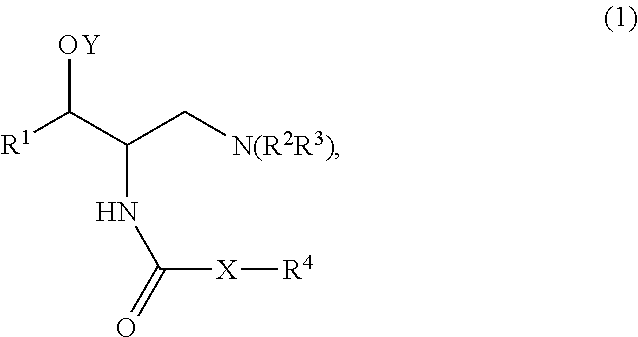Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "POLYCYSTIC RENAL DISEASE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for the treatment of polycystic kidney disease
The present invention provides a method for treating, inhibiting the progression of, or eradicating polycystic kidney disease of in a patient in need thereof which comprises providing to said patient an effective amount of a TACE inhibitor compound in combination with an effective amount of a Src kinase inhibitor, a HER-2 kinase inhibitor, or a combination of Src and HER-2 inhibitor.
Owner:WYETH LLC
Reverse-turn mimetics and method relating thereto
InactiveUS20070021425A1Efficient implementationBiocideNervous disorderUlcerative colitisPercent Diameter Stenosis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Reverse-turn mimetics and method relating thereto
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
3-cyanoquinolines, 3-cyano-1,6-naphthyridines, and 3-cyano-1,7-naphthyridines as protein kinase inhibitors
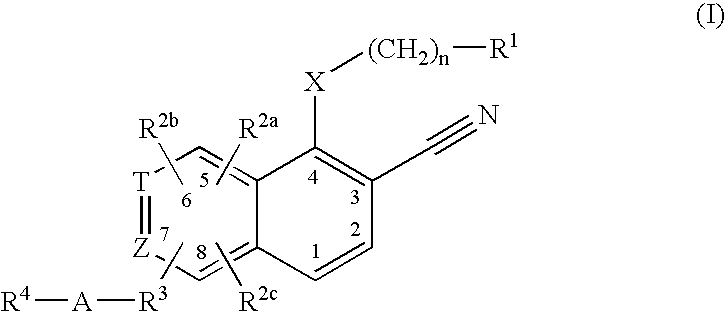
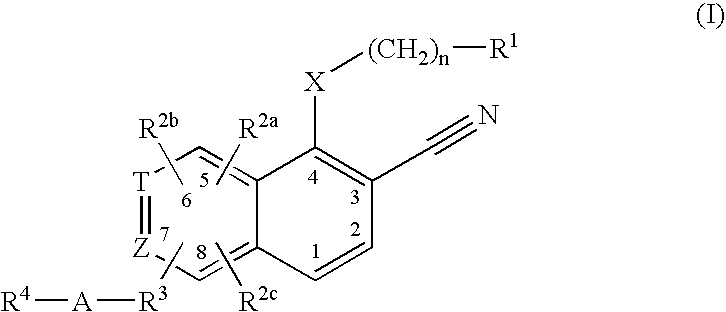
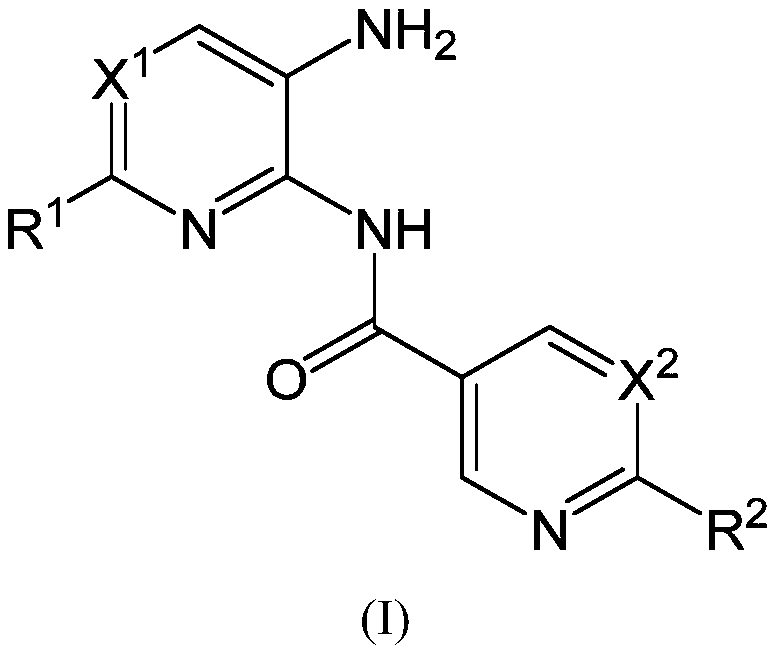
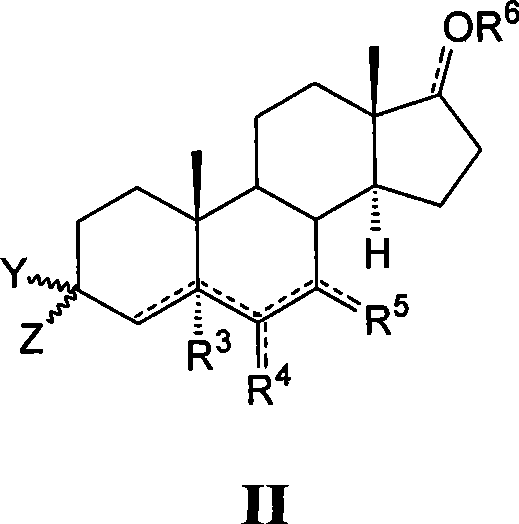
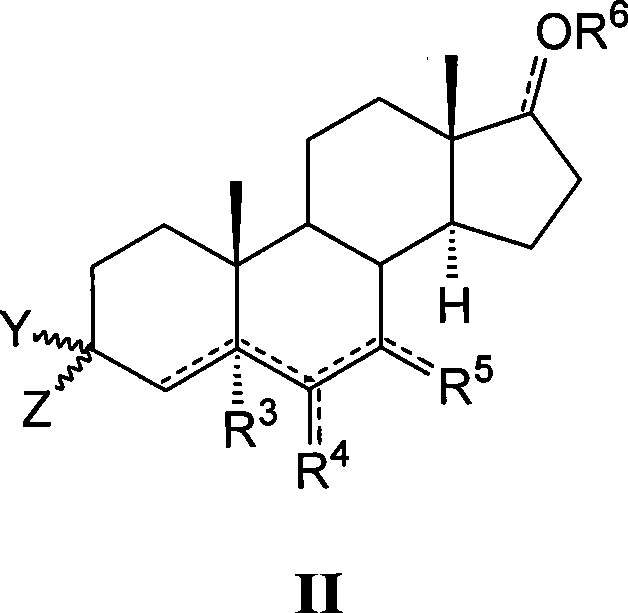
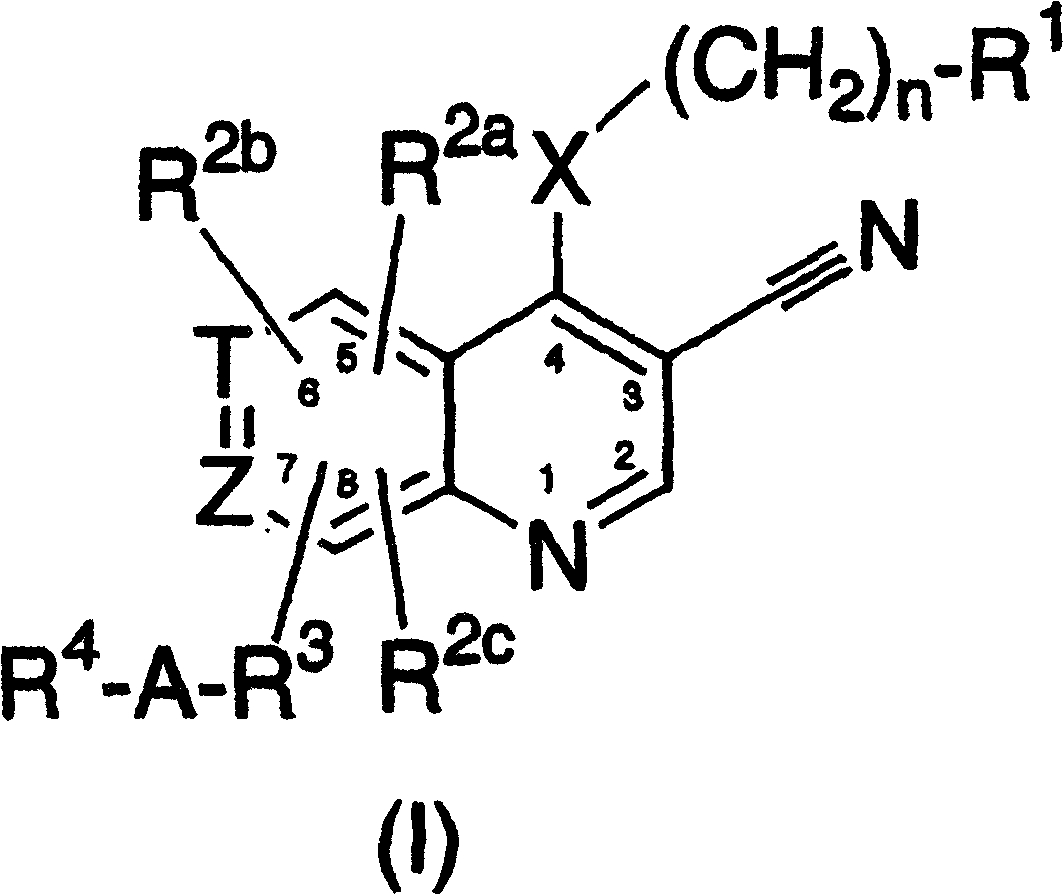
This invention provides compounds of Formula (I), having the structurewhere T, Z, X, A, R<1>, R<2a>, R<2b>, R<2c>, R<3>, R<4>, and n are defined herein, or a pharmaceutically acceptable salt thereof which are useful as antineoplastic agents and in the treatment of osteoporosis and polycystic kidney disease.
Owner:WYETH LLC
Reverse-turn mimetics and method relating thereto
ActiveUS20060084655A1Efficient implementationPromoting neurite outgrowthBiocideNervous disorderUlcerative colitisNodular sclerosis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Reverse-turn mimetics and method relating thereto
InactiveUS20070043052A1Efficient implementationPromoting neurite outgrowthBiocideOrganic chemistryUlcerative colitisNodular sclerosis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Treatment systems and methods for renal-related diseases
InactiveUS20090306625A1Increase risk of injuryAccurate and reliableOrganic active ingredientsBalloon catheterDiseaseMain vessel
Systems and methods are provided for locally delivering specific prophylactic, regenerative, or therapeutic agents within the body of a patient. Systems and methods can involve direct delivery of prophylactic, regenerative, or therapeutic agents into branch blood vessels or body lumens from a main vessel or lumen, respectively, and in particular into renal arteries extending from an aorta in a patient. Drug infusion techniques encompass specific treatment and prevention regimes for renal diseases including, but not limited to, Acute Kidney Injury, Renal Cell Carcinoma, Polycystic Kidney Disease, and Chronic Kidney Disease.
Owner:ANGIODYNAMICS INC
Endothelial cell specific antibodies and uses thereof
InactiveUS20070020271A1Prevent proliferationGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiabetic retinopathyDisease
Methods and composition provided herein relate to the inhibition of proliferation, migration, and tubule formation of cells and are thus useful in treating angiogenesis associated diseases, including cancer, polycystic kidney disease, diabetic retinopathy, rheumatoid arthritis, and psoriasis. Disclosed are methods of inhibiting endothelial cell proliferation, migration, and tubule formation by administering an antibody specific for Tumor EndothPelial Markers (TEMs). Also disclosed are methods of inhibiting angiogenesis and tumor growth by administering a TEM-specific antibody and antibody compositions useful in such methods.
Owner:GENZYME CORP +1
Polycystic kidney disease 12 gene and uses thereof
The present invention relates to the polycystic kidney disease 1 (PKD1) gene and its nucleic acid sequence, mutations thereof in patients having PKD1-associated disorders, the protein encoded by the PKD1 gene or its mutants, and their uses in disease diagnosis and therapy.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD +1
Reverse-turn mimetics and method relating thereto
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Reverse-turn mimetics and method relating thereto
ActiveUS20100222303A1Efficient implementationBiocideOrganic chemistryUlcerative colitisSignalling pathways
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins as well as their prodrugs are disclosed. Such reverse-turn mimetic structures and prodrugs have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds and prodrugs for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
Method of treating polycystic kidney diseases with ceramide derivatives
InactiveUS8912177B2Reduce needSymptoms improvedBiocideUrinary disorderPharmaceutical medicineBiological drugs
A method of treating polycystic kidney disease in a subject comprises administering to the subject an effective amount of a compound represented by Structural Formula (1): or a pharmaceutically acceptable salt thereof.
Owner:GENZYME CORP
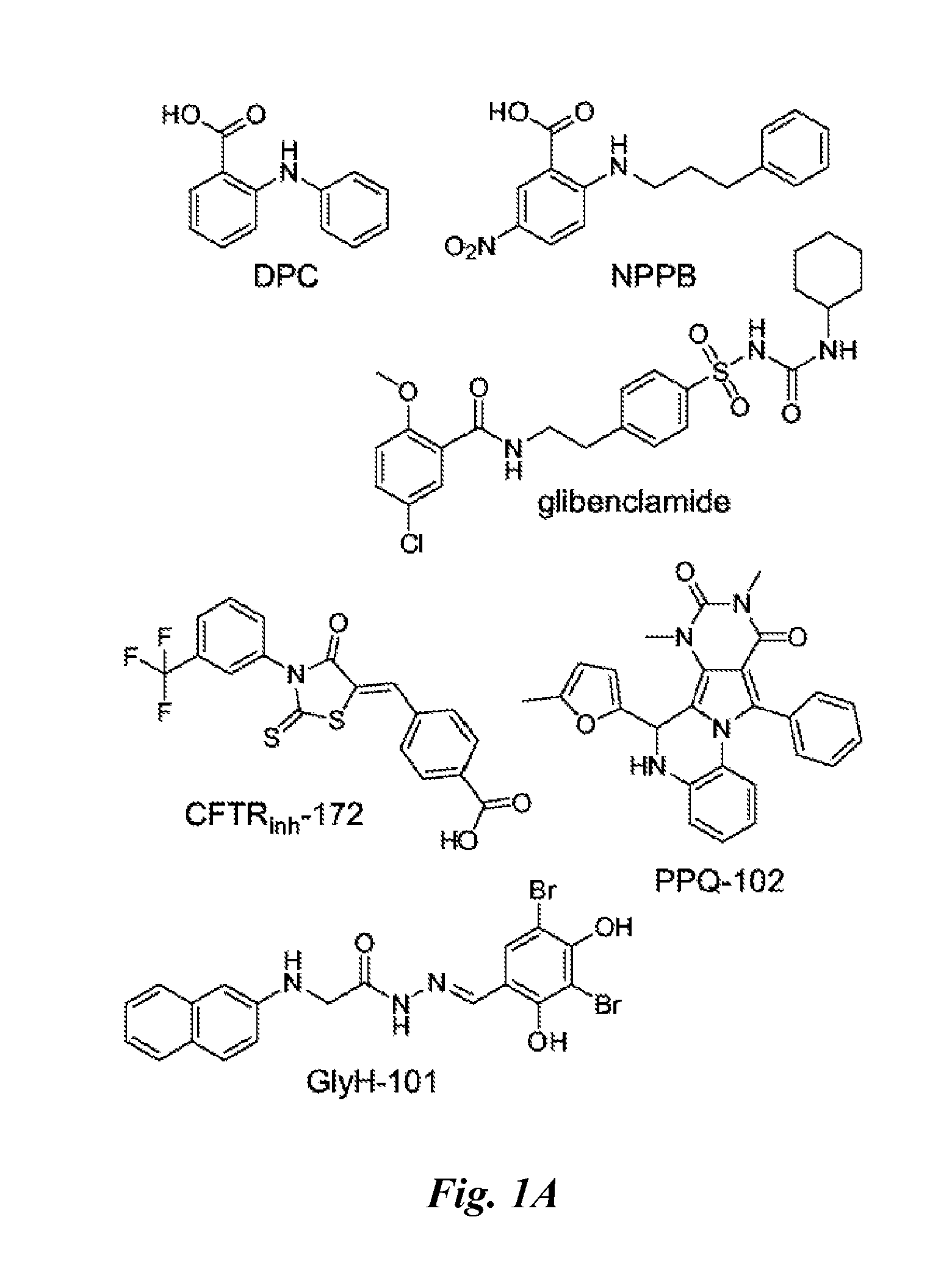
Water soluble small molecule inhibitors of the cystic fibrosis transmembrane conductance regulator
InactiveUS20110105565A1Inhibit overall enlargementInhibition formationBiocideOrganic chemistryDiseaseGlycine
Provided herein are highly water soluble, thiazolidinone derivative compounds and glycine hydrazide derivative compounds that inhibit the ion transport activity of the cystic fibrosis transmembrane conductance regulator (CFTR). The compounds, and compositions comprising the compounds, described herein are useful for treating diseases, disorders, and sequelae of diseases, disorders, and conditions that are associated with aberrantly increased CFTR activity, for example, secretory diarrhea. The compounds may also be used for inhibiting expansion or preventing formation of cysts in persons who have polycystic kidney disease.
Owner:RGT UNIV OF CALIFORNIA
Pyrimidine substituted benzenepropanoic acid derivative, its preparation method and use in curing polycystic kidney disease
ActiveCN101054372ATranscriptional activityGood treatment effectOrganic active ingredientsOrganic chemistry3-phenylpropanoic acidGeometric isomer
The present invention relates to a kind of pyrimidine substituted phenylpropionic acid derivative compounds of formula I which includes itsgeometric isomer, enantiomer, diastereoisomer, racemate, and mixture therefore or pharmaceutically acceptable salt thereof. The present invention also relates to the preparation method and use of such compound. Compounds of present invention are useful in the treatment of polycystic kidney disease.
Owner:SHENYANG SUNSHINE PHARMA
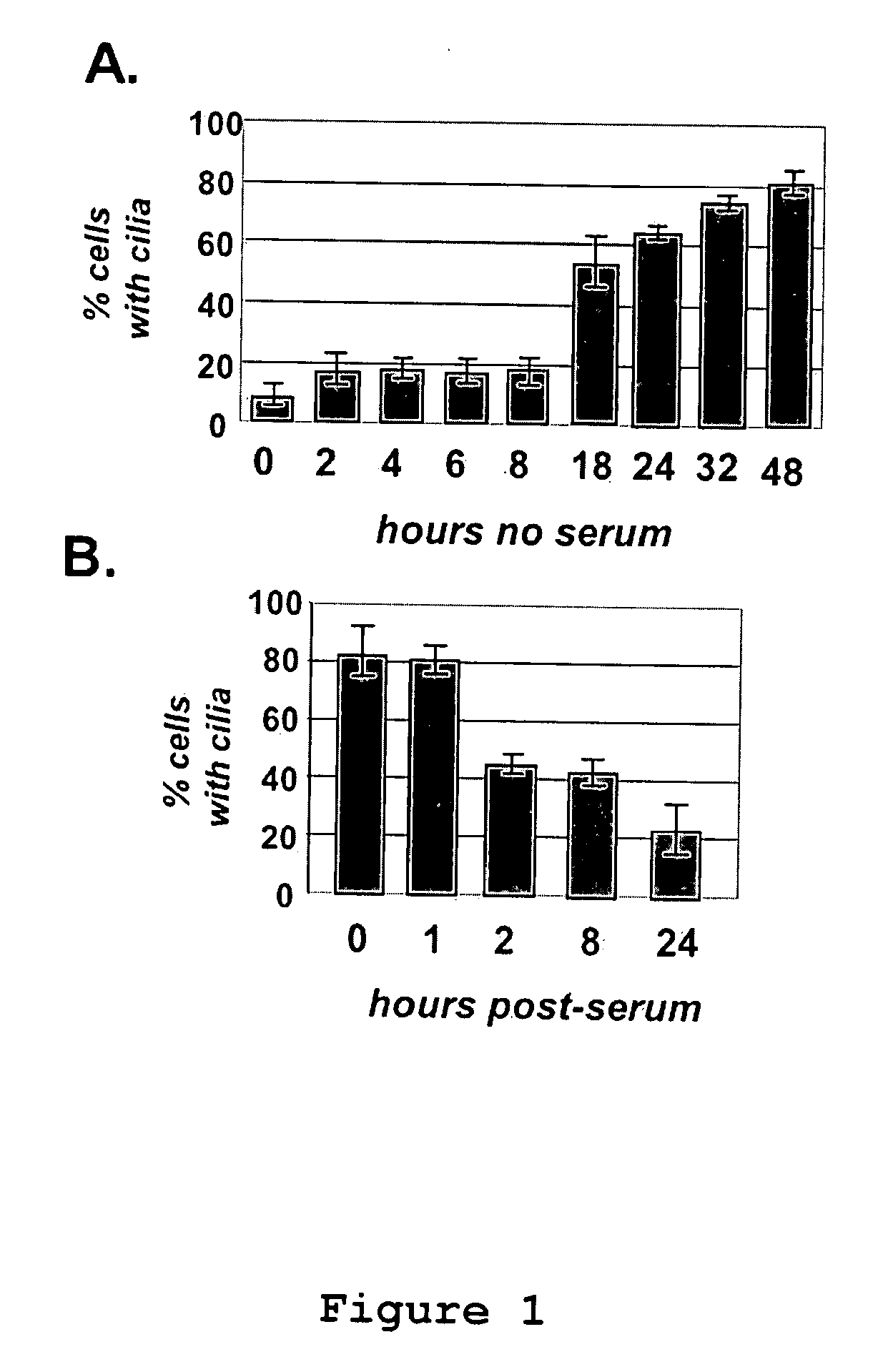
Compositions and Methods for the Treatment of Diseases Associated with Aberrant Cilia Assembly and Regulation
ActiveUS20090306183A1Organic active ingredientsMicrobiological testing/measurementStructure and functionDisease
Compositions and methods are provided for identifying agents which have efficacy for the treatment of disorders related to aberrant cilial structure and function, including polycystic kidney disease.
Owner:INST FOR CANCER RES
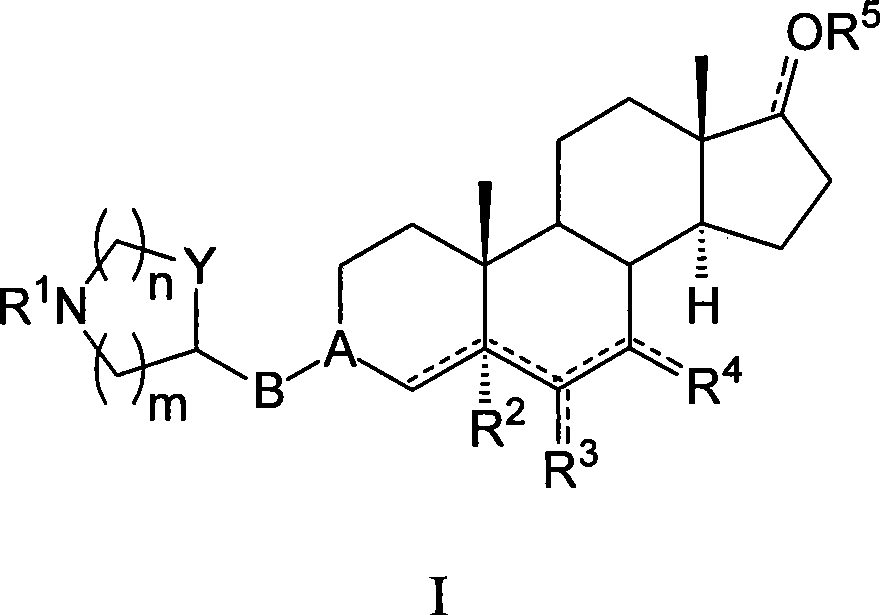
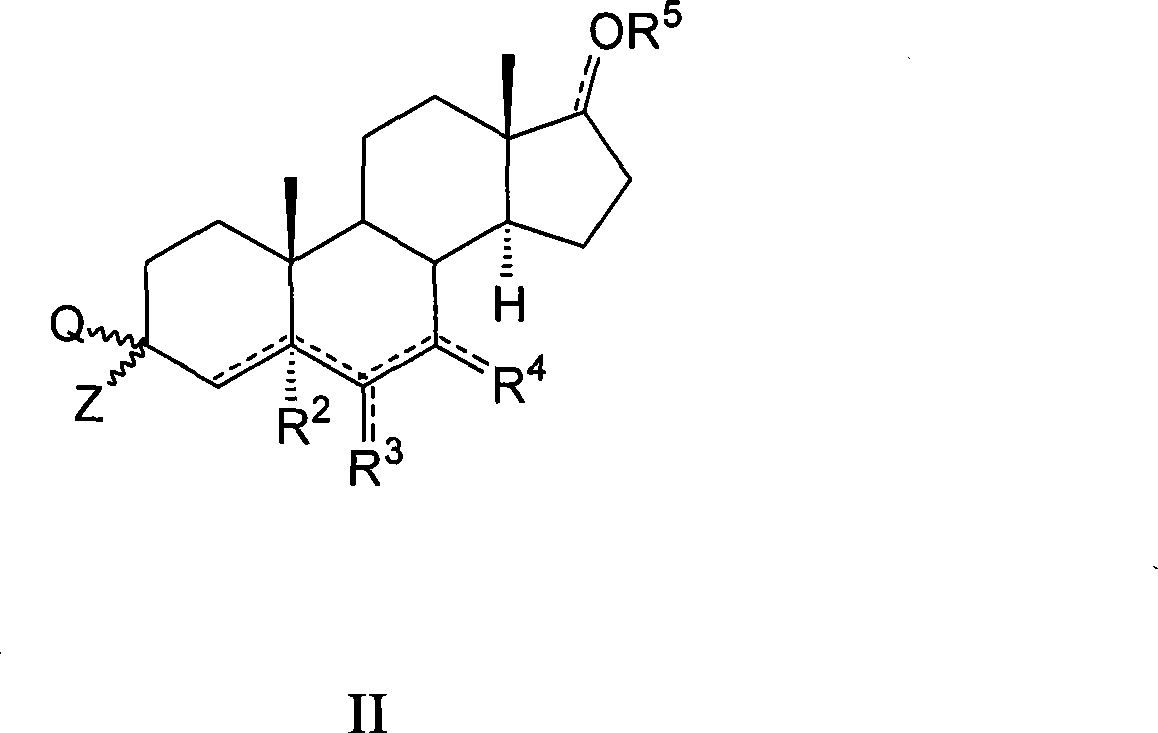
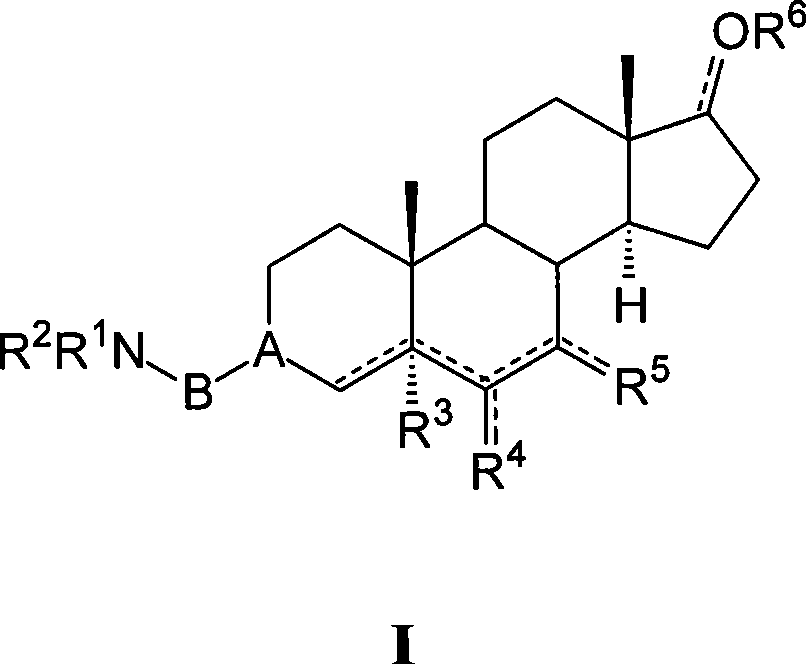
Azaheterocyclyl derivatives of androstanes and androstenes as medicaments for cardiovascular disorders
InactiveCN101466726AImprove efficacyGood cure rateOrganic active ingredientsSteroidsATPaseEndogenous ouabain
Compounds of formula (I) wherein: the groups are as defined in the description, are useful for the preparation of medicaments for the treatment of cardiovascular disorders, in particular heart failure and hypertension. The compounds are inhibitors of the enzymatic activity of the Na+,K+-ATPase. They are useful for the preparation of a medicament for the treatment of a disease caused by the hypertensive effects of endogenous ouabain, such as renal failure progression in autosomal dominant polycystic renal disease (ADPKD), preeclamptic hypertension and proteinuria and renal failure progression in patients with adducin polymorphisms.
Owner:CVIE THERAPEUTICS LTD
Method of treating polycystic kidney diseases with ceramide derivatives
InactiveUS20150216872A1Reduce needSymptoms improvedBiocideUrinary disorderPharmaceutical medicineBiological drugs
A method of treating polycystic kidney disease in a subject comprises administering to the subject an effective amount of a compound represented by Structural Formula (1):or a pharmaceutically acceptable salt thereof.
Owner:GENZYME CORP
Compounds, compositions and methods comprising pyridazine sulfonamide derivatives
The present invention relates to compositions and methods for treating a disease in an animal, which disease is responsive to inhibiting of functional cystic fibrosis transmembrane conductance regulator (CFTR) polypeptide by administering to a mammal in need thereof an effective amount of a compound defined herein (including those compounds set forth in Tables 1-3 or encompassed by formula I-III) or compositions thereof, thereby treating the disease. The present invention particularly, relates to a method of treating diarrhea and polycystic kidney disease.
Owner:INST FOR ONEWORLD HEALTH
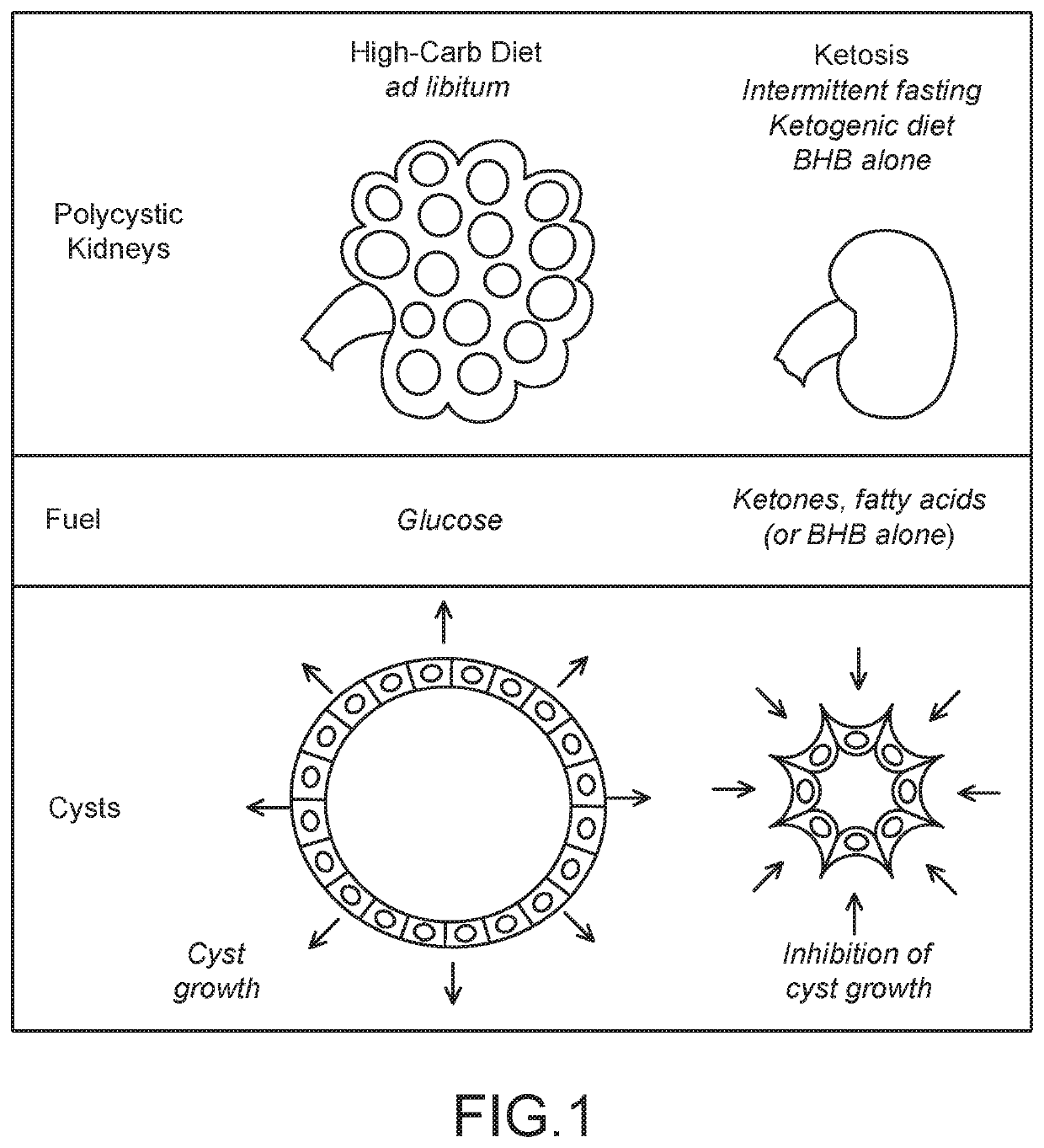
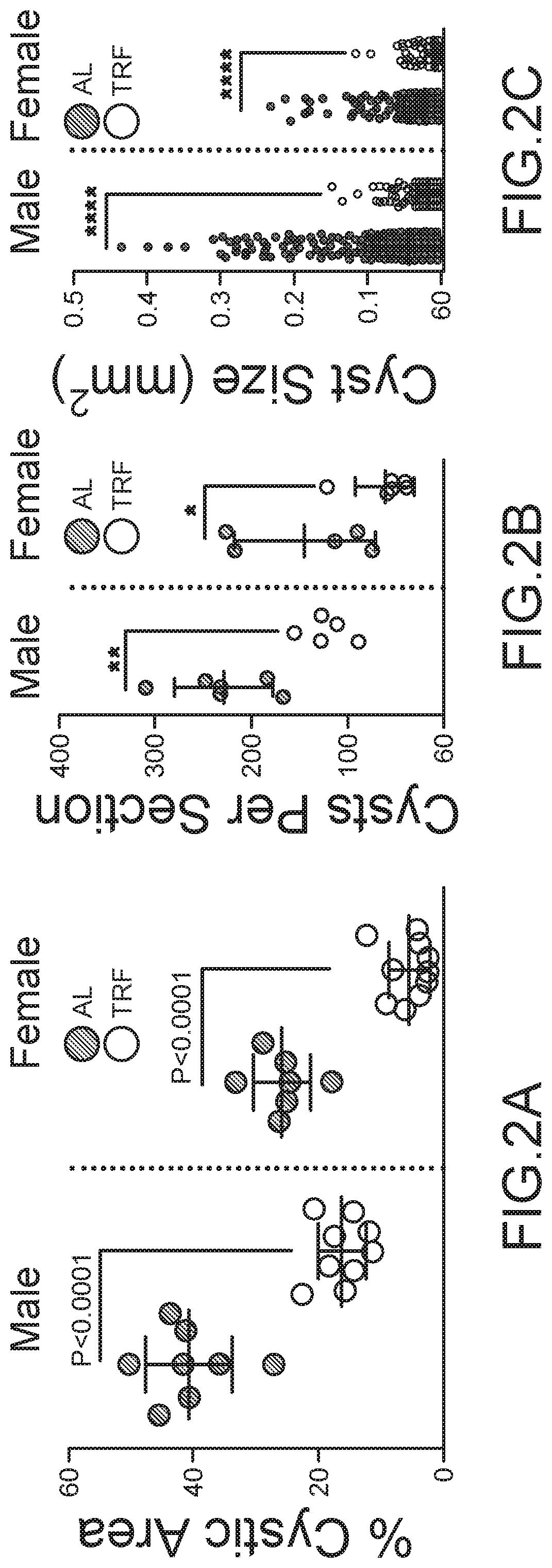
Methods and Compositions for Supporting Renal Health
ActiveUS20200289444A1Prevent and ameliorate severityAmeliorate riskHydroxy compound active ingredientsUrinary disorderCystic kidneyNephrosis
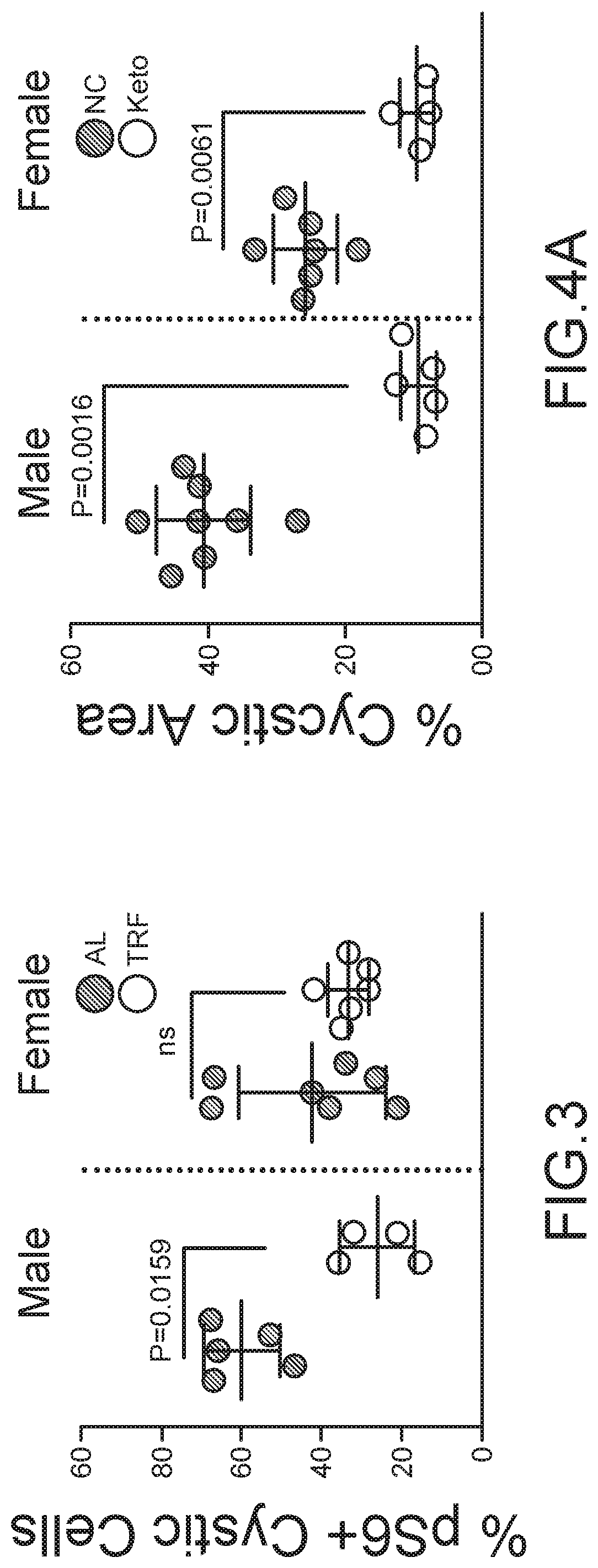
Compositions and methods for supporting health, especially renal health, comprising ketonic agents that recapitulate beneficial effects of ketosis by exogenously administered agents. The agents include BHB, analogs thereof, and GPR109A agonists. The agents may further include crystal precipitation inhibitors which synergistically improve treatment of certain renal conditions. The agents may be used in dietary supplements and therapeutic compositions for the treatment of cystic kidney diseases such as polycystic kidney disease, ciliopathies, and other conditions.
Owner:RGT UNIV OF CALIFORNIA +1
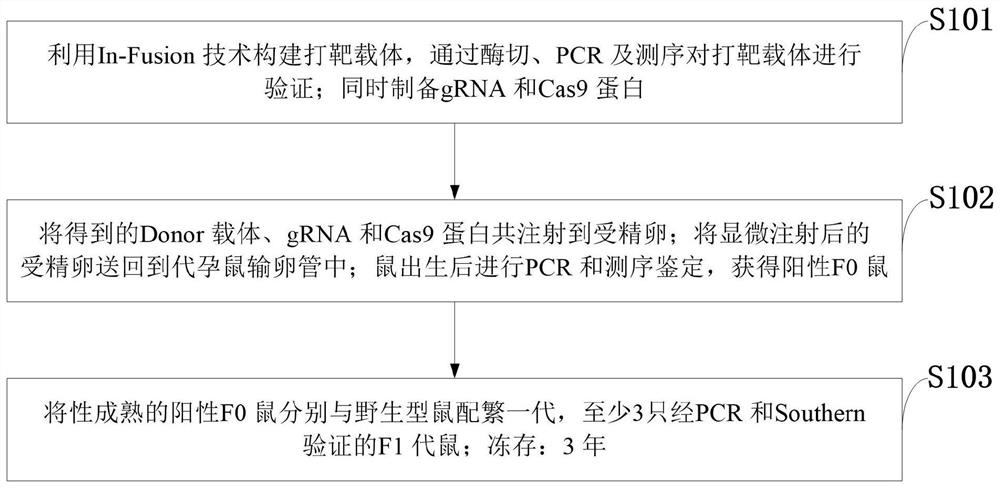
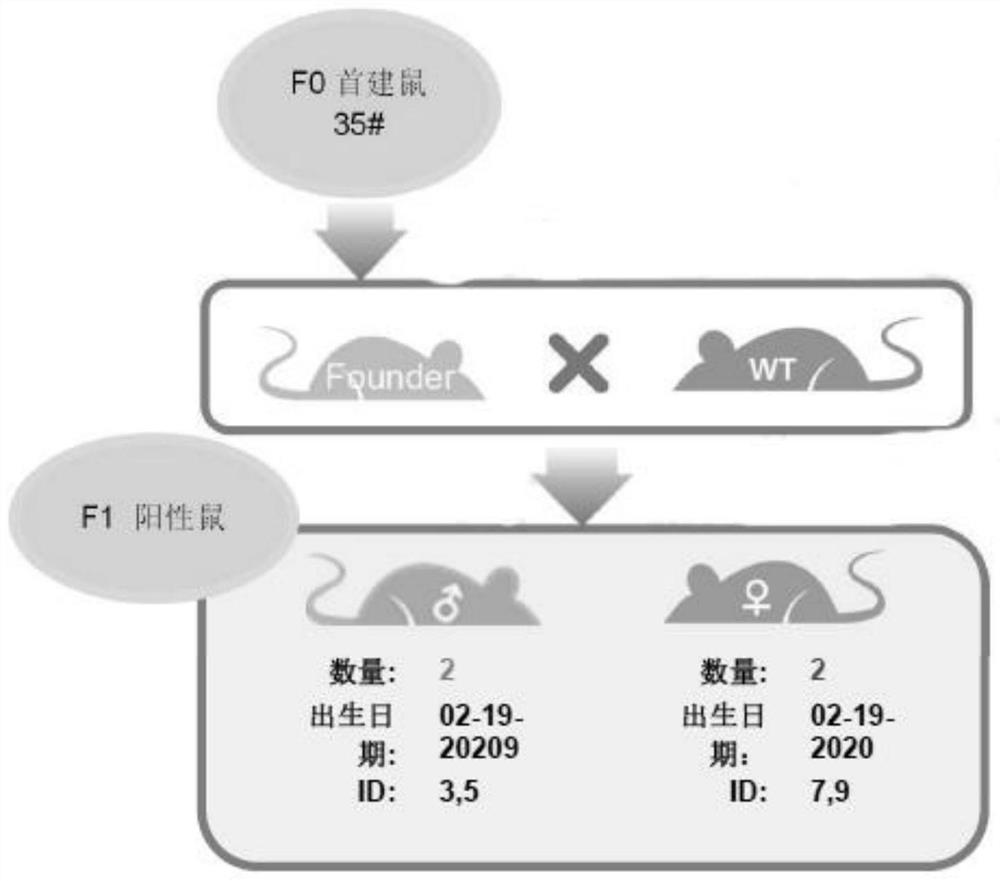
CRISPR Cas9 conditional gene knockout mouse and establishment method
PendingCN111961685AImprove knockout effectMicroinjection basedNucleic acid vectorKnockout animalSexual maturity
The invention belongs to the technical field of gene knockout, and discloses a CRISPR Cas9 conditional gene knockout mouse and an establishment method. According to the establishment method, a vectoris designed and constructed, and gRNA and Cas9 protein are prepared; a gRNA sequence and a targeting vector are designed; the targeting vector is constructed by the In-Fusion technology, and the targeting vector is verified by restriction digestion, PCR and sequencing; the gRNA and Cas9 protein are prepared at the same time; microinjection and F0 mouse identification are performed; the obtained Donor vector, gRNA and Cas9 protein are co-injected into a fertilized egg; the fertilized egg after microinjection is returned to the oviduct of a surrogate mouse; PCR and sequencing identification areperformed after a mouse is born, and a positive F0 mouse is obtained; the reproduction and identification of a F1 generation mouse are performed; the sexually mature positive F0 mice are matched withwild-type mice respectively for reproducing the first generation, and a polycystic kidney gene PKD1 knockout mouse is obtained successfully.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Reverse-turn mimetics and method relating thereto
InactiveUS20100029630A1Efficient implementationBiocideOrganic chemistryUlcerative colitisNodular sclerosis
Conformationally constrained compounds that mimic the secondary structure of reverse-turn regions of biologically active peptides and proteins are disclosed. Such reverse-turn mimetic structures have utility over a wide range of fields, including use as diagnostic and therapeutic agents. Libraries containing the reverse-turn mimetic structures of this invention are also disclosed as well as methods for screening the same to identify biologically active members. The invention also relates to the use of such compounds for inhibiting or treating disorders modulated by Wnt-signaling pathway, such as cancer, especially colorectal cancer, restenosis associated with angioplasty, polycystic kidney disease, aberrant angiogenesis disease, rheumatoid arthritis disease, tuberous sclerosis complex, Alzheimer's disease, excess hair growth or loss, or ulcerative colitis.
Owner:JW PHARMA CORP
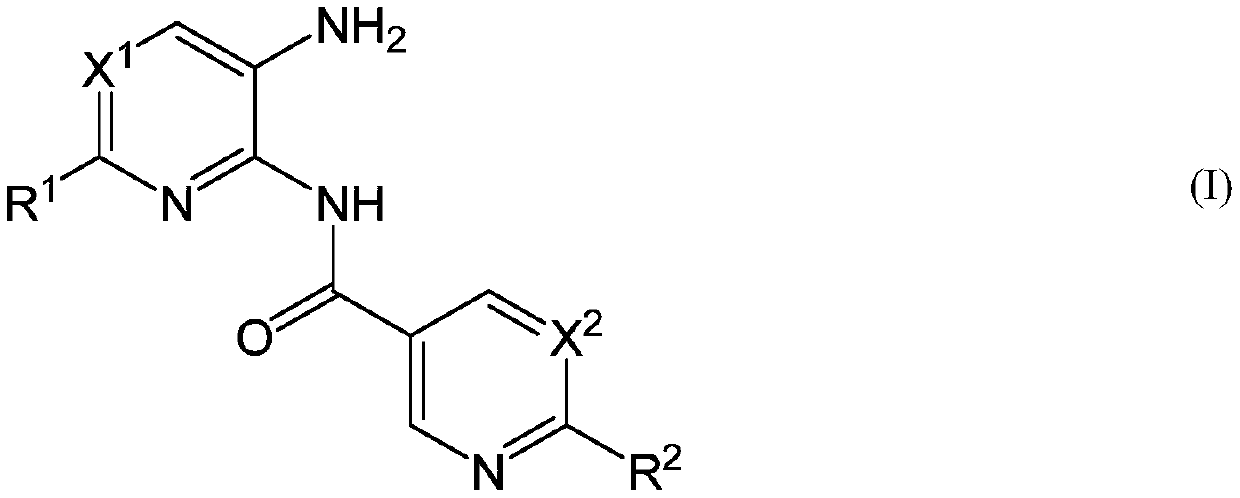
New heteroaryl amide derivatives as selective inhibitors of histone deacetylases 1 and 2 (hdac1-2)
The present invention relates to novel heteroaryl amide derivatives of formula (I), as selective inhibitors of histone deacetylase 1 and 2 (hdac1-2), to methods for the production of same, to pharmaceutical compositions comprising these compounds, and to the use of said compounds for the production of a medicament for the treatment of pathological conditions or diseases that can be improved by means of the inhibition of the activity of histone deacetylase class I, particularly HDAC1 and HDAC2, such as cancer, neurodegenerative diseases, infectious diseases, inflammatory diseases, heart failureand cardiac hypertrophy, diabetes, polycystic kidney disease, sickle cell disease and beta-thalassemia disease, and to methods for the treatment of the diseases mentioned above.
Owner:MEDIBIOFARMA SL
Non-oxidized, biological active parathyroid hormone determines mortality in hemodialysis patients
ActiveUS20160025748A1Reliable informationDisease diagnosisBiological testingParathyroid glandHormones regulation
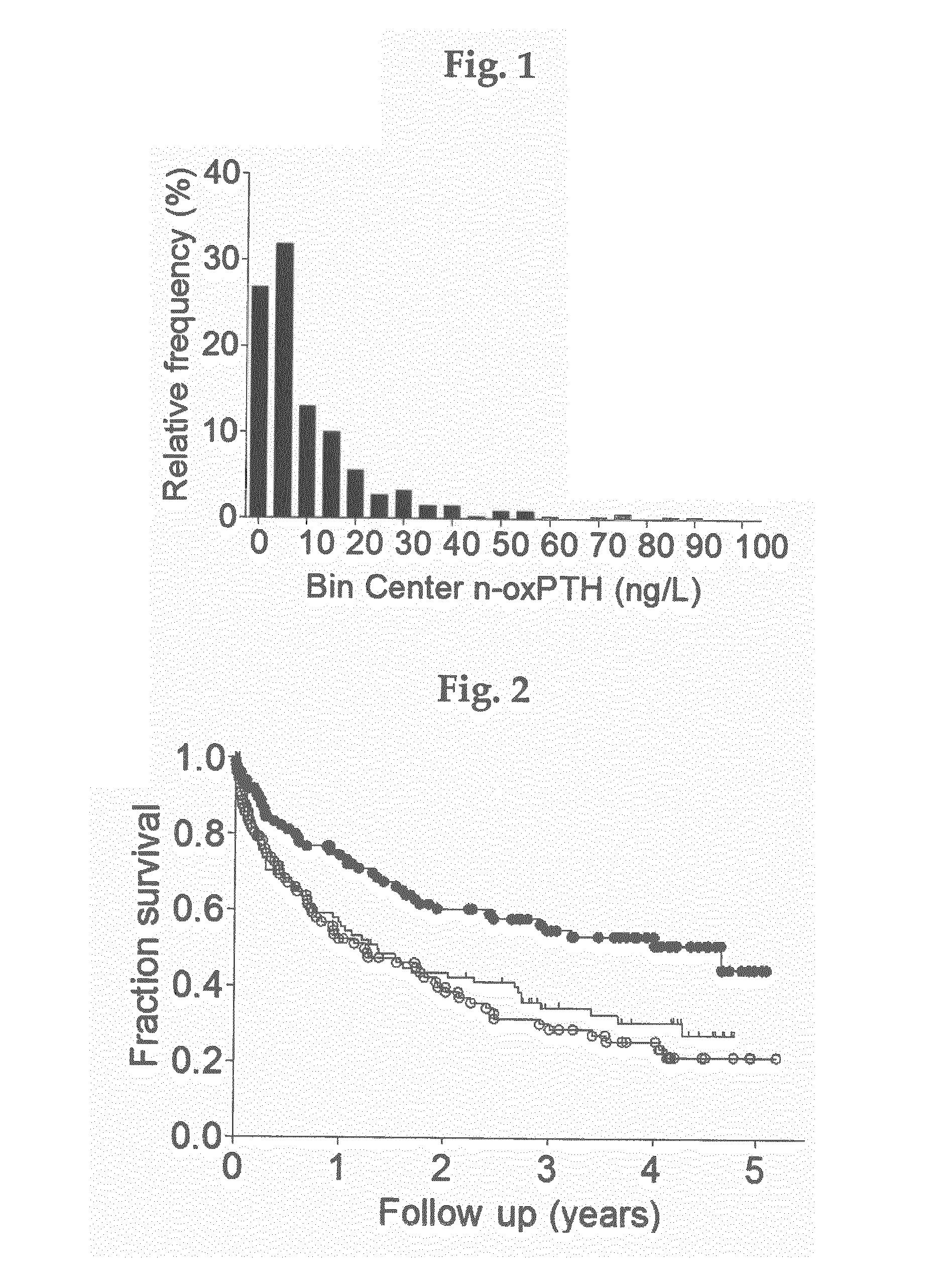
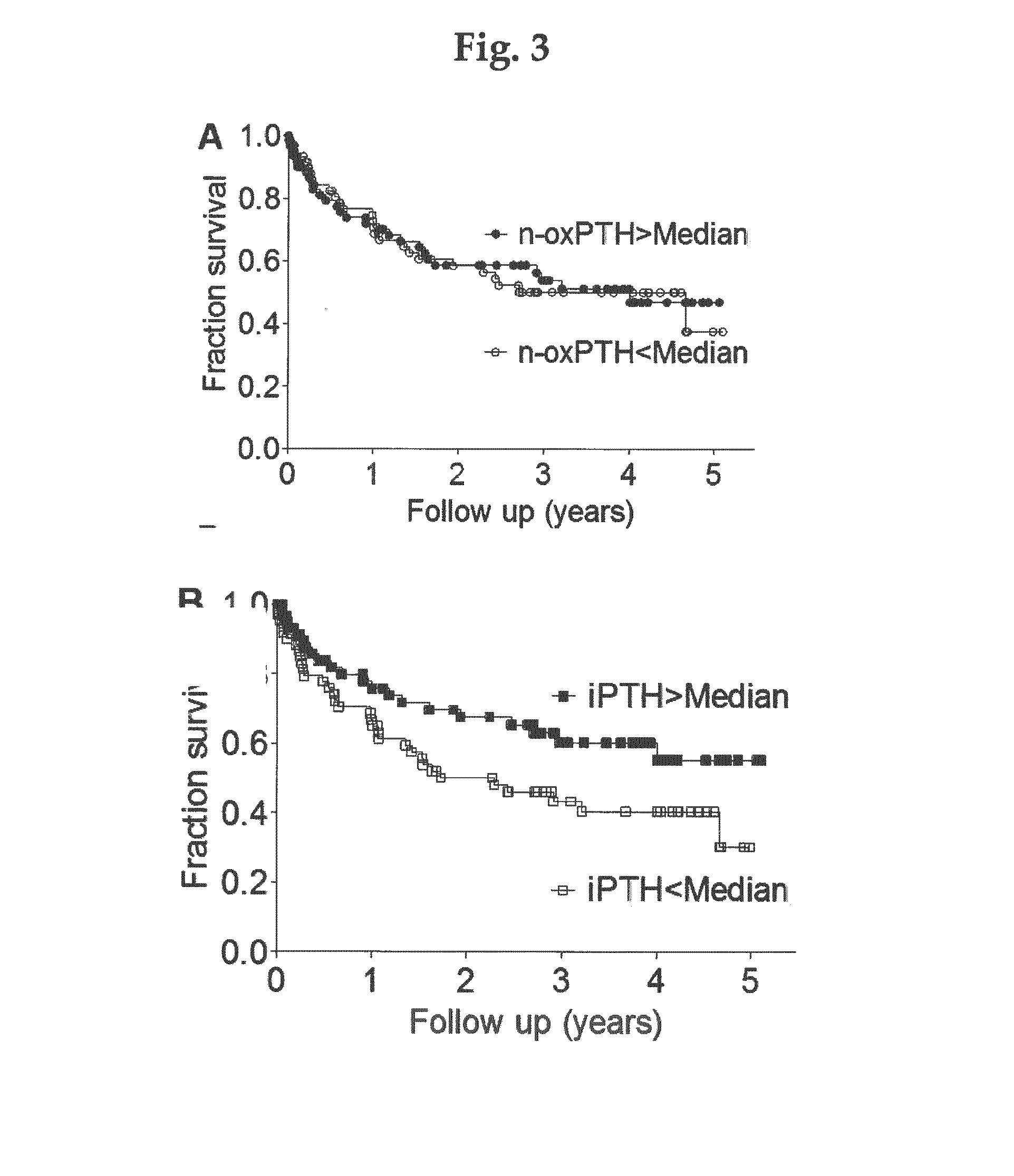
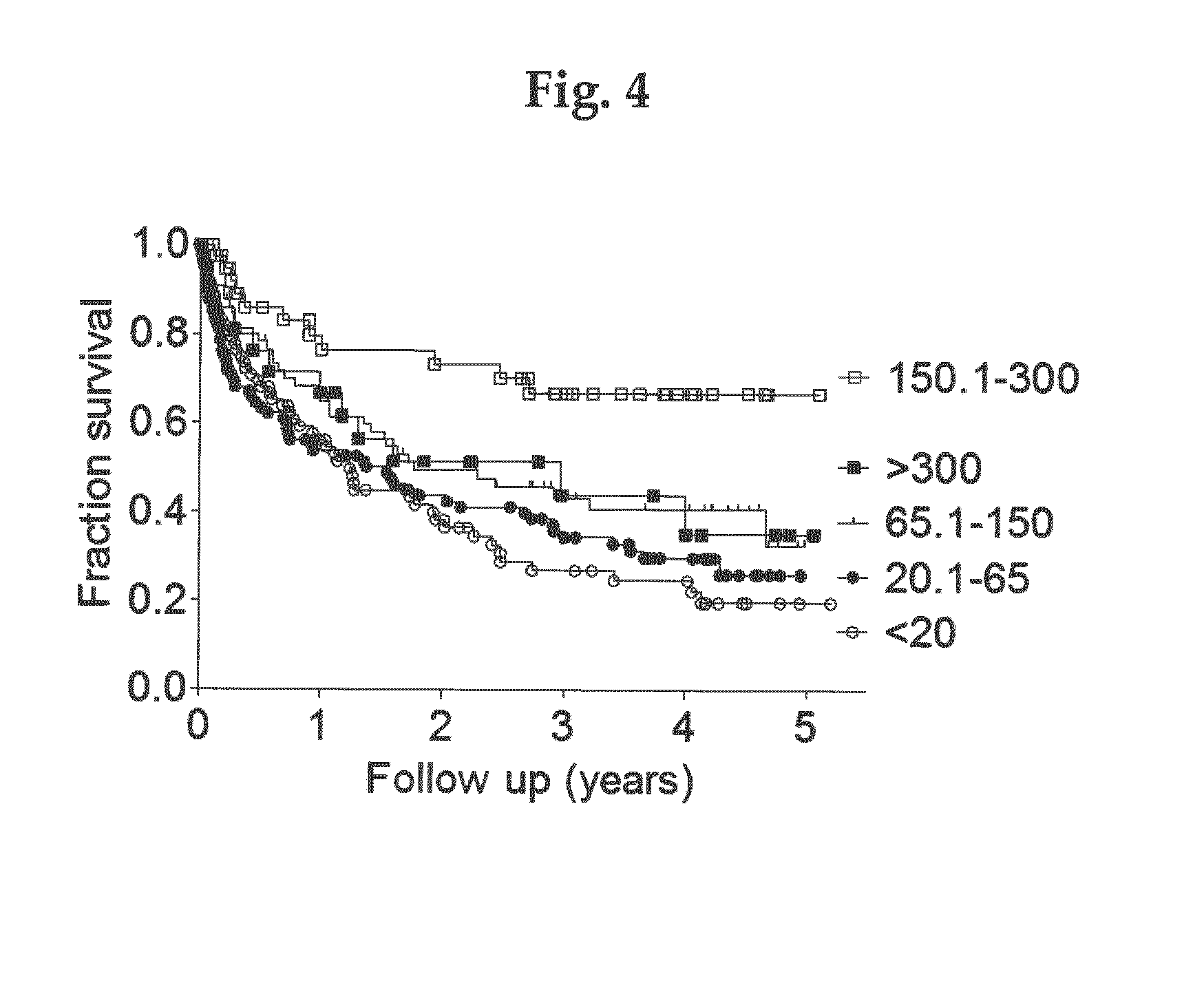
A new method of in vitro monitoring and assessing the need of a medication which interferes with the regulation of the parathyroid hormone level in a kidney patient subject to oxidative stress, notably hemodialysis patients. FIG. 1 shows the distribution of n-oxPTH concentrations in 340 hemodialysis patients (224 men and 116 women) with a median age of 66 years (IQR, 56 to 75 years), a median time since initiation of dialysis (dialysis vintage) of 266 days (IQR, 31 to 1209 days), and a median dialysis dose (kt / V) of 1.2 (IQR, 1.1 to 1.3). The cause of chronic kidney disease was nephrosclerosis in 113 cases (33%), diabetic nephropathy in 107 cases (31%), chronic glomerular nephritis in 29 cases (9%), polycystic kidney disease in 9 cases (3%) and other / unknown in 82 cases (24%). The median n-oxPTH concentration was 5.9 ng / L (IQR, 2.4 to 14.0 ng / L). n-oxPTH concentrations were not different in men and women (5.9 ng / L; IQR, 2.4 to 14.2 ng / L; n=224; vs. 5.5 ng / L; IQR, 2.4 to 14.0 ng / L; n=1 16; p=0.915).
Owner:IMMUNDIAGNOSTIK
Pyrimido-pyrrolo-quinoxalinedione inhibitors of cystic fibrosis transmembrane conductance regulator protein and uses therefor
Provided herein are pyrimido-pyrrolo-quinoxalinedione (PPQ) compounds, and compositions comprising these compounds, that inhibit cystic fibrosis transmembrane conductance regulator (CFTR) mediated ion transport and that are useful for treating diseases and disorders associated with aberrantly increased CFTR chloride channel activity. The compounds, and compositions comprising the compounds, described herein are useful for treating diseases, disorders, and sequelae of diseases, disorders, and conditions that are associated with aberrantly increased CFTR activity, for example, polycystic kidney disease. The compounds may be used for inhibiting expansion or preventing formation of cysts in persons who have polycystic kidney disease.
Owner:RGT UNIV OF CALIFORNIA
Amino derivatives of androstanes and androstenes as medicaments for cardiovascular disorders
Compounds of formula (I) wherein: the groups are as defined in the description, are useful for the preparation of medicaments for the treatment of cardiovascular disorders, in particular heart failure and hypertension. The compounds are inhibitors of the enzymatic activity of the Na<+>,K<+>-ATPase. Said compounds are used for the preparation of a medicament for the treatment of a disease caused by the hypertensive effects of endogenous ouabain, such as renal failure progression in autosomal dominant polycystic renal disease (ADPKD), preeclamptic hypertension and proteinuria and renal failure progression in patients with adducin polymorphisms.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
New uses of Shh signaling pathway inhibitor
InactiveCN106138050AReduce the degree of renal fibrosisReduce the degree of renal cystOrganic active ingredientsUrinary disorderCreatinine riseIsrapafant
The present invention discloses new uses of a Shh signaling pathway inhibitor. The new uses of the Shh signaling pathway inhibitor specifically comprises applications of the Shh signaling pathway inhibitor in preparation of autosomal domi-nant polycystic kidney disease (ADPKD) treatment product, wherein the Shh signaling pathway inhibitor is specifically Vismodegib. According to the present invention, a Vil-Cre:Pkd2f3 / f3 mouse as an ADPKD animal model, and the results show that with the Shh signaling pathway inhibitor Vismodegib, the renal cyst degree of the Vil-Cre:Pkd2f3 / f3 mouse can be reduced, the kidney weight ration of the Vil-Cre:Pkd2f3 / f3 mouse can be reduced, the contents of creatinine and urea nitrogen in the blood of the Vil-Cre:Pkd2f3 / f3 mouse can be reduced, and the renal fibrosis degree of the Vil-Cre:Pkd2f3 / f3 mouse can be reduced; and the important significance is provided for the analysis of the ADPKD pathogenesis and the research and development of the ADPKD treatment drug.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Medicine composition for treating polycystic kidney disease and use thereof
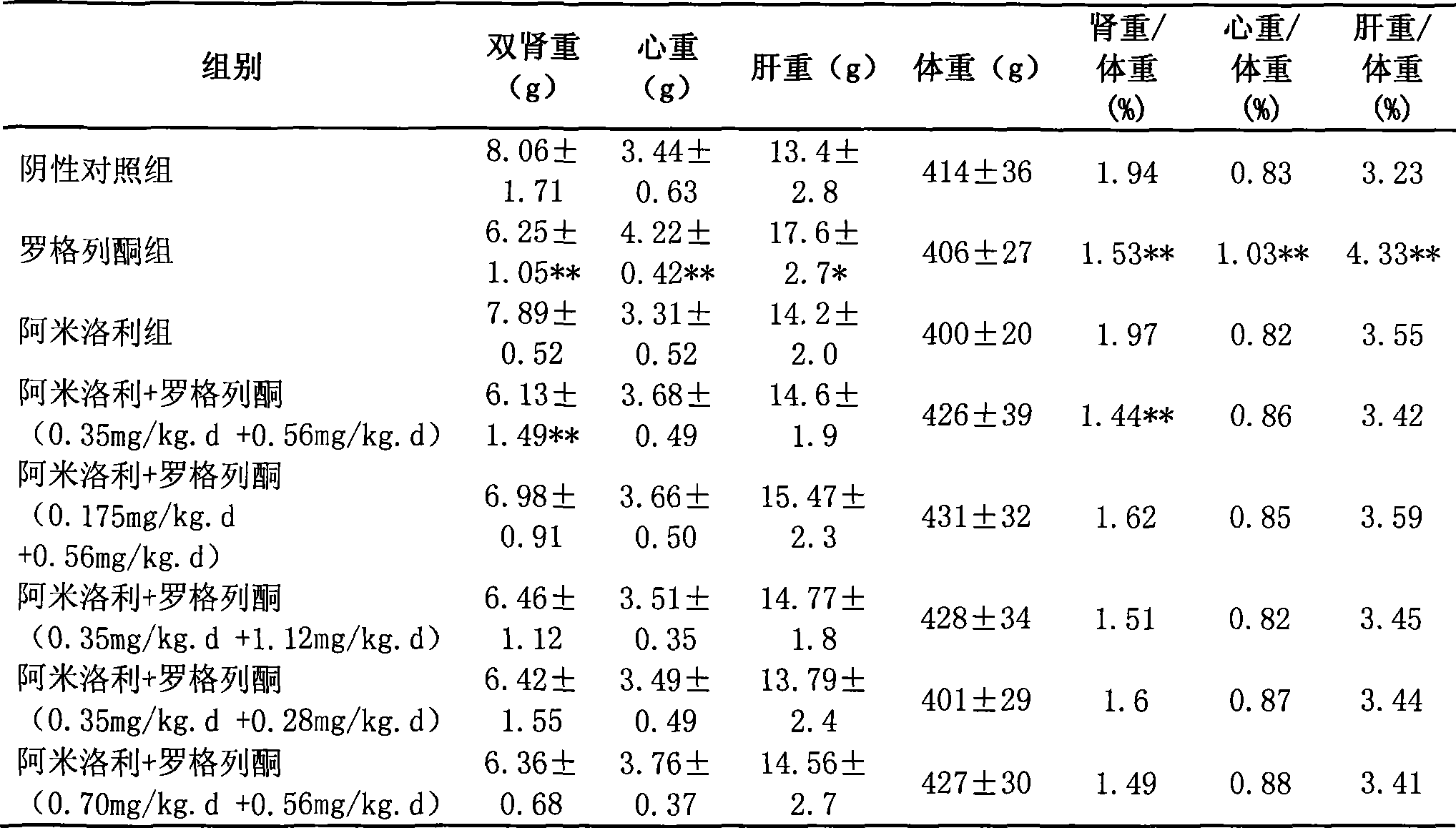
The invention relates to a pharmaceutical composition for treating polycystic kidney disease. The active ingredients of the pharmaceutical composition comprise effective doses of thiazolidinediones and diuretics. The preferential combination is as follows: the thiazolidinediones are selected from rosiglitazone and the diuretics are selected from amiloride hydrochloride. The animal experiments prove that the efficacy of the composition of the rosiglitazone and the diuretics on the treatment of the polycystic kidney disease is better than the single use of one drug. The effect of the pharmaceutical composition for treating the polycystic kidney disease is better than the single use of one drug, thereby being a drug for treating the polycystic kidney disease and being worth expecting.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
3-cyanoquinolines, 3-cyano-1,6-naphthyridines, and 3-cyano-1,7-naphthyridines as protein kinase inhibitors
InactiveCN100439340COrganic active ingredientsOrganic chemistryPTK InhibitorsTyrosine-kinase inhibitor
Compounds of structure formula (I) or a pharmaceutically salt thereof are useful as antineoplastic agents and in the treatment of osteoporosis and polycystic kidney disease.
Owner:WYETH LLC
Methods for treating polycystic kidney disease and polycystic liver disease
ActiveUS20150105361A1Reduces and avoids symptom and causeGood curative effectOrganic active ingredientsDigestive systemPhysiologyCyst
The present invention provides compounds of Formula (I) or (II), which are thought to be able to inhibit mTOR (mammalian target of rapamycin) signaling pathway, induce UPR (unfolded protein response), and / or perturb mitochondrial function of a cyst cell (e.g., a cyst cell causing polycystic kidney disease (PKD, e.g., autosomal dominant PKD (ADPKD) or autosomal recessive PKD (ARPKD)) or polycystic liver disease (PLD, e.g., autosomal dominant PLD (ADPLD) or autosomal recessive PLD (ARPLD)). The invention also provides pharmaceutical compositions, kits, and methods involving the compounds described herein for use in treating PKD or PLD, inhibiting the growth of a cyst cell, and / or killing a cyst cell.
Owner:YALE UNIV +1
Reagent composition and detection method for detecting autosomal dominant polycystic kidney disease
PendingCN114480603ASolve puzzlesThe experimental method is simpleMicrobiological testing/measurementGeneticsGenomic DNA
The reagent composition for detecting the autosomal dominant polycystic kidney disease comprises a primer group, a first reagent for extracting genomic DNA from a sample; the second reagent is used for carrying out long fragment PCR reaction by using the primer group; a third agent for processing the amplification product such that the amplification product can be used in a high-throughput sequencing technique; and the fourth reagent is used for performing high-throughput sequencing on the treated amplification product. Comprising the following steps: S1, amplifying a PKD1 gene by using a reagent composition according to any one of claims 1-7 through long-fragment PCR (Polymerase Chain Reaction); s2, performing high-throughput sequencing on the amplified sequence obtained in the step (1); s3, comparing the sequencing result obtained in the step (2) with a PKD1 gene reference sequence, and determining a mutation site; s4, eliminating false gene locus interference through bioinformatics analysis, and determining a PKD1 true gene mutation locus; and S5, comparing the true gene mutation site determined in the step S4 with a known PKD1 gene mutation site, and determining a new mutation site on the PKD1 gene.
Owner:广州源古纪科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com