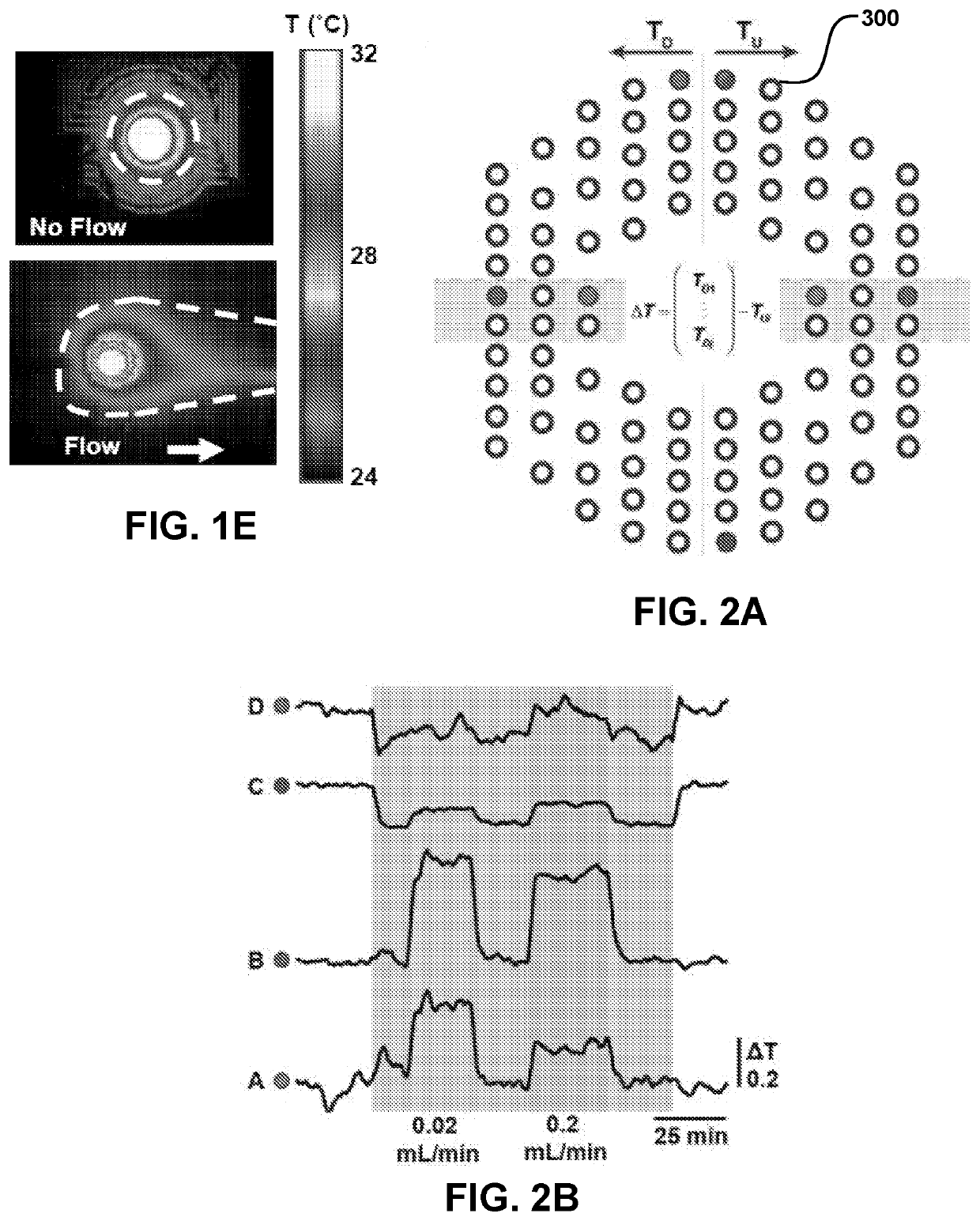
ActiveUS20210022686A1High precisionAdvantageously non-invasiveIntracranial pressure measurementTemperature sensorsElectronic communicationNon invasive
Provided are conformable devices to measure subdermal fluid flow and related methods. A soft, stretchable and flexible substrate supports a thermal actuator and various specially positioned temperature sensors. A microprocessor in electronic communication with sensors calculates subdermal fluid flow from the measured upstream and downstream temperatures, as well as various application-dependent parameters. Devices and methods provided herein are particularly useful for measuring cerebral spinal fluid in a ventricular shunt placed for treatment of hydrocephalus.
Owner:RHAEOS INC
Cyanocobalamin low viscosity aqueous formulations for intranasal delivery
ActiveUS7404489B1Prevent drynessAvoid stimulationOrganic active ingredientsIn-vivo radioactive preparationsIntramuscular injectionMedicine
A stable pharmaceutical mercury-free aqueous solution of cyanocobalamin comprised of cyanocobalamin and water wherein said solution of cyanocobalamin is suitable for intranasal administration, has a viscosity less than about 1000 cPs, and wherein said solution of cyanocobalamin has a bioavailability of cyanocobalamin when administered intranasally of at least about 7% relative to an intramuscular injection of cyanocobalamin with the proviso that the solution is essentially free of mercury and mercury-containing compounds. The present invention is also directed towards a method for elevating the vitamin B12 levels in the cerebral spinal fluid (CSF) comprising administering intranasally a sufficient amount of a mercury-free cyanocobalamin solution so as to increase the average ratio of vitamin B12 in the CSF to that in the blood serum (B12 CSF / B12 Serum×100) to at least about 1.1 comprising intranasally administering an aqueous solution of a cyanocobalamin, wherein said solution of cyanocobalamin has a bioavailability of at least 7% relative to an intramuscular injection of a cyanocobalamin.
Owner:ENDO PHARMA INC
Feedback Smart Injector
ActiveCN110507867BAccurate detectionImprove treatment efficiencyInfusion syringesMedical devicesHuman bodyHematological test
Owner:WUHAN ZKKL OPTOELECTRONICS TECH
Treatment liquid composition, treatment liquid kit and method for simultaneous biological organ transparentizing and immunolabeling
ActiveCN110763661AEasy to operateEnables immunofluorescent labelingPreparing sample for investigationBiological testingAntiendomysial antibodiesActive agent
The invention relates to a treatment liquid composition, a treatment liquid kit and a method for simultaneous biological organ transparentizing and immunolabeling. The treatment liquid composition according to the present invention comprises a transparent treatment liquid and a fluorescently labeled antibody in a volume ratio of 1: 10 to 1: 500 to the transparent treatment liquid, wherein the transparent treatment liquid comprises a saccharide, urea and a nonionic surfactant. Biological macromolecules such as antibodies are allowed to enter organs while transparentizing the whole organs (brain, spinal cord, articular cartilage, (tumor-bearing) organs and the like), and direct or indirect immunolabeling on the whole organ scale is achieved.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Generation of brain and spinal cord neurons, cardiac myocytes, renal nephrons and hepatocytes using reg peptides, peptidomimetics, small molecules and stimulatory antibodies to reg receptor
InactiveUS20170081375A1Improve solubilityImprove stabilityPeptide/protein ingredientsMammal material medical ingredientsProgenitorNephron
Peptides that are bioactive regions and optimized bioactive regions of the human and mammalian Reg gene proteins and that are capable of generation of tissues such as brain, spinal cord, heart, liver, and kidney are described. In particular, 7-15-amino acid Reg peptides and optimized Reg peptides are disclosed which are capable of in vivo and ex vivo transformation of progenitor cells, progenitor tissue and stem cells into specialized cells and tissues, including functioning brain and spinal cord neurons, cardiac myocytes, liver hepatocytes and renal nephrons. Methods of in vivo and ex vivo transformation of progenitor cells into differentiated cells and tissues are also described.
Owner:LEVETAN CLARESA
Chimeric autoantibody receptor (CAAR) that binds autoantibodies targeting the central nervous system in neurological autoimmune disease
PendingCN114008204APrevent or significantly reduce the risk of clinical relapseNo or reduced immunosuppressionOrganic active ingredientsNervous disorderAutoimmune conditionNmdar encephalitis
The invention relates to a chimeric autoantibody receptor (CAAR) that enables targeting of an immune cell to autoantibody producing B cells. The CAAR comprises an autoantigen or fragment thereof that is bound by autoantibodies associated with a neurological autoimmune disease primarily targeting the central nervous system. The invention relates to a nucleic acid molecule encoding a chimeric autoantibody receptor (CAAR), the nucleic acid molecule comprising a sequence encoding an autoantigen or fragment thereof that is bound by autoantibodies associated with a neurological autoimmune disease primarily targeting the central nervous system, a sequence encoding a transmembrane domain, and a sequence encoding an intracellular signaling domain. In one embodiment, the autoantigen encoded by the nucleic acid sequence comprises or consists of an N- methyl-D-aspartate receptor (NMDAR), or one or more NMDAR fragments. The invention further relates to the chimeric autoantibody receptor (CAAR) protein of the invention, a vector comprising a nucleic acid molecule encoding a chimeric autoantibody receptor (CAAR) of the invention, a genetically modified immune cell comprising the nucleic acid molecule encoding the CAAR and the use of the immune cell in the treatment or prevention of a neurological autoimmune disease primarily targeting the central nervous system, such as an autoimmune encephalopathy or encephalomyelopathy, preferably anti-NMDAR encephalitis.
Owner:德国神经退行性疾病中心 +1
Directional fibrin and polypeptide interpenetrating network composite hydrogel for nerve regeneration and preparation method thereof
PendingCN112516385AImprove adhesionBiochemically activePharmaceutical delivery mechanismTissue regenerationCell-Extracellular MatrixPhysisorption
The invention discloses a directional fibrin and polypeptide interpenetrating network composite hydrogel for nerve regeneration and a preparation method thereof, and belongs to the technical field ofbiomedical materials. The composite hydrogel is composed of a fibrin and polypeptide interpenetrating composite nanofiber structure. The directional fibrin hydrogel is prepared by electrostatic spinning, cross-linked polypeptide is interpenetrated with fibrin through swelling and physical adsorption, and then is stably combined with fibrin through a variety of cross-linking methods to form the directional interpenetrating network composite hydrogel. The composite hydrogel is a composite hydrogel with high water content, has the composition, structure and mechanical properties highly simulatingthe extracellular matrix of natural nerve tissues, contains polypeptide fragments simulating the function of growth factors, has excellent effects of promoting nerve cell adhesion and nerve fiber directional growth and inducing nerve and blood vessel regeneration, and can be applied to nerve regeneration repair of brain, spinal cord and peripheral nerve injury.
Owner:TSINGHUA UNIV
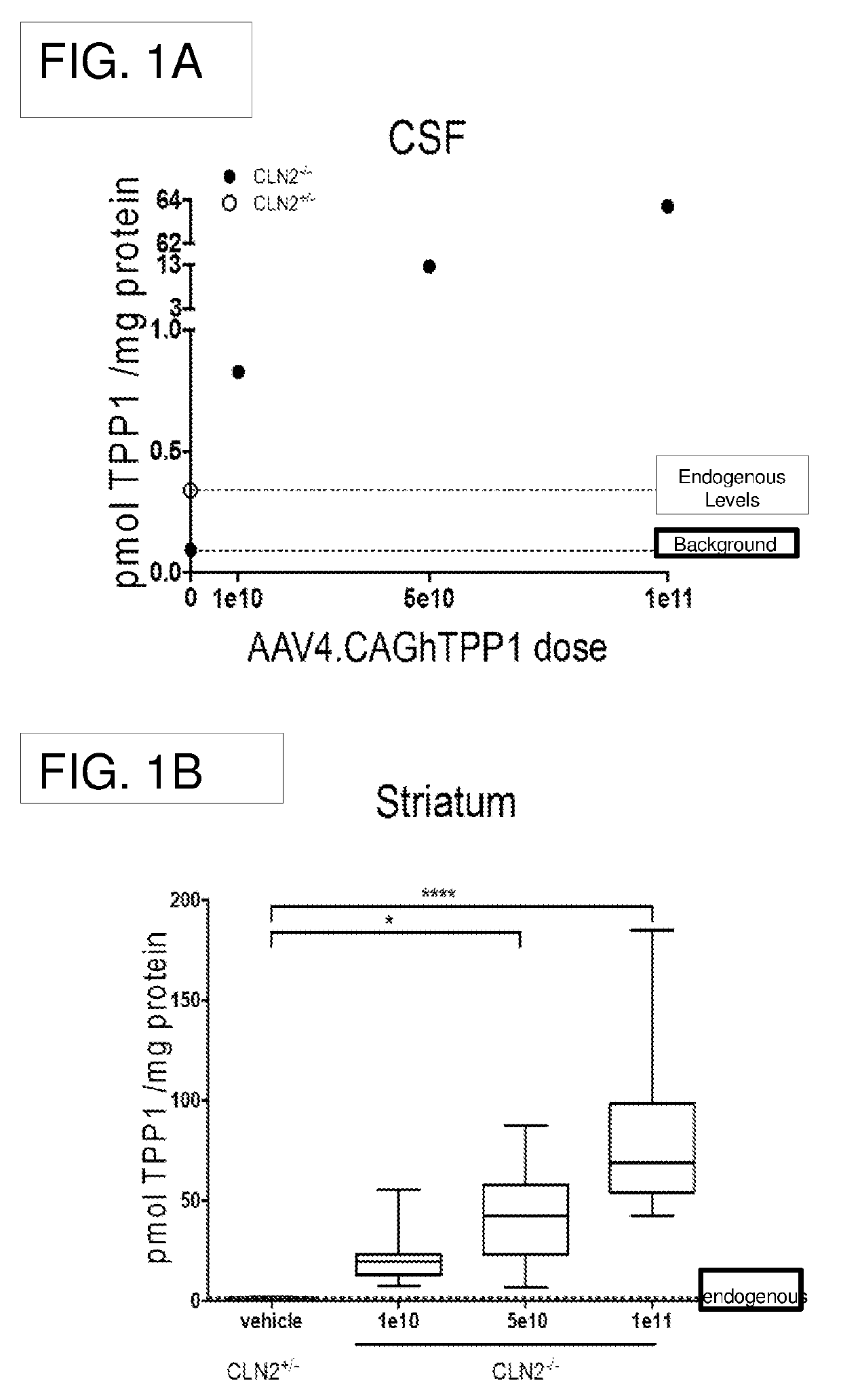
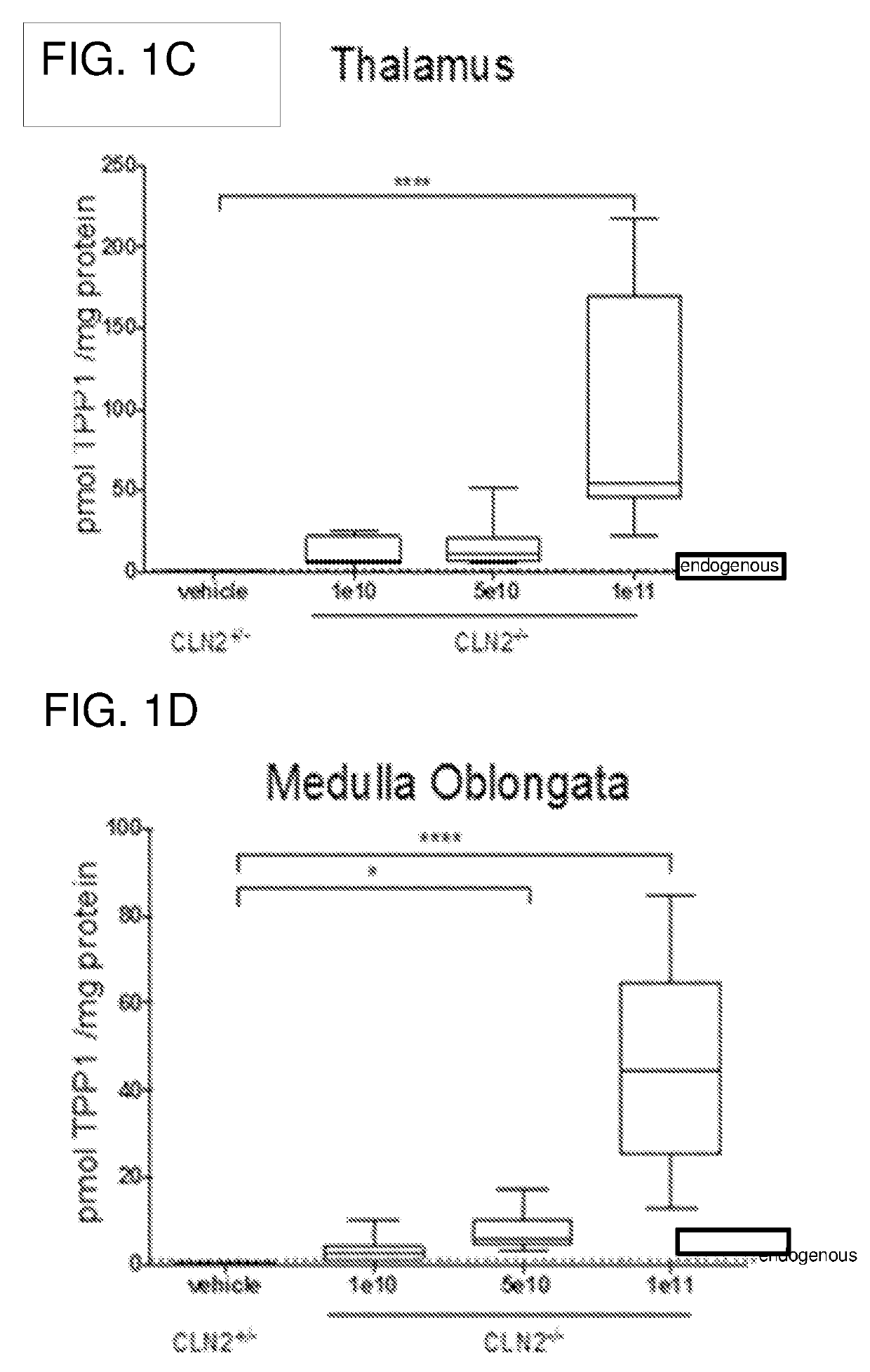
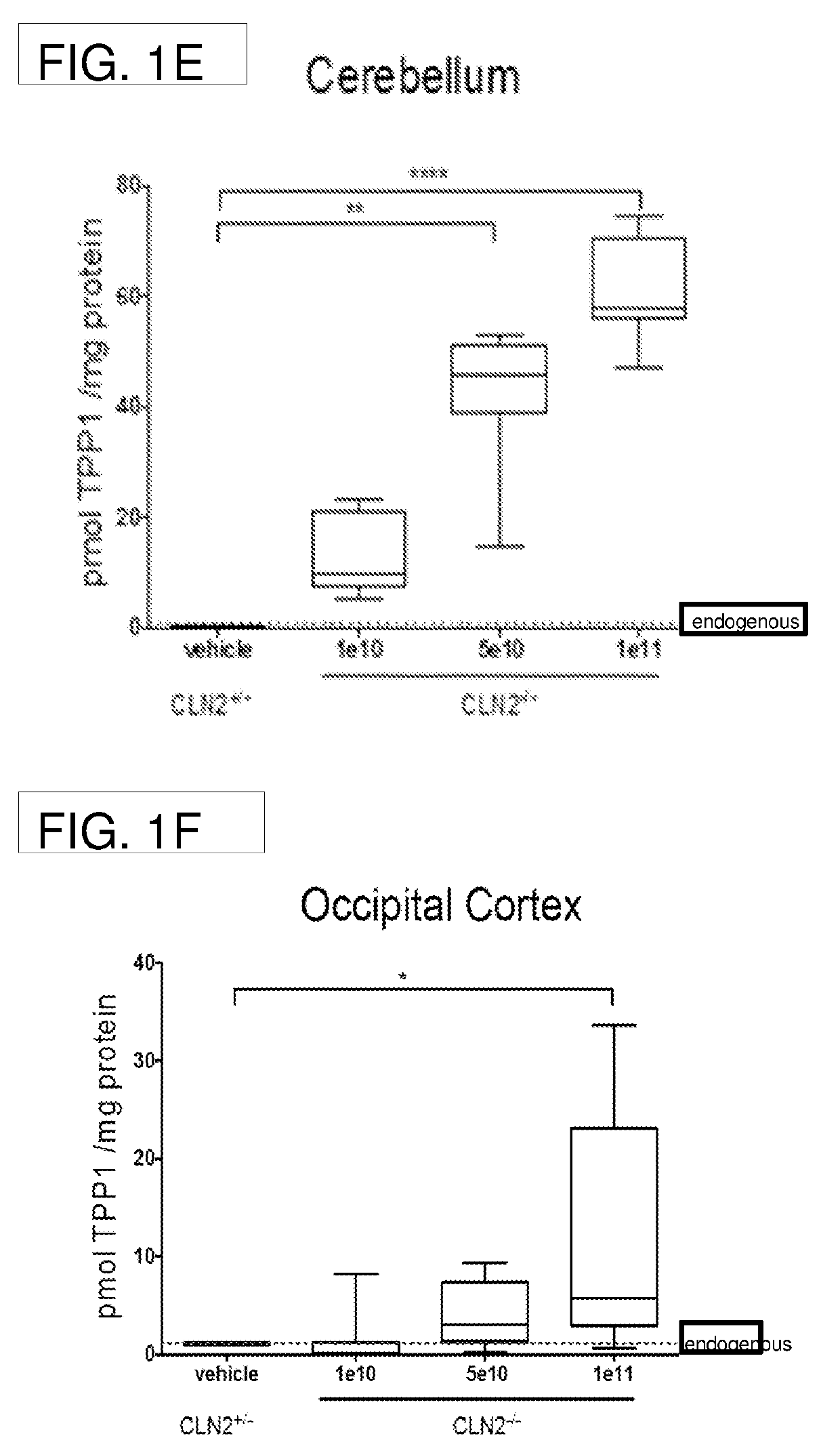
Methods for treating neurodegenerative diseases using gene therapy to delay disease onset and progression while providing cognitive protection
The present disclosure provides methods of treating a lysosomal storage disorder in a mammal which method comprises administering AAV particles encoding a polypeptide directly to the central nervous system of the mammal in conjunction with administering at least two immunosuppressive agents. AAV particles may be delivered by direct injection into the brain, spinal cord, cerebral spinal fluid or a portion thereof.
Owner:UNIV OF IOWA RES FOUND
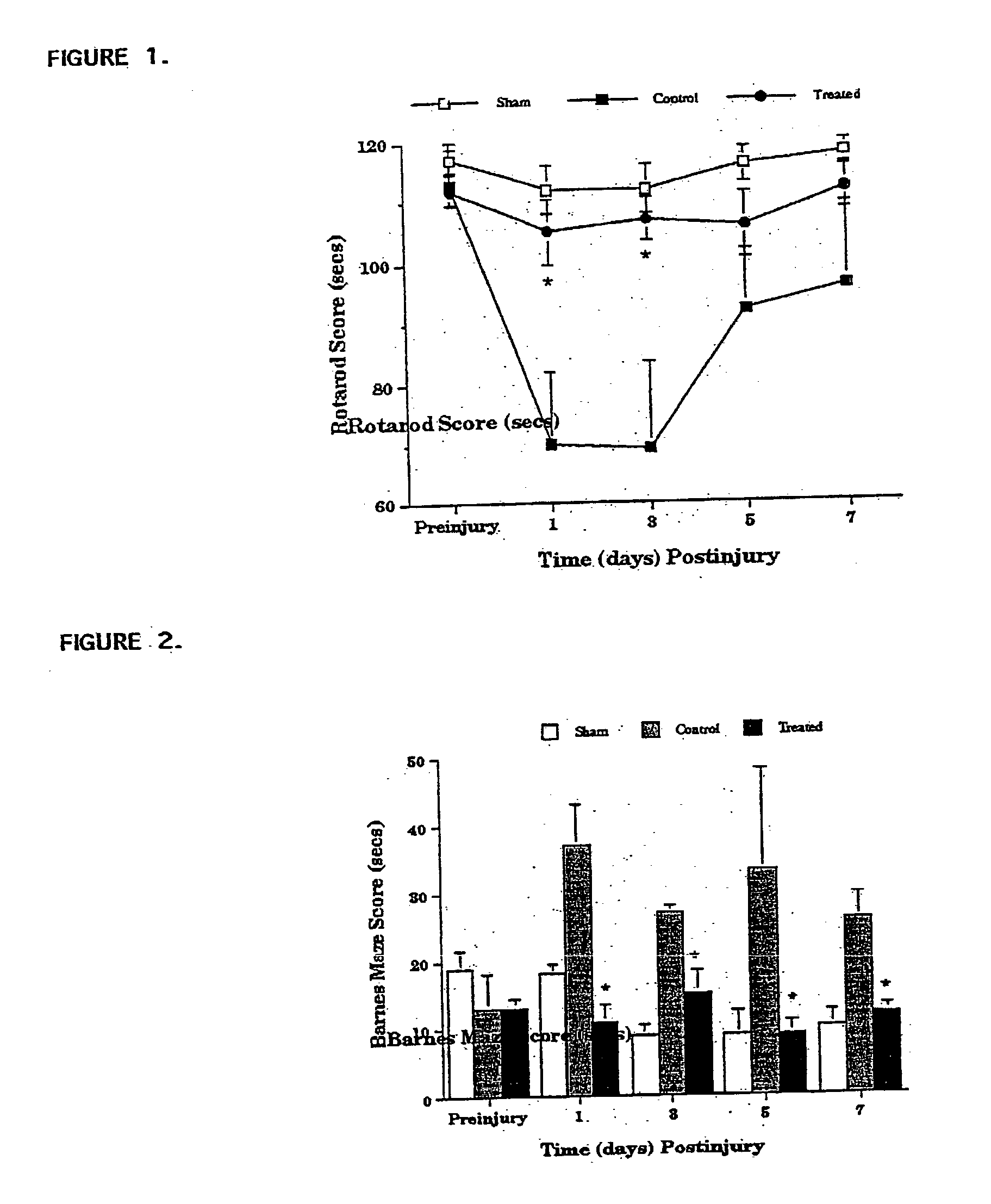
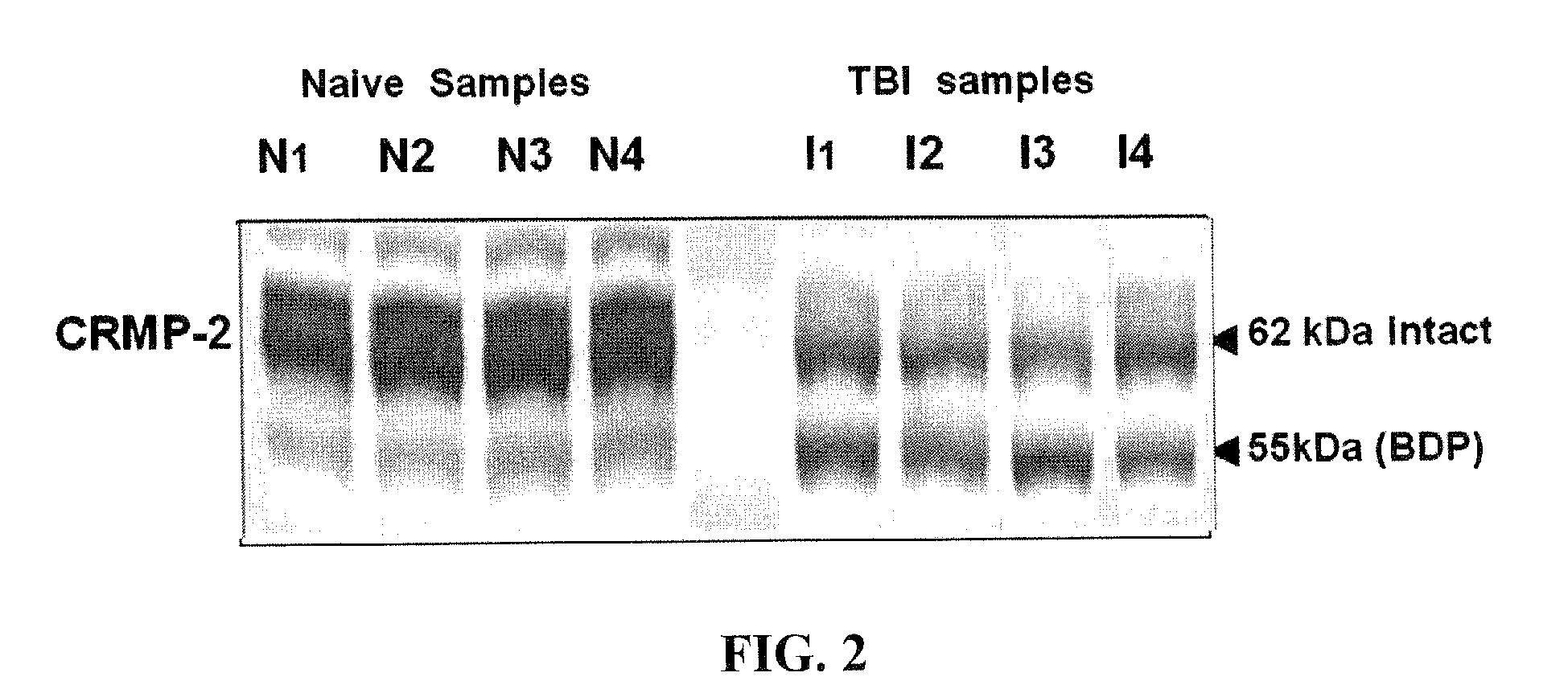
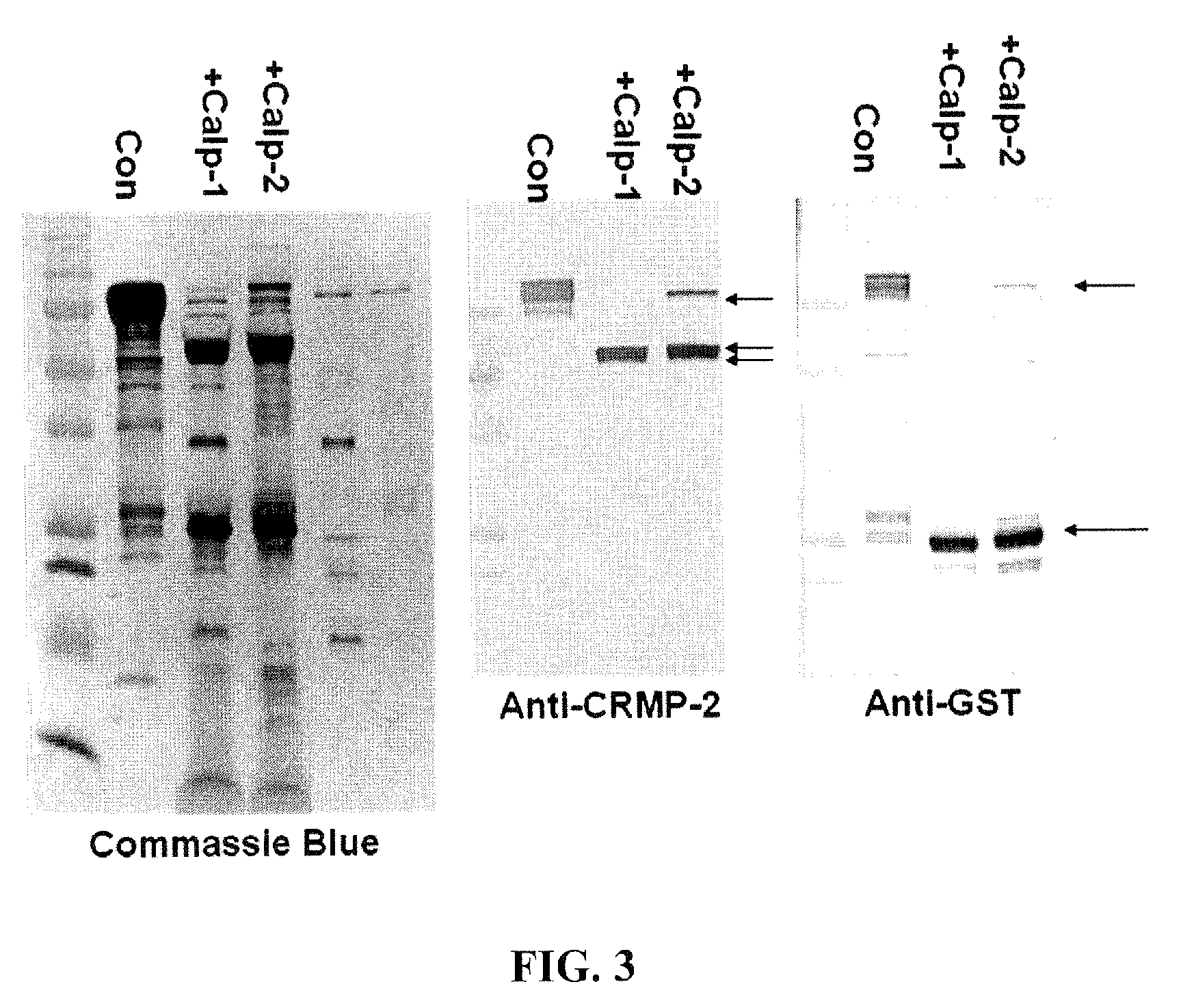
Synaptotagmin and Collapsin Response Mediator Protein as Biomarkers for Traumatic Brain Injury
ActiveUS20100047817A1Information can be usedReduce in quantityDisease diagnosisBiological testingIntact proteinBiomarker (petroleum)
Collapsin response mediator proteins (CRMPs) decreased in tissue and increased in biological fluids after neural injury from traumatic brain injury (TBI). Significant decreases of CRMP1, CRMP2, CRMP4 and CRMP5 were accompanied by the appearance of distinct 58 kDa (CRMP-2) or 55 kDa (CRMP-4) breakdown products from proteolytic cleavage by calpain. Synaptotagmin breakdown products were also associated with TBI and could be detected along with intact protein in human cerebral spinal fluid (CSF). Both biomarkers were detected in human biofluid and related to recovery from traumatic brain injury.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +1
Gene transfer compositions, methods and uses for treating neurodegenerative diseases
Provided are methods of treating a lysosomal storage disorder in a mammal which method includes administering AAV particles encoding a polypeptide to the central nervous system of the mammal. AAV particles may be delivered by direct injection into the brain, spinal cord, cerebral spinal fluid or a portion thereof for expression.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com