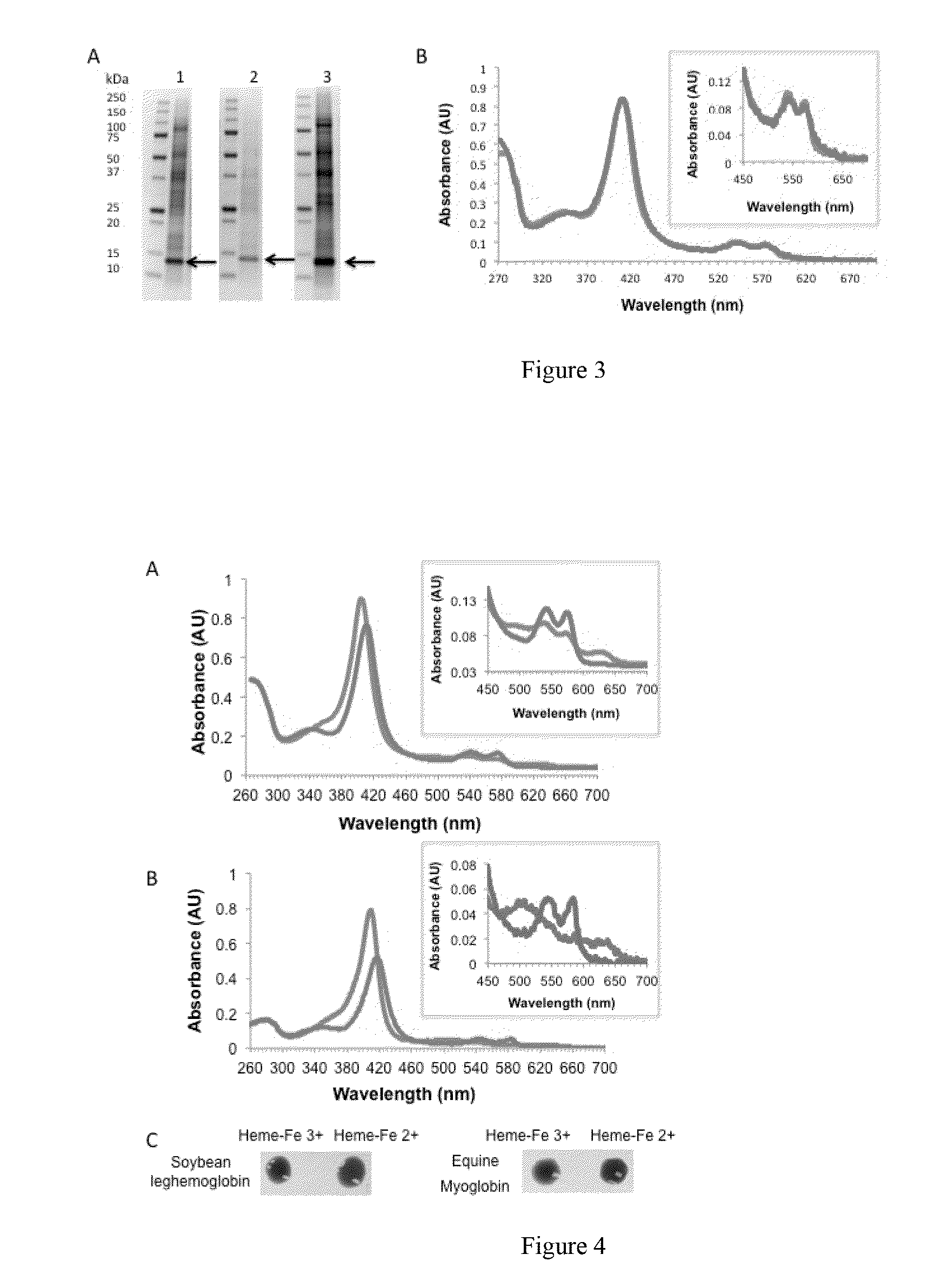
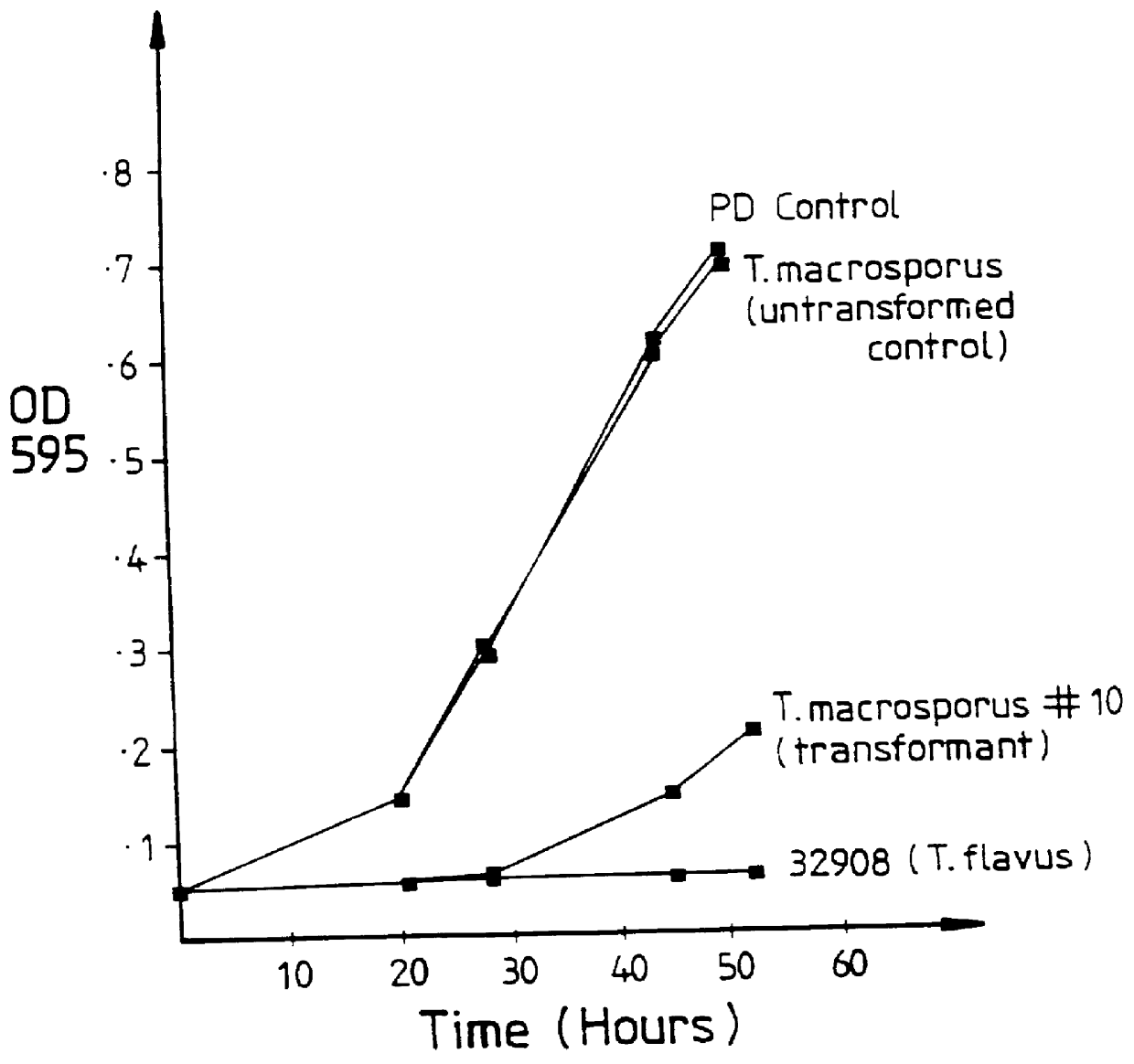
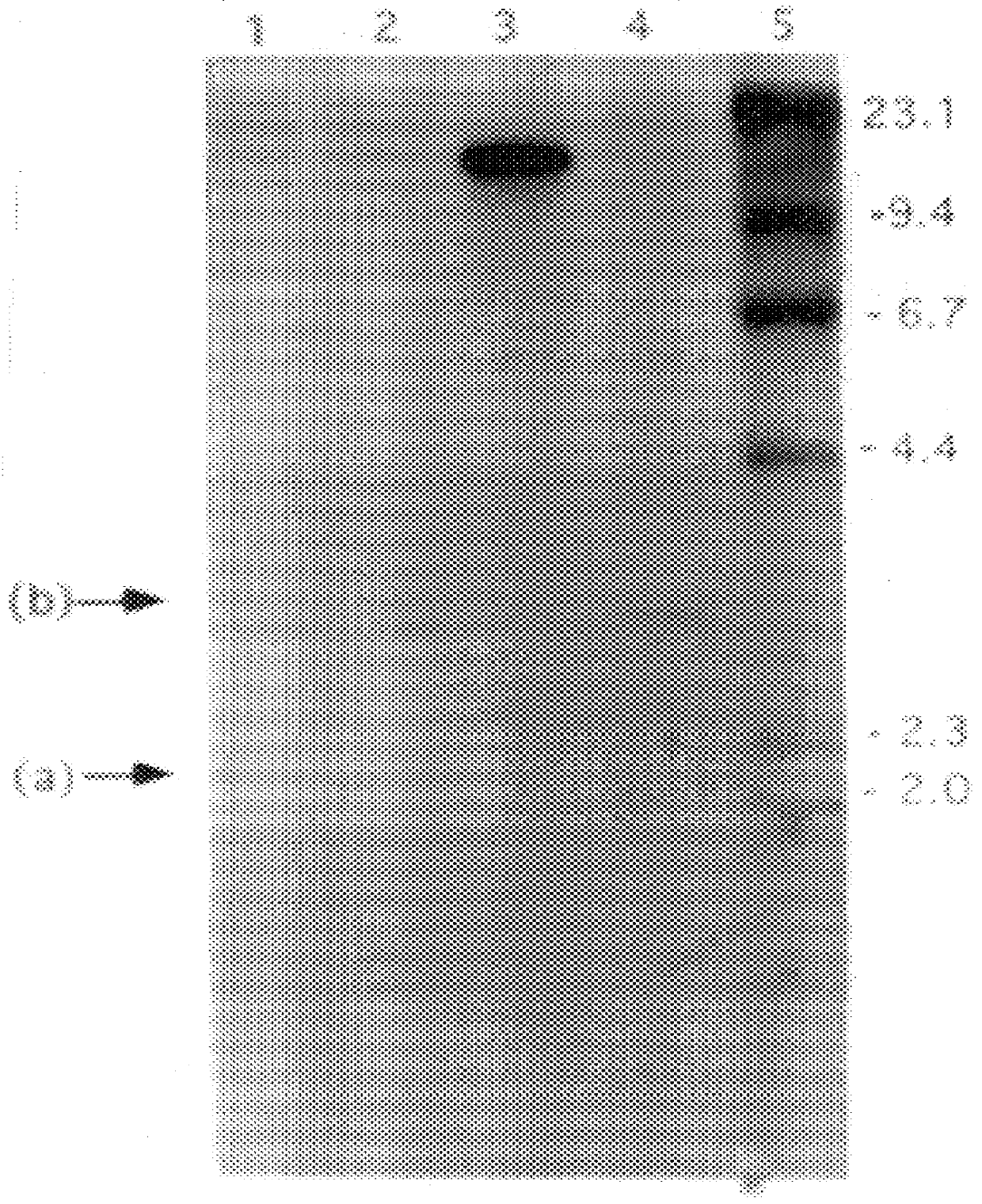
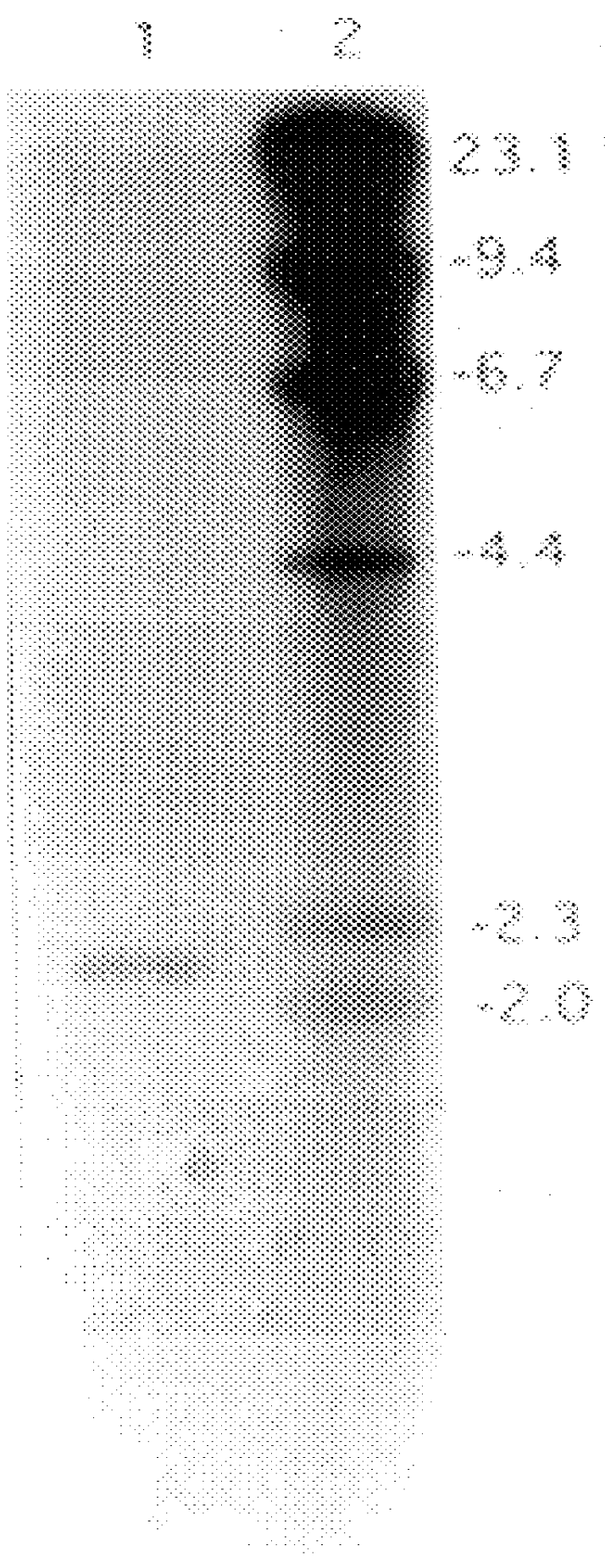
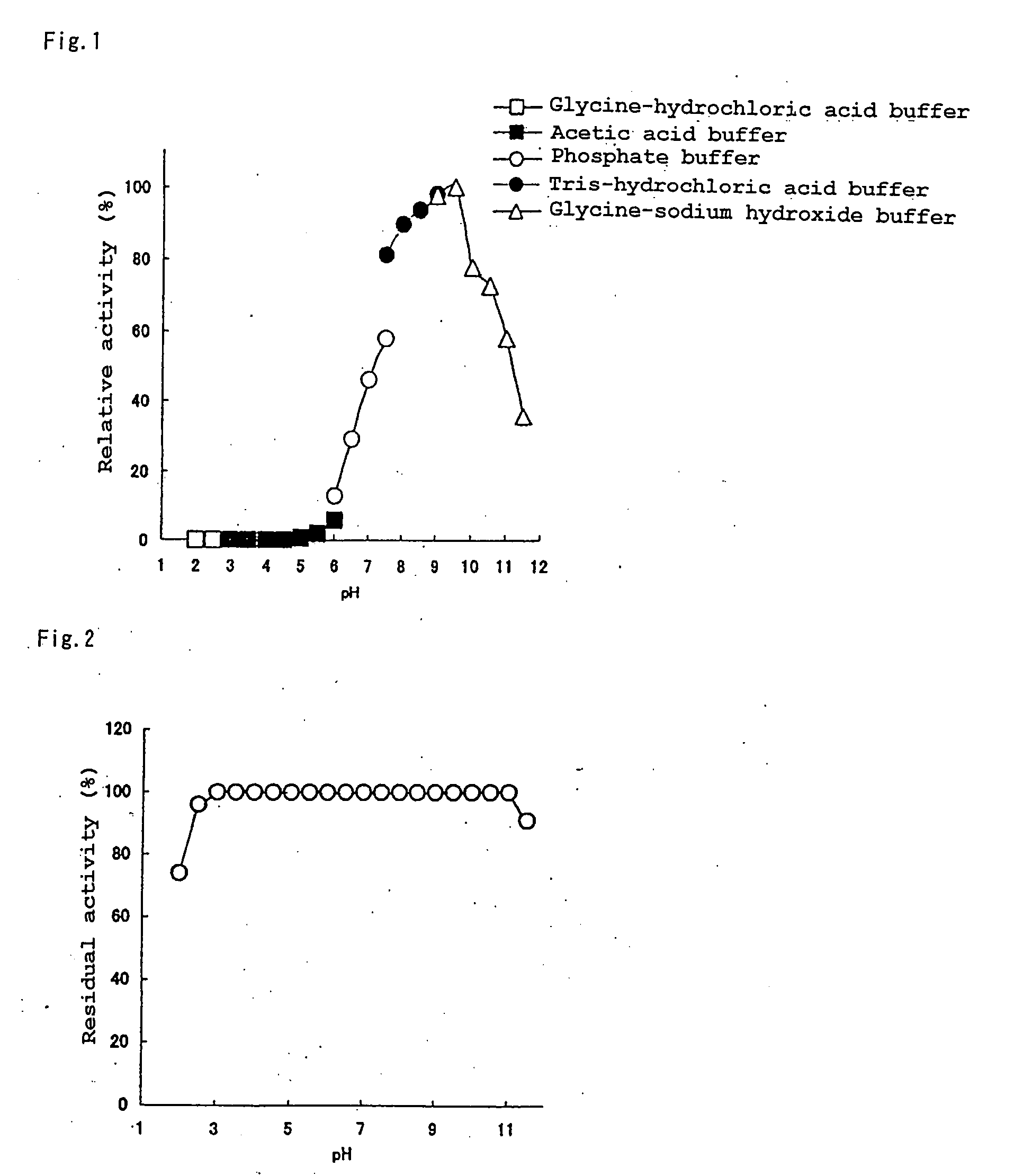
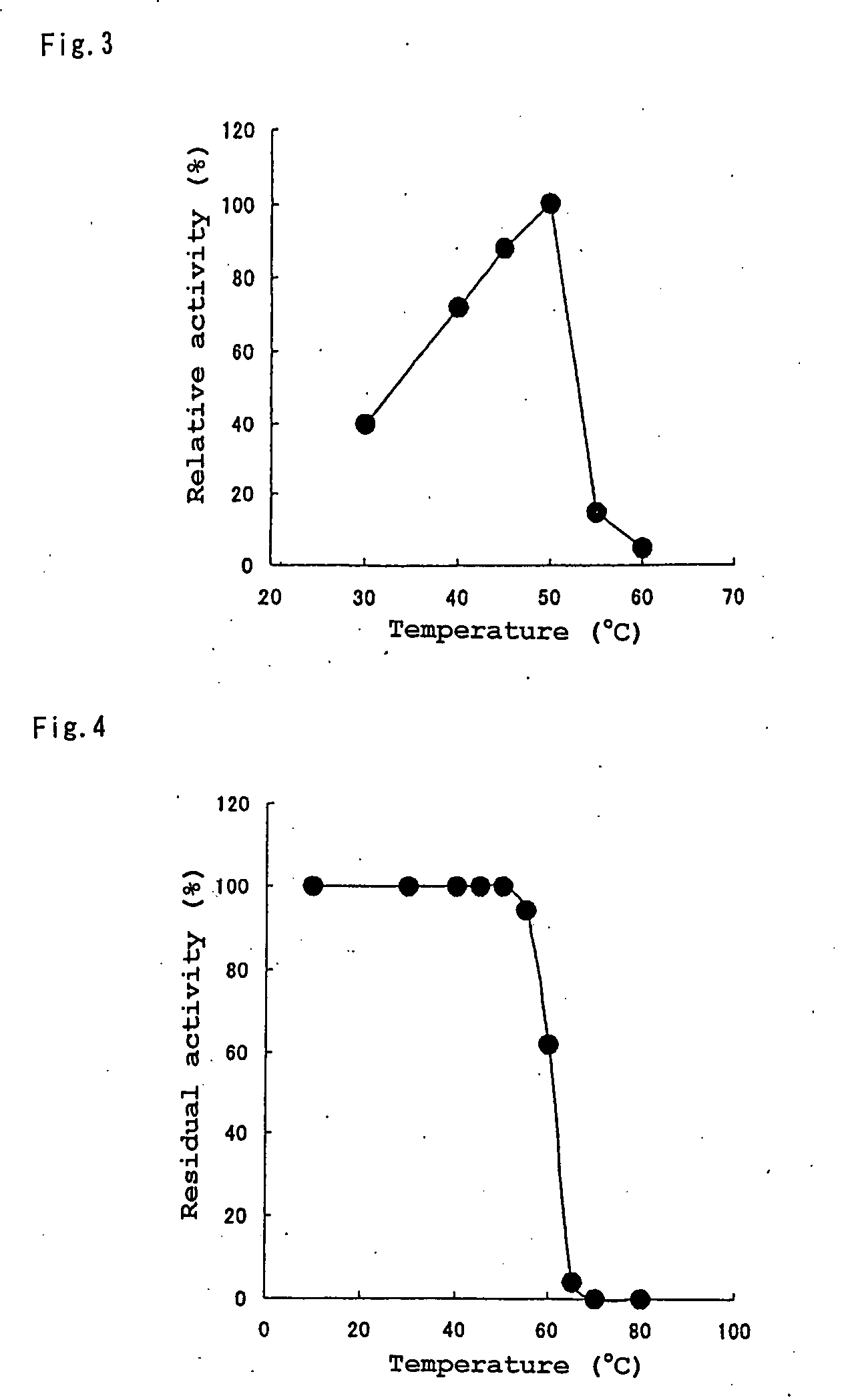
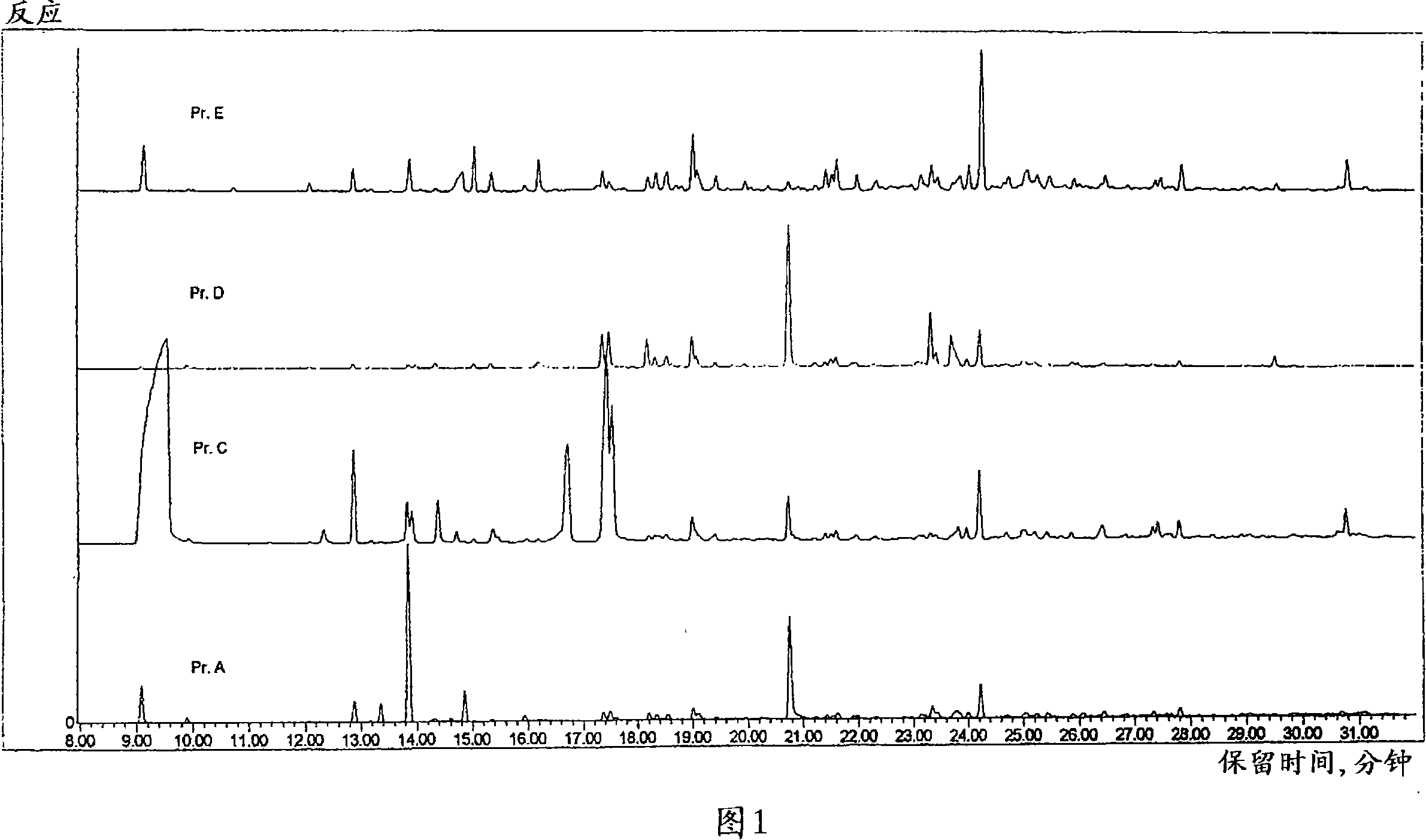
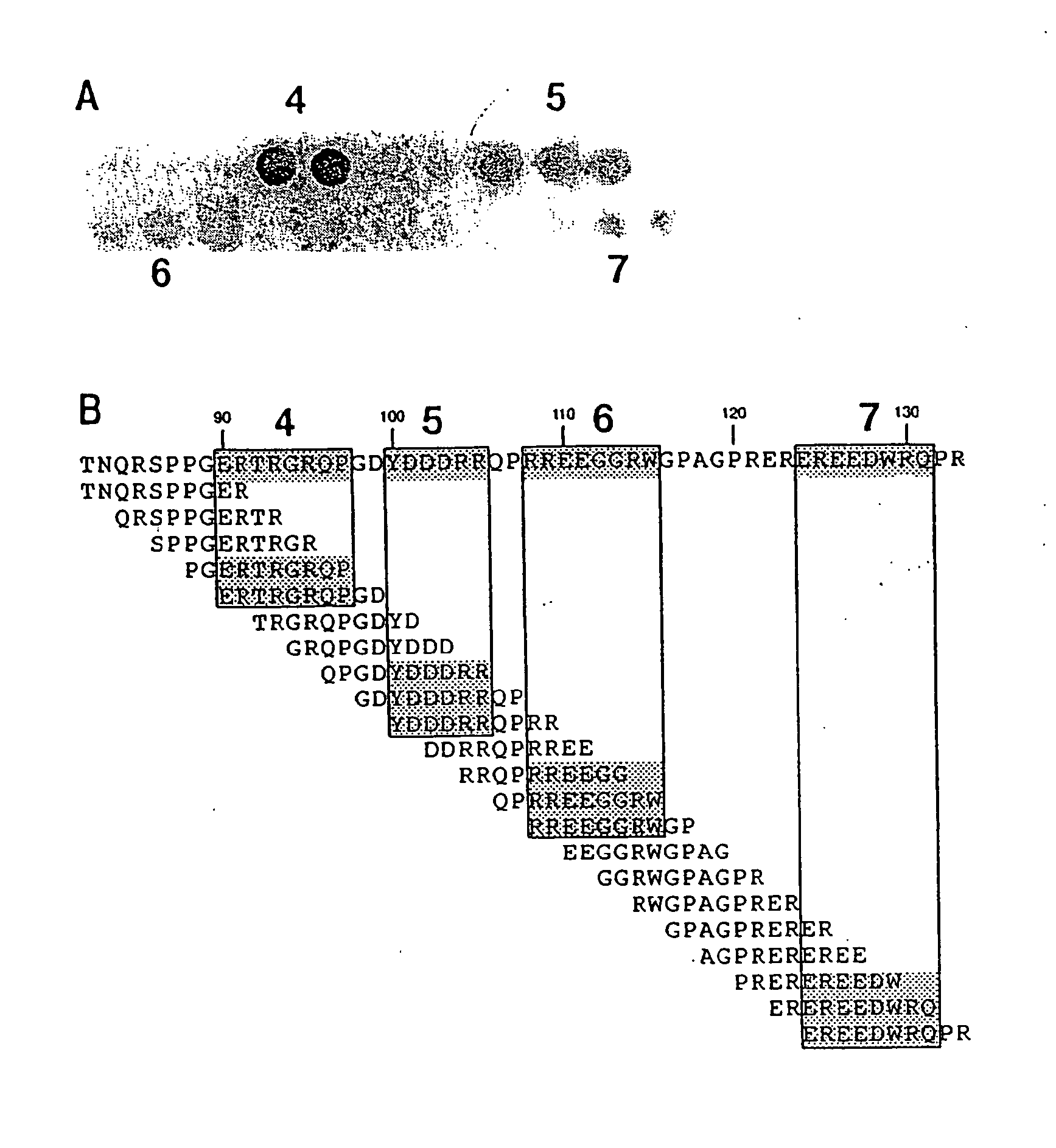
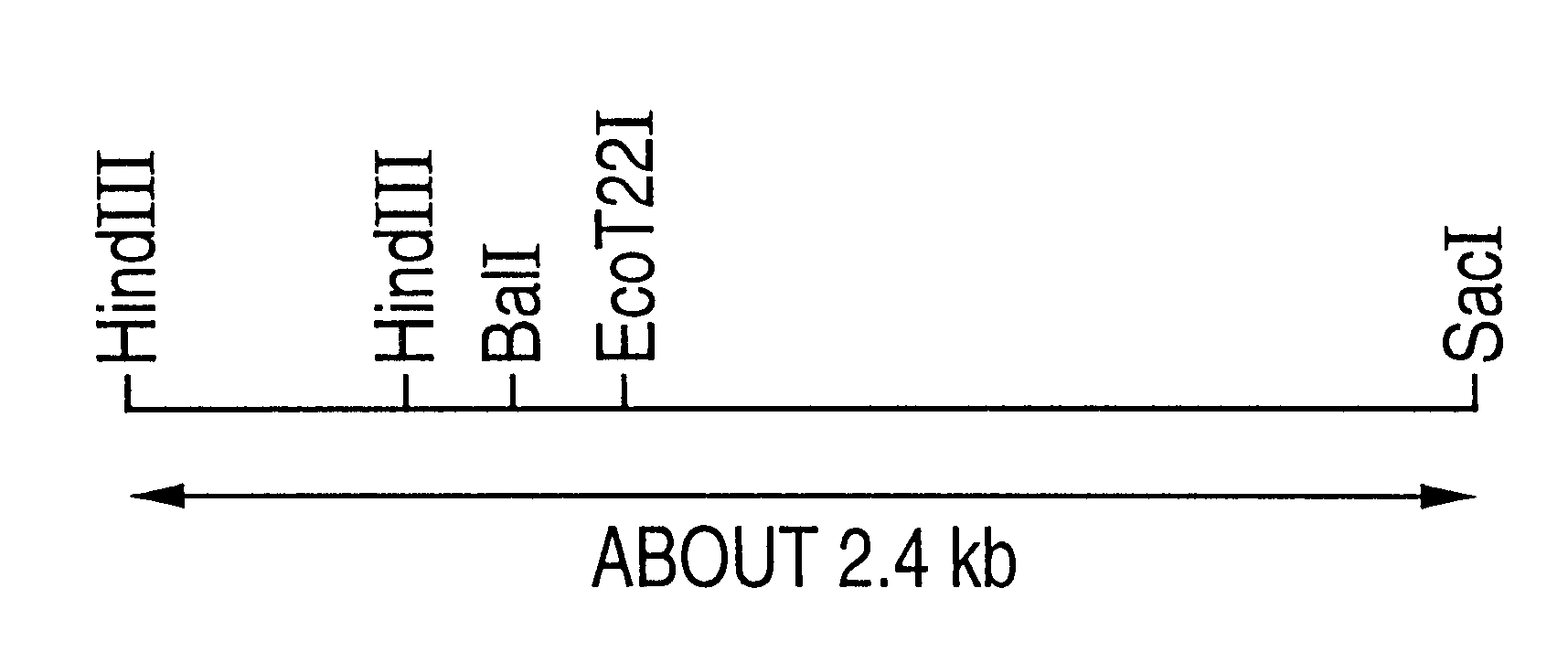
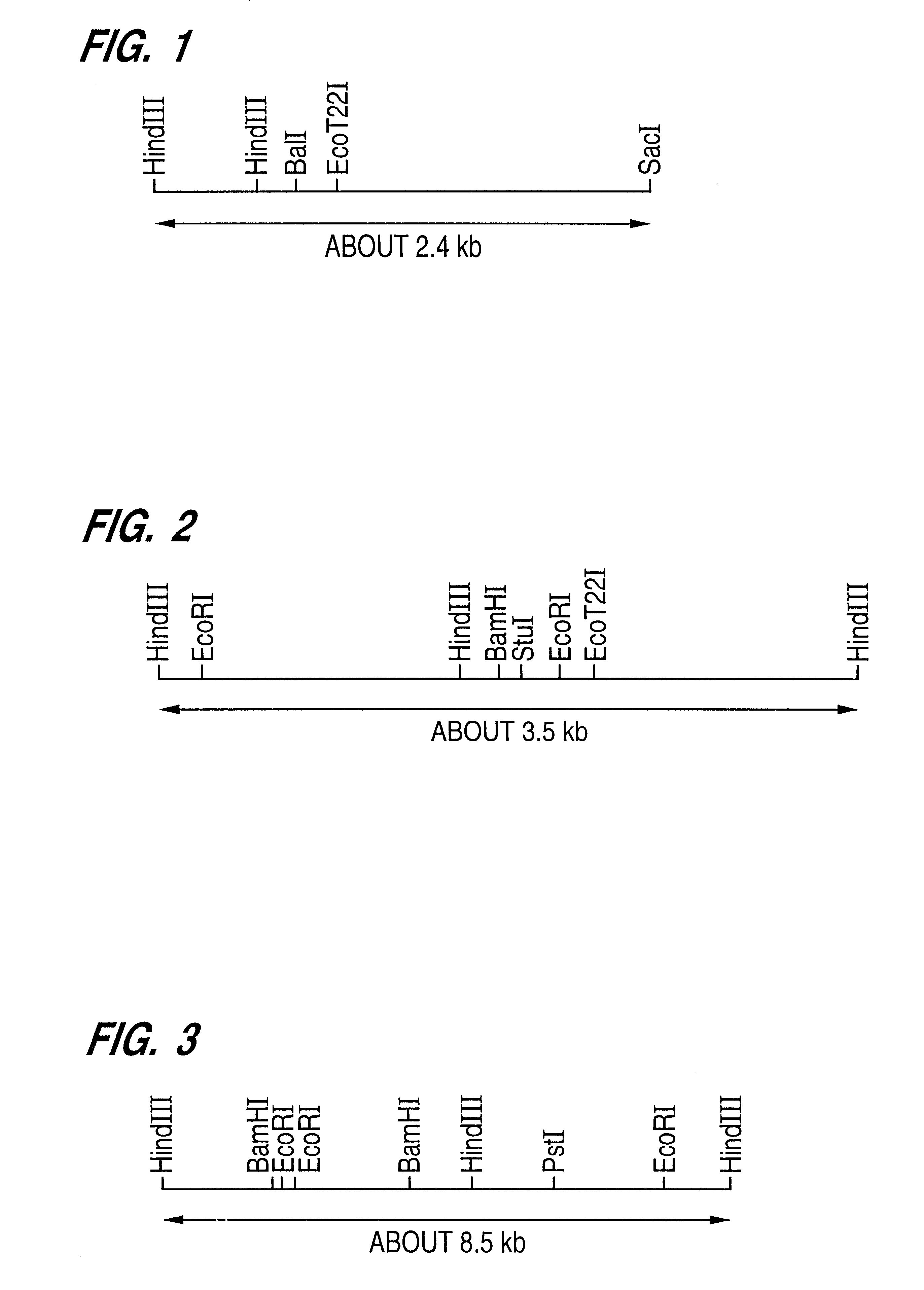
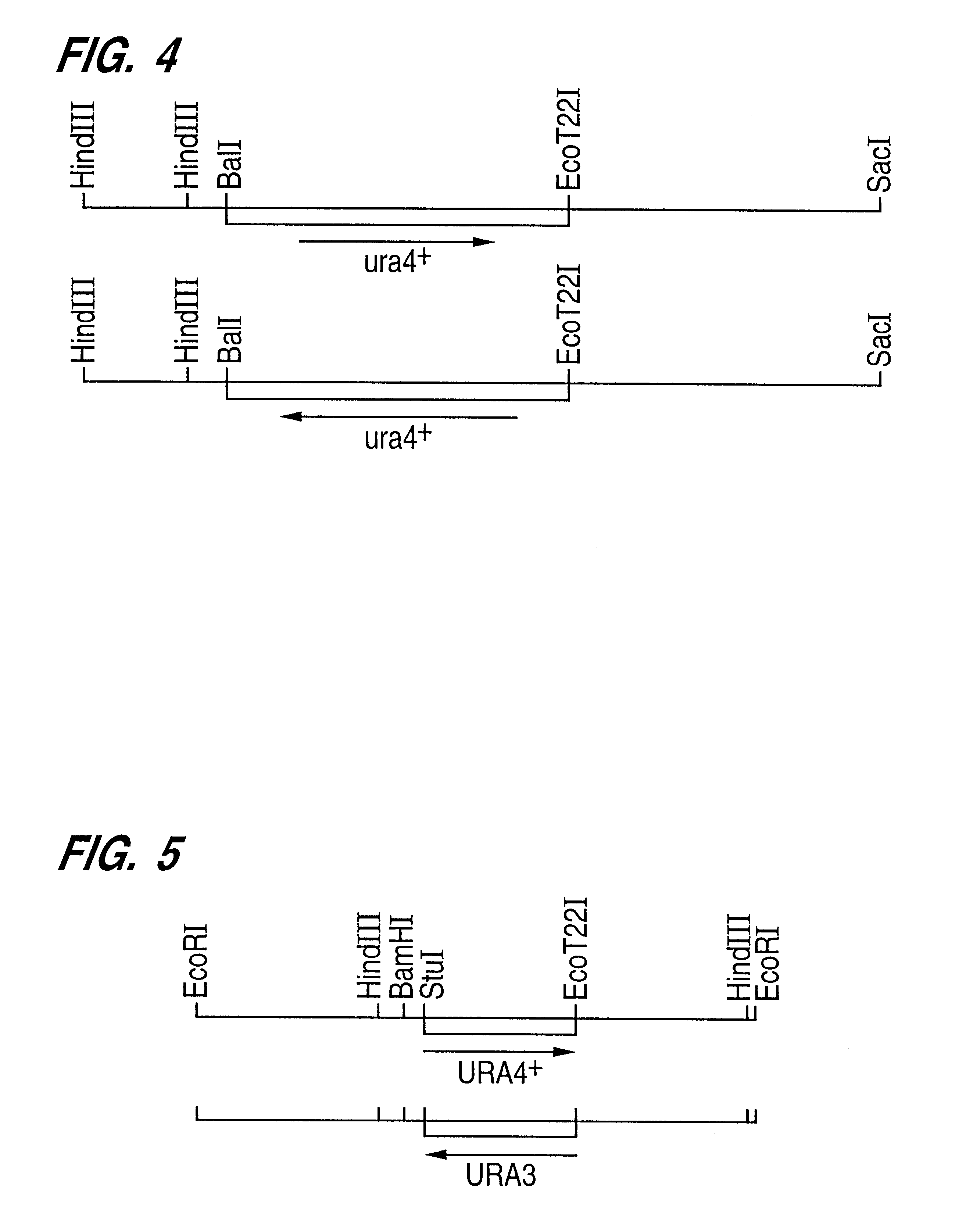
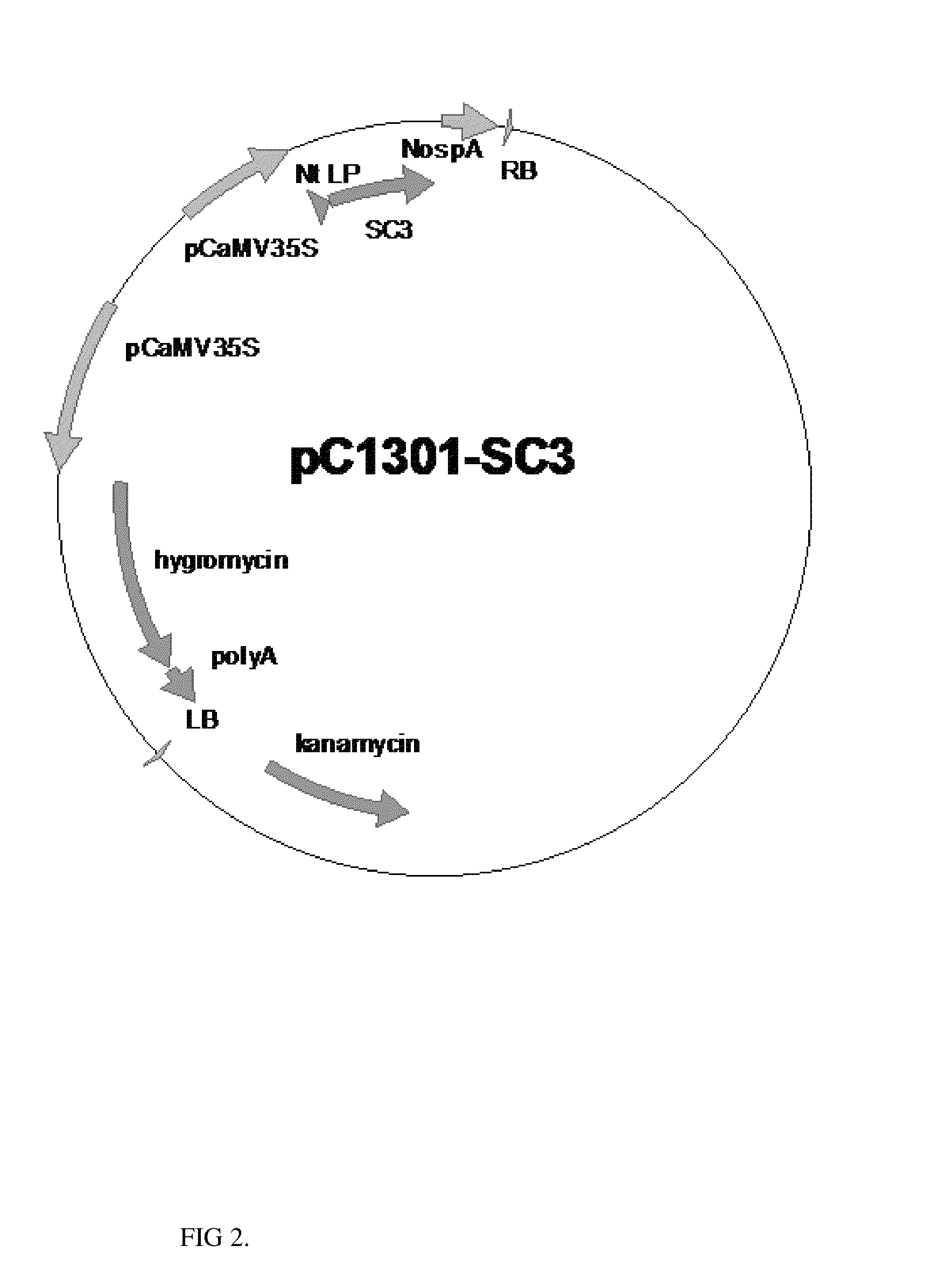
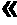Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
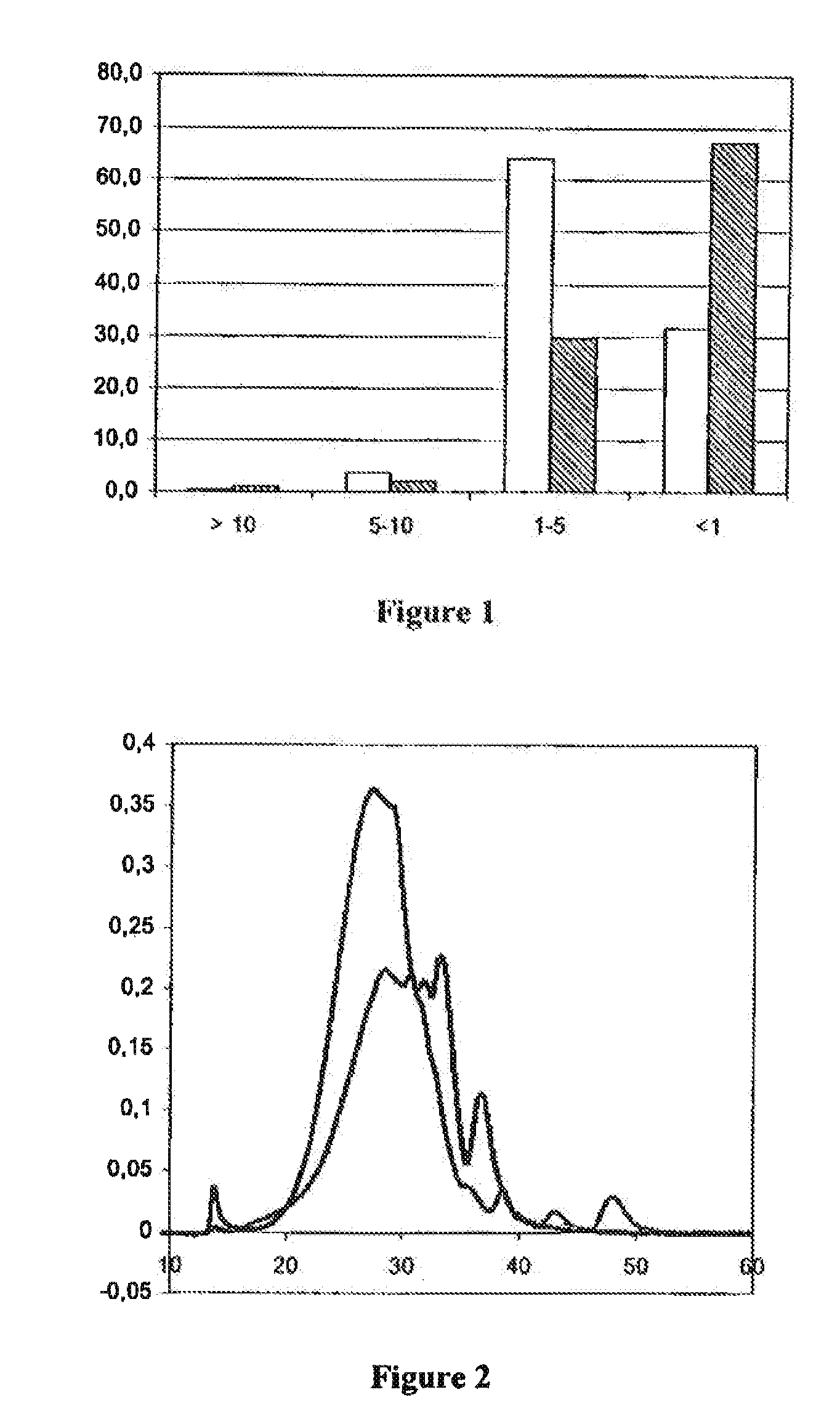
79results about "Protein composition from yeasts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Use of natural active substances in cosmetic or therapeutic compositions
InactiveUS20110052514A1Improved cosmetic and therapeutic activityEasy to implementCosmetic preparationsHydrolysed protein ingredientsYeast ProteinsMedicine
The invention relates to cosmetic or therapeutic compositions that contain hydrolysed yeast proteins as an active ingredient, to the use of said cosmetic or therapeutic compositions, and to a method for cosmetic treatment.
Owner:LESAFFRE & CIE
Mucorales fungi for use in preparation of foodstuffs
InactiveUS7045160B1Great tasteImproved meat-like propertyMilk preparationProtein composition from yeastsBiotechnologyFermentation
The preparation of a proteinaceous substance suitable for use in a foodstuff is described which comprises fungal fells of the order Mucorales. The cells are grown in a fermentor vessel in a liquid which is mixed during fermentation, after which the RNA content of the fungal cells is reduced to below 4% the fungal cells processed into an edible substance. This substance is then mechanically texturized into edible textured product for inclusion into foodstuffs, for example in the form of chunks as a meat substitute.
Owner:DSM IP ASSETS BV
Method for extracting selenium-containing protein from selenium-enriched yeast
InactiveCN102550795AImplement extractionPromote absorptionProtein composition from yeastsProtein solutionHydrogen
The invention discloses a method for extracting selenium-containing protein from selenium-enriched yeast, which is characterized by comprising the following steps: (1) choosing the selenium-enriched yeast as raw materials, using a sodium hydroxide or potassium hydroxide solution with potential of hydrogen (pH) of 11 to 14 to dissolve the selenium-enriched yeast to obtain mixed liquid A; (2) placing the mixed liquid in a vibration machine of 250 r / minute at the temperature of 35 DEG C to be vibrated for 8 hours to 12 hours, and performing ultrasound on the mixed liquid for 30 minutes for twice in vibration processes; and (3) arranging the mixed liquid in a centrifugal machine of 4,000 to 20,000 r / min for 30 minutes after vibration is finished to extract supernatant, and adjusting pH of the supernatant to 7 to obtain a selenium-containing protein solution. The method improves wall broken rate and protein dissolving rate of yeast cells, extraction rate of selenium in the selenium-enriched protein solution is more than 70% when the selenium-enriched solution is not filtered and purified, and selenium-containing protein with organic selenium content more than 95 % can be obtained after the selenium-enriched solution is filtered and purified.
Owner:SUZHOU SETEK
Methods and compositions for consumables
Provided herein are methods and compositions related to plant based meat substitutes which have properties similar to meat.
Owner:IMPOSSIBLE FOODS
Expression of the glucose oxidase gene in transgenic organisms
PCT No. PCT / AU95 / 00059 Sec. 371 Date Nov. 11, 1996 Sec. 102(e) Date Nov. 11, 1996 PCT Filed Feb. 10, 1995 PCT Pub. No. WO95 / 21924 PCT Pub. Date Aug. 17, 1995A genetic construct for use in production of transgenic plants with reduced susceptibility or increased resistance to pests or diseases comprises an isolated nucleotide sequence encoding the glucose oxidase enzyme of Talaromyces flavus, the nucleotide sequence being operably linked to a promoter capable of expression in a plant, plant cell or group of plant cells, and further comprises a nucleic acid segment having a nucleotide sequence encoding a signal sequence which directs secretion of the functional glucose oxidase enzyme of T. flavus from plant cells.
Owner:COMMONWEALTH SCI & IND RES ORG
Vegan simulated egg compositions and methods
InactiveUS20130084361A1Milk preparationProtein composition from yeastsAnimal scienceVegetarian diets
The present invention relates to compositions and methods for preparation of vegan simulated egg yolks, simulated egg whites, and simulated whole eggs consisting of the vegan simulated egg yolk and egg white compositions, for consumption. The compositions simulate sensorily an animal-derived egg, such as a chicken egg, and may have the same protein and vitamin content.
Owner:SHEPHEARD ROCKNEY A
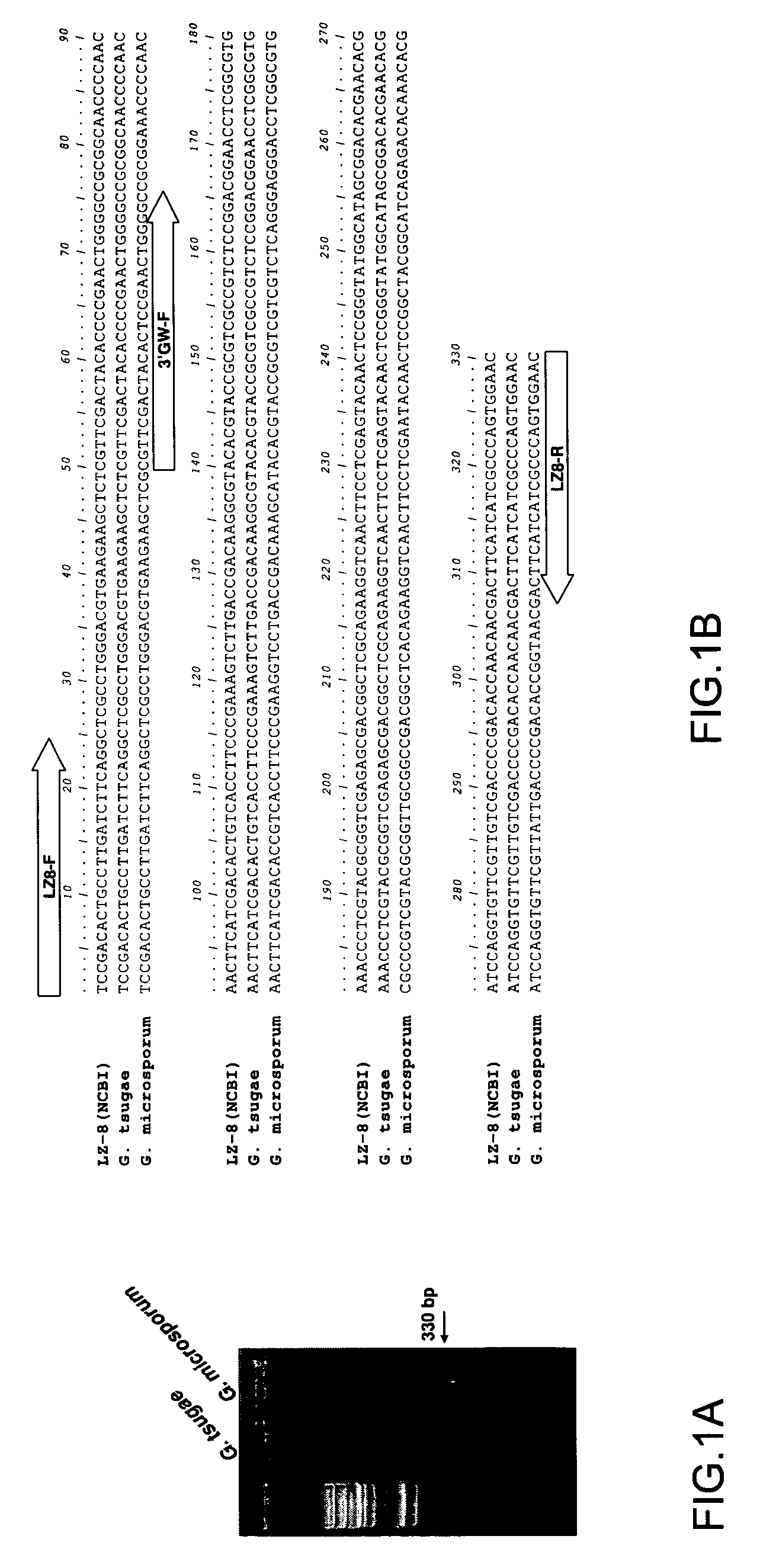
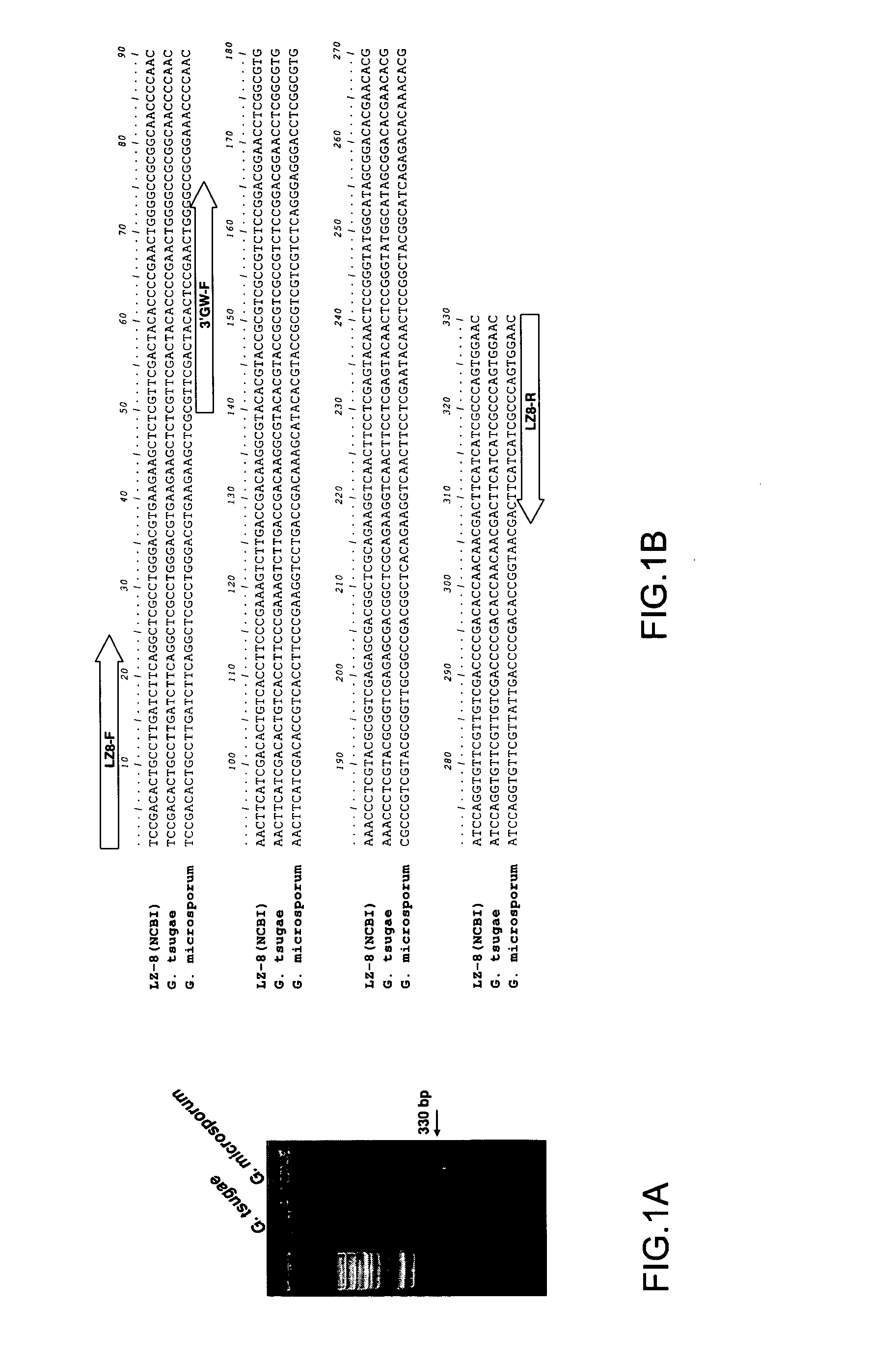
Immunomodulatory protein cloned from ganoderma microsporum
ActiveUS7601808B2Improve efficiencyPeptide/protein ingredientsProtein composition from yeastsSporeGanoderma pseudoferreum
An immunomodulatory protein is cloned from Ganoderma microsporum. Its molecular weight is 15863.79 Da. Its genome sequence and translated protein sequence are different from those protected by the known patent of glycoprotein LZ-8, which is isolated from the mycelium of G. lucidum and has immunomodulator effect, and its immunomodulator efficiency is better than that of LZ-8.
Owner:WORLD BIO TECH ALLIANCE
Yeast autolysates
InactiveUS20100183767A1Effective functionWider flavourMicroorganism lysisProtein composition from yeastsYeastHydrolysis
The present invention relates to a yeast autolysate with taste enhancing properties which is high in solubilised solids and which comprises a dry solids ratio of at least 50%. The present invention also relates to a process for preparing the autolysates of the invention. The process comprises subjecting a yeast or yeast fraction to autolysis or hydrolysis, immediately followed by concentration of the total reaction mixture, to form a yeast autolysate. Yeast autolysates according to the invention may advantageously be used in a flavour provider, in a flavour enhancer, in meat applications, in a flavour improver or in a top note carrier. The autolysate of the present invention is particularly useful in feed or food applications which do not permit the use of yeast autolysates with high solid contents, such as in meat injectors.
Owner:DSM IP ASSETS BV
Identification And Use Of Genes Encoding Amatoxin And Phallotoxin
The present invention relates to compositions and methods comprising genes and peptides associated with cyclic peptide toxins and toxin production in mushrooms. In particular, the present invention relates to using genes and proteins from Amanita species encoding Amanita peptides, specifically relating to amatoxins and phallotoxins. In a preferred embodiment, the present invention also relates to methods for detecting Amanita peptide toxin genes for identifying Amanita peptide-producing mushrooms and for diagnosing suspected cases of mushroom poisoning. Further, the present inventions relate to providing kits for diagnosing and monitoring suspected cases of mushroom poisoning in patients.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Treating compositions with lactoferrin
Owner:KRUZEL MARIAN L
Recombinant animal-free food compositions and methods of making them
ActiveUS20200138066A1Reduce allergiesImprove digestibilityDough treatmentTransferrinsBiotechnologyAnimal science
Provided herein are recombinant animal-free food compositions comprising egg-white proteins such as ovalbumin, ovotransferrin and lysozyme and methods of making such food compositions.
Owner:CLARA FOODS
Method for stabilization of proteins using non-natural amino acids
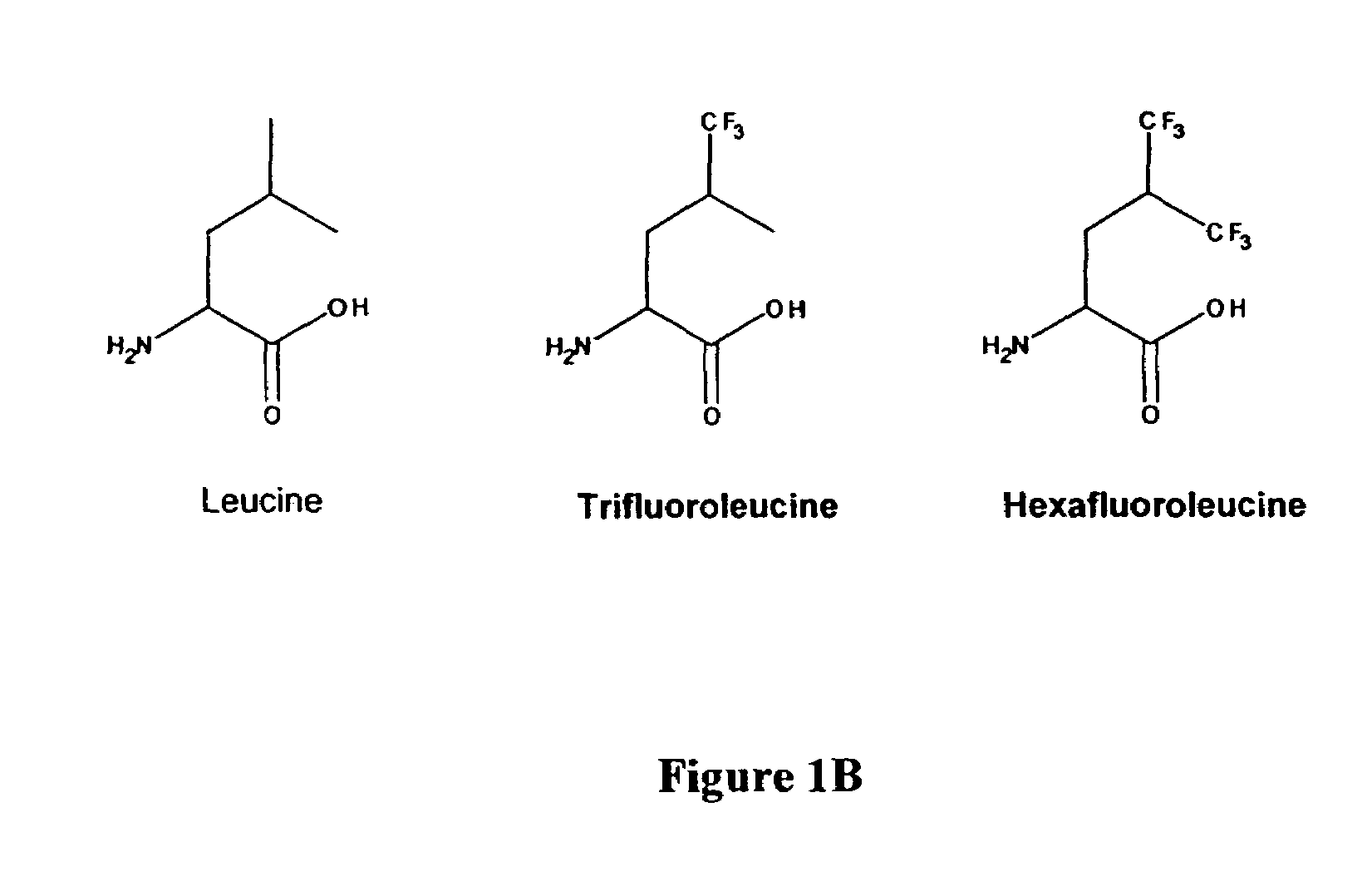
InactiveUS7449443B2Peptide/protein ingredientsProtein composition from yeastsCoiled coil proteinTert-leucine
The present invention provides a method for producing modified stable polypeptides introducing at least one non-natural amino acid into the hydrophobic region of the polypeptide. The thermal and chemical stability of such polypeptides is improved compared to those properties of its corresponding wild type proteins.The invention further provides purified leucine zipper and coiled-coil proteins in which the leucine residues have been replaced with 5,5,5-trifluoroleucines, and the modified proteins so produced demonstrate increased thermal and chemical stability compared to their corresponding wild-type natural proteins.
Owner:CALIFORNIA INST OF TECH
Carbonyl reductase, gene encoding the same, and process for producing optically active alcohols using the same
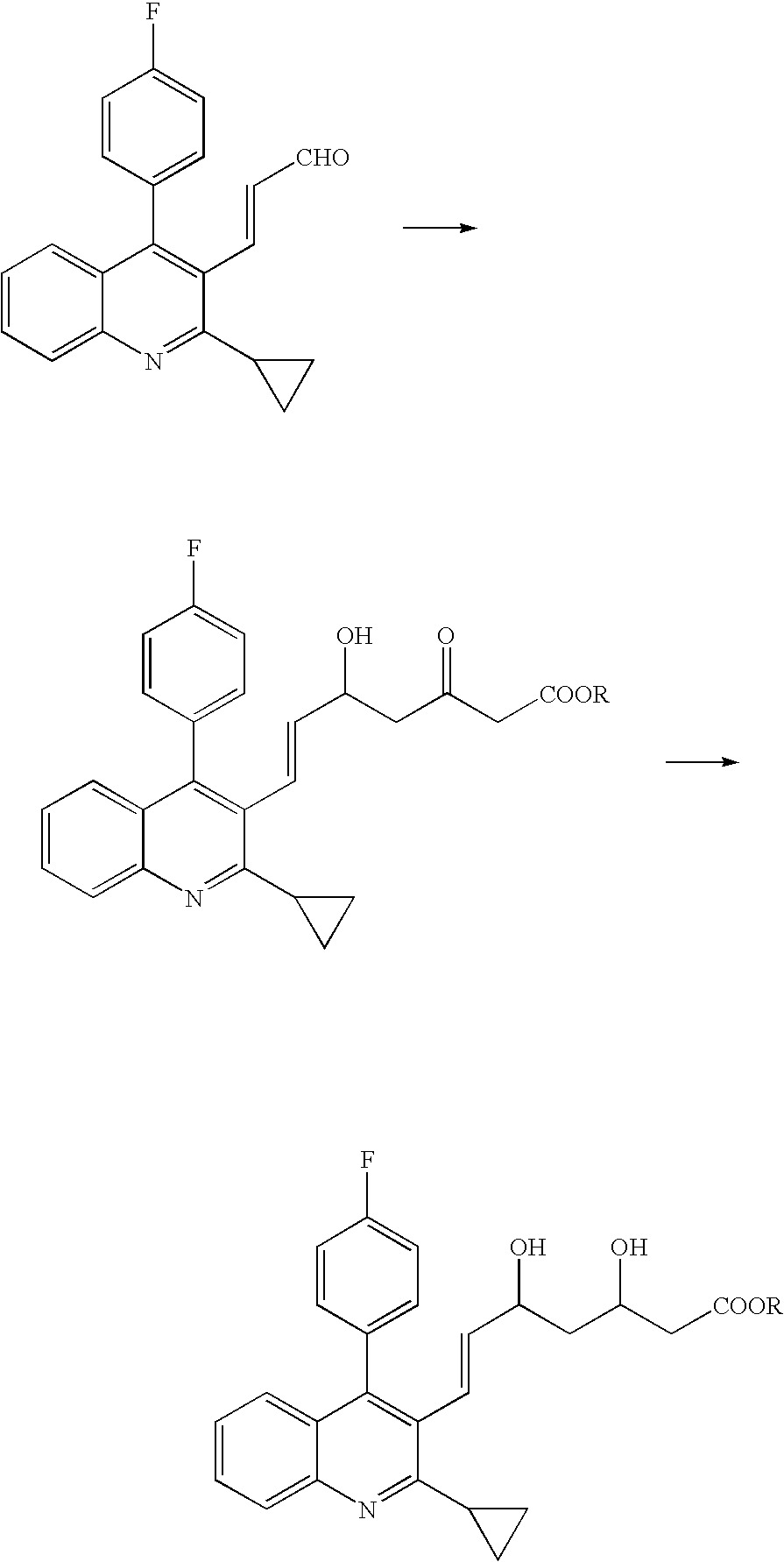
According to the present invention, there is provided a novel carbonyl reductase derived from a microbial belonging to the genus Ogataea and a DNA encoding the enzyme. By reducing ketones with the use of the carbonyl reductase, optically active alcohols, in particular, (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-quinolin-3-yl]-3,5-dihydroxy-hept-6-enoic acid esters can be produced. The carbonyl reductase according to the present invention is excellent in activity and stereoselectivity. Thus, according to the present invention, there is provided a process for producing optically active alcohols, which are industrially useful as intermediate materials for drugs, pesticides, etc.
Owner:MITSUBISHI CHEM CORP
Process for producing a flavor-enhancing material for foods
InactiveUS7118775B2Low applicabilityLow costProtein composition from yeastsFermentationYeastFood material
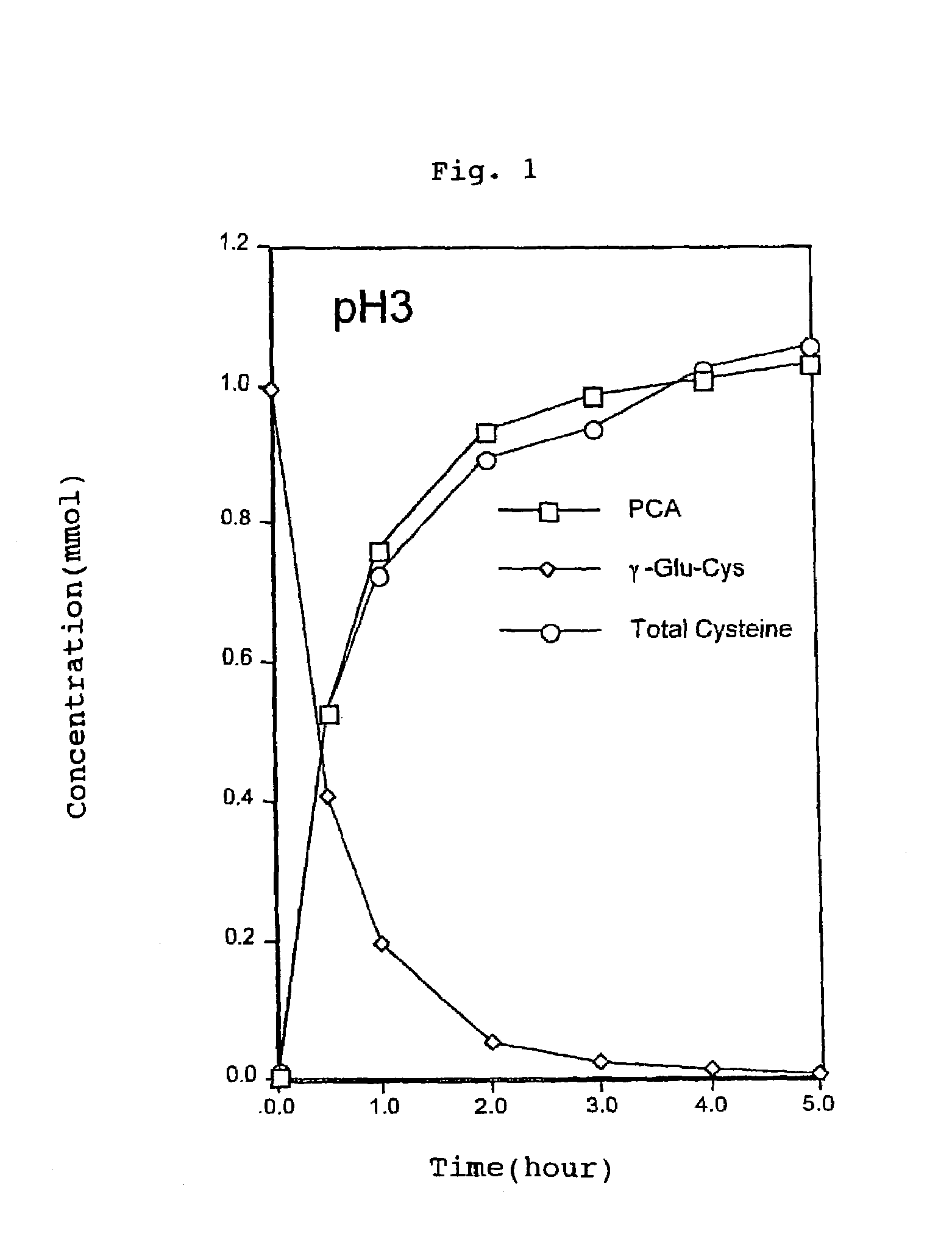
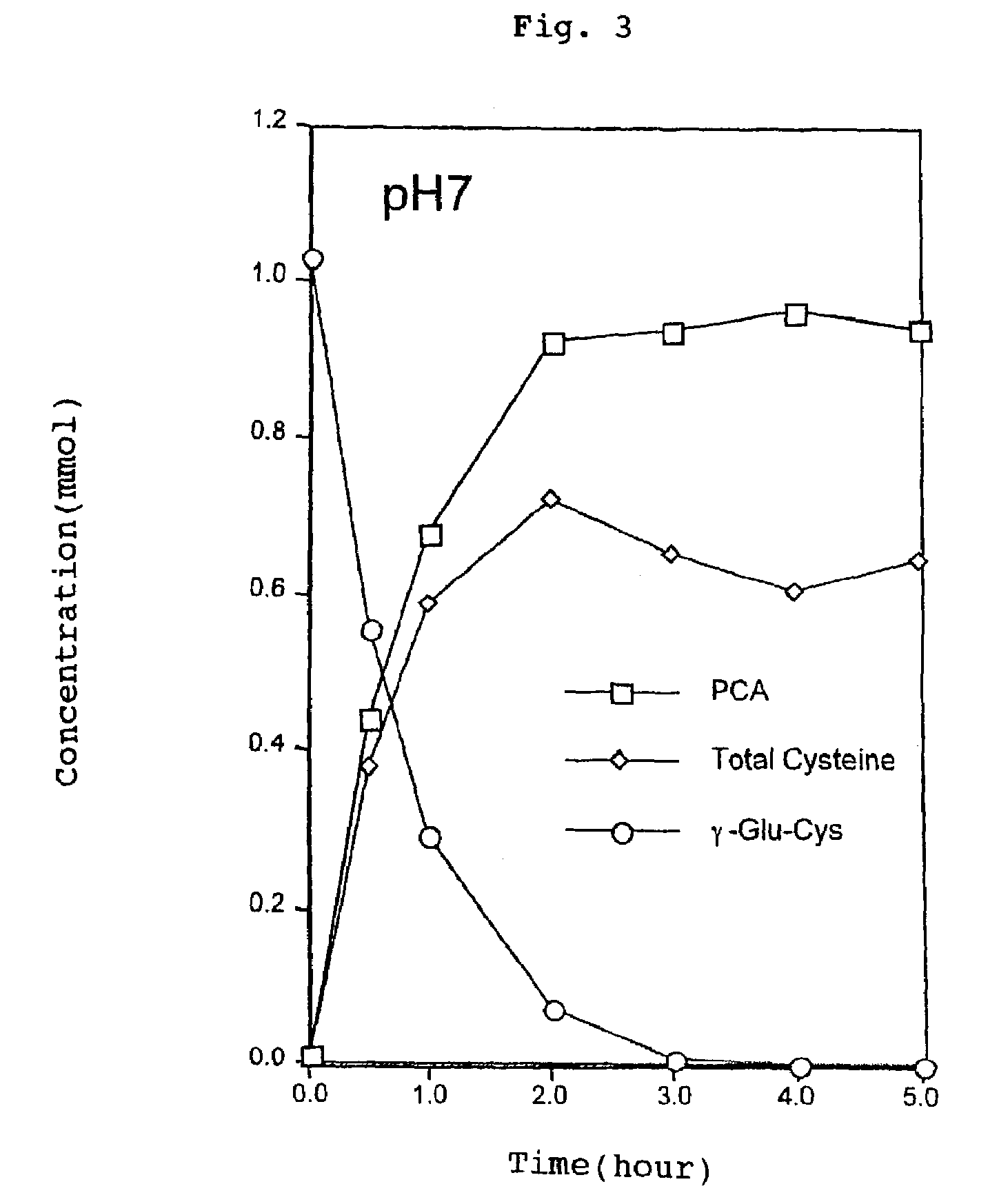
The present invention relates to a method for producing a cysteine-rich food material by maintaining a food material containing γ-glutamylcysteine at a ratio of at least 1 wt % to the solid content thereof at a temperature ranging from 50 to 120° C. and at a pH ranging from 1 to 7. This process is conducted in the absence of a sugar and in the presence of water. The present invention also relates to a method for producing a cysteine-rich food material by reacting the food material with a γ-glutamyl peptide hydrolase at a pH ranging from 3 to 9 and at a temperature ranging from 15 to 70° C. in the present of water. Further, the present invention relates to a method for producing a flavor-enhancing material for use in food, food products obtained by these processes, and yeast cells or extracts for use in food products.
Owner:AJINOMOTO CO INC
Method for producing edible nanoscale high-pressure spraying dried bear barm protein powder
InactiveCN101040655ASolve the problem of low nutrient absorption rateImprove digestion and absorption rateFungiProtein composition from yeastsYeastB Vitamin Family
The invention discloses a brewer's yeast albumen powder which comprises organic chromium Cr3+ 0.01-500mg / 100g, protein 45-60 wt%, dietary fiber 20-35 wt%, vitamin B 10-40mg / 100g. The invention also provides the process for preparing the albumen powder, which comprises nano wall-breaking, high temperature autolysis and high pressure spray-drying.
Owner:SHANGHAI GENON BIOENG
Method for preparing yeast peptide utilizing beer yeast
ActiveCN1934982AIncrease contentRetain nutritionProtein composition from yeastsYeastAdditive ingredient
The present invention discloses a preparation method capable of utilizing beer yeast produced in the beer production to make yeast peptide. Said method includes the following step: collecting beer yeast, extruding, mixing paste, zymolysis and breaking wall, secondary mixing paste, zymolyzing protein, concentrating, drying, pulverizing so as to obtain the invented yeast peptide. Said yeast peptide can be used as raw material for making multifunctional health-care product.
Owner:INFINITUS (CHINA) CO LTD
Novel Protease, Microorganism Producing the Same, and Application Thereof
InactiveUS20090155239A1Excellent thrombolytic activityWide applicationFungiPeptide/protein ingredientsMicroorganismProteinase activity
An object of the present invention are to provide a protease that is stable in a wide pH range from acidic to alkaline, and that has excellent thrombolytic activity; a protease-producing microorganism that produces the above protease; and a process for producing the protease.By culturing a novel filamentous fungus belonging to the genus Fusarium (Fusarium sp. strain BLB), a protease that is stable in a wide pH range from acidic to alkaline and that has excellent thrombolytic activity, is formed and accumulated in the culture medium, and recovered.
Owner:SODX
Fermented protein product
ActiveCN101155520AEnhance nutritional propertiesImprove sensory propertiesDough treatmentProtein composition from vegetable seedsYeastFermentation
The present invention relates to protein products with improved nutritional value, and enhanced organoleptic properties, and uses thereof. In particular, the present invention provides fermented protein rich products on basis of fermented pulses and yeast, wherein the fermentation is followed by a heating step.
Owner:HAMLET PROTEIN AS
Methods and reagents for decreasing clinical reaction to allergy
InactiveUS20070213507A1Less allergicEliminate IgE bindingPeptide/protein ingredientsFungi peptidesBinding siteT cell
It has been determined that allergens, which are characterized by both humoral (IgE) and cellular (T cell) binding sites, can be modified to be less allergenic by modifying the IgE binding sites. The IgE binding sites can be converted to non-IgE binding sites by masking the site with a compound that prevents IgE binding or by altering as little as a single amino acid within the protein, most typically a hydrophobic residue towards the center of the IgE-binding epitope, to eliminate IgE binding. The method allows the protein to be altered as minimally as possible, other than-within the IgE-binding sites, while retaining the ability of the protein to activate T cells, and, in some embodiments by not significantly altering or decreasing IgG binding capacity The examples use peanut allergens to demonstrate alteration of IgE binding sites. The critical amino acids within each of the IgE binding epitopes of the peanut protein that are important to immunoglobulin binding have been determined. Substitution of even a single amino acid within each of the epitopes led to loss of IgE binding. Although the epitopes shared no common amino acid sequence motif, the hydrophobic residues located in the center of the epitope appeared to be most critical to IgE binding.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Immunomodulatory protein cloned from ganoderma microsporum
ActiveUS20070207954A1Improve efficiencyPeptide/protein ingredientsProtein composition from yeastsSporeBiology
An immunomodulatory protein is cloned from Ganoderma microsporum. Its molecular weight is 15863.79 Da. Its genome sequence and translated protein sequence are different from those protected by the known patent of glycoprotein LZ-8, which is isolated from the mycelium of G. lucidum and has immunomodulator effect, and its immunomodulator efficiency is better than that of LZ-8.
Owner:WORLD BIO TECH ALLIANCE
Indole-Diterpene Biosynthesis
The present invention relates to the biosynthesis of indole diterpene compounds. In particular, the invention relates to genes encoding enzymes considered responsible for the synthesis of lolitrems.
Owner:AGRESEARCH LTD
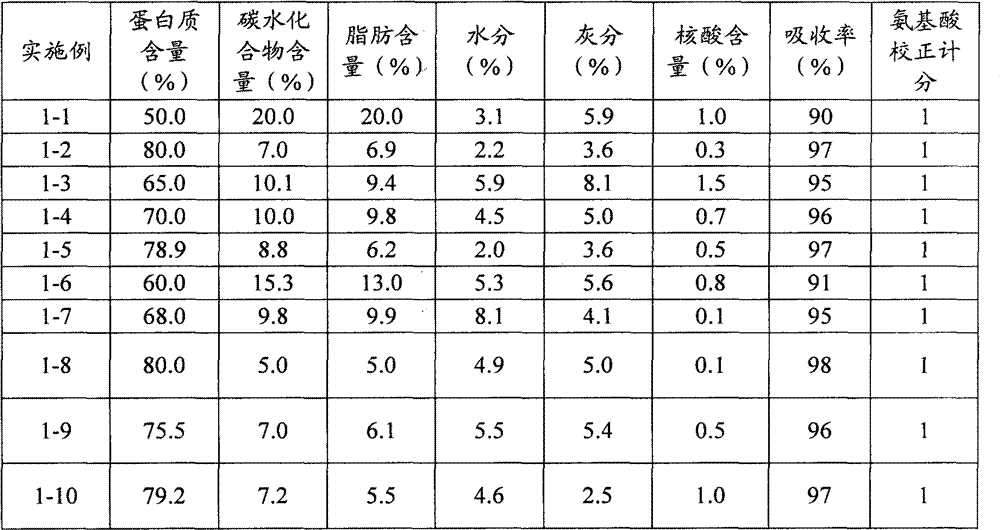
Yeast protein and preparation method thereof, food prepared from the protein as raw material and preparation method thereof
ActiveCN103082081AProtein composition from yeastsFood preparationYeast ProteinsAmino acid composition
The invention relates to a yeast protein and a preparation method thereof, a food prepared from the protein as a raw material and a preparation method thereof. The yeast protein comprises by weight, 50-80% of a protein, carbohydrate, fat, 0.1-1.5% of nucleic acid, moisture and ash. The preparation method of the yeast protein is as below: low nucleic acid yeast is used as a raw material, and subjected to processes comprising superfine grinding treatment, enzymatic hydrolysis, alkali treatment, acid precipitation, centrifugation, washing and spray drying. The food containing the yeast protein comprises the following raw materials by weight: 20-80% of a soybean protein, 1-40% of the yeast protein, and 1-40% of a whey protein. The food is prepared by steps of component mixing, granulation, drying, sieving and packaging. The food is an excellent protein nutrition, and has high protein biological utilization rate; and the protein has acid amino composition ratio quite similar to that of human body, and is a high-quality protein and suitable for body absorption.
Owner:ANGELYEAST CO LTD
Purified lactase
The present invention relates to a lactase solution comprising a lactase solution comprising less than 10 g / kg of poly and oligosaccharides, a process for the production of such a lactase solution from an untreated lactase solution, a sterilized lactase, whereby such lactose is filter sterilized in-line with the milk production process.
Owner:DSM IP ASSETS BV
Composition comprising protein and disperse fat
InactiveUS20110034394A1High nutritional valueImprove featuresCosmetic preparationsPeptide/protein ingredientsYeastFat proteins
The present invention relates to a method for the manufacture of a composition comprising proteins and fats, said method providing a simple and non-expensive way of obtaining fat-containing proteinaceous products of high nutritional value that are easy to handle and at the same time possess high stability towards coalescence and oxidation. More particularly, a method is provided for the manufacture of a composition comprising protein and fat in disperse form, the method comprising the following steps: (i) providing a suspension having a pH value higher than 7.0, said suspension comprising water, proteinaceous material, fat, and optionally alkali; (ii) incubating the suspension from (i) at a temperature in the interval 50-150° C.; (iii) homogenizing the suspension from (ii) to form a dispersion; and (iv), if desired, subjecting the dispersion from (iii) to a subsequent treatment; wherein the proteinaceous material in step (i) comprises vegetable-based proteinaceous material, and / or yeast-based proteinaceous material; wherein the suspension in step (i) comprises at least 5% fat by weight of dry matter. The current invention also relates to the compositions obtained by the method and to compositions obtainable by the method of the invention and, further, to the use of these compositions.
Owner:HAMLET PROTEIN AS
Gene regulating aubeobasidin sensitivity
The invention is directed to isolated DNAs having nucleic acid sequences which encode proteins which regulate aureobasidin sensitivity. Also disclosed are recombinant plasmids containing the DNAs, transformants containing the plasmids, and methods of producing the proteins.
Owner:TAKARA HOLDINGS
Method for hydrophobin production in plants and methods to produce hydrophobin multimers in plants and microbes
InactiveUS20130219559A1Produce highProduce high amounts of hydrophobinsProtein composition from yeastsPeptide preparation methodsMicroorganismBiotechnology
A novel method to produce hydrophobin monomers or multimers in plant tissues is disclosed. A novel method to produce hydrophobin multimers in E. coli cells is also described. The disclosure provides transgenic plants, transgenic seeds, a production system, and expression cassettes for making the transgenic plant carrying hydrophobin encoding gene sequences.
Owner:KOIVU KIMMO
Transcription regulatory factors for mannanases or cellulases, and genes for the transcription regulatory factor
Disclosed are: transcription regulatory factors capable of regulating the transcription or expression of genes for mannanases or cellulases, as mentioned below; and others. Specifically disclosed is a protein selected from the following proteins (a), (b) and (c): (a) a protein comprising the amino acid sequence depicted in SEQ ID NO:2; (b) a protein which comprises an amino acid sequence produced by deleting, substituting or adding one or several amino acid residues (e.g., 1 to 5 amino acid residues) in the amino acid sequence depicted in SEQ ID NO:2 and which is capable of regulating the transcription of genes for mannanases or cellulases; and (c) a protein which comprises an amino acid sequence having a 70% or higher sequence identity to the amino acid sequence depicted in SEQ ID NO:2 and which is capable of regulating the transcription of genes for mannanases or cellulases, or a partial fragment of the protein. Also specifically disclosed are a gene encoding the protein, and others.
Owner:NODA INST FOR SCI RES
Effective use of yeast and yeast extract residue
InactiveCN103857801ALess quantityAllergenicity reductionFungiProtein composition from yeastsPROTEASE MProtein content
To effectively use yeast extract residue that is produced excessively as a by-product of yeast extract, to reduce the amount of yeast extract residue, and to obtain a variety of useful products. By exposing yeast extract residue to a cell wall-decomposing enzyme that does not contain protease and then heat-treating the product for 10 to 20 minutes at 70-80 C, the residue can be separated into a fraction that is primarily cell wall and a fraction that is primarily protein. A yeast protein having a protein content of 60% or greater is obtained from the fraction that is primarily protein and a flavoring having high nitrogen content is obtained by enzyme decomposition of the yeast protein.
Owner:KOHJIN CO LTD
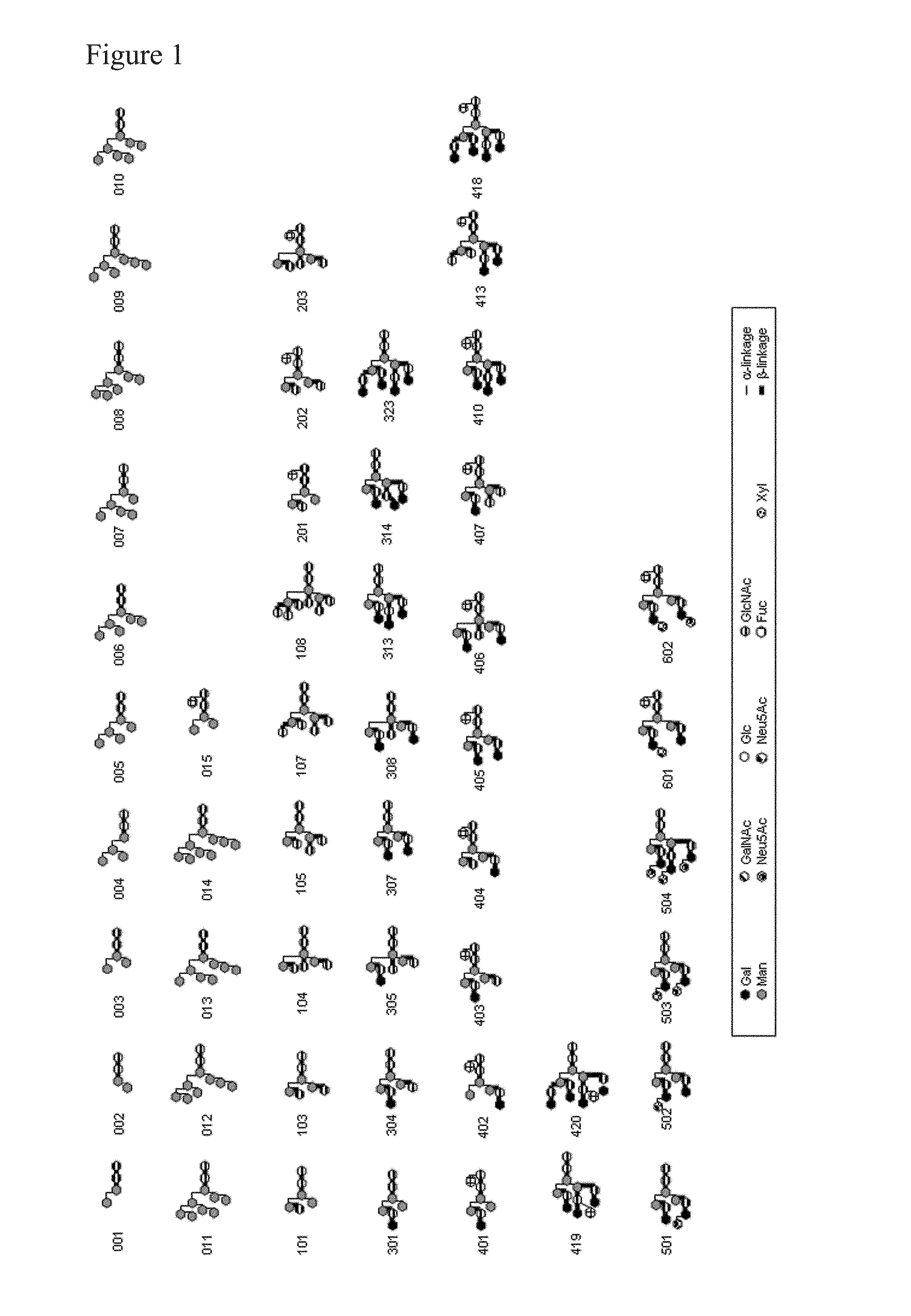
L-fucose α1→6 specific lectin
ActiveUS8461305B2Accurate detectionSelective specificityProtein composition from yeastsDepsipeptidesSpecific lectinSugar
Disclosed is a novel lectin which can bind specifically to an L-fucose α1→6 sugar chain. Also disclosed is use of the lectin. The L-fucose α1→6 specific lectin of the present invention is characterized in that: (1) the lectin is extracted from a basidiomycete or an ascomycete; (2) the lectin has a molecular weight by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of 4,000 to 40,000; and (3) the lectin has an affinity to an L-fucose α1→6 sugar chain, the affinity being represented by an association constant of 1.0×104M−1 or more (at 25 degrees C.). The lectin can be used for detecting an L-fucose α1→6 sugar chain specifically, and is effective for the purification of an L-fucose α1→6 sugar chain or a non L-fucose α1→6 sugar chain.
Owner:NAT INST OF ADVANCED IND SCI & TECH +2
Process for preparing nutritional, therapeutic or organoleptic products from crude glycerol
The present invention relates to a process for preparing a nutritional, therapeutic o organoleptic product by growing non-recombinant yeast under aerobic conditions, in medium that includes crude glycerol, as one possible carbon source to produce a yeas product. The yeast product can be processed to obtain such nutritional, therapeutic o organoleptic products as yeast paste, yeast metabolites, carbohydrates, proteins functional proteins, nucleotides, yeast autolysates, yeast extract, yeast cell walls, beta glucans, mannans or a product derived from a mineralized yeast product.
Owner:BIO PROCESSING AUSTRALIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com