Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108results about "Proteoglycans" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell-based fluorescence resonance energy transfer (FRET) assays for clostridial toxins
InactiveUS20070122858A1Decreased acceptor fluorescence intensityHigh fluorescence intensityAntibacterial agentsBacteriaClostridial toxinEnergy transfer
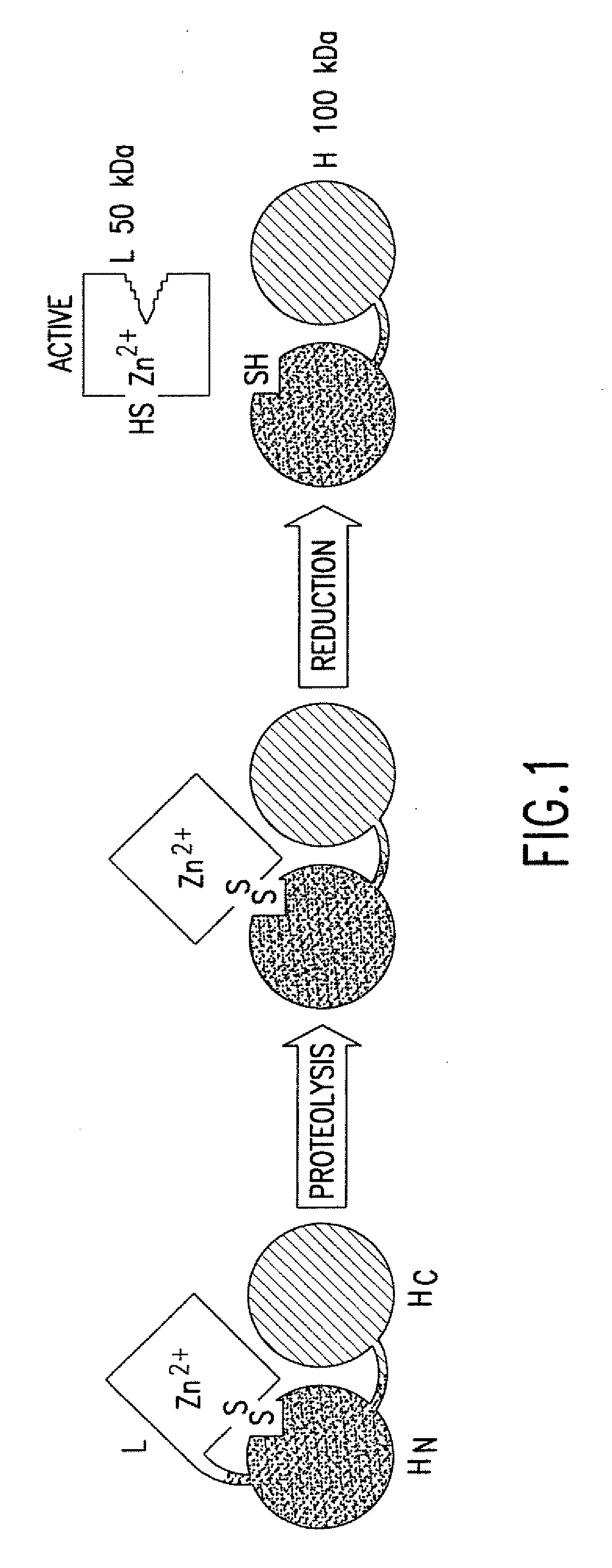
The present invention provides a method of determining clostridial toxin activity by (a) contacting with a sample a cell containing a clostridial toxin substrate that includes a donor fluorophore; an acceptor having an absorbance spectrum overlapping the emission spectrum of the donor fluorophore; and a clostridial toxin recognition sequence containing a cleavage site that intervenes between the donor fluorophore and the acceptor, where resonance energy transfer is exhibited between the donor fluorophore and the acceptor under the appropriate conditions; (b) exciting the donor fluorophore; and (c) determining resonance energy transfer of the contacted cell relative to a control cell, where a difference in resonance energy transfer of the contacted cell as compared to the control cell is indicative of clostridial toxin activity.
Owner:ALLERGAN INC
Bone/joint disease sensitivity gene and use thereof
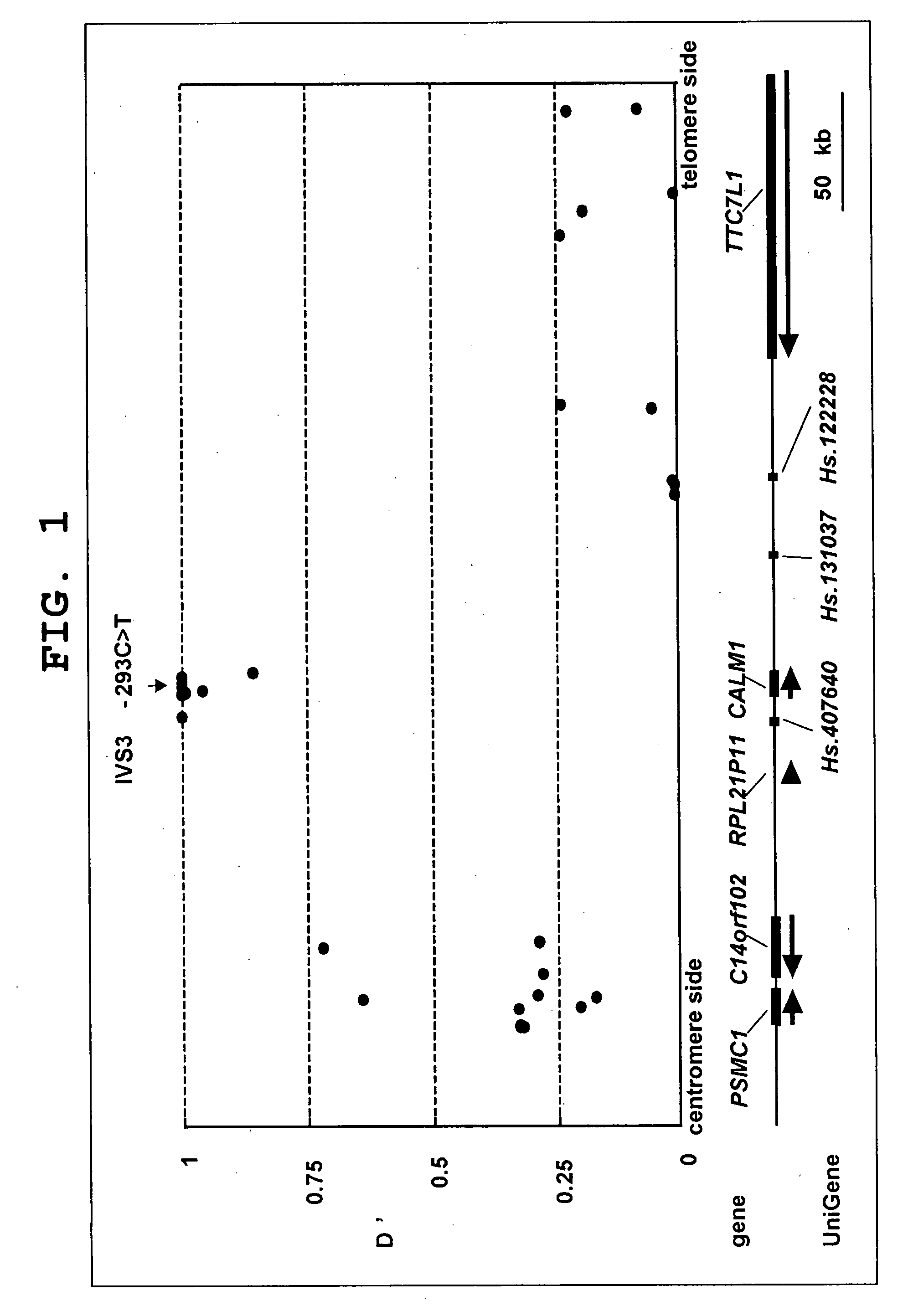
InactiveUS20090075921A1High expressionPromote differentiationOrganic active ingredientsPeptide/protein ingredientsDiseaseCALM1 Gene
The present invention provides the prophylaxis and treatment of bone and joint diseases by regulating the expression or activity of calmodulin, the prophylaxis and treatment of bone and joint diseases by regulating the expression or activity of asporin, and a diagnostic method for genetic susceptibility to bone and joint diseases by detecting polymorphisms in the CALM1 gene and / or the asporin gene, and the like.
Owner:TAKEDA PHARMA CO LTD +1
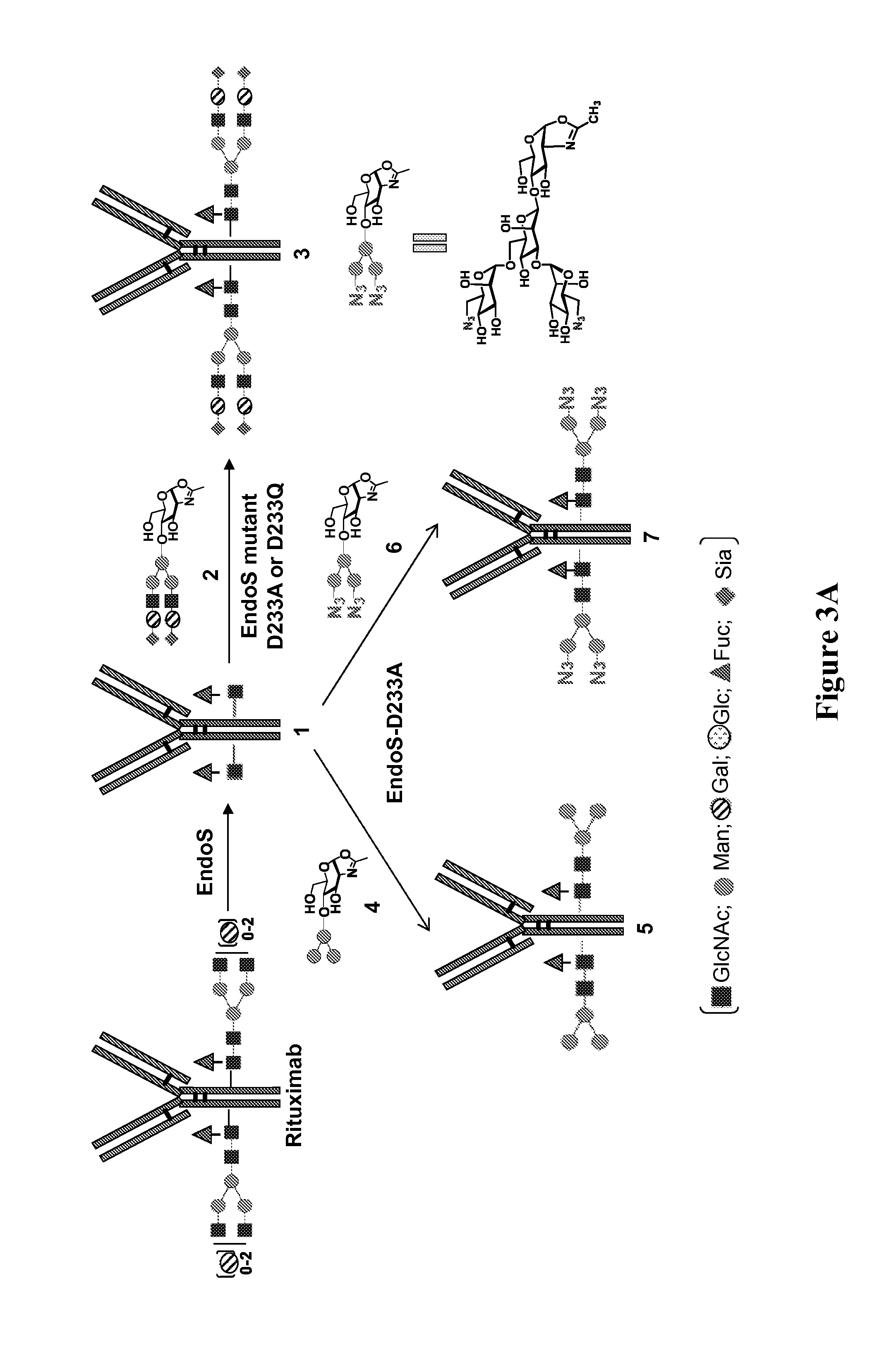
Chemoenzymatic glycoengineering of antibodies and fc fragments thereof
ActiveUS20150087814A1Reduced hydrolysis activityIncreased transglycosylation activityBacteriaMicroorganism based processesFucosylationFc domain
The present invention provides for recombinant Endo-S mutants that exhibit reduced hydrolysis activity and increased transglycosylation activity for the synthesis of glycoproteins wherein a desired sialylated oxazoline or synthetic oligosaccharide oxazoline is added to a core fucosylated or nonfucosylated GlcNAc-protein acceptor. Such recombinant Endo-S mutants are useful for efficient glycosylation remodeling of IgGl-Fc domain to provide different antibody glycoforms carrying structurally well-defined Fc N-glycans.
Owner:UNIV OF MARYLAND
Genes and proteins associated with angiogenesis and uses thereof
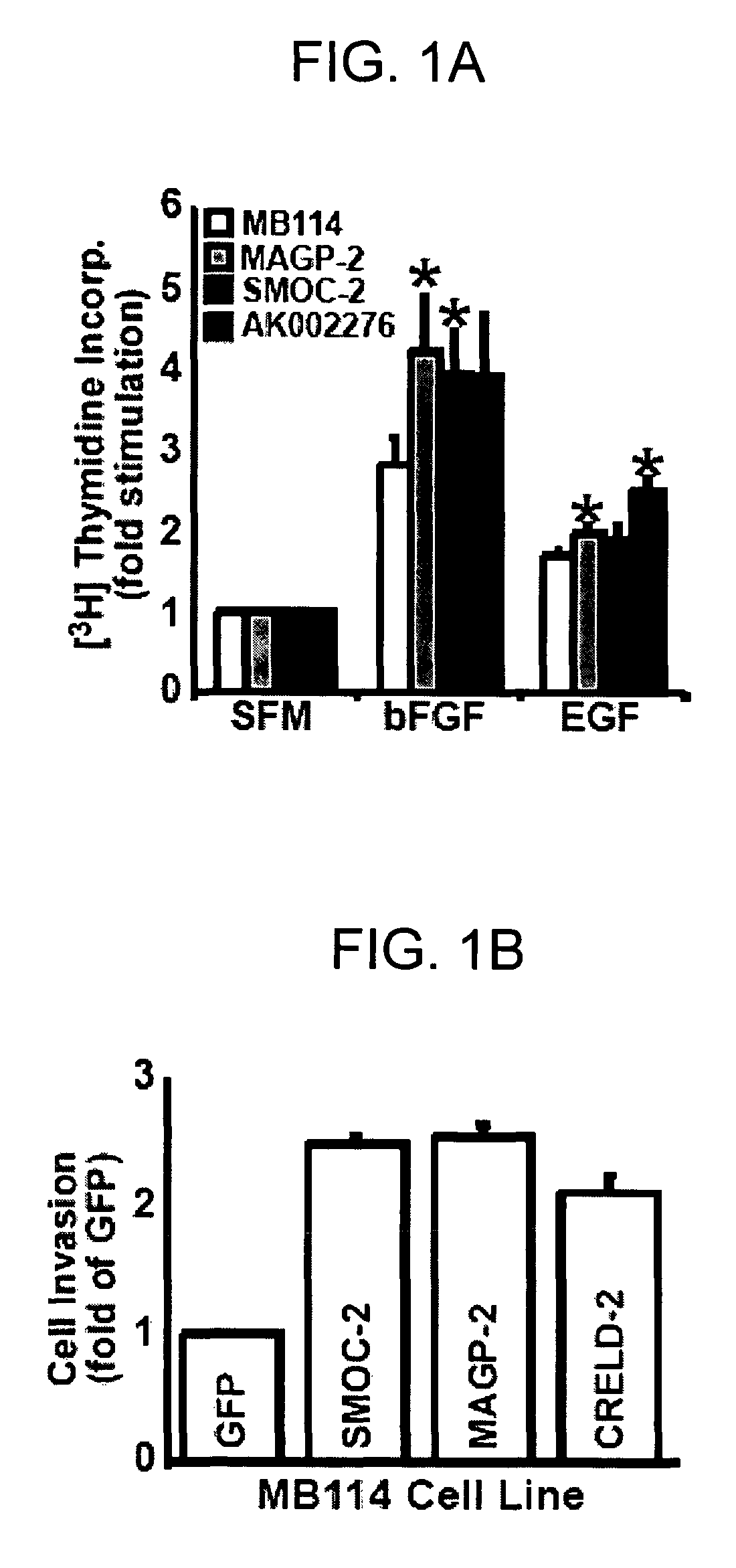
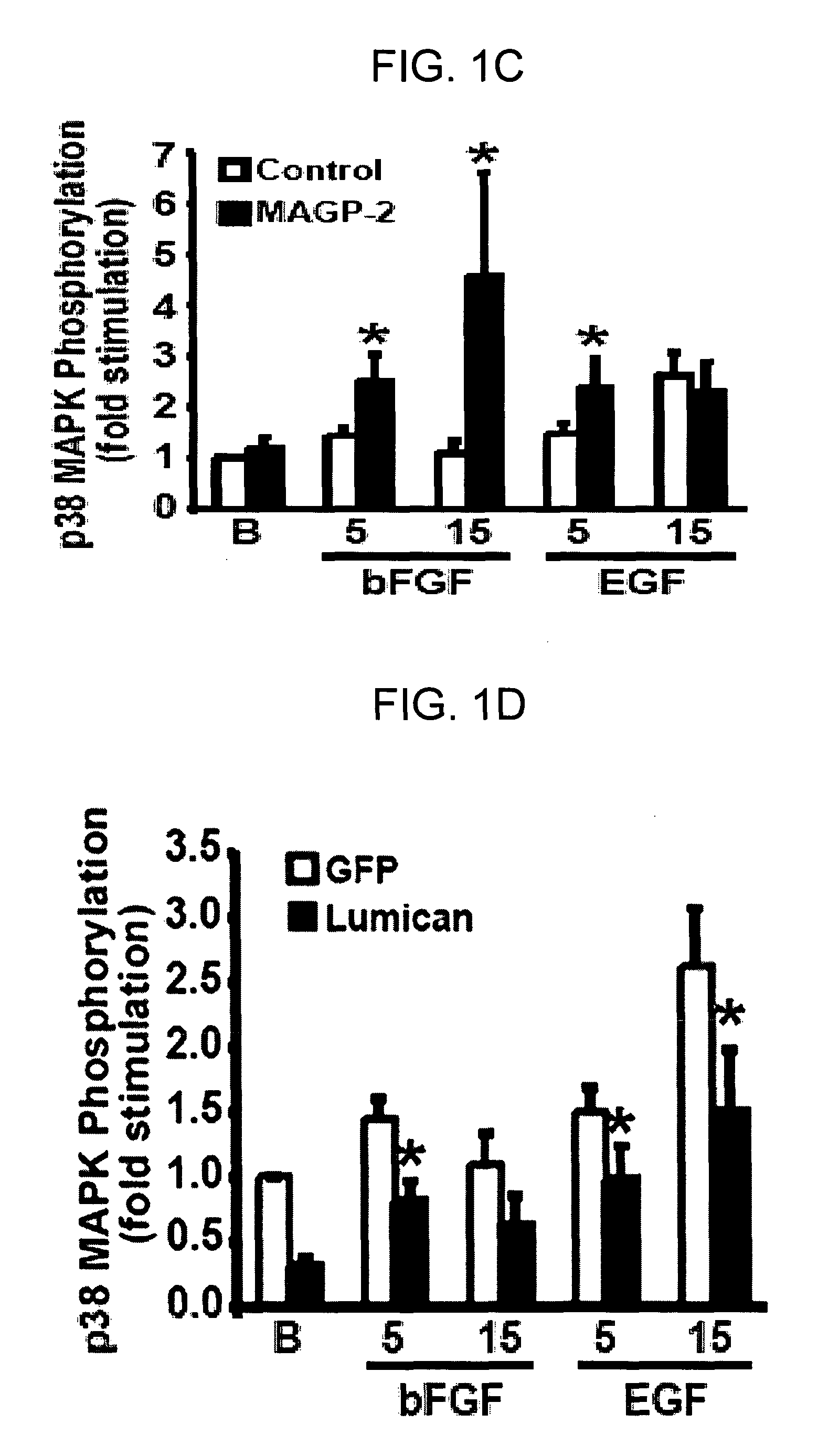
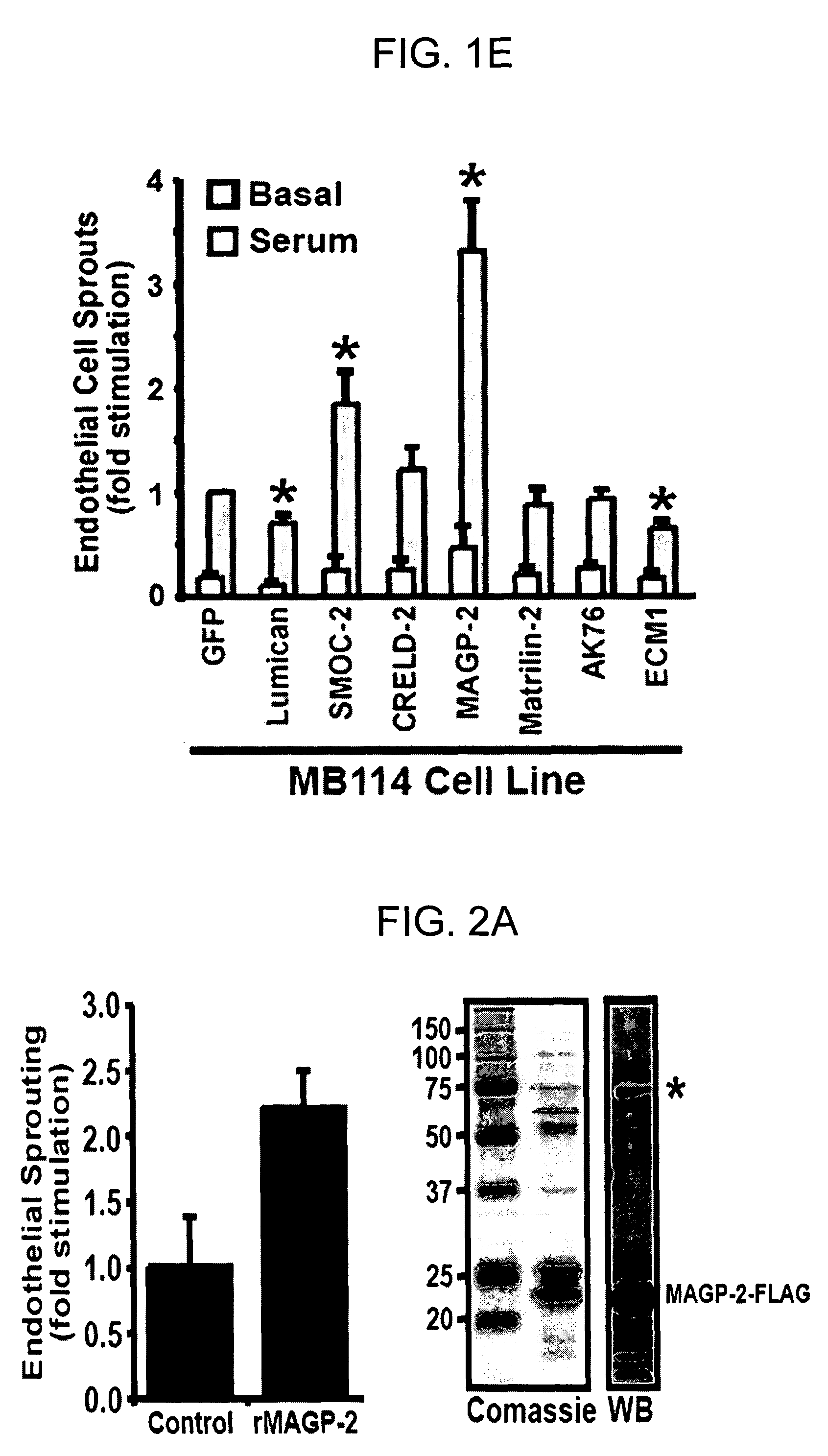
Disclosed is a panel of biomarkers associated with angiogenesis, and the use of such biomarkers (genes, proteins, homologues and analogs thereof) to regulate angiogenesis. Methods for identifying compounds useful for regulating angiogenesis and conditions related thereto are disclosed.
Owner:NAT JEWISH HEATH
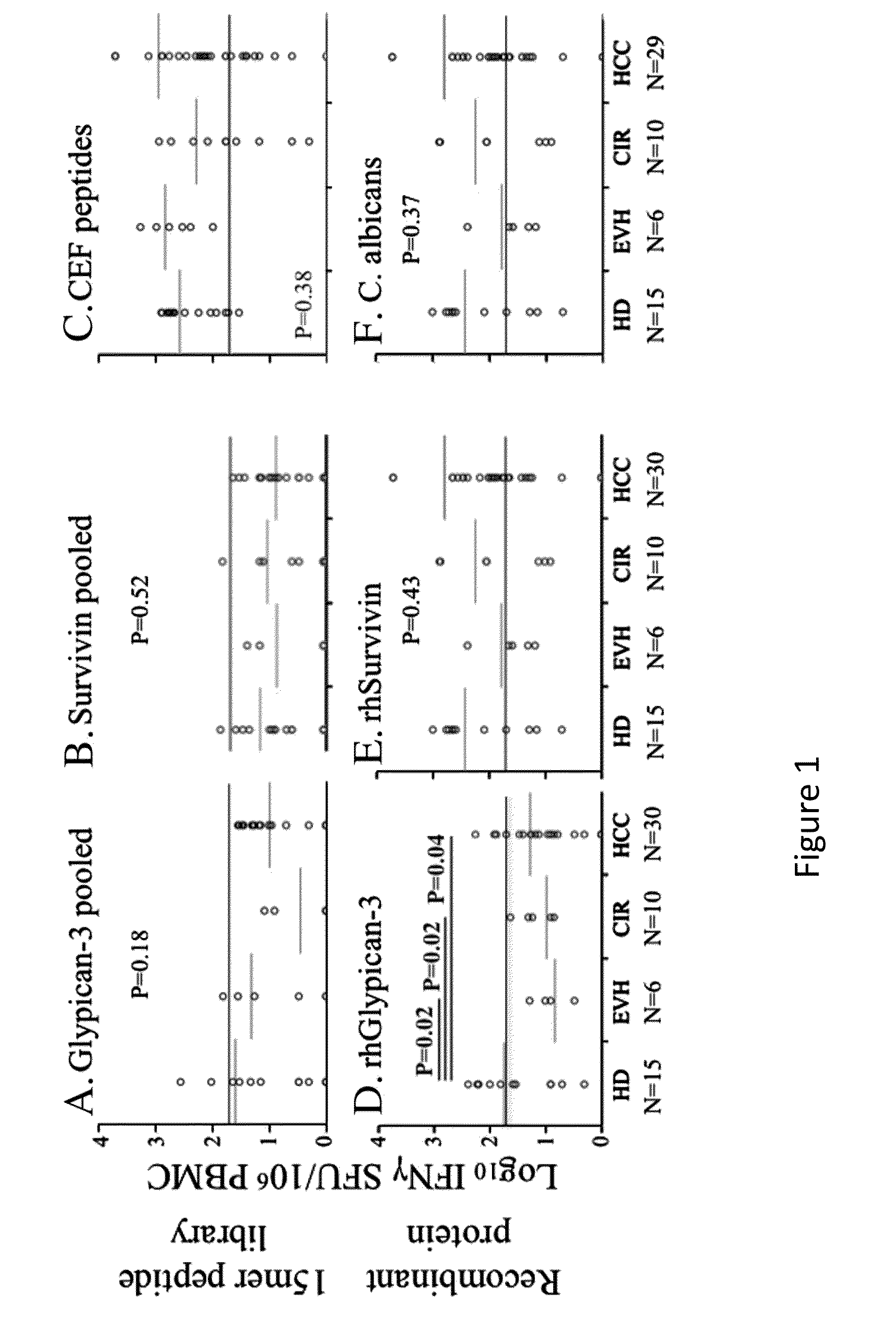
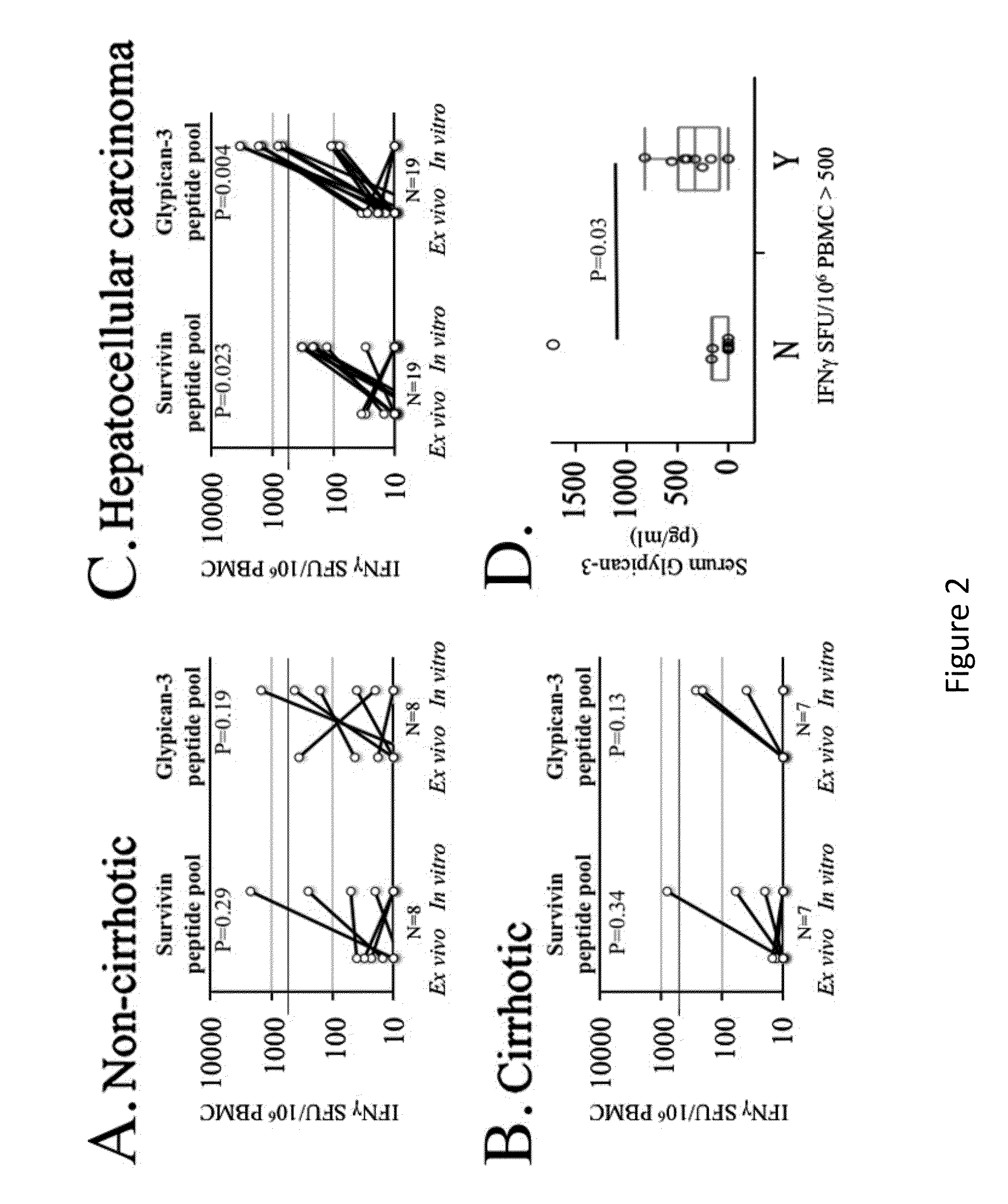
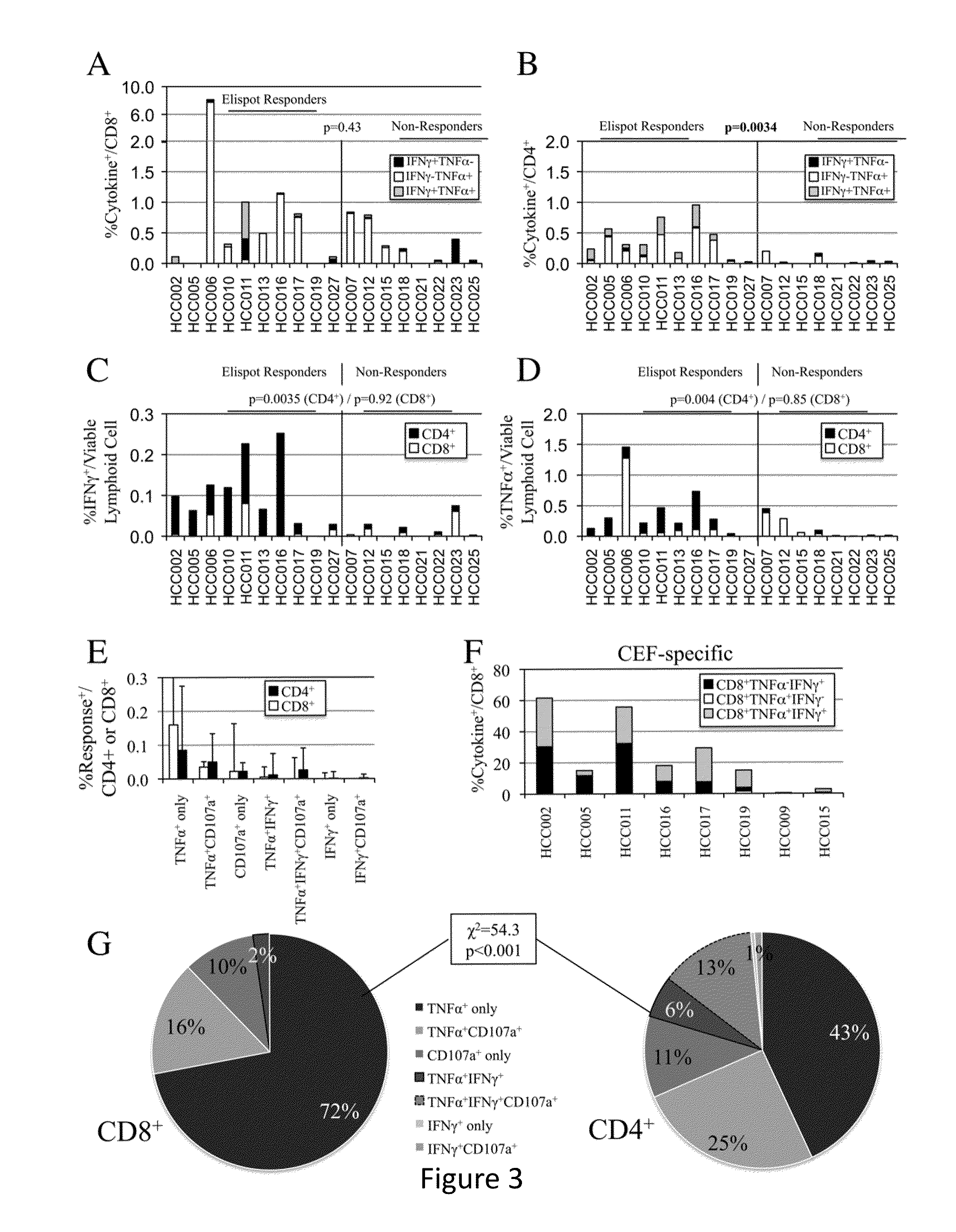
Expansion of Interferon-Gamma-Producing T-Cells Using Glypican-3 Peptide Library
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Methods for stabilizing corneal tissue
InactiveUS20090105127A1Promote resultsImprove acuityPeptide/protein ingredientsPharmaceutical delivery mechanismKeratoconusCollagen fibril
Methods of stabilizing collagen fibrils in a cornea are disclosed. The stabilization may be effected by treating the cornea with a protein that crosslinks collagen fibrils, such as decorin. The stablization methods include treatment of corneas before, during, or after a surgical procedure, treatment of keratectasia, and treatment of keratoconus.
Owner:EUCLID SYST CORP
Peptides of Syndecan-1 For Inhibiting Angiogenesis
InactiveUS20080020979A1Inhibit angiogenesisBiocidePeptide/protein ingredientsAngiogenesis growth factorSyndecan 1
The present invention provides a peptide derived from the extracellular domain of syndecan-1 that inhibits angiogenesis.
Owner:WISCONSIN ALUMNI RES FOUND
Production of recombinant lubricin
ActiveUS20160304572A1Improve abilitiesImprove the lubrication effectOrganic active ingredientsDispersion deliveryGlycoproteinLubrication
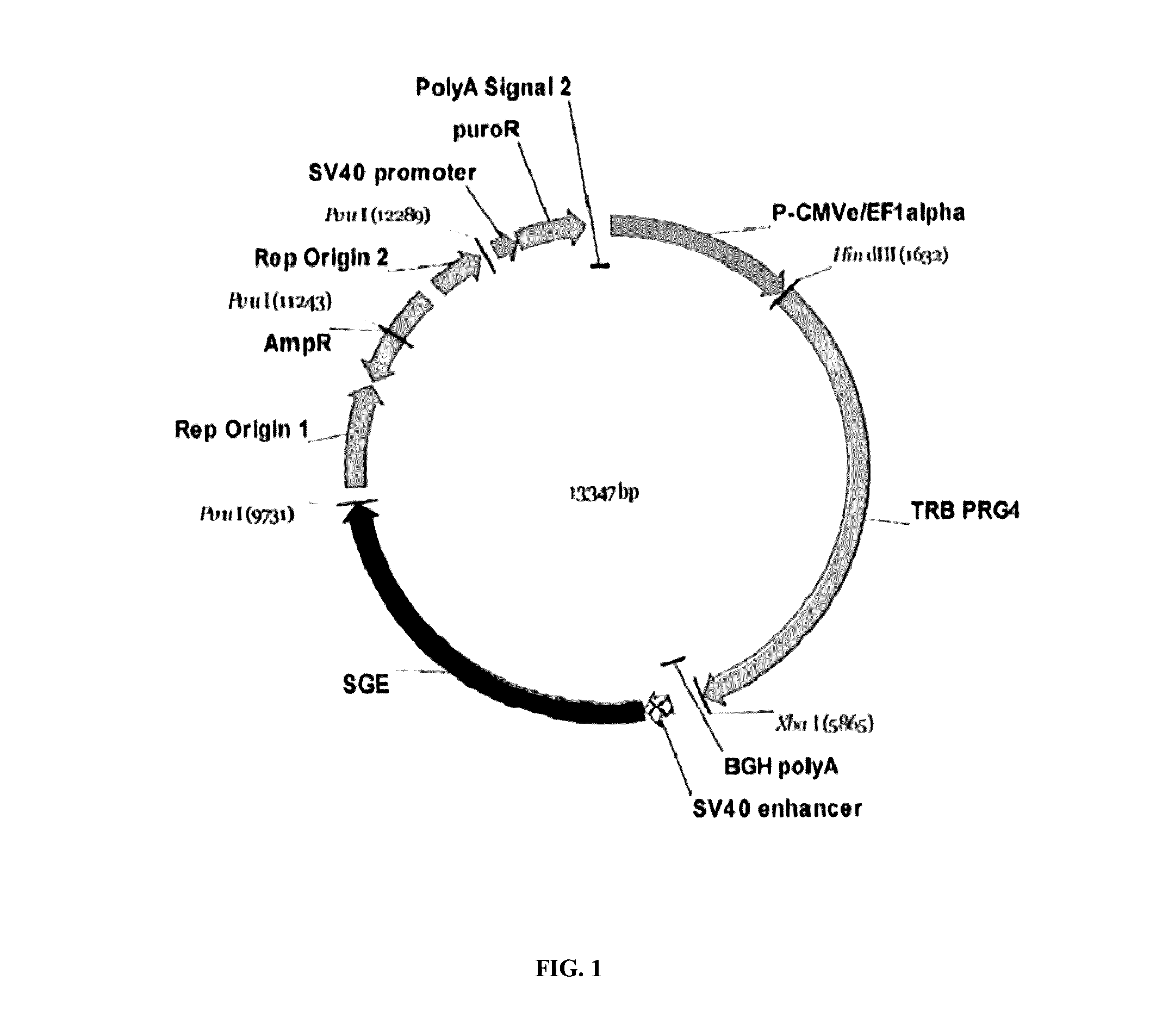
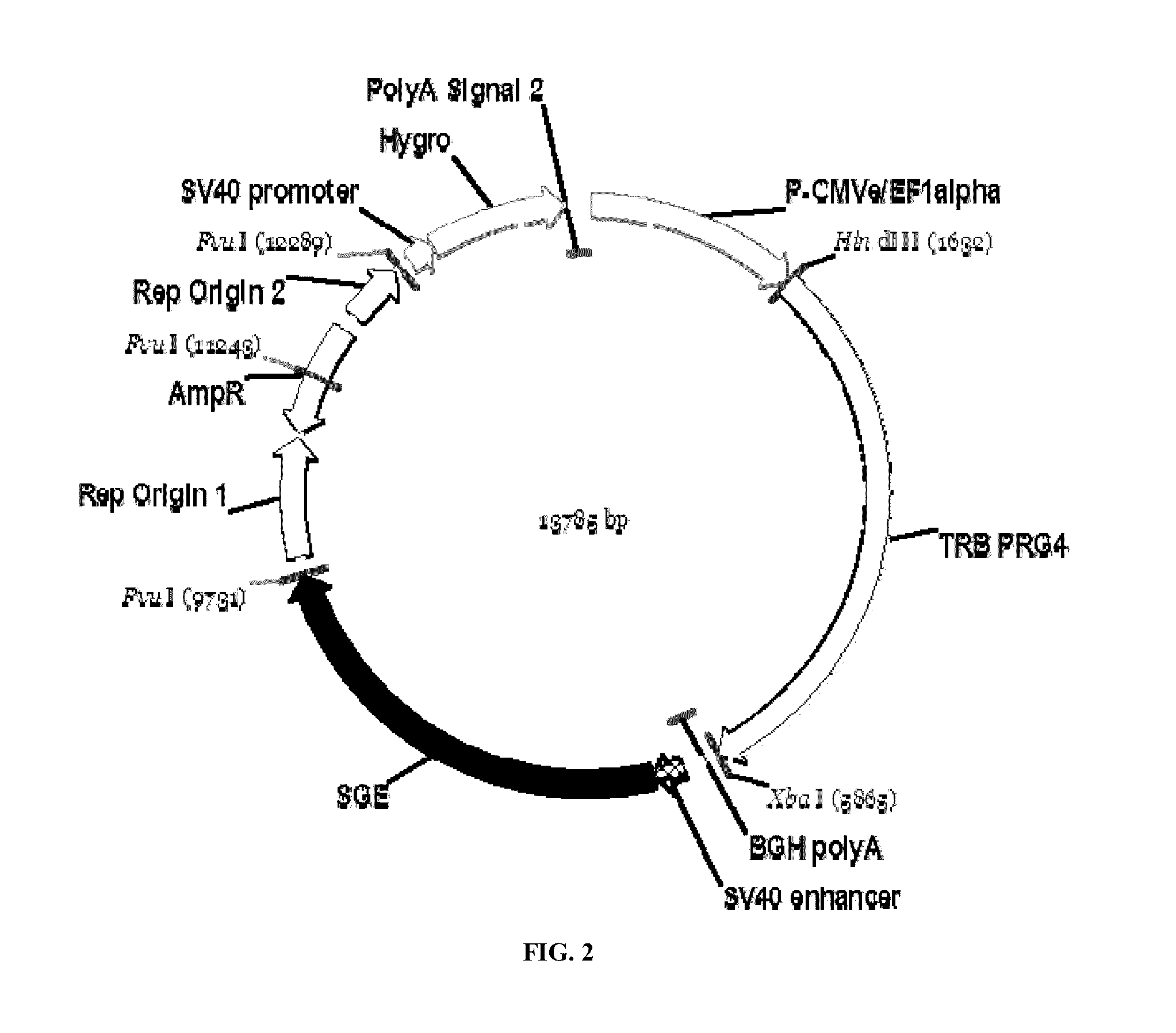
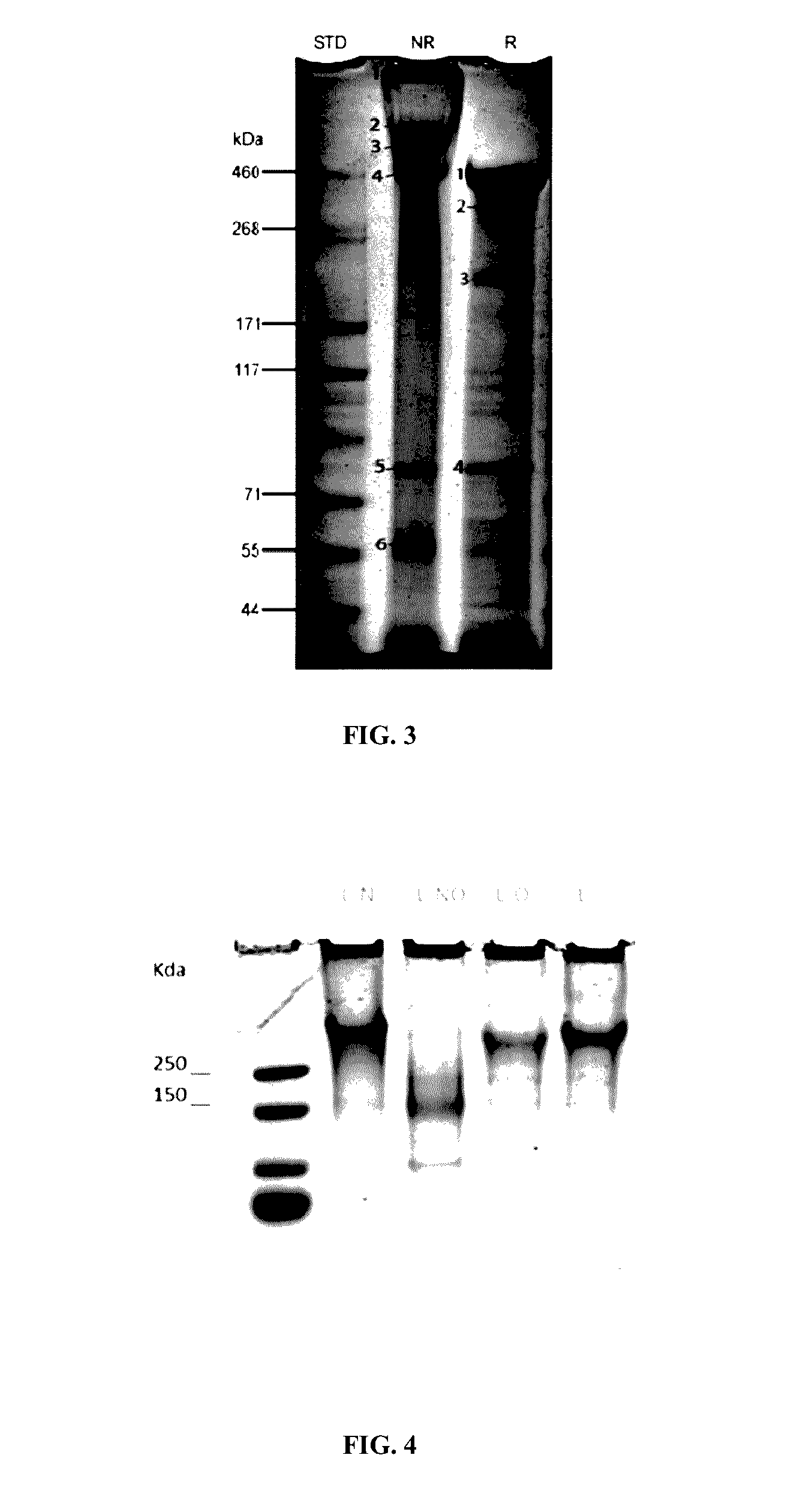
Disclosed are new recombinant isoforms of human-like lubricin or PRG4 glycoprotein having outstanding lubrication properties and a novel glycosylation pattern, and methods for their manufacture at high levels enabling commercial production.
Owner:LUBRIS
Hyaluronic acid-binding synthetic peptidoglycans, preparation, and methods of use
This invention pertains to the field of hyaluronic acid-binding synthetic peptidoglycans and methods of forming and using the same.
Owner:SYMIC OA CO
Lipidated glycosaminoglycan particles and their use in drug and gene delivery for diagnosis and therapy
InactiveUS20090155178A1Easy to manageAntibacterial agentsOrganic active ingredientsCross-linkLipid formation
Lipidated glycosaminoglycan particles, prepared by reacting a glycosaminoglycan with at least one lipid to cross-link the carboxylic acid groups in the glycosaminoglycan with a primary amine in the lipid, are used to encapsulate drugs for use in the treatment of pathological conditions in an animal.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Transgenically produced decorin
InactiveUS20050160483A1Reduce and preventGood for healthPeptide/protein ingredientsAlbumin peptidesTransgeneDecorin
Owner:GTC BIOTHERAPEUTICS INC
Compositions and methods for altering elastogenesis
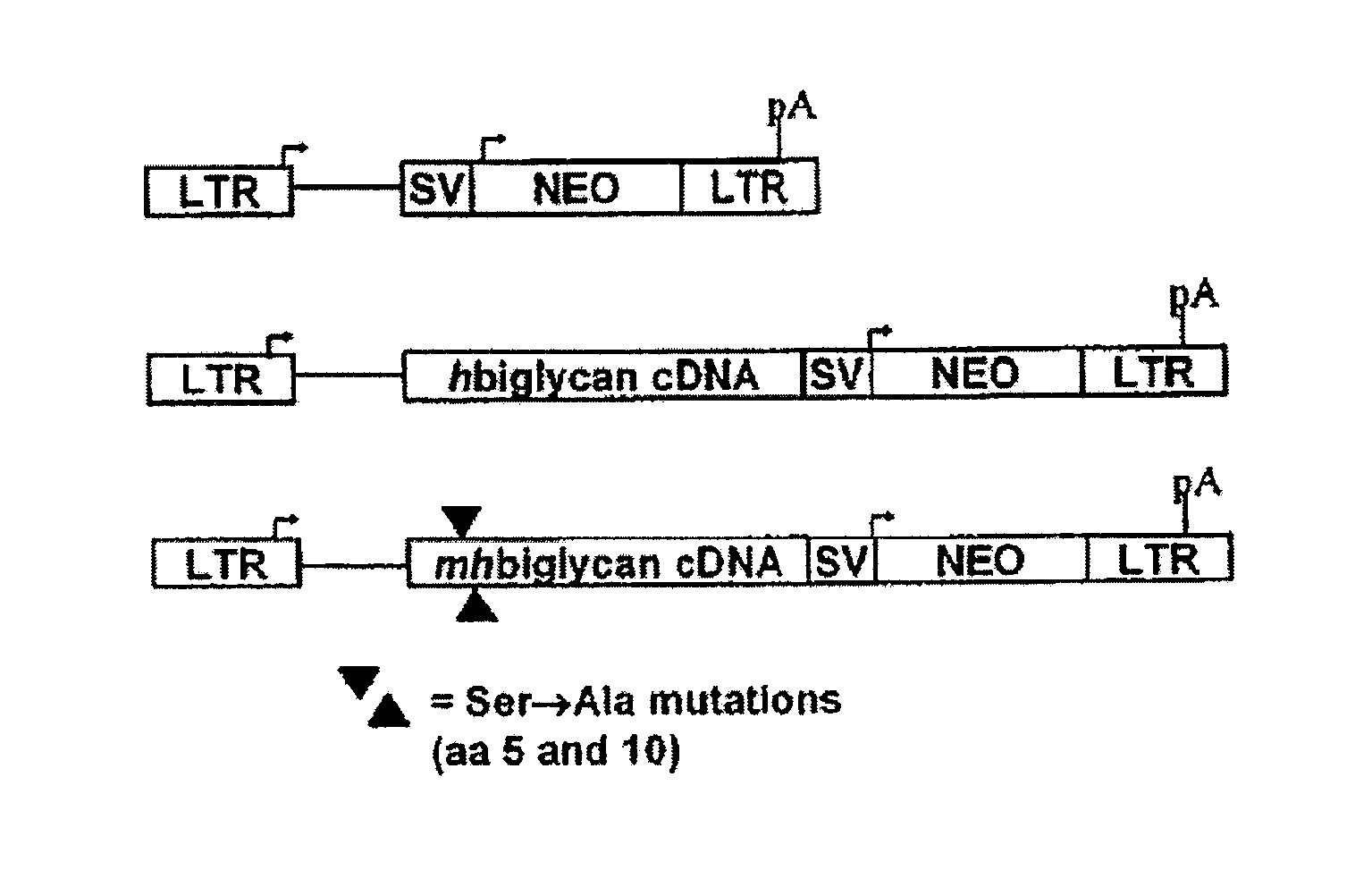
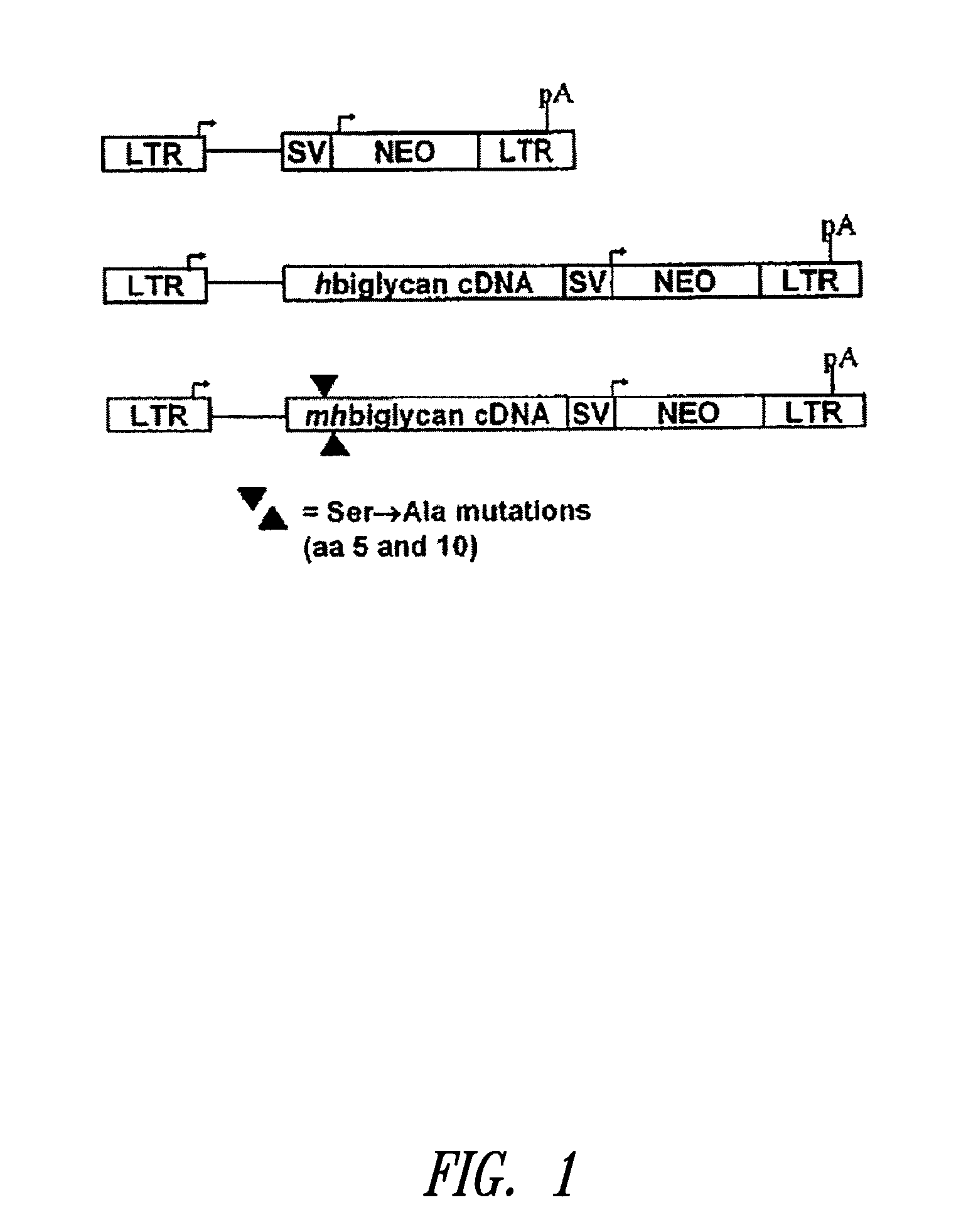
InactiveUS20100197563A1Promoting elastogenesisOrganic active ingredientsPeptide/protein ingredientsChondroitin sulphateBiglycan
Compositions and methods are provided for promoting elastin fiber formation (elastogenesis) in a cell, including methods that comprise contacting a cell that is capable of elastogenesis with (i) a mutated biglycan polypeptide that lacks chondroitin sulphate proteoglycan chains, (ii) a versican V3 isoform polypeptide that lacks most or all of the polypeptide regions encoded by one or more of exons 4, 5 or 6 or by exons 9-10 or 11-13, and / or with (iii) metastatin.
Owner:BENAROYA RES INST AT VIRGINIA MASON
Bioactive peptide for cell adhesion
InactiveUS7803905B2Sugar derivativesPeptide/protein ingredientsCell-Extracellular MatrixAdhesion process
The invention is directed to a polypeptide derived from domain IV of the extracellular matrix protein perlecan that can selectively adhere cells, nucleic acids encoding the inventive polypeptide, vectors comprising the nucleic acids, devices comprising a scaffold coated with the inventive polypeptide, and methods of adhering cells to a scaffold using the inventive polypeptide.
Owner:DELAWARE UNIV OF A DE
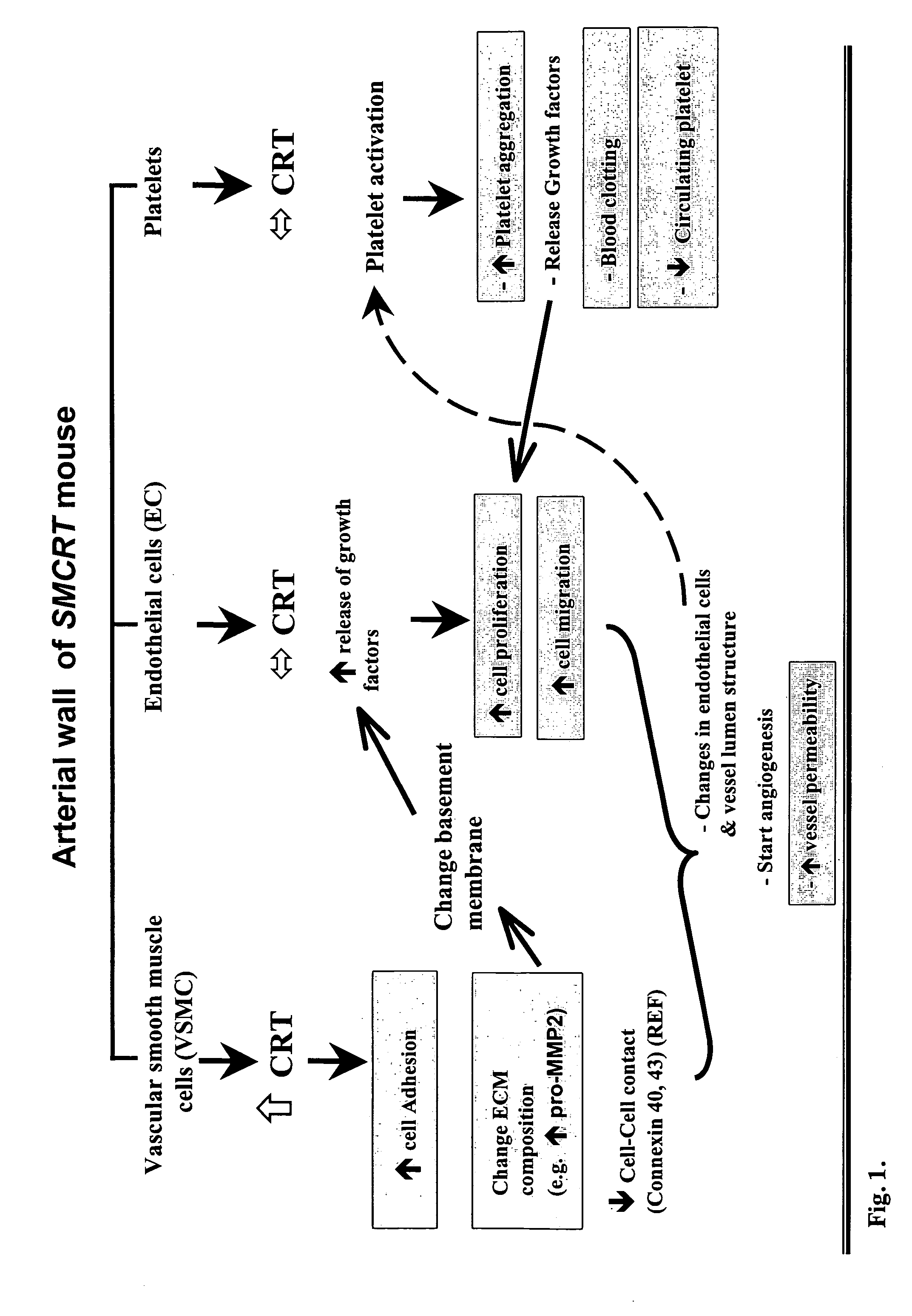
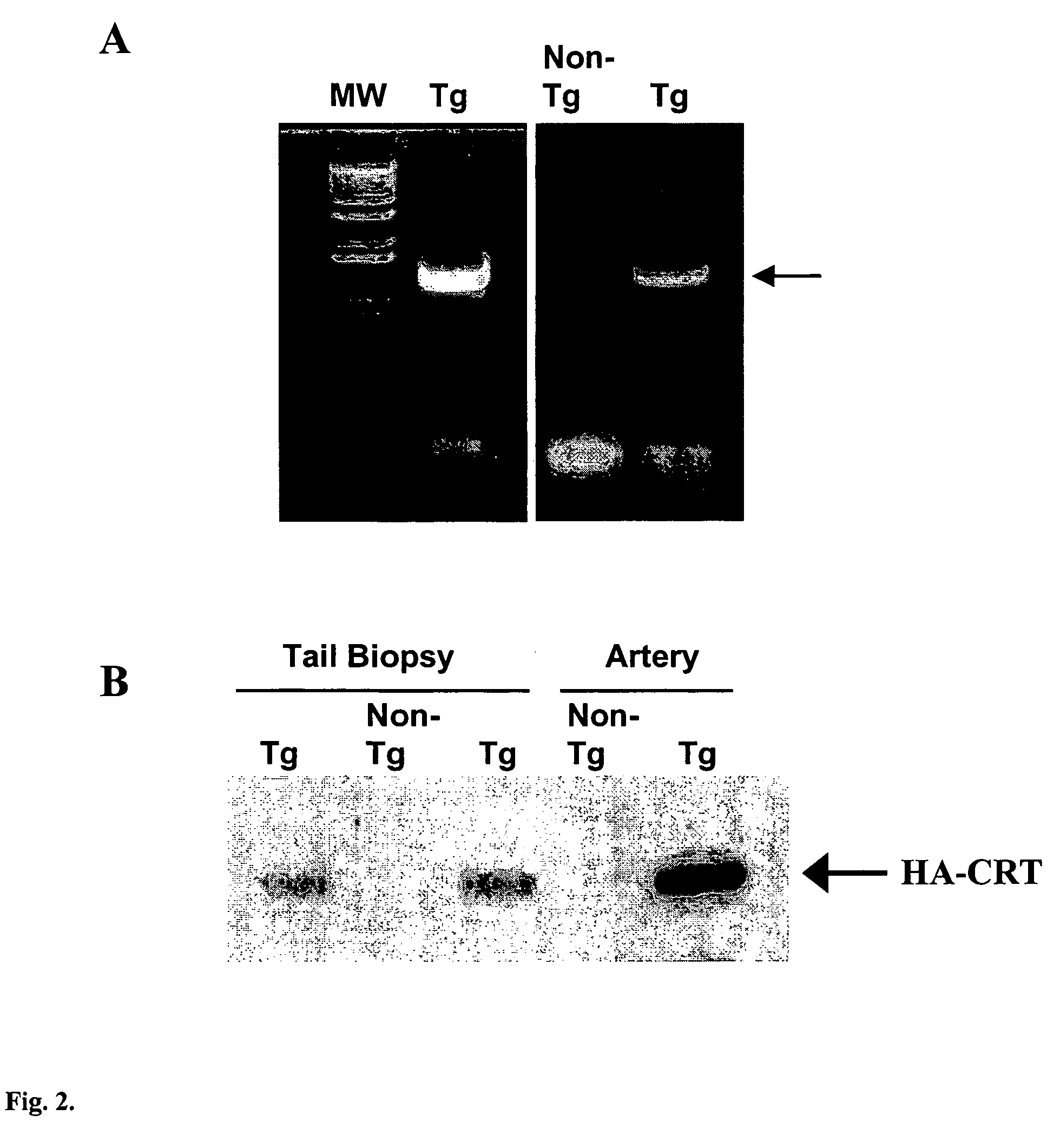
Transgenic mouse over-expressing calreticulin (CRT) in vascular smooth muscle cells
InactiveUS7186882B2Lower Level RequirementsReduce expressionVectorsSugar derivativesCalreticulinTranscription control
A transgenic mouse whose genome comprises a transcriptional control region operably linked to a cDNA encoding calreticulin (CRT) is described.
Owner:MESAELI NASRIN
Recombinant plasmid, recombinant malaria parasite and its application
ActiveCN106687592AConducive to long-term existenceFacilitated releaseTumor rejection antigen precursorsProtozoaBacteroidesMalaria
The present invention relates to the field of cellular immunotherapy for tumors and, more particularly, to a recombinant plasmid for the construction of recombinant malaria parasites and their use, wherein the recombinant plasmid is a tumorigenin-specific antigen gene inserted into the pL0017 plasmid, Recombinant malaria parasite, comprising the recombinant plasmid. Compared with plasmid DNA and RNA vector, the recombinant malaria parasite can be multiplied by the proliferation of Plasmodium, which is beneficial to the increase of the antigen in vivo. Compared with the defective virus and bacterial carrier, it survives in the red blood cells of the body longer, not short-term by the body's immune system to clear, long-term effective expression of exogenous tumor antigen, is conducive to long-term antigen and immune stimulation. The recombinant malaria parasite is capable of activating the high expression of Th1-related cytokines in vivo and increasing the proportion of CD8a + DCs in total CD11c + DCs and further activating specific cytotoxic T lymphocyte responses against tumor antigens, which is beneficial to the antitumor effect of the vaccine.
Owner:BLUE ELEGANT BIOTECH CO LTD
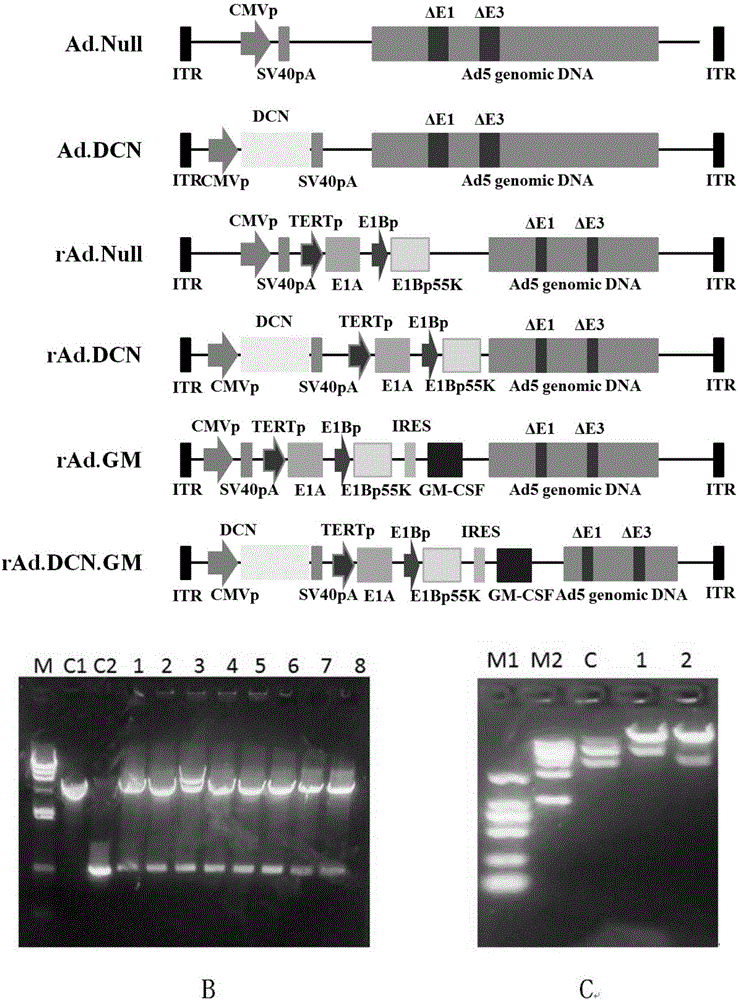
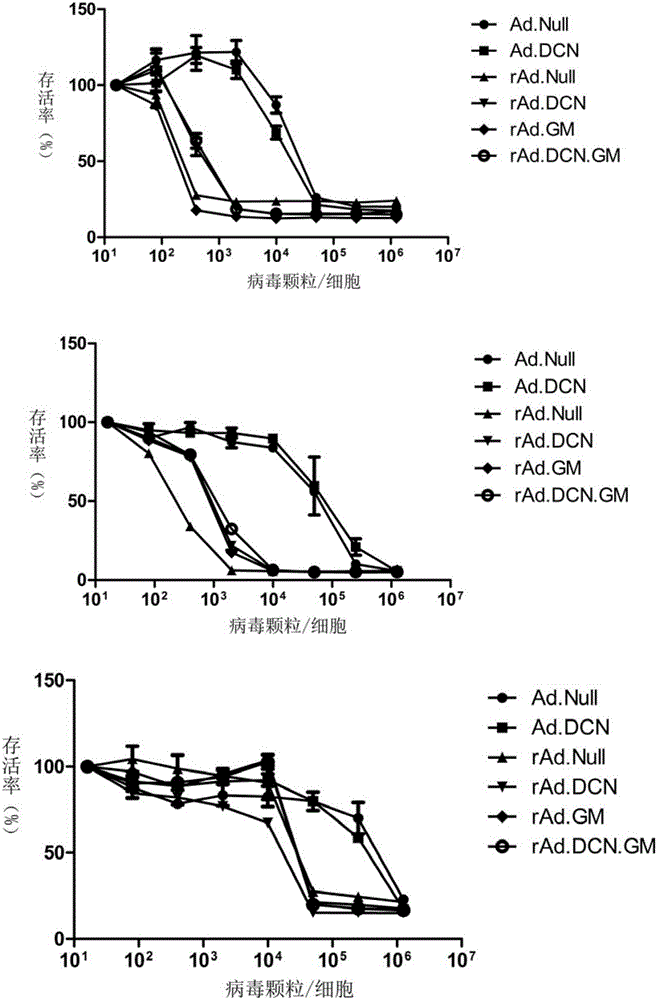
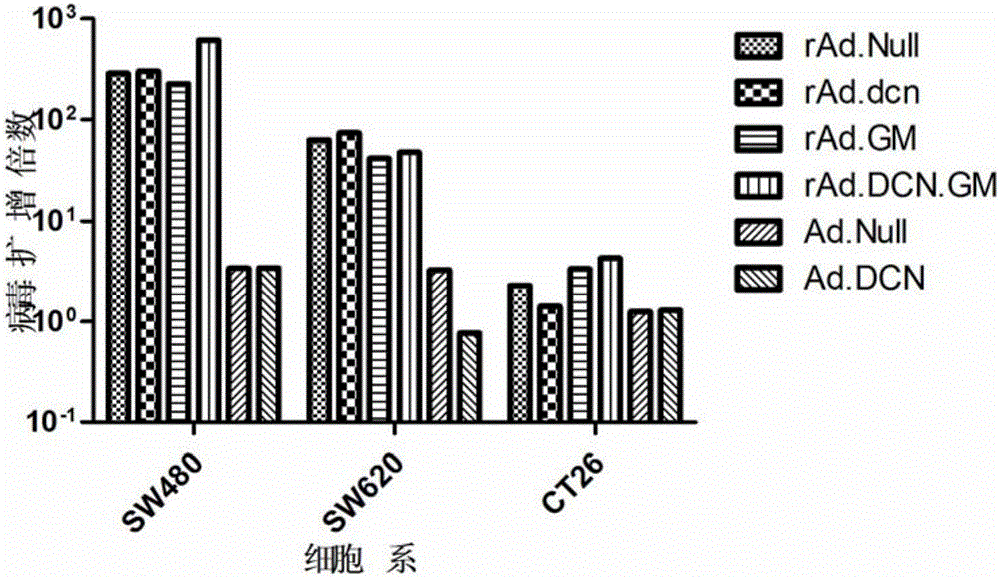
Oncolytic adenovirus, carrier for preparing same and application thereof
The invention relates to the field of biotechnology and gene treatment, and in particular to an oncolytic adenovirus, a carrier for preparing the same and an application thereof. The oncolytic adenovirus can coexpress decorin and GM-CSF proteins. The oncolytic adenovirus is applied to treating colon cancer, thereby providing an effectively immunogene treatment scheme for treatment of colon cancer.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA +1
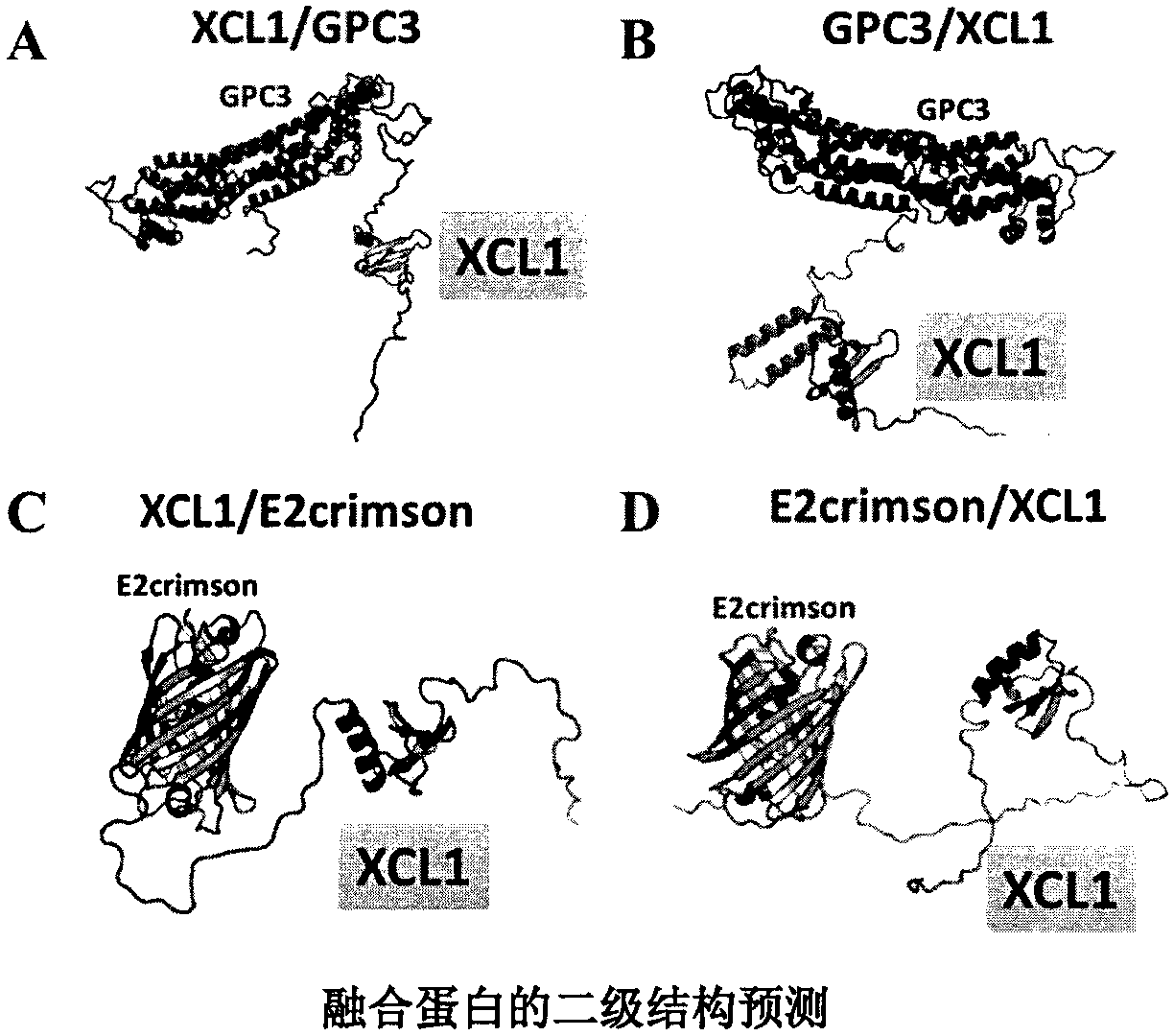
Hepatocellular carcinoma vaccine targeting secondary lymphoid tissues
ActiveCN109422816AOvercoming HLA restrictionChemokinesAntibody mimetics/scaffoldsHigh risk populationsCell membrane
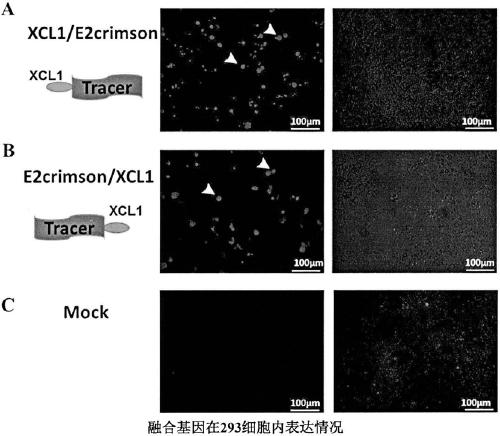
The invention provides a hepatocellular carcinoma vaccine targeting secondary lymphoid tissues. The vaccine comprises fusion protein comprising (a) phosphatidylinositol proteoglycan-3 (GPC3) and (b) lymphocyte chemotactic factor (XCL1) polypeptide of a specifically bonded chemokine receptor 1 (XCR1), nucleic acid encoding the fusion protein and vectors of the nucleic acid, wherein (a) is connectedwith (b) through a linker, and (a) lacks amino acid residues with a cell membrane anchoring effect. The vaccine comprises the fusion protein, the nucleic acid and / or the vectors, and application of the fusion protein, the nucleic acid and / or the expression vectors in prevention and treatment of liver cancers is provided. The vaccine has the effect of intervention of generation and development ofliver cancers, and can be applied to intervention of high-risk population of liver cancer, and for example, the vaccine can be applied to intervention of the generation progress of hepatitis B-relatedpatients to liver cancers.
Owner:NEWISH TECH (BEIJING) CO LTD +1
Vectors and yeast strains for protein production
InactiveUS20100311122A1High expressionReduce the amount of solutionFungiNucleic acid vectorATPaseMannosyltransferase
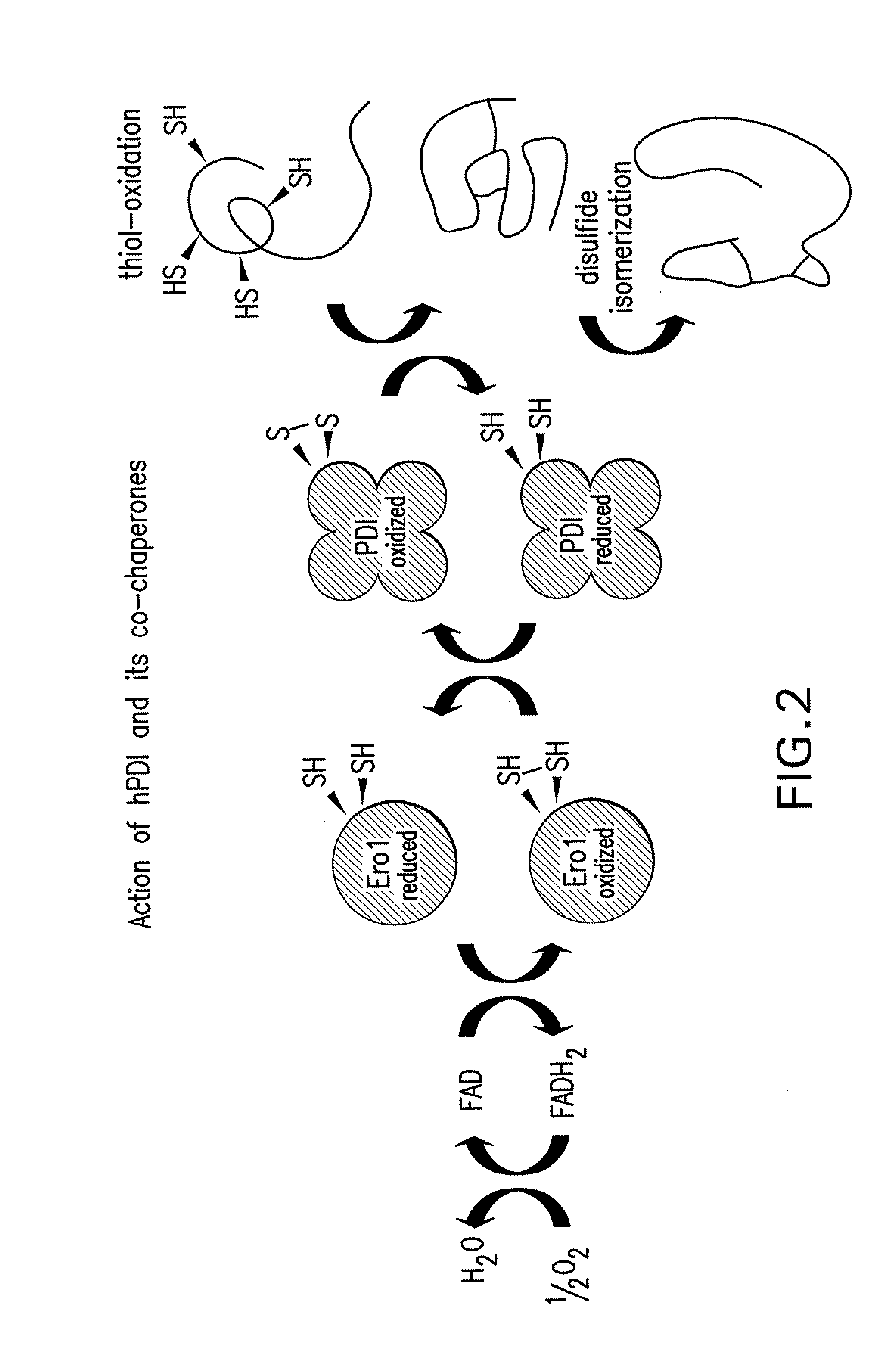
Lower eukaryote host cells in which the function of at least one endogenous gene encoding a chaperone protein, such as a Protein Disulphide Isomerase (PDI), has been reduced or eliminated and at least one mammalian homolog of the chaperone protein is expressed are described. In particular aspects, the host cells further include a deletion or disruption of one or more O-protein mannosyltransferase genes, and / or overexpression of an endogenous or exogenous Ca2+ ATPase. These host cells are useful for producing recombinant glycoproteins in large amounts and for producing recombinant glycoproteins that have reduced O-glycosylation.
Owner:GLYCOFI
Method for removing genomically unstable ips cells and synthetic peptide used therefor
ActiveUS20160252523A1Highly efficient and highly reliable evaluationGood effectPolypeptide with localisation/targeting motifMicrobiological testing/measurementGenomic StabilityCalreticulin
The present invention provides a method that allows highly efficient and highly reliable evaluation of genomic stability of pluripotent stem cells, a method for removing pluripotent stem cells that have been identified as genomically unstable by the evaluation method from a culture of pluripotent stem cells to be evaluated, and a synthetic peptide that can be used for the methods. The methods provided by the present invention include preparing a culture of pluripotent stem cells of interest and analyzing an expression level of calreticulin for the pluripotent stem cells in the culture followed by identifying genomic stability or genomic instability of the stem cells on the basis of the expression level of calreticulin.
Owner:TOAGOSEI CO LTD +1
Methods for Stabilizing Corneal Tissue
Methods of stabilizing collagen fibrils in a cornea are disclosed. The stabilization may be effected by treating the cornea with a protein that crosslinks collagen fibrils, such as decorin. The stablization methods include treatment of corneas before, during, or after a surgical procedure, treatment of keratectasia, and treatment of keratoconus.
Owner:THOMPSON VANCE +2
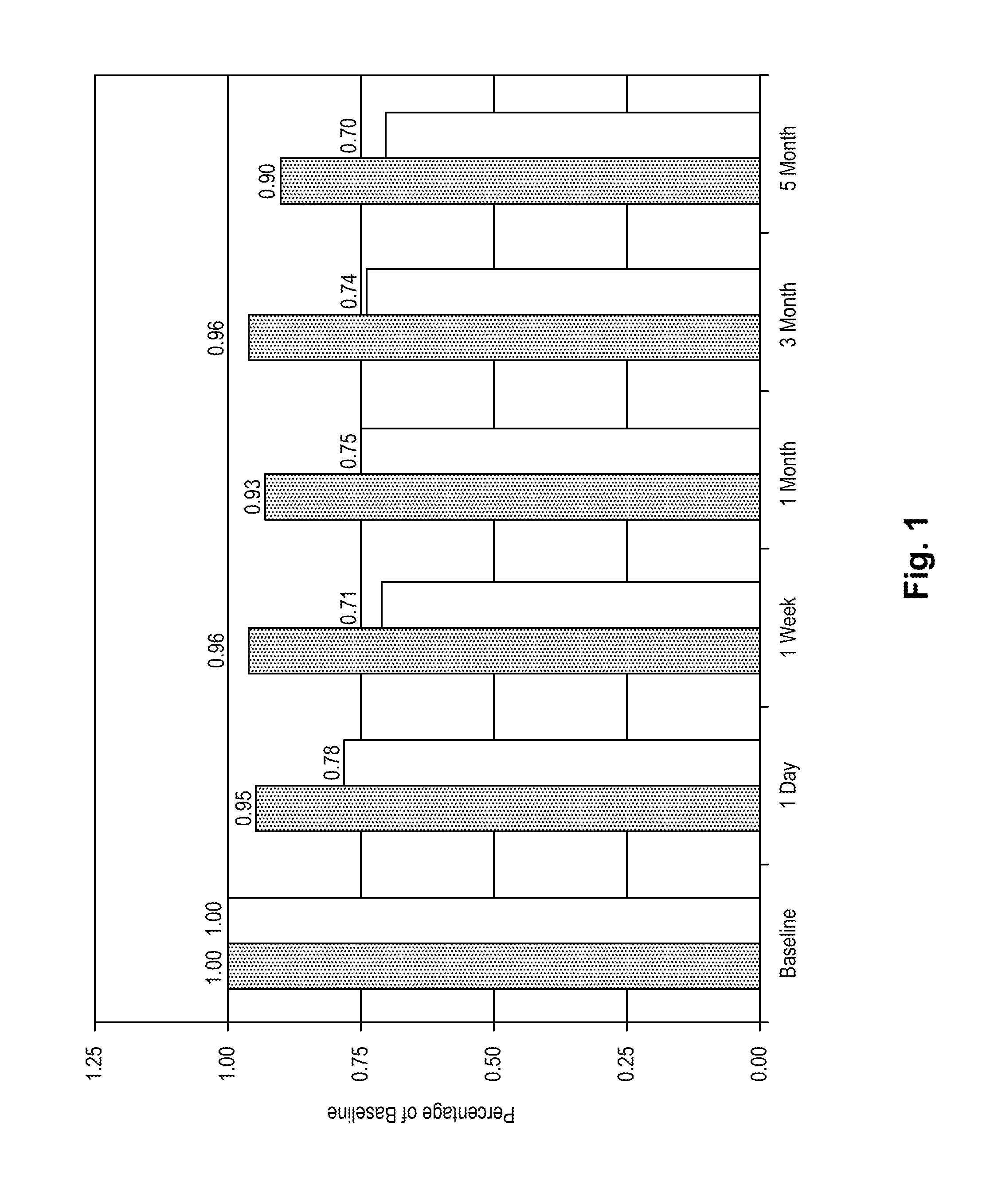
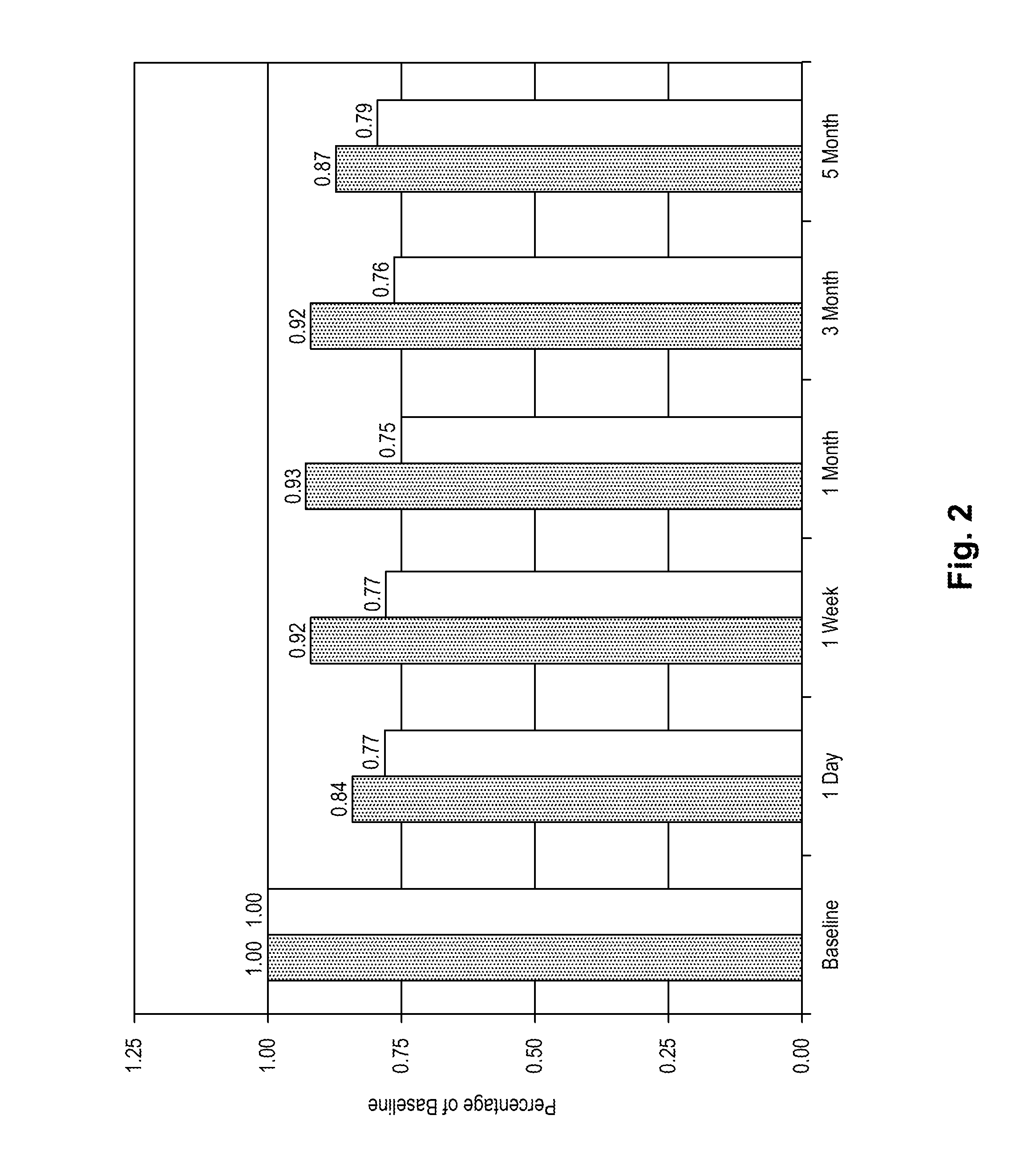
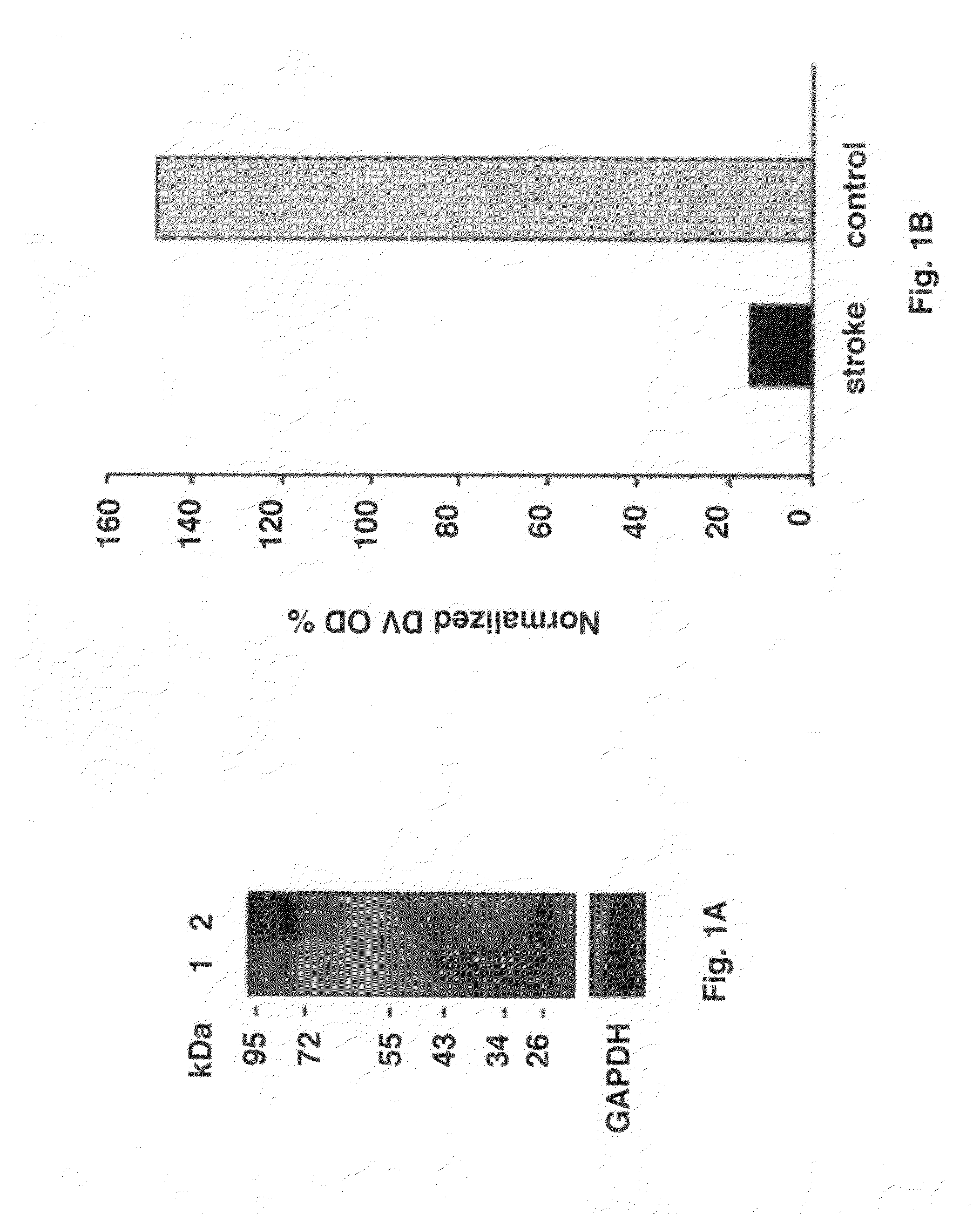
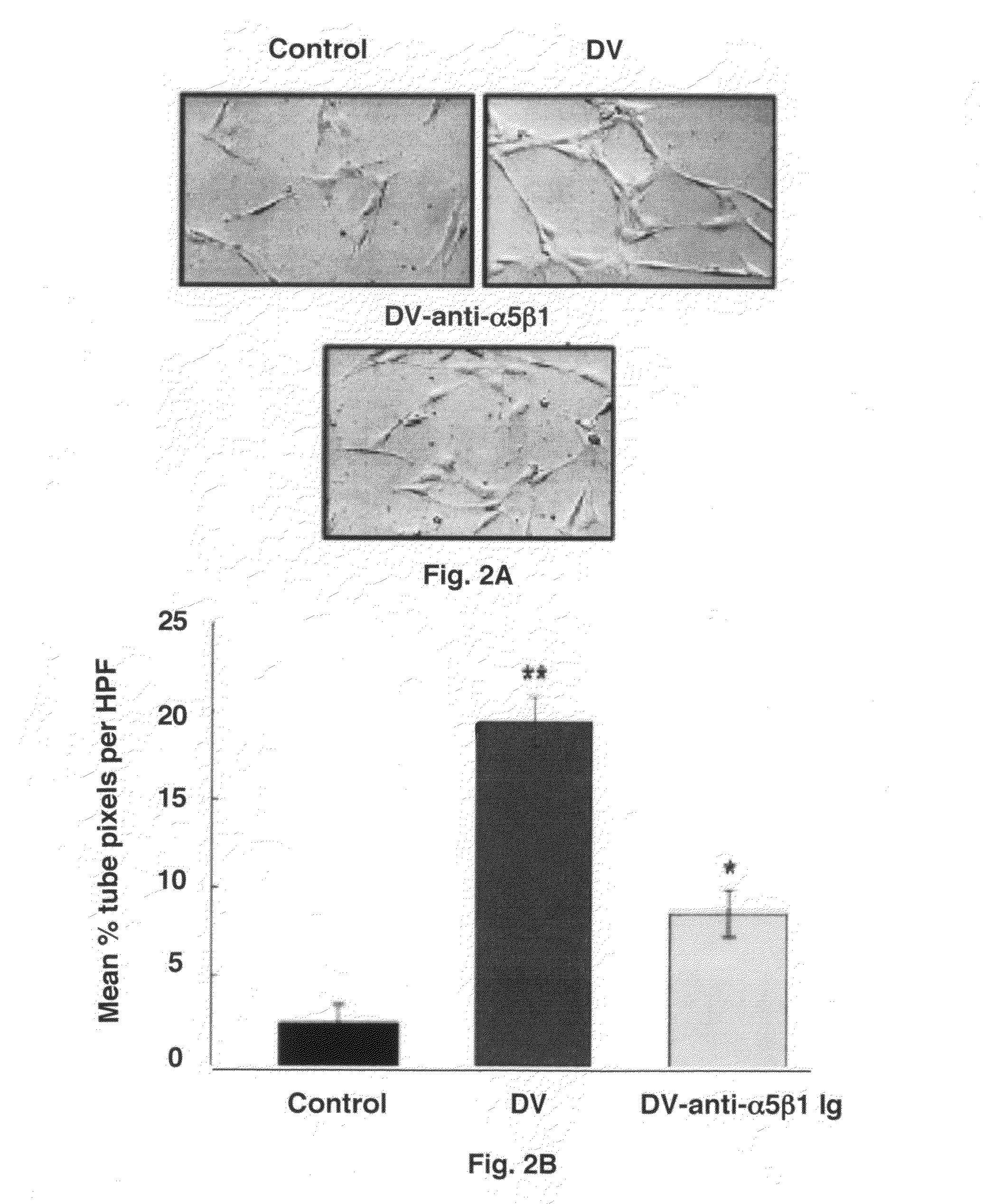
Stroke-generated angiogenesis enhancers and uses thereof
ActiveUS20100168025A1Enhances brain angiogenesisPromote recoveryBiocideOrganic active ingredientsAngiogenesis growth factorRisk stroke
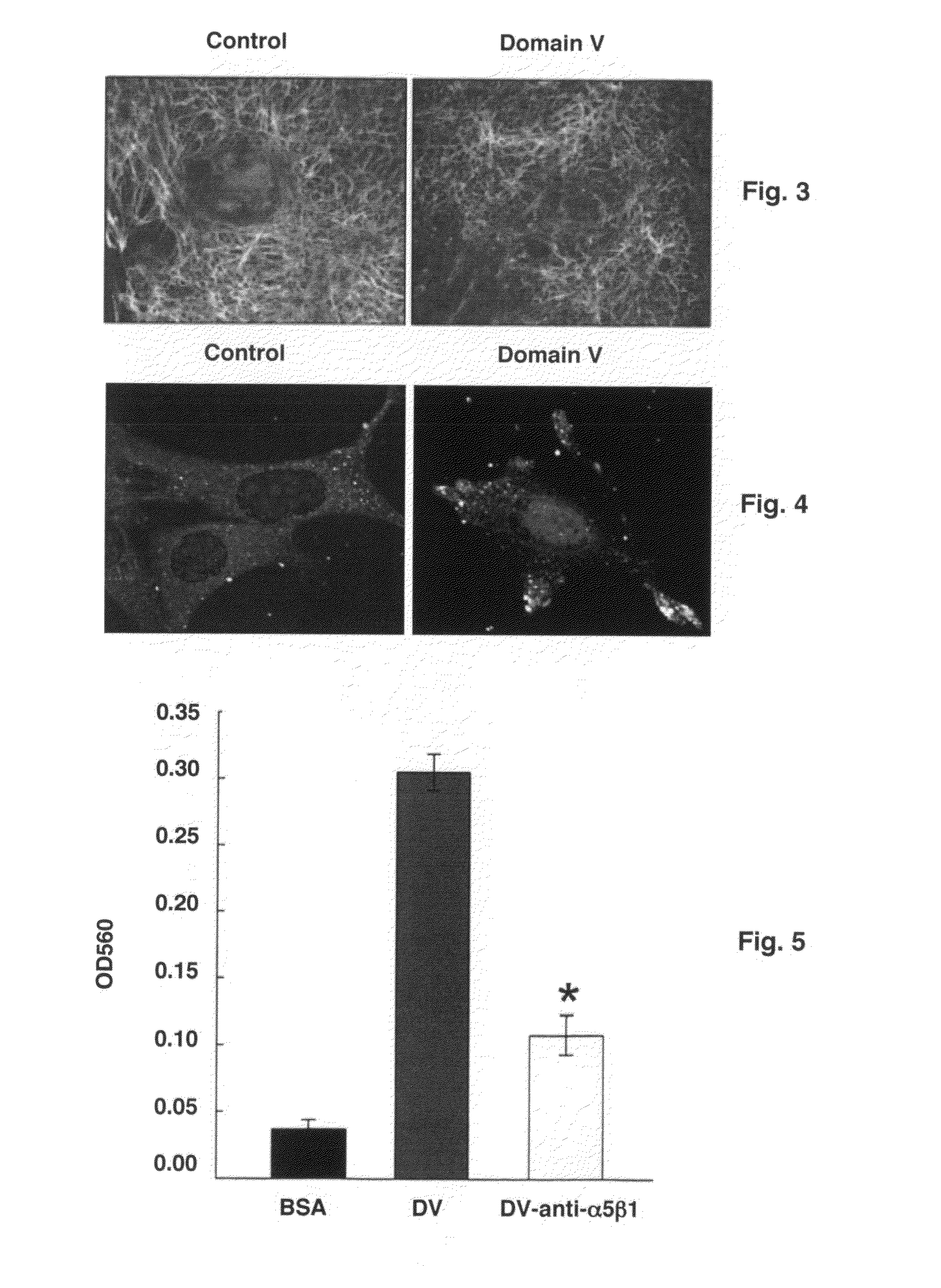
The present invention is drawn to methods of stimulating or enhancing angiogenesis in a patient comprising, administering to said patient a therapeutically effective amount of an endorepellin protein, wherein said endorepellin protein has an amino acid sequence of domain V of perlecan or fragments or derivatives, analogs thereof; and stimulating or enhancing generation of blood vessels. The present invention is drawn to compositions for enhancing angiogenesis.
Owner:TEXAS A&M UNIVERSITY
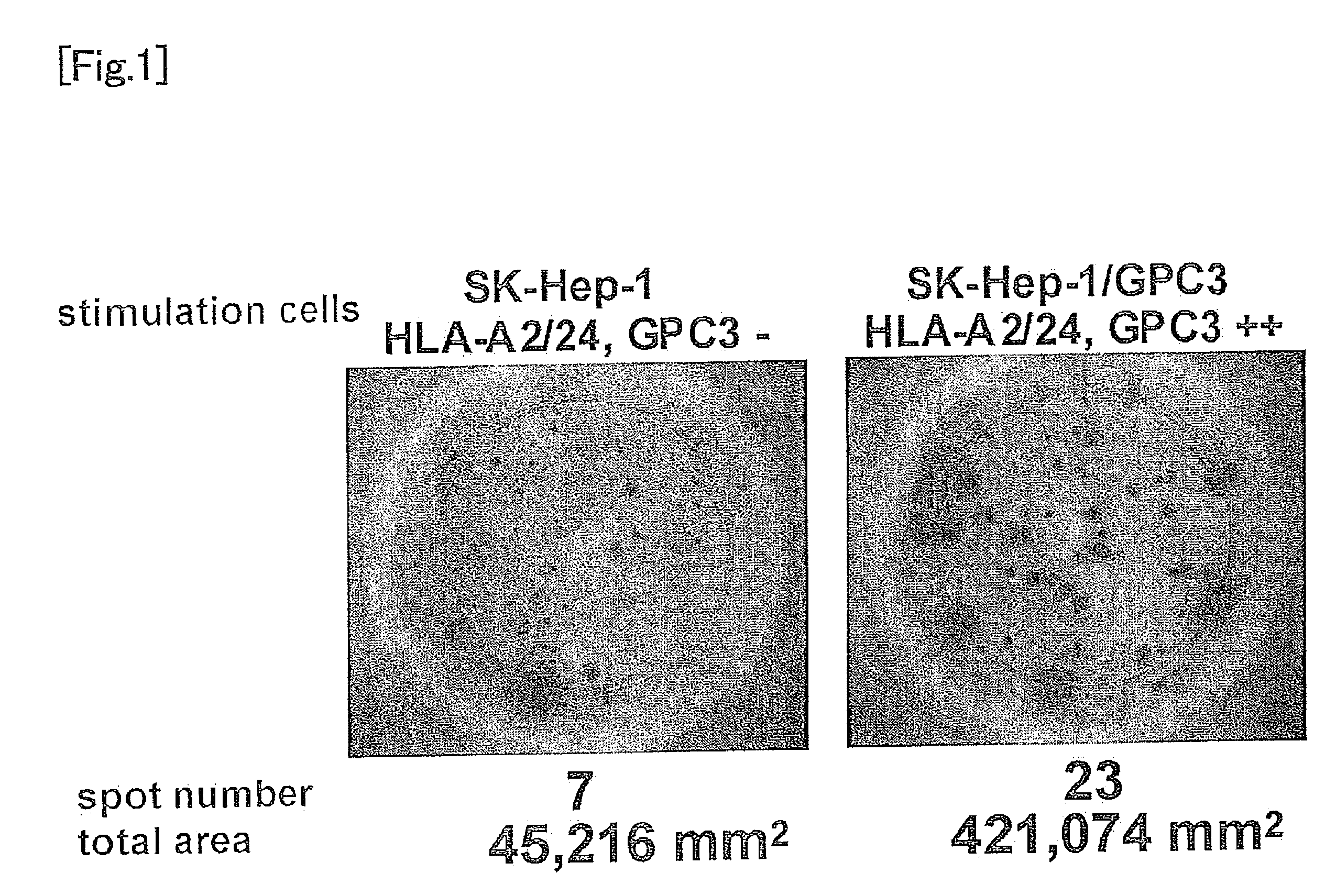
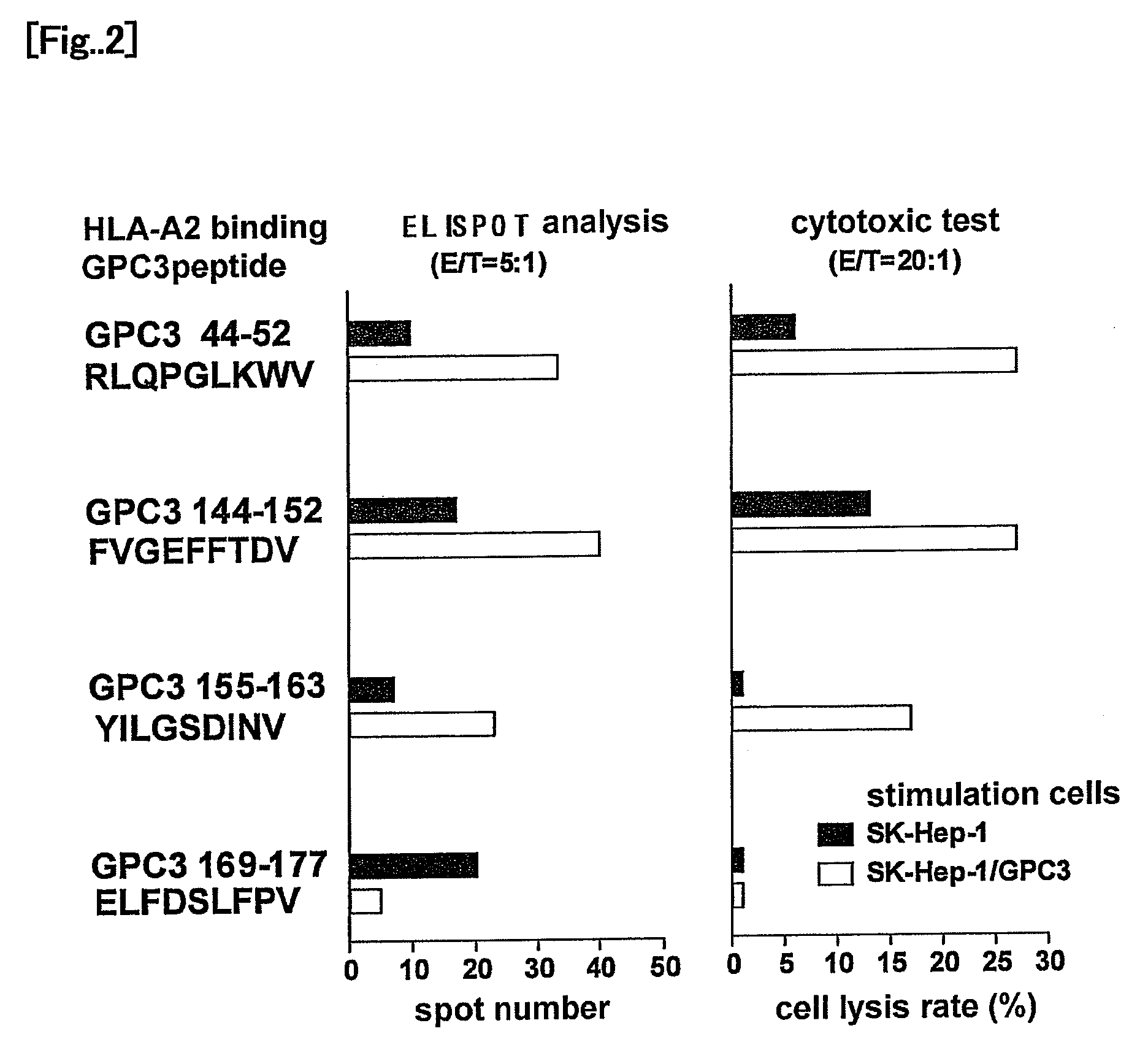
Glypican-3 (GPC3)-derived tumor rejection antigenic peptides useful for HLA-A2-positive patients and pharmaceutical comprising the same
ActiveUS8053556B2Cancer can be prevented and/or treatedEasy to useOrganic active ingredientsTumor rejection antigen precursorsImmunotherapyNatural killer T cell
It is an object of the present invention to identify a glypican-3-derived peptide which can bind to HLA-A2 and activate human killer T cells, so as to provide a means for carrying out an immunotherapy which is able to target approximately 40% of Japanese patients suffering from several types of cancers, which express GPC3 at a high level. The present invention provides a peptide of any of the following (A) or (B):(A) a peptide, which has the amino acid sequence as shown in any one of SEQ ID NOS: 1 to 3; or(B) a peptide, which has an amino acid sequence comprising a substitution or addition of one or two amino acids with respect to the amino acid sequence as shown in any one of SEQ ID NOS: 1 to 3, and which has ability to induce killer T cells.
Owner:ONCOTHERAPY SCI INC
PRO347 nucleic acids
The present invention is directed to novel polypeptides having homology to cysteine-rich secretory protein-3 and to nucleic acid molecules encoding those polypeptides. Also provided herein are vectors and host cell comprising those nucleic acid sequences, chimeric polypeptide molecules comprising the polypeptides of the present invention fused to heterologous polypeptide sequences, antibodies which bind to the polypeptides of the present invention and to methods for producing the polypeptides of the present invention.
Owner:GENENTECH INC
Methods for promoting elastogenesis and elastin fiber formation by increasing tropoelastin expression
InactiveUS8367619B2Promoting elastogenesisPeptide/protein ingredientsSkeletal disorderChondroitin sulphateExon
Compositions and methods are provided for promoting elastin fiber formation (elastogenesis) in a cell, including methods that comprise contacting a cell that is capable of elastogenesis with (i) a mutated biglycan polypeptide that lacks chondroitin sulphate proteoglycan chains, (ii) a versican V3 isoform polypeptide that lacks most or all of the polypeptide regions encoded by one or more of exons 4, 5 or 6 or by exons 9-10 or 11-13, and / or with (iii) metastatin.
Owner:BENAROYA RES INST AT VIRGINIA MASON
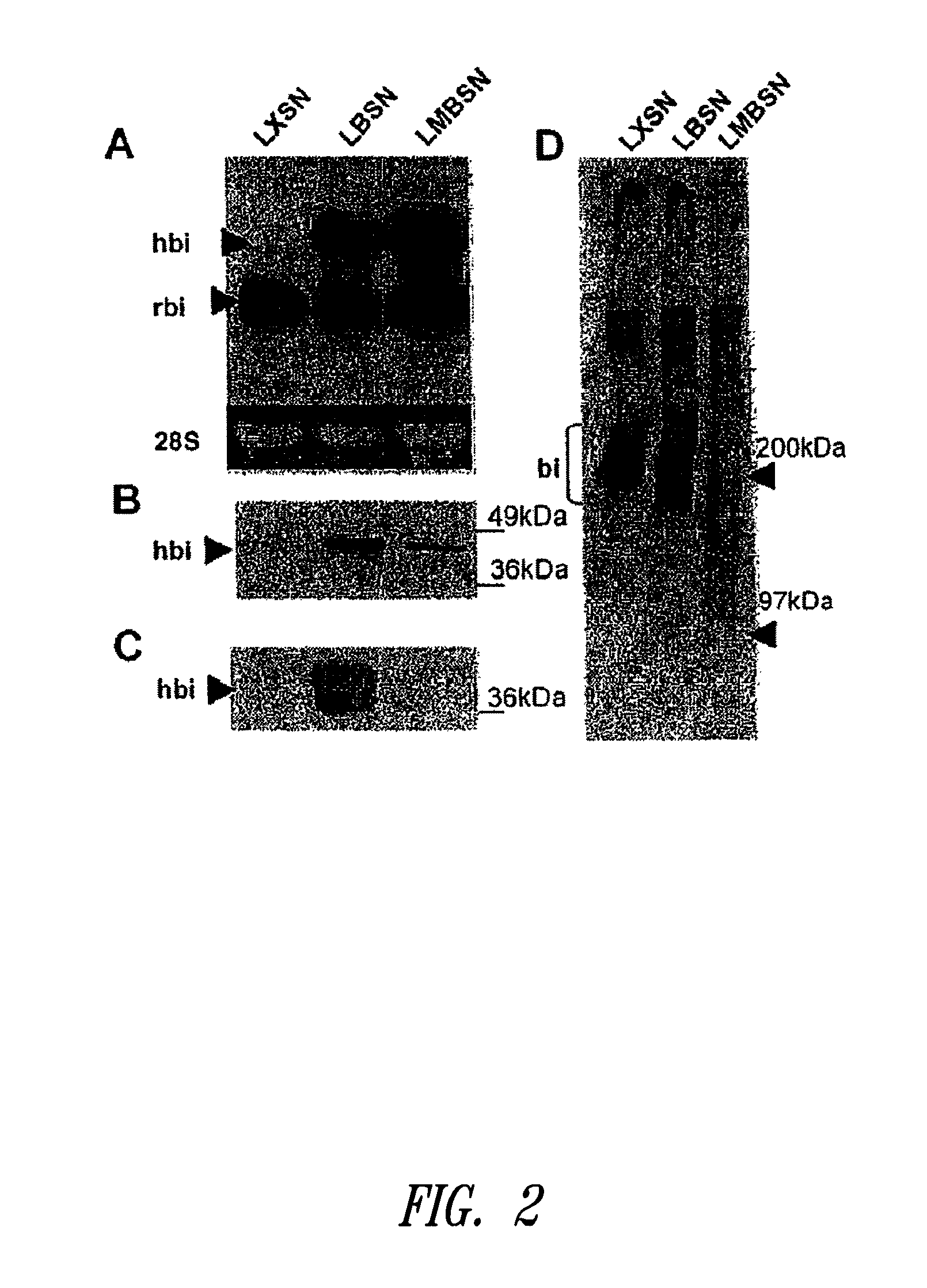
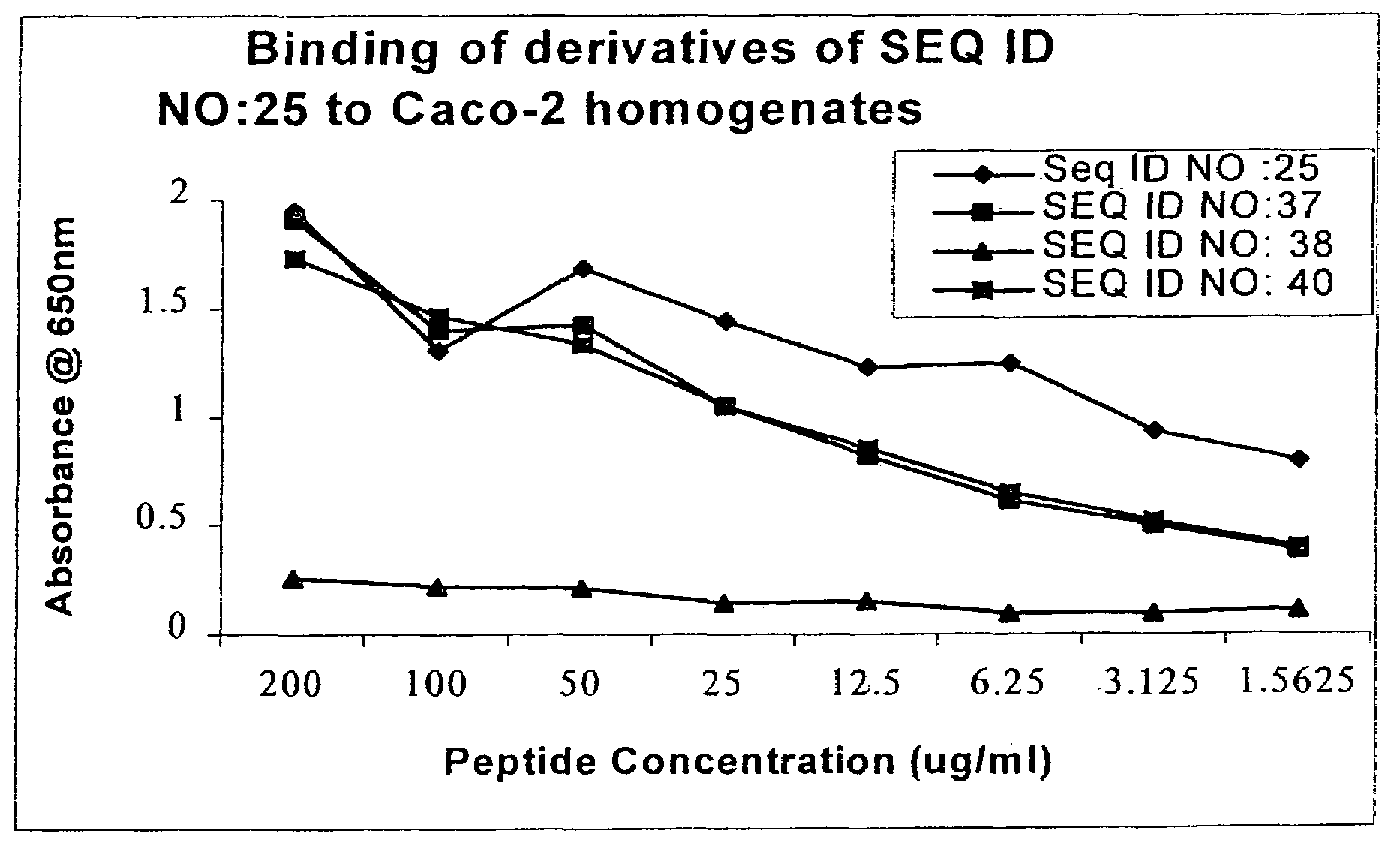
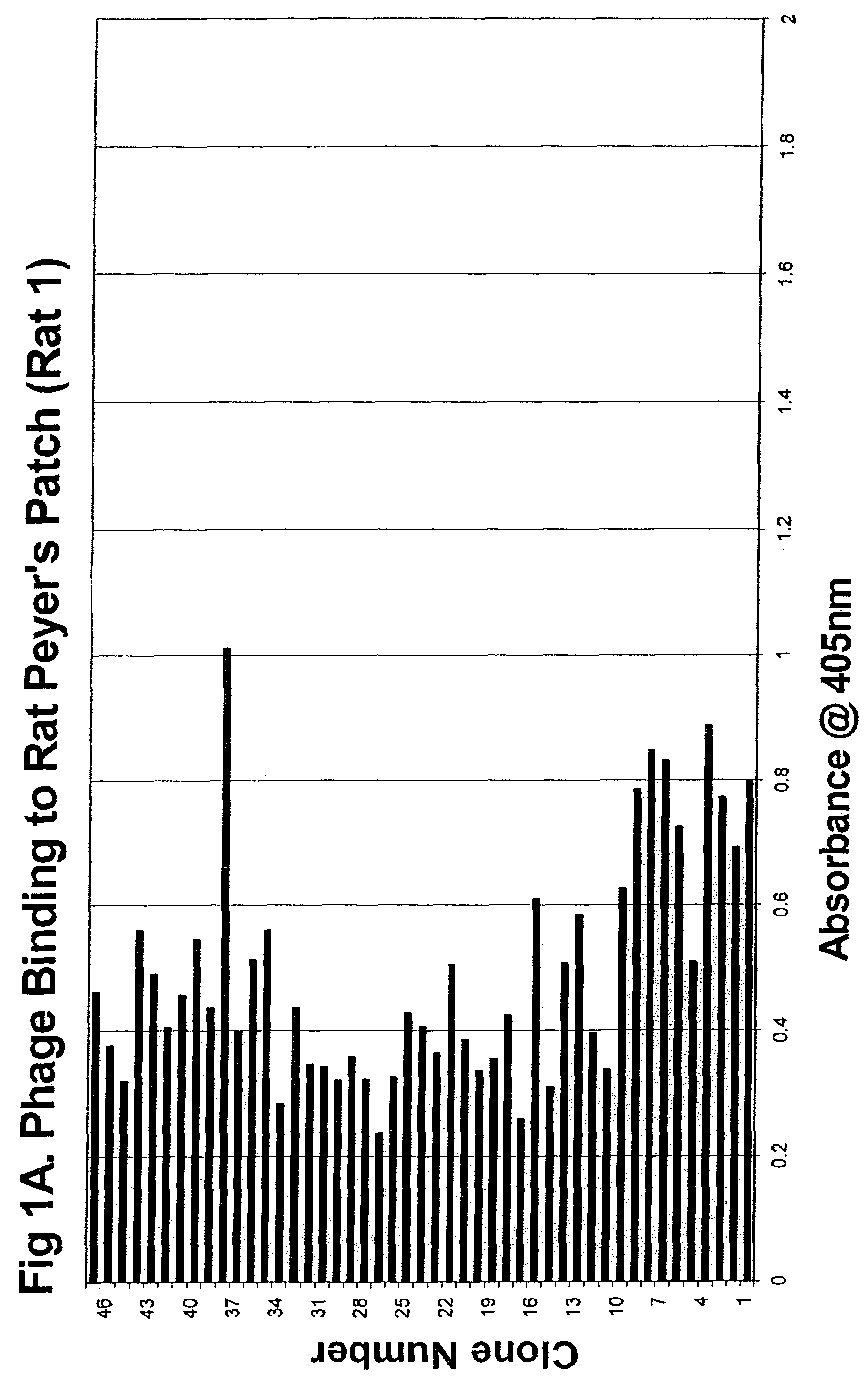
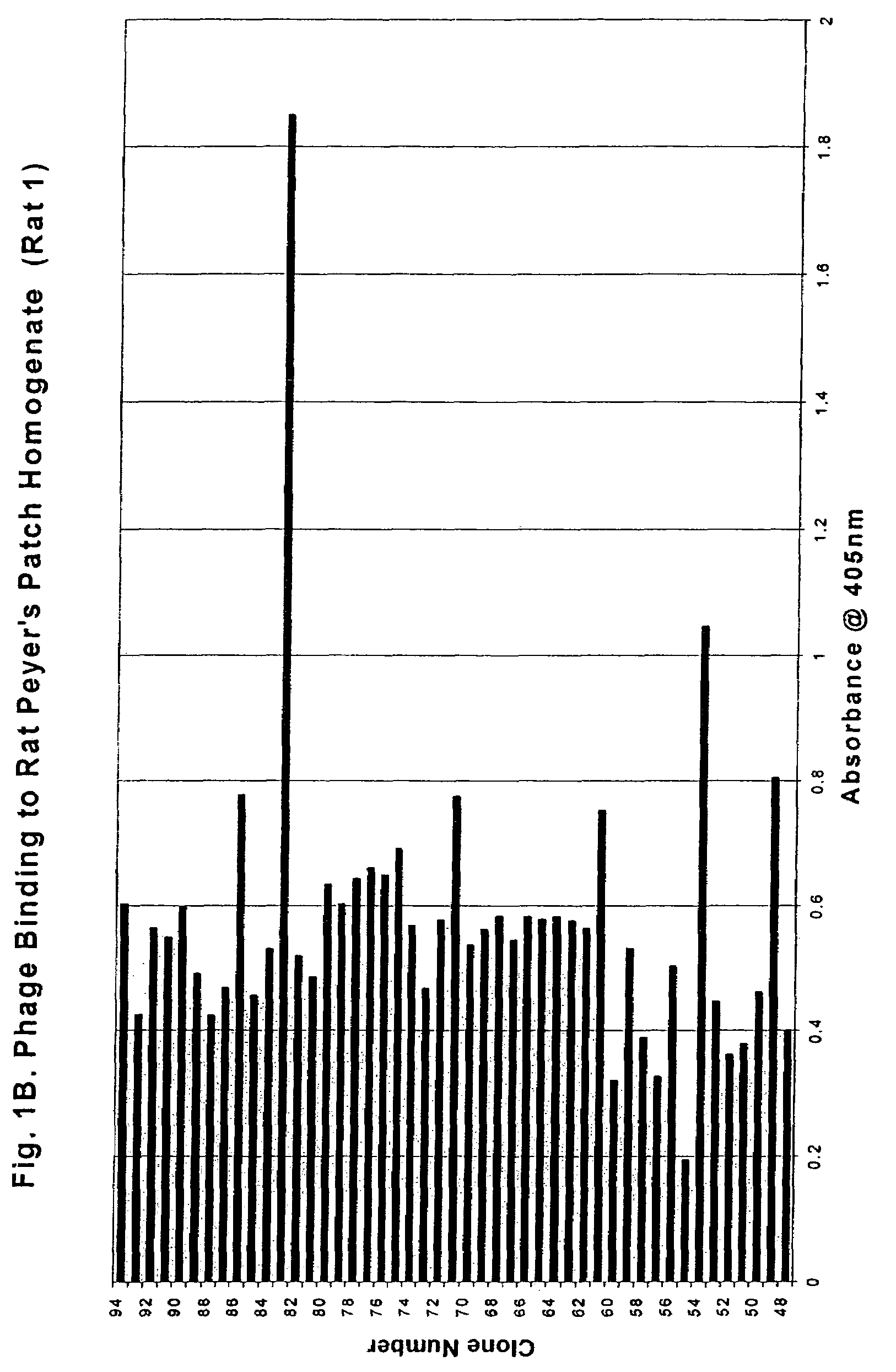
Peyer's patch and/or M-cell targeting ligands
Purified synthetic polypeptide ligands for targeting pharmaceutical agents and carriers comprising such agents to intestinal epithelial tissue, especially Peyer's patch and / or M-Cell tissue. Also methods of using the ligands.
Owner:NOVO NORDISK AS
Genes and proteins associated with angiogenesis and uses thereof
Disclosed is a panel of biomarkers associated with angiogenesis, and the use of such biomarkers (genes, proteins, homologues and analogs thereof) to regulate angiogenesis. Methods for identifying compounds useful for regulating angiogenesis and conditions related thereto are disclosed.
Owner:NAT JEWISH HEATH
Plants having enhanced yield-related traits and method for making same
The present application is directed to plants with improved properties where the expression of calreticulin, BET1-like polypeptides, DUS1-like polypeptides, ES43-like polypeptides, H0N5-like polypeptides or GSA1 polypeptides are modified. The improved property can be enhanced yield.
Owner:BASF PLANT SCI GMBH
Process for Producing Proteoglycan
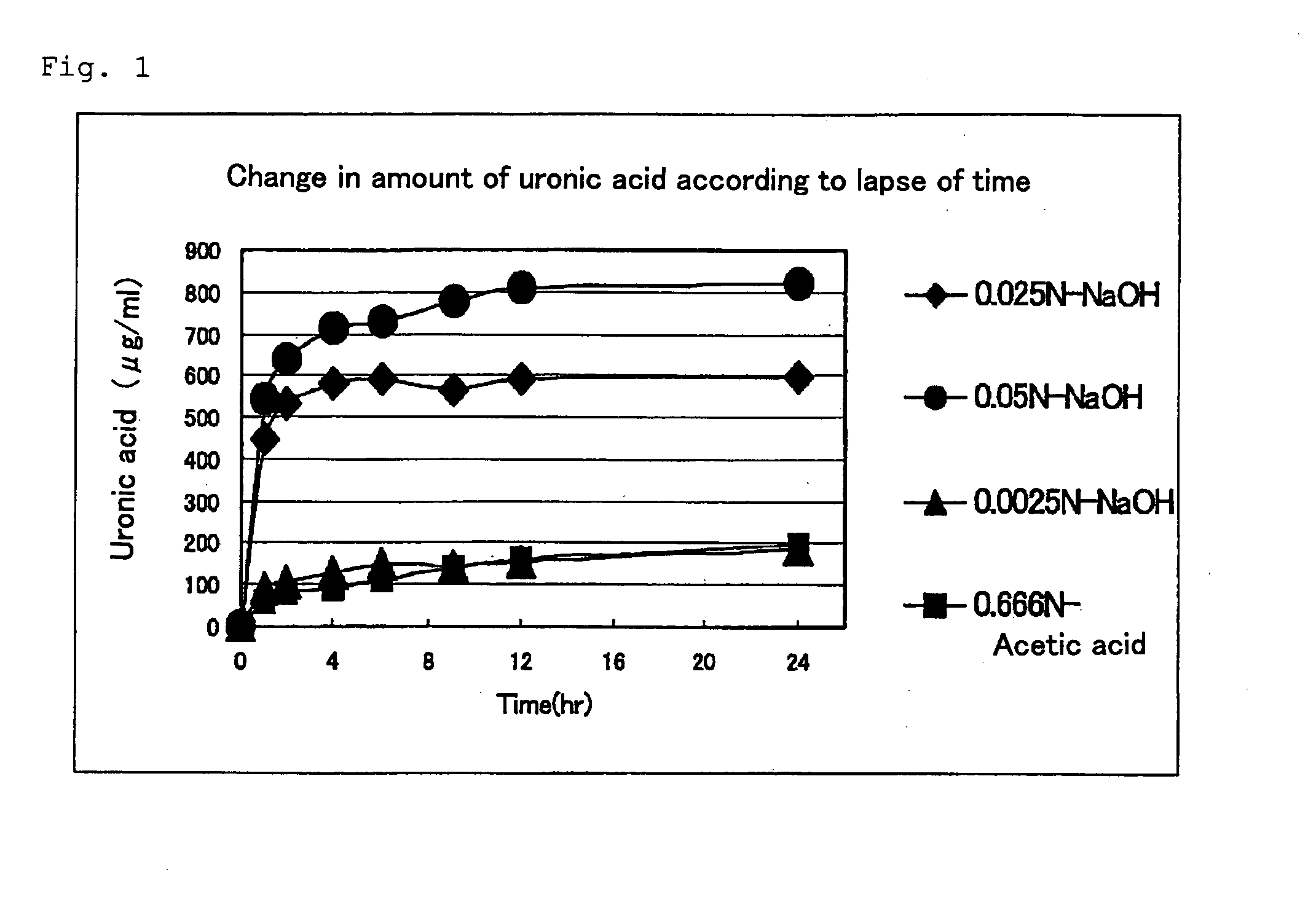
ActiveUS20100234580A1Reduction of proteoglycan production costEfficient use ofSkeletal disorderDepsipeptidesNatural resourcePolysaccharide
An efficient low-cost method of proteoglycan recovery from natural resources. There is provided a process for producing proteoglycan, comprising the steps of immersing a biological sample containing proteoglycan in an alkali solution of 0.0025 to 0.1 N and recovering the solution after the immersion. As compared with the conventional extraction method, proteoglycan can be recovered in unaltered undecomposed form easily within a short period of time, thereby attaining substantial reduction of proteoglycan production cost. Further, proteoglycan highly useful in industry can be recovered from wasted portions of fin, feather, mammal, etc. having mainly been discarded, thereby contributing toward effective utilization of industrial waste and reduction of the volume of industrial waste per se.
Owner:BIOMATEC JAPAN +1
Secreted and transmembrane polypeptides and nucleic acids encoding the same
The present invention is directed to secreted and transmembrane polypeptides and to nucleic acid molecules encoding those polypeptides. Also provided herein are vectors and host cells comprising those nucleic acid sequences, chimeric polypeptide molecules comprising the polypeptides of the present invention fused to heterologous polypeptide sequences, antibodies which bind to the polypeptides of the present invention and to methods for producing the polypeptides of the present invention.
Owner:GENENTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com