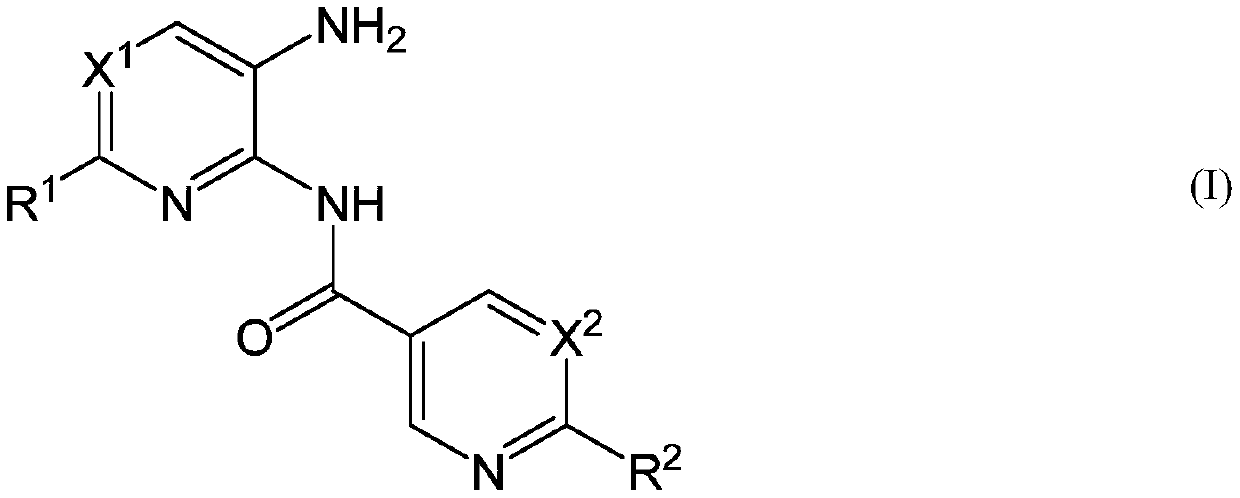
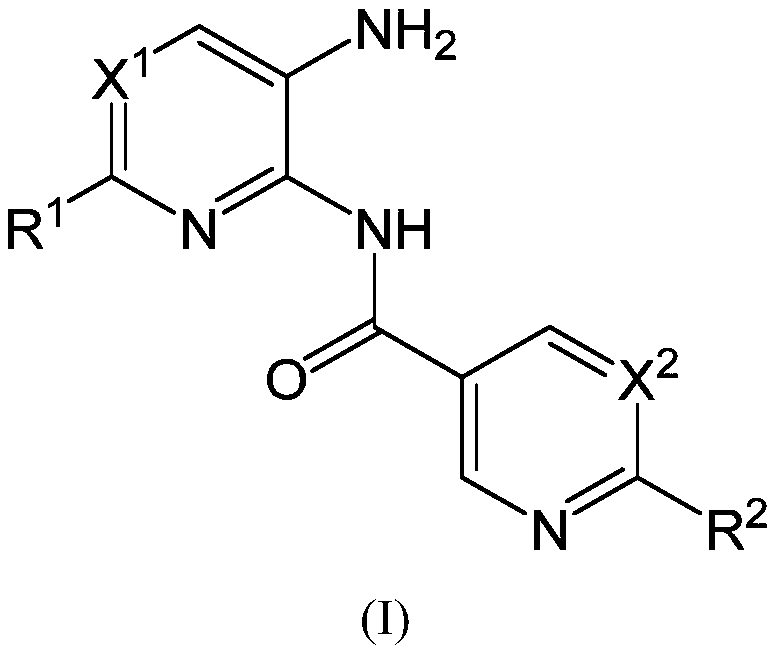
New heteroaryl amide derivatives as selective inhibitors of histone deacetylases 1 and 2 (hdac1-2)
A kind of heteroaryl, C1-C3 technology, applied in the field of new heteroaryl amide derivatives, can solve the problem of not showing HDAC isozyme selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] To a solution of Intermediate 5 (310 mg) in ethanol (20 ml) and ethyl acetate (35 ml) was added Pd / C (10%) (46 mg, 15% (w / w)) and the reaction was stirred under hydrogen overnight. After completion of the reaction as monitored by TLC, the reaction mixture was filtered through celite and evaporated to a residue. The residue was purified by preparative HPLC to obtain Example 1 as an off-white solid (20 mg, 10% yield).
[0226] 1 H-NMR (400MHz, DMSO-d 6 ):δ=10.25(br,s,1H),8.80(d,J=4.4Hz,1H),8.15(d,J=11.6Hz,1H),7.955(d,J=7.2Hz,2H),7.68 (d, J=8.0Hz, 1H), 7.42(t, J=7.6Hz, 2H), 7.31(m, 2H), 6.92(d, J=9.2Hz, 1H), 5.14(br,s, 2H) , 3.65 (t, J = 4.8Hz, 4H), 2.40 (t, J = 4.8Hz, 4H), 2.22 (s, 3H).
[0227] HPLC-MS: Rt 11.120m / z 389.6 (MH + ).
[0228] The following examples were synthesized using the procedure described in Scheme 6, starting from the corresponding pyridin-2-amine and nicotinic acid derivatives.
Embodiment 2
[0229] Example 2: N-(3-amino-6-phenylpyridin-2-yl)nicotinamide
[0230] 1 H-NMR (400MHz, DMSO-d 6 ):δ=10.60(s,1H),9.18(s,1H),8.77(dd,J=6.0,1.2Hz,1H),8.37(d,J=8.0Hz,1H),7.94(d,J= 7.6Hz, 2H), 7.71(d, J=8.4Hz, 1H), 7.58(m, 1H), 7.42(t, J=7.6Hz, H), 7.31(m, 2H), 5.29(br s, 2H ).
[0231] HPLC-MS: Rt 9.891m / z 291.0 (MH + ).
Embodiment 3
[0232] Example 3: N-(3-amino-6-(4-fluorophenyl)pyridin-2-yl)nicotinamide
[0233] 1 H-NMR (400MHz, DMSO-d 6)δ=10.59(s,1H),9.17(d,J=2.0Hz,1H),8.77(dd,J=6.8,1.6Hz,1H),8.37(m,1H),7.98(m,2H), 7.69 (d, J=8.4Hz, 1H), 7.58 (m, 1H), 7.26 (m, 3H), 5.29 (br, s, 2H).
[0234] HPLC-MS: Rt 10.590m / z 309.0 (MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com