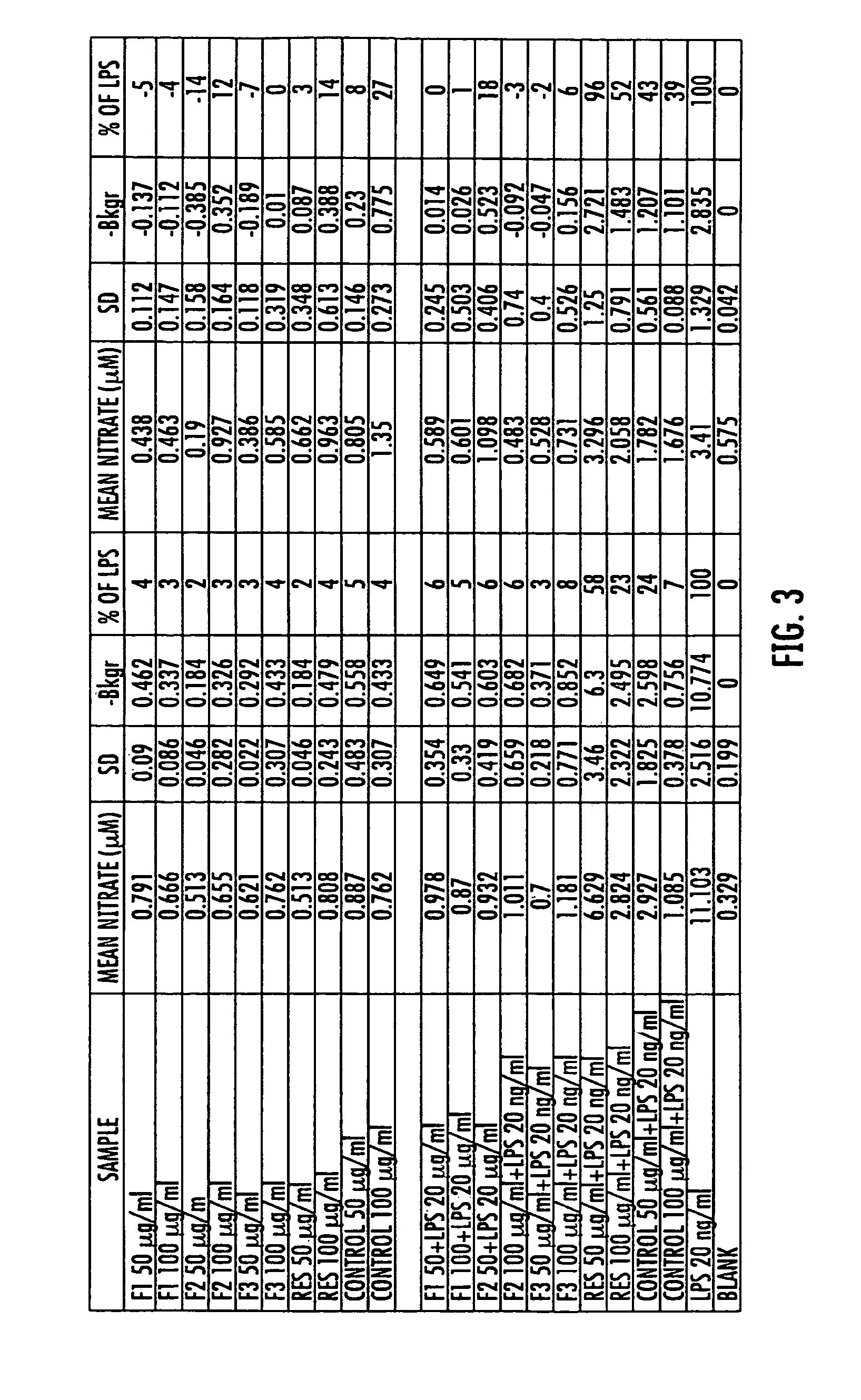
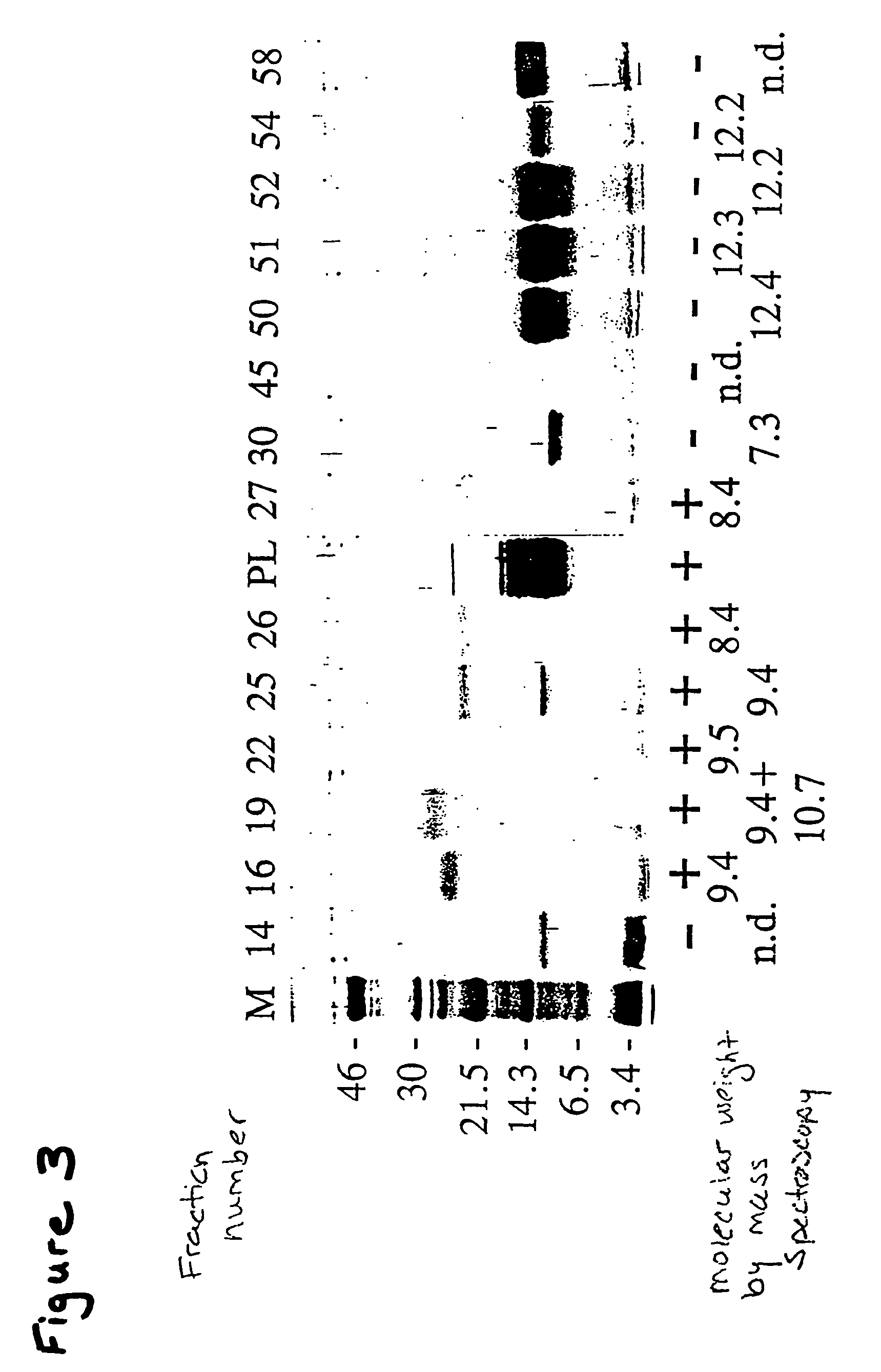
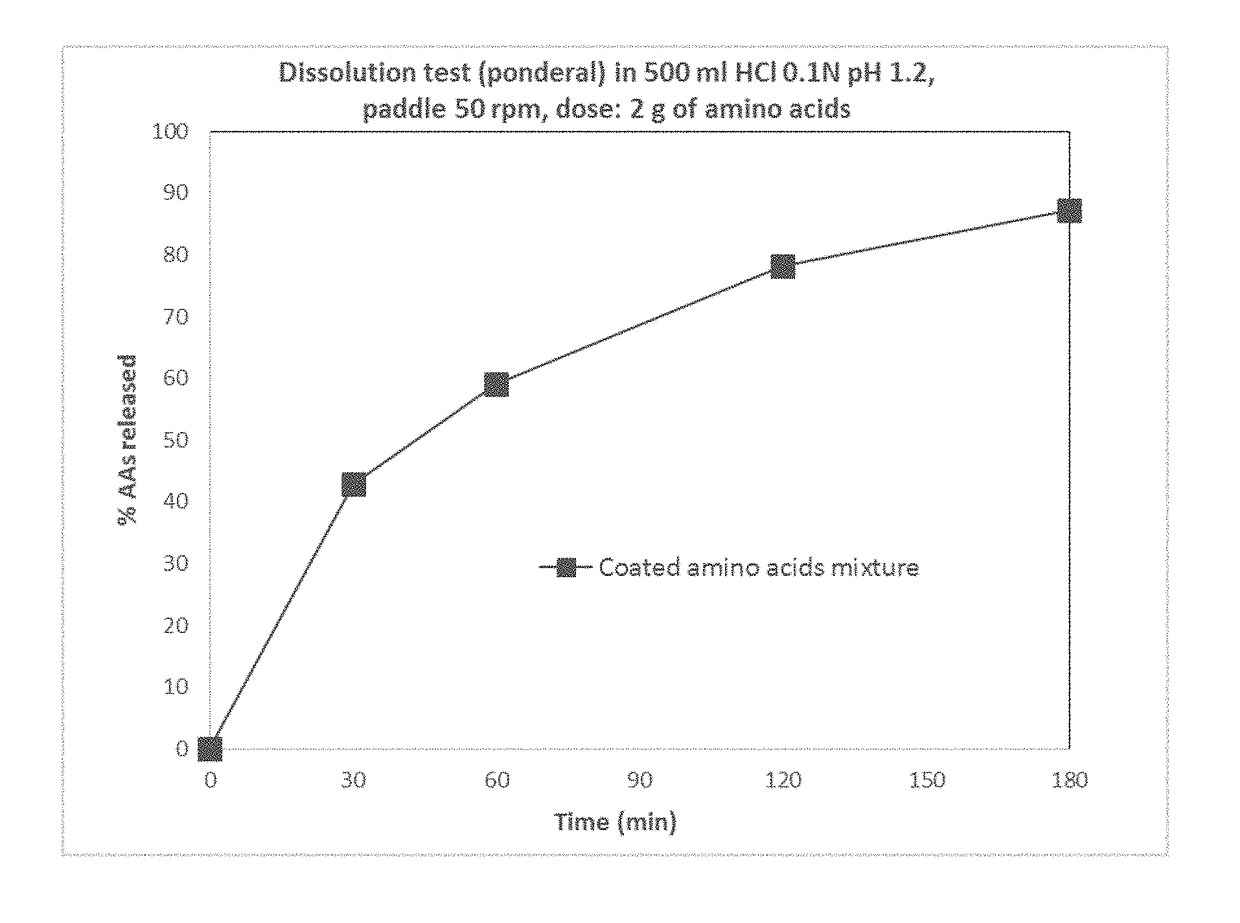
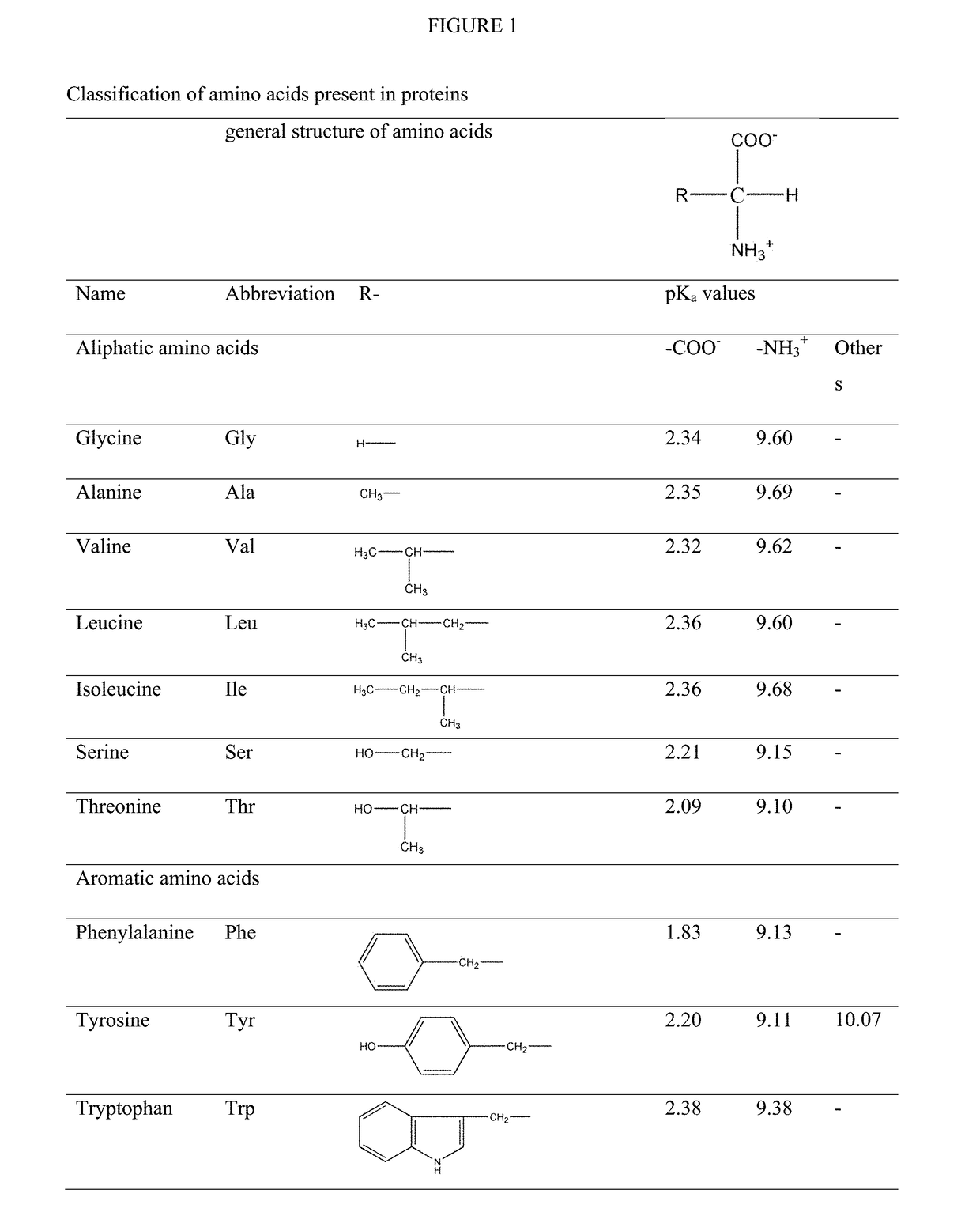
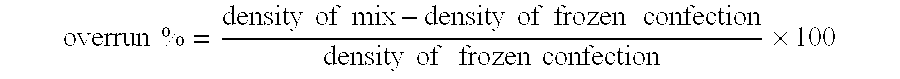Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "More palatable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
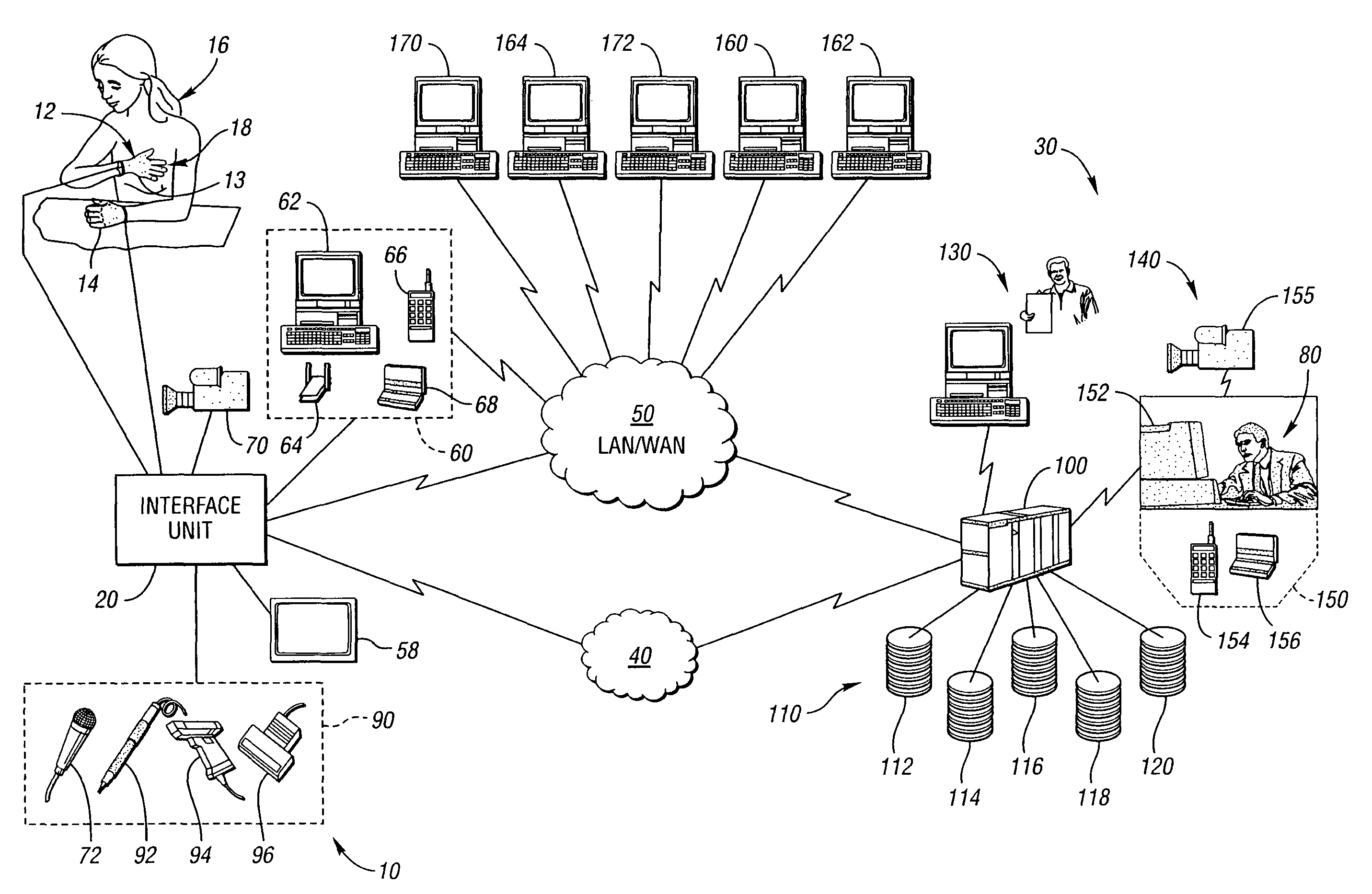
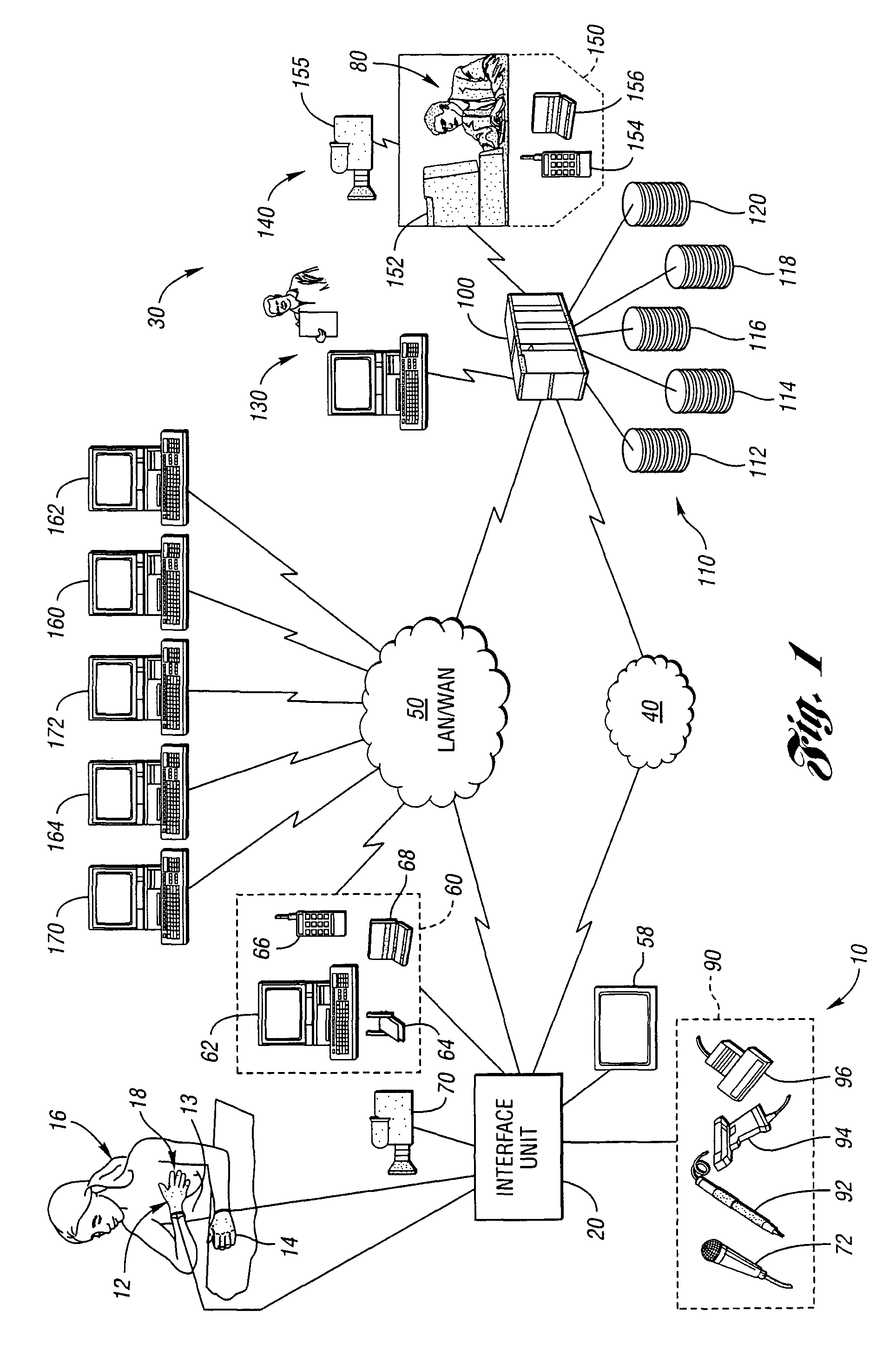
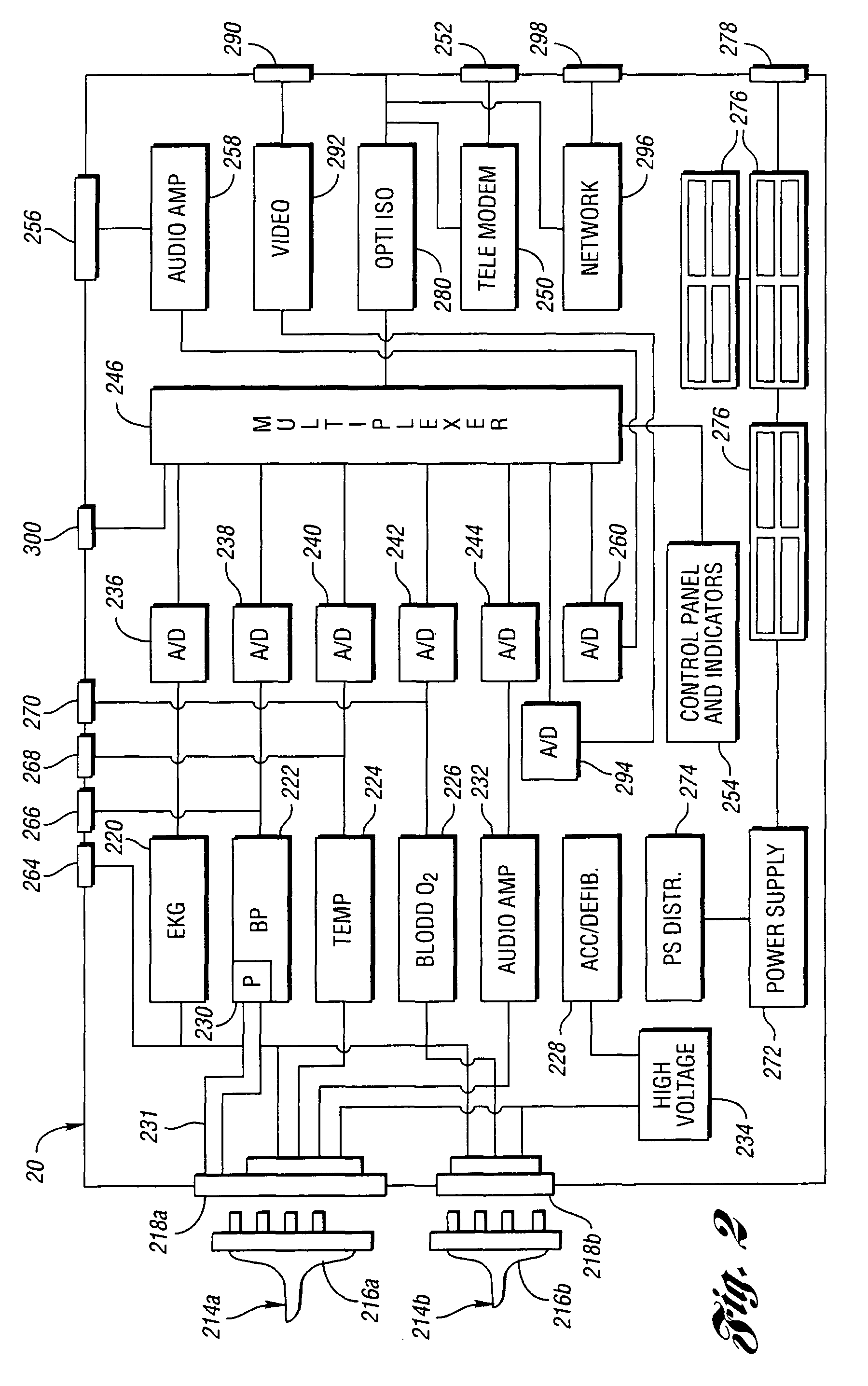
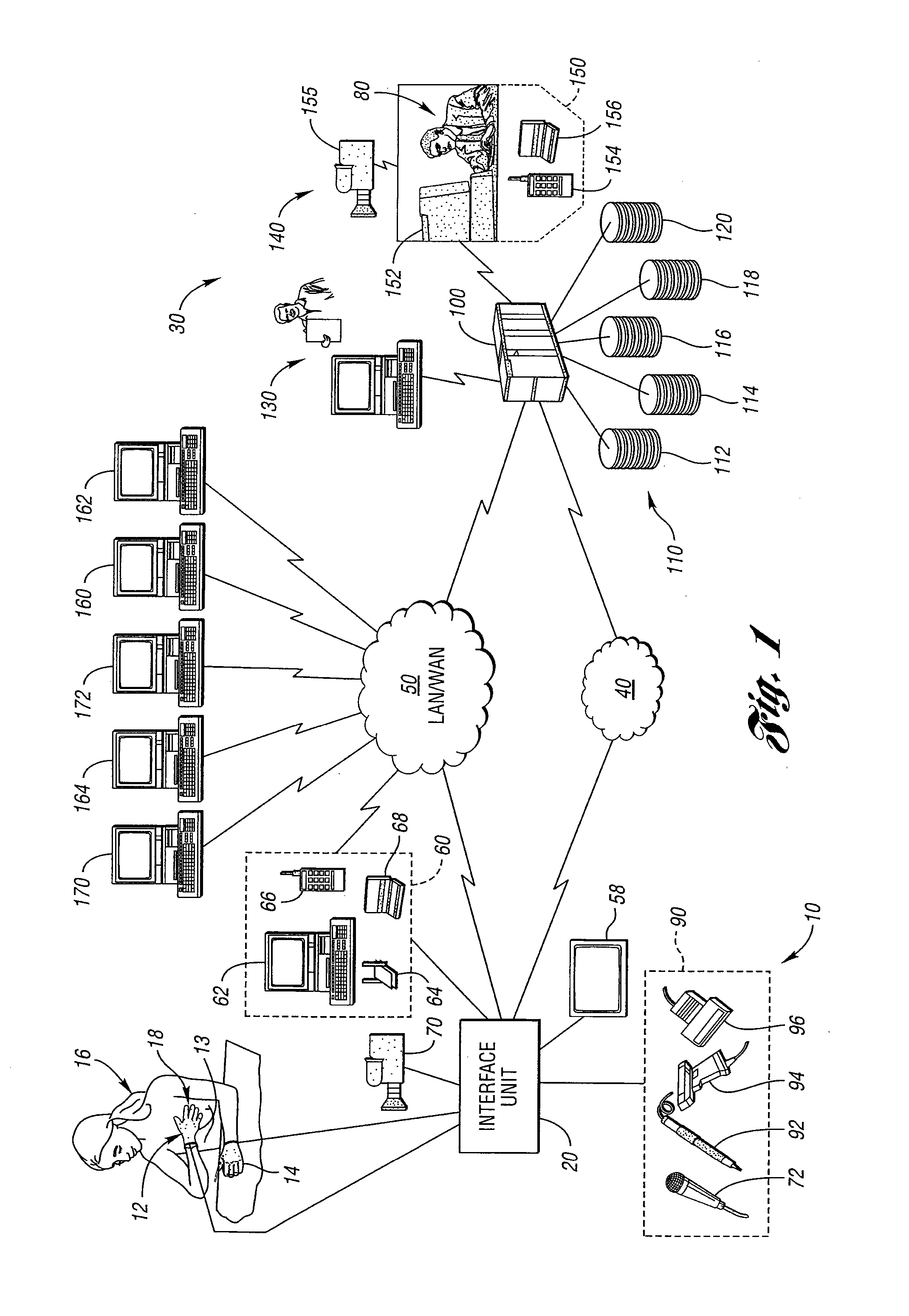
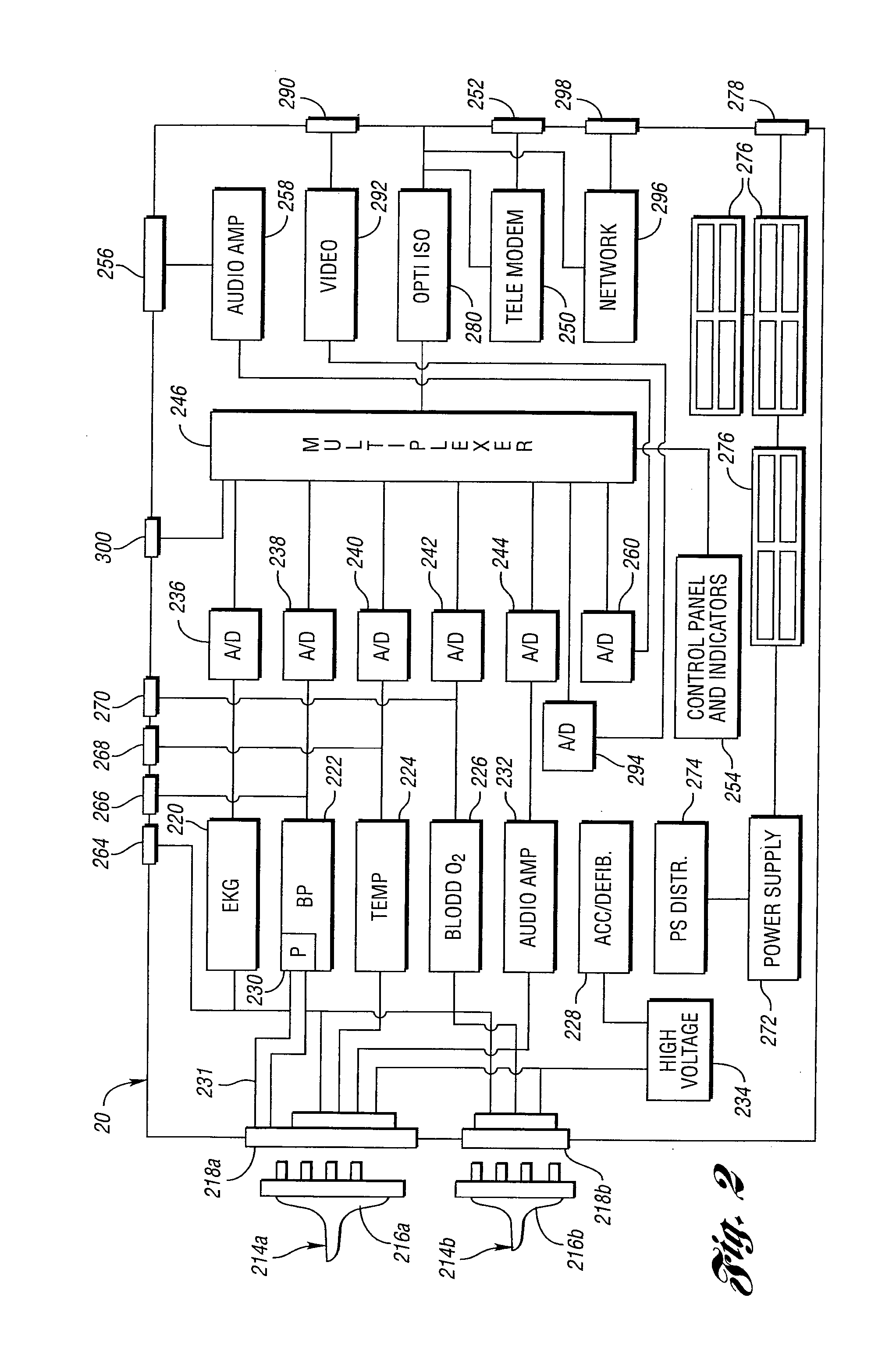
Method for remote medical consultation and care
InactiveUS7860725B2Easy to optimizeReduce paperworkTelevision conference systemsGlovesThe InternetVideo image
A method for remote medical consulting includes collecting diagnostic data using at least one wearable device contoured to at least a portion of a person's hand, transmitting the diagnostic data to a remote location, transmitting audio data and video images of the patient to the remote location, and communicating diagnosis and / or treatment information to the patient based at least in part on the diagnostic data. The treatment information may include a prescription electronically transmitted to the patient or a pharmacy. The method includes billing of the patient via credit or debit card, bank account, or a third party, such as an insurance company. The diagnostic data as well as the audio and video data may be transmitted wirelessly via cellular or satellite communication networks and / or using a wide area computer network such as the internet.
Owner:INEEDMD COM
Method for remote medical consultation and care
InactiveUS20110092825A1Easy to optimizeReduce paperworkTelevision conference systemsCatheterCredit cardWide area
A method for remote medical consulting includes collecting diagnostic data using at least one wearable device contoured to at least a portion of a person's hand, transmitting the diagnostic data to a remote location, transmitting audio data and video images of the patient to the remote location, and communicating diagnosis and / or treatment information to the patient based at least in part on the diagnostic data. The treatment information may include a prescription electronically transmitted to the patient or a pharmacy. The method includes billing of the patient via credit or debit card, bank account, or a third party, such as an insurance company. The diagnostic data as well as the audio and video data may be transmitted wirelessly via cellular or satellite communication networks and / or using a wide area computer network such as the internet.
Owner:INEEDMD COM
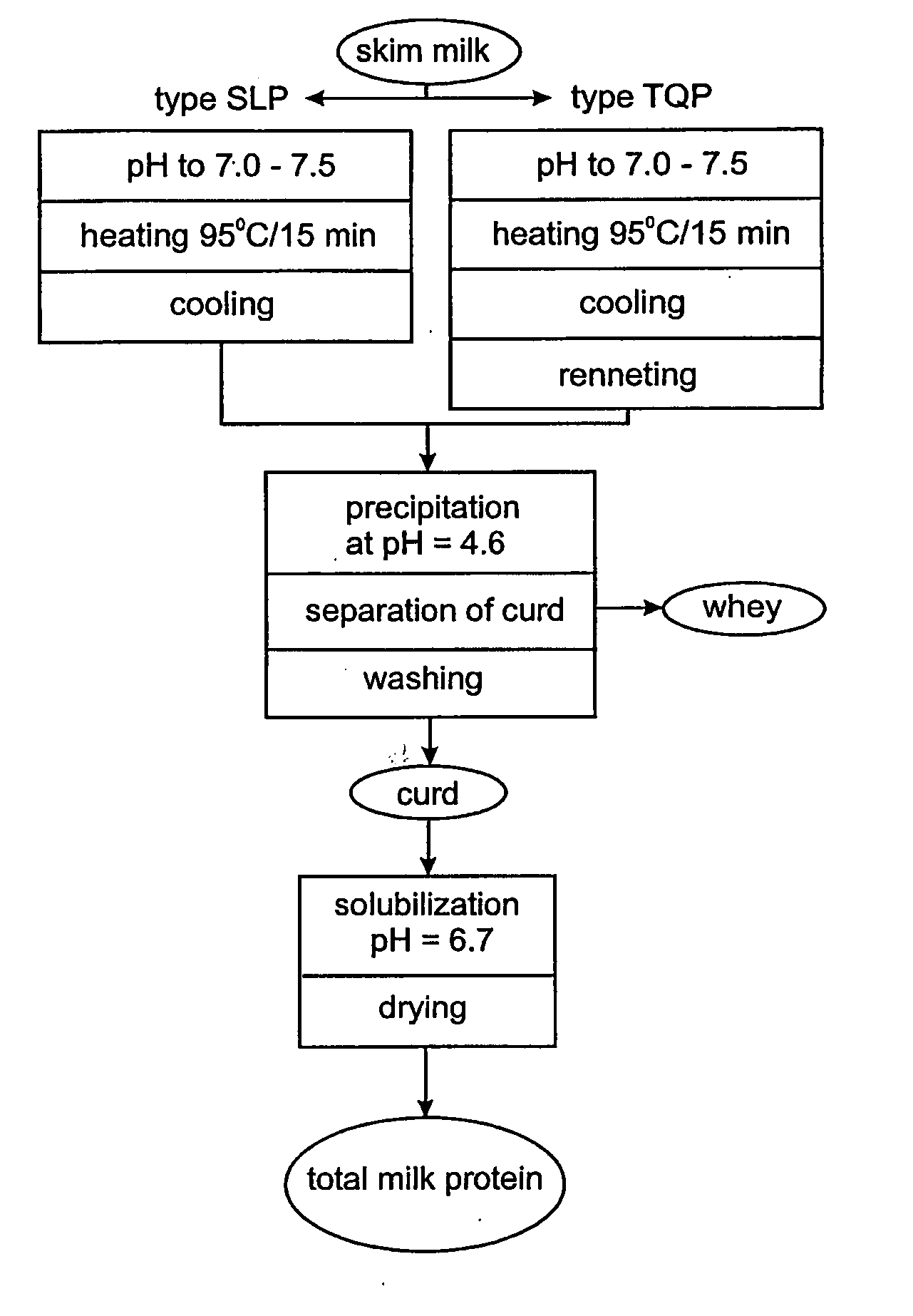
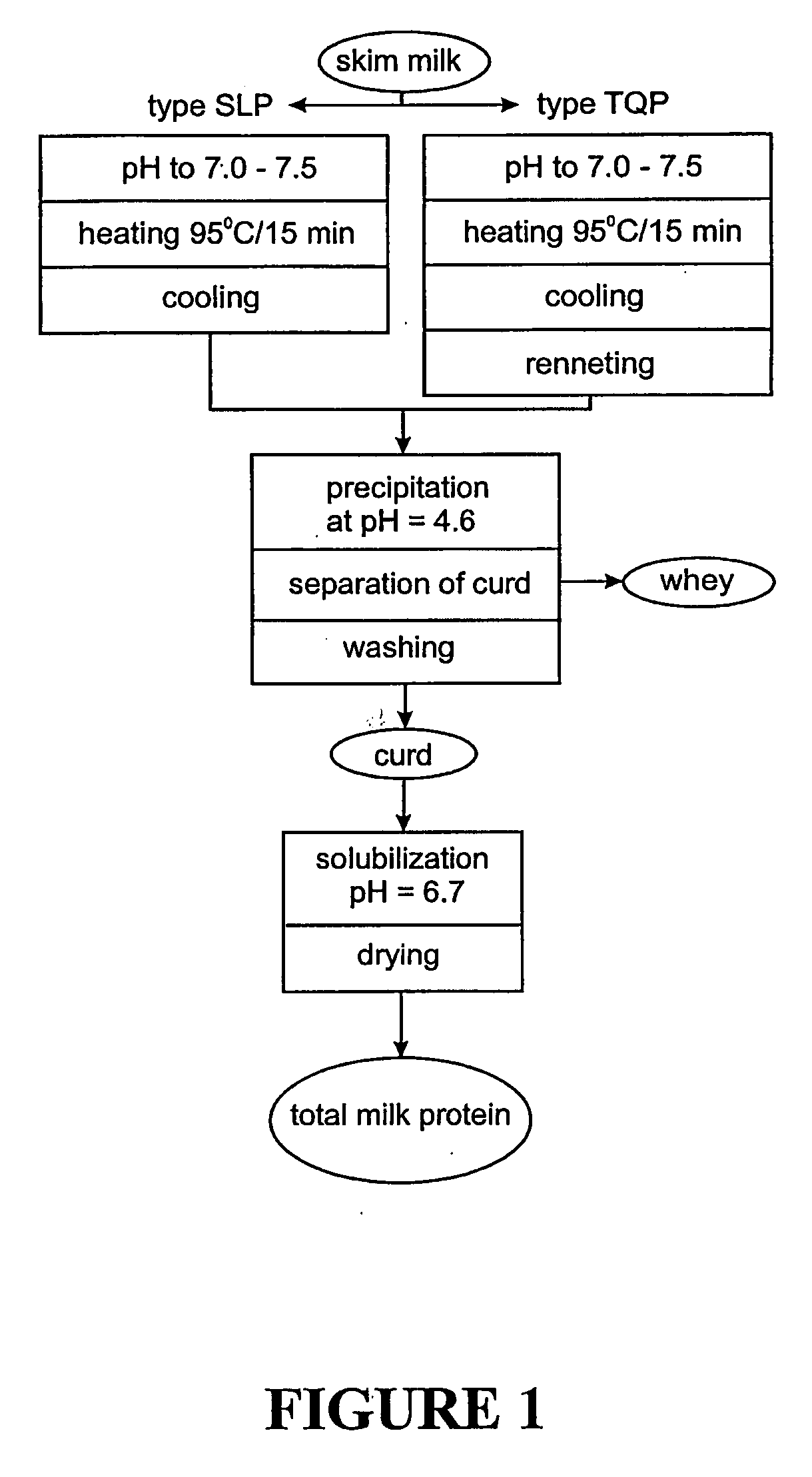
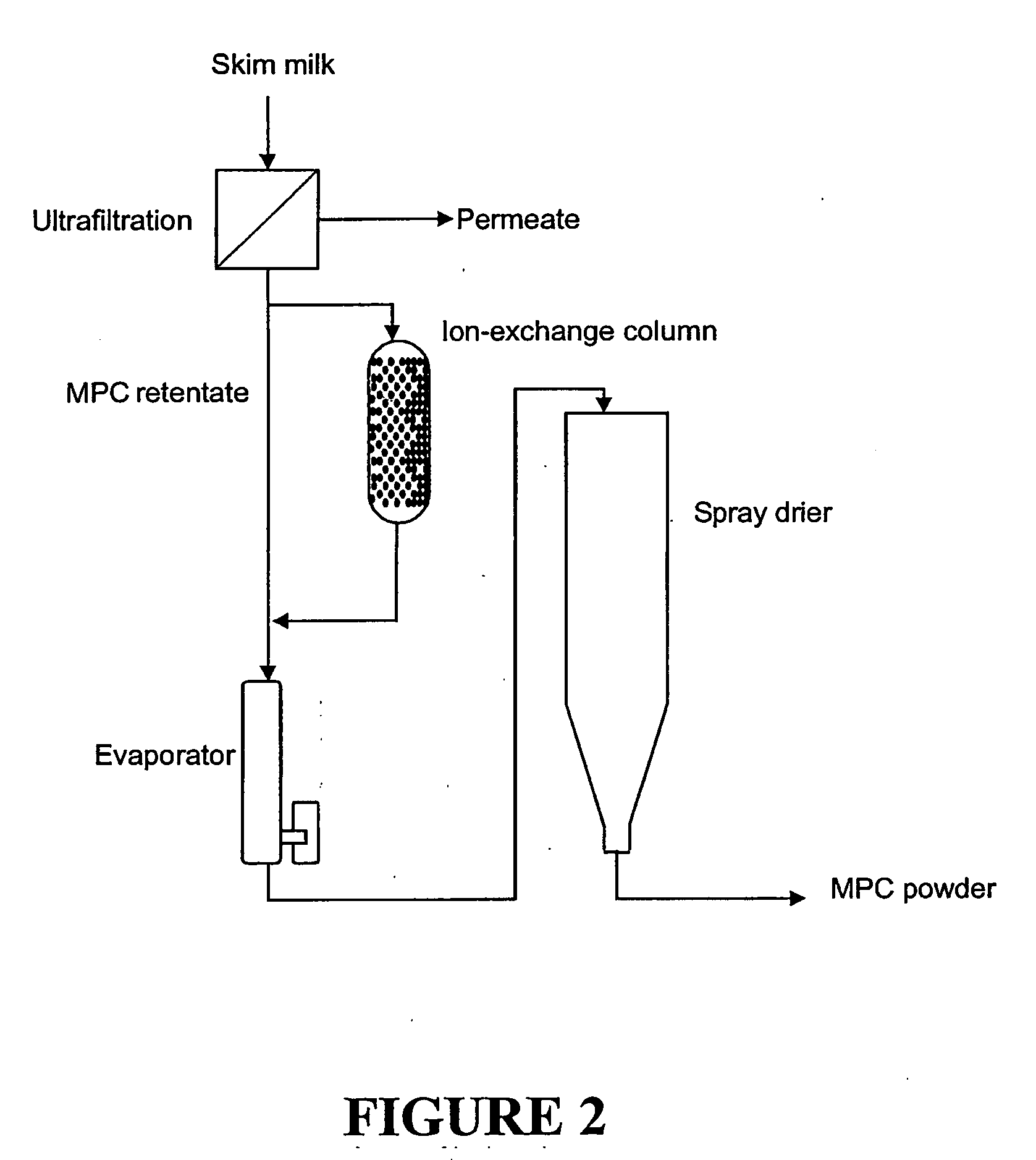
Milk protein products and processes
InactiveUS7157108B2Improve solubilityReduced nutritional valueProtein composition from fishMilk preparationCalcium depletionsCow milk
The invention relates to dried milk protein concentrates and their use. In particular the invention relates to such dried concentrates which have been calcium-depleted to an extent which allows improvements in the use of dried milk protein concentrates in cheese manufacture. The invention includes a method of cheese manufacture comprising: (a) dispersing in milk a dried MPC or MPI having at least 70% dry matter as milk protein; (b) treating the resulting mixture with one or more coagulating enzymes to produce a curd; and (c) processing the curd to make cheese; wherein the dried MPC or MPI is a calcium-depleted MPCF or MPI and the extend of calcium depletion is sufficient to allow manufacture of substantially nugget-free cheese.
Owner:FONTERRA COOP GRP LTD
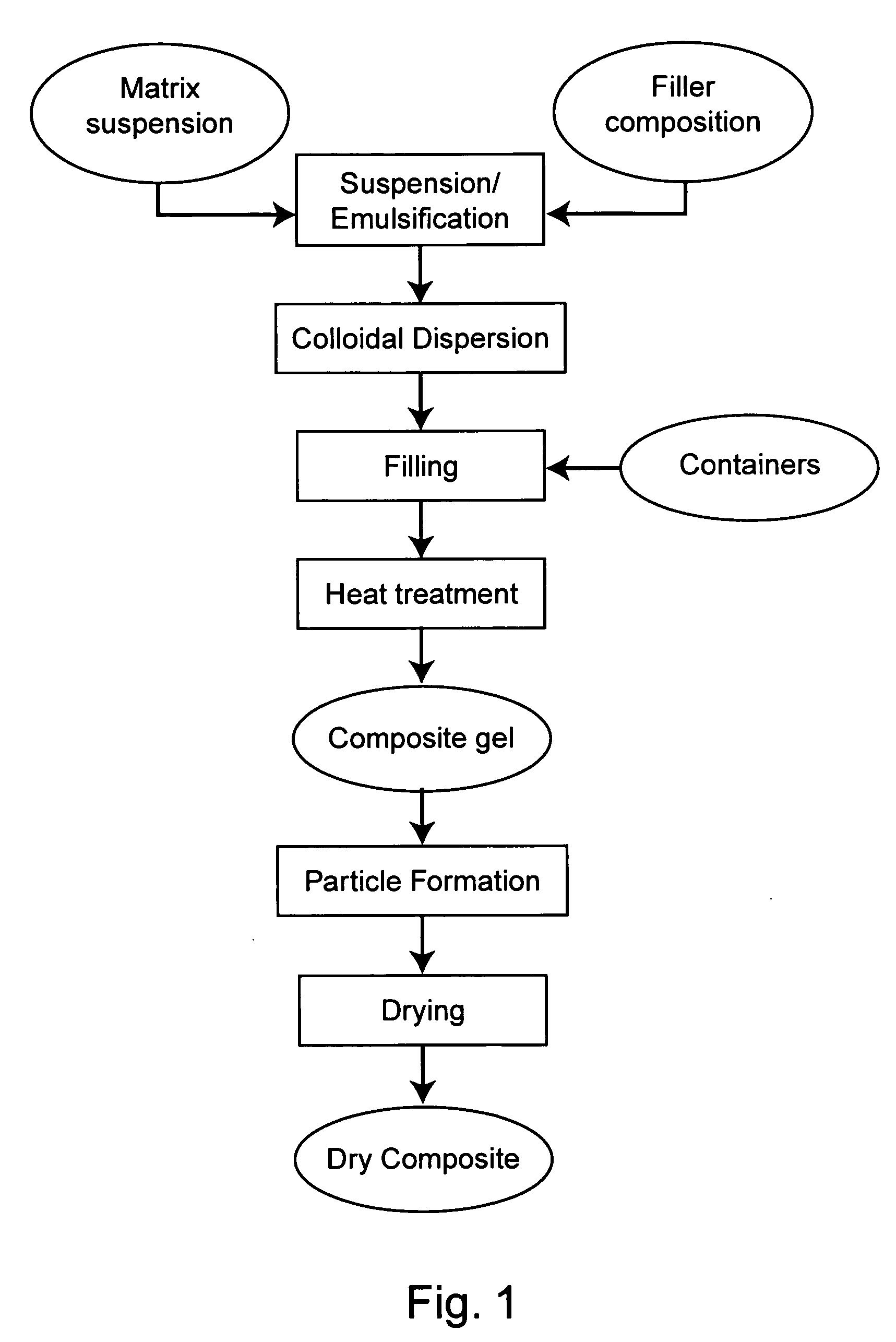
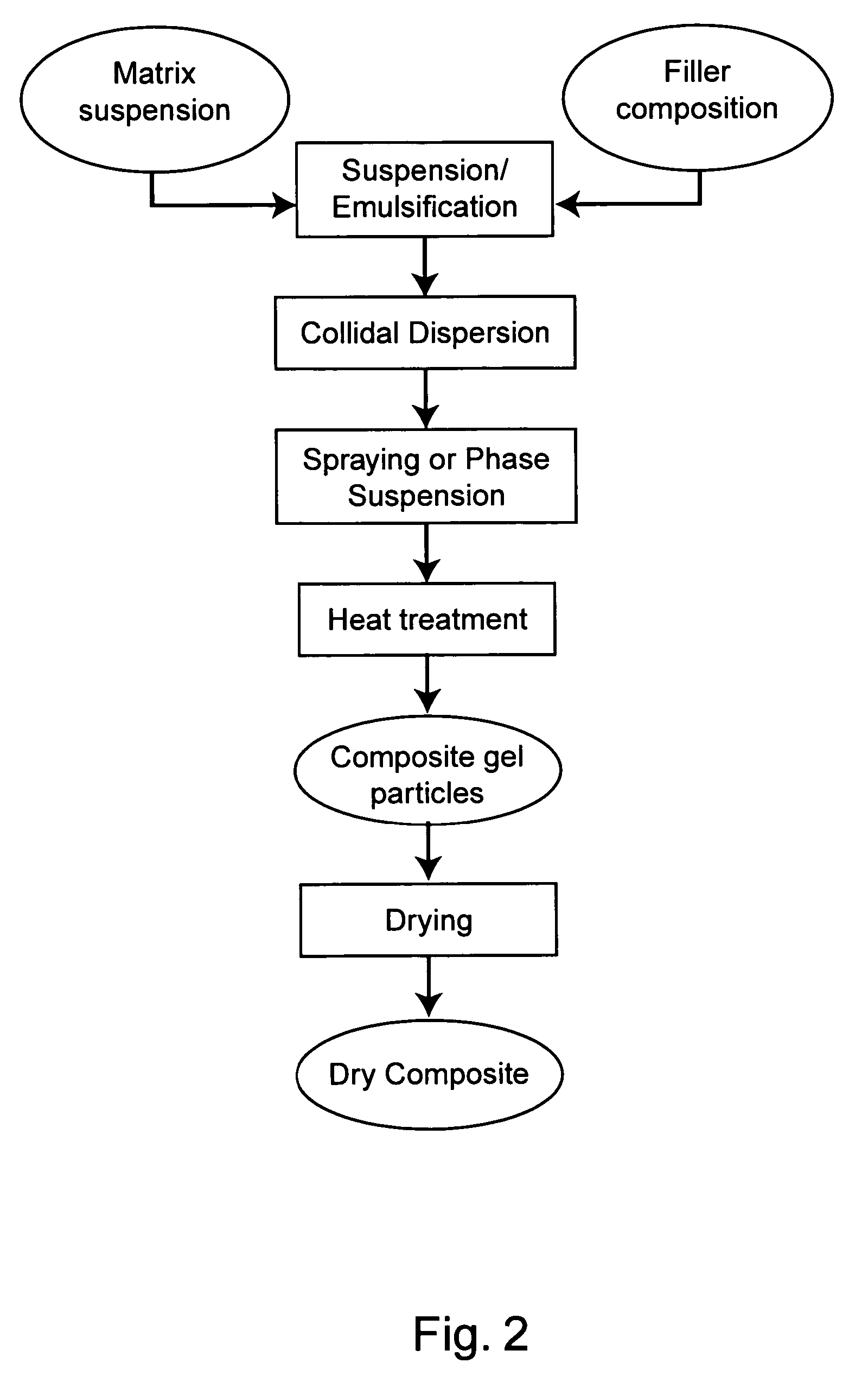
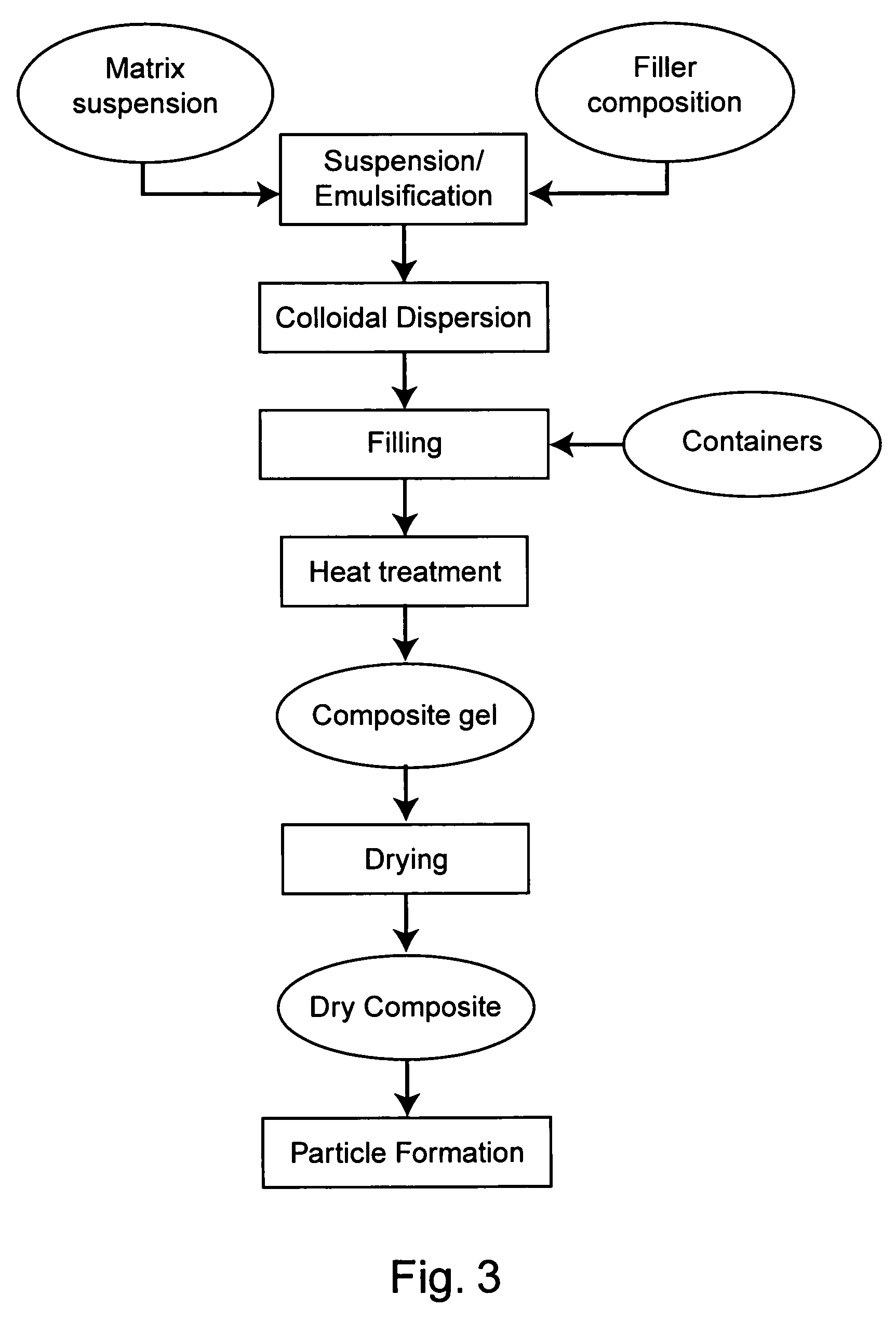
Protected dry composites
InactiveUS20050186305A1Reduce shrinkageReduce migrationAnimal feeding stuffAccessory food factorsBiologyAmino acid
This invention provides dry composites to, e.g., efficiently deliver unmodified amino acids, lipids, and / or feed supplements through the upper digestive tract of an animal. The invention also provides methods and systems to make and use protected dry composites.
Owner:RGT UNIV OF CALIFORNIA
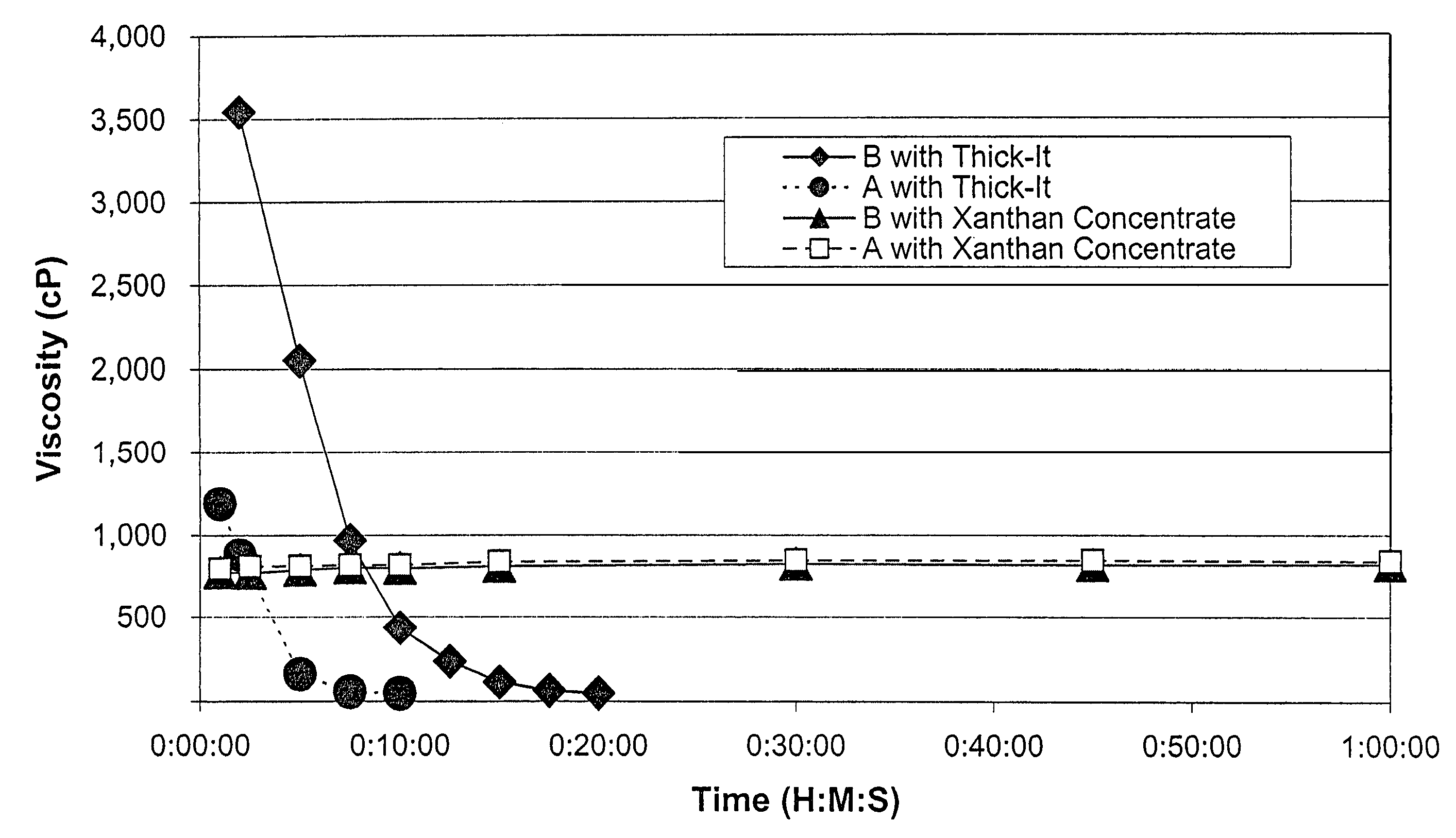
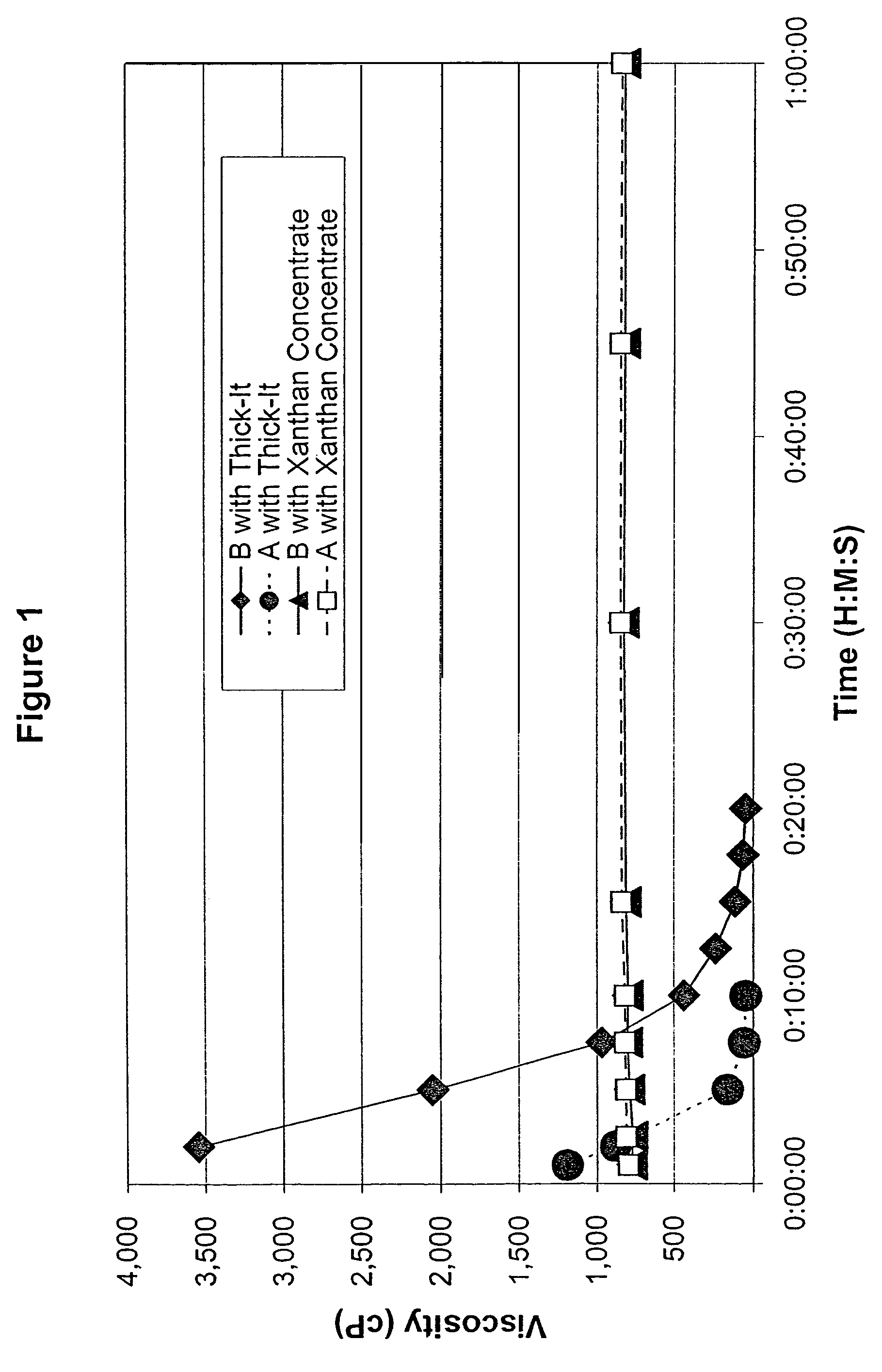
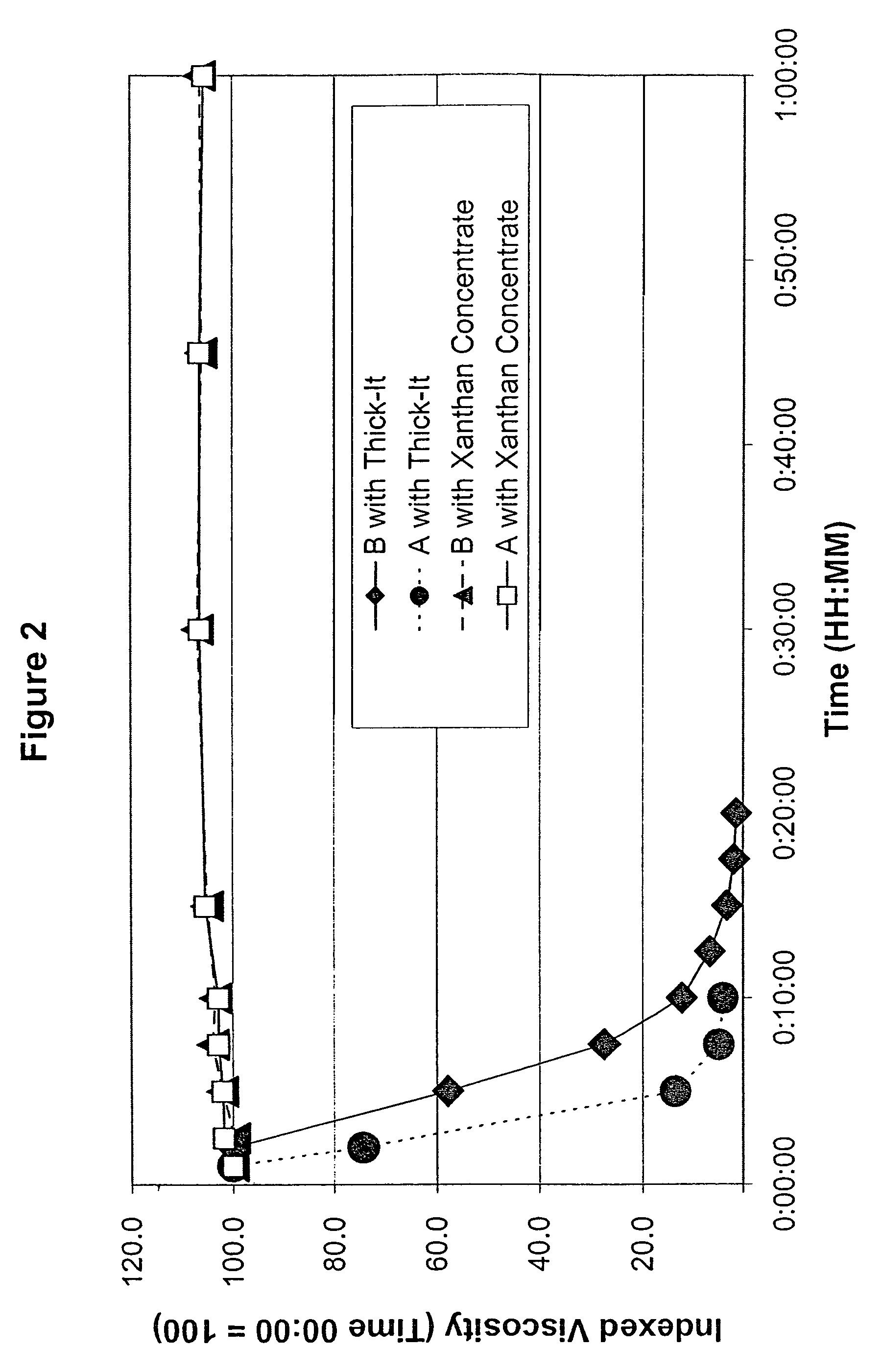
Process for preparing concentrate thickener compositions
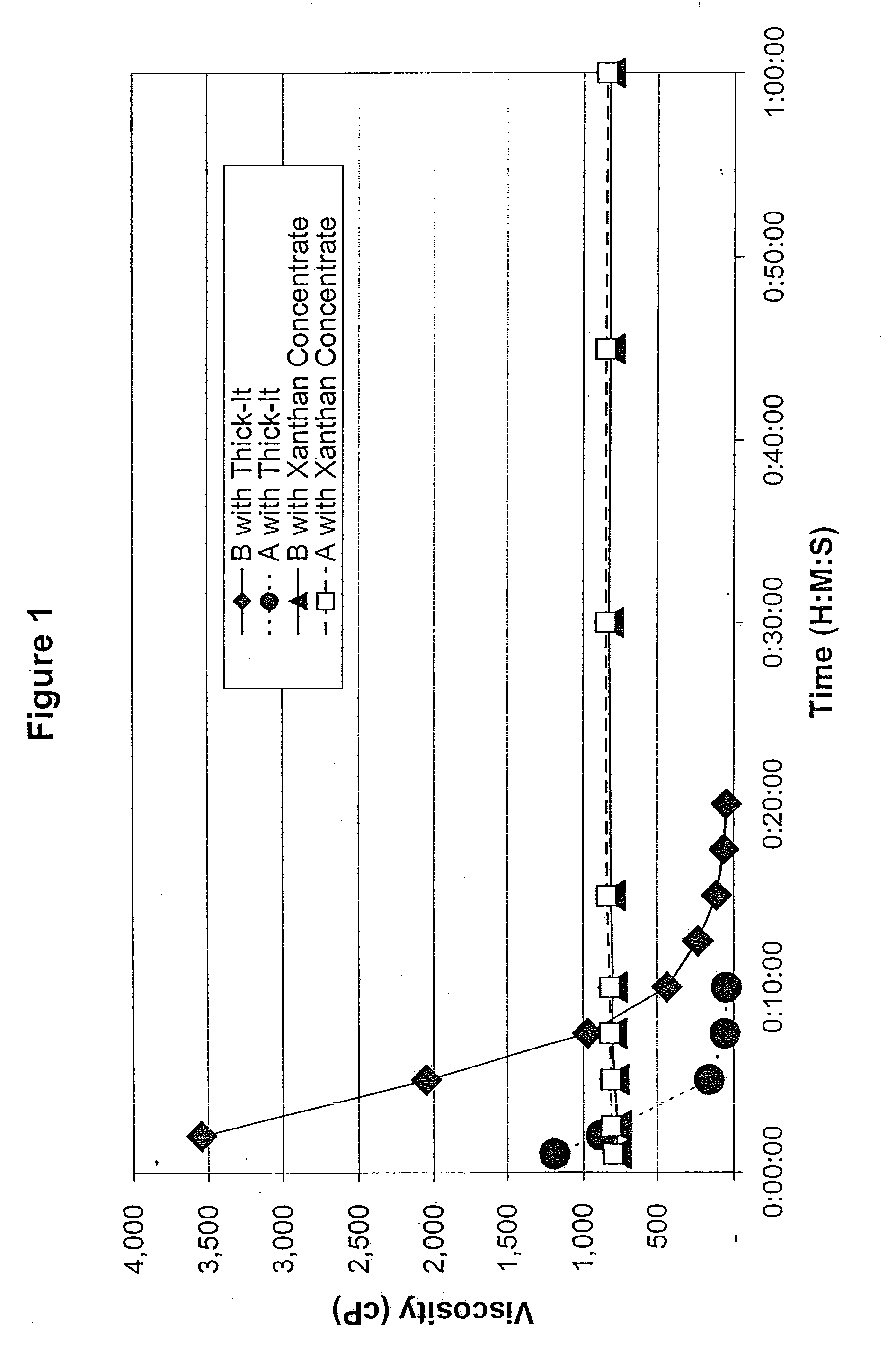
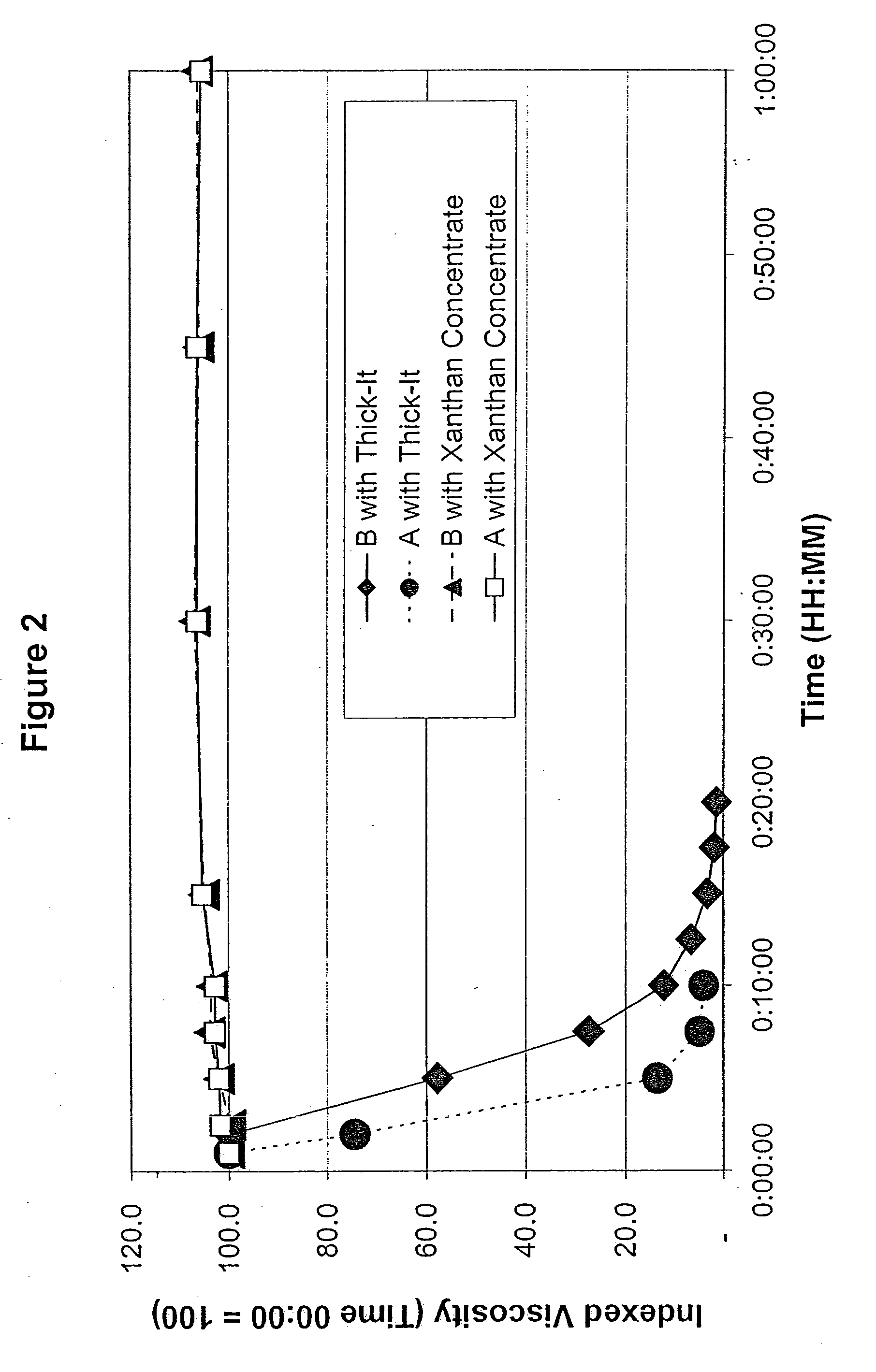
ActiveUS7638150B2Good dispersionMore palatableMilk preparationAnimal feeding stuffMedicineRadiologic Evaluation
Owner:SIMPLY THICK
Compositions methods for improving cardio vascular function
The present invention relates to compositions for supplementing the diet of subjects suffering from cardiovascular or peripheral vascular disease or those at risk for such conditions. Ribose is given alone or in combination with one or a combination of vasodilators, nutrients and vitamins. Preferred vitamins include Vitamins C, B6, B12 and folic acid. Preferred nutrients include glutamine and glucose.
Owner:BIOENERGY INC
Implement for speech therapy
A method and implement for use in speech therapy to assist with teaching a patient to correctly produce sounds, especially / r / sounds, designed to be used in a human mouth to support tongue tip and made of a palatable, non-toxic substance such as wax or candy.
Owner:HALL TRACY
Use of highly concentrated formulations of 4-phenylbutyrate for treatment of certain disorders
InactiveUS20080171792A1Improve complianceIncrease doseBiocideSurgical drugsTherapeutic treatmentSpinal muscular atrophies
A highly concentrated preparation of sodium 4-phenylbutyrate in an aqueous medium as an alternative for present high dosage therapeutic treatments of certain disorders is provided, specifically for the treatment of spinal muscular atrophy (SMA), central nervous system (CNS) cancer, myelodysplastic syndrome (MS), acute leukemia, glioblastoma multiforme, amyotrophic lateral sclerosis (ALS), and colon cancer.
Owner:NAVINTA +1
Systems and methods for providing products to animals
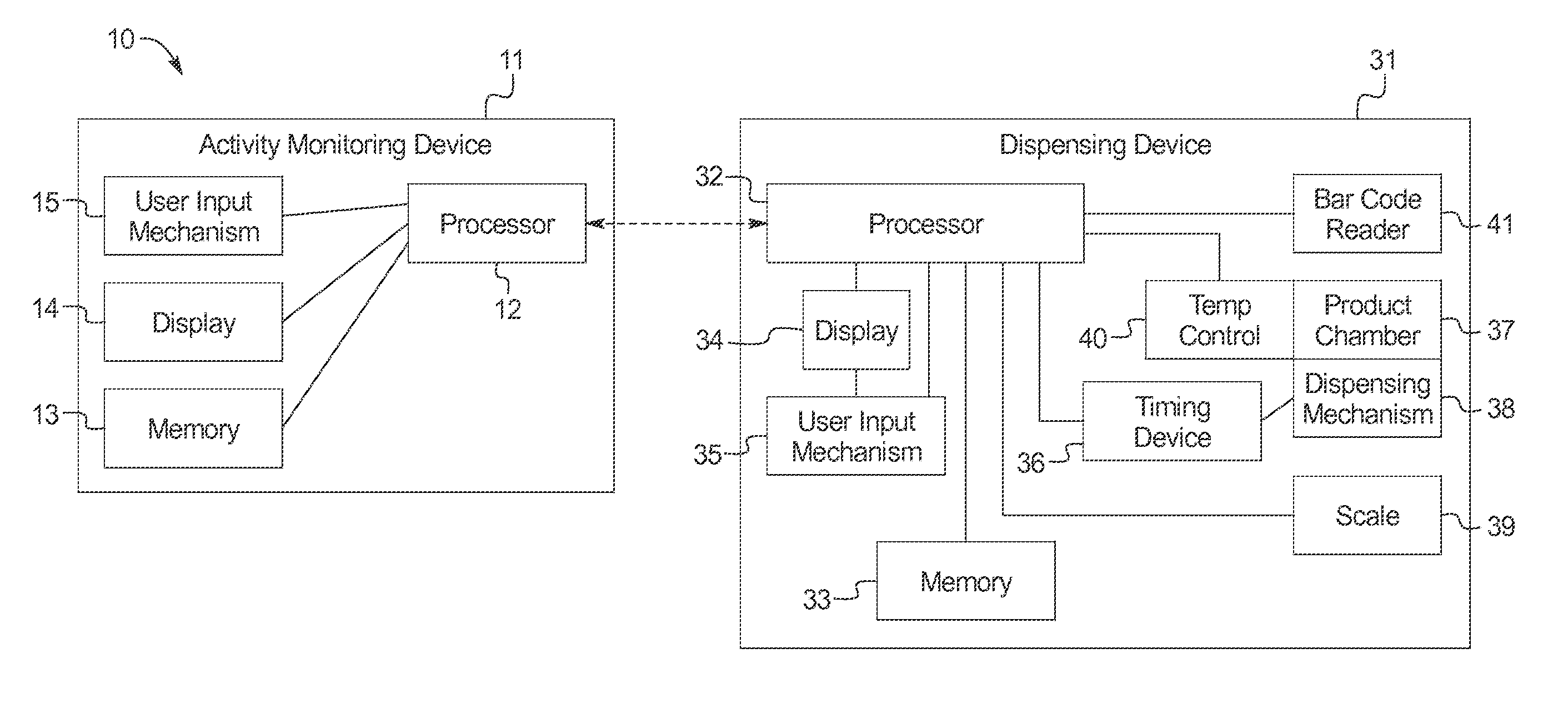
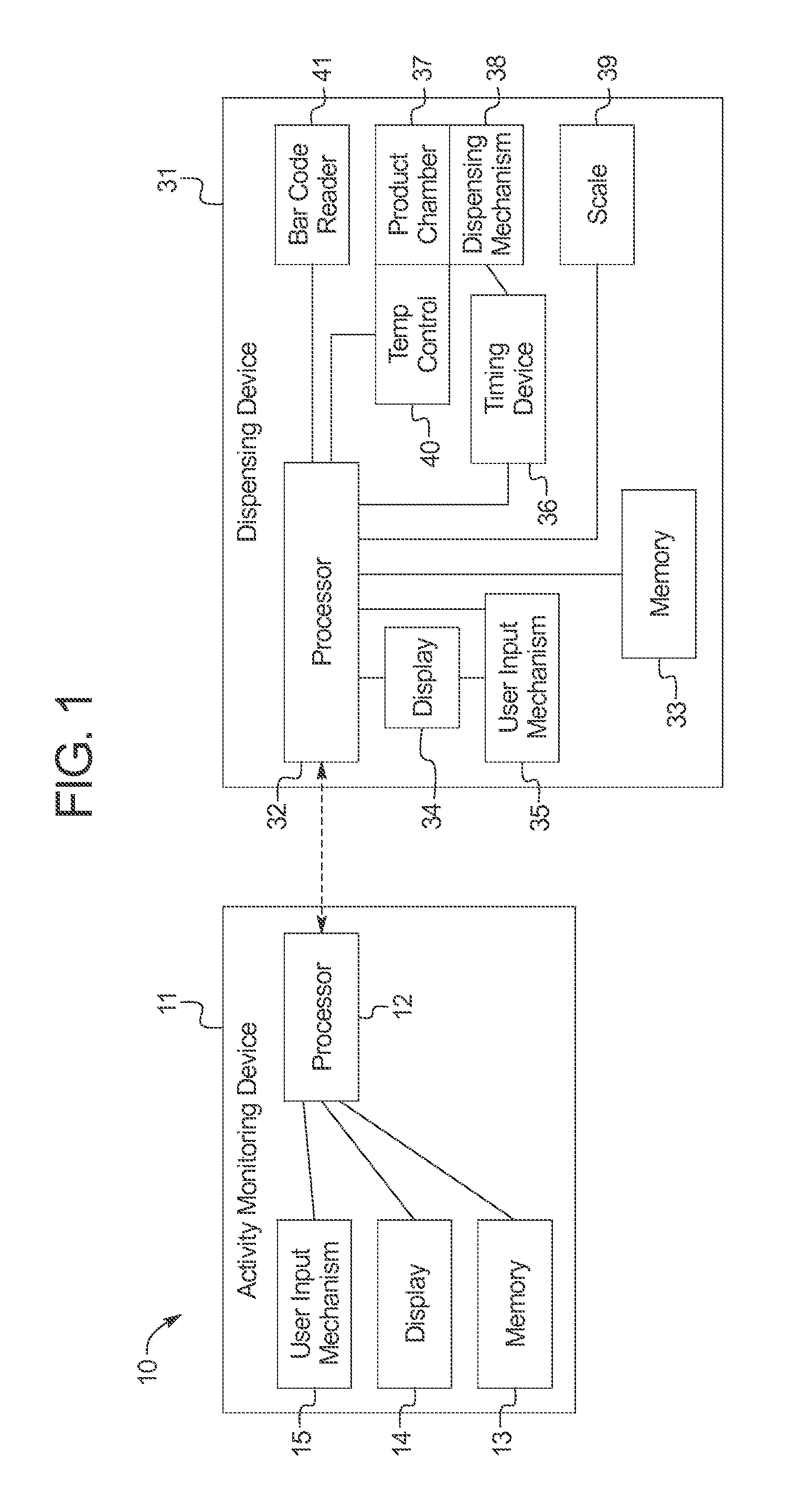
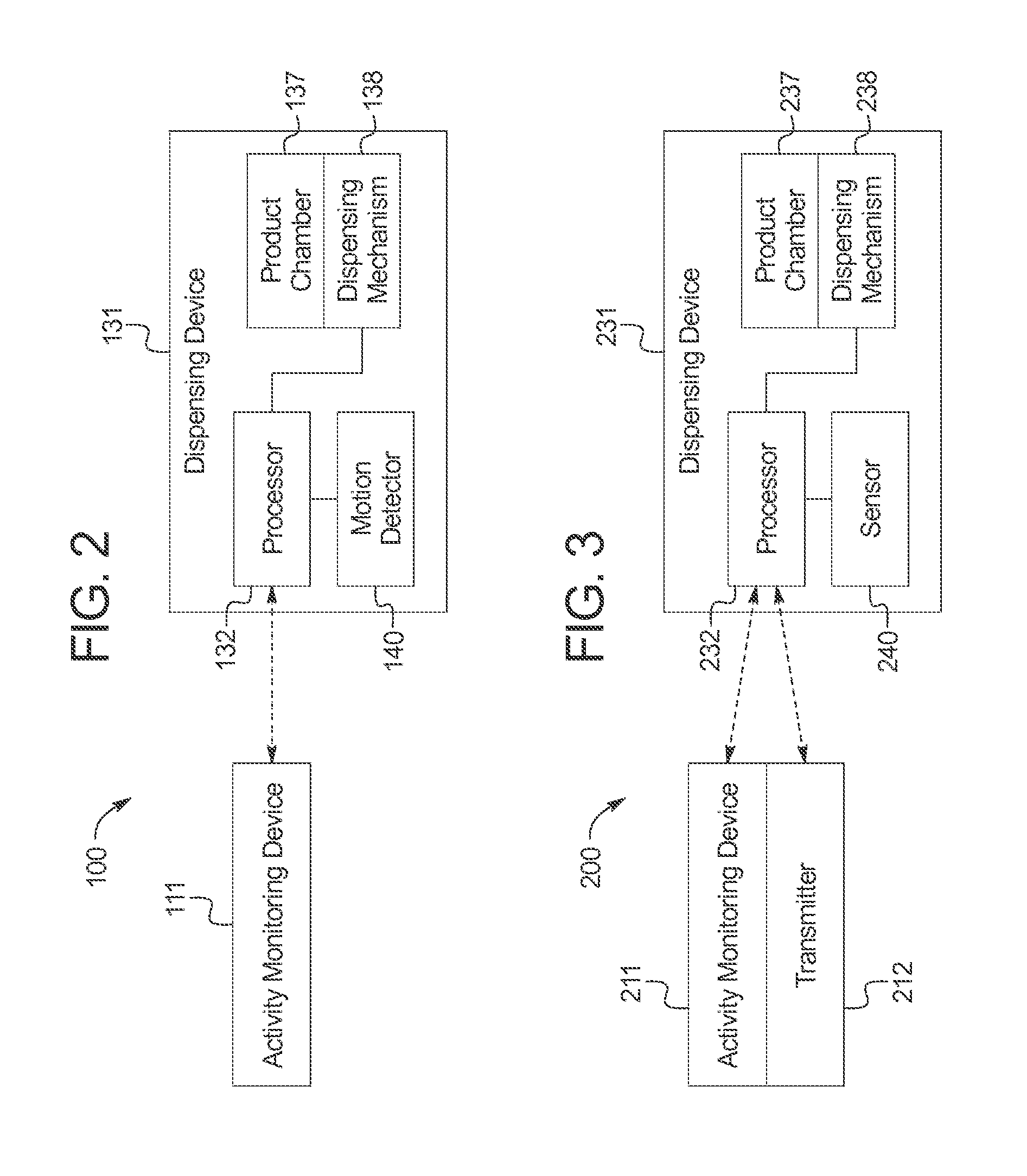
ActiveUS20110192351A1Simple methodMore palatableAnimal watering devicesAnimal feeding devicesActivity monitoringGerontology
Systems and methods for providing nutritional and other products to animals are provided. Generally, the invention provides a dispensing system comprising an activity monitoring device, and a dispensing device comprising a processor programmed to receive a communication generated by the activity monitoring device. The processor controls the dispensing device to dispense a nutritional or other product in response to the communication from the activity monitoring device. The activity monitoring device can be attached to an animal and communicate with the dispensing device information regarding the nutritional requirements of the animal.
Owner:SOC DES PROD NESTLE SA
Dairy protein process and applications thereof
InactiveUS20060159804A1More palatableRaise the pHMilk preparationProtein composition from milkWhey proteinConcentration protein
The invention provides a dried milk protein concentrate which has high denatured whey protein content and is calcium depleted. Processes for preparing the product are also provided. The product is useful in preparing cheese, particularly for reducing the formation of nuggets (thin protein rich gels of a different colour) in the cheese. In one embodiment the calcium content of a milk protein concentrate is reduced and whey proteins are denatured using heat treatment, prior to drying, to obtain the product.
Owner:FONTERRA COOP GRP LTD
Formulation for the administration of medicinal substances
InactiveUS20050208080A1Avoid heat damageAvoid problemsPharmaceutical delivery mechanismSandwich biscuitDrug
A formulation for administration of a medicinal substance, comprises a sandwich biscuit having two or more biscuit layers that support filler layer(s), in which the filler layer comprises a dosage unit form, or a multiple or sub-multiple thereof, of a medicament that is unpalatable in having, for example, a gritty texture or a chalky texture or other unpleasant mouth feel. The biscuit layer of the sandwich biscuit may be a plain, non-medicated, biscuit layer or may itself contain a medicament. In the latter instance, it is possible to select a different medicament for the filler layer from that in the biscuit layer, whereby the two medicaments have a co-operating or synergistic effect. The formulations also allow large dosages of drugs to be administered effectively in a palatable form and are suitable for the long-term administration of drugs.
Owner:HEIGHTMAN NICHOLAS JOHN +1
Packaged concentrate thickener compositions
InactiveUS20090291192A1Good dispersionMore palatableConfectionerySweetmeatsMedicineRadiologic Evaluation
A process for thickening liquid food and / or medications of people with swallowing problems which involves dilution of a concentrate thickener paste which has been thickened to several times its normally useful and cost-effective levels. The approach is beneficial in formulations intended for radiological evaluations of people with swallowing problems including those persons suffering from dysphagia.
Owner:SIMPLY THICK
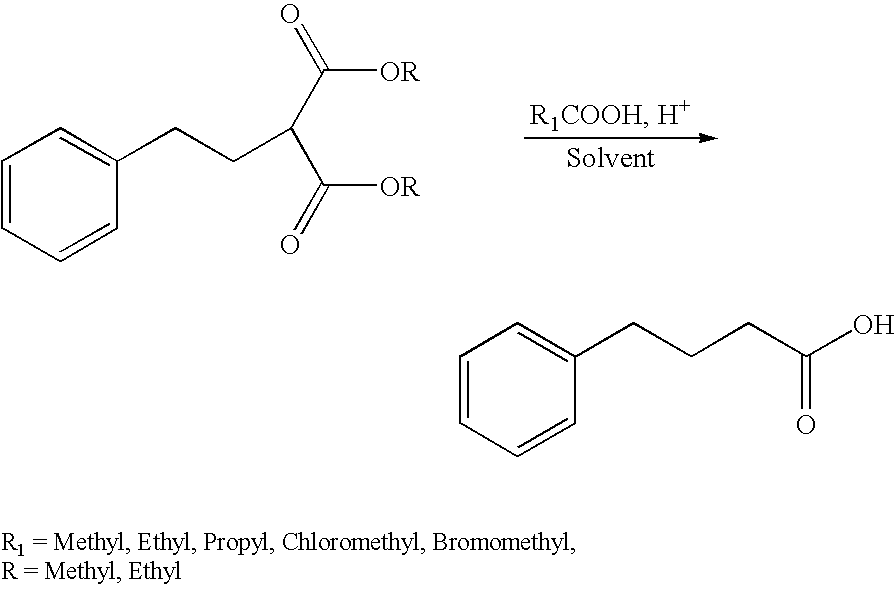
Process for preparation of liquid dosage form containing sodium 4-phenylbutyrate
InactiveUS20070004805A1Easy to manageMore palatableBiocideDispersion deliveryPHENYLBUTYRIC ACIDChemistry
A process for preparing a stable aqueous dosage form of sodium 4-phenylbutyrate, including such dosage forms in a highly concentrated solution, as well as methods for making 4-phenylbutyrate and 4-phenylbutyric acid, and for using 4-phenylbutyrate. The stable aqueous dosage forms do not freeze at 0° C.
Owner:NAVINTA
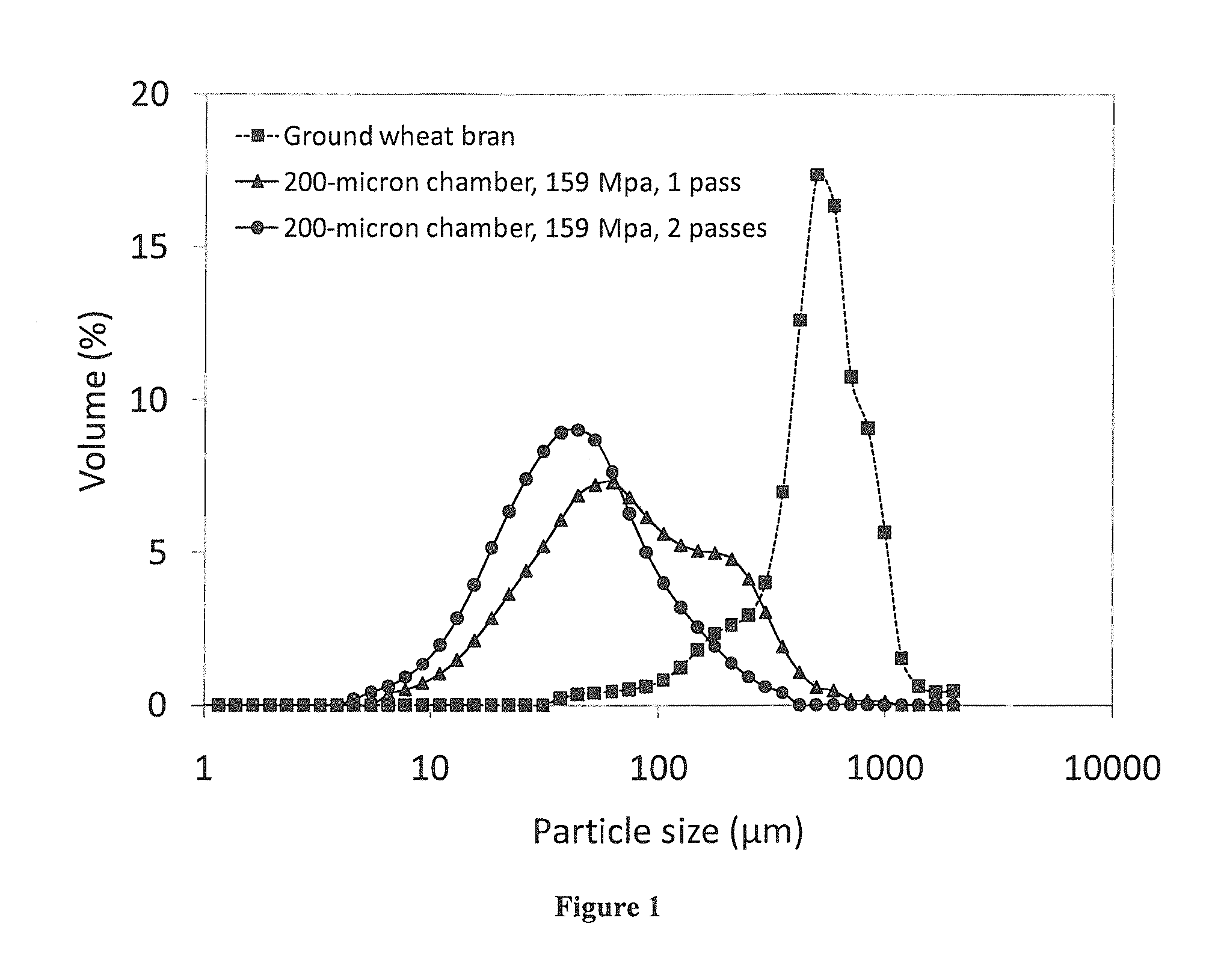
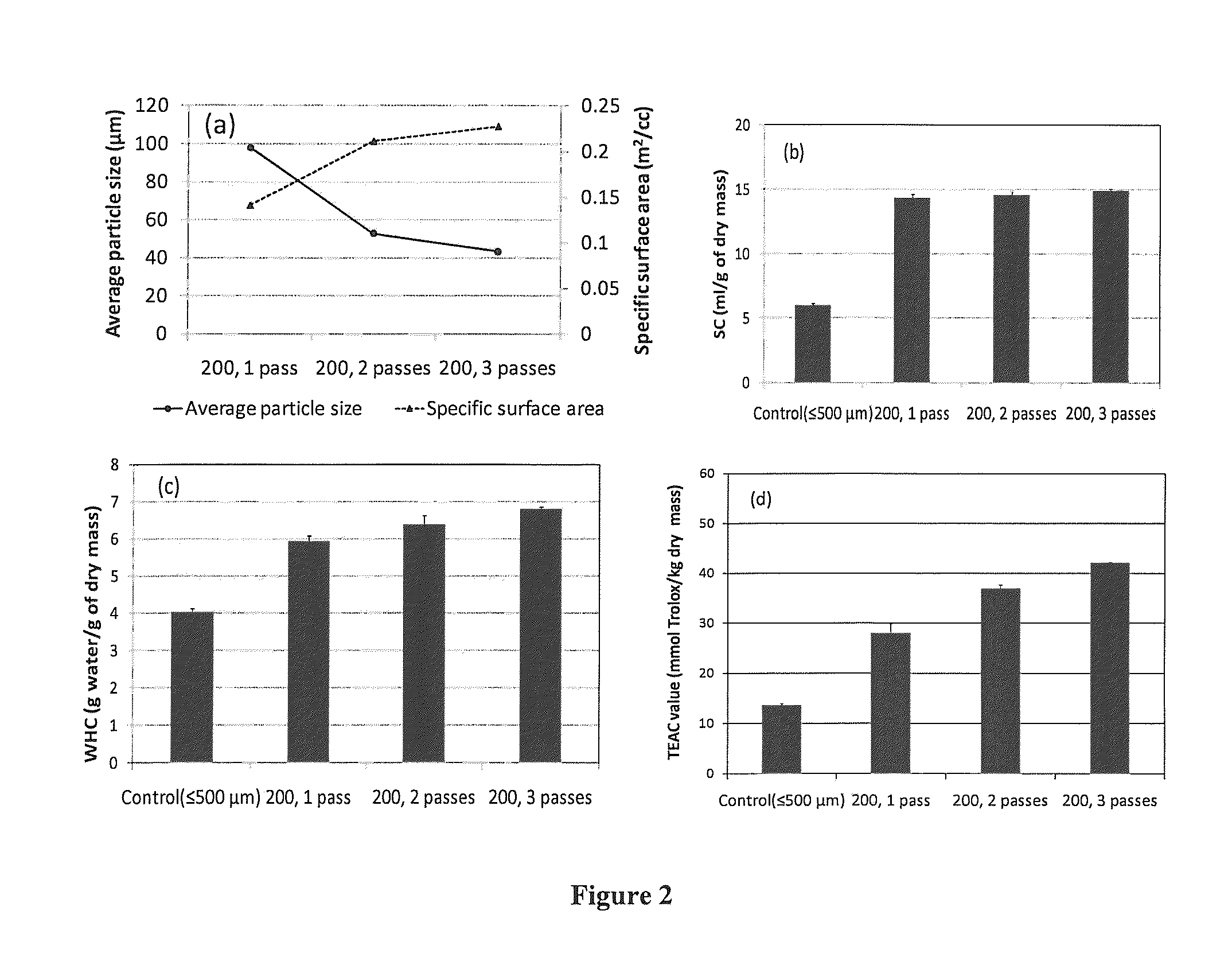
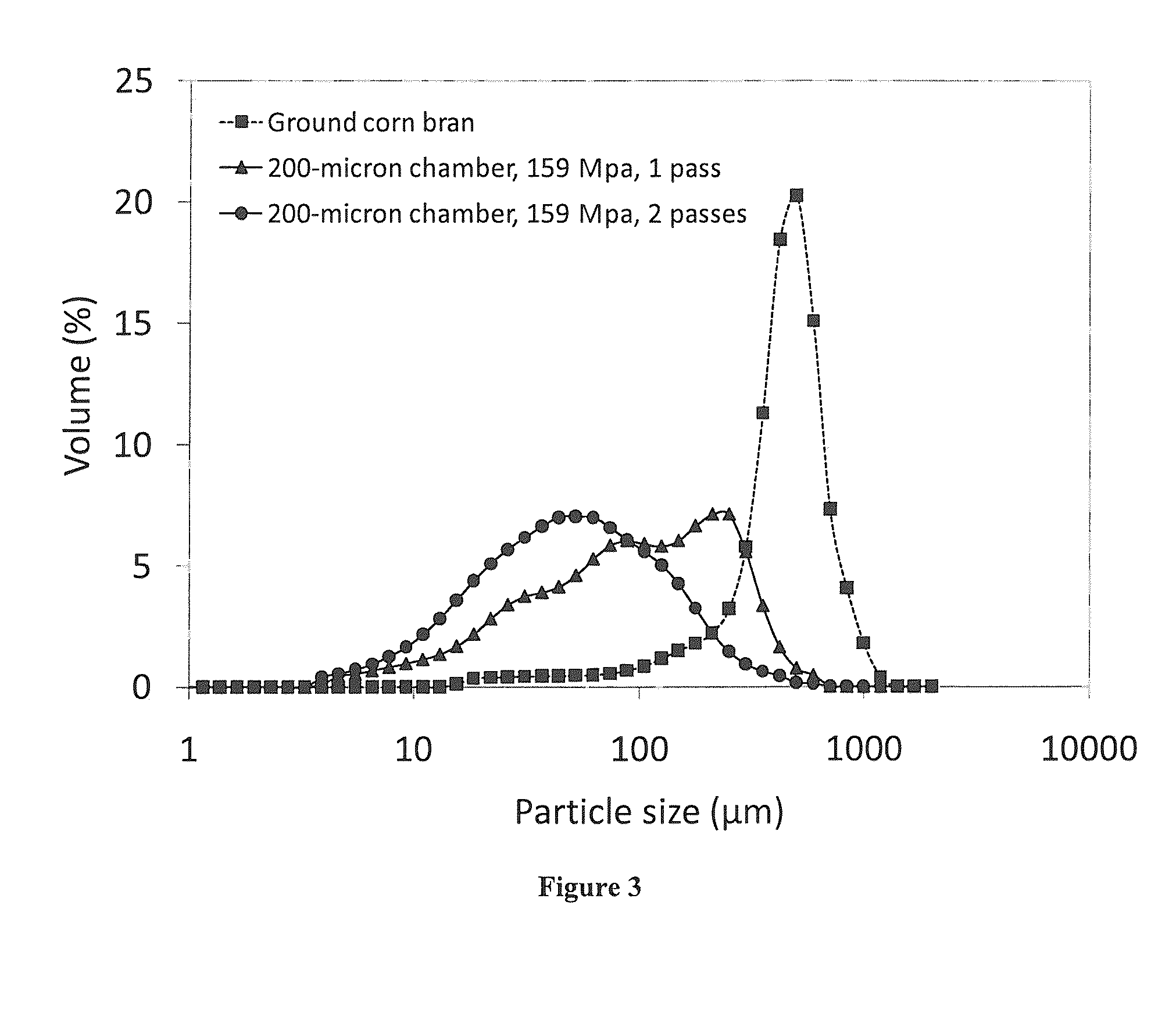
Microfluidization of Brans and Uses Thereof
InactiveUS20140242220A1Efficiently and effectively modifyIncrease intakeMilk preparationButtermilkFiberAdditive ingredient
The present application generally relates to the development of palatable fiber-enriched foodstuffs, including but not limited to soups, salad dressings, dips, sauces, baked goods and beverages, through modifying various dietary fiber ingredients using a novel mechanical approach.
Owner:NORTH CAROLINA AGRICULTURAL AND TECHNICAL STATE UNIVERSITY
Water-Soluble Aspirin Composition
InactiveUS20070092561A1Improve stabilityReduce and eliminate potential bleedingBiocideSalicyclic acid active ingredientsO-acetylsalicylic acidWater of crystallization
Compositions in which aspirin is present in combination with alkaline compounds, especially those containing water of crystallization (hydrates), deteriorate on standing. This deterioration may take several forms: It can be a physical deterioration in which such aspirin compositions become completely unmanageable, wet, gummy, sticky masses; or chemical decomposition in which aspirin loses its molecular structure chiefly by losing the acetyl group. The latter is accompanied by formation of acetic acid, the mixture developing its characteristic acetic odor. In both cases, such compositions become entirely unsuitable for all practical commercial and medicinal purposes. Yet, when preparation of water-soluble aspirin compositions is desired, it is impossible to avoid the use of alkaline compounds. This is because the only known method of converting aspirin into soluble form is by means of reacting it with an alkaline compound to form the soluble salt of aspirin. Unexpectedly, it was discovered and is the substance of the present invention that there are two compounds both of which are alkaline and contain water of crystallization (hydrates), and which, in combination with aspirin, give soluble compositions of outstanding stability. Unexpectedly, it was discovered and is also the substance of the present invention that there are several sugar substitutes which have demonstrated an acceleration of absorption into the bloodstream and have demonstrated a reduction in acididity which in turn should reduce or eliminate the potential for bleeding in the stomach. These two compounds are sodium citrate (tri) dihydrate, and potassium citrate (tri) monohydrate. Artificial sweeteners can also be combined in the soluble aspirin composition.
Owner:MILNE DON
Cysteine Protease from Ginger (Zingiber) as a Food Improver and Anti-Inflammatory
InactiveUS20070264311A1Improve filtration efficiencyImprove hydrolysis efficiencyBiocidePeptide/protein ingredientsFood technologyWaste treatment
The present invention relates generally to plant extracts and / or components isolated therefrom which exhibit desirable properties in relation to therapy and / or food technology. More particularly, the present invention relates to extracts and components isolated thereof from the plant genus Zingiber and in particular from the rhizome of the species Zingiber officinale (also known as ginger) which comprise activities having broad applicability in the fields of research reagents, inter alia pharmaceutical and / or nutraceutical product development, manufacture of improved high-value food and feed products, production of alcohol from cereals and waste treatment.
Owner:NATBIO
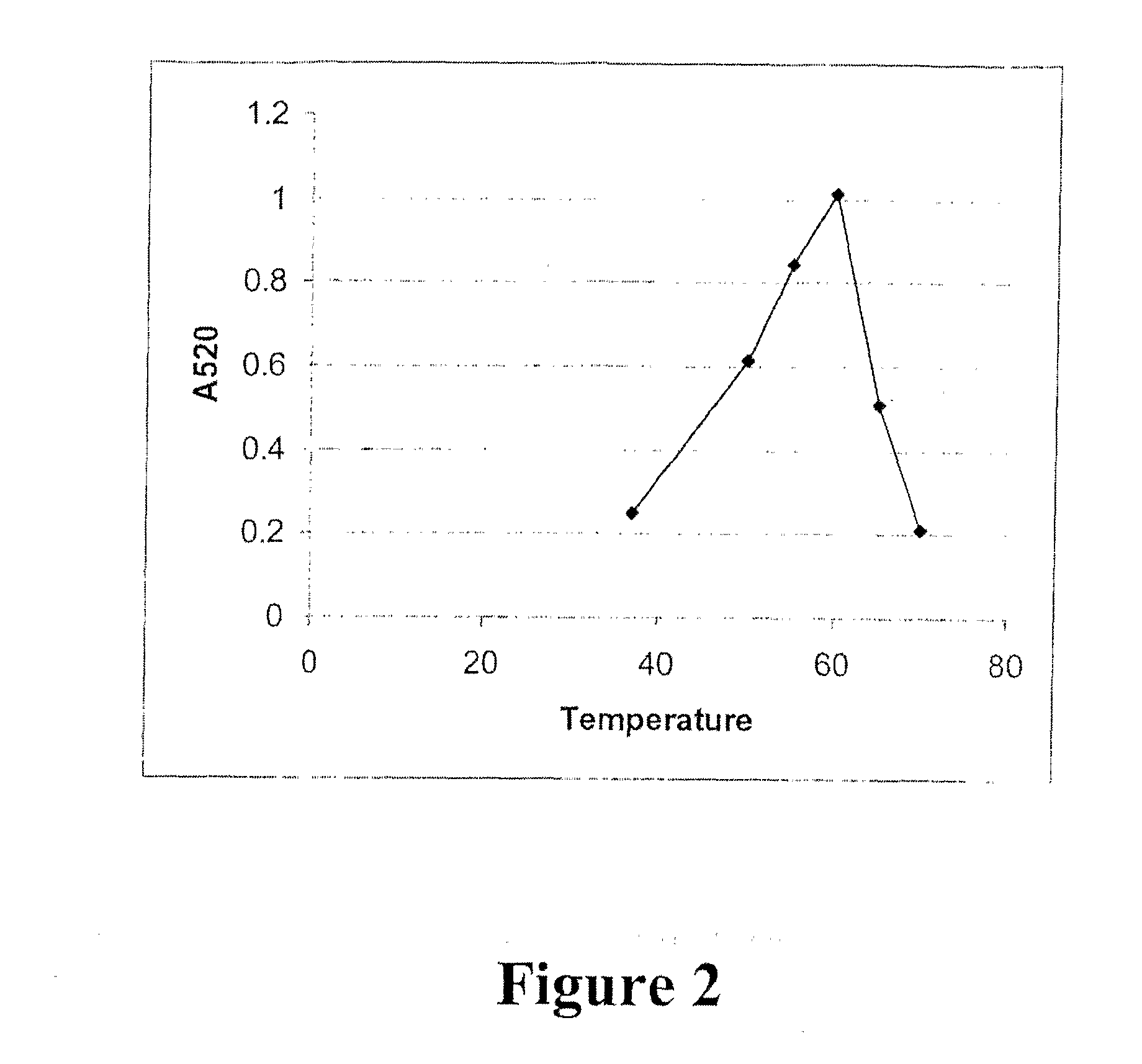
Frozen Confectionery and Beverage Products
A frozen confection or a beverage product is provided which contains at least 400 mg of theobromine and at least 40 mg of caffeine per 100 grams of the product. Also provided is a frozen confection or a beverage product for the enhancement of mood without transient elevation of blood pressure.
Owner:CONOPCO INC D B A UNILEVER
Edible animal chew resembling naturally occurring substantially unprocessed food source
InactiveUS20070022973A1Avoid discomfortMore palatableAnimal feeding stuffOther apparatusAnimal scienceCanis lupus familiaris
An edible animal chew for animals, particularly dogs, resembling a naturally occurring substantially unprocessed food source, such as a vegetable, grain, fruit, or animal, in its nutritional content and in one or more of its shape, color, or texture, and wherein the size, hardness, or flavoring of the chew may be changed to accommodate an animal with regard to, for example, the animal's type, age, or size.
Owner:BROWN THOMAS W
Detoxifier herbal formulation
InactiveUS20140147394A1Relieve pressureOvercome bitternessBiocideAerosol deliveryPatient complianceDrug herbal
The present invention provides a non-parenteral and non-ocular herbal synergistic detoxifier composition effective for blood purification, blood detoxification and in the treatment and management of disorders related to accumulation of toxins in the body having synergistic effect of its components in specific ratio, better bio availability wherein the amount of dose administered is remarkably low. The formulation of synergistic herbal composition has better patient compliance in the terms of palatability, ease of administration and is formulated in the form of syrup, lozenges / candies / jujubs / mouth freshener, sub-lingual tablets or chewable tablets. The present invention also provides a method of preparation of this formulation.
Owner:CHAUDHARY MANU
Systems and methods for providing products to animals
ActiveUS9192142B2Simple methodMore palatableAnimal watering devicesAnimal feeding devicesActivity monitoringGerontology
Systems and methods for providing nutritional and other products to animals are provided. Generally, the invention provides a dispensing system comprising an activity monitoring device, and a dispensing device comprising a processor programmed to receive a communication generated by the activity monitoring device. The processor controls the dispensing device to dispense a nutritional or other product in response to the communication from the activity monitoring device. The activity monitoring device can be attached to an animal and communicate with the dispensing device information regarding the nutritional requirements of the animal.
Owner:SOC DES PROD NESTLE SA
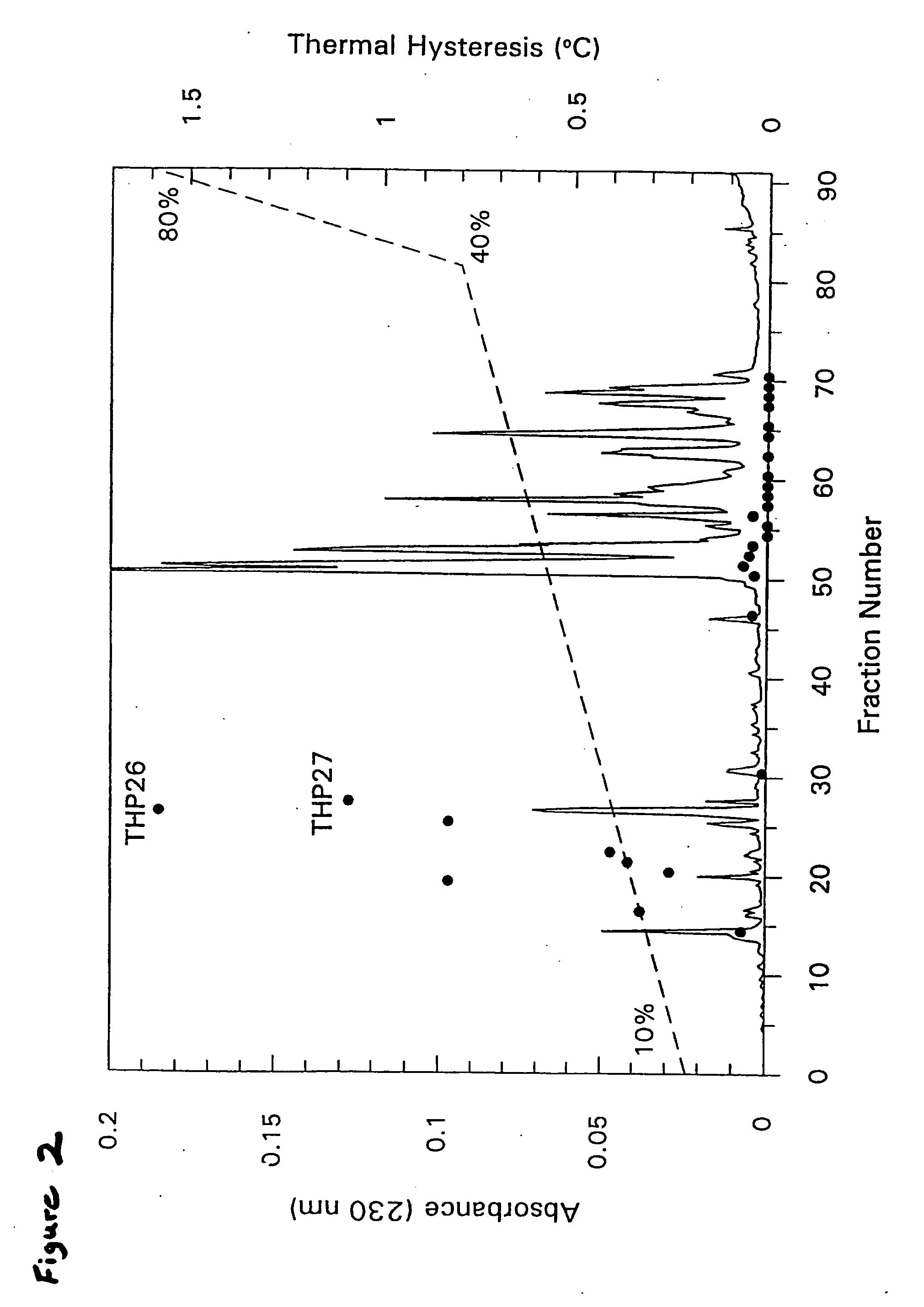
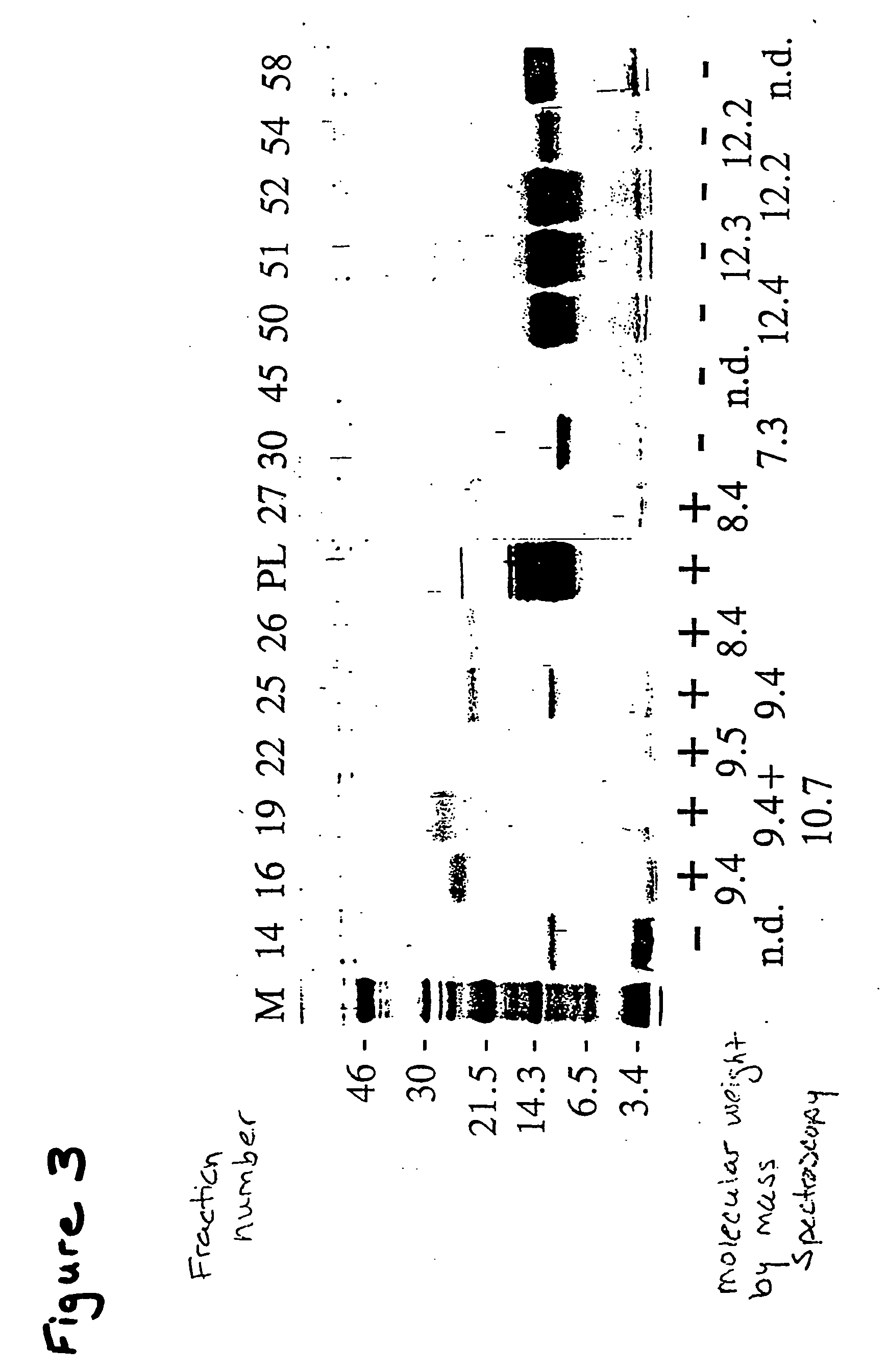
Tenebrio antifreeze proteins
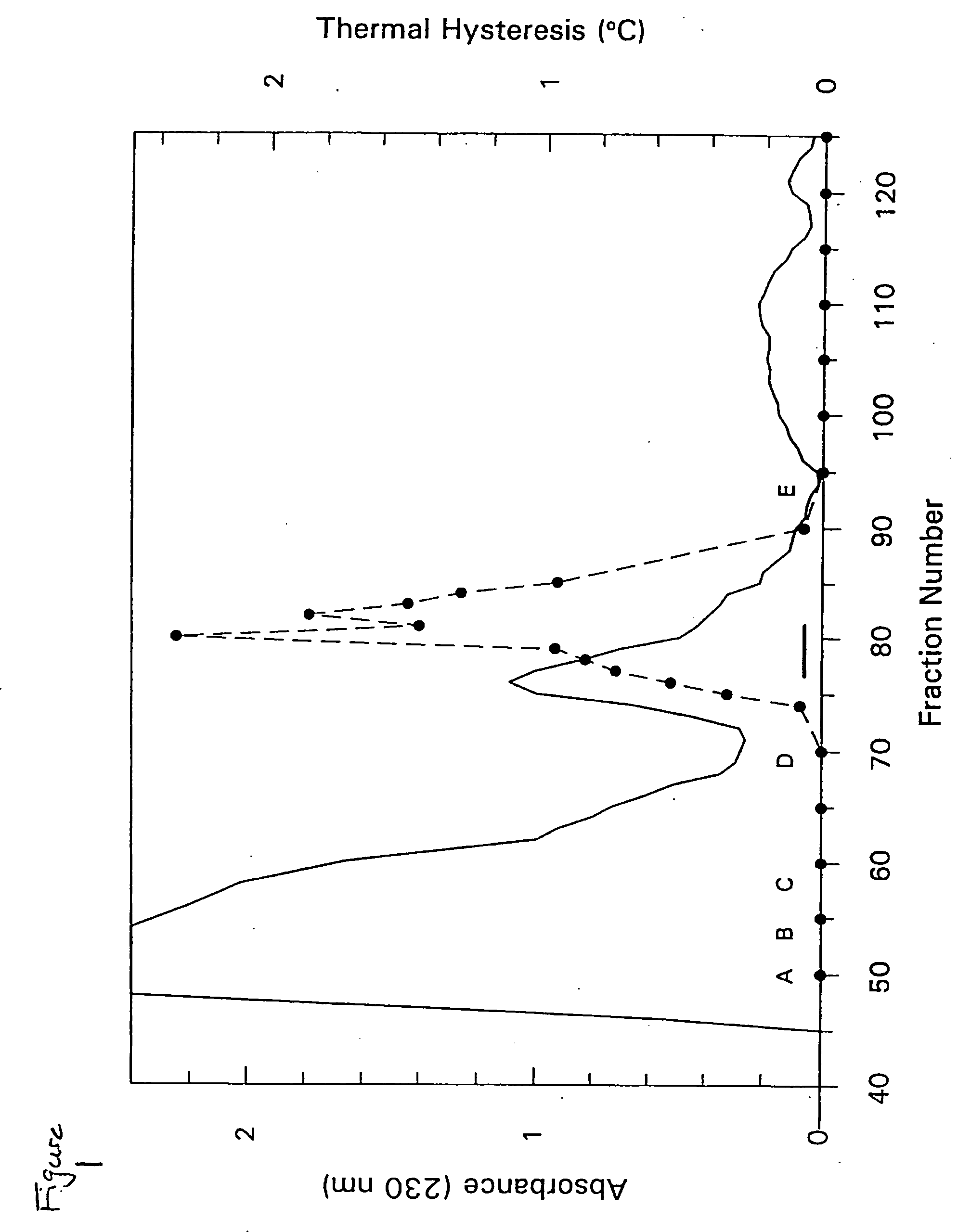
A novel class of thermal hysteresis (antifreeze) proteins (THP) that have up to 100 times the specific activity of fish antifreeze proteins has been isolated and purified from the mealworm beetle, Tenebrio molitor. Internal sequencing of the proteins, leading to cDNA cloning and production of the protein in bacteria has confirmed the identity and activity of the 8.4 to 10.7 kDa THP. They are novel Thr- and Cys-rich proteins composed largely of 12-amino-acid repeats of cys-thr-xaa-ser-xaa-xaa-cys-xaa-xaa-ala-xaa-thr. At a concentration of 55 μg / mL, the THP depressed the freezing point 1.6° C. below the melting point, and at a concentration of ˜1 mg / mL the THP or its variants can account for the 5.5° C. of thermal hysteresis found in Tenebrio larvae. The THP function by an adsorption-inhibition mechanism and produce oval-shaped ice crystals with curved prism faces.
Owner:QUEENS UNIV OF KINGSTON
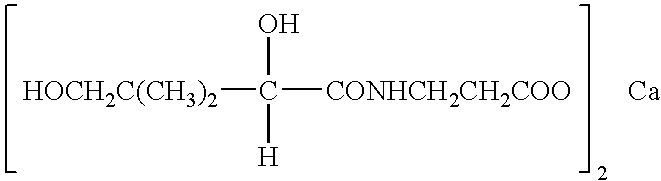
Calcium supplement for animals and method for making same
ActiveUS7105191B2Reduce hydroscopic effectMore palatableMeat/fish preservationAnimal feeding stuffChlorideFatty acid
A calcium supplement for animals comprising one or more coatings over a calcium-containing core. The coatings provide additional nutrients as well as providing a moisture-resistant outer coating to reduce the hygroscopic effects of the calcium-containing core. The supplement comprises calcium-containing core particles, calcium chloride for example, each particle having an animal nutrient coating over an outer surface of the core particles. The supplement further comprises a second coating, urea, deposited over the nutrient coating, and a final outer coating comprising one or more fatty acids or paraffinic hydrocarbons that are agreeable to animals. The method for making a calcium supplement for animals comprises coating calcium-containing granules with a layer of nutrients and then spraying molten soluble urea onto the previously coated calcium-containing granules which are sufficiently cool to solidify the molten material into second coating on the granules. A third coating of one or more fatty acids or paraffinic hydrocarbon is sprayed onto the urea coating.
Owner:TETRA TECH INC
Composition comprising L-arginine as a muscle growth stimulant and use thereof
InactiveUS20050043287A1Reduce morbidityMore palatableBiocidePeptide/protein ingredientsGrowth stimulantArginine
A composition for stimulating muscle growth, comprising an effective amount of L-arginine or a salt thereof, a pH control agent for controlling the pH at less than 7 and a pharmaceutically acceptable carrier. A method of using the composition to achieve muscle growth or an immune response is also described.
Owner:ALLEN ANN DE WEES
Detoxifier herbal formulation
InactiveUS9233133B2Relieve pressureOvercome bitternessBiocidePharmaceutical delivery mechanismPatient complianceDrug herbal
Owner:CHAUDHARY MANU
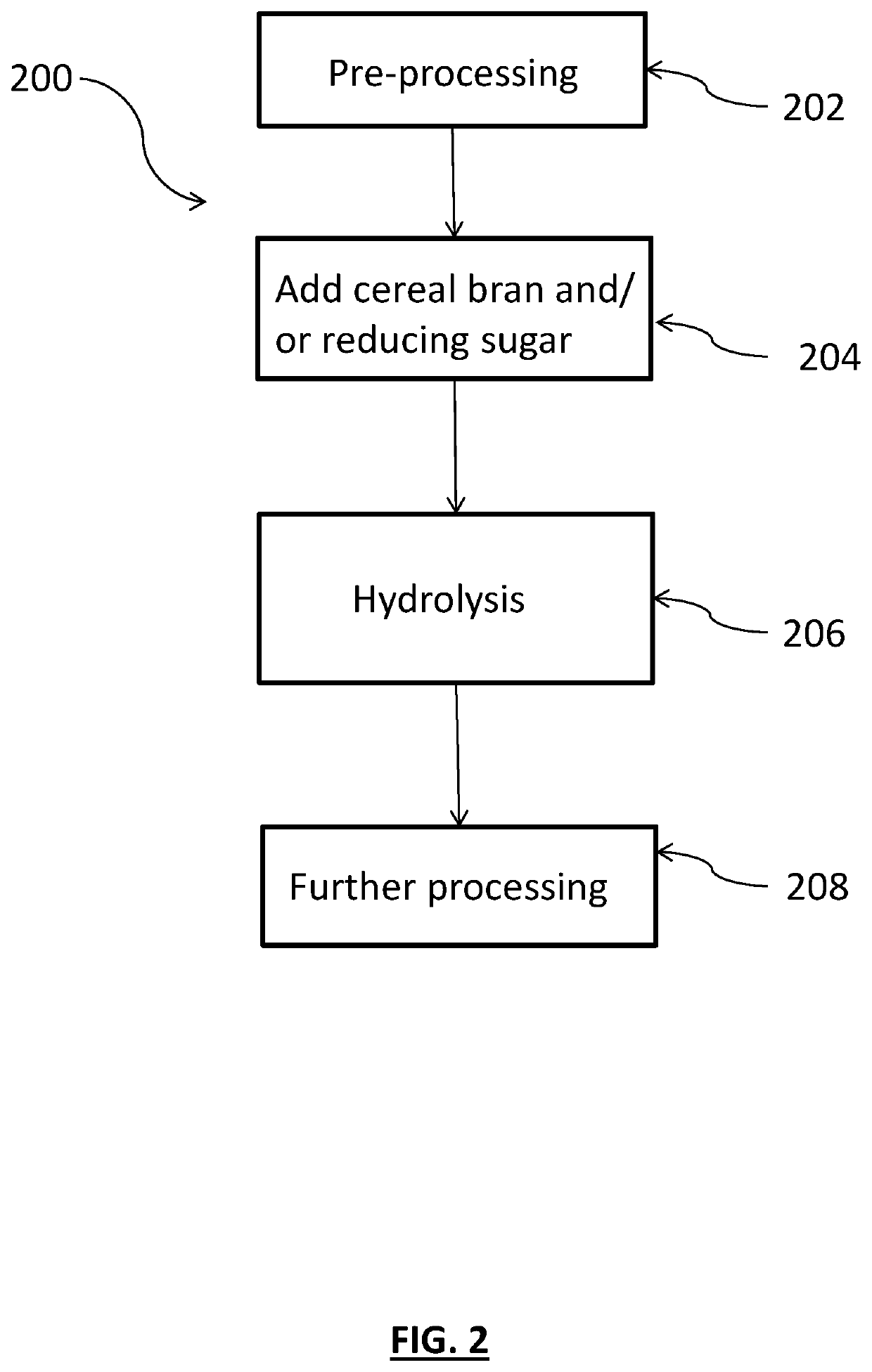
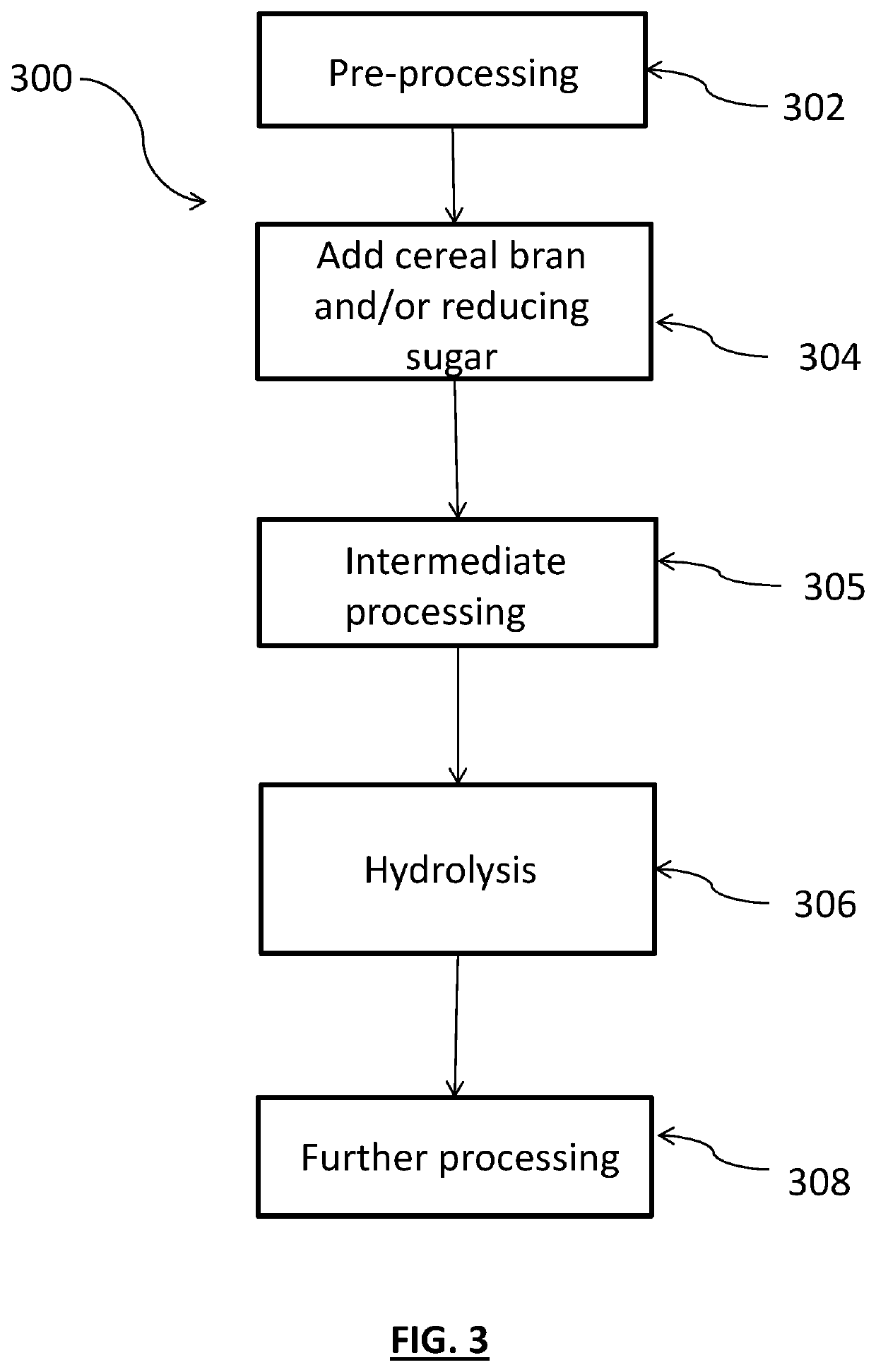
Methods for producing feather-based food products
PendingUS20200077676A1More palatableLightweight productionFood processingAnimal feeding stuffBiotechnologyOff-flavour
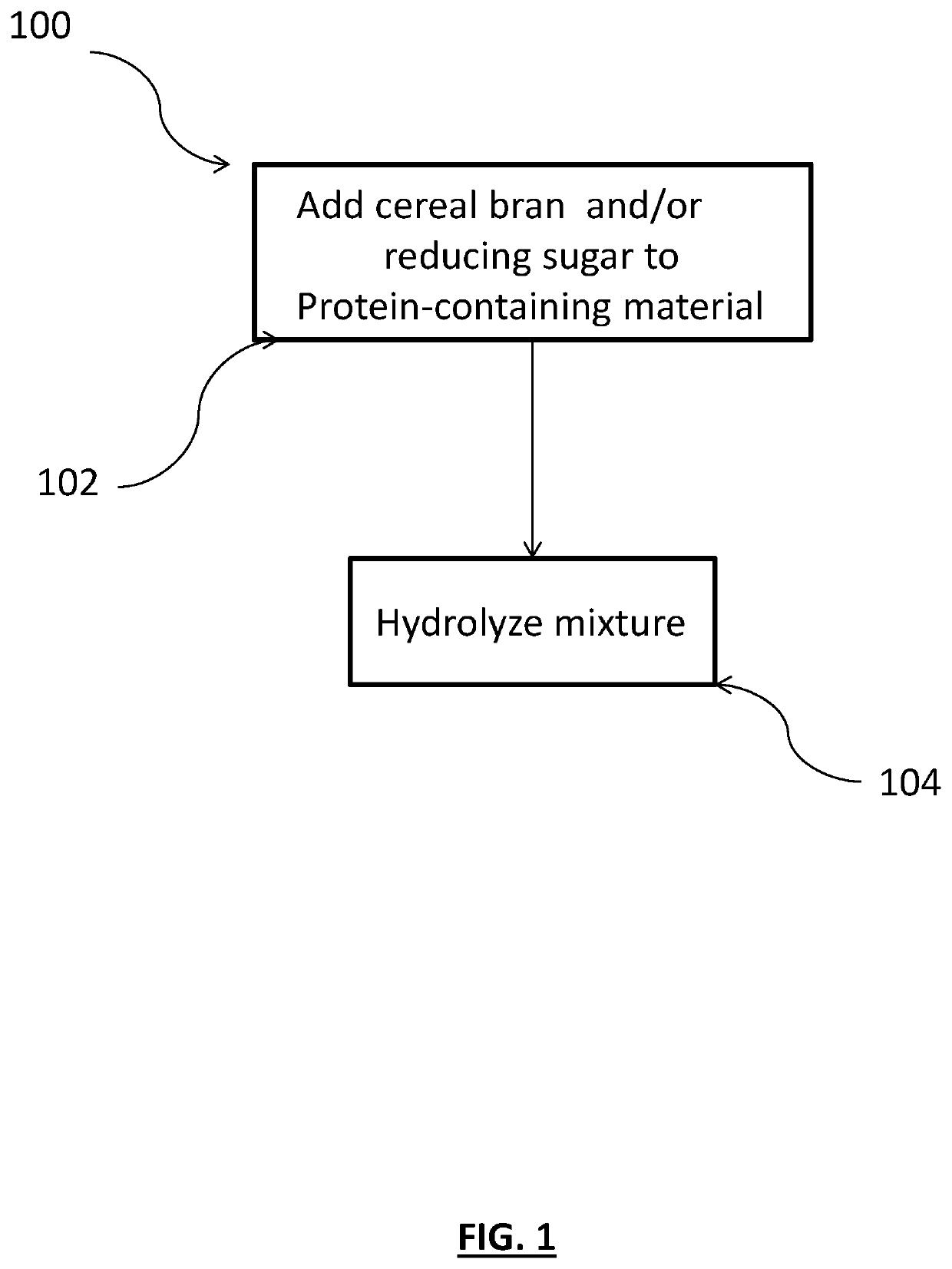
A method is provided for making food product ingredients from indigestible keratinous protein-containing material. The methods generally include adding an amount of cereal bran and / or one or more reducing sugars to the keratinous protein-containing material to provide a mixture and hydrolyzing the mixture. The methods generate fewer unpleasant odors, and food product ingredients produced by the method can similarly benefit. Antioxidants may also be added, and in such embodiments, even fewer off odors may be generated and / or palatability may be enhanced.
Owner:MARS INC
Process for manufacturing soft chewable free flowing granules and companion animal products thereof
PendingUS20220218717A1More palatableOrganic active ingredientsAccessory food factorsNutritionBULK ACTIVE INGREDIENT
The present invention relates to soft chewable composition and its method of manufacturing using pharmaceutical conventional equipment. The present invention relates to process for manufacturing soft chewable dosage form by manufacturing free flowing soft chewable granules and these granules are compressed to make soft chewable tablets using rotary compression machine. The free-flowing granules of the invention are formed of active ingredients or nutritional agents, diluents, binder, disintegrant, sugar component, oil component or humectant or combination of both, flavour, lubricant or plasticizers in intragranular or extra granular and other conventional tableting aids to help in making the tablet more palatable.
Owner:CUCKOS PHARMA PTE LTD
Oral solution comprising atomoxetine hydrochloride and methods thereof
ActiveUS9855228B1Effective to tasteEasy to swallowOrganic active ingredientsNervous disorderAttention deficitsPharmaceutical medicine
Disclosed herein is an oral pharmaceutical composition in the form of an aqueous solution of atomoxetine as an active ingredient. The aqueous solution of atomoxetine comprises a taste-masked liquid carrier comprising peppermint, orange flavor and a viscosity agent. The combined flavors successfully masked atomoxetine hydrochloride's bitter smell and / or taste which makes it a novel palatable pharmaceutical composition. The viscosity agent improves the oral pharmaceutical composition's consistency and provides a smooth texture which makes it easy to swallow. More specifically, the oral pharmaceutical composition comprises effective amounts of: (a) atomoxetine or the pharmaceutically acceptable salts thereof; and (b) a taste-masked liquid carrier. Also provided is a method for making the aqueous solution of atomoxetine. The present disclosure also provides methods of using oral pharmaceutical composition for the treatment of a subject having a disorder treatable by the administration of atomoxetine. In one embodiment, the disorder is attention deficit hyperactivity disorder (ADHD).
Owner:TAHO PHARMA
Compositions and methods for treating and preventing migrainous headaches and associated symptoms
ActiveUS8409637B2Not result in undesirable side effectsImprove stabilityBiocideNervous disorderHeadache severeHeadaches
Compositions and methods of treating migrainous headaches and their associated symptoms are provided. Methods of treating migrainous headaches and their associated symptoms include administering a lipid-based composition containing feverfew extract and ginger extract sublingually to a patient in need thereof. Treatments are effective using low total administered amounts of feverfew and ginger extracts.
Owner:DMR BIOLOGICS LLC
Tenebrio antifreeze proteins
A novel class of thermal hysteresis (antifreeze) proteins (THP) that have up to 100 times the specific activity of fish antifreeze proteins has been isolated and purified from the mealworm beetle, Tenebrio molitor. Internal sequencing of the proteins, leading to cDNA cloning and production of the protein in bacteria has confirmed the identity and activity of the 8.4 to 10.7 kDa THP. They are novel Thr- and Cys-rich proteins composed largely of 12-amino-acid repeats of cys-thr-xaa-ser-xaa-xaa-cys-xaa-xaa-ala-xaa-thr. At a concentration of 55 μg / mL, the THP depressed the freezing point 1.6° C. below the melting point, and at a concentration of ˜1 mg / mL the THP or its variants can account for the 5.5° C. of thermal hysteresis found in Tenebrio larvae. The THP function by an adsorption-inhibition mechanism and produce oval-shaped ice crystals with curved prism faces.
Owner:QUEENS UNIV OF KINGSTON
Modified release orally administered amino acid formulations
ActiveUS20180318244A1More palatableWell mixedOrganic active ingredientsDough treatmentDiseaseAmino acid metabolism
Methods and formulations of modified release amino acids are provided for the treatment or management of diseases defined by impaired amino acid metabolism, with improved pharmacokinetics, metabolism and utilization.
Owner:APR APPLIED PHARMA RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com