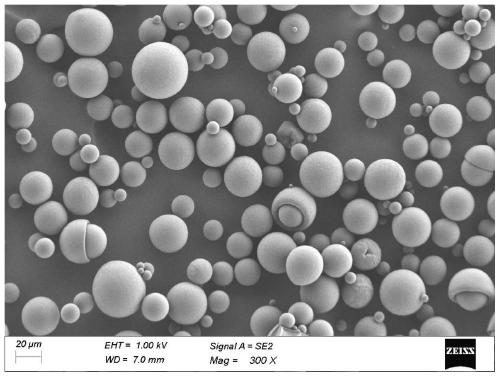
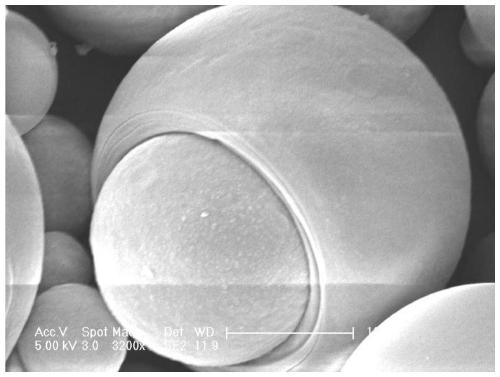
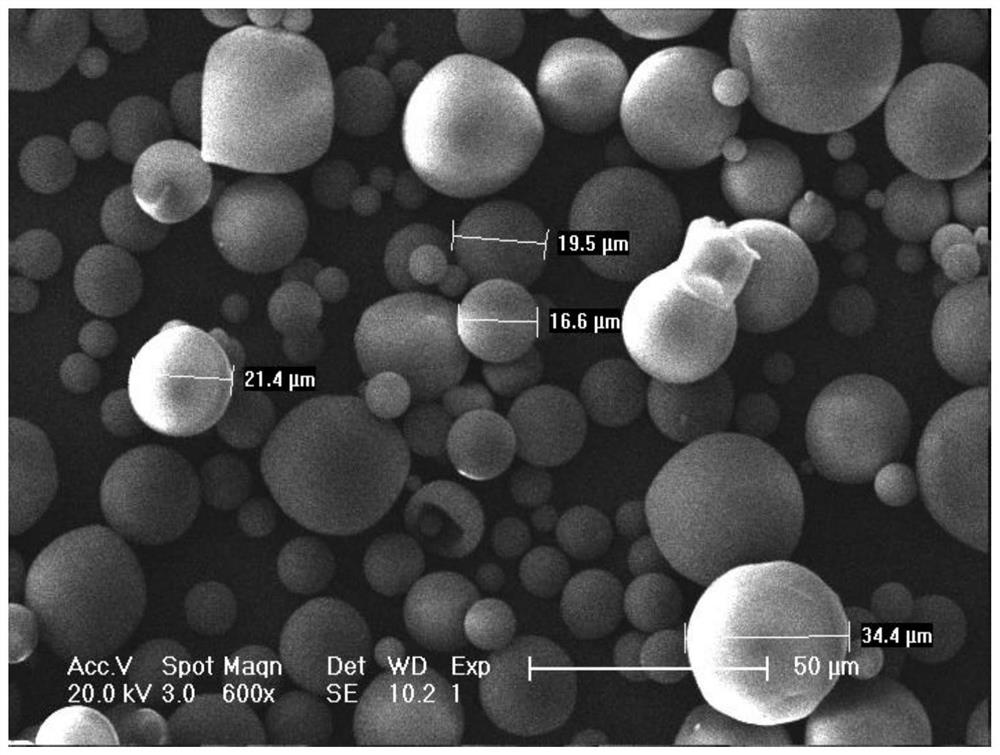
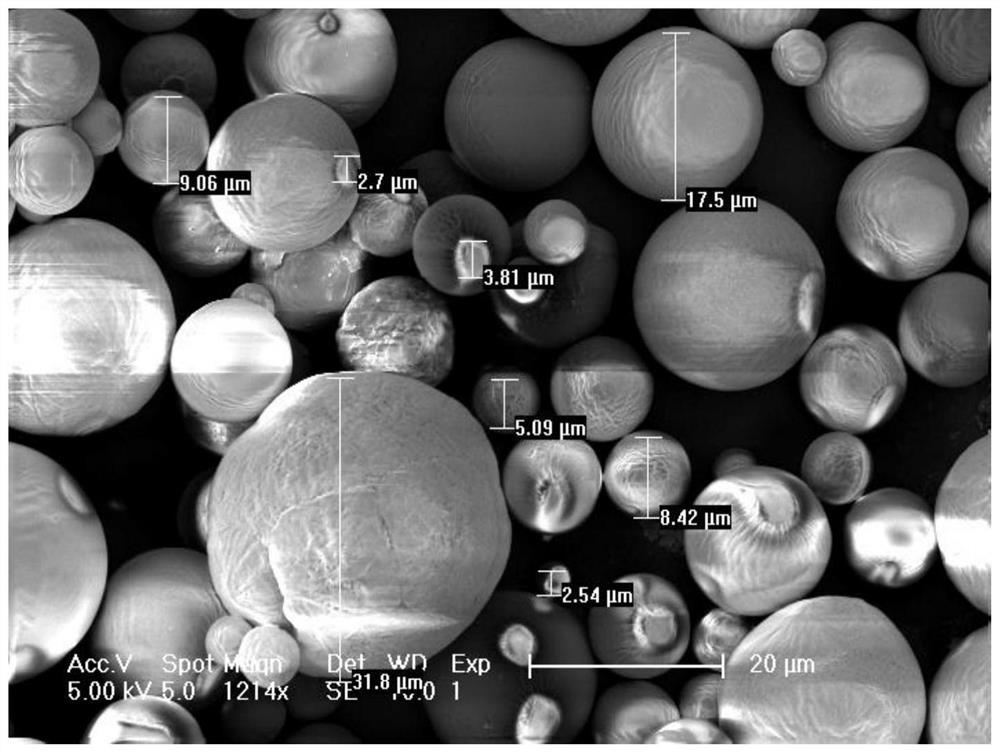
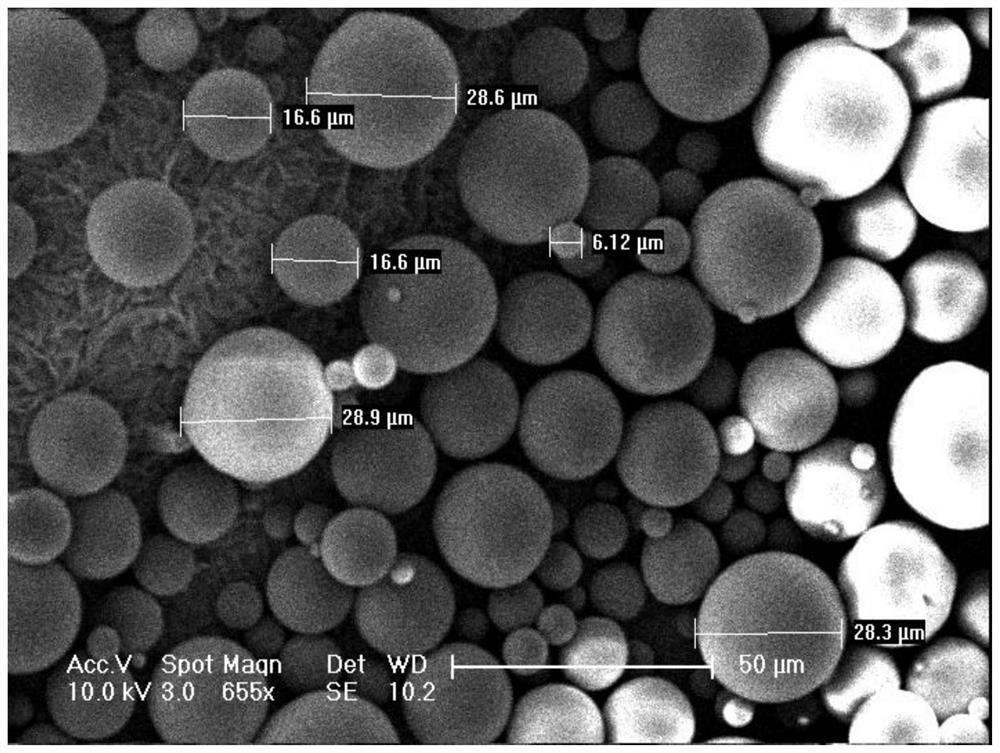
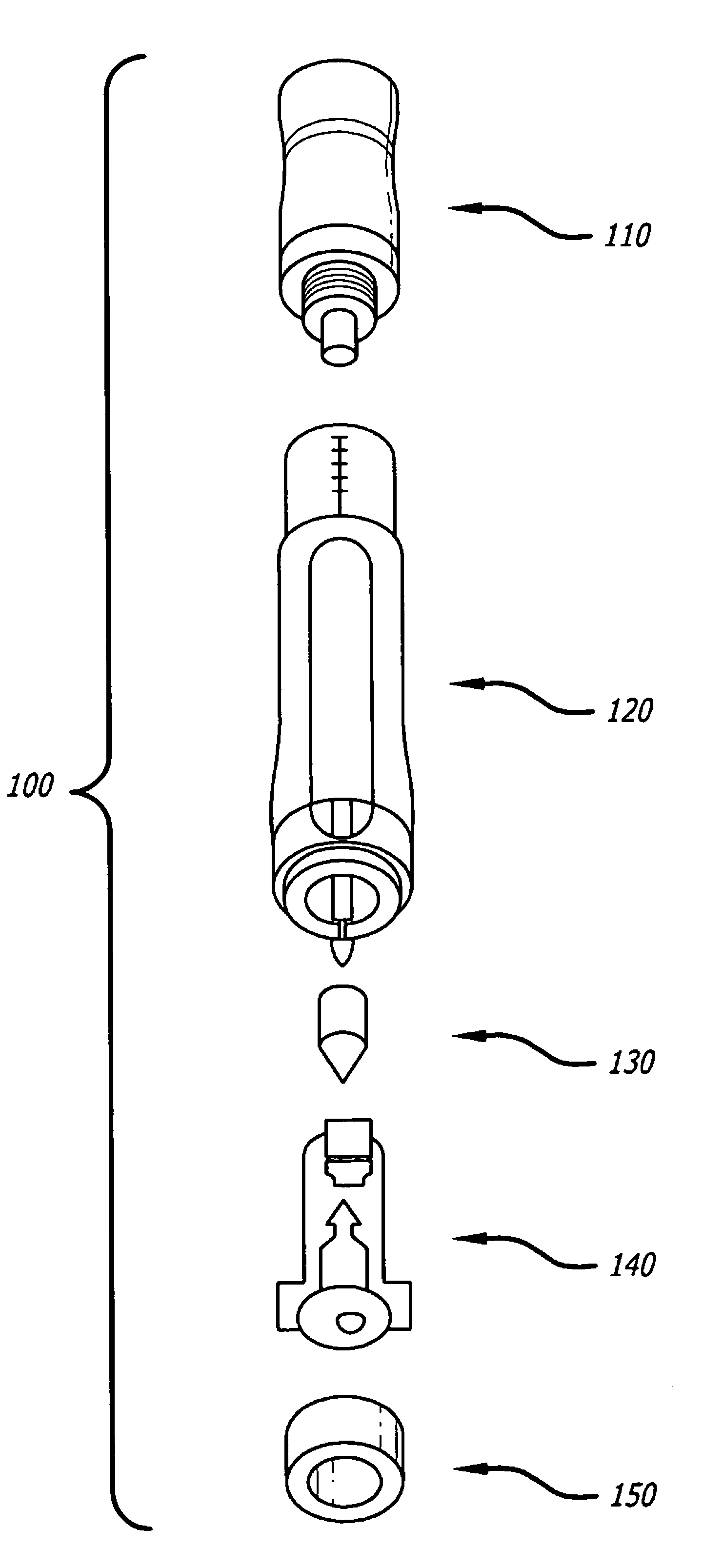
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61 results about "Skin Fillers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
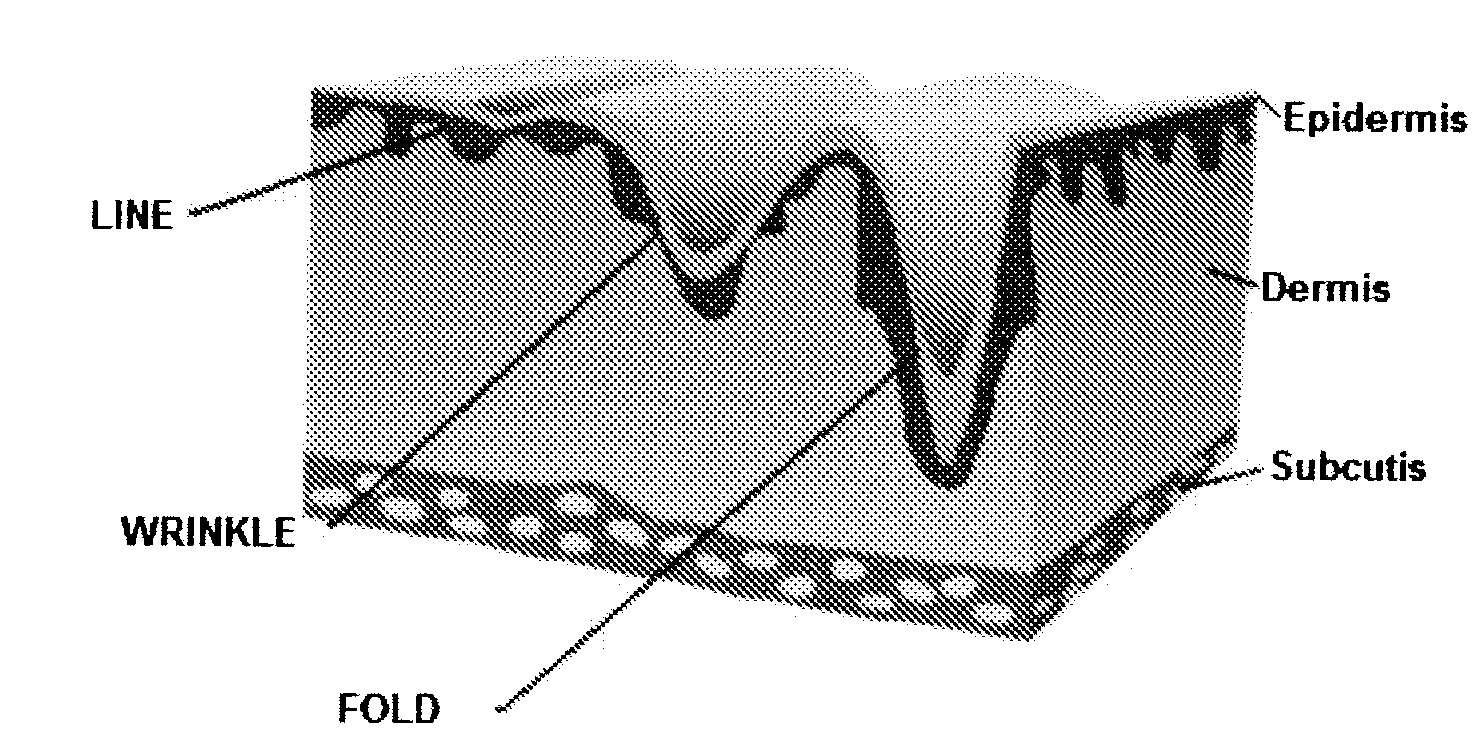
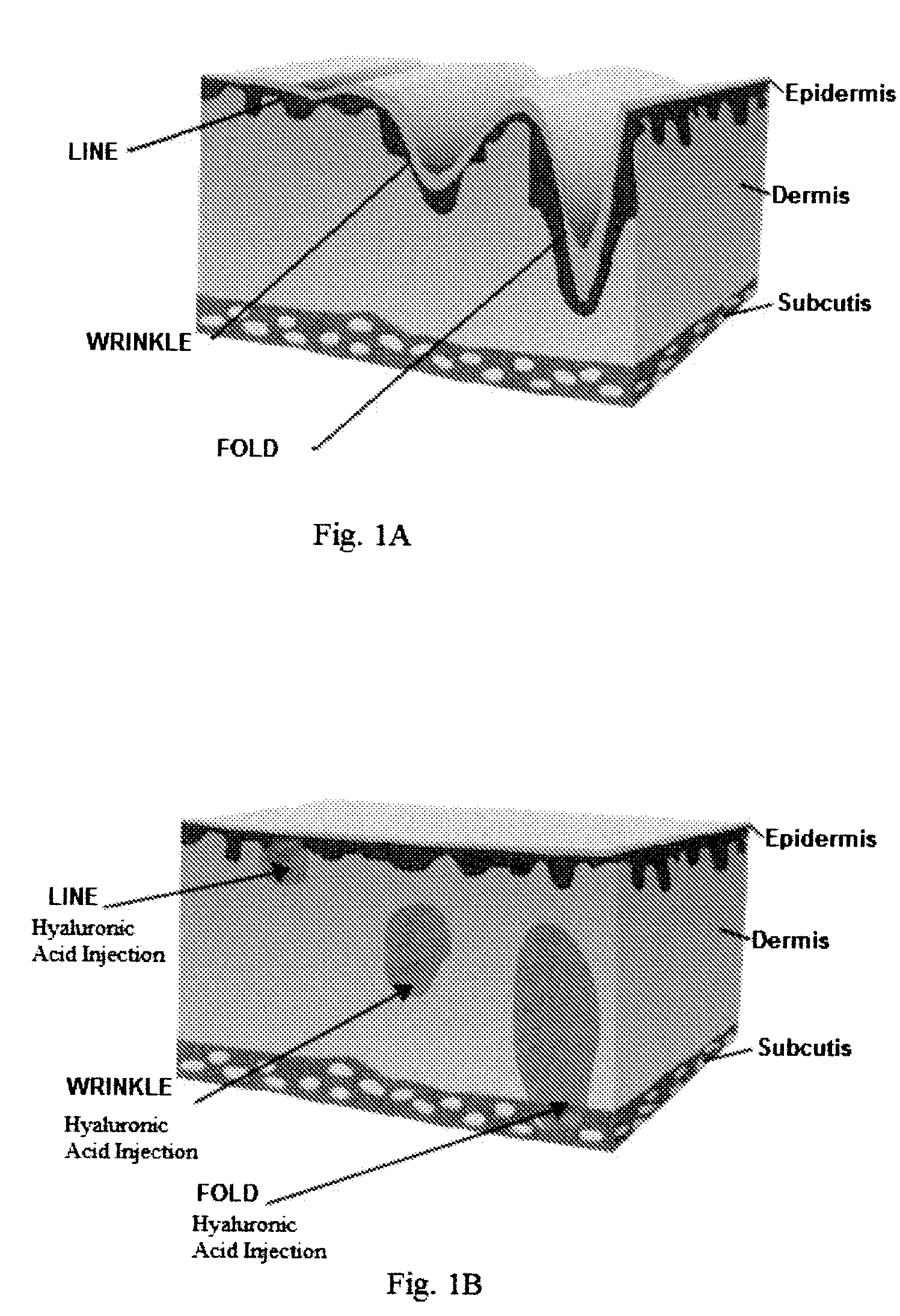
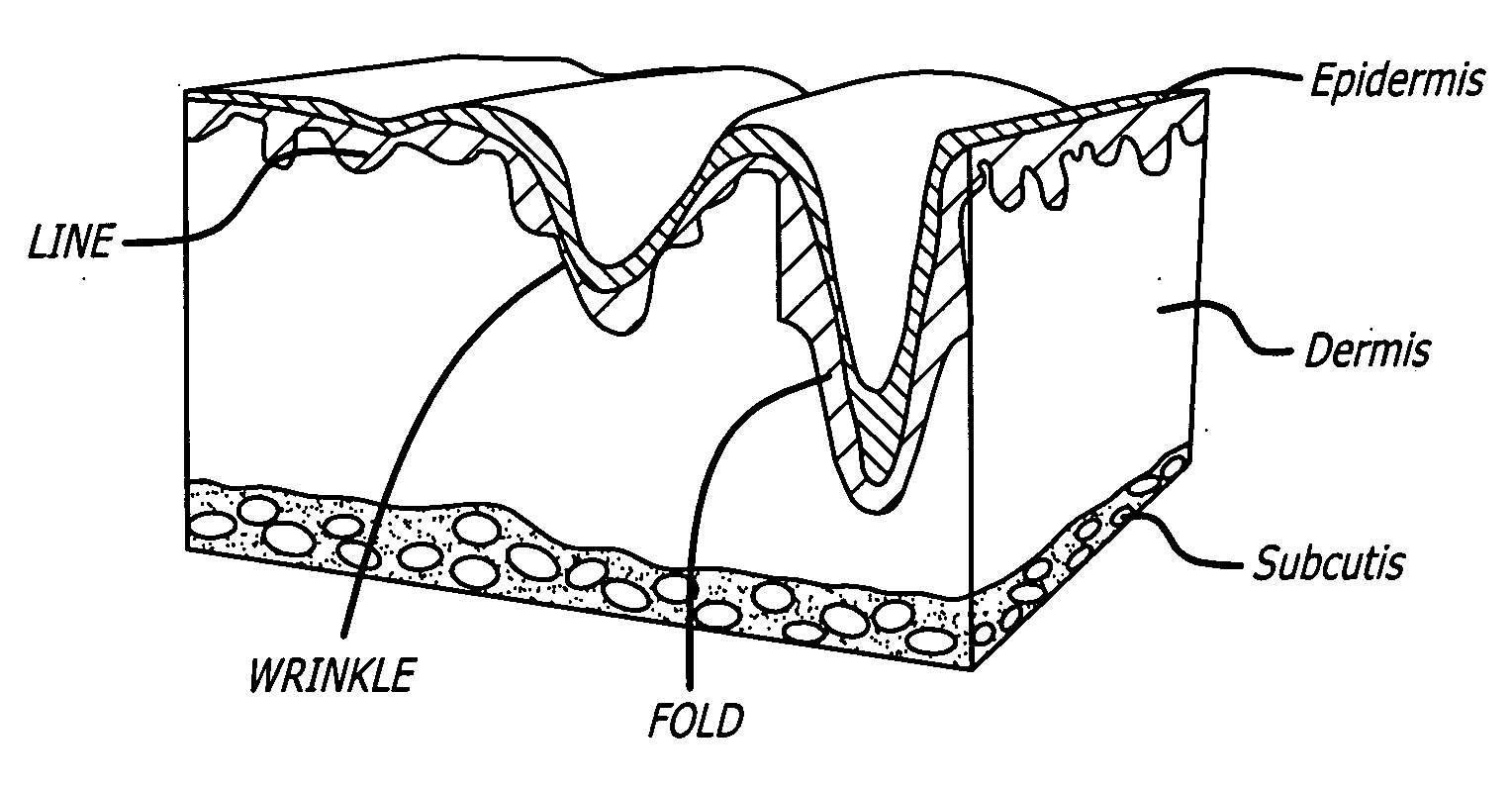
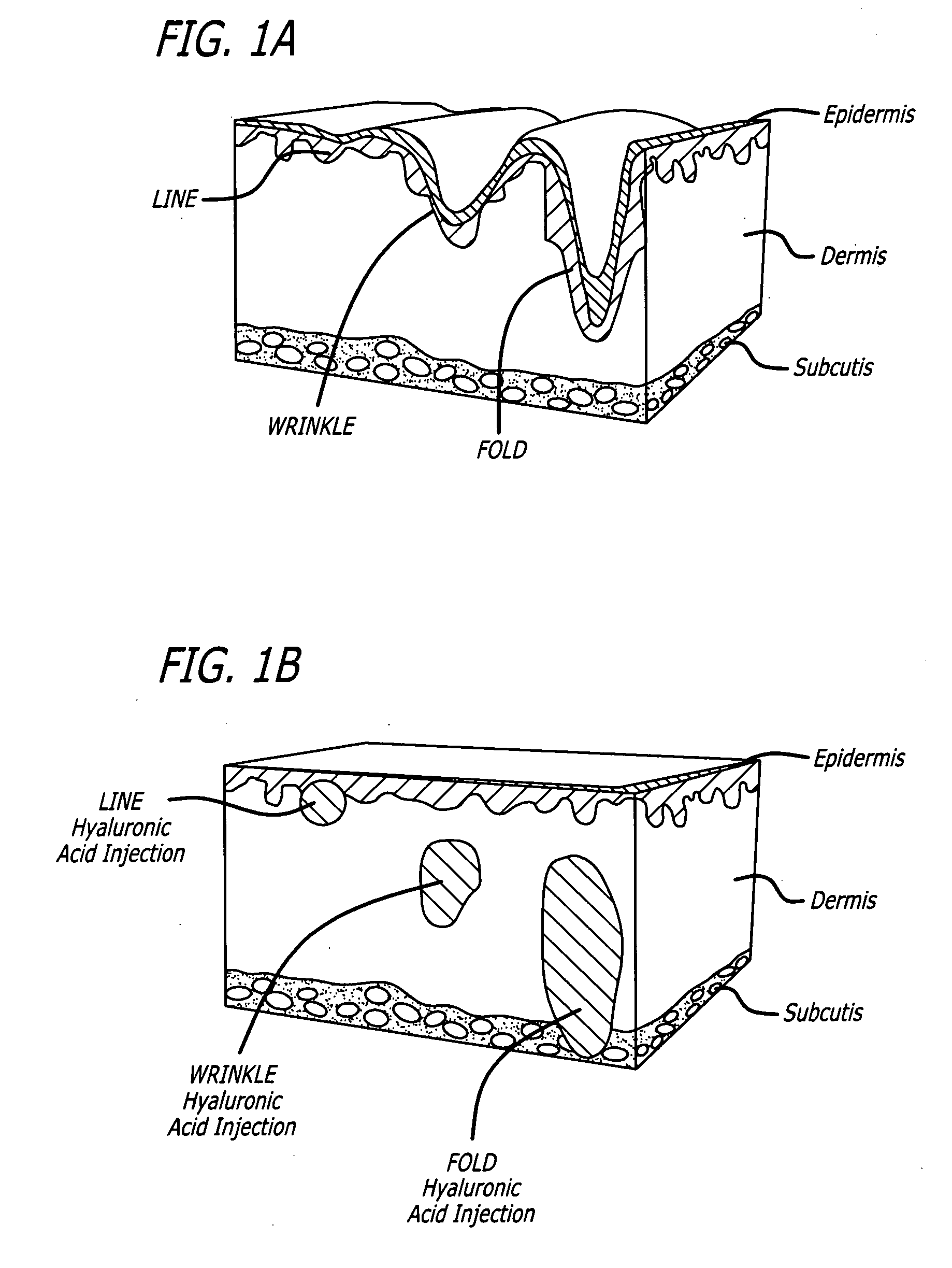
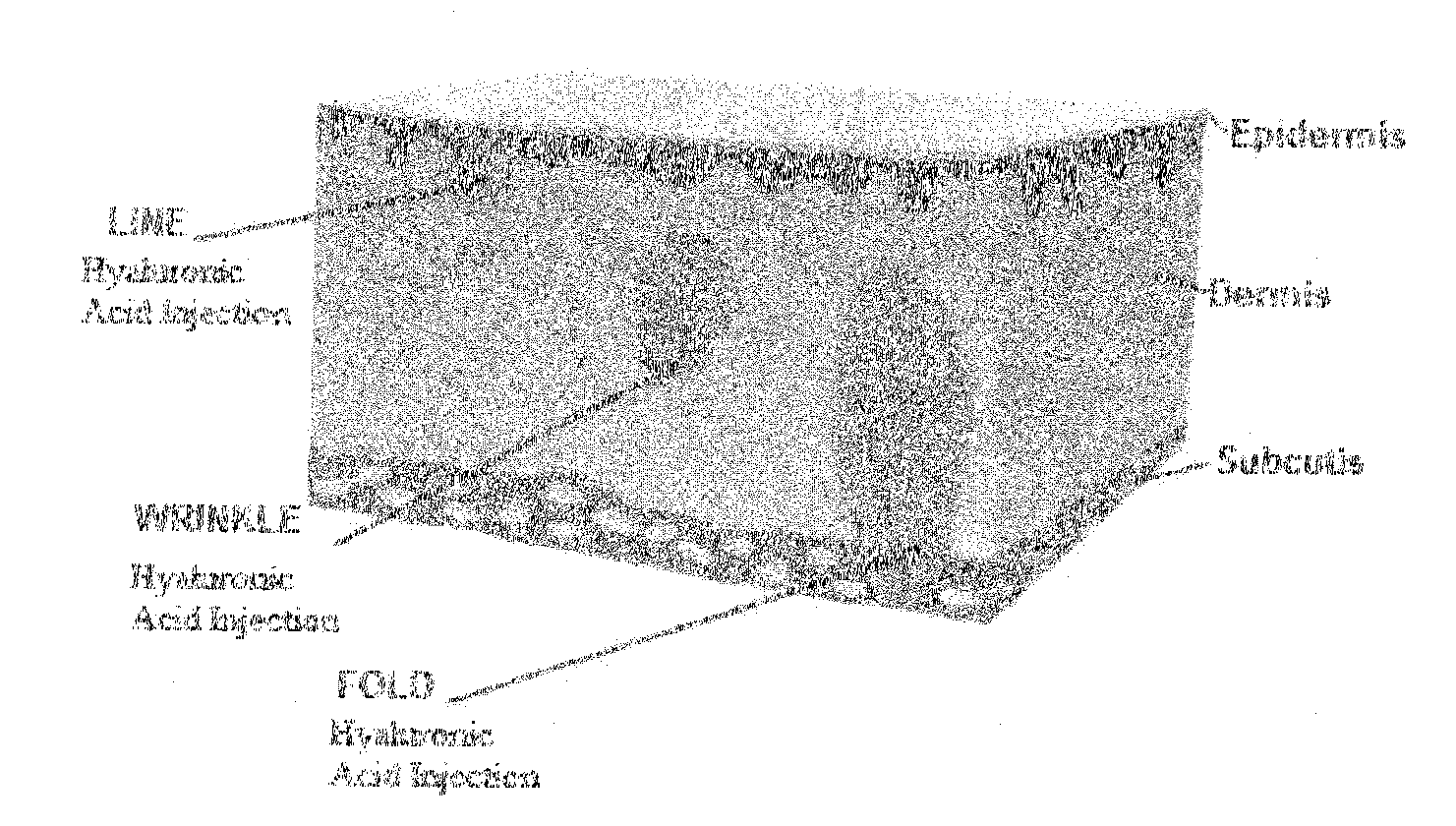
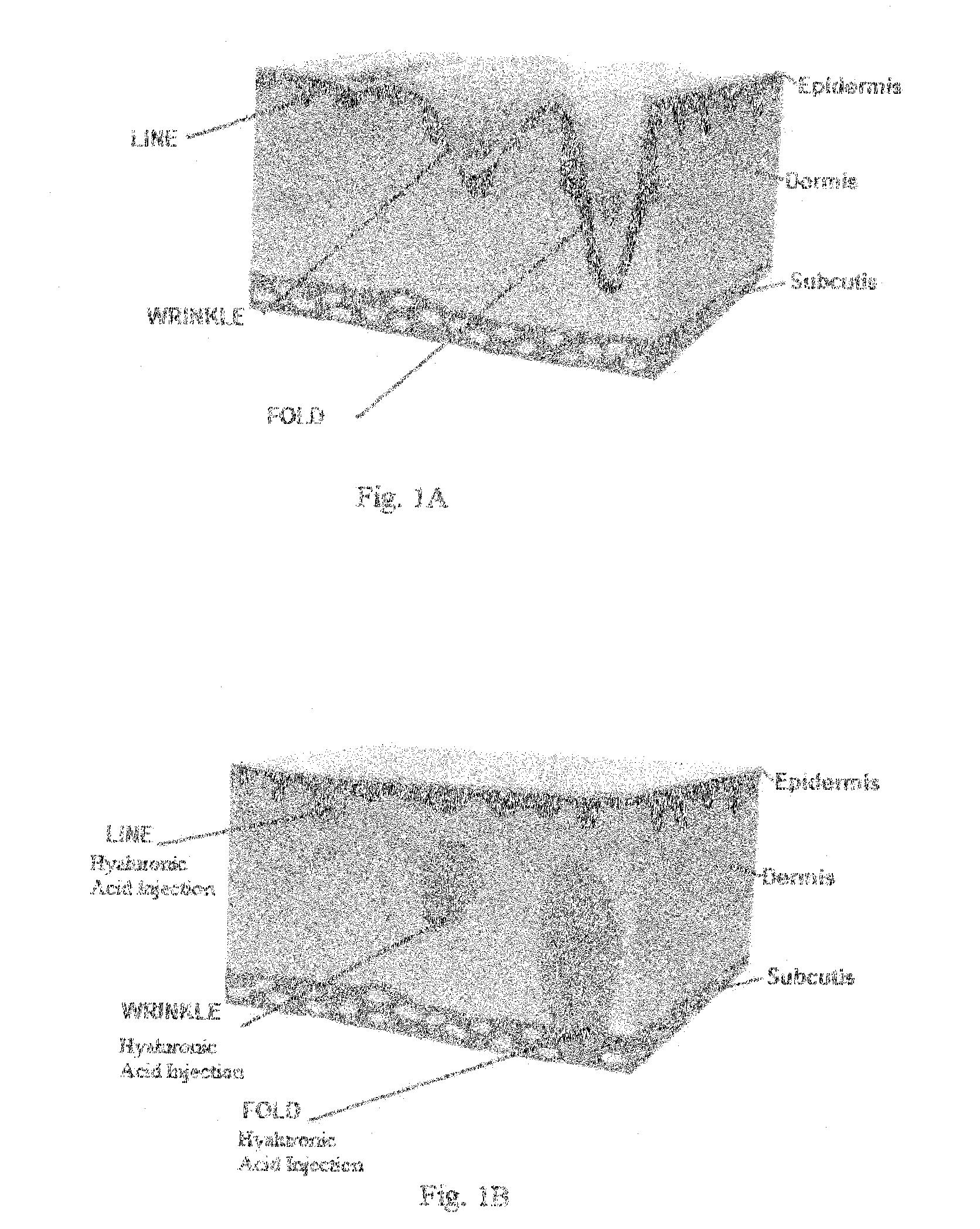
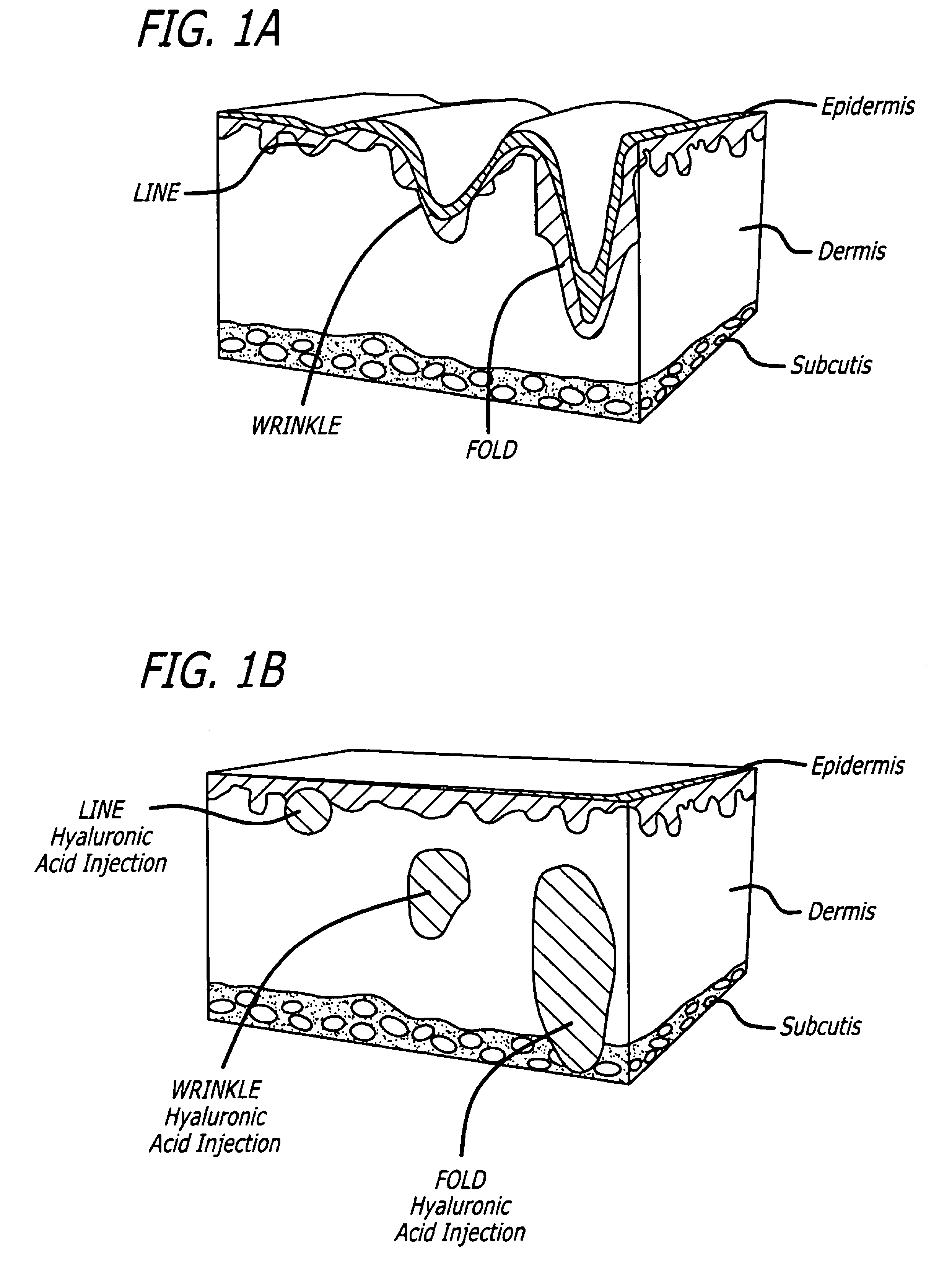
Facial fillers are products such as collagen, hyaluronic acid and calcium hydroxyl apatite that rejuvenate facial skin by reducing or eliminating wrinkles, raising scar depressions, enhancing lips and replacing soft-tissue volume loss through facial injections. With age, our skin becomes more susceptible to wrinkles and sagging.
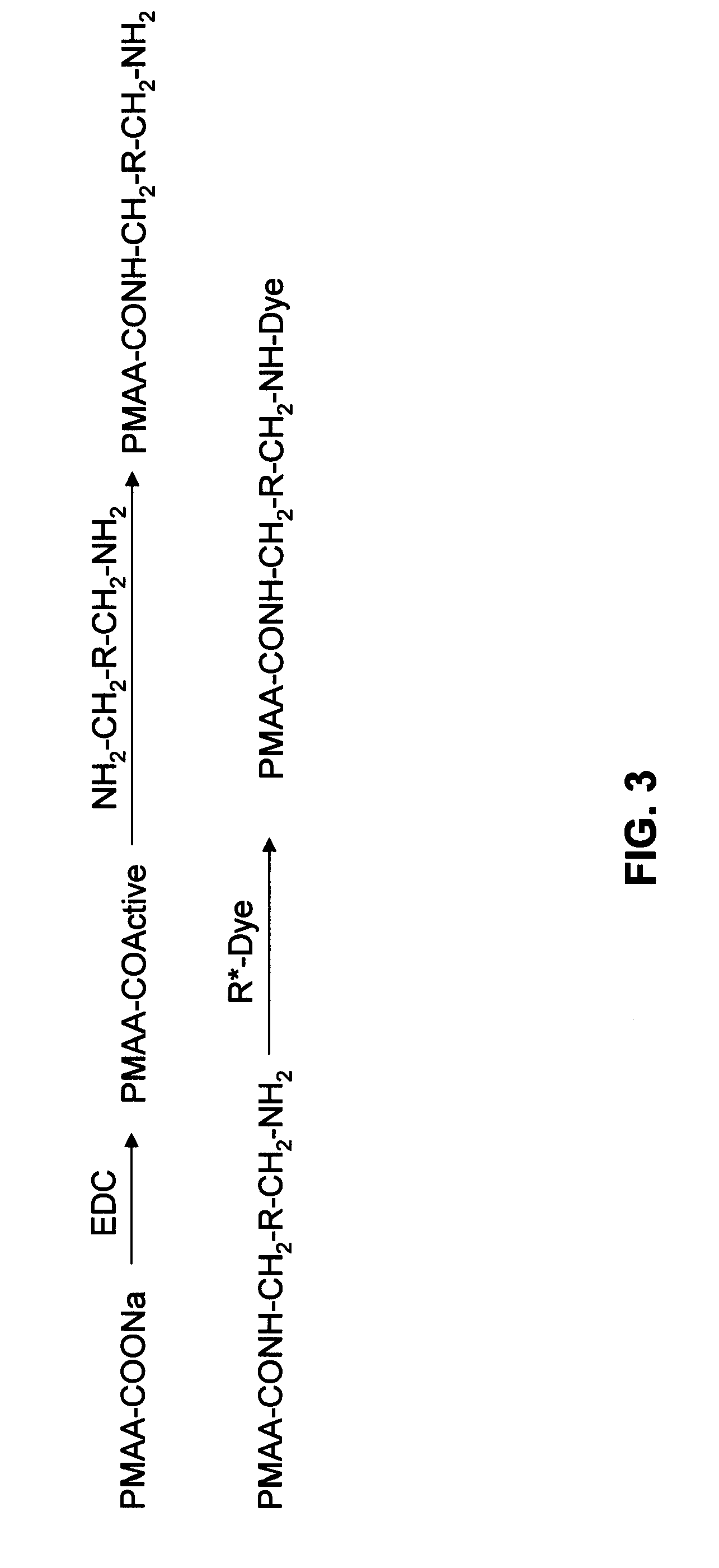
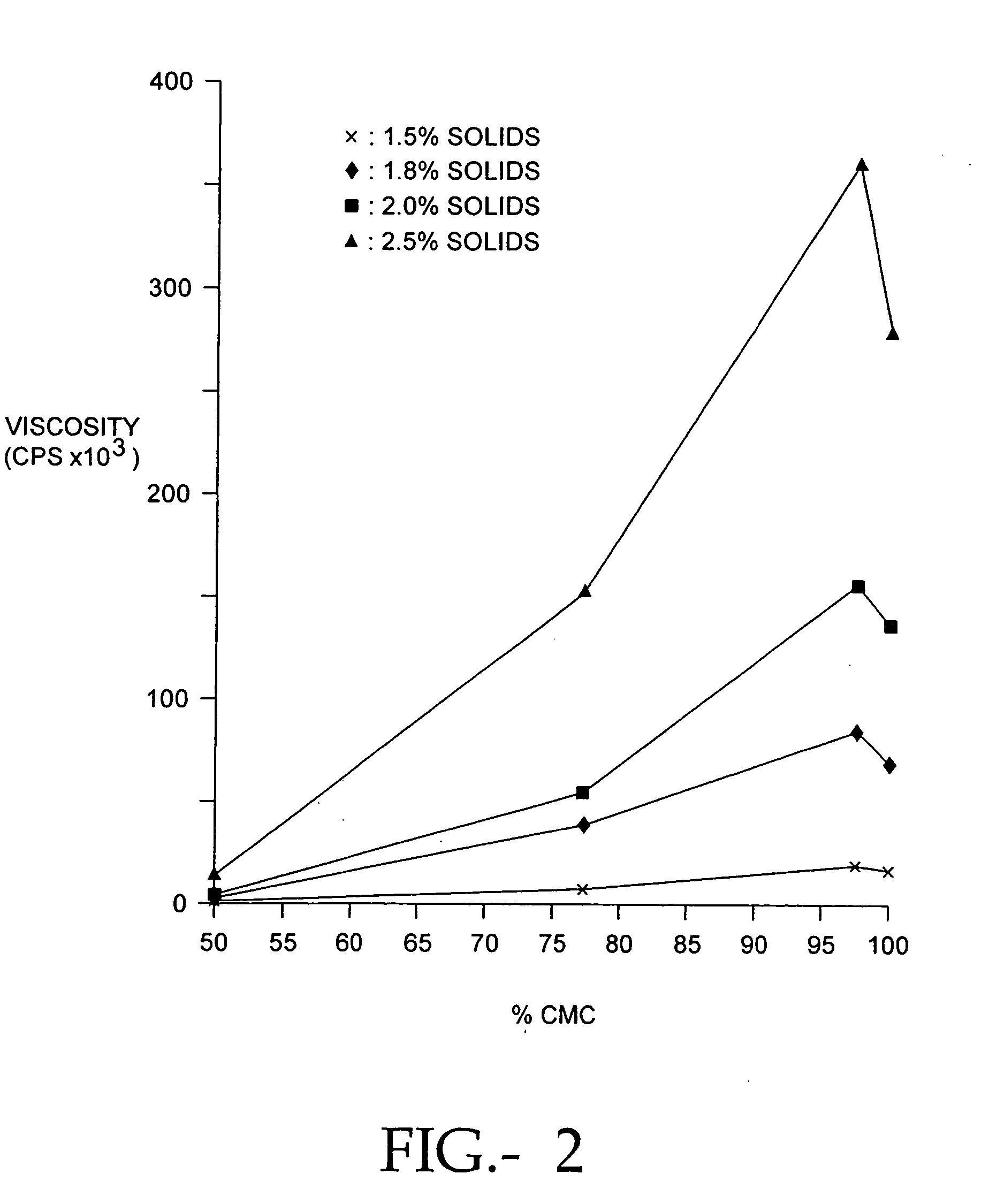
Compositions of polyacids and polyethers and methods for their use as dermal fillers
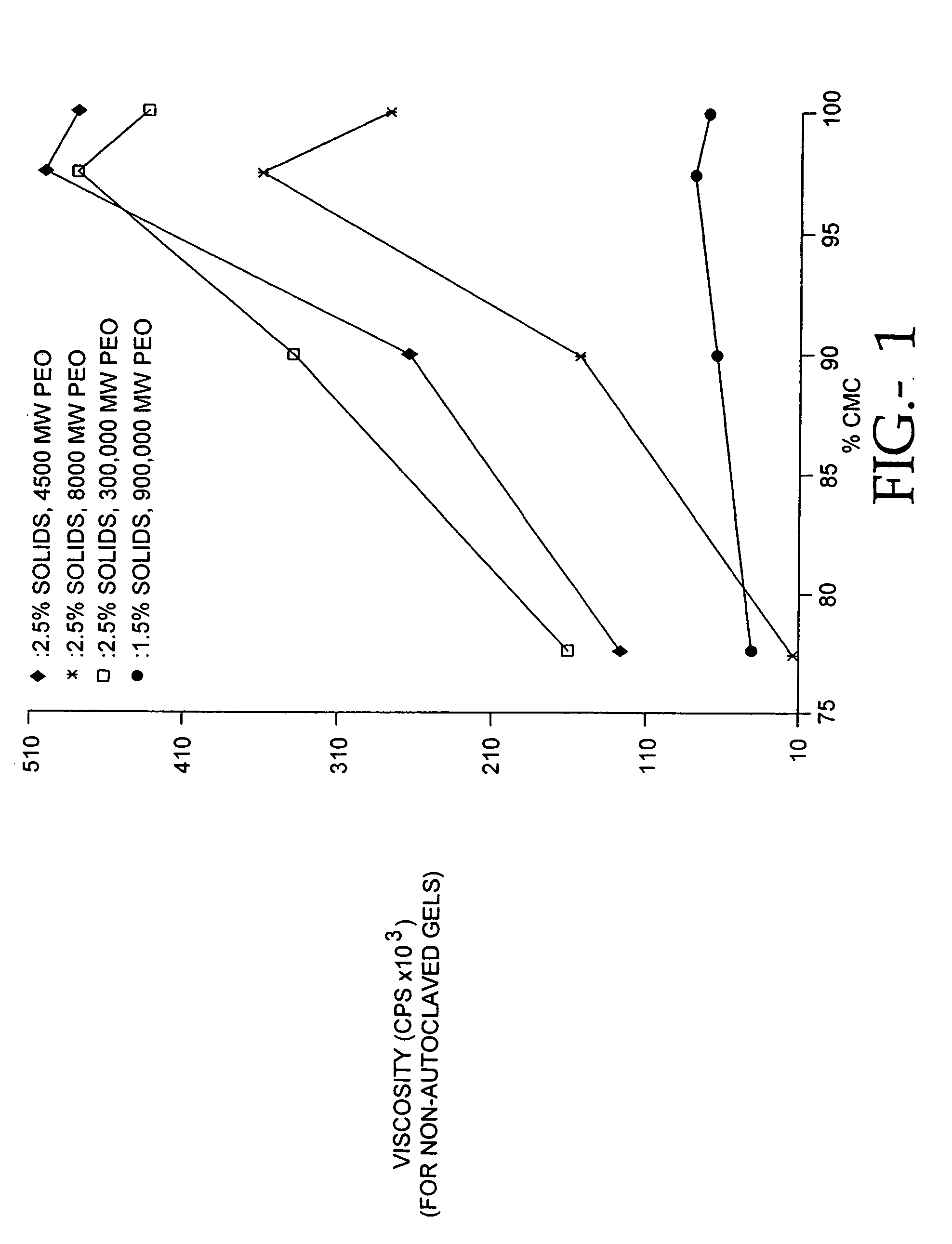
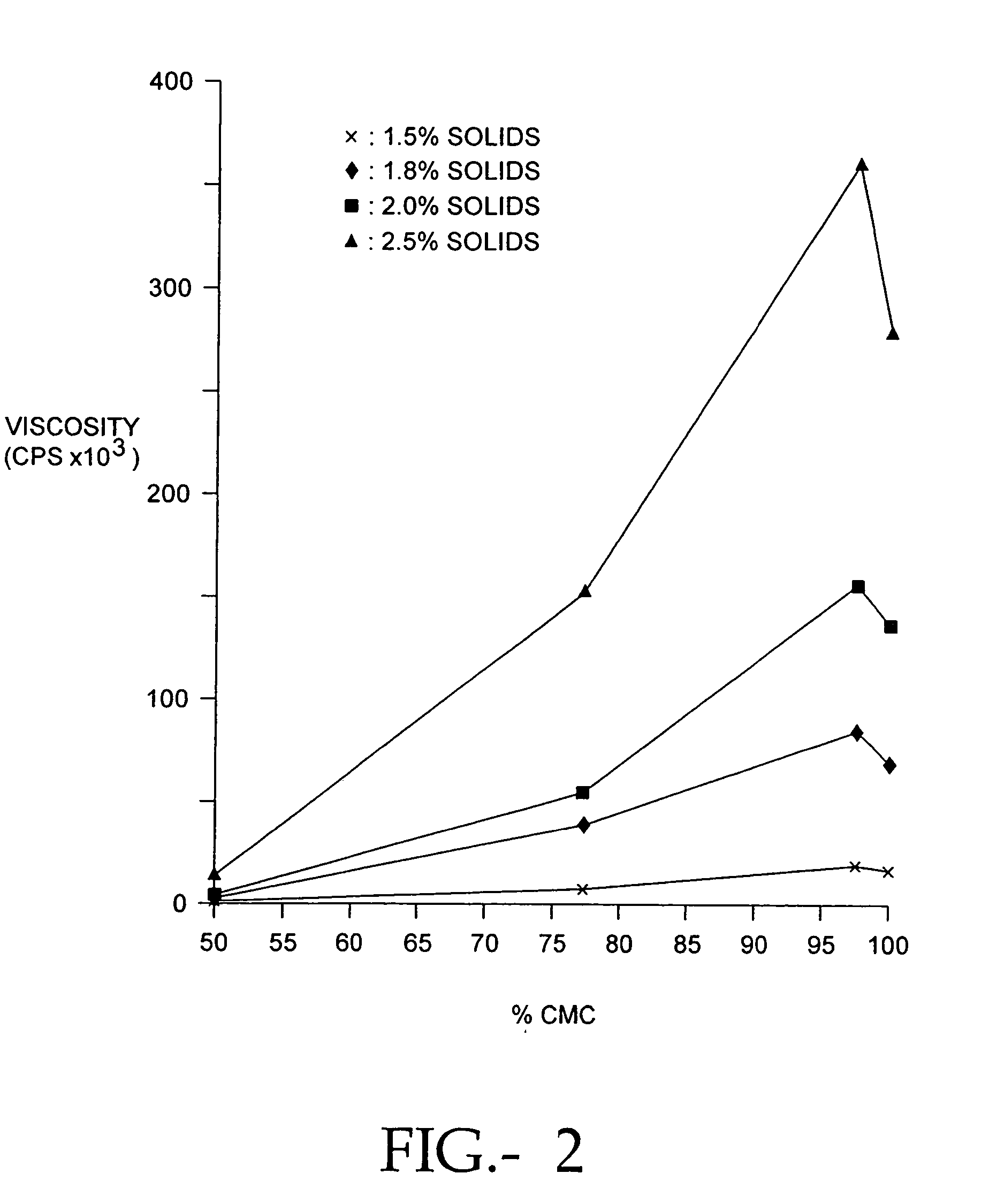
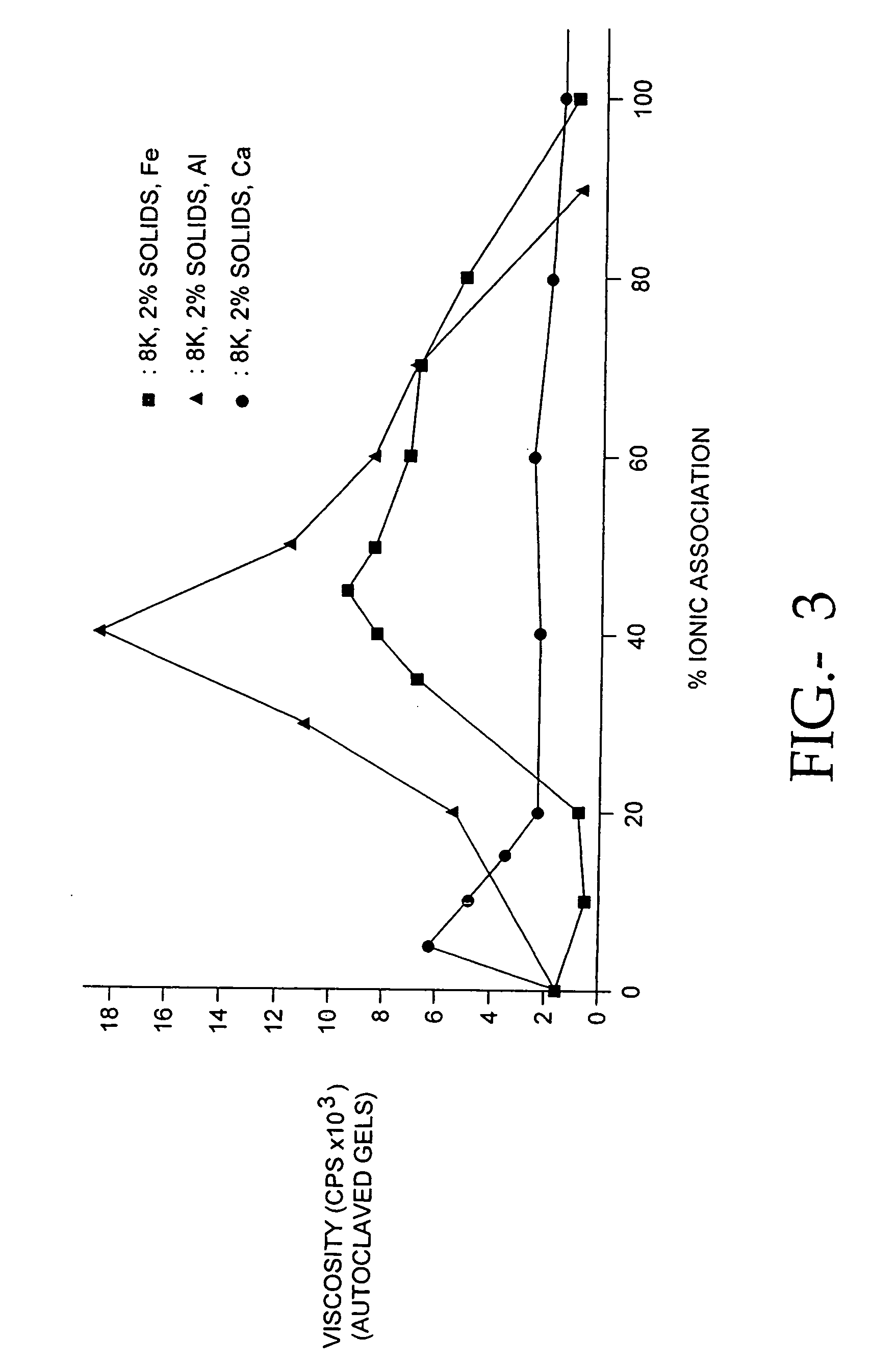
The present invention relates to improved methods for filling the skin for cosmetic or medical purposes. Compositions comprising carboxymethyl cellulose (CMC), polyethylene oxide (PEO) and calcium ions can be made and have physical properties that depend on the amounts and types of CMC, PEO, and calcium ions to form ioniclaly cross-linked gels. Compositions can be formed into microspheres, coascervates, gels, or membranes. Gels, microspheres and coascervates can be injected directly into a site for dermal filling. Membranes can be surgically introduced, where they swell to form hydrated gels. After introduction, the dermal filler persists for a period of time and then can disintegrate and be removed from the body.
Owner:FZIOMED
Alloplastic injectable dermal filler and methods of use thereof
InactiveUS20090110736A1Reduce wrinklesReduce scarsPowder deliveryCosmetic preparationsFiller ExcipientReticular Dermis
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
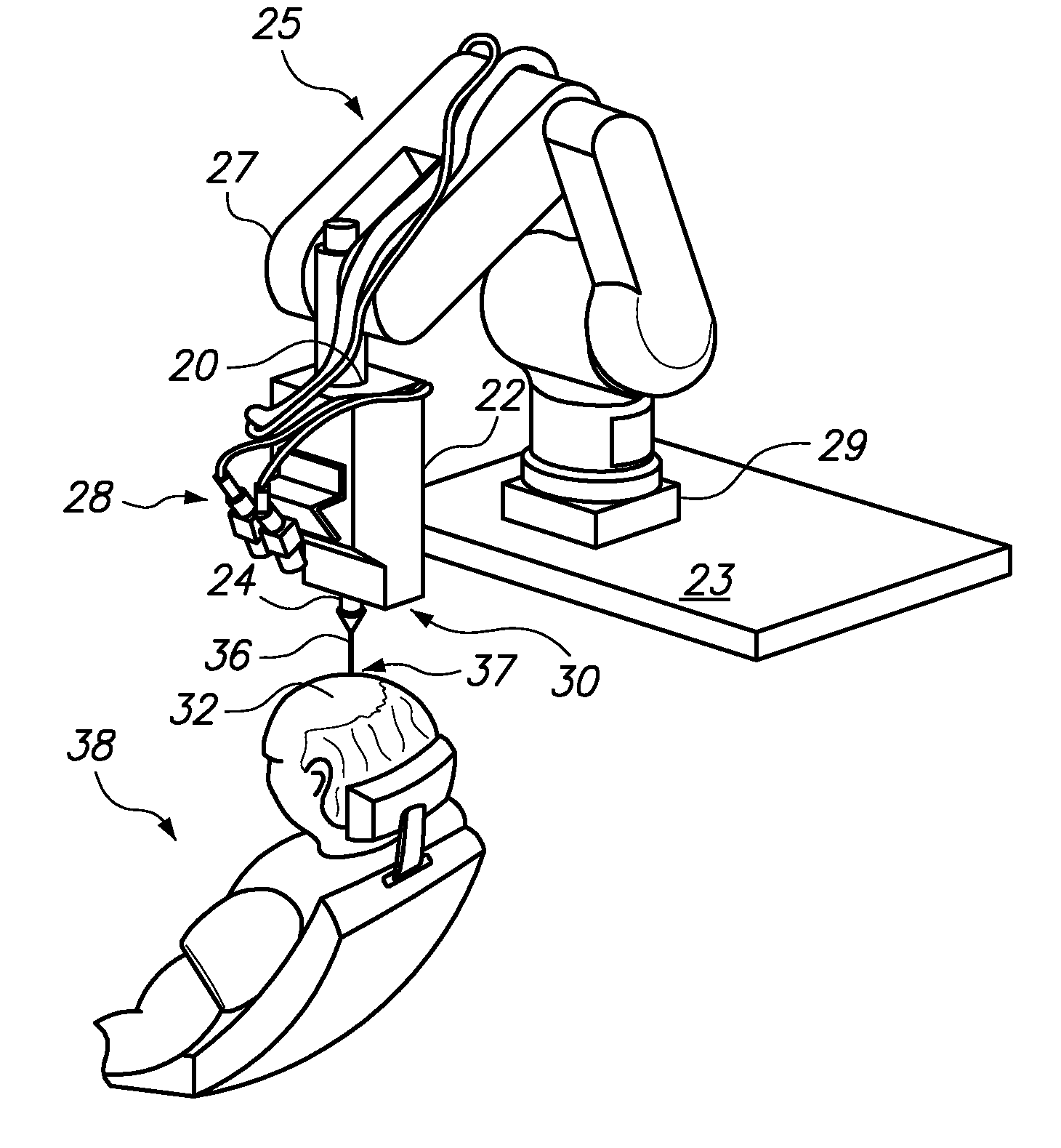
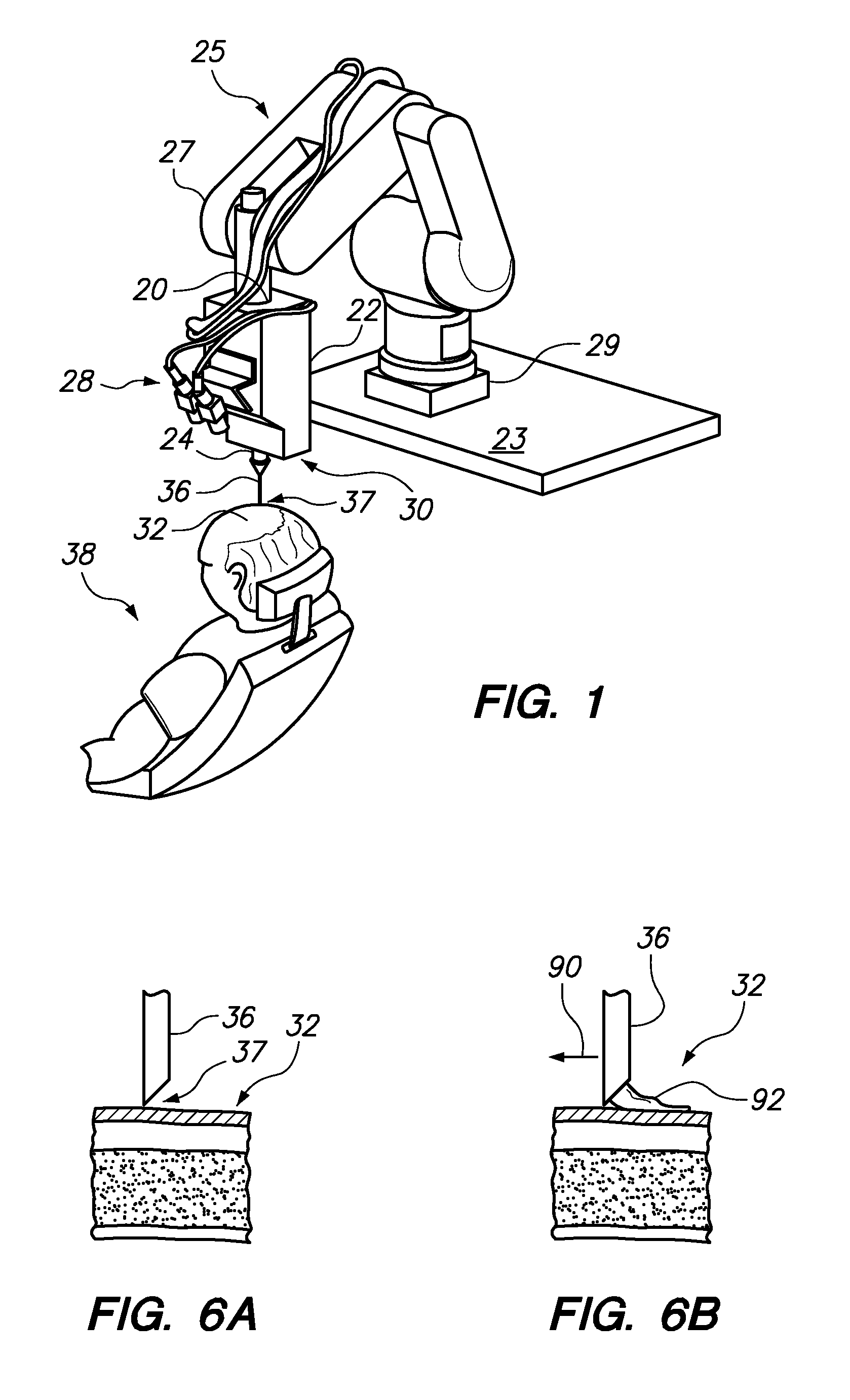
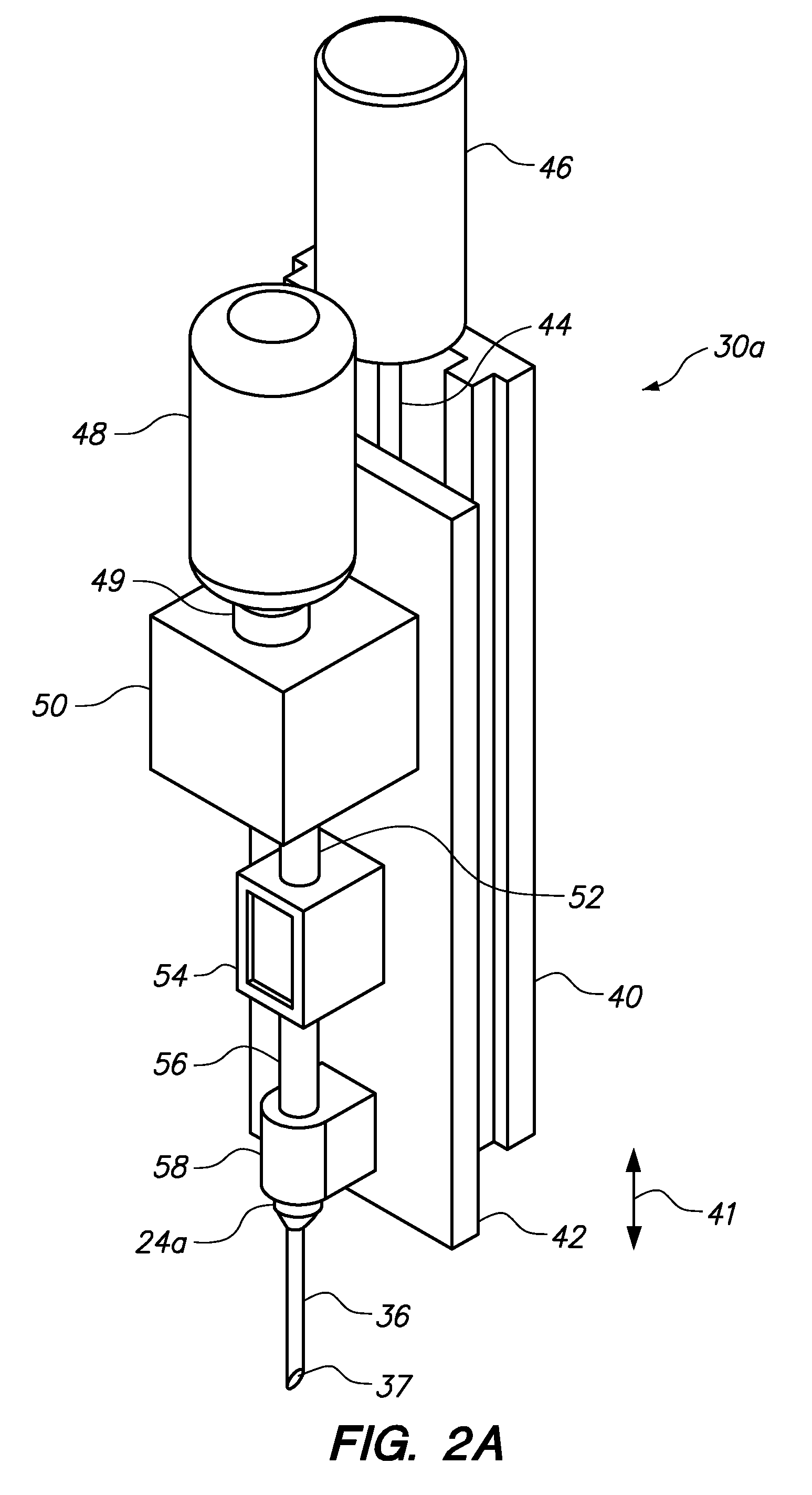
Automated delivery of a therapeutic or cosmetic substance to cutaneous, subcutaneous and intramuscular tissue regions
Owner:VENUS CONCEPT INC
Coated Hyaluronic Acid Particles
The present invention generally relates to particles comprising hyaluronic acid, wherein the particles are coated or encapsulated with a coating. The coating preferably comprises a polymer, protein, polysaccharide, or combination thereof that decreases the rate of degradation of the hyaluronic acid once the particles are placed in an aqueous environment, such as inside mammalian skin. The compositions of the present invention comprising such coated hyaluronic acid are useful for soft tissue augmentation, and are particularly useful as dermal fillers.
Owner:ALLERGAN INC
Tunable crosslinked polysaccharide compositions
InactiveUS20120071437A1Eliminate symptomsImprove the situationBiocideOrganic active ingredientsPolyethylene glycolPolysaccharide
The present specification generally relates to injectable dermal fillers including multifunctional polyethylene glycol-based crosslinking agents, hydrogel compositions comprising a matrix polymer crosslinked with such crosslinking agents, and methods of treating a soft tissue condition using such hydrogel compositions.
Owner:ALLERGAN INC
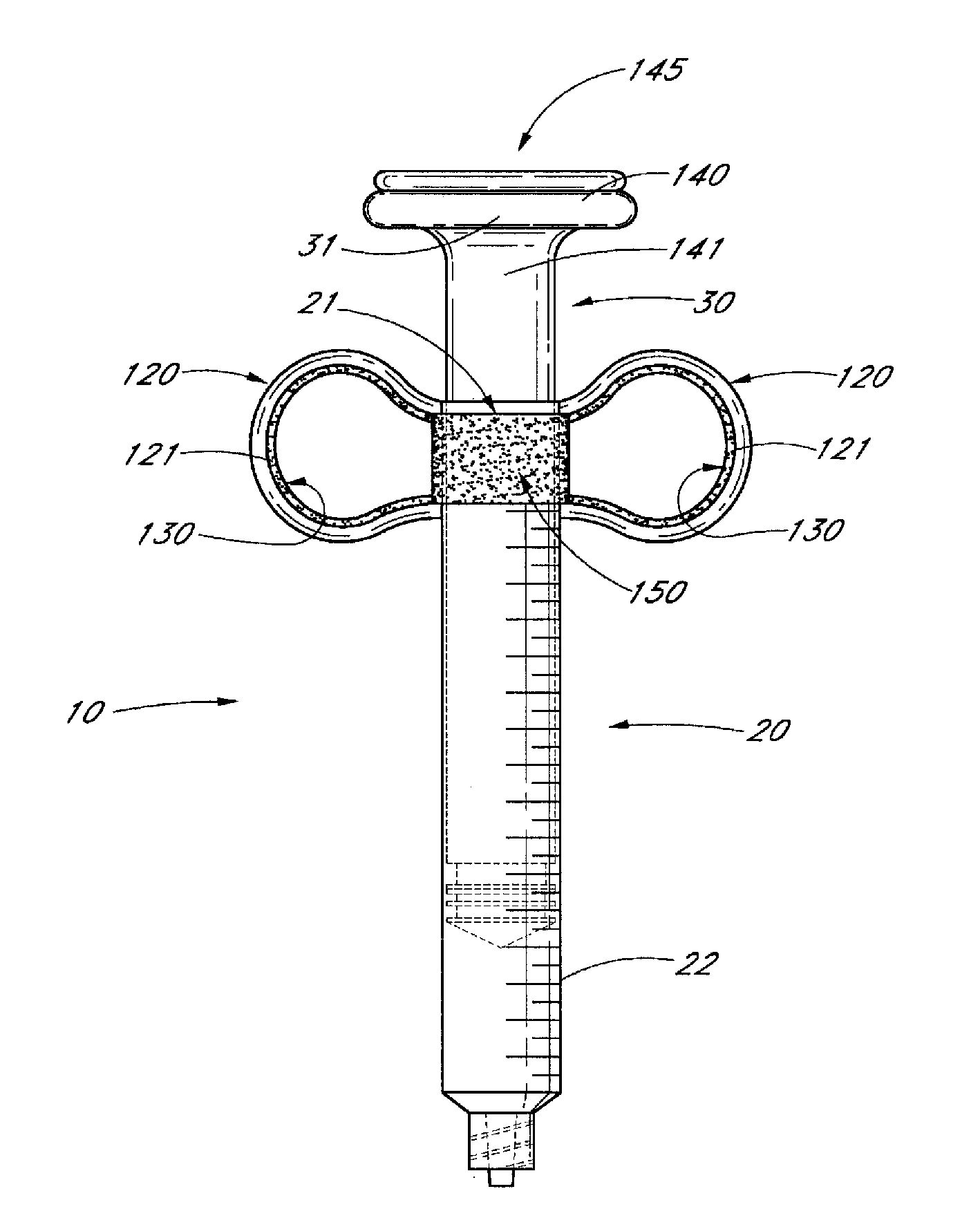
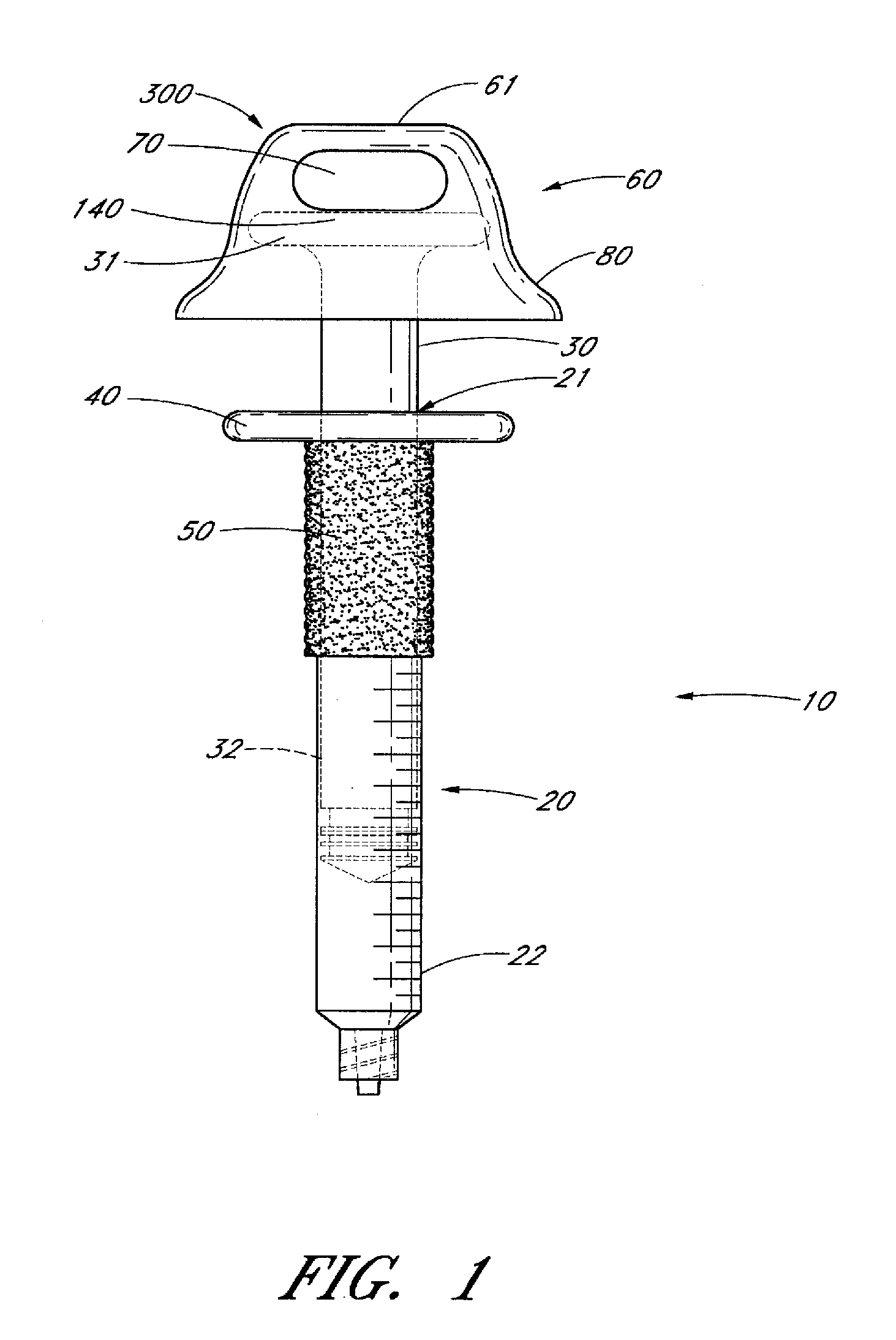
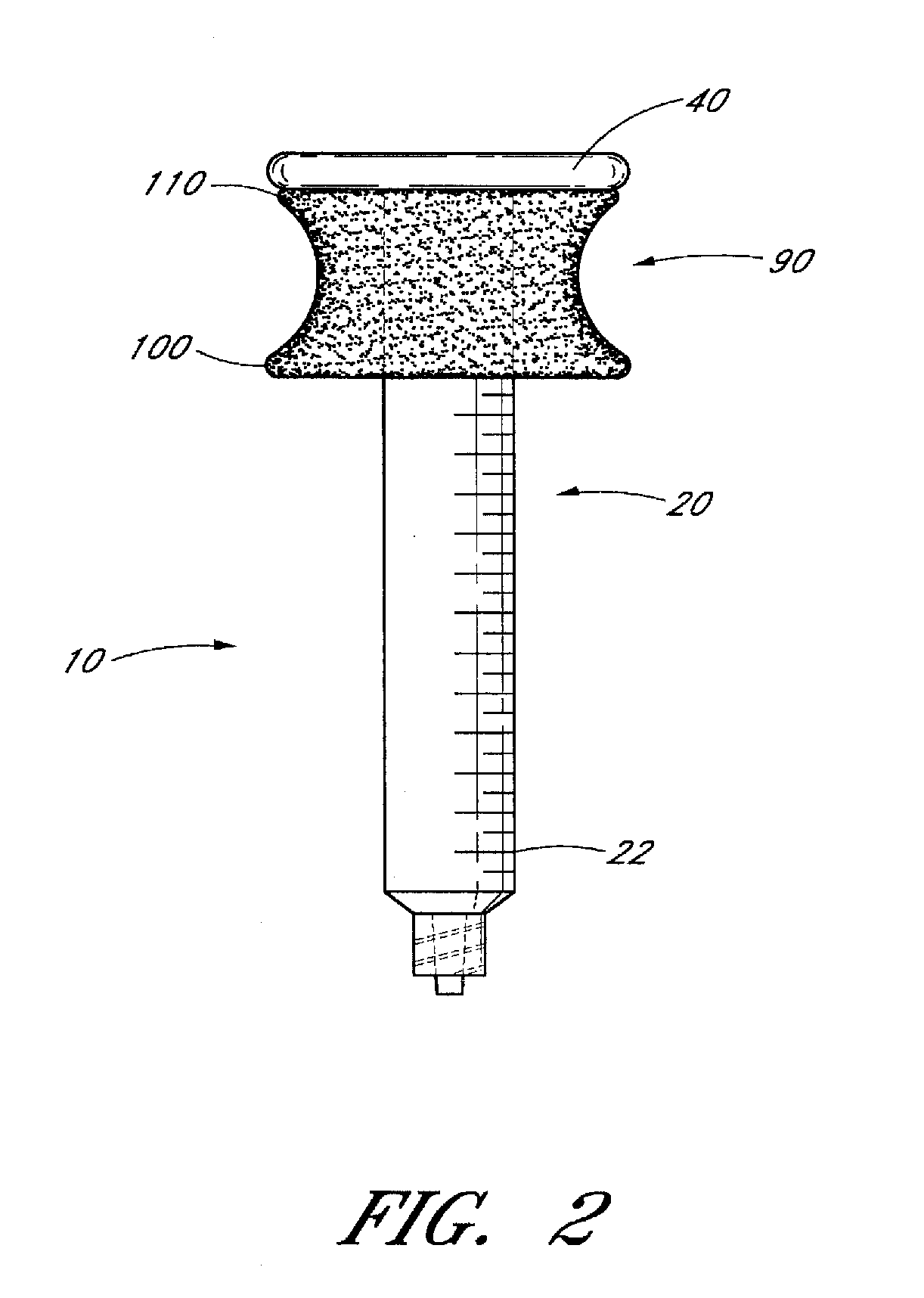
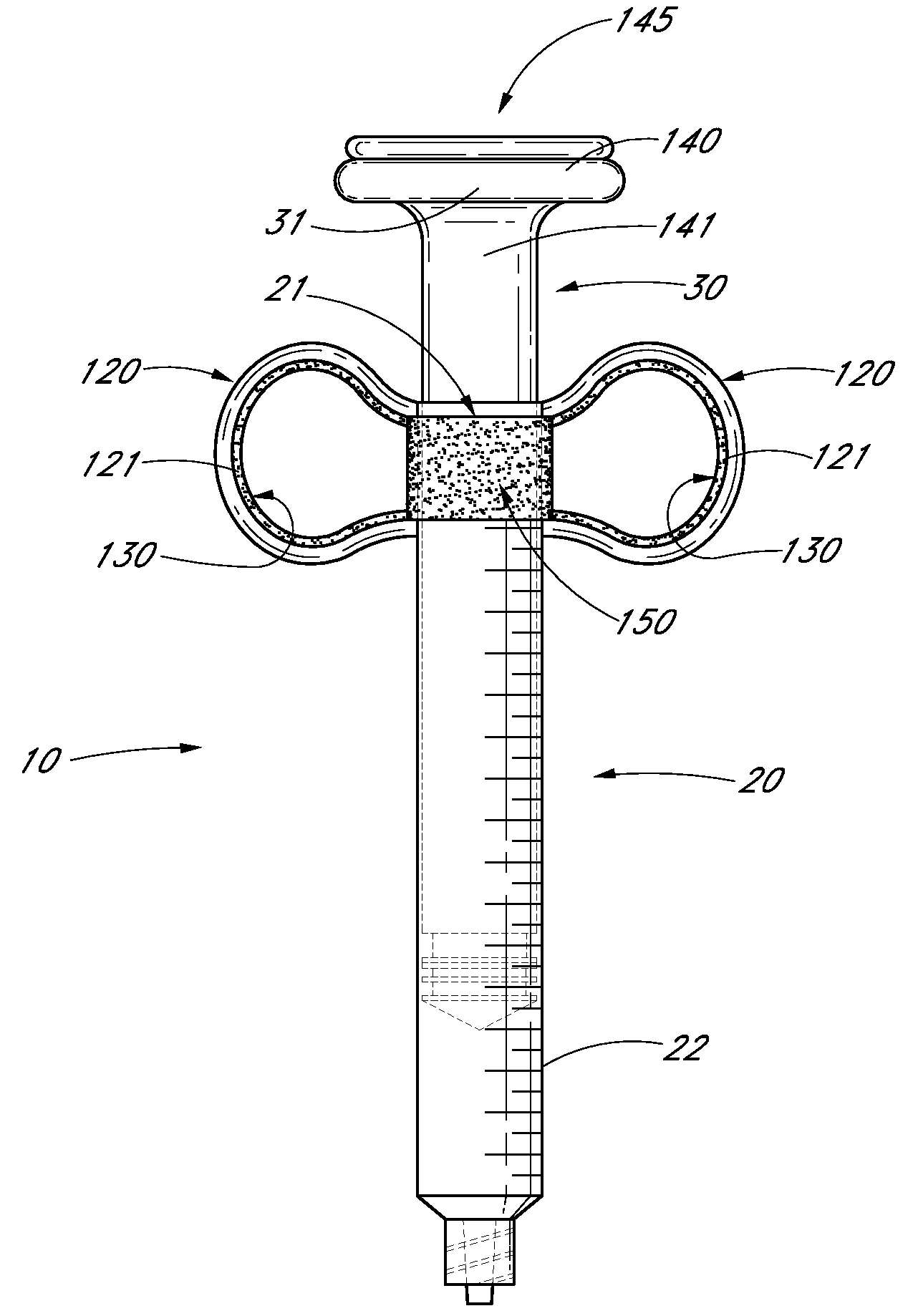
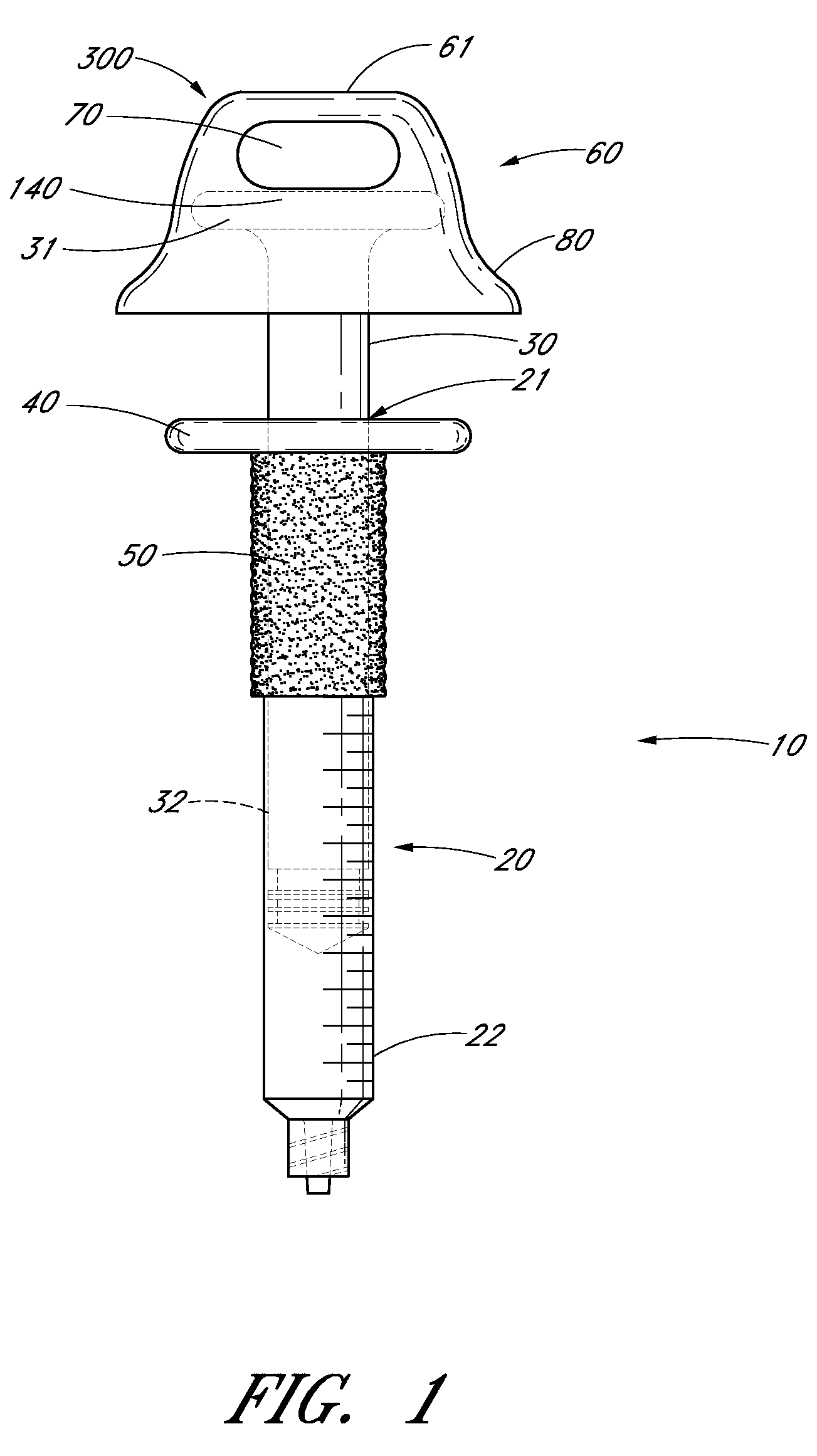
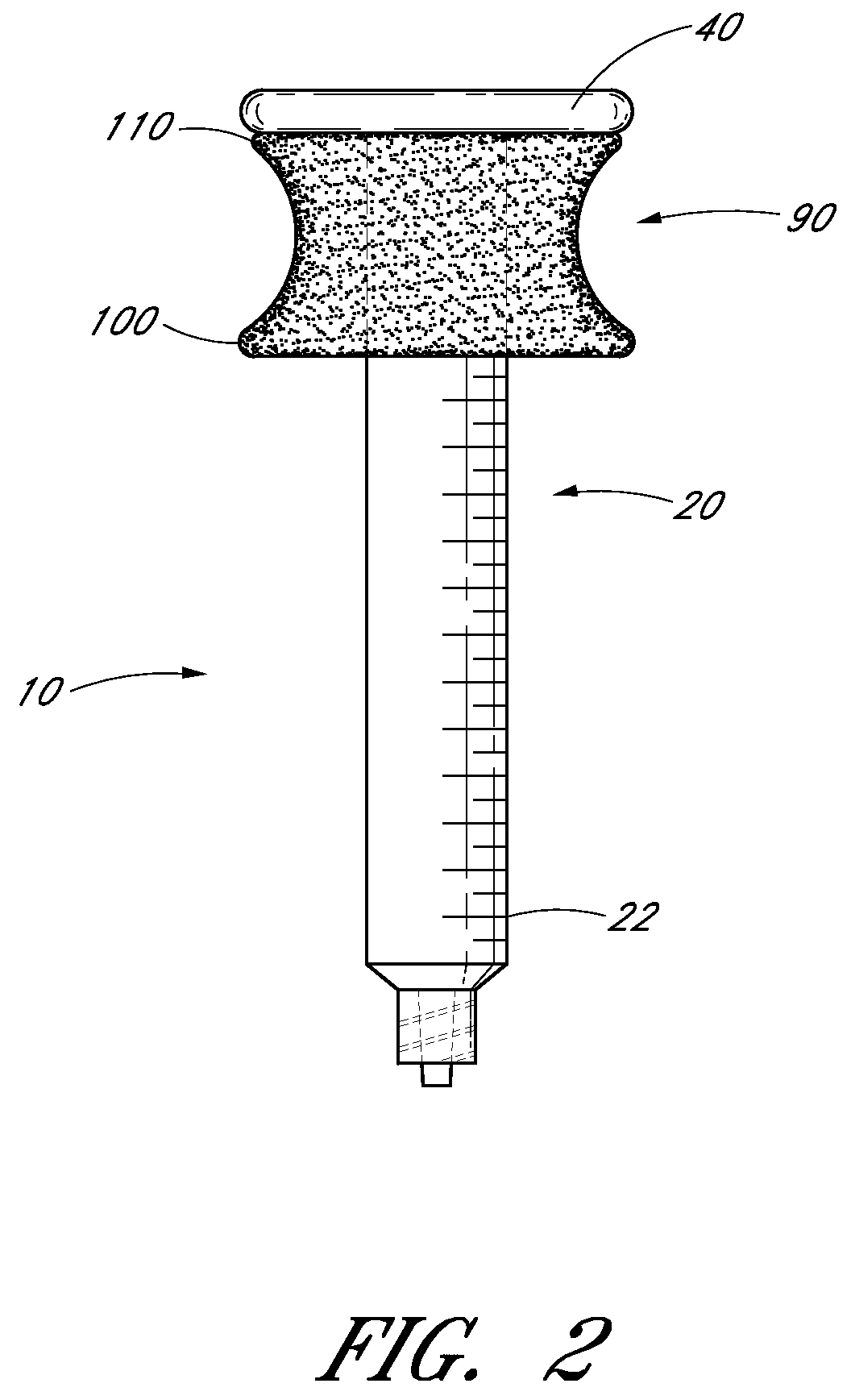
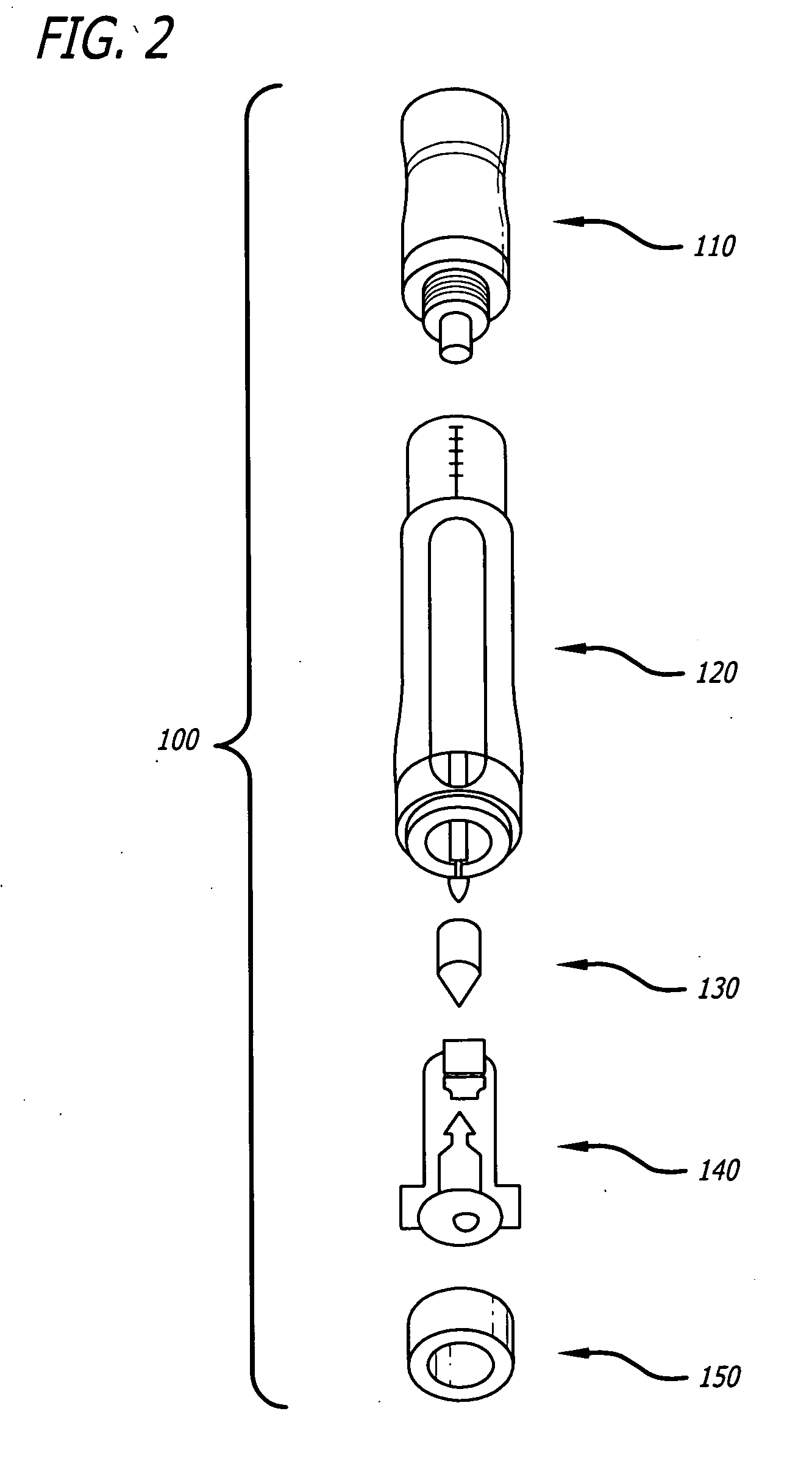
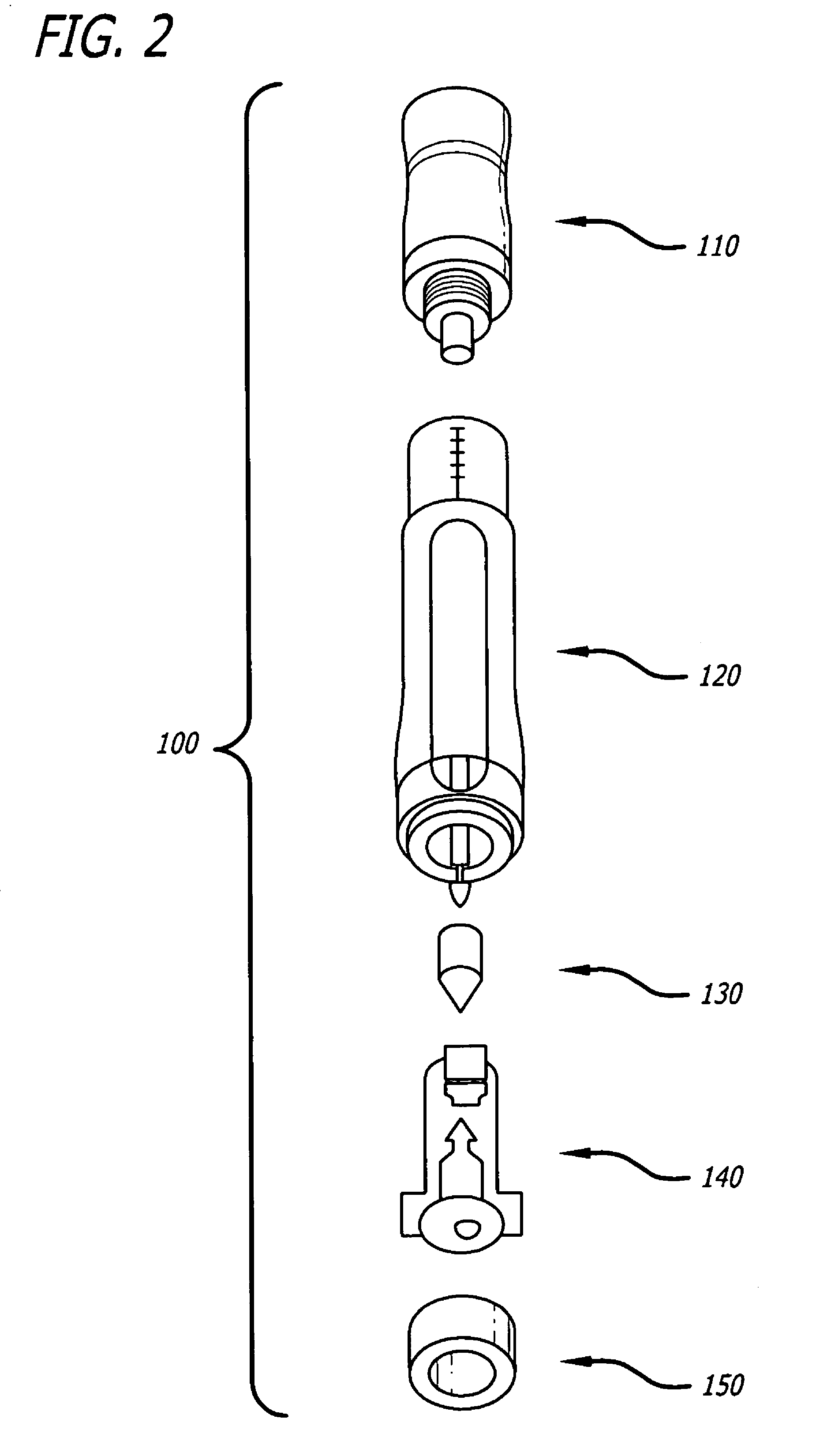
Ergonomic syringe
InactiveUS20120220948A1Reduce riskConvenient and accurate and comfortableInfusion syringesMedical devicesBiomedical engineeringLow volume
The present embodiments relate to devices and methods for injection. More specifically, the devices and methods provide an ergonomic aspect. In some embodiments, the devices and methods are especially useful in the administration of low volumes of dermal fillers to a subject.
Owner:IPSYRNG CAPITAL DEV
Ergonomic syringe
InactiveUS20090093787A1Reduce riskConvenient and accurate and comfortableInfusion syringesMedical devicesBiomedical engineeringLow volume
The present embodiments relate to devices and methods for injection. More specifically, the devices and methods provide an ergonomic aspect. In some embodiments, the devices and methods are especially useful in the administration of low volumes of dermal fillers to a subject.
Owner:BARBOUR JENNIFER
Preparations Derived From Placental Materials and Methods of Making and Using Same
Preparations derived from placental materials and methods of making and using same, the preparations including a first preparation composed of placental membranes digested in collagenase, a second preparation composed of multipotent cells derived from the collagenase digested placental membranes and that are grown in adherent culture beyond confluence and a third preparation composed of ground placental membranes re-suspended in a fluid containing hyaluronic acid. The preparations can be used for regenerating damaged or defective tissue including connective tissue, nerve tissue, muscle tissue, skin tissue, cartilage tissue and bone tissue. The preparations can also be used as dermal fillers in cosmetic and plastic surgery applications.
Owner:NUTECH MEDICAL
Automated delivery of a therapeutic or cosmetic substance to cutaneous, subcutaneous and intramuscular tissue regions
Automated systems and methods for delivery of a therapeutic or cosmetic substance into cutaneous, subcutaneous or intramuscular tissue, wherein an automated (e.g., robotic) arm is maneuvered to position a delivery device proximate a targeted location (e.g., an existing hair follicle, a location for implanting a skin filler, or a location for intradermal tattoo ink injection) on a patient's skin surface; and a substantially automated process is used to cause the delivery device to puncture the skin surface and penetrate to a desired depth into the tissue at the targeted location, and deliver the substance therein.
Owner:VENUS CONCEPT INC
Alloplastic injectable dermal filler and methods of use thereof
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent comprising particles of the unsubstituted acrylate / substituted acrylate copolymer with a diameter of less than about 100μ. Methods for making a composition comprising an alloplastic injectable suspension for use as a dermal filler are provided. Methods of augmenting soft tissue to provide long-term reduction of a skin defect and the stimulation of collagen production are also provided.
Owner:BOUTROS AYMAN
Color-Coded Polymeric Particles of Predetermined Size for Therapeutic and/or Diagnostic Applications and Related Methods
InactiveUS20100028260A1Minimizes probabilityPowder deliveryGenetic material ingredientsPolyphosphazeneMedicine
Various embodiments are directed to color-coded and size-calibrated polymeric particles comprising an acrylate-based hydrogel core incorporating one or more chromophores of interest, and an outer shell comprising polyphosphazenes of formula I, useful for various therapeutic and / or diagnostic procedures. In various embodiments, the color-coded and size-calibrated polymeric particles can be employed in any particle-mediated procedure, including as embolic agents, dermal fillers, and various implantable devices for a broad range of clinical and cosmetic applications. The incorporation of a particular chromophore formulation that correlates with a pre-determined size specificity for implantable and loadable polymeric particles (“color-coded and size-calibrated”) enables the visual detection and identification of particles exhibiting a particular size of interest, and minimizes the probability of user-introduced or procedural errors.
Owner:VARIAN MEDICAL SYSTEMS
Skin filler used for injection and preparing method and application thereof
InactiveCN105749359AHigh load rateRelease stabilitySurgeryCapsule deliveryMANNITOL/SORBITOLFreeze-drying
The invention discloses skin filler used for injection and a preparing method and application thereof.The skin filler used for injection is composed of sodium carboxymethylcellulose, mannitol and hydrophilic modified polylactic acid microspheres loading an antioxidant factor.Sodium carboxymethylcellulose, mannitol and hydrophilic modified polylactic acid microspheres loading the antioxidant factor are adopted to form the skin filler used for injection, wherein a polyethylene glycol-polylactic acid-hydroxyacetic acid segmented copolymer is adopted as a raw material of the hydrophilic modified polylactic acid microspheres loading the antioxidant factor, the material is good in biocompatibility and moderate in degradation time, and no residue exists in the human body; the preparing method of the skin filler used for injection is easy to operate and facilitates industrial production, activity of the antioxidant factor can be reserved by the process, and by the adoption of the freeze-drying process, the porous microspheres which are high in dissolution speed, uniform in morphology, high in encapsulation efficiency and stable in slow release can be prepared and used for meeting structural design requirements and clinical needs.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Compositions of polyacids and polyethers and methods for their use as dermal fillers
The present invention relates to improved methods for filling the skin for cosmetic or medical purposes. Compositions comprising carboxymethyl cellulose (CMC), polyethylene oxide (PEO) and calcium ions can be made and have physical properties that depend on the amounts and types of CMC, PEO, and calcium ions to form ioniclaly cross-linked gels. Compositions can be formed into microspheres, coascervates, gels, or membranes. Gels, microspheres and coascervates can be injected directly into a site for dermal filling. Membranes can be surgically introduced, where they swell to form hydrated gels. After introduction, the dermal filler persists for a period of time and then can disintegrate and be removed from the body.
Owner:FZIOMED
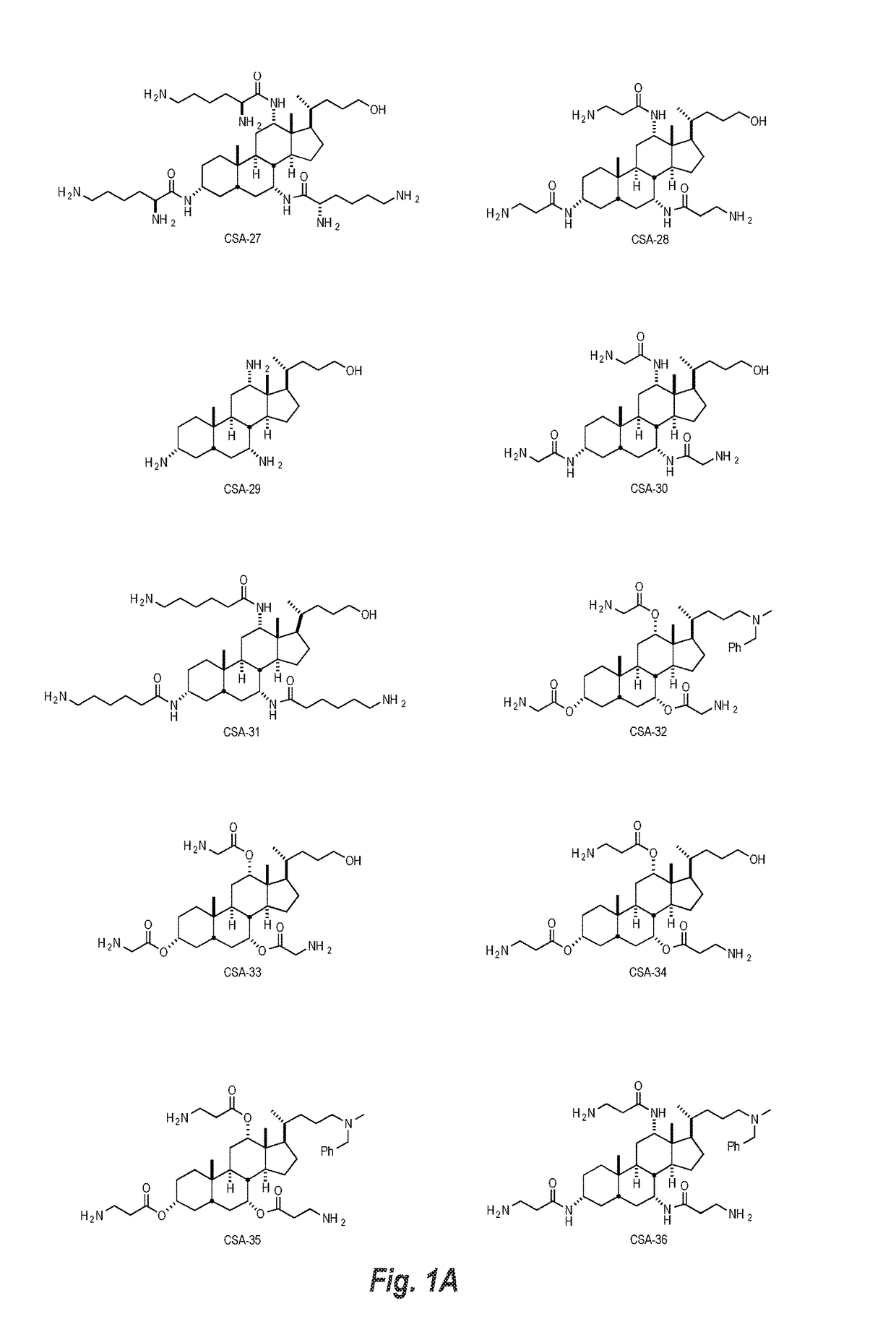
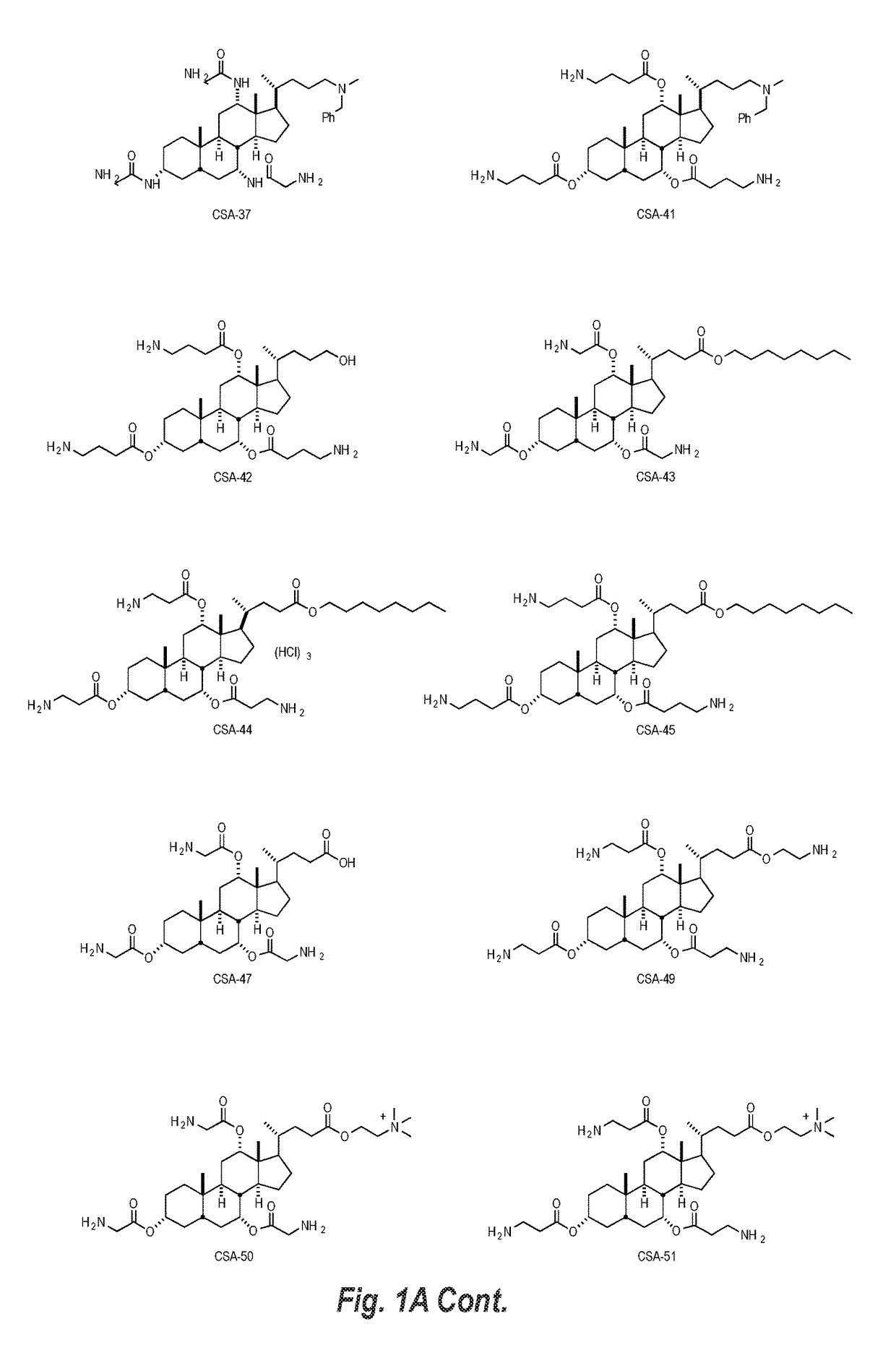
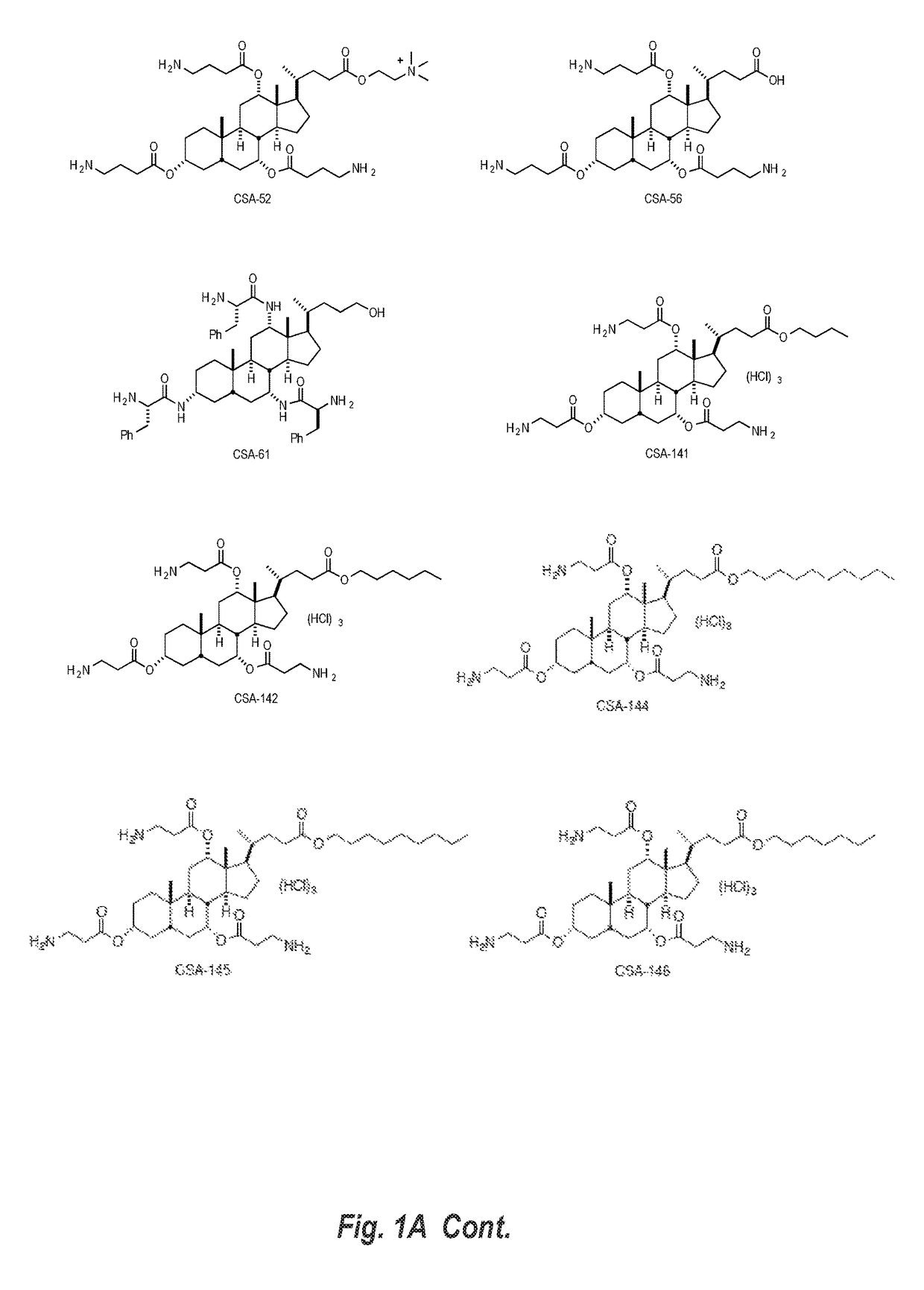
Cationic steroidal antimicrobial compositions for the treatment of dermal tissue
ActiveUS20170258963A1Reduce dirtReduce the risk of infectionAntibacterial agentsOrganic active ingredientsSkin treatmentsMedicine
This disclosure relates to dermal treatment compositions, such as dermal fillers and tissue glues, and injectable compositions that incorporate one or more cationic steroidal antimicrobials (CSAs). The CSAs are incorporated into the dermal treatment compositions to provide effective antimicrobial, anti-inflammatory, analgesic, anti-swelling and / or tissue-healing properties. A treatment composition includes a component formed from a biologically compatible material suitable for injection into and / or application onto tissue at a treatment site. One or more CSA compounds are mixed with the biologically compatible material so that the one or more CSA compounds are incorporated within the composition, forming a reservoir of CSA compounds within the resulting bolus of the treatment composition after injection and / or application.
Owner:BRIGHAM YOUNG UNIV
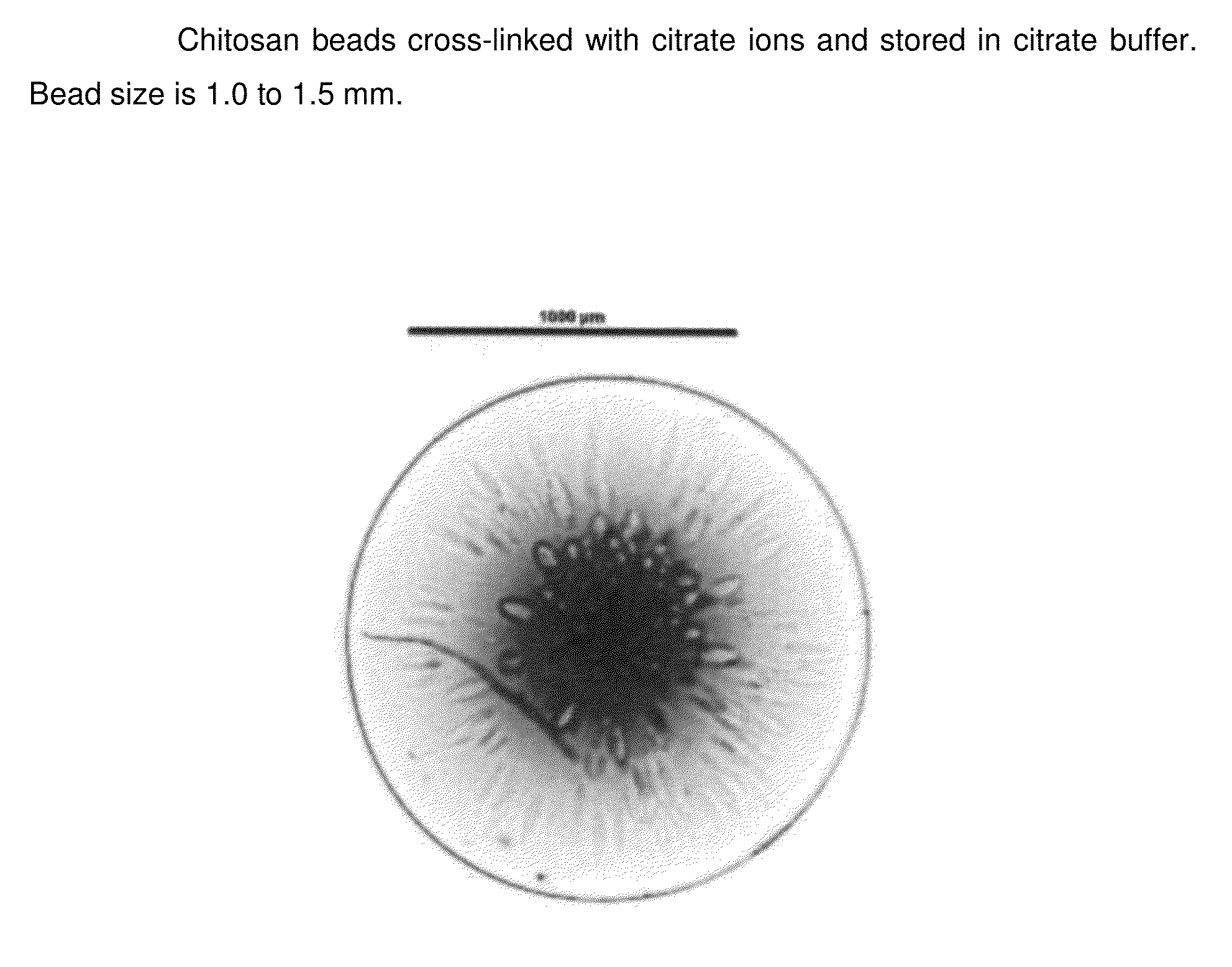
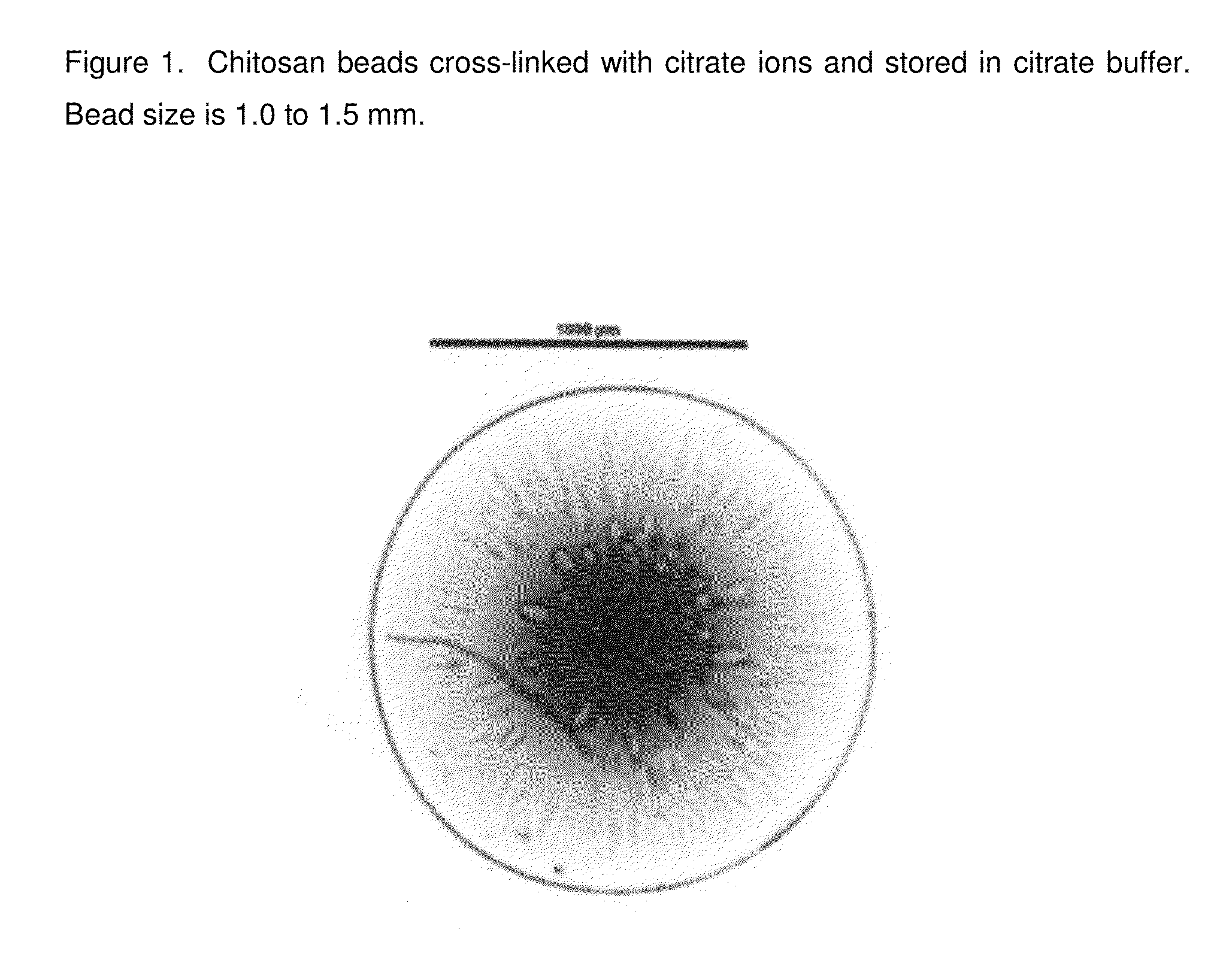
Chitosan beads and filler comprising such beads
InactiveUS20140377368A1Lasting effectRapidly volumeBiocideCosmetic preparationsCross-linkCITRATE ESTER
The present invention pertains to chitosan beads consisting of chitosan cross-linked with citrate ions. The present invention furthermore pertains to a filler comprising such chitosan-citrate beads. In one embodiment of the instant invention the filler is a dermal filler. In one further embodiment of the present invention the dermal filler is for the treatment of wrinkles and / or folds. In another embodiment of the instant invention the filler is for use in the treatment of a medical condition. The filler provided in the present invention may further comprise one or more active pharmaceutical ingredients. Further, the present invention pertains to a process for preparing the filler as claimed herein.
Owner:MERZ PHARMA GMBH & CO KGAA
Terminal sterilization of injectable collagen products
ActiveUS7902145B2Bioreactor/fermenter combinationsBiological substance pretreatmentsFluenceVolumetric Mass Density
Methods of sterilizing dermal fillers and injectable collagen material have been developed which reduce the level of active biological contaminants or pathogens without adversely affecting the material, i.e., wherein the dermal fillers and injectable collagen material retain their same properties before and after its terminal sterilization. In one embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation contains the steps of protecting the filler or material from radiation, and irradiating the filler or material with a suitable dose of radiation for a time and at a rate effective to sterilize the filler or injectable material. In a preferred embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation includes the steps of a) freezing the filler or material at a temperature below its freezing temperature, which is generally below 0° C. and b) irradiating the filler or material with a suitable dose of radiation at an effective rate for a time effective to sterilize the filler or material. The exposure of the radiation differs depending upon the density of the filler or material, but is preferably between 5 kGy and 12 kGy and more preferably between 6 kGy and 8 kGy. These doses result in a sterility assurance level (SAL) of 10−6 SAL for the filler or material.
Owner:MAM HLDG OF WEST FLORIDA L L C
Soft tissue augmentation by needle-free injection
ActiveUS20090030367A1Accurate placementJet injection syringesAutomatic syringesWrinkle skinNeedle free
The invention relates to needle-free apparatus that can be used to augment soft tissue. More specifically, the needle-free injectors of the present invention allow injection of more viscous materials such as collagen, hyaluronic acid, and other polymers that are useful as dermal fillers. The needle-free injectors of the present invention allow injection of such materials to fill the undesired lines, wrinkles, and folds of a patient. The present invention also relates to kits comprising such needle-free injectors and a quantity of dermal filling material. In addition, the present invention relates to methods of augmenting soft tissue using needle-free apparatus.
Owner:ALLERGAN INC
Alloplastic injectable dermal filler and methods of use thereof
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
Preparation method of novel skin filler
The present invention relates to a preparation method of a novel dermal filler. The method adopts low-toxic amino acid and its derivatives as cross-linking agent to prepare cross-linked sodium hyaluronate gel; Mild and easy to handle and other advantages, the basic amino acids-lysine and arginine or their derivatives among the essential amino acids for the human body are selected as the cross-linking agent to undergo a cross-linking reaction with the carboxyl end of the sodium hyaluronate molecule, and then partially seal the body The recognition site of hyaluronidase delays the recognition and degradation of the enzyme, and finally increases the residual time in the body. It provides a new technology for the development of safe and long-acting sodium hyaluronate injectable cosmetic fillers. Better market development prospects.
Owner:SHANGHAI QISHENG BIOLOGICAL PREPARATION CO LTD
Alloplastic injectable dermal filler and methods of use thereof
InactiveUS20100322982A1Reduce wrinklesReduce scarsCosmetic preparationsPowder deliveryFiller ExcipientReticular Dermis
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
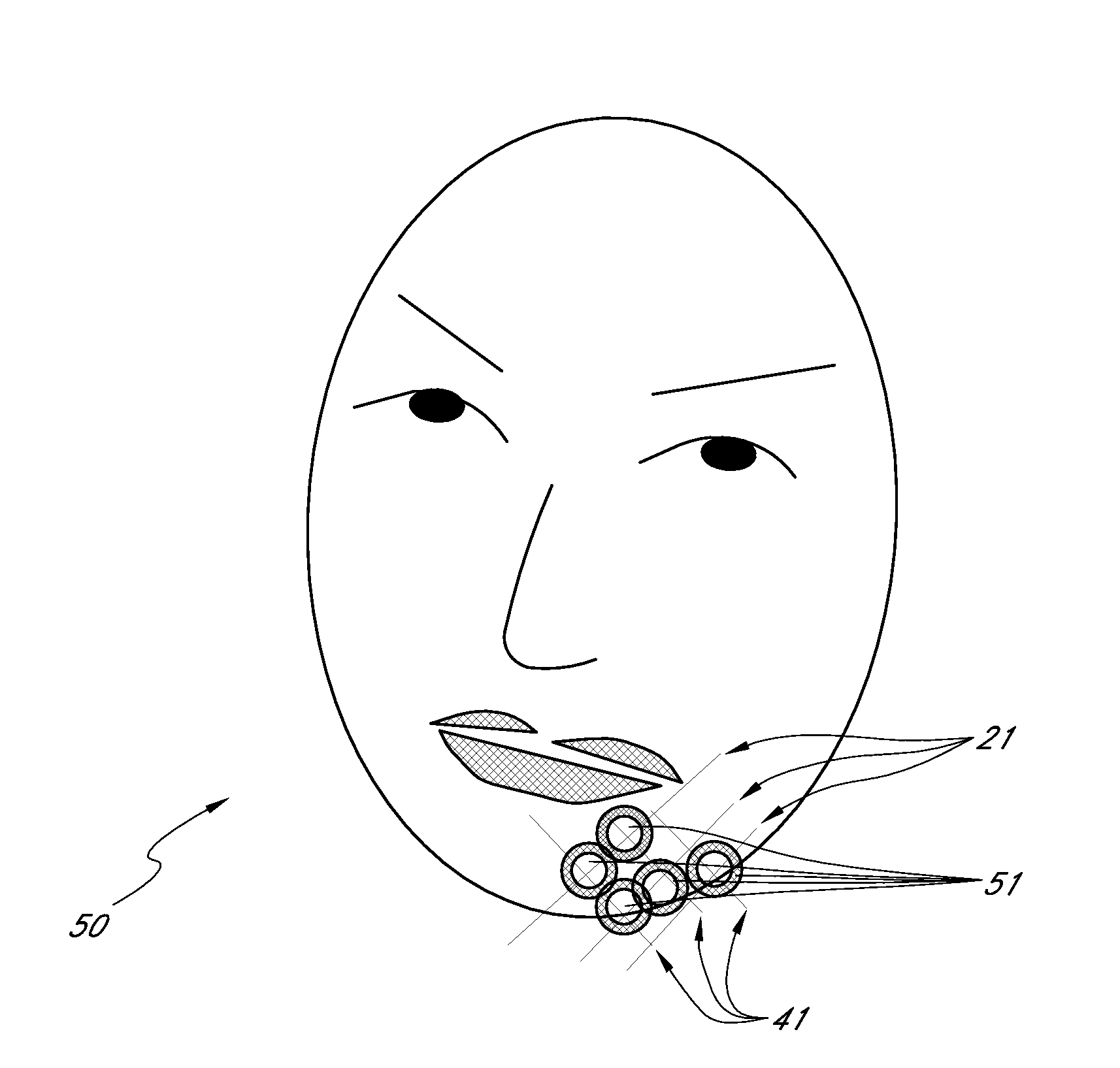
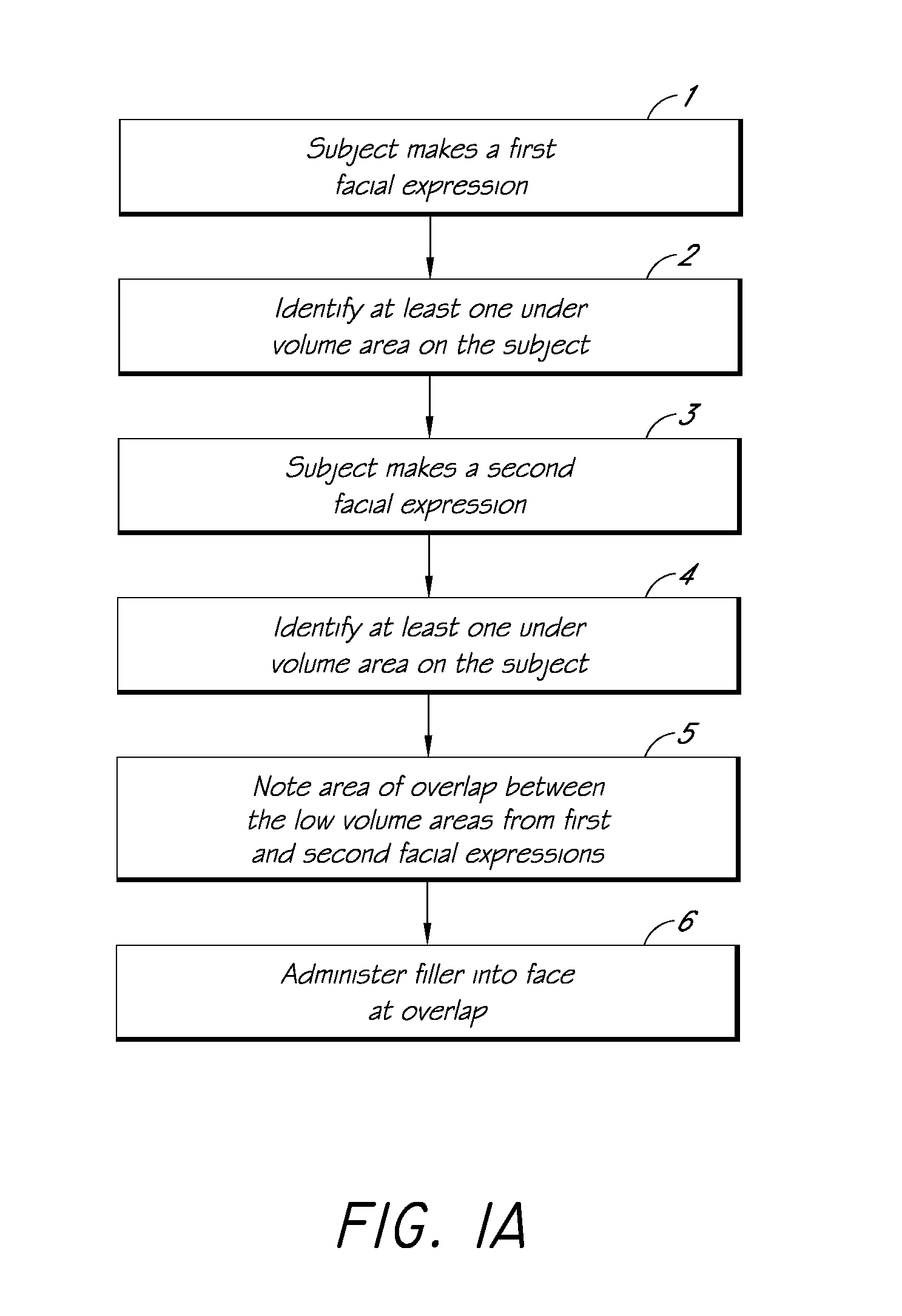
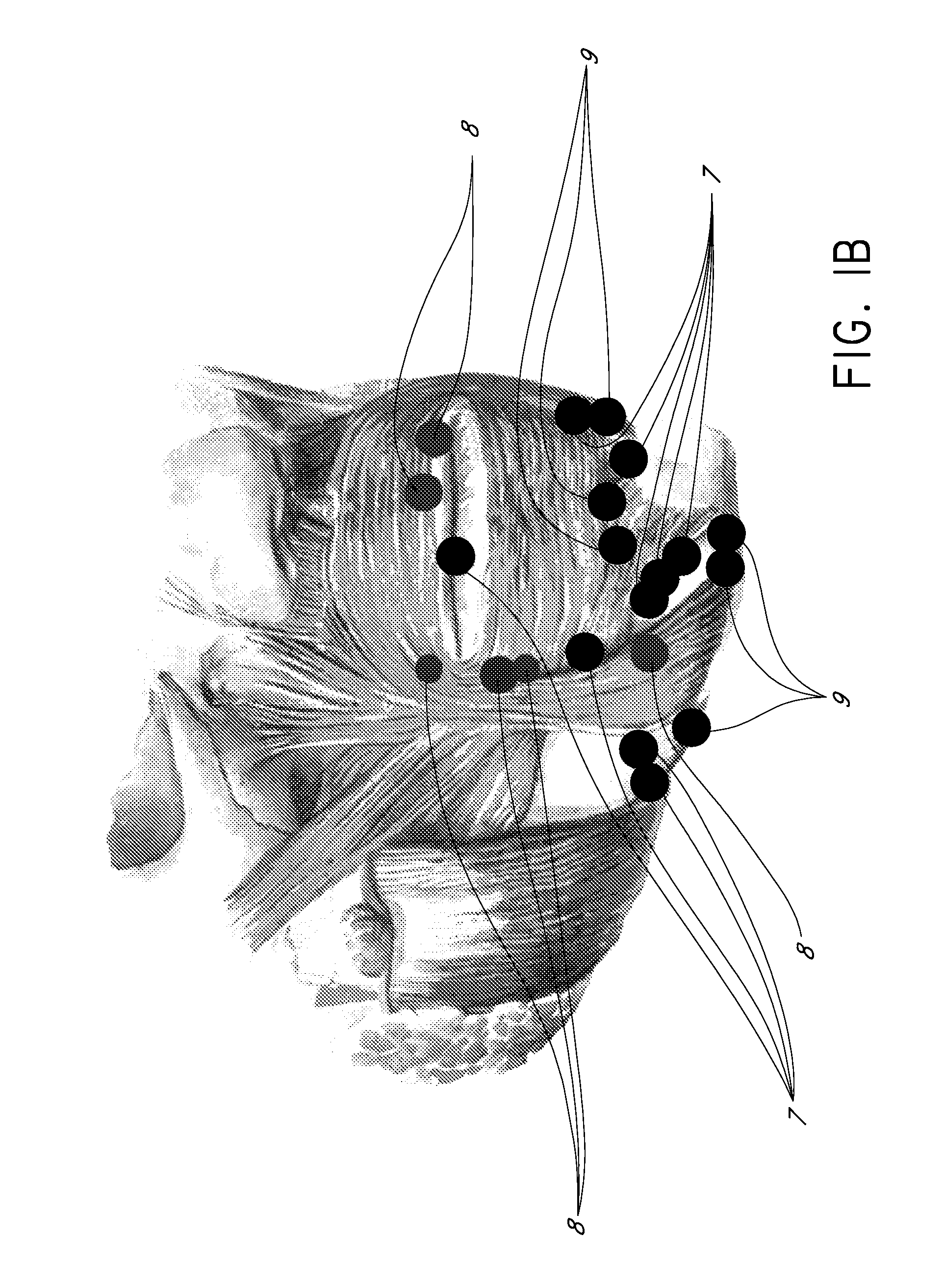
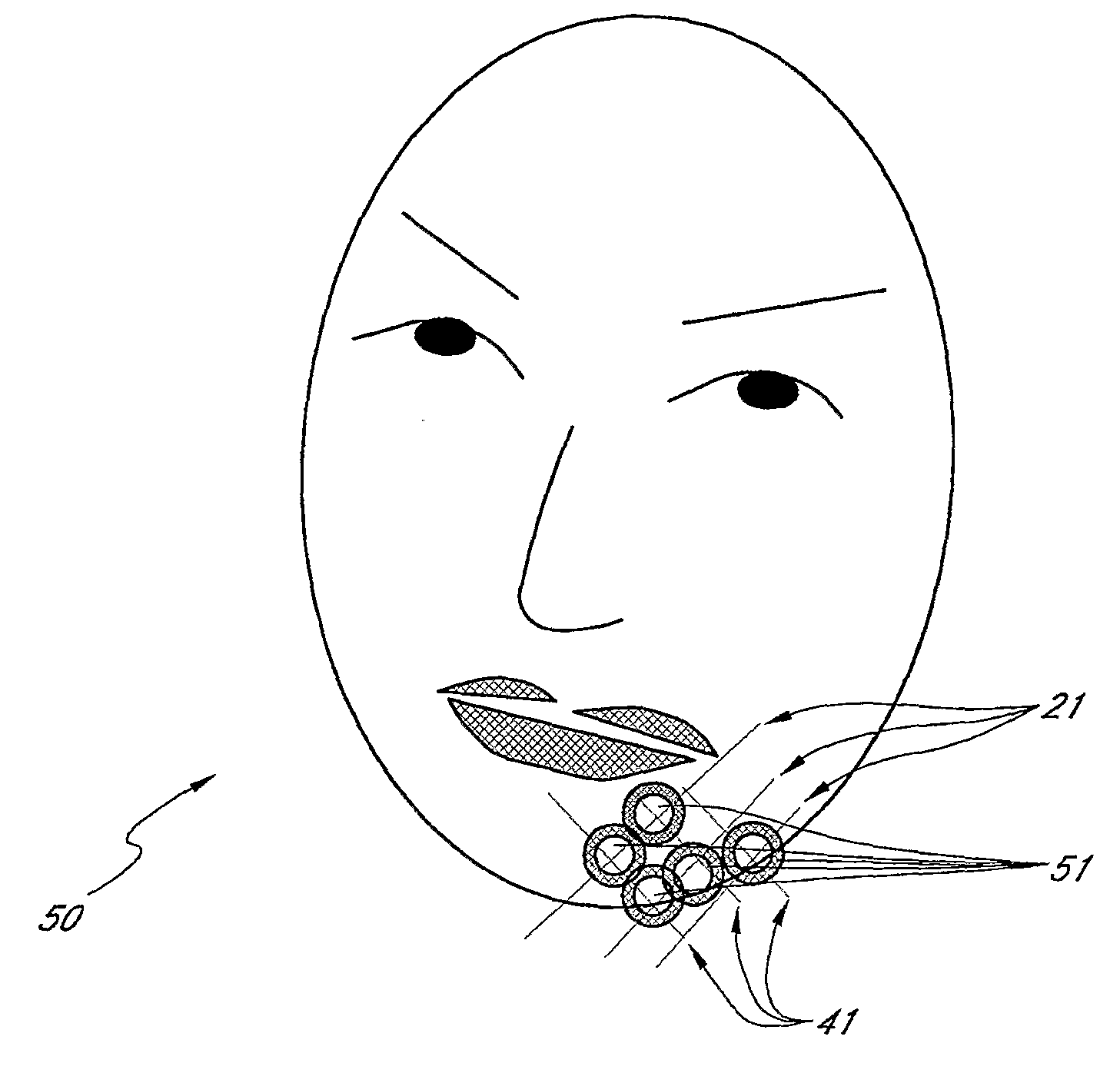
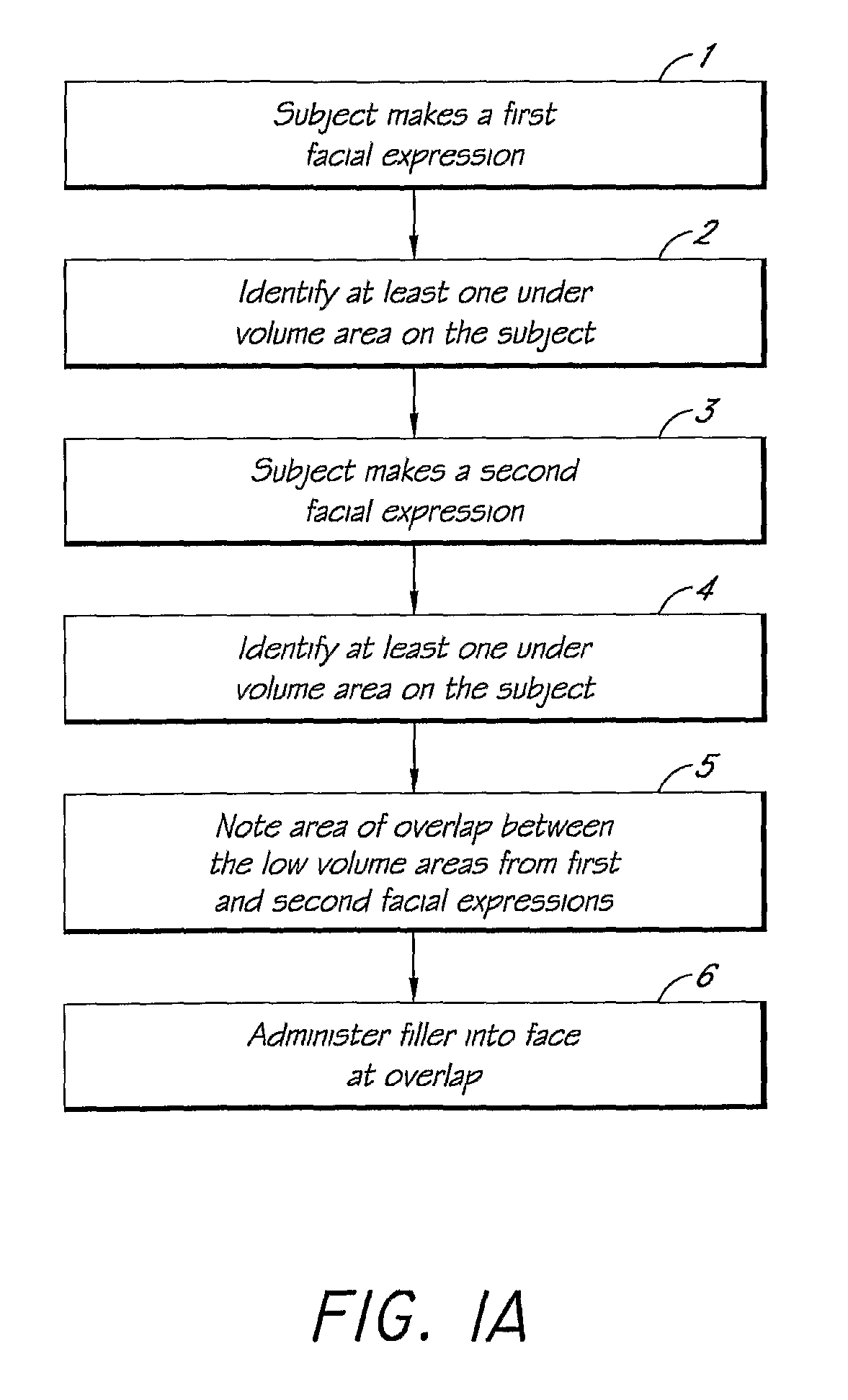
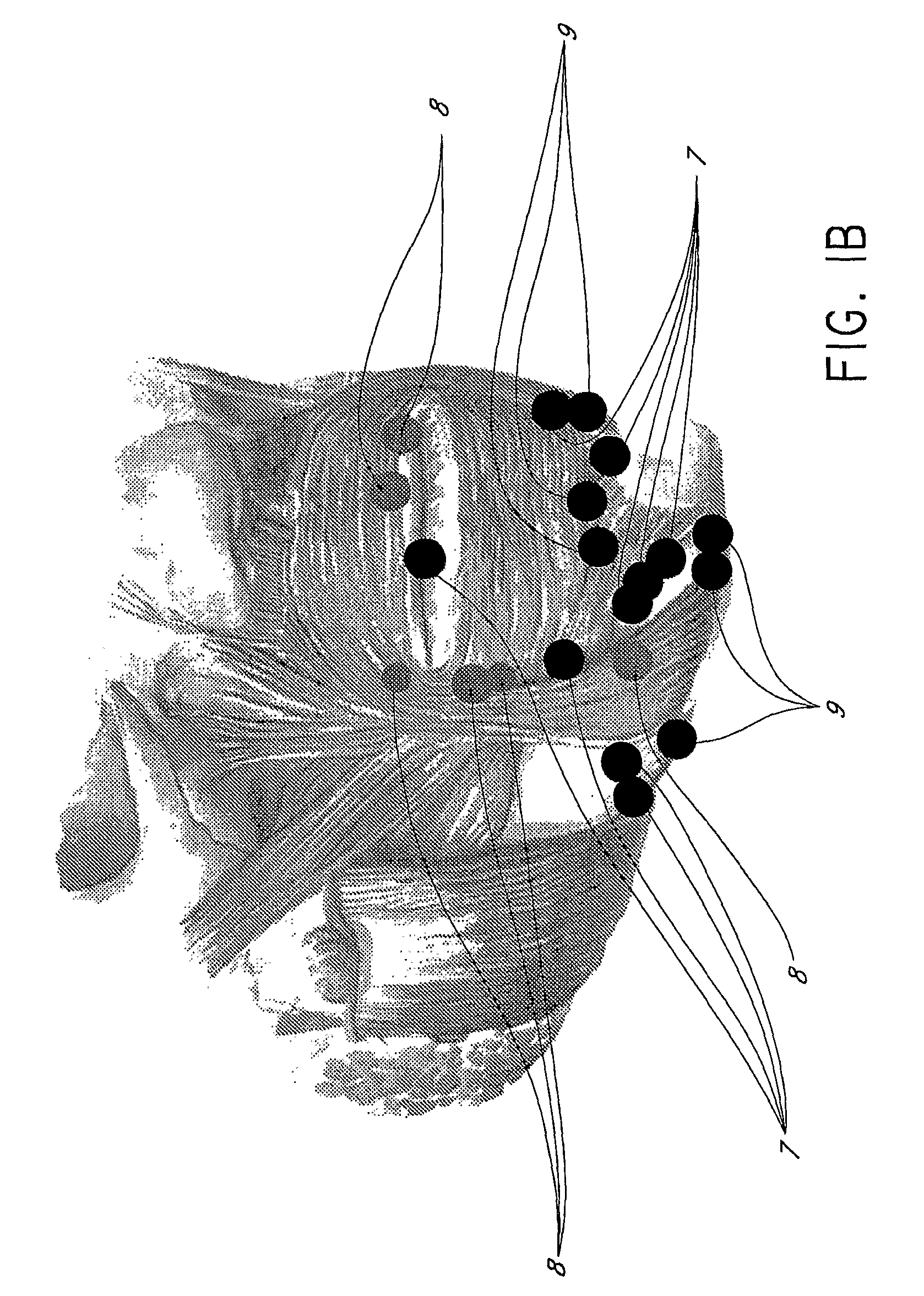
Methods for Identifying Areas of a Subject's Skin that Appear to Lack Volume
InactiveUS20100160849A1Increase aesthetic benefitImprove adverse effectsElectrotherapyPeptide/protein ingredientsMedicineSKIN REGIONS
The present embodiments relate to methods and systems for identifying areas of a subject's skin that lack sufficient volume. In some embodiments, this can be used to direct the administration of filler compositions or the application of techniques that increase the volume or firmness of the area. In some embodiments, the methods and systems provide for improved aesthetic benefit as well as suitability for instruction and training. In some embodiments, the methods are especially useful in the administration of dermal fillers to a subject.
Owner:IPSYRNG CAPITAL DEV
Swellable hyaluronic acid particles
InactiveUS20100217403A1Extend your lifeOrganic active ingredientsTissue regenerationMedicinePolysaccharide
Owner:ALLERGAN INC
Composite micron material, skin filling agent as well as preparation methods and application of composite micron material and skin filling agent
PendingCN111298195ALarge particle sizeImprove hydrophilicityPharmaceutical delivery mechanismProsthesisPolyesterPolymer science
The invention discloses a composite micro material, a skin filling agent as well as preparation methods and application of the composite micron material and the skin filling agent. The composite micron material comprises the preparation raw materials selected from at least one of a polyamino acid ester and aliphatic polyester blend, a polyamino acid-aliphatic polyester block copolymer and a polyamino acid-aliphatic polyester graft copolymer. The composite microsphere can be used for preparing the filling agent for treating and improving skin winkles, sag and scars, wherein the skin filling agent comprises the following raw materials: the composite micron material and medicinal auxiliary materials in a mass ratio being (0.01-2): (0.05-0.9). The skin filling agent of the composite microspheres or particles can effectively play a role in repairing wrinkles and sag and has good application prospects.
Owner:CHANGCHUN SINOBIOMATERIALS
Dermal filler composition
ActiveCN101426451ALong-term stable volume expansion effectPromote amplificationSkin implantsPenis implantsCross-linkFiller Excipient
Disclosed herein is a dermal filler composition. The composition includes polymethylmethacrylate (PMMA), cross-linked dextran, hydroxypropyl methylcellulose (HPMC), and physiological saline or distilled water. The composition rapidly restores volume at application sites by injection, does not require pre-testing, such as allergic skin testing, because it does not cause severe allergic reactions, is cheap, and is not easily degraded or absorbed in the body, thus ensuring a long-lasting volume augmentation effect. Due to the characteristics described above, the composition facilitates volume correction requiring a large amount (20 cc or greater) of dermal filler such as in augmentation phalloplasty.
Owner:曹康善 +1
Dermal filler composition
ActiveCN102247618ASimple production processReduce manufacturing costPharmaceutical delivery mechanismProsthesisPenisCross-link
The present invention relates to a novel dermal filler composition and to a method for preparing same. The composition of the present invention comprises, as a main component, cross-linked dextran, the molecular weight of which is 30,000 to 100,000. The composition can rapidly augment a defective area of the skin and maintain softness to the touch even when used alone. The composition eliminates the necessity of a pretreatment, such as an allergy test, which might otherwise be required prior to injection, is inexpensive, and is not easily decomposed or absorbed in vivo, thereby maintaining the tissue-volume augmentation effects thereof over a long period of time after injection. Therefore, the composition is suitable for use in a procedure such as penile augmentation or the like which requires the injection of a large amount of dermal filler, i.e. more than 20 cc. The composition of the present invention can be prepared through a simplified process to make the composition easily usable. The composition is soft to the touch when injected under the skin, and can thus be applicable not only to the skin of the penis but also to the skin of other parts of the human body, Including the face.
Owner:曹康善 +3
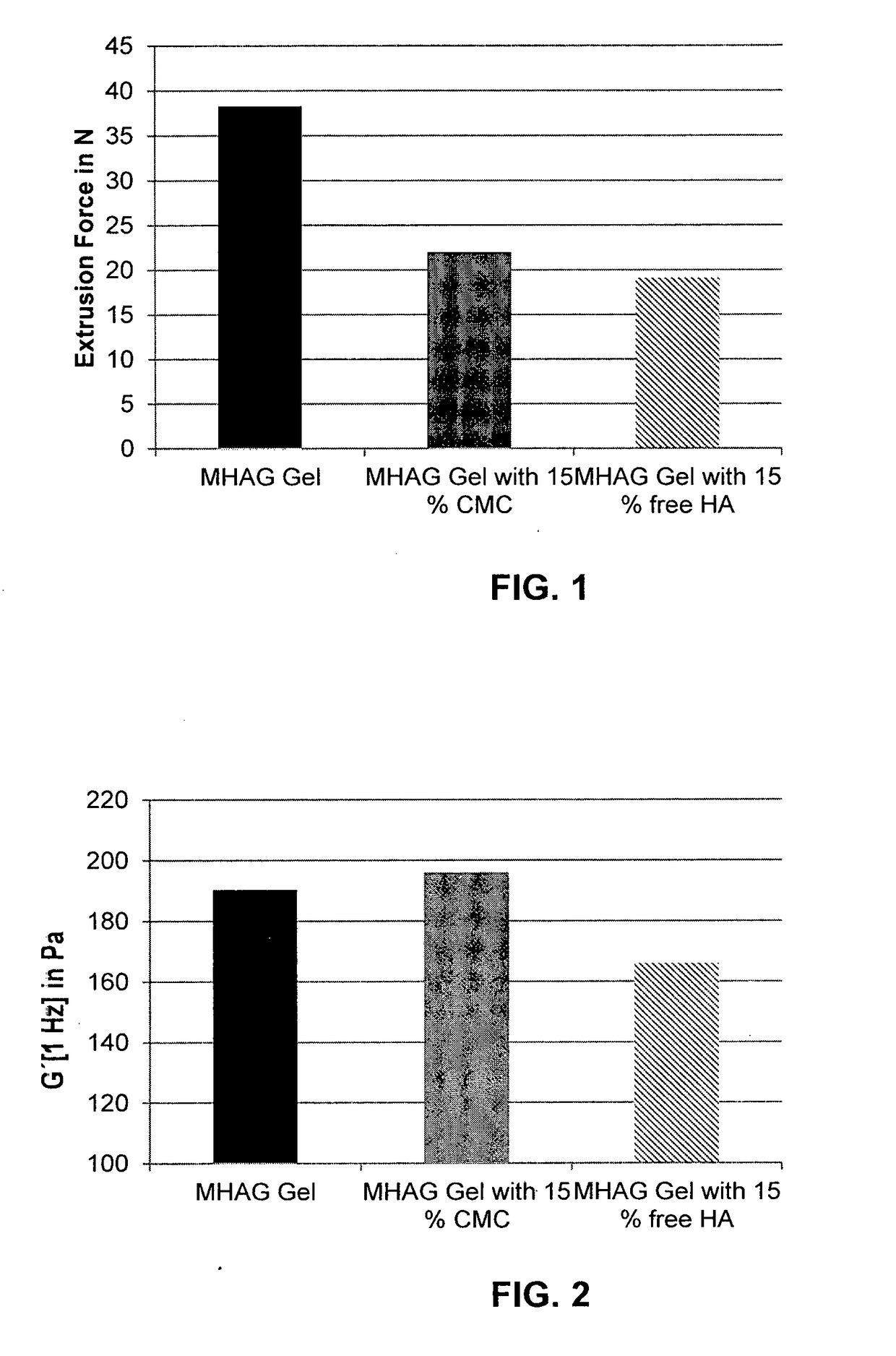
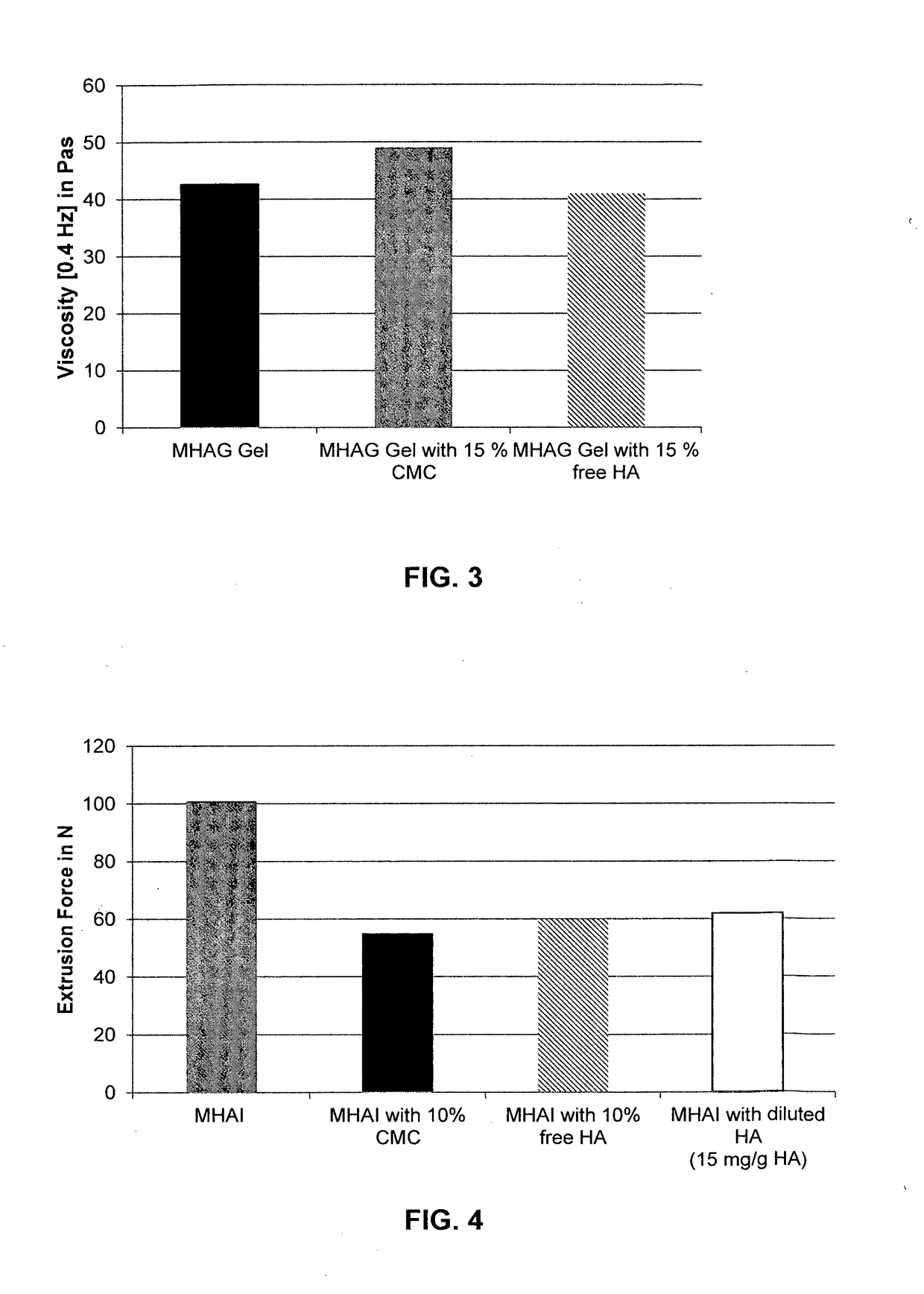
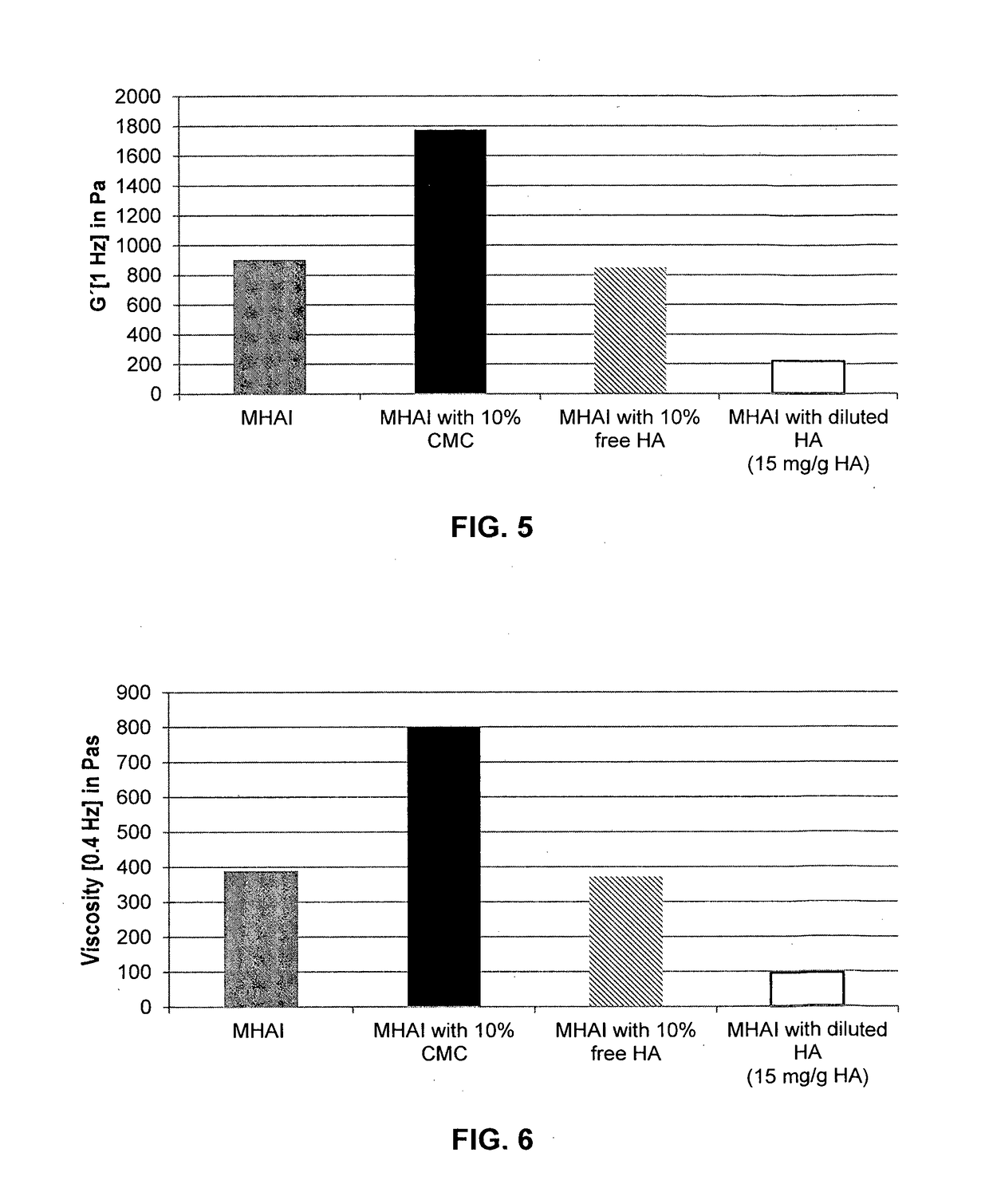
Dermal filler based on crosslinked hyaluronic acid and carboxymethyl cellulose lubricant
ActiveUS20170333596A1Improve rheologyEasy injectionPharmaceutical delivery mechanismAnaestheticsCarboxymethyl celluloseFiller Excipient
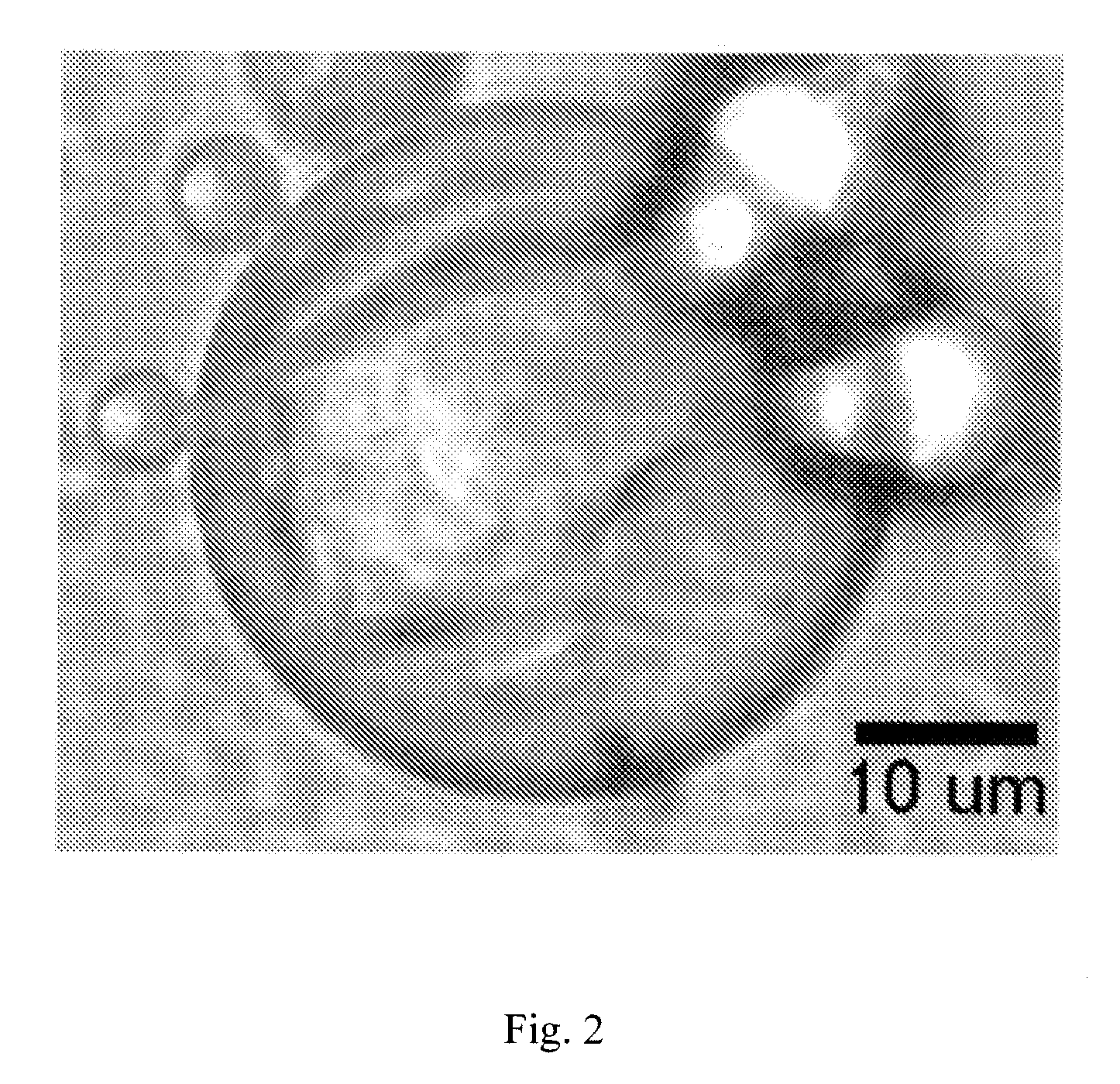
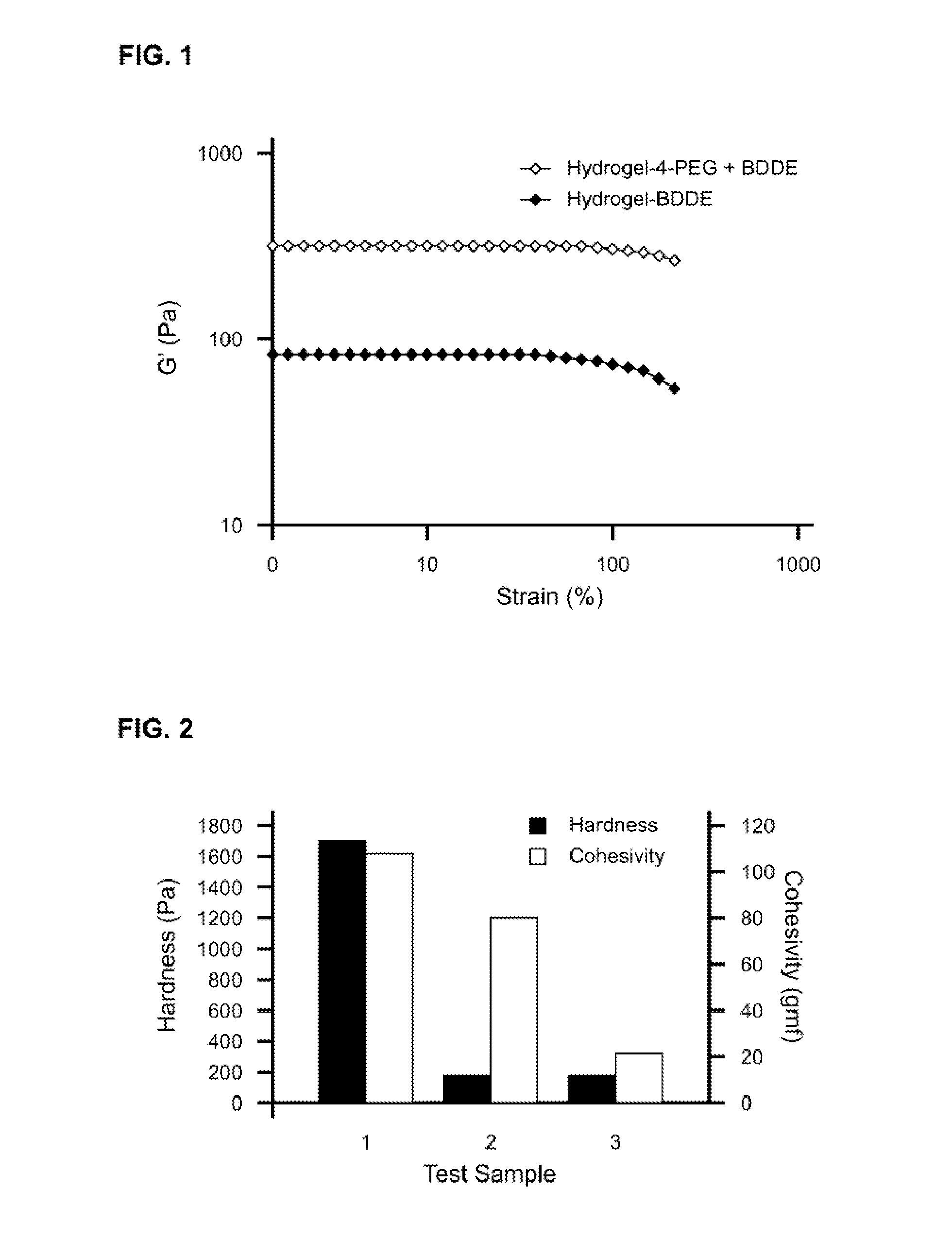
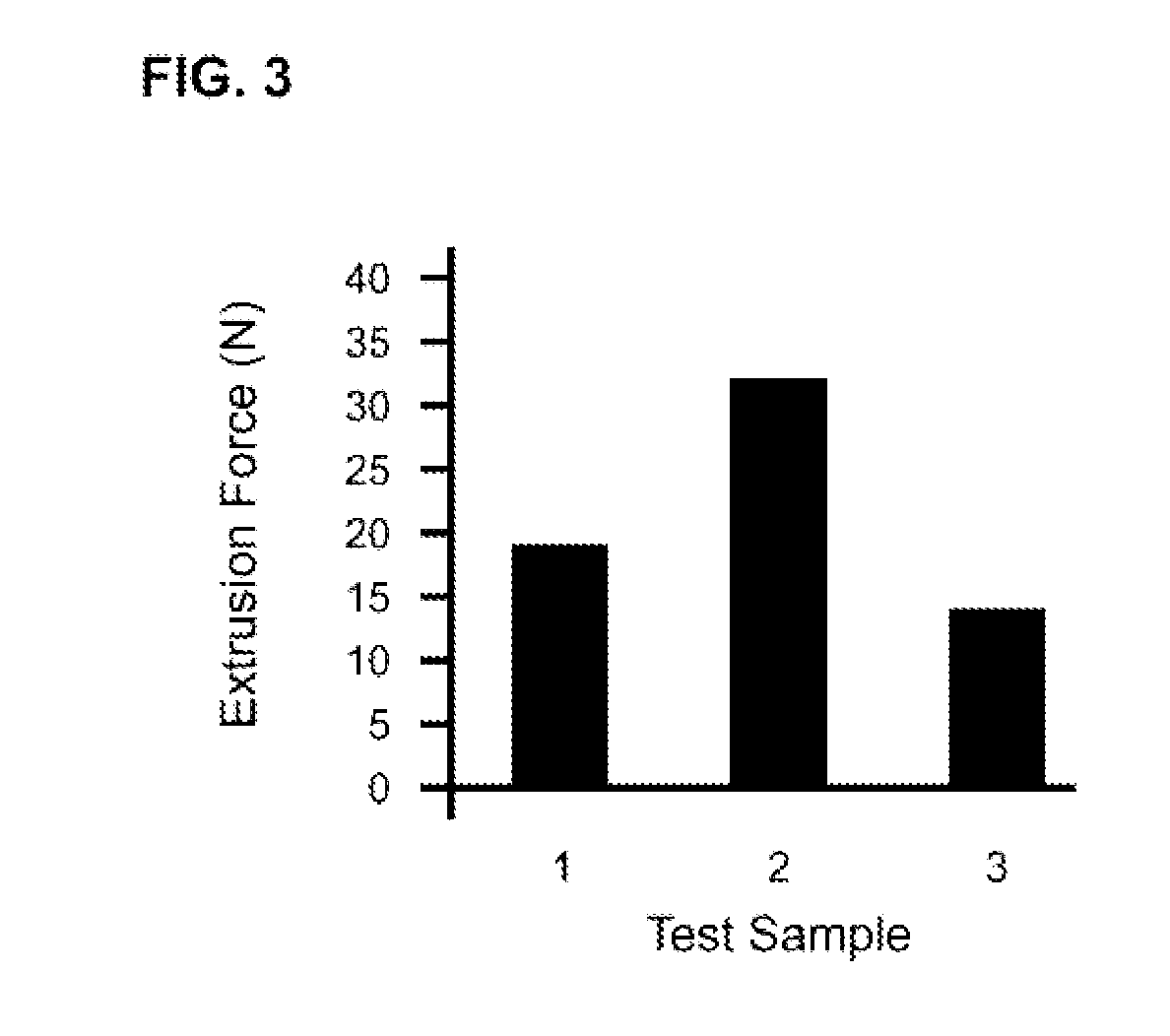
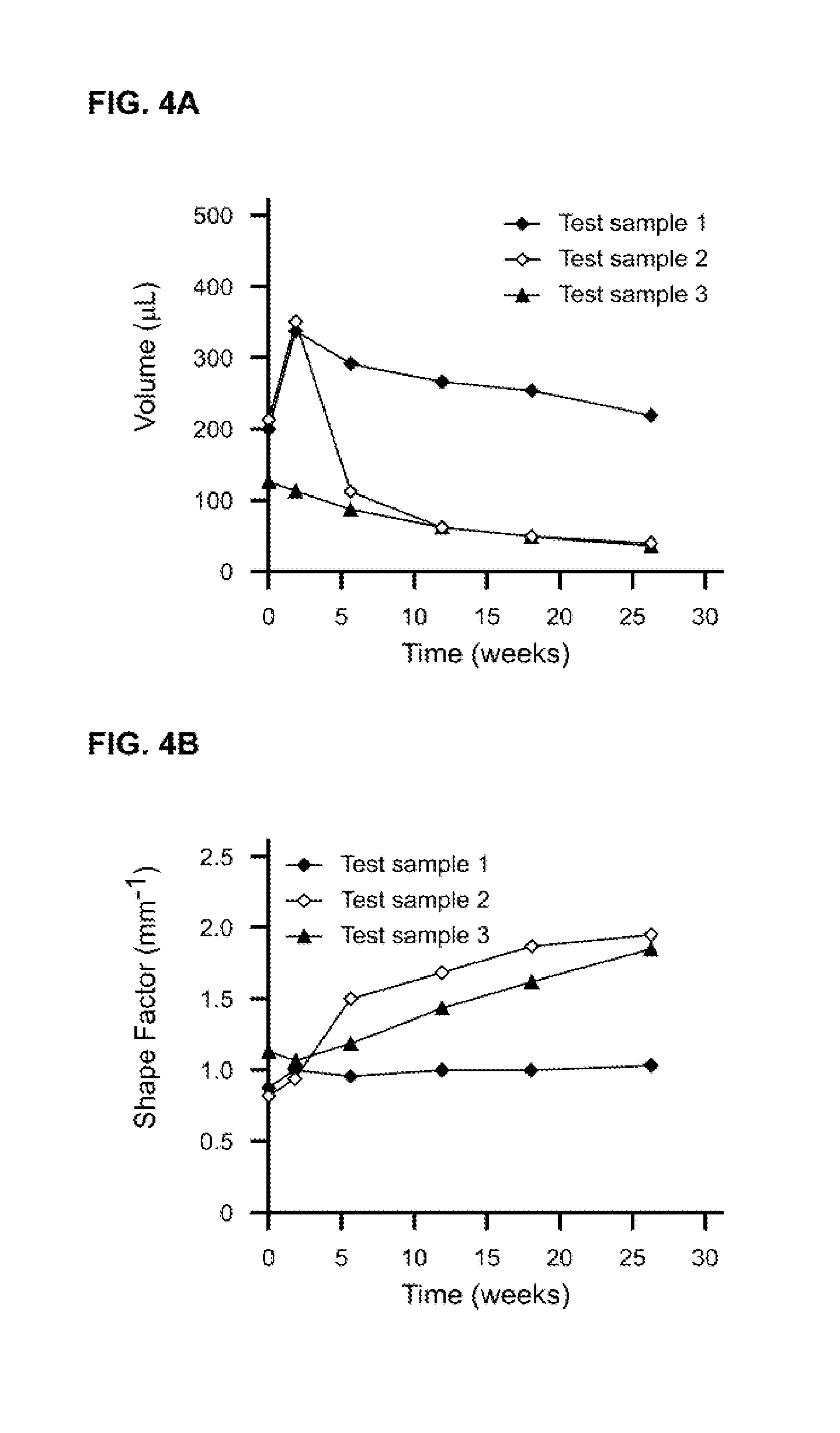
The present invention relates to injectable dermal filler compositions in the form of a gel, comprising hyaluronic acid (HA), carboxymethyl cellulose (CMC) and, optionally, microparticles such as calcium hydroxyapatite (CaHAP) microparticles. The injectable dermal filler compositions have improved rheological properties while at the same time have low extrusion forces. The present invention further relates to a method for preparing such injectable dermal filler compositions and their use for cosmetic and therapeutic purposes.
Owner:MERZ PHARMA GMBH & CO KGAA
Injectable skin filler and preparation method thereof
PendingCN113230451AAvoid the decontamination processEfficient repairPharmaceutical delivery mechanismProsthesisPolyesterMedicine
The invention discloses an injectable skin filler and a preparation method thereof. The skin filler is prepared from the following raw materials in percentage by mass: a polyester material, sodium hyaluronate and a dispersing agent. According to the embodiment of the invention, sodium hyaluronate is used as an emulsifier to prepare the skin filler; sodium hyaluronate in the skin filler not only can play roles in filling dermis tissues and keeping skin moisture, but also can be used as a suspending agent of particles. Compared with the existing technology for preparing the particles by adopting an emulsification method, the preparation method disclosed by the embodiment of the invention can realize synchronous preparation of the particles and the skin filler, and the preparation process is simplified.
Owner:CHANGCHUN SINOBIOMATERIALS
Soft tissue augmentation by needle-free injection
ActiveUS8021323B2Accurate placementJet injection syringesAutomatic syringesNeedle freeNeedle Free Injection
The invention relates to needle-free apparatus that can be used to augment soft tissue. More specifically, the needle-free injectors of the present invention allow injection of more viscous materials such as collagen, hyaluronic acid, and other polymers that are useful as dermal fillers. The needle-free injectors of the present invention allow injection of such materials to fill the undesired lines, wrinkles, and folds of a patient. The present invention also relates to kits comprising such needle-free injectors and a quantity of dermal filling material. In addition, the present invention relates to methods of augmenting soft tissue using needle-free apparatus.
Owner:ALLERGAN INC
Cationic steroidal antimicrobial compositions for the treatment of dermal tissue
ActiveUS10226550B2Reducing fouling of the injectedReduce the risk of infectionAntibacterial agentsOrganic active ingredientsSkin treatmentsMedicine
Owner:BRIGHAM YOUNG UNIV
Methods for Identifying Areas of a Subject's Skin that Appear to Lack Volume
InactiveUS20100262118A1Improve adverse effectsIncrease volumePeptide/protein ingredientsMedical devicesMedicineSKIN REGIONS
The present embodiments relate to methods and systems for identifying areas of a subject's skin that lack sufficient volume. In some embodiments, this can be used to direct the administration of filler compositions or the application of techniques that increase the volume or firmness of the area. In some embodiments, the methods and systems provide for improved aesthetic benefit as well as suitability for instruction and training. In some embodiments, the methods are especially useful in the administration of dermal fillers to a subject.
Owner:IPSYRNG CAPITAL DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com